Exhibit 99.1

1 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 SkinGun™ & CellMist™ Next Generation Ultra - Gentle Cell Spray Symbol: RCAR H.C. Wainwright Global Life Science Conference Alan L. Rubino Chairman & CEO Robin A. Robinson, PhD Chief Scientific Officer Robert W. Cook Chief Financial Officer March 9 - 10, 2021

2 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 This slide deck and the accompanying oral presentation contain forward - looking statements, including, but not limited to, statements related to RenovaCare Inc . ’s future financial and operating results, including 2020 financial guidance, 2021 goals and expectations for growth ; the company’s corporate development efforts ; the company’s growth strategy ; future product sales and volume ; planned sales and marketing and related efforts ; future inventory and supply challenges ; planned, ongoing and future clinical trials and other product development activities ; planned regulatory submissions ; ongoing and future product launches ; the timing of such events and activities ; and other statements that are not historical facts . These forward - looking statements are based on the company’s current plans, objectives, estimates, expectations and intentions and inherently involve significant risks and uncertainties . Actual results and the timing of events could differ materially from those anticipated in such forward - looking statements as a result of these risks and uncertainties, which including, without limitation, risks and uncertainties associated with : maintaining or increasing sales and revenue ; effectively commercializing the company’s products and product candidates ; the time - consuming and uncertain regulatory approval process, including the risk that the company’s current and planned regulatory submissions may not be submitted, accepted or approved by applicable regulatory authorities in a timely manner or at all ; the effectiveness of the license agreement ; costly and time - consuming product development and the uncertainty of clinical success, including risks related to failure or delays in initiating or completing clinical trials ; protecting and enhancing the company’s intellectual property rights ; delays or problems in the supply or manufacture of the company’s products or product candidates ; the company’s ability to maintain rights to its products and product candidates ; complying with applicable U . S . and non - U . S . regulatory requirements ; government investigations and other actions ; obtaining and maintaining adequate coverage and reimbursement for the company’s products ; identifying and acquiring, in - licensing or developing additional products or product candidates, financing these transactions and successfully integrating acquired businesses ; the company’s ability to realize the anticipated benefits of its collaborations with third parties for the development of product candidates ; the ability to achieve future financial performance and results and the uncertainty of future tax and other provisions and estimates ; and other risks and uncertainties affecting the company, including those described from time to time under the caption “Risk Factors” and elsewhere in RenovaCare Inc . ’s Securities and Exchange Commission filings and reports (Commission File No . 000 - 30156 , including the company’s Quarterly Report on Form 10 - Q for the quarter ended November 13 , 2020 and future filings and reports by the company . Other risks and uncertainties of which the company is not currently aware may also affect the company’s forward - looking statements and may cause actual results and the timing of events to differ materially from those anticipated . The forward - looking statements made in this slide deck and the accompanying oral presentations are made only as of the date hereof or as of the dates indicated in the forward - looking statements, even if they are subsequently made available by the company on its website or otherwise . The company undertakes no obligation to update or supplement any forward - looking statements to reflect actual results, new information, future events, changes in its expectations or other circumstances that exist after the date as of which the forward - looking statements were made . © 2020 RenovaCare Inc. All rights reserved. Forward - Looking Statements “SAFE HARBOR” STATEMENT UNDER THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995
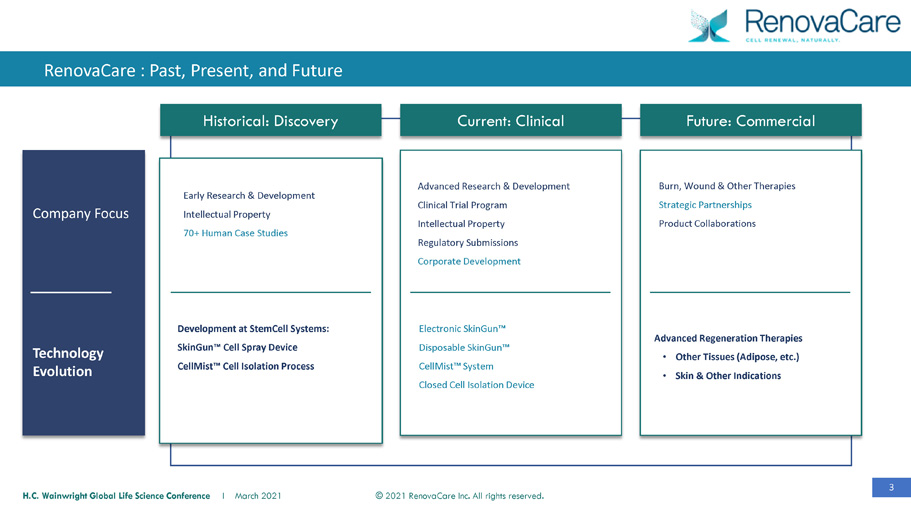
3 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Development at StemCell Systems: SkinGun™ Cell Spray Device CellMist™ Cell Isolation Process Historical: Discovery Current: Clinical Future: Commercial Technology Evolution Company Focus Electronic SkinGun™ Disposable SkinGun™ CellMist™ System Closed Cell Isolation Device Advanced Regeneration Therapies • Other Tissues (Adipose, etc.) • Skin & Other Indications Burn, Wound & Other Therapies Strategic Partnerships Product Collaborations Early Research & Development Intellectual Property 70+ Human Case Studies Advanced Research & Development Clinical Trial Program Intellectual Property Regulatory Submissions Corporate Development RenovaCare : Past, Present, and Future

4 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Next - Generation Biomedical Technologies Devices & Products

5 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Skin Grafting is Current Standard - of - Care Painful & Lengthy Treatment Cycle Expensive, Drug Dependent Psychologically Damaging Sheets of meshed skin for surgical stitching Mesh scars in skin graft patient Current Standard of Care: Expensive, Lengthy, Painful; Results: Multiple Surgeries, Extended Hospital Stays. INADEQUATE OPTIONS FOR BURN PATIENTS Skin Grafting is Inefficient, Painful, Expensive, and Leaves Scarring
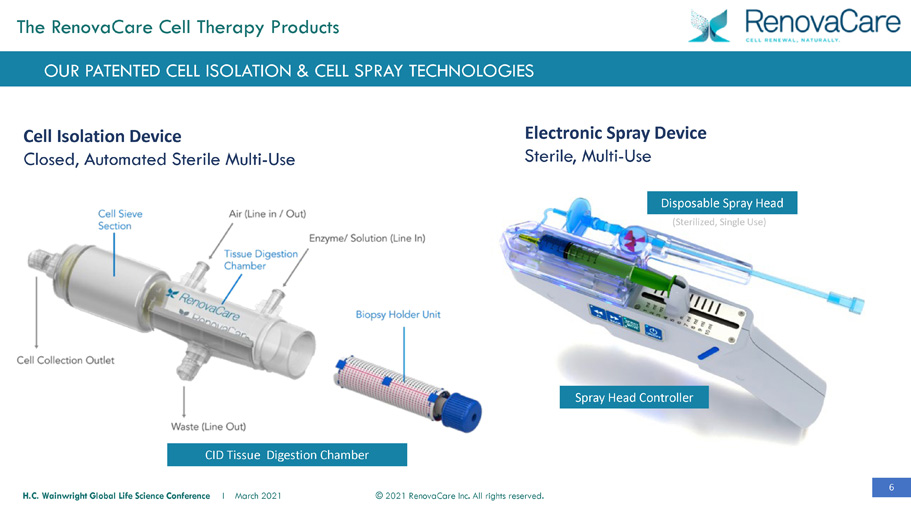
6 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Spray Head Controller Disposable Spray Head (Sterilized, Single Use) Electronic Spray Device Sterile, Multi - Use Cell Isolation Device Closed, Automated Sterile Multi - Use CID Tissue Digestion Chamber OUR PATENTED CELL ISOLATION & CELL SPRAY TECHNOLOGIES The RenovaCare Cell Therapy Products

7 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Step 1: Examine Wound/Harvest Donor Skin Tissue Performed bedside by the surgical burn team (15 min.) Step 2: Cell Isolation by Enzymatic Digestion in Cell Isolation Device Performed by burn tech (90 min.) Step 3: Cell Suspension Verification Performed by burn tech (5 min.) Step 4: Cell Spraying onto Wound with SkinGun Spray Device Performed in OR by burn surgeon (10 min.) 1 2 3 4 SIMPLE FOUR - STEP PROCESS IN LESS THAN THREE HOURS RenovaCare Cell Isolation & SkinGun Procedure

8 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 *Treated under special innovative practice approach at: Berlin - Brandenburg Center for Regenerative Therapies, Charité Berlin; an d under innovative practice approach at UPMC Medical Center, Pittsburgh, Pennsylvania. **Raw patient data available. 70+ Patients Successfully Treated* AUTOLOGOUS SKIN CELL THERAPY RAPID SELF - HEALING. SCAR - FREE. TOPICAL SPRAY WOUND CARE. RenovaCare CellMist™ System & SkinGun™: Clinical Experience
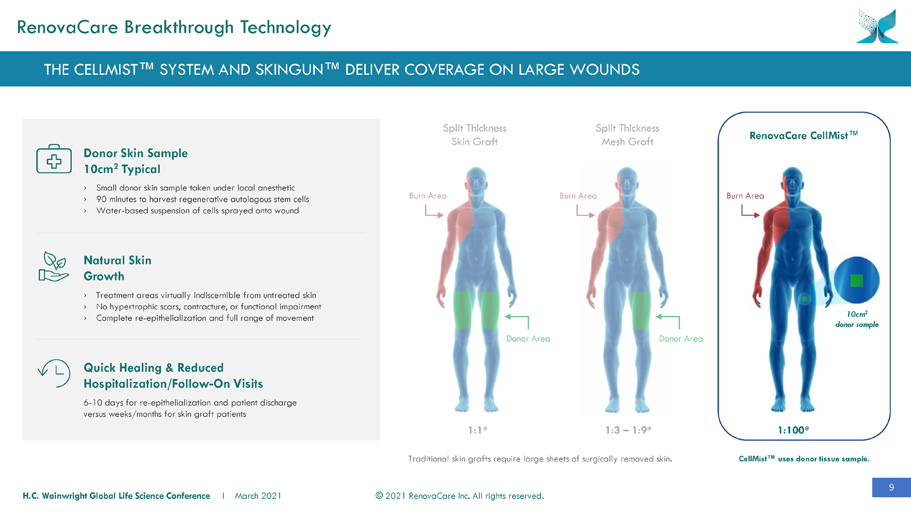
9 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 CellMist™ uses donor tissue sample. RenovaCare CellMist ™ Split Thickness Skin Graft 10cm 2 donor sample Donor Skin Sample 10cm 2 Typical › Small donor skin sample taken under local anesthetic › 90 minutes to harvest regenerative autologous stem cells › Water - based suspension of cells sprayed onto wound Natural Skin Growth › Treatment areas virtually indiscernible from untreated skin › No hypertrophic scars, contracture, or functional impairment › Complete re - epithelialization and full range of movement Quick Healing & Reduced Hospitalization/Follow - On Visits 6 - 10 days for re - epithelialization and patient discharge versus weeks/months for skin graft patients Split Thickness Mesh Graft 1:100* 1:3 – 1:9* 1:1* Donor Area Donor Area Burn Area Burn Area Burn Area Traditional skin grafts require large sheets of surgically removed skin. THE CELLMIST™ SYSTEM AND SKINGUN™ DELIVER COVERAGE ON LARGE WOUNDS RenovaCare Breakthrough Technology

10 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 IDE/PMA 510(k) 510(k) 510(k) CellMist System™ Electronic SkinGun™ Autologous Skin Stem Cell Therapy for Burns POTENTIAL APPROVALS FROM 2020 - 2025 Disposable SkinGun™ Cell Spray Deposition Device Electronic SkinGun™ Cell Spray Deposition Device Cell Isolation Device Multi - Tissue Automated Closed System onsite, rapid cell isolation and spray | multiple cell types | tissues and organs, beyond skin RENOVACARE PRODUCT PORTFOLIO: 3 SELECT MULTI - TISSUE CELL THERAPY PLATFORMS Our Execution Will Lead To Four Major Submissions for Approval
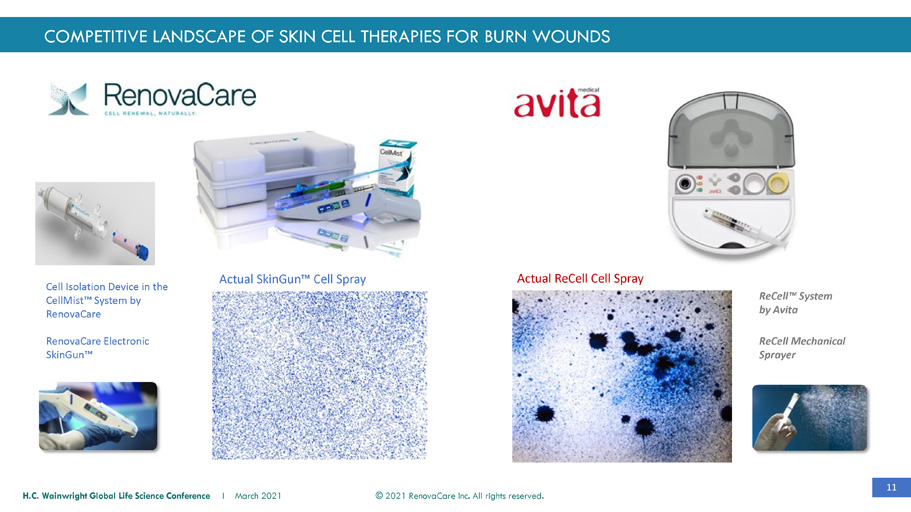
11 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Cell Isolation Device in the CellMist™ System by RenovaCare RenovaCare Electronic SkinGun™ Actual SkinGun™ Cell Spray ReCell™ System by Avita ReCell Mechanical Sprayer Actual ReCell Cell Spray COMPETITIVE LANDSCAPE OF SKIN CELL THERAPIES FOR BURN WOUNDS

12 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 IDE Submit (7/6/20) IDE Cond. Approval (8/5/20) IDE Full Approval Safety & Feasibility Clinical Study (Manual Method) Enrollment Commencement 2Q21 Bridging Clinical Study (Automated Method) Enrollment Commencement 3Q22 Pivotal Efficacy Clinical Study Enrollment Commencement 1Q24 PMA Submit PMA Approval ELECTRONIC SKINGUN™ SPRAY DEVICE Device Validation Testing 510(k) Submit 510(k) Approval PORTABLE SKINGUN™ SPRAY DEVICE Prototype Development Device Validation Testing 510(k) Submit 510(k) Approv al CLOSED AUTOMATED CELL ISOLATION DEVICE Prototype Development Device Validation Testing 510(k) Submit 510(k) Approval BURN WOUND SKIN CELL THERAPY RenovaCare Milestones & Timelines
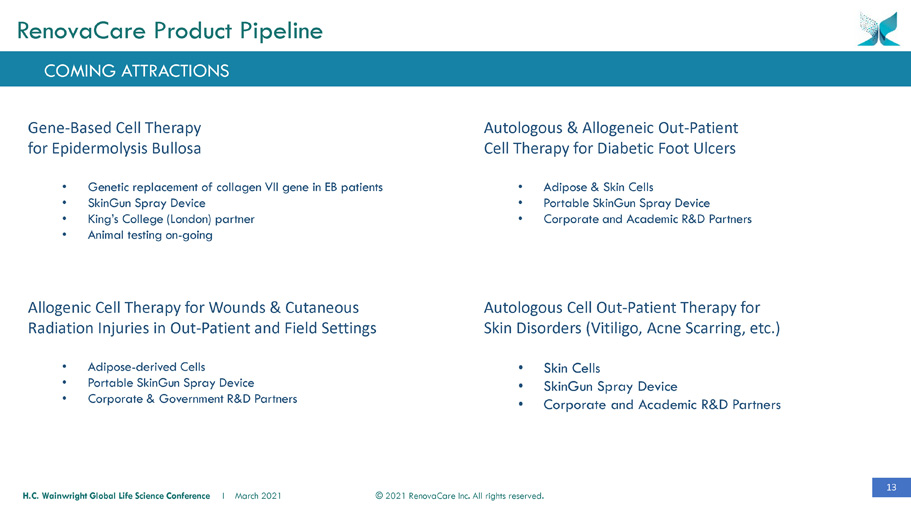
13 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Gene - Based Cell Therapy for Epidermolysis Bullosa • Genetic replacement of collagen VII gene in EB patients • SkinGun Spray Device • King’s College (London) partner • Animal testing on - going Allogenic Cell Therapy for Wounds & Cutaneous Radiation Injuries in Out - Patient and Field Settings • Adipose - derived Cells • Portable SkinGun Spray Device • Corporate & Government R&D Partners Autologous & Allogeneic Out - Patient Cell Therapy for Diabetic Foot Ulcers • Adipose & Skin Cells • Portable SkinGun Spray Device • Corporate and Academic R&D Partners Autologous Cell Out - Patient Therapy for Skin Disorders (Vitiligo, Acne Scarring, etc.) • Skin Cells • SkinGun Spray Device • Corporate and Academic R&D Partners COMING ATTRACTIONS RenovaCare Product Pipeline

14 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Research. Development. Innovation.

15 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Bioengineering & Cell Biology Regenerative Medicine Medical Device Engineering and Prototyping Regulatory - Centric IP Driven Engineering QMS Support Documentation RenovaCare R&D and Innovation Team 2 MD - PhDs 2 PhDs 1 MBA - Engineer 4 Engineers CellMist ™ and SkinGun ™ Invented, Prototyped, Patented & Clinically Translated RenovaCare R&D Innovation Center SURGERY BIOENGINEERING MANUFACTURING PRODUCT DESIGN QUALITY
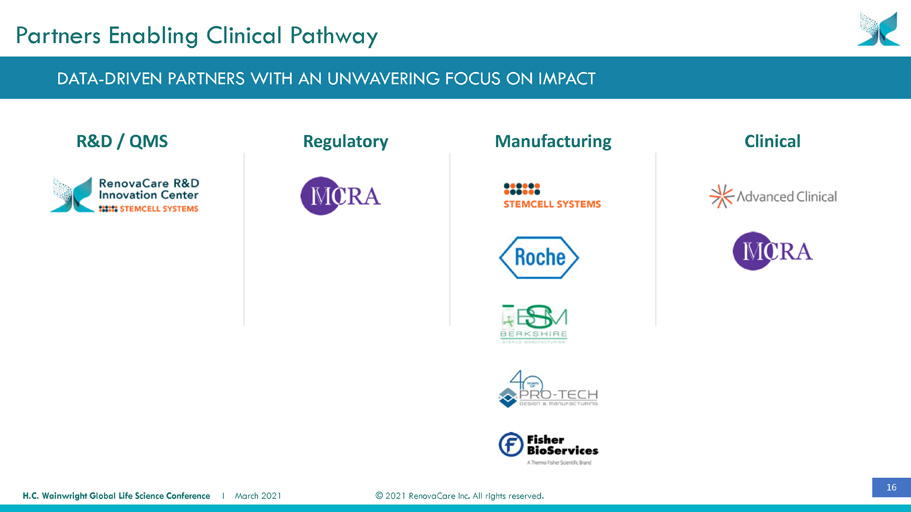
16 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 R&D / QMS Clinical Manufacturing Regulatory Partners Enabling Clinical Pathway DATA - DRIVEN PARTNERS WITH AN UNWAVERING FOCUS ON IMPACT

17 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 RenovaCare IP Summary
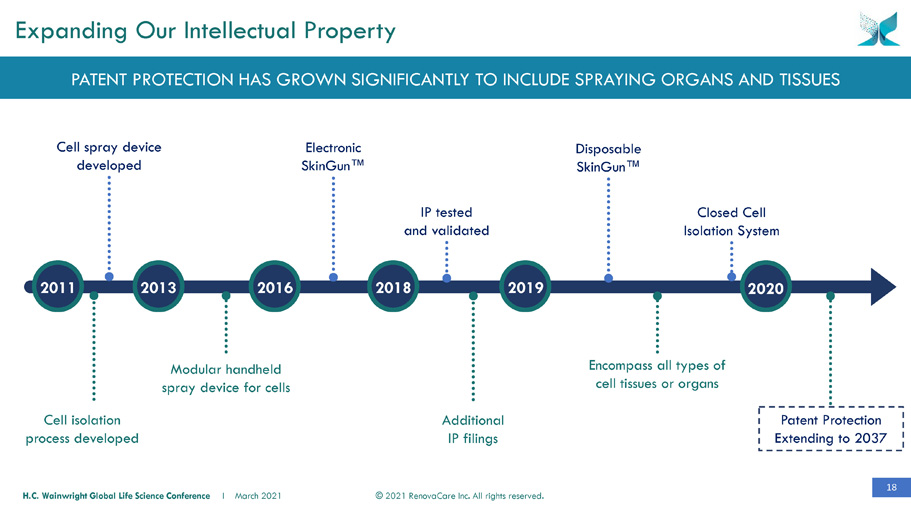
18 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Cell spray device developed Disposable SkinGun™ Encompass all types of cell tissues or organs Modular handheld spray device for cells Additional IP filings Electronic SkinGun™ IP tested and validated Cell isolation process developed Expanding Our Intellectual Property Closed Cell Isolation System PATENT PROTECTION HAS GROWN SIGNIFICANTLY TO INCLUDE SPRAYING ORGANS AND TISSUES Patent Protection Extending to 2037 2011 2013 2016 2018 2019 2020

19 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Building For Success Focused On Results

20 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 BUSINESS DEVELOPMENT OPERATIONS REGULATORY R&D MANUFACTURING CLINICAL FULLY - INTEGRATED CAPABILITIES Alan L. Rubino CEO / President / Chairman Robert Cook Chief Financial Officer Dr. Robin Robinson Chief Scientific Officer Dr. Jo Schweinle Chief Medical Officer Joseph Sierchio General Counsel Amit Singh Special Projects Dr. Roger Esteban - Vives VP R&D and Product Dev Dr. Rodney Sparks VP Intellectual Property Jean Krebs Quality Affairs MCRA Regulatory Consulting StemCell Systems GmbH Strategic Alliance Partner Mike Lerner Business Counsel Experienced Leadership Team INTELLECTUAL PROPERTY REGENERATIVE MEDICINE | BIOMEDICAL DEVICES | BURNS & WOUNDS
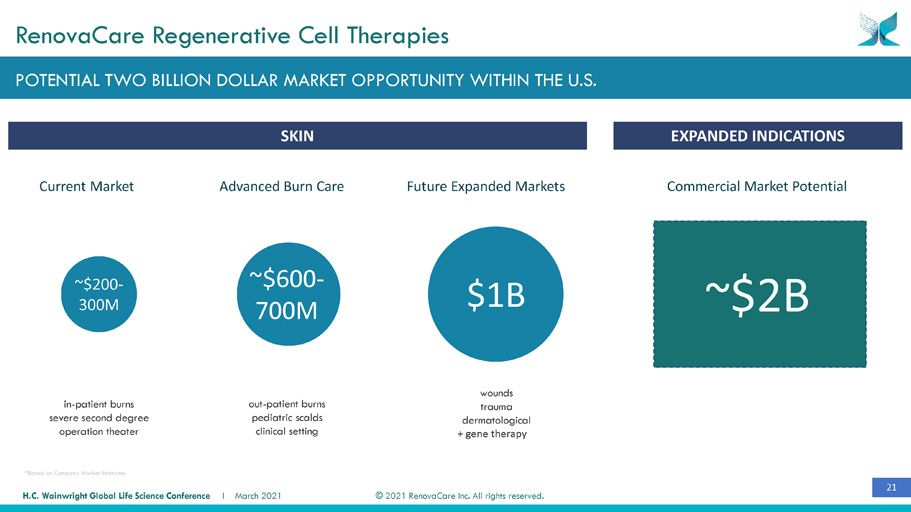
21 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 $1B ~$600 - 700M ~$200 - 300M ~$2B in - patient burns severe second degree operation theater POTENTIAL TWO BILLION DOLLAR MARKET OPPORTUNITY WITHIN THE U.S. RenovaCare Regenerative Cell Therapies Advanced Burn Care Current Market out - patient burns pediatric scalds clinical setting wounds trauma dermatological + gene therapy Future Expanded Markets SKIN EXPANDED INDICATIONS *Based on Company Market Estimates Commercial Market Potential

22 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Regulatory Priorities Planned Clinical Trials Corporate Development Activities Four Clinical Sites Selected: Q1 2020 Safety & Feasibility Clinical Study Start: Q2 2021 FDA IDE Submission: Jul 2020 FDA IDE Cond App: Aug 2020 FDA IDE Final App: Q1 2021 FDA 510(k) Submission: Q3 2021 Hired strategic talent to drive corporate activities, clinical trials, and FDA submissions Expand portfolio through multiple collaborative partnerships and strategic alliance activities 2020 – 2021 Milestones / Goals KEY TRANSITION ACTIVITIES FOR DEVELOPMENT - STAGE GROWTH
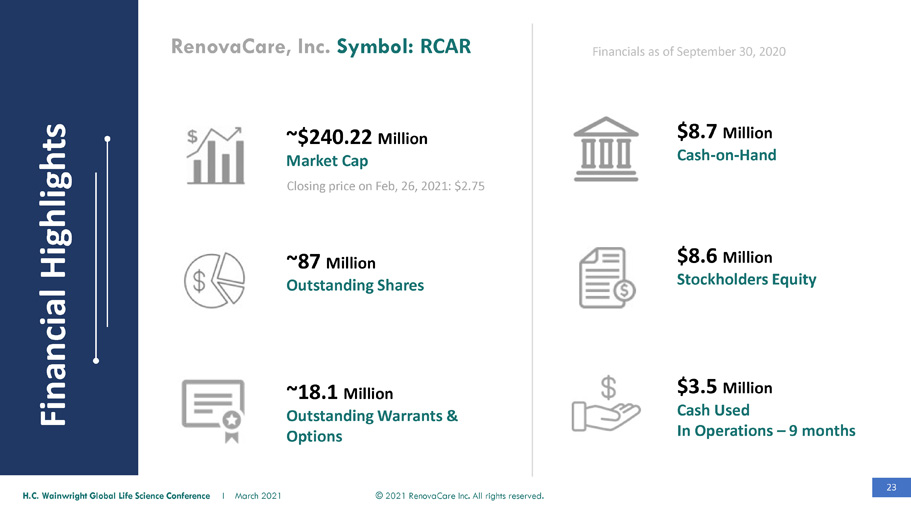
23 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 ~$240.22 Million Market Cap $8.7 Million Cash - on - Hand $8.6 Million Stockholders Equity Closing price on Feb, 26, 2021: $2.75 Financials as of September 30, 2020 RenovaCare, Inc. Symbol: RCAR $3.5 Million Cash Used In Operations – 9 months ~87 Million Outstanding Shares ~18.1 Million Outstanding Warrants & Options Financial Highlights

24 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 Building Shareholder Value RenovaCare: Focused, Growing, Disciplined & Proven ▪ cell isolation and spray technologies ▪ stem cell therapies for acute and chronic wounds, including burns ▪ highly - innovative, proven R&D group (IP, regulatory, clinical) 1 . Focused Portfolio : Cutting - Edge Stem - Cell Spray Therapies 3 . Robust, Expanding, Proven IP Estate 2 . Multiple Growth Drivers 4 . Demonstrated Record : Strategic Commercial Partnerships 5 . Disciplined Capital Allocation with World - Class Management Team ▪ worldwide market demand for self - donated stem cell therapies ▪ diverse therapeutic opportunities for tissue and organ regeneration ▪ portfolio growth in key therapeutic areas and strategic partnerships ▪ sole ownership of all technology with no in - licensing ▪ key technology protections covering methods and devices ▪ core areas focused on differentiated products for unmet needs ▪ clear and focused business development driven by experience ▪ established history of successful strategic longitudinal relationships ▪ well - positioned for government relationships with founding director, BARDA ▪ return on investment driven, time to market obsessive ▪ diversification and strengthening of immediate value - adds ▪ focused investment in strategic business drivers with operational efficiency

25 © 2021 RenovaCare Inc. All rights reserved. H.C. Wainwright Global Life Science Conference I March 2021 spray - on stem cells for rapid healing . SkinGun™ & CellMist™ Next Generation Ultra - Gentle Cell Spray Symbol: RCAR Thank you for your participation. RenovaCare Investor Forum RenovaCare, Inc. contact@renovacareinc.com +1 888 398 0202
























