
1 Third Quarter 2016 Financial Results and Business Update November 9, 2016

2 Impax Cautionary Statement Regarding Forward Looking Statements "Safe Harbor" statement under the Private Securities Litigation Reform Act of 1995: To the extent any statements made in this presentation contain information that is not historical; these statements are forward-looking in nature and express the beliefs and expectations of management. Such statements are based on current expectations and involve a number of known and unknown risks and uncertainties that could cause the Company’s future results, performance, or achievements to differ significantly from the results, performance, or achievements expressed or implied by such forward-looking statements. Such risks and uncertainties include, but are not limited to: fluctuations in revenues and operating income; the Company’s ability to successfully develop and commercialize pharmaceutical products in a timely manner; reductions or loss of business with any significant customer; the substantial portion of the Company’s total revenues derived from sales of a limited number of products; the impact of consolidation of the Company’s customer base; the impact of competition; the Company’s ability to sustain profitability and positive cash flows; any delays or unanticipated expenses in connection with the operation of the Company’s manufacturing facilities; the effect of foreign economic, political, legal, and other risks on the Company’s operations abroad; the uncertainty of patent litigation and other legal proceedings; the increased government scrutiny on the Company’s agreements with brand pharmaceutical companies; product development risks and the difficulty of predicting FDA filings and approvals; consumer acceptance and demand for new pharmaceutical products; the impact of market perceptions of the Company and the safety and quality of the Company’s products; the Company’s determinations to discontinue the manufacture and distribution of certain products; the Company’s ability to achieve returns on its investments in research and development activities; changes to FDA approval requirements ; the Company’s ability to successfully conduct clinical trials; the Company’s reliance on third parties to conduct clinical trials and testing; the Company’s lack of a license partner for commercialization of NUMIENT™ (IPX066) outside of the United States; impact of illegal distribution and sale by third parties of counterfeits or stolen products; the availability of raw materials and impact of interruptions in the Company’s supply chain; the Company’s policies regarding returns, allowances and chargebacks; the use of controlled substances in the Company’s products; the effect of current economic conditions on the Company’s industry, business, results of operations and financial condition; disruptions or failures in the Company’s information technology systems and network infrastructure caused by third party breaches or other events; the Company’s reliance on alliance and collaboration agreements; the Company’s reliance on licenses to proprietary technologies; the Company’s dependence on certain employees; the Company’s ability to comply with legal and regulatory requirements governing the healthcare industry; the regulatory environment; the effect of certain provisions in the Company’s government contracts; the Company’s ability to protect its intellectual property; exposure to product liability claims; risks relating to goodwill and intangibles; changes in tax regulations; the Company’s ability to manage growth, including through potential acquisitions and investments; risks related to the Company’s acquisitions of or investments in technologies, products or businesses; the restrictions imposed by the Company’s credit facility and indenture; the Company’s level of indebtedness and liabilities and the potential impact on cash flow available for operations; uncertainties involved in the preparation of the Company’s financial statements; the Company’s ability to maintain an effective system of internal control over financial reporting; the effect of terrorist attacks on the Company’s business; the location of the Company’s manufacturing and research and development facilities near earthquake fault lines; expansion of social media platforms and other risks described in the Company’s periodic reports filed with the Securities and Exchange Commission. Forward-looking statements speak only as to the date on which they are made, and the Company undertakes no obligation to update publicly or revise any forward-looking statement, regardless of whether new information becomes available, future developments occur or otherwise. Trademarks referenced herein are the property of their respective owners. ©2016 Impax Laboratories, Inc. All Rights Reserved.

3 Agenda • 3Q16 Financial Results • Business Update Fred Wilkinson President & CEO • 3Q16 Financial Review • 2016 Financial Guidance Update Bryan Reasons SVP & CFO • Q&A Executive Team

4 3Q16 Results and Business Update Fred Wilkinson President & Chief Executive Officer

5 • Quarter results essentially in line with our expectations › Marketing and operational strategies drove revenue growth of 112% in Epinephrine Auto-Injector and oxymorphone compared to 3Q15 › Recapturing generic Adderall XR® share but at significantly lower price › Launched 3 generic products • Short-term Albenza® supply interruption impacted Specialty Division results • Increased intensity of generic pricing impacting a number of products • Post Teva Transaction close: › Higher price concessions to retain key customers › Reduced price due to competition › Lower expected cash flows triggered a non-cash impairment charge 3Q16 and Recent Market Events
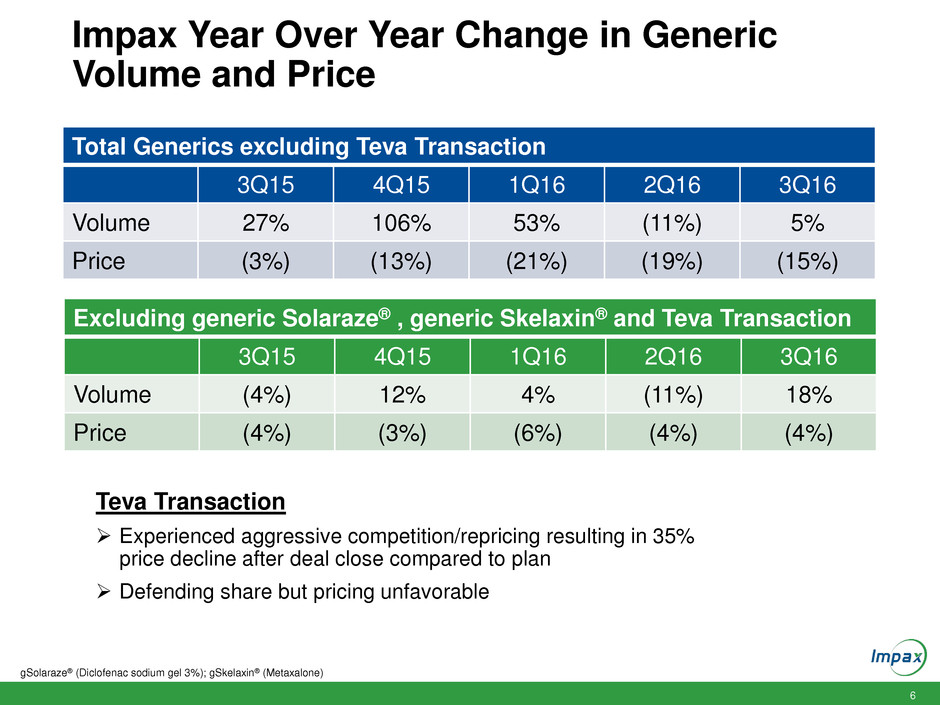
6 Impax Year Over Year Change in Generic Volume and Price Total Generics excluding Teva Transaction 3Q15 4Q15 1Q16 2Q16 3Q16 Volume 27% 106% 53% (11%) 5% Price (3%) (13%) (21%) (19%) (15%) gSolaraze® (Diclofenac sodium gel 3%); gSkelaxin® (Metaxalone) Excluding generic Solaraze® , generic Skelaxin® and Teva Transaction 3Q15 4Q15 1Q16 2Q16 3Q16 Volume (4%) 12% 4% (11%) 18% Price (4%) (3%) (6%) (4%) (4%) Teva Transaction Experienced aggressive competition/repricing resulting in 35% price decline after deal close compared to plan Defending share but pricing unfavorable
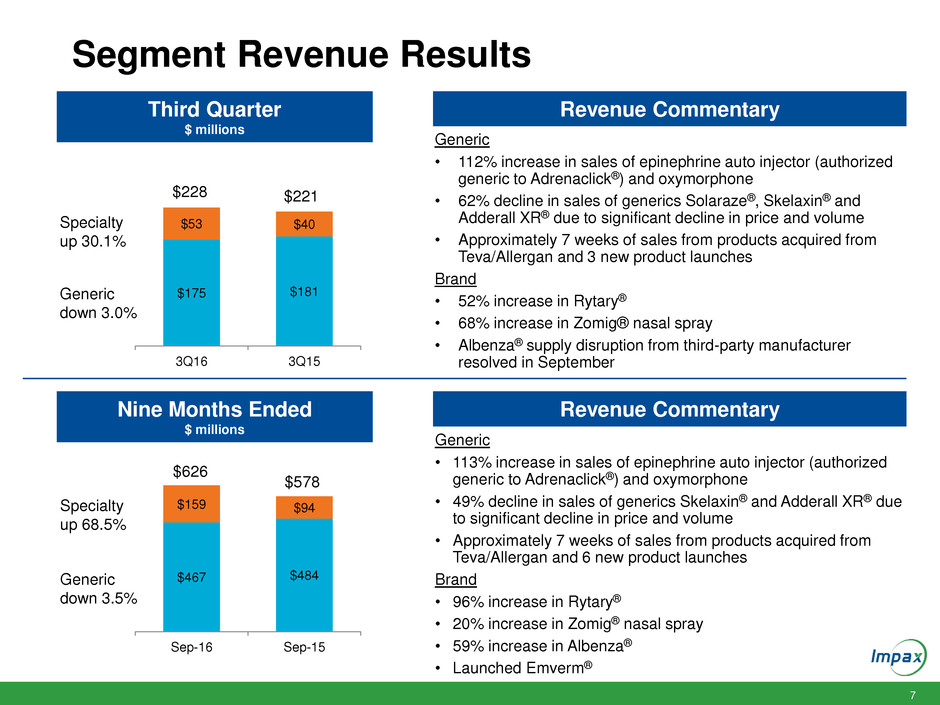
7 Segment Revenue Results $175 $181 $53 $40 3Q16 3Q15 Third Quarter $ millions Generic • 112% increase in sales of epinephrine auto injector (authorized generic to Adrenaclick®) and oxymorphone • 62% decline in sales of generics Solaraze®, Skelaxin® and Adderall XR® due to significant decline in price and volume • Approximately 7 weeks of sales from products acquired from Teva/Allergan and 3 new product launches Brand • 52% increase in Rytary® • 68% increase in Zomig® nasal spray • Albenza® supply disruption from third-party manufacturer resolved in September Generic • 113% increase in sales of epinephrine auto injector (authorized generic to Adrenaclick®) and oxymorphone • 49% decline in sales of generics Skelaxin® and Adderall XR® due to significant decline in price and volume • Approximately 7 weeks of sales from products acquired from Teva/Allergan and 6 new product launches Brand • 96% increase in Rytary® • 20% increase in Zomig® nasal spray • 59% increase in Albenza® • Launched Emverm® Nine Months Ended $ millions Revenue Commentary Revenue Commentary $228 $221 $467 $484 $159 $94 Sep-16 Sep-15 $626 $578 Specialty up 30.1% Generic down 3.0% Specialty up 68.5% Generic down 3.5%
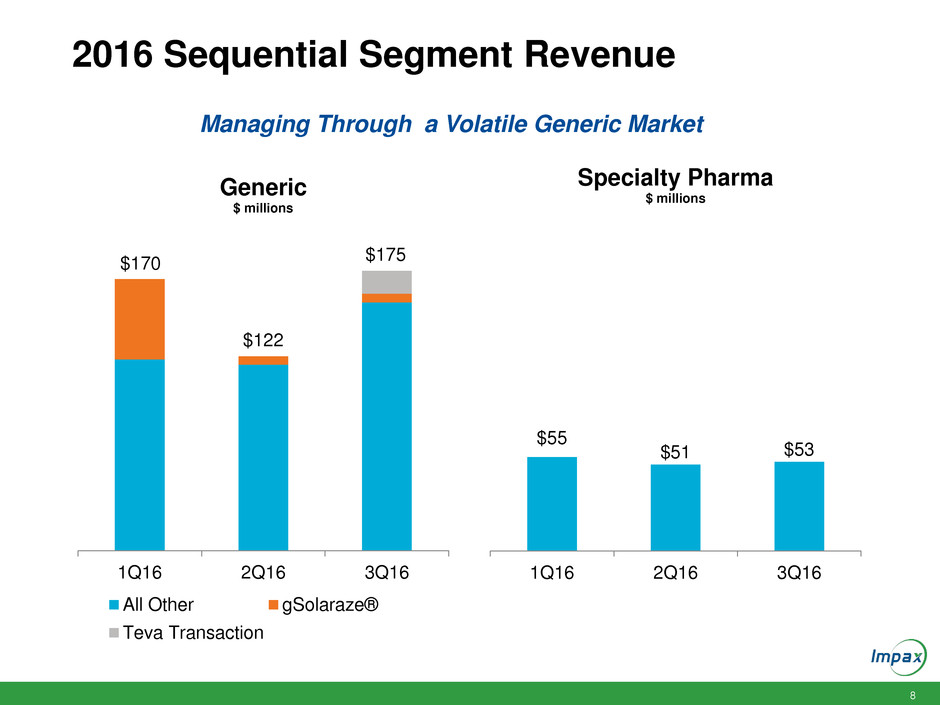
8 2016 Sequential Segment Revenue 1Q16 2Q16 3Q16 Generic $ millions All Other gSolaraze® Teva Transaction $55 $51 $53 1Q16 2Q16 3Q16 Specialty Pharma $ millions $170 $122 $175 Managing Through a Volatile Generic Market
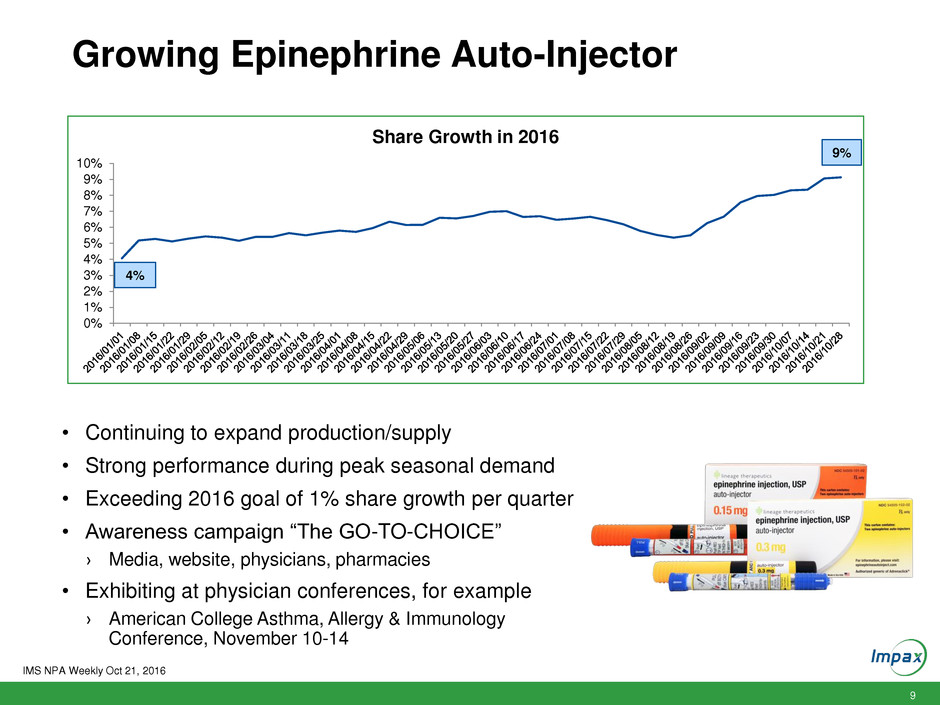
9 Growing Epinephrine Auto-Injector 0% 1% 2% 3% 4% 5% 6% 7% 8% 9% 10% Share Growth in 2016 4% 9% • Continuing to expand production/supply • Strong performance during peak seasonal demand • Exceeding 2016 goal of 1% share growth per quarter • Awareness campaign “The GO-TO-CHOICE” › Media, website, physicians, pharmacies • Exhibiting at physician conferences, for example › American College Asthma, Allergy & Immunology Conference, November 10-14 IMS NPA Weekly Oct 21, 2016
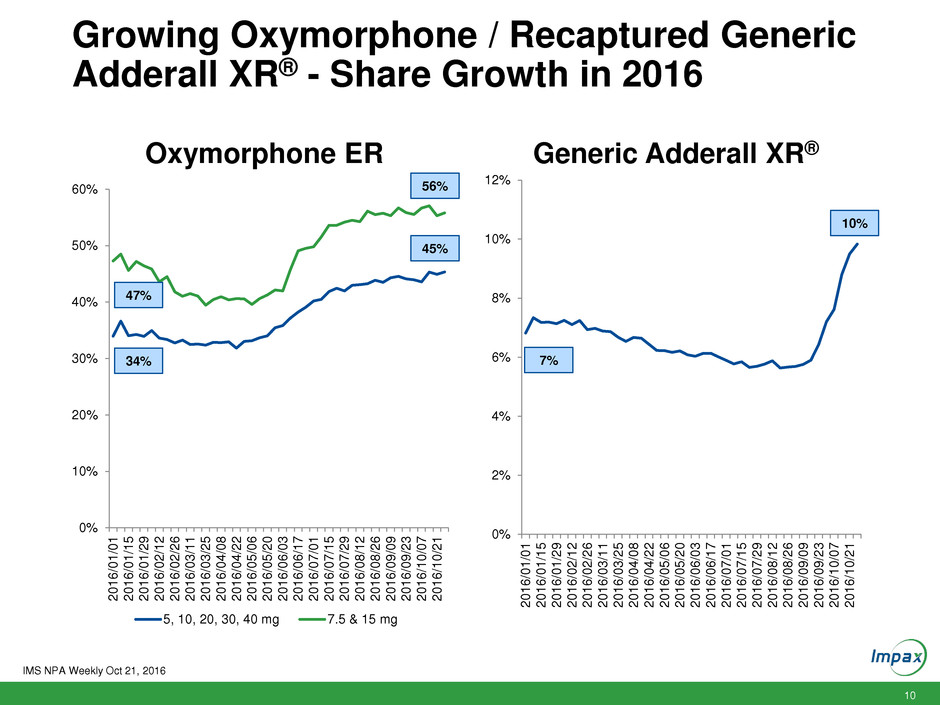
10 Growing Oxymorphone / Recaptured Generic Adderall XR® - Share Growth in 2016 0% 10% 20% 30% 40% 50% 60% 2 0 1 6 /0 1 /0 1 2 0 1 6 /0 1 /1 5 2 0 1 6 /0 1 /2 9 2 0 1 6 /0 2 /1 2 2 0 1 6 /0 2 /2 6 2 0 1 6 /0 3 /1 1 2 0 1 6 /0 3 /2 5 2 0 1 6 /0 4 /0 8 2 0 1 6 /0 4 /2 2 2 0 1 6 /0 5 /0 6 2 0 1 6 /0 5 /2 0 2 0 1 6 /0 6 /0 3 2 0 1 6 /0 6 /1 7 2 0 1 6 /0 7 /0 1 2 0 1 6 /0 7 /1 5 2 0 1 6 /0 7 /2 9 2 0 1 6 /0 8 /1 2 2 0 1 6 /0 8 /2 6 2 0 1 6 /0 9 /0 9 2 0 1 6 /0 9 /2 3 2 0 1 6 /1 0 /0 7 2 0 1 6 /1 0 /2 1 Oxymorphone ER 5, 10, 20, 30, 40 mg 7.5 & 15 mg 0% 2% 4% 6% 8% 10% 12% 2 0 1 6 /0 1 /0 1 2 0 1 6 /0 1 /1 5 2 0 1 6 /0 1 /2 9 2 0 1 6 /0 2 /1 2 2 0 1 6 /0 2 /2 6 2 0 1 6 /0 3 /1 1 2 0 1 6 /0 3 /2 5 2 0 1 6 /0 4 /0 8 2 0 1 6 /0 4 /2 2 2 0 1 6 /0 5 /0 6 2 0 1 6 /0 5 /2 0 2 0 1 6 /0 6 /0 3 2 0 1 6 /0 6 /1 7 2 0 1 6 /0 7 /0 1 2 0 1 6 /0 7 /1 5 2 0 1 6 /0 7 /2 9 2 0 1 6 /0 8 /1 2 2 0 1 6 /0 8 /2 6 2 0 1 6 /0 9 /0 9 2 0 1 6 /0 9 /2 3 2 0 1 6 /1 0 /0 7 2 0 1 6 /1 0 /2 1 Generic Adderall XR® IMS NPA Weekly Oct 21, 2016 34% 45% 47% 56% 10% 7%
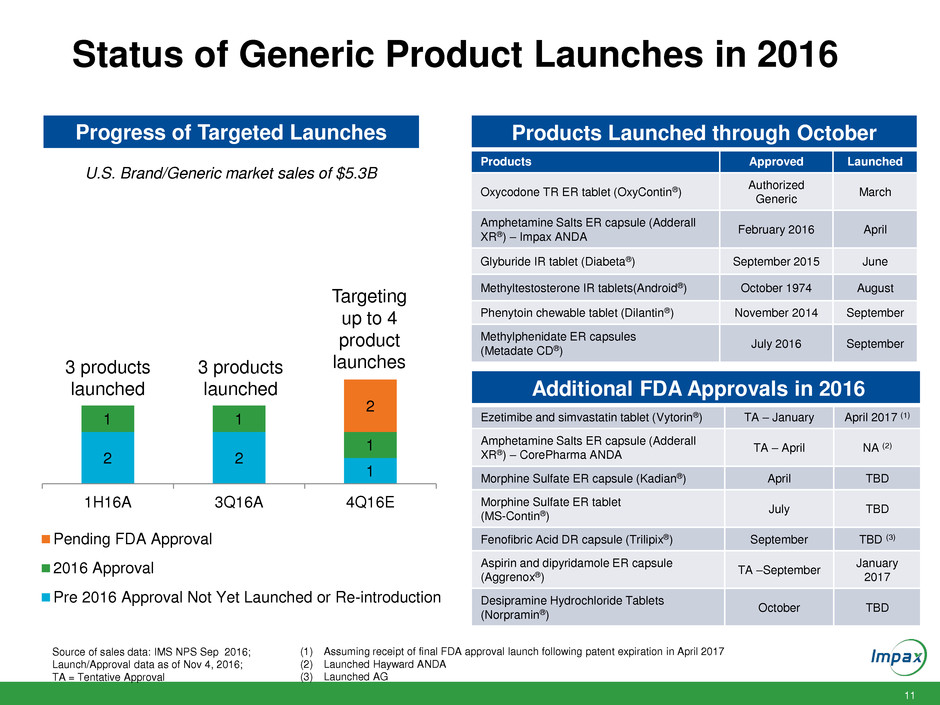
11 Status of Generic Product Launches in 2016 Source of sales data: IMS NPS Sep 2016; Launch/Approval data as of Nov 4, 2016; TA = Tentative Approval U.S. Brand/Generic market sales of $5.3B Progress of Targeted Launches Products Launched through October Products Approved Launched Oxycodone TR ER tablet (OxyContin®) Authorized Generic March Amphetamine Salts ER capsule (Adderall XR®) – Impax ANDA February 2016 April Glyburide IR tablet (Diabeta®) September 2015 June Methyltestosterone IR tablets(Android®) October 1974 August Phenytoin chewable tablet (Dilantin®) November 2014 September Methylphenidate ER capsules (Metadate CD®) July 2016 September Additional FDA Approvals in 2016 Ezetimibe and simvastatin tablet (Vytorin®) TA – January April 2017 (1) Amphetamine Salts ER capsule (Adderall XR®) – CorePharma ANDA TA – April NA (2) Morphine Sulfate ER capsule (Kadian®) April TBD Morphine Sulfate ER tablet (MS-Contin®) July TBD Fenofibric Acid DR capsule (Trilipix®) September TBD (3) Aspirin and dipyridamole ER capsule (Aggrenox®) TA –September January 2017 Desipramine Hydrochloride Tablets (Norpramin®) October TBD 2 2 1 1 1 1 2 1H16A 3Q16A 4Q16E Pending FDA Approval 2016 Approval Pre 2016 Approval Not Yet Launched or Re-introduction 3 products launched 3 products launched Targeting up to 4 product launches (1) Assuming receipt of final FDA approval launch following patent expiration in April 2017 (2) Launched Hayward ANDA (3) Launched AG
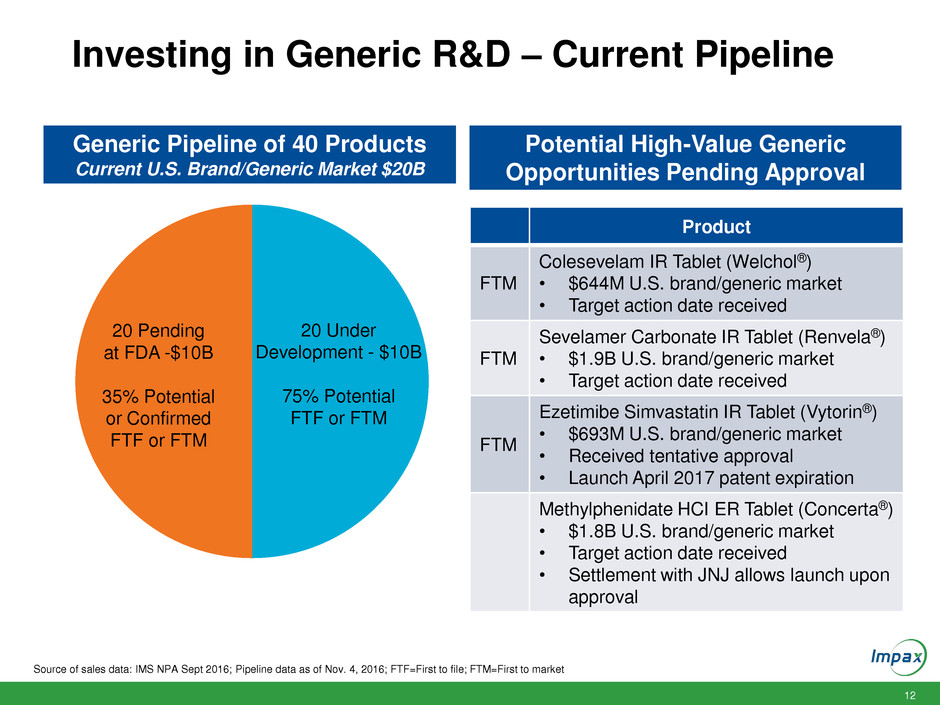
12 Investing in Generic R&D – Current Pipeline Source of sales data: IMS NPA Sept 2016; Pipeline data as of Nov. 4, 2016; FTF=First to file; FTM=First to market Generic Pipeline of 40 Products Current U.S. Brand/Generic Market $20B 20 Pending at FDA -$10B 35% Potential or Confirmed FTF or FTM 20 Under Development - $10B 75% Potential FTF or FTM Potential High-Value Generic Opportunities Pending Approval Product FTM Colesevelam IR Tablet (Welchol®) • $644M U.S. brand/generic market • Target action date received FTM Sevelamer Carbonate IR Tablet (Renvela®) • $1.9B U.S. brand/generic market • Target action date received FTM Ezetimibe Simvastatin IR Tablet (Vytorin®) • $693M U.S. brand/generic market • Received tentative approval • Launch April 2017 patent expiration Methylphenidate HCI ER Tablet (Concerta®) • $1.8B U.S. brand/generic market • Target action date received • Settlement with JNJ allows launch upon approval
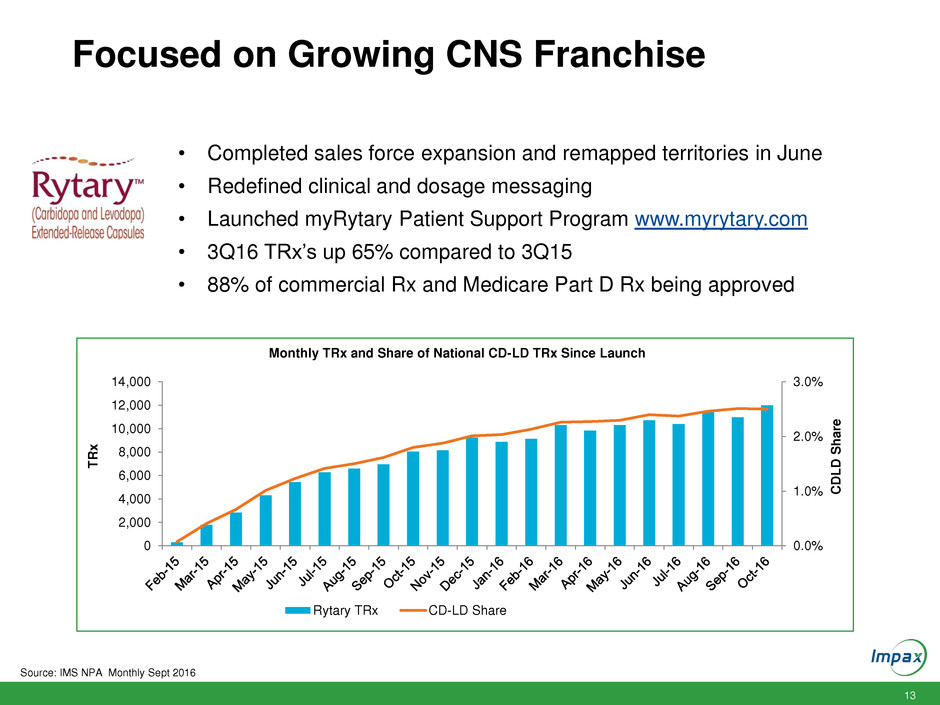
13 Focused on Growing CNS Franchise 0.0% 1.0% 2.0% 3.0% 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 CD L D S h a re T R x Monthly TRx and Share of National CD-LD TRx Since Launch Rytary TRx CD-LD Share • Completed sales force expansion and remapped territories in June • Redefined clinical and dosage messaging • Launched myRytary Patient Support Program www.myrytary.com • 3Q16 TRx’s up 65% compared to 3Q15 • 88% of commercial Rx and Medicare Part D Rx being approved Source: IMS NPA Monthly Sept 2016

14 Rytary® Patient Support Program
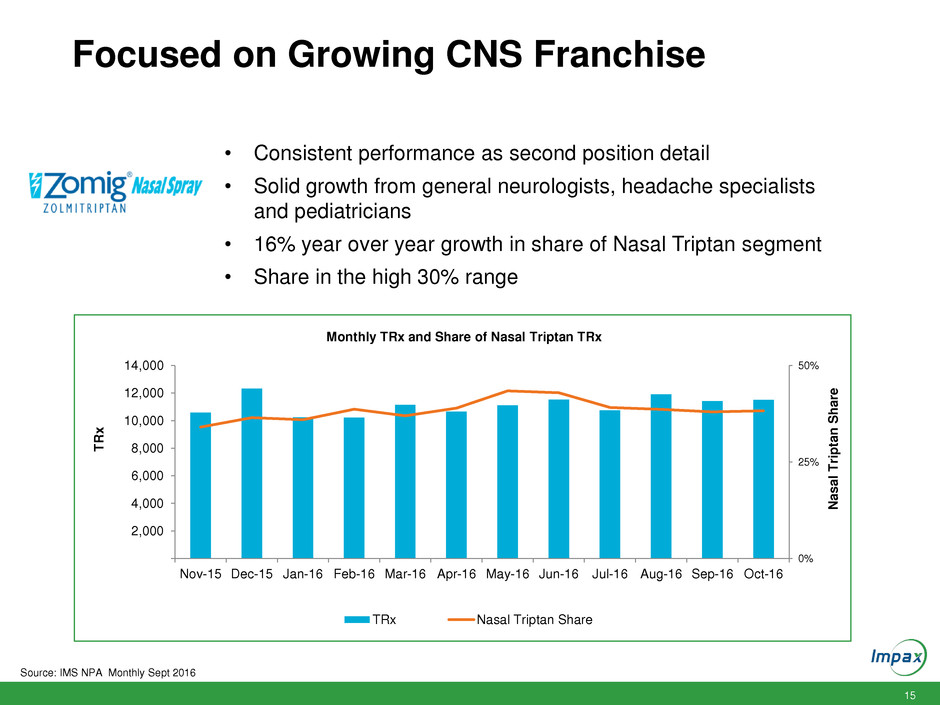
15 Focused on Growing CNS Franchise 0% 25% 50% 2,000 4,000 6,000 8,000 10,000 12,000 14,000 Nov-15 Dec-15 Jan-16 Feb-16 Mar-16 Apr-16 May-16 Jun-16 Jul-16 Aug-16 Sep-16 Oct-16 Nas a l T rip ta n S h a re T R x Monthly TRx and Share of Nasal Triptan TRx TRx Nasal Triptan Share • Consistent performance as second position detail • Solid growth from general neurologists, headache specialists and pediatricians • 16% year over year growth in share of Nasal Triptan segment • Share in the high 30% range Source: IMS NPA Monthly Sept 2016
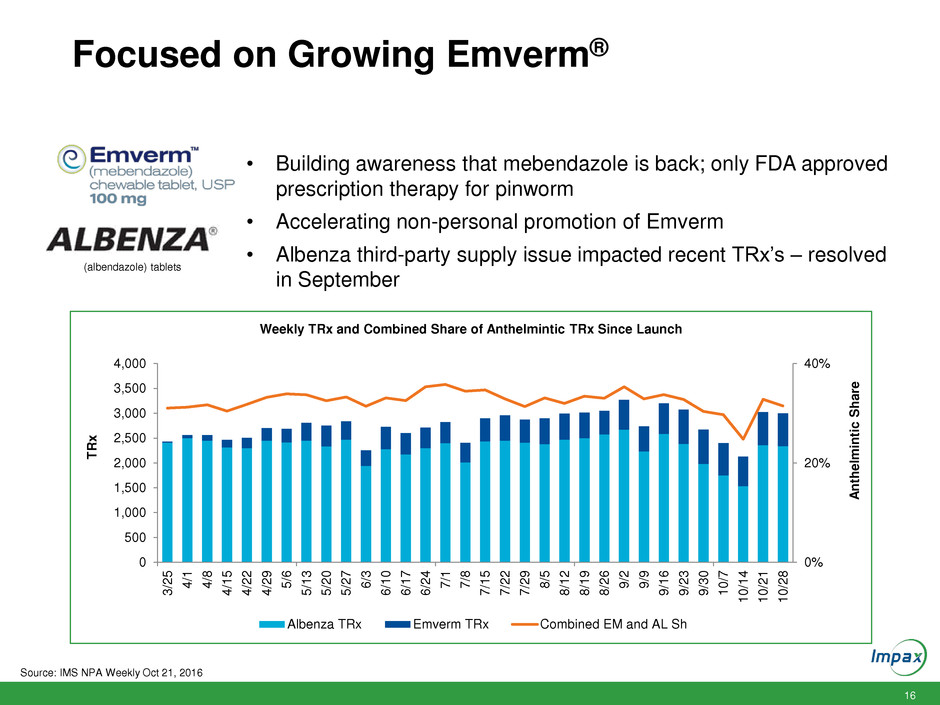
16 Focused on Growing Emverm® 0% 20% 40% 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 3 /2 5 4 /1 4 /8 4 /1 5 4 /2 2 4 /2 9 5 /6 5 /1 3 5 /2 0 5 /2 7 6 /3 6 /1 0 6 /1 7 6 /2 4 7 /1 7 /8 7 /1 5 7 /2 2 7 /2 9 8 /5 8 /1 2 8 /1 9 8 /2 6 9 /2 9 /9 9 /1 6 9 /2 3 9 /3 0 1 0 /7 1 0 /1 4 1 0 /2 1 1 0 /2 8 A n th elmin tic S h a re T R x Weekly TRx and Combined Share of Anthelmintic TRx Since Launch Albenza TRx Emverm TRx Combined EM and AL Sh (albendazole) tablets • Building awareness that mebendazole is back; only FDA approved prescription therapy for pinworm • Accelerating non-personal promotion of Emverm • Albenza third-party supply issue impacted recent TRx’s – resolved in September Source: IMS NPA Weekly Oct 21, 2016
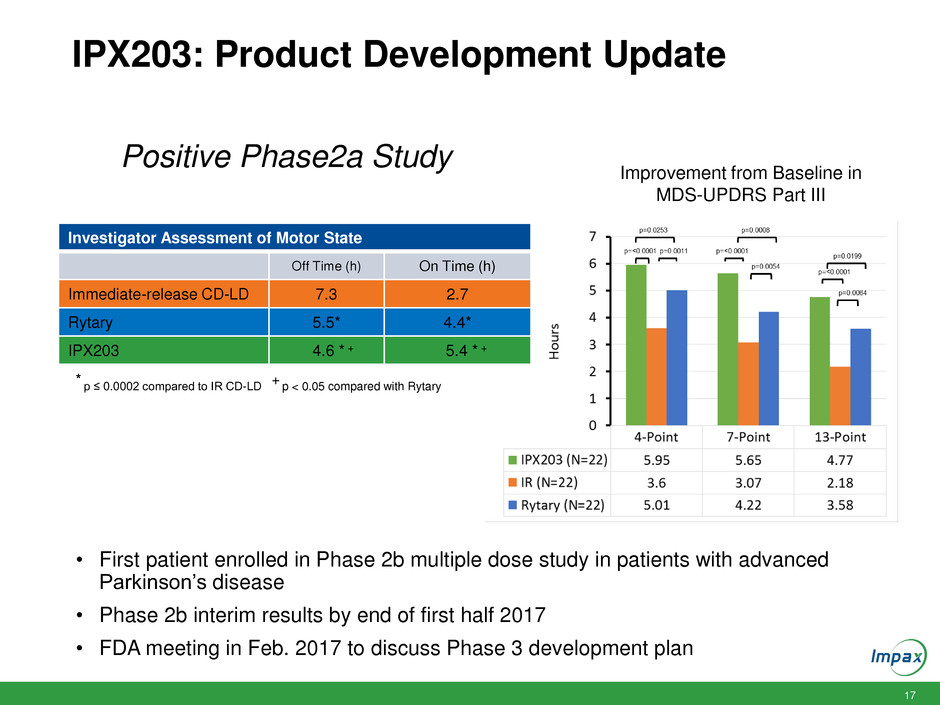
17 IPX203: Product Development Update Investigator Assessment of Motor State Off Time (h) On Time (h) Immediate-release CD-LD 7.3 2.7 Rytary 5.5* 4.4* IPX203 4.6 * + 5.4 * + * p ≤ 0.0002 compared to IR CD-LD + p < 0.05 compared with Rytary Improvement from Baseline in MDS-UPDRS Part III • First patient enrolled in Phase 2b multiple dose study in patients with advanced Parkinson’s disease • Phase 2b interim results by end of first half 2017 • FDA meeting in Feb. 2017 to discuss Phase 3 development plan Positive Phase2a Study

18 Financial Review and 2016 Financial Guidance Bryan Reasons Senior Vice President & Chief Financial Officer
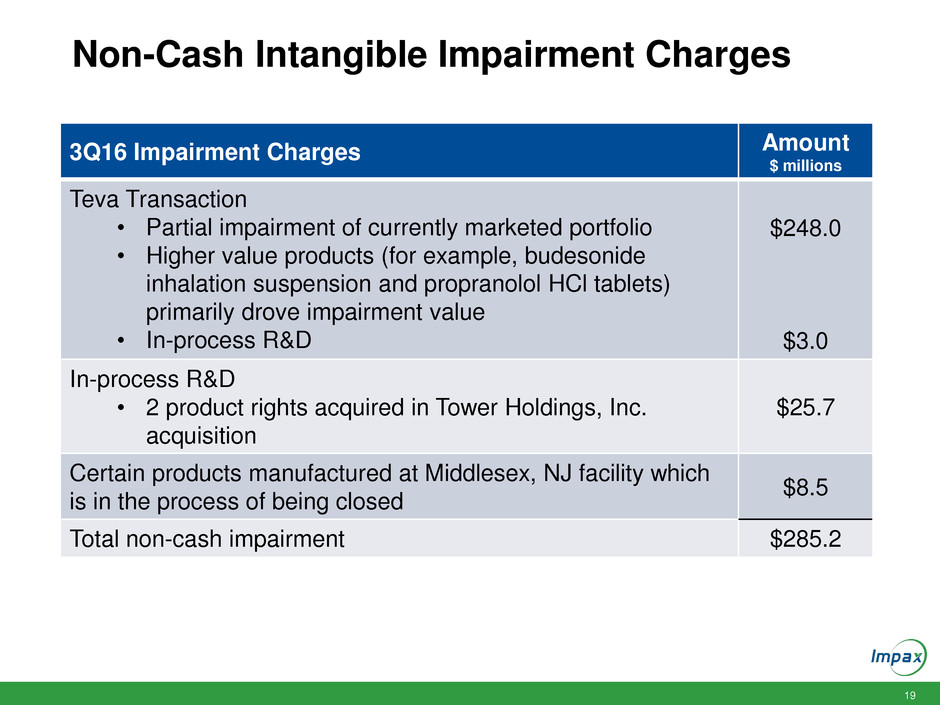
19 Non-Cash Intangible Impairment Charges 3Q16 Impairment Charges Amount $ millions Teva Transaction • Partial impairment of currently marketed portfolio • Higher value products (for example, budesonide inhalation suspension and propranolol HCl tablets) primarily drove impairment value • In-process R&D $248.0 $3.0 In-process R&D • 2 product rights acquired in Tower Holdings, Inc. acquisition $25.7 Certain products manufactured at Middlesex, NJ facility which is in the process of being closed $8.5 Total non-cash impairment $285.2

20 3Q16 Generic Division Results $ millions GAAP 3Q16 Adjustments Adjusted 3Q16 GAAP 3Q15 Adjustments Adjusted 3Q15 Adjusted Change Revenues $175.3 --- $175.3 $180.7 --- $180.7 (3.0%) Gross (Loss) Profit ($196.2) $273.1 $76.9 $68.0 $9.3 $77.3 (0.5%) Gross Margin (111.9%) --- 43.8% 37.6% --- 42.8% 1.0ppts SG&A Expense $6.1 ($0.1) $6.0 $5.1 ($0.1) $5.0 20.0% R&D Expense $15.4 ($0.6) $14.8 $14.3 ($0.8) $13.5 9.6% In-process R&D Impairment Charges $15.5 ($15.5) $0.0 $0.0 --- $0.0 0.0% Patent Litigation $0.1 --- $0.1 $0.4 --- $0.4 (75.0%) Operating (Loss) Income ($233.3) $289.3 $56.0 $48.1 $10.2 58.3 (3.9%) Operating Margin (133.1%) --- 31.9% 26.6% --- 32.3% (0.4)pts Note: The sum of the GAAP and Adjustments may not equal the Adjusted amount due to rounding Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results SG&A = Selling, general and administrative R&D = Research & development
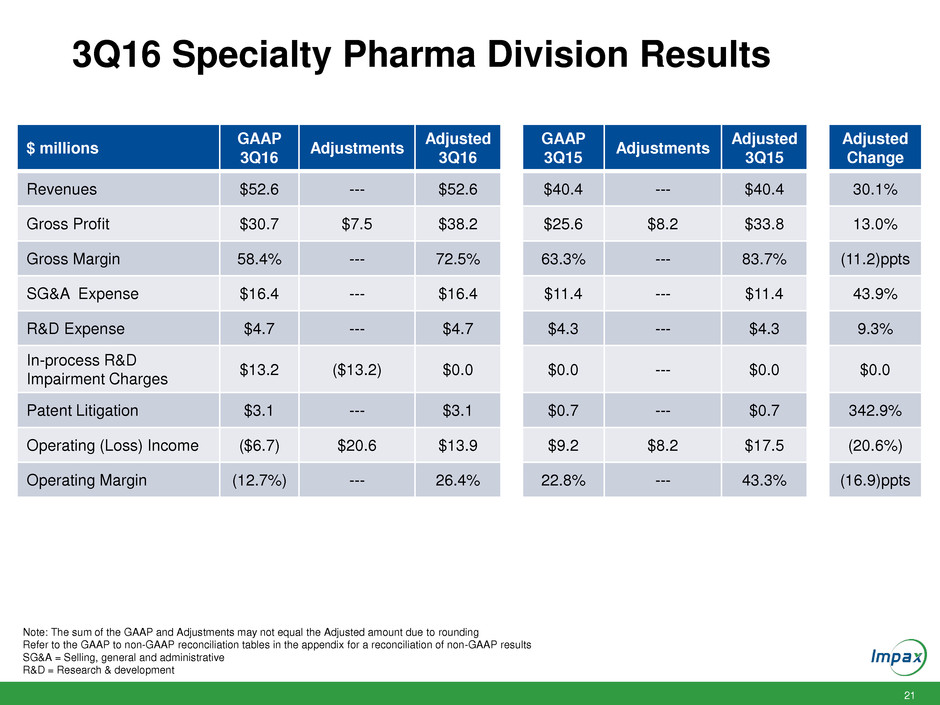
21 3Q16 Specialty Pharma Division Results $ millions GAAP 3Q16 Adjustments Adjusted 3Q16 GAAP 3Q15 Adjustments Adjusted 3Q15 Adjusted Change Revenues $52.6 --- $52.6 $40.4 --- $40.4 30.1% Gross Profit $30.7 $7.5 $38.2 $25.6 $8.2 $33.8 13.0% Gross Margin 58.4% --- 72.5% 63.3% --- 83.7% (11.2)ppts SG&A Expense $16.4 --- $16.4 $11.4 --- $11.4 43.9% R&D Expense $4.7 --- $4.7 $4.3 --- $4.3 9.3% In-process R&D Impairment Charges $13.2 ($13.2) $0.0 $0.0 --- $0.0 $0.0 Patent Litigation $3.1 --- $3.1 $0.7 --- $0.7 342.9% Operating (Loss) Income ($6.7) $20.6 $13.9 $9.2 $8.2 $17.5 (20.6%) Operating Margin (12.7%) --- 26.4% 22.8% --- 43.3% (16.9)ppts Note: The sum of the GAAP and Adjustments may not equal the Adjusted amount due to rounding Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results SG&A = Selling, general and administrative R&D = Research & development
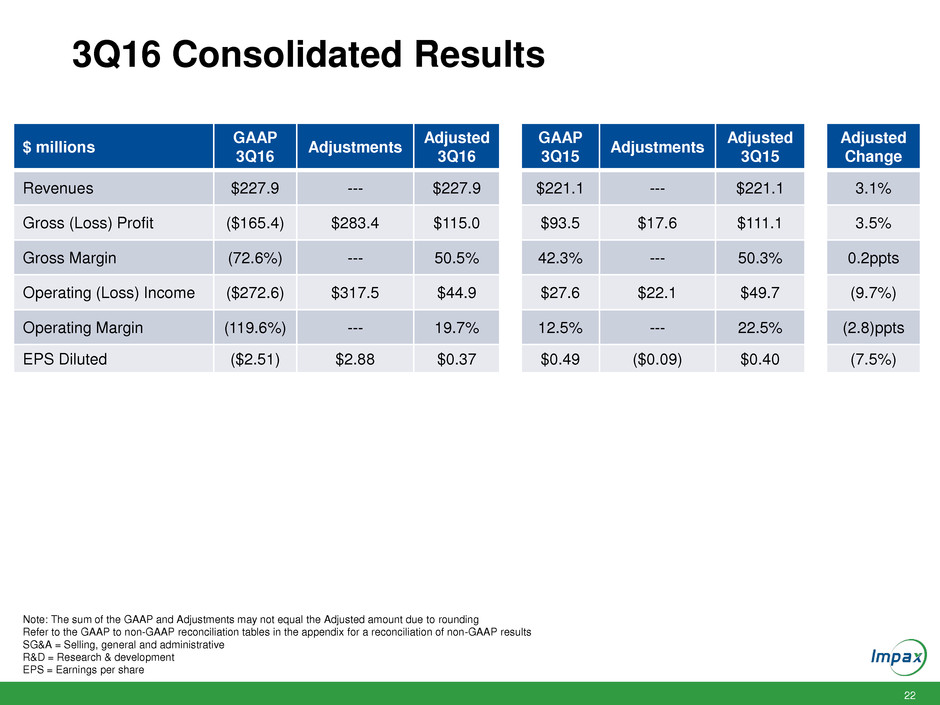
22 3Q16 Consolidated Results $ millions GAAP 3Q16 Adjustments Adjusted 3Q16 GAAP 3Q15 Adjustments Adjusted 3Q15 Adjusted Change Revenues $227.9 --- $227.9 $221.1 --- $221.1 3.1% Gross (Loss) Profit ($165.4) $283.4 $115.0 $93.5 $17.6 $111.1 3.5% Gross Margin (72.6%) --- 50.5% 42.3% --- 50.3% 0.2ppts Operating (Loss) Income ($272.6) $317.5 $44.9 $27.6 $22.1 $49.7 (9.7%) Operating Margin (119.6%) --- 19.7% 12.5% --- 22.5% (2.8)ppts EPS Diluted ($2.51) $2.88 $0.37 $0.49 ($0.09) $0.40 (7.5%) Note: The sum of the GAAP and Adjustments may not equal the Adjusted amount due to rounding Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results SG&A = Selling, general and administrative R&D = Research & development EPS = Earnings per share
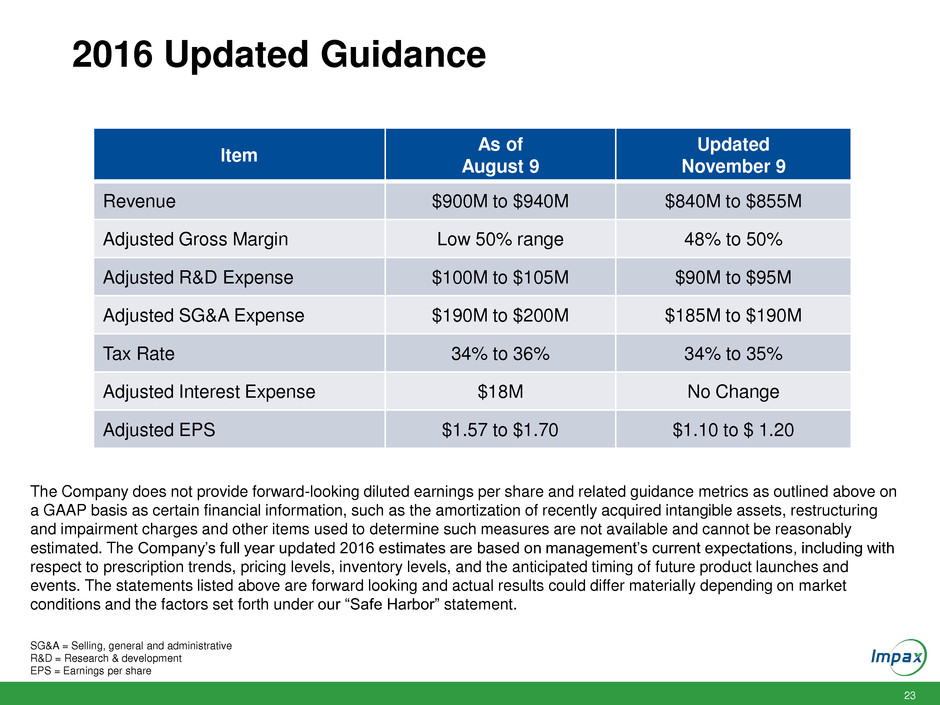
23 2016 Updated Guidance Item As of August 9 Updated November 9 Revenue $900M to $940M $840M to $855M Adjusted Gross Margin Low 50% range 48% to 50% Adjusted R&D Expense $100M to $105M $90M to $95M Adjusted SG&A Expense $190M to $200M $185M to $190M Tax Rate 34% to 36% 34% to 35% Adjusted Interest Expense $18M No Change Adjusted EPS $1.57 to $1.70 $1.10 to $ 1.20 SG&A = Selling, general and administrative R&D = Research & development EPS = Earnings per share The Company does not provide forward-looking diluted earnings per share and related guidance metrics as outlined above on a GAAP basis as certain financial information, such as the amortization of recently acquired intangible assets, restructuring and impairment charges and other items used to determine such measures are not available and cannot be reasonably estimated. The Company’s full year updated 2016 estimates are based on management’s current expectations, including with respect to prescription trends, pricing levels, inventory levels, and the anticipated timing of future product launches and events. The statements listed above are forward looking and actual results could differ materially depending on market conditions and the factors set forth under our “Safe Harbor” statement.
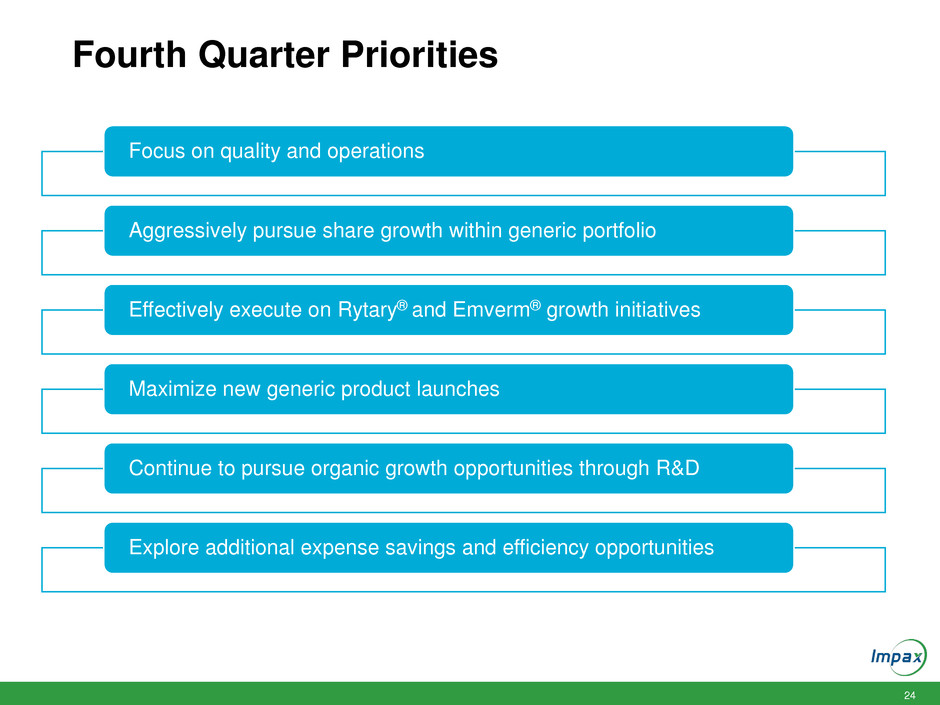
24 Fourth Quarter Priorities Focus on quality and operations Aggressively pursue share growth within generic portfolio Effectively execute on Rytary® and Emverm® growth initiatives Maximize new generic product launches Continue to pursue organic growth opportunities through R&D Explore additional expense savings and efficiency opportunities

25 Q&A

26 Appendix
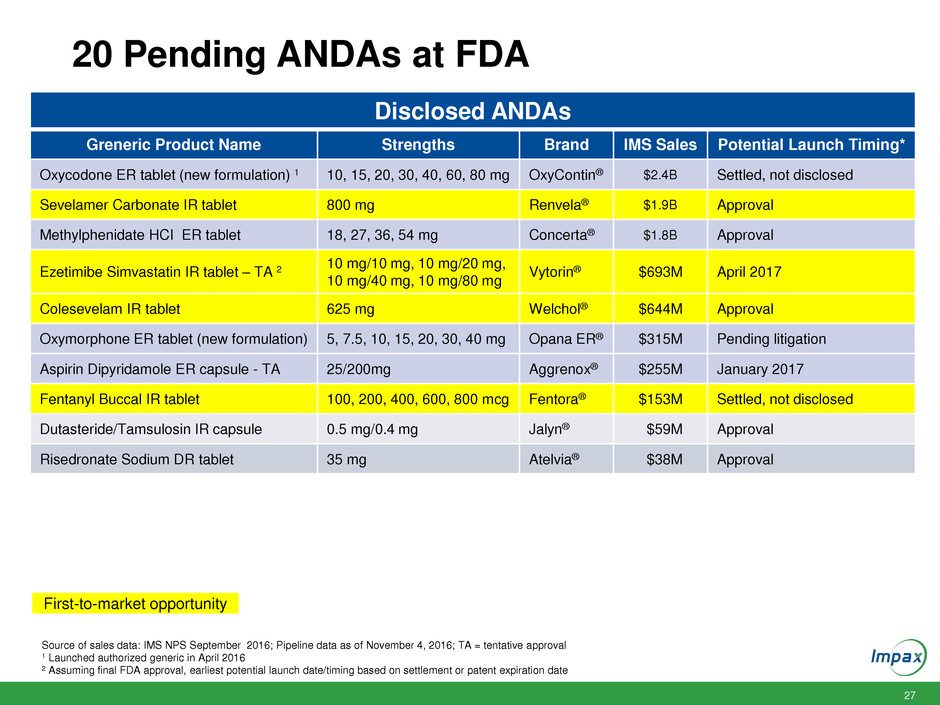
27 20 Pending ANDAs at FDA Source of sales data: IMS NPS September 2016; Pipeline data as of November 4, 2016; TA = tentative approval 1 Launched authorized generic in April 2016 2 Assuming final FDA approval, earliest potential launch date/timing based on settlement or patent expiration date First-to-market opportunity Disclosed ANDAs Greneric Product Name Strengths Brand IMS Sales Potential Launch Timing* Oxycodone ER tablet (new formulation) 1 10, 15, 20, 30, 40, 60, 80 mg OxyContin® $2.4B Settled, not disclosed Sevelamer Carbonate IR tablet 800 mg Renvela® $1.9B Approval Methylphenidate HCI ER tablet 18, 27, 36, 54 mg Concerta® $1.8B Approval Ezetimibe Simvastatin IR tablet – TA 2 10 mg/10 mg, 10 mg/20 mg, 10 mg/40 mg, 10 mg/80 mg Vytorin® $693M April 2017 Colesevelam IR tablet 625 mg Welchol® $644M Approval Oxymorphone ER tablet (new formulation) 5, 7.5, 10, 15, 20, 30, 40 mg Opana ER® $315M Pending litigation Aspirin Dipyridamole ER capsule - TA 25/200mg Aggrenox® $255M January 2017 Fentanyl Buccal IR tablet 100, 200, 400, 600, 800 mcg Fentora® $153M Settled, not disclosed Dutasteride/Tamsulosin IR capsule 0.5 mg/0.4 mg Jalyn® $59M Approval Risedronate Sodium DR tablet 35 mg Atelvia® $38M Approval
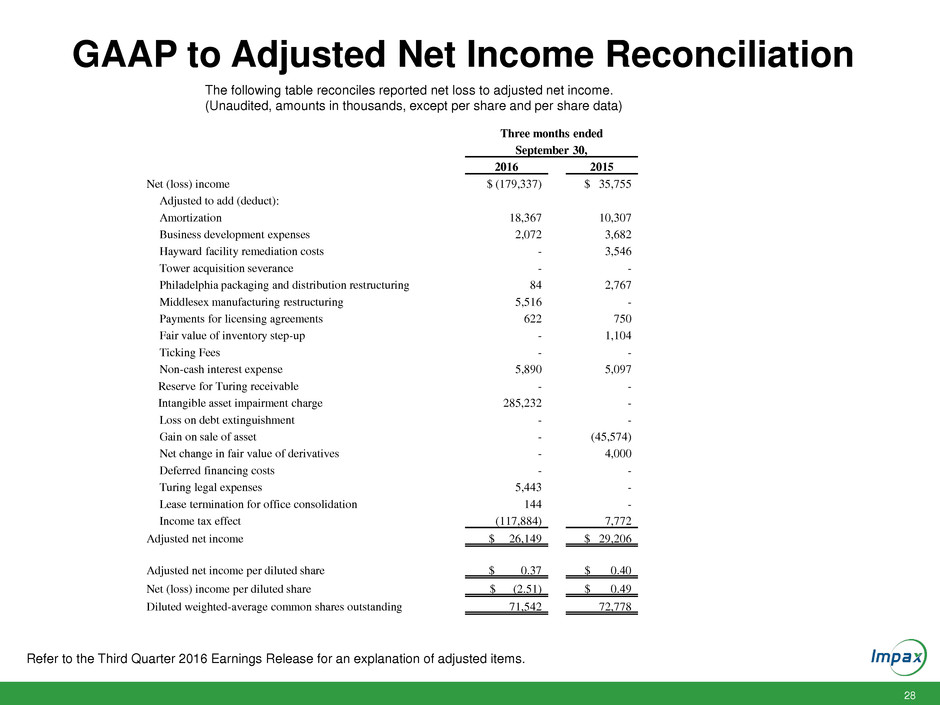
28 GAAP to Adjusted Net Income Reconciliation The following table reconciles reported net loss to adjusted net income. (Unaudited, amounts in thousands, except per share and per share data) Three months ended September 30, 2016 2015 Net (loss) income $ (179,337) $ 35,755 Adjusted to add (deduct): Amortization 18,367 10,307 Business development expenses 2,072 3,682 Hayward facility remediation costs - 3,546 Tower acquisition severance - - Philadelphia packaging and distribution restructuring 84 2,767 Middlesex manufacturing restructuring 5,516 - Payments for licensing agreements 622 750 Fair value of inventory step-up - 1,104 Ticking Fees - - Non-cash interest expense 5,890 5,097 Reserve for Turing receivable - - Intangible asset impairment charge 285,232 - Loss on debt extinguishment - - Gain on sale of asset - (45,574) Net change in fair value of derivatives - 4,000 Deferred financing costs - - Turing legal expenses 5,443 - Lease termination for office consolidation 144 - Income tax effect (117,884) 7,772 Adjusted net income $ 26,149 $ 29,206 Adjusted net income per diluted share $ 0.37 $ 0.40 Net (loss) income per diluted share $ (2.51) $ 0.49 Diluted weighted-average common shares outstanding 71,542 72,778 Refer to the Third Quarter 2016 Earnings Release for an explanation of adjusted items.
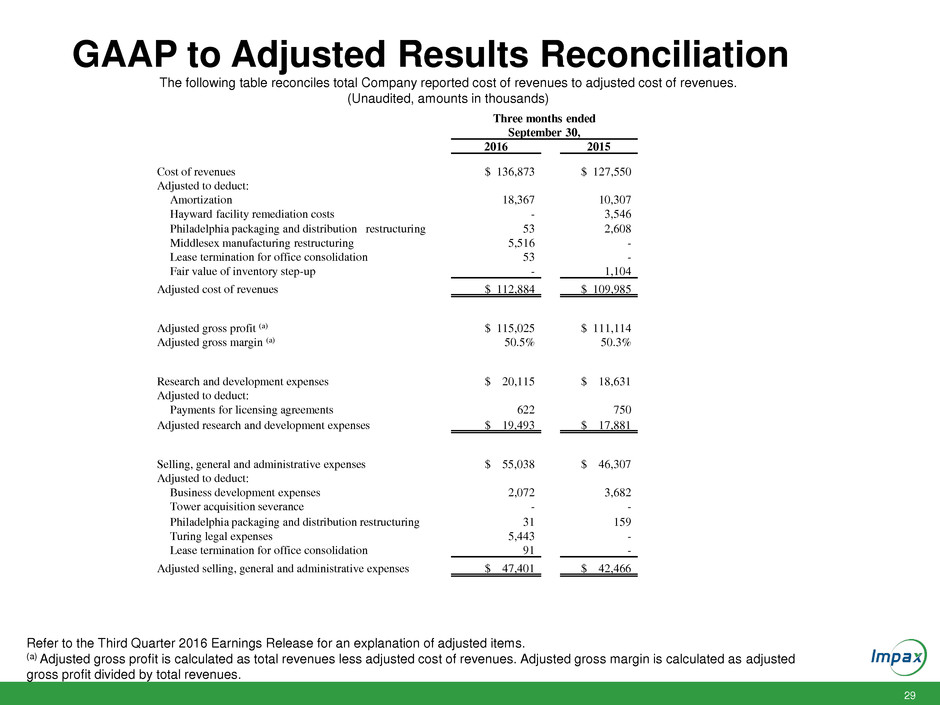
29 GAAP to Adjusted Results Reconciliation The following table reconciles total Company reported cost of revenues to adjusted cost of revenues. (Unaudited, amounts in thousands) Refer to the Third Quarter 2016 Earnings Release for an explanation of adjusted items. (a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by total revenues. Three months ended September 30, 2016 2015 Cost of revenues $ 136,873 $ 127,550 Adjusted to deduct: Amortization 18,367 10,307 Hayward facility remediation costs - 3,546 Philadelphia packaging and distribution restructuring 53 2,608 Middlesex manufacturing restructuring 5,516 - Lease termination for office consolidation 53 - Fair value of inventory step-up - 1,104 Adjusted cost of revenues $ 112,884 $ 109,985 Adjusted gross profit (a) $ 115,025 $ 111,114 Adjusted gross margin (a) 50.5% 50.3% Research and development expenses $ 20,115 $ 18,631 Adjusted to deduct: Payments for licensing agreements 622 750 Adjusted research and development expenses $ 19,493 $ 17,881 Selling, general and administrative expenses $ 55,038 $ 46,307 Adjusted to deduct: Business development expenses 2,072 3,682 Tower acquisition severance - - Philadelphia packaging and distribution restructuring 31 159 Turing legal expenses 5,443 - Lease termination for office consolidation 91 - Adjusted selling, general and administrative expenses $ 47,401 $ 42,466
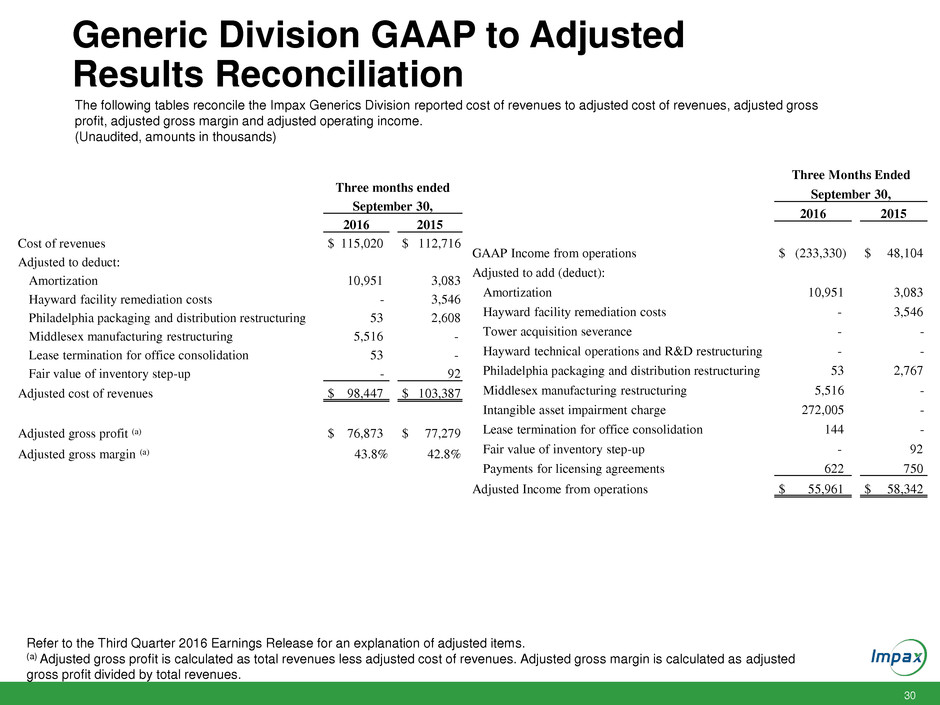
30 Generic Division GAAP to Adjusted Results Reconciliation The following tables reconcile the Impax Generics Division reported cost of revenues to adjusted cost of revenues, adjusted gross profit, adjusted gross margin and adjusted operating income. (Unaudited, amounts in thousands) Refer to the Third Quarter 2016 Earnings Release for an explanation of adjusted items. (a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by total revenues. Three Months Ended September 30, 2016 2015 GAAP Income from operations $ (233,330) $ 48,104 Adjusted to add (deduct): Amortization 10,951 3,083 Hayward facility remediation costs - 3,546 Tower acquisition severance - - Hayward technical operations and R&D restructuring - - Philadelphia packaging and distribution restructuring 53 2,767 Middlesex manufacturing restructuring 5,516 - Intangible asset impairment charge 272,005 - Lease termination for office consolidation 144 - Fair value of inventory step-up - 92 Payments for licensing agreements 622 750 Adjusted Income from operations $ 55,961 $ 58,342 Three months ended September 30, 2016 2015 Cost of revenues $ 115,020 $ 112,716 Adjusted to deduct: Amortization 10,951 3,083 Hayward facility remediation costs - 3,546 Philadelphia packaging and distribution restructuring 53 2,608 Middlesex manufacturing restructuring 5,516 - Lease termination for office consolidation 53 - Fair value of inventory step-up - 92 Adjusted cost of revenues $ 98,447 $ 103,387 Adjusted gross profit (a) $ 76,873 $ 77,279 Adjusted gross margin (a) 43.8% 42.8%
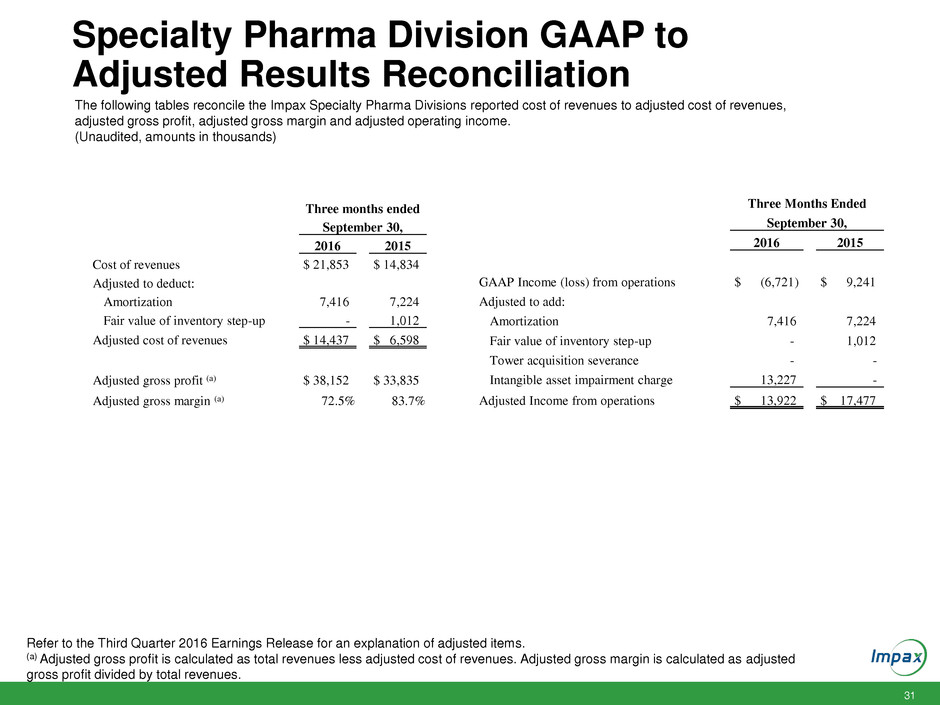
31 Specialty Pharma Division GAAP to Adjusted Results Reconciliation The following tables reconcile the Impax Specialty Pharma Divisions reported cost of revenues to adjusted cost of revenues, adjusted gross profit, adjusted gross margin and adjusted operating income. (Unaudited, amounts in thousands) Refer to the Third Quarter 2016 Earnings Release for an explanation of adjusted items. (a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by total revenues. Three months ended September 30, 2016 2015 Cost of revenues $ 21,853 $ 14,834 Adjusted to deduct: Amortization 7,416 7,224 Fair value of inventory step-up - 1,012 Adjusted cost of revenues $ 14,437 $ 6,598 Adjusted gross profit (a) $ 38,152 $ 33,835 Adjusted gross margin (a) 72.5% 83.7% Three Months Ended September 30, 2016 2015 GAAP Income (loss) from operations $ (6,721) $ 9,241 Adjusted to add: Amortization 7,416 7,224 Fair value of inventory step-up - 1,012 Tower acquisition severance - - Intangible asset impairment charge 13,227 - Adjusted Income from operations $ 13,922 $ 17,477






























