UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended January 1, 2011
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-33642
Masimo Corporation
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 33-0368882 |
(State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification Number) |
| |
| 40 Parker Irvine, California | | 92618 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(949) 297-7000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class: | | Name of each exchange on which registered: |
| Common Stock, par value $0.001 | | The NASDAQ Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one).
| | | | | | |
| Large accelerated filer | | x | | Accelerated filer | | ¨ |
| | | |
Non-accelerated filer | | ¨ (Do not check if a smaller reporting company) | | Smaller reporting company | | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of the registrant, based upon the closing sale price of the common stock on July 3, 2010, the last business day of the registrant’s most recently completed second fiscal quarter, as reported on the NASDAQ Global Select Market, was approximately $1.23 billion. Shares of stock held by officers, directors and 5 percent or more stockholders have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
At January 28, 2011, the registrant had 59,554,639 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III of this Form 10-K incorporate information by reference from the registrant’s proxy statement for the registrant’s 2011 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this annual report.
MASIMO CORPORATION
FISCAL YEAR 2010 FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, or this Form 10-K, contains “forward-looking statements” that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially and adversely from those expressed or implied by such forward-looking statements. The forward-looking statements are contained principally in Item 1—”Business,” Item 1A— “Risk Factors” and Item 7— “Management’s Discussion and Analysis of Financial Condition and Results of Operations” but appear throughout this Form 10-K. Examples of forward-looking statements include, but are not limited to any projection or expectation of earnings, revenue or other financial items; the plans, strategies and objectives of management for future operations; factors that may affect our operating results; our success in pending litigation; new products or services; the demand for our products; our ability to consummate acquisitions and successfully integrate them into our operations; future capital expenditures; effects of current or future economic conditions or performance; industry trends and other matters that do not relate strictly to historical facts or statements of assumptions underlying any of the foregoing. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “opportunity,” “plan,” “potential,” “predicts,” “seek,” “should,” “will,” or “would,” and similar expressions and variations or negatives of these words. These forward-looking statements are based on the expectations, estimates, projections, beliefs and assumptions of our management based on information currently available to management, all of which is subject to change. Such forward-looking statements are subject to risks, uncertainties and other factors that are difficult to predict and could cause our actual results and the timing of certain events to differ materially and adversely from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed under Item 1A. “Risk Factors” in this Form 10-K. Furthermore, such forward-looking statements speak only as of the date of this Form 10-K. We undertake no obligation to update or revise publicly any forward-looking statements to reflect events or circumstances after the date of such statements for any reason, except as otherwise required by law.
PART I
Overview
We are a global medical technology company that develops, manufactures, and markets noninvasive patient monitoring products that help clinicians improve patient care. We were incorporated in California in May 1989 and reincorporated in Delaware in May 1996. We invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of Measure-Through Motion and Low Perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. Pulse oximetry is the noninvasive measurement of the oxygen saturation level of arterial blood, or the blood that delivers oxygen to the body’s tissues and pulse rate. Our Masimo SET platform has addressed many of the previous technology limitations and has been referred to by several industry sources as the gold standard in pulse oximetry. The benefits of Masimo SET have been validated in over 100 independent clinical and laboratory studies.
We develop, manufacture, and market a family of noninvasive blood constituent patient monitoring solutions that consists of a monitor or circuit board and our proprietary single-patient use and reusable sensors and cables. In addition, we offer remote monitoring and clinician notification solutions, such as the Masimo Patient SafetyNet. Our solutions and related products are based upon our proprietary Masimo SET algorithms. This software-based technology is incorporated into a variety of product platforms depending on our customers’ specifications. We sell our products to end-users through our direct sales force and certain distributors, and some of our products to our original equipment manufacturer, or OEM, partners, for incorporation into their products. As of January 1, 2011, we estimate that the worldwide installed base of our pulse oximeters and OEM monitors that incorporate Masimo SET was 855,000 units, based on an estimated 10 year field life assumption. Our installed base is the primary driver for the recurring sales of our sensors, most notably, single-patient adhesive sensors. Based on industry reports, we estimate that the worldwide pulse oximetry market was over $1 billion in 2010, the largest component of which was the sale of sensors.
1
Our strategy is to utilize the reliability and accuracy of our Masimo SET platform, along with our Patient SafetyNet solutions, to facilitate the expansion of our pulse oximetry products into areas beyond critical care settings, including the general care areas of the hospital. Additionally, we have developed products that noninvasively monitor measurements beyond arterial blood oxygen saturation level and pulse rate, which create new market opportunities in both the critical care and non-critical care settings. In 2005, we launched our Masimo rainbow® SET platform utilizing licensed rainbow® technology, which we believe includes the first devices cleared by the U.S. Food and Drug Administration, or FDA, to noninvasively and continuously monitor multiple measurements that previously required invasive or complicated procedures. In 2005, we launched Masimo carboxyhemoglobin, or SpCO, allowing measurement of carbon monoxide levels in the blood. Carbon monoxide is the most common cause of poisoning in the world. When used with other clinical variables, SpCO may help clinicians and emergency responders detect carbon monoxide poisoning and help determine treatment and additional test options. In 2006, we launched Masimo methemoglobin, or SpMet, allowing for the measurement of methemoglobin levels in the blood. Methemoglobin in the blood leads to a dangerous condition known as methemoglobinemia, which occurs as a reaction to some common drugs used in hospitals and outpatient procedures. When used with other clinical variables, SpMet may help clinicians detect methemoglobinemia and help determine treatment and additional test options. In 2007, we launched Masimo Pleth Variability Index, or PVI. Fluid administration is critical to optimizing fluid status, but traditional methods to guide fluid administration often fail to predict fluid responsiveness. When used with other clinical variables, PVI may help clinicians assess fluid status and help determine treatment options. In May 2008, we received FDA clearance for total hemoglobin, or SpHb, and we began commercial release of our continuous monitoring total hemoglobin device in March 2009. Total hemoglobin is defined as the oxygen-carrying component of red blood cells, and is one of the most frequent invasive laboratory measurements in the world, often measured as part of a complete blood count. When used with other clinical variables, SpHb may help clinicians assess bleeding status and help determine treatment and additional test options. In November 2009, we received FDA clearance for Rainbow Acoustic MonitoringTM for continuous and noninvasive monitoring of respiration rate, or RRa, which is the number of breaths per minute, and often the leading indicator of patient distress. Traditional methods used to measure respiration rate are generally considered inaccurate or are not tolerated well by patients. When used with other clinical variables, RRa may help clinicians assess respiratory status and help determine treatment options. In December 2009, we announced initial limited market release of this parameter that allowed us to evaluate the product’s performance in the field and in June 2010, we began a full commercial release of Rainbow Acoustic MonitoringTM. Also, in June 2010, we received FDA clearance for a second spot-check hemoglobin device, Pronto-7. In October 2010, we debuted the Halo Index, which allows continuous global trending and assessment of multiple physiological measurements with a single number displayed on the Patient SafetyNet screen. In addition, we believe that we will develop and introduce additional measurements and products in the future based on our proprietary technology platforms.
Our technology is supported by a substantial intellectual property portfolio that we have built through internal development and, to a lesser extent, acquisitions and license agreements. As of January 1, 2011, we had 650 issued and pending patents worldwide. We have exclusively licensed from our development partner, Cercacor Laboratories, Inc., or Cercacor (formerly Masimo Laboratories, Inc.), the right to incorporate rainbow® technology into our products intended to be used by professional caregivers, including, but not limited to, hospital caregivers and alternate care market, facility caregivers.
Industry Background
Pulse oximetry has gained widespread clinical acceptance as a standard patient vital sign measurement because it can give clinicians an early warning of low arterial blood oxygen saturation levels, known as hypoxemia. Early detection is critical because hypoxemia can lead to a lack of oxygen in the body’s tissues, which can result in brain damage or death in a matter of minutes. Masimo pulse oximeters are used primarily in critical care settings, including emergency departments, surgery, recovery rooms, intensive care units, or ICUs, and alternative care settings, such as long-term care facilities and for home monitoring in patients with chronic conditions.
In addition, clinicians use pulse oximeters to estimate whether there is too much oxygen in the blood, a condition called hyperoxemia. In premature babies, hyperoxemia can lead to permanent eye damage or blindness. By ensuring that oxygen saturation levels in babies remain under 96%, clinicians believe they can lower the incidence of hyperoxemia. Hyperoxemia can also cause problems for adults, such as increased risk of postoperative infection and tissue damage. In adults, to prevent hyperoxemia, clinicians use pulse oximeters to administer the minimum level of oxygen necessary to maintain normal saturation levels.
Pulse oximeters use sensors attached to an extremity, typically the fingertip. These sensors contain two light emitting diodes that transmit red and infrared light from one side of the extremity through the tissue to a photodetector on the other side of the extremity. The photodetector in the sensor measures the amount of red and infrared light absorbed by the tissue. A microprocessor then analyzes the changes in light-absorption to provide a continuous, real-time measurement of the amount of oxygen in the patient’s arterial blood. Pulse oximeters typically give audio and visual alerts, or alarms, when the patient’s arterial blood oxygen saturation level or pulse rate falls outside of a designated range. As a result, clinicians are able to immediately initiate treatment to prevent the serious clinical consequences of hypoxemia and hyperoxemia.
2
Limitations of Conventional Pulse Oximetry
Conventional pulse oximetry is subject to technological limitations that reduce its effectiveness and the quality of patient care. In particular, when using conventional pulse oximetry, arterial blood signal recognition can be distorted by motion artifact, or patient movement, and low perfusion, or low arterial blood flow. Motion artifact can cause conventional pulse oximeters to inaccurately measure the arterial blood oxygen saturation level, due mainly to the movement and recognition of venous blood. Venous blood, which is partially depleted of oxygen, may cause falsely low oxygen saturation readings. Low perfusion can also cause conventional pulse oximeter to report inaccurate measurement, or some cases, no measurement at all. Conventional pulse oximeters cannot distinguish oxygenated hemoglobin, or the component of red blood cells that carries oxygen, from dyshemoglobin, which is hemoglobin that is incapable of carrying oxygen. In addition, conventional pulse oximetry readings can also be impacted by bright light and electrical interference from the presence of electrical surgical equipment. Independent, published research shows that conventional pulse oximeters are subject to operating limitations, including:
| | • | | inaccurate measurements, which can lead to the non-detection of a hypoxemic event or improper and unnecessary treatment; |
| | • | | false alarms, which occur when the pulse oximeter falsely indicates a drop in the arterial blood oxygen saturation level can lead to improper therapy, the inefficient use of clinical resources as clinicians respond to false alarms, or the non-detection of a true alarm if clinicians become desensitized to frequently occurring false alarms; and |
| | • | | signal drop-outs, which is the loss of a real-time signal as the monitor attempts to find or distinguish the pulse, which can lead to the non-detection of hypoxemic events. |
Published independent research shows that over 70% of the alarms outside the operating room are false, when using conventional pulse oximetry. In addition, in the operating room, conventional pulse oximeters failed to give measurements at all due to weak physiological signals, or low perfusion, in up to 9% of all cases studied. Manufacturers of conventional pulse oximeters have attempted to address some of these limitations, with varying degrees of success. Some devices have attempted to minimize the effects of motion artifact by repeating the last measurement before motion artifact is detected, until a new, clean signal is detected and a new measurement can be displayed, known as freezing values. Other devices have averaged the signal over a longer period of time, known as long-averaging, in an attempt to reduce the effect of brief periods of motion. These solutions, commonly referred to as alarm management techniques, mask the limitations of conventional pulse oximetry. Several published studies have demonstrated that some of these alarm management techniques have actually contributed to increased occurrences of undetected true alarms, or events where hypoxemia occurs, but is not detected by the pulse oximeter.
Conventional pulse oximetry technology also has several practical limitations. Because the technology cannot consistently measure oxygen saturation levels of arterial blood in the presence of motion artifact or low perfusion, the technology is not ideal to allow for its use in non-critical care settings of the hospital, such as general care areas, where the hospital staff-to-patient ratio is significantly lower. In order for pulse oximetry to become a standard patient monitor in these settings, these limitations must be overcome.
In addition, all pulse oximeters cannot distinguish oxygenated hemoglobin from dyshemoglobin. The most prevalent forms of dyshemoglobins are carboxyhemoglobin and methemoglobin. As a result of these dyshemoglobins, pulse oximeters will report falsely high oxygen levels when they are present in the blood. Prior to Masimo’s noninvasive and continuous carboxyhemoglobin and methemoglobin monitors, lab-based tests were the only way to detect dyshemoglobins, but these tests are invasive and do not provide immediate or continuous results.
Pulse Oximetry Market Opportunity
The pulse oximetry market consists of pulse oximeters and consumables, including single-patient use and reusable sensors, cables and other pulse oximetry accessories that are primarily sold to the hospital and alternative care markets. Based on available estimates for the U.S. and international market, we estimate that the worldwide pulse oximetry market was more than $1 billion in 2010.
According to a November 2010 market research report from iData, the total U.S. pulse oximetry market is $716 million. In this report:
| | • | | Masimo and Covidien had 50.3% and 38.1%, respectively, of the U.S. standalone pulse oximetry monitoring equipment market; |
| | • | | Masimo and Covidien had 45.2% and 37.4%, respectively, of the pulse oximeter OEM board market; and |
| | • | | Due to Covidien’s larger installed base of pulse oximeter standalone monitors and OEM boards, Masimo and Covidien held 32.1% and 60.1%, respectively, of the pulse oximetry sensor market. |
3
In a December 2008 report, Frost & Sullivan stated that it expects the growth in the U.S. pulse oximetry market to be driven by:
| | • | | ongoing adoption of low perfusion, motion-tolerant technology; |
| | • | | aggressive awareness campaigns; |
| | • | | rising patient acuity, or severity of illnesses, which increases the need for monitoring in the intermediate and sub-acute settings; |
| | • | | expansion of the market for pulse oximetry monitoring to the general surgical floor; |
| | • | | greater efficiencies for the health care worker through increased reliability, improved detection algorithms and the ability to reject false alarms; and |
| | • | | adoption of pulse oximetry outside the hospital and in the faster growing alternate care market. |
New Market Opportunities for Masimo SET
General Floor Monitoring Expansion
We believe there are opportunities to expand the market for pulse oximetry by applying Masimo SET’s proven benefits from critical care settings to non-critical care settings, as well as settings outside of the hospital. It is currently estimated that 87% of all U.S. hospital beds are located in non-critical care areas, where continuous monitoring is not widely used. A study published in July 2004 byHealthGrades showed that 264,000 hospital deaths over a three-year period were attributable to patient safety incidents, or generally preventable patient events in non-critical care areas. The study concluded that the failure to timely diagnose and treat patients accounted for over 70% of those deaths, suggesting that improved patient monitoring in non-critical care settings can alert clinicians of patient distress and help to improve patient care. A landmark study published in January 2010 by Dartmouth-Hitchcock Medical Center demonstrated that clinicians using Masimo SET and Masimo Patient SafetyNet identified patient distress earlier and decreasing rapid response team activations, ICU transfers, and ICU days.
The American Hospital Association estimated that there were 947,000 staffed beds in all U.S.-registered hospitals in 2004. In 2000, according to a study published in the Journal of Critical Care Medicine, 87% of all hospital beds in the U.S. were located in non-critical care settings which suggests a non-critical care market potential of 820,000 beds in the U.S. alone. While some of these non-critical care beds have some form of monitoring capabilities today, we believe that 15% or more of the 820,000 beds in the U.S. alone could become continuous monitoring beds. We believe that Masimo SET’s ability to dramatically minimize false alarms due to patient motion while maximizing the sensitivity of pulse oximeters to report true alarms will allow hospitals to reliably and continuously monitor their patients in the general floors.
Alternate Care
According to the November 2010 iData report, the fastest growing portion of the U.S. pulse oximetry equipment market is in the alternate care market. We believe that Masimo SET technology offers significant advantages in some segments of this market, including home care, post-acute care hospitals, and sleep diagnostics. The proven ability of Masimo SET to dramatically reduce false alarms and increase true event detection enables clinicians to make more reliable diagnoses of those who need oxygen therapy and Continuous Positive Airway Pressure. We plan to leverage this opportunity and expand our presence in this market.
New Market Opportunities for Masimo rainbow®SET
There are significant opportunities to expand Masimo’s hospital and alternate care markets by enabling the monitoring of additional noninvasive measurements beyond arterial blood oxygen saturation level and pulse rate.
Total Hemoglobin (SpHb®)
In May 2008, we received clearance from the FDA for our continuous total hemoglobin monitoring technology and in September 2008, we began shipping, in a limited market release, these monitors and sensors. In March 2009, we commercially launched our continuous noninvasive total hemoglobin device. Hemoglobin is the part of a red blood cell that carries oxygen to the body and therefore a measurement of the total hemoglobin parameter is an indicator of the oxygen carrying capacity of the blood. Because of its clinical importance, hemoglobin is one of the most commonly ordered lab diagnostic tests in the hospital and physician office. Each year in the U.S., over 400 million invasive hemoglobin tests are performed, which require multiple steps including collecting the patient’s blood sample, transferring the sample to the lab, analyzing the sample, documenting the results and reporting the results to the ordering clinician.
4
A low or falling hemoglobin measurement provides the primary indication for whether a patient receives a blood transfusion. Blood transfusions are common, with up to 20% of surgical patients and 35% of ICU patients receiving one or more units of blood. Transfusions have been shown in multiple studies to increase short and long-term morbidity and mortality. Blood transfusions are also costly, with blood being one of the highest cost items in a hospital. Each unit of blood is estimated to cost between $522 and $1,183, without including the additional cost of treatment associated with blood-transfusion-related complications. It is known that some blood transfusions are unnecessary, especially when given in stable anemia or when bleeding is perceived but not significant. Experts advocate implementing restrictive transfusion practices and use of appropriate indicators for blood transfusion. The decision to transfuse often requires the physician to either make an educated guess or wait for hemoglobin lab results to confirm that it is necessary. At the American Society of Anesthesiologists scientific meeting in October 2010, investigators from Massachusetts General Hospital presented the results of their study in which they evaluated the impact of SpHb monitoring in a randomized controlled trial in orthopedic surgery patients. Patients in the standard care group had a 4.5% transfusion rate and patients in the SpHb monitoring group had 0.6% transfusion rate, an 87% reduction in transfusion frequency with SpHb.
A low or falling hemoglobin measurement also helps determine whether a patient has internal bleeding that requires further investigation and significantly increases the cost of treatment. Significant bleeding occurs in up to 35% of surgical and critical care patients. A low hemoglobin measurement is associated with almost 90% of patients with bleeding. However, traditional laboratory measurements are infrequent and delayed.
When used with other clinical variables, Masimo SpHb may help clinicians assess bleeding status and help determine treatment and additional test options. While clinical research studies on SpHb are ongoing, clinicians inherently understand the value of continuous and noninvasive hemoglobin monitoring. A study by the consulting firm Capgemini concluded that the average 500 bed hospital would save $468,000 annually by implementing SpHb and other rainbow® measurements. Because of the potential clinical and cost advantages of measuring total hemoglobin noninvasively and continuously, we believe that a large number of hospitals will adopt Masimo rainbow® SET technology.
A significant portion of invasive hemoglobin measurements are made outside of hospital settings, in the physician office to aid patient assessment and treatment, and in the blood donation market to qualify potential donors for eligibility to donate blood. We believe that a significant number of the estimated 200,000 U.S. physician offices and estimated 15 million annual U.S. blood donations could be aided by the noninvasive and immediate assessment of hemoglobin.
Beginning in January 2010, the American Medical Association approved a Current Procedural Terminology, or CPT, code and Medicare implemented pricing on the Medicare Clinical Lab Fee Schedule for noninvasive hemoglobin, enabling U.S. hospitals and physician offices that perform testing to recover their costs, in addition to the clinical benefits they receive from this measurement.
Our new noninvasive technologies, such as total hemoglobin and Rainbow Acoustic MonitoringTM are new technologies. These new technologies have performance levels that we believe are acceptable for many clinical environments but may be insufficient in others. In addition, these noninvasive technologies may perform better in some patients and settings than others. The performance of these technologies shows a variability across a population that follows a gaussian distribution described in the accuracy specifications. Over time, we hope to reduce this variability and, if we do, we expect these new noninvasive technologies to become more useful in additional environments and to become more widely adopted. This is the adoption pattern we have experienced historically with our previously released measurements, such as oxygen saturation, and what we expect to experience with the new rainbow® measurements.
Carboxyhemoglobin (SpCO®)
Carbon monoxide is a colorless, odorless and tasteless gas that is undetectable by humans and is often unknowingly inhaled from combustion fumes, or during fires by victims and first responders. Carbon monoxide poisoning is the leading cause of accidental poisoning death in the U.S., responsible for up to 50,000 emergency department visits and 500 unintentional deaths annually. Carbon monoxide poisoning, which involves carbon monoxide binding with hemoglobin cells, thereby preventing them from carrying oxygen, can cause severe neurological damage, permanent heart damage, or death in a matter of minutes. Quick diagnosis and treatment of carbon monoxide poisoning is critical in saving lives and preventing long-term damage, but the condition is often misdiagnosed because symptoms are similar to the flu.
When used with other clinical variables, Masimo SpCO may help clinicians detect carbon monoxide poisoning and help determine treatment and additional test options. According to a study in March 2008 by Brown University, the emergency department using Masimo rainbow® SET carbon monoxide monitoring identified 60% more carbon monoxide poisoning cases than the conventional approach, and estimated that as many as 11,000 carbon monoxide poisoning cases per year in the U.S. were being missed with the conventional approach. Multiple leading emergency first responder associations, including the National Association of Emergency Medical Technicians, the National Association of EMS Educators, the International
5
Association of Fire Fighters, and the International Association of Fire Chiefs, now educate their members that noninvasive assessment for carbon monoxide poisoning is appropriate when exposure is suspected or when an individual presents symptoms that could indicate such poisoning. In addition, the National Fire Protection Association, or NFPA, included carbon monoxide screening by Pulse CO-Oximetry as part of a new national healthcare standard for firefighters potentially exposed to carbon monoxide poisoning. NFPA’s consensus codes and standards serve as the worldwide authoritative source on fire prevention and public safety.
In addition, the United Kingdom House of Commons All Party Parliamentary Gas Safety Group, in a report published in January 2009, aimed at increasing the awareness of carbon monoxide poisoning among medical professionals, recommended noninvasive carbon monoxide testing for Emergency Department and alternate care market providers as a way to improve the country’s rate of detection and diagnosis of carbon monoxide poisoning. For the preparation of this report, the United Kingdom Group used Masimo rainbow® SET Rad-57 devices for 12 months and reported successful cases with the Rad-57 devices.
Beginning January 2009, the American Medical Association approved a CPT code and Medicare implemented pricing on the Medicare Clinical Lab Fee Schedule for noninvasive carboxyhemoglobin, enabling U.S. hospitals that perform testing to recover their costs, in addition to the clinical benefits they receive.
We believe that the first and greatest opportunity for noninvasive blood carbon monoxide monitoring is in the alternate care market and emergency department settings. In the U.S. alone, there are 30,000 fire departments / alternate care market locations and 5,000 hospitals that would benefit from noninvasive carbon monoxide testing.
Methemoglobin (SpMet®)
Methemoglobinemia reduces the amount of oxygen bound to hemoglobin for delivery to tissues and forces normal hemoglobin to bind more tightly to oxygen, releasing less oxygen to the tissues. Methemoglobinemia is often unrecognized or diagnosed late, increasing risk to the patient. Commonly prescribed drugs can introduce methemoglobin into the blood and cause methemoglobinemia. Some of the 30 drugs that are known to cause methemoglobinemia are benzocaine, a local anesthetic, which is routinely used in procedures ranging from endoscopy to surgery; inhaled nitric oxide, routinely used in the Neonatal Intensive Care Unit; nitroglycerin used to treat cardiac patients and dapsone, used to treat infections for immune deficient patients, such as HIV patients.
According to a study published in September 2004 by researchers at Johns Hopkins University, over a 28 month period there were 414 cases, or 19% of all patients reviewed, of acquired methemoglobinemia. In these cases, the methemoglobinemia resulted in one fatality and three near-fatalities. Warnings, cautions and alerts regarding the clinical significance and prevalence of methemoglobinemia have been generated by the FDA, Veterans Administration, Institute for Safe Medication Practices, and the National Academy of Clinical Biochemistry. The American Academy of Pediatrics recommends monitoring methemoglobin levels in infants who receive nitric oxide therapy.
When used with other clinical variables, Masimo SpMet may help clinicians detect methemoglobinemia and help determine treatment and additional test options. We believe the initial opportunity for methemoglobin monitoring is in outpatient procedure labs in hospitals, such as esophageal echocardiography and gastrointestinal labs where use of caines, such as benzocaine, is prevalent, monitoring HIV patients who receive dapsone, as well as monitoring neonates who receive inspired nitric oxide in the neonatal ICU’s.
Beginning January 2009, the American Medical Association approved a CPT code and Medicare implemented pricing on the Medicare Clinical Lab Fee Schedule for noninvasive methemoglobin, enabling U.S. hospitals that perform testing to recover their costs, in addition to the clinical benefits they receive.
Pleth Variability Index (PVI®)
Fluid is administered through intravenous catheters to surgical and intensive care patients as part of a key objective to ensure that vital tissues are getting enough oxygen. However, unnecessary fluid may cause harm to patients. Therefore, the decision of whether to administer fluid is of fundamental importance in critically-ill and surgical patients. Ideally, a clinician would know prior to giving fluid whether the patient would respond favorably to the fluid, which is known as “fluid responsiveness.” However, traditional methods such as central venous pressure monitoring often fail to predict fluid responsiveness, and newer methods are invasive, complicated, and/or costly.
When used with other clinical variables, Masimo PVI may help clinicians assess fluid responsiveness in surgical and intensive care patients who are under mechanical ventilation. Mechanical ventilation means that a machine called a respirator is controlling patient breathing. PVI, which has been shown to help clinicians improve fluid management. has also been used
6
by clinicians in Goal Directed Therapy to reduce patient risk. Fluid management was improved by PVI by helping reduce the amount of fluid given to surgical patients, which lowered their patient risk as evidenced by a lowering of a key patient risk marker called lactate level.
We believe the primary opportunity for PVI monitoring is in mechanically ventilated adult patients during surgery and in the intensive care department in hospitals, but it is also possible that future studies may reveal application in non-mechanically ventilated adult patients in the hospital and other out-of-hospital patient populations.
Acoustic Respiration Rate (RRa TM)
We received FDA clearance for Rainbow Acoustic MonitoringTM of respiration rate in November 2009, announced initial market release of the parameter in December 2009 and announced full market release in June 2010.
Respiration rate is defined as the number of breaths per minute, and changes in respiration rate provide an early warning sign of deterioration in patient condition. Current methods to monitor respiration rate include end tidal CO2 monitoring, which requires a special tube inserted in the patient’s nose and therefore has low patient compliance, and impedance monitoring, which is considered unreliable. Masimo’s noninvasive respiration rate parameter will be available in our Masimo rainbow®SET platforms with the launch of MX-3 board, which was released in November 2009. These devices will be deployed through Masimo’s acoustic respiration sensor on the patient’s neck and connected to the bedside monitor with a separate cable. Should the respiration rate change or stop, an alarm will be displayed on the device and in addition, can be sent to the Masimo Patient SafetyNet system. Patient SafetyNet can then notify the attending clinician or nurse of the condition, directly on the monitor or remotely via a pager.
When used with other clinical variables, RRa may help clinicians assess respiratory status and help determine treatment options. We believe this noninvasive measurement will become a key and important measurement in the general floor environment and may also have important applications in the post-anesthesia care unit as well as non-mechanically ventilated surgical patients.
Halo IndexTM
In October 2010, we debuted Halo Index, which has received CE Marking in the European Union, but is subject to FDA clearance before commercialization in the U.S. Halo Index is a dynamic new indicator that facilitates continuous global trending and assessment of multiple physiological measurements to quantify changes in patient status. Currently, clinicians monitor multiple clinical measurements on each patient and respond independently to each of the measurement. Halo Index is a single displayed value on the Masimo Patient SafetyNet remote monitoring and notification system, which facilitates simple and comprehensive assessment within a single index. In the future, we expect Halo Index will also be available as part of Masimo standalone devices and OEM boards. As more clinical evidence is collected on Halo Index, its clinical utility in a variety of care areas and patient types will become more specific.
Current Technologies
In general, all of our new noninvasive technologies are new technologies. These new technologies have performance levels that we believe are acceptable for many clinical environments but may be insufficient in others. In addition, these noninvasive technologies may perform better in some patients and settings than others. The performance of these technologies shows a variability across a population that follows a gaussian distribution described in the accuracy specifications. Over time, we hope to reduce this variability and, if we do, we expect these new noninvasive technologies to become more useful in additional environments and to become more widely adopted. This is the adoption pattern we have experienced historically with our previously released measurements, such as oxygen saturation, and what we expect to experience in the future with our current and future technologies.
Future Measurements
We believe that our core signal processing and sensor technologies are widely applicable and may develop and launch future applications utilizing our proprietary technology platforms. However, we do not plan to communicate the priority, status, or timing of future measurements in development until such time that they have reached feasibility and/or received regulatory clearance.
The Masimo Solution
Our innovative and proprietary technologies and products are designed to overcome the primary limitations of conventional pulse oximetry, which involve maintaining accuracy in the presence of motion artifact and weak signal-to-noise situations. Our Masimo SET platform, which became available to hospitals in the U.S. in 1998, is the basis of our pulse oximetry products and we believe represented the first significant technological advancement in pulse oximetry since its introduction in the early 1980s. In addition, our products’ benefits have been validated in over 100 independent clinical and laboratory studies.
7
Masimo SET utilizes five signal processing algorithms, four of which are proprietary, in parallel, to deliver high precision, sensitivity and specificity in the measurement of arterial blood oxygen saturation levels. Sensitivity is the ability to detect true events and specificity is the ability to reject false alarms. One of our proprietary processing algorithms, Discrete Saturation Transform, separates the signal from noise in real-time through the use of adaptive filtering, and an iterative sampling technique that tests each possible saturation value for validity. Masimo SET signal processing can therefore identify the venous blood and other noise, isolate them, and extract the arterial signal.
To complement our Masimo SET platform, we have developed a wide range of proprietary single-patient use (disposable) and multi-patient (reusable) sensors, cables and other accessories designed specifically to work with Masimo SET software and hardware. Although our technology platforms operate solely with our proprietary sensor lines, our sensors have the capability to work with certain competitive pulse oximetry monitors through the use of adapter cables. Our neonatal adhesive sensors have been clinically proven to exhibit greater durability compared to competitive sensors. In response to the hospital market’s growing needs to implement environmentally friendly, or “green”, products and to decrease costs to remain competitive, Masimo developed the SpO2 ReSposable sensor system and began a limited market release in December 2010. The ReSposable sensor, part reusable and part disposable, combined the performance and comfort of single-use adhesive sensors with the economic and green advantages of reusable sensors.
In 2005, we introduced our Masimo rainbow® SET platform, leveraging our Masimo SET technology and incorporating licensed rainbow® technology to enable reliable, real-time monitoring of additional measurements beyond arterial blood oxygen saturation and pulse rate. The Masimo rainbow® SET platform has the unique ability to distinguish oxygenated hemoglobins from certain dyshemoglobins, hemoglobin incapable of transporting oxygen, and allows for the rapid, noninvasive monitoring of total hemoglobin, carboxyhemoglobin, methemoglobin, and pleth variability index, which we refer to as Pulse CO-Oximetry. Along with the release of our rainbow® Pulse CO-Oximetry products, we have developed multi-wavelength sensors that have the ability to monitor multiple measurements with a single sensor. We believe that the use of Masimo rainbow® Pulse CO-Oximetry products will become widely adopted for the noninvasive monitoring of these measurements. We believe the addition of Rainbow Acoustic MonitoringTM for noninvasive and continuous monitoring of respiration rate will strengthen the clinical demand for the rainbow® platform, especially in the growing general floor market.
Additionally, we market our Patient SafetyNet remote monitoring and clinician notification system for use with our Masimo SET pulse oximeters and rainbow® Pulse CO-Oximeters, which allow monitoring of the oxygen saturation and pulse rate of up to 80 patients simultaneously. We believe that the superior performance of the Masimo SET platform coupled with reliable, cost effective, and easy to use wireless remote monitoring will allow hospitals to create continuous surveillance solutions on general care floors where patients are at risk of avoidable adverse events and where direct patient observation by skilled clinicians is cost prohibitive.
Benefits of Our Products and Technology
We believe that our technology and products offer several key benefits, including:
| | • | | Accurate, Real-Time Measurement. We believe that the Masimo SET platform has the ability to provide more accurate measurements with fewer missed events and false alarms than other pulse oximeters in the market place. |
| | • | | Increased Quality of Patient Care. We believe that the proven accuracy and reliability of Masimo SET pulse oximetry allows for better clinical decisions, leading to fewer medical errors and better patient care. We believe that the noninvasive monitoring of carboxyhemoglobin will improve the quality of care based on the number of emergency room visits reported for carbon monoxide poisoning. We believe the noninvasive monitoring of methemoglobin will also improve patient care based on reported drug interactions that increase methemoglobin levels in the blood and that wireless remote alarm and monitoring on the general care floor will reduce avoidable adverse events through earlier detection and intervention. We believe Masimo rainbow® SET will allow earlier and better clinical decisions in a variety of care areas. |
| | • | | Reduced Cost of Care. Several independent studies have shown that hospitals can reduce their costs as a result of using Masimo SET products. We believe that factors contributing to lower costs include a reduction in sensor usage as a result of more durable sensors, fewer invasive arterial blood gas procedures needed, less oxygen administration and a reduction in length of stay as the result of weaning patients off of ventilators more quickly. In addition, we expect that the noninvasive monitoring of carboxyhemoglobin and methemoglobin will help |
8
| | reduce the cost of care by reducing the need for invasive blood tests and limiting the costs from complications caused by incorrect diagnoses. We believe early detection of avoidable adverse events will contribute to lower length of stay because such events will be treated earlier before patients decompensate to critical levels. We believe earlier and better clinical decisions from Masimo rainbow® SET will allow for more cost-effective care and in some cases reimbursable procedures for hospitals and non-hospital providers. |
| | • | | Masimo SET Platform Allows for Expansion into Non-Critical Care Settings. We believe the ability of Masimo SET products to provide reliable monitoring with fewer false alarms has expanded and will continue to expand the use of pulse oximetry into other settings where patient motion and false alarms have historically prevented its use. Since the introduction of Masimo SET, we believe that pulse oximetry has become a standard of care in the alternate care market. In addition, hospitals and other care centers can reduce their costs by moving less critically ill patients from the ICU to the general care areas where these patients can be continuously and accurately monitored in a more cost-effective manner. Many patients in the general care areas are at risk of dying due to inadequate oxygenation. To mitigate this risk, patients in the general care areas need to be continuously monitored. Our Patient SafetyNet systems enable the Masimo SET and rainbow® SET platforms to wirelessly and remotely monitor patients in the general care areas of the hospital that are not under the constant supervision of clinicians. |
| | • | | Upgradeable rainbow® Platform for Earlier and Better Decisions About Patient Care. Products with our MX circuit board contain our Masimo SET pulse oximetry technology as well as circuitry to support rainbow® measurements. At the time of purchase, or at any time in the future, our customers and our OEMs’ customers will have the option of purchasing a software measurement, which will allow the customer to expand their patient monitoring systems to monitor additional measurements with a cost-effective solution. The rainbow® platform enables breakthrough noninvasive monitoring of measurements that previously required invasive testing, which may lead to earlier and better clinical decisions and decreased costs compared to standard care. |
Our Strategy
Since inception, our mission has been to develop noninvasive blood constituent patient monitoring solutions that improve patient outcomes and reduce the cost of patient care. We intend to continue to grow our business and to improve our market position by pursuing the following strategies:
| | • | | Continue to Expand Our Market Share in Pulse Oximetry. We grew our product revenue to $356.4 million in 2010 from $200.2 million in 2007, representing a three year CAGR of 21.2%. This growth can be attributed to the increased access to pulse oximetry customers through our agreements with group purchasing organizations, or GPOs, our increased relationships with OEM partners, the expansion of our direct sales force, and strong, independent clinical evidence that demonstrates the benefits of our technology. We supplement our direct sales with sales through our distributors. Direct and distributor sales increased to $283.6 million, or 80%, of product revenue in 2010, from $143.7 million, or 72%, of product revenue in 2007. |
| | • | | Expand the Pulse Oximetry Market to Other Patient Care Settings. We believe the ability to continuously and accurately monitor patients outside of critical care settings, including the general care areas of the hospital, are currently unmet medical needs and have the potential to significantly improve patient care and increase the size of the pulse oximetry market. We believe the ability of Masimo SET to accurately monitor and address the limitations of conventional pulse oximetry has enabled, and will continue to enable, us to expand into non-critical care settings and thus significantly expand the market for our products. To further support our expansion into the general care areas, we market Masimo Patient SafetyNet, which enables continuous monitoring of up to 80 patients’ oxygen saturation, pulse rate, and with rainbow® SET, noninvasive hemoglobin and acoustic respiration rate. |
| | • | | Expand the use of rainbow® technology in the Hospital Setting. We believe the noninvasive measurement of rainbow® Pulse CO-Oximetry (total hemoglobin, carboxyhemoglobin, methemoglobin, pleth variability index) Rainbow Acoustic MonitoringTM (acoustic respiration rate), and the Halo Index, as well as future measurements, will provide an excellent opportunity to leverage existing customer relationships into new revenue opportunities, directly and through a greater ability to convert non-Masimo hospitals to Masimo hospitals due to our expanded measurement capabilities. |
| | • | | Expand the use of rainbow® technology in the Non-Hospital Setting.We believe the noninvasive measurement of hemoglobin creates a significant opportunity in markets such as the physician office, and noninvasive carboxyhemoglobin in the fire/alternate care market. To date, we have introduced a handheld product called Pronto and, in June 2010, we introduced, in a limited market release, a new handheld product called the Pronto-7, which is a handheld noninvasive multi-parameter testing device, that provides spot-check hemoglobin testing along with oxygen saturation, pulse rate and perfusion index results. With a touch screen for easy operation and |
9
| | wireless 802.11 and Bluetooth for printing and communication, the Pronto-7 is well suited for hemoglobin spot-check testing in almost any environment. The Pronto-7 allows users to simply and quickly perform a spot-check measurement of hemoglobin levels, one of the most common invasive laboratory measurements taken in physician offices. We believe that the ability to noninvasively measure total hemoglobin will increase efficiency and improve clinical decision making in physician offices and emergency departments by enabling quick determination of total hemoglobin levels. The new Pronto-7 product expedites the measurement process by quickly providing a noninvasive measurement thereby reducing the time required to take an invasive blood draw, create the labeling, sending the sample to the lab, waiting for the lab results (often not until the next day), and communicating these results to the patient. |
| | • | | Utilize Our Customer Base and OEM Relationships to Market Our Masimo rainbow® SET Products Incorporating Licensed rainbow® Technology. We sold our first Masimo rainbow Pulse CO-Oximetry products in September 2005. We are currently selling our rainbow SET products through our direct sales force and distributors. In addition, we plan to sell our MX circuit boards in our own pulse oximeters and to our OEM partners, equipped with circuitry to support rainbow® Pulse CO-Oximetry measurements which can be activated at time of sale or through a subsequent software upgrade. We believe that the clinical need of these measurements along with our installed customer base will help drive the adoption of our rainbow® Pulse CO-Oximetry products. |
| | • | | Continue to Innovate and Maintain Our Technology Leadership Position. We invented and pioneered what we believe is the first pulse oximeter to accurately measure arterial blood oxygen saturation level and pulse rate in the presence of motion artifact and low perfusion. In addition, through our license of rainbow® technology from Cercacor, we launched our rainbow® SET platform that enabled what we believe is the first FDA-cleared noninvasive monitoring of Carboxyhemoglobin, Methemoglobin, Hemoglobin and Pleth Variability Index, which previously required invasive testing. With our introduction of Rainbow Acoustic MonitoringTM, we believe we have launched the first platform to enable noninvasive and continuous monitoring of respiration rate through an easy to use single patient adhesive acoustic sensor. In October 2010, we debuted Halo Index. Halo Index is a dynamic new indicator that facilitates continuous global trending and assessment of multiple physiological measurements to quantify changes in patient status. We plan to continue to innovate and develop new technologies and products, internally and through our collaboration with Cercacor, for the noninvasive monitoring of other measurements. |
Our future growth strategy is also closely tied to our focus on international expansion opportunities. Since 2006, we have generated between 72% and 77% of our product revenue in the U.S. Since 2006, we have been aggressively expanding our sales and marketing presence in Europe, Asia, Canada and Latin America. We have accomplished this through both additional staffing and by adding or expanding sales offices in many of these territories. During the fourth quarter of 2008, we established a new international business structure designed to better serve and support our growing international business. By centralizing our international operations, including sales management, marketing, customer support, planning, logistics and administrative functions, we believe we have developed a more efficient and scalable international organization—capable of being even more responsive to the business needs of its international customers—all under one centralized management structure. As a result of these investments and focus on our international operations, we believe that our international product revenues, as a percent of total product revenues, will continue to increase.
Our Products
We develop, manufacture and market a patient monitoring solution that incorporates a monitor or circuit board and sensors including proprietary single-patient use, reusable and ReSposable sensors and patient cables. In addition, we offer remote alarm/monitoring solutions and software.
The following chart summarizes our principal product components and principal markets and methods of distribution:
| | | | |
Product Components | | Description | | Markets and Methods of Distribution |
| Patient Monitoring Solutions: | | | | |
Circuit Boards (e.g., MX-1, MX-3, MS-2011) | | • Signal processing apparatus for all Masimo SET and licensed Masimo rainbow® SET technology platforms | | Incorporated into our proprietary pulse oximeters and sold to OEM partners who incorporate our circuit boards into their patient monitoring systems |
| | |
Monitors / Devices (Pulse CO-Oximeters, Acoustic/Monitors, and Pulse Oximeters) | | • Bedside and handheld monitoring devices that incorporate Masimo SET with and without licensed Masimo rainbow® SET technology | | Sold directly to end-users and through distributors and in some cases to our OEM partners who sell to end-users |
| | |
Sensors (e.g., rainbow® and Non-rainbow Sensors) and Cables | | • Extensive line of both single-patient, reusable and ReSposable sensors | | Sold directly to end-users and through distributors and to OEM partners who sell to end-users |
10
| | | | |
Product Components | | Description | | Markets and Methods of Distribution |
| | |
| | • Patient cables, as well as adapter cables that enable the use of our sensors on certain competitive monitors | | |
| | |
Remote Alarm and Monitoring Solutions
(e.g., Patient SafetyNet) | | • Network-linked wired or wireless, multiple patient floor monitoring solutions • Standalone wireless alarm notification solutions | | Sold directly to end-users |
| | |
Software
(e.g., SpHb, SpCO, SpMet, PVI, RRa and Halo Index) | | • Rainbow measurements and other proprietary features sold to installed monitors | | Sold directly to end-users and through OEM partners who sell to end-users |
Circuit Boards
Masimo SET MS Circuit Boards. Our Masimo SET MS circuit boards perform all signal processing and other pulse oximetry functions incorporating the Masimo SET platform. Our MS circuit boards are included in our proprietary monitors for direct sale or sold to our OEM partners for incorporation into their monitors. Once incorporated into a pulse oximeter, the MS circuit boards perform all data acquisition processing and report the pulse oximetry levels to the host monitor. The circuit boards and related software interface directly with our proprietary sensors to calculate arterial blood oxygen saturation level and pulse rate. Our latest generation boards include the MS-2003, MS-2011 and MS-2013.
Masimo rainbow® SET MX Circuit Boards. Our next-generation circuit board is the foundation for our Masimo rainbow® Pulse CO-Oximetry platform, utilizing technology licensed from Cercacor. The MX circuit boards measure arterial blood oxygen saturation levels and pulse rate, and have the circuitry to enable the measurement of total hemoglobin, oxygen content, carboxyhemoglobin, methemoglobin, pleth variability index, acoustic respiration rate and halo index, along with other potential measurements in the future. Customers can choose to buy additional measurements beyond arterial blood oxygen saturation levels and pulse rate at the time of sale or at any time in the future through a field-installed software upgrade. As additional measurements are developed, each new measurement may be available as a software upgrade to the existing system.
Monitors / Devices
Radical-7.We believe that the Radical-7 is the most advanced and versatile pulse oximeter available. The Radical-7 incorporates the MX circuit board, which enables rainbow® SET measurements, and offers three-in-one capability to be used as:
| | • | | a standalone device for bedside monitoring; |
| | • | | a detachable, battery-operated handheld unit for easy portable monitoring; and |
| | • | | a monitor interface via SatShare, proprietary technology allowing our products to work with certain competitor products, to upgrade existing conventional multi-parameter patient monitors to Masimo SET while displaying rainbow® measurements on the Radical-7 itself. |
Radical-7 is a fully-equipped standalone Pulse CO-Oximeter with a detachable module, which functions as a battery-operated, handheld monitor. The handheld module can be connected with any other Radical-7 base station, which allows Radical-7 to stay with the patient, enabling continuous and reliable arterial blood oxygen saturation and blood constituent monitoring such as total hemoglobin as patients are transported within the hospital. For example, Radical-7 can continuously monitor a patient from the ambulatory environment, to the emergency room, to the operating room, to the general floor, and on until the patient is discharged. Radical-7 delivers the accuracy and reliability of Masimo rainbow® SET with multi-functionality, ease of use and a convenient upgrade path for existing monitors.
Our SatShare technology enables a conventional monitor to upgrade to Masimo SET through a simple cable connection from the back of Radical-7 to the sensor input port of the conventional monitor. No software upgrades or new modules are necessary for the upgrade, which can be completed in minutes. SatShare allows hospitals to standardize the technology and sensors used throughout the hospital while allowing them to gain more accurate monitoring capabilities and additional multi-functionality in a cost-effective manner. This has facilitated many hospital-wide conversions of previously installed competitor monitors to Masimo SET. In addition, Masimo rainbow® SET measurements such as total hemoglobin are available to clinicians on the Radical-7 itself while the device is being used in SatShare mode.
11
Rad-87. The Rad-87, which also contains Masimo rainbow® SET technology, is a compact, lightweight and easy-to-use device designed specifically for use in less acute settings than the Radical-7. The Rad-87 is available with a built-in bi-directional wireless radio for use as part of the Masimo Patient SafetyNet remote monitoring and clinician notification system. We began shipping the Rad-87 in July 2008.
Pronto. The Pronto is a handheld noninvasive multi-parameter testing device, not a continuous monitoring device, that uses Masimo rainbow® SET technology, to provide pulse oximetry, pulse rate, perfusion index and spot checking of total hemoglobin levels for both hospitals (i.e. emergency departments) and remote settings such as physician offices.
Pronto-7. The Pronto-7 is a noninvasive multi-parameter device, that provides spot-check hemoglobin testing along with oxygen saturation, pulse rate and perfusion index results. With a touch screen for easy operation and wireless 802.11 and Bluetooth for printing and communication, the Pronto-7 is well-suited for hemoglobin spot-check testing in almost any environment.
Rad-8. The Rad-8 is a bedside pulse oximeter featuring Masimo SET (but without rainbow® capability) with a low cost design and streamlined feature set, allowing it to be offered at a lower price point than the Radical-7 or Rad-87.
Rad-5. In addition to the bedside monitors, we have developed handheld pulse oximeters using Masimo SET. Our Rad-5 and Rad-5v handheld oximeters were the first dedicated handhelds with Masimo SET.
Rad-57. The Rad-57 is a fully featured handheld Pulse CO-Oximeter that provides continuous, noninvasive measurement of carboxyhemoglobin and methemoglobin in addition to oxygen saturation, pulse rate, perfusion and index. Its rugged and lightweight design makes it applicable for use in hospital and field settings, specifically for fire departments and emergency medical service units.
SEDLine monitor. The SEDLine monitor measures brain function on a continuous basis. SEDLine, an EEG-based brain function monitor, provides information about a patient’s response to anesthesia.
Sensors
Sensors and Cables. We have developed one of the broadest lines of single-patient use and reusable sensors and cables. In total, we have over 100 different types of sensors to meet virtually every clinical need. Masimo SET sensors are uniquely designed to reduce interference from physiological and non-physiological noise. Our proprietary technology platforms operate only with our proprietary sensor lines. However, through the use of adapter cables, we can connect our sensors to certain competitive pulse oximetry monitors. We sell our sensors and cables to end-users through our direct sales force and our distributors and OEM partners.
Our single-patient use sensors offer several advantages over reusable sensors, including improved performance, cleanliness, increased comfort and greater reliability. Our reusable sensors are primarily used for short-term, spot-check monitoring. Our new ReSposable sensors are expected to provide performance advantages for customers currently using reusable and reprocessed sensors.
SofTouch Sensors. We have developed SofTouch sensors, designed with less adhesive or no adhesive at all for compromised skin conditions. These include single-patient sensors for newborns and multi-site reusable sensors for pediatrics and adults.
Trauma and Newborn Sensors. We believe we were the first to develop two specialty sensor lines, specifically designed for trauma and resuscitation situations, as well as for newborns. These sensors contain an identifier which automatically sets the oximeter to monitor with maximum sensitivity and the shortest-averaging mode and allows for quick application, even in wet and slippery environments.
Blue Sensors. In 2005, we introduced what we believe to be the first FDA-cleared sensor to accurately monitor arterial blood oxygen saturation levels in cyanotic infants and children with abnormally low oxygen saturation levels.
Rainbow Sensors. We believe we were the first to develop proprietary, multi-wavelength sensors for use with our rainbow®Pulse CO-Oximetry products. As opposed to traditional sensors that only have the capability to monitor arterial blood oxygen saturation levels and pulse rate, our rainbow® sensors can also monitor carboxyhemoglobin, methemoglobin and total hemoglobin. Our licensed rainbow® SET sensors are the only sensors that are compatible with our licensed rainbow® SET products. Rainbow sensors are available in single-patient use, ReSposable, and reusable spot-check sensor types.
12
Rainbow Acoustic Sensors.We believe we were the first to develop a continuous respiration rate monitoring technology based on an acoustic sensor placed on the patient’s neck. Our Rainbow Acoustic Sensors detect the sounds associated with breathing, and convert the sounds into continuous respiration rate using proprietary signal processing that is based on Masimo’s signal extraction technology.
SEDLine sensor. Used exclusively with the SEDLine monitor, the SEDLine sensor is a disposable sensor that collects a high volume of brain function data from key areas of frontal lobe, for clinical assessment.
Remote Alarm and Monitoring Solutions
Patient SafetyNet. Patient SafetyNet is a remote monitoring and clinician notification system. It instantly routes bedside-generated alarms through a server to a qualified clinician’s handheld paging device in real-time. Each system can support up to 80 bedside monitors and can either be integrated into a hospital’s existing IT infrastructure or operate as a stand-alone wireless network.
Software
All of our monitors, including Radical-7 and certain future OEM products, which incorporate the MX board, will allow purchases of software for rainbow® measurements as well as other future measurements or features that can be field installed.
In addition, in October 2010 we debuted Halo Index, which is a dynamic new indicator that facilitates continuous global trending and assessment of multiple physiological measurements to quantify changes in patient status. Currently, clinicians monitor multiple clinical measurements on each patient and respond independently to each of the measurements. Halo Index is a single displayed value on the Masimo Patient SafetyNet remote monitoring and notification system, which facilitates simple and comprehensive assessment within a single index. In the future, subject to receipt of regulatory clearance, we expect Halo Index will also be available as part of Masimo standalone devices and OEM boards. As more clinical evidence is collected on Halo Index, its clinical utility in a variety of care areas and patient types will become more specific.
Sales and Marketing
We have sales and marketing employees in the U.S. and abroad. We expect to continue to increase our worldwide sales and sales support organizations as we continue to expand our presence throughout both the U.S. and throughout the world including Europe, the Middle East, Asia, Latin America, Canada and Australia. We currently sell all of our products both directly to hospitals and the alternate care market via our sales force, and certain distributors.
Our direct and distributor revenue accounted for 79.6% of our total product revenue in 2010. The primary focus of our sales representatives is to facilitate the conversion of competitor accounts to our Masimo SET pulse oximetry products. In addition to sales representatives, we employ clinical specialists to work with our sales representatives to educate end-users on the benefits of Masimo SET and assist with the introduction and implementation of our technology and products to their sites. Our sales and marketing strategy for pulse oximetry has been and will continue to be focused on building end-user awareness of the clinical and cost-saving benefits of our Masimo SET platform. More recently, we have expanded this communication and educational role to include our Masimo rainbow® Pulse CO-Oximetry and Rainbow Acoustic MonitoringTM products, including total hemoglobin, carboxyhemoglobin, methemoglobin, pleth variability index, Acoustic Respiration Rate, and Halo Index. For the year ended January 1, 2011, Owens & Minor, one of our just-in-time distributors, represented 13.8% of our total revenue and was the only customer that represented 10% or more of our revenue for the year ended January 1, 2011. Importantly, distributors such as Owens & Minor take and fulfill orders from our direct customers, many of whom have signed long-term sensor purchase agreements with us. As a result, in the event a specific just-in-time distributor is unable to fulfill these orders, the orders will be redirected to other distributors or fulfilled directly by us.
Additionally, we sell certain of our products through our OEM partners who both incorporate our boards into their monitors and resell our sensors to their customers’ installed base of Masimo SET products. Our OEM agreements allow us to expand the availability of Masimo SET through the sales and distribution channels of each OEM partner. To facilitate clinician awareness of Masimo SET installations, all of our OEM partners have agreed to place the Masimo SET logo prominently on their instruments.
In order to facilitate our direct sales to hospitals, we have signed contracts with companies that we believe to be the five largest GPOs, based on the total volume of negotiated purchases. In return for the GPOs to put our products on contract, we have agreed to pay the GPOs a percentage of our revenue from their member hospitals. In 2010 and 2009, revenue from the sale of our pulse oximetry products to hospitals that are associated with GPOs amounted to $183.8 million and $160.8 million, respectively.
13
Our marketing efforts are designed to build end-user awareness through advertising, direct mail and trade shows. In addition, we distribute published clinical studies, sponsor accredited educational seminars for doctors, nurses, biomedical engineers, and respiratory therapists and conduct clinical evaluations. During 2011, we expect to modestly increase the size of our sales and marketing force worldwide, as we continue to establish and expand our sales channels on a global basis.
Competition
The medical device industry is highly competitive and many of our competitors have substantially greater financial, technical, marketing and other resources than we do. While we regard any company that sells pulse oximeters as a potential customer, we also recognize that the companies selling pulse oximeters on an OEM basis and/or pulse oximetry sensors are also potential competitors. Our primary competitor, Covidien Ltd. and its subsidiary Nellcor Puritan Bennett, Inc., currently hold a substantial share of the pulse oximetry market. Covidien sells its own brand of Nellcor pulse oximeters to end-users, sells pulse oximetry modules to other monitoring companies on an OEM basis and licenses, to certain OEMs, the right to make their pulse oximetry platforms compatible with Nellcor sensors. We face substantial competition from larger medical device companies, including companies that develop products that compete with our proprietary Masimo SET. We believe that a number of companies have announced products which claim to offer Measure-Through motion accuracy. Based on those announcements and our investigations, we further believe that many of these products include technology that infringes our intellectual property rights. We have settled claims against some of these companies and intend to vigorously enforce and protect our proprietary rights with respect to the others whom we believe are infringing our technology. Some of the remaining companies, including GE Medical Systems and Mindray Medical International Ltd., are also currently OEM partners of ours.
We believe that the principal competitive factors in the market for pulse oximetry products include:
| | • | | accurate monitoring during both patient motion and low perfusion; |
| | • | | ability to introduce other clinically beneficial measurements related to oxygenation and respiration, such as noninvasive and continuous hemoglobin and acoustic respiration rate; |
| | • | | sales and marketing capability; |
| | • | | access to hospitals which are members of GPOs; |
| | • | | access to OEM partners; and |
Cercacor Laboratories, Inc. (formerly Masimo Laboratories, Inc.)
Cercacor Laboratories, Inc., or Cercacor, is an independent entity spun-off from us to our stockholders in 1998. Joe Kiani and Jack Lasersohn, members of our board of directors, are also members of the board of directors of Cercacor. Joe Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Cercacor.
We have a cross-licensing agreement with Cercacor for certain technologies. The following table outlines our rights under the Cross-Licensing Agreement relating to specific end user markets and the related technology applications of specific measurements.
| | | | |
| | | End User Markets |
Measurements | | Professional Caregiver and Alternate Care Market | | Patient and Pharmacist |
Vital Signs(1) | | Masimo (owns) | | Cercacor (non-exclusive license) |
Non-Vital Signs(2) | | Masimo (exclusive license) | | Cercacor (owns or exclusive license) |
| (1) | Vital Signs measurements include SpO2, peripheral venous oxygen saturation, mixed venous oxygen saturation, fetal oximetry, sudden infant death syndrome, ECG, blood pressure (noninvasive blood pressure, invasive blood pressure and continuous noninvasive blood pressure), temperature, respiration rate, CO2, pulse rate, cardiac output, EEG, perfusion index, depth of anesthesia, cerebral oximetry, tissue oximetry and/or EMG, and associated features derived from these measurements, such as 3-D alarms, PVI and other features. |
| (2) | Non-Vital Signs measurements include the body fluid constituents other than vital signs measurements and include, but are not limited to, carbon monoxide, methemoglobin, blood glucose, total hemoglobin and bilirubin. |
14
Our License to Cercacor. We granted Cercacor an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET owned by us for the monitoring of non-vital signs measurements and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs measurements in the Cercacor Market. We also granted Cercacor a non-exclusive, perpetual and worldwide license, with sublicense rights, to use Masimo SET for the measurement of vital signs in the Cercacor Market. In exchange, Cercacor pays us a 10% royalty on the amount of vital signs sensors and accessories sold by Cercacor.
The Cercacor Market is defined as any product market in which a product is intended to be used by a patient or pharmacist rather than a professional medical caregiver regardless of the particular location of the sale, including sales to doctors, hospitals, alternate care market professionals or otherwise, provided the product is intended to be recommended, or resold, for use by the patient or pharmacist.
Cercacor’s License to Us. We exclusively licensed from Cercacor the right to make and distribute products in the Masimo Market that utilize rainbow® technology for the measurement of carbon monoxide, methemoglobin, fractional arterial oxygen saturation, and total hemoglobin, which includes hematocrit. To date, we have developed and commercially released devices that measure carbon monoxide, methemoglobin and total hemoglobin using licensed rainbow® technology. We also have the option to obtain the exclusive license to make and distribute products in the Masimo Market that utilize rainbow® technology for the monitoring of other non-vital signs measurements, including blood glucose. These licenses are exclusive until the later of 20 years from the grant of the applicable license or the expiration of the last patent included in the rainbow® technology related to the applicable measurements.
The Masimo Market is defined as those product markets where the product is intended to be used by a professional medical caregiver, including hospital caregivers, surgicenter caregivers, paramedic vehicle caregivers, doctor’s offices caregivers, alternate care market, facility caregivers and vehicles where alternative care services are provided.
Our license to rainbow® technology for these measurements in these markets is exclusive on the condition that we continue to pay Cercacor royalties on our products incorporating rainbow® technology, subject to certain minimum aggregate royalty thresholds, and that we use commercially reasonable efforts to develop or market products incorporating the licensed rainbow® technology. The royalty is up to 10% of the rainbow® royalty base, which includes handhelds, tabletop and multi-parameter devices. Handheld products incorporating rainbow® technology will carry a 10% royalty rate. For other products, only the proportional amount attributable for that portion of our products used to monitor non-vital signs measurements, sensors and accessories, rather than for monitoring vital signs measurements, will be included in the 10% rainbow® royalty base. For multi-parameter devices, the rainbow® royalty base will include the percentage of the revenue based on the number of rainbow® enabled measurements. For hospital contracts where we place equipment and enter into a sensor contract, we pay a royalty to Cercacor on the total sensor contract revenue based on the ratio of rainbow® enabled devices to total devices.
We are also subject to certain specific annual minimum aggregate royalty payment obligations, including a $5.0 million per year payment that started in 2010.
From Cercacor’s inception in 1998 through January 1, 2011, we paid Cercacor $31.1 million for both exclusive options and minimum royalty payments. We have 180 days after proof of feasibility to exercise the above-referenced option to obtain a license to the remaining non-vital signs measurements, including bilirubin for an additional $500,000 and blood glucose for an additional $2.5 million. As of January 1, 2011, feasibility on these measurements has not been attained. From its inception in 1998 through January 1, 2011, Cercacor has incurred a total of $23.9 million in expenses.
Change in Control. The Cross-Licensing Agreement provides that, upon a change in control:
| | • | | if the surviving or acquiring entity ceases to use “Masimo” as a company name and trademark, all rights to the “Masimo” trademark will be assigned to Cercacor; |
| | • | | the option to license technology developed by Cercacor for use in blood glucose monitoring will be deemed automatically exercised and a $2.5 million license fee for this technology will become immediately payable to Cercacor; |
| | • | | per product minimum royalties, to the extent less than the annual minimums, will be payable to Cercacor; and |
| | • | | the minimum aggregate annual royalties for all licensed rainbow® measurements payable to Cercacor is $15.0 million per year until the exclusive period of the agreement ends, plus up to $2.0 million for each additional rainbow® measurements. |
A change in control includes any of the following with respect to us or Cercacor:
| | • | | the sale of all or substantially all of either company’s assets to a non-affiliated third party; |
15
| | • | | the acquisition by a non-affiliated third party of 50% or more of the voting power of either company; |
| | • | | Joe Kiani, our Chief Executive Officer and the Chief Executive Officer of Cercacor, resigns or is terminated from his position with either company; and |
| | • | | the merger or consolidation of either company with a non-affiliated third party. |
Ownership of Improvements. Any improvements to Masimo SET or rainbow® technology made by Cercacor, by us, or jointly by Cercacor with us or with any third party that relates to non-vital signs monitoring, and any new technology acquired by Cercacor, is and will be owned by Cercacor. Any improvements to the Masimo SET platform or rainbow® technology made by Cercacor, by us, or jointly by Cercacor with us or with any third party that relates to vital signs monitoring, and any new technology acquired by us, is and will be owned by us. However, for both non-vital signs and vital signs monitoring, any improvements to the technology, excluding acquired technology, will be assigned to the other party and be subject to the terms of the licenses granted under the Cross-Licensing Agreement. Any new non-vital signs monitoring technology utilizing Masimo SET that we develop will be owned by Cercacor and will be subject to the same license and option fees as if it had been developed by Cercacor. Also, we will not be reimbursed by Cercacor for our expenses relating to the development of any such technology.
Cercacor Services Agreement. We have also entered into a services agreement, or the Services Agreement, with Cercacor. Under this Services Agreement, we provide Cercacor with engineering services and accordingly charge Cercacor for these direct salary and payroll related expenses. In addition, at the end of each quarter, we charge Cercacor for its share of accounting, human resources and legal costs, which we collectively refer to as indirect expenses. We expect Cercacor to continue to engage us for these services. However, pursuant to the Services Agreement, Cercacor may terminate the agreement by providing us 30 days notice, while we may terminate with 180 days notice to Cercacor.
Cercacor’s Expenses related to Pronto-7. In February 2009, we and Cercacor agreed that in order to accelerate the development of the technology supporting this product, Cercacor would re-direct a substantial amount of its engineering development activities to focus on this project for our benefit. Accordingly, we and Cercacor agreed that from April 2009 through June 2010, the completion of this product development effort, 50% of Cercacor’s engineering and engineering related expenses, and all third party engineering supplies expense related to Pronto-7 development would be charged to us. During the six months ended January 1, 2011, Cercacor continued to assist us with other product development efforts and charged us accordingly. For the year ended January 1, 2011, the total funding for these additional Cercacor expenses was $2.6 million. Both companies agreed to maintain this structure until we notify Cercacor that we no longer require this engineering support.
Research and Product Development
We believe that ongoing research and development efforts are essential to our success. We expect to increase the size of our research and development staff during 2011. Our research and development efforts focus primarily on continuing to enhance our technical expertise in pulse oximetry, enabling the noninvasive monitoring of other measurements and developing remote alarm and monitoring solutions.
Although we and Cercacor each have separate research and development projects, we collaborate with Cercacor on multiple research and development activities related to rainbow® technology and other technologies. Under the Cross-Licensing Agreement, the parties have agreed to allocate proprietary ownership of technology developed by either party based on the functionality of the technology. We will have proprietary rights to all technology related to the noninvasive measurement of vital signs measurements, and Cercacor will have proprietary ownership of all technology related to the noninvasive monitoring of non-vital signs measurements.
Our total research and development expenditures for 2010 were $36.0 million, which included $1.7 million related to expenses incurred by Cercacor pursuant to the Cross-Licensing Agreement. In 2009, our total research and development expenditures were $31.7 million, which included $2.0 million related to expenses incurred by Cercacor. In 2008, our total research and development expenditures were $25.5 million, which included $2.4 million related to expenses incurred by Cercacor. We expect our research and development expenses to increase in 2011 and beyond as we expand our research and development force, enhance our existing products and technologies and develop new product candidates.
Intellectual Property
We believe that in order to maintain a competitive advantage in the marketplace, we must develop and maintain protection of the proprietary aspects of our technology. We rely on a combination of patent, trademark, trade secret, copyright and other intellectual property rights and measures to protect our intellectual property.
16
We have developed a patent portfolio internally, and to a lesser extent through acquisitions and licensing, that covers many aspects of our product offerings. As of January 1, 2011, we had 389 issued patents and 261 pending applications in the U.S., Europe, Japan, Australia, Canada and other countries throughout the world. In addition, as of January 1, 2011, technology we licensed from our development partner, Cercacor, was supported by 58 issued patents and 132 pending applications in the U.S. and internationally. Some of our earliest patents begin to expire in 2011. Some of Cercacor’s earliest patents begin to expire in 2015. Additionally, as of January 1, 2011, we owned 41 U.S. registered trademarks and 109 foreign registered trademarks, as well as trade names that we use in conjunction with the sale of our products.
Under the Cross-Licensing Agreement, we and Cercacor have agreed to allocate proprietary ownership of technology developed based on the functionality of the technology. We will have proprietary ownership, including ownership of all patents, copyrights and trade secrets, of all technology related to the noninvasive monitoring of vital signs measurements, and Cercacor will have proprietary ownership of all technology related to the noninvasive monitoring of non-vital signs measurements. We also rely upon trade secrets, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We seek to protect our trade secrets and proprietary know-how, in part, with confidentiality agreements with consultants, vendors and employees, although we cannot be certain that the agreements will not be breached, or that we will have adequate remedies for any breach.
There are risks related to our intellectual property rights. For further detail on these risks, see Item 1A— “Risk Factors.”
Government Regulation
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device that we wish to market in the U.S. must first receive either 510(k) clearance, by filing a 510(k) pre-market notification, or PMA approval, by filing a Premarket Approval Application, or PMA, from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA’s 510(k) clearance process usually takes from four to twelve months, but it can take longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from one to three years or even longer. We cannot be sure that 510(k) clearance or PMA approval will ever be obtained for any product we propose to market.
The FDA decides whether a device must undergo either the 510(k) clearance or PMA approval process based upon statutory criteria. These criteria include the level of risk that the agency perceives is associated with the device and a determination of whether the product is a type of device that is similar to devices that are already legally marketed. Devices deemed to pose relatively less risk are placed in either Class I or II, which generally requires the manufacturer to submit a pre-market notification requesting 510(k) clearance, unless an exemption applies.
Class I devices are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls, or General Controls, for medical devices, which include compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. Some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process.
Class II devices are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification procedure. All of our current devices are Class II devices.
Class III devices are those devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or deemed not substantially equivalent to a legally marketed predicate device. The safety and effectiveness of Class III devices cannot be assured solely by the General Controls and the other requirements described above. These devices almost always require formal clinical studies to demonstrate safety and effectiveness and must be approved through the premarket approval process described below. Premarket approval applications, and supplemental premarket approval applications, are subject to significantly higher user fees under Medical Device User Fee and Modernization Act of 2002, or MDUFMA, than are 510(k) premarket notifications, and generally take much longer for the FDA to review.
To obtain 510(k) clearance, a company must submit a premarket notification demonstrating that the proposed device is “substantially equivalent” in intended use and in technological and performance characteristics to a legally marketed “predicate device” that is either in Class I, Class II, or is a Class III device that was in commercial distribution before May 28, 1976, for which the FDA has not yet called for submission of a PMA application. Pursuant to the MDUFMA and the MDUFMA II provisions of the Food and Drug Amendments Act of 2007, unless a specific exemption applies, 510(k) premarket notification submissions are subject to user fees. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a
17
new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained. We have modified some of our 510(k) cleared devices, including our Masimo SET Software and Radical, but have determined that, in our view, based on FDA guidance as to when to submit a 510(k) notification for changes to a cleared device, new 510(k) clearances or PMA approvals are not required. We cannot assure you that the FDA would agree with any of our decisions not to seek additional 510(k) clearances or even PMA approval for these or future device modifications. If the FDA requires us to seek 510(k) clearance or PMA approval for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance or PMA approval.
Class III devices are required to undergo the PMA approval process in which the manufacturer must establish the safety and effectiveness of the device to the FDA’s satisfaction. A PMA application must provide extensive preclinical and clinical trial data as well as information about the device and its components regarding, among other things, device design, manufacturing and labeling. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR. A new PMA or a PMA Supplement is required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indications for use, manufacturing process, manufacturing facility, labeling and design. PMA Supplements often require submission of the same type of information as an original PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel. None of our products are currently approved under a PMA.
A clinical trial may be required in support of a 510(k) submission and generally is required for a PMA application. These trials generally require an Investigational Device Exemption, or IDE, application approved in advance by the FDA for a specified number of patients, unless the product is deemed a nonsignificant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results. Clinical trials may begin if the IDE application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance to market the product in the U.S.
We believe that our Original Equipment Manufacturer, or OEM, partners may be required to obtain 510(k) premarket clearance from the FDA for certain of their products that incorporate Masimo SET or Masimo rainbow® SET circuit boards and sensors. In order to facilitate our OEM partners in obtaining 510(k) clearance for their products that incorporate Masimo SET or Masimo rainbow® SET boards and sensors, we allow our OEM partners to reference the files from our cleared Masimo SET circuit boards, sensor, cable and notification system 510(k)s.
In the future, we may be required to submit additional 510(k) submissions to the FDA to address new claims, uses or products. We cannot assure you that the FDA will not deem one or more of our future products, or those of our OEM partners, to be a Class III device subject to the more burdensome PMA approval process. The FDA also may not approve or clear these products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or PMA of new products, new intended uses or modifications to existing products.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. Those regulatory requirements include:
| | • | | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
| | • | | QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| | • | | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses or indications; |
| | • | | clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
| | • | | approval of product modifications that affect the safety or effectiveness of one of our future approved devices; |
| | • | | medical device reporting, or MDR, regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
18
| | • | | post-approval restrictions or conditions, including post-approval study commitments; |
| | • | | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
| | • | | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
| | • | | regulations pertaining to voluntary recalls; and |
| | • | | notices of corrections or removals. |
We must also register with the FDA as a medical device manufacturer and must obtain all necessary state permits or licenses to operate our business. As a manufacturer, we are subject to announced and unannounced inspections by the FDA to determine our compliance with FDA’s QSR and other regulations.
Our OEM partners also are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we or one of our OEM partners have failed to comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| | • | | fines and civil penalties; |
| | • | | unanticipated expenditures to address or defend such actions; |
| | • | | delays in clearing or approving, or refusal to clear or approve, our products; |
| | • | | withdrawal or suspension of approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies; |
| | • | | product recall or seizure; |
| | • | | interruption of production; |
| | • | | operating restrictions; |
The FDA also has the authority to request repair, replacement or refund of the cost of any medical device manufactured or distributed by us. Our failure, or the failure of our OEM partners, to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our business, financial condition and results of operations.
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. If the FDA determines that our promotional materials or training constitutes promotion of an uncleared or unapproved use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. In that event, our reputation could be damaged and adoption of the products would be impaired.
Foreign Regulation
Many foreign countries in which we market or may market our products have regulatory bodies and restrictions similar to those of the FDA. International sales are subject to foreign government regulation, the requirements of which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance and the requirements may differ. Companies are now required to obtain the CE Mark prior to sale of some medical devices within the European Union. During this process, the sponsor must demonstrate compliance with the International Organization for Standardization’s manufacturing and quality requirements. We do have CE Marking on all of our products that require such markings. We cannot assure you that we or our OEM partners will be able to obtain necessary foreign government approvals or successfully comply with foreign regulations. Our failure to do so could hurt our business, financial condition and results of operations.
19
Other U.S. Regulation
We and our OEM partners also must comply with numerous federal, state and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection, fire hazard control and hazardous substance disposal. We cannot be sure that we will not be required to incur significant costs to comply with these laws and regulations in the future or that these laws or regulations will not hurt our business, financial condition and results of operations. Unanticipated changes in existing regulatory requirements or adoption of new requirements could hurt our business, financial condition and results of operations.
Environmental
Our manufacturing processes involve the use, generation and disposal of hazardous materials and wastes, including silicone adhesives, solder and solder paste, sealants, epoxies and various solvents such as methyl ethyl ketone, acetone and isopropyl alcohol. As such, we are subject to stringent federal, state and local laws relating to the protection of the environment, including those governing the use, handling and disposal of hazardous materials and wastes. Future environmental laws may require us to alter our manufacturing processes, thereby increasing our manufacturing costs. We believe that our products and manufacturing processes at our facilities comply in all material respects with applicable environmental laws and worker health and safety laws; however, the risk of environmental liabilities cannot be completely eliminated.
Health Care Fraud and Abuse
In the U.S., there are federal and state anti-kickback laws that generally prohibit the payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other health-related business. For example, the Federal Health Care Programs’ Anti-Kickback Law (42 U.S.C. § 1320a-7b(b)) prohibits anyone from, among other things, knowingly and willfully offering, paying, soliciting or receiving any bribe, kickback or other remuneration intended to induce the referral of patients for, or the purchase, order or recommendation of, health care products and services reimbursed by a federal health care program, including Medicare and Medicaid. Recognizing that the federal anti-kickback law is broad and potentially applicable to many commonplace arrangements, Congress and the Office of Inspector General within the Department of Health and Human Services, or OIG, has created statutory “exceptions” and regulatory “safe harbors.” Exceptions and safe harbors exist for a number of arrangements relevant to our business, including, among other things, payments to bona fide employees, certain discount and rebate arrangements, and certain payment arrangements involving GPOs. Although an arrangement that fits into one or more of these exceptions or safe harbors is immune from prosecution, arrangements that do not fit squarely within an exception or safe harbor do not necessarily violate the law and the OIG or other government enforcement authorities will examine the practice to determine whether it involves the sorts of abuses that the statute was designed to combat. Violations of this federal law can result in significant penalties, including imprisonment, monetary fines and assessments, and exclusion from Medicare, Medicaid and other federal health care programs. Exclusion of a manufacturer, like us, would preclude any federal health care program from paying for its products. In addition to the federal anti-kickback law, many states have their own laws that parallel and implicate antikickback restrictions analogous to the federal anti-kickback law, but may apply regardless of whether any federal health care program business is involved. Federal and state anti-kickback laws may affect our sales, marketing and promotional activities, educational programs, pricing and discount practices and policies, and relationships with health care providers by limiting the kinds of arrangements we may have with hospitals, alternate care market providers, GPOs, physicians and others in a position to purchase or recommend our products.
Federal and state false claims laws prohibit anyone from presenting, or causing to be presented, claims for payment to third-party payers that are false or fraudulent. For example, the federal Civil False Claims Act (31 U.S.C. § 3729 et seq.) imposes liability on any person or entity who, among other things, knowingly and willfully presents, or causes to be presented, a false or fraudulent claim for payment by a federal health care program, including Medicaid and Medicare. Some suits filed under the False Claims Act, known as “qui tam” actions, can be brought by a “whistleblower”, or “relator” on behalf of the government and such individuals may share in any amounts paid by the entity to the government in fines or settlement. Manufacturers, like us, can be held liable under false claims laws, even if they do not submit claims to the government, where they are found to have caused submission of false claims by, among other things, providing incorrect coding or billing advice about their products to customers that file claims, or by engaging in kickback arrangements with customers that file claims. A number of states also have false claims laws, and some of these laws may apply to claims for items or services reimbursed under Medicaid and/or commercial insurance. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, and imprisonment.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: health care fraud and false statements related to healthcare matters. The health care fraud statute prohibits, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program, including private payers. A violation
20
of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines and imprisonment.
The FCPA and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business.
Due to the breadth of some of these laws, it is possible that some of our current or future practices might be challenged under one or more of these laws. In addition, there can be no assurance that we would not be required to alter one or more of our practices to be in compliance with these laws. Evolving interpretations of current laws or the adoption of new federal or state laws or regulations could adversely affect many of the arrangements we have with customers and physicians. Our risk of being found in violation of these laws is increased by the fact that some of these laws are broad and open to interpretation. If our past or present operations are found to be in violation of any of these laws, we could be subject to civil and criminal penalties, which could hurt our business, financial condition and results of operations.
Privacy and Security of Health Information
Numerous federal, state and international laws and regulations govern the collection, use, and disclosure of patient-identifiable health information, including HIPAA. HIPAA applies to covered entities, which include most healthcare facilities that purchase and use our products. The HIPAA Privacy Rule restricts the use and disclosure of patient information, and requires covered entities to safeguard that information and to provide certain rights to individuals with respect to that information. The HIPAA Security Rule establishes elaborate requirements for safeguarding patient information transmitted or stored electronically. We are not a covered entity but due to activities that we perform for or on behalf of covered entities, we are sometimes deemed to be a business associate of covered entities.
In certain circumstances, the HIPAA rules require covered entities to contractually bind us, as a business associate, to protect the privacy and security of health information we may encounter during activities like training customers on the use of our products or investigating product performance. The Health Information Technology for Economic and Clinical Health Act, or HITECH, enacted in February 2009, made significant amendments to the HIPAA Privacy and Security Rules. Most provisions of HITECH were effective February 17, 2010; however, the new federal health data breach notice provision which requires business associates to notify covered entities of any breach of unsecured health information went into effect in September 2009. Prior to February 17, 2010, our business was not directly subject to the HIPAA Privacy and Security Rules. As a business associate, our privacy and security related obligations were solely contractual in nature and governed by the terms of each business associate agreement. HITECH fundamentally changed a business associate’s obligations by imposing a number of HIPAA Privacy Rule requirements and a majority of HIPAA Security Rule provisions directly on business associates and making business associates directly subject to HIPAA civil and criminal enforcement and the associated penalties for violation of the Privacy and Security Rule requirements. HITECH increased civil penalty amounts for violations of HIPAA by either covered entities or business associates and requires the U.S. Department of Health and Human Services to conduct periodic audits to confirm compliance. In addition, HITECH authorizes state attorneys general to bring civil actions in response to violations of HIPAA Privacy and Security Rules that threaten the privacy of state residents. Due to the very recent enactment of HITECH and expected implementing regulations, we are unable to predict what the extent of the impact on our business will be, but these new HITECH requirements may require us to incur additional costs and may restrict our business operations.
The HIPAA standards also apply to the use and disclosure of health information for research, and require the covered entity performing the research to obtain the written authorization of the research subject (or an appropriate waiver) before providing that subject’s health information to sponsors like us for purposes related to the research. These covered entities also typically impose contractual limitations on our use and disclosure of the health information they disclose to us. We may be required to make costly system modifications to comply with the privacy and security requirements that will be imposed on us and our failure to comply may result in liability and adversely affect our business.
Numerous other federal and state laws protect the confidentiality of patient information, including state medical privacy laws and federal and state consumer protection laws. These various laws in many cases are not preempted by the HIPAA rules and may be subject to varying interpretations by the courts and government agencies, creating complex compliance issues for us and our customers and potentially exposing us to additional expense, adverse publicity and liability. Other countries also have, or are developing, laws governing the collection, use and transmission of personal or patient information and these laws could create liability for us or increase our cost of doing business.
New health information standards, whether implemented pursuant to HIPAA, congressional action or otherwise, could have a significant effect on the manner in which we must handle health care related data, and the cost of complying with these standards could be significant. If we do not properly comply with existing or new laws and regulations related to patient health information we could be subject to criminal or civil sanctions.
21
Third-Party Reimbursement
Health care providers, including hospitals, that purchase our products generally rely on third-party payers, including the Medicare and Medicaid programs and private payers, such as indemnity insurers and managed care plans, to cover and reimburse all or part of the cost of the products and the procedures in which they are used. As a result, demand for our products is dependent in part on the coverage and reimbursement policies of these payers. No uniform coverage or reimbursement policy for medical technology exists among all third-party payers, and coverage and reimbursement can differ significantly from payer to payer.
Centers for Medicare and Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, along with its contractors, establish coverage and reimbursement policies for the Medicare program. Because a large percentage of the hospitals using our products treat elderly or disabled individuals who are Medicare beneficiaries, Medicare’s coverage and reimbursement policies are particularly significant to our business. In addition, private payers often follow the coverage and reimbursement policies of Medicare. We cannot assure you that government or private third-party payers will cover and reimburse the procedures using our products in whole or in part in the future or that payment rates will be adequate.
In general, Medicare will cover a medical product or procedure when the product or procedure is reasonable and necessary for the diagnosis or treatment of an illness or injury, or to improve the functioning of a malformed body part. Even if the medical product or procedure is considered medically necessary and coverage is available, Medicare may place restrictions on the circumstances where it provides coverage. For example, several Medicare local contractors have issued policies that restrict coverage for pulse oximetry in the hospital inpatient and outpatient settings to a limited number of conditions, including limiting coverage to patients who (i) exhibit signs of acute respiratory dysfunction, (ii) have chronic lung disease, severe cardiopulmonary disease or neuromuscular disease involving the muscles of respiration, (iii) are under treatment with a medication with known pulmonary toxicity, or (iv) have sustained multiple trauma or complaints of acute chest pain.
Reimbursement for our products may vary not only by the type of payer involved but also based upon the setting in which the product is furnished and utilized. For example, Medicare payment may be made, in appropriate cases, for patient stays in the hospital inpatient and outpatient settings involving the use of our products. Medicare generally reimburses hospitals based upon prospectively determined amounts. For hospital inpatient stays, the prospective payment generally is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay, using a classification system known as Medicare Severity Diagnosis-Related Groups, or MS-DRGs. Prospective rates are adjusted for, among other things, regional differences, co-morbidity, and complications. Hospitals generally do not receive separate Medicare reimbursement for the specific costs of purchasing our products for use in the inpatient setting. Rather, Medicare reimbursement for these costs is deemed to be included within the prospective payments made to hospitals for the inpatient services in which the products are utilized.
In contrast, some differences may be seen in the reimbursement for use of our products in hospital outpatient departments. In this setting, Medicare payments also are generally made under a prospective payment system based on the ambulatory payment classifications, or APCs, under which individual items and procedures are categorized. Hospitals receive the applicable APC payment rate for the procedure regardless of the actual cost for such treatment. Some outpatient services such as oximetry services do not receive separate reimbursement. Rather, their reimbursement is deemed packaged into the APC for an associated procedure, and the payment for that APC does not vary depending on whether the packaged procedure is performed. Some procedures also are paid through Composite APCs, which are APCs that establish a payment rate that applies when a specific combination of services is provided. Since January 1, 2007, reimbursement for certain pulse oximetry monitoring services, including those using our products, has not been packaged, but rather may receive a separate payment when no other separately payable services are provided. Effective January 1, 2011, these services may be separately payable when they are the only service provided to the patient on that day, packaged if provided with certain critical care services, or reimbursed through a composite APC when provided in connection with certain other services. This could result in changes to Medicare payments to our customers for the use of our products in the hospital outpatient setting.
Because payments through the Prospective Payment System in both the hospital inpatient and outpatient settings are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, hospitals have incentives to lower their operating costs by utilizing products that will reduce the length of inpatient stays, decrease labor or otherwise lower their costs. We cannot be certain that a hospital will purchase our products, despite the clinical benefits and opportunity for cost savings that we believe can be derived from their use. If hospitals cannot obtain adequate coverage and reimbursement for our products, or the procedures in which they are used, our business, financial condition and results of operations could suffer.
22
Our success with rainbow® SET technologies in U.S. care areas with reimbursable test procedures, such as hospital emergency department, hospital procedure labs, and the physician office will largely depend on the ability of providers to receive reimbursement for such testing procedures. Effective January, 1, 2011, the maximum rates for noninvasive carboxyhemoglobin, methemoglobin and total hemoglobin testing under the Medicare laboratory fee schedule are $7.06 per service. While private insurance payers generally follow Medicare coding and payment, we cannot be certain of this and in many cases, cannot control the coverage or payment rates that private insurance payers put in place. In addition, recently enacted health care reform legislation could affect future Medicare payment for these services.
Our success in non-U.S. markets depends largely upon the availability of coverage and reimbursement from the third-party payers through which health care providers are paid in those markets. Health care payment systems in non-U.S. markets vary significantly by country, and include single-payer, government managed systems as well as systems in which private payers and government managed systems exist side-by-side. Our ability to achieve market acceptance or significant sales volume in international markets we enter will be dependent in large part on the availability of reimbursement for procedures performed using our products under health care payment systems in such markets. There can be no assurance that reimbursement for our products, or the procedures in which our products are used, will be obtained or that such reimbursement will be adequate.
Manufacturing
Our strategy is to manufacture products in-house when it is efficient and cost-effective for us to do so. We currently manufacture internally our bedside and handheld pulse oximeters, our full line of disposable and reusable sensors and most of our patient cables. We maintain a 25,000 square foot International Organization for Standardization 13485:2003 certified manufacturing area in our facility in Irvine, California, and a 95,600 square foot facility in Mexicali, Mexico. We will continue to utilize third-party contract manufacturers for products and subassemblies that can be more efficiently manufactured by these parties, such as our circuit boards. We monitor our third-party manufacturers and perform inspections and product tests at various steps in the manufacturing cycle to ensure compliance with our specifications. We also do full functional testing of our circuit boards.
For raw materials, we and our contract manufacturers rely on sole source suppliers for some components, including digital signal processor chips and analog to digital converter chips. We and our contract manufacturers have taken steps to minimize the impact of a shortage or stoppage of shipments of digital signal processor chips or analog to digital converter chips, including maintaining a safety stock of inventory and designing software that may be easily ported to another digital signal processor chip. We believe that our sources of supply for components and raw materials are adequate. In the event of a delay or disruption in the supply of sole source components, we believe that we and our contract manufacturers will be able to locate additional sources of these sole source components on commercially reasonable terms and without experiencing material disruption in our business or operations.
We have agreements with certain major suppliers and each agreement provides for varying terms with respect to term, termination and pricing. The initial terms of some of these agreements have expired, however, and in each case the parties have either continued to perform under the agreement or the agreement provides for automatic renewal. Most of these agreements allow for termination upon specified notice, ranging from 120 days to six months, to the non-terminating party. Certain of these agreements with our major suppliers allows for pricing adjustments, each agreement provides for annual pricing negotiation, and one also guarantees us the most favorable pricing offered by the supplier to any of its other customers.
Operating Segment and Geographic Information
We operate in one business segment, using one measurement of profitability to manage our business. Sales and other financial information by geographic area is provided in Note 13 to our consolidated financial statements that are included in this Form 10-K.
Employees
As of January 1, 2011, we had 2,397 full-time employees and contract employees worldwide.
Address
Our principal executive offices are located at 40 Parker, Irvine, California 92618, and our telephone number at that address is (949) 297-7000. Our website address is www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this Form 10-K.
23
Executive Officers of the Registrant
Our executive officers, as of January 31, 2011, are set forth below:
| | | | |
Name | | Age1 | | Position(s) |
| Joe Kiani | | 46 | | Chief Executive Officer & Chairman of the Board of Directors |
| Tony Allan | | 45 | | Chief Operating Officer |
| Jon Coleman | | 47 | | President, International |
| Mark P. de Raad | | 51 | | Executive Vice President, Chief Financial Officer and Corporate Secretary |
| Rick Fishel | | 53 | | President Americas and Worldwide OEM Business |
| Paul Jansen | | 40 | | Executive Vice President, Marketing |
| Yongsam Lee | | 46 | | Chief Information Officer and Executive Vice President, Regulatory Affairs |
| Michael O’Reilly, M.D. | | 58 | | Chief Medical Officer |
| Stephen Paul | | 40 | | Executive Vice President of Acute Care Sales |
| Anand Sampath | | 44 | | Executive Vice President, Engineering |
Joe Kianiis the founder of Masimo and has served as Chief Executive Officer and Chairman of the Board of Directors since our inception in 1989. He is an inventor on more than 50 patents related to signal processing, sensors, and patient monitoring, including patents for the invention of measure-through motion and low perfusion pulse oximetry. Mr. Kiani is currently on the Board of Directors of Saba Software, Inc., a publicly-traded software company focused on human capital development and management solutions. Mr. Kiani holds a B.S.E.E. and an M.S.E.E. from San Diego State University.
Tony Allanhas served as our Chief Operating Officer since May 2010. From August 1995 to May 2010, he held various positions, including Vice President of Global Business Units, Instrumentation and Medical Sector and Senior Business Unit Director with Jabil Circuit Inc, an electronics design, manufacturing and product solutions company. Mr. Allan holds a Post Graduate diploma in Quality Engineering from Paisley University and a H.N.D. Engineering from Glasgow College.
Jon Colemanhas served as our President, International since August 2008. From October 2007 to August 2008, Mr. Coleman was President and Chief Executive Officer of You Take Control, Inc, a healthcare information technology start-up company. He served as General Manager, Americas of Targus Group International, a supplier of mobile computing cases and accessories, from March 2006 to February 2007. From March 1994 to February 2006, he held progressive leadership positions with Pfizer, Inc, most recently Vice President and General Manager, Canada & Caribbean Region. Mr. Coleman holds a M.B.A. from Harvard Business School, and a B.A. in International Relations from Brigham Young University.
Mark P. de Raad has served as our Executive Vice President and Chief Financial Officer since June 2006 and as our Corporate Secretary since December 2009. From November 2002 through May 2006, Mr. de Raad served as Vice President, Chief Financial Officer and Secretary for Avamar Technologies, Inc., a start-up enterprise software development company. He served as Chief Financial Officer, Quantum Storage Solutions Group, a division of Quantum Corporation from June 2001 through November 2002. From September 1997 through June 2001, Mr. de Raad was Vice President, Finance and Chief Financial Officer for ATL Products, Inc., a manufacturer of automated tape libraries. Mr. de Raad is a Certified Public Accountant (inactive) and holds a B.S. in Accounting from the University of Santa Clara.
Rick Fishel has served as President Americas and Worldwide OEM Business since February 2009 and was President of Masimo Americas from June 2004 to February 2009. From January 2003 to June 2004, Mr. Fishel was Regional Vice President of Sales for the Information Solutions segment of the McKesson Corporation, a provider of supply, information and care management products and services. From January 2001 to January 2003, he served as National Vice President of Sales for the Consulting Services division of GE Medical Systems, Inc., a provider of medical technology and productivity solutions. Mr. Fishel holds a B.S. in Marketing from Arizona State University.
Paul Jansen has served as our Executive Vice President of Marketing since April 2008, and was our Vice President of Marketing from January 2008 to April 2008. From August 1997 through December 2007, he served as Vice President, Marketing & Clinical Development for CardioDynamics, a cardiac monitoring and diagnostics company. Mr. Jansen holds a B.S. in Planning from Iowa State University and an M.B.A. from Arizona State University.
Yongsam Leehas served as our Chief Information Officer and Executive Vice President, Regulatory Affairs since November 2010. From March 1996 to October 2001 and from April 2002 to November 2010, Mr. Lee held various positions with us, including Chief Information Officer, Vice President, IT and Executive Vice President, Operations. From October 2001 to April 2002, he served as Director of IT at SMC Networks, Inc., a provider of networking solutions. Mr. Lee holds a B.S. in Applied Physics from the University of California, Irvine.
24
Michael O’Reilly, M.D. has served as our Chief Medical Officer since October 2010 and was our Executive Vice President, Medical Affairs from February 2008 to October 2010. Since April 2008, Dr. O’Reilly has been a Professor of Anesthesiology and Perioperative Care at the University of California, Irvine. He was an Associate Professor from September 2002 to February 2008, and an Assistant Professor from September 1996 to August 2002, in Anesthesiology at the University of Michigan. He was also the Director of the Liver Transplant Anesthesiology and Medical Director of Information Technology at the University of Michigan, and a member of various advisory boards. He has numerous publications in scientific journals, national and international invited presentations and earned various awards and grants. Dr. O’Reilly holds an M.D. and M.S. in Cell Biology from the University of Vermont.
Stephen Paul has served as our Executive Vice President of Acute Care Sales since January 2010, and was our Vice President of U.S. Hospital Sales from February 2009 to January 2010 and our Western Area Vice President of Sales from March 2008 to February 2009. From April 1996 to February 2008, he held various positions, including Western Area Sales Director and Regional Sales Manager, with Boston Scientific, a developer, manufacturer and marketer of medical devices. Mr. Paul holds a B.A. in Political Science from University of California at Santa Barbara.
Anand Sampath has served as our Executive Vice President, Engineering since March 2007. He is an inventor on more than four patents relating to patient monitoring, wireless networks and communications. From April 2006 to March 2007, Mr. Sampath was our Director of Systems Engineering. From October 1995 to March 2006, he held various positions, including Program Manager, Engineering Manager and Distinguished Member of Technical Staff, at Motorola, Inc. Mr. Sampath holds a B.S. in Engineering from Bangalore University.
Available Information
We are subject to the reporting requirements under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Consequently, we are required to file reports and information with the Securities and Exchange Commission, or SEC, including reports on the following forms: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. These reports and other information concerning us may be accessed through the SEC’s website at www.sec.gov and on our website at www.masimo.com. Such filings are placed on our website as soon as reasonably practical after they are filed with the SEC. Information contained in, or that can be accessed through, our website is not part of this Form 10-K.
The following risk factors and other information included in this Form 10-K should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations. If any of the following risks come to fruition, our business, financial condition, results of operations and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our stock could decline, and you could lose all or part of your investment.
Risks Related to Our Revenues
We currently derive substantially all of our revenue from our Masimo SET platform, Masimo rainbow® SET platform and related products. If this technology and the related products do not continue to achieve market acceptance, our business, financial condition and results of operations would be adversely affected.
We are dependent upon the success and market acceptance of our proprietary Masimo SET. Currently, our primary product offerings are based on the Masimo SET platform. Continued market acceptance of products incorporating Masimo SET will depend upon our ability to continue to provide evidence to the medical community that our products are cost-effective and offer significantly improved performance compared to conventional pulse oximeters. Health care providers that currently have significant investments in competitive pulse oximetry products may be reluctant to purchase our products. If hospitals and other health care providers do not believe our Masimo SET platform is cost-effective, safe or more accurate or reliable than competitive pulse oximetry products, they may not buy our products in sufficient quantities to enable us to be profitable. In addition, allegations regarding the safety and effectiveness of our products, whether or not substantiated, may impair or impede the acceptance of our products. If we are unable to achieve additional market acceptance of our core technology or products incorporating Masimo SET, we will not generate significant revenue growth from the sale of our products.
25
Some of our products, including those based on licensed rainbow® technology, are in development or have been recently introduced into the market and may not achieve market acceptance, which could limit our growth and adversely affect our business, financial condition and results of operations.
Our products that have been recently introduced into the market, including, but not limited to, those based on rainbow® technology, a technology that we license, may not be accepted in the market. In September 2008, we began our limited market release of total hemoglobin, and focused on obtaining data and clinical feedback on the performance of the product in the hospital. In October 2008, we received FDA clearance for Pronto, a handheld noninvasive multi-parameter testing device, not a continuous monitoring device, that uses our rainbow® SET technology, to provide pulse oximetry, pulse rate, perfusion index and spot checking of total hemoglobin levels. In the first quarter of 2009, we commercially launched our total hemoglobin product for continuous and noninvasive monitoring in the hospital. In June 2010, we received FDA clearance for Pronto-7, a handheld noninvasive multi-parameter testing device, that provides spot-check hemoglobin testing along with oxygen saturation, pulse rate and perfusion index results. This device is used for, among other things, spot-check testing of hemoglobin in almost any environment. Also, in June 2010, we initiated a full commercial release of Rainbow Acoustic MonitoringTM after a limited market release that allowed us to evaluate the product’s performance in the field.
Given that certain rainbow® technology products are new to the marketplace, we do not know to what degree the market will accept these products, if at all. Even if our customers recognize the benefits of our products, we cannot assure you that our customers will purchase them in quantities sufficient for us to be profitable or successful. We will need to invest in significant sales and marketing resources to achieve market acceptance of these products with no assurance of success. The degree of market acceptance of these products will depend on a number of factors, including:
| | • | | perceived advantages of our products and their sales prices; |
| | • | | perceived safety and effectiveness of our products; |
| | • | | reimbursement available through Centers for Medicare and Medicaid Services, or CMS, programs for using our products; and |
| | • | | introduction and acceptance of competing products or technologies. |
In general, all of our new noninvasive technologies are new technologies. These new technologies have performance levels that we believe are acceptable for many clinical environments but may be insufficient in others. In addition, these noninvasive technologies may perform better in some patients and settings than others. The performance of these technologies shows a variability across a population that follows a gaussian distribution described in the accuracy specifications. Over time, we hope to reduce this variability and, if we do, we expect these new noninvasive technologies to become more useful in additional environments and to become more widely adopted. This is the adoption pattern we have experienced historically with our previously released measurements, such as oxygen saturation, and what we expect to experience in the future with our current and future technologies. Although we will seek to reduce this variability over time, we may not be successful. If our products do not gain market acceptance or if our customers prefer our competitors’ products, our potential growth would be limited, which would adversely affect our business, financial condition and results of operations.
In addition, in December 2010, we announced our determination to voluntarily recall our Pronto-7 rainbow® 4D spot-check reusable sensors, which were in limited market release. The recall was initiated after we became aware that the thermistor on the sensor was not working as designed. As a result, we determined that the Pronto-7 multi-parameter spot-check device may have provided a SpHb measurement outside our specification range in certain settings. Although we are working to develop a replacement Pronto-7 rainbow® 4D sensor that will address the differences, there can be no assurance that we will be able to complete the development of a replacement sensor on a timely basis, that the replacement sensor will function properly in accordance with its design, or that the replacement sensor will achieve market acceptance. In the event of a prolonged delay in releasing a replacement Pronto-7 rainbow® 4D sensor, or if our replacement sensors receive limited market acceptance, our business, financial condition and results of operations could be adversely affected.
Our ability to commercialize new products, new or improved technologies and additional applications for Masimo SET and our right to use rainbow® technology are each limited to certain markets by our Cross-Licensing Agreement with Cercacor, which may impair our growth and adversely affect our financial condition and results of operations.
In May 1998, we spun-off a newly-formed entity, Cercacor Laboratories, Inc., or Cercacor (formerly Masimo Laboratories, Inc.), and provided it rights to use Masimo SET to commercialize non-vital signs monitoring applications while we retained the rights to Masimo SET to commercialize vital signs monitoring applications. On May 2, 1998, we entered into a cross-licensing agreement with Cercacor, which has been amended several times, most recently in an Amended and Restated Cross-Licensing Agreement, effective January 1, 2007, or the Cross-Licensing Agreement. Under the Cross-Licensing Agreement, we granted Cercacor:
| | • | | an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET owned by us, including all improvements on this technology, for the measurement of non-vital signs measurements and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs measurements in any product market in which a product is intended to be used by a patient or pharmacist rather than by a professional medical caregiver, which we refer to as the Cercacor Market, and |
26
| | • | | a non-exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET for measurement of vital signs in the Cercacor Market. |
Non-vital sign measurements consist of body fluid constituents other than vital sign measurements, including, but not limited to, carbon monoxide, methemoglobin, blood glucose, total hemoglobin and bilirubin.
Under the Cross-Licensing Agreement, we are only permitted to sell devices utilizing Masimo SET for the measurement of non-vital signs measurements in markets where the product is intended to be used by a professional medical caregiver, including, but not limited to, hospital caregivers and alternate care market facility caregivers, rather than by a patient or pharmacist, which we refer to as the Masimo Market. Accordingly, our ability to commercialize new products, new or improved technologies and additional applications for Masimo SET is limited. In particular, our inability to expand beyond the Masimo Market may impair our growth and adversely affect our financial condition and results of operations.
Pursuant to the Cross-Licensing Agreement, we have licensed from Cercacor the right to make and distribute products in the Masimo Market that utilize rainbow® technology for the measurement of only carbon monoxide, methemoglobin, fractional arterial oxygen saturation and total hemoglobin, which includes hematocrit. As a result, the opportunity to expand the market for our products incorporating rainbow® technology is limited, which could limit our ability to maintain or increase our revenue and impair our growth.
We face competition from other companies, many of which have substantially greater resources than we do. If we do not successfully develop and commercialize enhanced or new products that remain competitive with new products or alternative technologies developed by others, we could lose revenue opportunities and customers, and our ability to grow our business would be impaired.
A number of our competitors have substantially greater capital resources, larger customer bases, larger sales forces than ours, and have established stronger reputations with target customers and built relationships with GPOs that are more effective than ours. We face substantial competition from companies developing products that compete with our Masimo SET platform for use with third-party monitoring systems. We also face competition from companies currently marketing pulse oximetry monitors.
The medical device industry is characterized by rapid product development and technological advances, which places our products at risk of obsolescence. Our long-term success depends upon the development and successful commercialization of new products, new or improved technologies and additional applications for Masimo SET and licensed rainbow® technology. The research and development process is time-consuming and costly and may not result in products or applications that we can successfully commercialize. In particular, we may not be able to successfully commercialize our products for applications other than arterial blood oxygen saturation and pulse rate monitoring, including acoustic respiration rate, hemoglobin, carboxyhemoglobin and methemoglobin monitoring. If we do not successfully adapt our products and applications both within and outside these measurements, we could lose revenue opportunities and customers. Furthermore, one or more of our competitors may develop products that are substantially equivalent to our FDA-cleared products, or those of our original equipment manufacturer, or OEM, partners, whereby they may be able to use our products or those of our OEM partners, as predicate devices to more quickly obtain FDA clearance of their competing products. Competition could result in reductions in the price of our products, fewer orders for our products, a reduction of our gross margins and a loss of our market share.
We depend on our domestic and international OEM partners for a portion of our revenue. If they do not devote sufficient resources to the promotion of products that use Masimo SET and licensed rainbow® technology, our business would be harmed.
We are, and will continue to be, dependent upon our domestic and international OEM partners for a portion of our revenue through their marketing, selling and distribution of certain of their products that incorporate Masimo SET and licensed rainbow® technology. Although we expect that our OEM partners will accept and actively market, sell and distribute products that incorporate licensed rainbow® technology, they may not elect, and they have no contractual obligation, to do so. Because products that incorporate our technologies may represent a relatively small percentage of business for some of our OEM partners, they may have less incentive to promote these products rather than other products that do not incorporate these technologies. In addition, some of our OEM partners offer products that compete with ours. Therefore, we cannot guarantee that our OEM partners, or any company that might acquire any of our OEM partners, will vigorously promote products incorporating Masimo SET and licensed rainbow® technology, or at all. The failure of our OEM partners to successfully market, sell or distribute products incorporating these technologies, the termination of OEM agreements, the loss of OEM partners or the inability to enter into future OEM partnership agreements would have a material adverse effect on our business, financial condition and results of operations.
27
If we fail to maintain or develop relationships with GPOs, sales of our products would decline.
Our ability to sell our products to U.S. hospitals depends, in part, on our relationships with group purchasing organizations, or GPOs. Many existing and potential customers for our products become members of GPOs. GPOs negotiate beneficial pricing arrangements and contracts, which are sometimes exclusive, with medical supply manufacturers and distributors.
These negotiated prices are made available to a GPO’s affiliated hospitals and other members. If we are not one of the providers selected by a GPO, the GPO’s affiliated hospitals and other members may be less likely or unlikely to purchase our products. If a GPO has negotiated a strict sole source, market share compliance or bundling contract for another manufacturer’s products, we may be prohibited from making sales to members of the GPO for the duration of the contractual arrangement. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, shipments of our pulse oximetry products to customers that are members of GPOs represented $183.8 million, $160.8 million and $132.1 million, respectively, of our revenue from sales to U.S. hospitals. Our failure to renew our contracts with GPOs may cause us to lose market share and could have a material adverse effect on our sales, financial condition and results of operations. In addition, if we are unable to develop new relationships with GPOs, our competitive position would likely suffer and our business would be harmed.
Inadequate levels of coverage or reimbursement from governmental or other third-party payers for our products, or for procedures using our products, may cause our revenue to decline.
Sales of our products depend in part on the reimbursement and coverage policies of governmental and private health care payers. The ability of our health care provider customers, including hospitals, to obtain adequate coverage and reimbursement for our products, or for the procedures in which our products are used, may impact our customers’ purchasing decisions. Therefore, our customers’ inability to obtain adequate coverage and reimbursement for our products would have a material adverse effect on our business.
Third-party payers have adopted, and are continuing to adopt, health care policies intended to curb rising health care costs. These policies include, among others:
| | • | | controls on reimbursement for health care services and price controls on medical products and services; |
| | • | | limitations on coverage and reimbursement for new medical technologies and procedures; and |
| | • | | the introduction of managed care and prospective payment systems in which health care providers contract to provide comprehensive health care for a fixed reimbursement amount per person or per procedure. |
We cannot guarantee a governmental or third-party payer will reimburse, or continue to reimburse, a customer for the cost of our products. Some payers have indicated that they are not willing to reimburse for certain of our products or for the procedures in which our products are used. For example, some insurance carriers have issued policies denying coverage for transcutaneous hemoglobin measurement on the grounds that the technology is investigational in the outpatient setting. Other payers are continuing to investigate our products to determine if they will provide reimbursement to our customers. We are working with these payers to obtain reimbursement, but may not be successful. These trends could lead to pressure to reduce prices for our current products and product candidates and could cause a decrease in the size of the market or a potential increase in competition that could adversely affect our business, financial condition and results of operations.
Our customers may reduce, delay or cancel purchases due to a variety of factors, such as lower hospital census levels or third-party guidelines, which could adversely affect our business, financial condition and results of operations.
Our customers are facing a growing level of uncertainties, such as lower overall hospital census for paying patients and the impact of that lower census on hospital budgets.
In addition, there are specific portions of our business, such as our OEM customers, who, due to their capital equipment sales model, could be impacted by the ongoing economic uncertainties and the resulting constraints on hospital budgets. These hospital budget constraints could cause our OEMs more difficulty in selling their large, relatively high priced multi-parameter devices which, in turn, could reduce our board sales to our OEM customers. In addition, certain of our products, including our rainbow® measurements such as carbon monoxide, methemoglobin and total hemoglobin are sold with upfront license fees and more complex, and therefore, more expensive sensors could be impacted by hospital budget reductions.
In addition, states and other local regulatory authorities may issue guidelines regarding the appropriate scope and use of our products from time to time. For example, our SpCO monitoring devices may be subject to authorization by individual states as part of Emergency Medical Services, or EMS, scope of practice procedures. The State of California recently categorized SpCO as a laboratory test and therefore outside the scope of practice for EMS providers. Although a lack of inclusion into scope of practice procedures does not prohibit usage, it may limit adoption.
28
The loss of any large customer, or distributor, or any cancellation or delay of a significant purchase by a large customer could reduce our net sales and harm our operating results.
We also have a concentration of OEM, distribution and direct customers. If for any reason we were to lose our ability to sell to a specific group or class of customers, or through a distributor, we would experience a significant reduction in revenue, which would adversely impact our operating results. Also, we cannot provide any assurance that we will retain our current customers or groups of customers, or distributors, or that we will be able to attract and retain additional customers in the future. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, one of our just-in-time distributors represented 14%, 14% and 12%, respectively, of our total revenue. The loss of any large customer or distributor could have a material adverse effect on our financial condition and results of operations.
Medical device reprocessors that reprocess our single-use sensors and then resell them to hospitals at a cost lower than our new sensors may adversely affect our business, financial condition and results of operations.
Certain medical device reprocessors have been collecting our used single-use sensors from hospitals and then reprocessing, repackaging and reselling those sensors to hospitals at a price lower than our new sensors. These reprocessed sensors may lead to confusion with our authorized products, reduce our revenue and harm our customer relationships. In addition, this may increase time and expense spent investigating and addressing performance issues with the reprocessed sensors, and enforcing our proprietary rights and contracts against the reprocessors.
Despite our agreements and our customers’ acknowledged preference for disposable single patient adhesive sensors due to performance and risk of contamination, our customers that are concerned about finances could take desperate measures such as switching from disposable sensors to reusable sensors. In addition, our customers could also begin purchasing third party recycled sensors, rather than our new sensors, in an attempt to reduce their overall operating costs.
Covidien may seek to avoid paying any royalties to us after March 15, 2014, which would significantly reduce our royalty revenue, total revenues and adversely affect our business, financial condition and results of operations.
We are party to a settlement agreement with Covidien. Under the current settlement agreement, we earn royalties on Covidien’s total U.S. based pulse oximetry sales. In both 2010 and 2009, our royalties from the Covidien settlement totaled $49.0 million each year. Because these royalty payments do not carry any significant cost, they result in significant improvements to our reported gross profit, operating income levels and earnings per share. As a result, an elimination of royalties that we earn under the settlement agreement in the future will have a significant impact on our revenue, gross margins, operating income and earnings per share. In 2009, 2010 and through March 14, 2011, the remaining term of the settlement agreement, the royalty rate is 13%.
On January 28, 2011, we entered into a second amendment to this settlement agreement with Covidien. As part of this amendment, which will become effective as of March 14, 2011, Covidien has agreed to pay us a royalty at a rate of 7.75% of its United States pulse oximetry revenue, as that term is defined in the January 28, 2011 second amendment, at least through March 15, 2014. This second amendment provides Covidien with the option to stop paying the royalty on March 15, 2014. In exchange for this royalty payment, we have provided Covidien with a covenant not to sue for its current pulse oximetry products as long as Covidien is abiding by the terms of the second amendment. After March 15, 2014, Covidien may seek to stop paying us any royalties which would have a material adverse impact on our total revenue, gross margins, operating income and earnings per share.
Risks Related to Our Intellectual Property
If the patents we own or license, or our other intellectual property rights, do not adequately protect our technologies, we may lose market share to our competitors and be unable to operate our business profitably.
Our success depends significantly on our ability to protect our rights to the technologies used in our products, including Masimo SET and licensed rainbow® technology. We rely on patent protection, trade secrets, as well as a combination of copyright and trademark laws and nondisclosure, confidentiality and other contractual arrangements to protect our technology and rights. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or maintain any competitive advantage. In addition, we cannot be assured that any of our pending patent applications will result in the issuance of a patent to us. The U.S. Patent and Trademark Office, or PTO, may deny or require significant narrowing of claims in our pending patent applications, and patents issued as a result of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is advantageous to us. We could also incur substantial costs in proceedings before the PTO. Our issued and licensed patents and those that may be issued or licensed in the future may expire or may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related technologies. Some Masimo patents related to our Masimo SET algorithm technology begin to expire in March 2011. Additionally, upon expiration of our issued or licensed patents, we may lose some of our rights to exclude others from making, using, selling or importing products using the technology based on the expired patents. We also must rely on contractual rights with the third parties that license technology to us to protect our rights in the
29
technology licensed to us. There is no assurance that competitors will not be able to design around our patents. We also rely on unpatented proprietary technology. We cannot assure you that we can meaningfully protect all our rights in our unpatented proprietary technology or that others will not independently develop substantially equivalent proprietary products or processes or otherwise gain access to our unpatented proprietary technology.
We seek to protect our know-how and other unpatented proprietary technology with confidentiality agreements and intellectual property assignment agreements with our employees, our OEM partners, independent distributors and consultants. However, such agreements may not be enforceable or may not provide meaningful protection for our proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements or in the event that our competitors discover or independently develop similar or identical designs or other proprietary information. In addition, we rely on the use of registered and common law trademarks with respect to the brand names of some of our products. Common law trademarks provide less protection than registered trademarks. Loss of rights in our trademarks could adversely affect our business, financial condition and results of operations.
Furthermore, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the U.S. If we fail to apply for intellectual property protection or if we cannot adequately protect our intellectual property rights in these foreign countries, our competitors may be able to compete more effectively against us, which could adversely affect our competitive position, as well as our business, financial condition and results of operations.
If third parties claim that we infringe their intellectual property rights, we may incur liabilities and costs and may have to redesign or discontinue selling certain products.
Companies in the medical device industry have used intellectual property litigation to gain a competitive advantage in the marketplace. We face the risk of claims that we have infringed on third parties’ intellectual property rights. Searching for existing intellectual property rights may not reveal important intellectual property and our competitors may also have filed for patent protection, which is not publicly-available information, or claimed trademark rights that have not been revealed through our availability searches. In addition, many of our employees were previously employed at other medical device companies. We may be subject to claims that our employees have disclosed, or that we have used, trade secrets or other proprietary information of our employees’ former employers. Our efforts to identify and avoid infringing on third parties’ intellectual property rights may not always be successful. Any claims of patent or other intellectual property infringement against us, even those without merit, could:
| | • | | increase the cost of our products; |
| | • | | be expensive and time consuming to defend; |
| | • | | result in us being required to pay significant damages to third parties; |
| | • | | force us to cease making or selling products that incorporate the challenged intellectual property; |
| | • | | require us to redesign, reengineer or rebrand our products, product candidates and technologies; |
| | • | | require us to enter into royalty or licensing agreements in order to obtain the right to use a third party’s intellectual property on terms that may not be favorable or acceptable to us; |
| | • | | require us to indemnify third parties pursuant to contracts in which we have agreed to provide indemnification for intellectual property infringement claims; |
| | • | | divert the attention of our management and other key employees; |
| | • | | result in our customers or potential customers deferring or limiting their purchase or use of the affected products impacted by the claims until the claims are resolved; and |
| | • | | otherwise have a material adverse effect on our business, financial condition and results of operations. |
In addition, new patents obtained by our competitors could threaten the continued commercialization of our products in the market even after they have already been introduced. In 2009, Philips Electronics North America Corporation filed antitrust and patent infringement counterclaims against us, as further explained in Part 1, Item 3 of this Form 10-K.
We believe competitors may currently be violating and may in the future violate our intellectual property rights, and we may bring additional litigation to protect and enforce our intellectual property rights, which may result in substantial expense and may divert our attention from implementing our business strategy.
We believe that the success of our business depends, in significant part, on obtaining patent protection for our products and technologies, defending our patents and preserving our trade secrets. We were previously involved in significant litigation to protect our patent position and may be required to engage in further litigation. In 2006, we settled a costly, six-year lawsuit against Mallinckrodt, Inc., part of Tyco Healthcare (currently Covidien Ltd.), and one of its subsidiaries, Nellcor Puritan Bennett, Inc., in which we claimed that Covidien was infringing some of our pulse oximetry signal processing patents.
30
In February 2009, we filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH related to Philips FAST pulse oximetry technology and certain Philips patient monitors as described in Part 1, Item 3 of this Form 10-K, and Note 12 to the consolidated financial statements. Both Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH are associated with Philips Medical Systems, an OEM partner of ours. There is no guarantee that we will prevail in this suit or receive any damages or other relief if we do prevail.
We believe that other competitors of ours, including GE Medical Systems and Mindray Medical International Ltd., may be infringing at least one of our patents. Our failure to pursue any potential claim could result in the loss of our proprietary rights and harm our position in the marketplace. Therefore, we may be forced to pursue litigation to enforce our rights. Our ongoing and future litigation could result in significant additional costs and further divert the attention of our management and key personnel from our business operations and the implementation of our business strategy and may not be adequate to protect our intellectual property rights.
Risks Related to Our Regulatory Environment
Our failure to obtain and maintain FDA clearances or approvals on a timely basis, or at all, would prevent us from commercializing our current or upgraded products in the United States, which could severely harm our business.
Each medical device that we wish to market in the U.S. generally must first receive either 510(k) clearance from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act by filing a 510(k) pre-market notification, or PMA, through submitting a PMA application. Even if regulatory clearance or approval of a product is granted, the clearance or approval may be subject to limitations on the indicated uses for which the product may be marketed. We cannot assure you that the FDA will grant 510(k) clearance on a timely basis, if at all, for new products or uses that we propose for Masimo SET or licensed rainbow® technology. The FDA’s 510(k) clearance process of our products and uses has historically taken approximately four to six months. However, over the past year we have experienced a significantly longer 510(k) clearance review process. Our more recent experience in seeking FDA 510(k) clearance, along with information we have received from other medical device manufacturers, suggests that the FDA may have modified its 510(k) review protocol and process. Specifically, it appears that the FDA’s medical device product reviews currently require applicants to provide much more information and data than in prior periods, the FDA is not consistently relying upon prior precedents thereby leading to more review cycles or, in some cases, to non-substantially equivalent decisions, and that the FDA has broadened the scope of its reviews. As a result, we have experienced lengthier FDA 510(k) review periods over the past 12 months, which has delayed the 510(k) clearance process for our products and uses over this period compared to prior periods. In addition, in September 2009, the FDA commissioned the Institute of Medicine to study the premarket notification program used to review and clear certain medical devices marketed in the U.S. It is believed that this study may result in formal changes to the FDA’s 510(k) clearance review and approval processes which could potentially lengthen the review process of any future application of ours even further.
We have received FDA 510(k) clearance for the Pronto and Pronto-7 for noninvasive spot-checking of hemoglobin and other measurements in clinical and non-clinical settings, including blood donation facilities. Before commercializing either device in U.S. blood donation centers, we are also pursuing specific regulatory clearance from the FDA Center for Biologics Evaluation and Research, which regulates the collection of blood and blood components used for transfusion or for the manufacture of pharmaceuticals derived from blood and blood components.
To date, the FDA has regulated pulse oximeters incorporating Masimo SET and licensed rainbow® technology, and our sensors, cables and other products incorporating Masimo SET and licensed rainbow® technology for pulse oximetry under the 510(k) process. Although 510(k) clearances have been obtained for all of our current products, these clearances may be withdrawn by the FDA at any time if substantial safety or effectiveness problems develop with our devices. In November 2010, we voluntarily notified the FDA that we had received allegations regarding the safety and effectiveness of our Pronto and Pronto-7 products from certain former sales representatives of ours. As part of its review of this matter, the FDA may determine to reevaluate or reconsider its prior approval of our Pronto and Pronto-7 products. Furthermore, our new products or significantly modified marketed products could be denied 510(k) clearance and be required to undergo the more burdensome PMA process. The process of obtaining PMA is much more costly, lengthy and uncertain than the process for obtaining 510(k) clearance and generally takes one to three years, but may be longer.
31
The failure of our OEM partners to obtain required FDA clearances or approvals for products that incorporate our technologies could have a negative impact on our revenue.
Our OEM partners will be required to obtain their own FDA clearances for products incorporating Masimo SET and licensed rainbow® technology to market these products in the U.S. We cannot assure you that the FDA clearances we have obtained will make it easier for our OEM partners to obtain clearances of products incorporating these technologies, or that the FDA will ever grant clearances on a timely basis, if at all, for any future product incorporating Masimo SET and licensed rainbow® technology that our OEM partners propose to market.
If we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Our products, along with the manufacturing processes and promotional activities for such products, are subject to continual review and periodic inspections by the FDA and other regulatory bodies. In particular, we and our suppliers are required to comply with FDA’s Quality System Regulation, or QSR, which covers the methods and documentation of the design, testing, production, component suppliers control, quality assurance, labeling, packaging, storage and shipping of our products. The FDA enforces the QSR through announced and unannounced inspections. We are also subject to similar state requirements and licenses. Failure by us or one of our suppliers to comply with statutes and regulations administered by the FDA and other regulatory bodies, discovery of previously unknown problems with our products (including unanticipated adverse events or adverse events of unanticipated severity or frequency), manufacturing problems, or failure to comply with regulatory requirements, or failure to adequately respond to any FDA observations concerning these issues, could result in, among other things, any of the following actions:
| | • | | warning letters or untitled letters issued by the FDA; |
| | • | | fines, civil penalties, injunctions and criminal prosecution; |
| | • | | unanticipated expenditures to address or defend such actions; |
| | • | | delays in clearing or approving, or refusal to clear or approve, our products; |
| | • | | withdrawal or suspension of clearance or approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies; |
| | • | | product recall or seizure; |
| | • | | orders for physician notification or device repair, replacement or refund; |
| | • | | interruption of production; and |
| | • | | operating restrictions. |
Furthermore, our key component suppliers may not currently be, or may not continue to be, in compliance with applicable regulatory requirements. If any of these actions were to occur, it would harm our reputation and adversely affect our business, financial condition and results of operations.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing our products abroad.
We currently market and intend to continue to market our products internationally. Outside of the U.S., we can market a product only if we receive a marketing authorization and, in some cases, pricing approval, from the appropriate regulatory authorities. The regulatory registration/licensing process varies among international jurisdictions and may require additional testing. The time required for international registration of new products may differ from that required for obtaining FDA clearance. The foreign registration/licensing process may include all of the risks associated with obtaining FDA clearance in addition to other risks. We may not obtain foreign regulatory registration/licensing on a timely basis, if at all. FDA clearance does not ensure new product registration/licensing by foreign regulatory authorities. Approval by one foreign regulatory authority does not ensure approval by any other foreign regulatory authority or by the FDA. If we fail to receive necessary approvals to commercialize our products in foreign jurisdictions on a timely basis, or at all, our business, financial condition and results of operations could be adversely affected.
Modifications to our marketed devices may require new regulatory clearances or premarket approvals, or may require us to cease marketing or recall the modified devices until clearances or approvals are obtained.
Any modifications to an FDA-cleared device that could significantly affect its safety or effectiveness or that would constitute a major change in its intended use would require a new 510(k) clearance or possibly a PMA approval. We may not be able to obtain such clearances or approvals in a timely fashion, or at all. Delays in obtaining future clearances would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would have an adverse effect on our business, financial condition and results of operations. We have made modifications to our devices in the past and we may make additional modifications in the future.
32
If the FDA disagrees with our conclusion and requires new clearances or approvals for the modifications, we may be required to recall and to stop marketing the modified devices, which could have an adverse effect on our business, financial conditions and results of operations. In particular, we are working to develop a replacement Pronto-7 rainbow® 4D sensor and it may require additional clearance or approvals.
Federal regulatory reforms may reduce the profit we are able to earn on the sale of our products.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. However, any changes could make it more difficult for us to maintain or attain approval to develop and commercialize our products and technologies.
If our products cause or contribute to a death or serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions, including recall of our products.
Under the FDA medical device reporting regulations, we are required to report to the FDA any incident in which a product of ours may have caused or contributed to a death or serious injury or in which a product of ours malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. In addition, all manufacturers placing medical devices in European Union markets are legally required to report to the relevant authority in whose jurisdiction any serious or potentially serious incidents occurred involving devices produced or sold by the manufacturer.
The FDA and similar foreign governmental authorities have the authority to require the recall of our commercialized products in the event of material deficiencies or defects, in for example, design, labeling or manufacture. In the case of the FDA, the authority to require a recall generally must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found or we become aware of a safety issue involving a marketed product. A government-mandated or voluntary recall by us or by one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. We may initiate certain voluntary recalls involving our products in the future. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations.
From our inception through January 1, 2011, we initiated five voluntary recalls of our products, none of which was material to our operating results. Each of these recalls was reported to the FDA within the appropriate regulatory timeframes. Because of our dependence upon patient and physician perceptions, any negative publicity associated with these or any future voluntary recalls could materially and adversely affect our business, financial condition, results of operations and growth prospects.
Off-label promotion of our products or promotional claims deemed false or misleading could subject us to substantial penalties.
Obtaining 510(k) clearance only permits us to promote our products for the uses specifically cleared by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may use our products off-label because the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. Although we may request additional cleared indications for our current products, the FDA may deny those requests, require additional expensive clinical data to support any additional indications or impose limitations on the intended use of any cleared product as a condition of clearance. We must have adequate substantiation for our product performance claims. If the FDA determines that we or our OEM partners have promoted our products for off-label use or have made false or misleading or inadequately substantiated promotional claims, it could request that we or our OEM partners modify those promotional materials or take regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an uncleared or unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, we would be subject to extensive fines and penalties and our reputation could be damaged and adoption of our products would be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
33
We may be subject to or otherwise affected by federal and state health care laws, including fraud and abuse and health information privacy and security laws, and could face substantial penalties if we are unable to fully comply with these laws.
Although we do not provide health care services or receive payments directly from Medicare, Medicaid or other third-party payers for our products or the procedures in which our products are used, health care regulation by federal and state governments will impact our business. Health care fraud and abuse laws potentially applicable to our operations include, but are not limited to:
| | • | | the Federal Health Care Programs’ Anti-Kickback Law, which prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving any bribe, kickback or other remuneration intended to induce the purchase, order or recommendation of an item or service reimbursable under a federal health care program (such as the Medicare or Medicaid programs); |
| | • | | federal false claims laws which prohibit, among other things, knowingly and willfully presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; |
| | • | | the federal provisions of the HIPAA established federal crimes for knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services; |
| | • | | state laws analogous to each of the above federal laws, such as state anti-kickback and false claims laws that may apply to items or services reimbursed by non-governmental third-party payers, including commercial insurers, and state laws governing the privacy of certain health information. |
We have certain arrangements with hospitals that may be affected by these laws. For instance, under our standard customer arrangements, we provide hospitals with free pulse oximetry monitoring devices in exchange for their agreement to purchase future pulse oximetry sensor requirements from us. In addition, we occasionally provide our customers with rebates in connection with their annual purchases. While we believe that these arrangements are structured such that we are currently in compliance with applicable federal and state health care laws, one or more of these arrangements may not meet the Federal Anti-Kickback Law’s safe harbor requirements, which may result in increased scrutiny by government authorities that are responsible for enforcing these laws.
There can be no assurance that we will not be found to be in violation of any of such laws or other similar governmental regulations to which we are directly or indirectly subject, and as a result we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion of our products from reimbursement under Medicare, Medicaid and other federal health care programs, and the curtailment or restructuring of our operations. Any penalties could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Further, we are required to comply with federal and state laws governing the transmission, security and privacy of individually identifiable health information that we may obtain or have access to in connection with manufacture and sale of our products. We may be required to make costly system modifications to comply with the HIPAA privacy and security requirements. Our failure to comply may result in criminal and civil liability because the potential for enforcement action against business associates is greater as a result of the Health Information Technology for Economic and Clinical Health Act.
Numerous other federal and state laws protect the confidentiality of patient information including state medical information privacy laws, state social security number protection laws and state and federal consumer protection laws. In some cases, more protective state privacy and security laws are not preempted by HIPAA and may be subject to interpretation by various governmental authorities and courts resulting in potentially complex compliance issues for us and our customers.
State and federal human subject protection laws apply to our receipt of individually identifiable health information in connection with clinical research. These laws could create liability for us if one of our research collaborators uses or discloses research subject information without authorization and in violation of applicable laws.
34
Legislative and regulatory changes in the health care industry could have a negative impact on our financial performance. Furthermore, our business, financial condition, results of operations and cash flows could be significantly and adversely affected by recently enacted health care reform legislation in the U.S. or if reform programs are adopted in our key markets.
Changes in the health care industry in the U.S. and elsewhere could adversely affect the demand for our products as well as the way in which we conduct our business. Significantly, President Obama signed health care reform legislation into law that will require most individuals to have health insurance, establish new regulations on health plans, and create insurance pooling mechanisms and other expanded public health care measures. This legislation also will reduce Medicare spending on services provided by hospitals and other providers and after December 31, 2012, impose a 2.3% tax on the sale of a taxable medical device by the manufacturer, producer or importer. Our operating expenses resulting from this excise tax and results of operations will be materially and adversely affected, if we are unable to pass the excise tax onto our customers.
Many details of the recently enacted health care reform legislation will be addressed in the implementing regulations. We cannot predict the effect any future legislation or regulation will have on us or what health care initiatives, if any, will be implemented at the state level.
In general, an expansion in government’s role in the U.S. health care industry may lower reimbursements for our products, reduce demand for innovative products, reduce medical procedure volumes and adversely affect our business and results of operations, possibly materially.
Furthermore, many private payors look to Medicare’s coverage and reimbursement policies in setting their coverage policies and reimbursement amounts such that federal reforms could influence the private sector as well. Finally, many states also may attempt to reform their Medicaid programs such that either coverage for certain items or services may be narrowed or reimbursement for them could be reduced. These health care reforms may adversely affect our business.
Consistent with or in addition to Congressional or state reforms, the CMS, the federal agency that administers the Medicare and Medicaid programs could change its current policies that affect coverage and reimbursement for our products. CMS determined in 2007 that certain uses of pulse oximetry monitoring are eligible for separate Medicare payment in the hospital outpatient setting when no separately payable hospital outpatient services are reported on the same date of service. Each year, however, CMS re-examines the reimbursement rates for hospital inpatient and outpatient and physician office settings and could either increase or decrease the reimbursement rate for procedures utilizing our products. We are unable to predict when legislation or regulation that affects our business may be proposed or enacted in the future or what effect any such legislation or regulation would have on our business. Any such legislation, regulation or policies that affect the coverage and reimbursement of our current or future products, or the procedures utilizing our current or future products, could cause our sales to decrease and our revenue to decline.
In addition, the requirements or restrictions imposed on us or our products may change, either as a result of administratively adopted policies or regulations or as a result of the enactment of new laws. Such changes are particular possibilities in light of the 2008 elections in the U.S. There may be heightened scrutiny by federal and state regulators and legislators of the FDA’s device approval process, the agency’s efforts to assure the safety of marketed devices, and physician payments and promotional activities by manufacturers. Any new regulations or statutory provisions could result in delays or increased costs during the period of product development, clinical trials, and regulatory review and approval, as well as increased costs to assure compliance.
Further, our success in international markets also depends upon the eligibility of reimbursement for our products through government-sponsored health care payment systems and other third-party payers. Outside of the U.S., reimbursement systems vary by country. These systems are often subject to the same pressures to curb rising health care costs and control health care expenditures as those in the U.S. In addition, as economies of emerging markets develop, these countries may implement changes in their health care delivery and payment systems. If adequate levels of reimbursement from third-party payers outside of the U.S. are not obtained, sales of our products outside of the U.S. may be adversely affected.
Risks Related to Our Business and Operations
Cercacor has conducted most of the research and development of rainbow® technology and we are largely dependent upon Cercacor to develop improvements to certain rainbow® technologies.
Cercacor has conducted the substantial majority of research and development of certain rainbow® technologies. Although we expect Cercacor to continue its research and development activities related to certain rainbow® technology and specific noninvasive monitoring measurements, including blood glucose and total hemoglobin, we have no assurance that it will do so. In the event Cercacor does not continue to develop and improve selected rainbow® technologies, our business, financial condition and results of operations could be adversely affected.
We will experience conflicts of interest with Cercacor with respect to business opportunities and other matters.
Prior to our initial public offering in August 2007, our stockholders owned 99% of the outstanding shares of capital stock of Cercacor and we believe that as of January 1, 2011, a number of stockholders of Cercacor continued to own shares of our stock. Joe Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Cercacor.
35
Jack Lasersohn, another member of our board of directors, also serves on the board of directors of Cercacor. Due to the interrelated nature of Cercacor with us, conflicts of interest will arise with respect to transactions involving business dealings between us and Cercacor, potential acquisitions of businesses or products, development of products and technology, the sale of products, markets and other matters in which our best interests and the best interests of our stockholders may conflict with the best interests of the stockholders of Cercacor. We cannot assure you that any conflict of interest will be resolved in our favor, or that with respect to our transactions with Cercacor we will negotiate terms that are as favorable to us as if such transactions were with another third party.
We will be required to pay Cercacor for the right to use certain improvements to Masimo SET that we develop.
Under the Cross-Licensing Agreement, if we develop improvements to Masimo SET for the noninvasive measurement of non-vital signs measurements, we would be required to assign these developments to Cercacor and then license the technology back from Cercacor in consideration for a license fee and royalty obligations to Cercacor. Therefore, any improvement to this technology would be treated as if it had been developed exclusively by Cercacor. In addition, we will not be reimbursed by Cercacor for our expenses relating to the development of any such technology. As a result of these terms, we may not generate any revenue from the further development of Masimo SET for the measurement of non-vital signs measurements, which could adversely affect our business, financial condition and results of operations.
We are required to pay royalties to Cercacor for all products sold that contain rainbow® technology, including certain annual minimum royalty payments, and this may impact our gross margins if we discontinue consolidating Cercacor within our financial statements.
The Cross-Licensing Agreement requires us to pay Cercacor a royalty for all products that we sell which include their proprietary rainbow® technology. This includes handheld, table-top and multi-parameter products that incorporate licensed rainbow® technology. Beginning in 2009, for hospital contracts where we place equipment and enter into a sensor contract, we will pay a royalty to Cercacor on the total sensor contract revenue based on the ratio of rainbow® enabled devices to total devices. The agreement also requires that we make available to Cercacor, at its request, up to 10% of our annual board and sensor production volume at our total manufactured cost. In addition to these specific royalty and product obligations, our Cross-Licensing Agreement requires that we pay Cercacor specific annual minimum royalty payments.
Currently, we are required to consolidate Cercacor within our financial statements. Accordingly, the royalties that we owe to Cercacor are eliminated in our consolidated financial statements presented within this Annual Report on Form 10-K and our other periodic reports and the gross profit margins reported in our consolidated financial results do not include the royalty expense that we pay to Cercacor. We are also obligated to include, and have included, Cercacor’s engineering and administrative expenses in our reported engineering and administrative expenses. If our financial statements were not consolidated with Cercacor, our reported cost of goods sold would increase and our reported engineering and administrative expenses would decrease. To date, the amount of royalty expense has approximated the amount of engineering and administrative expense. In the future, depending upon the success of rainbow® products and the royalties earned by Cercacor on those revenues, it is possible that the royalty expense will grow at a rate higher than the growth of engineering and administrative expenses. Should this occur, and if we were not required to consolidate Cercacor’s financial results within our financial statements, then our unconsolidated cost of sales could grow at a faster rate than our unconsolidated engineering expenses.
Despite describing and reflecting this Cercacor consolidation requirement within our financial statements, failure to understand or appreciate the significance of our consolidation of Cercacor’s financial statements may lead current and prospective investors to draw inaccurate perspectives and conclusions regarding our historical and future financial condition and results of operations.
In the event that the Cross-Licensing Agreement is terminated for any reason, or Cercacor grants a license to rainbow® technology to a third party, our business would be materially and adversely affected.
Cercacor owns all of the proprietary rights to rainbow® technology developed with our proprietary Masimo SET for products intended to be used in the Cercacor Market, and all rights for any non-vital signs measurement for which we do not exercise an option pursuant to the Cross-Licensing Agreement. In addition, Cercacor has the right to terminate the Cross-Licensing Agreement or grant licenses covering rainbow® technology to third parties if we breach certain terms of the agreement, including any failure to meet our minimum royalty payment obligations or failure to use commercially reasonable efforts to develop or market products incorporating licensed rainbow® technology. If we lose our exclusive license to rainbow® technology, we would lose the ability to prevent others from making, using, selling or importing products using rainbow® technology in our market. As a result, we would likely be subject to increased competition within our market, and Cercacor or competitors who obtain a license to rainbow® technology from Cercacor would be able to offer related products.
36
We may not be able to commercialize our products incorporating licensed rainbow® technology cost-effectively or successfully.
It is generally more expensive for us to make products that incorporate rainbow® technology that we license than products that do not include licensed rainbow® technology, due to increased production costs and the royalties that we must pay to Cercacor for the licenses. In order to successfully commercialize products incorporating licensed rainbow® technology, we must be able to pass these higher costs on to the market. We cannot assure you that we will be able to sell products incorporating licensed rainbow® technology at a price the market is willing to accept. If we cannot commercialize our products incorporating licensed rainbow® technology successfully, we may not be able to generate sufficient product revenue from these products to be profitable, which could adversely affect our business, financial condition and results of operations.
Rights provided to Cercacor in the Cross-Licensing Agreement may impede a change in control of our company.
In the event we undergo a change in control, we are required to immediately pay a $2.5 million fee to exercise an option to license technology developed by Cercacor for use in blood glucose monitoring. Under the Cross-Licensing Agreement, a change in control includes, but is not limited to, the resignation or termination of Joe Kiani from his position of Chief Executive Officer of either Masimo or Cercacor. Additionally, our per product royalties payable to Cercacor will become subject to specified minimums, and the minimum aggregate annual royalties for all licensed rainbow® measurements payable to Cercacor is $15.0 million for carbon monoxide, methemoglobin, fractional arterial oxygen saturation, total hemoglobin and blood glucose, plus up to $2.0 million per other rainbow® measurements. Also, if the surviving or acquiring entity ceases to use “Masimo” as a company name and trademark following a change in control, all rights to the “Masimo” trademark will automatically be assigned to Cercacor. This could delay or discourage transactions involving an actual or potential change in control of us, including transactions in which our stockholders might otherwise receive a premium for their shares over our then-current trading price. In addition, our requirement to assign all future improvements for non-vital signs to Cercacor could impede a change in control of our company.
We may experience significant fluctuations in our quarterly results in the future and we may not maintain our recent profitability and changes to existing accounting pronouncements or taxation rules may affect how we conduct our business and affect our reported results of operations.
Our operating results have fluctuated in the past and are likely to fluctuate in the future. We may experience fluctuations in our quarterly results of operations as a result of:
| | • | | delays or interruptions in manufacturing and shipping of our products; |
| | • | | varying demand for and market acceptance of our technologies and products; |
| | • | | the effect of competing technological and market developments resulting in lower selling prices or significant promotional costs; |
| | • | | changes in the timing of product orders and the volume of sales to our OEM partners; |
| | • | | delays in hospital conversions to our products and declines in hospital patient census; |
| | • | | our legal expenses, particularly those related to litigation matters; |
| | • | | changes in our product or customer mix; |
| | • | | unanticipated delays or problems in the introduction of new products, including delays in obtaining clearance or approval from the FDA; |
| | • | | high levels of returns and repairs; and |
| | • | | change in reimbursement rates for SpHb, SpCO and SpMet parameters. |
If our operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly. Our expense levels are based, in part, on our expectations regarding future revenue levels and are relatively fixed in the short term. As a result, if our revenue for a particular period was below our expectations, we would not be able to proportionately reduce our operating expenses for that period. Any revenue shortfall would have a disproportionately negative effect on our operating results for the period. Due to these and other factors, you should not rely on our results for any one quarter as an indication of our future performance.
37
In addition, a change in accounting pronouncements or taxation rules or practices, or the interpretation of them by the SEC or other regulatory bodies, can have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New accounting pronouncements or taxation rules and varying interpretations of accounting pronouncements or taxation practice have occurred and may occur in the future. Changes to existing rules or the adoption of new rules may adversely affect our reported financial results or the way we conduct our business.
If we lose the services of our key personnel, or if we are unable to attract and retain other key personnel, we may not be able to manage our operations or meet our growth objectives.
We are highly dependent on our senior management, especially Joe Kiani, our Chief Executive Officer, and other key officers. We are also heavily dependent on our engineers and field sales team, including sales representatives and clinical specialists. Our success will depend on our ability to retain our current management, engineers and field sales team, and to attract and retain qualified personnel in the future, including scientists, clinicians, engineers and other highly skilled personnel. Competition for senior management, engineers and field sales personnel is intense and we may not be able to retain our personnel. In addition, some of our key personnel hold stock options with an exercise price that is greater than our recent closing prices, which may minimize the retention value of these options. The loss of the services of members of our key personnel could prevent the implementation and completion of our objectives, including the development and introduction of our products. In general, our officers may terminate their employment at any time without notice for any reason. We carry key person life insurance on only Mr. Kiani, who is also the Chief Executive Officer of Cercacor. Mr. Kiani devotes most of his time to us.
Existing or future acquisitions of businesses could negatively affect our business, financial condition and results of operations if we fail to integrate the acquired businesses successfully into our existing operations or if we discover previously undisclosed liabilities.
We have acquired four businesses since our inception and we may acquire additional businesses in the future. Successful acquisitions depend upon our ability to identify, negotiate, complete and integrate suitable acquisitions and to obtain any necessary financing. Even if we complete acquisitions, we may experience:
| | • | | difficulties in integrating any acquired companies, personnel, products and other assets into our existing business; |
| | • | | delays in realizing the benefits of the acquired company, products or other assets; |
| | • | | diversion of our management’s time and attention from other business concerns; |
| | • | | limited or no direct prior experience in new markets or countries we may enter; |
| | • | | higher costs of integration than we anticipated; and |
| | • | | difficulties in retaining key employees of the acquired business who are necessary to manage these acquisitions. |
In addition, an acquisition could materially impair our operating results by causing us to incur debt or requiring us to amortize acquisition expenses and acquired assets. We may also discover deficiencies in internal controls, data adequacy and integrity, product quality, regulatory compliance and product liabilities that we did not uncover prior to our acquisition of such businesses, which could result in us becoming subject to penalties or other liabilities. Any difficulties in the integration of acquired businesses or unexpected penalties or liabilities in connection with such businesses could have a material adverse effect on our business, financial condition and results of operations.
Our international business structure may not result in expected operational benefits.
In the fourth quarter of 2008, we implemented a new international business structure designed to better serve and support our growing international business. By centralizing our international operations, including sales management, marketing, customer support, planning, logistics and administrative functions, we believe we will be able to develop a more efficient and scalable international organization – capable of being even more responsive to the business needs of our international customers—all under one centralized management structure. We commenced the implementation of an international business structure to align our operations with the business needs of our non-U.S. customers and we believe that we may, in the long run, also benefit from certain operational benefits and achieve a lower overall tax rate. However, there can be no assurance that our efforts will produce any anticipated operational benefits or provide an overall lower tax rate. Realization of the expected benefits will depend on a number of factors, including our future business results and profitability, the effectiveness and timing of our implementation of our international business structure, changes in U.S. or international tax law and the geographic composition of our pre-tax income. Legislative action may be taken by the U.S. Congress which, if ultimately enacted, could adversely affect our effective tax rate and/or require us to take further action, at potentially significant expense, to seek to preserve our effective tax rate. We cannot predict the outcome of any specific legislative proposals. However, if proposals were enacted that had a negative effect on our international business structure, we could be subjected to increased taxation and/or potentially significant expense.
38
The risks inherent in operating internationally and the risks of selling and shipping our products and of purchasing our components and products internationally may adversely impact our business, financial condition and results of operations.
We derive a portion of our net sales from international operations. In the years ended January 1, 2011 and January 2, 2010, 27.9% and 25.8%, respectively, of our product revenue was derived from our international operations. In addition, we purchase a portion of our raw materials and components on the international market. The sale and shipping of our products across international borders, as well as the purchase of materials and components from international sources, subject us to extensive U.S. and foreign governmental trade regulations. Compliance with such regulations is costly and we would be exposed to potentially significant penalties for non-compliance. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, restrictions on certain business activities, and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our shipping, manufacturing and sales activities. Any material decrease in our international sales would adversely affect our business, financial condition and results of operations.
In addition, our international sales operations expose us and our representatives, agents and distributors to risks inherent in operating in foreign jurisdictions. These risks include, but are not limited to:
| | • | | the imposition of additional U.S. and foreign governmental controls or regulations; |
| | • | | the imposition of costly and lengthy new export licensing requirements; |
| | • | | a shortage of high-quality sales people and distributors; |
| | • | | loss of any key personnel that possess proprietary knowledge, or who are otherwise important to our success in certain international markets; |
| | • | | changes in duties and tariffs, license obligations and other non-tariff barriers to trade; |
| | • | | the imposition of new trade restrictions; |
| | • | | the imposition of restrictions on the activities of foreign agents, representatives and distributors; |
| | • | | scrutiny of foreign tax authorities which could result in significant fines, penalties and additional taxes being imposed on us; |
| | • | | pricing pressure that we may experience internationally; |
| | • | | laws and business practices favoring local companies; |
| | • | | political instability and actual or anticipated military or political conflicts; |
| | • | | longer payment cycles; and |
| | • | | difficulties in enforcing or defending intellectual property rights. |
In addition, the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Because of the predominance of government-sponsored health care systems around the world, many of our customer relationships outside of the U.S. are with governmental entities and are therefore subject to such anti-bribery laws. Our policies mandate compliance with these anti-bribery laws. Despite our training and compliance programs, our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could subject us to cash and non-cash penalties, disrupt our operations, involve significant management distraction and result in a material adverse effect on our business, financial condition and results of operations.
Our operations may be adversely impacted by our exposure to risks related to foreign currency exchange rates.
We market our products in certain foreign markets through our subsidiaries and other international distributors. The related sales agreements may provide for payments in a foreign currency. A majority of our sales and expenditures are transacted in U.S. dollars. We transact with foreign customers in currencies other than the U.S. dollar. These foreign currency revenues, when converted into U.S. dollars, can vary depending on average exchange rates during a respective period. In addition, we are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables. When converted to U.S. dollars, these receivables can vary depending on the monthly exchange rates at the end of the period. Certain of our foreign sales support subsidiaries transact in their respective country’s local currency, which is also their functional currency. As a result, expenses of these foreign subsidiaries when converted into U.S. dollars can vary depending on average monthly exchange rates during a respective period. Certain intercompany transactions may give rise to realized and unrealized foreign currency gains or losses. Accordingly, our operating results are subject to fluctuations in foreign currency exchange rates.
39
The balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar are translated into U.S. dollars at the rate of exchange at the balance sheet date and the statements of income and cash flows are translated into U.S. dollars using the average monthly exchange rate during the period. Any foreign exchange gain or loss as a result of translating the balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar is included in equity as a component of accumulated other comprehensive income.
We currently do not hedge against our foreign currency exchange rate risks and therefore believe our exposure to these risks may be higher than if we entered into hedging transactions, including forward exchange contracts or similar instruments. If we decide in the future to enter into forward foreign exchange contracts to attempt to reduce the risk related to foreign currency exchange rates, these contracts may not mitigate the potential adverse impact on our financial results due to the variability of timing and amount of payments under these contracts. In addition, these types of contracts may themselves cause financial harm to us and have inherent levels of counterparty risk over which we would have no control.
We currently manufacture our products at two locations and any disruption in or expansion of our manufacturing operations could adversely affect our business, financial condition and results of operations.
To date, we have relied on our manufacturing facilities in Irvine, California and Mexicali, Mexico. These facilities and the manufacturing equipment we use to produce our products would be difficult to replace and could require substantial time to repair. Our facilities may be affected by natural or man-made disasters. Earthquakes are of particular significance since both our Irvine, California and Mexicali, Mexico facilities are located in an earthquake-prone area. We are also vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fire, floods and similar events. In the event that one of our facilities was affected by a natural or man-made disaster, we would be forced to rely on third-party manufacturers if we could not shift production to our other manufacturing facility. Our insurance for damage to our property and the disruption of our business from casualties may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. If we are forced to seek alternative facilities, or if we voluntarily expand one or more of our manufacturing operations to new locations, we may incur additional transition costs and we may experience a disruption in the supply of our products until the new facilities are available and operating. We are also vulnerable to disruptions which may occur as a result of local, regional and worldwide health risks. Such disruptions may include the inability to manufacture and distribute our products due to the direct effects of illness on individuals or due to constraints on supply and distribution that may result from either voluntary or government imposed restrictions. Any disruption or delay at our manufacturing facilities and any expansion of our operations to additional locations could create operational hurdles and have an adverse impact on our ability to produce sufficient inventory of our products or may require us to incur additional expenses in order to produce sufficient inventory. In addition, any disruption, delay, transition or expansion of our manufacturing operations could impair our ability to meet the demand of our customers and our customers may cancel orders or purchase products from our competitors, which could adversely affect our business, financial condition and results of operations.
Our suppliers may not supply us with a sufficient amount of materials and components or materials and components of adequate quality.
We depend on sole or limited source suppliers for key materials and components of our noninvasive blood constituent patient monitoring solutions, and if we are unable to obtain these components on a timely basis, we will not be able to deliver our noninvasive blood constituent patient monitoring solutions to customers. Also, we cannot guarantee that any of the materials or components that we purchase, if available at all, will be of adequate quality. From time to time, there are industry-wide shortages of several electronic components that we use in our noninvasive blood constituent patient monitoring solutions. We may experience delays in production of our products if we fail to identify alternate vendors for materials and components, or any parts supply is interrupted or reduced or there is a significant increase in production costs, each of which could adversely affect our business, financial condition and results of operations.
If we fail to comply with the reporting obligations of the Securities Exchange Act of 1934 and Section 404 of the Sarbanes-Oxley Act of 2002, or if we fail to maintain adequate internal control over financial reporting, our business, results of operations and financial condition and investors’ confidence in us could be materially and adversely affected.
As a public company, we are required to comply with the periodic reporting obligations of the Securities Exchange Act of 1934, as amended, or the Exchange Act, including preparing annual reports, quarterly reports and current reports. Our failure to prepare and disclose this information in a timely manner and meet our reporting obligations in their entirety could subject us to penalties under federal securities laws and regulations of The Nasdaq Stock Market LLC expose us to lawsuits and restrict our ability to access financing on favorable terms, or at all.
40
In addition, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, we are required to evaluate and provide a management report of our systems of internal control over financial reporting and our independent registered public accounting firm is required to attest to, our internal control over financial reporting. During the course of the evaluation of our internal control over financial reporting, we may identify areas requiring improvement and may be required to design enhanced processes and controls to address issues identified through this review. This could result in significant delays and costs to us and require us to divert substantial resources, including management time from other activities. In addition, if we fail to maintain the adequacy of our internal controls over financial reporting, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with the Sarbanes-Oxley Act. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent fraud. Any failure to maintain the requirements of Section 404 on a timely basis could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business, negatively impact the trading price of our stock, and adversely affect investors’ confidence in our company and our ability to access capital markets for financing.
If product liability claims are brought against us, we could face substantial liability and costs.
The manufacture and sale of products using Masimo SET and licensed rainbow® technology expose us to product liability claims and product recalls, including, but not limited to, those that may arise from unauthorized off-label use, which is use of a device in a manner outside the measurement or measurements cleared by the FDA, or malfunction of, or design flaws or manufacturing defects in, our products or the use of our products with incompatible components or systems. Any losses that we may suffer from product liability claims, and the effect that any product liability litigation may have upon the reputation and marketability of our technology and products, together with the corresponding diversion of the attention of our key employees, may subject us to significant damages and could adversely affect our business, financial condition and results of operations. We cannot be certain that our product liability insurance will be sufficient to cover any or all damages or claims. Furthermore, we may not be able to obtain or maintain insurance in the future at satisfactory rates or in adequate amounts to protect us against any product liability claims.
We may incur environmental and personal injury liabilities related to certain hazardous materials used in our operations.
Our manufacturing processes involve the use, generation and disposal of certain hazardous materials and wastes, including silicone adhesives, solder and solder paste, sealants, epoxies and various solvents such as methyl ethyl ketone, acetone and isopropyl alcohol. As a result, we are subject to stringent federal, state and local laws relating to the protection of the environment, including those governing the use, handling and disposal of hazardous materials and wastes. We may incur significant costs to comply with environmental regulations.
Future environmental laws may significantly affect our operations because, for instance, our manufacturing processes may be required to be altered, which may increase our manufacturing costs. In our research and manufacturing activities, we use, and our employees, may be exposed to, materials that are hazardous to human health, safety or the environment. These materials and various wastes resulting from their use are stored at our facility pending ultimate use and disposal. The risk of accidental injury, including to our employees, or contamination from these materials cannot be eliminated. In the event of such an accident, we could be held liable for any resulting damages and any such liability could exceed our reserves. Although we maintain general liability insurance, we do not specifically insure against environmental liabilities. If an enforcement action were to occur, our reputation and our business and financial condition may be harmed, even if we were to prevail or settle the action on terms favorable to us.
Risks Related to Our Stock
Our stock price may be volatile, and your investment in our stock could suffer a decline in value.
There has been significant volatility in the market price and trading volume of equity securities, which is often unrelated to the financial performance of the companies issuing the securities. These broad market fluctuations may negatively affect the market price of our stock. From January 3, 2010 to January 1, 2011, our closing stock price ranged from $21.18 to $32.60. You may not be able to resell your shares at or above the price you paid for them due to fluctuations in the market price of our stock caused by changes in our operating performance or prospects and other factors.
Some specific factors, in addition to the other risk factors identified above, that may have a significant effect on our stock market price, many of which we cannot control. These include but are not limited to:
| | • | | actual or anticipated fluctuations in our operating results or future prospects; |
| | • | | our announcements or our competitors’ announcements of new products; |
41
| | • | | the public’s reaction to our press releases, our other public announcements and our filings with the SEC; |
| | • | | strategic actions by us or our competitors, such as acquisitions or restructurings; |
| | • | | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; |
| | • | | changes in accounting standards, policies, guidance, interpretations or principles; |
| | • | | changes in our growth rates or our competitors’ growth rates; |
| | • | | developments regarding our patents or proprietary rights or those of our competitors; |
| | • | | our inability to raise additional capital as needed; |
| | • | | concerns or allegations as to the safety or efficacy of our products; |
| | • | | changes in financial markets or general economic conditions; |
| | • | | sales of stock by us or members of our management team, our Board of Directors or certain institutional stockholders; and |
| | • | | changes in stock market analyst recommendations or earnings estimates regarding our stock, other comparable companies or our industry generally. |
Concentration of ownership among our existing directors, executive officers and principal stockholders may prevent new investors from influencing significant corporate decisions.
As of January 1, 2011, our current directors and executive officers and their affiliates, in the aggregate, beneficially owned 12.1% of our outstanding stock. Subject to any fiduciary duties owed to our other stockholders under Delaware law, the stockholders may be able to exercise a significant influence over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, and will have some control over our management and policies. Some of these persons or entities may have interests that are different from yours. For example, these stockholders may support proposals and actions with which you may disagree or which are not in your best interests. The concentration of ownership could delay or prevent a change in control of us or otherwise discourage a potential acquirer from attempting to obtain control of us, which in turn could reduce the price of our stock. In addition, these stockholders could use their voting influence to maintain our existing management and directors in office, delay or prevent changes in control of us, or support or reject other management and board proposals that are subject to stockholder approval, such as amendments to our employee stock plans and approvals of significant financing transactions.
You could experience substantial dilution of your investment as a result of subsequent exercises of our outstanding options or the grant of future equity awards by us.
As of January 1, 2011, an aggregate of 12,399,620 shares of our stock were reserved for future issuance under our three equity incentive plans, 6,941,182 of which were subject to options outstanding as of that date at a weighted average exercise price of $21.32 per share. To the extent outstanding options are exercised, our existing stockholders may incur dilution. We rely heavily on equity awards to motivate current employees and to attract new employees. The grant of future equity awards by us to our employees and other service providers may further dilute our stockholders.
Future resales of our stock, including those by our insiders and a few investment funds, may cause our stock price to decline.
A significant portion of our outstanding shares are held by directors, executive officers and a few investment funds. Resale by these stockholders of a substantial number of shares, announcements of the proposed resale of substantial amounts of our stock or the perception that substantial resales may be made, could significantly reduce the market price of our stock. Some of our directors and executive officers have entered into Rule 10b5-1 trading plans pursuant to which they have arranged to sell shares of our stock from time to time in the future. Generally, these sales require public filings. Actual or potential sales by these insiders, including those under a pre-arranged Rule 10b5-1 trading plan, could be interpreted by the market as an indication that the insider has lost confidence in our stock and reduce the market price of our stock.
We have registered and expect to continue to register shares reserved under our equity plans under a Registration Statement on Form S-8. All shares issued pursuant to a Registration Statement on Form S-8 can be freely sold in the public market upon issuance, subject to restrictions on our affiliates under Rule 144. If a large number of these shares are sold in the public market, the sales could reduce the trading price of our stock.
42
Our corporate documents and Delaware law contain provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our stock.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage, delay or prevent a merger or acquisition involving us that our stockholders may consider favorable. For example, our amended and restated certificate of incorporation authorizes our board of directors to issue up to five million shares of “blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire us. In addition, our amended and restated certificate of incorporation provides for a staggered board of directors, whereby directors serve for three year terms, with one third of the directors coming up for reelection each year. A staggered board will make it more difficult for a third party to obtain control of our board of directors through a proxy contest, which may be a necessary step in an acquisition of us that is not favored by our board of directors.
We are also subject to the anti-takeover provisions of the Delaware General Corporation Law. Under these provisions, if anyone becomes an “interested stockholder,” we may not enter into a “business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of us. An “interested stockholder” means, generally, someone owning 15% or more of our outstanding voting stock or an affiliate of ours that owned 15% or more of our outstanding voting stock during the past three years, subject to certain exceptions as described in the Delaware General Corporation Law.
In addition, we have adopted a stockholder rights plan. Under the stockholder rights plan if any person becomes the beneficial owner of 15% or more of the outstanding shares of stock, subject to a number of exceptions set forth in the plan, all of our stockholders other than the acquiring person will receive a right to purchase shares of our stock at a price of $136.00 per share. Our stockholder rights plan could discourage a takeover attempt and make an unsolicited takeover of our company more difficult. As a result, without the approval of our board of directors, you may not have the opportunity to sell your shares to a potential acquirer of us at a premium over prevailing market prices. This could reduce the market price of our stock.
We may elect to not declare cash dividends on our stock, or may elect to only pay dividends on an infrequent or irregular basis, and any return on your investment may be limited to the value of our stock.
Our board of directors may from time to time declare, and we may pay, dividends on our outstanding shares in the manner and upon the terms and conditions provided by law. However, we may elect to retain all future earnings for the operation and expansion of our business, rather than paying cash dividends on our stock. Any payment of cash dividends on our stock will be at the discretion of our board of directors and will depend upon our results of operations, earnings, capital requirements, financial condition, business prospects, contractual restrictions and other factors deemed relevant by our board of directors. In the event our board of directors declares any dividends, there is no assurance with respect to the amount, timing or frequency of any such dividends.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
We lease 162,700 square feet of space in Irvine, California, for our corporate headquarters, product manufacturing, research and development, warehousing and distribution operations. The leases covering most of this space expire in September 2014.
We also lease 95,600 square feet of space in Mexicali, Mexico, for the manufacture of our products under a shelter labor agreement with Industrial Vallera de Mexicali, S.A. de C.V., or IVEMSA. IVEMSA is a Mexican maquiladora, which is a shelter services provider incorporated in Mexico that is licensed to operate factories and plants in Mexico. The shelter program allows foreign companies to manufacture products in Mexico without being required to organize and operate their own subsidiary, for example, as a Mexican corporation. As a result, the risks of labor liability, ownership of facilities and legal presence of foreign corporations in Mexico are avoided. We entered into the agreement with IVEMSA to establish and run a facility to manufacture our products. IVEMSA leases the space directly from the owner of the property under an agreement that expires in August 2014.
For our international headquarters in Neuchatel, Switzerland, we lease 7,000 square feet of office space. This office space is focused on operations including sales, marketing, customer service and other administrative functions. In addition, we currently lease 10,000 square feet of space in Montreal, Canada, which we use primarily for research, development, sales and marketing activities. We also lease 7,400 square feet of space in Tokyo, Japan, which we use for sales, marketing, customer
43
service and administrative functions, as well as maintaining product inventory. We also maintain small sales offices in Europe, Asia, Australia and the United Kingdom. We believe that our existing facilities are adequate to meet our needs and that existing needs and future growth can be accommodated by leasing alternative or additional space.
On February 3, 2009, we filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH related to Philips FAST pulse oximetry technology and certain Philips patient monitors. The suit was brought in the U.S. District Court for the District of Delaware. Two patents originally asserted in this suit, related to our measure-through motion technology, were successfully enforced in our previous suit against Covidien. On June 15, 2009, Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH answered our complaint and Philips Electronics North America Corporation filed antitrust and patent infringement counterclaims against us as well as counterclaims seeking declaratory judgments of invalidity on the patents asserted by us against Philips. On July 9, 2009, we filed our answer denying Philips’ counterclaims and asserting various defenses. We also asserted counterclaims against Philips for fraud, intentional interference with prospective economic advantage and for declaratory judgments of noninfringement and invalidity with respect to the patents asserted by Philips against us. Philips later added a claim for infringement of one additional patent. Subsequently, the Court bifurcated Philips’ antitrust claims and its patent misuse defense, as well as stayed the discovery phase on those claims pending trial in the patent case. On October 4, 2010, the Court limited the number of patents to be construed to four for us and three for Philips. Further, on October 6, 2010, the Court denied Philips’ motion to bifurcate and stay damages in the patent case. In December 2010, the Court held a hearing regarding the construction of the patent claims and the parties are awaiting the resulting order from the Court. We believe that we have good and substantial defenses to the antitrust and patent infringement claims asserted by Philips. There is no guarantee that we will prevail in this suit or receive any damages or other relief if we do prevail.
From time to time, we are involved in legal proceedings in the normal course of business. We believe that currently we are not a party to any legal proceedings which, individually or in the aggregate, would have a material adverse effect on our consolidated financial position, results of operations or cash flows.
| ITEM 4. | (REMOVED AND RESERVED) |
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our stock is traded on the NASDAQ Global Market under the symbol “MASI”. The following table sets forth the high and low closing sales price of our stock for the periods indicated.
| | | | | | | | |
| | | Price Range | |
| | | High | | | Low | |
Fiscal 2010: | | | | | | | | |
First Quarter | | $ | 29.76 | | | $ | 25.73 | |
Second Quarter | | $ | 26.55 | | | $ | 21.18 | |
Third Quarter | | $ | 28.10 | | | $ | 21.86 | |
Fourth Quarter | | $ | 32.60 | | | $ | 27.47 | |
Fiscal 2009: | | | | | | | | |
First Quarter | | $ | 30.28 | | | $ | 22.35 | |
Second Quarter | | $ | 31.00 | | | $ | 22.70 | |
Third Quarter | | $ | 28.44 | | | $ | 22.13 | |
Fourth Quarter | | $ | 31.08 | | | $ | 26.21 | |
The above quotations reflect inter-dealer prices, without retail markup, markdown or commission and may not necessarily represent actual transactions.
As of January 28, 2011, the closing price of our stock on the NASDAQ Global Market was $27.58 per share, and the number of stockholders of record was 63. We believe that the number of beneficial owners is substantially greater than the number of record holders because a large portion of our stock is held of record through brokerage firms in “street name.”
44
Stock Performance Graph
The following stock performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act or Exchange Act, except to the extent that we specifically incorporate it by reference into such filing.
The following stock performance graph compares total stockholder returns for Masimo Corporation from the date of our initial public offering of August 8, 2007 through January 1, 2011 against the NASDAQ Market Composite Index and NASDAQ Medical Equipment Index, assuming a $100 investment made on August 8, 2007. Each of the two comparative measures of cumulative total return assumes reinvestment of dividends. The stock performance shown on the graph below is not necessarily indicative of future price performance.
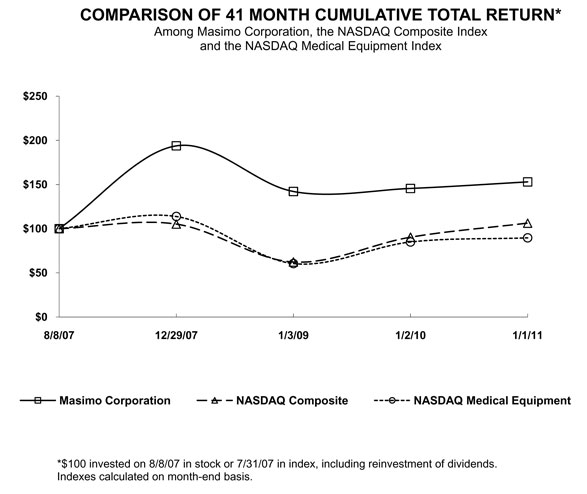
Dividend Policy
Future determination as to the payment of cash (or stock) dividends will depend upon many factors, including our financial condition and results of operations, the capital requirements of our businesses and any other relevant factors deemed relevant by our board of directors. The dividends declared in 2010 were deemed to be special dividends and there is no assurance, with respect to amount or frequency, that they will be declared again in the future.
45
| ITEM 6. | SELECTED FINANCIAL DATA |
The following tables reflect selected financial data derived from our consolidated financial statements for each of the last five years. The consolidated statement of income data for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 and the consolidated balance sheet data as of January 1, 2011 and January 2, 2010 are derived from our audited consolidated financial statements included in this Form 10-K. The consolidated statement of income data for the years ended December 29, 2007 and December 31, 2006, and the consolidated balance sheet data as of January 3, 2009, December 29, 2007 and December 31, 2006 are derived from our audited consolidated financial statements not included in this Form 10-K. Historical results are not necessarily indicative of future results. The selected financial data set forth below should be read in conjunction with our consolidated financial statements, the related notes and Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Form 10-K.
| | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | | | Year ended
December 29,
2007 | | | Year ended
December 31,
2006 | |
| | | (in thousands, except share information) | |
Statement of Income Data(1) : | | | | | | | | | | | | | | | | | | | | |
Revenue: | | | | | | | | | | | | | | | | | | | | |
Product | | $ | 356,422 | | | $ | 300,143 | | | $ | 259,592 | | | $ | 200,186 | | | $ | 155,542 | |
Royalty | | | 48,985 | | | | 48,972 | | | | 47,482 | | | | 56,100 | | | | 68,796 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 405,407 | | | | 349,115 | | | | 307,074 | | | | 256,286 | | | | 224,338 | |
Cost of goods sold | | | 119,825 | | | | 100,313 | | | | 89,454 | | | | 73,606 | | | | 61,640 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 285,582 | | | | 248,802 | | | | 217,620 | | | | 182,680 | | | | 162,698 | |
Operating (income) expenses: | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 174,089 | | | | 134,577 | | | | 120,069 | | | | 91,234 | | | | 91,384 | |
Research and development | | | 36,000 | | | | 31,701 | | | | 25,495 | | | | 22,960 | | | | 24,875 | |
Patent litigation proceeds(2) | | | — | | | | — | | | | — | | | | — | | | | (262,605 | ) |
Antitrust litigation expense (proceeds)(3) | | | (30,728 | ) | | | 298 | | | | 706 | | | | 1,537 | | | | 109 | |
| | | | | | | | | | | | | | | | | | | | |
Total operating (income) expenses | | | 179,361 | | | | 166,576 | | | | 146,270 | | | | 115,731 | | | | (146,237 | ) |
| | | | | | | | | | | | | | | | | | | | |
Operating income | | | 106,221 | | | | 82,226 | | | | 71,350 | | | | 66,949 | | | | 308,935 | |
Non-operating income (expense) | | | 1,348 | | | | (46 | ) | | | 1,041 | | | | 1,173 | | | | 5,468 | |
| | | | | | | | | | | | | | | | | | | | |
Income before provision for income taxes | | | 107,569 | | | | 82,180 | | | | 72,391 | | | | 68,122 | | | | 314,403 | |
Provision for income taxes | | | 34,164 | | | | 28,158 | | | | 40,464 | | | | 25,867 | | | | 132,577 | |
| | | | | | | | | | | | | | | | | | | | |
Net income including noncontrolling interests | | | 73,405 | | | | 54,022 | | | | 31,927 | | | | 42,255 | | | | 181,826 | |
Preferred stock dividend | | | — | | | | — | | | | — | | | | — | | | | (77,785 | ) |
Accretion of preferred stock | | | — | | | | — | | | | — | | | | (4,837 | ) | | | (7,985 | ) |
Undistributed income attributable to preferred stockholders | | | — | | | | — | | | | — | | | | (14,339 | ) | | | (34,275 | ) |
Net (income) loss attributable to noncontrolling interests | | | 125 | | | | (794 | ) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to Masimo Corporation | | $ | 73,530 | | | $ | 53,228 | | | $ | 31,927 | | | $ | 23,079 | | | $ | 61,781 | |
| | | | | | | | | | | | | | | | | | | | |
Net income per common share attributable to Masimo Corporation stockholders(4): | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 1.25 | | | $ | 0.92 | | | $ | 0.57 | | | $ | 0.71 | | | $ | 3.79 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | $ | 1.21 | | | $ | 0.88 | | | $ | 0.53 | | | $ | 0.60 | | | $ | 3.04 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average number of common shares: | | | | | | | | | | | | | | | | | | | | |
Basic – Two Class method | | | N/A | | | | N/A | | | | N/A | | | | 16,654,586 | | | | 16,319,898 | |
Diluted – Two Class method | | | N/A | | | | N/A | | | | N/A | | | | 20,732,872 | | | | 20,302,872 | |
Basic – Single Class method | | | 58,769,023 | | | | 57,602,646 | | | | 56,320,712 | | | | 54,660,216 | | | | N/A | |
Diluted – Single Class method | | | 60,609,087 | | | | 60,170,848 | | | | 60,190,335 | | | | 59,829,198 | | | | N/A | |
| (1) | Pursuant to authoritative accounting guidance, Cercacor is consolidated within our financial statements. Accordingly, all intercompany royalties, option and licensing fees, and other charges between us and Cercacor have been eliminated in the consolidation. Also, all direct engineering expenses that have been incurred by us and charged to Cercacor have not been eliminated and are included as research and development expense in our consolidated statements of income. For additional discussion of accounting for Cercacor, see Note 3 to the consolidated financial statements. |
46
| (2) | In January 2006, we entered into a settlement agreement with Nellcor (currently Covidien) under which we agreed to settle all pending patent litigation with them. In return, Nellcor agreed to pay us $263.0 million for damages incurred through January 2006. |
| (3) | During the year ended January 1, 2011, we completed negotiations to resolve the merits of our antitrust litigation with Covidien. As a result, we retained at total of $30.8 million from Covidien. |
| (4) | See Note 2 to the consolidated financial statements for a description of the method used to compute basic and diluted net income per common share. |
| | | | | | | | | | | | | | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | | | January 3,
2009 | | | December 29,
2007 | | | December 31,
2006 | |
| | | (in thousands, except dividends declared per common share) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 88,305 | | | $ | 189,043 | | | $ | 146,910 | | | $ | 96,733 | | | $ | 55,382 | |
Working capital | | | 147,408 | | | | 229,947 | | | | 166,595 | | | | 121,831 | | | | 30,125 | |
Total assets | | | 310,235 | | | | 356,345 | | | | 293,348 | | | | 235,511 | | | | 159,073 | |
Long term debt, including current portion | | | 172 | | | | 231 | | | | 622 | | | | 31,041 | | | | 21,042 | |
Total equity | | | 230,039 | | | | 289,688 | | | | 219,498 | | | | 150,105 | | | | 56,990 | |
Dividends declared per common share(5,6) | | $ | 2.75 | | | $ | — | | | $ | — | | | $ | — | | | $ | 4.09 | |
| (5) | During the year ended December 31, 2006, dividends were declared as a result of a one-time patent litigation settlement with Covidien in 2006. The dividends were declared for the same amount per share to both common and preferred stockholders, assuming the conversion of all outstanding shares of preferred stock into common stock on a 1:1 basis. |
| (6) | During the year ended January 1, 2011, our Board evaluated a variety of options to return value to shareholders, including acquisition opportunities, stock buy-back programs and dividends. After considering all available options, in 2010, the Board concluded that the best and most direct way to reward shareholders for their continued investment and confidence in Masimo was through the declaration of two cash dividends. In February 2010, the Board declared a special dividend of $2.00 per share, or $117.5 million, which was paid in March 2010. In November 2010, the Board declared a second special dividend of $0.75 per share, or $44.5 million, which was paid in December 2010. |
47
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read this discussion together with the financial statements, related notes and other financial information included in this Form 10-K. The following discussion may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under Item 1A—“Risk Factors” and elsewhere in this Form 10-K. These risks could cause our actual results to differ materially from any future performance suggested below.
Overview
We are a global medical technology company that develops, manufactures, and markets noninvasive patient monitoring products that help clinicians improve patient care. We invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of Measure-Through Motion and Low Perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. Pulse oximetry is the noninvasive measurement of the oxygen saturation level of arterial blood, or the blood that delivers oxygen to the body’s tissues, and pulse rate. Conventional pulse oximetry is subject to technological limitations that reduce its effectiveness and the quality of patient care. In particular, when using conventional pulse oximetry, arterial blood signal recognition can be distorted by motion artifact, or patient movement, and low perfusion, or low arterial blood flow. Low perfusion can also cause the failure of the conventional pulse oximeter to obtain an accurate measurement. Conventional pulse oximetry readings can also be impacted by bright light and electrical interference from the presence of electrical surgical equipment. Published independent research shows that over 70% of the alarms were false outside the operating room using conventional pulse oximetry. Our Masimo SET platform has addressed many of the previous technology limitations. The benefits of Masimo SET have been validated in over 100 independent clinical and laboratory studies.
We develop, manufacture and market a family of noninvasive blood constituent patient monitoring solutions that consists of a monitor or circuit board and our proprietary single-patient use and reusable sensors and cables. In addition, we offer remote-monitoring and clinician notification solutions. Although our Masimo SET platform is only operable with our proprietary sensors, our sensors have the capability to work with certain competitor pulse oximeters through the use of our adapter cables. In 2005, we launched our Masimo rainbow® Pulse CO-Oximetry platform utilizing licensed rainbow® technology from Cercacor Laboratories, Inc., or Cercacor (formerly Masimo Laboratories, Inc.) , which enables the noninvasive and continuous measurement of not only arterial blood oxygen saturation level and pulse rate, but also carboxyhemoglobin, or carbon monoxide levels in the blood, methemoglobin (released in 2006) saturation levels in the blood and total hemoglobin (released in 2008), or the oxygen-carrying component of red blood cells. In 2007, we launched PVI, which is a measurement that quantifies changes in the plethysmographic waveform over the respiration cycle. Along with the release of our Masimo rainbow® Pulse CO-Oximetry products, we have developed multi-wavelength sensors that have the ability to monitor multiple measurements with a single sensor. In October 2008, we received FDA clearance for Pronto, a handheld noninvasive multi-parameter testing device, not a continuous monitoring device, that uses Masimo rainbow® SET technology, to provide pulse oximetry, pulse rate, perfusion index and spot checking of total hemoglobin levels. In November 2009, we received FDA clearance for Rainbow Acoustic MonitoringTM for continuous and noninvasive monitoring of respiration rate, which is the number of breaths per minute. In December 2009, we announced initial limited market release of this measurement that allowed us to evaluate the product’s performance in the field. We began a full commercial release of Rainbow Acoustic MonitoringTM in June 2010. Also, in June 2010, we received FDA clearance for Pronto-7, a handheld noninvasive multi-parameter testing device, that provides spot-check hemoglobin testing along with oxygen saturation, pulse rate and perfusion index results.
We have focused on building our U.S. and international sales and marketing infrastructure to market our products to end-users, such as hospitals, and OEM partners for incorporation into their patient-monitoring products. We market our pulse oximetry products to hospitals and the alternate care market through our direct sales force, and market our circuit boards to our OEM partners. Today, the primary focus of our hospital sales force is to facilitate the conversion of hospitals to our Masimo SET or Masimo rainbow® SET products. In the U.S., we typically enter into long-term sales contracts with hospitals, pursuant to which we ship and install our pulse oximeters at no cost to the hospital in exchange for a commitment to purchase a minimum number of sensors from us over a specified period of time. With the introduction of Masimo rainbow® Pulse CO-Oximetry, we have established a sales force to concentrate on the alternate care market. Over the past year, we have expanded our sales and marketing staffing levels. We supplement our direct sales with sales through our distributors, who, within the U.S., function as inventory or fulfillment houses for our hospital customers. During 2010, we generated product revenue of $356.4 million, representing a compound annual growth rate, or CAGR, of 21.2% for the three years ended January 1, 2011.
48
The building of our installed base of pulse oximeters and pulse oximeter circuit boards generates recurring sales of our sensors, primarily single-patient use sensors. A user of one of our pulse oximeters or our OEMs’ pulse oximeters can obtain the benefit of the Masimo SET or Masimo rainbow® SET only by using our proprietary sensors that are designed for our system. We currently estimate that our worldwide installed base was 855,000 units, based on an estimated 10 year field life assumption, as of January 1, 2011, up from 724,000 units as of January 2, 2010. We estimate our installed base to be the number of pulse oximeters and pulse oximeter circuit boards that we have shipped in the past 10 years. We expect our worldwide installed base to continue to increase as we expand our market share and expand the pulse oximetry market to other patient care settings.
Antitrust Litigation Proceeds
During the year ended January 1, 2011, we completed negotiations to resolve the merits of our antitrust litigation with Covidien. As a result, we retained a total of $30.8 million from two payments from Covidien following the Ninth Circuit Court of Appeals’ October 2009 affirmance of a Federal District Court decision that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products. The gross payment amount from Covidien was $59.0 million, but excluding reimbursement of legal fees, costs and interest, the net amount was $43.5 million. Of this amount, we retained $30.1 million and the remainder was paid to the law firm that handled the trial for us. Subsequently, we received a second payment of $1.3 million from Covidien that related to our appeal attorneys’ fees and related expenses. Of this second amount, we retained $769,000, with the remainder paid to our attorneys.
Dividend Payments
Our Board continues to evaluate a variety of options to return value to shareholders, including acquisition opportunities, stock buy-back programs and dividends. After considering all available options, in 2010, the Board concluded that the best and most direct way to reward shareholders for their continued investment and confidence in Masimo was through the declaration of two cash dividends. In February 2010, the Board declared a special dividend of $2.00 per share, or $117.5 million, which was paid in March 2010. In November 2010, the Board declared a second special dividend of $0.75 per share, or $44.5 million, which was paid in December 2010.
The 2010 special dividends represented only a portion of our cash reserves, which the Board believed was sufficient to cover our current operational needs, and to fund continued research and development investments and current strategic initiatives.
Cercacor
Cercacor is an independent entity spun off from us to our stockholders in 1998. Joe Kiani and Jack Lasersohn, members of our board of directors, are also members of the board of directors of Cercacor. Joe Kiani, our Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Cercacor. We are a party to a cross-licensing agreement with Cercacor, which was amended and restated effective January 1, 2007, or the Cross-Licensing Agreement, that governs each party’s rights to certain intellectual property held by the two companies.
Under the Cross-Licensing Agreement, we granted Cercacor an exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET owned by us, including all improvements on this technology, for the monitoring of non-vital signs measurements and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs measurements in any product market in which a product is intended to be used by a patient or pharmacist, which we refer to as the Cercacor Market, rather than a professional medical caregiver. We also granted Cercacor a non-exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET for the measurement of vital signs in the Cercacor Market.
We exclusively license from Cercacor the right to make and distribute products in the professional medical caregiver markets, referred to as the Masimo Market, that utilize rainbow® technology for the measurement of carbon monoxide, methemoglobin, fractional arterial oxygen saturation and total hemoglobin, which includes hematocrit. To date, we have developed and commercially released devices that measure carbon monoxide, methemoglobin and total hemoglobin using licensed rainbow® technology. We also have the option to obtain the exclusive license to make and distribute products that utilize rainbow® technology for the monitoring of other non-vital signs measurements, including blood glucose, in product markets where the product is intended to be used by a professional medical caregiver.
In February 2009, in order to accelerate the product development of an improved total hemoglobin spot-check measurement device, Pronto-7, we agreed to fund additional Cercacor’s engineering expenses. Specifically, these expenses included third party engineering materials and supplies expense, as well as 50% of total Cercacor’s engineering and engineering related payroll expenses from April 2009 through June 2010, the original anticipated completion date of this product development effort. During the six months ended January 1, 2011, and until both parties agree to end these services, Cercacor has and will continue to assist us with continuing productization efforts of the new handheld noninvasive multi-parameter testing device, that provides spot-check hemoglobin testing. During the year ended January 1, 2011, the total expenses for these additional services, material and supplies totaled $2.6 million.
49
Pursuant to authoritative accounting guidance, Cercacor is consolidated within our financial statements for all periods presented. For the foreseeable future, we anticipate that we will continue to consolidate Cercacor pursuant to the current authoritative accounting guidance; however, in the event that Cercacor is no longer considered a variable interest entity, or VIE, or in the event that we are no longer the primary beneficiary of Cercacor, we may discontinue consolidating the entity.
SEDLine, Inc.
SEDLine, Inc., or SEDLine, a privately held entity that was formed in the fourth quarter of 2009, is a company that designs, manufactures, markets and sells brain function monitoring technology into the hospital marketplace. During 2009, we made loans to SEDLine totaling $3.0 million. These loans carried an interest rate of 7% and could be converted into equity upon certain predetermined conditions. Concurrently with the loans, we entered into a merger agreement with SEDLine, whereby we could acquire SEDLine at certain predetermined valuations.
Pursuant to authoritative accounting guidance, it was determined that SEDLine was a VIE and that we were the primary beneficiary during 2009 and the first six months of 2010. As a result, beginning in December 2009, we were required to consolidate SEDLine’s assets, liabilities and equity as a VIE. On July 2, 2010, upon conversion of our $3.0 million note receivable and the related accrued interest due from SEDLine into 100% of the authorized common stock of SEDLine, SEDLine became a wholly-owned subsidiary of ours. For additional discussion of SEDLine, see Note 3 to the consolidated financial statements.
Results of Operations
The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts and as a percentage of revenue.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 1, 2011 | | | Year ended
January 2, 2010 | | | Year ended
January 3, 2009 | |
| | | Amount | | | % of
Revenue | | | Amount | | | % of
Revenue | | | Amount | | | % of
Revenue | |
| | | (in thousands, except percentages) | |
Revenue: | | | | | | | | | | | | | | | | | | | | | | | | |
Product | | $ | 356,422 | | | | 87.9 | % | | $ | 300,143 | | | | 86.0 | % | | $ | 259,592 | | | | 84.5 | % |
Royalty | | | 48,985 | | | | 12.1 | | | | 48,972 | | | | 14.0 | | | | 47,482 | | | | 15.5 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 405,407 | | | | 100.0 | | | | 349,115 | | | | 100.0 | | | | 307,074 | | | | 100.0 | |
Cost of goods sold | | | 119,825 | | | | 29.6 | | | | 100,313 | | | | 28.7 | | | | 89,454 | | | | 29.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 285,582 | | | | 70.4 | | | | 248,802 | | | | 71.3 | | | | 217,620 | | | | 70.9 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 174,089 | | | | 42.9 | | | | 134,577 | | | | 38.5 | | | | 120,069 | | | | 39.1 | |
Research and development | | | 36,000 | | | | 8.9 | | | | 31,701 | | | | 9.1 | | | | 25,495 | | | | 8.3 | |
Antitrust litigation expense (proceeds) | | | (30,728 | ) | | | (7.6 | ) | | | 298 | | | | 0.1 | | | | 706 | | | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 179,361 | | | | 44.2 | | | | 166,576 | | | | 47.7 | | | | 146,270 | | | | 47.6 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 106,221 | | | | 26.2 | | | | 82,226 | | | | 23.6 | | | | 71,350 | | | | 23.3 | |
Non-operating income (expense) | | | 1,348 | | | | 0.3 | | | | (46 | ) | | | (0.1 | ) | | | 1,041 | | | | 0.4 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income before provision for income taxes | | | 107,569 | | | | 26.5 | | | | 82,180 | | | | 23.5 | | | | 72,391 | | | | 23.6 | |
Provision for income taxes | | | 34,164 | | | | 8.4 | | | | 28,158 | | | | 8.1 | | | | 40,464 | | | | 13.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income including noncontrolling interests | | | 73,405 | | | | 18.1 | | | | 54,022 | | | | 15.4 | | | | 31,927 | | | | 10.4 | |
Net (income) loss attributable to noncontrolling interests | | | 125 | | | | — | | | | (794 | ) | | | (0.2 | ) | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to Masimo Corporation | | $ | 73,530 | | | | 18.1 | % | | $ | 53,228 | | | | 15.2 | % | | $ | 31,927 | | | | 10.4 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comparison of the Year ended January 1, 2011 to the Year ended January 2, 2010
Revenue. Total revenue increased $56.3 million, or 16.1%, to $405.4 million for the year ended January 1, 2011 from $349.1 million for the year ended January 2, 2010.
Product revenue increased $56.3 million, or 18.8%, to $356.4 million in the year ended January 1, 2011 from $300.1 million in the year ended January 2, 2010. This increase was primarily due to an increase in our installed base of pulse oximeter circuit boards and pulse oximeters to 855,000 units, based on an estimated 10 year field life assumption, at January 1, 2011, from 724,000 units at January 2, 2010. Contributing to the increase in our product revenue was our rainbow® technology
50
product revenues which increased $13.4 million, or 69.0%, to $32.9 million in the year ended January 1, 2011 from $19.5 million in the year ended January 2, 2010. Product revenue generated by our direct and distribution sales channels increased $41.9 million, or 17.3%, to $283.6 million in the year ended January 1, 2011 from $241.7 million in the year ended January 2, 2010, while revenues from our OEM channel increased $14.3 million, or 24.6%, to $72.8 million in the year ended January 1, 2011 from $58.5 million in the year ended January 2, 2010. Our U.S. product revenue increased $34.3 million, or 15.4%, to $257.0 million in the year ended January 1, 2011 from $222.7 million in the year ended January 2, 2010. Additionally, our non-U.S. product revenue increased $22.1 million, or 28.4%, to $99.5 million in the year ended January 1, 2011, from $77.4 million for the year ended January 2, 2010.
Our royalty revenue was $49.0 million for the years ended January 1, 2011 and January 2, 2010. These amounts were based upon actual royalties received for the first nine months of each year, and an estimate of Covidien’s U.S. pulse oximeter sales for the last three months of each year, at the contractual royalty rate as prescribed by the 2006 settlement agreement.
Cost of Goods Sold.Cost of goods sold increased $19.5 million, or 19.5%, to $119.8 million for the year ended January 1, 2011 from $100.3 million for the year ended January 2, 2010. Our gross margin decreased to 70.4% for the year ended January 1, 2011 from 71.3% for the year ended January 2, 2010. Excluding royalties, product gross profit margins decreased by 0.2% to 66.4% for the year ended January 1, 2011 from 66.6% for the year ended January 2, 2010. This decrease was primarily due to the impact of increased sales of OEM boards and lower manufacturing absorption levels, partially offset by increased sales of rainbow® related products. We incurred $5.0 million and $4.0 million in Cercacor’s royalty expenses for the years ended January 1, 2011 and January 2, 2010, respectively, which have been eliminated in our consolidated financial results for the periods presented. Had these royalty expenses not been eliminated, our reported product gross profit margin would have been 65.0% and 65.2% for the years ended January 1, 2011 and January 2, 2010, respectively.
Selling, General and Administrative. Selling, general and administrative expenses increased $39.5 million, or 29.4%, to $174.1 million for the year ended January 1, 2011 from $134.6 million for the year ended January 2, 2010. The increase was primarily due to $14.7 million of one-time marketing related expenses related to the decision to reinvest a portion of the $30.1 million payment we received in January 2010, following the Ninth Circuit Court of Appeals’ October 2009 affirmance of a Federal District Court judgment in our favor that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products. Excluding the impact of these one-time expenses, total selling, general and administrative expenses increased $24.8 million, or 18.4%, to $159.4 million for the year ended January 1, 2011 compared to $134.6 million during the year ended January 2, 2010. This increase was primarily due to increased payroll and related expenses of $13.7 million as a result of an increase in selling, general and administrative staffing levels. In addition, travel and related expenses increased $4.5 million primarily due to additional sales representatives and their increased travel activities and legal expense for ongoing litigation activity increased $4.2 million, during the year ended January 1, 2011. Included in these total selling, general and administrative expenses are $2.7 million and $1.2 million of direct expenses incurred by our variable interest entities for the years ended January 1, 2011 and January 2, 2010, respectively.
Research and Development. Research and development expenses increased $4.3 million, or 13.6%, to $36.0 million for the year ended January 1, 2011 from $31.7 million for the year ended January 2, 2010. This increase was mainly due to increased payroll and payroll related costs of $3.7 million, associated with increased research and development staffing levels. In addition, new project costs and engineering supplies increased $1.5 million related to new product development and clinical trials. These increases were partially offset by $489,000 of grants received pursuant to a new federal government grant program. Included in these total research and development expenses are $2.0 million of engineering expenses incurred by our variable interest entities for both the years ended January 1, 2011 and January 2, 2010, respectively.
Antitrust litigation expenses (proceeds). Antitrust litigation proceeds were $30.7 million for the year ended January 1, 2011, as compared to expenses of $298,000 for the year ended January 2, 2010. The change was due to retaining a total of $30.8 million from payments from Covidien during the year ended January 1, 2011, relating to the antitrust litigation following the Ninth Circuit Court of Appeals’ October 2009 affirmance of a Federal District Court decision that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products.
Non-operating income (expense). Non-operating income was $1.3 million for the year ended January 1, 2011, as compared to non-operating expense of $46,000 for the year ended January 2, 2010. This net change of $1.4 million was primarily due to net foreign currency gains of $1.0 million during the year ended January 1, 2011, compared to net foreign currency losses of $236,000 during the year ended January 2, 2010. Net foreign currency gains during the year ended January 1, 2011 primarily include realized and unrealized gains on transactions denominated in Japanese yen, which resulted from the weakening of the U.S. dollar against the yen. These gains were partially offset by realized and unrealized losses on Euro denominated transactions, which resulted from the strengthening of the U.S. dollar against the Euro.
51
Provision for Income Taxes.Our provision for income taxes was $34.2 million for the year ended January 1, 2011, compared to $28.2 million for the year ended January 2, 2010. Our effective tax rate decreased to 31.8% in 2010 from 34.3% in 2009. This decrease in tax provision and effective tax rate was due primarily to the change in geographic composition of pre-tax income in jurisdictions in which we do business and the decrease in uncertain tax liabilities as a result of an expiring statute of limitations. Our future effective income tax rate will depend on various factors, including profits (losses) before taxes, changes to tax law, and the geographic composition of pre-tax income.
Comparison of the Year ended January 2, 2010 to the Year ended January 3, 2009
Revenue. Total revenue increased $42.0 million, or 13.7%, to $349.1 million for the year ended January 2, 2010 from $307.1 million for the year ended January 3, 2009.
Product revenue increased $40.5 million, or 15.6%, to $300.1 million in the year ended January 2, 2010, from $259.6 million for the year ended January 3, 2009. This increase was primarily due to an increase in our installed base of pulse oximeter circuit boards and pulse oximeters to 724,000 units, based on an estimated 10 year field life assumption, at January 2, 2010, from 625,000 units at January 3, 2009. Contributing to the increase in our product revenue was our rainbow® technology product revenues which increased $6.1 million, or 45.7%, to $19.5 million in the year ended January 2, 2010 from $13.4 million in the year ended January 3, 2009. Product revenue generated by our direct and distribution sales channels increased $40.6 million, or 20.2%, to $241.7 million from $201.1 million, while revenues from our OEM channel remained unchanged at $58.4 million. Our U.S. product revenue increased $28.3 million, or 14.6%, to $222.7 million in the year ended January 2, 2010, from $194.4 million for the year ended January 3, 2009. Additionally, our non-U.S. product revenue increased $12.2 million, or 18.7%, to $77.4 million in the year ended January 2, 2010, from $65.2 million for the year ended January 3, 2009.
Our royalty revenue increased $1.5 million, or 3.1%, to $49.0 million for the year ended January 2, 2010 from $47.5 million for the year ended January 3, 2009, based on actual royalties received and estimated Covidien U.S. pulse oximetry sales in the fourth quarter of 2009.
Cost of Goods Sold.Cost of goods sold increased $10.8 million, or 12.1%, to $100.3 million for the year ended January 2, 2010, from $89.5 million for the year ended January 3, 2009. Our gross margin increased to 71.3% for the year ended January 2, 2010, from 70.9% for the year ended January 3, 2009. Excluding royalties, product gross profit margins increased by 1.1% to 66.6% for the year ended January 2, 2010 from 65.5% for the year ended January 3, 2009. This increase was primarily due to a favorable product mix, including the impact of increased rainbow® product revenues, greater sensor sales and lower manufacturing costs resulting from the efficiencies derived from higher production levels. We incurred $4.0 million and $3.5 million in Cercacor’s royalty expenses for the years ended January 2, 2010 and January 3, 2009, respectively, which have been eliminated in our consolidated financial results for the periods presented. Had these royalty expenses not been eliminated, our reported product gross profit margin would have been 65.2% and 64.2% for the years ended January 2, 2010 and January 3, 2009, respectively.
Selling, General and Administrative. Selling, general and administrative expenses increased $14.5 million, or 12.1%, to $134.6 million for the year ended January 2, 2010, from $120.1 million in the year ended January 3, 2009. This increase was primarily due to increased payroll and related expenses of $12.4 million as a result of an increase in selling, general and administrative staffing. In addition, expenses related to new product samples increased $2.3 million during the year ended January 2, 2010 and travel and related expenses increased $1.7 million primarily due to additional sales representatives and their increased travel activities. These increases were partially offset by a reduction of $1.2 million in marketing related tradeshow expenses during the year ended January 2, 2010 as compared to January 3, 2009. Included in these total selling, general and administrative expenses are $1.2 million and $805,000 of activities performed by Cercacor for the years ended January 2, 2010 and January 3, 2009, respectively.
Research and Development. Research and development expenses increased $6.2 million, or 24.3%, to $31.7 million for the year ended January 2, 2010, from $25.5 million for the year ended January 3, 2009. This increase was mainly due to $2.8 million of additional engineering supplies and clinical trials research expense related to new product development, $1.0 million of increased payroll and payroll related costs associated with increased research and development staffing levels and an increase of $720,000 for additional outside service expense related to new product development. In addition, the reduction in expenses related to capitalized software development costs was $162,000 during the year ended January 2, 2010, as compared to an $844,000 reduction during the year ended January 3, 2009. Included in these total research and development expenses are $2.0 million and $2.4 million of engineering expenses incurred by Cercacor for the years ended January 2, 2010 and January 3, 2009, respectively.
52
Non-operating income (expense). Non-operating expense was $46,000 for the year ended January 2, 2010, as compared to non-operating income of $1.0 million the year ended January 3, 2009. This net change of $1.1 million was primarily due to the decline in interest income of $2.1 million resulting from the significant decline in interest rates earned on our invested cash. This decline was partially offset by lower interest expense of $678,000 resulting from the repayment of long-term debt balances in March 2008. The foreign currency losses were $236,000 during the year ended January 2, 2010, compared to currency losses of $414,000 during the year ended January 3, 2009. The foreign currency losses, primarily resulting from the weakening of the U.S. dollar as compared to the Canadian dollar, were partially offset by foreign currency gains primarily resulting from the weakening of the U.S. dollar compared to the Euro and Australian dollar.
Provision for Income Taxes.Our provision for income taxes was $28.2 million for the year ended January 2, 2010, compared to $40.5 million for the year ended January 3, 2009. Our effective tax rate decreased to 34.3% in 2009 from 55.9% in 2008. This decrease in tax provision and effective tax rate was due primarily to the implementation of a new international business structure in 2008, designed to ultimately align our operations, in a cost efficient manner, with the business needs of our non-U.S. customers, and which included a tax charge related to the prepayment of licensing commercial rights to utilize pre-existing intangibles. Our future effective income tax rate will depend on various factors, including profits (losses) before taxes, changes to tax law, and the geographic composition of pre-tax income.
Liquidity and Capital Resources
As of January 1, 2011, we had cash and cash equivalents of $88.3 million, of which $58.0 million was invested in U.S. Treasury bills, $12.2 million was in money market accounts with major financial institutions and $18.1 million was in checking accounts. These U.S. Treasury bills are classified as cash equivalents since they are highly liquid investments, with a maturity of three months or less at the date of purchase. We carry cash equivalents at cost which approximates fair value.
In 2010, 2009 and 2008, we received $48.5 million, $48.8 million and $49.9 million, respectively, in cash receipts from Covidien for royalties pursuant to our settlement agreement. Through March 14, 2011, we expect to receive a royalty payment based on a rate of 13% of Covidien’s U.S. pulse oximetry sales. On January 28, 2011, we entered into a second amendment to the settlement agreement with Covidien. As part of this amendment, which will become effective as of March 14, 2011, Covidien has agreed to pay us a royalty of 7.75% for its United States pulse oximetry revenue, as specifically defined in that second amendment, at least through March 15, 2014.
Cash Flows from Operating Activities. Cash provided by operating activities was $61.0 million in 2010. The source of cash consists primarily of net income including noncontrolling interests of $73.4 million, resulting from continued growth and profitability of our business. Additionally, non-cash share-based compensation was $12.3 million and depreciation and amortization expense was $6.6 million. In addition, income tax benefit from the exercise of stock options was $4.9 million, resulting from significant stock option exercises during 2010, and accounts payable increased $5.5 million due to continued growth of our business and recent inventory purchases in anticipation of future demand for our products. These sources of cash were partially offset by an increase in deferred cost of goods sold of $19.1 million as a result of continued shipments of equipment to customers pursuant to long-term sensor agreements, an increase in inventory of $14.1 million to meet the anticipated future demand for our products for both the sale and placement at hospitals under our long-term sensor agreements, an increase in accounts receivable of $10.9 million due to a slight increase in the timing of collections and the overall growth of our business.
Cash provided by operating activities was $47.1 million in 2009. This consists primarily of our net income including noncontrolling interests of $54.0 million, resulting from continued growth and profitability of our business. Additionally, share-based compensation expense was $10.7 million due to the continued granting of stock options, and depreciation and amortization was $6.0 million in 2009. These increases were partially offset by decreased income taxes payable of $10.2 million resulting from the 2009 payment of the prior year tax charge related to the prepaid license arising from our international business structure. Also, accounts receivable increased $9.0 million due to business growth, and inventory increased $3.9 million pursuant to our policy of having sufficient inventory to meet customer demand.
Cash Flows from Investing Activities. Cash provided by investing activities for 2010 was $45.5 million consisting of sales and maturities of short-term investments of $133.0 million, offset by purchases of short-term investments of $76.0 million, due to our investment goal focused primarily on preserving capital through investments in U.S. Treasury bills with maturities of three months or less. Additionally, we used $9.6 million of cash to purchase property and equipment to support our manufacturing operations.
Cash used in investing activities for 2009 was $64.5 million primarily due to the purchase of short-term investments of $57.0 million. These investment purchases were as a result of a change in our investment policy in 2009 to slightly longer term investments with a higher yield. In addition, property and equipment purchases were $3.6 million to support the growth of the business, and intangible assets increased $1.9 million relating primarily to capitalized legal expenses associated with our patent related activities. Cash paid for acquisitions was $2.0 million in 2009.
53
Cash Flows from Financing Activities. Cash used in financing activities for 2010 was $151.1 million, primarily due to $162.0 million of dividend payments, offset by $10.2 million of proceeds from the issuance of common stock associated with the exercise of stock options.
Cash provided by financing activities in 2009 was $2.3 million. This primarily consists of proceeds from issuance of stock of $2.6 million as a result of the exercise of stock options. These proceeds were partially offset by $450,000 of debt repayments in 2009. Unless we obtain additional third party financing, we anticipate that our current debt payments will be minimal in the future.
Future Liquidity Needs. In the future, in addition to funding our working capital requirements, we anticipate our primary use of cash to be the equipment that we provide to hospitals under our long-term sensor purchase agreements. We anticipate additional capital purchases related to expanding our worldwide international operations including manufacturing, sales, marketing and other areas of necessary infrastructure growth. Our focus on international expansion will also require both continuing and incremental investments in facilities and infrastructure in the Americas, Europe and Asia. We also anticipate possible uses of cash for the acquisition of technologies or the acquisition of technology companies. The amount and timing of our actual investing activities will vary significantly depending on numerous factors, such as the progress of our product development efforts, our timetable for international sales operations and manufacturing expansion and both domestic and international regulatory requirements. Despite these capital investment requirements, we anticipate that our existing cash and cash equivalents will be sufficient to meet our working capital requirements, capital expenditures and operations for at least the next 12 months.
Current Financing Arrangements. As of January 1, 2011, other than capital leases, we did not have any other long term borrowings. The capital lease amounts represent principal and interest due on leased office equipment.
Contractual Obligations. The following table summarizes our outstanding contractual obligations as of January 1, 2011 and the effect those obligations are expected to have on our cash liquidity and cash flow in future periods (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Payments Due By Period | |
| | | Less than
1 year | | | 1-3
years | | | 3-5
years | | | More than
5 years | | | Total | |
Operating Leases(1) | | $ | 3,223 | | | $ | 4,902 | | | $ | 1,506 | | | $ | — | | | $ | 9,631 | |
Capital Leases (including interest)(2) | | | 58 | | | | 102 | | | | 20 | | | | — | | | | 180 | |
Purchase Commitments(3) | | | 59,437 | | | | — | | | | — | | | | — | | | | 59,437 | |
| | | | | | | | | | | | | | | | | | | | |
Total Contractual Obligations | | $ | 62,718 | | | $ | 5,004 | | | $ | 1,526 | | | $ | — | | | $ | 69,248 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Facility, equipment and automobile leases. |
| (2) | Leased office equipment. |
| (3) | Certain inventory items under non cancellable purchase orders. |
Other obligation: As of January 1, 2011, the liability for uncertain tax positions including interest was $8.0 million. Due to the high degree of uncertainty regarding the timing of potential cash flows associated with these liabilities, we are unable to make a reasonably reliable estimate of the amounts and periods in which these liabilities might be made.
In addition to these contractual obligations, we have the following annual minimum royalty commitments to Cercacor, as of January 1, 2011 (in thousands):
| | | | | | | | | | | | | | | | |
| | | Payments Due By Period | |
| | | Less than
1 Year | | | 1-3
Years | | | 3-5
Years | | | More than
5 years | |
Minimum royalty commitment to Cercacor | | $ | 5,000 | | | $ | 10,000 | | | $ | 10,000 | | | | (1 | ) |
| (1) | Subsequent to 2015, the royalty arrangement requires a $5.0 million minimum annual royalty payment unless the agreement is amended, restated or terminated. |
Cercacor is consolidated within our financial statements for all periods presented. Accordingly, all intercompany royalties, option and license fees and other charges between us and Cercacor have been eliminated in the consolidation. For additional discussion of Cercacor, see Note 3 to the consolidated financial statements.
54
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts. As a result, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these relationships.
Critical Accounting Estimates
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenue and expenses for each reporting period. Management regularly evaluates its estimates and assumptions. These estimates and assumptions are based on historical experience and on various other factors that are believed to be reasonable under the circumstances, and form the basis for making management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain.
Inventory/Reserves for Excess or Obsolete Inventory
Inventories are stated at the lower of cost or market. Cost is determined using a standard cost method, which approximates FIFO (first-in, first-out). Inventory valuation allowances are recorded for materials that have become obsolete or are no longer used in current production and for inventory that has a market value less than the carrying value in inventory. We generally purchase raw materials in quantities that we anticipate will be fully used within one year. However, changes in operating strategy and customer demand, and frequent unpredictable fluctuations in market values for such materials can limit our ability to effectively utilize all of the raw materials purchased and sold through resulting finished goods to customers for a profit. We regularly monitor potential inventory excess, obsolescence and lower market values compared to standard costs and, when necessary, reduce the carrying amount of our inventory to its market value. Specific reserves are maintained to reduce the carrying value of inventory items on hand that we know may not be used in finished goods. A general inventory reserve is also maintained based on our estimate of future limitations on our ability to utilize the inventory on hand. Our inventory reserve was $4.0 million and $5.2 million at January 1, 2011 and January 2, 2010, respectively. If our estimates for potential inventory losses prove to be too low, then our future earnings will be affected when the related additional inventory losses are recorded.
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. This allowance is used to state trade receivables at a net estimated realizable value. We rely on prior experience to estimate the amount that we expect to collect on the gross receivables outstanding, which cannot be known with exact certainty as of the time of issuance of this report. We maintain a specific allowance for customer accounts that we know may not be collectible due to customer liquidity issues. We also maintain a general allowance for future collection losses that arise from customer accounts that do not indicate an inability, but may be unable, to pay. Although such losses have historically been within our expectations and the allowances we have established, we cannot guarantee that we will continue to experience the same loss rates that we have in the past, especially given the recent deterioration of the credit markets of the worldwide economy. A significant change in the liquidity or financial condition of our customers could cause unfavorable trends in our receivable collections and additional allowances may be required. Our accounts receivable balance was $49.7 million and $38.9 million, net of allowances for doubtful accounts of $1.7 million and $2.0 million at January 1, 2011 and January 2, 2010, respectively.
Share-Based Compensation
Effective January 1, 2006, we adopted an accounting standard for share-based compensation using the prospective method, which requires us to expense the estimated fair value of employee stock options and similar awards based on the fair value of the award on the date of grant. To calculate the fair value of stock options, we use the Black-Scholes option pricing model which requires the input of subjective assumptions. These assumptions include estimating the length of time employees will retain their vested stock options before exercising them, the estimated volatility of our stock price over the expected term and the number of options that will ultimately be forfeited prior to meeting their vesting requirements. Pursuant to the prospective transition method, stock options granted prior to January 1, 2006 continue to be accounted for under the prior existing guidance for stock issued to employees.
55
During the nine months ended September 27, 2008, we did not have sufficient information available that was indicative of future exercise and post-vesting behavior to estimate the expected term. As a result, we adopted the simplified method of estimating the expected term of a stock option. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term. The use of the simplified method requires our option plan to be consistent with a “plain vanilla” plan. Subsequent to September 27, 2008, we had sufficient Company specific and available external information to estimate our expected term and therefore we did not rely on the simplified method. As we obtain more historical data as a publicly traded company, we expect to rely increasingly on Company specific information for our estimate of expected term.
Additionally, during the nine months ended September 27, 2008, we did not have sufficient information available regarding the historic volatility for our shares. As a result, we estimated volatility based on a peer group of companies, which collectively provides a reasonable basis for estimating volatility. Subsequent to September 27, 2008 and after we had been publicly traded for more than one year, we were able to use Company specific as well as peer group information to estimate the volatility of our shares. As we obtain more historical data as a publicly traded company, we expect to rely increasingly on Company specific information for our estimate of volatility.
We are required to develop an estimate of the number of stock options that will be forfeited due to employee turnover. Adjustments in the estimated forfeiture rates can have a significant effect on our reported share-based compensation, as we recognize the cumulative effect of the rate adjustments for all expense amortization in the period the estimated forfeiture rates were adjusted. We estimate and adjust forfeiture rates based on a periodic review of recent forfeiture activity and expected future employee turnover. Adjustments in the estimated forfeiture rates could also cause changes in the amount of expense that we recognize in future periods.
Share-based compensation expense amounted to $12.3 million for the year ended January 1, 2011, $10.7 million for the year ended January 2, 2010 and $7.7 million for the year ended January 3, 2009. The fair market value of our stock may also increase the cost of future stock option grants. To the extent that the fair market value of our stock increases, the overall cost of granting these options will also increase. For further details regarding our share-based compensation see Note 11 of our consolidated financial statements.
Revenue Recognition and Deferred Revenue
We derive revenue primarily from four sources: (i) direct sales of pulse oximetry and related products to end user hospitals, emergency medical response organizations and other direct customers; (ii) direct sales of pulse oximetry and related products to distributors who then typically resell to end user hospitals, emergency medical response organizations and other direct customers; (iii) direct sales of integrated circuit boards and sensors to OEM customers who both incorporate our embedded software technology into their multi-parameter monitoring devices and, resell our sensors; (iv) long-term sales contracts to end user hospitals in which we may provide up front monitoring equipment at no charge in exchange for a multi-year sensor purchase commitment.
We enter into agreements to sell pulse oximetry and related products and services as well as multiple deliverable arrangements that include various combinations of products and services. While the majority of our sales transactions contain standard business terms and conditions, there are some transactions that contain non-standard business terms and conditions. As a result, contract interpretation is sometimes required to determine the appropriate accounting including: (i) whether an arrangement exists; (ii) how the arrangement consideration should be allocated among the deliverables if there are multiple deliverables; (iii) if fair value can be determined for each deliverable based on vendor specific objective evidence, or VSOE; (iv) when to recognize revenue on the deliverables; and (v) whether undelivered elements are essential to the functionality of the delivered elements. In situations where VSOE of fair value does not exist for certain undelivered elements, the contract product revenue and corresponding cost of goods sold are deferred until either the element is delivered, VSOE of fair value is established, or the obligation to deliver the element no longer exists. Changes in judgments on these assumptions and estimates could materially impact the timing of revenue recognition.
In situations where VSOE of fair value does not exist for certain undelivered elements, the entire contract product revenue and corresponding cost of goods sold are deferred until either the element is delivered, VSOE of fair value is established, or the obligation to deliver the element no longer exists. During the years ended January 1, 2011, January 2, 2010 and January 3, 2009, $0, $1.2 million and $3.6 million, respectively, of contract product revenue was deferred related to situations where VSOE of fair value did not exist for undelivered elements. Related deferred costs of $0, $451,000 and $1.3 million were deferred in the same 2010, 2009 and 2008 periods, respectively. During the years ended January 1, 2011, January 2, 2010 and January 3, 2009 previously deferred revenue of $0, $7.4 million and $3.5 million, respectively, was recognized related to such contracts. Related deferred costs of $0, $2.8 million and $1.3 million were expensed during the same 2010, 2009 and 2008 periods, respectively.
56
Accounting for Income Taxes
As part of the process of preparing our consolidated financial statements, we are required to determine our income taxes in each of the jurisdictions in which we operate. This process involves estimating our actual current tax expenses and assessing temporary differences resulting from recognition of items for income tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe that recovery is not likely, establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must reflect this increase as an expense within the tax provision in the statement of operations.
Management’s judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We continue to monitor the realizability of our deferred tax assets and adjust the valuation allowance accordingly.
At January 1, 2011, we have $21.0 million of net operating loss carryforwards from our operations in Switzerland, which will begin to expire in 2016. We also have $3.5 million, or $124,000 after tax effect, of net operating losses from various states, which will begin to expire in 2012, all of which will be recorded in equity when realized. We have state research and development credits of $1.7 million which will carryforward indefinitely. Additionally, we have $430,000 of investment tax credit on research and development expenditures from its operations in Canada which will begin to expire in 2018. We believe it is more likely than not that the deferred tax assets related to net operating loss carryforwards will not be realized. In making this determination, we consider all available positive and negative evidence, including scheduled reversals of liabilities, projected future taxable income, tax planning strategies and recent financial performances. A valuation allowance has been provided on such loss carryforwards. Our consolidated income tax provision or benefit and the net deferred tax assets include Cercacor’s income taxes provision or benefit and deferred tax assets. For income tax purposes, Cercacor is not a member of our consolidated group and files its separate federal and California income tax returns.
On January 1, 2007, we adopted an accounting standard which prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. As of January 1, 2011 and January 2, 2010, the balance of gross unrecognized tax benefits was $7.5 million and $8.1 million, respectively. The amount of unrecognized benefits which, if ultimately recognized, could favorably affect the tax rate in a future period was $6.5 million and $6.9 million as of January 1, 2011 and January 2, 2010, respectively. Both amounts are net of any federal and/or state benefits. It is reasonably possible that the amount of unrecognized tax benefits in various jurisdictions may change in the next 12 months due to the expiration of statutes of limitation or audit settlements. However, due to the uncertainty surrounding the timing of such events, an estimate of the change within the next 12 months cannot be made.
Interest and penalties related to unrecognized tax benefits are recognized in income tax expense. At January 1, 2011, we had accrued $600,000 for the payment of interest.
We conduct business in multiple jurisdictions, and as a result, one or more of our subsidiaries files income tax returns in the U.S. federal, various state, local and foreign jurisdictions. We have concluded on all U.S. federal income tax matters for years through 2007. All material state, local and foreign income tax matters have been concluded for years through 2003.
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board, or FASB issued Accounting Standards Update 2009-13, or ASU No. 09-13,Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements. ASU No. 09-13 modifies the requirements that must be met for an entity to recognize revenue from the sale of a delivered item that is part of a multiple-element arrangement when other items have not yet been delivered. This update shall be applied on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Earlier application as of the beginning of our fiscal year is permitted, provided we have not previously issued financial statements for any period within that year. We believe our prospective adoption of this update, in connection with the adoption of ASU No. 09-14,Software (Topic 985): Certain Revenue Arrangements That Include Software Elements, will eliminate the requirement to defer the entire contract product revenue and corresponding cost of goods sold on arrangements that contain undelivered tangible products for which VSOE of fair value has not been established.
In October 2009, the FASB issued Accounting Standards Update 2009-14, or ASU No. 09-14,Software (Topic 985): Certain Revenue Arrangements That Include Software Elements. ASU No. 09-14 modifies the software revenue recognition guidance to exclude from its scope tangible products that contain both software and non-software components that function together to deliver a product’s essential functionality. ASU No. 09-14 is effective for fiscal years beginning after June 15, 2010 and may
57
be applied retrospectively or prospectively for new or materially modified arrangements. Earlier application as of the beginning of our fiscal year is permitted, provided we have not previously issued financial statements for any period within that year. We believe our prospective adoption of this update, in connection with the adoption of ASU No. 09-13, will eliminate the requirement to defer the entire contract product revenue and corresponding cost of goods sold on arrangements that contain undelivered tangible products for which VSOE of fair value has not been established.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to various market risks that may arise from adverse changes in market rates and prices, such as interest rates, foreign exchange fluctuations and inflation. We do not enter into derivatives or other financial instruments for trading or speculative purposes.
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates to the increase or decrease in the amount of interest income we can earn on our investment portfolio and on the increase or decrease in the amount of interest expense we must pay with respect to our various outstanding debt instruments. Our risk associated with fluctuation in interest expense is limited to our outstanding capital lease arrangements, which have fixed interest rates. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. We ensure the safety and preservation of our invested principal funds by limiting default risk, market risk and reinvestment risk. We reduce default risk by investing in investment grade securities. A hypothetical 100 basis point drop in interest rates along the entire interest rate yield curve would not significantly affect the fair value of our interest-sensitive financial instruments at January 1, 2011. Declines in interest rates over time will, however, reduce our interest income and expense while increases in interest rates will increase our interest income and expense.
Foreign Currency Exchange Rate Risk
A majority of our assets and liabilities are maintained in the United States in U.S. dollars and a majority of our sales and expenditures are transacted in U.S. dollars. We transact with foreign customers in currencies other than the U.S. dollar. These foreign currency revenues, when converted into U.S. dollars, can vary depending on average exchange rates during a respective period. In addition, we are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables. Realized and unrealized foreign currency gains or losses on these transactions are included in our statements of income as incurred. Certain of our foreign sales support subsidiaries transact in their respective country’s local currency, which is also their functional currency. As a result, expenses of these foreign subsidiaries when converted into U.S. dollars can vary depending on average monthly exchange rates during a respective period. Certain intercompany transactions may give rise to realized and unrealized foreign currency gains or losses. These foreign currency gains or losses are included in our statements of income as incurred. In addition, any other transactions between us or our subsidiaries and a third party, denominated in a currency different from the functional currency, are a foreign currency transaction. Realized and unrealized foreign currency gains or losses on these transactions are included in our statements of income as incurred and are converted to U.S. dollars at average exchange rates for a respective period.
The balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar are translated into U.S. dollars at the rate of exchange at the balance sheet date and the statements of income and cash flows are translated into U.S. dollars using the average monthly exchange rate during the period. Any foreign exchange gain or loss as a result of translating the balance sheets of our foreign subsidiaries whose functional currency is not the U.S. dollar is included in equity as a component of accumulated other comprehensive income (loss).
Our primary foreign currency exchange rate exposures are with the Euro, the Japanese yen, the Canadian dollar, the British pound and the Australian dollar against the U.S. dollar. Foreign currency exchange rates have experienced significant movements recently and may continue in the future. We currently do not enter into forward exchange contracts to hedge exposures denominated in foreign currencies and do not use derivative financial instruments for trading or speculative purposes. The effect of a 10% change in foreign currency exchange rates could have a material effect on our future operating results or cash flows, depending on which foreign currency exchange rates change and depending on the directional change (either a strengthening or weakening against the U.S. dollar). As our foreign operations continue to grow, our exposure to foreign currency exchange rate risk may become more significant.
58
Inflation Risk
We do not believe that inflation has had a material effect on our business, financial condition or results of operations during the periods presented. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases. Our inability or failure to do so could have a material adverse effect on our business, financial condition and results of operations.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Our consolidated financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15(a)(1) and 15(a)(2), respectively.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) promulgated under the Exchange Act, as of the end of the period covered by this Annual Report on Form 10-K. We recognize that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) promulgated by the SEC under the Exchange Act. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework inInternal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of January 1, 2011.
Grant Thornton LLP, the independent registered public accounting firm that audited the financial statements included in this Form 10-K, has issued an attestation report on the effectiveness of our internal control over financial reporting as of January 1, 2011. This report, which expresses an unqualified opinion on the effectiveness of our internal control over financial reporting as of January 1, 2011, is included herein.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended January 1, 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item concerning our directors, compliance with Section 16 of the Exchange Act and our code of ethics that applies to our principal executive officer, principal financial officer and principal accounting officer is incorporated by reference from the information set forth in the sections under the headings “Election of Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Election of Directors—Information Regarding the Board of Directors and Corporate Governance” in our Definitive Proxy Statement to be filed with the SEC in connection with the Annual Meeting of Stockholders to be held in 2011, or the 2011 Proxy Statement.
59
Information regarding our executive officers is set forth in Item 1— “Business” of this Form 10-K under the caption “Executive Officers of the Registrant.”
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is incorporated by reference from the information in the 2011 Proxy Statement under the heading “Compensation of Executive Officers.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item is incorporated by reference from the information in the 2011 Proxy Statement under the headings “Equity Compensation Plan Information” and “Security Ownership of Certain Beneficial Owners and Management.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this item is incorporated by reference from the information in the 2011 Proxy Statement under the headings “Transactions with Related Persons” and “Election of Directors—Information Regarding the Board of Directors and Corporate Governance.”
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this item is incorporated by reference from the information in the 2011 Proxy Statement under the heading “Ratification of Selection of Independent Auditors—Principal Accountant Fees and Services.”
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1) Financial Statements
The Consolidated Financial Statements of Masimo Corporation and Report of Grant Thornton LLP, Independent Registered Public Accounting Firm, are included in a separate section of this Form 10-K beginning on page F-1.
(a)(2) Financial Statement Schedules
Schedules not listed above have been omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Consolidated Financial Statements or the Notes thereto.
60
(a)(3) Exhibits
| | |
Exhibit Number | | Description of Document |
| |
| 2.1(1)† | | Asset Purchase Agreement, dated December 21, 2005, between the Registrant, Masimo Canada ULC and Andromed Inc. (Exhibit 2.1) |
| |
| 2.1(a)(1) | | List briefly identifying the contents of schedules omitted from Exhibit 2.1 (Exhibit 2.1(a)) |
| |
| 3.1(1) | | Amended and Restated Certificate of Incorporation (Exhibit 3.2) |
| |
| 3.2(2) | | Certificate of Designation of Series A Junior Participating Preferred Stock (Exhibit 3.1) |
| |
| 3.3(6) | | Amended and Restated Bylaws adopted on October 9, 2008 (Exhibit 3.1) |
| |
| 4.1(1) | | Form of Common Stock Certificate (Exhibit 4.1) |
| |
| 4.2(1) | | Fifth Amended and Restated Registration Rights Agreement made and entered into as of September 14, 1999 between the Registrant and certain of its stockholders (Exhibit 4.2) |
| |
| 4.3(2) | | Rights Agreement, dated November 9, 2007, between the Registrant and Computershare Trust Company, N.A., as Rights Agent (Exhibit 4.1) |
| |
| 4.4(4)# | | Masimo Retirement Savings Plan (Exhibit 4.7) |
| |
| 10.1(1)# | | Form of Indemnity Agreement between the Registrant and its officers and directors (Exhibit 10.1) |
| |
| 10.2(1)# | | Employment Agreement, dated July 19, 2007, between Joe Kiani and the Registrant (Exhibit 10.2) |
| |
| 10.3(7)# | | Amendment No. 1 to Employment Agreement, dated December 31, 2008, between Joe Kiani and the Registrant (Exhibit 10.3) |
| |
| 10.4(1)# | | Offer Letter, dated February 15, 1996, between Yongsam Lee and the Registrant (Exhibit 10.7) |
| |
| 10.5(7)# | | Offer Letter, dated May 21, 2004, between Rick Fishel and the Registrant (Exhibit 10.13) |
| |
| 10.6(1)# | | Offer Letter, dated June 9, 2006, between Mark P. de Raad and the Registrant (Exhibit 10.9) |
| |
| 10.7(1)# | | Offer Letter, dated March 30, 2007, between Anand Sampath and the Registrant (Exhibit 10.8) |
| |
| 10.8(7)# | | Offer Letter, dated December 19, 2007, between Michael O’Reilly and the Registrant (Exhibit 10.12) |
| |
| 10.9(7)# | | Offer Letter, dated December 27, 2007, between Paul Jansen and the Registrant (Exhibit 10.11) |
| |
| 10.10(7)# | | Offer Letter, dated July 23, 2008, between Jon Coleman and the Registrant (Exhibit 10.9) |
| |
| 10.11(1)# | | Executive Annual Cash Bonus Award Plan, effective January 1, 2007 (Exhibit 10.40) |
| |
| 10.12(1)# | | Executive Multi-Year Cash Bonus Award Plan, effective January 1, 2008 (Exhibit 10.41) |
| |
| 10.13(7)# | | CEO and Executive Officer Equity Award Compensation Policy, effective January 4, 2008 (Exhibit 10.53) |
| |
| 10.14(7)# | | Amended and Restated 2007 Severance Protection Plan and Summary Plan Description, effective December 31, 2008 (Exhibit 10.54) |
| |
| 10.15(3)# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Mark P. de Raad (Exhibit 10.2) |
| |
| 10.16(3)# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Yongsam Lee (Exhibit 10.3) |
| |
| 10.17(7)# | | 2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Rick Fishel (Exhibit 10.57) |
| |
| 10.18(1)# | | Third Amended and Restated 1996 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.31) |
| |
| 10.19(1)# | | 2004 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.32) |
| |
| 10.20(1)# | | 2007 Stock Incentive Plan of the Registrant, and forms of agreements related thereto (Exhibit 10.33) |
61
| | |
Exhibit Number | | Description of Document |
| |
| 10.21(1)+ | | Purchase Agreement, dated July 26, 2001, between Jabil Circuit, Inc. and the Registrant (Exhibit 10.15) |
| |
| 10.22(1) | | ADSP-2136X Sharc ROM Agreement, dated July 19, 2004, between Analog Devices Inc. and the Registrant (Exhibit 10.36) |
| |
| 10.23(7)+ | | Manufacturing and Purchase Agreement, dated October 2, 2008, by and between Analog Devices, Inc. and the Registrant (Exhibit 10.21) |
| |
| 10.24(1) | | Manufacturing and Purchase Agreement, dated August 19, 2005, between Dowa Mining Co., Ltd. and the Registrant (Exhibit 10.10) |
| |
| 10.25(1)+ | | Supply Agreement, dated February 22, 2002, between Wintek Electro-Optics Corporation and the Registrant (Exhibit 10.24) |
| |
| 10.26(1)+ | | Shelter Labor Services Agreement, dated December 27, 2000, between Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant (Exhibit 10.11) |
| |
| 10.27(5)+ | | Lease Agreement effective as of September 1, 2007, by and among Industrias Asociadas Maquiladoras, S.A. de C.V., Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant, as guarantor (Exhibit 10.1) |
| |
| 10.28(1)+ | | Pulse Oximetry & Related Products Capital Equipment Supplier Agreement, dated December 16, 2005, between Novation, LLC (“Novation”) and the Registrant, as amended (the “Novation Agreement”) (Exhibit 10.22) |
| |
| 10.29(7)+ | | Letter Amendment to Exhibit A of the Novation Agreement, dated January 31, 2007, between Novation and the Registrant (Exhibit 10.28) |
| |
| 10.30(7)+ | | Letter Amendment to Exhibit A of the Novation Agreement, dated June 13, 2007, between Novation and the Registrant (Exhibit 10.29) |
| |
| 10.31(7)+ | | Letter Amendment to Exhibit A of the Novation Agreement, dated May 1, 2008, between Novation and the Registrant (Exhibit 10.30) |
| |
| 10.32(7) | | Extension and Amendment of the Novation Agreement, dated December 4, 2008, between Novation and the Registrant (Exhibit 10.31) |
| |
| 10.33(8)+ | | Letter Amendment to Exhibit A of the Pulse Oximetry & Related Products Capital Equipment Supplier Agreement, dated January 2, 2009, between Novation, LLC and the Registrant (Exhibit 10.30) |
| |
| 10.34* | | Extension letter to the Novation Agreement, dated October 21, 2010, between Novation and the Registrant |
| |
| 10.35(8)+ | | Group Purchasing Agreement—Capital Equipment, effective June 1, 2009, by and between Premier Purchasing Partners, L.P. and the Registrant (Exhibit 10.32) |
| |
| 10.36(9)+ | | Lease Agreement, relating the premises at 40 Parker, effective as of November 1, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.1) |
| |
| 10.37(9)+ | | Amendment No. 1 to Lease Agreement, relating the premises at 50 Parker, dated April 30, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.3) |
| |
| 10.38(9)+ | | Lease Agreement, relating the premises at 60 Parker, effective as of August 1, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.2) |
| |
| 10.39(1) | | Settlement Agreement and Release of Claims, dated January 17, 2006, between Cercacor Laboratories, Inc., Nellcor Puritan Bennett, Inc., Mallinckrodt, Inc., Tyco Healthcare Group LP, Tyco International Ltd., Tyco International (US) Inc. and the Registrant (Exhibit 10.30) |
| |
| 10.40(12) | | Second Amendment to the January 17, 2006 Settlement Agreement and Release of Claims, as amended pursuant to the January 24, 2006 Amendment to Settlement Agreement and Release of Claims, dated January 28, 2011, by and among Masimo Corporation, Masimo Laboratories, Inc., Nellcor Puritan Bennett LLC, Mallinckrodt Inc., Tyco Healthcare Group LP and Covidien Inc. (Exhibit 10.1) |
| |
| 10.41(1)+ | | Amended and Restated Cross-Licensing Agreement, effective January 1, 2007, between Cercacor Laboratories, Inc. and the Registrant (Exhibit 10.34) |
62
| | |
Exhibit Number | | Description of Document |
| |
| 10.42(1) | | Services Agreement, effective January 1, 2007, between Cercacor Laboratories, Inc. and the Registrant
(Exhibit 10.35) |
| |
| 10.43(1) | | Contribution and Assignment Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.16) |
| |
| 10.44(1) | | Sales and Distribution Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.17) |
| |
| 10.45(1) | | Occupancy Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.18) |
| |
| 10.46(1) | | Management Services Agreement, dated January 1, 2005, between Masimo Americas, Inc. and the Registrant (Exhibit 10.19) |
| |
| 10.47(7) | | Cost Sharing Agreement, effective September 29, 2008, by and between Masimo International Holdings and the Registrant (Exhibit 10.48) |
| |
| 10.48(7) | | Buy-in License Agreement, effective September 29, 2008, by and between Masimo International Holdings and the Registrant (Exhibit 10.49) |
| |
| 10.49(7) | | Assignment and Assumption of Cost Sharing Agreement and Buy-in License Agreement, effective November 21, 2008, by and between Masimo International Holdings and Masimo International Technologies SARL (Exhibit 10.50) |
| |
| 10.50(10)# | | Offer Letter, dated April 12, 2010, between the Registrant and Anthony Allan (Exhibit 10.1) |
| |
| 10.51(10)# | | Amended and Restated 2007 Severance Protection Plan Limited Participation Agreement, dated May 17, 2010, by and between the Registrant and Anthony Allan (Exhibit 10.3) |
| |
| 10.52(11) | | Confidential Settlement Agreement and Mutual Release, dated July 13, 2010, by and between the Company and Hygia Health Services, Inc. (Exhibit 10.1) |
| |
| 12.1* | | Statement Regarding the Computation of Ratio of Earnings to Fixed Charges |
| |
| 21.1* | | List of Registrant’s subsidiaries |
| |
| 23.1* | | Consent of Independent Registered Public Accounting Firm |
| |
| 31.1* | | Certification of Joe Kiani, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2* | | Certification of Mark P. de Raad, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1* | | Certification of Joe Kiani, Chief Executive Officer, and Mark P. de Raad, Chief Financial Officer, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 101.INS | | XBRL Instance Document |
| |
| 101.SCH | | XBRL Taxonomy Extension Schema Document |
| |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document |
| |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document |
| |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document |
| |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document |
Attached as Exhibit 101 to this report are documents formatted in XBRL (Extensible Business Reporting Language). Users of this data are advised that, pursuant to Rule 406T of Regulation S-T, the interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is otherwise not subject to liability under these sections.
63
| (1) | Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (No. 333-142171), originally filed on April 17, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form S-1, as amended. |
| (2) | Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K, filed on November 9, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (3) | Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K, filed on January 17, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (4) | Incorporated by reference to the exhibit to the Company’s Registration Statement on Form S-8, filed on February 11, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form S-8. |
| (5) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K, filed on June 5, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (6) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K, filed on October 10, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K. |
| (7) | Incorporated by reference to the exhibit to the Company’s Annual Report on Form 10-K, filed on March 4, 2009. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-K. |
| (8) | Incorporated by reference to the exhibit to the Company’s Quarterly Report on Form 10-Q, filed on May 6, 2009. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-Q. |
| (9) | Incorporated by reference to the exhibit to the Company’s Quarterly Report on Form 10-Q, filed on November 4, 2009. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-Q. |
| (10) | Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed on May 18, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| (11) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K filed on July 22, 2010. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| (12) | Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K filed on January 31, 2011. The number given in parentheses indicates the corresponding exhibit number in such Form 8-K. |
| # | Indicates management contract or compensatory plan. |
| + | The SEC has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| † | Pursuant to Item 601(b)(2) of Regulation S-K, the schedules to this agreement have been omitted. A list identifying the contents of the omitted schedules is included as Exhibit 2.1(a). The Registrant agrees to furnish supplementally a copy of any omitted schedule to the SEC upon request. |
(b) Exhibits
See Item 15(a)(3) above.
(c) Financial Statement Schedules
See Item 15(a)(2) above.
64
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | |
| Date: February 15, 2011 | | | | By: | | /s/ JOE KIANI |
| | | | | | Joe Kiani Chairman of the Board & Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
SIGNATURE | | TITLE(S) | | DATE |
| | |
/S/ JOE KIANI Joe Kiani | | Chairman of the Board & Chief Executive Officer (Principal Executive Officer) | | February 15, 2011 |
| | |
/S/ MARK P.DE RAAD Mark P. de Raad | | Executive Vice President & Chief Financial Officer (Principal Financial and Accounting Officer) | | February 15, 2011 |
| | |
/S/ STEVEN BARKER, M.D., PH.D. Steven Barker, M.D., Ph.D. | | Director | | February 15, 2011 |
| | |
/S/ EDWARD L. CAHILL Edward L. Cahill | | Director | | February 15, 2011 |
| | |
/S/ ROBERT COLEMAN, PH.D. Robert Coleman, Ph.D. | | Director | | February 15, 2011 |
| | |
/S/ SANFORD FITCH Sanford Fitch | | Director | | February 15, 2011 |
| | |
/S/ JACK LASERSOHN Jack Lasersohn | | Director | | February 15, 2011 |
65
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
MASIMO CORPORATION
| | | | |
| |
Reports of Independent Registered Public Accounting Firm | | | F-2 | |
| |
Consolidated Balance Sheets as of January 1, 2011 and January 2, 2010 | | | F-4 | |
| |
Consolidated Statements of Income for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 | | | F-5 | |
| |
Consolidated Statements of Equity for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 | | | F-6 | |
| |
Consolidated Statements of Cash Flows for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 | | | F-7 | |
| |
Notes to Consolidated Financial Statements | | | F-8 | |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Masimo Corporation
We have audited the accompanying consolidated balance sheets of Masimo Corporation as of January 1, 2011 and January 2, 2010, and the related consolidated statements of income, equity and cash flows for each of the three years in the period ended January 1, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Masimo Corporation as of January 1, 2011 and January 2, 2010, and the results of its operations and its cash flows for each of the three years in the period ended January 1, 2011, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Masimo Corporation’s internal control over financial reporting as of January 1, 2011, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated February 15, 2011 expressed an unqualified opinion on the effectiveness of internal control over financial reporting.
/s/ GRANT THORNTON LLP
Irvine, California
February 15, 2011
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Masimo Corporation
We have audited Masimo Corporation’s internal control over financial reporting as of January 1, 2011, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Masimo Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on Masimo Corporation’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Masimo Corporation maintained, in all material respects, effective internal control over financial reporting as of January 1, 2011, based on criteria established inInternal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the accompanying consolidated balance sheets of Masimo Corporation as of January 1, 2011 and January 2, 2010, and the related consolidated statements of income, equity and cash flows for each of the three years in the period ended January 1, 2011 and our report dated February 15, 2011 expressed an unqualified opinion on those consolidated financial statements.
/s/ GRANT THORNTON LLP
Irvine, California
February 15, 2011
F-3
MASIMO CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except share amounts)
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
ASSETS | | | | | | | | |
Current assets | | | | | | | | |
Cash and cash equivalents | | $ | 88,305 | | | $ | 132,054 | |
Short-term investments | | | — | | | | 56,989 | |
Accounts receivable, net of allowance for doubtful accounts of $1,720 and $1,972 at January 1, 2011 and January 2, 2010, respectively | | | 49,694 | | | | 38,897 | |
Royalties receivable | | | 12,000 | | | | 11,500 | |
Inventories | | | 45,028 | | | | 31,559 | |
Prepaid expenses | | | 4,535 | | | | 3,742 | |
Prepaid income taxes | | | 3,352 | | | | 1,705 | |
Deferred tax assets | | | 12,555 | | | | 11,585 | |
Other current assets | | | 2,136 | | | | 1,357 | |
| | | | | | | | |
Total current assets | | | 217,605 | | | | 289,388 | |
Deferred cost of goods sold | | | 47,184 | | | | 28,163 | |
Property and equipment, net | | | 15,951 | | | | 11,682 | |
Intangible assets, net | | | 10,497 | | | | 9,829 | |
Goodwill | | | 448 | | | | 448 | |
Deferred tax assets | | | 12,560 | | | | 11,500 | |
Other assets | | | 5,990 | | | | 5,335 | |
| | | | | | | | |
Total assets | | $ | 310,235 | | | $ | 356,345 | |
| | | | | | | | |
| | |
LIABILITIES AND EQUITY | | | | | | | | |
Current liabilities | | | | | | | | |
Accounts payable | | $ | 22,150 | | | $ | 16,716 | |
Accrued compensation | | | 21,074 | | | | 17,793 | |
Accrued liabilities | | | 9,832 | | | | 9,754 | |
Income taxes payable | | | 722 | | | | 477 | |
Deferred revenue | | | 16,369 | | | | 14,641 | |
Current portion of capital lease obligation | | | 50 | | | | 60 | |
| | | | | | | | |
Total current liabilities | | | 70,197 | | | | 59,441 | |
Deferred revenue | | | 1,554 | | | | 270 | |
Capital lease obligation, less current portion | | | 122 | | | | 171 | |
Other liabilities | | | 8,323 | | | | 6,775 | |
| | | | | | | | |
Total liabilities | | | 80,196 | | | | 66,657 | |
| | |
Commitments and contingencies | | | | | | | | |
Equity | | | | | | | | |
Masimo Corporation stockholders’ equity: | | | | | | | | |
Preferred stock, $0.001 par value; 5,000,000 shares authorized at January 1, 2011 and January 2, 2010; 0 shares issued and outstanding at January 1, 2011 and January 2, 2010 | | | — | | | | — | |
Common stock, $0.001 par value, 100,000,000 shares authorized at January 1, 2011 and January 2, 2010; 59,462,969 and 57,876,450 shares issued and outstanding at January 1, 2011 and January 2, 2010, respectively | | | 59 | | | | 58 | |
Treasury stock, 156,240 shares at January 1, 2011 and January 2, 2010 | | | (1,209 | ) | | | (1,209 | ) |
Additional paid-in capital | | | 222,206 | | | | 195,690 | |
Accumulated other comprehensive income | | | 925 | | | | 63 | |
Retained earnings | | | 5,664 | | | | 94,112 | |
| | | | | | | | |
Total Masimo Corporation stockholders’ equity | | | 227,645 | | | | 288,714 | |
Noncontrolling interest | | | 2,394 | | | | 974 | |
| | | | | | | | |
Total equity | | | 230,039 | | | | 289,688 | |
| | | | | | | | |
Total liabilities and equity | | $ | 310,235 | | | $ | 356,345 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share information)
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Revenue: | | | | | | | | | | | | |
Product | | $ | 356,422 | | | $ | 300,143 | | | $ | 259,592 | |
Royalty | | | 48,985 | | | | 48,972 | | | | 47,482 | |
| | | | | | | | | | | | |
Total revenue | | | 405,407 | | | | 349,115 | | | | 307,074 | |
Cost of goods sold | | | 119,825 | | | | 100,313 | | | | 89,454 | |
| | | | | | | | | | | | |
Gross profit | | | 285,582 | | | | 248,802 | | | | 217,620 | |
Operating expenses: | | | | | | | | | | | | |
Selling, general and administrative | | | 174,089 | | | | 134,577 | | | | 120,069 | |
Research and development | | | 36,000 | | | | 31,701 | | | | 25,495 | |
Antitrust litigation expense (proceeds) | | | (30,728 | ) | | | 298 | | | | 706 | |
| | | | | | | | | | | | |
Total operating expenses | | | 179,361 | | | | 166,576 | | | | 146,270 | |
| | | | | | | | | | | | |
Operating income | | | 106,221 | | | | 82,226 | | | | 71,350 | |
Non-operating income (expense) | | | 1,348 | | | | (46 | ) | | | 1,041 | |
| | | | | | | | | | | | |
Income before provision for income taxes | | | 107,569 | | | | 82,180 | | | | 72,391 | |
Provision for income taxes | | | 34,164 | | | | 28,158 | | | | 40,464 | |
| | | | | | | | | | | | |
Net income including noncontrolling interests | | | 73,405 | | | | 54,022 | | | | 31,927 | |
Net (income) loss attributable to noncontrolling interests | | | 125 | | | | (794 | ) | | | — | |
| | | | | | | | | | | | |
Net income attributable to Masimo Corporation | | $ | 73,530 | | | $ | 53,228 | | | $ | 31,927 | |
| | | | | | | | | | | | |
Net income per share attributable to Masimo Corporation stockholders: | | | | | | | | | | | | |
Basic | | $ | 1.25 | | | $ | 0.92 | | | $ | 0.57 | |
| | | | | | | | | | | | |
Diluted | | $ | 1.21 | | | $ | 0.88 | | | $ | 0.53 | |
| | | | | | | | | | | | |
Weighted average shares used in per share calculations: | | | | | | | | | | | | |
Basic | | | 58,769 | | | | 57,603 | | | | 56,321 | |
| | | | | | | | | | | | |
Diluted | | | 60,609 | | | | 60,171 | | | | 60,190 | |
| | | | | | | | | | | | |
Cash dividend declared per share | | $ | 2.75 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF EQUITY
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Masimo Corporation Stockholders | | | | | | | |
| | | Common Stock | | | Treasury Stock | | | Additional
Paid-In
Capital | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Retained
Earnings | | | Noncontrolling
Interest | | | Total
Equity | |
| | Shares | | | Amount | | | Shares | | | Amount | | | | | | |
Balance at December 29, 2007 | | | 54,692,232 | | | $ | 55 | | | | 156,240 | | | $ | (1,209 | ) | | $ | 143,297 | | | $ | (1,034 | ) | | $ | 8,957 | | | $ | 39 | | | $ | 150,105 | |
Stock options exercised | | | 2,634,295 | | | | 2 | | | | — | | | | — | | | | 9,753 | | | | — | | | | — | | | | — | | | | 9,755 | |
Income tax benefit from exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | 19,090 | | | | — | | | | — | | | | — | | | | 19,090 | |
Compensation related to stock option grants to employees | | | — | | | | — | | | | — | | | | — | | | | 7,526 | | | | — | | | | — | | | | 68 | | | | 7,594 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 31,927 | | | | — | | | | 31,927 | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,027 | | | | — | | | | — | | | | 1,027 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 32,954 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 3, 2009 | | | 57,326,527 | | | | 57 | | | | 156,240 | | | | (1,209 | ) | | | 179,666 | | | | (7 | ) | | | 40,884 | | | | 107 | | | | 219,498 | |
Stock options exercised | | | 549,923 | | | | 1 | | | | — | | | | — | | | | 2,574 | | | | — | | | | — | | | | — | | | | 2,575 | |
Income tax benefit from exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | 2,973 | | | | — | | | | — | | | | — | | | | 2,973 | |
Compensation related to stock option grants to employees | | | — | | | | — | | | | — | | | | — | | | | 10,477 | | | | — | | | | — | | | | 73 | | | | 10,550 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 53,228 | | | | 794 | | | | 54,022 | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 70 | | | | — | | | | — | | | | 70 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 54,092 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 2, 2010 | | | 57,876,450 | | | | 58 | | | | 156,240 | | | | (1,209 | ) | | | 195,690 | | | | 63 | | | | 94,112 | | | | 974 | | | | 289,688 | |
Stock options exercised | | | 1,586,519 | | | | 1 | | | | — | | | | — | | | | 10,238 | | | | — | | | | — | | | | — | | | | 10,239 | |
Income tax benefit from exercise of stock options | | | — | | | | — | | | | — | | | | — | | | | 5,558 | | | | — | | | | — | | | | — | | | | 5,558 | |
Reclassification of deficit noncontrolling interest upon acquisition | | | — | | | | — | | | | — | | | | — | | | | (1,466 | ) | | | — | | | | — | | | | 1,466 | | | | — | |
Compensation related to stock option grants to employees | | | — | | | | — | | | | — | | | | — | | | | 12,186 | | | | — | | | | — | | | | 79 | | | | 12,265 | |
Dividends declared | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (161,978 | ) | | | — | | | | (161,978 | ) |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 73,530 | | | | (125 | ) | | | 73,405 | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 862 | | | | — | | | | — | | | | 862 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income, net of tax | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 74,267 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at January 1, 2011 | | | 59,462,969 | | | $ | 59 | | | | 156,240 | | | $ | (1,209 | ) | | $ | 222,206 | | | $ | 925 | | | $ | 5,664 | | | $ | 2,394 | | | $ | 230,039 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
MASIMO CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income including noncontrolling interests | | $ | 73,405 | | | $ | 54,022 | | | $ | 31,927 | |
Adjustments to reconcile net income including noncontrolling interests to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 6,584 | | | | 5,979 | | | | 5,745 | |
Share-based compensation | | | 12,303 | | | | 10,674 | | | | 7,716 | |
Loss on disposal of property and equipment | | | — | | | | 5 | | | | 91 | |
Provision for doubtful accounts | | | 108 | | | | 733 | | | | 108 | |
Provision for obsolete inventory | | | 619 | | | | 232 | | | | 1,352 | |
Provision for warranty costs | | | 2,355 | | | | 2,220 | | | | 1,646 | |
Provision for (benefit from) deferred income taxes | | | (2,231 | ) | | | (3,566 | ) | | | 447 | |
Income tax benefit from exercise of stock options granted prior to January 1, 2006 | | | 4,851 | | | | 2,758 | | | | 17,201 | |
Excess tax benefits from share-based compensation arrangements | | | (707 | ) | | | (215 | ) | | | (1,889 | ) |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Increase in accounts receivable | | | (10,905 | ) | | | (8,982 | ) | | | (6,244 | ) |
(Increase) decrease in royalties receivable | | | (500 | ) | | | (125 | ) | | | 2,491 | |
Decrease in accounts receivable from related parties | | | — | | | | — | | | | 3,053 | |
Increase in inventories | | | (14,088 | ) | | | (3,929 | ) | | | (5,588 | ) |
(Increase) decrease in deferred cost of goods sold | | | (19,080 | ) | | | 309 | | | | (2,232 | ) |
(Increase) decrease in prepaid expenses | | | (743 | ) | | | 197 | | | | (54 | ) |
(Increase) decrease in prepaid income taxes | | | (1,648 | ) | | | (833 | ) | | | 2,376 | |
(Increase) decrease in other assets | | | (1,396 | ) | | | (3,080 | ) | | | 1,096 | |
Increase in accounts payable | | | 5,474 | | | | 777 | | | | 1,847 | |
Decrease in accounts payable to related parties | | | — | | | | — | | | | (583 | ) |
Increase in accrued compensation | | | 3,219 | | | | 1,926 | | | | 3,121 | |
Increase (decrease) in accrued liabilities | | | (2,281 | ) | | | 1,935 | | | | (2,485 | ) |
Increase (decrease) in income taxes payable | | | 940 | | | | (10,169 | ) | | | 12,754 | |
Increase (decrease) in deferred revenue | | | 3,012 | | | | (2,518 | ) | | | 111 | |
Increase (decrease) in other liabilities | | | 1,729 | | | | (1,244 | ) | | | 4,104 | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 61,020 | | | | 47,106 | | | | 78,111 | |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchase of short-term investments | | | (75,986 | ) | | | (56,989 | ) | | | — | |
Proceeds from sale and maturities of short-term investments | | | 132,975 | | | | — | | | | — | |
Purchases of property and equipment | | | (9,561 | ) | | | (3,636 | ) | | | (6,852 | ) |
Increase in intangible assets | | | (1,937 | ) | | | (1,851 | ) | | | (2,523 | ) |
Cash paid for acquisitions | | | — | | | | (1,981 | ) | | | — | |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | 45,491 | | | | (64,457 | ) | | | (9,375 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Repayments on long-term debt | | | (60 | ) | | | (450 | ) | | | (30,436 | ) |
Proceeds from issuance of common stock | | | 10,239 | | | | 2,575 | | | | 9,755 | |
Excess tax benefits from share-based compensation arrangements | | | 707 | | | | 215 | | | | 1,889 | |
Dividends paid | | | (161,978 | ) | | | — | | | | (13 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | (151,092 | ) | | | 2,340 | | | | (18,805 | ) |
| | | | | | | | | | | | |
Effect of foreign currency exchange rates on cash | | | 832 | | | | 155 | | | | 246 | |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (43,749 | ) | | | (14,856 | ) | | | 50,177 | |
Cash and cash equivalents at beginning of period | | | 132,054 | | | | 146,910 | | | | 96,733 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 88,305 | | | $ | 132,054 | | | $ | 146,910 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Cash paid for: | | | | | | | | | | | | |
Interest | | $ | 23 | | | $ | 78 | | | $ | 872 | |
Income taxes | | $ | 32,895 | | | $ | 39,075 | | | $ | 4,783 | |
Noncash investing and financing activities: | | | | | | | | | | | | |
Assets acquired under capital leases | | $ | — | | | $ | 59 | | | $ | 17 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of the Company
Masimo Corporation, or the Company, is a global medical technology company that develops, manufactures, and markets noninvasive patient monitoring products that help clinicians improve patient care. The Company invented Masimo Signal Extraction Technology, or Masimo SET, which provides the capabilities of Measure-Through Motion and Low Perfusion pulse oximetry to address the primary limitations of conventional pulse oximetry. The Company has also developed Masimo rainbow® SET products which monitor multiple blood measurements, including carboxyhemoglobin, methemoglobin, Pleth Variability Index, total hemoglobin, acoustic respiration rate and Halo Index. The Company develops, manufactures and markets a family of patient monitoring solutions which incorporate a monitor or circuit board and sensors, including both proprietary single-patient use and reusable sensors and cables. The Company considers the pulse oximetry device (monitor or circuit board), its sensors and cables and software fees to be products as defined in its consolidated statements of income. The Company sells to hospitals and the alternate care market through its direct sales force and distributors, and markets its circuit boards containing the Company’s proprietary algorithm and software architecture to original equipment manufacturer, or OEM, partners.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Masimo Corporation, its wholly-owned subsidiaries and the variable interest entities, or VIEs, in which the Company is the primary beneficiary. All intercompany accounts and transactions have been eliminated in consolidation. In accordance with accounting principles generally accepted in the United States of America, or GAAP, current authoritative guidance is applied when determining whether an entity is subject to consolidation.
Fiscal Periods
The Company follows a conventional 52/53 week fiscal year. In fiscal 2008, the Company followed a 53 week fiscal calendar in which its first, second and third quarters ended on Saturday, March 29, June 28 and September 27, 2008, respectively. The Company’s 2008 fiscal year ended on Saturday, January 3, 2009. For fiscal 2008, the first three quarters were 13 week quarters and the fourth fiscal quarter was a 14 week quarter. The additional week in fiscal 2008 did not have a significant impact on the Company’s results of operations.
In fiscal 2009, the Company followed a 52 week fiscal calendar in which the Company’s first, second and third quarters ended on Saturday, April 4, July 4 and October 3, 2009, respectively, and its fiscal year ended on Saturday, January 2, 2010. Each quarter in 2009 was a 13 week quarter.
Similar to fiscal 2009, fiscal 2010 followed a 52 week fiscal calendar in which the Company’s first, second and third quarters ended on Saturday, April 3, July 3 and October 2, 2010, respectively, and its fiscal year ended on Saturday, January 1, 2011. Each quarter in 2010 was a 13 week quarter.
Fiscal 2011 will be on a 52 week fiscal calendar in which the Company’s first, second and third quarters will end on Saturday, April 2, July 2 and October 1, 2011, respectively, and its fiscal year will end on Saturday, December 31, 2011. Each quarter in 2011 will be a 13 week quarter.
Use of Estimates
The Company prepares its financial statements in conformity with GAAP, which requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant estimates include: determination of accounts receivable allowances, inventory reserves, warranty reserves, rebate reserves, valuation of the Company’s stock options, distributor channel inventory, royalty revenues, deferred revenue, uncertain income tax positions and property taxes. Actual results could differ from those estimates.
F-8
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Reclassifications
Certain amounts in the consolidated financial statements for prior periods have been reclassified to conform with current period presentation.
Fair Value Measurements
Effective December 30, 2007, the Company adopted the authoritative guidance for fair value measurements issued by the Financial Accounting Standards Board, or FASB. This guidance defines fair value, establishes a framework for measuring fair value under GAAP and enhances disclosures about fair value measurements. In February 2008, the FASB provided a one year deferral of the effective date for non-financial assets and non-financial liabilities, except those that are recognized or disclosed in the financial statements at fair value at least annually. Effective January 4, 2009, the Company adopted the authoritative guidance for non-financial assets and liabilities.
This guidance describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
| | • | | Level 1—Quoted prices in active markets foridentical assets or liabilities. |
| | • | | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices forsimilar assets or liabilities; quoted prices in markets that are not active; or other inputs that can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | • | | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Pursuant to current authoritative guidance, entities are allowed an irrevocable option to elect fair value for the initial and subsequent measurement for specified financial assets and liabilities on a contract-by-contract basis. The Company did not elect the fair value option under this guidance as to specific assets or liabilities.
The following tables represent the Company’s fair value hierarchy for its financial assets and liabilities (in thousands):
| | | | | | | | | | | | | | | | |
| | | Fair Value Measurement as of January 1, 2011 using: | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets: | | | | | | | | | | | | | | | | |
Cash equivalents: | | | | | | | | | | | | | | | | |
U.S. Treasuries | | $ | 57,995 | | | $ | — | | | $ | — | | | $ | 57,995 | |
Money Market funds | | | 12,165 | | | | — | | | | — | | | | 12,165 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 70,160 | | | $ | — | | | $ | — | | | $ | 70,160 | |
| | | | | | | | | | | | | | | | |
| |
| | | Fair Value Measurement as of January 2, 2010 using: | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets: | | | | | | | | | | | | | | | | |
Cash equivalents: | | | | | | | | | | | | | | | | |
U.S. Treasuries | | $ | 118,986 | | | $ | — | | | $ | — | | | $ | 118,986 | |
Money Market funds | | | 1,691 | | | | — | | | | — | | | | 1,691 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 120,677 | | | $ | — | | | $ | — | | | $ | 120,677 | |
| | | | | | | | | | | | | | | | |
Short-term investments: | | | | | | | | | | | | | | | | |
U.S. Treasuries | | $ | 56,989 | | | $ | — | | | $ | — | | | $ | 56,989 | |
| | | | | | | | | | | | | | | | |
Short-term investments consisted of held-to-maturity securities with a maturity between three months and one year at the date of purchase. These held-to-maturity securities were recorded at amortized cost. The unrecognized gain or loss as of January 2, 2010 was de minimis, since the amortized cost and fair value were both $57.0 million.
In March 2010, the Company received $27.0 million from the sale of specific short-term investments classified as held-to-maturity, prior to their maturity date. The proceeds were used to pay a portion of the special dividend declared in February 2010. The carrying value of these short-term investments sold was their amortized cost basis of $27.0 million and the realized loss on the sale of these short-term investments was de minimis. The remaining short-term investments with a carrying value of $30.0 million were then reclassified as available-for-sale, but were redeemed at their maturity dates, which were prior to July 3, 2010. As of January 1, 2011, the Company did not have any held-to-maturity or available-for-sale securities.
F-9
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
In January 2010, the Company adopted a recently issued accounting standard which requires additional disclosure about the amounts of and reasons for significant transfers in and out of level 1 and level 2 fair value measurements. There have been no transfers between level 1 and level 2 inputs during the year ended January 1, 2011. This standard also clarifies existing disclosure requirements related to the level of disaggregation of fair value measurements for each class of assets and liabilities and disclosures about inputs and valuation techniques used to measure fair value for both recurring and nonrecurring level 2 and level 3 measurements. Since this accounting standard only requires enhanced disclosure, the adoption of this standard did not impact the Company’s consolidated financial statements.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity from date of purchase of three months or less, or highly liquid investments and readily convertible into known amounts of cash to be cash equivalents. As of January 1, 2011, the Company’s cash balance was $18.1 million, comprised of checking accounts. Additionally, the Company had cash equivalents of $70.2 million, consisting of $58.0 million of U.S. Treasury bills and $12.2 million of money market funds. As of January 2, 2010, the Company’s cash balance was $11.4 million, comprised of checking accounts. Additionally, the Company had cash equivalents of $120.7 million, consisting of $119.0 million of U.S. Treasury bills and $1.7 million of money market funds.
Short-term Investments
Short-term investments consist of U.S. Treasury bills with a maturity between three months and one year when acquired. These amounts are stated at cost which approximates fair value. As of January 2, 2010, the Company had $57.0 million in short-term investments, which were classified as held to maturity. During the year ended January 1, 2011, there were no realized gains or losses recognized. The Company did not have any short-term investments as of January 1, 2011.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable consist of trade receivables recorded upon recognition of revenue for product revenues, reduced by reserves for estimated bad debts and returns. Trade accounts receivable are recorded at the invoiced amount and do not bear interest. Credit is extended based on evaluation of the customer’s financial condition. Collateral is not required. The allowance for doubtful accounts is determined based on historical write-off experience, current customer information and other relevant factors, including specific identification of past due accounts, based on the age of the receivable in excess of the contemplated or contractual due date. Accounts are charged off against the allowance when the Company believes they are uncollectible.
Changes in the allowance for doubtful accounts were as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Allowance for doubtful accounts, beginning of period | | $ | 1,972 | | | $ | 1,300 | | | $ | 1,370 | |
Provision for doubtful accounts | | | 108 | | | | 733 | | | | 108 | |
Write off of uncollectible accounts | | | (360 | ) | | | (61 | ) | | | (178 | ) |
| | | | | | | | | | | | |
Allowance for doubtful accounts, end of period | | $ | 1,720 | | | $ | 1,972 | | | $ | 1,300 | |
| | | | | | | | | | | | |
As of January 1, 2011 and January 2, 2010, the accounts receivable balance was $49.7 million and $38.9 million, respectively, net of allowances for doubtful accounts.
Royalties Receivable
Pursuant to the settlement agreement with Nellcor Puritan Bennett, Inc. (currently Covidien Ltd., or Covidien), royalties are paid to the Company based on a percentage of sales of Covidien U.S. based pulse oximetry products. The Company estimates the royalty receivable based on the royalty rate per the settlement agreement multiplied by its estimate of Covidien’s sales for each quarter. Any adjustments to the quarterly estimated receivable are recorded prospectively in the following quarter when the Company receives the Covidien royalty report and payment, which is generally 60 days after the end of each of Covidien’s fiscal quarters. The royalty receivable of $12.0 million as of January 1, 2011 represents the Company’s estimated amount due for the three months ended January 1, 2011.
F-10
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using a standard cost method, which approximates FIFO (first in, first out) and includes material, labor and overhead. Inventory reserves are recorded for inventory items that have become excess or obsolete or are no longer used in current production and for inventory that has a market price less than the carrying value in inventory.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets, which range from three to five years. Leasehold improvements are amortized over the lesser of the lease term or the estimated useful life of the improvements. Normal repair and maintenance costs are expensed as incurred, whereas significant improvements that materially increase values or extend useful lives are capitalized and depreciated over the remaining estimated useful lives of the related assets. Upon sale or retirement of depreciable assets, the related cost and accumulated depreciation or amortization are removed from the accounts and any gain or loss on the sale or retirement is recognized in income. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, depreciation of property and equipment, which includes amortization of equipment under capital leases, was $5.3 million, $5.1 million and $5.0 million, respectively.
Intangible Assets
Intangible assets consist primarily of patents, trademarks and software development costs. Costs related to patents and trademarks, which include legal and application fees, are capitalized and amortized over the estimated useful lives using the straight-line method. Patent and trademark amortization commences once final approval of the patent or trademark has been obtained. Patent costs are amortized over the lesser of 10 years or the patent’s remaining legal life, which assumes renewals, and trademark costs over 17 years, and their associated amortization cost is included in selling, general and administrative expense in the accompanying consolidated statements of income. For intangibles purchased in an asset acquisition or business combination, which mainly include patents and trademarks, the useful life is determined in the same manner as noted above. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, amortization of patents and trademarks was $1.0 million, $708,000 and $584,000, respectively. As of January 1, 2011 and January 2, 2010, the total costs of patents not yet amortizing was $4.1 million and $3.2 million, respectively. As of January 1, 2011 and January 2, 2010, the total costs of trademarks not yet amortizing was $269,000 and $148,000, respectively. Costs to renew intangibles are capitalized and amortized over the remaining useful life of the intangible. For the year ended January 1, 2011, total renewal costs capitalized for patents and trademarks were $392,000 and $60,000, respectively. As of January 1, 2011, the weighted average number of years until the next renewal is one year for patents and six years for trademarks.
The Company’s policy is to renew its patents and trademarks. The Company continually evaluates the amortization period and carrying basis of patents and trademarks to determine whether any events or circumstances warrant a revised estimated useful life or reduction in value. Capitalized application costs are charged to operations when it is determined that the patent or trademark will not be obtained or is abandoned.
In accordance with authoritative accounting guidance, costs related to the research and development of new software products and enhancements to existing software products are expensed as incurred until technological feasibility of the product has been established, at which time such costs are capitalized, subject to expected recoverability. For the years ended January 1, 2011 and January 2, 2010, the Company capitalized $0 and $162,000 of software development costs, respectively. The capitalized costs are amortized over the estimated life of the products, or seven years. The Company amortized $224,000, $201,000 and $116,000 for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, respectively. The Company had unamortized software development costs of $910,000 and $1.1 million at January 1, 2011 and January 2, 2010, respectively, which is included within intangible assets, net on the consolidated balance sheets.
Goodwill and Impairment
The Company’s goodwill resulted from the acquisition of Andromed, Inc. in 2005. The impairment evaluation for goodwill is conducted annually as of the balance sheet date or more frequently if events or changes in circumstances indicate that an asset might be impaired. The evaluation is performed using a two-step process. In the first step, the estimated fair value of the reporting unit is compared with its carrying amount, including goodwill. Since the Company has one reporting unit, the estimated fair value of goodwill is determined by the Company’s stock market valuation compared to its net assets, excluding goodwill. If the estimated fair value is less than the carrying amount, then a second step must be completed in order to determine the amount of the goodwill impairment. In the second step, the implied fair value of the goodwill is determined by allocating the fair value of all of the reporting unit’s assets and liabilities other than goodwill in a manner similar to a purchase price allocation. The resulting implied fair value of the goodwill that results from the allocation is then compared to the carrying amount of the goodwill and an impairment charge is recorded for the difference.
F-11
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
The Company reviews long-lived assets and identifiable intangibles for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted operating cash flow expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount exceeds the fair value of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less costs to sell.
No impairment of goodwill, intangible assets, or other long-lived assets was recorded during the years ended January 1, 2011, January 2, 2010 or January 3, 2009.
Restricted Cash
As of January 1, 2011 and January 2, 2010, the Company had $512,000 and $593,000, respectively, of restricted cash that is included in other assets on the accompanying consolidated balance sheets. These restricted cash balances are primarily for restricted deposits established in connection with lease agreements for the Company’s corporate headquarters and international facilities.
Income Taxes
The Company accounts for income taxes in accordance with current authoritative accounting guidance, whereby deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which those temporary differences are expected to be recovered. The Company evaluates the need to establish a valuation allowance for deferred tax assets based on positive and negative evidence including past operating results, the amount of existing temporary differences to be recovered and expected future taxable income. A valuation allowance to reduce the deferred tax assets is established when it is “more likely than not” that some or all of the deferred tax assets will not be realized.
On January 1, 2007, the Company adopted an accounting standard which prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
Revenue Recognition and Deferred Revenue
The Company follows the current authoritative guidance for software revenue recognition. Based on these requirements, the Company recognizes revenue when: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable and (iv) collectibility is reasonably assured. Revenue from the sale of the Company’s products is generally recognized when title and risk of loss transfers to the customer upon shipment, the terms of which are shipping point or destination. The Company uses contracts and customer purchase orders to determine the existence of an arrangement. The Company uses shipping documents and/or third-party proof of delivery to verify that title has transferred. The Company assesses whether the fee is fixed or determinable based upon the terms of the agreement associated with the transaction. To determine whether collection is probable, the Company assesses a number of factors but primarily relies upon past transaction history with the customer, if available.
The Company derives revenue primarily from four sources: (i) direct sales of pulse oximetry and related products to end user hospitals, emergency medical response organizations and other direct customers; (ii) direct sales of pulse oximetry and related products to distributors who then typically resell to end user hospitals, emergency medical response organizations and other direct customers; (iii) direct sales of integrated circuit boards and sensors to OEM customers who both incorporate the Company’s embedded software technology into their multi-parameter monitoring devices and resell the Company’s sensors; (iv) long-term sales contracts to end user hospitals in which the Company may provide up front monitoring equipment at no charge in exchange for a multi-year sensor purchase commitment.
The Company enters into agreements to sell pulse oximetry and related products and services as well as multiple deliverable arrangements that include various combinations of products and services. While the majority of the Company’s sales transactions contain standard business terms and conditions, there are some transactions that contain non-standard business terms and conditions. As a result, contract interpretation is sometimes required to determine the appropriate accounting
F-12
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
including: (i) whether an arrangement exists; (ii) how the arrangement consideration should be allocated among the deliverables if there are multiple deliverables; (iii) if fair value can be determined for each deliverable based on vendor specific objective evidence, or VSOE; (iv) when to recognize revenue on the deliverables; and (v) whether undelivered elements are essential to the functionality of the delivered elements. Changes in judgments on these assumptions and estimates could materially impact the timing of revenue recognition.
The Company’s sales under long-term sales contracts are generally structured such that the Company agrees to provide up-front and at no charge certain monitoring equipment, installation, training and ongoing warranty support in exchange for the hospital’s agreement to purchase sensors over the term of the agreement, which ranges from three to six years. The Company has determined that its patented algorithm and software architecture, which resides within the monitors, is more than incidental to the product as a whole. The Company has also determined that the sensors are considered essential to the functionality of the delivered elements. Furthermore, no payments are due to the Company from the hospital customer until sensors are shipped or delivered to the hospital at fixed prices per sensor over the term of the arrangement. Accordingly, the Company does not recognize any revenue when the monitoring and related equipment is delivered to the hospitals and installation and training is complete. The Company recognizes revenue for all of the delivered elements, on a pro-rata basis, as the sensors are delivered under the long-term sales contract. The cost of the monitoring equipment initially placed at the hospitals is deferred and amortized to cost of goods sold over the life of the underlying long-term sales contract. In the event that the up-front monitoring equipment is delivered over a period of time, revenue is deferred and recognized on a proportional basis relative to the number of units of the monitoring equipment delivered over time. The Company provides certain end-user hospitals with the ability to purchase sensors under rebate programs. Under these programs, the end user hospitals may earn rebates based on their purchasing activity. The Company estimates and provides allowances for these programs at the time of sale as a reduction to revenue.
In situations where VSOE of fair value does not exist for certain undelivered elements, the entire contract product revenue and corresponding cost of goods sold are deferred until either the element is delivered, VSOE of fair value is established, or the obligation to deliver the element no longer exists. During the years ended January 1, 2011, January 2, 2010 and January 3, 2009, $0, $1.2 million and $3.6 million, respectively, of contract product revenue was deferred related to situations where VSOE of fair value did not exist for undelivered elements. Related deferred costs of $0, $451,000 and $1.3 million were deferred in the same 2010, 2009 and 2008 periods, respectively. During the years ended January 1, 2011, January 2, 2010 and January 3, 2009 previously deferred revenue of $0, $7.4 million and $3.5 million, respectively, was recognized related to such contracts. Related deferred costs of $0, $2.8 million and $1.3 million were expensed during the same 2010, 2009 and 2008 periods, respectively.
Sales to the Company’s distributors are recognized on the sell-through method. The Company’s distributors purchase primarily sensor products which they then resell to hospitals that are typically fulfilling their purchase obligations to the Company under the end-user hospitals’ long-term sensor purchase commitments. Upon shipment to the distributor, revenue is deferred until the Company’s commitment to its end-user hospital is fulfilled, which occurs when the sensors are sold by the distributor to the end-user hospital. Certain of the Company’s distributors purchase products at specified distributor pricing and then may resell the product to end-user hospitals with whom the Company has separate pricing agreements. Where distributor prices are higher than end-user hospital contracted prices, the Company provides rebates to these distributors for the difference between distributor prices and end-user hospital prices. The Company estimates and provides allowances for the rebate programs at the time of sales as a reduction to revenue and accounts receivable.
The Company also earns revenue from the sale of integrated circuit boards that use the Company’s software technology and license fees for allowing certain OEMs the right to use the Company’s technology in their products. The license fee is recognized upon shipment of the OEM’s product to its customers, as represented to the Company by the OEM.
In general, customers do not have a right of return for credit or refund. However, the Company allows returns under certain circumstances. At the end of each period, the Company estimates and accrues for these returns as a reduction to revenue and accounts receivable. The Company estimates returns based on several factors, including contractual limitations and past returns history.
In September 2005, the U.S. Federal Court of Appeals ruled that Mallinckrodt, Inc., now part of Covidien (formerly Tyco Healthcare), and one of its subsidiaries, Nellcor Puritan Bennett, Inc., collectively referred to as Nellcor, infringed Masimo patents and ordered the lower court to enjoin Nellcor’s infringing products. On January 17, 2006, the Company settled all existing patent litigation with Covidien. Under terms of the agreement, Covidien agreed to pay the Company royalties on its total U.S. pulse oximetry revenue at least through March 14, 2011, which the Company records as royalty revenue. The Covidien royalties are recognized by the Company based on U.S. sales of Covidien’s infringing products reported to the Company by Covidien. The Company recognizes royalty revenue based on the royalty rate per the settlement agreement multiplied by its estimate of Covidien’s sales for each quarter. This estimated revenue is adjusted prospectively when the Company receives the Covidien royalty report, approximately 60 days after the end of the quarter. For additional information about the Covidien settlement agreement, see Note 15 to the consolidated financial statements.
F-13
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Taxes Collected From Customers and Remitted to Governmental Authorities
Pursuant to authoritative guidance, the Company’s policy is to present revenue net of taxes collected from customers and remitted to governmental authorities.
Share-Based Compensation
On January 1, 2006, the Company began to expense the estimated fair value of employee stock options and similar awards based on the fair value of the stock option on the date of grant, in accordance with the current authoritative accounting guidance. The cost is recognized over the period during which an employee is required to provide services in exchange for the stock option, which is usually the vesting period. The Company adopted the accounting standard using the prospective transition method that applies to stock options granted, modified or canceled subsequent to the date of adoption. Prior periods were not revised for comparative purposes. The Company has elected to recognize share-based compensation expense on a straight-line basis over the requisite service period for the entire stock option.
Options granted prior to January 1, 2006, were accounted for using the intrinsic value method and using the minimum value method for its pro forma disclosures, unless such options are modified, repurchased or cancelled. The cash flows related to the reduction of income taxes paid as a result of the deduction triggered by employee exercise of stock options granted or modified prior to January 1, 2006 will continue to be presented as an operating cash flow.
Shipping and Handling Costs and Revenue
All shipping and handling costs are expensed as incurred and are recorded as a component of cost of sales. Charges for shipping and handling billed to customers are included as a component of product revenue in accordance with authoritative accounting guidance.
Product Warranty
The Company provides a warranty against defects in material and workmanship for a period ranging from six months to one year, depending on the product type. In the case of long-term sales agreements, the Company typically warranties the products for the term of the agreement, which ranges from three to six years. In traditional sales activities, including direct and OEM sales, the Company establishes an accrual for the estimated costs of warranty at the time of revenue recognition. Estimated warranty expenses are recorded as an accrued liability, with a corresponding provision to cost of sales. In long-term sales agreements, revenue related to extended warranty is recognized over the life of the contract, while the product warranty costs related to the long-term sales agreements are expensed as incurred.
Changes in the product warranty accrual were as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Warranty accrual, beginning of period | | $ | 354 | | | $ | 334 | | | $ | 649 | |
Provision for warranty costs | | | 2,355 | | | | 2,220 | | | | 1,646 | |
Warranty expenditures | | | (2,165 | ) | | | (2,200 | ) | | | (1,961 | ) |
| | | | | | | | | | | | |
Warranty accrual, end of period | | $ | 544 | | | $ | 354 | | | $ | 334 | |
| | | | | | | | | | | | |
Advertising Costs
Advertising costs are expensed as incurred. These costs are included in selling, general and administrative expense in the accompanying consolidated statements of income. Advertising costs for the years ended January 1, 2011, January 2, 2010, and January 3, 2009 were $5.9 million, $5.7 million and $7.7 million, respectively.
Research and Development
Costs related to research and development activities are expensed as incurred. These costs include personnel costs, materials, depreciation and amortization on associated tangible and intangible assets and an allocation of facility costs, all of which are directly related to research and development activities.
F-14
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Antitrust Litigation Expense (Proceeds)
The Company recorded proceeds from the antitrust litigation and related legal fees on a net basis in the consolidated statements of income under antitrust litigation expense (proceeds). During the year ended January 1, 2011, the Company received two payments totaling $44.8 million, of which it retained $30.8 million, and the remainder was paid to the law firm that handled the trial for the Company. These amounts represent the net payments from Covidien relating to the antitrust litigation following the Ninth Circuit Court of Appeals’ October 2009 affirmance of a Federal District Court decision that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products.
Foreign Currency Translation
The Company’s international headquarters is in Switzerland, and its functional currency is the U.S. dollar. The Company has several foreign sales support subsidiaries that maintain foreign offices, of which the most significant are in Japan and Europe. The functional currencies of these subsidiaries are the Japanese yen and Euro, respectively. The Company also has subsidiaries in Canada, Australia, Singapore, Hong Kong and China. The functional currencies of these subsidiaries are the Canadian dollar, Australian dollar, Singapore dollar, Hong Kong dollar and Chinese yuan, respectively.
The Company transacts with foreign customers in currencies other than the U.S. dollar. It experiences realized and unrealized foreign currency gains or losses on foreign denominated receivables. In addition, certain intercompany transactions give rise to realized and unrealized foreign currency gains or losses. Also, any other transactions between the Company or its subsidiaries and a third party, denominated in a currency different from the functional currency, are foreign currency transactions. Realized and unrealized foreign currency gains or losses are included as a component of non-operating income (expense) within the Company’s statements of income, as incurred and are converted to U.S. dollars at average exchange rates for a respective period. These transaction gains or (losses) were $1.0 million, $(236,000) and $(414,000) for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, respectively.
Assets and liabilities of foreign subsidiaries, whose functional currency is not the U.S. dollar, are translated into U.S. dollars at the rate of exchange at the balance sheet date. Statement of income amounts are translated at the average monthly exchange rates for the respective periods. For these foreign subsidiaries whose functional currency is not the U.S. dollar, translation gains and losses are included as a component of accumulated other comprehensive income (loss) within Masimo Corporation stockholders’ equity.
Comprehensive Income
Authoritative accounting guidance establishes requirements for reporting and disclosure of comprehensive income and its components. Comprehensive income includes foreign currency translation adjustments that have been excluded from net income including noncontrolling interests and reflected in Masimo Corporation stockholders’ equity.
Accumulated Other Comprehensive Income (Loss)
Accumulated other comprehensive income (loss) included in the Company’s consolidated statements of equity consists only of foreign currency translation adjustments. The change in accumulated other comprehensive income (loss) is summarized as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Other comprehensive income (loss), beginning of period | | $ | 63 | | | $ | (7 | ) | | $ | (1,034 | ) |
Foreign currency translation adjustment | | | 862 | | | | 70 | | | | 1,027 | |
Tax benefit (expense) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Other comprehensive income (loss), end of period | | $ | 925 | | | $ | 63 | | | $ | (7 | ) |
| | | | | | | | | | | | |
Net Income Per Share
Basic net income per share attributable to Masimo Corporation for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 is computed by dividing net income attributable to Masimo Corporation by the weighted average number of shares outstanding during each period. The diluted net income per share attributable to Masimo Corporation for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 is computed by dividing the net income attributable to Masimo
F-15
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Corporation by the weighted average number of shares and potential shares outstanding during each period, if the effect of potential shares is dilutive. Potential shares include incremental shares of stock issuable upon the exercise of stock options. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, options to purchase 4,497,698, 3,601,961 and 1,894,181 shares of common stock, respectively, were not included in the computation of diluted share equivalent because the options’ exercise prices were greater than the average market price for the period.
Based on authoritative accounting guidance, the Company reduced its net income including noncontrolling interests by the amount of net (income) loss attributable to noncontrolling interests for the years ended January 1, 2011 and January 2, 2010. A reconciliation of basic and diluted net income per share attributable to Masimo Corporation is as follows (in thousands, except share data):
| | | | | | | | | | | | |
| | | Year ended | |
| | | January 1,
2011 | | | January 2,
2010 | | | January 3,
2009 | |
Net income attributable to stockholders of Masimo Corporation: | | | | | | | | | | | | |
Net income including noncontrolling interests | | $ | 73,405 | | | $ | 54,022 | | | $ | 31,927 | |
Net (income) loss attributable to the noncontrolling interests | | | 125 | | | | (794 | ) | | | — | |
| | | | | | | | | | | | |
Net income attributable to Masimo Corporation | | $ | 73,530 | | | $ | 53,228 | | | $ | 31,927 | |
| | | | | | | | | | | | |
Basic net income per share attributable to Masimo Corporation: | | | | | | | | | | | | |
Net income attributable to Masimo Corporation | | $ | 73,530 | | | $ | 53,228 | | | $ | 31,927 | |
| | | | | | | | | | | | |
Weighted average shares outstanding | | | 58,769,023 | | | | 57,602,646 | | | | 56,320,712 | |
| | | | | | | | | | | | |
Basic net income per share attributable to Masimo Corporation | | $ | 1.25 | | | $ | 0.92 | | | $ | 0.57 | |
| | | | | | | | | | | | |
Diluted net income per share attributable to Masimo Corporation: | | | | | | | | | | | | |
Weighted average shares outstanding | | | 58,769,023 | | | | 57,602,646 | | | | 56,320,712 | |
Diluted share equivalent: stock options | | | 1,840,064 | | | | 2,568,202 | | | | 3,869,623 | |
| | | | | | | | | | | | |
| | | 60,609,087 | | | | 60,170,848 | | | | 60,190,335 | |
| | | | | | | | | | | | |
Diluted net income per share attributable to Masimo Corporation | | $ | 1.21 | | | $ | 0.88 | | | $ | 0.53 | |
| | | | | | | | | | | | |
Segment Information
The Company uses the “management approach” in determining reportable business segments. The management approach designates the internal organization used by management for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Based on this assessment, management has determined it operates in one reportable business segment, which is comprised of patient monitoring and related products.
New Accounting Pronouncements
In October 2009, the FASB issued Accounting Standards Update 2009-13, or ASU No. 09-13,Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements. ASU No. 09-13 modifies the requirements that must be met for an entity to recognize revenue from the sale of a delivered item that is part of a multiple-element arrangement when other items have not yet been delivered. This update shall be applied on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Earlier application as of the beginning of the Company’s fiscal year is permitted, provided it has not previously issued financial statements for any period within that year. The Company believes its prospective adoption of this update, in connection with the adoption of ASU No. 09-14,Software (Topic 985): Certain Revenue Arrangements That Include Software Elements, will eliminate the requirement to defer the entire contract product revenue and corresponding cost of goods sold on arrangements that contain undelivered tangible products for which VSOE of fair value has not been established.
In October 2009, the FASB issued Accounting Standards Update 2009-14, or ASU No. 09-14,Software (Topic 985): Certain Revenue Arrangements That Include Software Elements. ASU No. 09-14 modifies the software revenue recognition guidance to exclude from its scope tangible products that contain both software and non-software components that function together to deliver a product’s essential functionality. ASU No. 09-14 is effective for fiscal years beginning after June 15, 2010 and may be applied retrospectively or prospectively for new or materially modified arrangements. Earlier application as of the beginning of the Company’s fiscal year is permitted, provided it has not previously issued financial statements for any period
F-16
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
within that year. The Company believes its prospective adoption of this update, in connection with the adoption of ASU No. 09-13, will eliminate the requirement to defer the entire contract product revenue and corresponding cost of goods sold on arrangements that contain undelivered tangible products for which VSOE of fair value has not been established.
3. Variable Interest Entities (VIEs)
Effective January 3, 2010, the Company adopted a newly issued accounting standard which provides guidance for the consolidation of VIEs and requires an enterprise to determine whether its variable interest gives it a controlling financial interest in a VIE. This amended consolidation guidance for VIEs replaced the priorquantitative approach for identifying which enterprise should consolidate a VIE with aqualitative approach. The Company’s adoption of this standard did not change its initial assessment of its VIEs. Determination about whether an enterprise should consolidate a VIE is required to be evaluated continuously as changes to existing relationships or future transactions may result in consolidating or deconsolidating the VIE. The changes in noncontrolling interests for the consolidated VIEs are presented in the accompanying consolidated statements of equity.
Cercacor Laboratories, Inc. (formerly Masimo Laboratories, Inc.)
Cercacor Laboratories, Inc., or Cercacor, is an independent entity spun off from the Company to its stockholders in 1998. Joe Kiani and Jack Lasersohn, members of the Company’s board of directors, or Board, are also members of the board of directors of Cercacor. Joe Kiani, the Company’s Chairman and Chief Executive Officer, is also the Chairman and Chief Executive Officer of Cercacor. The Company is a party to a Cross-Licensing Agreement with Cercacor, which was most recently amended and restated effective January 1, 2007, that governs each party’s rights to certain intellectual property held by the two companies.
Under the Cross-Licensing Agreement, the Company granted Cercacor an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET owned by the Company, including all improvements on this technology, for the monitoring of non-vital signs measurements and to develop and sell devices incorporating Masimo SET for monitoring non-vital signs measurements in any product market in which a product is intended to be used by a patient or pharmacist rather than a professional medical caregiver. The Company refers to this market as the Cercacor Market. The Company also granted Cercacor a non-exclusive, perpetual and worldwide license, with sublicense rights to use all Masimo SET for the measurement of vital signs in the Cercacor Market. The Company exclusively licenses from Cercacor the right to make and distribute products in the professional medical caregiver markets, which the Company refers to as the Masimo Market, that utilize rainbow® technology for the measurement of carbon monoxide, methemoglobin, fractional arterial oxygen saturation, and total hemoglobin, which includes hematocrit. To date, the Company has developed and commercially released devices that measure carbon monoxide, methemoglobin and total hemoglobin using licensed rainbow® technology. The Company also has the option to obtain the exclusive license to make and distribute products that utilize rainbow® technology for the monitoring of other non-vital signs measurements, including blood glucose, in product markets where the product is intended to be used by a professional medical caregiver.
From May 1998 through May 2009, Cercacor contracted the services of the Company’s employees for the development of rainbow® technology. The Company paid Cercacor for the option to market and develop products based on Cercacor technology in defined markets. Through December 2005, the Company paid Cercacor $7.5 million in option fees. Nearly all these option fees were used by Cercacor to repay the Company for the services that the Company had provided to Cercacor. In addition, through September 2009, the Company exercised its options to three licenses, for $2.5 million each, for the right to market products based on the new carbon monoxide, methemoglobin and total hemoglobin parameter technologies developed by Cercacor. Effective as of January 1, 2007, the Company entered into a Services Agreement with Cercacor to govern the general and administrative services the Company provides to Cercacor.
The Company’s license to rainbow® technology for these parameters in these markets is exclusive on the condition that the Company continues to pay Cercacor royalties on its products incorporating rainbow® technology, subject to certain minimum aggregate royalty thresholds, and that the Company use commercially reasonable efforts to develop or market products incorporating the licensed rainbow® technology. The royalty is up to 10% of the rainbow® royalty base, which includes handhelds, tabletop and multi-parameter devices. Handheld products incorporating rainbow® technology will carry a 10% royalty rate. For other products, only the proportional amount attributable for that portion of the Company’s products used to monitor non-vital signs measurements, sensors and accessories, rather than for monitoring vital signs measurements, will be included in the 10% rainbow® royalty base. Effective January 2009, for multi-parameter devices, the rainbow® royalty base will include the percentage of the revenue based on the number of rainbow® enabled measurements. For hospital contracts where the Company places equipment and enters into a sensor contract, the Company pays a royalty to Cercacor on the total sensor contract revenues based on the ratio of rainbow® enabled devices to total devices.
F-17
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
The Company is also subject to certain specific annual minimum aggregate royalty payments. These minimum aggregate royalty payments were $5.0 million in 2010, $4.0 million for 2009 and $3.5 million for 2008. In 2011 and each year thereafter, the minimum aggregate royalty payment will be $5.0 million. In addition, in connection with a change in control, as defined in the Cross-Licensing Agreement, the minimum aggregate annual royalties for all licensed rainbow® measurements payable to Cercacor will increase to $15.0 million per year for 2010 and thereafter, and up to $2.0 million per year for other rainbow® measurements.
In February 2009, in order to accelerate the product development of an improved total hemoglobin spot-check measurement device, Pronto-7, the Masimo Board of Directors agreed to fund additional Cercacor’s engineering expenses. Specifically, these expenses included third party engineering materials and supplies expense as well as 50% of total Cercacor’s engineering and engineering related payroll expenses from April 2009 through June 2010, the original anticipated completion date of this product development effort. During the six months ended January 1, 2011, and until both parties agree to end these services, Cercacor has and will continue to assist the Company with continuing productization efforts of the new handheld noninvasive multi-parameter testing device, that provides spot-check hemoglobin testing. During the years ended January 1, 2011 and January 2, 2010, the total expenses for these additional services, material and supplies totaled $2.6 million and $2.7 million, respectively.
Pursuant to authoritative accounting guidance, Cercacor is consolidated within the Company’s financial statements for all periods presented. The Company was required to consolidate it since it was deemed to be the primary beneficiary of Cercacor’s activities. This determination was based on the Company’s significant influence over the operations and decision making of Cercacor. Accordingly, all intercompany royalties, option and license fees and other charges between the Company and Cercacor as well as all intercompany payables and receivables have been eliminated in the consolidation. Also, all direct engineering expenses that have been incurred by the Company and charged to Cercacor have not been eliminated and are included as research and development expense in the Company’s consolidated statements of income. Assets of Cercacor can only be used to settle obligations of Cercacor and creditors of Cercacor have no recourse to the general credit of the Company.
For the foreseeable future, the Company anticipates that it will continue to consolidate Cercacor pursuant to the current authoritative accounting guidance; however, in the event that Cercacor is no longer considered a VIE or in the event that the Company is no longer the primary beneficiary of Cercacor, the Company may discontinue consolidating the entity.
SEDLine, Inc.
SEDLine, Inc., a privately held entity that was formed in the fourth quarter of 2009, is a company that designs, manufactures, markets and sells brain function monitoring technology into the hospital marketplace. During 2009, the Company made loans to SEDLine totaling $3.0 million. These loans carried an interest rate of 7% and could be converted into equity upon certain predetermined conditions. Concurrently with the loans, the Company entered into a merger agreement with SEDLine, whereby the Company could acquire SEDLine at certain predetermined valuations. Also, concurrent with the Company’s loan to SEDLine, SEDLine purchased the assets of its neuromonitoring business from another company for $1.6 million. In December 2009, the Company purchased two patents from SEDLine for an aggregate of $500,000.
As of January 2, 2010, the Company had no equity ownership of SEDLine, but was considered to be the primary beneficiary of SEDLine as a VIE because SEDLine had insufficient equity investment at risk. Therefore the Company was required to consolidate SEDLine’s assets, liabilities and equity.
On July 2, 2010, the Company acquired 100% ownership of SEDLine, which became a wholly-owned subsidiary of the Company. The Company acquired all of SEDLine’s assets upon conversion of its $3.0 million note receivable and the related accrued interest, into all authorized shares of SEDLine. In connection with this acquisition, the Company also assumed all of SEDLine’s outstanding liabilities. As of July 3, 2010, SEDLine’s deficit noncontrolling interest balance of $1.5 million was consolidated with additional paid in capital of the Company on the consolidated balance sheet.
During the six months ended July 3, 2010, SEDLine was a VIE and the Company was deemed to be the primary beneficiary. Therefore, the Company has included SEDLine’s revenue and expenses incurred during the first six months of the year ended January 1, 2011, in its consolidated statement of income. However, during these six months ended July 3, 2010, SEDLine was a noncontrolling interest of the Company. Therefore, SEDLine’s net loss incurred during these six months ended July 3, 2010 was not included in the net income attributable to the Company for the year ended January 1, 2011.
As a result of the Company’s July 2, 2010 acquisition of SEDLine, SEDLine became a wholly-owned subsidiary and was no longer a noncontrolling interest of the Company. Due to SEDLine’s change in status, the Company included SEDLine’s revenue, expenses and results thereof, in its consolidated statement of income for the year ended January 1, 2011. As of January 1, 2011, all of SEDLine’s assets, liabilities and equity are consolidated with the Company, since SEDLine is a wholly-owned subsidiary.
F-18
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Below are condensed consolidating schedules of the Balance Sheets as of January 1, 2011 and January 2, 2010, and Statements of Income for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 reflecting Masimo Corporation, Cercacor, SEDLine and related eliminations (in thousands).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | January 1, 2011 | | | January 2, 2010 | |
Balance Sheets: | | Corp | | | Cercacor | | | Cercacor
Elim | | | Total | | | Corp | | | Cercacor | | | Cercacor
Elim | | | SEDLine | | | SEDLine
Elim | | | Total | |
ASSETS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 87,675 | | | $ | 630 | | | $ | — | | | $ | 88,305 | | | $ | 129,841 | | | $ | 464 | | | $ | — | | | $ | 1,749 | | | $ | — | | | $ | 132,054 | |
Short-term investments | | | — | | | | — | | | | — | | | | — | | | | 56,989 | | | | — | | | | — | | | | — | | | | — | | | | 56,989 | |
Accounts receivable, net | | | 61,694 | | | | 996 | | | | (996 | ) | | | 61,694 | | | | 50,397 | | | | 363 | | | | (363 | ) | | | — | | | | — | | | | 50,397 | |
Inventories | | | 45,028 | | | | — | | | | — | | | | 45,028 | | | | 30,687 | | | | 245 | | | | — | | | | 627 | | | | — | | | | 31,559 | |
Prepaid expenses | | | 7,741 | | | | 146 | | | | — | | | | 7,887 | | | | 5,370 | | | | 77 | | | | — | | | | — | | | | — | | | | 5,447 | |
Deferred tax asset, current | | | 11,490 | | | | 1,065 | | | | — | | | | 12,555 | | | | 10,406 | | | | 1,179 | | | | — | | | | — | | | | — | | | | 11,585 | |
Other current assets | | | 1,892 | | | | 244 | | | | — | | | | 2,136 | | | | 1,357 | | | | — | | | | — | | | | — | | | | — | | | | 1,357 | |
Deferred cost of goods sold | | | 47,184 | | | | — | | | | — | | | | 47,184 | | | | 28,163 | | | | — | | | | — | | | | — | | | | — | | | | 28,163 | |
Property and equipment, net | | | 14,788 | | | | 1,163 | | | | — | | | | 15,951 | | | | 11,087 | | | | 575 | | | | — | | | | 20 | | | | — | | | | 11,682 | |
Intangible assets, net | | | 14,074 | | | | 2,079 | | | | (5,656 | ) | | | 10,497 | | | | 13,373 | | | | 1,883 | | | | (6,031 | ) | | | 604 | | | | — | | | | 9,829 | |
Deferred tax asset, long term | | | 12,532 | | | | 28 | | | | — | | | | 12,560 | | | | 11,043 | | | | 457 | | | | — | | | | — | | | | — | | | | 11,500 | |
Other assets, long term | | | 6,399 | | | | 2,969 | | | | (2,930 | ) | | | 6,438 | | | | 8,747 | | | | 2,966 | | | | (2,930 | ) | | | — | | | | (3,000 | ) | | | 5,783 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 310,497 | | | $ | 9,320 | | | $ | (9,582 | ) | | $ | 310,235 | | | $ | 357,460 | | | $ | 8,209 | | | $ | (9,324 | ) | | $ | 3,000 | | | $ | (3,000 | ) | | $ | 356,345 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 21,262 | | | $ | 888 | | | $ | — | | | $ | 22,150 | | | $ | 15,702 | | | $ | 1,014 | | | $ | — | | | $ | — | | | $ | — | | | $ | 16,716 | |
Accrued liabilities and compensation | | | 31,249 | | | | 653 | | | | (996 | ) | | | 30,906 | | | | 27,466 | | | | 444 | | | | (363 | ) | | | — | | | | — | | | | 27,547 | |
Income taxes payable | | | 721 | | | | 1 | | | | — | | | | 722 | | | | 459 | | | | 18 | | | | — | | | | — | | | | — | | | | 477 | |
Deferred revenue, current | | | 16,369 | | | | 375 | | | | (375 | ) | | | 16,369 | | | | 14,641 | | | | 375 | | | | (375 | ) | | | — | | | | — | | | | 14,641 | |
Current portion of long-term debt | | | 50 | | | | — | | | | — | | | | 50 | | | | 60 | | | | — | | | | — | | | | — | | | | — | | | | 60 | |
Deferred revenue, long-term | | | 1,554 | | | | 5,281 | | | | (5,281 | ) | | | 1,554 | | | | 270 | | | | 5,656 | | | | (5,656 | ) | | | — | | | | — | | | | 270 | |
Long term debt, less current portion | | | 122 | | | | — | | | | — | | | | 122 | | | | 171 | | | | — | | | | — | | | | 3,000 | | | | (3,000 | ) | | | 171 | |
Other liabilities | | | 11,253 | | | | — | | | | (2,930 | ) | | | 8,323 | | | | 9,705 | | | | — | | | | (2,930 | ) | | | — | | | | — | | | | 6,775 | |
EQUITY (DEFICIT) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Common stock | | | 59 | | | | 10 | | | | (10 | ) | | | 59 | | | | 58 | | | | 10 | | | | (10 | ) | | | — | | | | — | | | | 58 | |
Treasury stock | | | (1,209 | ) | | | — | | | | — | | | | (1,209 | ) | | | (1,209 | ) | | | — | | | | — | | | | — | | | | — | | | | (1,209 | ) |
Additional paid-in capital | | | 222,206 | | | | 249 | | | | (249 | ) | | | 222,206 | | | | 195,690 | | | | 170 | | | | (170 | ) | | | — | | | | — | | | | 195,690 | |
Accumulated other comprehensive income | | | 925 | | | | — | | | | — | | | | 925 | | | | 63 | | | | — | | | | — | | | | — | | | | — | | | | 63 | |
Retained earnings (deficit) | | | 5,936 | | | | 1,863 | | | | (2,135 | ) | | | 5,664 | | | | 94,384 | | | | 522 | | | | (794 | ) | | | — | | | | — | | | | 94,112 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total Masimo Corporation stockholders’ equity (deficit) | | | 227,917 | | | | 2,122 | | | | (2,394 | ) | | | 227,645 | | | | 288,986 | | | | 702 | | | | (974 | ) | | | — | | | | — | | | | 288,714 | |
Noncontrolling interest | | | — | | | | — | | | | 2,394 | | | | 2,394 | | | | — | | | | — | | | | 974 | | | | — | | | | — | | | | 974 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total equity | | | 227,917 | | | | 2,122 | | | | — | | | | 230,039 | | | | 288,986 | | | | 702 | | | | — | | | | — | | | | — | | | | 289,688 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total liabilities and equity (deficit) | | $ | 310,497 | | | $ | 9,320 | | | $ | (9,582 | ) | | $ | 310,235 | | | $ | 357,460 | | | $ | 8,209 | | | $ | (9,324 | ) | | $ | 3,000 | | | $ | (3,000 | ) | | $ | 356,345 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-19
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended January 1, 2011 | | | Year ended January 2, 2010 | | | Year ended January 3, 2009 | |
Statements of Income: | | Corp | | | Cercacor | | | Cercacor
Elim | | | Sedline | | | Sedline
Elim | | | Total | | | Corp | | | Cercacor | | | Elim | | | Total | | | Corp | | | Cercacor | | | Elim | | | Total | |
Total revenue | | $ | 405,078 | | | $ | 5,620 | | | $ | (5,620 | ) | | $ | 329 | | | $ | — | | | $ | 405,407 | | | $ | 349,115 | | | $ | 4,375 | | | $ | (4,375 | ) | | $ | 349,115 | | | $ | 307,074 | | | $ | 3,875 | | | $ | (3,875 | ) | | $ | 307,074 | |
Cost of goods sold | | | 124,605 | | | | 245 | | | | (5,245 | ) | | | 220 | | | | — | | | | 119,825 | | | | 104,313 | | | | — | | | | (4,000 | ) | | | 100,313 | | | | 92,954 | | | | — | | | | (3,500 | ) | | | 89,454 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit (loss) | | | 280,473 | | | | 5,375 | | | | (375 | ) | | | 109 | | | | — | | | | 285,582 | | | | 244,802 | | | | 4,375 | | | | (375 | ) | | | 248,802 | | | | 214,120 | | | | 3,875 | | | | (375 | ) | | | 217,620 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 171,792 | | | | 1,557 | | | | (375 | ) | | | 1,115 | | | | | | | | 174,089 | | | | 133,781 | | | | 1,171 | | | | (375 | ) | | | 134,577 | | | | 119,264 | | | | 1,180 | | | | (375 | ) | | | 120,069 | |
Research and development | | | 33,967 | | | | 1,680 | | | | — | | | | 353 | | | | — | | | | 36,000 | | | | 29,656 | | | | 2,045 | | | | — | | | | 31,701 | | | | 23,065 | | | | 2,430 | | | | — | | | | 25,495 | |
Antitrust litigation expense (proceeds) | | | (30,728 | ) | | | — | | | | — | | | | — | | | | — | | | | (30,728 | ) | | | 298 | | | | — | | | | — | | | | 298 | | | | 706 | | | | — | | | | — | | | | 706 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 175,031 | | | | 3,237 | | | | (375 | ) | | | 1,468 | | | | — | | | | 179,361 | | | | 163,735 | | | | 3,216 | | | | (375 | ) | | | 166,576 | | | | 143,035 | | | | 3,610 | | | | (375 | ) | | | 146,270 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 105,442 | | | | 2,138 | | | | — | | | | (1,359 | ) | | | — | | | | 106,221 | | | | 81,067 | | | | 1,159 | | | | — | | | | 82,226 | | | | 71,085 | | | | 265 | | | | — | | | | 71,350 | |
Non-operating income (expense) | | | 1,451 | | | | 4 | | | | — | | | | (107 | ) | | | — | | | | 1,348 | | | | (46 | ) | | | — | | | | — | | | | (46 | ) | | | 1,038 | | | | 3 | | | | — | | | | 1,041 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income before provision for (benefit from) income taxes | | | 106,893 | | | | 2,142 | | | | — | | | | (1,466 | ) | | | — | | | | 107,569 | | | | 81,021 | | | | 1,159 | | | | — | | | | 82,180 | | | | 72,123 | | | | 268 | | | | — | | | | 72,391 | |
Provision for (benefit from) income taxes | | | 33,363 | | | | 801 | | | | — | | | | — | | | | — | | | | 34,164 | | | | 27,793 | | | | 365 | | | | — | | | | 28,158 | | | | 40,483 | | | | (19 | ) | | | — | | | | 40,464 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) including noncontrolling interests | | | 73,530 | | | | 1,341 | | | | — | | | | (1,466 | ) | | | — | | | | 73,405 | | | | 53,228 | | | | 794 | | | | — | | | | 54,022 | | | | 31,640 | | | | 287 | | | | — | | | | 31,927 | |
Net (income) loss attributable to noncontrolling interests | | | — | | | | — | | | | (1,341 | ) | | | — | | | | 1,466 | | | | 125 | | | | — | | | | — | | | | (794 | ) | | | (794 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) attributable to Masimo Corporation | | $ | 73,530 | | | $ | 1,341 | | | $ | (1,341 | ) | | $ | (1,466 | ) | | $ | 1,466 | | | $ | 73,530 | | | $ | 53,228 | | | $ | 794 | | | $ | (794 | ) | | $ | 53,228 | | | $ | 31,640 | | | $ | 287 | | | $ | — | | | $ | 31,927 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Note: For the year ended January 2, 2010, SEDLine’s Statement of Income is not separately disclosed because it did not have any revenues and its expenses were de minimis.
F-20
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
4. Related Party Transactions
As of January 1, 2011 and January 2, 2010, the Company had amounts due from employees of $180,000 and $242,000, respectively. These amounts are classified in other assets in the accompanying consolidated balance sheets.
The Company’s Chief Executive Officer is also the Chairman of the Masimo Foundation for Ethics, Innovation and Competition in Healthcare, or the Masimo Foundation, a non-profit organization which was founded during the first quarter of 2010 to benefit worldwide healthcare. The Company’s Chief Financial Officer is also a Director of the Masimo Foundation. For the year ended January 1, 2011, the Company contributed a total of $10.3 million to the Masimo Foundation, which has been recorded within selling, general and administrative expenses in the consolidated statements of income.
5. Inventories
Inventories consist of the following (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Raw materials | | $ | 28,028 | | | $ | 18,259 | |
Work in-process | | | 4,192 | | | | 3,949 | |
Finished goods | | | 12,808 | | | | 9,351 | |
| | | | | | | | |
Total | | $ | 45,028 | | | $ | 31,559 | |
| | | | | | | | |
Finished goods inventory held by distributors was $2.4 million and $1.8 million as of January 1, 2011 and January 2, 2010, respectively.
6. Property and Equipment
Property and equipment, net consists of the following (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Machinery and equipment | | $ | 20,059 | | | $ | 14,924 | |
Tooling | | | 8,977 | | | | 6,809 | |
Computer equipment | | | 7,058 | | | | 5,595 | |
Furniture and office equipment | | | 2,670 | | | | 2,236 | |
Vehicles | | | 45 | | | | 45 | |
Leasehold improvements | | | 5,401 | | | | 5,264 | |
Demonstration units | �� | | 2,785 | | | | 2,744 | |
| | | | | | | | |
| | | 46,995 | | | | 37,617 | |
Accumulated depreciation and amortization | | | (31,370 | ) | | | (26,034 | ) |
Construction-in-progress | | | 326 | | | | 99 | |
| | | | | | | | |
Total | | $ | 15,951 | | | $ | 11,682 | |
| | | | | | | | |
The gross value of furniture and office equipment under capital lease obligations was $278,000 and $272,000 as of January 1, 2011 and January 2, 2010, respectively, with accumulated amortization of $148,000 and $90,000, respectively.
7. Intangible Assets
Intangible assets, net consist of the following (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Cost | | | | | | | | |
Patents | | $ | 12,666 | | | $ | 11,140 | |
Trademarks | | | 1,189 | | | | 940 | |
Capitalized software development costs | | | 1,563 | | | | 1,563 | |
Other | | | 162 | | | | 40 | |
| | | | | | | | |
Total cost | | | 15,580 | | | | 13,683 | |
| | | | | | | | |
F-21
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Accumulated amortization | | | | | | | | |
Patents | | | (4,139 | ) | | | (3,171 | ) |
Trademarks | | | (275 | ) | | | (221 | ) |
Capitalized software development costs | | | (653 | ) | | | (429 | ) |
Other | | | (16 | ) | | | (33 | ) |
| | | | | | | | |
Total accumulated amortization | | | (5,083 | ) | | | (3,854 | ) |
| | | | | | | | |
Net carrying amount | | $ | 10,497 | | | $ | 9,829 | |
| | | | | | | | |
For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, total amortization expense was $1.3 million, $917,000 and $708,000, respectively.
Estimated amortization expense for each of the fiscal years are as follows (in thousands):
| | | | |
Fiscal year | | Amount | |
2011 | | $ | 1,148 | |
2012 | | | 956 | |
2013 | | | 887 | |
2014 | | | 841 | |
2015 | | | 595 | |
Thereafter | | | 6,070 | |
| | | | |
Total | | $ | 10,497 | |
| | | | |
During the year ended January 1, 2011, the Company did not acquire any intangible assets as part of an acquisition or business combination.
8. Capital Lease Obligations
Capital lease obligations consist of the following not including interest expense (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Capital lease obligations | | $ | 172 | | | $ | 231 | |
Less current portion of capital lease obligations | | | (50 | ) | | | (60 | ) |
| | | | | | | | |
Long-term portion | | $ | 122 | | | $ | 171 | |
| | | | | | | | |
As of January 1, 2011, the Company has six capital leases related to office equipment. These leases have interest rates ranging from 5.2% to 8.8% per year and mature on various dates between March 2011 and May 2014.
Future maturities of capital lease obligations for each of the fiscal years are as follows (in thousands):
| | | | |
Fiscal year | | Amount | |
2011 | | $ | 50 | |
2012 | | | 48 | |
2013 | | | 46 | |
2014 | | | 28 | |
2015 | | | — | |
| | | | |
Total | | $ | 172 | |
| | | | |
F-22
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
9. Other Liabilities, Long-Term
Other long-term liabilities consist of the following (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Unrecognized tax benefit | | $ | 8,020 | | | $ | 6,632 | |
Deferred rent, long-term | | | 186 | | | | 79 | |
Other | | | 117 | | | | 64 | |
| | | | | | | | |
Total other liabilities, long-term | | $ | 8,323 | | | $ | 6,775 | |
| | | | | | | | |
The unrecognized tax benefit relates to the Company’s long-term portion of tax liability, under recent authoritative accounting guidance which became effective on January 1, 2007. This guidance prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. See Note 14 of the consolidated financial statements for further details.
10. Equity
Series A Junior Participating Preferred Stock and Stockholder Rights Plan
On November 8, 2007, the Company authorized and declared a dividend of one preferred stock purchase right, or a Right, for each outstanding share of its common stock to stockholders of record at the close of business on November 26, 2007, or the Record Date. Each Right entitles the registered holder to purchase from the Company one one-thousandth of one share of the Company’s Series A junior participating preferred stock, par value $0.001 per share, at a purchase price equal to $136.00 per Right, subject to adjustment. In addition, one Right will be issued with each share of common stock that becomes outstanding after the Record Date, and prior to the earliest of the distribution date, the date the Rights are redeemed, or the Final Expiration Date of November 8, 2017. In connection with the stockholder rights plan described herein, the Board designated 100,000 shares of preferred stock as Series A junior participating preferred stock, as set forth in the Certificate of Designation of Series A Junior Participating Preferred Stock.
Until a Right is exercised, the holder of such Right will have no rights as a stockholder of the Company, beyond those as an existing stockholder, including, without limitation, the right to vote or to receive dividends. Subject to certain exceptions specified in the Rights Agreement, the Rights will separate from the common stock. The Rights have certain anti-takeover effects. The Rights will cause dilution to a person or group that attempts to acquire the Company in a transaction which the Board does not approve is in the best interests of the Company and its stockholders.
The shares of Series A junior preferred stock issuable upon exercise of the Rights have the following characteristics: they are not redeemable; the holders of preferred stock are entitled, when, as and if declared, to minimum preferential quarterly dividend payments of an amount equal to (i) $1.00 per share or (ii) 1,000 times the aggregate per share amount of all cash dividends and 1,000 times the aggregate per share amount of all non-cash dividends or other distributions; the holders of preferred stock are entitled, in the event of a liquidation, dissolution or winding up, to a minimum preferential payment equal to $1,000 per share, plus all accrued and unpaid dividends, provided that the holders shall be entitled to receive 1,000 times the aggregate payment made per common share; the holders of preferred stock are entitled to 1,000 votes per share, voting together with the common stock; and the holders of preferred stock are entitled, in the event of a merger, consolidation or other transaction in which outstanding shares of common stock are converted or exchanged, to receive 1,000 times the amount received per share of common stock.
Dividend Payments
In February 2010, the Company declared a special $2.00 per share cash dividend, payable on March 31, 2010 to stockholders of record as of the close of business on March 11, 2010. The total dividend payout was $117.5 million, which was made from retained earnings.
In November 2010, the Company declared a special $0.75 per share cash dividend, payable on December 21, 2010 to stockholders of record as of the close of business on December 7, 2010. The total dividend payout was $44.5 million, which was made from retained earnings.
F-23
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
11. Share-Based Compensation
In April 2004, the Company adopted the 2004 Incentive Stock Option, Nonqualified Stock Option, and Restricted Stock Purchase Plan, or the 2004 Plan, which initially provided for the issuance of options to purchase up to 3,000,000 shares of the Company’s common stock, plus any shares available under the prior year stock option plans, including shares that become available due to forfeitures at prices not less than the fair market value of the Company’s common stock on the date the option is granted, as determined by the Board. The options generally vest annually over five years using the straight-line method, unless otherwise provided, and expire ten years from the date of grant. The Board approved increases in the number of shares available for grant under the 2004 Plan to 4,500,000 shares on February 6, 2006, 6,000,000 shares on November 1, 2006 and 7,500,000 shares on May 24, 2007. The Company may terminate the 2004 Plan at any time. If not terminated sooner, the 2004 Plan will automatically terminate on April 29, 2014.
On August 7, 2007, in connection with the Company’s initial public offering, the 2007 Stock Incentive Plan, or the 2007 Plan, became effective. Under the 2007 Plan, 3,000,000 shares of common stock were initially reserved for future issuance, plus shares available under the prior year equity incentive plans, including shares that become available under the 2007 Plan due to forfeitures at prices not less than the fair market value of the Company’s common stock on the date the option is granted. The options generally vest annually over five years using the straight-line method, unless otherwise provided, and expire ten years from the date of grant. Options forfeited under the 2004 or 2007 Plans are automatically added to the share reserve of the 2007 Plan. Pursuant to the “evergreen” provision contained in the 2007 Plan, an additional 1,719,796 and 1,736,293 shares of common stock were added to the share reserve of the 2007 Plan on January 4, 2009 and January 3, 2010, respectively. These amounts represented 3% of the Company’s total shares outstanding as of the end of the prior fiscal years. The Company may terminate the 2007 Plan at any time. If not terminated sooner, the 2007 Plan will automatically terminate on August 7, 2017.
The number and weighted average exercise price of options issued and outstanding under all stock option plans are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 1, 2011 | | | Year ended
January 2, 2010 | | | Year ended
January 3, 2009 | |
| | | Shares | | | Average
Exercise
Price | | | Shares | | | Average
Exercise
Price | | | Shares | | | Average
Exercise
Price | |
Options outstanding, beginning of period | | | 8,125,871 | | | $ | 18.21 | | | | 7,329,474 | | | $ | 15.97 | | | | 8,321,191 | | | $ | 7.53 | |
Granted | | | 999,850 | | | $ | 26.20 | | | | 1,675,450 | | | $ | 25.30 | | | | 2,493,000 | | | $ | 32.05 | |
Canceled | | | (568,020 | ) | | $ | 27.93 | | | | (326,130 | ) | | $ | 27.24 | | | | (850,422 | ) | | $ | 18.53 | |
Expired | | | (30,000 | ) | | $ | 1.83 | | | | (3,000 | ) | | $ | 1.33 | | | | — | | | $ | — | |
Exercised | | | (1,586,519 | ) | | $ | 28.58 | | | | (549,923 | ) | | $ | 4.68 | | | | (2,634,295 | ) | | $ | 3.70 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Options outstanding, end of period | | | 6,941,182 | | | $ | 21.32 | | | | 8,125,871 | | | $ | 18.21 | | | | 7,329,474 | | | $ | 15.97 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Options exercisable, end of period | | | 3,146,602 | | | $ | 15.99 | | | | 3,491,917 | | | $ | 10.46 | | | | 2,760,046 | | | $ | 6.18 | |
Options available for grant, end of period | | | 5,458,438 | | | | | | | | 4,123,974 | | | | | | | | 834,234 | | | | | |
At January 1, 2011, an aggregate of 12,399,620 shares of stock were reserved for future issuance under the plans.
The Black-Scholes option pricing model is used to estimate the fair value of options granted under the Company’s share-based compensation plans. The range of assumptions used and the resulting weighted-average fair value of options granted at the date of grant were as follows:
| | | | | | |
| | | Year ended January 1, 2011 | | Year ended January 2, 2010 | | Year ended January 3, 2009 |
Risk-free interest rate | | 1.2% to 2.9% | | 1.2% to 3.1% | | 1.1% to 3.8% |
Expected term | | 5.3 years to 5.5 years | | 5.3 years to 5.5 years | | 5.3 years to 6.5 years |
Estimated volatility | | 37.4% to 41.4% | | 40.7% to 55.1% | | 36.6% to 53.4% |
Expected dividends | | 0% | | 0% | | 0% |
Weighted-average fair value of options granted | | $10.45 per share | | $11.66 per share | | $13.70 per share |
The risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues with a remaining term approximately equal to the expected life of the Company’s stock options.
F-24
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
During the nine months ended September 27, 2008, the Company did not have sufficient information available which is indicative of future exercise and post-vesting behavior to estimate the expected term. The Company adopted the simplified method of estimating the expected term of a stock option, as permitted by authoritative accounting guidance. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term. The use of the simplified method requires the Company’s option plan to be consistent with a “plain vanilla” plan. Subsequent to September 27, 2008, the Company had sufficient Company specific and available external information to estimate its expected term and therefore did not rely on the simplified method. As the Company obtains more historical data as a publicly traded company, it expects to rely increasingly on Company specific information for its estimate of expected term.
Additionally, during the nine months ended September 27, 2008, the Company did not have sufficient information available regarding the historic volatility for its shares. As a result, the Company estimated volatility based on a peer group of companies, which collectively provided a reasonable basis for estimating volatility. Subsequent to September 27, 2008, and after the Company had been publicly traded for more than one year, it was able to use Company specific as well as peer group information to estimate the volatility of its shares. As the Company obtains more historical data as a publicly traded company, it expects to rely increasingly on Company specific information for its estimate of volatility. The changes in the method of estimating the expected term and the volatility did not have a material impact on the results of operations.
The Company’s Board of Directors may from time to time declare, and the Company may pay, dividends on its outstanding shares in the manner and upon the terms and conditions provided by law. Any determination to declare and pay dividends will be made by the Company’s Board of Directors and will depend upon the Company’s results of operations, earnings, capital requirements, financial condition, business prospects, contractual restrictions and other factors deemed relevant by the Board of Directors. In the event a dividend is declared, there is no assurance with respect to the amount, timing or frequency of any such dividends. The dividends declared in 2010 were deemed to be special dividends and there is no assurance that they will be declared again during the expected term. Based on this uncertainty and unknown frequency, for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, no dividend rate was used in the assumptions to calculate the share-based compensation expense.
The Company is required to develop an estimate of the number of stock options that will be forfeited due to employee turnover. Adjustments in the estimated forfeiture rates can have a significant effect on its reported share-based compensation, as it recognizes the cumulative effect of the rate adjustments for all expense amortization in the period the estimated forfeiture rates were adjusted. The Company estimates and adjusts forfeiture rates based on a periodic review of recent forfeiture activity and expected future employee turnover. Adjustments in the estimated forfeiture rates could also cause changes in the amount of expense that it recognizes in future periods.
As of January 1, 2011, there was $42.8 million of total unrecognized share-based compensation expense related to unvested options granted or modified on or after January 1, 2006. That expense is expected to be recognized over a weighted average period of 3.0 years as of January 1, 2011. The Company has elected to recognize share-based compensation expense on a straight-line basis over the requisite service period for the entire award. The total fair market value on the respective vesting dates of all options vesting during 2010 and 2009, aggregated $27.6 million and $25.8 million, respectively. The aggregate intrinsic value of options outstanding, with an exercise price less than the closing price of the Company’s common stock, as of January 1, 2011 was $59.4 million. The aggregate intrinsic value of options exercisable, with an exercise price less than the closing price of the Company’s common stock, as of January 1, 2011 was $43.9 million. The aggregate intrinsic value of options exercised during 2010, 2009 and 2008 was $35.1 million, $12.2 million and $75.0 million, respectively. The aggregate intrinsic value is calculated as the difference between the market value of the Company’s common stock on the date of exercise or the respective period end, as appropriate, and the exercise price of the options. The weighted average remaining contractual term of options outstanding with an exercise price less than the closing price of the Company’s common stock, as of January 1, 2011 was 6.8 years. The weighted average remaining contractual term of options exercisable with an exercise price less than the closing price of the Company’s common stock, as of January 1, 2011 was 5.2 years. The total income tax benefit recognized in the consolidated statements of income for share-based compensation expense was $4.4 million, $3.8 million and $2.7 million for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, respectively.
The following table presents the total share-based compensation expense that is included in each functional line item of the consolidated statements of income (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Cost of goods sold | | $ | 483 | | | $ | 413 | | | $ | 257 | |
Selling, general and administrative | | | 9,033 | | | | 7,720 | | | | 5,223 | |
Research and development | | | 2,787 | | | | 2,541 | | | | 2,236 | |
| | | | | | | | | | | | |
Total | | $ | 12,303 | | | $ | 10,674 | | | $ | 7,716 | |
| | | | | | | | | | | | |
F-25
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
The schedule below reflects the number and weighted average exercise price of outstanding and exercisable options segregated by exercise price ranges:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | January 1, 2011 | | | January 2, 2010 | |
| | | Options Outstanding | | | Options
Exercisable | | | Options Outstanding | | | Options
Exercisable | |
Range of Exercise Prices | | Number of
Options | | | Average
Remaining
Contractual
Life | | | Number of
Options | | | Number of
Options | | | Average
Remaining
Contractual
Life | | | Number of
Options | |
$1.83 to $4.00 | | | 853,624 | | | | 3.09 | | | | 853,624 | | | | 1,907,197 | | | | 3.58 | | | | 1,720,123 | |
$4.67 to $12.00 | | | 953,155 | | | | 5.39 | | | | 803,365 | | �� | | 1,293,396 | | | | 6.37 | | | | 843,816 | |
$12.87 to $16.00 | | | 687,883 | | | | 6.37 | | | | 315,823 | | | | 940,488 | | | | 7.37 | | | | 354,348 | |
$21.18 to $28.99 | | | 2,751,570 | | | | 8.46 | | | | 465,110 | | | | 2,078,700 | | | | 9.08 | | | | 128,470 | |
$29.07 to $31.99 | | | 1,326,850 | | | | 7.22 | | | | 511,520 | | | | 1,344,950 | | | | 8.17 | | | | 265,860 | |
$32.09 to $38.30 | | | 110,100 | | | | 7.54 | | | | 45,280 | | | | 191,400 | | | | 8.51 | | | | 55,080 | |
$38.60 to $41.51 | | | 258,000 | | | | 7.47 | | | | 151,880 | | | | 369,740 | | | | 8.52 | | | | 124,220 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 6,941,182 | | | | 6.88 | | | | 3,146,602 | | | | 8,125,871 | | | | 6.97 | | | | 3,491,917 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
12. Commitments and Contingencies
Leases
The Company leases its facilities in North America, Europe and Asia under operating lease agreements expiring at various dates through September 2014. Certain facilities leases contain predetermined price escalations and in some cases renewal options. The Company recognizes the lease costs using a straight line method based on total lease payments. The Company also received certain leasehold improvement incentives totaling $650,000 for its headquarters facilities in the U.S. These leasehold improvement incentives have been recorded as deferred rent and are being amortized as a reduction to rent expense on a straight-line basis over the life of the lease. As of January 1, 2011 and January 2, 2010, rent expense accrued in excess of the amount paid aggregated $193,000 and $100,000, respectively, and is classified in other liabilities in the accompanying consolidated balance sheets. The Company also leases automobiles in Europe that are classified as operating leases and expire at various dates through April 2014. The majority of these leases are non-cancelable. The Company also has capital leases outstanding for office equipment all of which are non-cancelable.
Future minimum lease payments under operating and capital leases for each of the following fiscal years ending on or about December 31 are (in thousands):
| | | | | | | | | | | | |
| | | As of January 1, 2011 | |
Fiscal year | | Operating
Leases | | | Capital
Leases | | | Total | |
2011 | | $ | 3,223 | | | $ | 58 | | | $ | 3,281 | |
2012 | | | 2,540 | | | | 53 | | | | 2,593 | |
2013 | | | 2,362 | | | | 49 | | | | 2,411 | |
2014 | | | 1,506 | | | | 20 | | | | 1,526 | |
2015 | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total | | $ | 9,631 | | | $ | 180 | | | $ | 9,811 | |
| | | | | | | | | | | | |
Rental expense related to operating leases for the years ended January 1, 2011, January 2, 2010 and January 3, 2009 was $3.6 million, $3.2 million and $3.3 million, respectively. Included in the future minimum capital lease payments as of January 1, 2011 is interest aggregating $8,000.
F-26
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
Employee Retirement Savings Plan
In fiscal year 1996, the Company adopted the Masimo Retirement Savings Plan, or the Plan, which is a 401(k) plan covering all of the Company’s full-time U.S. employees who meet certain eligibility requirements. In general, the Company matches 100% of an employee’s contribution up to 3% of the employee’s compensation, subject to a maximum amount. The Company may also contribute to the Plan on a discretionary basis. The Company contributed $1.2 million, $1.1 million and $1.0 million to the plan for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, respectively, all in the form of matching contributions.
Employment and Severance Agreement
As of January 1, 2011, the Company had an employment agreement with one of its key employees that provides for an aggregate annual base salary of $707,000 plus other benefits, with annual increases at the discretion of the Compensation Committee of the Board. The agreement with the Company, which was restated effective July 14, 2009, also provides for an annual bonus based on the Company’s attainment of certain objectives and goals. The agreement has an initial term of three years, with automatic daily renewal, unless either the Company or the executive notifies the other party of non-renewal of the agreement. Also, under this employment agreement, the key employee may be entitled to receive certain salary, equity, tax, medical and life insurance benefits if he is terminated by the Company, if he terminates his employment for good reason under certain circumstances or if there is a change in control of the Company.
As of January 1, 2011, the Company had severance plan participation agreements with three of its executive officers. The participation agreements, or Agreements, are governed by the terms and conditions of the Company’s 2007 Severance Protection Plan, or Severance Plan, which became effective on July 19, 2007 and was amended effective December 31, 2008. Under the Agreements, each executive officer may be entitled to receive certain salary, equity, medical and life insurance benefits if he is terminated by the Company without cause or terminates his employment for good reason under certain circumstances. The executive officers are also required to give the Company six months advance notice of their resignation under certain circumstances.
As of January 1, 2011, the Company had limited severance plan participation agreements with four of its executive officers. These limited participation agreements, or Limited Agreements, are governed by the terms and conditions of the Severance Plan. Under the Limited Agreements, 50% of the executive officer’s unvested and outstanding stock options will immediately vest if the executive officer is terminated by the Company upon a change in control under certain circumstances. The executive officers are also required to give the Company six months advance notice of their resignation under certain circumstances.
Purchase Commitments
Pursuant to contractual obligations with vendors, the Company had $59.4 million of purchase commitments as of January 1, 2011, which is expected to be purchased within one year. These purchase commitments were made for certain inventory items to secure better pricing and to ensure the Company will have raw materials when necessary.
Concentrations of Risk
The Company is exposed to credit loss for the amount of cash deposits with financial institutions in excess of federally insured limits. As of January 1, 2011, the Company had $18.1 million of bank balances of which $12.0 million was covered by the Federal Deposit Insurance Corporation limit. The Company invests its excess cash deposits in U.S. Treasury bills and money market accounts with major financial institutions. As of January 1, 2011, the Company had $58.0 million in U.S. Treasury bills which are guaranteed by the U.S. federal government and $12.2 million in money market funds that are not guaranteed by the U.S. federal government.
While the Company and its contract manufacturers rely on sole source suppliers for certain components, steps have been taken to minimize the impact of a shortage or stoppage of shipments, such as maintaining a safety stock of inventory and designing products that may be easily modified to use a different component. However, there can be no assurance that a shortage or stoppage of shipments of the materials or components that the Company purchases will not result in a delay in production, or adversely affect the Company’s business.
The Company���s ability to sell its products to U.S. hospitals depends in part on its relationships with Group Purchasing Organizations, or GPOs. Many existing and potential customers for the Company’s products become members of GPOs. GPOs negotiate pricing arrangements and contracts, sometimes exclusively, with medical supply manufacturers and distributors, and these negotiated prices are made available to a GPO’s affiliated hospitals and other members. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, revenue from the sale of the Company’s pulse oximetry products to customers affiliated with GPOs amounted to $183.8 million, $160.8 million and $132.1 million, respectively.
F-27
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, sales through one of the Company’s just-in-time distributors represented 14%, 14% and 12%, respectively, of total revenue. For all periods, this particular just-in-time distributor takes and fulfills orders from the Company’s direct customers, many of whom have signed long-term sensor purchase agreements with the Company. In the event this distributor is unable to fulfill these orders, the orders would be redirected to other distributors or fulfilled directly by the Company.
As of January 1, 2011, one just-in-time distributor represented 5% of the accounts receivable balance and as of January 2, 2010, one just-in-time distributor and one OEM partner each represented 6% of the accounts receivable balance.
For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, the Company recorded $49.0 million, $49.0 million and $47.5 million, respectively, in royalty revenues from Covidien pursuant to the settlement agreement. The current royalty agreement provides Covidien with the option to stop paying the royalty on March 14, 2011. In exchange for this royalty payment, the Company has provided Covidien the ability to ship its patent infringing product with a covenant not to sue Covidien as long as they abide by the terms of the agreement. Should Covidien decide to discontinue abiding by the terms of the current settlement agreement, including paying the required royalty payment, then the royalty payments could decline to zero. For additional information on the Covidien settlement agreement, see Note 15 to the consolidated financial statements.
Litigation
On February 3, 2009, the Company filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH related to Philips FAST pulse oximetry technology and certain Philips patient monitors. The suit was brought in the U.S. District Court for the District of Delaware. Two patents originally asserted in this suit, related to the Company’s measure-through motion technology, were successfully enforced in its previous suit against Nellcor. On June 15, 2009, Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen GmbH answered the Company’s complaint and Philips Electronics North America Corporation filed antitrust and patent infringement counterclaims against the Company as well as counterclaims seeking declaratory judgments of invalidity on the patents asserted by the Company against Philips. On July 9, 2009, the Company filed its answer denying Philips’ counterclaims and asserting various defenses. The Company also asserted counterclaims against Philips for fraud, intentional interference with prospective economic advantage and for declaratory judgments of noninfringement and invalidity with respect to the patents asserted by Philips against the Company. Philips later added a claim for infringement of one additional patent. Subsequently, the Court bifurcated Philips’ antitrust claims and its patent misuse defense, as well as stayed the discovery phase on those claims pending trial in the patent case. On October 4, 2010, the Court limited the number of patents to be construed to four for the Company and three for Philips. Further, on October 6, 2010, the Court denied Philips’ motion to bifurcate and stay damages in the patent case. In December 2010, the Court held a hearing regarding the construction of the patent claims and the parties are awaiting the resulting order from the Court. The Company believes that it has good and substantial defenses to the antitrust and patent infringement claims asserted by Philips. There is no guarantee that the Company will prevail in this suit or receive any damages or other relief if it does prevail.
From time to time, the Company may be involved in other litigation relating to claims arising out of its operations in the normal course of business. The Company believes that it currently is not a party to any other legal proceedings which, individually or in the aggregate, would have a material adverse effect on its consolidated financial position, results of operations, or cash flows.
Voluntary Recall
On December 21, 2010, the Company initiated a voluntary recall of its Pronto-7 rainbow® 4D spot-check reusable sensors, which are currently in limited market release. Together with Pronto-7 Spot-Check Pulse CO-Oximeters, the sensors allow users to noninvasively measure hemoglobin, or SpHb, simply and quickly, thereby improving clinical decision making in physician offices and emergency departments. The Company has recently become aware that the thermistor on the sensor was not working as designed. As a result, the Pronto-7 Spot-Check Pulse CO-Oximeter may provide a SpHb measurement outside of its specification range in certain settings. Having identified this issue, the Company is working quickly and diligently to develop a replacement Pronto-7 rainbow® 4D sensor that will address the differences. The Company plans to have the revised Pronto-7 rainbow® 4D sensors available in the second quarter of 2011. As a result of this voluntary recall, the impact to the Company’s operating income in 2010 was a net reduction of $105,000. This is an estimate and the actual amount may differ. No other products, including the actual Pronto-7 handheld device, are affected by this voluntary recall.
F-28
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
13. Segment Information and Enterprise Reporting
The Company’s chief decision maker, the Chief Executive Officer, reviews financial information presented on a consolidated basis, accompanied by disaggregated information about revenues by geographic region for purposes of making operating decisions and assessing financial performance. Accordingly, the Company considers itself to be in a single reporting segment, specifically noninvasive patient monitoring solutions and related products. The Company does not assess the performance of its geographic regions on other measures of income or expense, such as depreciation and amortization, operating income or net income including noncontrolling interests. In addition, the Company’s assets are primarily located in the U.S. The Company does not produce reports for, or measure the performance of, its geographic regions on any asset-based metrics. Therefore, geographic information is presented only for revenues.
The following schedule presents an analysis of the Company’s product revenue based upon the geographic area to which the product was shipped (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended
January 1, 2011 | | | Year ended
January 2, 2010 | | | Year ended
January 3, 2009 | |
Geographic Area by Destination | | | | | | | | | | | | | | | | | | | | | | | | |
North and South America | | $ | 268,696 | | | | 75 | % | | $ | 229,940 | | | | 77 | % | | $ | 200,491 | | | | 77 | % |
Europe, Middle East and Africa | | | 49,698 | | | | 14 | | | | 41,459 | | | | 14 | | | | 37,684 | | | | 15 | |
Asia and Australia | | | 38,028 | | | | 11 | | | | 28,744 | | | | 9 | | | | 21,417 | | | | 8 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total product revenue | | $ | 356,422 | | | | 100 | % | | $ | 300,143 | | | | 100 | % | | $ | 259,592 | | | | 100 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
United States | | $ | 256,966 | | | | | | | $ | 222,708 | | | | | | | $ | 194,382 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
14. Income Taxes
The components of income before provision for income taxes are as follows (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
United States | | $ | 90,753 | | | $ | 76,344 | | | $ | 104,156 | |
Foreign | | | 16,816 | | | | 5,836 | | | | (31,765 | ) |
| | | | | | | | | | | | |
Total | | $ | 107,569 | | | $ | 82,180 | | | $ | 72,391 | |
| | | | | | | | | | | | |
The following table presents the current and deferred provision (benefit) for income taxes (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Current: | | | | | | | | | | | | |
Federal | | $ | 32,322 | | | $ | 26,952 | | | $ | 36,011 | |
State | | | 2,962 | | | | 4,081 | | | | 3,699 | |
Foreign | | | 1,111 | | | | 691 | | | | 307 | |
| | | | | | | | | | | | |
| | | 36,395 | | | | 31,724 | | | | 40,017 | |
| | | | | | | | | | | | |
Deferred: | | | | | | | | | | | | |
Federal | | | (3,102 | ) | | | (2,208 | ) | | | 2,176 | |
State | | | 1,358 | | | | (1,251 | ) | | | (1,447 | ) |
Foreign | | | (487 | ) | | | (107 | ) | | | (282 | ) |
| | | | | | | | | | | | |
| | | (2,231 | ) | | | (3,566 | ) | | | 447 | |
| | | | | | | | | | | | |
Total | | $ | 34,164 | | | $ | 28,158 | | | $ | 40,464 | |
| | | | | | | | | | | | |
Included in the 2009 and 2008 current tax provisions above are an increase of $872,000 and $4.1 million, respectively, for tax and accrued interest related to uncertain tax positions for each year. Also, included in the 2010 current tax provision is a net decrease of $450,000 for tax and accrued interest related to uncertain tax positions.
F-29
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
The temporary differences that give rise to the deferred tax provision (benefit) consist of (in thousands):
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Property and equipment | | $ | 809 | | | $ | 846 | | | $ | (358 | ) |
Capitalized research and development costs | | | (60 | ) | | | 95 | | | | 754 | |
Tax credits | | | 670 | | | | (781 | ) | | | (1,603 | ) |
Deferred revenue | | | (273 | ) | | | 926 | | | | 4,818 | |
Acquired intangibles | | | 57 | | | | 119 | | | | 88 | |
Net operating losses | | | 765 | | | | 343 | | | | 2,437 | |
Accrued liabilities | | | (539 | ) | | | (867 | ) | | | (1,686 | ) |
Share-based compensation | | | (3,227 | ) | | | (3,467 | ) | | | (2,033 | ) |
State taxes and other | | | 83 | | | | (165 | ) | | | 666 | |
Change in valuation allowance | | | (516 | ) | | | (615 | ) | | | (2,636 | ) |
| | | | | | | | | | | | |
Total | | $ | (2,231 | ) | | $ | (3,566 | ) | | $ | 447 | |
| | | | | | | | | | | | |
The reconciliation of the U.S. federal statutory tax rate to the Company’s effective tax rate is as follows:
| | | | | | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | | | Year ended
January 3,
2009 | |
Statutory regular federal income tax rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
State provision, net of federal benefit | | | 2.6 | | | | 2.2 | | | | 2.0 | |
Nondeductible items | | | 0.2 | | | | 0.3 | | | | 0.8 | |
Foreign tax rate differential | | | (4.9 | ) | | | (1.7 | ) | | | 0.2 | |
Prepaid licensing | | | — | | | | — | | | | 19.6 | |
Tax credits | | | (1.6 | ) | | | (1.4 | ) | | | (2.2 | ) |
Other | | | 0.5 | | | | (0.1 | ) | | | 0.5 | |
| | | | | | | | | | | | |
Total | | | 31.8 | % | | | 34.3 | % | | | 55.9 | % |
| | | | | | | | | | | | |
The provision for income taxes was $34.2 million, or an effective rate of 31.8%, during the year ended January 1, 2011 compared to $28.2 million, or an effective rate of 34.3%, during the year ended January 2, 2010. The effective tax rate differs from the statutory U.S. federal income tax rate of 35.0% primarily due to state taxes, permanent differences between financial pre-tax income and taxable income, research related tax credits, the recognition and derecognition of tax benefits related to uncertain tax positions and the mix of income across the jurisdictions in which the Company does business. The effective rate in 2010 differs from 2009 due primarily to the change in geographic composition of pre-tax income in jurisdictions in which the Company does business and the decrease in uncertain tax liabilities as a result of an expiring statute of limitations.
The components of the deferred tax assets are as follows (in thousands):
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
Deferred tax assets: | | | | | | | | |
Property and equipment | | $ | — | | | $ | 565 | |
Tax credits | | | 2,877 | | | | 3,769 | |
Deferred revenue | | | 3,505 | | | | 3,231 | |
Acquired intangibles | | | 799 | | | | 857 | |
Net operating losses | | | 1,034 | | | | 1,870 | |
Accrued liabilities | | | 7,751 | | | | 7,195 | |
Share-based compensation | | | 10,428 | | | | 7,201 | |
Other | | | 918 | | | | 965 | |
| | | | | | | | |
Total | | | 27,312 | | | | 25,653 | |
Valuation allowance | | | (1,410 | ) | | | (1,998 | ) |
| | | | | | | | |
Total deferred tax assets | | | 25,902 | | | | 23,655 | |
Property and equipment | | | (242 | ) | | | — | |
F-30
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
| | | | | | | | |
| | | January 1,
2011 | | | January 2,
2010 | |
State taxes and other | | | (545 | ) | | | (570 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | 25,115 | | | $ | 23,085 | |
| | | | | | | | |
Current net deferred tax asset | | | 12,555 | | | | 11,585 | |
Long-term net deferred tax asset | | | 12,560 | | | | 11,500 | |
| | | | | | | | |
Net deferred tax assets | | $ | 25,115 | | | $ | 23,085 | |
| | | | | | | | |
At January 1, 2011, the Company has $21.0 million of net operating loss carryforwards from its operations in Switzerland, which will begin to expire in 2016. The Company also has $3.5 million, or $124,000 after tax effect, of net operating losses from various states, which will begin to expire in 2012, all of which will be recorded in equity when realized. The Company has state research and development credits of $1.7 million which will carryforward indefinitely. Additionally, the Company has $430,000 of investment tax credit on research and development expenditures from its operations in Canada which will begin to expire in 2018. The Company believes that it is more likely than not that the deferred tax assets related to net operating loss carryforwards will not be realized. In making this determination, the Company considers all available positive and negative evidence, including scheduled reversals of liabilities, projected future taxable income, tax planning strategies and recent financial performances. A valuation allowance has been provided on such loss carryforwards.
During the years ended January 1, 2011, January 2, 2010 and January 3, 2009, the Company recorded a tax benefit of $5.6 million, $3.0 million and $19.1 million, respectively, from the exercise of non-qualified stock options and incentive stock options as a reduction of its income tax liability and an increase in equity. The tax benefit results from the difference between the fair value of the Company’s stock on the exercise dates and the exercise price of the option.
The Company has not provided for income taxes on undistributed earnings of foreign subsidiaries as such earnings are intended to be permanently reinvested in those operations. As of January 1, 2011, the Company’s foreign subsidiaries have cumulative losses which are offset by valuation allowances.
During the years ended January 1, 2011, January 2, 2010 and January 3, 2009, the Company included in its consolidated income tax provision, a current income tax provision of $258,000, $244,000 and $163,000, respectively, related to Cercacor. Also, for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, the Company included in its consolidated income tax provision, a deferred income tax provision of $543,000 and $125,000, and a deferred income tax benefit of $182,000, respectively, related to Cercacor. The temporary differences that give rise to the deferred tax provisions and benefits are mainly research and development credits, share-based compensation expense and deferred revenue for the years ended January 1, 2011, January 2, 2010 and January 3, 2009, respectively.
Cercacor’s deferred tax asset balance as of January 1, 2011 and January 2, 2010 was $1.1 million and $1.6 million, respectively, which consists of deferred revenue, fixed assets and intangibles, share-based compensation and research and experimentation credit carryforwards, which begin to expire in 2028. Management of Cercacor believes that it is more likely than not that part of deferred tax assets related to research and experimentation credit will not be realized. In making this determination, Cercacor considers all available positive and negative evidence, including scheduled reversals of liabilities, projected future taxable income, tax planning strategies and recent financial performances. For both of the years ended January 1, 2011 and January 2, 2010, a valuation allowance of $400,000, has been provided on such credit carryforwards.
Pursuant to authoritative guidance, the Company applies an accounting standard which prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits (in thousands):
| | | | | | | | |
| | | Year ended
January 1,
2011 | | | Year ended
January 2,
2010 | |
Unrecognized tax benefits, beginning of period | | $ | 8,094 | | | $ | 7,298 | |
Increase from tax positions in prior period | | | 94 | | | | 3 | |
Decrease from tax positions in prior period | | | — | | | | (27 | ) |
Increase from tax positions in current period | | | 1,129 | | | | 850 | |
Settlements | | | (143 | ) | | | — | |
Lapse of statute of limitations | | | (1,672 | ) | | | (30 | ) |
| | | | | | | | |
Unrecognized tax benefits, end of period | | $ | 7,502 | | | $ | 8,094 | |
| | | | | | | | |
F-31
MASIMO CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – (continued)
The amount of unrecognized benefits which, if ultimately recognized, could favorably affect the tax rate in a future period was $6.5 million and $6.9 million as of January 1, 2011 and January 2, 2010, respectively. Both amounts are net of any federal and/or state benefits. It is reasonably possible that the amount of unrecognized tax benefits in various jurisdictions may change in the next 12 months due to the expiration of statutes of limitation and audit settlements. However, due to the uncertainty surrounding the timing of these events, an estimate of the change within the next 12 months cannot be made currently.
Interest and penalties related to unrecognized tax benefits are recognized in income tax expense. For the years ended January 1, 2011, January 2, 2010 and January 3, 2009, the Company had accrued $600,000, $669,000 and $446,000, respectively, for the payment of interest.
The Company conducts business in multiple jurisdictions, and as a result, one or more of the Company’s subsidiaries files income tax returns in the U.S. federal, various state, local and foreign jurisdictions. The Company has concluded on all U.S. federal income tax matters for years through 2007. All material state, local and foreign income tax matters have been concluded for years through 2003.
15. Subsequent Event
On January 28, 2011, Masimo Corporation, Masimo Laboratories, Inc., Nellcor Puritan Bennett LLC, Mallinckrodt Inc., Tyco Healthcare Group LP and Covidien Inc. entered into the Second Amendment to the Settlement Agreement and Release of Claims, dated January 17, 2006, as amended by the Amendment to Settlement Agreement and Release of Claims, dated January 24, 2006. As part of this amendment, which will become effective as of March 14, 2011, Covidien has agreed to pay the Company a royalty of 7.75% of its United States pulse oximetry revenue, as that term is defined in the January 28, 2011 second amendment, at least through March 15, 2014.
16. Quarterly Financial Data (unaudited)
The following tables contain selected unaudited consolidated statements of income data for each quarter of 2010 and 2009 (in thousands, except per share data):
| | | | | | | | | | | | | | | | |
| | | Quarter ended | |
Fiscal 2010 | | April 3,
2010 | | | July 3,
2010 | | | October 2,
2010 | | | January 1,
2011 | |
Total revenue | | $ | 98,765 | | | $ | 100,080 | | | $ | 100,988 | | | $ | 105,574 | |
Gross profit | | | 69,537 | | | | 70,305 | | | | 71,612 | | | | 74,128 | |
Operating income | | | 40,784 | | | | 21,010 | | | | 22,838 | | | | 21,589 | |
Net income attributable to Masimo Corporation | | | 26,710 | | | | 14,287 | | | | 16,419 | | | | 16,114 | |
Net income per share attributable to Masimo Corporation Stockholders: | | | | | | | | | | | | | | | | |
Basic | | $ | 0.46 | | | $ | 0.24 | | | $ | 0.28 | | | $ | 0.27 | |
Diluted | | $ | 0.44 | | | $ | 0.24 | | | $ | 0.27 | | | $ | 0.26 | |
| |
| | | Quarter ended | |
Fiscal 2009 | | April 4,
2009 | | | July 4,
2009 | | | October 3,
2009 | | | January 2,
2010 | |
Total revenue | | $ | 85,492 | | | $ | 83,569 | | | $ | 87,441 | | | $ | 92,613 | |
Gross profit | | | 60,747 | | | | 59,995 | | | | 62,243 | | | | 65,817 | |
Operating income | | | 20,086 | | | | 19,948 | | | | 20,840 | | | | 21,352 | |
Net income attributable to Masimo Corporation | | | 13,021 | | | | 13,092 | | | | 13,055 | | | | 14,060 | |
Net income per share attributable to Masimo Corporation Stockholders: | | | | | | | | | | | | | | | | |
Basic(1) | | $ | 0.23 | | | $ | 0.23 | | | $ | 0.23 | | | $ | 0.24 | |
Diluted(1) | | $ | 0.22 | | | $ | 0.22 | | | $ | 0.22 | | | $ | 0.23 | |
| (1) | The sum of the quarterly basic and diluted net income per share amounts for the year ended January 2, 2010 does not equal the annual related per share amounts due to differences in the weighted average shares outstanding between the quarterly and annual computations. |
F-32
