
Antares Pharma, Inc.
Improving Pharmaceuticals Through Advanced Drug
Delivery Systems
AMEX: AIS
May 2007

This presentation may contain forward-looking statements which are made pursuant to the safe
harbor provisions of Section 21E of the Securities Exchange Act of 1934 and the Private Securities
Litigation Reform Act of 1995. Investors are cautioned that statements which are not strictly
historical statements, including, without limitation, statements regarding the plans, objectives and
future financial performance of Antares Pharma, constitute forward-looking statements which involve
risks and uncertainties. The Company’s actual results may differ materially from those anticipated in
these forward-looking statements based upon a number of factors, including anticipated operating
losses, uncertainties associated with research, development, testing and related regulatory
approvals, unproven markets, future capital needs and uncertainty of additional financing,
competition, uncertainties associated with intellectual property, complex manufacturing, high quality
requirements, dependence on third-party manufacturers, suppliers and collaborators, lack of sales
and marketing experience, loss of key personnel, uncertainties associated with market acceptance
and adequacy of reimbursement, technological change, and government regulation. For a more
detailed description of the risk factors associated with the Company, please refer to the Company’s
periodic reports filed with the U.S. Securities and Exchange Commission from time to time, including
its Annual Report on Form 10-K for the year ended December 31, 2006. Undue reliance should not
be placed on any forward-looking statements, which speak only as of the date of this presentation.
The Company undertakes no obligation to update any forward-looking information contained in t
his presentation.
Safe Harbor Statement

Antares Overview
WE ARE A LEADING PRODUCT COMPANY WITH:
THREE VALIDATED DRUG DELIVERY PLATFORMS
MULTIPLE PRODUCTS IN KEY THERAPEUTIC FIELDS
GROWING REVENUE STREAM
OUR OWN PRODUCT ENTERING PIVOTAL TRIALS
SIGNIFICANT DEVELOPMENT PIPELINE
GLOBAL PRESENCE / LOCATIONS IN BASEL,
SWITZERLAND; EWING, NJ; MINNEAPOLIS, MN

Experienced Management
Jack E. Stover, President & CEO / Sicor, Gynetics, B. Braun
Robert F. Apple, Sr. VP & CFO / InKine Pharmaceuticals
Dario N. R. Carrara, PhD, SVP & Managing Director / Permatec
Peter L. Sadowski, PhD, VP Device Division
James E. Hattersley, VP Corporate Business Development
Michael L. Kasprick, VP Business Development, Device Division

Antares is in the “Sweet Spot”
WE PROVIDE:
DIFFERENTIATION IN THE NEW GENERIC-
BIOLOGICS MARKETPLACE
NOVELTY AND PATENTS FOR EXISTING DRUGS
COST EFFECTIVE AT-HOME PATIENT SYSTEMS
LOWER EFFECTIVE DOSE AND SAFETY WHICH
THE FDA REQUIRES

AREAS OF THERAPEUTIC FOCUS
WOMENS’ HEALTH / UROLOGY
CENTRAL NERVOUS SYSTEM (CNS)
GENERIC BIOLOGICS

Long Term Growth Strategy
USING OUR VALIDATED TECHNOLOGIES WE WILL:
Develop products with our partners’ active
ingredients in our delivery systems
Utilize device division near-term revenues to
support longer term product development
Develop and commercialize our own niche
products

Validated Drug Delivery Platforms
1.
Transdermal
ATD™Gel
Technologies
Advanced Transdermal Delivery (ATDTM)
Systemic
Compatible with single or multiple actives
3. Fast Melt
Tablet
Technologies
EasyTec™ Orally Disintegrating Tablets
2. Injection
Devices
Vision / Valeo Needle-free Reusable Injection Devices
Vibex Mini-needle Disposable Injection Devices

Launch
Available
Nestorone ATD™
(contraception)
Available
Alprazolam ATD™
(anxiety / panic disorder)
Available
Testosterone ATD™
(FSD)
BioSante (US), EU
Available
Licensing
File / Appv
Phase III
Phase II
Phase I
Preclinical
ATD™ Gel Products
Product Pipeline
Easy TEC ™ (ODT)
US Mid ‘07
EU Available
Ibuprofen (pain)
Launch
Licensing
File / Appv
Phase III
Phase II
Phase I
Preclinical
Fast Melt Tablets
Licensing
Mfg Scale-Up & Launch
Partner Clin & Reg
Design/Development
Injection Devices
Launched
Licensed for hGH and
Insulin
Licensed in 2 fields,
available other fields
Medi-Jector VISION® /
VALEO™ (insulin, hGH)
Vibex™ Mini-Needle
(undisclosed fields)
Anturol™ ATD™
(overactive bladder)
Estradiol Gel (Elestrin ™)
(Menopausal symptoms)
EU

PARTNERED PRODUCTS

PARTNERS

Elestrin™ Market and Opportunity
MARKET MOVING TO TOPICALS
Transdermal estrogen products won 15.5% of the
$1.9 billion market (up 8.8% since 2002)
45 million American women currently in
menopause
One dose of Elestrin is 50% lower than the next
lowest dose
Compliance and ease of application: Elestrin is
fast-drying and administered in a pre-measured
dose

Elestrin™ Commercial Partnership
ATD™ Gel Drug Delivery Platform
Bradley signs as marketing partner
Up to $13 million in milestones
Payments of $2.6 million received
Royalty payments upon
commercialization
Bradley sales force of 70 specialty reps
Antares has the sole rights in Europe and
elsewhere

Low-Dose Testosterone for Women
ATD™ Gel
Phase II Study showed 238% increase in
satisfying sexual events
Phase III currently underway in US with BioSante,
our licensee
Antares has rights to all clinical data and
marketing rights in EU
P & G has received approval and launched their
FSD product Intrinsa™ in France, Germany and
the UK
Antares could obtain approval in EU next

TEVA Commercial Agreements
Three development and supply agreements to date
Teva actives (biologic, generic & branded generic)
in our novel delivery systems
Attractive economic terms for AIS
Upfronts, milestones, device sales & royalties
Products anticipated to launch over the next three
years
Products disclosed upon filing or approval

BASIS OF OUR TEVA
AGREEMENTS
Rapid injection
Elimination of sharps
disposal
High quality subcutaneous
injection
Pipeline Opportunities
Interferons,Oncology,
MS,Therapeutic
Monoclonal Antibodies,
Biogenerics
Needle-Free (Reusable) and
Mini-Needle (Disposable)
Injection Device Drug Delivery Platform
Advantages

JOINT DEVELOPMENT WITH POPULATION COUNCIL
UNIQUE ATD ™GEL CONTRACEPTIVE
Phase I scheduled to begin soon
24- 48 hour sustained release contraceptive in our
ATD™ gel
Using API acquired from Merck KG
Unique combination of products using natural
estradiol instead of synthetic estrogen
Minimal adverse events

Fast Melt Tablet Drug Delivery Platform
Multi-national pharmaceutical partner
Feasibility and development agreement signed
November 2006
Opioid analgesic to be delivered via Antares’
proprietary EasyTec (ODT) delivery platform
Opportunity for product life cycle extension
EASY TEC™ (ODT) Agreement

PIPELINE

UROLOGICAL ADVISORY BOARD
Alan J. Wein, MD, PhD- Professor & Chair, Urology /U of P
Roger R. Dmochowski, MD- Professor, Urology / Vanderbilt
Joseph F. Harryhill, MD, FACS- Asst. Clinical Professor, Urology /
U of P
Diane K. Newman, RNC, MSN- Co-Director, Penn Center for
Continence and Pelvic Health / U of P
Victor W. Nitti, MD- Assoc. Professor and Vice Chair NYU / School
of Medicine
Lauri J. Romanzi, MD,FACOG- Clinical Assoc. Professor / Weill
Cornell Medical College/New York Presbyterian Hospital
David R. Staskin, MD- Assoc. Professor, Urology / Weill Cornell
Medical College/ New York Presbyterian Hospital
David O. Sussman, DO- Clinical Assoc. Professor / UMDNJ

Our innovative Overactive Bladder
product will have…
Fast onset of therapeutic activity
Decreased incidence of systemic AE’s
Lower incidence of local/on-site irritation
Accurate, improved once-daily dosing
More flexibility and discretion than patch
ATD ™ Oxybutynin Gel: ANTUROL™
U.S. Market Potential: $200 million
Additional potential as first line therapy

Anturol™ Phase II Trial: Skin Erythema
Comparison to OXYTROL Patch
Overall, skin irritation due to the gel is about 1/3 lower than the patch, comparing data
from the marketed 3.9mg/d patch.

Dose proportionality across all doses tested
Steady state achieved after 3 applications (i.e., 3 days)
Efficacy expected to be comparable to marketed
products
Safety aspects
DEO / OXY similar to Oxytrol patch
Superior skin tolerance
Cmax not higher than oral products
Anturol™ Phase II Trial: Conclusions
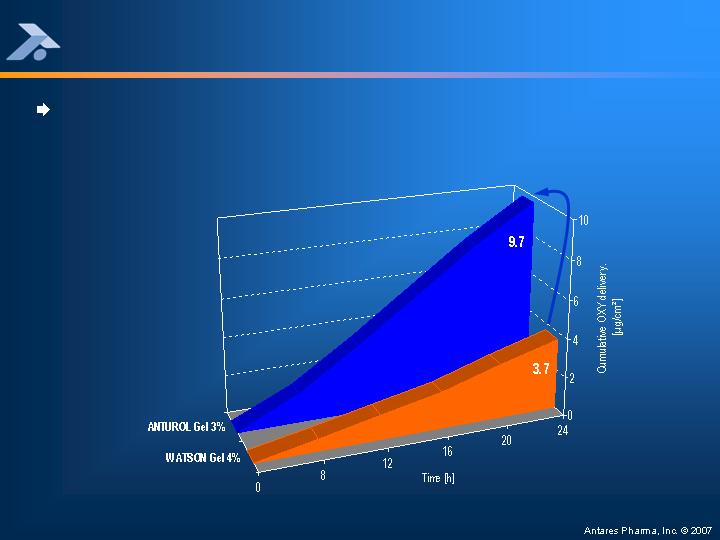
Anturol Gel Delivery Efficacy Compared to Watson Gel
Data from in vitro study 679/06 using excised pig skin (N=3)
ANTUROL Gel 3% delivers 2.6-fold higher OXY levels compared to WATSON
Gel 4%,
despite a 2-fold lower drug dose (0.18 mg/cm² versus 0.37 mg/cm²)
X 2.6
(p=0.02)

Design of pivotal trial and dosing protocol reviewed by FDA
180 patients per arm
12 week, multi-center
Selected dosing vs. Placebo
Clinical endpoint: # of incontinence episodes
Scale-up to 500 kg batch size initiated
Underway in Q3-2007
ATD patent notice of allowance issued Feb 2007
PHASE III TRIAL AND TIMELINES
UROLOGY: ANTUROL® ATD™ GEL

Selected Financial Information
Consolidated
Financial
Data
(In thousands)
December
3
1
, 2006
December 31, 2005
Cash and
investments
............................
$
7
,
700
$
2,7
00
Total assets
.
……………………………….
11,500
6,200
Total l
iabilities
................................
..........
6
,
500
5,4
00
Total stockholders’ equity ………………
5,100
760
Billings receiv
ed and/or accrued ………
5,300
2,200
Total revenue ………………………………
4,300
2,200

AIS Capital Overview
Market Cap: $82 M**
Shares Outstanding: 53 MM*
Fully Diluted Shares: 79 MM*
Avg Daily Volume (3 m): 238,000**
52-Week Range: $0.86-$1.75*
$10 Million Credit Facility
*as of 12.31.06
**as of 4.27.07

2007 Milestones
Receipt of additional milestones from Bradley Q1
Initiation of pivotal clinical trials for Anturol® Q3
Launch of Elestrin™ - royalty stream Q3
Additional partnerships, including Anturol® Q4
Approval of hGH in Needle-Free Injector Q4
Phase I results of Nestorone® contraceptive gel Q4
New EasyTec™(ODT) feasibility/development agreement 2007
Continued revenue build to cash flow break-even 2007

2006 Key Accomplishments
FDA approval of Elestrin™ for HRT / ATD™ platform
validated with this approval
Two Teva deals - revenues over the next three years
Positive Phase II results for Anturol™
Population Council contraceptive joint development
agreement
510k filed for hGH
ODT agreement / Life cycle planning opportunities
Significant financial improvement with manageable burn

Antares Pharma, Inc.
Improving Pharmaceuticals Through Advanced Drug
Delivery Systems
AMEX: AIS
May 2007
www.antarespharma.com