Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended March 30, 2012
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 0-23832

PSS WORLD MEDICAL, INC.
(Exact name of Registrant as specified in its charter)
| FLORIDA | 59-2280364 | |
| (State of incorporation) | (I.R.S. Employer Identification No.) | |
| 4345 Southpoint Boulevard Jacksonville, Florida | 32216 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code (904) 332-3000
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of Exchange on which registered | |||
| Common Stock, $0.01 par value per share | NASDAQ GS Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ¨ No x
The aggregate market value of common stock held by non-affiliates, computed by reference to the closing price as reported on the NASDAQ GS, as of September 30, 2011 was approximately $999,962,086.
The number of shares of Common Stock, $0.01 par value, of the Registrant outstanding as of May 18, 2012, was 50,427,436.
Table of Contents
Document Incorporated by Reference
Portions of the Registrant’s Definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission not later than 120 days after March 30, 2012, are incorporated by reference into Part III of this Annual Report on Form 10-K.
Table of Contents
Item | Page | |||||
| 1 | ||||||
| 1. | 3 | |||||
| 1A. | 12 | |||||
| 1B. | 23 | |||||
| 2. | 23 | |||||
| 3. | 25 | |||||
| 4. | 25 | |||||
| 5. | 25 | |||||
| 6. | 28 | |||||
| 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 29 | ||||
| 7A. | 57 | |||||
| 8. | F-1 | |||||
| 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 59 | ||||
| 9A. | 59 | |||||
| 9B. | 61 | |||||
| 10. | 62 | |||||
| 11. | 62 | |||||
| 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 62 | ||||
| 13. | Certain Relationships and Related Transactions, and Director Independence | 62 | ||||
| 14. | 62 | |||||
| 15. | 63 | |||||
| 68 | ||||||
Table of Contents
Forward-Looking Statements
Management may from time-to-time make written or oral forward-looking statements with respect to the Company’s annual or long-term goals, including statements contained in this Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, reports to shareholders, press releases, and other communications. These statements are subject to risks and uncertainties that could cause actual results to differ materially from historical earnings and those currently anticipated or projected. Management cautions readers not to place undue reliance on any of the Company’s forward-looking statements, which speak only as of the date made.
Words such as “anticipates,” “expects,” “intends,” “plans,” “purpose,” “mission,” “believes,” “seeks,” “estimates,” “may,” “could,” and similar expressions identify forward-looking statements. Forward-looking statements contained in this Annual Report on Form 10-K involve risks and uncertainties include, but may not be limited to:
| • | Management’s belief that the Company’s strategic restructuring will positively impact its financial results and better position the Company to participate among the fastest growing of the alternate-site healthcare market segments; |
| • | Management’s belief that the healthcare services industry will continue to be subject to extensive regulation at the federal, state, local and foreign levels and that enforcement activities will continue to increase; |
| • | Management’s belief that consolidation in the healthcare industry will continue among provider groups, long-term care facilities, and other alternate site providers, as well as health systems created through alliances between hospitals and long-term care facilities or physician practices; |
| • | Management’s belief that the outcome of legal proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations; |
| • | Management’s expectation that the remaining federal and state net operating loss carryforwards will be utilized prior to their expiration date, that the Company’s deferred tax assets as of March 30, 2012 will be realizable to reverse deferred tax liabilities and offset a portion of projected future taxable income, and that the Company’s unrecognized tax benefits will not change significantly over the next twelve months; |
| • | Management’s expectation to make strategic business acquisitions to increase market share and leverage distribution capabilities; |
| • | Management’s expectation that future working capital needs, capital expenditures, and the overall growth in the business will be funded through a combination of cash flows from operating activities, borrowings under the revolving line of credit, cash proceeds from the sale of the businesses outlined in the Company’s strategic restructuring plan, proceeds from the Company’s debt offerings, capital markets, and/or other financing arrangements; |
| • | Management’s belief that the Company’s global sourcing initiative will continue to impact net operating working capital levels and will continue to positively impact the Company’s results of operations; |
| • | Management’s expectation that the reorganization of the Company’s global sourcing subsidiaries will have a sustained positive impact on the Company’s effective tax rate; |
| • | Management’s intention to permanently reinvest undistributed earnings attributable to foreign operations; |
| • | Management’s intention to retain earnings for the growth and development of the Company’s business and not declare cash dividends in the immediate future; |
| • | Management’s belief that the Company may seek to retire a portion of its outstanding equity through cash purchases and may also seek to issue additional equity to meet its future liquidity requirements; |
1
Table of Contents
| • | Management’s belief that the medical products distribution industry is expected to experience continued growth due to the aging U.S. population, increased healthcare awareness, the introduction of new medical technology, the development of new pharmacology treatments, and recently enacted health care reform legislation; |
| • | Management’s belief that the extended care market is expected to benefit from the increasing growth rate of the U.S. elderly population, the increased incidence of chronic conditions, the growing acceptance of and emphasis on lower-cost alternate site treatment and the increased treatment of higher acuity patients in nursing homes; |
| • | Management’s belief that the physician market is expected to grow, due, in part, to the shift of procedures and diagnostic testing away from acute care settings to lower cost settings, such as the physician’s office, and from increased consumption of healthcare products and services, resulting from an aging U.S. population and the expected increase in utilization from the implementation of health care reform in 2014, offset by slower growth in the near term due to economic conditions and unemployment rates; |
| • | Management’s intent to either renegotiate existing leases or execute new leases upon the expiration date of such agreements, except for those that may be exited through the Company’s restructuring plan; |
| • | Management’s estimation and expectation of future payouts of long-term incentive compensation; |
| • | Management’s expectation that near term sales growth rates, excluding acquisitions, will remain lower than historical growth rates, due to economic conditions and lower patient utilization; |
| • | Management’s expectation that government funded programs will become more prevalent and that, as a result, reimbursements may become more reliant on federal and state budgets; and |
| • | Management’s belief that the Company’s distribution infrastructure is adequate to carry on its business as currently conducted and that, if necessary, the Company could find additional and/or replacement facilities to lease without suffering a material adverse effect on its business. |
In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, management has identified important factors that could affect the Company’s financial performance and could cause actual results for future periods to differ materially from those expressed in any forward-looking statements, including the risks described or referred to in Item 1A.Risk Factors.
The Company cautions readers not to place undue reliance on any of these forward-looking statements, which speak only as of the date made. The Company undertakes no duty and is under no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Third-Party Statistical Data
This report contains estimates and other information concerning the Company’s industry, including market size and growth rates, which are based on industry publications, analyst reports, surveys and forecasts. These estimates involve a number of assumptions and limitations, and you are cautioned not to give undue weight to these estimates. Although the Company believes the information in these industry publications, surveys and forecasts is reliable, it has not been independently verified and therefore the accuracy or completeness of the information cannot be guaranteed.
2
Table of Contents
| ITEM 1. | BUSINESS |
THE COMPANY
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation, began operations in 1983. The Company is a national distributor of medical products and supplies, diagnostic equipment, healthcare information technology and pharmaceutical products, and provides professional and consulting services to the physician, long-term care, assisted living, home health care, and hospice markets. The Company has full service distribution centers strategically located to efficiently serve all 50 states throughout the United States.
The Company’s business decisions and strategies are guided by its Purpose and Mission. The Company’s stated Purpose is to strengthen the clinical success and financial health of caregivers by solving their biggest problems. The Company’s Mission is to improve caregivers’ financial performance by 20%.
The Company currently conducts business through two operating segments, the Physician Business and the Extended Care Business, which serve a diverse customer base. During the year ended March 30, 2012, the Company rebranded its Elder Care Business to the “Extended Care Business” to more appropriately align with its customer base. A third reporting segment, Shared Services, consists of departments that support the operating segments through the delivery of standardized service. For information on comparative segment revenue, segment profit and related financial information, refer to Footnote 18,Segment Information, of the consolidated financial statements.
PSSI is a market leader in the two alternate-site customer segments it serves as a result of a high-touch, differentiated business model; value-added, solutions-based marketing programs; a consultative sales force with extensive product, disease state, reimbursement, and supply chain knowledge; a successfully expanded product and service offering including unique arrangements with manufacturers and a full line of the Company’s store brands; innovative information systems and customer-facing technologies that serve its core markets; and a culture of performance.
During the first quarter of fiscal year 2013, the Company’s Board of Directors approved a strategic restructuring plan (“the restructuring plan”), designed to transform the Company by focusing on four lines of business – Physician, Laboratory, In-Office Dispensing, and Home Care and Hospice. The restructuring plan will include the sale of two business units serving skilled nursing facilities within the Extended Care Business and specialty dental practices within the Physician Business. Additionally, the restructuring plan includes the integration of warehouse operations into one common distribution infrastructure, as well as a redesign of the shared services function. These efforts are expected to reduce operating costs as a percentage of net sales, while streamlining decision making and improving service. The Company expects to complete the restructuring plan within the next several fiscal years. Except as otherwise noted, the information contained in this Form 10-K reflects Company information as of March 30, 2012, before the announcement and commencement of the strategic restructuring plan.
THE INDUSTRY
The Company’s business strategies focus on the alternate site healthcare provider market, which is comprised of the estimated $12 billion physician office market, the $4 billion long-term care and assisted living market, and the $11 billion home health and hospice markets, in each case as estimated in industry analyst reports. The Company targets approximately 212,000 sites in the physician office market and 40,000 sites in the extended care market.
The medical products distribution industry is expected to experience continued growth due to the aging U.S. population, increased healthcare awareness, the introduction of new medical technology, new pharmaceutical treatments, and recently enacted health care reform legislation, which includes expanded insurance coverage. The physician market is expected to grow, due, in part, to the shift of procedures and diagnostic testing away from acute care settings to lower cost settings, such as the physician’s office, and from increased consumption of healthcare products and services, resulting from an aging U.S. population and the expected increase in utilization from the
3
Table of Contents
implementation of health care reform legislation. This growth may be slowed somewhat in the near term due to general economic conditions. The extended care markets are also expected to benefit from the increasing growth rate of the U.S. elderly population, the increased incidence of chronic conditions, the growing acceptance of and emphasis on lower-cost alternate site treatment and the increased treatment of higher acuity patients in nursing homes. As of August 2008, the U.S. Bureau of the Census report estimated the U.S. elderly population will more than double by 2050, with Americans age 85 years and older, the population in the greatest need of extended care services, projected to more than triple during this period.
According to industry analyst reports, the physician office end market is expected to grow at a long-term rate of 5% to 7%, the long-term care market is expected to grow at a rate of 1% to 3%, and the home health care market is expected to grow at a rate of 5% to 7%.
In recent years, the healthcare industry has experienced consolidation among provider groups, long-term care facilities, and other alternate site providers, as well as health systems created through alliances between hospitals and long-term care facilities or physician practices. The Company expects such consolidation activities to continue.
THE PHYSICIAN BUSINESS
The Physician Business operates through the Company’s Physician Sales & Service division and is a leading distributor of medical supplies, diagnostic equipment, healthcare information technology, pharmaceutical products and provider of professional and consulting services, based on revenues, number of customers and number of sales professionals. The Physician Business serves alternate site healthcare providers, including independent physicians and physician groups, community health centers, urgent care facilities, health system affiliated practices and other non-hospital based caregivers. The Physician Business has approximately 850 sales professionals trained in solution-focused selling, disease state management, and diagnostic and therapeutic products used by healthcare providers.
Customers
The Physician Business distributes products and offers services to office-based physicians who specialize in internal medicine, family practice, primary care, pediatrics, OB/GYN, cardiology, orthopedics, general practice, and other specialties. The Physician Business’ target market consists of approximately 498,000 independent physicians practicing at approximately 212,000 offices in the United States.
The Physician Business also distributes products and offers services to office-based physicians operating under the ownership of private or public companies or acute care facilities that range in size from small hospitals to large integrated delivery network systems (collectively “health systems”). Physicians employed by health systems are generally concentrated in the same specialties as independent physicians.
Customer pricing for each product is generally either negotiated directly with the physician office or contracted through group purchasing organizations (“GPOs”). GPOs negotiate directly with medical product manufacturers and distributors on behalf of their members, establishing exclusive or multi-supplier relationships.
Distribution Infrastructure
As of March 30, 2012, the Physician Business operated a distribution network consisting of 33 full-service distribution centers, 39 break-freight locations, 2 other operations-related facilities, and 2 redistribution facilities, some of which are shared with the Extended Care Business, to serve customers throughout the United States. The operations of a full-service distribution center include sales support and certain administrative functions, such as customer billing and customer service, as well as general warehousing functions, inventory management, and product delivery. Inventory purchasing is centralized at the Company’s Shared Services segment in Jacksonville, Florida. Full-service distribution centers receive inventory directly from manufacturers and redistribution centers. The distribution centers deliver product to customers and break-freight locations on a daily basis via the Company’s fleet of leased vehicles or third party transportation providers. Break-freight locations are warehouse facilities that receive packaged customer orders from full-service distribution centers and distribute them directly to customers on a daily basis. The Physician Business provides service to its customers through myPSS.com, a customer and sales force internet ordering portal, and SmartScan, a handheld inventory management device that allows customers to order product electronically and provides basic inventory management functions.
4
Table of Contents
Products
The Physician Business distributes approximately 164,000 different products consisting of disposable supplies, pharmaceuticals, diagnostic equipment, and non-diagnostic equipment. Additionally, the Physician Business offers healthcare information technology solutions and physician dispensing solutions.
Branded Medical-Surgical Disposable Supplies. This product category includes a broad range of medical supplies, including paper goods, needles and syringes, gauze and wound dressings, surgical instruments, sutures, examination gloves, orthopedic soft goods, tongue blades and applicators, sterilization and intravenous solutions, specimen containers, reagents for diagnostic equipment, and diagnostic rapid test kits. The Physician Business offers a broad array of branded products sourced from various medical product manufacturers.
Store Brand Medical-Surgical Disposable Supplies and Equipment. The Company offers its own products in connection with its strategy of sourcing through global channels to drive enhanced customer satisfaction and profitability. This product category includes a broad range of medical supplies, including paper goods, needles and syringes, gauze and wound dressings, surgical instruments, sutures, examination gloves, orthopedic soft goods, tongue blades and applicators, sterilization products, specimen containers, reagents for diagnostic equipment, and diagnostic rapid test kits marketed under various Company brand names.
Pharmaceutical Products. This product category includes various vaccines, injectables, inhalants, topicals, ophthalmic ointments and solutions, otic solutions and oral analgesics, antacids and antibiotics, and controlled pharmaceutical products, which are used or administered in the physician’s office. Controlled pharmaceutical products include injectable anesthesia agents, narcotics, and pain management drugs.
Diagnostic Equipment. This product category includes various equipment lines such as blood chemistry analyzers, automated cell and differential counters, immunoassay analyzers, bone densitometers, electrocardiograph monitors and defibrillators, cardiac stress systems, cardiac and OB/GYN ultrasound, holter monitors, flexible sigmoidoscopy scopes, and microscopes. Sales of certain diagnostic equipment generate recurring orders of disposable diagnostic reagents and supplies consumed in the operation of the equipment.
Non-Diagnostic Equipment.This category includes all other equipment used in a medical practice such as aesthetic lasers, autoclaves, examination tables, medical scales, and furniture.
Healthcare Information Technology. This category includes healthcare information technology products and services designed to improve the accuracy, efficiency, and effectiveness of physician business practices.
Physician Dispensing Solutions.The Physician Business provides dispensing solutions to physician business practices which include repackaging of medical products, dispensing software, claims processing services, formulary consultation services and compliance necessary for such practices to dispense medical products to their patients on-site. Medical products provided include pharmaceuticals repackaged for patient dispensing and equipment such as transcutaneous electrical nerve stimulation units.
Competition
The Physician Business operates in a highly competitive industry where products and services are readily available to customers from a number of manufacturers, distributors, and suppliers. Competitors of the Physician Business include large, national, full-line distributors, many smaller regional and local distributors, and manufacturers who sell directly to customers. Competitive factors within the medical/surgical supply distribution and services industry include pricing, product availability, sales force capabilities, delivery time, electronic commerce capabilities and relationships with customers, as well as the ability to meet customer-specific requirements.
5
Table of Contents
THE EXTENDED CARE BUSINESS
The Extended Care Business operates through the Gulf South Medical Supply, Inc. subsidiary and is a national distributor of medical supplies and related products and solutions to the extended care industry in the United States. The Extended Care Business serves the skilled nursing home, assisted living, home health care and hospice markets. In addition, the Extended Care Business provides Medicare Part B and Medicaid billing services. The home health care industry refers to providers (companies, agencies, and care givers) of medical services, medical supplies, and equipment to patients in a residential setting. The Extended Care Business has approximately 170 sales professionals trained in solution-focused selling, disease state management and diagnostic and therapeutic products used in extended care settings.
Customers
The Extended Care Business’ target market consists of approximately 40,000 independent, regional, and national nursing home facilities, home health agencies, assisted living centers, hospices, and home medical equipment dealers. Approximately 16%, 16%, and 15%, of the Extended Care Business’ net sales for fiscal years 2012, 2011, and 2010, respectively, represent net sales to its largest five customers.
Distribution Infrastructure
As of March 30, 2012, the Extended Care Business operated a distribution network consisting of 18 full-service distribution centers, 10 break-freight locations, 2 other operations-related facilities, and 2 redistribution centers, some of which are shared with the Physician Business, to serve customers throughout the United States. The operations of a full-service distribution center include general warehousing functions such as inventory management, warehouse management, and product delivery directly to customers on a daily basis. Full-service distribution centers receive inventory directly from manufacturers and redistribution centers and distribute product to customers and break-freight locations. Break-freight locations receive packaged customer orders from full-service distribution centers and distribute them directly to customers on a daily basis. Product is delivered using either the Company’s fleet of leased delivery vehicles or third party transportation providers. Accounts receivable collections, cash application, customer billing, and inventory purchasing are centralized in the Company’s Shared Services segment in Jacksonville, Florida, while customer order processing, customer service, and sales support are centralized in Jackson, Mississippi. Coupled with a team of sales professionals, myGSOnline, an automated customer internet platform, and SmartScan, the Extended Care Business provides service to customers ranging from nursing homes to providers of home health, hospice, sub-acute, rehabilitation, and transitional care.
Products
The Extended Care Business distributes approximately 46,000 different medical and related products consisting of medical supplies, incontinent supplies and personal care items, enteral feeding supplies, point of care testing devices, advanced wound care, home medical equipment, and other supplies required to provide long-term care. The Extended Care Business offers a broad array of branded products from various manufacturers. In addition, the Company offers its own store brands, including Select Medical Products and other specialty brand products, in connection with its strategy of sourcing through global channels to drive enhanced customer satisfaction and profitability.
Services
The Extended Care Business, through its wholly-owned subsidiary, Proclaim, Inc., provides Medicare Part B and Medicaid billing services to the nursing home and assisted living markets. The Extended Care Business also provides consulting services to extended care providers through its noncontrolling interest in Pathway Health Services, Inc. (“Pathway”).
6
Table of Contents
Competition
The Extended Care Business operates in a highly competitive industry where products and services are readily available to customers from a number of manufacturers, distributors, suppliers, and service providers. Competitors of the Extended Care Business include large, multinational, full-line distributors, many smaller regional and local distributors, manufacturers who sell directly to customers, and Medicare Part B billing service providers. Competitive factors within the extended care industry include pricing, product availability, delivery time, electronic commerce capabilities, as well as the ability to meet customer-specific requirements.
SHARED SERVICES
The Company’s Shared Services segment consists of departments that support the Company’s operating segments through the delivery of standardized service. Shared Services includes executive and administrative services; accounting and finance; information technology development and support; shared operations management; legal and regulatory compliance; human resources; training and development; supplier management; and sourcing and procurement of inventory. Shared Services allocates a portion of its operating costs and interest expense to the operating segments. The allocation of these costs is generally proportionate to the revenues of each operating segment. Interest expense is allocated based on an internal carrying value of historical capital used to acquire or develop the operating segments’ operations.
SUPPLIER RELATIONSHIPS
Supplier relationships are an integral part of the Company’s businesses. Sales support, performance incentives, product literature, samples, demonstration units, training, marketing intelligence, distributor discounts and rebates, and new products are important elements of developing successful supplier relationships. The Company seeks to increase profitability by purchasing certain medical supplies, pharmaceutical products, and equipment at the lowest available price through volume discounts, rebates, and product category consolidation under contracts with terms negotiated by the Company’s supplier management professionals.
The Company pursues opportunities to market and sell medical equipment and supplies through unique or exclusive marketing arrangements. Manufacturers of medical supplies and diagnostic equipment often seek to optimize the number of distributors selling their products to end users in order to reduce the cost associated with marketing and field sales support. The Company has been successful in obtaining unique or exclusive arrangements to sell certain products based on the size of its sales force and the effectiveness of its marketing programs.
GLOBAL PRODUCT SOURCING
The Company’s global sourcing activities include identification of manufacturers in foreign locations, selection and specification of products to be manufactured, management of quality assurance programs and controls, and alignment of product availability and customer needs. The Company’s global sourcing team, located in U.S. and foreign locations, consists of fully dedicated functional experts in areas such as product development, global sourcing, logistics, supply chain design and management, supplier relations, product management, quality assurance, and quality control.
As of March 30, 2012, the Company had approximately $49.6 million of globally-sourced product inventory, which represented approximately 1,200 SKUs and 200 product categories. These products were sourced from approximately 72 manufacturers located in countries including Canada, China, India, Japan, Malaysia, Mexico, Philippines, Poland, South Korea, Taiwan, and the United Kingdom.
INFORMATION SYSTEMS
The Physician and Extended Care Businesses operate the Oracle JD Edwards XE platform as the primary enterprise resource planning (“ERP”) system.
7
Table of Contents
The Physician Business’ internet portal, myPSS.com, provides its customers and sales representatives with sales history, accounts receivable detail, available inventory and supports a number of ordering methods. The Physician Business’ laptop-based sales force automation application, known as “ICON”, carries customer order history and accounts receivable detail, reflects on-hand inventory quantities for the local distribution center, and transmits orders over a secure wireless network. During the fourth quarter of fiscal year 2012, the Company enhanced the myPSS.com internet portal to allow for sales force ordering and began to phase out ICON. Online order processing is supplemented in the Physician Business with SmartScan, the Company’s handheld inventory management device. Approximately 75% of customer orders in the Physician Business are electronic orders.
The Extended Care Business’ internet portal, myGSOnline.com, provides its customers and sales representatives with sales history, accounts receivable detail, available inventory, and supports a number of ordering methods. The Extended Care Business offers its customers a wide variety of electronic data interchange (“EDI”) services whereby orders, order acknowledgments, invoices, and other industry standard EDI transactions are available electronically. This improves efficiency and timeliness for the Company and its customers. Approximately 83% of customer orders in the Extended Care Business are electronic orders.
During fiscal year 2012, the Company continued its focus on increasing its internet, e-commerce and EDI capabilities to connect more effectively with customers and suppliers. Additionally, the Company continued the implementation of a warehouse management system to streamline warehouse management, enhance inventory controls, and improve customer service.
REGULATORY MATTERS
General
Federal, state, local, and foreign government agencies extensively regulate the distribution and sale of medical devices, medical supplies and pharmaceutical products, and the billing of government-sponsored healthcare programs. Applicable federal, state and foreign statutes and regulations require the Company to meet various standards relating to, among other things, licensure, personnel, physical security, maintenance of proper records, privacy of health information, maintenance and repair of equipment, and quality assurance programs.
The Company’s costs associated with complying with the various applicable federal and state statutes and regulations, as they now exist and as they may be modified, could be material. Although the Company intends to comply with all applicable laws and regulations, many of them have been recently enacted, are broadly worded, and have not been interpreted by regulators and the courts. Consequently, they have been and may continue to be interpreted or applied by governmental authorities in a manner that differs from the Company’s interpretation, which has required and could continue to require the Company to make changes in its operating procedures which may increase operating costs. Future allegations by a state or the federal government that the Company has not complied with these laws could have a material adverse impact on the Company. If it is determined that the Company has not complied with these laws, or if the Company enters into settlement agreements to resolve allegations of non-compliance, the Company could be required to make settlement payments, quarantine or destroy inventory, or be subject to civil and criminal penalties, including fines and the loss of licenses or its ability to participate in Medicare, Medicaid, and other federal and state healthcare programs. In addition, the enforcement of these laws and regulations has increased and is expected to increase in the future. Any of the foregoing could have a material adverse impact on the Company. The Company believes that the healthcare services industry will continue to be subject to extensive regulation and enforcement at the federal, state, local, and foreign levels.
Healthcare Fraud and Abuse Laws
The Company is subject to extensive state and federal laws and regulations relating to healthcare fraud and abuse. Federal and state governments continue to scrutinize potentially fraudulent practices in the healthcare industry in an attempt to minimize the cost that such practices have on Medicare, Medicaid, and other government healthcare programs. Under Medicare, Medicaid, and other government-funded healthcare programs, the federal government enforces a federal law called the Anti-Kickback Statute. The Anti-Kickback Statute, and the related regulations prohibits any person from offering, paying, soliciting or receiving anything of value to or from another person to
8
Table of Contents
induce the referral of business, including the sale or purchase of items or services covered by Medicare, Medicaid, or other federally subsidized programs. Many states also have similar anti-kickback statutes. The Federal False Claims Act provides that those who knowingly submit, or cause another person or entity to submit, false claims for payment of government funds are liable for three times the government’s damages, plus civil penalties.
The Patient Protection and Affordable Care Act (“PPACA”), enacted in March 2010, known as “The Health Care Reform Bill,” significantly strengthened the Federal False Claims Act, and the Anti-Kickback provisions, which could lead to increased whistleblower or related suits. The Physician Payment Sunshine Act, which is a provision of the PPACA, imposed new reporting and disclosure requirements for pharmaceutical, medical device, and medical supply distributors and manufacturers with regard to payments or other transfers of value made to physicians and teaching hospitals. The Centers for Medicare and Medicaid Services (“CMS”) has delayed the implementation of the reporting requirements to an unspecified date allowing for the review of comments resulting from the published interim rules. Additional implemented or pending state legislation surrounding payments or other transfers of value made to certain practitioners requires disclosure and reporting.
State and Federal Drug Pedigree Laws
There have been increasing efforts by various levels of government to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated, or misbranded pharmaceuticals into the distribution system. Several states have enacted or proposed laws and regulations designed to protect the integrity of the supply channel for the distribution of pharmaceutical products. For example, several states have implemented drug pedigree requirements that require prescription drugs to be distributed with records or information documenting the prior distribution of the drug back to the manufacturers. In addition, California has proposed legislation that will require the implementation of an electronic drug pedigree system that provides “track and trace” chain of custody technologies, such as radio frequency identification (“RFID”) technologies by January 1, 2016 for wholesale distributors and repackagers. At the federal level, the FDA issued final regulations pursuant to the Prescription Drug Marketing Act that became effective in December 2006. The regulations impose drug pedigree and other chain of custody requirements that increase the costs and/or burden of selling products and handling product returns. There is currently a case pending in the Federal District Court for the Eastern District Court of New York enjoining the implementation of some of the federal drug pedigree requirements, in response to a case initiated by secondary distributors. Moreover, the United States Food and Drug Administration Amendments Act of 2007 require the FDA to establish standards to provide for the development of a standardized numerical identifier and include track and trace or authentication technologies, such as RFID and other technologies. In the future, Congress may implement legislation that would revise and expand the federal pedigree requirements. If enacted, these pedigree requirements could preempt existing state pedigree requirements and the Company may have to adopt or modify its operations to initiate and transmit electronically-coded pedigree information concerning the purchase and transmittal of prescription drugs in all 50 states.
The Food, Drug and Cosmetic Act, Prescription Drug Marketing Act of 1987, Safe Medical Devices Act of 1990, Controlled Substances Act and Various State Regulations
The Company’s business is subject to regulation under the Federal Food, Drug and Cosmetic Act, the Prescription Drug Marketing Act of 1987, the Safe Medical Devices Act of 1990, and state laws and regulations applicable to the manufacture, importation, and distribution of medical devices and over-the-counter pharmaceutical products, as well as the distribution of prescription pharmaceutical products or dispensing of pre-packaged prescription pharmaceuticals products. In addition, the Company is subject to regulations issued by the United States Food and Drug Administration (“FDA”), the Drug Enforcement Administration (“DEA”), and comparable state agencies.
The Federal Food, Drug, and Cosmetic Act generally regulates the manufacture and importation of drugs and medical devices shipped via interstate commerce, including such matters as labeling, packaging, storage, and handling of such products. The Prescription Drug Marketing Act of 1987, which amended the Federal Food, Drug and Cosmetic Act, establishes certain requirements applicable to the wholesale distribution of prescription drugs, including the requirement that wholesale drug distributors be registered with the Secretary of Health and Human Services or be licensed in each state in which business is conducted in accordance with federally established guidelines on storage, handling, and records maintenance. The Safe Medical Devices Act of 1990 imposes certain reporting requirements on distributors in the event of an incident involving serious illness, injury, or death caused by a medical device. The Company is also required to maintain licenses and permits for the distribution of pharmaceutical products and medical devices under the laws of the states in which it operates.
9
Table of Contents
The Health Insurance Portability and Accountability Act of 1996
The Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (collectively, “HIPAA”) establishes (i) national standards for some types of electronic health information transactions and the data elements used in those transactions, (ii) standards to protect the privacy of individually identifiable health information (“PHI”), and (iii) security standards to ensure the integrity and confidentiality of health information. Health plans, healthcare clearinghouses, and most healthcare providers, including the Company, are “Covered Entities” subject to HIPAA.
The Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), signed into law on February 17, 2009, expanded, among other things, (i) the scope of HIPAA to now apply directly to “business associates,” or independent contractors who receive or obtain PHI in connection with providing a service to a covered entity, including information exchange organizations, medical suppliers that ship to patient’s homes, and third-party billing service providers, (ii) substantive security and privacy obligations, including new federal security breach notification requirements to affected individuals of certain breaches of unsecured PHI, and (iii) the civil and criminal penalties that may be imposed for HIPAA violations. The HITECH Act may have a significant impact on the duties, responsibilities, and liabilities of the organization, particularly with regards to HIPAA compliance.
The United States Foreign Corrupt Practices Act of 1977
The Company conducts operations in a number of foreign countries making it subject to regulatory provisions under the United States Foreign Corrupt Practices Act (“FCPA”), as amended, and similar regulations in foreign jurisdictions. The FCPA prohibits U.S. and other business entities from making improper payments to foreign government officials, political parties, or political party officials for the purpose of furthering foreign business activities. In addition to anti-bribery laws, the Company is also subject to anti-corruption provisions under the FCPA which are enforced by the U.S. Department of Justice. The Securities and Exchange Commission requires strict observance and compliance with regulations set forth by the FCPA regarding certain accounting and internal control standards. Failure to comply with the provisions of the FCPA may result in severe penalties and other consequences for individual employees and/or the Company as a whole.
Government Procurement Contracts
The Company has multiple contracts with government entities. These contracts contain requirements and restrictions, that differ from those with commercial customers, and that could present significant risks if the Company does not maintain compliance. If the Company fails to comply with the terms of these contracts, penalties may be imposed including monetary damages and criminal and civil penalties. In addition contracts could be terminated and the Company could be prohibited from conducting future business with the governmental entity.
Other Laws
The Company is subject to various additional federal, state, and local laws and regulations in the United States, relating to the safe working conditions and the sales, use, and disposal of hazardous or potentially hazardous substances. In addition, laws that affect the Company’s foreign operations include U.S. and international import and export laws and regulations that require that the Company abide by certain standards relating to the importation and exportation of finished goods, raw materials, and supplies. Furthermore, the Department of Transportation regulates the conveyance of regulated materials, both in Company-leased delivery vehicles and via common carrier.
Impact of Changes in Healthcare Legislation
Federal, state, and foreign laws and regulations affecting the Company’s business are subject to change. The Company cannot predict what impact, if any, such changes might have on its business. Any new legislation or regulations, or new interpretations of existing statutes and regulations, governing the manner in which the Company conducts its business could have a material adverse impact on the Company and its results of operations.
10
Table of Contents
The extensive federal and state laws and regulations described above apply not only to the Company, but also to the manufacturers which supply the products distributed by the Company. For instance, medical product and device manufacturers are subject to design, manufacturing, labeling, promotion, and advertising standards imposed on, as well as registration and reporting requirements regarding their facilities and products. Likewise, pharmaceutical manufacturers are subject to development, manufacturing, and distribution regulation by the FDA, the DEA and other federal, state, and local authorities. Failure of a manufacturer to comply with these requirements, or changes in such requirements, could result in recalls, seizures, manufacturing suspensions, or other interruptions in the production, supply, and sale of its products. Such interruptions may result in a material adverse impact on the Company’s business. Similarly, changes in the extensive regulations or in their interpretation or enforcement applicable to the Company’s customers could adversely impact the Company’s business in ways which are difficult for the Company to predict.
See Item 1A “Risk Factors” for a discussion of additional regulatory developments that may affect the Company’s results of operations and financial condition.
PROPRIETARY RIGHTS
The Company has registered with the United States Patent and Trademark Office the marks PSS WORLD MEDICAL (and Design), PSS (and Design), GULF SOUTH MEDICAL (and Design), EXPERTISE DELIVERED, ANSWERS (and Design), SMARTSCAN, PHYSICIAN SELECT, NIGHTINGALE, SOUTHERN ANESTHESIA & SURGICAL, INC. (and Design), ADVANCE PLUS + by SOUTHERN ANESTHESIA & SURGICAL, INC. (and Design), GULF SOUTH MEDICAL SUPPLY (and Design), SELECT MEDICAL PRODUCTS (and Design) and SELECT MEDICAL PRODUCTS PSS GULF SOUTH MEDICAL SUPPLY (and Design), among others. The Company’s trademarks generally have a term of ten years. The Company believes that the PSS World Medical, Physician Sales & Service, and Gulf South Medical Supply names are well recognized in the medical supply industry and by healthcare providers and, therefore, are valuable assets of the Company.
EMPLOYEES
As of March 30, 2012, the Company employed approximately 4,100 full-time and part-time employees. The Company’s approximately 1,020 sales professionals are largely comprised of sales representatives. The Company believes ongoing employee training is critical to its success and, accordingly, invests significant resources in training, continuing professional education and leadership development. Management believes that relations with employees are strong and the Company’s long-term success depends upon its employees, including its sales professionals.
AVAILABLE INFORMATION
The Company files annual, quarterly and current reports, proxy statements, and other information with the Securities and Exchange Commission (“SEC”). Any documents that have been filed with the SEC may be read or copied, at prescribed rates contingent upon a written request, at its Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. These documents are also filed with the SEC electronically and are accessible on the SEC’s internet website found atwww.sec.gov. Copies of materials filed with the SEC may also be obtained free of charge from the Company’s internet website found atwww.pssworldmedical.com as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC.
The Company’s Code of Ethics, Audit Committee Charter, Corporate Governance Committee Charter, and Compensation Committee Charter are available to the public free of charge in the Investor Relations section of the Company’s websitewww.pssworldmedical.com or may be obtained by writing to: PSS World Medical, Inc., Investor Relations, 4345 Southpoint Blvd., Jacksonville, Florida 32216. The Company intends to post amendments to or waivers from its Code of Ethics (to the extent applicable to the Company’s principal executive officer, principal financial officer, or principal accounting officer) on its website.
11
Table of Contents
The Company’s continued success depends on management’s ability to identify, prioritize, and appropriately manage a wide range of enterprise risk exposures. Readers should carefully consider each of the following risks and additional information set forth in this Annual Report on Form 10-K. These risks and other factors may affect forward-looking statements, including those made by the Company in this document or elsewhere. The risks and uncertainties described herein may not be the only ones facing the Company and are not organized in order of priority. Additional risks and uncertainties not presently known to management or that management currently believes to be immaterial may also adversely affect the Company’s business. If any of the following risks and uncertainties develop into actual events, it could affect the Company’s business, financial condition, or results of operations, cause the trading price of the Company’s common stock to decline, or cause actual results to differ materially from those expected.
The recently announced strategic restructuring plan is subject to a number of risks and uncertainties.
During the first quarter of fiscal year 2013 the Company’s Board of Directors adopted a strategic restructuring plan. The restructuring plan will include, among other things, the sale of two business units serving skilled nursing facilities and specialty dental practices, the integration of warehouse operations into one common distribution infrastructure, as well as a redesign of the shared services function. The restructuring is subject to a number of risks and uncertainties that could adversely impact the Company’s financial condition, results of operations, and cash flows, and may otherwise cause disruption to the Company’s business. These risks and uncertainties include, but are not limited to:
| • | The Company may not be able to divest the business units on favorable terms, or at all; |
| • | The Company may not be able to retain key personnel (including sales representatives), customers, and suppliers during or after the restructuring; |
| • | The Company may not execute the restructuring plan in a timely or efficient manner; |
| • | The Company may experience a disruption to the Company’s IT systems; |
| • | The Company may not be able to find suitable acquisitions in the areas it has chosen to focus; and |
| • | The Company may incur substantial restructuring or impairment charges. |
The Company may not be able to continue to successfully compete with other medical supply companies and direct manufacturers.
Consolidation within the healthcare industry has resulted in increased competition by direct manufacturers, large national distributors, and drug wholesalers, and may result in lower customer pricing and/or higher operating costs. Additionally, changes in ownership of physician practices may erode the Company’s customer base. Continued consolidation in the healthcare industry could result in the following:
| • | potential new entrants to the markets the Company serves; |
| • | provider networks created through consolidation among physician provider groups, long-term care facilities, and other alternate site providers may shift purchasing decisions to entities or persons with whom the Company has no current relationship; |
| • | national hospital distributors, drug wholesale distributors, and healthcare manufacturers may focus their efforts more directly on the Company’s markets; |
| • | competitors obtaining exclusive rights to market products to the Company’s customers; and |
| • | hospitals forming alliances with long-term care facilities or physician practices to create health systems which may look to hospital distributors and manufacturers to supply their affiliates. |
12
Table of Contents
The medical supply distribution market is highly competitive. The Company’s results of operations could be materially adversely affected if competitors offer products similar to those distributed by the Company at significantly lower prices. Principal competitors of the Company include full-line and full-service, multi-market medical distributors, internet distributors, and direct selling manufacturers, many of which have a national presence and significantly greater resources than the Company. The Company also faces significant competition from regional and local distributors, telemarketing firms, internet companies, and mail order firms. The Company’s competition may have the following strengths:
| • | sales representatives that compete directly with the Company; |
| • | capability to market products directly to the Company’s customers; |
| • | exclusive access to unique products or services; |
| • | substantially greater financial resources than the Company; and |
| • | lower product and operating costs. |
There can be no assurance the Company will maintain operating margins and customer relationships and avoid increased competition and significant pricing pressure in the future if medical supply distributor consolidations, acquisitions of the Company’s customers by hospitals, and other customer consolidations occur. If the Company is unable to compete successfully with other medical supply distributors and direct manufacturers, the Company’s business, financial condition, and results of operations may be materially adversely affected.
General economic conditions, including the current global economy may materially adversely impact the Company’s operating results.
Current and future economic conditions and other factors including consumer confidence, unemployment levels, interest rates, tax rates, consumer debt levels, the threat or outbreak of terrorism, fuel and energy costs, the availability of consumer credit, and the impact of state and federal budget deficits on Medicaid and Medicare reimbursement can reduce consumer spending or change consumer purchasing habits, having a negative impact on the purchasing power of the Company’s customers. The current global economy has and may continue to materially adversely affect consumer spending habits and the Company’s operating results.
Trends in healthcare spending, as well as the recently enacted health care reform legislation, may impact the Company’s results of operations.
A significant portion of medical costs in the United States are funded by government and private insurance programs, such as Medicare, Medicaid, and corporate health insurance plans. In recent years, government-imposed limits on reimbursement to hospitals, physicians, nursing homes, home health providers, and other healthcare providers have significantly impacted spending in certain markets within the medical products industry. Future changes in Medicare and state administered Medicaid programs may limit payments to providers and customers served by the Company. Significant reductions in reimbursement levels and adjustments, in combination with rising costs, may negatively impact customers’ financial health and liquidity and may negatively affect the Company’s results of operations. Additionally, the Company’s Medicare Part B billing services are subject to a competitive bidding process. The inability of the Company to successfully compete in this environment could impact the Company’s net sales and results of operations.
Health care reform is a key priority of the current Administration. The Health Care Reform Bill included increased availability of insurance, provisions for healthcare information technology, increased efficiencies in Medicare and Medicaid, and provisions for additional taxes on medical devices. In addition, the Health Care Reform Bill provides for changes in how healthcare may be delivered to patients in the future, such as accountable care organizations and the use of healthcare information technology, which may impact the Company’s business. It is unclear at this time what impact such laws and regulations will have on the purchasing patterns of the Company’s customers, and as a result, the Company’s financial condition, results of operations, and cash flows.
13
Table of Contents
Numerous factors, many of which cannot be controlled by the Company, may cause the Company’s net sales and results of operations to fluctuate, which may adversely affect the market price of the Company’s common stock.
The Company’s net sales and operating results may fluctuate as a result of many factors, some of which are out of the Company’s control, including:
| • | general economic conditions; |
| • | demand for the products and services offered by the Company; |
| • | introduction of new products and services offered by the Company and its competitors; |
| • | seasonal and pandemic vaccine sales; |
| • | retention of sales representatives and other key employees; |
| • | acquisitions, dispositions, or other investments by the Company; |
| • | changes in manufacturers’ pricing policies, contract terms, and distribution strategies; |
| • | rapid or unexpected increases in product or operating costs; |
| • | changes in the Company’s business strategies, or those of its competitors; |
| • | product supply shortages; |
| • | product recalls by manufacturers; |
| • | changes in product mix; |
| • | fuel costs and third party shipping rates; |
| • | costs associated with the Company’s self-funded medical insurance program; |
| • | inclement weather; |
| • | disruptions resulting from implementing strategic business plans; |
| • | disruptions resulting from implementing ERP systems; and |
| • | changes by the government, including health care reform, changes in reimbursement rates to providers, regulatory requirements, and taxes related to the distribution of medical and pharmaceutical products. |
Accordingly, management believes that period-to-period comparisons of the results of operations should not be relied upon as an indication of future performance because these factors may cause the Company’s results of operations to differ from analysts’ and investors’ expectations in certain future periods. This could materially and adversely affect the trading price of the Company’s common stock.
The Company may face increasing competitive pricing pressures on sales to its customers.
The Company’s business strategy may not mitigate the effect of pricing pressures, which could adversely impact the Company’s net sales, gross margins, and results of operations. As a result of the current economic environment, the Company has experienced increased pressure on the price of its products and services, which may continue into future periods.
Additionally, sales to large accounts and provider groups, especially in the extended care market, represent a significant portion of the Company’s revenue base. Competitive pricing pressures may increase due to:
| • | change in ownership; |
| • | additional negotiating leverage of large customers; |
| • | supplier agreements containing volume discounts; |
| • | service specifications; |
| • | financial health of customers; |
| • | activity of competitors; and |
| • | activity of GPOs. |
14
Table of Contents
The viability of the Company’s customers may be threatened by various factors.
The Company has been negatively impacted in the past, and could be negatively impacted in the future, when customers experience disruptions resulting from tighter capital and credit markets, changes in reimbursement, or a loss of patient revenue due to changes in the general economy. Customers have, and may continue to modify, delay, or cancel plans to purchase the Company’s products or services. Additionally, if customers’ operating and financial performance deteriorate, or if they are unable to make scheduled payments or obtain credit, customers may not be able to pay, or may delay payment of, accounts receivable owed to the Company. Any inability of customers to pay for products and services may adversely affect the Company’s results of operations and cash flow.
The Company’s customers are also impacted by increasing costs of malpractice claims and liability insurance. As a result, customer financial viability may adversely impact the Company’s financial condition, net sales, results of operations, and cash flows from operations.
The Company’s future operating results are affected by its relationships with its customers, sales representatives, and senior management team.
The Company’s ability to retain existing customers and attract new customers is dependent upon hiring and retaining sales representatives. Customer relationships are at risk if a sales representative ceases employment with the Company, particularly where the representative seeks employment with a competitor. The Company uses employment agreements containing restrictive covenants, which protect the Company’s legitimate business interests. However, these agreements have not been obtained for all sales representatives. In addition, the terms of these agreements, in certain jurisdictions, may not be fully enforceable. The inability to adequately hire or retain sales representatives could limit the Company’s ability to expand its business and increase sales.
The Company’s success in executing its strategic objectives depends largely on the efforts and abilities of senior management, particularly the executive management team, as well as operations and sales leaders at each distribution center, as local leaders have significant decision-making authority. Although the Company maintains key man life insurance for certain officers, the loss of services of one or more of its members of senior management, the inability of the current management team to successfully execute the Company’s strategies, or the inability of the Company to attract and retain key personnel through appropriately aligned compensation and benefit plans may adversely affect the Company’s business.
The Company relies extensively on its relationships, significant distribution agreements, and other purchasing arrangements with suppliers.
The Company has distribution agreements and other purchasing arrangements with a substantial majority of its suppliers. The Company relies on these suppliers to manufacture and/or supply products for and to the Company for resale to the Company’s customers. If any distribution agreement or other purchasing arrangement between the Company and a supplier expires or is terminated, if the Company fails to meet the minimum requirements under the agreement, or if the Company and any supplier otherwise cease conducting business with each other, then the Company’s net sales and results of operations may be materially adversely affected.
Since the Company does not manufacture many of the products it sells, it is dependent on vendors and manufacturers for the supply of products. The Company relies on suppliers to provide, among other things:
| • | field sales representatives’ technical and selling support; |
| • | acceptable purchasing, pricing, and delivery terms; |
| • | sales performance and other financial incentives; |
| • | rebates for inventory purchases or sales volume; |
| • | support of sales and marketing programs; |
| • | promotional materials; |
| • | product availability; and |
| • | product indemnification on certain products. |
There can be no assurance that the Company will be successful in maintaining good relations with its suppliers. The Company’s global sourcing strategy may threaten relations with certain suppliers and risk the loss of key branded products. Additionally, there can be no assurance that the Company will meet forecasted inventory purchases, sales volume, or other criteria required to obtain the benefits outlined in supplier agreements.
15
Table of Contents
Cost increases for the Company’s products may impact the Company’s results of operations.
The Company’s suppliers, both domestic and foreign, may increase costs for products distributed by the Company. While the Company takes steps to mitigate the effect of these cost increases, there can be no assurance that these cost increases will not materially adversely impact the Company’s net sales, gross margins, financial conditions, and results of operations.
Expansion of GPO or hospital purchasing power and the multi-tiered costing structure may place the Company at a competitive disadvantage.
The medical products industry is subject to a multi-tiered costing structure, which can vary by manufacturer and/or product. Under this structure, certain competitors can obtain more favorable prices for medical products than the Company. The multi-tiered costing structure continues to expand as many large health systems and others with significant purchasing power, such as GPOs, demand more favorable pricing terms. This may threaten the Company’s ability to compete effectively, which would in turn negatively impact the Company’s results of operations. Although the Company seeks to obtain similar terms from manufacturers and obtain access to lower prices demanded by GPO contracts or other contracts, management cannot assure such terms will be obtained or contracts will be executed.
The operating costs of the Company’s delivery fleet could increase due to fuel price fluctuations and/or service interruptions by third parties.
The Company delivers its products to customers through either its Company-leased delivery fleet or third party transportation providers. Significant fluctuations in the cost of fuel have had and may continue to have an adverse impact on the Company’s cost to deliver product to customers. In addition, the Company’s operations may be impacted by events and conditions outside of its control, including strikes or other service interruptions by third party transportation providers which may increase the Company’s operating expenses and adversely affect the Company’s ability to deliver products on a timely basis.
The Company’s strategy for growth may not result in additional net sales or operating income and may have an adverse effect on working capital, operating cash flow, and results of operations.
The Company seeks to increase revenues and operating income by:
| • | developing innovative marketing and distribution programs; |
| • | expanding the sales force and increasing sales force productivity; |
| • | completing strategic acquisitions; |
| • | expanding e-commerce initiatives and development; |
| • | maintaining and expanding vendor incentive programs; |
| • | expanding product offerings; |
| • | expanding sales support services; |
| • | increasing healthcare information technology offerings; and |
| • | leveraging its infrastructure and information systems to improve sourcing, supply chain and distribution efficiency. |
These business strategies for growth may result in increased costs and expenses. There can be no assurance that the Company’s business strategy for growth will result in additional net sales or operating income. In addition, the implementation of the Company’s business strategy for growth may have an adverse effect on working capital, operating cash flow, and results of operations.
Execution of Company’s acquisition strategy could adversely affect the Company’s results of operations and financial condition.
16
Table of Contents
An element of the Company’s strategy is to identify, pursue, and consummate acquisitions that either expand or complement the Company’s business. Future acquisitions or investments may be financed by the issuance of equity securities that would increase the number of outstanding common shares and may decrease earnings per share, and through incurring additional debt. Additionally, changes in generally accepted accounting principles and general economic and market conditions may affect the profitability of acquisitions.
The Company has made numerous acquisitions over the past two fiscal years. The integration of acquisitions involves a number of risks. The Company may be unable to successfully integrate the operations of acquired companies and realize anticipated economic, operational, and other benefits in a timely manner. Integration of an acquired company may be difficult when the acquired business is in a market in which the Company has limited expertise. If the operations of acquired companies are not successfully integrated, the Company may:
| • | incur substantial unanticipated costs and delays; |
| • | experience operational, technical, or financial controls problems; and |
| • | damage relationships with key customers and employees. |
As a result of these operational, financing, and environmental factors, the Company’s business, financial condition, results of operations, and market price of the Company’s common stock may be adversely affected.
The Company’s ability to execute its global sourcing strategy, which includes sourcing products from foreign markets subject to political, economic and legal uncertainties, may affect the Company’s overall profitability.
The Company continues to expand its globally sourced product offerings, which are marketed under various brand names (collectively known as “store brand” or “store brands”). The Company’s global sourcing strategy revolves around sustaining sourcing channels to drive enhanced customer satisfaction and profitability. To attain its strategic objectives, the Company has focused on:
| • | expanding the store brand product offering; |
| • | strengthening the global sourcing infrastructure; |
| • | improving product sourcing processes and sourcing partner coordination; |
| • | ensuring the quality of store brand products; |
| • | supporting increases in volume of globally sourced products; |
| • | effectively marketing store brand products; and |
| • | providing appropriate incentives to its sales force in the form of commission and promotions. |
The Company’s global sourcing strategy involves purchasing certain products directly from foreign manufacturers. The Company’s business, financial condition, and results of operations may be adversely affected by changes in the political, social, or economic environment of certain foreign countries. Changes in laws and regulations, or their interpretation, the imposition of surcharges or any material increase in tax rates, restrictions on currency conversion, imports and sources of supply, or the nationalization or other expropriation of private enterprises could have a material adverse effect on the Company’s ability to conduct business and its results of operations. Additional risks related to the Company’s global sourcing strategy include:
| • | political unrest in certain regions; |
| • | intermittent supply interruptions with global manufacturers; |
| • | unfavorable changes in foreign currency exchange rates; |
| • | shipping disruptions due to transportation delays; |
| • | fluctuations in the cost of commodities; |
| • | fluctuations in labor costs; |
| • | potential quality issues; |
| • | shortages in facility capacity; |
| • | availability of raw materials; |
| • | increasing regulation of imports; |
| • | natural disasters in certain regions; |
17
Table of Contents
| • | regional tensions that adversely affect the development of ongoing agreements; |
| • | intellectual property violation claims; and |
| • | violations of the United States Foreign Corrupt Practices Act (“FCPA”). |
The Company’s failure or inability to execute any of its strategic global sourcing initiatives could adversely impact its future profitability.
The Company may not be able to effectively respond to changes in its systems and product-related technology.
The use of technology and e-commerce by the Company and its customers is expanding. E-commerce is an efficient system for customer ordering and inventory management functions and the use of technology applications is becoming more prevalent in the Company’s customers’ businesses. The Company provides multiple e-commerce and other technology options in order to meet the demands of its customers. Advancements in technology and e-commerce will require the Company to enhance existing services and introduce new services to meet customer demands. If the Company does not address the changing demands of customers on a timely basis, the Company could experience adverse results.
The Company’s business is dependent on data processing systems critical to the business operations.
The Company is reliant on its information systems for centralized customer support, operating, and administrative processes. Management relies on the capability, accuracy, timeliness, and stability of its data processing systems to:
| • | receive and ship customer orders, including those received electronically; |
| • | manage customer billings and collections; |
| • | provide accurate point-of-sale product cost information; |
| • | track and report third-party ancillary billing services; |
| • | provide product reporting, such as product purchases and sales by vendor and vendor incentives earned; |
| • | manage inventory procurement and processes; |
| • | track and report regulatory compliance related to certain pharmaceutical products and devices; |
| • | manage human resources information; |
| • | provide manufacturer rebate tracking, compliance, verification, and collection; |
| • | ensure payments to suppliers are made in accordance with negotiated terms; |
| • | ensure certain critical internal controls are operating properly; |
| • | prepare and present accurate financial statements and related information; and |
| • | integrate acquisitions. |
The Company’s business, financial condition, and results of operations may be materially adversely affected if, among other things:
| • | data errors are created by the information systems and remain undetected; |
| • | data processing capabilities are interrupted or fail to operate for an extended period of time; |
| • | the data processing system becomes unable to support the growth of the business; |
| • | data is lost or is unable to be restored; |
| • | data security is breached inadvertently or through malicious intent causing destruction or theft of the Company’s information; |
| • | problems occur with system upgrades and implementations, or such upgrades and implementations are not timely; |
| • | product maintenance and upgrades to the ERP system are no longer provided by suppliers; or |
| • | a revision is made to the estimated useful lives for certain computer software. |
18
Table of Contents
The Company’s future results of operations could be adversely affected by operational disruptions due to natural disasters, particularly in regions susceptible to hurricanes.
A natural disaster such as a hurricane, tornado, earthquake, or flood could cause severe damage and disruption to the Company’s operations, property, inventory, and the operations of its customers.
The Company has developed disaster recovery plans, which include the use of third party back-up facilities for information system infrastructure. In addition, the Company maintains business interruption insurance for instances of catastrophic loss. There is a risk the Company may fail to execute its disaster recovery plans and incur losses that exceed insurance policy limits or are excluded from policy provisions. Furthermore, the Company may have difficulty obtaining business interruption insurance in the future or similar types of coverage may not be available in the markets in which it operates. The Company’s failure to execute or inability to execute any of its disaster recovery plans and obtain adequate insurance coverage could materially adversely impact the Company’s business and results of operations.
The terms of the Company’s indebtedness may impose restrictions on the ability to engage in certain business activities, limit its ability to obtain additional financing, and limit its flexibility to react to industry or economic conditions.
Revolving Line of Credit
The Company maintains an asset-based revolving line of credit (the “RLOC”), which permits maximum borrowings of up to $300.0 million and may be increased to $400.0 million at the Company’s discretion. Availability depends on a borrowing base calculation consisting of accounts receivable and inventory, subject to satisfaction of certain eligibility requirements, and certain other reserves. Any deterioration in the amount or valuation of these assets, including the execution of the Company’s restructuring plan, could reduce the availability of borrowings under the RLOC. Increases in the level of the Company’s indebtedness or changes in the Company’s debt rating could adversely affect the Company’s liquidity and reduce the Company’s ability to:
| • | sell or transfer assets; |
| • | make certain permitted investments; and |
| • | incur additional indebtedness and liens. |
Operating cash requirements are normally funded by cash flows from operating activities and borrowings under the RLOC, which expires in 2016. The Company expects that sources of capital to fund future growth in the business will be provided by a combination of cash flows from operating activities, borrowings under the RLOC, cash proceeds from the sale the businesses outlined in the Company’s restructuring plan, proceeds from the Company’s debt offerings, capital markets, and/or other financing arrangements. However, changes in capital markets or adverse changes to the Company’s operations may disrupt the Company’s ability to maintain adequate levels of liquidity, including its ability to renew its RLOC in 2016 on terms acceptable to the Company.
If the Company is unable to generate sufficient cash flow from operating activities, the Company may be forced to adopt strategies that may include the following:
| • | sell assets; |
| • | restructure or refinance existing indebtedness; |
| • | seek additional equity capital; and |
| • | reduce or delay acquisitions and capital expenditures. |
2012 Notes
On February 24, 2012, the Company issued $250.0 million aggregate principal of 6.375% senior notes, which mature on March 1, 2022 (the “2012 Notes”). The operating and financial restrictions and covenants governing the RLOC and the indenture that governs the 2012 Notes may adversely affect the Company’s ability to finance future operations or capital needs or to engage in other business activities. Under the 2012 Notes, the Company is required
19
Table of Contents
to comply with certain operating and financial covenants, and, in certain circumstances, to satisfy and maintain specified financial ratios and tests. In addition, the indenture governing the 2012 Notes contains financial and other covenants that limit the Company’s ability to engage in certain activities, some of which may be in the Company’s long-term best interests, including the ability to:
| • | borrow money or sell preferred stock; |
| • | create liens; |
| • | pay dividends on or redeem or repurchase stock; |
| • | make certain types of investments, including acquisitions; |
| • | enter into agreements restricting the Company’s subsidiaries’ ability to pay dividends or make other payments to the Company; |
| • | enter into transactions with affiliates; |
| • | issue guarantees of debt; and |
| • | sell assets or merge with other companies. |
The Company’s failure to comply with any of the restrictions or covenants in the indenture governing the 2012 Notes could result in an event of default, which, if not cured or waived, would result in the acceleration of all of the indebtedness under the Company’s debt agreements, including the indenture governing the 2012 Notes.
2008 Notes
The Company’s common stock experiences price and volume fluctuations. Trading prices of the Company’s common stock may be influenced by operating results, projections, and economic, financial, regulatory, and other factors. In addition, general market conditions, including the level of, and fluctuations in, the trading prices of stocks generally, could affect the price of the Company’s common stock.
In August 2008, the Company issued $230.0 million aggregate principal of 3.125% senior convertible notes (“2008 Notes”), which mature on August 1, 2014. The market price of the 2008 Notes is expected to be significantly affected by the market price of the Company’s common stock as well as the general level of interest rates and the Company’s credit quality. This may result in a significantly greater volatility in the trading value of the 2008 Notes than would be expected for nonconvertible debt securities the Company may issue.
The price of the Company’s common stock may also be affected by possible sales of the Company’s common stock by investors who view the 2008 Notes as a more attractive means of equity participation in the Company and by hedging or arbitrage activity involving the Company’s common stock as a result of the issuance of the 2008 Notes. The hedging or arbitrage activity, in turn, could affect the trading prices of the 2008 Notes and common stock.
The Company faces potential litigation and liability exposure for product liability and other claims against the Company.
The Company is a distributor of medical products, equipment, and pharmaceutical products. As a result, there is a risk that injury or other liability arising from the use or transportation of the products may occur and result in litigation against the Company. Accordingly, the Company maintains various insurance policies, including product liability insurance, to cover such exposure at amounts that management considers adequate. However, there can be no assurance the coverage maintained by the Company under various insurance policies is sufficient to cover future claims or will be available in adequate amounts at a reasonable cost. In many cases, the manufacturer of the product for any product liability claims may indemnify the Company; however, these agreements may not apply to products sourced through alternate channels. Additionally, there can be no assurance that indemnification agreements provided by manufacturers will adequately protect the Company, particularly the enforceability of indemnification provisions provided by overseas suppliers for globally-sourced products. These risks increase as more of the Company’s sales relate to globally sourced products and products purchased through alternate channels. A successful claim brought against the Company in excess of available insurance or indemnification agreements, or any claim that results in significant adverse publicity against the Company, could harm the Company’s business, reputation, and results of operations.
20
Table of Contents
In addition to product liability claims, the Company is subject to various legal and administrative proceedings and claims arising in the normal course of business, which are described in Footnote 19,Commitments and Contingencies, of the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. The outcomes of such proceedings or claims that are unasserted, pending, or known to be threatened could have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
The Company faces risk that its proprietary rights may infringe on the rights of third parties and that the protection offered by its proprietary rights may not be adequate.
The Company relies on a combination of patent, copyright and trademark laws, nondisclosure and other contractual provisions to protect a number of its products, services, and intangible assets. These proprietary rights are important to the Company��s ongoing operations. There can be no assurance these protections will provide meaningful protection against competitive products or services or otherwise be commercially viable or the Company will be successful in obtaining additional intellectual property or enforcing its intellectual property rights against unauthorized users.
From time-to-time, outside parties may assert infringement claims against the Company. If the Company was found to be infringing on other’s rights, the Company may be required to pay substantial damage awards, obtain a license, or cease selling the products that contain the infringing property. Such actions may be significant and result in material losses to the Company.
Failure to comply with existing and future regulatory and legal requirements could adversely affect the Company’s results of operations and financial condition.
General
The healthcare industry is highly regulated and the Company is subject to various federal, state, local, and foreign laws and regulations, which include the DEA, the FDA, various state boards of pharmacy, state health departments, the United States Department of Health and Human Services (“HHS”), the Occupational Safety and Health Administration (“OSHA”), the CMS, various State Attorneys General, State Medicaid fraud units, and other comparable agencies. Certain of the Company’s distribution service centers may be required to register for permits and/or licenses with, and comply with operating and security standards of the DEA, the FDA, HHS, and various state boards of pharmacy, state health departments, and/or comparable state agencies as well as certain accrediting bodies depending upon the type of operations and location of product distribution, manufacturing, and sale. Enforcement activity with regards to these laws and regulations has increased recently and the Company expects it to continue to increase. In addition, the Company’s vehicle fleet is subject to extensive regulation by the Department of Transportation. Although the Company believes it is in compliance, in all material respects, with applicable laws and regulations, any non-compliance could have a material adverse effect on the Company.
The noncompliance by the Company with applicable laws and regulations or the failure to maintain, renew, or obtain necessary permits and licenses could have an adverse effect on the Company’s results of operations and financial condition. In addition, if changes were to occur to the laws and regulations applicable to the Company’s businesses, such changes could adversely affect many of the Company’s regulated operations or could otherwise restrict the Company’s existing operations, limit the expansion of the Company’s businesses, apply regulations to previously unregulated businesses, or otherwise affect the Company adversely. The costs associated with complying with federal and state regulations may be significant and failure to comply with any such laws and regulations could have a material adverse effect on the Company, including criminal and civil penalties, administrative sanctions, quarantine and destruction of inventory, fines, and other adverse actions.
The manufacture, distribution, and marketing of certain of the Company’s products are subject to extensive ongoing regulation by the FDA. Failure to comply with the requirements of the FDA could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution of products, civil or criminal sanctions, refusal of the government to grant approvals, restrictions on operations, or withdrawal of existing approvals. Any of these actions could cause a loss of customer confidence in the Company and its products which could adversely affect the Company’s sales. In addition, third parties may file claims against the Company in connection with these issues.
21
Table of Contents
Laws relating to physician dispensing
Certain physician medication dispensing solutions could be adversely affected by legislation that would provide restrictions and /or limitations that could affect profitability. In addition, the Company expects that an increase in enforcement activity of the laws and regulations surrounding this type of business activity will continue and could potentially inhibit or eliminate this business, impairing the Company’s goodwill balance.
Laws relating to healthcare fraud
The Company is subject to extensive and frequently changing federal and state laws and regulations relating to healthcare fraud. The federal government continues to increase enforcement of practices involving healthcare fraud. The Company’s relationships with manufacturers and healthcare providers subject the business to laws and regulations on fraud and abuse which, among other things, (i) prohibit persons from soliciting, offering, receiving, or paying any remuneration in order to induce the referral of a patient for treatment or to induce the ordering, purchasing, leasing, or arranging for or recommending ordering, purchasing or leasing of items or services that are in any way paid for by government-sponsored healthcare programs, and (ii) impose a number of restrictions upon referring physicians and providers of designated health services under government healthcare programs. While the Company believes that it is substantially compliant with all applicable laws, many of the applicable regulations are vague or indefinite and have not been interpreted by regulators or the courts. They may be interpreted or applied in a manner that could require changes in operations. In addition, the Federal False Claims Act creates a financial incentive for private individuals to bring suit on behalf of the government to recover funds paid pursuant to a false claim, which may include failure to comply with technical requirements for claim submission, coding, and billing. If the Company fails to comply with applicable laws and regulations, it could suffer civil and criminal penalties, including substantial fines or penalties, and other sanctions, including exclusion from participation in any federal healthcare program.
Laws affecting the Company’s foreign operations
The Company is subject to the FCPA, which generally prohibits United States companies from engaging in bribery or prohibited payments to foreign officials for the purpose of obtaining or retaining business. FCPA enforcement has increased significantly in recent years. The Company has implemented safeguards to prevent and discourage violations of the FCPA. There is no assurance, however, that these safeguards will be effective. If employees or other agents are found to have violated the FCPA, the Company could suffer severe penalties and other consequences that may have a material adverse effect on the Company’s business, financial condition, and results of operations.
Tax legislation initiatives and audits by tax authorities could adversely affect the Company’s net earnings and tax liabilities
The Company is subject to the tax laws and regulations of the United States federal, state, and local governments and certain foreign governments. Various legislative initiatives may be proposed, including those to alter the taxation of the Company’s earnings from foreign operations, which could adversely affect the Company’s tax positions. There can be no assurance that the Company’s effective tax rate will not be adversely affected by these initiatives. In addition, United States federal, state, and local tax laws and regulations are extremely complex and subject to varying interpretations. Although the Company believes that its historical tax positions are sound and consistent with applicable laws, regulations, and existing precedent, there can be no assurance that the Company’s tax positions will not be challenged by relevant tax authorities or that the Company would be successful in any such challenge.
From time to time the Company is audited by United States federal, state, local and foreign tax authorities. If these audits result in assessments different from recorded reserves, the Company’s future results may include unfavorable adjustments to tax liabilities.
See Item 1 “Business – Regulatory Matters” for additional information.
22
Table of Contents
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
The Company has received no written comments regarding its periodic or current reports from the staff of the Securities and Exchange Commission that were issued 180 days or more preceding the end of fiscal year 2012 and that remain unresolved.
| ITEM 2. | PROPERTIES |
The Company leases warehouse and office space for its full-service distribution centers, break-freight locations, redistribution facilities, and other operations-related facilities in various locations across the United States. In the normal course of business, management regularly assesses its business needs and makes changes to the capacity and location of these leased facilities. As of March 30, 2012, the Company believes its distribution infrastructure is adequate to carry on its business as currently conducted and that, if necessary, it could find additional and/or replacement facilities to lease without suffering a material adverse effect on its business.
23
Table of Contents
The following tables identify the full-service distribution centers, break-freight locations, redistribution facilities, and other operations-related facilities for each operating segment:
Physician Business | ||||||||
Full-Service Distribution Center Locations | ||||||||
| Aiea, HI | Fullerton, CA | Madison, TN | Santa Ana, CA | |||||
| Birmingham, AL | Gainesville, GA | Memphis, TN | Schertz, TX * | |||||
| Auburn, WA * | Grand Prairie, TX | New Hyde Park, NY | St. Rose, LA | |||||
| Charlotte, NC * | Houston, TX * | Olathe, KS * | Wareham, MA | |||||
| Colonial Heights, VA | Kennesaw, GA | Orlando, FL | West Columbia, SC | |||||
| Denver, CO | Kennesaw, GA | Phoenix, AZ | West Sacramento, CA | |||||
| Elgin, IL | Leetsdale, PA | Rochester, NY | ||||||
| Flowood, MS | Louisville, KY | Rogers, MN | ||||||
| Fairfield, NJ * | Lubbock, TX | Salt Lake City, UT | ||||||
Break-Freight Locations | ||||||||
| Albany, NY | Fresno, CA | Mesquite, TX * | Southfield, MI | |||||
| Baton Rouge, LA | Gahanna, OH * | Middletown, PA * | St. Charles, MO | |||||
| Big Bend, WI | Gresham, OR | Morrisville, NC * | St. Petersburg, FL | |||||
| Bloomfield, CT | Jacksonville, FL * | Nashville, TN | Tallahassee, FL | |||||
| Chatsworth, CA | Knoxville, TN | Newark, CA | Trussville, AL | |||||
| Chattanooga, TN | Lafayette, LA | Omaha, NE * | Tulsa, OK | |||||
| Chesapeake, VA | Lanham, MD | Pompano Beach, FL | Tyler, TX | |||||
| Cincinnati, OH * | Las Vegas, NV | Ridgeland, MS * | Warminster, PA * | |||||
| Columbia, SC | Macedonia, OH | Roanoke, VA | West Babylon, NY | |||||
| Fredericksburg, VA | Maumelle, AR * | San Diego, CA | ||||||
Redistribution Facilities | ||||||||
| Fullerton, CA * | Jacksonville, FL * | |||||||
Other | ||||||||
| Boise, ID | Channahon, IL | |||||||
Extended Care Business | ||||||||
Full-Service Distribution Center Locations | ||||||||
| Auburn, WA * | Gahanna, OH * | Omaha, NE * | Spokane, WA | |||||
| Augusta, GA | Londonderry, NH | Ontario, CA | Vancouver, WA | |||||
| Austell, GA | Mesquite, TX * | Orlando, FL | Windsor, WI | |||||
| Evanston, IL | Middletown, PA * | Ridgeland, MS * | ||||||
| Fort Lauderdale, FL | Morrisville, NC * | Sacramento, CA | ||||||
Break-Freight Locations | ||||||||
| Charlotte, NC * | Fairfield, NJ * | Maumelle, AR * | Warminster, PA * | |||||
| Cincinnati, OH * | Houston, TX * | Olathe, KS * | ||||||
| Eau Claire, WI | Indianapolis, IN | Schertz, TX * | ||||||
Redistribution Facilities | ||||||||
| Fullerton, CA * | Jacksonville, FL * | |||||||
Other | ||||||||
| Birmingham, AL | Redmond, WA | |||||||
| * | Facilities shared by Physician Business and Extended Care Business. |
24
Table of Contents
The Company’s Shared Services locations consist of approximately 150,000 square feet of leased office space located at 4345 Southpoint Boulevard and 4190 Belfort Road, Jacksonville, Florida 32216.
The Company also retains additional space for the purpose of providing support services to segment locations described in the previous table. These offices are located in the following cities: Charlotte, NC; Franklin, TN; Jacksonville, FL; Lake Forest, IL; Shanghai, China; and Woodstock, GA.
In the aggregate, the Company’s locations consist of approximately 2.9 million square feet of leased space. The lease agreements have expiration dates ranging from May 2012 to June 2021 and facilities ranging in size from approximately 1,000 square feet to 169,000 square feet.
| ITEM 3. | LEGAL PROCEEDINGS |
From time to time the Company is a party to various legal and administrative proceedings and claims arising in the normal course of business. While any litigation contains an element of uncertainty, the Company believes that the outcome of such other proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Shares of the Company’s common stock are quoted on Nasdaq Stock Market, Inc.’s Global Select Market (“NASDAQ GS”) under the ticker symbol “PSSI.” The following table presents, for the periods indicated, the range of high and low sale prices per share of the Company’s common stock as reported on NASDAQ GS:
Quarter Ended | High | Low | ||||||
Fiscal year ended March 30, 2012: | ||||||||
July 1, 2011 | $ | 29.47 | $ | 26.55 | ||||
September 30, 2011 | $ | 28.87 | $ | 19.14 | ||||
December 30, 2011 | $ | 25.05 | $ | 18.51 | ||||
March 30, 2012 | $ | 25.99 | $ | 22.67 | ||||
Fiscal year ended April 1, 2011: | ||||||||
July 2, 2010 | $ | 24.45 | $ | 20.51 | ||||
October 1, 2010 | $ | 21.79 | $ | 18.15 | ||||
December 31, 2010 | $ | 24.11 | $ | 20.50 | ||||
April 1, 2011 | $ | 27.36 | $ | 22.47 | ||||
Cash Dividends
Since inception, the Company has neither declared nor paid cash dividends, and intends to continue to retain earnings for the growth and development of the Company’s business; therefore, does not anticipate the declaration of a cash dividend in the immediate future. The Company’s revolving line of credit agreement contains certain covenants that limit the amount of cash dividends that may be declared by the Company.
25
Table of Contents
Holders of Common Stock
As of May 18, 2012, there were approximately 1,372 holders of record of the Company’s common stock.
Performance Graph
The graph below compares the cumulative total stockholder return on $100 invested, assuming reinvestment of dividends, if any, on March 30, 2007, the last trading day before the beginning of the Company’s fiscal year 2008 through the end of fiscal year 2012, with the cumulative return on $100 invested for the same period in the Nasdaq Stock Market (U.S. Companies) Composite Index.
The graph also compares the cumulative stockholder return to an index of companies management believes comprise the Company’s peer group, which includes the following: Amerisourcebergen Corporation, Baxter International, Inc., Cardinal Health, Inc., McKesson Corporation, Owens & Minor, Inc., Patterson Companies, Inc., and Henry Schein, Inc.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among PSS World Medical, Inc., The NASDAQ Composite Index
And A Peer Group
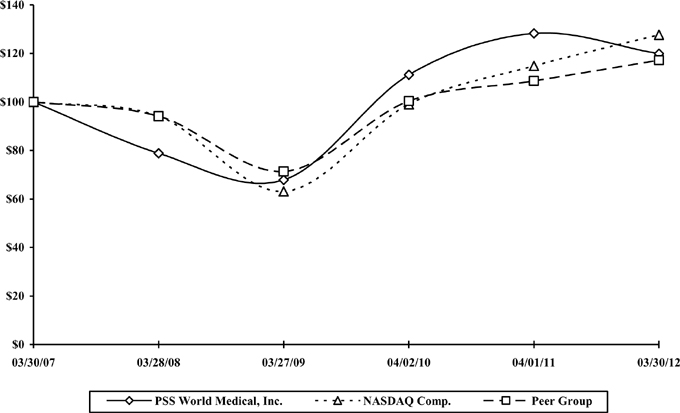
FISCAL YEAR ENDED MARCH 30, 2012 | ||||||||||||||||||||||||
| March 30, | March 28, | March 27, | April 2, | April 1, | March 30, | |||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |||||||||||||||||||
PSS World Medical, Inc. | 100.00 | 78.81 | 67.88 | 111.21 | 128.25 | 119.87 | ||||||||||||||||||
NASDAQ Composite | 100.00 | 94.11 | 63.12 | 99.02 | 114.84 | 127.66 | ||||||||||||||||||
Peer Group | 100.00 | 94.12 | 71.30 | 100.38 | 108.70 | 117.20 | ||||||||||||||||||
| * | Information presented above assumes $100 invested on March 30, 2007 and that dividends were reinvested. |
26
Table of Contents
Issuer Purchases of Equity Securities
From time to time, the Company’s Board of Directors authorizes the purchase of its outstanding common shares. The Company is authorized to repurchase a determined amount of its total common stock. Repurchases can be made in the open market, privately negotiated transactions, and other transactions publicly disclosed through filings with the SEC. The Company’s stock repurchase programs do not have an expiration date.
The following table summarizes the common stock repurchases and Board of Directors authorizations during the period from April 1, 2011 to March 30, 2012.
| (in thousands) | Shares | |||
Shares available for repurchase as of April 1, 2011 | 3,352 | |||
Additional shares authorized for repurchase | 2,680 | |||
Shares repurchased | (5,595 | ) | ||
|
| |||
Shares available for repurchase as of March 30, 2012 | 437 | |||
|
| |||
During fiscal year 2012, the Company repurchased approximately 5.6 million shares of common stock under these programs at an average price of $25.10 per common share for approximately $140.4 million.
The following table summarizes the Company’s repurchase activity during the three months ended March 30, 2012.
Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs | ||||||||||||||
January 2—January 30 | 444 | $ | 24.20 | 444 | 1,456,296 | |||||||||||||
January 31—February 29 | 329,406 | 24.55 | 329,406 | 1,126,890 | ||||||||||||||
March 1—March 30 | 689,696 | 24.04 | 689,696 | 437,194 | ||||||||||||||
|
|
|
| |||||||||||||||
Total fourth quarter | 1,019,546 | $ | 24.21 | 1,019,546 | 436,750 | |||||||||||||
|
|
|
| |||||||||||||||
27
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The selected financial data for fiscal years 2008 through 2012 have been derived from the Company’s consolidated financial statements, which give retroactive effect to the restatement related to adoption of the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) 470-20,Debt –Debt with Conversion and Other Options. The selected financial data below should be read in conjunction with the Company’s financial statements and the notes thereto and Item 7,Management’s Discussion and Analysis of Financial Condition and Results of Operations.
| Fiscal Year Ended | ||||||||||||||||||||
| 2012 | 2011 | 2010 | 2009 | 2008 | ||||||||||||||||
| (Dollars in thousands, except per share data) | ||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Net sales | $ | 2,102,002 | $ | 2,034,789 | $ | 2,055,171 | $ | 1,952,691 | $ | 1,855,791 | ||||||||||
Net income attributable to PSS World Medical, Inc. | $ | 74,319 | $ | 74,485 | $ | 69,363 | $ | 51,486 | $ | 53,133 | ||||||||||
Earnings per share: | ||||||||||||||||||||
Basic | $ | 1.43 | $ | 1.35 | $ | 1.20 | $ | 0.86 | $ | 0.82 | ||||||||||
Diluted | $ | 1.38 | $ | 1.32 | $ | 1.18 | $ | 0.85 | $ | 0.80 | ||||||||||
Weighted average shares outstanding: | ||||||||||||||||||||
Basic | 51,998 | 54,996 | 58,029 | 59,937 | 64,703 | |||||||||||||||
Diluted | 53,989 | 56,546 | 58,943 | 60,696 | 66,184 | |||||||||||||||
Ratio of earnings to fixed charges(a) | 5.1 | 5.7 | 4.2 | 3.3 | 4.5 | |||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
Working capital(b) | $ | 485,365 | $ | 345,402 | $ | 355,606 | $ | 323,545 | $ | 97,454 | ||||||||||
Total assets | $ | 1,155,970 | $ | 951,672 | $ | 872,066 | $ | 858,624 | $ | 813,236 | ||||||||||
Long-term liabilities(b) | $ | 564,832 | $ | 305,942 | $ | 277,994 | $ | 241,684 | $ | 65,198 | ||||||||||
| (a) | For the purpose of calculating the ratio of earnings to fixed charges, earnings consist of income from operations before provision for income taxes, plus fixed charges, less capitalized interest. Fixed charges consist of interest, whether expensed or capitalized, amortization of debt issuance costs, and the portion of rental expense estimated by management to be attributable to interest. |
| (b) | Fiscal year 2008 working capital and long-term liabilities reflect a reclassification of $150 million of 2.25% senior convertible notes from long-term to current liabilities made during fiscal year 2009. |
28
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
THE COMPANY
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation, began operations in 1983. The Company is a national distributor of medical products and supplies, diagnostic equipment, healthcare information technology and pharmaceutical products, and provides professional and consulting services to the physician, long-term care, assisted living, home health care, and hospice markets. The Company has full service distribution centers strategically located to efficiently serve all 50 states throughout the United States.
The Company’s business decisions and strategies are guided by its Purpose and Mission. The Company’s stated Purpose is to strengthen the clinical success and financial health of caregivers by solving their biggest problems. The Company’s Mission is to improve caregivers’ financial performance by 20%.
The Company currently conducts business through two operating segments, the Physician Business and the Extended Care Business, which serve a diverse customer base through full-service distribution centers, in all 50 states throughout the U.S. During the year ended March 30, 2012, the Company rebranded its Elder Care Business to the “Extended Care Business” to more appropriately align with its customer base. A third reporting segment, Shared Services, consists of departments that support the operating segments through the delivery of standardized service. For information on comparative segment revenue, segment profit and related financial information, refer to Footnote 18,Segment Information, of the consolidated financial statements.
PSSI is a market leader in the two alternate-site customer segments it serves as a result of a high-touch, differentiated business model; value-added, solutions-based marketing programs; a consultative sales force with extensive product, disease state, reimbursement, and supply chain knowledge; a successfully expanded product and service offering including unique arrangements with manufacturers and a full line of the Company’s store brands; innovative information systems and customer-facing technologies that serve its core markets; and a culture of performance.
During the first quarter of fiscal year 2013, the Company’s Board of Directors approved a strategic restructuring plan designed to transform the Company by focusing on four lines of business – Physician, Laboratory, In-Office Dispensing, and Home Care and Hospice. The restructuring plan will include the sale of two business units serving skilled nursing facilities within the Extended Care Business and specialty dental practices within the Physician Business. Additionally, the plan includes the integration of warehouse operations into one common distribution infrastructure, as well as a redesign of the shared services function. These efforts are expected to reduce operating costs as a percentage of net sales, while streamlining decision making and improving service. The Company expects to complete the restructuring plan within the next several fiscal years. Except as otherwise noted, the information contained in this Form 10-K reflects Company information as of March 30, 2012, before the announcement and commencement of the strategic restructuring plan.
THE COMPANY’S STRATEGY
The Company’s objective is to be the leading distributor and marketer of medical products and services to select customer segments in the U.S., with a goal to grow revenues at twice the market growth rate in the markets it serves. The key components of the Company’s strategy to achieve its Purpose and Mission include:
Reach and Strengthen:Grow sales through differentiated marketing programs, innovative products and services and new customer acquisitions.The Company believes its sales professionals, which consists of approximately 1,020 employees, and their customer relationships and knowledge are strategic competitive advantages. The Company has developed tailored sales force training focused on developing and building unique relationships with customers and providing solutions though innovative marketing programs, exclusive products, and new product and technology offerings that strengthen its customers’ clinical outcomes and financial health. The Company plans to continue to make fold-in acquisitions and grow its sales force through its sales representative expansion initiative.
29
Table of Contents
Our Health:Optimize the Company’s product offering and profitability.The Company has developed and implemented programs to increase its profitability at the product and customer level. The Company continues to develop its domestic and global product sourcing capabilities to optimize its product offering by integrating sourcing and branding initiatives with customer, product and Company strategies to increase profitability. The Company intends to broaden its reach and breadth of products by (i) expanding its store brand product offerings, which generally have higher margins, (ii) increasing product quality and category management, and (iii) leveraging its sourcing capabilities, both foreign and domestic.
LEAN:Simplify and improve business activities to provide only what our customers value.The Company is making significant investments in its distribution infrastructure, information systems, process reengineering, and training to simplify its distribution and administrative infrastructure, develop easy to use scalable processes and systems that enable growth, reduce costs to serve, and achieve the Company’s commitment to providing superior customer service.LEAN process improvement initiatives focus on process redesign, investments in automation and organizational commitments to customer service in the most efficient manner. During fiscal year 2012, the Company continued to leverage its existing infrastructure investments and process improvements resulting in operating efficiencies, improved customer service levels and operating margins.
Identify, develop and retain leaders capable of managing a growing corporation. The Company is committed to the effective recruitment, hiring and promotion of employees with outstanding performance, culture and leadership abilities. The Company provides leadership development opportunities, individual leadership assessment and development plans, education and coaching programs to its employees. The Company’s goal is to develop a diverse group of individuals capable of leading a growing corporation.
Conduct business in a legal and ethical manner.The Company believes each employee is responsible for personal integrity and the consequences of actions, and is expected to follow the highest standards of ethics, honesty, fairness and compliance with the law. The Company provides health, safety, and regulatory education training programs and a safe and positive work environment for its employees.
Be the employer of choice within the industry.The Company believes its management, sales force and employees are its most valuable assets. The Company seeks to foster a culture of performance and execution by designing employee incentive programs aligned with the Company’s Purpose and Mission, business strategies and objectives. The Company strives to be the employer of choice in the markets it serves, in terms of benefits offered to employees, availability of health and wellness programs, professional competency, growth opportunities, and personal development training.
Strategic acquisitions.The Company expects to continue to make strategic acquisitions to reach new customers, complement or expand its product offerings, leverage existing infrastructure and increase its market share and profitability.
EXECUTIVE OVERVIEW
During fiscal year 2012, the Company continued to grow sales despite continued low economic growth and weak utilization trends. Consolidated net sales increased 3.3% during fiscal year 2012 when compared to the prior year.
Consolidated income from operations decreased approximately $0.2 million, or 0.3% during the fiscal year ended March 30, 2012, and operating margins as a percentage of sales declined. The results for fiscal year 2012 were largely impacted by operating costs associated with acquired companies and acquisition-related expenses, partially offset by management’s focus on improving selling margin and reducing operating costs as a percentage of net sales through itsLEAN strategies, strategic acquisitions, and continued leverage of its distribution infrastructure. Additionally, net sales decreased during the year ended March 30, 2012 due to a historically light influenza season which resulted in an approximate $12.0 million to $16.0 million decline in net sales of influenza-related products.
Cash flows from operating activities during fiscal year 2012 was $128.3 million which, along with available cash balances, borrowings on its revolving line of credit, and proceeds from the issuance of its 6.375% senior notes, funded the Company’s stock repurchase program, investments in capital projects, and acquisitions during the fiscal year.
30
Table of Contents
The following significantly impacted the Company’s financial and operating results during fiscal years 2012, 2011, and 2010:
Acquisitions
During fiscal year 2012, the Company made a total of eight strategic acquisitions in both the Physician Business and Extended Care Business, with combined net sales of $16.6 million recorded in the Company’sConsolidated Statements of Operations as of March 30, 2012. Cash paid for acquisitions made during fiscal year 2012, 2011, and 2010 was $65.1 million, $65.9 million, and $14.8 million, respectively. Refer to Footnote 4,Purchase Business Combinations,for additional information.
Revolving Line of Credit
On November 16, 2011, the Company amended and restated the credit agreement related to its revolving line of credit (the “RLOC”) with the following features and key terms: (i) a five-year term, maturing on November 16, 2016; (ii) a facility size of $300.0 million, with increased borrowing capacity of $100.0 million via an accordion feature; and (iii) conditional covenants based on the Company’s borrowing availability and fixed charge coverage ratio requirements. See Footnote 12,Debt for additional information regarding the features and terms under the new RLOC.
Issuance of Senior Notes
On February 24, 2012, the Company issued $250.0 million aggregate principal 6.375% senior notes, which mature on March 1, 2022 (the “2012 Notes”). See Footnote 12,Debt for additional information regarding the features and terms of the 2012 Notes.
Global Sourcing Initiative
The Company’s global sourcing strategy involves purchasing products directly from contracted manufacturers and is a key initiative for the Company. Milestones reached during fiscal year 2012, 2011 and 2010 included (i) expanding the Company’s global sourcing resources in Asia and Europe, (ii) increasing the capacity of the redistribution infrastructure in the United States; (iii) expanding the store brand product offering, and (iv) designing and implementing a security assessment program for global manufacturers in compliance with the U.S. Customs Trade Partners Against Terrorism Act. As of March 30, 2012 and April 1, 2011, the Company had approximately $49.6 million and $46.0 million of globally-sourced product inventory, respectively. Management believes this initiative will continue to positively impact the Company’s results of operations in future years.
During fiscal year 2012, the Company completed a reorganization of its non-U.S. global sourcing subsidiaries. This reorganization increased the responsibilities and contributions of the non-U.S. subsidiaries, proportionally increasing their income and reducing the income of the U.S. subsidiaries. As the non-U.S. subsidiaries are generally subject to tax at rates lower than the U.S. subsidiaries, changes in the proportion of the Company’s taxable earnings originating outside the U.S. favorably impacts the effective tax rate. The Company expects this reorganization to continue to have a sustained positive impact on its effective tax rate; however, the Company cannot determine what impact, if any, the restructuring plan may have on the tax rate in future periods.
Change in Long-Term Incentive Compensation Estimate
During fiscal year 2012, the Company decreased its estimation of estimated achievement of performance targets related to long-term corporate incentive compensation plans based on actual and expected future financial performance. The change in estimate decreased Performance Share Units outstanding by approximately 98,000 shares. As a result of the change in performance estimate, stock based compensation expenses decreased $1.5 million.
31
Table of Contents
There were no material changes in estimates during fiscal year 2011.
During fiscal year 2010, management raised its estimation of expected achievement of performance targets related to long-term corporate incentive compensation plans. Due to the change in estimate, the Company recognized additional long-term incentive-based compensation expense of $11.4 million, offset by a decrease of $4.4 million in accruals for long-term incentive compensation related to the departure of the Company’s former Chairman and Chief Executive Officer.
See Footnote 15,Incentive and Stock-Based Compensation,for additional information.
Convertible Debt Transactions
During fiscal year 2009, the Company issued $230.0 million principal amount of 3.125% senior convertible notes (“2008 Notes”). As of March 30, 2012, April 1, 2011 and April 2, 2010, the Company was required to include shares underlying the 2008 Notes in its diluted weighted average shares outstanding, as the average stock price per share for the period exceeded $21.22 (the conversion price for the 2008 Notes). Prior to conversion, the purchased options from the convertible note hedge transaction are considered to be anti-dilutive. Refer to Footnote 12,Debt, for additional information.
H1N1 Influenza Pandemic
During fiscal year 2010, the Physician Business experienced increased sales of influenza test kits, surgical masks, medical gloves and hand sanitizers, and other product categories related to the H1N1 influenza pandemic. As a result, the Company recognized approximately $52.5 million in additional net sales during fiscal year 2010, which did not reoccur in fiscal year 2012 or 2011.
Investment in athenahealth, Inc.
During fiscal year 2010, the Company sold its investment in athenahealth, Inc. (“athena”), resulting in a gain of approximately $3.6 million ($2.3 million, net of tax). Refer to Footnote 7,Equity Investment,for additional information.
Subsequent Event—Restructuring Plan
During the first quarter of fiscal year 2013, the Company’s Board of Directors approved a strategic restructuring plan designed to transform the Company by focusing its efforts and investments on what it believes will be the fastest growing segments of non-acute, alternate site healthcare in the U.S. Specifically, the Company will focus on physician, laboratory, in-office dispensing, and the home care and hospice markets.
The restructuring plan will include the sale of two business units serving skilled nursing facilities and specialty dental practices, the integration of warehouse operations into one common distribution infrastructure, and a redesign of its shared services function. As such, current results of operations may not be indicative of future results.
32
Table of Contents
RESULTS OF OPERATIONS
FISCAL YEAR ENDED MARCH 30, 2012 VERSUS FISCAL YEAR ENDED APRIL 1, 2011
NET SALES
| For the Fiscal Year Ended | ||||||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||||||
| (dollars in millions) | Amount | Average Daily Net Sales | Amount | Average Daily Net Sales | Percent Change | |||||||||||||||
Physician Business | $ | 1,512.7 | $ | 6.0 | $ | 1,425.0 | $ | 5.6 | 6.2 | % | ||||||||||
Extended Care Business | 587.4 | 2.3 | 607.8 | 2.4 | (3.4 | ) | ||||||||||||||
Shared Services | 1.9 | — | 2.0 | — | (6.5 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total Company | $ | 2,102.0 | $ | 8.3 | $ | 2,034.8 | $ | 8.0 | 3.3 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Selling days | 253 | 253 | ||||||||||||||||||
Physician Business
Management evaluates the Physician Business by product category. The following table summarizes the growth rate by product category period over period.
| For the Fiscal Year Ended | ||||||||||||
| (dollars in millions) | March 30, 2012 | April 1, 2011 | Percent Change | |||||||||
Branded(a) | $ | 743.1 | $ | 751.5 | (1.1 | )% | ||||||
Store brand products and services(b) | 229.9 | 208.1 | 10.5 | |||||||||
Pharmaceutical products | 330.8 | 310.5 | 6.5 | |||||||||
Equipment(c) | 117.7 | 112.1 | 5.0 | |||||||||
Physician dispensing solutions | 84.3 | 36.9 | 128.5 | |||||||||
Other | 6.9 | 5.9 | 17.3 | |||||||||
|
|
|
| |||||||||
Total | $ | 1,512.7 | $ | 1,425.0 | 6.2 | % | ||||||
|
|
|
| |||||||||
Selling days | 253 | 253 | ||||||||||
| (a) | Branded products are comprised of disposables and lab diagnostics from branded manufacturers. |
| (b) | Store brand products and services are comprised of the Company’s brands of disposables, lab diagnostics, equipment, and laboratory consulting services. |
| (c) | Equipment from branded manufacturers. |
Overall, net sales during the fiscal year ended March 30, 2012 were positively impacted by revenue from acquisitions contributing to the physician dispensing solutions product category and continued success with the Company’sReach initiative resulting in the addition of new accounts during the period.
Net sales of branded products decreased during the year ended March 30, 2012 due to a historically light influenza season which resulted in an approximate $12.0 million to $16.0 million decline in net sales of influenza lab diagnostic test kits and other influenza-related products.
Net sales of store brand products and services increased during the year ended March 30, 2012 due to continued focus on the expansion of the store brands product category, resulting in new customer sales, as well as customer conversions from branded products to the Company’s store brands.
33
Table of Contents
Net sales of pharmaceutical increased during the fiscal year ended March 30, 2012 as a result of an existing manufacturer’s shift from a direct sales structure to a distribution-based structure, partially offset by a decrease in influenza vaccine and controlled pharmaceutical product sales compared to the prior fiscal year due to a historically light influenza season.
Net sales of equipment increased during the year ended March 30, 2012 due to increased demand, as prior fiscal year sales were negatively impacted by a decrease in discretionary spending, economic conditions, and tight credit markets which impacted the ability of physicians to obtain financing and delayed equipment purchases.
During fiscal years 2011 and 2012, the Physician Business made several strategic acquisitions of companies providing physician pharmaceutical dispensing products and services, establishing a new product category, physician dispensing solutions, which contributed approximately $84.3 million in net sales during the year ended March 30, 2012.
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan, which includes the sale of a business serving specialty dental practices representing approximately $46.5 million and $44.6 million of net sales as of March 30, 2012 and April 1, 2011, respectively.
Extended Care Business
Management evaluates the Extended Care business by customer category. During fiscal year 2012, certain customers were reclassified within these categories to better align with standard industry classifications. As a result, prior periods were recast to be consistent with current year presentation. The following table summarizes the change in net sales by customer segment period over period.
| For the Fiscal Year Ended | ||||||||||||
| (dollars in millions) | March 30, 2012 | April 1, 2011 | Percent Change | |||||||||
Nursing home and assisted living facilities | $ | 346.7 | $ | 356.4 | (2.7 | )% | ||||||
Hospice and home health care agencies | 178.2 | 187.0 | (4.7 | ) | ||||||||
Billing services | 10.5 | 12.0 | (11.8 | ) | ||||||||
Other | 52.0 | 52.4 | (0.7 | ) | ||||||||
|
|
|
| |||||||||
Total | $ | 587.4 | $ | 607.8 | (3.4 | )% | ||||||
|
|
|
| |||||||||
Selling days | 253 | 253 | ||||||||||
Net sales during the fiscal year ended March 30, 2012 compared to the prior year decreased approximately $20.4 million. Net sales in the nursing home and assisted living facilities and the hospice and home health care customer categories were negatively impacted by the loss of several regional and national chain customers.
Billing services net sales were negatively impacted by contractual billing adjustments related to Medicare and Medicaid billings and accounts lost due to competitive bidding.
Net sales of store brand products increased 7.3% during fiscal year 2012 when compared to fiscal year 2011 as a result of continued focus on the expansion of the store brands product category, resulting in new customer sales as well as customer conversions from branded products to the Company’s store brands.
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan, which includes the sale of a business serving skilled nursing facilities, representing a portion of the net sales within theNursing home and assisted living facilities andBilling services customer categories. As of the filing date, the Company could not determine the impact of the potential sale on future periods.
34
Table of Contents
GROSS PROFIT
Physician Business
Gross profit dollars for the Physician Business increased $44.2 million and gross margins increased 106 basis points during fiscal year 2012. The increase in gross profit dollars and gross margin was a result of growth in the Company’s store brand products and acquisitions in the physician dispensing solutions product category, which generally have higher gross margins than the Company’s other product categories.
Extended Care Business
Gross profit dollars in the Extended Care Business decreased $5.7 million while gross margin increased 4 basis points during fiscal year 2012. Gross profit dollars were negatively impacted by the reduction in net sales and competitive pricing pressures, while increased sales of store brand products positively impacted gross margin.
GENERAL AND ADMINISTRATIVE EXPENSES
| For the Fiscal Year Ended | ||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business(a) | $ | 229.6 | 15.2 | % | $ | 203.0 | 14.2 | % | ||||||||
Extended Care Business(a) | 124.4 | 21.2 | 119.5 | 19.7 | ||||||||||||
Shared Services(b) | 39.0 | 1.9 | 42.2 | 2.1 | ||||||||||||
|
|
|
| |||||||||||||
Total Company(b) | $ | 393.0 | 18.7 | % | $ | 364.7 | 17.9 | % | ||||||||
|
|
|
| |||||||||||||
| (a) | General and administrative expenses as a percentage of net sales are calculated based on reportable segment net sales. |
| (b) | General and administrative expenses as a percentage of net sales are calculated based on consolidated net sales. |
General and administrative expenses are impacted by (i) compensation and employee benefit costs; (ii) cost to deliver, which represents all costs associated with the warehousing, transportation and delivery of products to customers; (iii) shared services overhead costs; and (iv) general and administrative expenses of acquired companies and acquisition-related costs.
Physician Business
General and administrative expenses increased $26.6 million during the fiscal year ended March 30, 2012, when compared to the prior fiscal year. This increase was attributable to (i) an increase in payroll and payroll-related expenses of $13.7 million, $10.1 million of which was the result of the physician dispensing solutions acquisitions; (ii) an increase in depreciation and amortization expense of $4.4 million due to the addition of property and equipment and intangible assets related to acquisitions; (iii) an increase in cost to deliver of $3.0 million due to an increase in warehouse expense related to the growth in net sales during the year, and additional expenses from physician dispensing solutions acquisitions; (iv) an increase in consulting fees of $1.6 million related to acquired companies; and (v) an increase in allocated corporate expenses of $1.5 million, partially offset by a decrease in accrued incentive compensation expense of $2.2 million related to payout estimates based on performance.
Extended Care Business
General and administrative expenses increased $4.9 million during the fiscal year ended March 30, 2012, when compared to the prior fiscal year. The increase was attributable to (i) an increase in payroll and payroll-related expenses of $2.9 million due to acquisitions and the timing of the Company’s consolidation of Pathway; and (ii) an increase in corporate allocated expenses of $1.1 million, partially offset by a decrease in accrued incentive compensation expense of $0.7 million related to payout estimates based on performance.
35
Table of Contents
Shared Services
General and administrative expenses decreased $3.2 million during fiscal year 2012, when compared to the prior fiscal year. The decrease was attributable to (i) a decrease in accrued incentive and stock-based compensation expense of $11.4 million related to payout estimates based on performance; and (ii) a decrease in business insurance costs of $1.8 million, partially offset by (i) an increase in payroll and payroll-related expenses of $5.4 million; and (ii) and increase in consulting fees of $2.8 million.
SELLING EXPENSES
| For the Fiscal Year Ended | ||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business | $ | 127.4 | 8.4 | % | $ | 116.7 | 8.2 | % | ||||||||
Extended Care Business | 20.5 | 3.5 | 20.8 | 3.4 | ||||||||||||
|
|
|
| |||||||||||||
Total Company | $ | 147.9 | 7.0 | % | $ | 137.5 | 6.8 | % | ||||||||
|
|
|
| |||||||||||||
Selling expenses are principally driven by commission expenses, which are generally paid to sales representatives based on gross profit dollars and gross margin. The increase in Physician Business selling expenses was due to the impact of its sales representative expansion initiative, while the change in selling expenses as a percentage of net sales for the Physician Business and Extended Care Business was consistent with the change in gross profit dollars and gross margin year over year.
INCOME FROM OPERATIONS
| For the Fiscal Year Ended | ||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business | $ | 144.8 | 9.6 | % | $ | 138.0 | 9.7 | % | ||||||||
Extended Care Business | 27.6 | 4.7 | 37.8 | 6.2 | ||||||||||||
Shared Services | (39.0 | ) | — | (42.2 | ) | — | ||||||||||
|
|
|
| |||||||||||||
Total Company | $ | 133.4 | 6.3 | % | $ | 133.6 | 6.6 | % | ||||||||
|
|
|
| |||||||||||||
Income from operations for each business segment changed due to the factors discussed above. Business simplification andLEANstrategies, in conjunction with the successful integration of the Company’s strategic acquisitions, are expected to partially offset the effects of the lower net sales growth.
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan. The restructuring plan will include the sale of two business units serving: (i) specialty dental practices, representing approximately $7.0 million and $6.1 million of income from operations within the Physician Business during the fiscal years 2012 and 2011, respectively, and (ii) skilled nursing facilities within the Extended Care Business. Additionally, the restructuring plan includes the integration of warehouse operations into one common distribution infrastructure, and a redesign of its shared services function. As of the filing date, the Company could not determine the impact of the potential sale within the Extended Care Business and additional restructuring activities on future periods.
36
Table of Contents
INTEREST EXPENSE
The Company’s debt structure during fiscal year 2012 consisted of its 2012 Notes, 2008 Notes, and variable rate borrowings under its RLOC. The following table summarizes the various components of total interest expense and interest rates applicable to the borrowings outstanding under the RLOC:
| For the Fiscal Year Ended | Increase (Decrease) | |||||||||||
| (dollars in millions) | March 30, 2012 | April 1, 2011 | ||||||||||
Components of interest expense: | ||||||||||||
Interest on borrowings | $ | 20.0 | $ | 16.7 | $ | 3.3 | ||||||
Debt issuance costs | 1.1 | 0.9 | 0.2 | |||||||||
Less: Capitalized interest | (0.9 | ) | (0.5 | ) | (0.4 | ) | ||||||
|
|
|
|
|
| |||||||
Total interest expense | $ | 20.2 | $ | 17.1 | $ | 3.1 | ||||||
|
|
|
|
|
| |||||||
Weighted average interest rate-RLOC(a) | 2.27 | % | 2.37 | % | (0.10 | )% | ||||||
Average daily borrowings under the RLOC | $ | 44.4 | $ | 3.8 | $ | 40.6 | ||||||
| (a) | Weighted average interest rate excludes debt issuance costs and unused line fees. |
Interest expense increased during the fiscal year ended March 30, 2012 as average daily borrowings under the RLOC were higher, due to an increase in acquisition and share repurchase activity. In addition, approximately $1.3 million of the increase was a result of having both the 2008 Notes and 2012 Notes outstanding during fiscal year 2012, while only the 2008 Notes were outstanding during fiscal year 2011.
OTHER INCOME
| For the Fiscal Year Ended | ||||||||||||||||
| (dollars in millions) | March 30, 2012 | April 1, 2011 | Decrease | Percent Change | ||||||||||||
Total Company | $ | 2.1 | $ | 2.5 | $ | (0.4 | ) | (16.8 | )% | |||||||
Other income during the fiscal year ended March 30, 2012 remained relatively consistent with prior year and is mainly attributable to customer finance charges.
PROVISION FOR INCOME TAXES
| For the Fiscal Year Ended | ||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||
| (dollars in millions) | Amount | Effective Rate | Amount | Effective Rate | ||||||||||||
Total Company | $ | 41.1 | 35.6 | % | $ | 44.6 | 37.4 | % | ||||||||
The effective rate for the twelve months ended March 30, 2012 was impacted by a reorganization of the Company’s non-U.S. global sourcing subsidiaries. This reorganization increased the responsibilities and contributions of the non-U.S. subsidiaries, proportionally increasing their income and reducing the income of the U.S. subsidiaries. As the non-U.S. subsidiaries are generally subject to tax at rates lower than the U.S. subsidiaries, changes in the proportion of the Company’s taxable earnings originating outside the U.S. favorably impacts the effective tax rate. The Company expects this reorganization to continue to have a sustained positive impact on its effective tax rate; however, the Company cannot determine what impact, if any, the restructuring plan may have on the tax rate in future periods.
37
Table of Contents
RESULTS OF OPERATIONS
FISCAL YEAR ENDED APRIL 1, 2011 VERSUS FISCAL YEAR ENDED APRIL 2, 2010
NET SALES
| For the Fiscal Year Ended | ||||||||||||||||||||||||
| April 1, 2011 | April 2, 2010 | |||||||||||||||||||||||
| (dollars in millions) | Amount | Average Daily Net Sales | Amount | Average Daily Net Sales | Total Percent Change | Average Daily Net Sales Percent Change | ||||||||||||||||||
Physician Business | $ | 1,425.0 | $ | 5.6 | $ | 1,437.8 | $ | 5.6 | (0.9 | )% | 1.1 | % | ||||||||||||
Extended Care Business | 607.8 | 2.4 | 614.9 | 2.4 | (1.2 | ) | 0.8 | |||||||||||||||||
Shared Services | 2.0 | — | 2.5 | — | (15.9 | ) | (14.2 | ) | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
Total Company | $ | 2,034.8 | $ | 8.0 | $ | 2,055.2 | $ | 8.0 | (1.0 | )% | 1.0 | % | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
Physician Business
Management evaluates the Physician Business by product category. The following table summarizes the growth rate by product category period over period.
| For the Fiscal Year Ended | ||||||||||||
| (dollars in millions) | April 1, 2011 | April 2, 2010 | Percent Change | |||||||||
Branded(a) | $ | 751.5 | $ | 803.2 | (6.4 | )% | ||||||
Store brand products and services(b) | 208.1 | 199.3 | 4.4 | |||||||||
Pharmaceutical products | 310.5 | 312.8 | (0.7 | ) | ||||||||
Equipment | 112.1 | 118.8 | (5.6 | ) | ||||||||
Physician dispensing solutions | 36.9 | — | — | |||||||||
Other | 5.9 | 3.7 | 53.8 | |||||||||
|
|
|
| |||||||||
Total | $ | 1,425.0 | $ | 1,437.8 | (0.9 | )% | ||||||
|
|
|
| |||||||||
Selling days | 253 | 258 | ||||||||||
| (a) | Branded products are comprised of disposables and lab diagnostics from branded manufacturers. |
| (b) | Store brand products and services are comprised of the Company’s brands of disposables, lab diagnostics, equipment and laboratory consulting services. |
Net sales growth during the fiscal year ended April 1, 2011 decreased as a result of (i) fiscal year 2010 H1N1 related sales, (ii) five fewer selling days, (iii) the state of the overall economy resulting in decreased physician office visits, offset by (iv) sales from fiscal year 2011 acquisitions. During the fiscal year ended April 1, 2011, the Company continued to make strategic acquisitions in the Physician Business. Net sales from fiscal year 2011 acquisitions were $41.9 million, of which $36.9 million related to acquisitions in the physician dispensing solutions line of business. During the fiscal year ended April 2, 2010, the Physician Business increased sales in influenza test kits, surgical masks, medical gloves, hand sanitizer, and other products related to the H1N1 pandemic, recording approximately $52.5 million in additional sales across the branded and store brand product categories.
Store brand product sales increased 4.4% due to the Company’s continued focus on promoting its globally sourced products, which resulted in new customer sales as well as customer conversions from other manufacturer branded products to store brand products.
Equipment sales decreased as a result of general economic conditions and lower availability of credit for physician practices.
38
Table of Contents
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan, which includes the sale of a business serving specialty dental practices representing approximately $44.6 million and $38.1 million of revenue as of April 1, 2011 and April 2, 2010, respectively.
Extended Care Business
Management evaluates the Extended Care business by customer category. During fiscal year 2012, certain customers were reclassified amongst these categories to better align with standard industry classifications. As a result, prior periods were recast to be consistent with current year presentation. The following table summarizes the change in net sales by customer segment period over period.
| For the Fiscal Year Ended | ||||||||||||
| (dollars in millions) | April 1, 2011 | April 2, 2010 | Percent Change | |||||||||
Nursing home and assisted living facilities | $ | 356.4 | $ | 377.7 | (5.3 | )% | ||||||
Hospice and home health care agencies | 187.0 | 180.6 | 4.1 | |||||||||
Billing services | 12.0 | 13.3 | (9.6 | ) | ||||||||
Other | 52.4 | 43.3 | 13.7 | |||||||||
|
|
|
| |||||||||
Total | $ | 607.8 | $ | 614.9 | (1.2 | )% | ||||||
|
|
|
| |||||||||
Selling days | 253 | 258 | ||||||||||
Net sales during the fiscal year ended April 1, 2011 compared to the prior year decreased approximately $7.1 million, resulting from 5 fewer selling days. Net sales in the nursing home and assisted living customer segment was also negatively impacted by the loss of a few large regional nursing home customers, offset by continued growth in independent nursing homes and assisted living facilities.
Net sales growth in the hospice and home health care customer segments reflected the continued successful execution of strategies to diversify its customer base through expansion in the home health care market and other non-facility based care as well as net sales of approximately $9.3 million attributed to an acquisition made during fiscal year 2010, offset by five fewer selling days during fiscal year 2011. The Company’s net sales in billing services were negatively impacted by decreased Medicare and Medicaid reimbursements and accounts lost due to competitive bidding.
Net sales of store brand products increased 1.5% during fiscal year 2011, when compared to fiscal year 2010, due to the Company’s focus on promoting its globally sourced products which resulted in additional sales to new and existing customers.
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan, which includes the sale of a business serving skilled nursing facilities, representing a portion of the net sales within the Nursing home and assisted living facilities and Billing services customer categories. As of the filing date, the Company could not determine the impact of the potential sale on future periods.
GROSS PROFIT
Physician Business
Gross profit dollars for the Physician Business increased $6.9 million and gross margins increased 77 basis points during fiscal year 2011. The increase in gross profit was due to margin improvement initiatives, higher growth in the Company’s brand of products, and additional sales from the Company’s entry into the physician dispensing solutions market, which have higher margins than the Company’s existing product offerings.
Extended Care Business
Gross profit dollars in the Extended Care Business increased $1.4 million and gross margin increased 57 basis points during fiscal year 2011. The increase in gross profit was impacted by the consolidation of Pathway Healthcare Services, a consulting business consolidated as a variable interest entity, and gross margin improvement initiatives, including increased sales of store brand products.
39
Table of Contents
GENERAL AND ADMINISTRATIVE EXPENSES
| For the Fiscal Year Ended | ||||||||||||||||
| April 1, 2011 | April 2, 2010 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business(a) | $ | 203.0 | 14.2 | % | $ | 198.2 | 13.8 | % | ||||||||
Extended Care Business(a) | 119.5 | 19.7 | 118.1 | 19.2 | ||||||||||||
Shared Services(b) | 42.2 | 2.1 | 54.6 | 2.7 | ||||||||||||
|
|
|
| |||||||||||||
Total Company(b) | $ | 364.7 | 17.9 | % | $ | 370.9 | 18.0 | % | ||||||||
|
|
|
| |||||||||||||
| (a) | General and administrative expenses as a percentage of net sales are calculated based on reportable segment net sales. |
| (b) | General and administrative expenses as a percentage of net sales are calculated based on consolidated net sales. |
General and administrative expenses are impacted by (i) compensation and employee benefit costs; (ii) cost to deliver, which represents all costs associated with the warehousing, transportation and delivery of products to customers; and (iii) shared services overhead costs.
Physician Business
General and administrative expenses increased $4.8 million during the fiscal year ended April 1, 2011, when compared to the prior year. This increase was mainly attributable to (i) an increase in allocated corporate expenses of $3.8 million; (ii) an increase in depreciation expense of $1.0 million due to the addition of new assets; and (iii) an increase in general and administrative expenses of $6.9 million as a result of the DSI and Linear acquisitions partially offset by a reduction in incentive compensation expense of $8.4 million based on reduced achievement of performance targets.
Extended Care Business
General and administrative expenses increased $1.4 million during the fiscal year ended April 1, 2011, when compared to fiscal year 2010. This increase was mainly attributable to (i) an increase in allocated corporate expenses of $2.3 million; and (ii) an increase in insurance costs of $1.0 million, partially offset by a reduction in bad debt expense of $1.9 million.
Shared Services
General and administrative expenses decreased $12.4 million during fiscal year 2011 due to (i) decreased incentive and stock-based compensation expense of $7.3 million related to payout estimates based on performance; (ii) a reduction in separation expenses of $2.9 million, related to the departure of the Company’s former Chairman and Chief Executive Officer during fiscal year 2010; (iii) an increase in corporate expense allocations of $7.1 million, offset by (iv) an increase in business insurance of $1.4 million; and (v) an increase in payroll and payroll-related costs of $1.5 million related to general merit and benefit increases and a reduction in capitalized salaries related to internally developed software projects.
40
Table of Contents
SELLING EXPENSES
| For the Fiscal Year Ended | ||||||||||||||||
| April 1, 2011 | April 2, 2010 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business(a) | $ | 116.7 | 8.2 | % | $ | 115.1 | 8.0 | % | ||||||||
Extended Care Business(a) | 20.8 | 3.4 | 20.7 | 3.4 | ||||||||||||
|
|
|
| |||||||||||||
Total Company(b) | $ | 137.5 | 6.8 | % | $ | 135.8 | 6.6 | % | ||||||||
|
|
|
| |||||||||||||
| (a) | Selling expenses as a percentage of net sales are calculated based on divisional net sales. |
| (b) | Selling expenses as a percentage of net sales are calculated based on consolidated net sales. |
Selling expenses are principally driven by commission expenses, which are generally paid to sales representatives based on gross profit dollars and gross profit as a percentage of net sales. The change in selling expenses for the Physician Business and Extended Care Business was consistent with the increases in gross profit and gross margin year over year.
INCOME FROM OPERATIONS
| For the Fiscal Year Ended | ||||||||||||||||
| April 1, 2011 | April 2, 2010 | |||||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||||
Physician Business | $ | 138.0 | 9.7 | % | $ | 137.3 | 9.5 | % | ||||||||
Extended Care Business | 37.8 | 6.2 | 38.0 | 6.2 | ||||||||||||
Shared Services | (42.2 | ) | — | (54.3 | ) | — | ||||||||||
|
|
|
| |||||||||||||
Total Company | $ | 133.6 | 6.6 | % | $ | 121.0 | 5.9 | % | ||||||||
|
|
|
| |||||||||||||
Income from operations for each business segment changed due to the factors discussed above. Business simplification andLEANstrategies, in conjunction with the successful integration of the Company’s strategic acquisitions are expected to continue to offset the effects of the lower sales growth, resulting in continued growth in income from operations, as a percentage of revenues.
During the first quarter of fiscal year 2013, the Company announced a strategic restructuring plan. The restructuring plan will include the sale of two business units serving: (i) specialty dental practices, representing approximately $6.1 million and $4.6 million of income from operations within the Physician Business during the fiscal years 2011 and 2010, respectively, and (ii) skilled nursing facilities within the Extended Care Business. Additionally, the restructuring plan includes the integration of warehouse operations into one common distribution infrastructure, and a redesign of its shared services function. As of the filing date, the Company could not determine the impact of the potential sale within the Extended Care Business and additional restructuring activities on future periods.
INTEREST EXPENSE
The Company’s debt structure during fiscal year 2011 consisted of variable rate borrowings under its revolving line of credit (“RLOC”) agreement and its 2008 Notes. The Company adopted a new accounting pronouncement during fiscal year 2010, ASC 470-20,Debt – Debt with Conversion and Other Options and, as required by this new standard, the Company retrospectively applied this change in accounting to all prior periods for which the Company had applicable outstanding convertible debt. See Footnote 12,Debt, for additional information.
The following table summarizes the various components of total interest expense and interest rates applicable to the borrowings outstanding under the RLOC.
41
Table of Contents
| For the Fiscal Year Ended | ||||||||||||
| (dollars in millions) | April 1, 2011 | April 2, 2010 | Decrease | |||||||||
Components of interest expense: | ||||||||||||
Interest on borrowings | $ | 16.7 | $ | 17.4 | $ | (0.7 | ) | |||||
Debt issuance costs | 0.9 | 1.1 | (2.0 | ) | ||||||||
Less: Capitalized interest | (0.5 | ) | (1.2 | ) | 0.7 | |||||||
|
|
|
|
|
| |||||||
Total interest expense | $ | 17.1 | $ | 17.3 | $ | (0.2 | ) | |||||
|
|
|
|
|
| |||||||
Weighted average interest rate-RLOC(a) | 2.37 | % | 4.02 | % | (1.65 | )% | ||||||
Average daily borrowings under the RLOC | $ | 3.8 | $ | 44.2 | $ | (40.4 | ) | |||||
| (a) | Weighted average interest rate excludes debt issuance costs and unused line fees. |
During fiscal year 2008, the Company entered into a two-year $50.0 million variable-to-fixed interest rate swap, (“Swap Agreement”), which effectively fixed the interest rate on all or a portion of the borrowings under the RLOC at 3.95% (consisting of a fixed interest rate of 2.70% and a credit spread of 1.25%) for a notional amount of $50.0 million. The Swap Agreement expired on February 19, 2010.
During fiscal year 2010, as required by the Swap Agreement, the Company maintained a minimum balance of $50.0 million drawn on the RLOC. After expiration, the balance on the RLOC was paid down, resulting in the average daily balance decreasing to $3.8 million for the year ended April 1, 2011.
OTHER INCOME
| For the Fiscal Year Ended | ||||||||||||||||
| (dollars in millions) | April 1, 2011 | April 2, 2010 | Decrease | Percent Change | ||||||||||||
Total Company | $ | 2.5 | $ | 6.1 | $ | (3.6 | ) | (58.7 | )% | |||||||
The Company sold its investment in athena during fiscal year 2010, recognizing a gain of $3.6 million. Excluding the gains on the sale of athena stock,Other income during fiscal year 2011 remained consistent with prior year and is mainly attributable to customer finance charges. See Footnote 7,Equity Investment, for further information relating the Company’s investment in athena.
PROVISION FOR INCOME TAXES
| For the Fiscal Year Ended | ||||||||||||||||
| April 1, 2011 | April 2, 2010 | |||||||||||||||
| (dollars in millions) | Amount | Effective Rate | Amount | Effective Rate | ||||||||||||
Total Company | $ | 44.6 | 37.4 | % | $ | 40.8 | 37.0 | % | ||||||||
The increase in the provision for income taxes year over year is attributable to an increase in pre-tax income. The increase in the effective rate relates to a decrease in the proportion of income earned by the Company’s non-U.S. subsidiaries, which are generally subject to tax at rates lower than the United States.
42
Table of Contents
LIQUIDITY AND CAPITAL RESOURCES
Liquidity and Capital Resources Highlights
Cash flows from operations are impacted by profitability and changes in operating working capital. Management monitors operating working capital through the following metrics:
| Fiscal Year Ended | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Days Sales Outstanding:(a) | ||||||||||||
Physician Business | 40.7 | 38.7 | 38.3 | |||||||||
Extended Care Business | 46.3 | 47.3 | 48.4 | |||||||||
Days On Hand:(b) | ||||||||||||
Physician Business | 53.7 | 54.8 | 53.6 | |||||||||
Extended Care Business | 66.8 | 62.7 | 54.4 | |||||||||
Days in Accounts Payable:(c) | ||||||||||||
Physician Business | 37.2 | 37.5 | 37.3 | |||||||||
Extended Care Business | 24.6 | 21.3 | 22.6 | |||||||||
Cash Conversion Days:(d) | ||||||||||||
Physician Business | 57.3 | 56.0 | 54.6 | |||||||||
Extended Care Business | 88.4 | 88.7 | 80.2 | |||||||||
Inventory Turnover:(e) | ||||||||||||
Physician Business | 6.7x | 6.6x | 6.7x | |||||||||
Extended Care Business | 5.4x | 5.7x | 6.6x | |||||||||
Return on Committed Capital:(f) | ||||||||||||
Total Company | 34.8 | % | 35.5 | % | 33.5 | % | ||||||
| (a) | Days sales outstanding (“DSO”) is average accounts receivable divided by average daily net sales. Average accounts receivable is the sum of accounts receivable, net of the allowance for doubtful accounts, at the beginning and end of the most recent four quarters divided by five. Average daily net sales are net sales for the most recent four quarters divided by 360. |
| (b) | Days on hand (“DOH”) is average inventory divided by average daily cost of goods sold (“COGS”). Average inventory is the sum of inventory at the beginning and end of the most recent four quarters divided by five. Average daily COGS is COGS for the most recent four quarters divided by 360. |
| (c) | Days in accounts payable (“DIP”) is average accounts payable divided by average daily COGS. Average accounts payable is the sum of accounts payable at the beginning and end of the most recent four quarters divided by five. |
| (d) | Cash conversion days is the sum of DSO and DOH, less DIP. |
| (e) | Inventory turnover is 360 divided by DOH. |
| (f) | ROCC is defined as return divided by average committed capital. Return is calculated as net income less (i) provision for income taxes, (ii) amortization, (iii) interest expense, and (iv) interest and investment income. Committed capital is calculated as total assets less (i) cash, (ii) goodwill and intangibles, and (iii) liabilities, excluding current and long-term debt. |
43
Table of Contents
In addition to the cash flow metrics described above, the Company monitors and manages other components of liquidity, including the following:
| As of | ||||||||
| (dollars in thousands) | March 30, 2012 | April 1, 2011 | ||||||
Capital Structure: | ||||||||
Revolving line of credit (a) | $ | — | $ | — | ||||
2012 Notes (a) | 250,000 | — | ||||||
2008 Notes (a) | 204,916 | 195,643 | ||||||
Other debt (a) | — | 780 | ||||||
Cash and cash equivalents | (163,152 | ) | (29,348 | ) | ||||
|
|
|
| |||||
Net debt | 41,764 | 167,075 | ||||||
Total equity | 390,811 | 446,526 | ||||||
|
|
|
| |||||
Total capital | $ | 432,575 | $ | 613,601 | ||||
|
|
|
| |||||
Operating Working Capital: | ||||||||
Accounts receivable, net | $ | 257,700 | $ | 247,229 | ||||
Inventories | 213,586 | 213,211 | ||||||
Accounts payable | (146,533 | ) | (128,057 | ) | ||||
|
|
|
| |||||
| $ | 324,753 | $ | 332,383 | |||||
|
|
|
| |||||
| (a) | Outstanding debt is presented in order of seniority. |
Cash Flows from Operating Activities
The primary components cash flows from operating activities consist of net income adjusted to reflect the effect of non-cash expenses and changes in operating working capital. Net cash provided by operating activities during fiscal years 2012, 2011, and 2010 was impacted by net income adjusted for (i) depreciation of property and equipment and amortization of intangible assets of $35.8 million, $31.4 million, and $27.1 million, respectively, (ii) amortization of debt discount and issuance costs of $10.3 million, $9.4 million, and $8.9 million, respectively, (iii) operational working capital sources of approximately $13.0 million, and needs of $3.1 million and $14.6 million, respectively, and (iv) noncash compensation expense of $7.3 million, $10.2 million, and $12.8 million, respectively. The Company’s net operating working capital levels were impacted in fiscal years 2012, 2011, and 2010 by its sourcing initiatives, including global sourcing, which generally require longer supply chain lead times and different payment terms. Management expects to increase its global sourcing activities, which may be offset by the restructuring plan. The Company continues to focus on efforts to increase cash collections from customers, improve inventory turns without impacting customer service levels, and manage the cash disbursements process.
Cash flows from operating activities during fiscal years 2012, 2011, and 2010 reflect the Company’s utilization of $0.6 million (tax-effected), $0.9 million (tax-effected), and $1.1 million (tax-effected), respectively, of net operating loss (“NOL”) carryforwards to offset cash payments due for federal and state tax liabilities based on estimated taxable income. Cash flows from operating activities were also impacted by cash payments made to, and refunds received from, federal and state taxing authorities. During fiscal years 2012, 2011, and 2010, the Company paid taxes, net of refunds, of approximately $42.2 million, $36.4 million and $42.6 million, respectively, which related to federal and state tax payments.
As of March 30, 2012, the Company had a deferred income tax liability of $17.3 million (tax-effected) related to interest deductions taken for tax purposes on its 2.25% senior convertible notes issued in 2004 (“2004 Notes”). The liability will be fully deferred for five years and paid ratably from fiscal year 2014 to fiscal year 2018 in accordance with the American Recovery and Reinvestment Act of 2009.
During fiscal year 2012, the IRS completed an examination of the Company’s federal income tax return for the fiscal year ended March 27, 2009. As a result, no changes were made to the Company’s taxable income.
44
Table of Contents
During fiscal year 2010, the IRS completed an examination of the Company’s federal income tax return for the fiscal years ended March 28, 2008, March 30, 2007, and March 31, 2006. As a result, the Company agreed to minor adjustments to its taxable income that did not have a material impact on the Company’s financial condition or results of operations.
Cash Flows from Investing Activities
Payments for business acquisitions, net of cash acquired, were $65.1 million, $65.9 million, and $14.8 million during fiscal years 2012, 2011, and 2010, respectively. During fiscal years 2012 and 2010 the Company made acquisitions not deemed significant for individual disclosure. Refer to Footnote 4,Purchase Business Combinations, for further discussion. The Company expects to continue to make strategic business acquisitions in future periods to grow market share and leverage its existing distribution capabilities, which will impact cash flows from investing activities.
Capital expenditures totaled $23.9 million, $18.2 million, and $25.9 million, during fiscal years 2012, 2011, and 2010, respectively, of which approximately $17.9 million, $12.2 million, and $20.2 million, respectively, related to the development and enhancement of the Company’s ERP and supply chain systems, electronic commerce platforms, and internal productivity software. Capital expenditures related to distribution center expansions and enhancements were approximately $1.4 million, $1.4 million, and $0.9 million, during fiscal years 2012, 2011, and 2010, respectively. Prior to the announcement of the Company’s strategic restructuring plan, capital expenditures were estimated to be approximately $24.5 million during fiscal year 2013. The Company is currently unable to make a good faith estimate of the impact the strategic restructuring plan will have on the capital expenditure plan. Such expenditures are expected to be funded by existing cash balances, cash flows from operating activities, or borrowings under the Company’s RLOC.
During fiscal year 2011, the Company purchased a $3.3 million convertible note issued by Pathway. See Footnote 5,Variable Interest Entity, for further discussion.
During fiscal year 2010, the Company sold its investment in athenahealth, Inc. (“athena”), resulting in a gain of approximately $3.6 million ($2.3 million, net of tax) recorded inOther income, net on theConsolidated Statement of Operations. Cash proceeds of $10.7 million were received in fiscal year 2010. Refer to Footnote 7,Equity Investment, for additional discussion.
Cash Flows from Financing Activities
During fiscal years 2012, 2011, and 2010, the Company repurchased approximately $140.4 million, $54.8 million and $57.2 million of the Company’s common stock, respectively. The share repurchases represented approximately 5.6 million, 2.7 million, and 2.8 million shares, respectively. As of March 30, 2012, approximately 0.4 million common shares were available for repurchase under authorized share repurchase programs. Refer to Footnote 14,Equity, for additional discussion.
The Company recognized excess tax benefits from stock-based compensation arrangements of $2.1 million, $3.2 million, and $2.5 million during fiscal years 2012, 2011, and 2010, respectively. The increase in recognized excess tax benefits, defined as the amount by which the actual tax deduction exceeds recognized compensation expense, is due to increases in the Company’s stock price and timing of stock option exercises.
The Company issued $250.0 million of 6.375% senior notes during fiscal year 2012. In conjunction with the offering, the Company paid debt issuance costs of approximately $4.7 million. In addition, the Company paid approximately $1.8 million in debt issuance costs related to the amendment and restatement of the credit agreement for its RLOC during fiscal year 2012.
During fiscal years 2012 and 2011, the Company paid $9.5 million and $0.9 million in contingent consideration related to earn-outs from acquisitions, respectively.
45
Table of Contents
During fiscal years 2012, 2011, 2010, the Company used proceeds from borrowings on its RLOC to fund a portion of the Company’s share repurchases, acquisition activities, investment strategies, and operating activities during the periods.
Capital Resources
The Company closely monitors the capital and credit markets. While market conditions have improved, volatility remains that may restrict access to capital and the costs associated with issuing or refinancing debt may increase relative to the Company’s current position. While the Company believes it is well positioned, there can be no guarantee the recent disruptions in the overall economy and the financial markets will not adversely impact the business and results of operations.
The Company finances its business through cash from operating activities, the proceeds from the 2012 Notes and 2008 Notes offerings, and the $300.0 million RLOC. The ability to generate sufficient cash from operating activities is dependent on the continued demand for the Company’s products and services and its access to those products and services from suppliers. The Company’s capital structure provides the financial resources to support the Company’s core business strategies of customer service and revenue growth. The RLOC, which is an asset-based agreement, is collateralized by the Company’s accounts receivable and inventory. The Company’s long-term priorities for use of its capital include programs to grow sales, make fold-in and strategic acquisitions, and repurchase of its common stock.
As the Company’s business grows, its cash and working capital requirements are expected to increase. The Company expects the overall growth in the business will be funded through a combination of cash flows from operating activities, borrowings under the RLOC, cash proceeds from the sale of the businesses outlined in the Company’s strategic restructuring plan, proceeds from the issuance of its 2012 Notes, capital markets, and/or other financing arrangements.
The Company has not provided for U.S. income taxes on accumulated and undistributed earnings attributable to foreign operations as the Company intends to permanently reinvest these undistributed earnings. These earnings relate to ongoing operations and were $20.2 million and $10.5 million as of March 30, 2012 and April 1, 2011, respectively.
As of March 30, 2012, the Company has not entered into any material working capital commitments that require funding, other than the items discussed below and the obligations included in the future minimum obligation table.
Based on prevailing market conditions, liquidity requirements, contractual restrictions, and other factors, the Company may seek to retire a portion of its outstanding equity through cash purchases and/or reduce its debt. The Company may also seek to issue additional equity to meet its future liquidity requirements. Such transactions may occur in the open market, privately negotiated transactions, or otherwise. The amounts involved could be material.
2012 Notes
On February 24, 2012, the Company issued $250.0 million aggregate principal of 6.375% senior notes, which mature on March 1, 2022. Interest on the notes is payable semi-annually in arrears on March 1 and September 1, beginning September 1, 2012. The 2012 Notes are fully and unconditionally guaranteed on a joint and several basis by certain of the Company’s domestic subsidiaries (the “Guarantor Subsidiaries”). Refer to Footnote 22,Condensed Consolidating Financial Information, for further information regarding the Guarantor Subsidiaries.
The Company used a portion of the net proceeds of the offering to repay borrowings under the RLOC in the amount of $127.3 million. Remaining proceeds will be used to partially fund the retirement of the 2008 Notes, as well as for general corporate purposes, including potential acquisitions and share repurchases.
As of March 30, 2012, the fair value of the 2012 Notes was approximately $257.5 million. Refer to Footnote 12, Debt, for a detailed discussion regarding the 2012 Notes.
46
Table of Contents
2008 Notes
In August 2008, the Company issued $230.0 million principal amount of 3.125% senior convertible notes, which mature on August 1, 2014. Interest on the notes is payable semiannually in arrears on February 1 and August 1 of each year. The notes will be convertible into cash up to the principal amount of the notes and shares of the Company’s common stock for any conversion value in excess of the principal amount under the following circumstances: (i) if the Company has called the notes for redemption; (ii) in the event of a Fundamental Change, as defined in the indenture, such as a merger, acquisition, or liquidation; (iii) on or after May 1, 2014 and prior to the close of business on the second scheduled trading day immediately preceding August 1, 2014; (iv) prior to May 1, 2014, during the five consecutive business day period following any five consecutive trading day period in which the trading price for a note for each day of that trading period is less than 98% of the closing sale price of the Company’s common stock on such corresponding trading day multiplied by the applicable conversion rate; (v) prior to May 1, 2014, during any calendar quarter after September 30, 2008 in which the closing sale price of the Company’s common stock for at least 20 of the 30 consecutive trading days ending the day prior to such quarter is greater than 130% of the applicable conversion price of $21.22 per share (“Contingent Conversion Trigger”); or (vi) upon specified corporate events as discussed in the indenture governing the notes.
The ability of note holders to convert is assessed on a quarterly basis and is dependent on the trading price of the Company’s stock during the last 30 trading days of each quarter. The Contingent Conversion Trigger was not met during the three months ended March 30, 2012; therefore, the notes may not be converted during the Company’s first quarter of fiscal year 2013.
The Company used a portion of the net proceeds of the offering to repurchase approximately $35.0 million of its common stock in privately negotiated transactions with institutional investors concurrently with this offering. The Company also used $101.7 million of the net proceeds during fiscal year 2009, when holders of the 2004 Notes required the Company to redeem $149.98 million of the outstanding notes. Remaining proceeds have been used for general corporate purposes.
As of March 30, 2012, the fair value of the 2008 Notes was approximately $323.8 million. Refer to Footnote 12, Debt, for a detailed discussion regarding the 2008 Notes.
Convertible Note Hedge Transactions
In connection with the offering of the 2008 Notes, the Company also entered into convertible note hedge transactions with respect to its common stock (the “purchased options”) with a major financial institution, (the “counterparty”). The Company paid an aggregate amount of $54.1 million to the counterparty for the purchased options. The purchased options cover, subject to anti-dilution adjustments substantially identical to those in the notes, approximately 10.8 million shares of common stock at a strike price that corresponds to the initial conversion price of the notes, also subject to adjustment, and are exercisable at each conversion date of the notes. The purchased options will expire upon the earlier of (i) the last day the notes remain outstanding or (ii) the second scheduled trading day immediately preceding the maturity date of the notes.
The purchased options are intended to reduce the potential dilution upon conversion of the notes in the event that the market value per share of the common stock, as measured under the notes, at the time of exercise is greater than the conversion price of the notes.
The purchased options are separate transactions, entered into by the Company with the counterparty, and are not part of the terms of the notes. Holders of the notes will not have any rights with respect to the purchased options.
Warrant Transactions
The Company also entered into warrant transactions (the “warrants”), whereby the Company sold to the counterparty warrants in an aggregate amount of $25.4 million to acquire, subject to anti-dilution adjustments, up to 10.8 million shares of common stock at a strike price of $28.29 per share of common stock, also subject to adjustment. The warrants will expire after the purchased options in approximately ratable portions on a series of expiration dates commencing on November 3, 2014.
47
Table of Contents
The warrants are separate transactions, entered into by the Company with the counterparties, and are not part of the terms of the notes. Holders of the notes do not have any rights with respect to the warrants.
The purchased options will generally have the effect of increasing the conversion price of the 2008 Notes to approximately $28.29 per share, representing a 68.5% premium based on the closing sale price of the Company’s common stock of $16.79 per share on August 4, 2008.
Impact on Diluted Weighted Average Shares
In accordance with ASC 260,Earnings Per Share, and the Company’s stated policy of settling the principal amount in cash, the Company was required to include shares underlying the 2008 Notes in its diluted weighted average shares outstanding since the average stock price per share for the period exceeded $21.22 (the conversion price for the senior convertible notes). Only the number of shares that would be issuable under the treasury stock method of accounting for share dilution was included, which was based upon the amount by which the average stock price exceeded the conversion price. If the average stock price of the Company’s common stock exceeds $28.29 per share, it will also include the effect of the additional potential shares that may be issued related to the warrants, which may negatively impact the Company’s diluted weighted average shares and diluted earnings per share.
The purchased options are not included in the calculation of diluted earnings per share prior to the conversion of the 2008 Notes, as their effect is considered anti-dilutive. As of March 30, 2012, the purchased options were “in the money” and would have been convertible into approximately 1.9 million shares of the Company’s common stock. The exercise of the purchased options is restricted to each conversion date of the 2008 Notes.
Revolving Line of Credit
During fiscal year 2012, the Company amended and restated the credit agreement related to its RLOC, increasing the facility size to $300.0 million, with increased borrowing capacity of $100.0 million via an accordion feature. See Footnote 12,Debt for additional information regarding the features and terms under the new credit agreement.
The Company had no outstanding borrowings under the revolving line of credit as of March 30, 2012. After reducing availability for outstanding borrowings and letter of credit commitments, the Company has sufficient assets based on eligible accounts receivable and inventory to borrow $269.1 million (excluding the additional increase of $100.0 million) under the RLOC as of March 30, 2012. The average daily interest rate, excluding debt issuance costs and unused line fees, for the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010 was 2.27%, 2.37%, and 4.02%, respectively. Refer to Footnote 12,Debt, for a detailed discussion regarding the RLOC.
During the first quarter of fiscal year 2013, the Company’s Board of Directors approved a strategic restructuring plan. The restructuring plan will include the sale of two business units serving skilled nursing facilities within the Extended Care Business and specialty dental practices within the Physician Business. The sale of the businesses are expected to increase the Company’s available cash balances, while reducing the Company’s assets used to calculate its borrowing base under the RLOC. The Company estimates availability under the RLOC would be approximately $201.1 million as of March 30, 2012 as adjusted for the sale of these two businesses.
Debt Rating
The Company maintains relationships with two nationally recognized debt rating agencies: Standard & Poor’s Ratings Services (“S&P”) and Moody’s Investor Services (“Moody’s). Companies that have assigned ratings at the top end of the range have, in the opinion of the rating agency, the strongest capability for repayment of debt or payment of claims, while companies at the bottom end of the range have the weakest capacity.
48
Table of Contents
In conjunction with the issuance of the 2012 Notes, the Company sought an updated corporate rating and a new issuance rating from S&P and re-established its ratings relationship with Moody’s. The 2012 Notes require that the Company maintain ratings from two nationally recognized debt ratings services.
On February 10, 2012, S&P affirmed its corporate credit rating of “BB+” and outlook of Stable. Additionally, it initiated a rating of “BB-” on the 2012 Notes and downgraded the 2008 notes from “BB” to “BB-.”
Also on February 10, 2012, Moody’s initiated ratings coverage with the following ratings: Long Term Issuer Rating (Corporate Family) of Ba3 with an outlook of Stable; Senior Unsecured Issue Rating for the 2012 Notes of Ba3. Moody’s does not provide ratings coverage for the 2008 Notes.
Subsequent to March 30, 2012, in response to the Company’s announcement of its strategic restructuring plan, S&P affirmed its existing “BB+” corporate credit rating and “BB-” senior unsecured debt rating, and revised its outlook to Negative. Also in response to the strategic restructuring plan announcement, Moody’s affirmed its Long Term Issuer Rating (Corporate Family) of Ba3 with an outlook of Stable and its Senior Unsecured Issue Rating for the 2012 Notes of Ba3.
Agency ratings are subject to change, and there can be no assurance that a ratings agency will continue to rate the Company or its debt, and/or maintain its current ratings. Management cannot predict the effect that a change in debt ratings will have on the Company’s liquidity.
Off-Balance Sheet Arrangements
The Company’s most significant off-balance sheet financing arrangements as of March 30, 2012 are non-cancelable operating lease agreements for warehouse space and equipment rentals, and outstanding letters of credit. As of March 30, 2012, future minimum obligations under operating lease agreements are $72.0 million. The Company had no open letters of credit outstanding as of March 30, 2012. The Company does not participate in any off-balance sheet arrangements involving unconsolidated subsidiaries that provide financing or potentially expose the Company to unrecorded financial obligations.
49
Table of Contents
Future Contractual Obligations
In the normal course of business, the Company enters into obligations and commitments that require future contractual payments. The following table presents, in aggregate, scheduled payments under contractual obligations for the Physician Business, the Extended Care Business, and Shared Services:
Contractual Obligation | Payment Due By Fiscal Years | |||||||||||||||||||||||||||
| (in thousands) | 2013 | 2014 | 2015 | 2016 | 2017 | Thereafter | Total | |||||||||||||||||||||
Revolving line of credit (a) | $ | 750 | $ | 750 | $ | 750 | $ | 750 | $ | 469 | $ | — | $ | 3,469 | ||||||||||||||
Senior unsecured notes (b) | 15,938 | 15,938 | 15,938 | 15,938 | 15,938 | 273,904 | 353,594 | |||||||||||||||||||||
Convertible senior notes (b), (c) | 7,188 | 7,188 | 233,593 | — | — | — | 247,969 | |||||||||||||||||||||
Operating lease obligations (d) | 24,935 | 19,232 | 11,542 | 5,912 | 3,530 | 6,813 | 71,964 | |||||||||||||||||||||
Purchase commitments (e) | 812 | — | — | — | — | — | 812 | |||||||||||||||||||||
Obligations from acquisitions (f) | 2,159 | 1,620 | 1,188 | — | — | — | 4,967 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total (g) | $ | 51,782 | $ | 44,728 | $ | 263,011 | $ | 22,600 | $ | 19,937 | $ | 280,717 | $ | 682,775 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
| (a) | Amounts represent unused line fees on the revolving line of credit under the RLOC, which expires in November 2016. |
| (b) | Amounts include interest expense. |
| (c) | Under the terms of the convertible note agreement, the notes are convertible during any calendar quarter in which the closing sale price of the Company’s common stock for a certain number of days is greater than $27.59 per share. The 2008 Notes would be classified as a current liability during any such quarter. The 2008 Notes are discussed further in Footnote 12,Debt. |
| (d) | Amounts represent contractual obligations for operating leases of the Company as of March 30, 2012. Currently, it is management’s intent to either renegotiate existing leases or execute new leases upon the expiration date of such agreements, except for those that may be exited through the Company’s restructuring plan. |
| (e) | Amounts represent estimated obligations to be paid related to various shipping contracts and future purchases of certain vaccines. If a supply agreement for store brand products between a vendor and the Company were to be terminated, then the Company may be required to purchase from the vendor all remaining finished and unfinished products and product-materials held by the vendor. As of March 30, 2012, the Company had no material obligation to purchase remaining products or materials due to a termination of a supply agreement with a vendor who supplies store brand products to the Company. |
| (f) | Amounts represent estimated obligations to be paid to sellers of previously acquired businesses for contingent consideration, interest, and funds held to secure any adjustments or claims that may arise. |
| (g) | As of March 30, 2012, the Company had gross unrecognized tax benefits of $1.4 million. This amount is excluded from the table above as the Company cannot reasonably estimate the period of cash settlement with the respective taxing authorities. Additionally, the Company has a liability of $94.4 million related to a deferred compensation program recorded inOther noncurrent liabilities in the accompanyingConsolidated Balance Sheets. The amount is excluded from the table above as the Company cannot reasonably estimate the period of cash settlement and the liability is offset by the cash surrender value of corporate-owned life insurance policies recorded inOther assets in the accompanyingConsolidated Balance Sheets. |
CRITICAL ACCOUNTING ESTIMATES
In preparing the consolidated financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”), management is required to make certain estimates, judgments, and assumptions that affect the reported amounts of assets and liabilities, including the disclosure of contingent assets and liabilities, at the date of the financial statements and the reported amounts of revenues and expenses during the periods presented. The Company periodically evaluates the accounting policies and estimates it uses to prepare its financial statements, which are then reviewed by the Company’s audit committee. Management’s estimates are based on historical experience and other assumptions considered reasonable with the relevant facts and circumstances. Based on the uncertainty inherent in such estimates, actual results may differ.
The critical accounting estimates are those estimates that require the Company’s management to make assumptions about matters that are highly uncertain at the time the estimate is made and could have a material impact on the Company’s results due to changes in the estimates or the use of different estimates that could reasonably have been used. Additionally, the Company includes those accounting estimates whose initial application had a material impact on the Company’s financial presentation, unless the application resulted solely from the issuance of new accounting literature. The discussion below applies to each of the Company’s reportable segments (Physician Business, Extended Care Business, and Shared Services), unless otherwise noted.
50
Table of Contents
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses on trade receivables resulting from the inability to collect outstanding amounts due from its customers. The allowances include estimates of specific amounts for those accounts that are likely to be uncollectible, such as bankruptcies, and general allowances for those accounts that management currently believes to be collectible but may later become uncollectible. Management believes the estimates used in determining the allowance for doubtful accounts are critical accounting estimates because changes in credit worthiness and economic conditions, including bankruptcies, have had a material impact on operations in previous fiscal years and could have a material impact on the Company’s results from operations in the future.
The estimates used to determine the allowances for doubtful accounts are based on historical collection experience, current economic trends, credit-worthiness of customers, and changes in customer payment terms. The percentage of each aging category that is reserved is determined by analyzing historical write-offs and current trends in the credit quality of the customer base. Adjustments to credit limits and allowances for bad debts are made based upon payment history and the customer’s current credit worthiness. If the financial condition of the Company’s customers were to deteriorate or improve, allowances may be adjusted, impacting general and administrative expenses and the accounts receivable balance.
Physician Business
During fiscal years 2009 through 2011, the Physician Business’ allowance for doubtful accounts was reduced by customer deductions and write-offs ranging from $1.3 million to $2.3 million and was increased by additional provisions ranging from $1.5 million to $1.9 million. During fiscal year 2012, the Physician Business’ allowance for doubtful accounts was reduced by $2.1 million for customer deductions and write-offs and was increased by additional provisions of $2.3 million, remaining relatively consistent with prior years. During fiscal years 2009 through 2011, the Company’s allowance for doubtful accounts has represented between 1.6% and 1.9% of the Physician Business’ trade receivable balance. If management were to assume its reserve percentages as of March 30, 2012 were based on the fiscal year 2009 through 2011 historic ranges noted above, the allowance for doubtful accounts as of March 30, 2012 would range between $3.2 million and $3.8 million. As of March 30, 2012 the allowance for doubtful accounts for this business segment was $3.2 million.
Extended Care Business
During fiscal years 2009 through 2011, the Extended Care Business’ allowance for doubtful accounts was reduced by customer deductions and write-offs ranging from $0.9 million to $2.2 million, and was impacted by provisions ranging from a decrease of $0.1 million to an increase of $2.1 million. During fiscal year 2012, the Extended Care Business’ allowance for doubtful accounts was increased by $0.4 million for customer deductions and write-offs and was increased by additional provisions of $0.6 million. During fiscal years 2009 through 2011, the Company’s allowance for doubtful accounts represented between 3.5% and 4.7% of the Extended Care Business’ trade receivable balance. If management were to assume its reserve percentages as of March 30, 2012 were based on the fiscal year 2009 through 2011 historic ranges noted above, the allowance for doubtful accounts as of March 30, 2012 would range between $2.9 million and $3.9 million. As of March 30, 2012, the allowance for doubtful accounts for this business segment was $3.0 million.
Although the Company believes its judgments, estimates and/or assumptions related to allowances for doubtful accounts are reasonable, making material changes to such judgments, estimates and/or assumptions, and changes in customer’s credit worthiness could materially affect the Company’s financial results.
51
Table of Contents
Inventories
In order to state inventories (medical products, medical equipment, and other related products) at the lower of cost (determined using the first-in, first-out (“FIFO”) method) or market (net realizable value), the Company adjusts for excess or slow moving inventory based on the expectation that certain inventory will become obsolete, sold for less than cost, or become unsellable altogether. The adjustments are estimated based on factors such as historical trends, current market conditions, and management’s assessment of when the inventory would likely be sold and the quantities and prices at which the inventory would likely be sold in the normal course of business. Changes in product specifications, customer product preferences, or the loss of a customer may result in an unanticipated impairment in net realizable value that may have a material impact on cost of goods sold, gross margin, and net income. Obsolete or damaged inventory is disposed of or written down to net realizable value on a periodic basis. Additional adjustments, if necessary, are made based on management’s specific review of inventory on-hand. Management believes the estimates used in determining adjustments for excess and slow moving inventory are critical accounting estimates as changes in the estimates for both segments could have a material impact on net income and the estimates involve a high degree of judgment.
Inventory adjustments ranged from 1.3% to 1.8% of gross inventory for the Physician Business and 1.8% to 2.1% of gross inventory for the Extended Care Business during fiscal years 2009 through 2011. If management were to assume inventory adjustments were based on the fiscal years 2009 through 2011 historical ranges noted above, adjustments for excess and slow moving inventory as of March 30, 2012 would range from $2.0 million to $2.7 million for the Physician Business and $1.5 million to $1.8 million for the Extended Care Business, impacting the Company’sInventory balance andGross profit. As of March 30, 2012, management estimated adjustments for excess or slow moving inventory to be approximately $3.8��million and $2.8 million for the Physician Business and Extended Care Business, respectively. The increase in Physician Business inventory adjustments above the expected range relates to additional adjustments related to fiscal year 2012 acquisitions.
Although the Company believes its judgments, estimates and/or assumptions related to inventory adjustments are reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect the Company’s financial results.
Vendor Rebates
The Company receives transaction-based rebates from third party suppliers. Such rebates are classified as a reduction to cost of goods sold in the accompanying statements of operations.
Transaction-based rebates are generally associated with a specific customer contract and are recognized as a reduction to cost of goods sold at the time the transaction occurs. Management establishes a reserve for uncollectible transaction-based vendor rebates based on management’s judgment after considering the status of current outstanding rebate claims, historical denial experience with suppliers, and any other pertinent available information. Management believes the estimates used in determining the reserve for uncollectible transaction-based vendor rebates are critical accounting estimates because changes in the estimates could have a material impact on net income and the estimates involve a high degree of judgment.
Reserves for transaction-based rebates for the fiscal years ended March 30, 2012 and April 1, 2011 were $1.1 million and $1.5 million, respectively. Reserves ranged from 13.2% to 23.6% of rebates receivable during fiscal years 2009 through 2011. If management were to assume its reserve percentages as of March 30, 2012 were based on the fiscal year 2009 through 2011 historical ranges noted above, the transaction-based rebate reserve as of March 30, 2012 would range from $1.5 million to $2.7 million, impacting the Company’sPrepaid and other current assets balance andGross profit. The fiscal year 2012 transaction-based rebate reserve fell below the Company’s historical ranges. During fiscal year 2010, the Company implemented a contracts and rebates administration system which provided enhanced the accuracy of rebate filings and reduced rebate denials during fiscal years 2011 and 2012.
52
Table of Contents
Although the Company believes its judgments, estimates and/or assumptions related to vendor rebates are reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect the Company’s financial results.
Contractual Billing Adjustments
The Company provides medical claim billing services on a fee-for-service or a full-assignment basis and records claims receivable due from insurance carriers. A claim may become uncollectible in full due to denial or partially due to discounts taken. Contractual billing adjustments are estimated to record net revenues and claims receivables at their net realizable values. Management estimates contractual billing adjustments based on historical collection experience, and also considers voided claims and claims written off. Contractual billing adjustments are recorded as a reduction toAccounts receivable, netandNet sales. Management believes the estimates used in determining contractual billing adjustments are critical accounting estimates because changes in the estimates could have a material impact on net income and the estimates involve a high degree of judgment.
Physician Business
Contractual billing adjustments recorded to Physician Business claims receivables for the fiscal years ended March 30, 2012 and April 1, 2011 were $13.2 million and $2.9 million, respectively. Adjustments were 57.9% and 24.1% of gross claims receivable during the fiscal years ended March 30, 2012 and April 1, 2011, respectively. The increase in contractual billing adjustments in fiscal year 2012 as compared to prior year relates to increased internal claims adjudication which increased the claims receivable and related adjustments and physician dispensing acquisitions consummated during the year.
Extended Care Business
Contractual billing adjustments recorded to Extended Care Business claims receivables for the fiscal years ended March 30, 2012 and April 1, 2011 were $2.3 million and $3.1 million, respectively. Adjustments were 34.5% and 29.7% of gross claims receivable during the fiscal years ended March 30, 2012 and April 1, 2011, respectively. The increase in contractual billing adjustments in fiscal year 2012 as compared to prior year relates to additional adjustments on Medicare and Medicaid billings.
Although the Company believes its judgments, estimates and/or assumptions related to contractual billing adjustments are reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect the Company’s financial results.
Income Taxes
The Company uses the asset and liability method for determining its provision for income taxes and deferred tax assets and liabilities. Under this method, the amount of deferred tax assets and liabilities at the end of each period are determined using the tax rate expected to be in effect when taxes are actually paid or recovered. Valuation allowances are established to reduce deferred tax assets when it is more likely than not that some portion or all of the deferred tax assets will not be realized. In determining the need for valuation allowances, significant judgment and estimates are used as management considers short- and long-term forecasts of future taxable income as well as prudent and feasible tax planning strategies. These judgments and estimates include some degree of uncertainty and changes to these estimates could require management to adjust the valuation allowances for deferred tax assets.
The Company had gross deferred income tax assets of $75.6 million and $72.7 million as of March 30, 2012 and April 1, 2011, respectively. There were no valuation allowances as of March 30, 2012 and April 1, 2011, as management believes it will fully utilize the Company’s deferred tax assets before their expiration.
The Company has not provided for U.S. income taxes on accumulated and undistributed earnings attributable to foreign operations as the Company intends to permanently reinvest these undistributed earnings. These earnings relate to ongoing operations and were $20.2 million and $10.5 million as of March 30, 2012 and April 1, 2011, respectively.
53
Table of Contents
The Company’s tax filings are periodically subject to review by the Internal Revenue Service (“IRS”) and other taxing authorities, which may result in assessments of additional tax. Resolution of these assessments, either with the taxing authority or the courts, inevitably includes some degree of uncertainty; accordingly, the Company provides taxes only for the amounts management believes will ultimately result from these proceedings. Management’s experience has been that the estimates and assumptions used to provide for future tax assessments have proven to be appropriate. However, past experience is only a guide, and the potential exists, however limited, that adjustments resulting from the resolution of current and potential future tax controversies may differ materially from the amount accrued.
Current standards of accounting for uncertainty in income taxes provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes. This standard requires management to make significant judgments while assessing the probability of possible outcomes of future tax examinations. As of March 30, 2012 and April 1, 2011, the liability for uncertain tax positions was $1.4 million and $1.5 million, respectively. Management does not expect the amount of unrecognized tax benefits to change significantly over the next twelve months.
If the estimates or judgments described above were to change, a hypothetical 1% change in the Company’s effective tax rate would impact consolidated income from continuing operations by approximately $1.2 million in fiscal year 2012.
Valuation of Intangible Assets, Other Long-lived Assets, and Goodwill
Acquisitions
The Company allocates the purchase price of acquired companies to the tangible and intangible assets acquired and liabilities assumed based on estimated fair values. Such valuations require management to make significant estimates and assumptions. Critical estimates in the valuation of acquired assets include, but are not limited to: (i) expected future cash flows from existing customer contracts and relationships; (ii) assumptions relating to the impact of noncompete agreements on business operations; (iii) assumptions related to the impact on the timing of expected future cash flows; (iv) retention of customers and key business leaders; and (v) the risk inherent in investing in intangible assets. These estimates are inherently uncertain and unpredictable. Assumptions may be incomplete or inaccurate, and unanticipated events and circumstances may occur which may affect the accuracy or validity of such assumptions, estimates, or other actual results. For these reasons, management believes the estimates used in determining the fair value of assets acquired through an acquisition are critical accounting estimates.
During fiscal years 2009 through 2011, the Company made acquisitions with initial purchase prices totaling $84.4 million. During fiscal year 2012, the Company made acquisitions with initial, unadjusted purchase price totaling $70.0 million. Adjustments to the valuation of acquired assets and liabilities subsequent to the date of purchase based on changes in management’s original estimates were immaterial to the current and previous three fiscal years.
Impairment
Under ASC 350,Intangibles – Goodwill and Other(“ASC 350”), goodwill and indefinite-lived intangible assets are not amortized, but instead tested for impairment annually or whenever events or changes in circumstances indicate the carrying amount may be impaired. Goodwill and indefinite-lived intangible assets are reviewed for impairment at each reporting unit annually on the last day of each fiscal year.
The impairment and disposal of long-lived assets is accounted for in accordance with ASC 360-10,Property, Plant, and Equipment—Overall, (“ASC 360-10”). ASC 360-10 requires that long-lived assets, such as property and equipment and intangible assets subject to amortization, be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In the event that the carrying value of assets are determined to be unrecoverable, the Company would estimate the fair value of the assets or reporting unit and record an impairment charge for the excess of the carrying value over the fair value. In
54
Table of Contents
conducting the impairment analysis, the Company determines the fair value of its reporting units using valuation techniques which may include discounted cash flow analyses requiring management to make certain assumptions regarding estimated future cash flows, revenues, earnings, and other factors, including discount rates, to determine the fair value of these respective assets. The application of different assumptions about such matters as estimated future cash flows or discount rates, or the testing for impairment at a different level of the organization or on a different organizational structure, may produce materially different results. For these reasons, management believes the estimates used in evaluating the Company’s goodwill, indefinite-lived intangible assets, and long-lived assets are critical accounting estimates. Based on management’s review, goodwill, intangible assets, and other long-lived assets were not impaired during fiscal years 2011, 2010, and 2009. As of March 30, 2012 and April 1, 2011, the Company’s intangible asset, other long-lived asset, and goodwill balances totaled $357.4 million and $311.4 million, respectively.
Based on management’s review, goodwill, intangible assets, and other long-lived assets were not impaired during fiscal year 2012 and management does not believe there were any circumstances which indicated the carrying value of an asset might not be recoverable in the future. Additionally, a hypothetical 1% change in the discount rate utilized in the Company’s discounted cash flow analysis would not have indicated impairment for any of the Company’s reporting units.
Long-Term Incentive Compensation
Equity Incentive Plans
As of March 30, 2012, the Company has outstanding grants of nonqualified stock options, time-based restricted stock and performance-based restricted stock outstanding.
Estimates are required to determine the number of stock-based awards which will ultimately vest, and, in the case of performance-based restricted stock, estimates of the Company’s future performance. Changes in the estimated forfeiture rates and changes in estimates regarding the Company’s performance can have material effects on stock-based compensation expense. Accordingly, management has determined that the estimates used to determine equity-based compensation expense are critical accounting estimates.
When estimating forfeitures, the Company considers termination behaviors as well as trends of actual equity-based awards forfeited. Management periodically re-assesses the estimated forfeiture rate established upon grant date. Such estimates are revised if they differ materially from actual forfeitures. As required, forfeiture estimates are adjusted to reflect actual forfeitures when an award vests. Actual forfeitures in future reporting periods could be materially higher or lower than management’s current estimates, which could have a material impact on equity-based compensation expense recognized in future years.
When estimating the Company’s earnings per share goals for performance-based restricted stock, the Company reviews historical performance, internal plans and goals, economic conditions, and other performance metrics. These future performance estimates are re-assessed throughout the service period. Such estimates are revised, if necessary, if they differ materially from the original assessment and may have an impact on the vesting of an award. If actual performance differs significantly from management’s estimates, it could have a material impact on equity-based compensation expense recognized in future years.
During the fiscal year ended March 30, 2012, the Company changed its estimate of the number of shares to be delivered on its performance based awards. This change reflected a decrease in estimated achievement of performance conditions based on actual and expected future financial performance. The change in estimate decreased Performance Share Units outstanding by approximately 98,000 shares. As a result of the change in performance estimate, stock based compensation expenses decreased $1.5 million ($0.9 million, net of tax), or $0.02 per diluted share during the year ended March 30, 2012.
Based on the financial results during fiscal year 2010, management revised its assessment for probable achievement of performance conditions related to long-term incentive compensation plans. Management reviewed the fiscal year results impacted by: (i) the impact of revenue growth programs, (ii) the impact of implemented cost savings initiatives, (iii) the increase in sales of H1N1 related products, and (iv) the gain on sale of shares in athena. It was
55
Table of Contents
determined the cumulative impact of these events required the Company to adjust its estimates and adjust the accruals to these plans based on those estimates. The change in estimate for these awards resulted in an increase in stock-based compensation expense of $9.1 million ($5.6 million, net of tax), or $0.10 per diluted share during fiscal year 2010, offset by a decrease in expense of $4.4 million related to the departure of the Company’s former Chairman and Chief Executive Officer.
Total stock-based compensation expense during the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, was $6.4 million, $9.2 million, and $12.2 million, respectively. Current forfeiture rates average 1.0% per quarter, with actual rates ranging from 0.1% to 3.9% per quarter for fiscal years 2009-2011. If management had used the low and high end of these actual ranges during fiscal year 2012, equity-based compensation expense, included in general and administrative expenses, would have been $6.5 million and $6.4 million, respectively. Holding forfeiture rates static, if management had estimated the Company’s future performance at the minimum and maximum earnings per share ranges, since inception of the awards, equity-based compensation expense would have been $5.8 million and $8.4 million, respectively, during the fiscal year ended March 30, 2012. Refer to Footnote 15,Incentive and Stock-Based Compensation, for additional information.
Cash-based incentive plans
The Company maintains cash-based long-term incentive plans, the Shareholder Value Plans (“SVP”), for certain employees. The SVP provides incentive to enhance shareholder value through the achievement of cumulative earnings per share goals.
Estimates are required to determine the Company’s expected future performance and cumulative earnings per share at the end of the three-year performance period. Changes in estimates regarding the Company’s performance can have a material effect on cash-based incentive compensation expense. For this reason, management has determined that the performance estimates used for long-term cash-based compensation expense are critical accounting estimates.
When estimating the Company’s earnings per share goals for the SVP, the Company reviews historical performance, internal plans and goals, economic conditions, and other performance metrics. These future performance metrics are re-assessed throughout the service period. Such estimates are revised, if necessary, if they differ materially from the original assessment. If actual performance differs significantly from management’s estimates, it could have a material impact on cash-based compensation expense recognized in future years.
During fiscal year 2012, the Compensation Committee approved the 2011 Shareholder Value Plan (“2011 SVP”), a cash based performance award program for certain officers and management under the 2006 Incentive Plan. The performance period under the 2011 SVP is the 36-month period from April 1, 2011 to March 28, 2014. Target awards under the 2011 SVP were calculated as three times the participant’s base salary times an award factor ranging from 15% to 40% and performance goals were based on planned cumulative earnings per share. Due to a reduction in payout estimates based on performance, the Company has no accrued compensation cost related to the 2011 SVP recorded as of March 30, 2012.
During fiscal year 2009, the Compensation Committee approved the 2008 Shareholder Value Plan (“2008 SVP”). The performance period under the 2008 SVP was the three year period from March 31, 2008 to April 1, 2011. Based upon current results and expected future results as discussed above, the Company recognized an additional $2.3 million in corporate compensation expense during fiscal year 2010 related to the 2008 SVP due to a change in estimate. There were no material changes in estimates during fiscal year 2011. The Company accrued approximately $10.7 million of compensation cost related to the 2008 SVP, recorded inOther current liabilitiesin the accompanyingConsolidated Balance Sheets as of April 1, 2011, which was paid in June 2011.
Recent Accounting Pronouncements
In October 2009, the FASB issued an Accounting Standards Update (“ASU”) for multiple deliverable revenue arrangements. The update requires entities to allocate revenue in an arrangement using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The update eliminates the residual method of revenue allocation and requires revenues to be allocated using the relative selling price method. The Company
56
Table of Contents
adopted this update prospectively for revenue arrangements entered into or materially modified beginning in the first quarter of fiscal year 2012. The Company has evaluated this standard and determined it did not have a material effect on the Company’s statements of financial condition or results of operations.
In May 2011, the FASB issued an ASU with amendments to achieve common fair value measurement and disclosure requirements in GAAP. The amendments in this update clarified the language used to describe many of the requirements in GAAP for measuring fair value and for disclosing information about fair value measurements. The following areas were impacted by this ASU: (i) application of the highest and best use and valuation premise concepts; (ii) measuring the fair value of an instrument classified in shareholders’ equity; and (iii) additional quantitative disclosures regarding unobservable inputs used in Level 3 fair value measurements. The amendments are effective during interim and annual periods beginning after December 15, 2011, or the Company’s fourth quarter of fiscal year 2012. The Company has evaluated this standard and determined that, other than requiring additional disclosures, it will not have a material impact on the Company’s statements of financial condition or results of operations.
In June 2011, the FASB issued new guidance on the presentation of comprehensive income that requires changes in stockholders’ equity to be presented either (i) in a single continuous statement of comprehensive income, or (ii) in two separate consecutive statements. The ASU requires retrospective application and is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, or the Company’s fiscal year 2013. In December 2011, the FASB indefinitely deferred the effective date for amendments pertaining to the presentation of reclassification adjustments by component. The Company has evaluated this standard and determined it will not have a material effect on the Company’s statements of financial condition or results of operations.
In September 2011, the FASB issued amended guidance to simplify the method in which entities test goodwill for impairment. This ASU allows an entity to assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. Additional disclosure requirements were included with this update, including an explanation of qualitative factors used in the goodwill analysis. The amendments in this update are effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, or the Company’s fiscal year 2013. The Company has evaluated this standard and determined it will not have an effect on the Company’s statements of financial condition or results of operations.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Market Risk.The Company’s objective in managing market risk exposures is to identify and limit the potential impact of changes in interest rates, commodity availability, and access of capital on earnings and cash flow. The following assessment of the Company’s market risk does not include uncertainties that are either nonfinancial or nonquantifiable, such as political uncertainty, economic uncertainty, impact of future tax legislation, and credit risks.
Interest Rate Risk.The Company’s primary interest rate exposure relates to cash and cash equivalents and fixed and variable rate debt. During fiscal year 2012, the Company’s debt obligations consisted of (i) $250.0 million senior notes with a fixed rate of 6.375%, (ii) $230.0 million senior convertible notes with a fixed rate of 3.125%, and (iii) variable rate borrowings under the revolving line of credit, which bear interest at the bank’s base rate or at LIBOR plus an applicable margin.
Changes in interest rates affect interest payments under the Company’s variable rate revolving line of credit agreement. During fiscal year 2012, the Company had average daily variable rate borrowings under its line of credit of $44.4 million. A hypothetical 1% increase/decrease in prevailing interest rates as of March 30, 2012, would result in a corresponding increase/decrease in interest expense of less than $0.1 million.
During fiscal year 2011, the Company had average daily variable rate borrowings under its line of credit of $3.8 million. A hypothetical 1% increase/decrease in prevailing interest rates as of April 1, 2011, would result in a corresponding increase/decrease in interest expense of less than $0.1 million.
57
Table of Contents
Changes in interest rates also affect rates of return on the Company’s cash equivalents and short-term investments, which generally consist of money market accounts.
Currency Risk. The Company’s currency rate exposures relate to products that are globally sourced from manufacturers in Southeast Asia. Currently, the Company has negotiated settlement of payments to manufacturers in U.S. dollars. However, over time, local country currency fluctuations may increase or decrease the negotiated cost that the Company must pay for these products. In addition, the Company may in future periods negotiate settlement of payments to manufacturers in the local currency of the country providing a product which would then subject the Company to foreign currency risk.
Commodity Risk. The Company’s primary commodity exposures relate to fluctuations in the price of gasoline and diesel fuel and the procurement of certain medical supplies in which the product cost is dependent upon the price of raw materials, which may fluctuate significantly.
The Company’s direct fuel exposure relates to fluctuations in fuel costs that affect the Company-leased delivery fleet or third-party delivery charges. Significant increases in the cost of gasoline and diesel fuel may impact the Company’s gross margin, cost to deliver, and the operating costs of third party transportation providers. Common carriers have passed these increases through to the Company in the form of a fuel surcharge, which may adversely affect the Company’s results of operations. Beginning in fiscal year 2006, the Company implemented a fuel surcharge to its customers to pass on a portion of the increased cost of gasoline and diesel fuel with adjustments to the amount of surcharge based on market conditions. There can be no assurance that the Company will be able to fully pass along further significant increases in fuel costs to its customers due to the competitive nature of the medical supply distribution industry.
As of March 2012 and March 2011, the U.S. national average for unleaded gasoline was $3.98 and $3.74/gallon, respectively, and the U.S. national average for diesel fuel was $4.14 and $3.98/gallon, respectively. With respect to the Company’s direct fuel purchases, a hypothetical 10% increase/decrease in diesel and unleaded fuel costs during fiscal years 2012 and 2011 would have resulted in a corresponding increase/decrease in fuel expense of approximately $0.8 million and $0.6 million, respectively.
The Company purchases latex and vinyl gloves through agreements in which the pricing of gloves is based on the price of latex as traded on the Malaysian Rubber Exchange and the weighted price of the raw materials Poly Vinyl Chloride (“PVC”), Dioctylphthalate (“DOP”), and Nitrile Butadiene (“NDR”). Latex, PVC, DOP, and NDR in their raw form are only a few of many components used in the manufacture of gloves. Based on estimates of component mix, the following table presents the change in product cost of a hypothetical 10% increase/decrease in the underlying raw material cost during fiscal years 2012 and 2011:
| Hypothetical Change in Product Cost of 10% | ||||||||
| For the Fiscal Year Ended | ||||||||
| March 30, 2012 | April 1, 2011 | |||||||
| (dollars in millions) | Amount (+/-) | Amount (+/-) | ||||||
Latex gloves | $ | 1.4 | $ | 0.9 | ||||
Vinyl gloves | 1.7 | 1.1 | ||||||
Nitrile gloves | 0.7 | 0.3 | ||||||
58
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| F-2 | ||||
Consolidated Balance Sheets - March 30, 2012 and April 1, 2011 | F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-52 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
PSS World Medical, Inc.:
We have audited the accompanying consolidated balance sheets of PSS World Medical, Inc. and subsidiaries (the Company) as of March 30, 2012 and April 1, 2011, and the related consolidated statements of operations, equity and comprehensive income and cash flows for each of the years in the three-year period ended March 30, 2012. In connection with our audits of the consolidated financial statements, we also have audited the financial statement schedule. These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of PSS World Medical, Inc. and subsidiaries as of March 30, 2012 and April 1, 2011, and the results of their operations and their cash flows for each of the years in the three-year period ended March 30, 2012, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), PSS World Medical Inc.’s internal control over financial reporting as of March 30, 2012, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated May 25, 2012 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
May 25, 2012
Jacksonville, Florida
Certified Public Accountants
F-2
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
MARCH 30, 2012 AND APRIL 1, 2011
(Dollars in Thousands)
ASSETS |
| |||||||
| 2012 | 2011 | |||||||
Current Assets: | ||||||||
Cash and cash equivalents | $ | 163,152 | $ | 29,348 | ||||
Accounts receivable, net | 257,700 | 247,229 | ||||||
Inventories | 213,586 | 213,211 | ||||||
Deferred tax assets, net | 16,962 | 20,533 | ||||||
Prepaid expenses and other current assets | 34,292 | 34,285 | ||||||
|
|
|
| |||||
Total current assets | 685,692 | 544,606 | ||||||
Property and equipment, net | 101,036 | 102,401 | ||||||
Other Assets: | ||||||||
Goodwill | 201,752 | 167,094 | ||||||
Intangibles, net | 54,600 | 41,879 | ||||||
Other assets | 112,890 | 95,692 | ||||||
|
|
|
| |||||
Total assets(a) | $ | 1,155,970 | $ | 951,672 | ||||
|
|
|
| |||||
LIABILITIES AND EQUITY |
| |||||||
Current Liabilities: | ||||||||
Accounts payable | $ | 146,533 | $ | 128,057 | ||||
Accrued expenses | 41,753 | 37,175 | ||||||
Current portion of long-term debt | — | 761 | ||||||
Other current liabilities | 12,041 | 33,211 | ||||||
|
|
|
| |||||
Total current liabilities | 200,327 | 199,204 | ||||||
Revolving line of credit and long-term debt, excluding current portion | 454,916 | 195,662 | ||||||
Other noncurrent liabilities | 109,916 | 110,280 | ||||||
|
|
|
| |||||
Total liabilities(a) | 765,159 | 505,146 | ||||||
|
| �� |
| |||||
Commitments and contingencies (Notes 2, 11, 12, 14, 15, 16, 17 and 19) | ||||||||
Equity: | ||||||||
PSS World Medical Inc. shareholders’ equity: | ||||||||
Preferred stock, $0.01 par value; 1,000,000 shares authorized, no shares issued and outstanding | — | — | ||||||
Common stock, $0.01 par value; 150,000,000 shares authorized, 50,312,323 and 55,465,600 shares issued and outstanding as of March 30, 2012 and April 1, 2011, respectively | 495 | 546 | ||||||
Additional paid-in capital | — | 122,912 | ||||||
Retained earnings | 386,633 | 319,468 | ||||||
|
|
|
| |||||
Total PSS World Medical, Inc. shareholders’ equity | 387,128 | 442,926 | ||||||
Noncontrolling interest | 3,683 | 3,600 | ||||||
|
|
|
| |||||
Total equity | 390,811 | 446,526 | ||||||
|
|
|
| |||||
Total liabilities and equity | $ | 1,155,970 | $ | 951,672 | ||||
|
|
|
| |||||
| (a) | See Footnote 5,Variable Interest Entity, for discussion of the assets and liabilities related to the Company’s consolidated variable interest entity. |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED MARCH 30, 2012, APRIL 1, 2011 AND APRIL 2, 2010
(In Thousands, Except Per Share Data)
| 2012 | 2011 | 2010 | ||||||||||
Net sales | $ | 2,102,002 | $ | 2,034,789 | $ | 2,055,171 | ||||||
Cost of goods sold | 1,427,799 | 1,399,018 | 1,427,476 | |||||||||
|
|
|
|
|
| |||||||
Gross profit | 674,203 | 635,771 | 627,695 | |||||||||
General and administrative expenses | 392,990 | 364,749 | 370,871 | |||||||||
Selling expenses | 147,857 | 137,466 | 135,843 | |||||||||
|
|
|
|
|
| |||||||
Income from operations | 133,356 | 133,556 | 120,981 | |||||||||
|
|
|
|
|
| |||||||
Other (expense) income: | ||||||||||||
Interest expense | (20,148 | ) | (17,121 | ) | (17,295 | ) | ||||||
Interest income | 173 | 284 | 376 | |||||||||
Other income, net | 2,084 | 2,506 | 6,068 | |||||||||
|
|
|
|
|
| |||||||
Other expense, net | (17,891 | ) | (14,331 | ) | (10,851 | ) | ||||||
|
|
|
|
|
| |||||||
Income before provision for income taxes | 115,465 | 119,225 | 110,130 | |||||||||
Provision for income taxes | 41,063 | 44,561 | 40,767 | |||||||||
|
|
|
|
|
| |||||||
Net income | 74,402 | 74,664 | 69,363 | |||||||||
Net income attributable to noncontrolling interest | 83 | 179 | — | |||||||||
|
|
|
|
|
| |||||||
Net income attributable to PSS World Medical, Inc. | $ | 74,319 | $ | 74,485 | $ | 69,363 | ||||||
|
|
|
|
|
| |||||||
Earnings per common share attributable to | ||||||||||||
PSS World Medical, Inc.: | ||||||||||||
Basic | $ | 1.43 | $ | 1.35 | $ | 1.20 | ||||||
Diluted | $ | 1.38 | $ | 1.32 | $ | 1.18 | ||||||
Weighted average common shares outstanding: | ||||||||||||
Basic | 51,998 | 54,996 | 58,029 | |||||||||
Diluted | 53,989 | 56,546 | 58,943 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY AND COMPREHENSIVE INCOME
FOR THE YEARS ENDED MARCH 30, 2012, APRIL 1, 2011 AND APRIL 2, 2010
(Dollars in Thousands, Except Share Data)
| Shares | Amount | Additional Paid-In Capital | Retained Earnings | Accumulated Other Comprehensive (Loss) Income | Total PSS World Medical, Inc. Shareholders’ Equity | Noncontrolling Interest | Total Equity | |||||||||||||||||||||||||
Balance as of March 27, 2009 | 58,301,253 | $ | 583 | $ | 200,175 | $ | 175,620 | $ | 1,652 | $ | 378,030 | $ | — | $ | 378,030 | |||||||||||||||||
Net income | — | — | — | 69,363 | — | 69,363 | — | 69,363 | ||||||||||||||||||||||||
Unrealized holding gains on available-for-sale investments, net of taxes of $33 | — | — | — | — | 56 | 56 | — | 56 | ||||||||||||||||||||||||
Reclassification adjustment for gains on available-for-sale investments included in net income, net of taxes of $1,375 | — | — | — | — | (2,260 | ) | (2,260 | ) | — | (2,260 | ) | |||||||||||||||||||||
Impact of interest rate swap, net of taxes of $339 | — | — | — | — | 552 | 552 | — | 552 | ||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Total comprehensive income | 67,711 | — | 67,711 | |||||||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Repurchases and retirement of common stock | (2,767,093 | ) | (28 | ) | (57,148 | ) | — | — | (57,176 | ) | — | (57,176 | ) | |||||||||||||||||||
Exercise of stock options | 547,823 | 6 | 4,483 | — | — | 4,489 | — | 4,489 | ||||||||||||||||||||||||
Stock-based compensation | — | — | 11,887 | — | — | 11,887 | — | 11,887 | ||||||||||||||||||||||||
Vesting of restricted stock | 90,354 | 1 | (1 | ) | — | — | — | — | — | |||||||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | 2,516 | — | — | 2,516 | — | 2,516 | ||||||||||||||||||||||||
Employee benefits and other | 27,256 | — | 557 | — | — | 557 | — | 557 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance as of April 2, 2010 | 56,199,593 | $ | 562 | $ | 162,469 | $ | 244,983 | $ | — | $ | 408,014 | $ | — | $ | 408,014 | |||||||||||||||||
Net income | — | — | — | 74,485 | — | 74,485 | 179 | 74,664 | ||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Total comprehensive income | — | — | — | — | — | 74,485 | 179 | 74,664 | ||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Acquisition of variable interest entity | — | — | — | — | — | — | 3,421 | 3,421 | ||||||||||||||||||||||||
Repurchases and retirement of common stock | (2,728,300 | ) | (27 | ) | (54,734 | ) | — | — | (54,761 | ) | — | (54,761 | ) | |||||||||||||||||||
Exercise of stock options | 337,853 | 3 | 2,075 | — | — | 2,078 | — | 2,078 | ||||||||||||||||||||||||
Stock-based compensation | — | — | 9,285 | — | — | 9,285 | — | 9,285 | ||||||||||||||||||||||||
Vesting of restricted stock | 802,005 | 8 | (8 | ) | — | — | — | — | — | |||||||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | 3,187 | — | — | 3,187 | — | 3,187 | ||||||||||||||||||||||||
Employee benefits and other | 23,892 | — | 638 | — | — | 638 | — | 638 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance as of April 1, 2011 | 54,635,043 | $ | 546 | $ | 122,912 | $ | 319,468 | $ | — | $ | 442,926 | $ | 3,600 | $ | 446,526 | |||||||||||||||||
Net income | — | — | — | 74,319 | — | 74,319 | 83 | 74,402 | ||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Total comprehensive income | — | — | — | — | — | 74,319 | 83 | 74,402 | ||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||
Repurchases and retirement of common stock | (5,594,668 | ) | (56 | ) | (133,229 | ) | (7,154 | ) | — | (140,439 | ) | — | (140,439 | ) | ||||||||||||||||||
Exercise of stock options | 170,961 | 1 | 1,382 | — | — | 1,383 | — | 1,383 | ||||||||||||||||||||||||
Stock-based compensation | — | — | 6,430 | — | — | 6,430 | — | 6,430 | ||||||||||||||||||||||||
Vesting of restricted stock | 301,581 | 4 | (4 | ) | — | — | — | — | — | |||||||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | 2,057 | — | — | 2,057 | — | 2,057 | ||||||||||||||||||||||||
Employee benefits and other | 26,021 | — | 452 | — | — | 452 | — | 452 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance as of March 30, 2012 | 49,538,938 | $ | 495 | $ | — | $ | 386,633 | $ | — | $ | 387,128 | $ | 3,683 | $ | 390,811 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED MARCH 30, 2012, APRIL 1, 2011 AND APRIL 2, 2010
(Dollars in Thousands)
| 2012 | 2011 | 2010 | ||||||||||
Cash Flows From Operating Activities: | ||||||||||||
Net income | $ | 74,402 | $ | 74,664 | $ | 69,363 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation | 26,847 | 25,065 | 21,940 | |||||||||
Amortization of intangible assets | 8,930 | 6,378 | 5,121 | |||||||||
Amortization of debt discount and issuance costs | 10,289 | 9,447 | 8,852 | |||||||||
Noncash compensation expense | 7,302 | 10,227 | 12,772 | |||||||||
Provision for doubtful accounts | 2,858 | 1,741 | 3,795 | |||||||||
(Benefit) provision for deferred income taxes | (1,284 | ) | 3,251 | (8,264 | ) | |||||||
Provision for deferred compensation | 1,165 | 1,423 | 1,530 | |||||||||
(Gain) loss on sales of property and equipment | (102 | ) | 19 | 81 | ||||||||
Gain on sale of available for sale securities | — | — | (3,635 | ) | ||||||||
Changes in operating assets and liabilities, net of effects from business combinations: | ||||||||||||
Accounts receivable, net | 85 | (7,257 | ) | 221 | ||||||||
Inventories | 4,096 | 12,265 | (9,718 | ) | ||||||||
Prepaid expenses and other current assets | 833 | (6,433 | ) | (5,710 | ) | |||||||
Other assets | (10,885 | ) | (7,973 | ) | (4,685 | ) | ||||||
Accounts payable | 8,828 | (8,153 | ) | (5,129 | ) | |||||||
Accrued expenses and other liabilities | (5,080 | ) | 1,664 | 15,867 | ||||||||
|
|
|
|
|
| |||||||
Net cash provided by operating activities | 128,284 | 116,328 | 102,401 | |||||||||
|
|
|
|
|
| |||||||
Cash Flows From Investing Activities: | ||||||||||||
Payments for business combinations, net of cash acquired | (65,131 | ) | (65,934 | ) | (14,802 | ) | ||||||
Capital expenditures | (23,918 | ) | (18,227 | ) | (25,923 | ) | ||||||
Payment for investment in variable interest entity, net of cash | — | (3,277 | ) | — | ||||||||
Proceeds from sale of available for sale securities | — | — | 10,681 | |||||||||
Other | (163 | ) | (668 | ) | (541 | ) | ||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (89,212 | ) | (88,106 | ) | (30,585 | ) | ||||||
|
|
|
|
|
| |||||||
Cash Flows From Financing Activities: | ||||||||||||
Proceeds from issuance of debt | 250,000 | — | — | |||||||||
Proceeds from borrowings on the revolving line of credit | 405,056 | 106,400 | 5,350 | |||||||||
Repayments on the revolving line of credit | (405,056 | ) | (106,400 | ) | (55,350 | ) | ||||||
Purchase and retirement of common stock | (140,439 | ) | (54,761 | ) | (57,176 | ) | ||||||
Payment of contingent consideration on business acquisitions | (9,500 | ) | (862 | ) | — | |||||||
Payment for debt issue costs | (6,467 | ) | — | — | ||||||||
Excess tax benefits from stock-based compensation arrangements | 2,057 | 3,187 | 2,516 | |||||||||
Proceeds from exercise of stock options | 1,383 | 2,079 | 4,489 | |||||||||
Payments under capital lease obligations | (779 | ) | (834 | ) | (925 | ) | ||||||
Other | (1,523 | ) | (434 | ) | — | |||||||
|
|
|
|
|
| |||||||
Net cash provided by (used in) financing activities | 94,732 | (51,625 | ) | (101,096 | ) | |||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in cash and cash equivalents | 133,804 | (23,403 | ) | (29,280 | ) | |||||||
Cash and cash equivalents, beginning of period | 29,348 | 52,751 | 82,031 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents, end of period | $ | 163,152 | $ | 29,348 | $ | 52,751 | ||||||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
MARCH 30, 2012, APRIL 1, 2011 AND APRIL 2, 2010
(Dollars in Thousands, Except Per Share Data, Unless Otherwise Noted)
1. NATURE OF OPERATIONS
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation, began operations in 1983. The Company is a national distributor of medical products and supplies, diagnostic equipment, healthcare information technology and pharmaceutical products, and provides professional and consulting services to the physician, long-term care, assisted living, home health care, and hospice markets. The Company has full-service distribution centers strategically located to efficiently serve all 50 states throughout the United States.
The Company currently conducts business through two operating segments, the Physician Business and the Extended Care Business, which serve a diverse customer base. A third reporting segment, Shared Services, consists of departments that support the operating segments through the delivery of standardized service.
The Physician Business, or the Physician Sales & Service division, is a leading distributor of medical supplies, diagnostic equipment, pharmaceutical-related products, healthcare information technology, professional and consulting services and physician dispensing solutions to alternate site healthcare providers in the U.S. The Physician Business currently operates 33 full-service distribution centers, 39 break-freight locations, 2 service centers, and 2 redistribution facilities, some of which are shared with the Extended Care Business, serving physician offices in all 50 states.
The Extended Care Business, or the Gulf South Medical Supply division, is a national distributor of medical supplies and related products and solutions to the extended care industry in the United States. The Extended Care Business serves the skilled nursing home, assisted living, home health care, and hospice markets. In addition, the Extended Care Business also provides Medicare Part B billing services, either on a fee-for-service or a full-assignment basis and Medicaid billing services to the assisted living market. The Extended Care Business currently operates 18 full-service distribution centers, 10 break-freight locations, 2 service centers, and 2 redistribution facilities, some of which are shared with the Physician Business, serving independent and regional skilled nursing facilities, assisted living centers, home health care, and hospice providers in all 50 states.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and include the accounts of PSS World Medical, Inc. and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
The Company reports its year-end financial position, results of operations, and cash flows on the Friday closest to March 31. Fiscal years 2012 and 2011 each consisted of 52 weeks and 253 selling days and fiscal year 2010 consisted of 53 weeks or 258 selling days.
Use of Estimates
The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and assumptions include the carrying amount of inventories, property and equipment, goodwill, and intangibles; allowances for doubtful accounts receivables, contractual billing adjustments and vendor rebate receivables; valuation allowances for deferred income taxes; liabilities for loss contingencies; incentive and stock-based compensation expense; and valuations associated with business combinations. Actual results could differ from the estimates and assumptions used in preparing the consolidated financial statements.
F-7
Table of Contents
Fair Value of Financial Instruments
The carrying amounts of the Company’s current financial instruments, including cash and cash equivalents, short-term trade receivables, and accounts payable, approximate their fair values due to the short-term nature of these assets and liabilities. The gross carrying value of the Company’s 6.375% unsecured senior notes issued in 2012 as of March 30, 2012 was $250,000 and the fair value, estimated using a third party valuation model, was approximately $257,500. The gross carrying value of the Company’s 3.125% senior convertible notes issued in 2008 as of March 30, 2012 and April 1, 2011 was $230,000 and the fair value, estimated using a third party valuation model, was approximately $302,174 and $323,800, respectively.
Cash and Cash Equivalents
Cash and cash equivalents generally consist of demand deposits with financial institutions and highly liquid investment grade instruments having maturities of three months or less at the date of purchase. Cash and cash equivalents are stated at cost, which approximates market value.
Outstanding checks in excess of cash balances available for a legal right of offset are reclassified toAccounts payable on theConsolidated Balance Sheets. Amounts reclassified to accounts payable were $10,069 and $13,425 as of March 30, 2012 and April 1, 2011, respectively.
Accounts Receivable
Trade accounts receivable consists of amounts owed to the Company and is stated net of allowances, which approximates fair value due to the short-term nature of the asset. The Company’s outstanding accounts receivable balances are exposed to credit risk and valuation allowances are established for estimated losses resulting from non-collection of outstanding amounts due from customers. The valuation allowances include specific amounts for those accounts that are deemed likely to be uncollectible, such as disputed amounts and customers in bankruptcy, and general allowances for accounts that management currently believes to be collectible but that may later become uncollectible. Estimates are used to determine the valuation allowances and are generally based on historical collection results, current economic trends, credit-worthiness of customers, and changes in customer payment terms. Cash flows related to changes in accounts receivable balances are classified as operating activities within theConsolidated Statements of Cash Flows.
The Physician Business’ trade accounts receivable consists of many individual accounts, none of which is individually significant to the Company. The Physician Business had allowances for doubtful accounts of approximately $3,167 and $2,934 as of March 30, 2012 and April 1, 2011, respectively. During fiscal years 2012, 2011, and 2010, bad debt expense was less than 1% of net sales.
The Extended Care Business’ trade accounts receivable has a number of large customer accounts that are significant to its business. Approximately 16%, 16%, and 15%, of the Extended Care Business’ net sales for the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, respectively, represent net sales to its largest five customers. As of March 30, 2012 and April 1, 2011, the outstanding accounts receivable balances of these customers represented approximately 10% of accounts receivable, net of allowance for doubtful accounts, respectively. The Extended Care Business had allowances for doubtful accounts of approximately $3,047 and $2,875 as of March 30, 2012 and April 1, 2011, respectively. During fiscal years 2012, 2011, and 2010, bad debt expense was less than 1% of net sales.
Over the past three years, the Company’s average allowance for doubtful accounts has represented 2% of the Physician Business’ gross accounts receivable balance, and 4% of the Extended Care business’ gross accounts receivable balance.
F-8
Table of Contents
Contractual Billing Adjustments
The Company provides medical claim billing services on a fee-for-service or a full-assignment basis and records claims receivable due from insurance carriers. A claim may become uncollectible in full due to denial, or partially uncollectable due to discounts taken. Management estimates contractual billing adjustments based on historical collection experience, and also considers voided claims and claims written off. Contractual billing adjustments are recorded as a reduction toNet sales on theConsolidated Statements of Operations.
Inventories
Inventories consist of medical products, medical equipment, and other related products and are stated at the lower of cost or market. Cost is determined using the first-in, first-out (“FIFO”) method. Market is defined as net realizable value. The net realizable value of excess and slow moving inventory is determined using judgment as to when inventory will be sold and the quantities and prices at which inventory will be sold in the normal course of business. Obsolete or damaged inventory is disposed of or written down to net realizable value on a quarterly basis. Additional adjustments, if necessary, are made based on management’s specific review of inventory on-hand. Cash flows related to changes in inventory are classified as operating activities within theConsolidated Statements of Cash Flows.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the following estimated useful lives of the respective classes of assets:
| Useful Life | ||
Equipment | 2 to 10 years | |
Computer hardware and software | 3 to 15 years | |
Capitalized internal-use software costs | 5 to 15 years |
Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life. Management is required to use judgment in determining the estimated useful lives of such assets. Changes in circumstances such as technological advances, changes to the Company’s business model, changes in the Company’s business strategy, or changes in the planned use of property and equipment could result in the actual useful lives differing from the Company’s current estimates. In those cases where the Company determines the useful life of property and equipment should be shortened or extended, the Company depreciates the net book value in excess of the estimated salvage value over its revised remaining useful life.
The Company capitalizes the following costs associated with developing internal-use computer software: (i) external direct costs of materials and services consumed in developing or obtaining internal-use computer software; (ii) certain payroll and payroll-related costs for Company employees who are directly associated with the development of internal-use software, to the extent of time spent directly on the project; and (iii) interest costs incurred while developing internal-use computer software. According to ASC 835-20,Interest-Capitalization of Interest, interest cost may be capitalized as a part of the historical cost of acquiring certain assets, such as assets that are constructed or produced for a company’s own use. The amount of capitalized interest during fiscal years 2012, 2011, and 2010 was $897, $511, and $1,182, respectively.
Gains or losses upon retirement or disposal of property and equipment are recorded inOther income, net in the accompanyingConsolidated Statements of Operations. Normal repair and maintenance costs that do not substantially extend the life of property and equipment are expensed as incurred.
Goodwill
Goodwill represents the future economic benefits and synergies arising from other assets acquired in a business combination that are not individually identified and separately recognized. In accordance with the provisions of ASC 350-20,Intangibles – Goodwill and Other – Goodwill, goodwill is reviewed for impairment annually as of the
F-9
Table of Contents
last day of the fiscal year. An interim review is performed between annual tests whenever events or changes in circumstances indicate the carrying amount of the goodwill may be impaired. Because the estimated fair value of the reporting units exceeded the carrying amount of the goodwill, there was no impairment as of March 30, 2012 and April 1, 2011.
Intangibles
ASC 350-30,Intangibles – Goodwill and Other – General Intangibles Other Than Goodwill,requires intangible assets with finite useful lives be amortized over their respective estimated useful lives. Amortization is computed using the straight-line method.
Certain sales representatives employed by the Physician and Extended Care Businesses have executed employment agreements in exchange for a cash payment (“Nonsolicitation Agreements”). These employment agreements include nonsolicitation covenants, which state that the sales representative can neither solicit nor accept business from certain of the Company’s customers for a stated period of time subsequent to the date the sales representative ceases employment with the Company. The costs associated with these Nonsolicitation Agreements are capitalized and amortized on a straight-line basis over their estimated useful lives, plus the stated nonsolicitation period. If a sales representative terminates employment prior to the end of the estimated useful life of the agreement, the remaining net book value of the asset is amortized over the stated nonsolicitation period.
During the period the sales representatives remain employed with the Company, the nonsolicitation intangible asset is evaluated for impairment in accordance with the provisions of ASC 360-10,Property, Plant, and Equipment – Overall. This standard requires the Company to test for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Certain factors which may indicate an impairment exists include, but are not limited to: (i) a change in a state’s legal system that would impact any legal opinion relied upon when assessing enforceability of the nonsolicitation covenants, (ii) a decline in gross profit or sales volume, (iii) death, or (iv) full retirement by the sales representative. In the event the carrying value of the assets were to be determined unrecoverable, the Company would estimate the fair value of the assets and record an impairment charge for the excess of the carrying value over the fair value. There were no impairments as of March 30, 2012 or April 1, 2011.
Impairment of Long-Lived Assets
Long-lived assets, other than goodwill and indefinite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable in accordance with ASC 360-10,Property, Plant, and Equipment – Overall. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted future cash flows expected to result from the use and eventual disposition of the asset. The impairment loss is measured as the amount by which the carrying amount of the long-lived asset exceeds fair value.
The Company evaluates the recoverability of indefinite-lived intangible assets annually in accordance with ASC 350-30,Intangibles – Goodwill and Other – General Intangibles Other Than Goodwill. An interim review may be performed more frequently, if events or changes in circumstances, such as a decline in sales, earnings, or cash flows, or material adverse changes in the business climate, indicate that the carrying value of an asset might be impaired. There were no impairments as of March 30, 2012 or April 1, 2011.
Discontinued Operations
A business is classified as a discontinued operation when the operations and cash flows of the business can be clearly distinguished and have been or will be eliminated from the Company’s ongoing operations, the business has either been disposed of or is classified as held for sale, and the Company will not have any significant continuing involvement in the operations of the business after the disposal transaction. The results of discontinued operations (as well as the gain or loss on the disposal) are aggregated and separately presented in the Company’sConsolidated Statements of Operations, net of income taxes, and in theConsolidated Statements of Cash Flows.
F-10
Table of Contents
During the first quarter of fiscal year 2013 the Company’s Board of Directors approved a strategic restructuring plan designed to transform the Company. The restructuring plan will include the sale of two business units serving skilled nursing facilities and specialty dental practices, the integration of all warehouse operations into one common distribution infrastructure, as well as a redesign of the shared services function. The Company determined that certain held for sale criteria had not been met as of March 30, 2012 and therefore reports the assets, liabilities, and the related results of operations as continuing operations. See Footnote 23,Subsequent Events, for additional information.
Insurance Coverage
The Company has a self-funded program for employee and dependent health insurance. This program includes an administrator, a large provider network, and stop loss reinsurance to cover individual claims in excess of $250 per person, with an additional aggregate specific deductible of $190 annually, and up to $2,000 catastrophic loss maximum per lifetime benefit per person. Claims incurred but not reported are recorded based on estimates of claims provided by the third party administrator and are included inAccrued expenses in the accompanyingConsolidated Balance Sheets. The Company recognized $16,853, $13,153, and $13,452 in medical expenses, net of employee contributions, during the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, respectively.
The Company maintains a primary casualty insurance program for its automobile liability, employer’s liability, and general liability risks, which in general provides limits of up to $2,000, $1,000, and $2,000, respectively. The primary program contains a deductible of $350 for automobile liability, $500 for employer’s liability, and $100 for general liability, subject to a primary aggregate stop loss of approximately $8,000 for the current plan year. The Company also maintains workers compensation policies which have statutory limits that are based on state regulations and have a deductible of $500 per occurrence. In addition, the Company maintains an umbrella/excess liability program to cover occurrences in excess of the underlying primary limits.
Contingent Loss Accruals
In determining the accrual necessary for probable loss contingencies as defined by ASC 450-20,Contingencies – Loss Contingencies, the Company includes estimates for professional fees, such as legal, accounting, and consulting, and other related costs to be incurred, unless such fees and related costs are not probable of being incurred or are not reasonably estimable.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the tax consequences attributable to temporary differences between the financial statement carrying amounts and the respective tax basis in existing assets and liabilities. Deferred tax assets and liabilities are measured using the tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in theConsolidated Statements of Operations in the period that includes the enactment date. The Company has not provided for U.S. income taxes on accumulated and undistributed earnings attributable to foreign operations as the Company intends to permanently reinvest these undistributed earnings.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, evidence of delivery of products or services is obtained, the selling price is fixed or determinable, and collectability of the resulting accounts receivable is reasonably assured. The Company assesses collectability based upon a thorough evaluation of current and prospective customers’ credit history and ability to pay. The Company establishes and adjusts credit terms and limits to reflect customer credit worthiness based upon this evaluation. Customer credit evaluations are updated periodically and for specific events or circumstances such as deterioration in the aging of account balances, bankruptcy filings, or notice or knowledge of financial difficulties.
F-11
Table of Contents
Consolidated sales allowances are immaterial and generally represent less than 1% of gross sales.
Physician Business. The Physician Business has three primary sources of revenue: (i) the sale of consumable products; (ii) the sale of equipment; and (iii) claims processing services provided to physician dispensing customers.
Revenue from the sale of consumable products is recognized when products are shipped or delivered since at that time there is persuasive evidence that an arrangement exists, the price is fixed and determinable, and the collection of the resulting accounts receivable is reasonably assured. Revenue from the sale of single deliverable equipment is generally recognized when the equipment is shipped, unless there are multiple deliverables, in which case revenue is recognized when all obligations to the customer are fulfilled. Obligations to the customer are typically satisfied when installation and training are complete. Customers have the right to return consumable products and equipment. Sales allowances are recorded as a reduction of revenue for potential product returns and estimated billing errors. Management analyzes sales allowances quarterly using historical data adjusted for significant changes in volume and business conditions, as well as specific identification of significant returns or billing errors.
Revenue from claims processing services provided to physician dispensing customers is recognized when claims are processed. As the Company acts an agent in the arrangement, revenue is recorded on a net basis.
Extended Care Business. The Extended Care Business has three primary sources of revenue: (i) the sale of consumable products and services to skilled nursing home and assisted living facilities, hospice and home health care providers; (ii) service fees earned for providing Medicare Part B and Medicaid products and services; and (iii) consulting services to skilled nursing home and assisted living facilities, hospice and home health care providers.
Revenue from the sale of consumable products to skilled nursing home facilities, assisted living facilities, and home health care providers is recognized when products are shipped or delivered. Revenue for these products is recorded upon shipment since at that time there is persuasive evidence that an arrangement exists, the price is fixed and determinable, and the collection of the resulting accounts receivable is reasonably assured.
Revenue from providing ancillary medical supplies for Medicare Part B eligible patients and Medicaid eligible patients on a full assignment basis is recognized during the period the supplies are shipped to the eligible patients. The product is shipped to the facility patient specific and becomes the property of that specific patient. Revenue is recorded at the amounts expected to be collected from Medicare, Medicaid, other third-party payers, and directly from customers. Reimbursement from Medicare is subject to review by appropriate government regulators. Revenue from providing Medicare Part B and Medicaid billing services on a fee for service basis is recognized when billing services are rendered to the customer.
Revenue from the sale of consulting services to skilled nursing home and assisted living facilities, hospice and home health care providers is recognized when services are rendered to the customer.
Customers have the right to return consumable products and equipment. Sales allowances are recorded as a reduction of revenue for (i) potential product and equipment returns, (ii) patients that turn out to be ineligible to be billed to Medicare or other payor, and (iii) Medicare Part B and Medicaid reimbursement denials, capped rental of enteral pumps, and billing errors. Management analyzes actual revenue adjustments and Medicare Part B reimbursement denials using historical actual cash collection and actual adjustments to gross revenue. The historical percentage is used to estimate the future cash collections and required accounts receivable reserve. Additional allowances are recorded for any significant specific adjustments known to management.
Vendor Rebates
The Company receives transaction-based and performance-based rebates from third party suppliers. Transaction-based rebates are generally associated with a specific customer contract and are recognized as a reduction to cost of goods sold at the time the transaction occurs. Management establishes a reserve for uncollectible transaction-based vendor rebates based on management’s judgment after considering the status of current outstanding rebate claims, historical denial experience with suppliers, and any other pertinent available information.
F-12
Table of Contents
In accordance with ASC 605-50,Revenue Recognition – Customer Payments and Incentives, performance-based rebates are recognized based on a systematic estimation of the consideration to be received relative to the transaction that marks the progress of the Company toward earning vendor rebates, provided the collection of the amounts is, in the judgment of management, reasonably assured. The factors the Company considers in estimating performance-based rebates include actual inventory purchases or sales volumes, in conjunction with vendor rebate contract terms, which generally provide for increasing rebates based on either increased purchases or sales volume. Performance-based rebates are recognized in income only if the related inventory has been sold.
In accordance with ASC 605-50,Revenue Recognition – Customer Payments and Incentives, sales incentive arrangements that meet certain criteria are not recorded as a reduction of cost of sales. Accordingly, reimbursements from manufacturers under these arrangements are recognized by the Company as revenue rather than a reduction of cost of sales.
Transaction-based and performance-based rebate contracts are negotiated periodically with vendors.
The following table summarizes the financial statement impact of transaction-based and performance-based vendor rebates recognized by the Company and each of its segments during fiscal years 2012, 2011, and 2010. Such rebates are classified as either (i) a reduction to cost of goods sold or (ii) an increase to net sales in the accompanyingConsolidated Statements of Operations.
| (in thousands) | Physician Business | |||||||||||
Rebates included within: | 2012 | 2011 | 2010 | |||||||||
Net sales | $ | 759 | $ | 1,237 | $ | 2,433 | ||||||
Cost of goods sold | 124,792 | 107,924 | 108,143 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 125,551 | $ | 109,161 | $ | 110,576 | ||||||
|
|
|
|
|
| |||||||
| Extended Care Business | ||||||||||||
Rebates included within: | 2012 | 2011 | 2010 | |||||||||
Cost of goods sold | $ | 106,251 | $ | 108,718 | $ | 106,141 | ||||||
| Total Company | ||||||||||||
Rebates included within: | 2012 | 2011 | 2010 | |||||||||
Net sales | $ | 759 | $ | 1,237 | $ | 2,433 | ||||||
Cost of goods sold | 231,043 | 216,642 | 214,284 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 231,802 | $ | 217,879 | $ | 216,717 | ||||||
|
|
|
|
|
| |||||||
Shipping and Handling Costs
Shipping and handling costs billed to customers are included inNet sales and totaled approximately $15,832, $13,521, and $11,383, for fiscal years 2012, 2011, and 2010, respectively. Shipping and handling costs incurred by the Company, which are included inGeneral and administrative expenses, totaled approximately $110,142, $105,334, and $104,134, for fiscal years 2012, 2011, and 2010, respectively.
Convertible Debt Instruments
In accordance with ASC 470-20,Debt – Debt with Conversion and Other Options, issuers of convertible debt instruments that may be settled in cash upon conversion (including partial cash settlements) should separately account for the liability and equity components in a manner that reflects an estimate of the entity’s nonconvertible debt borrowing rate when interest expense is recognized in subsequent periods. The equity components of the Company’s senior convertible notes are included inAdditional paid in capital in theConsolidated Balance Sheets, with a corresponding reduction in the carrying values of these convertible notes as of the date of issuance or modification, as applicable. The reduced carrying values of the convertible notes are accreted to principal amounts through the recognition of non-cash interest expense. This accretion results in recognizing interest expense on these borrowings at effective rates approximating what would have been incurred had the Company issued nonconvertible debt with otherwise similar terms. See Footnote 12,Debt, for additional information.
F-13
Table of Contents
Derivative Financial Instruments
Derivative financial instruments are accounted for under ASC 815,Derivatives and Hedging.Accordingly, all derivatives are recorded on the balance sheet as assets or liabilities and measured at fair value. For derivatives designated as cash flow hedges, the effective portion of the changes in fair value of the derivatives are recorded inAccumulated other comprehensive income and subsequently recognized in earnings when the hedged items impact earnings, typically upon settlement. Changes in the fair value of derivatives not designated as hedges and the ineffective portion of cash flow hedges are recorded in current earnings.
Guidance within ASC 815,Derivatives and Hedging,requires enhanced disclosures about an entity’s derivative and hedging activities, including (i) how and why an entity uses derivative instruments, (ii) how derivative instruments and related hedged items are accounted for and its related interpretations, and (iii) how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows.
Derivative financial instruments are used principally in the management of the Company’s interest rate exposure. During the fiscal year ended March 28, 2008, the Company entered into an interest rate swap agreement to hedge the variable interest rate of its revolving line of credit. The interest rate swap was designated as a cash flow hedge. During fiscal year ended April 2, 2010, the interest rate swap matured. Amounts paid upon maturity of the interest rate swap agreement were recorded as additions to interest expense. Refer to Footnote 12,Debt, for additional information regarding the Company’s interest rate swap agreement.
Earnings Per Share
Basic and diluted earnings per share are presented in accordance with ASC 260,Earnings Per Share. Basic earnings per share is computed by dividing net income by the weighted average number of common shares outstanding during the period. Diluted earnings per share is computed by dividing net income by the weighted average number of common and common equivalent shares outstanding during the period adjusted for the potential dilutive effect of unvested restricted stock and stock options using the treasury stock method and the conversion of the senior convertible notes. Common equivalent shares are excluded from the computation in periods in which they have an anti-dilutive effect.
The following table sets forth computational data for the denominator in the basic and diluted earnings per share calculation for fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010:
| (in thousands) | 2012 | 2011 | 2010 | |||||||||
Denominator-weighted average shares outstanding used in computing basic earnings per share | 51,998 | 54,996 | 58,029 | |||||||||
Assumed exercise of stock options(a) | 63 | 198 | 324 | |||||||||
Assumed vesting of restricted stock | 453 | 650 | 514 | |||||||||
Assumed conversion of 2008 Notes | 1,475 | 702 | 76 | |||||||||
|
|
|
|
|
| |||||||
Denominator-weighted average shares outstanding used in computing diluted earnings per share | 53,989 | 56,546 | 58,943 | |||||||||
|
|
|
|
|
| |||||||
| (a) | There were no antidilutive options outstanding as of March 30, 2012, April 1, 2011, and April 2, 2010. |
In accordance with ASC 260,Earnings Per Share, and the Company’s stated policy of settling the principal amount in cash, the Company is required to include shares underlying the 2008 Notes in its diluted weighted average shares outstanding due to the average stock price per share for the period exceeding $21.22 (the conversion price for the senior convertible notes) during fiscal year ended March 30, 2012. Only the number of shares that would be issuable (under the treasury stock method of accounting for share dilution) was included, which was based upon the amount by which the average stock price exceeded the conversion price. If the price of the Company’s common
F-14
Table of Contents
stock exceeds $28.29 per share, it will also include the effect of the additional potential shares that may be issued related to the warrants transactions associated with the 2008 Notes, using the treasury stock method. Prior to conversion, the purchased options associated with the 2008 Notes are not considered for purposes of the dilutive earnings per share calculation as their effect is considered to be anti-dilutive. Refer to Footnote 12,Debt, for additional information regarding the 2008 Notes.
Stock-Based Compensation
Effective April 1, 2006, the Company adopted the provisions of ASC 718,Compensation – Stock Compensation, (“ASC 718”) using the modified prospective transition method, and therefore, has not restated results for prior periods. The Company applies the fair value recognition provisions of the guidance as it relates to the Company’s stock-based compensation, which requires the Company to recognize expense for the fair value of stock-based compensation awards. Refer to Footnote 15,Incentive and Stock-Based Compensation,for additional information.
Comprehensive Income
Comprehensive income represents all changes in equity of an enterprise that result from recognized transactions and other economic events during the period. Other comprehensive income refers to revenues, expenses, gains, and losses that under GAAP are included in comprehensive income but excluded from net income, such as the unrealized gain or loss on the interest rate swap and unrealized holding gain or loss on available-for-sale investments.
Marketable Securities
As of March 30, 2012, the Company held no investment in available for sale securities. Equity securities previously held by the Company were considered to be available for sale and carried at fair value as of the balance sheet dates. Fair values were based on quoted market prices.
Realized gains and losses on the sale of investments were determined on the basis of the cost of the specific investments sold and were credited or charged to income on a trade date basis. Unrealized gains or losses on equity securities which were classified as available for sale, net of applicable deferred income taxes (benefits), were excluded from earnings and credited or charged directly to a separate component of stockholders’ equity.
Share Repurchases
The Company repurchases its common stock under stock repurchase programs authorized by the Company’s Board of Directors. The Company retires shares upon repurchase. Payments to repurchase shares are recorded toCommon stock on theConsolidated Balance Sheets, with the amount in excess of par value recorded toAdditional paid-in capital on theConsolidated Balance Sheets.
During fiscal year 2012, the Company’s additional paid-in capital balance was reduced to zero as a result of share repurchases. In accordance with ASC 505,Equity, retirements of the Company’s shares may be recorded as a reduction of additional paid-in capital to the extent that previous net gains from sales or retirements of the same class of stock remain, and otherwise should be recorded to retained earnings. As a result, retained earnings was reduced by $7,154 during fiscal year 2012, which represented share repurchases occurring after the additional paid-in capital balance had been reduced to zero.
3. RECENT ACCOUNTING PRONOUNCEMENTS
In October 2009, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Update (“ASU”) for multiple deliverable revenue arrangements. The update requires entities to allocate revenue in an arrangement using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The update eliminates the residual method of revenue allocation and requires revenues to be allocated using the relative selling price method. The Company adopted this update prospectively for revenue arrangements entered into or materially modified beginning in the first quarter of fiscal year 2012. The Company has evaluated this standard and determined it will not have a material effect on the Company’s statements of financial condition or results of operations.
F-15
Table of Contents
In May 2011, the FASB issued an ASU with amendments to achieve common fair value measurement and disclosure requirements in GAAP. The amendments in this update clarified the language used to describe many of the requirements in GAAP for measuring fair value and for disclosing information about fair value measurements. The following areas were impacted by this ASU: (i) application of the highest and best use and valuation premise concepts; (ii) measuring the fair value of an instrument classified in shareholders’ equity; and (iii) additional quantitative disclosures regarding unobservable inputs used in Level 3 fair value measurements. The amendments are effective during interim and annual periods beginning after December 15, 2011, or the Company’s fourth quarter of fiscal year 2012. The Company has evaluated this standard and determined that, other than requiring additional disclosures, it did not have a material impact on the Company’s statements of financial condition or results of operations.
In June 2011, the FASB issued new guidance on the presentation of comprehensive income that requires changes in stockholders’ equity to be presented either (i) in a single continuous statement of comprehensive income, or (ii) in two separate consecutive statements. The ASU requires retrospective application and is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, or the Company’s fiscal year 2013. In December 2011, the FASB indefinitely deferred the effective date for amendments pertaining to the presentation of reclassification adjustments by component. The Company has evaluated this standard and determined it will not have a material effect on the Company’s statements of financial condition or results of operations.
In September 2011, the FASB issued amended guidance to simplify the method in which entities test goodwill for impairment. This ASU allows an entity to assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. Additional disclosure requirements were included with this update, including an explanation of qualitative factors used in the goodwill analysis. The amendments in this update are effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, or the Company’s fiscal year 2013. The Company has evaluated this standard and determined it will not have an effect on the Company’s statements of financial condition or results of operations.
4. PURCHASE BUSINESS COMBINATIONS
Acquisitions were accounted for under the purchase method of accounting; accordingly, the operations of the acquired companies have been included in the Company’s results of operations subsequent to the date of acquisition. The assets acquired and liabilities assumed were recorded at their estimated fair values at the date of the acquisition as determined by management based on information currently available and independent valuations.
The fair value of contingent consideration was determined using projected achievement of the earnings targets. See Footnote 6,Fair Value Measurements, for further discussion.
During fiscal year 2012, the Company completed acquisitions that were individually immaterial but material in the aggregate (the “2012 acquisitions”). Net sales and net loss attributable to the 2012 acquisitions since their respective acquisition dates was approximately $16,566 and ($120), respectively. These amounts do not include net sales and net loss attributable to acquisitions that have been integrated as discrete information is impracticable to obtain. Payments totaling $66,623, net of cash received of $26 and outstanding checks of $415, were made during fiscal year 2012 related to these acquisitions, of which $1,525 was held in escrow to secure certain adjustments or claims. In addition, $3,401 was held by the Company to secure certain adjustments or claims. Contingent consideration ranging from $0 to $667 may be paid based on the achievement of future earnings targets over a two year period. Goodwill represents the future economic benefits and synergies arising from other assets acquired in a business combination that are not individually identified and separately recognized. A portion of the goodwill related to the 2012 acquisitions in the amount of $19,285 is tax deductible. As of March 30, 2012, the purchase accounting associated with the 2012 acquisitions was not complete given the recent acquisition dates.
F-16
Table of Contents
During fiscal year 2011, the Physician Business acquired the assets of Linear Medical Solutions, Inc. (“Linear”) and all of the outstanding stock of Dispensing Solutions, Inc. (“DSI”), which market proprietary systems for dispensing medications to patients primarily within physician practices. Payments totaling $13,872, net of cash received of $359, were made related to the Linear acquisition during fiscal year 2011. Payments totaling $35,199, net of cash received of $801, were made related to the DSI acquisition during fiscal year 2011. During fiscal year 2012, the purchase accounting associated with these acquisitions was finalized and the fair value measurements of assets acquired and liabilities assumed as of the acquisition dates were revised.
Opening Balance Sheets
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as of the dates of the 2012 acquisitions, as adjusted:
| 2012 acquisitions | ||||||||||||
| Opening | ||||||||||||
| Balance | ||||||||||||
| Opening | Measurement | Sheet | ||||||||||
| Balance | Period | As Adjusted | ||||||||||
| (in thousands) | Sheet | Adjustment | March 30, 2012 | |||||||||
Current assets (a) | $ | 20,458 | (768 | ) | $ | 19,690 | ||||||
Goodwill | 37,858 | 8 | 37,866 | |||||||||
Intangible assets | 19,640 | 1,510 | 21,150 | |||||||||
Noncurrent assets (b) | 6,387 | — | 6,387 | |||||||||
Accounts payable and other current liabilities | (12,060 | ) | (387 | ) | (12,447 | ) | ||||||
Noncurrent liabilities (c) | (1,842 | ) | (679 | ) | (2,521 | ) | ||||||
Contingent consideration | (490 | ) | — | (490 | ) | |||||||
|
|
|
| |||||||||
Net assets acquired | $ | 69,951 | $ | (316 | ) | $ | 69,635 | |||||
|
|
|
| |||||||||
| (a) | The following represents balances withinCurrent assets as of March 30, 2012: accounts receivable, net of $13,103, inventory of $4,454, current deferred income taxes of $796, and other current assets of $1,337. |
| (b) | The following represents balances withinNoncurrent assets as of March 30, 2012: property and equipment, net of $1,674 and other noncurrent assets of $4,713. |
| (c) | The following represents balances withinNoncurrent liabilitiesas of March 30, 2012: noncurrent deferred tax liabilities of $2,381 and other noncurrent liabilities of $140. |
F-17
Table of Contents
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as of the dates of the Linear and the DSI acquisitions, as adjusted:
| Linear | DSI | |||||||||||||||||||||||
| Opening | Opening | Opening | Opening | |||||||||||||||||||||
| Balance | Balance | Balance | Balance | |||||||||||||||||||||
| Sheet | Measurement | Sheet | Sheet | Measurement | Sheet | |||||||||||||||||||
| As Adjusted | Period | As Adjusted | As Adjusted | Period | As Adjusted | |||||||||||||||||||
| (in thousands) | April 1, 2011 | Adjustment | March 30, 2012 | April 1, 2011 | Adjustment | March 30, 2012 | ||||||||||||||||||
Current assets(a) | $ | 12,711 | $ | — | $ | 12,711 | $ | 6,458 | $ | 431 | $ | 6,889 | ||||||||||||
Goodwill | 3,816 | 61 | 3,877 | 26,747 | (3,285 | ) | 23,462 | |||||||||||||||||
Intangible assets | 4,538 | — | 4,538 | 11,070 | — | 11,070 | ||||||||||||||||||
Noncurrent assets(b) | 1,734 | — | 1,734 | 2,090 | — | 2,090 | ||||||||||||||||||
Accounts payable and other current liabilities | (5,068 | ) | — | (5,068 | ) | (2,226 | ) | (50 | ) | (2,276 | ) | |||||||||||||
Noncurrent liabilities(c) | — | — | — | (2,639 | ) | — | (2,639 | ) | ||||||||||||||||
Contingent consideration | (3,500 | ) | — | (3,500 | ) | (5,500 | ) | — | (5,500 | ) | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
Net assets acquired | $ | 14,231 | $ | 61 | $ | 14,292 | $ | 36,000 | $ | (2,904 | ) | $ | 33,096 | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
| (a) | The following represents balances withinCurrent assets as of March 30, 2012: Linear – accounts receivable, net of $8,939, inventory of $3,182, and other current assets of $590; and DSI – accounts receivable, net of $2,615, inventory of $2,361, current deferred income taxes of $180, and other current assets of $1,733. |
| (b) | The following represents balances withinNoncurrent assets as of March 30, 2012: Linear – property and equipment, net of $1,690 and other noncurrent assets of $44; and DSI – property and equipment, net of $2,055 and other noncurrent assets of $35. |
| (c) | The following represents balances withinNoncurrent liabilitiesas of March 30, 2012: DSI – noncurrent deferred tax liabilities of $2,639. |
During fiscal year 2012, the Company recorded a $3,500 reduction in the purchase price of DSI related to circumstances outstanding at the acquisition date, as well as a working capital adjustment of $596. As of March 30, 2012, the purchase accounting related to DSI and Linear was finalized.
Unaudited Pro Forma Information
The following table presents unaudited pro forma financial information as if the closing of the 2012 acquisitions had occurred on the first day of fiscal year 2011, or April 3, 2010, and the acquisitions of Linear and DSI had occurred on the first day of fiscal year 2010, or March 28, 2009, after giving effect to certain purchase accounting adjustments.
F-18
Table of Contents
Pro forma information is not necessarily indicative of the results of operations that actually would have resulted had the acquisitions occurred on the dates indicated above or that may result in the future, and does not reflect potential synergies, integration costs or other such costs and savings. Supplemental net sales and supplemental net income figures are not comparable year over year as only pre-acquisition amounts are shown. Subsequent to acquisition, net sales and net income are included in the “as reported” figure.
| (in thousands) | 2012 | 2011 | 2010 | |||||||||
Net sales: | ||||||||||||
PSS World Medical, Inc. (as reported) | $ | 2,102,002 | $ | 2,034,789 | $ | 2,055,171 | ||||||
Supplemental net sales - 2012 acquisitions | 57,891 | 88,029 | N/A | |||||||||
Supplemental net sales - Linear | — | 53,339 | 70,633 | |||||||||
Supplemental net sales - DSI | — | 24,543 | 22,067 | |||||||||
|
|
|
|
|
| |||||||
Total pro forma net sales | $ | 2,159,893 | $ | 2,200,700 | $ | 2,147,871 | ||||||
Net income attributable to PSS World Medical, Inc.: | ||||||||||||
PSS World Medical, Inc. (as reported) | $ | 74,319 | $ | 74,485 | $ | 69,363 | ||||||
Supplemental net income - 2012 acquisitions | (1,525 | ) | (3,022 | ) | N/A | |||||||
Supplemental net income - Linear | — | 2,711 | 2,744 | |||||||||
Supplemental net income - DSI | — | 892 | (623 | ) | ||||||||
|
|
|
|
|
| |||||||
Total pro forma net income | $ | 72,794 | $ | 75,066 | $ | 71,484 | ||||||
Net income per common share: | ||||||||||||
Basic | $ | 1.40 | $ | 1.36 | $ | 1.23 | ||||||
Diluted | $ | 1.35 | $ | 1.33 | $ | 1.21 | ||||||
Contingent Consideration
During fiscal year 2012, the fair value of contingent consideration associated with the Linear, DSI, and other acquisitions completed prior to fiscal year 2012 was decreased by $594, $42, and $17, respectively, with the change in value reflected as a reduction inGeneral and administrative expenses on theConsolidated Statements of Operations. During fiscal year 2012, the Company paid final contingent consideration payments of $3,000 for Linear and $1,000 for other acquisitions. The Company also made a final contingent consideration payment related to DSI of $5,500, which was reduced by the purchase price adjustment of $3,500 discussed above.
Other Acquisitions
During fiscal years 2011 and 2010, the Company made cash payments of $16,777 and $13,609, respectively, related to other acquisitions not significant for additional disclosure individually or in the aggregate. During fiscal years 2012, 2011, and 2010, the Company made cash payments of $1,351, $85, and $1,193 for holdback payments and working capital adjustments related to prior year acquisitions. During fiscal year 2011, the Company recognized goodwill, including acquisition-related adjustments, of approximately $12,183.
5. VARIABLE INTEREST ENTITY
On June 25, 2010, the Company entered into an agreement with Pathway Health Services, Inc. (“Pathway”), a consulting services company within the Extended Care market, under which the Company purchased a $3,300 convertible note issued by Pathway. The note may be converted, at the Company’s discretion, into 73% of Pathway’s common stock. The Company also acquired a call option and issued a put option for Pathway’s common stock, both of which may be exercised if certain sales thresholds are met and time restrictions lapse. Under the agreement, the Company obtained a majority of seats and control of Pathway’s Board of Directors. The convertible note is considered a variable interest and the Company was determined to be the primary beneficiary of Pathway.
F-19
Table of Contents
The Company has consolidated Pathway under the purchase method of accounting and recorded noncontrolling interest under current accounting guidance for consolidations. The consolidated assets and liabilities, operating results and cash flows of Pathway are not considered significant to the Company’s financial position, operating results, or cash flows. Pathway’s assets cannot be used to settle the Company’s obligations and Pathway’s creditors have no recourse to the general credit of the Company.
The Company also holds an additional variable interest in an entity not considered material for disclosure.
6. FAIR VALUE MEASUREMENTS
Accounting standards on fair value measurement provide a framework for measuring fair value, expand disclosures about fair value measurements, and establish a fair value hierarchy which prioritizes the inputs used in measuring fair value summarized as follows:
Level 1: Inputs using unadjusted quoted prices for identical assets or liabilities in an active market that the Company has the ability to access.
Level 2: Inputs other than quoted prices in markets that are observable for the asset or liability, either directly or indirectly.
Level 3: Inputs that are both significant to the fair value measurement and unobservable.
As of March 30, 2012, the fair value of the Company’s financial assets and/or liabilities are measured using Level 1 or Level 3 inputs. The following table presents the Company’s assets and liabilities which are measured at fair value as of fiscal years ended March 30, 2012 and April 1, 2011, by level within the fair value hierarchy:
| (in thousands) | ||||||||||||
March 30, 2012 | Level 1 | Level 3 | Total | |||||||||
Assets: | ||||||||||||
Conversion option on VIE convertible note(a) | $ | — | $ | 701 | $ | 701 | ||||||
Liabilities: | ||||||||||||
Deferred compensation(b) | $ | 94,394 | $ | — | $ | 94,394 | ||||||
Contingent consideration(c) | — | 493 | 493 | |||||||||
|
|
|
|
|
| |||||||
Total liabilities | $ | 94,394 | $ | 493 | $ | 94,887 | ||||||
|
|
|
|
|
| |||||||
April 1, 2011 | Level 1 | Level 3 | Total | |||||||||
Assets: | ||||||||||||
Conversion option on VIE convertible note(a) | $ | — | $ | 845 | $ | 845 | ||||||
Liabilities: | ||||||||||||
Deferred compensation(b) | $ | 84,165 | $ | — | $ | 84,165 | ||||||
Contingent consideration(c) | — | 10,155 | 10,155 | |||||||||
|
|
|
|
|
| |||||||
Total liabilities | $ | 84,165 | $ | 10,155 | $ | 94,320 | ||||||
|
|
|
|
|
| |||||||
| (a) | Represents the Company’s conversion option to acquire 73% of the outstanding common stock in the Company’s consolidated variable interest entity (“VIE”), which is located inOther assets on the Company’sConsolidated Balance Sheets.See Footnote 5,Variable Interest Entity, for further information. The conversion option was calculated using an internal model that utilizes as its basis, unobservable inputs, including estimated interest rates based upon the estimated |
F-20
Table of Contents
| market interest rate which the VIE would have paid on a high-yield note in the open market. Significant increases (decreases) in any of those inputs would result in a significantly lower (higher) fair value measurement. The unobservable inputs are not considered to be interrelated. The remaining investment in Pathway has been eliminated in consolidation. |
| (b) | Represents the Company’s obligation to pay benefits under its non-qualified deferred compensation plans, which is included inOther noncurrent liabilities on the Company’sConsolidated Balance Sheets. The obligation to pay benefits is based on participants’ allocation percentages to plan investments. The investments are measured using quoted market prices. |
| (c) | Represents the estimated fair value of the additional variable cash consideration payable in connection with the Company’s acquisitions that are contingent upon the achievement of certain performance milestones. The Company estimated the fair value using expected future cash flows over the period in which the obligations are expected to be settled, and applied a discount rate that appropriately captures a market participant’s view of the risk associated with the obligation. Significant increases (decreases) to values of the unobservable inputs would result in a significantly lower (higher) fair value measurement. The unobservable inputs are not considered to be interrelated. The liabilities are included inOther current liabilitiesandOther noncurrent liabilities on the Company’sConsolidated Balance Sheets, depending on the period of expected payout. |
The following table summarizes the change in the fair value for Level 3 instruments for the fiscal year 2012.
| Level 3 Instruments | ||||
Assets: | ||||
Balance, April 1, 2011 | $ | 845 | ||
Fair value adjustment included in earnings | (144 | ) | ||
|
| |||
Balance, March 30, 2012 | $ | 701 | ||
|
| |||
Liabilities: | ||||
Balance, April 1, 2011 | $ | 10,155 | ||
Additions | 490 | |||
Settlement of obligation | (9,500 | ) | ||
Fair value adjustment included in earnings | (652 | ) | ||
|
| |||
Balance, March 30, 2012 | $ | 493 | ||
|
|
The Company has applied the requirement of ASC 820,Fair Value Measurement and Disclosure with respect to nonfinancial assets and liabilities not measured at fair value on a recurring basis with no material effect. The standard requires fair value disclosure of such nonfinancial assets only when there is an indication of potential impairment. See Footnote 2,Summary of Significant Accounting Policies,for disclosure of fair value of financial instruments.
The carrying amounts of the Company’s current financial instruments, including cash and cash equivalents, short-term trade receivables, and accounts payable, approximate their fair values due to the short-term nature of these assets and liabilities. The gross carrying value of the Company’s 2008 Notes as of March 30, 2012 and April 1, 2011 was $230,000 and the fair value, which is estimated using a third party valuation model, was approximately $302,174 and $323,800. The gross carrying value of the Company’s 2012 Notes as of March 30, 2012 was $250,000 and the fair value, which is estimated using a third party valuation model, was approximately $257,500.
7. EQUITY INVESTMENT
On June 29, 2007, the Company made a $24,064 investment (including $1,564 of legal and other professional fees) in athenahealth, Inc. (“athena”), a provider of internet-based healthcare information technology and business services to physician practices. During fiscal year 2010, the Company sold a portion of its investment in athena, resulting in a gain of approximately $3,635, or $2,260 net of tax. This gain was determined on a specific identification method and recognized inOther income on theConsolidated Statement of Operations. Proceeds of $10,681 were received during and related to a sale in fiscal year 2010. As of March 30, 2012, the Company did not hold any remaining investment in athena.
F-21
Table of Contents
8. PROPERTY AND EQUIPMENT
Property and equipment are summarized as follows:
| As of | ||||||||
| 2012 | 2011 | |||||||
Computer hardware and software | $ | 212,205 | $ | 191,459 | ||||
Equipment | 33,487 | 31,492 | ||||||
Leasehold improvements | 19,799 | 18,717 | ||||||
Computer hardware under capital leases | 2,516 | 2,516 | ||||||
Buildings | 1,114 | — | ||||||
Land | 236 | — | ||||||
Office equipment under capital leases | — | 452 | ||||||
|
|
|
| |||||
Property and equipment, gross | 269,357 | 244,636 | ||||||
Accumulated depreciation | (168,321 | ) | (142,235 | ) | ||||
|
|
|
| |||||
Property and equipment, net | $ | 101,036 | $ | 102,401 | ||||
|
|
|
| |||||
Depreciation expense, which includes amortization of capital leases, is included inGeneral and administrative expenses in the accompanyingConsolidated Statements of Operations, and approximated $26,847, $25,065, and $21,940, for fiscal years 2012, 2011, and 2010, respectively.
9. GOODWILL
The change in the carrying value of goodwill for the fiscal years ended March 30, 2012 and April 1, 2011 were as follows:
| Physician Business | Extended Care Business | Total | ||||||||||
Balance as of April 2, 2010 | $ | 31,693 | $ | 90,079 | $ | 121,772 | ||||||
Purchase business combinations | 40,500 | — | 40,500 | |||||||||
Purchase price allocation adjustments | 2,135 | — | 2,135 | |||||||||
Purchase of variable interest entity | — | 2,687 | 2,687 | |||||||||
|
|
|
|
|
| |||||||
Balance as of April 1, 2011 | $ | 74,328 | $ | 92,766 | $ | 167,094 | ||||||
Purchase business combinations | 22,162 | 16,160 | 38,322 | |||||||||
Purchase price allocation adjustments | (2,796 | ) | (868 | ) | (3,664 | ) | ||||||
|
|
|
|
|
| |||||||
Balance as of March 30, 2012 | $ | 93,694 | $ | 108,058 | $ | 201,752 | ||||||
|
|
|
|
|
| |||||||
F-22
Table of Contents
10. INTANGIBLES, NET
The following table summarizes the gross carrying amount and accumulated amortization for existing intangible assets by business segment and major asset class.
| As of | ||||||||||||||||||||||||
| March 30, 2012 | April 1, 2011 | |||||||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||||||||||||
INTANGIBLES SUBJECT TO AMORTIZATION | ||||||||||||||||||||||||
Customer Relationships: | ||||||||||||||||||||||||
Physician Business | $ | 33,430 | $ | (8,481 | ) | $ | 24,949 | $ | 24,390 | $ | (4,423 | ) | $ | 19,967 | ||||||||||
Extended Care Business | 14,209 | (2,672 | ) | 11,537 | 11,669 | (7,484 | ) | 4,185 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| 47,639 | (11,153 | ) | 36,486 | 36,059 | (11,907 | ) | 24,152 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Nonsolicitation Agreements: | ||||||||||||||||||||||||
Physician Business | 6,878 | (3,580 | ) | 3,298 | 8,475 | (4,444 | ) | 4,031 | ||||||||||||||||
Extended Care Business | 435 | (254 | ) | 181 | 424 | (188 | ) | 236 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| 7,313 | (3,834 | ) | 3,479 | 8,899 | (4,632 | ) | 4,267 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Noncompetition Agreements: | ||||||||||||||||||||||||
Physician Business | 4,162 | (942 | ) | 3,220 | 3,042 | (311 | ) | 2,731 | ||||||||||||||||
Extended Care Business | 5,369 | (1,581 | ) | 3,788 | 3,179 | (768 | ) | 2,411 | ||||||||||||||||
Shared Services | 1,224 | (1,050 | ) | 174 | 1,916 | (1,105 | ) | 811 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| 10,755 | (3,573 | ) | 7,182 | 8,137 | (2,184 | ) | 5,953 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Signing Bonuses: | ||||||||||||||||||||||||
Physician Business | 685 | (481 | ) | 204 | 580 | (317 | ) | 263 | ||||||||||||||||
Extended Care Business | 21 | (9 | ) | 12 | 26 | (12 | ) | 14 | ||||||||||||||||
Shared Services | 11 | (4 | ) | 7 | — | — | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| 717 | (494 | ) | 223 | 606 | (329 | ) | 277 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total intangible assets subject to amortization | $ | 66,424 | $ | (19,054 | ) | $ | 47,370 | $ | 53,701 | $ | (19,052 | ) | $ | 34,649 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
INTANGIBLES NOT SUBJECT TO AMORTIZATION | ||||||||||||||||||||||||
Tradename: | ||||||||||||||||||||||||
Physician Business | $ | 6,830 | $ | — | $ | 6,830 | $ | 6,830 | $ | — | $ | 6,830 | ||||||||||||
Extended Care Business | 400 | — | 400 | 400 | — | 400 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| 7,230 | — | 7,230 | 7,230 | — | 7,230 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total indefinite-lived intangible assets | $ | 7,230 | $ | — | $ | 7,230 | $ | 7,230 | $ | — | $ | 7,230 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total intangible assets | $ | 73,654 | $ | (19,054 | ) | $ | 54,600 | $ | 60,931 | $ | (19,052 | ) | $ | 41,879 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total amortization expense for intangible assets for the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, was $8,930, $6,378, and $5,121, respectively.
F-23
Table of Contents
The estimated amortization expense for the next five fiscal years is as follows:
Fiscal Year: | ||||
2013 | $ | 9,849 | ||
2014 | 9,197 | |||
2015 | 8,932 | |||
2016 | 7,742 | |||
2017 | 5,151 | |||
Thereafter | 6,499 | |||
|
| |||
Total | $ | 47,370 | ||
|
|
The remaining weighted-average amortization period, in total and by major asset class, is as follows:
| (in years) | March 30, 2012 | April 1, 2011 | ||||||
Nonsolicitation agreements | 8.5 | 9.5 | ||||||
Customer relationships | 5.9 | 5.5 | ||||||
Noncompetition agreements | 4.0 | 4.0 | ||||||
Signing bonuses | 1.1 | 1.4 | ||||||
|
|
|
| |||||
Total weighted-average amortization period | 5.0 | 4.8 | ||||||
|
|
|
| |||||
11. ACCRUED EXPENSES
Accrued expenses as of March 30, 2012 and April 1, 2011 were as follows:
| As of | ||||||||
| 2012 | 2011 | |||||||
Accrued payroll | $ | 16,788 | $ | 14,486 | ||||
Accrued interest | 2,598 | 1,245 | ||||||
Accrued incentive compensation | 1,204 | 8,085 | ||||||
Other(a) | 21,163 | 13,359 | ||||||
|
|
|
| |||||
Accrued expenses | $ | 41,753 | $ | 37,175 | ||||
|
|
|
| |||||
| (a) | Amounts within the “Other” category of total accrued expenses were not considered individually significant as of March 30, 2012 and April 1, 2011. |
12. DEBT
Outstanding debt consists of the following, in order of seniority:
| As of | ||||||||
| March 30, 2012 | April 1, 2011 | |||||||
Revolving line of credit | $ | — | $ | — | ||||
2012 Notes | 250,000 | — | ||||||
2008 Notes | 204,916 | 195,643 | ||||||
Capital lease obligations | — | 780 | ||||||
|
|
|
| |||||
Total debt | 454,916 | 196,423 | ||||||
Less: Current portion of debt | — | 761 | ||||||
|
|
|
| |||||
Long-term debt | $ | 454,916 | $ | 195,662 | ||||
|
|
|
| |||||
F-24
Table of Contents
2012 Notes
On February 24, 2012, the Company issued $250.0 million aggregate principal of 6.375% senior notes, which mature on March 1, 2022 (the “2012 Notes”). Interest on the notes is payable semi-annually in arrears on March 1 and September 1, beginning September 1, 2012. The 2012 Notes are fully and unconditionally guaranteed on a joint and several basis by certain of the Company’s domestic subsidiaries (the “Guarantor Subsidiaries”). Refer to Footnote 22,Condensed Consolidating Financial Information, for further information regarding the Guarantor Subsidiaries.
Prior to March 1, 2017, the Company may redeem some or all of the 2012 Notes at a redemption price equal to 100% of the aggregate principal amount of the notes to be redeemed, plus a make-whole premium, together with accrued and unpaid interest. The Company may redeem some or all of the notes at any time on or after March 1, 2017 at the redemption prices set forth in the Indenture, dated February 24, 2012 (the “Indenture”). In addition, the Company may redeem up to 35% of the aggregate principal amount of the notes prior to March 1, 2015 at 106.375% of their aggregate principal amount plus accrued interest with the net proceeds of certain qualified equity offerings.
If a change of control, as defined in the Indenture, occurs at any time, holders of the notes will have the right, at their option, to require the Company to repurchase all or a portion of such holder’s notes. The repurchase price for such a repurchase will be 101% of the aggregate principal amount of the notes to be repurchased plus accrued and unpaid interest to, but not including, the date of purchase.
The Indenture contains covenants that, among other things, limit the Company’s ability and the ability of the Company’s restricted subsidiaries to: borrow money or sell preferred stock; create liens; pay dividends on or redeem or repurchase stock; make certain types of investments; restrict dividends or other payments from subsidiaries; enter into transactions with affiliates; issue guarantees of debt; and sell assets or merge with other companies. Certain of these covenants will be suspended if the notes are assigned an investment grade rating by both Standard & Poor’s and Moody’s and no default has occurred and is continuing. If either rating on the notes should subsequently decline to below investment grade, the suspended covenants will be reinstated. These covenants are subject to important exceptions and qualifications as set forth in the Indenture.
The Company used a portion of the net proceeds of the offering to repay borrowings under the revolving line of credit in the amount of $127.3 million. Remaining proceeds will be used to partially fund the retirement of the 2008 Notes, as well as for general corporate purposes, including potential acquisitions and share repurchases.
The gross carrying value of the Company’s 2012 Notes as of March 30, 2012 was $250,000 and the fair value, which is estimated using a third party valuation model, was approximately $257,500.
2008 Notes
In August 2008, the Company issued $230.0 million principal amount of 3.125% senior convertible notes referred to as the 2008 Notes, which mature on August 1, 2014. Interest on the notes is payable semiannually in arrears on February 1 and August 1 of each year. The notes will be convertible into cash up to the principal amount of the notes and shares of the Company’s common stock for any conversion value in excess of the principal amount under the following circumstances: (i) if the Company has called the notes for redemption; (ii) in the event of a fundamental change, as defined in the indenture, such as a merger, acquisition, or liquidation; (iii) on or after May 1, 2014 and prior to the close of business on the second scheduled trading day immediately preceding August 1, 2014; (iv) prior to May 1, 2014, during the five consecutive business day period following any five consecutive trading day period in which the trading price for a note for each day of that trading period is less than 98% of the closing sale price of the Company’s common stock on such corresponding trading day multiplied by the applicable conversion rate; (v) prior to May 1, 2014, during any calendar quarter after September 30, 2008 in which the closing sale price of the Company’s common stock for at least 20 of the 30 consecutive trading days ending the day prior to such quarter is greater than 130% of the applicable conversion price of $21.22 per share (“Contingent Conversion Trigger”); or (vi) upon certain specified corporate events as discussed in the indenture governing the notes.
F-25
Table of Contents
A note holder may not exercise its conversion right with respect to all or any portion of a note, if such conversion would cause the note holder to become a beneficial owner of more than 9.9% of the Company’s outstanding voting stock. The initial conversion rate is 47.1342 shares of common stock per each $1 (in thousands) principal amount of notes and is equivalent to an initial conversion price of $21.22 per share. The conversion rate is subject to adjustment upon the occurrence of certain events. If the notes were converted as of March 30, 2012, the if-converted value would exceed the principal amounts of the 2008 Notes by $44,708.
As of March 30, 2012 and April 1, 2011, the fair value of the 2008 Notes was approximately $302,174 and $323,800, respectively.
The ability of note holders to convert is assessed on a quarterly basis and is dependent on the trading price of the Company’s stock during the last 30 trading days of each quarter. The Contingent Conversion Trigger was not met during the three months ended March 30, 2012; therefore, the notes may not be converted during the Company’s first quarter of fiscal year 2013.
The Company used a portion of the net proceeds of the offering to repurchase approximately $35.0 million of its common stock in privately negotiated transactions with institutional investors concurrently with this offering. The Company used $101.7 million of the net proceeds during fiscal year 2009, when holders of the senior convertible notes issued in 2004 required the Company to redeem approximately all of their outstanding notes plus accrued interest. Remaining proceeds were used for general corporate purposes.
The debt discount associated with the 2008 Notes will be amortized over periods that end on the scheduled maturity date and result in effective interest rates of approximately 8.25%. For the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010 interest expense was $7,168, $7,171, and $7,281, based on the contractual coupon rates, while debt discount amortization was $9,273, $8,543, and $7,948, respectively.
The principal balances, unamortized discounts and net carrying amounts of the liability components and the equity components for the Company’s 2008 Notes as of March 30, 2012 and April 1, 2011 are as follows:
| Liability Component | Equity Component | |||||||||||||||||
| (in thousands) | Principal | Unamortized | Net Carrying | Carrying Amount | ||||||||||||||
2008 Notes | Balance | Discount | Amount | Pretax(a) | ||||||||||||||
March 30, 2012 | $ | 230,000 | $ | (25,084 | ) | $ | 204,916 | $ | 55,636 | |||||||||
April 1, 2011 | $ | 230,000 | $ | (34,357 | ) | $ | 195,643 | $ | 55,636 | |||||||||
| (a) | The Company recognized a deferred tax liability of $20,523 related to the issuance of the 2008 Notes. |
Convertible Note Hedge Transactions
In connection with the offering of the notes, the Company also entered into convertible note hedge transactions with respect to its common stock (the “purchased options”) with a major financial institution (the “counterparty”). The Company paid an aggregate amount of $54.1 million to the counterparty for the purchased options. The purchased options cover, subject to anti-dilution adjustments substantially identical to those in the notes, approximately 10.8 million shares of common stock at a strike price that corresponds to the initial conversion price of the notes, also subject to adjustment, and are exercisable at each conversion date of the notes. The purchased options will expire upon the earlier of (i) the last day the notes remain outstanding or (ii) the second scheduled trading day immediately preceding the maturity date of the notes.
The purchased options are intended to reduce the potential dilution upon conversion of the notes in the event that the market value per share of the common stock, as measured under the notes, at the time of exercise is greater than the conversion price of the notes. The options have been accounted for as an adjustment to the Company’s equity, net of deferred tax assets of $21.0 million.
The purchased options are separate transactions, entered into by the Company with the counterparty, and are not part of the terms of the notes. Holders of the notes will not have any rights with respect to the purchased options.
F-26
Table of Contents
Warrant Transactions
The Company also entered into warrant transactions (the “warrants”), whereby the Company sold to the counterparty warrants in an aggregate amount of $25.4 million to acquire, subject to anti-dilution adjustments, up to 10.8 million shares of common stock at a strike price of $28.29 per share of common stock, also subject to adjustment. The warrants will expire after the purchased options in approximately ratable portions on a series of expiration dates commencing on November 3, 2014.
If the market value per share of the common stock, as measured under the warrants, exceeds the strike price of the warrants, the warrants will have a dilutive effect on the Company’s earnings per share. The warrants have been accounted for as an adjustment to the Company’s equity and recorded inAdditional paid-in capital on theConsolidated Balance Sheets.
The warrants are separate transactions, entered into by the Company with the counterparties, and are not part of the terms of the notes. Holders of the notes do not have any rights with respect to the warrants.
Revolving Line of Credit
The Company maintains an asset-based revolving line of credit (the “RLOC”) under a credit agreement (the “Credit Agreement”). As of April 1, 2011, the Credit Agreement permitted maximum borrowings of up to $200.0 million, with increased borrowing capacity to $250.0 million via an accordion feature. Availability of borrowings (“Availability”) was based on a borrowing base calculation consisting of accounts receivable and inventory, subject to satisfaction of certain eligibility requirements less any outstanding letters of credit. Borrowings under the RLOC bore interest at the bank’s prime rate plus an applicable margin based on a fixed charge coverage ratio, or at LIBOR plus an applicable margin based on a fixed charge coverage ratio. Additionally, the RLOC bore interest at a fixed rate of 0.25% for any unused portion of the facility.
On November 16, 2011, the Company amended and restated the Credit Agreement with the following features and key terms: (i) a five-year term, maturing on November 16, 2016; (ii) a facility size of $300.0 million, with increased borrowing capacity of $100.0 million via an accordion feature; and (iii) conditional covenants based on the Company’s borrowing availability and fixed charge coverage ratio requirements. Availability depends on a borrowing base calculation consisting of accounts receivable and inventory, subject to satisfaction of certain eligibility requirements, and certain other reserves. Borrowings under the RLOC bear interest at the bank’s base rate or at LIBOR plus applicable margins. Additionally, the RLOC incurs fees at a fixed rate of 0.25% for any unused portion of the facility.
Under the RLOC, the Company and certain of its subsidiaries are subject to certain covenants, including but not limited to, limitations on: (i) selling or transferring assets, (ii) making certain permitted investments, and (iii) incurring additional indebtedness and liens. However, these covenants may not apply if the Company maintains sufficient Availability under the credit facility and satisfies fixed charge coverage ratios.
Based on the amended terms of the Credit Agreement, and in accordance with ASC 470-10Debt – Overall, outstanding borrowings on the RLOC were classified withinRevolving line of credit and long-term debt, excluding current portion on theConsolidated Balance Sheets as of March 30, 2012. Prior to the amendment, the Credit Agreement contained both a subjective acceleration clause and a lock-box arrangement, and in accordance with ASC 470-10, borrowings were classified withinRevolving line of credit and current portion of long-term debton theConsolidated Balance Sheetsas of April 1, 2011.
Borrowings under the RLOC are anticipated to fund future requirements for working capital, capital expenditures, acquisitions, repurchases of the Company’s common stock, and the issuance of letters of credit, if necessary.
There were no outstanding borrowings under the RLOC as of March 30, 2012 and April 1, 2011. After reducing availability for outstanding borrowings and letter of credit commitments, the Company has sufficient assets based on eligible accounts receivable and inventory to borrow an additional $269.1 million (not including additional Availability via the accordion feature) under the RLOC. Average daily borrowings during fiscal years 2012 and 2011 were $44.4 million and $3.8 million, respectively. A hypothetical 1% increase/decrease in prevailing interest
F-27
Table of Contents
rates as of March 30, 2012 would result in a corresponding increase/decrease in interest expense of approximately than $0.4 million. The average daily interest rate, excluding debt issuance costs and unused line fees, for the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, was 2.27%, 2.37%, and 4.02%, respectively.
Interest Rate Swap Agreement
During fiscal year 2008, the Company entered into an interest rate swap agreement which was designated as a cash flow hedge and matured on February 19, 2010. The Company did not extend or enter into a new swap agreement, and as such, this agreement has expired. The purpose of the swap agreement was to hedge the variable interest rate of its RLOC, as such, the interest rate swap effectively fixed the interest rate on a portion of the revolving line of credit to 2.70%, plus an applicable margin as determined by the RLOC. The interest rate swap was disclosed in theConsolidated Statements of Equity and Comprehensive Income.
Capital Lease Obligations
During the fiscal year ended March 30, 2012, the Company leased certain computer hardware and office equipment at an aggregate annual rental of approximately $778. The equipment was capitalized at its fair market value, which approximated the present value of the future minimum lease payments, and was amortized over the useful life of the assets.
As of their term dates, the Company entered into new leasing agreements related to all equipment previously classified and accounted for as capital leases. Based on the terms of the new leasing agreements, these contractual obligations were reclassified as operating leases for accounting purposes. See Footnote 17,Operating Lease Commitments, for additional disclosures relating to the Company’s commitments under operating leases.
As of March 30, 2012, the Company had no outstanding capital lease obligations, while as of April 1, 2011, the Company had aggregate outstanding lease obligations of $754, net of imputed interest of $26.
13. INCOME TAXES
The provision for income taxes from continuing operations is detailed below:
| (in thousands) | 2012 | 2011 | 2010 | |||||||||
Current tax provision: | ||||||||||||
Federal | $ | 37,341 | $ | 36,384 | $ | 42,919 | ||||||
State | 5,006 | 4,926 | 6,112 | |||||||||
|
|
|
|
|
| |||||||
Total current provision | 42,347 | 41,310 | 49,031 | |||||||||
|
|
|
|
|
| |||||||
Deferred tax (benefit) provision: | ||||||||||||
Federal | (1,132 | ) | 2,863 | (7,234 | ) | |||||||
State | (152 | ) | 388 | (1,030 | ) | |||||||
|
|
|
|
|
| |||||||
Total deferred (benefit) provision | (1,284 | ) | 3,251 | (8,264 | ) | |||||||
|
|
|
|
|
| |||||||
Total income tax provision | $ | 41,063 | $ | 44,561 | $ | 40,767 | ||||||
|
|
|
|
|
| |||||||
F-28
Table of Contents
Total income tax expense for the years ended March 30, 2012, April 1, 2011, and April 2, 2010 was allocated as follows:
| (in thousands) | 2012 | 2011 | 2010 | |||||||||
Tax expense per Consolidated Statements of Operations | $ | 41,063 | $ | 44,561 | $ | 40,767 | ||||||
|
|
|
|
|
| |||||||
Other comprehensive income: | ||||||||||||
Unrealized holding gains on equity securities recognized for financial reporting purposes | — | — | (1,342 | ) | ||||||||
Unrealized losses on interest rate swap recognized for financial reporting purposes | — | — | 339 | |||||||||
|
|
|
|
|
| |||||||
Total income tax expense (benefit) allocated to other comprehensive income | — | — | (1,003 | ) | ||||||||
Benefit for compensation expense for tax purposes in excess of amounts recognized for financial reporting purposes | (2,057 | ) | (3,273 | ) | (2,516 | ) | ||||||
|
|
|
|
|
| |||||||
Total income tax expense | $ | 39,006 | $ | 41,288 | $ | 37,248 | ||||||
|
|
|
|
|
| |||||||
The difference between income tax computed at the federal statutory rate and the actual tax provision is shown below:
| (in thousands) | 2012 | 2011 | 2010 | |||||||||
Income before provision for income taxes | $ | 115,465 | $ | 119,225 | $ | 110,130 | ||||||
|
|
|
|
|
| |||||||
Tax provision at the 35% statutory rate | 40,413 | 41,729 | 38,546 | |||||||||
|
|
|
|
|
| |||||||
Increase (decrease) in taxes: | ||||||||||||
State income tax, net of federal benefit | 3,155 | 3,454 | 3,304 | |||||||||
Indefinitely invested earnings of foreign subsidiaries | (3,241 | ) | (1,289 | ) | (1,433 | ) | ||||||
Other, net | 736 | 667 | 350 | |||||||||
|
|
|
|
|
| |||||||
Total increase in taxes | 650 | 2,832 | 2,221 | |||||||||
|
|
|
|
|
| |||||||
Total income tax provision | $ | 41,063 | $ | 44,561 | $ | 40,767 | ||||||
|
|
|
|
|
| |||||||
Effective tax rate | 35.6 | % | 37.4 | % | 37.0 | % | ||||||
|
|
|
|
|
| |||||||
The effective rate for the fiscal year ended 2012 was impacted by a reorganization of the Company’s non-U.S. global sourcing subsidiaries. This reorganization increased the responsibilities and contributions of the non-U.S. subsidiaries, proportionally increasing their income and reducing the income of the U.S. subsidiaries. As the non-U.S. subsidiaries are generally subject to tax at rates lower than the U.S. subsidiaries, changes in the proportion of the Company’s taxable earnings originating outside the U.S. favorably impact the effective tax rate.
As of March 30, 2012 and April 1, 2011, the Company recorded an income tax receivable of $1,488 and an income tax payable of $1,233, respectively, related to current income tax filings.
F-29
Table of Contents
Deferred income taxes for fiscal years 2012 and 2011 reflect the impact of temporary differences between the financial statement and tax basis of assets and liabilities. The tax effect of temporary differences, which create deferred tax assets and liabilities, as of March 30, 2012 and April 1, 2011 are detailed below:
| (in thousands) | 2012 | 2011 | ||||||
Deferred tax assets: | ||||||||
Deferred compensation | $ | 35,224 | $ | 31,649 | ||||
Original issue discount on 2008 Notes | 9,653 | 13,197 | ||||||
Net operating loss and tax credit carryforwards | 10,591 | 4,722 | ||||||
Allowance for doubtful accounts and sales returns | 9,319 | 7,418 | ||||||
Accrued expenses and incentive compensation | 3,313 | 8,927 | ||||||
Inventory uniform cost capitalization | 4,171 | 3,725 | ||||||
Inventory obsolescence | 2,693 | 2,305 | ||||||
Other | 618 | 733 | ||||||
|
|
|
| |||||
Gross deferred tax assets | 75,582 | 72,676 | ||||||
Deferred tax liabilities: | ||||||||
Excess of tax depreciation over book depreciation | (23,762 | ) | (25,548 | ) | ||||
Interest on 2004 Notes | (17,303 | ) | (17,316 | ) | ||||
Discount on 2008 Notes related to ASC 470-20 | (9,362 | ) | (12,822 | ) | ||||
Excess of tax amortization over book amortization | (15,000 | ) | (11,043 | ) | ||||
Other | (998 | ) | (703 | ) | ||||
|
|
|
| |||||
Gross deferred tax liabilities | (66,425 | ) | (67,432 | ) | ||||
|
|
|
| |||||
Deferred tax assets, net | $ | 9,157 | $ | 5,244 | ||||
|
|
|
| |||||
The deferred tax accounts as of March 30, 2012 and April 1, 2011 include current deferred income tax assets of $16,962 and $20,533, respectively, included inCurrent assets and noncurrent deferred income tax liabilities of $7,805 and $15,289, respectively, included inOther noncurrent liabilities.
As of March 30, 2012 and April 1, 2011, the Company had federal and state net operating loss (“NOL”) carryforwards resulting in deferred tax assets of $10,269 and $4,593, respectively. The federal NOL carryforwards result in deferred tax assets as of March 30, 2012 and April 1, 2011 of $8,102 and $3,286, respectively, which expire in 2013 to 2032. The state NOL carryforwards result in deferred tax assets as of March 30, 2012 and April 1, 2011 of $2,167 and $1,307, respectively, which expire in 2013 to 2032. Management expects to utilize these NOL carryforwards prior to their expiration.
Management believes it is more likely than not that the deferred tax assets will be realized through the reversal of existing deferred tax liabilities and future taxable income and, therefore, no valuation allowance has been recorded as of March 30, 2012.
The Company has not provided for U.S. income taxes on accumulated and undistributed earnings attributable to foreign operations as the Company intends to permanently reinvest these undistributed earnings. These earnings relate to ongoing operations and were $20,225 and $10,547 as of March 30, 2012 and April 1, 2011, respectively.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
Unrecognized Tax Benefits as of April 2, 2010 | $ | 1,466 | ||
Gross Increases for tax positions of prior years | 295 | |||
Lapse of Statute of Limitations | (284 | ) | ||
|
| |||
Unrecognized Tax Benefits as of April 1, 2011 | 1,477 | |||
Gross Increases for tax positions of prior years | 342 | |||
Lapse of Statute of Limitations | (375 | ) | ||
|
| |||
Unrecognized Tax Benefits as of March 30, 2012 | $ | 1,444 | ||
|
|
F-30
Table of Contents
The Company classifies interest and penalties related to income tax matters as a component of income tax expense. As of March 30, 2012 and April 1, 2011 the Company had $192 and $179 of accrued interest related to uncertain tax positions, respectively.
The total amount of unrecognized tax benefits that would affect the effective income tax rate if recognized is $1,141 as of March 30, 2012. The unrecognized tax benefit with respect to certain of the Company’s tax positions may increase or decrease over the next twelve months; however, management does not expect the change, if any, to have a material effect on the Company’s financial position or results of operations within the next twelve months.
The Company files a United States federal income tax return and income tax returns in various states and foreign jurisdictions. With limited exceptions, the Company is no longer subject to income tax examinations for years prior to the fiscal year ended March 31, 2008.
During fiscal year 2012, the IRS completed an examination of the Company’s federal income tax return for the fiscal year ended March 27, 2009. As a result, no changes were made to the Company’s taxable income.
14. EQUITY
Stock Repurchase Programs
The Company repurchases its common stock under stock repurchase programs authorized by the Company’s Board of Directors. As of March 30, 2012, there were 0.4 million shares available for repurchase under existing stock repurchase programs.
From time to time, the Company’s Board of Directors authorizes the purchase of its outstanding common shares. Depending on current market conditions and other factors, the Company is authorized to repurchase a determined amount of its total common stock. Repurchases can be made in the open market, privately negotiated transactions, and other transactions that will be disclosed publicly through filings with the SEC. This authorization is in addition to any shares remaining available under existing repurchase programs.
The following table summarizes the common stock repurchases and Board of Directors authorizations during fiscal years 2012, 2011, 2010, as well as the shares available for repurchase under the stock repurchase program as of March 30, 2012, April 1, 2011, April 2, 2010, and March 27, 2009:
| (in thousands) | Number of Shares | |||
Shares available for repurchase at March 27, 2009 | 118 | |||
Shares authorized for repurchase | 5,966 | |||
Shares repurchased | (2,767 | ) | ||
|
| |||
Shares available for repurchase at April 2, 2010 | 3,317 | |||
Shares authorized for repurchase | 2,763 | |||
Shares repurchased | (2,728 | ) | ||
|
| |||
Shares available for repurchase at April 1, 2011 | 3,352 | |||
Shares authorized for repurchase | 2,680 | |||
Shares repurchased | (5,595 | ) | ||
|
| |||
Shares available for repurchase at March 30, 2012 | 437 | |||
|
| |||
During fiscal year 2012, the Company repurchased approximately 5.6 million shares of common stock at an average price of $25.10 per common share for approximately $140,439. During fiscal year 2011, the Company repurchased approximately 2.7 million shares of common stock at an average price of $20.07 per common share for approximately $54,761.
F-31
Table of Contents
During fiscal year 2012, the Company’s additional paid-in capital balance was reduced to zero as a result of share repurchases. In accordance with ASC 505,Equity, retirements of the Company’s shares may be recorded to additional paid-in capital to the extent that previous net gains from sales or retirements of the same class of stock remain, and otherwise should be recorded to retained earnings. As a result, retained earnings was reduced by $7,154 during fiscal year 2012, which represented share repurchases occurring after the additional paid-in capital balance had been reduced to zero.
15. INCENTIVE AND STOCK-BASED COMPENSATION
Equity Incentive Plans
The Company has equity incentive plans for the benefit of certain officers, directors, and employees. The Compensation Committee of the Board of Directors has the discretion to make grants under these plans in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, performance units, dividend equivalents, other stock-based awards, or other rights or interests relating to common stock or cash.
On June 7, 2006, the Board of Directors approved the PSS World Medical, Inc. 2006 Incentive Plan (the “2006 Plan”), a stock incentive plan under which equity may be granted to the Company’s officers, directors, and employees. The 2006 Plan became effective as of August 24, 2006, the date on which shareholders approved the plan. Grants under the 2006 Plan may be made in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted or deferred stock units, performance awards, dividend equivalents, performance-based cash awards, and other stock-based awards. Subject to adjustment as provided in the plan, the aggregate number of shares of common stock reserved and available for issuance pursuant to awards granted under the 2006 Plan is approximately 1,906 as of March 30, 2012.
In addition to the 2006 Plan, the Company maintains the 2004 Non-Employee Directors Compensation Plan (the “2004 Directors Plan”), which permits the grant of restricted stock to the Company’s non-employee directors. Subject to adjustment as provided in the plan, the aggregate number of shares of common stock reserved and available for issuance pursuant to awards granted under the 2004 Directors Plan is approximately 230 as of March 30, 2012. It is the Company’s policy to issue shares of common stock upon exercise of stock options or the grant of restricted stock from those shares reserved for issuance under the stock incentive plans.
Outstanding stock-based awards granted under equity incentive plans are as follows:
| (in thousands) | March 30, 2012 | April 1, 2011 | April 2, 2010 | |||||||||
Stock options(a) | 49 | 220 | 558 | |||||||||
Restricted stock(b) | 773 | 830 | 969 | |||||||||
Restricted stock units(a) | 575 | 670 | 593 | |||||||||
Deferred stock units(a) | 8 | 15 | 11 | |||||||||
|
|
|
|
|
| |||||||
Total outstanding stock based awards | 1,405 | 1,735 | 2,131 | |||||||||
|
|
|
|
|
| |||||||
| (a) | Amounts are excluded from shares of common stock issued and outstanding. Amounts for Performance Share units are based on the Company’s current estimate of shares expected to vest. |
| (b) | Amounts are included in shares of common stock issued and outstanding on the face of the balance sheet and in calculating weighted average shares outstanding, but are not considered outstanding for accounting purposes until restrictions lapse. |
ASC 718 requires companies to recognize the cost of employee services received in exchange for awards of equity instruments in the financial statements based on the grant date fair value of those awards, net of estimated forfeitures over the awards’ vesting period. ASC 718 requires forfeitures to be estimated at the time of grant and adjusted, if necessary, in subsequent periods if actual forfeitures differ from those estimates. When estimating forfeitures, the Company considers voluntary termination behaviors as well as trends of actual equity based awards forfeited.
F-32
Table of Contents
Stock Option Awards
On June 6, 2008, the Compensation Committee of the Company’s Board of Directors approved a retention award of 200 stock options under the Company’s 2006 Incentive Stock Plan to the Company’s former Chairman and Chief Executive Officer. The stock options awarded were to cliff-vest on the five-year anniversary of the grant date.
During the fiscal year ended 2010, the shares were forfeited due to the departure of the Company’s former Chairman and Chief Executive Officer. As a result, $580 ($358, net of tax) was recognized as an adjustment to reduce stock-based compensation during fiscal year 2010.
The following table summarizes the number of common shares to be issued upon exercise of outstanding options and the number of common shares remaining available for future issuance under the existing stock incentive plans as of March 30, 2012:
| (in thousands) | Number of securities to be issued upon exercise of outstanding options | Number of securities remaining available for future issuance | ||||||
Equity compensation plans approved by shareholders: 1999 Long-term Incentive Plan(a) | 10 | — | ||||||
Amended and Restated Directors’ Stock Plan(a) | 28 | — | ||||||
PSS World Medical, Inc. 2006 Incentive Plan(b) | — | 1,906 | ||||||
2004 Non-Employee Directors Compensation Plan(c) | — | 230 | ||||||
|
|
|
| |||||
| 38 | 2,136 | |||||||
Equity compensation plan not approved by shareholders: | ||||||||
1999 Broad Based Employee Stock Plan(a) | 11 | — | ||||||
|
|
|
| |||||
Total | 49 | 2,136 | ||||||
|
|
|
| |||||
| (a) | These plans are terminated; however, options remain outstanding as of March 30, 2012 which are exercisable. |
| (b) | This plan superseded the 1999 Long-term Incentive Plan and the 1999 Broad Based Employee Stock Plan and was approved by shareholders on August 24, 2006. |
| (c) | This plan superseded the Amended and Restated Directors’ Stock Plan and was approved by shareholders during fiscal year 2005. |
The following table summarizes the stock option activity during the period from March 27, 2009 to March 30, 2012:
| (share amounts in thousands) | Shares | Weighted Average Exercise Price | Weighted Average Contractual Term | Aggregate Intrinsic Value | ||||||||||||
Outstanding at, March 27, 2009 | 1,306 | $ | 9.20 | 3.4 | $ | 7,909 | ||||||||||
Exercised | (548 | ) | 8.15 | |||||||||||||
Expired | (200 | ) | 17.98 | |||||||||||||
|
| |||||||||||||||
Outstanding at, April 2, 2010 | 558 | $ | 7.05 | 1.9 | $ | 9,252 | ||||||||||
Exercised | (338 | ) | 6.15 | |||||||||||||
|
| |||||||||||||||
Outstanding at, April 1, 2011 | 220 | $ | 8.44 | 1.7 | $ | 4,115 | ||||||||||
Exercised | (171 | ) | 8.09 | |||||||||||||
|
| |||||||||||||||
Outstanding and Exercisable at, March 30, 2012 | 49 | $ | 9.63 | 1.5 | $ | 789 | ||||||||||
|
| |||||||||||||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (the difference between the Company’s closing stock price of $25.34 on the last trading day of the Company’s fiscal year end and the exercise price, multiplied by the number of outstanding stock options) that would have been received by the option holders had all option holders exercised their options on March 30, 2012. This amount changes over time based on changes in the fair market value of the Company’s stock.
F-33
Table of Contents
The total intrinsic value of stock options exercised during fiscal years ended March 30, 2012 and April 1, 2011 was $2,926 and $5,684, respectively. Cash received from stock option exercises during the fiscal year ended March 30, 2012 and April 1, 2011 was approximately $1,383 and $2,079, respectively. The actual tax benefit realized for the tax deductions from stock option exercises totaled approximately $1,115 and $2,161 during the fiscal years ended March 30, 2012 and April 1, 2011, respectively.
Restricted Stock Awards
The Company issues (i) restricted stock which vests based on the recipient’s continued service over time (“Time-Based Awards”) and (ii) restricted stock or restricted stock units which vest based on the Company achieving specified performance measurements (“Performance-Based Awards”).
Time-Based Awards
The Company measures the fair value of Time-Based Awards on the date of grant based on the closing stock price. The related compensation expense is recognized on a straight-line basis over the vesting period, net of estimated forfeitures.
Performance-Based Awards
The Company issues (i) performance-based restricted stock units (“Performance Shares”), (ii) performance-accelerated restricted stock (“PARS”), which were issued in fiscal years 2011 and 2010, and (iii) performance-accelerated restricted stock units (“PARS Units”), which were issued in fiscal years 2012 and 2011, under the Company’s 2006 Incentive Plan.
The Performance Shares cliff-vest three years from the date of grant and convert to shares of common stock based on the Company’s achievement of certain cumulative earnings per share growth targets. These awards, which are denominated in terms of a target number of shares, will be forfeited if performance falls below a designated threshold level and may increase up to 250% of the target number of shares for exceptional performance. The ultimate number of shares delivered to recipients and the related compensation cost recognized as expense will be based on actual performance. The Company recognizes compensation expense on a straight-line basis (net of estimated forfeitures) over the awards vesting period based on the Company’s estimate of what will ultimately vest. This estimate may be adjusted in future periods based on actual experience and changes in management assumptions.
The PARS and PARS Units awards vest on the five-year anniversary of the grant date, subject to accelerated vesting after three years if the Company achieves an earnings per share growth target. The Company measures stock-based compensation at the grant date, based on the estimated fair value of the award, and recognizes the cost as compensation expense on a straight-line basis (net of estimated forfeitures) over the awards’ vesting period of five years based on the Company’s estimate of its cumulative earnings per share growth rate. This estimate may be adjusted in future periods based on actual experience and changes in management assumptions.
Change in Estimate
Fiscal Year 2012
During the fiscal year ended March 30, 2012, the Company changed its estimate of the number of shares to be delivered on its performance based awards. This change reflected a decrease in estimated achievement of performance conditions based on actual and expected future financial performance. The change in estimate decreased Performance Share Units outstanding by approximately 98,000 shares.
As a result of the change in performance estimate, stock based compensation expenses decreased $1,464 ($908, net of tax), or $0.02 per diluted share during the year ended March 30, 2012.
F-34
Table of Contents
Fiscal Year 2010
During the fiscal year ended April 2, 2010, the Company changed its estimate of the number of shares to be delivered on its performance based awards. This change reflected an increase in estimated achievement of performance conditions based on actual and expected future financial performance. The change in estimate increased Performance Share Units outstanding by approximately 303,000 shares. Additionally, the expected vest date of PARS awards issued during fiscal year 2010 was accelerated to vest on the three-year anniversary of the grant date. As such, the Company adjusted the forfeiture rate related to certain PARS awards to reflect a reduction in expected forfeitures over the remaining vesting period.
As a result of the change in performance estimate, stock based compensation expenses increased $9,133 ($5,640, net of tax), or $0.10 per diluted share during the year ended April 2, 2010.
These estimates may be adjusted in future periods based on actual experience and changes in management assumptions.
Change in Management
During the fiscal year ended April 2, 2010, restricted stock awards were forfeited due to the departure of the Company’s former Chairman and Chief Executive Officer. As a result, $3,837 ($2,370, net of tax) was recognized as an adjustment to reduce stock-based compensation related to restricted stock during the year ended April 2, 2010.
Restricted Stock Activity
The following table summarizes the activity of restricted stock and restricted stock units during the period from March 27, 2009 to March 30, 2012:
| Performance-Based Awards | Time-Based Awards | |||||||||||||||||||||||||||||||
| Performance Shares | PARS | |||||||||||||||||||||||||||||||
| (share amounts in thousands) | Units | Weighted Average Grant Date Fair Value | Units | Weighted Average Grant Date Fair Value | Shares | Weighted Average Grant Date Fair Value | Shares | Weighted Average Grant Date Fair Value | ||||||||||||||||||||||||
Balance, March 27, 2009 | 221 | $ | 18.49 | — | $ | — | 767 | $ | 18.38 | 248 | $ | 19.23 | ||||||||||||||||||||
Granted | 241 | 17.00 | 132 | 17.00 | 7 | 21.84 | 171 | 20.06 | ||||||||||||||||||||||||
Addition from change in estimate | 303 | 18.39 | — | — | — | — | — | — | ||||||||||||||||||||||||
Vested | — | — | — | — | — | — | (90 | ) | 19.42 | |||||||||||||||||||||||
Forfeited | (250 | ) | 17.97 | (44 | ) | 17.00 | (122 | ) | 18.50 | (12 | ) | 19.50 | ||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance, April 2, 2010 | 515 | $ | 17.98 | 88 | $ | 17.00 | 652 | $ | 18.40 | 317 | $ | 19.68 | ||||||||||||||||||||
Granted | 94 | 22.23 | 103 | 22.23 | 460 | 21.85 | 87 | 20.00 | ||||||||||||||||||||||||
Addition from change in estimate | 34 | 23.41 | — | — | — | — | — | — | ||||||||||||||||||||||||
Vested | (164 | ) | 18.52 | — | — | (547 | ) | 18.40 | (91 | ) | 19.55 | |||||||||||||||||||||
Forfeited | — | — | — | — | (33 | ) | 19.11 | (15 | ) | 18.42 | ||||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance, April 1, 2011 | 479 | $ | 19.01 | 191 | $ | 19.82 | 532 | $ | 21.34 | 298 | $ | 19.88 | ||||||||||||||||||||
Granted | 87 | 27.29 | 87 | 27.29 | 47 | 22.55 | 72 | 23.61 | ||||||||||||||||||||||||
Reduction from change in estimate | (98 | ) | 19.34 | — | — | — | — | — | — | |||||||||||||||||||||||
Vested | (162 | ) | 18.47 | — | — | (81 | ) | 18.47 | (58 | ) | 19.37 | |||||||||||||||||||||
Forfeited | (5 | ) | 25.19 | (4 | ) | 25.19 | (27 | ) | 21.85 | (10 | ) | 22.10 | ||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Balance, March 30, 2012 | 301 | $ | 21.48 | 274 | $ | 22.11 | 471 | $ | 21.93 | 302 | $ | 20.79 | ||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Total compensation expense for restricted stock grants during the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, was $6,430, $9,198, and $12,164, respectively, with related income tax benefits of $2,443, $3,495, and $4,618, respectively. The total fair value of shares vested during the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, was $8,102, $17,581, and $1,897, respectively.
F-35
Table of Contents
Scheduled vesting for outstanding restricted stock and restricted stock units is as follows:
| (in thousands) | Number of Shares/Units | |||
Fiscal Year: | ||||
2013 | 425 | |||
2014 | 147 | |||
2015 | 145 | |||
2016 | 594 | |||
2017 and thereafter | 96 | |||
|
| |||
Total | 1,407 | |||
|
| |||
As of March 30, 2012, there was $16,535 of unrecognized compensation cost related to non-vested restricted stock and restricted stock units granted under the stock incentive plans. The estimated stock-based compensation expense for the next five fiscal years is expected to be recognized over a weighted average period of 1.7 years as follows:
Fiscal Year: | ||||
2013 | $ | 6,105 | ||
2014 | 4,648 | |||
2015 | 3,607 | |||
2016 | 2,128 | |||
2017 and thereafter | 47 | |||
|
| |||
Total | $ | 16,535 | ||
|
|
Corporate Long-Term Executive Cash-Based Incentive Plans
During fiscal year 2012, the Compensation Committee approved the 2011 Shareholder Value Plan (“2011 SVP”), a cash based performance award program for certain officers and management under the 2006 Incentive Plan. The performance period under the 2011 SVP is the 36-month period from April 1, 2011 to March 28, 2014. Target awards under the 2011 SVP were calculated as three times the participant’s base salary times an award factor ranging from 15% to 40% and performance goals were based on planned cumulative earnings per share. Due to a reduction in payout estimates based on performance, the Company has no accrued compensation cost related to the 2011 SVP recorded as of March 30, 2012.
During fiscal year 2009, the Compensation Committee approved the 2008 Shareholder Value Plan (“2008 SVP”) for non-executive officers and leaders. The performance period under the 2008 SVP was the 36-month period from March 31, 2008 to April 1, 2011. Target awards under the 2008 SVP were calculated as three times the participant’s base salary times an award factor ranging from 10% to 38% and performance goals were based on planned cumulative earnings per share. As a result of an increase in accounting estimate related to expected achievement of long-term performance measures related to the 2008 SVP, long-term incentive based compensation increased $2,276 ($1,405, net of tax), or $0.02 per diluted share during fiscal year 2010. The Company accrued approximately $10,697 of compensation cost related to the 2008 SVP, recorded inOther current liabilitiesin the accompanyingConsolidated Balance Sheets as of April 1, 2011, which was paid in June 2011.
16. EMPLOYEE BENEFIT PLANS
PSS World Medical, Inc. Savings Plan
The PSS World Medical, Inc. Savings Plan (the “Plan”) provides an opportunity for tax-deferred savings, enabling eligible employees to invest in a variety of investments, including an interest in the common stock of the Company. Employees become eligible to participate in the Plan upon the completion of 30 days of service. Employees may elect to defer up to 85% but not less than 1% of their compensation to the Plan, subject to certain limitations imposed by the Internal Revenue Code. The Company matches an amount equal to the lesser of (i) 50% of the
F-36
Table of Contents
employee deferrals up to 6% of their compensation or (ii) $1,750, previously $1,250 in fiscal years ended April 1, 2011 and April 2, 2010. This match can be invested in various mutual funds or the common stock of the Company at the discretion of the participant and vests over a six-year period. During the fiscal years ended March 30, 2012, April 1, 2011, and April 2, 2010, the Company contributed approximately $2,574, $1,914, and $1,821 respectively, to the Plan under this matching arrangement. The Plan owned approximately 1.1 million, 1.2 million, and 1.5 million shares of the Company’s common stock as of March 30, 2012, April 1, 2011, and April 2, 2010, respectively.
Employee Stock Purchase Plan
The Company also has an employee stock purchase plan available to all employees with at least six months of service. The plan allows eligible employees to purchase Company stock acquired in the open market through after-tax payroll deductions.
Deferred Compensation Program
The Company offers a deferred compensation program (the “Program”) to qualified executives, management, and sales representatives. The Program is a nonqualified plan governed by Sec. 409 of the IRS internal revenue code and consists of a deferred compensation plan and also previously consisted of a stock option program. Under the deferred compensation plan, participants can elect to defer up to 100% of their total compensation; however, the Company matching contribution program only applies to deferrals of up to 10% or 15% of the participant’s compensation. The Company’s matching contribution ranges from 10% to 125% of the participant’s deferral. Participant contributions are always 100% vested. The Company’s matching contribution vests in 20% increments beginning after participating in the plan for four years and becomes fully vested after participating in the plan for eight years.
Upon (i) retirement or termination from the Company and (ii) at age 60, or at age 55 with ten years of participation in the Program, the retirement benefit is distributed to participants in five equal annual installments, or in a lump sum payment if the vested account balance is less than $25. The retirement benefit is distributed in a lump sum upon death and over five years upon disability. In the event of termination of employment, 100% of the participant’s vested balance will be distributed in five equal installments or in a lump-sum payment if the vested account balance is less than $25. In the event of a change in control, if the successor terminates the plan, all participants become 100% vested in their accounts, including the Company’s matching contributions, discretionary Company contributions, and allocated return thereon. The Company has purchased corporate-owned life insurance policies for certain participants in the Program as the underlying assets will fund future payments related to the deferred compensation liability.
During fiscal years 2012, 2011, and 2010, the Company matched approximately $2,267, $2,230, and $1,919, respectively, of employee deferrals. The cash surrender value of the corporate-owned, life insurance policies, which is recorded inOther assets in the accompanyingConsolidated Balance Sheets, was approximately $99,162 and $89,132, as of March 30, 2012 and April 1, 2011, respectively. In addition, the deferred compensation liability, which is recorded at fair value inOther noncurrent liabilities in the accompanyingConsolidated Balance Sheets, was approximately $94,394 and $84,165, as of March 30, 2012 and April 1, 2011, respectively.
Directors’ Deferred Compensation Plan
Effective January 1, 2004, the Company offers a deferred compensation plan to non-employee members of the Board of Directors. Participants may elect to defer up to 100% of their annual retainer, meeting fees, other director’s fees, and other cash compensation and invest their deferrals in a variety of investment options. A participant’s deferred compensation account balance will be distributed, at the election of the participant, in a single lump sum payment following the participant’s termination of service on the board of directors, or in up to ten annual installments. The deferred compensation account balance will be distributed in a lump sum payment upon the death of the participant, or in the event of a change in control of the Company.
F-37
Table of Contents
17. OPERATING LEASE COMMITMENTS
The Company leases various facilities and equipment under operating leases. Certain lease commitments provide that the Company pays taxes, insurance, and maintenance expenses related to the leased assets. Many of the Company’s leases contain predetermined fixed escalations of the minimum rentals during the initial term. For these leases, the Company has recognized the related rental expense on a straight-line basis and has recorded the difference between the expense charged to income and amounts payable under the leases as other noncurrent liabilities in the accompanying balance sheets.
Rent expense for operating leases approximated $30,429, $30,149, and $29,803, for fiscal years 2012, 2011, and 2010, respectively. As of March 30, 2012, future minimum payments by fiscal year and in the aggregate, required under non-cancelable operating leases are as follows:
Fiscal Year: | ||||
2013 | $ | 24,935 | ||
2014 | 19,232 | |||
2015 | 11,542 | |||
2016 | 5,912 | |||
2017 | 3,530 | |||
Thereafter | 6,813 | |||
|
| |||
Total | $ | 71,964 | ||
|
|
18. SEGMENT INFORMATION
The Company’s reportable segments are strategic businesses that offer different products to different segments of the healthcare industry, and are the basis on which management regularly evaluates the Company. These segments are managed separately because of different customers and products. Refer to Footnote 1, Nature of Operations, for descriptive information about the Company’s operating segments. The Company evaluates the operating performance of its segments based on net sales and income from operations. Shared Services allocates a portion of its costs and interest expense to the operating segments. The allocation of shared operating costs is generally proportionate to the revenues of each operating segment. Interest expense is allocated based on an internal carrying value of historical capital used to acquire or develop the operating segments’ operations. The following tables present financial information about the Company’s business segments:
| 2012 | 2011 | 2010 | ||||||||||
NET SALES: | ||||||||||||
Physician Business | $ | 1,512,719 | $ | 1,425,012 | $ | 1,437,823 | ||||||
Extended Care Business | 587,388 | 607,750 | 614,937 | |||||||||
Shared Services | 1,895 | 2,027 | 2,411 | |||||||||
|
|
|
|
|
| |||||||
Total net sales | $ | 2,102,002 | $ | 2,034,789 | $ | 2,055,171 | ||||||
|
|
|
|
|
| |||||||
NET SALES BY PRODUCT TYPE: | ||||||||||||
Consumable products | $ | 1,497,146 | $ | 1,504,255 | $ | 1,563,462 | ||||||
Pharmaceutical products | 350,704 | 330,572 | 333,026 | |||||||||
Equipment | 146,030 | 138,414 | 134,067 | |||||||||
Physician dispensing solutions | 84,328 | 36,899 | — | |||||||||
Billing services | 10,549 | 11,956 | 13,219 | |||||||||
Customer freight charges | 15,544 | 13,382 | 10,926 | |||||||||
Vendor incentive and other income | (2,299 | ) | (689 | ) | 471 | |||||||
|
|
|
|
|
| |||||||
Total net sales | $ | 2,102,002 | $ | 2,034,789 | $ | 2,055,171 | ||||||
|
|
|
|
|
| |||||||
F-38
Table of Contents
| 2012 | 2011 | 2010 | ||||||||||
INCOME FROM OPERATIONS: | ||||||||||||
Physician Business | $ | 144,767 | $ | 137,995 | $ | 137,261 | ||||||
Extended Care Business | 27,620 | 37,782 | 38,017 | |||||||||
Shared Services | (39,031 | ) | (42,221 | ) | (54,297 | ) | ||||||
|
|
|
|
|
| |||||||
Total income from operations | $ | 133,356 | $ | 133,556 | $ | 120,981 | ||||||
|
|
|
|
|
| |||||||
DEPRECIATION: | ||||||||||||
Physician Business | $ | 11,100 | $ | 9,515 | $ | 7,983 | ||||||
Extended Care Business | 5,341 | 5,024 | 4,984 | |||||||||
Shared Services | 10,406 | 10,526 | 8,973 | |||||||||
|
|
|
|
|
| |||||||
Total depreciation | $ | 26,847 | $ | 25,065 | $ | 21,940 | ||||||
|
|
|
|
|
| |||||||
AMORTIZATION OF INTANGIBLE ASSETS: | ||||||||||||
Physician Business | $ | 5,798 | $ | 2,956 | $ | 2,343 | ||||||
Extended Care Business | 2,491 | 2,757 | 2,432 | |||||||||
Shared Services | 641 | 665 | 346 | |||||||||
|
|
|
|
|
| |||||||
Total amortization of intangible assets | $ | 8,930 | $ | 6,378 | $ | 5,121 | ||||||
|
|
|
|
|
| |||||||
PROVISIONS FOR DOUBTFUL ACCOUNTS AND NOTES RECEIVABLE: | ||||||||||||
Physician Business | $ | 2,288 | $ | 1,792 | $ | 1,944 | ||||||
Extended Care Business | 570 | (51 | ) | 1,851 | ||||||||
|
|
|
|
|
| |||||||
Total provision for doubtful accounts and notes receivable | $ | 2,858 | $ | 1,741 | $ | 3,795 | ||||||
|
|
|
|
|
| |||||||
INTEREST EXPENSE: | ||||||||||||
Physician Business | $ | 4,706 | $ | 4,631 | $ | 4,110 | ||||||
Extended Care Business | 8,391 | 8,157 | 8,192 | |||||||||
Shared Services | 7,051 | 4,333 | 4,993 | |||||||||
|
|
|
|
|
| |||||||
Total interest expense | $ | 20,148 | $ | 17,121 | $ | 17,295 | ||||||
|
|
|
|
|
| |||||||
PROVISION FOR INCOME TAXES: | ||||||||||||
Physician Business | $ | 50,248 | $ | 49,951 | $ | 49,982 | ||||||
Extended Care Business | 6,938 | 11,212 | 11,103 | |||||||||
Shared Services | (16,123 | ) | (16,602 | ) | (20,318 | ) | ||||||
|
|
|
|
|
| |||||||
Total provision for income taxes | $ | 41,063 | $ | 44,561 | $ | 40,767 | ||||||
|
|
|
|
|
| |||||||
CAPITAL EXPENDITURES: | ||||||||||||
Physician Business | $ | 2,864 | $ | 1,554 | $ | 1,282 | ||||||
Extended Care Business | 911 | 1,659 | 509 | |||||||||
Shared Services | 20,143 | 15,014 | 24,132 | |||||||||
|
|
|
|
|
| |||||||
Total capital expenditures | $ | 23,918 | $ | 18,227 | $ | 25,923 | ||||||
|
|
|
|
|
| |||||||
ASSETS: | ||||||||||||
Physician Business | $ | 606,725 | $ | 570,278 | $ | 440,916 | ||||||
Extended Care Business | 323,710 | 298,016 | 298,063 | |||||||||
Shared Services | 225,535 | 83,378 | 133,087 | |||||||||
|
|
|
|
|
| |||||||
Total assets | $ | 1,155,970 | $ | 951,672 | $ | 872,066 | ||||||
|
|
|
|
|
| |||||||
F-39
Table of Contents
19. COMMITMENTS AND CONTINGENCIES
Other Litigation Matters
The Company is party to various legal and administrative proceedings and claims arising in the normal course of business. While any litigation contains an element of uncertainty, the Company, after consultation with legal counsel, believes that the outcome of such other proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
The Company has various insurance policies, including product liability insurance, covering risks and in amounts it considers adequate. With respect to products manufactured by others and distributed by the Company, the manufacturer may provide indemnification. There can be no assurance that the insurance coverage maintained by the Company is sufficient or will be available in adequate amounts or at a reasonable cost, or that indemnification agreements will provide adequate protection for the Company, including agreements with foreign vendors.
Purchase Commitments
Periodically, the Company enters into various purchase agreements with vendors to ensure the availability and pricing of products and services. These commitments represent future obligations to purchase goods and services that are enforceable and legally binding on the Company and that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Included in these agreements are contracts that specify a minimum payment regardless of whether the Company takes delivery of the contracted products or services (take-or-pay) as well as commitments that involve a penalty in order to cancel the purchase commitment.
Commitments and Other Contingencies
The Company has employment agreements with certain executive officers which provide that in the event of their termination or resignation, under certain conditions, the Company may be required to pay severance to the executive officers in amounts ranging from one-fourth to two times their base salary and target annual bonus. In the event that a termination or resignation follows or is in connection with a change in control, the Company may be required to pay severance to the executive officers in amounts ranging from three-fourths to three times their base salary and target annual bonus. The Company may also be required to continue welfare benefit plan coverage for the executive officers following a termination or resignation for a period ranging from one month to two years.
If a supply agreement for store brand products between a vendor and the Company were to be terminated, then the Company may be required to purchase from the vendor all remaining finished and unfinished products and product-materials ordered or held by the vendor. As of March 30, 2012, the Company had no material obligation to purchase remaining products or materials due to a termination of a supply agreement with a vendor who supplies store brand products to the Company.
20. SUPPLEMENTAL CASH FLOW INFORMATION
The Company’s supplemental disclosures for the years ended March 30, 2012, April 1, 2011, and April 2, 2010 are as follows:
| Cash paid for: | 2012 | 2011 | 2010 | |||||||||||
Interest | $ | 8,644 | $ | 7,837 | $ | 9,620 | ||||||||
Income taxes, net | $ | 42,156 | $ | 36,382 | $ | 42,605 | ||||||||
During the fiscal years ended March 30, 2012 and April 1, 2011, the Company acquired cash from business combinations of $26 and $1,163, respectively. In the fiscal year ended March 30, 2012, the Company assumed outstanding checks from business combinations of $415. The Company did not assume outstanding checks from business combinations in the fiscal year ended April 1, 2011. During the fiscal year ended March 30, 2012, the Company had no material non-cash transactions. During the fiscal year ended April 1, 2011, the Company had approximately $171 in non-cash investing activities associated with acquisition-related adjustments.
F-40
Table of Contents
21. CONDENSED CONSOLIDATING FINANCIAL INFORMATION
The 2012 Notes of the Company (the “Parent”) are fully and unconditionally guaranteed on a joint and several senior unsecured basis by certain of its domestic subsidiaries (the “Guarantor Subsidiaries”). The guarantees made by the Guarantor Subsidiaries will rank senior in right of payment to all of their existing and future obligations expressly subordinated or junior in right of payment to the notes, equal with all of their existing and future unsecured unsubordinated obligations, and will be effectively subordinated to any of their existing and future secured obligations to the extent of the value of the assets securing such obligations.
The following tables present the condensed consolidating financial information of the Parent, the Guarantor Subsidiaries, and the subsidiaries that are not guarantors (the “Non-Guarantor Subsidiaries”) as of March 30, 2012 and April 1, 2011 and for the years ended March 30, 2012, April 1, 2011, and April 2, 2010.
F-41
Table of Contents
CONDENSED CONSOLIDATING BALANCE SHEET
MARCH 30, 2012
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries (a) | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
ASSETS | ||||||||||||||||||||
Current Assets: | ||||||||||||||||||||
Cash and cash equivalents | $ | 117,448 | $ | 13,530 | $ | 32,174 | $ | — | $ | 163,152 | ||||||||||
Accounts receivable, net | 139,502 | 116,574 | 1,624 | — | 257,700 | |||||||||||||||
Inventories | 117,684 | 95,611 | 291 | — | 213,586 | |||||||||||||||
Deferred tax assets, net | 12,552 | 4,410 | — | — | 16,962 | |||||||||||||||
Intercompany receivable | 257,349 | — | — | (257,349 | ) | — | ||||||||||||||
Prepaid expenses and other current assets | 22,879 | 11,295 | 118 | — | 34,292 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current assets | 667,414 | 241,420 | 34,207 | (257,349 | ) | 685,692 | ||||||||||||||
Property and equipment, net | 40,074 | 60,676 | 286 | — | 101,036 | |||||||||||||||
Other Assets: | ||||||||||||||||||||
Goodwill | 43,270 | 158,482 | — | — | 201,752 | |||||||||||||||
Intangibles, net | 18,518 | 36,082 | — | — | 54,600 | |||||||||||||||
Investment in subsidiaries | 390,811 | 24,084 | — | (414,895 | ) | — | ||||||||||||||
Other assets | 95,950 | 18,867 | 717 | (2,644 | ) | 112,890 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total assets | $ | 1,256,037 | $ | 539,611 | $ | 35,210 | $ | (674,888 | ) | $ | 1,155,970 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
LIABILITIES AND EQUITY | ||||||||||||||||||||
Current Liabilities: | ||||||||||||||||||||
Accounts payable | $ | 125,363 | $ | 20,462 | $ | 708 | $ | — | $ | 146,533 | ||||||||||
Accrued expenses | 20,833 | 20,449 | 471 | — | 41,753 | |||||||||||||||
Intercompany payable | — | 269,121 | 7,163 | (276,284 | ) | — | ||||||||||||||
Other current liabilities | (62,131 | ) | 74,200 | (28 | ) | — | 12,041 | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current liabilities | 84,065 | 384,232 | 8,314 | (276,284 | ) | 200,327 | ||||||||||||||
Revolving line of credit and long-term debt, excluding current portion | 454,916 | — | 2,579 | (2,579 | ) | 454,916 | ||||||||||||||
Other noncurrent liabilities | 85,401 | 24,515 | — | — | 109,916 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total liabilities | 624,382 | 408,747 | 10,893 | (278,863 | ) | 765,159 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total PSS World Medical, Inc. shareholders’ equity | 631,655 | 130,270 | 24,317 | (399,114 | ) | 387,128 | ||||||||||||||
Noncontrolling interest | — | 594 | — | 3,089 | 3,683 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total equity | 631,655 | 130,864 | 24,317 | (396,025 | ) | 390,811 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total liabilities and equity | $ | 1,256,037 | $ | 539,611 | $ | 35,210 | $ | (674,888 | ) | $ | 1,155,970 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (a) | Subsequent to March 30, 2012, the Company acquired companies that it intends to include as Guarantor Subsidiaries in future reporting periods. |
F-42
Table of Contents
CONDENSED CONSOLIDATING BALANCE SHEET
APRIL 1, 2011
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
ASSETS | ||||||||||||||||||||
Current Assets: | ||||||||||||||||||||
Cash and cash equivalents | $ | 13,901 | $ | 3,568 | $ | 11,879 | $ | — | $ | 29,348 | ||||||||||
Accounts receivable, net | 132,425 | 113,347 | 1,457 | — | 247,229 | |||||||||||||||
Inventories | 121,350 | 91,501 | 360 | — | 213,211 | |||||||||||||||
Deferred tax assets, net | 15,772 | 4,761 | — | — | 20,533 | |||||||||||||||
Intercompany receivable | 156,051 | — | 403 | (156,454 | ) | — | ||||||||||||||
Prepaid expenses and other current assets | 20,515 | 13,633 | 137 | — | 34,285 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current assets | 460,014 | 226,810 | 14,236 | (156,454 | ) | 544,606 | ||||||||||||||
Property and equipment, net | 44,456 | 57,588 | 357 | — | 102,401 | |||||||||||||||
Other Assets: | ||||||||||||||||||||
Goodwill | 37,518 | 129,576 | — | — | 167,094 | |||||||||||||||
Intangibles, net | 18,390 | 23,489 | — | — | 41,879 | |||||||||||||||
Investment in subsidiaries | 446,526 | 10,913 | — | (457,439 | ) | — | ||||||||||||||
Other assets | 80,396 | 16,975 | 741 | (2,420 | ) | 95,692 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total assets | $ | 1,087,300 | $ | 465,351 | $ | 15,334 | $ | (616,313 | ) | $ | 951,672 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
LIABILITIES AND EQUITY | ||||||||||||||||||||
Current Liabilities: | ||||||||||||||||||||
Accounts payable | $ | 91,936 | $ | 34,729 | $ | 1,392 | $ | — | $ | 128,057 | ||||||||||
Accrued expenses | 22,241 | 14,411 | 523 | — | 37,175 | |||||||||||||||
Current portion of long-term debt | 39 | 722 | — | — | 761 | |||||||||||||||
Intercompany payable | — | 166,317 | — | (166,317 | ) | — | ||||||||||||||
Other current liabilities | (39,191 | ) | 72,402 | — | — | 33,211 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current liabilities | 75,025 | 288,581 | 1,915 | (166,317 | ) | 199,204 | ||||||||||||||
Revolving line of credit and long-term debt, excluding current portion | 195,656 | 6 | 2,435 | (2,435 | ) | 195,662 | ||||||||||||||
Other noncurrent liabilities | 84,136 | 26,144 | — | — | 110,280 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total liabilities | 354,817 | 314,731 | 4,350 | (168,752 | ) | 505,146 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Equity: | ||||||||||||||||||||
PSS World Medical Inc. shareholders’ equity: | ||||||||||||||||||||
Total PSS World Medical, Inc. shareholders’ equity | 732,483 | 150,189 | 10,984 | (450,730 | ) | 442,926 | ||||||||||||||
Noncontrolling interest | — | 431 | — | 3,169 | 3,600 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total equity | 732,483 | 150,620 | 10,984 | (447,561 | ) | 446,526 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total liabilities and equity | $ | 1,087,300 | $ | 465,351 | $ | 15,334 | $ | (616,313 | ) | $ | 951,672 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
F-43
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF OPERATIONS
FOR THE YEAR ENDED MARCH 30, 2012
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries (a) | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Net sales | $ | 1,219,631 | $ | 861,179 | $ | 73,278 | $ | (52,086 | ) | $ | 2,102,002 | |||||||||
Cost of goods sold | 864,473 | 548,221 | 51,993 | (36,888 | ) | 1,427,799 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Gross profit | 355,158 | 312,958 | 21,285 | (15,198 | ) | 674,203 | ||||||||||||||
General and administrative expenses | 203,371 | 182,345 | 7,536 | (262 | ) | 392,990 | ||||||||||||||
Selling expenses | 100,732 | 47,125 | — | — | 147,857 | |||||||||||||||
Equity earnings of subsidiaries | (55,715 | ) | 13,171 | — | 42,544 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
(Loss) income from operations | (4,660 | ) | 96,659 | 13,749 | 42,562 | 133,356 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest expense | (19,605 | ) | (344 | ) | (275 | ) | 76 | (20,148 | ) | |||||||||||
Interest income | 9,553 | (9,304 | ) | — | (76 | ) | 173 | |||||||||||||
Other income, net | 1,334 | 759 | (9 | ) | — | 2,084 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other expense, net | (8,718 | ) | (8,889 | ) | (284 | ) | — | (17,891 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
(Loss) income before provision for income taxes | (13,378 | ) | 87,770 | 13,465 | 27,608 | 115,465 | ||||||||||||||
(Benefit) provision for income taxes | (18,226 | ) | 59,159 | 130 | — | 41,063 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income | (4,848 | ) | 28,611 | 13,335 | 27,608 | 74,402 | ||||||||||||||
Net income attributable to noncontrolling interest | — | 162 | — | (79 | ) | 83 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income attributable to PSS World Medical, Inc. | $ | (4,848 | ) | $ | 28,449 | $ | 13,335 | $ | 27,687 | $ | 74,319 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (a) | Subsequent to March 30, 2012, the Company acquired companies that it intends to include as Guarantor Subsidiaries in future reporting periods. |
F-44
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF OPERATIONS
FOR THE YEAR ENDED APRIL 1, 2011
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Net sales | $ | 1,302,655 | $ | 831,776 | $ | 15,658 | $ | (115,300 | ) | $ | 2,034,789 | |||||||||
Cost of goods sold | 965,219 | 537,263 | 5,695 | (109,159 | ) | 1,399,018 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Gross profit | 337,436 | 294,513 | 9,963 | (6,141 | ) | 635,771 | ||||||||||||||
General and administrative expenses | 204,804 | 155,275 | 4,812 | (142 | ) | 364,749 | ||||||||||||||
Selling expenses | 97,371 | 40,095 | — | — | 137,466 | |||||||||||||||
Equity earnings of subsidiaries | 38,512 | 3,607 | — | (42,119 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income from operations | 73,773 | 102,750 | 5,151 | (48,118 | ) | 133,556 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest expense | (16,668 | ) | (233 | ) | (220 | ) | — | (17,121 | ) | |||||||||||
Interest income | 9,303 | (9,019 | ) | — | — | 284 | ||||||||||||||
Other income, net | 1,730 | 776 | — | — | 2,506 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other expense, net | (5,635 | ) | (8,476 | ) | (220 | ) | — | (14,331 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income before provision for income taxes | 68,138 | 94,274 | 4,931 | (48,118 | ) | 119,225 | ||||||||||||||
(Benefit) provision for income taxes | (17,616 | ) | 61,991 | 186 | — | 44,561 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income | 85,754 | 32,283 | 4,745 | (48,118 | ) | 74,664 | ||||||||||||||
Net income attributable to noncontrolling interest | — | 71 | — | 108 | 179 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income attributable to PSS World Medical, Inc. | $ | 85,754 | $ | 32,212 | $ | 4,745 | $ | (48,226 | ) | $ | 74,485 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
F-45
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF OPERATIONS
FOR THE YEAR ENDED APRIL 2, 2010
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Net sales | $ | 1,345,645 | $ | 831,570 | $ | — | $ | (122,044 | ) | $ | 2,055,171 | |||||||||
Cost of goods sold | 1,004,697 | 544,823 | (6,795 | ) | (115,249 | ) | 1,427,476 | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Gross profit | 340,948 | 286,747 | 6,795 | (6,795 | ) | 627,695 | ||||||||||||||
General and administrative expenses | 218,248 | 151,200 | 1,423 | — | 370,871 | |||||||||||||||
Selling expenses | 97,292 | 38,551 | — | — | 135,843 | |||||||||||||||
Equity earnings of subsidiaries | 29,984 | 5,372 | — | (35,356 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income from operations | 55,392 | 102,368 | 5,372 | (42,151 | ) | 120,981 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest expense | (17,156 | ) | (139 | ) | — | — | (17,295 | ) | ||||||||||||
Interest income | 9,842 | (9,466 | ) | — | — | 376 | ||||||||||||||
Other income, net | 5,207 | 861 | — | — | 6,068 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Other expense, net | (2,107 | ) | (8,744 | ) | — | — | (10,851 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income before provision for income taxes | 53,285 | 93,624 | 5,372 | (42,151 | ) | 110,130 | ||||||||||||||
(Benefit) provision for income taxes | (21,127 | ) | 61,894 | — | — | 40,767 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income | 74,412 | 31,730 | 5,372 | (42,151 | ) | 69,363 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income attributable to PSS World Medical, Inc. | $ | 74,412 | $ | 31,730 | $ | 5,372 | $ | (42,151 | ) | $ | 69,363 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
F-46
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF CASH FLOWS
FOR THE YEARS ENDED MARCH 30, 2012
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries (a) | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Cash Flows From Operating Activities: | ||||||||||||||||||||
Net cash (used in) provided by operating activities | $ | (30,477 | ) | $ | 138,616 | $ | 20,184 | $ | (39 | ) | $ | 128,284 | ||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Investing Activities: | ||||||||||||||||||||
Payments for business combinations, net of cash acquired | (8,599 | ) | (56,532 | ) | — | — | (65,131 | ) | ||||||||||||
Capital expenditures | (2,597 | ) | (21,288 | ) | (33 | ) | — | (23,918 | ) | |||||||||||
Other | (268 | ) | (78 | ) | — | 183 | (163 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash used in investing activities | (11,464 | ) | (77,898 | ) | (32 | ) | 183 | (89,212 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Financing Activities: | ||||||||||||||||||||
Proceeds from issuance of debt | 250,000 | — | — | — | 250,000 | |||||||||||||||
Proceeds from borrowings on the revolving line of credit | 405,056 | — | 144 | (144 | ) | 405,056 | ||||||||||||||
Repayments on the revolving line of credit | (405,056 | ) | — | — | — | (405,056 | ) | |||||||||||||
Purchase and retirement of common stock | (140,439 | ) | — | — | — | (140,439 | ) | |||||||||||||
Payment of contingent consideration on business acquisition | — | (9,500 | ) | — | — | (9,500 | ) | |||||||||||||
Payment for debt issuance costs | (6,467 | ) | — | — | — | (6,467 | ) | |||||||||||||
Excess tax benefits from share-based compensation arrangements | 2,057 | — | — | — | 2,057 | |||||||||||||||
Proceeds from exercise of stock options | 1,383 | — | — | — | 1,383 | |||||||||||||||
Payments under capital lease obligations | (38 | ) | (741 | ) | — | — | (779 | ) | ||||||||||||
Intercompany dividend | 40,515 | (40,515 | ) | — | — | — | ||||||||||||||
Other | (1,523 | ) | — | — | — | (1,523 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash provided by (used in) financing activities | 145,488 | (50,756 | ) | 144 | (144 | ) | 94,732 | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net increase in cash and cash equivalents | 103,547 | 9,962 | 20,295 | — | 133,804 | |||||||||||||||
Cash and cash equivalents, beginning of period | 13,901 | 3,568 | 11,879 | — | 29,348 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash and cash equivalents, end of period | $ | 117,448 | $ | 13,530 | $ | 32,174 | $ | — | $ | 163,152 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (a) | Subsequent to March 30, 2012, the Company acquired companies that it intends to include as Guarantor Subsidiaries in future reporting periods. |
F-47
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF CASH FLOWS
FOR THE YEARS ENDED APRIL 1, 2011
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Cash Flows From Operating Activities: | ||||||||||||||||||||
Net cash (used in) provided by operating activities | $ | (852 | ) | $ | 116,284 | $ | 3,424 | $ | (2,528 | ) | $ | 116,328 | ||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Investing Activities: | ||||||||||||||||||||
Payments for business combinations, net of cash acquired | (10,177 | ) | (55,875 | ) | — | 118 | (65,934 | ) | ||||||||||||
Capital expenditures | (2,169 | ) | (15,784 | ) | (489 | ) | 215 | (18,227 | ) | |||||||||||
Payment for investment in variable interest entity, net of cash | — | (7,431 | ) | — | 4,154 | (3,277 | ) | |||||||||||||
Other | (527 | ) | (24 | ) | — | (117 | ) | (668 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash used in investing activities | (12,873 | ) | (79,114 | ) | (489 | ) | 4,370 | (88,106 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Financing Activities: | ||||||||||||||||||||
Proceeds from borrowings on the revolving line of credit | 106,400 | — | 1,429 | (1,429 | ) | 106,400 | ||||||||||||||
Repayments on the revolving line of credit | (106,400 | ) | — | — | — | (106,400 | ) | |||||||||||||
Purchase and retirement of common stock | (54,761 | ) | — | — | — | (54,761 | ) | |||||||||||||
Payment of contingent consideration on business acquisition | — | (862 | ) | — | — | (862 | ) | |||||||||||||
Excess tax benefits from share-based compensation arrangements | 3,187 | — | — | — | 3,187 | |||||||||||||||
Proceeds from exercise of stock options | 2,079 | — | — | — | 2,079 | |||||||||||||||
Payments under capital lease obligations | (44 | ) | (790 | ) | — | — | (834 | ) | ||||||||||||
Intercompany dividend | 41,811 | (41,812 | ) | — | 1 | — | ||||||||||||||
Other | (20 | ) | — | — | (414 | ) | (434 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash (used in) provided by financing activities | (7,748 | ) | (43,464 | ) | 1,429 | (1,842 | ) | (51,625 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (decrease) increase in cash and cash equivalents | (21,473 | ) | (6,294 | ) | 4,364 | — | (23,403 | ) | ||||||||||||
Cash and cash equivalents, beginning of period | 35,374 | 9,862 | 7,515 | — | 52,751 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash and cash equivalents, end of period | $ | 13,901 | $ | 3,568 | $ | 11,879 | $ | — | $ | 29,348 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
F-48
Table of Contents
CONDENSED CONSOLIDATING STATEMENT OF CASH FLOWS
FOR THE YEARS ENDED APRIL 2, 2010
(Dollars in Thousands)
| Parent | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Eliminations | Consolidated | ||||||||||||||||
Cash Flows From Operating Activities: | ||||||||||||||||||||
Net cash provided by operating activities | $ | 22,970 | $ | 75,076 | $ | 4,909 | $ | (554 | ) | $ | 102,401 | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Investing Activities: | ||||||||||||||||||||
Payments for business combinations, net of cash acquired | (1,647 | ) | (13,155 | ) | — | — | (14,802 | ) | ||||||||||||
Capital expenditures | (11,897 | ) | (14,325 | ) | (31 | ) | 330 | (25,923 | ) | |||||||||||
Proceeds from sale of available for sale securities | 10,681 | — | — | — | 10,681 | |||||||||||||||
Other | (735 | ) | — | — | 194 | (541 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash used in investing activities | (3,598 | ) | (27,480 | ) | (31 | ) | 524 | (30,585 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash Flows From Financing Activities: | ||||||||||||||||||||
Proceeds from borrowings on the revolving line of credit | 5,350 | — | — | — | 5,350 | |||||||||||||||
Repayments on the revolving line of credit | (55,350 | ) | — | — | — | (55,350 | ) | |||||||||||||
Purchase and retirement of common stock | (57,176 | ) | — | — | — | (57,176 | ) | |||||||||||||
Excess tax benefits from share-based compensation arrangements | 2,516 | — | — | — | 2,516 | |||||||||||||||
Proceeds from exercise of stock options | 4,489 | — | — | — | 4,489 | |||||||||||||||
Payments under capital lease obligations | (94 | ) | (861 | ) | — | 30 | (925 | ) | ||||||||||||
Intercompany dividends | 41,515 | (41,515 | ) | — | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net cash used in financing activities | (58,750 | ) | (42,376 | ) | — | 30 | (101,096 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (decrease) increase in cash and cash equivalents | (39,378 | ) | 5,220 | 4,878 | — | (29,280 | ) | |||||||||||||
Cash and cash equivalents, beginning of period | 74,752 | 4,642 | 2,637 | — | 82,031 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Cash and cash equivalents, end of period | $ | 35,374 | $ | 9,862 | $ | 7,515 | $ | — | $ | 52,751 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
F-49
Table of Contents
22. QUARTERLY RESULTS OF OPERATIONS (UNAUDITED)
The following tables present summarized unaudited quarterly results of operations for fiscal years 2012 and 2011. The Company believes all necessary adjustments have been included in the amounts stated below to present fairly the following selected information when read in conjunction with the consolidated financial statements of the Company. Future quarterly operating results may fluctuate depending on a number of factors, including the number of selling days in a quarter, the timing of business combinations, and changes in customer’s buying patterns of supplies, equipment, and pharmaceutical products. Results of operations for any particular quarter are not necessarily indicative of results of operations for a full year or any other quarter.
| Fiscal Year 2012 | ||||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Total | ||||||||||||||||
Net sales | $ | 513,682 | $ | 521,756 | $ | 527,695 | $ | 538,869 | $ | 2,102,002 | ||||||||||
Gross profit | 160,757 | 167,103 | 170,495 | 175,848 | 674,203 | |||||||||||||||
Net income attributable to PSS World Medical, Inc. | 14,189 | 20,031 | 20,132 | 19,967 | 74,319 | |||||||||||||||
Earnings per common share attributable to PSS World Medical, Inc.: | ||||||||||||||||||||
Basic | $ | 0.26 | $ | 0.38 | $ | 0.39 | $ | 0.40 | $ | 1.43 | ||||||||||
Diluted | $ | 0.25 | $ | 0.37 | $ | 0.38 | $ | 0.38 | $ | 1.38 | ||||||||||
Selling days | 64 | 63 | 62 | 64 | 253 | |||||||||||||||
| Fiscal Year 2011 | ||||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Total | ||||||||||||||||
Net sales | $ | 478,856 | $ | 496,188 | $ | 510,087 | $ | 549,658 | $ | 2,034,789 | ||||||||||
Gross profit | 147,846 | 157,125 | 157,895 | 172,905 | 635,771 | |||||||||||||||
Net income attributable to PSS World Medical, Inc. | 13,753 | 19,557 | 19,549 | 21,626 | 74,485 | |||||||||||||||
Earnings per common share attributable to PSS World Medical, Inc.: | ||||||||||||||||||||
Basic | $ | 0.25 | $ | 0.36 | $ | 0.36 | $ | 0.40 | $ | 1.35 | ||||||||||
Diluted | $ | 0.24 | $ | 0.35 | $ | 0.35 | $ | 0.38 | $ | 1.32 | ||||||||||
Selling days | 64 | 63 | 61 | 65 | 253 | |||||||||||||||
The Company reports its year-end and quarter-end financial position, results of operations, and cash flows as of the Friday closest to calendar month end, determined using the number of business days. As disclosed in the table above, the selling days may fluctuate between each quarter and/or differ from the previous fiscal year.
Fiscal Year 2012
During the three months ended March 30, 2012, the Company issued $250.0 million aggregate principal amount of 6.375% senior notes due 2022 in a private offering. The 2012 Notes were issued under an Indenture, dated February 24, 2012 among the Company, the Guarantors and U.S. Bank National Association, as trustee. Interest on the notes is payable semi-annually in arrears on March 1 and September 1, beginning September 1, 2012. Refer to 12,Debt, for further discussion. During the three months ended March 30, 2012, the Company recognized a decrease in accrued incentive and stock-based compensation expense of $7,358 related to payout estimates based on performance, which is reflected inNet income attributable to PSS World Medical, Inc.in the table above.
Fiscal Year 2011
During the three months ended April 1, 2011, the Company purchased 100% of the outstanding stock of Dispensing Solutions, Inc. (“DSI”). DSI, a formerly privately held company based in California, markets a proprietary system to primary care physicians for dispensing medications to patients on-site within their practices. The acquisition price for DSI was approximately $36,000, with additional consideration of up to $6,000 if the company achieved defined earnings targets over a one year period. Net sales related to the DSI acquisition during the three months ended April, 1, 2011, was approximately $6,595. Refer to Footnote 4,Purchase Business Combinations, for further discussion.
F-50
Table of Contents
23. SUBSEQUENT EVENTS
Restructuring Plan
During the first quarter of fiscal year 2013 the Company’s Board of Directors approved a strategic restructuring plan designed to transform the Company, focusing its efforts and investments on what it believes will be the fastest growing segments of non-acute, alternate site healthcare in the U.S. Specifically, the Company will focus on physician, laboratory, in-office dispensing, and the home care and hospice markets.
The restructuring plan will include the sale of two business units serving skilled nursing facilities and specialty dental practices, the integration of all warehouse operations into one common distribution infrastructure, as well as a redesign of the shared services function. The Company determined that certain held for sale criteria had not been met as of March 30, 2012, and therefore reports the assets, liabilities, and the related results of operations as continuing operations.
The Company is currently unable to make a good faith estimate of the amount or range of amounts expected to be incurred in connection with the strategic restructuring plan, both with respect to each major type of cost associated therewith and with respect to the total cost, or an estimate of the amount or range of amounts that will result in future cash expenditures for such transaction. The Company expects to complete the restructuring plan within the next several fiscal years.
Acquisitions
Subsequent to March 30, 2012, the Company completed two acquisitions that were individually immaterial, but material in the aggregate. The combined purchase price of the acquisitions was $72,400, of which $3,900 was held in escrow and $6,000 was held by the Company to secure certain adjustments or claims.
If the acquisitions of the companies had occurred on the first day of fiscal year 2011, consolidated net sales for the years ended March 30, 2012 and April 1, 2011 would have been $2,218,450 and $2,142,594, excluding the pro forma results of other acquisitions made during the current period. This pro forma information is unaudited and is not necessarily indicative of the results of operations that actually would have resulted had the acquisitions occurred on the date indicated above or that may result in the future, and does not reflect potential synergies.
Due to the proximity of the acquisitions’ closing to the Company’s filing date, the initial accounting for the acquisition has not been finalized. As a result, the Company has not provided additional disclosures required for business combinations.
F-51
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
SCHEDULE II–VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED March 30, 2012, April 1, 2011 and April 2, 2010
(Dollars in Thousands)
Description | Balance at Beginning of Period | Provision Charged to Expense | Write-offs (a) | Balance at End of Period | ||||||||||||
Valuation Allowance for Accounts Receivable: | ||||||||||||||||
Fiscal year ended March 30, 2012 | $ | 5,808 | $ | 2,858 | $ | 2,452 | $ | 6,214 | ||||||||
Fiscal year ended April 1, 2011 | $ | 6,310 | $ | 1,741 | $ | 2,243 | $ | 5,808 | ||||||||
Fiscal year ended April 2, 2010 | $ | 7,041 | $ | 3,795 | $ | 4,526 | $ | 6,310 | ||||||||
Other:(b) | ||||||||||||||||
Fiscal year ended March 30, 2012 | $ | 8,961 | N/A | N/A | $ | 17,796 | ||||||||||
Fiscal year ended April 1, 2011 | $ | 5,923 | N/A | N/A | $ | 8,961 | ||||||||||
Fiscal year ended April 2, 2010 | $ | 5,044 | N/A | N/A | $ | 5,923 | ||||||||||
| (a) | Uncollectible accounts written off, net of recoveries. |
| (b) | Includes (i) sales returns and allowance, (ii) reserve for rebates receivable, and (iii) contractual billing adjustments. |
See Report of Independent Registered Public Accounting Firm
F-52
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s Principal Executive Officer and Principal Financial Officer, has evaluated the effectiveness of the Company‘s disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this report (the “Evaluation Date”). Based on the evaluation, the Principal Executive Officer and the Principal Financial Officer concluded that, as of the Evaluation Date, the Company’s disclosure controls and procedures are effective in recording, processing, summarizing and reporting, on a timely basis, information required to be disclosed by the Company in the reports that it files or submits under the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) and are effective in ensuring that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act, is accumulated and communicated to the Company’s management, including the Company’s Principal Executive Officer and Principal Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Internal Control Over Financial Reporting
(a) Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining effective internal controls over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f).
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the Company’s financial statements.
Management, with the participation of the Company’s principal executive and principal financial officers, assessed the effectiveness of the Company’s internal control over financial reporting as of March 30, 2012. This assessment was performed using the criteria established under the Internal Control - Integrated Framework established by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations, including the possibility of human error or circumvention or overriding of internal control. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement preparation and reporting and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Based on the assessment performed using the criteria established by COSO, management has concluded that the Company maintained effective internal control over financial reporting as of March 30, 2012.
59
Table of Contents
KPMG LLP, the independent registered public accounting firm that audited the financial statements included in this Annual Report on Form 10-K for the fiscal year ended March 30, 2012, has issued an audit report on the effectiveness of the Company’s internal control over financial reporting. Such report appears immediately below.
(b) Attestation Report of the Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
PSS World Medical, Inc.:
We have audited PSS World Medical, Inc. and subsidiaries (the Company) internal control over financial reporting as of March 30, 2012, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). PSS World Medical, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying management’s annual report on internal control over financial reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, PSS World Medical, Inc. maintained, in all material respects, effective internal control over financial reporting as of March 30, 2012, based on criteria established inInternal Control – Integrated Framework issued by Committee of Sponsoring Organizations of the Treadway Commission (COSO).
60
Table of Contents
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of PSS World Medical, Inc. and subsidiaries as of March 30, 2012 and April 1, 2011and the related consolidated statements of operations, equity and comprehensive income and cash flows for each of the years in the three-year period ended March 30, 2012 and the financial statement schedule, and our report dated May 25, 2012, expressed an unqualified opinion on those consolidated financial statements and financial statement schedule.
/s/ KPMG LLP
May 25, 2012
Jacksonville, Florida
Certified Public Accountants
(c) Changes in Internal Control Over Financial Reporting
There were no changes in the Company’s internal control over financial reporting that occurred during the three months ended March 30, 2012 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
61
Table of Contents
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information called for by this Item 10 regarding the Company’s directors, executive officers and corporate governance is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC under the Exchange Act, relating to the Company’s fiscal year 2012 Annual Meeting of Shareholders under the sections“Corporate Governance”and“Management.”
The Company has adopted a Code of Ethics that applies to the Chief Executive Officer, Chief Financial Officer and Corporate Controller. The Code of Ethics may be viewed free of charge on the Company’s web sitewww.pssworldmedical.com. The Company intends to post amendments to or waivers from its Code of Ethics (to the extent applicable to the Company’s Chief Executive Officer, Chief Financial Officer, or Corporate Controller) on its web site.
Information required by this item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 is hereby incorporated by reference to the subsection entitled “Section 16(a) Beneficial Ownership Reporting Compliance.”
There have been no changes to the procedures by which stockholders may recommend nominees to the Company’s Board of Directors since the Company’s last disclosure of such procedures, which appeared in the Company’s definitive 2011 Proxy Statement filed pursuant to Regulation 14A on July 15, 2011.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information called for by this Item 11 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2012 Annual Meeting of Shareholders under the section“Executive Compensation.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information called for by this Item 12 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2012 Annual Meeting of Shareholders under the sections“Security Ownership of Certain Beneficial Owners and Management”and“Executive Compensation.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information called for by this Item 13 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2012 Annual Meeting of Shareholders under the section“Related Person Transactions”and the subsection “Director Independence.”
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information called for by this Item 14 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2012 Annual Meeting of Shareholders under the caption“Ratification of Independent Registered Public Accounting Firm.”
62
Table of Contents
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1) The following financial statements are included in Item 8 of this report:
| Page | ||||
Report of Independent Registered Public Accounting Firm. | F-2 | |||
Consolidated Balance Sheets—March 30, 2012 and April 1, 2011 | F-3 | |||
Consolidated Statements of Operations for the Years Ended March 30, 2012, April 1, 2011, and April 2, 2010 | F-4 | |||
Consolidated Statements of Equity and Comprehensive Income for the Years Ended March 30, 2012, April 1, 2011, and April 2, 2010 | F-5 | |||
Consolidated Statements of Cash Flows for the Years Ended March 30, 2012, April 1, 2011, and April 2, 2010 | F-6 | |||
Notes to Consolidated Financial Statements | F-7 | |||
(a)(2) The following supplemental schedule is included in this report:
| Page | ||||
Schedule II–Valuation and Qualifying Accounts for the Years Ended March 30, 2012, April 1, 2011, and April 2, 2010 | F-52 | |||
(a)(3) Exhibits required by Item 601 of Regulation S-K:
Exhibit Number | Description | |
| 3.1 | Restatement of Amended and Restated Articles of Incorporation, dated December 12, 2008. (18) | |
| 3.2 | Amended and Restated Bylaws, dated December 12, 2008. (18) | |
| 3.2a | First Amendment to the Amended and Restated Bylaws, dated August 25, 2011. (25) | |
| 4.1 | Indenture related to the 3.125% Convertible Senior Notes due 2014, dated as of August 4, 2008, among PSS World Medical, Inc., as issuer, U.S. Bank National Association, as trustee. (17) | |
| 4.2 | Form of 3.125% Convertible Senior Note due 2014. (17) | |
| 4.3 | Indenture, dated February 24, 2012, among the Company, the Guarantors, and U.S. Bank National Association, as trustee. (27) | |
| 4.4 | Registration Rights Agreement, dated February 24, 2012, among the Company, the Guarantors, Credit Suisse Securities (USA) LLC and Merrill Lynch, Pierce, Fenner & Smith Incorporated, as representatives of the several purchasers. (27) | |
| 10.1* | Amended and Restated Directors Stock Plan. (4) | |
| 10.2* | 1994 Employee Stock Purchase Plan. (1) | |
63
Table of Contents
Exhibit Number | Description | |
| 10.3* | 1999 Long-term Incentive Plan (Amended and Restated as of July 25, 2001). (4) | |
| 10.4 | 1999 Broad-Based Employee Stock Plan. (4) | |
| 10.5* | PSS World Medical, Inc. 2006 Incentive Plan. (13) | |
| 10.6* | Shareholder Value Plan.** (20) | |
| 10.7* | Description of Executive Officer Annual Incentive Bonus Program. (12) | |
| 10.8* | Conformed Amended and Restated Savings Plan. | |
| 10.8a* | May 2012 Amendment to the Conformed Amended and Restated Savings Plan. | |
| 10.9 | Amended and Restated Credit Agreement, dated as of May 20, 2003, by and among the Company, each of the Company’s subsidiaries therein named, the Lenders from time to time party thereto, Bank of America, N.A., as Agent, and Banc of America Securities LLC, as Arranger.** (23) | |
| 10.9a | Second Amendment to Credit Agreement, dated as of December 16, 2003, by and among the Company, each of the Company’s subsidiaries therein named, the Lenders from time to time party thereto, Bank of America, N.A., as Agent, and Banc of America Securities LLC, as Arranger. (23) | |
| 10.9b | Third Amendment to Credit Agreement, dated as of March 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (5) | |
| 10.9c | Fourth Amendment to Credit Agreement, dated as of June 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (7) | |
| 10.9d | Fifth Amendment to Credit Agreement, dated as of October 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (8) | |
| 10.9e | Sixth Amendment to Credit Agreement, dated as of June 30, 2005, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (11) | |
| 10.9f | Seventh Amendment to the Credit Agreement, dated as of January 23, 2008, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (16) | |
| 10.9g | Second Amended and Restated Credit and Security Agreement, dated as of November 16, 2011, by and among the Company, certain of the Company’s subsidiaries party thereto, the Lenders party thereto, Bank of America, N.A., as Administrative Agent, Merrill Lynch, Pierce, Fenner & Smith Incorporated, as Sole Arranger and Sole Book Runner, and Wells Fargo Bank, N.A. and JPMorgan Chase Bank, N.A., as Co-Syndication Agents.** (26) | |
| 10.9h | First Amendment to the Second Amended and Restated Credit and Security Agreement, dated as of February __, 2012, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as Administrative Agent, Merrill Lynch, Pierce, Fenner & Smith Incorporated, as Sole Arranger and Sole Book Runner, and Wells Fargo Bank, N.A. and JPMorgan Chase Bank, N.A., as Co-Syndication Agents. | |
64
Table of Contents
Exhibit Number | Description | |
| 10.9i | Second Amended and Restated Partnership Interest Pledge Agreement, dated as of November 16, 2011, by the Company and PSS Holding, Inc., in favor of Bank of America, N.A., as Agent.** (26) | |
| 10.9j | Second Amended and Restated Stock Pledge Agreement, dated as of November 16, 2011, by the Company and certain of the Company’s subsidiaries party thereto, in favor of Bank of America, N.A., as Agent.** (26) | |
| 10.10* | Employment Agreement, dated as of April 1, 2003, by and between the Company and David M. Bronson. (3) | |
| 10.10a* | Amended and Restated Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and David M. Bronson. (19) | |
| 10.11* | Employment Agreement, dated as of August 16, 2005, by and between the Company and Gary A. Corless. (10) | |
| 10.11a* | Amended and Restated Employment Agreement related to 409A, dated as of December 29, 2008, by and between the Company and Gary A. Corless. (19) | |
| 10.12* | Employment Agreement, dated as of April 1, 2004, by and between the Company and Kevin P. English. (6) | |
| 10.12a* | Amendment to Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and Kevin P. English. (19) | |
| 10.13* | Employment Agreement, dated as of January 1, 2002, by and between the Company and Bradley J. Hilton. (9) | |
| 10.13a* | Amendment to Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and Bradley J. Hilton. (19) | |
| 10.14* | Employment Agreement, dated as of April 1, 1998, by and between the Company and John F. Sasen, Sr. (2) | |
| 10.14a* | Amendment to Employment Agreement, dated as of April 17, 2000, by and between the Company and John F. Sasen, Sr. (2) | |
| 10.14b* | Amended and Restated Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and John F. Sasen Sr. (19) | |
| 10.15* | Separation Agreement, dated as of February 2, 2010, by and between the Company and David A. Smith. (22) | |
| 10.16* | PSS World Medical, Inc. Amended and Restated Officer Stock Option Grant Program, as amended through July 1, 2004. (8) | |
| 10.16a* | PSS World Medical Inc. Amended and Restated Director’s Deferred Compensation Plan, as amended and restated effective January 1, 2009. (19) | |
| 10.16b* | PSS World Medical Inc. Amended and Restated Officer Deferred Compensation Plan, as amended and restated effective January 1, 2009. (19) | |
| 10.16c* | Amendment to the Amended and Restated Officer Deferred Compensation Plan, effective December 9, 2010. (24) | |
65
Table of Contents
Exhibit Number | Description | |
| 10.16d* | Amendment to the Amended and Restated Officer Deferred Compensation Plan, effective March 27, 2012. | |
| 10.17* | PSS World Medical, Inc. Amended and Restated ELITe Stock Option Grant Program, as amended through July 1, 2004. (8) | |
| 10.17a* | PSS World Medical, Inc. Amended and Restated ELITe Deferred Compensation Plan, as amended and restated effective January 1, 2009. (19) | |
| 10.17b* | Amendment to the Amended and Restated ELITe Deferred Compensation Plan, effective December 9, 2010. (24) | |
| 10.17c* | Amendment to the Amended and Restated ELITe Deferred Compensation Plan, effective March 27, 2012. | |
| 10.18* | PSS World Medical, Inc. Leader’s Stock Option Grant Program, as amended through July 1, 2004. (8) | |
| 10.18a* | PSS World Medical Inc. Amended and Restated Leader’s Deferral Plan, as amended and restated effective January 1, 200 9 (19) | |
| 10.18b* | Amendment to the Amended and Restated Leader’s Deferred Compensation Plan, effective December 9, 2010. (24) | |
| 10.18c* | Amendment to the Amended and Restated Leader’s Deferred Compensation Plan, effective March 27, 2012. | |
| 10.19* | PSS World Medical, Inc. 2004 Non-Employee Directors’ Compensation Plan. (8) | |
| 10.19a* | Amendment No. 1 to the PSS World Medical, Inc. Amended and Restated 2004 Non-Employee Directors’ Compensation Plan, as amended through August 24, 2006. (14) | |
| 10.20* | Form of Restricted Stock Award Agreement. (10) | |
| 10.21* | Form of Performance-Accelerated Restricted Stock Award Agreement. (15) | |
| 10.22* | Form of Performance-Based Restricted Stock Unit Agreement. (15) | |
| 10.23* | Form of Non-statutory Stock Option Award. (17) | |
| 10.24 | Purchase Agreement dated as of July 29, 2008 among PSS World Medical and Goldman, Sachs & Co. (23) | |
| 10.25 | Convertible Bond Hedge Transaction Confirmation, dated July 29, 2008 between Goldman, Sachs & Co. and PSS World Medical, Inc. (17) | |
| 10.26 | Issuer Warrant Transaction Confirmation dated July 29, 2008 between Goldman, Sachs & Co. and PSS World Medical, Inc. (17) | |
| 10.27* | Form of Performance-Accelerated Restricted Stock Unit Agreement. (21) | |
| 12 | Computation of Consolidated Ratios of Earnings to Fixed Charges. | |
| 21 | List of Subsidiaries of PSS World Medical, Inc. | |
| 23 | Consent of Independent Registered Public Accounting Firm. | |
66
Table of Contents
Exhibit Number | Description | |
| 31.1 | Rule 13a-14(a) Certification of the Chief Executive Officer. | |
| 31.2 | Rule 13a-14(a) Certification of the Chief Financial Officer. | |
| 32.1 | Section 1350 Certification of the Chief Executive Officer. | |
| 32.2 | Section 1350 Certification of the Chief Financial Officer. | |
| 101.INS | XBRL Instance Document. | |
| 101.SCH | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |
| * | Represents a management contract or compensatory plan or arrangement. |
| ** | Certain portions of this Exhibit have been omitted upon a request for confidential treatment pursuant to 24b-2 of the Securities Exchange Act of 1934, as amended. The omitted portions have been filed separately with the Securities Exchange Commission. |
Footnote References |
(1) Incorporated by Reference to the Company’s Registration Statement on Form S-8, Registration No. 33-80657. (2) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 30, 2001. (3) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 28, 2003. (4) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. (5) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed March 9, 2004. (6) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended April 2, 2004. (7) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004. (8) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended October 1, 2004. (9) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended April 1, 2005. (10) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. (11) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed July 7, 2005. (12) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 31, 2006. (13) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed August 29, 2006. (14) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 29, 2006. (15) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 29, 2007. (16) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed January 29, 2008. (17) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 27, 2008. (18) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed December 17, 2008. (19) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended January 2, 2009. (20) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 27, 2009. (21) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 26, 2009. (22) Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended April 2, 2010. (23) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended October 1, 2010. (24) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended July 1, 2011. (25) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011. (26) Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 30, 2011. (27) Incorporated by Reference to the Company’s Current Report on Form 8-K, filed February 28, 2012. |
67
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Jacksonville, State of Florida, on May 25, 2012.
| PSS WORLD MEDICAL, INC. | ||
| By: | /s/ David M. Bronson | |
| David M. Bronson | ||
| Executive Vice President and Chief Financial Officer (Principal Financial Officer/Principal Accounting Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signatures | Title | Date | ||
/s/ Gary A. Corless Gary A. Corless | President, Chief Executive Officer, and Director (Principal Executive Officer) | May 25, 2012 | ||
/s/ Delores M. Kesler Delores M. Kesler | Chairman of the Board of Directors | May 25, 2012 | ||
/s/ Charles E. Adair Charles E. Adair | Director | May 25, 2012 | ||
/s/ Alvin R. Carpenter Alvin R. Carpenter | Director | May 25, 2012 | ||
/s/ Jeffrey C. Crowe Jeffrey C. Crowe | Director | May 25, 2012 | ||
/s/ A. Hugh Greene A. Hugh Greene | Director | May 25, 2012 | ||
/s/ Steven T. Halverson Steven T. Halverson | Director | May 25, 2012 | ||
/s/ Melvin L. Hecktman Melvin L. Hecktman | Director | May 25, 2012 | ||
/s/ Stephen H. Rogers Stephen H. Rogers | Director | May 25, 2012 | ||
/s/ David M. Bronson David M. Bronson | Executive Vice President and Chief Financial Officer (Principal Financial Officer/Principal Accounting Officer) | May 25, 2012 | ||
68