UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
QUARTERLY REPORT PURSUANT TO SECTION 13 or 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
| | | |
| For Quarterly Period Ended | | Commission File No. |
| September 30, 2005 | | 0-26770 |
NOVAVAX, INC.
(Exact name of registrant as specified in its charter)
| | | |
| Delaware | | 22-2816046 |
| (State or other jurisdiction of | | (I.R.S. Employer |
| incorporation or organization) | | Identification No.) |
| | | |
| 508 Lapp Road, Malvern, PA | | 19355 |
| (Address of principal executive offices) | | (Zip code) |
(484) 913-1200
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
þ Yeso No
Indicate by check mark whether the registrant is an accelerated filer (as defined in Rule 12b-2 of the Exchange Act).
þ Yeso No
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yesþ No
The number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date:
Shares of Common Stock Outstanding at November 4, 2005: 49,110,558
================================================================================
NOVAVAX, INC.
Form 10-Q
For the Quarter Ended September 30, 2005
Table of Contents
| | | | | |
| | | Page No. | |
Part I. Financial Information | | | | |
| | | | | |
| Item 1 Financial Statements | | | | |
| | | | | |
| Consolidated Balance Sheets as of September 30, 2005 and December 31, 2004 | | | 3 | |
| | | | | |
| Consolidated Statements of Operations for the three-month and nine-month periods ended September 30, 2005 and 2004 | | | 4 | |
| | | | | |
| Consolidated Statements of Cash Flows for the nine months ended September 30, 2005 and 2004 | | | 5 | |
| | | | | |
| Notes to the Consolidated Financial Statements | | | 6 | |
| | | | | |
| Item 2 Management’s Discussion and Analysis of Financial Condition and Results of Operations | | | 17 | |
| | | | | |
| Item 3 Quantitative and Qualitative Disclosures about Market Risk | | | 29 | |
| | | | | |
| Item 4 Controls and Procedures | | | 29 | |
| | | | | |
Part II. Other Information | | | | |
| | | | | |
| Item 1 Legal Proceedings | | | 30 | |
| | | | | |
| Item 2 Unregistered Sales of Equity Securities and Use of Proceeds | | | 30 | |
| | | | | |
| Item 3 Defaults upon Senior Securities | | | 30 | |
| | | | | |
| Item 4 Submission of Matters to a Vote of Security Holders | | | 30 | |
| | | | | |
| Item 5 Other Information | | | 30 | |
| | | | | |
| Item 6 Exhibits | | | 30 | |
| | | | | |
| Signature | | | 31 | |
| | | | | |
| Certifications | | | 32 | |
2
Part I. Financial Information
Item 1. Financial Statements
NOVAVAX, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share information)
| | | | | | | | | |
| | | September 30 | | | December 31, | |
| | | 2005 | | | 2004 | |
| | | (unaudited) | | | | | |
ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 6,944 | | | $ | 17,876 | |
| Trade accounts receivable, net of allowance for doubtful accounts of $387 and $752 as of September 30, 2005 and December 31, 2004, respectively | | | 1,147 | | | | 827 | |
| Inventory | | | 1,651 | | | | 3,464 | |
| Prepaid expenses and other current assets | | | 962 | | | | 1,770 | |
| | | | | | | |
| Total current assets | | | 10,704 | | | | 23,937 | |
| Property and equipment, net | | | 12,116 | | | | 14,147 | |
| Goodwill | | | 33,141 | | | | 33,141 | |
| Other intangible assets, net | | | 3,330 | | | | 5,048 | |
| Other non current assets | | | 1,255 | | | | 1,720 | |
| | | | | | | |
| Total assets | | $ | 60,546 | | | $ | 77,993 | |
| | | | | | | |
| | | | | | | | | |
LIABILITIES and STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 753 | | | $ | 3,242 | |
| Accrued expenses | | | 3,488 | | | | 4,140 | |
| Current portion of capital lease obligations and other liabilities | | | 467 | | | | 1,194 | |
| | | | | | | |
| Total current liabilities | | | 4,708 | | | | 8,576 | |
| | | | | | | |
| | | | | | | | | |
| Convertible notes | | | 35,000 | | | | 35,000 | |
| Deferred rent | | | 190 | | | | 166 | |
| Non-current portion of capital lease obligations and other liabilities | | | 746 | | | | 970 | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $.01 par value, 2,000,000 shares authorized; no shares issued and outstanding | | | –– | | | | –– | |
| Common stock, $.01 par value, 100,000,000 shares authorized; 44,107,724 shares issued and 43,853,876 outstanding at September 30, 2005, and 39,807,724 shares issued and 39,553,876 outstanding at December 31, 2004 | | | 441 | | | | 398 | |
| Additional paid-in capital | | | 171,403 | | | | 167,496 | |
| Notes receivable from directors | | | (1,480 | ) | | | (1,480 | ) |
| Accumulated deficit | | | (148,049 | ) | | | (130,720 | ) |
| Treasury stock 253,848 shares, cost basis | | | (2,413 | ) | | | (2,413 | ) |
| | | | | | | |
| Total stockholders’ equity | | | 19,902 | | | | 33,281 | |
| | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 60,546 | | | $ | 77,993 | |
| | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
3
NOVAVAX, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share information)
(unaudited)
| | | | | | | | | | | | | | | | | |
| | | Three months ended | | | Nine months ended | |
| | | September 30, | | | September 30, | |
| | | 2005 | | | 2004 | | | 2005 | | | 2004 | |
| Revenues: | | | | | | | | | | | | | | | | |
| Net product sales | | $ | 1,300 | | | $ | (664 | ) | | $ | 3,908 | | | $ | 4,190 | |
| Contract research and development | | | 417 | | | | 653 | | | | 1,086 | | | | 1,899 | |
| Milestone and licensing fees | | | 150 | | | | –– | | | | 150 | | | | 125 | |
| | | | | | | | | | | | | |
| Total revenues | | | 1,867 | | | | (11 | ) | | | 5,144 | | | | 6,214 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | |
| Cost of products sold | | | 1,068 | | | | 364 | | | | 5,074 | | | | 2,128 | |
| Research and development | | | 1,161 | | | | 1,552 | | | | 3,759 | | | | 5,828 | |
| Selling and marketing | | | 930 | | | | 8,931 | | | | 6,832 | | | | 17,261 | |
| General and administrative | | | 1,694 | | | | 1,901 | | | | 6,109 | | | | 5,992 | |
| Facility exit costs | | | 107 | | | | 723 | | | | 105 | | | | 723 | |
| Gain on sale of product assets | | | (856 | ) | | | –– | | | | (856 | ) | | | –– | |
| Gain on redemption of debt | | | –– | | | | (11,162 | ) | | | –– | | | | (11,162 | ) |
| | | | | | | | | | | | | |
| Total operating costs and expenses | | | 4,104 | | | | 2,309 | | | | 21,023 | | | | 20,770 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (2,237 | ) | | | (2,320 | ) | | | (15,879 | ) | | | (14,556 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Interest expense, net | | | (490 | ) | | | (337 | ) | | | (1,450 | ) | | | (1,075 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss | | $ | (2,727 | ) | | $ | (2,657 | ) | | $ | (17,329 | ) | | $ | (15,631 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Basic and diluted loss per share | | $ | (.06 | ) | | $ | (.07 | ) | | $ | (.42 | ) | | $ | (.43 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Basic and diluted weighted average number of common shares outstanding | | | 43,469,637 | | | | 38,577,458 | | | | 40,873,473 | | | | 36,040,465 | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
4
NOVAVAX, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| | | | | | | | | |
| | | Nine months ended | |
| | | September 30, | |
| | | 2005 | | | 2004 | |
Operating Activities: | | | | | | | | |
| Net loss | | $ | (17,329 | ) | | $ | (15,631 | ) |
| Reconciliation of net loss to net cash used in operating activities: | | | | | | | | |
| Amortization | | | 636 | | | | 562 | |
| Depreciation | | | 2,092 | | | | 1,602 | |
| Retirement of capital assets | | | 42 | | | | 30 | |
| Provision for bad debts | | | (38 | ) | | | 175 | |
| Amortization of deferred financing costs | | | 309 | | | | 131 | |
| Deferred rent | | | 24 | | | | 12 | |
| Non-cash stock compensation | | | 321 | | | | 25 | |
| Facility exit costs | | | 105 | | | | 723 | |
| Gain on redemption of debt | | | –– | | | | (11,162 | ) |
| Gain on sale of product assets | | | (856 | ) | | | –– | |
| Net proceeds from sale of product assets | | | 2,500 | | | | –– | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Trade accounts receivable | | | (282 | ) | | | 5 | |
| Inventory | | | 1,525 | | | | (1,423 | ) |
| Prepaid expenses and other current assets | | | 808 | | | | (1,328 | ) |
| Accounts payable and accrued expenses and other liabilities | | | (3,540 | ) | | | 5,643 | |
| Deferred revenue | | | –– | | | | (125 | ) |
| Other non current assets | | | 156 | | | | 57 | |
| | | | | | | |
| Net cash used in operating activities | | | (13,527 | ) | | | (20,704 | ) |
| | | | | | | |
| | | | | | | | | |
Investing Activities: | | | | | | | | |
| Capital expenditures | | | (103 | ) | | | (1,427 | ) |
| | | | | | | |
| Net cash used in investing activities | | | (103 | ) | | | (1,427 | ) |
| | | | | | | |
| | | | | | | | | |
Financing Activities: | | | | | | | | |
| Net proceeds from issuance of convertible notes | | | –– | | | | 32,943 | |
| Net payments associated with King transaction | | | –– | | | | (15,010 | ) |
| Principal payments of capital lease obligations | | | (931 | ) | | | (1,013 | ) |
| Net proceeds from sales of common stock | | | 3,629 | | | | 5,081 | |
| | | | | | | |
| Net cash provided by financing activities | | | 2,698 | | | | 22,001 | |
| | | | | | | |
| | | | | | | | | |
| Net change in cash and cash equivalents | | | (10,932 | ) | | | (130 | ) |
| Cash and cash equivalents at beginning of period | | | 17,876 | | | | 27,633 | |
| | | | | | | |
| | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 6,944 | | | $ | 27,503 | |
| | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
5
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. Organization
Novavax, Inc., a Delaware corporation (“Novavax” or “the Company”), was incorporated in 1987, and is a product development company focused on the research, development and commercialization of products utilizing its proprietary drug delivery and biological technologies for large and growing markets. The Company’s drug delivery technologies include the micellar nanoparticle (“MNP”) technology, which is the basis for the development of the Company’s first Food and Drug Administration approved product ESTRASORB®. In addition to MNP, the Company’s drug delivery technologies include Novasomes® (paucillamellar non-phospholipid liposomes) and Sterisomes®. The Company’s vaccine technologies include its virus-like particle (“VLP”) manufacturing utilizing the baculovirus expression system in insect cells as well as novel vaccine adjuvants based on Novasomes and dendrimer technologies.
In October 2005, the Company entered into license and supply agreements for ESTRASORB. Under the agreements, the Company will continue to manufacture ESTRASORB and the licensee, Esprit Pharma, Inc., which was granted an exclusive license, will sell ESTRASORB in North America.
The Company plans to continue leveraging its technologies to develop other new product candidates to be sold or licensed to pharmaceutical companies. While the Company’s main therapeutic areas of concentration have been women’s health and infectious diseases, the Company believes its technologies can be applied more broadly.
The products currently under development or in clinical trials by the Company will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval, prior to commercial use. There can be no assurance that the Company’s research and development efforts will be successful or that any potential products will prove to be safe and effective in clinical trials. Even if developed, these products may not receive regulatory approval or be successfully introduced and marketed at prices that would permit the Company to operate profitably. The Company also recognizes that the commercial launch of any product is subject to certain risks including, but not limited to, manufacturing scale-up and market acceptance. No assurance can be given that the Company can generate sufficient product revenue to become profitable or generate positive cash flow from operations on a sustained basis or at all.
The consolidated financial statements of Novavax for the three and nine months ended September 30, 2005 and 2004 are unaudited. These financial statements reflect all adjustments which, in the opinion of management, are necessary for a fair presentation of the results for the interim periods presented. All such adjustments are of a normal recurring nature. These interim results are not necessarily indicative of the results to be expected for the fiscal year ending December 31, 2005.
6
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Certain information in footnote disclosures normally included in financial statements prepared in accordance with generally accepted accounting principles has been condensed or omitted pursuant to the rules and regulations of the Securities and Exchange Commission, although the Company believes the disclosures herein are adequate to make the information presented not misleading. We suggest that these unaudited consolidated financial statements be read in conjunction with the audited consolidated financial statements and the notes thereto in the Company’s Annual Report on Form 10-K for the year ended December 31, 2004.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant inter-company accounts and transactions have been eliminated in consolidation. Certain amounts appearing in the prior year’s footnote disclosures have been re-classified to conform to the current year’s presentation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Inventories
Inventories consist of raw materials, work-in-process and finished goods, are priced at the lower of cost or market using the first-in-first-out method, and were as follows:
| | | | | | | | | |
| | | September 30, 2005 | | | December 31, 2004 | |
| | | (unaudited) | | | | | |
| | | (amounts in thousands) | |
| Raw materials | | $ | 213 | | | $ | 351 | |
| Work-in-process | | | 429 | | | | 700 | |
| Finished goods | | | 1,009 | | | | 2,413 | |
| | | | | | | |
| | | $ | 1,651 | | | $ | 3,464 | |
| | | | | | | |
7
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As part of the Pharmelle transaction (SeeAsset Purchase Agreement with Pharmelle, LLC), the Company sold $147,000 of finished goods and $200,000 of raw materials and eliminated $58,000 of inventory reserves in September 2005.
As part of the ESTRASORB transaction (SeeSubsequent Events), the Company sold $437,000 of finished goods in October 2005.
Revenue Recognition
The Company recognizes revenue in accordance with the provisions of Staff Accounting Bulletin No. 104,Revenue Recognition. For our product sales, revenue is recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, shipment of product to our distributor has occurred, the seller’s price to the buyer is fixed or determinable, and collectibility is reasonably assured. The Company recognizes these sales net of allowances for returns, rebates and chargebacks. A large part of our product sales are to distributors who resell the products to their customers. The Company provides rebates to members of certain buying groups who purchase from our distributors, to the distributors that sell to these customers at prices determined under contracts between us and the customer and to state agencies that administer various programs such as the federal Medicaid and Medicare programs. Rebate amounts are usually based upon the volume of purchases or by reference to a specific price for a product. The Company estimates the amount of the rebate that will be paid, and records the liability as a reduction of revenue when we record our sale of the products. Settlement of the rebate generally occurs from three to 12 months after sale. The Company regularly analyzes historical rebate trends and makes adjustments to recorded reserves for changes in trends and terms of rebate programs. In a similar manner, we estimate amounts for returns based on historical trends, distributor inventory levels and product prescription data and adjust those reserves as product returns occur. The shipping and handling costs the Company incurs are included in cost of sales in our accompanying statements of operations.
For nonrefundable up-front payment and licensing fees related to products sold for which no further performance obligations exist, the Company recognizes revenue on the earlier of when payments are received or collection is assured. Royalty and milestone payments are recognized as revenue upon achievement of contract-specified events and when there are no remaining performance obligations. Revenue earned under current research contracts is recognized per the contracts’ terms and conditions for invoicing of costs incurred and the achievement of defined milestones.
8
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Net Loss per Share
Basic loss per share is computed by dividing the net loss (the numerator) by the weighted average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period are weighted for the portion of the period that they were outstanding. The computation of diluted loss per share is similar to the computation of basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued (e.g. upon exercise of stock options). Potentially dilutive common shares are not included in the computation of diluted earnings per share if they are anti-dilutive. Net loss per share as reported was not adjusted for potential common shares, as they are anti-dilutive.
Property and Equipment
Property and equipment are recorded at cost. Manufacturing equipment is generally depreciated over seven to ten years. Manufacturing leaseholds are amortized over the remaining lease term of our manufacturing facility, under the straight line method. Depreciation of other fixtures and equipment is also provided under the straight line method over the estimated useful lives of the assets, generally three to seven years. Amortization of other leasehold improvements is provided over the shorter of the estimated useful lives of the improvements or the term of the respective lease. Repairs and maintenance costs are expensed as incurred.
Property and equipment is comprised of the following:
| | | | | | | | | |
| | | September 30, 2005 | | | December 31,2004 | |
| | | (unaudited) | | | | | |
| | | (amounts in thousands) | |
| Machinery and equipment | | $ | 11,477 | | | $ | 11,471 | |
| Leasehold improvements | | | 6,091 | | | | 6,048 | |
| Computer software and hardware | | | 481 | | | | 480 | |
| | | | | | | |
| | | | 18,049 | | | | 17,999 | |
| Less accumulated depreciation | | | (5,933 | ) | | | (3,852 | ) |
| | | | | | | |
| | | $ | 12,116 | | | $ | 14,147 | |
| | | | | | | |
9
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Accounting for Facility Exit Costs
In July 2004, the Company entered into a long-term agreement to lease a 32,900 square foot facility in Malvern, Pennsylvania, for the consolidation and expansion of corporate headquarters and product development activities. The lease, with a commencement date of September 15, 2004, has an initial term of ten years with two five year renewal options. Standard annual escalation rental rates are in effect during the initial lease term. With advance notice, the Company also has an option to lease adjoining space of 17,000 square feet, which could be built out for future manufacturing needs.
The Company applied the principles of SFAS No. 146,Accounting for Costs Associated with Exit or Disposal Activities,in accounting for contract termination costs and associated costs that will continue to be incurred under the operating lease expiring on October 31, 2006 related to the Company’s former corporate offices located in Columbia, Maryland. For the year ended December 31, 2004, $252,000 was included in facility exit costs on the consolidated statement of operations which represents the difference between the fair value of the remaining lease payments, and the current estimated sublease rentals that could be reasonably obtained. During the three month period ended September 30, 2005, the Company revised its estimate of sublease rentals that could reasonably be obtained before the expiration of the lease on October 31, 2006 and recorded an additional $105,000 in facility exit costs on the consolidated statements of operations.
A roll-forward of facility exit cost liability is as follows:
| | | | | | | | | |
| | | Current | | Non-Current |
| | | (in thousands) |
Original amount expensed and set up as a liability | | $ | 151 | | | $ | 101 | |
| Lease payments applied to the liability | | | (28 | ) | | | (24 | ) |
| | | |
Balance as of December 31, 2004 | | | 123 | | | | 77 | |
| Lease payments applied to the liability | | | (1 | ) | | | (124 | ) |
| Adjustment to original estimate | | | 45 | | | | 60 | |
| | | |
Balance as of September 30, 2005 | | $ | 167 | | | $ | 13 | |
| | | |
10
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Goodwill and Intangible Assets
Goodwill principally results from business acquisitions. Assets acquired and liabilities assumed are recorded at their fair values; the excess of the purchase price over the value of the identifiable net assets acquired is recorded as goodwill. Other intangible assets are the result of product acquisitions, non-compete arrangements, and internally-discovered patents. In accordance with SFAS No. 142,Goodwill and Other Intangible Assets(“SFAS No. 142”), goodwill and intangible assets deemed to have indefinite lives are not amortized but are subject to impairment tests annually, or more frequently should indicators of impairment arise. The Company utilizes a discounted cash flow analysis that includes profitability information, estimated future operating results, trends and other information in assessing whether the value of indefinite-lived intangible assets can be recovered. Under SFAS No. 142, goodwill impairment is deemed to exist if the carrying value of a reporting unit exceeds its estimated fair value.
Other intangible assets are amortized on a straight-line basis over their estimated useful lives, ranging from five to 15 years. Amortization expense was $205,000 and $235,000 for the three months ending September 30, 2005 and 2004, respectively, and $636,000 and $562,000 for the nine months ending September 30, 2005 and 2004, respectively.
As part of the Pharmelle transaction (SeeAsset Purchase Agreement with Pharmelle, LLC), $1,082,000 of net intangible assets pertaining to the AVC product acquisition were written off in September 2005.
As of September 30, 2005 and December 31, 2004, the Company’s other intangible assets and related accumulated amortization consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of September 30, 2005 | | | As of December 31, 2004 | |
| | | (unaudited) | | | | | | | | | | | |
| | | | | | | Accumulated | | | | | | | | | | | Accumulated | | | | |
| | | Gross | | | Amortization | | | Net | | | Gross | | | Amortization | | | Net | |
Other intangible assets, net | | | | | | | | | | | | | | | | | | | | | | | | |
| ESTRASORB rights | | $ | 2,514 | | | $ | (327 | ) | | $ | 2,187 | | | $ | 2,514 | | | $ | (136 | ) | | $ | 2,378 | |
| AVC product acquisition | | | –– | | | | –– | | | | –– | | | | 3,332 | | | | (1,904 | ) | | | 1,428 | |
| Patents | | | 2,525 | | | | (1,382 | ) | | | 1,143 | | | | 2,525 | | | | (1,283 | ) | | | 1,242 | |
| | | | | | | | | | | | | | | | | | | |
| | | $ | 5,039 | | | $ | (1,709 | ) | | $ | 3,330 | | | $ | 8,371 | | | $ | (3,323 | ) | | $ | 5,048 | |
| | | | | | | | | | | | | | | | | | | |
As part of the ESTRASORB transaction (SeeSubsequent Events), the net balance at the date of the transaction for ESTRASORB rights, totaling $2,175,000, was written off in October 2005.
11
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Stock-Based Compensation
In December 2004, the Financial Accounting Standards Board issued Statement No. 123 (revised),Share-Based Payment(“SFAS No. 123R”), which is a revision of Statement No. 123,Accounting for Stock-Based Compensation(“SFAS No. 123”). SFAS No. 123R supersedes APB No. 25,Accounting for Stock Issued to Employees(“APB No. 25”), and amends SFAS No. 95,Statement of Cash Flows. Generally, the approach in SFAS No. 123R is similar to the approach described in SFAS No. 123. However, SFAS No. 123R requires that all share-based payments made to employees, including grants of employee stock options, be recognized in the income statement based on their fair values. Pro forma disclosure is no longer an alternative.
SFAS No. 123R must be adopted in the first annual financial reporting period beginning after June 15, 2005. The Company will adopt SFAS No. 123R on January 1, 2006.
SFAS No. 123R permits public companies to adopt its requirements using one of two methods:
| | 1. | | A “modified prospective” method in which compensation cost is recognized beginning with the effective date (a) based on the requirements of SFAS No. 123R for all share-based payments granted after the effective date, and (b) based on the requirements of SFAS No. 123 for all awards granted to employees prior to the effective date of SFAS No. 123R that remain unvested on the effective date. |
| |
| | 2. | | A “modified retrospective” method which includes the requirements of the modified prospective method described above, but also permits entities to restate based on the amounts previously recognized under SFAS No. 123 for purposes of proforma disclosures either for (a) all prior periods presented, or (b) prior interim periods of the year of adoption. |
The Company plans to adopt SFAS No. 123R using the modified prospective method.
As permitted by SFAS No. 123, the Company currently accounts for share-based payments to employees using the intrinsic value method permitted by APB No. 25 and, as such, generally recognizes no compensation cost for employee stock options. Accordingly, the adoption of SFAS No. 123R’s fair value method will have a significant impact on the Company’s results of operations, although it will have no impact on the Company’s overall financial position. The impact of adoption of SFAS No. 123R cannot be predicted at this time because it will depend on levels of share-based payments granted in the future. However, had the Company adopted SFAS No. 123R in prior periods, the impact of that standard would have approximated the impact of SFAS No. 123 as described in the disclosure of pro forma net income and earnings per share below. SFAS No. 123R also requires the benefits of tax deductions in excess of recognized compensation cost to be reported as a financing cash flow, rather than as an operating cash flow as required under current literature.
12
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Stock-Based Compensation (continued):
This requirement will reduce net operating cash flows and increase net financing cash flows in periods after adoption. The Company cannot estimate what those amounts will be in the future because they depend, among other things, on when employees exercise stock options. For the nine months ended September 30, 2005 and 2004, the Company did not pay any taxes, therefore, there was no effect on operating cash flows for such tax deductions.
Had the Company applied the fair value principles of SFAS No. 123 for its employee options, its net loss for the three and nine months ending September 30, 2005 and 2004 would have increased as follows:
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended | | | Nine Months Ended | |
| | | September 30, | | | September 30, | |
| | | 2005 | | | 2004 | | | 2005 | | | 2004 | |
| | | (unaudited) | |
| | | (Amounts in thousands, except per share data) | |
| Net loss, as reported | | $ | (2,727 | ) | | $ | (2,657 | ) | | $ | (17,329 | ) | | $ | (15,631 | ) |
| Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards | | | (930 | ) | | | (1,272 | ) | | | (2,900 | ) | | | (3,911 | ) |
| | | | | | | | | | | | | |
| Pro forma net loss | | $ | (3,657 | ) | | $ | (3,929 | ) | | $ | (20,229 | ) | | $ | (19,542 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss per share: | | | | | | | | | | | | | | | | |
| Basic and diluted — as reported | | $ | (.06 | ) | | $ | (0.07 | ) | | $ | (.42 | ) | | $ | (.43 | ) |
| Basic and diluted — pro forma | | $ | (.08 | ) | | $ | (0.10 | ) | | $ | (.49 | ) | | $ | (.54 | ) |
These pro forma amounts are not necessarily indicative of the future effects of applying the fair value-based method due to, among other things, the vesting period of the stock options and the fair value of additional stock options issued in future years.
During the first nine months of 2005, the Company granted 552,434 restricted shares totaling $576,000 in value at the date of grant to various employees, officers and a board member of the Company, which vest over periods up to three years. In accordance with APB No. 25, for the three and nine months ended September 30, 2005, $89,000 and $104,000, respectively, of non — cash stock compensation expense was included in total operating costs and expenses related to this restricted stock and additional paid-in capital was increased accordingly.
13
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Sales and Issuance of Common Stock
In July 2005, the Company completed an agent-led offering of 4,000,000 shares of common stock at $1.00 per share for gross proceeds of $4,000,000. The stock was issued pursuant to an existing shelf registration statement. Net proceeds after deducting underwriter, legal, accounting and other miscellaneous fees were approximately $3,629,000.
In August 2005, the Company issued 250,000 shares of common stock in a private placement to its former chief executive officer for prior services.
Segment Information
The Company currently operates in one business segment, which is the research, development and commercialization of products utilizing proprietary drug delivery and biological technologies. The Company is managed and operated as one business. A single management team that reports to the Chief Executive Officer comprehensively manages the entire business. The Company does not operate separate lines of business with respect to its products or product candidates. Accordingly, the Company does not have separately reportable segments as defined by FASB Statement No. 131,Disclosure about Segments of an Enterprise and Related Information.
Related Party Transactions
In March 2002, pursuant to the Novavax, Inc. 1995 Stock Option Plan, the Company approved the payment of the exercise price of options by two of its directors, through the delivery of full-recourse, interest-bearing promissory notes in the aggregate amount of $1,479,268. The borrowings accrue interest at 5.07% per annum and are secured by an aggregate of 261,667 shares of common stock owned by the directors. The notes are payable upon the earlier to occur of the following: (i) the date upon which the director ceases for any reason to be a director of the Company, (ii) in whole, or in part, to the extent of net proceeds, upon the date on which the director sells all or any portion of the pledged shares, or (iii) on March 21, 2007. As of September 30, 2005 and December 31, 2004, accrued interest receivable related to the borrowing was $265,000 and $209,000, respectively.
In addition, in April 2002, we executed a conditional guaranty of a brokerage margin account for a director in the amount of $500,000. Prior to demanding payment from the Company, the brokerage firm must first make demand for payment to the director and then liquidate the account. Thereafter, if there remains a shortfall, the brokerage firm may demand payment from the Company. As of September 30, 2005 and December 31, 2004, the Company had not recorded any liability on its balance sheets related to this guarantee as we believe the possibility of required payment by the Company to be unlikely.
14
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Restructuring of the Sales Force
From March through August 2005, the Company implemented measures to reduce costs associated with its commercial operations by downsizing its sales force to correspond with the Company’s strategy of transitioning from a commercial business model to that of one focused on the Company’s core competency of new product development. The August restructuring eliminated the Company’s remaining sales force. The total estimated costs to be incurred in connection with these restructurings were approximately $444,000. Included in these amounts were (i) one-time termination benefits of $305,000, of which $40,000 is still included in accrued expenses as of September 30, 2005, (ii) auto lease contract termination costs of approximately $125,000, of which $92,000 is still included in accrued expenses as of September 30, 2005, and (iii) $14,000 of other associated costs, all of which were paid as of September 30, 2005. Included in the accompanying statements of operations for the three and nine months ended September 30, 2005 are $206,000 and $444,000 related to these two restructurings, respectively.
Opportunity Grant Funds
In July 2005, the Company received $400,000 from the Commonwealth of Pennsylvania for the reimbursement of certain costs incurred with the move of our corporate headquarters and product development activities to Malvern, Pennsylvania. These funds were included as an offset to general and administrative expenses included in the accompanying statements of operations for the three and nine months ended September 30, 2005.
Asset Purchase Agreement with Pharmelle, LLC
In September 2005, the Company entered into an Asset Purchase Agreement with Pharmelle, LLC for the sale of assets related to the AVC, NovaNatal and NovaStart products, as well as assets relating to certain formerly-marketed products Vitelle, Nestabs, Gerimed, Irospan and Nessentials. The assets sold included, but were not limited to, intellectual property, the New Drug Application for AVC products, inventory and sales and promotional materials. In connection with the sale, Pharmelle agreed to assume those liabilities and obligations arising after the closing date of the transaction in connection with the performance by Pharmelle of certain assumed contracts, those liabilities and obligations arising after the closing date in connection with products sold by Pharmelle after the closing date or the operation of the business relating to such products or the assets after such date (including any product liability claims associated with such products), and all liability and responsibility for returns of the products made after the closing date, regardless of when such products were produced, manufactured or sold.
15
NOVAVAX, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
In consideration for the sale of these assets, Pharmelle paid the Company $2,500,000 in cash and assumed the liabilities noted above. In addition, the Company is entitled to royalties on AVC for a five year period if net sales exceed certain levels. The Company wrote off $1,082,000, the net balance of its intangible assets related to the AVC product acquisition and $289,000 of inventory, recorded a $273,000 liability for future obligations and recorded a gain on the transaction of $856,000.
Subsequent Events
In October 2005, the Company entered into definitive license and supply agreements for ESTRASORB with Esprit Pharma, Inc. Under the license agreement, Esprit obtained exclusive rights to market ESTRASORB in North America and the Company will continue to manufacture ESTRASORB. In consideration for the rights granted, Esprit will pay the Company a minimum cash consideration of $12,500,000: $2,000,000 which was paid at closing, $8,000,000 by December 30, 2005, and the remaining $2,500,000 on the first anniversary date of the license agreement. The Company will also receive a royalty on all net sales of ESTRASORB as well as milestone payments based on specific pre-determined net sales levels of ESTRASORB.
As part of this transaction, Esprit paid the Company $273,000 for inventory and sales and promotional materials for which the Company had a book value of $437,000. The Company also wrote off the remaining balance of its intangible asset, ESTRASORB rights, which totaled $2,175,000, net at the date of the transaction.
In October 2005, certain holders of $6,000,0000 face amount of the Company’s 4.75% senior convertible notes due July 15, 2009 exercised their optional conversion right to convert their notes plus accrued interest of $81,000 into 1,070,635 shares of Novavax common stock, at the per share conversion price of $5.68. This reduces the aggregate principal amount of such notes outstanding from $35,000,000 to $29,000,000.
In November 2005, the Company completed an agent-led offering of 4,186,047 shares of common stock at $4.30 per share for gross proceeds of $18,000,000. The stock was issued pursuant to an existing shelf registration statement. Net proceeds after deducting underwriter, legal and other miscellaneous fees were approximately $17,000,000.
16
Item 2.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Certain statements contained herein or as may otherwise be incorporated by reference herein constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements regarding product sales, future product development and related clinical trials, and future research and development, including Food and Drug Administration approval. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Company, or industry results, to be materially different from those expressed or implied by such forward-looking statements.
Such factors include, among other things, the following: general economic and business conditions; competition; ability to enter into future collaborations with industry partners; unexpected changes in technologies and technological advances; ability to obtain rights to technology; ability to obtain and enforce patents; ability to commercialize and manufacture products; ability to maintain commercial-scale manufacturing capabilities; results of clinical studies; progress of research and development activities; business abilities and judgment of personnel; availability of qualified personnel; changes in, or failure to comply with, governmental regulations; ability to obtain adequate financing in the future through product licensing, co-promotional arrangements, public or private equity financing or otherwise; and other factors referenced herein.
All forward-looking statements contained in this quarterly report are based on information available to the Company on the date hereof, and the Company assumes no obligation to update any such forward-looking statements, except as specifically required by law. Accordingly, past results and trends should not be used to anticipate future results or trends.
Overview
Novavax is a product development company focused on the research, development and commercialization of products utilizing its proprietary drug delivery and biological technologies for large and growing markets. Our drug delivery technologies include the micellar nanoparticle (“MNP”) technology, which is the basis for the development of our first Food and Drug Administration approved product ESTRASORB®. In addition to MNP, our drug delivery technologies include Novasomes® (paucillamellar non-phospholipid liposomes) and Sterisomes®. Our vaccine technologies include functional virus-like particle (“VLP”) manufacturing utilizing the baculovirus expression system in insect cells as well as novel vaccine adjuvants based on Novasomes and dendrimer technologies.
17
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
We will continue to focus our efforts on the development of additional compounds that use our proprietary topical MNP drug delivery platform and which we believe have a high probability of technical success and large market potential. The table below summarizes our most advanced drug candidates currently in clinical or preclinical development.
We plan to continue leveraging our technologies to develop these and other new product candidates which will be marketed through sale or licensing to other pharmaceutical companies.
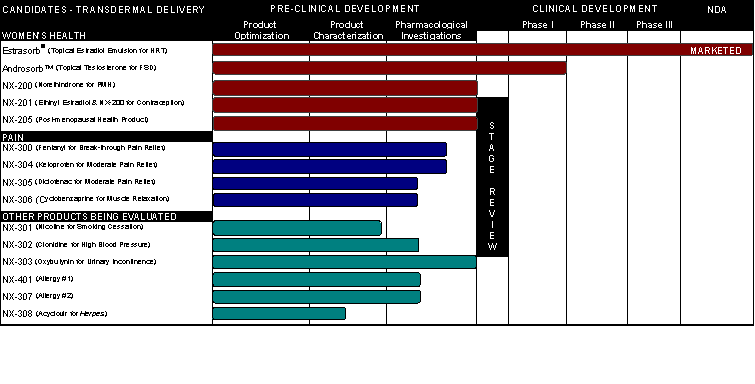
We also conduct research and development on preventative vaccines and proteins for infectious diseases and cancers, and tolerogens to prevent the initiation and progression of stroke, heart attack and other inflammatory diseases. Currently, the major impetus is the development and exploitation of our core virus-like particle technology for developing novel vaccines. VLPs are genetically-engineered particles that imitate the important three-dimensional structures of viruses but are composed of recombinant proteins and therefore are powerful immunogens but incapable of causing infection and disease. Our proprietary production technology for VLPs employs insect cells and the baculovirus expression system. We believe the main advantage of this technology is a more rapid production of a safe, effective, low-cost vaccine as compared to the current labor-intensive egg-based and cell culture based production processes. We believe that other key advantages of this technology are the ability to rapidly respond to emerging threats or new strains and the reduced risk of allergic reaction. Projects in development using our proprietary VLP technology include vaccines for pandemic and seasonal influenza, HIV and SARS. We are also developing E-selectin tolerogen for the prevention of secondary strokes.
18
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Recent Developments
In July 2005, we completed an agent-led offering of 4,000,000 shares of common stock at $1.00 per share, for gross proceeds of $4.0 million. The stock was issued pursuant to an existing shelf registration statement with net proceeds of approximately $3.6 million.
In October 2005, certain holders of $6.0 million face amount of our 4.75% senior convertible notes due July 15, 2009 exercised their optional conversion right to convert their notes plus accrued interest of $81,000 into 1,070,635 shares of Novavax common stock, at the per share conversion price of $5.68. This reduces the aggregate principal amount of Notes outstanding from $35.0 million to $29.0 million.
In November 2005, we completed an additional agent-led offering of 4,186,047 shares of common stock at $4.30 per share for gross proceeds of $18.0 million. The stock was issued pursuant to an existing registration statement with net proceeds of approximately $17.0 million.
During the third quarter of 2005, we made a strategic decision to transition the company from a commercial product sales business model to a company focused on new product development and the sale or out-licensing of those products to partners.
In accordance with that strategy, we completed the following programs and transactions over the past few months:
In August 2005, we implemented a measure to further reduce costs associated with our commercial operations. This restructuring eliminated our remaining sales force. The total estimated cost to be incurred in connection with this restructuring was approximately $0.3 million.
In September, 2005, we entered into an Asset Purchase Agreement with Pharmelle, LLC for the sale of assets related to our AVC, NovaNatal and NovaStart products, as well as assets relating to certain formerly-marketed products Vitelle, Nestabs, Gerimed, Irospan and Nessentials. In consideration for the sale of these assets, Pharmelle paid us $2.5 million in cash and assumed certain liabilities. In addition, we are entitled to royalties on AVC for a five year period if net sales exceed certain levels. This transaction resulted in recording a gain of $0.9 million, $1.1 million of net intangible assets were written off and the sale of $0.3 million of inventory and $0.2 million of liabilities were incurred.
19
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
In October 2005, we entered into definitive license and supply agreements for ESTRASORB with Esprit Pharma, Inc. Under the license agreement, Esprit obtained exclusive rights to market ESTRASORB in North America, and we will continue to manufacture the product. In consideration for the rights granted, Esprit will pay us a minimum cash consideration of $12.5 million: $2.0 million which was paid at closing, $8.0 million by December 30, 2005, and the remaining $2.5 million on the first anniversary date of the license agreement. We will also receive a royalty on all net sales of ESTRASORB as well as milestone payments based on specific pre-determined net sales. As part of this transaction, Esprit paid us $0.3 million for inventory and sales and promotional materials, and we wrote off the remaining balance of our intangible asset for ESTRASORB rights, totaling $2.2 million. We have retained the rights to ESTRASORB outside of North America and will continue to seek partners in order to maximize the value of this asset.
Critical Accounting Policies and Changes to Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
There have been no material changes in our critical accounting policies or critical accounting estimates since December 31, 2004, nor have we adopted any accounting policy that has or will have a material impact on our consolidated financial statements. For further discussion of our accounting policies see Note 2 —Summary of Significant Accounting Policiesin the Notes to the Consolidated Financial Statements included in this Quarterly Report on Form 10-Q and Note 3 in the Notes to the Consolidated Financial Statements for our Annual Report on Form 10-K for the fiscal year ended December 31, 2004.
Results of Operations
The following is a discussion of the historical consolidated financial condition and results of operations of Novavax, Inc. and its wholly-owned subsidiary and should be read in conjunction with the consolidated financial statements and notes thereto set forth in this Quarterly Report on Form 10-Q. Additional information concerning factors that could cause actual results to differ materially from those in the Company’s forward-looking statements is contained from time to time in the Company’s SEC filings, including but not limited to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2004.
20
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Three months ended September 30, 2005 (“2005”) compared to the three months ended September 30, 2004 (“2004”): (in thousands)
Revenues:
| | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | |
| | | (unaudited) | | | (unaudited) | | | | | |
| Product Sales: | | | | | | | | | | | | |
| Vitamins | | $ | 136 | | | $ | (1,018 | ) | | $ | 1,154 | |
| Gynodiol | | | 360 | | | | (18 | ) | | | 378 | |
| AVC Cream | | | 237 | | | | 15 | | | | 222 | |
| ESTRASORB | | | 574 | | | | 248 | | | | 326 | |
| Other | | | (7 | ) | | | 109 | | | | (116 | ) |
| | | | | | | | | | |
| Total product sales | | | 1,300 | | | | (664 | ) | | | 1,964 | |
| Contract research | | | 417 | | | | 653 | | | | (236 | ) |
| Milestone and licensing fees | | | 150 | | | | — | | | | 150 | |
| | | | | | | | | | |
| | | $ | 1,867 | | | $ | (11 | ) | | $ | 1,878 | |
| | | | | | | | | | |
Total revenues for the three months ended September 30, 2005 were $1.9 million, as compared to $(11,000) for 2004, a $1.9 million increase. Revenues for the third quarter of 2005 consisted of product sales of $1.3 million, compared to $(0.7) million in 2004; contract revenue of $0.4 million in 2005, compared to $0.7 million in 2004; and milestone and licensing fees of $0.2 million in 2005, compared to zero in 2004. The net increase in 2005 product sales compared to 2004 is attributed to a variety of factors including:
| | § | | During the third quarter of 2004, a non-recurring reserve of $1.3 million was recorded for anticipated returns of vitamins negatively impacted by generic competition. This reserve was for sales that occurred in 2002 and 2003. |
| |
| | § | | The initial commercial launch of ESTRASORBoccurred in June 2004. Sales for ESTRASORB were low for the third quarter of 2004 as wholesalers were filling orders from their initial launch inventories delivered in the second quarter of 2004. The major wholesalers depleted their initial launch inventories from June 2004 by the second quarter of 2005 and for the third quarter placed additional orders that corresponded with their anticipated monthly resale levels. ESTRASORB sales were $0.6 million in 2005 compared to $0.2 million in 2004. |
| |
| | § | | 2005 sales of Gynodiol and AVC products increased by $0.4 million and $0.2 million, respectively, over 2004 which was negatively affected by a high level of returns. |
As mentioned inRecent Developments,we have sold our prenatal vitamin line and AVC products and licensed our North American rights for ESTRASORB. Thus product sales will be reduced beginning in the fourth quarter of 2005.
21
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The decrease in contract revenue for 2005 as compared to 2004 is primarily attributable to the revision of several milestone payments from the National Institutes of Health that have resulted in the deferral of invoicing. The NIH has subsequently completed these revisions and these milestones are expected to be reached and revenue recognized during the fourth quarter of 2005.
Operating costs and expenses:
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | % Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Cost of products sold | | $ | 1,068 | | | $ | 364 | | | $ | 704 | | | | 193 | % |
| Research and development | | | 1,161 | | | | 1,552 | | | | (391 | ) | | | -25 | % |
| Selling and marketing | | | 930 | | | | 8,931 | | | | (8,001 | ) | | | -90 | % |
| General and administrative | | | 1,694 | | | | 1,901 | | | | (207 | ) | | | -10 | % |
| | | | | | | | | | | | | |
| Sub-total | | | 4,853 | | | | 12,748 | | | | (7,895 | ) | | | -62 | % |
| | | | | | | | | | | | | |
| Facility exit costs | | | 107 | | | | 723 | | | | (616 | ) | | | -85 | % |
| Gain on sale of assets | | | (856 | ) | | | –– | | | | (856 | ) | | | 100 | % |
| Gain on redemption of debt | | | –– | | | | (11,162 | ) | | | 11,162 | | | | -100 | % |
| | | | | | | | | | | | | |
| | | $ | 4,104 | | | $ | 2,309 | | | $ | 1,795 | | | | 78 | % |
| | | | | | | | | | | | | |
Cost of products sold, which includes fixed idle capacity costs at our manufacturing facility, increased to $1.1 million in 2005, compared to $0.4 million in 2004. Of the $1.1 million cost of products sold for 2005, $0.4 million was due to idle plant capacity costs at our manufacturing facility, which was operating at higher production levels at this time last year. The remaining $0.7 million is higher than the $0.4 million in 2004 by 75% and correlates to 2005 total product sales being higher than 2004 by $1.9 million or 296%.
Research and development costs decreased from $1.6 million in 2004 to $1.2 million in 2005, or 25%. The decrease of $0.4 million was due to the reduction of research facility costs compared to 2004.
Selling and marketing costs were $0.9 million in 2005 compared to $8.9 million in 2004. The decrease of $8.0 million, or 90%, was due to the elimination of the remaining sales force in August 2005 and the decision to discontinue marketing efforts other than telemarketing. This corresponds with our strategy of transitioning from a commercial business model to that of one focused on new product development.
General and administrative costs were $1.7 million in 2005 compared to $1.9 million in 2004. The decrease of $0.2 million, or 10%, is primarily due to $0.4 million of opportunity grant funds received from the Commonwealth of Pennsylvania in 2005 for the reimbursement of certain costs incurred with the move of our corporate headquarters and product development activities to Malvern, Pennsylvania, partially offset by increased business development costs.
22
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
A charge for facility exit costs of $0.7 million was recorded in 2004 for our move to Malvern, Pennsylvania from Columbia, Maryland. In addition, we recorded a liability of $0.2 million for contract termination costs and wrote-off the net value of the Columbia leasehold assets of $0.5 million. In 2005, we recorded an additional $0.1 million liability for the balance of lease obligations for this property.
In 2005 we also recorded a $0.9 million one-time gain related to the Pharmelle transaction (SeeRecent Developments), and in 2004 we recorded a one-time gain on the redemption of debt of $11.2 million related to our agreement with King Pharmaceuticals to terminate several license agreements and our co-promotion agreement for ESTRASORB.
Interest Income/(Expense):
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | % Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Interest income | | $ | 45 | | | $ | 93 | | | $ | (48 | ) | | | -52 | % |
| Interest expense | | | (535 | ) | | | (430 | ) | | | (105 | ) | | | -24 | % |
| | | | | | | | | | | | | |
| | | $ | (490 | ) | | $ | (337 | ) | | $ | (153 | ) | | | -45 | % |
| | | | | | | | | | | | | |
The 2005 increase in interest expense of $0.1 million is due to the increase in the amortization of deferred financing costs related to the issuance of new convertible notes in 2004.
Net loss:
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | %Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Net loss | | $ | (2,727 | ) | | $ | (2,657 | ) | | $ | 70 | | | | 3 | % |
| | | | | | | | | | | | | |
| Net loss per share | | $ | (.06 | ) | | $ | (.07 | ) | | $ | (.01 | ) | | | -14 | % |
| | | | | | | | | | | | | |
| Weighted shares outstanding | | | 43,469,637 | | | | 38,577,458 | | | | 4,892,179 | | | | 13 | % |
| | | | | | | | | | | | | |
The net loss for 2005 was $2.7 million or $(.06) per share, as compared to $2.6 million or $(.07) per share for 2004, an increase of $0.1 million, or $.01 per share. The increase is due to the increase in 2005 revenues of $1.9 million, the $7.9 million decrease in operating expenses and the increase in the gain related to the Pharmelle transaction, offset by the gain on redemption of debt from the King transaction in 2004 of $11.2 million.
23
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Nine months ended September 30, 2005 (“2005”) compared to the nine months ended September 30, 2004 (“2004”): (in thousands)
Revenues:
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | % Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Product Sales: | | | | | | | | | | | | | | | | |
| Vitamins | | $ | 888 | | | $ | 1,090 | | | $ | (202 | ) | | | -19 | % |
| Gynodiol | | | 695 | | | | 594 | | | | 101 | | | | 17 | % |
| AVC Cream | | | 708 | | | | 445 | | | | 263 | | | | 59 | % |
| ESTRASORB | | | 1,610 | | | | 1,729 | | | | (119 | ) | | | - 7 | % |
| Other | | | 7 | | | | 332 | | | | (325 | ) | | | -98 | % |
| | | | | | | | | | | | | |
| Total product sales | | | 3,908 | | | | 4,190 | | | | (282 | ) | | | - 7 | % |
| Contract research | | | 1,086 | | | | 1,899 | | | | (813 | ) | | | -43 | % |
| Milestone and licensing fees | | | 150 | | | | 125 | | | | 25 | | | | 20 | % |
| | | | | | | | | | | | | |
| | | $ | 5,144 | | | $ | 6,214 | | | $ | (1,070 | ) | | | -17 | % |
| | | | | | | | | | | | | |
Revenues for 2005 consisted of product sales of $3.9 million compared to $4.2 million in 2004, contract revenues of $1.1 million compared to $1.9 million in 2004, and $0.2 million of milestone and licensing fees in 2005, compared to $0.1 million in 2004. Total revenues for 2005 were $5.1 million, as compared to $6.2 million for 2004, a $1.1 million or 17% decrease.
Of the total decrease in revenues, product sales accounted for $0.3 million. This decrease is primarily due to generic competition for Analpram, a product we co-promoted, that is included in other product sales. The decrease in ESTRASORB of $0.1 million is offset by a collective increase in sales of our vitamin, Gynodiol and AVC products.
The decrease in contract revenue for 2005 as compared to 2004 is primarily due to the completion of several contracts during the first quarter of 2004, which resulted in final payments totaling $0.3 million, as well as $0.5 million of revenue recognized on an NIH contract that was later cancelled in July 2004. During 2005 there were revisions to several milestone payments with the NIH contracts which have resulted in the deferral of invoicing. The NIH has subsequently completed these revisions and these milestones are expected to be reached and revenue recognized during the fourth quarter of 2005.
We currently have research contracts totaling over $1.8 million for 2005 and we expect that remaining contract milestones will be met in the fourth quarter.
As mentioned inRecent Developments,we have sold our prenatal vitamin line and AVC products and licensed our North American rights for ESTRASORB. Thus product sales will be reduced beginning in the fourth quarter of 2005.
24
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Operating costs and expenses:
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | % Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Cost of products sold | | $ | 5,074 | | | $ | 2,128 | | | $ | 2,946 | | | | 138 | % |
| Research and development | | | 3,759 | | | | 5,828 | | | | (2,069 | ) | | | - 36 | % |
| Selling and marketing | | | 6,832 | | | | 17,261 | | | | (10,429 | ) | | | - 60 | % |
| General and administrative | | | 6,109 | | | | 5,992 | | | | 117 | | | | 2 | % |
| Facility exit costs | | | 105 | | | | 723 | | | | (618 | ) | | | 85 | % |
| | | | | | | | | | | | | |
| Sub-total | | | 21,879 | | | | 31,932 | | | | (10,053 | ) | | | -31 | % |
| | | | | | | | | | | | | |
| Gain on sale of assets | | | (856 | ) | | | –– | | | | (856 | ) | | | 100 | % |
| Gain on redemption of debt | | | –– | | | | (11,162 | ) | | | 11,162 | | | | -100 | % |
| | | | | | | | | | | | | |
| | | $ | 21,023 | | | $ | 20,770 | | | $ | 253 | | | $ | 1 | % |
| | | | | | | | | | | | | |
Cost of products sold, which includes fixed idle capacity costs at our manufacturing facility, increased to $5.0 million in 2005, compared to $2.1 million in 2004. Of the $5.0 million cost of products sold in 2005, $2.9 million was due to idle plant capacity at our ESTRASORB manufacturing facility, which was operating at higher production levels during the second and third quarters of 2004. The remaining $2.1 million for 2005 is comparable to 2004 levels. ESTRASORB sales in both 2005 and 2004 were 41% of net product sales. Sales for Gynodiol and AVC Cream were $0.4 million higher in 2005 than in 2004 and carry a higher cost of products sold than our vitamin products.
Research and development costs decreased from $5.8 million in 2004 to $3.8 million in 2005. The decrease of $2.0 million, or 36%, was primarily due to manufacturing start-up costs in 2004 being accounted for in the research and development category until April 2004. The 2004 manufacturing costs were incurred to prepare and validate the facility for GMP and FDA compliance and not to build inventory. Beginning in April 2004, manufacturing costs have been included in cost of sales, inventory or written off to idle capacity cost.
Selling and marketing costs were $6.8 million in 2005 compared to $17.3 million in 2004. The decrease of $10.5 million, or 60%, was primarily due to the elimination of a portion of our sales force in March 2005 and the remaining portion in August 2005. We also discontinued the majority of our marketing efforts. This corresponds with our strategy of transitioning from a commercial business model to that of one focused on our core competency of product development.
General and administrative costs were $6.1 million in 2005 compared to $6.0 million in 2004. The increase of $0.1 million is due to increased business development costs and was partially offset by $0.4 million of opportunity grant funds received from the Commonwealth of Pennsylvania in 2005 for the reimbursement of certain costs incurred in 2004 with the move of our corporate headquarters and product development activities to Malvern, Pennsylvania.
25
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Interest Income/(Expense):
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | % Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Interest income. | | $ | 148 | | | $ | 238 | | | $ | (90 | ) | | | -38 | % |
| Interest expense | | | (1,598 | ) | | | (1,313 | ) | | | (285 | ) | | | -22 | % |
| | | | | | | | | | | | | |
| | | $ | (1,450 | ) | | $ | (1,075 | ) | | $ | (375 | ) | | | -35 | % |
| | | | | | | | | | | | | |
Net interest expense was $1.5 million in 2005 compared to $1.1 million in 2004. Our 2004 interest expense related primarily to our promissory notes with King of $40.00 million through July 2004, at which time such notes were redeemed and we issued new convertible notes totaling $35.0 million to a group of institutional investors. The 2005 increase in interest expense of $0.3 million is due to the increase in the amortization of deferred financing costs related to the new notes.
Net loss:
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | $ Change | | | %Change | |
| | | (unaudited) | | | (unaudited) | | | | | | | | | |
| Net loss | | $ | (17,329 | ) | | $ | (15,631 | ) | | $ | 1,698 | | | | 11 | % |
| | | | | | | | | | | | | |
| Net loss per share | | $ | (.42 | ) | | $ | (.43 | ) | | $ | (.01 | ) | | | - 2 | % |
| | | | | | | | | | | | | |
| Weighted shares outstanding | | | 40,873,473 | | | | 36,040,465 | | | | 4,833,008 | | | | 13 | % |
| | | | | | | | | | | | | |
The net loss for 2005 was $17.3 million or $(.42) per share, as compared to $15.6 million or $(.43) per share for 2004, an increase of $1.7 million or 11%, with a $.01 decrease in net loss per share, due to the issuance of additional shares in the third quarter of 2005. The increase in net loss is primarily due to decreased revenues of $1.1 million and a decrease in operating expenses of $10.0 million, offset by the one-time gain of $11.2 million in 2004 as previously described.
26
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Liquidity and Capital Resources
Our capital requirements depend on numerous factors, including but not limited to the commitments and progress of our research and development programs, the progress of preclinical and clinical testing, the time and costs involved in obtaining regulatory approvals, the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights, competing technological and market developments, and manufacturing costs related to ESTRASORB. We plan to have multiple products and vaccines in various stages of product development and we believe our research and development as well as general administrative expenses and capital requirements will continue to exceed our revenues. Future activities, particularly product development, are subject to our ability to raise funds through debt or equity financing, or collaborative arrangements with industry partners.
| | | | | |
| | | Nine months ended | |
| | | September 30, | |
| | | 2005 | |
| | | (in thousands) | |
| Summary of Cash Flows: | | (unaudited) | |
| Net cash (used)/provided by: | | | | |
| Operating activities | | $ | (13,527 | ) |
| Investing activities | | | (103 | ) |
| Financing activities | | | 2,698 | |
| | | | |
| Net change in cash and cash equivalents | | | (10,932 | ) |
| Beginning cash and cash equivalents | | | 17,876 | |
| | | | |
| Ending cash and cash equivalents | | $ | 6,944 | |
| | | | |
27
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Cash and cash equivalents were $6.9 million at September 30, 2005, a decrease of $10.9 million from the December 31, 2004 balance of $17.9 million. The subsequent up-front payments from the ESTRASORB transaction and the funds received from the November 2005 financing transaction (SeeRecent Developments) will increase the cash position by approximately $27.0 million prior to fourth quarter operations. Of the $10.9 million of cash used in the first nine months of 2005, $13.5 million was used for operating activities, $0.1 million was used for investing activities and a net of $2.7 million was obtained from financing activities. Operating activities consisted of the net loss of $17.3 million, as previously discussed, non-cash activities of $2.5 million, $2.5 million received from the Pharmelle transaction, offset by $(1.2) million of net changes in balance sheet accounts. Working capital was $6.0 million at September 30, 2005 compared to $15.4 million at December 31, 2004. The decrease in working capital of $9.4 million was primarily due to the $13.5 million used for operating activities, offset by the $2.7 million obtained from financing activities during the nine months ended September 30, 2005.
As previously mentioned in theRecent Developmentssection, in July, 2005 and November 2005, the Company completed offerings of 4,000,000 shares of common stock at $1.00 per share and 4,186,047 shares of common stock at $4.30 per share, respectively. The shares were issued pursuant to an existing shelf registration statement. The net proceeds after deducting underwriter, legal, accounting and other miscellaneous fees were approximately $3.6 million and $17.0 million, respectively. We intend to use the proceeds from these offerings for general corporate purposes, including but not limited to our internal research and development programs, such as preclinical and clinical testing and studies of our product candidates and the development of new technologies, capital improvement and general working capital.
During the remainder of 2005, we anticipate lower cash requirements than we experienced during the first half of 2005 primarily due to the elimination of our commercial operations and related expense reductions. Partially offsetting this is an anticipated increase in research and development expenditures as new product candidates progress to the Investigational New Drug stage and we invest in the accelerated progression of our vaccine programs.
We will continue to pursue obtaining capital through product licensing, co-development arrangements on new products, or the public or private sale of securities of the Company. We have demonstrated our ability to obtain capital, as required; however, there can be no assurance that we will be able to obtain additional capital or, if such capital is available, that the terms of any financing will be satisfactory to the Company. Based on our assessment of the availability of capital and our business operations as currently contemplated, in the absence of new financings, licensing arrangements or partnership agreements, we believe following ourRecent Developmentswe will have adequate capital resources well into 2007. If we are unable to obtain additional capital, we will continue to assess our capital resources and we may be required to delay, reduce the scope of, or eliminate one or more of our product research and development programs, downsize our organization, or reduce general and administrative infrastructure.
28
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Item 3. Quantitative and Qualitative Disclosures about Market Risk
There have been no material changes during the period from the end of our last fiscal year through September 30, 2005 to the information concerning the Company’s quantitative and qualitative disclosures about market risk set forth in Item 7A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2004 as filed with the SEC.
Item 4. Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company’s reports (as defined in Rules 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended), is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Evaluation
The Company’s Chief Executive Officer and Chief Financial Officer, with the participation of certain members of management, conducted an evaluation of the effectiveness of the Company’s disclosure controls and procedures as of September 30, 2005. Based on that evaluation, the Company’s Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures were effective as of September 30, 2005. Such evaluation did not identify any change in the Company’s internal control over financial reporting during the quarter ended September 30, 2005 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
29
Part II. Other Information
Item 1 — Legal Proceedings
The Company is a defendant in a lawsuit filed by a former director alleging that the Company wrongfully terminated the former director’s stock options. Management believes that the termination and cancellation of the options was in accordance with the terms of the option agreements, following the director’s termination for cause in 1997 by a former parent company, IGI, Inc., and the lawsuit is without merit. Novavax intends to vigorously defend the claim. Management cannot reasonably estimate the liability, if any, related to this claim, or the likelihood of an unfavorable settlement. Accordingly, no liability related to this contingency is accrued in the consolidated balance sheet as of September 30, 2005. Any unfavorable determination, however, may have a material adverse impact on future operating results.
Item 2 — Unregistered Sales of Equity Securities and Use of Proceeds
In August 2005, the Company issued 250,000 shares of common stock in a private placement to its former chief executive officer for prior services. The shares were issued in reliance on Section 4(2) of the Securities Act of 1933, as amended. For more information, see the Company’s Form 8-K filed August 10, 2005.
Neither the Company nor any affiliated purchaser repurchased any Novavax equity securities during the period covered by this report.
Item 3 — Defaults upon Senior Securities
Not applicable.
Item 4 — Submission of Matters to a Vote of Security Holders
Not applicable.
Item 5 — Other Information
Not applicable.
Item 6 — Exhibits
| | 31.1 | | Certification of principal executive officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 31.2 | | Certification of principal financial officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 32.1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Rahul Singhvi, President and Chief Executive Officer of the Company. |
| | 32.2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Dennis W. Genge, Vice President and Chief Financial Officer of the Company. |
30
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | |
| | | NOVAVAX, INC.
(Registrant) | | |
| | | | | |
| Date: November 8, 2005 | | By: /s/ Dennis W. Genge | | |
| | | | | |
| | | Dennis W. Genge | | |
| | | Vice President and Chief Financial Officer | | |
| | | (Principal Financial and Accounting Officer) | | |
31
