
Leading development of novel therapeutics for men’s and women’s health Apricus Biosciences, Inc. (NASDAQ: APRI) Corporate Presentation May 15, 2014

Forward-Looking Statements This corporate presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act, as amended. Statements in this presentation that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things: references to the timing of planned launches and initial shipments of Vitaros® in various countries by Apricus’ commercial partners, particularly the United Kingdom, the planned commencement of a Phase 2a clinical trial and approved pathway for RayVa™ and the planned out-license of Femprox® in Europe; the potential for Vitaros to achieve commercial success generally or in any specific territory; and the Apricus’ 2014 financial outlook, including cash projections. Actual results could differ from those projected in any forward-looking statements due to a variety of reasons that are outside the control of Apricus, including, but not limited to: its ability to further develop its product Vitaros for the treatment of ED, such as the room temperature version of Vitaros, and its product candidate RayVa for the treatment of Raynaud’s phenomenon, as well as the timing of such events; Apricus’ ability to carry out clinical studies for RayVa, as well as the timing and success of the results of such studies; Apricus’ dependence on its commercial partners to carry out the commercial launch of Vitaros in various territories, and the potential for delays in the timing of commercial launch; competition in the ED market and other markets in which Apricus and its partners operate; Apricus’ ability to obtain and maintain intellectual property protection for Vitaros; Apricus’ ability to raise additional funding that it may need to continue to pursue its commercial and business development plans; Apricus’ ability to obtain the requisite governmental approval for Femprox and RayVa; and market conditions. These forward-looking statements are made as of the date of this press release, and Apricus assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements. Readers are urged to read the risk factors set forth in Apricus’ most recent annual report on Form 10-K, subsequent quarterly reports filed on Form 10-Q, and other filings made with the SEC. Copies of these reports are available from the SEC’s website at www.sec.gov or without charge from Apricus. 2

3 Our vision is to be a leader in the development and commercialization of innovative products in the field of men’s and women’s health Lead Products / Product Candidates: • Vitaros® - Topical Treatment for Erectile Dysfunction (ED) – Approved in Europe and Canada for entire ED patient population – Existing commercial partnerships with Abbott, Takeda, Sandoz, Majorelle, Recordati and Bracco – Takeda UK first to launch with KOL sampling program in May 2014 – Product launches expected in Europe and Canada throughout 2014 • RayVa™ - Topical Treatment for Secondary Raynaud’s Phenomenon – IND accepted by FDA in May 2014, granting clearance to begin Phase 2a clinical trial – Clear and defined clinical development plan and regulatory pathway to approval – Phase 2a trial expected to commence in H2 2014 • Femprox® - Topical Treatment for Female Sexual Interest / Arousal Disorder (FSIAD) – Successful completion of Phase 3 proof-of-concept trial in China – FDA and EU regulatory guidance received – Seeking a global strategic development partner for Femprox® Apricus Overview

Executive Management & Board of Directors Experienced team leading life science companies 4 Richard Pascoe Chief Executive Officer & Director Steve Martin Senior Vice President, Chief Financial Officer & Secretary Richard Martin, Ph.D. Vice President, Chemistry & Manufacturing Susan Meier-Davis, DVM, Ph.D. Vice President, Safety Edward Cox, MBA Vice President, Commercial Development Neil Morton, MBA Vice President, Business Development Kleanthis G. Xanthopoulos, Ph.D. Chairman of the Board of Directors Rusty Ray Director Deirdre Y. Gillespie, M.D. Director Paul V. Maier Director Wendell Wierenga, Ph.D. Director Management Team Board of Directors

5 First-in-class topical cream treatment for erectile dysfunction ®

Vitaros - Alprostadil/DDAIP.HCl • Only topically delivered cream for the treatment of erectile dysfunction • Formulated with our proprietary drug delivery system, NexACT® • Studied in over 3,300 patients • Significant efficacy and safety profile, including difficult to treat populations • Rapid onset (generally 5-15 minutes) • Addresses diabetics, hypertensives, patients with cardiac issues or on nitrates/alpha blockers, prostatectomy patients and PDE-5 (e.g. Viagra®) failures Attractive Commercial Opportunity • US PDE-5 market estimated at $2.5B1; Europe at $1.5B1 • Approved in Canada and Europe (under DCP) • Out-licensed in ex-US territories • Exploring re-acquiring rights in US from Actavis Second Generation Dispenser in Development • No refrigeration required; targeting 24+ month shelf-life • Complete the development of the Vitaros room temperature device (RTD) in 2014 • Key driver of Vitaros global market growth and expansion in 2016 and beyond Vitaros®: Treatment for Erectile Dysfunction 6
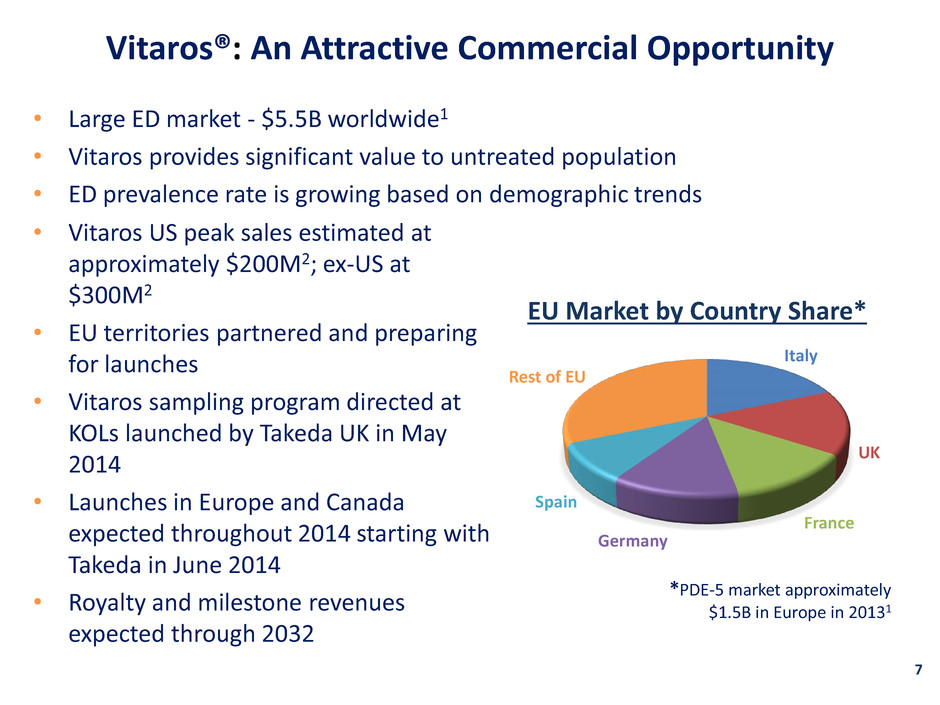
Vitaros®: An Attractive Commercial Opportunity 7 • Large ED market - $5.5B worldwide1 • Vitaros provides significant value to untreated population • ED prevalence rate is growing based on demographic trends $0.2B $0.3B Italy UK France Germany Spain Rest of EU EU Market by Country Share* • Vitaros US peak sales estimated at approximately $200M2; ex-US at $300M2 • EU territories partnered and preparing for launches • Vitaros sampling program directed at KOLs launched by Takeda UK in May 2014 • Launches in Europe and Canada expected throughout 2014 starting with Takeda in June 2014 • Royalty and milestone revenues expected through 2032 *PDE-5 market approximately $1.5B in Europe in 20131

Vitaros®: Targeting the Untreated 8 Contraindicated due to medications or concurrent diseases (18%)3 Non-responders (20%)4 Drop out after initial prescription (31%) or drop out after 3 years from start (48%)5 51% 18% 20% 11% ED Market Segmentation PDE5 Market Contraindicated Patients Non-Responders Other Dropouts (Present Existing Market) There is a significant ED patient population with an unmet need

Commercial Partnerships with Large Pharmaceutical Companies Partnered / Un-partnered Germany, Austria, Benelux & The Nordics United Kingdom Italy France, Monaco & Parts of Africa Spain, Russia, Turkey, CEE, CIS, Parts of Europe & Africa Canada USA (sold) Commercial Partnerships with Leading Pharmaceutical Companies 9
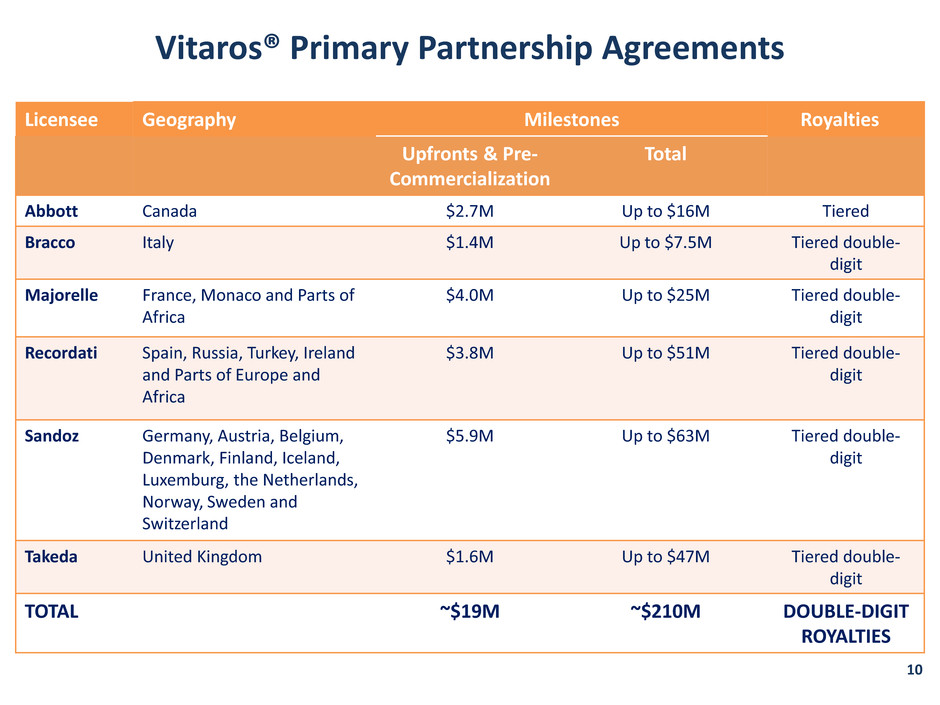
Vitaros® Primary Partnership Agreements Licensee Geography Milestones Royalties Upfronts & Pre- Commercialization Total Abbott Canada $2.7M Up to $16M Tiered Bracco Italy $1.4M Up to $7.5M Tiered double- digit Majorelle France, Monaco and Parts of Africa $4.0M Up to $25M Tiered double- digit Recordati Spain, Russia, Turkey, Ireland and Parts of Europe and Africa $3.8M Up to $51M Tiered double- digit Sandoz Germany, Austria, Belgium, Denmark, Finland, Iceland, Luxemburg, the Netherlands, Norway, Sweden and Switzerland $5.9M Up to $63M Tiered double- digit Takeda United Kingdom $1.6M Up to $47M Tiered double- digit TOTAL ~$19M ~$210M DOUBLE-DIGIT ROYALTIES 10

Initial production of commercial batches completed – Q1 2014 Joint EU Partner Marketing Summit – March 13, 2014 Vitaros Symposium held at the European Association of Urology (EAU) Congress in Stockholm – April 11, 2014 First Vitaros shipments accepted by commercial partner, Takeda UK – April 2014 Takeda Vitaros sampling program directed towards KOLs launched – May 2014 Product launches by commercial partners in Europe and Canada throughout 2014 starting with Takeda UK on June 16, 2014 Initial shipments of commercial product to other partners ongoing through Q4 2014 Initial royalty revenues expected in Q4 2014 11 Vitaros® “Road to Launch”

12 Potential first-in-class topical cream treatment for Raynaud’s Phenomenon Secondary to Scleroderma ™ (alprostadil cream)

RayVa – Alprostadil/DDAIP.HCl • Formulated with our proprietary drug delivery system, NexACT® • Topical, on-demand route of administration • RayVa-induced blood flow observed in preclinical models of Raynaud’s phenomenon using a cold challenge test Clear and Defined Pathway to Approval • Initial Phase 2a proof-of-concept trial in approximately 50 patients with Raynaud’s Phenomenon Secondary to Scleroderma expected to begin in second half of 2014 • Phase 2b trial in approximately 80 patients expected to begin in 2015 • Two Phase 3 pivotal trials, using validated endpoints accepted by FDA, following end of Phase 2 meeting • May qualify for priority review following NDA submission, which could occur as early as 2017 Attractive Commercial Opportunity • Currently no approved treatment options for Raynaud’s phenomenon in the United States, representing an unmet medical need • RayVa peak revenue estimated at approximately $100M6 • Over 4,500 rheumatologists in the US currently treating secondary Raynaud’s patients7 • Broad IP position with potential exclusivity out to 2032 RayVa™: Treatment for Raynaud’s Phenomenon Secondary to Scleroderma (SSc) 13

14 Raynaud’s Phenomenon Raynaud's Phenomenon is the episodic vasoconstriction to the distal extremities affecting an estimated 3-5% of the U.S. population8,9 Increased incidence in woman (approx. 80% of Scleroderma patients)10 Triggers include cold, emotional stress and vibration Condition is classified as either primary or secondary: • PRIMARY (7M-12M) 8,9,11 – Not associated with an underlying indication (such as Scleroderma or Lupus) – Not associated with gangrene, necrosis, ulceration – Often goes undiagnosed and untreated • SECONDARY (~500K) 9,11 Secondary to Scleroderma (~100,000)12 – Secondary Raynaud’s Phenomenon is driven by an underlying medical condition, such as Scleroderma, lupus or rheumatoid arthritis – Decreases blood supply to distal extremities, typically the fingers and toes – Symptoms: Pain, tingling, numbness, and coldness Affected areas show at least two color changes with a common pattern of progression: White (pallor), Blue (cyanosis) and Red (hyperemia) Brittle, ridged nails

• No approved treatment in the US • Current treatment includes the following: – Life-style changes, i.e. cold avoidance, smoking cessation, limiting caffeine, stress reduction – Pharmaceutical agents: Calcium channel blockers, Angiotensin receptor antagonists, Angiotensin converting enzyme inhibitors, serotonin re-uptake inhibitors, Phosphodiesterase Type V inhibitors, nitrates, prostanoids and others 8,9,13,14 • Pharmaceutical combinations and ‘add-ons’ applied dependent upon the patient’s response to therapy • All but the nitrates are administered systemically • Treatment success is variable and often associated with side effects 15 RayVa™: Unmet Need
16 Phase 2a Proof-of-Concept Trial • Randomized, double-blind, placebo-controlled, dose-ranging, crossover design • Target enrollment: 50 patients with Raynaud’s Phenomenon Secondary to Scleroderma • Evaluating hemodynamics and temperature at the site of application in response to a cold challenge, as measured by Laser Doppler and thermography • Other endpoints include safety and pharmacokinetic assessments • Results will inform Phase 2b study dose, formulations, frequency and efficacy • Anticipated initiation of trial in H2 2014 with enrollment expected to be completed in three months • Cost approximately $1 million Phase 2b Crossover Trial • Randomized, double-blind, placebo-controlled crossover design • Target enrollment: 60-80 patients with Raynaud’s Phenomenon Secondary to Scleroderma • Primary endpoint: Raynaud Composite Index (a validated primary endpoint) • Secondary endpoints: quality of life score (QoL), digital ulcer development/healing and safety assessments • Anticipated initiation in 2015 Fe b 20 201 4 RayVa™ Clinical Program
17 RayVa™ Clinical Program • Phase 3 Study Design • Two randomized, double-blind, placebo-controlled pivotal trials • Target enrollment: ~200 patients in each trial with Raynaud’s Phenomenon Secondary to Scleroderma • Primary endpoint: Raynaud Composite Index (a validated primary endpoint) • Secondary endpoints: Quality of Life score (QoL), digital ulcer development/healing and safety assessments • Flexibility to continue into open-label long-term safety assessment if required • Long-Term Safety Assessment • The need for long-term safety study (pre-registration) to be determined at End of Phase 2 meeting with the FDA • FDA cited ICH guidance but marketed use of alprostadil may be referenced • Safety of DDAIP may be supplemented with post-marketing data from Vitaros

Potential first-in-class topical treatment for FSIAD 18
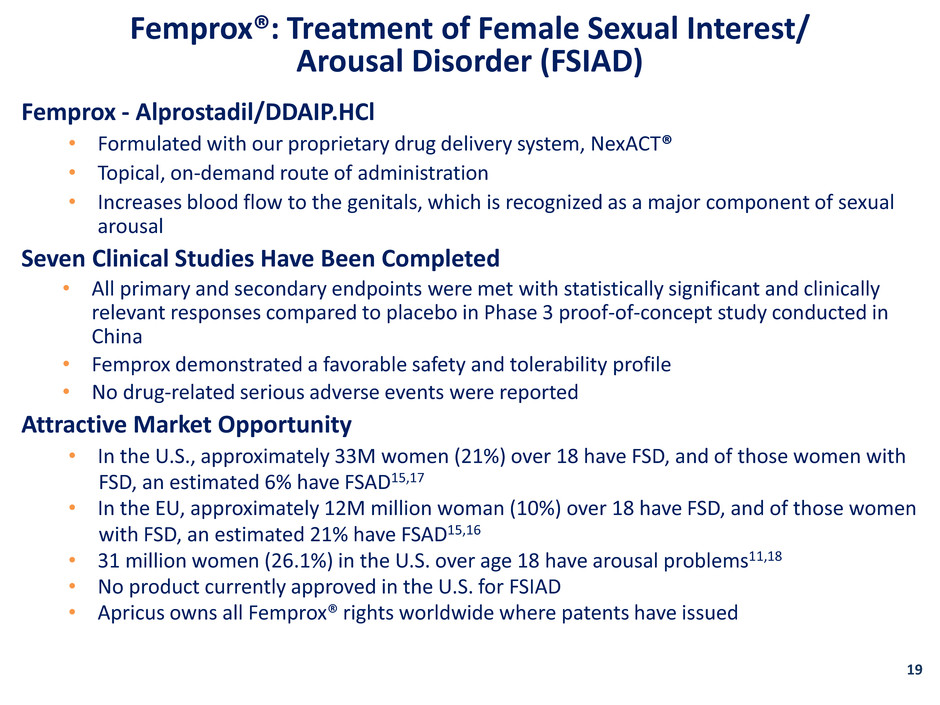
Femprox - Alprostadil/DDAIP.HCl • Formulated with our proprietary drug delivery system, NexACT® • Topical, on-demand route of administration • Increases blood flow to the genitals, which is recognized as a major component of sexual arousal Seven Clinical Studies Have Been Completed • All primary and secondary endpoints were met with statistically significant and clinically relevant responses compared to placebo in Phase 3 proof-of-concept study conducted in China • Femprox demonstrated a favorable safety and tolerability profile • No drug-related serious adverse events were reported Attractive Market Opportunity • In the U.S., approximately 33M women (21%) over 18 have FSD, and of those women with FSD, an estimated 6% have FSAD15,17 • In the EU, approximately 12M million woman (10%) over 18 have FSD, and of those women with FSD, an estimated 21% have FSAD15,16 • 31 million women (26.1%) in the U.S. over age 18 have arousal problems11,18 • No product currently approved in the U.S. for FSIAD • Apricus owns all Femprox® rights worldwide where patents have issued Femprox®: Treatment of Female Sexual Interest/ Arousal Disorder (FSIAD) 19

Uniform Guidance Received From US and Europe on Key Issues • Acceptance of Satisfying Sexual Event as the primary endpoint • Two 24-week duration Phase 3 clinical trials recommended • 500 mcg and 1000 mcg doses acceptable • Agreement on ICH-compliant long-term safety assessment Potential Upside in Pursuing a “Europe First” Strategy • One Phase 3 may be acceptable for filing if data is robust (p< 0.05) • EU clinical trial may serve as one of two trials for US NDA submission • Based upon results of PK study, safety database from Vitaros may be applicable to Femprox filing in Europe • Proprietary permeation enhancer (DDAIP.HCl) has been accepted in the EU demonstrating the benefit/risk of the excipient Strategic Plan for Femprox: • Femprox® licensing process initiated in March 2014 • Priority consideration will be given to global or multi-regional licenses • Apricus may seek to retain some commercial rights in US to preserve long-term asset value Femprox® Regulatory Feedback & Strategic Plan 20
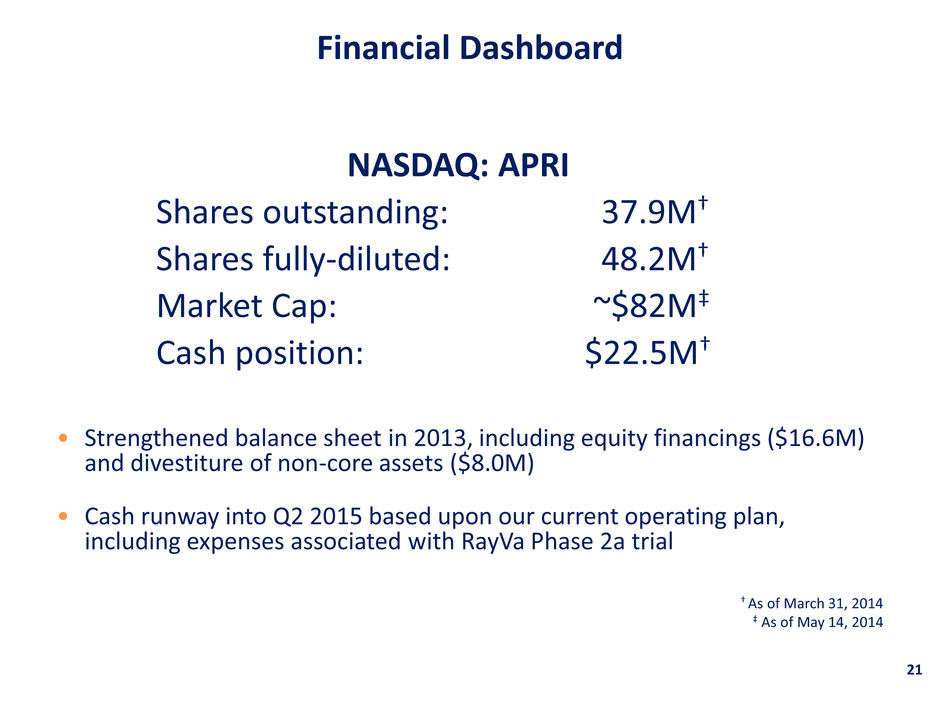
• Strengthened balance sheet in 2013, including equity financings ($16.6M) and divestiture of non-core assets ($8.0M) • Cash runway into Q2 2015 based upon our current operating plan, including expenses associated with RayVa Phase 2a trial † As of March 31, 2014 ‡ As of May 14, 2014 Financial Dashboard 21 NASDAQ: APRI Shares outstanding: 37.9M† Shares fully-diluted: 48.2M† Market Cap: ~$82M‡ Cash position: $22.5M†
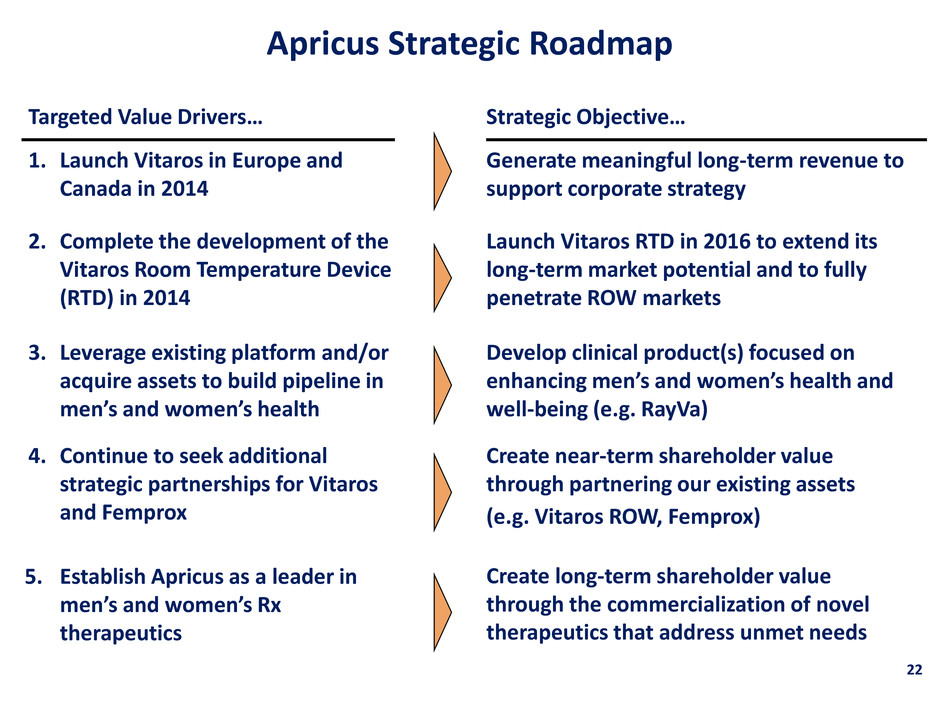
22 Apricus Strategic Roadmap Strategic Objective… Targeted Value Drivers… Launch Vitaros RTD in 2016 to extend its long-term market potential and to fully penetrate ROW markets 2. Complete the development of the Vitaros Room Temperature Device (RTD) in 2014 Generate meaningful long-term revenue to support corporate strategy 1. Launch Vitaros in Europe and Canada in 2014 3. Leverage existing platform and/or acquire assets to build pipeline in men’s and women’s health Develop clinical product(s) focused on enhancing men’s and women’s health and well-being (e.g. RayVa) Create near-term shareholder value through partnering our existing assets (e.g. Vitaros ROW, Femprox) 4. Continue to seek additional strategic partnerships for Vitaros and Femprox Create long-term shareholder value through the commercialization of novel therapeutics that address unmet needs 5. Establish Apricus as a leader in men’s and women’s Rx therapeutics

23 • Strategic focus in men’s and women’s health • Disciplined and experienced management team • Potential for multiple value creating milestones in 2014 • Vitaros launches in Europe and Canada generating long-term revenue • Exploring options to move Vitaros clinical development in the U.S • Initiating clinical development program for RayVa in H2 2014 • Pursuing Femprox out-licensing opportunity in Europe • Expected completion of Vitaros Room Temperature development program • Potential to create long-term shareholder value through: • Further leveraging our proprietary permeation enhancer technology • Building a focused commercial organization in the United States with the ability to launch novel therapeutics that address unmet needs while retaining strategic value for the benefit of our shareholders Investment Thesis

References 1 IMS Health 2013. 2 Analyst estimates (Cantor Fitzgerald, August 22, 2013). 3 D2 Market Research, June 2007. 4 J Sex Med 2012; 9: 2361–2369. 5 International Journal of Urology 2007; 14: 339-342. 6 Analyst estimates (Roth Capital, May 14, 2014). 7 American Medical Association 2011. 8 N Engl J Med 2002; 347: 1001–1008. 9 Drugs 2007; 67: 517-525. 10 Medicine 2013; 92: 191-205. 11 2012 U.S. Census Bureau: State and County QuickFacts (http://quickfacts.census.gov/qfd/states/00000.html). 12 Curr Opin Rheumatol 2012; 24: 165–170; American College of Rheumatology (http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Scleroderma). 13 Rheumatology 2005; 44: 587–596. 14 Brit J Health Assist 2011; 05: 130-132. 15 Based upon a market research study performed by a publically traded management consulting firm. 16 International Journal of Impotence Research 2004; 16: 261-269. 17 JAMA 1999; 281: 537-544. 18 JSM 2013; 10: 58-63. 24

Leading development of novel therapeutics for men’s and women’s health Apricus Biosciences, Inc. (NASDAQ: APRI) Corporate Presentation May 15, 2014
























