Filed pursuant to Rule 424(b)(4)
Registration No. 333-109034
PROSPECTUS
3,000,000 Shares

Common Stock
We are offering 2,400,000 shares and the selling stockholder is offering 600,000 shares of the common stock offered by this prospectus. We will not receive any proceeds from the sale of shares by the selling stockholder.
Our common stock is quoted on the Nasdaq National Market under the symbol “LCAV.” On December 3, 2003, the last reported sale price of our common stock on the Nasdaq National Market was $17.61 per share.
Investing in our common stock involves a high degree of risk. Before buying any shares, you should read the discussion of material risks of investing in our common stock described in “Risk factors” beginning on page 6.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| | | | | | | | | |
| | Per | | |
| | share | | Total |
|
|
| Public offering price | | $ | 16.50 | | | $ | 49,500,000 | |
|
| Underwriting discounts and commissions | | $ | 0.99 | | | $ | 2,970,000 | |
|
| Proceeds, before expenses, to us | | $ | 15.51 | | | $ | 37,224,000 | |
|
| Proceeds, before expenses, to the selling stockholder | | $ | 15.51 | | | $ | 9,306,000 | |
|
The underwriters may also purchase up to an additional 450,000 shares from the selling stockholder at the public offering price, less underwriting discounts and commissions, to cover over-allotments, if any, within 30 days from the date of this prospectus.
The underwriters are offering the shares of our common stock as described in “Underwriting.” Delivery of the shares will be made on or about December 9, 2003.
UBS Investment Bank
C.E. Unterberg, Towbin
The date of this prospectus is December 4, 2003.
TABLE OF CONTENTS
________________________________________________________________________________

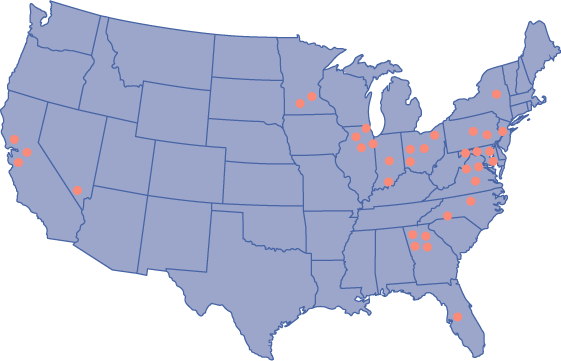
US LASIKPLUSLASER VISION CORRECTION CENTER LOCATIONS
| | | | | |
| |
CALIFORNIA
• Concord
• San Jose
• San Mateo
FLORIDA
• Tampa
GEORGIA
• Alpharetta
• Buckhead
• Galleria
• Gwinnett
ILLINOIS
• Naperville
• Oak Brook
• Riverwoods
• Schaumburg
INDIANA
• Indianapolis | | KENTUCKY
• Louisville
MARYLAND
• Annapolis
• Baltimore
• Columbia
• Rockville
MINNESOTA
• Edina
• Maple Grove
NEVADA
• Las Vegas
NORTH CAROLINA
• Charlotte
• Raleigh
NEW JERSEY
• Mt. Laurel | | NEW YORK
• Albany
OHIO
• Cincinnati
• Columbus
• Dayton
• Independence
PENSYLVANNIA
• Chadds Ford
• King of Prussia
VIRGINIA
• Alexandria
• Richmond
• Tysons Corner |
________________________________________________________________________________
________________________________________________________________________________
You should rely only on the information contained or incorporated by reference in this prospectus. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front cover of this prospectus.
TABLE OF CONTENTS
| | | | | |
| Prospectus summary | | | 1 | |
| Risk factors | | | 6 | |
| Forward-looking statements | | | 16 | |
| Use of proceeds | | | 17 | |
| Price range of common stock | | | 18 | |
| Dividend policy | | | 18 | |
| Capitalization | | | 19 | |
| Dilution | | | 20 | |
| Selected consolidated financial data | | | 21 | |
| Management’s discussion and analysis of financial condition and results of operations | | | 23 | |
| Business | | | 32 | |
| Management | | | 42 | |
| Certain transactions | | | 44 | |
| Principal and selling stockholders | | | 45 | |
| Description of capital stock | | | 47 | |
| Shares eligible for future sale | | | 48 | |
| Underwriting | | | 49 | |
| Legal matters | | | 52 | |
| Experts | | | 52 | |
| Where you can find more information | | | 52 | |
| Information incorporated by reference | | | 53 | |
| Index to consolidated financial statements | | | F-1 | |
The terms “LCA-Vision”, “Company”, “we”, “our” and “us” refer to LCA-Vision Inc. and its subsidiaries unless the context suggests otherwise. The term “you” refers to a prospective investor. LCA-Vision™, LasikPlusand the LCA-Vision logo are trademarks of LCA-Vision. All other trademarks or tradenames referred to in this prospectus are the property of their respective owners.
i
(Page Intentionally Left Blank)
Prospectus summary
This summary highlights information contained elsewhere or incorporated by reference in this prospectus. This summary highlights what we believe to be the most important information about us and the offering. Investors should read the entire prospectus carefully, including the “Risk factors” section, and other documents incorporated by reference into this prospectus for a complete understanding of our business and the offering and before making an investment decision.
ABOUT OUR COMPANY
We are a leading developer and operator of fixed-site laser vision correction centers under the brand name LasikPlus. Our vision centers provide the staff, facilities, equipment and support services for performing laser vision correction that employs advanced laser technologies to help correct nearsightedness, farsightedness and astigmatism. We currently utilize fixed-site excimer lasers manufactured by Bausch & Lomb, VISX and Alcon. Our vision centers are supported mainly by full-time credentialed board-certified ophthalmologists, optometrists and other health care professionals. The ophthalmologists perform the laser vision correction procedures in our vision centers, and either ophthalmologists or optometrists generally carry out the pre-procedure evaluations and post-procedure follow-ups in-center. We have performed over 300,000 laser vision correction procedures in our vision centers, in the United States and Canada, since 1991.
We currently operate 37 laser vision correction centers, including 34 wholly-owned vision centers located in large metropolitan markets throughout the United States, two joint ventures in Canada and one joint venture in Europe. We recently announced the opening of our newest vision center in Las Vegas.
Most people seeking vision correction suffer from nearsightedness, farsightedness and astigmatism, which often result from improper curvature of the cornea as related to the size and shape of the eye. Since the Food and Drug Administration approved the first laser to perform laser vision correction procedures in the United States in 1995, industry sources estimate that approximately 3 million patients have been treated. It is estimated that there are approximately 57.5 million potential patients for laser vision correction procedures in the United States, according to a report on the US refractive market published by MarketScope in November 2000. Laser vision correction is currently one of the most widely performed elective surgical procedures in the United States, with approximately 1.2 million laser vision correction procedures performed on approximately 600,000 patients in 2002. We believe we are well positioned to grow given the relatively low penetration of the large target market for laser vision correction. Laser vision correction is typically an elective, private pay procedure performed on an outpatient basis.
Laser vision correction procedures are designed to reshape the outer layers of the cornea to help correct refractive vision disorders by changing its curvature with an excimer laser, which may reduce the need for corrective lenses. The excimer laser emits energy in a series of pulses with each pulse typically lasting only a fraction of a second. High-energy ultraviolet light produced by the excimer laser creates a “non-thermal” process known as ablation, which removes tissue and reshapes the cornea.
We began performing LASIK, which now accounts for substantially all of the procedures performed in our vision centers, in the United States in 1997. In LASIK procedures, an automated microsurgical instrument called a microkeratome is used to create a thin flap, which remains hinged to the eye. The corneal flap is then laid back and excimer laser pulses are applied to the exposed surface of the cornea to treat the eye according to the patient’s prescription. The corneal flap is then folded back to its original position and inspected to ensure that it remains secured in position by the natural suction of the cornea. Since the surface layer of the cornea remains intact with LASIK, a bandage contact lens is normally not required and the patient typically experiences little discomfort. The LASIK procedure allows an ophthalmologist to treat both eyes of a patient during the same visit, involves little patient discomfort and produces prompt results, frequently enabling patients to see well postoperatively almost immediately.
The newest advance in laser vision correction procedures is LASIK using custom ablation. We provide custom ablation in all of our markets using state-of-the-art technology, including VISX CustomVue™ technology, Alcon’s Customcornea™ technology and Bausch & Lomb Zyoptix™ technology. To
1
perform a custom ablation procedure, we use digital technology to identify and measure imperfections in an individual’s eyes more precisely than with standard methods used for glasses and contact lenses and non-custom LASIK and non-custom PRK, a procedure we began performing in the United States in 1995. This information is then transferred to the laser, providing potentially greater precision and accuracy in the treatment.
OUR BUSINESS STRATEGY
Our business strategy is to provide quality laser vision correction services at an affordable price. In July 1999, we began converting our vision centers to closed access facilities from open access facilities in order to obtain increased control over the quality of care we provide to our patients and greater operational and financial control of our business. Under the open access model, we allowed qualified ophthalmologists to use our equipment and facilities in return for a facilities fee. Under our closed access model, in contrast, we either directly employ the ophthalmologist and the optometrist or contract for their services, and are responsible for marketing and patient acquisition.
We intend to grow our business through increased penetration in our current markets and expansion into new markets. Key elements of our business strategy include:
| |
| • | recruiting and retaining highly credentialed ophthalmologists and optometrists, |
| |
| • | providing patients with a “Continuum of Care,” |
| |
| • | opening and operating new laser vision correction centers, |
| |
| • | providing attractive patient financing alternatives, |
| |
| • | establishing relationships with leading managed care providers in the United States to source additional patients, and |
| |
| • | developing and implementing innovative direct marketing campaigns. |
RISK FACTORS
We commenced operations in 1991 and have posted net losses in every year other than 1999. Our net losses for 2000, 2001, and 2002 were $2,366,000, $23,375,000 and $3,826,000, respectively.
We face various risks and potential obstacles in implementing our business strategy. We derive substantially all of our revenues from laser vision correction services. If we are unable to continue to attract patients, our revenues would decline, and we might not have sufficient funds to implement certain elements of our business strategy. Several factors contribute to our ability to attract patients, including:
| |
| • | recruitment and retention of qualified ophthalmologists and optometrists, |
| |
| • | remaining competitive with better-financed or lower-cost providers of laser vision correction services, |
| |
| • | maintaining the newest technology in our centers for correcting refractive vision disorders, and |
| |
| • | continuing market acceptance of laser vision correction. |
An investment in our common stock involves a high degree of risk. You should consider the information contained in “Risk factors” beginning on page 6 before investing in our common stock.
The address of our principal executive office is 7840 Montgomery Road, Cincinnati, Ohio 45236, and our telephone number is (513) 792-9292. Our website is located at www.lasikplus.com. Information contained on our website does not constitute, and shall not be deemed to constitute, part of this prospectus.
2
The offering
| | |
| Shares offered by us | | 2,400,000 shares |
| |
| Shares offered by the selling stockholder | | 600,000 shares |
| |
| Common Stock to be outstanding after the offering | | 13,246,718 shares |
| |
| Use of proceeds | | To open additional laser vision correction centers, to purchase additional technology and equipment, to fund potential strategic transactions we may enter into in the future, and to provide working capital for general corporate purposes. |
| |
| | We will not receive any of the proceeds from the sale of common stock by the selling stockholder. |
| |
| Nasdaq National Market symbol | | LCAV |
The number of shares of our common stock to be outstanding after this offering is based on the number of shares outstanding on December 3, 2003, and does not include, as of that date:
| |
| • | an aggregate of 868,509 shares of common stock reserved for issuance upon exercise of outstanding options, at a weighted average exercise price of $11.20 per share; and |
| |
|
| • | an aggregate of 1,143,239 shares of common stock available for future issuance under our 1995, 1998 and 2001 stock option plans. |
|
Unless otherwise stated, all information contained in this prospectus assumes that the underwriters do not exercise their over-allotment option.
All share and per share information contained in this prospectus reflects a 1 for 4 reverse stock split effected by us effective as of November 11, 2002.
3
Summary consolidated financial data
The consolidated statement of operations data set forth below for the years ended December 31, 2000, 2001 and 2002 are derived from our audited consolidated financial statements included and incorporated by reference in this prospectus. The consolidated statement of operations data set forth below for the years ended December 31, 1998 and 1999 are derived from our audited consolidated financial statements not included or incorporated by reference in this prospectus. The following summary interim financial data as of September 30, 2003 and for the nine months ended September 30, 2002 and 2003 are unaudited and are derived from the interim financial statements included and incorporated by reference in this prospectus. In the opinion of management, the unaudited data have been prepared on the same basis as the audited consolidated financial statements and include all adjustments, consisting only of normal recurring adjustments, necessary for fair presentation. Results for interim periods are not indicative of results for a full year. The data set forth below should be read in conjunction with the consolidated financial statements and related notes and “Management’s discussion and analysis of financial condition and results of operations” included elsewhere in this prospectus.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | Nine months ended |
| | Year ended December 31, | | September 30, |
| Consolidated statement | |
| |
|
| of operations data: | | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | | 2002 | | 2003 |
| | |
| |
|
| | (unaudited) |
| | |
| | (in thousands, except for per share data) |
| Revenues: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Laser refractive surgery | | $ | 32,508 | | | $ | 56,358 | | | $ | 63,450 | | | $ | 68,096 | | | $ | 61,838 | | | $ | 48,538 | | | $ | 60,661 | |
| | Other | | | 2,692 | | | | 1,026 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total revenues | | | 35,200 | | | | 57,384 | | | | 63,450 | | | | 68,096 | | | | 61,838 | | | | 48,538 | | | | 60,661 | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Medical professional and license fees | | | 13,700 | | | | 22,930 | | | | 15,542 | | | | 13,626 | | | | 12,270 | | | | 9,791 | | | | 11,846 | |
| | Direct costs of services | | | 12,526 | | | | 16,113 | | | | 27,218 | | | | 33,616 | | | | 28,796 | | | | 22,127 | | | | 23,982 | |
| | General and administrative expenses | | | 6,198 | | | | 6,036 | | | | 9,394 | | | | 8,727 | | | | 8,327 | | | | 6,454 | | | | 6,073 | |
| | Marketing and advertising | | | 2,183 | | | | 5,671 | | | | 14,565 | | | | 12,732 | | | | 12,823 | | | | 10,179 | | | | 9,164 | |
| | Depreciation and amortization | | | 3,521 | | | | 2,964 | | | | 3,839 | | | | 5,625 | | | | 5,997 | | | | 4,458 | | | | 4,692 | |
| | Special charges | | | 10,500 | | | | (150 | ) | | | — | | | | 1,774 | | | | (174 | ) | | | (174 | ) | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total expenses | | | 48,628 | | | | 53,564 | | | | 70,558 | | | | 76,100 | | | | 68,039 | | | | 52,835 | | | | 55,757 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Operating (loss) income | | | (13,428 | ) | | | 3,820 | | | | (7,108 | ) | | | (8,004 | ) | | | (6,201 | ) | | | (4,297 | ) | | | 4,904 | |
| Equity in earnings from unconsolidated businesses | | | 354 | | | | 316 | | | | 49 | | | | 372 | | | | 241 | | | | 228 | | | | 245 | |
| Interest expense | | | (786 | ) | | | (169 | ) | | | (58 | ) | | | (17 | ) | | | (4 | ) | | | (3 | ) | | | (17 | ) |
| Interest income | | | 441 | | | | 1,633 | | | | 2,713 | | | | 924 | | | | 225 | | | | 110 | | | | 280 | |
| Litigation settlement | | | — | | | | — | | | | — | | | | — | | | | 2,282 | | | | 2,282 | | | | — | |
| Other income (expense) | | | 358 | | | | 6 | | | | 604 | | | | (61 | ) | | | (195 | ) | | | (160 | ) | | | (163 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income before taxes on income | | | (13,061 | ) | | | 5,606 | | | | (3,800 | ) | | | (6,786 | ) | | | (3,652 | ) | | | (1,840 | ) | | | 5,249 | |
| Income tax expense (benefit) | | | 157 | | | | (5,287 | ) | | | (1,434 | ) | | | 16,589 | | | | 174 | | | | 98 | | | | 181 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Net (loss) income | | | (13,218 | ) | | | 10,893 | | | | (2,366 | ) | | | (23,375 | ) | | | (3,826 | ) | | | (1,938 | ) | | | 5,068 | |
| Dividends to preferred shareholders | | | 518 | | | | 140 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income available to common shareholders | | $ | (13,736 | ) | | $ | 10,753 | | | $ | (2,366 | ) | | $ | (23,375 | ) | | $ | (3,826 | ) | | $ | (1,938 | ) | | $ | 5,068 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income per common share | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Basic | | $ | (1.46 | ) | | $ | 0.89 | | | $ | (0.19 | ) | | $ | (2.01 | ) | | $ | (0.35 | ) | | $ | (0.18 | ) | | $ | 0.47 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Diluted | | $ | (1.46 | ) | | $ | 0.84 | | | $ | (0.19 | ) | | $ | (2.01 | ) | | $ | (0.35 | ) | | $ | (0.18 | ) | | $ | 0.47 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Weighted average shares used in computation | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Basic | | | 9,417 | | | | 11,998 | | | | 12,741 | | | | 11,643 | | | | 10,794 | | | | 10,866 | | | | 10,753 | |
| | Diluted | | | 9,417 | | | | 12,729 | | | | 12,741 | | | | 11,643 | | | | 10,794 | | | | 10,866 | | | | 10,856 | |
Selected operating data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Laser vision correction procedures | | | 19,791 | | | | 33,266 | | | | 59,144 | | | | 72,032 | | | | 57,104 | | | | 44,900 | | | | 49,425 | |
continued on next page
4
| | | | | | | | | |
| | |
| | September 30, 2003 |
| |
|
| Consolidated balance sheet data: | | Actual | | As adjusted(2) |
|
|
| | |
| | (unaudited) |
| | |
| | (in thousands) |
Cash and cash equivalents(1) | | $ | 25,260 | | | $ | 62,259 | |
| Working capital | | | 22,131 | | | | 59,130 | |
| Total assets | | | 48,181 | | | | 85,180 | |
| Accumulated (deficit) | | | (39,270 | ) | | | (39,270 | ) |
| Total shareholders’ investment | | | 38,902 | | | | 75,901 | |
| |
| (1) | Includes $0.5 million of cash maintained by our consolidated captive insurance company pursuant to statutory requirements. |
| |
|
| (2) | The as adjusted presentation above gives effect to the sale by us of 2,400,000 shares of common stock offered hereby, after deducting underwriting discounts and commissions and estimated offering expenses. |
|
5
Risk factors
You should carefully consider the following risk factors, in addition to the other information set forth in this prospectus and the documents incorporated by reference in this prospectus before purchasing shares of our common stock. If any of the risks discussed below actually occur, our business, operating results or financial condition could be adversely affected. This could cause the trading price of our common stock to significantly decline, and you may lose all or part of your investment.
We have a history of operating losses.
We commenced operations in 1991 and have posted net losses in every year other than 1999. Our net losses for 2000, 2001 and 2002 were $2,366,000, $23,375,000 and $3,826,000, respectively. Our accumulated deficit as of September 30, 2003 was $39,270,000. Our ability to be profitable depends on, among other factors:
| |
| • | our ability to increase demand for our services, |
| |
| • | our ability to execute our planned business strategy, including further growth and expansion, and |
| |
| • | our ability to manage equipment and operating costs. |
Our quarterly and annual operating results are subject to significant fluctuations.
Our revenue and operating results have fluctuated and may continue to fluctuate significantly from quarter to quarter and from year to year, depending on many factors, including:
| |
| • | market acceptance of laser vision correction services, |
| |
| • | the number of laser vision correction procedures performed, |
| |
| • | the timing of new advancements by our suppliers and the purchase of such advancements or upgrade of equipment by us or our competitors, |
| |
| • | the impact of competitors, including those who compete by deeply discounting the price of laser vision correction services, in the geographic areas in which we operate, |
| |
| • | declining economic conditions in the geographic areas in which we operate, which can result in decreased demand for our laser vision correction services, |
| |
| • | the opening, closing or expansion of vision centers, |
| |
| • | regulatory matters, |
| |
| • | litigation, and |
| |
| • | acquisitions and other transactions. |
In addition, our revenue and operating results are subject to seasonal factors. In terms of number of procedures performed, our strongest quarter historically has been the first quarter of the year, and our weakest the fourth quarter. We believe these fluctuations are due to several factors, including:
| |
| • | the availability to potential patients of funds under typical corporate medical flexible spending plans, |
| |
| • | the general effect of the New Year season and New Year’s resolutions on the scheduling of procedures, and |
| |
| • | time constraints imposed by the holiday season and a desire by some individuals not to schedule procedures at that time of year. |
The revenue growth and recent profitability we have achieved in prior quarters might not continue, and revenues and net income in any particular quarter could be lower, or our losses greater, than those of the preceding quarters, including comparable quarters or prior fiscal years. Quarter-to-quarter comparisons of our operating results are not necessarily meaningful and should not be relied upon as
6
Risk factors
indications of likely future performance or annual operating results. Reductions in revenues or net income between quarters or our failure to achieve expected quarterly earnings per share could result in a decrease in the market price of our common stock.
We derive substantially all of our revenue from laser vision correction services and a decrease in the provision of these services could result in a significant decrease in our revenues and profitability.
We derive substantially all of our revenues from laser vision correction services and do not enjoy diversified revenue sources. If we are not able to provide laser vision correction services or the number of laser vision correction procedures we perform significantly decreases, our revenues and profitability could significantly decrease. We do not have other revenue sources which could offset such a significant decrease in revenues from our provision of laser vision correction services.
If we are unable to attract and retain qualified ophthalmologists, our ability to attract patients could be negatively affected.
Our revenues are generated by ophthalmologists who work with us, either as employees or independent contractors, to perform surgeries. In states where the corporate practice of medicine is prohibited, we may contract with professional corporations for ophthalmologists to perform surgeries at our facilities. In other states, we may directly hire ophthalmologists to work for us as our employees. The retention of qualified ophthalmologists is a critical factor in the success of our vision centers. However, it is sometimes difficult for us to hire or retain qualified ophthalmologists. If we are unable to consistently attract, hire and retain qualified ophthalmologists, our ability to attract patients could be negatively affected.
If technological improvements occur which render our equipment or services obsolete, we may need to make significant capital expenditures and might not have the financial resources to do so.
Newer technologies, techniques or products for the treatment of refractive vision disorders could be developed with better performance than the excimer laser technology we currently use. If new and better ophthalmic laser technology or other surgical or non-surgical methods for correcting refractive vision disorders is introduced that we would like to use in our vision centers, we may be unable to acquire such new technologies or may have to make significant capital expenditures to do so. If we do not have access to sufficient funds to acquire such new technologies, our ability to attract patients could be negatively affected.
If a better-financed or lower-cost provider of laser vision correction or a competing vision treatment forces us to lower our laser surgery prices in a particular geographic area, our revenues and profitability could decline.
Laser eye surgery competes with other surgical and non-surgical treatments for refractive vision disorders, including eyeglasses, contact lenses, other types of refractive surgery, corneal implants and other technologies currently under development. Among providers of laser vision correction, competition will come from firms similar to us and from hospitals, hospital-affiliated group entities, physician group practices and private ophthalmologists, among others, that, in order to offer laser vision correction to patients, purchase or rent excimer lasers. Suppliers of conventional eyeglasses and contact lenses, such as optometry chains, also may compete with us by purchasing laser systems and offering laser vision correction to their customers.
According to a report on the U.S. refractive market published by MarketScope in August 2003, as of the second quarter of 2003, independent surgeons represent 60% of the providers of laser vision correction services, corporate providers represent 32.9% and institutions represent 6%. According to the same report, among the corporate providers, our principal competitors are TLC Vision, which, based on the number of procedures performed, represents 49.5% of the corporate segment of the market for laser vision correction services and Laser Vision Institute, which represents 12.7%. The report indicated that we represent 18.3% of the corporate segment of the market.
7
Risk factors
Some of our competitors or companies that may choose to enter the industry in the future, including laser manufacturers themselves, may have substantially greater financial, technical, managerial, marketing or other resources and experience than we do and compete more effectively than us. Competition in the market for laser vision correction could increase as excimer laser surgery becomes more common and the number of ophthalmologists performing the procedure increases. Additional competition may develop, particularly as the price to purchase or rent excimer laser systems decreases. Our management, operations, strategy and marketing plans may not be successful in meeting this competition.
If more competitors offer laser vision correction or other competitive types of vision treatments in a given geographic market, we might find it necessary to reduce the prices we charge, particularly if such competitors offer the procedures at lower prices than we do. If that were to happen, we may not be able to make up for the reduced profit margin by increasing the number of procedures we perform, and our revenues and profitability could decrease, as we have experienced in prior fiscal periods.
Changes in general economic conditions may cause fluctuations in our revenues and profitability.
The cost of laser vision correction procedures is typically not reimbursed by third-party payors such as health care insurance companies or government programs. Accordingly, as we have experienced in prior fiscal periods, our operating results may vary based upon the impact of changes in economic conditions on the disposable income of consumers interested in laser vision correction. A significant decrease in consumer disposable income in a weakening economy may result in a decrease in the number of laser vision procedures performed and a decline in our revenues and profitability. In addition, weakening economic conditions may cause some of our customers to experience financial distress or declare bankruptcy, which may negatively impact our accounts receivable collection experience and adversely affect our results of operations and cash flow.
Our business has been adversely affected in the past by deeply-discounted pricing by some competitors, and it is possible that such competitive practices may adversely affect our business in the future.
In the past, certain competitors have utilized deeply-discounted pricing in an effort to generate procedural volume. These practices have in the past caused periods of intense price competition in our industry. As a result, we have lowered our prices in the past in order to remain competitive. While two of the larger heavily-discounted providers of laser vision correction services have ceased business, other competitors offer discounts in some of the geographic markets where we conduct business. It is possible that in the future, our revenues and profitability could decrease as a result of the discounting practices of competitors.
Our stock price has been and is likely to continue to be highly volatile, and an investment in our stock could suffer a decline in value.
The price of our common stock has been, and is likely to continue to be, highly volatile. In the period from January 1, 2001 through the date of this prospectus, our stock has traded at a high of $19.50 per share and a low of $1.86 per share. Our common stock has not historically had a large trading market, as evidenced by an average daily trading volume of 17,293 shares for the fiscal year ended 2002 and 67,811 shares for the nine months ended September 30, 2003. Our stock price may fluctuate substantially due to a number of factors, including:
| |
| • | actual or anticipated fluctuation in our results of operations, |
| |
| • | lack of institutional ownership in our common stock, |
| |
| • | illiquid nature of trading in our common stock, |
| |
| • | technological innovations, |
| |
| • | increased competition, |
| |
| • | conditions and trends in the laser refractive surgery industry, |
8
Risk factors
| |
| • | the lack of diversification of our revenues, |
| |
| • | general market conditions of the US economy, |
| |
| • | geopolitical risks that destabilize the world economy, and |
| |
| • | changes in or our failure to meet market or, to the extent securities analysts follow our common stock, securities analysts’ expectations. |
In addition, the stock market has from time to time experienced significant price and volume fluctuations that have affected the market prices for the securities of healthcare companies, including us. These broad market fluctuations may result in a material decline in the market price of our common stock, regardless of our operating performance. As a result, the value of your investment in our common stock could decline in value.
A listed company’s stock must maintain a minimum price in order to remain listed on the Nasdaq National Market. Our November 2002 1 for 4 reverse stock split followed receipt by us of a notice from the Nasdaq National Market that our stock was below the minimum price required to maintain listing.
In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against that company. We may become involved in this type of litigation in the future. Litigation is often expensive and diverts management’s attention and resources.
We have recently expanded our direct financing program and as a result may incur increased credit risk which could negatively affect our cash flow and results of operations.
We provide certain of our customers, including customers who could not otherwise obtain third-party financing, with the ability to pay for our procedures with direct financing. The terms of our direct financing typically require the customer to pay a set fee up front, which is intended to cover some or all of our variable costs, and pay the remaining balance in up to 36 equal monthly installments through direct withdrawal from his or her bank account. As a result of a recent expansion in the program, as of September 30, 2003, we had $3.0 million in accounts receivable, a substantial increase of approximately $2.6 million from our accounts receivable as of December 31, 2002. We are now exposed to increased credit risk than in the past, particularly given that some or all of our customers who participate in our direct financing program have not been deemed creditworthy by third-party financing companies with more experience in credit issues than we have. If the uncollectible amounts exceed the amounts we have reserved, we could be required to write down our accounts receivable, and our cash flow and results of operations could decrease.
If laser vision correction does not gain broader market acceptance, our profitability and growth would be severely limited.
We derive substantially all of our revenues from laser vision correction. As a result, we believe that our profitability and expansion depend to a large extent on the acceptance of laser vision correction as a safe and effective treatment. There can be no assurance that laser vision correction will be widely accepted by ophthalmologists, optometrists or the general population as an alternative to existing methods of treating refractive vision disorders. Acceptance of laser vision correction may be affected adversely by:
| |
| • | concerns about the safety and effectiveness of laser vision correction procedures, including procedures using new technologies or techniques such as custom ablation, |
| |
| • | general resistance to surgery of any type, and eye surgery in particular, |
| |
| • | its costs, particularly since laser vision correction is not typically covered by government or private insurers, |
| |
| • | the effectiveness of alternate methods of correcting refractive vision disorders, |
| |
| • | the lack of long-term follow-up data and the possibility of unknown side effects, |
9
Risk factors
| |
| • | regulatory developments, and |
| |
| • | reported adverse events or other unfavorable publicity involving patient outcomes from laser vision correction. |
Concerns about potential side effects and long-term results may negatively impact market acceptance of laser vision correction, result in potential liability for us and prevent us from growing our business.
Concerns have been raised with respect to the predictability and stability of results and potential complications or side effects of laser vision correction. Any complications or side effects of laser vision correction may call into question its safety and effectiveness, which in turn may negatively affect market acceptance of laser vision correction. Complications or side effects of laser vision correction could lead to professional liability, malpractice, product liability or other claims against us.
Some of the possible side effects of laser vision correction may include:
| |
| • | foreign body sensation, |
| |
| • | pain or discomfort, |
| |
| • | sensitivity to bright lights, |
| |
| • | blurred vision or haze, |
| |
| • | dryness or tearing, |
| |
| • | fluctuation in vision, |
| |
| • | night glare, |
| |
| • | poor or reduced visual quality, |
| |
| • | overcorrection or under correction, |
| |
| • | regression, and |
| |
| • | corneal flap or corneal healing complications. |
Laser vision correction may also involve the removal of “Bowman’s membrane,” an intermediate layer between the outer corneal layer and the middle corneal layer of the eye. The effect of the removal of Bowman’s membrane on patients is currently not clear.
We depend on limited sources for the excimer lasers, diagnostic equipment, microkeratomes and disposable blades we use, and shortages of these items could hinder our ability to increase our procedure volume.
We currently use several suppliers, including Bausch & Lomb, VISX and Alcon, for our excimer lasers and diagnostic equipment in the United States. If one or more of these companies becomes unwilling or unable to supply us with excimer lasers and diagnostic equipment, to repair or replace parts, or to provide services, our ability to open new centers or increase our capacity to perform laser vision correction services could be restricted.
We currently rely primarily on Bausch & Lomb to provide us with microkeratomes, the devices used to create the corneal flap in the LASIK procedure, as well as with microkeratome blades and other disposable items required for LASIK. There are a limited number of manufacturers of microkeratomes and microkeratome blades, and there can be no assurance that microkeratomes and microkeratome blades will be available in the quantities or within the time frames we require. Any shortages in our supplies of this equipment could limit our ability to maintain or increase the volume of procedures that we perform, which could result in a decrease in our revenues and profitability.
10
Risk factors
Our business may be impaired due to government regulations which could restrict our equipment, services and relationships with ophthalmologists, optometrists and other healthcare providers.
We, excimer laser manufacturers and our other business partners, including managed care companies and third-party patient financing companies, among others, are subject to extensive federal, state and foreign laws, rules and regulations, including:
| |
| • | restrictions on the approval, distribution and use of medical devices, |
| |
| • | anti-kickback statutes, |
| |
| • | fee-splitting laws, |
| |
| • | corporate practice of medicine restrictions, |
| |
| • | self-referral laws, |
| |
| • | anti-fraud provisions, |
| |
| • | facility license requirements and certificates of need, |
| |
| • | privacy laws and regulations, |
| |
| • | conflict of interest regulations, |
| |
| • | rules and regulations regarding advertising and marketing practices, and |
| |
| • | sales and use taxes. |
Some of these laws and regulations are vague or ambiguous, and courts and regulatory authorities have not always provided clarification. Moreover, state and local laws vary from jurisdiction to jurisdiction. As a result, some of our activities could be challenged, sometimes successfully.
The excimer lasers we use in our vision centers are medical devices that, in the United States, are subject to the jurisdiction of the Food and Drug Administration. In addition to FDA approval for the initial uses of these excimer lasers, new uses require separate approval. Obtaining such approval can be an expensive and time-consuming process, the success of which cannot be guaranteed. The failure of our suppliers to obtain regulatory approvals for any additional uses of excimer lasers or otherwise comply with regulatory requirements could limit the number of excimer lasers we have available for use and, therefore, limit the number of procedures we can perform.
Failure of the laser manufacturers to comply with applicable FDA requirements could subject us, ophthalmologists who practice in our vision centers or excimer laser manufacturers to enforcement actions, including product seizure, recalls, withdrawal of approvals and civil and criminal penalties. Further, failure to comply with regulatory requirements, or any adverse regulatory action, could result in limitations or prohibitions on our use of excimer lasers. Any such actions or proceedings could result in negative publicity which could result in decreased demand for our services and could result in a decrease in our capacity to perform laser vision correction services.
The use of an excimer laser to treat both eyes on the same day (bilateral treatment) has not been approved by the FDA. The FDA has stated that it considers the use of the excimer laser for bilateral treatment to be a practice of medicine decision, which the FDA is not authorized to regulate. Ophthalmologists, including those practicing in our vision centers, widely perform bilateral treatment in an exercise of professional judgment in connection with the practice of medicine. There can be no assurance that the FDA will not seek to challenge this practice in the future. Should the FDA choose to regulate this aspect of the use of excimer lasers in the future, any potential resulting inconvenience to patients could discourage potential patients from having laser vision correction services and could, therefore, cause the total number of procedures we perform to decrease.
Our business is heavily dependent on advertising, which is subject to regulation by the Federal Trade Commission. In 2002 the FTC conducted an extensive review of our advertising practices. Following this review, the FTC concluded that certain of our past advertisements contained claims that were not
11
Risk factors
properly substantiated. We elected to voluntarily settle with the FTC rather than incur the significant expense and distraction from our business that would have resulted from prolonged litigation. On July 18, 2003, the FTC formally entered a Complaint and an Agreement Containing Consent Order in which we agreed, among other things, that we would not represent in our advertising that our LASIK surgery services eliminate the need for glasses and contacts for life, pose significantly less risk to patients’ eye health than wearing glasses or contacts or eliminate the risk of glare and haloing, unless, at the time made, we possess and rely upon competent and reliable scientific evidence that substantiates the representation. No monetary penalties were imposed on us. We cannot be certain that this order, to which we agreed, will not substantially limit the nature and scope of our future marketing and advertising practices which is an important part of our strategy to generate demand for our laser vision correction services.
We are subject to lawsuits for patient injuries, which could subject us to significant judgments and damage our reputation.
The laser vision correction procedures performed in our vision centers involve the potential risk of injury to patients. Such risk could result in professional liability, malpractice, product liability, or other claims brought against us based upon injuries or alleged injuries associated with a defect in a product’s performance or malpractice by an ophthalmologist, optometrist, technician or other health care professional. Some injuries or defects may not become evident for a number of years. The operation of any excimer laser, diagnostic equipment, microkeratome or other equipment may result in substantial claims against us by patients who allege they were injured as a result of vision correction procedures. Significant lawsuits against us could subject us to significant judgments and damage our reputation. In addition, a partially or completely uninsured claim against us could have a material adverse effect on our business, financial condition and results of operations. We primarily rely and intend to continue to rely on ophthalmologists’ professional liability insurance policies and manufacturers’ insurance policies for product liability coverage, although we have limited umbrella product and professional liability insurance. We generally require the ophthalmologists who use our vision centers to maintain certain levels of professional liability insurance, although there can be no guarantee that the ophthalmologists will be successful in obtaining or maintaining such insurance coverage, particularly in the current insurance market.
The availability of professional liability insurance has decreased and its cost has increased significantly for a variety of reasons, including reasons outside our control. A future increase in cost could result in the reduced profitability of our business, and a future lack of availability of coverage for us or the doctors could result in increased exposure to liability.
We established a captive insurance company, and if significant claims are paid, it could affect our profitability and our financial condition.
Effective as of December 18, 2002, we established a captive insurance company to provide professional liability insurance coverage for claims brought against us after December 17, 2002. In addition, our captive insurance company’s charter allows it to provide professional liability insurance for our doctors, some of whom are currently insured by the captive. Our captive insurance company is managed by an independent insurance consulting and management firm and is capitalized and funded by us based on actuarial studies performed by an affiliate of the consulting and management firm. For the 12 months ending December 17, 2003, our captive insurance company purchased excess liability coverage for 80% of our losses in the year in excess of $1,000,000 per occurrence, up to $11,000,000. Under that arrangement, the coverage providers’ obligation arises only after our captive pays the first $1,000,000 of any loss and the coverage providers are only obligated to pay an aggregate of $8,000,000 in a given policy year. These excess liability coverage policies are currently up for renewal, and our management may elect to purchase similar, less, more or no excess liability coverage depending on the premiums quoted, among other factors. A number of claims covered by our captive insurance company are now pending. The payment of significant claims by our captive insurance company, an increase in our premiums or our assumption of incremental risk could negatively affect our profitability and our financial condition.
12
Risk factors
Our captive insurance company may not be able to successfully alleviate risk through excess liability coverage, which could result in material losses.
In order to reduce risk, our captive insurance company has to date purchased excess liability coverage. The availability and the cost of excess liability coverage is subject to market conditions, which are outside of our control. As a result, our captive insurance company may not be able to successfully alleviate risk through these arrangements in the future or these agreements may become significantly more expensive for us to obtain. In addition, we are still subject to credit risk with respect to our captive’s excess coverage providers given that the ceding of risk to such providers does not relieve the captive of its insurance liabilities. Such providers’ insolvency or inability to make payments under the terms of an excess coverage arrangement with our captive, therefore, could result in material losses.
We may have substantial future capital requirements, and our ability to obtain additional funding is uncertain.
We are unable to predict with certainty the timing or the amount of our future capital requirements. Operating losses or changes in our operations, expansion plans or capital requirements may consume available cash and other resources more rapidly than we anticipate and additional funding may be required. Our capital needs depend on many factors, including:
| |
| • | the rate and cost of purchases of equipment and other assets, |
| |
| • | the rate of opening new vision centers or expanding existing vision centers, |
| |
| • | market acceptance of laser vision correction, |
| |
| • | any strategic transactions we may make in the future, and |
| |
| • | actions by competitors. |
We may not have adequate resources to finance the growth in our business, and we may not be able to obtain additional capital through subsequent equity or debt financings on terms acceptable to us or at all. If we do not have adequate resources and cannot obtain additional capital, we will not be able to implement our expansion strategy successfully, our growth could be limited and our results of operations could decline.
Investors in this offering will experience immediate and substantial dilution.
The public offering price of our common stock is expected to be substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will incur immediate and substantial dilution in the pro forma net tangible book value per share of common stock from the price per share that you pay for the common stock. If the holders of outstanding options exercise those options at prices below the public offering price, you will incur further dilution.
Disputes with respect to intellectual property could result in a decrease in revenues and profitability.
There has been substantial litigation in the United States, Canada and elsewhere regarding the patents on ophthalmic lasers. If the use of an excimer laser or other procedure performed at any our vision centers is deemed to infringe a patent or other proprietary right, we may be named as a defendant in ensuing litigation, prohibited from using the equipment or performing the procedure that is the subject of the patent dispute, or required to obtain a royalty-bearing license, which may involve substantial costs, including ongoing royalty payments. If a license is not available on acceptable terms, we may be required to seek the use of products that do not infringe the patent. The unavailability of alternate products could cause us to cease operations in the United States, Canada or elsewhere or delay our expansion. If we are prohibited from performing laser vision correction at any of our vision centers, our revenues and profitability could decrease.
13
Risk factors
We are dependent on a small number of senior managers.
Our success depends, to a significant extent, upon the efforts and abilities of our Chairman and Chief Executive Officer, Stephen N. Joffe, and other members of senior management. We currently do not have employment agreements with senior key employees, other than short-term employment agreements with Kevin M. Hassey, our President, and Alan H. Buckey, our Executive Vice President/ Finance and Chief Financial Officer. We do not carry key man insurance on any of these key employees. The loss of the services of one or more of these key employees could have a material adverse effect on our business.
Stephen N. Joffe, our Chairman and Chief Executive Officer, and members of his family control a number of shares sufficient to influence corporate actions.
Members of the Joffe family together will own or control approximately 24.7% of our outstanding common stock after this offering (21.3% if the underwriters’ over-allotment option is exercised in full). The interests of the Joffe family may differ from those of our other stockholders, and they may take actions that advance their interests to the detriment of our other stockholders. If these persons acted together, they would have sufficient voting power to influence the outcome of corporate actions submitted to the stockholders for approval and to influence our management and affairs, including the election of our Board of Directors. In addition, this concentration of ownership may prevent attempts to remove or replace senior management. Stephen N. Joffe is the Chairman of our Board of Directors and our Chief Executive Officer. Craig P.R. Joffe, his son, is our Senior Vice President, General Counsel and Secretary.
Anti-takeover provisions under Delaware law and certain provisions of our certificate of incorporation may make a change in our management or an acquisition of us, which may be beneficial to our stockholders, more difficult.
We are incorporated in Delaware. Certain anti-takeover provisions of Delaware law, in addition to the control of a number of shares of our common stock by the Joffe family, may make changes in the management and control of our company more difficult, even if such changes would be beneficial to the stockholders. Delaware law also prohibits corporations from engaging in a business combination with any holders of 15% or more of their capital stock until the holder has held the stock for three years unless our board of directors or stockholders approve the transaction or certain other conditions are satisfied. Our board of directors may use these provisions to prevent changes in the management and control of our company. In addition, under applicable Delaware law, our board of directors may adopt additional anti-takeover measures in the future.
Pursuant to our certificate of incorporation, our board of directors is authorized to issue, without stockholder approval, any or all of our authorized but unissued shares of preferred stock with any dividend, redemption, conversion or exchange provisions as the board may designate. The rights of common stockholders would be subordinate and subject to and may be adversely affected by the rights of preferred stockholders. Issuance of preferred stock could have the effect of entrenching our board of directors and making it more difficult or discouraging for a third-party to acquire a majority of our outstanding voting stock.
Substantial future sales of our common stock by us or by our existing stockholders could cause our stock price to decline.
Additional equity financings or other securities issuances by us could adversely affect the market price of our common stock. In addition, sales by existing stockholders of a large number of shares of our common stock in the public market or the perception that additional sales could occur could cause the market price of our common stock to drop.
We have not historically paid dividends, and we do not presently anticipate declaring or paying any dividends.
We have not declared or paid any dividends on our common stock in the past and do not presently anticipate declaring or paying any dividends. As a result, you should assume that the return on an investment in our common stock will depend upon any future appreciation in value. Our common
14
Risk factors
stock may not appreciate in value or even maintain the price at which stockholders have purchased their shares, particularly given the dilution as a result of this offering.
We will have broad discretion as to the use of proceeds of this offering and may fail to use them effectively.
We expect to use a portion of the net proceeds of this offering to fund expansion of our business. However, our management will have broad discretion in utilizing the proceeds and may use them in ways with which you and our other shareholders may disagree, including diversifying our revenues into lines of business in which we are not currently engaged or do not have experience. We may not be able to invest the proceeds of this offering effectively, particularly given current market rates, which could adversely affect our profitability and financial condition.
15
Risk factors
Forward-looking statements
This prospectus and the documents incorporated by reference in this prospectus include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends affecting the financial condition of our business. These forward-looking statements are subject to a number of risks, uncertainties and assumptions about us, including, among other things:
| |
| • | our belief that the market for laser vision correction is growing, |
| |
| • | our strategy to expand through internal growth by adding new vision centers and through potential strategic transactions we may enter in the future, |
| |
| • | our belief that increases in marketing and advertising will cause higher demand for our laser vision correction services, |
| |
| • | our expectations and estimates concerning future financial performance, financing plans and the impact of competition, |
| |
| • | our belief that we will be able to conform our operations in all material respects to applicable healthcare laws and obtain any necessary licenses and certificates of need, |
| |
| • | general economic and business conditions, both nationally and in our markets, and |
| |
| • | other risk factors set forth under “Risk factors” in this prospectus and in the documents incorporated by reference in this prospectus. |
In addition, in this prospectus, the words “believe”, “may”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “plan”, “expect”, “hope” and similar expressions, as they relate to us, our business or our management, are intended to identify forward-looking statements.
We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus and in the documents incorporated by reference in this prospectus may not occur, and actual results could differ materially from those anticipated or implied in the forward-looking statements.
16
Use of proceeds
The net proceeds we will receive from the sale of common stock by us in this offering are estimated to be approximately $37.0 million after deducting underwriting discounts and commissions and estimated offering expenses. We will not receive any proceeds from the sale of shares by the selling stockholder. We intend to use the net proceeds as follows:
| |
| • | to open additional laser vision correction centers, |
| |
| • | to purchase additional technology and equipment, |
| |
| • | to fund potential strategic transactions we may enter in the future, and |
| |
| • | to provide working capital for general corporate purposes. |
We currently have no agreements or understandings with respect to any material strategic transactions. We have not allocated any portion of the net proceeds from this offering to a specific purpose and will have broad discretion in how to use our net proceeds. Until we use the net proceeds from this offering as described above, we intend to temporarily invest the net proceeds in short-term, investment-grade, interest-bearing securities or obligations of, or guaranteed by, the US government, which do not currently have significant yields given current market rates.
17
Price range of common stock
Since June 30, 1999 our common stock has been included for quotation on the Nasdaq National Market under the symbol “LCAV.” From 1996 through June 29, 1999, our common stock was included for quotation on the Nasdaq SmallCap Market under the Symbol “LCAV.” The following table sets forth the high and low bid prices of the common stock as reported by the Nasdaq National Market for the specified periods.
| | | | | | | | | |
| | High | | Low |
|
|
Fiscal year ended December 31, 2001 | | | | | | | | |
| First Quarter | | $ | 13.50 | | | $ | 4.38 | |
| Second Quarter | | | 13.75 | | | | 9.12 | |
| Third Quarter | | | 10.76 | | | | 4.04 | |
| Fourth Quarter | | | 4.76 | | | | 2.32 | |
Fiscal year ended December 31, 2002 | | | | | | | | |
| First Quarter | | $ | 7.96 | | | $ | 3.20 | |
| Second Quarter | | | 7.52 | | | | 3.60 | |
| Third Quarter | | | 5.08 | | | | 2.40 | |
| Fourth Quarter | | | 3.40 | | | | 1.86 | |
Fiscal year ending December 31, 2003 | | | | | | | | |
| First Quarter | | $ | 3.74 | | | $ | 2.25 | |
| Second Quarter | | | 9.75 | | | | 3.32 | |
| Third Quarter | | | 19.50 | | | | 8.57 | |
| Fourth Quarter (through December 3, 2003) | | | 18.75 | | | | 13.36 | |
As of December 3, 2003, there were approximately 2,425 holders of record of our common stock. As of December 3, 2003, the last reported sale price of our common stock on the Nasdaq National Market was $17.61 per share.
Dividend policy
We have not declared or paid any dividends in the past, and we do not presently anticipate declaring or paying any dividends in the future, on our common stock. Any future determination as to the declaration and payment of dividends will be at the discretion of our Board of Directors and will depend on then-existing conditions, including:
| |
| • | our financial condition, |
| |
| • | results of operations, |
| |
| • | contractual restrictions, |
| |
| • | capital requirements, |
| |
| • | business prospects, and |
| |
| • | any other factors our board of directors may deem relevant. |
18
Capitalization
The following table sets forth our capitalization as of September 30, 2003:
| |
| • | on an actual basis, and |
| |
|
| • | as adjusted to reflect this offering and the receipt of the estimated net proceeds to us from this offering. |
|
The following table should be read in conjunction with the “Management’s discussion and analysis of financial condition and results of operations” included elsewhere in this prospectus, our consolidated financial statements and related notes and the other financial information included elsewhere in this prospectus or incorporated herein by reference.
| | | | | | | | | | | | |
| | |
| | As of September 30, 2003 |
| |
|
| | Actual | | As adjusted |
|
|
| | |
| | (unaudited) |
| | |
| | (in thousands) |
| Debt maturing in one year | | | –0– | | | | –0– | |
| Shareholders’ investment: | | | | | | | | |
| | Preferred stock, par value $.001 per share, 5,000,000 shares authorized, no shares issued and outstanding, actual or as adjusted | | | –0– | | | | –0– | |
| | Common stock par value $.001 per share: 27,500,000 shares authorized, 13,169,923 (15,569,923, as adjusted) issued and 10,802,726 (13,202,726 as adjusted) outstanding | | | 13 | | | | 16 | |
| | Contributed capital | | | 91,800 | | | | 128,796 | |
| | Warrants | | | 1,982 | | | | 1,982 | |
| | Notes receivable from shareholders | | | (225 | ) | | | (225 | ) |
| | Treasury stock | | | (15,462 | ) | | | (15,462 | ) |
| | Accumulated deficit | | | (39,270 | ) | | | (39,270 | ) |
| | Accumulated other comprehensive income | | | 64 | | | | 64 | |
| | | |
| | | |
| |
| | | Total shareholders’ investment | | | 38,902 | | | | 75,901 | |
| | | |
| | | |
| |
| | | | Total capitalization | | $ | 38,902 | | | $ | 75,901 | |
| | | |
| | | |
| |
The number of shares of our common stock in the actual and as adjusted columns in the table above excludes:
| |
| • | an aggregate of 963,427 shares of common stock reserved for issuance upon exercise of outstanding options at a weighted average exercise price of $12.00 per share; and |
| |
| • | an aggregate of 1,091,863 shares of common stock available for future issuance under our 1995, 1998 and 2001 stock option plans. |
19
Dilution
Purchasers of the common stock in this offering will experience immediate and substantial dilution in the net tangible book value of the common stock from the initial public offering price. Net tangible book value per share represents the amount of our total tangible assets less total liabilities, divided by the total number of shares of our common stock outstanding.
At September 30, 2003, we had a net tangible book value of $38.6 million or $3.58 per share of common stock. After giving effect to the sale of 2,400,000 shares of common stock offered by this prospectus, and after the deduction of underwriting discounts and commissions and estimated offering expenses, our pro forma net tangible book value at September 30, 2003 would have been $75.6 million or $5.73 per share of common stock. This represents an immediate increase in such net tangible book value of $2.15 per share to existing stockholders and an immediate and substantial dilution of $10.77 per share to new investors purchasing common stock in this offering.
The following table illustrates this dilution per share:
| | | | | | | | | | |
| Public offering price | | | | | | $ | 16.50 | |
| | Net tangible book value per share as of September 30, 2003 | | $ | 3.58 | | | | | |
| | Increase attributable to new investors | | | 2.15 | | | | | |
| | | |
| | | | | |
| Pro forma net tangible book value after this offering | | | | | | | 5.73 | |
| | | | | | | |
| |
| Dilution in pro forma net tangible book value to new investors | | | | | | $ | 10.77 | |
| | | | | | | |
| |
The foregoing table does not take into effect further dilution to new investors that could occur upon the exercise of outstanding options. At September 30, 2003, there were:
| |
| • | an aggregate of 963,427 shares of common stock reserved for issuance upon exercise of outstanding options at a weighted average exercise price of $12.00 per share; and |
| |
| • | an aggregate of 1,091,863 shares of common stock available for future issuance under our 1995, 1998 and 2001 stock option plans. |
20
Selected consolidated financial data
The consolidated statement of operations data set forth below for the years ended December 31, 2000, 2001 and 2002 and the balance sheet data at December 31, 2001 and 2002 are derived from our audited consolidated financial statements included and incorporated by reference in this prospectus. The statement of operations data set forth below for the years ended December 31, 1998 and 1999 and the balance sheet data at December 31, 1998, 1999 and 2000 are derived from our audited financial statements not included or incorporated by reference in this prospectus. The following summary interim financial data as of September 30, 2003 and for the nine months ended September 30, 2002 and 2003 are unaudited and are derived from the interim financial statements included and incorporated by reference in this prospectus. In the opinion of our management, the unaudited data have been prepared on the same basis as the audited consolidated financial statements and include all adjustments, consisting only of normal recurring adjustments, necessary for fair presentation. Results for interim periods are not indicative of results for a full year. The data set forth below should be read in conjunction with the consolidated financial statements and related notes and “Management’s discussion and analysis of financial condition and results of operations” included elsewhere in this prospectus.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | Nine months ended |
| | Year ended December 31, | | September 30, |
| Consolidated statement of operations | |
| |
|
| data | | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | | 2002 | | 2003 |
|
|
| | |
| | (unaudited) |
| | |
| | (in thousands, except for per share data) |
| Revenues: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Laser refractive surgery | | $ | 32,508 | | | $ | 56,358 | | | $ | 63,450 | | | $ | 68,096 | | | $ | 61,838 | | | $ | 48,538 | | | $ | 60,661 | |
| | Other | | | 2,692 | | | | 1,026 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total revenues | | | 35,200 | | | | 57,384 | | | | 63,450 | | | | 68,096 | | | | 61,838 | | | | 48,538 | | | | 60,661 | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Medical professional and license fees | | | 13,700 | | | | 22,930 | | | | 15,542 | | | | 13,626 | | | | 12,270 | | | | 9,791 | | | | 11,846 | |
| | Direct costs of services | | | 12,526 | | | | 16,113 | | | | 27,218 | | | | 33,616 | | | | 28,796 | | | | 22,127 | | | | 23,982 | |
| | General and administrative expenses | | | 6,198 | | | | 6,036 | | | | 9,394 | | | | 8,727 | | | | 8,327 | | | | 6,454 | | | | 6,073 | |
| | Marketing and advertising | | | 2,183 | | | | 5,671 | | | | 14,565 | | | | 12,732 | | | | 12,823 | | | | 10,179 | | | | 9,164 | |
| | Depreciation and amortization | | | 3,521 | | | | 2,964 | | | | 3,839 | | | | 5,625 | | | | 5,997 | | | | 4,458 | | | | 4,692 | |
| | Special charges | | | 10,500 | | | | (150 | ) | | | — | | | | 1,774 | | | | (174 | ) | | | (174 | ) | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | Total expenses | | | 48,628 | | | | 53,564 | | | | 70,558 | | | | 76,100 | | | | 68,039 | | | | 52,835 | | | | 55,757 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Operating (loss) income | | | (13,428 | ) | | | 3,820 | | | | (7,108 | ) | | | (8,004 | ) | | | (6,201 | ) | | | (4,297 | ) | | | 4,904 | |
| Equity in earnings from unconsolidated businesses | | | 354 | | | | 316 | | | | 49 | | | | 372 | | | | 241 | | | | 228 | | | | 245 | |
| Interest expense | | | (786 | ) | | | (169 | ) | | | (58 | ) | | | (17 | ) | | | (4 | ) | | | (3 | ) | | | (17 | ) |
| Interest income | | | 441 | | | | 1,633 | | | | 2,713 | | | | 924 | | | | 225 | | | | 110 | | | | 280 | |
| Litigation settlement | | | — | | | | — | | | | — | | | | — | | | | 2,282 | | | | 2,282 | | | | — | |
| Other income (expense) | | | 358 | | | | 6 | | | | 604 | | | | (61 | ) | | | (195 | ) | | | (160 | ) | | | (163 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income before taxes on income | | | (13,061 | ) | | | 5,606 | | | | (3,800 | ) | | | (6,786 | ) | | | (3,652 | ) | | | (1,840 | ) | | | 5,249 | |
| Income tax expense (benefit) | | | 157 | | | | (5,287 | ) | | | (1,434 | ) | | | 16,589 | | | | 174 | | | | 98 | | | | 181 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Net (loss) income | | | (13,218 | ) | | | 10,893 | | | | (2,366 | ) | | | (23,375 | ) | | | (3,826 | ) | | | (1,938 | ) | | | 5,068 | |
| Dividends to preferred shareholders | | | 518 | | | | 140 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income available to common shareholders | | $ | (13,736 | ) | | $ | 10,753 | | | $ | (2,366 | ) | | $ | (23,375 | ) | | $ | (3,826 | ) | | $ | (1,938 | ) | | $ | 5,068 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (Loss) income per common share | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Basic | | $ | (1.46 | ) | | $ | 0.89 | | | $ | (0.19 | ) | | $ | (2.01 | ) | | $ | (0.35 | ) | | $ | (0.18 | ) | | $ | 0.47 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Diluted | | $ | (1.46 | ) | | $ | 0.84 | | | $ | (0.19 | ) | | $ | (2.01 | ) | | $ | (0.35 | ) | | $ | (0.18 | ) | | $ | 0.47 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Weighted average shares used in computation | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Basic | | | 9,417 | | | | 11,998 | | | | 12,741 | | | | 11,643 | | | | 10,794 | | | | 10,866 | | | | 10,753 | |
| | Diluted | | | 9,417 | | | | 12,729 | | | | 12,741 | | | | 11,643 | | | | 10,794 | | | | 10,866 | | | | 10,856 | |
| |
Selected operating data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Laser vision correction procedures | | | 19,791 | | | | 33,266 | | | | 59,144 | | | | 72,032 | | | | 57,104 | | | | 44,900 | | | | 49,425 | |
continued on next page
21
Selected consolidated financial data
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | |
| | At December 31, | | At September 30, |
| |
| |
|
| Balance sheet data: | | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | | 2003 |
|
|
| | (unaudited) |
| | |
| | (in thousands) |
| Cash and cash equivalents | | $ | 6,496 | | | $ | 11,891 | | | $ | 19,692 | | | $ | 16,609 | | | $ | 18,298 | | | $ | 25,260 | |
| Short-term investments | | | — | | | | 37,299 | | | | 8,626 | | | | — | | | | — | | | | — | |
| Working capital | | | 3,577 | | | | 49,212 | | | | 24,063 | | | | 14,378 | | | | 12,965 | | | | 22,131 | |
| Total assets | | | 31,377 | | | | 85,290 | | | | 75,597 | | | | 43,188 | | | | 39,996 | | | | 48,181 | |
| Debt maturing in one year | | | 787 | | | | 676 | | | | 178 | | | | 26 | | | | 10 | | | | — | |
| Total debt, excluding current portion | | | 2,724 | | | | 250 | | | | 48 | | | | 4 | | | | — | | | | — | |
| Preferred stock | | | 7,687 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Accumulated deficit | | | (25,664 | ) | | | (14,771 | ) | | | (17,137 | ) | | | (40,512 | ) | | | (44,338 | ) | | | (39,270 | ) |
| Total shareholders’ investment | | | 23,199 | | | | 80,045 | | | | 65,045 | | | | 38,202 | | | | 32,112 | | | | 38,902 | |
22
Management’s discussion and analysis of financial condition
and results of operations
You should read the following discussion and analysis in conjunction with the “Selected consolidated financial data” and the accompanying financial statements and related notes included elsewhere in this prospectus or incorporated herein by reference. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed here. Factors that could contribute to such differences include, but are not limited to, those discussed in “Risk factors.”
OVERVIEW
We are a leading developer and operator of fixed-site laser vision correction centers under the brand name LasikPlus. Our vision centers provide the facilities, equipment and support services for performing laser vision correction that employs advanced laser technologies to help correct nearsightedness, farsightedness and astigmatism. We currently utilize fixed-site excimer lasers manufactured by Bausch & Lomb, VISX and Alcon. Substantially all of our revenues currently are derived from LASIK procedures performed in our US vision centers. In July 1999, we began converting our vision centers to closed access facilities from open access facilities in order to obtain increased control over the quality of care we provide to our patients and greater operational growth and control of our business.
Our operating costs and expenses include:
| |
| • | medical professional and license fees, including per procedure fees for the ophthalmologists performing laser vision correction and the license fees per procedure paid to VISX, Bausch & Lomb and Alcon, |
| |
| • | direct costs of services, including center rent and utilities, equipment lease and maintenance costs, surgical supplies, center staff expense and costs related to other revenues, |
| |
| • | general and administrative costs, including headquarters staff expense and other overhead costs, |
| |
| • | marketing and advertising costs, and |
| |
| • | depreciation of equipment. |
Our centers have a relatively high degree of operating leverage due to the fact that many of our costs are fixed in nature. As a result, our level of procedure volume can have a significant impact on our level of profitability.
REVENUES
We derived substantially all of our revenues for the last three years from the delivery of LASIK laser vision correction services.
Our revenues in any period are primarily a function of the number of laser vision correction procedures performed and the pricing for such services.
Our revenues are impacted by a number of factors, including the following:
| |
| • | our ability to generate customers through our arrangements with managed care companies, direct to consumer advertising and word of mouth referrals, |
| |
| • | our mix of procedures among the different types of laser technology used by us, |
| |
| • | new center openings and our ability to increase procedure volume at existing centers, |
| |
| • | the availability of patient financing, |
| |
| • | general economic conditions and consumer confidence levels, |
23
Management’s discussion and analysis of financial condition and results of operations
| |
| • | the continued growth and increased acceptance of LASIK, and |
| |
| • | the effect of competition and discounting practices in our industry. |
Certain states prohibit us from practicing medicine, employing ophthalmologists to practice medicine on our behalf or employing optometrists to render optometry services on our behalf. In those states, we may contract with professional corporations to provide these services. Beginning in September 2002, we started a process to amend our management agreements with professional corporations. The new management agreements provide us with financial and operational control of the professional corporations. Therefore, we now consolidate the financial results for those professional corporations under the new management agreements in accordance with EITF 97-2. This change is not expected to have a material impact on our operating income (loss), as the increase in revenue resulting from consolidation of these professional corporations is offset by a corresponding increase in medical professional fees.
The following table details the number of laser vision correction procedures performed at our consolidated vision centers.
| | | | | | | | | | | | | | | | | |
| | 2000 | | 2001 | | 2002 | | 2003 |
|
|
| First quarter | | | 12,504 | | | | 25,061 | | | | 17,592 | | | | 17,028 | |
| Second quarter | | | 13,888 | | | | 22,940 | | | | 14,797 | | | | 16,432 | |
| Third quarter | | | 16,341 | | | | 13,347 | | | | 12,511 | | | | 15,965 | |
| Fourth quarter | | | 16,411 | | | | 10,684 | | | | 12,204 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| Year | | | 59,144 | | | | 72,032 | | | | 57,104 | | | | | |
Procedure volume declined in the second half of 2001, which we believe was a result of weakening consumer confidence and the tragedy of September 11th. Procedure volume rebounded in the first quarter of 2002 in a manner consistent with the seasonality experienced in prior years. We believe that falling procedure volume for the balance of 2002 was due to further weakening in consumer confidence and rising unemployment.
We believe that improved marketing and advertising effectiveness, together with third-party patient financing, and our own patient financing plan helped to grow procedure volume in the first nine months of 2003 over the first nine months of 2002.
In the first nine months of 2003, revenues increased by $12,123,000 from $48,538,000 in the first nine months of 2002 to $60,661,000 in the first nine months of 2003. Approximately $7,231,000 of this increase in revenues in the first nine months of 2003 was as a result of improved pricing, and approximately $4,892,000 of this increase was due to higher procedure volumes. In 2002, revenues declined by $6,258,000 from $68,096,000 in 2001 to $61,838,000 in 2002. In 2002, revenues decreased by $14,112,000 as a result of lower procedure volume as compared to 2001. The volume decrease more than offset the increase in revenue of $7,854,000 associated with improved price realization. In 2001, revenues increased by $4,646,000 from $63,450,000 in 2000 to $68,096,000 in 2001. In 2001, revenues increased by $13,826,000 as a result of higher procedure volumes as compared to 2000, which more than offset a decline in pricing which reduced revenues by $9,180,000.
We have raised our average price per procedure over the last ten quarters. Our average price per procedure has increased from $877 in the fourth quarter of 2000 to $1,281 in the quarter ended September 30, 2003. In the third quarter of 2003, we utilized the newest custom laser vision correction technology from Alcon in five of our markets, and we upgraded our VISX lasers in all our markets to perform custom procedures. LASIK procedures using custom ablation accounted for 7% of the third quarter procedure volume, and over 9% of third quarter revenues. We now offer LASIK using custom ablation in all of our markets and believe we can charge higher prices for this more advanced technology. As discussed elsewhere in this prospectus, from time to time we have lowered our prices in response to discounting by some of our competitors. While two of the larger heavily-discounted providers of laser vision correction services have ceased business, the market for laser vision correction services remains highly competitive.
24
Management’s discussion and analysis of financial condition and results of operations
Our strongest quarter in terms of number of procedures performed historically has been the first quarter of the year. We believe this is related to a number of factors, including the availability of funds under typical corporate medical flexible spending programs and the general effect of the New Year season. Our weakest quarter in this regard historically has been the fourth quarter. We believe this is due to time constraints imposed by the holiday season and the fact that medical flexible spending plans are often depleted by the end of the year.
EXPENSES
Nine months ended September 30, 2003 compared to nine months ended September 30, 2002
The following table shows the dollar amount of increase (decrease) in operating expenses from the nine months ended September 30, 2002 to the nine months ended September 30, 2003 and the percent of revenues for each period (dollars in thousands):
| | | | | | | | | | | | | |
| | | | |
| | % of Revenue | | |
| |
| | |
| | Nine months ended | | Nine months ended | | |
| | September 30, | | September 30, | | Increase |
| | 2002 | | 2003 | | (Decrease) |
|
|
| Medical professional and license fees | | | 20.2 | % | | | 19.5 | % | | | $2,055 | |
| Direct costs of services | | | 45.6 | | | | 39.5 | | | | 1,855 | |
| General and administrative expenses | | | 13.3 | | | | 10.0 | | | | (381 | ) |
| Marketing and advertising | | | 21.0 | | | | 15.1 | | | | (1,015 | ) |
| Depreciation and amortization | | | 9.2 | | | | 7.7 | | | | 234 | |
Medical professional expenses increased by approximately $2,327,000 in the nine months ended September 30, 2003 from the nine months ended September 30, 2002. Approximately $1,174,000 of this increase was due to higher revenues, and approximately $1,153,000 of this increase was due to the consolidation in 2003 for the first time of some professional corporations with whom we contract to provide medical services in those states where we cannot practice medicine. License fees decreased $272,000 from the nine months ended September 30, 2002, primarily as a result of a reduction in license fees payable in connection with enhancement procedures of $45,000 and purchasing rebates from suppliers of $811,000. The reductions in license fees were partially offset by an increase in license fees of $584,000 associated with higher procedure volumes.
Direct costs of services include the salary component of physician compensation for those physicians employed by us, staffing, equipment, medical supplies, and facility costs of operating laser vision correction centers. These direct costs increased in the first nine months of 2003 by $1,855,000 compared to the first nine months of 2002, largely because of an increase in provision for bad debt on patient financing of $1,074,000, due to our increased provision of direct financing, financing fees of $272,000 related to third-party patient financing, rent and utilities expense of $168,000 and salaries and benefits of $456,000.
General and administrative expenses decreased by $381,000 in the first nine months of 2003 from the first nine months of 2002, primarily as a result of the absence of warrant compensation expense of $510,000, a decrease in expenditures on professional services in 2003 of $268,000 and a decrease in other general and administrative expenses of $59,000. Warrant amortization relating to certain warrants granted to a former joint venture partner in our managed care business was completed in 2002. The decrease in warrant compensation expense, professional services expenditures and other general and administrative expenses was partially offset by an increase in insurance expense of $456,000.
Marketing and advertising expenses decreased by $1,015,000 in the first nine months of 2003 from the first nine months of 2002, primarily as a result of more cost-effective media buying.
Depreciation and amortization expense increased by $234,000 in the first nine months of 2003 from the first nine months of 2002, primarily as a result of laser upgrades and the acquisition of diagnostic
25
Management’s discussion and analysis of financial condition and results of operations
equipment to support custom LASIK and the purchase of diagnostic and surgical equipment in the fourth quarter of 2002 that previously had been leased.
Investment income increased by $170,000 in the first nine months of 2003 from the first nine months of 2002, primarily as a result of higher levels of invested cash.
Income tax expense of $181,000 was recorded in the first nine months of 2003 relating entirely to the current year income tax provision of our Canadian facilities. Revenue in Canada accounted for approximately 6% of consolidated revenue in the first nine months of 2003.
2002 compared to 2001
The following table shows the dollar amount of increase (decrease) in operating expenses from 2001 to 2002 and the percent of revenues for each year (dollars in thousands):
| | | | | | | | | | | | | |
| | | | |
| | % of Revenue | | |
| |
| | Increase |
| | 2001 | | 2002 | | (Decrease) |
|
|
| Medical professional and license fees | | | 20.0 | % | | | 19.8 | % | | | $(1,356 | ) |
| Direct costs of services | | | 49.4 | | | | 46.6 | | | | (4,820 | ) |
| General and administrative expenses | | | 12.8 | | | | 13.5 | | | | (400 | ) |
| Marketing and advertising | | | 18.7 | | | | 20.7 | | | | 91 | |
| Depreciation and amortization | | | 8.3 | | | | 9.7 | | | | 372 | |
Medical professional fees declined by $83,000 in 2002 as compared to 2001. Lower procedural volume in the United States was partially offset by increased procedural volume in Canada. License fees decreased by $1,457,000 in 2002 from the amount spent in 2001. The decrease in license fees is due to lower procedure volume in 2002 versus 2001. Enhancement expenses increased $123,000 as a result of a slightly higher enhancement rate.
Direct costs of services decreased $4,820,000 in 2002 versus 2001, because of cost reduction plans implemented in 2001, lower procedure volume in 2002, and decreased rent associated with formerly leased equipment purchased at the end of 2001.
General and administrative expenses decreased by $400,000 in 2002 from the amount expended in 2001. Salaries/benefits and travel and entertainment expenses decreased $494,000 and $198,000, respectively, as a result of cost reduction efforts started in the prior year. Warrant amortization was completed in 2002, resulting in a decrease of $192,000. Office equipment costs were reduced by $109,000 in 2002. State and local taxes were reduced by $98,000 in 2002. Offsetting these decreases were increases in insurance costs of $364,000 and shareholder communications of $290,000.
The increase in depreciation and amortization expense of $372,000 is primarily the result of the purchase of lasers and other equipment that had been previously leased.
During the third quarter of 2001, our management implemented a restructuring plan to close unprofitable locations and to reduce operating expenses, in part by reducing staffing levels in existing markets. We estimated the cost of the plan to be $1,375,000, comprised of a $535,000 restructuring charge and an $840,000 asset impairment charge for leasehold improvements, equipment and goodwill associated with the locations to be closed. The restructuring charges included $384,000 in lease termination costs and $151,000 in severance payments for 71 employees. By the end of the first quarter of 2002, the restructuring plan had been fully implemented and we released excess reserves of $174,000 relating to the restructuring plan.
Other
Investment income decreased by $699,000 in 2002 from 2001, primarily as a result of lower interest rates and lower average invested cash.
26
Management’s discussion and analysis of financial condition and results of operations
In August 2002, we received a settlement payment of $2,282,000 from Pillar Point Partners’ class-action litigation. Pillar Point Partners— a joint entity formed in 1995 by laser manufacturers VISX and Summit Technology Inc., now a subsidiary of Alcon Corporation— collected per-use royalties from all laser vision correction providers using their equipment. The manufacturers agreed to settle the various lawsuits for $37.8 million. Pillar Point was dissolved in July 1998 after the Federal Trade Commission filed an administrative complaint challenging the joint entity’s existence.
2001 compared to 2000
The following table shows the dollar amount of increase (decrease) in operating expenses from 2000 to 2001 and the percent of revenues for each year (dollars in thousands):
| | | | | | | | | | | | | |
| | | | |
| | % of Revenue | | |
| |
| | Increase |
| | 2001 | | 2002 | | (Decrease) |
|
|
| Medical professional and license fees | | | 24.5 | % | | | 20.0 | % | | | $(1,916 | ) |
| Direct costs of services | | | 42.9 | | | | 49.4 | | | | 6,398 | |
| General and administrative expenses | | | 14.8 | | | | 12.8 | | | | (667 | ) |
| Marketing and advertising | | | 23.0 | | | | 18.7 | | | | (1,833 | ) |
| Depreciation and amortization | | | 6.1 | | | | 8.3 | | | | 1,786 | |
License fees decreased in 2001 from the amount spent in 2000. The increase in license fees due to higher procedure volumes in 2001 was more than offset by lower expenditures on enhancements and the full-year effect of a reduction in the procedure license fee payable to the laser manufacturers from $260 to $110, which occurred in February 2000. In addition, physician contracts were generally renegotiated during the second quarter of 2000 to facilitate our remaining competitive at lower pricing levels for laser vision correction, resulting in lower medical professional fees.
Direct costs of services increased in 2001 as compared to 2000. Approximately $4,490,000 of the increase in direct costs of services related to more vision centers being in operation in 2001 than in 2000. An increase of $680,000 in medical supplies was generally the result of higher procedure volumes. The remaining increase in direct costs of services of $1,228,000 generally resulted from an increase in the average cost to run a center.
General and administrative expenses decreased by approximately $667,000 in 2001 from the amount expended in 2000. Warrant amortization and the repurchase of warrants from Cole National Corporation (a former joint venture partner) in the third quarter of 2000 decreased warrant related expenses by $463,000 in 2001 from 2000. Corporate employment and travel expenses decreased in 2001 by $443,000. Legal, accounting and consulting fees were reduced by $248,000 in 2001. Office equipment expenses were reduced by $75,000 in 2001. Offsetting a portion of these cost reductions was an increase in insurance costs of $172,000, an increase in national call center costs of $276,000 and an increase in information systems costs of $134,000.
Marketing and advertising costs decreased by $1,833,000 due to improved advertising effectiveness during the first half of 2001.
The increase in depreciation and amortization expense of $1,786,000 is a result of year 2001 capital expenditures associated with new center openings, and the placement of Bausch & Lomb Surgical lasers in most of our markets.
During the third quarter of 2001, our management implemented a restructuring plan to close unprofitable locations and to reduce operating expenses, in part by reducing staffing levels in existing markets. We estimated the cost of the plan to be $1,375,000, comprised of a $535,000 restructuring charge and an $840,000 asset impairment charge for leasehold improvements, equipment and goodwill associated with the locations to be closed. The restructuring charges included $384,000 in lease termination costs and $151,000 in severance payments for 71 employees.
As of December 31, 2001, the balance of the restructuring reserve was $262,000, which related to future facility rent payments.
27
Management’s discussion and analysis of financial condition and results of operations
Also during the third quarter of 2001, we provided for a reserve of $399,000 on a loan made to REI Corporation, which operated a licensed facility in Tokyo, Japan. We originally made this loan in 1999 as financing for REI’s purchase of a laser, which we expected at the time would be used by REI to generate a stream of license fees to us. REI was not ultimately successful in establishing a facility in Japan and in the third quarter of 2001, our management determined that the loan may not be recoverable.
As a result of our operating loss during the third quarter of 2001, and continuing uncertainties regarding the general economic conditions in the United States and the impact on ongoing operations, we recorded a $15,345,000 valuation reserve for our deferred tax assets as of September 30, 2001. This reserve was established according to the requirements of SFAS No. 109 “Accounting for Income Taxes.”
Other non-operating income decreased in 2001 over 2000 primarily as a result of the termination of a deferred compensation arrangement with a physician in the second quarter of 2000.
Other
Equity in income from unconsolidated affiliates increased by $323,000 in 2001, primarily as a result of growth in our managed care business, in which we provide discounted fees for laser vision correction services to participants in certain large managed care programs with which we have contractual arrangements.
Interest expense decreased $41,000 due to our reduction of capital lease obligations.
Interest income decreased $1,789,000 as a result of lower interest rates and lower average invested balances.
QUARTERLY FINANCIAL DATA (UNAUDITED)(1)
Financial results for interim periods do not necessarily indicate trends for any 12-month period. Quarterly results can be affected by the number of procedures performed and the timing of certain expense items (dollars in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | 2001 Quarters | | 2002 Quarters | | 2003 Quarters |
| |
| |
| |
|
| | First | | Second | | Third | | Fourth | | First | | Second | | Third | | Fourth | | First | | Second | | Third |
|
|
| Revenues | | | $22,490 | | | | $21,424 | | | | $13,288 | | | | $10,894 | | | | $18,808 | | | | $16,268 | | | | $13,462 | | | | $13,300 | | | | $19,982 | | | | $20,224 | | | | $20,455 | |
| Operating income (loss) | | | 1,720 | | | | 747 | | | | (5,718 | ) | | | (4,753 | ) | | | 966 | | | | (2,447 | ) | | | (2,814 | ) | | | (1,905 | ) | | | 1,641 | | | | 1,796 | | | | 1,468 | |
| Net Income (loss) | | | 1,299 | | | | 755 | | | | (20,794 | ) | | | (4,635 | ) | | | 1,151 | | | | (2,278 | ) | | | (810 | ) | | | (1,888 | ) | | | 1,757 | | | | 1,793 | | | | 1,517 | |
| Earnings (loss) per share | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | $0.11 | | | | $0.06 | | | | $(1.79 | ) | | | $(0.40 | ) | | | $0.11 | | | | $(0.21 | ) | | | $(0.08 | ) | | | $(0.18 | ) | | | $0.16 | | | | $0.17 | | | | $0.14 | |
| Diluted | | | $0.11 | | | | $0.06 | | | | $(1.79 | ) | | | $(0.40 | ) | | | $0.10 | | | | $(0.21 | ) | | | $(0.08 | ) | | | $(0.18 | ) | | | $0.16 | | | | $0.17 | | | | $0.14 | |
| |
| (1) | The quarterly amounts are not additive due to rounding. |
LIQUIDITY AND CAPITAL RESOURCES
Net cash provided by operating activities in the first nine months of 2003 was $8,350,000, which exceeded expenditures in investing activities during the period (consisting primarily of capital expenditures to open two new facilities). This resulted in an increase in cash and cash equivalents to $25,260,000 (including $0.5 million of cash maintained by our captive insurance company pursuant to statutory requirements) as of September 30, 2003, an increase of approximately 38% from $18,298,000 as of December 31, 2002.
Cash, cash-equivalent and short-term investments totaled $18,298,000 as of December 31, 2002, compared to $16,609,000 as of December 31, 2001. Net cash provided by operating activities in 2002 was $5,693,000. Major uses of cash in 2002 were $2,449,000 used under the common stock repurchase program and capital expenditures of $1,789,000.
28
Management’s discussion and analysis of financial condition and results of operations
During the second quarter of 2000, our directors initiated a program to encourage additional direct stock ownership by our senior management. We offered loans to nine key managers and directors for the purpose of purchasing shares in the open market. Each loan is an unsecured personal obligation of the borrower, and is evidenced by a promissory note. The interest rate on the notes is prime less one and one-half percent. The notes generally had a term of three years, and include provisions for early repayment under certain circumstances. A total of $1,541,000 was loaned under this program. The balance of the loans as of September 30, 2003 was $225,000. No loans were made after June 30, 2002, and no loans under this program will be made in the future.
We maintain a $10,000,000 revolving credit facility with The Provident Bank. In addition, we have a $10,000,000 credit commitment for the purpose of funding acquisitions, subject to the full and absolute discretion of The Provident Bank. The revolving credit line has a variable interest rate equal to the lender’s prime rate and is secured by a mortgage on our headquarters building in Cincinnati, which we own. As of September 30, 2003, no borrowings were outstanding under this credit facility. The acquisition funding commitment has no set terms and its availability is at the sole discretion of the lender. These credit arrangements are scheduled to expire July 31, 2004.
As of December 31, 2002, we had net operating loss carryforwards for federal income tax purposes of $48,765,000. These expire in varying amounts from 2007 until 2022. Approximately $18,000,000 of federal net operating loss carryforwards and $15,750,000 state net operating loss carryforwards were acquired through an acquisition consummated by us in 1997. Our ability to use these acquired net operating loss carryforwards is limited to approximately $2,500,000 per year under Code Section 382 of the Internal Revenue Code. Because we cannot be certain we will realize the full benefit of these loss carryforwards, we have established a valuation reserve equal in amount to the loss carryforwards.
In December 2002, we purchased medical equipment that was being previously leased for $672,000. We anticipate that this will result in a reduction in leased equipment expense of $734,000 and an increase in depreciation expense of $224,000 in 2003 as compared to 2002.
Our costs associated with the opening of a new center generally consist of capital expenditures such as the purchase or lease of lasers, diagnostic equipment, office equipment and leasehold improvements. In addition, we typically incur other startup expenses and pre-opening advertising expenses. Generally, we estimate the costs associated with opening a new center to be between $1.0 million and $1.5 million. Actual costs will vary from center to center based upon the location of the market, the number of lasers purchased for the center, the site of the center and the level of leasehold improvements required. Our capital expenditures consist primarily of investments incurred in connection with the opening of new centers and equipment purchases or upgrades at existing facilities.
In January 2003, we opened a new center in Cleveland, Ohio and in June 2003, we opened a new center in Indianapolis, Indiana. We recently announced the opening of our newest vision center in Las Vegas. We recently upgraded our VISX lasers and diagnostic equipment to enable us to offer custom ablation in all our markets. In addition, we are currently upgrading our Bausch & Lomb lasers to offer their recently approved Zyoptix™ custom technology. We estimate the cost of our laser and equipment upgrades and center expansion plans by year-end 2003 to be approximately $4,000,000 to $6,000,000. The planned capital expenditures for the fourth quarter are expected to be funded by cash flow from operations in combination with cash and cash equivalents on hand.
We recently expanded the use of our own sponsored patient financing. As of September 30, 2003, we had $3.0 million in accounts receivable, which is an increase of $2.6 million since December 31, 2002. Further growth in accounts receivable is expected over the balance of 2003 and beyond.
Our consolidated cash and cash equivalents includes $0.5 million of cash maintained by our consolidated captive insurance company pursuant to statutory requirements as of September 30, 2003. These funds are not available for general corporate purposes. We expect cash reserves in our captive insurance company to grow over time.
The ability to fund our marketing and advertising program, planned capital expenditures and new center rollouts depends on our future performance, which, to a certain extent, is subject to general
29
Management’s discussion and analysis of financial condition and results of operations
economic, competitive, legislative, regulatory and other factors that are beyond our control. Based upon our current level of operations and anticipated revenue growth, we currently believe that cash flow from operations, available cash and short-term investments, available borrowing under the credit facility from Provident Bank and the net proceeds to us from this offering should be adequate to meet these needs for at least the next 12 months.
CRITICAL ACCOUNTING POLICIES
In January 2002, the SEC issued FR-60 recommending that companies expand their disclosures related to critical accounting policies. Significant accounting policies are disclosed in Note 1 of the financial statements, and critical accounting policies are discussed in the following paragraphs.
Allowance for doubtful accounts
We provide patient financing to some of our customers, including those who could not otherwise obtain third-party financing. The terms of the financing require the patient to pay an up-front fee which is intended to cover some or all of our variable costs, and the remainder is deducted automatically from the patient’s checking account over a period of 12 to 36 months. We began our patient financing program in May 2002. As a result of a recent expansion of this program, we are currently exposed to more credit risk than we have experienced in the past. Based upon our own experience with patient financing and based upon the credit experience of lenders that provide financing to customers similar to ours, we have established bad debt reserves as of September 30, 2003 of $1,386,000 against accounts receivable of $4,413,000. To the extent that our actual bad debt write-offs are greater than our estimated bad debt reserve, it would adversely impact our results of operations and cash flows and to the extent that our actual bad debt write-offs are less than our estimated bad debt reserve, it would favorably impact our results of operations and cash flows.
Captive insurance company reserves
Effective December 18, 2002, we established a captive insurance company to provide professional liability insurance coverage for claims brought against us after December 17, 2002. In addition, our captive insurance company’s charter allows it to provide professional liability insurance for our doctors, some of whom are currently insured by the captive. Our captive insurance company is managed by an independent insurance consulting and management firm, and it is capitalized and funded by us based on actuarial studies performed by an affiliate of the consulting and management firm. For the 12 months ending December 17, 2003, our captive insurance company purchased excess liability coverage for 80% of our losses in the year in excess of $1,000,000 per occurrence, up to $11,000,000. Our captive insurance company is responsible for 20% of our aggregate losses in excess of $1,000,000, up to $11,000,000. Under that arrangement, the coverage providers’ obligation arises only after our captive pays the first $1,000,000 of any loss and the coverage providers are then only obligated to pay an aggregate of $8,000,000 in a given policy year. These excess liability coverage policies are currently up for renewal, and our management may elect to purchase similar, less, more or no excess liability coverage depending on the premiums quoted, among other factors. A number of claims covered by our captive insurance company are now pending. The financial statements of the captive insurance company are consolidated with our financial statements since it is a wholly-owned enterprise. As of September 30, 2003, we recorded an insurance reserve amount of $1,384,000, which primarily represents an actuarially determined estimate of claims incurred but not yet reported. To the extent that our actual claim experience is greater than our estimated insurance reserve, it would adversely impact our results of operations and cash flows and to the extent that our actual claim experience is less than our estimated insurance reserve, it would favorably impact our results of operations and cash flows.
30
Management’s discussion and analysis of financial condition and results of operations
Income taxes
As a result of our operating loss during the third quarter of 2001 and throughout 2002, and continuing uncertainties regarding the general economic conditions in the United States and the impact on our ongoing operations, we continue to record a full valuation reserve for deferred tax assets. This reserve was established according to the requirements of SFAS No. 109 “Accounting for Income Taxes.” Favorable changes in our operating profitability, as a result of improved general economic conditions in the United States or otherwise, could impact our determination as to whether reduction, in whole or in part, to the valuation reserve is necessary in the future. To the extent that such a reduction in the valuation reserve is necessary, an income tax benefit would be recorded in the consolidated statement of operations, which would favorably impact our results of operations. In addition, future taxable income may be absorbed by the net operating loss carryforward that we maintain, which would favorably impact our results of operations and cash flows. During the nine months ended September 30, 2003, we applied approximately $4,725,000 of our net operating losses against our taxable income for the period, which resulted in reduced federal income tax expenses and tax payments of approximately $1,654,000. The valuation reserve and net operating loss carryforward was $17,118,000 and $44,040,000, respectively, as of September 30, 2003.
Consolidation
We use the consolidation method to report our investment in our subsidiaries and other companies when we own a majority of the voting stock of the subsidiary. In addition, we consolidate the results of operations of professional corporations with which we contract to provide the services of ophthalmologists or optometrists at our vision centers, in accordance with EITF 97-2,Application of FASB Statement No. 94 and APB Opinion No. 16 to Physician Management Entities and Certain Other Entities with Contractual Management Arrangements. Prior to September 2002, our contractual management arrangements did not permit consolidation of our relationships with professional corporations pursuant to EITF 97-2 because we did not maintain a “controlling financial interest” in the professional corporations. Beginning in September 2002, we began a process of renewing our agreements with the professional corporations and opening new vision centers with agreements that meet the “controlling financial interest” criteria of EITF 97-2.
As a result of the FASB’s issuance of FIN 46,Consolidation of Variable Interest Entities, an interpretation of ARB No. 51,in January 2003, we have evaluated the contractual management arrangements entered into with professional corporations after January 1, 2003 and have determined that consolidation of these entities is appropriate under the FIN 46 guidance. With respect to the three remaining professional corporations with which we had a contractual management arrangement prior to January 1, 2003, we have determined that we should consolidate these entities under the FIN 46 guidance beginning July 1, 2003, which we do not believe will have a material impact on our consolidated financial statements. If modifications are made to existing management agreements, or if new agreements are made under different terms than existing management agreements, then the financial statements of the professional corporations may not be consolidated in the future.
31
Business
We are a leading developer and operator of fixed-site laser vision correction centers under the brand name LasikPlus. Our vision centers provide the staff, facilities, equipment and support services for performing laser vision correction that employs advanced laser technologies to help correct nearsightedness, farsightedness and astigmatism. We currently utilize fixed-site excimer lasers manufactured by Bausch & Lomb, VISX, and Alcon. Our vision centers are supported mainly by full-time credentialed board-certified ophthalmologists, optometrists and other health care professionals. The ophthalmologists perform the laser vision correction procedures in our vision centers, and either ophthalmologists or optometrists generally carry out the pre-procedure evaluations and post-procedure follow-ups in-center. We have performed over 300,000 laser vision correction procedures in our vision centers, in the United States and Canada, since 1991. Most of our patients currently receive a procedure called LASIK, which we began performing in the United States in 1997.
We currently operate 37 laser vision correction centers, including 34 wholly-owned vision centers located in large metropolitan markets throughout the United States, two joint ventures in Canada and one joint venture in Europe. We recently announced the opening of our newest vision center in Las Vegas.
THE LASER VISION CORRECTION MARKET
General
More than 150 million Americans, or approximately 50% of the US population, require eyeglasses or contact lenses to correct common vision problems. Most people seeking vision correction suffer from one or more refractive vision disorders, which often results from improper curvature of the cornea as related to the size and shape of the eye. If the cornea’s curvature is not precisely correct, it cannot properly focus the light passing through it onto the retina, and the viewer will see a blurred image. Three common refractive vision disorders are:
| |
| • | myopia (nearsightedness)—images are focused in front of the retina, resulting in the blurred perception of distant objects, |
| |
| • | hyperopia (farsightedness)—images are focused behind the retina, resulting in the blurred perception of near objects, and |
| |
| • | astigmatism—images are not focused on any point due to the varying curvature of the eye along different axes. |
Since the FDA approved the first laser to perform laser vision correction procedures in the United States in 1995, industry sources estimate that approximately 3 million patients have been treated. Laser vision correction is currently one of the most widely performed elective surgical procedures in the United States, with approximately 1.2 million laser vision correction procedures performed on approximately 600,000 patients in 2002. It is estimated that there are approximately 57.5 million potential patients for laser vision correction procedures in the United States, according to a report on the US refractive market published by MarketScope in November 2000. We believe we are well positioned to grow given the relatively low penetration of the large target market for laser vision correction.
Laser vision correction is typically an elective, private pay procedure performed on an outpatient basis. The current demand for laser vision correction procedures performed in the United States is widely believed to be attributable to many factors, including:
| |
| • | broader market acceptance—As the number of procedures performed increases, so does the number of patients able to attest to the perceived benefits of laser vision correction, |
| |
| • | improved technology—Compared to earlier laser vision correction procedures, the LASIK procedure typically results in less patient discomfort and a shorter recovery period, and |
32
Business
| |
| • | expanded applications—FDA approvals for the excimer laser used in LASIK laser vision correction procedures has been extended to the treatment of three of the most common types of refractive vision disorders: nearsightedness, farsightedness and astigmatism. |
Laser vision correction procedures
Laser vision correction procedures are designed to reshape the outer layers of the cornea to help correct refractive vision disorders by changing its curvature with an excimer laser, which may reduce the need for corrective lenses. Prior to the procedure, an assessment is made of the patient’s candidacy for the procedure and the correction required to program the excimer laser. The software of the excimer laser then calculates the number of pulses needed to achieve the intended correction using a specially developed algorithm. A speculum is inserted to prevent blinking and topical anesthetic eye drops are applied. The patient reclines in a chair, eyes focused on a fixed target, while the ophthalmologist positions the patient’s cornea for the procedure. The excimer laser emits energy in a series of pulses with each pulse typically lasting only a fraction of a second. High-energy ultraviolet light produced by the excimer laser creates a “non-thermal” process known as ablation, which removes tissue and reshapes the cornea. The amount of tissue removed depends upon the degree of the vision disorder being corrected. Following the procedure, the front surface of the eye is flatter when corrected for nearsightedness and steeper when corrected for farsightedness. In effect, the change made in the middle or periphery of the cornea is translated to the front surface of the cornea which results in helping correct vision. A series of patient follow-up visits are scheduled with an optometrist or ophthalmologist to monitor the corneal healing process, to check that there are no complications and to test the correction achieved by the laser vision correction procedure. The typical procedure takes 15 to 30 minutes from set-up to completion.
PRK. PRK has been approved by the FDA for commercial use in the United States since 1995. In PRK procedures, the ophthalmologist removes the thin layer of cells covering the outer surface of the cornea (the epithelium) in order to apply the excimer laser pulses to the surface of the cornea. Following the PRK procedure, a contact lens bandage is placed on the eye to protect it. The patient typically experiences discomfort and blurred vision until the epithelium heals. An ophthalmologist generally will prescribe certain topical pharmaceuticals for use by the patient post-procedure to assist in alleviating discomfort and to promote corneal healing. Although a patient generally experiences substantial improvement in clarity of vision within a few days following the procedure, it can take one to three months for the full benefits of the PRK procedure to be realized. Patients usually have one eye treated in one visit and the second eye treated at a later visit.
LASIK. We began performing LASIK, which now accounts for substantially all of the procedures performed in our vision centers, in the United States in 1997. In LASIK procedures, an automated microsurgical instrument called a microkeratome is used to create a thin flap, which remains hinged to the eye. The corneal flap is then laid back and excimer laser pulses are applied to the exposed surface of the cornea to treat the eye according to the patient’s prescription. The corneal flap is then folded back to its original position and inspected to ensure that it remains secured in position by the natural suction of the cornea. Since the surface layer of the cornea remains intact with LASIK, a bandage contact lens is normally not required and the patient typically experiences little discomfort. LASIK often has the advantage of more rapid recovery than PRK, with most patients seeing well enough to drive a car the next day and enjoying shorter recovery periods. The LASIK procedure allows an ophthalmologist to treat both eyes of a patient during the same visit, involves little patient discomfort and produces prompt results, frequently enabling patients to see well postoperatively almost immediately.
Custom ablation. The newest advance in laser vision correction procedures is LASIK using custom ablation. We provide custom ablation in all of our markets using state-of-the-art technology, including VISX CustomVue™ technology, Alcon’s Customcornea™ technology and Bausch & Lomb Zyoptix™ technology.
33
Business
To perform a custom ablation procedure, we use digital technology to identify and measure imperfections in an individual’s eyes more precisely than with standard methods used for glasses and contact lenses and non-custom LASIK and non-custom PRK, a procedure we began performing in the United States in 1995. This information in then transferred to the laser, providing potentially greater precision and accuracy in the treatment.
Prior to custom ablation, laser vision correction corrected only the visual distortions caused by nearsightedness, farsightedness, and astigmatism. However, these common types of vision distortions, called “lower order aberrations”, are typically responsible for only 85% to 90% of the overall quality of vision. There are other imperfections in the eye’s optical system that may affect the clarity of one’s vision and how well one sees at night or in low light. Doctors call these visual distortions “higher order aberrations” and they can cause glare, shadows, halos and other visual defects. Unless these higher order aberrations are addressed along with the lower order aberrations, the quality of vision may be compromised, even if it measures at 20/20.
With the new custom technology, it may now be possible to measure and, in some cases, help correct both lower and higher order aberrations. Flat waves of light are passed through the eye using a computerized wavefront-measuring instrument. As the light waves travel through the eye’s optical system, the distortions in vision are measured and compared against the flat light waves that would have been reflected if the optical system was perfect. A three dimensional map is then generated representing the unique visual distortions, including both lower and higher order aberrations. This map is a guide for the laser, instructing it how and where to reshape the cornea to correct vision. Other aspects of the custom LASIK procedure are similar to those described above for LASIK.
OUR BUSINESS STRATEGY
Our business strategy is to provide quality laser vision correction services at an affordable price. In July 1999, we began converting our vision centers to closed access facilities from open access facilities in order to obtain increased control over the quality of care we provide to our patients and greater operational and financial control of our business. Under the open access model, we allowed qualified ophthalmologists to use our equipment and facilities in return for a facilities fee. Under our closed access model, in contrast, we either directly employ the ophthalmologist and the optometrist or contract for their services, and are responsible for marketing and patient acquisition. By the first quarter of 2000, we changed the names of the majority of our vision centers to LasikPlusand began trying to build consumer awareness of the brand name through media campaigns that generally include newsprint, radio and direct mailings.
We intend to grow our business through increased penetration in our current markets and expansion into new markets. Key elements of our business strategy include:
| |
| • | recruiting and retaining highly credentialed ophthalmologists and optometrists, |
| |
| • | providing patients with a “Continuum of Care”, |
| |
| • | opening and operating new laser vision correction centers, |
| |
| • | providing attractive patient financing alternatives, |
| |
| • | establishing relationships with leading managed care providers in the United States to source additional patients, and |
| |
| • | developing and implementing innovative direct marketing campaigns. |
Recruiting and retaining highly credentialed ophthalmologists and optometrists. We generally focus our recruiting efforts on leading ophthalmologists and optometrists with a reputation for providing quality eye care within their respective markets and who are experienced in laser vision correction procedures. Our ophthalmologists have completed extensive FDA-mandated training and also have met our qualification criteria, including a review of state licensure, board certification, malpractice insurance, historical procedure experience and clinical outcomes.
34
Business
Providing patients with a “Continuum of Care”. We strive to achieve high patient satisfaction and have established a “Continuum of Care” program, the goal of which is to achieve the level of visual correction agreed to by the patient and physician. This program begins with our initial contact with the prospective patient. Our call center personnel are trained to answer questions regarding procedures and have access to both an ophthalmologist to address more difficult inquiries and past patients to relate procedure experience. Once in the vision center, potential patients can receive a free eye evaluation with the local center ophthalmologist or optometrist to determine their candidacy for laser vision correction as well as a consultation focused on educating the patient on vision correction procedures, how the procedure may help correct the patient’s specific refractive vision disorder and what results the patient may expect after the procedure. Additionally, our vision centers are designed to create a patient friendly environment and reduce any anxiety associated with getting laser vision correction. We schedule post-surgical follow-ups with patients who have received the procedure to monitor procedure results and evaluate potentially providing enhancements to those patients who do not receive the desired correction in the initial procedure. The vast majority of patients who respond to our customer satisfaction surveys indicate that they are satisfied with the care they received in our vision centers.
Opening and operating new laser vision correction centers.We plan to expand our business primarily through the development of new vision centers in desirable markets and within existing markets. In evaluating new and current markets for opening a laser vision correction center, we evaluate a number of factors, including population demographics, and attempt to determine the number of existing excimer lasers currently in that market. We also typically interview local ophthalmologists and optometrists. We target markets with potential to generate break-even procedure volume within the first six months of opening, including the necessary ophthalmologist and optometrist staffing to support such levels. We have developed what we believe to be cost-efficient standardized vision center plans and designs to be used in building each new vision center to efficiently manage patient flow and physician and staff productivity. In addition, from time to time we expect to evaluate strategic transactions as a means to supplement our growth.
Providing attractive patient financing alternatives. Because laser vision correction procedures are elective and generally not reimbursable by third party payors, we currently offer patients several financing alternatives. We have entered into an arrangement with an unaffiliated finance company, pursuant to which the finance company makes multiple payment plans available to qualifying customers. These payment plans typically provide for payments over a 12 month to 48 month period. We generally bear no credit risk for loans made under this program. For patients not qualifying for these plans, we also offer our own direct financing to customers under which we charge an up-front fee intended to cover some or all of our variable costs, with the remaining balance paid by the customer through direct withdrawals from the customer’s bank account in up to 36 equal monthly installments. We can also provide information to customers regarding other financing options, such as installment plans, home equity loans and payment through employer flexible benefit plans.
Establishing relationships with leading managed care providers in the United States to source additional patients.With the increasing number of employers adding vision services to their employee benefit packages, we hope to increase our US footprint and market share by developing contractual relationships with managed care organizations, through which we offer discounted rates to plan participants. Through our agreements with managed care providers to date, we believe we currently have access to approximately 75 million covered lives in over 100 major US markets. The plan participant, and not the managed care organization, is responsible for the payment of our fees under these arrangements. Customers from our managed care program have grown from representing 11% of our revenues in 2002 to representing approximately 18% of our revenues in the three-month period ended September 30, 2003. We believe we are particularly well-suited to offer such programs to managed care plans.
Developing and implementing innovative direct marketing campaigns.Our marketing programs seek to reinforce the LasikPlusbrand name in addition to raising awareness concerning laser vision correction and promoting our vision centers. In each market, we target a specific demographic group
35
Business
of potential patients that we believe may be interested in laser vision correction through the use of print media, radio, direct mail campaigns, internet marketing, brochures and videos. In most advertisements, prospective patients are provided our web site address, www.lasikplus.com, and a toll-free number, such as 1-888-529-2020, 1-800-LASIKPLUS or 1-800-243-EYES. Our call center representatives screen prospective patients, record patient names and information into our centralized computer system and attempt to schedule eye evaluation appointments for prospective patients with the local center ophthalmologist or optometrist to determine whether the prospective patient is a candidate for laser vision correction. We generally keep a prospective patient’s name on file if the patient elects not to proceed with a laser vision procedure following an initial evaluation. We continuously evaluate the effectiveness of our marketing programs and our marketing costs.
COMPETITION
Laser vision correction, whether performed at one of our vision centers or elsewhere, competes with several surgical and non-surgical treatments to correct refractive vision disorders, including eyeglasses, contact lenses, other types of refractive surgery and corneal implants, among other treatments. In addition, other technologies currently under development may ultimately prove to be more attractive and effective to consumers than current laser vision correction technology.
In addition, we face competition from other providers of laser vision correction. Eye care services in the United States are delivered through a fragmented system of local providers, including individual or small groups of opticians, optometrists and ophthalmologists and chains of retail optical stores and multi-site eye care vision centers. Laser vision correction corporate providers, such as us, are a specialized type of multi-site eye care center that primarily provide laser vision correction. Among the laser vision correction center providers, we believe we are one of the largest providers in terms of number of vision centers in the United States.
The market for providing laser vision correction service is highly competitive and fragmented. In most, if not all, of our markets, we compete with other laser vision correction center chains, including TLC Vision Corporation, which is also a public company, and Lasik Vision Institute, as well as with hospitals, surgical clinics, local operators of vision centers and ophthalmology practices, among others, that have purchased or rent their own lasers. According to a report on the U.S. refractive market published by MarketScope in August 2003, as of the second quarter of 2003 independent surgeons represent 60% of the providers of laser vision correction services, corporate providers represent 32.9% and institutions represent 6%. According to the same report, among the corporate providers, our principal competitors are TLC Vision, which, based on the number of procedures performed, represents 49.5% of the corporate segment of the market for laser vision correction services and Laser Vision Institute, which represents 12.7%. The report indicated that we represent 18.3% of the corporate segment of the market.
In the past certain competitors have utilized deeply-discounted pricing in an effort to generate procedural volume. These practices have in the past caused periods of intense price competition in our industry. As a result, we have lowered our prices in the past in order to remain competitive. While two of the larger heavily-discounted providers of laser vision correction services have ceased business, other competitors offer discounts in some geographic markets where we conduct business. It is possible that our business could be materially adversely affected in the future by discounting practices of competitors.
EMPLOYEES
As of November 12, 2003, we had 250 employees, 221 of whom were full-time. None of our employees are subject to a collective bargaining agreement nor have we experienced any work stoppages. We believe our relations with our employees are good.
36
Business
TRADEMARKS
Not all of our trademarked names have been formally registered yet. Where the trademark symbol is used, it is our intention to claim a trademark on such names under common law by using the “TM” symbol. The duration of such trademarks under common law is the length of time we continue to use them.
SUPPLIERS OF LASER EQUIPMENT
We are not directly involved in the research, development or manufacture of ophthalmic laser systems, diagnostic equipment, microkeratomes or microkeratome blades. There are at least five companies, Bausch & Lomb, VISX, Alcon, Nidek and LaserSight, whose excimer laser systems have been approved by the FDA for commercial sale in the United States. We currently use several suppliers, including Bausch & Lomb, VISX and Alcon, for our excimer lasers and diagnostic equipment in the United States. We rely primarily on Bausch & Lomb to provide us with microkeratomes, microkeratome blades and other disposable items required in LASIK procedures.
GOVERNMENT REGULATION
Our operations are subject to extensive federal, state and local laws, rules and regulations affecting the healthcare industry and the delivery of healthcare. Some of these include laws and regulations, which vary significantly from state to state, prohibiting unlawful rebates and division of fees, and limiting the manner in which prospective patients may be solicited. Furthermore, contractual arrangements with hospitals, surgery centers, ophthalmologists and optometrists, among others, are extensively regulated by state and federal law.
Failure to comply with applicable FDA requirements could subject excimer laser manufacturers and us to enforcement action, including product seizures, recalls, withdrawal of approvals and civil and criminal penalties, any one or more of which could have a material adverse affect on our business, financial condition and results of operations. In addition, clearance or approvals could be withdrawn in some circumstances. Failure by us or our principal suppliers to comply with regulatory requirements, or any adverse regulatory action, could result in us being named as a party in ensuing litigation or a limitation on or prohibition of our use of excimer lasers, which in turn would have a material adverse effect on our business, financial condition or results of operations. Discovery of problems, violations of current laws or future legislative or administrative action in the United States or elsewhere may adversely affect the manufacturers’ ability to obtain regulatory approval of laser equipment. Furthermore, the failure of VISX, Bausch & Lomb and Alcon, or any other manufacturers that supply or may supply excimer lasers to us to comply with applicable federal, state, or foreign regulatory requirements, or any adverse regulatory action against such manufacturers, could limit the supply of lasers or limit our ability to use the lasers.
The following is a more detailed description of certain laws and regulations that affect our operations.
Restrictions on medical devices
In the United States, the FDA regulates the manufacturing, labeling, distribution and marketing of medical devices, including excimer lasers, microkeratomes and certain equipment we provide for use in laser vision correction surgery. The excimer lasers and other major equipment that we use have been authorized by the FDA for certain uses.
Once FDA approval is obtained, however, medical device manufacturers are subject to continuing FDA obligations. For example, the FDA requires that medical devices be manufactured in accordance with its Quality System Regulations. In essence, this means that medical devices must be manufactured and records must be maintained in a prescribed manner with respect to production, testing and control activities. In addition, the FDA sometimes imposes restrictions and requirements regarding the labeling and promotion of medical devices, with which users such as us as well as manufacturers must comply.
37
Business
Non-compliance with FDA requirements could subject us and manufacturers to enforcement action, including:
| |
| • | product seizures, |
| |
| • | recalls, |
| |
| • | withdrawal of approvals, and |
| |
| • | civil and criminal penalties. |
Any such enforcement action could have a material adverse effect on our business, financial condition and results of operations.
The use of an excimer laser to treat both eyes on the same day (bilateral treatment) has not been approved by the FDA. The FDA has stated that it considers the use of the excimer laser for bilateral treatment to be a practice of medicine decision, which the FDA is not authorized to regulate. Ophthalmologists, including those practicing in our vision centers, widely perform bilateral treatment in an exercise of professional judgment in connection with the practice of medicine. There can be no assurance that the FDA will not seek to challenge this practice in the future.
To authorize new uses of medical devices, manufacturers are required to obtain a supplemental FDA authorization. Obtaining these authorizations is time consuming and expensive, and we cannot be sure that manufacturers of the devices we use will be able to obtain any such additional FDA authorizations. Further, later discovery of problems with the medical devices we use or manufacture or failure to comply with manufacturing or labeling requirements may result in restrictions on use of the devices or enforcement action against the manufacturers, including withdrawal of devices from the market. Changes in legislation or regulation could affect whether and how we can use the devices. These and other regulatory actions could limit the supply of devices we use or our ability to use them, which could have a material adverse effect on our business, financial condition and results of operations.
Anti-kickback statutes
In the United States, the federal anti-kickback statute prohibits the knowing and willful solicitation, receipt, offer or payment of any kickback in connection with:
| |
| • | the referral of patients, and |
| |
| • | the ordering or purchasing of items or services payable in whole or in part under Medicare, Medicaid or other federal healthcare programs. |
Some courts have interpreted the federal anti-kickback statute broadly to prohibit payments intended to induce the referral of Medicare or Medicaid business, regardless of any other legitimate motives. Sanctions for violations of the anti-kickback statute include:
| |
| • | criminal penalties, |
| |
| • | civil penalties of up to $50,000 per violation, and |
| |
| • | exclusion from Medicare, Medicaid and other federal programs. |
According to the US Office of the Inspector General, ophthalmologists and optometrists who engage in agreements to refer business may be violating the anti-kickback statute. Further, violations may occur even with respect to non-Medicare or Medicaid services if the arrangement has an impact on the referral pattern for Medicare or Medicaid services.
Some states have enacted statutes similar to the federal anti-kickback statute which are applicable to all referrals of patients. Although we have endeavored to structure our business operations to be in material compliance with such laws, authorities could determine that our business practices are in violation of such laws. This could have a material adverse effect on our business, financial condition and results of operations.
38
Business
Fee-splitting
Many states prohibit professionals (including ophthalmologists and optometrists) from paying a portion of a professional fee to another individual unless that individual is an employee or partner in the same professional practice. Violation of a state’s fee-splitting prohibition may result in civil or criminal fines, as well as loss of licensing privileges. Many states do not offer clear guidance on what relationships constitute fee-splitting, particularly in the context of providing management services for doctors. Although we have endeavored to structure our business operations in material compliance with these laws, state authorities could find that fee-splitting prohibitions apply to our business practices. This could have a material adverse effect on our business, financial condition and results of operations.
Corporate practice of medicine and optometry
The laws of many states prohibit business corporations, such as us, from practicing medicine and employing or engaging physicians to practice medicine. Some states prohibit business corporations from practicing optometry or employing or engaging optometrists to practice optometry. Such laws preclude companies that are not owned entirely by eye care professionals from:
| |
| • | employing eye care professionals, |
| |
| • | controlling clinical decision making, and |
| |
| • | engaging in other activities that are deemed to constitute the practice of optometry or ophthalmology. |
This prohibition is generally referred to as the prohibition against the corporate practice of medicine or optometry. Violation of this prohibition may result in civil or criminal fines, as well as sanctions imposed against the professional through licensing proceedings. Although we have endeavored to structure our contractual relationships to be in material compliance with these laws, if any aspect of our operations were found to violate state corporate practice of medicine or optometry prohibitions, this could have a material adverse effect on our business, financial condition and results of operations.
Self-referral laws
The US federal self-referral law (the “Stark Law”) prohibits physicians (including optometrists) from referring their Medicare or Medicaid patients for certain health services to any provider with which they (or their immediate family members) have a financial relationship. Certain referrals, however, fit within specific exceptions in the statute or regulations. The penalties for violating the Stark Law include:
| |
| • | denial of payment for the health services performed, |
| |
| • | civil fines of up to $15,000 for each service provided pursuant to a prohibited referral, |
| |
| • | a fine of up to $100,000 for participation in a circumvention scheme, and |
| |
| • | possible exclusion from Medicare and Medicaid programs. |
At this time it is unclear how ophthalmologists and optometrists are affected under the law. While we believe that our present business practices materially comply with the Stark Law and similar state laws to the extent we are subject to such laws, we cannot assure you that our business practices will not be challenged in the future. This could have a material adverse effect on our business, financial condition and results of operations.
Other Anti-fraud provisions
Certain federal and state laws impose penalties on healthcare providers and those who provide services to such providers (including businesses such as us) that fraudulently or wrongfully bill government or other third-party payors for healthcare services. Such penalties include substantial civil and criminal
39
Business
fines and imprisonment. In addition, the federal law prohibiting false Medicare/ Medicaid billings allows a private person to bring a civil action in the name of the US government for violations of its provisions. Such private individuals can obtain a portion of the false claims recovery if the action is successful. We believe that we operate in material compliance with these laws. If any of our activities are challenged or reviewed by governmental authorities or private parties asserting false claims, such actions could have a material adverse effect on our business, financial condition and results of operations.
Facility licensure and certificate of need
State Departments of Health may require us to obtain licenses in the various states in which we have or acquire laser vision correction centers or other business operations. We believe that we have obtained the necessary licensure in states where licensure is required and that we are not required to obtain licenses in other states. However, not all of the regulations governing the need for licensure are clear and there is little guidance available regarding certain interpretative issues. Therefore, it is possible that a state regulatory authority could determine that we are improperly conducting business operations without a license. This could subject us to significant fines or penalties, result in our being required to cease operations in that state and could otherwise have a material adverse effect on our business, financial condition and results of operations. We have no reason to believe that we will be unable to obtain necessary licenses without unreasonable expense or delay, but there can be no assurance that we will be able to obtain any required license.
Some states require permission by the State Department of Health in the form of a Certificate of Need (“CON”) prior to the construction or modification of an ambulatory care facility or the purchase of certain medical equipment in excess of a certain amount. We believe that we have obtained the necessary CONs in states where a CON is required. However, not all of the regulations governing the need for CONs are clear and there is little guidance to cover certain interpretive issues. Therefore, it is possible that a state regulatory authority could determine that we are improperly conducting business operations without a CON. This could have a material adverse effect on our business, financial condition and results of operations, and there can be no assurance that we will be able to acquire a CON in all states where it is required.
Health information practices
We, along with the health care industry in general, are impacted by the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which mandates, among other things, the adoption of standards, for the exchange of electronic health information in an effort to enhance the efficiency and simplify the administration of the healthcare system. In addition, HIPAA requires the Department of Health and Human Services to adopt standards for electronic transmissions and code sets; unique identifiers for providers, employers, health plans and individuals; security and electronic signatures; privacy; and enforcement. While HIPAA ultimately is designed to reduce administrative expenses within the healthcare system, the law likely will initially require significant, and possibly costly, changes for the industry. The Electronic Healthcare Transactions and Code Sets have gone into effect, and entities had until October 16, 2003 to comply with them, as long as they comply with the HIPAA privacy standards by April 14, 2003. On February 20, 2003, the Department of Health and Human Services published standards for the security of electronic health information. We must comply with the requirements of the security standards by April 21, 2005. Based on current information, we believe we will be able to fully comply with the HIPAA requirements by such date; however, we cannot at this time estimate the cost of compliance, whether in fact we will be successful in achieving such compliance or if implementation of the HIPAA standards will result in an adverse effect on our operations or profitability.
Healthcare reform
Healthcare reform is considered by many in the United States to be a national priority. Several states are also currently considering healthcare proposals. We cannot predict what additional action, if any,
40
Business
the federal government or any state may ultimately take with respect to healthcare reform. Healthcare reform may bring significant changes in the financing and regulation of the healthcare industry, which could have a material adverse effect on our business, financial condition and results of operations.
LEGAL PROCEEDINGS
In the first quarter of 2002, in response to an informal request for information we received from the Federal Trade Commission, we supplied the FTC with documentation related to a number of advertisements we ran in prior years. During the quarter ended September 30, 2002, the FTC informed us that they believed certain of our earlier advertisements violated the Federal Trade Commission Act because we lacked adequate substantiation for three specific claims made in the advertisements. We elected to voluntarily settle with the FTC rather than incur the significant expense and distraction from our business that would have resulted from prolonged litigation.
On December 10, 2002, we signed the Agreement Containing Consent Order. The FTC formally entered the Complaint and Agreement on July 18, 2003. This case is entitledIn the Matter of LCA-Vision Inc., d/b/a LASIKPLUS, File No. 022-3098. We agreed that in any future advertisements we would not represent in any manner that our LASIK surgery eliminates the need for glasses and contacts for life, poses significantly less risk to patients’ eye health than wearing glasses or contacts, or eliminates the risk of glare and haloing. The FTC did not impose any fine or other monetary penalty on us. We cannot be certain that this agreed order will not have a materially adverse effect on our business.
Our business routinely results in a number of medical malpractice lawsuits. Claims reported to us prior to December 18, 2002 were generally covered by external insurance policies and to date have not had a material financial impact on our business other than the cost of insurance and our deductibles under such policies. Due to substantial increases in insurance premiums, effective as of December 18, 2002, we established a captive insurance company to provide coverage for claims brought against us after December 17, 2002. Our captive insurance company is managed by an independent insurance consulting and management firm and is capitalized by us based on actuarial studies performed by an affiliate of the consulting and management firm. For the 12 months ending December 17, 2003, our captive insurance company purchased excess liability coverage for 80% of our losses in the year in excess of $1,000,000 per occurrence, up to $11,000,000. Under that arrangement, the coverage providers’ obligation arises only after our captive pays the first $1,000,000 of any loss and the coverage providers are only obligated to pay an aggregate of $8,000,000 in a given policy year. These excess liability coverage policies are currently up for renewal, and our management may elect to purchase similar, less, more or no excess liability coverage depending on the premiums quoted, among other factors. A number of claims covered by our captive insurance company are now pending.
We also are subject to other litigation from time to time, none of which we currently consider to be material.
We do not believe that any of the legal proceedings to which we are currently subject will have a material adverse effect on our business, financial position or results of operations.
41
Management
The directors, executive officers and key personnel of LCA-Vision and their positions with LCA-Vision are as follows:
| | | | | | | | | |
| Name | | Age | | Position |
|
|
| Stephen N. Joffe | | | 60 | | | Chairman of the Board and Chief Executive Officer, Director |
| Kevin M. Hassey | | | 46 | | | | President | |
| Alan H. Buckey | | | 45 | | | Executive Vice President/Finance and Chief Financial Officer |
| Craig P.R. Joffe | | | 31 | | | Senior Vice President, General Counsel and Secretary |
William O. Coleman(1) (2) | | | 74 | | | | Director | |
John H. Gutfreund(2) (3) | | | 73 | | | | Director | |
John C. Hassan(1) | | | 60 | | | | Director | |
| |
| (1) | Member of the Audit Committee |
| |
| (2) | Member of the Compensation Committee |
| |
| (3) | Mr. Gutfreund is the President of C.E. Unterberg, Towbin, one of the underwriters of this offering. See “Underwriting.” |
Stephen N. Joffe, M.D. is the Chairman of the Board, Chief Executive Officer, and a Director of LCA-Vision. He was the founder of the Company’s corporate predecessor, Laser Centers of America, Inc. and served as its Chairman of the Board and Chief Executive Officer from its founding in 1985 until its merger into our company in 1995. In addition, Dr. Joffe is an Esteemed Quondam Professor of Surgery at the University of Cincinnati Medical Center, a position he has held since 1990. He was a full-time Professor of Surgery at the University of Cincinnati Medical Center for nine years prior to 1990. He has held faculty appointments at the Universities of London, Glasgow and Cincinnati and holds fellowships of the American College of Surgeons and the Royal College of Surgeons of Edinburgh and Glasgow.
Kevin M. Hasseyhas been the President of LCA-Vision since August 1, 2003. Previously, he was the Vice President and General Manager of the Eyemed Managed Care Division of the Luxottica Group from July 1999 to July 2003 and the Vice President of Marketing of the Luxottica Group’s Lenscrafters division from July 1996 to July 1999. Prior to July 1996, Mr. Hassey held various marketing positions with Lenscrafters and spent a number of years in brand management with the Procter & Gamble Company.
Alan H. Buckeyis Executive Vice President/ Finance and Chief Financial Officer for LCA-Vision. He came to LCA-Vision from Pease Industries, a $70 million manufacturing company based in Fairfield, Ohio, where he served as Vice President, Finance from 1991. He also served as CFO of the Hilltop Companies, a contract laboratory research firm, and as a senior manager with Ernst & Young’s Great Lakes Consulting Group. While at Ernst & Young, he served as acting CFO of a start-up laser surgery management company which was the predecessor of LCA-Vision. He joined LCA-Vision in March 2000 and became Executive Vice President in January 2001.
Craig P.R. Joffehas been Senior Vice President, General Counsel and Secretary of LCA-Vision since March 2003. Previously, he served as Assistant General Counsel of USA Interactive (now named InterActive), a publicly traded leading multibrand interactive commerce company, from September 2000 to February 2003. Prior to joining USA Interactive, Mr. Joffe, a graduate of Harvard Law School, was a general practice associate in the New York and London offices of the international law firm Sullivan & Cromwell for over three years.
William O. Colemanis a director and formerly held positions at The Procter & Gamble Company from 1955 — 1988 that included General Sales Manager, Vice President Food Products, Vice President International/ Latin America, and Special Projects. After his retirement, Mr. Coleman served as a
42
Management
Trustee of the Procter & Gamble Retirement Trusts through June 2000. He currently serves on the board of the Touchstone family of funds. He has served as a director of our company since 1997.
John H. Gutfreundis the President of C.E. Unterberg, Towbin, an investment partnership for high-growth companies. Formerly, Mr. Gutfreund was with Salomon Brothers from 1953 — 1991, most recently as its Chairman. Mr. Gutfreund is: director, Montefiore Medical Center, New York City and a member of the Executive Committee; member, Council on Foreign Relations; member, the Board of Trustees, New York Public Library, Astor, Lenox and Tilden Foundations; honorary trustee, Oberlin (Ohio) College; chairman, Arperture Foundation; and director AccuWeather, Inc., Ascent Assurance, Inc., Evercel, Inc., Maxicare Health Plans, Inc. and Nutrition 21, Inc. He has served as a director of our company since 1997.
John C. Hassanis a director and has been the President of Champion Printing, Inc. for more than 11 years. Previously, he was Vice President, Marketing of the Drackett Company, a division of Bristol-Meyers Squibb. He currently serves on the boards of the Printing Industries of Ohio and Northern Kentucky, the Ohio Graphics Arts Health Fund and the Madiera/ Indian Hill Fire Co. He has served as a director of our company since 1996.
There are no family relationships between any of the directors or executive officers of LCA-Vision except that Stephen N. Joffe is Craig P.R. Joffe’s father.
BOARD OF DIRECTORS AND BOARD COMMITTEES
Our board of directors is currently comprised of four members who are elected annually. Our board of directors has two committees, the audit committee and the compensation committee. We do not currently have a nominating committee.
The audit committee reviews and reports to the board of directors with respect to various auditing and accounting matters, including the recommendation of independent auditors and review and consultation with the independent auditors on the audit, financial reporting and the adequacy of internal controls. Messrs. Coleman and Hassan are members of our audit committee. Pursuant to recently approved changes to the corporate governance rules of the Nasdaq National Market, our audit committee is required to have three independent members by our 2004 annual shareholders meeting.
The compensation committee recommends to our entire board of directors the compensation arrangements for our executive officers and administers our stock option plans. Messrs. Coleman and Gutfreund are members of our compensation committee.
43
Certain transactions
Stephen N. Joffe, our principal stockholder, Chairman of the Board and Chief Executive Officer, is the majority stockholder of an inactive ambulatory surgical center. Prior to January 1, 2000, we had made loans to Dr. Joffe and had advanced funds to the surgery center. At January 1, 2001 the note receivable from Dr. Joffe and the receivable from the affiliated company, net of allowance for doubtful accounts, was $360,000.
During 2001, all then remaining assets of the surgery center were sold for $138,000 and the proceeds were paid to us to reduce the note receivable. The remaining balance of the note was forgiven, and the receivable written off in 2001.
Stephen N. Joffe also owns Columbus Eye Associates, an Ohio professional corporation. In some states, we cannot employ ophthalmologists or optometrists. In such states, we contract with a professional corporation to provide medical services to us. In those states that allow a professional corporation to be qualified as a foreign corporation and provide such services, we contract with Columbus Eye Associates. We currently contract with Columbus Eye Associates for the professional services of ophthalmologists and optometrists in Kentucky, Maryland, Minnesota, North Carolina and Virginia and expect to contract with them soon for such services in Nevada. Patient fees are paid to Columbus Eye Associates, which in turn pays a facility fee to us. Amounts retained by Columbus Eye Associates are, by contract, limited to an amount equal to the cost of compensating its ophthalmologists and optometrists and covering its other operating expenses, and Dr. Joffe does not receive any compensation or other remuneration from Columbus Eye Associates.
In January, 2002, we and certain of our directors and then officers purchased 1,632,881 shares of our common stock at a price of $3.00 per share from Summit Autonomous Inc., a subsidiary of ALCON Holdings Inc., for an aggregate price of $4,898,643. At the time of the transaction Summit Autonomous Inc. was a beneficial owner of more than 10% of our outstanding common stock. Mr. Gutfreund and Mr. Hassan, current directors, purchased 25,000 and 6,250 shares, respectively. Dr. Joffe and Mr. Buckey, current executive officers, and Mr. Dzialo, our former President, purchased 743,940, 25,000 and 6,250 shares, respectively, and we purchased 816,440 shares of the common stock sold for an aggregate price of $2,449,000.
We will pay all of our expenses and the expenses of the selling stockholder, Stephen N. Joffe, in connection with this offering, except for the underwriting discounts and commissions. We and the selling stockholder will pay the underwriting discounts and commissions on a pro rata basis, based on the number of shares of common stock being sold by us and the selling stockholder in this offering.
44
Principal and selling stockholders
The following table and notes set forth certain information with respect to the beneficial ownership of our common stock as of November 12, 2003, both before and after giving effect to the sale of shares of common stock in this offering (excluding over-allotments, if any) for the following:
| |
| • | each of our directors and executive officers, |
| |
| • | all directors and executive officers as a group, |
| |
| • | the selling stockholder, and |
| |
| • | each person who is known by us to be the beneficial owner of more than 5% of our outstanding common stock. |
The table below sets forth the names of the selling stockholder and the number of shares which may be sold by the selling stockholder pursuant to this prospectus. “Shares Beneficially Owned” prior to and after the offering include those shares that could be acquired through the possible exercise of outstanding options granted under one or more of our stock option plans which are presently exercisable or will be exercisable within 60 days.
SEC rules provide that shares of common stock which an individual or group has a right to acquire within 60 days pursuant to the exercise of options or warrants are deemed to be outstanding for the purpose of computing the percentage of ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown on the table.
The information included below is based upon information provided by the individuals named below. Because this offering may not result in the sale of all additional shares pursuant to over-allotments, no definitive estimate as to the percentage of common stock that will be held by the selling stockholder after this offering can be provided. The table below has been prepared on the assumption that the over-allotment option has not been exercised, and that all of the shares offered under this prospectus will be sold to unaffiliated parties. See “Underwriting.”
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Shares Beneficially | | | | Shares Beneficially |
| | Owned | | | | Owned |
| | Prior to Offering(1) | | | | After the Offering |
| |
| | Shares | |
|
| Name | | Number | | Percent | | Offered | | Number | | Percent |
|
|
| Stephen N. Joffe, M.D | | | 3,386,412 | (2) | | | 31.2 | % | | | 600,000 | | | | 2,786,412 | (2) | | | 21.0 | % |
| William O. Coleman | | | 49,813 | (3) | | | * | | | | — | | | | 49,813 | (3) | | | * | |
| John H. Gutfreund | | | 50,938 | (4) | | | * | | | | — | | | | 50,938 | (4) | | | * | |
| John C. Hassan | | | 18,188 | (5) | | | * | | | | — | | | | 18,188 | (5) | | | * | |
| Kevin M. Hassey | | | 28,000 | (6) | | | * | | | | — | | | | 28,000 | (6) | | | * | |
| Alan H. Buckey | | | 94,700 | (7) | | | * | | | | — | | | | 94,700 | (7) | | | * | |
| Craig P.R. Joffe | | | 492,633 | (8) | | | 4.5 | % | | | — | | | | 492,633 | (8) | | | 3.7 | % |
| All directors and executive officers as a group (7 persons) | | | 4,120,684 | (9) | | | 37.7 | % | | | 600,000 | | | | 3,520,684 | (10) | | | 26.4 | % |
| | |
| | (1) | The persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws, where applicable, and the information contained in other footnotes to this table. |
| |
| | (2) | Stephen N. Joffe, MD is deemed to be the beneficial owner of all shares owned by himself and by his spouse, Sandra Joffe. The total shown consists of 1,663,257 shares of common stock held by the Stephen N. Joffe Grantor Retained Annuity Trust, dated December 31, 2002; 244,120 shares of common stock owned of record by Dr. Joffe; 624,585 shares of common stock held by the Sandra F.W. Joffe Grantor Retained Annuity Trust, dated December 31, 2002; 257,543 shares of common stock held by the Stephen N. Joffe Grantor Retained Annuity Trust, dated December 31, 2001 (157,363 shares after the offering); 96,713 shares of common stock held by the Sandra F.W. Joffe Grantor Retained Annuity Trust, dated |
45
Principal and selling stockholders
| | |
| | | December 31, 2001; 500,007 shares of common stock owned of record by Dr. and Mrs. Joffe jointly (187 shares after the offering); and 187 shares of common stock issuable to Mrs. Joffe in the event of her exercise of a currently exercisable stock option. If the underwriters exercise in full the over-allotment option granted to them by Dr. Joffe, the total number of shares beneficially owned by him will be 2,336,412, or 17.6% of our total outstanding shares. |
| | |
| | (3) | Includes 37,125 shares owned of record by Mr. Coleman; 1,750 held in trust for the benefit of certain family members (as to which shares Mr. Coleman disclaims beneficial ownership); and 10,938 shares of common stock issuable to Mr. Coleman upon the exercise of certain unexercised stock options. |
| |
| | (4) | Includes 40,000 shares owned of record by Mr. Gutfreund, and 10,938 shares of common stock issuable to Mr. Gutfreund upon the exercise of certain unexercised stock options. |
| |
| | (5) | Includes 7,250 shares owned of record by Mr. Hassan and 10,938 shares of common stock issuable to Mr. Hassan upon the exercise of certain unexercised stock options. |
| |
| | (6) | Includes 3,000 shares owned of record by Mr. Hassey and 25,000 shares of common stock issuable to Mr. Hassey upon the exercise of certain unexercised stock options. |
| |
| | (7) | Includes 50,000 shares owned of record by Mr. Buckey, 5,950 shares owned of record by his spouse and minor children, and 38,750 shares of common stock issuable to Mr. Buckey upon the exercise of certain unexercised stock options. |
| |
| | (8) | Includes 492,633 shares owned of record by Mr. Joffe. |
| |
| | (9) | Consists of 4,023,933 shares owned of record directly or indirectly by such persons and 96,751 shares issuable upon the exercise of stock options held directly or indirectly by such persons. |
| |
| (10) | Consists of 3,423,933 shares owned of record directly or indirectly by such persons and 96,751 shares issuable upon the exercise of stock options held directly or indirectly by such persons. |
46
Description of capital stock
Our authorized capital stock consists of 27,500,000 shares of common stock, $0.001 par value per share, and 5,000,000 shares of preferred stock, $0.001 par value per share. As of December 3, 2003, there were 10,846,718 shares of common stock outstanding held by approximately 2,425 holders of record. No shares of preferred stock are outstanding. Our common stock trades on the Nasdaq National Market.
The holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at times and in amounts as the board of directors may from time to time determine. Holders of common stock are entitled to one vote per share on all matters to be voted upon generally by the stockholders.
The common stock is not entitled to preemptive or preferential rights. There are no redemption or sinking fund provisions applicable to our common stock. Upon liquidation, dissolution or winding up of the company, the assets legally available for distribution to our stockholders are divided among the holders of the common stock in proportion to the number of shares of common stock held by each of them, following payment of all of our debts and liabilities and fulfillment of the rights of any outstanding class or series of preferred stock that has priority to distributed assets. The rights of holders of common stock are subordinate to those of holders of any series of preferred stock.
Our board of directors is authorized to issue from time to time, without stockholder authorization, in one or more designated series, any or all of our authorized but unissued shares of preferred stock with any dividend, redemption, conversion and exchange provision as may be provided in that particular series.
The rights of the holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of any preferred stock. Issuance of a new series of preferred stock, while providing desirable flexibility in connection with possible strategic transactions and other corporate purposes, could have the effect of entrenching our board of directors and making it more difficult for a third-party to acquire, or discouraging a third-party from acquiring, a majority of our outstanding voting stock.
47
Shares eligible for future sale
The market price of our common stock could drop due to sales of large number of shares of our common stock or the perception that such sales could occur. These factors could also make it more difficult to raise funds through future offerings of common stock.
After this offering, 13,246,718 shares of common stock will be outstanding. Of these shares, all shares sold in this offering will be freely tradeable without restriction under the Securities Act of 1933 except for any shares purchased by any of our “affiliates” as defined in Rule 144 under the Securities Act. After this offering, assuming no affiliates purchase shares in the offering and the underwriters do not exercise their over-allotment option, our affiliates will own approximately 3.5 million shares of our common stock.
Our officers and directors and the selling stockholder have entered into lock-up agreements pursuant to which they have agreed not to offer or sell any shares of common stock or securities convertible into or exercisable or exchangeable for shares of common stock for a period of 90 days after the date of this prospectus without the prior written consent of UBS Securities LLC, subject to certain permitted exceptions. See “Underwriting.” UBS Securities LLC may, at any time and without notice, waive any of the terms of these lock-up agreements. Following the lock-up period, these shares will not be eligible for sale in the public market without registration under the Securities Act unless such sales meet the conditions and restrictions of Rule 144 as described below.
In general, under Rule 144 as currently in effect, any person (or persons whose shares are aggregated), including an affiliate, who has beneficially owned shares for a period of at least one year is entitled to sell, within any three-month period, a number of shares that does not exceed the greater of (i) 1% of the then-outstanding shares of common stock and (ii) the average weekly trading volume in the common stock during the four calendar weeks immediately preceding the date on which the notice of such sale on Form 144 is filed with the SEC. Sales under Rule 144 are also subject to certain provisions relating to notice and manner of sale and the availability of current public information about us. The foregoing summary of Rule 144 is not intended to be a complete description.
48
Underwriting
We and the selling stockholder are offering the shares of our common stock described in this prospectus through the underwriters named below. UBS Securities LLC and C.E. Unterberg, Towbin are the representatives of the underwriters. We and the selling stockholder have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of common stock listed next to its name in the following table.
| | | |
| | Number of |
| Underwriters | | Shares |
|
|
| UBS Securities LLC | | 2,340,000 |
| C.E. Unterberg, Towbin | | 585,000 |
| Maxim Group LLC | | 75,000 |
| | |
|
| Total | | 3,000,000 |
| | |
|
The underwriting agreement provides that the underwriters must buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Our common stock and the common stock of the selling stockholder are offered subject to a number of conditions, including:
| |
| • | receipt and acceptance of our common stock by the underwriters, and |
| |
| • | the underwriters’ right to reject orders in whole or in part. |
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
OVER-ALLOTMENT OPTION
The selling stockholder has granted the underwriters an option to buy up to an aggregate of 450,000 additional shares of our common stock from the selling stockholder. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above.
COMMISSIONS AND DISCOUNTS
Shares sold by the underwriters to the public will initially be offered at the offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $0.59 per share from the public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $0.10 per share from the public offering price. If all the shares are not sold at the public offering price, the representatives may change the offering price and the other selling terms. Sales of shares made outside of the United States may be made by affiliates of the underwriters.
We will pay all of the expenses of this offering, except for the underwriting discounts and commissions. We and the selling stockholder will pay the underwriting discounts and commissions on a pro rata basis, based on the number of shares of common stock being sold by us and the selling stockholder in this offering. The following table shows the per share and total underwriting discounts and commissions we and the selling stockholder will pay to the underwriters. These amounts are
49
Underwriting
shown assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional 450,000 shares.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Paid by Us | | Paid by Selling Stockholder | | Total |
| |
| |
| |
|
| | No exercise | | Full exercise | | No exercise | | Full exercise | | No exercise | | Full exercise |
|
|
| Per Share | | $ | 0.99 | | | $ | 0.99 | | | $ | 0.99 | | | $ | 0.99 | | | $ | 0.99 | | | $ | 0.99 | |
| Total | | $ | 2,376,000 | | | $ | 2,376,000 | | | $ | 594,000 | | | $ | 1,039,500 | | | $ | 2,970,000 | | | $ | 3,415,500 | |
We estimate that the total expenses of the offering, payable by us, excluding our underwriting discounts and commissions, will be approximately $225,000.
NO SALES OF SIMILAR SECURITIES
We, our executive officers and directors and the selling stockholder have entered into lock-up agreements with the underwriters. Under these agreements, we and each of these persons may not, without the prior written consent of UBS Securities LLC, subject to limited exceptions, offer, sell, contract to sell, hedge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exercisable or exchangeable for shares of common stock. These restrictions will be in effect for a period of 90 days after the date of this prospectus. At any time and without public notice, UBS Securities LLC may in its sole discretion, release all or some of the securities from these lock-up agreements.
INDEMNIFICATION AND CONTRIBUTION
We and the selling stockholder have agreed to indemnify the underwriters against some liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect thereof.
NASDAQ NATIONAL MARKET QUOTATION
Our common stock is quoted on the Nasdaq National Market under the symbol “LCAV.”
PRICE STABILIZATION, SHORT POSITIONS, PASSIVE MARKET MAKING
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock including:
| |
| • | stabilizing transactions; |
| |
| • | short sales; |
| |
| • | purchases to cover positions created by short sales; |
| |
| • | imposition of penalty bids; |
| |
| • | syndicate covering transactions; and |
| |
| • | passive market making. |
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. Short sales involve the sale by the underwriters of a greater number of shares of our common stock than they are required to purchase in this offering. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position by either exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open
50
Underwriting
market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on the Nasdaq National Market or otherwise.
In addition, in connection with this offering certain of the underwriters (and selling group members) may engage in passive market making transactions in our common stock on the Nasdaq National Market prior to the pricing and completion of this offering. Passive market making consists of displaying bids on the Nasdaq National Market no higher than the bid prices of independent market makers and making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are limited to a specified percentage of the passive market maker’s average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of the common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
AFFILIATIONS
The underwriters and their affiliates may from time to time provide other services to us, including investment banking and financial advisory services, for which they will be entitled to receive separate compensation. In addition, John H. Gutfreund, who is a member of our board of directors, is the President of C.E. Unterberg, Towbin, one of the underwriters.
51
Legal matters
The validity of the common stock offered hereby and certain other legal matters will be passed upon for us by Dinsmore & Shohl LLP, Cincinnati, Ohio. Dewey Ballantine LLP, New York, New York, is counsel for the underwriters in connection with this offering.
Experts
The consolidated financial statements of LCA-Vision Inc. at December 31, 2002 and 2001, and for each of the years in the two year period ended December 31, 2002, appearing and as incorporated by reference in this prospectus and registration statement have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements included in this prospectus for the year ended December 31, 2000 have been so included in reliance on the report of PricewaterhouseCoopers LLP, independent accountants, given on the authority of said firm as experts in auditing and accounting.
Where you can find more information
We file reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on Form S-3 under the Securities Act of 1933 to register the offering of the shares of common stock offered hereby. This prospectus constitutes a part of the registration statement. You may read and copy the registration statement, its exhibits and schedules and any materials we file with the SEC without charge at the Public Reference Room maintained by the SEC at 450 Fifth Street, N.W., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, we are required to file electronic versions of these documents with the SEC through the SEC’s Electronic Data Gathering, Analysis, and Retrieval (EDGAR) system. The SEC maintains an Internet site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. We also maintain a web site, www.lasikplus.com, at which you may view information about us and some of the reports we file with the SEC. Information contained on our website does not constitute, and shall not be deemed to constitute, part of this prospectus.
52
Information incorporated by reference
The SEC allows us to “incorporate by reference” the information we file with the SEC, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered part of this prospectus, and information that we file later with the SEC will automatically update and supersede the information in this prospectus. The following documents, which were previously filed with the SEC pursuant to the Securities Exchange Act of 1934, are hereby incorporated by reference:
| |
| • | our Annual Report on Form 10-K for the year ended December 31, 2002, |
| |
| • | our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2003, June 30, 2003 and September 30, 2003, |
| |
| • | our Current Reports on Form 8-K filed with the Commission on January 13 and 30, February 4, 12 and 20, March 3 and 17, April 14, May 19 and 20, June 2, July 14 and 15, August 13 and 25, 2003, September 23 and October 7, 21 and 22 and |
| |
| • | the description of our common stock contained in our Registration Statement on Form 10-SB No. 0-27610, which became effective on January 25, 1996. |
All reports and other documents filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934 subsequent to the date of this prospectus and prior to the termination of this offering shall be deemed to be incorporated by reference into this prospectus and shall be a part hereof from the date of filing of such reports and documents.
We will provide without charge to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, upon such person’s written or oral request that includes the address (including title or department) and telephone number to which such information is to be directed, a copy of any and all information incorporated by reference in this prospectus (other than exhibits to the information that is incorporated by reference, unless such exhibits are specifically incorporated by reference herein). You can request such documents by writing or calling us at 7840 Montgomery Road, Cincinnati, Ohio 45236, (513) 792-9292, Attention: Alan H. Buckey.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus at the time you make your investment decision with respect to the common stock offered by this prospectus shall be deemed modified, superceded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus, or in any subsequently filed document that also is deemed to be incorporated by reference in this prospectus at the time you make your investment decision, modifies, supercedes or replaces such statement. Any statement so modified, superceded or replaced shall not be deemed, except as so modified, superceded or replaced, to constitute a part of this prospectus. Subject to the foregoing, all information appearing in this prospectus is qualified in its entirety by the information appearing in the documents incorporated by reference.
Statements contained in this prospectus as to the contents of any contract or other document are not necessarily complete, and in each instance we refer you to the copy of the contract or document filed as an exhibit to the registration statement or the documents incorporated by reference in this prospectus, each such statement being qualified in all respects by such reference.
53
LCA-Vision Inc.
Index to consolidated financial statements
| | | | | |
| | Page |
|
|
| Unaudited Consolidated Financial Statements | | | F-2 | |
| Condensed Consolidated Balance Sheet | | | F-2 | |
| Condensed Consolidated Statements of Income | | | F-3 | |
| Condensed Consolidated Statements of Cash Flow | | | F-4 | |
| Notes to Condensed Consolidated Financial Statements | | | F-5 | |
| |
| Audited Consolidated Financial Statements | | | F-11 | |
| Report of Independent Auditors | | | F-11 | |
| Consolidated Balance Sheets | | | F-13 | |
| Consolidated Statements of Operations | | | F-14 | |
| Consolidated Statements of Cash Flows | | | F-15 | |
| Consolidated Statements of Shareholders’ Investment | | | F-16 | |
| Notes to Consolidated Financial Statements | | | F-17 | |
F-1
LCA-Vision Inc.
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | | | | | | | | | |
| | September 30, | | December 31, |
| | 2003(1) | | 2002 |
| | |
| |
|
| | (dollars in thousands |
| | except per share data) |
ASSETS | | | | | | | | |
Current Assets | | | | | | | | |
| | Cash and cash equivalents | | $ | 25,260 | | | $ | 18,298 | |
| | Accounts receivable, net | | | 2,426 | | | | 393 | |
| | Receivables from vendors | | | 506 | | | | 337 | |
| | Prepaid expenses, inventory and other | | | 1,012 | | | | 1,462 | |
| | | |
| | | |
| |
| | | Total current assets | | | 29,204 | | | | 20,490 | |
| Accounts receivable noncurrent, net | | | 601 | | | | — | |
| Property and Equipment | | | 40,550 | | | | 37,301 | |
| Accumulated depreciation and amortization | | | (23,664 | ) | | | (18,868 | ) |
| | | |
| | | |
| |
| Property and equipment, net | | | 16,886 | | | | 18,433 | |
| Goodwill, net | | | 275 | | | | 275 | |
| Deferred compensation plan assets | | | 379 | | | | 127 | |
| Investment in unconsolidated businesses | | | 394 | | | | 263 | |
| Other assets | | | 442 | | | | 408 | |
| | | |
| | | |
| |
| | | Total assets | | $ | 48,181 | | | $ | 39,996 | |
| | | |
| | | |
| |
LIABILITIES AND SHAREHOLDERS’ INVESTMENT | | | | | | | | |
Current liabilities | | | | | | | | |
| | Accounts payable | | $ | 1,639 | | | $ | 3,855 | |
| | Accrued liabilities and other | | | 5,434 | | | | 3,605 | |
| | Debt maturing in one year | | | — | | | | 10 | |
| | | |
| | | |
| |
| | | Total current liabilities | | | 7,073 | | | | 7,470 | |
| Deferred compensation liability | | | 376 | | | | 129 | |
| Insurance reserve | | | 1,384 | | | | 55 | |
| Minority equity interest | | | 446 | | | | 230 | |
Shareholders’ investment | | | | | | | | |
| | Common stock ($0.01 par value; 13,169,923 and 13,110,306 shares and 10,802,726 and 10,743,109 shares issued and outstanding, respectively) | | | 13 | | | | 13 | |
| | Contributed capital | | | 91,800 | | | | 91,474 | |
| | Warrants | | | 1,982 | | | | 1,982 | |
| | Notes receivable from shareholders | | | (225 | ) | | | (1,532 | ) |
| | Common stock in treasury, at cost ( 2,367,197 shares and 2,367,197 shares) | | | (15,462 | ) | | | (15,462 | ) |
| | Accumulated deficit | | | (39,270 | ) | | | (44,338 | ) |
| | Foreign currency translation adjustment | | | 64 | | | | (25 | ) |
| | | |
| | | |
| |
| | | Total shareholders’ investment | | | 38,902 | | | | 32,112 | |
| | | |
| | | |
| |
| | | Total liabilities and shareholders’ investment | | $ | 48,181 | | | $ | 39,996 | |
| | | |
| | | |
| |
The notes to the Condensed Consolidated Financial Statements are an integral part of this statement.
F-2
LCA-Vision Inc.
CONDENSED CONSOLIDATED STATEMENTS OF INCOME
| | | | | | | | | | | | | | | | | | |
| | | | |
| | Three months ended | | Nine months ended |
| | September 30, | | September 30, |
| |
| |
|
| | 2003(1) | | 2002(1) | | 2003(1) | | 2002(1) |
| | |
| |
|
| | (dollars in thousands except per share data) |
| Revenues — Laser refractive surgery | | $ | 20,455 | | | $ | 13,462 | | | $ | 60,661 | | | $ | 48,538 | |
| Operating costs and expenses | | | | | | | | | | | | | | | | |
| | Medical professional and license fees | | | 3,873 | | | | 2,465 | | | | 11,846 | | | | 9,791 | |
| | Direct costs of services | | | 8,365 | | | | 7,341 | | | | 23,982 | | | | 22,127 | |
| | General and administrative expenses | | | 2,062 | | | | 1,994 | | | | 6,073 | | | | 6,454 | |
| | Marketing and advertising | | | 3,034 | | | | 2,968 | | | | 9,164 | | | | 10,179 | |
| | Depreciation and amortization | | | 1,653 | | | | 1,508 | | | | 4,692 | | | | 4,458 | |
| | Special charges (benefit) | | | — | | | | — | | | | — | | | | (174 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Operating income (loss) | | | 1,468 | | | | (2,814 | ) | | | 4,904 | | | | (4,297 | ) |
| Equity in earnings from unconsolidated businesses | | | 39 | | | | 23 | | | | 245 | | | | 228 | |
| Minority equity interest | | | (64 | ) | | | (44 | ) | | | (215 | ) | | | (157 | ) |
| Interest expense | | | (17 | ) | | | (1 | ) | | | (17 | ) | | | (3 | ) |
| Investment income (loss) | | | 172 | | | | (170 | ) | | | 280 | | | | 110 | |
| Other income (expense) | | | — | | | | (11 | ) | | | 52 | | | | (3 | ) |
| | | | | | | | | | | | | | | | 2 | |
| Litigation settlement | | | — | | | | 2,282 | | | | — | | | | ,282 | |
| | | |
| | | |
| | | |
| | | |
| |
| Income (loss) before taxes on income | | | 1,598 | | | | (735 | ) | | | 5,249 | | | | (1,840 | ) |
| Income tax expense | | | 81 | | | | 75 | | | | 181 | | | | 98 | |
| | | |
| | | |
| | | |
| | | |
| |
| Net income (loss) | | $ | 1,517 | | | $ | (810 | ) | | $ | 5,068 | | | $ | (1,938 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Income (loss) per common share | | | | | | | | | | | | | | | | |
| | Basic | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
| | Diluted | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
| Weighted average shares outstanding | | | | | | | | | | | | | | | | |
| | Basic | | | 10,771 | | | | 10,741 | | | | 10,753 | | | | 10,866 | |
| | Diluted | | | 11,044 | | | | 10,741 | | | | 10,856 | | | | 10,866 | |
The notes to the Condensed Consolidated Financial Statements are an integral part of this statement.
F-3
LCA-Vision Inc.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOW
| | | | | | | | | | | | |
| | |
| | Nine months ended |
| | September 30, |
| |
|
| | 2003(1) | | 2002(1) |
| | |
| |
|
| | (dollars in thousands |
| | except per share data) |
Cash flow from operating activities: | | | | | | | | |
| | Net income (loss) | | $ | 5,068 | | | $ | (1,938 | ) |
| | Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | | |
| | | Depreciation and amortization | | | 4,692 | | | | 4,458 | |
| | | Amortization of warrant | | | — | | | | 510 | |
| | | Provision for loss on doubtful accounts | | | 1,155 | | | | 81 | |
| | | Deferred compensation | | | 247 | | | | — | |
| | | Insurance reserve | | | 1,329 | | | | — | |
| | | Equity in earnings of unconsolidated affiliates | | | (245 | ) | | | (228 | ) |
| | | Special charges (benefit) | | | — | | | | (174 | ) |
| | | Other, net | | | (2 | ) | | | 3 | |
| | | Changes in assets and liabilities: | | | | | | | | |
| | | | Accounts receivable | | | (3,789 | ) | | | 29 | |
| | | | Receivable from vendor | | | (169 | ) | | | (39 | ) |
| | | | Prepaid expenses, inventory and other | | | 450 | | | | 960 | |
| | | | Accounts payable | | | (2,216 | ) | | | (239 | ) |
| | | | Accrued liabilities and other | | | 1,830 | | | | 862 | |
| | | |
| | | |
| |
| | Net cash provided by operations | | | 8,350 | | | | 4,285 | |
Cash flow from investing activities: | | | | | | | | |
| | | Purchase of property and equipment | | | (3,251 | ) | | | (986 | ) |
| | | Proceeds from sale of property and equipment | | | 2 | | | | 8 | |
| | | Deferred compensation plan | | | (252 | ) | | | — | |
| | | Other, net | | | 376 | | | | (114 | ) |
| | | |
| | | |
| |
| | Net cash used by investing activities | | | (3,125 | ) | | | (1,092 | ) |
Cash flows from financing activities: | | | | | | | | |
| | | Principal payments of long-term notes, debt and capital lease obligations | | | (10 | ) | | | (16 | ) |
| | | Loan payment made by shareholders | | | 1,329 | | | | — | |
| | | Loans to shareholders | | | (22 | ) | | | (33 | ) |
| | | Shares repurchased for treasury stock | | | — | | | | (2,449 | ) |
| | | Exercise of stock options and warrants | | | 326 | | | | 234 | |
| | | Distribution from (to) minority equity investees | | | 114 | | | | 183 | |
| | | |
| | | |
| |
| | Net cash provided by (used by) financing activities | | | 1,737 | | | | (2,081 | ) |
| | Increase in cash and cash equivalents | | | 6,962 | | | | 1,112 | |
| | Cash and cash equivalents at beginning of period | | | 18,298 | | | | 16,609 | |
| | | |
| | | |
| |
| | Cash and cash equivalents at end of period | | $ | 25,260 | | | $ | 17,721 | |
| | | |
| | | |
| |
The notes to the Consolidated Condensed Financial Statements are an integral part of this statement.
F-4
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
for the Three and Nine Months Ended September 30, 2003 and 2002
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This filing includes condensed consolidated Balance Sheets as of September 30, 2003 and December 31, 2002; condensed consolidated Statements of Income for the three and nine months ended September 30, 2003 and 2002; and condensed consolidated Statements of Cash Flow for the nine months ended September 30, 2003 and 2002. In the opinion of management, these unaudited consolidated financial statements contain all adjustments necessary to present fairly the financial position, results of operations and cash flows for the interim period reported. We suggest that these financial statements be read together with the financial statements and notes in our annual report on Form 10-K.
About our company
We are a leading developer and operator of fixed-site laser vision correction centers under the brand name LasikPlus. Our vision centers provide the staff, facilities, equipment and support services for performing laser vision correction that employs advanced laser technologies to help correct nearsightedness, farsightedness and astigmatism. We currently utilize fixed-site excimer lasers manufactured by Bausch & Lomb, VISX and Alcon. Our vision centers are supported mainly by full-time credentialed board-certified ophthalmologists, optometrists and other health care professionals. The ophthalmologists perform the laser vision correction procedures in our vision centers, and either ophthalmologists or optometrists generally carry out the pre-procedure evaluations and post-procedure follow-ups in-center as well. We have performed over 300,000 laser vision correction procedures in our vision centers, in the United States and Canada, since 1991.
We currently operate 37 laser vision correction centers, including 34 wholly-owned vision centers located in large metropolitan markets throughout the United States, two joint ventures in Canada and one joint venture in Europe. We recently announced the opening of our newest LasikPlusvision center in Las Vegas.
Consolidation policy
We use the consolidation method to report our investment in our subsidiaries and other companies when we own a majority of the voting stock of the subsidiary. In addition, we consolidate the results of operations of professional corporations with which we contract to provide the services of ophthalmologists or optometrists at our vision centers, in accordance with EITF 97-2,Application of FASB Statement No. 94 and APB Opinion No. 16 to Physician Management Entities and Certain Other Entities with Contractual Management Agreements.Prior to September 2002, our contractual management arrangements did not permit consolidation of our relationships with professional corporations pursuant to EITF 97-2 because we did not maintain a “controlling financial interest” in the professional corporations. Beginning in September 2002, we began a process of renewing our agreements with the professional corporations and opening new vision centers with agreements that meet the “controlling financial interest” criteria of EITF 97-2.
As a result of the FASB’s issuance of FIN 46,Consolidation of Variable Interest Entities, an interpretation of ARB No. 51, in January 2003, we have evaluated the contractual management arrangements entered into with professional corporations after January 1, 2003 and have determined that consolidation of these entities is appropriate under the FIN 46 guidance. With respect to the three remaining professional corporations with which we had a contractual management arrangement prior to January 1, 2003, we have determined that we should consolidate these entities under the FIN 46 guidance beginning July 1, 2003, which we do not believe will have a material impact on our
F-5
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
for the Three and Nine Months Ended September 30, 2003 and 2002
consolidated financial statement. Our condensed consolidated financial statements include the accounts of:
| |
| • | LCA-Vision Inc. |
| |
| • | LCA-Vision (Canada) Inc. and Subsidiaries |
| |
| • | The Baltimore Laser Sight Center, Ltd |
| |
| • | Columbus Eye Associates, Inc. (effective September 1, 2002) |
| |
| • | LasikPlus Medical Associates, Inc. (effective January 1, 2003) |
| |
| • | LasikPlus Medical Associates, S.C. (effective March 1, 2003) |
| |
| • | Lasik Insurance Company Ltd. |
| |
| • | LasikPlus Medical Associates, P.C. (effective July 1, 2003) |
| |
| • | LasikPlus Medical Associates, P.A. (effective July 1, 2003) |
| |
| • | Capital Region Vision Laser Associates, P.C. (effective July 1, 2003) |
Equity method
We use the equity method to report investments in businesses where we hold a 20% to 50% voting interest, giving us the ability to exercise significant influence, but not control, over operating and financial policies. Under the equity method we report:
| |
| • | our interest in the entity as an investment in our Condensed Consolidated Balance Sheets |
| |
| • | our percentage share of the earnings (losses) in our Condensed Consolidated Statements of Operations |
We own 43% of Silmalaseri Oy and 50% of both Cole LCA Vision LLC (through June 30, 2002) and Eyemed LCA Vision LLC and report our investments under the equity method.
Allowance for doubtful accounts
We provide patient financing to some of our customers, including those who could not otherwise obtain third-party financing. The terms of the financing require the patient to pay an up-front fee which is intended to cover some or all of our variable costs, and the remainder is deducted automatically from the patient’s checking account over a period of 12 to 36 months. We began our patient financing program in May, 2002. As a result of a recent expansion of this program, we are currently exposed to more credit risk than we have experienced in the past. Based upon our own experience with patient financing and based upon the credit experience of centers who provide financing to customers similar to ours, we have established bad debt reserves as of September 30, 2003 of $1,386,000 against accounts receivable of $4,413,000. To the extent that our actual bad debt write-offs are greater than our estimated bad debt reserve, it would adversely impact our results of operations and cash flows and to the extent that our actual bad debt write-offs are less than our estimated bad debt reserve, it would favorably impact our results of operations and cash flows.
Goodwill and other intangible assets
Goodwill is the excess of the acquisition cost of the businesses over the fair value of the identifiable net assets acquired. Through December 31, 2002, we amortized goodwill using the straight-line method over the estimated useful life. The Company adopted Financial Accounting Standards (SFAS) No. 142, “Goodwill and Other Intangible Assets” effective January 1, 2002. SFAS No. 142 discontinued the amortization of goodwill and requires companies to perform an annual impairment test of goodwill. Application of the non-amortization provision of the SFAS No. 142 resulted in a
F-6
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
for the Three and Nine Months Ended September 30, 2003 and 2002
decrease in annual operating expenses of $76,000. The impairment tests of goodwill as of December 31, 2002 indicated that the Company currently has no goodwill impairment.
Captive insurance company reserves
Effective December 18, 2002, we established a captive insurance company to provide professional liability insurance coverage for claims brought against us after December 17, 2002. In addition, our captive insurance company’s charter allows it to provide professional liability insurance for our doctors, some of whom are currently insured by the captive. Our captive insurance company is managed by an independent insurance consulting and management firm, and it is capitalized and funded by us based on actuarial studies performed by an affiliate of the consulting and management firm. For the 12 months ending December 17, 2003, our captive insurance company purchased excess liability coverage for 80% of our losses in the year in excess of $1,000,000 per occurrence, up to $11,000,000. Our captive insurance company is responsible for 20% of our aggregate losses in excess of $1,000,000 per claim, up to $11,000,000. Under that arrangement, the coverage providers’ obligation arises only after our captive pays the first $1,000,000 of any loss and the coverage providers are then only obligated to pay an aggregate of $8,000,000 in a given policy year. These excess liability coverage policies are currently up for renewal, and our management may elect to purchase similar, less, more or no excess liability coverage depending on the premiums quoted, among other factors. A number of claims covered by our captive insurance company are now pending. The financial statements of the captive insurance company are consolidated with our financial statements since it is a wholly-owned enterprise. As of September 30, 2003, we recorded an insurance reserve amount of $1,384,000, which primarily represents an actuarially determined estimate of claims incurred but not yet reported. To the extent that our actual claim experience is greater than our estimated insurance reserve, it would adversely impact our results of operations and cash flows and to the extent that our actual claim experience is less than our estimated insurance reserve, it would favorably impact our results of operations and cash flows.
Income taxes
As a result of our operating loss during the third quarter of 2001 and throughout 2002, and continuing uncertainties regarding the general economic conditions in the United States and the impact on our ongoing operations, we continue to record a full valuation reserve for deferred tax assets. This reserve was established according to the requirements of SFAS No. 109 “Accounting for Income Taxes.” Favorable changes in our operating profitability, as a result of improved general economic conditions in the United States or otherwise, could impact our determination as to whether reduction, in whole or in part, to the valuation reserve is necessary in the future. To the extent that such a reduction in the valuation reserve is necessary, an income tax benefit would be recorded in the consolidated statement of operations, which would favorably impact our results of operations. In addition, future taxable income may be absorbed by the net operating loss carryforward that we maintain, which would favorably impact our results of operations and cash flows. During the nine months ended September 30, 2003, we applied approximately $4,725,000 of our net operating losses against our taxable income for the period, which resulted in reduced federal income tax expenses and tax payments of approximately $1,654,000. The valuation reserve and net operating loss carryforward was $17,118,000 and $44,040,000, respectively, as of September 30, 2003.
Consolidation
We use the consolidation method to report our investment in our subsidiaries and other companies when we own a majority of the voting stock of the subsidiary. In addition, we consolidate the results of operations of professional corporations with which we contract to provide the services of ophthalmologists or optometrists at our vision centers, in accordance with EITF 97-2, Application of FASB Statement No. 94 and APB Opinion No. 16 to Physician Management Entities and Certain Other Entities with Contractual Management Arrangements. Prior to September 2002, our contractual
F-7
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
for the Three and Nine Months Ended September 30, 2003 and 2002
management arrangements did not permit consolidation of our relationships with professional corporations pursuant to EITF 97-2 because we did not maintain a “controlling financial interest” in the professional corporations. Beginning in September 2002, we began a process of renewing our agreements with the professional corporations and opening new vision centers with agreements that meet the “controlling financial interest” criteria of EITF 97-2.
As a result of the FASB’s issuance of FIN 46,Consolidation of Variable Interest Entities, an interpretation of ARB No. 51,in January 2003, we have evaluated the contractual management arrangements entered into with professional corporations after January 1, 2003 and have determined that consolidation of these entities is appropriate under the FIN 46 guidance. With respect to the three remaining professional corporations with which we had a contractual management arrangement prior to January 1, 2003, we have determined that we should consolidate these entities under the FIN 46 guidance beginning July 1, 2003, which we do not believe will have a material impact on our consolidated financial statements. If modifications are made to existing management agreements, or if new agreements are made under different terms than existing management agreements, then the financial statements of the professional corporations may not be consolidated in the future.
Use of estimates
Management makes estimates and assumptions when preparing financial statements under generally accepted accounting principles. Certain estimates are particularly sensitive due to their significance to the financial statements and the possibility that future events may differ significantly from management’s expectations. These estimates and assumptions affect various matters including:
| |
| • | Allowance for doubtful accounts — patient financing |
| |
| • | Deferred income taxes — valuation allowance |
| |
| • | Loss reserves — insurance captive |
Per share data
Basic per share data is income (loss) applicable to common shares divided by the weighted average common shares outstanding. Diluted per share data is income (loss) applicable to common shares divided by the weighted average common shares outstanding plus the potential issuance of common shares if stock options or warrants were exercised or convertible preferred stock were converted into common stock.
F-8
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
for the Three and Nine Months Ended September 30, 2003 and 2002
Following is a reconciliation of basic and diluted earnings per share for the three and nine months ended September 30, 2003 and 2002 (in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | | |
| | | | |
| | Three Months Ended | | Nine Months Ended |
| | September 30, | | September 30, |
| |
| |
|
| | 2003 | | 2002 | | 2003 | | 2002 |
|
|
| Basic earnings: | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | 1,517 | | | $ | (810 | ) | | $ | 5,068 | | | $ | (1,938 | ) |
| Weighted average shares outstanding | | | 10,771 | | | | 10,741 | | | | 10,753 | | | | 10,866 | |
| Basic earnings (loss) per share | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
| | Diluted earnings: | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | 1,517 | | | $ | (810 | ) | | $ | 5,068 | | | $ | (1,938 | ) |
| Weighted average shares outstanding | | | 10,771 | | | | 10,741 | | | | 10,753 | | | | 10,866 | |
| Effect of dilutive securities | | | | | | | | | | | | | | | | |
| | Stock options | | | 273 | | | | — | | | | 103 | | | | — | |
| Weighted average common shares and potential dilutive shares | | | 11,044 | | | | 10,741 | | | | 10,856 | | | | 10,866 | |
| Diluted earnings (loss) per share | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
Stock-based compensation
In December 2002, SFAS No. 148, “Accounting for Stock-Based Compensation — Transition and Disclosure,” which amends SFAS No. 123, “Accounting for Stock-Based Compensation,” was issued. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation and requires more prominent and more frequent disclosures in the financial statements of the effects of stock-based compensation. The provisions of SFAS No. 148 are effective for fiscal years ending after December 15, 2002.
We apply APB No. 25 and related interpretations utilizing the intrinsic value method in accounting for our stock option plans. We have adopted the disclosure-only provisions of SFAS No. 123. We recognize no compensation expense for our stock options granted to employees or directors. Compensation expense for options granted to non-employees in each of the three quarters ended September 30, 2002 and 2003 was immaterial. If we had elected to recognize compensation expense based on the fair value at the grant dates consistent with the provisions of SFAS No. 123, net income and income per share would have been changed to the pro forma amounts indicated below (dollars in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | | |
| | | | |
| | Three Months Ended | | Nine Months Ended |
| | September 30, | | September 30, |
| |
| |
|
| | 2003 | | 2002 | | 2003 | | 2002 |
|
|
| Net income (loss) | | | | | | | | | | | | | | | | |
| | As reported | | $ | 1,517 | | | $ | (810 | ) | | $ | 5,068 | | | $ | (1,938 | ) |
| | Pro forma | | | 1,275 | | | | (1,286 | ) | | | 4,365 | | | | (3,447 | ) |
| Basic per share income (loss) | | | | | | | | | | | | | | | | |
| | As reported | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
| | Pro forma | | | 0.12 | | | | (0.12 | ) | | | 0.41 | | | | (0.32 | ) |
| Diluted per share income (loss) | | | | | | | | | | | | | | | | |
| | As reported | | $ | 0.14 | | | $ | (0.08 | ) | | $ | 0.47 | | | $ | (0.18 | ) |
| | Pro forma | | | 0.12 | | | | (0.12 | ) | | | 0.40 | | | | (0.32 | ) |
F-9
LCA-Vision Inc.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
for the Three and Nine Months Ended September 30, 2003 and 2002
Shareholders’ investment
No material changes from the Company’s most recent Form 10-K.
Segment information
We operate in one segment: laser refractive surgery.
Commitments and contingencies
None.
Internet
The Company’s website is www.lasikplus.com. The Company makes available free of charge through a link provided at such website its Forms 10-K, 10-Q and 8-K, as well as any amendments thereto. Such reports are available as soon as reasonably practicable after they are filed or furnished to the Securities and Exchange Commission. To obtain a copy of Form 10-K by mail, please send a request to Investor Relations at LCA-Vision Inc., 7840 Montgomery Road, Cincinnati, Ohio 45236.
F-10
LCA-Vision Inc.
REPORT OF INDEPENDENT AUDITORS
To the Board of Directors and
Shareholders of LCA-Vision Inc.
We have audited the consolidated balance sheets of LCA-Vision Inc. as of December 31, 2002 and 2001, and the related consolidated statements of operations, shareholders’ investment, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the 2002 and 2001 financial statements referred to above present fairly, in all material respects, the consolidated financial position of LCA-Vision Inc. at December 31, 2002 and 2001, and the consolidated results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States.
/s/ Ernst & Young LLP
Cincinnati, Ohio
February 14, 2003
F-11
LCA-Vision Inc.
REPORT OF INDEPENDENT ACCOUNTANTS
To the Board of Directors and
Shareholders of LCA-Vision Inc.
In our opinion, the accompanying consolidated statements of operations, shareholders’ investment, and cash flows present fairly, in all material respects, the results of their operations and their cash flows of LCA-Vision Inc. for the year ended December 31, 2000, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management; our responsibility is to express an opinion on these financial statements based on our audit. We conducted our audit of these statements in accordance with auditing standards generally accepted in the United States of America, which require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Cincinnati, Ohio
February 13, 2001
F-12
LCA-Vision Inc.
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | | | |
| | |
| | At December 31, |
| |
|
| | 2002 | | 2001 |
| | |
| |
|
| | (dollars in thousands, |
| | except per share |
| | amounts) |
ASSETS | | | | | | | | |
Current assets | | | | | | | | |
| | Cash and cash equivalents | | $ | 18,298 | | | $ | 16,609 | |
| | Accounts receivable, net | | | 393 | | | | 517 | |
| | Receivable from vendors | | | 337 | | | | 234 | |
| | Prepaid expenses, inventory and other | | | 1,462 | | | | 1,959 | |
| | | |
| | | |
| |
| | | Total current assets | | | 20,490 | | | | 19,319 | |
| | Property and equipment | | | 37,301 | | | | 36,411 | |
| | Accumulated depreciation and amortization | | | (18,868 | ) | | | (13,753 | ) |
| | | |
| | | |
| |
| | Property and equipment, net | | | 18,433 | | | | 22,658 | |
| | Goodwill, net | | | 275 | | | | 275 | |
| | Deferred compensation plan assets | | | 127 | | | | — | |
| | Investment in unconsolidated businesses | | | 263 | | | | 290 | |
| | Other assets, net | | | 408 | | | | 646 | |
| | | |
| | | |
| |
| | | Total assets | | $ | 39,996 | | | $ | 43,188 | |
| | | |
| | | |
| |
LIABILITIES AND SHAREHOLDERS’ INVESTMENT | | | | | | | | |
Current liabilities | | | | | | | | |
| | Accounts payable | | $ | 3,855 | | | $ | 2,645 | |
| | Accrued liabilities and other | | | 3,660 | | | | 2,270 | |
| | Debt maturing in one year | | | 10 | | | | 26 | |
| | | |
| | | |
| |
| | | Total current liabilities | | | 7,525 | | | | 4,941 | |
| | Long-term debt | | | — | | | | 4 | |
| | Deferred Compensation Liability | | | 129 | | | | — | |
| | Commitments and Contingencies | | | — | | | | — | |
| | Minority equity interest | | | 230 | | | | 41 | |
Shareholders’ investment | | | | | | | | |
| | Common stock ($.001 par value; 13,110,306 and 13,064,056 shares and 10,743,109 and 11,513,299 shares issued and outstanding, respectively) | | | 13 | | | | 52 | |
| | Contributed capital | | | 91,474 | | | | 91,080 | |
| | Warrants | | | 1,982 | | | | 2,105 | |
| | Notes receivable from shareholders | | | (1,532 | ) | | | (1,488 | ) |
| | Common stock in treasury, at cost (2,367,197 shares and 1,550,757 shares) | | | (15,462 | ) | | | (13,013 | ) |
| | Accumulated deficit | | | (44,338 | ) | | | (40,512 | ) |
| | Accumulated other comprehensive loss | | | (25 | ) | | | (22 | ) |
| | | |
| | | |
| |
| | | Total shareholders’ investment | | | 32,112 | | | | 38,202 | |
| | | |
| | | |
| |
| | | Total liabilities and shareholders’ investment | | $ | 39,996 | | | $ | 43,188 | |
| | | |
| | | |
| |
See Notes to Consolidated Financial Statements
F-13
LCA-Vision Inc.
CONSOLIDATED STATEMENT OF OPERATIONS
| | | | | | | | | | | | | |
| | |
| | Years Ended December 31, |
| |
|
| | 2002 | | 2001 | | 2000 |
|
|
| | |
| | (dollars in thousands, |
| | except per share amounts) |
| Revenues — Laser refractive surgery | | $ | 61,838 | | | $ | 68,096 | | | $ | 63,450 | |
| Operating costs and expenses Medical professional and license fees | | | 12,270 | | | | 13,626 | | | | 15,542 | |
| Direct costs of services | | | 28,796 | | | | 33,616 | | | | 27,218 | |
| General and administrative expenses | | | 8,327 | | | | 8,727 | | | | 9,394 | |
| Marketing and advertising | | | 12,823 | | | | 12,732 | | | | 14,565 | |
| Depreciation and amortization | | | 5,997 | | | | 5,625 | | | | 3,839 | |
| Special charges | | | (174 | ) | | | 1,774 | | | | — | |
| | | |
| | | |
| | | |
| |
| Operating loss | | | (6,201 | ) | | | (8,004 | ) | | | (7,108 | ) |
| Equity in earnings from unconsolidated businesses | | | 241 | | | | 372 | | | | 49 | |
| Minority equity interest | | | (189 | ) | | | (10 | ) | | | (12 | ) |
| Interest expense | | | (4 | ) | | | (17 | ) | | | (58 | ) |
| Interest income | | | 225 | | | | 924 | | | | 2,713 | |
| Other (expense) income, net | | | (6 | ) | | | (51 | ) | | | 616 | |
| Litigation settlement | | | 2,282 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| |
| Loss before taxes on income | | | (3,652 | ) | | | (6,786 | ) | | | (3,800 | ) |
| Income tax expense (benefit) | | | 174 | | | | 16,589 | | | | (1,434 | ) |
| | | |
| | | |
| | | |
| �� |
| Net loss | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| | | |
| | | |
| | | |
| |
| Loss per common share Basic and Diluted | | $ | (0.35 | ) | | $ | (2.01 | ) | | $ | (0.19 | ) |
| Weighted average shares outstanding Basic and Diluted | | | 10,794 | | | | 11,643 | | | | 12,741 | |
See Notes to Consolidated Financial Statements
F-14
LCA-Vision Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | |
| | |
| | Years Ended December 31, |
| |
|
| | 2002 | | 2001 | | 2000 |
|
|
| | |
| | (dollars in thousands) |
Cash flow from operating activities | | | | | | | | | | | | |
| | Net loss | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| | Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
| | | Depreciation and amortization | | | 5,997 | | | | 5,625 | | | | 3,839 | |
| | | Warrant amortization | | | 510 | | | | 701 | | | | 855 | |
| | | Deferred income taxes | | | — | | | | 16,606 | | | | (1,454 | ) |
| | | Obligations due from shareholder and affiliate, net | | | — | | | | — | | | | 127 | |
| | | Restructuring/impairment provision | | | (174 | ) | | | 1,774 | | | | — | |
| | | Equity in earnings from unconsolidated businesses | | | (241 | ) | | | (372 | ) | | | (49 | ) |
| | | Deferred compensation | | | 129 | | | | — | | | | — | |
| | | Other, net | | | 6 | | | | 39 | | | | (17 | ) |
| | | Changes in working capital, net of effects from acquisition of businesses | | | | | | | | | | | | |
| | | Accounts receivable | | | 124 | | | | 900 | | | | 945 | |
| | | Receivable from vendor | | | (103 | ) | | | 2,046 | | | | (2,280 | ) |
| | | Prepaid expenses, inventory and other | | | 497 | | | | 29 | | | | (17 | ) |
| | | Accounts payable | | | 1,210 | | | | (4,942 | ) | | | 5,129 | |
| | | Accrued liabilities and other | | | 1,564 | | | | (1,024 | ) | | | 880 | |
| | | |
| | | |
| | | |
| |
| | Net cash provided by (used in) operating activities | | | 5,693 | | | | (1,993 | ) | | | 5,592 | |
Cash flows from investing activities | | | | | | | | | | | | |
| | | Purchase of property and equipment | | | (1,789 | ) | | | (7,061 | ) | | | (15,597 | ) |
| | | Proceeds from sale of property and equipment | | | 8 | | | | 5 | | | | 113 | |
| | | Purchase of held-to-maturity investments | | | — | | | | (4,378 | ) | | | (52,756 | ) |
| | | Maturities of held-to-maturity investments | | | — | | | | 13,004 | | | | 81,429 | |
| | | Deferred compensation plan | | | (127 | ) | | | — | | | | — | |
| | | Loans and advances to affiliated companies | | | — | | | | — | | | | (150 | ) |
| | | Loans to shareholders | | | (44 | ) | | | (475 | ) | | | (1,013 | ) |
| | | Other, net | | | (83 | ) | | | 610 | | | | (193 | ) |
| | | |
| | | |
| | | |
| |
| | Net cash (used in) provided by investing activities | | | (2,035 | ) | | | 1,705 | | | | 11,833 | |
| | | |
| | | |
| | | |
| |
Cash flows from financing activities | | | | | | | | | | | | |
| | | Principal payments of long-term debt and capital lease obligations | | | (20 | ) | | | (196 | ) | | | (704 | ) |
| | | Shares repurchased for treasury stock | | | (2,449 | ) | | | (3,138 | ) | | | (9,845 | ) |
| | | Exercise of stock options and warrants | | | 232 | | | | 162 | | | | 940 | |
| | | Distribution from minority equity investees | | | 268 | | | | 377 | | | | — | |
| | | Other, net | | | — | | | | — | | | | (15 | ) |
| | | |
| | | |
| | | |
| |
| | Net cash used in financing activities | | | (1,969 | ) | | | (2,795 | ) | | | (9,624 | ) |
| | | |
| | | |
| | | |
| |
| | Increase (decrease) in cash and cash equivalents | | | 1,689 | | | | (3,083 | ) | | | 7,801 | |
| | Cash and cash equivalents at beginning of year | | | 16,609 | | | | 19,692 | | | | 11,891 | |
| | | |
| | | |
| | | |
| |
| | Cash and cash equivalents at end of year | | $ | 18,298 | | | $ | 16,609 | | | $ | 19,692 | |
| | | |
| | | |
| | | |
| |
See Notes to Consolidated Financial Statement
F-15
LCA-Vision Inc.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ INVESTMENT
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| | Years Ended December 31, |
| |
|
| | | | | | |
| | 2002 | | 2001 | | 2000 |
| |
| |
| |
|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount |
|
|
| | |
| | (dollars in thousands) |
Common Stock | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | 13,064,056 | | | | 52 | | | | 13,030,019 | | | | 52 | | | | 12,880,415 | | | | 52 | |
| | Employee plans | | | 46,250 | | | | — | | | | 34,037 | | | | — | | | | 149,604 | | | | — | |
| | Stock split — par value effect | | | | | | | (39 | ) | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Balance at end of year | | | 13,110,306 | | | | 13 | | | | 13,064,056 | | | | 52 | | | | 13,030,019 | | | | 52 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Warrants | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | 127,734 | | | | 2,105 | | | | 127,734 | | | | 2,105 | | | | 227,734 | | | | 6,362 | |
| | Expired warrants | | | (27,734 | ) | | | (123 | ) | | | — | | | | — | | | | — | | | | — | |
| | Repurchased | | | | | | | | | | | — | | | | — | | | | (100,000 | ) | | | (248 | ) |
| | Revalued | | | | | | | | | | | — | | | | �� | | | | — | | | | (4,009 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Balance at end of year | | | 100,000 | | | | 1,982 | | | | 127,734 | | | | 2,105 | | | | 127,734 | | | | 2,105 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Notes Receivable from Shareholders | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | | | | | (1,488 | ) | | | | | | | (1,013 | ) | | | | | | | — | |
| | Increase in notes outstanding | | | | | | | (44 | ) | | | | | | | (475 | ) | | | | | | | (1,013 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Balance at end of year | | | | | | | (1,532 | ) | | | | | | | (1,488 | ) | | | | | | | (1,013 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Treasury Stock | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | (1,550,757 | ) | | | (13,013 | ) | | | (1,179,724 | ) | | | (9,875 | ) | | | (2,727 | ) | | | (30 | ) |
| | Shares repurchased | | | (816,440 | ) | | | (2,449 | ) | | | (371,033 | ) | | | (3,138 | ) | | | (1,176,997 | ) | | | (9,845 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | Balance at end of year | | | (2,367,197 | ) | | | (15,462 | ) | | | (1,550,757 | ) | | | (13,013 | ) | | | (1,179,724 | ) | | | (9,875 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Contributed Capital | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | | | | | 91,080 | | | | | | | | 90,918 | | | | | | | | 88,407 | |
| | Stock split — par value effect | | | | | | | 39 | | | | | | | | | | | | | | | | | |
| | Employee plans | | | | | | | 235 | | | | | | | | 161 | | | | | | | | 939 | |
| | Tax benefit from stock option exercise | | | | | | | — | | | | | | | | — | | | | | | | | 1,571 | |
| | Stock split fractional shares | | | | | | | (3 | ) | | | | | | | — | | | | | | | | — | |
| | Expired warrants | | | | | | | 123 | | | | | | | | — | | | | | | | | — | |
| | Other | | | | | | | — | | | | | | | | 1 | | | | | | | | 1 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
| | Balance at end of year | | | | | | | 91,474 | | | | | | | | 91,080 | | | | | | | | 90,918 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
Accumulated deficit | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | | | | | (40,512 | ) | | | | | | | (17,137 | ) | | | | | | | (14,771 | ) |
| | Net loss | | | | | | | (3,826 | ) | | | | | | | (23,375 | ) | | | | | | | (2,366 | ) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
| | Balance at end of year | | | | | | | (44,338 | ) | | | | | | | (40,512 | ) | | | | | | | (17,137 | ) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
Accumulated Other Comprehensive Loss (Income) | | | | | | | | | | | | | | | | | | | | | | | | |
| | Balance at beginning of year | | | | | | | (22 | ) | | | | | | | (5 | ) | | | | | | | 25 | |
| | Translation adjustments | | | | | | | (3 | ) | | | | | | | (17 | ) | | | | | | | (30 | ) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
| | Balance at end of year | | | | | | | (25 | ) | | | | | | | (22 | ) | | | | | | | (5 | ) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
| | | Total Shareholders’ Investment | | | | | | | 32,112 | | | | | | | | 38,202 | | | | | | | | 65,045 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| |
See Notes to Consolidated Financial Statements
F-16
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business
As of December 31, 2002, the Company owns and operates 32 LasikPluslaser vision correction facilities in the US, plus two centers in Canada and a joint venture in Europe.
We are a leading developer and operator of free-standing laser refractive surgery centers. Our laser refractive surgery centers provide the staff, facilities, equipment and support services for performing laser vision correction that employ state-of-the-art laser technologies to correct nearsightedness, farsightedness and astigmatism. The Company currently utilizes three primary excimer lasers: the Bausch & Lomb Technolas 217, the VISX Star S2/S3 and the Alcon LADARVision. Substantially all of the revenues from our laser vision correction procedures are derived from our North American Centers.
Operating costs and expenses consist of:
| |
| • | Medical professional and license fees, including per-procedure fees for the ophthalmologist performing laser vision correction and the license fee per procedure paid to Bausch & Lomb, VISX and Alcon |
| |
| • | Direct costs of services, including center rent and utilities, equipment lease and maintenance costs, surgical supplies, center staff expense, costs related to other revenue and all other costs associated with providing services in our centers |
| |
| • | General and administrative associated with corporate overhead costs |
| |
| • | Marketing and advertising costs |
| |
| • | Depreciation and amortization of equipment and intangible assets recorded in the balance sheet |
Consolidation policy
We use two different methods to report our investments in our subsidiaries and other companies — consolidation and the equity method.
Consolidation
We use consolidation when we own a majority of the voting stock of the subsidiary. In addition, we are in compliance with EITF 97-2, for Professional Corporations which requires consolidation where we have a controlling financial interest through a contractual management arrangement with the professional corporation.
Our condensed consolidated financial statements include the accounts of:
| |
| • | LCA-Vision Inc. |
| |
| • | LCA-Vision (Canada) Inc. and Subsidiaries |
| |
| • | The Baltimore Laser Sight Center, Ltd. |
| |
| • | Columbus Eye Associates, Inc. (contract effective September 1, 2002) |
| |
| • | Lasik Insurance Company, Ltd. (captive insurance entity) |
Equity method
We use the equity method to report investments in businesses where we hold a 20% to 50% voting interest, giving us the ability to exercise significant influence, but not control, over operating and financial policies. Under the equity method, we report:
| |
| • | our interest in the entity as an investment in our Consolidated Balance Sheets, and |
| |
| • | our percentage share of the earnings (losses) in our Consolidated Statements of Operations. |
F-17
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We own 43% of Oy and 50% of Cole/ LCA-Vision LLC (through June 30, 2002) and Eyemed/ LCA-Vision LLC, and report each of these investments under the equity method.
Use of estimates
Management makes estimates and assumptions when preparing financial statements under generally accepted accounting principles. Certain estimates are particularly sensitive due to their significance to the financial statements and the possibility that future events may differ significantly from management’s expectations. These estimates and assumptions affect various matters including:
| |
| • | Allowance for doubtful accounts—patient financing |
| |
| • | Enhancement accrual |
| |
| • | Deferred income taxes—valuation allowance |
| |
| • | Loss reserves—insurance captive |
Cash and cash equivalents
For the purpose of reporting our cash flows, we consider highly liquid investments with an original maturity of 90 days or less when purchased to be cash equivalents. Cash equivalents are stated at cost, which approximates market value.
Trade receivables
Trade receivables are comprised primarily of amounts owed to the Company from patients and from professional corporations— $624,000 at December 31, 2002 and $594,000 at December 31, 2001. Trade receivables are presented net of allowance for doubtful accounts of $231,000 and $77,000 at December 31, 2002 and 2001 respectively.
Property and equipment, goodwill, and depreciation and amortization
Property and Equipment
We show our property and equipment at its original cost, net of accumulated depreciation. At the time property or equipment is retired, sold, or otherwise disposed of, the related cost and accumulated depreciation or amortization are deducted from the amounts reported in the Consolidated Balance Sheet and any gains or losses on disposition are recognized in the Consolidated Statements of Operations. We expense repair and maintenance costs as incurred.
Goodwill and Other Intangible Assets
Goodwill is the excess of the acquisition cost of the business over the fair value of the identifiable net assets acquired. Through December 31, 2001, we amortized goodwill using the straight-line method over the estimated useful life. The Company adopted Financial Accounting Standards (SFAS) No. 142, “Goodwill and other Intangible Assets” effective January 1, 2002. SFAS No. 142 discontinued the amortization of goodwill and requires companies to perform an annual impairment test of goodwill. Application of the non-amortization provision of the SFAS No. 142 resulted in a decrease in annual operating expenses of $76,000 in 2002. The impairment tests of goodwill as of December 31, 2002 indicated that the Company currently has no goodwill impairment.
Depreciation and Amortization
We compute depreciation using the straight-line method which recognizes the cost of the asset over its estimated useful life. We use the following estimated useful lives for computing the annual depreciation expense: building, 5 to 31 years; furniture and fixtures, 3 to 7 years; medical equipment, 3 to 5 years; other equipment, 3 to 5 years. Amortization of leasehold improvements is recorded in the Consolidated Statements of Operations using the straight-line method based on the lesser of the useful life of the improvement or the lease term.
F-18
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We assess the impairment of property and equipment whenever events or circumstances indicate that the carrying value might not be recoverable. Estimates of future cash flows are used to determine if there is impairment; discounted cash flows are used to determine the amount of impairment.
Depreciation Expense
The amount of depreciation expense recorded in the consolidated statements of operations is as follows (dollars in thousands):
| | | |
| Year | | Depreciation |
|
|
| 2000 | | $3,541 |
| 2001 | | 5,497 |
| 2002 | | 5,997 |
Financial instruments
Concentration of Credit Risk
Financial instruments that subject us to concentrations of credit risk consist primarily of temporary cash investments. Our policy is to place our temporary cash investments in overnight repurchase agreements with The Provident Bank, the financial institution that provides our lines of credit.
Fair values of financial instruments
The fair values of our cash and cash equivalents, trade receivables and accounts payable approximate their fair values due to their short term maturities.
Income taxes
The provisions for income taxes are accounted for in accordance with SFAS No. 109,Accounting for Income Taxes. Under the asset and liability method of SFAS 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
As a result of the Company’s operating loss during the third quarter of 2001 and throughout 2002, and continuing uncertainties regarding the general economic conditions in the United States and the impact on ongoing operations, the Company continues to record a full valuation reserve for deferred tax assets, which reserve was established according to the requirements of SFAS No. 109. Favorable changes in the Company’s operating profitability as a result of improved general economic conditions in the United States or otherwise, could impact the Company’s determination as to whether reduction, in whole or in part, to the valuation reserve is necessary in the future.
Revenue recognition
We recognize revenues as services are performed. In states where the physicians are employed by professional corporations, the Company provides management, marketing and administrative services in return for management fees. Management fee revenue is equal to the net revenue of the professional corporation less amounts retained by the physician group. Management fee revenue accounted for 30% of total refractive surgery revenue in 2002, up from 25% in 2001.
Approximately 70% of the Company’s laser refractive surgery revenues in 2002 were generated in Company-owned laser centers. Net revenues in a Company-owned center represent the amount charged to patients less any applicable discounts. Company-owned centers accounted for 75% of laser refractive surgery revenues in 2001.
F-19
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Stock-Based compensation
In December 2002, SFAS No. 148, “Accounting for Stock-Based Compensation — Transition and Disclosure,” which amends SFAS No. 123, “Accounting for Stock-Based Compensation,” was issued. SFAS No. 148 provides alternative methods of transition for a voluntary change to the fair value based method of accounting for stock-based employee compensation and requires more prominent and more frequent disclosures in the financial statements of the effects of stock-based compensation. The provisions of SFAS No. 148 are effective for fiscal years ending after December 15, 2002.
We apply APB No. 25 and related interpretations utilizing the intrinsic value method in accounting for our stock option plans. We have adopted the disclosure-only provisions of SFAS No. 123. We recognize no compensation expense for our stock options granted to employees or directors. Compensation expense for options granted to non-employees in each of the three years ended December 31, 2000 was immaterial. If we had elected to recognize compensation expense based on the fair value at the grant dates consistent with the provisions of SFAS No. 123, net loss and loss per share would have been changed to the pro forma amounts indicated below (dollars in thousands, except per share amounts):
| | | | | | | | | | | | | | |
| | |
| | Years ended December 31, |
| |
|
| | 2002 | | 2001 | | 2000 |
|
|
| Net loss | | | | | | | | | | | | |
| | As reported | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| | Pro forma | | | (5,676 | ) | | | (25,690 | ) | | | (7,200 | ) |
| Diluted per share loss | | | | | | | | | | | | |
| | As reported | | $ | (0.35 | ) | | $ | (2.01 | ) | | $ | (0.19 | ) |
| | Pro forma | | | (0.53 | ) | | | (2.21 | ) | | | (0.57 | ) |
These results may not be representative of the effects on pro forma amounts for future years.
We determined the pro forma amounts using the Black-Scholes option-pricing model based on the following assumptions:
| | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
| Dividend yield | | | 0% | | | | 0% | | | | 0% | |
| Expected volatility | | | 84 – 124% | | | | 101% | | | | 97% | |
| Risk free interest rate | | | 2.03 – 4.58% | | | | 4.51 – 4.77% | | | | 5.26 – 6.69% | |
| Expected lives (in years) | | | 1 to 5 | | | | 3 to 5 | | | | 3 to 5 | |
Additional information on options is included in footnote 8, and additional information on warrants is included in footnote 2.
Marketing and advertising expenditures
Marketing and advertising expenditures are expensed as incurred, except for the costs associated with direct mail. Direct mail costs are expensed upon mailing.
New accounting pronouncements
In January 2002, the SEC issued FR-60 recommending that companies expand their disclosures related to Critical Accounting Policies. In response to FR-60, disclosure of accounting policies in the notes to the financial statements in MD&A has been enhanced. Note 1 to the financial statements and the Critical Accounting Policies section of MD&A will contain discussions regarding all significant accounting policies used.
Per share data
Basic per share data is earnings or loss applicable to common shareholders divided by weighted average common shares outstanding. Diluted per share data is earnings or loss applicable to common shareholders divided by weighted average common shares outstanding plus potential common shares
F-20
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
from dilutive securities such as options and convertible securities. The table shows how we calculated diluted earnings per share and diluted shares outstanding for the year ended December 31, 2002, 2001 and 2000 (amounts in thousands, except per share amounts).
| | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
Basic EPS | | | | | | | | | | | | |
| Net loss applicable to common stock | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| Weighted average shares outstanding | | | 10,794 | | | | 11,643 | | | | 12,741 | |
| Basic loss per share | | $ | (0.35 | ) | | $ | (2.01 | ) | | $ | (0.19 | ) |
| |
Diluted EPS | | | | | | | | | | | | |
| Net loss applicable to common stock | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| Weighted average common shares and potential dilutive shares | | | 10,794 | | | | 11,643 | | | | 12,741 | |
| Diluted loss per share | | $ | (0.35 | ) | | $ | (2.01 | ) | | $ | (0.19 | ) |
The weighted average shares for the years ended December 31, 2002, 2001 and 2000 diluted calculations do not assume exercise of any stock options or conversion of other securities since they would result in a reduced loss per share. The number of unexercised options and warrants as of December 31, 2000, December 31, 2001 and December 31, 2002 was:
| | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
| Options | | | 915,201 | | | | 914,819 | | | | 1,048,644 | |
| Warrants | | | 100,000 | | | | 127,734 | | | | 127,734 | |
| | | |
| | | |
| | | |
| |
| | | | 1,015,201 | | | | 1,042,553 | | | | 1,176,378 | |
| | | |
| | | |
| | | |
| |
2. SHAREHOLDERS’ INVESTMENT
Common Stock
We are authorized to issue up to 27.5 million shares of common stock. The number of shares reserved for future issuance is 2,214,907 which includes 2,114,907 shares under stock option plans and 100,000 shares for stock warrants.
Preferred stock
At December 31, 2002, there are no shares of preferred stock issued and outstanding.
Treasury stock
At December 31, 2002, there were 2,367,197 shares of common stock held in treasury. The Board of Directors previously authorized management to buy back 1,250,000 shares of common stock. As of December 31, 2002, 135,530 shares remain to be purchased.
On January 31, 2002, 1,632,881 shares of our common stock were purchased from Summit Autonomous, a subsidiary of Alcon Holdings Inc. for approximately $4,898,000. The transaction was split equally between the Company share repurchase program and members of our senior management and Board of Directors.
Notes receivable from shareholders
During the second quarter of 2000, the directors initiated a program to encourage additional direct stock ownership by senior management of the Company. The Company offered loans to nine key managers and directors for the purpose of purchasing shares in the open market. Each loan is a personal obligation of the borrower, and is evidenced by a promissory note. The interest rate on the notes is prime less one and one-half percent. Interest is accrued and added to the outstanding principal balance. The notes have a maximum term of three years, and contain provisions for early repayment. As of December 31, 2002, a total of $1,532,000 has been loaned under this program.
F-21
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Warrants
During 1999, we issued warrants to purchase a total of 231,250 shares of common stock at prices ranging from $8 to $48 per share. The warrants were issued to an investment banking firm and a joint venture partner.
Warrants for 3,516 shares have been exercised. Warrants to purchase 27,734 shares expired in 2002. Warrants to purchase 200,000 shares at $48 per share were issued during the third quarter of 1999 of which 100,000 are exercisable through June 30, 2003; the remaining warrants were repurchased in the third quarter of 2000 for $310,000.
The vested warrants result in prepaid expense for book purposes, using the Black-Scholes method, of $2,105,000. This amount was amortized over a three year period which coincides with the term of the agreement with Cole National Corporation to market laser vision correction as a managed care benefit.
Amortization of $510,000 is recorded in our 2002 Consolidated Statement of Operations, $701,000 is recorded in our 2001 Consolidated Statement of Operations, and $853,000 of amortization is recorded in our 2000 Consolidated Statement of Operations.
3. SPECIAL CHARGES
During the third quarter of 2001, management implemented a restructuring plan to close unprofitable locations and to reduce operating expenses. The cost of the plan was $1,375,000 which is comprised of a $535,000 restructuring charge and an $840,000 asset impairment charge for leasehold improvements, equipment and goodwill associated with the locations to be closed. The restructuring charges included $384,000 in lease termination costs and $151,000 in severance payments for 71 employees.
As of December 31, 2001, the balance of the restructuring accrual was $262,000 which related to future facility rent payments and is included in accrued liabilities and other in the Consolidated Balance Sheet. During the first quarter 2002, $88,000 was expended to buy out the remainder of a facility lease. With the restructuring plan fully implemented, the remaining reserve of $174,000 was reversed in the first quarter 2002.
Also during the third quarter of 2001, the Company provided a reserve of $399,000 on a loan made to REI Corporation which operated a licensed facility in Tokyo, Japan. During the quarter, management determined that the loan may not be recoverable.
4. CREDIT ARRANGEMENTS
On June 29, 1998 we entered into an $8,000,000 credit facility with The Provident Bank (“Provident”). At the same time we repaid our borrowing from, and terminated our relationship with, another lender. In May 1999, the credit facility was increased to $10,000,000. We also were granted a $10,000,000 line of credit for the purpose of funding acquisitions subject to Provident’s sole and absolute discretion.
The Provident facility, as amended, matures on June 30, 2003. The facility can be used to support up to $2,000,000 of letters of credit issued by Provident. Borrowings under the working capital portion of the facility bear interest at Provident’s prime rate. Substantially all of our assets at December 31, 2002 are pledged as collateral.
The credit facility, for which there is no formal compensating balance, requires us to pay a commitment fee of ..25% based on the unused portion. At December 31, 2002 we had $10,000,000 available to us under the credit facility.
The credit facility requires us to maintain tangible net worth of at least $30,000,000.
F-22
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
5. INVESTMENT IN UNCONSOLIDATED BUSINESSES
Our investment in unconsolidated businesses was $263,000 and $290,000 at December 31, 2002 and December 31, 2001, respectively. At December 31, 2002, we had investments in Silmalaseri Oy in Helsinki, Cole/ LCA-Vision LLC and Eyemed/ LCA-Vision LLC.
Combined summary financial information for these investments follows (dollars in thousands):
| | | | | | | | | | |
| | |
| | December 31, |
| |
|
| | 2002 | | 2001 |
|
|
Financial Position: | | | | | | | | |
| | Current assets | | $ | 377 | | | $ | 322 | |
| | Total assets | | | 545 | | | | 467 | |
| | Total liabilities | | | 267 | | | | 174 | |
| | Members’ equity | | | 278 | | | | 293 | |
| |
Operating Results: | | | | | | | | |
| | Revenue | | $ | 1,559 | | | $ | 2,019 | |
| | Net income | | | 497 | | | | 695 | |
6. INCOME TAXES
The components of income tax expense (benefit) for the three years ended December 31, 2002 are presented in the following table (dollars in thousands):
| | | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
Current: | | | | | | | | | | | | |
| | Federal | | $ | — | | | $ | — | | | $ | (1,441 | ) |
| | State and local | | | — | | | | — | | | | (568 | ) |
| | Foreign | | | 174 | | | | — | | | | 18 | |
| | | |
| | | |
| | | |
| |
| Total | | | 174 | | | | — | | | | (1,991 | ) |
| | | |
| | | |
| | | |
| |
Deferred: | | | | | | | | | | | | |
| | Federal | | | (880 | ) | | | (1,393 | ) | | | 443 | |
| | State and local | | | 8 | | | | (7 | ) | | | 114 | |
| | | |
| | | |
| | | |
| |
| Total | | | (872 | ) | | | (1,400 | ) | | | 557 | |
| | | |
| | | |
| | | |
| |
| Income tax (benefit) expense | | | (698 | ) | | | (1,400 | ) | | | (1,434 | ) |
| Valuation allowance increase | | | 872 | | | | 17,989 | | | | — | |
| | | |
| | | |
| | | |
| |
| Net income tax (benefit) expense | | $ | 174 | | | $ | 16,589 | | | $ | (1,434 | ) |
| | | |
| | | |
| | | |
| |
As a result of the Company’s operating loss during the third quarter of 2001, and continuing uncertainties regarding the general economic conditions in the United States and the impact on ongoing operations, the Company recorded a $15,345,000 valuation reserve for deferred tax assets as of September 30, 2001. The Company’s operating losses continued in 2002 and the uncertainties continued to exist, such that it was not more-likely-than-not that the deferred tax assets would be realized at this time. This valuation reserve was established according to the requirements of SFAS No. 109, “Accounting for Income Taxes.”
We are required to pay franchise taxes in most of the states in which we have operations due to our net operating loss carryforwards. We have included the franchise taxes paid in general and administrative expenses in our Consolidated Statements of Operations.
F-23
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The foreign tax provisions consist primarily of Canadian income taxes. We have made no provision for US income taxes on undistributed earnings from our international businesses because it is our intention to reinvest those earnings in those operations. If those earnings are distributed in the form of dividends, we may be subject to both foreign withholding taxes and US income taxes net of allowable foreign tax credits.
Pretax income (loss) for the last three years has been:
| | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
| Domestic | | $ | (3,925 | ) | | $ | (6,224 | ) | | $ | (3,588 | ) |
| Foreign | | | 273 | | | | (563 | ) | | | (212 | ) |
| | | |
| | | |
| | | |
| |
| Total | | $ | (3,652 | ) | | $ | (6,787 | ) | | $ | (3,800 | ) |
| | | |
| | | |
| | | |
| |
Deferred taxes arise because of differences in the book and tax bases of certain assets and liabilities. Significant components of our deferred tax assets are shown in the following table (dollars in thousands):
| | | | | | | | | | | |
| | |
| | December 31 |
| |
|
| | 2002 | | 2001 |
|
|
Deferred tax assets: | | | | | | | | |
| | Net operating loss carryforward | | $ | 18,772 | | | $ | 17,454 | |
| | Accounts receivable | | | 90 | | | | 283 | |
| | Equity investments | | | 293 | | | | 64 | |
| | Other | | | 251 | | | | 195 | |
| | | Total deferred tax assets | | | 19,406 | | | | 17,996 | |
| | | |
| | | |
| |
| | Valuation allowance | | | (18,861 | ) | | | (17,989 | ) |
| | | |
| | | |
| |
| | | Deferred tax assets, net of valuation allowance | | | 545 | | | | 7 | |
| Deferred tax liabilities — property and equipment | | | (545 | ) | | | (7 | ) |
| | | |
| | | |
| |
| Net deferred tax assets | | $ | — | | | $ | — | |
| | | |
| | | |
| |
The following table shows the principal reasons for the difference between the statutory federal income tax rate of 34% and the tax benefit shown in our Consolidated Statements of Operations (dollars in thousands):
| | | | | | | | | | | | | |
| | 2002 | | 2001 | | 2000 |
|
|
| Tax at statutory federal rate | | $ | (1,242 | ) | | $ | (2,307 | ) | | $ | (1,134 | ) |
| State income taxes, net of federal benefit | | | 5 | | | | (4 | ) | | | (294 | ) |
| Amortization of intangibles and other non-deductible items | | | 201 | | | | 401 | | | | — | |
| Foreign income tax | | | 174 | | | | — | | | | 18 | |
| Change in valuation allowance | | | 872 | | | | 17,989 | | | | — | |
| Other | | | 164 | | | | 510 | | | | (24 | ) |
| | | |
| | | |
| | | |
| |
| Income tax provision (benefit) | | $ | 174 | | | $ | 16,589 | | | $ | (1,434 | ) |
| | | |
| | | |
| | | |
| |
At December 31, 2002 and 2001 we have federal net operating loss carryforwards for income tax purposes of $48,765,000 and $45,033,000, respectively. These expire in varying amounts from 2007 until 2022. Approximately $18,000,000 of federal net operating loss carryforwards and $15,750,000 state net operating loss carryforwards were acquired when we bought Refractive Centers International, Inc. Our ability to use these acquired net operating loss carryforwards is limited to approximately $2,500,000 per year under Code Section 382 of the Internal Revenue Code.
F-24
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
7. LEASING ARRANGEMENTS
We lease office space for our centers and equipment for use in our operations under both capital and operating leases. Capital leases were primarily used for financing the lasers in our first five centers. Currently, we use a combination of operating leases and outright purchases for laser acquisition.
This table displays our aggregate minimal rental commitments under noncancellable leases for the periods shown (dollars in thousands):
| | | | | | | | | |
| | |
| | December 31, 2002 |
| |
|
| | Capital Leases | | Operating Leases |
|
|
| Year | | | | | | | | |
| 2003 | | $ | 10 | | | $ | 3,042 | |
| 2004 | | | — | | | | 3,070 | |
| 2005 | | | — | | | | 2,234 | |
| 2006 | | | — | | | | 1,157 | |
| 2007 | | | — | | | | 382 | |
| Beyond 2007 | | | — | | | | 408 | |
| | | |
| | | |
| |
| Total minimum rental commitment | | $ | 10 | | | $ | 10,293 | |
| | | |
| | | |
| |
Total rent expense under operating leases amounted to $4,352,000 in 2002; $6,770,000 in 2001; and $6,662,000 in 2000.
8. EMPLOYEE BENEFITS
Savings Plan
We sponsor a savings plan under Internal Revenue Code Section 401(k) to provide an opportunity for eligible employees to save for retirement on a tax-deferred basis. Under this plan, we make discretionary contributions to the participants’ accounts. We made contributions of $0 in 2002; $18,066 in 2001; and $20,000 in 2000.
Stock Option Plans
We have three stock incentive plans, the 1995 Long Term Stock Incentive Plan (“1995 Plan”), the 1998 Long Term Stock Incentive Plan (“1998 Plan”) and the 2001 Long Term Stock Incentive Plan (“2001 Plan). A maximum of 625,000 shares are reserved for the 1995 Plan, 1,250,000 shares are reserved for the 1998 Plan and 625,000 shares are reserved for the 2001 plan. The Compensation Committee of the Board of Directors administers all Stock Option Plans.
The Plans permit us to issue incentive and/or nonqualified stock options to purchase shares of common stock, stock appreciation rights, and stock awards to employees and consultants. The 1998 Plan is used to grant stock options to our non-employee directors. Non-employee directors receive an option to purchase 18,750 shares of our common stock upon initial election or appointment and an automatic option grant of 3,125 shares upon reelection. Prior to our shareholders approving the 1998 Plan, we granted our non-employee directors stock options under the LCA-Vision Inc. Directors’ Nondiscretionary Stock Option Plan which was discontinued in 1998.
Stock options are granted with an exercise price not less than fair market value on the date of grant. Stock options granted have generally been exercisable over 3 to 5 years and the maximum term is 10 years from the date of grant.
F-25
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table summarizes the status of our 1995, 1998 and 2001 Plans:
| | | | | | | | | |
| | Stock | | Exercise |
| | Options | | Price |
|
|
| Outstanding at 12/31/99 | | | 849,114 | | | $ | 9.76 | |
| Granted | | | 587,563 | | | | 13.12 | |
| Exercised | | | (149,104 | ) | | | 6.32 | |
| Cancelled/forfeited | | | (315,179 | ) | | | 4.12 | |
| | | |
| | | | | |
| Outstanding at 12/31/00 | | | 972,394 | | | | 13.92 | |
| Granted | | | 138,875 | | | | 10.76 | |
| Exercised | | | (33,788 | ) | | | 4.72 | |
| Cancelled/forfeited | | | (238,909 | ) | | | 12.84 | |
| | | |
| | | | | |
| Outstanding at 12/31/01 | | | 838,572 | | | | 14.08 | |
| Granted | | | 164,375 | | | | 5.25 | |
| Exercised | | | (46,250 | ) | | | 5.09 | |
| Cancelled/forfeited | | | (117,746 | ) | | | 14.47 | |
| | | |
| | | | | |
| Outstanding at 12/31/02 | | | 838,951 | | | | 12.80 | |
| | | |
| | | | | |
| Options exercisable, | | | | | | | | |
| December 31, | | | | | | | | |
| 2000 | | | 238,356 | | | | 12.36 | |
| 2001 | | | 405,941 | | | | 13.76 | |
| 2002 | | | 502,406 | | | | 14.37 | |
The following table summarizes information about the stock options granted under the 1995, 1998 and 2001 Plans that are outstanding at December 31, 2002:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | |
| | Stock Options Outstanding | | Options Exercisable |
| |
| |
|
| | | | Weighted-average | | | | Stock | | |
| Range of | | # Outstanding | | remaining | | Weighted-average | | # Exercisable | | Weighted-average |
| exercise prices | | as of 12/31/02 | | contractual life | | exercise price | | as of 12/31/02 | | exercise price |
|
|
| $ | 2.40-$5.00 | | | | 87,083 | | | | 5.69 | | | $ | 4.48 | | | | 65,833 | | | $ | 4.42 | |
| $ | 5.32-$5.32 | | | | 109,501 | | | | 9.16 | | | | 5.32 | | | | 27,767 | | | | 5.32 | |
| $ | 5.38-$6.00 | | | | 90,097 | | | | 7.13 | | | | 5.55 | | | | 45,080 | | | | 5.57 | |
| $ | 7.00-$11.00 | | | | 117,500 | | | | 8.11 | | | | 10.54 | | | | 59,834 | | | | 10.52 | |
| $ | 11.25-$12.00 | | | | 98,750 | | | | 7.59 | | | | 11.78 | | | | 68,746 | | | | 11.80 | |
| $ | 12.28-$16.00 | | | | 90,063 | | | | 7.25 | | | | 15.38 | | | | 40,233 | | | | 15.62 | |
| $ | 16.12-$18.75 | | | | 92,500 | | | | 6.94 | | | | 17.59 | | | | 74,996 | | | | 17.46 | |
| $ | 19.00-$27.75 | | | | 90,957 | | | | 7.05 | | | | 20.84 | | | | 57,417 | | | | 20.86 | |
| $ | 28.00-$28.00 | | | | 50,000 | | | | 6.68 | | | | 28.00 | | | | 50,000 | | | | 28.00 | |
| $ | 44.50-$44.50 | | | | 12,500 | | | | 6.52 | | | | 44.50 | | | | 12,500 | | | | 44.50 | |
| | | | | |
| | | | | | | | | | | |
| | | | | |
| $ | 2.40-$44.50 | | | | 838,951 | | | | 7.38 | | | | 12.80 | | | | 502,406 | | | | 14.37 | |
| | | | | |
| | | | | | | | | | | |
| | | | | |
At December 31, 2002 a total of 1,275,956 shares are available for granting stock options under the 1995, 1998 and 2001 Plans.
F-26
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table is a summary of the status of our discontinued Directors Nondiscretionary Stock Option Plan:
| | | | | | | | | |
| | | | Weighted-average |
| | Stock Options | | Exercise Price |
|
|
| Outstanding, | | | | | | | | |
| December 31, 2000, 2001, 2002 | | | 76,250 | | | | $17.68 | |
As of December 31, 2002, a total of 76,062 options with a weighted-average exercise price of $17.68 are exercisable under this discontinued plan.
The weighted-average fair value of options granted was $5.24 per option during 2002; $7.12 per option during 2001; and $10.48 per option during 2000.
9. COMMITMENTS AND CONTINGENCIES
In the opinion of management there are currently no commitments or contingencies that will have a material adverse effect on our financial position or results of operations.
10. LITIGATION SETTLEMENT
In August 2002, a settlement of $2,282,000 was received from Pillar Point Partner’s class-action litigation. Pillar Point Partners – a joint entity formed in 1995 by laser manufacturers VISX and Summit Technology Inc., now a subsidiary of Alcon Corporation – collected per-use royalties from all laser vision correction providers using their equipment. The manufacturers agreed to settle the various lawsuits for $37.8 million. Pillar Point was dissolved in July 1998 after the Federal Trade Commission filed an administrative complaint challenging the partnerships’ existence.
11. Additional Financial Information
The tables below provide additional financial information related to our consolidated financial statements (dollars in thousands):
Balance Sheet Information
| | | | | | | | | | |
| | |
| | At December 31, |
| |
|
| | 2002 | | 2001 |
|
|
| Property and Equipment | | | | | | | | |
| | Land | | $ | 375 | | | $ | 375 | |
| | Building and improvements | | | 5,660 | | | | 5,660 | |
| | Leasehold improvements | | | 5,385 | | | | 5,079 | |
| | Furniture and fixtures | | | 2,841 | | | | 3,087 | |
| | Equipment | | | 22,517 | | | | 21,711 | |
| | Equipment under capital leases | | | 494 | | | | 494 | |
| | | |
| | | |
| |
| | | | | 37,272 | | | | 36,406 | |
| | Accumulated depreciation | | | (18,868 | ) | | | (13,753 | ) |
| | Construction in progress | | | 29 | | | | 5 | |
| | | |
| | | |
| |
| | | | $ | 18,433 | | | $ | 22,658 | |
| | | |
| | | |
| |
F-27
LCA-Vision Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Cash Flow Information
| | | | | | | | | | | | | | |
| | |
| | For the Year Ended December 31, |
| |
|
| | 2002 | | 2001 | | 2000 |
|
|
| Cash paid during the year for | | | | | | | | | | | | |
| | Interest | | $ | 4 | | | $ | 17 | | | $ | 46 | |
| | Income taxes | | | 23 | | | | — | | | | 2 | |
| Other Comprehensive Loss Information | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | |
| Loss applicable to common stock | | $ | (3,826 | ) | | $ | (23,375 | ) | | $ | (2,366 | ) |
| Other comprehensive loss, net of income tax -currency translation adjustments | | | (3 | ) | | | (17 | ) | | | (30 | ) |
| | | |
| | | |
| | | |
| |
| Total comprehensive loss | | $ | (3,829 | ) | | $ | (23,392 | ) | | ($ | 2,396 | ) |
| | | |
| | | |
| | | |
| |
Segment Information
We operate in one segment – laser refractive surgery.
12. QUARTERLY FINANCIAL DATA (UNAUDITED)(1)
Financial results for interim periods do not necessarily indicate trends for any 12-month period. Quarterly results can be affected by the number of procedures performed and the timing of certain expense items (dollars in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | |
| | 2002 Quarters | | 2001 Quarters |
| |
| |
|
| | First | | Second | | Third | | Fourth | | First | | Second | | Third | | Fourth |
|
|
| Revenues | | $ | 18,808 | | | $ | 16,268 | | | $ | 13,462 | | | $ | 13,300 | | | $ | 22,490 | | | $ | 21,424 | | | $ | 13,288 | | | $ | 10,894 | |
| Operating (loss) income | | | 966 | | | | (2,447 | ) | | | (2,814 | ) | | | (1,905 | ) | | | 1,720 | | | | 747 | | | | (5,718 | ) | | | (4,753 | ) |
| Net Income (loss) | | | 1,151 | | | | (2,278 | ) | | | (810 | ) | | | (1,888 | ) | | | 1,299 | | | | 755 | | | | (20,794 | ) | | | (4,635 | ) |
| Earnings (loss) per share | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.11 | | | $ | (0.21 | ) | | $ | (0.08 | ) | | $ | (0.18 | ) | | $ | 0.11 | | | $ | 0.06 | | | $ | (1.79 | ) | | $ | (0.40 | ) |
| Diluted | | $ | 0.10 | | | $ | (0.21 | ) | | $ | (0.08 | ) | | $ | (0.18 | ) | | $ | 0.11 | | | $ | 0.06 | | | $ | (1.79 | ) | | $ | (0.40 | ) |
| |
| (1) | The quarterly amounts are not additive due to rounding. |
F-28


