EXHIBIT 99.1

The New Era of Biologic Revascularization for Refractory Myocardial Ischemia
Richard E. Otto
President and CEO
February 24, 2004
1

Forward Looking Statements
This presentation contains “forward-looking statements” within the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 with respect to Corautus Genetics’ financial condition, intellectual property, results of operations and businesses, research and development, approval of our product candidates and assessment of early stage trial results. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “could,” “would,” “will,” “may,” “can” and similar expressions identify forward-looking statements. These forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materially from the results contemplated by the forward-looking statements. Many of the important factors that will determine these results and values are beyond Corautus Genetics’ ability to control or predict. Prospective investors are cautioned not to put undue reliance on any forward-looking statements. Corautus Genetics does not assume any obligation to update any forward-looking statements. In evaluating the investment opportunity, you should carefully consider the discussion of risks factors in the section entitled “Risk Factors” beginning on page 2 in Amendment No. 1 to Corautus’ Registration Statement on Form S-3 (File No. 333-112239) filed February 2, 2004.
2
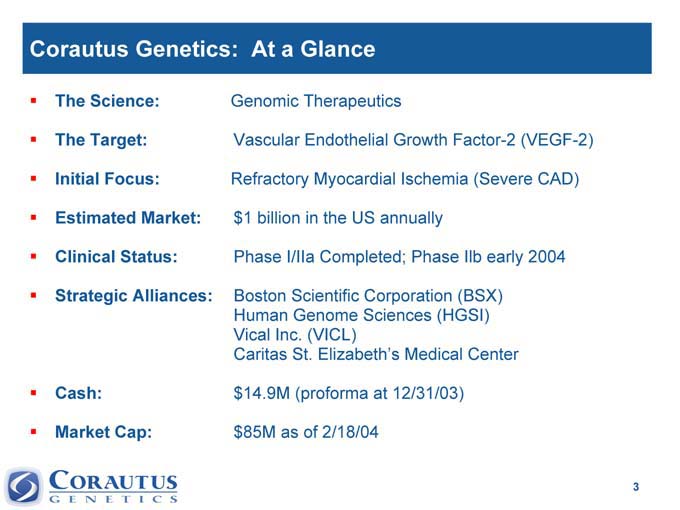
Corautus Genetics: At a Glance
The Science: Genomic Therapeutics
The Target: Vascular Endothelial Growth Factor-2 (VEGF-2)
Initial Focus: Refractory Myocardial Ischemia (Severe CAD)
Estimated Market: $1 billion in the US annually
Clinical Status: Phase I/IIa Completed; Phase Ilb early 2004
Strategic Alliances: Boston Scientific Corporation (BSX)
Human Genome Sciences (HGSI)
Vical Inc. (VICL)
Caritas St. Elizabeth’s Medical Center
Cash: $14.9M (proforma at 12/31/03)
Market Cap: $85M as of 2/18/04
3

Jeffery M. Isner, M.D. & Vascular Genetics Inc.
‘No discussion on therapeutic angiogenesis would be appropriate without honoring the person who was clearly the leader in the field. Dr. Jeffery M. IsnerJeff was the consummate Clinician/Scientist and without question the pioneer in this area.’
Cardiology Rounds
Harvard Medical School J
anuary 2002
4
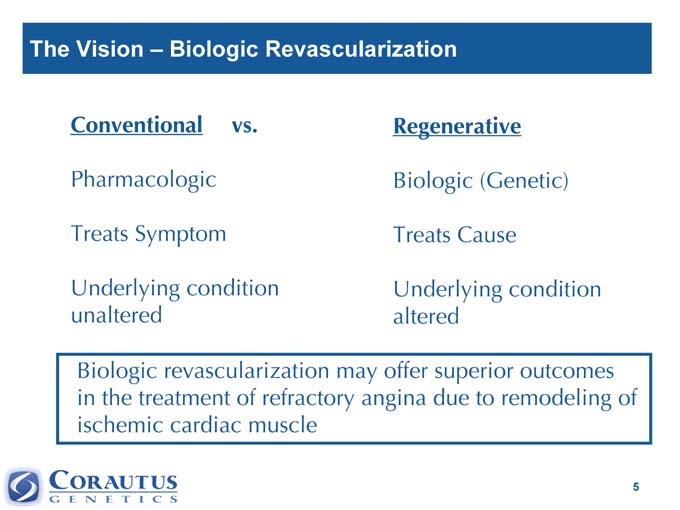
The Vision – Biologic Revascularization
Conventional vs. Regenerative
Pharmacologic Biologic (Genetic)
Treats Symptom Treats Cause
Underlying condition unaltered Underlying condition altered
Biologic revascularization may offer superior outcomes in the treatment of refractory angina due to remodeling of ischemic cardiac muscle
5

The Opportunity
6
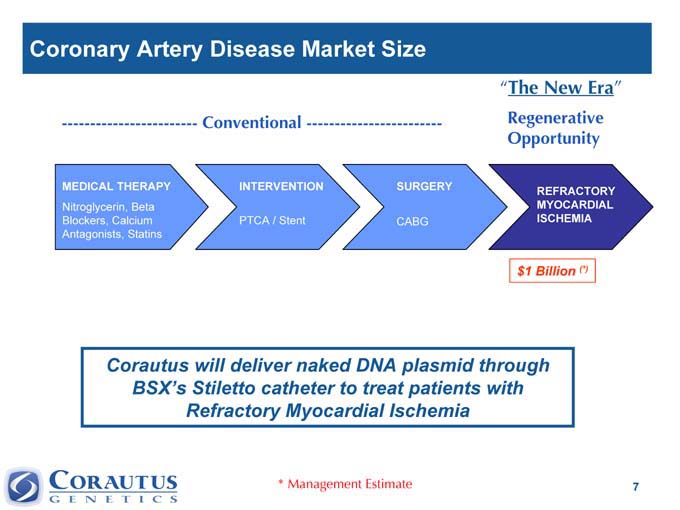
Coronary Artery Disease Market Size
“The New Era”
———————————— Conventional ———————————— Regenerative
Opportunity
MEDICAL THERAPY INTERVENTION SURGERY REFRACTORY
Nitroglycerin, Beta MYOCARDIAL
Blockers, Calcium PTCA / Stent CABG ISCHEMIA
Antagonists, Statins
$1 Billion (*)
Corautus will deliver naked DNA plasmid through BSX’s Stiletto catheter to treat patients with Refractory Myocardial Ischemia
* Management Estimate
7

Refractory Myocardial Ischemia
Class III-IV refractory angina
Cardiac muscle demand for 02 is greater than supply
Significant chest pain on minimal or no exertion
No proven therapy for Class III-IV refractory angina
Poor quality of life
Significant demand for effective treatment
8

Clinical Results – Phase I and IIa
Phase I – Mini Thoracotomy
30 Class III/IV angina patients injected directly into myocardium
Positive clinical results
– 74% patients reported a reduction in angina of greater than 2 classes
– Episodes of angina fell from 31 to 9/week
– Exercise time increased an average of 2 minutes
Single-Blind Placebo Controlled Pilot Study
6 patients; catheter-based injection into the myocardium Positive clinical results
? Patients experienced reduced angina (36.2 episodes/week before vs. 3.5 after)
? Reduced nitroglycerin consumption (33.8 versus 4.1 tablets/week) up to 360 days
Phase I/IIa – Double-Blind Placebo Controlled Study
12 patients received VEGF-2 catheter-based delivery (7 received placebo) Positive clinical results
? Data demonstrated safety of VEGF-2 catheter-based myocardial gene transfer
Source: Articles published by investigators in Circulation December 22/29, 1998, August 29, 2000 and April 30, 2002
9
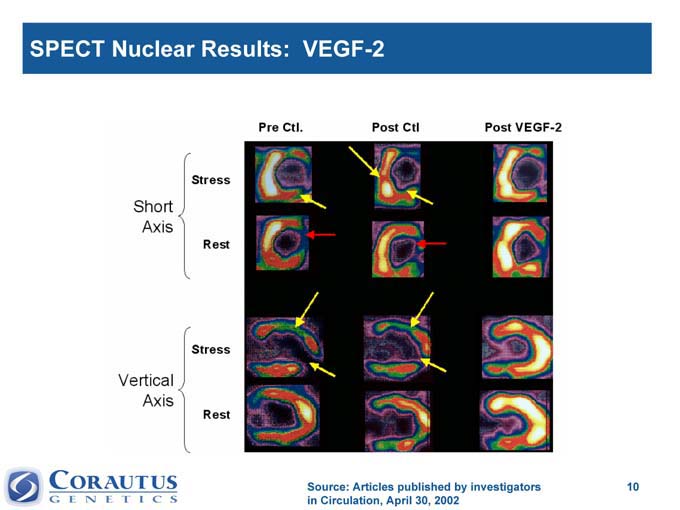
SPECT Nuclear Results: VEGF-2
Source: Articles published by investigators in Circulation, April 30, 2002
10
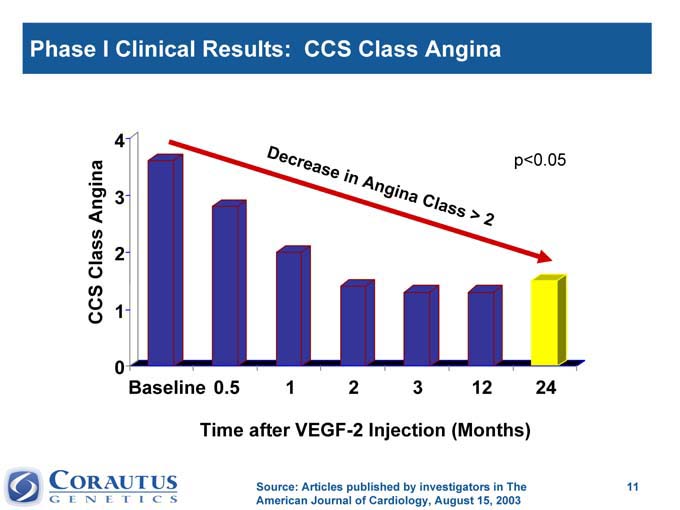
Phase I Clinical Results: CCS Class Angina
Time after VEGF-2 Injection (Months)
Source: Articles published by investigators in The American Journal of Cardiology, August 15, 2003
11

Phase IIb Trial
12

Physician Advisors
Douglas W. Losordo, M.D. – National Principal Investigator Involved in research with Dr. Isner for 14 years
Chief, Cardiovascular Research St. Elizabeth’s Medical Center Associate Professor of Medicine Tufts University School of Medicine Editorial Board, Circulation
Richard A. Schatz, M.D.
Identified Dr. Isner’s therapeutic for Vascular Genetics
Inventor and co-developer of Palmaz-Schatz stent
Co-Chairman of the Department of Cardiology, Cath Lab Director and research director of cardiovascular interventions at the Heart, Lung and Vascular Center at Scripps Clinic
Richard E. Kuntz, M.D., M.Sc. Designed Corautus’ Phase IIb trial
Harvard Clinical Research Institute
Chief, Division of Clinical Biometrics, Department of Medicine, Brigham and Women’s Hospital Associate Professor of Medicine, Harvard Medical School.
13

Phase Ilb Clinical Trial Design
National multi-center, double-blind, dose ranging N = 404, four cohorts (0, 20?g, 200?g, 800?g) Class III and IV angina “no op” patients Boston Scientific StilettoTM Catheter
Efficacy data measured at 3 months, safety data at 1 year 15 clinical sites in the United States
Primary Endpoint – Increase in Exercise Tolerance Time (“ETT”) Secondary Endpoint – SPECT Nuclear Imaging, Decreased angina
14

FDA Review Process of Combination Products
Center for Biologics Evaluation and Research (“CBER”)
Responsible for complete review of the entire submission
Reviews submission and refers device questions/concerns to CDRH Reports all CBER and CDRH comments to Sponsor of IND
Dialogue ongoing—additional guidance expected in the near term Trial can not commence until the submission is approved by the FDA
Center for Devices and Radiological Health (“CDRH”)
Reviews submission focusing on device components Delivers comments to CBER
15

Competitive Landscape
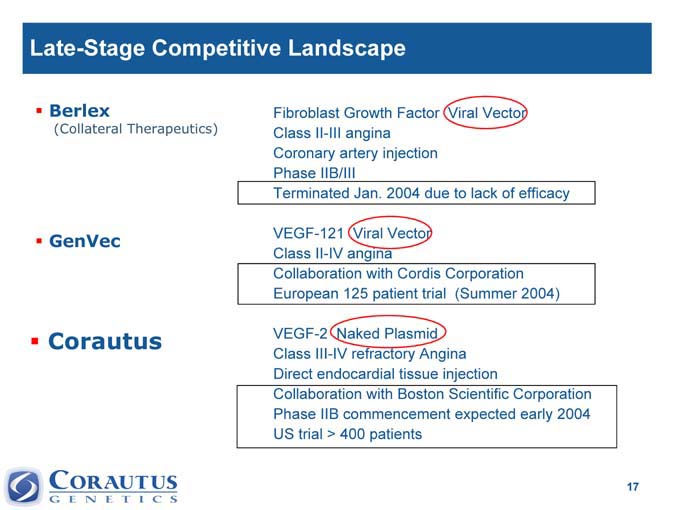
Late-Stage Competitive Landscape
Berlex Fibroblast Growth Factor Viral Vector
(Collateral Therapeutics) Class II-III angina
Coronary artery injection
Phase IIB/III
Terminated Jan. 2004 due to lack of efficacy
VEGF-121 Viral Vector
GenVec
Class II-IV angina
Collaboration with Cordis Corporation
European 125 patient trial(Summer 2004)
Corautus VEGF-2 Naked Plasmid
Class III-IV refractory Angina
Direct endocardial tissue injection
Collaboration with Boston Scientific Corporation
Phase IIB commencement expected early 2004
US trial > 400 patients
17

Company Highlights
18
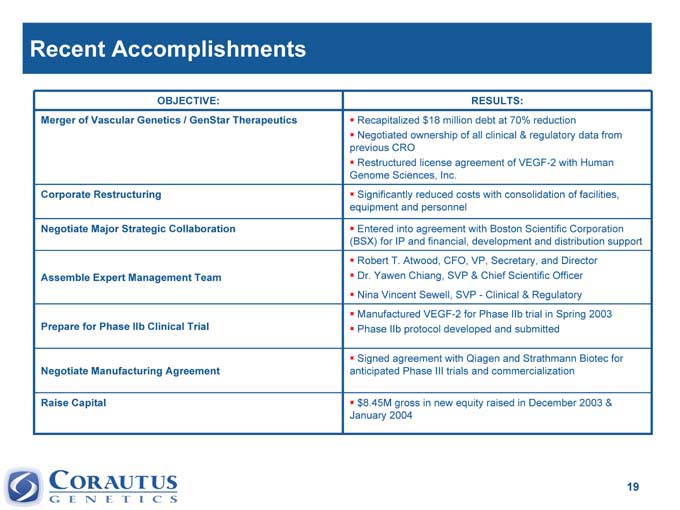
Recent Accomplishments
OBJECTIVE: RESULTS:
Merger of Vascular Genetics / GenStar Therapeutics Recapitalized $18 million debt at 70% reduction
Negotiated ownership of all clinical & regulatory data from
previous CRO
Restructured license agreement of VEGF-2 with Human
Genome Sciences, Inc.
Corporate Restructuring Significantly reduced costs with consolidation of facilities,
equipment and personnel
Negotiate Major Strategic Collaboration Entered into agreement with Boston Scientific Corporation
(BSX) for IP and financial, development and distribution support
Robert T. Atwood, CFO, VP, Secretary, and Director
Assemble Expert Management Team Dr. Yawen Chiang, SVP & Chief Scientific Officer
Nina Vincent Sewell, SVP -Clinical & Regulatory
Manufactured VEGF-2 for Phase IIb trial in Spring 2003
Prepare for Phase IIb Clinical Trial Phase IIb protocol developed and submitted
Signed agreement with Qiagen and Strathmann Biotec for
Negotiate Manufacturing Agreement anticipated Phase III trials and commercialization
Raise Capital 8.45M gross in new equity raised in December 2003 & $
January 2004
19
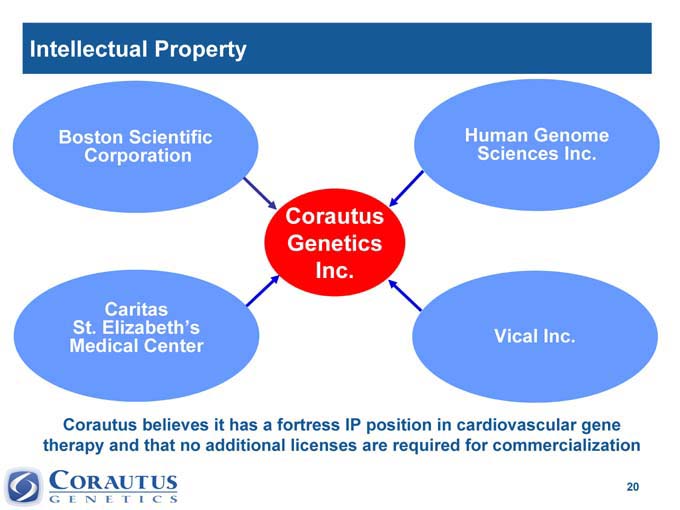
Intellectual Property
Boston Scientific Human Genome
Corporation Sciences Inc.
Corautus
Genetics
Inc.
Caritas
St. Elizabeth’s Vical Inc.
Medical Center
Corautus believes it has a fortress IP position in cardiovascular gene therapy and that no additional licenses are required for commercialization
20
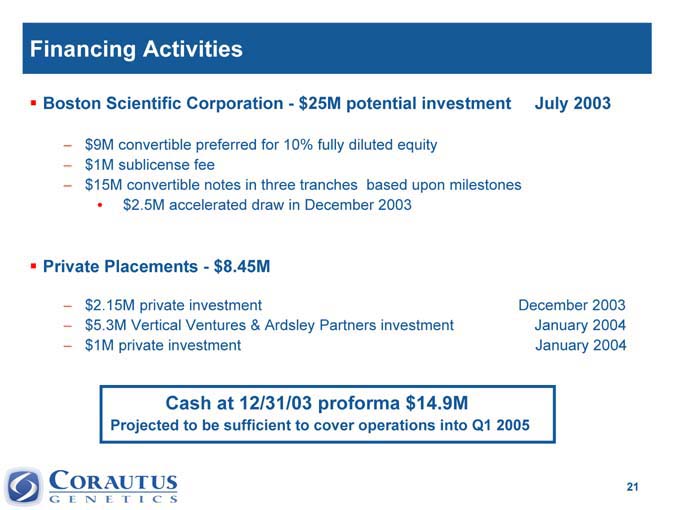
Financing Activities
Boston Scientific Corporation—$25M potential investment July 2003
?? 9M convertible preferred for 10% fully diluted equity
?? 1M sublicense fee
?? 15M convertible notes in three tranches based upon milestones
•$ 2.5M accelerated draw in December 2003
Private Placements—$8.45M
$2.15M private investment December 2003
$5.3M Vertical Ventures & Ardsley Partners investment January 2004
$1M private investment January 2004
Cash at 12/31/03 proforma $14.9M
Projected to be sufficient to cover operations into Q1 2005
21
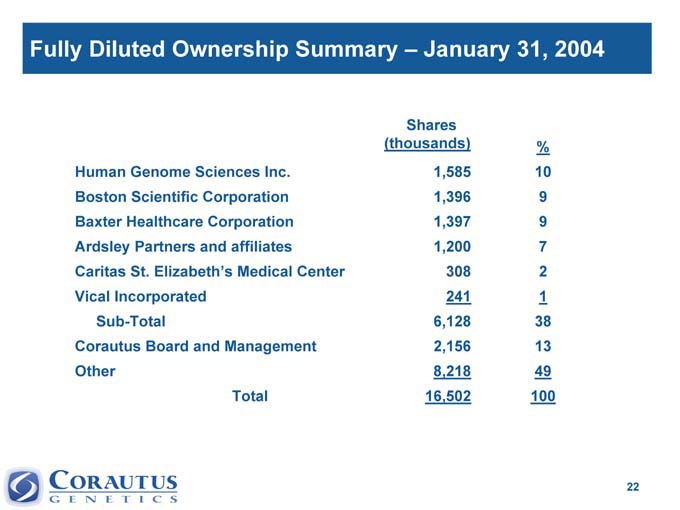
Fully Diluted Ownership Summary – January 31, 2004
Shares
(thousands) %
Human Genome Sciences Inc. 1,585 10
Boston Scientific Corporation 1,396 9
Baxter Healthcare Corporation 1,397 9
Ardsley Partners and affiliates 1,200 7
Caritas St. Elizabeth’s Medical Center 308 2
Vical Incorporated 241 1
Sub-Total 6,128 38
Corautus Board and Management 2,156 13
Other 8,218 49
Total 16,502 100
22
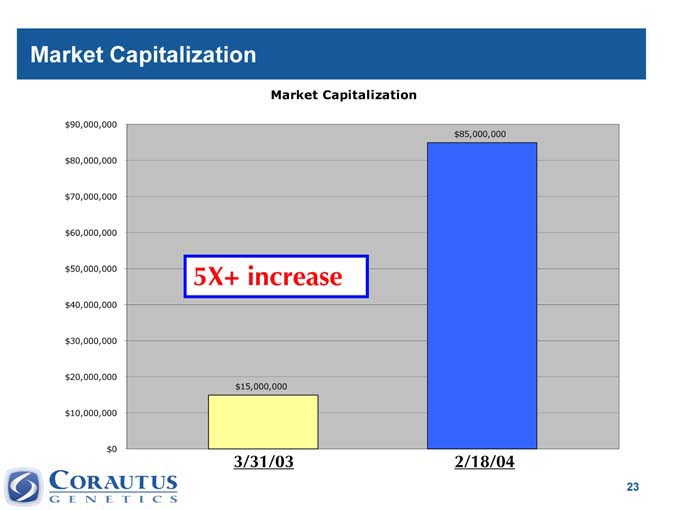
Market Capitalization
Market Capitalization
5X+ increase $15,000,000
3/31/03 $85,000,000
2/18/04
23

Next Steps
24

Management Goals 2004
Commence and Manage Phase IIB clinical trial
Initiate phase III-commercial product manufacturing
Efficiently deploy capital by controlling costs
Secure additional funding for 2005 and beyond
Focus R&D efforts on later-stage opportunities
Consider Investigational New Drug Application (IND) for Peripheral Artery Disease (PAD)
Achieve another year of significant valuation increase
25

Conclusion
26

Conclusion
“Later-stage” therapeutic serving an unmet medical condition
Support of key opinion leaders in cardiology
$1 billion estimated US annual market opportunity for Refractory CAD
Major collaboration agreement with Boston Scientific Corporation
Fortress of intellectual property
Recently strengthened cash position: $14.9M (12/31/03 pro forma)
Increased market capitalization from $15M at 3/31/03 to $85M in 2/04
Experienced and focused management team with proven record to efficiently enhance shareholder value
27

Conclusion
2003—Emerged as a Strong Survivor
2004—Significant Event-Driven Valuation Opportunities
28

75 Fifth Street, NW
Suite 313 Atlanta, GA 30308 404.526.6200 www.corautus.com
Richard E. Otto Robert T. Atwood
President and CEO Executive VP and CFO
29




























