
Apricus Biosciences, Inc. (NASDAQ: APRI) Corporate Presentation January 9, 2014

Apricus’ Forward-Looking Statement Safe Harbor Statements under the Private Securities Litigation Reform Act, as amended: with the exception of the historical information contained in this presentation, the matters described herein contain forward- looking statements that involve risks and uncertainties that may individually or mutually impact the matters herein described for a variety of reasons that are outside the control of the Company, including, but not limited to: estimated peak sales for any products or product candidates; its ability to further develop its product Vitaros® for erectile dysfunction, such as the room temperature version of Vitaros® and product candidate Femprox® for female sexual interest/arousal disorder; to have its product and product candidates receive additional patent protection and be approved by the relevant regulatory authorities in Europe, the United States, Canada and in other countries, such as additional national phase approvals for Vitaros® in the remaining CMS territories, including Belgium, Spain and Luxembourg, and guidance on development milestones and timing for Femprox®; our ability to enter into new licenses and partnering agreements; the ability of our partners, such as Abbott, Takeda, Sandoz, Bracco and Majorelle, to successfully launch Vitaros® in any market, the timing of any such launch and achievement of sales estimates, and our ability to realize revenue under existing license agreements; and to achieve its other development, commercialization and financial goals, including the successful manufacture and launch of Vitaros® in partnered territories. Readers are cautioned not to place undue reliance on these forward-looking statements as actual results could differ materially from the forward-looking statements contained herein. Readers are urged to read the risk factors set forth in the Company's most recent annual report on Form 10-K as initially filed and Form 10-K/A as amended, subsequent quarterly reports filed on Form 10-Q as initially filed and Form 10-Q/A as amended and other filings made with the SEC. Copies of these reports are available from the SEC's website or without charge from the Company. 2

• Our vision is to be a leader in the development and commercialization of innovative products that improve sexual health. • Our strategy is to develop and seek regulatory approval for our products and commercialize those products in major global markets by establishing partnerships with leading pharmaceutical companies. “The right strategy for the right products.” Our Vision and Strategy 3

Apricus Overview • Pharmaceutical company headquartered in San Diego, CA • Focused on development of prescription sexual health products • Disciplined leadership focused on execution – Experienced management team with solid regulatory, manufacturing, development and business development capabilities • Lead Products / Product Candidates: • Vitaros® - Topical Treatment for Erectile Dysfunction (“ED”) – Approved in Europe and Canada for entire ED patient population – Existing commercial partnerships with Abbott, Takeda, Sandoz, Majorelle and Bracco – Launches expected in Europe/Canada throughout 2014 • Femprox® - Topical Treatment for Female Sexual Interest / Arousal Disorder – Completed one successful Phase III proof-of-concept trial in China and moving towards additional late-stage trials – End of Phase II meeting with FDA completed with clear U.S. pathway for further clinical development – Seeking EU advice in Q1 2014 to determine if a “fast-track” regulatory path is possible – Strategic focus on global development and licensing 4

Our Team • CEO & Board Member- Richard Pascoe • Former CEO of Somaxon Pharmaceuticals - acquired by Pernix • Former COO ARIAD Pharmaceuticals – partnered lead asset with Merck • Former SVP at King Pharmaceuticals – acquired by Pfizer • Chairman of the Board- Kleanthis G. Xanthopoulos, Ph.D. • CEO & Board Member of Regulus Therapeutics • Former CEO of Anadys Pharmaceuticals - acquired by Roche • Former Vice President at Aurora Biosciences- acquired by Vertex Pharmaceuticals • SVP & CFO - Steve Martin • Former CFO, SVP and Interim CEO of BakBone Software – acquired by Quest • Former CFO of Stratagene Corporation – acquired by Agilent • VP Business Development- Edward Cox • Former President & Board member of Bio-Quant - acquired by Apricus • VP Chemistry & Manufacturing- Richard Martin, Ph.D. • Ph.D. from the University of Toronto • Postdoctoral studies at The Scripps Research Institute in San Diego • Former Senior Director of Chemistry at RetroVirox • VP Safety- Susan Meier-Davis, DVM, Ph.D. • DVM from the University of Wisconsin -Madison • Ph.D. in molecular pharmacology from the Stanford University School of Medicine • Former Director of nonclinical at Teikoku Pharma, Fibrogen and Theravance 5

Met Key Objectives in 2013 European approval received for Vitaros® cream for ED in June 2013 Partnership in France and Africa signed with Laboratoires Majorelle Up to $25M in upfront and milestone payments, plus tiered double-digit royalties Partnership through Northern Europe expanded with Sandoz Up to $60M in upfront and milestone payments, plus tiered double-digit royalties Femprox® End of Phase II FDA Meeting guidance received in late September 2013 Strengthened balance sheet in 2013 including equity financings ($16.6M) and divestiture of non-core assets ($5.7M) 6 Confidential

7

• Vitaros® (alprostadil/DDAIP.HCl) for the treatment of erectile dysfunction – Only topically delivered treatment for erectile dysfunction – Approved in Canada and Europe (under DCP) – Available in a single use 220 and 330 mcg dispenser • Attractive commercial opportunity – Ex-US PDE-5 market estimated at $2.0B1 – Vitaros® ex-US peak sales estimated at $300M2 • Significant efficacy and safety profile, including difficult to treat populations – Studied in over 3,300 patients – Rapid onset (generally 5-15 minutes) – Addresses diabetics, hypertensives, patients with cardiac issues or on nitrates/alpha blockers, prostatectomy patients and PDE-5 (e.g. Viagra®) failures • Strong IP Estate: issued patents through 2026 and patent applications filed for extended protection through 2032 • Second generation dispenser in development – No refrigeration required; targeting 24+ month shelf-life 8 Vitaros® Product Profile 1. IMS 2011 2. Analyst estimates (Cantor Fitzgerald, August 22, 2013)
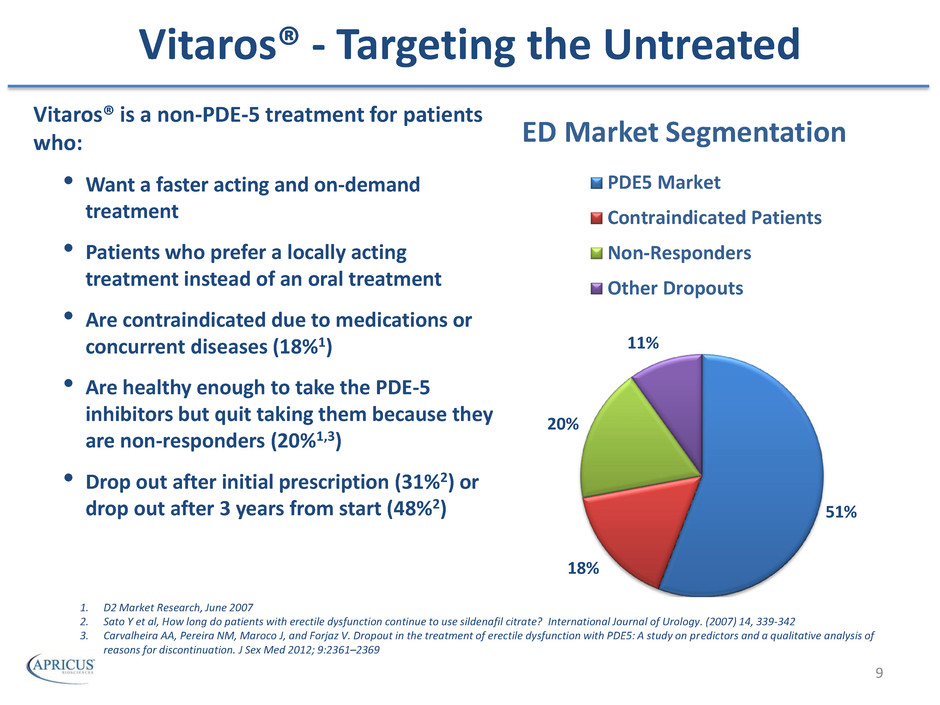
Vitaros® - Targeting the Untreated Vitaros® is a non-PDE-5 treatment for patients who: • Want a faster acting and on-demand treatment • Patients who prefer a locally acting treatment instead of an oral treatment • Are contraindicated due to medications or concurrent diseases (18%1) • Are healthy enough to take the PDE-5 inhibitors but quit taking them because they are non-responders (20%1,3) • Drop out after initial prescription (31%2) or drop out after 3 years from start (48%2) 51% 18% 20% 11% ED Market Segmentation PDE5 Market Contraindicated Patients Non-Responders Other Dropouts 1. D2 Market Research, June 2007 2. Sato Y et al, How long do patients with erectile dysfunction continue to use sildenafil citrate? International Journal of Urology. (2007) 14, 339-342 3. Carvalheira AA, Pereira NM, Maroco J, and Forjaz V. Dropout in the treatment of erectile dysfunction with PDE5: A study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med 2012; 9:2361–2369 9

Vitaros® Partnered with Major Pharmaceutical Companies Partnered / Available 10

11
Femprox® Overview • Femprox® (alprostadil/DDAIP.HCl) for the treatment of female sexual interest/arousal disorder (FSIAD) • Topical, on-demand route of administration • Increases blood flow to the genitals - this is recognized as a major component of sexual arousal • Favorable safety and tolerability profile • No drug related serious adverse events were reported • Attractive Market Opportunity • Over 53 million women (44.2%) in the U.S. over age 18 have some type of sexual dysfunction1 • 31 million women (26.1%) in the U.S. over age 18 have arousal problems1 • No product currently approved in the U.S. for FSIAD • Efficacy and safety demonstrated in ~400-patient Proof Of Concept Study • Femprox met all primary and secondary endpoints and resulted in statistically significant and clinically relevant response compared to placebo • Seven clinical studies completed to date • Strong IP Estate: issued patents through 2026 and patent applications filed for extended protection through 2032 • Confirming Development Strategy and Initiating Partnering Activities • End of Phase II meeting with FDA completed in August 2013 • Obtaining European Guidance in Q1 2014 • Initiating licensing process in early 2014 1US Census Bureau 2010 American Community Survey (121,078,439 women > age 18 US); Shrifren JL, et al. Obstet Gynecol.2008; 112:970-978 12

Financial Snapshot • NASDAQ: APRI • Shares outstanding: 37.5M† • Shares fully-diluted: 47.1M† • Market Cap: ~$90M † • Cash position: $23M† • Cash runway into 2015 based upon our current operating plan † As of January 7, 2014 13

14 2014 Strategic Focus Strategic Objective… Anticipated Value Drivers… Launch Vitaros® RTD in 2015 to extend its long-term market potential and to fully penetrate ROW markets 2. Complete the development of the Vitaros® Room Temperature Device (RTD) in 2014 Generate meaningful long-term revenue to support corporate strategy 1. Launch Vitaros® in Europe and Canada in 2014 3. Leverage existing platform to build pipeline in men’s and women’s health Develop existing and/or acquired assets focused on enhancing men’s and women’s health and well-being Create near-term shareholder value through partnering our existing commercial and development-stage assets (e.g. Vitaros® ROW, Femprox EU) 4. Continue to seek additional strategic partnerships for Vitaros® and Femprox® Position company to create shareholder value through the pursuit of strategic partnerships 5. Establish Apricus as a leader in the development of novel men’s and women’s Rx therapeutics

Why Invest In APRI? • Near-Term Value-Creating Milestones – National Phase Vitaros® Approvals continue into Q1 2014 – Additional Vitaros® Partnerships in 2014 – Vitaros® Product Launches throughout 2014 – Femprox® Strategic Plan and Partnering Initiative in early 2014 • Innovative Product Line – Unique products that improve male and female sexual health • Disciplined and Experienced Management Team • Focused Business Strategy • Long-Term Potential Value Drivers – Vitaros® royalty stream and product life cycle management – Ability to leverage our existing platform and our clinical development, regulatory and licensing competencies 15

For further information, please contact: Steve Martin, SVP & CFO ir@apricusbio.com +1-858-222-8041 Lourdes Catala, Argot Partners lourdes@argotpartners.com +1-646-439-0410 Apricus Biosciences, Inc. 11975 El Camino Real, Suite 300 San Diego, CA 92130 16















