
Annual Stockholders Meeting
May 7, 2004
Richard E. Otto
President and Chief Executive Officer
1

Forward-Looking Statements
This presentation may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements are subject to certain factors, risks and uncertainties that may cause actual results, events and performances to differ materially from those referred to in such statements. These risks include statements which address operating performance, events or developments that we expect or anticipate will occur in the future, such as projections about our future results of operations or our financial condition, benefits from the alliance with Boston Scientific, research, development and commercialization of our product candidates, anticipated trends in our business, manufacture of sufficient and acceptable quantities of our proposed products, approval of our product candidates, meeting additional capital requirements, and other risks that could cause actual results to differ materially. These risks are discussed in Corautus Genetics Inc.’s Securities and Exchange Commission filings, including, but not limited to, the risk factors in Corautus’ Annual Report on Form 10-K for the year ended December 31, 2003 (File No. 001-15833) filed March 30, 2004, which are incorporated by reference into this press release.
2

Company History
Vascular Genetics Inc.
GenStar Therapeutics Inc.
Corautus Genetics Inc.
The Merged Company – February 5, 2003
3
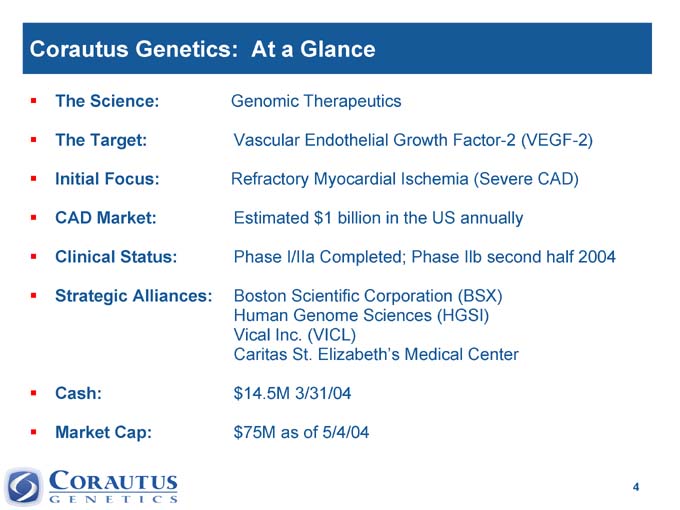
Corautus Genetics: At a Glance
The Science: Genomic Therapeutics
The Target: Vascular Endothelial Growth Factor-2 (VEGF-2)
Initial Focus: Refractory Myocardial Ischemia (Severe CAD)
CAD Market: Estimated $1 billion in the US annually
Clinical Status: Phase I/IIa Completed; Phase Ilb second half 2004
Strategic | | Alliances: Boston Scientific Corporation (BSX) Human Genome Sciences (HGSI) Vical Inc. (VICL) Caritas St. Elizabeth’s Medical Center |
Cash: $14.5M 3/31/04
Market Cap: $75M as of 5/4/04
4
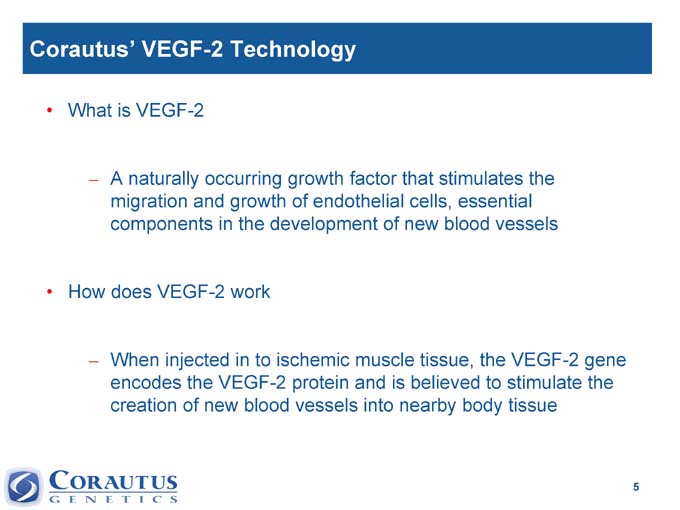
Corautus’ VEGF-2 Technology
• What is VEGF-2
– A naturally occurring growth factor that stimulates the migration and growth of endothelial cells, essential components in the development of new blood vessels
• How does VEGF-2 work
– When injected in to ischemic muscle tissue, the VEGF-2 gene encodes the VEGF-2 protein and is believed to stimulate the creation of new blood vessels into nearby body tissue
5

Jeffery M. Isner, M.D. & Vascular Genetics Inc.
‘No discussion on therapeutic angiogenesis would be appropriate without honoring the person who was clearly the leader in the field. Dr. Jeffery M. IsnerJeff was the consummate Clinician/Scientist and without question the pioneer in this area.’
Cardiology Rounds Harvard Medical School January 2002
6

The Vision – Biologic Revascularization
Conventional
Pharmacologic
Treats Symptom
Underlying condition unaltered
vs.
Regenerative
Biologic (Genetic)
Treats Cause
Underlying condition altered
Biologic revascularization may offer superior outcomes in the treatment of refractory angina due to remodeling of ischemic cardiac muscle
7

Phase Ilb Clinical Trial Design
National multi-center, double-blind, dose ranging
N = 404, four cohorts (0, 20?g, 200?g, 800?g)
Class III and IV angina “no op” patients
Boston Scientific StilettoTM Catheter
Efficacy data measured at 3 months, safety data at 1 year
15 clinical sites in the United States
Primary Endpoint – Increase in Exercise Tolerance Time (“ETT”)
Secondary Endpoint – SPECT Nuclear Imaging, Decreased angina
8

FDA Review Process of Combination Products
Center for Biologics Evaluation and Research (“CBER”)
Responsible for complete review of the entire submission
Reviews submission and refers device questions/concerns to CDRH
Reports all CBER and CDRH comments to Sponsor of IND
Dialogue ongoing—additional guidance expected in the near term
Trial can not commence until the submission is approved by the FDA
Center for Devices and Radiological Health (“CDRH”)
Reviews submission focusing on device components
Delivers comments to CBER
9
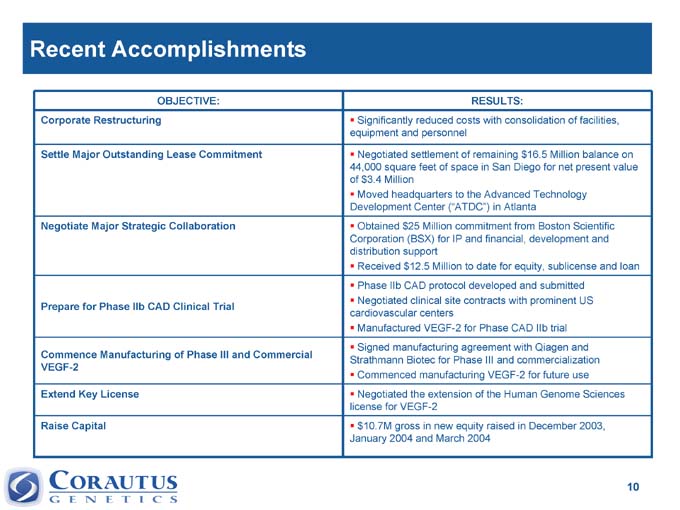
Recent Accomplishments
OBJECTIVE: RESULTS:
Corporate | | Restructuring Significantly reduced costs with consolidation of facilities, equipment and personnel |
Settle | | Major Outstanding Lease Commitment |
Negotiated | | settlement of remaining $16.5 Million balance on 44,000 square feet of space in San Diego for net present value of $3.4 Million |
Moved headquarters to the Advanced Technology Development Center (“ATDC”) in Atlanta
Negotiate Major Strategic Collaboration Obtained $25 Million commitment from Boston Scientific Corporation (BSX) for IP and financial, development and distribution support
Received $12.5 Million to date for equity, sublicense and loan
Prepare for Phase IIb CAD Clinical Trial Phase IIb CAD protocol developed and submitted
Negotiated clinical site contracts with prominent US cardiovascular centers
Commence Manufacturing of Phase III and Commercial VEGF-2 Manufactured VEGF-2 for Phase CAD IIb trial Signed manufacturing agreement with Qiagen and Strathmann Biotec for Phase III and commercialization
Commenced manufacturing VEGF-2 for future use
Extend Key License Negotiated the extension of the Human Genome Sciences license for VEGF-2
Raise Capital 10.7M gross in new equity raised in December 2003, $ January 2004 and March 2004
10
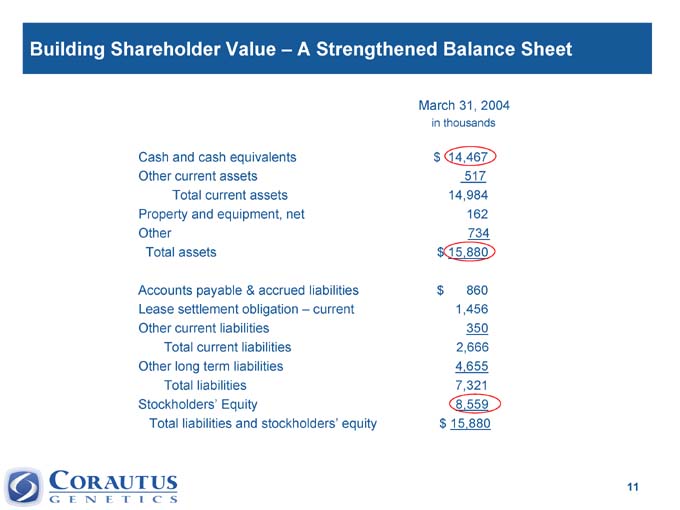
Building Shareholder Value – A Strengthened Balance Sheet
March 31, 2004
in thousands
Cash and cash equivalents $ 14,467
Other current assets 517
Total current assets 14,984
Property and equipment, net 162
Other 734
Total assets $ 15,880
Accounts payable & accrued liabilities $ 860
Lease settlement obligation – current 1,456
Other current liabilities 350
Total current liabilities 2,666
Other long term liabilities 4,655
Total liabilities 7,321
Stockholders’ Equity 8,559
Total liabilities and stockholders’ equity $ 15,880
11
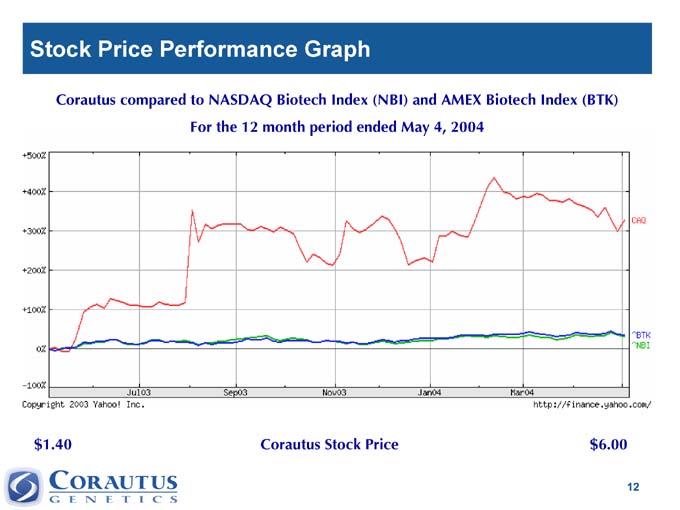
Stock Price Performance Graph
Corautus compared to NASDAQ Biotech Index (NBI) and AMEX Biotech Index (BTK)
For the 12 month period ended May 4, 2004
$1.40 Corautus Stock Price $6.00
12

Management Goals for 2004
Commence and Manage Phase IIb CAD clinical trial
Initiate phase III-commercial product manufacturing
Efficiently deploy capital by controlling costs
Secure additional funding for 2005 and beyond
Consider filing new protocol to existing Investigational New Drug Application (IND) for Peripheral Artery Disease (PAD)
Achieve another year of significant valuation increase
13

Conclusion
“Later-stage” therapeutic serving an unmet medical condition
Support of key opinion leaders in cardiology
$1 billion estimated US annual market opportunity for Refractory CAD
Major collaboration agreement with Boston Scientific Corporation
Strong intellectual property position Strengthened cash resources
Experienced and focused management team with proven record to efficiently enhance shareholder value
14













