Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning established by the Private Securities Litigation Reform Act of 1995 ("Act"). The forward-looking statements are intended to qualify under provisions of the federal securities laws for "safe harbor" treatment established by the Act. Forward-looking statements are based on currently available information, expectations, estimates, assumptions and projections, and management's judgment. Words such as would, expects, intends, plans, believes, estimates, assumes, anticipates, projects, predicts, forecasts or variations of such words or similar expressions are intended to identify forward-looking statements. The forward-looking statements are not guarantees of future performance. They are subject to risks, uncertainty and changes in circumstances. The statements that are not historical facts contained in this presentation are forward-looking statements, including, but not limited to, those related to Depomed’s strategy, plans, objectives, expectations (financial or otherwise) and intentions, product candidates, future financial results and growth potential, including projected revenues and earnings, expectations regarding periods of exclusivity for the NUCYNTA® franchise, Gralise®, CAMBIA® or Zipsor®, as well as any other statements that are not historical facts. These forward-looking statements are based on Depomed’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. Factors that may cause a result different than expected or anticipated include, but are not limited to: risks associated with the commercialization of our products, including competitive risks; risks relating to the pending ANDA litigation for Nucynta and Nucynta ER and generic entry into the market; pricing and third party payor reimbursement risks for our products; changes in regulatory policies, procedures, and laws, including FDA regulation of our products and regulation of our promotional practices; risks relating to the successful development of new product candidates; other risks and unforeseen events; as well as other risks related to Depomed's business detailed from time-to-time under the caption "Risk Factors" and elsewhere in Depomed's Securities and Exchange Commission filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2016, its most recent Quarterly Report on Form 10-Q. Depomed assumes no duty or obligation to provide public updates of any forward-looking statements. “Safe Harbor” Statement Under the Private Securities Litigation Reform Act of 1995 February 2018

Principles Guiding the Way We Do Business PUTTING the Patient First PRODUCTIVITY Aimed at Improving Effectiveness Across the Business PROFITABILITY in Line with Our Peer Group 3 February 2018
A Clear Strategy for Growth Executing on a Three Pillar Growth Strategy 4 MAINTAIN a Strong/Profitable NUCYNTA Franchise GROW Neurology and Pain Businesses BUILD a New Specialty Business Collegium Transaction December 2017 90 Rep Salesforce Operational October 2017 Cosyntropin Depot Transaction Announced November 2017 February 2018
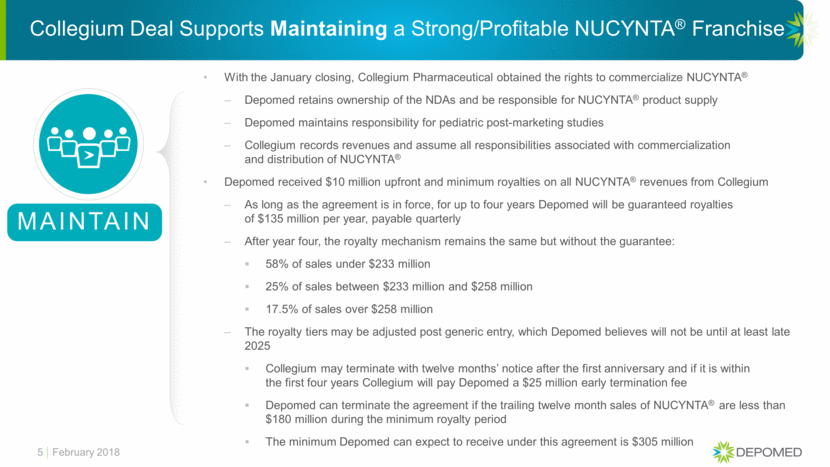
Collegium Deal Supports Maintaining a Strong/Profitable NUCYNTA® Franchise 5 MAINTAIN With the January closing, Collegium Pharmaceutical obtained the rights to commercialize NUCYNTA® Depomed retains ownership of the NDAs and be responsible for NUCYNTA® product supply Depomed maintains responsibility for pediatric post-marketing studies Collegium records revenues and assume all responsibilities associated with commercialization and distribution of NUCYNTA® Depomed received $10 million upfront and minimum royalties on all NUCYNTA® revenues from Collegium As long as the agreement is in force, for up to four years Depomed will be guaranteed royalties of $135 million per year, payable quarterly After year four, the royalty mechanism remains the same but without the guarantee: 58% of sales under $233 million 25% of sales between $233 million and $258 million 17.5% of sales over $258 million The royalty tiers may be adjusted post generic entry, which Depomed believes will not be until at least late 2025 Collegium may terminate with twelve months’ notice after the first anniversary and if it is within the first four years Collegium will pay Depomed a $25 million early termination fee Depomed can terminate the agreement if the trailing twelve month sales of NUCYNTA® are less than $180 million during the minimum royalty period The minimum Depomed can expect to receive under this agreement is $305 million February 2018
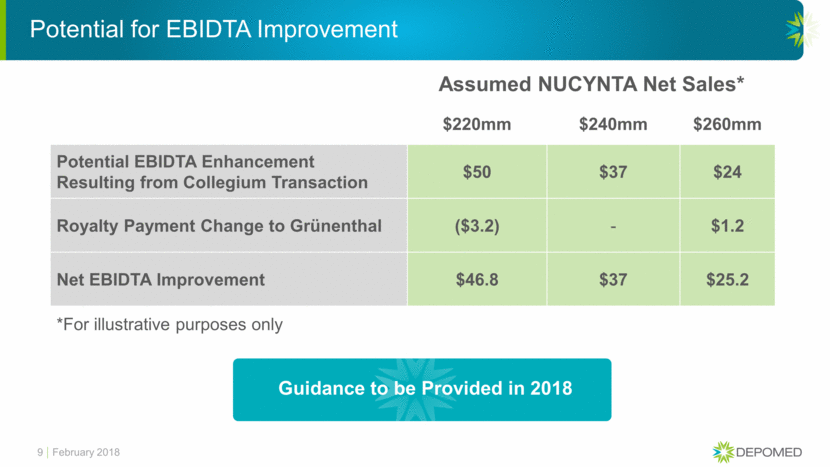
Industrial Logic is Clear and Compelling: Financially, Strategically and Patient-Focused Depomed Collegium Financially Immediately Accretive Improves Absolute EBIDTA Strategically Collegium as a Leader in Pain and Depomed Ability to Focus on Neurology/ Orphan Drugs Allows Significant Cost Synergies Patient-Focused Combined Portfolio Represents Best-In-Class Products That Improve Continuum of Care 6 February 2018
Collegium: Ideal Partner to Take NUCYNTA® to the Next Level Recognized as an emerging leader in pain management and committed to building a leading pain company Xtampza® ER is the fastest growing Extended Release branded opioid Strong home office, commercial and managed care infrastructures Salesforce focused on Pain Specialists with significant overlap with NUCYNTA customers Collegium desired an Immediate Release product and viewed NUCYNTA® IR as best-in-class Xtampza® ER and NUCYNTA® ER’s different MOAs will be positioned as complementary products: Xtampza® ER: Abuse deterrent oxycodone of choice NUCYNTA® ER: Dual MOA and Diabetic Peripheral Neuropathy (DPN) Offering improves the continuum of care for patients with chronic pain 7 Collegium and Depomed Share a Commitment to Putting the Patient First February 2018
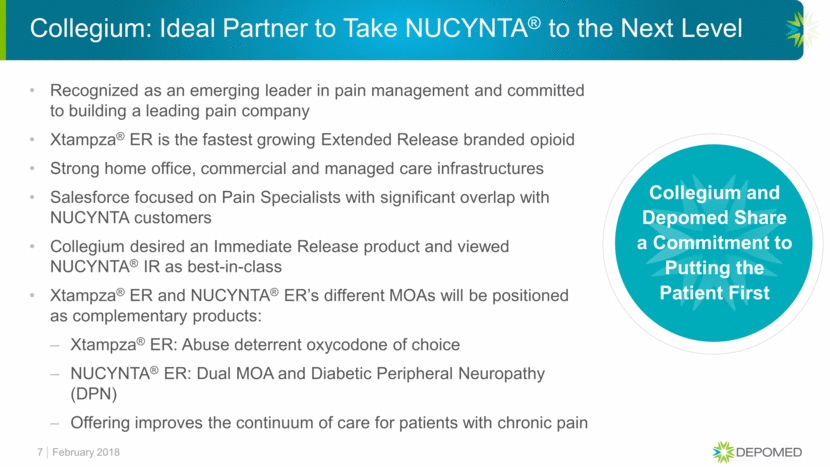
Depomed $135 Million Annual Payment - Highly Secure and Independent of Collegium’s Financial Position 8 100% 100% Gross Sales go into lock box Includes a $33.75M letter of credit should Collegium fall below the minimum Special Purpose Vehicle (SPV) Bankruptcy Protected 35% Daily sweep of Gross Sales into Depomed ** Approximately 70% of Net Sales assumes an approximately 50% gross to net * * 35% of Gross Sales until $33.75 million per quarter is reached February 2018 Equates to ~70% of Net Sales** Comfortably Covering Minimum Payments
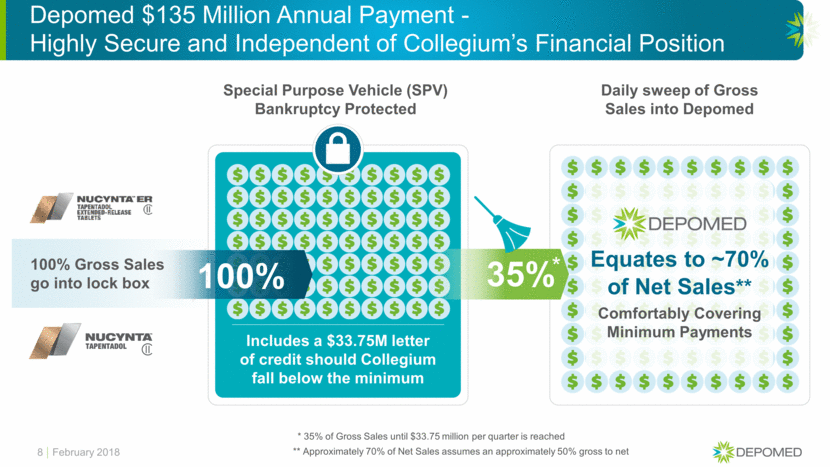
Potential for EBIDTA Improvement Assumed NUCYNTA Net Sales* 9 $220mm $240mm $260mm Potential EBIDTA Enhancement Resulting from Collegium Transaction $50 $37 $24 Royalty Payment Change to Grünenthal ($3.2) - $1.2 Net EBIDTA Improvement $46.8 $37 $25.2 Guidance to be Provided in 2018 *For illustrative purposes only February 2018
GROW Grow Our Neurology and Pain Businesses September marked the completed relaunch of our Neurology salesforce with 90 representatives Provides proper support to highly-promotionally sensitive products Gralise® and CAMBIA ® Creates credible platform to expand with new products Committed to adding at least one new product in 2018 10 February 2018

Build A New Specialty Business Establishment of a New Specialty Products Business Unit Mark Booth as General Manager brings 30+ years of biopharma experience Objective is to build a portfolio of specialty/orphan drugs addressing the unmet needs of the patient, physician and payors Provides a platform for new growth and diversification First product: Cosyntropin (Synthetic ACTH Depot) July 2017: Mallinckrodt grants license to West Therapeutic Development for Cosyntropin (Synthetic ACTH Depot); Forced FTC Divestiture 11 BUILD February 2018

Transformational Transaction Creates New Specialty Business Depomed/ Slán Medicinal Holdings Limited Acquisition of Cosyntropin (Synthetic ACTH Depo) provides a late-stage, high-value product Depomed is the exclusive licensee for United States Current U.S. market is led by H.P. Acthar® Gel (Natural ACTH Depot) 1st Indication: NDA filing expected in late 2018 Launch expected in 2H 2019 2nd Indication: Investigational New Drug (IND) trial enrolling in Infantile Spasms The Pediatric Epilepsy Research Consortium (Kelly Knupp, M.D., Principal Investigator) 15 sites enrolling approximately 400 patients Orphan Drug Status in infantile spasms granted August 2017 12 Next 12-18 Months: Expect to Bring Additional Products into This Business February 2018
Headquarters Restructuring and Relocation Aligns with New Business Model Headquarters Relocation in 2018: Evaluating Mid-West and East Coast Sites Provides Improved Access to Pharmaceutical Talent Current Footprint: 60,000 square feet housing 120 employees 380 person salesforce New Footprint: Approximately 30,000 square feet housing approximately 70 employees Approximately 100 person salesforce Reduction of total headcount by approximately 330 people Depomed Now Leaner, Nimbler, and Financially Stronger 13 February 2018
Gralise®: Differentiated within the 71MM Annual Prescription Highly Promotionally Sensitive Market Indication Management of post-herpetic neuralgia Performance 2016 net sales of $88 million 3Q 2017 net sales of $21 million Clinical Differentiation Only once-daily formulation of gabapentin Significantly less dizziness and somnolence 14 February 2018
Cambia®: Differentiated within the 15MM Annual Prescription Highly Promotionally Sensitive Market Indication Performance Clinical Differentiation Acute migraine attacks in adults 2016 net sales of $31 million 3Q 2017 net sales of $8 million Only single-agent in therapeutic class for acute migraine Rated as effective first-line treatment by the American Headache Society in 2015 February 2018 15
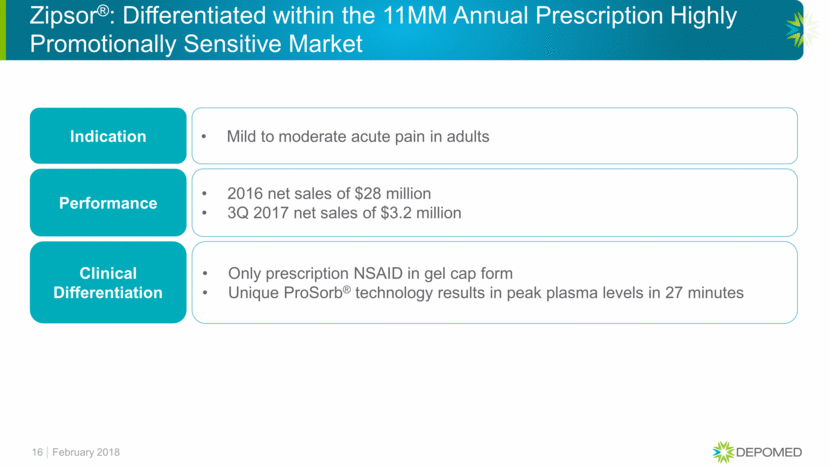
Zipsor®: Differentiated within the 11MM Annual Prescription Highly Promotionally Sensitive Market Indication Mild to moderate acute pain in adults Performance 2016 net sales of $28 million 3Q 2017 net sales of $3.2 million Clinical Differentiation Only prescription NSAID in gel cap form Unique ProSorb® technology results in peak plasma levels in 27 minutes 16 February 2018
NUCYNTA® Commercialization Agreement: Close Agreement with Collegium Pharmaceutical Synthetic Cosyntropin (Synthetic ACTH Depot): Commenced Investigational New Drug (IND) Trial in Infantile Spasms Purdue Litigation: Pre-Trial Conference to Set Trial Date Milestones Driving Growth in 2018 and Beyond 17 First Half 2018 Refinancing: Execute Refinancing of Deerfield and Pharmakon Advisors’ Secured Debt Synthetic Cosyntropin (Synthetic ACTH Depot): Submission of NDA by West for First Indication Business Development: Execute 1 to 2 New Opportunities Aimed at Accelerating Growth Second Half 2018 2018: A Year of Growth and Repositioning Setting up for a Breakout 2019/2020 February 2018

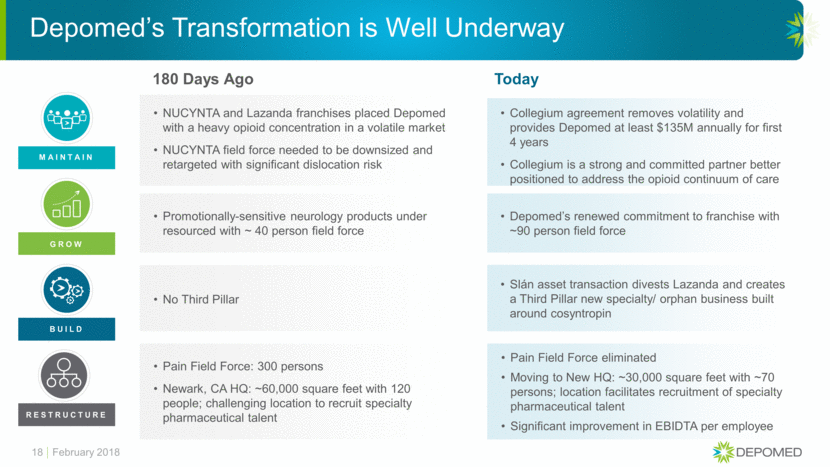
180 Days Ago NUCYNTA and Lazanda franchises placed Depomed with a heavy opioid concentration in a volatile market NUCYNTA field force needed to be downsized and retargeted with significant dislocation risk Promotionally-sensitive neurology products under resourced with ~ 40 person field force No Third Pillar Pain Field Force: 300 persons Newark, CA HQ: ~60,000 square feet with 120 people; challenging location to recruit specialty pharmaceutical talent Depomed’s Transformation is Well Underway 18 Today Collegium agreement removes volatility and provides Depomed at least $135M annually for first 4 years Collegium is a strong and committed partner better positioned to address the opioid continuum of care Depomed’s renewed commitment to franchise with ~90 person field force Slán asset transaction divests Lazanda and creates a Third Pillar new specialty/ orphan business built around cosyntropin Pain Field Force eliminated Moving to New HQ: ~30,000 square feet with ~70 persons; location facilitates recruitment of specialty pharmaceutical talent Significant improvement in EBIDTA per employee MAINTAIN GROW BUILD RESTRUCTURE February 2018
Our Commitment to Shareholders 19 Execute on a Clear Strategy of Maintain a Strong NUCYNTA Franchise, Grow Our Neurology and Pain Businesses and Build a New Specialty Business Leverage Opportunities to Drive Superior Profitability Committed to Putting the Patient First February 2018