UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended January 3, 2009
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-33642
Masimo Corporation
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 33-0368882 |
(State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification Number) |
| |
| 40 Parker Irvine, California | | 92618 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(949) 297-7000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class: | | Name of each exchange on which registered: |
Common Stock, par value $0.001 | | The NASDAQ Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
| | | | | | |
Large accelerated filer x | | Accelerated filer ¨ | | Non-accelerated filer ¨ | | Smaller reporting company ¨ |
| | | | (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of the registrant, based upon the closing sale price of the common stock on June 27, 2008, the last business day of the registrant’s most recently completed second fiscal quarter, as reported on the NASDAQ Global Market, was approximately $1.72 billion.
At February 13, 2009, the registrant had 57,394,949 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III of this Form 10-K incorporate information by reference from the registrant’s proxy statement for the registrant’s 2009 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this annual report.
MASIMO CORPORATION
FISCAL YEAR 2008 FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, or Form 10-K, contains “forward-looking statements” that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially and adversely from those expressed or implied by such forward-looking statements. The forward-looking statements are contained principally in Item 1—“Business,” Item 1.A—“Risk Factors” and Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations” but appear through the Form 10-K. Examples of forward-looking statements include, but are not limited to any projection or expectation of earnings, revenue or other financial items; the plans, strategies and objectives of management for future operations; factors that may affect our operating results; our success in pending litigation; new products or services; the demand for our products; our ability to consummate acquisitions and successfully integrate them into our operations; future capital expenditures; effects of current or future economic conditions or performance; industry trends and other matters that do not relate strictly to historical facts or statements of assumptions underlying any of the foregoing. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “opportunity,” “plan,” “potential,” “predicts,” “seek,” “should,” “will,” or “would,” and similar expressions and variations or negatives of these words. These forward-looking statements are based on the expectations, estimates, projections, beliefs and assumptions of our management based on information currently available to management, all of which is subject to change. Such forward-looking statements are subject to risks, uncertainties and other factors that are difficult to predict and could cause our actual results and the timing of certain events to differ materially and adversely from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed under Item 1.A. “Risk Factors” in this Form 10-K. Furthermore, such forward-looking statements speak only as of the date of this Form 10-K. We undertake no obligation to update or revise publicly any forward-looking statements to reflect events or circumstances after the date of such statements for any reason, except as otherwise required by law.
PART I
Overview
We are a global medical technology company that develops, manufactures and markets noninvasive patient monitoring products that improve patient care. We were incorporated in California in May 1989 and reincorporated in Delaware in May 1996. We invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of measure-through motion and low perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. Pulse oximetry is the noninvasive measurement of the oxygen saturation level of arterial blood, or the blood that delivers oxygen to the body’s tissues, and pulse rate. Our Masimo SET platform has addressed many of the previous technology limitations and has been referred to by several industry sources as the gold standard in pulse oximetry. The benefits of Masimo SET have been validated in over 100 independent clinical and laboratory studies. During fiscal 2008, we generated product revenue of $258.9 million and we increased our revenue at a compound annual growth rate, or CAGR, of approximately 34.0% for the three years ended January 3, 2009.
We develop, manufacture and market a family of noninvasive blood constituent patient monitoring solutions that consists of a monitor or circuit board and our proprietary single-patient use and reusable sensors and cables. In addition, we offer remote-alarm/monitoring solutions, such as the Masimo Patient SafetyNet. Our solutions and related products are based upon our proprietary Masimo SET algorithms. This software-based technology is incorporated into a variety of product platforms depending on our customers’ specifications. We sell our products to end-users through our direct sales force and certain distributors, and some of our products to our original equipment manufacturer, or OEM, partners, for incorporation into their products. We estimate that the worldwide installed base of our pulse oximeters and OEM monitors that incorporate Masimo SET was approximately 567,000 units as of January 3, 2009. Our installed base is the primary driver for the recurring sales of our sensors, most notably, single-patient adhesive sensors. Based on industry reports, we estimate that the worldwide pulse oximetry market was over $1.0 billion, as of January 3, 2009, the largest component of which was the sale of sensors.
Our strategy is to utilize the reliability and accuracy of our Masimo SET platform, along with our Patient SafetyNet solutions, to facilitate the expansion of our pulse oximetry products into areas beyond critical care settings, including the general care areas of the hospital. Additionally, we have developed products that noninvasively monitor parameters beyond arterial blood oxygen saturation level and pulse rate, which create new market opportunities in both the critical care and non-critical care settings. In 2005, we launched our Masimo Rainbow SET platform utilizing licensed Rainbow technology, which we believe includes the first devices cleared by the U.S. Food and Drug Administration, or FDA, to noninvasively measure select noninvasive blood parameters that previously required invasive procedures. In 2005, we launched carboxyhemoglobin, allowing measurement of carbon monoxide levels in the blood. In 2006, we launched methemoglobin, allowing for the measurement of a dangerous condition known as methemoglobinemia, which occurs as a reaction to some common drugs used in hospitals and in out patient procedures. In 2007, we launched Plethysmographic Variability Index, or PVI. Independent clinical studies have demonstrated that PVI can predict fluid responsiveness in surgical and intensive care patients. In May 2008, we received FDA approval for our most recent Rainbow measurement, total hemoglobin. Total hemoglobin is defined as the oxygen-carrying component of red blood cells, and is one of the
1
most frequent invasive laboratory measurements in the world, often measured as part of a complete blood count. We believe that the use of products incorporating Rainbow technology will become widely adopted for the noninvasive monitoring of these parameters. We also believe that we will develop and introduce additional parameters in the future based on our proprietary technology platforms.
Our technology is supported by a substantial intellectual property portfolio that we have built through internal development and, to a lesser extent, acquisitions and license agreements. As of January 3, 2009, we had 513 issued and pending patents worldwide. We have exclusively licensed from our development partner, Masimo Laboratories, Inc., or Masimo Labs, the right to incorporate Rainbow technology into our products intended to be used by professional caregivers, including, but not limited to, hospital caregivers and emergency medical services, or EMS, facility caregivers. On January 17, 2006, we settled a patent litigation dispute with Nellcor Puritan Bennett, Inc., a division of Tyco Healthcare (currently Covidien Ltd.). Under the terms of the settlement, Nellcor has agreed to discontinue the sale of its products found to infringe our patents and will pay us royalties at least through March 14, 2011 on the U.S. sales of its pulse oximetry products.
On August 13, 2007, we completed our initial public offering, or IPO, of common stock in which a total of 13,704,120 shares were sold, comprised of 10,416,626 shares sold by selling stockholders, 1,500,000 shares sold by us at the initial closing and 1,787,494 shares sold by us pursuant to the underwriters’ full exercise of their over-allotment option, at an issue price of $17.00 per share. We raised a total of $55.9 million in gross proceeds from the IPO, or approximately $47.8 million in net proceeds after deducting underwriting discounts and commissions of $3.9 million and estimated other offering costs of approximately $4.2 million. Upon the closing of the IPO, all shares of convertible preferred stock outstanding automatically converted into an aggregate of 34,612,503 shares of common stock. The consolidated financial statements as of and for the period ended December 29, 2007, including share and per share amounts, include the effects of the offering since it was completed prior to December 29, 2007.
Industry Background
Pulse oximetry has gained widespread clinical acceptance as a standard patient vital sign measurement because it can give clinicians an early warning of low arterial blood oxygen saturation levels, known as hypoxemia. Early detection is critical because hypoxemia can lead to a lack of oxygen in the body’s tissues, which can result in brain damage or death in a matter of minutes. Pulse oximeters are currently used primarily in critical care settings, including emergency departments, surgery, recovery rooms, intensive care units, or ICUs, and emergency medical services, or EMS market.
In addition, clinicians use pulse oximeters to estimate whether there is too much oxygen in the blood, a condition called hyperoxemia. In premature babies, hyperoxemia can lead to permanent eye damage or blindness. By ensuring that oxygen saturation levels in babies remain under 96%, clinicians believe they can lower the incidence of hyperoxemia. Hyperoxemia can also cause problems for adults, such as increased risk of postoperative infection and tissue damage. In adults, to prevent hyperoxemia, clinicians use pulse oximeters to administer the minimum level of oxygen necessary to maintain normal saturation levels.
Pulse oximeters use sensors attached to an extremity, typically the fingertip. These sensors contain two light emitting diodes, or LEDs, that in a transmittance sensor transmit red and infrared light from one side of the extremity through the tissue to a photodetector on the other side of the extremity. The photodetector in the sensor measures the amount of red and infrared light absorbed by the tissue. A microprocessor then analyzes the changes in light-absorption to provide a continuous, real-time measurement of the amount of oxygen in the patient’s arterial blood. Pulse oximeters typically give audio and visual alerts, or alarms, when the patient’s arterial blood oxygen saturation level or pulse rate falls outside of a designated range. As a result, clinicians are able to immediately initiate treatment to prevent the serious clinical consequences of hypoxemia and hyperoxemia.
Limitations of Conventional Pulse Oximetry
Conventional pulse oximetry is subject to technological limitations that reduce its effectiveness and the quality of patient care. In particular, when using conventional pulse oximetry, arterial blood signal recognition can be distorted by motion artifact, or patient movement, and low perfusion, or low arterial blood flow. Motion artifact can cause conventional pulse oximeters to inaccurately measure the arterial blood oxygen saturation level due mainly to the movement and recognition of venous blood. Venous blood, which is partially depleted of oxygen, may cause falsely low oxygen saturation readings. Low perfusion can also cause the failure of the conventional pulse oximeter to obtain an accurate measurement. Conventional pulse oximeters cannot distinguish oxygenated hemoglobin, or the component of red blood cells that carries oxygen, from dyshemoglobin, which is hemoglobin that is incapable of carrying oxygen. In addition, conventional pulse oximetry readings can also be impacted by bright light and electrical interference from the presence of electrical surgical equipment. Independent, published research shows that conventional pulse oximeters are subject to operating limitations, including:
| | • | | inaccurate measurements, which can lead to the non-detection of a hypoxemic event or improper and unnecessary treatment; |
2
| | • | | false alarms, which occur when the pulse oximeter falsely indicates a drop in the arterial blood oxygen saturation level which can lead to improper therapy, the inefficient use of clinical resources as clinicians respond to false alarms, or the non-detection of a true alarm if clinicians become desensitized to frequently occurring false alarms; and |
| | • | | signal drop-outs, which is the loss of a real-time signal as the monitor attempts to find or distinguish the pulse, which can lead to the non-detection of hypoxemic events. |
Published independent research shows that over 70% of the alarms were false outside the operating room using conventional pulse oximetry. In addition, in the operating room, conventional pulse oximeters failed to give measurements at all due to weak physiological signals, or low perfusion, in approximately 9% of all cases studied. Manufacturers of conventional pulse oximeters have attempted to address some of these limitations, with varying degrees of success. Some devices have attempted to minimize the effects of motion artifact by repeating the last measurement before motion artifact is detected, until a new, clean signal is detected and a new measurement can be displayed, known as freezing values. Other devices have averaged the signal over a longer period of time, known as long-averaging, in an attempt to reduce the effect of brief periods of motion. These solutions, commonly referred to as alarm management techniques, mask the limitations of conventional pulse oximetry. Several published studies have demonstrated that some of these alarm management techniques have actually contributed to increased occurrences of undetected true alarms, or events where hypoxemia occurs, but is not detected by the pulse oximeter.
Conventional pulse oximetry technology also has several practical limitations. Because the technology cannot consistently measure oxygen saturation levels of arterial blood in the presence of motion artifact or low perfusion, the technology is not sufficiently robust to allow for its use in non-critical care settings of the hospital, such as general care areas, where the hospital staff-to-patient ratio is significantly lower. In order for pulse oximetry to become a standard patient monitor in these settings, these limitations must be overcome.
In addition, conventional pulse oximeters cannot distinguish oxygenated hemoglobin, or the component of red blood cells that carries oxygen, from dyshemoglobin, which is hemoglobin that is incapable of carrying oxygen. The most prevalent forms of dyshemoglobins are carboxyhemoglobin and methemoglobin. As a result of these dyshemoglobins, pulse oximeters will report falsely high oxygen levels when they are present in the blood. Although currently there are lab-based tests that detect dyshemoglobins, they are invasive and do not provide immediate or continuous results.
Pulse Oximetry Market Opportunity
The pulse oximetry market consists of pulse oximeters and consumables, including single-patient use and reusable sensors, cables and other pulse oximetry accessories that are primarily sold to the hospital and EMS markets. According to a Frost & Sullivan report dated March 2004, it was estimated that U.S. pulse oximetrysensor market would increase to $622 million by 2010. According to a Frost & Sullivan report dated December 2008, it is estimated that the U.S. pulse oximetryequipment market would increase to $265 million by 2010. Based on these estimates, the total U.S. pulse oximetry market will be $887 million in 2010, with between 6% and 8% CAGR. Frost & Sullivan expects the growth in the U.S. pulse oximetry market to be driven by:
| | • | | ongoing adoption of low perfusion, motion-tolerant technology; |
| | • | | aggressive awareness campaigns; |
| | • | | rising patient acuity, or severity of illnesses, which increases the need for monitoring in the intermediate and sub-acute settings; |
| | • | | expansion of the market for pulse oximetry monitoring to the general surgical floor; |
| | • | | greater efficiencies for the health care worker through increased reliability, improved detection algorithms and the ability to reject false alarms; and |
| | • | | adoption of pulse oximetry outside the hospital and in the faster growing alternate care market. |
Based on this Frost & Sullivan estimate for the U.S. market and other available estimates for markets outside the U.S., we estimate that the worldwide pulse oximetry market will be more than $1.0 billion in 2010. According to the December 2008 Frost & Sullivan report, Masimo and Nellcor each comprised 37.4% of the total U.S. pulse oximetry monitoring equipment market in 2008. According to January 2009 market research report from iData, Masimo and Nellcor had 43.5% and 38.3% respectively of the U.S. pulse oximetry monitoring equipment market. We believe we will continue to grow our U.S. market share amount as more hospitals convert to Masimo technology.
New Market Opportunities for Masimo SET
General Floor Monitoring Expansion
We believe there are opportunities to expand the market for pulse oximetry by applying Masimo SET’s proven benefits from critical care settings to non-critical care settings, as well as settings outside of the hospital. It is currently estimated that over 86% of all U.S.
3
hospital beds are located in non-critical care areas, where continuous monitoring is not widely used. A study published in July 2004, byHealthGrades showed that approximately 264,000 hospital deaths over a three-year period were attributable to patient safety incidents, or generally preventable patient events in non-critical care areas. The study concluded that the failure to timely diagnose and treat patients accounted for over 70% of those deaths, suggesting that improved patient monitoring in non-critical care settings can alert clinicians of patient distress and help to improve patient care. As presented at the Society for Technology in Anesthesia in 2009, a recent study by Dartmouth-Hitchcock Medical Center demonstrated a measurable cost advantage with Patient SafetyNet’s ability to assist clinicians in the identification of the onset of patient distress earlier, contributing to decreases in patient costs on the general care floor.
The American Hospital Association estimated that there were approximately 947,000 staffed beds in all U.S.-registered hospitals in 2004. In 2000, approximately 87% of all hospital beds in the United States were located in non-critical care settings according to a study published in the Journal of Critical Care Medicine, which suggests a non-critical care market potential of approximately 820,000 beds in the U.S. alone. While some of these non-critical care beds have some form of monitoring capabilities today, we believe that approximately 15% of the 820,000 beds in the U.S. alone could become continuous monitoring beds. We believe that Masimo SET’s ability to dramatically minimize false alarms due to patient motion while maximizing the sensitivity of pulse oximeters to report true alarms will allow hospitals to reliably and continuously monitor their patients in the general floors.
Alternate Care
According to the June 2007 Frost & Sullivan report, the fastest growing portion of the U.S. pulse oximetry equipment market is in the alternate care market. We believe that Masimo SET technology offers significant advantages in some segments of this market, including home care and sleep diagnostics. The proven ability of Masimo SET to dramatically reduce false alarms and increase true event detection enables clinicians to make more reliable diagnoses of those who need oxygen therapy and Continuous Positive Airway Pressure, or CPAP, and we plan to leverage the opportunity and expand our presence in this market.
New Market Opportunities for Masimo Rainbow SET
There are opportunities to expand the market for patient monitoring by enabling the measurement of additional blood constituents beyond arterial blood oxygen saturation level and pulse rate by measuring total hemoglobin, carboxyhemoglobin and methemoglobin.
Total Hemoglobin (SpHb®)
In May 2008, we received clearance from the FDA for our total hemoglobin monitoring technology and in September 2008, we began shipping, in a limited market release, these monitors and sensors. Hemoglobin is the part of a red blood cell that carries oxygen to the body and therefore a measurement of total hemoglobin is an indicator of the oxygen carrying capacity of the blood. Because of its clinical importance, hemoglobin is one of the most commonly ordered lab diagnostic tests in the hospital and physician office. Each year in the United States, over 400 million invasive hemoglobin tests are performed, which require multiple steps including collecting the patient’s blood sample, and most often transferring the sample to the lab, analyzing the sample and documenting the results and reporting the results to the ordering clinician.
According to a 2007 study published inSurgery, unnecessary blood transfusions in a surgical setting account for between 9 - 44% of all transfusion costs. Bleeding during surgery may require a blood transfusion, which increases the length of hospital stays and the associated healthcare costs. The decision to transfuse often requires the physician to either make an educated guess or wait for lab results to confirm that it is necessary. Our Rainbow SET technology can provide noninvasive and continuous hemoglobin measurements to the clinician, typically within 90 seconds after placing our Rainbow sensor on a patient’s finger.
We believe that the ability to measure total hemoglobin on a noninvasive, immediate and continuous basis will, enable clinicians in surgery, ICU, emergency department, and other hospital settings to make earlier and better treatment decisions, including the detection of bleeding earlier and the decision of whether or not to transfuse, thus potentially decreasing costs. Because of the significant clinical and cost advantages of measuring total hemoglobin noninvasively and continuously, we believe that a large number of hospitals will adopt Masimo Rainbow SET technology because of our total hemoglobin measurement.
A significant portion of invasive hemoglobin measurements are made outside of hospital settings, in the physician office to aid diagnosis and treatment, and in the blood donation market to qualify potential donors for eligibility to donate blood. We believe that a significant number of the estimated 200,000 U.S. physician offices and estimated 15 million annual U.S. blood donations would be aided by the noninvasive and immediate assessment of hemoglobin. While we believe that these market opportunities will eventually become significant, we do not expect any expansion into these specific markets until 2010.
Carboxyhemoglobin (SpCO®)
Carbon monoxide is a colorless, odorless and tasteless gas that is undetectable by humans and is often unknowingly inhaled from combustion fumes, or during fires by victims and first responders. Carbon monoxide poisoning is the leading cause of accidental
4
poisoning death in the U.S., responsible for up to 50,000 emergency department visits and 500 unintentional deaths annually. Carbon monoxide poisoning, which involves carbon monoxide binding with hemoglobin cells, thereby preventing them from carrying oxygen, can cause severe neurological damage, permanent heart damage or death in a matter of minutes. Quick diagnosis and treatment of carbon monoxide poisoning is critical in saving lives and preventing long-term damage, but the condition is often misdiagnosed because symptoms are similar to the flu.
Masimo’s ability to noninvasively detect carbon monoxide has allowed clinicians and emergency professionals to identify carbon monoxide poisoning earlier, allowing faster triage and earlier intervention and treatment. A recent study in the emergency department using Masimo Rainbow SET carbon monoxide monitoring identified 60% more carbon monoxide poisoning cases than the conventional approach, and estimated that as many as 11,000 carbon monoxide poisoning cases per year in the U.S. were being missed with the conventional approach. Several leading emergency first responder associations, including the National Association of Emergency Medical Technicians, the National Association of EMS Educators and the International Association of Fire Fighters, have in the past 18 months issued recommendations to noninvasively screen for carbon monoxide poisoning when exposure is suspected or when an individual presents with symptoms of such poisoning. In addition the National Fire Protection Association, or NFPA, included carbon monoxide screening by Pulse CO-Oximetry as part of a new national healthcare standard for firefighters potentially exposed to carbon monoxide poisoning. NFPA’s consensus codes and standards serve as the worldwide authoritative source on fire prevention and public safety.
In addition, the United Kingdom House of Commons All Party Parliamentary Gas Safety Group, in a newly-published report aimed at increasing the awareness of carbon monoxide poisoning among medical professionals, and recommends noninvasive carbon monoxide testing for Emergency Department and EMS providers as a way to improve the country’s rate of detection and diagnosis of carbon monoxide poisoning. For the preparation of this report, the United Kingdom Group used Masimo Rainbow SET Rad-57 devices for 12 months and reported successful cases with the Rad-57 devices. In the U.S., Medicare recently approved a Current Procedural Terminology, or CPT, code and pricing for carboxyhemoglobin, enabling hospitals that perform testing to recoup their costs, in addition to the clinical benefits they receive.
We believe that the first opportunity for noninvasive blood carbon monoxide monitoring is in the EMS and emergency department settings. In the U.S. alone, there are approximately 30,000 fire departments / EMS locations and 5,000 hospitals that would benefit from noninvasive carbon monoxide testing. We believe other opportunities exist for Carboxyhemoglobin monitoring, including the pre-operative setting.
Methemoglobin (SpMet®)
Commonly prescribed drugs can introduce methemoglobin into the blood and cause methemoglobinemia. Some of the 30 drugs that are known to cause methemoglobinemia are benzocaine, a local anesthetic, which is routinely used in procedures ranging from endoscopy to surgery; inhaled nitric oxide, routinely used in the Neonatal Intensive Care Unit; nitroglycerin used to treat cardiac patients and dapsone, used to treat infections for immune deficient patients, such as HIV patients. Methemoglobinemia reduces the amount of oxygen bound to hemoglobin for delivery to tissues and forces normal hemoglobin to bind more tightly to oxygen, releasing less oxygen to the tissues. Methemoglobinemia is often unrecognized or diagnosed late, increasing risk to the patient.
According to a study published by researchers at Johns Hopkins University in September 2004, there were 414 cases, or 19% of all patients reviewed, of acquired methemoglobinemia, at two hospitals over a 28-month period. The methemoglobinemia resulted in one fatality and three near-fatalities. Warnings, cautions and alerts regarding the clinical significance and prevalence of methemoglobinemia have been generated by the FDA, Veterans Administration, Institute for Safe Medication Practices, and the National Academy of Clinical Biochemistry. The American Academy of Pediatrics recommends monitoring methemoglobin levels in infants who receive nitric oxide therapy. In the U.S., Medicare recently approved a CPT code and pricing for our methemoglobin monitoring, enabling hospitals who perform testing to recoup their costs, in addition to the clinical benefits they receive.
We believe the initial opportunity for methemoglobin monitoring is in outpatient procedure labs in hospitals, such as esophageal echocardiography and gastrointestinal labs where use of caines, such as benzocaine, is prevalent, monitoring HIV patients who receive dapsone, as well as monitoring neonates who receive inspired nitric oxide in the neonatal intensive care units.
Plethysmograph Variability Index (PVI®)
Plethysmograph Variability Index, or PVI, is a noninvasive measurement that quantifies changes in the plethysmographic waveform over the respiration cycle. PVI may help optimize fluid status, a critical factor during surgery and in intensive care. Traditional methods such as invasive pressure monitoring often fail to predict fluid responsiveness, and newer methods are also invasive and costly. PVI has been shown by one research group to predict fluid responsiveness in surgical and intensive care patients. Optimizing fluid status has been shown to improve patient outcomes in previously published studies, so we believe that PVI will be helpful in patient management as well.
We believe the primary opportunity for PVI monitoring is in surgery and intensive care in hospitals, but it is also possible that future studies may reveal application in identifying dehydration or in optimizing fluid in cardiac conditions such as heart failure.
5
Future Parameters
We believe that our core signal processing and sensor technologies are widely applicable and expect to develop and launch future applications utilizing our proprietary technology platforms.
In the second half of 2009, we expect to introduce, in limited market release, a noninvasive and continuous respiration rate parameter based on acoustic monitoring SET. Respiration rate is defined as the number of breaths per minute, and changes in respiration rate provide an early warning sign of deterioration in patient condition. Current methods to monitor respiration rate include end tidal CO2 monitoring, which requires a special tube inserted in the patient’s nose, and impedance monitoring, which is considered cumbersome to the clinician and patient and unreliable. Masimo’s noninvasive respiration rate parameter will be available in our Masimo Rainbow SET platforms such as Radical-7 and Rad 87 bedside monitoring devices, with the launch of MX-3 Board, slated for release in the second half of fiscal 2009. These devices will be deployed through an acoustic respiration sensor on the patient’s neck and connected to the bedside monitor with a special cable. Should the respiration rate change or stop, an alarm will be displayed on the device and in addition, can be sent to the Masimo Patent SafetyNet system, which can notify the attending clinician or nurse of the condition, directly on the monitor or remotely via a pager. We believe this noninvasive measurement will become a key and important measurement in both critical care and general floor environments.
The Masimo Solution
Our innovative and proprietary technologies and products are designed to overcome the primary limitations of conventional pulse oximetry, which involve maintaining accuracy in the presence of motion artifact and weak signal-to-noise situations. Our Masimo SET platform, which became available to hospitals in the United States in 1998, is the basis of our pulse oximetry products and we believe represented the first significant technological advancement in pulse oximetry since its introduction in the early 1980s. In addition, our products’ benefits have been validated in over 100 independent clinical and laboratory studies.
Masimo SET utilizes five signal processing algorithms, four of which are proprietary, in parallel, to deliver high precision, sensitivity and specificity in the measurement of arterial blood oxygen saturation levels. Sensitivity is the ability to detect true events and specificity is the ability to reject false alarms. One of our proprietary processing algorithms, Discrete Saturation Transform, separates signal from noise in real-time through the use of adaptive filtering, and an iterative sampling technique that tests each possible saturation value for validity. Masimo SET signal processing can therefore identify the venous blood and other noise, isolate them, and extract the arterial signal.
To complement our Masimo SET platform, we have developed a wide range of proprietary single-patient use and reusable sensors, cables and other accessories designed specifically to work with Masimo SET software and hardware. Although our technology platforms operate solely with our proprietary sensor lines, our sensors have the capability to work with certain competitive pulse oximetry monitors through the use of adapter cables. Our neonatal adhesive sensors have been clinically proven to exhibit greater durability compared to competitive sensors.
In 2005, we introduced our Masimo Rainbow SET platform, leveraging our Masimo SET technology and incorporating licensed Rainbow technology to enable reliable, real-time monitoring of additional parameters beyond arterial blood oxygen saturation and pulse rate. The Masimo Rainbow SET platform has the unique ability to distinguish oxygenated hemoglobins from certain dyshemoglobins, hemoglobin incapable of transporting oxygen, and allows for the rapid, noninvasive monitoring of total hemoglobin, carboxyhemoglobin, methemoglobin and PVI, which we refer to as Pulse CO-Oximetry. Along with the release of our Rainbow SET Pulse CO-Oximetry products, we have developed multi-wavelength sensors that have the ability to monitor multiple parameters with a single sensor. We believe that the use of Masimo Rainbow SET Pulse CO-Oximetry products will become widely adopted for the noninvasive monitoring of these parameters.
Additionally, we market our Patient SafetyNet and RadNet remote-alarm and monitoring systems for use with our Masimo SET pulse oximeters and Rainbow SET Pulse CO-Oximeters. These systems currently allow wireless and remote monitoring of the oxygen saturation and pulse rate of up to 40 patients simultaneously, and may facilitate the expansion of our products into areas beyond the critical care settings, such as the general care areas. We believe that the superior performance of the Masimo SET platform coupled with reliable, cost effective, and easy to use wireless remote monitoring will allow hospitals to create continuous surveillance solutions on general care floors where patients are at risk of avoidable adverse events and where direct patient observation by skilled clinicians is cost prohibitive.
Benefits of Our Products and Technology
We believe that our technology and products offer several key benefits, including:
| | • | | Accurate, Real-Time Measurement. We believe that the Masimo SET platform has the ability to provide more accurate measurements with fewer missed events and false alarms than other pulse oximeters in the market place. Many of the top hospitals in the United States, including four of the top five, according to “U.S. News and World Reports Honor Roll” for |
6
| | 2008, made Masimo SET their primary pulse oximetry platform. We believe Masimo Rainbow SET will allow noninvasive measurement of previously invasive parameters, such as total hemoglobin. |
| | • | | Increased Quality of Patient Care. We believe that the proven accuracy and reliability of Masimo SET pulse oximetry allows for better clinical decisions, leading to fewer medical errors and better patient care. We believe that the noninvasive monitoring of carboxyhemoglobin will improve the quality of care based on the number of emergency room visits reported for carbon monoxide poisoning. We believe the noninvasive monitoring of methemoglobin will also improve patient care based on reported drug interactions that increase methemoglobin levels in the blood. We believe wireless remote-alarm and monitoring on the general care floor will reduce avoidable adverse events through earlier detection and intervention. We believe Masimo Rainbow SET will allow earlier and better clinical decisions in a variety of care areas. |
| | • | | Reduced Cost of Care. Several independent studies have shown that hospitals can reduce their costs as a result of using Masimo SET products. We believe that factors contributing to lower costs include a reduction in sensor usage as a result of more durable sensors, fewer invasive arterial blood gas procedures needed, less oxygen administration and a reduction in length of stay as the result of weaning patients off of ventilators more quickly. In addition, we expect that the noninvasive monitoring of carboxyhemoglobin and methemoglobin will help reduce the cost of care by reducing the need for invasive blood tests and limiting the costs from complications caused by incorrect diagnoses. We believe early detection of avoidable adverse events will contribute to lower length of stay because such events will be treated earlier before patients decompensate to critical levels. We believe earlier and better clinical decisions from Masimo Rainbow SET will allow for more cost-effective care and in some cases reimbursable procedures for hospitals and non-hospital providers. |
| | • | | Masimo SET Platform Allows for Expansion into Non-Critical Care Settings. We believe the ability of Masimo SET products to provide reliable monitoring with fewer false alarms has expanded and will continue to expand the use of pulse oximetry into other settings where patient motion and false alarms have historically prevented its use. Since the introduction of Masimo SET, we believe that pulse oximetry has become a standard of care in the EMS market. In addition, hospitals and other care centers can reduce their costs by moving less critically ill patients from the ICU to the general care areas where these patients can be continuously and accurately monitored in a more cost-effective manner. Many patients in the general care areas are at risk of dying due to inadequate oxygenation. To mitigate this risk, patients in the general care areas need to be continuously monitored. Our Patient SafetyNet and RadNet systems enable the Masimo SET and Rainbow SET platforms to wirelessly and remotely monitor patients in the general care areas of the hospital that are not under the constant supervision of clinicians. |
| | • | | Upgradeable Rainbow Platform for the Monitoring of Additional Parameters. Products with our new MX circuit board contain our Masimo SET pulse oximetry technology as well as circuitry to support Rainbow parameters. At the time of purchase, or at any time in the future, our customers and our OEMs’ customers will have the option of purchasing a software parameter, which will allow the customer to expand their patient monitoring systems to monitor additional parameters with a cost-effective solution. |
Our Strategy
Since inception, our mission has been to develop noninvasive blood constituent patient monitoring solutions that improve patient outcomes and reduce the cost of patient care. We intend to continue to grow our business and to improve our market position by pursuing the following strategies:
| | • | | Continue to Expand Our Market Share in Pulse Oximetry. We grew our product revenue from $107.6 million in 2005 to $258.9 million in 2008, representing a three year CAGR of approximately 34.0%. This growth can be attributed to the increased access to pulse oximetry customers through our agreements with group purchasing organizations, or GPOs, and our increased relationships with OEM partners, the expansion of our direct sales force, and strong, independent clinical evidence that demonstrates the benefits of our technology. We supplement our direct sales with sales through our distributors. Direct and distributor sales increased to approximately $201.1 million, or 77.7%, of product revenue in 2008, from $69.1 million, or 64.2%, of product revenue in 2005. |
| | • | | Expand the Pulse Oximetry Market to Other Patient Care Settings. We believe the ability to continuously and accurately monitor patients outside of critical care settings, including the general care areas of the hospital, are currently unmet medical needs and have the potential to significantly improve patient care and increase the size of the pulse oximetry market. We believe the ability of Masimo SET to accurately monitor and address the limitations of conventional pulse oximetry has enabled, and will continue to enable, us to expand into non-critical care settings and thus significantly expand the market for our products. To further support our expansion into the general care areas, we market two wireless floor monitoring solutions, Patient SafetyNet and RadNet, that currently enable continuous monitoring of up to 40 patients’ oxygen saturation and pulse rate with one system, utilizing our Masimo SET or Masimo Rainbow SET platform. |
7
| | • | | Expand the use of Rainbow SET Pulse CO-Oximetry in the Hospital Setting. We believe the noninvasive measurement of total hemoglobin, carboxyhemoglobin, methemoglobin, PVI, and future parameters will provide an excellent opportunity to leverage existing customer relationships into new streams of revenue, directly and through a greater ability to convert non-Masimo hospitals to Masimo hospitals due to our expanded measurement capabilities. |
| | • | | Expand the use of Rainbow SET in the Non-Hospital Setting.We believe the noninvasive measurement of hemoglobin creates a significant opportunity in markets such as the physician office and blood donation centers, and noninvasive carboxyhemoglobin in the Fire/EMS market. By 2010, we expect to introduce a new handheld product called the Pronto into the Physician Office. The Pronto will allow non-hospital users to simply and quickly measure hemoglobin, one of the most common invasive laboratory measurements. We believe that the ability to noninvasively measure total hemoglobin will greatly facilitate the efficiency with which physicians will be able to quickly determine total hemoglobin levels of their patients, by reducing invasive blood draws, labeling, sending to the lab, waiting for the lab results, and communicating these results to the patient (usually the next day). |
| | • | | Utilize Our Customer Base and OEM Relationships to Market Our Masimo Rainbow SET Pulse CO-Oximetry Products Incorporating Licensed Rainbow Technology. We sold our first Masimo Rainbow SET Pulse CO-Oximetry products in September 2005. We are currently selling our Rainbow SET products through our direct sales force and distributors. In addition, we plan to sell our MX circuit boards in our own pulse oximeters and to our OEM partners, equipped with circuitry to support Rainbow SET Pulse CO-Oximetry parameters which can be activated at time of sale or through a subsequent software upgrade. We believe that the clinical need of these measurements along with our installed customer base will help drive the adoption of our Rainbow SET Pulse CO-Oximetry products. |
| | • | | Continue to Innovate and Maintain Our Technology Leadership Position. We invented and pioneered what we believe is the first pulse oximeter to accurately measure arterial blood oxygen saturation level and pulse rate in the presence of motion artifact and low perfusion. In addition, through our license of Rainbow technology from Masimo Labs, we launched our Rainbow SET Pulse CO-Oximetry platform that enabled what we believe are the first FDA-cleared noninvasive monitoring of carboxyhemoglobin and methemoglobin in the blood. We also developed PVI. We plan to continue to innovate and develop new technologies and products internally and through our collaboration with Masimo Labs, for the noninvasive monitoring of other parameters. |
Our future growth strategy is also closely tied to our focus on international expansion opportunities. Historically, we have generated between 75% to 80% of our revenues in the United States. Since 2006, we have been aggressively expanding our sales and marketing presence in Europe, Japan, Canada, Latin America and the rest of Asia. We have accomplished this through both additional staffing and by adding or expanding sales offices in many of these territories. During the fourth quarter of 2008, we established a new international business structure designed to better serve and support our growing international business. By centralizing our international operations, including sales management, marketing, customer support, planning, logistics and administrative functions, we believe it will be able to develop a more efficient and scalable international organization—capable of being even more responsive to the business needs of its international customers—all under one centralized management structure. As a result of these investments and focus on our international operations, we believe that our international product revenues, as a percent of total product revenues, will continue to increase.
Our Products
We develop, manufacture and market a patient monitoring solution that incorporates a monitor or circuit board and sensors including both proprietary single-patient use and reusable sensors and cables. In addition, we offer remote-alarm/monitoring solutions and software.
The following chart summarizes our principal product components and principal markets and methods of distribution:
| | | | |
Product Components | | Description | | Markets and Methods of Distribution |
Patient Monitoring Solutions: | | | | |
Circuit Boards (e.g. MX-1) | | • Signal processing apparatus for all Masimo SET and licensed Masimo Rainbow SET technology platforms | | Incorporated into our proprietary pulse oximeters and sold to OEM partners who incorporate our circuit boards into their patient monitoring systems |
| | |
Pulse CO-Oximeters/Monitors and Pulse Oximeters (e.g Radical-7) | | • Bedside and handheld monitoring devices that incorporate Masimo SET with and without licensed Masimo Rainbow SET technology | | Sold directly to end-users and through distributors and in some cases to our OEM partners who sell to end-users |
8
| | | | |
Product Components | | Description | | Markets and Methods of Distribution |
| | |
Sensors (e.g. Rainbow and Non-Rainbow Sensors) | | • Extensive line of both single-patient use and reusable sensors • Patient cables, as well as adapter cables that enable the use of our sensors on certain competitive monitors | | Sold directly to end-users and through distributors and to OEM partners who sell to end-users |
| | |
Remote Alarm and Monitoring Solutions (e.g. Patient Safety Net) | | • Network-linked wired or wireless, multiple patient floor monitoring solutions • Standalone wireless alarm notification solutions | | Sold directly to end-users |
| | |
| Software (e.g. SpHb, SpCO, SpMet, PVI) | | • Rainbow parameters and other proprietary features sold to installed monitors | | Sold directly to end-users and through OEM partners who sell to end-users |
Circuit Boards
Masimo SET MS Circuit Boards. Our Masimo SET MS circuit boards perform all signal processing and other pulse oximetry functions incorporating the Masimo SET platform. Our MS circuit boards are included in our proprietary monitors for direct sale or sold to our OEM partners for incorporation into their monitors. Once incorporated into a pulse oximeter, the MS circuit boards perform all data acquisition processing and report the pulse oximetry levels to the host monitor. The circuit boards and related software interface directly with our proprietary sensors to calculate arterial blood oxygen saturation level and pulse rate. Our latest generation boards include the MS-2003, MS-2011 and MS-2013. Our older generation boards, including the MS-1, MS-3, MS-5, MS-7, MS-11 and MS-13 circuit boards, which all vary in size and power consumption, have been made obsolete and are being transitioned out of our MS OEM Board family.
Masimo Rainbow SET MX Circuit Boards. Our next-generation circuit board is the foundation for our Masimo Rainbow SET Pulse CO-Oximetry platform, utilizing technology licensed from Masimo Labs. The MX circuit boards measure arterial blood oxygen saturation levels and pulse rate, and have the circuitry to enable the measurement of total hemoglobin, oxygen content, carboxyhemoglobin, methemoglobin, PVI and potentially other parameters. Customers can choose to buy additional parameters beyond arterial blood oxygen saturation levels and pulse rate at the time of sale or at any time in the future through a field-installed software upgrade. As additional parameters are developed, each new parameter may be available as a software upgrade to the existing system.
Pulse CO-Oximeters/Monitors and Pulse Oximeters
Radical-7. We believe that the Radical-7 pulse CO-Oximeter is the most advanced and versatile oximetry monitor available. The Radical-7 incorporates the MX circuit board, which enables all Rainbow SET parameters, and offers three-in-one capability to be used as:
| | • | | a standalone device for bedside monitoring; |
| | • | | a detachable, battery-operated handheld unit for easy portable monitoring; and |
| | • | | a monitor interface via SatShare, proprietary technology allowing our products to work with certain competitor products, to upgrade existing conventional multi-parameter patient monitors to Masimo SET while displaying Rainbow parameters on the Radical-7 itself. |
Radical-7 is a fully-equipped standalone pulse CO-Oximeter with a detachable module, which functions as a battery-operated, handheld monitor. The handheld module can be connected with any other Radical-7 base station, which allows Radical-7 to stay with the patient, enabling continuous and reliable arterial blood oxygen saturation and blood constituent monitoring such as total hemoglobin as patients are transported within the hospital. For example, Radical-7 can continuously monitor a patient from the ambulatory environment, to the emergency room, to the operating room, to the general floor, and on until the patient is discharged. Radical-7 delivers the accuracy and reliability of Masimo Rainbow SET with multi-functionality, ease of use and a convenient upgrade path for existing monitors.
Our SatShare technology enables a conventional monitor to upgrade to Masimo SET through a simple cable connection from the back of Radical-7 to the sensor input port of the conventional monitor. No software upgrades or new modules are necessary for the upgrade,
9
which can be completed in minutes. SatShare allows hospitals to standardize the technology and sensors used throughout the hospital while allowing them to gain more accurate monitoring capabilities and additional multi-functionality in a cost-effective manner. This has facilitated many hospital-wide conversions of previously installed competitor monitors to Masimo SET. In addition, Masimo Rainbow SET parameters such as total hemoglobin are available to clinicians on the Radical-7 itself while the device is being used in SatShare mode.
Rad-87. The Rad-87, which also contains Masimo Rainbow SET technology, is a compact, lightweight and easy-to-use device designed specifically for use in less acute settings than the Radical-7. The Rad-87 is available with a built-in bi-directional wireless radio for use as part of the Masimo Patient SafetyNet remote monitoring and clinician notification system. We began shipping the Rad-87 in July 2008.
Pronto. The Pronto is a handheld spot-check device, using Masimo Rainbow SET technology, specifically designed to noninvasively provide total hemoglobin levels in a physician office, clinic, or blood donation setting. Additionally, the device provides arterial oxygen saturation and pulse rate readings. The Pronto does not provide continuous monitoring. We received FDA clearance for the Pronto in December 2008 and expect to begin shipping the product, under a limited market release program, in the first half of 2009.
Rad-8. The Rad-8 is a bedside pulse oximeter featuring Masimo SET (but without Rainbow capability) with a low cost design and streamlined feature set, allowing it to be offered at a lower price point than the Radical-7 or Rad-87.
Rad-5. In addition to the bedside monitors, we have developed handheld pulse oximeters using Masimo SET. Our Rad-5 and Rad-5v handheld oximeters were the first dedicated handhelds with Masimo SET.
Rad-57. The Rad-57 is a fully featured handheld pulse CO-Oximeter that provides continuous, noninvasive measurement of carboxyhemoglobin and methemoglobin in addition to oxygen saturation, pulse rate, perfusion and index. Its rugged and lightweight design makes it applicable for use in hospital and field settings, specifically for fire departments and EMS units.
Sensors
Sensors and Cables. We have developed one of the broadest lines of single-patient use and reusable sensors and cables. Masimo SET sensors are uniquely designed to reduce interference from physiological and non-physiological noise. Our proprietary technology platforms operate only with our proprietary sensor lines. However, through the use of adapter cables, we can connect our sensors to certain competitive pulse oximetry monitors. We sell our sensors and cables to end-users through our direct sales force and our distributors and OEM partners.
Our single-patient use sensors offer several advantages over reusable sensors, including improved performance, cleanliness, increased comfort and greater reliability. In addition, our LNOP single-patient use sensors offer several advantages over competitive disposable sensors, including a more durable tape material that is less likely to tear and an adhesive that can be easily rejuvenated with an alcohol swab. As a result, the sensor can be moved and reapplied multiple times during a patient’s stay. Our LNOP single-patient neonatal adhesive sensors have been shown in independent, published studies to last approximately twice as long as the market-leading disposable sensor. Our reusable sensors, which include ear and forehead sensors, are primarily used for short-term hospital stays and spot checks. We currently sell over 40 different sensors for adults, children, infants and pre-term infants.
SofTouch Sensors. We have developed SofTouch sensors, designed with less adhesive or no adhesive at all for compromised skin conditions. These include single-patient sensors for babies and multi-site reusable sensors for pediatrics and adults.
Trauma and Newborn Sensors. We believe we were the first to develop two specialty sensor lines, specifically designed for trauma and resuscitation situations, as well as for newborns. These sensors contain an identifier which automatically sets the oximeter to monitor with maximum sensitivity and the shortest-averaging mode and allows for quick application, even in wet and slippery environments.
Blue Sensors. In 2005, we introduced what we believe to be the first FDA-cleared sensor to accurately monitor arterial blood oxygen saturation levels in cyanotic infants and children with abnormally low oxygen saturation levels.
Masimo Rainbow SET Sensors. We believe we were the first to develop proprietary, multi-wavelength sensors for use with our Rainbow SET Pulse CO-Oximetry products. As opposed to traditional sensors that only have the capability to monitor arterial blood oxygen saturation levels and pulse rate, our Rainbow sensors can also monitor carboxyhemoglobin, methemoglobin and total hemoglobin. Our licensed Rainbow SET sensors are the only sensors that are compatible with our licensed Rainbow SET products.
Remote-Alarm and Monitoring Solutions
Patient SafetyNet. Patient SafetyNet is a remote monitoring and clinician notification system. It instantly routes bedside-generated alarms through a server to a qualified clinician’s handheld paging device in real-time. Each system can support up to 40 bedside monitors and can either be integrated into a hospital’s existing IT infrastructure or operate as a stand-alone wireless network.
10
RadNet. RadNet enables Masimo SET and Rainbow SET monitors with a wired or wireless monitoring system to provide continuous, centralized monitoring of remotely located patients, with the ability to monitor up to 40 patients per system.
PPO+. PPO+, or Personal Pulse Oximeter, is a patient-wearable pulse oximeter and electrocardiogram, or ECG, monitor that can wirelessly transmit patients’ arterial blood oxygen saturation level, pulse rate and ECG to the RadNet. PPO+ is ideally suited for monitoring ambulatory patients in the general care areas, emergency department, emergency department waiting room and any other area where the patient is ambulatory.
Both RadNet and PPO+ are OEM products from Welch Allyn.
Software.
All of our monitors, including Radical-7 and certain future OEM products, which incorporate the MX board, will allow purchases of software for Rainbow parameters as well as other future parameters or features that can be field installed.
Geographic information
We are a global company with a geographically diverse market presence. See Note 14 to our consolidated financial statements for financial information relating to the geographic areas in which we currently engage in business.
Sales and Marketing
As of January 3, 2009, we had 324 employees in sales and marketing in the United States and abroad, including 138 sales representatives. We expect to continue to increase our worldwide sales and sales support organizations as we continue to expand our presence throughout both the United States and throughout the world including Europe, the Middle East, Japan, Asia, Latin America, Canada and Australia. We currently sell all of our products both directly to hospitals and the EMS market via our sales force, and certain distributors.
Our direct and distributor revenue accounted for approximately 77.7% of our total product revenue in 2008. The primary focus of our sales representatives is to facilitate the conversion of competitor accounts to our Masimo SET pulse oximetry products. In addition to sales representatives, we employ clinical specialists to work with our sales representatives to educate end-users on the benefits of Masimo SET and assist with the introduction and implementation of our technology and products to their sites. Our sales and marketing strategy for pulse oximetry has been and will continue to be focused on building end-user awareness of the clinical and cost-saving benefits of our Masimo SET platform. More recently, we have expanded this communication and educational role to include our Masimo Rainbow SET Pulse CO-Oximetry products, including total hemoglobin, carboxyhemoglobin, methemoglobin and PVI. For the year ended January 3, 2009, Owens & Minor, one of our distributors, represented 11.6% of our total revenue and was the only customer that represented 10% or more of our revenue for the year ended January 3, 2009. Importantly, distributors such as Owens & Minor take and fulfill orders from our direct customers, many of whom have signed long-term sensor agreements with us. As a result, in the event a specific distributor is unable to fulfill these orders, the orders will be redirected to other distributors or fulfilled directly by us.
Additionally, we sell certain of our products through our OEM partners who both incorporate our boards into their monitors and resell our sensors to their customers’ installed base of Masimo SET products. Our OEM agreements allow us to expand the availability of Masimo SET through the sales and distribution channels of each OEM partner. To facilitate clinician awareness of Masimo SET installations, all of our OEM partners have agreed to place the Masimo SET logo prominently on their instruments. As of January 3, 2009, we had agreements with 53 OEM partners whom we believe accounted for over 90% of worldwide shipments of pulse oximeters incorporated into multi-parameter monitors. As of January 3, 2009, our OEM partners had collectively launched a total of approximately 127 patient monitoring products worldwide incorporating Masimo SET.
In order to facilitate our direct sales to hospitals, we have signed contracts with companies that we believe to be the six largest GPOs, based on the total volume of negotiated purchases. In return for the GPOs to put our products on contract, we have agreed to pay the GPOs a percentage of our revenue from their member hospitals. In 2008 and 2007, revenue from the sale of our pulse oximetry products to hospitals that are associated with GPOs amounted to $132.1 million and $101.0 million, respectively.
Our marketing efforts are designed to build end-user awareness through advertising, direct mail and trade shows. In addition, we distribute published clinical studies, sponsor accredited educational seminars for doctors, nurses, biomedical engineers, and respiratory therapists and conduct clinical evaluations. We expect to increase the size of our sales and marketing force worldwide during 2009, as we continue to establish and expand our sales channels on a global basis.
11
Competition
The medical device industry is highly competitive and many of our competitors have substantially greater financial, technical, marketing and other resources than we do. While we regard any company that sells pulse oximeters as a potential customer, we also recognize that the companies selling pulse oximeters on an OEM basis and/or pulse oximetry sensors are also potential competitors. Our primary competitor, Covidien Ltd. (formerly Tyco Healthcare) and its subsidiary Nellcor Puritan Bennett, Inc., currently hold a substantial share of the pulse oximetry market. Covidien sells its own brand of Nellcor pulse oximeters to end-users, sells pulse oximetry modules to other monitoring companies on an OEM basis and licenses, to certain OEMs, the right to make their pulse oximetry platforms compatible with Nellcor sensors. Although Nellcor is still a competitor of ours, in 2006 we settled a patent infringement case against them following an appellate ruling which found that Nellcor had infringed three of our patents. See “—Nellcor Patent Litigation Settlement” in Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations.” We face substantial competition from larger medical device companies, including companies that develop products that compete with our proprietary Masimo SET. We believe that a number of companies have announced products which claim to offer measure-through motion accuracy. Based on those announcements and our investigations, we further believe that many of these products include technology that infringes our intellectual property rights. We have settled claims against some of these companies and intend to vigorously enforce and protect our proprietary rights with respect to the others whom we believe are infringing our technology. On February 3, 2009, we filed a patent infringement suit against Phillips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh, which are affiliates of Philips Medical Systems, one of our OEM partners. Some of the remaining companies, including GE Medical Systems and Mindray Medical International Ltd., are also currently OEM partners of ours.
We believe that the principal competitive factors in the market for pulse oximetry products include:
| | • | | accurate monitoring during both patient motion and low perfusion; |
| | • | | ability to introduce other clinically beneficial parameters related to oxygenation and respiration, such as carboxyhemoglobin and methemoglobin; |
| | • | | sales and marketing capability; |
| | • | | access to hospitals which are members of GPOs; |
| | • | | access to OEM partners; and |
Masimo Laboratories, Inc.
Masimo Laboratories, Inc., or Masimo Labs, is an independent entity spun-off from us to our stockholders in 1998. Joe E. Kiani and Jack Lasersohn, members of our board of directors, are also members of the board of directors of Masimo Labs. Joe E. Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Masimo Labs.
We have a cross-licensing agreement with Masimo Labs for certain technologies. The following table outlines our rights under the Cross-Licensing Agreement relating to specific end user markets and the related technology applications of specific parameters.
| | | | |
| | | End User Markets |
Parameters | | Professional Caregiver and EMS | | Patient and Pharmacist |
Vital Signs(1) | | Masimo (owns) | | Masimo Labs (non-exclusive license) |
Non-Vital Signs(2) | | Masimo (exclusive license) | | Masimo Labs (owns) |
(1) | Vital Signs parameters include SpO2, peripheral venous oxygen saturation, mixed venous oxygen saturation, fetal oximetry, sudden infant death syndrome, ECG, blood pressure (noninvasive blood pressure, invasive blood pressure and continuous non- invasive blood pressure), temperature, respiration rate, CO2, pulse rate, cardiac output, EEG, perfusion index, depth of anesthesia, cerebral oximetry, tissue oximetry and/or EMG, and associated features derived from these parameters, such as 3-D alarms, Pleth Variability Index and other features. |
(2) | Non-Vital Signs parameters include the body fluid constituents other than vital signs parameters and include, but are not limited to, carbon monoxide, methemoglobin, blood glucose, total hemoglobin and bilirubin. |
Our License to Masimo Labs. We granted Masimo Labs an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET owned by us for the measurement of non-vital signs parameters and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs parameters in the Labs Market. We also granted Masimo Labs a non-exclusive, perpetual
12
and worldwide license, with sublicense rights, to use Masimo SET for the measurement of vital signs in the Labs Market. In exchange, Masimo Labs pays us a 10% royalty on the amount of vital signs sensors and accessories sold by Masimo Labs.
The Labs Market is defined as any product market in which a product is intended to be used by a patient or pharmacist rather than a professional medical caregiver regardless of the particular location of the sale, including sales to doctors, hospitals, EMS professionals or otherwise, provided the product is intended to be recommended, or resold, for use by the patient or pharmacist.
Masimo Labs’ License to Us. We exclusively licensed from Masimo Labs the right to make and distribute products in the Masimo Market that utilize Rainbow technology for the measurement of carbon monoxide, methemoglobin, fractional arterial oxygen saturation, and total hemoglobin, which includes hematocrit. To date, we have developed and commercially released devices that measure carbon monoxide and methemoglobin using licensed Rainbow technology. We also have the option to obtain the exclusive license to make and distribute products in the Masimo Market that utilize Rainbow technology for the measurement of other non-vital signs parameters, including blood glucose. These licenses are exclusive until the later of 20 years from the grant of the applicable license or the expiration of the last patent included in the Rainbow technology related to the applicable parameter.
The Masimo Market is defined as those product markets where the product is intended to be used by a professional medical caregiver, including hospital caregivers, surgicenter caregivers, paramedic vehicle caregivers, doctor’s offices caregivers, EMS facility caregivers and vehicles where emergency medical services are provided.
Our license to Rainbow technology for these parameters in these markets is exclusive on the condition that we continue to pay Masimo Labs royalties on our products incorporating Rainbow technology, subject to certain minimum unit and aggregate royalty thresholds, and that we use commercially reasonable efforts to develop or market products incorporating the licensed Rainbow technology. The royalty is up to 10% of the Rainbow royalty base, which includes handhelds, tabletop and multi-parameter devices. Handheld products incorporating Rainbow technology will carry a 10% royalty rate. For other products, only the proportional amount attributable for that portion of our products used to measure non-vital signs parameters, sensors and accessories, rather than for measuring vital signs parameters, will be included in the 10% Rainbow royalty base. For multi-parameter devices, the Rainbow royalty base will include the percentage of the revenue based on the number of Rainbow-enabled parameters. Beginning in 2009, for hospital contracts where we place equipment and enter into a sensor contract, we will pay a royalty to Masimo Labs on the total sensor contract revenue based on the ratio of Rainbow enabled devices to total devices.
We are also subject to certain specific annual minimum aggregate royalty payments. The minimum aggregate royalty payment is $4.0 million for the 2009 fiscal year and $5.0 million per year thereafter.
From its inception in 1998 through January 3, 2009, we have agreed to pay Masimo Labs $22.1 million for both exclusive options and minimum royalty payments. We have 180 days after proof of feasibility to exercise the above-referenced option to obtain a license to the remaining non-vital signs parameters, including carbon monoxide, methemoglobin, total hemoglobin and bilirubin, for an additional $500,000 each, and blood glucose, for an additional $2.5 million.
Change in Control. The Cross-Licensing Agreement provides that, upon a change in control:
| | • | | if the surviving or acquiring entity ceases to use “Masimo” as a company name and trademark, all rights to the “Masimo” trademark will be assigned to Masimo Labs; |
| | • | | the option to license technology developed by Masimo Labs for use in blood glucose monitoring will be deemed automatically exercised and a $2.5 million license fee for this technology will become immediately payable to Masimo Labs; |
| | • | | per product minimum royalties, to the extent less than the annual minimums, will be payable to Masimo Labs; and |
| | • | | the minimum aggregate annual royalties for all licensed Rainbow parameters payable to Masimo Labs will increase to $10.0 million in the 2009 fiscal year and $15.0 million in each following year until the exclusive period of the agreement ends, plus up to $2.0 million per other Rainbow parameters. |
A change in control includes any of the following with respect to us or Masimo Labs:
| | • | | the sale of all or substantially all of either party’s assets to a non-affiliated third party; |
| | • | | the acquisition by a non-affiliated third party of 50% or more of the voting power of either party; |
| | • | | Joe E. Kiani, our Chief Executive Officer and the Chief Executive Officer of Masimo Labs, resigns or is terminated from his position with either party; and |
| | • | | the merger or consolidation of either party with a non-affiliated third party. |
Ownership of Improvements. Any improvements to Masimo SET or Rainbow technology made by Masimo Labs, by us, or jointly by Masimo Labs with us or with any third party that relates to non-vital signs monitoring, and any new technology acquired by Masimo
13
Labs, is and will be owned by Masimo Labs. Any improvements to the Masimo SET platform or Rainbow technology made by Masimo Labs, by us, or jointly by Masimo Labs with us or with any third party that relates to vital signs monitoring, and any new technology acquired by us, is and will be owned by us. However, in either case, any improvements to the technology, excluding acquired technology, will be assigned to the other party and be subject to the terms of the licenses granted under the Cross-Licensing Agreement. Any new non-vital signs monitoring technology utilizing Masimo SET that we develop will be owned by Masimo Labs and will be subject to the same license and option fees as if it had been developed by Masimo Labs. Also, we will not be reimbursed by Masimo Labs for our expenses relating to the development of any such technology.
Masimo Labs Services Agreement. We have also entered into a services agreement, or the Services Agreement, with Masimo Labs. Under this Services Agreement, we provide Masimo Labs with engineering services and accordingly charge Masimo Labs for these direct salary and payroll related expenses. In addition, at the end of each quarter, we charge Masimo Labs for its share of accounting, human resources, legal, facility and equipment costs, which we collectively refer to as indirect expenses. From its inception in 1998 through January 3, 2009, Masimo Labs has incurred approximately $17.4 million in both direct and indirect expenses. We expect Masimo Labs to continue to engage us for these services. However, pursuant to the Services Agreement, Masimo Labs may terminate the agreement by providing us 30 days notice, while we may terminate with 180 days notice to Masimo Labs.
Research and Product Development
We believe that ongoing research and development efforts are essential to our success. As of January 3, 2009, including Masimo Labs, we employed 136 engineers and engineering support staff. We expect to increase the size of our research and development staff during 2009. Our research and development efforts focus primarily on continuing to enhance our technical expertise in pulse oximetry, enabling the noninvasive monitoring of other parameters and developing remote-alarm and monitoring solutions.
Although we and Masimo Labs each have separate research and development projects, we collaborate with Masimo Labs on multiple research and development activities related to Rainbow technology and other technologies. Under the Cross-Licensing Agreement, the parties have agreed to allocate proprietary ownership of technology developed by either party based on the functionality of the technology. We will have proprietary rights to all technology related to the noninvasive measurement of vital signs parameters, and Masimo Labs will have proprietary ownership of all technology related to the noninvasive measurement of non-vital signs parameters. In addition, under our Services Agreement with Masimo Labs, we provide Masimo Labs with professional and management support services, including human resources, legal and accounting services. In January 2007, Masimo Labs realigned its development efforts and, as of January 3, 2009, it had nine full-time engineers supporting its development efforts.
Our total research and development expenditures for 2008 were $25.5 million, which included $2.4 million related to expenses incurred by Masimo Labs pursuant to the Cross-Licensing Agreement. In 2007, total research and development expenditures were $23.0 million, which included $1.9 million related to expenses incurred by Masimo Labs pursuant to the Cross-Licensing Agreement. In 2006, our total research and development expenditures were $24.9 million, which included $9.4 million of share-based payment expense, and $3.4 million related to expenses incurred by Masimo Labs pursuant to the Cross-Licensing Agreement. We expect our research and development expenses to increase in 2009 and beyond as we expand our research and development force, enhance our existing products and technologies and develop new ones.
Intellectual Property
We believe that in order to maintain a competitive advantage in the marketplace, we must develop and maintain protection of the proprietary aspects of our technology. We rely on a combination of patent, trademark, trade secret, copyright and other intellectual property rights and measures to protect our intellectual property.
We have developed a patent portfolio internally, and to a lesser extent through acquisitions and licensing, that covers many aspects of our product offerings. As of January 3, 2009, we had 327 issued patents and 186 pending applications in the United States, Europe, Japan, Australia, Canada and other countries throughout the world. In addition, as of January 3, 2009, technology we licensed from our development partner, Masimo Labs, was supported by 48 issued patents and 86 pending applications in the United States and internationally. Some of our earliest patents begin to expire in 2011. Some of Masimo Labs’ earliest patents begin to expire in 2015. Additionally, as of January 3, 2009, we owned 38 U.S. registered trademarks and 122 foreign registered trademarks, as well as trade names that we use in conjunction with the sale of our products.
Under the Cross-Licensing Agreement, we and Masimo Labs have agreed to allocate proprietary ownership of technology developed based on the functionality of the technology. We will have proprietary ownership, including ownership of all patents, copyrights and trade secrets, of all technology related to the noninvasive measurement of vital signs parameters, and Masimo Labs will have proprietary ownership of all technology related to the noninvasive measurement of non-vital signs parameters. We also rely upon trade secrets, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We seek to protect our trade secrets and proprietary know-how, in part, with confidentiality agreements with consultants, vendors and employees, although we cannot be certain that the agreements will not be breached, or that we will have adequate remedies for any breach.
14
There are risks related to our intellectual property rights. For further detail on these risks, see Item 1.A— “Risk Factors.”
Government Regulation
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device that we wish to market in the United States must first receive either 510(k) clearance, by filing a 510(k) pre-market notification, or PMA approval, by filing a PMA application, from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA’s 510(k) clearance process usually takes from four to twelve months, but it can take longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from one to three years or even longer. We cannot be sure that 510(k) clearance or PMA approval will ever be obtained for any product we propose to market.
The FDA decides whether a device must undergo either the 510(k) clearance or PMA approval process based upon statutory criteria. These criteria include the level of risk that the agency perceives is associated with the device and a determination whether the product is a type of device that is similar to devices that are already legally marketed. Devices deemed to pose relatively less risk are placed in either Class I or II, which requires the manufacturer to submit a pre-market notification requesting 510(k) clearance, unless an exemption applies.
Class I devices are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (General Controls). Some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process.
Class II devices are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification procedure. All of our current devices are Class II devices.
Class III devices are those devices which have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device. The safety and effectiveness of Class III devices cannot be assured solely by the General Controls and the other requirements described above. These devices almost always require formal clinical studies to demonstrate safety and effectiveness and must be approved through the premarket approval process described below. Premarket approval applications (and supplemental premarket approval applications) are subject to significantly higher user fees under Medical Device User Fee and Modernization Act of 2002, or MDUFMA, than are 510(k) premarket notifications.
To obtain 510(k) clearance, a company must submit a premarket notification demonstrating that the proposed device is “substantially equivalent” in intended use and in technological and performance characteristics to a legally marketed “predicate device” that is either in Class I, Class II, or is a Class III device that was in commercial distribution before May 28, 1976, for which the FDA has not yet called for submission of a PMA application. Pursuant to the MDUFMA and the MDUFMA II provisions of the Food and Drug Amendments Act of 2007, unless a specific exemption applies, 510(k) premarket notification submissions are subject to user fees. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained. We have modified some of our 510(k) cleared devices, including our Masimo SET Software and Radical, but have determined that, in our view, based on FDA guidance as to when to submit a 510(k) notification for changes to a cleared device, new 510(k) clearances or PMA approvals are not required. We cannot assure you that the FDA would agree with any of our decisions not to seek 510(k) clearance or PMA approval. If the FDA requires us to seek 510(k) clearance or PMA approval for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance or PMA approval.
Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or deemed not substantially equivalent to a legally marketed predicate device, are placed in Class III. These devices are required to undergo the PMA approval process in which the manufacturer must establish the safety and effectiveness of the device to the FDA’s satisfaction. A PMA application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR. New premarket approval, or PMA, applications or PMA application supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of
15
information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel. None of our products are currently approved under a PMA.
A clinical trial may be required in support of a 510(k) submission and generally is required for a PMA application. These trials generally require an Investigational Device Exemption, or IDE, application approved in advance by the FDA for a specified number of patients, unless the product is deemed a nonsignificant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results. Clinical trials may begin if the IDE application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S.
We believe that our OEM partners may be required to obtain 510(k) premarket clearance from the FDA for products that incorporate Masimo SET or Masimo Rainbow SET circuit boards and sensors. In order to facilitate our OEM partners in obtaining 510(k) clearance for their products that incorporate Masimo SET or Masimo Rainbow SET boards and sensors, we have submitted and received FDA clearance for 45 510(k) notices covering our Masimo SET circuit boards, sensors, cables and notification systems.
In the future, we may be required to submit additional 510(k) clearance to address new claims, uses or products. We cannot assure you that the FDA will not deem one or more of our future products (or those of our OEM partners) to be a Class III device subject to the more burdensome PMA approval process. The FDA also may not approve or clear these products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or PMA of new products, new intended uses or modifications to existing products.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. Those regulatory requirements include:
| | • | | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
| | • | | QSR, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| | • | | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
| | • | | clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
| | • | | approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
| | • | | medical device reporting, or MDR, regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
| | • | | post-approval restrictions or conditions, including post-approval study commitments; |
| | • | | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
| | • | | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
| | • | | regulations pertaining to voluntary recalls; and |
| | • | | notices of corrections or removals. |
We must also register with the FDA as a medical device manufacturer and must obtain all necessary state permits or licenses to operate our business. As a manufacturer, we are subject to announced and unannounced inspections by the FDA to determine our compliance with FDA’s QSR and other regulations.
Our OEM partners also are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that our OEM partners or we have failed to comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| | • | | fines and civil penalties; |
| | • | | unanticipated expenditures to address or defend such actions; |
16
| | • | | delays in clearing or approving, or refusal to clear or approve, our products; |
| | • | | withdrawal or suspension of approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies; |
| | • | | product recall or seizure; |
| | • | | interruption of production; |
| | • | | operating restrictions; |
The FDA also has the authority to request repair, replacement or refund of the cost of any medical device manufactured or distributed by us. Our failure (or the failure of our OEM partners) to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our business, financial condition and results of operations.
Other U.S. Regulation
We and our OEM partners also must comply with numerous federal, state and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection, fire hazard control and hazardous substance disposal. We cannot be sure that we will not be required to incur significant costs to comply with these laws and regulations in the future or that these laws or regulations will not hurt our business, financial condition and results of operations. Unanticipated changes in existing regulatory requirements or adoption of new requirements could hurt our business, financial condition and results of operations.
Environmental
Our manufacturing processes involve the use, generation and disposal of hazardous materials and wastes, including silicone adhesives, solder and solder paste, sealants, epoxies and various solvents such as methyl ethyl ketone, acetone and isopropyl alcohol. As such, we are subject to stringent federal, state and local laws relating to the protection of the environment, including those governing the use, handling and disposal of hazardous materials and wastes. Future environmental laws may require us to alter our manufacturing processes, thereby increasing our manufacturing costs. We believe that our products and manufacturing processes at our facilities comply in all material respects with applicable environmental laws and worker health and safety laws; however, the risk of environmental liabilities cannot be completely eliminated.
Health Care Fraud and Abuse
In the United States, there are federal and state anti-kickback laws that generally prohibit the payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other health-related business. For example, the Federal Health Care Programs’ Anti-Kickback Law (42 U.S.C. § 1320a-7b(b)) prohibits anyone from, among other things, knowingly and willfully offering, paying, soliciting or receiving any bribe, kickback or other remuneration intended to induce the referral of patients for, or the purchase, order or recommendation of, health care products and services reimbursed by a federal health care program (including Medicare and Medicaid). Recognizing that the federal anti-kickback law is broad and potentially applicable to many commonplace arrangements, the Office of Inspector General within the Department of Health and Human Services, or OIG, has issued regulations, known as the safe harbors, which identify permissible practices. If all of the requirements of an applicable safe harbor are met, an arrangement will not be prosecuted under this law. Safe harbors exist for a number of arrangements relevant to our business, including, among other things, payments to bona fide employees, certain discount and rebate arrangements, and certain payment arrangements involving GPOs. The failure of an arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal. However, conduct that does not fully satisfy each requirement of an applicable safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG or the Department of Justice. Violations of this federal law can result in significant penalties, including imprisonment, monetary fines and assessments, and exclusion from Medicare, Medicaid and other federal health care programs. Exclusion of a manufacturer, like us, would preclude any federal health care program from paying for its products. In addition to the federal anti-kickback law, many states have their own kickback laws. Often, these state laws closely follow the language of the federal law. Some state anti-kickback laws apply regardless of whether federal health care program payment is involved. Federal and state anti-kickback laws may affect our sales, marketing and promotional activities, educational programs, pricing and discount practices and policies, and relationships with health care providers by limiting the kinds of arrangements we may have with hospitals, EMS providers, GPOs, physicians and others in a position to purchase or recommend our products.
Federal and state false claims laws prohibit anyone from presenting, or causing to be presented, claims for payment to third-party payers that are false or fraudulent. For example, the federal Civil False Claims Act (31 U.S.C. § 3729 et seq.) imposes liability on any person or entity who, among other things, knowingly and willfully presents, or causes to be presented, a false or fraudulent claim for payment by a federal health care program (including Medicaid and Medicare). Manufacturers, like us, can be held liable under false
17
claims laws, even if they do not submit claims to the government, where they are found to have caused submission of false claims by, among other things, providing incorrect coding or billing advice about their products to customers that file claims, or by engaging in kickback arrangements with customers that file claims. A number of states also have false claims laws, and some of these laws may apply to claims for items or services reimbursed under Medicaid and/or commercial insurance. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, and imprisonment.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: health care fraud and false statements related to healthcare matters. The health care fraud statute prohibits, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program, including private payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines and imprisonment.
The FCPA and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business.
Due to the breadth of some of these laws, it is possible that some of our current or future practices might be challenged under one or more of these laws. In addition, there can be no assurance that we would not be required to alter one or more of our practices to be in compliance with these laws. Evolving interpretations of current laws or the adoption of new federal or state laws or regulations could adversely affect many of the arrangements we have with customers and physicians. Our risk of being found in violation of these laws is increased by the fact that some of these laws are broad and open to interpretation. If our past or present operations are found to be in violation of any of these laws, we could be subject to civil and criminal penalties, which could hurt our business, financial condition and results of operations.
Privacy and Security of Health Information
Numerous federal, state and international laws and regulations govern the collection, use, and disclosure of patient-identifiable health information, including HIPAA. HIPAA applies to covered entities, which include most healthcare facilities that purchase and use our products. The HIPAA Privacy Rule restricts the use and disclosure of patient information, and requires covered entities to safeguard that information and to provide certain rights to individuals with respect to that information. The HIPAA Security Rule establishes elaborate requirements for safeguarding patient information transmitted or stored electronically. We are not a covered entity and our business is not directly subject to HIPAA. In certain circumstances the HIPAA rules require covered entities to contractually bind us, as a business associate, to protect the privacy and security of health information we may encounter during activities like training customers on the use of our products or investigating product performance. The HIPAA standards also apply to the use and disclosure of health information for research, and require the covered entity performing the research to obtain the written authorization of the research subject (or an appropriate waiver) before providing that subject’s health information to sponsors like us for purposes related to the research. These covered entities also typically impose contractual limitations on our use and disclosure of the health information they disclose to us. We may be required to make costly system modifications to comply with the privacy and security requirements that will be imposed on us contractually by covered entities, and our failure to comply may result in liability and adversely affect our business.
Numerous other federal and state laws protect the confidentiality of patient information, including state medical privacy laws and federal and state consumer protection laws. These various laws in many cases are not preempted by the HIPAA rules and may be subject to varying interpretations by the courts and government agencies, creating complex compliance issues for us and our customers and potentially exposing us to additional expense, adverse publicity and liability. Other countries also have, or are developing, laws governing the collection, use and transmission of personal or patient information and these laws could create liability for us or increase our cost of doing business.
New health information standards, whether implemented pursuant to HIPAA, congressional action or otherwise, could have a significant effect on the manner in which we must handle health care related data, and the cost of complying with these standards could be significant. If we do not properly comply with existing or new laws and regulations related to patient health information we could be subject to criminal or civil sanctions.
Foreign Regulation
Many foreign countries in which we market or may market our products have regulatory bodies and restrictions similar to those of the FDA. International sales are subject to foreign government regulation, the requirements of which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval and the requirements may differ. Companies are now required to obtain the CE Mark prior to sale of some medical devices within the
18
European Union. During this process, the sponsor must demonstrate compliance with the International Organization for Standardization’s manufacturing and quality requirements. We do have CE Marking on all our products that require such markings. We cannot assure you that we or our OEM partners will be able to obtain necessary foreign government approvals or successfully comply with foreign regulations. Our failure to do so could hurt our business, financial condition and results of operations.
Third-Party Reimbursement
Health care providers, including hospitals, that purchase our products generally rely on third-party payers, including the Medicare and Medicaid programs and private payers, such as indemnity insurers and managed care plans, to cover and reimburse all or part of the cost of the products and the procedures in which they are used. As a result, demand for our products is dependent in part on the coverage and reimbursement policies of these payers. No uniform coverage or reimbursement policy for medical technology exists among all third-party payers, as coverage and reimbursement can differ significantly from payer to payer.
Centers for Medicare and Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, along with its contractors, establish coverage and reimbursement policies for the Medicare program. Because a large percentage of the hospitals using our products treat elderly or disabled individuals who are Medicare beneficiaries, Medicare’s coverage and reimbursement policies are particularly significant to our business. In addition, private payers often follow the coverage and reimbursement policies of Medicare. We cannot assure you that government or private third-party payers will cover and reimburse the procedures using our products in whole or in part in the future or that payment rates will be adequate.
In general, Medicare will cover a medical product or procedure when the product or procedure is reasonable and necessary for the diagnosis or treatment of an illness or injury, or to improve the functioning of a malformed body part. Even if the medical product or procedure is considered medically necessary and coverage is available, Medicare may place restrictions on the circumstances where it provides coverage. For example, several Medicare local contractors have issued policies that restrict coverage for pulse oximetry in the hospital inpatient and outpatient settings to a limited number of conditions, including limiting coverage to patients who (i) exhibit signs of acute respiratory dysfunction, (ii) have chronic lung disease, severe cardiopulmonary disease or neuromuscular disease involving the muscles of respiration, (iii) are under treatment with a medication with known pulmonary toxicity, or (iv) have sustained multiple trauma or complaints of acute chest pain.
Reimbursement for our products may vary not only by the type of payer involved but also based upon the setting in which the product is furnished and utilized. For example, Medicare payment may be made, in appropriate cases, for patient stays in the hospital inpatient and outpatient settings involving the use of our products. Medicare generally reimburses hospitals based upon prospectively determined amounts. For hospital inpatient stays, the prospective payment generally is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay, using a classification system known as diagnosis-related groups, or DRGs. Prospective rates are adjusted for, among other things, regional differences, co-morbidity, and complications. Hospitals generally do not receive separate Medicare reimbursement for the specific costs of purchasing our products for use in the inpatient setting. Rather, Medicare reimbursement for these costs is deemed to be included within the prospective payments made to hospitals for the inpatient services in which the products are utilized.
In contrast, some differences may be seen in the reimbursement for use of our products in hospital outpatient departments. In this setting, Medicare payments also are generally made under a prospective payment system based on the ambulatory payment classifications, or APCs, under which individual items and procedures are categorized. Hospitals receive the applicable APC payment rate for the procedure regardless of the actual cost for such treatment. Some outpatient services such as oximetry services do not receive separate reimbursement. Rather, their reimbursement is deemed packaged into the APC for an associated procedure. Effective January 1, 2007, however, reimbursement for certain pulse oximetry monitoring services, including those using our products, will no longer be packaged, but rather may receive a separate payment under APC 0443 (“Overnight Pulse Oximetry”) when no other separately payable services are provided. This could result in an increase in Medicare payments to our customers for the use of our products in the hospital outpatient setting.
Because PPS payments in both the hospital inpatient and outpatient settings are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, hospitals have incentives to lower their operating costs by utilizing products that will reduce the length of inpatient stays, decrease labor or otherwise lower their costs. We cannot be certain that a hospital will purchase our products, despite the clinical benefits and opportunity for cost savings that we believe can be derived from their use. If hospitals cannot obtain adequate coverage and reimbursement for our products, or the procedures in which they are used, our business, financial condition and results of operations could suffer.
Our success with Rainbow SET technologies in U.S. care areas with reimbursable test procedures, such as hospital emergency department, hospital procedure labs, and the physician office will largely depend on the ability of providers to receive reimbursement for such testing procedures. As of January 2009, Masimo received Medicare coding and pricing for noninvasive carboxyhemoglobin and methemoglobin, with a reimbursable amount of $7.33 per day. While private insurance payers generally follow Medicare coding and pricing, we cannot be certain of this and in many cases, cannot control the coverage or payment rates that private insurance payers
19
put in place. We applied for a CPT code for total hemoglobin and believe we will receive coding and pricing by the end of 2009, but we cannot be certain that we will receive such coding or a price that will favorable to providers wishing to include noninvasive total hemoglobin in their practice.
Our success in non-U.S. markets depends largely upon the availability of coverage and reimbursement from the third-party payers through which health care providers are paid in those markets. Health care payment systems in non-U.S. markets vary significantly by country, and include single-payer, government managed systems as well as systems in which private payers and government-managed systems exist side-by-side. Our ability to achieve market acceptance or significant sales volume in international markets we enter will be dependent in large part on the availability of reimbursement for procedures performed using our products under health care payment systems in such markets. There can be no assurance that reimbursement for our products, or the procedures in which our products are used, will be obtained or that such reimbursement will be adequate.
Manufacturing
Our strategy is to manufacture products in-house when it is efficient and cost-effective for us to do so. We currently manufacture internally our bedside and handheld pulse oximeters, our full line of disposable and reusable sensors and most of our patient cables. As of January 3, 2009, we had 1,188 employees and contract employees in manufacturing and quality worldwide. We maintain a 25,000 square foot International Organization for Standardization 13485:2003 certified manufacturing area in our facility in Irvine, California, and a 95,600 square foot facility in Mexicali, Mexico. We will continue to utilize third-party contract manufacturers for products and subassemblies that can be more efficiently manufactured by these parties, such as our circuit boards. We monitor our third-party manufacturers and perform inspections and product tests at various steps in the manufacturing cycle to ensure compliance with our specifications. We also do full functional testing of our circuit boards.
For raw materials, we and our contract manufacturers rely on sole source suppliers for some components, including digital signal processor chips and analog to digital converter chips. We and our contract manufacturers have taken steps to minimize the impact of a shortage or stoppage of shipments of digital signal processor chips or analog to digital converter chips, including maintaining excess inventory and designing software that may be easily ported to another digital signal processor chip. We believe that our sources of supply for components and raw materials are adequate. In the event of a delay or disruption in the supply of sole source components, we believe that we and our contract manufacturers will be able to locate additional sources of these sole source components on commercially reasonable terms and without experiencing material disruption in our business or operations.
We have four major suppliers, and our agreements with each provide for varying terms with respect to term, termination and pricing. The initial terms of each of these agreements have expired, however, in each case the parties have either continued to perform under the agreement or the agreement provides for automatic renewal. While one such agreement does not provide for express termination rights, the remaining three agreements allow for termination upon specified notice, ranging from 120 days to six months, to the non-terminating party. Each of these agreements allow for pricing adjustments: all four involve annual pricing negotiation, and one also assures us of the most favorable pricing offered to any other customer.
Operating Segment and Geographic Information
We operate in one business segment, using one measurement of profitability to manage our business. Sales and other financial information by geographic area is provided in Note 14 to our consolidated financial statements that are included in this Form 10-K.
Employees
As of January 3, 2009, including Masimo Labs, we had approximately 1,739 full-time employees and contract employees worldwide, 136 of which were engaged in research and development, 1,188 of which were engaged in manufacturing and quality assurance, 324 of which were engaged in sales and marketing and 91 of which were engaged in general and administrative functions.
Address
Our principal executive offices are located at 40 Parker, Irvine, California 92618, and our telephone number at that address is (949) 297-7000. Our website address is http://www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this Form 10-K.
Executive Officers of the registrant
Our executive officers, as of January 3, 2009, are set forth below:
| | | | |
Name | | Age1 | | Position(s) |
Joe E. Kiani | | 44 | | Chief Executive Officer & Chairman of the Board of Directors |
Ammar Al-Ali | | 45 | | Chief Technical Officer |
Jon Coleman | | 45 | | President, International |
20
| | | | |
Name | �� | Age1 | | Position(s) |
Mark P. de Raad | | 49 | | Executive Vice President & Chief Financial Officer |
Rick Fishel | | 50 | | President of Masimo Americas |
David Goodman, M.D. | | 52 | | Executive Vice President, Business Development |
Paul Jansen | | 38 | | Executive Vice President, Marketing |
Yongsam Lee | | 44 | | Executive Vice President, Operations & Chief Information Officer |
Stephen M. Moran, Esq. | | 52 | | Executive Vice President, General Counsel & Secretary, and Executive Vice President of Human Capital |
Michael O’Reilly, M.D. | | 56 | | Executive Vice President, Medical Affairs |
Anand Sampath | | 42 | | Executive Vice President, Engineering |
Joe E. Kianiis the founder of Masimo and has served as Chief Executive Officer and Chairman of the Board of Directors since our inception in 1989. He is an inventor on more than 50 patents related to signal processing, sensors, and patient monitoring, including patents for the invention of measure-through motion and low-perfusion pulse oximetry. Prior to founding Masimo, Mr. Kiani served as Regional Technical Manager for Anthem Electronics, Inc., a distributor of semiconductor and subsystem products, and as Field Applications Engineer for Bell Industries, Inc., which distributes advanced semiconductor components. He also previously served as Product Engineer at Unisys Corporation, a computer manufacturer. Mr. Kiani is currently on the Board of Directors of Saba Software, Inc., a publicly-traded software company focused on human capital development and management solutions and chairman of the Medical Device Manufacturers Association (MDMA). Mr. Kiani holds a B.S.E.E. and an M.S.E.E. from San Diego State University.
Ammar Al-Ali has served as our Chief Technical Officer since December 1996. He is an inventor on more than 48 patents related to signal processing, sensors and patient monitoring. From April 1995 to December 1996, Mr. Al-Ali held various positions with us, including Director of Software Development. From January 1992 to November 1994, he served as the Director of Research and Development, Electronics for Ami-Med Corporation, a medical device company that provides instruments for continuous cardiac output. Mr. Al-Ali holds a B.S.E.E. degree from the University of Arizona.
Jon Colemanhas served as our President, International since August 2008. From October 2007 to August 2008, Mr. Coleman was President and Chief Executive Officer of You Take Control, Inc, a healthcare information technology company. He served as General Manager, Americas of Targus Group International, a supplier of mobile computing cases and accessories, from March 2006 to February 2007. From March 1994 to February 2006, he held progressive leadership positions with Pfizer, Inc, most recently Vice President and General Manager, Canada & Caribbean Region. Mr. Coleman holds a M.B.A. from Harvard Business School, and a B.A. in International Relations from Brigham Young University.
Mark P. de Raad has served as our Executive Vice President and Chief Financial Officer since June 2006. From November 2002 through May 2006, Mr. de Raad served as Vice President, Chief Financial Officer and Secretary for Avamar Technologies, Inc., a start-up enterprise software development company. He served as Vice President, Finance and Chief Financial Officer for ATL Products, Inc., a manufacturer of automated tape libraries, from September 1997 through June 2001. From June 2001 through November 2002, Mr. de Raad was Chief Financial Officer, Quantum Storage Solutions Group, a division of Quantum Corporation, which acquired ATL Products, Inc. in 1998. From May 1987 to May 1997, Mr. de Raad was employed by AST Research, Inc., a personal computer manufacturer, where he held various financial management positions the last of which was Vice President Finance and Treasurer and Chief Accounting Officer. Mr. de Raad is a Certified Public Accountant and holds a B.S. in Accounting from the University of Santa Clara.
Rick Fishel has served as President of Masimo Americas since June 2004. From January 2003 to June 2004, Mr. Fishel was Regional Vice President of Sales for the Information Solutions segment of the McKesson Corporation, a provider of supply, information and care management products and services. From January 2001 to January 2003, he served as National Vice President of Sales for the Consulting Services division of GE Medical Systems, Inc., a provider of medical technology and productivity solutions. Mr. Fishel holds a B.S. in Marketing from Arizona State University.
David Goodman, M.D.has served as Executive Vice President of Business Development since September 2008. From September 2006 to September 2008, Dr. Goodman was a practicing physician in Preventative and Occupational Medicine, as well as a Principal with 0 to 1 Solutions, a professional consultancy. From September 2005 to June 2006, Dr. Goodman served as President and Chief Executive Officer of BaroSense, Inc., a medical device company. From January 2004 to September 2005, Dr. Goodman served as President and Chief Executive Officer of Interventional Therapeutic Solutions, Inc., an implantable drug delivery systems company. From January 2002 to December 2003, Dr. Goodman served as Chairman, President and Chief Executive Officer of Pherin Pharmaceuticals, a pharmaceutical discovery and development company. He was the founding Chief Executive Officer, Chief Medical Officer and Director of LifeMaster Supported SelfCare, a disease management services company from June 1994 to December 2001. Also, since June 2004, Dr. Goodman has been on the Board of Directors, as well as the Audit and Compensation Committee of NEUROMetrix, Inc, a medical device company. Dr. Goodman holds 18 U.S. patents. He holds a B.A.S. in Applied Science and
21
Bioengineering and a M.S.E. in Bioengineering from the University of Pennsylvania. He also received an M.D. cum laude from Harvard Medical School and the Harvard-M.I.T. Division of Health Sciences and Technology.
Paul Jansen has served as our Executive Vice President of Marketing since April 2008, and was our Vice President of Marketing from January 2008 to April 2008. From August 1997 through December 2007, he served as Vice President, Marketing & Clinical Development for CardioDynamics, a cardiac monitoring and diagnostics company. Mr. Jansen holds a B.S. in Urban Planning and Development from Iowa State University and an M.B.A. from Arizona State University.
Yongsam Leehas served as our Executive Vice President, Operations and Chief Information Officer since January 2003. From March 1996 to October 2001 and from April 2002 to December 2002, Mr. Lee held various positions with us, including Vice President, IT and Vice President, Operations. From October 2001 to April 2002, he served as Director of IT at SMC Networks, Inc., a provider of networking solutions. Mr. Lee holds a B.S. in Applied Physics from the University of California, Irvine.
Stephen M. Moran, Esq.has served in the dual roles as our Executive Vice President, General Counsel & Secretary, and Executive Vice President of Human Capital, since July 2008. From February 2003 until July 2008, Mr. Moran served as both Vice President, General Counsel & Secretary, and Vice President of Human Resources & Administration, for Toshiba America Business Solutions, Inc., a global copier, fax and printer company. From January 2000 through January 2003, he served as both Vice President, General Counsel, and Secretary and Vice President of Human Resources, of Intersil Corporation, a global semiconductor company. From February 1998 until January 2000, Mr. Moran served as Vice President and General Counsel for Toshiba America Electronic Components, Inc., a semiconductor company. Mr. Moran holds a B.A. in Political Science from Claremont McKenna College and a J.D. from Loyola Law School in Los Angeles. He is a member of the bars of California, Colorado, the District of Columbia and the Supreme Court of the United States.
Michael O’Reilly, M.D. has served as our Executive Vice President, Medical Affairs since February 2008. Dr. O’Reilly was an Associate Professor from September 2002 to February 2008, and an Assistant Professor from September 1996 to August 2002, in Anesthesiology at the University of Michigan. He was also the Director of the Liver Transplant Anesthesiology at the University of Michigan and a member of various advisory boards. He has numerous publications in scientific journals, national and international invited presentations and earned various awards and grants. Dr. O’Reilly holds an M.D. and M.S. in Cell Biology from the University of Vermont.
Anand Sampath has served as our Executive Vice President, Engineering since March 2007. He is an inventor on more than four patents relating to patient monitoring, wireless networks and communications. From April 2006 to March 2007, Mr. Sampath was our Director of Systems Engineering. From October 1995 to March 2006, he held various positions, including Program Manager, Engineering Manager and Distinguished Member of Technical Staff, at Motorola, Inc. Mr. Sampath holds a B.S. in Engineering from Bangalore University.
Available Information
We are subject to the reporting requirements under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Consequently, we are required to file reports and information with the Securities and Exchange Commission, or SEC, including reports on the following forms: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. These reports and other information concerning us may be accessed through the SEC’s website athttp://www.sec.gov and on our website atwww.masimo.com. Such filings are placed on our website as soon as reasonably practical after they are filed with the SEC. Information contained in, or that can be accessed through, our website is not part of this Form 10-K.
The following risk factors and other information included in this Form 10-K should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations. If any of the following risks come to fruition, our business, financial condition, results of operations and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business
We currently derive substantially all of our revenue from our Masimo SET platform and related products. If this technology and the related products do not continue to achieve market acceptance, our business, financial condition and results of operations would be adversely affected.
22
We are dependent upon the success and market acceptance of our proprietary Masimo SET. Currently, our primary product offerings are based on the Masimo SET platform. Continued market acceptance of products incorporating Masimo SET will depend upon our ability to continue to provide evidence to the medical community that our products are cost-effective and provide significantly improved performance compared to conventional pulse oximeters. Health care providers that currently have significant investments in competitive pulse oximetry products may be reluctant to purchase our products. If hospitals and other health care providers do not believe our Masimo SET platform is cost-effective, more accurate or reliable, they may not buy our products in sufficient quantities to enable us to be profitable. If we are unable to achieve additional market acceptance of our core technology or products incorporating Masimo SET, we will not generate significant revenue growth from the sale of our products.
If the patents we own or license, or our other intellectual property rights, do not adequately protect our products, we may lose market share to our competitors and be unable to operate our business profitably.
Our success depends significantly on our ability to protect our rights to the technologies used in our products, including Masimo SET and licensed Rainbow technology. We rely on patent protection, trade secrets, as well as a combination of copyright and trademark laws and nondisclosure, confidentiality and other contractual arrangements to protect our technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. In addition, we cannot be assured that any of our pending patent applications will result in the issuance of a patent to us. The United States Patent and Trademark Office, or PTO, may deny or require significant narrowing of claims in our pending patent applications, and patents issued as a result of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is advantageous to us. We could also incur substantial costs in proceedings before the PTO. These proceedings could result in adverse decisions as to the claims included in our patents.
Our issued and licensed patents and those that may be issued or licensed in the future may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products. Additionally, upon expiration of our issued or licensed patents, we may lose some of our rights to exclude others from making, using, selling or importing products using the technology based on the expired patents. We also must rely on contractual rights with the third parties that license technology to us to protect our rights in the technology licensed to us. There is no assurance that competitors will not be able to design around our patents. We also rely on unpatented proprietary technology. We cannot assure you that we can meaningfully protect all our rights in our unpatented proprietary technology or that others will not independently develop substantially equivalent proprietary products or processes or otherwise gain access to our unpatented proprietary technology. We seek to protect our know-how and other unpatented proprietary technology with confidentiality agreements and intellectual property assignment agreements with our employees, our OEM partners, independent distributors and consultants. However, such agreements may not be enforceable or may not provide meaningful protection for our proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements or in the event that our competitors discover or independently develop similar or identical designs or other proprietary information. In addition, we rely on the use of registered and common law trademarks with respect to the brand names of some of our products. Our common law trademarks provide less protection than our registered trademarks. Loss of rights in our trademarks could adversely affect our business, financial condition and results of operations.
Furthermore, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. If we fail to apply for intellectual property protection or if we cannot adequately protect our intellectual property rights in these foreign countries, our competitors may be able to compete more effectively against us, which could adversely affect our competitive position, as well as our business, financial condition and results of operations.
If third parties claim that we infringe their intellectual property rights, we may incur liabilities and costs and may have to redesign or discontinue selling certain products.
Companies in the medical device industry have used intellectual property litigation to gain a competitive advantage in the marketplace. Whether a technology or product infringes a patent involves complex legal and factual issues and is often difficult to determine. We face the risk of claims that we have infringed on third parties’ intellectual property rights. Searching for existing intellectual property rights may not reveal important intellectual property and our competitors may also have filed for patent protection, which is not publicly-available information, or claimed trademark rights that have not been revealed through our availability searches. Our efforts to identify and avoid infringing on third parties’ intellectual property rights may not always be successful. Any claims of patent or other intellectual property infringement against us, even those without merit, could:
| | • | | increase the cost of our products; |
| | • | | be expensive and time consuming to defend; |
| | • | | result in us being required to pay significant damages to third parties; |
| | • | | force us to cease making or selling products that incorporate the challenged intellectual property; |
| | • | | require us to redesign, reengineer or rebrand our products, product candidates and technologies; |
23
| | • | | require us to enter into royalty or licensing agreements in order to obtain the right to use a third party’s intellectual property on terms that may not be favorable or acceptable to us; |
| | • | | require us to indemnify third parties pursuant to contracts in which we have agreed to provide indemnification for intellectual property infringement claims; |
| | • | | divert the attention of our management and other key employees; |
| | • | | result in our customers or potential customers deferring or limiting their purchase or use of the affected products impacted by the claims until the claims are resolved; and |
| | • | | otherwise have a material adverse effect on our business, financial condition and results of operations. |
In addition, new patents obtained by our competitors could threaten the continued commercialization of our products in the market even after they have already been introduced.
We believe competitors may currently be violating and may in the future violate our intellectual property rights, and we may bring additional litigation to protect and enforce our intellectual property rights, which may result in substantial expense and may divert our attention from implementing our business strategy.
We believe that the success of our business depends, in significant part, on obtaining patent protection for our products and technologies, defending our patents and preserving our trade secrets. We were previously involved in significant litigation to protect our patent position and may be required to engage in further litigation. In 2006, we settled a costly, six-year lawsuit against Mallinckrodt, Inc., part of Tyco Healthcare (currently Covidien Ltd.), and one of its subsidiaries, Nellcor Puritan Bennett, Inc., in which we claimed that Nellcor was infringing some of our pulse oximetry signal processing patents. We believe that other competitors of ours, including some of our OEM partners, may be infringing at least one of our patents. Our failure to pursue any potential claim could result in the loss of our proprietary rights and harm our position in the marketplace. Therefore, we may be forced to pursue litigation to enforce our rights. On February 3, 2009, we filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh related to Philips FAST pulse oximetry technology and certain Philips patient monitors. Both Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh are associated with Philips Medical Systems, an OEM partner of ours. We cannot be certain that we will have the required financial resources to pursue this and other litigation or otherwise to protect these rights in the future. In addition, any future litigation could result in significant additional costs and further divert the attention of our management and key personnel from our business operations and the implementation of our business strategy and may not be adequate to protect our intellectual property rights.
Some of our products, including those based on licensed Rainbow technology, are in development or have been recently introduced into the market and may not achieve market acceptance, which could limit our growth and adversely affect our business, financial condition and results of operations.
Our products that have been recently introduced into the market, including, but not limited to those based on Rainbow technology, a technology that we license, may not be accepted in the market. Our first product incorporating licensed Rainbow technology was made commercially available in September 2005. Given that all Rainbow technologies are new to the marketplace, we do not know to what degree the market will accept these products, if at all. Even if our customers recognize the benefits of our products, we cannot assure you that our customers will purchase them in quantities sufficient for us to be profitable or successful. We will need to invest in significant sales and marketing resources to achieve market acceptance of these products with no assurance of success. The degree of market acceptance of these products will depend on a number of factors, including:
| | • | | perceived effectiveness of our products; |
| | • | | perceived advantages of our products over competing products; |
| | • | | introduction and acceptance of competing products or technologies; and |
| | • | | obtaining the required domestic and international regulatory approvals for our product candidates under development. |
In March 2008, we introduced our most recent Rainbow parameter, total hemoglobin. In May 2008, we received FDA clearance to begin to market this new parameter. In September 2008, we began our limited market release of the product and focused on obtaining data and clinical feedback on the performance of the product in the hospital. Since September 2008, we have continued to gather data and input on the device, and expect to continue this activity through the first quarter of 2009. In the first or second quarter of 2009, we intend to commercially launch our total hemoglobin product for continuous monitoring in the hospital. While we are enthusiastic about the products’ long-term market potential, we cannot determine how quickly or successfully the product will be received within its initial targeted market. As with any new technology, there are significant barriers to market adoption and we cannot be assured that the marketplace will respond favorably to this new technology and product.
24
In the second half of 2009, we intend to release our latest parameter, acoustic respiration monitoring, or ARM. As with our total hemoglobin release, we will initially provide this new measurement to the market in a limited market release which will allow us to evaluate the product’s performance in the field. While we believe that we should be able to achieve this market timing, there can be no assurances of this timing and or the regulatory approvals still required. Also, while we expect this product will have an important role within the hospital general floor environment, there can be no assurance that this product will receive be successful within this initial target market. As with any new technology, there are significant barriers to market adoption and we cannot be assured that the marketplace will respond favorably to this new technology and product.
In order for any of our products to be accepted in the marketplace, we must demonstrate that they are effective and commercially beneficial. Even if customers accept these products, this acceptance may not result in sales if our competitors develop similar products that our customers prefer. If our products do not gain market acceptance or if our customers prefer our competitors’ products, our potential growth would be limited, which would adversely affect our business, financial condition and results of operations.
If our products cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting, or MDR, regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. In addition, all manufacturers placing medical devices in European Union markets are legally bound to report to the relevant authority in whose jurisdiction any serious or potentially serious incidents occurred involving devices produced or sold by the manufacturer. We may experience events that may require reporting to the FDA pursuant to the MDR regulations. Any adverse event involving our products could result in future voluntary corrective actions, including recalls or customer notifications, or agency action, including inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as any litigation resulting from any adverse event, would require the dedication of our time and capital, distract management from operating our business and could harm our reputation, business operations and financial results.
Our products have been, and may in the future be, subject to product recalls that could harm our reputation, business operations and financial results.
After a device is placed on the market, numerous regulatory requirements apply, including MDR regulations, that require us to report to the FDA or similar governmental bodies in other countries if our products cause or contribute to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects, in for example, design, labeling or manufacture. In the case of the FDA, the authority to require a recall generally must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or by one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. We may initiate certain voluntary recalls involving our products in the future. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. If we determine that a recall does not require notification of the FDA, the FDA may disagree with our determination and require us to report the recall to the FDA.
From our inception through January 3, 2009, we initiated four voluntary recalls of our products, none of which was material. Each of these recalls was reported to the FDA within the appropriate regulatory timeframes. Because of our dependence upon patient and physician perceptions, any negative publicity associated with these voluntary recalls could materially and adversely affect our business, financial condition, results of operations and growth prospects.
Recent deterioration in the credit markets and the financial services industry may negatively impact our business, results of operations, financial condition or liquidity.
Recently, the credit markets and the financial services industry have been experiencing a period of unprecedented turmoil and upheaval characterized by the bankruptcy, failure, collapse or sale of various financial institutions and an unprecedented level of intervention from the United States federal government. While the ultimate outcome of these events cannot be predicted, they may have a material adverse effect on our liquidity, financial condition and results of operations if our customers’ ability to borrow money from their existing lenders, or to obtain credit from other sources to purchase our products under long term sensor agreements, were to be impaired. This credit market deterioration could affect our ability to acquire new customers for our products. In addition, the recent economic crisis could also adversely impact our suppliers’ ability to provide us with materials and components, either of which may negatively impact our business, financial condition and results of operations.
25
Although we maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments and such losses have historically been within our expectations and the allowances we have established, we cannot guarantee that we will continue to experience the same loss rates that we have in the past, especially given the recent deterioration of the credit markets of the worldwide economy. A significant change in the liquidity or financial condition of our customers could cause unfavorable trends in our receivable collections and additional allowances may be required. These additional allowances could materially affect our financial results.
Our ability to commercialize new products, new or improved technologies and additional applications for Masimo SET and our right to use Rainbow technology are each limited to certain markets by our Cross-Licensing Agreement with Masimo Labs, which may impair our growth and adversely affect our financial condition and results of operations.
In May 1998, we created a newly-formed entity, Masimo Laboratories, Inc., or Masimo Labs, and provided it rights to use Masimo SET to commercialize non-vital signs monitoring applications while we retained the rights to Masimo SET to commercialize vital signs monitoring applications. On May 2, 1998, we entered into a cross-licensing agreement with Masimo Labs, which has been amended several times, most recently in an Amended and Restated Cross-Licensing Agreement, effective January 1, 2007, or the Cross-Licensing Agreement. Under the Cross-Licensing Agreement, we granted Masimo Labs:
| | • | | an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET owned by us, including all improvements on this technology, for the measurement of non-vital signs measurements and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs measurements in any product market in which a product is intended to be used by a patient or pharmacist rather than by a professional medical caregiver, which we refer to as the Labs Market, and |
| | • | | a non-exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET for measurement of vital signs in the Labs Market. |
Non-vital signs measurements consist of body fluid constituents other than vital signs measurements, including but not limited to carbon monoxide, methemoglobin, blood glucose, total hemoglobin and bilirubin.
Under the Cross-Licensing Agreement, we are only permitted to sell devices utilizing Masimo SET for the measurement of non-vital signs measurements in markets where the product is intended to be used by a professional medical caregiver, including but not limited to hospital caregivers and emergency medical services, or EMS, facility caregivers, rather than by a patient or pharmacist, which we refer to as the Masimo Market. Accordingly, our ability to commercialize new products, new or improved technologies and additional applications for Masimo SET is limited. In particular, our inability to expand beyond the Masimo Market may impair our growth and adversely affect our financial condition and results of operations.
Pursuant to the Cross-Licensing Agreement, we have licensed from Masimo Labs the right to make and distribute products in the Masimo Market that utilize Rainbow technology for the measurement of only carbon monoxide, methemoglobin, fractional arterial oxygen saturation and total hemoglobin, which includes hematocrit. As a result, the opportunity to expand the market for our products incorporating Rainbow technology is limited, which could limit our ability to maintain or increase our revenue and impair our growth.
We will be required to pay Masimo Labs for the right to use certain improvements to Masimo SET that we develop.
Under the Cross-Licensing Agreement, if we develop improvements to Masimo SET for the noninvasive measurement of non-vital signs measurements, we would be required to assign these developments to Masimo Labs and then license the technology back from Masimo Labs in consideration for a license fee and royalty obligations to Masimo Labs. Therefore, any improvement to this technology would be treated as if it had been developed exclusively by Masimo Labs. In addition, we will not be reimbursed by Masimo Labs for our expenses relating to the development of any such technology. As a result of these terms, we may not generate any revenue from the further development of Masimo SET for the measurement of non-vital signs measurements, which could adversely affect our business, financial condition and results of operations.
In the event that the Cross-Licensing Agreement is terminated for any reason, or Masimo Labs grants a license to Rainbow technology to a third party, our business would be materially and adversely affected.
Masimo Labs owns all of the proprietary rights to Rainbow technology developed with our proprietary Masimo SET for products intended to be used in the Labs Market, and all rights for any non-vital signs measurement for which we do not exercise an option pursuant to the Cross-Licensing Agreement. In addition, Masimo Labs has the right to terminate the Cross-Licensing Agreement or grant licenses covering Rainbow technology to third parties if we breach certain terms of the agreement, including any failure to meet our minimum royalty payment obligations or failure to use commercially reasonable efforts to develop or market products incorporating licensed Rainbow technology. If we lose our exclusive license to Rainbow technology, we may not be able to develop comparable technology or license similar technology on commercially favorable terms or at all, and we would lose the ability to prevent others from making, using, selling or importing products using Rainbow technology in our market. As a result, we would likely be subject to increased competition within our market, and Masimo Labs or competitors who obtain a license to Rainbow technology from Masimo Labs would be able to offer related products.
26
We are required to pay royalties to Masimo Labs for all products sold that contain Rainbow technology, including certain annual minimum royalty payments, and this may impact our gross margins, if we discontinue consolidating Masimo Labs within our financial statements.
The Cross-Licensing Agreement requires us to pay Masimo Labs a royalty for all products that we sell which include their proprietary Rainbow technology. This includes handheld, table-top and multi-measurement products that incorporate licensed Rainbow technology. Beginning in 2009, for hospital contracts where we place equipment and enter into a sensor contract, we will pay a royalty to Masimo Labs on the total sensor contract revenue based on the ratio of Rainbow enabled devices to total devices. The agreement also requires that we provide to Masimo Labs, at its request, up to 10% of our annual board and sensor production volume at our total manufactured cost. In addition to these specific royalty and product obligations, our Cross-Licensing Agreement requires that we pay Masimo Labs specific annual minimum royalty payments.
Currrently, in accordance with Financial Accounting Standards Board Interpretation No. 46(R),“Consolidation of Variable Interest Entities—an Interpretation of ARB No. 51,” or FIN 46(R), we are currently required to consolidate Masimo Labs within our financial statements. Accordingly, the royalties that we owe to Masimo Labs are eliminated in our consolidated financial statements presented within this Form 10-K. As a result, the gross profit margins reported in our consolidated financial results do not include the royalty expense that we pay to Masimo Labs. Also in accordance with FIN 46(R), we are obligated to include Masimo Labs’ engineering and administrative expenses in our reported engineering and administrative expenses. As a result, our reported engineering and administrative expenses include Masimo Labs’s engineering and administrative expenses. If our financial statements were not consolidated with Masimo Labs, our reported cost of goods sold would increase and our reported engineering and administrative expenses would decrease. As a result of this requirement to consolidate, we report within our public filings, both our reported gross profit, engineering expense and administrative expenses as if we were not required to consolidate. To date, the amount of royalty expense has approximated the amount of engineering and administrative expense and therefore, the net impact to our consolidated financial statements has not been significant. However, in the future, depending upon the success of Rainbow products and the royalties earned by Labs on those revenues, it is possible that the royalty expense will grow at a rate higher than the growth of engineering and administrative expenses. In the event the net impact on our consolidated results is material, we will reflect the amount of Labs income through our profit and loss statement as an element of minority interest income. As a result, despite the current requirement to consolidate, any profit in our consolidated financial results that is attributable to Masimo Labs will be separately identified.
Despite describing and reflecting this Masimo Labs consolidation requirement within our financial statements, it is possible that, if the reader of our financial statements do not understand or appreciate the significant of our consolidation of Masimo Labs financial statements in our FIN 46(R), they may draw inaccurate perspectives and conclusions regarding our financial condition and results of operations.
We may not be able to commercialize our products incorporating licensed Rainbow technology cost-effectively or successfully.
It is more expensive for us to make products that incorporate Rainbow technology than products that do not due to increased production costs and the royalties that we must pay to Masimo Labs. In order to successfully commercialize products incorporating Rainbow technology, we must be able to pass these higher costs on to the market. We cannot assure you that we will be able to sell products incorporating Rainbow technology at a price the market is willing to accept. If we cannot commercialize our products incorporating licensed Rainbow technology successfully, we may not be able to generate sufficient product revenue from these products to be profitable, which could adversely affect our business, financial condition and results of operations.
Rights provided to Masimo Labs in the Cross-Licensing Agreement may impede a change in control of our company.
In the event we undergo a change in control, we are required to immediately pay a $2.5 million fee to exercise an option to license technology developed by Masimo Labs for use in blood glucose monitoring. Under the Cross-Licensing Agreement, a change in control includes, but is not limited to, the resignation or termination of Joe E. Kiani from his position of Chief Executive Officer of either Masimo or Masimo Labs. Additionally, our per product royalties payable to Masimo Labs will become subject to specified minimums, and the minimum aggregate annual royalties for all licensed Rainbow measurements payable to Masimo Labs will increase to up to $15.0 million for carbon monoxide, methemoglobin, fractional arterial oxygen saturation, total hemoglobin and blood glucose, plus up to $2.0 million per other Rainbow measurements. Also, if the surviving or acquiring entity ceases to use “Masimo” as a company name and trademark following a change in control, all rights to the “Masimo” trademark will automatically be assigned to Masimo Labs. This could delay or discourage transactions involving an actual or potential change in control of us, including transactions in which our stockholders might otherwise receive a premium for their shares over our then-current trading price. In addition, our requirement to assign all future improvements for non-vital signs to Masimo Labs could impede a change in control.
Masimo Labs has conducted most of the research and development of Rainbow technology and we are dependent upon Masimo Labs to develop improvements to Rainbow technology.
Masimo Labs has conducted the research and development of Rainbow technology. Although we expect Masimo Labs to continue its research and development activities related to Rainbow technology and specific noninvasive monitoring measurements, including
27
blood glucose and total hemoglobin, no assurance can be given that it will do so. In the event Masimo Labs does not continue to develop and improve Rainbow technology, our business, financial condition and results of operations could be adversely affected.
We will experience conflicts of interest with Masimo Labs with respect to business opportunities and other matters.
Prior to our initial public offering in August 2007, our stockholders owned approximately 99% of the outstanding shares of capital stock of Masimo Labs and we believe that as of January 3, 2009, a number of stockholders of Masimo Labs, including some of our executive officers and directors, continued to own shares of our common stock. In addition, Joe E. Kiani and Jack Lasersohn, members of our board of directors, are also members of the board of directors of Masimo Labs. Joe E. Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Masimo Labs. Due to the interrelated nature of Masimo Labs with us, conflicts of interest will arise with respect to transactions involving business dealings between us and Masimo Labs, potential acquisitions of businesses or products, development of products and technology, the sale of products, markets and other matters in which our best interests and the best interests of our stockholders may conflict with the best interests of the stockholders of Masimo Labs. We cannot assure you that any conflict of interest will be resolved in our favor, or that with respect to our transactions with Masimo Labs we will negotiate terms that are as favorable to us as if such transactions were with an unaffiliated third party.
We may experience significant fluctuations in our quarterly results and we may not maintain our recent profitability in the future.
We incurred net losses attributable to common stockholders in each year from our inception through 2004. Our net losses attributable to common stockholders were approximately $8.6 million, $15.4 million and $12.3 million in 2002, 2003 and 2004, respectively. We expect our expenses to increase as we continue to expand our research and development and sales and marketing activities. As a result, if we are unable to maintain or increase our revenue, we may incur net losses in the future.
Our operating results have fluctuated in the past and are likely to fluctuate in the future. We may experience fluctuations in our quarterly results of operations as a result of:
| | • | | delays or interruptions in manufacturing and shipping of our products; |
| | • | | varying demand for and market acceptance of our technologies and products; |
| | • | | the effect of competing technological and market developments resulting in lower selling prices or significant promotional costs; |
| | • | | changes in the timing of product orders and the volume of sales to our OEM partners; |
| | • | | actions taken by group purchasing organizations, or GPOs; |
| | • | | delays in hospital conversions to our products; |
| | • | | declines in hospital patient census; |
| | • | | our legal expenses, particularly those related to litigation matters; |
| | • | | changes in our product or customer mix; |
| | • | | unanticipated delays or problems in the introduction of new products, including delays in obtaining clearance or approval from the FDA; |
| | • | | high levels of returns and repairs. |
These factors, some of which are not within our control, may cause the price of our stock to fluctuate substantially. To respond to these and other factors, we may need to make business decisions that could result in our failure to meet financial expectations. If our quarterly operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly. Most of our expenses, including our employee compensation, inventory and debt repayment obligations, are relatively fixed in the short term. Moreover, our expense levels are based, in part, on our expectations regarding future revenue levels. As a result, if our revenue for a particular period were below our expectations, we would not be able to proportionately reduce our operating expenses for that period. Any revenue shortfall would have a disproportionately negative effect on our operating results for the period.
Due to these and other factors, we believe that quarter-to-quarter comparisons of our operating results may not be meaningful. You should not rely on our results for any one quarter as an indication of our future performance. In future quarters, our operating results may be below the expectations of securities analysts or investors.
We depend on our OEM partners for a portion of our revenue. If they do not devote sufficient resources to the promotion of products that use Masimo SET and licensed Rainbow technology, our business would be harmed.
We are, and will continue to be, dependent upon our OEM partners for a portion of our revenue through their marketing, selling and distribution of certain of their products that incorporate Masimo SET and licensed Rainbow technology. Although we expect that our OEM partners will accept and actively market, sell and distribute products that incorporate licensed Rainbow technology, they may
28
elect not, and they have no contractual obligation, to do so in the near future or at all. The failure of our OEM partners to successfully market, sell or distribute products incorporating these technologies, the termination of OEM agreements, the loss of OEM partners or the inability to enter into future OEM partnership agreements would have a material adverse effect on our business, financial condition and results of operations. Our success will depend in part upon whether our OEM partners devote sufficient resources to the promotion of products that incorporate these technologies. These products may represent a relatively small percentage of business for some of our OEM partners and therefore they may have less incentive to promote these products rather than other products that do not incorporate these technologies; the desire and ability of our OEMs to commit these resources could be negatively impacted by the current turbulent and volatile economic turmoil. In addition, some of our OEM partners offer products that compete with ours. Therefore, we cannot guarantee that our OEM partners will vigorously promote products incorporating Masimo SET and licensed Rainbow technology, or at all. If any of our OEM partners were to be acquired, we cannot assure you that an acquiring company would devote sufficient resources to promote products that incorporate technology we own or license.
The loss of any large customer or any cancellation or delay of a significant purchase by a large customer could reduce our net sales and harm our operating results.
We also have a concentration of OEM, distribution and direct customers. If for any reason we were to lose our ability to sell to a specific group or class of customers, we would experience a significant reduction in revenue, which would adversely impact our operating results. Also, we cannot assure you that we will retain our current customers or groups of customers or that we will be able to attract and retain additional customers in the future. For the years ended January 3, 2009 and December 29, 2007, one of our distributors represented 12% and 11%, respectively, of our total revenue. No distributors represented over 10% of our total revenue for the year ended December 31, 2006.
Our royalty agreement with Covidien provides for a royalty rate schedule that could decline over the term of the settlement agreement which could significantly harm our total sales and operating results.
In fiscal 2008, our royalties from the Covidien settlement totaled $47.5 million. Because these royalty payments do not carry any significant cost, they result in significant improvements to our reported gross profit and operating income levels. As a result, any decline in royalties that we earn under the settlement agreement in the future will have a significant impact on our revenue, gross margins and operating income. Under the agreement, we earn royalties on Covidien’s total U.S.-based pulse oximetry sales. The royalty rate in 2006 was nearly 20% if averaged over the entire year. The royalty rate for fiscal 2007 declined to 15%. In 2008 and through the term of the royalty agreement, at least through March 14, 2011, the royalty rate is 13%, but may decline to 10%, subject to Covidien’s ability to develop new products that avoid some of our current patent coverage as negotiated in the settlement agreement. As a result, there is a significant financial risk to our operating income if we are unable to generate sufficient revenue and gross margins to offset the impact of declining royalty rates on sales of Covidien’s pulse oximetry products in the United States.
If we fail to maintain relationships with GPOs, sales of our products would decline.
Our ability to sell our products to U.S. hospitals depends in part on our relationships with GPOs. Many existing and potential customers for our products become members of GPOs. GPOs negotiate beneficial pricing arrangements and contracts, which are sometimes exclusive, with medical supply manufacturers and distributors. These negotiated prices are made available to a GPO’s affiliated hospitals and other members. If we are not one of the providers selected by a GPO, the GPO’s affiliated hospitals and other members may be less likely or unlikely to purchase our products. If a GPO has negotiated a strict sole source, market share compliance or bundling contract for another manufacturer’s products, we may be prohibited from making sales to members of the GPO for the duration of the contractual arrangement. In the fiscal years ended January 3, 2009, December 29, 2007 and December 31, 2006, shipments of our pulse oximetry products related to GPOs were $132.1 million, $101.0 million and $66.6 million, respectively. Our failure to renew our contracts with GPOs may cause us to lose market share and could have a material adverse effect on our sales, financial condition and results of operations. In addition, if we are unable to develop new relationships with GPOs, our competitive position would likely suffer and our business would be harmed.
If we do not successfully develop and commercialize enhanced or new products that remain competitive with new products or alternative techniques developed by others, we could lose revenue opportunities and customers, and our ability to grow our business would be impaired.
The medical device industry is characterized by rapid product development and technological advances, which places our products at risk of obsolescence. Our long-term success depends upon the development and successful commercialization of new products, new or improved technologies and additional applications for Masimo SET and licensed Rainbow technology. The research and development process is time-consuming and costly and may not result in products or applications that we can successfully commercialize. In particular, we may not be able to successfully commercialize our products for applications other than arterial blood oxygen saturation and pulse rate monitoring, including carboxyhemoglobin and methemoglobin monitoring. If we do not successfully adapt our products and applications both within and outside these measurements, we could lose revenue opportunities and customers. In addition, we may not be able to improve our products or develop new products or technologies quickly enough to maintain a competitive position in our
29
markets and continue to grow our business. Furthermore, one or more of our competitors may develop products that are substantially equivalent to our FDA-cleared products, or those of our OEM partners, whereby they may be able to use our products or those of our OEM partners, as predicate devices to more quickly obtain FDA clearance of their competing products.
We face competition from other companies, many of which have substantially greater resources than we do and may be able to develop products perceived as more effective or easier to use than ours or are more readily accepted, or offer their products at lower prices than we can, which could adversely affect our business, financial condition and results of operations.
We face substantial competition from companies developing products that compete with our Masimo SET platform for use with third-party monitoring systems. We also face competition from companies currently marketing pulse oximetry monitors. One company, Covidien, currently holds a substantial share of the pulse oximetry market. Our revenue and profit are significantly smaller than our primary competitors. A number of the companies in the pulse oximetry market have substantially greater capital resources, larger customer bases, larger sales forces, greater marketing and management resources, larger research and development staffs and larger facilities than ours, and have established stronger reputations with our target customers and built relationships with GPOs and worldwide distribution channels that are more effective than ours. Competition could result in reductions in the price of our products, fewer orders for our products, a reduction of our gross margins and a loss of our market share. Reliance on clinical studies is an important means of demonstrating the effectiveness of products in our industry. If clinical studies supporting our competitors’ products are perceived to be accurate and reliable, market acceptance and sales of our products could be adversely impacted and we could lose market share to our competitors.
Our suppliers may not supply us with a sufficient amount of materials and components or materials and components of adequate quality.
We depend on sole or limited source suppliers for key materials and components of our noninvasive blood constituent patient monitoring solutions, and if we are unable to obtain these components on a timely basis, we will not be able to deliver our noninvasive blood constituent patient monitoring solutions to customers. Also, we cannot guarantee that any of the materials or components that we purchase, if available at all, will be of adequate quality or that the prices we pay for these materials or components will not increase. From time to time, there are industry-wide shortages of several electronic components that we use in our noninvasive blood constituent patient monitoring solutions. We may experience delays in production of our products if we fail to identify alternate vendors for materials and components, or any parts supply is interrupted or reduced or there is a significant increase in production costs, each of which could adversely affect our business, financial condition and results of operations.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at other medical device companies. We may be subject to claims that our employees have disclosed, or that we have used, trade secrets or other proprietary information of our employees’ former employers. Defending against these claims could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research and development or sales personnel could limit our ability to sell our existing products and develop new products and technologies, which could adversely affect our business, financial condition and results of operations.
If product liability claims are brought against us, we could face substantial liability and costs.
The manufacture and sale of products using Masimo SET and licensed Rainbow technology expose us to product liability claims and product recalls, including those that may arise from misuse, including but not limited to unauthorized off-label use, which is use of a device in a manner outside the measurement or measurements cleared by the FDA, or malfunction of, or design flaws or manufacturing defects in, our products or the use of our products with incompatible components or systems. Any losses that we may suffer from future liability claims, and the effect that any product liability litigation may have upon the reputation and marketability of our technology and products, together with the corresponding diversion of the attention of our key employees, could adversely affect our business, financial condition and results of operations. Any product liability claims could require significant cost and management resources and may subject us to significant damages. We currently have product liability insurance that we believe to be adequate, but we cannot be certain that it will be sufficient to cover any or all damages or claims. Furthermore, we may not be able to obtain or maintain insurance in the future at satisfactory rates or in adequate amounts to protect us against any product liability claims.
Our failure to obtain and maintain FDA clearances or approvals on a timely basis, or at all, would prevent us from commercializing our current or upgraded products in the United States, which could severely harm our business.
Each medical device that we wish to market in the United States generally must first receive either 510(k) clearance from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act by filing a 510(k) pre-market notification, or pre-market approval, or PMA, through submitting a PMA application. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed. We cannot assure you that the FDA will grant 510(k) clearance on a
30
timely basis, if at all, for new products or uses that we propose for Masimo SET or licensed Rainbow technology. The FDA’s 510(k) clearance process usually takes from four to six months, although it can take longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain and generally takes from one to three years or even longer.
To date, the FDA has regulated pulse oximeters incorporating Masimo SET and licensed Rainbow technology, and our sensors, cables and other products incorporating Masimo SET and licensed Rainbow technology for pulse oximetry under the 510(k) process. Although 510(k) clearances have been obtained for all of our current products, these clearances may be revoked by the FDA at any time if safety or effectiveness problems develop with our devices. Furthermore, our new products or significantly modified marketed products could be denied 510(k) clearance and be required to undergo the more burdensome PMA process. In that case, our ability to upgrade our products in a timely fashion could be limited. The withdrawal of existing 510(k) clearances or the inability to obtain new ones on a timely basis, or at all, could severely harm our business, financial condition and results of operations.
The failure of our OEM partners to obtain required FDA clearances or approvals for products that incorporate our products or technologies could have a negative impact on our revenue.
Our OEM partners will be required to obtain their own FDA clearances for products incorporating Masimo SET and licensed Rainbow technology to market these products in the United States. We cannot assure you that the FDA clearances we have obtained will make it easier for our OEM partners to obtain clearances of products incorporating these technologies, or that the FDA will ever grant clearances on a timely basis, if at all, for any future product incorporating Masimo SET and licensed Rainbow technology that our OEM partners propose to market.
If we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Our products, along with the manufacturing processes and promotional activities for such products, are subject to continual review and periodic inspections by the FDA and other regulatory bodies. In particular, we and our suppliers are required to comply with the QSR which covers the methods and documentation of the design, testing, production, component suppliers control, quality assurance, labeling, packaging, storage and shipping of our products. The FDA enforces the QSR through unannounced inspections. We are also subject to similar state requirements and licenses. Failure by us or one of our suppliers to comply with statutes and regulations administered by the FDA and other regulatory bodies, discovery of previously unknown problems with our products (including unanticipated adverse events or adverse events of unanticipated severity or frequency), manufacturing problems, or failure to comply with regulatory requirements, or failure to adequately respond to any FDA observations concerning these issues, could result in, among other things, any of the following actions:
| | • | | warning letters or untitled letters issued by the FDA; |
| | • | | fines and civil penalties; |
| | • | | unanticipated expenditures to address or defend such actions; |
| | • | | delays in clearing or approving, or refusal to clear or approve, our products; |
| | • | | withdrawal or suspension of clearance or approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies; |
| | • | | product recall or seizure; |
| | • | | orders for physician notification or device repair, replacement or refund; |
| | • | | interruption of production; |
| | • | | operating restrictions; |
If any of these actions were to occur, it would harm our reputation and adversely affect our business, financial condition and results of operations. Furthermore, our key component suppliers may not currently be, or may not continue to be, in compliance with applicable regulatory requirements.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing our products abroad.
We currently market and intend to continue to market our products internationally. Outside the United States, we can market a product only if we receive a marketing authorization and, in some cases, pricing approval, from the appropriate regulatory authorities. The approval procedure varies among international jurisdictions and may require additional testing. The time required to obtain approval internationally may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval in addition to other risks. We may not obtain foreign regulatory approvals on a timely
31
basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. If we fail to receive necessary approvals to commercialize our products in foreign jurisdictions on a timely basis, or at all, our business, financial condition and results of operations could be adversely affected.
Modifications to our marketed devices may require new regulatory clearances or premarket approvals, or may require us to cease marketing or recall the modified devices until clearances or approvals are obtained.
Any modifications to an FDA-cleared device that could significantly affect its safety or effectiveness or that would constitute a major change in its intended use would require a new 510(k) clearance or possibly a PMA approval. We may not be able to obtain such clearances or approvals in a timely fashion, or at all. Delays in obtaining future clearances would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would have an adverse effect on our business, financial condition and results of operations. We have made modifications to our devices in the past and we may make additional modifications in the future, some of which we may believe do not or will not require additional clearances or approvals. If the FDA disagrees with our conclusion and requires new clearances or approvals for the modifications, we may be required to recall and to stop marketing the modified devices, which could have an adverse effect on our business, financial conditions and results of operations.
Off-label promotion of our products or promotional claims deemed false or misleading could subject us to substantial penalties.
Obtaining 510(k) clearance only permits us to promote our products for the uses specifically cleared by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may use our products off-label because the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. Although we may request additional cleared indications for our current products, the FDA may deny those requests, require additional expensive clinical data to support any additional indications or impose limitations on the intended use of any cleared product as a condition of clearance. We must have adequate substantiation for our product performance claims. If the FDA determines that we or our OEM partners have promoted our products for off-label use or have made false or misleading or inadequately substantiated promotional claims, it could request that we or our OEM partners modify those promotional materials or take regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of our products would be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. However, any changes could make it more difficult for us to maintain or attain approval to develop and commercialize our products and product candidates.
The Food and Drug Administration Amendments Act of 2007, or the Amendments, requires, among other things, that the FDA propose and ultimately implement regulations that will require manufacturers to label medical devices with unique identifiers unless a waiver is received from the FDA. Once implemented, compliance with those regulations may require us to take additional steps in the manufacture of our products and labeling. These steps may require additional resources and could be costly. In addition, the Amendments will require us to, among other things, pay annual establishment registration fees to the FDA for each of our FDA-registered facilities.
If we are unable to increase our sales, marketing and distribution capabilities or maintain or establish arrangements with third parties to sell, market, manufacture and distribute our pulse oximetry and Rainbow technology products, our business, financial condition and results of operations could be adversely affected.
We have limited sales and marketing experience both in the United States and internationally and may not be successful in developing and implementing our business strategy. In addition, we currently have a small sales organization compared to many of our competitors. To increase our commercial capabilities, we need to:
| | • | | increase our sales and marketing forces; |
32
| | • | | continue to maintain domestic and international OEM partners; |
| | • | | ensure that distributors and OEM partners provide the technical and educational support customers need to use products incorporating Masimo SET and Rainbow technology successfully; |
| | • | | promote monitoring systems using Masimo SET and Rainbow technology so that sales of those systems and our sensors increase; and |
| | • | | be prepared to provide services, as necessary, to geographically dispersed users of monitoring systems using Masimo SET and Rainbow technology. |
Failure to accomplish any of these requirements could have a material adverse effect on our business, financial condition and results of operations.
We currently plan to increase the size of our direct sales force to further market our products in the United States and internationally. Our sales force will be competing with the experienced and well-funded sales and marketing operations of our competitors. Increasing our direct sales capabilities will be expensive and time consuming. We may not be able to further develop this capacity on a timely basis or at all. If we are unable to expand our sales and marketing capabilities, we will need to continue to contract with third parties to market and sell our approved products in the United States and internationally. To the extent that we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenue could be lower than if we directly marketed and sold our products. Furthermore, to the extent that we enter into co-promotion or other sales and marketing arrangements with other companies, any revenue received will depend on the skills and efforts of others, and we do not know whether these efforts will be successful. If we are unable to maintain adequate sales, marketing, manufacturing and distribution capabilities, independently or with others, we may not be able to generate sufficient product revenue to be profitable.
If we are unable to manufacture an adequate supply of our products, we could lose customers and our revenue and growth could be limited.
Our anticipated growth may strain our ability to manufacture an increasingly large supply of our products. Manufacturing facilities often experience difficulties in scaling up production, including problems with production yields and quality control and assurance. If we cannot scale our manufacturing operations appropriately, maintain control over expenses or otherwise adapt to anticipated growth, or if we have underestimated our future growth, we may not have the capability to satisfy market demand, which would have an adverse effect on our business, financial condition and results of operations.
We anticipate and plan for significant growth, which we may not be able to effectively manage.
Both domestically and internationally, we expect to rapidly expand our operations and our research and development, product development, sales, marketing and administrative organizations. This growth and activity will likely result in new and increased responsibilities for management and place a significant strain upon our operating and financial systems and resources. To accommodate our expected growth and compete effectively, we will be required to improve our current infrastructure, including information systems, as well as create additional processes, procedures and controls and expand, train, motivate and manage our work force. We also may need to expand our manufacturing resources.
We cannot be certain that our personnel, infrastructure, financial systems, processes, procedures, asset management, facilities and controls will be adequate to support our future operations. Any failure to effectively manage our growth could impede our ability to successfully develop, market and sell our products and materially and adversely affect our business, financial condition and results of operations.
We manufacture our products at two locations. Any disruption in these manufacturing facilities could adversely affect our business, financial condition and results of operations.
To date, we have relied on our manufacturing facilities in Irvine, California and Mexicali, Mexico. These facilities and the manufacturing equipment we use to produce our products would be difficult to replace and could require substantial lead-time to repair or replace. Our facilities may be affected by natural or man-made disasters. Earthquakes are of particular significance since our Irvine, California facility is located in an earthquake-prone area. We are also vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fire, floods and similar events. In the event that one of our facilities was affected by a natural or man-made disaster, we would be forced to rely on third-party manufacturers if we could not shift production to our other manufacturing facility. Although we believe we possess adequate insurance for damage to our property and the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. If we are forced to seek alternative facilities, we may incur additional costs and we may experience a disruption in the supply of our products until those facilities are available. Any disruption in our manufacturing capacity could have an adverse impact on our ability to produce sufficient inventory of our products or may require us to incur additional expenses in order to produce sufficient inventory, and, therefore, may adversely affect our revenue, gross margins and results of operations. Any disruption or delay at our manufacturing facilities could impair our ability to meet the demand of our customers and
33
our customers may cancel orders or purchase products from our competitors, which could adversely affect our business, financial condition and results of operations.
In the future, we may choose to add new manufacturing capabilities in either our existing facilities or in new facilities throughout the world. If we expand our worldwide manufacturing locations, there can be no assurance that this expansion will occur without implementation difficulties, or at all, or that such expansion will ultimately lower our overall cost of production.
If we lose the services of our key personnel, or if we are unable to attract and retain other key personnel, we may not be able to manage our operations or meet our growth objectives.
We are highly dependent on our senior management, especially Joe E. Kiani, our Chief Executive Officer, and other key officers. We are also heavily dependent on our engineers and field sales team, including sales representatives and clinical specialists. Our success will depend on our ability to retain our current management, engineers and field sales team, and to attract and retain qualified personnel in the future, including scientists, clinicians, engineers and other highly skilled personnel. Competition for senior management, engineers and field sales personnel is intense and we may not be able to retain our personnel. The loss of the services of members of our key personnel could prevent the implementation and completion of our objectives, including the development and introduction of our products. In general, our officers may terminate their employment at any time without notice for any reason. In some specific situations, officers who signed a severance agreement, have agreed to provide the company up to six months of notice in the event they elect to terminate their employment for any reason. We carry key person life insurance on only Mr. Kiani, who is also the Chief Executive Officer of Masimo Labs. Mr. Kiani devotes most of his time to us.
Existing or future acquisitions of businesses could negatively affect our business, financial condition and results of operations if we fail to integrate the acquired businesses successfully into our existing operations or if we discover previously undisclosed liabilities.
In order to expand our products and technology platform, we have acquired four businesses since our inception and we may acquire additional businesses in the future. Successful acquisitions depend upon our ability to identify, negotiate, complete and integrate suitable acquisitions and to obtain any necessary financing. Even if we complete acquisitions, we may experience:
| | • | | difficulties in integrating any acquired companies, personnel and products into our existing business; |
| | • | | delays in realizing the benefits of the acquired company or products; |
| | • | | diversion of our management’s time and attention from other business concerns; |
| | • | | limited or no direct prior experience in new markets or countries we may enter; |
| | • | | higher costs of integration than we anticipated; and |
| | • | | difficulties in retaining key employees of the acquired business who are necessary to manage these acquisitions. |
In addition, an acquisition could materially impair our operating results by causing us to incur debt or requiring us to amortize acquisition expenses and acquired assets. We may also discover deficiencies in internal controls, data adequacy and integrity, product quality, regulatory compliance and product liabilities that we did not uncover prior to our acquisition of such businesses, which could result in us becoming subject to penalties or other liabilities. Any difficulties in the integration of acquired businesses or unexpected penalties or liabilities in connection with such businesses could have a material adverse effect on our business, financial condition and results of operations.
We may be subject to or otherwise affected by federal and state health care laws, including fraud and abuse and health information privacy and security laws, and could face substantial penalties if we are unable to fully comply with these laws.
Although we do not provide health care services or receive payments directly from Medicare, Medicaid or other third-party payers for our products or the procedures in which our products are used, health care regulation by federal and state governments will impact our business. Health care fraud and abuse and health information privacy and security laws potentially applicable to our operations include, but are not limited to:
| | • | | the Federal Health Care Programs’ Anti-Kickback Law, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or providing remuneration intended to induce the purchase, order or recommendation of an item or service reimbursable under a federal health care program (such as the Medicare or Medicaid programs); |
| | • | | federal false claims laws which prohibit, among other things, knowingly and willfully presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; |
| | • | | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which established federal crimes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or |
34
| | services, as well as imposed certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| | • | | state laws analogous to each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by non-governmental third-party payers, including commercial insurers, and state laws governing the privacy of certain health information. |
We have certain arrangements with hospitals that may be affected by these laws. For instance, under our standard customer arrangements, we provide hospitals with free pulse oximetry monitoring devices in exchange for their agreement to purchase future pulse oximetry sensor requirements from us. In addition, we occasionally provide our customers with rebates in connection with their annual purchases. While we believe that we are currently in compliance with applicable federal and state health care laws, one or more of these arrangements may not meet the Federal Anti-Kickback Law’s safe harbor requirements, which may result in increased scrutiny by government authorities that are responsible for enforcing these laws.
There can be no assurance that we will not be found to be in violation of any of such laws or other similar governmental regulations to which we are directly or indirectly subject, and as a result we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion of our products from reimbursement under Medicare, Medicaid and other federal health care programs, and the curtailment or restructuring of our operations. Any penalties could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
We face environmental and personal injury liabilities related to certain hazardous materials used in our operations.
Our manufacturing processes involve the use, generation and disposal of certain hazardous materials and wastes, including silicone adhesives, solder and solder paste, sealants, epoxies and various solvents such as methyl ethyl ketone, acetone and isopropyl alcohol. As a result, we are subject to stringent federal, state and local laws relating to the protection of the environment, including those governing the use, handling and disposal of hazardous materials and wastes. We may incur significant costs to comply with environmental regulations. Future environmental laws may significantly affect our operations because, for instance, our manufacturing processes may be required to be altered, which may increase our manufacturing costs. In our research and manufacturing activities, we use, and our employees, may be exposed to, materials that are hazardous to human health, safety or the environment. These materials and various wastes resulting from their use are stored at our facility pending ultimate use and disposal. The risk of accidental injury, including to our employees, or contamination from these materials cannot be eliminated. In the event of such an accident, we could be held liable for any resulting damages and any such liability could exceed our reserves. Although we maintain general liability insurance, we do not specifically insure against environmental liabilities. If an enforcement action were to occur, our reputation and our business and financial condition may be harmed, even if we were to prevail or settle the action on terms favorable to us.
The risks inherent in operating internationally and the risks of selling and shipping our products and of purchasing our components and products internationally may adversely impact our business, financial condition and results of operations.
We derive a portion of our net sales from international operations. In fiscal 2008, 2007 and 2006, 25.1%, 23.6% and 22.6%, respectively, of our product revenue was derived from our international operations. In addition, we purchase a portion of our raw materials and components on the international market. The sale and shipping of our products across international borders, as well as the purchase of materials and components from international sources, subject us to extensive U.S. and foreign governmental trade regulations. Compliance with such regulations is costly and we would be exposed to potentially significant penalties for non-compliance. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, restrictions on certain business activities, and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our shipping and sales activities.
In addition, our international sales operations expose us and our representatives, agents and distributors to risks inherent in operating in foreign jurisdictions. These risks include, but are not limited to:
| | • | | the imposition of additional U.S. and foreign governmental controls or regulations; |
| | • | | the imposition of costly and lengthy new export licensing requirements; |
| | • | | a shortage of high-quality sales people and distributors; |
| | • | | loss of any key personnel that possess proprietary knowledge, or who are otherwise important to our success in certain international markets; |
| | • | | changes in duties and tariffs, license obligations and other non-tariff barriers to trade; |
| | • | | the imposition of new trade restrictions; |
| | • | | the imposition of restrictions on the activities of foreign agents, representatives and distributors; |
35
| | • | | scrutiny of foreign tax authorities which could result in significant fines, penalties and additional taxes being imposed on us; |
| | • | | pricing pressure that we may experience internationally; |
| | • | | laws and business practices favoring local companies; |
| | • | | possible failure to comply with anti-bribery laws such as the FCPA and similar anti-bribery laws in other jurisdictions; |
| | • | | political instability and actual or anticipated military or political conflicts; |
| | • | | longer payment cycles; and |
| | • | | difficulties in enforcing or defending intellectual property rights. |
We cannot assure you that one or more of these factors will not harm our business. Any material decrease in our international sales would adversely affect our business, financial condition and results of operations.
Our new international business structure may not result in expected operational benefits.
In the fourth quarter of 2008, we implemented a new international business structure designed to better serve and support our growing international business. By centralizing our international operations, including sales management, marketing, customer support, planning, logistics and administrative functions, we believe we will be able to develop a more efficient and scalable international organization – capable of being even more responsive to the business needs of our international customers—all under one centralized management structure. We commenced the implementation of an international business structure to align our operations with the business needs of our non-U.S. customers and we believe that we may, in the long run, also benefit from certain operational benefits as well as a lower overall tax rate. However, there can be no assurance that our efforts will produce any anticipated operational benefits or provide an overall lower tax rate. Realization of the expected benefits will depend on a number of factors, including our future business results and profitability, the effectiveness and timing of our implementation of our international business structure, changes in tax law and the geographic composition of pre-tax income.
Our operations may be adversely impacted by our exposure to risks related to foreign currency exchange rates.
We market our products in certain foreign markets through our subsidiaries and other international distributors. The related sales agreements may provide for payments in a foreign currency. Accordingly, our operating results are subject to fluctuations in foreign currency exchange rates. When the United States dollar weakens against these currencies, the dollar value of the foreign-currency denominated expense increases, and when the dollar strengthens against these currencies, the dollar value of the foreign-currency denominated expense decreases. Changes in exchange rates, and in particular a weakening of the United States dollar, may adversely affect our results of operations. Our primary foreign currency exchange rate exposures are with the Euro, the Japanese yen, the Canadian dollar and the Australian dollar against the U.S. dollar.
We currently do not hedge against our foreign currency exchange rate risks and therefore believe our exposure to these risks may be higher than if we entered into hedging transactions, including forward exchange contracts or similar instruments. If we decide in the future to enter into forward foreign exchange contracts to attempt to reduce the risk related to foreign currency exchange rates, these contracts may not mitigate the potential adverse impact on our financial results due to the variability of timing and amount of payments under these contracts. In addition, these types of contracts may themselves cause financial harm to us and have inherent levels of counterparty risk over which we would have no control.
Our international operations could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws in non-U.S. jurisdictions.
The FCPA and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Because of the predominance of government-sponsored healthcare systems around the world, many of our customer relationships outside of the United States are with governmental entities and are therefore subject to such anti-bribery laws. Our policies mandate compliance with these anti-bribery laws. Despite our training and compliance programs, our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our results of business, financial condition and results of operations.
Inadequate levels of coverage or reimbursement from governmental or other third-party payers for our products, or for procedures using our products, may cause our revenue to decline.
Sales of our products depend in part on the reimbursement and coverage policies of governmental and private health care payers. The ability of our health care provider customers, including hospitals, to obtain adequate coverage and reimbursement for our products, or for the procedures in which our products are used, may impact our customers’ purchasing decisions. Therefore, our customers’ inability to obtain adequate coverage and reimbursement for our products would have a material adverse effect on our business.
36
Third-party payers have adopted, and are continuing to adopt, health care policies intended to curb rising health care costs. These policies include, among others:
| | • | | controls on reimbursement for health care services and price controls on medical products and services; |
| | • | | limitations on coverage and reimbursement for new medical technologies and procedures; and |
| | • | | the introduction of managed care and prospective payment systems in which health care providers contract to provide comprehensive health care for a fixed reimbursement amount per person or per procedure. |
These trends could lead to pressure to reduce prices for our current products and product candidates and could cause a decrease in the size of the market or a potential increase in competition that could adversely affect our business, financial condition and results of operations.
Legislative and regulatory changes in the health care industry could have a negative impact on our financial performance.
Changes in the health care industry in the United States and elsewhere could adversely affect the demand for our products as well as the way in which we conduct our business. Significantly, the new administration and Congressional and state leaders have expressed a strong desire to reform the U.S. health care system. Included in this reform could be laws that narrow Medicare coverage or reduce reimbursement levels for healthcare services or items provided by physicians and hospitals. Furthermore, many private payors look to Medicare’s coverage and reimbursement policies in setting their coverage policies and reimbursement amounts such that federal reforms could influence the private sector as well. Finally, many states also may attempt to reform their Medicaid programs such that either coverage for certain items or services may be narrowed or reimbursement for them could be reduced. These healthcare reforms may adversely affect our business.
Consistent with or in addition to Congressional or state reforms, the Centers for Medicare and Medicaid Services, or CMS, the federal agency that administers the Medicare and Medicaid programs could change its current policies that affect reimbursement for our products. CMS determined in 2007 that certain uses of pulse oximetry monitoring are eligible for separate Medicare payment in the hospital outpatient setting when no separately payable hospital outpatient services are reported on the same date of service. Each year, however, CMS re-examines the reimbursement rates for hospital inpatient and outpatient and physician office settings and could either increase or decrease the reimbursement rate for procedures utilizing our products Overall, we are unable to predict when legislation or regulation that affects our business may be proposed or enacted in the future or what effect any such legislation or regulation would have on our business. Any such legislation, regulation or policies that affect the coverage and reimbursement of our current or future products, or the procedures utilizing our current or future products, could cause our sales to decrease and, as a result, our revenue to decline.
In addition, the requirements or restrictions imposed on us or our products may change, either as a result of administratively adopted policies or regulations or as a result of the enactment of new laws. Such changes are particular possibilities in light of the 2008 elections in the United States. There may be heightened scrutiny by federal and state regulators and legislators of the FDA’s device approval process, the agency’s efforts to assure the safety of marketed devices, and physician payments and promotional activities by manufacturers. Any new regulations or statutory provisions could result in delays or increased costs during the period of product development, clinical trials, and regulatory review and approval, as well as increased costs to assure compliance.
Further, our success in international markets also depends upon the eligibility of reimbursement for our products through government-sponsored health care payment systems and other third-party payers. Outside of the United States, reimbursement systems vary by country. These systems are often subject to the same pressures to curb rising health care costs and control health care expenditures as those in the United States. In addition, as economies of emerging markets develop, these countries may implement changes in their health care delivery and payment systems. If adequate levels of reimbursement from third-party payers outside of the United States are not obtained, sales of our products outside of the United States may be adversely affected.
Our ongoing antitrust litigation against Tyco Healthcare (currently Covidien) could result in significant additional costs and further divert the attention of our management and key personnel from our business operations.
In May 2002, we filed a lawsuit against Tyco Healthcare, parent company of Nellcor, in the United States District Court for the Central District of California, alleging damage to our business as a result of the anti-competitive business practices of Tyco Healthcare in connection with its Nellcor pulse oximetry brand in violation of federal antitrust laws. Specifically, we alleged that we had incurred damages as a result of a series of illegal exclusionary and anti-competitive acts by Tyco Healthcare that were designed to maintain its monopoly in the pulse oximetry market.
In March 2005, a jury found that Tyco Healthcare’s use of sole-source contracts, product bundling and market share-based compliance pricing contracts, among other conduct violated the federal antitrust laws and awarded damages on that basis. Tyco Healthcare filed post-trial motions requesting that the District Court either override the jury decision or grant a new trial. In March 2006, the District Court upheld a portion of the jury verdict and vacated the remaining verdict. In addition, the District Court vacated the jury’s damages award and granted Tyco Healthcare a new trial on damages. After a retrial of damages, on July 2, 2007, the District Court entered its final judgment, awarding us damages which were trebled as is mandatory under federal antitrust law to $43.5 million and denying our
37
request for a permanent injunction with respect to the Tyco Healthcare business practices found to be anti-competitive. We and Tyco Healthcare each filed a notice of appeal from the judgment. We filed our opening brief on December 17, 2007 with the United States Court of Appeals for the 9th Circuit. On December 27, 2007, the Consumer Federation of America and the Medical Device Manufacturers Association filed an Amicus brief supporting us. Tyco filed its opposition and appeal brief on March 3, 2008. A group of law professors filed an Amicus brief supporting Tyco on March 10, 2008. We filed our response and reply brief on May 19, 2008. The Consumer Federation of America and the Medical Device Manufacturers Association filed an additional Amicus brief in support of us on May 29, 2008. Tyco filed its second appeal brief on July 17, 2008. We are seeking reinstatement of the jury’s verdict on bundling and an affirmance of the liability findings concerning sole-source and market share-based compliance contracts. We are also asking the appellate court to increase the amount of damages awarded by the trial court. Oral argument took place on December 8, 2008. Even if we are ultimately awarded damages in this litigation, the amount will be subject to a 50% legal fee contingency agreement, in which case we would receive 50% of the net (of costs) proceeds from any award.
We believe that Covidien continues to enter into sole-source contracts, product bundling agreements and market share-based agreements. In bundling agreements, the customer is able to obtain discounts on unrelated products when they purchase Covidien pulse oximeters for most of their pulse oximetry needs. Co-marketing agreements also provide significant impediments to competition in that Covidien pays large patient monitoring companies to integrate Covidien pulse oximetry products into their products.
Continued litigation could result in substantial costs and diversion of resources that would harm our business. In addition, there can be no assurance that we will receive any cash award or any equitable relief from the litigation.
We may require additional capital in the future, which may not be available on favorable terms, if at all. To raise capital, we may issue additional securities, including shares, debt or equity-linked debt, which may dilute our existing stockholders and depress our stock price.
To the extent that our existing capital is insufficient to meet our requirements and cover any losses, we will need to raise additional funds through financings or borrowings or curtail our growth and reduce our assets. Any issuance of equity securities or convertible debt or other equity-linked securities to raise financing could:
| | • | | cause substantial dilution of the percentage ownership of our security holders at the time of the issuance; |
| | • | | cause substantial dilution of our earnings per share; |
| | • | | subject us to the risks associated with increased leverage, including a reduction in our ability to obtain financing or an increase in the cost of any financing we obtain; |
| | • | | subject us to restrictive covenants that could limit our flexibility in conducting future business activities; and |
| | • | | adversely affect the prevailing market price for our outstanding securities. |
Securities issued in future financings may have rights, preferences and privileges that are senior to those of our common stock. These rights, preferences and privileges may include, among others, dividend rights, conversion rights, voting rights and liquidation rights. As a result, the rights of holders of our common stock will be subject to, and could be adversely affected by, the rights of holders of any preferred stock or other senior securities that may be issued in the future. We do not intend to seek stockholder approval for any such security issuance unless required by applicable law or regulation or the terms of existing securities.
In addition, any financing may not be on terms that are favorable to us, if at all. If our need for capital arises because of significant losses, the occurrence of these losses may make it more difficult for us to raise the necessary capital. If we cannot raise funds on acceptable terms, if and when needed, or if the funds are not available to us, we may not be able to develop or enhance our products or technologies, take advantage of future opportunities, grow our business or respond to competitive pressures or unanticipated requirements.
If we fail to comply with the reporting obligations of the Securities Exchange Act of 1934 and Section 404 of the Sarbanes-Oxley Act of 2002, or if we fail to maintain adequate internal control over financial reporting, our business, results of operations and financial condition and investors’ confidence in us could be materially and adversely affected.
As a public company, we are required to comply with the periodic reporting obligations of the Securities Exchange Act of 1934, as amended, or the Exchange Act, including preparing annual reports, quarterly reports and current reports. Our failure to prepare and disclose this information in a timely manner and meet our reporting obligations in their entirety could subject us to penalties under federal securities laws and regulations of The Nasdaq Stock Market, LLC, or Nasdaq, expose us to lawsuits and restrict our ability to access financing on favorable terms, or at all.
In addition, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, we are required to evaluate and provide a management report of our systems of internal control over financial reporting and our independent registered public accounting firm is required to attest to, our internal control over financial reporting. We have dedicated, and we expect to continue to dedicate, significant management, financial and other resources in connection with maintaining compliance with Section 404 based on the standards adopted by the Public Company Accounting Oversight Board. These efforts include reviewing our existing internal control structure and performing system and process evaluation and testing (and any necessary remediation). As a result of these
38
ongoing activities, we may either hire or outsource additional personnel to expand and strengthen our finance function. In addition, during the course of the evaluation of our internal control over financial reporting, we may identify areas requiring improvement and may be required to design enhanced processes and controls to address issues identified through this review. This could result in significant delays and costs to us and require us to divert substantial resources, including management time from other activities.
We cannot provide any assurance that we will be able to successfully maintain the certification requirements of Section 404 or that our independent registered public accounting firm will be able to provide the attestation report required under the regulations. If we fail to maintain the adequacy of our internal control over financial reporting, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with the Sarbanes-Oxley Act. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent fraud. Our failure to maintain the requirements of Section 404 may subject us to investigations by regulatory authorities, including the SEC or Nasdaq, and sanctions. As a result, our failure to maintain the requirements of Section 404 on a timely basis could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business, negatively impact the trading price of our common stock, and adversely affect investors’ confidence in our company and our ability to access capital markets for financing.
Changes to existing accounting pronouncements or taxation rules or practices may affect how we conduct our business and affect our reported results of operations.
A change in accounting pronouncements or taxation rules or practices, or the interpretation of them by the SEC or other regulatory bodies, can have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New accounting pronouncements or taxation rules and varying interpretations of accounting pronouncements or taxation practice have occurred and may occur in the future. Changes to existing rules or the adoption of new rules may adversely affect our reported financial results or the way we conduct our business.
Risks Related to Our Common Stock
Our stock price may be volatile, and your investment in our common stock could suffer a decline in value.
There has been significant volatility in the market price and trading volume of equity securities, which is unrelated to the financial performance of the companies issuing the securities. These broad market fluctuations may negatively affect the market price of our common stock. From December 30, 2007 to January 3, 2009, our closing stock price ranged from $22.04 to $41.95.You may not be able to resell your shares at or above the price you paid for them due to fluctuations in the market price of our common stock caused by changes in our operating performance or prospects and other factors.
Some specific factors, in addition to the other risk factors identified above, that may have a significant effect on our common stock market price, many of which we cannot control, include but are not limited to:
| | • | | actual or anticipated fluctuations in our operating results or future prospects; |
| | • | | our announcements or our competitors’ announcements of new products; |
| | • | | the public’s reaction to our press releases, our other public announcements and our filings with the SEC; |
| | • | | strategic actions by us or our competitors, such as acquisitions or restructurings; |
| | • | | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; |
| | • | | changes in accounting standards, policies, guidance, interpretations or principles; |
| | • | | changes in our growth rates or our competitors’ growth rates; |
| | • | | developments regarding our patents or proprietary rights or those of our competitors; |
| | • | | our inability to raise additional capital as needed; |
| | • | | concern as to the efficacy of our products; |
| | • | | changes in financial markets or general economic conditions; |
| | • | | sales of common stock by us or members of our management team or our Board of Directors; and |
| | • | | changes in stock market analyst recommendations or earnings estimates regarding our common stock, other comparable companies or our industry generally. |
In particular, the current decline of the financial markets and related factors beyond our control, including the credit and mortgage crisis in both the U.S. and worldwide, may cause our stock price to decline rapidly and unexpectedly.
Concentration of ownership among our existing directors, executive officers and principal stockholders may prevent new investors from influencing significant corporate decisions.
39
As of January 3, 2009, our current directors and executive officers and their affiliates, in the aggregate, beneficially owned approximately 12.1% of our outstanding common stock. Subject to any fiduciary duties owed to our other stockholders under Delaware law, the stockholders may be able to exercise a significant influence over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, and will have some control over our management and policies. Some of these persons or entities may have interests that are different from yours. For example, these stockholders may support proposals and actions with which you may disagree or which are not in your best interests. The concentration of ownership could delay or prevent a change in control of us or otherwise discourage a potential acquirer from attempting to obtain control of us, which in turn could reduce the price of our common stock. In addition, these stockholders, some of whom have representatives sitting on our board of directors, could use their voting influence to maintain our existing management and directors in office, delay or prevent changes in control of us, or support or reject other management and board proposals that are subject to stockholder approval, such as amendments to our employee stock plans and approvals of significant financing transactions.
You could experience substantial dilution of your investment as a result of subsequent exercises of our outstanding options or the grant of future equity awards by us.
As of January 3, 2009, an aggregate of 8,681,630 shares of our common stock were reserved for future issuance under our three equity incentive plans, 7,329,474 of which were subject to options outstanding as of that date at a weighted average exercise price of $15.97 per share. To the extent outstanding options are exercised, our existing stockholders may incur dilution. We rely heavily on equity awards to motivate current employees and to attract new employees. The grant of future equity awards by us to our employees and other service providers may further dilute our stockholders.
Future resales of our common stock, including those by our insiders, may cause our stock price to decline.
As of January 3, 2009, there were 57,326,527 shares of our common stock outstanding, including 3,287,494 shares sold by us and 10,416,626 shares sold by our selling stockholders in our initial public offering, or IPO, in August 2007. A significant portion of our shares of common stock outstanding prior to our IPO that were not sold by selling stockholders became eligible for sale in the public market on February 4, 2008 upon expiration of lock-up agreements entered into in connection with our IPO, although as of January 3, 2009, 5,856,989 of these shares were held by directors, executive officers and other affiliates and subject to volume limitations under Rule 144 promulgated by the SEC under the Securities Act of 1933, as amended, or Rule 144, as of that date. A large portion of our outstanding shares are held by a small number of persons and investment funds. Resale by these stockholders of a substantial number of shares, announcements of the proposed resale of substantial amounts of our common stock or the perception that substantial resale may be made, could significantly reduce the market price of our common stock. Moreover, the holders of 318,562 shares of common stock at January 3, 2009 have rights, subject to some conditions, to require us to file registration statements covering the shares they currently hold or to include these shares in registration statements that we may file for ourselves or other stockholders from time to time.
Certain of our directors and executive officers have entered into Rule 10b5-1 trading plans pursuant to which they have sold and will continue to sell shares of our common stock. Generally, these sales require public filings. Actual or potential sales by these insiders, including those under a pre-arranged Rule 10b5-1 trading plan, could be viewed negatively by the market and adversely affect the market price of our common stock.
In December 2007, we registered an aggregate of 11,218,285 shares reserved under our equity plans under a Registration Statement on Form S-8 and we anticipate filing one or more additional Registration Statements on Form S-8 to register additional shares reserved under our equity plans. All shares issued pursuant to a Registration Statement on Form S-8 can be freely sold in the public market upon issuance, subject to restrictions on our affiliates under Rule 144. If a large number of these shares are sold in the public market, the sales could reduce the trading price of our common stock and impede our ability to raise future capital.
Our corporate documents and Delaware law contain provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our common stock.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage, delay or prevent a merger or acquisition involving us that our stockholders may consider favorable. For example, our amended and restated certificate of incorporation authorizes our board of directors to issue up to five million shares of “blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire us. In addition, our amended and restated certificate of incorporation provides for a staggered board of directors, whereby directors serve for three year terms, with one third of the directors coming up for reelection each year. A staggered board will make it more difficult for a third party to obtain control of our board of directors through a proxy contest, which may be a necessary step in an acquisition of us that is not favored by our board of directors.
We are also subject to the anti-takeover provisions of the Delaware General Corporation Law. Under these provisions, if anyone becomes an “interested stockholder,” we may not enter into a “business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of us. An
40
“interested stockholder” means, generally, someone owning 15% or more of our outstanding voting stock or an affiliate of ours that owned 15% or more of our outstanding voting stock during the past three years, subject to certain exceptions as described in the Delaware General Corporation Law.
In addition, our board of directors has adopted a stockholder rights plan. Under the stockholder rights plan if any person becomes the beneficial owner of 15% or more of the outstanding shares of common stock, subject to a number of exceptions set forth in the plan, all of our stockholders other than the acquiring person will receive a right to purchase shares of our common stock at a price of $136.00 per share. Our stockholder rights plan could discourage a takeover attempt and make an unsolicited takeover of our company more difficult. As a result, without the approval of our board of directors, you may not have the opportunity to sell your shares to a potential acquirer of us at a premium over prevailing market prices. This could reduce the market price of our common stock.
We will incur significant increased costs as a result of operating as a public company, and our management and key employees will be required to devote substantial time to new compliance initiatives.
Prior to August 2007, we operated as a private concern. As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, we are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act. These requirements may place a strain on our people, systems and resources. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, significant resources and management oversight will be required. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the SEC and Nasdaq are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time-consuming. This may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition and results of operations.
We do not intend to declare cash dividends on our stock, and any return on investment may be limited to the value of our stock.
We currently intend to retain all future earnings for the operation and expansion of our business and do not anticipate declaring or paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends on our common stock will be at the discretion of our board of directors and will depend upon our results of operations, earnings, capital requirements, financial condition, business prospects, contractual restrictions and other factors deemed relevant by our board of directors.
Securities analysts may not cover our common stock or may issue negative reports, which may have a negative impact on the market price of our common stock.
Securities analysts may elect not to provide research coverage of our common stock. If securities analysts do not cover our common stock, the lack of research coverage may cause the market price of our common stock to decline. The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about our business or the pulse oximetry market. If one or more of the analysts who elects to cover us downgrades our stock, our stock price could decline rapidly. If one or more of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our stock price to decline. In addition, recently adopted rules mandated by the Sarbanes-Oxley Act, and a global settlement reached in 2003 between the SEC, other regulatory agencies and a number of investment banks, has led to a number of fundamental changes in how analysts are reviewed and compensated. In particular, many investment banking firms are required to contract with independent financial analysts for their stock research. As long as we have a smaller market capitalization, it may be difficult for us to attract independent financial analysts who will cover our common stock, which could have a negative effect on the market price of our stock.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
We lease approximately 120,400 square feet of space in Irvine, California, for our corporate headquarters and product manufacturing, research and development, warehousing and distribution operations. The lease covering 70,200 square feet of this space expires in October 2009. We have the right to renew this lease for an additional five-year period at the end of the lease term. In February 2006, we entered into a lease for an additional 50,200 square feet of space adjacent to our current facility for office space and research and development. This lease expires in March 2010. We have an option to renew this lease for an additional five-year period at the end of the lease term.
41
We also lease approximately 95,600 square feet of space in Mexicali, Mexico, for the manufacture of our sensors and accessories under a shelter labor agreement with Industrial Vallera de Mexicali, S.A. de C.V., or IVEMSA. IVEMSA is a Mexican maquiladora, which is a shelter services provider incorporated in Mexico that is licensed to operate factories and plants in Mexico. The shelter program allows foreign companies to manufacture in Mexico without being required to organize and operate their own subsidiary, for example, as a Mexican corporation. As a result, the risks of labor liability, ownership of facilities and legal presence of foreign corporations in Mexico are avoided. We entered into the agreement with IVEMSA to establish and run a facility to manufacture our sensors and accessory products. IVEMSA leases the space directly from the owner of the property under an agreement that expires in August 2014.
On January 1, 2009, Masimo International Sarl entered into a five year lease for approximately 7,000 square feet of office space in Neuchatel, Switzerland. This office space will house our new international headquarters and will be focused on operations including sales, marketing, customer service and other administrative functions. In addition, Masimo Europe, Ltd. leases approximately 6,900 square feet as its headquarters in Limonest, France to support its sales, marketing, customer service and administrative functions. Masimo Japan, K.K. leases approximately 3,700 square feet of space as its headquarters in Tokyo, Japan, which it uses for sales, marketing, customer service and administrative functions, as well as maintaining product inventory. In addition, Masimo Canada ULC leases approximately 23,700 square feet of space as its headquarters in Montreal, Canada, which it uses primarily for research and development activities. We also maintain small sales offices in Germany, the United Kingdom, Italy, Spain, Japan, Australia, Singapore and China. We believe that our existing facilities are adequate to meet our needs and that existing needs and future growth can be accommodated by leasing alternative or additional space.
In May 2002, we filed a lawsuit against Tyco Healthcare, parent company of Nellcor, in the United States District Court for the Central District of California, alleging damage to our business as a result of the anti-competitive business practices of Tyco Healthcare. Specifically, we alleged that we had incurred damages as a result of a series of illegal exclusionary and anti-competitive acts by Tyco Healthcare that were designed to maintain its monopoly in the pulse oximetry market in violation of federal antitrust laws.
In March 2005, a jury found that Tyco Healthcare’s use of sole-source contracts, product bundling and market share-based compliance pricing contracts, among other conduct, violated the federal antitrust laws and awarded damages on that basis. Tyco Healthcare filed post-trial motions requesting that the District Court either override the jury decision or grant a new trial. In March 2006, the District Court upheld a portion of the jury verdict and vacated the remaining verdict. In addition, the District Court vacated the jury’s damages award and granted Tyco Healthcare a new trial on damages. After a retrial of damages, on July 2, 2007, the District Court entered its final judgment awarding us damages which were trebled as is mandatory under federal antitrust law to $43.5 million and denying our request for a permanent injunction with respect to Tyco Healthcare’s business practices found to be anti-competitive. We and Tyco Healthcare each filed a notice of appeal from the judgment. We filed our opening brief on December 17, 2007 with the United States Court of Appeals for the 9th Circuit. On December 27, 2007, the Consumer Federation of America and the Medical Device Manufacturers Association filed an Amicus brief supporting Masimo. Tyco filed its opposition and appeal brief on March 3, 2008 and a group of law professors filed an Amicus brief supporting Tyco on March 10, 2008. We filed our response and reply brief on May 19, 2008. The Consumer Federation of America and the Medical Device Manufacturers Association filed an additional Amicus brief in support of us on May 29, 2008. Tyco filed its second appeal brief on July 17, 2008. We are seeking reinstatement of the jury’s verdict on bundling and an affirmance of the liability findings concerning sole-source and market share-based compliance contracts. We are also asking the appellate court to increase the amount of damages awarded by the trial court. Oral argument took place on December 8, 2008. Even if we are ultimately awarded damages in this litigation, the amount will be subject to a 50% legal fee contingency agreement, in which case we would receive 50% of the net (of costs) proceeds from the award. Even though most of the legal expenses to date have been on a contingency basis, we expect to incur expenses related to the appellate work, which will be treated as operating expense, as incurred.
We believe the jury verdict we received in the Tyco Healthcare antitrust litigation has been important in our efforts to increase our market share among certain large hospital systems and GPOs that were formerly closed as a result of Tyco Healthcare’s anti-competitive conduct. However, the lawsuit has been and will continue to be a diversion of management’s attention from the implementation of our business strategy. See “Risk Factors” for a description of the risks related to our litigation against Tyco Healthcare.
On February 19, 2008, we filed a lawsuit in the United Stated District Court for the Central District of California against Respironics, Inc. for breach of contract, breach of the covenant of good faith and fair dealing, and interference with prospective economic advantage, based on a January 16, 2006, contract between Respironics and us. On April 7, 2008, Respironics filed a demurrer seeking to dismiss the lawsuit on the grounds that our complaint fails to state sufficient facts to constitute valid claims. The court subsequently denied Respironics’ demurrer. On July 16, 2008, Respironics answered our complaint and filed a cross-complaint. We answered the cross-complaint on August 15, 2008, denying all material allegations. There is no guarantee that we will prevail in this suit or receive any damages or other relief if we do prevail.
42
On February 3, 2009, we filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh related to Philips FAST pulse oximetry technology and certain Philips patient monitors. The suit was brought in the United States District Court for the District of Delaware. Two patents at issue in this suit, related to our measure-through-motion technology, were successfully enforced in our previous suit against Nellcor. There is no guarantee that we will prevail in this suit or receive any damages or other relief if we do prevail.
From time to time, we are involved in legal proceedings in the ordinary course of business. Other than the proceedings described above, we are not currently involved in any material legal proceedings.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
We did not submit any matters to a vote of our stockholders during the quarter ended January 3, 2009.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
We had our initial public offering on August 7, 2007. Our common stock is traded on the NASDAQ Global Market under the symbol “MASI”. The following table sets forth the high and low closing sales price of our common stock for the periods indicated.
| | | | | | |
| | | Price Range |
| | | High | | Low |
Fiscal 2007: | | | | | | |
Third Quarter (from August 8, 2007) | | $ | 26.64 | | $ | 19.55 |
Fourth Quarter | | $ | 41.79 | | $ | 26.24 |
Fiscal 2008: | | | | | | |
First Quarter | | $ | 39.50 | | $ | 25.86 |
Second Quarter | | $ | 35.00 | | $ | 25.06 |
Third Quarter | | $ | 41.95 | | $ | 33.49 |
Fourth Quarter | | $ | 38.51 | | $ | 22.04 |
The above quotations reflect inter-dealer prices, without retail markup, markdown or commission and may not necessarily represent actual transactions.
As of February 13, 2009, the closing price of our common stock on the NASDAQ Global Market was $27.08 per share, and the number of stockholders of record was approximately 363. We believe that the number of beneficial owners is substantially greater than the number of record holders because a large portion of our common stock is held of record through brokerage firms in “street name.”
Use of Proceeds
On August 13, 2007, we completed our initial public offering, or IPO, of common stock in which a total of 13,704,120 shares were sold, comprised of 10,416,626 shares sold by selling stockholders, 1,500,000 shares sold by us at the initial closing and 1,787,494 shares sold by us pursuant to the underwriters’ full exercise of their over-allotment option, at an issue price of $17.00 per share. We raised a total of $55.9 million in gross proceeds from the IPO, or approximately $47.8 million in net proceeds after deducting underwriting discounts and commissions of $3.9 million and estimated other offering costs of approximately $4.2 million. Upon the closing of the IPO, all shares of convertible preferred stock outstanding automatically converted into an aggregate of 34,612,503 shares of common stock.
As of January 3, 2009, we used over $33.9 million of the net proceeds from our IPO for working capital, purchase and installation of equipment and general corporate purposes. We anticipate that we will continue to use the remaining net proceeds from our IPO for additional working capital, purchase and installation of equipment and general corporate purposes. The timing and amount of our actual expenditures of the remaining IPO net proceeds will be based on many factors, including without limitation, cash flows from operations and the anticipated growth of our business. We have invested portions of the net proceeds from our IPO in U.S. Treasury bills with maturities of 3 months or less and short-term money market securities. There has been no material change in the planned use of proceeds from our IPO as described in the final prospectus filed with the SEC on August 8, 2007 pursuant to Rule 424(b) promulgated by the SEC under the Securities Act of 1933, as amended.
43
Stock Performance Graph
The following stock performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act or Exchange Act, except to the extent that we specifically incorporate it by reference into such filing.
The following stock performance graph compares total stockholder returns for Masimo Corporation from the date of our initial public offering of August 8, 2007 through January 3, 2009 against the NASDAQ Market Composite Index and NASDAQ Medical Equipment Index, assuming a $100 investment made on August 8, 2007. Each of the two comparative measures of cumulative total return assumes reinvestment of dividends. The stock performance shown on the graph below is not necessarily indicative of future price performance.
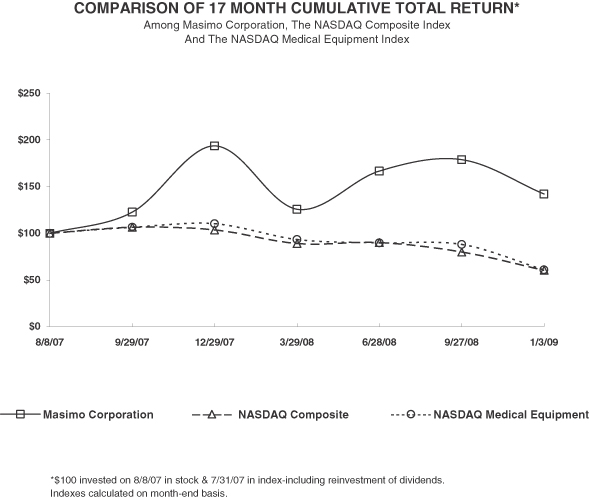
44
| ITEM 6. | SELECTED FINANCIAL DATA |
The following tables reflect selected financial data derived from our consolidated financial statements for each of the last five years. The consolidated statement of operations data for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 and the consolidated balance sheet data as of January 3, 2009 and December 29, 2007 are derived from our audited consolidated financial statements included in this Form 10-K. The consolidated statement of operations data for the years ended December 31, 2005 and 2004, and the consolidated balance sheet data as of December 31, 2006, 2005 and 2004 are derived from our audited consolidated financial statements not included in this Form 10-K. Historical results are not necessarily indicative of future results. The selected financial data set forth below should be read in conjunction with our consolidated financial statements, the related notes and Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Form 10-K.
| | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | | | Year ended
December 31,
2005 | | | Year ended
December 31,
2004 | |
| | | (in thousands, except share information) | |
Statement of Operations Data(1): | | | | | | | | | | | | | | | | | | | | |
Revenue: | | | | | | | | | | | | | | | | | | | | |
Product | | $ | 258,895 | | | $ | 199,684 | | | $ | 155,131 | | | $ | 107,613 | | | $ | 69,069 | |
Royalty and license fee | | | 48,179 | | | | 56,602 | | | | 69,207 | | | | 277 | | | | 288 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 307,074 | | | | 256,286 | | | | 224,338 | | | | 107,890 | | | | 69,357 | |
Cost of goods sold | | | 89,454 | | | | 73,606 | | | | 61,640 | | | | 42,717 | | | | 29,354 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 217,620 | | | | 182,680 | | | | 162,698 | | | | 65,173 | | | | 40,003 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 25,495 | | | | 22,960 | | | | 24,875 | | | | 8,548 | | | | 6,044 | |
Selling, general and administrative | | | 120,069 | | | | 91,234 | | | | 91,384 | | | | 42,807 | | | | 29,880 | |
Patent litigation expenses (proceeds) | | | — | | | | — | | | | (262,605 | ) | | | 1,736 | | | | 6,204 | |
Purchased in-process research and development | | | — | | | | — | | | | — | | | | 2,800 | | | | — | |
Antitrust litigation | | | 706 | | | | 1,537 | | | | 109 | | | | 278 | | | | 238 | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 146,270 | | | | 115,731 | | | | (146,237 | ) | | | 56,169 | | | | 42,366 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | 71,350 | | | | 66,949 | | | | 308,935 | | | | 9,004 | | | | (2,363 | ) |
Non-operating income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest income | | | 2,305 | | | | 2,361 | | | | 6,741 | | | | 224 | | | | 107 | |
Interest expense | | | (753 | ) | | | (2,475 | ) | | | (1,824 | ) | | | (1,851 | ) | | | (1,434 | ) |
Other | | | (511 | ) | | | 1,287 | | | | 551 | | | | (8 | ) | | | 8 | |
| | | | | | | | | | | | | | | | | | | | |
Total non-operating income (expense) | | | 1,041 | | | | 1,173 | | | | 5,468 | | | | (1,635 | ) | | | (1,319 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision for (benefit from) income taxes | | | 72,391 | | | | 68,122 | | | | 314,403 | | | | 7,369 | | | | (3,682 | ) |
Provision for (benefit from) income taxes | | | 40,464 | | | | 25,867 | | | | 132,577 | | | | (26,012 | ) | | | 161 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | 31,927 | | | | 42,255 | | | | 181,826 | | | | 33,381 | | | | (3,843 | ) |
Preferred stock dividend | | | — | | | | — | | | | (77,785 | ) | | | — | | | | — | |
Accretion of preferred stock | | | — | | | | (4,837 | ) | | | (7,985 | ) | | | (8,278 | ) | | | (8,477 | ) |
Undistributed income attributable to preferred stockholders | | | — | | | | (14,339 | ) | | | (34,275 | ) | | | (19,599 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to common stockholders | | $ | 31,927 | | | $ | 23,079 | | | $ | 61,781 | | | $ | 5,504 | | | $ | (12,320 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) per common share(2): | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 0.57 | | | $ | 0.71 | | | $ | 3.79 | | | $ | 0.57 | | | $ | (1.31 | ) |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | $ | 0.53 | | | $ | 0.60 | | | $ | 3.04 | | | $ | 0.42 | | | $ | (1.31 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average number of common shares: | | | | | | | | | | | | | | | | | | | | |
Basic – Two Class method | | | N/A | | | | 16,654,586 | | | | 16,319,898 | | | | 9,717,882 | | | | 9,378,741 | |
Diluted – Two Class method | | | N/A | | | | 20,732,872 | | | | 20,302,872 | | | | 13,102,611 | | | | 9,378,741 | |
Basic – Single Class method | | | 56,320,712 | | | | 54,660,216 | | | | N/A | | | | N/A | | | | N/A | |
Diluted – Single Class method | | | 60,190,335 | | | | 59,829,198 | | | | N/A | | | | N/A | | | | N/A | |
45
(1) | Pursuant to FIN 46(R), Masimo Labs is consolidated within our financial statements. Accordingly, all inter-company royalties, option and licensing fees, and other charges between us and Masimo Labs have been eliminated in the consolidation. Also in accordance with FIN 46(R), all direct engineering expenses that have been incurred by us and charged to Masimo Labs have not been eliminated and are included as research and development expense in our consolidated statements of operations. For additional discussion of accounting for Masimo Labs, see Note 3 to the Consolidated Financial Statements. |
(2) | See Note 2 to the Consolidated Financial Statements for a description of the method used to compute basic and diluted net income (loss) per common share. |
| | | | | | | | | | | | | | | | | |
| | | January 3,
2009 | | December 29,
2007 | | December 31,
2006 | | December 31,
2005 | | | December 31,
2004 | |
| | | (in thousands, except dividends declared per common share) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 146,910 | | $ | 96,733 | | $ | 55,382 | | $ | 14,172 | | | $ | 11,794 | |
Working capital | | | 166,595 | | | 121,831 | | | 30,125 | | | 34,213 | | | | 6,030 | |
Total assets | | | 293,348 | | | 235,511 | | | 159,073 | | | 100,589 | | | | 54,221 | |
Long term debt, including current portion | | | 622 | | | 31,041 | | | 21,042 | | | 29,060 | | | | 23,828 | |
Convertible preferred stock | | | — | | | — | | | — | | | 143,959 | | | | 135,681 | |
Total stockholders’ equity (deficit) | | | 219,391 | | | 150,066 | | | 56,961 | | | (101,082 | ) | | | (127,573 | ) |
Dividends declared per common share(3) | | | — | | | — | | | 4.09 | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
(3) | Dividends declared as a result of a one-time patent litigation settlement with Nellcor in 2006. See Item 7 – “Management’s Discussion and Analysis of Financial Condition and Results of Operations” for further details regarding the settlement. The dividends were declared for the same amount per share to both common and preferred stockholders, assuming the conversion of all outstanding shares of preferred stock into common stock on a 1:1 basis. See Note 11 to the consolidated financial statements for further details regarding the dividends declared. |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read this discussion together with the financial statements, related notes and other financial information included in this Form 10-K. The following discussion may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under Item 1.A—“Risk Factors” and elsewhere in this Form 10-K. These risks could cause our actual results to differ materially from any future performance suggested below.
Overview
We are a global medical technology company that develops, manufactures and markets noninvasive patient monitoring products that improve patient care. We invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of measure-through motion and low perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. Pulse oximetry is the noninvasive measurement of the oxygen saturation level of arterial blood, or the blood that delivers oxygen to the body’s tissues, and pulse rate. Conventional pulse oximetry is subject to technological limitations that reduce its effectiveness and the quality of patient care. In particular, when using conventional pulse oximetry, arterial blood signal recognition can be distorted by motion artifact, or patient movement, and low perfusion, or low arterial blood flow. Low perfusion can also cause the failure of the conventional pulse oximeter to obtain an accurate measurement. Conventional pulse oximetry readings can also be impacted by bright light and electrical interference from the presence of electrical surgical equipment. Published independent research shows that over 70% of the alarms were false outside the operating room using conventional pulse oximetry. Our Masimo SET platform has addressed many of the previous technology limitations. The benefits of Masimo SET have been validated in over 100 independent clinical and laboratory studies. During 2008, we generated product revenue of $258.9 million, representing a compound annual growth rate, or CAGR, of 34.0% for the three years ended January 3, 2009.
We develop, manufacture and market a family of noninvasive blood constituent patient monitoring solutions that consists of a monitor or circuit board and our proprietary single-patient use and reusable sensors and cables. In addition, we offer a remote-alarm/monitoring solution, such as the Masimo Patient SafetyNet. Although our Masimo SET platform is only operable with our proprietary sensors, our sensors have the capability to work with certain competitor pulse oximeters through the use of our adapter cables. In 2005, we launched our Masimo Rainbow SET Pulse CO-Oximetry platform utilizing licensed Rainbow technology from Masimo Labs, which enables the noninvasive measurement of not only arterial blood oxygen saturation level and pulse rate, but also carboxyhemoglobin, or carbon monoxide levels in the blood, methemoglobin saturation levels in the blood and total hemoglobin, or
46
the oxygen-carrying component of red blood cells. Also, in 2007 we launched Plethysmographic Variability Index, or PVI, which is a measurement that quantifies changes in the plethysmographic waveform over the respiration cycle. Along with the release of our Masimo Rainbow SET Pulse CO-Oximetry products, we have developed multi-wavelength sensors that have the ability to monitor multiple parameters with a single sensor.
We have focused on building our U.S. and international sales and marketing infrastructure to market our products to end-users, such as hospitals, and OEM partners for incorporation into their patient-monitoring products. We market our pulse oximetry products to hospitals and the EMS market through our direct sales force, and market our circuit boards to our OEM partners. Today, the primary focus of our hospital sales force is to facilitate the conversion of hospitals to our Masimo SET or Masimo Rainbow SET products. In the United States, we typically enter into long-term sales contracts with hospitals, pursuant to which we ship and install our pulse oximeters at no cost to the hospital in exchange for a commitment to purchase a minimum number of sensors from us over a specified period of time. With the introduction of Masimo Rainbow SET Pulse CO-Oximetry, we have established a small sales force to concentrate on the EMS market. Over the past year, we have expanded our sales and marketing staffing levels to 324 as of January 3, 2009, from 292 as of December 29, 2007. We supplement our direct sales with sales through our distributors. During this period, direct and distributor sales have increased to $201.1 million, or 77.7%, of product revenue for 2008, from $143.7 million, or 71.9%, of product revenue for 2007. We expect the percentage of our revenue from direct sales to continue to increase as we expand our worldwide direct sales force.
The building of our installed base of pulse oximeters and pulse oximeter circuit boards generates recurring sales of our sensors, primarily single-patient use sensors. A user of one of our pulse oximeters or our OEMs’ pulse oximeters can obtain the benefit of the Masimo SET or Masimo Rainbow SET only by using our proprietary sensors that are designed for our system. We currently estimate that our worldwide installed base was approximately 567,000 units as of January 3, 2009, up from 470,000 units as of December 29, 2007. We estimate our installed base to be the number of pulse oximeters and pulse oximeter circuit boards that we have shipped in the past seven years. In the event we increase this assessment period beyond seven years in the future, our estimated installed base may increase materially. We expect our worldwide installed base to continue to increase as we expand our market share and expand the pulse oximetry market to other patient care settings.
On August 13, 2007, we completed our initial public offering, or IPO, of common stock in which a total of 13,704,120 shares were sold, comprised of 10,416,626 shares sold by selling stockholders, 1,500,000 shares sold by us at the initial closing and 1,787,494 shares sold by us pursuant to the underwriters’ full exercise of their over-allotment option, at an issue price of $17.00 per share. We raised a total of $55.9 million in gross proceeds from the IPO, or approximately $47.8 million in net proceeds after deducting underwriting discounts and commissions of $3.9 million and estimated other offering costs of approximately $4.2 million. Upon the closing of the IPO, all shares of convertible preferred stock outstanding automatically converted into an aggregate of 34,612,503 shares of common stock. The consolidated financial statements as of and for the period ended January 3, 2009, including share and per share amounts, include the effects of the offering since it was completed prior to January 3, 2009.
Masimo Laboratories, Inc.
Masimo Laboratories, Inc., or Masimo Labs, is an independent entity spun off from us to our stockholders in 1998. Joe E. Kiani and Jack Lasersohn, members of our board of directors, are also members of the board of directors of Masimo Labs. Joe E. Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Masimo Labs. We are a party to a cross-licensing agreement with Masimo Labs, which was amended and restated effective January 1, 2007, or the Cross-Licensing Agreement, that governs each party’s rights to certain of the intellectual property held by the two companies.
Under the Cross-Licensing Agreement, we granted Masimo Labs an exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET owned by us, including all improvements on this technology, for the measurement of non-vital signs parameters and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs parameters in any product market in which a product is intended to be used by a patient or pharmacist rather than a professional medical caregiver, which we refer to as the Labs Market. We also granted Masimo Labs a non-exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET for the measurement of vital signs in the Labs Market.
From May 1998 through December 2008, Masimo Labs contracted the services of our employees for the development of Rainbow technology. We paid Masimo Labs for the option to market and develop products based on Masimo Labs technology in defined markets. Through December 2005, we had paid Masimo Labs $7.5 million in option fees and nearly all these option fees were used by Masimo Labs to repay us for the services that we had provided to Masimo Labs. In addition, through December 2006, we exercised two licenses, for $2.5 million each, for the right to market products based on the new carbon monoxide and methemoglobin parameter technologies developed by Masimo Labs. As of January 3, 2009, the entire $5.0 million in fees had been used by Masimo Labs to repay us for the shared engineering and other services that we provided to Masimo Labs. We also entered into a Services Agreement with Masimo Labs to govern the services we will provide to Masimo Labs going forward, effective as of January 1, 2007. As part of the Cross-Licensing Agreement, we exercised an additional license for total hemoglobin for a fee of $2.5 million, on January 1, 2007.
47
The Cross-Licensing Agreement requires us to pay certain royalties on products incorporating the licensed Rainbow technology. The royalty is up to 10% of the Rainbow royalty base, which will include handhelds, tabletop and multi-parameter devices. Handheld products incorporating Rainbow technology will carry a 10% royalty rate. For other products, only the proportional amount attributable for that portion of our products used to measure non-vital sign parameters, sensors and accessories, rather than for measuring vital sign parameters, will be included in the 10% Rainbow royalty base. For multi-parameter devices, the Rainbow royalty base will include the percentage of the revenues based on the number of Rainbow-enabled parameters. Beginning in 2009, for hospital contracts where we place equipment and enter into a sensor contract, we will pay a royalty to Masimo Labs on the total sensor contract revenues based on the ratio of Rainbow enabled devices to total devices.
We are also subject to certain specific annual minimum aggregate royalty payments. These minimum aggregate royalty payments were $481,000 for 2006, $3.2 million for 2007, and $3.5 million for 2008. In 2009 and each year thereafter, the minimum aggregate royalty payments are $4.0 million and $5.0 million, respectively. In addition, in connection with a change in control, as defined in the Cross-Licensing Agreement, the minimum aggregate annual royalties for all licensed Rainbow parameters payable to Masimo Labs will increase to $10.0 million and $15.0 million in 2009 and 2010, respectively, and $15.0 million per year thereafter, and up to $2.0 million per year for each additional Rainbow parameter.
Pursuant to FIN 46(R), Masimo Labs is consolidated within our financial statements for all periods presented. Accordingly, all royalties, option and license fees and other charges between us and Masimo Labs have been eliminated in the consolidation. Also in accordance with FIN 46(R), all direct engineering expenses that have been incurred by us and charged to Masimo Labs have not been eliminated and are included as research and development expense in our consolidated statements of operations. For additional discussion of Masimo Labs, see Note 3 to the Consolidated Financial Statements.
Nellcor Patent Litigation Settlement
In October 1999, we filed a patent infringement lawsuit in the United States District Court for the Central District of California against Mallinckrodt, Inc., now part of Covidien (formerly Tyco Healthcare), and one of its subsidiaries, Nellcor Puritan Bennett, Inc., collectively referred to as Nellcor. Nellcor is one of the largest manufacturers and distributors of pulse oximetry products in the world. The lawsuit was filed for infringement of our pulse oximetry signal processing patents. Nellcor denied our claims and made counterclaims alleging infringement of its patents by us. This lawsuit resulted in a jury verdict that Nellcor had infringed several of our patents, including one of our measure-through motion pulse oximeter patents. In September 2005, the U.S. Federal Court of Appeals ruled that Nellcor infringed several Masimo patents and ordered the lower court to enjoin Nellcor’s infringing products. Prior to the issuance of a permanent injunction, Nellcor entered into a settlement agreement with us on January 17, 2006, under which we agreed to settle all pending patent litigation with Nellcor. In return, Nellcor agreed to pay us $263.0 million for damages incurred through January 2006. We granted Nellcor a covenant not to sue on certain new products and Nellcor agreed to pay us royalties on its total U.S. pulse oximetry revenue at least through March 14, 2011. In addition, in January 2006, Nellcor made an advance royalty payment to us of $67.5 million for estimated sales of its products in the United States during the remainder of calendar 2006. Throughout 2006, we received a total of $330.5 million in cash from Nellcor pursuant to the settlement agreement.
We recorded the $263.0 million lump sum payment as patent litigation proceeds in January 2006 and we recognized approximately $68.8 million of royalty revenue in 2006 based on Nellcor’s total 2006 U.S. pulse oximetry revenue as report to us. We recognize royalty revenue based on the royalty rate per the settlement agreement multiplied by our estimate of Nellcor’s sales for each quarter. This estimate is adjusted prospectively when we receive the Nellcor royalty report, approximately 60 days after the end of each quarter. Per our settlement agreement, the 2007 royalty rate declined significantly from the 2006 rates and declined again in 2008. However, the 2008 rates will remain consistent through March 14, 2011, the remainder of the settlement agreement period, unless Nellcor is able to develop new products that avoid some of our current patent coverage as negotiated in the settlement agreement.
Cash Dividends and Special Bonus Payments
In March 2006, we paid a cash dividend of $3.365 per share, in the aggregate amount of approximately $171.8 million, to holders of our common and preferred stock, assuming the conversion of all outstanding shares of preferred stock into an aggregate of 34,612,503 shares of common stock. Of this amount, $21.7 million relates to dividend payments made to stockholders who exercised stock options by delivering us a promissory note. In accordance with Emerging Issues Task Force, or EITF, 95-16, the $21.7 million in cash dividends have been classified as compensation expense in the accompanying consolidated financial statements, under cost of goods sold, research and development and selling, general and administrative expenses. In February 2007, we paid additional cash dividends of $0.468 per share and $0.257 per share, in the aggregate amount of approximately $37.1 million, to holders of our common and preferred stock assuming conversion into common stock. In March 2006 and March 2007, we also made special bonus payments in the aggregate amount of approximately $9.7 million and $2.0 million, respectively, to our employees and directors who held vested stock options as of March 1, 2006. These cash dividends and special bonus payments were made from the after-tax proceeds that we received from our patent infringement lawsuit against Nellcor and interest earned thereon. We do not intend to distribute any future royalties received from Nellcor under the settlement agreement to our stockholders or our option holders.
48
The following is share-based payment expense associated with the dividend and special bonus payment discussed above, as well as related to implementation of FASB 123(R), and other stock related compensation for the years ended December 31, 2006, December 29, 2007 and January 3, 2009.
| | | | | | | | | | | | |
| | | Functional Expense |
| | | Cost of
Goods Sold | | Research
and
Development | | Selling,
General and
Administrative | | Total |
| | | (in thousands) |
Dividends declared on common shares securing the outstanding non recourse notes | | $ | 308 | | $ | 5,101 | | $ | 16,264 | | $ | 21,673 |
Special bonus payments to holders of vested options to purchase common stock | | | 1,822 | | | 3,990 | | | 5,900 | | | 11,712 |
Stock option compensation pursuant to adoption of SFAS 123(R) | | | 249 | | | 287 | | | 794 | | | 1,330 |
Other | | | — | | | — | | | 355 | | | 355 |
| | | | | | | | | | | | |
For the year ended December 31, 2006 | | $ | 2,379 | | $ | 9,378 | | $ | 23,313 | | $ | 35,070 |
| | | | | | | | | | | | |
Stock option compensation pursuant to adoption of SFAS 123(R) | | $ | 264 | | $ | 670 | | $ | 2,541 | | $ | 3,475 |
Other | | | — | | | — | | | 417 | | | 417 |
| | | | | | | | | | | | |
For the year ended December 29, 2007 | | $ | 264 | | $ | 670 | | $ | 2,958 | | $ | 3,892 |
| | | | | | | | | | | | |
Stock option compensation pursuant to adoption of SFAS 123(R), for the year ended January 3, 2009 | | $ | 257 | | $ | 2,236 | | $ | 5,223 | | $ | 7,716 |
| | | | | | | | | | | | |
Results of Operations
The following tables provide a comparison of our earnings per share calculated under Emerging Issues Task Force Issue No. 03-6, “Participating Securities and the Two-Class Method under FASB Statement No. 128”, or EITF 03-6, and Financial Accounting Standards Board No. 128 “Earnings per Share”, or FASB 128, in accordance with GAAP and the non-GAAP if converted method based upon FASB 128 for the year ended December 29, 2007, as compared to our earnings per share for the year ended January 3, 2009. The non-GAAP if converted method assumes conversion of all shares of our preferred stock into common stock as of December 31, 2006.
Upon the closing of our initial public offering on August 13, 2007, all outstanding shares of our prior Series A through Series G convertible preferred stock were converted into an aggregate of 34,612,503 shares of common stock. Therefore, effective August 13, 2007, we transitioned from computing earnings per share from the two class method in accordance with EITF 03-6 to the if converted method in accordance with FASB 128. Net income for the year ended December 29, 2007 was allocated between the periods during which two classes of equity securities were outstanding and during which a single class of equity securities was outstanding based on the respective number of days. For the year ended December 29, 2007, two classes of equity securities were outstanding for 224 days and a single class of equity securities was outstanding for 139 days, or 61.7% and 38.3% of the total days in the year end reporting period, respectively.
We believe that the following non-GAAP earnings per share information is relevant and useful information that can be used by analysts, investors and other interested parties to assess our performance on a comparable basis to future reported earnings per share. Accordingly, we are disclosing this information to permit additional analysis of our performance (in thousands, except share data):
| | | | | | | | |
| | | Year Ended
January 3, 2009 | | Year Ended December 29, 2007 |
| | | As Reported | | As Reported | | | Non-GAAP |
Net income attributable to common stockholders: | | | | | | | | |
Net income – two class method (1) | | N/A | | $ | 26,075 | | | |
Accretion of preferred stock | | N/A | | | (4,837 | ) | | |
Income attributable to preferred stockholders | | N/A | | | (14,339 | ) | | |
| | | | | | | | |
Net income attributable to common stockholders | | N/A | | $ | 6,899 | | | |
| | | | | | | | |
Basic net income per common share: | | | | | | | | |
Weighted average common shares outstanding – two class method | | N/A | | | 16,654,586 | | | |
| | | | | | | | |
Basic earnings per share for period during which two classes of equity securities were outstanding | | N/A | | $ | 0.41 | | | |
| | | | | | | | |
49
| | | | | | | | | |
Net income for period during which single class of equity securities was outstanding (1) | | $ | 31,927 | | $ | 16,180 | | $ | 42,255 |
| | | | | | | | | |
Weighted average common shares outstanding – single class (2) | | | 56,320,712 | | | 54,660,216 | | | 52,611,674 |
| | | | | | | | | |
Basic net income per share for period during which single class of equity securities was outstanding | | $ | 0.57 | | $ | 0.30 | | | |
| | | | | | | | | |
Basic net income per common share | | $ | 0.57 | | $ | 0.71 | | $ | 0.80 |
| | | | | | | | | |
Diluted net income per common share: | | | | | | | | | |
Weighted average common shares outstanding – two class method | | | N/A | | | 16,654,586 | | | |
Diluted common share equivalent: stock options | | | N/A | | | 4,078,286 | | | |
| | | | | | | | | |
| | | N/A | | | 20,732,872 | | | |
| | | | | | | | | |
Diluted earnings per share for period during which two classes of equity securities were outstanding | | | N/A | | $ | 0.33 | | | |
| | | | | | | | | |
Net income for period during which single class of equity securities was outstanding (1) | | $ | 31,927 | | $ | 16,180 | | $ | 42,255 |
| | | | | | | | | |
Weighted average common shares outstanding – single class (2) | | | 56,320,712 | | | 54,660,216 | | | 52,611,674 |
Diluted common share equivalent: stock options | | | 3,869,623 | | | 5,168,982 | | | 4,615,833 |
| | | | | | | | | |
| | | 60,190,335 | | | 59,829,198 | | | 57,227,507 |
| | | | | | | | | |
Diluted net income per share for period during which single class of equity securities was outstanding | | $ | 0.53 | | $ | 0.27 | | | |
| | | | | | | | | |
Diluted net income per common share | | $ | 0.53 | | $ | 0.60 | | $ | 0.74 |
| | | | | | | | | |
| (1) | Net income for the year ended December 29, 2007 was allocated between the periods during which two classes of equity securities were outstanding and during which a single class of equity securities was outstanding based on the respective number of days. The convertible preferred stock was converted into common stock on August 13, 2007, the closing date of our initial public offering. For the year ended December 29, 2007, two classes of equity securities were outstanding for 224 days and a single class of equity securities was outstanding for 139 days, or 61.7% and 38.3% of the total days in the reporting period, respectively. |
| (2) | Weighted average shares outstanding used to compute basic net income per share after conversion of convertible preferred stock; one class of common shares was outstanding for the period from August 13, 2007 to December 29, 2007. |
50
The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts and as a percentage of revenue. The patent litigation proceeds and the royalty received from Nellcor in 2006 have significantly affected our revenue, results of operations and financial position. Accordingly, our results of operations for the year ended December 31, 2006 are difficult to compare to our results of operations for the years ended January 3, 2009 and December 29, 2007.
| | | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 3, 2009 | | | Year ended
December 29, 2007 | | | Year ended
December 31, 2006 | |
| | | Amount | | | % of
Revenue | | | Amount | | | % of
Revenue | | | Amount | | | % of
Revenue | |
| | | (in thousands, except percentages) | |
Revenue: | | | | | | | | | | | | | | | | | | | | | |
Product | | $ | 258,895 | | | 84.3 | % | | $ | 199,684 | | | 77.9 | % | | $ | 155,131 | | | 69.2 | % |
Royalty and license fee | | | 48,179 | | | 15.7 | | | | 56,602 | | | 22.1 | | | | 69,207 | | | 30.8 | |
| | | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 307,074 | | | 100.0 | | | | 256,286 | | | 100.0 | | | | 224,338 | | | 100.0 | |
Cost of goods sold | | | 89,454 | | | 29.1 | | | | 73,606 | | | 28.7 | | | | 61,640 | | | 27.5 | |
| | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 217,620 | | | 70.9 | | | | 182,680 | | | 71.3 | | | | 162,698 | | | 72.5 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 25,495 | | | 8.3 | | | | 22,960 | | | 9.0 | | | | 24,875 | | | 11.1 | |
Selling, general and administrative | | | 120,069 | | | 39.1 | | | | 91,234 | | | 35.6 | | | | 91,384 | | | 40.7 | |
Patent litigation proceeds | | | — | | | — | | | | — | | | — | | | | (262,605 | ) | | (117.1 | ) |
Antitrust litigation | | | 706 | | | 0.2 | | | | 1,537 | | | 0.6 | | | | 109 | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 146,270 | | | 47.6 | | | | 115,731 | | | 45.2 | | | | (146,237 | ) | | (65.2 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 71,350 | | | 23.3 | | | | 66,949 | | | 26.1 | | | | 308,935 | | | 137.7 | |
Non-operating income (expense): | | | | | | | | | | | | | | | | | | | | | |
Interest income | | | 2,305 | | | 0.8 | | | | 2,361 | | | 0.9 | | | | 6,741 | | | 3.0 | |
Interest expense | | | (753 | ) | | (0.2 | ) | | | (2,475 | ) | | (1.0 | ) | | | (1,824 | ) | | (0.8 | ) |
Other | | | (511 | ) | | (0.2 | ) | | | 1,287 | | | 0.5 | | | | 551 | | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | |
Total non-operating income (expense) | | | 1,041 | | | 0.4 | | | | 1,173 | | | 0.4 | | | | 5,468 | | | 2.4 | |
| | | | | | | | | | | | | | | | | | | | | |
Income before provision for income taxes | �� | | 72,391 | | | 23.6 | | | | 68,122 | | | 26.5 | | | | 314,403 | | | 140.1 | |
Provision for income taxes | | | 40,464 | | | 13.2 | | | | 25,867 | | | 10.0 | | | | 132,577 | | | 59.1 | |
| | | | | | | | | | | | | | | | | | | | | |
Net income | | | 31,927 | | | 10.4 | | | | 42,255 | | | 16.5 | | | | 181,826 | | | 81.0 | |
Preferred stock dividend | | | — | | | — | | | | — | | | — | | | | (77,785 | ) | | (34.7 | ) |
Accretion of preferred stock | | | — | | | — | | | | (4,837 | ) | | (1.9 | ) | | | (7,985 | ) | | (3.6 | ) |
Undistributed income attributable to preferred stockholders | | | — | | | — | | | | (14,339 | ) | | (5.6 | ) | | | (34,275 | ) | | (15.3 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Net income attributable to common stockholders | | $ | 31,927 | | | 10.4 | % | | $ | 23,079 | | | 9.0 | % | | $ | 61,781 | | | 27.4 | % |
| | | | | | | | | | | | | | | | | | | | | |
Comparison of the Year ended January 3, 2009 to the Year ended December 29, 2007
Revenue. Total revenue increased $50.8 million, or 19.8%, to $307.1 million for the year ended January 3, 2009 from $256.3 million for the year ended December 29, 2007.
Product revenue increased $59.2 million, or 29.7%, to $258.9 million in the year ended January 3, 2009, from $199.7 million for the year ended December 29, 2007. This increase was primarily due to an increase in our installed base of pulse oximeter circuit boards and pulse oximeters to 567,000 units at January 3, 2009, from 470,000 units at December 29, 2007. Product revenue generated by our direct and distribution sales channels increased $57.4 million, or 40.0%, to $201.1 million from $143.7 million, while revenues from our OEM channel increased $1.8 million, or 3.2%, to $57.8 million from $56.0 million. Contributing to the year over year increase in product revenues were increased sales of our Rainbow products which rose by 92.0% to $13.4 million in 2008 from $7.0 in 2007.
Our royalty and license fee revenue decreased $8.4 million, or 14.9%, to $48.2 million in 2008 from $56.6 million in 2007, primarily due to the expected decline in the 2008 royalty rate from 15% to 13% under the terms of our settlement agreement with Nellcor.
Cost of Goods Sold.Cost of goods sold increased $15.9 million, or 21.5% to $89.5 million for the year ended January 3, 2009, from $73.6 million for the year ended December 29, 2007. Our gross margin decreased to 70.9% for the year ended January 3, 2009, from 71.3% for the year ended December 29, 2007. This decrease in gross margin was primarily due to the expected $8.4 million decrease in Nellcor royalty revenue. Excluding royalties and license fees, product gross profit margins increased by 2.3% to 65.4% for the year
51
ended January 3, 2009 from 63.1% for the year ended December 29, 2007. This increase was primarily due to the impact of increased Rainbow products revenues and lower manufacturing costs resulting from the efficiencies derived from higher production levels. We incurred $3.5 million and $3.2 million in Masimo Lab’s royalty expenses for the years ended January 3, 2009 and December 29, 2007, respectively, which, in accordance with FIN 46(R), have been eliminated in our consolidated financial results for the periods presented. Had these royalty expenses not been eliminated, our reported product gross profit margin would have been 64.1% and 61.6% for the years ended January 3, 2009 and December 29, 2007, respectively.
Research and Development. Research and development expenses increased $2.5 million, or 11.0%, to $25.5 million for the year ended January 3, 2009, from $23.0 million for the year ended December 29, 2007. This increase was mainly due to $3.1 million of increased payroll and payroll related costs associated with increased research and development staffing levels as total employees rose to 136 at January 3, 2009 from 130 at December 29, 2007. This increase in payroll and payroll related costs were partially offset by a reduction in software costs capitalized of $570,000, net of amortization. Share based compensation expense related to SFAS 123(R), which is included in payroll and payroll related costs, was $2.2 million and $670,000 for the years ended January 3, 2009 and December 29, 2007, respectively. Included in these total research and development expenses are $2.4 million and $1.9 million of engineering expenses incurred by Masimo Labs for the years ended January 3, 2009 and December 29, 2007, respectively.
Selling, General and Administrative. Selling, general and administrative expenses increased $28.9 million, or 31.6%, to $120.1 million for the year ended January 3, 2009, from $91.2 million in the year ended December 29, 2007. This increase was primarily due to increased payroll and related expenses of $14.6 million as a result of an increase in staffing to 415 at January 3, 2009 from 360 at December 29, 2007. Additionally, travel and related expenses increased $4.6 million primarily due to additional sales representatives and their increased travel activities. Also, professional fees increased $3.4 million mainly due to increases in expenses related to the implementation of our new international business structure, Sarbanes-Oxley Act compliance efforts and other activities related to tax compliance, audit and financial reporting. Tradeshow and related marketing expenses rose by $1.8 million due to additional tradeshows during 2008 as compared to 2007, while legal expenses increased $1.2 million related to the Shaklee litigation which concluded in 2008. Share based compensation expense related to SFAS 123(R), which is included in payroll and payroll related costs, was $5.2 million and $3.0 million for the years ended January 3, 2009 and December 29, 2007, respectively. Included in these total selling, general and administrative expenses are $805,000 and $530,000 of activities performed by Masimo Labs for the years ended January 3, 2009 and December 29, 2007, respectively.
Non-operating income. Non-operating income declined to $1.0 million for the year ended January 3, 2009, from $1.2 million for the year ended December 29, 2007. This decline in income was due to the increase in other expense of $1.8 million for the year ended January 3, 2009, which resulted primarily from $1.6 million change in foreign currency transaction gain (loss). The foreign currency loss was $(414,000) during the year ended January 3, 2009, as compared to $1.2 million of currency gain during the year ended December 29, 2007, resulting from the strengthening of the U.S. dollar as compared to the Euro, British pound and Australian dollar for most of 2008. This increase in other expense was partially offset by a decrease in interest expense of $1.7 million, as a result of the payoff of $26.7 million in debt during the year ended January 3, 2009.
Provision for Income Taxes.Our provision for income taxes was $40.5 million for the year ended January 3, 2009, compared to $25.9 million for the year ended December 29, 2007. Our effective tax rate increased to 55.9% in 2008 from 38.0% in 2007. This increase in tax provision and effective tax rate was due primarily to the implementation of a new international business structure, designed to ultimately align our operations, in a cost efficient manner, with the business needs of our non-US customers. The tax charges related to expenses for sharing in the costs of our ongoing research and development efforts as well as the prepayment of licensing commercial rights to utilize pre-existing intangibles. Absent this implementation, our effective tax rate would have been 35.3% for 2008; that rate being reduced as compared to 2007 due primarily to increased research and development related tax credits. Our future effective income tax rate will depend on various factors, including profits (losses) before taxes, changes to tax law, and the geographic composition of pre-tax income.
Comparison of the Year ended December 29, 2007 to the Year ended December 31, 2006
Revenue. Total revenue increased $32.0 million, or 14.2%, to $256.3 million for the year ended December 29, 2007 from $224.3 million for the year ended December 31, 2006.
Product revenue increased $44.6 million, or 28.7%, to $199.7 million in the year ended December 29, 2007, from $155.1 million for the year ended December 31, 2006. This increase was primarily from the result of an increase in our installed base of pulse oximeter circuit boards and pulse oximeters to 470,000 units at December 29, 2007 from 377,000 units at December 31, 2006. Product revenue generated by our direct and distribution sales channels increased $39.7 million, or 38.1%, to $143.7 million from $104.0 million, while revenues from our OEM channel increased $4.9 million, or 9.6%, to $56.0 million from $51.1 million. Contributing to the year over year increase in product revenues were increased sales of our Rainbow products which rose by 89.2% to $7.0 million in 2007 from $3.7 million in 2006.
52
Our royalty and license fee revenue decreased $12.6 million, or 18.2%, to $56.6 million in 2007 from $69.2 million in 2006, primarily due to a decline in the 2007 royalty rate under the terms of our settlement agreement with Nellcor.
Cost of Goods Sold.Cost of goods sold increased $12.0 million, or 19.4% to $73.6 million for the year ended December 29, 2007, from $61.6 million for the year ended December 31, 2006. Our gross margin decreased to 71.3% for the year ended December 29, 2007, from 72.5% for the year ended December 31, 2006. This decrease in gross margin was due to the expected decrease in Nellcor royalty revenue of $12.7 million, although partially offset by $2.1 million of special bonus payments in 2006. Notwithstanding the Nellcor royalty and special bonus payments, the gross margin would have increased by 1.5%. During 2007, our product gross margins increased to 63.1% from 61.6% in 2006, after adjusting for the special bonus payments. This increase was primarily due to the impact of increased sales of our Rainbow products and lower manufacturing costs resulting from the efficiencies derived from higher production levels. We incurred $3.2 million and $481,000 in Masimo Lab’s royalty expenses for the years ended December 29, 2007 and December 31, 2006, respectively, which, in accordance with FIN 46(R), have been eliminated in our consolidated financial results for the periods presented. Had these royalty expenses not been eliminated, our reported product gross profit margin would have been 61.6% and 61.3% for the years ended December 29, 2007 and December 31, 2006, respectively.
Research and Development. Research and development expenses decreased $1.9 million, or 7.7%, to $23.0 million for the year ended December 29, 2007, from $24.9 million for the year ended December 31, 2006. The 2006 amount included a charge of $9.1 million in special bonus payments. Notwithstanding that charge, research and development expenses increased $7.2 million, mainly due to $5.3 million of increased payroll and payroll related costs associated with increased research and development staffing levels. Research and development staffing increased to 130 at December 29, 2007 from 98 at December 31, 2006. In addition, engineering supplies expense increased $1.3 million, due to additional project development activities. Included in these total research and development expenses are $1.9 million and $3.4 million of engineering expenses incurred by Masimo Labs for the years ended December 29, 2007 and December 31, 2006, respectively. Share based compensation expense related to SFAS 123(R) which is included in payroll and payroll related costs, was $670,000 and $287,000 for the years ended December 29, 2007 and December 31, 2006, respectively.
Selling, General and Administrative. Selling, general and administrative expenses decreased $0.2 million, or 0.2%, to $91.2 million for the year ended December 29, 2007, from $91.4 million in the year ended December 31, 2006. The 2006 amount included a charge of $22.2 million in special bonus payments. Notwithstanding that charge, selling, general and administrative expenses increased $22.0 million, primarily due to increased payroll and related expenses of $11.9 million, increased marketing and related expenses of $6.9 million, increased professional fees of $1.4 million and $1.8 million of other expense. The primary reason for the increased payroll and related expenses was an increase in selling, general and administrative staffing to 360 at December 29, 2007 from 292 at December 31, 2006. Increased marketing and related expenses resulted from additional trade show expense of $1.4 million, higher promotional and sample materials of $1.4 million, increased customer training activities of $1.3 million and increased group purchasing organization fees of $1.2 million. Increased professional fees related to increased litigation costs, tax planning and audit work, as well as consulting to document compliance with the Sarbanes-Oxley Act. Included in these total selling, general and administrative expenses are $530,000 and $147,000 of activities performed by Masimo Labs for the years ended December 29, 2007 and December 31, 2006, respectively. Share based compensation expense related to SFAS 123(R) which is included in payroll and payroll related costs, was $2.5 million and $794,000 for the years ended December 29, 2007 and December 31, 2006, respectively.
Non-operating income. Non-operating income was $1.2 million for the year ended December 29, 2007, compared to $5.5 million for the year ended December 31, 2006. This change was primarily due to the decrease in interest income of $4.4 million resulting from lower overall 2007 cash levels as compared to 2006. Average cash levels throughout 2006 were higher than 2007 due to the investment of patent litigation settlement proceeds and prepaid royalties received in January 2006. In addition, other income was $1.3 million for the year ended December 29, 2007, resulting primarily from foreign currency transaction gains relating to the impact of the strength in the Euro and British Pound as compared to the US dollar for most of 2007.
Provision for Income Taxes.Our provision for income taxes was $25.9 million for the year ended December 29, 2007, compared to $132.6 million for the year ended December 31, 2006. This decline in provision for income taxes was due primarily to the decline in our 2007 taxable income as a result of the one-time gain from our patent litigation settlement in 2006. Our effective tax rate declined to 38.0% in 2007 from 42.2% in 2006. This decline in rate was due to the non-deductibility of dividends classified as compensation expense in 2006, which we did not incur in 2007, a reduction of foreign losses not benefited in the US and a reduction in state tax obligations.
Liquidity and Capital Resources
Since our inception, we have financed our operations primarily through the sale of equity securities. Through January 3, 2009, we raised $81.7 million through seven preferred stock private equity financings, approximately $47.8 million from our August 2007 initial public offering and $26.6 million from the exercise of stock options, of which $10.1 million was from exercises after our initial public offering.
53
As of January 3, 2009, we had cash and cash equivalents of $146.9 million, of which $125.5 million was invested in U.S. Treasury bills, $13.6 million was in money market accounts with major financial institutions and $7.8 million was in checking accounts and certificates of deposit. These U.S. Treasury bills are classified as cash equivalents since they are highly liquid investments, with a maturity of three months or less at the date of purchase. We carry cash equivalents at cost which approximates fair value.
Under the terms of our patent litigation settlement with Nellcor, Nellcor paid us $263.0 million for damages incurred through January 2006 and made an advance royalty payment to us of $67.5 million related to sales of Nellcor’s products for the remainder of 2006. In total, we have received $330.5 million in cash from Nellcor through December 2006. In March 2006 and February 2007, we declared dividends in the aggregate amount of approximately $208.9 million to holders of our common and preferred stock. In addition, in March 2006 and March 2007, we made special bonus payments in the aggregate amount of approximately $11.7 million to our employees and directors who held vested stock options as of March 1, 2006. The majority of these cash dividends and special bonus payments were made from the after-tax proceeds that we received from our settlement with Nellcor and interest earned thereon. In the future, we do not intend to distribute any royalties received from Nellcor under the settlement agreement to our stockholders or our option holders. Additionally, in 2008 and 2007, we received $49.9 million and $43.5 million, respectively, in cash receipts from Nellcor for royalties pursuant to our settlement agreement. The increase in cash received was due to the 2006 prepayment of a portion of the royalties actually earned in 2007. For further details on the litigation settlement, see “Business—Nellcor Patent Litigation Settlement.”
Cash Flows from Operating Activities. Cash provided by operating activities was $78.2 million in 2008. This consists primarily of our net income of $31.9 million, resulting from continued growth and profitability of our business. Income tax benefit from the exercise of stock options was $17.2 million, resulting from significant stock option exercises during 2008. Income taxes payable increased by $12.9 million as the result of increased operating income and a higher effective tax rate in 2008 primarily due to the implementation of a new international business structure. Additionally, share based compensation was $7.7 million and depreciation and amortization was $5.7 million in 2008. Also, other liabilities increased $4.1 million due to increased unrecognized tax liability. These increases to cash flow from operating activities were offset by an increase of $6.2 million in accounts receivable due to business growth which was partially offset by continued improvement in the timing of cash receipts and a $5.6 million increase in inventory pursuant to our policy of having sufficient inventory to meet customer demand.
Cash provided by operating activities was $28.8 million in 2007. This consisted primarily of our net income of $42.3 million, resulting from overall growth and profitability of our business. In addition, depreciation and amortization increased to $5.3 million in 2007 due to the purchase of additional fixed assets and deferred revenue increased $2.8 million in 2007 due to continued growth of our business. These increases to cash flow from operating activities were offset by an increase in royalties receivable of $12.6 million, related to the timing of Nellcor royalty payments which had been prepaid in 2006, but beginning in 2007 are paid approximately 60 days after the end of each quarter. Additional offsets included a $7.0 million increase in inventory pursuant to our policy of having sufficient inventory to meet customer demand, a $4.3 million increase in deferred cost of goods consistent with the increase in equipment placed at hospitals under long-term sensor purchase agreements, and a $4.2 million increase in accounts receivable due to business growth.
Cash Flows from Investing Activities. Cash used in investing activities for 2008 was $9.4 million primarily consisting of $6.9 million in purchases of property and equipment and $2.5 for the increase in intangible assets relating primarily to capitalized legal expenses associated with our patent related activities. The property and equipment purchases included manufacturing equipment and tools of $3.7 million, leasehold improvements of $1.2 million and $1.0 million for additional construction in process, all in order to support the growth of the business.
Cash used in investing activities for 2007 was $7.2 million primarily consisting of $5.3 million in purchases of property and equipment and $1.6 for the increase in intangible assets relating primarily to capitalized legal expenses associated with patent related activities. The property and equipment purchases included manufacturing equipment of $1.7 million and computer hardware and software of $1.6 million, both in order to support the growth of the business.
Cash Flows from Financing Activities. Cash used by financing activities in 2008 was $18.8 million. This primarily consists of debt repayments of $30.4 million throughout 2008, including $26.7 million, or the entire then outstanding balance on one financing arrangement. This was partially offset by proceeds from issuance of common stock of $9.8 million and $1.9 million of excess tax benefit from share based payment arrangements, due to exercises of stock options in 2008. Unless we obtain additional third party financing, we anticipate that our current debt payments will be minimal in the future.
Cash provided by financing activities in 2007 was $20.8 million. This primarily consists of net proceeds from the initial public offering of $47.8 million and $20.1 million in proceeds from long-term debt borrowings resulting from financing equipment placed at hospitals under long term sensor purchase agreements. These sources of funds were partially offset by $37.4 million of dividends paid and debt repayments throughout 2007 of $10.2 million.
Future Liquidity Needs. In the future, in addition to funding our working capital requirements, we anticipate our primary use of cash to be the equipment that we provide to hospitals under our long-term sensor purchase agreements. We anticipate additional capital
54
purchases related to expanding our worldwide international operations including manufacturing, sales, marketing and other areas of necessary infrastructure growth. We also expect to continue to invest in productivity enhancing tools, primarily within our manufacturing and information technology organizations. Our focus on international expansion will also require both continuing and incremental investments in facilities and infrastructure in the Americas, Europe and Asia. The amount and timing of our actual investing activities will vary significantly depending on numerous factors, such as the progress of our product development efforts, our timetable for international sales operations and manufacturing expansion and both domestic and international regulatory requirements. Despite these capital investment requirements, we anticipate that our existing cash and cash equivalents will be sufficient to meet our working capital requirements, capital expenditures, and operations for at least the next 12 months.
Current Financing Arrangements. As of December 29, 2007, we had two long term borrowings that allowed for the financing of the equipment placed with hospitals in connection with the related long-term sensor purchase agreements. In March 2008, we repaid $26.7 million, or the entire then outstanding balance, on one of the arrangements. This is a non-recurring event and is not expected to occur in the future. In addition, we paid $168,000 in prepayment fees which are included in other non-operating income (expense). Therefore, as of January 3, 2009, we had only one remaining financing arrangement with an outstanding balance of $395,000. As of January 3, 2009, the total monthly principal and interest payment under this remaining financing agreement was $52,000 based on an average interest rate of 7.0%. There are no additional amounts available for future borrowing, under this remaining arrangement.
Contractual Obligations. The following table summarizes our outstanding contractual obligations as of January 3, 2009, and the effect those obligations are expected to have on our cash liquidity and cash flow in future periods:
| | | | | | | | | | | | | | | |
| | | Payments Due By Period |
| | | Less than
1 year | | 1-3
years | | 3-5 years | | More than
5 years | | Total |
| | | (in thousands) |
Long-Term Debt(1) | | $ | 395 | | $ | — | | $ | — | | $ | — | | $ | 395 |
Operating Leases(2) | | | 2,833 | | | 1,790 | | | 1,280 | | | 353 | | | 6,256 |
Purchase Commitments(3) | | | 21,749 | | | 1,656 | | | — | | | — | | | 23,405 |
Capital Leases (including interest)(4) | | | 82 | | | 128 | | | 41 | | | — | | | 251 |
Interpretation 48 Obligations including interest and penalties(5) | | | 30 | | | 2,338 | | | 4,863 | | | 67 | | | 7,298 |
| | | | | | | | | | | | | | | |
Total Contractual Obligations | | $ | 25,089 | | $ | 5,912 | | $ | 6,184 | | $ | 420 | | $ | 37,605 |
| | | | | | | | | | | | | | | |
(1) | Principal and interest payments owed on our equipment financing arrangements. |
(2) | Facility, equipment and automobile leases. |
(3) | Certain inventory items under non cancellable purchase orders to secure better pricing and ensure we will have materials on hand. |
(4) | Leased office equipment. |
(5) | FIN 48 obligations shown in the table above represent uncertain tax positions related to temporary differences. The years for which the temporary differences related to the uncertain tax positions will reverse have been estimated in scheduling the obligations within the table. In addition to the Interpretation 48 obligations in the table above, approximately $7.3 million of unrecognized tax benefits have been recorded as liabilities in accordance with Interpretation 48, and we are uncertain as to if or when such amounts may be settled. Related to the unrecognized tax benefits not included in the table above, we have also recorded a liability for potential interest of $446,000. |
In addition to these contractual obligations, we have the following annual minimum royalty commitments to Masimo Labs, as of January 3, 2009:
| | | | | | | | | | | | |
| | | Payments Due By Period | |
| | | Less than
1 Year | | 1-3
Years | | 3-5 Years | | More than
5 years | |
| | | (in thousands) | |
Minimum royalty commitment to Masimo Labs | | $ | 4,000 | | $ | 10,000 | | $ | 10,000 | | (1 | ) |
(1) | Subsequent to 2013, the royalty agreement requires a $5.0 million minimum annual royalty payment unless the agreement is amended, restated or terminated. |
Pursuant to FIN 46(R), Masimo Labs is consolidated within our financial statements for all periods presented. Accordingly, all inter-company royalties, option and license fees and other charges between us and Masimo Labs have been eliminated in the consolidation. Also in accordance with FIN 46(R), all direct engineering expenses that have been incurred by us and charged to Masimo Labs have not been eliminated and are included as research and development expense in our consolidated statements of operations. For additional discussion of Masimo Labs, see Note 3 to the Consolidated Financial Statements.
55
For the foreseeable future, we anticipate that we will continue to be required by FIN 46(R) to consolidate Masimo Labs; however, in the event that Masimo Labs secures additional external financing and/or expands its customer base or is no longer financially dependent upon us and we are no longer the primary beneficiary of Masimo Labs activities, we may be able to discontinue consolidating Masimo Labs.
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts. As a result, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these relationships.
Critical Accounting Estimates
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses for each reporting period. Management regularly evaluates its estimates and assumptions. These estimates and assumptions are based on historical experience and on various other factors that are believed to be reasonable under the circumstances, and form the basis for making management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain.
Inventory/Reserves for Excess or Obsolete Inventory
Inventories are stated at the lower of cost or market. Cost is determined using a standard cost method, which approximates FIFO (first-in, first-out). Inventory valuation allowances are recorded for materials that have become obsolete or are no longer used in current production and for inventory that has a market value less than the carrying value in inventory. We generally purchase raw materials in quantities that we anticipate will be fully used within one year. However, changes in operating strategy and customer demand, and frequent unpredictable fluctuations in market values for such materials can limit our ability to effectively utilize all of the raw materials purchased and sold through resulting finished goods to customers for a profit. We regularly monitor potential inventory excess, obsolescence and lower market values compared to standard costs and, when necessary, reduce the carrying amount of our inventory to its market value. Specific reserves are maintained to reduce the carrying value of inventory items on hand that we know may not be used in finished goods. A general inventory reserve is also maintained based on our estimate of future limitations on our ability to utilize the inventory on hand. Our inventory reserves were $5.2 million and $4.1 million at January 3, 2009 and December 29, 2007, respectively. If our estimates for potential inventory losses are low, our earnings will be affected.
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. This allowance is used to state trade receivables at a net estimated realizable value. We rely on prior experience to estimate the amount that we expect to collect on the gross receivables outstanding, which cannot be known with exact certainty as of the time of issuance of this report. We maintain a specific allowance for customer accounts that we know may not be collectible due to customer liquidity issues. We also maintain a general allowance for future collection losses that arise from customer accounts that do not indicate an inability, but may be unable, to pay. Although such losses have historically been within our expectations and the allowances we have established, we cannot guarantee that we will continue to experience the same loss rates that we have in the past, especially given the recent deterioration of the credit markets of the worldwide economy. A significant change in the liquidity or financial condition of our customers could cause unfavorable trends in our receivable collections and additional allowances may be required. Our accounts receivable balance, including those from related parties was $30.7 million and $27.0 million, net of allowances for doubtful accounts of $1.3 million and $1.4 million at January 3, 2009 and December 29, 2007, respectively. The allowance for doubtful accounts declined due to an improvement in the aging of customer receivables at the end of 2008 as compared to the end of 2007.
Share-Based Payment
We have historically issued stock options to reward our employees and directors. Prior to December 31, 2005, we accounted for these option grants under the recognition and measurement principles of Accounting Principles Board, or APB, Opinion No. 25,“Accounting for Stock Issued to Employees,” and related interpretations, and applied the disclosure provisions of Statement of Financial Accounting Standards, or SFAS, No. 123,“Accounting for Stock-Based Compensation,”as amended by SFAS No. 148,“Accounting for Stock-Based Compensation—Transition and Disclosure—an amendment of Financial Accounting Standards Board, or FASB, Statement No. 123.” This accounting treatment resulted in a pro forma stock option expense that was estimated and reported in the footnotes to our consolidated financial statements for those years.
56
For option grants made on or prior to December 31, 2005, we recorded stock-based compensation, typically associated with options granted to non-employees or directors based upon the difference, if any, between the estimated fair value of common stock underlying the options on the date of grant and the option exercise price. The fair value of the common stock for options granted prior to December 31, 2004 was originally estimated solely by our board of directors, with input from management. We believe the members of our board of directors have extensive experience in the medical device market and many of our directors were accredited venture capital investors. For grants made prior to December 31, 2004, we did not obtain contemporaneous valuations by an unrelated valuation specialist. Since there was no public market for our shares, our board of directors exercised judgment in determining the estimated fair value of our common stock on the date of grant based on several objective and subjective factors, including our operating and financial performance, corporate milestones, product development and market acceptance, the superior rights and preferences of our convertible preferred stock and the risk and non-liquid nature of our common stock. For grants made after December 31, 2004 and prior to our initial public offering, our board of directors also relied on valuations performed by an unrelated valuation specialist.
Effective January 1, 2006, we adopted the provisions of SFAS No. 123(R), “Share Based Payment” using the prospective method, which requires us to expense the estimated fair value of employee stock options and similar awards based on the fair value of the award on the date of grant. In March 2005, the SEC issued Staff Accounting Bulletin 107, or SAB 107, relating to SFAS No. 123(R). We have applied the provisions of SAB 107 in the adoption of SFAS No. 123(R) for 2006 and 2007. In December 2007, the SEC issued Staff Accounting Bulletin 110, or SAB 110, relating to SFAS No. 123(R). Beginning in January 2008, we applied the provisions of SAB 110.
Effective January 1, 2006, the fair value of each option is estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions used for grants:
| | | | | | |
| | | Year ended
January 3, 2009 | | Year ended
December 29, 2007 | | Year ended
December 31, 2006 |
Risk-free interest rate | | 1.1% to 3.8% | | 3.4% to 4.8% | | 4.4% to 4.9% |
Expected term | | 5.3 years to 6.5 years | | 6.5 years | | 6.5 years |
Estimated volatility | | 36.6% to 53.4% | | 36.7% to 41.6% | | 44.8% to 48.6% |
Expected dividends | | 0% | | 0% | | 0% |
The Black-Scholes option pricing model requires the use of certain assumptions, including fair value, expected term, expected volatility, expected dividends, risk-free interest rate, and expected pre-vesting forfeiture rate to calculate the fair value of share-based payment awards.
The risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues with a remaining term approximately equal to the expected life of our stock options. The estimated pre-vesting forfeiture rate is based on our historical experience and the composition of option plan participants, among other factors, and reduces the compensation expense recognized. If the actual forfeitures differ from the estimates, adjustments to compensation expense may be required in future periods.
During the year ended December 29, 2007 and the nine months ended September 27, 2008, we did not have sufficient information available which was indicative of future exercise and post-vesting behavior to estimate the expected term. As a result, we adopted the simplified method of estimating the expected term of a stock option, as permitted by SAB 107. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term. The use of the simplified method requires our option plan to be consistent with a “plain vanilla” plan. Subsequent to September 27, 2008, we had sufficient Company specific and available external information to estimate our expected term and therefore we did not rely on the simplified method. As we obtain more historical data as a publicly traded company, we expect to rely increasingly on Company specific information for our estimate of expected term.
Additionally, during the year ended December 29, 2007 and the nine months ended September 27, 2008, we did not have sufficient information available regarding the historic volatility for our shares. As a result, we estimated volatility based on a peer group of companies, which collectively provides a reasonable basis for estimating volatility. Subsequent to September 27, 2008 and after we had been publicly traded for more than one year, we were able to use Company specific as well as peer group information to estimate the volatility of our shares. As we obtain more historical data as a publicly traded company, we expect to rely increasingly on Company specific information for our estimate of volatility.
We do not expect to declare dividends in the future. As part of a one-time patent settlement, our board of directors declared a dividend in March 2006 and declared two dividends in December 2006. These dividends were declared only due to the receipt of settlement proceeds in connection with patent infringement litigation with Nellcor. Absent this settlement and the corresponding receipt of settlement proceeds, we would not have declared and paid any of these dividends.
57
Share-based payment expense related to the adoption of SFAS 123(R) amounted to $7.7 million for the year ended January 3, 2009 and $3.5 million for the year ended December 29, 2007. We expect share-based payment expense to increase significantly in future years as we intend to continue to grant stock options to both new and existing employees and directors consistent with our stock option policy. The fair market value of our common stock may also increase the cost of future stock option grants in the future. To the extent that the fair market value of our common stock increases, the overall cost of granting these options will also increase. For further details regarding our share based compensation see Note 11 of our consolidated financial statements.
Accounting for Income Taxes
As part of the process of preparing our combined consolidated financial statements, we are required to determine our income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax expenses together with assessing temporary differences resulting from recognition of items for income tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe that recovery is not likely, establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must reflect this increase as an expense within the tax provision in the statement of operations.
Management’s judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We continue to monitor the realizability of our deferred tax assets and adjust the valuation allowance accordingly. At January 3, 2009, we have $33.4 million of net operating loss carryforwards from our foreign jurisdictions which begin to expire in 2015 and $8.9 million of net operating losses from various states, which begin to expire in 2012. We believe that it is more likely than not that the deferred tax assets related to foreign and state net operating losses will not be realized. A valuation allowance has been provided on such loss carryforwards.
Under FIN 46(R), our consolidated income tax provision or benefit and the net deferred tax assets include Masimo Labs’ income taxes provision or benefit and deferred tax assets. For income tax purposes, Masimo Labs is not a member of our consolidated group and files its separate federal and California income tax returns.
In July 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109,” or FIN 48, which became effective on January 1, 2007. FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. The adoption of FIN 48 resulted in a reduction of our beginning retained earnings as of January 1, 2007 of $618,000. As of January 3, 2009 and December 29, 2007, the balance of gross unrecognized tax benefits was $7.3 million and $3.3 million, respectively. The amount of unrecognized benefits which, if ultimately recognized, could favorably affect the tax rate in a future period was $6.1 million and $2.2 million as of January 3, 2009 and December 29, 2007, respectively. Both amounts are net of any federal and/or state benefits and the remaining balance relates to timing differences. It is reasonably possible that the amount of unrecognized tax benefits will decrease in the next 12 months by $30,000 primarily related to certain state taxes.
Interest and penalties related to unrecognized tax benefits are recognized in income tax expense. At January 3, 2009 we had accrued $446,000 for the payment of interest (net of tax benefits).
We conduct business in multiple jurisdictions, and as a result, one or more of our subsidiaries files income tax returns in the U.S. federal, various state, local and foreign jurisdictions. Due to the utilization of net operating loss carryforwards, all years since 1994 are open for examination by major taxing authorities.
Recent Accounting Pronouncements
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 141(R), or SFAS 141(R),“Business Combinations”. This Statement provides greater consistency in the accounting and financial reporting of business combinations. It requires the acquiring entity in a business combination to record all assets acquired and liabilities assumed at their respective acquisition-date fair values and changes other practices under SFAS 141. SFAS 141(R) also requires additional disclosure of information surrounding a business combination, such that users of the entity’s financial statements can fully understand the nature and financial impact of the business combination. SFAS 141(R) is effective for fiscal years beginning after December 15, 2008. We do not expect the adoption of this statement to have a material impact on our consolidated financial statements.
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 160, or SFAS 160, “Noncontrolling Interests in Consolidated Financial Statements”. This Statement amends Accounting Research Bulletin No. 51, “Consolidated Financial Statements”, to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. SFAS 160 is effective for fiscal years beginning after December 15, 2008. We do not expect the adoption of this statement to have a material impact on our consolidated financial statements.
58
In April 2008, the FASB issued FASB Staff Position No. FAS 142-3, or FSP 142-3, “Determination of the Useful Life of Intangible Assets”, that amends the factors considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets”. FSP 142-3 requires a consistent approach between the useful life of a recognized intangible asset under SFAS No. 142 and the period of expected cash flows used to measure the fair value of an asset under SFAS No. 141 (R), “Business Combinations”. The FSP also requires enhanced disclosures when an intangible asset’s expected future cash flows are affected by an entity’s intent and/or ability to renew or extend the arrangement. FSP No. 142-3 is effective for fiscal years beginning after December 15, 2008 and is applied prospectively. We do not expect the adoption of this statement to have a material impact on our consolidated financial statements.
In November 2008, the FASB ratified EITF Issue No. 08-7, or EITF 08-7, “Accounting for Defensive Intangible Assets”. EITF 08-7 applies to defensive intangible assets, which are acquired intangible assets that the acquirer does not intend to actively use but intends to hold to prevent its competitors from obtaining access to them. As these assets are separately identifiable, EITF 08-7 requires an acquiring entity to account for defensive intangible assets as a separate unit of accounting. Defensive intangible assets must be recognized at fair value in accordance with SFAS 141(R) and SFAS 157, “Fair Value Measurements.” EITF 08-7 is effective for defensive intangible assets acquired in fiscal years beginning on or after December 15, 2008. We do not expect the adoption of this statement to have a material impact on our consolidated financial statements.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to various market risks that may arise from adverse changes in market rates and prices, such as interest rates, foreign exchange fluctuations and inflation. We do not enter into derivatives or other financial instruments for trading or speculative purposes.
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates to the increase or decrease in the amount of interest income we can earn on our investment portfolio and on the increase or decrease in the amount of interest expense we must pay with respect to our various outstanding debt instruments. Our risk associated with fluctuation to interest expense is limited to our outstanding financing arrangements, which have fixed interest rates. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. We ensure the safety and preservation of our invested principal funds by limiting default risk, market risk and reinvestment risk. We reduce default risk by investing in investment grade securities. A hypothetical 100 basis point drop in interest rates along the entire interest rate yield curve would not significantly affect the fair value of our interest-sensitive financial instruments at January 3, 2009. Declines in interest rates over time will, however, reduce our interest income and expense while increases in interest rates will increase our interest income and expense.
Foreign Currency Exchange Rate Risk
A majority of our assets and liabilities are maintained in the United States in U.S. dollars and a majority of our sales and expenditures are transacted in U.S. dollars. However, certain of our foreign subsidiaries transact in their respective country’s local currency, which is also their functional currency. As a result, revenues and expenses of these foreign subsidiaries when converted into US dollars can vary depending on average monthly exchange rates during a respective period. Intercompany transactions of certain of our foreign subsidiaries with Masimo Corporation are denominated in U.S. dollars and are considered foreign currency denominated transactions by the subsidiary. In addition, any other transactions between Masimo Corporation or its subsidiaries and a third party, denominated in a currency different from the functional currency, is a foreign currency transaction. Realized and unrealized foreign currency gains or losses on these transactions are included in our statements of income as incurred and are converted to U.S. dollars at average exchange rates for a respective period.
The balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar are translated into U.S. dollars at the rate of exchange at the balance sheet date and the statements of income and cash flows are translated into U.S. dollars using the average monthly exchange rate during the period. Any foreign exchange gain or loss as a result of translating the balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar is included in equity as a component of accumulated other comprehensive income (loss).
Our primary foreign currency exchange rate exposures are with the Euro, the Japanese yen, the Canadian dollar and the Australian dollar against the U.S. dollar. We currently do not enter into forward exchange contracts to hedge exposures denominated in foreign currencies and do not use derivative financial instruments for trading or speculative purposes. The effect of an immediate 10% change in foreign currency exchange rates could have a material effect on our future operating results or cash flows, depending on which foreign currency exchange rates change and depending on the directional change (either a strengthening or weakening against the U.S. dollar). As our foreign operations continue to grow, our exposure to foreign currency exchange rate risk may become more significant.
59
Inflation Risk
We do not believe that inflation has had a material effect on our business, financial condition or results of operations during the periods presented, and we do not anticipate that it will have a material adverse effect in the future.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Our consolidated financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15(a)(1) and 15(a)(2), respectively.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) promulgated under the Exchange Act, as of the end of the period covered by this Annual Report. We recognize that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) promulgated by the SEC under the Exchange Act. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework inInternal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of January 3, 2009.
Grant Thornton LLP, the independent registered public accounting firm that audited the financial statements included in this Form 10-K, has issued an attestation report on the effectiveness of our internal control over financial reporting as of January 3, 2009. This report, which expresses an unqualified opinion on the effectiveness of our internal control over financial reporting as of January 3, 2009, is included herein.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended January 3, 2009 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item concerning our directors, compliance with Section 16 of the Exchange Act and our code of ethics that applies to our principal executive officer, principal financial officer and principal accounting officer is incorporated by reference from the information set forth in the sections under the headings “Election of Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Election of Directors—Information Regarding the Board of Directors and Corporate Governance” in our Definitive Proxy Statement to be filed with the SEC in connection with the Annual Meeting of Stockholders to be held in 2009, or the 2009 Proxy Statement.
Information regarding our executive officers is set forth in Item 1— “Business” of this Form 10-K under the caption “Executive Officers of the Registrant.”
60
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is incorporated by reference from the information in the 2009 Proxy Statement under the heading “Compensation of Executive Officers.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item is incorporated by reference from the information in the 2009 Proxy Statement under the headings “Equity Compensation Plan Information” and “Security Ownership of Certain Beneficial Owners and Management.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this item is incorporated by reference from the information in the 2009 Proxy Statement under the headings “Transactions with Related Persons” and “Election of Directors—Information Regarding the Board of Directors and Corporate Governance.”
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item is incorporated by reference from the information in the 2009 Proxy Statement under the heading “Ratification of Selection of Independent Auditors—Principal Accountant Fees and Services.”
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1) Financial Statements
The Consolidated Financial Statements of Masimo Corporation and Report of Grant Thornton LLP, Independent Registered Public Accounting Firm, are included in a separate section of this Form 10-K beginning on page F-1.
(a)(2) Financial Statement Schedules
Schedules not listed above have been omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Consolidated Financial Statements or the Notes thereto.
(a)(3) Exhibits
| | |
Exhibit
Number | | Description of Document |
| 2.1(1)† | | Asset Purchase Agreement, dated December 21, 2005, between the Registrant, Masimo Canada ULC and Andromed Inc. (Exhibit 2.1) |
| |
| 2.1(a)(1) | | List briefly identifying the contents of schedules omitted from Exhibit 2.1 (Exhibit 2.1(a)) |
| |
| 3.1(1) | | Amended and Restated Certificate of Incorporation (Exhibit 3.2) |
| |
| 3.2(2) | | Certificate of Designation of Series A Junior Participating Preferred Stock (Exhibit 3.1) |
| |
| 3.3(7) | | Amended and Restated Bylaws adopted on October 9, 2008 (Exhibit 3.1) |
| |
| 4.1(1) | | Form of Common Stock Certificate (Exhibit 4.1) |
| |
| 4.2(1) | | Fifth Amended and Restated Registration Rights Agreement made and entered into as of September 14, 1999 between the Registrant and certain of its stockholders (Exhibit 4.2) |
| |
| 4.3(2) | | Rights Agreement, dated November 9, 2007, between the Company and Computershare Trust Company, N.A., as Rights Agent (Exhibit 4.1) |
| |
| 4.4(4)# | | Masimo Retirement Savings Plan (Exhibit 4.7) |
| |
| 10.1(1)# | | Form of Indemnity Agreement between the Registrant and its officers and directors (Exhibit 10.1) |
| |
| 10.2(1)# | | Employment Agreement, dated July 19, 2007, between Joe E. Kiani and the Registrant (Exhibit 10.2) |
| |
| 10.3*# | | Amendment No. 1 to Employment Agreement, dated December 31, 2008, between Joe E. Kiani and the Registrant |
| |
| 10.4(1)# | | Offer Letter, dated March 31, 1995, between Ammar Al-Ali and the Registrant (Exhibit 10.4) |
61
| | |
Exhibit
Number | | Description of Document |
| 10.5(1)# | | Offer Letter, dated February 15, 1996, between Yongsam Lee and the Registrant (Exhibit 10.7) |
| |
| 10.6(1)# | | Offer Letter, dated March 30, 2007, between Anand Sampath and the Registrant (Exhibit 10.8) |
| |
| 10.7(1)# | | Offer Letter, dated June 9, 2006, between Mark P. de Raad and the Registrant (Exhibit 10.9) |
| |
| 10.8*# | | Offer Letter, dated June 27, 2008, between Stephen M. Moran and the Registrant |
| |
| 10.9*# | | Offer Letter, dated July 23, 2008, between Jon C. Coleman and the Registrant |
| |
| 10.10*# | | Offer Letter, dated September 12, 2008, between David Goodman and the Registrant |
| |
| 10.11*# | | Offer Letter, dated December 27, 2007, between Paul Jansen and the Registrant |
| |
| 10.12*# | | Offer Letter, dated December 19, 2007, between Michael O’Reilly and the Registrant |
| |
| 10.13*# | | Offer Letter, dated May 21, 2004, between Rick Fishel and the Registrant |
| |
| 10.14(5)+ | | Purchasing Agreement, effective as of February 1, 2008, between HealthTrust Purchasing Group, L.P. and the Registrant (Exhibit 10.3) |
| |
| 10.15(1) | | Manufacturing and Purchase Agreement, dated August 19, 2005, between Dowa Mining Co., Ltd. and the Registrant (Exhibit 10.10) |
| |
| 10.16(1)+ | | Shelter Labor Services Agreement, dated December 27, 2000, between Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant (Exhibit 10.11) |
| |
| 10.17(6)+ | | Lease Agreement effective as of September 1, 2007, by and among Industrias Asociadas Maquiladoras, S.A. de C.V., Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant, as guarantor (Exhibit 10.1) |
| |
| 10.18(1)+ | | Purchase Agreement, dated July 26, 2001, between Jabil Circuit, Inc. and the Registrant (Exhibit 10.15) |
| |
| 10.19(1) | | Contribution and Assignment Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.16) |
| |
| 10.20(1) | | ADSP-2136X Sharc ROM Agreement, dated July 19, 2004, between Analog Devices Inc. and the Registrant (Exhibit 10.36) |
| |
| 10.21*++ | | Manufacturing and Purchase Agreement, dated October 2, 2008, by and between Analog Devices, Inc. and the Registrant |
| |
| 10.22(1) | | Sales and Distribution Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.17) |
| |
| 10.23(1) | | Occupancy Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.18) |
| |
| 10.24(1) | | Management Services Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.19) |
| |
| 10.25(1)+ | | Sublease Agreement, dated January 31, 2004, between Multilayer Technology, Inc. and the Registrant (Exhibit 10.20) |
| |
| 10.26(1)+ | | Standard Industrial/Commercial Multi-Tenant Lease-Net, dated February 8, 2006, between The Northwestern Mutual Life Insurance Company and the Registrant (Exhibit 10.21) |
| |
| 10.27(1)++ | | Pulse Oximetry & Related Products Capital Equipment Supplier Agreement, dated December 16, 2005, between Novation, LLC (“Novation”) and the Registrant, as amended (the “Novation Agreement”) (Exhibit 10.22) |
| |
| 10.28*++ | | Letter Amendment to Exhibit A of the Novation Agreement, dated January 31, 2007, between Novation and the Registrant |
| |
| 10.29*++ | | Letter Amendment to Exhibit A of the Novation Agreement, dated June 13, 2007, between Novation and the Registrant |
| |
| 10.30*++ | | Letter Amendment to Exhibit A of the Novation Agreement, dated May 1, 2008, between Novation and the Registrant |
| |
| 10.31* | | Extension and Amendment of the Novation Agreement, dated December 4, 2008, between Novation and the Registrant |
| |
| 10.32(1)+ | | Group Purchasing Agreement—Capital Equipment, effective as of March 1, 2006, between Premier Purchasing Partners, L.P. (“Premier”) and the Registrant, as amended (the “Group Purchasing Agreement”) (Exhibit 10.23) |
62
| | |
Exhibit
Number | | Description of Document |
| 10.33*++ | | Amendment Number 3 to the Group Purchasing Agreement, effective as of August 15, 2007, between Premier and the Registrant |
| |
| 10.34* | | Amendment Number 4 to the Group Purchasing Agreement, effective as of December 1, 2007, between Premier and the Registrant |
| |
| 10.35*++ | | Amendment Number 5 to the Group Purchasing Agreement, effective as of March 17, 2008, between Premier and the Registrant |
| |
| 10.36* | | Amendment Number 6 to the Group Purchasing Agreement, effective as of January 1, 2009, between Premier and the Registrant |
| |
| 10.37*++ | | Amendment Number 7 to the Group Purchasing Agreement, effective as of May 1, 2008, between Premier and the Registrant |
| |
| 10.38*++ | | Amendment Number 8 to the Group Purchasing Agreement, effective as of November 1, 2008, between Premier and the Registrant |
| |
| 10.39*++ | | Amendment Number 9 to the Group Purchasing Agreement, effective as of February 28, 2009, between Premier and the Registrant |
| |
| 10.40(1)+ | | Supply Agreement, dated February 22, 2002, between Wintek Electro-Optics Corporation and the Registrant (Exhibit 10.24) |
| |
| 10.41(1)+ | | Form of Equipment Purchase and Assignment of Proceeds, between the Registrant and Med One Capital Funding LLC (Exhibit 10.25) |
| |
| 10.42(1) | | Settlement Agreement and Release of Claims, dated January 17, 2006, between Masimo Laboratories, Inc., Nellcor Puritan Bennett, Inc., Mallinckrodt, Inc., Tyco Healthcare Group LP, Tyco International Ltd., Tyco International (US) Inc. and the Registrant (Exhibit 10.30) |
| |
| 10.43(1)# | | Third Amended and Restated 1996 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.31) |
| |
| 10.44(1)# | | 2004 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.32) |
| |
| 10.45(1)# | | 2007 Stock Incentive Plan of the Registrant, and forms of agreements related thereto (Exhibit 10.33) |
| |
| 10.46(1)+ | | Amended and Restated Cross-Licensing Agreement, effective January 1, 2007, between Masimo Laboratories, Inc. and the Registrant (Exhibit 10.34) |
| |
| 10.47(1) | | Services Agreement, effective January 1, 2007, between Masimo Laboratories, Inc. and the Registrant (Exhibit 10.35) |
| |
| 10.48* | | Cost Sharing Agreement, effective September 29, 2008, by and between Masimo International Holdings and the Registrant |
| |
| 10.49* | | Buy-in License Agreement, effective September 29, 2008, by and between Masimo International Holdings and the Registrant |
| |
| 10.50* | | Assignment and Assumption of Cost Sharing Agreement and Buy-in License Agreement, effective November 21, 2008, by and between Masimo International Holdings and Masimo International Technologies SARL |
| |
| 10.51(1)# | | Executive Annual Cash Bonus Award Plan, effective January 1, 2007 (Exhibit 10.40) |
| |
| 10.52(1)# | | Executive Multi-Year Cash Bonus Award Plan, effective January 1, 2008 (Exhibit 10.41) |
| |
| 10.53*# | | CEO and Executive Officer Equity Award Compensation Policy, effective January 4, 2008 |
| |
| 10.54*# | | Amended and Restated 2007 Severance Protection Plan and Summary Plan Description, effective December 31, 2008 |
| |
| 10.55(3)# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between Masimo Corporation and Mark P. de Raad (Exhibit 10.2) |
| |
| 10.56(3)# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between Masimo Corporation and Yongsam Lee (Exhibit 10.3) |
63
| | |
Exhibit
Number | | Description of Document |
| 10.57*# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between Masimo Corporation and Rick Fishel |
| |
| 21.1* | | List of Registrant’s subsidiaries |
| |
| 23.1* | | Consent of Independent Registered Public Accounting Firm |
| |
| 31.1* | | Certification of Joe E. Kiani, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2* | | Certification of Mark P. de Raad, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1* | | Certification of Joe E. Kiani, Chief Executive Officer, and Mark P. de Raad, Chief Financial Officer, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| (1) | Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (No. 333-142171), originally filed on April 17, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form S-1, as amended. |
| (2) | Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K, filed on November 9, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (3) | Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K, filed on January 17, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (4) | Incorporated by reference to the exhibit to the Company’s Registration Statement on Form S-8, filed on February 11, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form S-8. |
| (5) | Incorporated by reference to the exhibit to the Company’s Annual Report on Form 10-K, filed on March 4, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-K. |
| (6) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K, filed on June 5, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (7) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K, filed on October 10, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K |
| # | Indicates management contract or compensatory plan. |
| + | The SEC has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| ++ | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| † | Pursuant to Item 601(b)(2) of Regulation S-K, the schedules to this agreement have been omitted. A list identifying the contents of the omitted schedules is included as Exhibit 2.1(a). The Company agrees to furnish supplementally a copy of any omitted schedule to the SEC upon request. |
See Item 15(a)(3) above.
| (c) | Financial Statement Schedules |
See Item 15(a)(2) above.
64
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| | | | |
| | | |
| Date: March 3, 2009 | | | | By: | | /s/ JOE E. KIANI |
| | | | | | | | Joe E. Kiani Chairman of the Board & Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
SIGNATURE | | TITLE(S) | | DATE |
| | |
/S/ JOE E. KIANI Joe E. Kiani | | Chairman of the Board & Chief Executive Officer (Principal Executive Officer) | | March 3, 2009 |
| | |
/S/ MARK P.DE RAAD Mark P. de Raad | | Executive Vice President & Chief Financial Officer (Principal Financial and Accounting Officer) | | March 3, 2009 |
| | |
/S/ STEVEN BARKER, M.D., PH.D. Steven Barker, M.D., Ph.D. | | Director | | March 3, 2009 |
| | |
/S/ EDWARD L. CAHILL Edward L. Cahill | | Director | | March 3, 2009 |
| | |
/S/ ROBERT COLEMAN, PH. D. Robert Coleman, Ph.D. | | Director | | March 3, 2009 |
| | |
/S/ SANFORD FITCH Sanford Fitch | | Director | | March 3, 2009 |
| | |
/S/ JACK LASERSOHN Jack Lasersohn | | Director | | March 3, 2009 |
65
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
MASIMO CORPORATION
| | |
Reports of Independent Registered Public Accounting Firm | | F-2 |
| |
Consolidated Balance Sheets as of January 3, 2009 and December 29, 2007 | | F-4 |
| |
Consolidated Statements of Income for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 | | F-5 |
| |
Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 | | F-6 |
| |
Consolidated Statements of Cash Flows for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 | | F-8 |
| |
Notes to Consolidated Financial Statements | | F-9 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Masimo Corporation
We have audited the accompanying consolidated balance sheets of Masimo Corporation (the Company) as of January 3, 2009 and December 29, 2007, and the related consolidated statements of income, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended January 3, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Masimo Corporation as of January 3, 2009 and December 29, 2007, and the consolidated results of its operations and its cash flows for each of the three years in the period ended January 3, 2009, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Notes 2 and 14, effective January 1, 2007, the Company adopted FIN No. 48, Accounting for Uncertainty in Income Taxes—an Interpretation of Statement No. 109.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Masimo Corporation’s internal control over financial reporting as of January 3, 2009, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 3, 2009 expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Irvine, California
March 3, 2009
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Masimo Corporation
We have audited Masimo Corporation’s internal control over financial reporting as of January 3, 2009, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Masimo Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on Masimo Corporation’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Masimo Corporation maintained, in all material respects, effective internal control over financial reporting as of January 3, 2009, based on criteria established inInternal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Masimo Corporation as of January 3, 2009 and December 29, 2007, and the related consolidated statements of income, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended January 3, 2009 and our report dated March 3, 2009 expressed an unqualified opinion on those financial statements.
/s/ GRANT THORNTON LLP
Irvine, California
March 3, 2009
F-3
MASIMO CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except share amounts)
| | | | | | | | |
| | | January 3,
2009 | | | December 29,
2007 | |
ASSETS | | | | | | | | |
Current assets | | | | | | | | |
Cash and cash equivalents | | $ | 146,910 | | | $ | 96,733 | |
Accounts receivable, net of allowance for doubtful accounts of $1,300 and $1,370 at January 3, 2009 and December 29, 2007, respectively | | | 30,715 | | | | 23,917 | |
Royalties receivable | | | 11,375 | | | | 13,866 | |
Accounts receivable from related parties | | | — | | | | 3,053 | |
Inventories | | | 27,400 | | | | 23,110 | |
Prepaid expenses | | | 3,908 | | | | 3,837 | |
Prepaid income taxes | | | 872 | | | | 3,247 | |
Deferred tax assets | | | 10,511 | | | | 14,334 | |
Other current assets | | | 551 | | | | 1,543 | |
| | | | | | | | |
Total current assets | | | 232,242 | | | | 183,640 | |
Deferred cost of goods sold | | | 28,431 | | | | 26,249 | |
Property and equipment, net | | | 12,979 | | | | 11,164 | |
Deferred tax assets | | | 8,781 | | | | 5,332 | |
Restricted cash | | | 577 | | | | 513 | |
Intangible assets, net | | | 7,410 | | | | 5,589 | |
Goodwill | | | 448 | | | | 448 | |
Other assets | | | 2,480 | | | | 2,576 | |
| | | | | | | | |
Total assets | | $ | 293,348 | | | $ | 235,511 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities | | | | | | | | |
Accounts payable | | $ | 15,914 | | | $ | 14,057 | |
Accounts payable to related parties | | | — | | | | 583 | |
Accrued compensation | | | 15,607 | | | | 12,409 | |
Accrued liabilities | | | 5,396 | | | | 6,211 | |
Dividends payable | | | 170 | | | | 183 | |
Income taxes payable | | | 10,862 | | | | — | |
Deferred revenue | | | 17,233 | | | | 16,827 | |
Current portion of long-term debt | | | 395 | | | | 11,470 | |
Current portion of long-term debt to related parties | | | — | | | | 7 | |
Current portion of capital lease obligation | | | 70 | | | | 62 | |
| | | | | | | | |
Total current liabilities | | | 65,647 | | | | 61,809 | |
Deferred revenue | | | 213 | | | | 366 | |
Long-term debt, less current portion | | | — | | | | 19,294 | |
Capital lease obligation, less current portion | | | 157 | | | | 208 | |
Other liabilities | | | 7,940 | | | | 3,768 | |
| | | | | | | | |
Total liabilities | | | 73,957 | | | | 85,445 | |
| | |
Commitments and contingencies | | | | | | | | |
| | |
Stockholders’ equity | | | | | | | | |
Preferred stock, $0.001 par value; 5,000,000 shares authorized at January 3, 2009 and December 29, 2007; 0 shares issued and outstanding at January 3, 2009 and December 29, 2007 | | | — | | | | — | |
Common stock, $0.001 par value, 100,000,000 shares authorized at January 3, 2009 and December 29, 2007, 57,326,527 and 54,692,232 shares issued and outstanding at January 3, 2009 and December 29, 2007, respectively | | | 57 | | | | 55 | |
Treasury stock, 156,240 shares at January 3, 2009 and December 29, 2007 | | | (1,209 | ) | | | (1,209 | ) |
Additional paid-in capital | | | 179,666 | | | | 143,297 | |
Accumulated other comprehensive loss | | | (7 | ) | | | (1,034 | ) |
Retained earnings | | | 40,884 | | | | 8,957 | |
| | | | | | | | |
Total stockholders’ equity | | | 219,391 | | | | 150,066 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 293,348 | | | $ | 235,511 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except share information)
| | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Revenue: | | | | | | | | | | | | |
Product(1) | | $ | 258,895 | | | $ | 199,684 | | | $ | 155,131 | |
Royalty and license fee(2) | | | 48,179 | | | | 56,602 | | | | 69,207 | |
| | | | | | | | | | | | |
Total revenue | | | 307,074 | | | | 256,286 | | | | 224,338 | |
Cost of goods sold | | | 89,454 | | | | 73,606 | | | | 61,640 | |
| | | | | | | | | | | | |
Gross profit | | | 217,620 | | | | 182,680 | | | | 162,698 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 25,495 | | | | 22,960 | | | | 24,875 | |
Selling, general and administrative | | | 120,069 | | | | 91,234 | | | | 91,384 | |
Patent litigation proceeds | | | — | | | | — | | | | (262,605 | ) |
Antitrust litigation | | | 706 | | | | 1,537 | | | | 109 | |
| | | | | | | | | | | | |
Total operating expenses | | | 146,270 | | | | 115,731 | | | | (146,237 | ) |
| | | | | | | | | | | | |
Operating income | | | 71,350 | | | | 66,949 | | | | 308,935 | |
Non-operating income (expense): | | | | | | | | | | | | |
Interest income | | | 2,305 | | | | 2,361 | | | | 6,741 | |
Interest expense | | | (753 | ) | | | (2,475 | ) | | | (1,824 | ) |
Other | | | (511 | ) | | | 1,287 | | | | 551 | |
| | | | | | | | | | | | |
Total non-operating income (expense) | | | 1,041 | | | | 1,173 | | | | 5,468 | |
| | | | | | | | | | | | |
Income before provision for income taxes | | | 72,391 | | | | 68,122 | | | | 314,403 | |
Provision for income taxes | | | 40,464 | | | | 25,867 | | | | 132,577 | |
| | | | | | | | | | | | |
Net income | | | 31,927 | | | | 42,255 | | | | 181,826 | |
Preferred stock dividend | | | — | | | | — | | | | (77,785 | ) |
Accretion of preferred stock | | | — | | | | (4,837 | ) | | | (7,985 | ) |
Undistributed income attributable to preferred stockholders | | | — | | | | (14,339 | ) | | | (34,275 | ) |
| | | | | | | | | | | | |
Net income attributable to common stockholders | | $ | 31,927 | | | $ | 23,079 | | | $ | 61,781 | |
| | | | | | | | | | | | |
Net income per common share: | | | | | | | | | | | | |
Basic | | $ | 0.57 | | | $ | 0.71 | | | $ | 3.79 | |
| | | | | | | | | | | | |
Diluted | | $ | 0.53 | | | $ | 0.60 | | | $ | 3.04 | |
| | | | | | | | | | | | |
Weighted-average number of common shares: | | | | | | | | | | | | |
Basic – Two class method | | | N/A | | | | 16,654,586 | | | | 16,319,898 | |
Diluted – Two class method | | | N/A | | | | 20,732,872 | | | | 20,302,872 | |
Basic – Single class method | | | 56,320,712 | | | | 54,660,216 | | | | N/A | |
Diluted – Single class method | | | 60,190,335 | | | | 59,829,198 | | | | N/A | |
| | | | | | | | | | | | |
(1) | Includes related party product revenue of $0, $20,100 and $18,516 for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively. See Note 4. |
(2) | Includes related party royalty revenue of $0, $321 and $323 for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively. See Note 4. |
The following table presents details of the share-based payment expense (Notes 11 and 12) that is included in each functional line item in the consolidated statements of income above (in thousands):
| | | | | | | | | |
| | | Year ended
January 3,
2009 | | Year ended
December 29,
2007 | | Year ended
December 31,
2006 |
Cost of goods sold | | $ | 257 | | $ | 264 | | $ | 2,379 |
Research and development | | $ | 2,236 | | $ | 670 | | $ | 9,378 |
Selling, general and administrative | | $ | 5,223 | | $ | 2,958 | | $ | 23,313 |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Convertible
Preferred Stock | | | Common Stock | | Treasury Stock | | | Additional
Paid In
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Retained
Earnings
(Deficit) | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | | Amount | | | Shares | | | Amount | | Shares | | Amount | | | | | |
Balance at December 31, 2005 | | — | | | $ | — | | | 9,940,410 | | | $ | 10 | | — | | $ | — | | | $ | — | | | $ | (134 | ) | | $ | (100,958 | ) | | $ | (101,082 | ) |
Stock options exercised | | — | | | | — | | | 6,739,722 | | | | 7 | | — | | | — | | | | 14,412 | | | | — | | | | — | | | | 14,419 | |
Income tax benefit from exercise of stock options | | — | | | | — | | | — | | | | — | | — | | | — | | | | 4,201 | | | | — | | | | — | | | | 4,201 | |
Dividends declared in excess of (i) amounts previously accreted to holders of preferred stock and (ii) amount included in stock compensation expense | | — | | | | — | | | — | | | | — | | — | | | — | | | | (20,298 | ) | | | — | | | | (103,322 | ) | | | (123,620 | ) |
Reclassification of convertible preferred stock | | 11,537,501 | | | | 143,959 | | | — | | | | — | | — | | | — | | | | — | | | | — | | | | — | | | | 143,959 | |
Reclassification of cumulative dividends accreted to dividends payable | | — | | | | (63,616 | ) | | — | | | | — | | — | | | — | | | | — | | | | — | | | | — | | | | (63,616 | ) |
Accretion of redemption value on convertible preferred stock | | — | | | | 7,985 | | | — | | | | — | | — | | | — | | | | — | | | | — | | | | (7,985 | ) | | | — | |
Compensation related to stock options granted to consultants | | — | | | | — | | | — | | | | — | | — | | | — | | | | 43 | | | | — | | | | — | | | | 43 | |
Compensation related to stock option grants to employees | | — | | | | — | | | — | | | | — | | — | | | — | | | | 1,329 | | | | — | | | | — | | | | 1,329 | |
Repurchase of common stock from employees | | — | | | | — | | | (114,600 | ) | | | — | | 114,600 | | | (628 | ) | | | 313 | | | | — | | | | — | | | | (315 | ) |
Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | — | | | | — | | | — | | | | — | | — | | | — | | | | — | | | | — | | | | 181,826 | | | | 181,826 | |
Foreign currency translation adjustment | | — | | | | — | | | — | | | | — | | — | | | — | | | | — | | | | (183 | ) | | | — | | | | (183 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 181,643 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | 11,537,501 | | | | 88,328 | | | 16,565,532 | | | | 17 | | 114,600 | | | (628 | ) | | | — | | | | (317 | ) | | | (30,439 | ) | | | 56,961 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Stock options exercised | | — | | | | — | | | 268,343 | | | | — | | — | | | — | | | | 831 | | | | — | | | | — | | | | 831 | |
Income tax benefit from exercise of stock options | | — | | | | — | | | — | | | | — | | — | | | — | | | | 204 | | | | — | | | | — | | | | 204 | |
Conversion of preferred stock to common stock | | (11,537,501 | ) | | | (93,165 | ) | | 34,612,503 | | | | 35 | | — | | | — | | | | 93,130 | | | | — | | | | — | | | | — | |
Issuance of common stock in initial public offering | | — | | | | — | | | 3,287,494 | | | | 3 | | — | | | — | | | | 47,846 | | | | — | | | | — | | | | 47,849 | |
Accretion of redemption value on convertible preferred stock | | — | | | | 4,837 | | | — | | | | — | | — | | | — | | | | (2,596 | ) | | | — | | | | (2,241 | ) | | | — | |
Cumulative impact of change in accounting for uncertainties in income taxes | | — | | | | — | | | — | | | | — | | — | | | — | | | | — | | | | — | | | | (618 | ) | | | (618 | ) |
Compensation related to stock option grants to employees | | — | | | | — | | | — | | | | — | | — | | | — | | | | 3,475 | | | | — | | | | — | | | | 3,475 | |
Repurchase of common stock from employees | | — | | | | — | | | (41,640 | ) | | | — | | 41,640 | | | (581 | ) | | | 417 | | | | — | | | | — | | | | (164 | ) |
Reclassification of Masimo Labs additional paid in capital to minority interest | | — | | | | — | | | — | | | | — | | — | | | — | | | | (10 | ) | | | — | | | | — | | | | (10 | ) |
Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | — | | | | | | | | | |
Net income | | — | | | | — | | | — | | | | — | | — | | | — | | | | — | | | | — | | | | 42,255 | | | | 42,255 | |
Foreign currency translation adjustment | | — | | | | — | | | — | | | | — | | — | | | — | | | | — | | | | (717 | ) | | | — | | | | (717 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 41,538 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 29, 2007 | | — | | | $ | — | | | 54,692,232 | | | $ | 55 | | 156,240 | | $ | (1,209 | ) | | $ | 143,297 | | | $ | (1,034 | ) | | $ | 8,957 | | | $ | 150,066 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-6
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Convertible
Preferred Stock | | Common Stock | | Treasury Stock | | | Additional
Paid In
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Retained
Earnings
(Deficit) | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | | | | |
Balance at December 29, 2007 | | — | | $ | — | | 54,692,232 | | $ | 55 | | 156,240 | | $ | (1,209 | ) | | $ | 143,297 | | | $ | (1,034 | ) | | $ | 8,957 | | $ | 150,066 | |
Stock options exercised | | — | | | — | | 2,634,295 | | | 2 | | — | | | — | | | | 9,753 | | | | — | | | | — | | | 9,755 | |
Income tax benefit from exercise of stock options | | — | | | — | | — | | | — | | — | | | — | | | | 19,090 | | | | — | | | | — | | | 19,090 | |
Compensation related to stock option grants to employees | | — | | | — | | — | | | — | | — | | | — | | | | 7,594 | | | | — | | | | — | | | 7,594 | |
Reclassification of Masimo Labs additional paid in capital to minority interest | | — | | | — | | — | | | — | | — | | | — | | | | (68 | ) | | | — | | | | — | | | (68 | ) |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | — | | | — | | — | | | — | | — | | | — | | | | — | | | | — | | | | 31,927 | | | 31,927 | |
Foreign currency translation adjustment | | — | | | — | | — | | | — | | — | | | — | | | | — | | | | 1,027 | | | | — | | | 1,027 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 32,954 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 3, 2009 | | — | | $ | — | | 57,326,527 | | $ | 57 | | 156,240 | | $ | (1,209 | ) | | $ | 179,666 | | | $ | (7 | ) | | $ | 40,884 | | $ | 219,391 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income | | $ | 31,927 | | | $ | 42,255 | | | $ | 181,826 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 5,745 | | | | 5,263 | | | | 3,669 | |
Share-based payment | | | 7,716 | | | | 3,892 | | | | 1,685 | |
(Gain) loss on disposal of property and equipment | | | 91 | | | | — | | | | (19 | ) |
Provision for doubtful accounts | | | 108 | | | | (25 | ) | | | 1,187 | |
Provision for obsolete inventory | | | 1,352 | | | | 1,155 | | | | 901 | |
Provision for warranty costs | | | 1,646 | | | | 1,482 | | | | 1,445 | |
Provision for deferred income taxes | | | 447 | | | | 2,696 | | | | 6,400 | |
Income tax benefit from exercise of stock options | | | 17,201 | | | | 60 | | | | 4,133 | |
Excess tax benefits from share based payment arrangements | | | (1,889 | ) | | | (144 | ) | | | (68 | ) |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Increase in accounts receivable | | | (6,244 | ) | | | (3,269 | ) | | | (7,179 | ) |
(Increase) decrease in royalties receivable | | | 2,491 | | | | (12,577 | ) | | | (1,289 | ) |
(Increase) decrease in accounts receivable from related parties | | | 3,053 | | | | (953 | ) | | | (860 | ) |
Increase in inventories | | | (5,588 | ) | | | (7,021 | ) | | | (4,981 | ) |
Increase in deferred cost of goods sold | | | (2,232 | ) | | | (4,306 | ) | | | (6,072 | ) |
Increase in prepaid expenses | | | (54 | ) | | | (1,727 | ) | | | (776 | ) |
(Increase) decrease in prepaid income taxes | | | 2,376 | | | | (3,247 | ) | | | — | |
(Increase) decrease in other assets | | | 1,163 | | | | (2,210 | ) | | | (1,023 | ) |
Increase in accounts payable | | | 1,847 | | | | 4,345 | | | | 1,734 | |
Increase (decrease) in accounts payable to related parties | | | (583 | ) | | | 99 | | | | 117 | |
Increase in accrued compensation | | | 3,121 | | | | 53 | | | | 5,041 | |
Increase (decrease) in accrued liabilities | | | (2,485 | ) | | | 19 | | | | (154 | ) |
Increase (decrease) in income taxes payable | | | 12,754 | | | | (1,110 | ) | | | 1,131 | |
Increase in deferred revenue | | | 111 | | | | 2,784 | | | | 6,749 | |
Increase in other liabilities | | | 4,104 | | | | 1,309 | | | | 167 | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 78,178 | | | | 28,823 | | | | 193,764 | |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchases of property and equipment | | | (6,852 | ) | | | (5,325 | ) | | | (5,921 | ) |
Proceeds from disposition of property and equipment | | | — | | | | — | | | | 6 | |
Increase in intangible assets | | | (2,523 | ) | | | (1,641 | ) | | | (1,048 | ) |
Increase in restricted cash | | | (67 | ) | | | — | | | | — | |
Cash paid for acquisition | | | — | | | | (187 | ) | | | (1,326 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (9,442 | ) | | | (7,153 | ) | | | (8,289 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from initial public offering, net of proceeds | | | — | | | | 47,849 | | | | — | |
Proceeds from issuance of long-term debt | | | — | | | | 20,075 | | | | — | |
Repayments on long-term debt | | | (30,436 | ) | | | (10,158 | ) | | | (8,202 | ) |
Proceeds from issuance of common stock | | | 9,755 | | | | 831 | | | | 14,419 | |
Excess tax benefits from share based payment arrangements | | | 1,889 | | | | 144 | | | | 68 | |
Dividends paid | | | (13 | ) | | | (37,350 | ) | | | (149,703 | ) |
Purchase of treasury stock | | | — | | | | (581 | ) | | | (628 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | (18,805 | ) | | | 20,810 | | | | (144,046 | ) |
| | | | | | | | | | | | |
Effect of foreign currency exchange rates on cash | | | 246 | | | | (1,129 | ) | | | (219 | ) |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | 50,177 | | | | 41,351 | | | | 41,210 | |
Cash and cash equivalents at beginning of period | | | 96,733 | | | | 55,382 | | | | 14,172 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 146,910 | | | $ | 96,733 | | | $ | 55,382 | |
| | | | | | | | | | | | |
| | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Cash paid for: | | | | | | | | | | | | |
Interest | | $ | 872 | | | $ | 2,355 | | | $ | 1,545 | |
Income taxes | | $ | 4,783 | | | $ | 25,911 | | | $ | 120,954 | |
Noncash investing and financing activities: | | | | | | | | | | | | |
Accretion of redemption value of convertible preferred stock | | $ | — | | | $ | 4,837 | | | $ | 7,985 | |
Assets acquired under capital leases | | $ | 17 | | | $ | 83 | | | $ | 182 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
January 3, 2009
| 1. | Description of the Company |
Masimo Corporation, or the Company, is a global medical technology company that develops, manufactures and markets noninvasive patient monitoring products that improve patient care. The Company invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of Measure-Through Motion and Low Perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. The Company also invented Masimo Rainbow SET which measures multiple blood parameters, including carboxyhemoglobin, methemoglobin, plethysmographic variability index and total hemoglobin. The Company develops, manufactures and markets a family of patient monitoring solutions which incorporate a monitor or circuit board and sensors, including both proprietary single-patient use and reusable sensors and cables. The Company considers both the pulse oximetry device and its sensors and cables to be products as defined in its statements of income. The Company sells to hospitals and the emergency medical services, or EMS, market through its direct sales force and distributors, and markets its circuit boards containing the Company’s proprietary algorithm and software architecture to original equipment manufacturer, or OEM, partners.
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of Masimo Corporation and Masimo Laboratories, Inc., which has been consolidated pursuant to FIN 46(R),“Consolidation of Variable Interest Entities—an Interpretation of ARB No. 51.” In addition, these consolidated financial statements include the accounts of Masimo Corporation’s wholly-owned subsidiaries, Masimo Americas, Inc., Masimo Europe Ltd., Masimo Japan, Masimo Canada ULC, Masimo Australia Pty. Ltd., Masimo Holdings L.P., Masimo International Sarl and Masimo International Technologies Sarl. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant estimates include: determination of accounts receivable allowances, inventory reserves, warranty reserves, rebate reserves, valuation of the Company’s common stock and stock options, distributor channel inventory, royalty revenues, property tax and uncertain income tax positions. Actual results could differ from those estimates.
Fair Value Measurements
Effective December 30, 2007, the Company adopted Statement of Financial Accounting Standard No. 157, or SFAS 157, “Fair Value Measurements”. SFAS 157 defines fair value, establishes a framework for measuring fair value under Generally Accepted Accounting Principles, or GAAP, and enhances disclosures about fair value measurements. In February 2008, the Financial Accounting Standards Board, or FASB, issued FASB Staff Position No. FAS 157-2, “Effective Date of FASB Statement No. 157”, which provides a one year deferral of the effective date of SFAS 157 for non-financial assets and non-financial liabilities, except those that are recognized or disclosed in the financial statements at fair value at least annually. Therefore, effective December 30, 2007, the Company adopted the provisions of SFAS 157 with respect to its financial assets and liabilities only.
SFAS 157 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
| | • | | Level 1—Quoted prices in active markets foridentical assets or liabilities. |
| | • | | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices forsimilar assets or liabilities; quoted prices in markets that are not active; or other inputs that can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | • | | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Effective December 30, 2007, the Company adopted SFAS No. 159 “The Fair Value Option for Financial Assets and Financial Liabilities,” or SFAS 159. SFAS 159 allows an entity the irrevocable option to elect fair value for the initial and subsequent measurement for specified financial assets and liabilities on a contract-by-contract basis. The Company did not elect the fair value
F-9
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
option under this statement as to specific assets or liabilities. Therefore, through January 3, 2009, the Company has not recognized the net change in fair value of its financial assets and liabilities.
The estimated fair values of the Company’s financial instruments, which include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, income taxes payable and long-term debt, approximate their carrying values due to their short maturities and because the weighted average borrowing rate of the long-term debt approximates the current market rates for similar borrowings. These items are classified as either Level 1 or Level 2 inputs. Cash equivalents consist of highly liquid investments, with a maturity of three months or less at the date of purchase, including U.S. Treasury bills and money market funds.
The Company records U.S. Treasury bills at cost and continues to carry those amounts at cost, which approximates fair value. The cost and fair value of the U.S. Treasury bills at January 3, 2009, excluding accrued interest, were both $125.5 million. The fair value is based on quoted market prices in active markets for identical assets, level 1 input.
The Company records money market funds at cost and continues to carry those amounts at cost which equals fair value. The fair value is based on quoted market prices in active markets for identical assets, level 1 input. As of January 3, 2009, the cost and fair value of the Company’s money market funds was equal to $13.6 million.
Fiscal Periods
The Company follows a conventional 52/53 week fiscal year and fiscal 2007 was designated as a 52 week fiscal year. Under a conventional 52/53 week fiscal year, a 52 week year includes four quarters of 13 fiscal weeks while a 53 week fiscal year includes three 13 week quarters and one 14 week quarter. Under the Company’s fiscal year policy, each quarter and the year end will end on the Saturday corresponding to either the 13 or 14 week quarter. As a result of the adoption of the 52/53 week convention in fiscal 2007, the Company’s first, second and third quarters ended on Saturday, March 31, June 30 and September 29, 2007, respectively, and its fiscal year ended on Saturday, December 29, 2007. In fiscal 2008, the Company was on a 53 week fiscal calendar in which its first, second and third quarters ended on Saturday, March 29, June 28 and September 27, 2008, respectively. The Company’s 2008 fiscal year ended on Saturday, January 3, 2009. For fiscal 2008, the first three quarters were 13 week quarters and the fourth fiscal quarter was a 14 week quarter. The additional week in fiscal 2008 did not have a significant impact on the Company’s results of operations. In fiscal 2009, the Company will be on a 52 week fiscal calendar in which the Company’s first, second and third quarters will end on Saturday, April 4, July 4 and October 3, 2009, respectively, and its fiscal year will end on Saturday, January 2, 2010. Each quarter in 2009 will be 13 week quarters.
Cash and Cash Equivalents
Cash equivalents include all highly liquid investments that are readily convertible into known amounts of cash and have an original maturity of three months or less when acquired. These amounts are stated at cost which approximates fair value. The Company had $5.9 million and $1.5 million held in foreign countries as of January 3, 2009 and December 29, 2007, respectively. Interest income on cash and cash equivalents is accrued and recognized monthly.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable consist of trade receivables recorded upon recognition of revenue for product revenues, reduced by reserves for estimated bad debts and returns. Trade accounts receivable are recorded at the invoiced amount and do not bear interest. Credit is extended based on evaluation of the customer’s financial condition. Collateral is not required. The allowance for doubtful accounts is determined based on historical write-off experience, current customer information and other relevant factors, including specific identification of past due accounts, based on the age of the receivable in excess of the contemplated or contractual due date. Accounts are charged off against the allowance when the Company believes they are uncollectible.
Changes in the allowance for doubtful accounts for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, were as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Allowance for doubtful accounts, beginning of period | | $ | 1,370 | | | $ | 1,625 | | | $ | 444 | |
Provision for doubtful accounts | | | 108 | | | | (25 | ) | | | 1,187 | |
Write off of uncollectible accounts | | | (178 | ) | | | (230 | ) | | | (6 | ) |
| | | | | | | | | | | | |
Allowance for doubtful accounts, end of period | | $ | 1,300 | | | $ | 1,370 | | | $ | 1,625 | |
| | | | | | | | | | | | |
F-10
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Our accounts receivable balance, including those from related parties was $30.7 million and $27.0 million, net of allowances for doubtful accounts of $1.3 million and $1.4 million at January 3, 2009 and December 29, 2007, respectively.
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using a standard cost method, which approximates FIFO (first in, first out) and includes material, labor and overhead. Inventory reserves are recorded for materials that have become excess or obsolete or are no longer used in current production and for inventory that has a market price less than the carrying value in inventory.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets, which range from three to five years. Leasehold improvements are amortized over the lesser of the lease term or the estimated useful life of the improvements. Normal repair and maintenance costs are expensed as incurred, whereas significant improvements that materially increase values or extend useful lives are capitalized and depreciated over the remaining estimated useful lives of the related assets. Upon sale or retirement of depreciable assets, the related cost and accumulated depreciation or amortization are removed from the accounts and any gain or loss on the sale or retirement is recognized in income. For the years ended January 3, 2009, December 29, 2007 and December 31, 2006, depreciation of property and equipment, which includes amortization of capital leases, was $5.0 million, $4.6 million and $3.3 million, respectively.
Intangible Assets and Goodwill
Intangible assets consist primarily of patents and trademarks, and goodwill resulting from the acquisition of Andromed, Inc. in 2006. Costs related to patents and trademarks, which include legal and application fees, are capitalized and amortized over the estimated useful lives using the straight-line method. Patent and trademark amortization commences once final approval of the patent or trademark has been obtained. Patent costs are amortized over 10 years and trademark costs over 17 years, and their associated amortization cost is included in general and administrative expense. For the years ended January 3, 2009, December 29, 2007 and December 31, 2006, amortization of patents and trademarks was $584,000, $594,000 and $349,000, respectively. As of January 3, 2009 and December 29, 2007, the total costs of patents not yet amortizing was $2.7 million and $2.0 million, respectively. As of January 3, 2009 and December 29, 2007, the total costs of trademarks not yet amortizing was $180,000 and $226,000, respectively. Management continually evaluates the amortization period and carrying basis of patents and trademarks to determine whether any events or circumstances warrant a revised estimated useful life or reduction in value. Capitalized application costs are charged to operations when it is determined that the patent or trademark will not be obtained or is abandoned.
Impairment
The impairment evaluation for goodwill is conducted annually as of the balance sheet date or more frequently if events or changes in circumstances indicate that an asset might be impaired. The evaluation is performed using a two-step process. In the first step, the estimated fair value of the reporting unit is compared with its carrying amount, including goodwill. Since the Company has one reporting unit, the estimated fair value of goodwill is determined by the Company’s stock market valuation compared to its net assets, excluding goodwill. If the estimated fair value is less than the carrying amount, then a second step must be completed in order to determine the amount of the goodwill impairment. In the second step, the implied fair value of the goodwill is determined by allocating the fair value of all of the reporting unit’s assets and liabilities other than goodwill in a manner similar to a purchase price allocation. The resulting implied fair value of the goodwill that results from the allocation is then compared to the carrying amount of the goodwill and an impairment charge is recorded for the difference.
The Company reviews long-lived assets and identifiable intangibles for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted operating cash flow expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount exceeds the fair value of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less costs to sell.
No impairment of goodwill, intangible assets, or other long lived assets was recorded during the years ended January 3, 2009, December 29, 2007 or December 31, 2006.
Restricted Cash
In May 2004, the Company entered into a facilities sublease which required the Company to deliver an irrevocable standby letter of credit in the amount of $450,000 to the sub-landlord. In connection with the letter of credit issued by Comerica Bank, the Company
F-11
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
was required to deposit $450,000 into a restricted account. In December 2005, the Company entered into a facilities lease in France which required the Company to deliver an irrevocable standby letter of credit in the amount of EUR €43,000 (USD $60,000 as of January 3, 2009 and USD $63,000 as of December 29, 2007) to the landlord. In connection with the letter of credit issued by Banque Nationale de Paris, the Company was required to deposit EUR €43,000 into a restricted account. In December 2008, the Company entered into a facilities lease in Switzerland which required the Company to deposit CHF $22,000 (USD $21,000 as of January 3, 2009) in a restricted bank account on behalf of the landlord. Additionally, in January and July 2008, the Company was required to deposit $9,000 and $37,000, respectively, into a restricted bank account by various governmental agencies. All of these amounts are shown as restricted cash on the accompanying consolidated balance sheets.
Income Taxes
The Company accounts for income taxes in accordance with Statement of Financial Accounting Standards, or SFAS, No. 109, “Accounting for Income Taxes,” whereby deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which those temporary differences are expected to be recovered. The Company evaluates the need to establish a valuation allowance for deferred tax assets based on positive and negative evidence including past operating results, the amount of existing temporary differences to be recovered and expected future taxable income. A valuation allowance to reduce the deferred tax assets is established when it is “more likely than not” that some or all of the deferred tax assets will not be realized.
In July 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109,” or FIN 48, which became effective for the Company on January 1, 2007. FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. The adoption of FIN 48 on January 1, 2007, resulted in a reduction of the Company’s beginning retained earnings by $618,000.
Revenue Recognition and Deferred Revenue
The Company recognizes revenue pursuant to the requirements of American Institute of Certified Public Accountants, or AICPA, Statement of Position, or SOP, 97-2, “Software Revenue Recognition,” as amended by SOP 98-9, “Software Revenue Recognition, With Respect to Certain Transactions,” Financial Accounting Standards Board, or FASB, Emerging Issues Task Force, or EITF, Issue No. 03-5, “Applicability of AICPA Statement of Position 97-2 to Non-Software Deliverables in an Arrangement Containing More-Than-Incidental Software,” and other authoritative accounting guidance.
The Company recognizes revenue when: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable and (iv) collectibility is reasonably assured. Revenue from the sale of the Company’s products is generally recognized when title and risk of loss transfers to the customer upon shipment, the terms of which are shipping point or destination. The Company uses contracts and customer purchase orders to determine the existence of an arrangement. The Company uses shipping documents and/or third-party proof of delivery to verify that title has transferred. The Company assesses whether the fee is fixed or determinable based upon the terms of the agreement associated with the transaction. To determine whether collection is probable, the Company assesses a number of factors but primarily relies upon past transaction history with the customer, if available.
The Company derives revenue primarily from four sources: (i) direct sales of pulse oximetry and related products to end user hospitals, emergency medical response organizations and other direct customers; (ii) direct sales of pulse oximetry and related products to distributors who then typically resell to end user hospitals, emergency medical response organizations and other direct customers; (iii) direct sales of integrated circuit boards to OEM customers who incorporate the Company’s embedded software technology into their multi-parameter monitoring devices and (iv) long-term sales contracts to end user hospitals in which the Company typically provides up front monitoring equipment at no charge in exchange for a multi-year sensor purchase commitment.
The Company enters into agreements to sell pulse oximetry and related products and services as well as multiple deliverable arrangements that include various combinations of products and services. While the majority of the Company’s sales transactions contain standard business terms and conditions, there are some transactions that contain non-standard business terms and conditions. As a result, contract interpretation is sometimes required to determine the appropriate accounting including: (i) whether an arrangement exists; (ii) how the arrangement consideration should be allocated among the deliverables if there are multiple deliverables; (iii) if fair value can be determined for each deliverable based on vendor specific objective evidence, or VSOE; (iv) when to recognize revenue on the deliverables; and (v) whether undelivered elements are essential to the functionality of the
F-12
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
delivered elements. Changes in judgments on these assumptions and estimates could materially impact the timing of revenue recognition.
The Company’s sales under long-term sales contracts are generally structured such that the Company agrees to provide up-front and at no charge certain monitoring equipment, installation, training and ongoing warranty support in exchange for the hospital’s agreement to purchase sensors over the term of the agreement, which ranges from three to six years. Under SOP 97-2, the Company has determined that its patented algorithm and software architecture, which resides within the monitors, is more than incidental to the product as a whole. In accordance with EITF Issue No. 03-05, the Company has also determined that the non-software deliverables (i.e. sensors, adapter cables, etc.) are considered essential to the functionality of the delivered elements. Furthermore, no payments are due to the Company from the hospital customer until sensors are shipped or delivered to the hospital at fixed prices per sensor over the term of the arrangement. Accordingly, the Company does not recognize any revenue when the monitoring and related equipment is delivered to the hospitals and installation and training is complete. The Company recognizes revenue for all of the delivered elements, on a pro-rata basis, as the sensors are delivered under the long-term sales contract. The cost of the monitoring equipment initially placed at the hospitals is deferred and amortized to cost of goods sold over the life of the underlying long-term sensor contract.
In situations where VSOE does not exist for undelivered elements, the contract product revenue and corresponding cost of goods sold are deferred until either the element is delivered or VSOE is established. For the years ended January 3, 2009, December 29, 2007 and December 31, 2006, $3.6 million, $4.5 million and $3.3 million, respectively, of contract product revenue was deferred related to contracts for which VSOE was not established for undelivered elements. During the year ended, January 3, 2009, $3.5 million of such previously deferred revenue was recognized, including $1.9 million related to an agreement in which VSOE was established as a result of a contract amendment providing the Company with the option to substitute a deliverable with VSOE in place of a deliverable with no VSOE, and $1.6 million, as a result of a contract amendment to remove a requirement to provide an upgrade which had no established VSOE. Deferred costs associated with this revenue aggregating $1.3 million were expensed in the same 2008 periods. During the year ended December 29, 2007, $2.0 million of previously deferred revenue was recognized related to the delivery of an upgrade. Deferred costs associated with this revenue aggregating $718,000 were expensed in the same 2007 period. During the year ended December 31, 2006, no previously deferred revenue relating to contract product revenue was recognized and no previously deferred costs relating to contract product revenue was expensed. Pursuant to the Company’s revenue recognition policy, it expects to continue to defer revenue and the related costs of goods sold, and recognize previously deferred revenue in future periods as required under applicable accounting guidance.
The Company provides certain end-user hospitals with the ability to purchase sensors under rebate programs. Under these programs, the end user hospitals may earn rebates based on their purchasing levels. The Company estimates and provides allowances for these programs at the time of sales as a reduction to revenue and an increase to deferred revenues.
Sales to the Company’s distributors are recognized on the sell-through method. The Company’s distributors purchase primarily sensor products which they then resell to hospitals that are typically fulfilling their purchase obligation to the Company under the end-user hospital’s long-term sensor sales contract. Because of the underlying contractual relationship between the Company and the end-user hospital, revenue is deferred until the Company’s commitment to its end user consumer is fulfilled. In the distribution channel, the Company believes this fulfillment occurs when the sensors are sold by the distributor to the end-user hospital and, accordingly, believes the use of the sell-through method properly reflects the completion of the sales process.
Certain of the Company’s distributors purchase products at specified distributor pricing and then may resell the product to end-user hospitals with whom the Company has separate pricing agreements. Where distributor prices are higher than end-user hospital contracted prices, the Company provides rebates to these distributors for the difference between distributor prices and end-user hospital prices. The Company estimates and provides allowances for the rebate programs at the time of sales as a reduction to revenue and accounts receivable.
In general, customers do not have a right of return for credit or refund. However, the Company allows returns under certain circumstances. At the end of each period, the Company estimates and accrues for these returns as a reduction to revenue and accounts receivable. The Company estimates returns based on several factors, including contractual limitations and past returns history.
In September 2005, the U.S. Federal Court of Appeals ruled that Mallinckrodt, Inc., now part of Covidien (formerly Tyco Healthcare), and one of its subsidiaries, Nellcor Puritan Bennett, Inc., collectively referred to as Nellcor, infringed Masimo patents and ordered the lower court to enjoin Nellcor’s infringing products. On January 17, 2006, the Company settled all existing patent litigation with Nellcor. Under terms of the agreement, Nellcor agreed to stop selling its infringing products and to pay the Company $263.0 million for damages through January 2006. The proceeds of the settlement were recorded to patent litigations expense (proceeds) in the consolidated statement of income for the year ended December 31, 2006. In addition, in exchange for the Company’s covenant not to sue Nellcor on future sales of its new products, Nellcor agreed to pay the Company royalties on its total U.S. pulse oximetry revenue at least through March 14, 2011, which the Company records as royalty revenue.
F-13
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In January 2006, Nellcor made an advance royalty payment to the Company of $67.5 million related to the settlement agreement. Based on sales information provided by Nellcor, the Company’s total 2006 Nellcor royalties were $68.8 million. The Nellcor royalties are recognized by the Company based on sales of Nellcor’s infringing products reported to the Company by Nellcor. We recognize royalty revenue based on the royalty rate per the settlement agreement multiplied by our estimate of Nellcor’s sales for each quarter. This estimate is adjusted prospectively when we receive the Nellcor royalty report, approximately 60 days after the end of the year. As a result, at January 3, 2009 and December 29, 2007, the Company has recorded a receivable of $11.4 million and $13.9 million, respectively, related to royalty payments owed by Nellcor to the Company.
The Company also earns revenue from the sale of integrated circuit boards that use the Company’s software technology and royalties and licensing fees for allowing others the right to use the Company’s technology in their products. The royalty revenue is recognized upon shipment of the OEM’s product, as represented to the Company by the OEM. Licensing fees are fixed in amount and recognized over the term of the license agreements on a straight-line basis.
Taxes Collected From Customers and Remitted to Governmental Authorities
Accordingto EITF 06-3 “How Taxes Collected from Customers and Remitted to Governmental Authorities Should Be Presented in the Income Statement (That Is, Gross Versus Net Presentation)”, such presentation is an accounting policy decision. The Company’s policy is to present taxes collected from customers and remitted to governmental authorities on a net basis.
Product Warranty Expense
The Company provides a product warranty against defects in material and workmanship for a period ranging from six months to one year, depending on the product type. In the case of long-term sales agreements, the Company typically warranties the products for the term of the agreement, which ranges from three to six years. In traditional sales activities, including direct and OEM sales, the Company establishes an accrual for the estimated costs of the product warranty at the time of revenue recognition. Estimated product warranty expenses are recorded as an accrued liability, with a corresponding provision to cost of sales. In end-user hospital contracts, revenue related to an extended product warranty is recognized over the life of the contract, while the product warranty costs related to the end-user hospital contracts are expensed as incurred.
Changes in the product warranty accrual were as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Warranty accrual, beginning of period | | $ | 649 | | | $ | 599 | | | $ | 415 | |
Provision for warranty costs | | | 1,646 | | | | 1,482 | | | | 1,445 | |
Warranty expenditures | | | (1,961 | ) | | | (1,432 | ) | | | (1,261 | ) |
| | | | | | | | | | | | |
Warranty accrual, end of period | | $ | 334 | | | $ | 649 | | | $ | 599 | |
| | | | | | | | | | | | |
Shipping and Handling Costs and Revenue
All shipping and handling costs are expensed as incurred and are recorded as a component of cost of sales. Charges for shipping and handling billed to customers are included as a component of product revenue in accordance with EITF Issue No. 00-10, “Accounting for Shipping and Handling Fees and Costs.”
Advertising Costs
Advertising costs are expensed as incurred. These costs are included in selling, general and administrative expense in the accompanying consolidated statements of income. Advertising costs for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 were $7.7 million, $6.4 million and $3.2 million, respectively.
Research and Development
Costs related to research and development activities are expensed as incurred. These costs include personnel costs, materials, depreciation and amortization on associated tangible and intangible assets and an allocation of facility costs, all of which are directly related to research and development activities.
F-14
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Software Development Costs
In accordance with SFAS No. 86, “Accounting for the Costs of Computer Software to Be Sold, Leased, or Otherwise Marketed,” costs related to the research and development of new software products and enhancements to existing software products are expensed as incurred until technological feasibility of the product has been established, at which time such costs are capitalized, subject to expected recoverability. For the years ended January 3, 2009 and December 29, 2007, the Company capitalized $844,000 and $273,000 of software development costs, respectively. The capitalized costs are being amortized over the estimated life of the products, or seven years. No software development costs were capitalized for the year ended December 31, 2006. The Company amortized $116,000, $58,000 and $41,000 for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively. The Company has unamortized software development costs of $1.2 million and $444,000 at January 3, 2009 and December 29, 2007, respectively, which is classified as intangible assets in the accompanying consolidated balance sheets.
Foreign Currency Translation
The Company has foreign subsidiaries that maintain foreign offices in Japan, Europe, Canada and Australia, for its sales, marketing, customer service and administrative functions. The functional currencies of these subsidiaries are the Japanese yen, Euro, Canadian dollar and Australian dollar, respectively. In addition, the Company has recently opened its new international headquarters in Switzerland. The functional currency of the Company’s Switzerland subsidiaries is the U.S. dollar.
Intercompany transactions of certain of our foreign subsidiaries with Masimo Corporation are denominated in U.S. dollars and are considered foreign currency denominated transactions by the subsidiary. In addition, any other transactions between Masimo Corporation or its subsidiaries and a third party, denominated in a currency different from the functional currency, is a foreign currency transaction. Realized and unrealized foreign currency gains or losses on these transactions are included in our statements of income as incurred and are converted to U.S. dollars at average exchange rates for a respective period,. These transaction gains or (losses) were $(414,000), $1.2 million and $293,000 for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively.
Assets and liabilities of foreign subsidiaries, whose functional currency is not the U.S. dollar, are translated into U.S. dollars at the rate of exchange at the balance sheet date. Statement of income amounts are translated at the average monthly exchange rates for the respective periods. Translation gains and losses are included as a component of accumulated other comprehensive income (loss) within stockholders’ equity.
Comprehensive Income (Loss)
SFAS No. 130, “Reporting Comprehensive Income,” establishes requirements for reporting and disclosure of comprehensive income (loss) and its components. Comprehensive income (loss) includes foreign currency translation adjustments and other items that have been excluded from net income and is presented in the accompanying consolidated statements of stockholders’ equity (deficit).
Accumulated Other Comprehensive Income (Loss)
Accumulated other comprehensive income (loss) included in the Company’s consolidated statements of stockholders’ equity (deficit) consists only of foreign currency translation adjustments. The change in accumulated other comprehensive income (loss), net of taxes is summarized as follows (in thousands):
| | | | |
| | | Accumulated Other
Comprehensive Income
(Loss), net of Taxes | |
Balance at December 31, 2005 | | $ | (134 | ) |
Other comprehensive loss | | | (183 | ) |
Tax benefit (expense) | | | — | |
| | | | |
Balance at December 31, 2006 | | | (317 | ) |
Other comprehensive loss | | | (717 | ) |
Tax benefit (expense) | | | — | |
| | | | |
Balance at December 29, 2007 | | | (1,034 | ) |
Other comprehensive income | | | 1,027 | |
Tax benefit (expense) | | | — | |
| | | | |
Balance at January 3, 2009 | | $ | (7 | ) |
| | | | |
F-15
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Segment Information
In accordance with SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information”, the Company uses the “management approach” in determining reportable business segments. The management approach designates the internal organization used by management for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Based on this assessment, management has determined it operates in one reportable business segment, comprised of patient monitoring related products.
Net Income Per Common Share
Basic net income per common share is computed by dividing net income attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Net income attributable to common stockholders in 2007 and 2006 is calculated using the two class method under EITF Issue No. 03-06, “Participating Securities and the Two-Class Method under FASB Statement No. 128.” EITF No. 03-06 establishes standards regarding the computation of earnings per share by companies that have issued securities other than common stock that contractually entitle the holder of such securities to participate in dividends and earnings of the Company. Pursuant to EITF 03-6, the two-class method of computing basic earnings per share is required when an entity has participating securities. Dividends must be calculated for the participating security on undistributed earnings and are a reduction in the net income attributable to common shareholders. The Company’s Series A through G preferred stock were participating securities as they had the right to dividends in the event dividends were declared on common stock. Assumed dividends on undistributed earnings were allocated as if the entire net income were distributed and were based on the relationship of the weighted average of common shares outstanding and the weighted average of common shares outstanding if the preferred stock were converted into common stock.
Upon closing of the Company’s initial public offering on August 13, 2007, all of the outstanding convertible preferred shares were converted into common shares. Therefore, subsequent to this stock conversion, the Company used the if-converted method under SFAS No. 128, “Earnings Per Share,” to calculate earnings per share. Accordingly, for the year ended December 29, 2007, the Company calculated net income per share using the two-class method for the first 224 days of the period and the if-converted method for the remainder of the period. Income was allocated between these periods on a straight-line basis over the number of days of the respective periods. The Company calculated net income per share for the year ended December 31, 2006 using the two-class method.
Diluted net income per common share is computed by dividing the net income attributable to common stockholders for the period by the weighted average number of common and potential common shares outstanding during the period, if the effect of potential common shares is dilutive. Potential common shares include incremental shares of common stock issuable upon the exercise of stock options and warrants. A reconciliation of the numerator and denominator used in the calculation of basic and diluted net income per common share follows (in thousands, except share and per share data):
F-16
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Net income attributable to common stockholders: | | | | | | | | | | | |
Net income—two class method (1) | | | N/A | | $ | 26,075 | | | $ | 181,826 | |
Preferred stock dividend | | | N/A | | | — | | | | (77,785 | ) |
Accretion of preferred stock | | | N/A | | | (4,837 | ) | | | (7,985 | ) |
Undistributed income attributable to preferred stockholders | | | N/A | | | (14,339 | ) | | | (34,275 | ) |
| | | | | | | | | | | |
Net income attributable to common stockholders (A) | | | N/A | | $ | 6,899 | | | $ | 61,781 | |
| | | | | | | | | | | |
Basic net income per common share: | | | | | | | | | | | |
Weighted average common shares outstanding – two class method (B) | | | N/A | | | 16,654,586 | | | | 16,319,898 | |
| | | | | | | | | | | |
Basic earnings per share for period during which two classes of equity securities were outstanding (A/B) | | | N/A | | $ | 0.41 | | | $ | 3.79 | |
| | | | | | | | | | | |
Net income for period during which single class of equity securities was outstanding (1) (C) | | $ | 31,927 | | $ | 16,180 | | | | N/A | |
Weighted average common shares outstanding – single class (2) (D) | | | 56,320,712 | | | 54,660,216 | | | | N/A | |
Basic net income per share for period during which single class of equity securities was outstanding (C/D) | | | 0.57 | | $ | 0.30 | | | | N/A | |
| | | | | | | | | | | |
Basic net income per common share | | $ | 0.57 | | $ | 0.71 | | | $ | 3.79 | |
| | | | | | | | | | | |
Diluted net income per common share: | | | | | | | | | | | |
Weighted average common shares outstanding – two class method | | | N/A | | | 16,654,586 | | | | 16,319,898 | |
Diluted common share equivalent: stock options | | | N/A | | | 4,078,286 | | | | 3,982,974 | |
| | | | | | | | | | | |
Total diluted common share and share equivalents – two class (E) | | | N/A | | | 20,732,872 | | | | 20,302,872 | |
| | | | | | | | | | | |
Diluted earnings per share for period during which two classes of equity securities were outstanding (A/E) | | | N/A | | $ | 0.33 | | | $ | 3.04 | |
| | | | | | | | | | | |
Net income for period during which single class of equity securities was outstanding (1) (F) | | $ | 31,927 | | $ | 16,180 | | | | N/A | |
Weighted average common shares outstanding – single class (2) | | | 56,320,712 | | | 54,660,216 | | | | N/A | |
Diluted common share equivalent: stock options | | | 3,869,623 | | | 5,168,982 | | | | N/A | |
| | | | | | | | | | | |
Total diluted common share and share equivalents – single class (G) | | | 60,190,335 | | | 59,829,198 | | | | N/A | |
| | | | | | | | | | | |
Diluted net income per share for period during which single class of equity securities was outstanding (F/G) | | | 0.53 | | $ | 0.27 | | | | N/A | |
| | | | | | | | | | | |
Diluted net income per common share | | $ | 0.53 | | $ | 0.60 | | | $ | 3.04 | |
| | | | | | | | | | | |
| (1) | Net income for the year ended December 29, 2007 was allocated between the periods during which two classes of equity securities were outstanding and during which a single class of equity securities was outstanding based on the respective number of days. The convertible preferred stock was converted to common stock on August 13, 2007 the closing date of the Company’s initial public offering. For year ended December 29, 2007, two classes of equity securities were outstanding for 224 days and a single class of equity securities was outstanding for 139 days. |
| (2) | Weighted average shares outstanding used to compute basic net income per share after conversion of convertible preferred stock; one class of common shares was outstanding for the period from August 13, 2007 to December 29, 2007. |
Share Based Payment
On January 1, 2006, the Company adopted the provisions of SFAS No. 123(R), “Share Based Payment,” which require companies to expense the estimated fair value of employee stock options and similar awards based on the fair value of the award on the date of grant. The cost is recognized over the period during which an employee is required to provide services in exchange for the award, which is usually the vesting period.
The Company adopted SFAS No. 123(R) using the prospective transition method that applies to awards granted, modified or canceled subsequent to the date of adoption. Prior periods were not revised for comparative purposes, and existing options continue to be accounted for in accordance with Accounting Principles Board, or APB, Opinion No. 25, “Accounting for Stock Issued to Employees,”
F-17
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
unless such options are modified, repurchased or canceled after the adoption date. Prior to January 1, 2006, the Company accounted for employee stock options using the intrinsic value method and using the minimum value method for its pro forma disclosures under SFAS No. 123, “Accounting for Stock Based Compensation.” As a result, options granted prior to the adoption of SFAS No. 123(R) will continue to be accounted for using the intrinsic value method in accordance with APB No. 25 unless such options are modified, repurchased or cancelled. In accordance with EITF Issue No. 00-15, “Classification in the Statement of Cash Flows of the Income Tax Benefit Received by a Company upon Exercise of a Nonqualified Employee Stock Option”, which was nullified by SFAS No. 123(R) except for companies that continue to account for awards under Opinion No. 25, the cash flows related to the reduction of income taxes paid as a result of the deduction triggered by employee exercise of stock options granted or modified prior to the adoption of SFAS No. 123(R) will continue to be presented as an operating cash flow.
New Accounting Pronouncements
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 141(R), or SFAS 141(R),“Business Combinations”. This Statement provides greater consistency in the accounting and financial reporting of business combinations. It requires the acquiring entity in a business combination to record all assets acquired and liabilities assumed at their respective acquisition-date fair values and changes other practices under SFAS 141. SFAS 141(R) also requires additional disclosure of information surrounding a business combination, such that users of the entity’s financial statements can fully understand the nature and financial impact of the business combination. SFAS 141(R) is effective for fiscal years beginning after December 15, 2008. The Company does not expect the adoption of this statement to have a material impact on its consolidated financial statements.
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 160, or SFAS 160, “Noncontrolling Interests in Consolidated Financial Statements”. This Statement amends Accounting Research Bulletin No. 51, “Consolidated Financial Statements”, to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. SFAS 160 is effective for fiscal years beginning after December 15, 2008. The Company does not expect the adoption of this statement to have a material impact on its consolidated financial statements.
In April 2008, the FASB issued FASB Staff Position No. FAS 142-3, or FSP 142-3, “Determination of the Useful Life of Intangible Assets”, that amends the factors considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets”. FSP 142-3 requires a consistent approach between the useful life of a recognized intangible asset under SFAS No. 142 and the period of expected cash flows used to measure the fair value of an asset under SFAS No. 141 (R), “Business Combinations”. The FSP also requires enhanced disclosures when an intangible asset’s expected future cash flows are affected by an entity’s intent and/or ability to renew or extend the arrangement. FSP No. 142-3 is effective for fiscal years beginning after December 15, 2008 and is applied prospectively. The Company does not expect the adoption of this statement to have a material impact on its consolidated financial statements.
In November 2008, the FASB ratified EITF Issue No. 08-7, or EITF 08-7, “Accounting for Defensive Intangible Assets”. EITF 08-7 applies to defensive intangible assets, which are acquired intangible assets that the acquirer does not intend to actively use but intends to hold to prevent its competitors from obtaining access to them. As these assets are separately identifiable, EITF 08-7 requires an acquiring entity to account for defensive intangible assets as a separate unit of accounting. Defensive intangible assets must be recognized at fair value in accordance with SFAS 141(R) and SFAS 157, “Fair Value Measurements.” EITF 08-7 is effective for defensive intangible assets acquired in fiscal years beginning on or after December 15, 2008. The Company does not expect the adoption of this statement to have a material impact on its consolidated financial statements.
| 3. | Masimo Laboratories, Inc. |
Masimo Laboratories, Inc., or Masimo Labs, is an independent entity spun off from the Company to its stockholders in 1998. Joe E. Kiani and Jack Lasersohn, members of the Company’s board of directors, or Board, are also members of the board of directors of Masimo Labs. Joe E. Kiani, the Company’s Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Masimo Labs. The Company is a party to a cross-licensing agreement with Masimo Labs, which was recently amended and restated effective January 1, 2007, or the Cross-Licensing Agreement, that governs each party’s rights to certain of the intellectual property held by the two companies.
Under the Cross-Licensing Agreement, the Company granted Masimo Labs an exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET owned by the Company, including all improvements on this technology, for the measurement of non-vital signs parameters and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs parameters in any product market in which a product is intended to be used by a patient or pharmacist rather than a professional medical caregiver, or the Labs Market. The Company also granted Masimo Labs a non-exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET for the measurement of vital signs in the Labs Market.
F-18
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Vital signs parameters include peripheral venous oxygen saturation, arterial oxygen saturation, or SpO2, mixed venous oxygen saturation, fetal oximetry, sudden infant death syndrome, electrocardiogram, or ECG, blood pressure (noninvasive blood pressure, invasive blood pressure and continuous noninvasive blood pressure), temperature, respiration rate, carbon dioxide, or CO2, pulse rate, cardiac output, electroenchephalogram, or EEG, perfusion index, depth of anesthesia, cerebral oximetry, tissue oximetry and/or electromyography, or EMG, and associated features derived from these parameters, such as 3-D alarms, Pleth Variability Index and other features. Non-vital signs parameters are body fluid constituents other than vital signs parameters, and include, but are not limited to, carbon monoxide, methemoglobin, blood glucose, total hemoglobin and bilirubin.
The Company exclusively licenses from Masimo Labs the right to make and distribute products in the professional medical caregiver markets, or the Masimo Market, that utilize Rainbow technology for the measurement of carbon monoxide, methemoglobin, fractional arterial oxygen saturation, and total hemoglobin, which includes hematocrit. To date, the Company has developed and commercially released devices that measure carbon monoxide and methemoglobin using licensed Rainbow technology. Additionally, the Company has developed total hemoglobin and released it on a limited market basis. The Company also has the option to obtain the exclusive license to make and distribute products that utilize Rainbow technology for the measurement of other non-vital signs parameters, including blood glucose, in product markets where the product is intended to be used by a professional medical caregiver.
From May 1998 through December 2008, Masimo Labs contracted the services of the Company’s employees for the development of Rainbow technology. The Company paid Masimo Labs for the option to market and develop products based on Masimo Labs technology in defined markets. Through December 2005, the Company had paid Masimo Labs $7.5 million in option fees and nearly all these option fees were used by Masimo Labs to repay the Company for the services that the Company had provided to Masimo Labs. In addition, through December 2006, the Company exercised two licenses, for $2.5 million each, for the right to market products based on the new carbon monoxide and methemoglobin parameter technologies developed by Masimo Labs. Effective as of January 1, 2007, the Company entered into a Services Agreement with Masimo Labs to govern the services the Company will provide to Masimo Labs going forward. As part of the Cross-Licensing Agreement, the Company exercised an option to purchase an additional license for total hemoglobin for a fee of $2.5 million on January 1, 2007.
The Cross-Licensing Agreement requires the Company to pay certain royalties on products incorporating the licensed Rainbow technology. The royalty is up to 10% of the Rainbow royalty base, which will include handhelds, tabletop and multi-parameter devices. Handheld products incorporating Rainbow technology will carry a 10% royalty rate. For other products, only the proportional amount attributable for that portion of the Company’s products used to measure non-vital signs parameters, sensors and accessories, rather than for measuring vital signs parameters, will be included in the 10% Rainbow royalty base. For multi-parameter devices, the Rainbow royalty base will include the percentage of the revenue based on the number of Rainbow-enabled parameters. Beginning in 2009, for hospital contracts where Masimo places equipment and enters into a sensor contract, the Company will pay a royalty to Masimo Labs on the total sensor contract revenues based on the ratio of Rainbow enabled devices to total devices.
The Company is also subject to certain specific annual minimum aggregate royalty payments. These minimum aggregate royalty payments were $3.5 million for 2008, $3.2 million for 2007 and $481,000 for 2006. In 2009 and thereafter, the minimum aggregate royalty payments are $4.0 million and $5.0 million, respectively. In addition, in connection with a change in control, as defined in the Cross-Licensing Agreement, the minimum aggregate annual royalties for all licensed Rainbow parameters payable to Masimo Labs will increase to $10.0 million and $15.0 million for 2009 and 2010, respectively, and $15.0 million per year thereafter and up to $2.0 million per year for other Rainbow parameters. For 2008, 2007 and 2006, the Company paid the minimum royalties of $3.5 million, $3.2 million and $481,000, respectively.
Through January 3, 2009, the Company had agreed to pay Masimo Labs a total of $22.1 million, exclusive of amounts agreed to be paid to Masimo Labs subsequent to January 3, 2009. The $22.1 million included $7.5 million in option fees, $7.5 million in license fees for Rainbow parameter technologies and $7.1 million for minimum royalties for licensed Rainbow products. Of this total amount, approximately $17.4 million had been used by Masimo Labs to repay the Company for the shared engineering and other services that the Company provided to Masimo Labs through January 3, 2009. In addition, Masimo Labs has used approximately $1.7 million of the $22.1 million from the Company in order to fund purchases of fixed assets, the costs associated with capitalized patents and trademarks, to pay income taxes and for working capital needs. The remaining unpaid amount of $3.0 million, represents the net amount owed by the Company to Masimo Labs.
Pursuant to FIN 46(R), Masimo Labs is consolidated within the Company’s financial statements for all periods presented. The Company was required to consolidate since it was deemed to be the primary beneficiary. This determination was based on the obligation to absorb the expected losses as defined under FIN 46(R), as well as exercising significant influence over the operations and decision making of Masimo Labs. Accordingly, all inter-company royalties, option and license fees and other charges between the Company and Masimo Labs as well as all intercompany payables and receivable have been eliminated in the consolidation. Also in accordance with FIN 46(R), all direct engineering expenses that have been incurred by the Company and charged to Masimo Labs have not been eliminated and are included as research and development expense in the Company’s consolidated statements of income.
F-19
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
For the foreseeable future, the Company anticipates that it will continue to be required by FIN 46(R) to consolidate Masimo Labs; however, in the event that Masimo Labs secures additional external financing and/or expands its customer base or is no longer financially dependent upon the Company and the Company is no longer the primary beneficiary of Masimo Labs activities, the Company may be able to discontinue consolidating Masimo Labs.
The Consolidated Financial Statements of the Company include a minority interest in Masimo Labs of $107,000 and $39,000 as of January 3, 2009 and December 29, 2007, respectively, which represents the value of common stock and additional paid in capital of Masimo Labs, which is not available to Masimo Corporation and is classified in other long-term liabilities. Masimo Corporation has not been required to collateralize any of Masimo Lab’s obligations and creditors of Masimo Labs have no recourse to the general credit of Masimo Corporation. Retained earnings of Masimo Labs, which is not available to the Company, was a $273,000 deficit as of January 3, 2009. However, because the Company has agreed to absorb any losses of Masimo Labs, under FIN 46(R) this retained deficit is included in the Consolidated Financial Statements of the Company as a reduction of total retained earnings.
Below are condensed consolidating schedules of the Balance Sheets as of January 3, 2009 and December 29, 2007, and Statements of Operations for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 reflecting Masimo Corporation, Masimo Labs and related eliminations (in thousands).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | January 3, 2009 | | | December 29, 2007 | |
Balance Sheets: | | Corp | | | Labs | | | Elim | | | Total | | | Corp | | | Labs | | | Elim | | | Total | |
ASSETS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 146,684 | | | $ | 226 | | | $ | — | | | $ | 146,910 | | | $ | 96,717 | | | $ | 16 | | | $ | — | | | $ | 96,733 | |
Accounts receivable, net | | | 42,090 | | | | — | | | | — | | | | 42,090 | | | | 40,836 | | | | — | | | | — | | | | 40,836 | |
Inventories | | | 27,400 | | | | — | | | | — | | | | 27,400 | | | | 23,110 | | | | — | | | | — | | | | 23,110 | |
Prepaid expenses | | | 3,869 | | | | 39 | | | | — | | | | 3,908 | | | | 3,820 | | | | 17 | | | | — | | | | 3,837 | |
Prepaid income taxes | | | 754 | | | | 118 | | | | — | | | | 872 | | | | 2,971 | | | | 276 | | | | — | | | | 3,247 | |
Deferred tax asset, current | | | 9,336 | | | | 1,175 | | | | — | | | | 10,511 | | | | 13,286 | | | | 1,048 | | | | — | | | | 14,334 | |
Other current assets | | | 551 | | | | 3,002 | | | | (3,002 | ) | | | 551 | | | | 1,543 | | | | 3,314 | | | | (3,314 | ) | | | 1,543 | |
Deferred cost of goods sold | | | 28,431 | | | | — | | | | — | | | | 28,431 | | | | 26,249 | | | | — | | | | — | | | | 26,249 | |
Property and equipment, net | | | 12,566 | | | | 413 | | | | — | | | | 12,979 | | | | 11,019 | | | | 145 | | | | — | | | | 11,164 | |
Deferred tax asset, long term | | | 8,196 | | | | 585 | | | | — | | | | 8,781 | | | | 4,801 | | | | 531 | | | | — | | | | 5,332 | |
Restricted cash | | | 577 | | | | — | | | | — | | | | 577 | | | | 513 | | | | — | | | | — | | | | 513 | |
Intangible assets, net | | | 12,771 | | | | 1,045 | | | | (6,406 | ) | | | 7,410 | | | | 11,474 | | | | 896 | | | | (6,781 | ) | | | 5,589 | |
Goodwill | | | 448 | | | | — | | | | — | | | | 448 | | | | 448 | | | | — | | | | — | | | | 448 | |
Other assets | | | 2,447 | | | | 33 | | | | — | | | | 2,480 | | | | 2,548 | | | | 28 | | | | — | | | | 2,576 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 296,120 | | | $ | 6,636 | | | $ | (9,408 | ) | | $ | 293,348 | | | $ | 239,335 | | | $ | 6,271 | | | $ | (10,095 | ) | | $ | 235,511 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 15,776 | | | $ | 138 | | | $ | — | | | $ | 15,914 | | | $ | 14,631 | | | $ | 9 | | | $ | — | | | $ | 14,640 | |
Accrued compensation | | | 15,354 | | | | 253 | | | | — | | | | 15,607 | | | | 12,409 | | | | — | | | | — | | | | 12,409 | |
Accrued liabilities | | | 8,396 | | | | 2 | | | | (3,002 | ) | | | 5,396 | | | | 9,523 | | | | 2 | | | | (3,314 | ) | | | 6,211 | |
Dividends payable | | | 170 | | | | — | | | | — | | | | 170 | | | | 183 | | | | — | | | | — | | | | 183 | |
Income taxes payable | | | 10,862 | | | | — | | | | — | | | | 10,862 | | | | — | | | | — | | | | — | | | | — | |
Deferred revenue, current | | | 17,233 | | | | 375 | | | | (375 | ) | | | 17,233 | | | | 16,827 | | | | 375 | | | | (375 | ) | | | 16,827 | |
Current portion of long-term debt | | | 465 | | | | — | | | | — | | | | 465 | | | | 11,539 | | | | — | | | | — | | | | 11,539 | |
Deferred revenue, long-term | | | 213 | | | | 6,031 | | | | (6,031 | ) | | | 213 | | | | 366 | | | | 6,406 | | | | (6,406 | ) | | | 366 | |
Long term debt, less current portion | | | 157 | | | | — | | | | — | | | | 157 | | | | 19,502 | | | | — | | | | — | | | | 19,502 | |
Other liabilities | | | 7,830 | | | | 3 | | | | 107 | | | | 7,940 | | | | 3,729 | | | | — | | | | 39 | | | | 3,768 | |
STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Common stock | | | 57 | | | | 10 | | | | (10 | ) | | | 57 | | | | 55 | | | | 10 | | | | (10 | ) | | | 55 | |
Treasury stock | | | (1,209 | ) | | | — | | | | — | | | | (1,209 | ) | | | (1,209 | ) | | | — | | | | — | | | | (1,209 | ) |
Additional paid in capital | | | 179,666 | | | | 97 | | | | (97 | ) | | | 179,666 | | | | 143,297 | | | | 29 | | | | (29 | ) | | | 143,297 | |
Accumulated other comprehensive income (loss) | | | (7 | ) | | | — | | | | — | | | | (7 | ) | | | (1,034 | ) | | | — | | | | — | | | | (1,034 | ) |
Retained earnings (deficit) | | | 41,157 | | | | (273 | ) | | | — | | | | 40,884 | | | | 9,517 | | | | (560 | ) | | | — | | | | 8,957 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total liabilities and stockholders’ equity (deficit) | | $ | 296,120 | | | $ | 6,636 | | | $ | (9,408 | ) | | $ | 293,348 | | | $ | 239,335 | | | $ | 6,271 | | | $ | (10,095 | ) | | $ | 235,511 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-20
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended January 3, 2009 | | Year ended December 29, 2007 | | Year ended December 31, 2006 | |
Statement of Operations: | | Corp | | Labs | | | Elim | | | Total | | Corp | | Labs | | Elim | | | Total | | Corp | | | Labs | | | Elim | | | Total | |
Total revenue | | $ | 307,074 | | $ | 3,875 | | | $ | (3,875 | ) | | $ | 307,074 | | $ | 256,286 | | $ | 3,527 | | $ | (3,527 | ) | | $ | 256,286 | | $ | 224,338 | | | $ | 660 | | | $ | (660 | ) | | $ | 224,338 | |
Cost of good sold | | | 92,954 | | | — | | | | (3,500 | ) | | | 89,454 | | | 76,760 | | | — | | | (3,154 | ) | | | 73,606 | | | 61,640 | | | | — | | | | — | | | | 61,640 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | 214,120 | | | 3,875 | | | | (375 | ) | | | 217,620 | | | 179,526 | | | 3,527 | | | (373 | ) | | | 182,680 | | | 162,698 | | | | 660 | | | | (660 | ) | | | 162,698 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 23,065 | | | 2,430 | | | | — | | | | 25,495 | | | 21,065 | | | 1,895 | | | — | | | | 22,960 | | | 21,495 | | | | 3,380 | | | | — | | | | 24,875 | |
Selling, general and administrative | | | 119,264 | | | 1,180 | | | | (375 | ) | | | 120,069 | | | 90,702 | | | 905 | | | (373 | ) | | | 91,234 | | | 91,870 | | | | 147 | | | | (633 | ) | | | 91,384 | |
Patent litigation proceeds | | | — | | | — | | | | — | | | | — | | | — | | | — | | | — | | | | — | | | (262,605 | ) | | | — | | | | — | | | | (262,605 | ) |
Antitrust litigation | | | 706 | | | — | | | | — | | | | 706 | | | 1,537 | | | — | | | — | | | | 1,537 | | | 109 | | | | — | | | | — | | | | 109 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 143,035 | | | 3,610 | | | | (375 | ) | | | 146,270 | | | 113,304 | | | 2,800 | | | (373 | ) | | | 115,731 | | | (149,131 | ) | | | 3,527 | | | | (633 | ) | | | (146,237 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | 71,085 | | | 265 | | | | — | | | | 71,350 | | | 66,222 | | | 727 | | | — | | | | 66,949 | | | 311,829 | | | | (2,867 | ) | | | (27 | ) | | | 308,935 | |
Non-operating income | | | 1,038 | | | 3 | | | | — | | | | 1,041 | | | 1,173 | | | — | | | — | | | | 1,173 | | | 5,468 | | | | — | | | | — | | | | 5,468 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision for (benefit from) income taxes | | | 72,123 | | | 268 | | | | — | | | | 72,391 | | | 67,395 | | | 727 | | | — | | | | 68,122 | | | 317,297 | | | | (2,867 | ) | | | (27 | ) | | | 314,403 | |
Provision for (benefit from) income taxes | | | 40,483 | | | (19 | ) | | | — | | | | 40,464 | | | 25,555 | | | 312 | | | — | | | | 25,867 | | | 133,867 | | | | (1,290 | ) | | | — | | | | 132,577 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | 31,640 | | $ | 287 | | | $ | — | | | $ | 31,927 | | $ | 41,840 | | $ | 415 | | $ | — | | | $ | 42,255 | | $ | 183,430 | | | $ | (1,577 | ) | | $ | (27 | ) | | $ | 181,826 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-21
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 4. | Related Party Transactions |
Two of the Company’s customers and one third-party vendor are stockholders of the Company, and were considered to be related parties for the two years ended December 29, 2007. They were not considered to be related parties during the year ended January 3, 2009, as a result of their disposal of Masimo common stock in 2008.
Sales to these two customers for the years ended December 29, 2007 and December 31, 2006 were $20.4 million and $18.8 million, respectively. At December 29, 2007, aggregate accounts receivable from these two customers was $3.1 million. Also, the Company purchased certain inventory from one of the stockholders referred to in the preceding paragraph. Total purchases from this stockholder for the years ended December 29, 2007 and December 31, 2006 were $2.4 million and $2.7 million, respectively. At December 29, 2007, aggregate accounts payable to this stockholder was $110,000.
The Company also had amounts outstanding under a term loan (See Note 8) with one of the Company’s stockholders noted above. As of December 29, 2007, the amount outstanding on this term loan was $7,000. For the years ended December 29, 2007 and December 31, 2006, interest expense under this term loan was $9,000 and $68,000, respectively.
The Company made payments of $3.6 million and $3.8 million for the years ended December 29, 2007 and December 31, 2006, respectively, to one of the Company’s stockholders noted above for legal services. At December 29, 2007, accounts payable to this stockholder was $473,000.
During the year ended December 31, 2006, the Company declared dividends totaling $10.8 million to these three stockholders, of which $8.9 million was paid in March 2006 and $1.9 million was paid in February 2007.
As of January 3, 2009 and December 29, 2007, the Company had amounts due from employees of $239,000 and $598,000, respectively. As of January 3, 2009, these amounts are classified in other assets in the accompanying consolidated balance sheets. No loans were outstanding to officers of the Company as of both January 3, 2009 and December 29, 2007.
The Company’s Chief Executive Officer has been a member of the board of directors of Saba Software, Inc., a human capital development and management solutions provider, since 1997. The Company has paid Saba Software $84,000, $51,000 and $19,000 for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively, for various software products and services.
In the first quarter of 2006, the Company made $12.0 million in loans to certain of its directors and executive officers and $1.6 million in loans to employees in connection with their exercise of stock options. All of the loans, plus accrued interest, were repaid in full in March 2006. As a result of exercising stock options with non-recourse loans, dividends paid of $21.7 million on the related shares of common stock were recorded as stock compensation expense, pursuant to EITF Issue No. 95-16 “Accounting for Stock Compensation Arrangements with Employer Loan Features Under APB 25”, during the year ended December 31, 2006.
Inventories consist of the following (in thousands):
| | | | | | |
| | | January 3,
2009 | | December 29,
2007 |
Raw materials | | $ | 17,678 | | $ | 13,173 |
Work in-process | | | 2,001 | | | 1,956 |
Finished goods | | | 7,721 | | | 7,981 |
| | | | | | |
Total | | $ | 27,400 | | $ | 23,110 |
| | | | | | |
Finished goods inventory held by distributors was $1.7 million as of both January 3, 2009 and December 29, 2007.
Property and equipment, net consists of the following (in thousands):
| | | | | | |
| | | January 3,
2009 | | December 29,
2007 |
Machinery and equipment | | $ | 13,537 | | $ | 11,688 |
Tooling | | | 5,979 | | | 4,174 |
Computer equipment | | | 5,338 | | | 4,614 |
Furniture and office equipment | | | 2,088 | | | 1,817 |
F-22
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| | | | | | | | |
| | | January 3,
2009 | | | December 29,
2007 | |
Vehicles | | | 45 | | | | 45 | |
Leasehold improvements | | | 3,513 | | | | 2,325 | |
Demonstration units | | | 2,853 | | | | 3,105 | |
| | | | | | | | |
| | | 33,353 | | | | 27,768 | |
Accumulated depreciation and amortization | | | (21,467 | ) | | | (16,843 | ) |
Construction-in-progress | | | 1,093 | | | | 239 | |
| | | | | | | | |
Total | | $ | 12,979 | | | $ | 11,164 | |
| | | | | | | | |
The gross value of furniture and office equipment under capital lease obligations was $446,000 and $422,000 as of January 3, 2009 and December 29, 2007, respectively, with accumulated amortization of $264,000 and $185,000.
Intangible assets consist of the following (in thousands):
| | | | | | | | |
| | | January 3,
2009 | | | December 29,
2007 | |
Cost | | | | | | | | |
Patents | | $ | 8,074 | | | $ | 6,460 | |
Trademarks | | | 833 | | | | 768 | |
Capitalized software development costs | | | 1,401 | | | | 557 | |
Covenant not to compete | | | 40 | | | | 40 | |
| | | | | | | | |
Total cost | | | 10,348 | | | | 7,825 | |
| | | | | | | | |
Accumulated amortization | | | | | | | | |
Patents | | | (2,510 | ) | | | (1,967 | ) |
Trademarks | | | (174 | ) | | | (139 | ) |
Capitalized software development costs | | | (229 | ) | | | (113 | ) |
Covenant not to compete | | | (25 | ) | | | (17 | ) |
| | | | | | | | |
Total accumulated amortization | | | (2,938 | ) | | | (2,236 | ) |
| | | | | | | | |
Net carrying amount | | $ | 7,410 | | | $ | 5,589 | |
| | | | | | | | |
For the years ended January 3, 2009, December 29, 2007 and December 31, 2006, total amortization expense was $708,000, $660,000 and $398,000, respectively.
Estimated amortization expense for each of the fiscal years ended are as follows (in thousands):
| | | |
2009 | | $ | 788 |
2010 | | | 734 |
2011 | | | 650 |
2012 | | | 561 |
2013 | | | 480 |
Thereafter | | | 4,197 |
| | | |
Total | | $ | 7,410 |
| | | |
During the year ended January 3, 2009, the Company purchased patents in the amount of $335,000. The weighted average amortization period for these patents purchased is eight years.
F-23
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 8. | Long-Term Debt and Capital Lease Obligations |
Long-term debt consists of the following (in thousands):
| | | | | | | | |
| | | January 3,
2009 | | | December 29,
2007 | |
Financing arrangements | | $ | 395 | | | $ | 30,764 | |
Term loan with stockholder | | | — | | | | 7 | |
| | | | | | | | |
Total debt | | | 395 | | | | 30,771 | |
Less current portion of long-term debt | | | (395 | ) | | | (11,477 | ) |
| | | | | | | | |
Long-term portion | | $ | — | | | $ | 19,294 | |
| | | | | | | | |
The Company has established various credit facilities with third-party medical equipment financing companies. The Company had two arrangements which allow for the financing of the equipment placed with hospitals in connection with the related long-term sensor purchase agreements. These agreements provide for an equipment line whereby some draws are collateralized by (i) equipment and (ii) either a future revenue stream associated with the long-term sensor purchase agreement or a defined repayment schedule associated with the long-term sensor purchase agreement. The related equipment securing these borrowings is recorded on the Company’s consolidated financial statements as deferred cost of goods sold and is depreciated on a straight-line basis over the life of the sensor contract to which they are related. Both financing arrangements are non-recourse to the Company. In the event the hospital was unable to continue performing under the terms of the long-term sensor agreement, the Company would be required to write-down the remaining deferred cost of goods sold and the related financing obligation reflected in long-term debt. To date, no hospitals have defaulted under this program. During the year ended December 29, 2007, the Company borrowed $20.1 million under these facilities. During the year ended January 3, 2009, the Company repaid $26.7 million, or the entire amount owed, on one of the arrangements plus $168,000 in prepayment fees which are included in other non-operating income (expense). As of January 3, 2009, the total monthly principal and interest payment under the remaining financing arrangement was $52,000 based on an average interest rate of 7.0% per year. At January 3, 2009, no equipment collateralized these borrowings. There are no additional amounts available for future borrowing under this remaining arrangement.
Capital lease obligations consist of the following (in thousands):
| | | | | | | | |
| | | January 3,
2009 | | | December 29,
2007 | |
Capital lease obligations | | $ | 227 | | | $ | 270 | |
Less current portion of capital lease obligations | | | (70 | ) | | | (62 | ) |
| | | | | | | | |
Long-term portion | | $ | 157 | | | $ | 208 | |
| | | | | | | | |
The Company currently has seven capital leases outstanding, all for office equipment. The interest rates on these capital leases range from 5.2% to 8.8%. These capital leases mature on various dates between December 2009 and April 2013.
Future maturities of long-term debt and capital lease obligations for each of the years ended are as follows (in thousands):
| | | |
| | | As of
January 3, 2009 |
2009 | | $ | 465 |
2010 | | | 64 |
2011 | | | 53 |
2012 | | | 39 |
2013 | | | 1 |
| | | |
Total | | $ | 622 |
| | | |
F-24
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 9. | Other Liabilities, Long-term |
Other long-term liabilities consist of the following:
| | | | | | |
| | | January 3,
2009 | | December 29,
2007 |
Unrecognized tax benefit | | $ | 7,206 | | $ | 3,319 |
Minority interest in Masimo Labs | | | 107 | | | 39 |
Deferred rent, long term | | | 49 | | | 277 |
Other | | | 578 | | | 133 |
| | | | | | |
Other liabilities, long-term | | $ | 7,940 | | $ | 3,768 |
| | | | | | |
The unrecognized tax benefit relates to the Company’s long term portion of tax liability for FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109,” or FIN 48, which became effective on January 1, 2007. FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. See Note 15 for further details.
Stock Split
In May 2007 and June 2007, the Company’s Board of Directors and stockholders, respectively, approved a forward stock split of the Company’s common stock at a ratio of three shares for every one share previously outstanding. The forward stock split was effective on June 25, 2007. As a result of the stock split, the conversion price of each outstanding share of the Company’s preferred stock was reduced to one-third of the pre-stock split conversion price of such preferred stock, and effectively increased the conversion ratio to three shares of common stock for one share of preferred stock. All common stock share and per share data included in these consolidated financial statements reflect the forward stock split.
Authorized Number of Common Shares
In May 2007 and June 2007, the Company’s board of directors and stockholders, respectively, approved an increase in the authorized number of shares of common stock to 77,500,000 shares to accommodate the June 25, 2007 stock split.
Initial Public Offering
In August 2007, the Company completed its IPO of common stock in which a total of 13,704,120 shares were sold and issued, comprised of 10,416,626 shares sold by selling stockholders, 1,500,000 shares sold by the Company at the initial closing and 1,787,494 shares sold by the Company pursuant to the underwriters’ full exercise of their over-allotment option, at an issue price of $17.00 per share. The Company raised a total of $55.9 million in gross proceeds from the IPO, or approximately $47.8 million in net proceeds after deducting underwriting discounts and commissions of $3.9 million and estimated other offering costs of approximately $4.2 million. The consolidated financial statements, including share and per share amounts, include the effects of the offering.
Upon closing of the IPO, an Amended and Restated Certificate of Incorporation which had been approved in May 2007 and June 2007 by the Company’s Board of Directors and stockholders, respectively, became effective. The Amended and Restated Certificate of Incorporation increased the authorized number of shares of common stock to 100,000,000 and authorized 5,000,000 shares of preferred stock of the Company, known as “blank check” preferred stock, to be issued in the future at the full discretion of the Board of Directors.
Convertible Preferred Stock
Upon the closing of the IPO in 2007, all outstanding shares of convertible preferred stock, Series A through G, automatically converted into an aggregate of 34,612,503 shares of common stock. Also, the convertible preferred stockholders rights to receive annual cumulative dividends and the related accretion were eliminated. Voting rights of the preferred stockholders was eliminated.
In March 2006, in connection with a dividend declaration resulting from the settlement of the Company’s patent litigation with Nellcor (See Note 11), the Board and the Company’s stockholders approved an amendment to the Company’s Certificate of Incorporation that eliminated all mandatory redemption provisions of the Preferred Stock. The amendment was adopted concurrently with the Board’s declaration of a dividend, in an amount of up to $3.83 per share, on the Company’s outstanding Common Stock and Preferred Stock, assuming conversion of the preferred stock into 34,612,503 shares of common stock. Upon elimination of the mandatory redemption feature, the preferred stock was reclassified to the stockholders’ equity (deficit) section of the Company’s
F-25
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
consolidated balance sheet as of March 31, 2006. The amendment to the Certificate of Incorporation permitted the payment of the dividends on all shares of the Company’s capital stock on a pro-rata basis.
When the dividends were declared in March and December 2006, cumulative dividends accreted through each date were reclassified from the carrying value of preferred stock to dividends payable. The reclassification aggregated $55.6 million and $8.0 million in March and December 2006, respectively. Dividends declared and paid in excess of dividends accreted through each of the dividend declaration dates are reflected as dividends declared in the accompanying consolidated statements of stockholders’ equity (deficit).
Series A Junior Participating Preferred Stock and Stockholder Rights Plan
On November 8, 2007, the Company authorized and declared a dividend of one preferred stock purchase right, or a Right, for each outstanding share of its Common Stock to stockholders of record at the close of business on November 26, 2007, or the Record Date. Each Right entitles the registered holder to purchase from the Company one one-thousandth of one share of the Company’s Series A junior participating preferred stock, par value $0.001 per share, at a purchase price equal to $136.00 per Right, subject to adjustment. In addition, one Right will be issued with each share of Common Stock that becomes outstanding after the Record Date, and prior to the earliest of the distribution date, the date the Rights are redeemed, or the Final Expiration Date. In connection with the stockholder rights plan described herein, the Board designated 100,000 shares of preferred stock as Series A junior participating preferred stock, as set forth in the Certificate of Designation of Series A Junior Participating Preferred Stock.
Until a Right is exercised, the holder of such Right will have no rights as a stockholder of the Company, beyond those as an existing stockholder, including, without limitation, the right to vote or to receive dividends. Subject to certain exceptions specified in the Rights Agreement, the Rights will separate from the Common Stock and a distribution date will occur upon the earlier of (i) ten business days following a public announcement that a person or group of affiliated persons or an Acquiring Person has acquired beneficial ownership of 15% or more of the Company’s outstanding Common Stock, other than as a result of repurchases of stock by the Company or actions determined to be inadvertent by the Board of Directors of the Company, or Board, by a person or group of affiliated or associated persons and such person or group promptly sells shares of the Company’s Common Stock until such person or group owns less than 15% of the Company’s outstanding Common Stock, or (ii) ten business days following the announcement of an intention to make a tender offer or exchange offer that would result in a person or group becoming an Acquiring Person. The Rights have certain anti-takeover effects. The Rights will cause dilution to a person or group that attempts to acquire the Company in a transaction which the Board does not approve as in the best interests of the Company and its stockholders, as discussed in detail below.
In the event that a person becomes an Acquiring Person, each holder of a Right, other than the Acquiring Person, will thereafter have the right to receive, upon exercise, Common Stock having a market value equal to two times the exercise price of the Right. However, Rights are not exercisable following the occurrence of the event set forth above until such time as the Rights are no longer redeemable by the Board as set forth below. All Rights that are or were beneficially owned by any Acquiring Person will be null and void. In the event any person or group becomes an Acquiring Person and the Company merges into or engages in certain other business combinations with an Acquiring Person, or 50% or more of the Company’s consolidated assets or earning power are sold to an Acquiring Person, each holder of a Right will thereafter have the right to receive, upon exercise, common stock of the acquiring company that at the time of such transaction will have a market value of two times the exercise price of the Right.
At any time after a person becomes an Acquiring Person, the Board may exchange the Rights, in whole or in part, at an exchange ratio of one share of Common Stock, or, under certain circumstances, cash, property or other securities of the Company, including fractions of a share of preferred stock, per Right. The Rights will not be exercisable until the Distribution Date and will expire on November 8, 2017, the ten-year anniversary of the date the Rights Agreement was approved by the Pricing Committee, unless such date is extended or the Board redeems or exchanges them before that time.
At any time before a person or group becomes an Acquiring Person, the Board may redeem the Rights in whole, but not in part, at a price of $0.001 per Right and on such terms and conditions as the Board may establish. Immediately upon the action of the Board ordering redemption of the Rights, the right to exercise the Rights will terminate and the only right of the holders of Rights will be to receive the redemption price. The terms of the Rights may be amended by a resolution of the Board without the consent of the holders of the Rights, except that after a person or group becomes an Acquiring Person, no such amendment may adversely affect the interests of the holders of the Rights. After the period for redemption of the Rights has expired, the Board may not amend the Rights Agreement to extend the period for redemption of the Rights.
The shares of Preferred Stock issuable upon exercise of the Rights have the following characteristics: they are not redeemable; the holders of Preferred Stock are entitled, when, as and if declared, to minimum preferential quarterly dividend payments of an amount equal to (i) $1.00 per share or (ii) 1,000 times the aggregate per share amount of all cash dividends and 1,000 times the aggregate per share amount of all non-cash dividends or other distributions; the holders of Preferred Stock are entitled, in the event of a liquidation,
F-26
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
dissolution or winding up, to a minimum preferential payment equal to $1,000 per share, plus all accrued and unpaid dividends, provided that the holders shall be entitled to receive 1,000 times the aggregate payment made per common share; the holders of Preferred Stock are entitled to 1,000 votes per share, voting together with the Common Stock; and the holders of Preferred Stock are entitled, in the event of a merger, consolidation or other transaction in which outstanding shares of Common Stock are converted or exchanged, to receive 1,000 times the amount received per share of Common Stock.
| 11. | Cash Dividends and Special Bonus Payments |
In March 2006, the Company declared a cash dividend of $3.365 per share, in the aggregate amount of approximately $171.8 million, to holders of the Company’s common and preferred stock, assuming the conversion of all outstanding shares of preferred stock into an aggregate of 34,612,503 shares of common stock. Of this amount, $21.7 million relates to dividend payments made to stockholders who exercised stock options by delivering a non-recourse promissory note for the full exercise price. In accordance EITF, 95-16, the $21.7 million in cash dividends have been classified as compensation expense in the accompanying consolidated financial statements, under cost of goods sold, research and development and selling, general and administrative expenses. In December 2006, the Company declared additional cash dividends of $0.468 per share and $0.257 per share, in the aggregate amount of approximately $37.1 million, to holders of the Company’s common and preferred stock assuming conversion into common stock. In March 2006 and December 2006, the Company also recorded special bonus charges in the aggregate amount of approximately $9.7 million and $2.0 million, respectively, to employees and directors who held vested stock options as of March 1, 2006. These cash dividends and special bonus payments were made from the after-tax proceeds that the Company received from the Company’s patent infringement lawsuit against Nellcor and interest earned thereon.
The following table identifies, from December 31, 2005 through January 3, 2009, the activity in dividends payable and convertible preferred stock resulting from the accretion, dividends declared and dividends paid during this period (in thousands).
| | | | | | | | |
| | | Dividends
Payable | | | Convertible
Preferred
Stock | |
Balance as of December 31, 2005 | | $ | — | | | $ | (143,959 | ) |
Accretion of redemption value on convertible preferred stock | | | — | | | | (7,985 | ) |
Dividends declared: | | | | | | | | |
Reclassification of cumulative dividends accreted to dividends payable | | | (63,616 | ) | | | 63,616 | |
Common shares securing the outstanding non recourse notes | | | (21,673 | ) | | | — | |
Dividends declared in excess of (i) amounts previously accreted to holders of preferred stock and (ii) amount included in stock compensation expense | | | (123,620 | ) | | | — | |
| | | | | | | | |
Total dividends declared | | | (208,909 | ) | | | 63,616 | |
Dividends paid in 2006 | | | 171,376 | | | | — | |
| | | | | | | | |
Balance as of December 31, 2006 | | | (37,533 | ) | | | (88,328 | ) |
Accretion of redemption value on convertible preferred stock | | | — | | | | (4,837 | ) |
Dividends paid in 2007 | | | 37,350 | | | | — | |
Conversion of preferred stock into common shares upon completion of initial public offering | | | — | | | | 93,165 | |
| | | | | | | | |
Balance as of December 29, 2007 | | | (183 | ) | | | — | |
Dividends paid in 2008 | | | 13 | | | | — | |
| | | | | | | | |
Balance as of January 3, 2009 | | $ | (170 | ) | | $ | — | |
| | | | | | | | |
| 12. | Share Based Compensation |
The Company’s 1989 Incentive Stock Option, Nonqualified Stock Option, and Restricted Stock Purchase Plan, or the 1989 Plan, provided for the issuance of options to purchase up to 3,000,000 shares of the Company’s common stock to eligible officers, key employees, non-employee directors and consultants of the Company at prices not less than the fair market value of the Company’s common stock on the date the option is granted, as determined by the Board. The options vested annually over five years using the straight-line method, unless otherwise provided, and expire five or ten years from the date of grant. The 1989 Plan terminated on September 26, 1999.
In May 1996, the Company adopted the 1996 Incentive Stock Option, Nonqualified Stock Option, and Restricted Stock Purchase Plan, or the 1996 Plan, which initially provided for the issuance of options to purchase up to 600,000 shares of the Company’s common stock, to eligible officers, key employees, non-employee directors and consultants of the Company at prices not less than the fair
F-27
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
market value of the Company’s common stock on the date the option is granted, as determined by the Board. The options vested annually over five years using the straight-line method, unless otherwise provided, and expire ten years from the date of grant. The Board voted in October 1999 to amend the 1996 Plan to increase the number of shares authorized for issuance to include the unissued options from the 1989 Plan prior to its expiration, as well as any additional options that would become available through future forfeitures. The Board approved increases in the number of shares available for grant under the 1996 Plan to 3,600,000 shares in December 1997, to 4,200,000 shares in August 1999, to 7,200,000 shares in March 2000, and to 9,450,000 shares in March 2003. The Company terminated the 1996 Plan on May 4, 2006.
In April 2004, the Company adopted the 2004 Incentive Stock Option, Nonqualified Stock Option, and Restricted Stock Purchase Plan, or the 2004 Plan, which initially provided for the issuance of options to purchase up to 3,000,000 shares of the Company’s common stock, plus any shares available under the prior year stock option plans, including shares that become available due to forfeitures at prices not less than the fair market value of the Company’s common stock on the date the option is granted, as determined by the Board. The options generally vest annually over five years using the straight-line method, unless otherwise provided, and expire ten years from the date of grant. The Board approved increases in the number of shares available for grant under the 2004 Plan to 4,500,000 shares on February 6, 2006, to 6,000,000 shares on November 1, 2006 and to 7,500,000 shares on May 24, 2007. The Company may terminate the 2004 Plan at any time. If not terminated sooner, the 2004 Plan will automatically terminate on April 29, 2014.
On August 7, 2007, in connection with the Company’s IPO, the 2007 Stock Incentive Plan, or the 2007 Plan, became effective. Under the 2007 Plan, 3,000,000 shares of common stock are reserved for future issuance, plus any shares available under the prior year stock option plans, including shares that become available due to forfeitures at prices not less than the fair market value of the Company’s common stock on the date the option is granted. The options generally vest annually over five years using the straight-line method, unless otherwise provided, and expire ten years from the date of grant.
The number and weighted average exercise price of options issued and outstanding under all stock option plans, at exercise prices ranging between $1.34 and $41.51 per share, are as follows:
| | | | | | | | | | | | | | | | | | |
| | | Year ended
January 3, 2009 | | Year ended
December 29, 2007 | | Year ended
December 31, 2006 |
| | | Shares | | | Average
Exercise
Price | | Shares | | | Average
Exercise
Price | | Shares | | | Average
Exercise
Price |
Options outstanding, beginning of period | | 8,321,191 | | | $ | 7.53 | | 7,691,388 | | | $ | 4.95 | | 13,036,320 | | | $ | 2.58 |
Granted | | 2,493,000 | | | $ | 32.05 | | 1,676,150 | | | $ | 19.40 | | 2,209,620 | | | $ | 10.72 |
Canceled | | (850,422 | ) | | $ | 18.53 | | (776,504 | ) | | $ | 9.14 | | (814,830 | ) | | $ | 5.82 |
Expired | | — | | | $ | — | | (1,500 | ) | | $ | 1.00 | | — | | | $ | — |
Exercised | | (2,634,295 | ) | | $ | 3.70 | | (268,343 | ) | | $ | 3.10 | | (6,739,722 | ) | | $ | 2.14 |
| | | | | | | | | | | | | | | | | | |
Options outstanding, end of period | | 7,329,474 | | | $ | 15.97 | | 8,321,191 | | | $ | 7.53 | | 7,691,388 | | | $ | 4.95 |
| | | | | | | | | | | | | | | | | | |
Options exercisable, end of period | | 2,760,046 | | | $ | 6.18 | | 4,052,329 | | | $ | 3.48 | | 3,197,682 | | | $ | 2.72 |
Options available for grant, end of period | | 1,352,156 | | | | | | 5,396,076 | | | | | | 1,752,582 | | | | |
The weighted-average fair value of options granted was $13.70 for the year ended January 3, 2009, $9.01 for the year ended December 29, 2007 and $5.74 for the year ended December 31, 2006. At January 3, 2009, an aggregate of 8,681,630 shares of common stock were reserved for future issuance under the plans.
Effective January 1, 2007, the fair value of each option is estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions used for grants:
| | | | | | |
| | | Year ended
January 3, 2009 | | Year ended
December 29, 2007 | | Year ended
December 31, 2006 |
Risk-free interest rate | | 1.1% to 3.8% | | 3.4% to 4.8% | | 4.4% to 4.9% |
Expected term | | 5.3 years to 6.5 years | | 6.5 years | | 6.5 years |
Estimated volatility | | 36.6% to 53.4% | | 36.7% to 41.6% | | 44.8% to 48.6% |
Expected dividends | | 0% | | 0% | | 0% |
F-28
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The Black-Scholes option pricing model requires the use of certain assumptions, including fair value, expected terms, expected volatility, expected dividends, risk-free interest rate and expected forfeiture rate to calculate the fair value of share-based payment awards.
As a non-public company prior to August 13, 2007, the date of completion of its IPO, the Company estimated the current price of the underlying shares based on valuations established by the Board. Historically, the Board has used various sources to establish the value of the Company’s stock. As a publicly traded entity, the Company relies on daily reported prices of the Company’s shares.
The risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues with a remaining term approximately equal to the expected life of the Company’s stock options.
During the year ended December 29, 2007 and the nine months ended September 27, 2008, the Company did not have sufficient information available which is indicative of future exercise and post-vesting behavior to estimate the expected term. The Company adopted the simplified method of estimating the expected term of a stock option, as permitted by Staff Accounting Bulletin 107. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term. The use of the simplified method requires the Company’s option plan to be consistent with a “plain vanilla” plan. Subsequent to September 27, 2008, we had sufficient Company specific and available external information to estimate its expected term and therefore did not rely on the simplified method. As the Company obtains more historical data as a publicly traded company, it expects to rely increasingly on Company specific information for its estimate of expected term.
Additionally, during the year ended December 29, 2007 and the nine months ended September 27, 2008, the Company did not have sufficient information available regarding the historic volatility for its shares. As a result, the Company estimated volatility based on a peer group of companies, which collectively provided a reasonable basis for estimating volatility. Subsequent to September 27, 2008, and after the Company had been publicly traded for more than one year, we were able to use Company specific as well as peer group information to estimate the volatility of its shares. As the Company obtains more historical data as a publicly traded company, it expects to rely increasingly on Company specific information for its estimate of volatility. The changes in the method of estimating the expected term and the volatility did not have a material impact on the results of operations.
The Company does not expect to issue dividends in the future. As part of an unusual, one-time patent settlement, the Board declared a dividend in March 2006 and declared two dividends in December 2006. These dividends were declared only due to the receipt of settlement proceeds in connection with patent infringement litigation with a competitor. Absent such a settlement, the Company would not have declared and paid either dividend.
The estimated pre-vesting forfeiture rate is based on the Company’s historical experience and the composition of option plan participants, among other factors, and reduces the compensation expense recognized. If the actual forfeitures differ from the estimates, adjustments to compensation expense may be required in future periods.
As a result of adopting SFAS 123(R), the Company recorded share-based payment expense of $7.7 million, $3.5 million, and $1.3 million during the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively. The related deferred tax asset was $3.7 million as of January 3, 2009 and $1.7 million as of December 29, 2007. For the year ended December 31, 2006, income before taxes, income tax expense and net income were lower by $1.3 million, $500,000 and $800,000, respectively, than if the Company had continued to account for share-based payment expense under APB Opinion No. 25. Basic and diluted net income per common share was lower by $0.05 and $0.04, respectively. The Company has elected to recognize share-based payment expense on a straight-line basis over the requisite service period for the entire award.
As of January 3, 2009, there was $44.4 million of total unrecognized share-based payment expense related to unvested options granted or modified on or after January 1, 2006. That expense is expected to be recognized over a weighted average period of 3.9 years as of January 3, 2009. The total fair market value on the respective vesting dates of all options vesting during 2008 and 2007, aggregated $21.9 million and $24.5 million, respectively.
The aggregate intrinsic value of options outstanding, with an exercise price greater than their fair market values, as of January 3, 2009 was $107.6 million. The aggregate intrinsic value of options exercisable, with an exercise price greater than their fair market values, as of January 3, 2009 was $65.6 million. The aggregate intrinsic value of options exercised during 2008, 2007 and 2006 was $75.0 million, $4.1 million and $23.7 million, respectively. The intrinsic value is calculated as the difference between the market value of the Company’s common stock on the date of exercise or the respective period end, as appropriate, and the exercise price of the options. The weighted average remaining contractual term of options outstanding as of January 3, 2009 was 6.5 years. The weighted average remaining contractual term of options exercisable as of January 3, 2009 was 5.2 years. No options were granted to consultants during the years ended January 3, 2009 and December 29, 2007. For the year ended December 31, 2006, deferred compensation amortized to expense related to options granted to consultants was $43,000. No amount of deferred compensation was amortized to
F-29
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
expense for the years ended January 3, 2009 and December 29, 2007. At January 3, 2009 and December 29, 2007, the remaining deferred compensation related to options granted to consultants was $0.
The schedule below reflects the number and weighted average exercise price of outstanding and exercisable options segregated by exercise price ranges:
| | | | | | | | | | | | |
| | | January 3, 2009 | | December 29, 2007 |
| | | Options Outstanding | | Options
Exercisable | | Options Outstanding | | Options
Exercisable |
Range of Exercise Prices | | Number of
Options | | Average
Remaining
Contractual
Life | | Number of
Options | | Number of
Options | | Average
Remaining
Contractual
Life | | Number of
Options |
$1.34 to $4.00 | | 2,315,509 | | 4.50 | | 1,858,531 | | 4,632,785 | | 4.87 | | 3,477,923 |
$4.67 to $12.00 | | 1,444,995 | | 7.34 | | 604,935 | | 2,135,156 | | 8.37 | | 549,776 |
$12.87 to $16.00 | | 1,008,600 | | 8.36 | | 194,180 | | 1,136,000 | | 9.38 | | 16,650 |
$24.32 to $28.98 | | 564,470 | | 9.44 | | 20,760 | | 202,300 | | 9.77 | | 2,280 |
$29.83 to $31.99 | | 1,289,800 | | 9.11 | | 9,300 | | 169,250 | | 9.86 | | — |
$32.09 to $38.30 | | 293,600 | | 9.52 | | 400 | | — | | — | | — |
$38.44 to $41.51 | | 412,500 | | 9.47 | | 71,940 | | 45,700 | | 9.95 | | 5,700 |
| | | | | | | | | | | | |
Total | | 7,329,474 | | 7.26 | | 2,760,046 | | 8,321,191 | | 6.63 | | 4,052,329 |
| | | | | | | | | | | | |
The weighted-average exercise price of all options outstanding as of January 3, 2009 and December 29, 2007, was $15.97 and $7.53 per option, respectively.
The Company repurchased 41,640 shares of common stock from former employees for $581,000 during the year ended December 29, 2007 and 114,600 shares of common stock for $628,000 during the year ended December 31, 2006. These shares were recorded in treasury stock, using the cost method, and are available for reissue. The difference between the repurchase prices and the original option exercise prices totaled $417,000 and $313,000 and was recorded as an operating expense for the year ended December 29, 2007 and the year ended December 31, 2006, respectively. No shares were repurchased during the year ended January 3, 2009.
| 13. | Commitments and Contingencies |
Leases
The Company leases its manufacturing and headquarters facilities in the United States under non-cancelable operating leases that expire in October 2009 and March 2010. These leases contain pre-determined price escalations. The Company also leases its manufacturing facilities in Mexico and offices in France, Germany, United Kingdom, Japan, Singapore, Australia, and China under operating lease agreements, almost all of which are non-cancelable, expiring at various dates through August 2014. The Company also recently entered into a lease agreement for its new international headquarters in Switzerland. Certain leases contain pre-determined price escalations and in some cases renewal options. The Company recognizes the lease costs using a straight line method based on total lease payments. The Company also received certain leasehold improvement incentives totaling approximately $650,000 for its manufacturing and headquarters facilities in the United States. These leasehold improvement incentives have been recorded as deferred rent and are being amortized as a reduction to rent expense on a straight-line basis over the life of the lease. As of January 3, 2009 and December 29, 2007, rent expense accrued in excess of the amount paid and the remaining unamortized leasehold improvement incentive aggregated $282,000 and $625,000, respectively. The Company also leases automobiles in Europe and Japan that are classified as operating leases and expire at various dates through October 2010. The majority of these leases are non-cancelable. The Company also has capital leases outstanding for office equipment all of which are non-cancelable.
Future minimum lease payments under operating and capital leases for each of the following fiscal years ending on or about December 31 are as follows (in thousands):
| | | | | | | | | |
| | | As of January 3, 2009 |
| | | Operating
Leases | | Capital
Leases | | Total |
2009 | | $ | 2,833 | | $ | 82 | | $ | 2,915 |
2010 | | | 1,132 | | | 71 | | | 1,203 |
2011 | | | 658 | | | 57 | | | 715 |
2012 | | | 645 | | | 40 | | | 685 |
2013 | | | 635 | | | 1 | | | 636 |
Thereafter | | | 353 | | | — | | | 353 |
| | | | | | | | | |
Total | | $ | 6,256 | | $ | 251 | | $ | 6,507 |
| | | | | | | | | |
F-30
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Rental expense related to operating leases for the years ended January 3, 2009, December 29, 2007 and December 31, 2006 was $3.3 million, $2.2 million and $1.6 million, respectively. Included in the capital lease obligations as of January 3, 2009 is interest aggregating $24,000.
Employee Benefit Plan
In fiscal year 1996, the Company adopted the Masimo Retirement Savings Plan, or the Plan, which is a 401(k) plan covering all of the Company’s full-time U.S. employees who meet certain eligibility requirements. In general, the Company matches 100% of an employee’s contribution up to 3% of the employee’s compensation, subject to a maximum amount. The Company may also contribute to the Plan on a discretionary basis. The Company contributed $1.1 million, $977,000 and $491,000 to the plan for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively, all in the form of matching contributions.
Employment and Severance Agreement
As of January 3, 2009, the Company had an employment agreement with one of its key employees that provides for an aggregate annual base salary of $686,400 plus other benefits, with annual increases at the discretion of the Board of its Compensation Committee. The agreement with the Company also provides for an annual bonus based on the Company’s attainment of certain objectives and goals. The agreement had an initial term of three years, with automatic renewal, unless either the Company or the executive notifies the other party of non-renewal of the agreement. Also, under this employment agreement, the key employee may be entitled to receive certain salary, equity, tax, medical and life insurance benefits if he is terminated by the Company, terminates his employment for good reason under certain circumstances or there is a change in control of the Company. As of January 3, 2009, the Company had properly not recorded any accrual for severance under this agreement.
Another employment agreement provides for an annual base salary of EUR €150,000 ($209,000 as of January 3, 2009). The agreement also contemplates an annual bonus based on the attainment of certain revenue, profit and gross margin milestones. The agreement also contains a non-compete provision. If the Company enforces this provision following the employee’s termination of employment, the employee would be entitled to receive a lump sum payment equal to 50% of his annual base salary as of the date of his termination, which shall be paid in equal installments over the term of the non-competition period. As of January 3, 2009, the Company had properly not recorded any accrual for severance under this agreement.
On January 11, 2008, the Company entered into a severance plan participation agreement with three of its executive officers. The participation agreements, or Agreements, are governed by the terms and conditions of the Company’s 2007 Severance Protection Plan, or Severance Plan, which became effective on July 19, 2007. Under the Agreements, the executive officer may be entitled to receive certain salary, equity, medical and life insurance benefits if he is terminated by the Company without cause or terminates his employment for good reason under certain circumstances. The executive officers are also required to give the Company six months advance notice of their resignation under certain circumstances. As of January 3, 2009, the Company had properly not recorded any accrual for these severance agreements.
Purchase Commitments
Pursuant to contractual obligations with vendors, the Company had $23.4 million of purchase commitments as of January 3, 2009, of which at least $21.7 million is expected to be purchased within 1 year. The remaining $1.7 million may be purchased within the next 1 to 2 years. The Company does not have any purchase commitments for more than 2 years. These purchase commitments were made for certain inventory items to secure better pricing and to ensure the Company will have raw materials when necessary.
Concentrations of Risk
The Company is exposed to credit loss for the amount of cash deposits with financial institutions in excess of federally insured limits. As of January 3, 2009, the Company had $7.8 million of bank balances of which $884,000 was covered by the Federal Deposit Insurance Corporation limit. The Company invests its excess cash deposits in U.S. Treasury bills and money market accounts with major financial institutions. As of January 3, 2009, the Company had $125.5 million in U.S. Treasury bills which are backed by the U.S. federal government. Also, as of January 3, 2009, the Company had $13.6 million in money market accounts, of which $11.8 million was covered under the U.S. Treasury Department Temporary Guarantee Program for Money Market Funds effective on September 19, 2008.
F-31
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
While the Company and its contract manufacturers rely on sole source suppliers for certain components, steps have been taken to minimize the impact of a shortage or stoppage of shipments, such as maintaining excess inventory and designing products that may be easily modified to use a different component. There can be no assurance that a shortage or stoppage of shipments of the materials or components that the Company purchases will not result in a delay in production, or adversely affect the Company’s business.
The Company’s ability to sell its products to U.S. hospitals depends in part on its relationships with Group Purchasing Organizations, or GPOs. Many existing and potential customers for the Company’s products become members of GPOs. GPOs negotiate pricing arrangements and contracts, sometimes exclusive, with medical supply manufacturers and distributors, and these negotiated prices are made available to a GPO’s affiliated hospitals and other members. For the years ended January 3, 2009, December 29, 2007 and December 31, 2006, revenue from the sale of the Company’s pulse oximetry products to customers affiliated with GPOs amounted to $132.1 million, $101.0 million and $66.6 million, respectively, representing, 68.1%, 89.4% and 80.7%, respectively, of its revenue from sales to U.S. hospitals.
For the years ended January 3, 2009 and December 29, 2007, one customer represented 12% and 11% of total revenue. This particular customer was a distributor, which takes and fulfills orders from our direct customers, many of whom have signed long-term sensor agreements with us. In the event this distributor was unable to fulfill these orders, the orders would be redirected to other distributors of ours or fulfilled directly by us. For the year ended December 31, 2006, no individual customer represented over 10% of total revenue.
Two customers represented 10% and 7% of accounts receivable at January 3, 2009 and 8% and 5% of accounts receivable at December 29, 2007, respectively. A third customer represented 6% of accounts receivable at December 29, 2007.
Litigation
In May 2002, the Company filed a lawsuit against Tyco Healthcare, parent company of Nellcor, in the United States District Court for the Central District of California, alleging damage to the Company’s business as a result of the anti-competitive business practices of Tyco Healthcare. Specifically, the Company alleges that it had incurred damages as a result of a series of illegal exclusionary and anti-competitive acts by Tyco Healthcare that were designed to maintain its monopoly in the pulse oximetry market in violation of federal antitrust laws.
In March 2005, a jury found that Tyco Healthcare’s use of sole-source contracts, product bundling and market share-based compliance pricing contracts, among other conduct, violated the federal antitrust laws and awarded damages on that basis. Tyco Healthcare filed post-trial motions requesting that the District Court either override the jury decision or grant a new trial. In March 2006, the District Court upheld a portion of the jury verdict and vacated the remaining verdict. In addition, the District Court vacated the jury’s damages award and granted Tyco Healthcare a new trial on damages. After a retrial of damages to the court, on July 2, 2007, the District Court entered its final judgment awarding the Company damages which were trebled as is mandatory under federal antitrust law to $43.5 million and denying the Company’s request for a permanent injunction with respect to Tyco Healthcare’s business practices found to be anti-competitive. The Company and Tyco Healthcare each filed a notice of appeal from the judgment. The Company filed its opening brief on December 17, 2007 with the United States Court of Appeals for the 9th Circuit. On December 27, 2007, the Consumer Federation of America and Medical Device Manufacturers Association filed an Amicus brief supporting Masimo. Tyco filed its opposition and appeal brief on March 3, 2008 and a group of law professors filed an Amicus brief supporting Tyco on March 10, 2008. We filed our response and reply brief on May 19, 2008. The Consumer Federation of America and Medical Device Manufacturers Association filed an additional Amicus brief in support of us on May 29, 2008. Tyco filed its second appeal brief on July 17, 2008. We are seeking reinstatement of the jury’s verdict on bundling and an affirmance of the liability findings concerning sole-source and market share-based compliance contracts. We are also asking the appellate court to increase the amount of damages awarded by the trial court. Oral argument took place on December 8, 2008. Even if the Company is ultimately awarded damages in this litigation, the amount will be subject to a 50% legal fee contingency agreement, in which case the Company would receive 50% of the net (of costs) proceeds from the award. Even though most of the legal expenses to date have been on a contingency basis, the Company expects to incur expenses related to the appellate work, which will be reported as operating expense within the Company’s statements of income.
The Company believes the jury verdict it received in the Tyco Healthcare antitrust litigation has been important in its efforts to increase its market share among certain large hospital systems and GPOs that were formerly closed as a result of Tyco Healthcare’s anti-competitive conduct. However, the lawsuit has been and will continue to be a diversion of management’s attention from the implementation of the Company’s business strategy. See “Risk Factors” for a description of the risks related to the Company’s litigation against Tyco Healthcare.
On February 19, 2008, the Company filed a lawsuit against Respironics, Inc. for breach of contract, breach of the covenant of good faith and fair dealing, and interference with prospective economic advantage, based on a January 16, 2006, contract between
F-32
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Respironics and the Company. On April 7, 2008, Respironics filed a demurrer seeking to dismiss the lawsuit on the grounds that the Company’s complaint fails to state sufficient facts to constitute valid claims. The court subsequently denied Respironics’ demurrer. On July 16, 2008, Respironics answered the Company’s complaint and filed a cross-complaint. The Company answered the cross-complaint on August 15, 2008, denying all material allegations. There is no guarantee that the Company will prevail in this suit or receive any damages or other relief if it does prevail.
From time to time, the Company may be involved in litigation relating to claims arising out of its operations in the normal course of business. The Company believes that it currently is not a party to any legal proceedings which, individually or in the aggregate, would have a material adverse effect on its consolidated financial position, results of operations, or cash flows.
Voluntary Recall
On July 31, 2007, the Company determined to initiate a voluntary recall of its Rad-9 pulse oximeter, a standalone bedside pulse oximeter product, sales of which represented less than 0.2%, 0.3% and 0.6% of the Company’s product revenue in 2008, 2007 and 2006, respectively. In accordance with its original design and similar to other pulse oximeter devices, the Rad-9 gives a visual alarm if there is a sensor fault; under other circumstances, the Rad-9 gives both a visual and audio alarm. In late 2006, the Company sent notice to owners of the Rad-9 that a free upgrade was available to add an audio alarm to the Rad-9 when a sensor fault is detected. In July 2007, the Company decided to voluntarily recall the Rad-9 to implement this upgrade. The Company does not believe that a non-upgraded Rad-9 poses a significant risk to health. The Company decided to voluntarily recall the Rad-9 because it believes it has the possibility of improving the care of patients. This decision follows a customer report that an elderly patient, who may have damaged her pulse oximeter sensor, had died after removing her tracheostomy tube. Based on what is currently known, the Rad-9 appears to have been operating in accordance with its specifications. Originally, the Company estimated that the total costs resulting from this voluntary recall would be approximately $300,000. The Company incurred this charge in the quarter ended September 29, 2007. As of January 3, 2009, the Company had incurred actual upgrade costs of $292,000 and does not expect to incur any additional costs in the future related to this recall.
| 14. | Segment Information and Enterprise Reporting |
In accordance with SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information”, the Company’s chief decision maker, the Chief Executive Officer, reviews financial information presented on a consolidated basis, accompanied by disaggregated information about revenues by geographic region for purposes of making operating decisions and assessing financial performance. Accordingly, the Company considers itself to be in a single reporting segment, specifically patient monitoring and related products. The Company does not assess the performance of its geographic regions on other measures of income or expense, such as depreciation and amortization, operating income or net income. In addition, the Company’s assets are primarily located in the United States and are not allocated to any specific region. The Company does not produce reports for, or measure the performance of, its geographic regions on any asset-based metrics. Therefore, geographic information is presented only for revenues.
The following schedule presents an analysis of the Company’s product revenue based upon the geographic area to which the product was shipped (in thousands):
| | | | | | | | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Geographic Area by Destination | | | | | | | | | | | | | | | | | | |
North and South America | | $ | 200,090 | | 77 | % | | $ | 155,782 | | 78 | % | | $ | 123,193 | | 79 | % |
Europe, Middle East and Africa | | | 37,446 | | 15 | | | | 28,201 | | 14 | | | | 19,496 | | 13 | |
Asia and Australia | | | 21,359 | | 8 | | | | 15,701 | | 8 | | | | 12,442 | | 8 | |
| | | | | | | | | | | | | | | | | | |
Total product revenue | | $ | 258,895 | | 100 | % | | $ | 199,684 | | 100 | % | | $ | 155,131 | | 100 | % |
| | | | | | | | | | | | | | | | | | |
Sales to customers located in the United States were $194.0 million, $152.1 million and $120.0 million, for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively.
The components of income (loss) before provision for (benefit from) income taxes are as follows (in thousands):
F-33
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | Year ended
December 31,
2006 | |
United States | | $ | 104,156 | | | $ | 67,008 | | $ | 321,766 | |
Foreign | | | (31,765 | ) | | | 1,114 | | | (7,363 | ) |
| | | | | | | | | | | |
| | $ | 72,391 | | | $ | 68,122 | | $ | 314,403 | |
| | | | | | | | | | | |
The following table presents the current and deferred provision (benefit) for income taxes (in thousands):
| | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 |
Current: | | | | | | | | | | | |
Federal | | $ | 36,011 | | | $ | 20,654 | | | $ | 111,284 |
State | | | 3,699 | | | | 2,449 | | | | 14,893 |
Foreign | | | 307 | | | | 68 | | | | — |
| | | | | | | | | | | |
| | | 40,017 | | | | 23,171 | | | | 126,177 |
| | | | | | | | | | | |
Deferred: | | | | | | | | | | | |
Federal | | | 2,176 | | | | 2,747 | | | | 3,296 |
State | | | (1,447 | ) | | | (51 | ) | | | 3,104 |
Foreign | | | (282 | ) | | | — | | | | — |
| | | | | | | | | | | |
| | | 447 | | | | 2,696 | | | | 6,400 |
| | | | | | | | | | | |
Total | | $ | 40,464 | | | $ | 25,867 | | | $ | 132,577 |
| | | | | | | | | | | |
Included in the 2008 and 2007 current tax provisions above are $4.1 million and $1.7 million, respectively, of tax and accrued interest for the years with respect to FIN 48.
The temporary differences that give rise to the deferred tax provision (benefit) consist of (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Property and equipment | | $ | (358 | ) | | $ | (114 | ) | | $ | (8 | ) |
Capitalized research and development costs | | | 754 | | | | (217 | ) | | | (24 | ) |
Tax credits | | | (1,603 | ) | | | (241 | ) | | | 4,283 | |
Deferred revenue | | | 4,818 | | | | 1,046 | | | | 96 | |
Acquired intangibles | | | 88 | | | | 101 | | | | 86 | |
Net operating losses | | | 2,437 | | | | 1,787 | | | | 5,585 | |
Accrued liabilities | | | (1,686 | ) | | | 52 | | | | (1,873 | ) |
Share based compensation | | | (2,033 | ) | | | (1,121 | ) | | | (424 | ) |
State taxes and other | | | 666 | | | | 2,941 | | | | (5,090 | ) |
Change in valuation allowance | | | (2,636 | ) | | | (1,538 | ) | | | 3,769 | |
| | | | | | | | | | | | |
Total | | $ | 447 | | | $ | 2,696 | | | $ | 6,400 | |
| | | | | | | | | | | | |
The reconciliation of the U.S. federal statutory tax rate to the Company’s effective tax rate is as follows:
| | | | | | | | | |
| | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
Statutory regular federal income tax rate | | 35.0 | % | | 35.0 | % | | 35.0 | % |
State provision, net of federal benefit | | 2.0 | | | 2.3 | | | 3.7 | |
Nondeductible items | | 0.8 | | | 0.9 | | | 2.7 | |
Intercompany cost sharing and prepaid licensing | | 20.6 | | | — | | | — | |
Tax credits | | (2.2 | ) | | (0.5 | ) | | (0.1 | ) |
Other | | (0.3 | ) | | 0.3 | | | 0.9 | |
| | | | | | | | | |
Total | | 55.9 | % | | 38.0 | % | | 42.2 | % |
| | | | | | | | | |
F-34
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The provision for income taxes was $40.5 million, or an effective tax rate of 55.9%, during the year ended January 3, 2009 compared to $25.9 million, or an effective tax rate of 38.0 %, during the year ended December 29, 2007. The effective tax rate differs from the statutory U.S. federal income tax rate of 35.0% primarily due to state taxes, permanent differences between GAAP pre-tax income and taxable income, the mix of income and losses not benefited across the jurisdictions in which we do business and research related tax credits. The effective rate in 2008 differs from 2007 due primarily to the implementation of a new international business structure, designed to ultimately align our operations, in a cost efficient manner, with the business needs of our non-US customers. The tax charges related to expenses for sharing in the costs of ongoing research and development efforts as well as the prepayment of licensing commercial rights to utilize pre-existing intangibles.
The components of the deferred tax assets are as follows (in thousands):
| | | | | | | | |
| | | January 3, 2009 | | | December 29, 2007 | |
Deferred tax assets: | | | | | | | | |
Property and equipment | | $ | 1,411 | | | $ | 1,053 | |
Capitalized research and development costs | | | — | | | | 447 | |
Tax credits | | | 2,845 | | | | 1,163 | |
Deferred revenue | | | 4,156 | | | | 8,974 | |
Acquired intangibles | | | 975 | | | | 1,063 | |
Net operating losses | | | 2,092 | | | | 4,529 | |
Accrued liabilities | | | 6,246 | | | | 4,565 | |
Share based compensation | | | 3,734 | | | | 1,701 | |
State taxes | | | — | | | | 794 | |
Other | | | 934 | | | | 505 | |
| | | | | | | | |
Total | | | 22,393 | | | | 24,794 | |
Valuation allowance | | | (2,492 | ) | | | (5,128 | ) |
| | | | | | | | |
Total deferred tax assets | | | 19,901 | | | | 19,666 | |
State taxes and other | | | (609 | ) | | | — | |
| | | | | | | | |
Net deferred tax assets | | $ | 19,292 | | | $ | 19,666 | |
| | | | | | | | |
Current net deferred tax asset | | | 10,511 | | | | 14,334 | |
Long term net deferred tax asset | | | 8,781 | | | | 5,332 | |
| | | | | | | | |
Net deferred tax assets | | $ | 19,292 | | | $ | 19,666 | |
| | | | | | | | |
At January 3, 2009, the Company has $33.4 million of net operating loss carryforwards from its foreign jurisdictions which will begin to expire in 2015. Also, the Company has $13.1 million of net operating losses from various states, which will begin to expire in 2012, of which $4.2 million, or $150,000 after tax effect, will be recorded in stockholders’ equity when realized. The Company has state research and development credits of $1.5 million which will carryforward indefinitely. Additionally, the Company has $282,000 of investment tax credit on research and development expenditures from its operations in Canada which will begin to expire in 2018. Management believes that it is more likely than not the deferred tax assets related to foreign and state net operating losses will not be realized. In making this determination, the Company considers all available positive and negative evidence, including scheduled reversals of liabilities, projected future taxable income, tax planning strategies and recent financial performances. A valuation allowance has been provided on such loss carryforwards.
During the year ended January 3, 2009, December 29, 2007 and December 31, 2006, the Company recorded a tax benefit of $19.1 million, $204,000 and $4.2 million, respectively, from the exercise of non-qualified stock options and incentive stock options as a reduction of its income tax liability and an increase in stockholders’ equity. The tax benefit results from the difference between the fair value of the Company’s common stock on the exercise dates and the exercise price of the option.
F-35
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The Company has not provided for income taxes on undistributed earnings of foreign subsidiaries as such earnings are intended to be permanently reinvested in those operations. As of January 3, 2009, the Company’s foreign subsidiaries have cumulative losses. Net deferred tax assets in the foreign subsidiaries relate primarily to net operating losses and are offset in full by valuation allowances.
Included in the Company’s consolidated income tax provision of $40.5 million, $25.9 million and $132.6 million for the years ended January 3, 2009, December 29, 2007 and December 31, 2006, respectively, are the following related to Masimo Labs: current income tax provision of $163,000, current income tax benefit of $36,000 and current income tax provision of $113,000, respectively, and deferred income tax benefit of $182,000, deferred income tax provision of $348,000 and deferred income tax benefit of $1.4 million, respectively. The temporary differences that give rise to the deferred tax benefit of $182,000 and deferred tax provision of $348,000 are mainly research and development credits, share-based payment expense and deferred revenue for the years ended January 3, 2009 and December 29, 2007, respectively. Masimo Labs’ deferred tax asset balance as of January 3, 2009 and December 29, 2007, was approximately $1.8 million and $1.6 million, respectively, which consists of deferred revenue, fixed assets and intangibles, share based compensation and research and experimentation credit carryforwards, which begin to expire in 2020. Management of Masimo Labs believes that it is more likely than not that part of deferred tax assets related to research and experimentation credit will not be realized. In making this determination, Masimo Labs considers all available positive and negative evidence, including scheduled reversals of liabilities, projected future taxable income, tax planning strategies and recent financial performances. For the years ended January 3, 2009 and December 29, 2007, a valuation allowance of $400,000 has been provided on such credit carryforwards.
In July 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109,” or FIN 48, which became effective on January 1, 2007. FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities.
The adoption of FIN 48 resulted in a reduction of the Company’s beginning retained earnings as of January 1, 2007, of $618,000. As of the adoption date, the balance of gross unrecognized tax benefits is $3.6 million.
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits for the year ended January 3, 2009 (in thousands):
| | | | |
Unrecognized tax benefits, December 30, 2007 | | $ | 3,340 | |
Increase from tax positions in prior period | | | 73 | |
Decrease from tax positions in prior period | | | (176 | ) |
Increase from tax positions in current period | | | 4,061 | |
Settlements | | | — | |
Lapse of statute of limitations. | | | — | |
| | | | |
Unrecognized tax benefits, January 3, 2009 | | $ | 7,298 | |
| | | | |
The amount of unrecognized benefits which, if ultimately recognized, could favorably affect the tax rate in a future period was $6.1 million and $2.2 million as of January 3, 2009 and December 29, 2007, respectively. Both amounts are net of any federal and/or state benefits, and the remaining balance relates to timing differences. It is reasonably possible that the amount of unrecognized tax benefits will decrease in the next 12 months by $30,000 primarily related to certain state taxes.
Interest and penalties related to unrecognized tax benefits are recognized in income tax expense. At January 3, 2009, the Company had accrued $446,000 for the payment of interest.
The Company conducts business in multiple jurisdictions, and as a result, one or more of the Company’s subsidiaries files income tax returns in the U.S. federal, various state, local and foreign jurisdictions. Due to the utilization of net operating loss carryforwards, all years since 1994 are open for examination by major taxing authorities.
Additional shares of common stock reserved
Pursuant to the “evergreen” provision contained in the 2007 Plan, an additional 1.7 million shares of common stock were added to the Company’s share reserve on January 4, 2009. The additional shares are 3% of the Company’s total shares outstanding as of the close of business on January 3, 2009.
F-36
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Philips Patent Infringement litigation
On February 3, 2009, the Company filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh related to Philips FAST pulse oximetry technology and certain Philips patient monitors. The suit was brought in the United States District Court for the District of Delaware. Two patents at issue in this suit, related to the Company’s measure-through-motion technology, were successfully enforced in a previous suit by the Company against Nellcor. There is no guarantee that the Company will prevail in this suit or receive any damages or other relief if it does prevail.
| 17. | Quarterly Financial Data (unaudited) |
The following tables contain selected unaudited Consolidated Statements of Operations data for each quarter of 2008 and 2007 (in thousands, except per share data):
| | | | | | | | | | | | | |
| | | Quarter ended | |
Fiscal 2008 | | March 29,
2008 | | June 28,
2008 | | September 27,
2008 | | January 3,
2009 | |
Total revenue | | $ | 71,110 | | $ | 74,766 | | $ | 78,132 | | $ | 83,066 | |
Gross profit | | | 49,989 | | | 53,363 | | | 55,739 | | | 58,529 | |
Operating income | | | 13,994 | | | 16,740 | | | 20,506 | | | 20,110 | |
Net income (loss)(1) | | | 8,791 | | | 10,601 | | | 13,065 | | | (530 | ) |
Net income (loss) per share: | | | | | | | | | | | | | |
Basic | | $ | 0.16 | | $ | 0.19 | | $ | 0.23 | | $ | (0.01 | ) |
Diluted(2) | | $ | 0.15 | | $ | 0.18 | | $ | 0.22 | | $ | (0.01 | ) |
| |
| | | Quarter ended | |
Fiscal 2007 | | March 31,
2007 | | June 30,
2007 | | September 29,
2007 | | December 29,
2007 | |
Total revenue | | $ | 58,954 | | $ | 63,680 | | $ | 64,376 | | $ | 69,276 | |
Gross profit(3) | | | 42,053 | | | 45,761 | | | 45,567 | | | 49,300 | |
Operating income(3) | | | 15,187 | | | 18,259 | | | 16,925 | | | 16,581 | |
Net income(3) | | | 9,097 | | | 10,556 | | | 10,550 | | | 12,055 | |
Net income attributable to common stockholders | | | 2,312 | | | 2,798 | | | 6,886 | | | 11,083 | |
Net income per share: | | | | | | | | | | | | | |
Basic | | $ | 0.14 | | $ | 0.17 | | $ | 0.18 | | $ | 0.22 | |
Diluted | | $ | 0.11 | | $ | 0.13 | | $ | 0.16 | | $ | 0.20 | |
(1) | During the quarter ended January 3, 2009, the Company incurred an income tax expense of $20.5 million, or 102.7% of income before provision for income taxes. This increase in tax expense was due to the implementation of an international restructuring during the quarter ended January 3, 2009 offset by benefit from increased research and development tax credits. The tax charges related to expenses for sharing in the costs of ongoing research and development efforts as well as the prepayment of licensing commercial rights to utilize pre-existing intangibles. |
(2) | The sum of the quarterly diluted net income (loss) per common share amounts for the year ended January 3, 2009 does not equal the annual related per common share amounts due to differences in the weighted average common shares outstanding between the quarterly and annual computations. |
(3) | The sum of the quarterly amounts for 2007 does not equal the annual related amount due to rounding within the individual quarters. |
F-37
