
Delivering Value Now and Into The Future David Hung, M.D. Founder, President and CEO Exhibit 99.1

Additional Information Forward-Looking Statements This presentation contains forward-looking statements. All statements relating to events or results that may occur in the future, including but not limited to statements regarding our future results of operations and financial position, estimated future sales of XTANDI®, market opportunity for our products and product candidates, potential regulatory approvals or events, and clinical trial events or progress, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Words such as “believe,” “opportunity,” “potential,” “expected,” “potentially,” “may,” “goals,” and similar expressions are intended to identify these forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors, including risks inherent in obtaining regulatory approvals, that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, you should not rely on these forward -looking statements as predictions of future events. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in forward-looking statements, including risks relating to relating to our business in general, see our Quarterly Report on Form 10-Q for the quarter ended March 31, 2016, filed with the SEC on May 5, 2016, under the caption “Risk Factors.” Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Additional Information This presentation is neither an offer to buy nor a solicitation of an offer to sell any securities of Medivation. No tender offer for the shares of Medivation has commenced at this time. In connection with its proposed transaction, Sanofi may file tender offer documents, consent solicitation documents or other documents with the U.S. Securities and Exchange Commission (“SEC”). If a tender offer and/or consent solicitation is commenced, Medivation will file with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to such tender offer and may file a solicitation of revocation in connection with such consent solicitation. Once filed, stockholders will be able to obtain, as applicable, the tender offer statement on Schedule TO, the offer to purchase, the Solicitation/Recommendation Statement of Medivation on Schedule 14D-9, any consent solicitation, any solicitation of revocation and related materials with respect to any tender offer or consent solicitation, free of charge, at the website of the SEC at www.sec.gov, and from any information agent and/or dealer manager named in the tender offer materials. Stockholders may also obtain, at no charge, any such documents filed with or furnished to the SEC by Medivation under the “SEC Filings” tab in the “Investor Relations” section of Medivation’s website atwww.medivation.com. Stockholders are advised to read these documents, if and when they become available, including any amendments thereto, as well as any other documents relating to any tender offer and/or consent solicitation that are filed with the SEC, carefully and in their entirety prior to making any decisions with respect to whether to tender shares or submit consents because the documents will contain important information. Certain Information Regarding Participants Medivation, its directors and certain of its executive officers may be deemed to be participants in the solicitation of revocations in connection with any Sanofi solicitation. Information regarding the names of Medivation’s directors and executive officers and their respective interests in Medivation by security holdings or otherwise is set forth in Medivation’s proxy statement for the 2016 Annual Meeting of Shareholders, as amended, filed with the SEC on April 29, 2016. Additional information can also be found in Medivation’s Annual Report on Form 10-K for the year ended December 31, 2015, filed with the SEC on February 26, 2016 and in Medivation’s latest Quarterly Report on Form 10-Q.

Non-GAAP Financial Measures
Table of Contents Medivation’s Compelling Value Proposition XTANDI Commercial Success and Runway for Growth in Prostate Cancer and Beyond Innovative Late-Stage Pipeline: Talazoparib and Pidilizumab Sanofi’s Substantially Inadequate and Opportunistically-Timed Proposal Conclusion

Medivation’s Compelling Value Proposition

Why We Are Here Graeme Today (with 3rd grandchild) Graeme in 2010 (given 3 weeks to live, enrolled in XTANDI AFFIRM trial) We seek to meaningfully improve the quality of life of patients and families suffering from serious disease by identifying and rapidly and efficiently developing and delivering medically innovative therapies
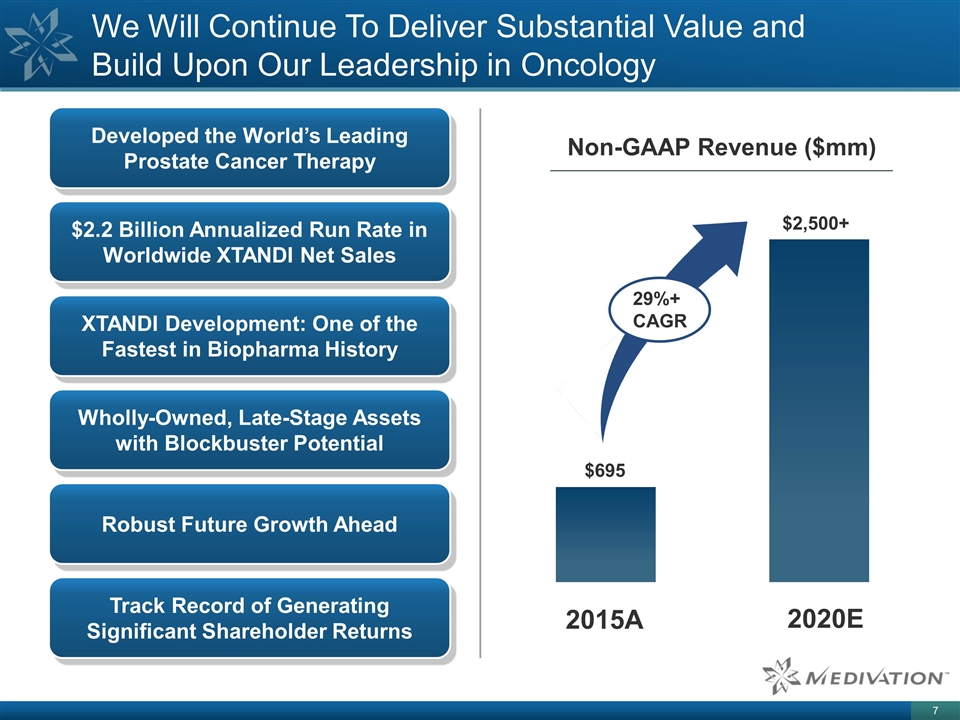
We Will Continue To Deliver Substantial Value and Build Upon Our Leadership in Oncology Developed the World’s Leading Prostate Cancer Therapy $2.2 Billion Annualized Run Rate in Worldwide XTANDI Net Sales Wholly-Owned, Late-Stage Assets with Blockbuster Potential XTANDI Development: One of the Fastest in Biopharma History Track Record of Generating Significant Shareholder Returns 2015A 2020E $695 $2,500+ Non-GAAP Revenue ($mm) 29%+ CAGR Robust Future Growth Ahead
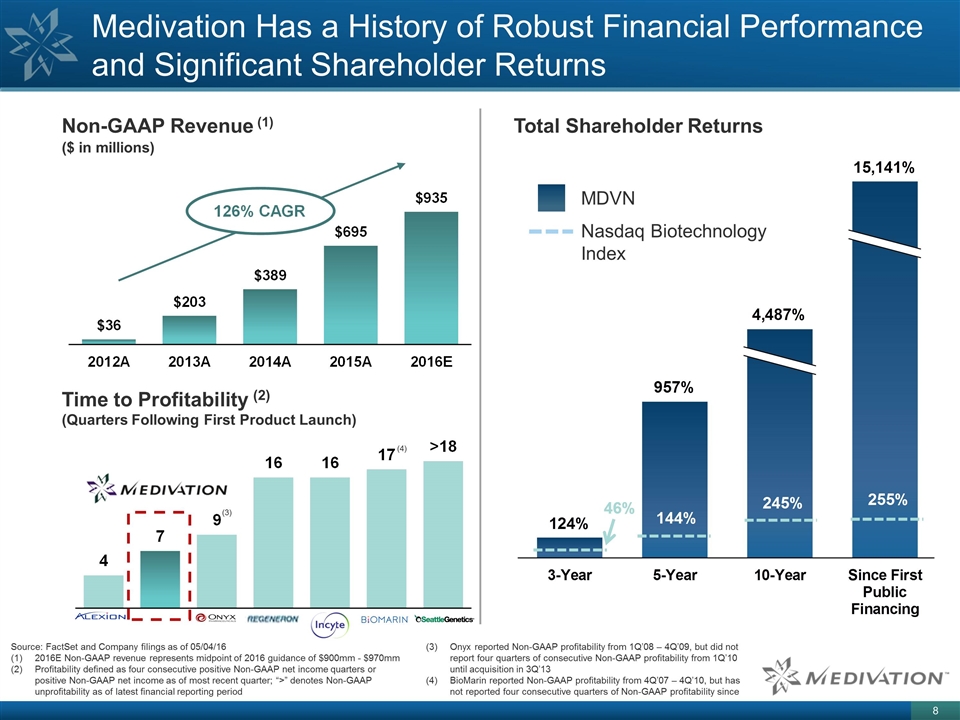
Medivation Has a History of Robust Financial Performance and Significant Shareholder Returns Source: FactSet and Company filings as of 05/04/16 2016E Non-GAAP revenue represents midpoint of 2016 guidance of $900mm - $970mm Profitability defined as four consecutive positive Non-GAAP net income quarters or positive Non-GAAP net income as of most recent quarter; “>” denotes Non-GAAP unprofitability as of latest financial reporting period Non-GAAP Revenue (1) Time to Profitability (2) (Quarters Following First Product Launch) ($ in millions) Total Shareholder Returns Onyx reported Non-GAAP profitability from 1Q’08 – 4Q’09, but did not report four quarters of consecutive Non-GAAP profitability from 1Q’10 until acquisition in 3Q’13 BioMarin reported Non-GAAP profitability from 4Q’07 – 4Q’10, but has not reported four consecutive quarters of Non-GAAP profitability since MDVN Nasdaq Biotechnology Index (3) (4)
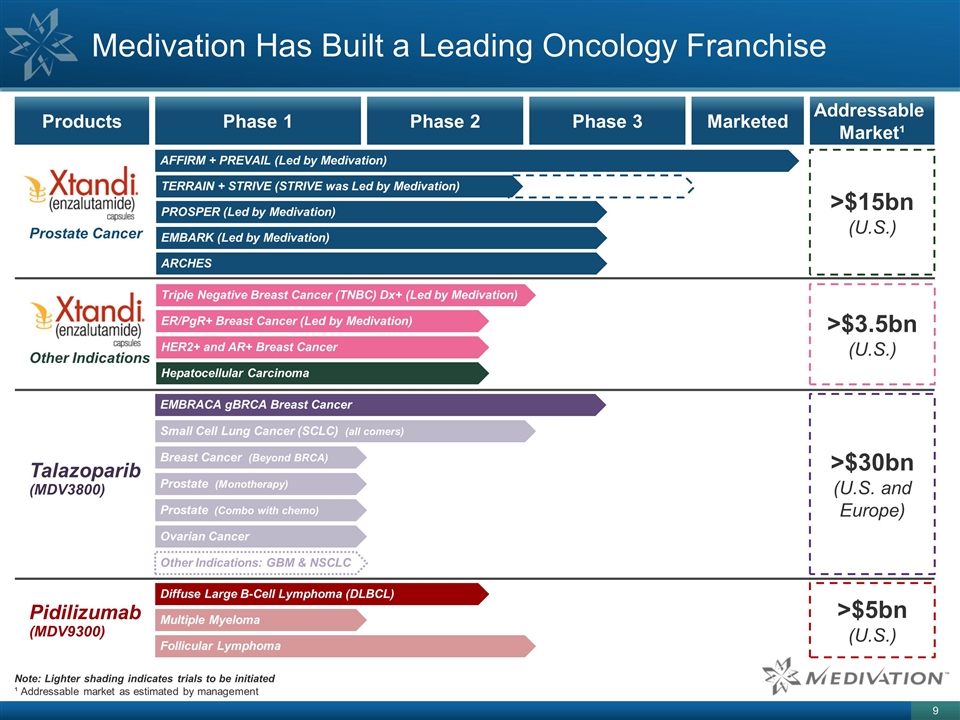
Talazoparib (MDV3800) Other Indications Medivation Has Built a Leading Oncology Franchise Products Phase 1 Phase 2 Phase 3 Marketed XTANDI Prostate Cancer AFFIRM + PREVAIL (Led by Medivation) TERRAIN + STRIVE (STRIVE was Led by Medivation) PROSPER (Led by Medivation) EMBARK (Led by Medivation) ARCHES Triple Negative Breast Cancer (TNBC) Dx+ (Led by Medivation) ER/PgR+ Breast Cancer (Led by Medivation) Hepatocellular Carcinoma EMBRACA gBRCA Breast Cancer Breast Cancer (Beyond BRCA) Prostate (Monotherapy) Prostate (Combo with chemo) Other Indications: GBM & NSCLC Small Cell Lung Cancer (SCLC) (all comers) Ovarian Cancer Pidilizumab (MDV9300) Diffuse Large B-Cell Lymphoma (DLBCL) Multiple Myeloma Follicular Lymphoma Note: Lighter shading indicates trials to be initiated ¹ Addressable market as estimated by management Addressable Market¹ >$15bn (U.S.) >$3.5bn (U.S.) >$30bn (U.S. and Europe) >$5bn (U.S.) HER2+ and AR+ Breast Cancer
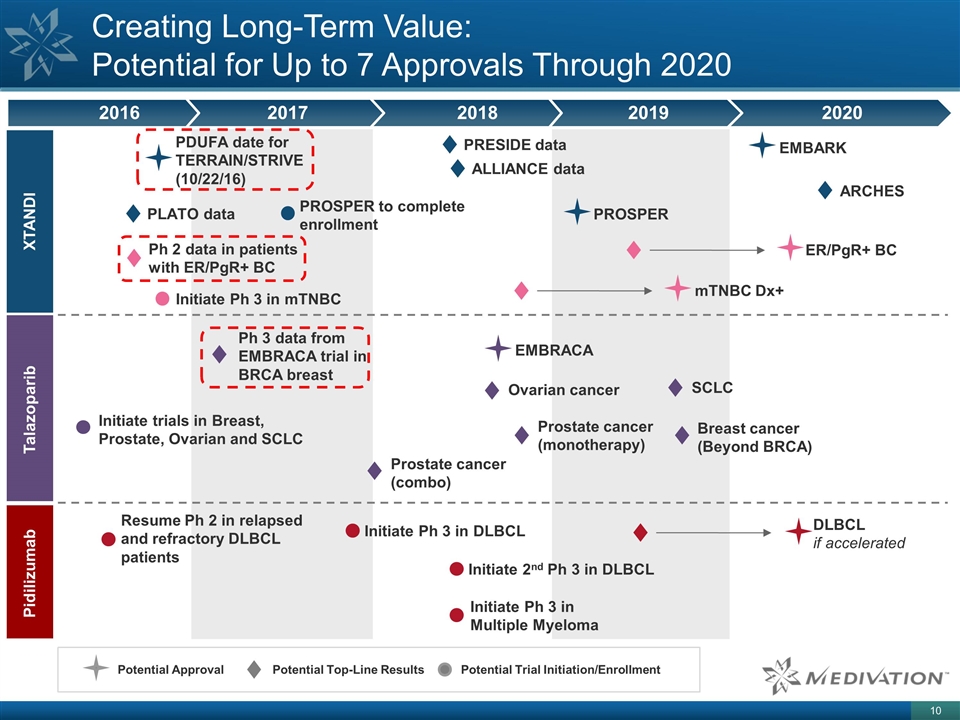
Creating Long-Term Value: Potential for Up to 7 Approvals Through 2020 Potential Top-Line Results Potential Approval Potential Trial Initiation/Enrollment XTANDI Talazoparib Pidilizumab 2016 2017 2018 2019 2020 mTNBC Dx+ ARCHES PROSPER EMBARK DLBCL if accelerated Ph 2 data in patients with ER/PgR+ BC PDUFA date for TERRAIN/STRIVE (10/22/16) EMBRACA Ph 3 data from EMBRACA trial in BRCA breast Initiate trials in Breast, Prostate, Ovarian and SCLC Initiate Ph 3 in mTNBC Resume Ph 2 in relapsed and refractory DLBCL patients PROSPER to complete enrollment ER/PgR+ BC PLATO data Initiate Ph 3 in DLBCL Initiate 2nd Ph 3 in DLBCL Initiate Ph 3 in Multiple Myeloma Breast cancer (Beyond BRCA) Ovarian cancer Prostate cancer (monotherapy) Prostate cancer (combo) SCLC PRESIDE data ALLIANCE data

XTANDI Commercial Success and Runway for Growth in Prostate Cancer and Beyond
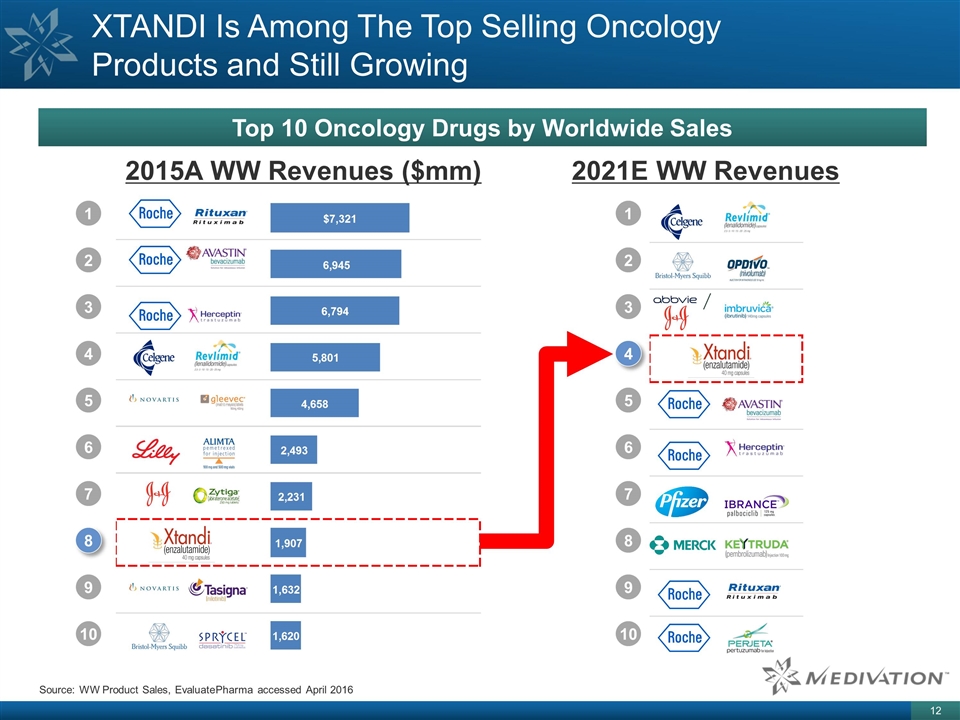
XTANDI Is Among The Top Selling Oncology Products and Still Growing Top 10 Oncology Drugs by Worldwide Sales Source: WW Product Sales, EvaluatePharma accessed April 2016 2015A WW Revenues ($mm) 2021E WW Revenues 1 2 3 4 5 6 7 8 9 10 1 2 3 4 5 6 7 8 9 10
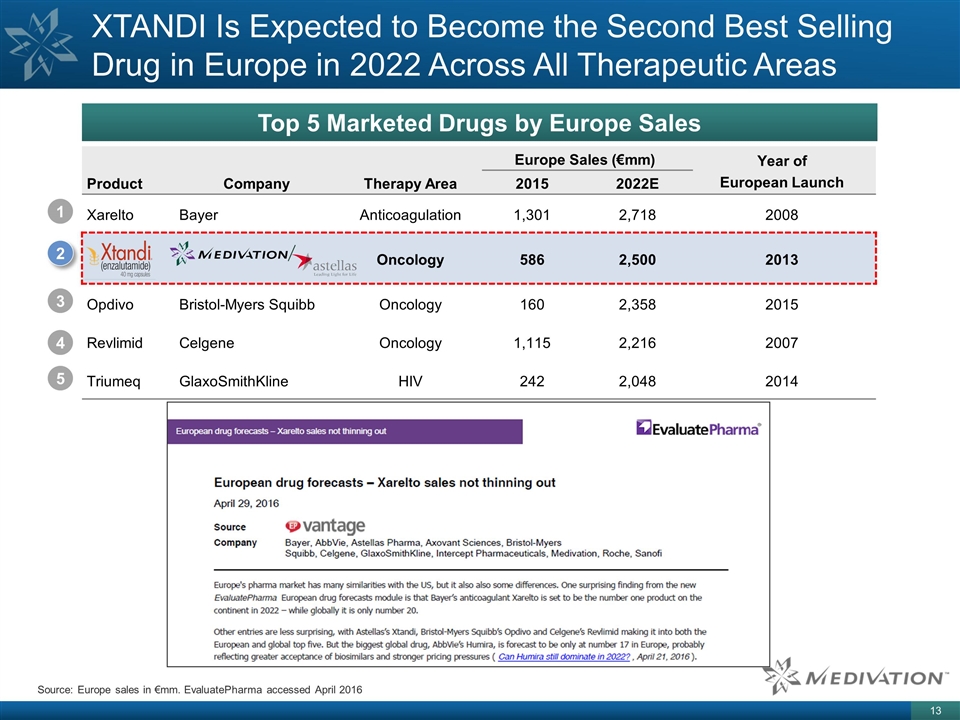
Europe Sales (€mm) Year of European Launch Product Company Therapy Area 2015 2022E Xarelto Bayer Anticoagulation 1,301 2,718 2008 Oncology 586 2,500 2013 Opdivo Bristol-Myers Squibb Oncology 160 2,358 2015 Revlimid Celgene Oncology 1,115 2,216 2007 Triumeq GlaxoSmithKline HIV 242 2,048 2014 XTANDI Is Expected to Become the Second Best Selling Drug in Europe in 2022 Across All Therapeutic Areas Top 5 Marketed Drugs by Europe Sales 1 2 3 4 5 Source: Europe sales in €mm. EvaluatePharma accessed April 2016
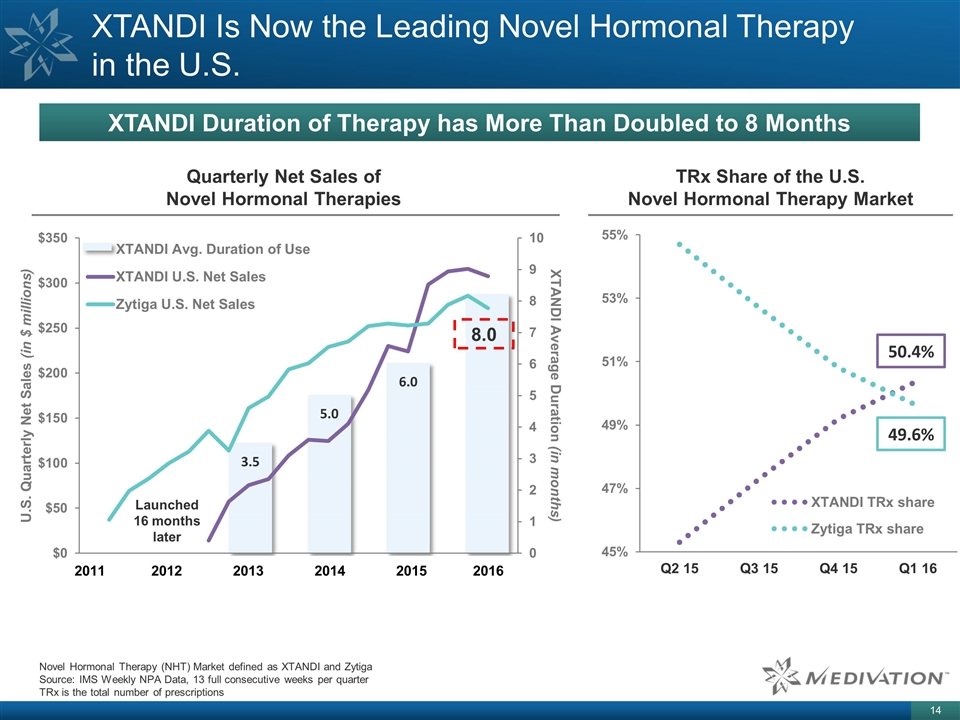
3.5 5.0 6.0 XTANDI Is Now the Leading Novel Hormonal Therapy in the U.S. Novel Hormonal Therapy (NHT) Market defined as XTANDI and Zytiga Source: IMS Weekly NPA Data, 13 full consecutive weeks per quarter TRx is the total number of prescriptions 2011 2012 2013 2014 2015 2016 Launched 16 months later 50.4% TRx Share of the U.S. Novel Hormonal Therapy Market Quarterly Net Sales of Novel Hormonal Therapies 8.0 49.6% XTANDI Duration of Therapy has More Than Doubled to 8 Months
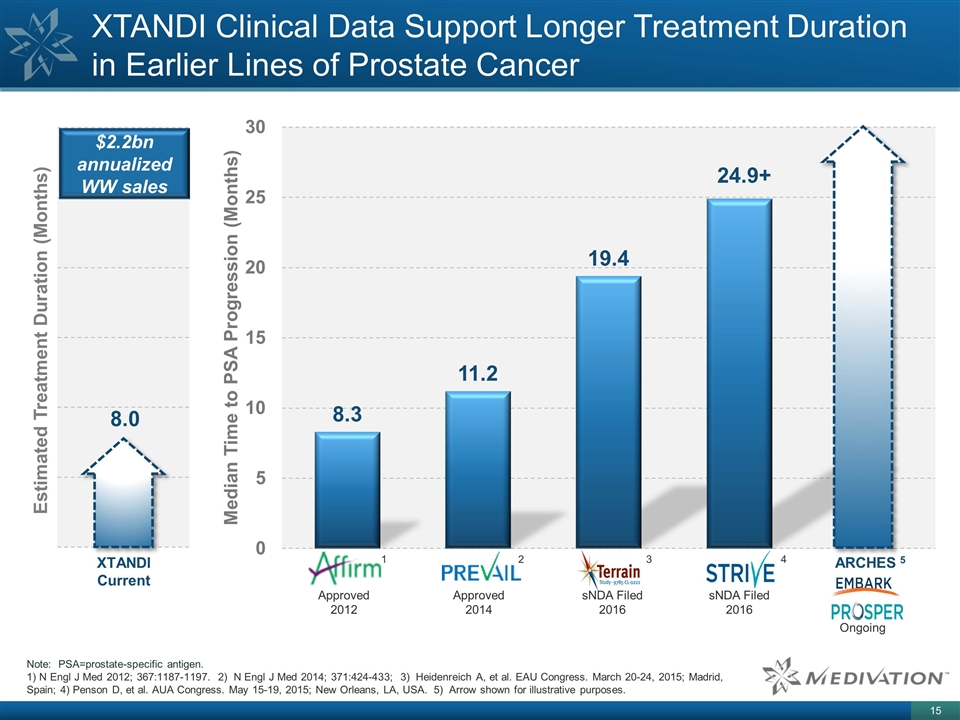
XTANDI Clinical Data Support Longer Treatment Duration in Earlier Lines of Prostate Cancer ARCHES 5 Note: PSA=prostate-specific antigen. N Engl J Med 2012; 367:1187-1197. 2) N Engl J Med 2014; 371:424-433; 3) Heidenreich A, et al. EAU Congress. March 20-24, 2015; Madrid, Spain; 4) Penson D, et al. AUA Congress. May 15-19, 2015; New Orleans, LA, USA. 5) Arrow shown for illustrative purposes. Approved 2014 Ongoing Approved 2012 sNDA Filed 2016 sNDA Filed 2016 24.9+ 1 2 3 4 8.0 XTANDI Current $2.2bn annualized WW sales Estimated Treatment Duration (Months)
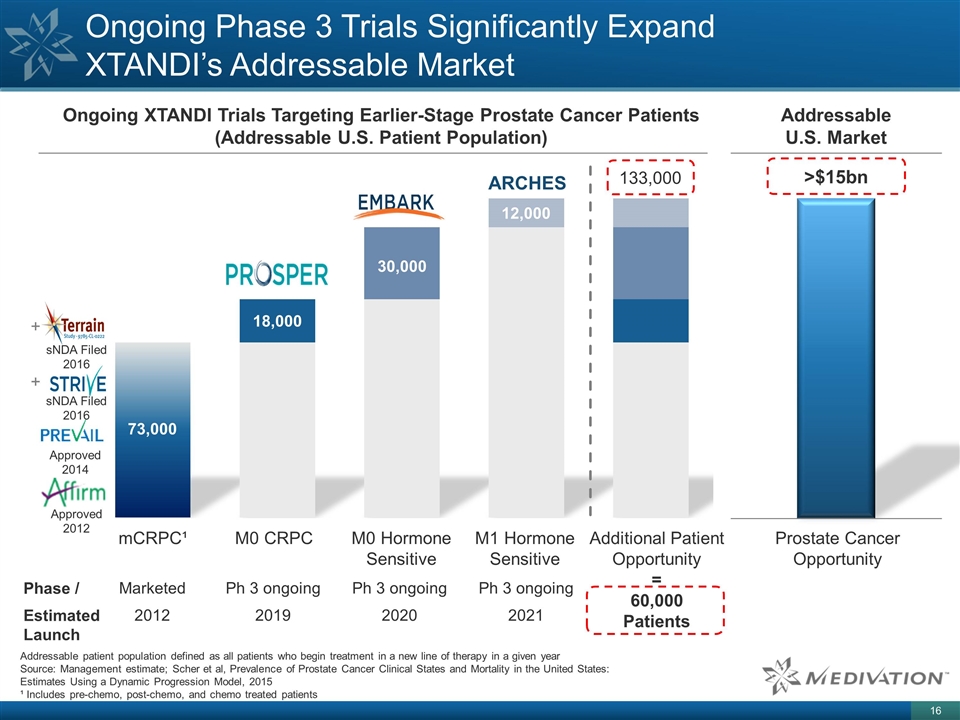
Ongoing Phase 3 Trials Significantly Expand XTANDI’s Addressable Market 73,000 18,000 30,000 12,000 mCRPC¹ M0 CRPC M0 Hormone Sensitive M1 Hormone Sensitive Additional Patient Opportunity = 60,000 Patients ARCHES Addressable patient population defined as all patients who begin treatment in a new line of therapy in a given year Source: Management estimate; Scher et al, Prevalence of Prostate Cancer Clinical States and Mortality in the United States: Estimates Using a Dynamic Progression Model, 2015 ¹ Includes pre-chemo, post-chemo, and chemo treated patients Approved 2014 Ph 3 ongoing 2019 Ph 3 ongoing 2020 Ph 3 ongoing 2021 133,000 + + Approved 2012 sNDA Filed 2016 sNDA Filed 2016 Ongoing XTANDI Trials Targeting Earlier-Stage Prostate Cancer Patients (Addressable U.S. Patient Population) Phase / Estimated Launch Marketed 2012 Addressable U.S. Market Prostate Cancer Opportunity >$15bn
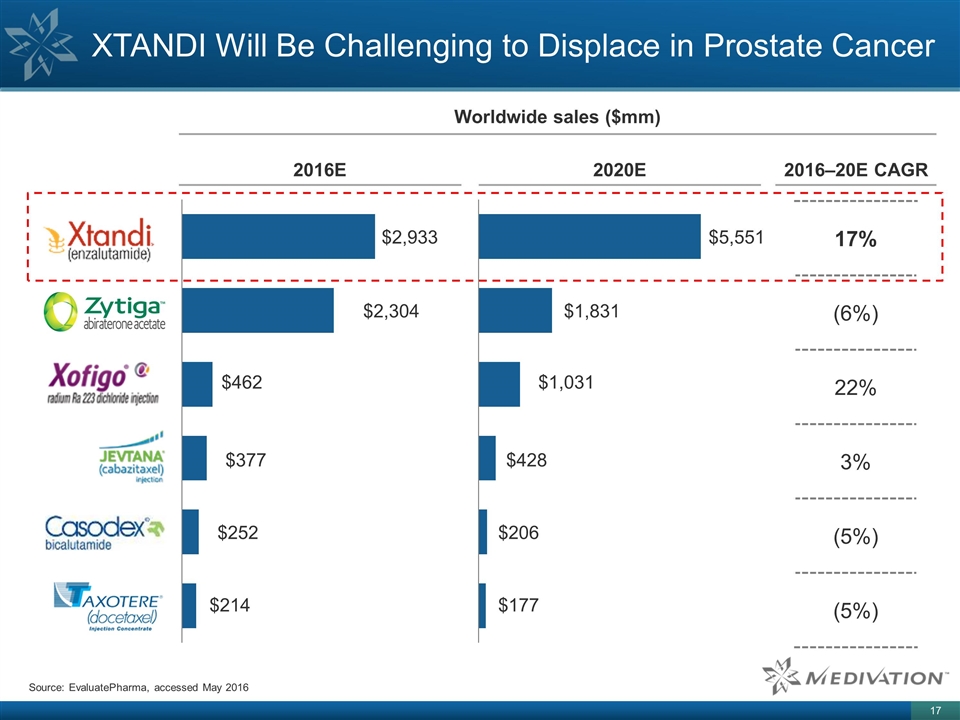
XTANDI Will Be Challenging to Displace in Prostate Cancer Source: EvaluatePharma, accessed May 2016 Worldwide sales ($mm) 2016E 2020E 2016–20E CAGR 17% (6%) 22% 3% (5%) (5%)
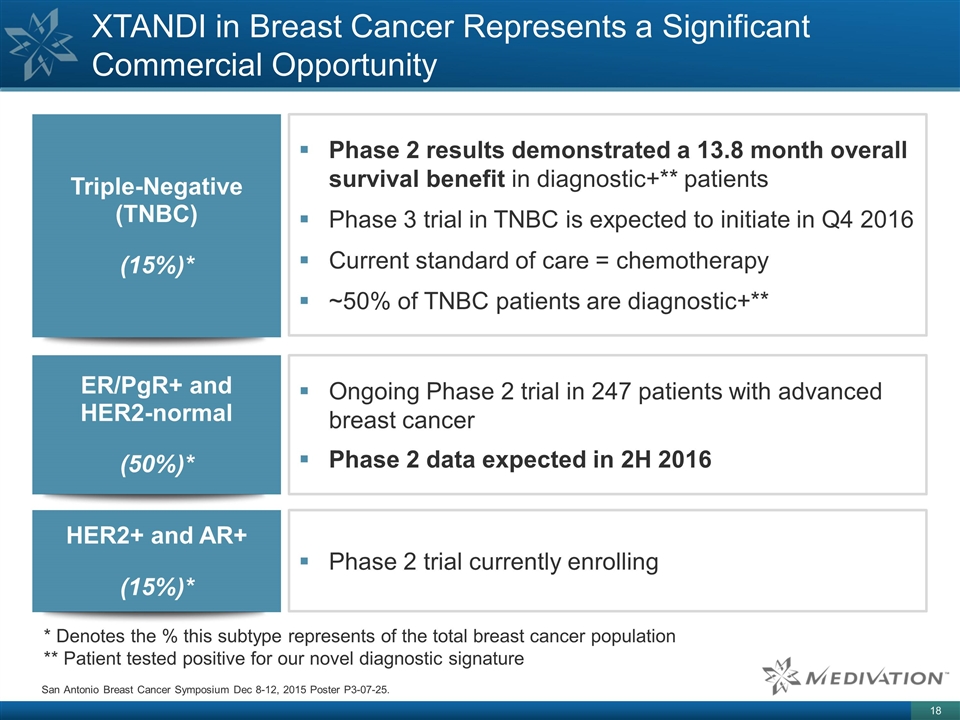
XTANDI in Breast Cancer Represents a Significant Commercial Opportunity Triple-Negative (TNBC) (15%)* ER/PgR+ and HER2-normal (50%)* HER2+ and AR+ (15%)* San Antonio Breast Cancer Symposium Dec 8-12, 2015 Poster P3-07-25. * Denotes the % this subtype represents of the total breast cancer population ** Patient tested positive for our novel diagnostic signature Phase 2 results demonstrated a 13.8 month overall survival benefit in diagnostic+** patients Phase 3 trial in TNBC is expected to initiate in Q4 2016 Current standard of care = chemotherapy ~50% of TNBC patients are diagnostic+** Ongoing Phase 2 trial in 247 patients with advanced breast cancer Phase 2 data expected in 2H 2016 Phase 2 trial currently enrolling
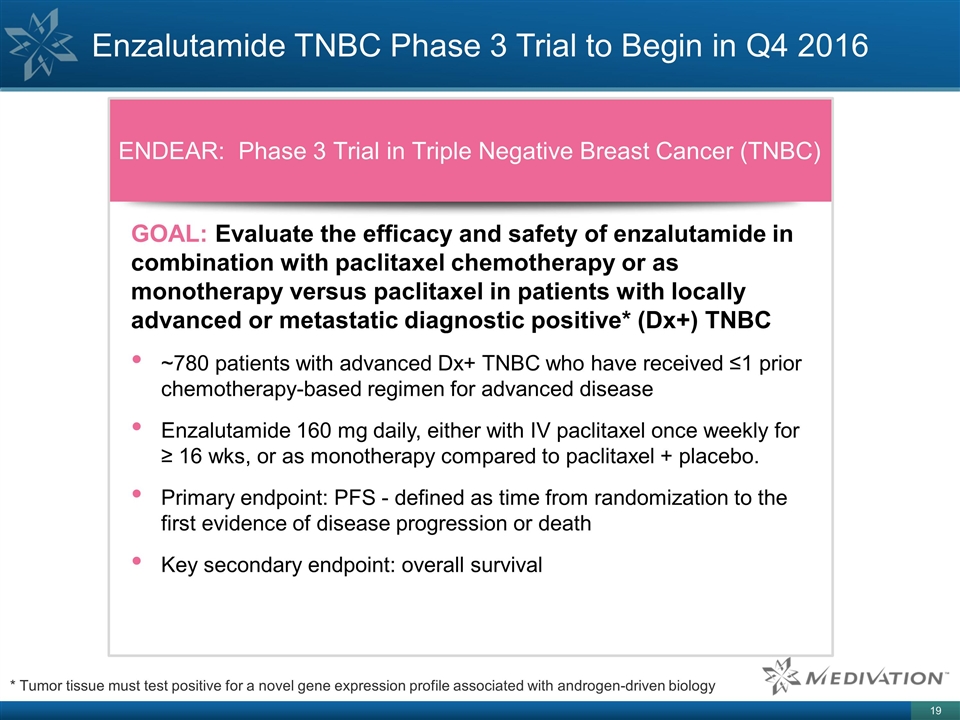
Enzalutamide TNBC Phase 3 Trial to Begin in Q4 2016 ENDEAR: Phase 3 Trial in Triple Negative Breast Cancer (TNBC) GOAL: Evaluate the efficacy and safety of enzalutamide in combination with paclitaxel chemotherapy or as monotherapy versus paclitaxel in patients with locally advanced or metastatic diagnostic positive* (Dx+) TNBC ~780 patients with advanced Dx+ TNBC who have received ≤1 prior chemotherapy-based regimen for advanced disease Enzalutamide 160 mg daily, either with IV paclitaxel once weekly for ≥ 16 wks, or as monotherapy compared to paclitaxel + placebo. Primary endpoint: PFS - defined as time from randomization to the first evidence of disease progression or death Key secondary endpoint: overall survival * Tumor tissue must test positive for a novel gene expression profile associated with androgen-driven biology
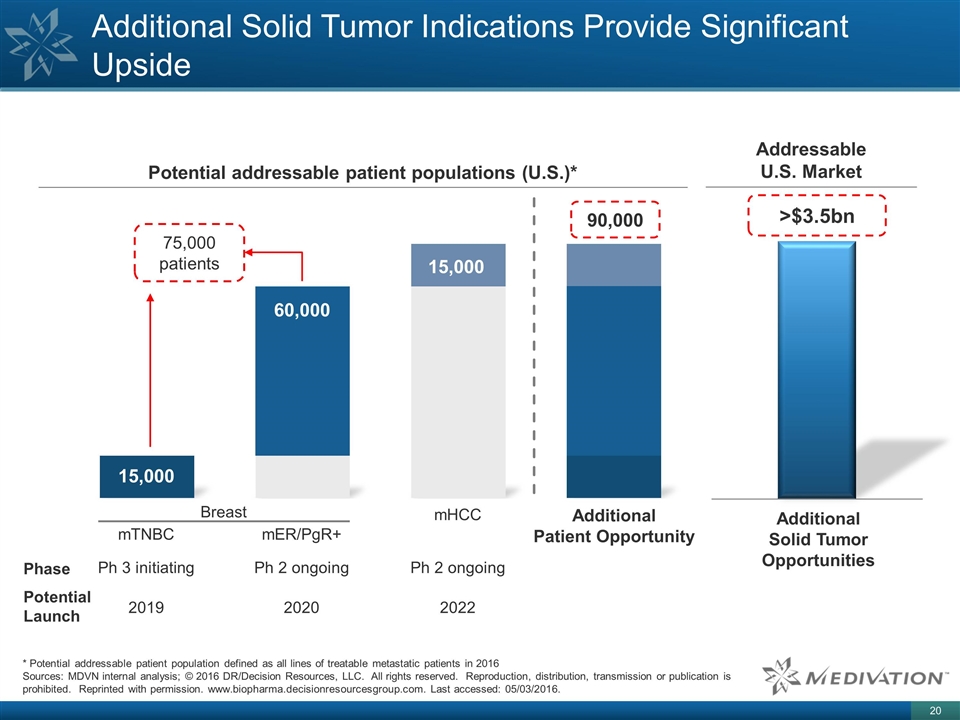
Additional Solid Tumor Indications Provide Significant Upside * Potential addressable patient population defined as all lines of treatable metastatic patients in 2016 Sources: MDVN internal analysis; © 2016 DR/Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. www.biopharma.decisionresourcesgroup.com. Last accessed: 05/03/2016. Potential addressable patient populations (U.S.)* Addressable U.S. Market 90,000 60,000 15,000 Additional Solid Tumor Opportunities >$3.5bn 75,000 patients Additional Patient Opportunity 15,000 Potential Launch Phase Ph 2 ongoing 2022 Ph 3 initiating 2019 mHCC mTNBC Ph 2 ongoing 2020 mER/PgR+ Breast
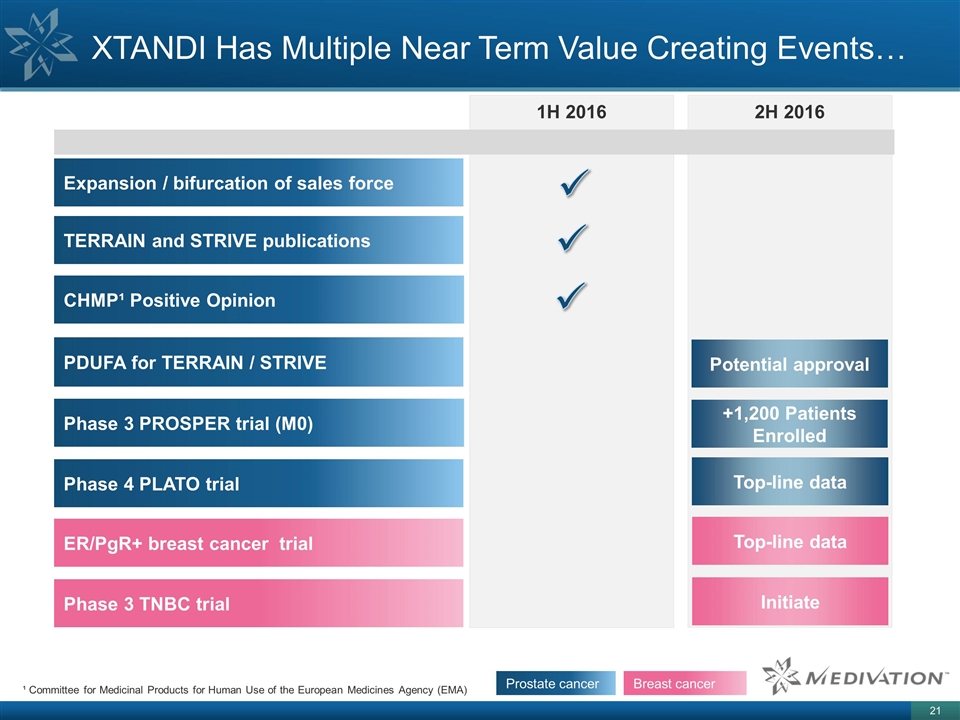
1H 2016 2H 2016 XTANDI Has Multiple Near Term Value Creating Events… CHMP¹ Positive Opinion Phase 4 PLATO trial Phase 3 TNBC trial Initiate ER/PgR+ breast cancer trial Top-line data TERRAIN and STRIVE publications Expansion / bifurcation of sales force PDUFA for TERRAIN / STRIVE Potential approval Phase 3 PROSPER trial (M0) Top-line data +1,200 Patients Enrolled ü ü ü Prostate cancer Breast cancer ¹ Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA)
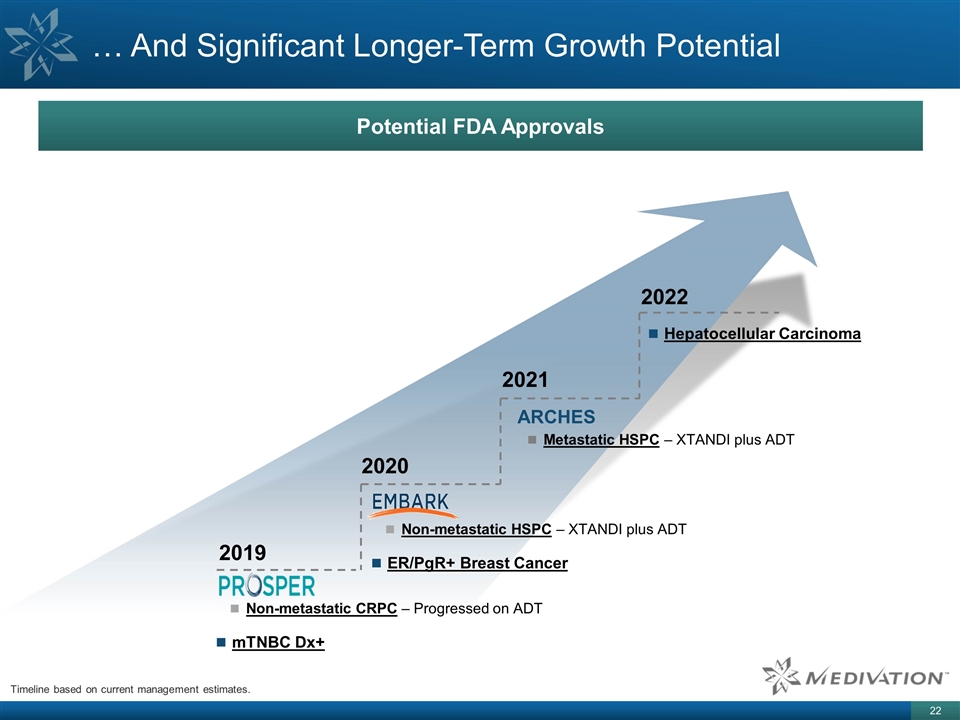
… And Significant Longer-Term Growth Potential Timeline based on current management estimates. 2019 2020 2021 Non-metastatic CRPC – Progressed on ADT mTNBC Dx+ 2022 Non-metastatic HSPC – XTANDI plus ADT ER/PgR+ Breast Cancer ARCHES Metastatic HSPC – XTANDI plus ADT Hepatocellular Carcinoma Potential FDA Approvals

Innovative Late-Stage Pipeline: Talazoparib and Pidilizumab
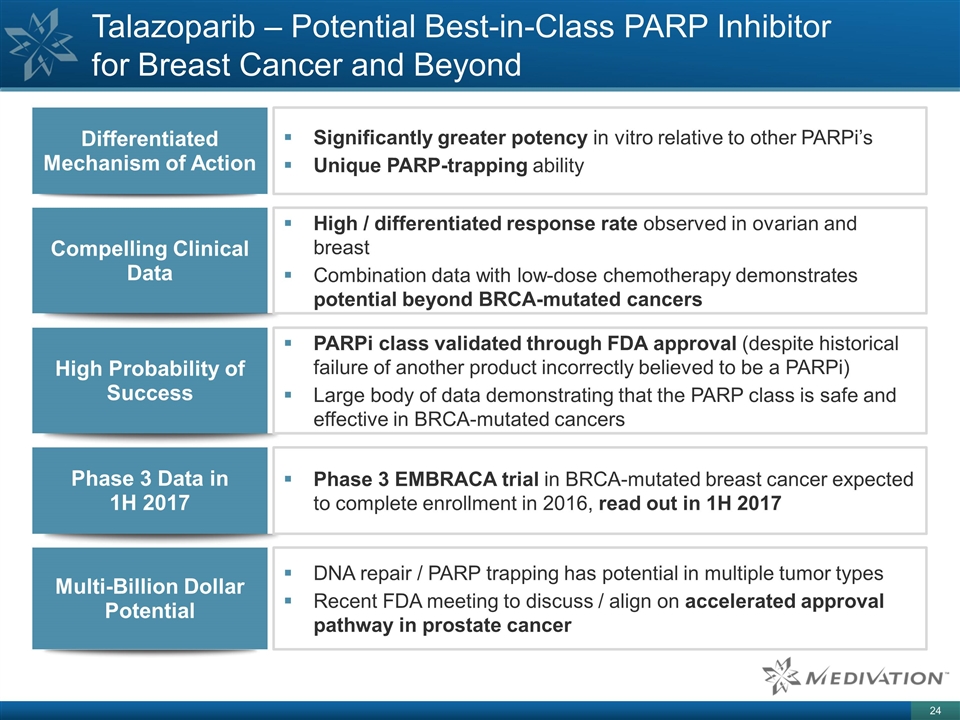
Talazoparib – Potential Best-in-Class PARP Inhibitor for Breast Cancer and Beyond Significantly greater potency in vitro relative to other PARPi’s Unique PARP-trapping ability Differentiated Mechanism of Action Compelling Clinical Data High / differentiated response rate observed in ovarian and breast Combination data with low-dose chemotherapy demonstrates potential beyond BRCA-mutated cancers Phase 3 Data in 1H 2017 Phase 3 EMBRACA trial in BRCA-mutated breast cancer expected to complete enrollment in 2016, read out in 1H 2017 Multi-Billion Dollar Potential DNA repair / PARP trapping has potential in multiple tumor types Recent FDA meeting to discuss / align on accelerated approval pathway in prostate cancer High Probability of Success PARPi class validated through FDA approval (despite historical failure of another product incorrectly believed to be a PARPi) Large body of data demonstrating that the PARP class is safe and effective in BRCA-mutated cancers
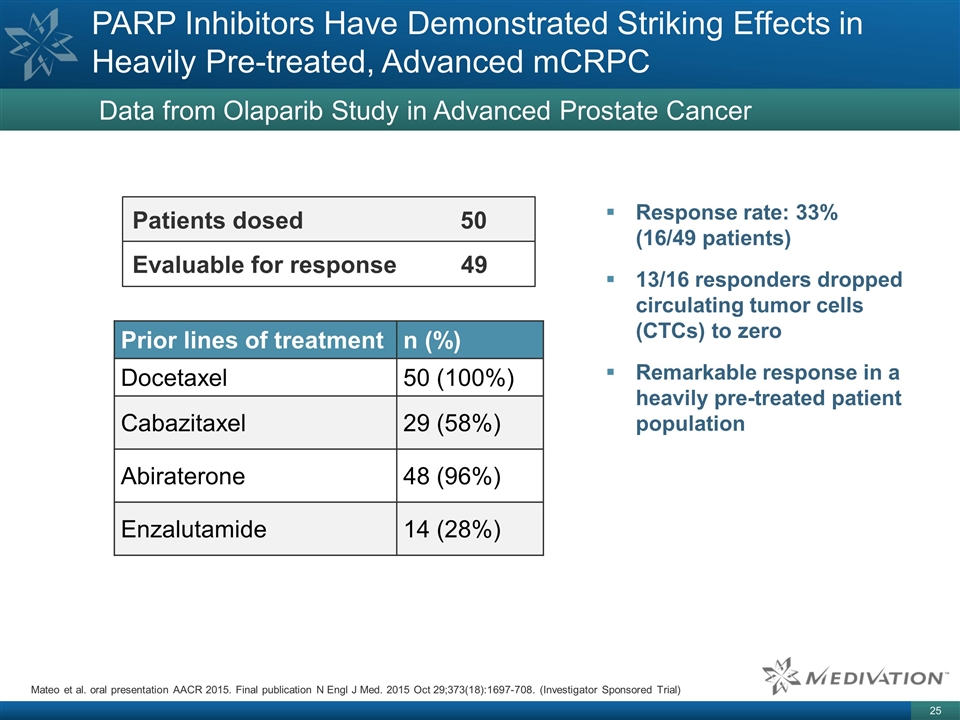
PARP Inhibitors Have Demonstrated Striking Effects in Heavily Pre-treated, Advanced mCRPC Data from Olaparib Study in Advanced Prostate Cancer Patients dosed 50 Evaluable for response 49 Prior lines of treatment n (%) Docetaxel 50 (100%) Cabazitaxel 29 (58%) Abiraterone 48 (96%) Enzalutamide 14 (28%) Mateo et al. oral presentation AACR 2015. Final publication N Engl J Med. 2015 Oct 29;373(18):1697-708. (Investigator Sponsored Trial) Response rate: 33% (16/49 patients) 13/16 responders dropped circulating tumor cells (CTCs) to zero Remarkable response in a heavily pre-treated patient population
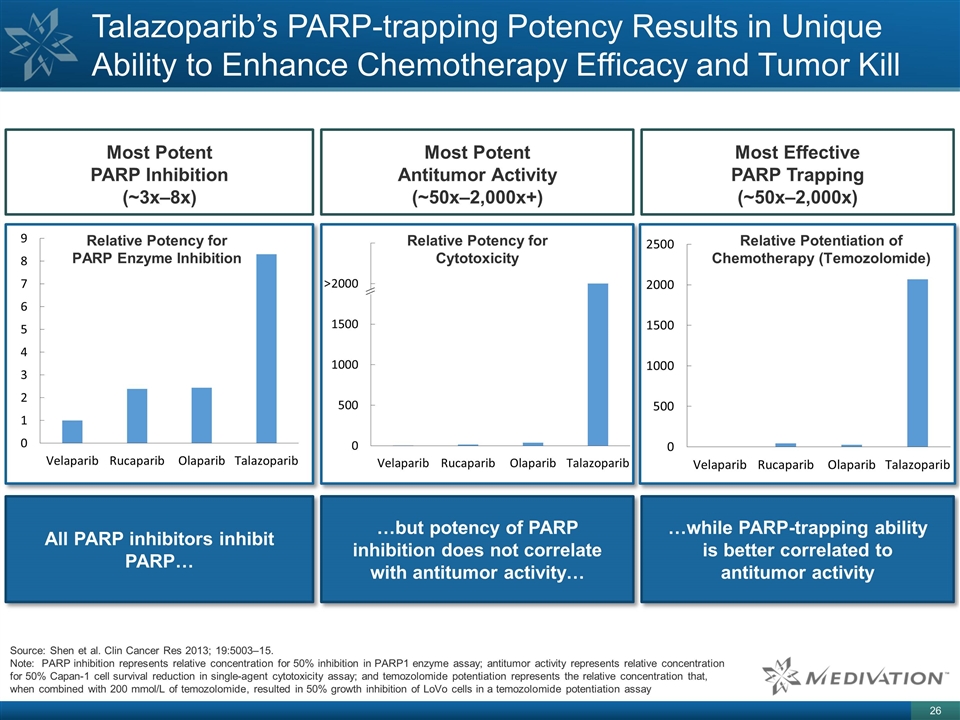
Source: Shen et al. Clin Cancer Res 2013; 19:5003–15. Note: PARP inhibition represents relative concentration for 50% inhibition in PARP1 enzyme assay; antitumor activity represents relative concentration for 50% Capan-1 cell survival reduction in single-agent cytotoxicity assay; and temozolomide potentiation represents the relative concentration that, when combined with 200 mmol/L of temozolomide, resulted in 50% growth inhibition of LoVo cells in a temozolomide potentiation assay All PARP inhibitors inhibit PARP… …but potency of PARP inhibition does not correlate with antitumor activity… …while PARP-trapping ability is better correlated to antitumor activity Most Potent PARP Inhibition (~3x–8x) Most Potent Antitumor Activity (~50x–2,000x+) Most Effective PARP Trapping (~50x–2,000x) Relative Potency for PARP Enzyme Inhibition Relative Potency for Cytotoxicity Relative Potentiation of Chemotherapy (Temozolomide) Talazoparib’s PARP-trapping Potency Results in Unique Ability to Enhance Chemotherapy Efficacy and Tumor Kill
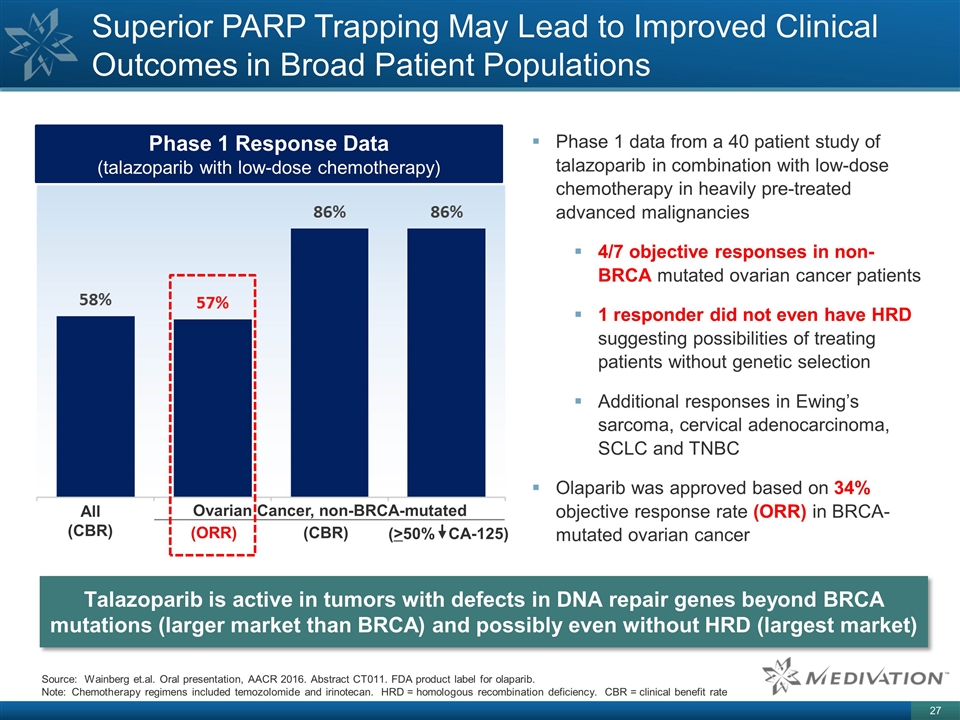
Phase 1 Response Data (talazoparib with low-dose chemotherapy) Superior PARP Trapping May Lead to Improved Clinical Outcomes in Broad Patient Populations Phase 1 data from a 40 patient study of talazoparib in combination with low-dose chemotherapy in heavily pre-treated advanced malignancies 4/7 objective responses in non-BRCA mutated ovarian cancer patients 1 responder did not even have HRD suggesting possibilities of treating patients without genetic selection Additional responses in Ewing’s sarcoma, cervical adenocarcinoma, SCLC and TNBC Olaparib was approved based on 34% objective response rate (ORR) in BRCA-mutated ovarian cancer Talazoparib is active in tumors with defects in DNA repair genes beyond BRCA mutations (larger market than BRCA) and possibly even without HRD (largest market) All (CBR) Ovarian Cancer, non-BRCA-mutated (ORR) (CBR) (>50% CA-125) Source: Wainberg et.al. Oral presentation, AACR 2016. Abstract CT011. FDA product label for olaparib. Note: Chemotherapy regimens included temozolomide and irinotecan. HRD = homologous recombination deficiency. CBR = clinical benefit rate
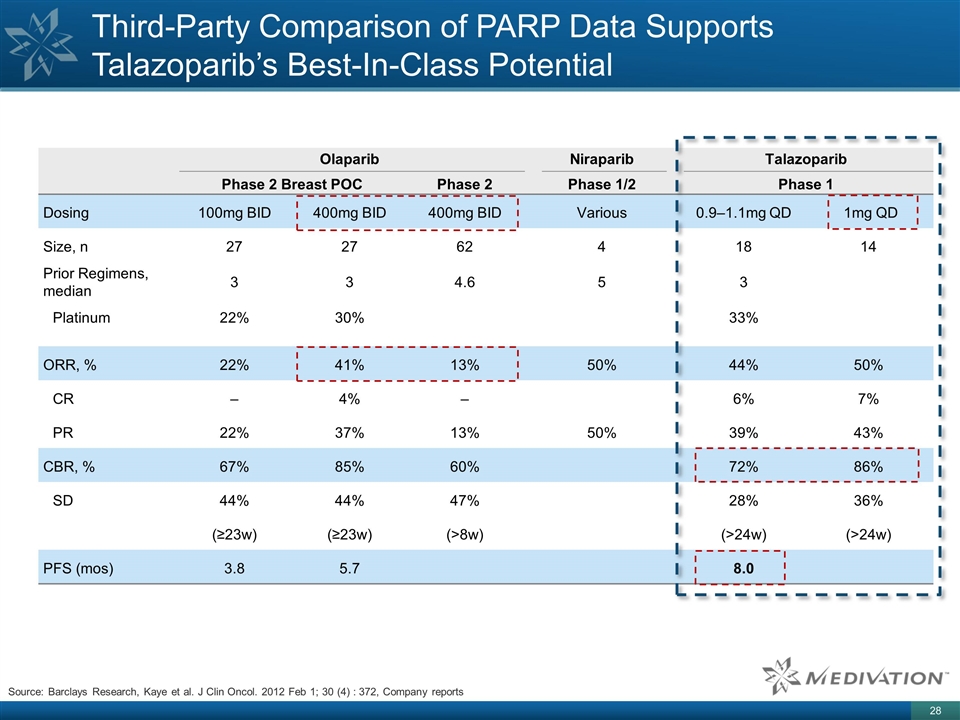
Third-Party Comparison of PARP Data Supports Talazoparib’s Best-In-Class Potential Olaparib Niraparib Talazoparib Phase 2 Breast POC Phase 2 Phase 1/2 Phase 1 Dosing 100mg BID 400mg BID 400mg BID Various 0.9–1.1mg QD 1mg QD Size, n 27 27 62 4 18 14 Prior Regimens, median 3 3 4.6 5 3 Platinum 22% 30% 33% ORR, % 22% 41% 13% 50% 44% 50% CR – 4% – 6% 7% PR 22% 37% 13% 50% 39% 43% CBR, % 67% 85% 60% 72% 86% SD 44% 44% 47% 28% 36% (≥23w) (≥23w) (>8w) (>24w) (>24w) PFS (mos) 3.8 5.7 8.0 Source: Barclays Research, Kaye et al. J Clin Oncol. 2012 Feb 1; 30 (4) : 372, Company reports
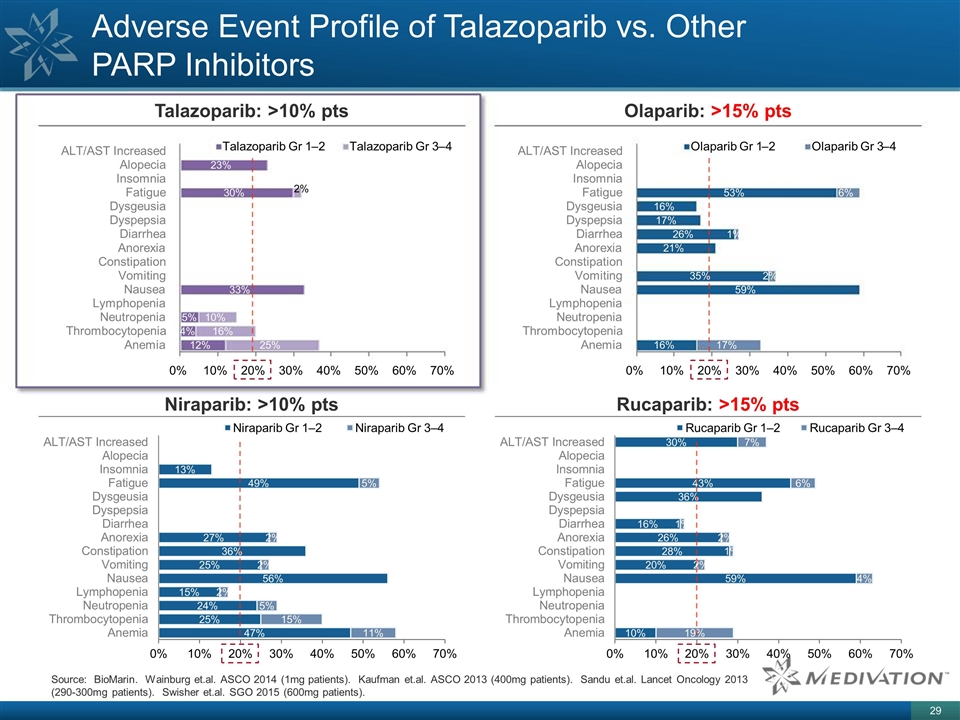
Adverse Event Profile of Talazoparib vs. Other PARP Inhibitors Talazoparib: >10% pts Niraparib: >10% pts Olaparib: >15% pts Rucaparib: >15% pts Source: BioMarin. Wainburg et.al. ASCO 2014 (1mg patients). Kaufman et.al. ASCO 2013 (400mg patients). Sandu et.al. Lancet Oncology 2013 (290-300mg patients). Swisher et.al. SGO 2015 (600mg patients). 12% 4% 5% 33% 30% 23% 25% 16% 10% 2% Anemia Thrombocytopenia Neutropenia Lymphopenia Nausea Vomiting Constipation Anorexia Diarrhea Dyspepsia Dysgeusia Fatigue Insomnia Alopecia ALT/AST Increased 0% 10% 20% 30% 40% 50% 60% 70% Talazoparib Gr 1 – 2 Talazoparib Gr 3 – 4 16% 59% 35% 21% 26% 17% 16% 53% 17% 2% 1% 6% Anemia Thrombocytopenia Neutropenia Lymphopenia Nausea Vomiting Constipation Anorexia Diarrhea Dyspepsia Dysgeusia Fatigue Insomnia Alopecia ALT/AST Increased 0% 10% 20% 30% 40% 50% 60% 70% Olaparib Gr 1 – 2 Olaparib Gr 3 – 4
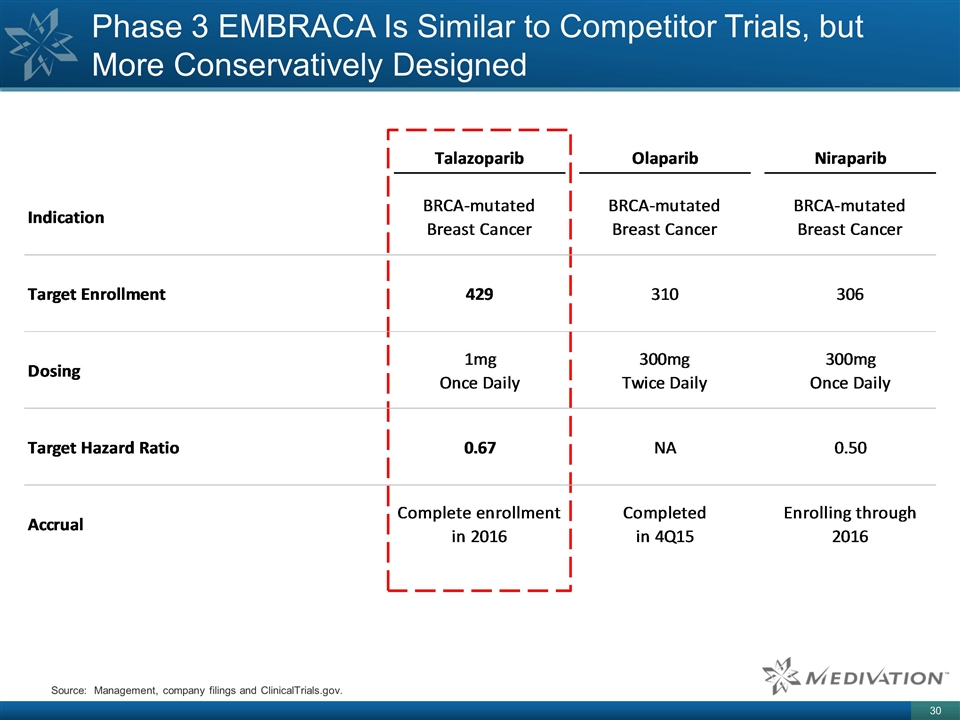
Phase 3 EMBRACA Is Similar to Competitor Trials, but More Conservatively Designed Source: Management, company filings and ClinicalTrials.gov.
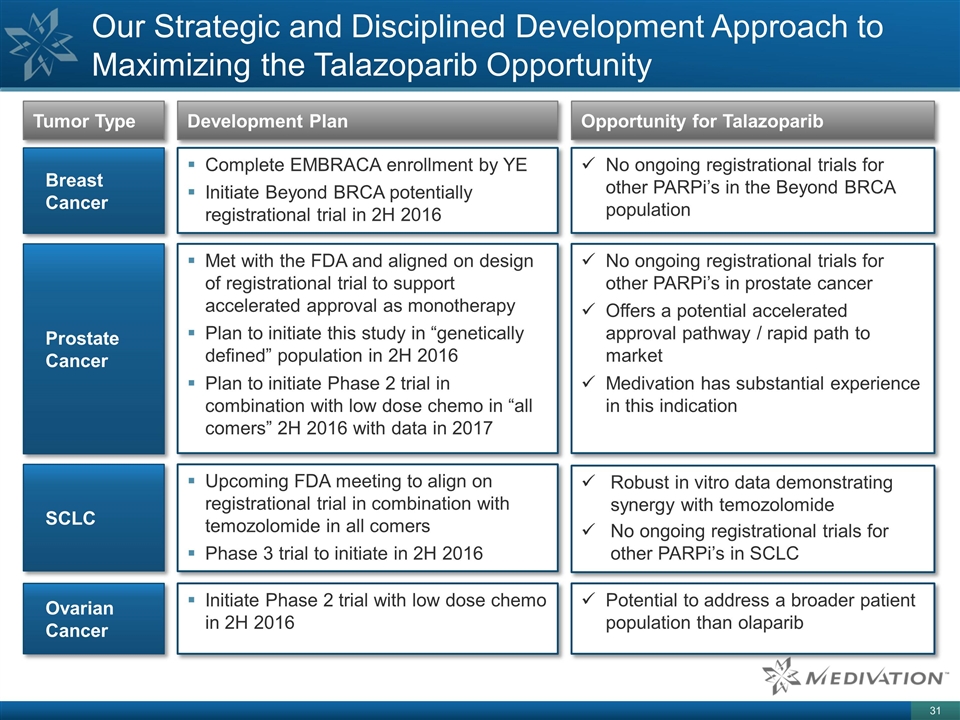
Our Strategic and Disciplined Development Approach to Maximizing the Talazoparib Opportunity Tumor Type Development Plan Opportunity for Talazoparib Breast Cancer Complete EMBRACA enrollment by YE Initiate Beyond BRCA potentially registrational trial in 2H 2016 No ongoing registrational trials for other PARPi’s in the Beyond BRCA population Prostate Cancer Met with the FDA and aligned on design of registrational trial to support accelerated approval as monotherapy Plan to initiate this study in “genetically defined” population in 2H 2016 Plan to initiate Phase 2 trial in combination with low dose chemo in “all comers” 2H 2016 with data in 2017 No ongoing registrational trials for other PARPi’s in prostate cancer Offers a potential accelerated approval pathway / rapid path to market Medivation has substantial experience in this indication SCLC Upcoming FDA meeting to align on registrational trial in combination with temozolomide in all comers Phase 3 trial to initiate in 2H 2016 Robust in vitro data demonstrating synergy with temozolomide No ongoing registrational trials for other PARPi’s in SCLC Ovarian Cancer Initiate Phase 2 trial with low dose chemo in 2H 2016 Potential to address a broader patient population than olaparib
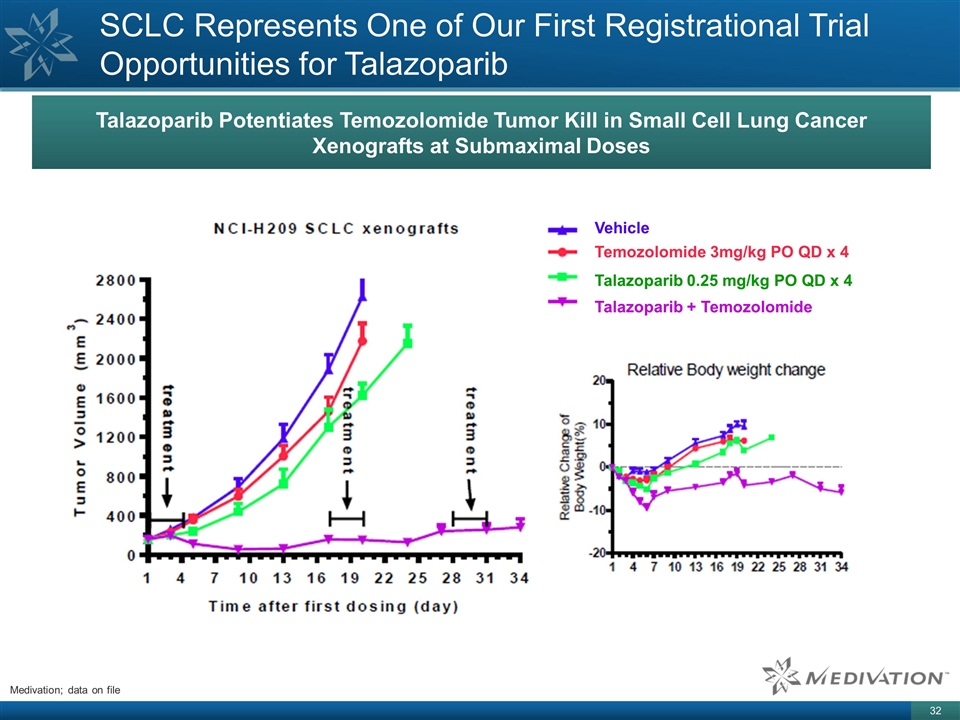
Talazoparib Potentiates Temozolomide Tumor Kill in Small Cell Lung Cancer Xenografts at Submaximal Doses SCLC Represents One of Our First Registrational Trial Opportunities for Talazoparib Vehicle Temozolomide 3mg/kg PO QD x 4 Talazoparib 0.25 mg/kg PO QD x 4 Talazoparib + Temozolomide Medivation; data on file
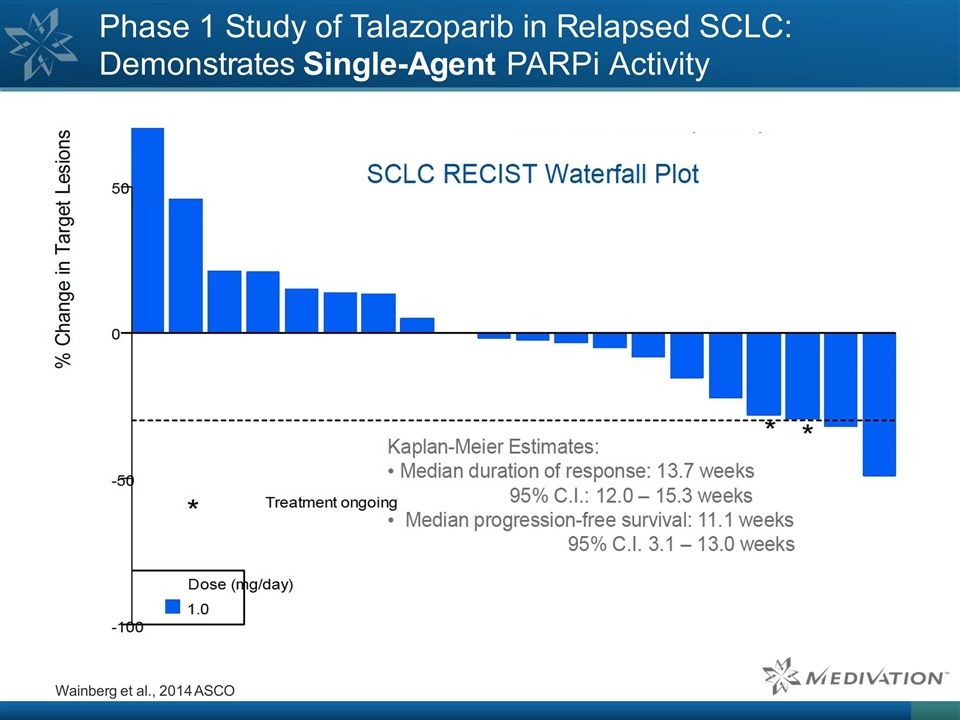
Phase 1 Study of Talazoparib in Relapsed SCLC: Demonstrates Single-Agent PARPi Activity Wainberg et al., 2014 ASCO
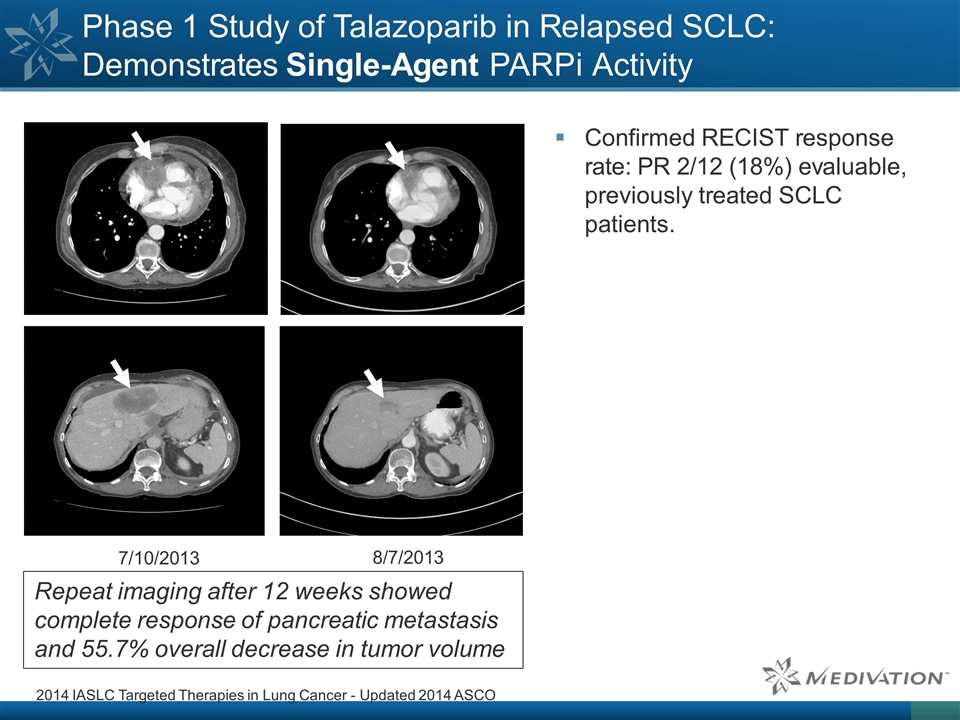
Phase 1 Study of Talazoparib in Relapsed SCLC: Demonstrates Single-Agent PARPi Activity Confirmed RECIST response rate: PR 2/12 (18%) evaluable, previously treated SCLC patients. 2014 IASLC Targeted Therapies in Lung Cancer - Updated 2014 ASCO 7/10/20138/7/2013 Repeat imaging after 12 weeks showed complete response of pancreatic metastasis and 55.7% overall decrease in tumor volume
Talazoparib in Other Indications Talazoparib arm of Phase 2 trial activated in May 2016 Sponsored by QuantumLeap Healthcare Collaborative I-SPY 2 Trial: Talazoparib + Irinotecan in Neoadjuvant Setting Goal: Compare pCR rates in women with early breast cancer when treated in the neoadjuvant setting with either talazoparib + irinotecan or paclitaxel followed by standard chemotherapy Up to 75 women with HER-2 negative breast cancer will be randomized into the talazoparib arm in an adaptive manner Talazoparib treatment arm: Talazoparib (1 mg daily) + low dose irinotecan (25 mg/m2 IV every two weeks) for 12 weeks followed by 8-12 wks of treatment with doxorubicin and cyclophosphamide treatment Control treatment arm: Patients in the comparator arm will receive 12 wks of standard paclitaxel therapy followed by 8-12 wks of doxorubicin and cyclophosphamide treatment Primary endpoint: Pathologic complete response (pCR) rate pCR defined as absence of clinical and pathological evidence of invasive tumor in breast or lymph nodes
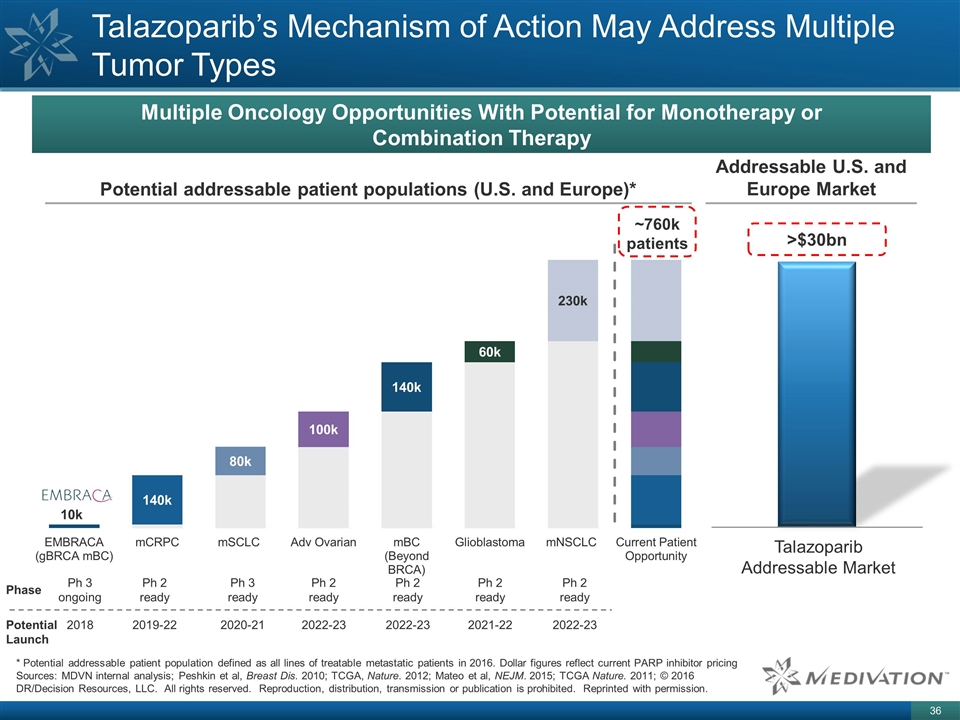
Talazoparib’s Mechanism of Action May Address Multiple Tumor Types * Potential addressable patient population defined as all lines of treatable metastatic patients in 2016. Dollar figures reflect current PARP inhibitor pricing Sources: MDVN internal analysis; Peshkin et al, Breast Dis. 2010; TCGA, Nature. 2012; Mateo et al, NEJM. 2015; TCGA Nature. 2011; © 2016 DR/Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. Multiple Oncology Opportunities With Potential for Monotherapy or Combination Therapy Potential addressable patient populations (U.S. and Europe)* Phase Potential Launch Ph 2 ready 2022-23 2022-23 Ph 2 ready Ph 3 ready 2020-21 Ph 2 ready 2019-22 Ph 3 ongoing 2018 ~760k patients Ph 2 ready 2021-22 Ph 2 ready 2022-23 Addressable U.S. and Europe Market Talazoparib Addressable Market >$30bn
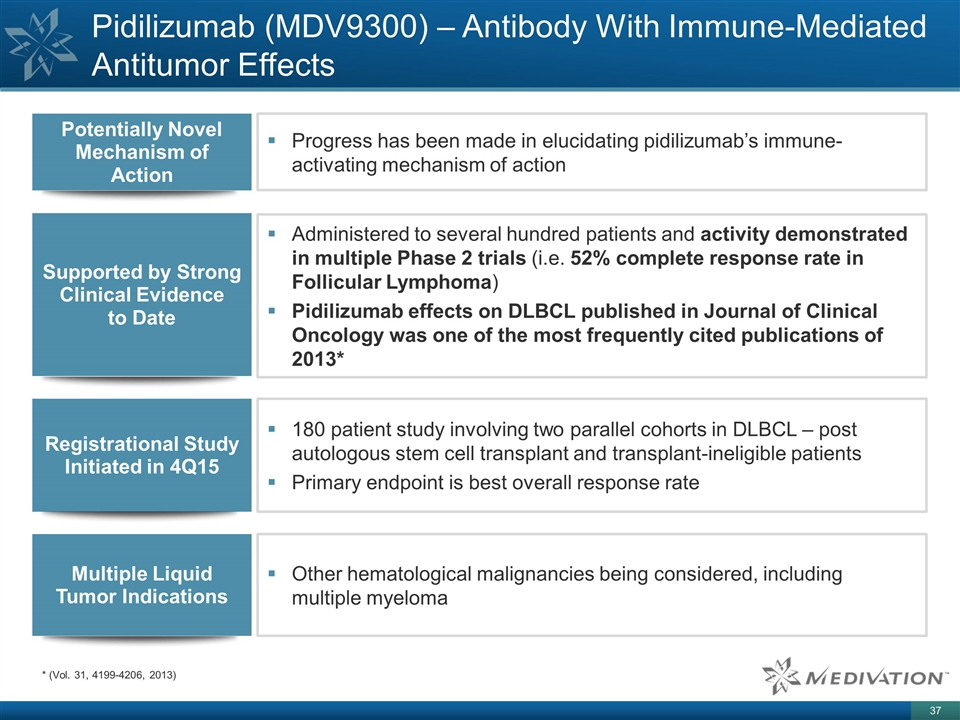
Pidilizumab (MDV9300) – Antibody With Immune-Mediated Antitumor Effects Progress has been made in elucidating pidilizumab’s immune-activating mechanism of action Potentially Novel Mechanism of Action Supported by Strong Clinical Evidence to Date Administered to several hundred patients and activity demonstrated in multiple Phase 2 trials (i.e. 52% complete response rate in Follicular Lymphoma) Pidilizumab effects on DLBCL published in Journal of Clinical Oncology was one of the most frequently cited publications of 2013* Registrational Study Initiated in 4Q15 180 patient study involving two parallel cohorts in DLBCL – post autologous stem cell transplant and transplant-ineligible patients Primary endpoint is best overall response rate Multiple Liquid Tumor Indications Other hematological malignancies being considered, including multiple myeloma * (Vol. 31, 4199-4206, 2013)

Sanofi’s Substantially Inadequate and Opportunistically-Timed Proposal

Sanofi’s Opportunistic Proposal Substantially Undervalues Medivation Sanofi’s proposed price of $52.50 per share substantially undervalues Medivation: Medivation is a unique opportunity as one of the few profitable and sizeable oncology companies Medivation has built XTANDI into a rapidly-growing, multi-billion dollar oncology product Medivation is leveraging its expertise to develop and bring to market additional products from its wholly-owned, differentiated late-stage pipeline Talazoparib represents another blockbuster opportunity as a potentially best-in-class PARP inhibitor targeting a wide range of oncology indications Sanofi's timing is designed to benefit Sanofi – not Medivation’s shareholders: Sanofi approached Medivation following a period of significant market dislocation, particularly in biotech The proposed price is 21% below Medivation's 52-week trading high Sanofi did not wait for a response from Medivation’s Board with respect to its non-binding proposal before rushing to make the same substantially inadequate proposal public The Board believes that the continued successful execution of our well-defined strategic plan will deliver greater value to Medivation's shareholders than Sanofi's substantially inadequate proposal
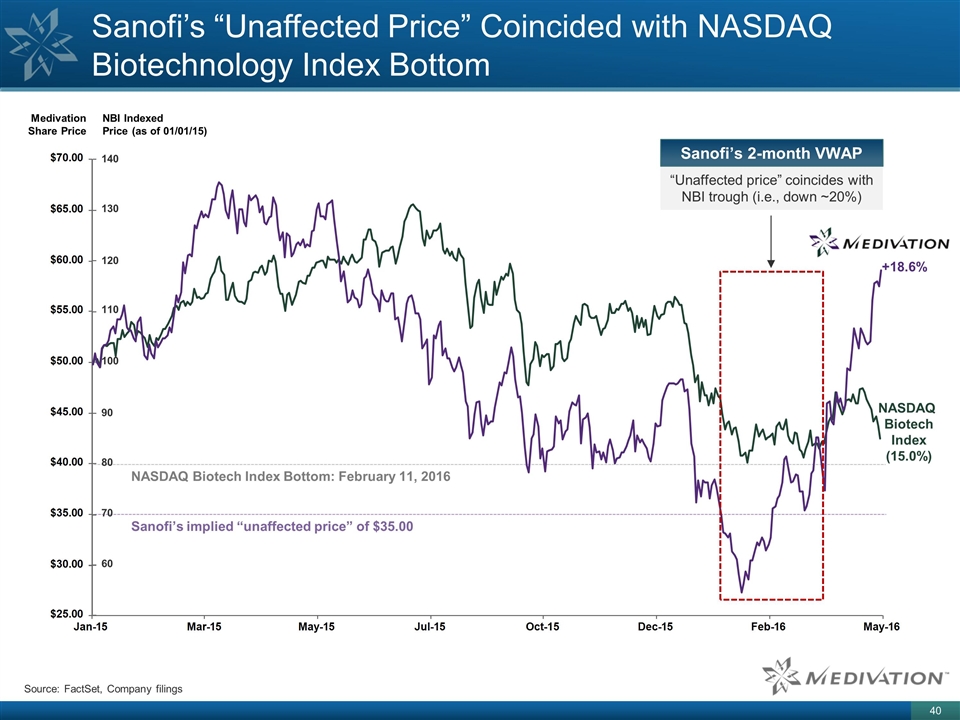
Sanofi’s “Unaffected Price” Coincided with NASDAQ Biotechnology Index Bottom Source: FactSet, Company filings Medivation Share Price NBI Indexed Price (as of 01/01/15) 140 130 120 110 100 90 80 70 60 NASDAQ Biotech Index Bottom: February 11, 2016 Sanofi’s implied “unaffected price” of $35.00 “Unaffected price” coincides with NBI trough (i.e., down ~20%) +18.6% NASDAQ Biotech Index (15.0%) Sanofi’s 2-month VWAP
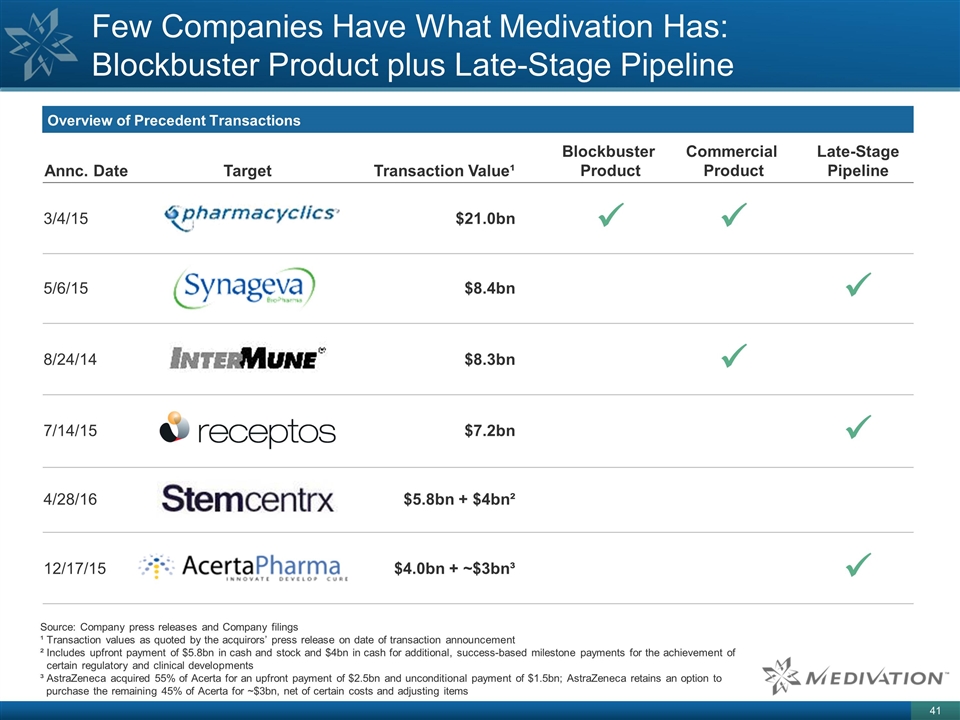
Few Companies Have What Medivation Has: Blockbuster Product plus Late-Stage Pipeline Overview of Precedent Transactions Source: Company press releases and Company filings ¹ Transaction values as quoted by the acquirors’ press release on date of transaction announcement ² Includes upfront payment of $5.8bn in cash and stock and $4bn in cash for additional, success-based milestone payments for the achievement of certain regulatory and clinical developments ³ AstraZeneca acquired 55% of Acerta for an upfront payment of $2.5bn and unconditional payment of $1.5bn; AstraZeneca retains an option to purchase the remaining 45% of Acerta for ~$3bn, net of certain costs and adjusting items Target Transaction Value¹ Annc. Date 4/28/16 $5.8bn + $4bn² Blockbuster Product Commercial Product Late-Stage Pipeline 3/4/15 ü $21.0bn ü 8/24/14 $8.3bn ü 5/6/15 $8.4bn ü 7/14/15 $7.2bn ü 12/17/15 $4.0bn + ~$3bn³ ü
“There are several lines of evidence that PARP inhibition may be effective in prostate cancer and up to 50% of men with prostate cancer may be eligible for niraparib… We view the Janssen deal as highly validating of niraparib and providing a pathway to a substantial commercial opportunity” ̶ Wells Fargo 04/06/16 Recent Third-Party Transactions Validate the Value of Medivation’s Key Assets Royalty Pharma recently announced a $1.14bn+(1) acquisition of a portion of UCLA’s right to the 4% royalty on sales of XTANDI payable to the University The deal represents strong third-party validation of the long-term growth prospects ahead of XTANDI Johnson & Johnson (JNJ) recently announced a prostate cancer collaboration with Tesaro for its PARP inhibitor, niraparib For only a single indication, in ex-Japan territories, Tesaro may receive up to $500 million and double-digit royalties This transaction demonstrates the clear potential value that PARP inhibitors may have in prostate cancer “We believe that the $1.14B price tag implies at least somewhere in the $5-$7B range in peak WW sales, depending on ramp, etc.” ̶ RBC 03/04/16 “We think the purchase price paid validates the more bullish commercial scenarios… [and implies] global peak XTANDI sales of no less than $6bn (using low discount rates) and as high as $8bn. Keep in mind that in order to make money on the stream, actual sales would need to be above these numbers.” ̶ UBS 03/04/16 Source: Company filings, company websites, press releases, Wall Street research The transaction includes a cash payment of $1.14 billion and potential additional payments based on future XTANDI sales
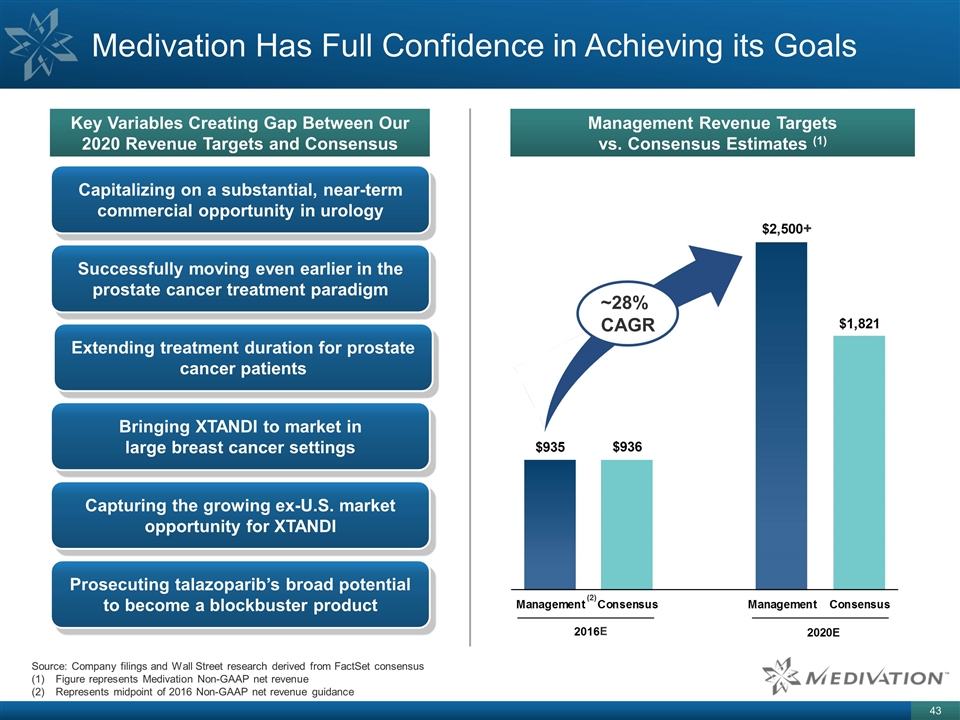
Medivation Has Full Confidence in Achieving its Goals Key Variables Creating Gap Between Our 2020 Revenue Targets and Consensus Management Revenue Targets vs. Consensus Estimates (1) (2) ~28% CAGR Capitalizing on a substantial, near-term commercial opportunity in urology Successfully moving even earlier in the prostate cancer treatment paradigm Extending treatment duration for prostate cancer patients Bringing XTANDI to market in large breast cancer settings Capturing the growing ex-U.S. market opportunity for XTANDI Prosecuting talazoparib’s broad potential to become a blockbuster product Source: Company filings and Wall Street research derived from FactSet consensus Figure represents Medivation Non-GAAP net revenue Represents midpoint of 2016 Non-GAAP net revenue guidance + E

Conclusion
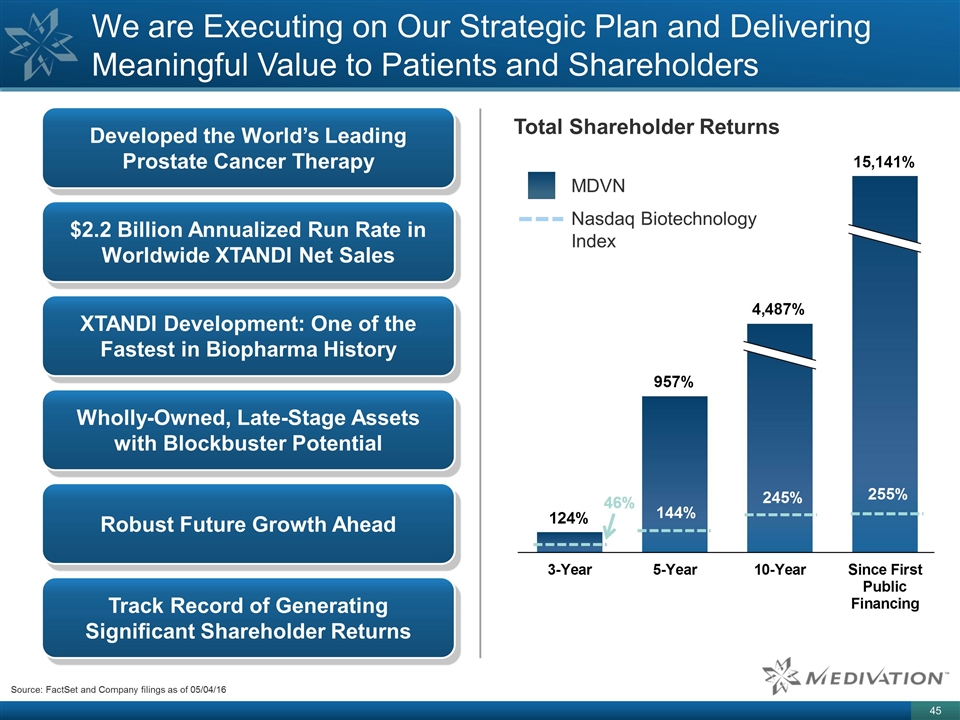
We are Executing on Our Strategic Plan and Delivering Meaningful Value to Patients and Shareholders Developed the World’s Leading Prostate Cancer Therapy $2.2 Billion Annualized Run Rate in Worldwide XTANDI Net Sales Wholly-Owned, Late-Stage Assets with Blockbuster Potential XTANDI Development: One of the Fastest in Biopharma History Track Record of Generating Significant Shareholder Returns Robust Future Growth Ahead Total Shareholder Returns MDVN Nasdaq Biotechnology Index Source: FactSet and Company filings as of 05/04/16

Appendix
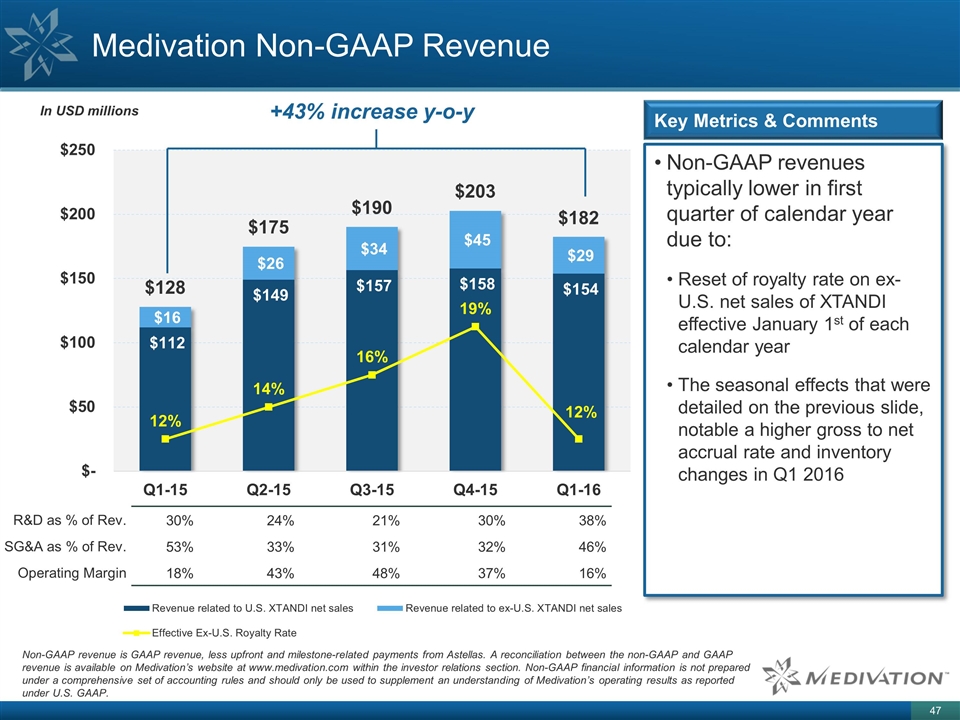
Medivation Non-GAAP Revenue Non-GAAP revenues typically lower in first quarter of calendar year due to: Reset of royalty rate on ex-U.S. net sales of XTANDI effective January 1st of each calendar year The seasonal effects that were detailed on the previous slide, notable a higher gross to net accrual rate and inventory changes in Q1 2016 Key Metrics & Comments +43% increase y-o-y R&D as % of Rev. 30% 24% 21% 30% 38% SG&A as % of Rev. 53% 33% 31% 32% 46% Operating Margin 18% 43% 48% 37% 16% Non-GAAP revenue is GAAP revenue, less upfront and milestone-related payments from Astellas. A reconciliation between the non-GAAP and GAAP revenue is available on Medivation’s website at www.medivation.com within the investor relations section. Non-GAAP financial information is not prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of Medivation’s operating results as reported under U.S. GAAP.
Enzalutamide Earlier in the Prostate Cancer Disease Continuum ~1,560 non-metastatic CRPC patients Primary endpoint: MFS* 2 arm study: enzalutamide + continued ADT vs placebo + continued ADT GOAL: Evaluate potential clinical benefit in patients with M0 CRPC 1,860 non-metastatic hormone-sensitive patients Primary endpoint: MFS* 3 arm study: enzalutamide alone vs enzalutamide + leuprolide acetate vs placebo + leuprolide acetate PROSPER initiated first patient in Dec. 2013; EMBARK in Jan. 2015. * MFS = Metastasis-free survival GOAL: Evaluate in patients with hormone-sensitive, M0 prostate cancer with rising PSA after definitive therapy
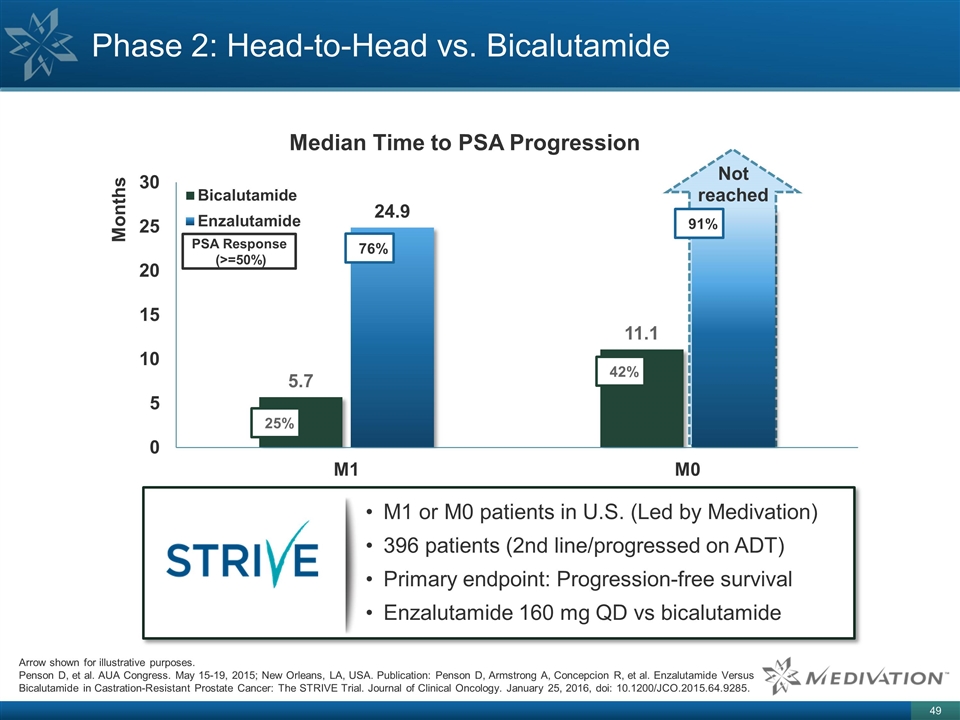
Phase 2: Head-to-Head vs. Bicalutamide TBD M1 or M0 patients in U.S. (Led by Medivation) 396 patients (2nd line/progressed on ADT) Primary endpoint: Progression-free survival Enzalutamide 160 mg QD vs bicalutamide 25% 42% 76% 91% PSA Response (>=50%) Arrow shown for illustrative purposes. Penson D, et al. AUA Congress. May 15-19, 2015; New Orleans, LA, USA. Publication: Penson D, Armstrong A, Concepcion R, et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. Journal of Clinical Oncology. January 25, 2016, doi: 10.1200/JCO.2015.64.9285.
















































