UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
____________________
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
OR
o TRANSACTION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM TO .
Commission File Number 0-26068
____________________
(Exact name of registrant as specified in its charter)
|
| |
| DELAWARE | 95-4405754 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporation organization) | Identification No.) |
| | |
| 520 NEWPORT CENTER DRIVE, | |
| NEWPORT BEACH, CA | 92660 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (949) 480-8300
Securities registered pursuant to Section 12(b) of the Act:
|
| |
| Title of Each Class | Name of Each Exchange on Which Registered |
| Common Stock, $0.001 par value | The NASDAQ Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Act: None
____________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes R No £
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes £ No R
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to filing requirements for the past 90 days. Yes R No £
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T(§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes R No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
| | |
Large accelerated filer R | | Accelerated filer £ |
Non-accelerated filer £ (Do not check if a smaller reporting company) | | Smaller reporting company £ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes £ No R
The aggregate market value of the registrant’s voting and non-voting common stock held by non-affiliates of the registrant on June 30, 2014, the last business day of the registrant’s most recently completed second fiscal quarter, computed by reference to the last sale price of the registrant’s common stock as reported by The Nasdaq Global Select Market on such date, was approximately $871,822,000. This computation assumes that all executive officers and directors are affiliates of the registrant. Such assumption should not be deemed conclusive for any other purpose.
As of February 25, 2015, 50,948,316 shares of common stock were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
In accordance with General Instruction G(3) to Form 10-K, portions of the registrant’s Definitive Proxy Statement on Schedule 14A for its Annual Meeting of Stockholders to be filed with the Commission within 120 days after the close of the fiscal year covered by this Annual Report on Form 10-K are incorporated by reference into Part III of this Annual Report on Form 10-K. Only those portions of the proxy statement that are specifically incorporated by reference herein shall constitute a part of this Annual Report on Form 10-K.
ACACIA RESEARCH CORPORATION
ANNUAL REPORT ON FORM 10-K
FISCAL YEAR ENDED DECEMBER 31, 2014
TABLE OF CONTENTS
|
| | |
| | | Page |
| PART I |
| | | |
| Item 1. | | |
| Item 1A. | | |
| Item 1B. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | | |
| | | |
| PART II |
| | | |
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| | | |
| | | |
| PART III |
| | | |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | | |
| | | |
| PART IV |
| | | |
| Item 15. | | |
PART I
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
As used in this Annual Report on Form 10-K, “we,” “us” and “our” refer to Acacia Research Corporation and/or its wholly and majority-owned operating subsidiaries. All patent portfolio investments, development, licensing and enforcement activities are conducted solely by certain of our wholly owned operating subsidiaries.
This Annual Report on Form 10-K, or the annual report, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which include, without limitation, statements about our future business operations and results, our strategies and competition, and other forward-looking statements included in this annual report. Such statements may be identified by the use of forward-looking terminology such as “may,” “will,” “expect,” “believe,” “estimate,” “anticipate,” “intend,” “continue,” or similar terms, variations of such terms or the negative of such terms. Such statements are based on management’s current expectations and are subject to a number of risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. Such statements address future events and conditions concerning earnings, capital expenditures, litigation, competition, regulatory matters, stock price volatility, liquidity and capital resources and accounting matters. Actual results in each case could differ materially from those anticipated in such statements by reason of factors such as future economic conditions, legislative, regulatory and competitive developments in markets in which we and our subsidiaries operate, and other circumstances affecting anticipated revenues and costs, as more fully disclosed in our discussion of “Risk Factors” in Item 1A of Part I of this annual report. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. Additional factors that could cause such results to differ materially from those described in the forward-looking statements are set forth in connection with the forward-looking statements.
ITEM 1. BUSINESS
General
Our operating subsidiaries partner with inventors and patent owners, applying our legal and technology expertise to patent assets to unlock the financial value in their patented inventions. We are an intermediary in the patent marketplace, bridging the gap between invention and application, facilitating efficiency and delivering monetary rewards to patent owners.
Our operating subsidiaries generate revenues and related cash flows from the granting of intellectual property rights for the use of patented technologies that our operating subsidiaries control or own. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, where necessary, with the enforcement against unauthorized users of their patented technologies through the filing of patent infringement litigation. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to multiple patent portfolios, which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries.
We are a leader in licensing and enforcing patented technologies and have established a proven track record of licensing success with over 1,430 license agreements executed to date, across 181 of our patent portfolio licensing and enforcement programs. To date, we have generated gross licensing revenue of approximately $1.1 billion, and have returned more than $665 million to our patent partners.
Other
We were originally incorporated in California in January 1993 and reincorporated in Delaware in December 1999. Our website address is www.acaciaresearch.com. Reference in this annual report to this website address does not constitute incorporation by reference of the information contained on the website. We make our filings with the Securities and Exchange Commission, or the SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, other reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, and amendments to the foregoing reports, available free of charge on or through our website as soon as reasonably practicable after we file these reports with, or furnish such reports to, the SEC. In addition, we post the following information on our website:
| |
| • | our corporate code of conduct, our code of conduct for our board of directors and our fraud policy; |
| |
| • | charters for our audit committee, nominating and corporate governance committee, disclosure committee and compensation committee; and |
| |
| • | applicable dividend related tax forms. |
The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Also, the SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers, including us, that file electronically with the SEC. The public can obtain any documents that we file with the SEC at http://www.sec.gov.
Patent Licensing and Enforcement Business
Our operating subsidiaries invest in, license and enforce patented technologies. Our operating subsidiaries partner with inventors and patent owners, applying our legal and technology expertise to patent assets to unlock the financial value in their patented inventions. We are an intermediary in the patent marketplace, bridging the gap between invention and application, facilitating efficiency and delivering monetary rewards to patent owners.
Our operating subsidiaries generate revenues and related cash flows from the granting of intellectual property rights for the use of patented technologies that our operating subsidiaries control or own. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, where necessary, with the enforcement against unauthorized users of their patented technologies through the filing of patent infringement litigation.
Refer to the section entitled “Patented Technologies” below for a partial summary of patent portfolios owned or controlled by certain of our operating subsidiaries.
Patents are an important asset class worldwide. Due to legislative and regulatory changes, licensing and enforcing patents has become increasingly difficult for patent holders, necessitating an experienced, well-capitalized, licensing partner. We focus solely on the patent marketplace, and have emerged as the leading outsource patent licensing and enforcement company for patent owners that have made the important choice to outsource their patent licensing and enforcement activities.
We are a leader in patent licensing and enforcement, and our operating subsidiaries have established a proven track record of licensing success with more than 1,430 license agreements executed to date. On a consolidated basis, to date, we have generated revenues from 181 of our patent portfolio licensing and enforcement programs. Our professional staff includes in-house patent attorneys, licensing executives, engineers and business development executives.
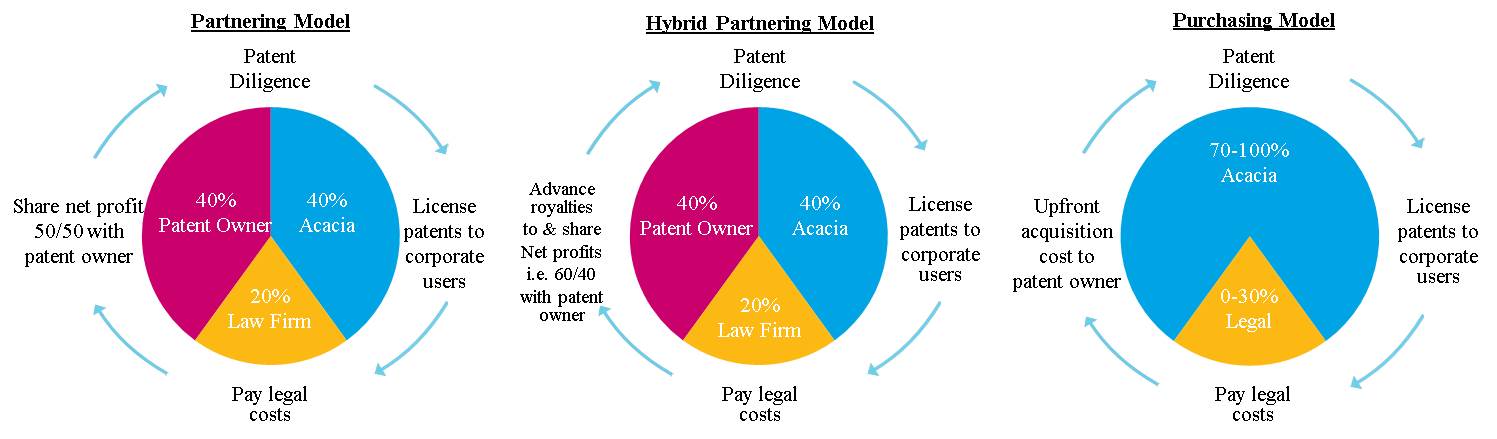
We partner with the disenfranchised patent owner, including individual inventors, universities, and large multi-national corporations in the technology, medical technology, energy, and industrial sectors. A disenfranchised patent owner owns patents that are being infringed by third-parties in connection with the design, manufacture, use, or distribution of products and/or services, but is not receiving fair compensation for the unauthorized use of his or her patented inventions by those third-parties. We strive to reward inventors and patent owners for their creative technological contributions. We also partner with patent owners, including individual inventors, universities, and domestic and multi-national corporations who may have limited internal resources and/or expertise to effectively address the unauthorized use of their patented technologies, and those that are seeking to effectively and efficiently monetize their portfolio of patented technologies on an outsourced basis. In a typical arrangement, our operating subsidiary will partner with a patent portfolio owner, acquiring rights in the patent portfolio or acquiring the patent portfolio outright, and in exchange, the original patent portfolio owner receives (i) a percentage of our operating subsidiary’s net recoveries from the licensing and enforcement of the patent portfolio, which we refer to as our Partnering Model, or (ii) an upfront payment for the purchase of the patent portfolio rights or the patent portfolio, which we refer to as our Purchasing Model, or (iii) a combination of the two, which we refer to as our Hybrid Partnering Model.
Under U.S. law, a patent owner has the right to exclude others from making, selling or using their patented invention. A third-party infringes a patent by making, offering for sale, selling, or using a patented invention without a license from the patent owner. Unfortunately, in the majority of cases, infringers are generally unwilling, at least initially, to negotiate or pay reasonable license fees for their unauthorized use of third-party patents and will typically fight any allegations of patent infringement. Inventors and/or patent holders without sufficient legal, financial and/or expert technical resources to bring and continue the pursuit of costly and complex patent infringement actions are often blatantly ignored.
As a result of the common reluctance of patent infringers to negotiate and ultimately take a patent license for the use of patented technologies without at least the threat of legal action, patent licensing and enforcement often begins with the filing of patent enforcement litigation. However, most patent infringement litigation settles out of court at amounts that are related to the strength of the patent and the value of the invention in the infringer’s products or services. We execute agreements that grant rights in our patents to users of our patented technologies. Our agreements can be negotiated without the filing of patent litigation, or negotiated in the shadow of ongoing patent litigation, depending on the specific facts and circumstances.
Patents are a complex and highly technical subject area. Our professionals actively seek to identify high-quality but undervalued patent portfolios in a variety of industries. We combine our legal expertise, technology expertise, and our extensive knowledge of, and experience in, the patent licensing ecosystem, to continually uncover important patent assets and bring needed proficiency to patent licensing and enforcement.
Our partnership with patent owners is the cornerstone of our operating subsidiaries’ corporate strategy. We assume all responsibility for advancing operational expenses while pursuing a patent licensing and enforcement program, and then share net licensing revenue with our patent partners as that program matures, on a pre-arranged and negotiated basis. We may also provide upfront capital to the patent owner as an advance against future licensing revenue. We are a principal in the licensing and enforcement effort, with our operating subsidiaries obtaining control of the rights in the patent portfolio, or control of the patent portfolio outright.
Business Model and Strategy - Overview
We have the flexibility to structure arrangements in a number of ways to address the needs and specific sets of circumstances presented by each of our unique patent partners, examples of which include the following:
Partnering Model:
| |
| • | 50/50 net profit sharing of revenue after legal costs and other licensing and enforcement costs. Profit sharing percentages can vary. |
| |
| • | Typical partners include major corporations, research labs and universities and individual inventors. |
| |
| • | Upon return of advanced costs, net profit revenue share with patent partner commences. |
Hybrid Partnering Model:
| |
| • | Hybrid Partnership with up-front capital infusion to our patent partners as an advance on future licensing revenue streams. |
| |
| • | Increases our total addressable market providing an advantage over competitors. |
| |
| • | Typical partners include major corporations seeking to effectively and efficiently monetize their patent portfolios. |
| |
| • | We maintain at least a 100% preferred rate of return until all deployed capital is returned. |
| |
| • | Upon return of capital infusion, net profit revenue share with patent partner commences. |
| |
| • | Target recovery of advanced capital in 18 months. |
Purchasing Model:
| |
| • | We invest in 100% of the patents for 100% of the profits, with no backed participation for the patent owner. |
| |
| • | Typical partners include distressed corporations and other corporations with limited success controlled by venture capitalists. |
| |
| • | Target recovery of advanced capital in 18 months. |
Key Elements of Business Strategy
Patent licensing and enforcement can be an effective and efficient way to maximize the profit potential of a patent. A patent license agreement grants a third-party user of an invention specific patent rights to the patented invention in exchange for patent license fees. Patent licensing is especially suitable for patents that are owned by the patent-disenfranchised. Patent disenfranchised owners of patents are those that have not successfully converted their patented invention into a profitable product or service, and therefore, are not generating revenues from their patented inventions. The patent-disenfranchised, for example, include owners of the following categories of patented inventions:
| |
| • | Inventions that were so far ahead of the technology curve that there is no existing ecosystem to support the patented products or services at the time they are introduced to market; |
| |
| • | Inventions that can only be deployed in very capital-intensive industries, such as semiconductor fabrication, energy, or medical sectors, but whose owners do not have sufficient amounts of capital to deploy; and |
| |
| • | Inventions that, for one reason or another, including the shifting of cost-effective manufacturing overseas, are no longer being practiced by the patent owner. |
Our patent licensing business provides patent holders with an opportunity to generate income from their patented inventions being practiced by third-parties without authorization. Our patent licensing and enforcement business strategy, conducted solely by our operating subsidiaries, includes three fundamental elements, as follows:
| |
| • | Patent Discovery - Discover potentially valuable patents or patent portfolios. |
| |
| • | Assessment of Economic Value - Work internally and with external experts to evaluate the use of the patented invention(s) in the relevant marketplace and assess a patents or patent portfolios’ expected economic value. |
| |
| • | Licensing and Enforcement - For unauthorized users of the patented invention, enter into license negotiations and, if necessary, litigation to monetize the patent based on its assessed value. |
Patent Discovery. The patent process breeds, encourages and sustains innovation and invention by granting a limited monopoly to the inventor in exchange for sharing the invention with the public. Certain technologies, including several of the technologies controlled by our operating subsidiaries, some of which are summarized below, become core technologies in the way products and services are manufactured, sold or delivered by companies across a wide array of industries. Our operating subsidiaries identify core, patented technologies that have been or are anticipated to be widely adopted by third-parties in connection with the manufacture, sale or use of products and services. Patent discovery occurs when we reach out to patent holders who may be disenfranchised, or when patent holders approach us seeking assistance with the monetization and enforcement of their patent portfolios.
Assessment of Economic Value. Subsequent to the patent discovery process, our executives work internally and/or with external industry experts in the specific technology field, to evaluate the patented invention and its adoption and implementation in the marketplace. There are several key factors to consider when analyzing a patent and determining a patent’s value: (1) Infringement, (2) Validity and (3) Enforceability.
| |
| • | Infringement. To determine infringement, we must first identify third-parties that are practicing the invention(s) covered by the patent without obtaining permission from the patent owner to do so. A key tool in determining whether or not a company is infringing a patent is a claim chart. A claim chart demonstrates how the manufacture, sale, or use of an existing product compares against the claims of the patent. |
| |
| • | Invalidity. The three main factors analyzed to determine invalidity are (1) anticipation, (2) obviousness, and (3) the existence of non-patentable subject matter. |
| |
| ◦ | Anticipation occurs when the claims of the patent are entirely revealed within a single piece of prior art. “Prior art” is a technical term that generally refers to an invention that existed prior to the grant of the patent being analyzed. |
| |
| ◦ | Even if the claims of the patent are not entirely revealed within a single piece of prior art, the patent may still be invalid if determined to be “obvious” under the law. “Obvious” essentially means that the |
differences between prior art and the patented invention are so slight such that they would have been obvious at the time of invention to one who is skilled in the subject matter being patented.
| |
| ◦ | Even if the patent lacks anticipation and obviousness, it may still be invalid if its subject matter is un-patentable by law. Un-patentable subject matter includes naturally occurring things, abstract concepts, or algorithms that perform an ordinary function. |
| |
| • | Enforceability. A myriad of factors are analyzed to determine whether or not a patent is enforceable, including whether or not there has been patent misuse, or whether or not there are antitrust violations associated with the patent. Due to the inherently complex nature of patent law, only a court or specific administrative body, such as the International Trade Commission, can make a decision whether a patent is infringed, valid and enforceable; however, we employ our wealth of expertise to make the best assessment possible given a specific fact pattern and set of circumstances. |
We estimate a patent’s economic value by evaluating the expected value of the license revenue stream based on past, present and future revenue of infringing products or services, and the risk that a court will disagree with our infringement, validity or enforcement assessments of the patent.
The processes and procedures employed in connection with the evaluation of a specific patent portfolio for future investment, licensing and enforcement are tailored and unique to each specific situation and can vary widely based on the specific facts and circumstances of a specific patent portfolio, such as the related technology, related industry and certain other factors. Some of the key components of our processes and procedures may include:
| |
| • | Utilizing our staff of in-house business development executives, patent attorneys, patent licensing executives, and technology engineers to conduct our tailored patent investment and evaluation processes and procedures. We may also leverage the expertise of external specialists and technology consultants. |
| |
| • | Identifying emerging growth areas where patented technologies will play a vital role in connection with the manufacture or sale of products and services. |
| |
| • | Identifying core, patented technologies that have been or are anticipated to be widely adopted by third-parties in connection with the manufacture or sale of products and services. |
| |
| • | Considering the impact of subtleties in the language of a patent, recorded interactions with the patent office, evaluating prior art and literature and considering the impact on the potential licensing and enforcement revenue that can be derived from a patent or patent portfolio. |
| |
| • | Evaluating the strength of a patent portfolio, including consideration of the types of claims and the number of claims potentially infringed by third-parties, and the results of any prior art searches or analysis, before the decision is made to allocate resources to a patent portfolio investment or an effective licensing and enforcement effort. |
| |
| • | Identifying and considering potential problem areas, if any, and determining whether potential problem areas can be overcome prior to acquiring a patent portfolio or launching an effective licensing program. |
| |
| • | Identifying potential infringers, industries within which the potential infringers exist, longevity of the patented technology, and a variety of other factors that directly impact the magnitude and potential success of a licensing and enforcement program. |
Licensing and Enforcement. The final step in the patent licensing and enforcement process is to monetize the patent by securing license agreements based on the patents estimated value. While we prefer to convince unauthorized users of our patented inventions of the value of the patented invention and secure a license agreement in a non-litigious manner, many infringers refuse to take such licenses even when confronted with substantial and persuasive evidence of infringement, validity, enforceability and significant economic value. As a result, often we must resort to litigation to demonstrate and prove infringement and ultimately induce infringers to take a license. We have found it effective to negotiate licenses concurrently with litigation due to the fact that litigation necessitates and facilitates an information exchange that helps both sides assess the value of a patent and make informed decisions. Also, litigation eventually leads to a court’s judgment. When a court agrees with our assessment of a patent, this judgment stops recalcitrant infringers from indefinitely profiting from the patent they are infringing.
Our operating subsidiaries engage highly competent and experienced patent lawyers to prosecute their patent portfolio litigation. It is imperative to be persistent and patient throughout the litigation process as it typically takes 18-36 months from the filing date of a lawsuit to yield a license agreement from a potential licensee. Often, it takes longer to secure a final court judgment.
Patent license negotiations and litigation initiated by our operating subsidiaries usually lead to serious and thoughtful discussions with the unauthorized users of the patented inventions. The result can be quite favorable with the user being granted rights under the patents for the patented invention in its products and services in exchange for financial remuneration. This remuneration is typically shared between our operating subsidiary and the patent holder.
Patent Prosecution. Concurrent with our patent litigation and licensing negotiation activities, we often assist patent holders with the acquisition of additional rights associated with their inventions both in the United States and across the globe. This is referred to as “continued prosecution,” and is done to further define the boundaries of an invention. It can also be effective to correct technical deficiencies discovered within a patent that may have been identified in the negotiation and litigation process. These deficiencies, if not appropriately addressed, can limit the value of patents that are otherwise infringed, valid, enforceable.
Our specialists, along with third-party experts that we engage, are trained and skilled in the areas of patent discovery, assessment of a patent or patent portfolios expected economic value, acquiring additional patent rights via continued patent prosecution, and patent licensing and enforcement. In applying our legal and technology expertise to high quality patent assets, we bridge the gap between invention and application, facilitating efficiency and delivering monetary rewards to the patent disenfranchised and other patent owners with whom we partner.
Patented Technologies
Currently, on a consolidated basis, our operating subsidiaries own or control the rights to patent portfolios with future patent expiration dates ranging from 2015 to approximately 2033, covering technologies used in a wide variety of industries, a sample of which includes the following:
|
| | |
| Operating Subsidiary | Industry | Description |
| 3 Degrees, LLC | Social Networking | Patents relate to professional and social media networking technology. |
| 3D Design Solutions, LLC | Software | Patents relating to Computer-Aided Design Technology. |
| Adaptix, Inc. | Telecommunications / Smartphones | Portfolio relates to air interface technology used in modern 4G wireless networks. The patents relate to both infrastructure and user equipment. |
| American Vehicular Sciences, LLC | Transportation And Automotive | Patents from Automotive Technologies International, or ATI and Intelligent Technologies International, or ITI, relating to numerous automotive safety, navigation and diagnostics technologies. |
| Body Science, LLC | Peripheral Vascular Devices | Patents relating to apparatus for use in wireless physiological monitoring. |
| Bonutti Skeletal Innovations, LLC | Orthopedic Implants And Sports Medicine Market | Issued and pending patents and applications in the orthopedic field covering, among other things, suture anchors, biologics, total knee replacements, total hip replacements, minimally invasive surgery, partial knee and hip replacement, spinal implants, and surgical instruments and methods of use. |
| Brandywine Communications Technologies, LLC | Communications | Patents related to Broadband Communications Technology. |
| Brilliant Optical Solutions, LLC | Semiconductor/MEMS | Patent relates to Core Fiber Optic Network Architectures. |
| Cell and Network Selection, LLC | Telecommunications / Smartphones | Patent family generally relates to LTE user equipment (phones, tablets, dongles). |
| Cellular Communications Equipment, LLC | Telecommunications / Smartphones | Portfolio covers Wireless Infrastructure and User Equipment Technology relating to second (2G), third (3G) and fourth (4G) generation wireless technologies and to air interface technology used in 2G, 3G and 4G wireless networks. |
| CeraMedic, LLC | Medical | U.S. patent plus foreign patent relating to Ceramic Hip Replacement technology. |
| Computer Software Protection, LLC | Software | Patent for Software Activation Technology, which generally relates to preventing software from running on unlicensed systems. |
| Data Engine Technologies, LLC | Software | Patent portfolio covering a wide range of Software Technology. |
| Delaware Display Group, LLC | Transportation And Automotive | Portfolio relates to certain display technologies used in smartphones, tablets, computers, HDTVs and other devices. |
| Dynamic 3D Geosolutions, LLC | Software | Patent related to Geological Interpretation and Modeling Technology. |
| Dynamic Transmissions Tech, LLC | Wireless Communications | Patent portfolios relating to on line collaboration, data networking, cellular communications and digital cameras. |
|
| | |
| Endotach, LLC | Peripheral Vascular Devices | Patents relating to stent grafts. |
| EVM Systems, LLC | Peripheral Vascular Devices | Patents and applications covering a series of medical instruments utilizing a slotted, shape memory tube. |
| GT Gaming, LLC | Internet/Ecommerce/Business Methods | Patent relating to Online Gaming Technology. |
| In-Depth Test, LLC | Semiconductor/MEMS | Patent portfolio relating to Semiconductor Testing Technology. |
| Industrial Print Technologies, LLC | Computers/Peripherals/Printers | Patent portfolio covering ink jet printer and ink jet printing technologies and other printer and printing technologies. |
| Innovative Display Technologies, LLC | Telecommunications / Smartphones | Portfolio generally relates to back-lighting for displays and the patented technology covers various improvements to LCD displays. |
| Labyrinth Optical Technologies, LLC | Communications | Patents relating to Optical Networking Technology. |
| Lambda Optical Solutions, LLC | Communications | Patents relating to Optical Switching Technology. |
| LifePort Sciences, LLC | Peripheral Vascular Devices | Multiple patents and applications relating to, among other things, stent grafts, stent graft delivery systems and stent placement procedures. |
| LifeScreen Sciences, LLC | Peripheral Vascular Devices | Portfolio consists of multiple patents and applications relating to, among other things, vena cava filters, embolic protection and associated delivery systems. |
| LifeShield Sciences, LLC | Peripheral Vascular Devices | Portfolio consists of multiple patents and applications relating to stent grafts, and stent graft delivery systems. |
| Light Transformation Technologies, LLC | Energy/Lighting | Patents relating to Improved Lighting Technology. |
| Mobile Enhancement Solutions, LLC | Telecommunications / Smartphones | This portfolio relates to enhanced mobile communications and covers many features found in smartphones today. |
| Nexus Display Technologies, LLC | Consumer Electronics | Patent portfolio relating to high speed digital display interface technology used in industry standards such as DisplayPort and DisplayPort-related technologies and also MIPI DSI.
|
| Online News Link, LLC | Internet/Ecommerce/Business Methods | Patents relate to embedded links in on-line newsletters. |
| Optimum Content Protection, LLC and Super Interconnect Technologies, LLC | Telecommunications / Smartphones | Portfolios relate to high speed circuit interconnect, display control technology and content security used in consumer electronics, PCs and mobile devices such as smartphones, tablets, and laptops. |
| Parthenon Unified Memory Architecture, LLC | Semiconductor | Patents relate to the use of shared memory in multimedia processing systems such as mobile phones, tablets and other consumer electronic devices. |
| Progressive Semiconductor Solutions, LLC | Semiconductor/MEMS | Patent portfolio covering Microprocessor and Memory Technology. |
| Promethean Insulation Technology, LLC | Energy/Lighting | Patent relates to insulation material used in building construction. |
| Saint Lawrence Communications, LLC | Wireless | Patents relating to Speech Codecs used in Wireless and Wireline Systems. |
| Signal Enhancement Technologies, LLC | Telecommunications / Smartphones | Portfolio covers radio frequency modulation technology used in mobile devices such as smartphones, tablets, and laptops from a major technology company. |
| Smartphone Technologies, LLC | Telecommunications / Smartphones | Portfolio includes patents from Palmsource and Geoworks, amongst others, that resulted from the merging of personal digital assistants and cell phones, a space in which Palm was the undisputed leader. Specifically, the patents are directed towards various interface and synchronization technologies which are used on modern smartphones today. |
| Super Resolution Technologies, LLC | Imaging And Diagnostics | Portfolio comprises U.S. and foreign patents relating to super resolution microscopy, also referred to as nanoscopy. |
| Unified Messaging Solutions, LLC | Communications | Patent for Messaging Technology. |
| Wireless Mobile Devices, LLC | Telecommunications / Smartphones | Portfolio includes patents that cover a wide range of wireless services such as Location Based Services technology and navigation that can be found on all smartphones today. |
Revenues for the periods presented include revenues generated from several of the portfolios summarized above and other technology patent portfolios owned or controlled by us. Refer to Item 7. “Management’s Discussion and Analysis of
Financial Condition and Results of Operations - Overview” for a summary of patent portfolios generating revenues for the applicable periods presented.
Competition
We expect to encounter increased competition in the area of patent portfolio investments and enforcement. This includes an increase in the number of competitors seeking to invest in the same or similar patents and technologies that we may seek to invest in. Non-practicing entities such as RPX, AST, Intellectual Ventures, Wi-LAN, Conversant, Round Rock Research LLC, IPvalue Management Inc., Vringo Inc. and Pendrell Corporation compete in acquiring rights to patents, and we expect more entities to enter the market.
We also compete with financial firms, corporate buyers and others acquiring IP. Many of these competitors may have more financial and human resources than our operating subsidiaries. As we become more successful, we may find more companies entering the market for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
Companies or other entities may develop competing technologies that offer better or less expensive alternatives to our patented technologies that we may invest in and license. Many potential competitors may have significantly greater resources than the resources that our operating subsidiaries possess. Such technological advances or entirely different approaches developed by one or more of our competitors could render certain of the technologies owned or controlled by our operating subsidiaries obsolete and/or uneconomical.
Employees
As of December 31, 2014, on a consolidated basis, we had 57 full-time employees. Neither we, nor any of our subsidiaries, are a party to any collective bargaining agreement. We consider our employee relations to be good.
ITEM 1A. RISK FACTORS
The following is a summary of certain risks we face in our business. They are not the only risks we face. Additional risks that we do not yet know of or that we currently believe are immaterial may also impair our business operations. If any of the following risks actually occur, our business, financial condition and results of operations could be materially adversely affected, and the trading price of our common stock could decline significantly. All patent portfolio investments, development, licensing and enforcement activities are conducted solely by certain of our wholly and majority-owned operating subsidiaries.
Risks Related to Our Business
We have a history of losses and may incur additional losses in the future.
We reported a net loss of $66.0 million and $56.4 million for the years ended December 31, 2014 and 2013 and net income of $59.5 million for the year ended December 31, 2012, and on a cumulative basis, we have sustained substantial losses since our inception. As of December 31, 2014, our accumulated deficit was $128.1 million. As of December 31, 2014, we had approximately $193.0 million in cash and cash equivalents and short-term investments and working capital of $172.8 million. We expect to continue incurring significant legal, marketing and general and administrative expenses in connection with our operations. As a result, we anticipate that we may incur losses in the future. We believe, however, that our current cash and cash equivalents and investments will be sufficient to finance our anticipated capital and operating requirements for at least the next twelve months.
If we encounter unforeseen difficulties with our business or operations in the future that require us to obtain additional working capital, and we cannot obtain additional working capital on favorable terms, or at all, our business may suffer.
Our consolidated cash and cash equivalents and short-term investments totaled $193.0 million and $256.7 million at December 31, 2014 and 2013, respectively. To date, we have relied primarily upon net cash flows from our operations and from the public and private sale of equity securities to generate the working capital needed to finance our operations.
We may encounter unforeseen difficulties with our business or operations in the future that may deplete our capital resources more rapidly than anticipated. As a result, we may be required to obtain additional working capital in the future through bank credit facilities, public or private debt or equity financings, or otherwise. If we are required to raise additional
working capital in the future, such financing may be unavailable to us on favorable terms, if at all, or may be dilutive to our existing stockholders. If we fail to obtain additional working capital, as and when needed, such failure could have a material adverse impact on our business, results of operations and financial condition.
Failure to effectively manage our growth could place strains on our managerial, operational and financial resources and could adversely affect our business and operating results.
Our growth has placed, and is expected to continue to place, a strain on our managerial, operational and financial resources and systems. Further, as our subsidiary companies’ businesses grow, we will be required to continue to manage multiple relationships. Any further growth by us or our subsidiary companies, or an increase in the number of our strategic relationships, may place additional strain on our managerial, operational and financial resources and systems. Although we may not grow as we expect, if we fail to manage our growth effectively or to develop and expand our managerial, operational and financial resources and systems, our business and financial results will be materially harmed.
Our future success depends on our ability to expand our organization to match the growth of our subsidiaries.
As our operating subsidiaries grow, the administrative demands upon us and our operating subsidiaries will grow, and our success will depend upon our ability to meet those demands. These demands include increased accounting, management, legal services, staff support, and general office services. We may need to hire additional qualified personnel to meet these demands, the cost and quality of which is dependent in part upon market factors outside of our control. Further, we will need to effectively manage the training and growth of our staff to maintain an efficient and effective workforce, and our failure to do so could adversely affect our business and operating results.
Potential patent portfolio investments may present risks, and we may be unable to achieve the financial or other goals intended at the time of any potential investment.
Our future growth depends, in part, on our ability to invest in patented technologies, patent portfolios, or companies holding such patented technologies and patent portfolios. Accordingly, we have engaged in patent portfolio investments to expand our patent portfolios and we intend to continue to explore such investments. Such investments are subject to numerous risks, including the following:
| |
| • | our inability to enter into a definitive agreement with respect to any potential patent portfolio investment, or if we are able to enter into such agreement, our inability to consummate the potential investment transaction; |
| |
| • | difficulty integrating the operations, technology and personnel of the acquired entity; |
| |
| • | our inability to achieve the anticipated financial and other benefits of the specific patent portfolio investment; |
| |
| • | our inability to retain key personnel from the acquired company, if necessary; |
| |
| • | difficulty in maintaining controls, procedures and policies during the transition and integration process; |
| |
| • | diversion of our management’s attention from other business concerns; and |
| |
| • | failure of our due diligence process to identify significant issues, including issues with respect to patented technologies and patent portfolios, and other legal and financial contingencies. |
If we are unable to manage these risks effectively as part of any patent portfolio investment, our business could be adversely affected.
Our revenues are unpredictable, and this may harm our financial condition.
From January 2005 to the present, our operating subsidiaries have executed our business strategy of partnering with inventors and patent owners, applying our legal and technology expertise to patent assets to unlock the financial value in their patented inventions. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to multiple patent portfolios which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries. These patent portfolio investments continue to expand and diversify our revenue generating opportunities. We believe that our cash and cash equivalents and short-term investment balances, anticipated cash flow from operations, proceeds from prior offerings of our common stock (refer to “Liquidity and Capital Resources” below) and other external sources of
available credit, will be sufficient to meet our cash requirements through at least March 2016 and for the foreseeable future. However, due to the nature of our licensing business and uncertainties regarding the amount and timing of the receipt of license and other fees from potential infringers, stemming primarily from uncertainties regarding the outcome of enforcement actions, rates of adoption of our patented technologies, the growth rates of our existing licensees and certain other factors, our revenues may vary significantly from quarter to quarter and period to period, which could make our business difficult to manage, adversely affect our business and operating results, cause our quarterly and periodic results to fall below market expectations and adversely affect the market price of our common stock.
Our operating subsidiaries depend upon relationships with others to provide technology-based opportunities that can develop into profitable royalty-bearing licenses, and if they are unable to maintain and generate new relationships, then they may not be able to sustain existing levels of revenue or increase revenue.
Neither we nor our operating subsidiaries invent new technologies or products; rather, we depend upon the identification and investment in new patents and inventions through our relationships with inventors, universities, research institutions, technology companies and others. If our operating subsidiaries are unable to maintain those relationships and to continue to grow new relationships, then they may not be able to identify new technology-based opportunities for sustainable revenue and growth.
Our current or future relationships may not provide the volume or quality of technologies necessary to sustain our business. In some cases, universities and other technology sources may compete against us as they seek to develop and commercialize technologies. Universities may receive financing for basic research in exchange for the exclusive right to commercialize resulting inventions. These and other strategies may reduce the number of technology sources and potential clients to whom we can market our services. If we are unable to maintain current relationships and sources of technology or to secure new relationships and sources of technology, such inability may have a material adverse effect on our operating results and financial condition.
The success of our operating subsidiaries depends in part upon their ability to retain the best legal counsel to represent them in patent enforcement litigation.
The success of our licensing business depends upon our operating subsidiaries’ ability to retain the best legal counsel to prosecute patent infringement litigation. As our operating subsidiaries’ patent enforcement actions increase, it will become more difficult to find the best legal counsel to handle all of our cases because many of the best law firms may have a conflict of interest that prevents their representation of our subsidiaries.
We spend a significant amount of our financial and management resources to pursue our current litigation matters. We believe that these litigation matters and others that we may in the future determine to pursue could continue for years and continue to consume significant financial and management resources. The counterparties to our litigation are sometimes large, well-financed companies with substantially greater resources than us. We cannot assure that any of our current or future litigation matters will result in a favorable outcome for us. In addition, in part due to the appeals process and other legal processes, even if we obtain favorable interim rulings or verdicts in particular litigation matters, they may not be predictive of the ultimate resolution of the dispute. Also, we cannot assure that we will not be exposed to claims or sanctions against us which may be costly or impossible for us to defend. Unfavorable or adverse outcomes may result in losses, exhaustion of financial resources or other adverse effects which could encumber our ability to develop and commercialize products.
In connection with any of our patent enforcement actions, it is possible that a defendant may request and/or a court may rule that we have violated statutory authority, regulatory authority, federal rules, local court rules, or governing standards relating to the substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against us or our operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material, and if required to be paid by us or our operating subsidiaries, could materially harm our operating results and our financial position.
Our operating subsidiaries, in certain circumstances, rely on representations, warranties and opinions made by third-parties that, if determined to be false or inaccurate, may expose us and our operating subsidiaries to certain material liabilities.
From time to time, our operating subsidiaries may rely upon representations and warranties made by third-parties from whom our operating subsidiaries acquired patents or the exclusive rights to license and enforce patents. We also may rely upon the opinions of purported experts. In certain instances, we may not have the opportunity to independently investigate and verify the facts upon which such representations, warranties, and opinions are made. By relying on these representations, warranties
and opinions, our operating subsidiaries may be exposed to liabilities in connection with the licensing and enforcement of certain patents and patent rights which could have a material adverse effect on our operating results and financial condition.
In connection with patent enforcement actions conducted by certain of our subsidiaries, a court may rule that we or our subsidiaries have violated certain statutory, regulatory, federal, local or governing rules or standards, which may expose us and our operating subsidiaries to certain material liabilities.
In connection with any of our patent enforcement actions, it is possible that a defendant may request and/or a court may rule that we have violated statutory authority, regulatory authority, federal rules, local court rules, or governing standards relating to the substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against us or our operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material, and if we or our operating subsidiaries are required to pay such monetary sanctions, attorneys’ fees and/or expenses, such payment could materially harm our operating results and our financial position.
In connection with patent enforcement actions conducted by certain of our subsidiaries, a court may find the patents invalid, not infringed or unenforceable and/or the U.S. Patent and Trademark Office, or the USPTO, or other relevant patent office, may either invalidate the patents or materially narrow the scope of their claims during the course of a reexamination, opposition or other such proceeding.
Patent litigation is inherently risky and the outcome is uncertain. Some of the parties that we believe infringe on our patents are large and well-financed companies with substantially greater resources than ours. We believe that these parties would devote a substantial amount of resources in an attempt to avoid or limit a finding that they are liable for infringing on our patents or, in the event liability is found, to avoid or limit the amount of associated damages. In addition, there is a risk that these parties may file reexaminations or other proceedings with the USPTO or other government agencies in the United States or abroad in an attempt to invalidate, narrow the scope or render unenforceable the patents we own or control. If this were to occur, it may have a material adverse effect on the viability of our company and our operations.
In addition, it is difficult to predict the outcome of patent enforcement litigation at any level. In the United States, there is a higher rate of appeals in patent enforcement litigation than standard business litigation. The defendant to any case we bring, may file as many appeals as allowed by right, including to the first, second and/or final courts of appeal (in the United States those courts would be the Federal Circuit and Supreme Court, respectively). Such appeals are expensive and time-consuming, and the outcomes of such appeals are sometimes unpredictable, resulting in increased costs and reduced or delayed revenue.
Our licensing cycle is lengthy and costly, and our marketing, legal and sales efforts may be unsuccessful.
We expect our operating subsidiaries to incur significant marketing, legal and sales expenses prior to entering into license agreements and generating license revenues. We will also spend considerable resources educating prospective licensees on the benefits of a license arrangement with us. As such, we may incur significant losses in any particular period before any associated revenue stream begins.
If our efforts to educate prospective licensees on the benefits of a license arrangement are unsuccessful, we may need to pursue litigation or other enforcement action to protect our patent rights. We may also need to litigate to enforce the terms of our existing license agreements, protect our trade secrets, or determine the validity and scope of the proprietary rights of others. Enforcement proceedings are typically protracted and complex. The costs are typically substantial, and the outcomes are unpredictable. Enforcement actions will divert our managerial, technical, legal and financial resources from business operations.
Risks Related to Our Industry
Our exposure to uncontrollable outside influences, including new legislation, court rulings or actions by the United States Patent and Trademark Office, could adversely affect our licensing and enforcement business and results of operations.
Our licensing and enforcement business is subject to numerous risks from outside influences, including the following:
New legislation, regulations or rules related to obtaining patents or enforcing patents could significantly increase our operating costs and decrease our revenue.
Our operating subsidiaries invest in patents with enforcement opportunities and spend a significant amount of resources to enforce those patents. If new legislation, regulations or rules are implemented by Congress, the USPTO or the courts that impact the patent application process, the patent enforcement process or the rights of patent holders, such changes could negatively affect our business. Recently, United States patent laws were amended with the enactment of the Leahy-Smith America Invents Act, or the America Invents Act, which took effect on March 16, 2013. The America Invents Act includes a number of significant changes to U.S. patent law. In general, the legislation attempts to address issues surrounding the enforceability of patents and the increase in patent litigation by, among other things, establishing new procedures for patent litigation. For example, the America Invents Act changes the way that parties may be joined in patent infringement actions, increasing the likelihood that such actions will need to be brought against individual allegedly-infringing parties by their respective individual actions or activities. In addition, the America Invents Act enacted a new inter-partes review process at the USPTO which can be, and often is, used by defendants, and other individuals and entities, to separately challenge the validity of any patent. At this time, it is not clear what, if any, overall impact the America Invents Act will have on the operation of our enforcement business. However, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the enforcement of our patented technologies, which could have a material adverse effect on our business and financial condition.
The U.S. Department of Justice, or the DOJ, has conducted reviews of the patent system to evaluate the impact of patent assertion entities on industries in which those patents relate. It is possible that the findings and recommendations of the DOJ could impact the ability to effectively license and enforce standards-essential patents and could increase the uncertainties and costs surrounding the enforcement of any such patented technologies. Also, in 2014, the Federal Trade Commission, or FTC, initiated a study under Section 6(b) of the Federal Trade Commission Act to evaluate the patent assertion practice and market impact of Patent Assertion Entities, or PAEs. The FTC’s initial notice and request for public comment relating to the PAE study appeared in the Federal Register on October 3, 2013. Acacia Research Corporation received and responded to a request for information as part of this FTC study. It is expected that the results of the PAE study by the FTC will be provided to Congress and other agencies, such as the DOJ, who could take action, including legislative proposals, based on the results of the study.
Finally, new rules regarding the burden of proof in patent enforcement actions could significantly increase the cost of our enforcement actions, and new standards or limitations on liability for patent infringement could negatively impact our revenue derived from such enforcement actions. In addition, recent federal court decisions have lowered the threshold for obtaining attorneys’ fees in patent infringement cases and increased the level of deference given to a district court’s fee-shifting determination. These decisions may make it easier for district courts to shift a prevailing party’s attorneys' fees to a non-prevailing party if the district court believes that the case was weak or conducted in an abusive manner. As a result, defendants in patent infringement actions brought by non-practicing entities may elect not to settle because these decisions make it much easier for defendants to get attorneys’ fees.
Changes in patent law could adversely impact our business.
Patent laws may continue to change, and may alter the historically consistent protections afforded to owners of patent rights. Such changes may not be advantageous for us and may make it more difficult to obtain adequate patent protection to enforce our patents against infringing parties. Increased focus on the growing number of patent-related lawsuits may result in legislative changes which increase our costs and related risks of asserting patent enforcement actions. For instance, the United States Congress is considering a bill that would require, among other things, non-practicing entities that bring patent infringement lawsuits to pay legal costs of the defendants, if the lawsuits are unsuccessful and certain standards are not met.
Trial judges and juries often find it difficult to understand complex patent enforcement litigation, and as a result, we may need to appeal adverse decisions by lower courts in order to successfully enforce our patents.
It is difficult to predict the outcome of patent enforcement litigation at the trial level. It is often difficult for juries and trial judges to understand complex, patented technologies, and as a result, there is a higher rate of successful appeals in patent enforcement litigation than more standard business litigation. Such appeals are expensive and time consuming, resulting in increased costs and delayed revenue. Although we diligently pursue enforcement litigation, we cannot predict with significant reliability the decisions made by juries and trial courts.
More patent applications are filed each year resulting in longer delays in getting patents issued by the USPTO.
Certain of our operating subsidiaries hold and continue to invest in pending patents. We have identified a trend of increasing patent applications each year, which we believe is resulting in longer delays in obtaining approval of pending patent applications. The application delays could cause delays in recognizing revenue from these patents and could cause us to miss opportunities to license patents before other competing technologies are developed or introduced into the market.
Federal courts are becoming more crowded, and as a result, patent enforcement litigation is taking longer.
Our patent enforcement actions are almost exclusively prosecuted in federal court. Federal trial courts that hear our patent enforcement actions also hear criminal cases. Criminal cases always take priority over our actions. As a result, it is difficult to predict the length of time it will take to complete an enforcement action. Moreover, we believe there is a trend in increasing numbers of civil lawsuits and criminal proceedings before federal judges and, as a result, we believe that the risk of delays in our patent enforcement actions will have a greater effect on our business in the future unless this trend changes.
Any reductions in the funding of the USPTO could have an adverse impact on the cost of processing pending patent applications and the value of those pending patent applications.
The assets of our operating subsidiaries consist of patent portfolios, including pending patent applications before the USPTO. The value of our patent portfolios is dependent upon the issuance of patents in a timely manner, and any reductions in the funding of the USPTO could negatively impact the value of our assets. Further, reductions in funding from Congress could result in higher patent application filing and maintenance fees charged by the USPTO, causing an unexpected increase in our expenses.
Competition is intense in the industries in which our subsidiaries do business and as a result, we may not be able to grow or maintain our market share for our technologies and patents.
We expect to encounter competition in the area of patent portfolio investments and enforcement as the number of companies entering this market is increasing. This includes competitors seeking to invest in the same or similar patents and technologies that we may seek to invest in. Entities including RPX, AST, Intellectual Ventures, Wi-LAN, Conversant, Round Rock Research LLC, IPvalue Management Inc., Vringo Inc. and Pendrell Corporation compete in acquiring rights to patents, and we expect more entities to enter the market. As new technological advances occur, many of our patented technologies may become obsolete before they are completely monetized. If we are unable to replace obsolete technologies with more technologically advanced patented technologies, then this obsolescence could have a negative effect on our ability to generate future revenues.
Our licensing business also competes with venture capital firms and various industry leaders for patent licensing opportunities. Many of these competitors may have more financial and human resources than we do. As we become more successful, we may find more companies entering the market for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
Our patented technologies face uncertain market value.
Our operating subsidiaries have invested in patents and technologies that may be in the early stages of adoption in the commercial and consumer markets. Demand for some of these technologies is untested and is subject to fluctuation based upon the rate at which our licensees will adopt our patents and technologies in their products and services.
Further, significant judgment is required in connection with estimates of the recoverability of the carrying value of our intangible patent assets, including estimates of market values, estimates of the amount and timing of future cash flows, and estimates of other factors that are used to determine the fair value and recoverability of the respective patent asset values. Developments with respect to ongoing patent litigation, patent challenges and re-exams, legislative and judicial decisions and other factors outside of our control, may unfavorably impact the validity, applicability, and enforceability of our patent assets, and therefore, negatively impact the future value of our patent portfolios. If certain of these unfavorable events occur, our estimates or related projections may change materially in future periods, and future intangible asset impairment tests may result in material charges to earnings.
As patent enforcement litigation becomes more prevalent, it may become more difficult for us to voluntarily license our patents.
We believe that the more prevalent patent enforcement actions become, the more difficult it will be for us to voluntarily license our patents. As a result, we may need to increase the number of our patent enforcement actions to cause infringing companies to license the patent or pay damages for lost royalties. This may increase the risks associated with an investment in our company.
The markets served by our operating subsidiaries are subject to rapid technological change, and if our operating subsidiaries are unable to develop and invest in new technologies and patents, our ability to generate revenues could be substantially impaired.
The markets served by our operating subsidiaries and their licensees frequently undergo transitions in which products rapidly incorporate new features and performance standards on an industry-wide basis. Products for communications applications and high-speed computing applications, as well as other applications covered by our operating subsidiaries’ intellectual property, are based on continually evolving industry standards. In addition, the communications industry is intensely competitive and has been impacted by price erosion, rapid technological change, short product life cycles, cyclical market patterns and increasing foreign and domestic competition. Our ability to compete in the future will depend on our ability to identify and ensure compliance with evolving industry standards. This will require our continued efforts and success in acquiring new patent portfolios with licensing and enforcement opportunities. While we expect for the foreseeable future to have sufficient liquidity and capital resources to maintain the level of patent portfolio investments necessary to keep pace with these technological advances, various factors may require us to have greater liquidity and capital resources than we currently expect. If we are unable to invest in new patented technologies and patent portfolios, or to identify and ensure compliance with evolving industry standards, our ability to generate revenues could be substantially impaired and our business and financial condition could be materially harmed.
Uncertainty in global economic conditions could negatively affect our business, results of operations and financial condition.
Our revenue-generating opportunities depend on the use of our patented technologies by existing and prospective licensees, the overall demand for the products and services of our licensees, and on the overall economic and financial health of our licensees. Although economic conditions appear to be improving, recent uncertainties in global economic conditions have resulted in the tightening of the credit markets, a low level of liquidity in many financial markets, and extreme volatility in the credit, equity and fixed income markets. If economic conditions do not continue to improve, or if they further deteriorate, many of our licensees’ customers, which may rely on credit financing, may delay or reduce their purchases of our licensees’ products and services. In addition, the use or adoption of our patented technologies is often based on current and forecasted demand for our licensees’ products and services in the marketplace and may require companies to make significant initial commitments of capital and other resources. If negative conditions in the global credit markets delay or prevent our licensees’ and their customers’ access to credit, overall consumer spending on the products and services of our licensees may decrease and the adoption or use of our patented technologies may slow, respectively. Further, if the markets in which our licensees’ participate do not continue to improve, or deteriorate further, this could negatively impact our licensees’ long-term sales and revenue generation, margins and operating expenses, which could in turn have an adverse effect on our business, results of operations and financial condition.
In addition, we have significant patent-related intangible assets recorded on our consolidated balance sheets. We will continue to evaluate the recoverability of the carrying amount of our patent-related intangible assets on an ongoing basis, and we may incur substantial impairment charges, which would adversely affect our consolidated financial results. There can be no assurance that the outcome of such reviews in the future will not result in substantial impairment charges. Impairment assessment inherently involves judgment as to assumptions about expected future cash flows and the impact of market conditions on those assumptions. Future events and changing market conditions may impact our assumptions as to prices, costs, holding periods or other factors that may result in changes in our estimates of future cash flows. Although we believe the assumptions we used in testing for impairment are reasonable, significant changes in any one of our assumptions could produce a significantly different result.
Risks Related to Our Common Stock
The availability of shares for sale in the future could reduce the market price of our common stock.
In the future, we may issue securities to raise cash for operations and patent portfolio investments. We may also pay for interests in additional subsidiary companies by using shares of our common stock or a combination of cash and shares of our common stock. We may also issue securities convertible into our common stock. Any of these events may dilute stockholders’ ownership interests in our company and have an adverse impact on the price of our common stock.
In addition, sales of a substantial amount of our common stock in the public market, or the perception that these sales may occur, could reduce the market price of our common stock. This could also impair our ability to raise additional capital through the sale of our securities.
Delaware law and our charter documents contain provisions that could discourage or prevent a potential takeover of our company that might otherwise result in our stockholders receiving a premium over the market price of their shares.
Provisions of Delaware law and our certificate of incorporation and bylaws could make the acquisition of our company by means of a tender offer, proxy contest or otherwise, and the removal of incumbent officers and directors, more difficult. These provisions include:
| |
| • | Section 203 of the Delaware General Corporation Law, which prohibits a merger with a 15%-or-greater stockholder, such as a party that has completed a successful tender offer, until three years after that party became a 15%-or-greater stockholder; |
| |
| • | amendment of our bylaws by the stockholders requires a two-thirds approval of the outstanding shares; |
| |
| • | the authorization in our certificate of incorporation of undesignated preferred stock, which could be issued without stockholder approval in a manner designed to prevent or discourage a takeover; |
| |
| • | provisions in our bylaws eliminating stockholders’ rights to call a special meeting of stockholders, which could make it more difficult for stockholders to wage a proxy contest for control of our board of directors or to vote to repeal any of the anti-takeover provisions contained in our certificate of incorporation and bylaws; and |
| |
| • | the division of our board of directors into three classes with staggered terms for each class, which could make it more difficult for an outsider to gain control of our board of directors. |
Together, these provisions may make the removal of management more difficult and may discourage transactions that could otherwise involve payment of a premium over prevailing market prices for our common stock.
We may fail to meet market expectations because of fluctuations in quarterly operating results, which could cause the price of our common stock to decline.
Our reported revenues and operating results have fluctuated in the past and may continue to fluctuate significantly from quarter to quarter in the future. It is possible that in future periods, revenues could fall below the expectations of securities analysts or investors, which could cause the market price of our common stock to decline. The following are among the factors that could cause our operating results to fluctuate significantly from period to period:
| |
| • | the dollar amount of agreements executed in each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee; |
| |
| • | the specific terms and conditions of agreements executed in each period and the periods of infringement contemplated by the respective payments; |
| |
| • | fluctuations in the total number of agreements executed; |
| |
| • | fluctuations in the sales results or other royalty-per-unit activities of our licensees that impact the calculation of license fees due; |
| |
| • | the timing of the receipt of periodic license fee payments and/or reports from licensees; |
| |
| • | fluctuations in the net number of active licensees period to period; |
| |
| • | costs related to investments, alliances, licenses and other efforts to expand our operations; |
| |
| • | the timing of payments under the terms of any customer or license agreements into which our operating subsidiaries may enter; |
| |
| • | expenses related to, and the timing and results of, patent filings and other enforcement proceedings relating to intellectual property rights, as more fully described in this section; and |
| |
| • | new litigation or developments in current litigation and the unpredictability of litigation results or settlements or appeals. |
Technology company stock prices are especially volatile, and this volatility may depress the price of our common stock.
The stock market has experienced significant price and volume fluctuations, and the market prices of technology companies have been highly volatile. We believe that various factors may cause the market price of our common stock to fluctuate, perhaps substantially, including, among others, the following:
| |
| • | announcements of developments in our patent enforcement actions; |
| |
| • | developments or disputes concerning our patents; |
| |
| • | our or our competitors’ technological innovations; |
| |
| • | developments in relationships with licensees; |
| |
| • | variations in our quarterly operating results; |
| |
| • | our failure to meet or exceed securities analysts’ expectations of our financial results; |
| |
| • | a change in financial estimates or securities analysts’ recommendations; |
| |
| • | changes in management’s or securities analysts’ estimates of our financial performance; |
| |
| • | changes in market valuations of similar companies; |
| |
| • | the current sovereign debt crises affecting several countries in the European Union and concerns about sovereign debt of the United States; |
| |
| • | announcements by us or our competitors of significant contracts, investments, strategic partnerships, joint ventures, capital commitments, new technologies, or patents; and |
| |
| • | failure to complete significant transactions. |
For example, the NASDAQ-100 Technology Sector Index (NDXT) had a range of $1,834.00 - $2,426.26 during the 52-weeks ended December 31, 2014 and the NASDAQ Composite Index (IXIC) had a range of $3,946.03-$4,814.95 over the same period. Over the same period, our common stock fluctuated within a range of $13.11 - $19.93.
The recent financial crisis affecting the banking system and financial markets and the uncertainty in global economic conditions have resulted in a tightening in the credit markets, a low level of liquidity in many financial markets, and extreme volatility in the credit, equity and fixed income markets. As noted above, our stock price, like many others, has fluctuated significantly in recent periods and if investors have concerns that our business, operating results and financial condition will be negatively impacted by global economic conditions, our stock price could continue to fluctuate significantly in future periods.
In addition, we believe that fluctuations in our stock price during applicable periods can also be impacted by court rulings and/or other developments in our patent licensing and enforcement actions. Court rulings in patent enforcement actions
are often difficult to understand, even when favorable or neutral to the value of our patents and our overall business, and we believe that investors in the market may overreact, causing fluctuations in our stock prices that may not accurately reflect the impact of court rulings on our business operations and assets.
In the past, companies that have experienced volatility in the market price of their stock have been the objects of securities class action litigation. If our common stock was the object of securities class action litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could materially harm our business and financial results.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our principal executive, corporate and administrative offices are located in Newport Beach, California, where we lease approximately 17,758 square feet of office space, under a lease agreement that expires in December 2019. Our primary operating subsidiary, Acacia Research Group, LLC, and its subsidiaries, are headquartered in Plano, Texas, where we lease approximately 12,137 square feet of office space, under a lease agreement that expires in June 2020. Certain of our operating subsidiaries also maintain additional leased office space in Woodcliff Lake, New Jersey, Houston, Texas, Tokyo, Japan and Munich, Germany. We believe that our facilities are adequate, suitable and of sufficient capacity to support our immediate needs.
ITEM 3. LEGAL PROCEEDINGS
In the ordinary course of business, we are the subject of, or party to, various pending or threatened legal actions, including various counterclaims in connection with our patent enforcement activities. We believe that any liability arising from these actions will not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
In connection with any of our patent enforcement actions, it is possible that a defendant may request and/or a court may rule that we have violated statutory authority, regulatory authority, federal rules, local court rules, or governing standards relating to the substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against us or our operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material, and if required to be paid by us or our operating subsidiaries, could materially harm our operating results and our financial position.
Our operating subsidiaries are often required to engage in litigation to enforce their patents and patent rights. Certain of our operating subsidiaries are parties to ongoing patent enforcement related litigation, alleging infringement by third-parties of certain of the patented technologies owned or controlled by our operating subsidiaries.
ITEM 4. MINE SAFETY DISCLOSURES
None.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
General
Our common stock trades on The NASDAQ Global Select Market under the symbol “ACTG.”
Price Range of Common Stock
The high and low sales prices for our common stock as reported by The NASDAQ Global Select Market for the periods indicated are shown in the table below. Such prices are inter-dealer prices without retail markups, markdowns or commissions and may not necessarily represent actual transactions.
|
| | | | | | | | | | | | | | | | |
| | | 2014 | | 2013 |
| | | Fourth Quarter | | Third Quarter | | Second Quarter | | First Quarter | | Fourth Quarter | | Third Quarter | | Second Quarter | | First Quarter |
| | | | | | | | | | | | | | | | | |
| High | | $19.93 | | $18.74 | | $18.29 | | $16.46 | | $23.21 | | $25.74 | | $30.74 | | $32.59 |
| Low | | $13.93 | | $14.65 | | $14.68 | | $13.11 | | $12.23 | | $21.26 | | $20.37 | | $24.52 |
Dividend Policy
On April 23, 2013, we announced that our Board of Directors approved the adoption of a cash dividend policy that calls for the payment of an expected total annual cash dividend of $0.50 per common share, payable in the amount of $0.125 per share per quarter. Under the policy, we paid quarterly cash dividends totaling $25.0 million and $18.6 million during 2014 and 2013, respectively. In addition, on February 19, 2015, we announced that our Board of Directors approved a quarterly cash dividend payable in the amount of $0.125 per share, which will be paid on March 30, 2015 to stockholders of record at close of business on March 2, 2015. While we paid dividends to holders of our common stock on a quarterly basis during fiscal year 2014 and 2013, the declaration and payment of future dividends will depend on many factors, including, but not limited to, our earnings and financial condition, and any future dividends will be made solely at the discretion of our Board of Directors.
Holders of Common Stock
On February 25, 2015, there were approximately 112 owners of record of our common stock. The majority of the outstanding shares of our common stock are held by a nominee holder on behalf of an indeterminable number of ultimate beneficial owners.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
On November 16, 2012, we announced that our Board of Directors authorized a program for repurchases of shares of our outstanding common stock. Under the stock repurchase program, effective November 16, 2012, we were authorized to purchase in the aggregate up to $100 million of our common stock through the period ended August 15, 2013.
On November 15, 2013, our Board of Directors authorized a program for repurchases of shares of our outstanding common stock. We were authorized to purchase in the aggregate up to $70 million of our outstanding common stock through the period ending May 14, 2014. Repurchases may be made from time to time by us in the open market or in block purchases in compliance with applicable SEC rules. The following are our monthly stock repurchases for the periods presented, all of which were purchased as part of publicly announced plans or programs:
|
| | | | | | | | | | | |
| | Total Number of Shares Purchased | Average Price paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Approximate Dollar Value of Shares that May Yet be Purchased under the Plans or Programs | Plan Expiration |
| | | | | | |
| Plan Announced November 2012 | | | | | |
| November 16, 2012 - November 30, 2012 | 256,262 |
| $ | 21.58 |
| 256,262 |
| $ | — |
| August 15, 2013 |
| December 1, 2012 - December 31, 2012 | 873,146 |
| $ | 24.26 |
| 873,146 |
| $ | — |
| August 15, 2013 |
| Totals for 2012 | 1,129,408 |
| | 1,129,408 |
| | |
| | | | | | |
| Plan Announced November 2013 | | | | | |
| December 4, 2013 - December 11, 2013 | 600,000 |
| $ | 13.18 |
| 600,000 |
| $ | — |
| May 14, 2014 |
| Totals for 2013 | 600,000 |
| | 600,000 |
| | |
The repurchases were made using existing cash resources and occurred in the open market.
Stock Price Performance Graph
The following stock price performance graph shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities under that Section and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act.
The Stock Performance Graph depicted below compares the yearly change in our cumulative total stockholder return for the last five fiscal years with the cumulative total return of The NASDAQ Stock Market (U.S.) Composite Index and the NASDAQ-100 Technology Sector Index.
|
| | | | | | | | | | |
| | | 2010 | | 2011 | | 2012 | | 2013 | | 2014 |
| | | | | | | | | | | |
| Acacia Research Corporation common stock | | $285 | | $401 | | $282 | | $160 | | $186 |
| Nasdaq Composite Index (IXIC) | | $117 | | $115 | | $133 | | $184 | | $209 |
| NASDAQ-100 Technology Sector Index (NDXT) | | $122 | | $114 | | $123 | | $168 | | $208 |
The graph covers the period from December 31, 2009 to December 31, 2014. Cumulative total returns are calculated assuming that $100 was invested on December 31, 2009, in our common stock, in the NASDAQ Composite Index, and in the NASDAQ-100 Technology Sector Index, and that all dividends, if any, were reinvested. Stockholder returns over the indicated period should not be considered indicative of future stock prices or stockholder returns.
ITEM 6. SELECTED FINANCIAL DATA
The consolidated selected balance sheet data as of December 31, 2014 and 2013 and the consolidated selected statements of operations data for the years ended December 31, 2014, 2013 and 2012 set forth below have been derived from our audited consolidated financial statements included elsewhere herein, and should be read in conjunction with those financial statements (including notes thereto). The consolidated selected balance sheet data as of December 31, 2012, 2011 and 2010 and the consolidated selected statements of operations data for the years ended December 31, 2011 and 2010 have been derived from audited consolidated financial statements not included herein, but which were previously filed with the SEC.
Consolidated Statements of Operations Data
(In thousands, except share and per share data)
|
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2014 | | 2013 | | 2012 | | 2011 | | 2010 |
| | | | | | | | | | | |
Revenues and other operating income(1) | | $ | 130,876 |
| | $ | 130,556 |
| | $ | 250,727 |
| | $ | 184,707 |
| | $ | 131,829 |
|
Inventor royalties and contingent legal fees expense(1) | | 44,233 |
| | 54,508 |
| | 50,679 |
| | 91,669 |
| | 45,198 |
|
| Litigation and licensing expenses - patents | | 37,614 |
| | 39,335 |
| | 21,591 |
| | 13,005 |
| | 13,891 |
|
| Amortization of patents | | 57,242 |
| | 53,658 |
| | 39,019 |
| | 9,745 |
| | 6,931 |
|
| Marketing, general and administrative expenses (excluding non-cash stock compensation expense) | | 30,439 |
| | 31,335 |
| | 28,426 |
| | 22,114 |
| | 17,946 |
|
| Non-cash stock compensation expense (included in MG&A in the statements of operations) | | 18,115 |
| | 27,894 |
| | 25,657 |
| | 13,579 |
| | 7,121 |
|
| Research, consulting and other expenses - business development | | 3,840 |
| | 3,251 |
| | 4,943 |
| | 4,338 |
| | 2,121 |
|
| Other | | 1,548 |
| | 3,506 |
| | — |
| | — |
| | — |
|
| Operating income (loss) | | $ | (62,155 | ) | | $ | (82,931 | ) | | $ | 80,412 |
| | $ | 30,257 |
| | $ | 38,621 |
|
| | | | | | | | | | | |
| Income (loss) from continuing operations before (provision for) benefit from income taxes | | $ | (62,750 | ) | | $ | (80,800 | ) | | $ | 81,349 |
| | $ | 30,353 |
| | $ | 38,756 |
|
| (Provision for) benefit from income taxes | | (3,912 | ) | | 21,958 |
| | (22,060 | ) | | (8,708 | ) | | (1,740 | ) |
| Net income (loss) from continuing operations including noncontrolling interests in operating subsidiaries | | $ | (66,662 | ) | | $ | (58,842 | ) | | $ | 59,289 |
| | $ | 21,645 |
| | $ | 37,016 |
|
| Net income (loss) attributable to Acacia Research Corporation | | $ | (66,029 | ) | | $ | (56,434 | ) | | $ | 59,453 |
| | $ | 21,106 |
| | $ | 34,051 |
|
| | | | | | | | | | | |
| Diluted income (loss) per common share | | $ | (1.37 | ) | | $ | (1.18 | ) | | $ | 1.21 |
| | $ | 0.50 |
| | $ | 0.95 |
|
| Cash dividends declared per common share | | $ | 0.500 |
| | $ | 0.375 |
| | $ | — |
| | $ | — |
| | $ | — |
|
Consolidated Balance Sheet Data (In thousands)
|
| | | | | | | | | | | | | | | | | | | | |
| | | At December 31, |
| | | 2014 | | 2013 | | 2012 | | 2011 | | 2010 |
| | | | | | | | | | | |
| Cash and cash equivalents and investments | | $ | 193,024 |
| | $ | 256,702 |
| | $ | 311,279 |
| | $ | 323,286 |
| | $ | 104,516 |
|
| Patents, net of accumulated amortization | | 286,636 |
| | 288,432 |
| | 313,529 |
| | 25,188 |
| | 19,803 |
|
| Total assets | | 536,348 |
| | 593,393 |
| | 668,717 |
| | 352,877 |
| | 134,784 |
|
| Total liabilities | | 47,300 |
| | 31,195 |
| | 50,239 |
| | 30,765 |
| | 20,931 |
|
| Noncontrolling interests in operating subsidiaries | | 5,491 |
| | 6,488 |
| | 6,976 |
| | 2,163 |
| | 2,982 |
|
| Acacia Research Corporation stockholders’ equity | | 483,557 |
| | 555,710 |
| | 611,502 |
| | 319,949 |
| | 110,871 |
|
__________________________________
(1) Includes verdict insurance proceeds and related costs reflected separately in the statement of operations for the year ended December 31, 2011.
Factors Affecting Comparability:
| |
| • | As of December 31, 2011, we maintained a full valuation allowance against our net deferred tax assets. The net deferred tax liability resulting from the acquisition of ADAPTIX, Inc., or ADAPTIX, in January 2012 created an additional source of income to utilize against the majority of our existing consolidated net deferred tax assets. In addition, we estimated that certain of our other foreign tax credit and state tax related deferred tax assets were more likely than not realizable in |
future periods. Accordingly, the valuation allowance on the majority of our net deferred tax assets was released, resulting in a financial statement income tax benefit of $10.7 million during the year ended December 31, 2012. At December 31, 2013, we recorded a partial valuation allowance for certain tax attribute carryforwards and other deferred tax assets totaling $7.6 million, due to uncertainty regarding future realizability. We recorded a full valuation allowance for net deferred tax assets generated during fiscal year 2014, due to uncertainty regarding future realizability.
| |
| • | For the years ended December 31, 2014, 2013, 2012, 2011 and 2010, we paid patent related investment costs totaling $42.7 million, $25.1 million, $178.3 million (excluding the investment in ADAPTIX of $150.0 million), $14.7 million and $8.2 million, respectively. Patent related investment costs are amortized using the straight-line method over the estimated economic useful life of the underlying patents. |
| |
| • | ADAPTIX, Inc. Acquisition. In January 2012, we acquired ADAPTIX, a pioneer in the development of 4G technologies for wireless systems, for $150.0 million, net of cash acquired, as described below and at Note 7 to the consolidated financial statements elsewhere herein. Under the acquisition method of accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangible assets and liabilities assumed, based on their estimated fair market values on the date of acquisition. Amounts attributable to the patents acquired, totaling $150.0 million, are being amortized using the straight-line method over an estimated weighted average economic useful life of the underlying patents, which was estimated to be approximately 10 years. |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our consolidated financial statements included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors including the risks we discuss in Item 1A, “Risk Factors,” and elsewhere herein.
General
Our operating subsidiaries invest in, license and enforce patented technologies. Our operating subsidiaries partner with inventors and patent owners, applying our legal and technology expertise to patent assets to unlock the financial value in their patented inventions. We are an intermediary in the patent marketplace, bridging the gap between invention and application, facilitating efficiency and delivering monetary rewards to patent owners.
Our operating subsidiaries generate revenues and related cash flows from the granting of patent rights for the use of patented technologies that our operating subsidiaries control or own. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, where necessary, with the enforcement against unauthorized users of their patented technologies through the filing of patent infringement litigation.
We are a leader in licensing patented technologies and have established a proven track record of licensing success with over 1,430 license agreements executed to date, across 181 of our patent portfolio licensing and enforcement programs. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to multiple patent portfolios, which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries. To date, we have generated gross licensing revenue of approximately $1.1 billion, and have returned more than $665 million to our patent partners.
The patent portfolio investment, development, licensing and enforcement business conducted by our operating subsidiaries is described more fully in Item 1. “Business,” of this annual report.
Executive Overview
During the periods presented, we continued our business of empowering patent owners and rewarding invention by providing a path to patent monetization for the people and companies who have contributed valuable patented inventions to an industry, but who require a professional, experienced independent third-party licensing partner to get rewarded for those inventions. These people and companies are our customers, and in many cases, components of the patent disenfranchised. In so doing, we have placed ourselves at the forefront of an emerging secondary market in patent assets under which holders of high quality patents, including the patent disenfranchised, may be rewarded for the use of their patented inventions by others, even if they do not have the capital and expertise to engage in lengthy, costly and risky patent litigation.
Our operating activities for the periods presented were principally focused on the continued investment in and development of our patent licensing and enforcement business, including the continued pursuit of our ongoing patent licensing and enforcement programs and the commencement of new patent licensing and enforcement programs. In addition, we continued our focus on business development, including the investment in several additional high quality patent portfolios by certain of our operating subsidiaries and the continued pursuit of additional opportunities to partner with patent owners and invest in patent portfolios, and continue our industry leading patent licensing and enforcement activities.
We have strategically chosen to shift the focus of our operating business to increasingly serve a smaller number of customers each having higher quality patent portfolios. High quality patent portfolios are typically associated with higher numbers of varied defensible claims, higher revenue potential, originating from high-pedigreed patent owners and/or possessing a relatively large number of prospective licensees. In this regard, commencing in the later portion of 2013 and early 2014, we have continued the shift in our focus at our point of patent intake, from quantity to quality. As we have continued to shift our focus as described above, we continue to see a growing need for our services, which we believe will maintain the strength of our high quality patent portfolio intake pipeline.
We continue to identify and explore opportunities for partnering with companies in the technology, energy, medical technology and other sectors for the licensing and enforcement of their high quality patented technologies, and are also
expanding our activity in international markets, both of which we expect will expand and diversify our future revenue generating opportunities.
Operating activities during the periods presented included the following: |
| | | | | | | | | | | |
| | 2014 | | 2013 | | 2012 |
| | | | | | |
| Revenues (in thousands) | $ | 130,876 |
| | $ | 130,556 |
| | $ | 250,727 |
|
| New agreements executed | 88 |
| | 120 |
| | 138 |
|
| Licensing and enforcement programs generating revenues - during the respective period | 46 |
| | 53 |
| | 68 |
|
| Licensing and enforcement programs with initial revenues | 15 |
| | 23 |
| | 31 |
|
| New patent portfolios | 6 |
| | 25 |
| | 55 |
|
| Cumulative number of licensing and enforcement programs generating revenues - inception to date | 181 |
| | 166 |
| | 143 |
|
We measure and assess the performance and growth of the patent licensing and enforcement businesses conducted by our operating subsidiaries based on consolidated revenues (including other operating income) recognized across all of our patent licensing and enforcement programs on a trailing twelve-month basis. Trailing twelve-month revenues during the periods presented were as follows (in thousands, except percentage change values): |
| | | | | | | |
| As of Date: | | Trailing Twelve -Month Revenues | | % Change |
| | | | | |
| December 31, 2014 | | $ | 130,876 |
| | 14 | % |
| September 30, 2014 | | 114,911 |
| | 23 | % |
| June 30, 2014 | | 93,239 |
| | 41 | % |
| March 31, 2014 | | 66,273 |
| | (49 | )% |
| December 31, 2013 | | 130,556 |
| | (48 | )% |
| December 31, 2012 | | 250,727 |
| | — |
|
Our revenues historically have fluctuated period to period, and can vary significantly, based on a number of factors including the following:
| |
| • | the dollar amount of agreements executed each period, which can be driven by the nature and characteristics of the technology or technologies being licensed and the magnitude of infringement associated with a specific licensee; |
| |
| • | the specific terms and conditions of agreements executed each period including the nature and characteristics of rights granted, and the periods of infringement or term of use contemplated by the respective payments; |
| |
| • | fluctuations in the total number of agreements executed each period; |
| |
| • | the number of, timing, results and uncertainties associated with patent licensing negotiations, mediations, patent infringement actions, trial dates and other enforcement proceedings relating to our patent licensing and enforcement programs; |
| |
| • | the relative maturity of licensing programs during the applicable periods; |
| |
| • | other external factors, including the periodic status or results of ongoing negotiations, the status or results of ongoing litigations and appeals, actual or perceived shifts in the regulatory environment, impact of unrelated patent related judicial proceedings and other macroeconomic factors; and |
| |
| • | historically, based on the merits and strength of our operating subsidiary’s patent infringement claims and other factors, many prospective licensees have elected to settle significant patent infringement cases and pay reasonable license fees for the use of our patented technology, as those patent infringement cases approached a court determined trial date. |
Management does not attempt to manage for smooth sequential periodic growth in revenues period to period, and therefore, periodic results can be uneven. Unlike most operating businesses and industries, licensing revenues not generated in a current period are not necessarily foregone but, most likely, depending on whether negotiations, litigation or both continue into subsequent periods, and depending on a number of other factors, such potential revenues may be pushed into subsequent fiscal periods.
Summary of Results of Operations - For Fiscal Years 2014, 2013 and 2012
(In thousands, except percentage change values)
|
| | | | | | | | | | | | | | | | | |
| | Fiscal Year | | % Change |
| | 2014 | | 2013 | | 2012 | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | | | | | | | | | |
| Revenues | $ | 130,876 |
| | $ | 130,556 |
| | $ | 250,727 |
| | — | % | | (48 | )% |
| Inventor royalties and contingent legal fees | 44,233 |
| | 54,508 |
| | 50,679 |
| | (19 | )% | | 8 | % |
| Litigation and licensing expenses - patents | 37,614 |
| | 39,335 |
| | 21,591 |
| | (4 | )% | | 82 | % |
| Amortization expense | 57,242 |
| | 53,658 |
| | 39,019 |
| | 7 | % | | 38 | % |
Other operating costs and expenses(1) | 53,942 |
| | 65,986 |
| | 59,026 |
| | (18 | )% | | 12 | % |
| Operating income (loss) | (62,155 | ) | | (82,931 | ) | | 80,412 |
| | (25 | )% | | (203 | )% |
| Benefit from (provision for) income taxes | (3,912 | ) | | 21,958 |
| | (22,060 | ) | | (118 | )% | | (200 | )% |
Net loss (income) attributable to noncontrolling interests(2) | 633 |
| | 2,408 |
| | 164 |
| | (74 | )% | | * |
|
| Net income (loss) attributable to Acacia Research Corporation | (66,029 | ) | | (56,434 | ) | | 59,453 |
| | 17 | % | | (195 | )% |
* Percentage change in excess of 300%
(1) Includes non-cash stock compensation charges of $18.1 million, $27.9 million and $25.7 million in fiscal years 2014, 2013 and 2012, respectively, included in Marketing, general and administrative expense in the statements of operations.
(2) Refer to Note 1 to the notes to consolidated financial statements included elsewhere in this annual report for additional information.
Overview - Fiscal year 2014 compared with Fiscal Year 2013
| |
| • | Revenues were relatively flat in fiscal year 2014, as compared to fiscal year 2013. |
| |
| • | Inventor royalties and contingent legal fees, on a combined basis, decreased $10.3 million, or 19%, due primarily to a greater percentage of revenues generated in fiscal year 2014 having no inventor royalty obligations, and lower average inventor royalty rates, as compared to the portfolios generating revenues in fiscal year 2013. |
| |
| • | Litigation and licensing expenses-patents decreased $1.7 million, or 4%, to $37.6 million, due primarily to a net decrease in litigation support and third-party technical consulting expenses associated with ongoing and new licensing and enforcement programs commenced during fiscal year 2014. |
| |
| • | Patent amortization increased $3.6 million, or 7%, to $57.2 million, due primarily to an increase in amortization expense related to new patent portfolio investments during 2014 and accelerated patent amortization for patent portfolio dispositions, partially offset by a decrease in accelerated patent amortization related to patent portfolio impairment charges during fiscal year 2014. |
| |
| • | Other Operating Expenses: |
| |
| ◦ | Marketing, general and administrative expenses decreased $10.7 million, or 18%, to $48.6 million, due primarily to a net decrease in personnel costs in connection with the net reduction in headcount during 2014, a decrease in other non-recurring personnel severance costs including the impact of non-recurring cash and non-cash charges associated with the board approved CEO retirement package in 2013, and a net decrease in non-cash stock compensation expense. |
| |
| ◦ | Fiscal year 2014 operating expenses included an expense accrual for court determined attorney fees related to matters initiated in 2010 and 2011 totaling $1.5 million. The respective operating subsidiaries have filed notices of appeal. Fiscal year 2013 operating expenses included a one-time, non-recurring charge related to the resolution of a dispute concerning legal fees associated with a prior matter totaling $3.5 million |
| |
| • | Tax expense for fiscal year 2014 reflects the impact of a full valuation allowance recorded against our net deferred tax assets generated during the period, including a benefit from the reversal of the net deferred tax liability of $1,735,000 at the beginning of the year. As such, no tax benefit was recognized for net operating loss and foreign tax credit related tax benefits generated during the 2014 periods. Tax expense for fiscal year 2014 primarily reflects foreign taxes withheld on |
revenue agreements with licensees in foreign jurisdictions and other state taxes. See below for discussion of 2013 tax benefit.
Overview - Fiscal year 2013 compared with Fiscal Year 2012
| |
| • | Revenues decreased $120.2 million, or 48%, due primarily to a decrease in the average revenue per executed agreement and a decrease in the total number of agreements executed in fiscal year 2013. |
| |
| • | Inventor royalties and contingent legal fees, on a combined basis, increased $3.8 million, or 8%, as compared to the 48% decrease in related revenues for the same periods, due primarily to a greater percentage of revenues generated in fiscal year 2012 having no inventor royalty or contingent legal fee arrangement obligations, and lower average inventor royalty and contingent legal fee rates, as compared to the portfolios generating revenues in fiscal year 2013. |
| |
| • | Litigation and licensing expenses-patents increased $17.7 million, or 82%, to $39.3 million, due primarily to an increase in international enforcement costs, an increase in strategic patent portfolio prosecution costs, and a net increase in litigation support and third-party technical consulting expenses associated with ongoing and new licensing and enforcement programs commenced during 2013. |
| |
| • | Patent amortization increased $14.6 million, or 38%, to $53.7 million, due primarily to amortization expense related to new patent portfolio investments during the fourth quarter of 2012 and a net increase in accelerated patent amortization related to patent portfolio impairment charges totaling $4.6 million and other dispositions during fiscal year 2013, partially offset by a decrease in accelerated patent amortization related to recoupable up-front patent portfolio investment costs recovered during fiscal year 2013. |
| |
| • | Other Operating Expenses: |
| |
| ◦ | Marketing, general and administrative expenses increased $5.1 million, or 10%, to $59.2 million, due primarily to a net increase in personnel costs in connection with the enhancement of our business development, licensing and engineering teams, an increase in other non-recurring personnel severance costs including the impact of non-recurring cash and non-cash charges associated with Paul Ryan’s retirement severance package, approved by the board of directors, and a net increase in corporate legal, facilities, general and administrative costs, partially offset by a decrease in variable performance-based compensation costs. |
| |
| ◦ | Operating expenses for fiscal year 2013 included a one-time, non-recurring charge related to the resolution of a dispute concerning legal fees associated with a prior matter totaling $3.5 million. |
| |
| • | We recorded a pre-tax net loss and a tax benefit for fiscal year 2013, compared to pre-tax net income and tax expense for fiscal year 2012, as shown above. Our effective tax rate was 27% for fiscal years 2013 and 2012, respectively. The fiscal year 2013 effective tax benefit rate was lower than the U.S. Federal statutory rate primarily due to an increase in the valuation allowance related to foreign tax credits generated in 2013 and certain permanent nondeductible items. The fiscal year 2012 effective tax rate was lower than the U.S. federal statutory rate primarily due to $10.7 million of tax benefits recognized resulting from the release of valuation allowance on the majority of our net deferred tax assets in the first quarter of 2012, as discussed below. |
Revenues in fiscal year 2014 included fees from the following licensing and enforcement programs:
|
| | | | |
| • | 3G & 4G Cellular Air Interface and Infrastructure technology | | • | Multi-Display Content Delivery and Data Aggregation technology(1) |
| • | 4G Wireless technology | | • | Oil and Gas Production technology (1) |
| • | Audio Communications Fraud Detection technology | | • | Online Auction Guarantee technology |
| • | Automotive Safety, Navigation and Diagnostics technology | | • | Online Gaming technology |
| • | Broadband Communications technology | | • | Online newsletters with links technology |
| • | Cardiology and Vascular Device technology(1) | | • | Optical Networking technology |
| • | Computer Aided Design Tools technology | | • | Optimized Microprocessor Operation technology(1) |
| • | Computer-Aided Design technology(1) | | • | Radio Frequency Modulation technology(1) |
| • | Core Fiber Optic Network Architectures technology | | • | Reflective and Radiant Barrier Insulation technology |
| • | Distributed Data Management & Synchronization technology(1) | | • | Semiconductor Packaging technology |
| • | DMT technology | | • | Software Activation technology |
| • | Electronic Access Control technology(1) | | • | Software Technology |
|
| | | | |
| • | Enhanced Mobile Communications technology | | • | Speech codes used in wireless and wireline systems technology(1) |
| • | Gas Modulation Control Systems technology | | • | Spinning and Jousting Toy Game technology (1) |
| • | High Speed Circuit Interconnect and Display Control technology(1) | | • | Super Resolutions Microscopy technology(1) |
| • | Improved Lighting technology | | • | Suture Anchors technology |
| • | Innovative Display technology(1) | | • | Telematics technology |
| • | Intercarrier SMS technology | | • | Video Analytics for Security technology |
| • | Interstitial and Pop-Up Internet Advertising technology | | • | Voice-Over-IP technology |
| • | Location Based Services technology | | • | Wireless Data Synchronization & Data Transfer technology |
| • | Messaging technology | | • | Wireless Infrastructure and User Equipment technology(1) |
| • | Microprocessor and Memory technology(1) | | • | Wireless Location Based Services technology |
| • | Mobile Computer Synchronization technology | | • | Wireless Monitoring technology |
Revenues in fiscal year 2013 included fees from the following licensing and enforcement programs:
|
| | | | |
| • | 3G & 4G Wireless technology(1) | | • | Memory Circuit and Packaging technology(1) |
| • | Audio Communications Fraud Detection technology | | • | Messaging technology |
| • | Automotive Safety, Navigation and Diagnostics technology | | • | Mobile Computer Synchronization technology |
| • | Broadband Communications technology(1) | | • | Mobile Enhancement Solutions technology |
| • | Business Process Modeling technology | | • | MRI technology(1) |
| • | Camera Support technology | | • | NOR Flash technology |
| • | Catheter Ablation technology(1) | | • | Online Auction Guarantees technology |
| • | Computer Aided Design Tools technology(1) | | • | Online Gaming technology |
| • | Computer Architecture and Power Management technology | | • | Online newsletters with links technology |
| • | Core Fiber Optic Network Architectures technology(1) | | • | Optical Networking technology(1) |
| • | Digital Imaging technology(1) | | • | Power Management within Integrated Circuits technology |
| • | Digital Signal Processing Architecture technology | | • | Prescription Lens technology(1) |
| • | DMT® technology | | • | Reflective and Radiant Barrier Insulation technology(1) |
| • | Domain Name Redirection technology | | • | Semiconductor Memory and Process technology(1) |
| • | Dynamic Transmissions technology(1) | | • | Semiconductor Packaging technology(1) |
| • | Electronic spreadsheet, data analysis and software development technology(1) | | • | Software Activation technology |
| • | Facilities Operation Management System technology | | • | Surgical Access technology |
| • | Gas Modulation Control Systems technology(1) | | • | Suture Anchors technology |
| • | Greeting Card technology(1) | | • | Telematics technology |
| • | Improved Memory Manufacturing technology | | • | User Programmable Engine Control technology |
| • | Information Portal Software technology | | • | Video Analytics for Security technology |
| • | Information Storage, Searching & Retrieval technology | | • | Video Delivery and Processing technology |
| • | Inhaler Drug Delivery technology(1) | | • | Web Collaboration technology(1) |
| • | Intercarrier SMS technology(1) | | • | Wireless Data Synchronization & Data Transfer technology(1) |
| • | Interstitial and Pop-Up Internet Advertising technology | | • | Wireless Location Based Services technology(1) |
| • | Lighting Ballast technology | | • | X-Ray Powder Diffraction technology(1) |
| • | Location Based Services technology | | | |
Revenues in fiscal year 2012 included fees from the following licensing and enforcement programs:
|
| | | | |
| • | 4G Wireless technology(1) | | • | Messaging technology |
| • | Application Authentication technology(1) | | • | Minimally Invasive Surgery technology(1) |
| • | Audio Communications Fraud Detection technology | | • | Mobile Computer Synchronization technology |
| • | Automotive Safety, Navigation and Diagnostics technology(1) | | • | Network Monitoring technology |
| • | Bone Graft Harvesting technology(1) | | • | NOR Flash technology |
| • | Bone Spacer Devices technology(1) | | • | Online Ad Tracking technology |
| • | Bone Wedge technology(1)(2) | | • | Online Auction Guarantee technology |
| • | Camera Support technology | | • | Online Gaming technology(1) |
|
| | | | |
| • | Consumer Rewards technology(1) | | • | Optical Networking technology |
| • | Data Compression technology | | • | Optical Recording technology |
| • | DDR SDRAM technology | | • | Optical Switching technology |
| • | Digital Signal Processing Architecture technology | | • | Pop-up Internet Advertising technology |
| • | Disk Array Systems & Storage Area Network technology | | • | Power Management Within Integrated Circuits technology |
| • | DMT® technology | | • | Power-over-Ethernet technology |
| • | Document Assembly Technology for Printers(1) | | • | Radiation Therapy technology(1) |
| • | Document Generation technology | | • | Rule Based Monitoring technology |
| • | Domain Name Redirection technology(1) | | • | Semiconductor Memory and Process Patents(1) |
| • | Dynamic Random Access Memory technology(1) | | • | Shape Memory Alloys technology |
| • | Enhanced Mobile Communications technology(1) | | • | Software Activation technology(1) |
| • | Facilities Operation Management System technology | | • | Storage technology |
| • | Hearing Aid technology(1) | | • | Surgical Access technology(1) |
| • | Impact Instrument technology | | • | Suture Anchors technology(1) |
| • | Improved Anti-Trap Safety Technology for Vehicles(1) | | • | Targeted Content Delivery & Network File Transfer technology |
| • | Improved Lighting technology | | • | Telematics technology |
| • | Improved Memory Manufacturing technology(1) | | • | Unicondylar Knee Replacement technology(1) |
| • | Information Portal Software technology | | • | User Programmable Engine Control technology |
| • | Information Storage, Searching and Retrieval technology(1) | | • | Video Analytics for Security technology(1) |
| • | Integrated Access technology(1) | | • | Video Delivery and Processing technology(1) |
| • | Intraluminal Device technology(1) | | • | Video Encoding technology |
| • | Lighting Ballast technology | | • | Videoconferencing technology(1) |
| • | Location Based Services technology | | • | Visual Data Evaluation technology |
| • | Medical Image Manipulation technology(1) | | • | Voice-Over-IP Technology(1) |
| • | Medical Monitoring technology | | • | Website Crawling technology |
| • | MEMS technology | | • | Wireless Monitoring technology(1) |
_________________________________________
|
| |
(1) | Initial revenues recognized during the applicable period. |
Although revenues from one or more of our patents or patent portfolios may be significant in a specific reporting period, we believe that none of our individual patents or patent portfolios is individually significant to our licensing and enforcement business as a whole.
Patent Licensing and Enforcement
We expect patent-related legal expenses to continue to fluctuate from period to period based on the factors summarized herein, in connection with future trial dates, international enforcement, strategic patent portfolio prosecution and our current and future patent portfolio investment, prosecution, licensing and enforcement activities. The pursuit of enforcement actions in connection with our licensing and enforcement programs can involve certain risks and uncertainties, including the following:
| |
| • | Increases in patent-related legal expenses associated with patent infringement litigation, including, but not limited to, increases in costs billed by outside legal counsel for discovery, depositions, economic analyses, damages assessments, expert witnesses and other consultants, re-exam and inter partes review costs, case-related audio/video presentations and other litigation support and administrative costs could increase our operating costs and decrease our profit generating opportunities; |
| |
| • | Our patented technologies and enforcement actions are complex and, as a result, we may be required to appeal adverse decisions by trial courts in order to successfully enforce our patents; |
| |
| • | New legislation, regulations or rules related to enforcement actions, including any fee or cost shifting provisions, could significantly increase our operating costs and decrease our profit generating opportunities. Increased focus on the growing number of patent-related lawsuits may result in legislative changes which increase our costs and related risks of asserting patent enforcement actions. For instance, the United States House of Representatives |
passed a bill that would require non-practicing entities that bring patent infringement lawsuits to pay legal costs of the defendants, if the lawsuits are unsuccessful and certain standards are not met;
| |
| • | Courts may rule that our subsidiaries have violated certain statutory, regulatory, federal, local or governing rules or standards by pursuing such enforcement actions, which may expose us and our operating subsidiaries to material liabilities, which could harm our operating results and our financial position; and |
| |
| • | The complexity of negotiations and potential magnitude of exposure for potential infringers associated with higher quality patent portfolios may lead to increased intervals of time between the filing of litigation and potential revenue events (i.e. markman dates, trial dates), which may lead to increased legal expenses, consistent with the higher revenue potential of such portfolios. |
Investments in Patent Portfolios
Our operating subsidiaries intend to sustain the long term growth of our patent licensing and enforcement business through the continued identification of opportunities to partner with patent owners with high-quality patent assets, across a wide range of technology areas that have been, or are anticipated to be, widely adopted by third-parties in connection with the manufacture or sale of products and services. Going forward, we have strategically chosen to shift the focus of the company to increasingly serve a smaller number of customers each having higher quality patent portfolios. In this regard, during the later portion of 2013 and early 2014, we continued the shift in our focus at our point of patent intake, from quantity to quality.
In fiscal year 2014, we obtained control, primarily through partnering arrangements, of 6 new patent portfolios with applications over a wide range of technology areas, compared to 25 (including the acquisition of ADAPTIX) new patent portfolios, and 55 new patent portfolios in fiscal years 2013 and 2012, respectively. Patent portfolio investment costs paid in fiscal year 2014 totaled $42.7 million, compared to $25.1 million and $328.3 million (including the $150.0 million acquisition of ADAPTIX) in fiscal years 2013 and 2012, respectively. Accrued patent investment costs, to be paid in 2015, totaled $16.7 million at December 31, 2014.
Patent portfolio intake in fiscal years 2014, 2013 and 2012 were comprised of the following:
|
| | | | | | | | | | | | | | | | | | |
| | | Number of Patent Portfolios |
| | | 2014 | | % | | 2013 | | % | | 2012 | | % |
| | | | | | | | | | | | | |
| Partnering - revenue share with upfront cash advance and preferred returns | | 4 |
| | 67 | % | | 18 |
| | 72 | % | | 25 |
| | 45 | % |
| Partnering - revenue share with no upfront cash advance | | — |
| | — | % | | 4 |
| | 16 | % | | 19 |
| | 35 | % |
| Outright purchase | | 2 |
| | 33 | % | | 3 |
| | 12 | % | | 10 |
| | 18 | % |
| Acquisition of ADAPTIX, Inc. | | — |
| | — | % | | — |
| | — | % | | 1 |
| | 2 | % |
| | | 6 |
| | 100 | % | | 25 |
| | 100 | % | | 55 |
| | 100 | % |
ADAPTIX, Inc. Acquisition. In January 2012, we acquired ADAPTIX, a pioneer in the development of 4G technologies for wireless systems, for $150.0 million, net of cash acquired, as described below and at Note 7 to the consolidated financial statements elsewhere herein. With patents filed as early as 2000, ADAPTIX’s research and development efforts have resulted in one of the most significant intellectual property portfolios focused on 4G technologies. With its rapidly growing portfolio of 230 issued and pending patents in 13 countries, ADAPTIX’s innovations extend across a broad range of 4G technologies including OFDMA and MIMO.
In general, the majority of patent portfolio investment costs incurred for patent portfolios with future inventor royalty obligations are subject to contractual provisions providing for higher percentage returns to our operating subsidiaries early in the licensing and enforcement program until such initial upfront patent portfolio investment costs are fully recovered.
Fiscal year 2014 patent portfolio intake included the following:
| |
| • | In February 2014, partnered with a leading research institute to monetize the institute’s patents relating to ceramics and associated manufacturing processes for medical devices. |
| |
| • | In March 2014, invested in US patents and foreign counterparts related to the use of shared memory in |
multimedia processing systems such as mobile phones, tablets and other consumer electronic devices.
| |
| • | In April 2014, we partnered with a leading semiconductor company on patents related to high speed digital display interface technology used in industry standards such as DisplayPort and DisplayPort-related |
technologies and also MIPI DSI. DisplayPort is widely deployed in today’s PCs, laptops, tablets, monitors
while DisplayPort-related technologies and MIPI DSI are used in smart phones and other consumer
electronic devices with smaller displays.
| |
| • | In June 2014, we announced that Renesas Electronics Corporation, a premier supplier of advanced semiconductor solutions, and Acacia Research Group LLC agreed to a new phase in their strategic patent licensing alliance. Pursuant to this new agreement, we will receive broad and lengthy access to the worldwide patent portfolio of Renesas Electronics. |
| |
| • | In December 2014, sourced rights in additional patent portfolios from Nokia Networks. With these new portfolios, our subsidiary now controls high quality patent portfolios relating to 2G/3G/LTE and LTE-Advanced technologies. |
As of December 31, 2014, certain of our operating subsidiaries have entered into option agreements with third-party patent portfolio owners regarding the potential partnering and / or the investment in additional patent portfolios for future licensing and enforcement. Future patent portfolio investments will continue to expand and diversify our future revenue generating opportunities.
Critical Accounting Policies
Our consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America. In preparing these financial statements, we make assumptions, judgments and estimates that can have a significant impact on amounts reported in our consolidated financial statements. We base our assumptions, judgments and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates under different assumptions or conditions. On a regular basis, we evaluate our assumptions, judgments and estimates and make changes accordingly.
We believe that, of the significant accounting policies discussed in Note 2 to our notes to consolidated financial statements, the following accounting policies require our most difficult, subjective or complex judgments:
| |
| • | stock-based compensation expense; |
| |
| • | valuation of long-lived and intangible assets; |
| |
| • | accounting for business combinations - acquisition method of accounting; and |
| |
| • | accounting for income taxes. |
We discuss below the critical accounting assumptions, judgments and estimates associated with these policies. Historically, our assumptions, judgments and estimates relative to our critical accounting policies have not differed materially from actual results. For further information on our critical accounting policies, refer to Note 2 to the notes to consolidated financial statements included herein.
Revenue Recognition
As described below, significant management judgment must be made and used in connection with the revenue recognized in any accounting period. Material differences may result in the amount and timing of revenue recognized or deferred for any period, if management made different judgments.
Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
We make estimates and judgments when determining whether the collectibility of fees receivable from licensees is reasonably assured. We assess the collectibility of fees receivable based on a number of factors, including past transaction history and the credit-worthiness of licensees. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash for transactions where collectibility may have been an issue. Management’s estimates regarding
collectibility impact the actual revenues recognized each period and the timing of the recognition of revenues. Our assumptions and judgments regarding future collectibility could differ from actual events and thus materially impact our financial position and results of operations.
In general, our revenue arrangements provide for the payment of contractually determined fees in consideration for the grant of certain intellectual property rights for patented technologies owned or controlled by our operating subsidiaries. These rights typically include some combination of the following: (i) the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by patented technologies owned or controlled by our operating subsidiaries, (ii) a covenant-not-to-sue, (iii) the release of the licensee from certain claims, and (iv) the dismissal of any pending litigation. The intellectual property rights granted may be perpetual in nature, extending until the expiration of the related patents, or can be granted for a defined, relatively short period of time, with the licensee possessing the right to renew the agreement at the end of each contractual term for an additional minimum upfront payment. Pursuant to the terms of these agreements, our operating subsidiaries have no further obligation with respect to the grant of the non-exclusive retroactive and future licenses, covenants-not-to-sue, releases, and other deliverables, including no express or implied obligation on our operating subsidiaries’ part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the licenses, covenants-not-to-sue, releases, and other significant deliverables upon execution of the agreement, or upon receipt of the minimum upfront payment for term agreement renewals. As such, the earnings process is complete and revenue is recognized upon the execution of the agreement, when collectibility is reasonably assured, or upon receipt of the minimum upfront fee for term agreement renewals, and when all other revenue recognition criteria have been met.
Depending on the complexity of the underlying revenue arrangement and related terms and conditions, significant judgments, assumptions and estimates may be required to determine when substantial delivery of contract elements has occurred, whether any significant ongoing obligations exist subsequent to contract execution, whether amounts due are collectible and the appropriate period or periods in which, or during which, the completion of the earnings process occurs. Depending on the magnitude of specific revenue arrangements, if different judgments, assumptions and estimates are made regarding contracts executed in any specific period, our periodic financial results may be materially affected.
Our operating subsidiaries are responsible for the licensing and enforcement of their respective patented technologies and pursue third-parties that are utilizing their intellectual property without a license or who have under-reported the amount of royalties owed under a license agreement. As a result of these activities, from time to time, our operating subsidiaries may recognize revenues in a current period that relate to infringements by licensees that occurred in prior periods. These recoveries may cause revenues to be higher than expected during a particular reporting period and may not occur in subsequent periods. Differences between amounts initially recognized and amounts subsequently audited or reported as an adjustment to those amounts, are recognized in the period such adjustment is determined as a change in accounting estimate.
The economic terms of the inventor agreements, operating agreements and contingent legal fee arrangements associated with the patent portfolios owned or controlled by our operating subsidiaries, if any, including royalty rates, contingent fee rates and other terms, vary across the patent portfolios owned or controlled by our operating subsidiaries. Inventor royalties, noncontrolling interests and contingent legal fees expenses fluctuate period to period, based on the amount of revenues recognized each period, the terms and conditions of revenue agreements executed each period and the mix of specific patent portfolios with varying economic terms and obligations generating revenues each period. Inventor royalties, noncontrolling interests and contingent legal fees expenses will continue to fluctuate and may continue to vary significantly period to period, based primarily on these factors.
For fiscal years 2014, 2013 and 2012, the majority of our revenue agreements provided for the payment to us of one-time, paid-up license fees in consideration for the grant of certain intellectual property rights for patented technology rights owned by our operating subsidiaries. These rights were primarily granted on a perpetual basis, extending until the expiration of the underlying patents. Pursuant to the terms of these agreements, our operating subsidiaries have no further obligation with respect to the grant of the non-exclusive licenses, covenants-not-to-sue, releases, and other deliverables, including no express or implied obligation on our operating subsidiaries’ part to maintain or upgrade the technology, or provide future support or services. The agreements provided for the grant of the licenses, covenants-not-to-sue, releases, and other significant deliverables upon execution of the agreement. As such, the earnings process was determined to be complete and revenue was recognized upon the execution of the agreements, when all other revenue recognition criteria were met. Historically, term license agreements have not been a material component of our operating revenues, with the majority of license agreements being paid-up, perpetual license agreements.
During the year ended December 31, 2012, we entered into significant agreements with unrelated third-parties resolving pending patent matters that resulted in the grant of certain intellectual property rights and recognition of revenues, portions of which were not subject to inventor royalty and contingent legal fee arrangements, as well as the grant of licenses
from certain of our operating subsidiaries and recognition of revenues that were subject to inventor royalties and contingent legal fee arrangements. Revenues recognized subject to inventor royalties and contingent legal fees are based on a determination by the respective operating subsidiaries. Depending on the magnitude of specific revenue arrangements, if different judgments are made regarding revenues subject to inventor royalties and contingent legal fees in any specific period, our periodic financial results may be materially affected.
Stock-based Compensation Expense
Stock-based compensation payments to employees and non-employee directors are recognized as expense in the statements of operations. The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award (determined using a Black-Scholes option pricing model for stock options and intrinsic value on the date of grant for nonvested restricted stock), and is recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity award). Determining the fair value of stock-based awards at the grant date requires significant estimates and judgments, including estimating the market price volatility of our common stock, future employee stock option exercise behavior and requisite service periods.
Stock-based compensation expense is recorded only for those awards expected to vest using an estimated pre-vesting forfeiture rate. As such, we are required to estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures on compensation expense recognized. Estimates of pre-vesting forfeitures must be periodically revised in subsequent periods if actual forfeitures differ from those estimates. We consider several factors in connection with our estimate of pre-vesting forfeitures, including types of awards, employee class, and historical pre-vesting forfeiture data. The estimation of stock awards that will ultimately vest requires judgment, and to the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are revised. If actual results differ significantly from these estimates, stock-based compensation expense and our results of operations could be materially impacted. Refer to Notes 2 and 10 to our notes to consolidated financial statements included elsewhere herein.
Valuation of Long-lived and Intangible Assets Including Goodwill
We review long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Factors we consider important, which could trigger an impairment review, include the following:
| |
| • | significant underperformance relative to expected historical or projected future operating results; |
| |
| • | significant changes in the manner of our use of the assets or the strategy for our overall business; |
| |
| • | significant negative industry or economic trends; |
| |
| • | significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments; and |
| |
| • | significant decline in our stock price for a sustained period. |
If a potential impairment exists, a calculation is performed to determine the estimated fair value of the long-lived asset. This calculation is based on a valuation model, which considers the estimated future undiscounted cash flows resulting from the use of the asset, and a discount rate commensurate with the risks involved. Third party appraised values may also be used in determining whether impairment potentially exists. The estimated fair value is compared to the long-lived asset’s carrying value to determine whether impairment exists.
Goodwill is tested for impairment at the reporting unit level (operating segment or one level below an operating segment) on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value. We consider our market capitalization and the carrying value of our assets and liabilities, including goodwill, when performing goodwill impairment tests. When conducting our annual goodwill impairment assessment, we initially perform a qualitative evaluation of whether it is more likely than not that goodwill is impaired. If it is determined by a qualitative evaluation that it is more likely than not that goodwill is impaired, we then apply a two-step impairment test. The two-step impairment test first compares the fair value of our reporting unit to its carrying or book value. If the fair value of the reporting unit exceeds its carrying value, goodwill is not impaired and we are not required to perform further testing. If the carrying value of the reporting unit exceeds its fair value, we are required to determine the implied fair value of the reporting unit’s goodwill and if the carrying value of the reporting unit’s goodwill
exceeds its implied fair value, then an impairment loss equal to the difference is recorded in the consolidated statement of operations.
Fair value is generally estimated using the “Income Approach,” as described by ASC 820, “Fair Value Measurements and Disclosures,” focusing on the estimated future income-producing capability of the patent portfolios over the remaining economic useful life of the patent portfolios. The underlying premise of this approach is that the value of an asset can be measured by the present worth of the net economic benefit (cash receipts less cash outlays) to be received over the remaining life of the asset. The steps followed in applying this approach include estimating the expected after-tax cash flows attributable to the asset over its remaining life and converting these after-tax cash flows to present value through “discounting.” The discounting process contemplates an estimated rate of return that accounts for both the time value of money and investment risk factors. The cash inflows considered are comprised of an estimate of licensee fees expected to be generated over the remaining estimated economic useful life of the patent portfolio from potential future licensees. Estimated license fees are typically estimated based on a general estimated reasonable royalty rate for the applicable technology applied to estimated market share data for potential future licensees. Estimated cash outflows are based on existing contractual obligations, such as contingent legal fee and inventor royalty obligations, applied to estimated license fee revenues, in addition to other estimates of out-of-pocket expenses associated with a specific patent portfolio’s licensing and enforcement program. The analysis also contemplates consideration of current information about the patent portfolio including, status and stage of litigation, periodic results of the litigation process, strength of the patent portfolio, technology coverage and other pertinent information that could impact future net cash flows.
As described above, in assessing the recoverability of intangible assets and goodwill, significant judgment is required in connection with estimates of market values, estimates of the amount and timing of future cash flows, and estimates of other factors that are used to determine the fair value of the respective assets. If these estimates or related projections change in future periods, future intangible asset impairment tests may result in charges to earnings.
Accounting for Business Combinations - Acquisition Method of Accounting
Acquisitions are accounted for in accordance with the acquisition method of accounting under Financial Accounting Standards Board, or FASB, ASC Topic 805, “Business Combinations,” or Topic 805. Topic 805 requires, among other things, that identifiable assets acquired and liabilities assumed be recognized at their fair values as of the acquisition date. Under the acquisition method of accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangible assets and liabilities assumed, based on their estimated fair market values on the date of acquisition. Any excess purchase price after the initial allocation to identifiable net tangible and identifiable intangible assets is assigned to goodwill. Amounts attributable to patents are amortized using the straight-line method over the estimated economic useful life of the underlying patents. Acquisition accounting includes the establishment of a net deferred tax asset or liability resulting from book tax basis differences related to assets acquired and liabilities assumed on the date of acquisition.
We assess fair value for financial statement purposes using a variety of methods, including the use of present value models and may also reference independent analyses. Amounts recorded as intangible assets, including patents and patent rights, are based on assumptions and estimates, as of the date of acquisition, regarding the amount and timing of projected revenues and costs associated with the licensing and enforcement of patents and patent rights acquired, appropriate risk-adjusted discount rates, rates of technology adoption, market penetration, technological obsolescence, product launch timing, the impact of competition or lack of competition in the market place, tax implications and other factors. Also, upon acquisition, based on several of the estimates and assumptions previously described, we determine the estimated economic useful lives of the acquired intangible assets for amortization purposes.
Management is responsible for determining the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed as of the acquisition date, solely for purposes of allocating the purchase price to the assets acquired and liabilities assumed. Fair value measurements can be highly subjective, and it is possible that other professionals for other purposes, applying reasonable judgment and criteria to the same facts and circumstances, could develop and support a range of alternative estimated amounts. Actual results may vary from projected results.
Accounting for Income Taxes
As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves the estimating of our actual current tax exposure together with assessing temporary differences resulting from differing treatment of items, such as deferred revenue, amortization of intangibles and asset depreciation for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheets. We must then assess the likelihood that our
deferred tax assets will be recovered from future taxable income and to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must include an expense within the tax provision in the consolidated statements of operations.
Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and our valuation allowance. Due to uncertainties related to our ability to utilize certain deferred tax assets in future periods, we have recorded a full valuation allowance against our net deferred tax assets totaling $7.6 million as of December 31, 2014. These assets primarily consist of foreign tax credits, capital loss carryforwards and net operating loss carryforwards.
In assessing the need for a valuation allowance, management has considered both the positive and negative evidence available, including but not limited to, estimates of future taxable income and related probabilities, estimates surrounding the character of future income and the timing of realization, consideration of the period over which our deferred tax assets may be recoverable, our recent history of net income and prior history of losses, projected future outcomes, industry and market trends and the nature of existing deferred tax assets. In management’s estimate, any positive indicators, including forecasts of potential future profitability of our businesses, are outweighed by the uncertainties surrounding our estimates and judgments of potential future taxable income, primarily due to uncertainties surrounding the timing of realization of future taxable income and the character of such income in particular future periods (i.e. foreign or domestic). In the event that actual results differ from these estimates or we adjust these estimates should we believe we would be able to realize these deferred tax assets in the future, an adjustment to the valuation allowance would increase income in the period such determination was made. For example, a similar analysis was performed in the first quarter of 2012, resulting in the release of the valuation allowance on the majority of our net deferred tax assets and a related tax benefit of $10.7 million recognized in the first quarter of 2012. The release of the valuation allowance contemplated the net deferred tax liability resulting from the acquisition of ADAPTIX in January 2012, which created an additional source of income to utilize against the majority of the existing consolidated net deferred tax assets, and our estimate that certain other deferred tax assets related to foreign tax credits and other state related deferred taxes were more likely than not realizable in future periods. In 2014, based on management’s assessment, a full valuation allowance was recorded against the company’s net deferred tax assets during the period, due to uncertainty regarding future realizability of such tax assets pursuant to guidance set forth in ASC 740, “Income Taxes.” In future periods, if we determine that the company will more likely than not be able to realize certain of these amounts, the applicable portion of the benefit from the release of the valuation allowance will generally be recognized in the statement of operations in the period the determination is recorded.
Any changes in the judgments, assumptions and estimates associated with our analysis of the need for a valuation allowance in any future periods could materially impact our financial position and results of operations in the periods in which those determinations are made.
Consolidated Results of Operations
Comparison of the Results of Operations for Fiscal Years 2014, 2013 and 2012
Revenues
|
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | | 2014 | | 2013 | | 2012 | | $ Change | | % Change | | $ Change | | % Change |
| | | (in thousands, except percentage change values and number of agreements) |
| Revenues | | $ | 130,876 |
| | $ | 130,556 |
| | $ | 250,727 |
| | $ | 320 |
| | — | % | | $ | (120,171 | ) | | (48 | )% |
| New revenue agreements executed | | 88 |
| | 120 |
| | 138 |
| | | | | | | | |
| Average revenue per agreement | | $ | 1,487 |
| | $ | 1,088 |
| | $ | 1,817 |
| | | | | | | | |
A reconciliation of the change in revenues (based on average revenue per agreement) for the periods presented, in relation to the revenues reported for the comparable prior year period, is as follows:
|
| | | | | | | | |
| | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | | (in thousands) |
| Increase (decrease) in number of agreements executed | | $ | (34,815 | ) | | $ | (32,706 | ) |
| Increase (decrease) in average revenue per agreement executed | | 35,135 |
| | (87,465 | ) |
| Total | | $ | 320 |
| | $ | (120,171 | ) |
Two licensees individually accounted for 22% and 22%, respectively, of revenues recognized in fiscal year 2014, two licensees individually accounted for 38% and 16%, respectively, of revenues recognized in fiscal year 2013, and four licensees individually accounted for 21%, 14%, 10% and 10%, respectively, of revenues recognized in fiscal year 2012.
Management does not attempt to manage for smooth sequential periodic growth in revenues, and therefore, periodic results can be uneven. Unlike most operating businesses and industries, licensing revenues not generated in a current period are not necessarily foregone, but most likely, depending on whether negotiations, litigation or both continue into subsequent periods, and depending on a number of other factors, such potential revenues may be pushed into subsequent fiscal periods.
Net Income (Loss)
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | | 2014 | | 2013 | | 2012 | | $ Change | | % Change | | $ Change | | % Change |
| | | (in thousands, except percentages) |
| Net income (loss) attributable to Acacia Research Corporation | | (66,029 | ) | | (56,434 | ) | | 59,453 |
| | $ | (9,595 | ) | | 17 | % | | $ | (115,887 | ) | | (195 | )% |
A reconciliation of the change in net income (loss) for the periods presented is as follows:
|
| | | | | | | | | | | | | |
| | 2014 vs. 2013 | | % | | 2013 vs. 2012 | | % |
| | (in thousands, except percentage values) |
| Increase (decrease) in revenues | $ | 320 |
| | (3 | )% | | $ | (120,171 | ) | | 104 | % |
| (Increase) decrease in inventor royalties and contingent legal fees combined | 10,275 |
| | (107 | )% | | (3,829 | ) | | 3 | % |
| (Increase) decrease in marketing, general and administrative expenses | 10,675 |
| | (111 | )% | | (5,146 | ) | | 4 | % |
| (Increase) decrease in litigation and licensing expenses | 1,721 |
| | (18 | )% | | (17,744 | ) | | 15 | % |
| (Increase) in patent amortization expenses | (3,584 | ) | | 37 | % | | (14,639 | ) | | 13 | % |
| Change in (provision for) benefit from income taxes | (25,870 | ) | | 270 | % | | 44,018 |
| | (38 | )% |
| Other | (3,132 | ) | | 32 | % | | 1,624 |
| | (1 | )% |
| Net change in net income (loss) | $ | (9,595 | ) | | 100 | % | | $ | (115,887 | ) | | 100 | % |
Cost of Revenues
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | 2014 | | 2013 | | 2012 | | $ Change | | % Change | | $ Change | | % Change |
| | (in thousands, except percentages) |
| Inventor royalties | $ | 20,670 |
| | $ | 29,724 |
| | $ | 26,028 |
| | $ | (9,054 | ) | | (30 | )% | | $ | 3,696 |
| | 14 | % |
| Contingent legal fees | 23,563 |
| | 24,784 |
| | 24,651 |
| | (1,221 | ) | | (5 | )% | | 133 |
| | 1 | % |
| Litigation and licensing expenses - patents | 37,614 |
| | 39,335 |
| | 21,591 |
| | (1,721 | ) | | (4 | )% | | 17,744 |
| | 82 | % |
| Amortization of patents | 57,242 |
| | 53,658 |
| | 39,019 |
| | 3,584 |
| | 7 | % | | 14,639 |
| | 38 | % |
Inventor Royalties and Contingent Legal Fees Expense. The economic terms of patent partnering agreements, operating agreements and contingent legal fee arrangements, associated with the patent portfolios owned or controlled by our operating subsidiaries, if any, including royalty obligations, if any, royalty rates, contingent fee rates and other terms and conditions, vary across the patent portfolios owned or controlled by our operating subsidiaries. In certain instances, we have invested in certain patent portfolios without future inventor royalty obligations. These costs fluctuate period to period, based on the amount of revenues recognized each period, the terms and conditions of revenue agreements executed each period and the mix of specific patent portfolios with varying economic terms, conditions and obligations generating revenues each period.
A summary of the main drivers of the change in inventor royalties expense and contingent legal fees expense, in relation to the change in total revenues, for the comparable periods presented, is as follows:
|
| | | | | | | | | | | | | |
| | 2014 vs. 2013 | | % of Prior Period Balance | | 2013 vs. 2012 | | % of Prior Period Balance |
| Inventor Royalties: | (in thousands, except percentage change values) |
| Increase (decrease) in inventor royalty rates | $ | (5,044 | ) | | (16 | )% | | $ | 4,499 |
| | 17 | % |
| Increase (decrease) in total revenues | 82 |
| | — | % | | (26,382 | ) | | (101 | )% |
| Decrease (increase) in revenues without inventor royalty obligations | (4,092 | ) | | (14 | )% | | 25,579 |
| | 98 | % |
| Total change - inventor royalties expense | $ | (9,054 | ) | | (30 | )% | | $ | 3,696 |
| | 14 | % |
|
| | | | | | | | | | | | | |
| | 2014 vs. 2013 | | % of Prior Period Balance | | 2013 vs. 2012 | | % of Prior Period Balance |
| Contingent Legal Fees: | (in thousands, except percentage change values) |
| Increase (decrease) in contingent legal fee rates | $ | (601 | ) | | (2 | )% | | $ | 10,355 |
| | 42 | % |
| Increase (decrease) in total revenues | 61 |
| | — | % | | (13,463 | ) | | (55 | )% |
| Decrease (increase) in revenues without contingent legal fee obligations | (681 | ) | | (3 | )% | | 3,241 |
| | 14 | % |
| Total change - contingent legal fees | $ | (1,221 | ) | | (5 | )% | | $ | 133 |
| | 1 | % |
Certain revenue agreements with unrelated third-parties entered into during fiscal 2012 resulted in the grant of certain intellectual property rights and recognition of revenues, portions of which were not subject to inventor royalty and contingent legal fee arrangements, as well as the grant of licenses from certain of our operating subsidiaries and recognition of revenues that were subject to inventor royalties and contingent legal fee arrangements. Certain of the revenues recognized subject to inventor royalties and contingent legal fees are based on a determination by the respective operating subsidiaries.
Litigation and Licensing Expenses - Patents. Litigation and licensing expenses-patents include patent-related prosecution and enforcement costs incurred by outside patent attorneys engaged on an hourly basis and the out-of-pocket expenses incurred by law firms engaged on a contingent fee basis. Litigation and licensing expenses-patents also includes licensing and enforcement related third-party patent research, development, prosecution, consulting, and other costs incurred in connection with the licensing and enforcement of patent portfolios. Litigation and licensing expenses-patents fluctuate from period to period based on patent enforcement and prosecution activity associated with ongoing licensing and enforcement programs and the timing of the commencement of new licensing and enforcement programs in each period.
Fiscal year 2014 litigation and licensing expenses-patents decreased, as compared to fiscal year 2013, due primarily to a net decrease in litigation support and third-party technical consulting expenses associated with ongoing and new licensing and enforcement programs commenced during fiscal year 2014.
Fiscal year 2013 litigation and licensing expenses-patents increased, as compared to fiscal year 2012, due primarily to an increase in international enforcement costs, an increase in strategic patent portfolio prosecution costs, and a net increase in litigation support and third-party technical consulting expenses associated with ongoing and new licensing and enforcement programs commenced during fiscal year 2013.
We expect patent-related legal expenses to continue to fluctuate period to period as we incur increased costs related to upcoming scheduled and/or anticipated trial dates, international enforcement activities and strategic patent portfolio prosecution activities over the next several fiscal quarters, as we continue to focus on our investments in these areas.
Amortization of Patents. The change in amortization expense for the comparable periods presented was due to the following:
|
| | | | | | | |
| | 2014 vs. 2013 | | 2013 vs. 2012 |
| | (in thousands) |
| Amortization of patent portfolio investments made since the end of the prior year | $ | 2,534 |
| | $ | 1,790 |
|
| Scheduled amortization related to patent portfolios owned or controlled as of the end of the prior year | 562 |
| | 19,088 |
|
| Accelerated amortization related to recovery of upfront advances | 655 |
| | (9,982 | ) |
| Acquisition of Adaptix, Inc. | — |
| | 411 |
|
| Patent portfolio dispositions | 955 |
| | (1,287 | ) |
| Patent portfolio impairment charges | (1,122 | ) | | 4,619 |
|
| Total change in patent amortization expense | $ | 3,584 |
| | $ | 14,639 |
|
Patent portfolio impairment charges included in patent amortization expense in the statement of operations totaled $3.5 million and $4.6 million in fiscal years 2014 and 2013, respectively. The impairment charges related to partial impairment of a portfolio due to a reduction in expected estimated future net cash flows (2014 only) and the impairment of certain patent portfolios that management determined it would no longer allocate future resources to in connection with the licensing and enforcement of such portfolios, due primarily to potential prior art related complexities in two of the programs (2013 only), and/or the overall determination that future resources would be allocated to other licensing and enforcement programs with higher potential return profiles.
Operating Expenses
|
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | 2014 vs. 2013 | | 2013 vs. 2012 |
| | | 2014 | | 2013 | | 2012 | | $ Change | | % Change | | $ Change | | % Change |
| | | (in thousands, except percentages) |
| Marketing, general and administrative | | $ | 30,439 |
| | $ | 31,335 |
| | $ | 28,426 |
| | $ | (896 | ) | | (3 | )% | | $ | 2,909 |
| | 10 | % |
| Non-cash stock compensation | | 18,115 |
| | 27,894 |
| | 25,657 |
| | (9,779 | ) | | (35 | )% | | 2,237 |
| | 9 | % |
| Total marketing, general and administrative expenses | | $ | 48,554 |
| | $ | 59,229 |
| | $ | 54,083 |
| | $ | (10,675 | ) | | (18 | )% | | $ | 5,146 |
| | 10 | % |
| | | | | | | | | | | | | | | |
| Research, consulting and other expenses - business development | | 3,840 |
| | 3,251 |
| | 4,943 |
| | 589 |
| | 18 | % | | (1,692 | ) | | (34 | )% |
Marketing, General and Administrative Expenses. Marketing, general and administrative expenses include employee compensation and related personnel costs, including variable performance based compensation and non-cash stock compensation expenses, office and facilities costs, legal and accounting professional fees, public relations, marketing, stock administration, state taxes based on gross receipts and other corporate costs. A summary of the main drivers of the change in marketing, general and administrative expenses for the periods presented, is as follows (in thousands):
|
| | | | | | | |
| | 2014 vs. 2013 | | 2013 vs. 2012 |
| | (in thousands) |
| Net change in licensing, business development, engineering related personnel costs and other personnel costs | $ | (1,142 | ) | | $ | 1,715 |
|
| Variable performance-based compensation costs | 274 |
| | (4,019 | ) |
| Corporate, general and administrative costs | 1,353 |
| | 2,764 |
|
| Non-cash stock compensation expense | (7,956 | ) | | 414 |
|
| Non-recurring CEO retirement and other employee severance costs | (165 | ) | | 1,131 |
|
| Nonrecurring non-cash stock compensation - CEO retirement package | (1,823 | ) | | 1,823 |
|
| Other | (1,216 | ) | | 1,318 |
|
| Total change in marketing, general and administrative expenses | $ | (10,675 | ) | | $ | 5,146 |
|
The decrease in non-cash stock compensation expense, excluding the impact of the CEO retirement package, was due primarily to a decrease in the average grant date fair value of the shares expensed in fiscal year 2014, and a decrease in the number of shares expensed resulting from a net reduction in employee headcount and a decrease in the number of shares vesting for current employees. Refer to Note 10 to the consolidated financial statements elsewhere herein.
Research, Consulting and Other Expenses - Business Development. Research, consulting and other expenses include third-party business development related research, development, consulting, and other costs incurred in connection with business development activities. These costs fluctuate period to period based on business development related activities in each period.
Other Operating Expenses
During 2014, a federal court held that a lawsuit initiated in 2010 was exceptional. Additionally, in a separate matter, a federal court held that a lawsuit initiated in 2011 was exceptional. The total amount requested by these two defendants in these two matters was $2.8 million. The respective operating subsidiaries have filed notices of appeal. Operating expenses for fiscal 2014 included an expense accrual for these matters totaling $1.5 million. Operating expenses for fiscal 2013 included a one-time, non-recurring charge related to the resolution of a dispute concerning legal fees associated with a prior matter totaling $3.5 million.
Income Taxes
|
| | | | | | | | | | | |
| | 2014 | | 2013 | | 2012 |
| (Provision for) benefit from income taxes (in thousands) | $ | (3,912 | ) | | $ | 21,958 |
| | $ | (22,060 | ) |
| Effective tax rate | 6 | % | | (27 | )% | | (27 | )% |
Fiscal Year 2014. Our effective tax rate for fiscal year 2014 was mainly comprised of foreign and state income taxes, a full valuation allowance recorded against the company’s net deferred tax assets generated in 2014, the reversal of the net deferred tax liability at the beginning of the year totaling $1.7 million and certain nondeductible permanent items. The foreign taxes withheld are related to revenue agreements executed with third-party licensees domiciled in certain foreign jurisdictions totaling $5.2 million.
Fiscal Year 2013. Our effective tax rate for fiscal year 2013 was mainly comprised of U.S. federal and state income tax benefits and nondeductible permanent expenses. The benefit for income taxes for fiscal year 2013 reflects realization of net operating loss carryforward related tax benefits generated during the period. In 2013, the rate at which we recorded the tax benefit associated with the pretax loss for the period was reduced from the statutory rate primarily due to certain nondeductible permanent items and expired capital loss carryforwards. In fiscal year 2013, we recorded a valuation allowance against foreign tax credits generated in fiscal 2013 totaling $4.6 million.
Fiscal Year 2012. We generated pretax income in 2012 resulting in tax expense for the period. The fiscal year 2012 effective tax rate was lower than the U.S. federal statutory rate primarily due to $10.7 million of tax benefits recognized resulting from the release of valuation allowance on the majority of our net deferred tax assets in the first quarter of 2012, as discussed at Note 9 to the consolidated financial statements contained elsewhere herein.
Fiscal year 2012 tax expense included noncash tax expense calculated as a result of excluding excess tax benefits related to the exercise and vesting of equity-based incentive awards from the calculation of tax expense for financial reporting purposes, totaling approximately $13.2 million. This amount was credited to additional paid-in capital, not taxes payable, as the expense does not reflect cash taxes payable. Fiscal year 2012 tax expense included foreign withholding taxes, totaling $11.9 million.
In general, foreign taxes withheld may be claimed as a deduction on future U.S. corporate income tax returns, or as a credit against future U.S. income tax liabilities, subject to certain limitations. At December 31, 2014 and 2013, we established a full valuation allowance against foreign tax credit related deferred tax assets generated in the periods, due to uncertainty regarding future realizability. The tax provision for fiscal year 2012 provides for the utilization of the foreign taxes withheld as a credit against fiscal year 2012 income tax expense calculated for financial statement purposes.
Inflation
Inflation has not had a significant impact on us or any of our subsidiaries in the current or prior periods.
Liquidity and Capital Resources
General
Our primary sources of liquidity are cash, cash equivalents and investments on hand generated from our operating activities and proceeds from recent equity financings. Refer to “Cash Flows from Financing Activities” below for information
regarding recent equity financings. We retain broad discretion over the use of the net proceeds from recent equity offerings and intend to use the net proceeds for operations and for other general corporate purposes, including, but not limited to, working capital, strategic investments and other transactions.
Our management believes that our cash and cash equivalent balances, investments, and anticipated cash flow from operations, will be sufficient to meet our cash requirements through at least March 2016 and for the foreseeable future. We may, however, encounter unforeseen difficulties that may deplete our capital resources more rapidly than anticipated, including those set forth under Item 1A, “Risk Factors”, above. Any efforts to seek additional funding could be made through issuances of equity or debt, or other external financing. However, additional funding may not be available on favorable terms, if at all. The capital and credit markets have experienced extreme volatility and disruption since late 2007 and the volatility and impact of the disruption has continued into 2014. At times during this period, the volatility and disruption has reached unprecedented levels. In several cases, the markets have exerted downward pressure on stock prices and credit capacity for certain issuers, and there can be no assurance that the commercial paper markets will be a reliable source of short-term financing for us. If we fail to obtain additional funding when needed, we may not be able to execute our business plans and our business, conducted by our operating subsidiaries, may suffer.
Certain of our operating subsidiaries are often required to engage in litigation to enforce their patents and patent rights. In connection with any of our operating subsidiaries’ patent enforcement actions, it is possible that a defendant may request and/or a court may rule that an operating subsidiary has violated statutory authority, regulatory authority, federal rules, local court rules, or governing standards relating to the substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against us or our operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material.
Cash, Cash Equivalents and Investments
Our consolidated cash, cash equivalents and investments on hand totaled $193.0 million at December 31, 2014, compared to $256.7 million at December 31, 2013. The net change in cash and cash equivalents for the periods presented was comprised of the following (in thousands):
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Net cash provided by (used in): | | | | | | |
| Operating activities | | $ | 4,184 |
| | $ | (3,509 | ) | | $ | 104,603 |
|
| Investing activities | | 29,297 |
| | (66,059 | ) | | (408,792 | ) |
| Financing activities | | (25,700 | ) | | (25,551 | ) | | 211,260 |
|
Cash Flows from Operating Activities. Cash receipts from licensees totaled $117.0 million, $133.5 million and $243.8 million in fiscal years 2014, 2013 and 2012, respectively. The fluctuations in cash receipts for the periods presented primarily reflects the corresponding fluctuations in revenues recognized during the same periods, as described above, and the related timing of payments received from licensees. Cash outflows from operations totaled $112.9 million, $137.0 million and $139.2 million in fiscal years 2014, 2013 and 2012, respectively. The fluctuations in cash outflows for the periods presented reflects the fluctuations in revenue related inventor royalties and contingent legal fees and other operating costs and expenses during the same periods, as discussed above, and the impact of the timing of payments to inventors, attorneys and other vendors.
Cash Flows from Investing Activities. Cash flows from investing activities and related changes were comprised of the following for the periods presented (in thousands):
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| | | | | | | |
| Purchase of ADAPTIX, Inc., net of cash acquired | | $ | — |
| | $ | — |
| | $ | (150,000 | ) |
| Patent portfolio investment costs | | (42,746 | ) | | (25,061 | ) | | (178,260 | ) |
| Net sale (purchase) of available-for-sale investments | | 72,152 |
| | (40,323 | ) | | (80,264 | ) |
| Other | | (109 | ) | | (675 | ) | | (268 | ) |
| Net cash provided by (used in) investing activities | | $ | 29,297 |
| | $ | (66,059 | ) | | $ | (408,792 | ) |
Cash Flows from Financing Activities. Cash flows from financing activities and related changes included the following for the periods presented (in thousands): |
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Dividends paid to stockholders | | $ | (25,039 | ) | | $ | (18,633 | ) | | $ | — |
|
| Distributions to noncontrolling interests - Acacia IP Fund | | (867 | ) | | — |
| | (312 | ) |
| Proceeds from the exercise of stock options | | 206 |
| | 486 |
| | 340 |
|
| Repurchases of common stock | | — |
| | (7,926 | ) | | (26,732 | ) |
| Contributions from noncontrolling interests - Acacia IP Fund | | — |
| | 1,920 |
| | 5,793 |
|
| Excess tax benefits (shortfalls) from stock-based compensation | | — |
| | (1,398 | ) | | 13,210 |
|
| Proceeds from sale of common stock, net of issuance costs | | — |
| | — |
| | 218,961 |
|
| Net cash provided by financing activities | | $ | (25,700 | ) | | $ | (25,551 | ) | | $ | 211,260 |
|
Stock Repurchase Programs. On November 16, 2012, we announced that our Board of Directors authorized a program for repurchases of shares of our outstanding common stock. Under the stock repurchase program, effective November 16, 2012, we were authorized to purchase in the aggregate up to $100.0 million of our common stock through the period ended August 15, 2013. This repurchase program expired on August 15, 2013.
On November 15, 2013, our Board of Directors authorized a program for repurchases of shares of our outstanding common stock. We were authorized to purchase in the aggregate up to $70.0 million of our outstanding common stock through the period ending May 14, 2014. Repurchases may be made from time to time by us in the open market or in block purchases in compliance with applicable SEC rules.
In fiscal year 2013, we acquired 600,000 shares of our common stock at an average price of $13.18. In fiscal year 2012, we acquired 1,129,408 shares of our common stock at an average price of $23.65 per share. Repurchases to date were made using existing cash resources and occurred in the open market.
Proceeds from the Sale of Common Stock. In February 2012, we raised net proceeds of $219.0 million through the sale of 6,122,449 shares of our common stock at a price of $36.75 per share in a private placement offering with certain institutional accredited investors. The net proceeds will continue to be used to finance future patent related investments, other patent licensing vehicles, and for working capital and general corporate purposes.
Dividends to Stockholders. On April 23, 2013, we announced that our Board of Directors approved the adoption of a cash dividend policy that calls for the payment of an expected total annual cash dividend of $0.50 per common share, payable in the amount of $0.125 per share per quarter. Under the policy, we paid four quarterly cash dividends totaling $25.0 million in 2014 and three quarterly dividends totaling $18.6 million in 2013. In addition, on February 19, 2015, we announced that our
Board of Directors approved a quarterly cash dividend payable in the amount of $0.125 per share. The quarterly cash dividend will be paid on March 30, 2015 to stockholders of record at close of business on March 2, 2015. While we paid dividends to holders of our common stock on a quarterly basis during fiscal year 2014, the declaration and payment of future dividends will depend on many factors, including, but not limited to, our earnings and financial condition, and any future dividends will be made solely at the discretion of our Board of Directors.
Working Capital
The primary components of working capital are cash and cash equivalents, accounts receivable, prepaid expenses, accounts payable, accrued expenses, and royalties and contingent legal fees payable. Working capital at December 31, 2014 was $172.8 million, compared to $247.7 million at December 31, 2013.
Consolidated accounts receivable from licensees increased to $20.2 million at December 31, 2014, compared to $6.3 million at December 31, 2013. Accounts receivable balances fluctuate based on the timing, magnitude and payment terms associated with revenue agreements executed during the year, and the timing of cash receipts on accounts receivable balances recorded in previous periods. Three licensees individually represented approximately 30%, 17% and 15%, respectively, of accounts receivable at December 31, 2014. Two licensees individually represented approximately 60% and 22%, respectively, of accounts receivable at December 31, 2013.
Accounts payable and accrued expenses increased to $14.9 million at December 31, 2014, from $11.6 million at December 31, 2013, due primarily to the related timing of payments to vendors in the ordinary course.
Consolidated royalties and contingent legal fees payable increased to $14.4 million at December 31, 2014, compared to $10.4 million at December 31, 2013. Royalties and contingent legal fees payable balances fluctuate based on the magnitude and timing of the execution of related license agreements, the timing of cash receipts for the related license agreements, and the timing of payment of current and prior period royalties and contingent legal fees payable to inventor and outside attorneys, respectively.
The majority of accounts receivable from licensees at December 31, 2014 were collected or scheduled to be collected in the first quarter of 2015, in accordance with the terms of the related underlying license agreements. The majority of royalties and contingent legal fees payable are scheduled to be paid in the first and second quarter of 2015 in accordance with the underlying contractual arrangements.
Off-Balance Sheet Arrangements
We have not entered into off-balance sheet financing arrangements, other than operating leases.
Contractual Obligations
We have no significant commitments for capital expenditures in 2015. We have no committed lines of credit or other committed funding or long-term debt. The following table lists our material known future cash commitments as of December 31, 2014, and any material known commitments arising from events subsequent to year end:
|
| | | | | | | | | | | | | | | | |
| | | Payments Due by Period (In thousands) |
| | | Total | | Less than 1 year | | 1-3 years | | More than 3 years |
| | | | | | | | | |
| Operating leases | | $ | 8,163 |
| | $ | 1,564 |
| | $ | 3,152 |
| | $ | 3,447 |
|
| Scheduled patent investment related payments | | 16,700 |
| | 16,700 |
| | — |
| | — |
|
| Total contractual obligations | | $ | 24,863 |
| | $ | 18,264 |
| | $ | 3,152 |
| | $ | 3,447 |
|
Uncertain Tax Positions. At December 31, 2014, we had total unrecognized tax benefits of approximately $2.1 million, including a recorded noncurrent liability of $85,000 related to unrecognized tax benefits primarily associated with state taxes. No interest and penalties have been recorded for the unrecognized tax benefits as of December 31, 2014. If recognized, approximately $2.1 million would impact our effective tax rate. We do not expect that the liability for unrecognized tax benefits will change significantly within the next 12 months. Activity related to the gross unrecognized tax benefits for the periods presented was as follows (in thousands):
|
| | | | |
| Balance at January 1, 2012 | | $ | 85 |
|
| 2012 Additions for tax positions related to prior years | | 772 |
|
| 2012 Additions resulting from the acquisition of ADAPTIX | | 1,270 |
|
| Balance at December 31, 2014, 2013 and 2012 | | $ | 2,127 |
|
Recent Accounting Pronouncements
Refer to Note 2 to our notes to consolidated financial statements included elsewhere herein.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The primary objective of our investment activities is to preserve principal while concurrently maximizing the income we receive from our investments without significantly increasing risk. In addition, we sometimes invest in marketable equity securities for strategic purposes related to our patent monetization-based businesses. Some of the securities that we invest in may be subject to interest rate risk and/or market risk. This means that a change in prevailing interest rates, with respect to interest rate risk, or a change in the value of the United States equity markets, with respect to market risk, may cause the principal amount or market value of the investments to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the current value of the principal amount of our investment may decline. To minimize these risks in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds, high-grade corporate bonds, government and non-government debt securities and certificates of deposit. However, to the extent that our marketable equity securities have strategic value, we typically do not attempt to reduce or eliminate market risk with respect to such securities through hedging activities.
At December 31, 2014 and 2013, our short-term investments were comprised of AAA rated money market funds that invest in first-tier only securities, which primarily include domestic commercial paper, securities issued or guaranteed by the U.S. government or its agencies, U.S. bank obligations, and fully collateralized repurchase agreements (included in cash and cash equivalents in the accompanying consolidated balance sheets), and direct investments in highly liquid, AAA, U.S. government securities (included in short term investments in the accompanying consolidated balance sheets).
In general, money market funds are not subject to market risk because the interest paid on such funds fluctuates with the prevailing interest rate. Accordingly, a 100 basis point increase in interest rates or a 10% decline in the value of the United States equity markets would not be expected to have a material impact on the value of such money market funds. Investments in U.S. government fixed income securities are subject to interest rate risk and will decline in value if interest rates increase. However, due to the relatively short duration of our investment portfolio, an immediate 10% change in interest rates would have no material impact on our financial condition, results of operations or cash flows. Declines in interest rates over time will, however, reduce our interest income.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and related financial information required to be filed hereunder are indexed under Item 15 of this report and are incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2014, our disclosure controls and procedures were effective to ensure that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure, and that such information is recorded, processed, summarized and reported within the time periods prescribed by the SEC.
(b) Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the 2013 framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2014.
Grant Thornton LLP, the independent registered public accounting firm who audited our consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the effectiveness of our internal control over financial reporting as of December 31, 2014, which is included herein.
Changes in Internal Controls. There were no changes in our internal control over financial reporting during the fourth fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Except as provided below, in accordance with General Instruction G(3) to Form 10-K, certain information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2015.
Code of Conduct.
We have adopted a Code of Conduct that applies to all employees, including our chief executive officer, chief financial and accounting officer, president and any persons performing similar functions. Our Code of Conduct is provided on our internet website at www.acaciaresearch.com.
ITEM 11. EXECUTIVE COMPENSATION
In accordance with General Instruction G(3) to Form 10-K, the information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2015.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
In accordance with General Instruction G(3) to Form 10-K, certain information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2015.
Equity Compensation Plan Information
The following table provides information with respect to shares of our common stock issuable under our equity compensation plans as of December 31, 2014:
|
| | | | | | | | | | |
Plan Category | | (a) Number of securities to be issued upon exercise of outstanding options | | (b) Weighted-average exercise price of outstanding options | | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
| | | | | | | |
| Equity compensation plans approved by security holders | | | | | | |
2002 Acacia Technologies Stock Incentive Plan(1) | | 150,000 |
| | $ | 7.59 |
| | — |
|
2007 Acacia Technologies Stock Incentive Plan(2) | | — |
| | — |
| | — |
|
2013 Acacia Research Stock Incentive Plan(3) | | — |
| | — |
| | 3,243,000 |
|
| Subtotal | | 150,000 |
| | $ | 7.59 |
| | 3,243,000 |
|
| Equity compensation plans not approved by security holders | | |
| | | | |
|
Grants to New Employees Outside of the Plans(4) | | — |
| | — |
| | — |
|
| Total | | 150,000 |
| | 7.59 |
| | 3,243,000 |
|
____________________
| |
| (1) | The 2002 Stock Plan expired in December 2012. Column (a) excludes 97,000 in nonvested restricted stock awards and restricted stock units outstanding at December 31, 2014. Refer to Note 10 to our notes to consolidated financial statements included elsewhere herein. |
| |
| (2) | The initial share reserve under the 2007 Acacia Technologies Stock Incentive Plan, or the 2007 Plan, was 560,000 shares of our common stock. The share reserve under the 2007 Plan automatically increased on January 1, 2008 and 2009, by an amount equal to two percent (2%) of the total number of shares of our common stock outstanding on the last trading day of December in the prior calendar year. After January 1, 2009, no new additional shares will be added to the 2007 Plan without security holder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2007 Plan). Column (a) excludes 8,000 in nonvested restricted stock awards and restricted stock units outstanding at December 31, 2014. Refer to Note 10 to our notes to consolidated financial statements included elsewhere herein. |
| |
| (3) | The initial share reserve under the 2013 Acacia Research Stock Incentive Plan, or the 2013 Plan, was 4,750,000 shares of our common stock. No new additional shares will be added to the 2013 Plan without security holder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2013 Plan). Column (a) excludes 1,001,000 in nonvested restricted stock awards and restricted stock units outstanding at December 31, 2014. Refer to Note 10 to our notes to consolidated financial statements included elsewhere herein. |
| |
| (4) | Column (a) excludes 19,000 in nonvested restricted stock awards outstanding at December 31, 2014 that were granted to new employees outside of existing approved plans, pursuant to and in accordance with applicable SEC guidelines. Refer to Note 10 to our notes to consolidated financial statements included elsewhere herein. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
In accordance with General Instruction G(3) to Form 10-K, the information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2015.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
In accordance with General Instruction G(3) to Form 10-K, the information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than April 30, 2015.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
| |
| (a) | The following documents are filed as part of this report. |
|
| | |
| (1) Financial Statements | | Page |
| | | |
| Acacia Research Corporation Consolidated Financial Statements | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
| (2) Financial Statement Schedules | |
| | | |
| Financial statement schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or the Notes thereto. |
| | | |
| (3) Exhibits | |
| | | |
| Refer to Item 15(b) below. | |
|
| |
| (b) | Exhibits. The following exhibits are either filed herewith or incorporated herein by reference: |
|
| |
Exhibit Number | Description |
| | |
| 2.1 | Agreement and Plan of Merger, dated November 22, 2011, by and among Acacia Research Group LLC, Apollo Patent Corp., Adaptix, Inc., and Baker Communications Fund II (QP), L.P., solely in its capacity as representative for the shareholders of Adaptix, Inc.(15) |
| 3.1 | Amended and Restated Certificate of Incorporation (1) |
| 3.2 | Amended and Restated Bylaws (17) |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (2) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (3) |
| 10.3* | 2002 Acacia Technologies Stock Incentive Plan (4) |
| 10.4* | 2007 Acacia Technologies Stock Incentive Plan (5) |
| 10.5* | Form of Acacia Technologies Stock Option Agreement under the 2007 Acacia Technologies Stock Incentive Plan (6) |
| 10.6* | Form of Acacia Technologies Stock Issuance Agreement under the 2002 Acacia Technologies Stock Incentive Plan (6) |
| 10.7* | Form of Acacia Technologies Stock Issuance Agreement under the 2007 Acacia Technologies Stock Incentive Plan (6) |
| 10.8 | Office Space Lease dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (7) |
| 10.9 | Form of Indemnification Agreement (8) |
| 10.10 | Third Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (9) |
|
| |
| 10.11* | Employment Agreement, dated April 12, 2004, by and between Acacia Media Technologies Corporation and Edward Treska (10) |
| 10.11.1* | Addendum, dated March 31, 2008, to Employment Agreement by and between Acacia Media Technologies Corporation and Edward Treska (11) |
| 10.12 | Fourth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10) |
| 10.13 | Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10) |
| 10.14* | Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Paul Ryan (12) |
| 10.14.1* | Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Paul Ryan (12) |
| 10.15* | Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (11) |
| 10.15.1* | Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (12) |
| 10.16* | Amended Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (11) |
| 10.16.1* | Amendment to Amended Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (12) |
| 10.17* | Acacia Research Corporation Amended and Restated Executive Severance Policy (12) |
| 10.18 | Sixth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (14) |
| 10.19 | Form of Purchase Agreement (16) |
| 10.20* | 2013 Acacia Research Corporation Stock Incentive Plan (18) |
| 10.21* | Form of Stock Issuance Agreement under the 2013 Acacia Research Corporation Stock Incentive Plan (19) |
| 10.22* | Employment Agreement, dated October 28, 2006, by and between Acacia Technologies Services Corporation and Matthew Vella (20) |
| 18.1 | Preferability Letter dated February 25, 2010 from Grant Thornton LLP, independent registered public accounting firm, regarding change in accounting principle (13) |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of Independent Registered Public Accounting Firm |
| 24.1 | Power of Attorney (included in the signature page hereto). |
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 |
| 32.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350 |
| 32.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350 |
| 101 | Interactive Date Files Pursuant to Rule 405 of Regulation S-T. |
___________________________
| |
| * | The referenced exhibit is a management contract, compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(c) of Form 10-K. |
| |
| (1) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on June 5, 2008 (File No. 000-26068). |
| |
| (2) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 20, 2000 (File No. 000-26068). |
| |
| (3) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 26, 1996 (File No. 000-26068). |
| |
| (4) | Incorporated by reference to Annex E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (File No. 333-87654) which became effective on November 8, 2002. |
| |
| (5) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-8 (File No. 333-144754) which became effective on July 20, 2007. |
| |
| (6) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2007, filed on November 2, 2007 (File No. 000-26068). |
| |
| (7) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10‑K for the year ended December 31, 2001, filed on March 27, 2002 (File No. 000‑26068). |
| |
| (8) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 2012, filed on July 30, 2012 (File No. 000-26068). |
| |
| (9) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended March 31, 2006, filed on May 10, 2006 (File No. 000‑26068). |
| |
| (10) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2007, filed on March 14, 2008 (File No. 000-26068). |
| |
| (11) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on April 2, 2008 ( File No. 000-26068). |
| |
| (12) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008, filed on February 26, 2009 (File No. 000-26068). |
| |
| (13) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2009, filed on February 26, 2010, as amended on March 1, 2010 (File No. 000-26068) |
| |
| (14) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, as amended on March 24, 2011 (File No. 000-26068). |
| |
| (15) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K/A filed on January 19, 2012 (File No. 000-26068). Portions of this exhibit have been omitted pursuant to a request for confidential treatment under Rule 24-b-2 of the Securities Exchange Act of 1934, as amended. The omitted material has been separately filed with the Securities and Exchange Commission. |
| |
| (16) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on February 16, 2012 (File No. 000-26068). |
| |
| (17) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2012, filed on February 28, 2013 (File No. 000-26068). |
| |
| (18) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 24, 2013 (File No. 000-26068). |
| |
| (19) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on May 22, 2013 (File No. 000-26068). |
| |
| (20) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on July 5, 2013 (File No. 000-26068). |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | | |
| | | | ACACIA RESEARCH CORPORATION | |
| | | | | |
| Dated: | February 27, 2015 | By: | /s/ Matthew Vella | |
| | | | Matthew Vella | |
| | | | Chief Executive Officer (Authorized Signatory) | |
POWER OF ATTORNEY
We, the undersigned directors and officers of Acacia Research Corporation, do hereby constitute and appoint Matthew Vella and Clayton J. Haynes, and each of them, as our true and lawful attorneys-in-fact and agents with power of substitution, to do any and all acts and things in our name and behalf in our capacities as directors and officers and to execute any and all instruments for us and in our names in the capacities indicated below, which said attorney-in-fact and agent may deem necessary or advisable to enable said corporation to comply with the Securities Exchange Act of 1934, as amended, and any rules, regulations and requirements of the Securities and Exchange Commission, in connection with this Annual Report on Form 10-K, including specifically but without limitation, power and authority to sign for us or any of us in our names in the capacities indicated below, any and all amendments hereto; and we do hereby ratify and confirm all that said attorney-in-fact and agent, shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and the capacities and on the dates indicated.
|
| | | | | |
Signature | | Title | | Date |
| | | | | | |
| /s/ | Matthew Vella | | Chief Executive Officer | | February 27, 2015 |
| | Matthew Vella | | (Principal Executive Officer) | | |
| | | | | | |
| | | | | | |
| /s/ | Robert L. Harris, II | | Executive Chairman | | February 27, 2015 |
| | Robert L. Harris, II | | | | |
| | | | | | |
| /s/ | Clayton J. Haynes | | Chief Financial Officer and Treasurer | | February 27, 2015 |
| | Clayton J. Haynes | | (Principal Financial and Accounting Officer) | | |
| | | | | | |
| /s/ | Fred A. de Boom | | Director | | February 27, 2015 |
| | Fred A. de Boom | | | | |
| | | | | | |
| /s/ | Edward W. Frykman | | Director | | February 27, 2015 |
| | Edward W. Frykman | | | | |
| | | | | | |
| /s/ | G. Louis Graziadio, III | | Director | | February 27, 2015 |
| | G. Louis Graziadio, III | | | | |
| | | | | | |
| /s/ | William S. Anderson | | Director | | February 27, 2015 |
| | William S. Anderson | | | | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Acacia Research Corporation
We have audited the accompanying consolidated balance sheets of Acacia Research Corporation (the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Acacia Research Corporation as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2014, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 27, 2015 expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Los Angeles, California
February 27, 2015
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Acacia Research Corporation
We have audited the internal control over financial reporting of Acacia Research Corporation (the “Company”) as of December 31, 2014, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on criteria established in the 2013 Internal Control-Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements of the Company as of and for the year ended December 31, 2014, and our report dated February 27, 2015 expressed an unmodified on those financial statements.
/s/ GRANT THORNTON LLP
Los Angeles, California
February 27, 2015
ACACIA RESEARCH CORPORATION
CONSOLIDATED BALANCE SHEETS
As of December 31, 2014 and 2013
(In thousands, except share and per share information)
|
| | | | | | | | |
| | | 2014 | | 2013 |
| ASSETS | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 134,466 |
| | $ | 126,685 |
|
| Short-term investments | | 58,558 |
| | 130,017 |
|
| Accounts receivable | | 20,168 |
| | 6,341 |
|
| Deferred income tax | | 1,161 |
| | 3,139 |
|
| Prepaid expenses and other current assets | | 4,355 |
| | 7,546 |
|
| Total current assets | | 218,708 |
| | 273,728 |
|
| Furniture and equipment, net of accumulated depreciation and amortization | | 500 |
| | 766 |
|
| Patents, net of accumulated amortization | | 286,636 |
| | 288,432 |
|
| Goodwill | | 30,149 |
| | 30,149 |
|
| Other assets | | 355 |
| | 318 |
|
| | | $ | 536,348 |
| | $ | 593,393 |
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | |
| | |
|
| Current liabilities: | | |
| | |
|
| Accounts payable and accrued expenses | | $ | 14,860 |
| | $ | 11,555 |
|
| Accrued patent investment costs | | 16,700 |
| | 4,000 |
|
| Royalties and contingent legal fees payable | | 14,351 |
| | 10,447 |
|
| Total current liabilities | | 45,911 |
| | 26,002 |
|
| Deferred income taxes | | 1,161 |
| | 4,874 |
|
| Other liabilities | | 228 |
| | 319 |
|
| Total liabilities | | 47,300 |
| | 31,195 |
|
| Commitments and contingencies (Note 11) | |
|
| |
|
|
| Stockholders’ equity: | | |
| | |
|
| Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; no shares issued or outstanding | | — |
| | — |
|
| Common stock, par value $0.001 per share; 100,000,000 shares authorized; 50,065,382 shares issued and outstanding as of December 31, 2014 and 49,385,057 shares issued and outstanding as of December 31, 2013 | | 50 |
| | 49 |
|
| Treasury stock, at cost, 1,729,408 shares as of December 31, 2014 and December 31, 2013 | | (34,640 | ) | | (34,640 | ) |
| Additional paid-in capital | | 646,595 |
| | 653,314 |
|
| Accumulated comprehensive loss | | (353 | ) | | (947 | ) |
| Accumulated deficit | | (128,095 | ) | | (62,066 | ) |
| Total Acacia Research Corporation stockholders’ equity | | 483,557 |
| | 555,710 |
|
| Noncontrolling interests in operating subsidiaries | | 5,491 |
| | 6,488 |
|
| Total stockholders’ equity | | 489,048 |
| | 562,198 |
|
| | | $ | 536,348 |
| | $ | 593,393 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
For the Years Ended December 31, 2014, 2013 and 2012
(In thousands, except share and per share information)
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Revenues | | $ | 130,876 |
| | $ | 130,556 |
| | $ | 250,727 |
|
| Operating costs and expenses: | | |
| | |
| | |
|
| Cost of revenues: | | |
| | |
| | |
|
| Inventor royalties | | 20,670 |
| | 29,724 |
| | 26,028 |
|
| Contingent legal fees | | 23,563 |
| | 24,784 |
| | 24,651 |
|
| Litigation and licensing expenses - patents | | 37,614 |
| | 39,335 |
| | 21,591 |
|
| Amortization of patents | | 57,242 |
| | 53,658 |
| | 39,019 |
|
| Marketing, general and administrative expenses (including non-cash stock compensation expense of $18,115 in 2014, $27,894 in 2013 and $25,657 in 2012) | | 48,554 |
| | 59,229 |
| | 54,083 |
|
| Research, consulting and other expenses - business development | | 3,840 |
| | 3,251 |
| | 4,943 |
|
| Other | | 1,548 |
| | 3,506 |
| | — |
|
| | | | | | | |
| Total operating costs and expenses | | 193,031 |
| | 213,487 |
| | 170,315 |
|
| | | | | | | |
| Operating income (loss) | | (62,155 | ) | | (82,931 | ) | | 80,412 |
|
| | | | | | | |
| Other income (expense): | | | | | | |
| Other income | | — |
| | — |
| | 500 |
|
| Interest and other investment income (loss) | | (595 | ) | | 2,131 |
| | 482 |
|
| Write off of investment | | — |
| | — |
| | (45 | ) |
| Total other income (expense) | | (595 | ) | | 2,131 |
| | 937 |
|
| Income (loss) from operations before (provision for) benefit from income taxes | | (62,750 | ) | | (80,800 | ) | | 81,349 |
|
| (Provision for) benefit from income taxes | | (3,912 | ) | | 21,958 |
| | (22,060 | ) |
| Net income (loss) including noncontrolling interests in operating subsidiaries | | (66,662 | ) | | (58,842 | ) | | 59,289 |
|
| Net loss attributable to noncontrolling interests in operating subsidiaries | | 633 |
| | 2,408 |
| | 164 |
|
| Net income (loss) attributable to Acacia Research Corporation | | $ | (66,029 | ) | | $ | (56,434 | ) | | $ | 59,453 |
|
| | | | | | | |
| Net income (loss) attributable to common stockholders - basic | | $ | (66,755 | ) | | $ | (56,945 | ) | | $ | 57,564 |
|
| Net income (loss) attributable to common stockholders - diluted | | $ | (66,755 | ) | | $ | (56,945 | ) | | $ | 57,577 |
|
| | | |
| | |
| | |
|
| Basic income (loss) per common share | | $ | (1.37 | ) | | $ | (1.18 | ) | | $ | 1.22 |
|
| Diluted income (loss) per common share | | $ | (1.37 | ) | | $ | (1.18 | ) | | $ | 1.21 |
|
| | | | | | | |
| Weighted-average number of shares outstanding, basic | | 48,658,088 |
| | 48,155,832 |
| | 47,251,061 |
|
| Weighted-average number of shares outstanding, diluted | | 48,658,088 |
| | 48,155,832 |
| | 47,584,120 |
|
| | | | | | | |
| Cash dividends declared per common share | | $ | 0.50 |
| | $ | 0.375 |
| | — |
|
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
For the Years Ended December 31, 2014, 2013 and 2012
(In thousands)
|
| | | | | | | | | | | |
| | 2014 | | 2013 | | 2012 |
| Net income (loss) including noncontrolling interests in operating subsidiaries | $ | (66,662 | ) | | $ | (58,842 | ) | | $ | 59,289 |
|
| Other comprehensive income (loss): | | | | | |
| Unrealized gain (loss) on short-term investments, net of tax of $0 | (1,488 | ) | | 26 |
| | 657 |
|
| Unrealized gain (loss) on foreign currency translation, net of tax of $0 | (128 | ) | | — |
| | 7 |
|
| Add: reclassification adjustment for losses included in net income | 2,210 |
| | 193 |
| | 277 |
|
| Total other comprehensive income (loss) | (66,068 | ) | | (58,623 | ) | | 60,230 |
|
| Comprehensive income attributable to noncontrolling interests | 633 |
| | 2,408 |
| | 164 |
|
| Comprehensive income (loss) attributable to Acacia Research Corporation | $ | (65,435 | ) |
| $ | (56,215 | ) | | $ | 60,394 |
|
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2014, 2013 and 2012
(In thousands, except share information)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Shares | | Common Stock | | Treasury Stock | | Additional Paid-in Capital | | Other Comprehensive (Loss) Income | | Accumulated Deficit | | Noncontrolling Interests in Operating Subsidiaries | | Total |
| | | | | | | | | | | | | | | | | |
| Balance at December 31, 2011 | | 42,928,001 |
| | $ | 43 |
| | $ | — |
| | $ | 386,821 |
| | $ | (1,830 | ) | | $ | (65,085 | ) | | $ | 2,163 |
| | $ | 322,112 |
|
| Net income attributable to Acacia Research Corporation | | — |
| | — |
| | — |
| | — |
| | — |
| | 59,453 |
| | — |
| | 59,453 |
|
| Sale of common stock, net of issuance costs of $6,039 | | 6,122,449 |
| | 6 |
| | — |
| | 218,955 |
| | — |
| | — |
| | — |
| | 218,961 |
|
| Repurchase of common stock | | (1,129,408 | ) | | (1 | ) | | (26,731 | ) | | — |
| | — |
| | — |
| | — |
| | (26,732 | ) |
| Stock options exercised | | 71,272 |
| | — |
| | — |
| | 340 |
| | — |
| | — |
| | — |
| | 340 |
|
| Compensation expense relating to restricted stock awards | | 1,168,530 |
| | 1 |
| | — |
| | 25,656 |
| | — |
| | — |
| | — |
| | 25,657 |
|
| Excess tax benefits from stock-based compensation | | — |
| | — |
| | — |
| | 13,210 |
| | — |
| | — |
| | — |
| | 13,210 |
|
| Net loss attributable to noncontrolling interests in operating subsidiaries | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (164 | ) | | (164 | ) |
| Contributions from noncontrolling interests in operating subsidiary, net | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 5,793 |
| | 5,793 |
|
| Distributions to noncontrolling interests in operating subsidiary | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (816 | ) | | (816 | ) |
| Unrealized gain on foreign currency translation | | — |
| | — |
| | — |
| | — |
| | 7 |
| | — |
| | — |
| | 7 |
|
| Unrealized gain on short-term investments | | — |
| | — |
| | — |
| | — |
| | 657 |
| | — |
| | — |
| | 657 |
|
| Balance at December 31, 2012 | | 49,160,844 |
| | 49 |
| | (26,731 | ) | | 644,982 |
| | (1,166 | ) | | (5,632 | ) | | 6,976 |
| | 618,478 |
|
| Net loss attributable to Acacia Research Corporation | | — |
| | — |
| | — |
| | — |
| | — |
| | (56,434 | ) | | — |
| | (56,434 | ) |
| Dividends paid to stockholders | | — |
| | — |
| | — |
| | (18,633 | ) | | — |
| | — |
| | — |
| | (18,633 | ) |
| Repurchase of common stock | | (600,000 | ) | | (1 | ) | | (7,909 | ) | | — |
| | — |
| | — |
| | — |
| | (7,910 | ) |
| Repurchase of restricted common stock | | (666 | ) | | — |
| | — |
| | (16 | ) | | | | | | | | (16 | ) |
| Stock options exercised | | 115,346 |
| | — |
| | — |
| | 486 |
| | — |
| | — |
| | — |
| | 486 |
|
| Compensation expense relating to restricted stock awards | | 709,533 |
| | 1 |
| | — |
| | 27,893 |
| | — |
| | — |
| | — |
| | 27,894 |
|
| Excess tax benefits from stock-based compensation | | — |
| | — |
| | — |
| | (1,398 | ) | | — |
| | — |
| | — |
| | (1,398 | ) |
| Net loss attributable to noncontrolling interests in operating subsidiaries | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (2,408 | ) | | (2,408 | ) |
| Contributions from noncontrolling interests in operating subsidiary, net | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 1,920 |
| | 1,920 |
|
| Unrealized gain on short-term investments | | — |
| | — |
| | — |
| | — |
| | 219 |
| | — |
| | — |
| | 219 |
|
| Balance at December 31, 2013 | | 49,385,057 |
| | $ | 49 |
| | $ | (34,640 | ) | | $ | 653,314 |
| | $ | (947 | ) | | $ | (62,066 | ) | | $ | 6,488 |
| | $ | 562,198 |
|
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| |
| (Continued on next page) |
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ACACIA RESEARCH CORPORATION |
| CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (Continued) |
| For the Years Ended December 31, 2014, 2013 and 2012 |
| (In thousands, except share information) |
| | | | | | | | | | | | | | | | | |
| | | Common Shares | | Common Stock | | Treasury Stock | | Additional Paid-in Capital | | Other Comprehensive (Loss) Income | | Accumulated Deficit | | Noncontrolling Interests in Operating Subsidiaries | | Total |
| Balance at December 31, 2013 | | 49,385,057 |
| | $ | 49 |
| | $ | (34,640 | ) | | $ | 653,314 |
| | $ | (947 | ) | | $ | (62,066 | ) | | $ | 6,488 |
| | $ | 562,198 |
|
| Net loss attributable to Acacia Research Corporation | | — |
| | — |
| | — |
| | — |
| | — |
| | (66,029 | ) | | — |
| | (66,029 | ) |
| Dividends paid to stockholders | | — |
| | — |
| | — |
| | (25,039 | ) | | — |
| | — |
| | — |
| | (25,039 | ) |
| Stock options exercised | | 44,506 |
| | — |
| | — |
| | 206 |
| | — |
| | — |
| | — |
| | 206 |
|
| Compensation expense relating to restricted stock awards | | 635,819 |
| | 1 |
| | — |
| | 18,114 |
| | — |
| | — |
| | — |
| | 18,115 |
|
| Net loss attributable to noncontrolling interests in operating subsidiaries | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (633 | ) | | (633 | ) |
| Distributions to noncontrolling interests in operating subsidiary | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (364 | ) | | (364 | ) |
| Unrealized loss on foreign currency translation | | — |
| | — |
| | — |
| | — |
| | (99 | ) | | — |
| | — |
| | (99 | ) |
| Unrealized gain on short-term investments | | — |
| | — |
| | — |
| | — |
| | 693 |
| | — |
| | — |
| | 693 |
|
| Balance at December 31, 2014 | | 50,065,382 |
| | $ | 50 |
| | $ | (34,640 | ) | | $ | 646,595 |
| | $ | (353 | ) | | $ | (128,095 | ) | | $ | 5,491 |
| | $ | 489,048 |
|
| | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31, 2014, 2013 and 2012
(In thousands)
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Cash flows from operating activities: | | | | | | |
| Net income (loss) including noncontrolling interests in operating subsidiaries | | $ | (66,662 | ) | | $ | (58,842 | ) | | $ | 59,289 |
|
| Adjustments to reconcile net income (loss) including noncontrolling interests in operating subsidiaries to net cash provided by (used in) operating activities: | | |
| | |
| | |
|
| Depreciation and amortization | | 57,546 |
| | 53,894 |
| | 39,168 |
|
| Non-cash stock compensation | | 18,115 |
| | 27,894 |
| | 25,657 |
|
| Excess tax benefits from stock-based compensation | | — |
| | 1,398 |
| | (13,210 | ) |
| Provision for deferred income taxes | | (1,736 | ) | | (26,746 | ) | | 9,889 |
|
| Other | | (28 | ) | | 12 |
| | 777 |
|
| Changes in assets and liabilities: | | |
| | |
| | |
|
| Accounts receivable | | (13,827 | ) | | 3,502 |
| | (6,928 | ) |
| Prepaid expenses and other assets | | 3,154 |
| | (5,300 | ) | | (1,294 | ) |
| Accounts payable and accrued expenses / costs | | 3,718 |
| | 2,740 |
| | 2,255 |
|
| Royalties and contingent legal fees payable | | 3,904 |
| | (2,061 | ) | | (11,000 | ) |
| | | | | | | |
| Net cash provided by (used in) operating activities | | 4,184 |
| | (3,509 | ) | | 104,603 |
|
| | | | | | | |
| Cash flows from investing activities: | | |
| | |
| | |
|
| Purchases of furniture and equipment |
| (109 | ) |
| (675 | ) |
| (268 | ) |
| Purchases of available-for-sale investments |
| (109,963 | ) |
| (279,693 | ) |
| (402,500 | ) |
| Sales and maturities of available-for-sale investments |
| 182,115 |
|
| 239,370 |
|
| 322,236 |
|
| Purchase of ADAPTIX, Inc., net of cash acquired |
| — |
|
| — |
|
| (150,000 | ) |
| Patent portfolio investment costs |
| (42,746 | ) |
| (25,061 | ) |
| (178,260 | ) |
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) investing activities |
| 29,297 |
|
| (66,059 | ) |
| (408,792 | ) |
| | | | | | | |
| Cash flows from financing activities: | | |
| | |
| | |
|
| Dividends paid to stockholders | | (25,039 | ) | | (18,633 | ) | | — |
|
| Distributions to noncontrolling interests in operating subsidiary | | (867 | ) |
| — |
|
| (312 | ) |
| Proceeds from the exercise of stock options | | 206 |
|
| 486 |
|
| 340 |
|
| Repurchases of common stock | | — |
|
| (7,926 | ) |
| (26,732 | ) |
| Contributions from noncontrolling interests in operating subsidiary, net of issuance costs | | — |
|
| 1,920 |
|
| 5,793 |
|
| Excess tax benefits (shortfalls) from stock-based compensation |
| — |
|
| (1,398 | ) |
| 13,210 |
|
| Proceeds from sale of common stock, net of issuance costs | | — |
|
| — |
|
| 218,961 |
|
| | | | | | | |
| Net cash provided by (used in) financing activities | | (25,700 | ) | | (25,551 | ) | | 211,260 |
|
| | | | | | | |
| Increase (decrease) in cash and cash equivalents | | 7,781 |
| | (95,119 | ) | | (92,929 | ) |
| | | | | | | |
| Cash and cash equivalents, beginning | | 126,685 |
| | 221,804 |
| | 314,733 |
|
| | | | | | | |
| Cash and cash equivalents, ending | | $ | 134,466 |
| | $ | 126,685 |
| | $ | 221,804 |
|
| | | | | | | |
| Supplemental schedule of noncash investing activities: | | | | | | |
| Patent portfolio investment costs included in accrued expenses / costs | | $ | 16,700 |
| | $ | 4,000 |
| | $ | — |
|
The accompanying notes are an integral part of these consolidated financial statements.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Description of Business. As used herein, “Acacia” and the “Company” refer to Acacia Research Corporation and/or its wholly and majority-owned and controlled operating subsidiaries, and/or where applicable, its management. All patent investment, prosecution, licensing and enforcement activities are conducted solely by certain of Acacia’s wholly and majority-owned and controlled operating subsidiaries.
Acacia’s operating subsidiaries invest in, license and enforce patented technologies. Acacia’s operating subsidiaries partner with inventors and patent owners, applying their legal and technology expertise to patent assets to unlock the financial value in their patented inventions. Acacia is an intermediary in the patent marketplace, bridging the gap between invention and application, facilitating efficiency and delivering monetary rewards to patent owners.
Acacia’s operating subsidiaries generate revenues and related cash flows from the granting of intellectual property rights for the use of patented technologies that its operating subsidiaries control or own. Acacia’s operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, where necessary, with the enforcement against unauthorized users of their patented technologies through the filing of patent infringement litigation.
Acacia’s operating subsidiaries are principals in the licensing and enforcement effort, obtaining control of the rights in the patent portfolio, or control of the patent portfolio outright. Acacia’s operating subsidiaries own or control the rights to multiple patent portfolios, which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries.
In January 2012, a wholly owned operating subsidiary of Acacia acquired ADAPTIX, Inc. (“ADAPTIX”), a pioneer in the development of 4G technologies for wireless systems, for cash consideration of $150,000,000, net of cash acquired, as described at Note 7 to these consolidated financial statements.
Acacia was incorporated on January 25, 1993 under the laws of the State of California. In December 1999, Acacia changed its state of incorporation from California to Delaware.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Accounting Principles and Fiscal Year End. The consolidated financial statements and accompanying notes are prepared on the accrual basis of accounting in accordance with generally accepted accounting principles in the United States of America.
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of Acacia and its wholly and majority-owned and controlled subsidiaries. Material intercompany transactions and balances have been eliminated in consolidation.
Noncontrolling interests in Acacia’s majority-owned and controlled operating subsidiaries (“noncontrolling interests”) are separately presented as a component of stockholders’ equity. Consolidated net income or (loss) is adjusted to include the net (income) or loss attributed to noncontrolling interests in the consolidated statements of operations. Refer to the accompanying consolidated statements of stockholders’ equity for total noncontrolling interests.
In August 2010, a wholly owned subsidiary of Acacia became the general partner of the Acacia Intellectual Property Fund, L.P. (the “Acacia IP Fund”), which was formed in August 2010. The Acacia IP Fund is included in the Company’s consolidated financial statements since 2010, as Acacia’s wholly owned subsidiary, as the general partner, has the ability to control the operations and activities of the Acacia IP Fund. Refer to Note 11 to these consolidated financial statements.
Revenue Recognition. Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been substantially performed pursuant to the terms of the arrangement, (iii) amounts are fixed or determinable, and (iv) the collectibility of amounts is reasonably assured.
In general, revenue arrangements provide for the payment of contractually determined fees in consideration for the grant of certain intellectual property rights for patented technologies owned or controlled by Acacia’s operating subsidiaries. These rights typically include some combination of the following: (i) the grant of a non-exclusive, retroactive and future license
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
to manufacture and/or sell products covered by patented technologies owned or controlled by Acacia’s operating subsidiaries, (ii) a covenant-not-to-sue, (iii) the release of the licensee from certain claims, and (iv) the dismissal of any pending litigation. The intellectual property rights granted may be perpetual in nature, extending until the expiration of the related patents, or can be granted for a defined, relatively short period of time, with the licensee possessing the right to renew the agreement at the end of each contractual term for an additional minimum upfront payment. Pursuant to the terms of these agreements, Acacia’s operating subsidiaries have no further obligation with respect to the grant of the non-exclusive retroactive and future licenses, covenants-not-to-sue, releases, and other deliverables, including no express or implied obligation on Acacia’s operating subsidiaries’ part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the licenses, covenants-not-to-sue, releases, and other significant deliverables upon execution of the agreement, or upon receipt of the minimum upfront payment for term agreement renewals. As such, the earnings process is complete and revenue is recognized upon the execution of the agreement, when collectibility is reasonably assured, or upon receipt of the minimum upfront fee for term agreement renewals, and when all other revenue recognition criteria have been met.
For the periods presented herein, the majority of the revenue agreements executed by the Company provided for the payment of one-time, paid-up license fees in consideration for the grant of certain intellectual property rights for patented technology rights owned by our operating subsidiaries. These rights were primarily granted on a perpetual basis, extending until the expiration of the underlying patents.
Certain of the Company’s revenue arrangements provide for future royalties or additional required payments based on future licensee activities. Additional royalties are recognized in revenue upon resolution of the related contingency provided that all revenue recognition criteria, as described above, have been met. Amounts of additional royalties due under these license agreements, if any, cannot be reasonably estimated by management.
Certain of the Company’s revenue arrangements provide for the calculation of fees based on a licensee’s actual quarterly sales or actual per unit activity, applied to a contractual royalty rate. Licensees that pay fees on a quarterly basis generally report actual quarterly sales or actual per unit activity information and related quarterly fees due within 30 days to 45 days after the end of the quarter in which such sales or activity takes place. The amount of fees due under these revenue arrangements each quarter cannot be reasonably estimated by management. Consequently, Acacia’s operating subsidiaries recognize revenue from these revenue arrangements on a three-month lag basis, in the quarter following the quarter of sales or per unit activity, provided amounts are fixed or determinable and collectibility is reasonably assured. The lag method described above allows for the receipt of licensee royalty reports prior to the recognition of revenue.
Amounts related to revenue arrangements that do not meet the revenue recognition criteria described above are deferred until the revenue recognition criteria are met.
Acacia assesses the collectibility of fees receivable based on a number of factors, including past transaction history and credit-worthiness of licensees. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash.
Cost of Revenues. Cost of revenues include the costs and expenses incurred in connection with Acacia’s patent licensing and enforcement activities, including inventor royalties paid to original patent owners, contingent legal fees paid to external patent counsel, other patent-related legal expenses paid to external patent counsel, licensing and enforcement related research, consulting and other expenses paid to third-parties and the amortization of patent-related investment costs. These costs are included under the caption “Cost of revenues” in the accompanying consolidated statements of operations.
Inventor Royalties and Contingent Legal Expenses. Inventor royalties are expensed in the consolidated statements of operations in the period that the related revenues are recognized. In certain instances, pursuant to the terms of the underlying inventor agreements, upfront advances paid to patent owners by Acacia’s operating subsidiaries are recoverable from future net revenues. Patent costs that are recoverable from future net revenues are amortized over the estimated economic useful life of the related patents, or as the prepaid royalties are earned by the inventor, as appropriate, and the related expense is included in amortization expense in the consolidated statements of operations. Any unamortized patent portfolio investment costs recovered from net revenues are expensed in the period recovered, and included in amortization expense in the consolidated statements of operations. Refer to Note 11 for additional information.
Contingent legal fees are expensed in the consolidated statements of operations in the period that the related revenues are recognized. In instances where there are no recoveries from potential infringers, no contingent legal fees are paid; however, Acacia’s operating subsidiaries may be liable for certain out of pocket legal costs incurred pursuant to the underlying legal services agreement.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Fair Value Measurements. U.S. generally accepted accounting principles define fair value as the price that would be received for an asset or the exit price that would be paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date, and also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs, where available. The three-level hierarchy of valuation techniques established to measure fair value is defined as follows:
|
| | | |
| | ● | Level 1 - | Observable Inputs: Quoted prices in active markets for identical investments; |
| | ● | Level 2 - | Pricing Models with Significant Observable Inputs: Other significant observable inputs, including quoted prices for similar investments, interest rates, credit risk, etc.; and |
| | ● | Level 3 - | Unobservable Inputs: Significant unobservable inputs, including the entity’s own assumptions in determining the fair value of investments. |
Whenever possible, the Company is required to use observable market inputs (Level 1 - quoted market prices) when measuring fair value. Acacia has not elected the fair value option for recording non-financial assets and liabilities, and therefore no fair value measurements are performed on a recurring basis.
Cash and Cash Equivalents. Acacia considers all highly liquid, short-term investments with original maturities of three months or less when purchased to be cash equivalents. For the periods presented, Acacia’s cash equivalents are comprised of investments in AAA rated money market funds that invest in first-tier only securities, which primarily includes: domestic commercial paper, securities issued or guaranteed by the U.S. government or its agencies, U.S. bank obligations, and fully collateralized repurchase agreements. Acacia’s cash equivalents are measured at fair value using quoted prices that represent Level 1 inputs.
Investments in Marketable Securities. Investments in securities with original maturities of greater than three months and less than one year and other investments representing amounts that are available for current operations are classified as short-term investments, unless there are indications that such investments may not be readily sold in the short term. The fair values of these investments approximate their carrying values. At December 31, 2014 and 2013, all of Acacia’s investments were classified as available-for-sale, which are reported at fair value on a recurring basis using significant observable inputs (Level 1), with related unrealized gains and losses in the value of such securities recorded as a separate component of other comprehensive income (loss) in stockholders’ equity until realized. Realized and unrealized gains and losses are recorded based on the specific identification method. Interest on all securities is included in interest and other investment income (loss).
Impairment of Marketable Securities. Acacia evaluates its investments in marketable securities for potential impairment, employing a systematic methodology on a quarterly basis that considers available quantitative and qualitative evidence. If the cost or carrying value of an investment exceeds its estimated fair value, the Company evaluates, among other factors, general market conditions, credit quality of instrument issuers, the duration and extent to which the fair value is less than cost, and the Company’s intent and ability to hold, or plans or ability to sell. Fair value is estimated based on publicly available market information or other estimates determined by management. Investments are considered to be impaired when a decline in fair value is estimated to be other-than-temporary. Acacia reviews impairments associated with its investments in marketable securities and determines the classification of any impairment as temporary or other-than-temporary. An impairment is deemed other-than-temporary unless (a) Acacia has the ability and intent to hold an investment for a period of time sufficient for recovery of its carrying amount and (b) positive evidence indicating that the investment’s carrying amount is recoverable within a reasonable period of time outweighs any evidence to the contrary. All available evidence, both positive and negative, is considered to determine whether, based on the weight of such evidence, the carrying amount of the investment is recoverable within a reasonable period of time. For investments classified as available-for-sale, unrealized losses that are other-than-temporary are recognized in the consolidated statements of operations.
Concentration of Credit Risks. Financial instruments that potentially subject Acacia to concentrations of credit risk are cash equivalents, investments and accounts receivable. Acacia places its cash equivalents and investments primarily in highly rated money market funds and investment grade marketable securities. Cash equivalents are also invested in deposits with certain financial institutions and may, at times, exceed federally insured limits. Acacia has not experienced any significant losses on its deposits of cash and cash equivalents.
Two licensees individually accounted for 22% and 22%, respectively, of revenues recognized during the year ended December 31, 2014. Two licensees individually accounted for 38% and 16%, respectively, of revenues recognized during the year ended December 31, 2013. Four licensees individually accounted for 21%, 14%, 10% and 10%, respectively, of revenues recognized during the year ended December 31, 2012. Three licensees individually represented approximately 30%, 17% and 15%, respectively, of accounts receivable at December 31, 2014. Two licensees individually represented approximately 60%
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
and 22% of accounts receivable at December 31, 2013. For 2014, 2013 and 2012, 43%, 24% and 43%, respectively, of revenues were attributable to licensees domiciled in foreign jurisdictions, based on the jurisdiction of the entity obligated to satisfy payment obligations pursuant to the applicable revenue arrangement. The Company does not have any material foreign operations.
Acacia performs credit evaluations of its licensees with significant receivable balances, if any, and has not experienced any significant credit losses. Accounts receivable are recorded at the executed contract amount and generally do not bear interest. Collateral is not required. An allowance for doubtful accounts may be established to reflect the Company’s best estimate of probable losses inherent in the accounts receivable balance, and is reflected as a contra-asset account on the balance sheet and a charge to operating expenses in the statement of operations for the applicable period. The allowance is determined based on known troubled accounts, historical experience, and other currently available evidence. There was no allowance for doubtful accounts established for the periods presented.
Furniture and Equipment. Furniture and equipment are recorded at cost. Major additions and improvements that materially extend useful lives of furniture and equipment are capitalized. Maintenance and repairs are charged against the results of operations as incurred. When these assets are sold or otherwise disposed of, the asset and related depreciation are relieved, and any gain or loss is included in the consolidated statements of operations for the period of sale or disposal. Depreciation and amortization is computed on a straight-line basis over the following estimated useful lives of the assets:
|
| |
| Furniture and fixtures | 3 to 5 years |
| Computer hardware and software | 3 to 5 years |
| Leasehold improvements | 2 to 5 years (Lesser of lease term or useful life of improvement) |
Rental payments on operating leases are charged to expense in the consolidated statements of operations on a straight-line basis over the lease term.
Patents. Patents include the cost of patents or patent rights (hereinafter, collectively “patents”) acquired from third-parties or obtained in connection with business combinations. Patent costs are amortized utilizing the straight-line method over their remaining economic useful lives, ranging from one to ten years. Certain patent application and prosecution costs incurred to secure additional patent claims, that based on management’s estimates are deemed to be recoverable, are capitalized and amortized over the remaining estimated economic useful life of the related patent portfolio.
Goodwill. Goodwill is tested for impairment at the reporting unit level (operating segment or one level below an operating segment) on an annual basis (December 31) and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value. Acacia considers its market capitalization and the carrying value of its assets and liabilities, including goodwill, when performing its goodwill impairment test. When conducting its annual goodwill impairment assessment, the Company initially performs a qualitative evaluation of whether it is more likely than not that goodwill is impaired. If it is determined by a qualitative evaluation that it is more likely than not that goodwill is impaired, the Company then applies a two-step impairment test. The two-step impairment test first compares the fair value of the Company’s reporting unit to its carrying or book value. If the fair value of the reporting unit exceeds its carrying value, goodwill is not impaired and the Company is not required to perform further testing. If the carrying value of the reporting unit exceeds its fair value, the Company determines the implied fair value of the reporting unit’s goodwill and if the carrying value of the reporting unit’s goodwill exceeds its implied fair value, then an impairment loss equal to the difference is recorded in the consolidated statement of operations.
Impairment of Long-lived Assets. Acacia reviews long-lived assets and intangible assets for potential impairment annually (quarterly for patents) and when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss is recorded equal to the excess of the asset’s carrying value over its fair value. If an asset is determined to be impaired, the loss is measured based on quoted market prices in active markets, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows.
Fair value is generally estimated using the “Income Approach,” focusing on the estimated future net income-producing capability of the patent portfolios over the estimated remaining economic useful life. Estimates of future after-tax cash flows are converted to present value through “discounting,” including an estimated rate of return that accounts for both the time value of money and investment risk factors. Estimated cash inflows are typically based on estimates of reasonable royalty rates for
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
the applicable technology, applied to estimated market share data. Estimated cash outflows are based on existing contractual obligations, such as contingent legal fee and inventor royalty obligations, applied to estimated license fee revenues, in addition to other estimates of out-of-pocket expenses associated with a specific patent portfolio’s licensing and enforcement program. The analysis also contemplates consideration of current information about the patent portfolio including, status and stage of litigation, periodic results of the litigation process, strength of the patent portfolio, technology coverage and other pertinent information that could impact future net cash flows.
Fair Value of Financial Instruments. The carrying value of cash and cash equivalents, investments, accounts receivables, accounts payable and accrued expenses approximates their fair values due to their short-term maturities.
Contingent Liabilities. The Company, from time to time, is involved in certain legal proceedings. Based upon consultation with outside counsel handling its defense in these matters and the Company’s analysis of potential outcomes, if the Company determines that a loss arising from such matters is probable and can be reasonably estimated, an estimate of the contingent liability is recorded in its consolidated financial statements. If only a range of estimated loss can be determined, an amount within the range that, based on estimates, assumptions and judgments, reflects the most likely outcome, is recorded as a contingent liability in the consolidated financial statements. In situations where none of the estimates within the estimated range is a better estimate of probable loss than any other amount, the Company records the low end of the range. Any such accrual would be charged to expense in the appropriate period. Litigation expenses for these types of contingencies are recognized in the period in which the litigation services were provided.
Stock-Based Compensation. The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award, and is recognized as an expense on a straight-line basis over the employee’s requisite service period (generally the vesting period of the equity award) which is generally two to four years. The fair value of restricted stock and restricted stock unit awards is determined by the product of the number of shares or units granted and the grant date market price of the underlying common stock. The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. Stock-based compensation expense is recorded only for those awards expected to vest using an estimated forfeiture rate. Refer to Note 10 to these notes to consolidated financial statements for information on stock-based awards granted for the periods presented.
Income Taxes. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in Acacia’s consolidated financial statements or consolidated income tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized, or if it is determined that there is uncertainty regarding future realizability of such assets.
Under U.S. generally accepted accounting principles, a tax position is a position in a previously filed tax return or a position expected to be taken in a future tax filing that is reflected in measuring current or deferred income tax assets and liabilities. Tax positions are recognized only when it is more likely than not (likelihood of greater than 50%), based on technical merits, that the position will be sustained upon examination. Tax positions that meet the more likely than not threshold are measured using a probability weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement.
If a deduction reported on a tax return for an equity-based incentive award exceeds the cumulative compensation cost for those instruments recognized for financial reporting purposes, any resulting realized tax benefit that exceeds the previously calculated deferred tax asset for those instruments is considered an excess tax benefit, and is recognized as additional paid-in capital. If the tax deduction is less than the cumulative book compensation cost, the tax effect of the resulting difference is charged first to APIC, to the extent of the available pool of windfall tax benefits, with any remainder recognized in income tax expense.
Segment Reporting. Acacia uses the management approach, which designates the internal organization that is used by management for making operating decisions and assessing performance as the basis of Acacia’s reportable segments. Acacia’s patent licensing and enforcement business constitutes its single reportable segment.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Acacia believes that, of the significant accounting policies described herein, the accounting policies associated with revenue recognition, stock-based compensation expense, impairment of marketable securities and intangible assets, the determination of
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
the economic useful life of amortizable intangible assets, income taxes and valuation allowances against net deferred tax assets and the application of the acquisition method of accounting for business combinations, require its most difficult, subjective or complex judgments.
Income (Loss) Per Share. The Company computes net income (loss) attributable to common stockholders using the two-class method required for capital structures that include participating securities. Under the two-class method, securities that participate in non-forfeitable dividends, such as the Company’s outstanding unvested restricted stock, are considered “participating securities.”
In applying the two-class method, (i) basic net income (loss) per share is computed by dividing net income (loss) (less any dividends paid on participating securities) by the weighted average number of shares of common stock and participating securities outstanding for the period and (ii) diluted earnings per share may include the additional effect of other securities, if dilutive, in which case the dilutive effect of such securities is calculated by applying the two-class method and the treasury stock method to the assumed exercise or vesting of potentially dilutive common shares. The method yielding the more dilutive result is ultimately reported for the applicable period. Potentially dilutive common stock equivalents primarily consist of employee stock options, and restricted stock units for calculations utilizing the two-class method, and also include unvested restricted stock, when utilizing the treasury method.
The following table presents the weighted-average number of common shares outstanding used in the calculation of basic and diluted income per share:
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| Numerator (in thousands): | | | | | | |
| Basic | | | | | | |
| Net income (loss) | | $ | (66,029 | ) | | $ | (56,434 | ) | | $ | 59,453 |
|
| Undistributed earnings allocated to participating securities | | — |
| | — |
| | (1,889 | ) |
| Total dividends paid | | (25,039 | ) | | (18,633 | ) | | — |
|
| Dividends attributable to common stockholders | | 24,313 |
| | 18,122 |
| | — |
|
| Net income (loss) attributable to common stockholders – basic | | $ | (66,755 | ) | | $ | (56,945 | ) | | $ | 57,564 |
|
| | | | | | | |
| Diluted | | | | | | |
| Net income (loss) | | $ | (66,029 | ) | | $ | (56,434 | ) | | $ | 59,453 |
|
| Undistributed earnings allocated to participating securities | | — |
| | — |
| | (1,876 | ) |
| Total dividends paid | | (25,039 | ) | | (18,633 | ) | | — |
|
| Dividends attributable to common stockholders | | 24,313 |
| | 18,122 |
| | — |
|
| Net income (loss) attributable to common stockholders – diluted | | $ | (66,755 | ) | | $ | (56,945 | ) | | $ | 57,577 |
|
| | | | | | | |
| Denominator: | | | | | | |
| Weighted-average shares used in computing net income (loss) per share attributable to common stockholders – basic | | 48,658,088 |
| | 48,155,832 |
| | 47,251,061 |
|
| Effect of potentially dilutive securities: | | | | | | |
| Common stock options and restricted stock units | | — |
| | — |
| | 333,059 |
|
| Weighted-average shares used in computing net income (loss) per share attributable to common stockholders – diluted | | 48,658,088 |
| | 48,155,832 |
| | 47,584,120 |
|
| | | | | | | |
| Basic net income (loss) per common share | | $ | (1.37 | ) | | $ | (1.18 | ) | | $ | 1.22 |
|
| Diluted net income (loss) per common share | | $ | (1.37 | ) | | $ | (1.18 | ) | | $ | 1.21 |
|
| Anti-dilutive equity-based incentive awards excluded from the computation of diluted income (loss) per share | | 27,760 |
| | 27,760 |
| | 30,812 |
|
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Revision of Prior Period Earnings (Loss) Per Share - Two-Class Method. In connection with the preparation of the Company’s Quarterly Report on Form 10-Q as of and for the three and nine months ended September 30, 2013, the Company determined that its basic and diluted net income (loss) per share calculations should have been prepared using the “two-class method.” Pursuant to guidance set forth in Staff Accounting Bulletin (“SAB”) No. 99, “Materiality,” the Company concluded that the errors were not material to any of its prior period financial statements. Although the errors were immaterial to prior periods, the prior period financial statements presented herein were revised, in accordance with SAB No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements.” The impact of the revision for the comparable prior period earnings (loss) per share calculations using the two-class method were as follows: |
| | | | |
| | | 2012 |
| Numerator: | | |
| Net income attributable to common stockholders – basic and diluted - As Reported | | $ | 59,453 |
|
| Net income attributable to common stockholders – basic - As Adjusted | | $ | 57,564 |
|
| Net income attributable to common stockholders – diluted - As Adjusted | | $ | 57,577 |
|
| | | |
| Denominator: | | |
| Weighted-average shares used in computing net income per share attributable to common stockholders – basic - As Reported | | 47,251,061 |
|
| Weighted-average shares used in computing net income per share attributable to common stockholders – basic - As Adjusted | | 47,251,061 |
|
| Weighted-average shares used in computing net income per share attributable to common stockholders – diluted - As Reported | | 48,060,647 |
|
| Weighted-average shares used in computing net income per share attributable to common stockholders – diluted - As Adjusted | | 47,584,120 |
|
| | | |
| Basic net income per common share - As Reported | | $ | 1.26 |
|
| Basic net income per common share - As Adjusted | | $ | 1.22 |
|
| Diluted net income per common share - As Reported | | $ | 1.24 |
|
| Diluted net income per common share - As Adjusted | | $ | 1.21 |
|
Treasury Stock. Repurchases of the Company’s outstanding common stock are accounted for using the cost method. The applicable par value is deducted from the appropriate capital stock account on the formal or constructive retirement of treasury stock. Any excess of the cost of treasury stock over its par value is charged to additional paid-in capital, and reflected as Treasury Stock on the consolidated balance sheets.
Recent Accounting Pronouncements - Not Yet Adopted. In June 2014, the Financial Accounting Standards Board (the “FASB”) issued a new accounting standard which requires that a performance target that affects vesting and could be achieved after the requisite service period shall be treated as a performance condition. Adoption of this standard is required for annual periods beginning after December 15, 2015. Early adoption is permitted. The Company is currently evaluating the impact the pronouncement will have on its consolidated financial statements and related disclosures.
In May 2014, the FASB issued a new accounting standards update addressing revenue from contracts with customers, which clarifies existing accounting literature relating to how and when a company recognizes revenue. Under the standard, a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods and services. The amendments for this standard update are effective for interim and annual reporting periods beginning after December 15, 2016, and are to be applied
retrospectively or the cumulative effect as of the date of adoption, with early application not permitted. The Company is currently evaluating the impact and method of adoption the pronouncement will have on its consolidated financial statements and related disclosures.
In August 2014, the FASB issued a new accounting standard which requires management to assess an entity’s ability to continue as a going concern every reporting period including interim periods, and to provide related footnote disclosure in certain circumstances. Adoption of this standard is required for annual periods beginning after December 15, 2016 and are to be applied retrospectively or the cumulative effect as of the date of adoption. Early adoption is permitted. The Company is currently evaluating the impact the pronouncement will have on its consolidated financial statements and related disclosures.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Recently Adopted Accounting Pronouncements - Adopted Effective January 1, 2014. In July 2013, the FASB issued a new accounting standard addressing when unrecognized tax benefits should be presented as reductions to deferred tax assets for net operating loss carryforwards in the financial statements. This standard was adopted effective January 1, 2014. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements and related disclosures.
In March 2013, the FASB issued a new accounting standard addressing the accounting for the cumulative translation adjustment when a parent either sells a part or all of its investment in a foreign entity or no longer holds a controlling financial interest in a subsidiary or group of assets that is a nonprofit activity or a business within a foreign entity. This standard was adopted effective January 1, 2014. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements and related disclosures.
3. SHORT-TERM INVESTMENTS
Short-term marketable securities for the periods presented were comprised of the following (in thousands): |
| | | | | | | | | | | | | | | |
| | December 31, 2014 |
| Security Type | Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| U.S. government fixed income securities | $ | 58,819 |
| | $ | 2 |
| | $ | (263 | ) | | $ | 58,558 |
|
| Total short-term investments | $ | 58,819 |
| | $ | 2 |
| | $ | (263 | ) | | $ | 58,558 |
|
|
| | | | | | | | | | | | | | | |
| | December 31, 2013 |
| Security Type | Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| U.S. government fixed income securities | $ | 130,971 |
| | $ | 21 |
| | $ | (975 | ) | | $ | 130,017 |
|
| Total short-term investments | $ | 130,971 |
| | $ | 21 |
| | $ | (975 | ) | | $ | 130,017 |
|
Short-term investments at December 31, 2014 and 2013 were comprised of investments in highly liquid, AAA, U.S. government fixed income securities with maturity dates in 2015, and ranging from 2014 to 2015, respectively. Short-term marketable securities in unrealized loss positions at December 31, 2014 and 2013 have been in continuous unrealized loss positions for less than one year.
U.S. government fixed income securities. The gross unrealized loss can be primarily attributed to a combination of market conditions as well as the demand for and duration of the U.S. government fixed income securities. The Company has the ability to hold these securities until maturity, currently has no intent to sell, there is no requirement to sell and the Company believes that it can recover the amortized cost of these investments. The Company has found no evidence of impairment due to credit losses in its portfolio. Therefore, these unrealized losses were recorded in other comprehensive income (loss). However, the Company cannot provide any assurance that its portfolio of short-term marketable securities will not be impacted by adverse conditions in the financial markets, which may require the Company in the future to record an impairment charge for credit losses which could adversely impact its financial results.
For the year ended December 31, 2014, proceeds from the sale of short-term marketable securities classified as available-for-sale were $182,115,000 and gross realized losses were $2,188,000. Gross realized losses are recorded in the statements of operations in interest and other investment income (loss). For the year ended December 31, 2013, proceeds from the sale of short-term marketable securities classified as available-for-sale were $239,370,000, gross realized gains were $1,174,000 and gross realized losses were $981,000. For the year ended December 31, 2012, proceeds from the sale of short-term marketable securities classified as available-for-sale were $319,811,000, gross realized gains were $31,000 and gross realized losses were $555,000.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
4. FURNITURE AND EQUIPMENT
Furniture and equipment consists of the following at December 31, 2014 and 2013 (in thousands):
|
| | | | | | | | |
| | | 2014 | | 2013 |
| | | | | |
| Furniture and fixtures | | $ | 761 |
| | $ | 783 |
|
| Computer hardware and software | | 650 |
| | 687 |
|
| Leasehold improvements | | 144 |
| | 306 |
|
| | | 1,555 |
| | 1,776 |
|
| Less: accumulated depreciation and amortization | | (1,055 | ) | | (1,010 | ) |
| | | $ | 500 |
| | $ | 766 |
|
Depreciation expense was $304,000, $236,000 and $149,000 for the years ended December 31, 2014, 2013 and 2012, respectively. In 2014 and 2013, the Company retired $330,000 and $130,000, respectively, of items held in furniture and equipment and recorded a $71,000 and $12,000, respectively, loss on disposal.
5. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consist of the following at December 31, 2014 and 2013 (in thousands):
|
| | | | | | | | |
| | | 2014 | | 2013 |
| | | | | |
| Accounts payable | | $ | 121 |
| | $ | 128 |
|
| Payroll and other employee benefits | | 1,481 |
| | 1,039 |
|
| Accrued vacation | | 806 |
| | 813 |
|
| Accrued legal expenses - patent | | 8,410 |
| | 5,900 |
|
| Accrued attorney's fees | | 1,548 |
| | — |
|
| Accrued consulting and other professional fees | | 1,530 |
| | 2,948 |
|
| Accrued distribution to noncontrolling interests | | — |
| | 504 |
|
| Other accrued liabilities | | 964 |
| | 223 |
|
| | | $ | 14,860 |
| | $ | 11,555 |
|
6. PATENTS
Acacia’s only identifiable intangible assets are patents and patent rights, with estimated remaining economic useful lives ranging from one to nine years. For all periods presented, all of Acacia’s identifiable intangible assets were subject to amortization. The gross carrying amounts and accumulated amortization related to investments in intangible assets as of December 31, 2014 and 2013 are as follows (in thousands):
|
| | | | | | | | |
| | | 2014 | | 2013 |
| | | | | |
| Gross carrying amount - patents | | $ | 453,201 |
| | $ | 400,755 |
|
| Accumulated amortization - patents | | (166,565 | ) | | (112,323 | ) |
| Patents, net | | $ | 286,636 |
| | $ | 288,432 |
|
The weighted-average remaining estimated economic useful life of Acacia’s patents and patent rights is 6 years. Scheduled annual aggregate amortization expense is estimated to be $52,019,000 in 2015, $49,332,000 in 2016, $48,245,000 in 2017, $44,117,000 in 2018, $38,679,000 in 2019 and $54,244,000 thereafter.
For the years ended December 31, 2014, 2013 and 2012, Acacia paid patent investment costs totaling $42,746,000, $25,061,000 and $178,260,000 (excluding the investment in ADAPTIX), respectively. The patents have estimated economic useful lives ranging from three to ten years. Included in net additions to capitalized patent costs during the years ended
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2014 and 2013 are accrued patent investment costs totaling $16,700,000 and $4,000,000, respectively, which are amortized over the estimated economic useful life of the related patents.
Refer to Note 7 to these consolidated financial statements for additions to patents and goodwill in connection with Acacia’s acquisition of ADAPTIX and the related application of the acquisition method of accounting.
During the periods presented, certain operating subsidiaries recovered up-front patent portfolio advances from applicable net licensing proceeds prior to the scheduled amortization of such up-front patent portfolio advances, resulting in the acceleration of amortization expense for the applicable patent-related assets. For the years ended December 31, 2014, 2013 and 2012, accelerated amortization expense related to the recovery of up-front patent portfolio advances totaled $1,247,000, $592,000 and $10,574,000, respectively.
For the years ended December 31, 2014, 2013 and 2012, pursuant to the terms of the respective inventor agreements, certain Acacia operating subsidiaries elected to terminate or sell their rights to patent portfolios, resulting in the acceleration of amortization expense for the patent-related assets totaling $2,702,000, $1,747,000 and $3,034,000, respectively. Included in amortization of patents for the year ended December 31, 2014 and 2013 are patent impairment charges totaling $3,497,000 and $4,619,000, respectfully. There was no impairment charge in 2012. The impairment charges related to partial impairment of a portfolio due to a reduction in expected estimated future net cash flows (2014 only) and certain patent portfolios that management determined it would no longer allocate future resources to in connection with the licensing and enforcement of such portfolios, due primarily to potential prior art related complexities in two of the programs (2013 only) and/or the overall determination that future resources would be allocated to other licensing and enforcement programs with higher potential return profiles. The impairment charges consisted of the excess of the asset’s carrying value over its estimated fair value as of December 31, 2014 and 2013.
For the years ended December 31, 2014, 2013 and 2012, capitalized patent costs and accumulated amortization, and sales proceeds and other costs, related to patent-related sales and disposals are as follows (in thousands):
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Capitalized patent costs | | $ | 3,000 |
| | $ | 3,500 |
| | $ | 5,500 |
|
| Accumulated amortization | | 298 |
| | 1,753 |
| | 2,466 |
|
| Sales proceeds | | 3,500 |
| | 1,000 |
| | 2,792 |
|
7. ACQUISITION
On January 12, 2012 (the “Acquisition Date”), pursuant to the terms and conditions of the Agreement and Plan of Merger dated as of November 22, 2011 (the “Merger Agreement”) among Acacia Research Group LLC (“ARG”), a wholly-owned subsidiary of Acacia, Apollo Patent Corp., a newly-formed, wholly-owned subsidiary of ARG (“Merger Sub”), ADAPTIX, a Delaware corporation, and Baker Communications Fund II (QP), L.P. solely in its capacity as shareholder representative, ARG completed its acquisition of ADAPTIX, which held no material assets other than its portfolio of patents and $10,000,000 in cash, through a merger of Merger Sub with and into ADAPTIX, with ADAPTIX as the surviving corporation (the “Merger”). Upon completion of the Merger, the separate corporate existence of Merger Sub ceased and ADAPTIX became a wholly-owned subsidiary of ARG.
ADAPTIX, a pioneer in the development of 4G technologies for wireless systems, is a technology company recognized in the industry as one of the first developers of 4G wireless systems. With patents filed as early as 2000, ADAPTIX’s research and development efforts have resulted in a significant intellectual property portfolio focused on 4G technologies. With its growing portfolio of 230 issued and pending patents in 13 countries, ADAPTIX’s innovations extend across a broad range of 4G technologies including OFDMA and MIMO.
The Merger was being accounted for in accordance with the acquisition method of accounting under FASB ASC Topic 805, “Business Combinations” (“Topic 805”). Topic 805 requires, among other things, that identifiable assets acquired and liabilities assumed be recognized at their fair values as of the Acquisition Date. Under the acquisition method of accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangible assets and liabilities assumed, based on their estimated fair market values on the date of acquisition. Any excess purchase price after the initial allocation to identifiable net tangible and identifiable intangible assets is assigned to goodwill. Amounts
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
attributable to patents are amortized using the straight-line method over the estimated economic useful life of the underlying patents.
The total consideration paid by ARG in connection with the Merger was approximately $160,000,000, in cash. Based on the total purchase consideration and the estimate of the assets acquired and the liabilities assumed by ARG as of the Acquisition Date, the purchase price allocation was as follows ($ amounts in thousands):
|
| | | | | | | | | | |
| | | | | Amortization Period | | Annual Amortization |
| Assets Acquired and Liabilities Assumed: | | | | | | |
| | | | | | | |
| Fair value of net tangible assets acquired | | $ | 10,000 |
| | | | |
| Intangible assets acquired - patents | | 150,000 |
| | 10 years | | $ | 15,000 |
|
| Goodwill | | 30,149 |
| | | | |
| Net deferred income tax liability | | (30,149 | ) | | | | |
| Total | | $ | 160,000 |
| | | | |
Amounts attributable to the patents acquired are being amortized using the straight-line method over an estimated weighted average economic useful life of the underlying patents, which was estimated to be approximately 10 years. Goodwill is calculated as the residual after recording the identifiable net assets acquired and associated net deferred tax assets and liabilities.
Management is responsible for determining the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed as of the Acquisition Date. Management considered a number of factors, including reference to an analysis under Topic 805 solely for the purpose of allocating the purchase price to the assets acquired and liabilities assumed. The analysis included a discounted cash flow which estimated future net cash flows resulting from the licensing and enforcement of the patent portfolio based on information as of the date of acquisition, considering assumptions and estimates related to potential infringers of the patents, applicable industries, usage of the underlying patented technologies, estimated license fee revenues, contingent legal fee arrangements, other estimated costs, tax implications and other factors. A discount rate consistent with the risks associated with achieving the estimated net cash flows was used to estimate the present value of estimated net cash flows.
The Merger was treated for tax purposes as a nontaxable transaction and as such, the historical tax bases of the acquired assets and assumed liabilities, net operating losses, and other tax attributes of ADAPTIX will carryover. As a result, no new tax goodwill will be created in connection with the Merger as there is no step-up to fair value of the underlying tax bases of the acquired net assets. Acquisition accounting includes the establishment of a net deferred tax asset or liability resulting from book tax basis differences related to assets acquired and liabilities assumed on the date of acquisition. Acquisition date deferred tax assets primarily relate to certain net operating loss carryforwards of ADAPTIX. Acquisition date deferred tax liabilities relate to specifically identified non-goodwill intangibles acquired. The estimated net deferred tax liability was determined as follows ($ amounts in thousands):
|
| | | | | | | | | | | | |
| | | Book Basis | | Tax Basis | | Difference |
| | | | | | | |
| Intangible assets acquired - patents | | $ | 150,000 |
| | $ | — |
| | $ | (150,000 | ) |
| Estimated acquired deferred tax assets (including net operating loss carryforwards) - ADAPTIX | | — |
| | 63,860 |
| | 63,860 |
|
| Net deferred tax liability - pretax | | | | | | (86,140 | ) |
| Estimated tax rate | | | | | | 35 | % |
| Estimated net deferred tax liability | | | | | | $ | (30,149 | ) |
The following unaudited pro forma combined results of operations for periods presented are provided for illustrative purposes only and assume the acquisition occurred as of January 1, 2012. The unaudited pro forma combined financial results do not purport to be indicative of the results of operations for future periods or the results that actually would have been realized had the entities been a single entity during these periods. The unaudited pro forma combined results are presented in thousands, except share and per share information.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
| | | |
| | 2012 |
| | |
| Revenues | $ | 250,727 |
|
| Total operating costs and expenses | 170,953 |
|
| Operating income | 79,774 |
|
| Interest and investment income | 937 |
|
| Income from operations before provision for income taxes | 80,711 |
|
| Provision for income taxes | (22,060 | ) |
| Net income including noncontrolling interests in operating subsidiaries | 58,651 |
|
| Net loss (income) attributable to noncontrolling interests in operating subsidiaries | 164 |
|
| Net income attributable to Acacia Research Corporation | $ | 58,815 |
|
|
| | | |
| | 2012 |
| | |
| Pro forma income per common share attributable to Acacia Research Corporation: | |
|
| Basic earnings per share | $ | 1.24 |
|
| Diluted earnings per share | $ | 1.22 |
|
| Weighted average number of shares outstanding, basic | 47,251,061 |
|
| Weighted average number of shares outstanding, diluted | 48,060,647 |
|
Pro forma adjustments primarily relate to the amortization of identifiable intangible assets acquired over an estimated economic useful life of ten years, historical operating expenses of ADAPTIX for 2012, and the expensing of acquisition costs incurred by ARG in connection with the Merger.
The unaudited pro forma combined statements of income for the periods presented herein have been adjusted to give effect to pro forma events that are expected to have a continuing impact on the combined results. As such, the income tax benefit related to the release of valuation allowance reflected in the statement of operations for 2012, as described at Note 9, is not reflected in the accompanying unaudited pro forma combined statements of operations for the periods presented.
8. STOCKHOLDERS’ EQUITY
Equity Offerings. In February 2012, Acacia raised gross proceeds of $225,000,000 through the sale of 6,122,449 shares of Acacia’s common stock at a price of $36.75 per share in a private placement offering with certain institutional accredited investors. Net proceeds, net of placement agent fees and estimated offering expenses, totaled approximately $218,961,000. The use of proceeds included the finance of pending and future acquisitions of patents and patent royalties and other patent licensing vehicles and companies with patent assets, and for working capital and general corporate purposes.
Repurchases of Common Stock. On November 16, 2012, Acacia’s Board of Directors authorized a program for repurchases of shares of Acacia’s outstanding common stock. Under the stock repurchase program, effective November 16, 2012, Acacia was authorized to purchase in the aggregate up to $100,000,000 of its outstanding common stock through the period ended May 15, 2013. On April 23, 2013, Acacia’s Board of Directors approved an extension of the stock repurchase program from May 15, 2013 until August 15, 2013. The November 16, 2012 program expired on August 15, 2013.
On November 15, 2013, Acacia’s Board of Directors authorized a program for repurchases of shares of Acacia’s outstanding common stock. Under the stock repurchase program, effective November 15, 2013, Acacia was authorized to purchase in the aggregate up to $70,000,000 of its of its outstanding common stock through the period ending May 14, 2014.
Repurchases were made from time to time by Acacia in the open market or in block purchases in compliance with applicable Securities and Exchange Commission rules. Repurchases to date were made using existing cash resources and occurred in the open market. The authorization to repurchase shares presented an opportunity to reduce the outstanding share count and enhance stockholder value. The following is the monthly stock repurchases for the periods presented, all of which were purchased as part of publicly announced plans or programs:
|
| | | | | | | | | |
| �� | Total Number of Shares Purchased | Average Price paid per Share | Approximate Dollar Value of Shares that May Yet be Purchased under the Program | Plan Expiration |
| | | | | |
| November 16, 2012 - November 30, 2012 | 256,262 |
| $ | 21.58 |
| $ | — |
| August 15, 2013 |
| December 1, 2012 - December 31, 2012 | 873,146 |
| $ | 24.26 |
| $ | — |
| August 15, 2013 |
| Totals for 2012 | 1,129,408 |
| $ | 23.65 |
| | |
| | | | | |
| December 4, 2013 - December 11, 2013 | 600,000 |
| $ | 13.18 |
| $ | — |
| May 14, 2014 |
| Totals for 2013 | 600,000 |
| | | |
Cash Dividends. On April 23, 2013, Acacia announced that its Board of Directors approved the adoption of a cash dividend policy that calls for the payment of an expected total annual cash dividend of $0.50 per common share, payable in the amount of $0.125 per share per quarter. Under the policy, the Company paid four quarterly cash dividends totaling $25,039,000 in 2014 and three quarterly cash dividends totaling $18,633,000 in 2013. While the Company paid dividends to holders of its common stock on a quarterly basis during fiscal years 2014 and 2013, the declaration and payment of future dividends will depend on many factors, including, but not limited to, earnings and financial condition, and any future dividends will be made solely at the discretion of the Board of Directors.
On February 19, 2015, Acacia announced that its Board of Directors approved a quarterly cash dividend payable in the amount of $0.125 per share. The quarterly cash dividend will be paid on March 30, 2015 to stockholders of record at close of business on March 2, 2015.
9. INCOME TAXES
Acacia’s provision for income taxes consists of the following for the years ended December 31, (in thousands):
|
| | | | | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Current: | | | | | | |
| Federal | | $ | — |
| | $ | — |
| | $ | — |
|
| State taxes | | 289 |
| | 113 |
| | 281 |
|
| Foreign taxes | | 5,359 |
| | 4,405 |
| | 11,890 |
|
| Total current | | 5,648 |
| | 4,518 |
| | 12,171 |
|
| Deferred: | | | | | | |
| Federal | | (1,867 | ) | | (26,151 | ) | | 10,085 |
|
| State taxes | | 131 |
| | (325 | ) | | (196 | ) |
| Total deferred | | (1,736 | ) | | (26,476 | ) | | 9,889 |
|
| Provision for (benefit from) income taxes | | $ | 3,912 |
| | $ | (21,958 | ) | | $ | 22,060 |
|
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The tax effects of temporary differences and carryforwards that give rise to significant portions of deferred tax assets and liabilities consist of the following at December 31, 2014 and 2013 (in thousands):
|
| | | | | | | | |
| | | 2014 | | 2013 |
| | | | | |
| Deferred tax assets: | | | | |
| Net operating loss and capital loss carryforwards and credits | | $ | 59,427 |
| | $ | 34,679 |
|
| Stock compensation | | 1,800 |
| | 3,052 |
|
| Basis of investments in affiliates | | 1,437 |
| | 867 |
|
| Accrued liabilities and other | | 409 |
| | 387 |
|
| Unrealized loss on short-term investments | | 92 |
| | 337 |
|
| State taxes | | 26 |
| | 18 |
|
| Total deferred tax assets | | 63,191 |
| | 39,340 |
|
| Valuation allowance | | (35,927 | ) | | (7,585 | ) |
| Total deferred tax assets, net of valuation allowance | | 27,264 |
| | 31,755 |
|
| | | | | |
| Deferred tax liabilities: | | | | |
| Fixed assets and intangibles | | (27,157 | ) | | (33,378 | ) |
| Other | | (107 | ) | | (112 | ) |
| Total deferred tax liabilities | | (27,264 | ) | | (33,490 | ) |
| | | | | |
| Net deferred tax assets (liabilities) | | $ | — |
| | $ | (1,735 | ) |
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows:
|
| | | | | | | | | |
| | | 2014 | | 2013 | | 2012 |
| | | | | | | |
| Statutory federal tax rate - (benefit) expense | | (35 | )% | | (35 | )% | | 35 | % |
| State income and foreign taxes, net of federal tax effect | | 9 | % | | 5 | % | | 15 | % |
| Foreign tax credit | | (8 | )% | | (6 | )% | | (15 | )% |
| Noncontrolling interests in operating subsidiaries | | — | % | | 1 | % | | — | % |
| Nondeductible permanent items | | 1 | % | | 2 | % | | 5 | % |
| Expired net operating loss carryforwards | | — | % | | 2 | % | | — | % |
| Valuation allowance | | 39 | % | | 4 | % | | (13 | )% |
| | | 6 | % | | (27 | )% | | 27 | % |
At December 31, 2013, the Company recorded valuation allowances for certain tax attribute carryforwards and other deferred tax assets due to uncertainty regarding future realizability, as follows:
|
| | | | |
| | | 2013 |
| | | |
| Capital loss carryforwards | | $ | 1,562 |
|
| Net operating loss carryforwards | | 1,281 |
|
| Foreign tax credits | | 4,405 |
|
| Unrealized losses on short-term investments and other deferred tax assets | | 337 |
|
| Total valuation allowance | | $ | 7,585 |
|
At December 31, 2014, the Company recorded a full valuation allowance against its net deferred tax assets due to uncertainty regarding future realizability pursuant to guidance set forth in ASC 740, “Income Taxes.” In future periods, if the Company determines it will more likely than not be able to realize certain of these amounts, the applicable portion of the benefit from the release of the valuation allowance will generally be recognized in the statement of operations in the period the determination is made.
Capital loss carryforwards and certain net operating loss carryforwards included in the valuation allowances for the periods presented expire in varying amounts from 2015 through 2034. Foreign tax credits included in the valuation allowance were generated during the years ended December 31, 2013 and 2014, and expire in 2023 and 2024, respectively.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2011, Acacia maintained a full valuation allowance against its net deferred tax assets. The net deferred tax liability resulting from the acquisition of ADAPTIX in January 2012 created an additional source of income to utilize against the majority of Acacia’s existing consolidated net deferred tax assets. In addition, Acacia estimated that certain other deferred tax assets, primarily related to foreign tax credits and other state related deferred tax assets, were more likely than not realizable in future periods. Accordingly, the valuation allowance on the majority of the Company’s net deferred tax assets was released, resulting in a financial statement income tax benefit of $10,651,000 for the year ended December 31, 2012.
At December 31, 2014, Acacia had U.S. federal and state income tax net operating loss carryforwards (“NOLs”) totaling approximately $118,715,000 and $129,632,000, expiring between 2025 and 2034, and 2015 and 2034, respectively, for which $0 and $441,000 of federal and state net operating losses are included as a deferred tax asset related to the tax benefits of stock option deductions and which will be credited to additional paid-in capital when realized as a reduction of taxes payable on Acacia’s tax return. In addition, $1,928,000 and $37,771,000 of federal and state net operating losses are not included as a deferred tax asset and will be credited to additional paid-in capital when realized as a reduction of taxes payable on Acacia’s tax return as they relate to unrecognized excess tax benefits (see additional information regarding the ordering of windfall tax benefits and use of the “with-and-without” approach below).
At December 31, 2014, approximately $29,318,000 of the U.S. federal NOLs, acquired in connection with the acquisition of ADAPTIX, are subject to an annual utilization limitation of approximately $14,100,000, pursuant to the “change in ownership” provisions under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”).
As of December 31, 2014, Acacia has approximately $29,877,000 of foreign tax credits, expiring between 2015 and 2024, of which $20,313,000 has been utilized for financial statement purposes. Future realization of the credits as a reduction of taxes payable on Acacia’s tax return will result in an income tax benefit recognizable through additional paid in capital since the entire amount of the credits have been utilized for financial statement purposes under the “with-and-without approach.” In general, foreign taxes withheld may be claimed as a deduction on future U.S. corporate income tax returns, or as a credit against future U.S. income tax liabilities, subject to certain limitations.
Tax expense for fiscal year 2014 primarily reflects foreign taxes withheld on revenue agreements with licensees in foreign jurisdictions, the benefit, totaling $1,735,000, from the reversal of the net deferred tax liability that existed at the beginning of the year and other state taxes. Excluding the impact of the change in valuation allowance in fiscal years 2014, 2013 and 2012, annual effective tax rates were (33)%, (31%), and 40%, respectively. In 2014, the rate at which the Company recorded the tax benefit associated with the pre-tax loss for the period was reduced from the statutory rate primarily due to the full valuation allowance and certain nondeductible permanent items. The Company recorded a full valuation allowance on its net deferred tax assets, as discussed above, and therefore, did not recognize the related tax benefit in fiscal year 2014, other than the benefit from the reversal of the deferred tax liability that existed at the beginning of the year. In 2013, the rate at which the Company recorded the tax benefit associated with the pre-tax loss for the period was reduced from the statutory rate primarily due to certain nondeductible permanent items and expired capital loss carryforwards. The Company recorded a valuation allowance on foreign tax credits generated in fiscal year 2013 totaling $4,605,000, as discussed above, and therefore, did not recognize the related tax benefit for these tax assets in fiscal year 2013. The Company generated pretax income in 2012, resulting in tax expense for the period, and had significant nondeductible permanent items which increased the effective tax rate as shown above. The fiscal year 2012 effective tax rate was lower than the U.S. federal statutory rate primarily due to $10,651,000 million of tax benefits recognized resulting from the release of valuation allowance on the majority of the net deferred tax assets in the first quarter of 2012, as discussed above.
The Company has elected to utilize the “with-and-without approach” regarding ordering of windfall tax benefits to determine whether the windfall tax benefit has reduced taxes payable. Under this approach, the windfall tax benefits would be recognized in additional paid in capital only if an incremental tax benefit is realized after considering all other tax benefits presently available to the Company. The deductions related to the exercise and vesting of equity-based incentive awards during the periods presented are, in general, available to offset taxable income on Acacia’s consolidated tax returns. Accordingly, the excess tax benefit related to the exercise and vesting of equity-based incentive awards for the periods presented was credited to additional paid-in capital, not taxes payable. The actual tax benefit realized for excess tax deductions resulting from the exercise and vesting of equity-based incentive awards (noncash tax expense) totaled $13,210,000 for the year ended December 31, 2012. For the year ended December 31, 2013, the Company incurred approximately $1,398,000 of net short falls from the exercise and vesting of equity-based incentive awards, of which $1,398,000 was recorded against its additional paid-in capital pool with no impact to the income statement. For the year ended December 31, 2014, the Company incurred approximately $2,713,000 of net short falls from the exercise and vesting of equity-based incentive awards, of which $2,713,000 was recorded against its additional paid-in capital, subject to a full valuation allowance, with no impact to the income statement.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Acacia is subject to taxation in the U.S. and in various state jurisdictions and incurs foreign tax withholdings on revenue agreements with licensees in certain foreign jurisdictions. With no material exceptions, Acacia is no longer subject to U.S. federal or state examinations by tax authorities for years before 1998. The California Franchise Tax Board is auditing the 2011 and 2012 California combined income tax returns. The audit is in process and no findings or adjustments have been proposed.
At December 31, 2014, the Company had total unrecognized tax benefits of approximately $2,127,000, including a recorded noncurrent liability of $85,000, related to unrecognized tax benefits primarily associated with state taxes. No interest and penalties have been recorded for the unrecognized tax benefits as of December 31, 2014. If recognized, approximately $2,127,000 would impact the Company’s effective tax rate. The Company does not expect that the liability for unrecognized tax benefits will change significantly within the next 12 months. Activity related to the gross unrecognized tax benefits for the periods presented was as follows (in thousands):
|
| | | | |
| Balance at January 1, 2012 | | $ | 85 |
|
| Additions based on tax positions related to the current year | | — |
|
| Additions for tax positions related to prior years | | 772 |
|
| Additions resulting from the acquisition of ADAPTIX | | 1,270 |
|
| Reductions | | — |
|
| Balance at December 31, 2012 | | $ | 2,127 |
|
| Balance at December 31, 2013 | | $ | 2,127 |
|
| Balance at December 31, 2014 | | $ | 2,127 |
|
Acacia recognizes interest and penalties with respect to unrecognized tax benefits in income tax expense. Acacia has identified no uncertain tax position for which it is reasonably possible that the total amount of unrecognized tax benefits will significantly increase or decrease within 12 months.
10. STOCK-BASED INCENTIVE PLANS
The 2002 Acacia Technologies Stock Incentive Plan (“2002 Plan”), the 2007 Acacia Technologies Stock Incentive Plan (“2007 Plan”) and the 2013 Acacia Research Corporation Stock Incentive Plan (“2013 Plan”) (collectively, the “Plans”) were approved by the stockholders of Acacia in December 2002, May 2007 and May 2013, respectively. All Plans allow grants of stock options, stock awards and performance shares with respect to Acacia common stock to eligible individuals, which generally includes directors, officers, employees and consultants. Except as noted below, the terms and provisions of the Plans are identical in all material respects. The term of the 2002 Plan expired in December 2012.
Acacia’s compensation committee administers the discretionary option grant and stock issuance programs. The compensation committee determines which eligible individuals are to receive option grants or stock issuances under those programs, the time or times when the grants or issuances are to be made, the number of shares subject to each grant or issuance, the status of any granted option as either an incentive stock option or a non-statutory stock option under the federal tax laws, the vesting schedule to be in effect for the option grant or stock issuance and the maximum term for which any granted option is to remain outstanding. The exercise price of options is generally equal to the fair market value of Acacia’s common stock on the date of grant. Options generally begin to be exercisable six months to one year after grant and generally expire ten years after grant. Stock options generally vest over two to three years and restricted shares generally vest in full after two to three years (generally representing the requisite service period). The Plans terminate no later than the tenth anniversary of the approval of the incentive plans by Acacia’s stockholders.
The Plans provide for the following separate programs:
| |
| • | Discretionary Option Grant Program. Under the discretionary option grant program, Acacia’s compensation committee may grant (1) non-statutory options to purchase shares of common stock to eligible individuals in the employ or service of Acacia or its subsidiaries (including employees, non-employee board members and consultants) at an exercise price not less than 85% of the fair market value of those shares on the grant date, and (2) incentive stock options to purchase shares of common stock to eligible employees at an exercise price not less than 100% of the fair market value of those shares on the grant date (not less than 110% of fair market value if such employee actually or constructively owns more than 10% of Acacia’s voting stock or the voting stock of any of its subsidiaries). |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| |
| • | Stock Issuance Program. Under the stock issuance program, eligible individuals may be issued shares of common stock directly, upon the attainment of performance milestones or the completion of a specified period of service or as a bonus for past services. Under this program, the purchase price for the shares shall not be less than 100% of the fair market value of the shares on the date of issuance, and payment may be in the form of cash or past services rendered. The eligible individuals shall have full stockholder rights with respect to any shares of Common Stock issued to them under the Stock Issuance Program, whether or not their interest in those shares is vested. Accordingly, the eligible individuals shall have the right to vote such shares and to receive any regular cash dividends paid on such shares. |
| |
| • | Automatic Option Grant Program (2002 and 2013Plans only). Commencing in fiscal 2008, each non-employee director will receive restricted stock units for the number of shares determined by dividing the annual retainer by the closing price of Acacia’s common stock on the grant date, provided that such individual has served as a non-employee director for at least 6 months. In addition, as of May 2007, each new non-employee director will receive restricted stock units for the number of shares determined by dividing the annual board of directors retainer by the closing price of Acacia’s common stock on the commencement date. Restricted stock units vest in a series of twelve quarterly installments over the three year period following the grant date, subject to immediate acceleration upon a change in control. Acacia will deliver shares corresponding to the vested restricted stock units within thirty (30) days after the first to occur of the following events: (i) the fifth (5th) anniversary of the grant date; or (ii) termination of the non-employee director’s service as a member of the Company’s Board of Directors. The non-employee directors do not have any rights, benefits or entitlements with respect to any shares unless and until the shares have been delivered. |
The number of shares of common stock available for issuance under the 2002 Plan automatically increased on the first trading day of January each calendar year during the term of the Plan by an amount equal to three percent (3%) of the total number of shares of common stock outstanding on the last trading day in December of the immediately preceding calendar year, not to exceed 500,000 shares. The aggregate number of shares of common stock available for issuance under the 2002 Plan could not exceed 20,000,000 shares. At December 31, 2014, there were no shares available for grant under the expired 2002 Plan.
The initial share reserve under the 2007 Plan was 560,000 shares. The number of shares of common stock available for issuance under the 2007 Plan automatically increased on January 1, 2008 and 2009, by an amount equal to two percent (2%) of the total number of shares of common stock outstanding on the last trading day of December in the prior calendar year. After January 1, 2009, no new additional shares will be added to the 2007 Plan without stockholder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2007 Plan). At December 31, 2014, there were no shares available for grant under the 2007 Plan.
The number of shares of Common Stock initially reserved for issuance under the 2013 Plan was 4,750,000 shares. No new additional shares will be added to the 2013 Plan without security holder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2013 Plan). The stock issuable under the 2013 Plan shall be shares of authorized but unissued or reacquired Common Stock, including shares repurchased by the Company on the open market. At December 31, 2014, there were 3,243,000 shares available for grant under the 2013 Plan.
Upon the exercise of stock options, the granting of restricted stock, or the delivery of shares pursuant to vested restricted stock units, it is Acacia’s policy to issue new shares of common stock. Acacia’s board of directors may amend or modify the Plans at any time, subject to any required stockholder approval.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table summarizes stock option activity for the Plans for the year ended December 31, 2014:
|
| | | | | | | | | | | | | |
| | | | | Weighted-Average | | |
| | | Options | | Exercise Price | | Remaining Contractual Term | | Aggregate Intrinsic Value |
| | | | | | | | | |
| Outstanding at December 31, 2013 | | 195,000 |
| | $ | 6.91 |
| | | | |
| Exercised | | (45,000 | ) | | $ | 4.60 |
| | | | |
| Outstanding at December 31, 2014 | | 150,000 |
| | $ | 7.59 |
| | 1 year | | $ | 1,402,000 |
|
| Vested | | 150,000 |
| | $ | 7.59 |
| | 1 year | | $ | 1,402,000 |
|
| Exercisable at December 31, 2014 | | 150,000 |
| | $ | 7.59 |
| | 1 year | | $ | 1,402,000 |
|
The aggregate intrinsic value of options exercised during the years ended December 31, 2014, 2013 and 2012 was $518,000, $2,024,000, and $1,796,000, respectively. No options vested during the years ended December 31, 2014, 2013 and 2012.
The following table summarizes nonvested restricted share activity for the year ended December 31, 2014:
|
| | | | | | | |
| | | Nonvested Restricted Shares | | Weighted Average Grant Date Fair Value |
| | | | | |
| Nonvested restricted stock at December 31, 2013 | | 1,237,000 |
| | $ | 28.23 |
|
| Granted | | 887,000 |
| | $ | 14.41 |
|
| Vested | | (814,000 | ) | | $ | 26.40 |
|
| Canceled | | (292,000 | ) | | $ | 24.55 |
|
| Nonvested restricted stock at December 31, 2014 | | 1,018,000 |
| | $ | 18.71 |
|
The weighted-average grant date fair value of nonvested restricted stock granted during the years ended December 31, 2014, 2013 and 2012 was $14.41, $24.31, and $32.17, respectively. The aggregate fair value of restricted stock that vested during the years ended December 31, 2014, 2013 and 2012 was $21,490,000, $22,317,000, $28,865,000, respectively. As of December 31, 2014, the total unrecognized compensation expense related to nonvested restricted stock awards was $15,012,000, which is expected to be recognized over a weighted-average period of approximately 1.7 years.
The following table summarizes restricted stock unit activity for the year ended December 31, 2014:
|
| | | | | | | |
| | | Restricted Stock Units | | Weighted Average Grant Date Fair Value |
| | | | | |
| Nonvested restricted stock units outstanding at December 31, 2013 | | 20,000 |
| | $ | 27.83 |
|
| Granted | | 33,000 |
| | $ | 14.33 |
|
| Vested | | (20,000 | ) | | $ | 22.99 |
|
| Nonvested restricted stock units outstanding at December 31, 2014 | | 33,000 |
| | $ | 17.10 |
|
| Vested restricted stock units outstanding at December 31, 2014 | | 74,000 |
| | $ | 21.14 |
|
The weighted-average grant date fair value of restricted stock units granted during the years ended December 31, 2014 and 2012 was $14.33 and $29.39, respectively. There were no restricted units granted during the year ended December 31, 2013. The aggregate fair value of restricted stock units that vested during the years ended December 31, 2014, 2013 and 2012 was $460,000, $469,000 and $363,000, respectively. As of December 31, 2014, the total unrecognized compensation expense related to restricted stock unit awards was $475,000, which is expected to be recognized over a weighted-average period of approximately 1.7 years.
As of December 31, 2014, there are 3,500,000 shares of common stock are reserved for issuance under the Plans.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
11. COMMITMENTS AND CONTINGENCIES
Operating Leases
Acacia leases certain office space under various operating lease agreements expiring at various dates from 2015 through 2020. Minimum annual rental commitments for operating leases of continuing operations having initial or remaining noncancellable lease terms in excess of one year are as follows (in thousands):
|
| | | |
| Years ending December 31, | |
| 2015 | $ | 1,564 |
|
| 2016 | 1,595 |
|
| 2017 | 1,557 |
|
| 2018 | 1,612 |
|
| 2019 | 1,669 |
|
| Thereafter | 166 |
|
| Total minimum lease payments | $ | 8,163 |
|
Rent expense for the years ended December 31, 2014, 2013 and 2012 approximated $1,523,000, $1,312,000 and $898,000, respectively. Rental payments are expensed in the statements of operations in the period to which they relate. Scheduled rent increases are amortized on a straight-line basis over the lease term.
Inventor Royalties and Contingent Legal Expenses
In connection with the investment in certain patents and patent rights, certain of Acacia’s operating subsidiaries executed related agreements which grant to the former owners of the respective patents or patent rights, the right to receive inventor royalties based on future net revenues (as defined in the respective agreements) generated as a result of licensing and otherwise enforcing the respective patents or patent portfolios.
Acacia’s operating subsidiaries may retain the services of law firms that specialize in patent licensing and enforcement and patent law in connection with their licensing and enforcement activities. These law firms may be retained on a contingent fee basis whereby such law firms are paid on a scaled percentage of any negotiated fees, settlements or judgments awarded based on how and when the fees, settlements or judgments are obtained.
The economic terms of the inventor agreements, operating agreements and contingent legal fee arrangements associated with the patent portfolios owned or controlled by Acacia’s operating subsidiaries, if any, including royalty rates, contingent fee rates and other terms, vary across the patent portfolios owned or controlled by such operating subsidiaries. Inventor royalties, payments to noncontrolling interests and contingent legal fees expenses fluctuate period to period, based on the amount of revenues recognized each period, the terms and conditions of revenue agreements executed each period and the mix of specific patent portfolios with varying economic terms and obligations generating revenues each period. Inventor royalties and contingent legal fees expenses will continue to fluctuate and may continue to vary significantly period to period, based primarily on these factors.
During the years ended December 31, 2012, Acacia entered into significant agreements with unrelated third-parties resolving pending patent matters that resulted in the grant of certain intellectual property rights and recognition of revenues, portions of which were not subject to inventor royalty and contingent legal fee arrangements, as well as the grant of licenses from certain of Acacia’s operating subsidiaries and recognition of revenues that were subject to inventor royalties and contingent legal fee arrangements. Certain revenues recognized subject to inventor royalties and contingent legal fees are based on a determination by the respective operating subsidiaries.
Patent Enforcement and Other Litigation
Acacia is subject to claims, counterclaims and legal actions that arise in the ordinary course of business. Management believes that the ultimate liability with respect to these claims and legal actions, if any, will not have a material effect on Acacia’s consolidated financial position, results of operations or cash flows.
Certain of Acacia’s operating subsidiaries are often required to engage in litigation to enforce their patents and patent rights. In connection with any of Acacia’s operating subsidiaries’ patent enforcement actions, it is possible that a defendant may
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
request and/or a court may rule that an operating subsidiary has violated statutory authority, regulatory authority, federal rules, local court rules, or governing standards relating to the substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against Acacia or its operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material, and if required to be paid by Acacia or its operating subsidiaries, could materially harm the Company’s operating results and financial position.
During fiscal year 2014, a federal court held that a lawsuit initiated in 2010 was exceptional. Additionally, in a separate matter, a federal court held that a lawsuit initiated in 2011 was exceptional. The total amount requested by these two defendants in these two matters was $2,800,000. The respective operating subsidiaries have filed notices of appeal. Operating expenses for the year ended December 31, 2014 included an accrual for these matters totaling $1,548,000.
Operating expenses for the year ended December 31, 2013 included a one-time, non-recurring charge related to the resolution of a dispute concerning legal fees associated with a prior matter totaling $3,506,000.
Guarantees and Indemnifications
Certain of Acacia’s operating subsidiaries have made guarantees and indemnities under which they may be required to make payments to a guaranteed or indemnified party, in relation to certain transactions, including revenue transactions in the ordinary course of business. In connection with certain facility leases, Acacia and certain of its operating subsidiaries have indemnified lessors for certain claims arising from the facilities or the leases. Acacia indemnifies its directors and officers to the maximum extent permitted under the laws of the State of Delaware. However, Acacia has a directors and officers insurance policy that may reduce its exposure in certain circumstances and may enable it to recover a portion of future amounts that may be payable, if any. The duration of the guarantees and indemnities varies and, in many cases is indefinite but subject to statute of limitations. The majority of guarantees and indemnities do not provide any limitations of the maximum potential future payments that Acacia could be obligated to make. To date, Acacia has made no payments related to these guarantees and indemnities. Acacia estimates the fair value of its indemnification obligations to be insignificant based on this history and therefore, have not recorded any liability for these guarantees and indemnities in the accompanying consolidated balance sheets. Additionally, no events or transactions have occurred that would result in a material liability at December 31, 2014.
Other
In August 2010, a wholly owned subsidiary of Acacia became the general partner of the Acacia IP Fund, which was formed in August 2010. The Acacia IP Fund is authorized to raise up to $250,000,000. The Acacia IP Fund invests in, licenses and enforces intellectual property consisting primarily of patents, patent rights, and patented technologies. Refer to Note 2 to these notes to consolidated financial statements for information regarding the consolidation of majority-owned subsidiaries and the presentation of related noncontrolling interests. At December 31, 2014 and 2013, the Acacia IP Fund net assets and net loss were primarily comprised of the following (in thousands):
|
| | | | | | | | |
| | | 2014 | | 2013 |
| Cash and other assets | | $ | 2,823 |
| | $ | 2,927 |
|
| Patents, net of accumulated amortization | | 967 |
| | 1,373 |
|
| Investments - noncurrent | | 8,281 |
| | 11,120 |
|
| Total assets | | $ | 12,071 |
| | $ | 15,420 |
|
| Accrued expenses and contributions | | $ | 1,876 |
| | $ | 2,437 |
|
| Total liabilities | | 1,876 |
| | 2,437 |
|
| Net assets | | $ | 10,195 |
| | $ | 12,983 |
|
|
| | | | | | | | |
| | | 2014 | | 2013 |
| Revenues | | $ | 1,560 |
| | $ | 1,448 |
|
| Operating expenses | | 1,724 |
| | 6,907 |
|
| Gain from operations | | (164 | ) | | (5,459 | ) |
| Net loss in equity method investments | | (1,807 | ) | | (542 | ) |
| Net loss | | $ | (1,971 | ) | | $ | (6,001 | ) |
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
12. RETIREMENT SAVINGS PLAN AND EXECUTIVE SEVERANCE POLICY
Retirement Savings Plan. Acacia has an employee savings and retirement plan under section 401(k) of the Code (the “Plan”). The Plan is a defined contribution plan in which eligible employees may elect to have a percentage of their compensation contributed to the Plan, subject to certain guidelines issued by the Internal Revenue Service. Acacia may contribute to the Plan at the discretion of the board of directors. There were no contributions made by Acacia during the periods presented.
Executive Severance Policy. Under Acacia’s Amended Executive Severance Policy, full-time employees with the title of Senior Vice President and higher (“SVP and higher”) are entitled to receive certain benefits upon termination of employment. If employment of an SVP and higher employee is terminated for other than cause or other than on account of death or disability, Acacia will (i) promptly pay to the SVP and higher employee a lump sum amount equal to the aggregate of (a) accrued obligations (i.e., annual base salary through the date of termination to the extent not theretofore paid and any compensation previously deferred (together with any accrued interest or earnings thereon) and any accrued vacation pay, and reimbursable expenses, in each case to the extent not theretofore paid) and (b) three (3) months of base salary for each full year that the SVP and higher employee was employed by the Company (the “Severance Period”), up to a maximum of twelve (12) months (eighteen (18) months for executive officers of Acacia Research Corporation) of base salary, and (ii) provide to the SVP and higher employee, Acacia paid COBRA coverage for the medical and dental benefits selected in the year in which the termination occurs, for the duration of the Severance Period.
13. SUPPLEMENTAL CASH FLOW INFORMATION
Estimated federal taxes paid totaled $0, $3,000,000 and $0 for the years ended December 31, 2014, 2013 and 2012, respectively. At December 31, 2013, prepaid expenses and other current assets included federal and state income taxes receivable totaling $3,251,000. Cash paid for state income taxes totaled $172,000, $516,000 and $771,000 for the years ended December 31, 2014, 2013 and 2012, respectively. Foreign taxes withheld totaled $5,159,000, $4,605,000 and $11,890,000 for the years ended December 31, 2014, 2013 and 2012.
Refer to Note 6 to these notes to consolidated financial statements for information regarding noncash investing activity related to the investment in patent portfolios for the periods presented. Cash flows from financing activities for the year ended December 31, 2012 exclude $504,000 of accrued distributions payable to noncontrolling interests.
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
14. QUARTERLY FINANCIAL DATA (unaudited)
The following table sets forth unaudited consolidated statements of operations data for the eight quarters in the period ended December 31, 2014. This information has been derived from Acacia’s unaudited condensed consolidated financial statements that have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments, necessary for a fair statement of the information when read in conjunction with the audited consolidated financial statements and related notes thereto. Acacia’s quarterly results have been, and may in the future be, subject to significant fluctuations. As a result, Acacia believes that results of operations for interim periods should not be relied upon as any indication of the results to be expected in any future periods.
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Quarter Ended |
| | | Mar. 31, | | Jun. 30, | | Sept. 30, | | Dec. 31, | | Mar. 31, | | Jun. 30, | | Sept. 30, | | Dec. 31, |
| | | 2014 | | 2014 | | 2014 | | 2014 | | 2013 | | 2013 | | 2013 | | 2013 |
| | | (Unaudited, in thousands, except share and per share information) |
| Revenues | | $ | 12,578 |
| | $ | 50,076 |
| | $ | 37,192 |
| | $ | 31,030 |
| | $ | 76,861 |
| | $ | 23,110 |
| | $ | 15,520 |
| | $ | 15,065 |
|
| Operating costs and expenses: | | |
| | |
| | | | | | |
| | |
| | |
| | |
| Cost of revenues: | | |
| | |
| | | | | | |
| | |
| | |
| | |
| Inventor royalties | | 951 |
| | 10,694 |
| | 4,667 |
| | 4,358 |
| | 18,481 |
| | 5,610 |
| | 2,353 |
| | 3,280 |
|
| Contingent legal fees | | 1,527 |
| | 7,077 |
| | 7,663 |
| | 7,296 |
| | 15,032 |
| | 4,024 |
| | 2,547 |
| | 3,181 |
|
| Litigation and licensing expenses - patents | | 8,994 |
| | 10,820 |
| | 9,592 |
| | 8,208 |
| | 9,648 |
| | 9,918 |
| | 10,870 |
| | 8,899 |
|
| Amortization of patents | | 14,472 |
| | 15,532 |
| | 13,511 |
| | 13,727 |
| | 11,730 |
| | 12,578 |
| | 12,615 |
| | 16,735 |
|
| Marketing, general and administrative expenses (including non-cash stock compensation expense) | | 11,693 |
| | 13,181 |
| | 11,636 |
| | 12,044 |
| | 13,844 |
| | 13,184 |
| | 18,235 |
| | 13,988 |
|
| Research, consulting and other expenses - business development | | 992 |
| | 1,003 |
| | 1,208 |
| | 637 |
| | 1,031 |
| | 726 |
| | 730 |
| | 742 |
|
| Other | | — |
| | — |
| | 1,548 |
| | — |
| | — |
| | — |
| | 3,506 |
| | — |
|
| Total operating costs and expenses | | 38,629 |
| | 58,307 |
| | 49,825 |
| | 46,270 |
| | 69,766 |
| | 46,040 |
| | 50,856 |
| | 46,825 |
|
| Operating income (loss) | | (26,051 | ) | | (8,231 | ) | | (12,633 | ) | | (15,240 | ) | | 7,095 |
| | (22,930 | ) | | (35,336 | ) | | (31,760 | ) |
| Total other income (expense) | | 109 |
| | (196 | ) | | (57 | ) | | (451 | ) | | 1,290 |
| | 400 |
| | 280 |
| | 161 |
|
| Income (loss) before (provision for) benefit from income taxes | | (25,942 | ) | | (8,427 | ) | | (12,690 | ) | | (15,691 | ) | | 8,385 |
| | (22,530 | ) | | (35,056 | ) | | (31,599 | ) |
| Benefit from (provision for) income taxes | | 1,372 |
| | (4,689 | ) | | (145 | ) | | (450 | ) | | (3,272 | ) | | 9,050 |
| | 19,570 |
| | (3,390 | ) |
| Net income (loss) including noncontrolling interests | | (24,570 | ) | | (13,116 | ) | | (12,835 | ) | | (16,141 | ) | | 5,113 |
| | (13,480 | ) | | (15,486 | ) | | (34,989 | ) |
| Net (income) loss attributable to noncontrolling interests in operating subsidiaries | | 149 |
| | 167 |
| | 420 |
| | (103 | ) | | — |
| | 977 |
| | (225 | ) | | 1,656 |
|
| Net income (loss) attributable to Acacia Research Corporation | | $ | (24,421 | ) | | $ | (12,949 | ) | | $ | (12,415 | ) | | $ | (16,244 | ) | | $ | 5,113 |
| | $ | (12,503 | ) | | $ | (15,711 | ) | | $ | (33,333 | ) |
| Net income (loss) per common share attributable to Acacia Research Corporation: | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
|
Basic income (loss) per share - As Adjusted(1) | | $ | (0.51 | ) | | $ | (0.27 | ) | | $ | (0.26 | ) | | $ | (0.34 | ) | | $ | 0.10 |
| | $ | (0.26 | ) | | $ | (0.33 | ) | | $ | (0.69 | ) |
Diluted income (loss) per share - As Adjusted(1) | | $ | (0.51 | ) | | $ | (0.27 | ) | | $ | (0.26 | ) | | $ | (0.34 | ) | | $ | 0.10 |
| | $ | (0.26 | ) | | $ | (0.33 | ) | | $ | (0.69 | ) |
Weighted-average number of shares outstanding, basic - As Adjusted(1) | | 48,329,375 |
| | 48,543,334 |
| | 48,806,334 |
| | 48,944,914 |
| | 47,859,774 |
| | 48,008,998 |
| | 48,330,149 |
| | 48,415,684 |
|
Weighted-average number of shares outstanding, diluted - As Adjusted(1) | | 48,329,375 |
| | 48,543,334 |
| | 48,806,334 |
| | 48,944,914 |
| | 48,104,242 |
| | 48,008,998 |
| | 48,330,149 |
| | 48,415,684 |
|
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
| | | | | | | | | | | | | | | | | | | | |
| | | Quarter Ended |
| | | Mar. 31, | | Jun. 30, | | Sept. 30, | | Dec. 31, | | Mar. 31, | | Jun. 30, | | Sept. 30, | | Dec. 31, |
| | | 2014 | | 2014 | | 2014 | | 2014 | | 2013 | | 2013 | | 2013 | | 2013 |
| | | (Unaudited, In thousands, except share and per share information) |
Basic income (loss) per share - As Reported(1) | | N/A | | N/A | | N/A | | N/A | | $ | 0.11 |
| | $ | (0.26 | ) | | N/A | | N/A |
Diluted income (loss) per share - As Reported(1) | | N/A | | N/A | | N/A | | N/A | | $ | 0.11 |
| | $ | (0.26 | ) | | N/A | | N/A |
Weighted-average number of shares outstanding, basic - As Reported(1) | | N/A | | N/A | | N/A | | N/A | | 47,859,774 |
| | 48,008,998 |
| | N/A | | N/A |
Weighted-average number of shares outstanding, diluted - As Reported(1) | | N/A | | N/A | | N/A | | N/A | | 48,354,444 |
| | 48,008,998 |
| | N/A | | N/A |
_______________________
(1) - Revision of Prior Period Earnings (Loss) Per Share - Two-Class Method. In connection with the preparation of the Company’s Quarterly Report on Form 10-Q as of and for the three months ended September 30, 2013, the Company determined that its basic and diluted net income (loss) per share calculations should have been prepared using the “two-class method.” Under the two-class method, securities that participate in dividends are considered “participating securities.” The Company’s unvested restricted shares outstanding are considered “participating securities” because they include non-forfeitable rights to dividends.
Pursuant to guidance set forth in Staff Accounting Bulletin (“SAB”) No. 99, “Materiality,” the Company concluded that the errors were not material to any of its prior period financial statements. Although the errors were immaterial to prior periods, the prior period financial statements presented herein were revised, in accordance with SAB No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements.”
EXHIBIT INDEX
|
| |
Exhibit Number | Description |
| | |
| 2.1 | Agreement and Plan of Merger, dated November 22, 2011, by and among Acacia Research Group LLC, Apollo Patent Corp., Adaptix, Inc., and Baker Communications Fund II (QP), L.P., solely in its capacity as representative for the shareholders of Adaptix, Inc.(15) |
| 3.1 | Amended and Restated Certificate of Incorporation (1) |
| 3.2 | Amended and Restated Bylaws |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (2) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (3) |
| 10.3* | 2002 Acacia Technologies Stock Incentive Plan (4) |
| 10.4* | 2007 Acacia Technologies Stock Incentive Plan (5) |
| 10.5* | Form of Acacia Technologies Stock Option Agreement under the 2007 Acacia Technologies Stock Incentive Plan (6) |
| 10.6* | Form of Acacia Technologies Stock Issuance Agreement under the 2002 Acacia Technologies Stock Incentive Plan (6) |
| 10.7* | Form of Acacia Technologies Stock Issuance Agreement under the 2007 Acacia Technologies Stock Incentive Plan (6) |
| 10.8 | Office Space Lease dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (7) |
| 10.9 | Form of Indemnification Agreement (8) |
| 10.10 | Third Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (9) |
| 10.11* | Employment Agreement, dated April 12, 2004, by and between Acacia Media Technologies Corporation and Edward Treska (10) |
| 10.11.1* | Addendum, dated March 31, 2008, to Employment Agreement by and between Acacia Media Technologies Corporation and Edward Treska (11) |
| 10.12 | Fourth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10) |
| 10.13 | Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10) |
| 10.14* | Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Paul Ryan (12) |
| 10.14.1* | Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Paul Ryan (12) |
| 10.15* | Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (11) |
| 10.15.1* | Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (12) |
| 10.16* | Amended Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (11) |
| 10.16.1* | Amendment to Amended Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (12) |
| 10.17* | Acacia Research Corporation Amended and Restated Executive Severance Policy (12) |
| 10.18 | Sixth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (14) |
| 10.19 | Form of Purchase Agreement (16) |
| 10.20* | 2013 Acacia Research Corporation Stock Incentive Plan (18) |
| 10.21* | Form of Stock Issuance Agreement under the 2013 Acacia Research Corporation Stock Incentive Plan (19) |
| 10.22* | Employment Agreement, dated October 28, 2006, by and between Acacia Technologies Services Corporation and Matthew Vella (20) |
| 18.1 | Preferability Letter dated February 25, 2010 from Grant Thornton LLP, independent registered public accounting firm, regarding change in accounting principle (13) |
|
| |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of Independent Registered Public Accounting Firm |
| 24.1 | Power of Attorney (included in the signature page hereto). |
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934 |
| 32.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350 |
| 32.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350 |
| 101 | Interactive Date Files Pursuant to Rule 405 of Regulation S-T. |
___________________________
| |
| * | The referenced exhibit is a management contract, compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(c) of Form 10-K. |
| |
| (1) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on June 5, 2008 (File No. 000-26068). |
| |
| (2) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 20, 2000 (File No. 000-26068). |
| |
| (3) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 26, 1996 (File No. 000-26068). |
| |
| (4) | Incorporated by reference to Annex E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (File No. 333-87654) which became effective on November 8, 2002. |
| |
| (5) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-8 (File No. 333-144754) which became effective on July 20, 2007. |
| |
| (6) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2007, filed on November 2, 2007 (File No. 000-26068). |
| |
| (7) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10‑K for the year ended December 31, 2001, filed on March 27, 2002 (File No. 000‑26068). |
| |
| (8) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 2012, filed on July 30, 2012 (File No. 000-26068). |
| |
| (9) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended March 31, 2006, filed on May 10, 2006 (File No. 000‑26068). |
| |
| (10) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2007, filed on March 14, 2008 (File No. 000-26068). |
| |
| (11) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on April 2, 2008 ( File No. 000-26068). |
| |
| (12) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008, filed on February 26, 2009 (File No. 000-26068). |
| |
| (13) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2009, filed on February 26, 2010, as amended on March 1, 2010 (File No. 000-26068) |
| |
| (14) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, as amended on March 24, 2011 (File No. 000-26068). |
| |
| (15) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K/A filed on January 19, 2012 (File No. 000-26068). Portions of this exhibit have been omitted pursuant to a request for confidential treatment under Rule 24-b-2 of the Securities Exchange Act of 1934, as amended. The omitted material has been separately filed with the Securities and Exchange Commission. |
| |
| (16) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on February 16, 2012 (File No. 000-26068). |
| |
| (17) | Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2012, filed on February 28, 2013 (File No. 000-26068). |
| |
| (18) | Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 24, 2013 (File No. 000-26068). |
| |
| (19) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on May 22, 2013 (File No. 000-26068). |
| |
| (20) | Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on July 5, 2013 (File No. 000-26068). |