UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended May 29, 2011, or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition period for _________ to _________.
Commission file number: 0-27446
LANDEC CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 94-3025618 |
| (State or other jurisdiction of | (IRS Employer |
| incorporation or organization) | Identification Number) |
3603 Haven Avenue
Menlo Park, California 94025
(Address of principal executive offices)
Registrant's telephone number, including area code:
(650) 306-1650
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
| Common Stock | The NASDAQ Global Select Stock Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer” and “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large Accelerated Filer ¨ | Accelerated Filer x |
Non Accelerated Filer ¨ | Smaller Reporting Company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ¨ No x
The aggregate market value of voting stock held by non-affiliates of the Registrant was approximately $157,209,000 as of November 28, 2010, the last business day of the registrant’s most recently completed second fiscal quarter, based upon the closing sales price on The NASDAQ Global Select Market reported for such date. Shares of Common Stock held by each officer and director and by each person who owns 10% or more of the outstanding Common Stock have been excluded from such calculation in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of July 20, 2011, there were 26,405,799 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement relating to its October 2011 Annual Meeting of Stockholders which statement will be filed not later than 120 days after the end of the fiscal year covered by this report, are incorporated by reference in Part III hereof.
LANDEC CORPORATION
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
Item No. | | Description | | Page |
| | | | | |
| Part I | | | | |
| 1. | | Business | | 3 |
| | | | | |
| 1A. | | Risk Factors | | 20 |
| | | | | |
| 1B. | | Unresolved Staff Comments | | 27 |
| | | | | |
| 2. | | Properties | | 27 |
| | | | | |
| 3. | | Legal Proceedings | | 27 |
| | | | | |
| 4. | | [Removed and reserved] | | 27 |
| | | | | |
| Part II | | | | |
| 5. | | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | | 28 |
| | | | | |
| 6. | | Selected Financial Data | | 29 |
| | | | | |
| 7. | | Management’s Discussion and Analysis of Financial Condition and Results of Operations | | 30 |
| | | | | |
| 7A. | | Quantitative and Qualitative Disclosures about Market Risk | | 48 |
| | | | | |
| 8. | | Financial Statements and Supplementary Data | | 48 |
| | | | | |
| 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | 48 |
| | | | | |
| 9A. | | Controls and Procedures | | 48 |
| | | | | |
| 9B. | | Other Information | | 49 |
| | | | | |
| Part III | | | | |
| 10. | | Directors, Executive Officers and Corporate Governance | | 50 |
| | | | | |
| 11. | | Executive Compensation | | 50 |
| | | | | |
| 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | | 50 |
| | | | | |
| 13. | | Certain Relationships and Related Transactions, and Director Independence | | 50 |
| | | | | |
| 14. | | Principal Accountant Fees and Services | | 50 |
| | | | | |
| Part IV | | | | |
| 15. | | Exhibits and Financial Statement Schedules | | 51 |
PART I
Item 1. Business
This report contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. Words such as “projected,” “expects,” “believes,” “intends” and “assumes” and similar expressions are used to identify forward-looking statements. These statements are made based upon current expectations and projections about our business and assumptions made by our management and are not guarantees of future performance, nor do we assume any obligation to update such forward-looking statements after the date this report is filed. Our actual results could differ materially from those projected in the forward-looking statements for many reasons, including the risk factors listed in Item 1A. “Risk Factors” and the factors discussed below.
Corporate Overview
Landec Corporation and its subsidiaries (“Landec” or the “Company”) design, develop, manufacture and sell polymer products for food and agricultural products, medical devices and licensed partner applications that incorporate Landec’s patented polymer technologies. The Company has two proprietary polymer technology platforms: 1) Intelimer® polymers, and 2) hyaluronan (“HA”) biopolymers. The Company’s HA biopolymers are proprietary in that they are specially formulated for specific customers to meet strict regulatory requirements. The Company’s polymer technologies, along with its customer relationships and trade names, are the foundation and a key differentiating advantage upon which Landec has built its business.
Following the acquisition of Lifecore Biomedical, Inc. (“Lifecore”) on April 30, 2010, Landec has four core businesses – Food Products Technology, Food Export, Hyaluronan-based Biomaterials and Technology Licensing, each of which is described below. Financial information concerning the industry segments for which the Company reported its operations during fiscal years 2009, 2010 and 2011 is summarized in Note 14 to the Consolidated Financial Statements.
Our wholly-owned subsidiary, Apio, Inc. (“Apio”), operates our Food Products Technology business, which combines our proprietary food packaging technology with the capabilities of a large national food supplier and value-added produce processor. In Apio’s value-added operations, produce is processed by trimming, washing, mixing, and packaging into bags and trays that incorporate Landec’s BreatheWay® membrane technology. The BreatheWay membrane increases shelf life and reduces shrink (waste) for retailers and, for certain products, eliminates the need for ice during the distribution cycle and helps to ensure that consumers receive fresh produce by the time the product makes its way through the supply chain. Apio also licenses the BreatheWay technology to partners such as Chiquita Brands International, Inc. (“Chiquita”) for packaging and distribution of bananas and avocados and to Windset Farms (“Windset”) for packaging of greenhouse grown cucumbers, peppers and tomatoes.
Apio also operates the Food Export business through its subsidiary, Cal Ex Trading Company (“Cal-Ex”). The Export business purchases and sells whole fruit and vegetable products to predominantly Asian markets.
Our wholly-owned subsidiary, Lifecore Biomedical, Inc. (“Lifecore”), operates our Hyaluronan-based Biomaterials business and is principally involved in the development and manufacture of products utilizing hyaluronan, a naturally occurring polysaccharide that is widely distributed in the extracellular matrix of connective tissues in animals and humans. Lifecore’s products are primarily sold to three medical areas: (1) Ophthalmic, (2) Orthopedic and (3) Veterinary. Lifecore also supplies hyaluronan to customers pursuing other medical applications, such as aesthetic surgery, medical device coatings, tissue engineering and pharmaceuticals. Lifecore leverages its fermentation process to manufacture premium, pharmaceutical-grade hyaluronan, and uses its aseptic filling capabilities to also deliver proprietary HA finished goods to its customers. Lifecore also manufactures and sells it own HA-based finished goods. Lifecore is known in the medical segments as a premium supplier of HA. Its name recognition allows Lifecore to acquire new customers and sell new products with only a small targeted marketing or sales capability.
Landec’s Technology Licensing business develops proprietary polymer technologies and applies them in a wide range of applications including seed coatings and treatments, temperature indicators, controlled release systems for drug delivery, pressure sensitive adhesives and personal care products. These applications are commercialized through partnerships with third parties resulting in licensing and royalty revenues, as well as reimbursed R&D funding. For example, Monsanto Company (“Monsanto”) has an exclusive license to use our Intellicoat® seed coating technology for certain seed treatment applications, Air Products and Chemicals, Inc. (“Air Products”) has an exclusive license to use our Intelimer polymers for personal care products and Nitta Corporation (“Nitta”) licenses Landec’s proprietary pressure sensitive adhesives for use in the manufacture of electronic components by their customers.
Landec was incorporated in California on October 31, 1986 and reincorporated as a Delaware corporation on November 6, 2008. Our common stock is listed on The NASDAQ Global Select Market under the symbol “LNDC”.
Technology Overview
Landec has two polymer technology platforms. The first platform is its Intelimer polymer. With the acquisition of Lifecore, Landec added its second polymer technology platform.
A) Intelimer Polymers
The Intelimer polymer is a crystalline, hydrophobic polymer that has unique characteristics and benefits. The first unique feature of this polymer system is the way that it uses a temperature switch to control and modulate properties such as viscosity, permeability and adhesion when varying the materials’ temperature above and below the temperature switch. The sharp temperature switch is adjustable between 0-100°C. For instance, Intelimer polymers can change within the range of one or two degrees Celsius from a non-adhesive state to a highly tacky, adhesive state; from an impermeable state to a highly permeable state; or from a solid state to a viscous liquid state. These abrupt changes can be irreversible or repeatedly reversible and can be tailored by Landec to occur at specific temperatures, thereby offering substantial competitive advantages in the Company's target markets.
A second unique feature of the Intelimer polymer materials is its unique controlled release properties. The polymer is able to deliver active ingredients with low or no burst, with a sustained release over periods of time. Finally, Intelimer polymers can be designed to contain up to 80% renewable materials from components of natural raw materials such as rapeseed oil, palm oil or coconut oil, and can be supplied in biocompatible and bioerodible forms.
Polymers are important and versatile materials found in many of the products of modern life. Certain polymers, such as cellulose and natural rubber, occur in nature. Man-made or synthetic polymers include nylon fibers used in carpeting and clothing, coatings used in paints and finishes, plastics such as polyethylene, and elastomers used in automobile tires and latex gloves. Historically, synthetic polymers have been designed and developed primarily for improved mechanical and thermal properties, such as strength and the ability to withstand high temperatures. Improvements in these and other properties and the ease of manufacturing synthetic polymers have allowed these materials to replace wood, metal and natural fibers in many applications over the last 50 years. More recently, scientists have focused their efforts on identifying and developing sophisticated polymers with novel properties for a variety of commercial and industrial applications.
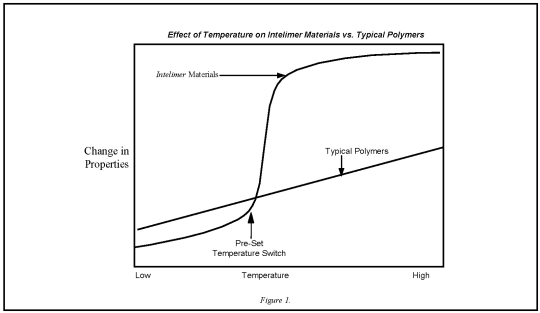
Landec's Intelimer polymers are a proprietary class of synthetic polymeric materials that respond to temperature changes in a controllable, predictable way. Typically, polymers gradually change in adhesion, permeability and viscosity over broad temperature ranges. Landec's Intelimer materials, in contrast, can be designed to exhibit abrupt changes in permeability, adhesion and/or viscosity over temperature ranges as narrow as 1°C to 2°C. These changes can be designed to occur at relatively low temperatures (0°C to 100°C) that are relatively easy to maintain in industrial and commercial environments. Figure 1 illustrates the effect of temperature on Intelimer materials as compared to typical polymers.
Landec's proprietary polymer technology is based on the structure and phase behavior of Intelimer materials. The abrupt thermal transitions of specific Intelimer materials are achieved through the controlled use of hydrocarbon side chains that are attached to a polymer backbone. Below a pre-determined switch temperature, the polymer's side chains align through weak hydrophobic interactions resulting in a crystalline structure. When this side chain crystallizable polymer is heated to, or above, this switch temperature, these interactions are disrupted and the polymer is transformed into an amorphous, viscous state. Because this transformation involves a physical and not a chemical change, this process is irreversible or repeatedly reversible. Landec can set the polymer switch temperature anywhere between 0°C to 100°C by varying the average length of the side chains. The reversible transitions between crystalline and amorphous states are illustrated in Figure 2 below.
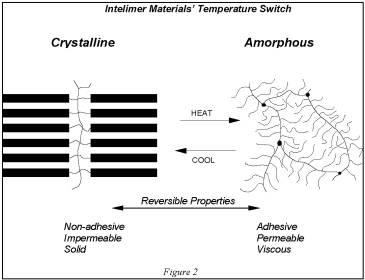
This chemical structure provides an additional benefit. Spatially distinct regions of the Intelimer polymer confer different physical properties on the material. Each part can be tuned independently to meet the needs of a given application. For example, switching temperature (which arises from one part of the chain) can be adjusted independently of adhesive properties (which arise from another part of the chain). In addition to temperature, the pH and other environmental parameters can be used as the “switch” to trigger a significant change in physical properties. Also, side chain crystallizable polymers when mixed with any active material, for example a therapeutic drug, can control the release of the active materials by the crystalline structure of the Intelimer polymer while in the crystalline state. In this manner therapeutic drugs can be delivered over a sustained and long period of time. Or, a fragrance can be emitted steadily over a long period of time from a crystalline Intelimer polymer.
Side chain crystallizable polymers were first discovered by academic researchers in the mid-1950's. These polymers were initially considered to be merely of scientific curiosity from a polymer physics perspective and, to the Company's knowledge, no significant commercial applications were pursued. In the mid-1980's, Dr. Ray Stewart, the Company's founder, became interested in the idea of using the temperature-activated permeability properties of these polymers to deliver various materials such as catalysts and pesticides. After forming Landec in 1986, Dr. Stewart subsequently discovered broader utility for these polymers. After several years of basic research, commercial development efforts began in the early 1990's, resulting in initial products in the mid 1990’s.
Landec's Intelimer materials are generally synthesized from long side-chain acrylic monomers that are derived primarily from natural materials such as coconut and palm oils that are highly purified and designed to be manufactured economically through known synthetic processes. These acrylic-monomer raw materials are then polymerized by Landec leading to many different side-chain crystallizable polymers whose properties vary depending upon the initial materials and the synthetic process. Intelimer materials can be made into many different forms, including films, coatings, microcapsules and discrete forms.
B) Hyaluronan Biopolymers
Hyaluronan is a non-crystalline, hydrophilic polymer that exists naturally within the human body, most notably within the aqueous humor of the eye, synovial fluid, skin and umbilical cord. The viscoelastic properties and water solubility of HA make it ideal for medicinal applications where lubricity and protection are critical. Due to its widespread presence in tissues, its critical role in normal physiology, and its high degree of biocompatibility, the Company believes that hyaluronan will continue to be used for an increasing variety of medical applications.
Hyaluronan can be produced in two ways, either through bacterial fermentation or through extraction from rooster combs. Lifecore produces HA only from fermentation, using an extremely efficient microbial fermentation process and a highly effective purification operation.
Hyaluronan was first demonstrated to have commercial medical utility as a viscoelastic solution in cataract
surgery. In this application, it is used for maintaining the shape of the anterior chamber and protecting corneal tissue during the removal and implantation of intraocular lenses. The first ophthalmic hyaluronan product, produced by extraction from rooster comb tissue, became commercially available in the United States in 1981. Hyaluronan-based products, produced either by rooster comb extraction or by fermentation processes such as Lifecore’s, have since gained widespread acceptance in ophthalmology and are currently used in the majority of cataract extraction procedures in the world. Lifecore’s hyaluronan is also used as an orthopedic carrier vehicle for allogeneic freeze-dried demineralized bone as the active component of devices to treat the symptoms of osteoarthritis, and as a formulation component to provide increased lubricity to medical devices. Lifecore’s hyaluronan has also been utilized in veterinary drug applications to treat traumatic arthritis.
Trademarks/Trade names
Intelimer®, Landec®, Apio™, Eat Smart®, BreatheWay®, Clearly Fresh™, Intellicoat®, Early Plant®, Pollinator Plus®, Relay® Cropping, Lifecore®, Revitalure™, LUROCOAT® and Ortholure™ are trademarks or registered trademarks and trade names of the Company in the United States and other countries. This Annual Report on Form 10-K also refers to the trademarks of other companies.
Description of Core Business
Landec participates in four core business segments: Apio, Inc. with the Food Products Technology and Food Export businesses, Lifecore Biomedical, Inc., with Hyaluronan-based Biomaterials business and Landec’s Technology Licensing business.
A) Food Products Technology Business
The Company began marketing its proprietary Intelimer-based BreatheWay membranes in 1996 for use in the fresh-cut produce packaging market, historically one of the fastest growing segments in the food industry. Landec’s proprietary BreatheWay packaging technology is used to package fresh-cut or whole produce, the result is a convenient, ready-to-eat finished product that achieves increased shelf life and reduced shrink (waste) without the need for ice during the distribution cycle. These products are referred to as “value-added” products. In 1999, the Company acquired Apio, its then largest customer in the Food Products Technology business and one of the nation’s leading marketers and packers of produce and specialty packaged fresh-cut vegetables. Apio utilizes a state-of-the-art fresh-cut processing facility and year-round access to quality vegetable sourcing to produce products which Apio distributes to top U.S. retail grocery chains, major club stores and foodservice customers. The Company’s proprietary BreatheWay packaging business has been combined with Apio into a subsidiary that retains the Apio name. This vertical integration within the Food Products Technology business gives Landec direct access to the large and growing fresh-cut and whole produce market.
The Technology: BreatheWay Membranes
Certain types of fresh-cut and whole produce can spoil or discolor rapidly when packaged in conventional packaging materials and, therefore, are limited in their ability to be distributed broadly to markets. The Company’s proprietary BreatheWay packaging technology extends the shelf life and quality of fresh-cut and whole produce.
Fresh-cut produce is cut, washed, and packaged in a form that is ready to use by the consumer and is thus typically sold at premium price levels compared to unpackaged produce. The total U.S. fresh produce market is estimated to be $100 billion to $120 billion. Of this, U.S. retail sales of fresh-cut produce is estimated to comprise 10% of the fresh produce market.
Although fresh-cut produce companies have had success in the salad market, the industry has been slower to diversify into other fresh-cut vegetables or fruits because of limitations in film and plastic tray materials used to package these products. After harvesting, vegetables and fruit continue to respire, consuming oxygen and releasing carbon dioxide. Too much or too little oxygen can result in premature spoilage and decay. Conventional packaging films used today, such as polyethylene and polypropylene, can be made with modest permeability to oxygen and carbon dioxide, but often do not provide the optimal atmosphere for the produce packaged. Shortcomings of conventional packaging materials have not significantly hindered the growth in the fresh-cut salad market because lettuce, unlike many vegetables and fruit, has low respiration requirements.
The respiration rate of produce varies from vegetable to vegetable and from fruit to fruit. To achieve optimal product performance, each fruit or vegetable requires its own unique package atmosphere conditions. The challenge facing the industry is to develop packaging that meets the highly variable needs that each product requires in order to achieve value creating performance. The Company believes that its BreatheWay packaging technology possesses all of the critical functionalities required to serve this diverse market. In creating a product package, a BreatheWay membrane is applied over a small cutout section or an aperture of a flexible film bag or plastic tray. This highly permeable “window” acts as the mechanism to provide the majority of the gas transmission requirements for the entire package. These membranes are designed to provide three principal benefits:
High Permeability. Landec's BreatheWay packaging technology is designed to permit transmission of oxygen and carbon dioxide at 300 to 1,000 times the rate of conventional packaging films. The Company believes that these higher permeability levels will facilitate the packaging diversity required to market many types of fresh-cut and whole produce in many package sizes and configurations.
Ability to Adjust Oxygen and Carbon Dioxide Permeability. BreatheWay packaging can be tailored with carbon dioxide to oxygen transfer ratios ranging from 1.0 to 12.0 and selectively transmit oxygen and carbon dioxide at optimum rates to sustain the quality and shelf life of packaged produce. Other high permeability packaging materials, such as micro-perforated films cannot differentially control carbon dioxide permeability resulting in sub-optimal package atmosphere conditions for many produce products.
Temperature Responsiveness. Landec has developed breathable membranes that can be designed to increase or decrease permeability in response to environmental temperature changes. The Company has developed packaging that responds to higher oxygen requirements at elevated temperatures but is also reversible, and returns to its original state as temperatures decline. As the respiration rate of fresh produce also increases with temperature, the BreatheWay membrane’s temperature responsiveness allows packages to compensate for the change in produce respiration by automatically adjusting gas permeation rates. By doing so, detrimental package atmosphere conditions are avoided and improved quality is maintained through the distribution chain.
The Company believes that the growth of the fresh-cut produce market has been driven by consumer demand and the willingness to pay for convenience, freshness, uniform quality, and safety delivered to the point of sale. Landec believes that growth of the overall produce market will be driven by the increasing demand for the convenience and nutrition of fresh-cut produce. This demand will in turn require packaging that facilitates the quality and shelf life of produce transported to fresh-cut distributors in bulk and pallet quantities. The Company thinks that in the future its BreatheWay packaging technology will be useful for packaging a diverse variety of fresh-cut and whole produce products. Potential opportunities for using Landec’s technology outside of the produce market exist in cut flowers and in other respiring products.
Landec is working with leaders in club stores and retail grocery chains. The Company thinks it will have growth opportunities for the next several years through new customers and products in the United States, expansion of its existing customer relationships, and through export and shipments of specialty packaged produce.
Landec manufactures its BreatheWay packaging through selected qualified contract manufacturers. In addition to using BreatheWay packaging for its value-added produce business, the Company markets and sells BreatheWay packaging directly to food distributors.
The Business: Food Products Technology
Our Food Products Technology business, which operates through our Apio subsidiary, had revenues of approximately $176 million for the fiscal year ended May 29, 2011, $175 million for the fiscal year ended May 30, 2010 and $168 million for the fiscal year ended May 31, 2009.
Based in Guadalupe, California, Apio’s primary business is fresh-cut and whole value-added products packaged in our proprietary BreatheWay packaging. The fresh-cut value-added products business markets a variety of fresh-cut and whole vegetables to the top retail grocery chains and club stores. During the fiscal year ended May 29, 2011, Apio shipped nearly sixteen million cartons of produce to leading supermarket retailers, wholesalers, food service suppliers and club stores throughout North America, primarily in the United States.
There are four major distinguishing characteristics of Apio that provide competitive advantages in the Food Products Technology market:
Value-Added Supplier: Apio has structured its business as a marketer and seller of fresh-cut and whole value-added produce. It is focused on selling products under its Eat Smart brand and other brands for its fresh-cut and whole value-added products. As retail grocery and club store chains consolidate, Apio is well positioned as a single source of a broad range of products.
Reduced Farming Risks: Apio reduces its farming risk by not taking ownership of farmland, and instead, contracts with growers for produce and enters into joint ventures with growers for produce. The year-round sourcing of produce is a key component to the fresh-cut and whole value-added processing business.
Lower Cost Structure: Apio has strategically invested in the rapidly growing fresh-cut and whole value-added business. Apio’s 136,000 square foot value-added processing plant, recently expanded from 96,000 square feet, is automated with state-of-the-art vegetable processing equipment. Virtually all of Apio’s value-added products utilize Apio’s proprietary BreatheWay packaging technology. Apio’s primary strategy is to operate one large central processing facility in one of California’s largest, lowest cost growing regions, the Santa Maria Valley, and use packaging technology that allows for the nationwide delivery of fresh produce products.
Expanded Product Line Using Technology: Apio, through the use of its BreatheWay packaging technology, is introducing on average fifteen new value-added products each year. These new product offerings range from various sizes of fresh-cut bagged products, to vegetable trays, to whole produce, to vegetable salads and snack packs. During the last twelve months, Apio has introduced 14 new products.
Apio established its Apio Packaging division in 2005 to advance the sales of BreatheWay packaging technology for shelf-life sensitive vegetables and fruit. The Company’s specialty packaging for case liner products extends the shelf life of certain produce commodities up to 50%. This shelf life extension can enable the utilization of alternative distribution strategies to gain efficiencies or reach new markets while maintaining product quality to the end customer.
Apio Packaging’s first program has concentrated on bananas and was formally consummated when Apio entered into an agreement to supply Chiquita with its proprietary banana packaging technology on a worldwide basis for the ripening, conservation and shelf-life extension of bananas for most applications on an exclusive basis and for other applications on a non-exclusive basis. In addition, Apio provides Chiquita with ongoing research and development and process technology support for the BreatheWay membranes and bags, and technical service support throughout the customer chain in order to assist in the development and market acceptance of the technology.
Chiquita provides marketing, distribution and retail sales support for Chiquita® bananas sold worldwide in BreatheWay packaging. To maintain the exclusive license, Chiquita must meet quarterly minimum purchase thresholds of BreatheWay banana packages.
In fiscal year 2008, the Company expanded the use of its BreatheWay technology to include avocados and mangos under an expanded licensing agreement with Chiquita. Commercial sales of avocados packaged in Landec’s BreatheWay packaging into the food service industry began late in fiscal year 2008 and commercial retail sales began in fiscal 2010.
In June 2008, Apio entered into a collaboration agreement with Seminis Vegetable Seeds, Inc., a wholly-owned subsidiary of Monsanto, to develop novel broccoli and cauliflower products for the exclusive sale by Apio in the North American market. These novel products will be packaged in Landec’s proprietary BreatheWay packaging and will be sold to retail grocery chains, club stores and the food service industry. Field trials for the initial target varieties began in the Fall of 2008. Consumer test markets began in April 2011.
In June 2010, Apio entered into an exclusive license agreement with Windset Farms (“Windset”) for Windset to utilize Landec’s proprietary breathable packaging to extend the shelf life of greenhouse grown cucumbers, peppers and tomatoes.
On February 15, 2011, Apio entered into a share purchase agreement (the “Purchase Agreement”) with Windset Holdings 2010 Ltd., a Canadian corporation (“Windset”). Pursuant to the Purchase Agreement, Apio purchased 150,000 senior preferred shares for $15 million and 201 common shares for $201 that were issued by Windset (the “Purchased Shares”). The Company’s common shares represent a 20.1% interest in Windset. The non-voting senior preferred shares yield a cash dividend of 7.5% annually. The dividend is payable within 90 days of each anniversary of the execution of the Purchase Agreement. The Purchase Agreement includes a put and call option, which can be exercised on the sixth anniversary of the Purchase Agreement whereby Apio can exercise the put to sell its Purchased Shares to Windset, or Windset can exercise the call to purchase the Purchased Shares from Apio, in either case, at a price equal to 20.1% of the appreciation in the fair market value of Windset from the date of the Company’s investment through the put/call date, plus the purchase price of the Purchased Shares. Under the terms of the arrangement with Windset, the Company is entitled to designate one of five members on the Board of Directors of Windset.
B) Food Export Business
Food Export revenues consist of revenues generated from the purchase and sale of primarily whole commodity fruit and vegetable products to Asia through Apio’s export company, Cal-Ex. The Food Export business is a buy/sell business that realizes a commission-based margin on average in the 6-7% range.
The Business: Food Export
The Food Export business had revenues of approximately $62 million for the fiscal year ended May 29, 2011, $55 million for the fiscal year ended May 30, 2010 and $60 million for the fiscal year ended May 31, 2009.
Apio is strategically positioned to benefit from the growth in export sales to Asia and other parts of the world over the next decade with Cal-Ex. Through Cal-Ex, Apio is currently one of the largest U.S. exporters of broccoli to Asia.
C) Hyaluronan-based Biomaterials Business
Our Hyaluronan-based Biomaterials business operates through our Lifecore subsidiary, which we acquired on April 30, 2010. Lifecore had revenues of approximately $32.5 million for the fiscal year ended May 29, 2011 and $1.5 million for the one month included in the fiscal year ended May 30, 2010.
The Technology: Hyaluronan-based Biomaterials
Lifecore uses its fermentation process and aseptic formulation and filling expertise to be a leader in the development of hyaluronan-based products for multiple applications and to take advantage of non-hyaluronan device and drug opportunities which leverage its expertise in manufacturing and aseptic syringe filling capabilities. Elements of Lifecore’s strategy include the following:
· Establish strategic relationships with market leaders. Lifecore will continue to develop applications for products with partners who have strong marketing, sales and distribution capabilities to end-user markets. Through its strong reputation and history of providing premium HA products, Lifecore has been able to establish long-term relationships with the market leading companies such as Alcon, Inc. (Alcon) and Abbott Medical Optics (Abbott) in ophthalmology, and Musculoskeletal Transplant Foundation (MTF) and Novartis AG in orthopedics.
· Expand medical applications for hyaluronan. Due to the growing knowledge of the unique characteristics of hyaluronan and the role it plays in normal physiology, Lifecore continues to identify and pursue further uses for hyaluronan in other medical applications, such as wound care, aesthetic surgery, adhesion prevention, drug delivery, device coatings and pharmaceuticals. Further applications may involve expanding process development activity and/or additional licensing of technology.
· License hyaluronan technology from third parties. Lifecore currently has no commercial products using cross-linking technology. In 2007, Lifecore entered into a world-wide exclusive license and development agreement withThe Cleveland Clinic Foundation to develop and commercialize hyaluronan-based products and related applications. The license is for Corgel ™ Biohydrogel using patented hyaluronan-based cross-linking technology, that can be used for products in aesthetics, orthopedics, ophthalmology and other medical fields. Lifecore has not yet identified any potential commercial products for this technology; however Landec will continue to investigate potential applications.
· Utilize manufacturing infrastructure to pursue contract aseptic filling and fermentation opportunities. Lifecore will continue to evaluate providing contract services for opportunities that are suited for the capital and facility investment related to aseptic filling equipment, fermentation and purification.
· Maintain flexibility in product development and supply relationships. Lifecore’s vertically integrated development and manufacturing capabilities allow it to establish a variety of relationships with global corporate partners. Lifecore’s role in these relationships extends from supplying hyaluronan raw materials to manufacturing of aseptically-packaged, finished sterile products to developing and manufacturing its own proprietary products.
Hyaluronan Products
The following table summarizes the principal products of the Hyaluronan-based Biomaterials business, along with their applications, and the companies with which Lifecore has related strategic relationships:
| PRODUCT | | DESCRIPTION | | MARKET | | STATUS+ |
| OPHTHALMIC | | | | | | |
Viscoat® Intraocular Viscoelastic | | Lifecore supplies hyaluronan powder for inclusion in Alcon’s Viscoat® Ophthalmic Viscoelastic. | | Cataract surgery | | Commercial sales since 1986 |
| | | | | | | |
| LUROCOAT Ophthalmic Viscoelastic | | Lifecore supplies its private label product for marketing on a non-exclusive basis to multiple distribution partners. | | Cataract surgery | | Commercial sales since June 1997 |
| ORTHOPEDIC | | | | | | |
| Hyaluronan Solution for DBX® Demineralized Bone Matrix | | Lifecore supplies a sterile hyaluronan solution to MTF for use as a carrier vehicle for its allogeneic demineralized, freeze-dried bone. | | Grafting material for restoration of bone defects | | Commercial sales since 2000 |
| | | | | | | |
| Hyaluron HEXAL® Orthopedic Viscosupplement | | Lifecore supplies a finished orthopedic viscosupplement for Novartis AG’s distribution network. | | Injections for the local treatment of pain associated with osteoarthritis | | Commercial sales since 2005 |
VETERINARY | | | | | | |
| HY-50® | | Lifecore supplies a finished veterinary viscosupplement to Bexco Pharma, Inc. for use as an equine injectable. | | Veterinary drug/device | | Commercial sales since 1993 |
+ For all products listed above, government regulatory approvals were required before commercial sales could commence in the United States or elsewhere. See “Government Regulation.” No assurance can be given that such products will be successfully approved in new markets.
Ophthalmic Applications
Cataract Surgery. Currently a primary commercial application for Lifecore’s hyaluronan is in cataract surgery. Hyaluronan, in the form of a viscoelastic solution, is used to maintain a deep chamber during anterior segment surgeries (including cataract extraction and intraocular lens implantation) and to protect the corneal endothelium and other ocular tissue. These solutions have been shown to reduce surgical trauma and thereby contribute to more rapid recovery with fewer complications than were experienced prior to the use of viscoelastics. Hyaluronan-based products are used in the majority of cataract surgeries in the world.
Lifecore currently sells hyaluronan for this application to Alcon, the leading producer of ophthalmic surgical products in the world, for inclusion in Alcon’s proprietary viscoelastic solutions. Lifecore’s relationship with Alcon and its predecessors commenced in 1983. Since that time, sales of hyaluronan to Alcon have continued to be made pursuant to supply agreements. The current supply agreements are non-cancelable, non-exclusive and encompass a term through December 2017.
Lifecore has developed its own viscoelastic solution, LUROCOAT Ophthalmic Viscoelastic. Lifecore received CE marking for LUROCOAT Ophthalmic Viscoelastic in 1997, allowing LUROCOAT Ophthalmic Viscoelastic to be marketed and sold outside the United States. Lifecore also has distribution agreements with multiple companies to supply its hyaluronan-based LUROCOAT Ophthalmic Viscoelastic under private label.
Lifecore signed an agreement with Abbott to supply Lifecore’s hyaluronan-based viscoelastic under private label with sales commencing in 2004. The current supply agreement with Abbott is non-cancelable, non-exclusive and incorporates a term through May 2013 with renewal provisions.
Lifecore estimates that its hyaluronan has been used in over 50 million ophthalmic patients globally since 1983.
Orthopedic Applications
Lifecore supplies an aseptic hyaluronan solution to BioCon, Inc., the non-profit affiliate of MTF, which utilizes the solution as a carrier vehicle for its allogeneic demineralized, freeze-dried bone in a final putty composition trademarked as “DBX Demineralized Bone Matrix”. This bone putty is provided by MTF to orthopedic surgeons through MTF’s distribution channels. Lifecore has a non-cancelable, exclusive supply agreement with MTF through December 2014.
Lifecore also supplies a private-labeled finished orthopedic viscosupplement for Novartis AG’s distribution network under a non-cancelable supply agreement through 2014.
Veterinary Applications
Lifecore manufactures Bexco Pharma, Inc.’s HY-50 product, an aseptically packaged hyaluronan solution for use as a veterinary viscosupplement as an equine injectable drug, under a non-cancelable, exclusive supply agreement through June 2015 with renewal provisions.
Lifecore estimates that its veterinary hyaluronan product has been used in over 700,000 equine procedures worldwide.
Product Development
Lifecore undertakes its own product development activities for hyaluronan-based applications, as well as on a contract basis with certain clients. The majority of the projects are intended to demonstrate that Lifecore’s hyaluronan is suitable for a particular medical application. Suitability is often measured by detailed specifications for product characteristics such as purity, stability, viscosity and molecular weight, as well as efficacy for a particular medical application in a clinical setting.
In addition, since 2007, Lifecore has licensed a hyaluronate cross-linking technology from The Cleveland Clinic Foundation designed to provide a development vehicle for possible new products for the existing medical segments, as well as potentially new market segments. To date, Lifecore has yet to identify potential commercial products or attract potential third party partners in developing the technology.
There can be no assurance that products currently under development by Lifecore or in partnership with others will be successfully developed or, if so developed, will be successfully and profitably marketed.
D) Technology Licensing Business
Our Technology Licensing Business, which includes our seed coating subsidiary Landec Ag LLC (“Landec Ag”), had revenues of $6.9 million for the fiscal year ended May 29, 2011, $6.1 million for the fiscal year ended May 30, 2010 and $5.4 million for the fiscal year ended May 31, 2009.
Seeds Business – Intellicoat Seed Coatings and Landec Ag
Following the sale of Fielder’s Choice Direct (“FCD”), Landec Ag’s strategy has been to work closely with Monsanto to further develop our patented, functional polymer coating technology for sale and/or licensing to the seed industry. In accordance with its License, Supply and R&D agreement with Monsanto, Landec Ag is currently focused on commercializing products for the soybean and seed corn market and plans to broaden the technology to other seed crop applications.
The Technology: Intellicoat Seed Coatings
Landec's Intellicoat seed coating applications are designed to control seed germination timing, increase crop yields, reduce risks and extend crop-planting windows. These coatings are currently available on male inbred corn used for seed production. In fiscal year 2000, Landec Ag launched Pollinator Plusâ coatings, which is a coating application used by seed companies as a method for spreading pollination to increase yields and reduce risk in the production of hybrid seed corn. In 2011, Pollinator Plus was used by eight seed companies on approximately 20% of the seed corn production acres in the U.S.
Monsanto announced in 2008 that it had formed a new business called the Seed Treatment Business which will allow Monsanto to develop its seed treatment requirements internally. The concept of seed treatments is to place an insecticide or fungicide directly onto the seed surface in order to protect the seed and the seedling as it emerges. Landec’s Intellicoat seed coating technology could be an integral and proprietary part of Monsanto’s commitment to building a major position in seed treatments worldwide by using Landec’s seed coatings as a “carrier” of insecticides/fungicides which can be dispensed at the appropriate time based on time or soil temperature. During fiscal year 2010, we amended our agreement with Monsanto and as a result our development activities are focused on a specific technology of interest to Monsanto. During fiscal year 2011, we have focused on validating the use of Landec’s coating technology for these applications.
Monsanto License
In December 2006, Landec entered into a five-year co-exclusive technology license and polymer supply agreement (“the Monsanto Agreement”) with Monsanto for the use of Landec’s Intellicoat polymer seed coating technology. Under the terms of the Monsanto Agreement, Monsanto agreed to pay Landec Ag $2.6 million per year. The Monsanto Agreement was amended in November 2009. Under the terms of the amended Monsanto Agreement, Monsanto continues to have an exclusive license to use Landec’s Intellicoat polymer technology for specific seed treatment applications.
Along with regaining the use of the Intellicoat technology outside of the specific applications licensed to Monsanto under the amended Monsanto Agreement in November 2009, Landec has assumed responsibility for Landec Ag’s operating expenses and realizes all the revenues and profits from the sales of existing and new Intellicoat seed coating products.
The Monsanto Agreement also provides for a fee payable to Landec Ag of $4 million if Monsanto elects to terminate the Monsanto Agreement or $10 million if Monsanto elects to purchase the rights to the exclusive field. If Monsanto does not exercise its purchase option by December 2011, Landec Ag will receive the termination fee and all rights to the Intellicoat seed coating technology will revert to Landec. Under the Monsanto Agreement, Landec will receive aggregate minimum guaranteed payments of $17 million for license fees and polymer supply payments over five years or $23 million in aggregate maximum payments if Monsanto elects to purchase the rights to the exclusive field. The incremental $6 million to be received in the event Monsanto exercises the purchase option has been deferred and will be recognized on the exercise of the purchase option. The fair value of the purchase option was determined by management to be less than the amount of the deferred revenue.
If Monsanto elects to purchase the rights to the exclusive field, a gain on the sale will be recognized at the time of purchase. If Monsanto exercises its purchase option, we expect to enter into a new long-term supply agreement and/or a technology service fee agreement with Monsanto.
Non-Seed Business
We think our technology has commercial potential in a wide range of industrial, consumer and medical applications beyond those identified in our other segments. For example, our core patented technology, Intelimer materials, can be used to trigger release of catalysts, insecticides or fragrances just by changing the temperature of the Intelimer materials or to activate adhesives through controlled temperature change. In order to exploit these opportunities, we have entered into and will enter into licensing and collaborative corporate agreements for product development and/or distribution in certain fields. However, given the infrequency and unpredictability of when the Company may enter into any such licensing and research and development arrangements, the Company is unable to disclose its financial expectations in advance of entering into such arrangements.
Industrial Materials and Adhesives
Landec’s industrial product development strategy focuses on coatings, catalysts, resins, additives and adhesives in the polymer materials market. During the product development stage, the Company identifies corporate partners to support the ongoing development and testing of these products, with the ultimate goal of licensing the applications at the appropriate time.
Intelimer Latent Catalyst Polymer Systems
Landec has developed latent catalysts useful in extending pot-life, extending shelf life, reducing waste and improving thermoset cure methods. Some of these latent catalysts are currently being distributed by Akzo-Nobel Chemicals B.V. through our licensing agreement with Air Products. The rights to develop and sell Landec’s latent catalysts and personal care technologies were licensed to Air Products in March 2006.
Personal Care and Cosmetic Applications
Landec’s personal care and cosmetic applications strategy is focused on supplying Intelimer materials to industry leaders for use in lotions and creams, as well as color cosmetics, lipsticks and hair care. The Company's partner, Air Products, is currently shipping products to L’Oreal, Mentholatum and other companies for use in lotions and creams. The rights to develop and sell Landec’s polymers for personal care products were licensed to Air Products in March 2006 along with the latent catalyst rights. The Company’s Intelimer polymers are currently in over 50 personal care products worldwide.
Intelimer Drug Delivery Polymers
Landec has been developing both biodegradable and non-biodegradable polymers for use in drug delivery applications targeting the use of its highly crystalline polymers and the tunable physical properties to minimize or eliminate burst, extend drug release profiles and deliver novel valuable properties to the pharma industry.
Sales and Marketing
Each of the Company’s core businesses is supported by dedicated sales and marketing resources. The Company intends to develop its internal sales capacity as more products progress toward commercialization and as business volume expands geographically. During fiscal years 2011, 2010 and 2009, sales to the Company’s top five customers accounted for approximately 44%, 48% and 46%, respectively, of its revenues, with the top customer, Costco Wholesale Corp., accounting for approximately 16%, 20% and 21%, respectively, of the Company’s revenues.
Apio
Apio has 21 sales and marketing employees, located in central California and throughout the U.S., supporting the Food Products Technology business and the Food Export business.
Seasonality
The Company’s sales are moderately seasonal. All of Landec Ag’s product sales are generated in our fiscal fourth quarter during the spring planting season. In addition, the Food Products Technology business can be heavily affected by seasonal weather factors which have impacted quarterly results, such as high cost of sourcing product due to a shortage of essential value-added produce items and the change in fair value in Apio’s investment in Windset. The Food Export business also typically recognized a much higher percentage of its revenues and profit during the first half of Landec’s fiscal year compared to the second half. Lifecore’s business is not significantly affected by seasonality.
Manufacturing and Processing
Food Products Technology Business
The manufacturing process for the Company's proprietary BreatheWay packaging products is comprised of polymer manufacturing, membrane manufacturing and label package conversion. A third party toll manufacturer currently makes virtually all of the polymers for the BreatheWay packaging system. Select outside contractors currently manufacture the breathable membranes and Landec has transitioned virtually all of the label package conversion to Apio’s Guadalupe facility to meet the increasing product demand and to provide additional developmental capabilities.
Apio processes virtually all of its fresh-cut value-added products in its state-of-the-art processing facility located in Guadalupe, California. Cooling of produce is done through third parties and Apio Cooling LP, a separate consolidated subsidiary in which Apio has a 60% ownership interest and is the general partner.
Hyaluronan-based Biomaterials Business
The commercial production of hyaluronan by Lifecore requires fermentation, separation and purification capabilities. Products are supplied in a variety of bulk and single dose configurations.
Lifecore produces its hyaluronan through a bacterial fermentation process. In the early 1980’s, Lifecore introduced the bacterial fermentation process to manufacture premium pharmaceutical-grade hyaluronan, and received patent protection in 1985. Lifecore’s fermentation process patent expired in 2002. Previously, medical grade hyaluronan was commercially available through an extraction process from rooster combs. Lifecore thinks that the fermentation manufacturing approach is superior to rooster comb extraction because of greater efficiency and flexibility, a more favorable long-term regulatory environment, and better economies of scale in producing large commercial quantities.
Lifecore’s 112,000 square foot facility in Chaska, Minnesota is used primarily for the hyaluronan manufacturing process, formulation and aseptic syringe and bulk filling. The Company considers that the current inventory on-hand, together with its manufacturing capacity, will be sufficient to allow it to meet the needs of its current customers for the foreseeable future.
Lifecore provides versatility in the manufacturing of various types of finished products. Currently, it supplies several different forms of hyaluronan in a variety of molecular weight fractions as powders, solutions and gels, and in a variety of bulk and single-use finished packages. Lifecore continues to conduct development work designed to improve production efficiencies and expand its capabilities to achieve a wider range of hyaluronan product specifications in order to address the broadening opportunities for using hyaluronan in medical applications.
The FDA inspects the Company’s manufacturing systems periodically and requires conformance to the FDA’s Quality System Regulation (“QSR”). In addition, Lifecore’s corporate partners conduct intensive quality audits of the facility and its operations. Lifecore also periodically contracts with independent regulatory consultants to conduct audits of its operations. As a result, similar to other manufacturers of HA subject to regulatory and customer specific requirements, Lifecore’s facility was designed to meet applicable regulatory requirements and has been cleared for the manufacturing of both device and pharmaceutical products. The Company maintains a Quality System which assures conformance to all applicable current standards (21 CFR820, 21 CFR210-211, ISO 13485:2003, 93/42/EEC, and Canadian Medical Device Regulation: 1998). These approvals represent international symbols of quality system assurance and compliance with applicable European Medical Device Directives, which greatly assist in the marketing of Lifecore’s products in the European Union.
Technology Licensing Business
Landec performs its batch seed coating operations in a leased facility in Oxford, Indiana. This facility is being used to coat other seed companies’ inbred seed corn with the Company’s Pollinator Plus seed corn coatings.
Landec has a pilot manufacturing facility in Indiana to support process development, scale-up and commercialization of the Company’s seed coating programs. This facility utilizes a continuous coating process that has increased seed coating capabilities by over tenfold compared to the previous system using batch coaters.
General
Several of the raw materials used in manufacturing certain of the Company’s products are currently purchased from a single source. Upon manufacturing scale-up of seed coating operations, the Company may enter into alternative supply arrangements. Although to date the Company has not experienced difficulty acquiring materials for the manufacture of its products, no assurance can be given that interruptions in supplies will not occur in the future, that the Company will be able to obtain substitute vendors, or that the Company will be able to procure comparable materials at similar prices and terms within a reasonable time. Any such interruption of supply could have a material adverse effect on the Company’s ability to manufacture and distribute its products and, consequently, could materially and adversely affect the Company’s business, operating results and financial condition.
Research and Development
Landec is focusing its research and development resources on both existing and new applications of its polymer technologies. Expenditures for research and development for the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009 were $9.3 million, $4.4 million and $3.7 million, respectively. Research and development expenditures funded by corporate or governmental partners were $0 for both the fiscal years ended May 29, 2011 and May 30, 2010 and $152,000 for the fiscal year ended May 31, 2009. The Company may continue to seek funds for applied materials research programs from U.S. government agencies as well as from commercial entities. The Company anticipates that it will continue to have significant research and development expenditures in order to maintain its competitive position with a continuing flow of innovative, high-quality products and services. As of May 29, 2011, Landec had 53 employees engaged in research and development with experience in polymer and analytical chemistry, product application, product formulation, mechanical and chemical engineering.
Competition
The Company operates in highly competitive and rapidly evolving fields, and new developments are expected to continue at a rapid pace. Competition from large food processors, packaging companies, agricultural companies, medical and pharmaceutical companies is intense. In addition, the nature of the Company's collaborative arrangements and its technology licensing business may result in its corporate partners and licensees becoming competitors of the Company. Many of these competitors have substantially greater financial and technical resources and production and marketing capabilities than the Company, and many have substantially greater experience in conducting field trials, obtaining regulatory approvals and manufacturing and marketing commercial products. There can be no assurance that these competitors will not succeed in developing alternative technologies and products that are more effective, easier to use or less expensive than those which have been or are being developed by the Company or that would render the Company's technology and products obsolete and non-competitive.
Patents and Proprietary Rights
The Company's success depends in large part on its ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of third parties. The Company has had 37 U.S. patents issued of which 26 remain active as of May 29, 2011 with expiration dates ranging from 2012 to 2023. The Company's issued and pending patents include claims relating to compositions, devices and use of a class of temperature and time sensitive polymers that exhibit distinctive properties of permeability, adhesion and viscosity control. There can be no assurance that any of the pending patent applications will be approved, that the Company will develop additional proprietary products that are patentable, that any patents issued to the Company will provide the Company with competitive advantages or will not be challenged by any third parties or that the patents of others will not prevent the commercialization of products incorporating the Company's technology. Furthermore, there can be no assurance that others will not independently develop similar products, duplicate any of the Company's products or design around the Company's patents. Any of the foregoing results could have a material adverse effect on the Company's business, operating results and financial condition.
The commercial success of the Company will also depend, in part, on its ability to avoid infringing patents issued to others. If the Company were determined to be infringing any third-party patent, the Company could be required to pay damages, alter its products or processes, obtain licenses or cease certain activities. In addition, if patents are issued to others which contain claims that compete or conflict with those of the Company and such competing or conflicting claims are ultimately determined to be valid, the Company may be required to pay damages, to obtain licenses to these patents, to develop or obtain alternative technology or to cease using such technology. If the Company is required to obtain any licenses, there can be no assurance that the Company will be able to do so on commercially favorable terms, if at all. The Company's failure to obtain a license to any technology that it may require to commercialize its products could have a material adverse impact on the Company's business, operating results and financial condition.
Litigation, which could result in substantial costs to the Company, may also be necessary to enforce any patents issued or licensed to the Company or to determine the scope and validity of third-party proprietary rights. If competitors of the Company prepare and file patent applications in the United States that claim technology also claimed by the Company, the Company may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention, which could result in substantial cost to and diversion of effort by the Company, even if the eventual outcome is favorable to the Company. Any such litigation or interference proceeding, regardless of outcome, could be expensive and time consuming and could subject the Company to significant liabilities to third parties, require disputed rights to be licensed from third parties or require the Company to cease using such technology and consequently, could have a material adverse effect on the Company's business, operating results and financial condition.
In addition to patent protection, the Company relies on trade secrets, proprietary know-how and technological advances which the Company seeks to protect, in part, by confidentiality agreements with its collaborators, employees and consultants. There can be no assurance that these agreements will not be breached, that the Company will have adequate remedies for any breach, or that the Company's trade secrets and proprietary know-how will not otherwise become known or be independently discovered by others.
Government Regulation
Government regulation in the United States and other countries is a significant factor in the marketing of certain of the Company’s products and in the Company’s ongoing research and development activities. Some of the Company’s products are subject to extensive and rigorous regulation by the FDA, which regulates some of the products as medical devices and which, in some cases, requires Pre-Market Approval (“PMA”), and by foreign countries, which regulate some of the products as medical devices or drugs. Under the Federal Food, Drug, and Cosmetic Act (“FDC Act”), the FDA regulates the clinical testing, manufacturing, labeling, distribution, sale and promotion of medical devices in the United States.
Following the enactment of the Medical Device Amendments of 1976 to the FDC Act, the FDA classified medical devices in commercial distribution at the time of enactment (“pre-Amendment devices”) into one of three classes - Class I, II or III. This classification is based on the controls necessary to reasonably assure the safety and effectiveness of medical devices. Class I devices are those whose safety and effectiveness can reasonably be assured through general controls, such as establishment registration and labeling, and adherence to FDA-mandated current QSR requirements for devices. Most Class I devices are exempt from FDA premarket review, but some require premarket notification (“510(k) Notification”). Class II devices are those whose safety and effectiveness can reasonably be assured through the use of special controls, such as performance standards, postmarket surveillance, patient registries and FDA guidelines. Class III devices are devices that require a PMA from the FDA to assure their safety and effectiveness. A PMA ordinarily must contain data from a multi-center clinical study demonstrating the device’s safety and effectiveness for the intended use and patient population. Class III devices are generally life-sustaining, life-supporting or implantable devices, and also include most devices that were not on the market before May 28, 1976 (“new devices”) and for which the FDA has not made a finding of substantial equivalence based upon a 510(k) Notification. A pre-Amendment Class III device does not require a PMA unless and until the FDA issues a regulation requiring submission of a PMA application for the device.
The FDA requires clinical data for a PMA application and has the authority to require such data for a 510(k) Notification. If clinical data are necessary, the company that sponsors the study must follow the FDA’s Investigational Device Exemption (“IDE”) regulations governing the conduct of human studies. The FDA’s regulations require institutional review board approval of the study and the informed consent of the study subjects. In addition, for a “significant risk” device, the FDA must approve an IDE application before the study can begin. Non-significant risk devices do not require FDA approval of an IDE application, and are conducted under the “abbreviated IDE” requirements. Once in effect, an IDE or abbreviated IDE permits evaluation of devices under controlled clinical conditions. After a clinical evaluation process, the resulting data may be included in a PMA application or a 510(k) Notification. The PMA may be approved or the 510(k) Notification may be cleared by the FDA only after a review process that may include FDA requests for additional data, sometimes requiring further studies.
If a manufacturer or distributor of medical devices can establish to the FDA’s satisfaction through a 510(k) Notification that a new device is substantially equivalent to what is called a “predicate device,” i.e., a legally marketed Class I or Class II medical device or a legally marketed pre-Amendment Class III device for which the FDA has not required a PMA, the manufacturer or distributor may market the new device. In the 510(k) Notification, a manufacturer or distributor makes a claim of substantial equivalence, which the FDA may require to be supported by various types of information, including data from clinical studies, showing that the new device is as safe and effective for its intended use as the predicate device.
Following submission of the 510(k) Notification, the manufacturer or distributor may not place the new device into commercial distribution until the FDA issues a “substantial equivalence” determination finding the new device to be substantially equivalent to a predicate device. The FDA has a 90 day period in which to respond to a 510(k) Notification (30 days for a Special 510(k)). Depending on the specific submission and subsequent agency information requests, the 510(k) Notification process can take significantly longer to complete. The FDA may agree with the manufacturer or distributor that the new device is substantially equivalent to a predicate device and allow the new device to be marketed in the United States. The FDA may, however, determine that the new device is not substantially equivalent and require the manufacturer or distributor to submit a PMA or require further information, such as additional test data, including data from clinical studies, before it is able to make a determination regarding substantial equivalence. Although the PMA process is significantly more complex, time-consuming and expensive than the 510(k) Notification process, the latter process can also be expensive and substantially delay the market introduction of a product. Modifications to a device that is marketed under a 510(k) Notification might require submission of a new 510(k) prior to their implementation, although some modifications can be made through a “note to file” procedure described in FDA guidance.
For devices that cannot be found “substantially equivalent” to a predicate device, the manufacturer must submit a PMA application, petition for reclassification, or submit a PMA application via the de novo process. A PMA must contain information on the materials and manufacturing process for the device, results of preclinical testing, clinical data, and labeling for the device. The FDA has 180 days to review a PMA application, but may request additional information, which could include additional studies. The FDA might refer a PMA to an advisory committee of outside experts to review and make recommendation on whether a device should be approved. After considering the data in the PMA application and the recommendations of an advisory committee, the FDA can approve the device, approve the device with conditions or refuse approval. Devices approved by the FDA are subject to periodic reporting requirements, and may be subject to restrictions on sale, distribution or use.
Hyaluronan products are generally Class III devices. In cases where the Company is supplying hyaluronan to a corporate partner as a raw material or producing a finished product under a license for the partner, the corporate partner will be responsible for obtaining the appropriate FDA clearance or approval. Export of the Company’s hyaluronan products generally requires approval of the importing country and compliance with the export provisions of the FDC Act.
Other regulatory requirements are placed on the manufacture, processing, packaging, labeling, distribution, recordkeeping and reporting of a medical device and on the quality control procedures, such as the FDA’s device QSR regulations. Manufacturing facilities are subject to periodic inspections by the FDA to assure compliance with device QSR requirements. Lifecore’s facility is subject to inspections as both a device and a drug manufacturing operation. For PMA devices, the Company is required to submit an annual report and to obtain approval of a PMA supplement for modifications to the device or its labeling. Other applicable FDA requirements include the medical device reporting (“MDR”) regulation, which requires that the Company provide information to the FDA regarding deaths or serious injuries alleged to have been associated with the use of its devices, as well as product malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur. The FDA also requires reporting regarding notices of correction and the removal of a medical device.
If the Company is not in compliance with FDA requirements, the FDA or the federal government can order a recall, detain the Company’s devices, refuse to grant 510(k) Notification clearances or PMA approvals, withdraw or limit product approvals, institute proceedings to seize the Company’s devices, seek injunctions to control or prohibit marketing and sales of the Company’s devices, assess civil money penalties and impose criminal sanctions against the Company, its officers or its employees.
There can be no assurance that any of the Company’s clinical studies will show safety or effectiveness; that 510(k) Notifications or PMA applications or supplemental applications will be submitted or, if submitted, accepted for filing; that any of the Company’s products that require clearance of a 510(k) Notification or approval of a PMA application or PMA supplement will obtain such clearance or approval on a timely basis, on terms acceptable to the Company for the purpose of actually marketing the products, or at all; or that following any such clearance or approval previously unknown problems will not result in restrictions on the marketing of the products or withdrawal of clearance or approval.
Product Liability
Product liability claims may be asserted with respect to the Company’s products. The Company maintains product liability insurance coverage in amounts the Company deems to be adequate There can be no assurance that the Company will have sufficient resources to satisfy product claims if they exceed available insurance coverage.
As of May 29, 2011, Landec had 255 full-time employees, of whom 185 were dedicated to research, development, manufacturing, quality control and regulatory affairs and 70 were dedicated to sales, marketing and administrative activities. Landec intends to recruit additional personnel in connection with the development, manufacturing and marketing of its products. None of Landec's employees is represented by a union, and Landec believes its relationship with its employees is good.
Available Information
Landec’s website is http://www.landec.com. Landec makes available free of charge its annual, quarterly and current reports, and any amendments to those reports, as soon as reasonably practicable after electronically filing such reports with the SEC. Information contained on our website is not part of this Report.
Item 1A. Risk Factors
Landec desires to take advantage of the “Safe Harbor” provisions of the Private Securities Litigation Reform Act of 1995 and of Section 21E and Rule 3b-6 under the Securities Exchange Act of 1934. Specifically, Landec wishes to alert readers that the following important factors could in the future affect, and in the past have affected, Landec’s actual results and could cause Landec’s results for future periods to differ materially from those expressed in any forward-looking statements made by or on behalf of Landec. Landec assumes no obligation to update such forward-looking statements.
The Global Economy is Currently Undergoing a Period of Slowdown and Unprecedented Volatility, Which May Have an Adverse Effect on Our Business
The U.S. and international economy and financial markets have experienced significant slowdown and volatility due to uncertainties related to the availability of credit, energy prices, difficulties in the banking and financial services sectors, softness in the housing market, severely diminished market liquidity, geopolitical conflicts, falling consumer confidence and high unemployment rates. This slowdown has and could further lead to reduced demand for our products, which in turn, would reduce our revenues and adversely affect our business, financial condition and results of operations. In particular, the slowdown and volatility in the global markets have resulted in softer demand and more conservative purchasing decisions by customers, including a tendency toward lower-priced products, which could negatively impact our revenues, gross margins and results of operations. In addition to a reduction in sales, our profitability may decrease during downturns because we may not be able to reduce costs at the same rate as our sales decline. These slowdowns are expected to worsen if current economic conditions are prolonged or deteriorate further. We cannot predict the ultimate severity or length of the current economic crisis, or the timing or severity of future economic or industry downturns.
Given the current unfavorable economic environment, our customers may have difficulties obtaining capital at adequate or historical levels to finance their ongoing business and operations, which could impair their ability to make timely payments to us. This may result in lower sales and/or inventory that may not be saleable or bad debt expense for Landec. In addition to the impact of the economic downturn on our customers, some of our vendors and growers may experience a reduction in their availability of funds and cash flows, which could negatively impact their business as well as ours. A continuing or deepening downturn of the U.S. economy, including increased volatility in the credit markets, could adversely impact our customers’ and vendors’ ability or willingness to conduct business with us on the same terms or at the same levels as they have historically.
We are unable to predict the likely duration and severity of the current disruption in the financial markets and adverse economic conditions in the U.S. and other countries and whether such conditions, if they persist or worsen, will further adversely impact our business, operating results, and financial condition. Further, these conditions and uncertainty about future economic conditions make it challenging for Landec to forecast its operating results, make business decisions, and identify the risks that may affect its business, sources and use of cash, financial condition and results of operations.
Our Future Operating Results Are Likely to Fluctuate Which May Cause Our Stock Price to Decline
In the past, our results of operations have fluctuated significantly from quarter to quarter and are expected to continue to fluctuate in the future. Apio can be heavily affected by seasonal and weather factors which have impacted financial results due to a shortage of essential value-added produce items, including the $5.0 million negative impact on net income which occurred in fiscal year 2011 due to weather-related produce sourcing issues. Our earnings may also fluctuate based on our ability to collect accounts receivable from customers and notes receivable from growers and on price fluctuations in the fresh vegetables and fruits markets. Other factors that affect our operations include:
| | the seasonality of our supplies, |
| | our ability to process produce during critical harvest periods, |
| | the timing and effects of ripening, |
| | the degree of perishability, |
| | the effectiveness of worldwide distribution systems, |
| | total worldwide industry volumes, |
| | the seasonality and timing of consumer demand, |
| | foreign currency fluctuations, and |
| | foreign importation restrictions and foreign political risks. |
As a result of these and other factors, we expect to continue to experience fluctuations in quarterly operating results.
Uncertainty Relating To Integration Of New Business Acquisitions.
The successful integration of new business acquisitions may require substantial effort from the Company's management. The diversion of the attention of management and any difficulties encountered in the transition process could have a material adverse effect on the Company's ability to realize the anticipated benefits of the acquisitions. The successful combination of new businesses also requires coordination of research and development activities, manufacturing, and sales and marketing efforts. In addition, the process of combining organizations could cause the interruption of, or a loss of momentum in, the Company's activities. There can be no assurance that the Company will be able to retain key management, technical, sales and customer support personnel, or that the Company will realize the anticipated benefits of any acquisitions, and the failure to do so would have a material adverse effect on the Company's business, results of operations and financial condition.
We May Not Be Able to Achieve Acceptance of Our New Products in the Marketplace
Our success in generating significant sales of our products depends in part on our ability and our partners and licensees to achieve market acceptance of our new products and technology. The extent to which, and rate at which, we achieve market acceptance and penetration of our current and future products is a function of many variables including, but not limited to:
| | marketing and sales efforts, and |
| | general economic conditions affecting purchasing patterns. |
We may not be able to develop and introduce new products and technologies in a timely manner or new products and technologies may not gain market acceptance. We are in the early stage of product commercialization of certain Intelimer-based specialty packaging, Intellicoat seed coatings, HA-based products and other Intelimer polymer products and many of our potential products are in development. We believe that our future growth will depend in large part on our ability to develop and market new products in our target markets and in new markets. In particular, we expect that our ability to compete effectively with existing food products, agricultural, industrial, medical and pharmaceutical companies will depend substantially on successfully developing, commercializing, achieving market acceptance of and reducing the cost of producing our products. In addition, commercial applications of our temperature switch polymer technology are relatively new and evolving. Our failure to develop new products or the failure of our new products to achieve market acceptance would have a material adverse effect on our business, results of operations and financial condition.
We Face Strong Competition in the Marketplace
Competitors may succeed in developing alternative technologies and products that are more effective, easier to use or less expensive than those which have been or are being developed by us or that would render our technology and products obsolete and non-competitive. We operate in highly competitive and rapidly evolving fields, and new developments are expected to continue at a rapid pace. Competition from large food products, agricultural, industrial, medical and pharmaceutical companies is expected to be intense. In addition, the nature of our collaborative arrangements may result in our corporate partners and licensees becoming our competitors. Many of these competitors have substantially greater financial and technical resources and production and marketing capabilities than we do, and may have substantially greater experience in conducting clinical and field trials, obtaining regulatory approvals and manufacturing and marketing commercial products.
We Have a Concentration of Manufacturing in One Location for Apio and Lifecore and May Have to Depend on Third Parties to Manufacture Our Products
Any disruptions in our primary manufacturing operation at Apio’s facility in Guadalupe, California or Lifecore’s facility in Chaska, Minnesota would reduce our ability to sell our products and would have a material adverse effect on our financial results. Additionally, we may need to consider seeking collaborative arrangements with other companies to manufacture our products. If we become dependent upon third parties for the manufacture of our products, our profit margins and our ability to develop and deliver those products on a timely basis may be adversely affected. Failures by third parties may impair our ability to deliver products on a timely basis and impair our competitive position. We may not be able to continue to successfully operate our manufacturing operations at acceptable costs, with acceptable yields, and retain adequately trained personnel.
Our Dependence on Single-Source Suppliers and Service Providers May Cause Disruption in Our Operations Should Any Supplier Fail to Deliver Materials
We may experience difficulty acquiring materials or services for the manufacture of our products or we may not be able to obtain substitute vendors. We may not be able to procure comparable materials at similar prices and terms within a reasonable time. Several services that are provided to Apio are obtained from a single provider. Several of the raw materials we use to manufacture our products are currently purchased from a single source, including some monomers used to synthesize Intelimer polymers, substrate materials for our breathable membrane products and raw materials for our HA products. Any interruption of our relationship with single-source suppliers or service providers could delay product shipments and materially harm our business.
We May Be Unable to Adequately Protect Our Intellectual Property Rights
We may receive notices from third parties, including some of our competitors, claiming infringement by our products of patent and other proprietary rights. Regardless of their merit, responding to any such claim could be time-consuming, result in costly litigation and require us to enter royalty and licensing agreements which may not be offered or available on terms acceptable to us. If a successful claim is made against us and we fail to develop or license a substitute technology, we could be required to alter our products or processes and our business, results of operations or financial position could be materially adversely affected. Our success depends in large part on our ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of third parties. Any pending patent applications we file may not be approved and we may not be able to develop additional proprietary products that are patentable. Any patents issued to us may not provide us with competitive advantages or may be challenged by third parties. Patents held by others may prevent the commercialization of products incorporating our technology. Furthermore, others may independently develop similar products, duplicate our products or design around our patents.
Our Operations Are Subject to Regulations that Directly Impact Our Business
Our products and operations are subject to governmental regulation in the United States and foreign countries. The manufacture of our products is subject to periodic inspection by regulatory authorities. We may not be able to obtain necessary regulatory approvals on a timely basis or at all. Delays in receipt of or failure to receive approvals or loss of previously received approvals would have a material adverse effect on our business, financial condition and results of operations. Although we have no reason to believe that we will not be able to comply with all applicable regulations regarding the manufacture and sale of our products and polymer materials, regulations are always subject to change and depend heavily on administrative interpretations and the country in which the products are sold. Future changes in regulations or interpretations relating to matters such as safe working conditions, laboratory and manufacturing practices, environmental controls, and disposal of hazardous or potentially hazardous substances may adversely affect our business.
We are subject to FDA rules and regulations concerning the safety of the food products handled and sold by Apio, and the facilities in which they are packed and processed. Failure to comply with the applicable regulatory requirements can, among other things, result in:
fines, injunctions, civil penalties, and suspensions,
withdrawal of regulatory approvals,
product recalls and product seizures, including cessation of manufacturing and sales,
operating restrictions, and
We may be required to incur significant costs to comply with the laws and regulations in the future which may have a material adverse effect on our business, operating results and financial condition.
Our food packaging products are subject to regulation under the Food, Drug and Cosmetic Act (the “FDC Act”). Under the FDC Act, any substance that when used as intended may reasonably be expected to become, directly or indirectly, a component or otherwise affect the characteristics of any food may be regulated as a food additive unless the substance is generally recognized as safe. We believe that food packaging materials are generally not considered food additives by the FDA because these products are not expected to become components of food under their expected conditions of use. We consider our breathable membrane product to be a food packaging material not subject to regulation or approval by the FDA. We have not received any communication from the FDA concerning our breathable membrane product. If the FDA were to determine that our breathable membrane products are food additives, we may be required to submit a food additive petition for approval by the FDA. The food additive petition process is lengthy, expensive and uncertain. A determination by the FDA that a food additive petition is necessary would have a material adverse effect on our business, operating results and financial condition.
Our agricultural operations are subject to a variety of environmental laws including, the Food Quality Protection Act of 1966, the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, the Federal Insecticide, Fungicide and Rodenticide Act, and the Comprehensive Environmental Response, Compensation and Liability Act. Compliance with these laws and related regulations is an ongoing process. Environmental concerns are, however, inherent in most agricultural operations, including those we conduct. Moreover, it is possible that future developments, such as increasingly strict environmental laws and enforcement policies could result in increased compliance costs. Our Food Products Technology business is subject to the Perishable Agricultural Commodities Act (“PACA”) law. PACA regulates fair trade standards in the fresh produce industry and governs all the products sold by Apio. Our failure to comply with the PACA requirements could among other things, result in civil penalties, suspension or revocation of a license to sell produce, and in the most egregious cases, criminal prosecution, which could have a material adverse effect on our business.
Lifecore’s existing products and its products under development are considered to be medical devices and therefore, require clearance or approval by the FDA before commercial sales can be made in the United States. The products also require the approval of foreign government agencies before sales may be made in many other countries. The process of obtaining these clearances or approvals varies according to the nature and use of the product. It can involve lengthy and detailed laboratory and clinical testing, sampling activities and other costly and time-consuming procedures. There can be no assurance that any of the required clearances or approvals will be granted on a timely basis, if at all.
In addition, most of the existing products being sold by Lifecore and its customers are subject to continued regulation by the FDA, various state agencies and foreign regulatory agencies which regulate manufacturing, labeling and record keeping procedures for such products. Marketing clearances or approvals by these agencies can be withdrawn due to failure to comply with regulatory standards or the occurrence of unforeseen problems following initial clearance or approval. These agencies can also limit or prevent the manufacture or distribution of Lifecore’s products. A determination that Lifecore is in violation of such regulations could lead to the imposition of civil penalties, including fines, product recalls or product seizures, injunctions, and, in extreme cases, criminal sanctions.
Federal, state and local regulations impose various environmental controls on the use, storage, discharge or disposal of toxic, volatile or otherwise hazardous chemicals and gases used in some of the manufacturing processes. Our failure to control the use of, or to restrict adequately the discharge of, hazardous substances under present or future regulations could subject us to substantial liability or could cause our manufacturing operations to be suspended and changes in environmental regulations may impose the need for additional capital equipment or other requirements.
Adverse Weather Conditions and Other Acts of God May Cause Substantial Decreases in Our Sales and/or Increases in Our Costs
Our Food Products Technology business is subject to weather conditions that affect commodity prices, crop quality and yields, and decisions by growers regarding crops to be planted. Crop diseases and severe conditions, particularly weather conditions such as unexpected or excessive rain or other precipitation, unseasonable temperature fluctuations, floods, droughts, frosts, windstorms, earthquakes and hurricanes, may adversely affect the supply of vegetables and fruits used in our business, which could reduce the sales volumes and/or increase the unit production costs. In fiscal year 2011, the Company’s net income was negatively impacted by $5.0 million due to weather-related produce sourcing issues in the Food Products Technology business. Because a significant portion of the costs are fixed and contracted in advance of each operating year, volume declines due to production interruptions or other factors could result in increases in unit production costs which could result in substantial losses and weaken our financial condition.
We Depend on Strategic Partners and Licenses for Future Development
Our strategy for development, clinical and field testing, manufacture, commercialization and marketing for some of our current and future products includes entering into various collaborations with corporate partners, licensees and others. We are dependent on our corporate partners to develop, test, manufacture and/or market some of our products. Although we believe that our partners in these collaborations have an economic motivation to succeed in performing their contractual responsibilities, the amount and timing of resources to be devoted to these activities are not within our control. Our partners may not perform their obligations as expected or we may not derive any additional revenue from the arrangements. Our partners may not pay any additional option or license fees to us or may not develop, market or pay any royalty fees related to products under the agreements. Moreover, some of the collaborative agreements provide that they may be terminated at the discretion of the corporate partner, and some of the collaborative agreements provide for termination under other circumstances. Our partners may pursue existing or alternative technologies in preference to our technology. Furthermore, we may not be able to negotiate additional collaborative arrangements in the future on acceptable terms, if at all, and our collaborative arrangements may not be successful. Our International Operations and Sales May Expose Our Business to Additional Risks
For fiscal year 2011, approximately 38% of our total revenues were derived from product sales to international customers. A number of risks are inherent in international transactions. International sales and operations may be limited or disrupted by any of the following:
| | regulatory approval process, |
| | export license requirements, |
| | difficulties in staffing and managing international operations. |
Foreign regulatory agencies have or may establish product standards different from those in the United States, and any inability to obtain foreign regulatory approvals on a timely basis could have a material adverse effect on our international business, and our financial condition and results of operations. While our foreign sales are currently priced in dollars, fluctuations in currency exchange rates may reduce the demand for our products by increasing the price of our products in the currency of the countries to which the products are sold. Regulatory, geopolitical and other factors may adversely impact our operations in the future or require us to modify our current business practices.
Cancellations or Delays of Orders by Our Customers May Adversely Affect Our Business
During fiscal year 2011, sales to our top five customers accounted for approximately 44% of our revenues, with our largest customer, Costco Wholesale Corporation, accounting for approximately 16% of our revenues. We expect that, for the foreseeable future, a limited number of customers may continue to account for a substantial portion of our revenues. We may experience changes in the composition of our customer base as we have experienced in the past. The reduction, delay or cancellation of orders from one or more major customers for any reason or the loss of one or more of our major customers could materially and adversely affect our business, operating results and financial condition. In addition, since some of the products processed by Apio at its Guadalupe, California facility and by Lifecore at its Chaska, Minnesota facility are sole sourced to customers, our operating results could be adversely affected if one or more of our major customers were to develop other sources of supply. Our current customers may not continue to place orders, orders by existing customers may be canceled or may not continue at the levels of previous periods or we may not be able to obtain orders from new customers.
Our Sale of Some Products May Increase Our Exposure to Product Liability Claims
The testing, manufacturing, marketing, and sale of the products we develop involve an inherent risk of allegations of product liability. If any of our products were determined or alleged to be contaminated or defective or to have caused a harmful accident to an end-customer, we could incur substantial costs in responding to complaints or litigation regarding our products and our product brand image could be materially damaged. Either event may have a material adverse effect on our business, operating results and financial condition. Although we have taken and intend to continue to take what we believe are appropriate precautions to minimize exposure to product liability claims, we may not be able to avoid significant liability. We currently maintain product liability insurance. While we believe the coverage and limits are consistent with industry standards, our coverage may not be adequate or may not continue to be available at an acceptable cost, if at all. A product liability claim, product recall or other claim with respect to uninsured liabilities or in excess of insured liabilities could have a material adverse effect on our business, operating results and financial condition. Our Stock Price May Fluctuate in Accordance with Market Conditions
The following events may cause the market price of our common stock to fluctuate significantly:
| | technological innovations applicable to our products, |
| | our attainment of (or failure to attain) milestones in the commercialization of our technology, |
| | our development of new products or the development of new products by our competitors, |
| | new patents or changes in existing patents applicable to our products, |
| | our acquisition of new businesses or the sale or disposal of a part of our businesses, |
| | development of new collaborative arrangements by us, our competitors or other parties, |
| | changes in government regulations applicable to our business, |
| | changes in investor perception of our business, |
| | fluctuations in our operating results, and |
| | changes in the general market conditions in our industry. |
| | These broad fluctuations may adversely affect the market price of our common stock. |
We May Be Exposed to Employment Related Claims and Costs that Could Materially Adversely Affect Our Business
We have been subject in the past, and may be in the future, to claims by employees based on allegations of discrimination, negligence, harassment and inadvertent employment of illegal aliens or unlicensed personnel, and we may be subject to payment of workers' compensation claims and other similar claims. We could incur substantial costs and our management could spend a significant amount of time responding to such complaints or litigation regarding employee claims, which may have a material adverse effect on our business, operating results and financial condition.
We Are Dependent on Our Key Employees and if One or More of Them Were to Leave, We Could Experience Difficulties in Replacing Them and Our Operating Results Could Suffer
The success of our business depends to a significant extent upon the continued service and performance of a relatively small number of key senior management, technical, sales, and marketing personnel. The loss of any of our key personnel would likely harm our business. In addition, competition for senior level personnel with knowledge and experience in our different lines of business is intense. If any of our key personnel were to leave, we would need to devote substantial resources and management attention to replace them. As a result, management attention may be diverted from managing our business, and we may need to pay higher compensation to replace these employees.
We May Issue Preferred Stock with Preferential Rights that Could Affect Your Rights
Our Board of Directors has the authority, without further approval of our stockholders, to fix the rights and preferences, and to issue shares, of preferred stock. In November 1999, we issued and sold shares of Series A Convertible Preferred Stock and in October 2001 we issued and sold shares of Series B Convertible Preferred Stock. The Series A Convertible Preferred Stock was converted into 1,666,670 shares of Common Stock in November 2002 and the Series B Convertible Preferred Stock was converted into 1,744,102 shares of Common Stock in May 2004.
The issuance of new shares of preferred stock could have the effect of making it more difficult for a third party to acquire a majority of our outstanding stock, and the holders of such preferred stock could have voting, dividend, liquidation and other rights superior to those of holders of our Common Stock.
We Have Never Paid any Dividends on Our Common Stock
We have not paid any dividends on our Common Stock since inception and do not expect to in the foreseeable future. Any dividends may be subject to preferential dividends payable on any preferred stock we may issue. Our Profitability Could Be Materially and Adversely Affected if it Is Determined that the Book Value of Goodwill is Higher than Fair Value
Our balance sheet includes an amount designated as “goodwill” that represents a portion of our assets and our stockholders’ equity. Goodwill arises when an acquirer pays more for a business than the fair value of the tangible and separately measurable intangible net assets. In accordance with accounting guidance, the amortization of goodwill has been replaced with an “impairment test” which requires that we compare the fair value of goodwill to its book value at least annually and more frequently if circumstances indicate a possible impairment. If we determine at any time in the future that the book value of goodwill is higher than fair value then the difference must be written-off, which could materially and adversely affect our profitability.
1B. Unresolved Staff Comments
None.
As of May 29, 2011, the Company owned or leased properties in Menlo Park, Arroyo Grande and Guadalupe, California; West Lebanon and Oxford, Indiana and Chaska, Minnesota.
These properties are described below:
| | | | | | | | | | | |
| Menlo Park, CA | | Technology Licensing | | Leased | | 14,600 square feet of office and laboratory space | | — | | 12/31/14 | |
| Chaska, MN | | Hyaluronan-based Biomaterials | | Owned | | 112,000 square feet of office, laboratory and manufacturing space | | 27.5 | | — | |
| West Lebanon, IN | | Technology Licensing | | Owned | | 4,000 square feet of warehouse and manufacturing space | | — | | — | |
| Oxford, IN | | Technology Licensing | | Leased | | 13,400 square feet of laboratory and manufacturing space | | — | | 6/30/12 | |
| Guadalupe, CA | | Food Products Technology | | Owned | | 199,000 square feet of office space, manufacturing and cold storage | | 17.7 | | — | |
| Arroyo Grande, CA | | Food Export | | Leased | | 1,100 square feet of office space | | — | | Month-to Month | |
The obligations of the Company under its credit agreement with Wells Fargo Bank, N.A. are secured by a lien on the Chaska, MN land and building.
| Item 3. | Legal Proceedings |
As of the date of this report, the Company is not a party to any legal proceedings.
| Item 4. | [Removed and Reserved] |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
The Common Stock is traded on The NASDAQ Global Select Market under the symbol “LNDC”. The following table sets forth for each period indicated the high and low sales prices for the Common Stock.
| Fiscal Year Ended May 29, 2011 | | High | | | Low | |
| | | | | | | |
4th Quarter ending May 29, 2011 | | $ | 6.61 | | | $ | 5.52 | |
| | | | | | | | | |
3rd Quarter ending February 27, 2011 | | $ | 7.08 | | | $ | 5.66 | |
| | | | | | | | | |
2nd Quarter ending November 28, 2010 | | $ | 6.50 | | | $ | 5.30 | |
| | | | | | | | | |
1st Quarter ending August 29, 2010 | | $ | 6.89 | | | $ | 5.40 | |
| Fiscal Year Ended May 30, 2010 | | High | | | Low | |
| | | | | | | |
4th Quarter ending May 30, 2010 | | $ | 7.45 | | | $ | 5.50 | |
| | | | | | | | | |
3rd Quarter ending February 28, 2010 | | $ | 6.63 | | | $ | 5.81 | |
| | | | | | | | | |
2nd Quarter ending November 29, 2009 | | $ | 7.03 | | | $ | 6.00 | |
| | | | | | | | | |
1st Quarter ending August 30, 2009 | | $ | 7.17 | | | $ | 5.99 | |
Holders
There were approximately 70 holders of record of 26,405,799 shares of outstanding Common Stock as of July 20, 2011. Since certain holders are listed under their brokerage firm’s names, the actual number of stockholders is higher.
Dividends
The Company has not paid any dividends on the Common Stock since its inception. The Company presently intends to retain all future earnings, if any, for its business and does not anticipate paying cash dividends on its Common Stock in the foreseeable future.
Issuer Purchases of Equity Securities
There were no shares repurchased by the Company during the fiscal quarter ended on May 29, 2011. During fiscal year 2011, the Company repurchased and retired 215,648 shares of Common Stock for $1.2 million. The Company may still repurchase up to $8.8 million of the Company’s Common Stock under the Company’s stock repurchase plan announced on July 14, 2010.
| Item 6. | Selected Financial Data |
The information set forth below is not necessarily indicative of the results of future operations and should be read in conjunction with the information contained in Item 7 – “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and Notes to Consolidated Financial Statements contained in Item 8 of this report.
| | | Year Ended May 29, 2011 | | | Year Ended May 30, 2010 | | | Year Ended May 31, 2009 | | | Year Ended May 25, 2008 | | | Year Ended May 27, 2007 | |
| Statement of Income Data: | | | | | | | | | | | | | | | |
| (in thousands) | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | |
| Product sales | | $ | 267,121 | | | $ | 228,390 | | | $ | 224,404 | | | $ | 227,550 | | | $ | 201,892 | |
| Service revenues | | | 3,391 | | | | 3,699 | | | | 4,145 | | | | 3,640 | | | | 3,539 | |
| License fees | | | 5,400 | | | | 5,400 | | | | 6,000 | | | | 6,231 | | | | 4,013 | |
| R&D and royalty revenues | | | 817 | | | | 735 | | | | 1,389 | | | | 1,106 | | | | 1,054 | |
| Total revenues | | | 276,729 | | | | 238,224 | | | | 235,938 | | | | 238,527 | | | | 210,498 | |
| | | | | | | | | | | | | | | | | | | | | |
| Cost of revenue: | | | | | | | | | | | | | | | | | | | | |
| Cost of product sales | | | 227,167 | | | | 201,466 | | | | 198,369 | | | | 197,288 | | | | 175,252 | |
| Cost of service revenue | | | 2,867 | | | | 2,992 | | | | 3,289 | | | | 3,011 | | | | 2,860 | |
| Total cost of revenue | | | 230,034 | | | | 204,458 | | | | 201,658 | | | | 200,299 | | | | 178,112 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 46,695 | | | | 33,766 | | | | 34,280 | | | | 38,228 | | | | 32,386 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 9,275 | | | | 4,361 | | | | 3,665 | | | | 3,251 | | | | 3,074 | |
| Selling, general and administrative | | | 24,608 | | | | 17,698 | | | | 18,017 | | | | 19,801 | | | | 21,616 | |
| Other operating expenses | | | 4,780 | | | | 3,725 | | | | — | | | | — | | | | — | |
| Income from sale of FCD | | | — | | | | — | | | | — | | | | — | | | | (22,669 | ) |
| Total operating costs and expenses | | | 38,663 | | | | 25,784 | | | | 21,682 | | | | 23,052 | | | | 2,021 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating profit | | | 8,032 | | | | 7,982 | | | | 12,598 | | | | 15,176 | | | | 30,365 | |
| | | | | | | | | | | | | | | | | | | | | |
| Dividend income | | | 328 | | | | — | | | | — | | | | — | | | | — | |
| Interest income | | | 430 | | | | 834 | | | | 1,306 | | | | 2,219 | | | | 1,945 | |
| Interest expense | | | (820 | ) | | | (88 | ) | | | (8 | ) | | | (22 | ) | | | (251 | ) |
| Other income | | | 472 | | | | — | | | | ― | | | | ― | | | | ― | |
| Net income before taxes | | | 8,442 | | | | 8,728 | | | | 13,896 | | | | 17,373 | | | | 32,059 | |
| Income tax expense | | | (4,181 | ) | | | (4,262 | ) | | | (5,611 | ) | | | (3,354 | ) | | | (2,456 | ) |
| Consolidated net income | | | 4,261 | | | | 4,466 | | | | 8,285 | | | | 14,019 | | | | 29,603 | |
| Non controlling interest | | | (341 | ) | | | (482 | ) | | | (555 | ) | | | (477 | ) | | | (414 | ) |
| Net income applicable to Common Stockholders | | $ | 3,920 | | | $ | 3,984 | | | $ | 7,730 | | | $ | 13,542 | | | $ | 29,189 | |
| | | | | | | | | | | | | | | | | | | | | |
| Basic net income per share | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.30 | | | $ | 0.52 | | | $ | 1.16 | |
| Diluted net income per share | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.29 | | | $ | 0.50 | | | $ | 1.07 | |
| | | | | | | | | | | | | | | | | | | | | |
| Shares used in per share computation: | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 26,397 | | | | 26,382 | | | | 26,202 | | | | 26,069 | | | | 25,260 | |
| Diluted | | | 26,626 | | | | 26,633 | | | | 26,751 | | | | 26,935 | | | | 26,558 | |
| | | May 29, 2011 | | | May 30, 2010 | | | May 31, 2009 | | | May 25, 2008 | | | May 27, 2007 | |
| Balance Sheet Data: | | | | | | | | | | | | | | | |
| (in thousands) | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 8,135 | | | $ | 27,817 | | | $ | 43,459 | | | $ | 44,396 | | | $ | 62,556 | |
| Total assets | | | 206,312 | | | | 200,197 | | | | 153,498 | | | | 149,957 | | | | 141,368 | |
| Debt | | | 19,830 | | | | 23,770 | | | | — | | | | — | | | | — | |
| Retained earnings (deficit) | | | 17,126 | | | | 13,206 | | | | 9,222 | | | | 1,492 | | | | (19,332 | ) |
| Total stockholders’ equity | | $ | 136,055 | | | $ | 130,784 | | | $ | 125,406 | | | $ | 114,466 | | | $ | 110,228 | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion should be read in conjunction with the Company’s Consolidated Financial Statements contained in Item 8 of this report. Except for the historical information contained herein, the matters discussed in this report are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. These forward-looking statements involve certain risks and uncertainties that could cause actual results to differ materially from those in the forward-looking statements. Potential risks and uncertainties include, without limitation, those mentioned in this report and, in particular, the factors described in Item 1A. "Risk Factors.” Landec undertakes no obligation to revise any forward-looking statements in order to reflect events or circumstances that may arise after the date of this report.
Overview
Since its inception in October 1986, the Company has been engaged in the research and development of its Intelimer technology and related products. The Company has launched four product lines from this core development – QuickCast™ splints and casts in April 1994, which was subsequently sold to Bissell Healthcare Corporation in August 1997; BreatheWay packaging technology for the fresh-cut and whole produce packaging market in September 1995; Intelimer Polymer Systems that includes polymer materials for various industrial applications in June 1997 and for personal care applications in November 2003; and Intellicoat coated corn seeds in the Fall of 1999. In addition, on April 30, 2010, the Company acquired Lifecore which develops and manufactures products utilizing hyaluronan, a naturally occurring polysaccharide that is widely distributed in the extracellular matrix of connective tissues in both animals and humans.
With the acquisition of Lifecore, Landec has four core businesses – Food Products Technology, Food Export, Hyaluronan-based Biomaterials and Technology Licensing. The Food Products Technology segment combines the Company’s Intelimer packaging technology with Apio’s fresh-cut and whole produce business. The Food Export business is operated through Apio’s Cal-Ex export company which purchases and sells whole fruit and vegetable products to predominantly Asian markets. The Hyaluronan-based Biomaterials business sells products utilizing hyaluronan in the ophthalmic, orthopedic and veterinary segments and also supplies hyaluronan to customers pursuing other medical applications, such as aesthetic surgery, medical device coatings, tissue engineering and pharmaceuticals. The Technology Licensing business includes our proprietary Intellicoat seed coating technology in which certain fields of application have been licensed to Monsanto and our Intelimer polymer business that licenses and/or supplies products to companies such as Air Products and Nitta. See "Business - Description of Core Business".
From inception through May 29, 2011, the Company’s retained earnings were $17.1 million. The Company may incur losses in the future. The amount of future net profits, if any, is uncertain and there can be no assurance that the Company will be able to sustain profitability in future years.
Critical Accounting Policies and Use of Estimates
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make certain estimates and judgments that affect the amounts reported in the financial statements and accompanying notes. The accounting estimates that require management’s most significant, difficult and subjective judgments include revenue recognition; sales returns and allowances; recognition and measurement of current and deferred income tax assets and liabilities; the assessment of recoverability of long-lived assets; the valuation of intangible assets and inventory; the valuation and nature of impairments of investments; and the valuation and recognition of stock-based compensation.
These estimates involve the consideration of complex factors and require management to make judgments. The analysis of historical and future trends can require extended periods of time to resolve, and are subject to change from period to period. The actual results may differ from management’s estimates.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. The allowance for doubtful accounts is based on review of the overall condition of accounts receivable balances and review of significant past due accounts. If the financial condition of the Company’s customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. Bad debt losses are partially mitigated due to the fact that the Company’s customers are predominantly large financially low-risk national and international companies.
Inventories
Inventories are stated at the lower of cost or market. If the cost of the inventories exceeds their expected market value, provisions are recorded currently for the difference between the cost and the market value. These provisions are determined based on specific identification for unusable inventory and an additional reserve, based on historical losses, for inventory currently considered to be usable.
Revenue Recognition
Revenue from product sales is recognized when there is persuasive evidence that an arrangement exists, title has transferred, the price is fixed and determinable, and collectibility is reasonably assured. Allowances are established for estimated uncollectible amounts, product returns, and discounts based on specific identification and historical losses.
The Company takes title to all produce it trades and/or packages, and therefore, records revenues and cost of sales at gross amounts in the Consolidated Statements of Income.
Licensing revenue is recognized in accordance with prevailing accounting guidance. Initial license fees are deferred and amortized to revenue over the period of the agreement when a contract exists, the fee is fixed and determinable, and collectibility is reasonably assured. Noncancellable, nonrefundable license fees are recognized over the period of the agreement, including those governing research and development activities and any related supply agreement entered into concurrently with the license when the risk associated with commercialization of a product is non-substantive at the outset of the arrangement.
Contract revenue for research and development (R&D) is recorded as earned, based on the performance requirements of the contract. Non-refundable contract fees for which no further performance obligations exist, and there is no continuing involvement by the Company, are recognized on the earlier of when the payments are received or when collection is assured.
Goodwill and Other Intangibles
The Company’s intangible assets are comprised of customer relationships with an estimated useful life of twelve years and trademarks/trade names and goodwill with indefinite lives (collectively, “intangible assets”), which the Company recognized in accordance with accounting guidance (i) upon the acquisition of Lifecore in April 2010, our HA-based Biomaterials reporting unit, (ii) upon the acquisition of Apio in December 1999, which consists of our Food Products Technology and Food Export reporting units and (iii) from the repurchase of all non controlling interests in the common stock of Landec Ag in December 2006. Accounting guidance defines goodwill as “the excess of the cost of an acquired entity over the net of the estimated fair values of the assets acquired and the liabilities assumed at date of acquisition.” All intangible assets, including goodwill, associated with the acquisitions of Lifecore and Apio were allocated to our HA-based Biomaterials reporting unit and our Food Products Technology reporting unit, respectively, pursuant to accounting guidance based upon the allocation of assets and liabilities acquired and consideration paid for each reporting unit. The consideration paid for the Food Export reporting unit approximated its fair market value at the time of acquisition, and therefore, no intangible assets were recorded in connection with the Company’s acquisition of this reporting unit. Goodwill associated with the Technology Licensing reporting unit consists entirely of goodwill resulting from the repurchase of the Landec Ag non controlling interests. As of May 29, 2011, the HA-based Biomaterials reporting unit had $13.9 million of goodwill and the Food Products Technology reporting unit had $22.6 million of goodwill. As described below, the $4.8 million of goodwill in the Technology Licensing reporting unit was written off as of May 29, 2011.
The Company tests its intangible assets for impairment at least annually, in accordance with accounting guidance. When evaluating indefinite-lived intangible assets for impairment, accounting guidance requires the Company to compare the fair value of the asset to its carrying value to determine if there is an impairment loss. When evaluating goodwill for impairment, accounting guidance requires the Company to first compare the fair value of the reporting unit to its carrying value to determine if there is an impairment loss. If the fair value of the reporting unit exceeds its carrying value, goodwill is not considered impaired; thus application of the second step of the two-step approach under accounting guidance is not required. Application of the intangible assets impairment tests requires significant judgment by management, including identification of reporting units, assignment of assets and liabilities to reporting units, assignment of intangible assets to reporting units, and the determination of the fair value of each indefinite-lived intangible asset and reporting unit based upon projections of future net cash flows, discount rates and market multiples, which judgments and projections are inherently uncertain.
Property, plant and equipment and finite-lived intangible assets are reviewed for possible impairment whenever events or changes in circumstances occur that indicate that the carrying amount of an asset (or asset group) may not be recoverable. The Company’s impairment review requires significant management judgment including estimating the future success of product lines, future sales volumes, revenue and expense growth rates, alternative uses for the assets and estimated proceeds from the disposal of the assets. The Company conducts quarterly reviews of idle and underutilized equipment, and reviews business plans for possible impairment indicators. Impairment occurs when the carrying amount of the asset (or asset group) exceeds its estimated future undiscounted cash flows and the impairment is viewed as other than temporary. When impairment is indicated, an impairment charge is recorded for the difference between the asset’s book value and its estimated fair value. Depending on the asset, estimated fair value may be determined either by use of a discounted cash flow model or by reference to estimated selling values of assets in similar condition. The use of different assumptions would increase or decrease the estimated fair value of assets and would increase or decrease any impairment measurement.
The Company tested its indefinite-lived intangible assets and goodwill for impairment as of July 24, 2011 and determined that no adjustments to the carrying values of the intangible assets were necessary as of that date for its HA-based Biomaterials and Food Products Technology reporting units. For the Technology Licensing reporting unit during the fourth quarter of fiscal year 2011 it became apparent to the Company that acceptable biological test results are probably not achievable in the four months left in the agreement before Monsanto has to make its purchase option decision (see Note 4 to the Consolidated Financial Statements). The uncertainty related to whether Monsanto will exercise its purchase option for the licensed fields of technology, and the fact that Landec Ag is projected to be unprofitable for several years absent any ongoing relationship with Monsanto, were the main factors contributing to the significant decrease in the estimated fair value of the Landec Ag business and as a result the goodwill of the Technology Licensing reporting unit was determined to be fully impaired as of May 29, 2011, and therefore, the Company wrote off the entire $4.8 million of goodwill associated with the Technology Licensing reporting unit. As of May 29, 2011, there were no impairment indicators identified by the Company in its analysis of impairment associated with the acquired indefinite-lived intangible assets. On a quarterly basis, the Company considers the need to update its most recent annual tests for possible impairment of its intangible assets, based on management’s assessment of changes in its business and other economic factors since the most recent annual evaluation. Such changes, if significant or material, could indicate a need to update the most recent annual tests for impairment of the intangible assets during the current period. The results of these tests could lead to write-downs of the carrying values of the intangible assets in the current period. The Company uses the discounted cash flow (“DCF”) approach to develop an estimate of fair value. The DCF approach recognizes that current value is premised on the expected receipt of future economic benefits. Indications of value are developed by discounting projected future net cash flows to their present value at a rate that reflects both the current return requirements of the market and the risks inherent in the specific investment. The market approach was not used to value the Food Products Technology, Hyaluronan-based Biomaterials and Technology Licensing reporting units (the “Reporting Units”) because insufficient market comparables exist to enable the Company to develop a reasonable fair value of its intangible assets due to the unique nature of each of the Company’s Reporting Units.
The DCF approach requires the Company to exercise judgment in determining future business and financial forecasts and the related estimates of future net cash flows. Future net cash flows depend primarily on future product sales, which are inherently difficult to predict. These net cash flows are discounted at a rate that reflects both the current return requirements of the market and the risks inherent in the specific investment.
The DCF associated with the Food Products Technology reporting unit is based on management’s five-year projection of revenues, gross profits and operating profits by fiscal year and assumes a 38% effective tax rate for each year. Management takes into account the historical trends of Apio and the industry categories in which Apio operates along with inflationary factors, current economic conditions, new product introductions, cost of sales, operating expenses, capital requirements and other relevant data when developing its projection. The estimated fair value of the Food Products Technology reporting units as of July 24, 2011 exceeded its book value by 55% and therefore, no intangible asset impairment was deemed to exist. For the test performed as of July 25, 2010, the projected cash flow from operations for determining the DCF for fiscal year 2011 was $16.6 million for the Food Products Technology reporting unit. The actual cash flow from operations for fiscal year 2011 was $12.1 million. The difference of $4.5 million was a result of increased produce sourcing costs due to weather-related produce shortages which the Company had no way of foreseeing.
The fair value of indefinite and finite-lived intangible assets associated with our acquisition of Lifecore on April 30, 2010, was determined using a DCF model based on management’s five-year projections of revenues, gross profits and operating profits by fiscal year and assumes a 38% effective tax rate for each year. Management takes into account the historical trends of Lifecore and the industry categories in which Lifecore operates along with inflationary factors, current economic conditions, new product introductions, cost of sales, operating expenses, capital requirements and other relevant data when developing its projection. The trade name intangible asset was valued using the relief from royalty valuation method and the customer relationship intangible asset was valued using the multi-period excess earnings method. The fair value of goodwill was calculated as the excess of consideration paid, including the fair value of contingent consideration under the terms of the purchase agreement, over the fair value of the tangible and intangible assets acquired less liabilities assumed. The Company updated its analysis of the fair value of the indefinite-lived intangible assets and goodwill as of its annual impairment analysis date, concluding that the fair value of the Hyaluronan-based Biomaterials reporting unit, as determined by the DCF approach, exceeded its book value by 71%, and therefore, no intangible asset impairment was deemed to exist. For the test performed as of July 25, 2010, the projected cash flow from operations for determining the DCF for fiscal year 2011 was $3.1million for the Hyaluronan-based Biomaterials reporting unit. The actual cash flow from operations for fiscal year 2011 was $9.0 million. The difference of $5.9 million is due to Lifecore exceeding its planned net income by $2.0 million and the change in working capital being much more favorable than planned.
Income Taxes
The Company accounts for income taxes in accordance with accounting guidance which requires that deferred tax assets and liabilities be recognized using enacted tax rates for the effect of temporary differences between the book and tax bases of recorded assets and liabilities. The Company maintains valuation allowances when it is likely that all or a portion of a deferred tax asset will not be realized. Changes in valuation allowances from period to period are included in the Company’s income tax provision in the period of change. In determining whether a valuation allowance is warranted, the Company takes into account such factors as prior earnings history, expected future earnings, unsettled circumstances that, if unfavorably resolved, would adversely affect utilization of a deferred tax asset, carryback and carryforward periods, and tax strategies that could potentially enhance the likelihood of realization of a deferred tax asset. At May 29, 2011, the Company had a valuation allowance of $383,000 against deferred tax assets. In addition to valuation allowances, the Company establishes accruals for certain tax contingencies, when, despite the belief that the Company’s tax return positions are fully supported, the Company believes that certain tax positions are likely to be challenged and that the Company’s positions may not be fully sustained. The tax-contingency accruals are adjusted in light of changing facts and circumstances, such as the progress of tax audits, case law and emerging legislation. The Company recognizes interest and penalties related to uncertain tax positions as a component of income tax expense. The Company’s effective tax rate includes the impact of tax-contingency accruals as considered appropriate by management.
A number of years may elapse before a particular matter, for which the Company has accrued, is audited and finally resolved. The number of years with open tax audits varies by jurisdiction. While it is often difficult to predict the final outcome or the timing of resolution of any particular tax matter, the Company believes its tax-contingency accruals are adequate to address known tax contingencies. Favorable resolution of such matters could be recognized as a reduction to the Company’s effective tax rate in the year of resolution. Unfavorable settlement of any particular issue could increase the effective tax rate. Any resolution of a tax issue may require the use of cash in the year of resolution. The Company’s tax-contingency accruals are presented in the balance sheet within accrued liabilities.
Stock-Based Compensation
The Company’s stock-based awards include stock option grants and restricted stock unit awards (RSUs).
The Company adopted accounting guidance using the modified prospective transition method, which requires the application of the accounting standard to (i) all stock-based awards issued on or after May 29, 2006 and (ii) any outstanding stock-based awards that were issued but not vested as of May 29, 2006.
The estimated fair value for stock options, which determines the Company’s calculation of compensation expense, is based on the Black-Scholes pricing model. Upon the adoption of new accounting guidance, the Company changed its method of calculating and recognizing the fair value of stock-based compensation arrangements to the straight-line, single-option method. Compensation expense for all stock option and restricted stock unit awards granted prior to May 29, 2006 will continue to be recognized using the straight-line, multiple-option method. In addition, the new accounting guidance requires the estimation of the expected forfeitures of stock-based awards at the time of grant. As a result, the Company uses historical data to estimate pre-vesting forfeitures and records stock-based compensation expense only for those awards that are expected to vest and revises those estimates in subsequent periods if the actual forfeitures differ from the prior estimates.
Fair Value Measurements
The Company uses fair value measurement accounting for financial assets and liabilities and for financial instruments and certain other items at fair value. The Company has elected the fair value option for its investment in a non-public company (see Note 3 to the Consolidated Financial Statements). The Company has not elected the fair value option for any of its other eligible financial assets or liabilities.
The accounting guidance established a three-tier hierarchy for fair value measurements, which prioritizes the inputs used in measuring fair value as follows:
| | Level 1 – | observable inputs such as quoted prices for identical instruments in active markets. |
| | Level 2 – | inputs other than quoted prices in active markets that are observable either directly or indirectly through corroboration with observable market data. |
| | Level 3 – | unobservable inputs in which there is little or no market data, which would require the Company to develop its own assumptions. |
As of May 29, 2011, the Company held certain assets and liabilities that are required to be measured at fair value on a recurring basis, including cash equivalents, marketable securities, interest rate swap and liability for contingent consideration in connection with the acquisition of Lifecore.
The fair value of the Company’s cash equivalents and marketable securities is determined based on observable inputs that are readily available in public markets or can be derived from information available in publicly quoted markets. Therefore, the Company has categorized its cash equivalents and marketable securities as Level 1.
The fair value of the Company’s interest rate swap is determined based on model inputs that can be observed in a liquid market and key inputs include yield curves and are categorized as Level 2 inputs. As of May 29, 2011, the Company recorded to other comprehensive loss on the consolidated balance sheets an unrealized loss of $267,000, net of taxes of $159,000, representing the cumulative change in the interest rate swap since inception. If the interest rate swap is terminated or the debt borrowed is paid off prior to April 30, 2015, the amount of unrealized loss or gain included in other comprehensive income (loss) would be reclassified to earnings. The Company has no intentions of terminating the interest rate swap or prepaying the debt in the next twelve months. The interest rate swap liability is included in other non-current liabilities as of May 29, 2011 and May 30, 2010.
The fair value of the Company’s liability for contingent consideration is based on significant inputs not observed in the market and thus represents a Level 3 measurement. The Company determined the fair value of the liability for the contingent consideration based on a probability-weighted discounted cash flow analysis, as further discussed in Note 2 to the Consolidated Financial Statements.
The Company has elected the fair value option of accounting for its investment in Windset. The fair value of the Company’s investment in Windset utilizes significant unobservable inputs in the discounted cash flow models, including projected cash flows, growth rates and the discount rate, and is therefore considered Level 3, as further discussed in Note 3 to the Consolidated Financial Statements.
Imprecision in estimating unobservable market inputs can affect the amount of gain or loss recorded for a particular position. Furthermore, the Company believes its valuation methods are appropriate and consistent with those of other market participants. The use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
The Company has no other financial assets or liabilities for which fair value measurement has been adopted.
Recent Accounting Pronouncements
Recently Adopted Pronouncements
Fair Value Measurements
In January 2010, the FASB issued new accounting guidance related to the disclosures for transfers in and out of Levels 1 and 2 fair value measurements and the activity in Level 3 fair value measurements. The amendment recommends a reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. Further, in the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances and settlements (that is, on a gross basis rather than as one net number). Also, the amendment requires clarification in existing disclosures for disaggregation of fair value measurement disclosures for each class of assets and liabilities and disclosures about inputs and valuation techniques. The effective date is for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company adopted all the amended provisions of new guidance in the first quarter of fiscal year 2011 and such adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
Variable Interest Entities
In June 2009, the FASB issued new guidance which amends the evaluation criteria to identify the primary beneficiary of a VIE. Additionally, the new guidance requires ongoing reassessments of whether an enterprise is the primary beneficiary of the VIE. The Company adopted the new guidance on May 31, 2010 and such adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
Revenue Recognition
In October 2009, the FASB issued new guidance in relation to "Multiple-Deliverable Revenue Arrangements". The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration to each deliverable to be based on the relative selling price. The Company early adopted these standards as of May 31, 2010. There have been no materially modified agreements since the adoption of the standard. The adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
Recently Issued Pronouncements
Comprehensive Income
In June 2011, the FASB issued new guidance that improves the comparability, consistency, and transparency of financial reporting and increases the prominence of items reported in other comprehensive income by eliminating the option to present components of other comprehensive income as part of the statement of changes in stockholders' equity. The amendments in this standard require that all nonowner changes in stockholders' equity be presented either in a single continuous statement of comprehensive income or in two separate but consecutive statements. Under either method, adjustments must be displayed for items that are reclassified from other comprehensive income ("OCI") to net income, in both net income and OCI. The standard does not change the current option for presenting components of OCI gross or net of the effect of income taxes, provided that such tax effects are presented in the statement in which OCI is presented or disclosed in the notes to the financial statements. Additionally, the standard does not affect the calculation or reporting of earnings per share. For public entities, the amendments in this ASU are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011 and are to be applied retrospectively, with early adoption permitted. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
Revenue Recognition
In April 2010, the FASB issued guidance on applying the milestone method of revenue recognition in arrangements with research and development activities. These amendments are effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. The Company's adoption of the provisions of this update is not expected to have a material impact on its revenue recognition in the Company’s fiscal year beginning on May 30, 2011.
Subsequent Events
In February 2010, the FASB issued updates to the subsequent events guidance requiring an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement for an SEC filer to disclose a date, in both issued and revised financial statements, through which the filer had evaluated subsequent events. The adoption is not expected to have an impact on the Company's financial position, results of operations or cash flows upon adoption in the Company’s fiscal year beginning on May 30, 2011.
Business Combinations
In December 2010, the FASB issued an update requiring a public entity to disclose pro forma information for business combinations that occurred in the current reporting period. The disclosures include pro forma revenue and earnings of the combined entity for the current reporting period as though the acquisition date for all business combinations that occurred during the year had been as of the beginning of the annual reporting period. If comparative financial statements are presented, the pro forma revenue and earnings of the combined entity for the comparable prior reporting period should be reported as though the acquisition date for all business combinations that occurred during the current year had been as of the beginning of the comparable prior annual reporting period. These amendments affect any public entity as defined by US GAAP that enters into business combinations that are material on an individual or aggregate basis, and are effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. The Company does not expect the provisions of this update to have a material effect on its financial position, results of operations or cash flows upon adoption in its fiscal year beginning on May 30, 2011.
Results of Operations
Fiscal Year Ended May 29, 2011 Compared to Fiscal Year Ended May 30, 2010
Revenues (in thousands):
| | | Fiscal Year ended May 29, 2011 | | | Fiscal Year ended May 30, 2010 | | | Change | |
| Apio Value Added | | $ | 172,683 | | | $ | 172,416 | | | | 0 | % |
| Apio Packaging | | | 2,981 | | | | 2,630 | | | | 13 | % |
| Food Technology | | | 175,664 | | | | 175,046 | | | | 0 | % |
| Apio Export | | | 61,663 | | | | 54,926 | | | | 12 | % |
| Total Apio | | | 237,327 | | | | 229,972 | | | | 3 | % |
| HA | | | 32,505 | | | | 1,457 | | | | N/M | |
| Technology Licensing | | | 6,897 | | | | 6,795 | | | | 2 | % |
| Total Revenues | | $ | 276,729 | | | $ | 238,224 | | | | 16 | % |
Apio Value Added
Apio’s value-added revenues consist of revenues generated from the sale of specialty packaged fresh-cut and whole value-added processed vegetable products that are washed and packaged in our proprietary packaging and sold under Apio’s Eat Smart brand and various private labels. In addition, value-added revenues include the revenues generated from Apio Cooling, LP, a produce cooling operation in which Apio is the general partner with a 60% ownership position.
Apio's value-added revenues for the fiscal year ended May 29, 2011 were unchanged compared to the same period last year. This was primarily due to increased unit sales volumes related to new product introductions which increased unit sales volumes by 1% and from a larger percentage of Apio's value-added revenues being generated from sales to club stores rather than retail grocery chains. These increases in revenue were virtually completely offset by less promotional activity in Apio’s value-added business which reduced unit sales volumes by 2% and due to Apio exiting business that would have resulted in less than acceptable gross profit margins which reduced unit sales volumes by another 2%. On a net basis, Apio’s value added unit sales volumes decreased 3% in fiscal year 2011 compared to fiscal year 2010.
Apio Packaging
Apio packaging revenues consist of Apio’s packaging technology business using its BreatheWay membrane technology. The first commercial application included in Apio packaging is our banana packaging technology and during fiscal year 2011 the addition of a second application for avocados.
The increase in Apio packaging revenues for the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to the sale of BreatheWay membranes for packaged avocados to Chiquita.
Apio export revenues consist of revenues generated from the purchase and sale of primarily whole commodity fruit and vegetable products to Asia by Cal-Ex. Apio records revenue equal to the sale price to third parties because it takes title to the product while in transit.
The increase in revenues in Apio’s export business for the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to an 8% increase in export sales volumes as a result of an increased supply of fruit to export and due to more favorable pricing of fruit in fiscal year 2011 compared to fiscal year 2010.
Hyaluronan-based Biomaterials (“HA”)
Lifecore principally generates revenue through the sale of products containing HA. Lifecore primarily sells products to customers in three medical areas: (1) Ophthalmic, which represented over 70% of Lifecore’s revenues in fiscal year 2011, (2) Orthopedic, which represented nearly 20% of Lifecore’s revenues in fiscal year 2011 and (3) Veterinary/Other. Lifecore was acquired on April 30, 2010. From the acquisition date through May 29, 2011, Lifecore has added new customers and has expanded its product offerings to existing customers.
Technology Licensing
Technology licensing revenues consist of revenues generated from the licensing agreements with Monsanto, Air Products and Nitta.
The increase in technology licensing revenues for the fiscal year ended May 29, 2011 compared to the same period last year was not significant to consolidated Landec revenues.
Gross Profit (in thousands):
| | | Fiscal Year ended May 29, 2011 | | | Fiscal Year ended May 30, 2010 | | | Change | |
| Apio Value Added | | $ | 16,564 | | | $ | 20,261 | | | | (18 | )% |
| Apio Packaging | | | 2,324 | | | | 2,253 | | | | 3 | % |
| Food Technology | | | 18,888 | | | | 22,514 | | | | (16 | )% |
| Apio Export | | | 3,901 | | | | 3,906 | | | | 0 | % |
| Total Apio | | | 22,789 | | | | 26,420 | | | | (14 | )% |
| HA | | | 17,231 | | | | 815 | | | | N/M | |
| Technology Licensing | | | 6,675 | | | | 6,531 | | | | 2 | % |
| Total Gross Profit | | $ | 46,695 | | | $ | 33,766 | | | | 38 | % |
General
There are numerous factors that can influence gross profit including product mix, customer mix, manufacturing costs, volume, sale discounts and charges for excess or obsolete inventory, to name a few. Many of these factors influence or are interrelated with other factors. The Company includes in cost of sales all of the costs related to the sale of products in accordance with U.S. generally accepted accounting principles. These costs include the following: raw materials (including produce, casein, seeds and packaging), direct labor, overhead (including indirect labor, depreciation, and facility related costs) and shipping and shipping-related costs. The following are the primary reasons for the changes in gross profit for the fiscal year ended May 29, 2011 compared to the same period last year as outlined in the table above.
Apio Value-Added
The decrease in gross profit for Apio’s value-added specialty packaging vegetable business for the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to shortages of produce resulting from cold and wet weather in California during the Company’s third and fourth fiscal quarters which reduced gross profit by approximately $5.0 million. These decreases were partially offset by a year over year mix change to greater sales of higher margin tray products from lower margin products and fewer promotions on bag products compared to the same period last year.
Apio Packaging
The increase in gross profit for Apio packaging for the fiscal year ended May 29, 2011 compared to the same period last year was not significant to consolidated Landec gross profit.
Apio Export
Apio’s export business is a buy/sell business that realizes a commission-based margin in the 6-7% range. Gross profit during the fiscal year ended May 29, 2011 was flat compared to the prior fiscal year. The 12% increase in revenues was higher than the growth in gross profits because of an unfavorable product mix changes to lower margin products which resulted in a lower gross margin during fiscal year 2011 of 6.3% compared to a gross margin of 7.1% in fiscal year 2010.
Hyaluronan-based Biomaterials
Lifecore operates in the higher margin medical devices industry and has historically realized an overall gross margin of approximately 50%. For fiscal year 2011, Lifecore’s overall gross margin was 53% due to a favorable sales mix. Lifecore was acquired on April 30, 2010.
Technology Licensing
The increase in Technology Licensing gross profit for the fiscal year ended May 29, 2011 compared to the same period of the prior year was not significant to consolidated Landec gross profit.
Operating Expenses (in thousands):
| | | Fiscal Year ended May 29, 2011 | | | Fiscal Year ended May 30, 2010 | | | Change | |
| Research and Development: | | | | | | | | | |
| Apio | | $ | 1,023 | | | $ | 1,182 | | | | (13 | )% |
| HA | | | 4,272 | | | | 395 | | | | N/M | |
| Technology Licensing | | | 3,980 | | | | 2,784 | | | | 43 | % |
| Total R&D | | $ | 9,275 | | | $ | 4,361 | | | | 113 | % |
| | | | | | | | | | | | | |
| Selling, General and Administrative: | | | | | | | | | | | | |
| Apio | | $ | 12,722 | | | $ | 12,128 | | | | 5 | % |
| HA | | | 4,838 | | | | 339 | | | | N/M | |
| Technology Licensing | | | 419 | | | | 100 | | | | 319 | % |
| Corporate | | | 6,629 | | | | 5,131 | | | | 29 | % |
| Total S,G&A | | $ | 24,608 | | | $ | 17,698 | | | | 39 | % |
| | | | | | | | | | | | | |
| Other operating expenses: | | | | | | | | | | | | |
| Technology Licensing | | $ | 4,780 | | | $ | 1,000 | | | | 378 | % |
| Corporate | | | — | | | | 2,725 | | | | N/M | |
| Total Other Operating Expenses | | $ | 4,780 | | | $ | 3,725 | | | | 28 | % |
Research and Development
Landec’s research and development expenses consist primarily of expenses involved in product development and commercialization initiatives. Research and development efforts at Apio are focused on the Company’s proprietary BreatheWay membranes used for packaging produce, with recent focus on extending the shelf life of bananas and other shelf-life sensitive vegetables and fruit. In the HA business, the research and development efforts primarily relate to technical development efforts to support customer specific product requests or process improvements, under which technical modifications are made to existing products or processes to meet customer specific needs and such work can also lead to new products or processes. In the Technology Licensing business, the research and development efforts are focused on uses for the proprietary Intelimer polymers outside of food and HA.
The increase in research and development expenses for the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to the research and development expenses from Lifecore and from an increase in scientific staff in our Technology Licensing business to support the development of new product applications.
Selling, General and Administrative
Selling, general and administrative expenses consist primarily of sales and marketing expenses associated with Landec’s product sales and services, business development expenses and staff and administrative expenses.
The increase in selling, general and administrative expenses for the fiscal year ended May 29, 2011 compared to the same periods last year was primarily due to (1) the selling, general and administrative expenses for Lifecore, (2) a $472,000 increase in the share-based compensation expense as a result of option and RSU grants in May 2010 and (3) a $700,000 increase in accounting and tax fees associated with the initial audit of Lifecore and from several tax projects.
Other Operating Expenses
Other operating expenses consists of impairment charges and transaction costs associated with the Lifecore acquisition. The $4.8 million in fiscal year 2011 in Technology Licensing is from the write off of Landec Ag’s goodwill. The $1.0 million amount in fiscal year 2010 in Technology Licensing is from the partial write down of the Company’s investment in Aesthetic Sciences Corporation. The $2.7 million amount in fiscal year 2010 in Corporate is for the transaction costs from the acquisition of Lifecore on April 30, 2010.
Non-operating income/(expense) (in thousands):
| | | Fiscal Year ended May 29, 2011 | | | Fiscal Year ended May 30, 2010 | | | Change | |
| Dividend Income | | $ | 328 | | | $ | — | | | | N/M | |
| Interest Income | | $ | 430 | | | $ | 834 | | | | (48 | )% |
| Interest Expense | | $ | (820 | ) | | $ | (88 | ) | | | 832 | % |
| Other Income | | $ | 472 | | | $ | ― | | | | N/M | |
| Income Taxes | | $ | (4,181 | ) | | $ | (4,262 | ) | | | (2 | )% |
| Noncontrolling Interest | | $ | (341 | ) | | $ | (482 | ) | | | (29 | )% |
Dividend Income
The increase in dividend income was due to dividends accrued from the $15 million preferred stock investment in Windset (see Note 3) which yields a cash dividend of 7.5% annually. The $328,000 represents dividends for the period February 15, 2011 through May 29, 2011.
Interest Income
The decrease in interest income for the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to having less cash to invest because of the cash used to acquire Lifecore and purchase our minority investment in Windset and from lower yields on investments due to declines in interest rates.
Interest Expense
The increase in interest expense during the fiscal year ended May 29, 2011 compared to the same period last year was primarily due to the interest expense on the credit facility entered into on April 30, 2010 in connection with the acquisition of Lifecore.
Other Income
The other income is for the $662,000 increase in the fair market value of our Windset investment (see Note 3 to the Consolidated Financial Statements) partially offset by a $190,000 expense for the amortization of the discount on Lifecore’s earnout obligation (see Note 2 to the Consolidated Financial Statements) .
Income Taxes
The decrease in the income tax expense in fiscal year 2011 compared to fiscal years 2010 is due to a 2% decrease in taxable income partially offset by an increase in the Company’s effective tax rate to 52% in fiscal year 2011 up from 51% in fiscal year 2010. The effective tax rates for fiscal year 2011 differ from the statutory federal income tax rate of 35 percent as a result of several factors, including state taxes, non-deductible stock-based compensation expense, tax exempt interest and the goodwill impairment charge. In addition to the above, the Company was able to further reduce the effective tax rate for fiscal year 2011 as a result of being a recipient of a therapeutic drug credit award and the extension of the federal research and development credit (see Note 11 to the Consolidated Financial Statement for the material components of the Company’s effective tax rates).
Noncontrolling Interest
The noncontrolling interest consists of the limited partners’ equity interest in the net income of Apio Cooling, LP.
The decrease in the noncontrolling interest for the fiscal year ended May 29, 2011 compared to the same periods last year was not significant.
Fiscal Year Ended May 30, 2010 Compared to Fiscal Year Ended May 31, 2009
Revenues (in thousands):
| | | Fiscal Year ended May 30, 2010 | | | Fiscal Year ended May 31, 2009 | | | Change | |
| Apio Value Added | | $ | 172,416 | | | $ | 165,648 | | | | 4 | % |
| Apio Packaging | | | 2,630 | | | | 2,608 | | | | 1 | % |
| Food Technology | | | 175,046 | | | | 168,256 | | | | 4 | % |
| Apio Export | | | 54,926 | | | | 60,445 | | | | (9 | )% |
| Total Apio | | | 229,972 | | | | 228,701 | | | | 1 | % |
| HA | | | 1,457 | | | | — | | | | N/M | |
| Technology Licensing | | | 6,795 | | | | 7,237 | | | | (6 | )% |
| Total Revenues | | $ | 238,224 | | | $ | 235,938 | | | | 1 | % |
Apio Value Added
Apio’s value-added revenues consist of revenues generated from the sale of specialty packaged fresh-cut and whole value-added processed vegetable products that are washed and packaged in our proprietary packaging and sold under Apio’s Eat Smart brand and various private labels. In addition, value-added revenues include the revenues generated from Apio Cooling, LP, a produce cooling operation in which Apio is the general partner with a 60% ownership position.
The increase in Apio's value-added revenues for the fiscal year ended May 30, 2010 compared to the same period last year was primarily due to increased unit sales volumes related to gaining increased market share, increased promotional activity, and new product introductions which increased unit sales volumes by 14%, 4%, and 3%, respectively. These increases in revenue were partially offset by a decrease in value-added unit sales volumes of 4% related to existing business and a year over year mix change to greater sales of lower priced bag products from higher priced tray products. The decrease in unit sales volumes from existing business during fiscal year 2010 was due to Apio exiting business that would have resulted in less than acceptable gross profit margins.
Apio Packaging
Apio packaging revenues consist of Apio’s packaging technology business using its BreatheWay membrane technology. The first commercial application included in Apio packaging is our banana packaging technology and more recently the addition of a second application for avocados.
The increase in Apio packaging revenues for the fiscal year ended May 30, 2010 compared to the same period last year was not significant to consolidated Landec revenues.
Apio Export
Apio export revenues consist of revenues generated from the purchase and sale of primarily whole commodity fruit and vegetable products to Asia through Apio’s export company, Cal-Ex, and from the purchase and sale of whole commodity fruit and vegetable products domestically.
The decrease in revenues in Apio's export business for the fiscal year ended May 30, 2010 compared to the same period last year was due to a 9% decrease in unit volume sales due to a shortage of produce to export.
Hyaluronan-based Biomaterials (“HA”)
The Company’s Lifecore subsidiary generates revenue through sale of products containing hyaluronan, Lifecore was acquired on April 30, 2010.
Technology Licensing
Technology licensing revenues consist of revenues generated from the licensing agreements with Monsanto, Air Products and Nitta.
The decrease in technology licensing revenues for the fiscal year ended May 30, 2010 compared to the same period last year was not significant to consolidated Landec revenues.
Gross Profit (in thousands):
| | | Fiscal Year ended May 30, 2010 | | | Fiscal Year ended May 31, 2009 | | | Change | |
| Apio Value Added | | $ | 20,261 | | | $ | 21,020 | | | | (4 | )% |
| Apio Packaging | | | 2,253 | | | | 2,366 | | | | (5 | )% |
| Food Technology | | | 22,514 | | | | 23,386 | | | | (4 | )% |
| Apio Export | | | 3,906 | | | | 3,657 | | | | 7 | % |
| Total Apio | | | 26,420 | | | | 27,043 | | | | (2 | )% |
| HA | | | 815 | | | | — | | | | N/M | |
| Technology Licensing | | | 6,531 | | | | 7,237 | | | | (10 | )% |
| Total Gross Profit | | $ | 33,766 | | | $ | 34,280 | | | | (1 | )% |
General
There are numerous factors that can influence gross profit including product mix, customer mix, manufacturing costs, volume, sale discounts and charges for excess or obsolete inventory, to name a few. Many of these factors influence or are interrelated with other factors. The Company includes in cost of sales all of the costs related to the sale of products in accordance with U.S. generally accepted accounting principles. These costs include the following: raw materials (including produce, casein, seeds and packaging), direct labor, overhead (including indirect labor, depreciation, and facility related costs) and shipping and shipping-related costs. The following are the primary reasons for the changes in gross profit for the fiscal year ended May 30, 2010 compared to the same period last year as outlined in the table above.
Apio Value-Added
The decrease in gross profit for Apio’s value-added specialty packaged vegetable business for the fiscal year ended May 30, 2010 compared to the same period last year was primarily due to an increase in the cost for sourcing produce during the fourth quarter of fiscal year 2010 which resulted in lower gross margins coupled with a sales mix change to lower margin bag products from higher margin tray products. These decreases in gross profit were partially offset by decreased packaging costs. The gross margin for Apio’s value-added business for fiscal year 2010 was 11.8% compared to a gross margin of 12.7% for fiscal year 2009.
Apio Packaging
The decrease in gross profit for Apio packaging for the fiscal year ended May 30, 2010 compared to the same period last year was not significant to consolidated Landec gross profit.
Apio Export
Apio’s export business is a buy/sell business that realizes a commission-based margin in the 5-7% range. The increase in gross profit during the fiscal year ended May 30, 2010 compared to the prior fiscal year was primarily due to product mix changes to higher margin products in our export business which resulted in a higher gross margin during fiscal year 2010 of 7.1% compared to a gross margin of 6.1% in fiscal year 2009.
Hyaluronan-based Biomaterials
Lifecore was acquired on April 30, 2010.
Technology Licensing
The decrease in technology licensing gross profit for the fiscal year ended May 30, 2010 compared to the same period of the prior year was primarily due to completion of license payments by Air Products in fiscal year 2009 which resulted in $600,000 being recognized last fiscal year and none this fiscal year.
Operating Expenses (in thousands):
| | | Fiscal Year ended May 30, 2010 | | | Fiscal Year ended May 31, 2009 | | | Change | |
| Research and Development: | | | | | | | | | |
| Apio | | $ | 1,182 | | | $ | 1,321 | | | | (11 | )% |
| HA | | | 395 | | | | — | | | | N/M | |
| Technology Licensing | | | 2,784 | | | | 2,344 | | | | 19 | % |
| Total R&D | | $ | 4,361 | | | $ | 3,665 | | | | 19 | % |
| | | | | | | | | | | | | |
| Selling, General and Administrative: | | | | | | | | | | | | |
| Apio | | $ | 12,128 | | | $ | 12,709 | | | | (5 | )% |
| HA | | | 339 | | | | — | | | | N/M | |
| Technology Licensing | | | 100 | | | | — | | | | N/M | |
| Corporate | | | 5,131 | | | | 5,308 | | | | (3 | )% |
| Total S,G&A | | $ | 17,698 | | | $ | 18,017 | | | | (2 | )% |
| | | | | | | | | | | | | |
| Other operating expenses: | | | | | | | | | | | | |
| Technology Licensing | | $ | 1,000 | | | $ | — | | | | N/M | |
| Corporate | | | 2,725 | | | | — | | | | N/M | |
| Total other operating expenses | | $ | 3,725 | | | $ | — | | | | N/M | |
Research and Development
Landec’s research and development expenses consist primarily of expenses involved in product development and commercialization initiatives. Research and development efforts at Apio are focused on the Company’s proprietary BreatheWay membranes used for packaging produce, with recent focus on extending the shelf life of bananas and other shelf-life sensitive vegetables and fruit. In the HA business, the research and development efforts are focused on new products and applications for Hyaluronan-based biomaterials. In the Technology Licensing business, the research and development efforts are focused on uses for the proprietary Intelimer polymers outside of food and HA.
The increase in research and development expenses for the fiscal year ended May 30, 2010 compared to the same period last year was primarily due to increased headcount, increased consulting fees surrounding development work in our Technology Licensing business and the acquisition of Lifecore on April 30, 2010.
Selling, General and Administrative
Selling, general and administrative expenses consist primarily of sales and marketing expenses associated with Landec’s product sales and services, business development expenses and staff and administrative expenses.
The decrease in selling, general and administrative expenses for the fiscal year ended May 30, 2010 compared to the same period last year was not significant.
Other Operating Expenses
Other operating expenses consists of impairment charges and transaction costs associated with the Lifecore acquisition. The $1.0 million amount in fiscal year 2010 in Technology Licensing is from the partial write down of the Company’s investment in Aesthetic Sciences Corporation. The $2.7 million amount in fiscal year 2010 in Corporate is for the transaction costs from the acquisition of Lifecore on April 30, 2010.
Non-operating income/(expense) (in thousands):
| | | Fiscal Year ended May 30, 2010 | | | Fiscal Year ended May 31, 2009 | | | Change | |
| Interest Income | | $ | 834 | | | $ | 1,306 | | | | (36 | )% |
| Interest Expense | | $ | (88 | ) | | $ | (8 | ) | | | 1000 | % |
| Income Taxes | | $ | (4,262 | ) | | $ | (5,611 | ) | | | (24 | )% |
| Noncontrolling Interest | | $ | (482 | ) | | $ | (555 | ) | | | (13 | )% |
The decrease in interest income for the fiscal year ended May 30, 2010 compared to the same period last year was due to lower yields on investments.
Interest Expense
The increase in interest expense during the fiscal year ended May 30, 2010 compared to the prior year was due to the new credit facility entered into on April 30, 2010 in conjunction with the acquisition of Lifecore.
Income Taxes
The decrease in the income tax expense for the fiscal year ended May 30, 2010 is due to a 37% decrease in net income before taxes compared to the fiscal year ended May 31, 2009. The effective tax rate in fiscal year 2010 increased to 51% from 42% in fiscal year 2009. The effective tax rates for the fiscal years 2010 and 2009 differ from the statutory federal income tax rate of 35 percent as a result of several factors, including state taxes, non-deductible stock-based compensation expense, and tax exempt interest (see Note 11 to the Consolidated Financial Statement for the material components of the Company’s effective tax rates).
Noncontrolling Interest
The noncontrolling interest expense consists of the minority interest associated with the limited partners’ equity interest in the net income of Apio Cooling, LP.
The decrease in the noncontrolling interest expense in fiscal year 2010 compared to fiscal year 2009 was not significant.
Liquidity and Capital Resources
As of May 29, 2011, the Company had cash and cash equivalents of $8.1 million, a net decrease of $19.7 million from $27.8 million at May 30, 2010.
Cash Flow from Operating Activities
Landec generated $14.5 million of cash flow from operating activities during the fiscal year ended May 29, 2011 compared to $7.5 million during the fiscal year ended May 30, 2010. The primary sources of cash from operating activities during fiscal year 2011 were $4.3 million of net income and non-cash related expenses of $13.1 million, partially offset by a net decrease of $2.9 million in working capital. The primary changes in working capital during fiscal year 2011 which decreased working capital were (a) a $3.0 million increase in trade accounts receivable primarily due to a $1.7 million increase in receivables at Apio as a result of revenues for May 2011 being $2.0 million higher than May 2010 and from a $484,000 increase at Lifecore as a result of revenues for May 2011 being $1.0 million higher than May 2010, (b) a $4.1 million increase in inventories due to a $1.6 million increase in export inventories that were in transit at fiscal year end due to the timing of shipments, a $900,000 increase in raw material and finished goods inventories for Apio’s value-added vegetable business as a result of anticipated increases in demand and a $1.8 million increase in inventories at Lifecore due to the inventory build for expected new business, and (c) a $1.7 million decrease in deferred revenue associated with the Monsanto Agreement. The primary changes in working capital during fiscal year 2011 which increased working capital were a $3.9 million increase in current liabilities primarily due to a $2.3 million increase in accounts payable resulting from increased cost of sales from the increase in revenues and increase in operating expenses and a $1.0 million increase in accrued compensation primarily from bonuses earned by Lifecore employees.
Cash Flow from Investing Activities
Net cash used in investing activities for the fiscal year ended May 29, 2011 was $29.4 million compared to net cash used in investing activities of $42.8 million for the same period last year. The primary uses of cash in investing activities during fiscal year 2011 were from (a) the purchase of $6.7 million of property, plant and equipment primarily for the further expansion of Apio’s value-added processing facility and the further automation of Apio’s value-added processing facility and facility modifications and equipment purchased at Lifecore to support business growth, (b) the net purchase of $7.7 million of marketable securities and (c) the purchase of $15 million of preferred and common shares in Windset. The primary uses of cash from investing activities during fiscal year 2010 were from the acquisition of Lifecore for $39.7 million (net of cash acquired), from the purchase of $5.2 million of property and equipment primarily for the growth of Apio’s value-added vegetable business partially offset by net proceeds of $2.1 million from the sale of marketable securities.
Cash Flow from Financing Activities
Net cash used in financing activities for the fiscal year ended May 29, 2011 was $4.8 million compared to net cash provided by financing activities of $19.7 million for the same period last year. The use of cash in financing activities during the fiscal year 2011 was primarily from $3.9 million of long-term debt payments and the repurchase of $1.2 million of the Company’s outstanding Common Stock, partially offset by the tax benefit from stock-based compensation of $764,000. The primary source of cash from financing activities during fiscal year 2010 was from the $20.0 million of proceeds from long-term debt entered into in connection with the acquisition of Lifecore.
Capital Expenditures
During the fiscal year ended May 29, 2011, Landec continued its expansion of Apio’s value-added processing facility and purchased vegetable processing equipment to support the further automation of Apio’s value- added processing facility and facility modifications and equipment purchased at Lifecore to support business growth. These expenditures represented the majority of the $6.7 million of capital expenditures.
Debt
On April 30, 2010 in connection with the acquisition of Lifecore, Lifecore entered into a $20 million Credit Agreement with Wells Fargo Bank N.A. (“Wells Fargo”) with a five year term that provides for equal monthly principal payments plus interest. The Credit Agreement contains certain restrictive covenants, which require Lifecore to meet certain financial tests, including minimum levels of net income, minimum quick ratio, minimum fixed coverage ratio and maximum capital expenditures. All of Lifecore’s assets have been pledged to secure the debt incurred pursuant to the Credit Agreement. Landec is the guarantor of the debt. On August 9, 2010 and September 14, 2010, the Company amended its Credit Agreement with Wells Fargo to modify certain financial covenants. The amendment on August 9, 2010 amended the definition of the net income of Lifecore to exclude non-recurring expenses incurred in connection with the acquisition of Lifecore and to exclude expenses related to the impact of adjustments from purchase accounting (e.g. inventory step-up, discount on the earn out, etc.) as they relate to the minimum net income covenant. The aforementioned adjustments were made to the initial forecast that the Company had previously provided Wells Fargo which was the basis for the covenants in the Credit Agreement and which were necessary for compliance as of May 30, 2010. The amendment on September 14, 2010 amended the definition of the net income of Lifecore to include only the current fiscal year to date results as compared to the previous trailing four quarter basis, so as to exclude results prior to the acquisition. In addition, the minimum net income requirement for the first quarter ended August 29, 2010 was changed to $1.00 from $500,000. The Company was in compliance with all financial covenants as of May 29, 2011.
On May 4, 2010, the Company entered into an interest rate swap agreement that has the economic effect of modifying the variable interest obligations associated with the $20 million Credit Agreement so that the interest payable is effectively fixed at a rate of 4.24% (see Note 10 to the Consolidated Financial Statemets).
On August 19, 2004, Lifecore issued variable rate industrial revenue bonds (“IRB”). These bonds were assumed by Landec in the acquisition of Lifecore (see Note 2 to the Consolidated Financial Statements). The bonds are collateralized by a bank letter of credit which is secured by a first mortgage on the Company’s facility in Chaska, Minnesota. In addition, the Company pays an annual remarketing fee equal to 0.125% and an annual letter of credit fee of 0.50%.
Contractual Obligations
The Company’s material contractual obligations for the next five years and thereafter as of May 29, 2011, are as follows (in thousands):
| | | Due in Fiscal Year Ended May | |
| | | | | | | | | | | | | | | | | | | | | |
| Income taxes | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Long-term debt | | | 19,830 | | | | 4,330 | | | | 4,340 | | | | 4,355 | | | | 4,365 | | | | 375 | | | | 2,065 | |
| Interest payments | | | 1,818 | | | | 677 | | | | 499 | | | | 321 | | | | 142 | | | | 55 | | | | 124 | |
| Unrealized loss on interest rate swap | | | 430 | | | | 430 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Operating leases | | | 2,571 | | | | 734 | | | | 607 | | | | 509 | | | | 307 | | | | 261 | | | | 153 | |
| Licensing obligation | | | 50 | | | | 50 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Purchase commitments | | | 2,194 | | | | 2,194 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Total | | $ | 26,893 | | | $ | 8,415 | | | $ | 5,446 | | | $ | 5,185 | | | $ | 4,814 | | | $ | 691 | | | $ | 2,342 | |
The income tax amounts above exclude liabilities associated with the accounting for uncertainty in income taxes as we are unable to reasonably estimate the ultimate amount or timing of settlement. See Note 11 in the Notes to Consolidated Financial Statements for further discussion.
The interest payment amounts above include the fixed interest rate payments for the Credit Agreement with Wells Fargo and an estimated interest rate payment on the variable rate IRB based on the five year historical interest rate average for the Municipal Swap Index plus 20 basis points plus the letter of credit and remarketing fees of 62.5 basis points resulting in a estimated rate of 2.515%.
Landec is not a party to any agreements with, or commitments to, any special purpose entities that would constitute material off-balance sheet financing other than the operating lease commitments.
Landec’s future capital requirements will depend on numerous factors, including the progress of its research and development programs; the continued development of marketing, sales and distribution capabilities; the ability of Landec to establish and maintain new collaborative and licensing arrangements; any decision to pursue additional acquisition opportunities; weather conditions that can affect the supply and price of produce, the timing and amount, if any, of payments received under licensing and research and development agreements; the costs involved in preparing, filing, prosecuting, defending and enforcing intellectual property rights; the ability to comply with regulatory requirements; the emergence of competitive technology and market forces; the effectiveness of product commercialization activities and arrangements; and other factors. If Landec’s currently available funds, together with the internally generated cash flow from operations are not sufficient to satisfy its capital needs, Landec would be required to seek additional funding through other arrangements with collaborative partners, additional bank borrowings and public or private sales of its securities. There can be no assurance that additional funds, if required, will be available to Landec on favorable terms, if at all.
Landec believes that its cash from operations, along with existing cash, cash equivalents and marketable securities will be sufficient to finance its operational and capital requirements for at least the next twelve months.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not significant.
Item 8. Financial Statements and Supplementary Data
See Item 15 of Part IV of this report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management evaluated, with participation of our Chief Executive Officer and our Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures are effective in ensuring that information required to be disclosed in reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified by the Securities and Exchange Commission, and are effective in providing reasonable assurance that information required to be disclosed by the Company in such reports is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended). Our management assessed the effectiveness of our internal control over financial reporting as of May 29, 2011. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control - Integrated Framework. Our management has concluded that, as of May 29, 2011, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected.
Our independent registered public accounting firm, Ernst & Young LLP, has issued an audit report on our internal control over financial reporting, which is included herein.
Changes in Internal Controls over Financial Reporting
On April 30, 2010, the Company acquired Lifecore. The Company has expanded the scope of a number of internal processes to include Lifecore and is fully integrated as of May 29, 2011. There were no other changes in our internal controls over financial reporting during the fiscal year ended May 29, 2011 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Landec Corporation
We have audited Landec Corporation and subsidiaries’ internal control over financial reporting as of May 29, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Landec Corporation and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Landec Corporation and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of May 29, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Landec Corporation and subsidiaries as of May 29, 2011 and May 30, 2010, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended May 29, 2011 of Landec Corporation and subsidiaries and our report dated August 5, 2011 expressed an unqualified opinion thereon.
San Francisco, California
August 5, 2011
Item 9B. Other Information
PART III
| Item 10. | Directors, Executive Officers and Corporate Goverance |
| | This information required by this item will be contained in the Registrant’s definitive proxy statement which the Registrant will file with the Commission no later than September 26, 2011 (120 days after the Registrant’s fiscal year end covered by this Report) and is incorporated herein by reference. |
| Item 11. | Executive Compensation |
| | This information required by this item will be contained in the Registrant’s definitive proxy statement which the Registrant will file with the Commission no later than September 26, 2011 (120 days after the Registrant’s fiscal year end covered by this Report) and is incorporated herein by reference. |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
| | This information required by this item will be contained in the Registrant’s definitive proxy statement which the Registrant will file with the Commission no later than September 26, 2011 (120 days after the Registrant’s fiscal year end covered by this Report) and is incorporated herein by reference. |
| Item 13. | Certain Relationships and Related Transactions and Director Independence |
| | This information required by this item will be contained in the Registrant’s definitive proxy statement which the Registrant will file with the Commission no later than September 26, 2011 (120 days after the Registrant’s fiscal year end covered by this Report) and is incorporated herein by reference. |
| Item 14. | Principal Accountant Fees and Services |
| | This information required by this item will be contained in the Registrant’s definitive proxy statement which the Registrant will file with the Commission no later than September 26, 2011 (120 days after the Registrant’s fiscal year end covered by this Report) and is incorporated herein by reference. |
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) | 1. | Consolidated Financial Statements of Landec Corporation | | |
| | | | | |
| | | | | Page |
| | | | | |
| | | Report of Ernst & Young LLP, Independent Registered Public Accounting Firm | | 52 |
| | | | | |
| | | Consolidated Balance Sheets at May 29, 2011 and May 30, 2010 | | 53 |
| | | | | |
| | | Consolidated Statements of Income for the Years Ended May 29, 2011, May 30, 2010 and May 31, 2009 | | 54 |
| | | | | |
| | | Consolidated Statements of Changes in Stockholders’ Equity for the Years Ended May 29, 2011, May 30, 2010 and May 31, 2009 | | 55 |
| | | | | |
| | | Consolidated Statements of Cash Flows for the Years Ended May 29, 2011, May 30, 2010 and May 31, 2009 | | 56 |
| | | | | |
| | | Notes to Consolidated Financial Statements | | 57 |
| | | | | |
| | 2. | All schedules provided for in the applicable accounting regulations of the Securities and Exchange Commission have been omitted since they pertain to items which do not appear in the financial statements of Landec Corporation and its subsidiaries or to items which are not significant or to items as to which the required disclosures have been made elsewhere in the financial statements and supplementary notes and such schedules. | | |
| | | | | |
| | 3. | Index of Exhibits | | 86 |
| | | | | |
| | | The exhibits listed in the accompanying Index of Exhibits are filed or incorporated by reference as part of this report. | | |
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Landec Corporation
We have audited the accompanying consolidated balance sheets of Landec Corporation and subsidiaries as of May 29, 2011 and May 30, 2010, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended May 29, 2011. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Landec Corporation and subsidiaries at May 29, 2011 and May 30, 2010, and the consolidated results of their operations and their cash flows for each of the three years in the period ended May 29, 2011, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 1 to the consolidated financial statements, under the heading ‘Business Combinations,’ the Company adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, Business Combinations, effective June 1, 2009.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Landec Corporation’s internal control over financial reporting as of May 29, 2011, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 5, 2011 expressed an unqualified opinion thereon.
San Francisco, California
August 5, 2011
LANDEC CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share amounts)
| | | May 29, 2011 | | | May 30, 2010 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 8,135 | | | $ | 27,817 | |
| Marketable securities | | | 28,124 | | | | 20,421 | |
| Accounts receivable, less allowance for doubtful accounts of $342 and $189 at May 29, 2011 and May 30, 2010, respectively | | | 21,653 | | | | 18,637 | |
| Accounts receivable, related party | | | 453 | | | | 729 | |
| Income taxes receivable | | | 571 | | | | 739 | |
| Inventories, net | | | 20,161 | | | | 16,107 | |
| Deferred taxes | | | 542 | | | | 1,262 | |
| Prepaid expenses and other current assets | | | 5,987 | | | | 3,230 | |
| Total current assets | | | 85,626 | | | | 88,942 | |
| | | | | | | | | |
| Investment in non-public company, non-fair value | | | 793 | | | | 793 | |
| Investment in non-public company, fair value | | | 15,662 | | | | — | |
| Property and equipment, net | | | 51,779 | | | | 50,161 | |
| Goodwill, net | | | 36,462 | | | | 41,154 | |
| Trademarks/ trade names, net | | | 12,428 | | | | 12,428 | |
| Customer relationships, net | | | 3,366 | | | | 3,674 | |
| Other assets | | | 196 | | | | 3,045 | |
| Total Assets | | $ | 206,312 | | | $ | 200,197 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 16,747 | | | $ | 14,354 | |
| Related party payables | | | 300 | | | | 349 | |
| Accrued compensation | | | 3,080 | | | | 2,043 | |
| Other accrued liabilities | | | 3,581 | | | | 3,277 | |
| Deferred revenue | | | 2,657 | | | | 3,391 | |
| Current portion of long-term debt | | | 4,330 | | | | 4,521 | |
| Total current liabilities | | | 30,695 | | | | 27,935 | |
| | | | | | | | | |
| Long-term debt | | | 15,500 | | | | 19,249 | |
| Deferred revenue | | | — | | | | 1,000 | |
| Deferred taxes | | | 11,338 | | | | 8,801 | |
| Other non-current liabilities | | | 11,053 | | | | 10,737 | |
| Total liabilities | | | 68,586 | | | | 67,722 | |
| Commitments and contingencies (Note 12) | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock, $0.001 par value; 50,000,000 shares authorized; 26,405,799 and 26,490,259 shares issued and outstanding at May 29, 2011 and May 30, 2010, respectively | | | 27 | | | | 27 | |
| Additional paid-in capital | | | 119,169 | | | | 117,730 | |
| Accumulated other comprehensive loss | | | (267 | ) | | | (179 | ) |
| Retained earnings | | | 17,126 | | | | 13,206 | |
| Total stockholders’ equity | | | 136,055 | | | | 130,784 | |
| Non-controlling interest | | | 1,671 | | | | 1,691 | |
| Total Equity | | | 137,726 | | | | 132,475 | |
| Total Liabilities and Stockholders’ Equity | | $ | 206,312 | | | $ | 200,197 | |
See accompanying notes.
LANDEC CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share amounts)
| | | Year Ended | | | Year Ended | | | Year Ended | |
| | | 2011 | | | 2010 | | | 2009 | |
| Revenues: | | | | | | | | | |
| Product sales | | $ | 267,121 | | | $ | 228,390 | | | $ | 224,404 | |
| Services revenue, related party | | | 3,391 | | | | 3,699 | | | | 4,145 | |
| License fees | | | 5,400 | | | | 5,400 | | | | 6,000 | |
| Research, development and royalty revenues | | | 817 | | | | 735 | | | | 1,389 | |
| Total revenues | | | 276,729 | | | | 238,224 | | | | 235,938 | |
| | | | | | | | | | | | | |
| Cost of revenue: | | | | | | | | | | | | |
| Cost of product sales | | | 223,613 | | | | 198,075 | | | | 195,180 | |
| Cost of product sales, related party | | | 3,554 | | | | 3,391 | | | | 3,189 | |
| Cost of services revenue | | | 2,867 | | | | 2,992 | | | | 3,289 | |
| Total cost of revenue | | | 230,034 | | | | 204,458 | | | | 201,658 | |
| | | | | | | | | | | | | |
| Gross profit | | | 46,695 | | | | 33,766 | | | | 34,280 | |
| | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | |
| Research and development | | | 9,275 | | | | 4,361 | | | | 3,665 | |
| Selling, general and administrative | | | 24,608 | | | | 17,698 | | | | 18,017 | |
| Other operating expenses | | | 4,780 | | | | 3,725 | | | | — | |
| Total operating costs and expenses | | | 38,663 | | | | 25,784 | | | | 21,682 | |
| | | | | | | | | | | | | |
| Operating income | | | 8,032 | | | | 7,982 | | | | 12,598 | |
| | | | | | | | | | | | | |
| Dividend income | | | 328 | | | | — | | | | — | |
| Interest income | | | 430 | | | | 834 | | | | 1,306 | |
| Interest expense | | | (820 | ) | | | (88 | ) | | | (8 | ) |
| Other income | | | 472 | | | | — | | | | ― | |
| Net income before taxes | | | 8,442 | | | | 8,728 | | | | 13,896 | |
| Income tax expense | | | (4,181 | ) | | | (4,262 | ) | | | (5,611 | ) |
| Consolidated net income | | | 4,261 | | | | 4,466 | | | | 8,285 | |
| Non controlling interest | | | (341 | ) | | | (482 | ) | | | (555 | ) |
| Net Income applicable to Common Stockholders | | $ | 3,920 | | | $ | 3,984 | | | $ | 7,730 | |
| | | | | | | | | | | | | |
| Basic net income per share | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.30 | |
| Diluted net income per share | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.29 | |
| | | | | | | | | | | | | |
| Shares used in per share computation: | | | | | | | | | | | | |
| Basic | | | 26,397 | | | | 26,382 | | | | 26,202 | |
| Diluted | | | 26,626 | | | | 26,633 | | | | 26,751 | |
See accompanying notes.
LANDEC CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN
STOCKHOLDERS’ EQUITY
(in thousands, except share and per share amounts)
| | | | | | Additional Paid-in | | | Retained Earnings | | | Other Comprehensive | | | Total Stockholders’ | | | Non- controlling | |
| | | Shares | | | Amount | | | Capital | | | (Deficit) | | | Loss | | | Equity | | | interest | |
| Balance at May 25, 2008 | | | 26,156,323 | | | $ | 26 | | | $ | 112,948 | | | $ | 1,492 | | | | ― | | | $ | 114,466 | | | $ | 1,550 | |
| Issuance of common stock at $2.82 to $5.34 per share | | | 160,570 | | | | — | | | | 379 | | | | — | | | | ― | | | | 379 | | | | — | |
| Issuance of common stock for vested restricted stock units | | | 9,996 | | | | — | | | | — | | | | — | | | | ― | | | | — | | | | — | |
| Stock-based compensation | | | — | | | | — | | | | 933 | | | | — | | | | ― | | | | 933 | | | | — | |
| Tax benefit from stock-based compensation expense | | | — | | | | — | | | | 1,898 | | | | — | | | | ― | | | | 1,898 | | | | — | |
| Non-controlling interest expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 555 | |
| Payments to non-controlling interest | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (315 | ) |
| Net income and comprehensive income | | | — | | | | — | | | | — | | | | 7,730 | | | | ― | | | | 7,730 | | | | — | |
| Balance at May 31, 2009 | | | 26,326,889 | | | | 26 | | | | 116,158 | | | | 9,222 | | | | ― | | | | 125,406 | | | | 1,790 | |
| Issuance of common stock at $1.89 to $6.75 per share, net of taxes paid by Landec on behalf of employees | | | 121,442 | | | | 1 | | | | 378 | | | | — | | | | ― | | | | 379 | | | | — | |
| Issuance of common stock for vested restricted stock units | | | 41,928 | | | | — | | | | ― | | | | — | | | | ― | | | | — | | | | — | |
| Taxes paid by Company for stock swaps and RSUs | | | ― | | | | ― | | | | (339 | ) | | | — | | | | ― | | | | (339 | ) | | | — | |
| Stock-based compensation | | | — | | | | — | | | | 1,016 | | | | — | | | | ― | | | | 1,016 | | | | — | |
| Tax benefit from stock-based compensation expense | | | — | | | | — | | | | 517 | | | | — | | | | ― | | | | 517 | | | | — | |
| Non-controlling interest expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 482 | |
| Payments to non-controlling interest | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (581 | ) |
| Net income and comprehensive loss | | | — | | | | — | | | | — | | | | 3,984 | | | | (179 | ) | | | 3,805 | | | | — | |
| Balance at May 30, 2010 | | | 26,490,259 | | | | 27 | | | | 117,730 | | | | 13,206 | | | | (179 | ) | | | 130,784 | | | | 1,691 | |
| Issuance of common stock at $3.38 to $3.80 per share, net of taxes paid by Landec on behalf of employees | | | 91,091 | | | | — | | | | 126 | | | | — | | | | ― | | | | 126 | | | | — | |
| Issuance of common stock for vested restricted stock units | | | 40,133 | | | | — | | | | ― | | | | — | | | | ― | | | | — | | | | — | |
| Common stock repurchased on the open market | | | (215,684 | ) | | | — | | | | (1,184 | ) | | | — | | | | — | | | | (1,184 | ) | | | | |
| Taxes paid by Company for stock swaps and RSUs | | | ― | | | | ― | | | | (218 | ) | | | — | | | | ― | | | | (218 | ) | | | — | |
| Stock-based compensation | | | — | | | | — | | | | 1,951 | | | | — | | | | ― | | | | 1,951 | | | | — | |
| Tax benefit from stock-based compensation expense | | | — | | | | — | | | | 764 | | | | — | | | | ― | | | | 764 | | | | — | |
| Non-controlling interest expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 341 | |
| Payments to non-controlling interest | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (361 | ) |
| Net income and comprehensive loss | | | — | | | | — | | | | — | | | | 3,920 | | | | (88 | ) | | | 3,832 | | | | — | |
| Balance at May 29, 2011 | | | 26,405,799 | | | $ | 27 | | | $ | 119,169 | | | $ | 17,126 | | | $ | (267 | ) | | $ | 136,055 | | | $ | 1,671 | |
See accompanying notes
LANDEC CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | Year Ended | | | Year Ended | | | Year Ended | |
| | | May 29, | | | May 30, | | | May 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
| Cash flows from operating activities: | | | | | | | | | |
| Consolidated net income | | $ | 4,261 | | | $ | 4,466 | | | $ | 8,285 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 5,313 | | | | 3,364 | | | | 3,139 | |
| Stock-based compensation expense | | | 1,951 | | | | 1,016 | | | | 933 | |
| Deferred taxes | | | 3,257 | | | | 3,248 | | | | 2,569 | |
| Change in investment in non-public company | | | (662 | ) | | | ― | | | | ― | |
| Increase in long-term receivable | | | (800 | ) | | | (800 | ) | | | (800 | ) |
| Tax benefit from stock based compensation | | | (764 | ) | | | (517 | ) | | | (1,898 | ) |
| Net loss on disposal of property and equipment | | | 26 | | | | ― | | | | ― | |
| Impairment charges | | | 4,780 | | | | 1,000 | | | | ― | |
| Changes in assets and liabilities, net of effects from acquisitions: | | | | | | | | | | | | |
| Accounts receivable, net | | | (3,016 | ) | | | (1,506 | ) | | | 4,189 | |
| Accounts receivable, related party | | | 276 | | | | (97 | ) | | | (221 | ) |
| Income taxes receivable | | | 878 | | | | (764 | ) | | | — | |
| Inventories, net | | | (4,054 | ) | | | (1,269 | ) | | | 1,500 | |
| Issuance of notes and advances receivable | | | (3,073 | ) | | | (3,030 | ) | | | (3,055 | ) |
| Collection of notes and advances receivable | | | 3,314 | | | | 2,975 | | | | 3,269 | |
| Prepaid expenses and other current assets | | | 602 | | | | (1,172 | ) | | | (184 | ) |
| Accounts payable | | | 2,393 | | | | 957 | | | | (5,929 | ) |
| Related party accounts payable | | | (49 | ) | | | 50 | | | | 26 | |
| Income taxes payable | | | — | | | | 1,162 | | | | 2,005 | |
| Accrued compensation | | | 1,038 | | | | (264 | ) | | | (1,085 | ) |
| Other accrued liabilities | | | 532 | | | | 703 | | | | (1,125 | ) |
| Deferred revenue | | | (1,734 | ) | | | (2,039 | ) | | | (2,183 | ) |
| Net cash provided by operating activities | | | 14,469 | | | | 7,483 | | | | 9,435 | |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Purchases of property and equipment | | | (6,684 | ) | | | (5,192 | ) | | | (4,576 | ) |
| Acquisition of Lifecore, net of cash acquired (Note 2) | | | — | | | | (39,682 | ) | | | — | |
| Acquisition related earnout payments | | | — | | | | — | | | | (7 | ) |
| Issuance of notes and advances receivable | | | ― | | | | — | | | | (2 | ) |
| Collection of notes and advances receivable | | | ― | | | | — | | | | 103 | |
| Purchase of marketable securities | | | (59,833 | ) | | | (67,433 | ) | | | (45,808 | ) |
| Proceeds from maturities and sales of marketable securities | | | 52,130 | | | | 69,510 | | | | 37,953 | |
| Investment in non-public company (fair market value) | | | (15,000 | ) | | | — | | | | — | |
| Net cash used in investing activities | | | (29,387 | ) | | | (42,797 | ) | | | (12,337 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Repurchase of outstanding common stock | | | (1,184 | ) | | | — | | | | — | |
| Proceeds from sale of common stock | | | 126 | | | | 379 | | | | 379 | |
| Taxes paid by Company for stock swaps and RSUs | | | (218 | ) | | | (339 | ) | | | ― | |
| Tax benefit from stock-based compensation expense | | | 764 | | | | 517 | | | | 1,898 | |
| Net change in other assets/liabilities | | | 49 | | | | — | | | | 3 | |
| Proceeds from long term debt | | | — | | | | 20,000 | | | | — | |
| Payments on long term debt | | | (3,940 | ) | | | (387 | ) | | | — | |
| Payments to non controlling interest. | | | (361 | ) | | | (498 | ) | | | (315 | ) |
| Net cash provided by (used in) financing activities | | | (4,764 | ) | | | 19,672 | | | | 1,965 | |
| Net decrease in cash and cash equivalents | | | (19,682 | ) | | | (15,642 | ) | | | (937 | ) |
| Cash and cash equivalents at beginning of year | | | 27,817 | | | | 43,459 | | | | 44,396 | |
| Cash and cash equivalents at end of year | | $ | 8,135 | | | $ | 27,817 | | | $ | 43,459 | |
| Supplemental disclosure of cash flows information: | | | | | | | | | | | | |
| Cash paid during the period for interest | | $ | 761 | | | $ | 88 | | | $ | 8 | |
| Cash paid during the period for income taxes | | $ | 146 | | | $ | 652 | | | $ | 1,135 | |
| | | | | | | | | | | | | |
| Supplemental schedule of noncash operating and financing activities: | | | | | | | | | | | | |
| Long-term receivable from Monsanto | | $ | 800 | | | $ | 800 | | | $ | 800 | |
| Income tax expense not payable | | $ | 784 | | | $ | 517 | | | $ | 1,898 | |
| Accrued non controlling interest distribution | | $ | — | | | $ | 250 | | | $ | — | |
| Impairment charges | | $ | 4,780 | | | $ | 1,000 | | | $ | — | |
See accompanying notes.
LANDEC CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies
Organization
Landec Corporation and its subsidiaries (“Landec” or the “Company”) design, develop, manufacture and sell polymer products for food and agricultural products, medical devices and licensed partner applications that incorporate Landec’s patented polymer technologies. The Company has two proprietary polymer technology platforms: 1) Intelimer® polymers, and 2) hyaluronan (“HA”) biopolymers. The Company’s HA biopolymers are proprietary in that they are specially formulated for specific customers to meet strict regulatory requirements. The Company’s polymer technologies, along with its customer relationships and trade names, are the foundation, and a key differentiating advantage upon which Landec has built its business. The Company sells specialty packaged fresh-cut vegetables and whole produce to retailers and club stores, primarily in the United States and Asia through its Apio, Inc. (“Apio”) subsidiary, Hyaluronan-based biomaterials through its Lifecore Biomedical, Inc. (“Lifecore”) subsidiary, and Intellicoat® coated seed products through its Landec Ag LLC (“Landec Ag”) subsidiary.
Basis of Presentation
Basis of Consolidation
The consolidated financial statements are presented on the accrual basis of accounting in accordance with U.S. generally accepted accounting principles and include the accounts of Landec Corporation and its subsidiaries, Apio, Lifecore and Landec Ag. All material inter-company transactions and balances have been eliminated.
Arrangements that are not controlled through voting or similar rights are reviewed under the guidance of variable interest entities (“VIEs”). A company is required to consolidate the assets, liabilities and operations of a VIE if it is determined to be the primary beneficiary of the VIE.
In June 2009, the FASB changed the consolidation analysis for VIEs to require a qualitative analysis to determine the primary beneficiary of the VIE. The determination of the primary beneficiary of a VIE is based on whether the entity has the power to direct matters which most significantly impact the activities of the VIE and has the obligation to absorb losses, or the right to receive benefits, of the VIE which could potentially be significant to the VIE. The guidance requires an ongoing reconsideration of the primary beneficiary and also amends the events triggering a reassessment. The new guidance was effective for the Company beginning May 31, 2010. Additional disclosures for VIEs are required, including a description about a reporting entity’s involvement with VIEs, how a reporting entity’s involvement with a VIE affects the reporting entity’s financial statements, and significant judgments and assumptions made by the reporting entity to determine whether it must consolidate the VIE.
Under the new guidance, an entity is a VIE and subject to consolidation, if by design: a) the total equity investment at risk is not sufficient to permit the entity to finance its activities without additional subordinated financial support provided by any parties, including equity holders or b) as a group the holders of the equity investment at risk lack any one of the following three characteristics: (i) the power, through voting rights or similar rights to direct the activities of an entity that most significantly impact the entity’s economic performance, (ii) the obligation to absorb the expected losses of the entity, or (iii) the right to receive the expected residual returns of the entity. The Company reviewed the consolidation guidance and concluded that the non-public companies in which the Company holds equity investments are not VIEs. The Company has concluded that there is no impact on the financial statements as a result of the adoption of the new guidance.
Under applicable accounting guidance, a Company also considered the requirements to consolidate an entity in which it holds voting control and concluded that due to the lack of voting control, the Company is not required to consolidate the investments in non-public companies.
| 1. | Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued) |
Reclassifications
Certain reclassifications have been made to prior year financial statements to conform to the current year presentation.
Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make certain estimates and judgments that affect the amounts reported in the financial statements and accompanying notes. The accounting estimates that require management’s most significant, difficult and subjective judgments include revenue recognition; sales returns and allowances; recognition and measurement of current and deferred income tax assets and liabilities; the assessment of recoverability of long-lived assets; the valuation of intangible assets and inventory; the valuation and nature of impairments of investments; and the valuation and recognition of stock-based compensation.
These estimates involve the consideration of complex factors and require management to make judgments. The analysis of historical and future trends, can require extended periods of time to resolve, and are subject to change from period to period. The actual results may differ from management’s estimates.
Concentrations of Risk
Cash and cash equivalents, marketable securities, trade accounts receivable, grower advances and notes receivable are financial instruments that potentially subject the Company to concentrations of credit risk. Corporate policy limits, among other things, the amount of credit exposure to any one issuer and to any one type of investment, other than securities issued or guaranteed by the U.S. government. The Company routinely assesses the financial strength of customers and growers and, as a consequence, believes that trade receivables, grower advances and notes receivable credit risk exposure is limited. Credit losses for bad debt are provided for in the consolidated financial statements through a charge to operations. A valuation allowance is provided for known and anticipated credit losses. The recorded amounts for these financial instruments approximate their fair value.
Several of the raw materials we use to manufacture our products are currently purchased from a single source, including some monomers used to synthesize Intelimer® polymers, substrate materials for our breathable membrane products and raw materials for our HA products.
During the fiscal year ended May 29, 2011, sales to the Company’s top five customers accounted for approximately 44% of total revenue, with the top customer, Costco Wholesale Corporation from the Food Products Technology segment, accounting for approximately 16% of total revenues. In addition, approximately 38% of the Company’s total revenues were derived from product sales to international customers, two of whom individually accounted for more than 5% of total revenues. As of May 29, 2011, Costco Wholesale Corporation represented approximately 14% of total accounts receivable.
During the fiscal year ended May 30, 2010, sales to the Company’s top five customers accounted for approximately 48% of total revenue, with the top customer, Costco Wholesale Corporation from the Food Products Technology segment, accounting for approximately 20% of total revenues. In addition, approximately 29% of the Company’s total revenues were derived from product sales to international customers, none of whom individually accounted for more than 5% of total revenues. As of May 30, 2010, Costco Wholesale Corporation represented approximately 15% of total accounts receivable.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that their carrying amounts may not be recoverable. Recoverability of assets is measured by comparison of the carrying amount of the asset to the net undiscounted future cash flow expected to be generated from the asset. If the future undiscounted cash flows are not sufficient to recover the carrying value of the assets, the assets’ carrying value is adjusted to fair value.
The Company regularly evaluates its long-lived assets for indicators of possible impairment. On July 16, 2010, Aesthetic Science sold the rights to its Smartfil™ Injector System. The Company evaluated its cost method investment for impairment, utilizing a discounted cash flow analysis under the terms of the purchase agreement. Based on the terms of the agreement, the Company has determined that its investment is other than temporarily impaired and therefore recorded an impairment loss of $1.0 million as of May 30, 2010.
Financial Instruments
The Company’s financial instruments are primarily composed of marketable debt securities, commercial-term trade payables, grower advances, and notes receivable, as well as long-term notes receivables and debt instruments. For short-term instruments, the historical carrying amount is a reasonable estimate of fair value. Fair values for long-term financial instruments not readily marketable are estimated based upon discounted future cash flows at prevailing market interest rates. Based on these assumptions, management believes the fair market values of the Company’s financial instruments are not materially different from their recorded amounts as of May 29, 2011.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments and sales discounts. The allowance for doubtful accounts is based on review of the overall condition of accounts receivable balances and review of significant past due accounts. The allowance for doubtful accounts is based on specific identification of past due amounts and a general reserve for accounts over 90-days past due. The changes in the Company’s allowances for doubtful accounts are summarized in the following table (in thousands).
| | | Balance at beginning of period | | | Additions charged to costs and expenses | | | | | | | |
Year ended May 31, 2009 Allowance for doubtful accounts receivable | | $ | 169 | | | $ | - | | | $ | (4 | ) | | $ | 165 | |
| | | | | | | | | | | | | | | | | |
Year ended May 30, 2010 Allowance for doubtful accounts receivable | | $ | 165 | | | $ | 68 | | | $ | (44 | ) | | $ | 189 | |
| | | | | | | | | | | | | | | | | |
Year ended May 29, 2011 Allowance for doubtful accounts receivable | | $ | 189 | | | $ | 209 | | | $ | (56 | ) | | $ | 342 | |
Revenue Recognition
Revenue from product sales is recognized when there is persuasive evidence that an arrangement exists, title has transferred, the price is fixed and determinable, and collectibility is reasonably assured. Allowances are established for estimated uncollectible amounts, product returns, and discounts based on specific identification and historical losses.
| 1. | Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued) |
The Company takes title to all produce it trades and/or packages, and therefore, records revenues and cost of sales at gross amounts in the Consolidated Statements of Income.
Licensing revenue is recognized in accordance with accounting guidance. Initial license fees are deferred and amortized to revenue over the period of the agreement when a contract exists, the fee is fixed and determinable,
and collectibility is reasonably assured. Noncancellable, nonrefundable license fees are recognized over the period of the agreement, including those governing research and development activities and any related supply agreement entered into concurrently with the license when the risk associated with commercialization of a product is non-substantive at the outset of the arrangement.
Contract revenue for research and development (R&D) is recorded as earned, based on the performance requirements of the contract. Non-refundable contract fees for which no further performance obligations exist, and there is no continuing involvement by the Company, are recognized on the earlier of when the payments are received or when collection is assured.
Other Accounting Policies and Disclosures
Cash, Cash Equivalents and Marketable Securities
The Company records all highly liquid securities with three months or less from date of purchase to maturity as cash equivalents and consists mainly of certificate of deposits (CDs), money market funds and U.S. Treasuries. Short-term marketable securities consist of CDs that are FDIC insured and single A or better rated municipal bonds with original maturities of more than three months at the date of purchase regardless of the maturity date as the Company views the funds within its portfolio as available for use in its current operations. The aggregate amount of CDs included in marketable securities at May 29, 2011 and May 30, 2010 was zero and $1.5 million, respectively. The Company classifies all debt securities with readily determined market values as “available for sale”. The contractual maturities of the Company's marketable securities that are due in less than one year represent $21.5 million of its marketable securities and those due in one to two years represent the remaining $6.6 million of the Company’s marketable securities as of May 29, 2011. These investments are classified as marketable securities on the consolidated balance sheet as of May 29, 2011 and May 30, 2010 and are carried at fair market value. Unrealized gains and losses are reported as a component of stockholders’ equity. The cost of debt securities is adjusted for amortization of premiums and discounts to maturity. This amortization is recorded to interest income. Realized gains and losses on the sale of available-for-sale securities are also recorded to interest income and were not significant for the fiscal years ended May 29, 2011 and May 30, 2010. During fiscal years 2011 and 2010, the Company received proceeds of $27.3 million and $9.5 million, respectively, from the sale of marketable securities. The cost of securities sold is based on the specific identification method.
Inventories
Inventories are stated at the lower of cost (using the first-in, first-out method) or market. As of May 29, 2011 and May 30, 2010 inventories consisted of (in thousands):
| | | May 29, 2011 | | | May 30, 2010 | |
| Finished goods | | $ | 10,261 | | | $ | 7,226 | |
| Raw materials | | | 7,999 | | | | 6,868 | |
| Work in progress | | | 1,901 | | | | 2,013 | |
| Inventories, net | | $ | 20,161 | | | $ | 16,107 | |
If the cost of the inventories exceeds their expected market value, provisions are recorded currently for the difference between the cost and the market value. These provisions are determined based on specific identification for unusable inventory and an additional reserve, based on historical losses, for inventory currently considered to be useable.
| 1. | Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued) |
Advertising Expense
Advertising expenditures for the Company are expensed as incurred. Advertising expense for the Company for fiscal years 2011, 2010 and 2009 was $458,000, $557,000 and $475,000, respectively.
Notes and Advances Receivable
Apio has made advances to produce growers for crop and harvesting costs. Notes and advances receivable related to operating activities are for the sourcing of crops for Apio’s business and notes and advances receivable related to investing activities are for financing transactions with third parties. Typically operating advances are paid off within the growing season (less than one year) from harvested crops. Advances not fully paid during the current growing season are converted to interest bearing obligations, evidenced by contracts and notes receivable. These notes and advances receivable are secured by perfected liens on land and/or crops and have terms that range from six to twelve months. Notes receivable are periodically reviewed (at least quarterly) for collectibility. A reserve is established for any note or advance deemed to not be fully collectible based upon an estimate of the crop value or the fair value of the security for the note or advance.
Related Party Transactions
Apio provides cooling and distributing services for farms in which the Chairman of Apio (the “Apio Chairman”) has a financial interest and purchases produce from those farms. Apio also purchases produce from Beachside Produce LLC for sale to third parties. Beachside Produce is owned by a group of entities and persons, including the Apio Chairman, that supply produce to Apio. Revenues and the resulting accounts receivable and cost of product sales and the resulting accounts payable are classified as related party items in the accompanying financial statements as of May 29, 2011 and May 30, 2010 and for the three years ended May 29, 2011.
Prior to the expiration of the leases in December 2009, Apio leased, for approximately $310,000 on an annual basis, agricultural land that is owned by the Apio Chairman. Apio, in turn, subleased that land at cost to growers who were obligated to deliver product from that land to Apio for value added products. There was generally no net statement of income impact to Apio as a result of these leasing activities but Apio created a guaranteed source of supply for the value added business. Apio had loss exposure on the leasing activity to the extent that it was unable to sublease the land. For the years ended May 30, 2010 and May 31, 2009, the Company subleased all of the land leased from the Apio Chairman and received sublease income of $150,000 and $316,000, respectively, which is substantially equal to the amount the Company paid to lease that land for such periods.
Apio's domestic commodity vegetable business was sold to Beachside Produce in 2003. The Apio Chairman is a 12.5% owner in Beachside Produce. During fiscal years 2011, 2010 and 2009, the Company recognized revenues of $726,000, $853,000 and $1.3 million, respectively, from the sale of products to Beachside Produce. The related accounts receivable from Beachside Produce are classified as related party in the accompanying Consolidated Balance Sheets as of May 29, 2011 and May 30, 2010.
The Apio Chairman and Windset Holdings 2010 Ltd., a Canadian corporation (“Windset”), entered into a land lease in July 2009, which included an option to purchase the land in Santa Maria, California for $10.5 million. Windset exercised its option to purchase the land from the Apio Chairman on March 2, 2011. Windset intends to initially construct 64 acres of indoor vegetable production along with the required support facilities for growing, harvesting, grading and selling numerous varieties of hydroponically grown tomatoes.
Apio purchases produce from Windset for sale to third parties. Apio has a 20.1% equity interest in Windset (see Note 3). During fiscal year 2011, Apio purchased $153,000 of produce from Windset.
All related party transactions are monitored quarterly by the Company and approved by the Audit Committee of the Board of Directors.
| 1. | Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued) |
Property and Equipment
Property and equipment are stated at cost. Expenditures for major improvements are capitalized while repairs and maintenance are charged to expense. Depreciation is expensed on a straight-line basis over the estimated useful lives of the respective assets, generally three to thirty years for buildings and leasehold improvements and three to seven years for furniture and fixtures, computers, capitalized software, machinery, equipment and autos. Leasehold improvements are amortized over the lesser of the economic life of the improvement or the life of the lease on a straight-line basis.
The Company capitalizes software development costs for internal use in accordance with accounting guidance. Capitalization of software development costs begins in the application development stage and ends when the asset is placed into service. The Company amortizes such costs using the straight-line basis over estimated useful lives of three to seven years. The Company did not capitalize any software development costs during fiscal years 2011 or 2010.
Intangible Assets
The Company’s intangible assets are comprised of customer relationships with an estimated useful life of twelve years and trademarks/trade names and goodwill with indefinite lives (collectively, “intangible assets”), which the Company recognized in accordance with accounting guidance (i) upon the acquisition of Lifecore in April 2010, our HA-based Biomaterials reporting unit, (ii) upon the acquisition of Apio in December 1999, which consists of our Food Products Technology and Export reporting units and (iii) from the repurchase of all non controlling interests in the common stock of Landec Ag in December 2006. Accounting guidance defines goodwill as “the excess of the cost of an acquired entity over the net of the estimated fair values of the assets acquired and the liabilities assumed at date of acquisition.” All intangible assets, including goodwill, associated with the acquisitions of Lifecore and Apio were allocated to our HA-based Biomaterials reporting unit and our Food Products Technology reporting unit, respectively, pursuant to accounting guidance based upon the allocation of assets and liabilities acquired and consideration paid for each reporting unit. The consideration paid for the Export reporting unit approximated its fair market value at the time of acquisition, and therefore, no intangible assets were recorded in connection with the Company’s acquisition of this reporting unit. Goodwill associated with the Technology Licensing reporting unit consists entirely of goodwill resulting from the repurchase of the Landec Ag non controlling interests. As of May 29, 2011, the HA-based Biomaterials reporting unit had $13.9 million of goodwill and the Food Products Technology reporting unit had $22.6 million of goodwill. As described below, the $4.8 million of goodwill in the Technology Licensing reporting unit was written off as of May 29, 2011.
The Company tests its intangible assets for impairment at least annually, in accordance with accounting guidance. When evaluating indefinite-lived intangible assets for impairment, accounting guidance requires the Company to compare the fair value of the asset to its carrying value to determine if there is an impairment loss. When evaluating goodwill for impairment, accounting guidance requires the Company to first compare the fair value of the reporting unit to its carrying value to determine if there is an impairment loss. If the fair value of the reporting unit exceeds its carrying value, goodwill is not considered impaired; thus application of the second step of the two-step approach under accounting guidance is not required. Application of the intangible assets impairment tests requires significant judgment by management, including identification of reporting units, assignment of assets and liabilities to reporting units, assignment of intangible assets to reporting units, and the determination of the fair value of each indefinite-lived intangible asset and reporting unit based upon projections of future net cash flows, discount rates and market multiples, which judgments and projections are inherently uncertain.
Property, plant and equipment and finite-lived intangible assets are reviewed for possible impairment whenever events or changes in circumstances occur that indicate that the carrying amount of an asset (or asset group) may not be recoverable. The Company’s impairment review requires significant management judgment including estimating the future success of product lines, future sales volumes, revenue and expense growth rates, alternative uses for the assets and estimated proceeds from the disposal of the assets. The Company conducts quarterly reviews of idle and underutilized equipment, and reviews business plans for possible impairment indicators. Impairment occurs when the carrying amount of the asset (or asset group) exceeds its estimated future undiscounted cash flows and the impairment is viewed as other than temporary. When impairment is indicated, an impairment charge is recorded for the difference between the asset’s book value and its estimated fair value. Depending on the asset, estimated fair value may be determined either by use of a discounted cash flow model or by reference to estimated selling values of assets in similar condition. The use of different assumptions would increase or decrease the estimated fair value of assets and would increase or decrease any impairment measurement.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
The Company tested its indefinite-lived intangible assets and goodwill for impairment as of July 24, 2011 and determined that no adjustments to the carrying values of the intangible assets were necessary as of that date for its HA-based Biomaterials and Food Products Technology reporting units. For the Technology Licensing reporting unit during the fourth quarter of fiscal year 2011 it became apparent to the Company that acceptable biological test results are probably not achievable in the four months left in the agreement before Monsanto has to make its purchase option decision (see Note 4). The uncertainty related to whether Monsanto will exercise its purchase option for the licensed fields of technology, and the fact that Landec Ag is projected to be unprofitable for several years absent any ongoing relationship with Monsanto, were the main factors contributing to the significant decrease in the estimated fair value of the Landec Ag business and as a result the goodwill of the Technology Licensing reporting unit was determined to be fully impaired as of May 29, 2011, and therefore, the Company wrote off the entire $4.8 million of goodwill associated with the Technology Licensing reporting unit. As of May 29, 2011, there were no impairment indicators identified by the Company in its analysis of impairment associated with the acquired indefinite-lived intangible assets. On a quarterly basis, the Company considers the need to update its most recent annual tests for possible impairment of its intangible assets, based on management’s assessment of changes in its business and other economic factors since the most recent annual evaluation. Such changes, if significant or material, could indicate a need to update the most recent annual tests for impairment of the intangible assets during the current period. The results of these tests could lead to write-downs of the carrying values of the intangible assets in the current period.
The Company uses the discounted cash flow (“DCF”) approach to develop an estimate of fair value. The DCF approach recognizes that current value is premised on the expected receipt of future economic benefits. Indications of value are developed by discounting projected future net cash flows to their present value at a rate that reflects both the current return requirements of the market and the risks inherent in the specific investment. The market approach was not used to value the Food Products Technology, Hyaluronan-based Biomaterials and Technology Licensing reporting units (the “Reporting Units”) because insufficient market comparables exist to enable the Company to develop a reasonable fair value of its intangible assets due to the unique nature of each of the Company’s Reporting Units.
The DCF approach requires the Company to exercise judgment in determining future business and financial forecasts and the related estimates of future net cash flows. Future net cash flows depend primarily on future product sales, which are inherently difficult to predict. These net cash flows are discounted at a rate that reflects both the current return requirements of the market and the risks inherent in the specific investment.
The DCF associated with the Food Products Technology reporting unit is based on management’s five-year projection of revenues, gross profits and operating profits by fiscal year and assumes a 38% effective tax rate for each year. Management takes into account the historical trends of Apio and the industry categories in which Apio operates along with inflationary factors, current economic conditions, new product introductions, cost of sales, operating expenses, capital requirements and other relevant data when developing its projection. The estimated fair value of the Food Products Technology reporting units as of July 24, 2011 exceeded its book value by 55%, and therefore, no intangible asset impairment was deemed to exist. For the test performed as of July 25, 2010, the projected cash flow from operations for determining the DCF for fiscal year 2011 was $16.6 million for the Food Products Technology reporting unit. The actual cash flow from operations for fiscal year 2011 was $12.1 million. The difference of $4.5 million was a result of increased produce sourcing costs due to weather-related produce shortages which the Company had no way of foreseeing.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
The fair value of indefinite and finite-lived intangible assets associated with our acquisition of Lifecore on April 30, 2010, was determined using a DCF model based on management’s five-year projections of revenues, gross profits and operating profits by fiscal year and assumes a 38% effective tax rate for each year. Management takes into account the historical trends of Lifecore and the industry categories in which Lifecore operates along with inflationary factors, current economic conditions, new product introductions, cost of sales, operating expenses, capital requirements and other relevant data when developing its projection. The trade name intangible asset was valued using the relief from royalty valuation method and the customer relationship intangible asset was valued using the multi-period excess earnings method. The fair value of goodwill was calculated as the excess of consideration paid, including the fair value of contingent consideration under the terms of the purchase agreement, over the fair value of the tangible and intangible assets acquired less liabilities assumed. The Company updated its analysis of the fair value of the indefinite-lived intangible assets and goodwill as of its annual impairment analysis date, concluding that the fair value of the Hyaluronan-based Biomaterials reporting unit, as determined by the DCF approach, exceeded its book value by 71%, and therefore, no intangible asset impairment was deemed to exist. For the test performed as of July 25, 2010, the projected cash flow from operations for determining the DCF for fiscal year 2011 was $3.1million for the Hyaluronan-based Biomaterials reporting unit. The actual cash flow from operations for fiscal year 2011 was $9.0 million. The difference of $5.9 million is due to Lifecore exceeding its planned net income by $2.0 million and the change in working capital being much more favorable than planned.
Investment in Non-Public Company
The Company’s investment in Aesthetic Science is carried at cost and adjusted for impairment losses. Since there is no readily available market value information, the Company periodically reviews this investment to determine if any other than temporary declines in value have occurred based on the financial stability and viability of Aesthetic Science. Aesthetic Science sold the rights to its Smartfil™ Injector System on July 16, 2010. Landec evaluated its cost method investment for impairment, utilizing a discounted cash flow analysis under the terms of the purchase agreement. Based on the terms of the agreement, the Company determined that its investment was other than temporarily impaired and therefore recorded an impairment loss of $1.0 million as of May 30, 2010, which is classified as part of general and administrative expenses in the Consolidated Statements of Income. The Company’s carrying value of its investment in Aesthetic Sciences, net of the impairment loss, is $793,000 at May 29, 2011 and May 30, 2010 and is reported as a component of other non current assets.
On February 15, 2011, the Company made an investment in Windset Holdings 2010 Ltd., a Canadian corporation (“Windset”), which is reported as an investment in non-public company, fair value, in the accompanying consolidated balance sheets as of May 29, 2011. The Company has elected to account for its investment in Windset under the fair value option (see Note 3).
Deferred Revenue
Cash received in advance of services performed (principally revenues related to upfront license fees) are recorded as deferred revenue. At May 29, 2011, $2.3 million has been recognized as a liability for deferred license fee revenues and $357,000 for advances from customers. At May 30, 2010, $4.3 million has been recognized as a liability for deferred license fee revenues and $91,000 for advances from customers.
Comprehensive Loss
Comprehensive loss consists of net income and other comprehensive income for which Landec includes changes in unrealized gains and losses on its interest rate swap with Wells Fargo Bank, N.A. Accumulated other comprehensive loss is reported as a component of stockholders’ equity. For the fiscal year ended May 29, 2011, the comprehensive loss from the unrealized loss on the interest rate swap, net of $159,000 of income taxes, was $267,000. For the fiscal year ended May 30, 2010, the comprehensive loss from the unrealized loss on the interest rate swap, net of $105,000 of income taxes, was $179,000. There was no comprehensive income or loss in the fiscal year ended May 31, 2009.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
Non Controlling Interest
The Company reports all non controlling interests as a separate component of stockholders’ equity, the reporting of consolidated net income (loss) as the amount attributable to both the parent and the non controlling interests and the separate disclosure of net income (loss) attributable to the parent and to the non controlling interests. Changes in a parent’s ownership interest while the parent retains its controlling interest will be accounted for as equity transactions and any retained non controlling equity investment upon the deconsolidation of a subsidiary will be initially measured at fair value.
In connection with the acquisition of Apio, Landec acquired Apio’s 60% general partner interest in Apio Cooling, a California limited partnership. Apio Cooling is included in the consolidated financial statements of Landec for all periods presented. The non-controlling interest balance of $1.7 million at both May 29, 2011 and May 30, 2010 is comprised of the limited partners’ interest in Apio Cooling.
Income Taxes
The Company accounts for income taxes in accordance with accounting guidance which requires that deferred tax assets and liabilities be recognized using enacted tax rates for the effect of temporary differences between the book and tax bases of recorded assets and liabilities. The Company maintains valuation allowances when it is likely that all or a portion of a deferred tax asset will not be realized. Changes in valuation allowances from period to period are included in the Company’s income tax provision in the period of change. In determining whether a valuation allowance is warranted, the Company takes into account such factors as prior earnings history, expected future earnings, unsettled circumstances that, if unfavorably resolved, would adversely affect utilization of a deferred tax asset, carryback and carryforward periods, and tax strategies that could potentially enhance the likelihood of realization of a deferred tax asset. At May 29, 2011, the Company had $383,000 valuation allowance against deferred tax assets.
In addition to valuation allowances, the Company establishes accruals for uncertain tax positions. The tax-contingency accruals are adjusted in light of changing facts and circumstances, such as the progress of tax audits, case law and emerging legislation. The Company recognizes interest and penalties related to uncertain tax positions as a component of income tax expense. The Company’s effective tax rate includes the impact of tax-contingency accruals as considered appropriate by management.
A number of years may elapse before a particular matter, for which the Company has accrued, is audited and finally resolved. The number of years with open tax audits varies by jurisdiction. While it is often difficult to predict the final outcome or the timing of resolution of any particular tax matter, the Company believes its tax-contingency accruals are adequate to address known tax contingencies. Favorable resolution of such matters could be recognized as a reduction to the Company’s effective tax rate in the year of resolution. Unfavorable settlement of any particular issue could increase the effective tax rate. Any resolution of a tax issue may require the use of cash in the year of resolution. The Company’s tax-contingency accruals are presented in the balance sheet within accrued liabilities.
Per Share Information
Accounting guidance requires the presentation of basic and diluted earnings per share. Basic earnings per share excludes any dilutive effects of options, warrants and convertible securities and is computed using the weighted average number of common share outstanding. Diluted earnings per share reflects the potential dilution if securities or other contracts to issue common stock were exercised or converted into common stock. Diluted common equivalent shares consist of stock options using the treasury stock method.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
The following table sets forth the computation of diluted net income per share (in thousands, except per share amounts):
| | | Fiscal Year Ended May 29, 2011 | | | Fiscal Year Ended May 30, 2010 | | | Fiscal Year Ended May 31, 2009 | |
| Numerator: | | | | | | | | | |
| Net income applicable to Common Stockholders | | $ | 3,920 | | | $ | 3,984 | | | $ | 7,730 | |
| Denominator: | | | | | | | | | | | | |
| Weighted average shares for basic net income per share | | | 26,397 | | | | 26,382 | | | | 26,202 | |
| Effect of dilutive securities: | | | | | | | | | | | | |
| Stock options | | | 229 | | | | 251 | | | | 549 | |
| Weighted average shares for diluted net income per share | | | 26,626 | | | | 26,633 | | | | 26,751 | |
| | | | | | | | | | | | | |
| Diluted net income per share | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.29 | |
Options to purchase 2,032,867, 1,016,239 and 357,514 shares of Common Stock at a weighted average exercise price of $6.67, $7.62 and $9.72 per share were outstanding during fiscal years ended May 29, 2011, May
30, 2010 and May 31, 2009, respectively, but were not included in the computation of diluted net income per share because the options’ exercise price were greater than the average market price of the Common Stock and, therefore, the effect would be antidilutive.
Cost of Sales
The Company includes in cost of sales all the costs related to the sale of products in accordance with generally accepted accounting principles. These costs include the following: raw materials (including produce, seeds, packaging, syringes and fermentation and purification supplies), direct labor, overhead (including indirect labor, depreciation, and facility related costs) and shipping and shipping related costs.
Research and Development Expenses
Costs related to both research contracts and Company-funded research is included in research and development expenses. Costs to fulfill research contracts generally approximate the corresponding revenue. Research and development costs are primarily comprised of salaries and related benefits, supplies, travel expenses, consulting expenses and corporate allocations.
Accounting for Stock-Based Compensation
The Company records compensation expense for stock-based awards issued to employees and directors in exchange for services provided based on the estimated fair value of the awards on their grant dates and is recognized over the required service periods. The cash flows resulting from the tax benefit due to tax deductions in excess of the compensation expense recognized for those options (excess tax benefit) are classified as financing activities with the statement of cash flows. The Company’s stock-based awards include stock option grants and restricted stock unit awards (RSUs).
During the fiscal year ended May 29, 2011, the Company recognized stock-based compensation expense of $1,951,000 which included $857,000 for restricted stock unit awards and $1,094,000 for stock option grants. During the fiscal year ended May 30, 2010, the Company recognized stock-based compensation expense of $1,016,000 which included $474,000 for restricted stock unit awards and $542,000 for stock option grants. During the fiscal year ended May 31, 2009, the Company recognized stock-based compensation expense of $933,000 which included $308,000 for restricted stock unit awards and $625,000 for stock option grants.
| 1. | Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued) |
The following table summarizes the stock-based compensation by income statement line item:
| | | Fiscal Year Ended May 29, 2011 | | | Fiscal Year Ended May 30, 2010 | | | Fiscal Year Ended May 31, 2009 | |
| Research and development | | $ | 565,000 | | | $ | 185,000 | | | $ | 171,000 | |
| Sales, general and administrative | | | 1,386,000 | | | | 831,000 | | | | 762,000 | |
| Total stock-based compensation expense | | $ | 1,951,000 | | | $ | 1,016,000 | | | $ | 933,000 | |
The estimated fair value for stock options, which determines the Company’s calculation of compensation expense, is based on the Black-Scholes option pricing model. The Company uses the straight line single option method to calculate and recognize the fair value of stock-based compensation arrangements. Compensation expense for all stock option and restricted stock awards granted prior to May 29, 2006 will continue to be recognized using the straight-line, multiple-option method. In addition, the Company uses historical data to estimate pre-vesting forfeitures and records stock-based compensation expense only for those awards that are expected to vest and revises those estimates in subsequent periods if the actual forfeitures differ from the prior estimates.
As of May 29, 2011, May 30, 2010 and May 31, 2009, the fair value of stock option grants was estimated using the Black-Scholes option pricing model. The following weighted average assumptions were used:
| | | Fiscal Year Ended May 29, 2011 | | | Fiscal Year Ended May 30, 2010 | | | Fiscal Year Ended May 31, 2009 | |
| Expected life (in years) | | | 3.76 | | | | 3.56 | | | | 3.78 | |
| Risk-free interest rate | | | 1.16 | % | | | 1.47 | % | | | 2.35 | % |
| Volatility | | | 0.52 | | | | 0.52 | | | | 0.52 | |
| Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
The Black-Scholes option pricing model requires the input of highly subjective assumptions, including the expected stock price volatility.
The weighted average estimated fair value of Landec employee stock options granted at grant date market prices during the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009 was $2.42, $2.52 and $2.74 per share, respectively. No stock options were granted above or below grant date market prices during the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009.
Fair Value Measurements
The Company uses fair value measurement accounting for financial assets and liabilities and for financial instruments and certain other items at fair value. The Company has elected the fair value option for its investment in a non-public company (see Note 3). The Company has not elected the fair value option for any of its other eligible financial assets or liabilities.
The accounting guidance established a three-tier hierarchy for fair value measurements, which prioritizes the inputs used in measuring fair value as follows:
| | Level 1 – | observable inputs such as quoted prices for identical instruments in active markets. |
| | Level 2 – | inputs other than quoted prices in active markets that are observable either directly or indirectly through corroboration with observable market data. |
| | Level 3 – | unobservable inputs in which there is little or no market data, which would require the Company to develop its own assumptions. |
As of May 29, 2011, the Company held certain assets and liabilities that are required to be measured at fair value on a recurring basis, including cash equivalents, marketable securities, interest rate swap, liability for contingent consideration in connection with the acquisition of Lifecore and its minority interest investment in Windset.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
The fair value of the Company’s cash equivalents and marketable securities is determined based on observable inputs that are readily available in public markets or can be derived from information available in publicly quoted markets. Therefore, the Company has categorized its cash equivalents and marketable securities as Level 1.
The fair value of the Company’s interest rate swap is determined based on model inputs that can be observed in a liquid market and key inputs include yield curves and are categorized as Level 2 inputs. As of May 29, 2011, the Company recorded to other comprehensive loss on the consolidated balance sheets an unrealized loss of $267,000, net of taxes of $159,000, representing the cumulative change in the interest rate swap since inception. If the interest rate swap is terminated or the debt borrowed is paid off prior to April 30, 2015, the amount of unrealized loss or gain included in other comprehensive income (loss) would be reclassified to earnings. The Company has no intentions of terminating the interest rate swap or prepaying the debt in the next twelve months. The interest rate swap liability is included in other non-current liabilities as of May 29, 2011 and May 30, 2010.
The fair value of the Company’s liability for contingent consideration is based on significant inputs not observed in the market and thus represents a Level 3 measurement. The Company determined the fair value of the liability for the contingent consideration based on a probability-weighted discounted cash flow analysis, as further discussed in Note 2.
The Company has elected the fair value option of accounting for its investment in Windset. The fair value of the Company’s investment in Windset utilizes significant unobservable inputs in the discounted cash flow models, including projected cash flows, growth rates and the discount rate, and is therefore considered Level 3, as further discussed in Note 3.
Imprecision in estimating unobservable market inputs can affect the amount of gain or loss recorded for a particular position. Furthermore, the Company believes its valuation methods are appropriate and consistent with those of other market participants. The use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
The Company has no other financial assets or liabilities that fair value measurement has been adopted.
Recent Accounting Pronouncements
Recently Adopted Pronouncements
Fair Value Measurements
In January 2010, the FASB issued new accounting guidance related to the disclosures for transfers in and out of Levels 1 and 2 fair value measurements and the activity in Level 3 fair value measurements. The amendment recommends a reporting entity should disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and describe the reasons for the transfers. Further, in the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuances and settlements (that is, on a gross basis rather than as one net number). Also, the amendment requires clarification in existing disclosures for disaggregation of fair value measurement disclosures for each class of assets and liabilities and disclosures about inputs and valuation techniques. The effective date is for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company adopted all the amended provisions of new guidance in the first quarter of fiscal year 2011 and such adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
Variable Interest Entities
In June 2009, the FASB issued new guidance which amends the evaluation criteria to identify the primary beneficiary of a VIE. Additionally, the new guidance requires ongoing reassessments of whether an enterprise is the primary beneficiary of the VIE. The Company adopted the new guidance on May 31, 2010 and such adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
1. Organization, Basis of Presentation, and Summary of Significant Accounting Policies (continued)
Revenue Recognition
In October 2009, the FASB issued new guidance in relation to "Multiple-Deliverable Revenue Arrangements". The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration to each deliverable to be based on the relative selling price. The Company early adopted these standards as of May 31, 2010. There have been no materially modified agreements since the adoption of the standard. The adoption did not have an impact on the Company’s results of operations or financial position for the fiscal year ended May 29, 2011.
Recently Issued Pronouncements
Comprehensive Income
In June 2011, the FASB issued new guidance that improves the comparability, consistency, and transparency of financial reporting and increases the prominence of items reported in other comprehensive income by eliminating the option to present components of other comprehensive income as part of the statement of changes in stockholders' equity. The amendments in this standard require that all nonowner changes in stockholders' equity be presented either in a single continuous statement of comprehensive income or in two separate but consecutive statements. Under either method, adjustments must be displayed for items that are reclassified from other comprehensive income ("OCI") to net income, in both net income and OCI. The standard does not change the current option for presenting components of OCI gross or net of the effect of income taxes, provided that such tax effects are presented in the statement in which OCI is presented or disclosed in the notes to the financial statements. Additionally, the standard does not affect the calculation or reporting of earnings per share. For public entities, the amendments in this ASU are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011 and are to be applied retrospectively, with early adoption permitted. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
Revenue Recognition
In April 2010, the FASB issued guidance on applying the milestone method of revenue recognition in arrangements with research and development activities. These amendments are effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. The Company's adoption of the provisions of this update is not expected to have a material impact on its revenue recognition in the Company’s fiscal year beginning on May 30, 2011.
Subsequent Events
In February 2010, the FASB issued updates to the subsequent events guidance requiring an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement for an SEC filer to disclose a date, in both issued and revised financial statements, through which the filer had evaluated subsequent events. The adoption is not expected to have an impact on the Company's financial position, results of operations or cash flows upon adoption in the Company’s fiscal year beginning on May 30, 2011.
Business Combinations
In December 2010, the FASB issued an update requiring a public entity to disclose pro forma information for business combinations that occurred in the current reporting period. The disclosures include pro forma revenue and earnings of the combined entity for the current reporting period as though the acquisition date for all business combinations that occurred during the year had been as of the beginning of the annual reporting period. If comparative financial statements are presented, the pro forma revenue and earnings of the combined entity for the comparable prior reporting period should be reported as though the acquisition date for all business combinations that occurred during the current year had been as of the beginning of the comparable prior annual reporting period. These amendments affect any public entity as defined by US GAAP that enters into business combinations that are material on an individual or aggregate basis, and are effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. The Company does not expect the provisions of this update to have a material effect on its financial position, results of operations or cash flows upon adoption in its fiscal year beginning on May 30, 2011.
2. Acquisition of Lifecore Biomedical, Inc.
On April 30, 2010 (the “Acquisition Date”), the Company acquired all of the common stock of Lifecore Biomedical, Inc. (“Lifecore”) under a Stock Purchase Agreement (“Purchase Agreement”) in order to expand its product offerings and enter into new markets. Lifecore was a privately-held hyaluronan-based biomaterials company located in Chaska, Minnesota. Lifecore is principally involved in the development and manufacture of products utilizing hyaluronan, a naturally occurring polysaccharide that is widely distributed in the extracellular matrix of connective tissues in both animals and humans. In 2007, Lifecore entered into a world-wide exclusive license and development agreement withThe Cleveland Clinic Foundation to develop and commercialize hyaluronan-based products and related applications. The license is for patented hyaluronan-based cross-linking technology, Corgel ™ Biohydrogel products, that can be used for products in aesthetics, orthopedics, ophthalmology and other medical fields. Lifecore has not yet identified any potential commercial products for this technology; however Landec will continue to investigate potential applications.
Under the Purchase Agreement, the aggregate consideration payable by the Company to the former Lifecore stockholder at closing consisted of $40.0 million in cash, which included $6.6 million that is held in an escrow account to secure the indemnification rights of Landec and other indemnities with respect to certain matters, including breaches of representations, warranties and covenants included in the Purchase Agreement. The escrow account is in the name of the seller and Landec’s right under the escrow agreement consist solely of its ability to file a claim against the escrow. Half of the escrow or $3.3 million was released and paid to the former Lifecore shareholder in May 2011. In addition, the Company may be required to pay in cash up to an additional $10.0 million in earnout payments in the event that Lifecore achieves certain revenue targets in calendar years 2011 and 2012.
The acquisition date fair value of the total consideration transferred was $49.65 million, which consisted of the following (in thousands):
| Cash | | $ | 40,000 | |
| Contingent consideration | | | 9,650 | |
| Total | | $ | 49,650 | |
The assets and liabilities of Lifecore were recorded at their respective estimated fair values as of the date of the acquisition using generally accepted accounting principles for business combinations. The excess of the purchase price over the fair value of the net identifiable assets acquired has been allocated to goodwill. Goodwill represents a substantial portion of the acquisition proceeds because of the workforce in-place at acquisition and because of Lifecore’s long history and future prospects. Management believes that there is further growth potential by extending Lifecore’s product lines into new channels.
2. Acquisition of Lifecore Biomedical, Inc. (continued)
The following table summarizes the estimated fair values of Lifecore’s assets acquired and liabilities assumed and related deferred income taxes, effective April 30, 2010, the date the Company obtained control of Lifecore (in thousands).
| Cash and cash equivalents | | $ | 318 | |
| Accounts receivable, net | | | 1,860 | |
| Inventories, net | | | 9,009 | |
| Property and equipment | | | 25,529 | |
| Other tangible assets | | | 1,455 | |
| Intangible assets | | | 7,900 | |
| Total identifiable assets acquired | | | 46,071 | |
| Accounts payable and other liabilities | | | (2,983 | ) |
| Long-term debt | | | (4,157 | ) |
| Deferred taxes | | | (3,162 | ) |
| Total liabilities assumed | | | (10,302 | ) |
| Net identifiable assets acquired | | | 35,769 | |
| Goodwill | | | 13,881 | |
| Net assets acquired | | $ | 49,650 | |
The Company used a combination of the market and cost approaches to estimate the fair values of the Lifecore assets acquired and liabilities assumed. During the measurement period (which is not to exceed one year from the acquisition date), the Company is required to retrospectively adjust the provisional assets or liabilities if new information is obtained about facts and circumstances that existed as of the acquisition date that, if known, would have resulted in the recognition of those assets or liabilities as of that date. The Company has finalized the fair values of the acquired assets and assumed liabilities and has completed the purchase price allocation as of April 30, 2011.
A step-up in the value of inventory of $523,000 was recorded in the allocation of the purchase price based on valuation estimates. During fiscal year 2011, the remaining $496,000 of step-up was charged to cost of products sold as the inventory was sold.
The Company identified two intangible assets in connection with the Lifecore acquisition: trade names valued at $4.2 million, which is considered to be an indefinite life asset and therefore will not be amortized; and customer base valued at $3.7 million with a twelve year useful life. The trade name intangible asset was valued using the relief from royalty valuation method and the customer relationship intangible asset was valued using the multi-period excess earnings method.
The excess of the consideration transferred over the fair values assigned to the assets acquired and liabilities assumed was $13.9 million, which represents the goodwill amount resulting from the acquisition which can be attributable to its work force in place at the time of the acquisition and to Lifecore’s long history and future prospects. None of the goodwill is expected to be deductible for income tax purposes. The Company will test goodwill for impairment on an annual basis or sooner, if deemed necessary. During fiscal year 2011, goodwill increased $88,000 primarily due to adjustments in Lifecore’s deferred tax balance as of the acquisition date.
2. Acquisition of Lifecore Biomedical, Inc. (continued)
Liability for Contingent Consideration
In addition to the cash consideration paid to the former shareholder of Lifecore, the Company may be required to pay up to an additional $10.0 million in earnout payments based on Lifecore achieving certain revenue targets in calendar years 2011 and 2012. The fair value of the liability for the contingent consideration recognized on the acquisition date was $9.84 million and $9.65 million, as of May 29, 2011 and May 30, 2010, respectively, and is classified as a non current liability in the Consolidated Balance Sheets and the $190,000 change in the fair value of the liability is netted against other income in the Consolidated Statements of Income. The Company projects that it will pay the entire $10 million earn out during the third quarter of fiscal year 2012.
| 3. | Investments in non-public companies |
In December 2005, Landec entered into an exclusive licensing agreement with Aesthetic Sciences for the exclusive rights to use Landec's IntelimerÒ materials technology for the development of dermal fillers worldwide under the agreement. The Company received shares of preferred stock in exchange for the license with a valuation of $1.8 million. Aesthetic Sciences sold the rights to its Smartfil™ Injector System on July 16, 2010. Landec has evaluated its investment in Aesthetic Sciences for impairment, utilizing a discounted cash flow analysis under the terms of the purchase agreement. Based on the terms of the sale, the Company determined that its investment was other than temporarily impaired and therefore recorded an impairment charge of $1.0 million as of May 30, 2010. The Company’s carrying value of its investment in Aesthetic Sciences is $793,000 as of May 29, 2011 and May 30, 2010. No additional impairment has been determined for the Company’s investment in Aesthetic Sciences.
On February 15, 2011, Apio entered into a share purchase agreement (the “Purchase Agreement”) with Windset. Pursuant to the Purchase Agreement, Apio purchased 150,000 senior preferred shares for $15 million and 201 common shares for $201 that were issued by Windset (the “Purchased Shares”). The Company’s common shares represent a 20.1% interest in Windset. The non-voting senior preferred shares yield a cash dividend of 7.5% annually. The dividend is payable within 90 days of each anniversary of the execution of the Purchase Agreement. The Purchase Agreement includes a put and call option, which can be exercised on the sixth anniversary of the Purchase Agreement whereby Apio can exercise the put to sell its Purchased Shares to Windset, or Windset can exercise the call to purchase the Purchased Shares from Apio, in either case, at a price equal to 20.1% of the appreciation in the fair market value of Windset from the date of the Company’s investment through the put and call date, plus the purchase price of the Purchased Shares. Under the terms of the arrangement with Windset, the Company is entitled to designate one of five members on the Board of Directors of Windset.
In accordance with accounting guidance, the investment in Windset does not qualify for equity method accounting as the investment does not meet the criteria of in-substance common stock due to returns through the annual dividend on the non-voting senior preferred shares that are not available to the common stock holders. As the put and call options require the Purchased Shares to be put or called in equal proportions, the Company has deemed that the investment, in substance, should be treated as a single security for purposes of accounting. The Company has adopted fair value option in the accounting for its investment in Windset effective on the acquisition date. The Company believes that reporting its investment at fair value provides its investors with useful information on the performance of the Company’s investment and the anticipated appreciation in value as Windset expands its business.
The Company also entered into an exclusive license agreement with Windset, which was executed in June 2010, prior to contemplation of Apio’s investment in Windset (see Note 5).
The fair value of the Company’s investment in Windset was determined utilizing a discounted cash flow model based on projections developed by Windset, and considers the put and call conversion options. These features impact the duration of the cash flow utilized to derive the estimated fair value of the investment. The Company has concluded that the estimated fair value of its investment in Windset approximates the cash consideration paid for the Purchased Shares at the date of acquisition. Assumptions included in the discounted cash flow model will be evaluated quarterly based on Windset’s actual and projected operating results to determine the change in fair value.
| 3. | Investments in non-public companies (continued) |
From the close of the Purchase Agreement to May 29, 2011, the Company recorded $328,000 in dividend income. The change in the fair market value of the Company’s investment in Windset as of May 29, 2011 was determined to be $662,000 which is recorded as other income.
4. Sale of Fielder’s Choice Direct and License Agreement
On December 1, 2006, Landec entered into a five-year co-exclusive technology license and polymer supply agreement (“the Monsanto Agreement”) with Monsanto for the use of Landec’s Intellicoat polymer seed coating technology. Under the terms of the Monsanto Agreement, Monsanto agreed to pay Landec Ag $2.6 million per year. The Monsanto Agreement was amended in November 2009. Under the terms of the amended Monsanto Agreement, Monsanto continues to have an exclusive license to use Landec’s Intellicoat polymer technology for specific seed treatment applications. Over the remaining two-year term of the amended Monsanto Agreement, Monsanto will investigate uses of Landec’s Intellicoat technology in a variety of seed categories in the field exclusively licensed to Monsanto.
Along with regaining the use of the Intellicoat technology outside of the specific applications licensed to Monsanto under the amended Monsanto Agreement, Landec has assumed responsibility for Landec Ag’s operating expenses and realizes all the revenues and profits from the sales of existing and new Intellicoat seed coating products.
The Monsanto Agreement also provides for a fee payable to Landec Ag of $4 million if Monsanto elects to terminate the Monsanto Agreement or $10 million if Monsanto elects to purchase the rights to the exclusive field. If the purchase option is exercised before December 2011, or if Monsanto elects to terminate the Monsanto Agreement, all annual license fees and supply payments that have not been paid to Landec Ag will become due upon the purchase or termination. If Monsanto does not exercise its purchase option by December 2011 Landec Ag will receive the termination fee and all rights to the Intellicoat seed coating technology will revert to Landec. Accordingly, we will receive aggregate minimum guaranteed payments of $17 million for license fees and polymer supply payments over five years or $23 million in aggregate maximum payments if Monsanto elects to purchase the rights to the exclusive field. The minimum guaranteed payments and the deferred gain of $2 million per year described above will result in Landec recognizing revenue and operating income of $5.4 million per year for fiscal years 2008 through 2011 and $2.7 million per year for fiscal years 2007 and 2012. The incremental $6 million to be received in the event Monsanto exercises the purchase option has been deferred and will be recognized upon the exercise of the purchase option. The fair value of the purchase option was determined by management to be less than the amount of the deferred revenue.
If Monsanto elects to purchase the rights to the exclusive field, a gain or loss on the sale will be recognized at the time of purchase. If Monsanto exercises its purchase option, we expect to enter into a new long-term supply agreement with Monsanto pursuant to which Landec would continue to be the exclusive supplier of Intellicoat polymer materials to Monsanto.
5. Other License Agreements
In March 2006, Landec entered into an exclusive license and research and development agreement with Air Products and Chemicals, Inc. (“Air Products”). Landec will provide research and development support to Air Products for three years with a mutual option for two additional years. The license fees were recognized as license revenue over a three year period beginning March 2006. In addition, in accordance with the agreement, Landec receives 40% of the gross profit generated from the sale of products by Air Products occurring after April 1, 2007, that incorporate Landec’s Intelimer materials.
In September 2007, the Company amended its licensing and supply agreement with Chiquita Brands International, Inc. (“Chiquita”). Under the terms of the amendment, the license for bananas was expanded to include additional exclusive fields using Landec’s BreatheWay® packaging technology, and a new exclusive license was added for the sale and marketing of avocados and mangos using Landec’s BreatheWay packaging technology. The agreement with Chiquita, which terminates in December 2011 (subject to Chiquita’s five year renewal option), requires Chiquita to pay annual gross profit minimums to Landec in order for Chiquita to maintain its exclusive license for bananas, avocados and mangos. Under the terms of the agreement, Chiquita must notify Landec before December 1st of each year whether it is going to maintain its exclusive license for the following calendar year and thus agree to pay the minimums for that year. Landec was notified by Chiquita in November 2010 that Chiquita wanted to maintain its exclusive license for calendar year 2011 and thus agreed at that time to pay the minimum gross profit for calendar year 2011.
5. Other License Agreements (continues)
In June 2010, Apio entered into an exclusive license agreement with Windset for Windset to utilize Landec’s proprietary breathable packaging to extend the shelf life of greenhouse grown cucumbers, peppers and tomatoes (“exclusive products”). In accordance with the agreement, Apio received and recorded a one-time upfront research and development fee of $100,000 and will receive license fees equal to 3% of net revenue of the exclusive products utilizing the proprietary breathable packaging technology, with or without the BreatheWay® trademark. The ongoing license fees are subject to annual minimums of $150,000 for each of the three types of exclusive product as each is added to the agreement. As of May 29, 2011, only one product has been added to the agreement and the first year minimum payment period had an original payment date of June 2011. However, the original payment date of June 2011 has been deferred until April 2012 due to delays in obtaining the required packaging materials.
6. Property and Equipment
Property and equipment consists of the following (in thousands):
| | | Years of | | | | | | | |
| | | Useful Life | | | May 29, 2011 | | | May 30, 2010 | |
| Land and building | | 15-30 | | | $ | 43,885 | | | $ | 41,990 | |
| Leasehold improvements | | 3-20 | | | | 1,082 | | | | 1,111 | |
| Computer, capitalized software, machinery, equipment and auto | | 3-7 | | | | 35,024 | | | | 31,869 | |
| Furniture and fixtures | | 5-7 | | | | 513 | | | | 411 | |
| Construction in process | | | | | | 6 | | | | 618 | |
| Gross property and equipment | | | | | | 80,510 | | | | 75,999 | |
| Less accumulated depreciation and amortization | | | | | | (28,731 | ) | | | (25,838 | ) |
| Net property and equipment | | | | | $ | 51,779 | | | $ | 50,161 | |
Depreciation and amortization expense for property and equipment for the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009 was $5.0 million, $3.4 million and $3.1 million, respectively. There was no equipment under capital leases at May 29, 2011 or May 30, 2010. Amortization related to capitalized software was $136,000, $39,000 and $175,000 for fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009, respectively. The unamortized computer software costs at May 29, 2011 and May 30, 2010 were $491,000 and $260,000, respectively.
7. Intangible Assets
Changes in the carrying amount of goodwill for the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009 by reportable segment, are as follows (in thousands):
| | | Food Products Technology | | | Technology Licensing | | | Hyaluronan- based Biomaterials | | | Total | |
| | | | | | | | | | | | | |
| Balance as of May 25, 2008 | | $ | 22,574 | | | $ | 4,780 | | | $ | — | | | $ | 27,354 | |
| Goodwill acquired during the period | | | 7 | | | | — | | | | — | | | | 7 | |
| Balance as of May 31, 2009 | | | 22,581 | | | | 4,780 | | | | — | | | | 27,361 | |
| Goodwill acquired during the period | | | — | | | | — | | | | 13,793 | | | | 13,793 | |
| Balance as of May 30, 2010 | | | 22,581 | | | | 4,780 | | | | 13,793 | | | | 41,154 | |
| Goodwill acquired/reclassed during the period | | | — | | | | — | | | | 88 | | | | 88 | |
| Goodwill impaired during the period | | | — | | | | (4,780 | ) | | | — | | | | (4,780 | ) |
| Balance as of May 29, 2011 | | $ | 22,581 | | | $ | — | | | $ | 13,881 | | | $ | 36,462 | |
Information regarding Landec’s other intangible assets is as follows (in thousands):
| | | Trademarks & Trade names | | | Customer Relationships | | | Total | |
| Balance as of May 25, 2008 | | $ | 8,228 | | | $ | — | | | $ | 8,228 | |
| Amortization expense | | | — | | | | — | | | | — | |
| Balance as of May 31, 2009 | | | 8,228 | | | | — | | | | 8,228 | |
| Acquired during the period | | | 4,200 | | | | 3,700 | | | | 7,900 | |
| Amortization expense | | | — | | | | (26 | ) | | | (26 | ) |
| Balance as of May 30, 2010 | | | 12,428 | | | | 3,674 | | | | 16,102 | |
| Amortization expense | | | — | | | | (308 | ) | | | (308 | ) |
| Balance as of May 29, 2011 | | $ | 12,428 | | | $ | 3,366 | | | $ | 15,794 | |
Accumulated amortization as of May 29, 2011 was $3.8 million and accumulated amortization as of May 30, 2010 and May 31, 2009 was $3.4 million. The amortization expense for fiscal years 2011 through 2022 was and will be $308,000 per year. Accumulated impairment losses through May 29, 2011 were $4.8 million.
8. Stockholders’ Equity
Holders of Common Stock are entitled to one vote per share.
Convertible Preferred Stock
The Company has authorized two million shares of preferred stock, and as of May 29, 2011 has no outstanding preferred stock.
Common Stock and Stock Option Plans
At May 29, 2011, the Company had 3.4 million common shares reserved for future issuance under Landec equity incentive plans.
On October 15, 2009, following stockholder approval at the Annual Meeting of Stockholders of the Company, the 2009 Stock Incentive Plan (the “Plan”) became effective and replaced the Company’s 2005 Stock Incentive Plan. Employees (including officers), consultants and directors of the Company and its subsidiaries and affiliates are eligible to participate in the Plan.
8. Stockholders’ Equity (continued)
The Plan provides for the grant of stock options (both nonstatutory and incentive stock options), stock grants, stock units and stock appreciation rights. Awards under the Plan will be evidenced by an agreement with the Plan participants and 1.9 million shares of the Company’s Common Stock (“Shares”) were initially available for award under the Plan. Under the Plan, no recipient may receive awards during any fiscal year that exceeds the following amounts: (i) stock options covering in excess of 500,000 Shares; (ii) stock grants and stock units covering in excess of 250,000 Shares in the aggregate; or (iii) stock appreciation rights covering more than 500,000 Shares. In addition, awards to non-employee directors are discretionary. However, a non-employee director may not be granted awards in excess of 30,000 Shares in the aggregate during any fiscal year. The exercise price of the options was the fair market value of the Company’s Common Stock on the date the options were granted.
On October 14, 2005, following stockholder approval at the Annual Meeting of Stockholders of the Company, the 2005 Stock Incentive Plan (“2005 Plan”) became effective. The 2005 Plan replaced the Company’s four then existing equity plans and no shares remain available for grant under those plans. Employees (including officers), consultants and directors of the Company and its subsidiaries and affiliates eligible to participate in the 2005 Plan. The 2005 Plan provided for the grant of stock options (both nonstatutory and incentive stock options), stock grants, stock units and stock appreciation rights. Under the 2005 Plan, 861,038 Shares were initially available for awards, and as of May 29, 2011, 715,916 shares and options to purchase shares were outstanding. The exercise price of the options was the fair market value of the Company’s Common Stock on the date the options were granted.
The 1995 Directors’ Stock Option Plan (the “Directors’ Plan”) provided that each person who became a non- employee director of the Company, who had not received a previous grant, be granted a nonstatutory stock option to purchase 20,000 shares of Common Stock on the date on which the optionee first became a non-employee director of the Company. Thereafter, on the date of each annual meeting of the stockholders each non-employee director was granted an additional option to purchase 10,000 shares of Common Stock if, on such date, he or she had served on the Company’s Board of Directors for at least six months prior to the date of such annual meeting. The exercise price of the options was the fair market value of the Company’s Common Stock on the date the options were granted. Options granted under this plan were exercisable and vested upon grant.
The 1996 Non-Executive Stock Option Plan authorized the Board of Directors to grant non-qualified stock options to employees, including executive officers, and outside consultants of the Company. The exercise price of the options was equal to the fair market value of the Company’s Common Stock on the date the options were granted. Options were generally exercisable upon vesting and generally vested ratably over four years and were subject to repurchase if exercised before being vested.
The 1996 Stock Option Plan authorized the Board of Directors to grant stock purchase rights, incentive stock options or non-statutory stock options to Landec executives. The exercise price of the stock purchase rights, incentive stock options and non-statutory stock options could be no less than 100% of the fair market value of Landec’s Common Stock on the date the options were granted. Options generally were exercisable upon vesting, generally vested ratably over four years and were subject to repurchase if exercised before being vested.
The New Executive Stock Option Plan authorized the Board of Directors to grant non-statutory stock options to officers of Landec or officers of Apio or Landec Ag whose employment with each of those companies began after October 24, 2000. The exercise price of the non-statutory stock options could be no less than 100% and 85%, for named executives and non-named executives, respectively, of the fair market value of Landec's Common Stock on the date the options were granted. Options generally were exercisable upon vesting, generally vested ratably over four years and were subject to repurchase if exercised before being vested.
On July 14, 2010, the Board of Directors of the Company approved the establishment of a stock repurchase plan which allows for the repurchase of up to $10 million of the Company’s Common Stock. The Company may repurchase its common stock from time to time in open market purchases or in privately negotiated transactions. The timing and actual number of shares repurchased is at the discretion of management of the Company and will depend on a variety of factors, including stock price, corporate and regulatory requirements, market conditions, the relative attractiveness of other capital deployment opportunities and other corporate priorities. The stock repurchase program does not obligate Landec to acquire any amount of its common stock and the program may be modified, suspended or terminated at any time at the Company's discretion without prior notice. During fiscal year 2011, the Company repurchased on the open market 215,684 shares of its Common Stock for $1.2 million and retired those shares.
8. Stockholders’ Equity (continued)
Activity under all Landec equity incentive plans is as follows:
Stock-Based Compensation Activity
| | | Restricted Stock Outstanding | | | Stock Options Outstanding | |
| | | RSU’s and Options Available for Grant | | | Number of Restricted Shares | | | Weighted Average Grant Date Fair Value | | | Number of Stock Options | | | Weighted Average Exercise Price | |
| Balance at May 25, 2008 | | | 567,396 | | | | 63,168 | | | $ | 11.32 | | | | 1,896,950 | | | $ | 5.68 | |
| Granted | | | (506,254 | ) | | | 127,504 | | | $ | 6.62 | | | | 378,750 | | | $ | 6.62 | |
| Awarded/Exercised | | | — | | | | (10,002 | ) | | $ | 13.32 | | | | (331,950 | ) | | $ | 3.66 | |
| Forfeited | | | 14,875 | | | | (3,666 | ) | | $ | 7.45 | | | | (11,209 | ) | | $ | 8.59 | |
| Plan shares expired | | | (209 | ) | | | — | | | | — | | | | — | | | | — | |
| Balance at May 31, 2009 | | | 75,808 | | | | 177,004 | | | $ | 7.88 | | | | 1,932,541 | | | $ | 6.19 | |
| Additional shares reserved | | | 1,900,000 | | | | ― | | | | | | | | | | | | | |
| Granted | | | (1,193,022 | ) | | | 307,272 | | | $ | 5.80 | | | | 885,750 | | | $ | 5.78 | |
| Awarded/Exercised | | | — | | | | (51,671 | ) | | $ | 8.11 | | | | (190,962 | ) | | $ | 4.32 | |
| Forfeited | | | — | | | | (1,000 | ) | | $ | 13.32 | | | | (1,000 | ) | | $ | 13.32 | |
| Plan shares expired | | | ― | | | | — | | | | — | | | | (169,500 | ) | | $ | 7.01 | |
| Terminated plan | | | (12,475 | ) | | | ― | | | | ― | | | | ― | | | | ― | |
| Balance at May 30, 2010 | | | 770,311 | | | | 431,605 | | | $ | 6.35 | | | | 2,456,829 | | | $ | 6.13 | |
| Granted | | | (129,335 | ) | | | 32,335 | | | $ | 6.00 | | | | 97,000 | | | $ | 6.00 | |
| Awarded/Exercised | | | — | | | | (48,855 | ) | | $ | 9.48 | | | | (217,076 | ) | | $ | 3.46 | |
| Forfeited | | | — | | | | — | | | | — | | | | (18,000 | ) | | $ | 10.63 | |
| Balance at May 29, 2011 | | | 640,976 | | | | 415,085 | | | $ | 5.96 | | | | 2,318,753 | | | $ | 6.34 | |
Upon vesting of certain RSUs and the exercise of certain options during the period ended May 29, 2011, certain RSUs and exercised options were net share-settled to cover the required exercise price and withholding tax and the remaining amount were converted into an equivalent number of shares of Common Stock. The Company withheld shares with value equivalent to the exercise price for options and the employees' minimum statutory obligation for the applicable income and other employment taxes, and remitted the cash to the appropriate taxing authorities. The total shares withheld for fiscal years 2011, 2010 and 2009 were 136,374, 79,263 and 171,386 RSUs and options, respectively,.which was based on the value of the option and/or RSUs on their exercise or vesting date as determined by the Company's closing stock price. Total payments for the employees' tax obligations to the taxing authorities were approximately $218,000. These net-share settlements had the effect of share repurchases by the Company as they reduced and retired the number of shares that would have otherwise have been issued as a result of the vesting and did not represent an expense to the Company.
8. Stockholders’ Equity (continued)
The following table summarizes information concerning stock options outstanding and exercisable at May 29, 2011:
| | | Options Outstanding | | | Options Exercisable | |
Range of Exercise Prices | | Number of Shares Outstanding | | | Weighted Average Remaining Contractual Life | | | Weighted Average Exercise Price | | | Aggregate Intrinsic Value | | | Number of Shares Exercisable | | | Weighted Average Exercise Price | | | Aggregate Intrinsic Value | |
| | | | | | (in years) | | | | | | | | | | | | | | | | |
| $ 1.66 - $2.55 | | | 35,000 | | | | 1.76 | | | $ | 2.42 | | | $ | 119,250 | | | | 35,000 | | | $ | 2.42 | | | $ | 119,250 | |
| $ 2.82 - $4.67 | | | 151,809 | | | | 1.55 | | | $ | 3.58 | | | $ | 341,525 | | | | 151,809 | | | $ | 3.58 | | | $ | 341,525 | |
| $ 5.63 - $5.63 | | | 677,750 | | | | 6.00 | | | $ | 5.63 | | | $ | 135,550 | | | | 228,395 | | | $ | 5.63 | | | $ | 45,679 | |
| $ 5.65 - $6.22 | | | 785,694 | | | | 3.97 | | | $ | 6.15 | | | $ | 3,660 | | | | 561,761 | | | $ | 6.15 | | | $ | 2,100 | |
| $ 6.35 - $8.86 | | | 580,500 | | | | 5.53 | | | $ | 7.32 | | | $ | — | | | | 523,222 | | | $ | 7.41 | | | $ | — | |
| $ 13.32 - $13.32 | | | 88,000 | | | | 3.08 | | | $ | 13.32 | | | $ | — | | | | 88,000 | | | $ | 13.32 | | | $ | — | |
| $ 1.66 - $13.32 | | | 2,318,753 | | | | 4.23 | | | $ | 6.34 | | | $ | 599,985 | | | | 1,588,187 | | | $ | 6.56 | | | $ | 508,554 | |
The weighted average remaining contractual life of options exercisable as of May 29, 2011 was 3.46 years.
At May 29, 2011 and May 30, 2010 options to purchase 1,588,187 and 1,413,160 shares of Landec’s Common Stock were vested, respectively. No options have been exercised prior to being vested. The aggregate intrinsic value in the table above represents the total pretax intrinsic value, based on the Company’s closing stock price of $5.83 on May 27, 2011, which would have been received by holders of stock options had all holders of stock options exercised their stock options that were in-the-money as of that date. The total number of in-the-money stock options exercisable as of May 29, 2011, was approximately 450,000 shares. The aggregate intrinsic value of stock options exercised during the fiscal year 2011 was $750,000
Shares Subject to Vesting
The following table summarizes the activity relating to unvested stock option grants and RSUs during the fiscal year ended May 29, 2011:
| | | Stock Options | | | Restricted Stock | |
| | | Shares | | | Weighted Average Fair Value | | | Shares | | | Weighted Average Fair Value | |
| Unvested at May 30, 2010 | | | 1,043,669 | | | $ | 2.72 | | | | 431,605 | | | $ | 6.35 | |
| Granted | | | 97,000 | | | $ | 2.42 | | | | 32,335 | | | $ | 6.00 | |
| Vested/Awarded | | | (392,103 | ) | | $ | 2.56 | | | | (48,855 | ) | | $ | 9.48 | |
| Forfeited | | | (18,000 | ) | | $ | 5.78 | | | | — | | | | — | |
| Unvested at May 29, 2011 | | | 730,566 | | | $ | 2.69 | | | | 415,085 | | | $ | 5.96 | |
As of May 29, 2011, there was $3.1 million of total unrecognized compensation expense related to unvested equity compensation awards granted under the Company’s incentive stock plans. Total expense is expected to be recognized over the weighted-average period of 1.9 years for stock options and 1.8 years for restricted stock awards.
9. Debt
On April 30, 2010 in conjunction with the acquisition of Lifecore, Lifecore entered into a $20 million Credit Agreement with Wells Fargo Bank N.A. (“Wells Fargo”) with a five year term that provides for equal monthly principal payments plus interest. The Credit Agreement contains certain restrictive covenants, which require Lifecore to meet certain financial tests, including minimum levels of net income, minimum quick ratio, minimum fixed coverage ratio and maximum capital expenditures. All of Lifecore’s assets have been pledged to secure the debt incurred pursuant to the Credit Agreement. Landec is the guarantor of the debt. On August 9, 2010 and September 14, 2010, the Company amended its Credit Agreement with Wells Fargo to modify certain financial covenants. The amendment on August 9, 2010 amended the definition of the net income of Lifecore to exclude non-recurring expenses incurred in connection with the acquisition of Lifecore and to exclude expenses related to the impact of adjustments from purchase accounting (e.g. inventory step-up, discount on the earn out, etc.) as they relate to the minimum net income covenant. The aforementioned adjustments were made to the initial forecast that the Company had previously provided Wells Fargo which was the basis for the covenants in the Credit Agreement and which were necessary for compliance as of May 30, 2010. The amendment on September 14, 2010 amended the definition of the net income of Lifecore to include only the current fiscal year to date results as compared to the previous trailing four quarter basis, so as to exclude results prior to the acquisition. In addition, the minimum net income requirement for the first quarter ended August 29, 2010 was changed to $1.00 from $500,000. The Company was in compliance with all financial covenants as of May 29, 2011.
On August 19, 2004, Lifecore issued variable rate industrial revenue bonds (“IRB”). These bonds were assumed by Landec in the acquisition of Lifecore (see Note 2). The bonds are collateralized by a bank letter of credit which is secured by a first mortgage on the Company’s facility in Chaska, Minnesota. In addition, the Company pays an annual remarketing fee equal to 0.125% and an annual letter of credit fee of 0.50%.
Long-term debt consists of the following (in thousands):
| | | May 29, 2011 | | | May 30, 2010 | |
| Credit agreement with Wells Fargo; due in monthly payments of $333,333 through April 30, 2015 with interest payable monthly at Libor plus 2% per annum | | $ | 16,000 | | | $ | 19,667 | |
| Industrial revenue bond issued by Lifecore; due in annual payments through 2020 with interest at a variable rate set weekly by the bond remarketing agent (.40% and 2.56% at May 29, 2011 and May 30, 2010, respectively) | | | 3,830 | | | | 4,103 | |
| Total | | | 19,830 | | | | 23,770 | |
| Less current portion | | | (4,330 | ) | | | (4,521 | ) |
| Long-term portion | | $ | 15,500 | | | $ | 19,249 | |
The future minimum principal payments of the Company’s debt for each year presented are as follows (in thousands):
| | | Wells Fargo | | | IRB | | | Total | |
| FY2012 | | $ | 4,000 | | | $ | 330 | | | $ | 4,330 | |
| FY2013 | | | 4,000 | | | | 340 | | | | 4,340 | |
| FY2014 | | | 4,000 | | | | 355 | | | | 4,355 | |
| FY2015 | | | 4,000 | | | | 365 | | | | 4,365 | |
| FY2016 | | | — | | | | 375 | | | | 375 | |
| Thereafter | | | | | | | 2,065 | | | | 2,065 | |
| | | $ | 16,000 | | | $ | 3,830 | | | $ | 19,830 | |
The maturities on the IRB are held in a sinking fund account, recorded in Other Current Assets in the accompanying Consolidated Balance Sheets, and are paid out each year on September 1st.
10. Derivative Financial Instruments
The Company is exposed to interest rate risks primarily through borrowings under its Credit Agreement with Wells Fargo (see Note 9). Interest on all of the Company’s borrowings under its Credit Agreement is based upon variable interest rates. As of May 29, 2011, the Company had borrowings of $16.0 million outstanding under its Credit Agreement which bear interest at a rate equal to the one-month LIBOR plus 2%. As of May 29, 2011, the interest rate on borrowings under the Credit Agreement was accruing at 2.25%.
In May 2010, the Company entered into a five-year interest rate swap agreement under the Credit Agreement which expires on April 30, 2015. The interest rate swap was designated as a cash flow hedge of future interest payments of LIBOR and has a notional amount of $20 million. As a result of the interest rate swap transaction, the Company fixed for a five-year period the interest rate at 4.24% subject to market based interest rate risk on $20 million of borrowings under its Credit Agreement. The Company’s obligations under the interest rate swap transaction as to the scheduled payments were guaranteed and secured on the same basis as is its obligations under the Credit Agreement. As of May 29, 2011 and May 30, 2010, the Company recorded to Other Comprehensive Loss on the Consolidated Balance Sheets an unrealized loss of $267,000, net of taxes of $159,000 and $179,000, net of taxes of $105,000, respectively, as a result of the interest rate swap. The unrealized loss was based on Level 2 hierarchy for fair value measurements. If the interest rate swap is terminated or the debt borrowed is paid off prior to April 30, 2015, the amount of unrealized loss or gain included in Other Comprehensive Income (Loss) would be reclassified to earnings. The Company has no intentions of terminating the interest rate swap or prepaying the debt in the next twelve months. The interest rate swap liability is included in other non current liabilities as of May 29, 2011 and May 30, 2010.
11. Income Taxes
The provision for income taxes consisted of the following (in thousands):
| | | Year ended | | | Year ended | | | Year ended | |
| | | May 29, 2011 | | | May 30, 2010 | | | May 31, 2009 | |
| Current: | | | | | | | | | |
| Federal | | $ | 881 | | | $ | 844 | | | $ | 2,217 | |
| State | | | 176 | | | | 170 | | | | 883 | |
| Total | | | 1,057 | | | | 1,014 | | | | 3,100 | |
| Deferred: | | | | | | | | | | | | |
| Federal | | | 3,140 | | | | 3,186 | | | | 2,060 | |
| State | | | (16 | ) | | | 62 | | | | 451 | |
| Total | | | 3,124 | | | | 3,248 | | | | 2,511 | |
| Income tax expense | | $ | 4,181 | | | $ | 4,262 | | | $ | 5,611 | |
The actual provision for income taxes differs from the statutory U.S. federal income tax rate as follows (in thousands):
| | | Year Ended May 29, 2011 | | | Year Ended May 30, 2010 | | | Year Ended May 31, 2009 | |
| Provision at U.S. statutory rate (1) | | $ | 2,835 | | | $ | 2,886 | | | $ | 4,669 | |
| State income taxes, net of federal benefit | | | 213 | | | | 217 | | | | 1,023 | |
| Goodwill impairment charge | | | 1,849 | | | | — | | | | — | |
| Change in valuation allowance | | | (7 | ) | | | 390 | | | | — | |
| Tax-exempt interest | | | (115 | ) | | | (209 | ) | | | (196 | ) |
| Tax credit carryforwards | | | (637 | ) | | | (102 | ) | | | (159 | ) |
| Transaction Costs | | | — | | | | 982 | | | | — | |
| Other | | | 43 | | | | 98 | | | | 274 | |
| Total | | $ | 4,181 | | | $ | 4,262 | | | $ | 5,611 | |
(1) Statutory rate was 35% for fiscal years 2011, 2010 and 2009.
11. Income Taxes (continued)
The decrease in the income tax expense in fiscal year 2011 compared to fiscal years 2010 is due a 2% decrease in taxable income partially offset by an increase in the Company’s effective tax rate to 52% in fiscal year 2011 up from 51% in fiscal year 2010. The decrease in the income tax expense in fiscal year 2010 compared to fiscal year 2009 is due to a 38% decrease in taxable income partially offset by an increase in the Company’s effective tax rate to 51% in fiscal year 2010 up from 42% in fiscal year 2009. The effective tax rates for fiscal year 2011 differ from the statutory federal income tax rate of 35 percent as a result of several factors, including state taxes, non-deductible stock-based compensation expense, tax exempt interest and the goodwill impairment charge. In addition to the above, the Company was able to further reduce the effective tax rate for fiscal year 2011 as a result of being a recipient of a therapeutic drug credit award and the extension of the federal research and development credit. The effective tax rates for the fiscal year ended May 30, 2010 differ from the statutory federal income tax rate of 35 percent as a result of several factors, including state taxes, non-deductible stock-based compensation expense, tax exempt interest and accounting for transaction costs associated with the Lifecore acquisition in fiscal year 2010.
Significant components of deferred tax assets and liabilities consisted of the following (in thousands):
| | | May 29, 2011 | | | May 30, 2010 | |
| Deferred tax assets: | | | | | | |
| Net operating loss carryforwards | | $ | 6 | | | $ | — | |
| Research and AMT credit carryforwards | | | 892 | | | | 705 | |
| Accruals and reserves, not currently deductible for tax | | | 627 | | | | 1,528 | |
| Stock-based compensation | | | 837 | | | | 605 | |
| Other | | | 396 | | | | 344 | |
| Gross deferred tax assets | | | 2,758 | | | | 3,182 | |
| Valuation allowance | | | (383 | ) | | | (390 | ) |
| Net deferred tax assets | | | 2,375 | | | | 2,792 | |
| | | | | | | | | |
| Deferred tax liabilities: | | | | | | | | |
| Basis difference in trading securities | | | (253 | ) | | | — | |
| Depreciation and amortization | | | (4,293 | ) | | | (2,425 | ) |
| Goodwill and other indefinite life intangibles | | | (8,625 | ) | | | (7,906 | ) |
| Deferred tax liabilities | | | (13,171 | ) | | | (10,331 | ) |
| | | | | | | | | |
| Net deferred tax (liabilities) assets | | $ | (10,796 | ) | | $ | (7,539 | ) |
Valuation allowances are reviewed each period on a tax jurisdiction by jurisdiction basis to analyze whether there is sufficient positive or negative evidence to support a change in judgment about the realizability of the related deferred tax assets. Based on this analysis and considering all positive and negative evidence, we determined that a valuation allowance of $383,000 and $390,000 as of May 29, 2011 and May 30, 2010, respectively, should be recorded as a result of a book impairment loss on the Company’s investment in Aesthetic Sciences as it is more likely than not that a portion of the deferred tax asset will not be realized in the foreseeable future. The valuation allowance decrease of $7,000 from the prior year was due to a change in the Company’s apportionment.
As of May 29, 2011, the Company had federal and state net operating loss carryforwards of approximately $7.7 million and $5.0 million, respectively. These losses expire in different periods through 2030, if not utilized. Such net operating losses consist of excess tax benefits from employee stock option exercises and have not been recorded in the Company’s deferred tax assets. The Company will record a credit to additional paid in capital as and when such excess tax benefits are ultimately realized.
11.. Income Taxes (continued)
The Company also had federal and state research and development tax credits carryforwards of approximately $2.2 million and $1.6 million, respectively. The research and development tax credit carryforwards expire in different periods through 2031 for federal purposes and have an unlimited carryforward period for state purposes. The Company also has a federal therapeutic drug tax credit carryforward of $244,000 that will expire in 2030. Furthermore, the Company has federal alternative minimum tax credits of approximately $800,000 that can be carried forward indefinitely. Certain tax credit carryovers are attributable to excess tax benefits from employee stock option exercises and have not been recorded in the Company’s deferred tax assets. The Company will record a credit to additional paid in capital as and when such excess tax benefits are ultimately realized.
The accounting for uncertainty in income taxes recognized in an enterprise’s financial statements prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return, and the derecognition of tax benefits, classification on the balance sheet, interest and penalties, accounting in interim periods, disclosure, and transition.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| | | As of | |
| | | May 29, 2011 | | | May 30, 2010 | | | May 31, 2009 | |
| Unrecognized tax benefits – beginning of the period | | $ | 868 | | | $ | 619 | | | $ | 679 | |
| Gross increases – tax positions in prior period | | | 280 | | | | 138 | | | | 16 | |
| Gross decreases – tax positions in prior period | | | (310 | ) | | | (203 | ) | | | (51 | ) |
| Gross increases – current-period tax positions | | | 75 | | | | 332 | | | | 14 | |
| Settlements | | | — | | | | (18 | ) | | | (39 | ) |
| Lapse of statute of limitations | | | (153 | ) | | | — | | | | — | |
| | | | | | | | | | | | | |
| Unrecognized tax benefits – end of the period | | $ | 760 | | | $ | 868 | | | $ | 619 | |
The unrecognized tax benefits at May 29, 2011, May 30, 2010 and May 31, 2009 were $760,000, $868,000 and $619,000, of which $601,000, $708,000 and $549,000, respectively, will impact the effective tax rate. The Company accrues interest and penalties related to unrecognized tax benefits in its provision for income taxes. The total amount of penalties and interest is not significant as of May 29, 2011. Additionally, the Company expects its unrecognized tax benefits to change by $240,000 within the next 12 months related to the expiration of tax attributes.
Due to tax attribute carryforwards, the Company is subject to examination for tax years 1996 forward for U.S. tax purposes. The Company was also subject to examination in various state jurisdictions for tax years 1998 forward, none of which were individually material.
| 12. | Commitments and Contingencies |
Landec leases facilities and equipment under operating lease agreements with various terms and conditions, which expire at various dates through fiscal year 2017.
The approximate future minimum lease payments under these operating leases, excluding land leases, at May 29, 2011 are as follows (in thousands):
| | | Amount | |
| FY2012 | | $ | 734 | |
| FY2013 | | | 607 | |
| FY2014 | | | 509 | |
| FY2015 | | | 307 | |
| FY2016 | | | 261 | |
| Thereafter | | | 153 | |
| | | $ | 2,571 | |
| 12. | Commitments and Contingencies (continued) |
Rent expense for operating leases, including month to month arrangements was $1.2 million for the fiscal year ended May 29, 2011, $1.5 million for the fiscal year ended May 30, 2010 and $1.6 million for the fiscal year ended May 31, 2009.
Employment Agreements
Landec has entered into employment agreements with certain key employees. These agreements provide for these employees to receive incentive bonuses based on the financial performance of certain divisions in addition to their annual base salaries. The accrued incentive bonuses amounted to $347,000 at May 29, 2011 and $359,000 at May 30, 2010.
Licensing Agreement
In fiscal year 2001, the Company entered into an agreement for the exclusive worldwide rights to market grapes under certain brand names. Under the terms of the amended agreement (amended in fiscal year 2004), the Company is obligated to make one final payment of $50,000 in fiscal year 2012.
Purchase Commitments
At May 29, 2011, the Company was committed to purchase $2.2 million of produce during fiscal year 2012 in accordance with contractual terms. Payments of $10.4 million were made in fiscal year 2011 under these arrangements.
Loss Contingencies
As of May 29, 2011, the Company is not a party to any legal proceedings.
| 13. | Employee Savings and Investment Plans |
The Company sponsors a 401(k) plan which is available to substantially all of the Company’s employees. Landec’s Corporate Plan, which is available to all Landec employees (“Landec Plan”), allows participants to contribute from 1% to 50% of their salaries, up to the Internal Revenue Service (IRS) limitation into designated investment funds. Beginning in fiscal year 2001, the Company amended the plan so that it contributes an amount equal to 50% of the participants’ contribution up to 3% of the participants’ salary. In May 2003, the Company again amended the plan to make the Company’s matching contribution to the plan on behalf of participants voluntary, and to make employees participation in the plan voluntary. In June 2006, the Company again amended the plan to increase the company match from 50% on the first 6% contributed by an employee to 67% on the first 6% contributed. Participants are at all times fully vested in their contributions. The Company's contribution vests annually over a four-year period at a rate of 25% per year. The Company retains the right, by action of the Board of Directors, to amend, modify, or terminate the plan. For the fiscal years ended May 29, 2011, May 30, 2010 and May 31, 2009, the Company contributed $720,000, $368,000 and $341,000, respectively, to the Landec Plan.
14. Business Segment Reporting
The Company manages its business operations through four strategic business units. Based upon the information reported to the chief operating decision maker, who is the Chief Executive Officer, the Company has the following reportable segments: the Food Products Technology segment, the Commodity Trading segment, the Hyaluronan-based Biomaterials segment and the Technology Licensing segment. The Food Products Technology segment markets and packs specialty packaged whole and fresh-cut vegetables that incorporate the BreatheWay specialty packaging for the retail grocery, club store and food services industry. In addition, the Food Products Technology segment sells BreatheWay packaging to partners for non-vegetable products. The Food Export segment consists of revenues generated from the purchase and sale of primarily whole commodity fruit and vegetable products to Asia and domestically. The Hyaluronan-based Biomaterials segment sells products utilizing hyaluronan, a naturally occurring polysaccharide that is widely distributed in the extracellar matrix of connective tissues in both animals and humans for medical use primarily in the Ophthalmic, Orthopedic and Veterinary markets. The Technology Licensing segment licenses Landec’s patented Intellicoat seed coatings to the farming industry and licenses the Company’s Intelimer polymers for personal care products and other industrial products. Corporate includes corporate general and administrative expenses, non Food Products Technology interest income and Company-wide income tax expenses. All of the assets of the Company are located within the United States of America. The Company’s international sales are primarily to Canada, Taiwan, Belgium, Indonesia, China and Japan. Operations and identifiable assets by business segment consisted of the following (in thousands):
| Fiscal Year Ended May 29, 2011 | | | | | Food Export | | | Hyaluronan- based Biomaterials | | | | | | | | | | |
| Net sales | | $ | 175,664 | | | $ | 61,663 | | | $ | 32,505 | | | $ | 6,897 | | | $ | — | | | $ | 276,729 | |
| International sales | | $ | 18,580 | | | $ | 61,214 | | | $ | 24,024 | | | $ | ¾ | | | $ | ¾ | | | $ | 103,818 | |
| Gross profit | | $ | 18,888 | | | $ | 3,901 | | | $ | 17,231 | | | $ | 6,675 | | | $ | — | | | $ | 46,695 | |
| Net income (loss) | | $ | 8,200 | | | $ | 1,617 | | | $ | 7,278 | | | $ | (2,504 | ) | | $ | (10,671 | ) | | $ | 3,920 | |
| Identifiable assets | | $ | 88,241 | | | $ | 16,320 | | | $ | 83,954 | | | $ | 7,527 | | | $ | 10,270 | | | $ | 206,312 | |
| Depreciation and amortization | | $ | 3,174 | | | $ | 8 | | | $ | 1,972 | | | $ | 159 | | | $ | — | | | $ | 5,313 | |
| Capital expenditures | | $ | 3,620 | | | $ | — | | | $ | 2,817 | | | $ | 247 | | | $ | — | | | $ | 6,684 | |
| Dividend income | | $ | 328 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 328 | |
| Interest income | | $ | 129 | | | $ | — | | | $ | 164 | | | $ | — | | | $ | 137 | | | $ | 430 | |
| Interest expense | | $ | 2 | | | $ | — | | | $ | 818 | | | $ | — | | | $ | — | | | $ | 820 | |
| Income tax expense | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 4,181 | | | $ | 4,181 | |
| Impairment charges | | $ | — | | | $ | — | | | $ | — | | | $ | 4,780 | | | $ | — | | | $ | 4,780 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Fiscal Year Ended May 30, 2010 | | | | | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 175,046 | | | $ | 54,926 | | | $ | 1,457 | | | $ | 6,795 | | | $ | — | | | $ | 238,224 | |
| International sales | | $ | 15,714 | | | $ | 52,319 | | | $ | 603 | | | $ | ¾ | | | $ | ¾ | | | $ | 68,636 | |
| Gross profit | | $ | 22,514 | | | $ | 3,906 | | | $ | 815 | | | $ | 6,531 | | | $ | — | | | $ | 33,766 | |
| Net income (loss) | | $ | 11,051 | | | $ | 1,789 | | | $ | 13 | | | $ | 2,647 | | | $ | (11,516 | ) | | $ | 3,984 | |
| Identifiable assets | | $ | 75,280 | | | $ | 13,979 | | | $ | 79,604 | | | $ | 11,848 | | | $ | 19,486 | | | $ | 200,197 | |
| Depreciation and amortization | | $ | 3,055 | | | $ | 8 | | | $ | 141 | | | $ | 160 | | | $ | — | | | $ | 3,364 | |
| Capital expenditures | | $ | 4,212 | | | $ | — | | | $ | 739 | | | $ | 241 | | | $ | — | | | $ | 5,192 | |
| Interest income | | $ | 223 | | | $ | — | | | $ | 8 | | | $ | — | | | $ | 603 | | | $ | 834 | |
| Interest expense | | $ | 12 | | | $ | — | | | $ | 76 | | | $ | — | | | $ | — | | | $ | 88 | |
| Income tax expense | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 4,262 | | | $ | 4,262 | |
| Impairment charges | | $ | — | | | $ | — | | | $ | — | | | $ | 1,000 | | | $ | — | | | $ | 1,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Fiscal Year Ended May 31, 2009 | | | | | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 168,256 | | | $ | 60,445 | | | $ | ― | | | $ | 7,237 | | | $ | — | | | $ | 235,938 | |
| International sales | | $ | 14,393 | | | $ | 55,267 | | | $ | ― | | | $ | ¾ | | | $ | ¾ | | | $ | 69,660 | |
| Gross profit | | $ | 23,386 | | | $ | 3,657 | | | $ | ― | | | $ | 7,237 | | | $ | — | | | $ | 34,280 | |
| Net income (loss) | | $ | 11,279 | | | $ | 1,555 | | | $ | ― | | | $ | 4,893 | | | $ | (9,997 | ) | | $ | 7,730 | |
| Identifiable assets | | $ | 75,466 | | | $ | 14,329 | | | $ | ― | | | $ | 12,278 | | | $ | 51,425 | | | $ | 153,498 | |
| Depreciation and amortization | | $ | 2,947 | | | $ | 14 | | | $ | ― | | | $ | 178 | | | $ | — | | | $ | 3,139 | |
| Capital expenditures | | $ | 4,367 | | | $ | — | | | $ | ― | | | $ | 209 | | | $ | — | | | $ | 4,576 | |
| Interest income | | $ | 385 | | | $ | — | | | $ | ― | | | $ | — | | | $ | 921 | | | $ | 1,306 | |
| Interest expense | | $ | 8 | | | $ | — | | | $ | ― | | | $ | — | | | $ | — | | | $ | 8 | |
| Income tax expense | | $ | — | | | $ | — | | | $ | ― | | | $ | — | | | $ | 5,611 | | | $ | 5,611 | |
15. Quarterly Consolidated Financial Information (unaudited)
The following is a summary of the unaudited quarterly results of operations for fiscal years 2011, 2010 and 2009 (in thousands, except for per share amounts):
| | 1st Quarter | | | 2nd Quarter | | | 3rd Quarter | | | 4th Quarter | | | FY 2011 | |
| Revenues | | $ | 64,953 | | | $ | 70,168 | | | $ | 73,508 | | | $ | 68,100 | | | $ | 276,729 | |
| Gross profit | | $ | 11,817 | | | $ | 11,855 | | | $ | 12,477 | | | $ | 10,546 | | | $ | 46,695 | |
| Net income (loss) | | $ | 2,304 | | | $ | 2,055 | | | $ | 2,298 | | | $ | (2,737 | ) | | $ | 3,920 | |
| Net income (loss) per basic share | | $ | 0.09 | | | $ | 0.08 | | | $ | 0.09 | | | $ | (0.10 | ) | | $ | 0.15 | |
| Net income (loss) per diluted share | | $ | 0.09 | | | $ | 0.08 | | | $ | 0.09 | | | $ | (0.10 | ) | | $ | 0.15 | |
| | | | | | | | | | | | | | | | | | | | | |
| | 1st Quarter | | | 2nd Quarter | | | 3rd Quarter | | | 4th Quarter | | | FY 2010 | |
| Revenues | | $ | 60,943 | | | $ | 60,933 | | | $ | 58,133 | | | $ | 58,215 | | | $ | 238,224 | |
| Gross profit | | $ | 8,870 | | | $ | 7,417 | | | $ | 8,127 | | | $ | 9,352 | | | $ | 33,766 | |
| Net income (loss) | | $ | 2,184 | | | $ | 1,534 | | | $ | 1,734 | | | $ | (1,468 | ) | | $ | 3,984 | |
| Net income (loss) per basic share | | $ | 0.08 | | | $ | 0.06 | | | $ | 0.07 | | | $ | (0.06 | ) | | $ | 0.15 | |
| Net income (loss) per diluted share | | $ | 0.08 | | | $ | 0.06 | | | $ | 0.07 | | | $ | (0.06 | ) | | $ | 0.15 | |
| | | | | | | | | | | | | | | | | | | | | |
| | 1st Quarter | | | 2nd Quarter | | | 3rd Quarter | | | 4th Quarter | | | FY 2009 | |
| Revenues | | $ | 71,753 | | | $ | 58,038 | | | $ | 53,911 | | | $ | 52,236 | | | $ | 235,938 | |
| Gross profit | | $ | 10,123 | | | $ | 7,557 | | | $ | 7,591 | | | $ | 9,009 | | | $ | 34,280 | |
| Net income | | $ | 2,839 | | | $ | 1,498 | | | $ | 1,540 | | | $ | 1,853 | | | $ | 7,730 | |
| Net income per basic share | | $ | 0.11 | | | $ | 0.06 | | | $ | 0.06 | | | $ | 0.07 | | | $ | 0.30 | |
| Net income per diluted share | | $ | 0.11 | | | $ | 0.06 | | | $ | 0.06 | | | $ | 0.07 | | | $ | 0.29 | |
16. Subsequent Events
The Company has evaluated subsequent events through the date these consolidated financial statements were filed with the Securities and Exchange Commission.
(b) Index of Exhibits.
Exhibit Number: | | Exhibit Title |
| 3.1 | | Certificate of Incorporation of Registrant, incorporated herein by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K dated November 7, 2008. |
| | | |
| 3.2 | | Amended and Restated Bylaws of Registrant, incorporated herein by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K dated December 16, 2008. |
| | | |
| 10.1 | | Form of Indemnification Agreement, incorporated herein by reference to Exhibit 10.1 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended May 29, 2005. |
| | | |
| 10.2* | | Form of Option Agreement for 1995 Directors’ Stock Option Plan, incorporated herein by reference to Exhibit 10.4 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended October 31, 1996. |
| | | |
| 10.3 | | Industrial Real Estate Lease dated March 1, 1993 between the Registrant and Wayne R. Brown & Bibbits Brown, Trustees of the Wayne R. Brown & Bibbits Brown Living Trust dated December 30, 1987, incorporated by reference to Exhibit 10.6 to the Registrant’s Registration Statement on Form S-1 (File No. 33-80723) declared effective on February 12, 1996. |
| | | |
| 10.4* | | Form of Option Agreement for the 1996 Non-Executive Stock Option Plan, as amended, incorporated herein by reference to Exhibit 10.16 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended October 31, 1996. |
| | | |
| 10.5* | | 1996 Amended and Restated Stock Option Plan, incorporated herein by reference to Exhibit 10.17 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended April 29, 2001. |
| | | |
| 10.6* | | Form of Option Agreement for 1996 Amended and Restated Stock Option Plan, incorporated herein by reference to Exhibit 10.17 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended April 30, 1997. |
| | | |
| 10.7* | | New Executive Stock Option Plan, incorporated herein by reference to Exhibit 10.30 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended October 29, 2000. |
| | | |
| 10.8* | | 1996 Non-Executive Stock Option Plan, as amended, incorporated herein by reference to Exhibit 10.35 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended October 28, 2001. |
| | | |
| 10.9* | | Employment Agreement between the Registrant and Gary T. Steele effective as of January 1, 2009, incorporated herein by reference to Exhibit 99.1 to the Registrant’s Current Report on Form 8-K dated December 16, 2008 |
| | | |
| 10.10 | | Supply Agreement between the Registrant and Apio Fresh LLC and the Growers listed therein, dated as of July 3, 2003, incorporated herein by reference to Exhibit 2.3 to the Registrant’s Current Report on Form 8-K dated July 3, 2003. |
| | | |
| 10.11* | | 1995 Directors’ Stock Option Plan, as amended, incorporated herein by reference to Exhibit 10.53 to the Registrant’s Annual Report on Form 10-Q for the fiscal quarter ended May 25, 2003. |
Exhibit Number: | | Exhibit Title |
| 10.12# | | License and research and development agreement between the Registrant and Air Products and Chemicals, Inc. dated March 14, 2006, incorporated herein by reference to Exhibit 10.63 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended May 28, 2006. |
| | | |
| 10.13* | | 2005 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.1 to the Registrant's Current Report on Form 8-K dated October 14, 2005. |
| | | |
| 10.14* | | Form of Stock Grant Agreement for 2005 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.2 to the Registrant's Current Report on Form 8-K dated October 14, 2005. |
| | | |
| 10.15* | | Form of Notice of Stock Option Grant and Stock Option Agreement for 2005 Stock Incentive Plan, incorporated herein by reference to Exhibit 10.66 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended May 28, 2006. |
| | | |
| 10.16* | | Form of Stock Unit Agreement for 2005 Stock Incentive Plan, incorporated herein by reference to Exhibit 10.67 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended May 28, 2006. |
| | | |
| 10.17* | | Form of Stock Appreciation Right Agreement for 2005 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.5 to the Registrant's Current Report on Form 8-K dated October 14, 2005. |
Exhibit Number: | | Exhibit Title |
| | | |
| 10.20 | | Agreement and Plan of Merger between Landec Corporation, a California corporation, and the Registrant, dated as of November 6, 2008, incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K dated November 7, 2008. |
| | | |
| 10.21* | | 2009 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.1 to the Registrant's Current Report on Form 8-K dated October 19, 2009. |
| | | |
| 10.22* | | Form of Stock Grant Agreement for 2009 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.2 to the Registrant's Current Report on Form 8-K dated October 19, 2009. |
| | | |
| 10.23* | | Form of Notice of Stock Option Grant and Stock Option Agreement for 2009 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.3 to the Registrant's Current Report on Form 8-K dated October 19, 2009. |
| | | |
| 10.24* | | Form of Stock Unit Agreement for 2009 Stock Incentive Plan, incorporated herein by reference to Exhibit 99.4 to the Registrant's Current Report on Form 8-K dated October 19, 2009. |
| | | |
| 10.25* | | Form of Stock Appreciation Right Agreement for 2009 Stock Incentive Plan, |
| | | incorporated herein by reference to Exhibit 99.5 to the Registrant's Current Report on Form 8-K dated October 19, 2009. |
| 10.26* | | |
| | | First Amendment to Executive Employment between the Registrant and Gary Steele dated as of December 10, 2009, incorporated herein by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated December 15, 2009. |
| 10.27 | | |
| | | Stock Purchase Agreement by and among the Registrant, Lifecore Biomedical, Inc., Lifecore Biomedical, LLC and Warburg Pincus Private Equity IX, L.P., dated April 30, 2010, incorporated herein by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated May 5, 2010. |
| | | |
| 10.28 | | Credit Agreement by and between Lifecore Biomedical, LLC and Wells Fargo Bank, N.A. dated April 30, 2010, incorporated herein by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K dated May 5, 2010. |
| | | |
| 10.29 | | Continuing Guaranty Agreement by and between the Registrant and Wells Fargo Bank, N.A., dated April 30, 2010, incorporated herein by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K dated May 5, 2010. |
| | | |
| 10.30 | | Amendment No. 1 to the Credit Agreement by and between Lifecore Biomedical, LLC and Wells Fargo Bank, N.A. dated August 9, 2010. |
| | | |
| 10.31 | | Amended and Restated License, Supply and R&D Agreement dated November 27, 2009 by and among the Registrant, Landec Ag, LLC and Monsanto Company, incorporated by reference to Exhibit 10.25 to the Registrant’s Current Report on Form 8-K dated December 3, 2009. |
| | | |
| 10.32 | | Amendment No. 2 to the Credit Agreement by and between Lifecore Biomedical, LLC and Wells Fargo Bank, N.A. dated September 14, 2010, incorporated herein by reference to Exhibit 10.32 to the Registrant’s Current Report on Form 10-Q for the fiscal quarter ended August 29, 2010. |
Exhibit Number: | | Exhibit Title |
| | | |
| 10.33 | | Share Purchase Agreement, dated February 15, 2011, by and between Apio, Inc. and Windset Holdings 2010 Ltd., incorporated herein by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated February 18, 2011. |
| | | |
| 10.34* | | 2012 Cash Bonus Plan, incorporated herein by reference to the Registrant’s Current Report on Form 8-K dated May 27, 2011. |
| | | |
| 21.1 | | Subsidiaries of the Registrant | State of Incorporation |
| | | Landec Ag, LLC | Delaware |
| | | Apio, Inc. | Delaware |
| | | Lifecore Biomedical, Inc. | Delaware |
| | | |
| 23.1+ | | Consent of Independent Registered Public Accounting Firm |
| | | |
| 24.1+ | | Power of Attorney – See page 90 |
| | | |
| 31.1+ | | CEO Certification pursuant to section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 31.2+ | | CFO Certification pursuant to section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.1+ | | CEO Certification pursuant to section 906 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.2+ | | CFO Certification pursuant to section 906 of the Sarbanes-Oxley Act of 2002 |
| | * | Represents a management contract or compensatory plan or arrangement required to be filed as an exhibit to this report pursuant to Item 15(b) of Form 10-K. |
| | # | Confidential treatment requested as to certain portions. The term “confidential treatment” and the mark “*” as used throughout the indicated Exhibit means that material has been omitted. |
SIGNATURES
Pursuant to the requirements of section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Menlo Park, State of California, on August 5, 2011.
| LANDEC CORPORATION |
| |
| By: | /s/ Gregory S. Skinner |
| | Gregory S. Skinner |
| | Vice President of Finance and Administration |
| | and Chief Financial Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Gary T. Steele and Gregory S. Skinner, and each of them, as his attorney-in-fact, with full power of substitution, for him in any and all capacities, to sign any and all amendments to this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming our signatures as they may be signed by our said attorney to any and all amendments to said Report on Form 10-K.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed by the following persons in the capacities and on the dates indicated:
| Signature | | Title | | Date |
| /s/ Gary T. Steele | | | | |
| Gary T. Steele | | President and Chief Executive Officer and Director (Principal Executive Officer) | | August 5, 2011 |
| | | | | |
| /s/ Gregory S. Skinner | | | | |
| Gregory S. Skinner | | Vice President of Finance and Administration and Chief Financial Officer (Principal Financial and Accounting Officer) | | August 5, 2011 |
| | | | | |
| /s/ Nicholas Tompkins | | | | |
| Nicholas Tompkins | | Chairman of the Board of Apio, Inc. and Director | | August 5, 2011 |
| | | | | |
| /s/ Robert Tobin | | | | |
| Robert Tobin | | Director | | August 5, 2011 |
| | | | | |
| /s/ Duke K. Bristow, Ph.D | | | | |
| Duke K. Bristow, Ph.D | | Director | | August 5, 2011 |
| | | | | |
| /s/ Frederick Frank | | | | |
| Frederick Frank | | Director | | August 5, 2011 |
| | | | | |
| /s/ Stephen E. Halprin | | | | |
| Stephen E. Halprin | | Director | | August 5, 2011 |
| | | | | |
| /s/ Richard S. Schneider, Ph.D | | | | |
| Richard S. Schneider, Ph.D | | Director | | August 5, 2011 |
| | | | | |
| /s/ Steven Goldby | | | | |
| Steven Goldby | | Director | | August 5, 2011 |
| | | | | |
| /s/ Richard Dean Hollis | | | | |
| Richard Dean Hollis | | Director | | August 5, 2011 |
EXHIBIT INDEX
Exhibit Number | | Exhibit Title |
| | | |
| 23.1 | | Consent of Independent Registered Public Accounting Firm |
| | | |
| 24.1 | | Power of Attorney. See page 90 |
| | | |
| 31.1 | | CEO Certification pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | CFO Certification pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | CEO Certification pursuant to section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.2 | | CFO Certification pursuant to section 906 of the Sarbanes-Oxley Act of 2002. |