Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
IPXL similar filings
- 13 Feb 13 Entry into a Material Definitive Agreement
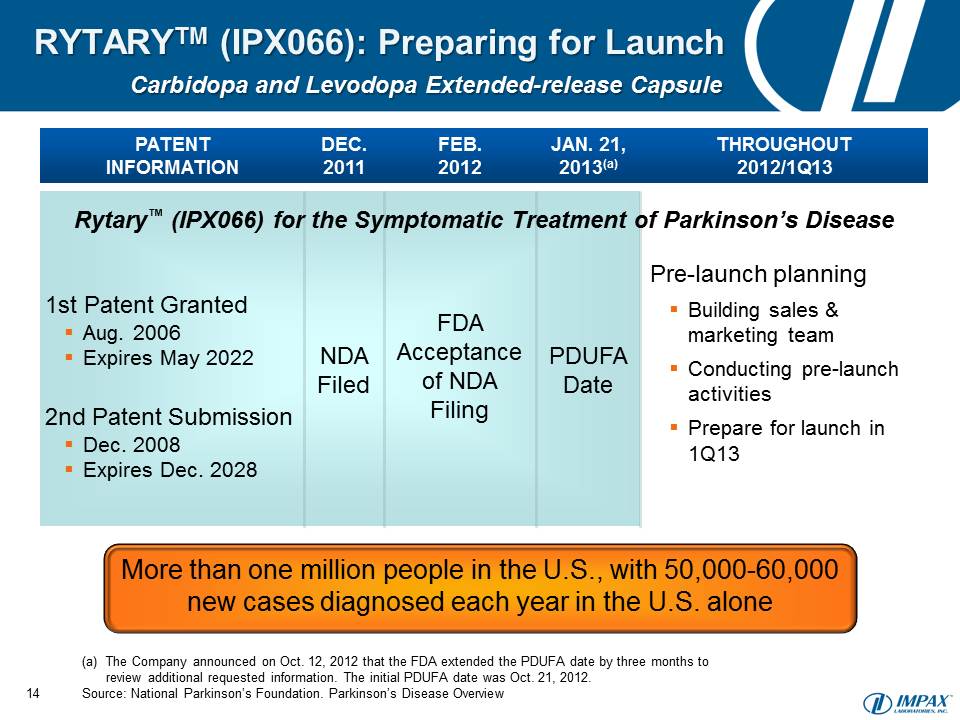
- 22 Jan 13 FDA Issues Complete Response Letter for RYTARY™ (Carbidopa and Levodopa) Extended-Release Capsules (IPX066) New Drug Application
- 10 Jan 13 Entry into a Material Definitive Agreement
- 8 Jan 13 Regulation FD Disclosure
- 13 Dec 12 Departure of Directors or Certain Officers
- 30 Oct 12 Impax Laboratories Reports Third Quarter 2012 Results
- 28 Aug 12 Amendments to Articles of Incorporation or Bylaws
Filing view
External links