Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF
THE SECURITIES EXCHANGE ACT OF 1934
For the month ended November 30, 2016
Commission File Number 0-28564
QIAGEN N.V.
Hulsterweg 82
5912 PL Venlo
The Netherlands
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
Form 20-F ☒ Form 40-F ☐
Indicate by check mark whether the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T
Rule 101(b)(1): ☐
Indicate by check mark whether the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T
Rule 101(b)(7): ☐
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ☐ No ☒
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-
Table of Contents
QIAGEN N.V.
Form 6-K
Item | Page | |||
| 3 | ||||
| 4 | ||||
| 5 | ||||
2
Table of Contents
On November 15, 2016, QIAGEN N.V. held an Analyst & Investor Day in New York, New York. The presentations discussed that day are furnished herewith as Exhibit 99.1 through 99.9 and are incorporated by reference herein.
USE OF NON-GAAP FINANCIAL MEASURES
QIAGEN reports adjusted results, as well as results on a constant exchange rate basis, and other non-U.S. GAAP (generally accepted accounting principles) figures, to provide additional insight on performance. Adjusted results included in the Exhibits reflect adjusted operating expenses, adjusted earnings interest, taxes, depreciation and amortization (EBITDA), adjusted diluted earnings per share and free cash flow. Adjusted results are non-GAAP financial measures that we believe should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. We believe certain items should be excluded from adjusted results when they are outside of our ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the appendices provided in the Exhibits entitled “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. We do not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on our GAAP results.
FORWARD-LOOKING STATEMENTS
Exhibits 99.1 through 99.9 contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to our business, goals, strategy and financial and operational outlook. These “forward-looking statements” are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by forward-looking statements. These risks and uncertainties include, but are not limited to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for our products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting our products to integrated solutions and producing such products; our ability to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of our new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that we have filed with, or furnished to, the U.S. Securities and Exchange Commission. We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
3
Table of Contents
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
QIAGEN N.V.
| By: | /s/ Roland Sackers | |
| Roland Sackers | ||
| Chief Financial Officer | ||
| Date: | November 23, 2016 | |
4
Table of Contents
Exhibit No. | Exhibit | |
| 99.1 | Introduction 2016 Analyst & Investor Day | |
| 99.2 | Strategy Update | |
| 99.3 | Life Sciences | |
| 99.4 | Molecular Diagnostics | |
| 99.5 | Bioinformatics | |
| 99.6 | Commercial Operations | |
| 99.7 | Finance | |
| 99.8 | QuantiFeron-TB | |
| 99.9 | GeneReader NGS System | |
5
Table of Contents
Exhibit 99.1
|
QIAGEN
Welcome:
2016 Analyst & Investor Day
John Gilardi
Vice President, Corporate Communications and Investor Relations November 15, 2016
Sample to Insight
2016 Analyst & Investor Day
Table of Contents
|
QIAGEN
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
Table of Contents
|
QIAGEN
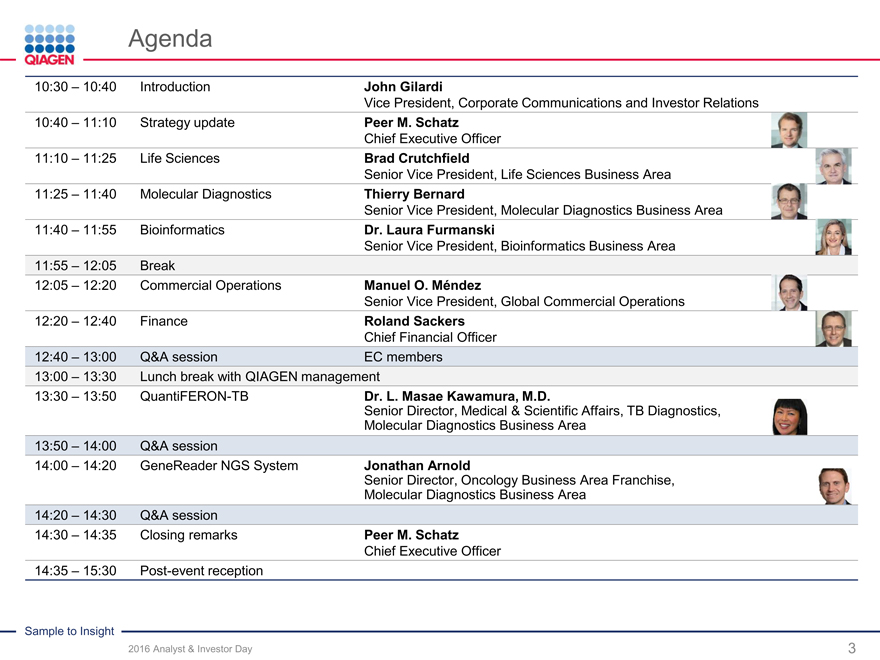
10:30 – 10:40 Introduction John Gilardi
Vice President, Corporate Communications and Investor 10:40 – 11:10 Strategy update Peer M. Schatz Chief Executive Officer 11:10 – 11:25 Life Sciences Brad Crutchfield Senior Vice President, Life Sciences Business Area 11:25 – 11:40 Molecular Diagnostics Thierry Bernard Senior Vice President, Molecular Diagnostics Business Area 11:40 – 11:55 Bioinformatics Dr. Laura Furmanski Senior Vice President, Bioinformatics Business Area 11:55 – 12:05 Break 12:05 – 12:20 Commercial Operations Manuel O. Méndez Senior Vice President, Global Commercial Operations 12:20 – 12:40 Finance Roland Sackers Chief Financial Officer 12:40 – 13:00 Q&A session EC members 13:00 – 13:30 Lunch break with QIAGEN management 13:30 – 13:50 QuantiFERON-TB Dr. L. Masae Kawamura, M.D.
Senior Director, Medical & Scientific Affairs, TB Diagnostics, Molecular Diagnostics Business Area 13:50 – 14:00 Q&A session 14:00 – 14:20 GeneReader NGS System Jonathan Arnold Senior Director, Oncology Business Area Franchise, Molecular Diagnostics Business Area 14:20 – 14:30 Q&A session 14:30 – 14:35 Closing remarks Peer M. Schatz Chief Executive Officer 14:35 – 15:30 Post-event reception
Sample to Insight
2016 Analyst & Investor Day
Table of Contents
Exhibit 99.2
|
QIAGEN
Setting a new trajectory for growth and greater value creation
Peer M. Schatz
Chief Executive Officer
November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Table of Contents
|
QIAGEN
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day 2
Table of Contents
|
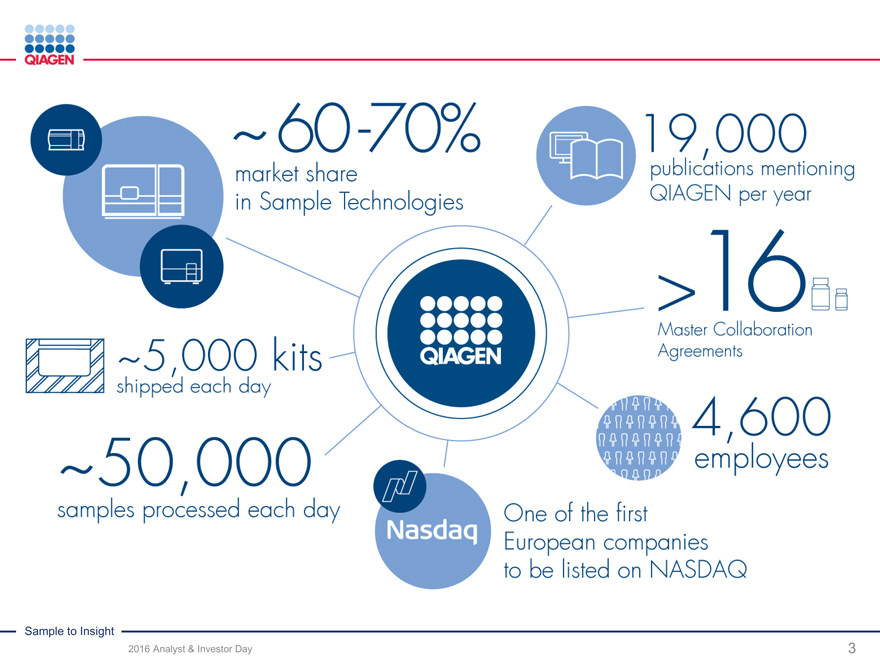
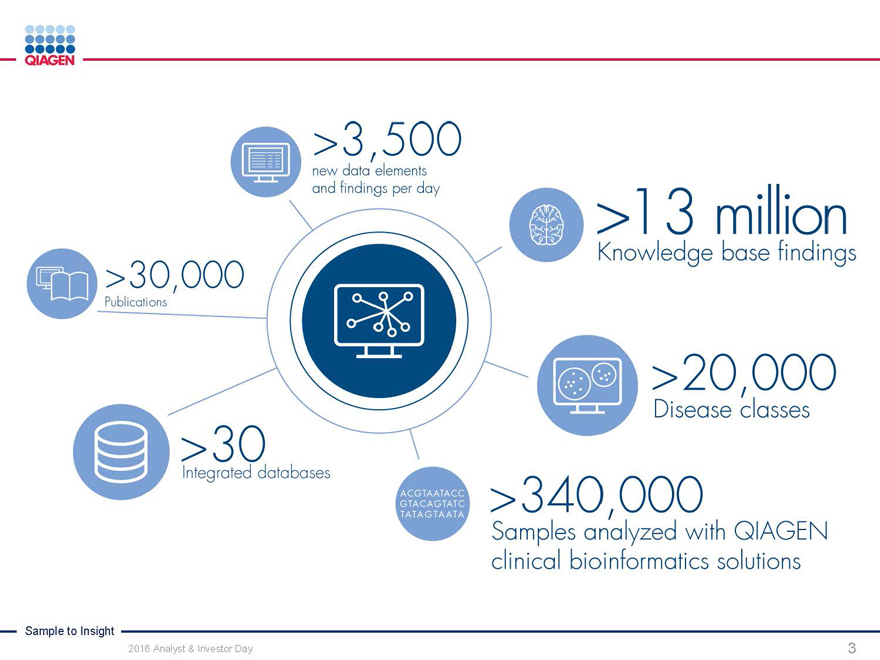
~60-70%
Market share in Sample Technologies
19,000
Publications mentioning QIAGEN per year
>16
Master Collaboration Agreements
~5,000 kits shipped each day
~50,000
Samples processed each day
Nasdaq
One of the first European companies to be listed on NASDAQ
4,600 employees
Sample to Insight
2016 Analyst & Investor Day 3
Table of Contents
|
~$1.3 billion ConsumablesEurope /Americas
>500,000 (incl. bioinformatics)Middle East /
~87% Africa
>35 countries ~32%
~4,600
~47%
NASDAQ / Frankfurt
Venlo (Netherlands)
~20%
~13%
Instruments andAsia-Pacific /
automation systemsJapan / Rest of world
Situation
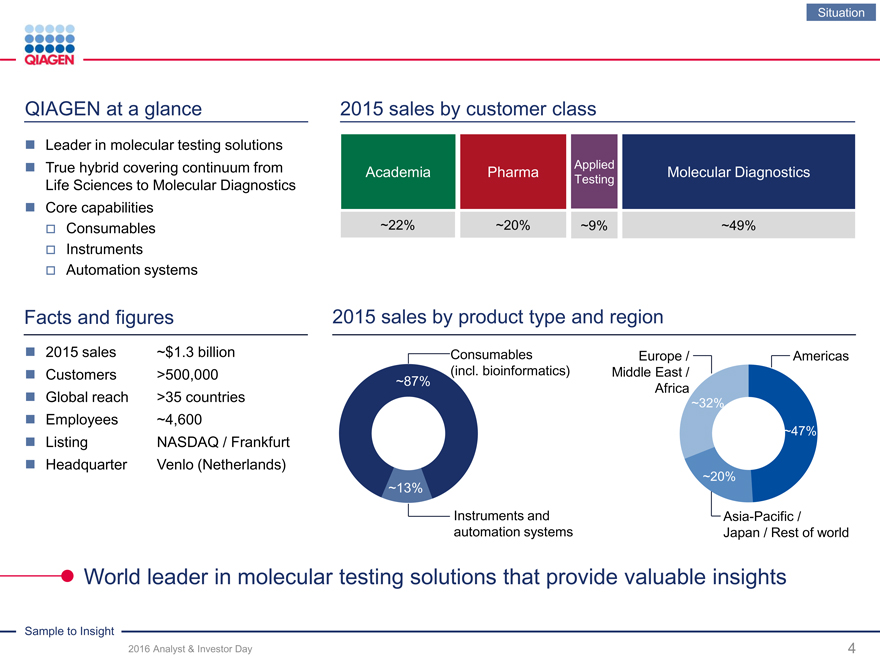
QIAGEN at a glance
2015 sales by customer class
Leader in molecular testing solutions
True hybrid covering continuum from Life Sciences to Molecular Diagnostics
Core capabilities
Consumables
Instruments
Automation systems
Facts and figures
Applied
Academia Pharma Molecular Diagnostics
Testing
~22% ~20% ~9%~49%
2015 sales by product type and region
2015 sales
Customers
Global reach
Employees
Listing
Headquarter
World leader in molecular testing solutions that provide valuable insights
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
Situation
Customers want meaningful molecular insights
Faster Better More efficiently
Sample Technologies Assay Technologies
Bioinformatics Automation systems
VALUABLE
BIOLOGICAL SAMPLE TO INSIGHT SOLUTIONS MOLECULAR
SAMPLE INSIGHTS
Moving from tools and components to solutions that provide valuable insights
Sample to Insight
2016 Analyst & Investor Day 5
Table of Contents
|
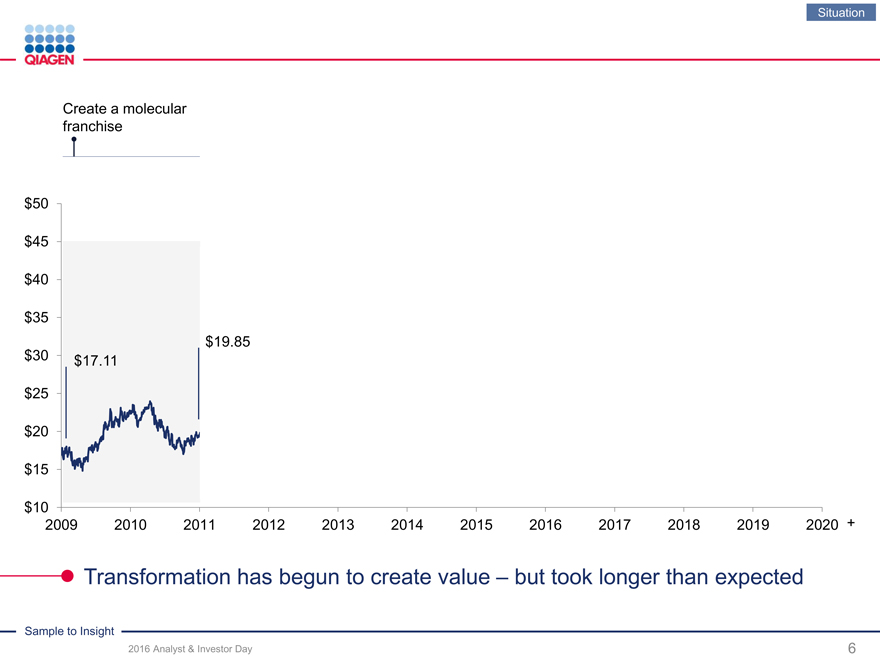
Situation
Create a molecular franchise
$50 $45 $40
$35
$19.85
$30 $17.11 $25
$20
$15
$10
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 +
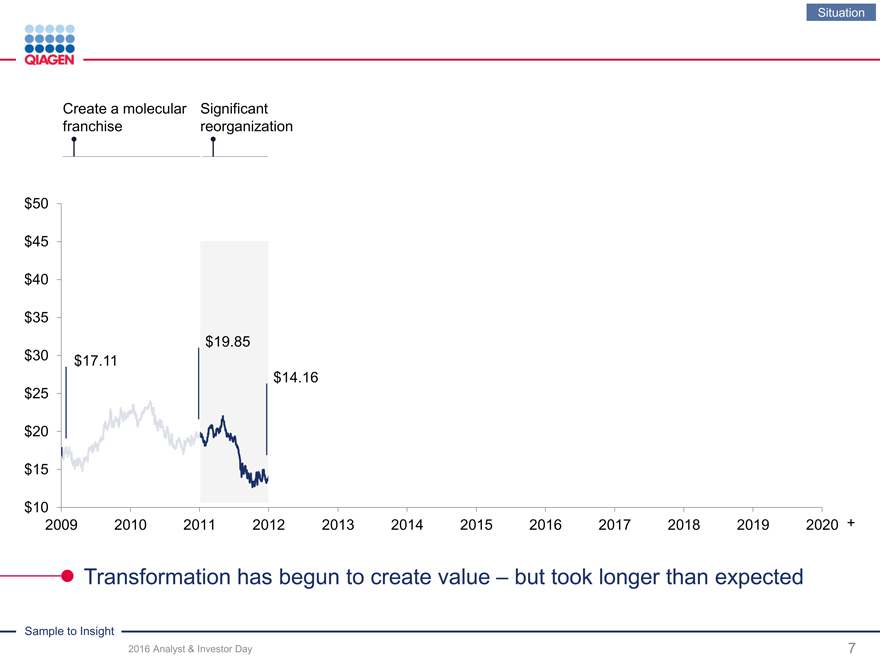
Transformation has begun to create value – but took longer than expected
Sample to Insight
2016 Analyst & Investor Day 6
Table of Contents
|
$50 $45 $40
$35
$19.85
$30 $17.11
$14.16 $25
$20
$15
$10
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 +
Transformation has begun to create value – but took longer than expected
Sample to Insight
2016 Analyst & Investor Day 7
Situation
Create a molecular Significant
franchise reorganization
Table of Contents
|
$50 $45 $40
$23.37 $35
$19.85
$30 $17.11
$14.16 $25
$20
$15
$10
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 +
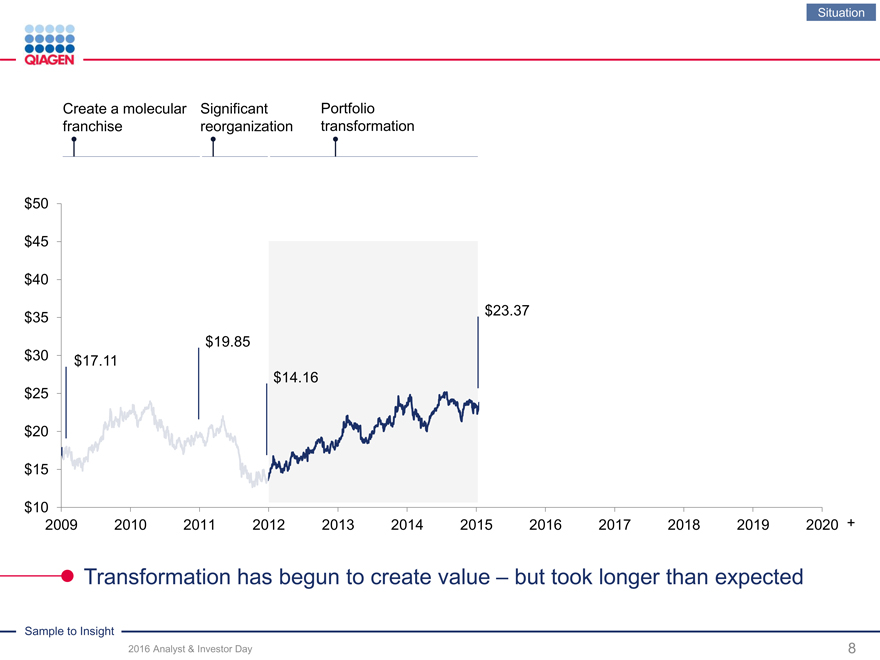
Transformation has begun to create value – but took longer than expected
Sample to Insight
2016 Analyst & Investor Day 8
Situation
Create a molecular Significant Portfolio
franchise reorganization transformation
Table of Contents
|
$50
$45
$40 $27.51
$23.37 $35
$19.85
$30 $17.11
$14.16 $25
$20
$15
$10
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 +
Transformation has begun to create value – but took longer than expected
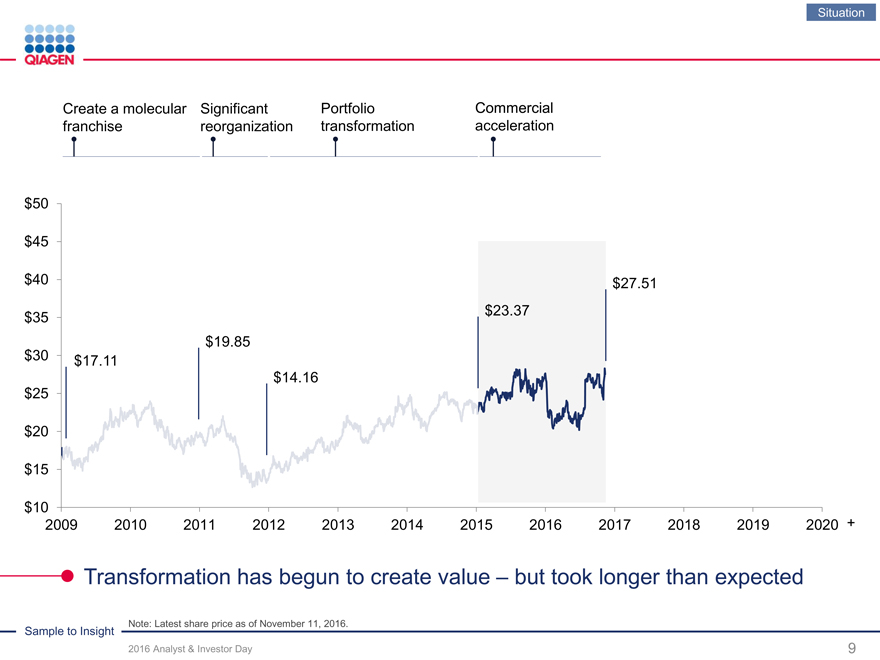
Situation
Create a molecular Significant PortfolioCommercial
franchise reorganization transformationacceleration
Note: Latest share price as of November 11, 2016.
Sample to Insight
2016 Analyst & Investor Day 9
Table of Contents
|
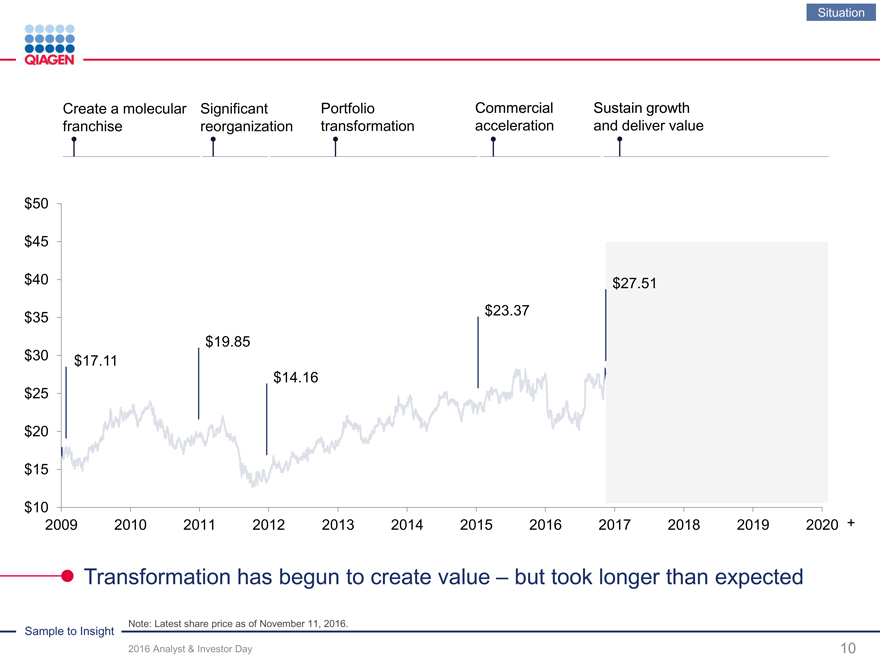
$50
$45
$40 $27.51
$23.37 $35
$19.85
$30 $17.11
$14.16 $25
$20
$15
$10
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 +
Transformation has begun to create value – but took longer than expected
Situation
Create a molecular Significant PortfolioCommercialSustain growth
franchise reorganization transformationaccelerationand deliver value
Note: Latest share price as of November 11, 2016.
Sample to Insight
2016 Analyst & Investor Day 10
Table of Contents
|
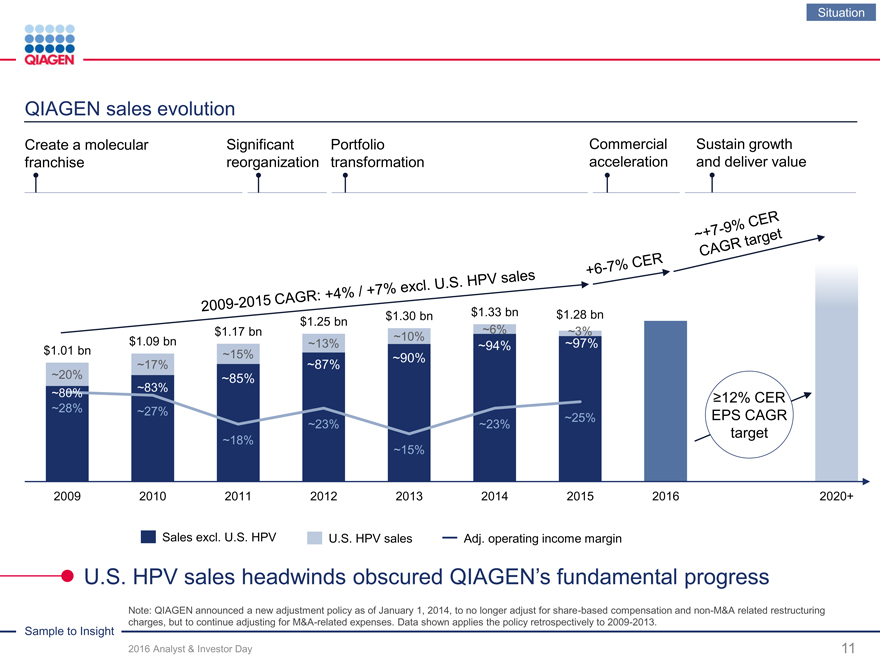
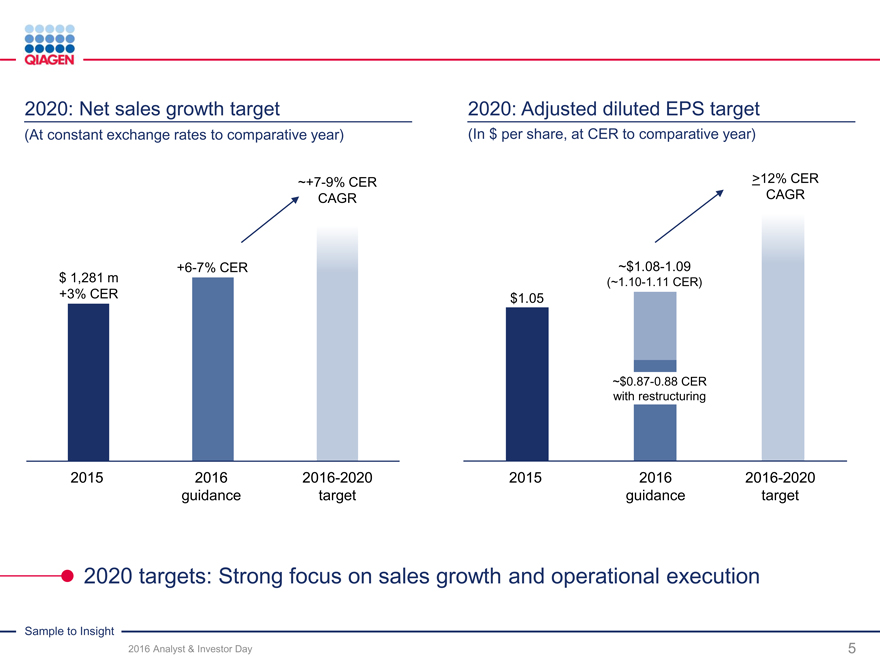
QIAGEN sales evolution
Create a molecular Significant PortfolioCommercialSustain growth
franchise reorganization transformationaccelerationand deliver value
$1.30 bn $1.33 bn $1.28 bn
$1.25 bn
$1.17 bn ~6% ~3%
$1.09 bn ~10%
~13% ~94% ~97%
$1.01 bn ~15%
~90%
~17% ~87%
~20% ~85% ~83%
~80% ?12% CER
~28% ~27%
~25% EPS CAGR
~23% ~23%
target
~18%
~15%
2009 2010 2011 2012 2013 2014 2015 2016 2020+
Sales excl. U.S. HPV U.S. HPV sales Adj. operating income margin
U.S. HPV sales headwinds obscured QIAGEN’s fundamental progress
Note: QIAGEN announced a new adjustment policy as of January 1, 2014, to no longer adjust for share-based compensation and non-M&A related restructuring Sample to Insight charges, but to continue adjusting for M&A-related expenses. Data shown applies the policy retrospectively to 2009-2013.
2016 Analyst & Investor Day 11
Situation
Table of Contents
|
Situation
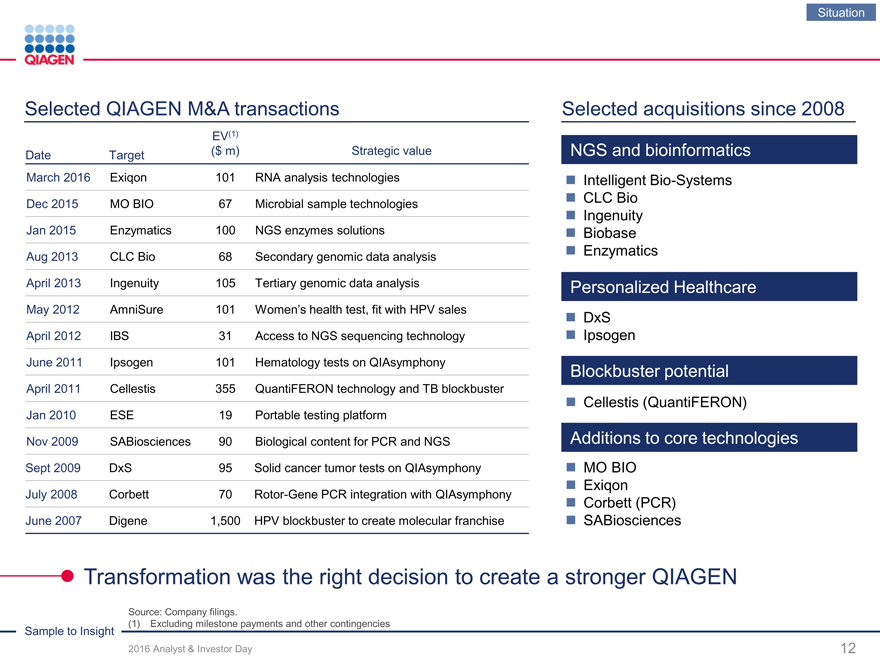
Selected QIAGEN M&A transactions Selected acquisitions since 2008
EV(1)
Date Target ($ m)Strategic valueNGS and bioinformatics
March 2016 Exiqon 101RNA analysis technologiesIntelligent Bio-Systems
Dec 2015 MO BIO 67Microbial sample technologiesCLC Bio
Ingenuity
Jan 2015 Enzymatics 100NGS enzymes solutionsBiobase
Aug 2013 CLC Bio 68Secondary genomic data analysisEnzymatics
April 2013 Ingenuity 105Tertiary genomic data analysisPersonalized Healthcare
May 2012 AmniSure 101Women’s health test, fit with HPV salesDxS
April 2012 IBS 31Access to NGS sequencing technologyIpsogen
June 2011 Ipsogen 101Hematology tests on QIAsymphonyBlockbuster potential
April 2011 Cellestis 355QuantiFERON technology and TB blockbuster
Cellestis (QuantiFERON)
Jan 2010 ESE 19Portable testing platform
Nov 2009 SABiosciences 90Biological content for PCR and NGSAdditions to core technologies
Sept 2009 DxS 95Solid cancer tumor tests on QIAsymphonyMO BIO
Exiqon
July 2008 Corbett 70Rotor-Gene PCR integration with QIAsymphonyCorbett (PCR)
June 2007 Digene 1,500HPV blockbuster to create molecular franchiseSABiosciences
Transformation was the right decision to create a stronger QIAGEN
Source: Company filings.
(1) Excluding milestone payments and other contingencies
Sample to Insight
2016 Analyst & Investor Day 12
Table of Contents
|
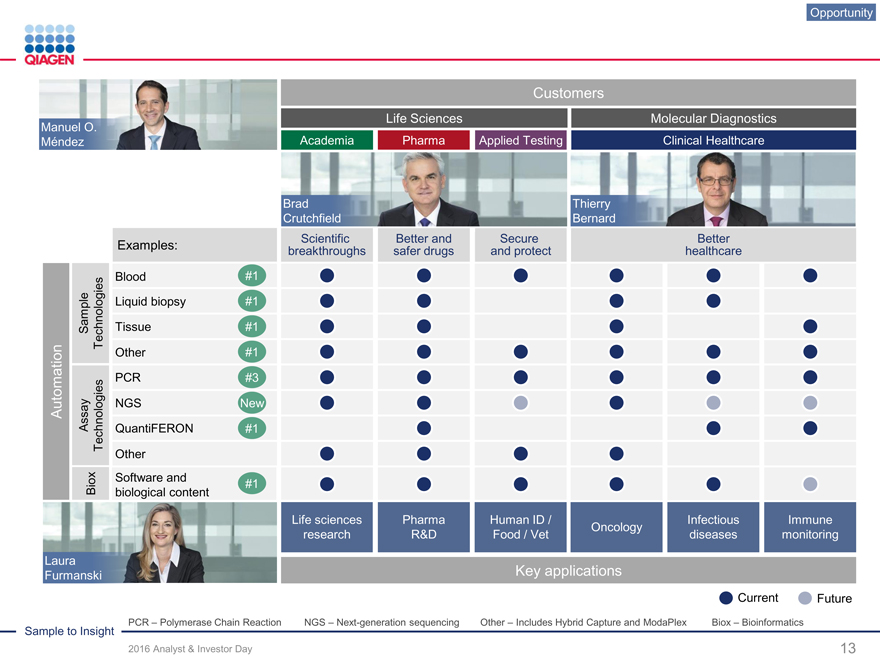
Brad Thierry Crutchfield Bernard
Scientific Better and Secure Better
Examples: breakthroughs safer drugs and protect healthcare
Blood #1 Liquid biopsy #1 Sample Technologies Tissue #1 Other #1
PCR #3 Automation Assay NGS New Technologies QuantiFERON #1 Other Software and Biox #1 biological content
Life sciences Pharma Human ID / Infectious Immune Oncology research R&D Food / Vet diseases monitoring
Laura
Furmanski Key applications
Current Future
PCR – Polymerase Chain Reaction NGS – Next-generation sequencing Other – Includes Hybrid Capture and ModaPlex Biox – Bioinformatics
Opportunity
Customers
Life Sciences Molecular Diagnostics
Manuel O.
Méndez Academia Pharma Applied Testing Clinical Healthcare
Sample to Insight
2016 Analyst & Investor Day 13
Table of Contents
|
Opportunity
AMP 2016: Early movers on new trends in Molecular Diagnostics
Sample to Insight
2016 Analyst & Investor Day 14
Table of Contents
|
Analyst & Investor Day:
November 2013
Sales Improving trends while addressing challenges
targets Reallocating resources to best opportunities
Growth Building momentum
drivers QIAsymphony
Personalized Healthcare (PHC)
QuantiFERON-TB
Bioinformatics
Next-generation sequencing
Operating Further improve operating margin
efficiency Significant operational improvements
Capital More leverage with healthy financial position
structure Completed first $100 m buyback,
new program started
M&A Continue consistent M&A strategy
Reviewing our progress since 2013
Sample to Insight
2016 Analyst & Investor Day
Opportunity
15
Table of Contents
|
Strategy
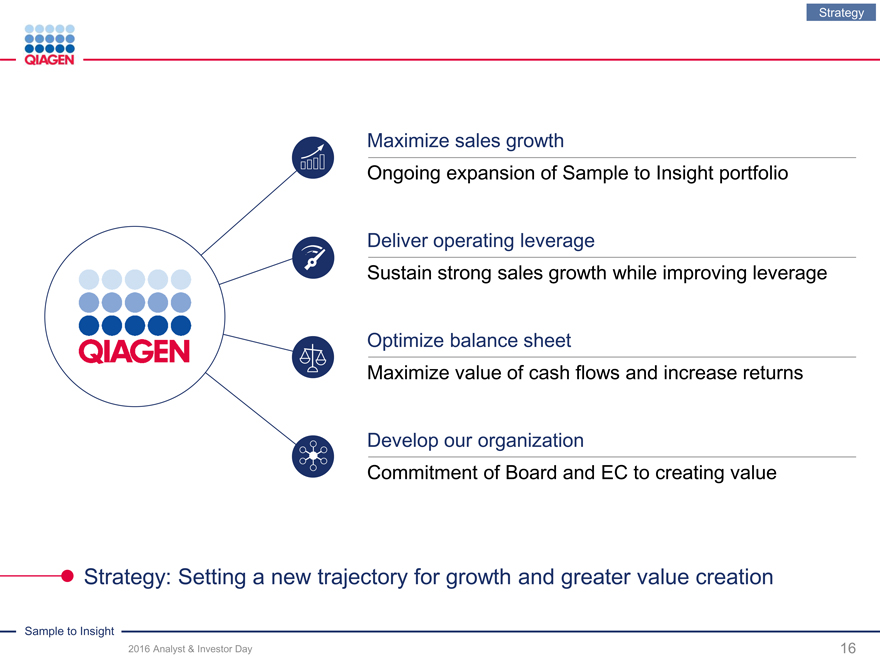
Maximize sales growth
Ongoing expansion of Sample to Insight portfolio
Deliver operating leverage
Sustain strong sales growth while improving leverage
Optimize balance sheet
Maximize value of cash flows and increase returns
Develop our organization
Commitment of Board and EC to creating value
Strategy: Setting a new trajectory for growth and greater value creation
Sample to Insight
2016 Analyst & Investor Day 16
Table of Contents
|
Strategy
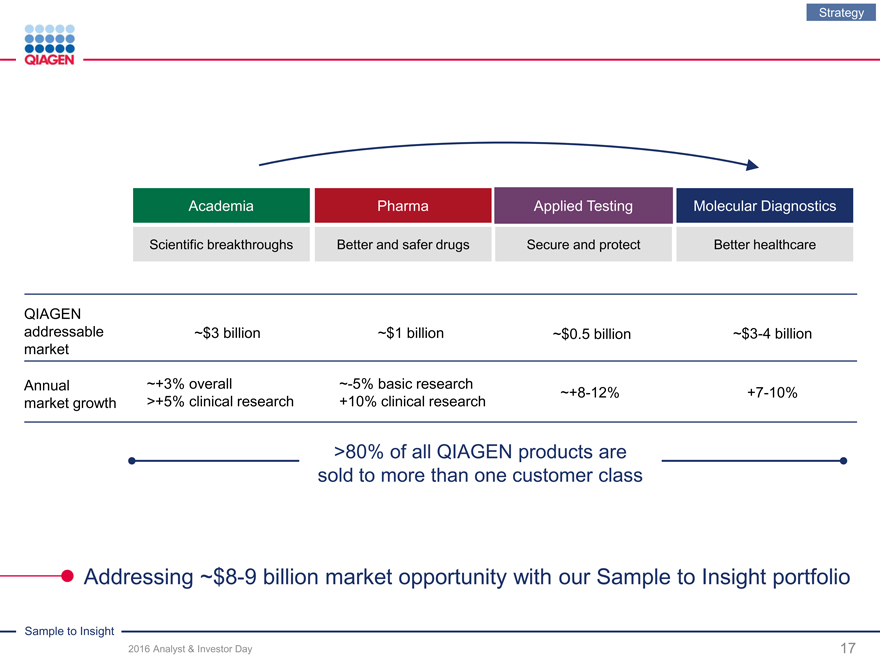
Academia Pharma Applied TestingMolecular Diagnostics
Scientific breakthroughs Better and safer drugs Secure and protectBetter healthcare
QIAGEN
addressable ~$3 billion ~$1 billion~$0.5 billion~$3-4 billion
market
Annual �� ~+3% overall ~-5% basic research~+8-12%+7-10%
market growth >+5% clinical research +10% clinical research
>80% of all QIAGENproducts are
sold to more than one customer class
Addressing ~$8-9 billion market opportunity with our Sample to Insight portfolio
Sample to Insight
2016 Analyst & Investor Day
17
Table of Contents
|
Strategy
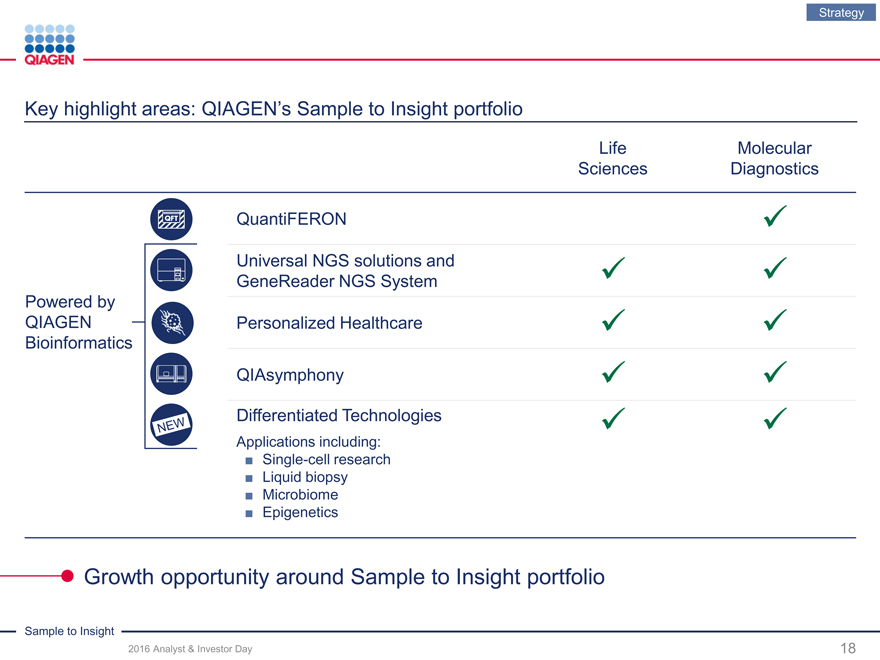
Key highlight areas: QIAGEN’s Sample to Insight portfolio
LifeMolecular
SciencesDiagnostics
QuantiFERON
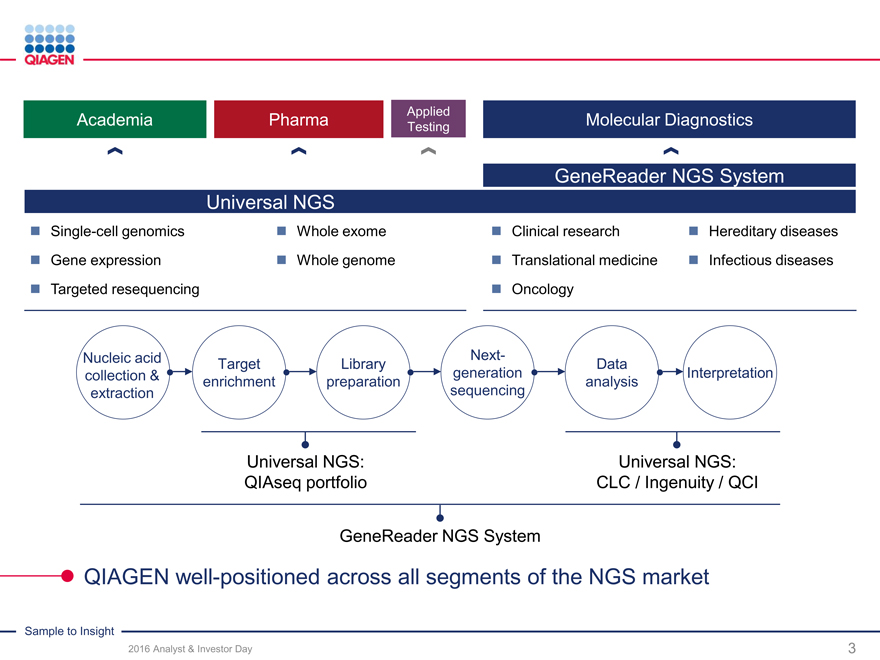
Universal NGS solutions and
GeneReader NGS System
Powered by
QIAGEN Personalized Healthcare
Bioinformatics
QIAsymphony
Differentiated Technologies
Applications including:
? Single-cell research
? Liquid biopsy
? Microbiome
? Epigenetics
Growth opportunity around Sample to Insight portfolio
Sample to Insight
2016 Analyst & Investor Day 18
Table of Contents
|
Strategy
QuantiFERON
Opportunity
QuantiFERON: “Pre-molecular” technology
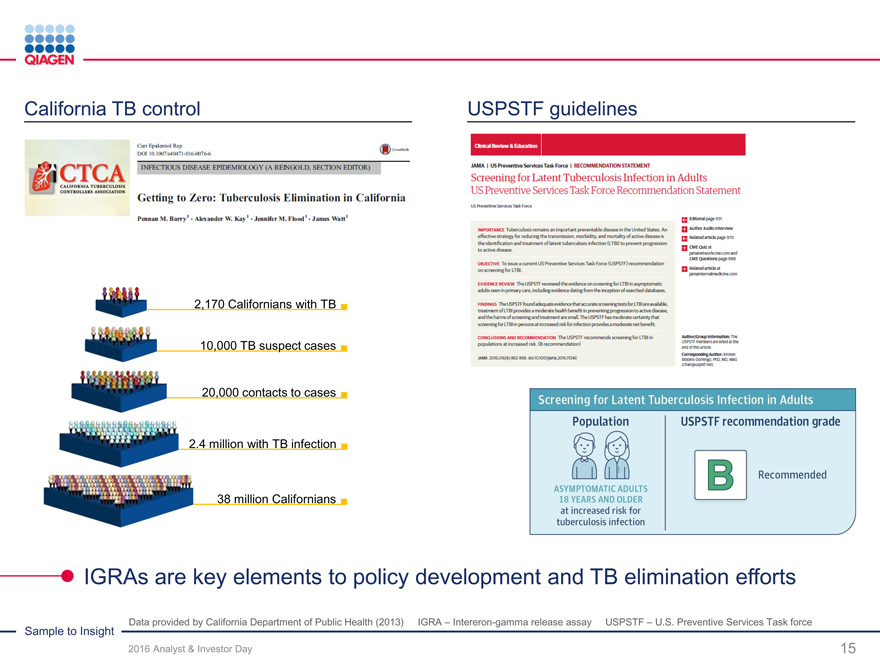
Lead $1 billion latent TB test market conversion
Attractive menu expansion options
Trends
Clinical needs for latent and active disease detection
Global push for TB prevention and elimination
2016 developments
2016 goal: >$140 million of QuantiFERON-TB sales
Ongoing conversion from tuberculin skin test (TST)
Support from new TB guidelines (USPSTF)
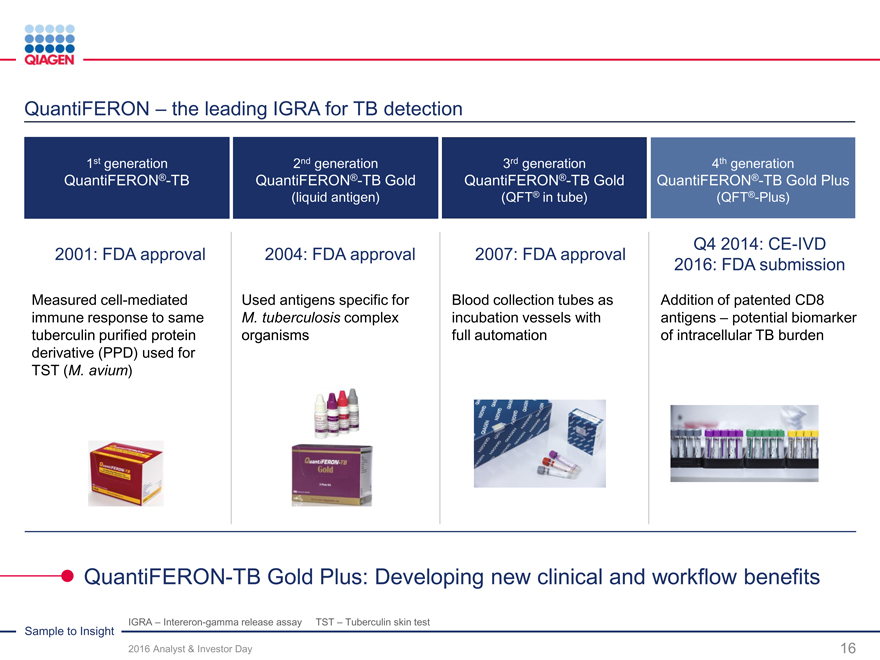
QFT-Plus 4th generation test:
CE-IVD launch momentum
U.S. submission in Q4 2016
Fully addressed issues in U.S. FDA warning letter
QIAGEN value proposition 2017-2020 ambitions
QuantiFERON-TB: Modern gold standard TB test 2020 target: >$300 million of QuantiFERON-TB sales
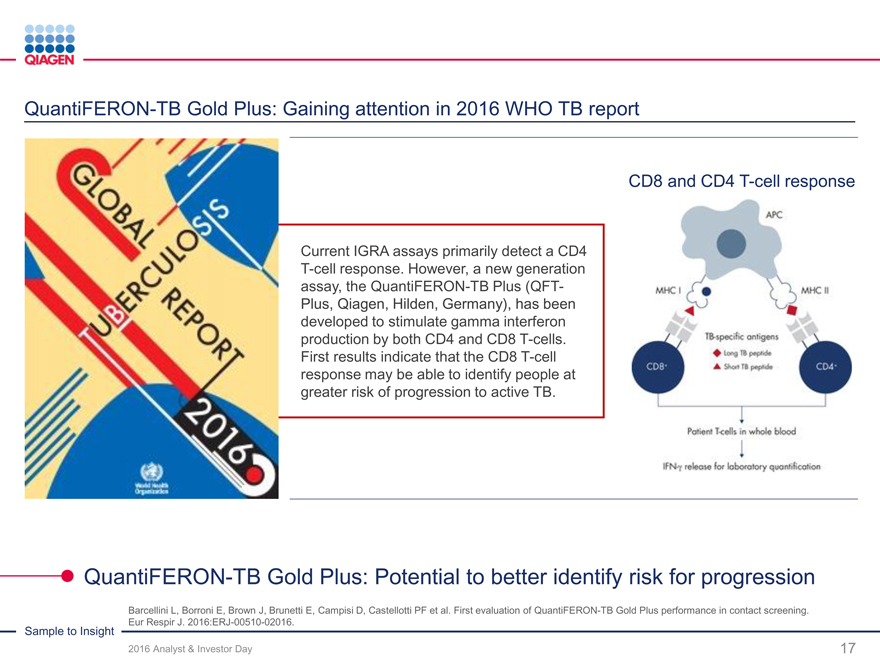
QuantiFERON Monitor and CMV tests (CE-IVD) Advance TB detection with QFT-Plus clinical benefits
Scalable automation solutions Expand QuantiFERON to other latent diseases
QuantiFERON: Setting new standards in the global fight against TB
Sample to Insight
2016 Analyst & Investor Day
19
Table of Contents
|
Strategy
Next-generation sequencing
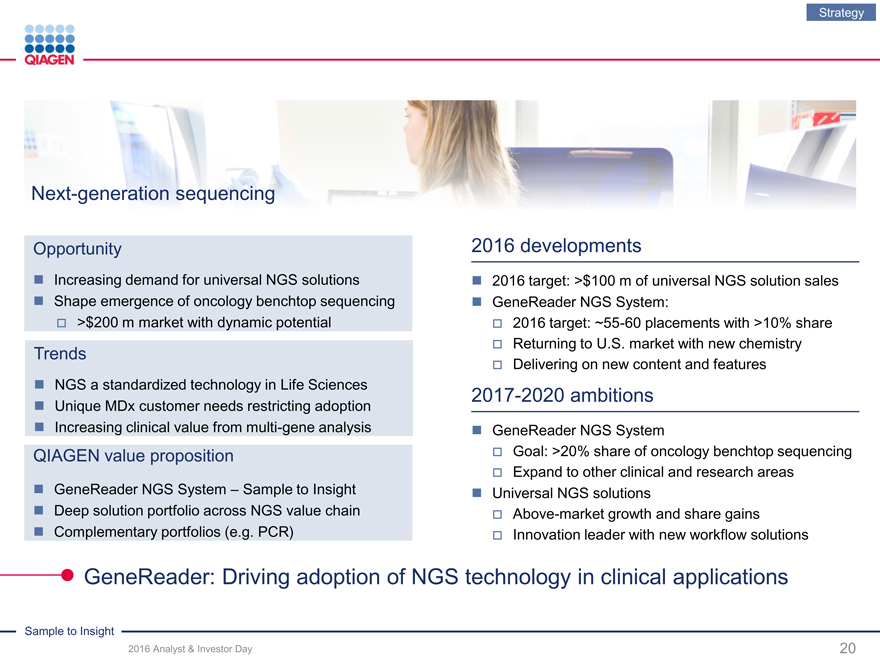
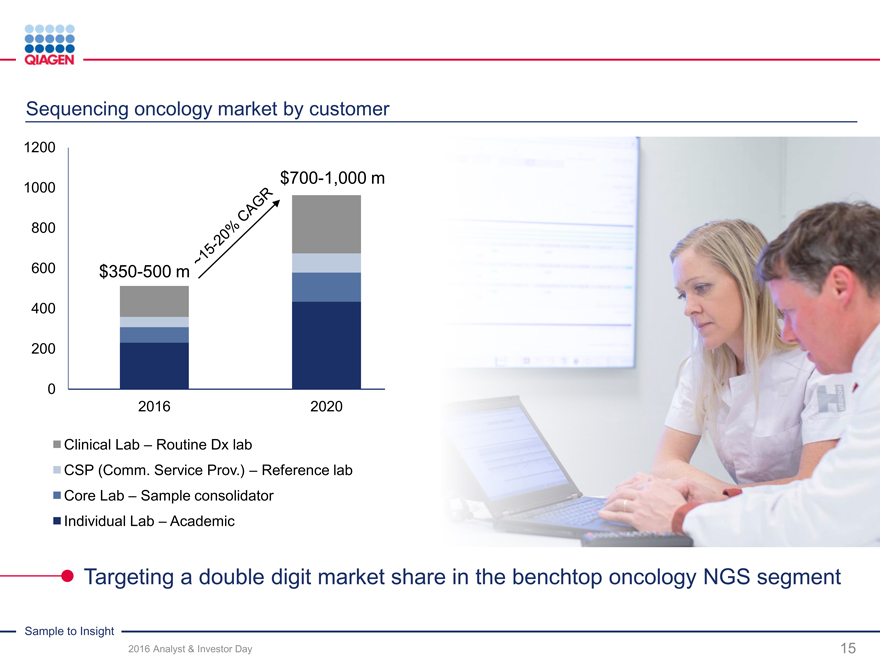
Opportunity
Increasing demand for universal NGS solutions
Shape emergence of oncology benchtop sequencing ? >$200 m market with dynamic potential
Trends
NGS a standardized technology in Life Sciences
Unique MDx customer needs restricting adoption
Increasing clinical value from multi-gene analysis
QIAGEN value proposition
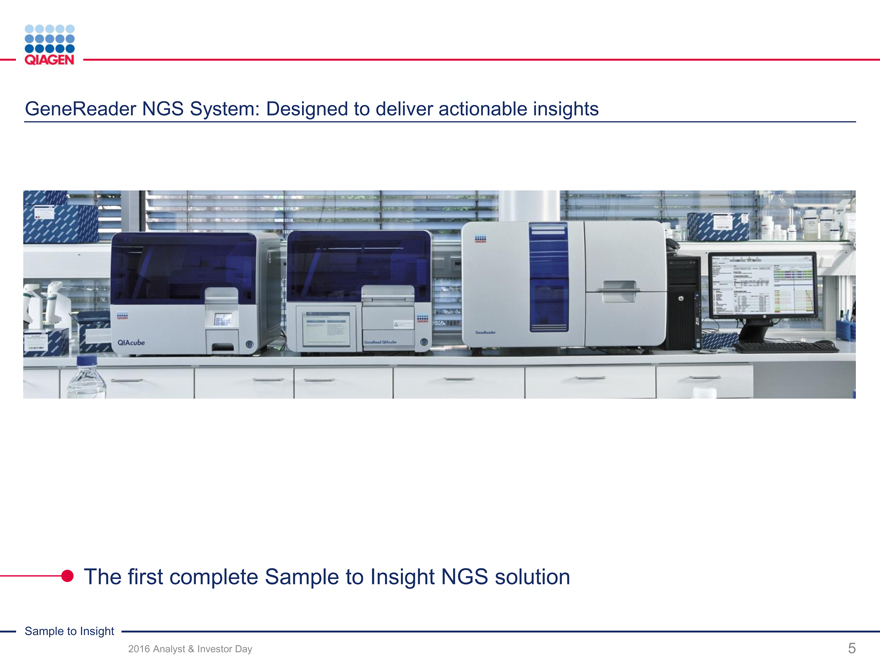
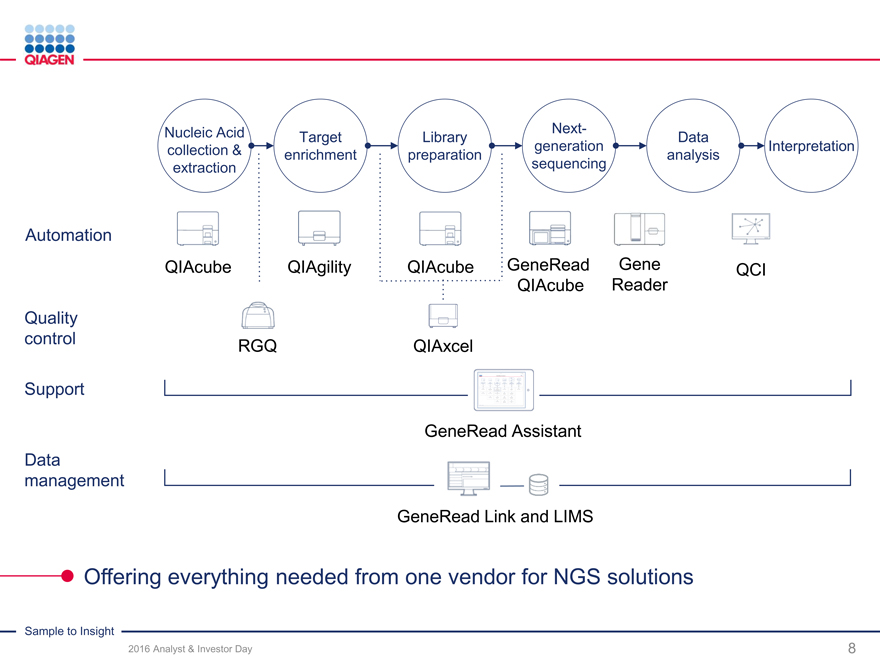
GeneReader NGS System – Sample to Insight
Deep solution portfolio across NGS value chain
Complementary portfolios (e.g. PCR)
2016 developments
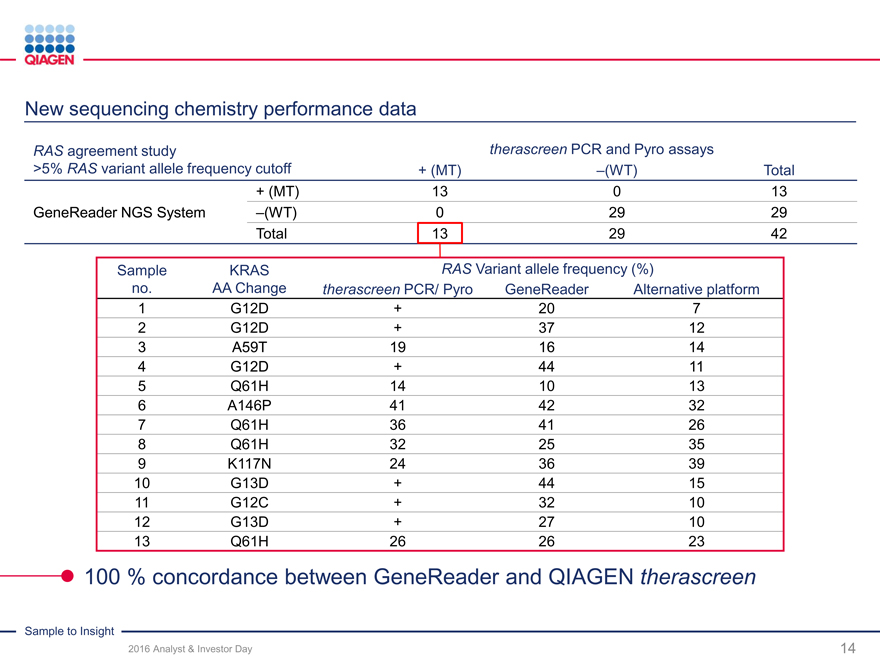
2016 target: >$100 m of universal NGS solution sales
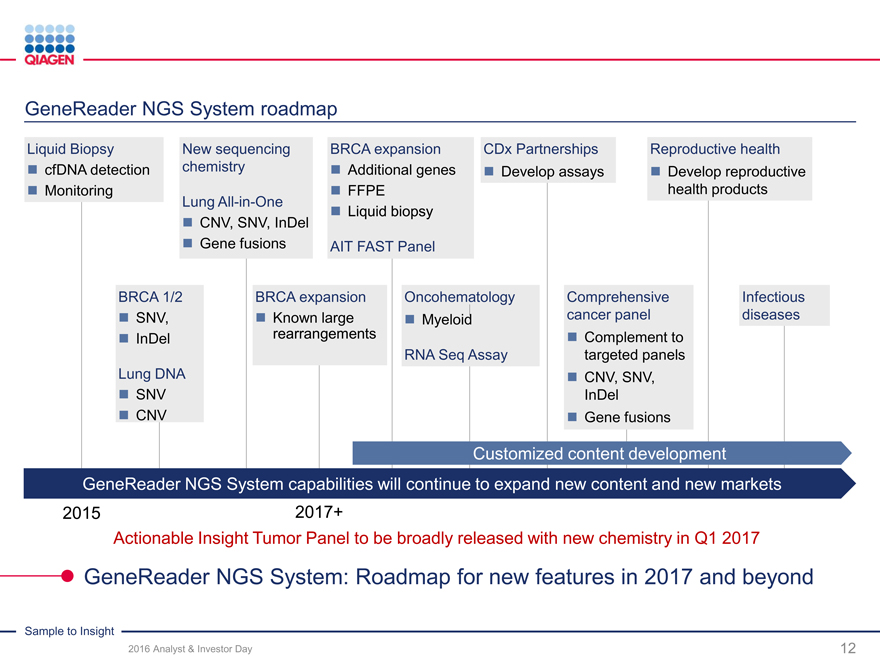
GeneReader NGS System:
2016 target: ~55-60 placements with >10% share
Returning to U.S. market with new chemistry
Delivering on new content and features
2017-2020 ambitions
GeneReader NGS System
Goal: >20% share of oncology benchtop sequencing
Expand to other clinical and research areas
Universal NGS solutions
Above-market growth and share gains
Innovation leader with new workflow solutions
GeneReader: Driving adoption of NGS technology in clinical applications
Sample to Insight
2016 Analyst & Investor Day
20
Table of Contents
|
Strategy
Personalized Healthcare
Opportunity 2016 developments
Precision medicine changing cancer treatment Milestone: >16 master collaboration agreements in 2016
Scientific advances: New tests and technologies Two new collaborations
Daiichi Sankyo – new extensive collaboration
Trends Confidential partner – immuno-oncology therapies
Expanded portfolio with GeneReader NGS System
Significant growth in molecular testing (PCR and NGS) Only supplier offering PCR and NGS technologies
Increasing acceptance of CDx value 2017-2020 ambitions
Varying regulatory conditions worldwide
QIAGEN value proposition Ambition to capture >50% of all CDx partnering deals
Expand CDx test portfolio to labs worldwide:
No. 1 worldwide in molecular companion diagnostics PCR
No. 1 in pharma co-development collaborations NGS
Proven track record of CE-IVD and FDA approvals Other molecular technologies
Personalized Healthcare: Expanding our leadership with NGS solutions
Sample to Insight
2016 Analyst & Investor Day
21
Table of Contents
|
Strategy
QIAsymphony
Opportunity 2016 developments
Medium-throughput testing: >$750 m market On track for >1,750 cumulative placements in 2016
Customer demand for robust automation systems Front-end use with GeneReader NGS System
? Modular components for different applications Workflow automation of QIAGEN liquid biopsy kits
Trends Major tender wins (viral load and transplantation)
Increasing test volumes requires more automation
Both IVD and laboratory-developed tests increasing 2017-2020 ambitions
2017 goal: >2,000 cumulative placements
QIAGEN value proposition Geographic strategy:
QIAsymphony: Highly flexible, proven automation Europe and rest of world: Broaden test menu
Largest IVD test menu in Europe U.S.: Address LDT and sample processing demand
Standard for sample processing for any application
QIAsymphony: Highly versatile automation solution providing solid growth
Sample to Insight
2016 Analyst & Investor Day 22
Table of Contents
|
Strategy
Differentiated Technologies
Opportunity
Improving Life Sciences funding trends
Demands for workflow solutions – not technologies
Trends
Cycle of scientific breakthroughs gaining momentum
Faster transition into clinical applications (IVD / LDT)
2016 developments
Developing Sample to Insight workflows:
Liquid biopsy
Digital NGS
Microbiomes
Epigenetics
Single-cell research
More to come
QIAGEN value proposition 2017-2020 ambitions
Sample to Insight solutions Expand portfolio by at least 2 emerging technologies
Trusted brand per year
Ability to standardize and support breakthroughs Drive double-digit CER sales growth
[Graphic Appears Here]
Differentiated Technologies: Turning innovations into attractive workflows
Sample to Insight
2016 Analyst & Investor Day
23
Table of Contents
|
Strategy
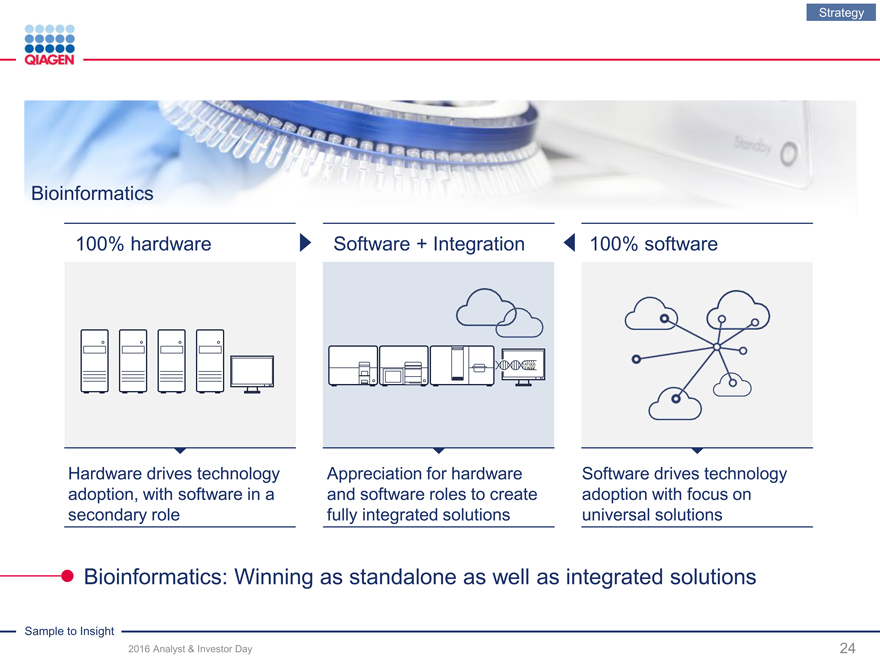
Bioinformatics
100% hardware Software + Integration 100% software
TACGT ATGCA
Hardware drives technology Appreciation for hardware Software drives technology adoption, with software in a and software roles to create adoption with focus on secondary role fully integrated solutions universal solutions
Bioinformatics: Winning as standalone as well as integrated solutions
Sample to Insight
2016 Analyst & Investor Day 24
Table of Contents
|
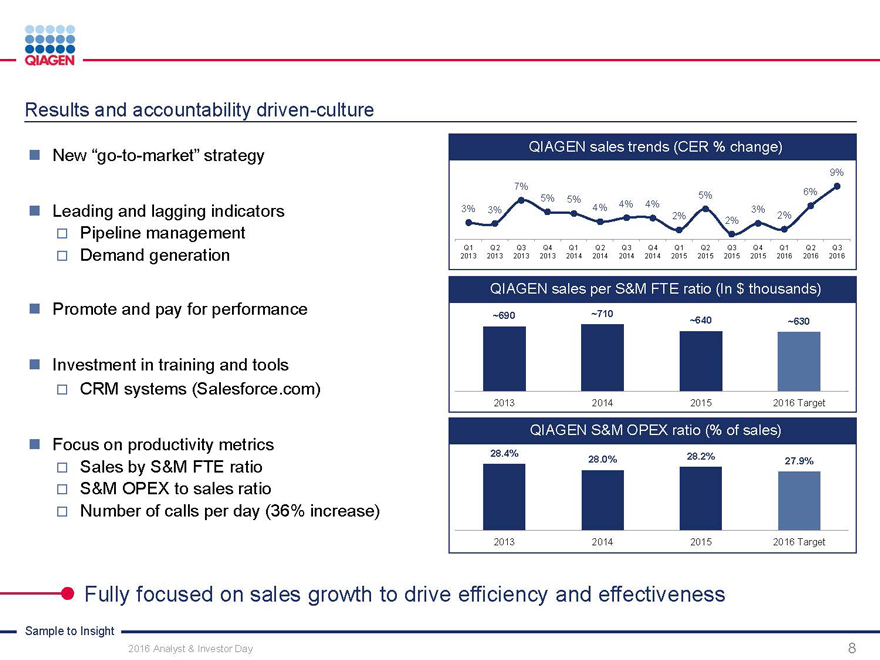
Strategy
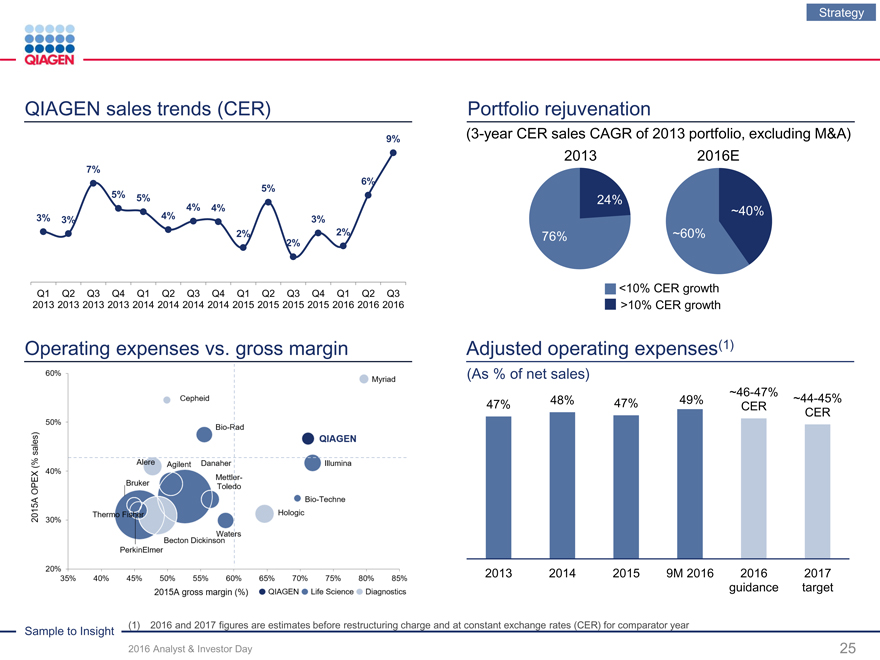
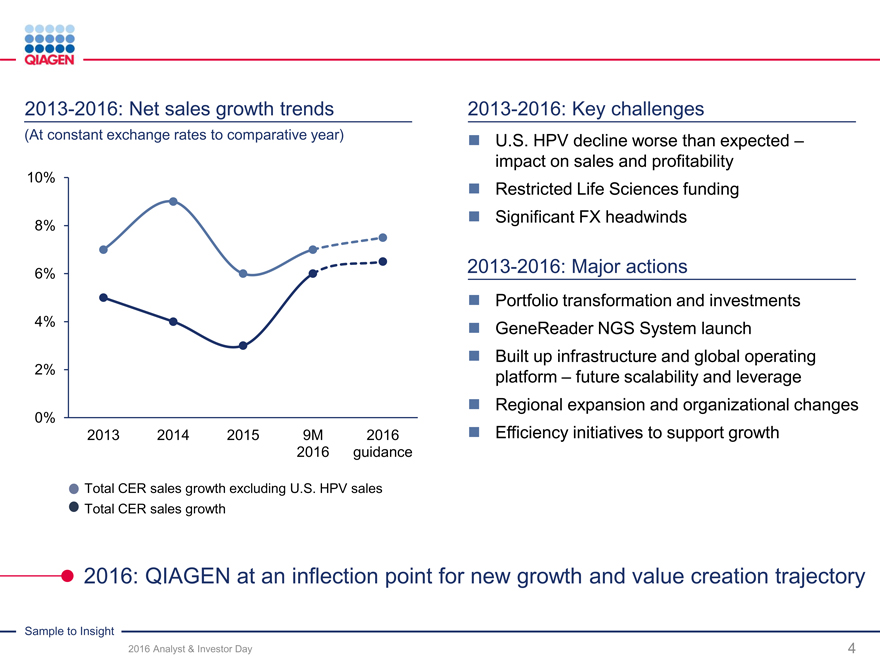
QIAGEN sales trends (CER)
9%
7%
6%
5%
5% 5%
4% 4%
3% 3% 4% 3%
2% 2%
2%
Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3
2013 2013 2013 2013 2014 2014 2014 2014 2015 2015 2015 2015 2016 2016 2016
Operating expenses vs. gross margin
Portfolio rejuvenation
(3-year CER sales CAGR of 2013 portfolio, excluding M&A)
2013 2016E
24%
~40%
76% ~60%
<10% CER growth
>10% CER growth
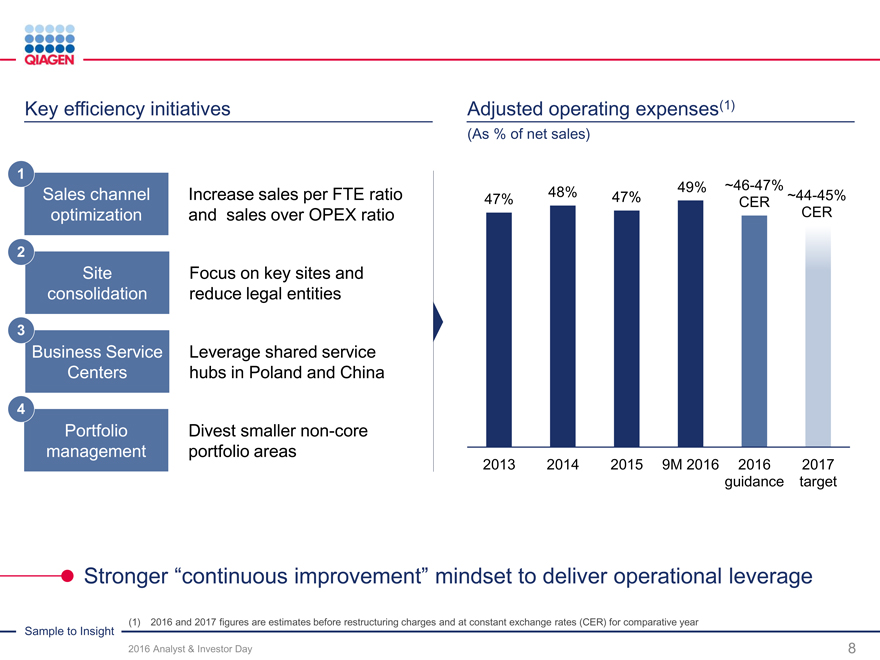
Adjusted operating expenses(1)
(As % of net sales)
48% ~46-47%
47% 47% 49%CER~44-45%
CER
2013 201420159M 201620162017
guidancetarget
Sample to Insight (1) 2016 and 2017 figures are estimates before restructuring charge and at constant exchange rates (CER) for comparator year
2016 Analyst & Investor Day 25
Table of Contents
|
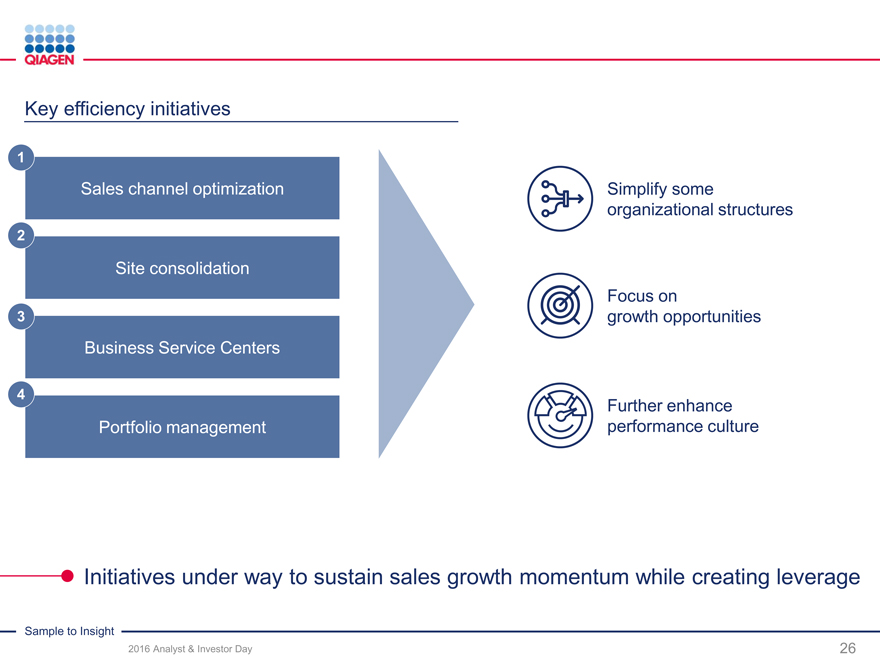
Key efficiency initiatives
1
Sales channel optimization Simplify some organizational structures
2
Site consolidation
Focus on
3 growth opportunities Business Service Centers
4
Further enhance Portfolio management performance culture
Initiatives under way to sustain sales growth momentum while creating leverage
Sample to Insight
2016 Analyst & Investor Day 26
Table of Contents
|
Strategy
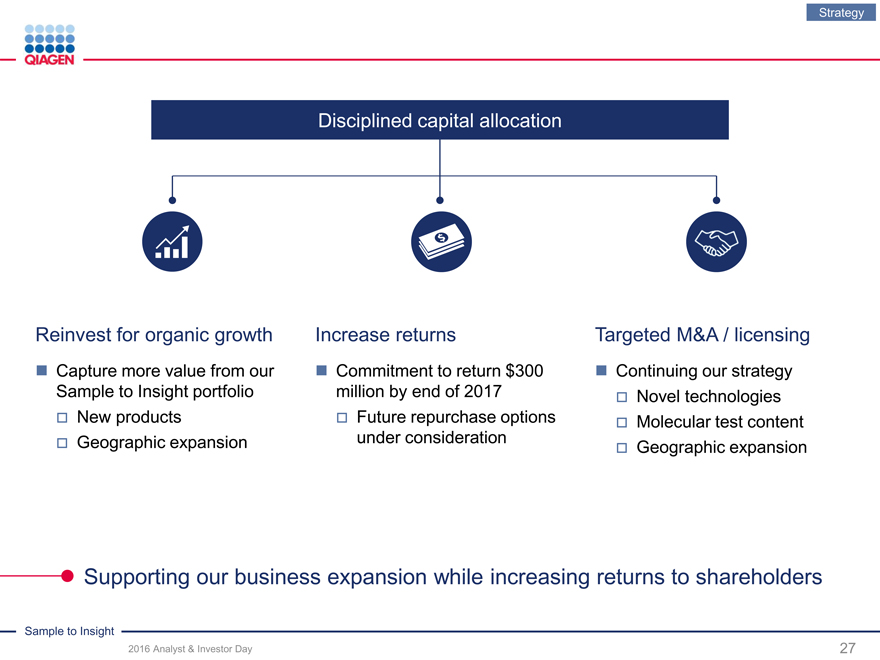
Disciplined capital allocation
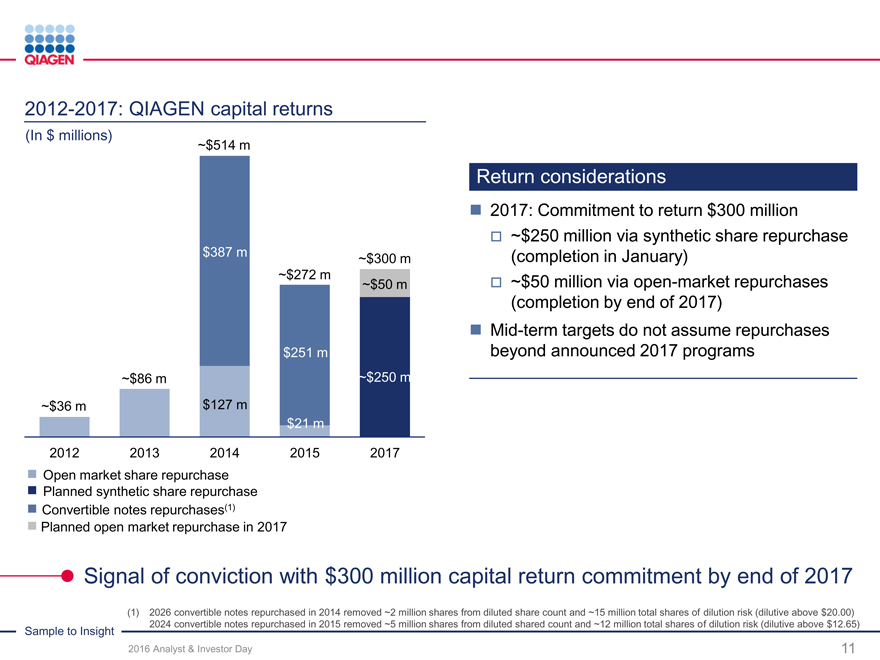
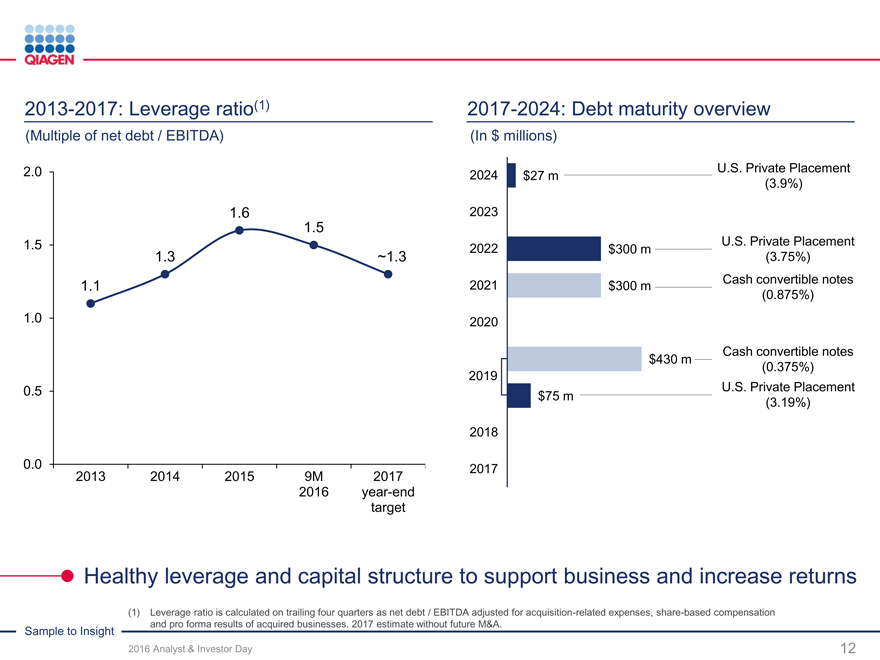
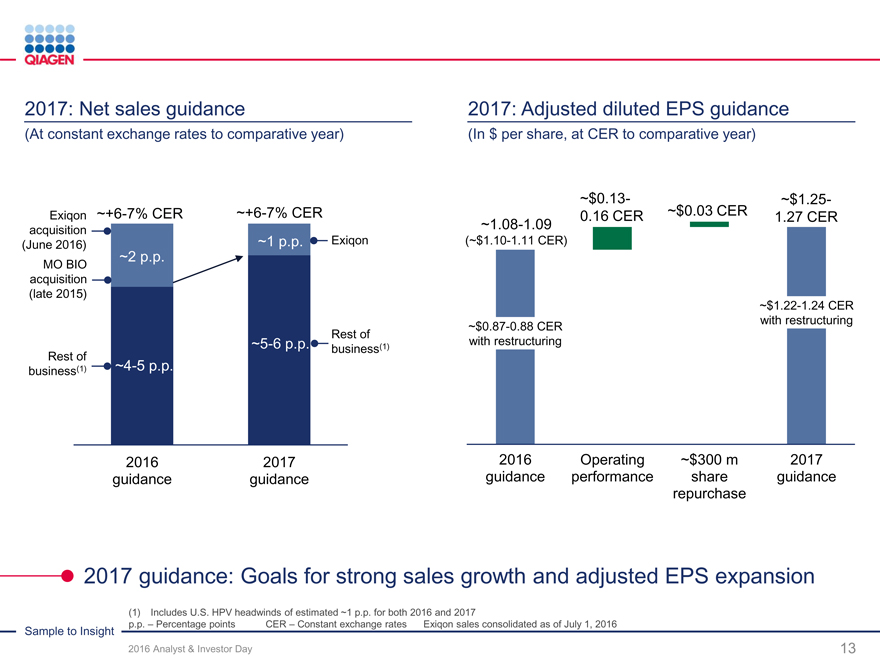
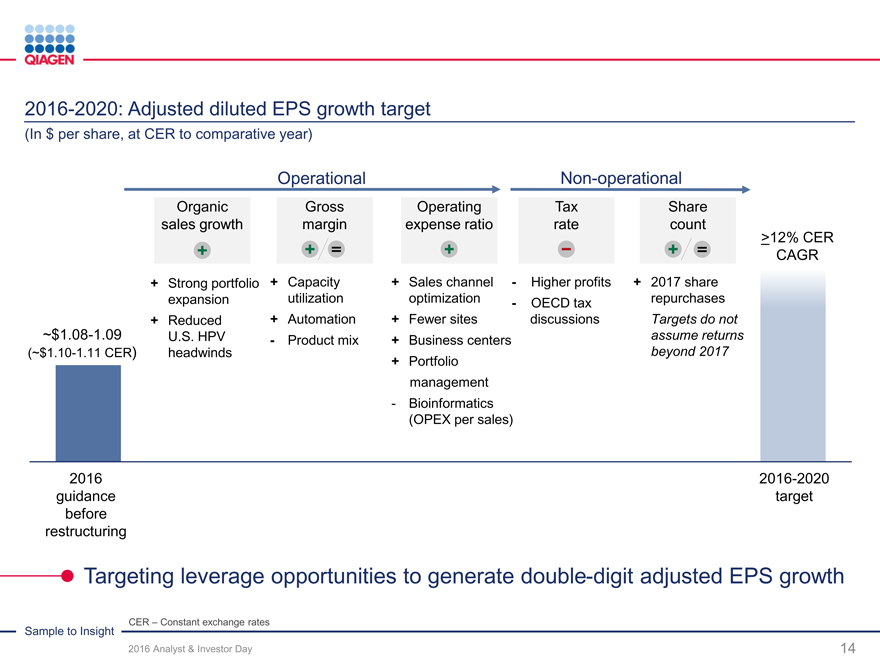
Reinvest for organic growth Increase returns Targeted M&A / licensing
Capture more value from our Commitment to return $300Continuing our strategy
Sample to Insight portfolio million by end of 2017Novel technologies
New productsFuture repurchase optionsMolecular test content
Geographic expansionunder considerationGeographic expansion
Supporting our business expansion while increasing returns to shareholders
Sample to Insight
2016 Analyst & Investor Day
27
Table of Contents
|
Strategy
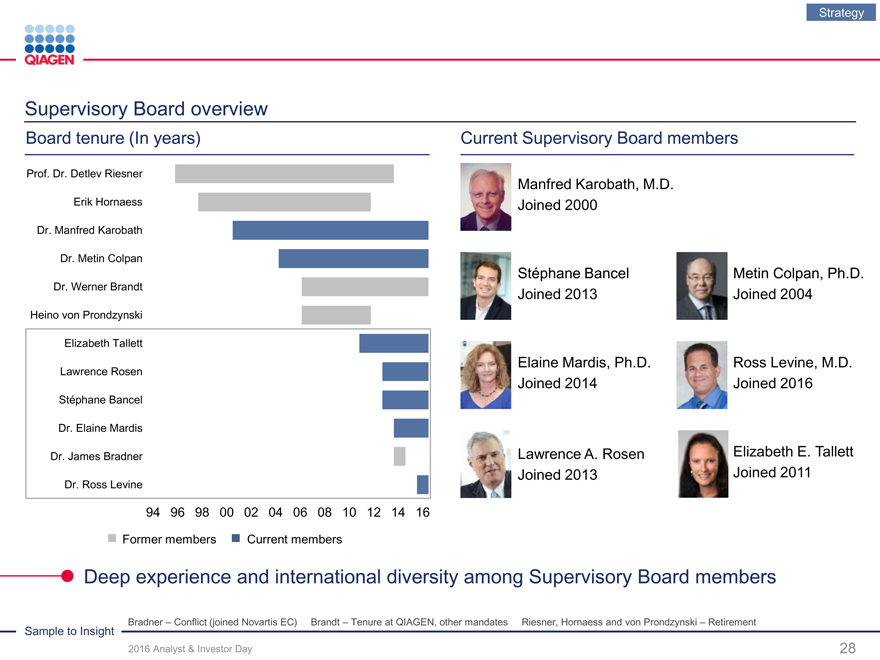
Supervisory Board overview
Board tenure (In years) Current Supervisory Board members
Prof. Dr. Detlev Riesner
Manfred Karobath, M.D.
Erik Hornaess Joined 2000
Dr. Manfred Karobath
Dr. Metin Colpan
Stéphane Bancel Metin Colpan, Ph.D.
Dr. Werner Brandt Joined 2013 Joined 2004
Heino von Prondzynski
Elizabeth Tallett
Lawrence Rosen
Stéphane Bancel
Dr. Elaine Mardis
Dr. James Bradner
Dr. Ross Levine
94 96 98 00 02 04 06 08 10 121416
Former members Current members
Elaine Mardis, Ph.D. Ross Levine, M.D.
Joined 2014 Joined 2016
Lawrence A. Rosen Elizabeth E. Tallett
Joined 2013 Joined 2011
Deep experience and international diversity among Supervisory Board members
Sample to Insight Bradner – Conflict (joined Novartis EC) Brandt – Tenure at QIAGEN, other mandates Riesner, Hornaess and von Prondzynski – Retirement
2016 Analyst & Investor Day 28
Table of Contents
|
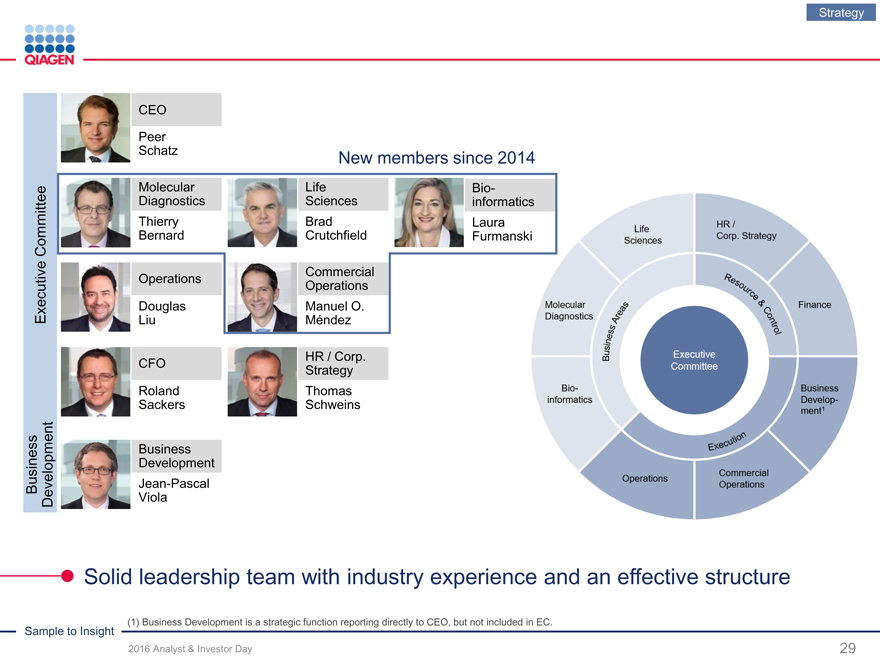
Strategy
CEO
Peer
Schatz
New members since 2014
Molecular LifeBio-
Diagnostics Sciencesinformatics
Thierry BradLaura
Committee Bernard CrutchfieldFurmanski
Commercial
Operations
Operations
Executive DouglasManuel O.
LiuMéndez
HR / Corp.
CFO
Strategy
RolandThomas
SackersSchweins
Business
Development
Jean-Pascal
Business Development
Viola
Solid leadership team with industry experience and an effectivestructure
(1) Business Development is a strategic function reporting directly to CEO, but not included in EC.
Sample to Insight
2016 Analyst & Investor Day29
Table of Contents
|
Strategy
Committed employees worldwide helping customers to gain valuable insights
Sample to Insight
2016 Analyst & Investor Day 30
Table of Contents
|
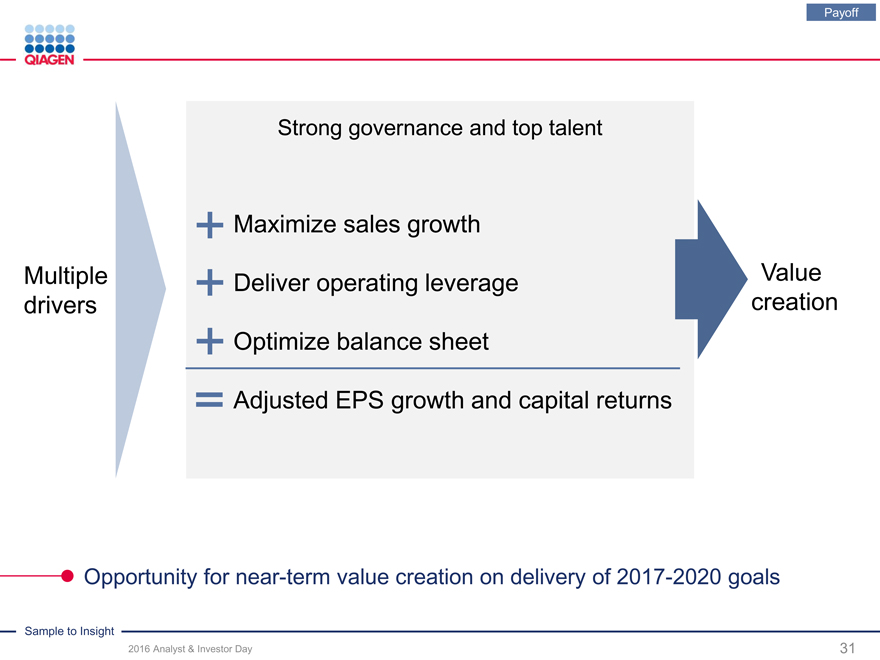
Payoff
Strong governance and top talent
Maximize sales growth
Multiple Deliver operating leverage Value drivers creation Optimize balance sheet
Adjusted EPS growth and capital returns
Opportunity for near-term value creation on delivery of 2017-2020 goals
Sample to Insight
2016 Analyst & Investor Day 31
Table of Contents
|
Summary
Reaccelerating performance after transformation Sustainable sales growth and leverage trajectory Emerging as a stronger, focused, differentiated leader Committed to higher returns and greater value creation
Sample to Insight
2016 Analyst & Investor Day 32
Table of Contents
Exhibit 99.3
|
Life Sciences:
Innovator at the leading edge of research
Brad Crutchfield
Senior Vice President, Head of Life Sciences Business Areas November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Table of Contents
|
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Sample to Insight
2016 Analyst & Investor Day 3
Table of Contents
|
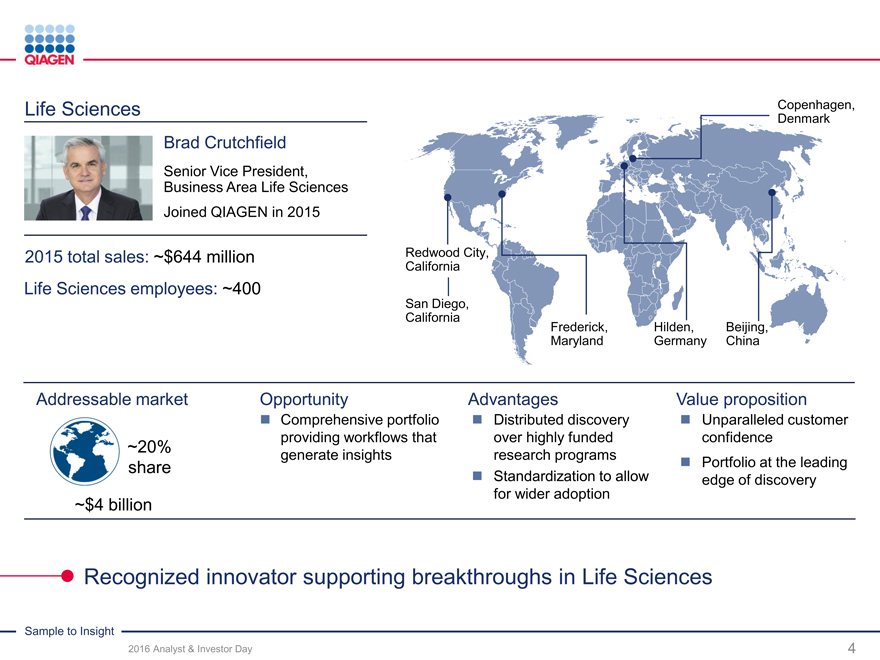
Life Sciences Copenhagen,
Denmark
Brad Crutchfield
Senior Vice President,
Business Area Life Sciences
Joined QIAGEN in 2015
2015 total sales: ~$644 million Redwood City,
California
Life Sciences employees: ~400
San Diego,
California
Frederick,Hilden,Beijing,
MarylandGermanyChina
Addressable market Opportunity AdvantagesValue proposition
Comprehensive portfolio Distributed discovery Unparalleled customer
providing workflows thatover highly fundedconfidence
~20% generate insightsresearch programs
share Portfolio at the leading
Standardization to allowedge of discovery
for wider adoption
~$4 billion
Recognized innovator supporting breakthroughs in Life Sciences
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
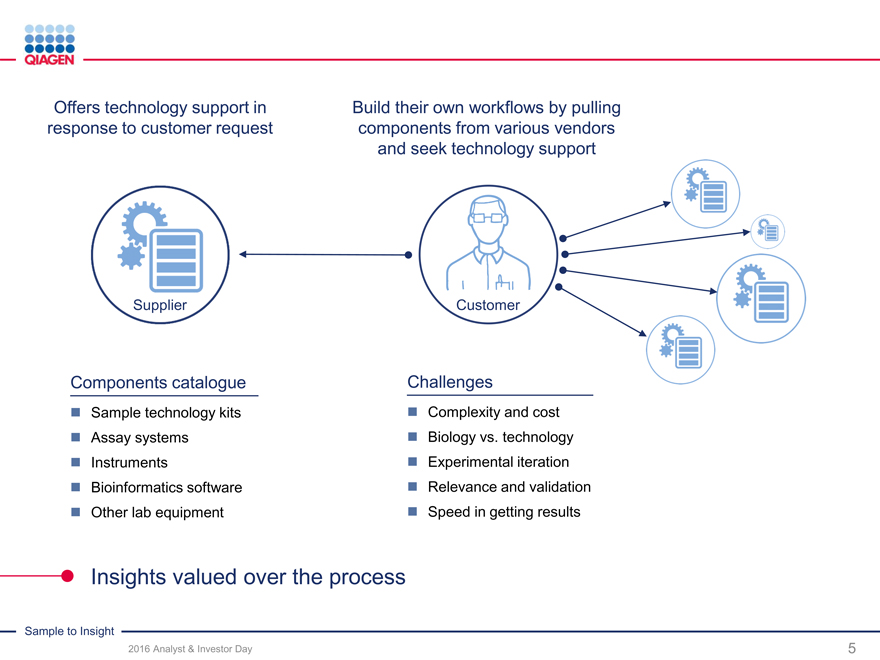
Offers technology support in Build their own workflows by pulling
response to customer request components from various vendors
and seek technology support
Supplier Customer
Components catalogue Challenges
Sample technology kits Complexity and cost
Assay systems Biology vs. technology
Instruments Experimental iteration
Bioinformatics software Relevance and validation
Other lab equipment Speed in getting results
Insights valued over the process
Sample to Insight
2016 Analyst & Investor Day
5
Table of Contents
|
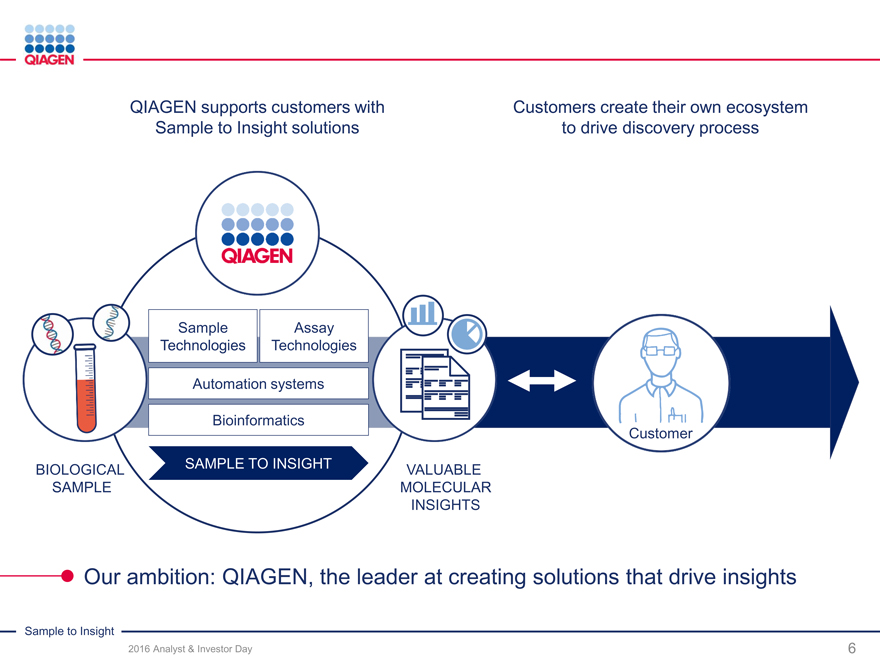
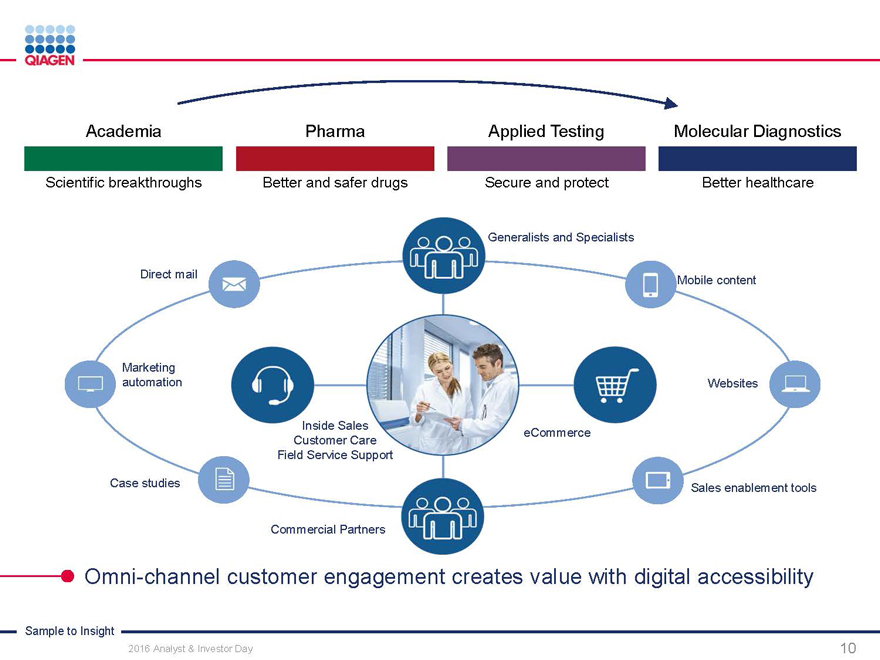
QIAGEN supports customers with Customers create their own ecosystem
Sample to Insight solutions to drive discovery process
Sample Assay
Technologies Technologies
Automation systems
Bioinformatics
Customer
BIOLOGICAL SAMPLE TO INSIGHT VALUABLE
SAMPLE MOLECULAR
INSIGHTS
Our ambition: QIAGEN, the leader at creating solutions that drive insights
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
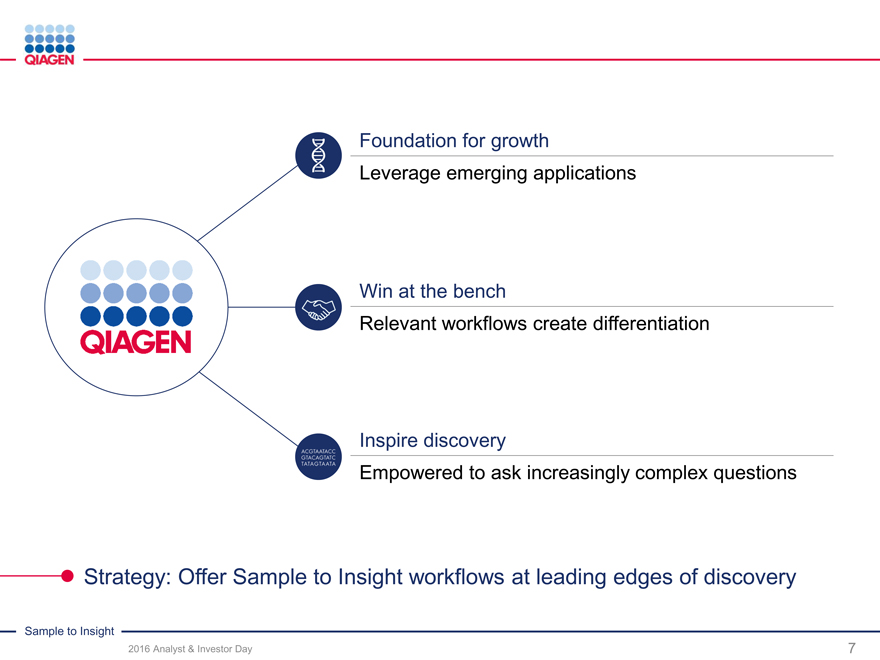
Foundation for growth
Leverage emerging applications
Win at the bench
Relevant workflows create differentiation
Inspire discovery
Empowered to ask increasingly complex questions
Strategy: Offer SampletoInsight workflows at leading edges of discovery
Sample to Insight
2016 Analyst & Investor Day
7
Table of Contents
|
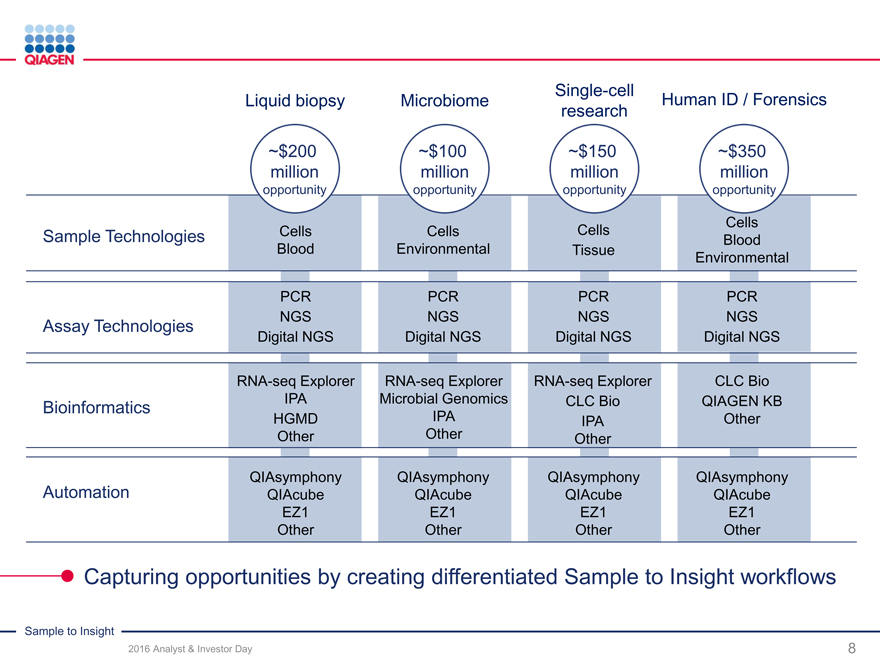
Single-cell
Liquid biopsy MicrobiomeHuman ID / Forensics
research
~$200 ~$100~$150~$350
million millionmillionmillion
opportunity opportunityopportunityopportunity
Cells
Sample Technologies Cells CellsCellsBlood
Blood EnvironmentalTissueEnvironmental
PCR PCRPCRPCR
Assay Technologies NGS NGSNGSNGS
Digital NGS Digital NGSDigital NGSDigital NGS
RNA-seq Explorer RNA-seq ExplorerRNA-seq ExplorerCLC Bio
Bioinformatics IPA Microbial GenomicsCLC BioQIAGEN KB
HGMD IPAIPAOther
Other OtherOther
QIAsymphony QIAsymphonyQIAsymphonyQIAsymphony
Automation QIAcube QIAcubeQIAcubeQIAcube
EZ1 EZ1EZ1EZ1
Other OtherOtherOther
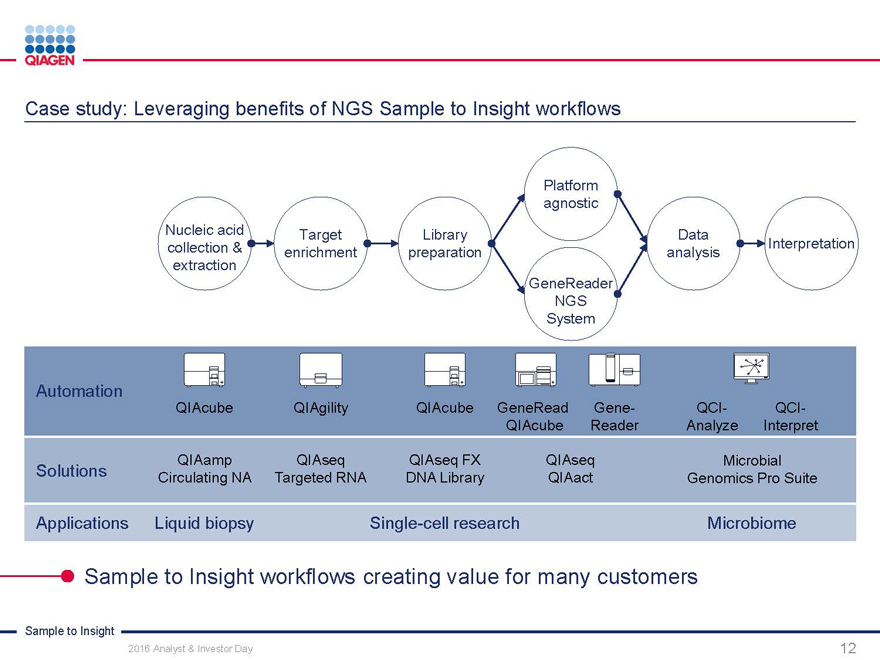
Capturing opportunities by creating differentiated Sample to Insight workflows
Sample to Insight
2016 Analyst & Investor Day
8
Table of Contents
|
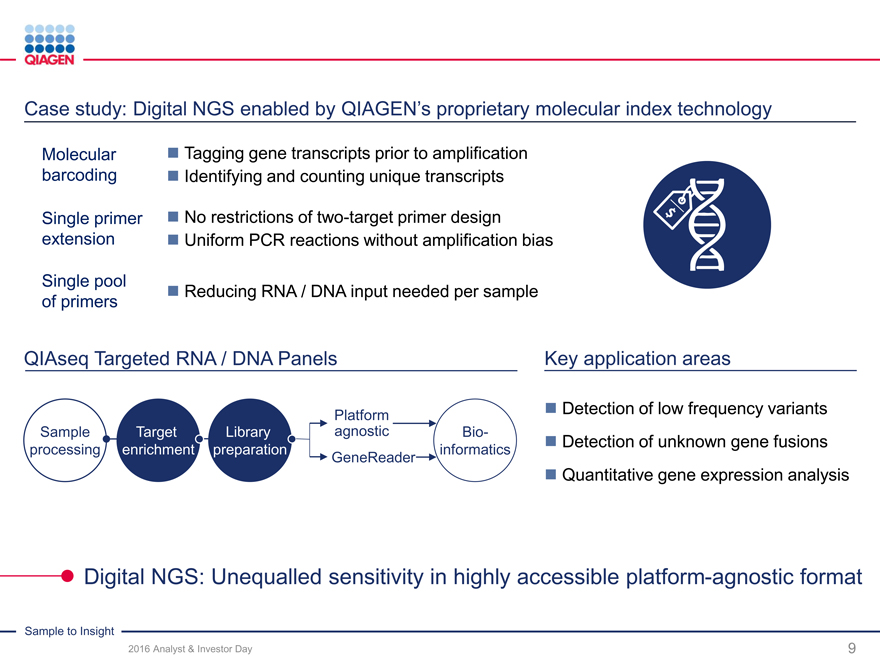
Case study: Digital NGS enabled by QIAGEN’s proprietary molecular index technology
Molecular Tagging gene transcripts prior to amplification
barcoding Identifying and counting unique transcripts
Single primer No restrictions of two-target primer design
extension Uniform PCR reactions without amplification bias
Single pool Reducing RNA / DNA input needed per sample
of primers
QIAseq Targeted RNA / DNA Panels Key application areas
Platform
Sample Target LibraryagnosticBio-
processing enrichment preparationinformatics
GeneReader
Detection of low frequency variants
Detection of unknown gene fusions
Quantitative gene expression analysis
Digital NGS: Unequalled sensitivity in highly accessible platform-agnostic format
Sample to Insight
2016 Analyst & Investor Day
9
Table of Contents
|
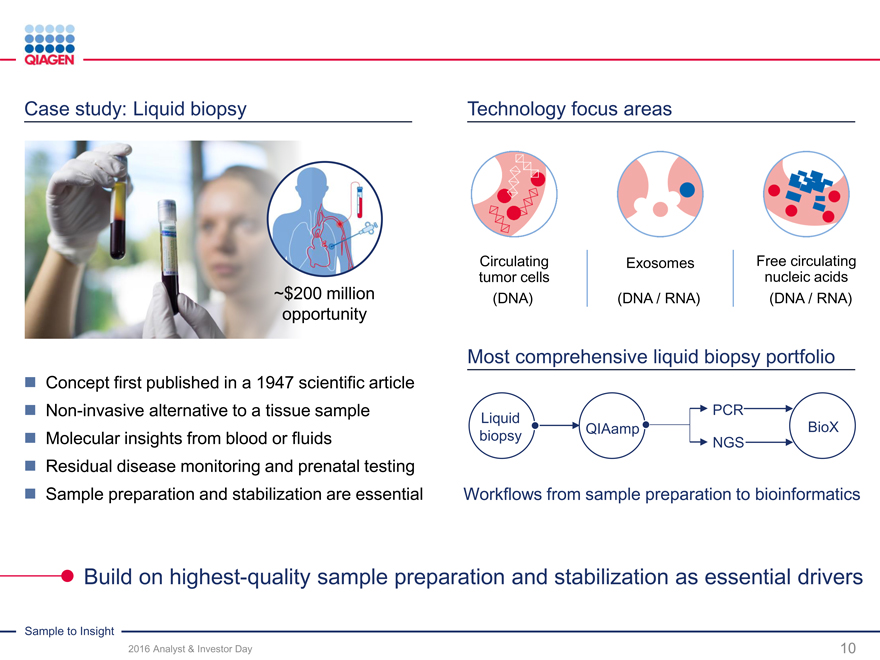
Case study: Liquid biopsy Technology focus areas
CirculatingExosomesFree circulating
tumor cellsnucleic acids
~$200 million (DNA)(DNA / RNA)(DNA / RNA)
opportunity
Most comprehensive liquid biopsy portfolio
Concept first published in a 1947 scientific article
Non-invasive alternative to a tissue sample PCR
LiquidQIAampBioX
Molecular insights from blood or fluids biopsyNGS
Residual disease monitoring and prenatal testing
Sample preparation and stabilization are essential Workflows from sample preparation to bioinformatics
Build on highest-quality sample preparation and stabilization as essential drivers
Sample to Insight
2016 Analyst & Investor Day 10
Table of Contents
|
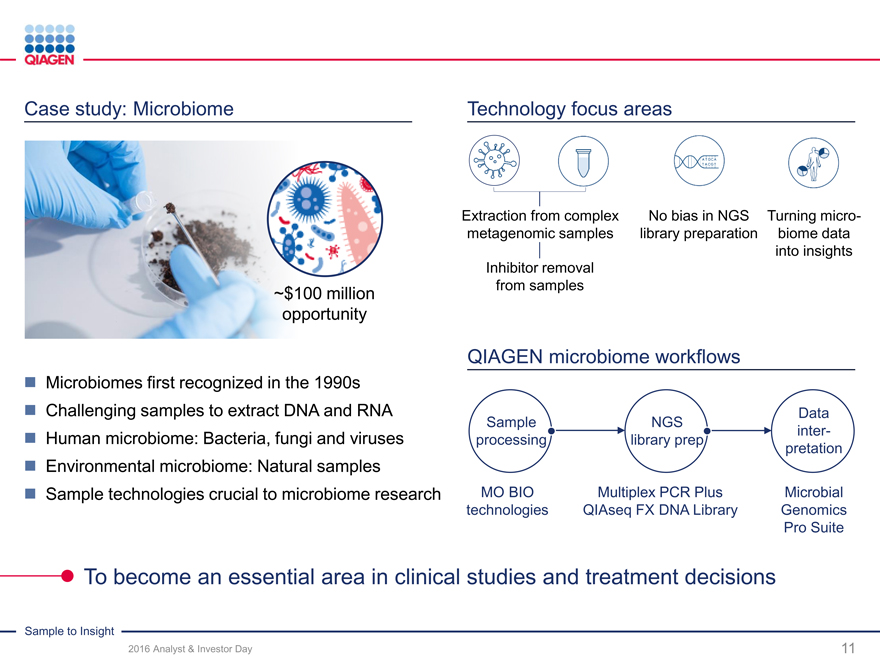
Case study: Microbiome
~$100 million opportunity
Technology focus areas
Microbiomes first recognized in the 1990s
Challenging samples to extract DNA and RNA
Human microbiome: Bacteria, fungi and viruses
Environmental microbiome: Natural samples
Sample technologies crucial to microbiome research
Extraction from complex No bias in NGS Turning micro-
metagenomic samples library preparation biome data
into insights
Inhibitor removal
from samples
QIAGEN microbiome workflows
Data
Sample NGSinter-
processing library preppretation
MO BIO Multiplex PCR Plus Microbial
technologies QIAseq FX DNA Library Genomics
Pro Suite
To become an essential area in clinical studies and treatment decisions
Sample to Insight
2016 Analyst & Investor Day
11
Table of Contents
|
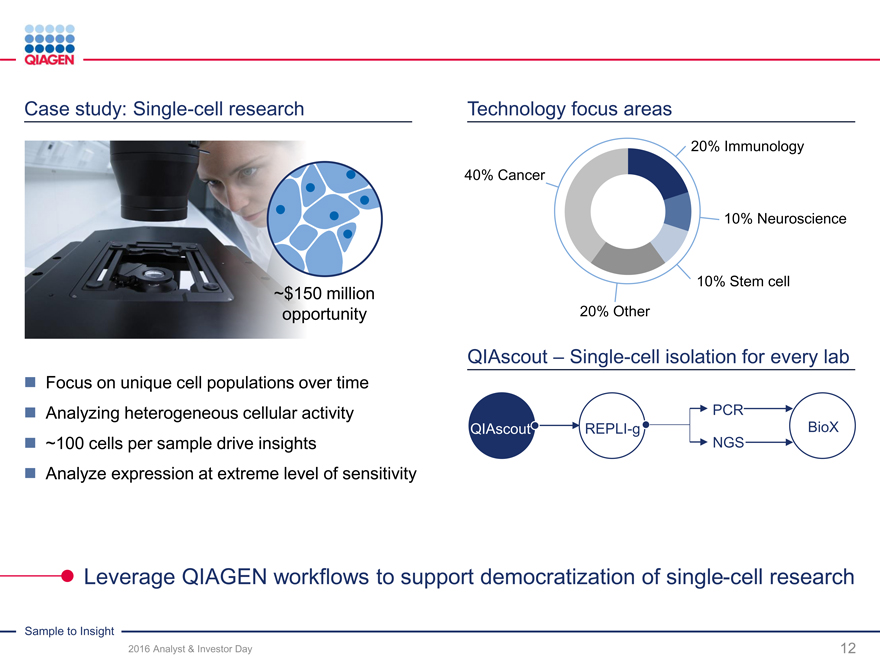
Case study: Single-cell research Technology focus areas
20% Immunology
40% Cancer
10% Neuroscience
10% Stem cell
~$150 million
opportunity 20% Other
QIAscout – Single-cell isolation for every lab
Focus on unique cell populations over time
Analyzing heterogeneous cellular activity PCR
QIAscoutREPLI-gBioX
~100 cells per sample drive insights NGS
Analyze expression at extreme level of sensitivity
Leverage QIAGEN workflows to support democratization of single-cell research
Sample to Insight
2016 Analyst & Investor Day 12
Table of Contents
|
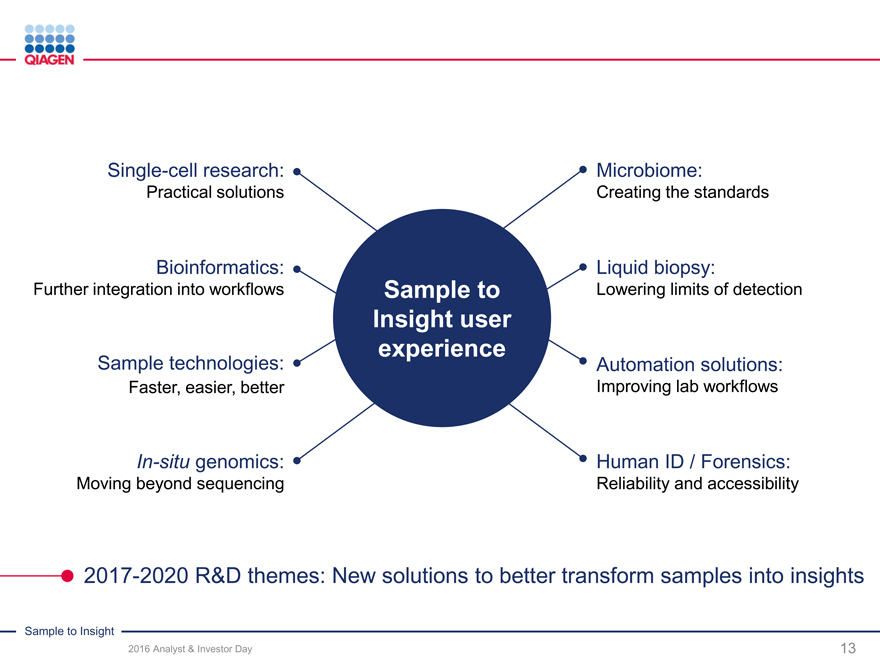
Single-cell research: Microbiome:
Practical solutions Creating the standards
Bioinformatics: Liquid biopsy:
Further integration into workflows Sample to Lowering limits of detection
Insight user
experience
Sample technologies: Automation solutions:
Faster, easier, better Improving lab workflows
In-situ genomics: Human ID / Forensics:
Moving beyond sequencing Reliability and accessibility
2017-2020 R&D themes: New solutions to better transform samples into insights
Sample to Insight
2016 Analyst & Investor Day
13
Table of Contents
|
A trusted innovator in Life Science Research Unique position to deliver solutions Differentiation with the best user experience Transforming samples into valuable insights
Sample to Insight
2016 Analyst & Investor Day 14
Table of Contents
Exhibit 99.4
|
Molecular Diagnostics:
Addressing urgent healthcare issues with innovative solutions
Thierry Bernard
Senior Vice President, Head of Molecular Diagnostics Business Area November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Disclaimer
Table of Contents
|
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
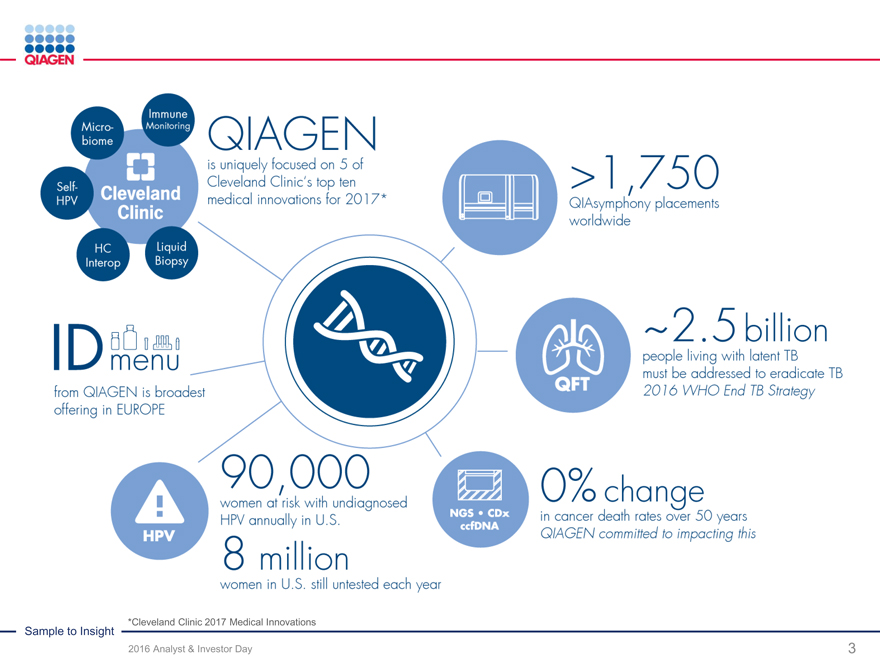
Sample to Insight *Cleveland Clinic 2017 Medical Innovations
2016 Analyst & Investor Day 3
Table of Contents
|
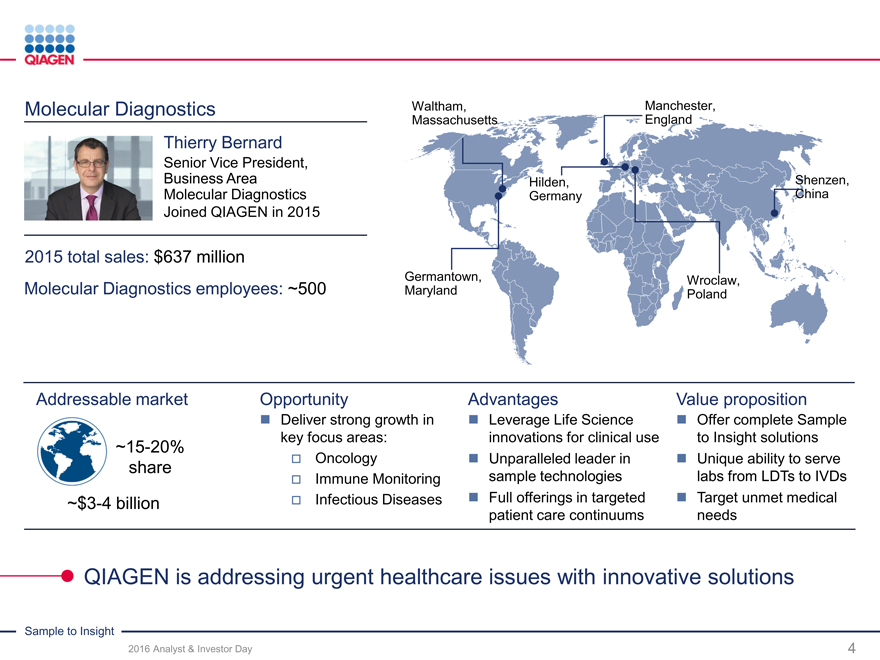
Molecular Diagnostics Waltham, Manchester,
Massachusetts England
Thierry Bernard
Senior Vice President,
Business Area Hilden,Shenzen,
Molecular Diagnostics GermanyChina
Joined QIAGEN in 2015
2015 total sales: $637 million
Germantown, Wroclaw,
Molecular Diagnostics employees: ~500 Maryland Poland
Addressable market Opportunity AdvantagesValue proposition
Deliver strong growth in Leverage Life Science Offer complete Sample
~15-20% key focus areas: innovations for clinical useto Insight solutions
share Oncology Unparalleled leader in Unique ability to serve
Immune Monitoringsample technologieslabs from LDTs to IVDs
~$3-4 billion Infectious Diseases Full offerings in targeted Target unmet medical
patient care continuumsneeds
QIAGEN is addressing urgent healthcare issues with innovative solutions
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
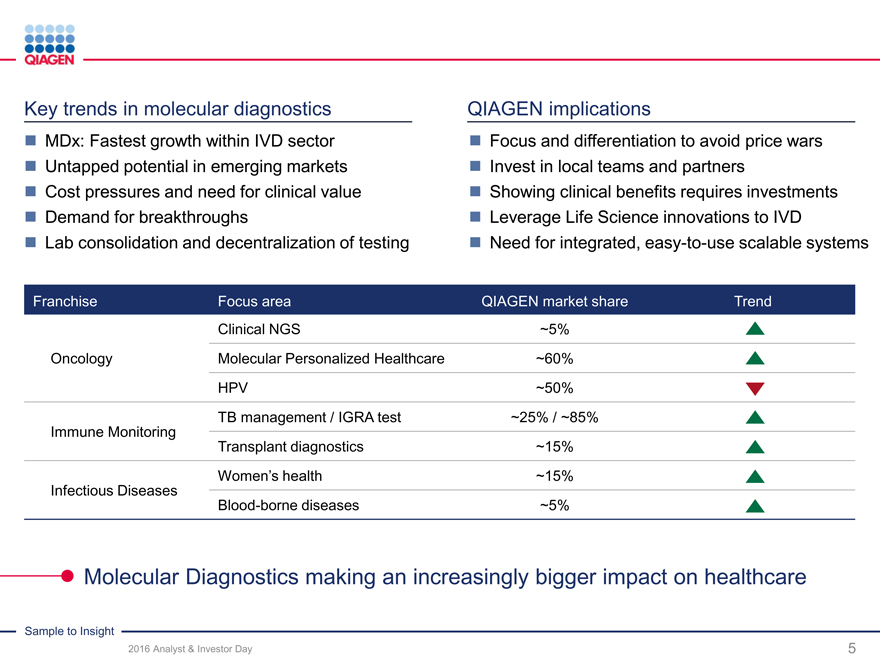
Key trends in molecular diagnostics
MDx: Fastest growth within IVD sector
Untapped potential in emerging markets
Cost pressures and need for clinical value
Demand for breakthroughs
Lab consolidation and decentralization of testing
QIAGEN implications
Focus and differentiation to avoid price wars
Invest in local teams and partners
Showing clinical benefits requires investments
Leverage Life Science innovations to IVD
Need for integrated, easy-to-use scalable systems
Franchise Focus area QIAGEN market shareTrend
Clinical NGS ~5%
Oncology Molecular Personalized Healthcare ~60%
HPV ~50%
TB management / IGRA test ~25% / ~85%
Immune Monitoring
Transplant diagnostics ~15%
Women’s health ~15%
Infectious Diseases
Blood-borne diseases ~5%
Molecular Diagnostics making an increasingly bigger impact on healthcare
Sample to Insight
2016 Analyst & Investor Day
5
Table of Contents
|
Covering the most attractive Molecular Diagnostics segments
QIAGEN’s differentiated offering addressing urgent healthcare needs:
Immune Monitoring
Oncology
Infectious Diseases
Complete Sample to Insight solutions: Scalable workflows using different technologies
Our ambition: A QIAGEN Sample to Insight solution in every MDx lab worldwide
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
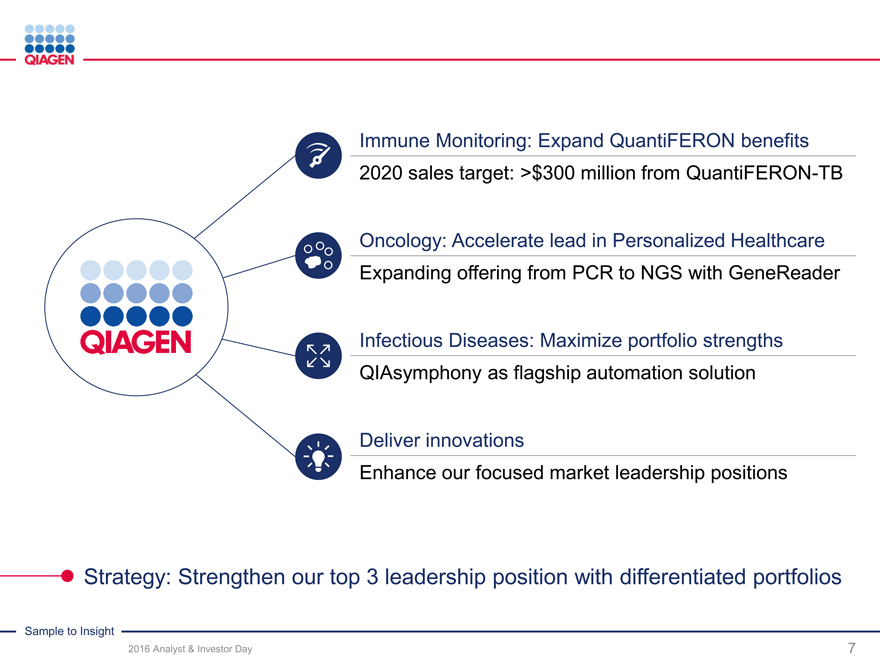
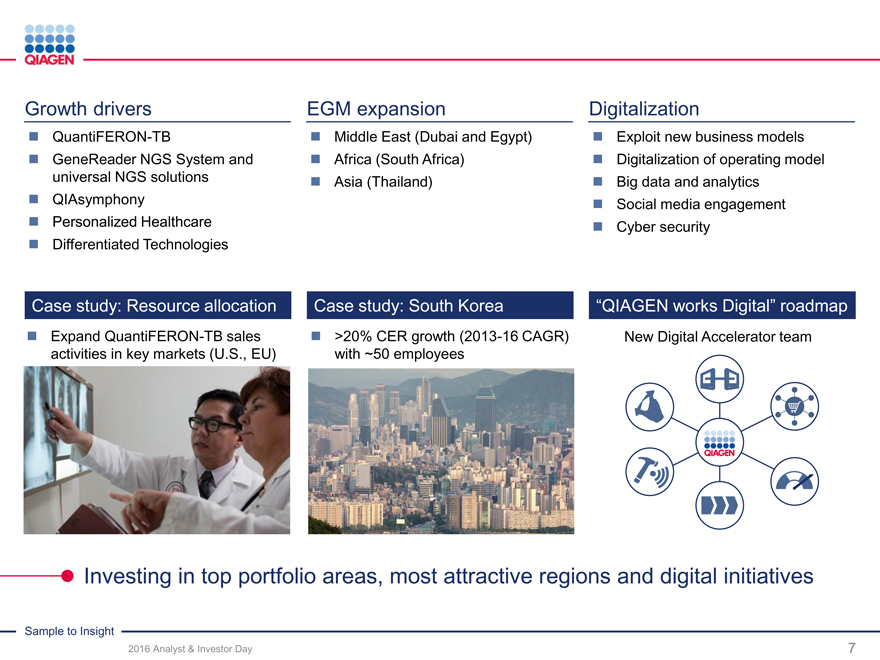
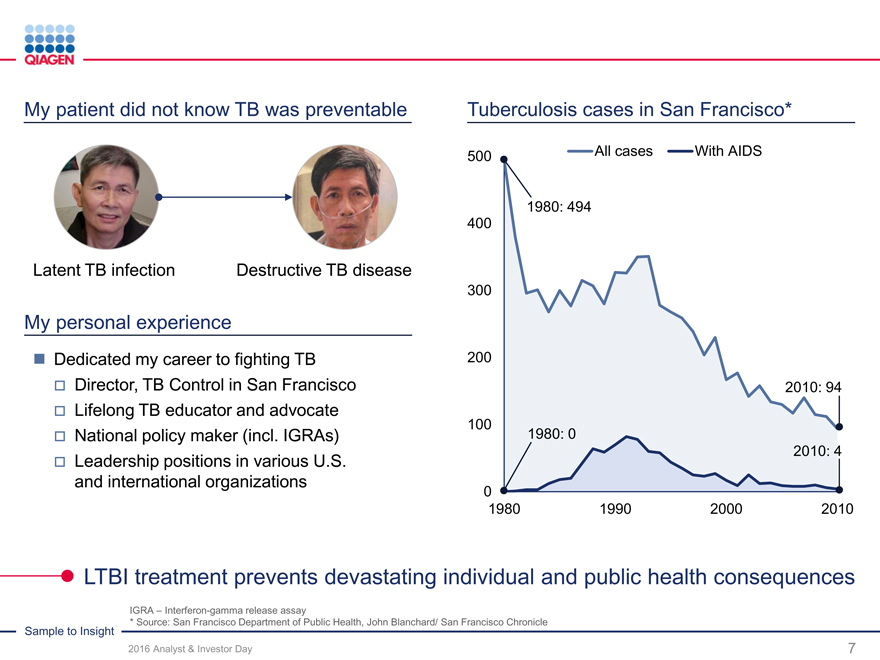
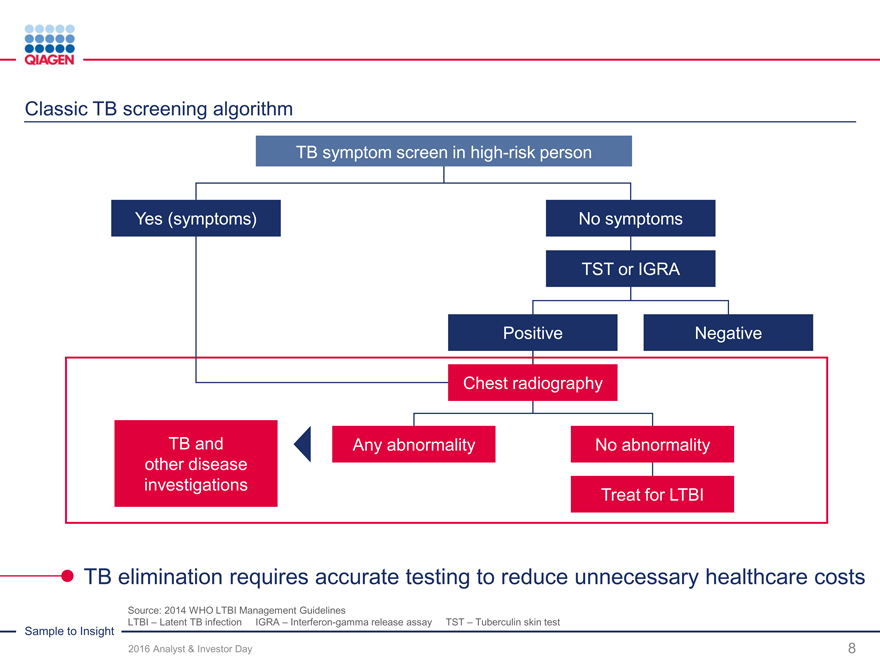
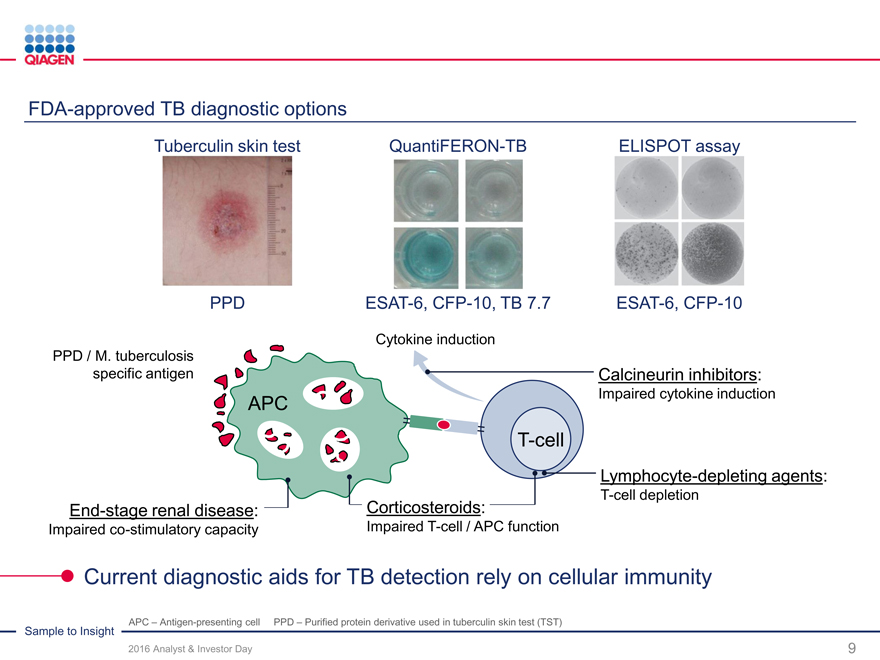
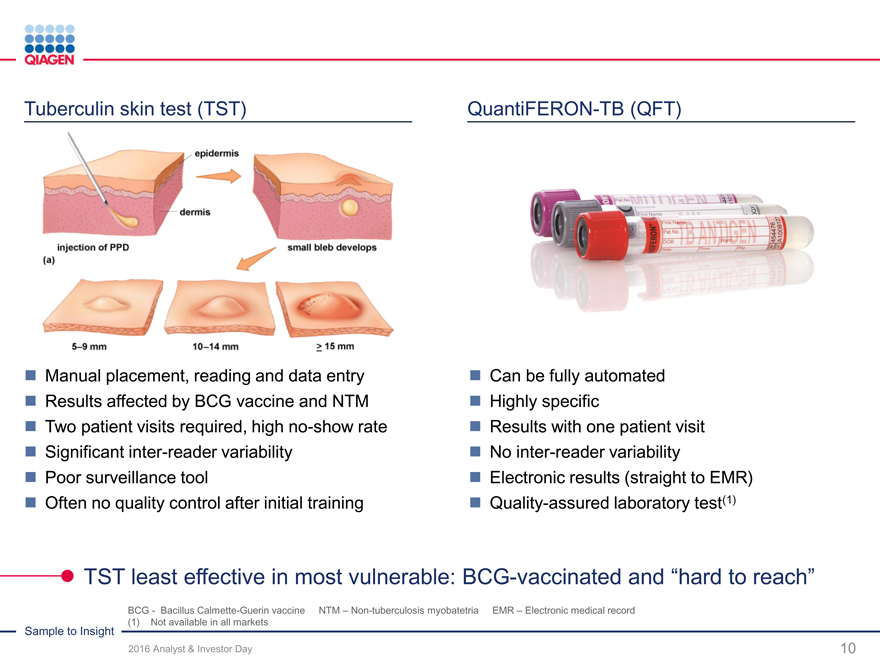
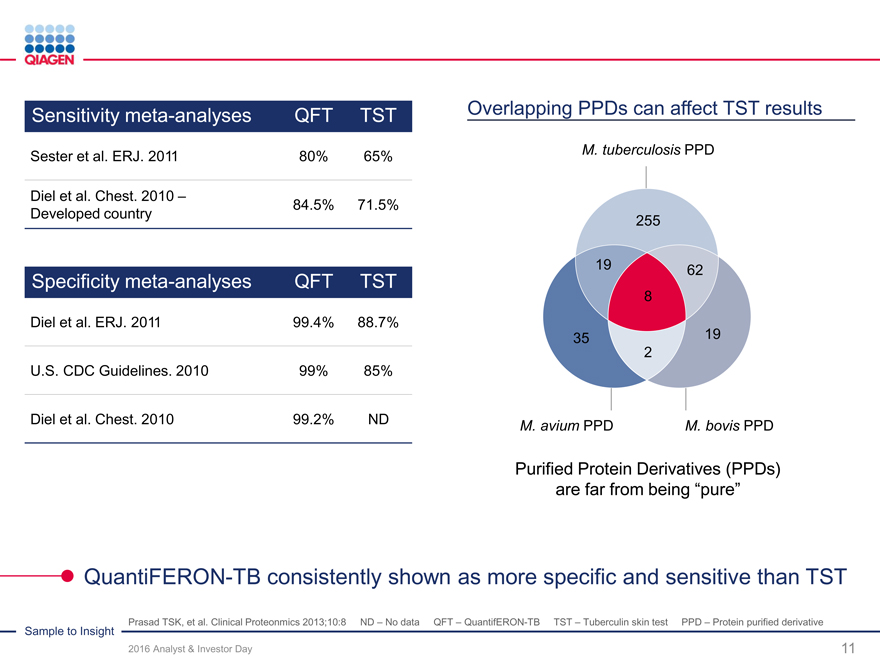
Immune Monitoring: Expand QuantiFERON benefits 2020 sales target: >$300 million from QuantiFERON-TB
Oncology: Accelerate lead in Personalized Healthcare Expanding offering from PCR to NGS with GeneReader
Infectious Diseases: Maximize portfolio strengths
QIAsymphony as flagship automation solution
Deliver innovations
Enhance our focused market leadership positions
Strategy: Strengthen our top 3 leadership position with differentiated portfolios
Sample to Insight
2016 Analyst & Investor Day
7
Table of Contents
|
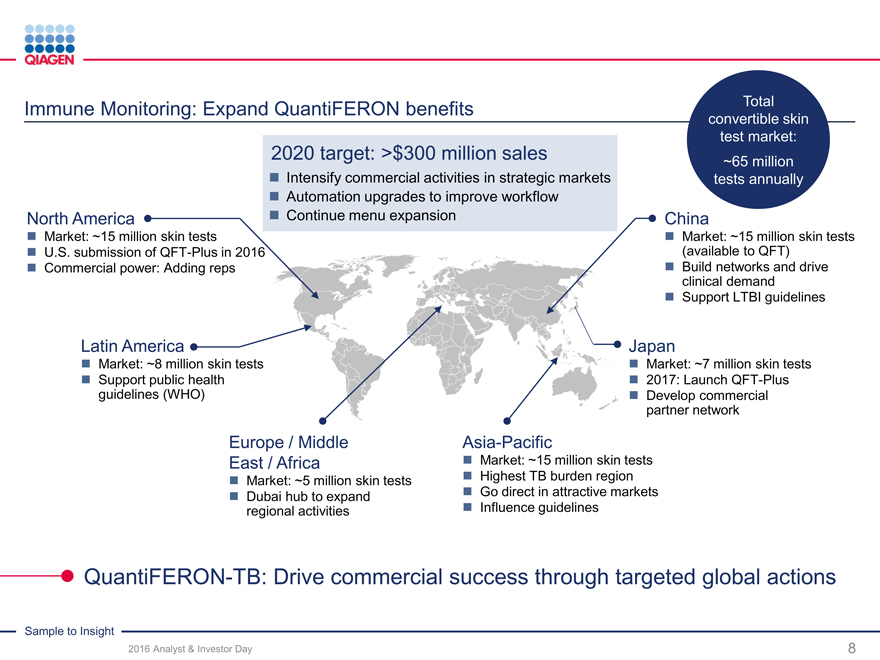
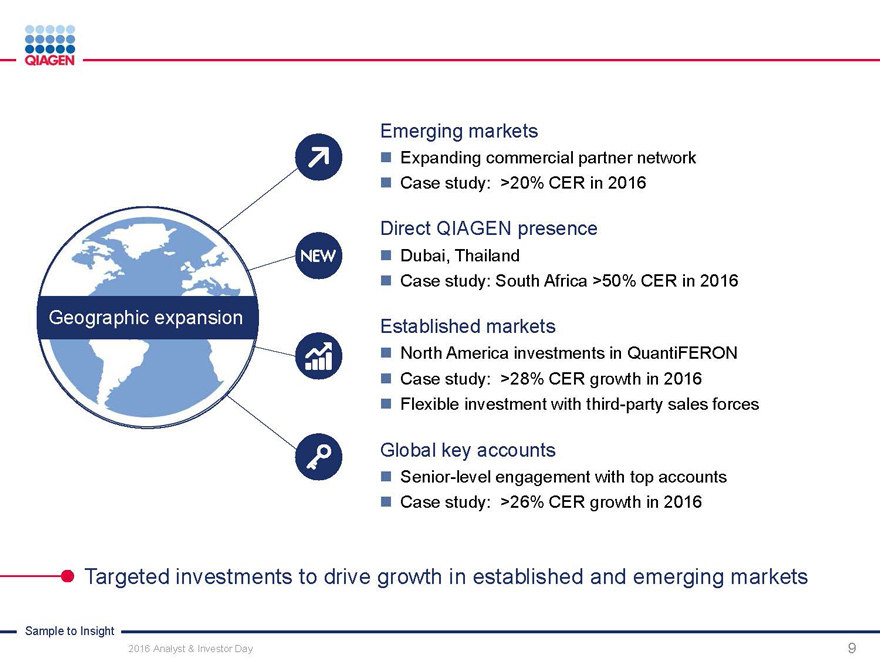
Immune Monitoring: Expand QuantiFERON benefits Total
convertible skin
test market:
2020 target: >$300 million sales ~65 million
Intensify commercial activities in strategic marketstests annually
Automation upgrades to improve workflow
North America Continue menu expansionChina
Market: ~15 million skin tests Market: ~15 million skin tests
U.S. submission of QFT-Plus in 2016 (available to QFT)
Commercial power: Adding reps Build networks and drive
clinical demand
Support LTBI guidelines
Latin America Japan
Market: ~8 million skin tests Market: ~7 million skin tests
Support public health 2017: Launch QFT-Plus
guidelines (WHO) Develop commercial
partner network
Europe / Middle Asia-Pacific
East / Africa Market: ~15 million skin tests
Market: ~5 million skin tests Highest TB burden region
Dubai hub to expand Go direct in attractive markets
regional activities Influence guidelines
QuantiFERON-TB: Drive commercial success through targeted global actions
Sample to Insight
2016 Analyst & Investor Day
8
Table of Contents
|
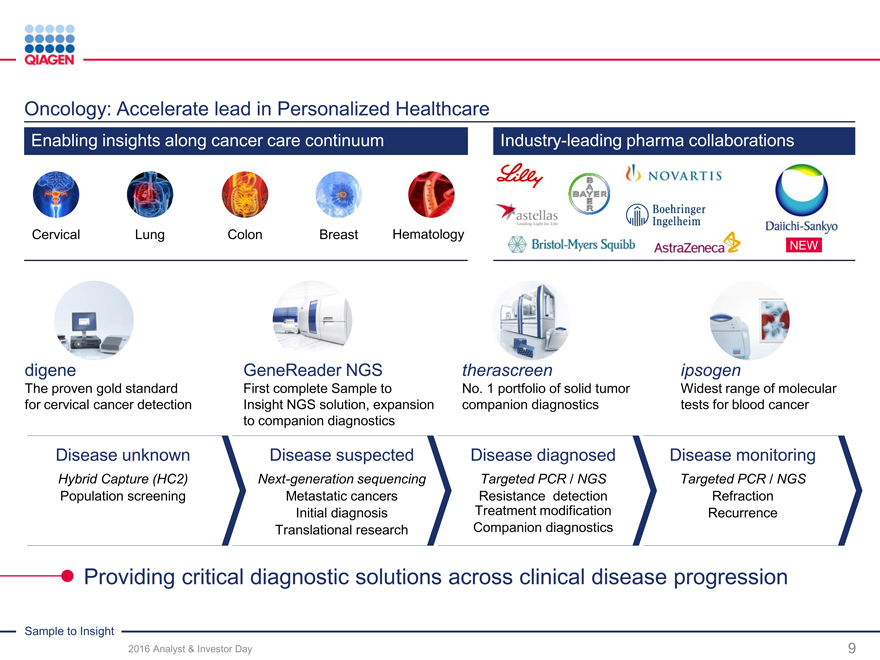
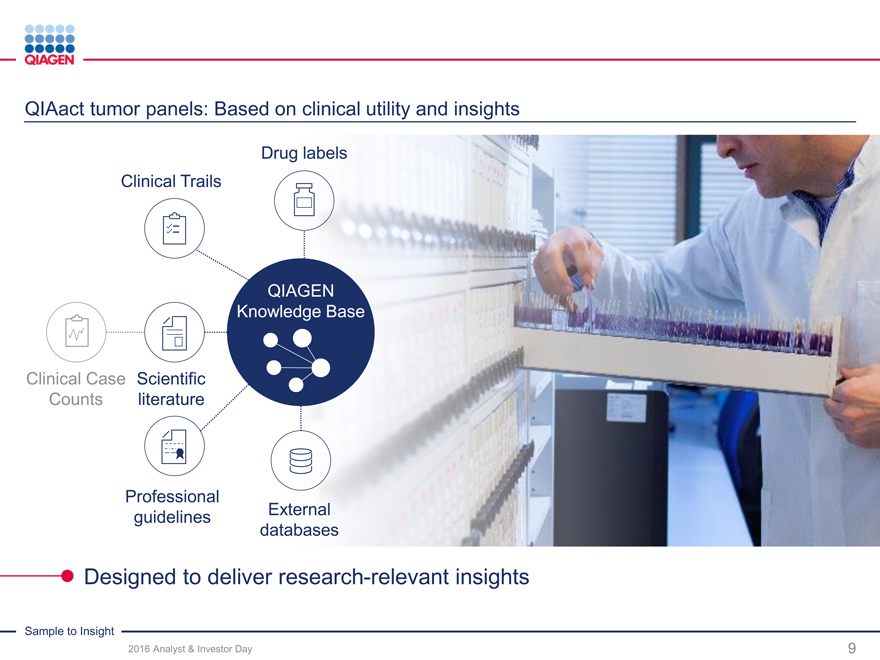
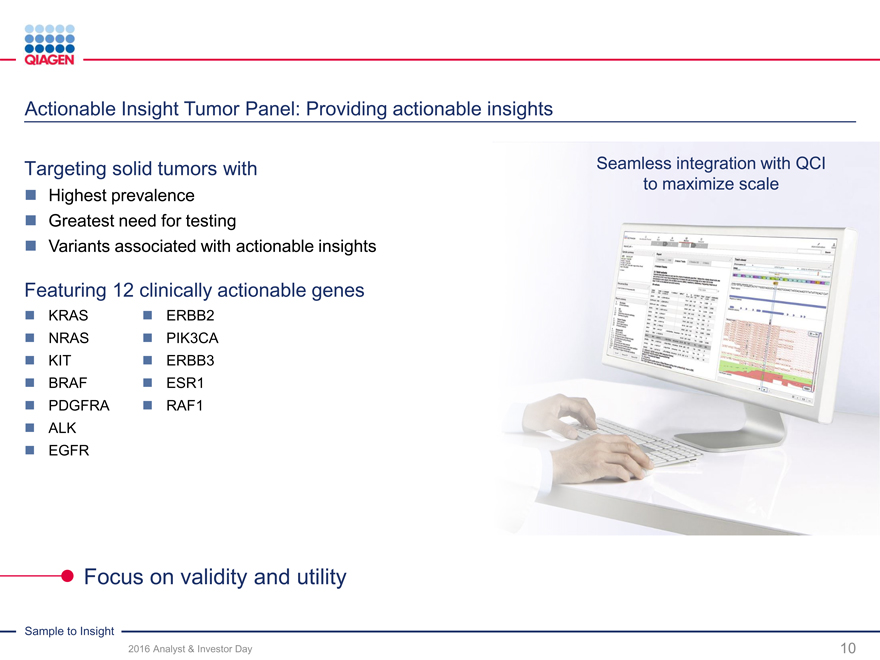
Oncology: Accelerate lead in Personalized Healthcare
Enabling insights along cancer care continuum Industry-leading pharma collaborations
Cervical Lung ColonBreastHematology
NEW
digene GeneReader NGS therascreenipsogen
The proven gold standard First complete Sample to No. 1 portfolio of solid tumorWidest range of molecular
for cervical cancer detection Insight NGS solution, expansion companion diagnosticstests for blood cancer
to companion diagnostics
Disease unknown Disease suspected Disease diagnosedDisease monitoring
Hybrid Capture (HC2) Next-generation sequencing Targeted PCR / NGSTargeted PCR / NGS
Population screening Metastatic cancers Resistance detectionRefraction
Initial diagnosis Treatment modificationRecurrence
Translational research Companion diagnostics
Providing critical diagnostic solutions across clinical disease progression
Sample to Insight
2016 Analyst & Investor Day 9
Table of Contents
|
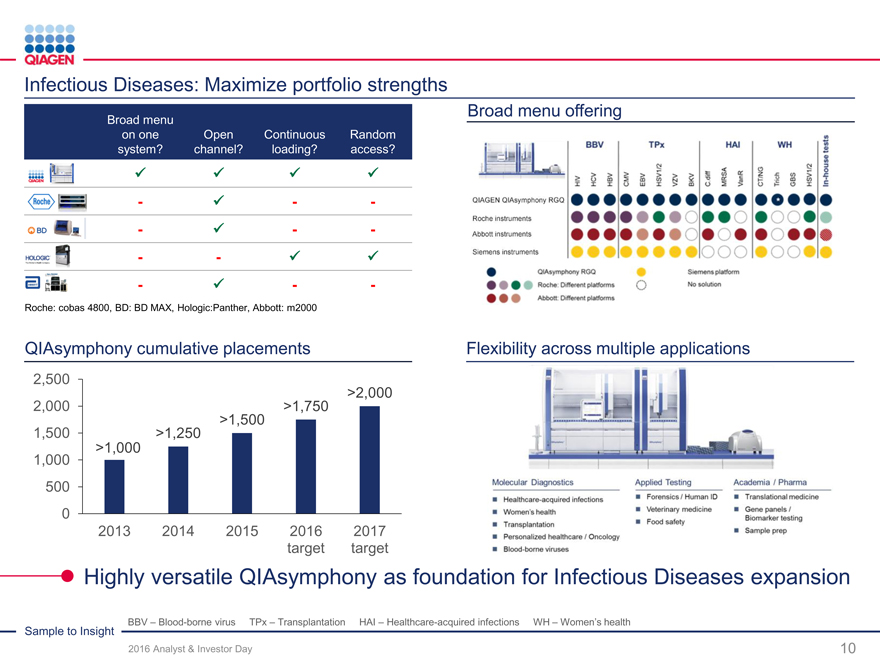
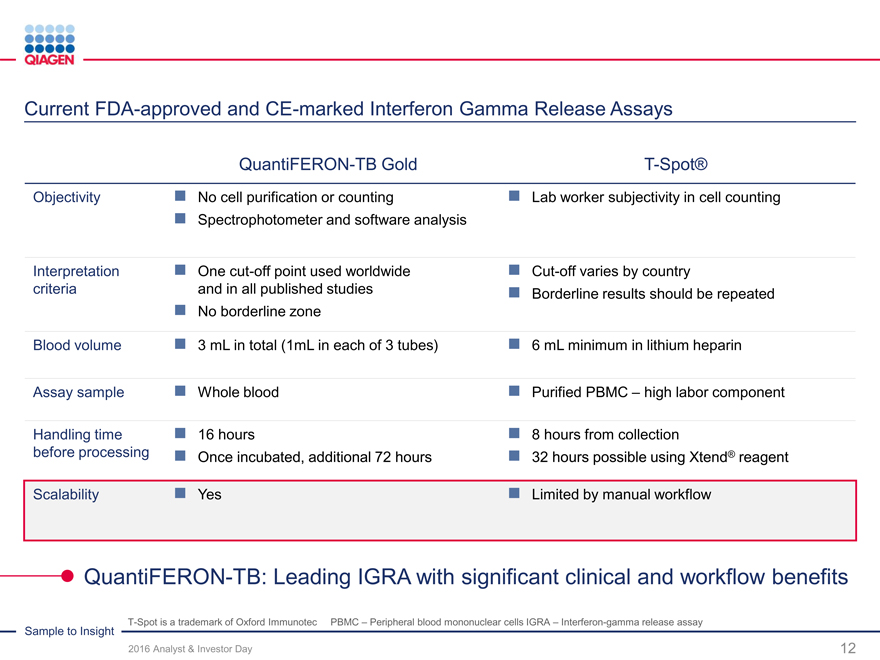
Infectious Diseases: Maximize portfolio strengths
Broad menu Broad menu offering
on one OpenContinuousRandom
system? channel? loading?access?
??
- ?--
- ?--
- -??
- ?--
Roche: cobas 4800, BD: BD MAX, Hologic:Panther, Abbott: m2000
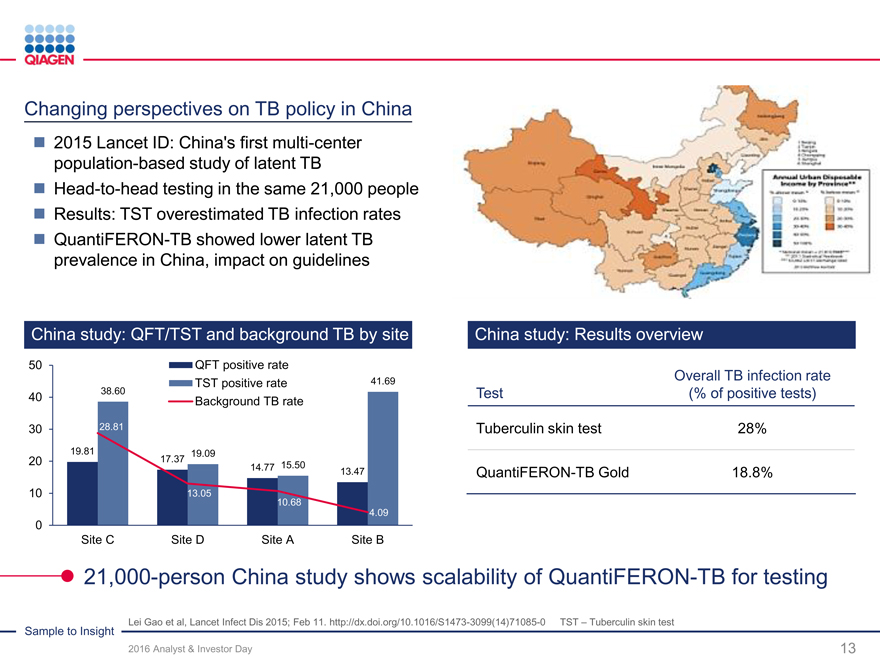
QIAsymphony cumulative placements Flexibility across multiple applications
2,500
>2,000
2,000 >1,750
>1,500
1,500 >1,250
>1,000
1,000
500
0
2013 2014 201520162017
targettarget
Highly versatile QIAsymphony as foundation for Infectious Diseasesexpansion
BBV – Blood-borne virus TPx – Transplantation HAI – Healthcare-acquired infections WH – Women’s health
Sample to Insight
2016 Analyst & Investor Day 10
Table of Contents
|
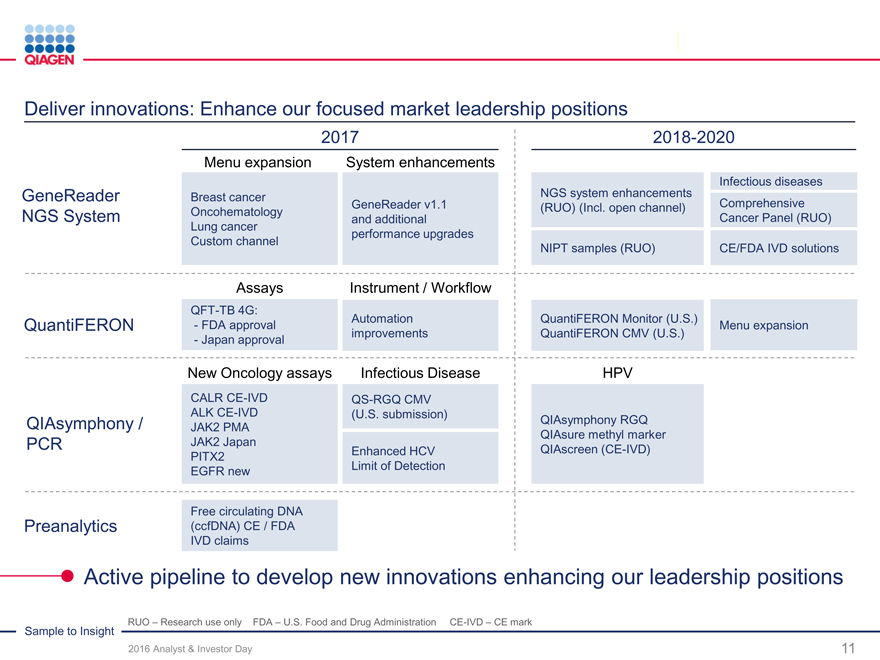
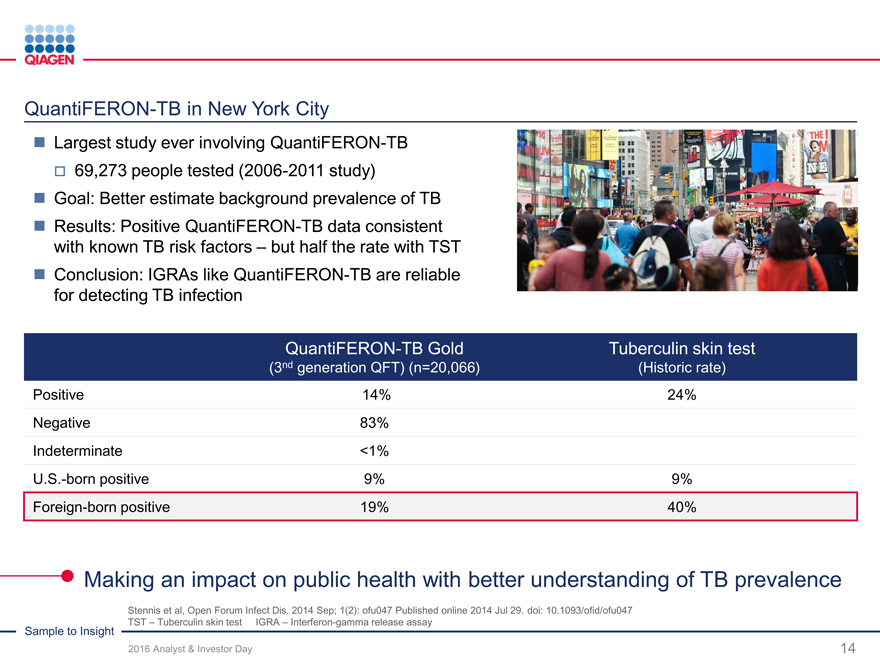
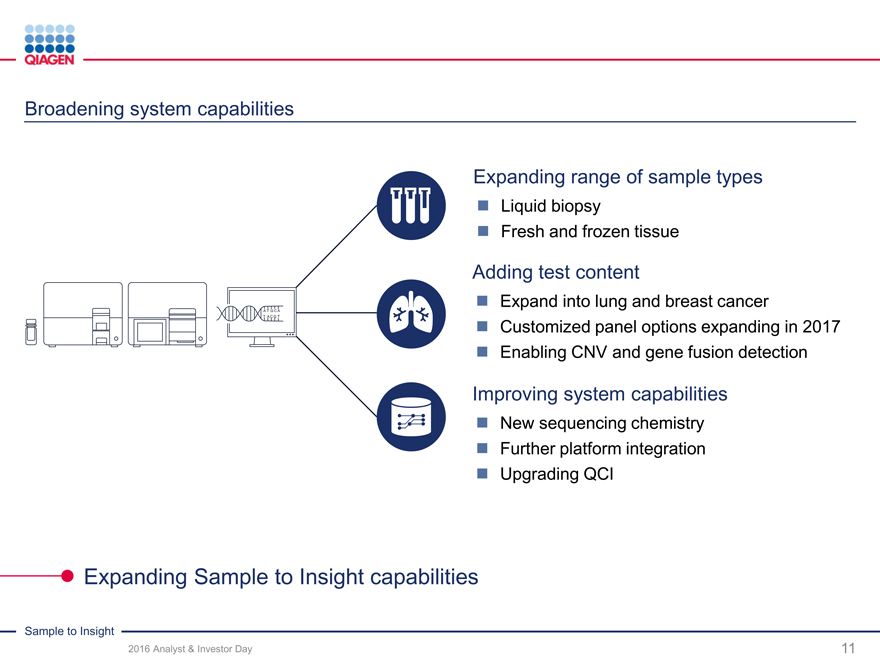
Deliver innovations: Enhance our focused market leadership positions
2017 2018-2020
Menu expansion System enhancements
Infectious diseases
GeneReader Breast cancer NGS system enhancements
GeneReader v1.1(RUO) (Incl. open channel)Comprehensive
NGS System Oncohematology and additionalCancer Panel (RUO)
Lung cancer performance upgrades
Custom channel NIPT samples (RUO)CE/FDA IVD solutions
Assays Instrument / Workflow
QFT-TB 4G:
QuantiFERON - FDA approval AutomationQuantiFERON Monitor (U.S.)Menu expansion
- Japan approval improvementsQuantiFERON CMV (U.S.)
New Oncology assays Infectious DiseaseHPV
CALR CE-IVD QS-RGQ CMV
ALK CE-IVD (U.S. submission)
QIAsymphony / JAK2 PMA QIAsymphony RGQ
QIAsure methyl marker
PCR JAK2 Japan Enhanced HCVQIAscreen (CE-IVD)
PITX2
EGFR new Limit of Detection
Free circulating DNA
Preanalytics (ccfDNA) CE / FDA
IVD claims
Active pipeline to develop new innovations enhancing our leadership positions
Sample to Insight RUO – Research use only FDA – U.S. Food and Drug Administration CE-IVD – CE mark
2016 Analyst & Investor Day 11
Table of Contents
|
Unique portfolio of solutions from LDT to IVD tests Focus on significant unmet needs with differentiation Offering complete, scalable solutions across continuum Delivering to labs a one-stop shop for molecular solutions
Sample to Insight
2016 Analyst & Investor Day 12
Table of Contents
Exhibit 99.5
|
Bioinformatics:
Partner of choice for insights from complex genomic data
Dr. Laura Furmanski, Ph.D.
Senior Vice President, Head of Bioinformatics Business Area November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Table of Contents
|
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section 21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Sample to Insight
2016 Analyst & Investor Day 3
Table of Contents
|
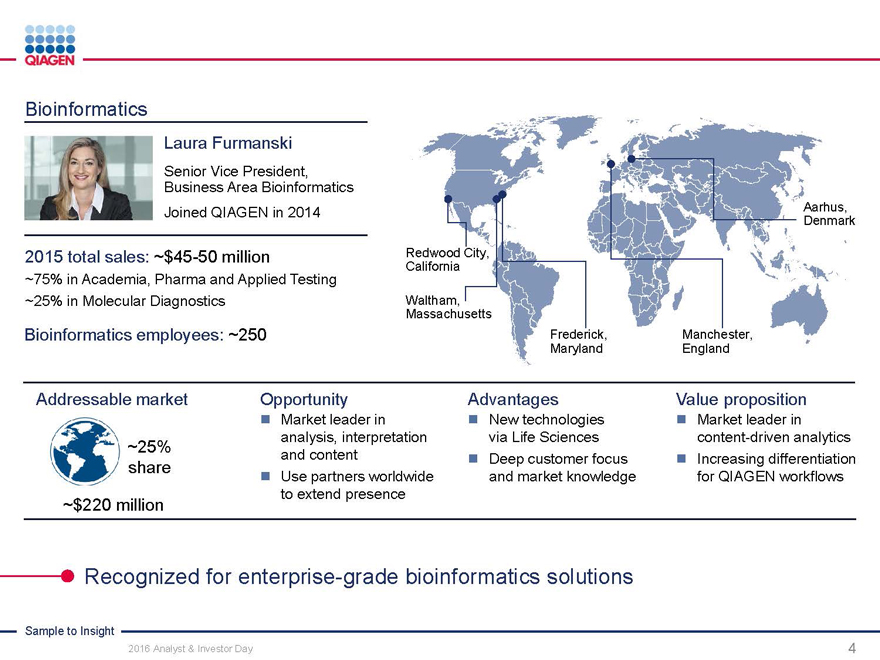
Bioinformatics
Laura Furmanski
Senior Vice President, Business Area Bioinformatics Joined QIAGEN in 2014
2015 total sales: ~$45-50 million Redwood City,
California
~75% in Academia, Pharma and Applied Testing
~25% in Molecular Diagnostics Waltham,
Massachusetts
Aarhus, Denmark
Bioinformatics employees: ~250 Frederick,Manchester,
MarylandEngland
Addressable market Opportunity AdvantagesValue proposition
Market leader in New technologies Market leader in
analysis, interpretationvia Life Sciencescontent-driven analytics
~25% and content Deep customer focus Increasing differentiation
share Use partners worldwideand market knowledgefor QIAGEN workflows
~$220 million to extend presence
Recognized for enterprise-grade bioinformatics solutions
Sample to Insight
2016 Analyst & Investor Day 4
Table of Contents
|
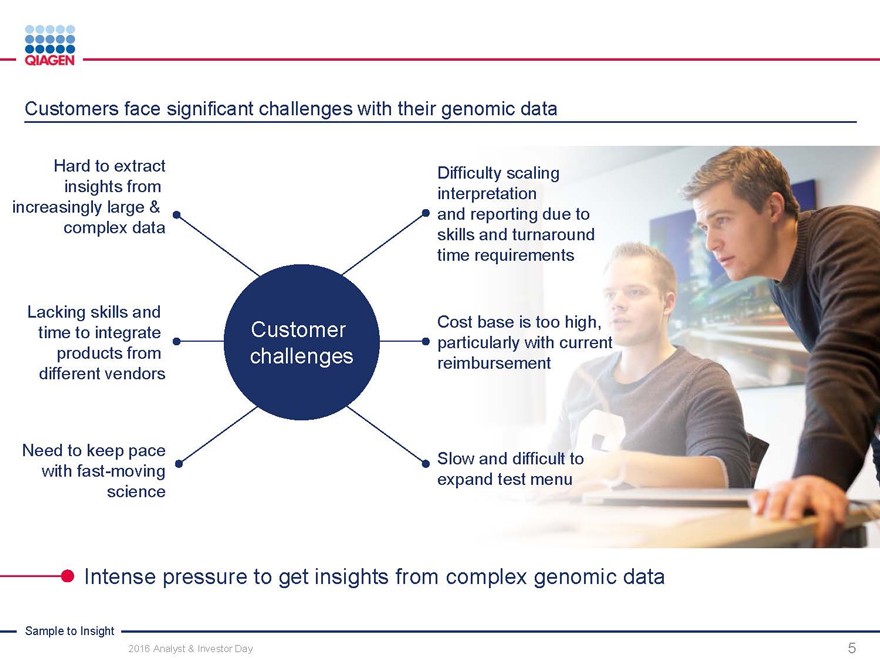
Customers face significant challenges with their genomic data
Hard to extract Difficulty scaling
insights from interpretation
increasingly large & and reporting due to
complex data skills and turnaround
time requirements
Lacking skills and
time to integrate Customer Cost base is too high,
particularly with current
products from challenges reimbursement
different vendors
Need to keep pace Slow and difficult to
with fast-moving expand test menu
science
Intense pressure to get insights from complex genomic data
Sample to Insight
2016 Analyst & Investor Day
5
Table of Contents
|
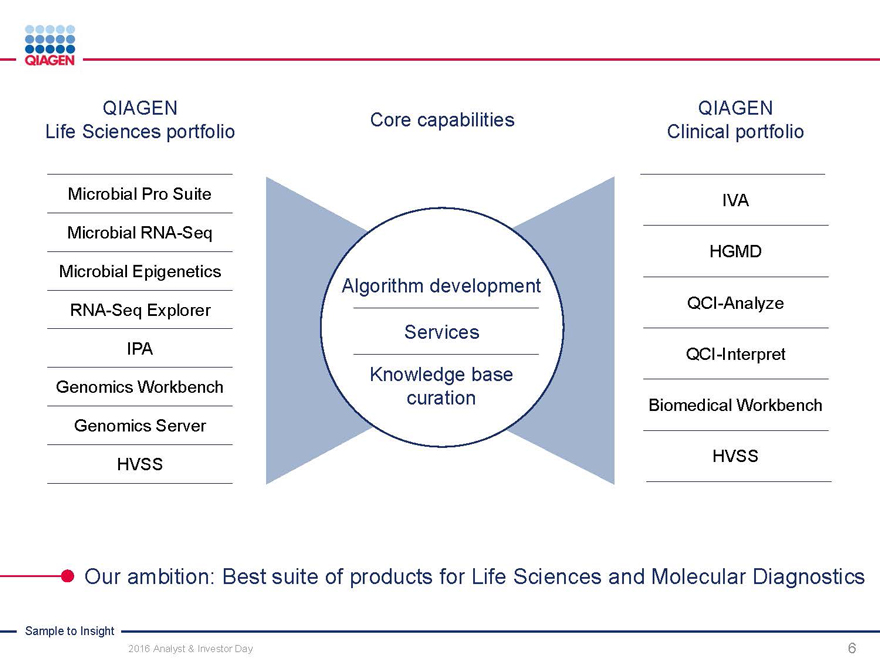
QIAGEN QIAGEN
Core capabilities
Life Sciences portfolio Clinical portfolio
Microbial Pro Suite IVA
Microbial RNA-Seq
HGMD
Microbial Epigenetics
Algorithm development
RNA-Seq Explorer QCI-Analyze
Services
IPA QCI-Interpret
Knowledge base
Genomics Workbench curation Biomedical Workbench
Genomics Server
HVSS HVSS
Our ambition: Best suite of products for Life Sciences and Molecular Diagnostics
Sample to Insight
2016 Analyst & Investor Day 6
Table of Contents
|
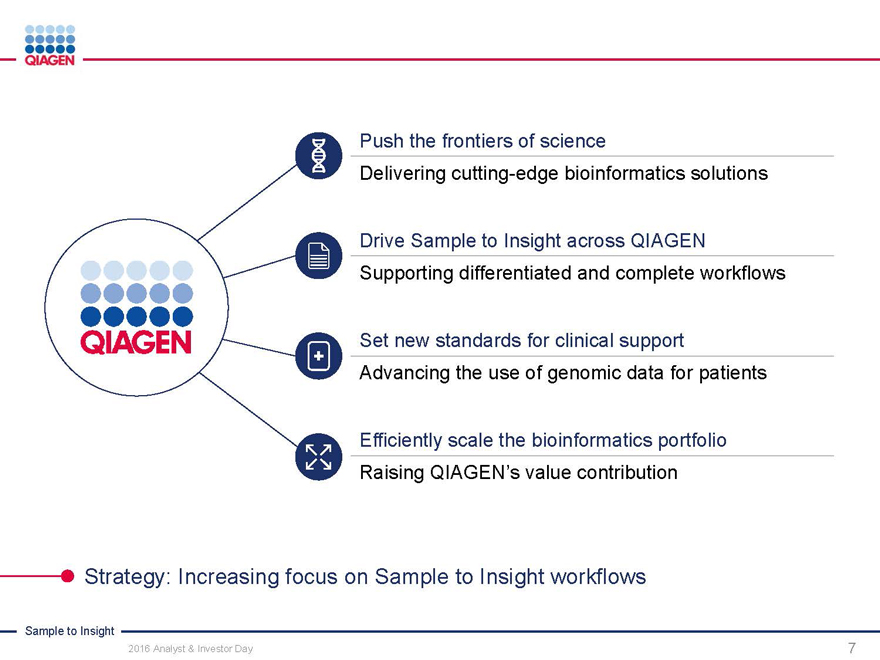
Push the frontiers of science
Delivering cutting-edge bioinformatics solutions
Drive Sample to Insight across QIAGEN Supporting differentiated and complete workflows
Set new standards for clinical support
Advancing the use of genomic data for patients
Efficiently scale the bioinformatics portfolio Raising QIAGEN’s value contribution
Strategy: Increasing focus on Sample to Insight workflows
Sample to Insight
2016 Analyst & Investor Day
7
Table of Contents
|
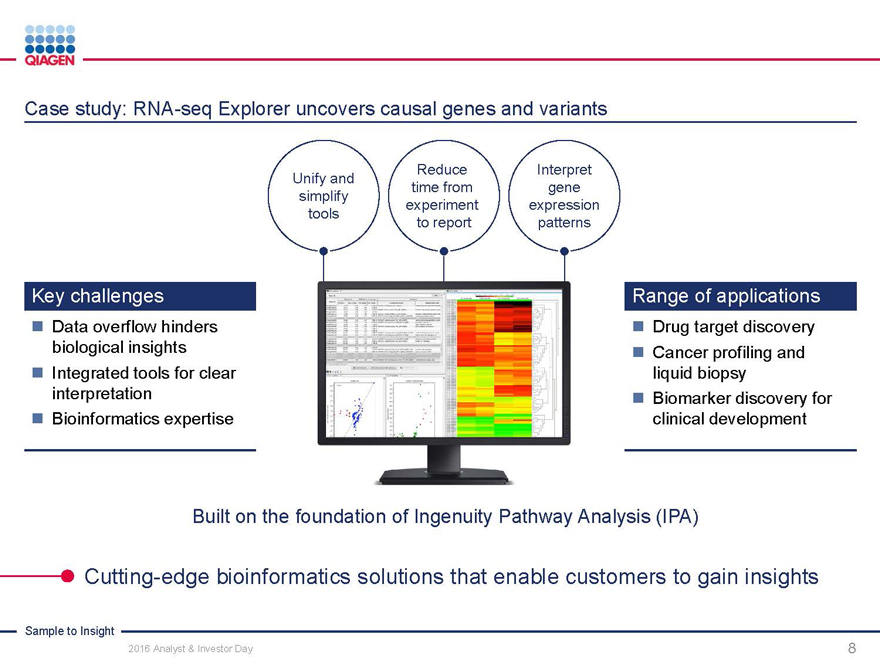
Case study: RNA-seq Explorer uncovers causal genes and variants
Unify and Reduce Interpret
simplify time from gene
experiment expression
tools to report patterns
Key challenges Range of applications
Data overflow hinders Drug target discovery
biological insights Cancer profiling and
Integrated tools for clear liquid biopsy
interpretation Biomarker discovery for
Bioinformatics expertise clinical development
Built on the foundation of Ingenuity Pathway Analysis (IPA)
Cutting-edge bioinformatics solutions that enable customers to gain insights
Sample to Insight
2016 Analyst & Investor Day
8
Table of Contents
|
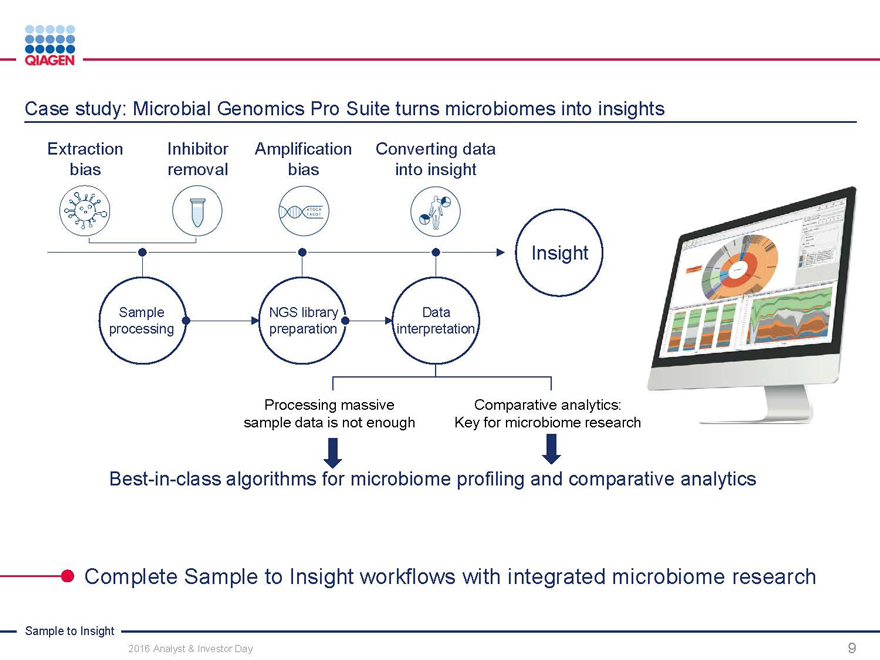
Case study: Microbial Genomics Pro Suite turns microbiomes into insights
Extraction Inhibitor AmplificationConverting data
bias removal biasinto insight
Insight
Sample NGS library Data processing preparation interpretation
Processing massive Comparative analytics: sample data is not enough Key for microbiome research
Best-in-class algorithms for microbiome profiling and comparative analytics
Complete Sample to Insight workflows with integrated microbiome research
Sample to Insight
2016 Analyst & Investor Day 9
Table of Contents
|
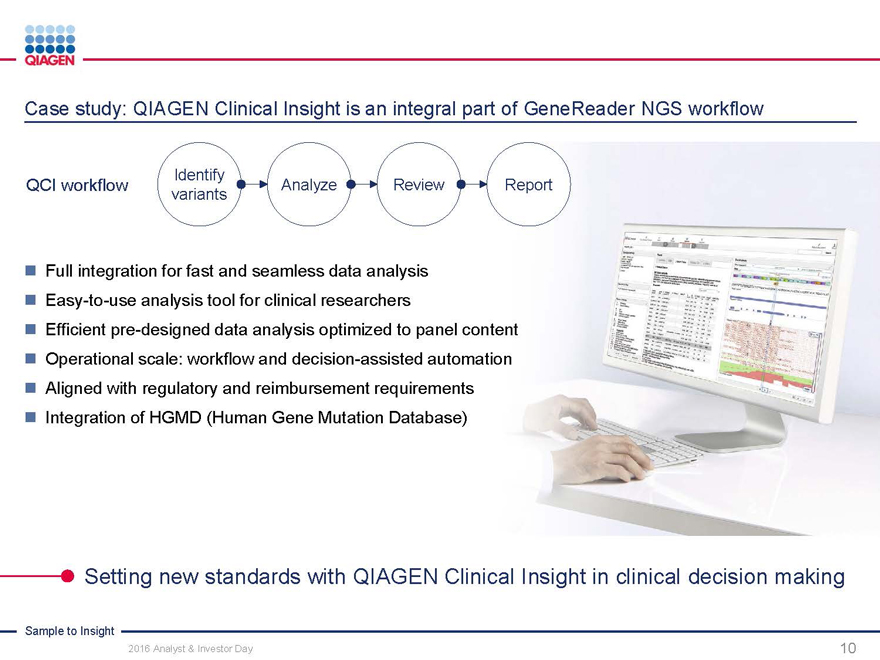
Case study: QIAGEN Clinical Insight is an integral part of GeneReader NGS workflow
Identify
QCI workflow AnalyzeReviewReport
variants
Full integration for fast and seamless data analysis
Easy-to-use analysis tool for clinical researchers
Efficient pre-designed data analysis optimized to panel content
Operational scale: workflow and decision-assisted automation
Aligned with regulatory and reimbursement requirements
Integration of HGMD (Human Gene Mutation Database)
Setting new standards with QIAGEN Clinical Insight in clinical decision making
Sample to Insight
2016 Analyst & Investor Day
10
Table of Contents
|
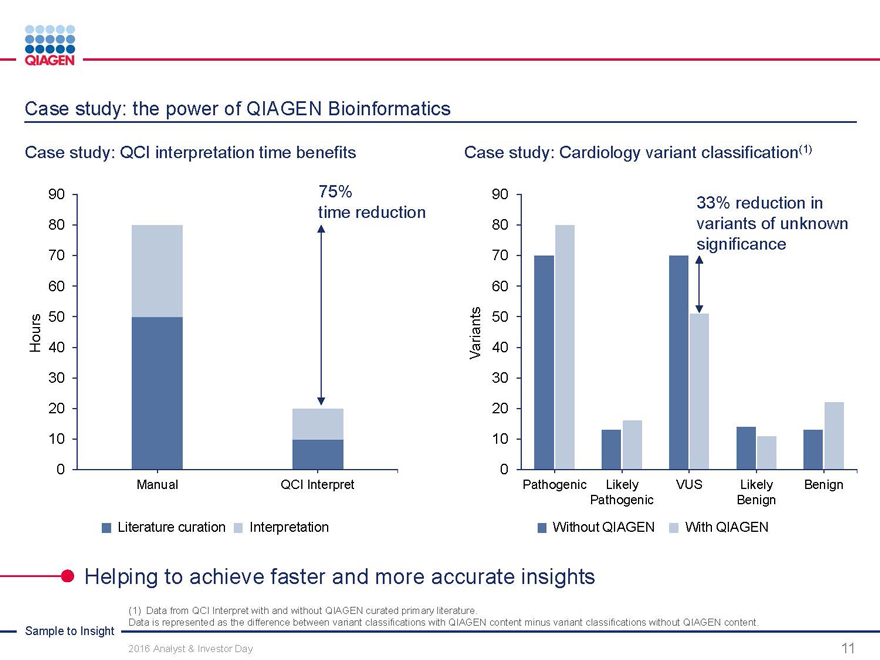
Case study: the power of QIAGEN Bioinformatics
Case study: QCI interpretation time benefits Case study: Cardiology variant classification(1)
90 75%90
time reduction33% reduction in
80 80variants of unknown
70 70significance
60 60
50 50
Hours 40 Variants40
30 30
20 20
10 10
0 0
Manual QCI InterpretPathogenic LikelyVUSLikelyBenign
PathogenicBenign
Literature curation InterpretationWithout QIAGENWith QIAGEN
Helping to achieve faster and more accurate insights
Sample to Insight
(1) Data from QCI Interpret with and without QIAGEN curated primary literature.
Data is represented as the difference between variant classifications with QIAGEN content minus variant classifications without QIAGEN content.
2016 Analyst & Investor Day
11
Table of Contents
|
“We truly are a Sample to Insight laboratory. As a result, we are bringing greater understanding to a wide range of important medical issues, particularly in cancer.”
Dr. Lars Joensen
Head of NGS Core Unit, Center for Genomic Medicine Rigshospitalet, Copenhagen
“Analysis is a bottleneck and it is not going to get easier in the future. The only solution is to analyze the data quicker and QIAGEN allows us to do this – faster and better.”
Indresh Singh
Senior Bioinformatics Engineer, JCVI
“Assembling allele frequency information in a comprehensive fashion over many different populations is a critical element of genome interpretation, and making it available as a public resource is the right way to do it.”
Dr. Eric Schadt
Director of the Icahn Institute for Genomics and Multiscale Biology at Mount Sinai
40,000 active users across the QIAGEN bioinformatics product offering
Sample to Insight
2016 Analyst & Investor Day
12
Table of Contents
|
Market leader in analysis, interpretation and content Portfolio with strong commercial traction Solving challenges of complex genomic data Focusing on delivering Sample to Insight solutions
Sample to Insight
2016 Analyst & Investor Day 13
Table of Contents
Exhibit 99.6
|
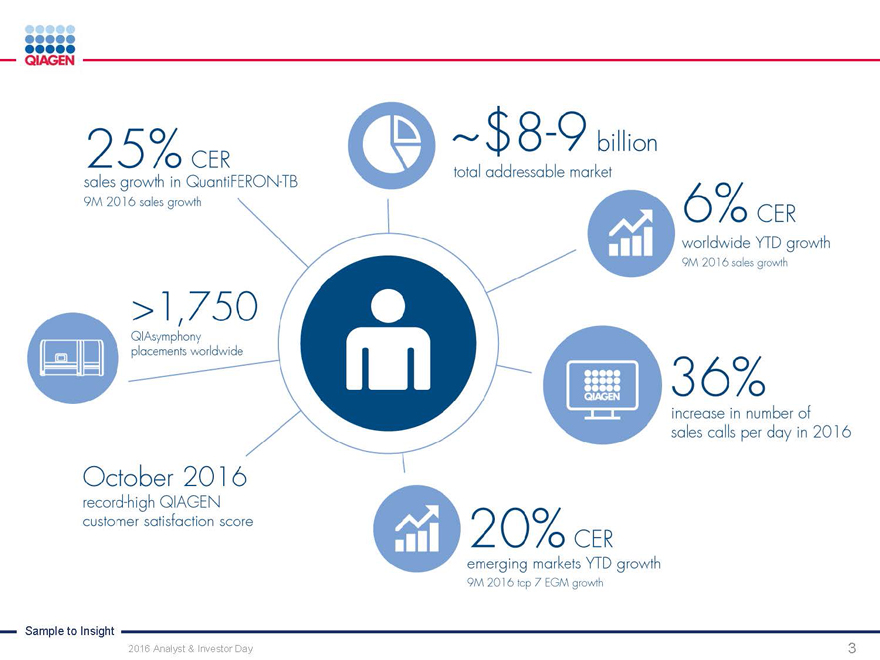
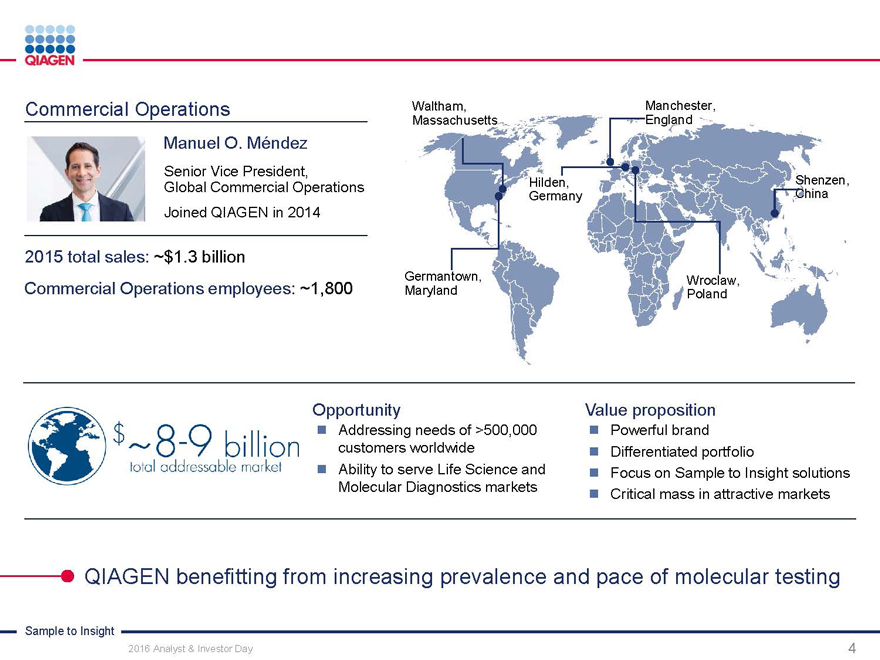
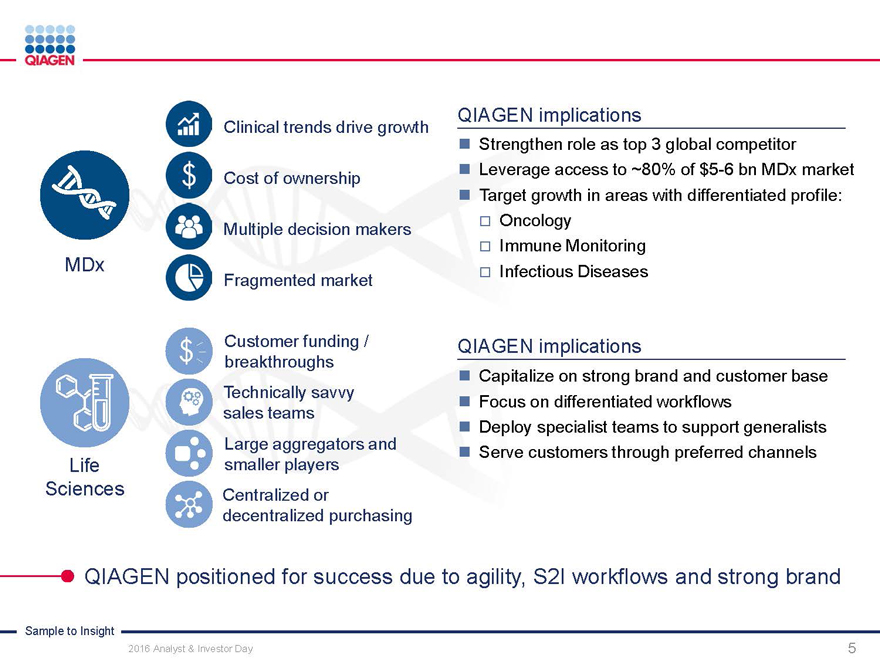
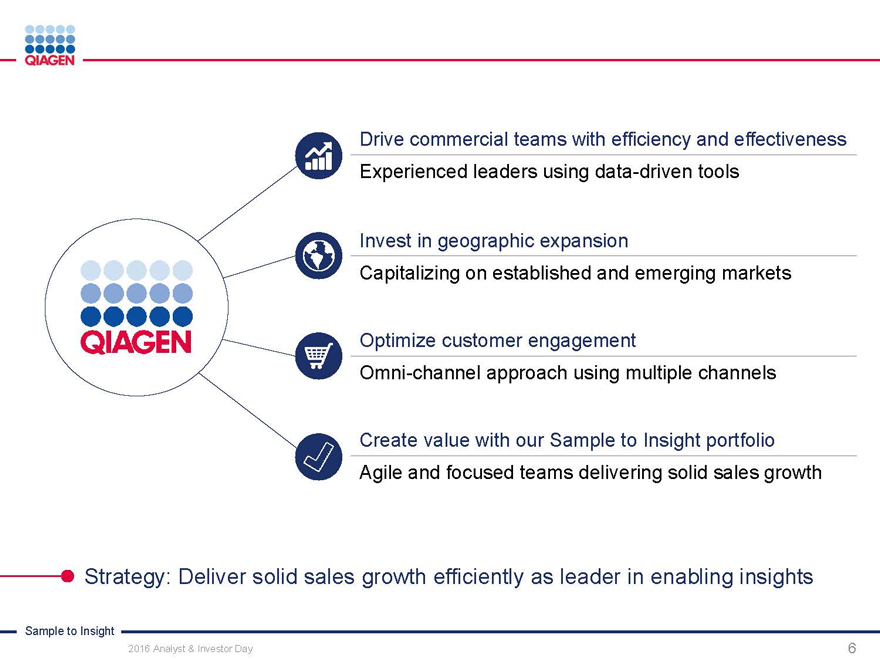
Commercial Operations:
Engaging customers with our Sample to Insight portfolio
Manuel O. Méndez
Senior Vice President, Head of Global Commercial Operations November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Disclaimer
Table of Contents
|
Safe are, or Harbor may be Statement: deemed toThis be forward presentation looking contains statements both within historical theand meaning forward of-Section looking statements 27A of the .UAll .S.statements Securities Act other ofthan 1933, statements as amended, of historical and Section fact 21E assumptions of the U.prove S. Securities inaccurate Exchange or unknown Act of risks 1934,or asuncertainties amended. These materialize, statements actual are results basedcould on current vary materially expectations from of future our own events expectations . If underlying and competition; projections. Some risks associated of the factors withthat managing could cause growthactual and international results to differ operations include, (including but are the not effects limited,of tocurrency the following: fluctuations, general regulatory industry conditions processes and and products dependence to customers on logistics), in academia, variability of pharma, operating applied results testing and allocations and molecular between diagnostics; customer changing classes,relationships and the commercial with customers, development suppliers of markets and strategic for our partners; economiccompetition; conditions, the rapid level or unexpected and timing of changes customers’ in technologies; funding, budgets fluctuations and other in demand factors);for our QIAGEN’s ability to products obtain regulatory (including approval factors such of ouras products; general technological producing such advances products; of our the competitors ability of QIAGEN and related to identify legal disputes; and develop difficulties new products in successfully and toadapting differentiate QIAGEN’s and protect products ourto products integrated from solutions competitor and to products; “Risk Factors” market acceptance section of reports of QIAGEN’s that QIAGEN new products has filed and with, theor integration furnishedof to, acquired the U.Stechnologies . Securities and andExchange businesses Commission . For further(SEC) information, . We undertake please refer no extent obligation, required and do by not law.intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the accepted Regulation accounting G: QIAGEN principles), reports adjusted to provide results, additional as well insight as results on performance on a constant . In this exchange presentation, rate (CER) adjusted basis,results and other include non-adjusted U.S. GAAP operating figuresexpenses, (generally adjusted addition to EBITDA, reported adjusted results diluted prepared EPS in and accordance free cash with flow GAAP, . Adjusted but should results not are be non considered -GAAP financial as a substitute measures . QIAGEN QIAGEN believes believes should certainbe items considered should be in results excluded with from itsadjusted competitors results and when its own theyprior are outside periodsof . Please its ongoing see core the Appendix operations, provided vary significantly in this presentation from period“Reconciliation to period, or affect of Non the-GAAP comparability to GAAP of QIAGEN Measures” does for not reconciliations reconcile forward of historical -lookingnon non -GAAP -GAAPmeasures financial measures to comparable to the GAAP corresponding measures GAAP andmeasures the definitions due to ofthe terms highused variability in the and presentation difficulty in. making GAAP financial accuratemeasures forecasts to and the projections corresponding that are GAAP impacted measures by future are not decisions available and without actions unreasonable . Accordingly,effort reconciliations . However,of the these actual forward amounts -looking of these non-excluded items will have a significant impact on QIAGEN’s GAAP results.
QIAGEN GeneReader GeneReader® NGS System: NGS The System QIAGEN with new GeneReader® sequencing chemistry NGS System will be is made currently available available in the forUnited marketing States and starting salesinoutside December the 2016 United . The States QIAGEN . The GeneReader® QIAGEN Clinical NGS Insight® System is is anintended evidence for -based Research decision Usesupport Only. This software product intended is not intended as an aid forinthe thediagnosis, interpretation prevention of variants or treatment observed ofin a genomic disease. available sequencing databases data. The and software annotations, evaluates drug genomic labels and variants clinical in -the trials context . Based ofon published this evaluation, biomedical theliterature, software professional proposes a association classification guidelines, and bibliographic publicly references substitute for to aid professional in the interpretation healthcare of advice observed . Each variants laboratory . Theissoftware responsible is not for intended ensuring ascompliance a primary diagnostic with applicable tool by international, physicians or national to be used and local as a clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Sample to Insight
2016 Analyst & Investor Day 3
Table of Contents
|
Commercial Operations Waltham, Manchester,
Massachusetts England
Manuel O. Méndez
Senior Vice President,
Global Commercial Operations Hilden,Shenzen,
GermanyChina
Joined QIAGEN in 2014
2015 total sales: ~$1.3 billion
Germantown, Wroclaw,
Commercial Operations employees: ~1,800 Maryland Poland
Opportunity Value proposition
Addressing needs of >500,000 Powerful brand
customers worldwide Differentiated portfolio
Ability to serve Life Science and Focus on Sample to Insight solutions
Molecular Diagnostics markets Critical mass in attractive markets
QIAGEN benefitting from increasing prevalence and pace of molecular testing
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
Clinical trends drive growth
Cost of ownership
Multiple decision makers
MDx
Fragmented market
Customer funding / breakthroughs Technically savvy sales teams Large aggregators and Life smaller players Sciences Centralized or decentralized purchasing
QIAGEN implications
Strengthen role as top 3 global competitor
Leverage access to ~80% of $5-6 bn MDx market
Target growth in areas with differentiated profile:
Oncology
Immune Monitoring
Infectious Diseases
QIAGEN implications
Capitalize on strong brand and customer base
Focus on differentiated workflows
Deploy specialist teams to support generalists
Serve customers through preferred channels
QIAGEN positioned for success due to agility, S2I workflows and strong brand
Sample to Insight
2016 Analyst & Investor Day
5
Table of Contents
|
Drive commercial teams with efficiency and effectiveness Experienced leaders using data-driven tools
Invest in geographic expansion
Capitalizing on established and emerging markets
Optimize customer engagement
Omni-channel approach using multiple channels
Create value with our Sample to Insight portfolio Agile and focused teams delivering solid sales growth
Strategy: Deliver solid sales growth efficiently as leader in enabling insights
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
NEW TO QIAGEN
Manuel O. Méndez Mohammad El Khoury
President (Interim) President
Commercial Operations Commercial Operations
North America Europe / Middle East / Africa
Formerly bioMerieux, Abbott, Thermo Formerly GE, bioMerieux
NEW TO QIAGEN NEW ROLE
Enrique Razzeto Ian Martin
Vice President President
Commercial Operations Commercial Operations
Latin America Asia-Pacific
Formerly Abbott, Dako Formerly Abbott
NEW TO QIAGEN NEW ROLE NEW ROLE
Scott MacPherson Wolfgang Leibinger, Ph.D. Hans Peter Fatscher, Ph.D.
Vice President Vice President Vice President
Global Key & Corporate Accounts Global Marketing ComOps Custom by QIAGEN
Formerly Abbott, Bayer Formerly Millipore Formerly Boehringer Ingelheim
NEW TO QIAGEN NEW TO QIAGEN
Kesava Nagar-Anthal, Ph.D. Vera M. Bökenbrink
Vice President Senior Director ComOps Finance
Commercial Excellence and Business Analytics
& Effectiveness Formerly Kirchhoff Automotive, FEV Motorentechnik
Formerly bioMerieux, BD, Thermo
Manuel O. Méndez In last 12-18 months
Senior Vice President NEW TO QIAGEN
Global Commercial Operations
Formerly bioMerieux, Abbott, Thermo NEW ROLE
Significant executive leadership changes implemented while growing sales
Sample to Insight
2016 Analyst & Investor Day 7
Table of Contents
|
Results and accountability driven-culture
New “go-to-market” strategy QIAGEN sales trends (CER % change)
9%
7%5% 5%5%6%
Leading and lagging indicators 3% 3%4% 4%4% 2%3% 2%
2%
Pipeline management
Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3
Demand generation2013 2013 2013 2013 2014 2014 20142014 2015 2015 20152015 2016 2016 2016
QIAGEN sales per S&M FTE ratio (In $ thousands)
Promote and pay for performance ~690~710~640~630
Investment in training and tools
CRM systems (Salesforce.com)
2013201420152016 Target
QIAGEN S&M OPEX ratio (% of sales)
Focus on productivity metrics
Sales by S&M FTE ratio28.4%28.0%28.2%27.9%
S&M OPEX to sales ratio
Number of calls per day (36% increase)
2013201420152016 Target
Fully focused on sales growth to drive efficiency and effectiveness
Sample to Insight
2016 Analyst & Investor Day
8
Table of Contents
|
Geographic expansion
Emerging markets
Expanding commercial partner network
Case study: >20% CER in 2016
Direct QIAGEN presence
Dubai, Thailand
Case study: South Africa >50% CER in 2016
Established markets
North America investments in QuantiFERON
Case study: >28% CER growth in 2016
Flexible investment with third-party sales forces
Global key accounts
Senior-level engagement with top accounts
Case study: >26% CER growth in 2016
Targeted investments to drive growth in established and emerging markets
Sample to Insight
2016 Analyst & Investor Day
9
Table of Contents
|
Academia Pharma Applied TestingMolecular Diagnostics
Scientific breakthroughs Better and safer drugs Secure and protectBetter healthcare
Generalists and Specialists
Direct mail Mobile content
Marketing
automation Websites
Inside Sales eCommerce
Customer Care
Field Service Support
Case studies Sales enablement tools
Commercial Partners
Omni-channel customer engagement creates value with digital accessibility
Sample to Insight
2016 Analyst & Investor Day
10
Table of Contents
|
Academia Pharma Applied TestingMolecular Diagnostics
Scientific breakthroughs Better and safer drugs Secure and protectBetter healthcare
Case study: New York
Memorial Sloan Kettering Pfizer Pharmaceuticals
New York University (NYU) NYC Chief Medical Examiner
Leverage Sample to Insight portfolio for customer types and preferred channels
Sample to Insight
2016 Analyst & Investor Day 11
Table of Contents
|
Case study: Leveraging benefits of NGS Sample to Insight workflows
Platform
agnostic
Nucleic acid TargetLibraryData
collection & enrichmentpreparationanalysisInterpretation
extraction
GeneReader
NGS
System
Automation
QIAcube QIAgilityQIAcubeGeneReadGene-QCI-QCI-
QIAcubeReaderAnalyzeInterpret
QIAamp QIAseqQIAseq FXQIAseqMicrobial
Solutions Circulating NA Targeted RNADNA LibraryQIAactGenomics Pro Suite
Applications Liquid biopsy Single-cell researchMicrobiome
Sample to Insight workflows creating value for many customers
Sample to Insight
2016 Analyst & Investor Day 12
Table of Contents
|
Experienced commercial team using data-driven tools
Targeted geographic investments to accelerate growth
Optimizing customer engagement in multiple channels
Agile and focused teams with improved sales efficiency
Sample to Insight
2016 Analyst & Investor Day
13
Table of Contents
Exhibit 99.7
|
Multiple drivers to improve returns and create greater value
Roland Sackers Chief Financial Officer November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Table of Contents
|
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements.
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Sample to Insight
2016 Analyst & Investor Day 3
Table of Contents
|
2013-2016: Net sales growth trends
(At constant exchange rates to comparative year)
10%
8%
6%
4%
2%
0%
2013
2014
2015
9M
2016
2016
guidance
Total CER sales growth excluding U.S. HPV sales Total CER sales growth
2013-2016: Key challenges
U.S. HPV decline worse than expected – impact on sales and profitability
Restricted Life Sciences funding
Significant FX headwinds
2013-2016: Major actions
Portfolio transformation and investments
GeneReader NGS System launch
Built up infrastructure and global operating platform – future scalability and leverage
Regional expansion and organizational changes
Efficiency initiatives to support growth
2016: QIAGEN at an inflection point for new growth and value creation trajectory
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
2020: Net sales growth target
(At constant exchange rates to comparative year)
~+7-9% CER
CAGR
2020: Adjusted diluted EPS target
(In $ per share, at CER to comparative year)
>12% CER
CAGR
+6-7% CER ~$1.08-1.09
$ 1,281 m (~1.10-1.11 CER)
+3% CER $1.05
~$0.87-0.88 CER
with restructuring
2015 2016 2016-2020201520162016-2020
guidance targetguidancetarget
2020 targets: Strong focus on sales growth and operational execution
Sample to Insight
2016 Analyst & Investor Day
5
Table of Contents
|
Invest in growth opportunities
Allocate more resources to most attractive areas
Generate operating leverage
Optimize cost base while sustaining sales growth
Step up operating cash flow
Achieve ~$600 million target for 2020
Ensure disciplined capital deployment Invest in QIAGEN while increasing returns
Strategy: Multiple drivers to improve returns and create greater value
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
Growth drivers
EGM expansion
Digitalization
QuantiFERON-TB
GeneReader NGS System and universal NGS solutions
QIAsymphony
Personalized Healthcare
Differentiated Technologies
Middle East (Dubai and Egypt)
Africa (South Africa)
Asia (Thailand)
Exploit new business models
Digitalization of operating model
Big data and analytics
Social media engagement
Cyber security
Case study: Resource allocation Case study: South Korea “QIAGEN works Digital” roadmap
Expand QuantiFERON-TB sales >20% CER growth (2013-16 CAGR)New Digital Accelerator team
activities in key markets (U.S., EU) with ~50 employees
Investing in top portfolio areas, most attractive regions and digital initiatives
Sample to Insight
2016 Analyst & Investor Day
7
Table of Contents
|
Key efficiency initiatives Adjusted operating expenses(1)
(As % of net sales)
1
Sales channel Increase sales per FTE ratio 47%48%47%49%~46-47% CER~44-45%
optimization and sales over OPEX ratio CER
2
Site Focus on key sites and
consolidation reduce legal entities
3
Business Service Leverage shared service
Centers hubs in Poland and China
4
Portfolio Divest smaller non-core
management portfolio areas
2013201420159M 201620162017
guidancetarget
Stronger “continuous improvement” mindset to deliver operationalleverage
Sample to Insight (1) 2016 and 2017 figures are estimates before restructuring charges and at constant exchange rates (CER) for comparative year
2016 Analyst & Investor Day 8
Table of Contents
|
Manufacturing productivity gains Development cost structure
Product cost index (As % of 2013 costs) MDX development employees (As % of total)
Hilden GermantownEmployees at other sites
~40%
100 90 ~80-8510095~85-90~75%~60%Employees in
Centers of Excellence
~40%~60%
~25%
2013 2016 2020 target201320162020 target201320162020
target
Insourcing initiatives and network consolidation
QIAzen
Supply chain management optimization
Sales & Marketing efficiency
Net sales per S&M FTE Net sales share in digital channels
(In $ thousands) (In % of total sales)
~$690 ~$720 CER~50%
~$630 ~30%
~25%
2013 2016 2020 target201320162020 target
Invest in Centers of Excellence for product development:
>~200 employees in Manchester targeted for 2017
Building new integrated software development group
Improve simplicity and agility, realize economies of scale
Business Service Center functions
Leverage investments in teams and digital infrastructure
Efficiently manage cost per touchpoint across channel mix
Improve Marketing efficiency and effectiveness with alignment and new Centers of Excellence
Sample to Insight
2016 Analyst & Investor Day
2013 20162020 target
BSC employees: ~50 ~200> ~400
Major functions and shared service potential:
Finance
Procurement
Human Resources
Customer Care
SCM/ Logistics
9
Table of Contents
|
Cash flow considerations 2020: Operating cash flow target(1)
(In $ millions)
1 ~$600 m
Working capital
management
2 ~$350 m
Benefits of $318 m
$288 m
efficiency gains $259 m
3
Maintain DSOs with
geographic expansion
4
Disciplined capex
investments 2013 2014201520162020
guidancetarget
Setting a 2020 target to reach ~$600 million of operating cashflow
Sample to Insight (1) 2016 and 2020 targets before restructuring charges and based on current exchange rates.
2016 Analyst & Investor Day 10
Table of Contents
|
2012-2017: QIAGEN capital returns
(In $ millions)
~$514 m
$387 m~$300 m
~$272 m
~$50 m
$251 m
~$ 86 m~$250 m
~$36 m $127 m
$21 m
2012 2013 201420152017
Open market share repurchase
Planned synthetic share repurchase
Convertible notes repurchases(1)
Planned open market repurchase in 2017
Return considerations
2017: Commitment to return $300 million
~$250 million via synthetic share repurchase (completion in January)
~$50 million via open-market repurchases (completion by end of 2017)
Mid-term targets do not assume repurchases beyond announced 2017 programs
Signal of conviction with $300 million capital return commitment by end of 2017
(1) 2026 convertible notes repurchased in 2014 removed ~2 million shares from diluted share count and ~15 million total shares of dilution risk (dilutive above $20.00) 2024 convertible notes repurchased in 2015 removed ~5 million shares from diluted shared count and ~12 million total shares of dilution risk (dilutive above $12.65)
Sample to Insight
2016 Analyst & Investor Day
11
Table of Contents
|
2013-2017: Leverage ratio(1)
(Multiple of net debt / EBITDA)
2.0
1.6
1.5
1.5
1.3 ~1.3
1.1
1.0
0.5
0.0
2013 2014 20159M2017
2016year-end
target
2017-2024: Debt maturity overview
(In $ millions)
2024 $27 m U.S. Private Placement
(3.9%)
2023
2022 $300 mU.S. Private Placement
(3.75%)
2021 $300 mCash convertible notes
(0.875%)
2020
Cash convertible notes
$430 m(0.375%)
2019
U.S. Private Placement
$75 m (3.19%)
2018
2017
Healthy leverage and capital structure to support business and increase returns
(1) Leverage ratio is calculated on trailing four quarters as net debt / EBITDA adjusted for acquisition-related expenses, share-based compensation and pro forma results of acquired businesses. 2017 estimate without future M&A.
Sample to Insight
2016 Analyst & Investor Day
12
Table of Contents
|
2017: Net sales guidance 2017: Adjusted diluted EPS guidance
(At constant exchange rates to comparative year) (In $ per share, at CER to comparative year)
~$0.13-~$1.25-
Exiqon ~+6-7% CER ~+6-7% CER0.16 CER~$0.03 CER1.27 CER
acquisition ~1.08-1.09
(June 2016) ~1 p.p.Exiqon(~$1.10-1.11 CER)
MO BIO ~2 p.p.
acquisition
(late 2015)
~$1.22-1.24 CER
~$0.87-0.88 CERwith restructuring
Rest of
~5-6 p.p.business(1)with restructuring
Rest of
business(1) ~4-5 p.p.
2016 2017 2016Operating~$300 m2017
guidance guidance guidanceperformanceshareguidance
repurchase
2017 guidance: Goals for strong sales growth and adjusted EPS expansion
(1) Includes U.S. HPV headwinds of estimated ~1 p.p. for both 2016 and 2017
p.p. – Percentage points CER – Constant exchange rates Exiqon sales consolidated as of July 1, 2016
Sample to Insight
2016 Analyst & Investor Day 13
Table of Contents
|
2016-2020: Adjusted diluted EPS growth target
(In $ per share, at CER to comparative year)
OperationalNon-operational
Organic GrossOperatingTaxShare
sales growth marginexpense ratioratecount
>12% CER
CAGR
+ Strong portfolio + Capacity+Sales channel-Higher profits+ 2017 share
expansion utilizationoptimization-OECD taxrepurchases
+ Reduced + Automation+Fewer sitesdiscussionsTargets do not
~$1.08-1.09 U.S. HPV - Product mix+Business centersassume returns
(~$1.10-1.11 CER) headwinds beyond 2017
+Portfolio
management
-Bioinformatics
(OPEX per sales)
2016 2016-2020
guidance target
before
restructuring
Targeting leverage opportunities to generate double-digit adjusted EPS growth
CER – Constant exchange rates
Sample to Insight
2016 Analyst & Investor Day 14
Table of Contents
|
Key assumptions: 2016-2020
(As of November 15, 2016)
Capital expenditure (PP&E)
Capital expenditure (Intangibles) (Excluding acquired IP) Depreciation and amortization (Excluding acquired IP)
Amortization of acquired IP (Excluding any future M&A)
Restructuring charges (Included in adjusted results) Net interest expense (Included in adjusted results)
Adjusted tax rate
Repurchase considerations Weighted average diluted number of shares(1) FX assumptions
2016 2017 2018-2020
~5% of sales ~5% of sales ~4-5% of sales
~1-2% of sales ~2-3% of sales ~2-3% of sales
~7% of sales ~7% of sales ~6-7% of sales
2018: ~$90 million
~$120 million ~$105-110 million 2019: ~$70 million
2020: ~$50 million
~$75 million ~$10 million
~$12-14 million ~$13-15 million ~$15-20 million
~12-13% / ~16-17% ~18% ~19-20%
(With / without restructuring charge)
– ~$300 m Under consideration
~238 million ~232 million Depending on
(Includes $300 m repurchase) capital returns
Net sales: ~-1-2 ppts Net sales: ~-1 ppt
Adjusted EPS: ~-$0.01-0.02 Adjusted EPS: ~-$0.01-0.02
(1) 2017 includes planned $300 m share repurchase. Every $1.00 change in market price per share of common stock above $30.00 share price results in an increase / decrease of ~700,000 in dilutive shares due to call-spread overlay (CSO) related to convertible notes.
Sample to Insight
2016 Analyst & Investor Day
15
Table of Contents
|
Multiple drivers to improve returns and create greater value
Sustain strong growth through targeted investments
Sharpen focus on operational and financial leverage
Maintain disciplined capital allocation strategy
Sample to Insight
16
Table of Contents
|
Appendix
Sample to Insight
17
Table of Contents
|
Q3 2016: Consolidated Statements of Income (unaudited)
Three months ended Three months ended
(In $ thousands, except share data) September 30, 2016 September 30, 2015
Net sales 338,685 314,561
Cost of sales 117,879 109,915
Gross profit 220,806 204,646
Adjusted gross profit 241,429 222,300
Operating expenses:
Research and development 36,202 35,568
Sales and marketing 94,442 88,759
General and administrative, integration and other 33,339 24,562
Acquisition-related intangible amortization 9,851 9,586
Total operating expenses 173,834 158,475
Income from operations 46,972 46,171
Adjusted income from operations 86,763 78,333
Other income (expense):
Interest income 1,658 1,283
Interest expense (9,626) (9,206)
Other expense, net (2,374) (684)
Total other expense, net (10,342) (8,607)
Income before income taxes 36,630 37,564
Adjusted income before income taxes 82,197 75,121
Income taxes 2,922 3,550
Adjusted income tax 14,014 11,868
Net income 33,708 34,014
Net (loss) income attributable to non-controlling interest (53)84
Net income attributable to the owners of QIAGEN N.V. 33,761 33,930
Adjusted net income attributable to the owners of QIAGEN N.V. 68,236 63,169
Diluted net income per common share attributable to the owners of QIAGEN N.V. $0.14$0.14
Adjusted diluted net income per common share attributable to the owners of QIAGEN N.V. $0.29$0.27
Diluted shares used in computing diluted net income per common share (in thousands) 238,343 237,105
Sample to Insight
Third quarter and first nine months 2016 results 18
Table of Contents
|
First nine months 2016: Consolidated Statements of Income (unaudited)
Nine months ended Nine months ended
(In $ thousands, except share data) September 30, 2016 September 30, 2015
Net sales 971,476 932,446
Cost of sales 355,025 329,388
Gross profit 616,451 603,058
Adjusted gross profit 683,248 666,076
Operating expenses:
Research and development 118,082 107,471
Sales and marketing 287,185 267,200
General and administrative, integration and other 89,943 78,210
Acquisition-related intangible amortization 29,387 28,888
Total operating expenses 524,597 481,769
Income from operations 91,854 121,289
Adjusted income from operations 209,297 224,594
Other income (expense):
Interest income 4,676 3,028
Interest expense (28,370) (27,746)
Other expense, net (41)(10,585)
Total other expense, net (23,735) (35,303)
Income before income taxes 68,119 85,986
Adjusted income before income taxes 201,937 213,970
Income taxes (1,426) 7,502
Adjusted income tax 32,863 38,402
Net income 69,545 78,484
Net loss attributable to non-controlling interest (101) (46)
Net income attributable to the owners of QIAGEN N.V. 69,646 78,530
Adjusted net income attributable to the owners of QIAGEN N.V. 169,175 175,614
Diluted net income per common share attributable to the owners of QIAGEN N.V. $0.29$0.33
Adjusted diluted net income per common share attributable to the owners of QIAGEN N.V. $0.71$0.74
Diluted shares used in computing diluted net income per common share (in thousands) 237,453 237,172
Sample to Insight
Third quarter and first nine months 2016 results 19
Table of Contents
|
Q3 2016 and 9M 2016: Reconciliation adjusted results
Net GrossOperatingPretaxIncomeTaxNetDiluted
(In $ millions, except EPS) sales profitincomeincometaxrateincomeEPS(1)
Third quarter 2016
Reported results 338.7 220.847.036.6-2.98%33.80.14
Adjustments
Business integration and acquisition-related items 0.49.79.7-2.86.90.03
Purchased intangibles amortization 20.230.130.1-10.120.00.09
Non-cash interest expense charges 5.05.00.02
Other special income and expense 0.81.82.50.01
Total adjustments 20.639.845.6-11.134.40.15
Adjusted results 338.7 241.486.882.2-14.017%68.20.29
First nine months 2016
Reported results 971.5 616.591.968.11.4NM69.60.29
Adjustments
Business integration and acquisition-related items 6.728.028.0-8.119.90.09
Purchased intangibles amortization 60.089.489.4-30.059.40.25
Non-cash interest expense charges 14.814.80.06
Other special income and expense 1.63.95.50.02
Total adjustments 66.7117.4133.8-34.299.60.42
Adjusted results 971.5 683.2209.3201.9-32.916%169.20.71
(1) Weighted number of diluted shares (Q3 2016: 238.3 million) (9M 2016: 237.5 million) NM – Not meaningful
Table may have rounding differences. Net income and diluted EPS based on net income attributable to owners of QIAGEN N.V.
Sample to Insight
Third quarter and first nine months 2016 results 20
Table of Contents
|
Q3 and 9M 2016: Currency impact
Net sales Net salesCurrency exposureChange
(In $ m) (CER)(As % of CER sales)(In $ m)
Third quarter 2016
U.S. dollar 143.6 143.642%0.0
Euro 72.0 71.821%0.2
British pound 25.7 30.29%-4.5
Japanese yen 12.6 10.63%2.0
Other currencies 84.8 85.625%-0.8
Total net sales 338.7 341.8100%-3.1
First nine months 2016
U.S. dollar 409.9 409.942%0.0
Euro 217.5 216.922%0.6
British pound 69.8 77.38%-7.5
Japanese yen 37.1 33.43%3.7
Other currencies 237.2 248.025%-10.8
Total net sales 971.5 985.5100%-14.0
Sample to Insight
CER—Constant exchange rates
Other currencies include CAD, DKK, TRY, SEK, CHF, AUD, BRL, CNY, MYR, SGD, KRW, HKD, MXN, INR, TWD, RUB and ZAR
Third quarter and first nine months 2016 results
21
Table of Contents
|
2016: Quarterly and YTD income statement summary
(In $ millions, unless indicated)
(Diluted EPS in $ per share) Q1 2016 Q2 2016Q3 2016Q4 2016YTD 2016
Net sales 298.4 334.4338.7971.5
Net sales (CER) 305.6 338.1341.8985.5
Gross profit 184.7 210.9220.8616.5
Gross profit margin 62% 63%65%63%
Adjusted gross profit 207.5 234.4241.4683.2
Adjusted gross profit margin 70% 70%71%70%
Operating income 15.4 29.547.091.9
Operating margin 5% 9%14%9%
Adjusted operating income 53.4 69.286.8209.3
Adjusted operating margin 18% 21%26%22%
Tax rate -66% 7%8%-2%
Adjusted tax rate 15% 16%17%16%
Net income 14.9 21.033.869.6
Adjusted net income 44.0 56.968.2169.2
Diluted EPS 0.06 0.090.140.29
Adjusted diluted EPS (CER) ($ per share) 0.19 (0.19) 0.24 (0.24)0.29 (0.29)0.71 (0.73)
Diluted shares outstanding for EPS calculation 236.9 237.2238.3237.5
Sample to Insight
CER—Constant exchange rates Table may have rounding differences.
Net income, adjusted net income, diluted EPS and adjusted diluted EPS based on net income attributable to owners of QIAGEN N.V.
Third quarter and first nine months 2016 results
22
Table of Contents
|
2015: Quarterly and YTD income statement summary
(In $ millions, unless indicated)
(Diluted EPS in $ per share) Q1 2015 Q2 2015Q3 2015Q4 20159M 2015FY 2015
Net sales 298.4 319.5314.6348.5932.41,281.0
Adjusted net sales 298.7 319.5314.6348.5932.71,281.2
Adjusted net sales (CER) 324.8 348.9342.5372.61,016.21,388.8
Gross profit 197.9 200.5204.6223.3603.1826.4
Gross profit margin 66% 63%65%64%65%65%
Adjusted gross profit 218.1 225.7222.3246.0666.1912.0
Adjusted gross profit margin 73% 71%71%71%71%71%
Operating income 35.1 40.046.254.4121.3175.7
Operating margin 12% 13%15%16%13%14%
Adjusted operating income 67.4 78.978.390.0224.6314.5
Adjusted operating margin 23% 25%25%26%24%25%
Tax rate -2% 15%10%-4%9%4%
Adjusted tax rate 19% 19%16%15%18%17%
Net income 19.5 25.133.948.678.5127.1
Adjusted net income 51.5 60.963.273.9175.6249.5
Diluted EPS 0.08 0.110.140.200.330.54
Adjusted diluted EPS (CER) ($ per share) 0.22 (0.24) 0.26 (0.28)0.27 (0.29)0.31 (0.33)0.74 (0.81)1.05 (1.13)
Diluted shares outstanding for EPS calculation 237.4 237.0237.1237.1237.2237.2
Sample to Insight
CER—Constant exchange rates Table may have rounding differences
Net income, adjusted net income, diluted EPS and adjusted diluted EPS based on net income attributable to owners of QIAGEN N.V.
Third quarter and first nine months 2016 results
23
Table of Contents
|
Consolidated Balance Sheets
Sept 30, Dec 31,Sept 30,Dec 31,
(In $ thousands, except par value) 2016 2015(In $ thousands, except par value)20162015
Assets (unaudited) Liabilities and Equity(unaudited)
Current assets: Current liabilities:
Cash and cash equivalents 354,646 290,011Accounts payable40,87452,306
Short-term investments 109,693 130,817Accrued and other current liabilities196,963192,069
Accounts receivable, net 260,039 273,853Income taxes payable12,28421,515
Income taxes receivable 26,443 26,940Deferred income taxes—2,463
Total current liabilities250,121268,353
Inventories, net 144,710 136,586Long-term liabilities:
Prepaid expenses and other current 67,529 70,121
assets Long-term debt1,069,4401,049,026
Deferred income taxes — 33,068Deferred income taxes40,35775,726
Total current assets 963,060 961,396Other long-term liabilities302,526224,058
Long-term assets: Total long-term liabilities1,412,3231,348,810
Property, plant and equipment, net 460,509 442,944Equity:
Goodwill 1,962,514 1,875,698Common shares, EUR 0.01 par value:2,8122,812
Intangible assets, net 623,272 636,421Additional paid-in capital1,764,6531,741,167
Deferred income taxes 7,671 2,036Retained earnings1,273,6051,227,509
Accumulated other comprehensive
Other long-term assets 311,118 260,622loss(249,070)(259,156)
Total long-term assets 3,365,084 3,217,721Less treasury shares at cost(126,300)(152,412)
Total assets 4,328,144 4,179,117Total equity attributable to the
owners of QIAGEN N.V.2,665,7002,559,920
Non-controlling interest—2,034
Total equity2,665,7002,561,954
Total liabilities and equity4,328,1444,179,117
Sample to Insight
Third quarter and first nine months 2016 results
24
Table of Contents
|
Consolidated Statements of Cash Flows (unaudited)
Nine months ended Nine months ended
Sept 30, Sept 30,Sept 30,Sept 30,
(In $ thousands) 2016 2015(In $ thousands)20162015
Cash flows from operating activities: Cash flows from financing activities:
Net income 69,545 78,484Net proceeds from issuance of cash
Adjustments to reconcile net income to net convertible notes and cash paid for
cash provided by operating activities, net of issuance costs—(86)
effects of businesses acquired: Repayment of long-term debt(6,738)(251,398)
Depreciation and amortization 156,695 139,666Principal payments on capital leases(949)(809)
Non-cash impairments — 2,189Proceeds from subscription receivables—97
Deferred income taxes (11,695) (7,899)
Excess tax benefits from share-based
Other items, net including fair value compensation4482,513
changes in derivatives 35,355 50,790
Change in operating assets 17,911 19,560Proceeds from issuance of common shares2,5629,117
Change in operating liabilities (26,232) (52,055)Purchase of treasury shares—(20,818)
Net cash provided by operating activities 241,579 230,735Other financing activities(11,156)(836)
Net cash used in financing activities(15,833)(262,220)
Cash flows from investing activities:
Effect of exchange rate changes on cash and
Purchases of property, plant and equipment (54,846) (67,683)cash equivalents2,143(13,088)
Proceeds from sale of equipment 22 103
Net increase (decrease) in cash and cash
Purchases of intangible assets (12,694) (14,432)equivalents64,635(61,716)
Purchases of investments (21,375) (5,596)Cash and cash equivalents, beginning of
Cash paid for acquisitions, net of cash period290,011392,667
acquired (90,490) (7,097)Cash and cash equivalents, end of period354,646330,951
Purchases of short-term investments (436,249) (190,508)
Proceeds from sales of short-term Reconciliation of Free Cash Flow1
investments 458,883 274,125Net cash provided by operating activities241,579230,735
Other investing activities (6,505) (6,055)Purchases of property, plant and equipment(54,846)(67,683)
Net cash used in investing activities (163,254) (17,143)Free Cash Flow186,733163,052
(1) Free cash flow is a non-GAAP financial measure and is calculated from cash provided by operations reduced by the investments in fixed assets. QIAGEN believes this is a common financial measure useful to further evaluate the results of operations.
Sample to Insight
Third quarter and first nine months 2016 results
25
Table of Contents
Exhibit 99.8
|
QuantiFERON-TB:
Changing the way the world looks at TB prevention and elimination
Dr. L. Masae Kawamura, M.D.
Senior Director, Medical & Scientific Affairs, TB Diagnostics November 15, 2016
Sample to Insight
2016 Analyst & Investor Day 1
Table of Contents
|
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Tuberculosis: A stealth foe and global killer
CAVITY –Millions to billions of bacilli
Etiology Transmission Impact Global Incidence
Non-motile slow growing bacteria
May persist in the body for decades
Airborne disease
Spread mainly through cough
A person dies of TB every 18 seconds
More deaths from TB than HIV or malaria
10.4 million active TB cases in 2015
2-3 billion latent TB infections globally
TB is preventable, but persists due to ignorance, apathy and poor detection
Sample to Insight
2016 Analyst & Investor Day
3
Table of Contents
|
The world is waking up to the importance of latent TB infection and prevention
2,000
1,770 1,600
Active TB case detection and treatment million 1,200 per ases 800
C Mass LTBI detection 400 and treatment Active and latent TB
0 detection and treatment 2010 2020 2030 2040 2050 2015
Multiple studies show addressing latent TB essential to eliminating the disease
LTBI – Latent TB infection
Sample to Insight
Adapted from Abu-Raddad et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. PNAS. 2009; 106; 13980-5.
2016 Analyst & Investor Day
4
Table of Contents
|
Latent TB market endorsement
2015: UN approves global goal to reduce TB incidence by 80% by 2030
2014: WHO End TB Strategy: Prevention is an essential component
2014: WHO issues guidelines on management of latent TB infection
Scaling up the fight against latent TB
International guidelines expanding latent TB testing beyond developed countries
Targeting 115 countries with TB rates of <100 cases per 100,000
Diagnostic aids: IGRA or TST (skin test)
Eliminating TB requires scaling up the fight on a global basis
Sample to Insight IGRA – Interferon-gamma release assay TST – Tuberculin skin test
2016 Analyst & Investor Day 5
Table of Contents
|
Targeting high-risk groups for TB detection
Disease progression TB infection and transmission
? HIV infected ?Healthcare workers
? Immunosuppressive therapies ?Immigrants from high
? Growing use of biologics TB-burden countries
? End-stage renal disease ?Prisoners
? Children under age 5 ?Homeless
? Elderly ?Military
Cumulative risks
Immigrants with co-morbidities
Diabetes
Tobacco use
Malnourishment
Globalization and travel
Urgent need for stricter TB testing due to fragmented landscape of risk groups
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
My patient did not know TB was preventable Tuberculosis cases in San Francisco*
500 All cases With AIDS
1980: 494 400
Latent TB infection Destructive TB disease
300
My personal experience
? Dedicated my career to fighting TB 200
? Director, TB Control in San Francisco 2010: 94? Lifelong TB educator and advocate
100
? National policy maker (incl. IGRAs) 1980: 0
2010: 4
? Leadership positions in various U.S. and international organizations
0
1980 1990 2000 2010
LTBI treatment prevents devastating individual and public health consequences
IGRA – Interferon-gamma release assay
Sample to Insight * Source: San Francisco Department of Public Health, John Blanchard/ San Francisco Chronicle
2016 Analyst & Investor Day 7
Table of Contents
|
Classic TB screening algorithm
TB symptom screen in high-risk person
Yes (symptoms) No symptoms
TST or IGRA
Positive Negative
Chest radiography
TB and Any abnormality No abnormality
other disease
investigations Treat for LTBI
TB elimination requires accurate testing to reduce unnecessary healthcare costs
Source: 2014 WHO LTBI Management Guidelines
Sample to Insight LTBI – Latent TB infection IGRA – Interferon-gamma release assay TST – Tuberculin skin test
2016 Analyst & Investor Day 8
Table of Contents
|
FDA-approved TB diagnostic options
Tuberculin skin test QuantiFERON-TB ELISPOT assay
PPD ESAT-6, CFP-10, TB 7.7 ESAT-6, CFP-10
Cytokine induction PPD / M. tuberculosis
specific antigen Calcineurin inhibitors:
Impaired cytokine induction
APC
T-cell
Lymphocyte-depleting agents:
T-cell depletion
End-stage renal disease: Corticosteroids:
Impaired co-stimulatory capacity Impaired T-cell / APC function
Current diagnostic aids for TB detection rely on cellular immunity
Sample to Insight APC – Antigen-presenting cell PPD – Purified protein derivative used in tuberculin skin test (TST)
2016 Analyst & Investor Day 9
Table of Contents
|
Tuberculin skin test (TST) QuantiFERON-TB (QFT)
? Manual placement, reading and data entry ?Can be fully automated
? Results affected by BCG vaccine and NTM ?Highly specific
? Two patient visits required, high no-show rate ?Results with one patient visit
? Significant inter-reader variability ?No inter-reader variability
? Poor surveillance tool ?Electronic results (straight to EMR)
? Often no quality control after initial training ?Quality-assured laboratory test(1)
TST least effective in most vulnerable: BCG-vaccinated and “hard to reach”
BCG—Bacillus Calmette-Guerin vaccine NTM – Non-tuberculosis myobatetria EMR – Electronic medical record Sample to Insight (1) Not available in all markets
2016 Analyst & Investor Day 10
Table of Contents
|
Sensitivity meta-analyses QFT TST Overlapping PPDs can affect TST results
M. tuberculosis PPD Sester et al. ERJ. 2011 80% 65%
Diel et al. Chest. 2010 –
84.5% 71.5% Developed country
255
19 62
Specificity meta-analyses QFT TST 8
Diel et al. ERJ. 2011 99.4% 88.7%
35 19 2 U.S. CDC Guidelines. 2010 99% 85%
Diel et al. Chest. 2010 99.2% ND M. avium PPD M. bovis PPD
Purified Protein Derivatives (PPDs) are far from being “pure”
QuantiFERON-TB consistently shown as more specific and sensitive than TST
Sample to Insight Prasad TSK, et al. Clinical Proteonmics 2013;10:8 ND – No data QFT – QuantifERON-TB TST – Tuberculin skin test PPD – Protein purified derivative
2016 Analyst & Investor Day 11
Table of Contents
|
Current FDA-approved and CE-marked Interferon Gamma Release Assays
QuantiFERON-TB GoldT-Spot®
Objectivity ? No cell purification or counting?Lab worker subjectivity in cell counting
? Spectrophotometer and software analysis
Interpretation ? One cut-off point used worldwide?Cut-off varies by country
criteria and in all published studies?Borderline results should be repeated
? No borderline zone
Blood volume ? 3 mL in total (1mL in each of 3 tubes)?6 mL minimum in lithium heparin
Assay sample ? Whole blood?Purified PBMC – high labor component
Handling time ? 16 hours?8 hours from collection
before processing ? Once incubated, additional 72 hours?32 hours possible using Xtend® reagent
Scalability ? Yes?Limited by manual workflow
QuantiFERON-TB: Leading IGRA with significant clinical and workflow benefits
Sample to Insight T-Spot is a trademark of Oxford Immunotec PBMC – Peripheral blood mononuclear cells IGRA – Interferon-gamma release assay
2016 Analyst & Investor Day 12
Table of Contents
|
Changing perspectives on TB policy in China
2015 Lancet ID: China’s first multi-center population-based study of latent TB
Head-to-head testing in the same 21,000 people
Results: TST overestimated TB infection rates
QuantiFERON-TB showed lower latent TB prevalence in China, impact on guidelines
China study: QFT/TST and background TB by site China study: Results overview
50 QFT positive rate
41.69 Overall TB infection rate
TST positive rate
38.60 Test (% of positive tests)
40 Background TB rate
30 28.81 Tuberculin skin test 28%
19.81 19.09
20 17.37
14.77 15.50
13.47 QuantiFERON-TB Gold 18.8%
10 13.05 10.68
4.09
0
Site C Site D Site A Site B
21,000-person China study shows scalability of QuantiFERON-TB for testing
Sample to Insight Lei Gao et al, Lancet Infect Dis 2015; Feb 11. http://dx.doi.org/10.1016/S1473-3099(14)71085-0 TST – Tuberculin skin test
2016 Analyst & Investor Day 13
Table of Contents
|
QuantiFERON-TB in New York City
Largest study ever involving QuantiFERON-TB ? 69,273 people tested (2006-2011 study)
Goal: Better estimate background prevalence of TB
Results: Positive QuantiFERON-TB data consistent with known TB risk factors – but half the rate with TST
Conclusion: IGRAs like QuantiFERON-TB are reliable for detecting TB infection
QuantiFERON-TB Gold Tuberculin skin test
(3nd generation QFT) (n=20,066) (Historic rate)
Positive 14% 24%
Negative 83%
Indeterminate <1%
U.S.-born positive 9% 9%
Foreign-born positive 19% 40%
Making an impact on public health with better understanding of TB prevalence
Stennis et al, Open Forum Infect Dis. 2014 Sep; 1(2): ofu047 Published online 2014 Jul 29. doi: 10.1093/ofid/ofu047 Sample to Insight TST – Tuberculin skin test IGRA – Interferon-gamma release assay
2016 Analyst & Investor Day 14
Table of Contents
|
California TB control
2,170 Californians with TB
10,000 TB suspect cases
20,000 contacts to cases
million with TB infection 38 million Californians
USPSTF guidelines
IGRAs are key elements to policy development and TB elimination efforts
Sample to Insight Data provided by California Department of Public Health (2013) IGRA – Intereron-gamma release assay USPSTF – U.S. Preventive Services Task force
2016 Analyst & Investor Day 15
Table of Contents
|
QuantiFERON – the leading IGRA for TB detection
1st generation 2nd generation 3rd generation4th generation
QuantiFERON®-TB QuantiFERON®-TB Gold QuantiFERON®-TB GoldQuantiFERON®-TB Gold Plus
(liquid antigen) (QFT® in tube)(QFT®-Plus)
Q4 2014: CE-IVD
2001: FDA approval 2004: FDA approval 2007: FDA approval2016: FDA submission
Measured cell-mediated Used antigens specific for Blood collection tubes asAddition of patented CD8
immune response to same M. tuberculosis complex incubation vessels withantigens – potential biomarker
tuberculin purified protein organisms full automationof intracellular TB burden
derivative (PPD) used for
TST (M. avium)
QuantiFERON-TB Gold Plus: Developing new clinical and workflow benefits
Sample to Insight IGRA – Intereron-gamma release assay TST – Tuberculin skin test
2016 Analyst & Investor Day 16
Table of Contents
|
QuantiFERON-TB Gold Plus: Gaining attention in 2016 WHO TB report
CD8 and CD4 T-cell response
Current IGRA assays primarily detect a CD4
T-cell response. However, a new generation
assay, the QuantiFERON-TB Plus (QFT-
Plus, Qiagen, Hilden, Germany), has been
developed to stimulate gamma interferon
production by both CD4 and CD8 T-cells.
First results indicate that the CD8 T-cell
response may be able to identify people at
greater risk of progression to active TB.
QuantiFERON-TB Gold Plus: Potential to better identify risk for progression
Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening.
Sample to Insight
Eur Respir J. 2016:ERJ-00510-02016.
2016 Analyst & Investor Day
17
Table of Contents
|
TB remains a stealth foe and a global killer
QuantiFERON-TB changing the way the world looks at TB prevention and elimination
Leading IGRA test used to date in >30 million patients
Key clinical benefits with new QFT 4th generation test
Sample to Insight
2016 Analyst & Investor Day 18
Table of Contents
Exhibit 99.9
|
GeneReader NGS System:
The world’s first truly complete Sample to Insight NGS system
Jonathan Arnold
Senior Director, Business Leader for Oncology Franchise, MDx Business Area
November 15, 2016
Table of Contents
|
Disclaimer
Safe Harbor Statement: This presentation contains both historical and forward-looking statements. All statements other than statements of historical fact are, or may be deemed to be forward looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, and Section
21E of the U.S. Securities Exchange Act of 1934, as amended. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our own expectations and projections. Some of the factors that could cause actual results to differ include, but are not limited, to the following: general industry conditions and competition; risks associated with managing growth and international operations (including the effects of currency fluctuations, regulatory processes and dependence on logistics), variability of operating results and allocations between customer classes, and the commercial development of markets for our products to customers in academia, pharma, applied testing and molecular diagnostics; changing relationships with customers, suppliers and strategic partners; competition; rapid or unexpected changes in technologies; fluctuations in demand for QIAGEN’s products (including factors such as general economic conditions, the level and timing of customers’ funding, budgets and other factors); our ability to obtain regulatory approval of our products; technological advances of our competitors and related legal disputes; difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products; the ability of QIAGEN to identify and develop new products and to differentiate and protect our products from competitor products; market acceptance of QIAGEN’s new products and the integration of acquired technologies and businesses. For further information, please refer to “Risk Factors” section of reports that QIAGEN has filed with, or furnished to, the U.S. Securities and Exchange Commission (SEC). We undertake no obligation, and do not intend, to update these forward-looking statements as a result of new information or future events or developments unless and to the extent required by law.
Regulation G: QIAGEN reports adjusted results, as well as results on a constant exchange rate (CER) basis, and other non-U.S. GAAP figures (generally accepted accounting principles), to provide additional insight on performance. In this presentation, adjusted results include adjusted operating expenses, adjusted EBITDA, adjusted diluted EPS and free cash flow. Adjusted results are non-GAAP financial measures QIAGEN believes should be considered in addition to reported results prepared in accordance with GAAP, but should not be considered as a substitute. QIAGEN believes certain items should be excluded from adjusted results when they are outside of its ongoing core operations, vary significantly from period to period, or affect the comparability of results with its competitors and its own prior periods. Please see the Appendix provided in this presentation “Reconciliation of Non-GAAP to GAAP Measures” for reconciliations of historical non-GAAP measures to comparable GAAP measures and the definitions of terms used in the presentation. QIAGEN does not reconcile forward-looking non-GAAP financial measures to the corresponding GAAP measures due to the high variability and difficulty in making accurate forecasts and projections that are impacted by future decisions and actions. Accordingly, reconciliations of these forward-looking non-GAAP financial measures to the corresponding GAAP measures are not available without unreasonable effort. However, the actual amounts of these excluded items will have a significant impact on QIAGEN’s GAAP results.
GeneReader NGS System: The QIAGEN GeneReader® NGS System is currently available for marketing and sales outside the United States. The QIAGEN GeneReader® NGS System with new sequencing chemistry will be made available in the United States starting in December 2016. The QIAGEN GeneReader® NGS System is intended for Research Use Only. This product is not intended for the diagnosis, prevention or treatment of a disease. QIAGEN Clinical Insight® is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic sequencing data. The software evaluates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases and annotations, drug labels and clinical-trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is not intended as a primary diagnostic tool by physicians or to be used as a substitute for professional healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national and local clinical laboratory regulations and other accreditation requirements
Sample to Insight
2016 Analyst & Investor Day
2
Table of Contents
|
Applied
Academia Pharma Testing
Universal NGS
Single-cell genomics Whole exome
Gene expression Whole genome
Targeted resequencing
Molecular Diagnostics
GeneReader NGS System
Clinical research
Translational medicine
Oncology
Hereditary diseases
Infectious diseases
Nucleic acid Target LibraryNext-Data
collection & enrichment preparationgenerationanalysisInterpretation
extraction sequencing
Universal NGS: Universal NGS:
QIAseq portfolio CLC / Ingenuity / QCI
GeneReader NGS System
QIAGEN well-positioned across all segments of theNGS market
Sample to Insight
2016 Analyst & Investor Day
3
Table of Contents
|
Key challenges
Simple Seamless Cost-
workflow bioinformatics effective
Labs want solutions to issues preventing them from realizing NGS promise
Sample to Insight
2016 Analyst & Investor Day
4
Table of Contents
|
GeneReader NGS System: Designed to deliver actionable insights
The first complete Sample to Insight NGS solution
Sample to Insight
2016 Analyst & Investor Day 5
Table of Contents
|
GeneReader NGS System: Key benefits and unique selling proposition
The first truly complete NGS workflow
Seamlessly integrated flexible work?ow
Delivered by a single vendor you can trust
Actionable insights
Create relevant reports
Proven gene panels and bioinformatics
Flexibility to fit your needs Predictable costs Expertise and service
Scalable batch sizes Price-per Insight modelGlobal team support
Continuous loading Affordable and efficientEfficient operation help
Delivering on the promise of NGS to improve lives
Sample to Insight
2016 Analyst & Investor Day
6
Table of Contents
|
Best-in-class consumables pull-through with flexible and transparent pricing models
Capital purchase
Flexible Price-Per-Insight pricing
Reagent rental
QIAGEN is a true NGS partner willing to share operational risk
Sample to Insight
2016 Analyst & Investor Day 7
Table of Contents
|
Nucleic Acid Next-
Target Library Data collection & generation Interpretation enrichment preparation analysis extraction sequencing
Automation
QIAcube QIAgility QIAcube GeneRead Gene QCI QIAcube Reader Quality control RGQ
QIAxcel
Support
GeneRead Assistant Data management
GeneRead Link and LIMS
Offering everything needed from one vendor for NGS solutions
Sample to Insight
2016 Analyst & Investor Day 8
Table of Contents
|
QIAact tumor panels: Based on clinical utility and insights
Drug labels
Clinical Trails
QIAGEN Knowledge Base
Clinical Case Scientific
Counts literature
Professional
External guidelines databases
Designed to deliver research-relevant insights
Sample to Insight
2016 Analyst & Investor Day
9
Table of Contents
|
Actionable Insight Tumor Panel: Providing actionable insights
Targeting solid tumors with Seamless integration with QCI
Highest prevalence to maximize scale
Greatest need for testing
Variants associated with actionable insights
Featuring 12 clinically actionable genes
KRAS ERBB2
NRAS PIK3CA
KIT ERBB3
BRAF ESR1
PDGFRA RAF1
ALK
EGFR
Focus on validity and utility
Sample to Insight
2016 Analyst & Investor Day
10
Table of Contents
|
Broadening system capabilities
Expanding range of sample types
Liquid biopsy
Fresh and frozen tissue
Adding test content
Expand into lung and breast cancer
A T G CA
T ACG T
Customized panel options expanding in 2017
Enabling CNV and gene fusion detection
Improving system capabilities
New sequencing chemistry
Further platform integration
Upgrading QCI
Expanding Sample to Insight capabilities
Sample to Insight
2016 Analyst & Investor Day 11
Table of Contents
|
GeneReader NGS System roadmap
Liquid Biopsy New sequencingBRCA expansionCDx PartnershipsReproductive health
? cfDNA detection chemistry Additional genes? Develop assaysDevelop reproductive
? Monitoring FFPEhealth products
Lung All-in-OneLiquid biopsy
CNV, SNV, InDel
Gene fusionsAIT FAST Panel
BRCA 1/2 BRCA expansionOncohematologyComprehensiveInfectious
SNV, Known largeMyeloidcancer paneldiseases
InDel rearrangementsComplement to
RNA Seq Assaytargeted panels
Lung DNA CNV, SNV,
SNV InDel
CNV Gene fusions
Customized content development
GeneReader NGS System capabilities will continue to expand new content and new markets
2015 2017+
Actionable Insight Tumor Panel to be broadly released with new chemistry in Q1 2017
GeneReader NGS System: Roadmap for new features in 2017 and beyond
Sample to Insight
2016 Analyst & Investor Day 12
Table of Contents
|
AMP 2016: GeneReader returning to the U.S. with new chemistry
Sample to Insight
2016 Analyst & Investor Day 13
Table of Contents
|
New sequencing chemistry performance data
RAS agreement study therascreen PCR and Pyro assays
>5% RAS variant allele frequency cutoff + (MT)–(WT)Total
+ (MT) 13013
GeneReader NGS System –(WT) 02929
Total 132942
Sample KRAS RAS Variant allele frequency (%)
no. AA Change therascreen PCR/ PyroGeneReaderAlternative platform
1 G12D +207
2 G12D +3712
3 A59T 191614
4 G12D +4411
5 Q61H 141013
6 A146P 414232
7 Q61H 364126
8 Q61H 322535
9 K117N 243639
10 G13D +4415
11 G12C +3210
12 G13D +2710
13 Q61H 262623
100 % concordance between GeneReader and QIAGEN therascreen
Sample to Insight
2016 Analyst & Investor Day
14
Table of Contents
|
Sequencing oncology market by customer
1200
$700-1,000 m
1000
800
600 $350-500 m
400
200
0
2016 2020
Clinical Lab – Routine Dx lab
CSP (Comm. Service Prov.) – Reference lab Core Lab – Sample consolidator Individual Lab – Academic
Targeting a double digit market share in the benchtop oncology NGS segment
Sample to Insight
2016 Analyst & Investor Day 15
Table of Contents
|
GeneReader value proposition resonating extremely well
Roadmap to strengthen competitive positioning
Leveraging synergies of platform-agnostic solutions and GeneReader NGS System
Sample to Insight
2016 Analyst & Investor Day 16