UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 for the fiscal year ended December 31, 2006 |
OR
| [_] | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 0-27264
CORAUTUS GENETICS INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 33-0687976 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification no.) |
70 Mansell Court, Suite 100, Roswell, Georgia 30076
(Address of principal executive offices) (Zip code)
Registrant’s Telephone Number, Including Area Code:
(404) 526-6200
Securities Registered Pursuant to Section 12(b) of the Act
NONE
Securities Registered Pursuant to Section 12(g) of the Act:
| | |
Title of Class | | Name of Each Exchange Where Registered |
| Common Stock, $.001 par value | | NASDAQ Capital Market |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by checkmark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by checkmark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form-10-K. [ X ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and larger accelerated filer” in Rule 12b-2 of the Exchange Act. Check one:
Large accelerated filer ¨ Accelerated filer ¨ Non-accelerated filer x
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s outstanding common stock held by non-affiliates of the registrant as of June 30, 2006 was $11,505,467. As of March 26, 2007, there were 19,728,854 shares of the registrant’s common stock ($0.001 par value) outstanding.
Documents Incorporated by Reference: Specifically identified portions of the Proxy Statement for the 2007 Annual Meeting of Stockholders, to be filed not later than 120 days after the end of the fiscal year covered by this Report, are incorporated by reference in Part III hereof.
TABLE OF CONTENTS
CORAUTUS GENETICS INC.
(A DEVELOPMENT-STAGE ENTERPRISE)
FORM 10-K
-i-
CAUTIONARY FACTORS THAT MAY AFFECT FUTURE RESULTS
We believe it is important to communicate our expectations to investors. However, there may be events in the future that we are not able to predict accurately or that we do not fully control that could cause actual results to differ materially from those expressed or implied. This annual report and the documents incorporated by reference in this annual report may contain forward–looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements are subject to certain factors, risks and uncertainties that may cause actual results, events and performances to differ materially from those referred to in such statements. These risks include statements that address operating performance, events or developments that we expect or anticipate will occur in the future, such as such as the likelihood of identifying and securing life sciences opportunities upon which to focus our resources and the adequacy of our cash position to transition to new opportunities if found, and other risks that could cause actual results to differ materially. The forward–looking statements are based on information available to us on the date hereof, and we assume no obligation to update any such forward–looking statements.
PART I
The following discussion must be read in conjunction with, and is subject to, the risks and uncertainties set forth in “Risk Factors” in Item 1A of this report.
Recent Events
On October 30, 2006, our board of directors determined that it was in the best interests of the company and its stockholders to not conduct further clinical trials for Vascular Endothelial Growth Factor 2, or VEGF-2, for the treatment of cardiovascular and peripheral vascular disease. The board of directors authorized management to immediately reduce its cash spending and to continue identifying other life sciences technologies that it could acquire or in-license, or other life sciences opportunities. We have been actively seeking new product opportunities, as well as a merger or business combination, due to the refocus of our business away from our VEGF-2 product candidates.
On February 7, 2007, we entered into an Agreement and Plan of Merger and Reorganization with VIA Pharmaceuticals, Inc., a Delaware corporation that currently is privately-held, and our wholly owned subsidiary, Resurgens Merger Corp. Pursuant to the merger agreement, Resurgens will merge with and into VIA, with VIA surviving as a wholly-owned subsidiary of Corautus. In exchange for their VIA capital stock, VIA stockholders will receive shares of Corautus common stock. Currently, we expect that former VIA stockholders will hold approximately 78% of our common stock post-transaction. Additionally, if the transaction with VIA is consummated, Corautus’ clinical operations will focus on VIA’s product candidates.
We expect that a proxy statement for a special meeting of the stockholders will be mailed to all Corautus stockholders of record on a date to be set by our Board of Directors. Interested parties should review carefully this proxy statement when available for terms of the merger with VIA.
We expect the merger to be completed in the second quarter of 2007. If the merger is not completed, we will need to consider other alternatives, which will include liquidation and dissolution. Due to liquidation preferences of our Series C, Series D, and Series E Preferred Stock, in the event of liquidation, all cash held by Corautus would be distributed to the holders of preferred stock.
1
On May 25, 2006, we received a notice from the NASDAQ Stock Market indicating that we were not in compliance with the NASDAQ Stock Market’s requirements for continued listing because, for the previous 30 consecutive business days, the bid price of our common stock had closed below the minimum $1.00 per share requirement for continued inclusion under NASDAQ Marketplace Rule 4310(c)(4). We had until November 21, 2006 to achieve compliance with the minimum requirements for continued listing. On November 22, 2006, we received a notice from the NASDAQ Stock Market indicating that we remained in non-compliance with the NASDAQ Stock Market’s requirements for continued listing because we had failed to achieve a bid price of our common stock above $1.00 per share. However, because we met all criteria for initial listing on the NASDAQ Capital Market at that time (other than the bid price requirement), we were granted an additional 180 days, or until May 21, 2007, to regain compliance with the NASDAQ Stock Market’s requirements for continued listing.
On December 8, 2006, we received a further notification from NASDAQ that our common stock would be delisted from the NASDAQ Capital Market on December 19, 2006 unless we requested an appeal no later than December 15, 2006. In its notification, NASDAQ stated that it believes we currently are not engaged in active business operations and are therefore a “public shell,” which, in NASDAQ’s determination, could be detrimental to the interests of the investing public. Marketplace Rule 4300 provides NASDAQ with discretionary authority to apply more stringent criteria for continued listing and terminate the inclusion of particular securities based on any event that occurs, that in the opinion of NASDAQ, makes inclusion of the securities in NASDAQ inadvisable or unwarranted, even though the securities meet all enumerated criteria for continued inclusion on NASDAQ.
On December 15, 2006, Corautus appealed the NASDAQ Staff’s “public shell” determination to a NASDAQ Listing Qualifications Panel. On February 8, 2007, Corautus presented its arguments to the Qualifications Panel. During the oral hearing, we requested relief from the “public shell” determination for a 90-day period, or until May 9, 2007, in order to complete the proposed merger. On March 23, 2007, we received a decision from the Qualifications Panel providing us until April 17, 2007 to mail proxy materials to our stockholders regarding the transaction with VIA Pharmaceuticals, Inc., and until May 21, 2007 to consummate such transaction. There can be no assurance that we can meet these deadlines.
Overview
Corautus is a development stage company dedicated to the development of innovative products in the life sciences industry. Corautus, formerly known as GenStar Therapeutics Corporation and Urogen Corp., was formed as a Delaware corporation on June 30, 1995. Prior to November 1, 2006, Corautus was primarily focused on the clinical development of gene therapy products using a vascular growth factor gene, Vascular Endothelial Growth Factor 2 (VEGF-2), for the treatment of severe cardiovascular disease. Corautus was the sponsor of a Phase IIb clinical trial to study the efficacy of VEGF-2 for the treatment of severe cardiovascular disease, known as the GENASIS trial. In addition, Corautus supported initial clinical trials studying the efficacy of VEGF-2 for the treatment of peripheral artery disease and diabetic neuropathy.
Our gene therapy treatment approach was based upon the research of the late Dr. Jeffrey Isner, former Chief of Vascular Medicine and Cardiovascular Research at Caritas St. Elizabeth’s Medical Center of Boston, Inc. Our VEGF-2 product candidates were designed to induce therapeutic angiogenesis, which is the stimulation of blood vessel formation, to restore blood flow to ischemic, or oxygen deprived, muscle caused by cardiovascular or peripheral vascular disease. In this way, we sought to treat a cause of the underlying condition rather than the symptoms of the disease. Our approach to therapeutic angiogenesis involved the injection of naked plasmid DNA, or DNA that is formulated without modifying materials such as liposomes or pegylation, directly into the ischemic muscle.
2
We enrolled 30 Class III and Class IV angina patients in our Phase I clinical trial. From our investigator’s published data, a significant improvement in angina class was noted at the two-year follow-up; no patients evaluated as having Class IV angina; only three were evaluated as having Class III angina; and the remaining were evaluated as having Class I or II angina. Our 19 patient Phase I/IIa trial was a multicenter, randomized study testing VEGF-2 in two dosages (200 and 800 microgram) plus a placebo. The investigators reported a significant improvement in symptoms of angina, including an average increase in exercise tolerance time of almost two minutes in the VEGF-2 treated patients, a reduction of angina class, and a reduction in frequency of angina episodes in the VEGF-2 treated patients compared to the placebo patients.
We initiated the GENASIS trial on August 31, 2004. GENASIS was a multicenter, randomized, four-arm, double-blind study of up to 404 patients in approximately 30 institutions in the United States evaluating the efficacy and safety of three doses (20, 200, 800 micrograms) of VEGF-2 versus placebo percutaneously delivered via the Stiletto™ catheter. Prior to cessation, 295 patients were treated in the GENASIS clinical trial. The primary endpoint of the GENASIS trial was the improvement in exercise tolerance time on a treadmill 90 days following patient treatment, with additional follow-up at 6 and 12 months.
On March 14, 2006, we announced that in conjunction with Boston Scientific Corporation, we had temporarily suspended patient treatments in our GENASIS clinical trial. Boston Scientific requested the voluntary suspension as a result of three reported serious adverse events of pericardial effusion, which did not appear to be related to the VEGF-2 biologic. We notified the FDA of the voluntary suspension prior to making the public announcement, and in a subsequent teleconference, we were informed that the FDA had placed the trial on clinical hold.
As part of the process to address the clinical hold, the independent Data Monitoring Committee, or DMC, reviewed safety information. To better consider safety in the context of risk/benefit in this trial, the DMC also requested and subsequently reviewed a limited amount of available unblinded summary efficacy data related to the increase in exercise tolerance time, or ETT, which is measured by a patient’s performance on a treadmill. The DMC recommended to us that, based on available efficacy data, enrollment should be terminated under the current protocol as the DMC saw very little chance for significant efficacy as to the primary endpoint. On April 10, 2006, we announced that we had accepted the DMC’s recommendation that enrollment in the GENASIS trial be terminated and notified the FDA of the termination of enrollment.
Corautus locked the database on August 14, 2006 for the 295 patients treated in the GENASIS clinical trial. The efficacy and safety analyses performed on the database included 295 patients at 3 months, 241 patients at 6 months and 103 patients at 12 months. On October 10, 2006 Corautus announced that the final efficacy results from its GENASIS clinical trial did not achieve a statistically significant difference from placebo in any active dose group for the primary efficacy endpoint. The primary efficacy endpoint in the GENASIS clinical trial was an improvement of at least one minute in ETT from baseline to three months. The data indicated considerable overlap in results between the active and placebo groups for the secondary endpoints as well, and no clear dose effect was seen. In general, a majority of patients in all treatment arms (active and placebo) significantly improved from their baseline status in both primary and secondary efficacy endpoints; however, there was no significant separation from the placebo in any active dose group.
Collaborative Efforts and Intellectual Property
We established a portfolio of licensed patents, intellectual property rights and technologies relating to the development and use of the VEGF-2 technology for the gene therapy treatment. We do not own any of
3
the patents or patent applications relating to our core VEGF-2 technology. We hold exclusive licenses, through our wholly-owned subsidiary Vascular Genetics Inc., from Human Genome Sciences, Inc. and Vical Incorporated, and non-exclusive licenses from Caritas St. Elizabeth’s and Boston Scientific to certain U.S. patents, pending U.S. patent applications, and corresponding foreign patent applications.
In connection with the anticipated refocus of our business away from VEGF-2 and towards VIA’s product candidates, we expect to terminate all of our material agreements relating to VEGF-2.
Government Regulation
New drugs and biologics, including gene therapy products, are subject to regulation under the Federal Food, Drug, and Cosmetic Act. In addition to being subject to certain provisions of that Act, biologics are also regulated under the Public Health Service Act. We believe that any pharmaceutical products developed by us will be regulated either as biological products or as new drugs. Both statutes and their corresponding regulations govern, among other things, the testing, manufacturing, distribution, safety, efficacy, labeling, storage, record keeping, advertising and other promotional practices involving biologics or new drugs. FDA approval or other clearances must be obtained before clinical testing, manufacturing and marketing of biologics and drugs. Obtaining FDA approval has historically been a costly and time-consuming process.
In addition, any gene therapy products developed by us will require regulatory approvals prior to human trials and additional regulatory approvals prior to marketing. New human gene therapy products are subject to extensive regulation by the FDA and the Center for Biological Evaluation and Research and comparable agencies in other countries. Currently, each human-study protocol is reviewed by the FDA and, in some instances, the National Institutes of Health, on a case-by-case basis. The FDA and the National Institutes of Health have published guidance documents with respect to the development and submission of gene therapy protocols.
In order to commercialize any proposed products, we must sponsor and file an investigational new drug application and be responsible for initiating and overseeing the human studies to demonstrate the safety and efficacy and, for a biologic product, the potency, which are necessary to obtain FDA approval of any such products. For Corautus-sponsored investigational new drug applications, we will be required to select qualified investigators (usually physicians within medical institutions) to supervise the administration of the products, and we will be required to ensure that the investigations are conducted and monitored in accordance with FDA regulations and the general investigational plan and protocols contained in the investigational new drug application.
The FDA receives reports on the progress of each phase of testing, and it may require the modification, suspension, or termination of trials if an unwarranted risk is presented to patients. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA. The investigational new drug application process can thus result in substantial delay and expense. Human gene therapy products, which is the primary area in which we were seeking to develop products, are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy and potency of human gene therapy products, or that the data generated in these studies will be acceptable to the FDA to support marketing approval.
After the completion of clinical trials of a new drug or biologic product, FDA marketing approval must be obtained. If the product is regulated as a biologic, the Center for Biological Evaluation and Research will require the submission and approval, depending on the type of biologic, of either a biologic license
4
application or a product license application and an establishment license application before commercial marketing of the biologic. If the product is classified as a new drug, we must file a new drug application with the Center for Drug Evaluation and Research and receive approval before commercial marketing of the drug. In limited circumstances, such as in our trial, both divisions are involved. The new drug application or biologic license applications must include results of product development, laboratory, animal and human studies, and manufacturing information. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the new drug application or biologic license applications for filing and, even if filed, that any approval will be granted on a timely basis, if at all. In the past, new drug applications and biologic license applications submitted to the FDA have taken, on average, one to two years to receive approval after submission of all test data. If questions arise during the FDA review process, approval can take more than two years.
Notwithstanding the submission of relevant data, the FDA may ultimately decide that the new drug application or biologic license application does not satisfy its regulatory criteria for approval and require additional studies. In addition, the FDA may condition marketing approval on the conduct or specific post-marketing studies to further evaluate safety and effectiveness. Rigorous and extensive FDA regulation of pharmaceutical products continues after approval, particularly with respect to compliance with current good manufacturing practices, or “GMPs,” reporting of adverse effects, advertising, promotion and marketing. Discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
Ethical, social and legal concerns about gene therapy, genetic testing and genetic research could result in additional regulations restricting or prohibiting the processes we or our suppliers may use. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. More restrictive regulations or claims that our products are unsafe or pose a hazard could prevent us from commercializing any products.
In addition to the foregoing, state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservancy and Recovery Act and the Toxic Substances Control Act, affect our business. These and other laws govern our use, handling and disposal of various biological, chemical and radioactive substances used in, and wastes generated by, our operations. If our operations result in contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and governmental fines. We believe that we are in material compliance with applicable environmental laws and that continued compliance therewith will not have a material adverse effect on our business. We cannot predict, however, how changes in these laws may affect our future operations.
Competition
The pharmaceutical and biotechnology industries are intensely competitive. Any product candidate developed by us would compete with existing drugs and therapies and with others under development. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in research and development of products for the treatment of cardiovascular and vascular disease. Many of these organizations have financial, technical, research, clinical, manufacturing and marketing resources that are greater than ours. If a competing company develops or acquires rights to a more efficient, more effective, or safer competitive therapy for treatment of the same diseases we have targeted, or one that offers significantly lower costs of treatment, our business, financial condition and results of operations could be materially adversely affected. We believe that the most significant competitive factor in the gene therapy field is the effectiveness and safety of a product due to the relatively early stage of the industry.
5
We believe that our business will be subject to significant competition from companies using alternative technologies, as well as to increasing competition from companies that develop and apply technologies similar to ours. Other companies may succeed in developing products earlier than we do, obtaining approvals for these products from the FDA more rapidly than we do or developing products that are safer and more effective than those under development or proposed to be developed by us. We cannot assure you that research and development by others will not render our technology or potential products obsolete or non-competitive or result in treatments superior to any therapy developed by us, or that any therapy developed by us will be preferred to any existing or newly developed technologies.
Research and Development Expenses
Research and development expenses totaled approximately $9,614,000, $15,949,000, and $6,593,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
Employees
During the fourth quarter of 2006, in connection with our board of directors’s determination to reduce cash spending, we significantly reduced our workforce. We currently have two employees, both in the administrative operations. In addition, we have consulting arrangements with three of our former officers.
Available Information
A copy of this Annual Report on Form 10-K, as well as our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to these reports, are available free of charge on the Internet at our website, www.corautus.com, as soon as reasonably practicable after we electronically file these reports with, or furnish these reports to, the Securities and Exchange Commission. The reference to our website address does not constitute incorporation by reference of the information contained on the website and the information on our website should not be considered part of this document. Copies of any materials we file with, or furnish to, the SEC can also be obtained free of charge through the SEC’s website at http//www.sec.gov or at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
You should carefully consider the risks described below before making an investment decision. You should also refer to the other information in this annual report, including the information incorporated by reference into this annual report. The risks and uncertainties we describe below are those that we currently believe may materially affect us. Additional risks and uncertainties that we are unaware of or that we currently deem immaterial also may become important factors that affect us.
Risks Related to the Proposed Transaction with VIA
The number of shares that VIA stockholders will be entitled to receive at closing of the merger is dependent upon the net cash balance of Corautus at closing. The lesser the amount of Corautus’ net cash at closing, the greater the number of shares of Corautus common stock VIA stockholders will be entitled to receive in the merger and the more the present ownership in Corautus will be diluted.
As of the date of this report and assuming the merger closes in the second quarter of 2007, Corautus anticipates having approximately $11.25 million of net cash at closing. As a result, Corautus currently
6
anticipates that holders of VIA’s equity securities will be entitled to receive in the aggregate approximately 124,279,574 shares of Corautus common stock and options to purchase Corautus common stock at closing and will own approximately 77.59% of the capital stock of the combined company (on a fully-diluted basis), with current Corautus equityholders owning approximately 22.41% of the capital stock of the combined company (on a fully-diluted basis) at closing. The merger agreement provides that the exchange ratio is subject to upward and downward adjustment based on the net cash balance of Corautus (as calculated pursuant to the merger agreement) at the closing of the merger and the aggregate fully-diluted number of shares of each of Corautus and VIA outstanding immediately prior to the closing of the merger. If the net cash balance of Corautus at the closing of the merger is below $11.25 million, the exchange ratio will be adjusted to increase the number of shares of Corautus common stock that holders of VIA’s equity securities will be entitled to receive pursuant to the merger, which would further dilute current Corautus stockholders’ ownership in the combined company. If the net cash balance of Corautus at the closing of the merger is below $11.0 million, Corautus would be unable to satisfy a closing condition for the merger, and VIA may elect not to consummate the merger. If the net cash balance of Corautus at the closing of the merger is greater than $11.25 million, the exchange ratio will be adjusted to decrease the number of shares of Corautus common stock that holders of VIA’s equity securities will be entitled to receive pursuant to the merger, which would dilute current VIA equity holders’ ownership in the combined company. The items that will constitute Corautus’ net cash balance at the closing of the merger are subject to many factors, many of which are outside of Corautus’ control. The following table sets forth the approximate percentage ownership of Corautus that VIA equity holders and current Corautus equity holders would be expected to hold immediately following the closing of the merger, assuming net cash balances of Corautus at the closing of the merger of $11.0 million, $11.25 million, $11.5 million, $11.75 million, $12.0 million, $12.25 million and $12.5 million.
| | | | | | |
Corautus’ Net Cash Balance at Closing | | Corautus Stockholders’
Approximate
Ownership Percentage
in the Combined
Company at Closing | | | VIA Stockholders’
Approximate Ownership Percentage
in the Combined
Company at Closing | |
$11,000,000 | | 22.02 | % | | 77.98 | % |
$11,250,000 | | 22.41 | % | | 77.59 | % |
$11,500,000 | | 22.79 | % | | 77.21 | % |
$11,750,000 | | 23.17 | % | | 76.83 | % |
$12,000,000 | | 23.55 | % | | 76.45 | % |
$12,250,000 | | 23.92 | % | | 76.08 | % |
$12,500,000 | | 24.29 | % | | 75.71 | % |
The ownership percentages set forth above represent ownership percentages of Corautus at the time of closing of the merger. Corautus will require substantial additional funding shortly after consummation of the merger. Any such financing will further dilute ownership interest in Corautus.
Because the lack of a public market for VIA’s capital stock makes it difficult to evaluate the fairness of the merger, the stockholders of VIA may receive consideration in the merger that is greater than or less than the fair market value of VIA’s capital stock.
The outstanding capital stock of VIA is privately-held and is not traded in any public market. The lack of a public market makes it extremely difficult to determine the fair market value of VIA. Since the percentage of Corautus equity to be issued to VIA stockholders was determined based on negotiations between the parties, it is possible that the value of Corautus common stock to be issued to VIA stockholders in connection with the merger will be greater than the fair market value of VIA. Alternatively, it is possible that the value of the shares of Corautus common stock to be issued in connection with the merger will be less than the fair market value of VIA.
7
The exchange ratio is not adjustable based on the market price of Corautus common stock and if the market price of Corautus common stock increases, the aggregate value of the shares of Corautus common stock that VIA stockholders will be entitled to receive pursuant to the merger could be significantly higher.
The merger agreement has set the exchange ratio for the VIA capital stock and such exchange ratio is only adjustable upward or downward depending upon Corautus’ net cash balance, as calculated pursuant to the merger agreement, at the closing of the merger, not the market price of Corautus common stock. Accordingly, any changes in the market price of Corautus common stock will not affect the number of shares that VIA stockholders will be entitled to receive pursuant to the merger.
Some of Corautus’ officers and directors have interests in the merger that may be different from yours and may influence them to support the merger without regard to your interests.
Certain officers and directors of Corautus participate in arrangements that provide them with interests in the merger that may be different from yours, including, among others, severance benefits, the acceleration of stock and stock option vesting and continued indemnification. These interests, among others, may influence the officers and directors of Corautus to support or approve the merger.
Failure to complete the merger may result in Corautus paying a termination fee to VIA and could harm Corautus’ future business and operations and cause the price of Corautus’ common stock to decline.
If the merger is not completed, Corautus may be subject to the following risks:
| • | | if the merger agreement is terminated, under certain circumstances Corautus will be required to pay VIA a termination fee of $425,000, plus an amount equal to VIA’s reasonable documented out-of-pocket expenses incurred in connection with the merger agreement up to $425,000; |
| • | | the price of Corautus stock may decline; and |
| • | | our costs related to the merger, such as legal, accounting and certain financial advisory fees, must be paid, even if the merger is not completed. |
In addition, if the merger agreement is terminated and Corautus’ or VIA’s board of directors determines to seek another business combination, there can be no assurance that Corautus will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided by VIA in the merger. In such circumstances, Corautus’ board of directors may elect to attempt to complete another strategic transaction like the merger or take the steps necessary to liquidate all of Corautus’ remaining assets, and in such cases, the consideration that Corautus receives for its remaining assets may be less attractive than the consideration to be received by Corautus’ stockholders pursuant to the merger agreement.
If the conditions to the merger are not met, the merger may not occur.
Even if the necessary approvals are obtained from the stockholders of Corautus and VIA, specified conditions must be satisfied or waived for the merger to be consummated. These conditions include, among others, Corautus having a net cash balance of at least $11.0 million at closing, and are described in detail in the merger agreement. We cannot assure you that all of the conditions will be satisfied. If the conditions are not satisfied or waived, the merger will not occur or will be delayed, and we may lose some or all of the intended benefits of the merger.
8
VIA is a development stage company subject to risks related to, among others, technology efficacy, intellectual property and financing. Because VIA’s business will constitute the business of the combined company after the closing of the merger, if any negative events occur with respect to VIA’s technology, intellectual property, or financing, those events could cause the potential benefits of the merger not to be realized.
Corautus is in the process of winding-down its operations related to its terminated GENASIS Phase II trial, and, as a result, the business of the combined company immediately following the merger will be the business conducted by VIA immediately prior to the merger. As a result, if a negative event should occur with respect to VIA’s technology or intellectual property, those events could cause the potential benefits of the merger not to be realized, a difficulty in identifying and obtaining financing, and the market price of Corautus’ common stock to decline.
Risks Related to Our Business
If the merger with VIA is not completed, Corautus may not have continuing business operations.
Our board of directors has authorized the termination of our operations and clinical trials related to VEGF-2, which was our only product candidate. Additionally, we are in the process of terminating many of our intellectual property license agreements. Should we not complete the merger with VIA, we may not have any ongoing business operations, and our board of directors will need to consider other alternatives, including liquidation and dissolution.
Corautus’ business to date has been largely dependent on the success of VEGF-2 in the treatment of cardiovascular and vascular disease, which were the subjects of the Phase IIb GENASIS clinical trial that we terminated in 2006. Although we ceased the development of VEGF-2 and have reduced our workforce, we may be unable to successfully manage our remaining resources, including available net cash, while we seek to wind-down operations related to VEGF-2 and implement the merger with VIA.
Corautus’s Phase IIb GENASIS clinical trial testing the safety and efficiency of VEGF-2 in patients with cardiovascular and vascular disease was discontinued during 2006 based upon the recommendation of the independent Data and Safety Monitoring Board, or DSMB, with oversight responsibility for these clinical trials. The DSMB concluded that the clinical trial data were unlikely to provide significant evidence of achievement of the primary endpoint for VEGF-2-treated patients versus patients who received placebo. We had previously devoted substantially all of our research, development and clinical efforts and financial resources to the development of VEGF-2. In connection with the termination of our clinical trial for VEGF-2, we announced a realignment of our business activities, including significant workforce reductions, and incurred approximately $1.4 million of severance and related costs in 2006, the substantial majority of which were cash expenditures. As a result of the discontinuation of our clinical trials, we are in the process of terminating many of our remaining intellectual property license agreements, development programs and manufacturing operations for VEGF-2. We may be unable to successfully reduce expenses associated with our existing manufacturing agreement, intellectual property agreements, clinical trial agreements and other commitments related to VEGF-2.
If the merger with VIA is not consummated and we were to liquidate and dissolve, no distributions would be made to common stockholders.
If the merger with VIA is not completed and we were to liquidate and dissolve, we cannot predict when, or if, we would be able to make a distribution to our stockholders. However, our Series C Preferred Stock, Series D Preferred Stock, and Series E Preferred Stock have preferences to distributions upon liquidation that exceed the amount of cash now available, and we anticipate that upon liquidation all of our assets would be distributed to the holders of our preferred stock, and the holders of common stock would receive no distributions.
9
If the merger with VIA is not consummated, we will need substantial additional funding to identify and develop product candidates or life science opportunities and for our future operations.
In the event the merger with VIA is not consummated, we will need to identify other business opportunities or develop other product candidates, which will require a commitment of substantial funds to conduct the costly and time-consuming research and due diligence, pre-clinical and clinical testing necessary to obtain regulatory approvals and bring our product candidates to market. We will need to raise substantial additional capital to fund our future operations. We cannot be certain that additional financing will be available on acceptable terms, or at all. To the extent we raise additional funds by issuing equity securities, our existing stockholders will be diluted. If additional funds are raised through the issuance of debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and preferred stock. Moreover, we may enter into financing transactions at prices that represent a substantial discount to market price. A negative reaction by investors and securities analysts to any discounted sale of our equity securities could result in a decline in the market price of our common stock.
If the merger with VIA is not consummated, we may continue to incur the expense of complying with public company reporting requirements.
We have an obligation to continue to comply with the applicable reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, even though compliance with such reporting requirements is economically burdensome. If the merger with VIA is not completed and we were to liquidate and dissolve, then to curtail such expenses, we might seek relief from the Securities and Exchange Commission, or the SEC, for a substantial portion of the periodic reporting requirements under the Exchange Act after filing our certificate of dissolution upon stockholder approval of a plan of liquidation. There can be no assurance that we would be able to obtain such relief and that we would not deplete our cash in an effort to comply.
We may not have the staff or infrastructure necessary to develop or exploit life science opportunities or product candidates that we may identify.
On October 30, 2006, our board of directors determined that it was in the best interests of the company to significantly reduce our workforce. That workforce has now been reduced to two employees and one part-time consultant. Identifying life sciences opportunities, conducting clinical trials, and obtaining development, manufacturing and distribution partners requires knowledgeable and experienced employees as well as a developed infrastructure. In the event the merger with VIA is not consummated, we may not have sufficient staff or infrastructure to identify new life sciences opportunities or develop and commercialize product candidates based upon such life science opportunities, and we may be unsuccessful in outsourcing the work that could have been performed by employees.
We have substantially reduced our workforce as part of our wind-down of operations.
During 2006, we significantly reduced our work-force to two employees and one part-time consultant. If the merger with VIA is not completed, it may be difficult for us to identify new business opportunities or efficiently implement the staged wind-down of our business.
We are highly dependent on key management personnel.
We are highly dependent on the principal members of our management staff, particularly our President and Chief Executive Officer and Chief Financial Officer. Our President and Chief Executive Officer ceased being a full-time employee on January 1, 2007 and serves as a part-time consultant. As a result of the termination of our VEGF-2 business, it may be difficult to adequately incent our remaining two
10
employees to continue working for us. The loss of either of these employees or our consultant could have a material adverse effect on our ability to identify new business opportunities if the merger with VIA is not consummated.
We may be unable to maintain our listing on the NASDAQ Capital Market. Failure to maintain our listing could adversely affect our business, and the liquidity of our common stock and stock price would be negatively affected.
Our common stock is currently traded on the NASDAQ Capital Market. To maintain a listing on NASDAQ, we must maintain minimum listing requirements, including certain levels of stockholders’ equity, market capitalization and minimum bid price for our common stock. Currently, we do not satisfy certain of these requirements. Additionally, NASDAQ may consider, among other things, the nature of the business, financial integrity and future outlook of a company in considering whether to de-list a company. If our common stock is de-listed from NASDAQ, it would reduce the liquidity of our common stock, and could decrease the market price of our common stock and negatively impact our ability to obtain additional capital.
We are a development stage company with a history of insignificant revenues and significant net losses. We currently do not have a lead product candidate in clinical trials. We expect continued net losses for the foreseeable future, and we may never become profitable.
We are a development stage company, and we have not yet generated significant revenues. From our inception in July 1991 to December 31, 2006, we have incurred net losses of approximately $125 million, including net losses of approximately $15 million in 2006, almost all of which consisted of research and development, clinical trials and general and administrative expenses. Our board of directors determined in the fourth quarter of 2006 that it was in our best interest and the best interest of our stockholders at the present time to not conduct further clinical trials of VEGF-2 for the treatment of cardiovascular and peripheral vascular disease, and we instead are redirecting our focus to other life science opportunities. In addition, we do not expect to generate revenues from sales for a number of years, if at all. As a result, we expect our net losses from operations to continue for the foreseeable future.
Our ability to generate revenues and become profitable will depend on our ability, alone or with collaborators, to timely, efficiently and successfully identify and develop product candidates, conduct pre-clinical and clinical tests, obtain necessary regulatory approvals, and manufacture and market product candidates. We may not be successful in identifying and securing life sciences opportunities with commercial promise. We may never generate profits and, even if we do achieve profitability, we cannot predict the level or sustainability of such profitability.
We currently do not have a product candidate.
We previously have focused our operations on clinical trials related to VEGF-2 product candidates. Because of our board of directors’ determination to cease VEGF-2 clinical trials, we currently do not have a product candidate. Therefore, we will need to identify new technology in order to have a product candidate. Failure to develop a product candidate will significantly limit our ability to generate revenue.
Any product candidate requires additional research, development, testing and regulatory approvals prior to marketing. If any product candidates are delayed or fail, our financial condition will be negatively affected, and we may have to curtail or cease our operations.
We are in the early stage of product development. We currently do not sell any products, do not have any product candidates, and do not expect to have any product candidates commercially available for several years, if at all. Any proposed product candidates require additional research and development, clinical testing and regulatory clearances prior to marketing. There are many reasons that proposed product candidates may fail or not advance beyond clinical testing, including the possibility that:
| | • | | proposed product candidates may be ineffective, unsafe or associated with unacceptable side effects; |
11
| | • | | proposed product candidates may fail to receive necessary regulatory approvals or otherwise fail to meet applicable regulatory standards; |
| | • | | proposed product candidates may be too expensive to develop, manufacture or market; |
| | • | | physicians, patients, third-party payers or the medical community in general may not accept or use proposed product candidates; |
| | • | | collaborators may withdraw support for or otherwise impair the development and commercialization of proposed product candidates; |
| | • | | other parties may hold or acquire proprietary rights that could prevent us or our collaborators from developing or marketing proposed product candidates; or |
| | • | | others may develop equivalent or superior products candidates. |
In addition, proposed product candidates are subject to the risks of failure inherent in the development of medical therapy products based on innovative technologies. As a result, we are not able to predict whether our research, development and testing activities will result in any commercially viable products or applications. If product candidates are delayed or we fail to successfully identify, develop and commercialize product candidates, our financial condition may be negatively affected, and we may have to curtail or cease our operations.
Because we cannot predict whether or when we will obtain regulatory approval to commercialize any product candidates, we cannot predict the timing of any future revenue from these product candidates.
We cannot commercialize any product candidates to generate revenue until the appropriate regulatory authorities have reviewed and approved the applications for the product candidates. We cannot assure you that the regulatory agencies will complete their review processes in a timely manner or that we will obtain regulatory approval for any product candidate we or our collaborators develop. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Outside the United States, the ability to market a product is also contingent upon receiving clearances from appropriate foreign regulatory authorities. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. If the delays are significant, they would negatively affect our financial results, ability to raise capital and our commercial prospects for future product candidates.
We may not successfully establish and maintain collaborative and licensing arrangements, which could adversely affect our ability to develop and commercialize product candidates. We are also limited by the collaboration and marketing arrangements on which we rely.
Our strategy for the development, testing, manufacturing and commercialization of product candidates relies on establishing and maintaining collaborations with corporate partners, licensors and other third parties. We may not be able to maintain or expand these licenses and collaborations or establish additional licensing and collaboration arrangements necessary to develop and commercialize product candidates. Even if we are able to maintain or establish licensing or collaboration arrangements, these arrangements may not be on favorable terms. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize product candidates.
12
We rely on third parties to manufacture any product candidates. There can be no guarantee that we can obtain sufficient and acceptable quantities of any product candidates on acceptable terms, which may delay or impair our ability to develop, test and market such product candidates.
We currently do not have any manufacturing capabilities. Our business strategy relies on third parties to manufacture and produce any product candidates. These third party manufacturers are subject to extensive government regulation and must receive FDA approval before they can produce clinical material or commercial product. Regulatory difficulties experienced by others could affect our ability to obtain products when needed. Any product candidates may be subject to delays if third party manufacturers give other products greater priority than our product candidates. These third parties may also not deliver sufficient quantities of our product candidates, manufacture our product candidates in accordance with specifications, or comply with applicable government regulations. Additionally, if the manufactured product candidates fail to perform as specified, our business and reputation could be severely impacted.
We depend on clinical trial arrangements with medical institutions to advance our technology, and the loss of these arrangements could impair the development of product candidates.
We may rely upon medical institutions for the conduct of any clinical trials. The early termination of any of these clinical trial arrangements or the failure of these institutions to comply with the regulations and requirements governing clinical trials would hinder the progress of our clinical trial program. If any of these relationships are terminated, the clinical trial might not be completed, and the results might not be able to be evaluated.
If we are unable to maintain agreements with third parties to perform sales, marketing and distribution functions, we will be required to develop these capabilities to commercialize any proposed product candidates.
We currently have no sales, marketing or distribution capabilities. Therefore, to commercialize any product candidates, if and when such products have been approved and are ready for marketing, we must collaborate with third parties to perform these functions. We cannot assure you that we will be able to enter into arrangements with companies to commercialize any product candidates. We have no experience in developing, training or managing a sales force, and we will incur substantial additional expenses if we are forced to market our product candidates directly. Developing a marketing and sales force is also time consuming and could delay launch of our product candidates. In addition, we will compete with many companies that currently have extensive and well-funded marketing and sales operations. Our marketing and sales efforts may be unable to compete successfully against these companies.
If we do not comply with applicable regulatory requirements in the manufacture and distribution of product candidates, we may incur penalties that may inhibit our ability to commercialize our product candidates and adversely affect our revenue.
Our failure or the failure of our collaborators or third party manufacturers to comply with applicable FDA or other regulatory requirements including manufacturing, quality control, labeling, safety surveillance, promoting and reporting may result in criminal prosecution, civil penalties, recall or seizure of our product candidates, total or partial suspension of production or an injunction, as well as other regulatory action against our product candidates or us. Discovery of previously unknown problems with a product, supplier, manufacturer or facility may result in restrictions on the sale of our product candidates, including a withdrawal of the product candidates from the market.
13
We are exposed to potential risks resulting from Section 404 of the Sarbanes-Oxley Act of 2002.
We are evaluating our internal controls to allow management to report on, and our independent registered certified public accounting firm to attest to, our internal controls, as required by Section 404 of the Sarbanes-Oxley Act of 2002. We may encounter unexpected delays in implementing the requirements relating to internal controls; therefore, we cannot be certain about the timing of completion of our evaluation, testing and remediation actions or the impact that these activities will have on our operations since there is no precedent available by which to measure the adequacy of our compliance. We also expect to incur additional expenses and diversion of management’s time as a result of performing the system and process evaluation, testing and remediation required in order to comply with the management certification and independent registered certified public accounting firm attestation requirements. If we are not able to timely comply with the requirements set forth in Section 404, we might be subject to sanctions or investigation by regulatory authorities. Any such action could adversely affect our business and financial results. The requirement to comply with Section 404 of the Sarbanes-Oxley Act of 2002 is expected to become effective, at the earliest, for our fiscal year ending December 31, 2007.
In addition, in our system of internal controls we may rely on the internal controls of third parties. In our evaluation of our internal controls, we will consider the implication of our reliance on the internal controls of third parties. Until we have completed our evaluation, we are unable to determine the extent of our reliance on those controls, the extent and nature of the testing of those controls, and remediation actions necessary where that reliance cannot be adequately evaluated and tested.
Risks Related to Intellectual Property
If our right to use intellectual property we license from third parties is terminated or adversely affected, our financial condition, operations or ability to develop and commercialize any proposed product candidates will be materially harmed.
We do not own any patents and have not filed any patent applications. We may rely on licenses to use certain technologies that are material to our operations, both to have freedom to carry out our business and to protect our market from competitors. The success of our operations will depend in part on our ability and that of our licensors to:
| | • | | obtain patent protection for our methods of gene therapy, therapeutic genes and/or gene-delivery methods both in the United States and in other countries with substantial markets; |
| | • | | defend patents once obtained against third party infringers; |
| | • | | maintain trade secrets and operate without infringing patents and proprietary rights of others; and |
| | • | | obtain and maintain appropriate licenses upon reasonable terms to patents or proprietary rights held by others that are necessary or useful to us in commercializing our technology, both in the United States and in other countries with substantial markets. |
In addition, the license agreements may include certain milestones that we must meet in order to maintain these licenses. There is no assurance that we can meet such upcoming milestones in either license or that we can obtain any necessary extensions of the milestones in the future. Our licensors may terminate these licenses if we fail to meet the applicable milestones.
If we or our licensors are not able to obtain and maintain adequate patent protection for our product candidates, we may be unable to commercialize our product candidates or to prevent others from using our technology in competitive products.
14
Our success will depend in part on our ability to obtain patent protection both in the United States and in other countries for our product candidates and their use. We have no patents in our own name. We have, however, licensed patents and patent applications and other proprietary rights from third parties that cover our product candidates. Our ability to protect our product candidates from unauthorized or infringing use by third parties depends at least initially on the willingness and cooperation of our licensors to assert the patent rights licensed to us. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering biotechnology inventions and the scope of claims made under these patents, the ability of our licensors to obtain and enforce patents is uncertain and involves complex legal and factual considerations. Accordingly, rights under any issued patents may not provide us with sufficient protection for our product candidates or provide sufficient protection to afford us a commercial advantage against our competitors or their competitive products or processes. In addition, we cannot guarantee that any patents will be issued from any pending or future patent applications licensed to us. Even if issued, we cannot guarantee that the claims of these patents are, or will be, valid or enforceable, or provide us with any significant protection against competitive products or otherwise be commercially valuable to us. In addition, others may challenge, seek to invalidate, infringe or circumvent any patents or patent applications we license, and rights we receive under those patents or patent applications may not provide competitive advantages to us. Further, the manufacture, use or sale of our product candidates may infringe the patent rights of others.
We may not have identified all patents, published applications or published literature that affect our business either by blocking our ability to commercialize our drugs, by negatively impacting the patentability of our product candidates to our licensees, or by disclosing the same or similar technologies on which basis our licensors’ patents may not be valid, limit the scope of our or our licensors’ future patent claims or adversely affect our ability to market our product candidates. For example, patent applications in the United States are maintained in confidence for up to 18 months after the filing of their earliest priority application. In some cases, however, patent applications, such as those filed prior to November 29, 2000, remain confidential in the United States Patent and Trademark Office, or USPTO, for the entire time prior to issuance as a U.S. patent. Similarly, patent applications filed in foreign countries are typically published 18 months after the filing date of their earliest priority application. Publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Therefore, we cannot be certain that our licensors were the first to invent, or the first to file, patent applications covering our product candidates or their use. In the event that a third party has also filed a U.S. patent application covering our product candidates or a similar invention, we may have to participate in an adversarial proceeding, known as an interference, in the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and our efforts could be unsuccessful, resulting in a loss of our U.S. patent position. The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we or our licensors encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
We may not have adequate protection for our unpatented proprietary information, which could adversely affect our competitive position.
We also rely on unpatented trade secrets and know-how to maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with employees, consultants and others. These parties may breach or terminate these agreements, and we may not have adequate remedies for any breach. Our trade secrets may also be independently discovered by competitors. We rely on certain technologies to which we do not have exclusive rights or which may not be patentable or proprietary and thus may be available to competitors.
15
Risks Related to Our Industry
Negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may adversely affect our ability to conduct our business or obtain regulatory approvals for our product candidates.
Ethical, social and legal concerns about gene therapy and genetic research could result in additional regulations restricting or prohibiting the products and processes we may use. Even with the requisite approvals, the commercial success of any product candidates will depend in part on public acceptance of the use of such therapies for the treatment of human disease. Public attitudes may be influenced by claims that gene therapy is unsafe, and gene therapy may not gain the acceptance of the public or the medical community. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates.
We are subject to significant government regulation with respect to our product candidates. Compliance with government regulation can be a costly and time-consuming process, with no assurance of ultimate regulatory approval. If these approvals are not obtained, we will not be able to sell our product candidates.
We and our collaborators are subject to extensive and rigorous government regulation in the United States and foreign countries. The FDA, the National Institutes of Health and comparable agencies in foreign countries impose many requirements on the introduction of new pharmaceutical products through lengthy and detailed clinical testing procedures and other costly and time consuming compliance procedures. These requirements vary widely from country to country and make it difficult to estimate when our product candidates will be commercially available, if at all. In addition, gene therapies such as those being developed by us are relatively new and are only beginning to be tested in humans. Regulatory authorities may require us or our collaborators to demonstrate that product candidates are improved treatments relative to other therapies or may significantly modify the requirements governing gene therapies, which could result in regulatory delays or rejections. If we are delayed or fail to obtain required approvals for our product candidates, our operations and financial condition would be damaged. We may not sell product candidates without applicable regulatory approvals.
Numerous regulations in the United States and abroad also govern the manufacturing, safety, labeling, storage, record keeping, reporting and marketing of any product candidates. Compliance with these regulatory requirements is time consuming and expensive. If we fail to comply with regulatory requirements, either prior to approval or in marketing our product candidates after approval, we could be subject to regulatory or judicial enforcement actions. These actions could result in withdrawal of existing approvals, product recalls, injunctions, civil penalties, criminal prosecution, and enhanced exposure to product liabilities.
We face the risk of product liability claims, which could adversely affect our business and financial condition.
Our operations will expose us to potential product liability risks that are inherent in the testing, manufacturing and marketing of gene therapy products and could prevent or delay the commercialization of our product candidates or negatively affect our financial condition. Regardless of the merit or eventual outcome, product liability claims may result in withdrawal of proposed product candidates from clinical trials, costs of litigation, and substantial monetary awards to plaintiffs and decreased demand for products.
16
Product liability may result from harm to patients using our product candidates as a result of mislabeling, misuse or product failure. While we require all patients enrolled in our clinical trials to sign consents, which explain the risks involved with participating in the trial, the consents provide only a limited level of protection. Additionally, we indemnify the clinical centers and related entities in connection with losses they may incur through their involvement in the clinical trials. Product liability insurance is expensive and we may not be able to maintain our product liability coverage on acceptable terms or obtain adequate coverage against potential liabilities.
Risks Related to Our Common Stock
Our stock price has been and may continue to be volatile, and an investment in our common stock could significantly decline in value.
The market prices for securities of pharmaceutical and biotechnology companies in general, have been highly volatile and may continue to be highly volatile in the future. The market price of our common stock has been and may continue to be volatile based on the following factors, in addition to other risk factors described herein and in light of the low volume of trades in our common stock:
| | • | | developments concerning any research and development, clinical trials, manufacturing, and marketing collaborations; |
| | • | | announcements of technological innovations or new commercial products by our competitors or us; |
| | • | | developments concerning proprietary rights, including patents; |
| | • | | publicity regarding actual or potential results relating to medicinal products under development by our competitors or us; |
| | • | | regulatory developments in the United States and other countries; |
| | • | | economic and other external factors, including disasters or crises; or |
| | • | | period-to-period fluctuations in financial results. |
You may have difficulty selling your shares of common stock at or above the price paid for such shares or at the time you would like to sell.
We have a low volume of daily trades in our common stock on the NASDAQ Capital Market, which could make it difficult to conduct large transactions in our common stock. In addition, any sales by stockholders of a large number of shares of our common stock, or the perception that the holders of a large number of our shares intend to sell our common stock, may cause a significant reduction in the price of our common stock. In this regard, we have a significant number of shares of common stock underlying outstanding options and warrants that are currently exercisable and eligible for immediate sale in the public market. The issuance of common stock upon the exercise of stock options and warrants would also dilute existing investors.
Our quarterly operating results may fluctuate, causing volatility in our stock price.
17
We do not receive any revenues from sales. Our results of operations historically have fluctuated on a quarterly basis, which we expect to continue. Our results of operations at any given time will be based primarily on the following factors:
| | • | | the status of development of product candidates; |
| | • | | whether we enter into collaboration agreements and the timing and accounting treatment of payments, if any, to us under those agreements; |
| | • | | whether and when we achieve specified development or commercialization milestones; and |
| | • | | the addition or termination of research programs or funding support. |
We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of future performance. These fluctuating results may cause the price of our stock to fluctuate, perhaps substantially.
We may be unable to maintain our listing on the NASDAQ Capital Market. Failure to maintain our listing could adversely affect our business, and the liquidity of our common stock would be seriously limited.
Our common stock is currently traded on the NASDAQ Capital Market. To maintain a listing on NASDAQ, we must maintain minimum listing requirements, including certain levels of stockholders’ equity, market capitalization, and minimum bid price for our common stock. Currently, we do not satisfy certain of these requirements. Additionally, NASDAQ may consider, among other things, nature of the business, financial integrity and future outlook of a company in considering whether to de-list a company. If our common stock is de-listed from NASDAQ, it could reduce the liquidity of our common stock, decrease the market price of our common stock and negatively impact our ability to obtain additional capital. As discussed above, we currently are not in compliance with the NASDAQ Stock Market’s requirements for continued listing because the bid price of our common stock had closed below the minimum $1.00 per share requirement for continued inclusion under NASDAQ Marketplace Rule 4310(c)(4). We have until May 21, 2007, to regain compliance with the NASDAQ Stock Market’s requirements for continued listing.
Additionally, we received a notification from NASDAQ that our common stock would be delisted from the NASDAQ Capital Market because NASDAQ believes we currently are not engaged in active business operations and are therefore a “public shell.” On March 23, 2007, we received a decision from the hearing panel providing us until April 17, 2007 to mail proxy materials to our stockholders regarding the transaction with VIA Pharmaceuticals, Inc., and until May 21, 2007 to consummate such transaction. There are no assurances that we can meet these deadlines, and failure to meet these deadlines could result in the loss of our listing on the NASDAQ Capital Market.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
NONE
We currently lease approximately 375 square feet of administrative offices in Roswell, Georgia. The lease for this office is on a month to month basis with a forty-five day notice period.
18
We also currently lease approximately 2,200 square feet of laboratory and office space in San Jose, California. The lease for this space expires in July 2007.
We believe that our current leased facilities are adequate for our current needs.
NONE
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
No matters were submitted to a vote of security holders during the fourth quarter of the fiscal year ended December 31, 2006.
| ITEM 4A. | EXECUTIVE OFFICERS OF THE REGISTRANT |
Set forth below, in accordance with General Instruction G(3) of Form 10-K and Instruction 3 of Item 401(b) of Regulation S-K, are the names, ages and positions of our executive officers along with their business experience during the past five years. Unless otherwise indicated, the information set forth is as of December 31, 2006. The age of each officer listed is as of the date of the filing of this report. Unless otherwise indicated, all of our executive officers have served continuously since the dates indicated. There are no family relationships among the officers.
| | |
Name, Age and Position with the Company | | Dates Elected or Appointed |
Richard E. Otto, Age 57 | | |
Chief Executive Officer | | February 7, 2003 |
President | | April 11, 2003 (1) |
| |
Jack W. Callicutt, Age 40 | | |
Senior Vice President and Chief Financial Officer | | January 1, 2007 (2) |
| (1) | Richard E. Otto is our Chief Executive Officer, President and director and has served as Chief Executive Officer and director since the merger between GenStar and Vascular Genetics in February 2003. Mr. Otto became President of Corautus in April 2003. Effective December 31, 2006, Mr. Otto’s employment with Corautus was terminated, and Mr. Otto entered into a consulting agreement whereunder Mr. Otto will continue to serve as President and Chief Executive Officer of Corautus. Prior to the merger, he served as Chief Executive Officer, President and a director of Vascular Genetics since January 2002. From March 1998 through January 2002, Mr. Otto served as a consultant to various charitable and commercial organizations. Mr. Otto has spent the past 35 years in the cardiac therapy industry. From September 1995 to March 1998 he served as Chief Executive Officer and a director of CardioDynamics International Corporation, a publicly traded company listed on NASDAQ that develops, manufactures and markets noninvasive heart-monitoring devices. Mr. Otto has served as a consultant to the founder of WebMD and as a consultant to the Cardiac Rhythm Management division of St. Jude Medical. His career includes key management positions with Cardiac Pacemakers Inc. (now a Guidant company). Mr. Otto also held positions at Intermedics, Inc., Medtronic Inc., and Eli Lilly and Company. Mr. Otto has served on the Georgia board of directors of the Juvenile Diabetes Foundation, and Leukemia Society. Mr. Otto was a semifinalist in the 1997 Entrepreneur of the Year Award for the Southern California Region. He received a B.S. in chemistry from the University of Georgia. |
19
| (2) | Jack W. Callicutt was elected as Chief Financial Officer of Corautus as of January 1, 2007. Prior to such time, Mr. Callicutt served as Vice President—Finance and Administration, Chief Accounting Officer and Assistant Secretary since September 2003. Prior to joining Corautus, Mr. Callicutt spent 14 years with Deloitte & Touche, the last six years as an audit senior manager. Mr. Callicutt is a Certified Public Accountant and graduated, with honors, from Delta State University in 1989 with degrees in Accounting and Computer Information Systems. |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Our common stock is currently traded on the NASDAQ Capital Market under the symbol “VEGF.” Prior to October 13, 2004, our common stock was traded on the American Stock Exchange under the symbol “CAQ.” As of March 8, 2007, there were approximately 469 registered holders of record of common stock.
As of December 31, 2006, there were outstanding options to purchase 5,419,439 shares of our common stock and warrants to purchase 1,169,024 shares of our common stock. There are 2,000 shares of our Series C preferred stock, 1,385,377 shares of our Series D Preferred Stock, and 2,475,659 shares of our Series E Preferred Stock outstanding.
We have never paid any cash dividends on our common stock to date. We currently anticipate that we will retain all future earnings, if any, to fund the development and growth of our business and do not anticipate paying any cash dividends for at least the next five years, if ever.
The following table shows for the periods indicated the high and low closing prices for our common stock on the NASDAQ Capital Market.
| | | | | | |
| | | HIGH | | LOW |
FISCAL YEAR ENDED December 31, 2005: | | | | | | |
First Quarter | | $ | 5.74 | | $ | 3.99 |
Second Quarter | | $ | 5.00 | | $ | 3.58 |
Third Quarter | | $ | 5.85 | | $ | 3.79 |
Fourth Quarter | | $ | 4.61 | | $ | 3.88 |
FISCAL YEAR ENDED December 31, 2006: | | | | | | |
First Quarter | | $ | 5.26 | | $ | 3.32 |
Second Quarter | | $ | 3.51 | | $ | 0.67 |
Third Quarter | | $ | 0.88 | | $ | 0.65 |
Fourth Quarter | | $ | 0.68 | | $ | 0.32 |
FISCAL YEAR ENDED December 31, 2007: | | | | | | |
First Quarter (through March 23, 2007) | | $ | 0.72 | | $ | 0.36 |
Set forth below is a line graph comparing the annual percentage change in the cumulative return to the shareholders of our common stock with the cumulative return of the NASDAQ Stock Market and of the NASDAQ Biotechnology Index for the period commencing December 31, 2001 and ending on December 31, 2006. Our common stock first began trading on the NASDAQ Capital Market (formerly NASDAQ SmallCap Market) on October 13, 2004. Prior to such time, our common stock traded on the American Stock Exchange. Returns for the indices are weighted based on market capitalization at the beginning of each measurement point.
20
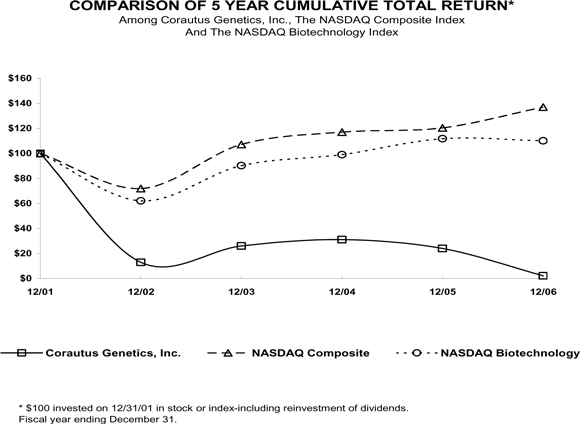
| ITEM 6. | SELECTED FINANCIAL DATA |
The selected financial data as of December 31, 2006 and 2005 and for the years ended December 31, 2006, 2005 and 2004 and for the period from July 1, 1991 (inception) to December 31, 2006 are derived from Corautus’ consolidated financial statements prepared using U.S. generally accepted accounting principles, referred to as GAAP, which have been audited by Ernst & Young LLP, independent registered public accounting firm, and are included in this report. The selected historical consolidated financial data as of December 31, 2004, 2003 and 2002 and for the years ended December 31, 2003 and 2002 are derived from Corautus’ consolidated financial statements, which have been audited by Ernst & Young LLP, independent registered public accounting firm, and are not included in this report. The financial data should be read in conjunction with “Corautus’ Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Corautus’ consolidated financial statements and related notes appearing elsewhere in this report. The historical results are not necessarily indicative of results to be expected in any future period.
21
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Period
from
July 1, 1991
(inception)
to Dec. 31, | |
| | | 2006 | | | 2005 | | | 2004 | | | 2003 | | | 2002 | | | 2006 | |
| | | (in thousands except per share data) | |
Statements of Operation Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Net revenues | | $ | 83 | | | $ | 83 | | | $ | 83 | | | $ | 35 | | | $ | 1,120 | | | $ | 2,827 | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of sales | | | — | | | | — | | | | — | | | | — | | | | — | | | | 822 | |
Research and development | | | 9,614 | | | | 15,949 | | | | 6,593 | | | | 3,217 | | | | 9,067 | | | | 67,625 | |
General and administrative | | | 6,214 | | | | 3,897 | | | | 3,681 | | | | 7,939 | | | | 3,670 | | | | 33,477 | |
Write-off of acquired in-process technology | | | — | | | | — | | | | — | | | | 17,295 | | | | — | | | | 24,405 | |
Write-off of property and equipment | | | 93 | | | | — | | | | — | | | | 1,946 | | | | 636 | | | | 2,687 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total costs and expenses | | | 15,921 | | | | 19,846 | | | | 10,274 | | | | 30,397 | | | | 13,373 | | | | 129,016 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (15,838 | ) | | | (19,763 | ) | | | (10,191 | ) | | | (30,362 | ) | | | (12,253 | ) | | | (126,189 | ) |
Other income, net | | | — | | | | 43 | | | | 10 | | | | 19 | | | | 75 | | | | 138 | |
Interest expense | | | (498 | ) | | | (832 | ) | | | (452 | ) | | | (123 | ) | | | (462 | ) | | | (3,721 | ) |
Interest income | | | 977 | | | | 815 | | | | 251 | | | | 58 | | | | 586 | | | | 4,878 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | (15,359 | ) | | | (19,737 | ) | | | (10,382 | ) | | | (30,408 | ) | | | (12,054 | ) | | | (124,894 | ) |
Series E preferred stock dividend | | | (498 | ) | | | — | | | | — | | | | — | | | | — | | | | (498 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss available to common stockholders | | $ | (15,857 | ) | | $ | (19,737 | ) | | $ | (10,382 | ) | | $ | (30,408 | ) | | $ | (12,054 | ) | | $ | (125,392 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (0.81 | ) | | $ | (1.16 | ) | | $ | (0.79 | ) | | $ | (3.31 | ) | | $ | (3.54 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average shares outstanding | | | 19,722 | | | | 16,954 | | | | 13,140 | | | | 9,196 | | | | 3,408 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | December 31, |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 |
| | | (in thousands) |
Balance Sheet Data: | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 15,964 | | $ | 30,962 | | $ | 24,487 | | $ | 8,912 | | $ | 4,912 |
Working capital | | | 13,631 | | | 27,291 | | | 22,626 | | | 6,751 | | | 2,061 |
Total assets | | | 16,417 | | | 31,511 | | | 26,015 | | | 10,534 | | | 10,621 |
Long-term debt and capital lease obligations, net of current portions | | | — | | | 16,120 | | | 10,329 | | | 2,500 | | | 506 |
Lease settlement obligation, net of current portion | | | — | | | — | | | 1,079 | | | 1,815 | | | — |
Stockholders’ equity | | | 13,047 | | | 10,562 | | | 11,232 | | | 2,491 | | | 6,097 |
22
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements that are subject to a variety of risks and uncertainties including those set forth under “Risk Factors” in Item 1A of this report. The statements that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, including without limitation statements regarding our expectations, beliefs, intentions or strategies regarding the future. All forward-looking statements included in this document are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Our actual results could differ materially from those anticipated in these forward-looking statements. See the section at the beginning of this report titled “Cautionary Factors That May Affect Future Results.”
Introduction
Corautus is a development stage company dedicated to the development of innovative products in the life sciences industry. Corautus, formerly known as GenStar Therapeutics Corporation and Urogen Corp., was formed as a Delaware corporation on June 30, 1995. Prior to November 1, 2006, Corautus was primarily focused on the clinical development of gene therapy products using a vascular growth factor gene, Vascular Endothelial Growth Factor 2 (VEGF-2), for the treatment of severe cardiovascular disease. Corautus was the sponsor of a Phase IIb clinical trial to study the efficacy of VEGF-2 for the treatment of severe cardiovascular disease, known as the GENASIS trial. In addition, Corautus supported initial clinical trials studying the efficacy of VEGF-2 for the treatment of peripheral artery disease and diabetic neuropathy.
Our gene therapy treatment approach was based upon the research of the late Dr. Jeffrey Isner, former Chief of Vascular Medicine and Cardiovascular Research at Caritas St. Elizabeth’s Medical Center of Boston, Inc. Our VEGF-2 product candidates were designed to induce therapeutic angiogenesis, which is the stimulation of blood vessel formation, to restore blood flow to ischemic, or oxygen deprived, muscle caused by cardiovascular or peripheral vascular disease. In this way, we sought to treat a cause of the underlying condition rather than the symptoms of the disease. Our approach to therapeutic angiogenesis involved the injection of naked plasmid DNA, or DNA that is formulated without modifying materials such as liposomes or pegylation, directly into the ischemic muscle.
We enrolled 30 Class III and Class IV angina patients in our Phase I clinical trial. From our investigator’s published data, a significant improvement in angina class was noted at the two-year follow-up; no patients evaluated as having Class IV angina; only three were evaluated as having Class III angina; and the remaining were evaluated as having Class I or II angina. Our 19 patient Phase I/IIa trial was a multicenter, randomized study testing VEGF-2 in two dosages (200 and 800 microgram) plus a placebo. The investigators reported a significant improvement in symptoms of angina, including an average increase in exercise tolerance time of almost two minutes in the VEGF-2 treated patients, a reduction of angina class, and a reduction in frequency of angina episodes in the VEGF-2 treated patients compared to the placebo patients.
We initiated the GENASIS trial on August 31, 2004. GENASIS was a multicenter, randomized, four-arm, double-blind study of up to 404 patients in approximately 30 institutions in the United States evaluating the efficacy and safety of three doses (20, 200, 800 micrograms) of VEGF-2 versus placebo
23
percutaneously delivered via the Stiletto™ catheter. Prior to cessation, 295 patients were treated in the GENASIS clinical trial. The primary endpoint of the GENASIS trial was the improvement in exercise tolerance time on a treadmill 90 days following patient treatment, with additional follow-up at 6 and 12 months.
On March 14, 2006, we announced that in conjunction with Boston Scientific Corporation, we had temporarily suspended patient treatments in our GENASIS clinical trial. Boston Scientific requested the voluntary suspension as a result of three reported serious adverse events of pericardial effusion, which did not appear to be related to the VEGF-2 biologic. We notified the FDA of the voluntary suspension prior to making the public announcement, and in a subsequent teleconference, we were informed that the FDA had placed the trial on clinical hold.
As part of the process to address the clinical hold, the independent Data Monitoring Committee, or DMC, reviewed safety information. To better consider safety in the context of risk/benefit in this trial, the DMC also requested and subsequently reviewed a limited amount of available unblinded summary efficacy data related to the increase in exercise tolerance time, or ETT, which is measured by a patient’s performance on a treadmill. The DMC recommended to us that, based on available efficacy data, enrollment should be terminated under the current protocol as the DMC saw very little chance for significant efficacy as to the primary endpoint. On April 10, 2006, we announced that we had accepted the DMC’s recommendation that enrollment in the GENASIS trial be terminated and notified the FDA of the termination of enrollment.
Corautus locked the database on August 14, 2006 for the 295 patients treated in the GENASIS clinical trial. The efficacy and safety analyses performed on the database included 295 patients at 3 months, 241 patients at 6 months and 103 patients at 12 months. On October 10, 2006 Corautus announced that the final efficacy results from its GENASIS clinical trial did not achieve a statistically significant difference from placebo in any active dose group for the primary efficacy endpoint. The primary efficacy endpoint in the GENASIS clinical trial was an improvement of at least one minute in ETT from baseline to three months. The data indicated considerable overlap in results between the active and placebo groups for the secondary endpoints as well, and no clear dose effect was seen. In general, a majority of patients in all treatment arms (active and placebo) significantly improved from their baseline status in both primary and secondary efficacy endpoints; however, there was no significant separation from the placebo in any active dose group.
On October 30, 2006, our board of directors determined that it was in the best interests of the company and its stockholders to not conduct further clinical trials for VEGF-2 for the treatment of cardiovascular and peripheral vascular disease. The board of directors authorized management to immediately reduce its cash spending and to continue identifying other life sciences technologies that it could acquire or in-license, or other life sciences opportunities.
We have been actively seeking new product opportunities, as well as a merger or business combination, due to the refocus of our business away from our VEGF-2 product candidates. On February 7, 2007, we entered into an Agreement and Plan of Merger and Reorganization with VIA Pharmaceuticals, Inc., a Delaware corporation that currently is privately-held, and our wholly owned subsidiary, Resurgens Merger Corp. Pursuant to the merger agreement, Resurgens will merge with and into VIA, with VIA surviving as a wholly-owned subsidiary of Corautus. In exchange for their VIA capital stock, VIA stockholders will receive shares of Corautus common stock. Currently, we expect that former VIA stockholders will hold approximately 78% of our common stock post-transaction. Additionally, if the transaction with VIA is consummated, Corautus’ clinical operations will focus on VIA’s product candidates.
24
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, accrued liabilities and stock-based compensation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect the significant judgments and estimates used in the preparation of our consolidated financial statements (see Note 1 to our consolidated financial statements).
Revenue Recognition
Grant revenue is recognized as the research expenses related to the grants are incurred. Contract revenue arising from collaborative research agreements is recognized either (i) ratably over the term of the agreement, which approximates the performance of services, for contracts specifying payment for services over a given period, or (ii) as services are performed under the agreement, for contracts specifying payment on a per full-time employee basis. All amounts received under collaborative research agreements or research grants are not refundable, regardless of the success of the underlying research.
Accrued Liabilities
The Company estimates expenses related to costs that have been incurred, but have not yet been billed. This process involves identifying services and activities that have been performed by third-party vendors on our behalf and estimating the level to which they have been performed and the associated cost incurred for such service as of each balance sheet date. These costs include fees for professional services, such as those provided by certain clinical research organizations and investigators in conjunction with clinical trials, and fees owed to contract manufacturers in conjunction with the manufacture of material for planned clinical trials and commercial use.
Stock Based Compensation
On January 1, 2006, we adopted Statement of Financial Accounting Standards No. 123R (revised 2004),Share-Based Payment, (“SFAS 123(R)”) which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors including employee stock options and employee stock purchases under the Employee Stock Purchase Plan (“ESPP”) based on estimated fair values. SFAS 123(R) requires companies to estimate the fair value of stock-based awards on the date of grant using an option pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in our Consolidated Statements of Operations. We adopted SFAS 123(R) using the modified prospective transition method
25
which requires the application of the accounting standard starting from January 1, 2006. Prior to the adoption of SFAS 123(R), we accounted for stock-based awards to employees and directors using the intrinsic value method in accordance with Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees and under this method, no stock-based compensation expense for employee stock options was recognized in the prior periods in our Consolidated Statements of Operations because the exercise price of our stock options granted to employees and directors equaled the fair market value of the underlying stock at the date of grant. In accordance with the modified prospective transition method we used in adopting SFAS 123(R), our results of operations prior to fiscal year 2006 have not been restated to reflect, and do not include, the impact of SFAS 123(R).
The value of options, warrants, or stock awards issued to non-employees have been determined in accordance with SFAS No. 123(R),Accounting for Stock Based Compensation, and Emerging Issues Task Force Issue No. 96-18,Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods and Services, and are periodically remeasured as the options vest, if required. Warrants for common stock are initially valued at the date of issuance and are recorded to the expense category related to the services provided.
Use of Estimates
The preparation of the financial statements in accordance with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Results of Operations
Revenues
Total revenues for the years ended December 31, 2006, 2005 and 2004 were approximately $83,000 for each year. These revenues were from the sublicense of certain patents to Boston Scientific Corporation as part of Boston Scientific’s investment in Corautus. Product revenues are contingent on, among other matters, the success of our product candidates in clinical trials, and currently we do not have any product candidates. Therefore, we do not anticipate revenues from product sales unless we acquire product candidates.
Research and Development and Acquired In-Process Technology
Research and development expenses decreased $6.3 million to $9.6 million for the year ended December 31, 2006 compared to approximately $15.9 million for the year ended December 31, 2005. Research and development expenses increased $9.3 million to $15.9 million for the year ended December 31, 2005 compared to approximately $6.6 million for the year ended December 31, 2004.
The decrease in research and development expense in 2006 from 2005 was primarily caused by the termination in 2006 of our GENASIS Phase IIb clinical trial that began in Fall of 2004, including reduction in costs for patient treatment costs and clinical research organization costs and by costs incurred under the manufacturing agreement in developing processes and procedures for the intended production of our clinical material for planned Phase III clinical trial and commercial use, which will no longer occur. Also included in 2006 were severance expenses associated with the reduction in workforce of research and development personnel of approximately $582,000 and non-cash stock based compensation expense of approximately $200,000.
26
The increase in research and development expense in 2005 over 2004 was primarily caused by our GENASIS Phase IIb clinical trial that began in Fall of 2004, including patient treatment costs and clinical research organization costs and by costs incurred under the manufacturing agreement in developing processes and procedures for the intended production of our clinical material for planned Phase III clinical trial and commercial use.
We currently do not have any products in clinical testing, nor do we have any plans to initiate clinical trials outside of continuing VIA’s clinical trials if the merger is consummated. We estimate that it will take many years to complete clinical testing of any products and obtain regulatory approvals, if we are to initiate clinical trials and are able to obtain approvals. We anticipate that research and development expenses will increase in the event we conduct clinical testing in the future.
General and Administrative Expenses
General and administrative expenses increased $2.3 million for the year ended December 31, 2006 to approximately $6.2 million compared to approximately $3.9 million for the year ended December 31, 2005. General and administrative expenses increased $216,000 for the year ended December 31, 2005 to approximately $3.9 million compared to approximately $3.7 million for the year ended December 31, 2004.
General and administrative expenses include the costs of our administrative personnel and consultants, office lease expenses and other overhead costs, including legal and accounting costs. The increase in general and administrative expense for the year ended December 31, 2006 compared to the year ended December 31, 2005 relates primarily to costs associated with our planned common stock offering in March 2006, which was terminated due to the suspension of enrollment in our GENASIS clinical trial, in addition to non-cash stock-based compensation costs and severance expenses relating to the reduction in workforce in the fourth quarter of 2006. The terminated offering costs primarily consisted of legal, accounting, printing, filing fees, and travel totaling approximately $695,000. We recorded non-cash stock-based compensation costs of $994,000 in 2006 and none in 2005. Severance expense in the fourth quarter of 2006 for general and administrative personnel was approximately $811,000. The increase in general and administrative expense in 2005 over 2004 relates primarily to increases in personnel costs.
Write-off of Property and Equipment
The write-off of property and equipment of approximately $93,000 in 2006 is related to the write down and disposal of certain office furniture and equipment and lab equipment subsequent to our board of directors decision to pursue other life sciences opportunities, and the relocation to smaller office space.
Interest Income and Expense
Interest income increased $162,000 for the year ended December 31, 2006 to approximately $977,000 compared to approximately $815,000 for the year ended December 31, 2005. Interest income increased $564,000 for the year ended December 31, 2005 to approximately $815,000 compared to approximately $251,000 for the year ended December 31, 2004. Interest income is a result of investment of excess cash in auction rate securities, money market accounts, and certificates of deposit. The increase in interest income is due to increases in the balances in those investments and in the investment yield.
Interest expense decreased $333,000 for the year ended December 31, 2006 to approximately $498,000 compared to approximately $831,000 for the year ended December 31, 2005. Interest expense increased
27
$379,000 for the year ended December 31, 2005 to approximately $831,000 compared to approximately $452,000 for the year ended December 31, 2004. The decrease in interest expense in 2006 compared to 2005 is primarily due to Boston Scientific Corporation’s exchange of all of its Promissory Notes, with an aggregate principal amount of $15.0 million, together with accrued interest of $1.6 million on June 30, 2006, for 2.5 million shares of the Company’s Series E Preferred Stock. The increase in interest expense in 2005 over 2004 is due to the timing of issuance of the notes payable partially offset by lower interest on capital lease obligations.
Series E Preferred Stock Dividend
On June 30, 2006, the Company entered into a Recapitalization Agreement with Boston Scientific Corporation, pursuant to which Boston Scientific exchanged all of its Promissory Notes, with an aggregate principal amount of $15,000,000, together with accrued interest of $1,608,103 through June 30, 2006, for 2,475,659 shares of the Company’s Series E Preferred Stock with a liquidation preference of $6.71 per share and is entitled to a cumulative annual dividend of 6%. The Series E Preferred Stock is convertible into 2,475,659 shares of Corautus’ common stock, subject to adjustment, and votes with the common stock. The agreement was executed and closed on June 30, 2006. The dividends on the Series E Preferred Stock, $498,350 as of December 31, 2006, are payable upon declaration by the board of directors on January 1 and July 1 of each year and are payable in additional shares of Series E Preferred Stock until January 1, 2009, when such dividends will be payable in cash from then forward. The Company’s board of directors has not declared any dividends on the Series E Preferred Stock as of December 31, 2006. The Series E Preferred Stock dividends represent an increase in net loss available to common stockholders.
Liquidity and Capital Resources
From inception through December 31, 2006, we have primarily financed our operations through private placements of equity and convertible debt securities, our strategic alliance with Boston Scientific Corporation and through our partnership with Baxter Healthcare. We have raised approximately $16.4 million in convertible debt offerings (including $15.0 million from Boston Scientific Corporation), $55.5 million in private equity financings, $16.0 million in net proceeds from the Boston Scientific Corporation investment in our common and preferred stock and received equity funding of $14.9 million related to our relationship with Baxter Healthcare.
Net cash used by operating activities in 2006 was approximately $15.4 million and primarily consists of expenditures for research and development and general and administrative expenses. Net cash provided by investing activities of $15.3 million during 2006 consists of sales of short-term investments, purchases of short-term investments and the purchase of property and equipment. Net cash provided by financing activities of $400,000 during 2006 consists primarily of proceeds from the exercise of warrants and interest payable on convertible debt, offset by repayments of capital leases.
As of December 31, 2006, we had cash, cash equivalents and short-term investments totaling approximately $16.0 million. Our operations to date have consumed substantial amounts of cash and have generated insignificant revenues. The negative cash flow from operations is expected to continue. The development of any products will require a commitment of substantial funds to conduct the costly and time-consuming research, preclinical and clinical testing necessary to bring our products to market and to establish manufacturing and marketing capabilities. Our future capital requirements will depend on many factors including the progress of our research and development programs; the progress, scope and results of our preclinical and clinical testing; the time and cost involved in obtaining regulatory approvals; the
28
cost of manufacturing for our proposed products; the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; competing technological and market developments; and our ability to establish and maintain collaborative and other arrangements with third parties, such as licensing and manufacturing agreements, and the cost of such arrangements.
We will be seeking this additional funding either through collaborative arrangements or through public or private equity or debt financings. We cannot be certain that additional financing will be available on acceptable terms, or at all. We do not have any commitments or arrangements assuring us of any additional funds in the future, and there is no assurance that we will be able to obtain additional capital on acceptable terms, or at all. Failure to successfully address ongoing liquidity requirements will have a material adverse effect upon our business. In the event that we are unable to obtain additional capital, we will be required to take actions that will harm our business and our ability to achieve cash flow in the future. To the extent we raise additional cash by issuing equity securities, our existing stockholders will be diluted. If additional funds are raised through the issuance of debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and preferred stock.
Contractual Obligations
The following is a summary of our contractual obligations as of December 31, 2006:
| | | | | | | | | | | | | | | |
| | | Payments due by Period |
Contractual Obligations | | Total | | Less than 1 year | | 1–3 years | | 3–5 years | | More than 5 years |
Capital lease | | $ | 2,000 | | $ | 2,000 | | $ | — | | $ | — | | $ | — |
| | | | | | | | | | | | | | | |
Operating lease | | | 21,000 | | | 21,000 | | | — | | | — | | | — |
| | | | | | | | | | | | | | | |
Total | | $ | 23,000 | | $ | 23,000 | | $ | — | | $ | | | $ | |
| | | | | | | | | | | | | | | |
New Accounting Pronouncements
In March 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154,Accounting Changes and Error Corrections (“SFAS 154”), which replaces Accounting Principles Board (“APB”) Opinion No. 20,Accounting Changes, and SFAS 3,Reporting Accounting Changes in Interim Financial Statement, and changes the requirements for the accounting for and the reporting of a change in accounting principle. SFAS 154, which became effective for all accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005, had no effect on the Company’s financial position or results of operations.
In February 2006, the FASB issued SFAS No. 155,Accounting for Certain Hybrid Financial Instruments (“SFAS 155”), which amends SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities and SFAS No. 140,Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities, aimed at improving the financial reporting of certain hybrid financial instruments by requiring more consistent accounting that eliminates exemptions and provides a means to simplify the accounting for these instruments. SFAS 155 is effective for all financial instruments acquired or issued in fiscal years beginning after September 15, 2006. The Company does not expect that the adoption of SFAS 155 will have any impact on its financial position and results of operations.
In June 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes (“FIN 48”), which clarifies when tax benefits should be recorded in financial statements, requires certain disclosures of uncertain tax matters and indicates how any tax reserves should be classified in a balance sheet. The Company has not determined the impact, if any, the adoption of FIN 48, which is effective for fiscal years beginning after December 15, 2006, will have on its financial position and results of operations.
In September 2006, the FASB issued SFAS No. 157,“Fair Value Measurements” (“SFAS 157”). SFAS 157 provides guidance for using fair value to measure assets and liabilities. It also responds to investors’ requests for expanded information about the extent to which companies measure assets and liabilities at fair value, the information used to measure fair value, and the effect of fair value measurements on earnings. SFAS 157 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, and does not expand the use of fair value in any new circumstances. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and is required to be adopted by the Company in its first quarter of fiscal 2008. The Company has not determined the impact, if any, the adoption of SFAS 157 will have on its financial position and results of operations.
29
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Some of the securities that we invest in may have market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the market value of our investment will probably decline. To minimize this risk in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including auction-rate securities, commercial paper, money market funds, government and non-government debt securities and certificates of deposit. The average duration of the majority of our investments in 2006 and 2005 was less than two months. Due to the short-term nature of these investments, we believe that we have no material exposure to interest rates arising from our investments. Therefore, no quantitative tabular disclosure is included in this report.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
SEE ITEM 15. “Exhibits and Financial Statement Schedules.”
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
NONE
| ITEM 9A. | CONTROLS AND PROCEDURES |
| | (a) | Evaluation of disclosure controls and procedures. Our chief executive officer and chief financial officer are responsible for establishing and maintaining “disclosure controls and procedures” (as defined in the Securities and Exchange Act of 1934 Rules 13a-15(e) and 15d-15(e)) for the company. Our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures as of the end of the period covered by this annual report, have concluded that our disclosure controls and procedures were effective in timely alerting them to material information relating to us (including our consolidated subsidiaries) required to be included in our periodic SEC filings. |
| | (b) | Changes in internal controls over financial reporting. There were no changes in our internal controls over financial reporting that occurred during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting. |
| ITEM 9B. | OTHER INFORMATION |
NONE
31
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item with respect to directors is set forth under the captions “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters,” “Proposal 1—Election of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” in the 2007 Proxy Statement and is incorporated herein by reference. The information required by this item with respect to the executive officers is, pursuant to Instruction 3 of Item 401(b) of Regulation S-K and General Instruction G (3) of Form 10-K, set forth at Part I, Item 4(A) of this report under the caption “Executive Officers of the Registrant.”
We have adopted a code of business conduct and ethics, which applies to our chief executive officer and our senior financial officers. Our code of business conduct and ethics is posted on our website at www.corautus.com under the headings “Investor Relations- Corporate Governance- Code of Business Conduct and Ethics.” We will also provide a copy of the code of business conduct and ethics to stockholders upon request. Any amendments to or waivers from any provision of our code of business conduct and ethics will be disclosed by posting such information on our website.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is set forth under the captions “Compensation of Directors and Executive Officers” and “Corporate Governance” in the 2007 Proxy Statement and is incorporated herein by reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
Information required by this item is set forth under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in the 2007 Proxy Statement and is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this item is set forth under the captions “Certain Relationships and Related Transactions” and “Corporate Governance” in the 2007 Proxy Statement and is incorporated by reference.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item is set forth under the caption “Proposal 2—Ratification of Re-Appointment of Independent Registered Public Accounting Firm” in the 2007 Proxy Statement and is incorporated by reference.
32
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The following documents are filed as a part of this report:
| (a) | Index to Financial Statements |
| | |
| | | Page |
Report of Ernst & Young LLP, Independent Registered Public Accounting Firm | | F-1 |
| |
Consolidated Balance Sheets as of December 31, 2006 and 2005 | | F-2 |
| |
Consolidated Statements of Operations for the years ended December 31, 2006, 2005, and 2004 and the period from July 1, 1991 (inception) to December 31, 2006 | | F-3 |
| |
Consolidated Statements of Stockholders’ Equity (Deficit) for the period from July 1, 1991 (inception) to December 31, 2006 | | F-4 |
| |
Consolidated Statements of Cash Flows for the years ended December 31, 2006, 2005, and 2004 and the period from July 1, 1991 (inception) to December 31, 2006 | | F-9 |
| |
Notes to Consolidated Financial Statements | | F-10 |
| 2. | Schedules to Financial Statements |
All schedules are omitted because they are not applicable or the required information is included in the financial statements or notes thereto.
| | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit
Number | | Exhibit Description | | Form | | Date | | Filed
Herewith |
| 2.1 | | Agreement and Plan of Merger and Reorganization, dated February 7, 2007, by and among Corautus Genetics Inc., Resurgens Merger Corp., and VIA Pharmaceuticals, Inc. | | 8-K | | 02/07/07 | | |
| | | | |
| 3.1 | | Restated Certificate of Incorporation | | 10-K | | 03/22/05 | | |
| | | | |
| 3.2 | | Certificate of Amendment to the Restated Certificate of Incorporation | | 10-KSB | | 03/30/00 | | |
| | | | |
| 3.3 | | Certificate of Amendment to the Restated Certificate of Incorporation | | 10-K | | 03/28/03 | | |
| | | | |
| 3.4 | | Certificate of Amendment to the Restated Certificate of Incorporation | | 10-K | | 03/28/03 | | |
| | | | |
| 3.5 | | Certificate of Amendment to the Restated Certificate of Incorporation | | 10-K | | 03/28/03 | | |
| | | | |
| 3.6 | | Amended and Restated Certificate of Designation of Preferences and Rights of Series A Preferred Stock | | S-4/A* | | 12/19/02 | | |
33
| | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Description | | Form | | Date | | Filed
Herewith |
| 3.7 | | Amended and Restated Certificate of Designation of Preferences and Rights of Series B Preferred Stock | | S-4/A* | | 12/19/02 | | |
| | | | |
| 3.8 | | Amended and Restated Certificate of Designation of Preferences and Rights of Series C Preferred Stock | | S-4/A* | | 12/19/02 | | |
| | | | |
| 3.9 | | Certificate of Designation of Preferences and Rights of Series D Preferred Stock | | 10-Q | | 08/14/03 | | |
| | | | |
| 3.10 | | Certificate of Amendment to Certificate of Designation of Preferences and Rights of Series D Preferred Stock | | 10-Q | | 08/12/05 | | |
| | | | |
| 3.11 | | Certificate of Amendment to Certificate of Designation of Preferences and Rights of Series E Preferred Stock | | 8-K | | 07/06/06 | | |
| | | | |
| 3.12 | | Second Amended and Restated Bylaws | | 10-K | | 03/22/05 | | |
| | | | |
| 4.1 | | Investor Rights Agreement, dated July 8, 1998, between UroGen Corp. and Baxter Healthcare Corporation | | 8-K | | 07/23/98 | | |
| | | | |
| 4.2 | | First Amendment to the Investor Rights Agreement, effective February 5, 2003, between GenStar Therapeutics Corporation and Baxter Healthcare Corporation | | S-4/A* | | 12/19/02 | | |
| | | | |
| 4.3 | | Investor Rights Agreement, dated July 30, 2003, between Corautus and Boston Scientific Corporation | | 10-Q | | 08/14/03 | | |
| | | | |
| 4.4 | | Registration Rights Agreement, dated as of June 27, 2005, between Corautus and Boston Scientific Corporation | | 10-Q | | 08/12/05 | | |
| | | | |
| 4.5 | | Amendment to Recapitalization Agreement, dated October 30, 2006, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 8-K | | 11/06/06 | | |
| | | | |
| 4.6 | | Voting Agreement, dated February 7, 2007, by and among Corautus Genetics Inc. and the persons named therein | | 8-K | | 02/08/07 | | |
| | | | |
| 10.1 | | Form of Indemnification Agreement | | 10-Q | | 08/16/04 | | |
| | | | |
| 10.2 | | Employment Agreement, dated November 2, 2006, by and between Corautus Genetics Inc. and Jack W. Callicutt | | | | | | X |
| | | | |
| 10.3 | | Separation Agreement, dated November 2, 2006, by and between Corautus Genetics Inc. and Richard E. Otto | | | | | | X |
| | | | |
| 10.4 | | Separation Agreement, dated November 2, 2006, by and between Corautus Genetics Inc. and Robert T. Atwood | | | | | | X |
34
| | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit
Number | | Exhibit Description | | Form | | Date | | Filed
Herewith |
| 10.5 | | Consulting Services Agreement, dated December 28, 2006, by and between Corautus Genetics Inc. and Richard E. Otto | | | | | | X |
| | | | |
| 10.6 | | Employment Agreement, dated October 1, 2005, by and between Corautus Genetics Inc. and Jack W. Callicutt | | 8-K | | 10/05/05 | | |
| | | | |
| 10.7 | | Employment Agreement, dated October 1, 2005, by and between Corautus Genetics Inc. and Yawen D. Chiang | | 8-K | | 10/05/05 | | |
| | | | |
| 10.8 | | Employment Agreement, dated May 1, 2005, by and between Corautus Genetics Inc. and Michael K. Steele | | 8-K | | 05/03/05 | | |
| | | | |
| 10.9 | | Investment Agreement, dated July 30, 2003, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/14/03 | | |
| | | | |
| 10.10 | | Development Agreement, dated July 30, 2003, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/14/03 | | |
| | | | |
| 10.11 | | First Amendment to Development Agreement, dated July 22, 2004, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/16/04 | | |
| | | | |
| 10.12 | | Second Amendment to Development Agreement, dated June 27, 2005, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/12/05 | | |
| | | | |
| 10.13 | | Third Amendment to Development Agreement, dated February 2, 2006, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 5/12/06 | | |
| | | | |
| 10.14 | | Distribution Agreement, dated July 30, 2003, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/14/03 | | |
| | | | |
| 10.15 | | First Amendment to Distribution Agreement, dated as of June 27, 2005, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/12/05 | | |
| | | | |
| 10.16 | | Investment Agreement, dated as of June 27, 2005, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/12/05 | | |
| | | | |
| 10.17 | | Amended and Restated License Agreement, dated February 28, 2001, by and between Vascular Genetics Inc. and Human Genome Sciences, Inc. | | 10-Q | | 05/14/03 | | |
| | | | |
| 10.18 | | First Amendment to Amended and Restated License Agreement, dated October 10, 2002, by and between Vascular Genetics Inc. and Human Genome Sciences, Inc. | | 10-Q | | 05/14/03 | | |
35
| | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit
Number | | Exhibit Description | | Form | | Date | | Filed
Herewith |
| 10.19 | | Second Amendment to Amended and Restated License Agreement, dated April 26, 2004, by and between Vascular Genetics Inc. and Human Genome Sciences, Inc. | | 10-Q | | 04/30/04 | | |
| | | | |
| 10.20 | | Third Amendment to Amended and Restated License Agreement, dated March 21, 2005 by and between Vascular Genetics Inc. and Human Genome Sciences, Inc. | | 10-K | | 03/22/05 | | |
| | | | |
| 10.21 | | Fourth Amendment to Amended and Restated License Agreement, dated January 31, 2006 by and between Vascular Genetics Inc. and Human Genome Sciences, Inc. | | 10-K | | 03/30/06 | | |
| | | | |
| 10.22 | | License Agreement, dated February 24, 2000, by and between Vascular Genetics Inc. and Vical Incorporated | | 10-Q | | 05/14/03 | | |
| | | | |
| 10.23 | | Employee Stock Purchase Plan | | 10-KSB | | 03/29/02 | | |
| | | | |
| 10.24 | | License Agreement, dated August 10, 2005, by and between Corautus Genetics Inc. and Caritas St. Elizabeth’s Medical Center of Boston, Inc. | | 10-Q | | 11/14/05 | | |
| | | | |
| 10.25 | | Material Transfer Agreement, dated August 10, 2005, by and between Corautus Genetics Inc. and Caritas St. Elizabeth’s Medical Center of Boston, Inc. | | 10-Q | | 11/14/05 | | |
| | | | |
| 10.26 | | Material Transfer Agreement, dated January 6, 2006, by and between Corautus Genetics Inc. and Caritas St. Elizabeth’s Medical Center of Boston, Inc. | | 10-K | | 03/30/06 | | |
| | | | |
| 10.27 | | Common Stock and Warrant Purchase Agreement, dated June 24, 2005, by and among Corautus Genetics Inc. and the Purchasers named therein | | 10-Q | | 08/12/05 | | |
| | | | |
| 10.28 | | Corautus Genetics Inc. 2002 Stock Plan, as amended and restated | | 10-Q | | 08/16/04 | | |
| | | | |
| 10.29 | | Master Services Agreement, dated March 7, 2005, by and between Corautus Genetics Inc. and PPD Development, L.P. | | 10-K | | 03/22/05 | | |
| | | | |
| 10.30 | | Project Addendum, dated March 7, 2005, by and between Corautus Genetics Inc. and PPD Development, L.P. | | 10-K | | 03/22/05 | | |
| | | | |
| 10.31 | | Agreement for the Transfer of Sponsors Obligations, dated March 7, 2005, by and between Corautus Genetics Inc. and PPD Development, L.P. | | 10-K | | 03/22/05 | | |
36
| | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit
Number | | Exhibit Description | | Form | | Date | | Filed
Herewith |
| 10.32 | | Manufacturing Agreement for Clinical Trial and Commercial Supply, dated May 13, 2005, by and between Corautus Genetics Inc. and Boehringer Ingelheim Austria GmbH | | 8-K | | 05/19/05 | | |
| | | | |
| 10.33 | | Underwriting Agreement, dated March 9, 2006, by and between Lazard Capital Markets LLC (for itself and as representative for Jefferies & Company, Inc.). | | 8-K | | 03/14/06 | | |
| | | | |
| 10.34 | | Recapitalization Agreement, dated June 30, 2006, by and between Corautus Genetics Inc. and Boston Scientific Corporation | | 10-Q | | 08/10/06 | | |
| | | | |
| 21.1 | | Subsidiaries of Corautus Genetics Inc. | | | | | | X |
| | | | |
| 23.1 | | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | | | | | | X |
| | | | |
| 24.1 | | Power of Attorney (included with signature page hereto) | | | | | | X |
| | | | |
| 31.1 | | Section 302 Certification of Chief Executive Officer | | | | | | X |
| | | | |
| 31.2 | | Section 302 Certification of Chief Financial Officer | | | | | | X |
| | | | |
| 32.1 | | Section 906 Certification of Chief Executive Officer | | | | | | X |
| | | | |
| 32.2 | | Section 906 Certification of Chief Financial Officer | | | | | | X |
| * | Form S-4/A file no. 333-101606 |
| ** | Form S-3 file no. 333-112239 |
37
SIGNATURES
Pursuant to the requirements of Section 13 or 15 (d) of Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized on March 30, 2007.
| | |
| CORAUTUS GENETICS INC. |
| A Delaware Corporation |
| |
| By: | | /s/ Jack W. Callicutt |
| | Jack W. Callicutt |
| | Senior Vice President and Chief Financial Officer |
| | (Principal Accounting and Financial Officer) |
Power of Attorney
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Richard E. Otto and Jack W. Callicutt, and each of them his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K for the calendar year ended December 31, 2006, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or ay of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated as of March 30, 2007.
| | |
Signatures | | Title |
| |
/s/ Richard E. Otto | | Chief Executive Officer (Principal Executive Officer), |
| Richard E. Otto | | President and Director |
| |
/s/ Robert T. Atwood | | Director |
| Robert T. Atwood | | |
| |
/s/ James C. Gilstrap | | Chairman of the Board of Directors |
| James C. Gilstrap | | |
38
| | |
/s/ Eric N. Falkenberg | | Director |
| Eric N. Falkenberg | | |
| |
/s/ John R. Larson | | Director |
| John R. Larson | | |
| |
/s/ B. Lynne Parshall | | Director |
| B. Lynne Parshall | | |
| |
/s/ F. Richard Nichol | | Director |
| F. Richard Nichol | | |
| |
/s/ Ivor Royston, M.D. | | Director |
| Ivor Royston, M.D. | | |
| |
/s/ Victor W. Schmitt | | Director |
| Victor W. Schmitt | | |
39
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Corautus Genetics Inc.
We have audited the accompanying consolidated balance sheets of Corautus Genetics Inc. (formerly GenStar Therapeutics Corporation) (a development stage enterprise) (the “Company”) as of December 31, 2006 and 2005, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2006 and for the period from July 1, 1991 (inception) to December 31, 2006. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principals used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Corautus Genetics Inc. (a development stage enterprise) at December 31, 2006 and 2005, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2006 and for the period from July 1, 1991 (inception) to December 31, 2006, in conformity with U. S. generally accepted accounting principles.
As discussed in Note 1 of the Notes to the Consolidated Financial Statements, the Company adopted Statement of Financial Accounting Standards No. 123R,Share-Based Payment, in 2006.
Atlanta, Georgia
February 26, 2007
F-1
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED BALANCE SHEETS
| | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 1,873,586 | | | $ | 1,636,601 | |
Short-term investments | | | 14,090,000 | | | | 29,325,275 | |
Prepaid and other current assets | | | 405,040 | | | | 443,234 | |
| | | | | | | | |
Total current assets | | | 16,368,626 | | | | 31,405,110 | |
Property and equipment, net | | | 48,447 | | | | 83,915 | |
Other assets | | | — | | | | 22,388 | |
| | | | | | | | |
TOTAL ASSETS | | $ | 16,417,073 | | | $ | 31,511,413 | |
| | | | | | | | |
| | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 190,054 | | | $ | 538,193 | |
Accrued employee benefits | | | 1,068,200 | | | | 410,842 | |
Accrued clinical trial costs | | | 1,249,025 | | | | 3,003,666 | |
Other accrued liabilities | | | 147,304 | | | | 77,809 | |
Deferred revenue, current portion | | | 83,333 | | | | 83,333 | |
| | | | | | | | |
Total current liabilities | | | 2,737,916 | | | | 4,113,843 | |
Notes and interest payable | | | — | | | | 16,118,464 | |
Other long-term liabilities | | | — | | | | 1,596 | |
Deferred revenue, net of current portion | | | 631,945 | | | | 715,278 | |
| | | | | | | | |
TOTAL LIABILITIES | | | 3,369,861 | | | | 20,949,181 | |
| | |
Stockholders’ equity: | | | | | | | | |
Convertible Preferred Stock—$0.001 par value, 5,000,000 shares authorized: | | | | | | | | |
Series A Preferred Stock, no shares issued and outstanding | | | — | | | | — | |
Series B Preferred Stock, no shares issued and outstanding | | | — | | | | — | |
Series C Preferred Stock, 2,000 shares issued and outstanding, liquidation preference of $2,000,000 | | | 2 | | | | 2 | |
Series D Preferred Stock, 1,385,377 shares issued and outstanding, liquidation preference of $9,004,951 | | | 1,385 | | | | 1,385 | |
Series E Preferred Stock, 2,475,659 shares issued and outstanding, liquidation preference of $16,608,103 | | | 2,476 | | | | — | |
Common Stock—$0.001 par value, 100,000,000 shares authorized; 19,759,078 issued, 19,728,854 outstanding, as of December 31, 2006; and 19,728,703 issued, 19,698,479 outstanding, as of December 31, 2005 | | | 19,759 | | | | 19,729 | |
Additional paid-in capital | | | 134,348,855 | | | | 116,507,630 | |
Treasury stock – 30,224 shares at cost | | | (157,029 | ) | | | (157,029 | ) |
Deficit accumulated during development stage | | | (121,168,236 | ) | | | (105,809,485 | ) |
| | | | | | | | |
TOTAL STOCKHOLDERS’ EQUITY | | | 13,047,212 | | | | 10,562,232 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 16,417,073 | | | $ | 31,511,413 | |
| | | | | | | | |
See accompanying notes.
F-2
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | | | July 1, 1991
(inception) to
December 31, 2006 | |
| | | 2006 | | | 2005 | | | 2004 | | |
Revenues | | $ | 83,333 | | | $ | 83,333 | | | $ | 83,333 | | | $ | 2,827,068 | |
Costs and expenses: | | | | | | | | | | | | | | | | |
Cost of sales | | | — | | | | — | | | | — | | | | 821,878 | |
Research and development | | | 9,614,175 | | | | 15,949,455 | | | | 6,593,395 | | | | 67,625,094 | |
General and administrative | | | 6,213,722 | | | | 3,896,674 | | | | 3,681,173 | | | | 33,477,095 | |
Write-off of acquired in-process technology | | | — | | | | — | | | | — | | | | 24,405,005 | |
Write-off of property and equipment | | | 92,843 | | | | — | | | | — | | | | 2,686,885 | |
| | | | | | | | | | | | | | | | |
Total costs and expenses | | | 15,920,740 | | | | 19,846,129 | | | | 10,274,568 | | | | 129,015,957 | |
| | | | | | | | | | | | | | | | |
Loss from operations | | | (15,837,407 | ) | | | (19,762,796 | ) | | | (10,191,235 | ) | | | (126,188,889 | ) |
Other income (expense), net | | | — | | | | 42,821 | | | | 10,101 | | | | 137,708 | |
Interest expense | | | (497,860 | ) | | | (831,471 | ) | | | (451,705 | ) | | | (3,720,858 | ) |
Interest income | | | 976,516 | | | | 814,603 | | | | 250,866 | | | | 4,878,010 | |
| | | | | | | | | | | | | | | | |
Net loss | | $ | (15,358,751 | ) | | $ | (19,736,843 | ) | | $ | (10,381,973 | ) | | $ | (124,894,029 | ) |
Series E Preferred Stock dividend | | | (498,350 | ) | | | — | | | | — | | | | (498,350 | ) |
| | | | | | | | | | | | | | | | |
Net loss available to common shareholders | | $ | (15,857,101 | ) | | $ | (19,736,843 | ) | | $ | (10,381,973 | ) | | $ | (125,392,379 | ) |
| | | | | | | | | | | | | | | | |
Basic and diluted loss per share | | $ | (0.80 | ) | | $ | (1.16 | ) | | $ | (0.79 | ) | | | | |
| | | | | | | | | | | | | | | | |
Number of shares used in the computation of basic and diluted loss per share | | | 19,722,002 | | | | 16,954,398 | | | | 13,140,362 | | | | | |
| | | | | | | | | | | | | | | | |
See accompanying notes.
F-3
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Period July 1, 1991 (Inception) To December 31, 2006
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | Common Stock | | Additional
Paid-in
Capital | | Note
Receivable
From
Stockholder | | | Deficit
Accumulated
During
Development
Stage | | | Advances
From
Medstone | | | Divisional
Accumulated
Deficit | | | Accumulated
Other
Comprehensive
Income (loss) | | Total | |
| | | Number
of Shares | | Amount | | Number of Shares | | Amount | | | | | | | |
Advances and contributions from Medstone July 1, 1991 to December 31, 1997 | | | — | | $ | — | | — | | $ | — | | $ | — | | $ | — | | | $ | — | | | $ | 4,388,875 | | | $ | — | | | $ | — | | $ | 4,388,875 | |
Distribution of stock dividend and net assets February 9, 1996 | | | — | | | — | | 802,361 | | | 802 | | | 662,280 | | | — | | | | — | | | | (4,388,875 | ) | | | 3,725,793 | | | | — | | | — | |
Distribution of Common Stock for Services at $0.35 per share | | | — | | | — | | 51,857 | | | 52 | | | 18,098 | | | — | | | | — | | | | — | | | | — | | | | — | | | 18,150 | |
Issuance of Common Stock for cash upon exercise of options at $0.35 per share | | | — | | | — | | 201,429 | | | 201 | | | 70,299 | | | — | | | | — | | | | — | | | | — | | | | — | | | 70,500 | |
Issuance of Common Stock for cash and note receivable at $0.35 per share | | | — | | | — | | 21,113 | | | 21 | | | 7,369 | | | (7,242 | ) | | | — | | | | — | | | | — | | | | — | | | 148 | |
Net loss and comprehensive loss July 1, 1991 to December 31, 1997 | | | — | | | — | | — | | | — | | | — | | | — | | | | (743,175 | ) | | | — | | | | (3,725,793 | ) | | | — | | | (4,468,968 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1997 | | | — | | $ | — | | 1,076,760 | | $ | 1,076 | | $ | 758,046 | | $ | (7,242 | ) | | $ | (743,175 | ) | | $ | — | | | $ | — | | | $ | | | $ | 8,705 | |
Issuance of Preferred and Common Stock for equipment and acquired in-process technology at $630 and $4.41 per share, respectively, net of issuance costs of $83,366 | | | 5,830 | | | 6 | | 263,031 | | | 263 | | | 5,715,806 | | | — | | | | — | | | | — | | | | — | | | | — | | | 5,716,075 | |
Issuance of warrants for Common Stock valued at $4.13 per share | | | — | | | — | | — | | | — | | | 305,910 | | | — | | | | — | | | | — | | | | — | | | | — | | | 305,910 | |
Interest and other related to note receivable from stockholder | | | — | | | — | | — | | | — | | | 11,250 | | | (13,280 | | | | — | | | | — | | | | — | | | | — | | | (2,030 | ) |
Net loss and comprehensive loss | | | — | | | — | | — | | | — | | | — | | | — | | | | (7,962,388 | ) | | | — | | | | — | | | | — | | | (7,962,388 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1998 | | | 5,830 | | $ | 6 | | 1,339,791 | | $ | 1,339 | | $ | 6,791,012 | | $ | (20,522 | ) | | $ | (8,705,563 | ) | | $ | — | | | $ | — | | | $ | — | | $ | (1,933,728 | ) |
Issuance of Preferred Stock from conversion of advance from related party at $1,000 per share | | | 2,998 | | | 3 | | — | | | — | | | 2,997,997 | | | — | | | | — | | | | — | | | | — | | | | — | | | 2,998,000 | |
Issuance of Common Stock upon conversion of notes payable plus accrued interest at $7.00 and $2.10 per share | | | — | | | — | | 351,544 | | | 352 | | | 1,512,067 | | | — | | | | — | | | | — | | | | — | | | | — | | | 1,512,419 | |
Collection of note receivable | | | — | | | — | | — | | | — | | | — | | | 20,522 | | | | — | | | | — | | | | — | | | | — | | | 20,522 | |
Issuance of Common Stock for cash upon exercise of options at $0.35 per share | | | — | | | — | | 5,188 | | | 5 | | | 1,506 | | | — | | | | — | | | | — | | | | — | | | | — | | | 1,511 | |
Issuance of warrants valued at $1.26 and $1.40 per share | | | — | | | — | | — | | | — | | | 311,667 | | | — | | | | — | | | | — | | | | — | | | | — | | | 311,667 | |
Issuance of Common Stock for cash upon exercise of warrants at $0.007 per share | | | — | | | — | | 31,205 | | | 31 | | | 187 | | | — | | | | — | | | | — | | | | — | | | | — | | | 218 | |
Issuance of Common Stock for services at $2.03 per share | | | — | | | — | | 1,429 | | | 2 | | | 2,898 | | | — | | | | — | | | | — | | | | — | | | | — | | | 2,900 | |
Net loss and comprehensive loss | | | — | | | — | | — | | | — | | | — | | | — | | | | (3,818,827 | ) | | | — | | | | — | | | | — | | | (3,818,827 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1999 | | $ | 8,828 | | $ | 9 | | 1,729,157 | | $ | 1,729 | | $ | 11,617,334 | | $ | — | | | $ | (12,524,390 | ) | | $ | — | | | $ | — | | | $ | — | | $ | (905,318 | ) |
Continued on next page
F-4
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Period July 1, 1991 (Inception) To December 31, 2006 (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | Common Stock | | | Additional
Paid-in
Capital | | | Note
Receivable
From
Stockholder | | Deficit
Accumulated
During
Development
Stage | | | Advances
From
Medstone | | Divisional
Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income (loss) | | | Total | |
| | | Number
of Shares | | Amount | | Number of Shares | | | Amount | | | | | | | | |
Balance at December 31, 1999 | | 8,828 | | $ | 9 | | 1,729,157 | | | $ | 1,729 | | | $ | 11,617,334 | | | $ | — | | $ | (12,524,390 | ) | | $ | — | | $ | — | | $ | — | | | $ | (905,318 | ) |
Issuance of Preferred Stock from conversion of advance from related party at $1,000 per share | | 4,043 | | | 4 | | — | | | | — | | | | 4,043,075 | | | | — | | | — | | | | — | | | — | | | — | | | | 4,043,079 | |
Issuance of Common Stock, net of offering costs | | — | | | — | | 1,194,686 | | | | 1,195 | | | | 23,423,194 | | | | — | | | — | | | | — | | | — | | | — | | | | 23,424,389 | |
Issuance of Common Stock for cash upon exercise of options and warrants | | — | | | — | | 138,457 | | | | 138 | | | | 394,548 | | | | — | | | — | | | | — | | | — | | | — | | | | 394,686 | |
Net exercises of warrants for Common Stock | | — | | | — | | 141,707 | | | | 142 | | | | (142 | ) | | | — | | | — | | | | — | | | — | | | — | | | | — | |
Issuance of options at less than Fair Market Value | | — | | | — | | — | | | | — | | | | 217,200 | | | | — | | | — | | | | — | | | — | | | — | | | | 217,200 | |
Issuance of Common Stock for purchase of technology | | — | | | — | | 41,143 | | | | 41 | | | | 1,458,679 | | | | — | | | — | | | | — | | | — | | | — | | | | 1,458,720 | |
Issuance of Common Stock for services | | — | | | — | | 1,429 | | | | 1 | | | | 64,999 | | | | — | | | — | | | | — | | | — | | | — | | | | 65,000 | |
Issuance of stock options to consultant | | — | | | — | | — | | | | — | | | | 47,436 | | | | — | | | — | | | | — | | | — | | | — | | | | 47,436 | |
Issuance of warrants valued at $14.00 per share in connection with a loan agreement | | — | | | — | | — | | | | — | | | | 687,000 | | | | — | | | — | | | | — | | | — | | | — | | | | 687,000 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | — | | — | | | | — | | | | — | | | | — | | | (9,609,540 | ) | | | — | | | — | | | — | | | | (9,609,540 | ) |
Unrealized gain on short-term investments | | — | | | — | | — | | | | — | | | | — | | | | — | | | — | | | | — | | | — | | | 360,586 | | | | 360,586 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | — | | | — | | — | | | | — | | | | — | | | | — | | | — | | | | — | | | — | | | — | | | | (9,248,954 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2000 | | 12,871 | | $ | 13 | | 3,246,579 | | | $ | 3,246 | | | $ | 41,953,323 | | | $ | — | | $ | (22,133,930 | ) | | $ | — | | $ | — | | $ | 360,586 | | | $ | 20,183,238 | |
Issuance of Common Stock for cash upon exercise of options and warrants | | — | | | — | | 114,131 | | | | 114 | | | | 421,311 | | | | — | | | — | | | | — | | | — | | | — | | | | 421,425 | |
Issuance of Series B Preferred Stock from conversion of advance from related party at $1,000 per share | | 5,849 | | | 6 | | — | | | | — | | | | 5,848,355 | | | | — | | | — | | | | — | | | — | | | — | | | | 5,848,361 | |
Issuance of Series C Preferred Stock for cash at $1,000 per share | | 2,000 | | | 2 | | — | | | | — | | | | 1,999,998 | | | | — | | | — | | | | — | | | — | | | — | | | | 2,000,000 | |
Net exercises of warrants for Common Stock | | — | | | — | | 27,634 | | | | 28 | | | | (28 | ) | | | — | | | — | | | | — | | | — | | | — | | | | — | |
Proceeds from investor’s short-swing profit | | — | | | — | | — | | | | — | | | | 123,820 | | | | — | | | — | | | | — | | | — | | | — | | | | 123,820 | |
Extension of stock option exercise period for terminated employees | | — | | | — | | — | | | | — | | | | 35,504 | | | | — | | | — | | | | — | | | — | | | — | | | | 35,504 | |
Repurchase of restricted stock | | — | | | — | | (1,488 | ) | | | (1 | ) | | | (2,811 | ) | | | — | | | — | | | | — | | | — | | | — | | | | (2,812 | ) |
Stock grants to employees | | — | | | — | | 4,428 | | | | 4 | | | | 94,146 | | | | — | | | — | | | | — | | | — | | | — | | | | 94,150 | |
Issuance of options and warrants for services | | — | | | — | | — | | | | — | | | | 250,705 | | | | — | | | — | | | | — | | | — | | | — | | | | 250,705 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | — | | — | | | | — | | | | — | | | | — | | | (11,095,010 | ) | | | — | | | — | | | — | | | | (11,095,010 | ) |
Unrealized loss on short-term investments | | — | | | — | | — | | | | — | | | | — | | | | — | | | — | | | | — | | | — | | | (74,686 | ) | | | (74,686 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | — | | | — | | — | | | | — | | | | — | | | | — | | | — | | | | — | | | — | | | — | | | | (11,169,696 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2001 | | 20,720 | | $ | 21 | | 3,391,284 | | | $ | 3,391 | | | $ | 50,724,323 | | | $ | — | | $ | (33,228,940 | ) | | $ | — | | $ | — | | $ | 285,900 | | | $ | 17,784,695 | |
Continued on next page
F-5
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Period July 1, 1991 (Inception) To December 31, 2006 (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | | Common Stock | | Additional
Paid-in
Capital | | | Note
Receivable
From
Stockholder | | Deficit
Accumulated
During
Development
Stage | | | Advances
From
Medstone | | Divisional
Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income (loss) | | | Total | |
| | | Number of Shares | | | Amount | | | Number of Shares | | | Amount | | | | | | | |
Balance at December 31, 2001 | | 20,720 | | | $ | 21 | | | 3,391,284 | | | $ | 3,391 | | $ | 50,724,323 | | | $ | — | | $ | (33,228,940 | ) | | $ | — | | $ | — | | $ | 285,900 | | | $ | 17,784,695 | |
Issuance of Common Stock for cash upon exercise of options | | — | | | | — | | | 31,549 | | | | 31 | | | 67,939 | | | | — | | | — | | | | — | | | — | | | — | | | | 67,970 | |
Net exercises of warrants for Common Stock | | — | | | | — | | | 1,607 | | | | 2 | | | (2 | ) | | | — | | | — | | | | — | | | — | | | — | | | | — | |
Extension of stock option exercise period for terminated employees | | — | | | | — | | | — | | | | — | | | 47,564 | | | | — | | | — | | | | — | | | — | | | — | | | | 47,564 | |
Repurchase of restricted stock | | — | | | | — | | | (119 | ) | | | — | | | (268 | ) | | | — | | | — | | | | — | | | — | | | — | | | | (268 | ) |
Issuance of options and warrants for services and other | | — | | | | — | | | 7,072 | | | | 7 | | | 537,265 | | | | — | | | — | | | | — | | | — | | | — | | | | 537,272 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | | — | | | — | | | | — | | | — | | | | — | | | (12,053,583 | ) | | | — | | | — | | | — | | | | (12,053,583 | ) |
Unrealized loss on short-term investments | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | (286,821 | ) | | | (286,821 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | — | | | | (12,340,404 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2002 | | 20,720 | | | $ | 21 | | | 3,431,393 | | | $ | 3,431 | | $ | 51,376,821 | | | $ | — | | $ | (45,282,523 | ) | | $ | — | | $ | — | | $ | (921 | ) | | $ | 6,096,829 | |
Issuance of Common Stock for cash upon exercise of options | | — | | | | — | | | 132,365 | | | | 133 | | | 86,133 | | | | — | | | — | | | | — | | | — | | | — | | | | 86,266 | |
Net exercises of warrants for Common Stock | | — | | | | — | | | 10,436 | | | | 10 | | | (10 | ) | | | — | | | — | | | | — | | | — | | | — | | | | — | |
Conversion of Preferred Stock into Common stock | | (18,720 | ) | | | (19 | ) | | 1,120,131 | | | | 1,120 | | | (1,101 | ) | | | — | | | — | | | | — | | | — | | | — | | | | — | |
Issuance of options and warrants for services and other | | — | | | | — | | | — | | | | — | | | 724,407 | | | | — | | | — | | | | — | | | — | | | — | | | | 724,407 | |
Issuance of options at less than fair market value | | — | | | | — | | | — | | | | — | | | 164,490 | | | | — | | | — | | | | — | | | — | | | — | | | | 164,490 | |
Issuance of Common Stock for Services at $5.75 per share | | — | | | | — | | | 8,696 | | | | 9 | | | 49,991 | | | | — | | | — | | | | — | | | — | | | — | | | | 50,000 | |
Extension of stock option exercise period for terminated employees | | — | | | | — | | | — | | | | — | | | 68,340 | | | | — | | | — | | | | — | | | — | | | — | | | | 68,340 | |
Issuance of Common Stock and Stock Purchase Warrants for Cash | | — | | | | — | | | 541,690 | | | | 542 | | | 2,149,426 | | | | — | | | — | | | | — | | | — | | | — | | | | 2,149,968 | |
Issuance of Common Stock in connection with Merger | | — | | | | — | | | 5,320,166 | | | | 5,320 | | | 15,468,429 | | | | — | | | — | | | | — | | | — | | | — | | | | 15,473,749 | |
Issuance of Preferred Stock for Cash, net of issuance costs of $915,624 | | 1,385,377 | | | | 1,385 | | | — | | | | — | | | 8,082,991 | | | | — | | | — | | | | — | | | — | | | — | | | | 8,084,376 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | — | | | | — | | | — | | | | — | | | — | | | | — | | | (30,408,146 | ) | | | — | | | — | | | — | | | | (30,408,146 | ) |
Unrealized loss on short-term investments reclassified into earnings | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | 921 | | | | 921 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | | — | | | — | | | — | | | | (30,407,225 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2003 | | 1,387,377 | | | $ | 1,387 | | | 10,564,877 | | | $ | 10,565 | | $ | 78,169,917 | | | $ | — | | $ | (75,690,669 | ) | | $ | — | | $ | — | | $ | — | | | $ | 2,491,200 | |
Continued on next page.
F-6
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Period July 1, 1991 (Inception) To December 31, 2006 (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | Common Stock | | | | | Treasury Stock | | | | | | | |
| | | Number of
Shares | | Amount | | Number of
Shares | | Amount | | Additional
Paid-in Capital | | | Number of Shares | | Amount | | | Deficit
Accumulated
During
Development
Stage | | | Total | |
Balance at December 31, 2003 | | 1,387,377 | | $ | 1,387 | | 10,564,877 | | $ | 10,565 | | $ | 78,169,917 | | | — | | | — | | | $ | (75,690,669 | ) | | $ | 2,491,200 | |
Issuance of Common Stock for cash upon exercise of options and warrants | | — | | | — | | 122,550 | | | 122 | | | 224,512 | | | — | | | — | | | | — | | | | 224,634 | |
Net exercises of warrants for Common Stock | | — | | | — | | 22,003 | | | 22 | | | (22 | ) | | — | | | — | | | | — | | | | — | |
Issuance of options and warrants for services and other | | — | | | — | | — | | | — | | | 479,000 | | | — | | | — | | | | — | | | | 479,000 | |
Issuance of options at less than fair market value | | — | | | — | | — | | | — | | | 6,720 | | | — | | | — | | | | — | | | | 6,720 | |
Extension of stock option exercise period for terminated employees | | — | | | — | | — | | | — | | | 43,992 | | | — | | | — | | | | — | | | | 43,992 | |
Issuance of Common Stock and Stock Purchase Warrants for Cash, net | | — | | | — | | 3,812,811 | | | 3,813 | | | 17,986,879 | | | — | | | — | | | | — | | | | 17,990,692 | |
Issuance of Common Stock pursuant to indemnification agreement from merger | | — | | | — | | 13,576 | | | 14 | | | 77,641 | | | — | | | — | | | | — | | | | 77,655 | |
Issuance of Common Stock pursuant to lease settlement agreement | | — | | | — | | 53,180 | | | 53 | | | 299,947 | | | — | | | — | | | | — | | | | 300,000 | |
Net loss and comprehensive loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | | (10,381,973 | ) | | | (10,381,973 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2004 | | 1,387,377 | | $ | 1,387 | | 14,588,997 | | $ | 14,589 | | $ | 97,288,586 | | | — | | | — | | | $ | (86,072,642 | ) | | $ | 11,231,920 | |
Issuance of Common Stock for cash or exchange for common stock owned upon exercise of options and warrants | | — | | | — | | 204,228 | | | 204 | | | 409,458 | | | 30,224 | | | (157,029 | ) | | | — | | | | 252,633 | |
Net exercises of warrants for Common Stock | | — | | | — | | 4,152 | | | 5 | | | (5 | ) | | | | | — | | | | — | | | | — | |
Issuance of Common Stock for Cash, net | | — | | | — | | 4,745,319 | | | 4,745 | | | 17,909,777 | | | | | | — | | | | — | | | | 17,914,522 | |
Issuance of Common Stock pursuant to lease settlement agreement | | — | | | — | | 186,007 | | | 186 | | | 899,814 | | | | | | — | | | | — | | | | 900,000 | |
Net loss and comprehensive loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | | (19,736,843 | ) | | | (19,736,843 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | 1,387,377 | | $ | 1,387 | | 19,728,703 | | $ | 19,729 | | $ | 116,507,630 | | | 30,224 | | $ | (157,029 | ) | | $ | (105,809,485 | ) | | $ | 10,562,232 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Continued on next page.
F-7
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Period July 1, 1991 (Inception) To December 31, 2006 (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Preferred Stock | | Common Stock | | | | | Treasury Stock | | | | | | | |
| | | Number of Shares | | Amount | | Number of Shares | | Amount | | Additional
Paid-in Capital | | | Number
of Shares | | Amount | | | Deficit
Accumulated
During
Development
Stage | | | Total | |
Balance at December 31, 2005 | | 1,387,377 | | $ | 1,387 | | 19,728,703 | | $ | 19,729 | | $ | 116,507,630 | | | 30,224 | | $ | (157,029 | ) | | $ | (105,809,485 | ) | | $ | 10,562,232 | |
Issuance of Common Stock for cash upon exercise of warrants and through Employee Stock Purchase Plan | | — | | | — | | 18,471 | | | 18 | | | 29,480 | | | — | | | — | | | | — | | | | 29,498 | |
Issuance of options and warrants for services and other | | — | | | — | | — | | | — | | | 12,250 | | | — | | | — | | | | — | | | | 12,250 | |
Net exercises of warrants for Common Stock | | — | | | — | | 12,653 | | | 13 | | | (13 | ) | | — | | | — | | | | — | | | | — | |
Non-cash stock based compensation expense | | — | | | — | | — | | | — | | | 1,193,881 | | | — | | | — | | | | — | | | | 1,193,881 | |
Conversion of long-term debt into Series E Preferred Stock | | 2,475,659 | | | 2,476 | | — | | | — | | | 16,605,627 | | | — | | | — | | | | — | | | | 16,608,103 | |
Net loss and comprehensive loss | | — | | | — | | — | | | — | | | — | | | — | | | — | | | | (15,358,751 | ) | | | (15,358,751 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | 3,863,036 | | $ | 3,863 | | 19,759,078 | | $ | 19,759 | | $ | 134,348,855 | | | 30,224 | | $ | (157,029 | ) | | $ | (121,168,236 | ) | | $ | 13,047,212 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-8
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | | | July 1, 1991
(inception) to
December 31,
2006 | |
| | | 2006 | | | 2005 | | | 2004 | | |
Cash flows from operating activities: | | | | | | | | | | | | | | | | |
Net loss | | $ | (15,358,751 | ) | | $ | (19,736,843 | ) | | $ | (10,381,973 | ) | | $ | (124,894,029 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | |
Write-off of in-process technology acquired with stock | | | — | | | | — | | | | 77,655 | | | | 23,560,174 | |
Expenses satisfied via advances from related party | | | — | | | | — | | | | — | | | | 695,557 | |
Depreciation and amortization | | | 61,785 | | | | 105,886 | | | | 215,088 | | | | 3,787,721 | |
Non-cash stock based compensation expense | | | 1,193,881 | | | | — | | | | — | | | | 1,193,882 | |
Accrued interest satisfied through issuance of Common Stock | | | — | | | | — | | | | — | | | | 40,245 | |
Stock, stock options and warrants issued for services, extension of stock option exercise period and issuance of options below fair market value | | | 12,250 | | | | — | | | | 154,711 | | | | 2,147,334 | |
Charge related to lease termination settlement | | | — | | | | — | | | | — | | | | 3,423,791 | |
Loss (Gain) on disposal of property and equipment | | | 92,843 | | | | (31,027 | ) | | | (9,091 | ) | | | 3,090,323 | |
Amortization of debt discount and loan fee | | | — | | | | — | | | | — | | | | 1,261,297 | |
Deferred rent | | | — | | | | — | | | | (13,160 | ) | | | — | |
Deferred revenue | | | (83,333 | ) | | | (83,333 | ) | | | (83,333 | ) | | | 715,278 | |
Change in operating assets and liabilities, net of acquisition: | | | | | | | | | | | | | | | | |
Prepaids and other current assets | | | 38,194 | | | | 272,102 | | | | (31,186 | ) | | | (303,899 | ) |
Other assets | | | 22,388 | | | | (6,391 | ) | | | (6,053 | ) | | | (780,276 | ) |
Accounts payable | | | (348,139 | ) | | | (479,424 | ) | | | 874,912 | | | | (130,629 | ) |
Other current liabilities | | | (1,021,581 | ) | | | 2,782,299 | | | | (20,235 | ) | | | 2,503,287 | |
Other long-term liabilities | | | — | | | | — | | | | — | | | | (13,160 | ) |
Lease settlement obligation | | | — | | | | (955,901 | ) | | | (925,000 | ) | | | (1,880,901 | ) |
| | | | | | | | | | | | | | | | |
Net cash used in operating activities | | | (15,390,463 | ) | | | (18,132,632 | ) | | | (10,147,665 | ) | | | (85,584,005 | ) |
| | | | | | | | | | | | | | | | |
| | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | |
Purchase of short-term investments | | | (35,515,000 | ) | | | (54,874,969 | ) | | | (56,050,000 | ) | | | (191,438,269 | ) |
Sale of short-term investments | | | 50,750,275 | | | | 50,364,969 | | | | 31,935,000 | | | | 178,048,544 | |
Purchase of property and equipment | | | (7,698 | ) | | | (48,147 | ) | | | (24,720 | ) | | | (5,746,887 | ) |
Proceeds from sale of property and equipment | | | — | | | | 32,678 | | | | 47,039 | | | | 241,870 | |
| | | | | | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | 15,227,577 | | | | (4,525,469 | ) | | | (24,092,681 | ) | | | (18,894,742 | ) |
| | | | | | | | | | | | | | | | |
| | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | |
Advances from related party | | | — | | | | — | | | | — | | | | 12,193,883 | |
Repayment of note receivable from stockholder | | | — | | | | — | | | | — | | | | 20,000 | |
Proceeds from investor’s short-swing profit | | | — | | | | — | | | | — | | | | 123,820 | |
Proceeds from notes payable | | | — | | | | 5,000,000 | | | | 7,500,000 | | | | 18,415,292 | |
Interest payable | | | 489,639 | | | | 788,987 | | | | 329,477 | | | | 1,608,103 | |
Repayment of capital lease and notes payable | | | (114,766 | ) | | | (33,322 | ) | | | (344,569 | ) | | | (3,414,719 | ) |
Proceeds from issuance of Common Stock upon exercise of options and warrants | | | 24,998 | | | | 252,633 | | | | 224,634 | | | | 1,543,251 | |
Proceeds from sale of Common Stock, net of issuance costs | | | — | | | | 17,914,522 | | | | 17,990,692 | | | | 61,397,942 | |
Proceeds from issuance of Series C Preferred Stock | | | — | | | | — | | | | — | | | | 2,000,000 | |
Proceeds from issuance of Series D Preferred Stock, net of issuance costs | | | — | | | | — | | | | — | | | | 8,084,376 | |
Repurchase of restricted shares of common stock | | | — | | | | — | | | | — | | | | (3,080 | ) |
Net advances from Medstone | | | — | | | | — | | | | — | | | | 3,883,465 | |
Capital contribution by Medstone | | | — | | | | — | | | | — | | | | 500,000 | |
| | | | | | | | | | | | | | | | |
Net cash provided by financing activities | | | 399,871 | | | | 23,922,820 | | | | 25,700,234 | | | | 106,352,333 | |
| | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 236,985 | | | | 1,264,719 | | | | (8,540,112 | ) | | | 1,873,586 | |
Cash and cash equivalents, beginning of period | | | 1,636,601 | | | | 371,882 | | | | 8,911,994 | | | | — | |
| | | | | | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 1,873,586 | | | $ | 1,636,601 | | | $ | 371,882 | | | $ | 1,873,586 | |
| | | | | | | | | | | | | | | | |
See Notes 3, 4, 6 and 11 for supplemental cash flow information.
See accompanying notes.
F-9
CORAUTUS GENETICS INC.
(A DEVELOPMENT STAGE ENTERPRISE)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Organization
Corautus Genetics Inc. (the “Company”) is a development stage company dedicated to the development of innovative products in the life sciences industry.
The Company was incorporated as UroGen Corp. in the State of Delaware on June 30, 1995, as a wholly-owned subsidiary of Medstone International, Inc. Between July 1, 1991 (Inception) and June 30, 1995, the Company operated as a division of Medstone. On December 29, 1995, Medstone declared a dividend of all of the stock of UroGen Corp. to be distributed to all Medstone stockholders. In March 2000, the Company’s name was changed to GenStar Therapeutics Corporation.
On February 5, 2003, the Company completed a merger with Vascular Genetics Inc. and concurrently changed the Company’s name from GenStar Therapeutics Corporation to Corautus Genetics Inc. The Company’s focus from February 2003 through October 30, 2006 was the clinical development of gene therapy products using a vascular growth factor gene known as Vascular Endothelial Growth Factor 2, or VEGF-2, for the treatment of severe cardiovascular and vascular disease.
On October 30, 2006, the Board of Directors of Corautus determined that it was in the best interests of the Company and its stockholders at the present time to not conduct further clinical trials for VEGF-2 for the treatment of cardiovascular and peripheral vascular disease. Corautus began to immediately reduce its cash spending and continued identifying other life sciences technologies that it could acquire or in-license, or other life sciences opportunities. In connection with the realignment of our business activities, the Company incurred approximately $1.4 million in severance and related costs. See Note 14 for more information about the Company’s activities.
Basis of Presentation
The consolidated financial statements include the accounts of Corautus Genetics Inc. and its wholly-owned subsidiary, Vascular Genetics Inc. All significant intercompany transactions and balances have been eliminated in consolidation. As the Company is devoting its efforts to research and the development of its products and there has been no revenue generated from product sales, the Company’s financial statements are presented as statements of a development stage enterprise.
Revenue Recognition
Grant revenue is recognized as the research expenses related to the grants are incurred. Contract and license revenue arising from collaborative research agreements is recognized either (i) ratably over the term of the agreement, which approximates the performance of services, for contracts specifying payment for services over a given period, or (ii) as services are performed under the agreement, for contracts specifying payment on a per full-time employee basis. All amounts received under research grants or from collaborative research agreements are not refundable, regardless of the success of the underlying research. For the years ended December 31, 2006, 2005, and 2004, all revenue was from the license fee related to certain intellectual property licensed to Boston Scientific Corporation, which is being recognized as revenue over the licenses’ expected life of 12 years.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. The carrying amount of cash and cash equivalents approximates fair value at December 31, 2006 and 2005.
Short-Term Investments
In accordance with the Statement of Financial Accounting Standards (“SFAS”) No. 115, Accounting for Certain Investments in Debt and Equity Securities, management determines the appropriate classification of short-term investment securities at the time of purchase and reevaluates such designation as of each balance sheet date. Securities classified as available-for-sale are carried at fair value based upon quoted market prices, with gains and losses, if any, reported as a separate component of stockholders’ equity and included in comprehensive loss. Realized gains and losses are calculated on the specific identification method and are recorded as
F-10
interest income. As of December 31, 2006 and 2005, the Company had short-term investments in Auction Rate Securities, or ARS. ARS generally have long-term stated maturities of 20 to 30 years. However, these securities have certain economic characteristics of short-term investments due to a rate-setting mechanism and the ability to liquidate them through a Dutch auction process that occurs on pre-determined intervals of less than 90 days. As such, these investments are classified as short-term investments. These investments are classified as available-for-sale securities due to management’s intent regarding these securities. As of December 31, 2006 and 2005, there were no unrealized gains or losses associated with these investments and the adjusted fair market value equaled the adjusted cost.
Property and Equipment
Property and equipment is stated at cost. Depreciation is computed using the straight-line method over the useful lives of the assets, estimated at three to five years, or the term of the lease if shorter. Depreciation expense for the years ended December 31, 2006, 2005 and 2004 was $61,785, $61,939, and $78,278, respectively.
Patents
Costs related to filing and pursuing patent applications are expensed as incurred as recoverability of such expenditures is uncertain.
Accrued Liabilities
The Company estimates expenses related to costs that have been incurred, but have not yet been billed. This process involves identifying services and activities that have been performed by third-party vendors on our behalf and estimating the level to which they have been performed and the associated cost incurred for such service as of each balance sheet date. These costs include fees for professional services, such as those provided by certain clinical research organizations and investigators in conjunction with clinical trials, and fees owed to contract manufacturers in conjunction with the manufacture of material for planned clinical trials and commercial use.
Stock Based Compensation
On January 1, 2006, the Company adopted Statement of Financial Accounting Standards No. 123R (revised 2004),Share-Based Payment, (“SFAS 123(R)”) which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors including employee stock options and employee stock purchases under the Employee Stock Purchase Plan (“ESPP”) based on estimated fair values. SFAS 123(R) supersedes previous accounting under Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees (“APB 25”) for periods beginning in fiscal year 2006. In March 2005, the SEC issued Staff Accounting Bulletin No. 107 (“SAB 107”) providing supplemental implementation guidance for SFAS 123(R). The Company has applied the provisions of SAB 107 in its adoption of SFAS 123(R).
SFAS 123(R) requires companies to estimate the fair value of stock-based awards on the date of grant using an option pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service period in the Company’s Consolidated Statements of Operations. The Company adopted SFAS 123(R) using the modified prospective transition method which requires the application of the accounting standard effective January 1, 2006. The Company’s Consolidated Financial Statements, as of December 31, 2006 and for the year then ended reflect the impact of SFAS 123(R). Stock-based compensation expense for the year ended December 31, 2006, was approximately $1,194,000, which consisted primarily of stock-based compensation expense related to employee stock options and the ESPP recognized under SFAS 123(R). Of the total for the year ended December 31, 2006, approximately $200,000 is included in research and development costs and approximately $994,000 is included in general and administrative costs.
Prior to the adoption of SFAS 123(R), the Company accounted for stock-based awards to employees and directors using the intrinsic value method in accordance with APB 25 as allowed under Statement of Financial Accounting Standards No. 123,Accounting for Stock-Based Compensation (“SFAS 123”). Under the intrinsic value method, no stock-based compensation expense for employee stock options had been recognized in the Consolidated Statements of Operations because the exercise price of the Company’s stock options granted to employees and directors equaled the fair market value of the underlying stock at the date of grant. In accordance with the modified prospective transition method the Company used in adopting SFAS 123(R), the results of operations prior to fiscal year 2006 have not been restated to reflect, and do not include, the impact of SFAS 123(R).
Generally, the Company grants stock options with a contractual term of ten years from the date of grant with a vesting term of 1 to 2 years.
The table below reflects net loss and net loss per share for the year ended December 31, 2006 compared with the pro forma information for the years ended December 31, 2005 and 2004:
| | | | | | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
Net loss available to common stockholders, as reported | | $ | (15,857,101 | ) | | $ | (19,736,843 | ) | | $ | (10,381,973 | ) |
Add: Stock based employee compensation included in net loss | | | 1,193,881 | | | | — | | | | 50,712 | |
Deduct: Total stock based employee compensation expense determined under fair value based methods for all awards | | | (1,193,881 | ) | | | (2,014,828 | ) | | | (1,923,999 | ) |
| | | | | | | | | | | | |
Net loss available to common stockholders, pro forma | | $ | (15,857,101 | ) | | $ | (21,751,671 | ) | | $ | (12,255,260 | ) |
| | | | | | | | | | | | |
Basic and diluted loss per share, as reported | | $ | (0.80 | ) | | $ | (1.16 | ) | | $ | (0.79 | ) |
| | | | | | | | | | | | |
Basic and diluted loss per share, pro forma | | $ | (0.80 | ) | | $ | (1.28 | ) | | $ | (0.93 | ) |
| | | | | | | | | | | | |
F-11
As of December 31, 2006, total unamortized stock-based compensation cost related to non-vested stock options was approximately $260,000, which is expected to be recognized over the remaining vesting period of each grant over the next 18 months.
Upon the adoption of SFAS No. 123R, the Company selected the Black-Scholes option-pricing model as the most appropriate model for determining the estimated fair value for stock-based awards. The use of the Black-Scholes model requires the use of extensive actual employee exercise behavior data and the use of a number of complex assumptions including expected volatility, risk-free interest rate, and expected dividends. The following are the weighted-average assumptions for 2006, 2005, and 2004: a risk-free interest rate of 5.1%, 3.8%, and 2.7%, respectively, a dividend yield of 0% for all periods, a volatility factor of the expected market price of the Company’s common stock of 93%, 54%, and 93%, respectively, and an expected life of the options ranging from two to five years.
The Company estimated the volatility of its stock using historical volatility. The Company will continue to monitor relevant factors used to measure expected volatility for future option grants. Prior to the adoption of SFAS 123(R), the Company also had used its historical stock price volatility in accordance with SFAS 123 for purposes of pro forma information disclosed in the Notes to Consolidated Financial Statements.
The risk-free interest rate assumption is based upon observed interest rates appropriate for the expected term of the Company’s employee stock options. The dividend yield assumption is based on the Company’s history and expectation of dividend payouts.
After January 1, 2006, the expected term of employee stock options is the average between the contractual term and the vesting term as permitted by guidance in SFAS 123(R) and SAB 107. Prior to the adoption of SFAS 123(R), the Company used an estimate of expected term for the purposes of pro forma information under SFAS 123, as disclosed in the Notes to Consolidated Financial Statements for the related periods.
As stock-based compensation expense recognized in the results for the year ended December 31, 2006 is based on awards ultimately expected to vest, the amount has been reduced for estimated forfeitures. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on our historical experience. Prior to fiscal year 2006, the Company accounted for forfeitures as they occurred for the purposes of its pro forma information under SFAS 123, as disclosed in the Notes to Consolidated Financial Statements for the related periods.
The value of options, warrants, or stock awards issued to non-employees have been determined in accordance with SFAS No. 123R, and Emerging Issues Task Force Issue No. 96-18,Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods and Services, and are periodically remeasured as the options vest, if required. Warrants for common stock are valued at the date of issuance and are recorded to the expense category related to the services provided.
Segment Reporting
SFAS No. 131, Disclosures About Segments of an Enterprise and Related Information, requires the use of a management approach in identifying segments of an enterprise. Management has determined that the Company operates in one business segment, which is scientific research and development activities.
Net Loss Per Share
Net loss per share is computed on the basis of the weighted average number of common shares outstanding during the periods presented. Loss per share assuming dilution is computed on the basis of the weighted-average number of common shares outstanding and the dilutive effect of all common stock equivalents and convertible securities. Net loss per share assuming dilution for the years ended December 31, 2006, 2005 and 2004 is equal to net loss per share since the effect of common stock equivalents outstanding during the periods, including stock options, warrants and convertible, are antidilutive. Dividends on the Series E Preferred Stock are deducted from net loss to compute net loss and per share net loss available to common stockholders from July 1, 2006 forward. See Note 4 below.
F-12
Income Taxes
The Company accounts for income taxes under SFAS No. 109,Accounting for Income Taxes, which requires that provision be made for taxes currently due and for the expected future tax effects of temporary differences between book and tax bases of assets and liabilities.
Research and Development
Research and development costs are expensed as incurred.
Comprehensive Income (Loss)
SFAS No. 130, Reporting Comprehensive Income, requires that all components of comprehensive income or loss, including net income or loss, be reported in the financial statements in the period in which they are recognized. Comprehensive income or loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and other comprehensive income (loss), including foreign currency translation adjustments and unrealized gains and losses on investments, shall be reported, net of their related tax effect, to arrive at comprehensive loss. Comprehensive loss for the years ended December 31, 2006, 2005, and 2004 was the same as the net loss for those periods.
Use of Estimates
The preparation of financial statements in accordance with U. S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
New Accounting Pronouncements
In March 2005, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 154,Accounting Changes and Error Corrections(“SFAS 154”), which replaces Accounting Principles Board (“APB”) Opinion No. 20,Accounting Changes, and SFAS 3,Reporting Accounting Changes in Interim Financial Statement, and changes the requirements for the accounting for and the reporting of a change in accounting principle. SFAS 154, which became effective for all accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005, had no effect on the Company’s financial position or results of operations.
In February 2006, the FASB issued SFAS No. 155,Accounting for Certain Hybrid Financial Instruments (“SFAS 155”), which amends SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities and SFAS No. 140,Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities, aimed at improving the financial reporting of certain hybrid financial instruments by requiring more consistent accounting that eliminates exemptions and provides a means to simplify the accounting for these instruments. SFAS 155 is effective for all financial instruments acquired or issued in fiscal years beginning after September 15, 2006. The Company does not expect that the adoption of SFAS 155 will have any impact on its financial position and results of operations.
In June 2006, the FASB issued FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes (“FIN 48”), which clarifies when tax benefits should be recorded in financial statements, requires certain disclosures of uncertain tax matters and indicates how any tax reserves should be classified in a balance sheet. The Company has not determined the impact, if any, the adoption of FIN 48, which is effective for fiscal years beginning after December 15, 2006, will have on its financial position and results of operations.
In September 2006, the FASB issued SFAS No. 157,“Fair Value Measurements” (“SFAS 157”). SFAS 157 provides guidance for using fair value to measure assets and liabilities. It also responds to investors’ requests for expanded information about the extent to which companies measure assets and liabilities at fair value, the information used to measure fair value, and the effect of fair value measurements on earnings. SFAS 157 applies whenever other standards require (or permit) assets or liabilities to be measured at fair value, and does not expand the use of fair value in any new circumstances. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and is required to be adopted by the Company in its first quarter of fiscal 2008. The Company has not determined the impact, if any, the adoption of SFAS 157 will have on its financial position and results of operations.
F-13
2. Property and Equipment
Property and equipment are comprised of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
Equipment | | $ | 367,792 | | | $ | 394,860 | |
Furniture and fixtures | | | 17,527 | | | | 19,723 | |
| | | | | | | | |
| | | 385,319 | | | | 414,583 | |
Accumulated depreciation | | | (336,872 | ) | | | (330,668 | ) |
| | | | | | | | |
| | $ | 48,447 | | | $ | 83,915 | |
| | | | | | | | |
Computer and laboratory equipment with a net book value of approximately $23,000 was written off and disposed of in December 2006 in connection with the Company’s reduction in workforce and relocation of its offices to a smaller office.
3. Capital Lease Obligation
During 2005, the Company financed $6,526 of computer equipment under a capital lease arrangement that originally had a maturity date in April 2007. This capital lease was paid in full in January 2007.
In April 2006, the Company executed a capital lease for approximately $114,000 of office furniture. Total payments of approximately $146,000 were scheduled to be made over a five year period. In December 2006, due to the reduction in employees and the Company’s relocation to a smaller office, the Company paid off the remaining balance of the lease and wrote the furniture down by approximately $70,000 to its estimated fair value of $27,500. The Company sold the furniture in January 2007 and recorded no gain or loss at that time on the disposal.
4. Notes and Interest Payable
The Company entered into 4 senior convertible promissory notes payable with Boston Scientific Corporation (“BSC”), each of which bore interest at 6% and were only convertible into shares of common stock upon a change in control. Each note was to be repaid in three equal, annual payments beginning on the fifth anniversary of the note, additional details are as follows:
| | | | | | | | | | | | |
| | | Note 1 | | Note 2 | | Note 3 | | Note 4 |
Date of note | | | June 30, 2005 | | | September 22, 2004 | | | July 22, 2004 | | | December 31, 2003 |
Proceeds from note | | $ | 5,000,000 | | $ | 2,500,000 | | $ | 5,000,000 | | $ | 2,500,000 |
Convertible into number of common shares | | | 933,911 | | | 376,288 | | | 675,740 | | | 250,011 |
On June 30, 2006, the Company entered into a Recapitalization Agreement with BSC, pursuant to which BSC exchanged all of its Promissory Notes, with an aggregate principal amount of $15,000,000, together with accrued interest of $1,608,103 through June 30, 2006, for 2,475,659 shares of the Company’s Series E Preferred Stock with a liquidation preference of $6.71 per share and is entitled to a cumulative annual dividend of 6%. The Series E Preferred Stock is convertible into 2,475,659 shares of Corautus’ common stock, subject to adjustment, and votes with the common stock. The agreement was executed and closed on June 30, 2006. The dividends on the Series E Preferred Stock are payable upon declaration by the board of directors on January 1 and July 1 of each year and are payable in additional shares of Series E Preferred Stock until January 1, 2009, when such dividends will be payable in cash from then forward. The Company’s board of directors has not declared any dividends on the Series E Preferred Stock as of December 31, 2006.
5. Acquisition of Technology and Related Agreements
On February 5, 2003, in connection with the merger with Vascular Genetics, a total of 5,320,166 shares of the Company’s common stock were issued to Vascular Genetics stockholders. Additionally, the Company assumed options to purchase 229,648
F-14
shares of common stock and warrants to purchase 19,164 shares of common stock. In connection with the acquisition, the Company also reserved 556,904 shares of common stock for potential indemnity obligations to former Vascular Genetics stockholders. In September 2004, the indemnity period for the merger ended and Corautus issued an additional 13,576 shares of common stock to the former Vascular Genetics stockholders resulting from claims asserted against Corautus under the indemnity provisions. Corautus recorded an expense in the third quarter of 2004 of $77,655 representing the fair value of the 13,576 shares computed in accordance with the merger agreement. The acquisition was accounted for as a purchase of assets by the Company for financial reporting purposes, in accordance with U. S. generally accepted accounting principles. Pursuant to the Emerging Issues Task Force’s Issue No. 98-3,Determining Whether a Nonmonetary Transaction Involves the Receipt of Productive Assets or of a Business, Vascular Genetics did not meet the criteria necessary to qualify as a business. Therefore, the Company’s acquisition of Vascular Genetics did not qualify as a business combination under SFAS No. 141,Business Combinations, and no goodwill resulted from the recording of the transaction. The value of the in-process research and development was charged to operations upon the close of the merger. The value allocated to acquired workforce of $80,000 was amortized over two years. Amortization expense in 2005, 2004, and 2003 was $3,334, $40,000, and $36,667, respectively, and is included in general and administrative expenses.
In May 2000, the Company acquired all of the outstanding shares of Allegro Cell Systems, Inc. in exchange for 41,143 shares of common stock and an obligation to issue an additional 1,714 shares of common stock. The additional 1,714 shares of common stock were issued during 2002. Allegro’s sole asset was a license to certain technologies for the treatment and prevention of AIDS and for lentiviral gene therapy. Allegro had no products, revenues, employees, facilities or other assets. The shares issued to acquire the technologies were valued at $1,458,720 based on the fair value of the common stock on the date of the agreement which was charged to acquired in-process technology due to the early stage of development of the technology and the lack of alternative future uses for it.
In July 1998, the Company executed various agreements with Baxter Healthcare pursuant to which the Company acquired certain rights and assets from Baxter Healthcare. Under the terms of the agreements, the Company obtained the rights to Baxter Healthcare’s adenoviral-based gene transfer technologies and certain equipment in exchange for 5,830 shares of non-voting convertible Series A Preferred Stock and 263,031 shares of common stock. The shares issued to acquire the gene transfer technologies were valued at $5,455,505 based upon a discounted cash flow analysis, estimated research and development costs for six years and product revenues commencing in year seven, after the assumed completion of clinical trials and product approval by the U.S. Food and Drug Administration, or FDA. The value of the technology was charged to acquired in-process technology because technological feasibility had not been achieved nor had alternative future uses of the technology been identified. The value of the stock issued for property and equipment was $343,937 based upon the fair value of those assets.
Under the terms of the Company’s Developmental Collaboration Agreement and Credit Agreement with Baxter Healthcare, Baxter Healthcare was required to provide funding for pre-clinical development of the hemophilia product. When the Company treated the first patient in a Phase I clinical trial for the hemophilia product, a milestone payment of $2,000,000 was due from Baxter Healthcare. On June 13, 2001, the first patient was treated using the hemophilia product, and Baxter Healthcare paid the $2,000,000 milestone payment in exchange for 2,000 shares of Series C Preferred Stock. Additionally, the remaining balance under the Credit Agreement of approximately $5,848,000 was converted to Series B Preferred Stock in August 2001. The Series B Preferred Stock was convertible at Baxter Healthcare’s option into 287,274 shares of common stock and was converted to common stock in February 2003 in connection with the closing of the merger with Vascular Genetics. (See Note 6).
The Company entered into a Distribution Agreement with Baxter Healthcare whereby Baxter Healthcare has an exclusive, worldwide right to market, sell and distribute all products that may be developed under the Developmental Collaboration Agreement. The term of the Distribution Agreement is the longer of ten years from the date of regulatory approval of the first product or the expiration of the last to expire of any related patents issued on or before ten years from the date of regulatory approval.
The Company, Baxter Healthcare and certain founding stockholders of the Company entered into an Investor Rights Agreement under which the shares held by these entities were subject to certain restrictions on transfer until July 8, 2003 and these entities have certain registration rights. Additionally, under this agreement, Baxter Healthcare has the obligation to purchase Series C Preferred Stock at a price of $1,000 per share upon the Company’s achievement of the following milestones: (i) $2,000,000 upon treatment of the first patient in a Phase I clinical trial for a product developed under the Developmental Collaboration Agreement; (ii) $5,000,000 upon commencement of Phase III clinical trials of a product developed under the Developmental Collaboration Agreement; and (iii) $10,000,000 upon approval by the FDA of a product developed under the Developmental Collaboration Agreement. (See related discussion in Note 6).
6. Stockholders’ Equity
Convertible Preferred Stock
F-15
The Company is authorized to issue 5,000,000 shares of Preferred Stock, $0.001 par value. As of December 31, 2006, 40,000, 13,000, 17,000, 1,400,000, and 3,500,000 shares were designated Series A, Series B, Series C, Series D and Series E Preferred Stock, respectively.
As of December 31, 2006, there were no shares of Series A or Series B Preferred Stock issued and outstanding.
As of December 31, 2006, there were 2,000 shares of Series C Preferred Stock issued and outstanding. The Company anticipates that Series C Preferred Stock will be sold only to Baxter Healthcare upon the Company meeting certain specified milestones, referred to as the “Series C Milestones.” Holders of the Series C Preferred Stock are not entitled to receive dividends, have a liquidation preference amount of one thousand dollars ($1,000.00) per share prior to any distribution to holders of Series A Preferred Stock and to holders of common stock and have no voting rights, except as to the issuance of additional Series C Preferred Stock. Each share of Series C Preferred Stock becomes convertible into common stock upon the earlier of (i) the first business day following the approval by the FDA of the right to market, sell or distribute any product using the MAXIMUM-AD Vector Technology for treatment of blood clotting disorders in humans relating to Hemophilia A, which product has been developed pursuant to the Company’s Developmental Collaboration Agreement with Baxter Healthcare and (ii) the date seven years after the achievement of the most recently achieved Series C Milestone. The Series C Preferred Stock is convertible into common stock in an amount equal to (a) the quotient of (i) the Liquidation Value (adjusted for Recapitalizations), divided by (ii) one hundred and ten percent (110%) of the per share Fair Market Value of the Company’s common stock (as defined), multiplied by (b) the number of shares of Series C Preferred converted. In February 2003, the stockholders approved an amendment to the certificate of designation for the Series C Preferred Stock in which the Series C Preferred Stock would be convertible upon the earlier of (i)��the first business day following the approval by the FDA of the right to market, sell or distribute any product using the MAXIMUM-AD Vector Technology for treatment of blood clotting disorders in humans relating to Hemophilia A, which product has been developed pursuant to the Developmental Collaboration Agreement with Baxter Healthcare and (ii) June 13, 2010.
On July 31, 2003, Boston Scientific Corporation made a $9,000,000 investment in exchange for 1,385,377 shares of Series D Preferred Stock, which was initially convertible into the same number of shares of common stock, subject to adjustment. As of December 31, 2006, the 1,385,377 shares of Series D Preferred Stock were convertible into 1,420,339 shares of common stock. The Series D Preferred Stock is convertible into common shares at any time at the option of the holder and votes with the common stock on an “as adjusted basis.” The Series D Preferred Stock has a liquidation preference of $9,004,951 that is payable prior to any distribution to holders of Series A Preferred Stock and common stock.
See Note 4 above for description of Series E Preferred Stock.
Warrants
In conjunction with the lease settlement discussed in Note 9, in November 2003 the Company issued a warrant, exercisable for 5 years, to acquire 100,000 shares of Corautus common stock at an exercise price of $4.50 per share. The value of the warrant totaled approximately $490,000 in 2003, which was recorded in general and administrative expense.
In October 2003, the Company issued a warrant to purchase 1,000 shares of common stock in partial consideration for past public relations services. The warrant is fully vested and exercisable for seven years at an exercise price of $5.87. The warrant was valued at $4,660 and recorded in general and administrative expense in 2003.
In March 2003, a warrant to purchase 100,000 shares of common stock at $1.53 was issued as part of the consideration paid for investor relations and public relations services. One-fourth of the common stock under the warrant vested upon issuance, with the remaining portion vesting over the next nine months. The warrant was exercisable for ten years from the issuance date and was exercised by the holder in 2004. The value of the vested portion of the warrant totaled approximately $104,000 and $230,000 in 2004 and 2003, respectively, and was recorded in general and administrative expense.
In October 2001, the Company issued a warrant to purchase 12,785 shares of common stock to a service provider. The warrant had an exercise price of $16.45 per share and expired in October 2006. The warrant was valued at $134,250 and was recorded to general and administrative expense in 2001.
Holders of the convertible notes payable issued in 1998 and 1999 also were granted warrants to purchase 73,571 and 194,881 shares of common stock at an exercise price of $5.18 and $2.10 per share, respectively. The remaining 48,208 warrants issued in 1998 with an exercise price of $5.18 per share expired unexercised during 2005. The remaining 59,519 warrants issued in 1999 to purchase 97,611 shares of common stock at $2.10 per share expired unexercised in April, 2006.
F-16
Private Sale of Common Stock and Warrants
On June 27, 2005, Corautus entered into an investment agreement whereby BSC agreed to purchase 2,105,264 shares of restricted common stock for a per share purchase price of $3.80. The $8,000,000 in proceeds resulting from the sale of shares of common stock to BSC were held in escrow at June 30, 2005, subject to stockholder approval at a special meeting that occurred on August 11, 2005.
Also, on June 27, 2005, Corautus and a group of investors closed a private placement offering. Pursuant to the agreement with the investors, Corautus sold 2,640,055 shares of restricted common stock for a purchase price of $3.80 per share. The proceeds of the offering, which closed on August 11, 2005 (totaling approximately $10,032,000 less direct expenses of $17,000), will be used to fund projected operations, including expenditures related to the GENASIS trial, the manufacturing costs for VEGF-2 for a Phase III trial, and the initial costs related to conducting a planned Phase III clinical trial.
On July 7, 2004, Corautus and thirteen investors entered into the Common Stock and Warrant Purchase Agreement, whereunder the investors agreed to purchase an aggregate of 1,886,952 shares of Corautus common stock and warrants exercisable for an aggregate of 471,732 shares of Corautus common stock for net cash proceeds to Corautus of $9,320,540. The transaction closed in two tranches. The first tranche in the amount of $4,666,572 closed on July 7, 2004. The commitment for the second tranche in the amount of $4,653,968 was initially placed into escrow and then closed on September 14, 2004 after the treatment of the first patient in Corautus’ Phase IIb clinical trial. The common stock in both tranches was issued at a price of $5.22 per share, which equals 90% of the average closing price during the thirteen trading days prior to execution of the definitive agreement. The warrants for each tranche are exercisable for five years at a price equal to 120% of the closing price on the day prior to the closing of that tranche (e.g. $8.064 and $6.30 for the warrants issued in the first and second tranches, respectively). The proceeds of the offering were used for working capital and other general corporate purposes.
On May 10, 2004, thirteen current or former directors, advisors and officers of Corautus or its subsidiaries purchased an aggregate of 97,909 shares of unregistered common stock from the company at $5.50 per share for aggregate net cash proceeds to Corautus of $538,500. The subscription agreements were entered into effective May 7, 2004 and the price per share was the closing price of the publicly traded stock of the Company on that date. The proceeds of the offering were used for working capital and other general corporate purposes.
In March 2004, Corautus and two separate investors entered into Common Stock and Warrant Purchase Agreements, whereunder the investors agreed to purchase an aggregate of 376,000 shares of Corautus common stock and warrants exercisable for 18,800 shares of Corautus common stock. The common stock was issued at a price equal to 88% of the closing market price of Corautus common stock on the day immediately preceding the agreement execution date (i.e., $5.9835 per share). The warrants are exercisable at a price equal to 125% of the closing market price of Corautus common stock on the day immediately preceding the date the transaction closed (i.e., $8.375 per share). Additionally, the warrants are exercisable by the holder for up to five years immediately following issuance. The transaction closed on March 18, 2004. The proceeds of the offering (totaling $2,249,984) were used in funding Corautus’ Phase IIb clinical trial.
On January 8, 2004, Corautus and a group of investors entered into the Common Stock and Warrant Purchase Agreement, whereunder the investors agreed to purchase an aggregate of 1,200,000 shares of Corautus common stock and warrants exercisable for 240,000 shares of Corautus common stock. The common stock was issued at a price equal to 90% of the closing market price of Corautus common stock on the day immediately preceding the agreement execution date (i.e., $4.3819 per share). The warrants are exercisable at a price equal to 120% of the closing market price of Corautus common stock on the day immediately preceding the date the transaction closed (i.e., $6.72 per share). Additionally, the warrants are exercisable by the holder for up to five years immediately following issuance. The transaction closed on January 16, 2004. The proceeds of the offering (totaling $5,260,680 less $289,337 in finder’s fees) were used for working capital and other general corporate purposes.
On December 19, 2003, Corautus and three investors entered into the Common Stock and Warrant Purchase Agreement, whereunder the investors agreed to purchase in two tranches an aggregate of 793,640 shares of Corautus common stock and warrants exercisable for 160,639 shares of Corautus common stock. The common stock was issued at a price equal to 90% of the closing market price of Corautus common stock on the day immediately preceding the agreement execution date (i.e., $3.967 per share). The warrants are exercisable at a price equal to 125% of the closing market price of Corautus common stock on the day immediately preceding the date each transaction closed (i.e., $5.4375 per share for tranche one (108,338 shares) and $6.7625 per share for tranche two (50,390 shares)). Additionally, the warrants are exercisable by the holder for up to five years immediately following issuance. The transaction closed on December 31, 2003 with respect to 541,690 shares and January 27, 2004 with respect to the remaining shares. In connection with this offering, the Company issued warrants to purchase an aggregate of 30,420 shares of its common stock at an exercise price of $1.00 per share as a finder’s fee for the offering. The proceeds of the offering (totaling $3,149,957 with no underwriting discounts or commissions) were used for working capital and other general corporate purposes.
F-17
Employee Stock Purchase Plan
In May 2001, the Company adopted an Employee Stock Purchase Plan (the “Purchase Plan”), which was approved by the stockholders in July 2001. A total of 142,857 shares of common stock have been authorized and reserved for issuance under this Purchase Plan. The Purchase Plan permits all eligible employees to purchase common stock through payroll deductions (which cannot exceed 15% of each employee’s compensation) at the lower of 85% of fair market value at the beginning or the end of a 24 month offering period. No shares have been issued under this plan. As of December 31, 2006, there were no participants in the plan.
Stock Options
In November 2002, the Board of Directors adopted the 2002 Stock Plan, which was approved by the stockholders in February 2003 and was amended by stockholder approval in May 2004. Under the 2002 Stock Plan, the Board or a committee has the authority to grant options and rights to purchase common stock to officers, key employees, consultants and certain advisors to the Company. Options granted under the 2002 Stock Plan may be either incentive stock options or nonstatutory stock options, as determined by the Board or a committee. The 2002 Stock Plan, as amended in May 2004, reserves 3,500,000 shares for issuance under the Plan plus (a) any shares of common stock which have been reserved but not issued under the 1999 Stock Plan, the 1995 Stock Plan and the 1995 Directors’ Option Plan as of the date of stockholder approval of the 2002 Stock Plan, (b) any shares of common stock returned to the 1999 Stock Plan, the 1995 Stock Plan and the 1995 Directors’ Option Plan as a result of the termination of options or repurchase of shares of common stock issued under those plans and (c) an annual increase on the first day of each year by the lesser of (i) 300,000 shares, (ii) the number of shares equal to two percent of the total outstanding common shares or (iii) a lesser amount determined by the Board of Directors. Generally, options are granted with vesting periods from one to two years and expire ten years from date of grant or 90 days after termination of employment, if sooner.
The following table summarizes the activity of the Company’s stock options:
| | | | | | |
| | | Employee and
Director Stock
Options | | | Weighted
Average
Exercise
Price |
Balance at December 31, 2003 | | 2,062,177 | | | $ | 4.43 |
Granted | | 1,048,000 | | | $ | 5.37 |
Exercised | | (23,334 | ) | | $ | 3.30 |
Canceled | | (395,707 | ) | | $ | 9.23 |
| | | | | | |
Balance at December 31, 2004 | | 2,691,136 | | | $ | 4.10 |
Granted | | 1,504,200 | | | $ | 4.85 |
Exercised | | (204,228 | ) | | $ | 2.01 |
Canceled | | (133,014 | ) | | $ | 14.61 |
| | | | | | |
Balance at December 31, 2005 | | 3,858,094 | | | $ | 4.14 |
Granted | | 1,914,337 | | | $ | 0.84 |
Exercised | | — | | | | |
Canceled | | (352,992 | ) | | $ | 2.71 |
| | | | | | |
Balance at December 31, 2006 | | 5,419,439 | | | $ | 3.07 |
| | | | | | |
Options outstanding as of December 31, 2006:
| | | | | | | | | | |
Exercise Price | | Options Outstanding | | Weighted Average
Contractual Life in
Years | | Weighted Average
Exercise Price | | Options Exercisable | | Weighted Average
Exercise Price of Options Exercisable |
$0.75 - $0.85 | | 1,726,851 | | 9.37 | | $0.83 | | 931,488 | | $0.81 |
$1.30 | | 1,270,568 | | 6.33 | | $1.30 | | 1,270,568 | | $1.30 |
$2.24 - $2.94 | | 49,783 | | 6.03 | | $2.80 | | 49,783 | | $2.80 |
$3.98 - $4.83 | | 552,092 | | 6.63 | | $4.40 | | 517,092 | | $4.39 |
$5.01 - $7.56 | | 1,771,377 | | 6.99 | | $5.45 | | 1,771,377 | | $5.45 |
$14.35 - $68.25 | | 48,768 | | 4.54 | | $27.14 | | 48,768 | | $27.14 |
| | | | | | | | | | |
| | 5,419,439 | | 7.53 | | $3.07 | | 4,589,076 | | $3.44 |
| | | | | | | | | | |
F-18
The weighted-average grant date fair value of options granted in 2006, 2005, and 2004 was $0.84, $1.70, and $3.06, respectively. The weighted-average grant date fair value of options granted at less than fair value on the grant date in 2004 was $4.64. There were no options granted at less than fair value in 2006 or 2005. The intrinsic value of outstanding and vested options was zero at December 31, 2006.
In the fourth quarter of 2006, the Company’s board of directors approved termination agreements for two senior officers which included acceleration of vesting of all their options. The modification of these awards resulted in $18,000 of non-cash expense in 2006.
During 2004, the Company accelerated the vesting of certain employee stock options upon the employees’ termination. Also, the Company extended the period during which certain vested employee stock options could be exercised upon employee termination. Because these changes qualified as stock option modifications under FASB Interpretation No. 44,Accounting for Certain Transactions involving Stock Compensation, an interpretation of APB Opinion No. 25, the Company recorded compensation expense of approximately $44,000 in general and administrative expense in 2004 representing the excess of the intrinsic value of the options on the modification date over the options’ original intrinsic value.
During 2004, the Company granted stock options to certain individuals, which had exercise prices below the market price of the Company’s common stock on the date of grant. In accordance with APB No. 25, the Company recorded compensation expense over the vesting period of approximately $6,700 in general and administrative expense in 2004 based on the intrinsic value of the options on the date of grant.
The following common stock is reserved for future issuance at December 31, 2006:
| | |
Stock options | | |
Granted and outstanding | | 5,419,439 |
Reserved for future grants | | 399,364 |
Common stock warrants | | 1,169,024 |
Series D Preferred Stock | | 1,420,339 |
Series E Preferred Stock | | 2,475,659 |
Employee stock purchase plan | | 142,857 |
| | |
Total | | 11,026,682 |
| | |
The number of shares of common stock into which Series C Preferred Stock will be converted will not be known until the date of conversion because the conversion factor is based on fair value of the Company’s common stock on the date the Series C Preferred Stock becomes convertible, June 13, 2010.
7. Income Taxes
There is no income tax provision (benefit) for federal or state income taxes as the Company has incurred operating losses since inception.
A reconciliation of the statutory income tax rate to the effective income tax rate as recognized in the statements of operations for the years ended December 31, 2006, 2005 and 2004, is as follows:
| | | | | | | | | |
| | | For the year ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
Federal statutory rate | | 35 | % | | 35 | % | | 35 | % |
State tax rate, net of federal benefit | | 5 | % | | 5 | % | | 5 | % |
Change in valuation allowance | | (40 | )% | | (40 | )% | | (40 | )% |
| | | | | | | | | |
| | — | | | — | | | — | |
| | | | | | | | | |
As of December 31, 2006, the Company has federal and state net operating loss carryforwards of approximately $138,000,000 and $100,000,000, respectively, which will begin to expire in 2011 and 2007, respectively, unless previously utilized. The Company has federal and state research and development credit carryforwards of approximately $2,135,000 and $1,786,000, respectively. The federal research and development credit carryforwards will begin to expire in 2011 unless previously utilized. The Company has a California manufacturer’s investment credit of approximately $60,000 that will begin to expire in 2008 unless previously utilized.
F-19
Under Internal Revenue Code Sections 382 and 383, the Company’s use of net operating loss and tax credit carryforwards could be limited in the event of certain cumulative changes in the Company’s stock ownership.
Deferred tax assets are comprised of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
Net operating loss carryforwards | | $ | 53,954,000 | | | $ | 48,088,000 | |
Research and development and other state credit carryforwards | | | 3,597,000 | | | | 2,637,000 | |
Employee compensation accruals | | | 403,000 | | | | — | |
Other, net | | | 322,000 | | | | 379,000 | |
| | | | | | | | |
Total deferred tax assets | | | 58,276,000 | | | | 51,104,000 | |
Valuation allowance | | | (58,276,000 | ) | | | (51,104,000 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
8. Related Party Balances and Transactions
During 2001, the Company entered into a note receivable with an officer and stockholder in the amount of $37,000. The note was originally to be repaid over a four year period, however in April, 2002, the Company agreed to forgive the loan. Under the terms of the forgiveness arrangement, the loan was forgiven over a four-year period beginning April, 2002. The amount of the loan that was forgiven in 2006, 2005, and 2004 was $3,198, $9,633, and $9,633, respectively. The balance of this loan was $0 and $3,198 as of December 31, 2006 and 2005, respectively.
9. Commitments
Facilities
The Company leases approximately 365 square feet of office space under an operating lease that may be terminated by the Company at any time with forty five days notice. Also, the Company leases approximately 2,200 square feet of office and laboratory space under an operating lease that expires on July 31, 2007.
In May 2003, the Company entered into an agreement to terminate the lease for its manufacturing facility (Barnes Canyon) and to surrender possession of such facility without prejudice to any remedies of the landlord for the recovery of rent. As of the date of such termination, future payments due under this operating lease for the remaining eight year term were approximately $16.5 million. The Company entered into a settlement and release agreement with the landlord, dated as of November 7, 2003, for the settlement of the remaining payments that would have been due for the original term of such lease. Pursuant to the settlement and release agreement, the Company was obligated to pay or deliver to the landlord the following:
| | • | | immediately upon execution of the agreement, a warrant to acquire 100,000 shares of Corautus common stock at an exercise price of $4.50 per share; |
| | • | | $650,000 in cash on or before April 15, 2004, in one or more installments; |
| | • | | an aggregate of $550,000 in cash in twenty-two equal consecutive monthly installments beginning February 2004; |
| | • | | for twenty-four months beginning November 2003, that number of shares of Corautus common stock which on the first day of each such month shall have a trading value of $50,000; and |
| | • | | $681,000 in cash on or before January 5, 2006, subject to reduction for certain amounts received by the landlord prior to December 1, 2004 from re-leasing the property. |
The Company had delivered to the landlord a standby letter of credit, secured by a restricted certificate of deposit, for $680,901, securing a portion of its obligations under the agreement. The Company agreed to file a registration statement to register the shares delivered to the landlord, including the shares underlying the warrants, pursuant to the Securities Act of 1933, on or before June 1, 2004. Such registration statement was filed on January 27, 2004 and was amended on February 3, 2004. Effective with the $650,000
F-20
cash payment due on or before April 15, 2004, the Company was released from its obligations under the lease. The net present value of this settlement amount of $3.4 million was charged to expense in 2003. The estimated fair value of the warrants of $490,000 was recorded to additional paid in capital. In 2005 and 2004, the Company made payments of $955,901 and $925,000, respectively, and issued 186,007 and 53,180 shares of common stock valued at $900,000 and $300,000, respectively, to the landlord pursuant to the settlement agreement. As of December 31, 2005, all obligations under this settlement agreement had been met, eliminating the liability.
For the years ended December 31, 2006, 2005, and 2004, rent expense was approximately $222,000, $151,000, and $305,000, respectively.
Future minimum lease payments under the Company’s leases are as follows:
Purchase Commitments
On May 13, 2005, the Company entered into a Manufacturing Agreement with Boehringer Ingelheim Austria GmbH (“BI”) regarding BI’s provision of certain development, optimization, and manufacturing services for the Company’s VEGF-2 material. The Manufacturing Agreement with BI was a long-term agreement for manufacturing VEGF-2 for commercial use, the Company’s planned Phase III cardiovascular clinical trial and any potential clinical trials related to peripheral artery disease. The Manufacturing Agreement had a term extending from its execution until seven years after the first licensing of VEGF-2, renewable thereafter on successive four-year terms unless either party terminated with 24 months notice. The Company originally estimated that its total payments under the agreement would be approximately 11.1 million euros (equating to approximately $14.6 million in U.S. dollars as of December 31, 2006), of which approximately 6 million euros (equating to approximately $7.6 million in U.S. dollars) have been paid through December 31, 2006. However due to the results of the Company’s Phase IIb clinical trial using VEGF-2 and the Company’s decision to not pursue new trials using VEGF-2, the Company expects to terminate the Manufacturing Agreement in 2007. Any costs incurred under the Manufacturing Agreement related to termination are expected to be minimal.
NASDAQ De-Listing
On December 8, 2006, the Company received notification from NASDAQ that its common stock would be delisted from NASDAQ on December 19, 2006 unless it requested an appeal no later than December 15, 2006 because, in NASDAQ’s determination, it was not currently engaged in active business operations and is therefore a “public shell.” On December 15, 2006, the Company appealed the “public shell” determination and requested an oral hearing, which occurred on February 8, 2007. During the oral hearing, it requested relief from the “public shell” determination for a 90-day period, or until May 9, 2007, in order to complete the proposed merger. The NASDAQ hearing panel has not rendered a decision as of this date. The maximum amount of relief time the Company can receive from NASDAQ is 180 days from the date NASDAQ originally sent the delisting determination letter (December 8, 2006), which means the 180-day period expires on June 5, 2007.
10. Profit Sharing Plan
In 1998, the Company established a savings plan which covers all employees working more than 1,000 hours per year which has been established pursuant to the provisions of Section 401(k) of the Internal Revenue Code. Contributions to the plan are discretionary and contributions made prior to September 2005 vest over a four-year period. Beginning September 1, 2005, contributions made to eligible employees under the Safe Harbor Matching Contribution are 100% vested. Employer contributions during the years ended December 31, 2006, 2005 and 2004 were $60,622, $58,946, and $49,774, respectively.
11. Supplemental Cash Flow Information
Cash paid for income taxes was $1,600 for each of the years ended December 31, 2006, 2005 and 2004. Cash paid for interest was $8,221, $1,904, and $23,948 for the years ended December 31, 2006, 2005 and 2004, respectively.
In October 2003, the Company issued common stock with a market value of $50,000 (8,696 common shares) to a vendor in settlement for outstanding obligations. The expense was recorded in general and administrative expense.
During the years ended December 31, 2001 and 2000, amounts due Baxter Healthcare under the Credit Agreement of $5,848,361 and $4,043,079 were converted into 5,849 and 4,043 shares of Series B Preferred Stock, respectively. Additionally, during 2001, 2,000 shares of Series C Preferred Stock were issued to Baxter Healthcare in exchange for a $2,000,000 milestone payment (Note 5).
F-21
During 2000, the Company issued 41,143 shares of common stock valued at $1,519,500 to acquire the license for technologies for the treatment and prevention of AIDS and for lentiviral gene therapy (Note 5). During 2002, an additional 1,714 shares of common stock were issued in connection with this transaction.
During 2000, a warrant for 14,286 shares of common stock was issued to Baxter Healthcare in conjunction with Baxter Healthcare’s guarantee of a note payable. The warrant was valued at $687,000 and classified as deferred loan fees and was amortized to interest expense over the term of the note payable, which matured in 2003. Additionally, the Company acquired $200,000 of leasehold improvements under a note payable to a related party.
As more fully described in Note 5, in 1998 the Company acquired certain technology and equipment in exchange for 5,830 shares of Series A Preferred Stock and 263,031 shares of common stock valued at $5,799,442. The equipment acquired was valued at $343,937 based on an appraisal.
12. Selected Quarterly Data (Unaudited)
The following tables set forth certain unaudited quarterly information for each of the eight fiscal quarters in the two-year period ended December 31, 2006. This quarterly information has been prepared on a consistent basis with the audited financial statements and, in the opinion of management, includes all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of the information for the periods presented. The Company’s quarterly operating results may fluctuate significantly as a result of a variety of factors and operating results for any quarter are not necessarily indicative of results for a full fiscal year or future quarters.
| | | | | | | | | | | | | | | | |
2006 Quarter Ended | | December 31 | | | September 30 | | | June 30 | | | March 31 | |
Total revenue | | $ | 20,834 | | | $ | 20,833 | | | $ | 20,833 | | | $ | 20,833 | |
Operating expenses | | | 3,584,090 | | | | 3,178,054 | | | | 3,637,416 | | | | 5,521,180 | |
Net loss available to common shareholders | | | (3,610,527 | ) | | | (3,167,487 | ) | | | (3,593,844 | ) | | | (5,485,243 | ) |
Basic and diluted net loss available to common shareholders per common share | | $ | (0.18 | ) | | $ | (0.16 | ) | | $ | (0.18 | ) | | $ | (0.28 | ) |
| | | | | | | | | | | | | | | | |
2005 Quarter Ended | | December 31 | | | September 30 | | | June 30 | | | March 31 | |
Total revenue | | $ | 20,834 | | | $ | 20,833 | | | $ | 20,833 | | | $ | 20,833 | |
Operating expenses | | | 5,481,737 | | | | 4,616,115 | | | | 5,474,950 | | | | 4,273,327 | |
Net loss | | | (5,418,484 | ) | | | (4,562,143 | ) | | | (5,476,351 | ) | | | (4,279,865 | ) |
Basic and diluted net loss per common share | | $ | (0.28 | ) | | $ | (0.25 | ) | | $ | (0.37 | ) | | $ | (0.29 | ) |
13. Litigation
The Company is involved in various litigation and administrative proceedings arising in the normal course of business. In the opinion of management, any liabilities that may result from these claims will not, individually or in the aggregate, have a material adverse effect on the Company’s financial position, results of operations, or cash flows.
14. Subsequent Event
On February 7, 2007, the Company executed a definitive merger agreement (“Agreement”) with VIA Pharmaceuticals, Inc. (“VIA”), a privately-held drug development company headquartered in San Francisco, California. The merger is expected to create a drug development company focused on compounds that target inflammation in the blood vessel wall as an innovative approach to the treatment of cardiovascular disease. Under the terms of the Agreement, the Company will issue, and the holders of VIA’s equity
F-22
securities will receive (or purchase in the case of option holders), shares of the Company’s common stock in exchange for their shares of VIA stock. It is expected that holders of VIA’s equity securities will own approximately 77.59% and existing Company’s stockholders will own approximately 22.41% of the combined company on a pro forma, fully-diluted basis based on the delivery of $11.25 million of net cash at closing (the actual percentages to be determined based on the Company’s net cash at closing). The merger is intended to qualify for federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the U.S. Internal Revenue Code of 1986, as amended. The transaction is subject to the Company maintaining certain minimum cash levels, as well as certain other customary conditions, including obtaining stockholder approval. The transaction is expected to close in the second quarter of 2007.
F-23
