- SEELQ Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Seelos Therapeutics (SEELQ) 8-KOther Events
Filed: 18 Apr 13, 12:00am
 Apricus Biosciences, Inc. (NASDAQ: APRI) Corporate Presentation April 18, 2013 1 Exhibit 99.1 |
 Safe-Harbor Statement Statements under the Private Securities Litigation Reform Act, as amended: With the exception of the historical information contained in this presentation, the matters described herein contain forward-looking statements that involve risks and uncertainties that may individually, mutually, or materially impact the matters herein described. These forward-looking statements include, but are not limited to: the ability to obtain regulatory approval for Vitaros® in Europe and other markets in the time frames estimated, if at all; the ability to successfully develop other products in clinical development, including a room- temperature formulation of Vitaros® and Femprox®; our ability to enter into new licenses and partnering agreements; the ability of our partners to launch Vitaros® in any market, our ability to realize revenue under existing license agreements; and our ability to successfully commercialize Vitaros®. Attendees and readers of these materials are cautioned not to place undue reliance on these forward-looking statements as actual results could differ materially from the forward-looking statements contained herein. Attendees and readers of these materials are urged to read the risk factors set forth in the Company’s most recent annual report on Form 10-K, subsequent quarterly reports filed on Form 10-Q and its most recent SEC filings for additional risks and considerations that could cause actual results to differ materially from expected results. Company disclaims any intention to update this presentation to reflect actual subsequent events. 2 |
 Our Vision and Strategy • Our vision is to be a leader in the development and commercialization of innovative products that improve sexual health • Our strategy is to develop and seek regulatory approval for our products and commercialize those products in major global markets by establishing partnerships with leading pharmaceutical companies 3 |
 Company Highlights • Established in 1995 as a pharmaceutical company. Headquartered in San Diego, CA • NASDAQ Listed: APRI • Proprietary NexACT® drug delivery technology • Lead Products / Product Candidates: • Vitaros® - Topical Treatment for Erectile Dysfunction – Approved in Canada for entire ED patient population & awaiting approval decision in Europe – Existing commercial partnerships with Abbott, Takeda, Sandoz, Warner Chilcott and Bracco – Strategic focus is on establishing new partnerships in the territories available for licensing • Femprox® - Topical Treatment for Female Sexual Arousal Disorder (FSAD) – Potential best and first-in-class treatment for FSAD – Completed one successful Phase III proof-of-concept trial in China and moving towards additional late-stage trials – Strategic focus is on developing and licensing Femprox® throughout the world 4 |
 Upcoming Expected Milestones Vitaros® approval decision: Europe Switzerland & other territories Femprox® regulatory & clinical design plan communicated Commence sales of Vitaros® in Canada by Abbott Additional Vitaros partnerships 5 |
 6 • NASDAQ: APRI • Shares outstanding: 29.9M † • Shares fully-diluted: 37.0M † • Cash position: $15.1M † • Share-price: $3.00 ‡ • Market Cap: $89.7M ‡ † As of December 31, 2012 ‡ As of April 10, 2013 Financial Snapshot |
 Vitaros® 7 |
 Vitaros® 8 • Vitaros® (alprostadil/DDAIP.HCl) for the treatment of erectile dysfunction • Significant efficacy, including difficult to treat populations • Approved in Canada – launch date pending • Filed in Europe using DCP – approval decision expected Q2 2013 • Patent (issued and pending) coverage thru 2031 • Seeking commercial partners: Europe, Asia Pacific & Emerging Markets – Rapid onset (generally 5-15 minutes) – Studied in over 3,300 patients – Diabetics, hypertensives, patients with cardiac issues or on nitrates/alpha blockers, prostatectomy patients, Sildenafil (Viagra®) failures Second Generation • Cold Chain (2 C - 8 C) • 7 Days Room Temp • Room Temperature • Expected 24-36 month shelf-life |
 Vitaros® - Target Patient Population 9 1. D2 Market Research, June 2007 2. Sato Y et al, How long do patients with erectile dysfunction continue to use sildenafil citrate? International Journal of Urology. (2007) 14, 339-342 3. Carvalheira AA, Pereira NM, Maroco J, and Forjaz V. Dropout in the treatment of erectile dysfunction with PDE5: A study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med 2012;9:2361–2369 (Present Existing Market) $2.6B Ex-US 51% 18% 20% 11% ED Market Segmentation PDE5 Market Contraindicated Patients Non- Responders Other Dropouts Vitaros® is a non-PDE-5 treatment for patients who: • Want a faster acting and on-demand treatment • Patients who prefer a locally acting treatment instead of an oral treatment • Are contraindicated due to medications or concurrent diseases (18% 1 ) • Are healthy enough to take the PDE5 inhibitors but quit taking them because they are non-responders (20% 1,3 ) and • Drop out after initial prescription (31% 2 ) or drop out after 3 years from start (48% 2 ) |
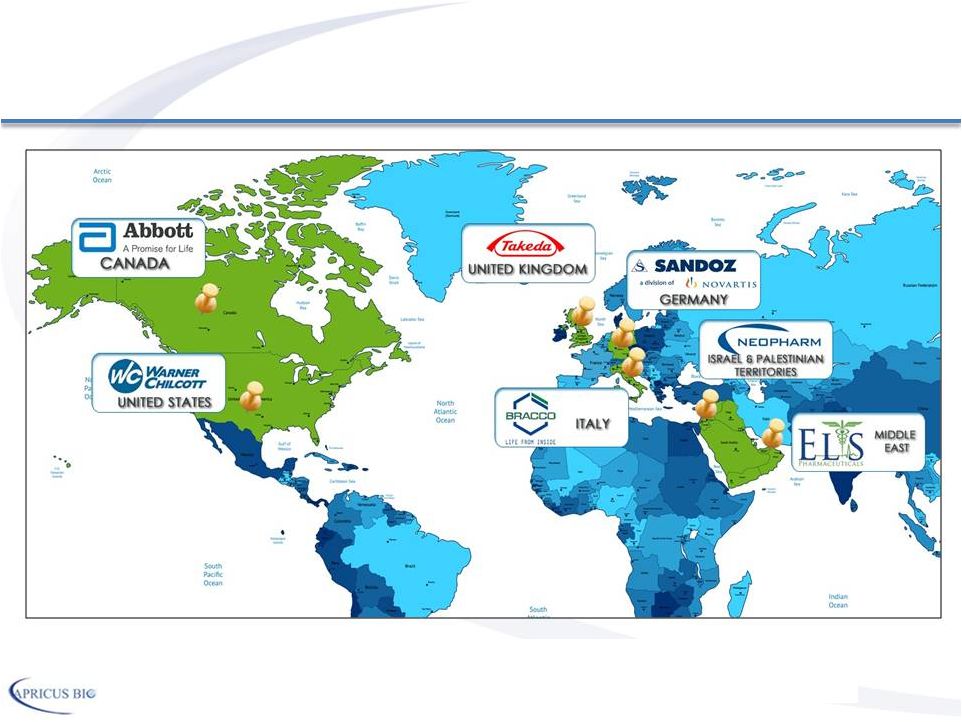 Leading Commercial & Development Partners 10 Partnerships throughout the world for Vitaros® |
 Femprox® 11 |
 Femprox® - Product Overview • Femprox ® (alprostadil/DDAIP.HCl) intended for the treatment of female sexual arousal disorder (FSAD) • FSAD affects as many as 10M women in the US and is different than Female Sexual Dysfunction • Achieved statistically significant efficacy in primary and secondary endpoints with favorable safety and tolerability • No product currently approved in the US for FSAD • Regulatory guidance meetings (US, Europe, Canada) have occurred and are planned • Patent (issued and pending) coverage through 2031 12 Seven clinical studies completed to date, including one, ~100-patient Phase II study in the U.S. and a ~400-patient Phase III study in China |
 Femprox® - Profile 13 • Indications and Usage – First-line topical treatment for Female Sexual Arousal Disorder • Mechanism of Action – Alprostadil induces vasodilation by means of a direct effect on vascular smooth muscle, clitoral engorgement – DDAIP.HCl as a patented proprietary permeation enhancer opens the cellular tight junctions, allowing drugs to permeate through epidermal barriers, enabling rapid absorption of high concentrations of alprostadil cream directly to the target site • Proof of Concept Phase III Clinical Trial and Result – Phase III study in ~400 patients completed in China – Femprox met all primary and secondary endpoints and resulted in statistically significant and clinically relevant response compared to placebo – Favorable safety and tolerability profile. Only 5 patients (1.2%) withdrawn from the Phase III study because of local adverse events – No drug related serious adverse events were reported |
 Femprox® - Safety & Efficacy • Safety – Both alprostadil (US, CDN) and DDAIP.HCl (CDN) are approved agents – Administered topical and locally – effects are local only and of short duration – Other than Femprox®, other drug candidates are given systemically (orally, transdermal, subcutaneous) resulting in an increased likelihood of adverse events and reduced tolerability (which is being confirmed clinically). • Hormones (testosterone) • Anti-depressants (SSRI’s) • Efficacy – Direct route of administration – Increases blood flow to the genitals - this is recognized as a major component of sexual arousal 14 |
 Development Strategy • Completion of Femprox® Product Plan – CMC – Non-clinical – Clinical – Commercial assessment – Publication Strategy – Life cycle management plan – IP strategy • Obtain US FDA Regulatory Guidance – End-of Phase II meeting with US FDA • Key Clinical Development Initiatives – Complete Femprox room temperature device design – Complete required supportive studies – Conduct pivotal Phase III trial(s) in the U.S. 15 APRI- Confidential |
 Investment Thesis • Re-calibrated business model: – Focus on sexual medicine – Disciplined leadership – Complete divesture of non-core assets – Strengthen development and partnering core competencies • Vitaros® - Topical Treatment for Erectile Dysfunction – Approval decision in Europe in 2013 – Additional ex-US partnering opportunities – Progress on Vitaros room temperature device • Femprox® - Topical Treatment for Female Sexual Arousal Disorder (FSAD) – Potential first and best-in-class FSAD treatment – Completed one successful Phase III trial – Obtain FDA regulatory guidance in 2013 16 • Potential near-term milestones: |
 For further information, please contact: Steve Martin, SVP & CFO smartin@apricusbio.com +1-858-222-8041 Lourdes Catala, Argot Partners lourdes@argotpartners.com +1-646-439-0410 Apricus Biosciences 11975 El Camino Real, Suite 300 San Diego, CA 92130 17 |