Exhibit 10.10
LICENSE AGREEMENT
by and between
RUSH-PRESBYTERIAN-ST. LUKE’S MEDICAL CENTER
and
ACORDA THERAPEUTICS, INC.
1
Certain portions of this Exhibit have been omitted pursuant to a request for confidentiality.
Such omitted portions, which are marked with brackets [ ] and an asterisk *, have been separately filed with the Commission.
THIS LICENSE AGREEMENT effective as of September 26, 2003 (“Effective Date”), by and between RUSH-PRESBYTERIAN-ST. LUKE’S MEDICAL CENTER, an Illinois not-for-profit corporation and having its principal office at 1725 W. Harrison St. Chicago, Ill. 60612 (“RUSH”) and ACORDA THERAPEUTICS, INC., a corporation organized and existing under the laws of the State of Delaware and having its principal office at 15 Skyline Drive, Hawthorne, New York 10532 (“ACORDA”).
W I T N E S S E T H:
WHEREAS, RUSH has conducted investigations of the compound known as 4-aminopyridine for treatment of the symptoms of multiple sclerosis and has accordingly developed know-how in relation thereto;
WHEREAS, RUSH has received a notice of designation (the “Rush Orphan Designation”) from the FDA stating that the Licensed Product (as defined herein) “qualifies for orphan designation for the relief of symptoms of multiple sclerosis;”
WHEREAS, RUSH’s right and title to the Rush Orphan Designation for the Licensed Product has been assigned to ACORDA and RUSH has consented to such assignment;
WHEREAS, RUSH has the right to grant licenses in respect of the RUSH Know-How (as defined herein) and has granted no licenses thereto except (i) the option agreement, dated September 7, 1990 (the “Option Agreement”), between RUSH and Elan Pharmaceutical Research Corp. (“EPRC”), a predecessor corporation of Elan Drug Delivery Inc., a wholly-owned subsidiary of Elan Corporation plc (“ELAN”) and (ii) the license agreement dated November 13, 1990 (the “Rush/Elan License”), between RUSH and EPRC, (the Option Agreement and the Rush/Elan License being collectively referred to herein as the “Rush/Elan Agreements”);
WHEREAS, pursuant to the Side Agreement, as defined below, RUSH and ELAN and EPRC have, among other things terminated the Rush/Elan Agreements as of the Effective Date;
WHEREAS, ACORDA desires to obtain exclusive license rights, with a right to grant sublicenses, under and to the RUSH Know-How (as defined herein), and RUSH desires to grant such license to ACORDA, upon the terms and conditions set forth herein; and
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound, the Parties hereby agree as follows:
2
ARTICLE I
DEFINITIONS
Unless specifically set forth to the contrary herein, the following terms, where used in the singular or plural, shall have the respective meanings set forth below:
1.1. “Act” shall mean the Federal Food Drug and Cosmetic Act of 1934, and the rules and regulations promulgated thereunder, or any successor act, as the same shall be in effect from time to time.
1.2. “Affiliate” shall mean (i) any corporation or business entity of which more than fifty percent (50%) of the securities or other ownership interests representing the equity, the voting stock or general partnership interest are owned, controlled or held, directly or indirectly, by a Party; (ii) any corporation or business entity which, directly or indirectly, owns, controls or holds more than fifty percent (50%) (or the maximum ownership interest permitted by law) of the securities or other ownership interests representing the equity, voting stock or general partnership interest of a Party or (iii) any corporation or business entity of which a Party has the right to acquire, directly or indirectly, at least fifty percent (50%) of the securities or other ownership interests representing the equity, voting stock or general partnership interest thereof.
1.3. “Base Royalty Term” shall mean, in any country in the Territory, the period beginning with the date of the First Commercial Sale in such country and continuing until the earlier of (i) expiration of the last to expire Elan Patent in such country; or (ii) ten (10) years from the date of First Commercial Sale in such country; provided however, that, in the event that ACORDA receives Regulatory Approval in the United States for Licensed Product with an Orphan Designation for the treatment of multiple sclerosis, then the Base Royalty Term in the United States shall not be less than seven years from the date of First Commercial Sale in the United States. In the event that RUSH’s further development of the RUSH Know-How results in the issuance to RUSH of a patent in any country or additional Orphan Drug Designation following the effective date of this Agreement that provides for a greater period of market exclusivity of the Product in such country, the Base Royalty Term in such country will continue for that period of market exclusivity provided by such patent or Orphan Drug Designation.
1.4. “Business Day(s)” shall mean any day that is not a Saturday or a Sunday or a day on which the New York Stock Exchange is closed.
1.5. “Calendar Quarter” shall mean the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31.
1.6. “Calendar Year” shall mean each successive period of twelve (12) months commencing on January 1 and ending on December 31.
1.7. “Compound” shall mean the chemical compound known as 4-aminopyridine, as diagrammed on Schedule 1.7 hereto.
1.8. “CFR” shall mean the United States Code of Federal Regulations.
1.9. “Effective Date” shall mean the date first above written.
3
1.10. “Elan/Acorda License” shall mean the Amended and Restated License Agreement effective as the Effective Date by and between ACORDA and ELAN.
1.11. “Elan Patent” shall mean any patent included in the Elan Patent Rights as set forth on Schedule 1.11 hereto
1.12. “End of Phase 2 Meeting” shall mean the first end of Phase 2 meeting with the FDA, as defined in 21 CFR Section 312.47, intended to determine the safety of proceeding to a Phase 3 Clinical Trial, evaluate the Phase 3 plan and protocols and identify any additional information necessary to support the NDA.
1.13. “FDA” shall mean the United States Food and Drug Administration and any successor agency having substantially the same functions.
1.14. “First Commercial Sale” shall mean the first commercial sale of Product by ACORDA, its Affiliate or its sublicensees in a country, for end use or consumption, after all required Regulatory Approvals have been granted by the governing health authority of such country. Sales for test marketing, clinical trial purposes, research and development, or compassionate or similar use where Acorda does not receive revenue from the sale other than cost recovery, shall not be deemed to constitute a commercial sale.
1.15. “GAAP” shall mean generally accepted accounting principles in the United States, consistently applied.
1.16. “Improvement” shall mean any and all improvements and enhancements, patentable or otherwise, related to the Compound or Product including, without limitation, in the manufacture, formulation, ingredients, preparation, presentation, means of delivery or administration, dosage, indication, use or packaging of Compound or Product.
1.17. “Licensed Product” shall mean any Product that utilizes or exploits the RUSH Know-How in the treatment of multiple sclerosis.
1.18. “NDA” shall mean a new drug application as defined in the Act and applicable regulations promulgated thereunder that is filed with the FDA to obtain Regulatory Approval of Licensed Product in the United States.
1.19. “Neurological Indications” shall mean indications concerning disorders and conditions of the neuromuscular system, central, peripheral and autonomic nervous systems, the neuromuscular junction and/or muscle. Such indications shall include, but not be limited to, multiple sclerosis and spinal cord injury.
1.20. “Net Sales” shall mean the gross amount invoiced for commercial sales of Product in the Territory by ACORDA or its Affiliates to Third Parties commencing upon the date of First Commercial Sale in any country in the Territory, after deducting the following:
(i) trade, cash and quantity discounts;
4
(ii) credits and allowances on account of returned or rejected Product, including allowance for breakage or spoilage, recalls or Product destruction (whether voluntarily made or requested or made by a Regulatory Authority)
(iii) chargebacks, rebates or similar payments granted to customers, including, but not limited to, managed health care organizations, wholesalers, distributors, buying groups, retailers, health care insurance carriers, pharmacy benefit management companies, health maintenance organizations or other institutions or health care organizations or to federal, state/provincial, local and other governments, their agencies and purchasers and reimbursers;
(iv) sales or excise taxes, VAT or other taxes, and transportation, freight, postage, shipping and insurance charges and additional special transportation, custom duties, and other governmental charges;
(v) retroactive price reductions; and
(vi) write-offs or allowances for bad debts, to the extent permitted by GAAP.
Sales or other transfers between ACORDA and its Affiliates shall be excluded from the computation of Net Sales and no payments will be payable on such sales or transfers except where such Affiliates are end users, but Net Sales shall include the subsequent sales to Third Parties by such Affiliates.
1.21. “Orphan Designation” shall mean the designation of a drug as a drug for a rare disease or condition pursuant to Section 526 of the Act.
1.22. “Party” shall mean RUSH or ACORDA.
1.23. “Phase 3 Clinical Trial” shall mean a clinical trial in patients with multiple sclerosis conducted after an End of Phase 2 Meeting and conducted on a sufficient number of patients that is designed to establish that Licensed Product is safe and efficacious for its intended use, and to define warnings, precautions and adverse reactions that are associated with Licensed Product in the dosage range to be prescribed, and supporting Regulatory Approval of Licensed Product in the treatment of multiple sclerosis.
1.24. “Product” shall mean any finished pharmaceutical formulation for prescription use for the treatment of any human Neurological Indications which contains Compound as the therapeutically active ingredient.
1.25. “Proprietary Information” shall mean any and all scientific, clinical, regulatory, marketing, financial and commercial information or data, whether communicated in writing, orally or by any other means, which is owned and under the protection of one
5
Party and is being provided by that Party to the other Party in connection with this Agreement.
1.26. “Reduced Royalty Term” shall mean, in any country in the Territory, the period of time beginning with the date following the expiration of the Base Royalty Term in such country and continuing until the fifteenth anniversary of the Effective Date.
1.27. “Regulatory Authority” shall mean the FDA in the U.S., the EMEA or any agency in the European Union and any health regulatory authority(ies) in any country(ies) in the Territory that holds responsibility for granting Regulatory Approval for a Product in such country(ies), and any successor(s) agency thereto having substantially the same functions.
1.28. “Regulatory Approval” shall mean all approvals (including pricing and reimbursement approvals required for marketing authorization), product and/or establishment licenses, registrations or authorizations of all regional, federal, state or local regulatory agencies, departments, bureaus or other governmental entities, necessary for the manufacture, use, storage, import, export, transport and sale of Product in a regulatory jurisdiction.
1.29. “Royalty Year” shall mean, (i) for the year in which the First Commercial Sale occurs (the “First Royalty Year”), the period commencing with the first day of the Calendar Quarter in which the First Commercial Sale occurs and expiring on the last day of the Calendar Year in which the First Commercial Sale occurs; and (ii) for each subsequent year commencing after the end of the First Royalty Year, each successive Calendar Year.
1.30. “RUSH Know-How” shall mean all information and materials, including but not limited to, discoveries, information, Improvements, processes, formulas, data, inventions, know-how and trade secrets, patentable or otherwise, which as of the Effective Date or at any time during the term of this Agreement:
(a) relate to Compound or Product; and
(b) were developed by or on behalf of RUSH, are owned by RUSH or are in RUSH’s possession or control.
Such know-how shall include, without limitation, all chemical, pharmaceutical, toxicological, preclinical, clinical, assay control, regulatory submissions, designations and approvals, and any other information used or useful for the development, manufacturing and/or regulatory approval of Compound or Product, including such rights which RUSH may have to information developed by Third Parties.
1.31. “Side Agreement” shall mean the Side Agreement by and among RUSH, ACORDA and ELAN executed as of the Effective Date, a copy of which is attached hereto as Exhibit 1.31.
1.32. “Territory” shall mean all of the countries in the world.
1.33. “Third Party(ies)” shall mean a person or entity who or which is neither a Party nor an Affiliate of a Party.
6
ARTICLE II
LICENSE; SUBLICENSES
2.1. License Grant. RUSH hereby grants to ACORDA an exclusive (even as to RUSH) license, including the right to grant sublicenses, under the RUSH Know-How, to develop, make, have made, use, import, offer for sale, market, commercialize, distribute and sell and otherwise dispose of Product in the Territory and to use and practice the RUSH Know-How. Notwithstanding the foregoing grant, Rush is expressly permitted to use its 4-AP know how for internal development and research efforts; provided, however, that (i) such use is for non-commercial academic purposes only, and (ii) that RUSH shall promptly notify ACORDA of any intellectual property, discovery or invention, once conceived and/or reduced to practice by RUSH in the course of conducting or performing such non-commercial activity, which shall be deemed RUSH Know-How for purposes of this Agreement.
2.2. Improvements by ACORDA. All rights and title to and interest in any Improvement developed or discovered by ACORDA in connection with the license granted under Section 2.1 above or ACORDA’s activities hereunder shall be vested solely in ACORDA. Notwithstanding the provisions of 2.2, Acorda will continue to have royalty obligations set forth in Article V, to the extent applicable, with respect to any Product that contains an Improvement and which includes the Compound as the primary therapeutically active ingredient.
2.3. Sublicenses. ACORDA shall have the right to grant sublicenses of the licenses granted to it under Section 2.1 of this Agreement to Affiliates or any Third Party. ACORDA shall provide written notice to RUSH of any such sublicenses.
ARTICLE III
DEVELOPMENT AND COMMERCIALIZATION
3.1. Exchange of Information. Following execution of this Agreement, RUSH shall utilize good faith reasonable efforts to disclose to ACORDA in English and in writing, all Rush Know-How not previously available or made available to ACORDA, in electronic format, where available, and hard copies (or, upon ACORDA’s request, originals), with the intention to make such information available to ACORDA as soon as reasonably practicable Throughout the term of this Agreement, and in addition to the other communications required under this Agreement, RUSH shall also promptly disclose to ACORDA in English and in writing on an ongoing basis all Rush Know-How, and any and all additions or revisions thereto. To the extent not previously assigned to ACORDA, RUSH hereby conveys, assigns and transfers to ACORDA, free and clear of all claims, liens and encumbrances and contractually imposed restrictions, all right, title and interest in and to the Rush Orphan Designation. RUSH shall assist and cooperate with ACORDA in the submission of any letters or other documents to the FDA required or requested in connection with the change in ownership of the Rush Orphan Designation from RUSH to ACORDA. RUSH shall notify ACORDA promptly of any request for, or
7
any expression of interest in using, Compound for research or any other purpose and shall refer any such requests or expressions of interest directly to ACORDA. RUSH shall also promptly notify ACORDA of any intellectual property, discovery or invention, once conceived and/or reduced to practice by RUSH or any employee or agent of RUSH, in the course of conducting or performing any activity relating to Compound or Product.
3.2. Development and Commercialization. ACORDA shall use commercially reasonable efforts to develop and commercialize Licensed Product. As used herein, “commercially reasonable efforts” shall mean efforts and resources normally used by ACORDA for a product owned by it or to which it has exclusive rights, which is of similar market potential at a similar stage in its development or product life, taking into account issues of safety and efficacy, product profile, the competitiveness of the marketplace, the proprietary position of the compound or product, the regulatory and reimbursement structure involved, the profitability of the applicable products, and other relevant factors. ACORDA shall provide RUSH with an annual written report summarizing the status of ACORDA’S clinical development and regulatory activities with respect to Licensed Product, with the delivery to RUSH of the summary of the annual report to an IND submitted by ACORDA to the FDA in connection with the periodic reporting requirements of the IND to be in satisfaction of the foregoing requirement. The obligations set forth in this Section 3.2 are expressly conditioned upon the absence of any serious adverse conditions or event relating to the safety or efficacy of Compound or Product including the absence of any action by any regulatory authority limiting the development or commercialization of Compound or Product.
3.3. Regulatory Matters.
(a) ACORDA shall own, control and retain primary legal responsibility for the preparation, filing and prosecution of all filings and regulatory applications required to obtain Regulatory Approvals. ACORDA shall notify RUSH upon the receipt of Regulatory Approvals and of the date of First Commercial Sale.
(b) Upon ACORDA’S request, RUSH shall consult and cooperate with ACORDA in connection with obtaining Regulatory Approval of Product.
3.4. Trademark. ACORDA shall select, own and maintain trademarks for Product in the Territory.
ARTICLE IV
CONFIDENTIALITY AND PUBLICITY
4.1. Non-Disclosure and Non-Use Obligations. All Proprietary Information disclosed by one Party to the other Party hereunder shall be maintained in confidence and shall not be disclosed to any Third Party or used for any purpose except as expressly permitted herein without the prior written consent of the Party that disclosed the Proprietary Information to the other Party during the term of this Agreement. The foregoing non-disclosure and non-use obligations shall not apply to the extent that such Proprietary Information:
8
(a) is known by the receiving Party at the time of its receipt, and not through a prior disclosure by the disclosing Party, as documented by business records;
(b) is or becomes properly in the public domain or knowledge;
(c) is subsequently disclosed to a receiving Party by a Third Party who may lawfully do so and is not under an obligation of confidentiality to the disclosing Party; or
(d) is developed by the receiving Party independently of Proprietary Information received from the other Party, as documented by research and development records.
4.2. Permitted Disclosure of Proprietary Information. Notwithstanding Section 4.1, a Party receiving Proprietary Information of another Party may disclose such Proprietary Information:
(a) by ACORDA to governmental or other regulatory agencies in order to obtain patents or to gain approval to conduct clinical trials or to market Product;
(b) by ACORDA or its agents, consultants, Affiliates, sublicensees and/or other Third Parties for the research and development, manufacturing and/or marketing of the Compound and/or Product (or for such parties to determine their interests in performing such activities) on the condition that such Third Parties agree to be bound by the confidentiality obligations consistent with this Agreement; or
(c) if required to be disclosed by law or court order, provided that notice is promptly delivered to the non-disclosing Party in order to provide an opportunity to challenge or limit the disclosure obligations; provided, however, without limiting any of the foregoing, it is understood that ACORDA or its Affiliates may make disclosure of this Agreement and the terms hereof in any filings required by the Securities and Exchange Commission (“SEC”) or any other governmental agency, may file this Agreement as an exhibit to any filing with the SEC or such agency and may distribute any such filing in the ordinary course of its business.
4.3. Publication Neither RUSH nor any Affiliate or employee of or consultant to RUSH shall make any publication relating to Compound or Product without the prior consent of ACORDA. If RUSH proposes to submit for written or oral publication any manuscript, abstract or the like relating to Compound or Product, it shall first deliver the proposed publication to ACORDA at least thirty (30) Business Days prior to planned submission. At the request of ACORDA, the submission of such publication may be delayed for up to fourteen (14) days in addition to the said thirty Business Days, including for issues of patent protection or other matters relating to the development of Compound or Product. If ACORDA requests modifications to the publication, RUSH shall edit such publication as
9
Certain portions of this Exhibit have been omitted pursuant to a request for confidentiality.
Such omitted portions, which are marked with brackets [ ] and an asterisk *, have been separately filed with the Commission.
reasonably necessary to prevent disclosure of trade secret or proprietary business information prior to submission of the publication or presentation.
ARTICLE V
PAYMENTS; ROYALTIES AND REPORTS
5.1. Up-front License Fee. In consideration of the rights granted by RUSH hereunder, ACORDA shall pay RUSH an up-front license fee of $200,000 within five (5) Business Days after the Effective Date.
5.2. Milestone Payments. In further consideration of the rights granted by RUSH hereunder, ACORDA or its designees shall pay RUSH the following milestone payments, contingent upon occurrence of the specified event, with each milestone payment to be made no more than once with respect to the achievement of such milestone (but payable the first time such milestone is achieved) for Licensed Product:
(a) US $[* *] upon the commencement (first dosing of the first patient) of the first Phase 3 Clinical Trial;
(b) US $[* *] upon the completion of the first Phase 3 Clinical Trial;
(c) US $[* *] upon the FDA’s acceptance for filing of the NDA; and
(d) US $[* *] upon receipt of first written Regulatory Approval of the NDA for marketing in the United States by the FDA.
ACORDA shall notify RUSH in writing within thirty (30) Business Days after the achievement of each milestone and such notice shall be accompanied by the appropriate milestone payment. The milestone payments described in this Section 5.2 shall be payable only upon the initial achievement of each milestone, and no amounts shall be due hereunder for any subsequent or repeated achievement of such milestones, regardless of the number of Licensed Products for which such milestone may be achieved.
5.3. Royalties and Other Payments.
5.3.1. Royalties
(a) Subject to the terms and conditions of this Agreement, and in further consideration of the rights granted by RUSH hereunder, ACORDA or its designees shall pay to RUSH royalties during the Base Royalty Term in an amount equal to (i) [* *] of Net Sales in each Royalty Year in the United States; and (ii) [* *] of Net Sales in each Royalty Year in each country in the Territory other than the United States. Royalties on Net Sales at the rates set forth in this Section 5.3.1(a) shall accrue as of the date of First Commercial Sale of Product in the applicable country and shall continue and accrue on Net Sales on a country-by-country basis until the expiration of the Base Royalty Term in such country. Thereafter, ACORDA shall be relieved of any royalty payment under this Section 5.3.1(a).
10
Certain portions of this Exhibit have been omitted pursuant to a request for confidentiality.
Such omitted portions, which are marked with brackets [ ] and an asterisk *, have been separately filed with the Commission.
(b) Subject to the terms and conditions of this Agreement, and in further consideration of the rights granted by RUSH hereunder, ACORDA or its designees shall pay to RUSH royalties during the Reduced Royalty Term in an amount equal to (i) [* *] of Net Sales in each Royalty Year in the United States; and (ii) [* *] of Net Sales in each Royalty Year in each country in the Territory other than the United States. Royalties on Net Sales at the rates set forth in this Section 5.3.1(b) shall accrue as of the commencement of the Reduced Royalty Term in the applicable country and shall continue and accrue on Net Sales on a country-by-country basis until the expiration of the Reduced Royalty Term in such country. Thereafter, ACORDA shall be relieved of any royalty payment under this Agreement.
(c) The payment of royalties set forth above shall be subject to the following conditions:
(A) only one payment shall be due with respect to the same unit of Product;
(B) no royalties shall accrue on the disposition of Product by ACORDA, Affiliates or sublicensees as samples (promotion or otherwise) or as donations (for example, to non-profit institutions or government agencies) or to clinical trials or for research and and/or development or for compassionate or similar use where ACORDA does not receive revenue other than cost recovery; and
(C) RUSH shall be responsible for payment of any royalties or other obligations owed by RUSH to any Third Party.
5.3.2. Affiliate and Sublicensee Sales. In the event that ACORDA transfers Compound or Product to one of its Affiliates or sublicensees, there shall be no royalty due at the time of transfer. Subsequent sales of Product by the Affiliates or sublicensees to Third Parties such as patients, hospitals, medical institutions, health plans or funds, wholesalers (which are not sublicensees), pharmacies or other retailers, shall be reported as Net Sales hereunder.
5.3.3. Third Party Licenses. If one or more licenses from a Third Party or Third Parties are obtained by ACORDA in order to develop, make, have made, use, sell or import Compound or Product in a particular country, [* *] of any royalties or other payments paid under such Third Party patent licenses by ACORDA in such country for such Calendar Quarter shall be creditable against the royalty or other payments payable to RUSH by ACORDA in such country; provided, however, that the amount credited in any Calendar Quarter shall not exceed [* *] of the royalties that would have otherwise been payable to RUSH for such Calendar Quarter.
5.3.4. Combination Product. Notwithstanding the provisions of Section 5.3.1, in the event a Product is sold as a combination product with other biologically active components, Net Sales, for purposes of royalty payments on the combination product, shall be calculated by multiplying the Net Sales of that combination product by the
11
fraction A/B, where A is the gross selling price of the Product sold separately and B is the gross selling price of the combination product. If no such separate sales are made, Net Sales for royalty determination shall be calculated by multiplying Net Sales of the combination product by the fraction C/(C+D), where C (excluding the fully allocated cost of the other biologically active component in question) is the fully allocated cost of the Compound and D is the fully allocated cost of such other biologically active components.
5.4. Reports; Payment of Royalty. During the term of the Agreement for so long as royalty payments are due, ACORDA shall furnish to RUSH a written report for each Calendar Quarter showing the Net Sales of all Products subject to royalty payments during the reporting period and the calculation of the royalties payable to RUSH under this Agreement, including deductions from Net Sales. Reports shall be due on the forty-fifth (45th) day following the close of each Calendar Quarter. Royalties shown to have accrued by each royalty report, if any, shall be due and payable on the date such report is due. ACORDA shall keep complete and accurate records in sufficient detail to enable the royalties hereunder to be determined. ACORDA shall retain such records for twenty-four (24) months after submission of the corresponding report.
5.5. Audits. Upon the written request of RUSH and not more than once during the twelve (12) month period next following the expiration of each Royalty Year during the term of the Agreement, ACORDA shall, at RUSH’s expense, permit an independent certified public accounting firm selected by RUSH and reasonably acceptable to ACORDA to have access during normal business hours, upon thirty (30) days prior notice to ACORDA, to such of the records of ACORDA as may be reasonably necessary to verify the accuracy of the royalty reports hereunder for any Royalty Year ending not more than twenty-four (24) months prior to the date of such request. The accounting firm shall provide a written report as soon as practicable, which shall disclose only whether the royalty reports are correct or incorrect and the specific details concerning any discrepancies. This Section 5.5 shall survive the expiration or termination of this Agreement for a period of two years.
5.5.1. If such accounting firm concludes that additional royalties were owed during such period, ACORDA shall pay the additional royalties within sixty (60) days of the date RUSH delivers to ACORDA such accounting firm’s written report so concluding; provided however, that, in the event that ACORDA shall not be in agreement with the conclusion of such report (a) ACORDA shall not be required to pay such additional royalties and (b) such matter shall be resolved pursuant to the provisions of Section 9.6 herein. In the event such accounting firm concludes that amounts were overpaid by ACORDA during such period, such over payment will be credited against future royalties; provided, however, that, in the event that RUSH shall not be in agreement with the conclusion of such report (x) such matter shall be resolved pursuant to the provisions of Section 9.6 herein and (y) in the event that the overpayment to RUSH exceeds royalties due and owing to Rush over the term of the agreement, RUSH shall reimburse ACORDA within 60 days for any remaining overpayment. The fees charged by such accounting firm shall be paid by RUSH; provided, however, that if an error in favor of RUSH of more than five percent (5%) of the royalties due hereunder for the period being reviewed is discovered, then
12
ACORDA shall pay the reasonable fees and expenses charged by such accounting firm.
5.5.2. Upon the expiration of twenty-four (24) months following the end of any Royalty Year (subject to tolling of such period during the pendency of an audit relating to such period under Section 5.5.1 above) the calculation of royalties payable with respect to such year shall be binding and conclusive upon RUSH, and ACORDA shall be released from any liability or accountability with respect to royalties for such year.
5.5.3. RUSH shall treat all financial information subject to review under this Section 5.5 in accordance with the confidentiality provisions of this Agreement.
5.6. Payment Exchange Rate. All payments to RUSH under this Agreement shall be made in United States dollars. In the case of sales outside the United States, the rate of exchange to be used in computing Net Sales shall be calculated monthly in accordance with the conversion rates published in the Wall Street Journal, Eastern edition (if available).
5.7. Tax Withholding. If laws, rules or regulations require withholding of income taxes or other taxes imposed upon payments set forth in this Article V, RUSH shall provide ACORDA, prior to any such payment, annually or more frequently if required, with all forms or documentation required by any applicable taxation laws, treaties or agreements to such withholding or as necessary to claim a benefit thereunder (including, but not limited to Form W-8BEN or any successor forms) and ACORDA shall make such withholding payments as required and subtract such withholding payments from the payments set forth in this Article V. ACORDA will use commercially reasonable efforts consistent with its usual business practices and cooperate with RUSH to ensure that any withholding taxes imposed are reduced as far as possible under the provisions of the current or any future taxation treaties or agreements between foreign countries.
5.8. Exchange Controls. Notwithstanding any other provision of this Agreement, if at any time legal restrictions prevent the prompt remittance of part or all of the royalties with respect to Net Sales in any country, payment shall be made through such lawful means or methods as ACORDA may determine. When in any country the law or regulations prohibit both the transmittal and deposit of royalties on sales in such a country, royalty payments shall be suspended for as long as such prohibition is in effect (and such suspended payments shall not accrue interest), and promptly after such prohibition ceases to be in effect, all royalties or other payments that ACORDA or its Affiliates would have been obligated to transmit or deposit, but for the prohibition, shall be deposited or transmitted, as the case may be, to the extent allowable (with any interest earned on such suspended royalties which were placed in an interest-bearing bank account in that country, less any transactional costs). If the royalty rate specified in this Agreement should exceed the permissible rate established in any country, the royalty rate for sales in such country shall be adjusted to the highest legally permissible or government-approved rate.
13
ARTICLE VI
REPRESENTATIONS AND WARRANTIES
6.1. RUSH Representations and Warranties. RUSH represents and warrants to ACORDA that as of the Effective Date:
(a) Each of this Agreement and the Side Agreement has been duly executed and delivered by RUSH and constitutes legal, valid, and binding obligations enforceable against RUSH in accordance with their respective terms;
(b) no approval, authorization, consent, or other order or action of or filing with any court, administrative agency or other governmental authority is required for the execution and delivery by RUSH of this Agreement or the Side Agreement or the consummation by RUSH of the transactions contemplated hereby or thereby except such consents or filings as are contemplated by this Agreement;
(c) RUSH has the full corporate power and authority to enter into and deliver this Agreement and the Side Agreement, to perform and to grant the licenses granted under Article II hereof and to consummate the transactions contemplated hereby and by the Side Agreement; all corporate acts and other proceedings required to be taken to authorize such execution, delivery, and consummation have been duly and properly taken and obtained;
(d) With the exception of the Rush/Elan Agreements, which have terminated in their entirety pursuant to the Side Agreement, RUSH has not previously assigned, transferred, conveyed or otherwise encumbered its right, title and interest in the Compound or Product or the RUSH Know-How or entered into any agreement with any Third Party which is in conflict with the rights granted to ACORDA pursuant to this Agreement;
(e) RUSH is the sole and exclusive owner of the RUSH Know-How, all of which are free and clear of any security interests, liens, charges, encumbrances or restrictions on license, and no Third Party has any claim of ownership or other rights with respect to the RUSH Know-How, whatsoever, except that RUSH agrees and acknowledges that the Orphan Designation has been assigned to ACORDA;
(f) RUSH has the sole and exclusive authority to grant the rights and licenses granted under Article II and, with the exception of the Rush/Elan Agreements, which have terminated in their entirety pursuant to the Side Agreement, RUSH has not previously granted, and will not grant, or engage in any discussions to grant, during the term of this Agreement, any right, license or interest in and to the Compound or Product or the RUSH
14
Know-How, or any portion thereof, inconsistent with the license granted to ACORDA herein;
(g) there are no claims, judgments or settlements against or owed by RUSH or pending or, to the best of its knowledge, threatened claims or litigation relating to the Compound or the Rush Know-How;
(h) RUSH will use reasonable efforts to disclose to ACORDA all relevant information known by it regarding the Rush Know-How reasonably related to the activities contemplated under this Agreement to the extent such Rush know-how has not previously been disclosed;
(i) in connection with development of the Rush Know-How, RUSH has complied in all material respects with applicable U.S. laws and regulations;
(j) RUSH has not filed and is not the owner in any country in the Territory of any patents or patent applications or of any certificates of invention or applications for certificates of invention, relating to Compound or Product; and
(k) With the exception of the Rush/Elan Agreements, which have terminated in their entirety pursuant to the Side Agreement, there are no contracts, agreements or any other arrangements between RUSH and any Third Party relating to the research, development or commercialization of the Compound or Product.
6.2. ACORDA Representations and Warranties. ACORDA represents and warrants to RUSH that as of the Effective Date:
(a) Each of this Agreement and the Side Agreement have been duly executed and delivered by it and constitutes legal, valid, and binding obligations enforceable against ACORDA in accordance with their respective terms;
(b) �� it has full corporate power and authority to execute and deliver this Agreement and the Side Agreement and to consummate the transactions contemplated hereby and thereby. All corporate acts and other proceedings required to be taken to authorize such execution, delivery, and consummation have been duly and properly taken and obtained;
(c) no approval, authorization, consent, or other order or action of or filing with any court, administrative agency or other governmental authority is required for the execution and delivery by it of this Agreement or the Side Agreement or the consummation by it of the transactions contemplated hereby or thereby.
15
ARTICLE VII
7.1. Indemnification. ACORDA shall defend, indemnify and hold harmless RUSH from and against any and all loss, cost and liability, including RUSH’s reasonable attorneys fees and costs (“Losses”), arising in connection with claims made by Third Parties respecting the manufacture, sale or use of any Product by such Third Party (“Claims”). RUSH shall give ACORDA prompt notice of any such Loss or claim, shall cooperate in its defense, and shall give ACORDA full authority to defend and settle such claim on RUSH’s behalf.
7.2. The indemnity obligation set forth in Section 7.1 above shall not apply in the case of Losses or Claims caused by or based on (i) RUSH’s gross negligence or willful misconduct; (ii) any breach of this Agreement by RUSH; or (iii) any violation of RUSH’s representations or warranties hereunder.
ARTICLE VIII
TERM AND TERMINATION
8.1. Term and Expiration. This Agreement shall be effective as of the Effective Date and unless terminated earlier pursuant to Section 8.2 below, the term of this Agreement shall continue in effect until expiration of all royalty or other payment obligations hereunder.
8.2. Termination.
8.2.1 Termination for Cause. Either Party may terminate this Agreement by notice to the other Party at any time during the term of this Agreement as follows:
(a) if the other Party is in breach of any material obligation hereunder by causes and reasons within its control, or has breached, in any material respect, any representations or warranties set forth in Article VI, and has not cured such breach within ninety (90) days after notice requesting cure of the breach, provided, however, that if the breach is not capable of being cured within ninety (90) days of such written notice, the Agreement may not be terminated so long as the breaching Party commences and is taking commercially reasonable actions to cure such breach as promptly as practicable; or
(b) upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other Party; provided, however, in the case of any involuntary bankruptcy, reorganization, liquidation, receivership or assignment proceeding such right to terminate shall only become effective if the Party consents to the involuntary proceeding or such proceeding is not dismissed within ninety (90) days after the filing thereof.
16
8.2.2 Licensee Rights Not Affected.
All rights and licenses granted pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the Bankruptcy Code licenses of rights to “intellectual property” as defined under Section 101(35A) of the Bankruptcy Code. The Parties agree that ACORDA and RUSH shall retain and may fully exercise all of their respective rights, remedies and elections under the Bankruptcy Code. The Parties further agree that, in the event of the commencement of a bankruptcy or reorganization case by or against a Party under the Bankruptcy Code, the other Party shall be entitled to all applicable rights under Section 365 (including 365(n)) of the Bankruptcy Code. Upon rejection of this Agreement by a Party or a trustee in bankruptcy for such Party, pursuant to Section 365(n), the other Party may elect (i) to treat this Agreement as terminated by such rejection or (ii) to retain its rights (including any right to enforce any exclusivity provision of this Agreement) to intellectual property (including any embodiment of such intellectual property) under this Agreement and under any agreement supplementary to this Agreement for the duration of this Agreement and any period for which this Agreement could have been extended by such other Party, subject, however, to the continued payment of all amounts owing under Section 5.3 of this Agreement, all of which amounts shall be deemed to be royalties for purposes of Section 365(n) of the Bankruptcy Code. Upon written request to the trustee in bankruptcy or bankrupt Party, the trustee or Party, as applicable, shall (i) provide to the other Party any intellectual property (including such embodiment) held by the trustee or the bankrupt Party and shall provide to the other Party a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property and (ii) not interfere with the rights of the other Party to such intellectual property as provided in this Agreement or any agreement supplementary to this Agreement, including any right to obtain such intellectual property (or such embodiment or duplicates thereof) from a Third Party.
8.3. Effect of Expiration or Termination. Expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing prior to such expiration or termination. ACORDA and its Affiliates and sublicensees shall have the right to sell or otherwise dispose of the stock of any Product subject to this Agreement then on hand or in process of manufacture and ACORDA will continue to pay Rush royalties pursuant to Article V after the expiration or termination of this Agreement for any such Product sold. In addition to any other provisions of this Agreement which by their terms continue after the expiration of this Agreement, the provision of Article IV shall survive the expiration or termination of this Agreement and shall continue in effect for five (5) years from the date of expiration or termination and the provisions of Article IX shall survive the expiration or termination of this Agreement. Upon any termination of this Agreement, each party shall promptly return to the other party all Proprietary Information received from the other party (except one copy of which may be retained for archival purposes). In addition, any other provision required to interpret and enforce the Parties’ rights and obligations under this Agreement shall also survive, but only to the extent required for the full observation and performance of this Agreement. Any expiration or early termination of this Agreement shall be without prejudice to the rights of any Party against the other
17
accrued or accruing under this Agreement prior to termination. In the event ACORDA breaches any of the financial provisions contained in this Agreement, in lieu of any other remedy that may be available, RUSH shall be entitled to pursue its remedies at law, but shall not be entitled to injunctive relief.
ARTICLE IX
MISCELLANEOUS
9.1. Right to Develop Independently. Nothing in this Agreement will impair ACORDA’s right to independently acquire, license, develop, or have others develop for it, products similar to or performing functions similar to Product, or similar technology performing similar functions to the Products or to market and distribute products based on other technology.
9.2. Force Majeure. Neither Party shall be held liable or responsible to the other Party nor be deemed to have defaulted under or breached the Agreement for failure or delay in fulfilling or performing any term of the Agreement during the period of time when such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party including, but not limited to, fire, flood, embargo, war, acts of war (whether war be declared or not), insurrection, riot, civil commotion, strike, lockout or other labor disturbance, act of God or act, omission or delay in acting by any governmental authority or the other Party. The affected Party shall notify the other Party of such force majeure circumstances as soon as reasonably practicable.
9.3. Assignment. The Agreement may not be assigned or otherwise transferred without the prior written consent of the other Party; provided, however, that ACORDA may assign this Agreement to an Affiliate or in connection with the transfer or sale of its business or all or substantially all of its assets related to Compound or Product or in the event of a merger, consolidation, change in control or similar corporate transaction. Any permitted assignee shall assume all obligations of its assignor under this Agreement.
9.4. Severability. In the event that any of the provisions contained in this Agreement are held invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein shall not in any way be affected or impaired thereby, unless the absence of the invalidated provision(s) adversely affect the substantive rights of the Parties. In such event, the Parties shall replace the invalid, illegal or unenforceable provision(s) with valid, legal and enforceable provision(s) which, insofar as practical, implement the purposes of this Agreement.
9.5. Notices. All notices or other communications which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by nationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows:
18
if to ACORDA to:
ACORDA THERAPEUTICS, INC.
15 Skyline Drive
Hawthorne, New York 10532
Attention: : President
Fax No.: 914.347.4560
if to RUSH to:
RUSH-PRESBYTERIAN-ST. LUKE’S MEDICAL CENTER
1725 W. Harrison Street
Chicago, Illinois 60612
Attention: Intellectual Property Office/General Counsel’s Office
Fax No.: 312-942-2055
or to such other address as the Party to whom notice is to be given may have furnished to the other Parties in writing in accordance herewith. Any such communication shall be deemed to have been given when delivered if personally delivered or sent by facsimile on a Business Day, upon confirmed delivery by nationally-recognized overnight courier if so delivered and on the third Business Day following the date of mailing if sent by registered or certified mail.
9.6. Applicable Law and Dispute Resolution. The Agreement shall be governed by and construed in accordance with the laws of the United States of America and State of New York without reference to any rules of conflict of laws.
(a) The Parties agree to attempt initially to solve all claims, disputes, or controversies arising under, out of, or in connection with this Agreement (a “Dispute”) by conducting good faith negotiations. Any Disputes which cannot be resolved by good faith negotiation within twenty (20) Business Days, shall be referred, by written notice from either Party to the other, to the Chief Executive Officer of each Party. Such Chief Executive Officers shall negotiate in good faith to achieve a resolution of the Dispute referred to them within twenty (20) Business Days after such notice is received by the Party to whom the notice was sent. If the Chief Executive Officers are unable to settle the Dispute between themselves within twenty (20) Business Days, they shall so report to the Parties in writing. The Dispute shall then be referred to mediation as set forth in the following subsection (b).
(b) Upon the Parties receiving the Chief Executive Officers’ report that the Dispute referred to them pursuant to subsection (a) has not been resolved, the Dispute shall be referred to mediation by written notice from either Party to the other. The mediation shall be conducted pursuant to the American Arbitration Association (“AAA”) procedures. The place of the mediation shall be Chicago, Illinois. If the Parties have not reached a settlement within twenty (20) Business Days of the date of the notice of mediation, the Dispute shall be referred to arbitration pursuant to subsection (c) below.
19
(c) If after the procedures set forth in subsections (a) and (b) above, the Dispute has not been resolved, a Party shall decide to institute arbitration proceedings, it shall give written notice to that effect to the other Party. The Parties shall refrain from instituting the arbitration proceedings for a period of sixty (60) days following such notice. During such period, the Parties shall continue to make good faith efforts to amicably resolve the dispute without arbitration. If the Parties have not reached a settlement during that period the arbitration proceedings shall go forward and be governed by the AAA rules then in force. Each such arbitration shall be conducted by a panel of three arbitrators: one arbitrator shall be appointed by each of RUSH and ACORDA and the third arbitrator, who shall be the Chairman of the tribunal, shall be appointed by the two-Party appointed arbitrators. Any such arbitration shall be held in Chicago, Illinois, USA.
The arbitrators shall have the authority to direct the Parties as to the manner in which the Parties shall resolve the disputed issues, to render a final decision with respect to such disputed issues, or to grant specific performance with respect to any such disputed issue. Judgment upon the award so rendered may be entered in any court having jurisdiction or application may be made to such court for judicial acceptance of any award and an order of enforcement, as the case may be. Nothing in this Section shall be construed to preclude either Party from seeking provisional remedies, including but not limited to, temporary restraining orders and preliminary injunctions, from any court of competent jurisdiction, in order to protect its rights pending arbitration, but such preliminary relief shall not be sought as a means of avoiding arbitration. In no event shall a demand for arbitration be made after the date when institution of a legal or equitable proceeding based on such claim, dispute or other matter in question would be barred by the applicable statute of limitations. Each Party shall bear its own costs and expenses incurred in connection with any arbitration proceeding and the Parties shall equally share the cost of the mediation and arbitration levied by the AAA.
Any mediation or arbitration proceeding entered into pursuant to this Section 9.6 shall be conducted in the English language. Subject to the foregoing, for purposes of this Agreement, each Party consents, for itself and its Affiliates, to the jurisdiction of the courts of the State of New York, county of New York and the U.S. District Court for the Southern District of New York.
9.7. Entire Agreement. This Agreement, together with the exhibits and schedules hereto, contains the entire understanding of the Parties with respect to the subject matter hereof and supersedes all previous writings and understandings between the Parties. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by all Parties hereto.
9.8. Independent Contractors. It is expressly agreed that the Parties shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency. Neither Party shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other Party, without the prior consent of such other Party.
20
9.9 Waiver. The waiver by a Party hereto of any right hereunder or the failure to perform or of a breach by another Party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other Party whether of a similar nature or otherwise.
9.10. Further Assurances. At any time or from time to time on and after the Effective Date, RUSH shall at the request of ACORDA (i) deliver to ACORDA such records, data or other documents consistent with the provisions of this Agreement, (ii) execute, and deliver or cause to be delivered, all such consents, documents or further instruments of transfer or license, and (iii) take or cause to be taken all such actions as ACORDA may reasonably deem necessary or desirable in order for ACORDA to obtain the full benefits of this Agreement and the transactions contemplated hereby.
9.11. Headings. The captions to the several Articles and Sections hereof are not a part of the Agreement, but are merely guides or labels to assist in locating and reading the several Articles and Sections hereof.
9.12. Counterparts. The Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
9.13. Use of Names. Except as otherwise provided in this Agreement, neither Party shall not use the name of the other Party in relation to this transaction in any public announcement, press release or other public document without the consent of the other Party (which consent shall not be unreasonably withheld or delayed), except as may be required by applicable law.
9.14. LIMITATION OF LIABILITY. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY INDIRECT CONSEQUENTIAL DAMAGES ARISING OUT OF THIS AGREEMENT, HOWEVER CAUSED, UNDER ANY THEORY OF LIABILITY.
21
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the date first set forth above.
RUSH-PRESBYTERIAN-ST. LUKE’S MEDICAL CENTER
By: | /s/ James T. Frankenbach |
|
|
| Name: James T. Frankenbach |
| |
| Title: Senior Vice President |
| |
|
|
| |
|
|
| |
ACORDA THERAPEUTICS, INC. |
| ||
|
|
| |
|
|
| |
By: | /s/ Ron Cohen |
|
|
| Name: Ron Cohen |
| |
| Title: President and Chief Executive Officer |
| |
22
SCHEDULE 1.7
DIAGRAM OF COMPOUND
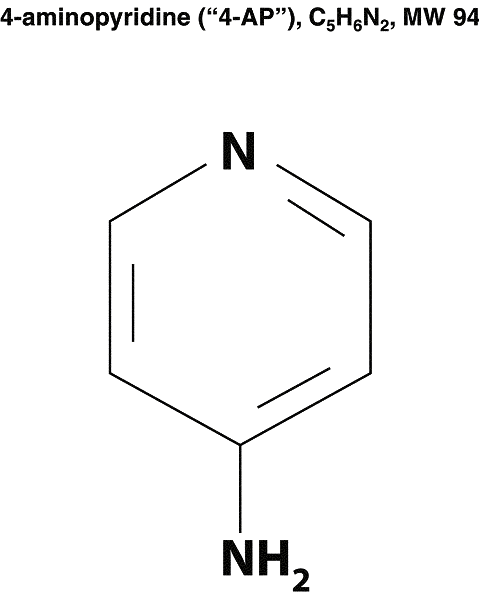
23
Certain portions of this Exhibit have been omitted pursuant to a request for confidentiality.
Such omitted portions, which are marked with brackets [ ] and an asterisk *, have been separately filed with the Commission.
SCHEDULE 1.11
ELAN PATENT RIGHTS
For purposes of this Agreement, Elan Patent Rights shall mean any and all rights under any and all patents and patent applications now existing, currently pending or hereafter filed, owned or acquired or licensed by Elan (and/or its Affiliates) which would be infringed by the manufacture, use or sale of the Product, the current status of which is set forth below. Elan Patent Rights shall also include all continuations, continuations-in-part, divisionals and re-issues of such patents and patent applications and any patents issuing thereon and extensions of any patents licensed hereunder. Elan Patent Rights shall further include any patents or patent applications covering any improved methods of making or using the Product invented or acquired by Elan (and/or its Affiliates) during the term of the Elan/Acorda Agreement and under which Elan (and/or its Affiliates) has a right to grant a licence under the Elan/Acorda Agreement, and Elan’s (and/or its Affiliates) interest in any intellectual property conceived reduced to practice or otherwise developed in connection with the Project (as defined in the Elan/Acorda Agreement).
1806 |
| Formulations and their use in the treatment of neurological diseases |
| Pending: |
| [* *] |
|
|
|
|
|
|
|
|
|
|
|
|
| Issued: |
|
|
|
|
|
|
| Australia |
| 657706 |
|
24
EXHIBIT 1.31
SIDE AGREEMENT
25