QuickLinks -- Click here to rapidly navigate through this document
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2001 COMMISSION FILE NUMBER:000-21429
ARQULE, INC.
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
DELAWARE
(STATE OR OTHER JURISDICTION OF INCORPORATION OR ORGANIZATION) | | 04-3221586
(I.R.S. EMPLOYER IDENTIFICATION NO.) |
19 PRESIDENTIAL WAY, WOBURN, MASSACHUSETTS 01801
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES INCLUDING ZIP CODE)
REGISTRANT'S TELEPHONE NUMBER, INCLUDING AREA CODE:
(781) 994-0300
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| (TITLE OF EACH CLASS) | | NAME OF EACH EXCHANGE ON WHICH REGISTERED |
| None | | None |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
COMMON STOCK, $.01 PAR VALUE
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
The aggregate market value of voting stock held by non-affiliates of the registrant as of March 1, 2002 was: $250,782,374.
There were 20,140,238 shares of the registrant's Common Stock outstanding as of March 1, 2002.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive proxy statement of the Registrants 2001 Annual Meeting of Shareholder to be held on May 16, 2002, which definitive proxy statement will be filed with the Securities and Exchange Commission not later that 120 days after the registrant's fiscal year of December 31, 2001, are incorporated by reference into Part III of the Form 10-K.
PART I
ITEM 1. BUSINESS
ArQule, Inc. is a drug discovery company with approximately 385 employees in four locations worldwide (Woburn and Medford, Massachusetts, Menlo Park, California and Cambridge, England). ArQule engages in drug discovery programs with partners and for its own account under agreements providing for shared and exclusive commercial rights to chemical compounds with therapeutic potential.
IMPORTANT FACTORS REGARDING FORWARD-LOOKING STATEMENTS
You should carefully consider the risks described below together with all of the other information included in this Form 10-K before making an investment decision. An investment in our common stock involves a high degree of risk. We operate in a dynamic and rapidly changing industry that involves numerous uncertainties. The risks and uncertainties described below are not the only ones we face. Other risks and uncertainties, including those that we do not currently consider material, may impair our business. If any of the risks discussed below actually occur, our business, financial condition, operating results or cash flows could be materially adversely affected. This could cause the trading price of our common stock to decline, and you may lose all or part of your investment.
This Form 10-K contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those projected in the forward-looking statements or historical performance due to numerous risks and uncertainties that exist in ArQule's operations, development efforts and the business environment, including without limitation: the ability to transition successfully from chemistry services to drug discovery, to satisfy milestones, and to deliver compounds to corporate collaborators; the ability to predict consistently and successfully absorption, distribution, metabolism, elimination, and toxicity ("ADMET") properties and to design optimized chemical entities; the progress of product research and development activities and projected expenditures; the ability to enter into future collaborations with pharmaceutical and biotechnology companies; and difficulties and costs associated with the integration of the acquired businesses and the risks and uncertainties described this document. The forward-looking statements contained herein represent the judgment of ArQule as of the date of this Form 10-K. ArQule disclaims any intent or obligation to update any forward-looking statement except to the extent required by law.
BUSINESS OVERVIEW
With the possibility of fundamental molecular understanding of the nature and causes of diseases a reality, and recent scientific advances in both the chemistry and biological aspects of drug design, ArQule has broadened the scope of its objectives. In the past we sought to become the premier partner of pharmaceutical companies for lead generation, qualification and optimization programs. While we will continue to forge strategic alliances that encompass these elements, our primary goal will be to design and produce independently small molecules that become medicines.
Before a molecule can become a medicine it must effectively interact with proteins or other large molecules. In order to be valid biological targets, these large molecules must play fundamental roles in the onset or progression of particular diseases. It is not enough that a small molecule drug candidate effectively interacts with one of these targets. It must also have a combination of characteristics that produce an acceptable risk-benefit profile in humans.
Until recently, pharmaceutical researchers were limited to studying how approximately 400 biological targets interact with chemical compounds. Genomics, the science of identifying genes and their role in biological processes, including disease, have created a wealth of potential new targets for drug discovery. Scientists now use genomics to identify specific genes and the proteins they encode,
2
which they believe are involved in a disease process and, therefore, may be suitable drug discovery targets.
The drug discovery and development process includes:
- •
- target identification and validation—confirmation that a specific target plays an important role in a given disease process;
- •
- compound discovery;
- •
- lead generation—identification of chemical compounds that interact with the target;
- •
- lead qualification—selection of compounds which have sufficient drug-like characteristics to justify further evaluation;
- •
- lead optimization—refinement and evaluation of compounds for potential as clinical candidates, that is:
- •
- effectiveness against the target;
- •
- specificity for the target;
- •
- "ADMET" characteristics:
- •
- Absorption: whether the compound will be absorbed properly in the body;
- •
- Distribution: once absorbed, how the compound will be distributed throughout the body;
- •
- Metabolism: how the compound will change within the body;
- •
- Elimination: whether the compound will be removed from the body in a harmless way; and
- •
- Toxicity: whether the compound might be toxic to the body and cause harmful side effects.
- •
- minimization or elimination of adverse characteristics and maximization of potency and selectivity.
ArQule's opportunity to participate fully in the process of discovery and optimization of small molecules that will become medicines arises from its collaborative relationships with preeminent pharmaceutical, biotechnology, and genomics-based drug discovery companies. By combining the demonstrated expertise of our partners in human disease pathways and processes with our own biologically relevant chemistry capabilities, we are well positioned to address the twin industry challenges of producing significantly greater numbers of chemically relevant drug candidates affecting novel and traditional targets and substantially reducing the time and cost of bringing a drug to market. Overcoming these challenges is a pre-requisite for success in drug discovery.
As a drug discovery company we are focused on:
- •
- continued development of our internal portfolio of research and development programs aimed at production of small molecules with optimal, drug-like characteristics;
- •
- expansion of our technology platform and process improvements;
- •
- integration of high-throughput, automated chemistry technology with predictive, computational design and experimental biology techniques;
3
- •
- initiation of additional strategic collaborations centered around our integrated technology platform including:
- •
- delivery of relevant chemical compounds,
- •
- predictive modeling for ADMET characteristics,
- •
- program rescue for drug candidates which have failed because of poor ADMET qualities, and
- •
- technology transfer of our computational and informatics compound design tools.
Strategic collaborations, such as the one we have with Pfizer, are important to ArQule. We believe the exchanges of information and technology between collaborators inherent in these relationships enhance the internal capabilities of both parties and create opportunities for breakthroughs in the drug discovery process. In addition, such alliances generate free cash flow for us to reinvest in our drug discovery portfolio and technology platform.
ArQule's Competitive Advantage
We believe that the fundamental processes of drug development must be changed. We believe ArQule's primary competitive advantage lies in our integrated technology platform which consists of the following:
- (1)
- high-throughput automated chemistry;
- (2)
- predictive ADMET models;
- (3)
- computational and informatics capabilities for intelligent design of compounds; and experimental biology techniques incorporating in-vitro ADMET screening.
(1) High-throughput Automated Chemistry
Our Automated Molecular Assembly Plant ("AMAP") Chemistry Operating System, which forms the foundation of our compound production technology, is a highly automated and integrated series of chemistry workstations and processes designed to enable rapid, parallel generation of thousands of novel, pure, diverse and spatially-addressed arrays of compounds. It allows us to perform high-throughput, automated production of new chemical compounds. The drug discovery process requires efficient production of a vast number and wide range of chemical compounds. Lead generation requires a large number of screening compounds; lead optimization requires the rapid creation of structurally similar compounds. The growth in available targets will only increase these needs.
�� The AMAP System represents the integration of proprietary and patented technologies in seven areas that results in a consistent, well-defined, well-monitored, reproducible and flexible process consisting of the following steps:
- •
- library design;
- •
- process chemistry;
- •
- production;
- •
- purification;
- •
- quality control;
- •
- culling and reformatting; and
- •
- replication.
4
With the AMAP system we believe we can:
- •
- deliver discrete compounds of known structure and high purity in sufficient quantity for lead optimization;
- •
- quickly understand how variations in chemical structure alter compound profiles;
- •
- design and produce compound libraries with pre-selected characteristics for increased likelihood of generating marketable drugs;
- •
- reduce screening costs by utilizing smaller, more focused compound libraries; and
- •
- perform cost-efficient profiling of compounds for desirable ADMET and other characteristics.
Because the AMAP Chemistry Operating System incorporates proprietary processes, software and equipment and relies on highly trained operators, we believe that duplication of the system by others would be difficult.
(2) Predictive ADMET Models
We have developed various computational models for ADMET characterization of compounds based on their structure. We use our ADMET models to screen compounds to predict their ADMET properties before they are synthesized. Early access to predictive data is designed to optimize compounds for multiple ADMET properties in parallel, in order to expedite the optimization process and reduce late stage failures. These models will enable ArQule to profile "virtual libraries" of compounds in order to determine which compounds to make from among the thousands or millions of compounds that we could make. These models are also useful for compound redesign during lead optimization.
Currently, our suite of computer programs model human intestinal absorption, blood brain barrier penetration and metabolism by the isoenzymes of cytochrome P450. Additional components are constantly being added to this integrated suite.
(3)(a) Computational and Informatics Capabilities
Our library design and lead optimization capabilities are supported by several software products that have been developed at ArQule. Currently, these consist of MapMaker, ALOFT (ArQule Lead Optimization Framework and Toolkit) and AQUIRE (ArQule United Information Repository and Exchange).
MapMaker allows scientists to design combinatorial libraries in an efficient manner, automating a series of tasks often performed by a computational chemist. Once presented with a novel chemistry, MapMaker provides easy access to reagent selection algorithms, creates a combinatorial expansion based on those selections, calculates a number of physical properties, and then selects the most diverse set of molecules from the resulting set for actual synthesis.
ALOFT provides our scientists with a powerful and extensible framework for the design of new chemical compounds with drug-like properties. A modular design allows new computational approaches to be easily included in the suite of tools provided. ALOFT presents not only access to all relevant experimental information, but through the use of an increasing number of predictive models, provides guidance in "what to make next."
AQUIRE tracks all information generated on any ArQule compound throughout its design, creation and use against a biological target. A key component of AQUIRE is a suite of tools to query the growing database of information generated by ArQule and its drug discovery partners. AQUIRE supports a number of tools for both structured and ad hoc queries by ArQule scientists to mine this
5
information and to generate further insights into the interaction of ArQule chemistry and biological targets.
(3)(b) Experimental Biology Techniques
In addition to the computational models we have developed, we have produced "in vitro" experimental ADMET ("eADMET") models to rapidly screen compounds for ADMET characterization. Our platform has capabilities for high-throughput screening of compounds for activity against selected targets. ArQule's biochemistry function supports these screening efforts and provides the additional capability of in-depth characterization of the mechanism of action and biological effects for specific compounds.
While many companies possess some or all of this technology, or technology of similar function, we believe that our advantage is the high degree of integration being incorporated in our platform.
Chemistry in the Post-Genomic Era
Producing small molecule drugs requires expertise in biologically relevant chemistry. Access to small molecules and chemistry expertise is essential to target-rich companies without small molecule chemistry capabilities, because what ultimately is approved for market is the molecule - i.e., the chemistry - not a target or a gene sequence. Most prescription medicines are (and we believe will continue to be) small molecules due to ease of administration and relatively low production cost. These qualities compare favorably to injectible proteins and monoclonal antibodies which are larger in size and more costly to manufacture.
Small molecules are chemical structures. Most medicines will therefore derive from designing and producing small molecules which effectively interact with the selected biological target and have an appropriate mix of characteristics required to produce an acceptable risk-benefit profile in humans. Before a molecule can become a medicine, it must have:
- •
- Appropriate physicochemical and biochemical properties;
- •
- Affinity, potency, and selectivity for the intended target or disease pathways, and a favorable ADMET profile in humans.
Optimizing a selected chemical entity requires using a variety of technologies that together comprise biologically relevant chemistry. These technologies should give us the ability to recognize early in the drug discovery process, and therefore cost-effectively, which chemical entity should be optimized for drug development. Such technologies should also reveal those circumstances in which no acceptable optimized chemical entity can be made. In our view, this should permit early reallocation of resources to a different discovery program with an increased therapeutic potential.
Currently, the number of small molecules that, in theory, could be made into medicines is estimated at 1060. This number is so large that predictive/computer modeling, including predictive ADMET and structure-based design approaches, will be a prerequisite for selection of the right molecule for the right target.
In summary, biologically relevant chemistry focused on the abundance of targets generated from genomics efforts will turn molecules into medicines.
ArQule's Integrated Science and Technology Strategy:
- 1)
- Invest in ArQule's drug discovery platform;
- 2)
- Advance our wholly-owned and partnered drug discovery portfolio;
- 3)
- Improve the drug discovery process;
6
- 4)
- Leverage existing relationships; and
- 5)
- Out-license or sell drug candidates at key value points on the inflection curve.
1) Invest in ArQule's Discovery Platform
We believe ArQule has built a strong scientific foundation. We believe that we possess a broad set of core competencies: established leadership in small molecule chemistry; expertise in high throughput automated chemistry; high quality design tools; and integrated technology platform including predictive ADMET modeling. (Fig 1.)
Fig 1. Summary of ArQule's Core Competencies
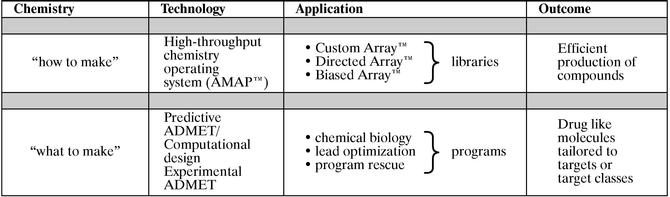
With our mature "how to make" technology and applications, ArQule can develop, synthesize, purify, and characterize large numbers of molecules rapidly and reliably with reproducibility. With our emerging "what to make" technology and applications, ArQule aims to predict the structure and characteristics of select drug-like small molecules that will have an increased likelihood of surviving through the lead optimization process. Integrating our mature and emerging technologies will be required to achieve the purpose of ArQule's technology—the efficient production of potent and selective drug candidates. We will continue to improve our drug-discovery technology platform through in-licensing opportunities, alliances and acquisitions.
2) Advance our wholly-owned and partnered drug discovery portfolio
To improve our design capabilities for optimal chemical entities ArQule uses a multi-disciplinary approach that combines high-throughput, automated chemistry and intelligent formulation of molecules with parallel evaluation of ADMET parameters.
These optimal chemical entities are intended to have the right balance of physicochemical characteristics, affinity for a relevant biological target, selectivity for the target and appropriate ADMET characteristics in humans. These optimal chemical entities will be target-relevant chemical structures whose drug-like characteristics have been optimized prior to their entry into pre-clinical studies and, as such, potentially have a greater likelihood of success in clinical trials.
Through this parallel approach to optimal chemical entity design, ArQule intends to avoid the typical causes of compound failure and compress the current discovery timelines in order to develop a portfolio of drug candidates for less than the average industry cost.
We also hope to embark on drug rescue programs with partners for compounds that have been shelved due to poor ADMET characteristics. By utilizing our predictive ADMET models we propose to analyze existing data to optimize alternative compounds in order to reduce or eliminate the ADMET liabilities. This strategy is advantageous because generally these compounds have already demonstrated selectivity and potency for their biological targets.
7
3) Improve the discovery process
The shortcomings in the current compound discovery process create two major problems for pharmaceutical researchers. First, due in part to the lack of early information about lead compounds and the lack of alternative chemotypes, very few lead compounds meet the minimum criteria to become clinical candidates. Second, of the lead compounds that meet the minimum criteria, too many are only marginally acceptable and are therefore more likely to fail during clinical development. Without improvements in this process, the current failure rate of drug candidates will continue and there will not be enough new drugs to fuel continued revenue and profit growth of the major pharmaceutical companies.
Because researchers conduct lead optimization in sequential rather than parallel steps, the traditional compound discovery process is long and expensive. In the conventional lead optimization process, medicinal chemists analyze a lead compound's structure and use their experience to suggest changes that might produce the desired result for potency or ADMET characteristics. Because changes to a compound's structure that enhance one desired feature may impair other features, the traditional, sequential lead optimization process is inefficient, time-consuming and unpredictable.
ArQule is increasing the efficiency of drug discovery by complementing internal chemistry competencies with newly-acquired biology competencies. By utilizing computational design, predictive ADMET modeling and other aspects of our integrated technology platform we believe we can render obsolete the sequential process described above used in drug discovery today and implement a parallel process for identifying and optimizing lead compounds.
We believe that efficiently generating drug-like molecules requires moving from random, trial and error, time consuming, sequential drug discovery to a predictive, parallel approach. A predictive, parallel approach should rule out potential failure of drug candidates earlier in the discovery process as well as save time and cost.
4) Leverage existing relationships
ArQule engages in both partnered and wholly-owned drug discovery programs resulting in shared and exclusive commercial rights for molecules that may advance into clinical trials. In addition, ArQule has collaborations with partners centered on our delivery of custom chemical compounds. We will utilize the cash flow, if any, generated by these alliances to invest further in our drug discovery portfolio and platform.
5) Out-license or sale at key value inflection points
We intend to capitalize on our internal drug discovery efforts and certain external collaborations to capture more value by advancing drug candidates into clinical trials through third party licensees or purchasers of our rights. If this effort is successful, ArQule could receive lump sum payments, milestone payments and/or royalties.
ARQULE'S BUSINESS STRATEGY
ArQule's strategy is based on aligning internal goals with industry demand in a manner that will sustain growth for ArQule over the long term. Our internal goals include the following:
- •
- improving lead generation position i.e., produce biologically relevant and diverse compound libraries that increase hit rates for selected targets;
- •
- advancing lead optimization efforts by developing programs with significant ownership interest;
- •
- integrating our technologies to design and implement an improved process for delivering IND candidates.
8
We believe this business strategy will enable ArQule to pursue a higher value position over time. ArQule's short and medium term focus is on core business performance with efforts towards chemistry-based drug discovery, and investment in building a drug discovery portfolio while improving aspects of efficiency in drug discovery and investing in our technology platform. In the longer term, advances in efficiency can then be applied to improving the overall productivity of pharmaceutical research and development, which we believe could transition ArQule to a leadership position.
Transition from a chemistry service based business to a drug discovery company
ArQule will continue to pursue strategic collaborations with biotechnology and pharmaceutical companies. While we generate revenue from chemistry capabilities (compound libraries and technology transfer), we will continue improvement of our integrated technology platform, i.e., target-biased arrays of chemical compounds, in-vitro-biology for ADMET screening, computational design tools and predictive ADMET modeling, with the intent to develop a broader based platform.
Partnerships and shared technology arrangements provide ArQule with access to targets, supplementing internal chemistry expertise with external biology expertise. Using this combined experience with its diversified "what to make" technology, ArQule will attempt to integrate its efforts to deliver chemically relevant biological candidates with drug-like qualities.
Process Redesign
By expanding our biology expertise in connection with our chemistry strength, we believe we will broaden our technology platform for integrated drug discovery. Our focus in the medium term will be to use this broader platform to build and enhance our drug discovery portfolio.
By building and driving the integration of our technology platform, we are attempting to redesign a more efficient drug discovery process. A better process for drug discovery could result in more innovation and a competitive advantage.
MARKET OPPORTUNITY
The challenge that lies in the post-genomic era is for drug discovery efforts to deliver chemically relevant drug candidates for novel and traditional targets.
Industry demands improvement in the productivity of pharmaceutical research and development. ArQule's primary focus is on efficiency in drug discovery. We believe by establishing a fully integrated drug discovery company yielding a more efficient drug discovery process we will raise the industry standard, raise ArQule's current position from industry player/contributor to potential industry leader, and potentially attract sustainable revenue-generating strategic alliances. In addition we believe if we are successful we can potentially establish ArQule as a supplier for large pharmaceutical companies and biotechnology companies which may cost effectively deliver an increased volume of low risk IND candidates. These candidates may address unmet medical needs benefiting patients, doctors and the medical community, and provide stability, growth, and profitability for the Company over the long term.
Pharmaceutical Industry
We believe pharmaceutical companies may be unable to commit the chemistry resources for internal efforts that will be optimal in the post-genomic era. As a result, they may collaborate with companies like ArQule to access integrated drug discovery capabilities. While medicinal chemistry might be considered the core competency of the largest pharmaceutical companies, there has been a significant trend towards outsourcing to the best chemistry companies.
9
Biotechnology Industry
We believe many biotechnology companies depend on co-development efforts and partners for lead discovery/optimization, diversity and depth of pipeline in order to thrive independently. ArQule is and should be a good partner in such efforts.
KEY DRUG DISCOVERY PRODUCTS & PROGRAMS
To support ArQule's strategy, ArQule's existing and new drug discovery programs encompass several phases of drug discovery; assay development, lead generation, lead optimization, hit to advanced lead, and qualification of GLP tox and IND candidates.
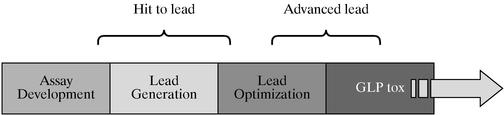
To sustain growth and profitability over the long term drug discovery efforts include multiple programs with multiple entry points.
Compound Discovery Products
ArQule is an established market leader for producing compounds with high throughput technology. In 2001, ArQule added computational design tools and predictive modeling capabilities. Using a platform approach, ArQule has collaborations in three defined areas that we believe have enhanced our ability to become an emerging drug discovery company:
- •
- chemistry operating system (AMAP™);
- •
- diverse libraries of chemical compounds; and
- •
- technology for intelligent design of compounds.
Lead Generation/Optimization Programs
ArQule has discovery programs that encompass utilization of our integrated technology platform (biased libraries of compounds, computational design tools, and predictive modeling). These consist of wholly owned programs focused on ion channel and kinase target classes and shared risk programs with genomic based companies which give ArQule access to targets and assays to match with our chemically diverse libraries. Additionally, ArQule has shared and partnered programs that may result in advanced leads providing ArQule the opportunity to pursue discovery in defined therapeutic areas.
ArQule also is engaged in co-development efforts with a partner giving us access to the GPCR target class area and relevant structural target information. The GPCR target class area represents the largest target class of known drugs on the market.
To advance our portfolio ArQule may collaborate with target rich biotechnology companies which should give ArQule access to different classes of targets.
Additionally, ArQule is seeking a strategic alliance with a large pharmaceutical company to progress late stage compounds to investigational new drug (IND) status.
10
ArQule intends to access certain targets from the public domain for certain disease states or target classes and/or in-license or generate internal targets, all in order to progress compounds towards IND status & Phase I development.
Process Redesign Programs
ArQule is attempting to redesign integrated systems and processes that improve the overall efficiency and cost effectiveness of drug discovery. Integrating drug discovery science and technology provides the opportunity to redefine a new process that should generate an internal pipeline of IND candidates.
OUR COLLABORATIONS
The following table summarizes our collaborations with pharmaceutical companies:
COMPANY
| | PRODUCTS/SERVICES PROVIDED
|
|---|
| Pfizer Inc | | Technology transfer of AMAP Chemistry Operating System, Design Tools, Lead Generation Capabilities and Custom Array libraries |
| Bayer AG | | Custom Array libraries |
| Wyeth Pharmaceuticals | | Mapping Array and Directed Array libraries |
| Solvay Pharmaceuticals B.V. | | Mapping Array, Compass Array and Directed Array Libraries and a non-exclusive license to our AMAP Chemistry Operating System |
| G.D. Searle, a division of Pharmacia Corp. | |
Mapping Array, Compass Array and Directed Array libraries and lead optimization services |
| Sankyo Company, Ltd. | | Mapping Array, Compass Array and Directed Array libraries |
| Johnson & Johnson, Inc. | | Mapping Array libraries and Compass Array libraries |
| GlaxoSmithKline | | Compass Array libraries and Lead Optimization services |
| Abbott Laboratories | | Mapping Array and Directed Array libraries |
| Roche Bioscience | | Directed Array libraries |
Pfizer. In July 1999, we entered into a four and one-half year technology acquisition agreement with Pfizer Inc. We manage and staff a dedicated facility containing an AMAP Chemistry Operating System for Pfizer in Medford, Massachusetts. The facility produces Custom Array libraries exclusively for Pfizer. Pfizer owns all rights in compounds produced at this facility. In addition, we have trained Pfizer staff to use our AMAP Chemistry Operating System. On December 31, 2003, Pfizer will receive a non- exclusive license to the AMAP Chemistry Operating System. In December 2001, we entered into a new seven year agreement with Pfizer which superceded our July 1999 agreement. In addition to continuing to provide custom array libraries for Pfizer, we will also transfer our library design tools on a non-exclusive basis to Pfizer. Pfizer has also committed to perform one lead optimization program with ArQule. We may earn up to $345 million over the length of the contract, depending on the achievement of certain delivery milestones. Pfizer has committed to maintain certain cash payments resulting from our prior collaboration as it relates to the transfer of our AMAP chemistry operation system. As of March 1, 2002, we have received $80.5 million under these agreements. Pfizer has also agreed to make equity investments totaling $18 million depending on the achievement of certain delivery milestones. Pfizer made a $10 million equity investment in December 2001. Pfizer may terminate the new agreement after four years for any reason. Pfizer will pay no milestones or royalties to us on compounds that they develop and market.
11
Bayer. In October 1999, we entered into a three-year collaboration with Bayer AG to produce Custom Array libraries. In October 2001, we extended the production period until June 30, 2003. Bayer will own all rights in compounds for an initial period, after which we will co-own rights in compounds that Bayer has not claimed in a patent application. We received a $3 million upfront payment and will receive up to an additional $27 million during the term of the agreement for delivery and success fees. As of March 1, 2002, we have received $17.5 million under this agreement. Bayer will pay no milestones or royalties to us on compounds that they develop and market.
Wyeth Pharmaceuticals. In July 1997, we entered into a four and one half year agreement with Wyeth Pharmaceuticals ("Wyeth"). Under this agreement, Wyeth subscribed to our Mapping Array and Directed Array Programs. We discontinued our Mapping Array program as of 2002, and as a consequence and in agreement with Wyeth, we did not renew our collaboration. Wyeth made a $2 million equity investment in ArQule in June 1998. As of March 1, 2002, we have received all $26.1 million that we are due under this agreement. Wyeth has agreed to pay us development milestones and royalties from the sales of products resulting from compounds we shipped during the collaboration. To date, we have not received any milestone or royalty payments.
Solvay. In November 1995, we entered into a five-year agreement with Solvay Duphar B.V. Under this agreement, Solvay subscribed to our Mapping Array and Directed Array programs and received a non-exclusive license to our AMAP Chemistry Operating System. This agreement was superseded by an amended and restated agreement with Solvay Pharmaceuticals B.V., which became effective on January 1, 2001. The amended agreement extends the collaboration through December 31, 2003. Under the amended agreement, Solvay receives our Compass Array libraries and continues to access our Mapping Array libraries and Directed Array programs. We discontinued our Mapping Array program as of 2002 as planned. The remainder of the contract was not affected. As of March 1, 2002, we have received $19.9 million under these agreements. Solvay has also agreed to make additional payments if we achieve certain development milestones and to pay royalties on sales of any drugs that result from the relationship. To date, we have not received any milestone or royalty payments. In connection with the original collaboration, signed in November 1995, an affiliate of Solvay, Physica B.V., made a $7 million equity investment in ArQule.
Pharmacia. We entered into a five-year collaboration with Monsanto Company (now Pharmacia Corporation) in December 1996. Under this agreement, we provided Monsanto with access to our Mapping and Directed Array programs for use in the development of agrochemicals. In January 2000, we expanded this collaboration to cover life science applications, including pharmaceutical use by Monsanto's G.D. Searle division, and extended the term until December 2002. We also agreed to provide Monsanto with Compass Array and Mapping Array libraries through 2001 and Compass Array libraries only through December 2002. We also converted the Monsanto agrochemical Directed Array Program into a credit for pharmaceutical lead optimization services. Pharmacia is committed to make payments totaling $13.0 million under this agreement. In addition, Monsanto has agreed to pay us development milestones and royalties from the sales of products resulting from the collaboration. In July 1998, we received a milestone payment for a Mapping Array compound selected by Monsanto for entry into field trials. On June 30, 2000, in connection with the merger between Monsanto and Pharmacia, we replaced our existing collaboration agreement with a new collaboration agreement with G.D. Searle & Co., a division of Pharmacia. The financial terms of the new agreement are substantially the same as the prior agreement. However, we expanded the scope of the agreement to enable Pharmacia and its affiliates to screen our compounds, which may result in milestone and royalty payments in the future. To date, we have received $12.4 million under this agreement. In March 2002, we entered into a one year technical access agreement with Pharmacia which granted them non-exclusive access to our proprietary ADMET simulation technology.
12
Sankyo. In November 1997, we entered into a three-year agreement with Sankyo Company, Ltd. to discover and optimize drug candidates. Under the terms of the agreement, Sankyo received a subscription to our Mapping Array program to discover new lead compounds. Sankyo also committed to a minimum number of Directed Array Programs during the term of the agreement. In April 2001, we extended our agreement with Sankyo through 2004 to include the Compass Array libraries in addition to continuing to use our Directed Array program. The total value of the extended agreement is up to $14.8 million in committed payments. To date, we have received $11.1 million under both agreements. Sankyo has also agreed to pay us developmental milestones and royalties resulting from sales of any products resulting from this collaboration. To date, we have not received any milestone or royalty payments under this agreement.
Johnson & Johnson. In December 1998, we entered into a three-year collaboration with R.W. Johnson Pharmaceutical Research Institute, a division of Johnson & Johnson, Inc., in which R.W. Johnson subscribed to our Mapping Array program. We discontinued our Mapping Array program as of 2002, and, as a consequence and in agreement with R.W. Johnson, we did not renew our collaboration. As of March 1, 2002, we have received all $8.6 million that we are due under this agreement. In addition, R.W. Johnson has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. To date, we have not received any milestone or royalty payments.
GlaxoSmithKline. In November 2000, we entered into a five-year collaboration and license agreement with SmithKline Beecham Corporation (now GlaxoSmithKline). Under the terms of the agreement, GlaxoSmithKline receives access to our Compass Array libraries and Mapping Array libraries for screening primarily in the anti-infective field. In addition, we have initiated the first of potentially two drug discovery programs based on a lead compound discovered in a GlaxoSmithKline compound library. GlaxoSmithKline receives all rights in compounds developed in these drug discovery programs. As of March 1, 2002, we have received $1.2 million under this collaboration. GlaxoSmithKline may terminate the agreement before the end of the five-year term. GlaxoSmithKline has agreed to pay us development milestones and royalties on sales of products resulting from the collaboration. To date, we have not received any milestone or royalty payments.
Abbott Laboratories. In June 1995, we entered into an agreement with Abbott Laboratories. Under this agreement Abbott subscribed to our Mapping Array and Directed Array programs. This collaboration was extended on two occasions and ended successfully in March 1999. Abbott has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. We have not received any milestone or royalty payments from Abbott.
Roche Bioscience. In September 1996, we entered into an agreement with Roche Bioscience. Under this agreement, we synthesized Directed Array compounds. Our obligations under this agreement ended in March 1999. Roche Bioscience has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. In May 1999, we received a milestone payment from Roche Bioscience for a Directed Array compound that was chosen for Investigational New Drug application, or IND, enabling toxicology studies.
In the past, we have entered into a number of collaborations with biotechnology companies, primarily providing them with access to our Mapping Array libraries. Going forward, we intend to enter into only a select number of focused biotechnology collaborations with companies that can contribute significant numbers of important targets, generally around a specific target class or therapeutic area. These collaborations should pool the necessary resources from each company to conduct a drug discovery program aimed at delivering at least one IND candidate per collaboration.
13
Genome Therapeutics Corporation. On October 17, 2000, we entered into a collaborative drug discovery agreement with Genome Therapeutics Corporation to discover and develop anti-infective drug candidates. Under the agreement, we will use our Parallel Track Drug Discovery program to screen and optimize compounds against a significant number of proprietary validated anti-infective targets which Genome Therapeutics has derived from its PathoGenome(TM) Database. We will share equally in all downstream value created by the collaboration, including future milestone, royalty and upfront payments resulting from the outlicensing of clinical candidates or later stage compounds derived from the collaboration.
ACADIA Pharmaceuticals. On December 18, 2000, ArQule and ACADIA Pharmaceuticals entered into a drug discovery collaboration. Under the agreement, ACADIA offers its functional genomics platform and ArQule contributes its Parallel Track™ Drug Discovery program to discover novel small molecule drug candidates directed at individual G-protein coupled receptor (GPCR) targets. We will share intellectual property resulting from the collaboration, and equally contribute to at least one joint drug discovery program. We will share any revenues resulting from the commercialization of joint drug discovery programs. In addition to these joint drug discovery programs, each of us will receive exclusive rights to certain compounds that we have decided not to develop in a joint drug discovery program, subject to a royalty payment to the other party. On April 7, 1998, we entered into a material transfer and screening agreement with ACADIA. Under this agreement, we provided to ACADIA access to certain ArQule compound arrays for screening against their target collection. On May 10, 2000, we entered into a compound license agreement with ACADIA. Under this agreement, we granted to ACADIA an exclusive license to certain of our compounds having activity against certain of their targets, in return for milestone payments and royalties.
PATENTS AND PROPRIETARY RIGHTS
We have sixteen issued U.S. utility patents, one issued U.S. design patent, seven granted foreign patents, and numerous patent applications in the U.S. and other countries. We depend, in part, on these patents to protect our technology and products. We also rely upon our trade secrets, know-how and continuing technological advances to develop and maintain our competitive position. In an effort to maintain the confidentiality and ownership of our trade secrets and proprietary information, we require our employees and consultants to sign confidentiality and invention assignment agreements. We intend these agreements to protect our proprietary information by controlling the disclosure and use of technology to which we have rights. These agreements also provide that we will own all the proprietary technology developed at ArQule or developed using our resources.
14
COMPETITION
The biotechnology industry is highly competitive. Our services and products face competition based on several factors, including size, diversity and ease of use of compound libraries. We also face competition related to the speed and costs of identifying and optimizing potential lead compounds and our patent position. We compete with many organizations that are engaged in attempting to identify and optimize compounds. Our competitors include Discovery Partners International, Array Biopharma and Albany Molecular Research Institute. In addition we compete with Vertex Pharmaceuticals, Neurogen, and 3-Dimensional Pharmaceuticals for chemistry-based drug discovery. We also compete with academic and scientific institutions, governmental agencies and public and private research organizations.
Smaller companies may also prove to be significant competitors, particularly through arrangements with large corporate collaborators. In addition to competition for our customers, these organizations also compete with us in recruiting and retaining highly qualified scientific and management personnel.
Historically, pharmaceutical companies have maintained close control over their research activities, including the synthesis, screening and optimization of chemical compounds. Many of these companies, which represent a significant potential market for our products and services, are developing in-house combinatorial chemistry and other methodologies to improve productivity, including major investments in robotics technology to permit the automated parallel synthesis of compounds and computational chemistry skills. In addition, these companies may already have large collections of compounds previously synthesized or ordered from chemical supply catalogs or other sources against which they may screen new targets. Other sources of compounds include extracts from natural products such as plants and microorganisms and compounds created using rational design. Academic institutions, governmental agencies and other research organizations are also conducting research in areas in which we are working either on their own or through collaborative efforts.
GOVERNMENT REGULATION
Our research and development processes involve the controlled use of hazardous materials and controlled substances. Although we are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and waste products, the license or sale of our products is not subject to significant government regulations. Our future profitability, however, depends on our collaborators selling pharmaceuticals and other products developed from our compounds that may be subject to government regulation.
Virtually all pharmaceutical and biotechnology products developed by our collaborative partners will require regulatory approval by governmental agencies prior to commercialization. The nature and the extent to which these regulations apply to our collaborative partners varies depending on the nature of their products. In particular, human pharmaceutical products and biologics are subject to rigorous preclinical and clinical testing and other approval procedures by the FDA and by foreign regulatory authorities. Various federal and, in some cases, state statutes and regulations also govern or influence the manufacturing, safety, labeling, storage, record keeping and marketing of these products. The process of obtaining these approvals and the subsequent compliance with appropriate federal and foreign statutes and regulations are time consuming and require substantial resources.
Generally, in order to gain FDA approval, a company first must conduct preclinical studies in the laboratory and in animal models to gain preliminary information on a compound's efficacy and to identify any safety problems. The results of these studies are submitted as a part of an IND that the FDA must review before human clinical trials of an investigational drug can start. In order to commercialize any products, we or our collaborator will be required to sponsor and file an IND and will be responsible for initiating and overseeing the clinical studies to demonstrate the safety and efficacy that are necessary to obtain FDA approval. Clinical trials are normally done in three phases and generally take several years, but may take longer to complete. After completion of clinical trials of
15
a new product, FDA and foreign regulatory authority marketing approval must be obtained. If the product is classified as a new pharmaceutical, we or our collaborator will be required to file a New Drug Application, or NDA, and receive approval before commercial marketing of the drug. Similarly, if the product is a new biologic, a biological license application, BLA, must be filed and must receive approval prior to commercial marketing of the product. The testing and approval processes require substantial time and effort. NDAs and BLAs submitted to the FDA can take several years to obtain approval.
Even if FDA regulatory clearances are obtained, a marketed product is subject to continual review. If and when the FDA approves any of our or our collaborators' products under development, the manufacture and marketing of these products will be subject to continuing regulation, including compliance with current Good Manufacturing Practices, known as GMPs, adverse event reporting requirements and prohibitions on promoting a product for unapproved uses. Later discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
For marketing outside the United States, we or our partners will be subject to foreign regulatory requirements governing human clinical trials and marketing approval for pharmaceutical products and biologics. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary widely from country to country.
EMPLOYEES
As of March 1, 2002, we employed 385 people worldwide, 325 in Woburn and Medford, Massachusetts, 40 in Menlo Park, California, and 20 in Cambridge, United Kingdom. Of the 385 employees, 105 have Ph.D. degrees. 198 of our employees were engaged in operations, 131 were engaged in research and development, and 56 were engaged in marketing and general administration. None of our employees are covered by collective bargaining agreements. We believe that we have good relations with our employees.
OUR TRADEMARKS
The terms "ArQule", "Mapping Array", and "Directed Array" are trademarks of ArQule that are registered in the U.S. Patent and Trademark Office. The terms "Compass Array", "AMAP", "Custom Array", "ArQule Reactor", "MapMaker", "Parallel Track", and "PrepQule" are trademarks of ArQule.
16
ITEM 1A. EXECUTIVE OFFICERS AND DIRECTORS OF THE REGISTRANT
Set forth below is certain information regarding our current executive officers and directors, including their respective ages, as of March 1, 2002. L. Patrick Gage, a director since January 1998, tendered his resignation from the Board of Directors effective January 4, 2002.
NAME
| | AGE
| | POSITION
|
|---|
| Dr. Stephen A. Hill | | 43 | | President, Chief Executive Officer and a Director |
Philippe Bey, Ph.D |
|
59 |
|
Senior Vice President of Research and Development and Chief Scientific Officer |
David C. Hastings |
|
40 |
|
Vice President, Chief Financial Officer and Treasurer |
Harold E. Selick, Ph.D |
|
47 |
|
President, Chief Executive Officer of Camitro Corporation |
Michael Rosenblatt, M.D. |
|
53 |
|
Director |
Werner Cautreels, Ph.D. |
|
49 |
|
Director |
Laura Avakian |
|
56 |
|
Director |
Tuan Ha-Ngoc |
|
49 |
|
Director |
Ariel Elia |
|
66 |
|
Director, Chairman of the Board |
Timothy C. Barabe |
|
49 |
|
Director |
STEPHEN A. HILL, M.D. Stephen A. Hill, B.M., B.Ch., M.A., F.R.C.S. has served as our President and CEO since April 1999. Prior to his employment with us, and since 1997, Dr. Hill was the Head of Global Drug Development at F. Hoffmann-La Roche Ltd. He joined Roche in 1989 as Medical Adviser to Roche Products in the United Kingdom. He held several senior positions there, including that of Medical Director, with responsibility for clinical trials of compounds across a broad range of therapeutic areas, including those of CNS, HIV, cardiovascular, metabolic, and oncology products. Dr. Hill also served as Head of International Drug Regulatory Affairs at Roche headquarters in Basel, Switzerland, where he led the regulatory submissions for seven major new chemical entities globally. He also was a member of Roche's Portfolio Management, Research, Development and Pharmaceutical Division Executive Boards. Prior to Roche, Dr. Hill served for seven years with the National Health Service in the United Kingdom, in General and Orthopedic Surgery. Dr. Hill is a Fellow of the Royal College of Surgeons of England, and holds his scientific and medical degrees from St. Catherine's College at Oxford University.
PHILIPPE BEY, PH.D. Philippe Bey, Ph.D. has served as our Chief Scientific Officer and Senior Vice President of Research and Development since August 1999. Dr. Bey has previously held various senior management positions at Hoechst Marion Roussel (HMR), Marion Merrell Dow, Inc. and Selectide, a combinatorial chemistry company fully owned by HMR. While at HMR, he coordinated U.S. research & development programs and participated in a task force that defined HMR's strategic plans (1996–1999). At Marion Merrell Dow, where he served as Vice President of Global Research, he designed research strategies to incorporate new technologies and internal organizational competencies while improving productivity. Dr. Bey also served as President of Selectide. Dr. Bey earned his BS and Ph.D. Chemistry qualifications at the Louis Pasteur University in Strasbourg, France, and conducted post-doctoral training at the California Institute of Technology in Pasadena.
DAVID C. HASTINGS David C. Hastings has served as our Vice President and Chief Financial Officer since February 2000. Prior to his employment with us, Mr. Hastings was at Genzyme, Inc. (1997–2000) where his final position was Vice President and Corporate Controller, responsible for the management of the finance department. Prior to his employment with Genzyme, Mr. Hastings was the
17
Director of Finance at Sepracor, Inc. where he was primarily responsible for Sepracor's internal and external reporting. Mr. Hastings is a Certified Public Accountant and received his BA in Economics at the University of Vermont.
HAROLD E. SELICK, PH.D Harold E. Selick has served as President and Chief Executive Officer of Camitro, which we acquired in January 2001, since November 1999. Dr. Selick has tendered his resignation and will be leaving the Company effective March 31, 2002. Prior to his employment with Camitro, Dr., Selick was, from January 1995 to July 1997, a Director of Research Affymax Research Institute. From July 1997 to December 1998, he was Vice President of Research of Affymax where he directed activities in combinatorial chemistry-based drug discovery, with particular emphasis on the development of technologies for improving the process of lead optimization. Prior to joining Affymax, Dr. Selick held scientific positions in two other biotechnology companies, one of which was Protein Design Labs, where he was co-inventor of the technology underlying the creation of fully humanized antibodies. He applied this technology to the creation of the "Smart anti-TAC" antibody, which was successfully developed by Roche as "Zenapax", for treating kidney transplant rejection. Prior to working at Protein Design Labs, he was a Damon Runyon-Walter Winchell Cancer Fund Fellow with Professor Bruce Alberts and an American Cancer Society Fellow at the University of California, San Francisco, School of Medicine. Dr. Selick holds a Ph.D. in Molecular Biology and a B.A. in Biophysics from the University of Pennsylvania.
MICHAEL ROSENBLATT, M.D. Michael Rosenblatt, M.D. has been a director since April 1998. From 1992-1998, Dr. Rosenblatt served as the Robert H. Ebert Professor of Molecular Medicine at the Harvard Medical School, Chief of the Division of Bone and Mineral Metabolism at Beth Israel Hospital, and the director of the Harvard MIT Division of Health Sciences and Technology. From 1996-1999, he was the executive director of the Carl J. Shapiro Institute for Education and Research at Harvard Medical School and Beth Israel Deaconess Medical Center. From 1996-1999, he was Harvard faculty dean for academic programs at the Beth Israel Deaconess Medical Center. From 1999 - 2000, he was President of Beth Israel Deaconess Medical Center. He currently serves as the George R. Minot Professor of Medicine at Harvard Medical School. Prior to 1992, Dr. Rosenblatt was the Senior Vice President for Research at Merck Research Laboratories, a pharmaceutical company. Dr. Rosenblatt serves as a director of several privately held companies.
WERNER CAUTREELS, PH.D. Werner Cautreels, Ph.D. has been a director since September 1999. Since May 1998, Dr. Cautreels has been the Global Head of Research and Development of Solvay Pharmaceuticals. Prior to that, Dr. Cautreels had been employed by Nycomed Amersham Ltd., Sterling Winthrop, and Sanofi in a variety of positions in Research and Development. Dr. Cautreels received his Ph.D. in Chemistry from the University of Antwerp, Belgium.
LAURA AVAKIAN Laura Avakian has been a director since March 2000. Ms. Avakian is currently and since 1999 has been Vice President for Human Resources for the Massachusetts Institute of Technology where she directs all human resource programs and oversees the institution's Medical Department. Prior to joining MIT, she was Senior Vice President, Human Resources, for Beth Israel Deaconess Medical Center and for its parent corporation CareGroup (1996–1999). She has previously served as President of the American Society for Healthcare Human Resources Administration, and has received the distinguished service award, literature award and chapter leadership award from that society. She received the 1996 Award for Professional Excellence in Human Resources Management from the Society for Human Resource Management. She has also served as editor of the Yearbook of Healthcare Management and authored numerous chapters and articles on human resources management. Ms. Avakian received her BA degree from the University of Missouri at Columbia and her MA degree from Northwestern University.
TUAN HA-NGOC Tuan Ha-Ngoc has been a director since March 2000. Dr. Ha-Ngoc is the founder, and was Chief Executive Officer and a director of deNovis, Inc., an advanced business to
18
business financial transactions platform for health care benefits administration. Mr. Ha-Ngoc was previously the Vice President of Strategic Development at American Home Products Corporation. Prior to joining AHP, he was an Executive Vice President for Genetics Institute, Inc. Dr. Ha-Ngoc is a member of the Board of Fellows and Chairman of the Executive Research Committee at the Harvard School of Dental Medicine. He received an MBA from INSEAD and received a Master's Degree in Pharmacy from the University of Paris, France.
ARIEL ELIA Ariel Elia has been a director since September 2000, and was named Chairman of the Board in March 2001. Currently, and since 1999, Mr. Elia serves as Chairman of the European Advisory Board of E.Med Securities, a private, U.S.-based company providing investment banking services to emerging growth companies in the life science industry. Mr. Elia is and since 1995 has been a director of Altamir S.A., a French venture capital company, and is and since 1999 has been a director of Yissum, the research and development company of the Hebrew University of Jerusalem in Israel. Mr. Elia also serves as a Governor of both the Ben Gurion University (since 1992) and the Hebrew University of Jerusalem (since 1998), in Israel. Prior to his current positions, Mr. Elia was the Chief Executive Officer of Jouveinal Laboratories, a privately held, French pharmaceutical company. Mr. Elia also spent 17 years with Merck & Co., serving both in Europe and in the U.S., most recently as Senior Vice President, International Division. Before joining Merck & Co., Mr. Elia spent 12 years with American Home Products Corp., serving as President of the International Household Products Division prior to his departure. Mr. Elia is a U.S. citizen born in Alexandria, Egypt. He graduated from Victoria College in Alexandria, Egypt with an Oxford and Cambridge degree as a Bachelor of Arts. His honors include Knight of the Order of the Crown in Belgium, and Doctor of Philosophy Honoris Causa of Ben Gurion University, Israel.
TIMOTHY C. BARABE Timothy C. Barabe has been a director since November 2001. Mr. Barabe has been employed by Novartis AG since April 1982 in various capacities. From 1993 through January 2002, Mr. Barabe was the Chief Financial Officer of CIBA Vision Corp., a subsidiary of Novartis. Since February 2002, Mr. Barabe has been Group Vice President and President, Specialty Lenses of CIBA Vision. Mr. Barabe received his B.B.A. degree from the University of Massachusetts (Amherst) and his M.B.A. degree from the University of Chicago. Mr. Barabe is a Trustee and Treasurer of Fernbank Natural History Museum.
RISKS RELATING TO OUR BUSINESS AND STRATEGY
We are at an early stage of development.
From our inception in 1993 through December 31, 2001, we have incurred cumulative losses of approximately $71.7 million. These losses have resulted principally from the costs of our research activities and enhancements to our technology. We have derived our revenue primarily from:
- •
- license fees;
- •
- payments for product deliveries;
- •
- milestone payments; and
- •
- research and development funding paid under our agreements with our collaboration partners.
To date, except for 1997 and 2000, these revenues have not generated profits, nor have we realized any revenue from royalties from the sale by any of our collaboration partners of a commercial product developed using our technology. We cannot be certain that we will ever become profitable on a sustainable basis.
We cannot guarantee that our strategy of using our integrated compound discovery technologies in the development of new drugs and other products will ever be commercially successful.
19
Our approach to compound discovery has not yet yielded a commercially successful drug.
Our strategy is to use our technology platform to rapidly identify, optimize and obtain financial interest in as many compounds with commercial potential as possible. This approach has not yet yielded a commercially successful drug, either in our collaborations or in our internal development programs. In addition, we have recently reoriented our business and technology strategies to offer an integrated compound discovery solution, in addition to combinatorial chemistry products and services. Our new strategy may not be accepted by our potential customers. In particular, we have not proven that we can use our products successfully to assist our customers to conduct lead optimization. Our ability to succeed depends in the near term on our potential customers accepting our approach to combinatorial chemistry and integrated compound discovery as an effective tool in the discovery and development of compounds with commercial potential. If we cannot demonstrate that our approach can result in successful products, we may not be able to attract additional customers or to retain our existing customers.
Furthermore, since our suite of predictive ADMET models are still in the early stage of development, we do not know if we can successfully integrate these models into our technology platform.
Because of the specialized nature and high price of our services, our potential customer base is limited, and these potential customers may decide to try to use our approach themselves without our assistance or try other methods.
Because we offer specialized assistance in the development of drugs, our potential customer base consists of a limited number of pharmaceutical and biotechnology companies and research institutions. These organizations have historically conducted lead compound identification and optimization within their own research departments. Because of the high cost of our products and programs, they may decide to conduct these activities without our assistance.
RISKS RELATING TO COLLABORATORS
We depend on collaboration arrangements with third parties for our revenue and cannot be sure whether our collaborations will succeed or whether we will realize much of the potential revenue from our collaborations.
We depend on our collaborations for all of our revenues, and we will only realize much of the potential revenue under these collaborations if we meet compound delivery targets, satisfy milestones and earn royalties.
Our revenue stream and our business strategy depend largely on the formation of collaborative arrangements with third parties, initially pharmaceutical and biotechnology companies and research institutions. In implementing our strategy, we have concentrated on a limited number of collaborations involving extensive, multi-faceted research and development activities. Much of the potential revenue from our collaborations consists of contingent payments, such as payments for achieving development milestones and royalties payable on sales of drugs developed using our products. We cannot guarantee that these milestones will be achieved or that commercial drugs or other products will be developed on which royalties will be payable.
If we do not achieve milestones as expected, our revenue will be reduced and our stock price may decline. For planning purposes, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other milestones, such as the commencement or completion of scientific studies and clinical trials and the submission of regulatory filings. These estimates are based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in many cases for reasons beyond our control.
20
Our ability to realize potential revenue from milestones and royalties from our collaborations depends, in large part, on the efforts of our partners, over which we have little control.
Much of the revenue from milestones and royalties that we may receive under these collaborations will depend upon our partners' ability to successfully develop, license, introduce, market and sell new drugs developed using our products. Each of our collaborators has significant discretion in determining the efforts and resources that it will apply to the alliance. Our products will result in commercialized drugs generating milestone payments and royalties only after, among other things:
- •
- significant preclinical and clinical development efforts or the completion of preliminary field trials;
- •
- the receipt of the required regulatory approvals;
- •
- developing manufacturing capabilities; and
- •
- successful marketing efforts.
With the exception of certain aspects of preclinical drug development, we do not currently intend to perform any of these activities. Accordingly, we will depend on our partners having the necessary expertise and dedicating sufficient resources to develop and commercialize products. Our collaboration partners may fail to develop or commercialize a compound or product to which they have obtained rights from us, because, among other reasons:
- •
- they decide not to devote the necessary resources because of internal constraints or other development priorities;
- •
- they decide to pursue a competitive potential drug or compound developed outside of the collaboration;
- •
- they cannot obtain the necessary regulatory approvals; or
- •
- they may exercise limited rights to terminate these collaborations with us.
To be successful, we must expand the number of our collaborations both by maintaining existing collaborations, as well as continuing to enter into agreements with new partners to use our technology to develop potential drugs. We face significant competition in seeking appropriate collaborators. Moreover, these collaborative arrangements are complex to negotiate and time-consuming to document. We may not be able to maintain our existing collaborations or establish new collaborations, and we cannot guarantee that any collaboration will be on commercially acceptable terms. Moreover, such collaborations or other arrangements may not be scientifically or commercially successful.
RISKS ASSOCIATED WITH COMPETITION
Our competitors may have greater resources or research and development capabilities than we have and we may not have the resources required to successfully compete with them.
Our competitors may have greater research and development capabilities for drug discovery.
The drug development business is highly competitive. We compete with many organizations that are engaged in attempting to identify and optimize compounds as potential drugs. Many of these competitors have greater financial and human resources and more experience in research and development than we have. Competitors include:
- •
- biotechnology, pharmaceutical, combinatorial chemistry and other companies;
- •
- academic and scientific institutions;
- •
- governmental agencies; and
21
- •
- public and private research organizations.
Historically, pharmaceutical companies have maintained close control over their research activities, including the synthesis, screening and optimization of potential drugs. Many of these companies, which represent a significant potential market for our products and services, have developed or are developing internal combinatorial chemistry and other capabilities to improve productivity. We anticipate that we will face increased competition in the future as new companies enter the market and alternative technologies become available.
Our competitors may have greater resources for recruiting and business development.
These organizations also compete with us to attract qualified personnel and for acquisitions, joint ventures or other collaborations. They also compete with us to attract academic research institutions as partners and to license these institutions' proprietary technology. If our competitors successfully enter into partnering arrangements or license agreements with academic research institutions, we will then be precluded from pursuing those specific opportunities. Since each of these opportunities is unique, we may not be able to find a substitute. Many biotechnology companies have already begun drug development programs, some of which may target diseases that we are also targeting, and have already entered into partnering and licensing arrangements with academic research institutions, reducing the pool of available opportunities.
While universities and public and private research institutions primarily have educational or basic research objectives, they may develop proprietary technology and acquire patents that we may need for the development of our products. We will attempt to license this proprietary technology, if available. These licenses may not be available to us on acceptable terms, if at all.
We compete with many organizations for scientific and managerial personnel.
Competition for scientific and managerial personnel in our industry is intense; we will not be able to execute our strategy, grow and manage our growth if we are not able to attract additional qualified personnel.
We face intense competition for qualified personnel in our industry. Our future success will depend heavily on our ability to continue to hire, train, retain and motivate additional skilled managerial and scientific personnel. The pool of personnel with the skills that we require is limited. Competition to hire from this limited pool is intense. Our success depends on the expansion and proper management of our operations. To be cost-effective in our delivery of services and products, we must enhance productivity by further automating our processes and technology. We may not succeed in our efforts to further automate these processes. We also must successfully structure and manage multiple collaborative relationships. Further, we may not succeed in managing and meeting the staffing requirements of additional collaborative relationships.
RISKS RELATING TO OUR FINANCIAL RESULTS AND NEED FOR FINANCING
We face uncertainty in raising additional funds that may be necessary to fund our operations.
Our capital requirements depend on many factors. If our operations do not become profitable on a sustainable basis before we exhaust existing resources, we will need to obtain additional financing, either through public or private financings, including debt or equity financings, or through collaboration or other arrangements with corporate partners. We may not be able to obtain adequate funds for our operations from these sources when needed or on acceptable terms. If we raise additional capital through the sale of equity, or securities convertible into equity, your proportionate ownership in ArQule may be diluted. If we cannot obtain additional financing, we could be forced to delay or scale back our research and development programs. If adequate funds are not available, we may be required
22
to curtail operations significantly or to obtain funds by entering into arrangements with collaboration partners or others that may require that we relinquish rights to certain technologies, product candidates, products or potential markets.
Moreover, our fixed expenses such as rent, license payments and other contractual commitments are substantial and will increase in the future. These fixed expenses will increase because we may enter into:
- •
- Additional construction or lease expense for new facilities and capital equipment;
- •
- Additional licenses and collaborative agreements;
- •
- Additional contracts for consulting, maintenance and administrative services; and
- •
- Additional contracts for product manufacturing.
We believe that our cash, cash equivalents and short-term investment securities balances as of March 1, 2002 will be sufficient to meet our operating and capital requirements for the next several years. This estimate is based on assumptions and estimates, which may prove to be wrong. As a result, we may need or choose to obtain additional financing during that time.
RISKS ASSOCIATED WITH TECHNOLOGY DEVELOPMENT
We will not be able to successfully grow our business if we are unable to expand and integrate new technologies as we acquire them.
Our success depends on the integration and expansion of various chemistry-based drug discovery technologies in both our internal and collaborative programs. In order for us to achieve our goal of reducing materially the cost and time currently incurred by the pharmaceutical industry for identifying investigational new drug candidates, or IND candidates, we will have to integrate and acquire complimentary technologies. We also must successfully structure and manage multiple collaborative relationships. If we do not, we could fail to achieve such goal and lose significant amounts of revenues. Further, we may not succeed in managing and meeting the staffing requirements of additional collaborative relationships.
Integration will include several technical and administrative challenges, including managing information transfer, integrating certain of technical staff into our research and development structure and managing multiple operations in different countries. If we do not accomplish this integration effectively, our programs could be delayed and our operating and research and development expenditures could increase beyond anticipated levels. Additionally, the integration could require a significant time commitment from our senior management.
One aspect of our plan to obtain access to new technologies involves acquisition of other companies. We will not be successful if we are unable to consummate the acquisition of other companies, integrate acquired companies with our other operations or if the acquired technology or personnel of acquired companies do not meet our expectations.
We may not be able to identify appropriate companies and technologies for acquisition or we may not be able to consummate the transactions to acquire them. If we cannot, the resultant lack of access to new external technologies may delay or prevent implementation of our strategy. We may not be able to successfully integrate or profitably manage acquired businesses. In addition, the combination of our business with these businesses may not achieve revenues, net income or loss levels, efficiencies or synergies that justify the acquisitions. The combined company may experience slower rates of growth as compared to our historical rates of growth and of these businesses independently.
23
RISKS RELATING TO INTELLECTUAL PROPERTY
We depend on patents and other proprietary rights that may fail to protect our business.
Our success will depend on our ability to obtain and protect patents on our technology and to protect our trade secrets. We cannot be certain that we will receive any additional patents, that the claims of our patents will offer significant protection of our technology, or that our patents will not be challenged, narrowed, invalidated or circumvented. In order to protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. These lawsuits could be expensive, take significant time and divert management's attention from other business concerns. We may also provoke these third parties to assert claims against us. The patent position of biotechnology firms is generally highly uncertain, involves complex legal and factual questions, and has recently been the subject of much litigation. No consistent policy has emerged from the U.S. Patent and Trademark Office or the courts regarding the breadth of claims allowed or the degree of protection afforded under many biotechnology patents. In addition, there is a substantial backlog of biotechnology patent applications at the U.S. Patent and Trademark Office, and the approval or rejection of patent applications may take several years.
In an effort to protect our trade secrets, we require our employees, consultants and advisors to execute confidentiality agreements. We cannot guarantee, however, that these agreements will provide us with adequate protection against improper use or disclosure of confidential information. Also, others may independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets.
Our success will depend partly on our ability to operate without infringing on or misappropriating the proprietary rights of others.
Others may sue us for infringing on their patent rights. Intellectual property litigation is costly, and, even if we prevail, the cost of such litigation could adversely affect our business, financial condition and results of operations. In addition, litigation is time consuming and could divert management attention and resources away from our business. If we do not prevail in litigation, in addition to any damages we might have to pay, we could be required to stop the infringing activity or obtain a license. Any required license may not be available to us on acceptable terms, or at all. In addition, some licenses may be non-exclusive and, accordingly, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license or are unable to design around a patent, we may be unable to sell some of our products. Any of these occurrences will result in lost revenues and profits for us.
Forward-looking statements
This Form 10-K contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, principally in the sections entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business." Generally, any statement that is not a statement of historical fact should be considered a forward-looking statement. These statements can usually be identified by the use of words or phrases like "believe," "expect," "anticipate," "plan," "may," "will," "could," "estimate," "potential," "opportunity," "future," "project, "will likely result," and similar expressions. They will include statements about our:
- •
- implementation of our corporate strategy;
- •
- financial performance;
- •
- ability to deliver our products to our corporate collaborators and to satisfy milestones so that we can earn future payments under our collaboration agreements;
24
- •
- collaborators' ability to develop and commercialize products using our technology and pay us royalties on the sales of those products;
- •
- ability to enter into future collaborations with pharmaceutical and biotechnology companies and academic institutions;
- •
- product research and development activities and projected expenditures;
- •
- cash needs;
- •
- potential acquisitions of technologies and other companies;
- •
- plans for sales and marketing; and
- •
- results of scientific research.
These forward-looking statements involve risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed in "Risk Factors." You should carefully consider that information before you make an investment decision. You should not place undue reliance on our forward-looking statements that speak only as of the date on which they are made.
We will not undertake and specifically declines any obligation to publicly release the result of any revisions which may be made to any forward-looking statements to reflect events or circumstances after the date of such statements or.
Similarly, we are under no duty to update or revise any of the forward looking statements after the date of this Form 10-K to conform such statements to actual results or to reflect the occurrence of anticipated or unanticipated events which may cause management to re-evaluate its forward-looking statements and will not do so, unless required by law.
Although we believe that the expectations reflected in the forward looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of such statements.
ITEM 2. PROPERTIES
In November 1999, we moved our main operations to a new facility in Woburn, Massachusetts, which includes approximately 128,000 square feet of laboratory and office space. This facility was designed to our specific requirements. On November 28, 2000, we exercised our options to purchase the entire building and the adjacent lot, and on March 2, 2001 we closed the transaction. The total consideration paid for the properties was $20.5 million, of which $18.2 million represented the purchase price for the entire building and the land on which it sits and $2.3 million represented the purchase price for the adjacent lot. The purchase price was determined through an arms-length negotiation with Metro North Corporate Center LLC and Metro North Corporate Center LLC II, which are unaffiliated with us and any of our directors or executive officers.
The land upon which our facility sits is approximately 7.2 acres, including a parking lot, while the adjacent parcel of land represents approximately 5 acres. We plan to continue to use our facility in its current capacity and may develop the adjacent parcel at a presently undetermined time in the future. Our research facilities also include approximately 56,000 square feet of laboratory and office space in Medford, Massachusetts, the majority of which is dedicated to the Pfizer collaboration. We lease these facilities under two lease agreements, one of which expires on July 30, 2005 and one of which expires on July 30, 2006. We sublease the majority of this facility pursuant to two sublease agreements. We have approximately 10,000 square feet of unoccupied space. We are currently evaluating the facility resources that we will require to meet the demands of the expanded Pfizer collaboration.
25
On August 1, 2001, Cummings Properties, LLC ("Cummings") significantly raised our rent on the lease that expires July 30, 2006. We believe this increase to be in excess of market rates and, as a result, we are litigating the increase. We have accrued the difference between our estimate of market rate rent and amounts due to us under the subleases. We are currently disputing the amount of the rent increase with the landlord. If the amount of rent we must pay is in excess of our current estimate of market rates, we will need to recognize additional expense. See Item 3, Legal Proceedings, for additional information.
In connection with our acquisition of Camitro Corporation on January 29, 2001, we assumed Camitro's existing lease for approximately 25,000 square feet of office space in Menlo Park, California, which will expire on September 30, 2002. Prior to expiration, we have the option to renew this lease for a term of 18 months, which we will not exercise. Our lease payments are $46,282 per month.
In January 2001, Camitro Corporation, through its wholly-owned subsidiary Camitro UK, Ltd. leased approximately 10,000 square feet of office and laboratory space in Cambridge, England for 15,416 British Pounds per month ($21,891 per month as of March 1, 2002). We lease this facility under an agreement which expires in December 2005. In March 2001, we executed a two year sublease with a third party for approximately 4,000 square feet of the premises for 7,333 British Pounds per month ($10,413 per month as of March 1, 2002). This sublease extends until March 2003.
ITEM 3. LEGAL PROCEEDINGS
On January 16, 2001, we brought a complaint for declaratory relief and damages against Cummings Properties arising out of Cummings' attempts to avoid its contractual obligations under our two Medford, Massachusetts leases and Cummings' attempts to increase our lease rates to what we believe is a higher amount than is due under the lease agreement. We seek recovery of the funds that we have already paid under protest, without waiver of any rights, to Cummings in response to Cummings' threats to accelerate rental payments and commence eviction proceedings against us.
We are prosecuting these actions and, considering our reserves, we are of the opinion that these actions should not have a material adverse effect on our financial position or results of operations.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to stockholders for a vote during the fourth quarter of 2001.
26
PART II
ITEM 5. MARKET FOR THE REGISTRANT'S COMMON STOCK AND RELATED STOCKHOLDER MATTERS
ArQule's common stock is traded on The Nasdaq National Market under the symbol "ARQL".
The following table sets forth, for the periods indicated, the range of the high and low closing sale prices for ArQule's common stock:
| | HIGH
| | LOW
|
|---|
| 2000 | | | | |
| First Quarter | | 37.50 | | 8.25 |
| Second Quarter | | 19.88 | | 6.25 |
| Third Quarter | | 24.13 | | 16.63 |
| Fourth Quarter | | 33.75 | | 13.00 |
| 2001 | | | | |
| First Quarter | | 31.25 | | 10.00 |
| Second Quarter | | 21.66 | | 10.88 |
| Third Quarter | | 21.45 | | 7.95 |
| Fourth Quarter | | 17.78 | | 8.50 |
| 2002 | | | | |
| First Quarter (through March 1, 2002) | | 16.90 | | 10.75 |
As of March 1, 2002, there were approximately 177 holders of record and approximately 7,085 beneficial shareholders of our common stock.
We have never paid cash dividends on our common stock and we do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, for use in our business.
ITEM 6. SELECTED FINANCIAL DATA
The following data, insofar as it relates to the years 1997, 1998, 1999, 2000 and 2001, have been derived from ArQule's audited financial statements, including the balance sheet as of December 31, 2000 and 2001 and the related statements of operations and of cash flows for the three years ended December 31, 2001 and notes thereto appearing elsewhere in this Annual Report on Form 10-K. This data should be read in conjunction with the Financial Statements and the Notes thereto and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing
27
elsewhere in this Annual Report on Form 10-K. The historical results are not necessarily indicative of the results of operations to be expected in the future. This data is in thousands, except per share data.
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 1997
| | 1998
| | 1999
| | 2000
| | 2001
| |
|---|
| STATEMENT OF OPERATIONS DATA: | | | | | | | | | | | | | | | | |
| Revenue | | $ | 17,420 | | $ | 22,193 | | $ | 18,582 | | $ | 50,296 | | $ | 58,396 | |
| Cost and expenses: | | | | | | | | | | | | | | | | |
| | Cost of revenue | | | 10,218 | | | 14,036 | | | 17,457 | | | 21,343 | | | 29,441 | |
| | Research and development | | | 4,284 | | | 10,214 | | | 13,774 | | | 17,699 | | | 28,446 | |
| | Marketing, general and administrative | | | 4,670 | | | 6,387 | | | 6,022 | | | 8,293 | | | 12,353 | |
| | Stock-based compensation | | | 420 | | | 213 | | | 486 | | | 880 | | | 6,949 | |
| | Amortization of intangibles | | | — | | | — | | | — | | | — | | | 7,104 | |
| | In-process research and development | | | — | | | — | | | — | | | — | | | 18,000 | |
| | |
| |
| |
| |
| |
| |
| | | | | | Total costs and expenses | | | 19,592 | | | 30,850 | | | 37,739 | | | 48,215 | | | 102,293 | |
| | |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | (2,172 | ) | | (8,657 | ) | | (19,157 | ) | | 2,081 | | | (43,897 | ) |
| Interest income (expense), net | | | 2,463 | | | 2,195 | | | 1,724 | | | 1,774 | | | 2,870 | |
| | |
| |
| |
| |
| |
| |
| Net income (loss) | | $ | 291 | | $ | (6,462 | ) | $ | (17,433 | ) | $ | 3,855 | | $ | (41,027 | ) |
| | |
| |
| |
| |
| |
| |
| Basic net income (loss) per share | | $ | .03 | | $ | (0.54 | ) | $ | (1.38 | ) | $ | 0.28 | | $ | (2.06 | ) |
| | |
| |
| |
| |
| |
| |
| Weighted average common shares outstanding — basic | | | 11,282 | | | 12,031 | | | 12,606 | | | 13,911 | | | 19,905 | |
| | |
| |
| |
| |
| |
| |
| | Diluted net income (loss) per share | | $ | .02 | | $ | (0.54 | ) | $ | (1.38 | ) | $ | 0.25 | | $ | (2.06 | ) |
| | |
| |
| |
| |
| |
| |
| Weighted average common shares outstanding — diluted | | | 12,394 | | | 12,031 | | | 12,606 | | | 15,208 | | | 19,905 | |
| | |
| |
| |
| |
| |
| |
| | DECEMBER 31,
|
|---|
| | 1997
| | 1998
| | 1999
| | 2000
| | 2001
|
|---|
| BALANCE SHEET DATA: | | | | | | | | | | | | | | | |
| Cash, cash equivalents and marketable securities | | $ | 49,282 | | $ | 33,870 | | $ | 36,421 | | $ | 110,019 | | $ | 98,002 |
| Working capital | | | 46,023 | | | 35,546 | | | 17,371 | | | 93,437 | | | 69,365 |
| Total assets | | | 66,925 | | | 60,480 | | | 77,346 | | | 149,476 | | | 208,475 |
| Long-term debt | | | 1,213 | | | 306 | | | 10,700 | | | 7,200 | | | 11,700 |
| Total stockholders' equity | | | 57,340 | | | 54,267 | | | 38,753 | | | 120,420 | | | 166,739 |
28
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
OVERVIEW
We are engaged in the production and development of novel chemical compounds with commercial potential in the pharmaceutical and biotechnology industries. We primarily manufacture arrays of synthesized compounds for delivery to our customers for use in lead compound generation and lead compound optimization activities. In addition, we have established a number of joint drug discovery programs with biotechnology companies and academic institutions, and are pursuing a limited number of our own internal drug discovery programs.
We primarily generate revenue through our collaborative agreements for production and delivery of compound arrays and other research and development services. Under many of these collaborative agreements, we are also entitled to receive milestone and royalty payments if the customer develops products resulting from the collaboration. To date, we have received two milestone payments and no royalty payments. In addition, we have not yet realized any revenue from our joint discovery programs with biotechnology companies and academic institutions, or from our internal drug discovery programs. While we expect our revenue to increase in 2002, our financial performance may vary from expectations, including quarterly variations in performance, because levels of revenue are dependent on expanding or continuing existing collaborations, entering into additional corporate collaborations, receiving future milestones and royalty payments, and realizing value from ongoing drug discovery programs, all of which are difficult to anticipate. See the discussion of our revenue recognition policy in our Critical Accounting Policies below.
We will continue to invest in technologies that enhance and expand our capabilities in drug discovery. These continued investments in technology are intended to enhance the novelty, diversity, and medical relevance of our compound arrays and to augment the power and scope of our chemistry capabilities. In addition to investments in technology, we may invest in internal lead optimization programs with the goal of delivering clinical candidates. Investments of this nature may result in near term earnings fluctuations or impact the magnitude of profitability or loss.
In November 2000, we sold 3,358,000 shares of common stock at $22.50 per share in a follow-on public offering. This included the exercise of the overallotment option of 438,000 shares. The offering resulted in net proceeds of approximately $70.7 million.
In November 2000, we exercised our options to purchase our building and the adjacent lot in Woburn, Massachusetts and in March 2001 we closed the transaction. The total consideration paid for the properties was $20.5 million, of which $18.2 million represented the purchase price for the entire building and the land on which it sits and $2.3 million represented the purchase price for the adjacent lot. The purchase price was determined through an arms-length negotiation with Metro North Corporate Center LLC and Metro North Corporate Center LLC II, which are unaffiliated with us or any of our directors or executive officers. We paid $4.5 million in cash and we amended and extended our term loan agreement with Fleet National Bank to borrow an additional $16.0 million in order to finance this facility and land purchase. Principal amounts are payable in 16 equal quarterly installments beginning on April 1, 2001. We made four $1.0 million payments in 2001. Interest payments are made monthly in arrears beginning on the first day of the month following commencement of the amended agreement. Interest accrues at the rate of one month LIBOR plus 1.75%, 3.68% as of December 31, 2001.
In January 2001, we acquired Camitro Corporation, a privately held predictive modeling company based in Menlo Park, California. At the time of the acquisition, Camitro had developed various computational models for ADMET characterization of compounds based on their structure. During the past year, ArQule has been advancing and integrating a suite of these predictive models into its chemistry-based technology platform to aid in the design, selection and optimization of pharmaceutical
29
drug candidates. We believe these models will enable ArQule to profile "virtual libraries" of compounds to decide which compounds to make from among the thousands or millions of compounds that we could make. The models are also useful for compound redesign during lead optimization. For example, the metabolism models are unique in their ability to direct a chemist to change particular sites on a compound to improve its metabolic stability. As more data are developed using the technology, the models themselves are refined. Consequently, the process of developing and integrating the models is an ongoing one. As we seek to change the nature of the drug discovery process, we will continue to improve the predictive modeling and other aspects of our integrated technology platform.
Under the terms of the merger agreement, we issued approximately 3.4 million shares of our common stock and $1.7 million in cash in exchange for all of Camitro's outstanding shares and the assumption of all of Camitro's outstanding stock options and warrants. The merger transaction was valued at $84.3 million based on our share price on the measurement date for the merger. The transaction was accounted for as a purchase transaction.
Upon consummation of the Camitro acquisition in January 2001, we immediately charged to income $18.0 million representing the estimated fair value of purchased in-process technology that had not yet reached technological feasibility and had no alternative future use (see note 6 of notes to consolidated financial statements). The value was determined by estimating the costs to develop the purchased in-process technology into commercially viable products, estimating the resulting net cash flows from such projects, and discounting the net cash flows back to their present values. The discount rate in each project takes into account the uncertainty surrounding the successful development and commercialization of the purchased in-process technology.
The resulting net cash flows from such projects were based on our management's best estimates of revenue, cost of sales, research and development costs, selling, general and administrative costs and income taxes from such projects. The net cash flows reflect the assumptions that would be used by market participants.
If these projects are not successfully developed, our revenue and profitability may be adversely affected in future periods. Additionally, the value of other intangible assets acquired may become impaired. We are continuously monitoring our development projects. Management believes that the assumptions used in the valuation of purchased in-process technology represent a reasonably reliable estimate of the future benefits attributable to the purchased in-process technology. No assurance can be given that actual results will not deviate from those assumptions in future periods.
We plan to use our existing cash to develop the purchased in-process technology related to the acquisition of Camitro into commercially viable products. This primarily consists of the completion of all planning, designing, prototyping, high-volume manufacturing verification and testing activities that are necessary to establish that a product can be produced to meet its design specifications, including functions, features and technical performance requirements. We are actively engaged in combining this Camitro technology, including predictive ADMET modeling, into our integrated science and technology platform.
We have incurred a cumulative net loss of $71.7 million through December 31, 2001. Losses have resulted principally from costs incurred in research and development activities related to our efforts to develop our technologies and from the associated administrative costs required to support those efforts. While we were profitable in fiscal year 2000, we were not profitable in 2001, we expect that we will not be profitable in 2002 and our ability to achieve sustained profitability is dependent on a number of factors, including our ability to perform under our collaborations at the expected cost, expand or continue existing collaborations, timing of additional investments in technology and the realization of value from the development and commercialization of products in which we have an economic interest, all of which are difficult to anticipate.
The Management's Discussion and Analysis of Financial Condition and Results of Operation contains forward-looking statements reflecting management's current expectations regarding our future
30
performance. Such expectations are based on certain assumptions regarding the progress of product development efforts under collaborative agreements, the executions of new collaborative agreements and other factors relating to our growth. Such expectations may not materialize if product development efforts are delayed or suspended, if negotiations with potential collaborators are delayed or unsuccessful or if other assumptions prove incorrect.
RESULTS OF OPERATIONS
Years Ended December 31, 2000 and 2001
Revenue. Total revenues for 2001 were $58.4 million as compared to $50.3 million in 2000, an increase of $8.1 million or approximately 16 percent. This increase is primarily due to increased fees of approximately $10.1 million for delivery of Custom Array(TM) sets from 2000 to 2001 to Pfizer and Bayer.
Cost of revenue. Cost of revenue in 2001 totaled $29.4 million, an increase of $8.1 million or 38 percent as compared to 2000. The increase in costs of revenue was attributable to increased costs for producing Custom Array(TM) sets for Pfizer and Bayer. Our gross margin as a percentage of sales was 50 percent for the year ended December 31, 2001 as compared to 58 percent for the prior year. Our gross margin as a percentage of sales was lower in 2001 due to increased costs relating to the Bayer collaboration.
Research and development expenses. Research and development expenses in 2001 were $28.5 million, an increase of $9.9 million or 53 percent as compared to $18.6 million in 2000. This increase is the result of our ongoing efforts to augment and enhance our chemistry, predictive and computational modeling capabilities and related proprietary technologies, including increased personnel, as we expand our drug discovery programs and technology platform.
Marketing, general and administrative expenses. Marketing, general and administrative expenses were $12.4 million in 2001, an increase of $4.1 million or 49 percent as compared to $8.3 million in 2000. The increase was primarily associated with increased support costs, including increased personnel, to support our growth during 2001.
Amortization of intangibles and other merger-related charges. We recorded an $18.0 million in-process research and development charge, $7.0 million in stock-based compensation and $7.1 million in amortization of intangibles in 2001 as a result of our acquisition of Camitro in 2001.
Net investment income. Net investment income consists primarily of interest income partially offset by interest expense and other non-operating income and expenses. Investment income in 2001 was $4.5 million as compared to $2.9 million in 2000, an increase of $1.6 million or approximately 55 percent. Our higher investment income in 2001 is a result of our higher average cash balance in 2001 due to our $70.7 million stock offering in November 2000, offset by lower average interest rates on our investment portfolio when compared to 2000. Interest expense in 2001 was $1.6 million as compared to $1.1 million in 2000, resulting primarily from our higher average debt balance on our term loan with Fleet National Bank.
Net income (loss). Our net loss for the year ended December 31, 2001 was $41.0 million, compared to our net income of $3.9 million for the same period in 2000. Our net loss in 2001 was primarily attributable to our increased investments in drug discovery programs and the merger-related charges due to our acquisition of Camitro Corporation.
Years Ended December 31, 1999 and 2000
Revenue. Total revenues for 2000 were $50.3 million as compared to $18.6 million in 1999, an increase of $31.7 million or approximately 171 percent. This increase is primarily due to the amortization of upfront fees of approximately $15.3 million and fees for delivery of Custom Array(TM)
31
sets of approximately $10.7 million to Pfizer Inc and Bayer AG and from other delivery fees earned from our collaborations.
Cost of revenue. Cost of revenue in 2000 totaled $21.3 million, an increase of $3.8 million or 22 percent as compared to 1999. The increase in costs of revenue was attributable to increased costs for producing Custom Array(TM) sets for Pfizer Inc and Bayer AG. Our gross margin as a percentage of sales was 58 percent for the year ended December 31, 2000 as compared to 6 percent for the prior year. Our gross margin as a percentage of sales was higher in 2000 due to the higher gross margin on the Pfizer collaboration and other economies of scale.
Research and development expenses. Research and development expenses in 2000 were $18.6 million, an increase of $4.3 million or 30 percent as compared to $14.3 million in 1999. This increase is the result of our ongoing efforts to augment and enhance our chemistry capabilities and related proprietary technologies, including increased personnel, as we expand our lead optimization programs.
Marketing, general and administrative expenses. Marketing and general administrative expenses in 2000 were $8.3 million in 2000, an increase of $2.3 million or 38 percent as compared to 1999. The increase was due primarily associated with increased administrative costs, including increased personnel, to support our growth during 2000.
Net investment income. Net investment income consists primarily of interest income partially offset by interest expense and other non-operating income and expenses. Investment income in 2000 was $2.9 million as compared to $1.9 million in 1999, an increase of $1.0 million or approximately 50 percent. Interest expense in 2000 was $1.1 million as compared to $0.2 million in 1999, resulting primarily from our higher average debt balance on our term loan with Fleet National Bank.
Net income (loss). Our net income for the year ended December 31, 2000 was $3.9 million, compared to a net loss of $17.4 million for the same period in 1999. Our net income in 2000 has been primarily attributable to increased revenue from our collaborator base.
LIQUIDITY AND CAPITAL RESOURCES
At December 31, 2001, we held cash, cash equivalents and marketable securities with a value of $98.0 million, compared to $110.0 million at December 31, 2000. Our working capital at December 31, 2001 was $69.4 million. We have funded operations through December 31, 2001 with sales of common stock, payments from corporate collaborators, and the utilization of bank financing. In March of 1999, we consummated a term loan agreement with Fleet National Bank, which we expanded in March of 2001, to support our facilities expansion. Under this expanded agreement, we have borrowed $30.0 million. As of December 31, 2001, we have made principal payments of $10.8 million and had an outstanding balance of $19.2 million.
Cash flows from operating activities for the year ended December 31, 2001 decreased $2.1 million to $4.4 million from $6.5 million for the same period in 2000. This decrease is a result of our net operating loss, excluding non-cash charges, in 2001.
Cash flows from investing activities for the year ended December 31, 2001 decreased $66.8 million to a use of cash of $65.5 million from $1.3 million provided by investing activities for the same period in 2000. During 2001, we purchased our Woburn facility for $20.5 million, made a $5.0 million investment in a privately-owned proteomics company, and shifted a significant portion of our cash raised in the November 2000 follow-on stock offering from cash equivalents into marketable securities.
Cash flows from financing activities for the year ended December 31, 2001 decreased $55.9 million to $18.2 million from $74.1 million for the same period in 2000. In November 2000, we completed a follow-on offering of 3.4 million shares of our common stock that raised $70.7 million net of expenses. In December 2001, Pfizer made an equity investment of $10.0 million.
32
We expect that our available cash and marketable securities, together with operating revenues and investment income will be sufficient to finance our working capital and capital requirements for the next several years. We have been cash flow positive from operations for three consecutive years, and coupled with our existing cash and marketable securities, we believe that even without additional funding we would have sufficient funds to operate for at least the next several years. Our cash requirements may vary materially from those now planned depending upon the results of our drug discovery and development strategies, our ability to enter into any additional corporate collaborations in the future and the terms of such collaborations, the results of research and development, the need for currently unanticipated capital expenditures, competitive and technological advances, acquisitions and other factors. We cannot guarantee that we will be able to obtain additional customers for our products and services, or that such products and services will produce revenues adequate to fund our operating expenses. If we experience increased losses, we may have to seek additional financing from public or private sales of our securities, including equity securities. There can be no assurance that additional funding will be available when needed or on acceptable terms.
CRITICAL ACCOUNTING POLICIES
Our significant accounting policies are described in Note 1 to the consolidated financial statements included in Item 8 of this Form 10-K. We believe our most critical accounting policies include revenue recognition for agreements entered into with various collaborators and our intangible assets.
ArQule has entered into various collaborative agreements with pharmaceutical and biotechnology companies. Revenue from collaborative agreements includes non-refundable technology transfer fees, funding of compound development work, the delivery of compounds, payments based upon delivery of specialized compounds meeting the collaborators specified criteria, and certain milestones and royalties on net product sales. Non-refundable technology transfer fees are recognized as revenue when we have the contractual right to receive such payment, provided a contractual arrangement exists, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and we have no further performance obligations under the license agreement. When we have performance obligations under the terms of a contract, non-refundable fees are recognized as revenue as we complete our obligations. Where our level of effort is relatively constant over the performance period, the revenue is recognized on a straight-line basis. Funding of compound development work is recognized over the term of the applicable contract using the percentage of completion method using the proportional achievement of deliveries against a compound delivery schedule or the development labor is expended against a total research and development labor plan as the measure of progress toward completion. Any significant changes in the assumptions underlying our estimates to complete could impact our revenue recognition. Payments based upon delivery of specialized compounds meeting the collaborator's specified criteria are recognized as revenue once the collaborator has accepted the delivery of these compounds and collection is reasonably assured. Revenues from milestone payments related to arrangements under which we have no continuing performance obligations are recognized upon achievement of the related milestone. Revenues from milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as we complete our performance obligations. Payments received under these arrangements prior to the completion of the related work are recorded as deferred revenue. We believe our revenue recognition policies are in full compliance with Staff Accounting Bulletin 101, "Revenue Recognition in Financial Statements" ("SAB 101").
33
Intangible assets primarily relate to the value of the core technology and goodwill from our acquisition of Camitro Corporation. The cost of the core technology and goodwill is being amortized on a straight-line basis over seven years. Intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2001, the Financial Accounting Standards Board ("FASB") issued Statement of Financial Accounting Standards ("SFAS") No. 141, "Business Combinations" and SFAS No. 142, "Goodwill and Other Intangible Assets." SFAS No. 141 requires business combinations initiated after June 30, 2001 to be accounted for using the purchase method of accounting. It also specifies the types of acquired intangible assets that are required to be recognized and reported separately from goodwill. SFAS 142 will require that goodwill and certain intangibles no longer be amortized, but instead tested for impairment at least annually. SFAS 142 is required to be applied starting with fiscal years beginning after December 15, 2001, with early application permitted in certain circumstances. We will adopt SFAS 141 and SFAS 142 in fiscal 2002. The balance of goodwill is approximately $25.9 million at December 31, 2001. We do not expect any impairment of goodwill upon adoption. Goodwill will not be amortized beginning on January 1, 2002. Goodwill amortization expense was $4.0 million for the year ended December 31, 2001.
In June 2001, the FASB issued Statement of Financial Accounting Standards No. 143 ("SFAS 143"), "Accounting for Asset Retirement Obligations," which is effective January 1, 2003. SFAS 143 addresses the financial accounting and reporting for obligations and retirement costs related to the retirement of tangible long-lived assets. We do not expect that the adoption of SFAS 143 will have a significant impact on our financial statements.
In August 2001, the FASB issued Statement of Financial Accounting Standards No. 144 ("SFAS 144"), "Accounting for the Impairment or Disposal of Long-Lived Assets," which is effective January 1, 2002. SFAS 144 supersedes FASB Statement No 121, "Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of," and the accounting and reporting provisions relating to the disposal of long-lived assets. We do not expect that the adoption of SFAS 144 will have a significant impact on our financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We own financial instruments that are sensitive to market risk as part of our investment portfolio. Our investment portfolio is used to preserve our capital until it is used to fund operations, including our research and development activities. None of these market - risk sensitive instruments are held for trading purposes. We invest our cash primarily in money market mutual funds and U.S. Government and other investment grade debt securities. These investments are evaluated quarterly to determine the fair value of the portfolio. Our investment portfolio includes only marketable securities with active secondary or resale markets to help insure liquidity. We have implemented policies regarding the amount and credit ratings of investments. Due to the conservative nature of these policies, we do not believe we have material exposure due to market risk.
Our use of derivative financial instruments is limited to the utilization of two interest rate swap agreements. Settlement accounting is used for these interest rate swaps, whereby amounts to be paid or received under the interest rate swap agreements are accrued as interest rates change and are recognized as an adjustment to interest expense.
See Notes 2 and 9 to the Consolidated Financial Statements for a description of our use of derivatives and other financial instruments. The carrying amounts reflected in the consolidated balance sheet of cash and cash equivalents, trade receivables, and trade payables approximates fair value at December 31, 2001 due to the short-term maturities of these instruments.
34
ITEM 8. CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | Page
|
|---|
| Report of Independent Accountants | | 36 |
Consolidated Balance Sheet at December 31, 2000 and 2001 |
|
37 |
Consolidated Statement of Operations for the three years ended December 31, 2001 |
|
38 |
Consolidated Statement of Stockholders' Equity for the three years ended December 31, 2001 |
|
39 |
Consolidated Statement of Cash Flows for the three years ended December 31, 2001 |
|
40 |
Notes to Consolidated Financial Statements |
|
42 |
Consolidated Financial Statement Schedules: |
|
|
Schedules are not included because they are not applicable or the information is included in the Notes to Consolidated Financial Statements |
35
REPORT OF INDEPENDENT ACCOUNTANTS
To the Board of Directors and Stockholders of ArQule, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of stockholders' equity and of cash flows present fairly, in all material respects, the financial position of ArQule, Inc. and its subsidiaries at December 31, 2000 and 2001, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2001, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States of America, which require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PRICEWATERHOUSECOOPERS LLP
Boston, Massachusetts
January 18, 2002
36
ARQULE, INC.
CONSOLIDATED BALANCE SHEET
| | DECEMBER 31,
| |
|---|
| | 2000
| | 2001
| |
|---|
| | (IN THOUSANDS, EXCEPT
SHARE AND PER SHARE DATA)
| |
|---|
| ASSETS | | | | | | | |
| Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 86,079 | | $ | 43,260 | |
| | Marketable securities | | | 23,940 | | | 51,070 | |
| | Accounts receivable | | | 1,564 | | | 1,772 | |
| | Accounts receivable—related parties | | | 718 | | | 820 | |
| | Inventory | | | 400 | | | 625 | |
| | Prepaid expenses and other current assets | | | 1,326 | | | 1,440 | |
| | |
| |
| |
| | | | | | Total current assets | | | 114,027 | | | 98,987 | |
| Property and equipment, net | | | 33,699 | | | 54,018 | |
| Non-current marketable securities | | | — | | | 3,672 | |
| Intangible assets | | | — | | | 46,400 | |
| Other assets | | | 1,750 | | | 5,398 | |
| | |
| |
| |
| | | $ | 149,476 | | $ | 208,475 | |
| | |
| |
| |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
| Current liabilities: | | | | | | | |
| | Current portion of long term debt | | | 3,500 | | | 7,500 | |
| | Accounts payable and accrued expenses | | | 4,171 | | | 7,515 | |
| | Deferred revenue | | | 12,919 | | | 14,607 | |
| | |
| |
| |
| | | | | | Total current liabilities | | | 20,590 | | | 29,622 | |
| Deferred revenue | | | 1,266 | | | 414 | |
| Long term debt | | | 7,200 | | | 11,700 | |
| | |
| |
| |
| | | | | | Total liabilities | | | 29,056 | | | 41,736 | |
| | |
| |
| |
| Commitments (Note 11) | | | — | | | — | |
| Stockholders' equity: | | | | | | | |
| | Preferred stock, $0.01 par value; 1,000,000 shares authorized; no shares issued or outstanding | | | — | | | — | |
| | Common stock, $0.01 par value; 50,000,000 shares authorized; 17,072,727 and 21,116,419 shares issued and outstanding at December 31, 2000 and 2001, respectively | | | 171 | | | 211 | |
| | Additional paid-in capital | | | 151,084 | | | 242,776 | |
| | Unrealized gain on marketable securities | | | 29 | | | 78 | |
| | Cumulative translation adjustment | | | - | | | (107 | ) |
| | Deferred compensation | | | (181 | ) | | (4,509 | ) |
| | Accumulated deficit | | | (30,683 | ) | | (71,710 | ) |
| | |
| |
| |
| | | | | | Total stockholders' equity | | | 120,420 | | | 166,739 | |
| | |
| |
| |
| | | $ | 149,476 | | $ | 208,475 | |
| | |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
37
ARQULE, INC.
CONSOLIDATED STATEMENT OF OPERATIONS
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 1999
| | 2000
| | 2001
| |
|---|
| | (IN THOUSANDS, EXCEPT PER SHARE DATA)
| |
|---|
| Revenue | | | | | | | | | | |
| | Compound development revenue | | $ | 9,421 | | $ | 40,507 | | $ | 53,266 | |
| | Compound development revenue — related parties | | | 9,161 | | | 9,789 | | | 5,130 | |
| | |
| |
| |
| |
| | | | 18,582 | | | 50,296 | | | 58,396 | |
| | |
| |
| |
| |
| Costs and expenses: | | | | | | | | | | |
| | Cost of revenue | | | 8,851 | | | 13,888 | | | 22,657 | |
| | Cost of revenue — related parties | | | 8,606 | | | 7,455 | | | 6,784 | |
| | Research and development | | | 13,774 | | | 17,699 | | | 28,446 | |
| | Marketing, general and administrative | | | 6,022 | | | 8,293 | | | 12,353 | |
| | Stock-based compensation | | | 486 | | | 880 | | | 6,949 | |
| | Amortization of intangibles | | | — | | | — | | | 7,104 | |
| | In-process research and development | | | — | | | — | | | 18,000 | |
| | |
| |
| |
| |
| | | | 37,739 | | | 48,215 | | | 102,293 | |
| | |
| |
| |
| |
| | | Income (loss) from operations | | | (19,157 | ) | | 2,081 | | | (43,897 | ) |
| Investment income | | | 1,915 | | | 2,865 | | | 4,491 | |
| Interest expense | | | (191 | ) | | (1,091 | ) | | (1,621 | ) |
| | |
| |
| |
| |
| | | Net income (loss) | | $ | (17,433 | ) | $ | 3,855 | | $ | (41,027 | ) |
| | |
| |
| |
| |
| Basic net income (loss) per share | | $ | (1.38 | ) | $ | 0.28 | | $ | (2.06 | ) |
| | |
| |
| |
| |
| Weighted average common shares outstanding — basic | | | 12,606 | | | 13,911 | | | 19,905 | |
| | |
| |
| |
| |
| Diluted net income (loss) per share | | $ | (1.38 | ) | $ | 0.25 | | $ | (2.06 | ) |
| | |
| |
| |
| |
| Weighted average common shares outstanding — diluted | | | 12,606 | | | 15,208 | | | 19,905 | |
| | |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
38
ARQULE, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
| | COMMON STOCK
| |
| | UNREALIZED
GAIN
MARKETABLE
SECURITIES
| |
| |
| |
| |
| |
|---|
| | ADDITIONAL
PAID-IN
CAPITAL
| | CUMULATIVE
TRANSLATION
ADJUSTMENT
| | DEFERRED
COMPENSATION
| | ACCUMULATED
DEFICIT
| | TOTAL
STOCKHOLDERS'
EQUITY
| |
|---|
| | SHARES
| | PAR VALUE
| |
|---|
| | (IN THOUSANDS, EXCEPT SHARE DATA)
| |
|---|
| Balance at December 31, 1998 | | 12,171,335 | | $ | 122 | | $ | 71,432 | | $ | 0 | | $ | 0 | | $ | (182 | ) | $ | (17,105 | ) | $ | 54,267 | |
| Stock option exercises | | 536,473 | | | 5 | | | 888 | | | | | | | | | | | | | | | 893 | |
| Employee stock purchase plan | | 156,417 | | | 2 | | | 538 | | | | | | | | | | | | | | | 540 | |
| Compensation related to the grant of common stock options | | | | | | | | 309 | | | | | | | | | (309 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | | | | | | | 486 | | | | | | 486 | |
| Net loss | | | | | | | | | | | | | | | | | | | | (17,433 | ) | | (17,433 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 1999 | | 12,864,225 | | | 129 | | | 73,167 | | | 0 | | | 0 | | | (5 | ) | | (34,538 | ) | | 38,753 | |
| Stock option exercises | | 758,403 | | | 8 | | | 5,670 | | | | | | | | | | | | | | | 5,678 | |
| Employee stock purchase plan | | 92,099 | | | 1 | | | 533 | | | | | | | | | | | | | | | 534 | |
| Issuance of common stock in connection with secondary public offering, net of insurance costs of $4,864 | | 3,358,000 | | | 33 | | | 70,658 | | | | | | | | | | | | | | | 70,691 | |
| Compensation related to the grant of common stock options | | | | | | | | 1,056 | | | | | | | | | (1,056 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | | | | | | | 880 | | | | | | 880 | |
| Unrealized gain on marketable securities | | | | | | | | | | | 29 | | | | | | | | | | | | 29 | |
| Net income | | | | | | | | | | | | | | | | | | | | 3,855 | | | 3,855 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2000 | | 17,072,727 | | | 171 | | | 151,084 | | | 29 | | | 0 | | | (181 | ) | | (30,683 | ) | | 120,420 | |
| Stock option exercises | | 90,310 | | | 1 | | | 753 | | | | | | | | | | | | | | | 754 | |
| Employee stock purchase plan | | 93,958 | | | 1 | | | 827 | | | | | | | | | | | | | | | 828 | |
| Issuance of common stock in connection with the Camitro acquisition | | 3,103,567 | | | 31 | | | 81,871 | | | | | | | | | (12,552 | ) | | | | | 69,350 | |
| Issuance of common stock in connection with Pfizer investment in ArQule, Inc | | 755,857 | | | 7 | | | 9,516 | | | | | | | | | | | | | | | 9,523 | |
| Forfeiture of deferred compensation in connection with the Camitro acquisition | | | | | | | | (1,404 | ) | | | | | | | | 1,404 | | | | | | — | |
| Compensation related to the grant of common stock options | | | | | | | | 129 | | | | | | | | | (129 | ) | | | | | — | |
| Amortization of deferred compensation | | | | | | | | | | | | | | | | | 6,949 | | | | | | 6,949 | |
| Unrealized gain on marketable securities | | | | | | | | | | | 49 | | | | | | | | | | | | 49 | |
| Cumulative translation adjustment | | | | | | | | | | | | | | (107 | ) | | | | | | | | (107 | ) |
| Net loss | | | | | | | | | | | | | | | | | | | | (41,027 | ) | | (41,027 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2001 | | 21,116,419 | | $ | 211 | | $ | 242,776 | | $ | 78 | | $ | (107 | ) | $ | (4,509 | ) | $ | (71,710 | ) | $ | 166,739 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
39
ARQULE, INC.
CONSOLIDATED STATEMENT OF CASH FLOWS
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 1999
| | 2000
| | 2001
| |
|---|
| | (IN THOUSANDS)
| |
|---|
| INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | | | | | | | | | |
| Cash flows from operating activities: | | | | | | | | | | |
| | Net income (loss) | | $ | (17,433 | ) | $ | 3,855 | | $ | (41,027 | ) |
| | Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | |
| | | Depreciation and amortization | | | 7,690 | | | 7,350 | | | 8,971 | |
| | | Amortization of deferred compensation | | | 486 | | | 880 | | | 6,949 | |
| | | Amortization of goodwill and purchased intangibles | | | — | | | — | | | 7,104 | |
| | | Purchase of in-process research and development | | | — | | | — | | | 18,000 | |
| | Changes in assets and liabilities, net of acquisition: | | | | | | | | | | |
| | | Decrease (increase)in accounts receivable | | | 1,755 | | | 1,671 | | | (303 | ) |
| | | Decrease (increase) in inventory | | | 40 | | | 86 | | | (225 | ) |
| | | Decrease (increase) in prepaid expenses and other current assets | | | 290 | | | (747 | ) | | 35 | |
| | | (Increase) decrease in other assets | | | (158 | ) | | 64 | | | 1,883 | |
| | | Decrease in notes receivable from related parties | | | 30 | | | — | | | — | |
| | | Increase (decrease) in accounts payable and accrued expenses | | | 3,625 | | | (1,548 | ) | | 2,217 | |
| | | Increase (decrease) in deferred revenue | | | 16,427 | | | (5,148 | ) | | 836 | |
| | |
| |
| |
| |
| | | | Net cash provided by operating activities | | | 12,752 | | | 6,463 | | | 4,440 | |
| | |
| |
| |
| |
| Cash flows from investing activities: | | | | | | | | | | |
| | Purchases of marketable securities | | | (57,731 | ) | | (58,302 | ) | | (66,272 | ) |
| | Proceeds from sale or maturity of marketable securities | | | 53,608 | | | 66,604 | | | 35,519 | |
| | Investment in unconsolidated affiliates | | | — | | | — | | | (5,000 | ) |
| | Acquisitions, net of cash acquired | | | — | | | — | | | (1,834 | ) |
| | Proceeds from tenant improvement allowance | | | — | | | 2,335 | | | — | |
| | Additions to property and equipment | | | (23,962 | ) | | (9,291 | ) | | (28,095 | ) |
| | |
| |
| |
| |
| | | | Net cash (used in) provided by investing activities | | | (28,085 | ) | | 1,346 | | | (65,682 | ) |
| | |
| |
| |
| |
| Cash flows from financing activities: | | | | | | | | | | |
| | Principal payments of capital lease obligations | | | (897 | ) | | (316 | ) | | (1,182 | ) |
| | Borrowings of long term debt | | | 14,000 | | | — | | | 16,000 | |
| | Principal payments of long term debt | | | (775 | ) | | (2,525 | ) | | (7,500 | ) |
| | Proceeds from issuance of common stock, net | | | 1,433 | | | 76,903 | | | 11,105 | |
| | |
| |
| |
| |
| | | | Net cash provided by financing activities | | | 13,761 | | | 74,062 | | | 18,423 | |
| | |
| |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | (1,572 | ) | | 81,871 | | | (42,819 | ) |
| Cash and cash equivalents, beginning of period | | | 5,780 | | | 4,208 | | | 86,079 | |
| | |
| |
| |
| |
| Cash and cash equivalents, end of period | | $ | 4,208 | | $ | 86,079 | | $ | 43,260 | |
| | |
| |
| |
| |
40
Supplemental Disclosure of Cash Flow Information:
During 1999, 2000, and 2001 the Company paid approximately $191, $1,091 and $1,621, respectively, for interest expense.
Net assets and liabilities assumed in Camitro acquisition - see Note 6.
The accompanying notes are an integral part of these consolidated financial statements.
41
ARQULE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(IN THOUSANDS, EXCEPT SHARE AND PER SHARE DATA)
1. ORGANIZATION AND NATURE OF OPERATIONS
ArQule, Inc. is engaged in the discovery, development and production of novel chemical compounds primarily for the pharmaceutical and biotechnology industries. Our operations are focused on the integration of high throughput automated chemistry, structure-guided rational drug design, computational and predictive models of drug-like compound characteristics and other proprietary technologies which automate the process of chemical synthesis to produce arrays of novel small organic chemical compounds used to generate and optimize drug development and product development candidates. We are subject to risks common to companies in the biotechnology, medical device and diagnostic industries, included, but not limited to, development by the Company or our competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, and compliance with government regulations.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Significant accounting policies followed in the preparation of these financial statements are as follows:
The consolidated financial statements include the accounts of ArQule, Inc. and its wholly-owned subsidiary Camitro Corporation, including Camitro, U.K. Ltd., which was acquired on January 29, 2001 (collectively, "we", "us", "our" and the "Company"). All intercompany transactions and balances have been eliminated.
We consider all highly liquid investments purchased within three months of maturity date to be cash equivalents. We invest our available cash primarily in money market mutual funds and U.S. government and other investment grade debt securities that have strong credit ratings. As a matter of policy, we determine on a quarterly basis the fair market value of its investment portfolio. Our securities are recorded on our balance sheet at fair market value. Unrealized gains and losses on securities are included in shareholders equity, net of related tax effects. If the fair market value of a marketable security declines below its cost basis, and, based upon our consideration of all available evidence, we conclude such decline is "other than temporary", we mark the investment to market through a charge to current earnings. At December 31, 2000 and 2001, we have classified these investments as available-for-sale.
At December 31, 2000 and 2001, our financial instruments consist of cash, cash equivalents, marketable securities, accounts receivable, accounts payable, accrued expenses, debt and interest rate swaps. The carrying amounts of these instruments approximate their fair values.
Intangible assets primarily relate to the value of the core technology and goodwill from our acquisition of Camitro Corporation. The cost of the core technology is being amortized on a straight-
42
line basis over seven years. Intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Our foreign subsidiary, Camitro, U.K. Ltd., has designated the Great Britain Pound Sterling as its functional currency. Financial statements of this foreign subsidiary are translated to U.S. dollars for consolidation purposes using current rates of exchange for monetary assets and liabilities and historical rates of exchange for nonmonetary assets and related elements of expense. Revenue and other expense elements are translated at rates that approximate the rates in effect on the transaction dates. Related cumulative translation adjustments are included as a component of equity in the consolidated balance sheet.
Property and equipment are recorded at cost and depreciated using the straight-line method over their estimated useful lives. Assets under capital leases and leasehold improvements are amortized over the shorter of their estimated useful lives or the term of the respective leases by use of the straight-line method. Maintenance and repair costs are expensed as incurred.
ArQule has entered into various collaborative agreements with pharmaceutical and biotechnology companies. Revenue from collaborative agreements includes non-refundable technology transfer fees, funding of compound development work, the delivery of compounds, payments based upon delivery of specialized compounds meeting the collaborators specified criteria, and certain milestones and royalties on net product sales. Non-refundable technology transfer fees are recognized as revenue when we have the contractual right to receive such payment, provided a contractual arrangement exists, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and we have no further performance obligations under the license agreement. When we have performance obligations under the terms of a contract, non-refundable fees are recognized as revenue as we complete our obligations. Where our level of effort is relatively constant over the performance period, the revenue is recognized on a straight-line basis. Funding of compound development work is recognized over the term of the applicable contract using the percentage of completion method using the proportional achievement of deliveries against a compound delivery schedule or the development labor is expended against a total research and development labor plan as the measure of progress toward completion. Any significant changes in the assumptions underlying our estimates to complete could impact our revenue recognition. Payments based upon delivery of specialized compounds meeting the collaborator's specified criteria are recognized as revenue once the collaborator has accepted the delivery of these compounds and collection is reasonably assured. Revenues from milestone payments related to arrangements under which we have no continuing performance obligations are recognized upon achievement of the related milestone. Revenues from milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: the milestone payments are non-refundable; achievement of the milestone was not reasonably assured at the inception of the arrangement; substantive effort is involved in achieving the milestone; and the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as we complete our performance obligations. Payments received under these arrangements prior to the completion of the related work are recorded as deferred revenue. We believe our revenue recognition policies are in full compliance with Staff Accounting Bulletin 101, "Revenue Recognition in Financial Statements" ("SAB 101").
43
Cost of revenue represents the actual costs incurred in connection with performance pursuant to collaborative agreements and the costs incurred to develop and produce compounds. These costs consist primarily of payroll and payroll-related costs, chemicals, supplies and overhead expenses.
Costs of internal Research & development and Research & development done pursuant to collaborations are charged to operations as incurred. Development costs incurred in connection with funded development collaborations are included in cost of revenue. We incurred research and development expenses of $13,774, $17,699 and $46,446 in 1999, 2000 and 2001, respectively, including amounts assigned to acquired in-process technology of $18,000 in 2001. The value assigned to acquired in-process technology was determined by identifying and valuing those acquired in-process research projects for which (a) technological feasibility had not been established at the acquisition date, (b) there was no alternative future use, and (c) the fair value was estimable with reasonable reliability. See Note 6 for additional information.
Options granted to employees and members of the Board of Directors are accounted for in accordance with Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees" (APB No. 25) and related interpretations, including FASB Interpretation No.44, "Accounting for Certain Transactions Involving Stock Compensation." Under APB No. 25, no compensation expense is recognized for options granted at fair market value with fixed terms. We have adopted the disclosure requirements of Statement of Financial Accounting Standards No. 123, "Accounting for Stock Based Compensation" (SFAS No. 123). All stock-based awards granted to nonemployees, including members of the Scientific Advisory Board, are accounted for as prescribed by SFAS No. 123 and Emerging Issues Task Force 96-18, "Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods or Services."
Inventory, consisting only of raw materials used in producing our compound libraries is stated at the lower of cost, on a first-in, first-out basis, or market.
Management uses consolidated financial information in determining how to allocate resources and assess performance. For this reason, we have determined that we are principally engaged in one industry segment. We operate three facilities in the United States and we have a small research and development facility in Cambridge, UK, as a result of our acquisition of Camitro Corporation. See Note 15 with respect to significant customers. Substantially all of our revenue since inception has been generated in the United States and substantially all of our long lived assets are located in the United States.
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
44
Certain reclassifications have been made to the 1999 and 2000 financial statements to conform to the 2001 presentation.
The computations of basic and diluted earnings (loss) per common share are based upon the weighted average number of common shares outstanding and potentially dilutive securities. Potentially dilutive securities include stock options and warrants. Options to purchase of 2,604,493 and 3,397,277 shares of common stock were not included in the 1999 and 2001 computation of diluted net income (loss) per share, respectively, because inclusion of such shares would have an anti-dilutive effect on net loss per share.
Other comprehensive income (loss) refers to revenues, expenses, gains and losses that under accounting principles generally accepted in the United States are included in comprehensive income (loss) but are excluded from net income (loss) as these amounts are recorded directly as an adjustment to stockholders' equity, net of tax. Our other comprehensive income (loss) is composed of unrealized gains and losses on available-for-sale securities and foreign currency translation amounts relating to realized investment gains and losses and investment impairment charges are reclassified from other comprehensive income as they are included in net income.
The asset and liability approach is used to account for income taxes by recognizing deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. We record a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized.
In June 2001, the Financial Accounting Standards Board ("FASB") issued Statement of Financial Accounting Standards ("SFAS") No. 141, "Business Combinations" and SFAS No. 142, "Goodwill and Other Intangible Assets." SFAS No. 141 requires business combinations initiated after June 30, 2001 to be accounted for using the purchase method of accounting. It also specifies the types of acquired intangible assets that are required to be recognized and reported separately from goodwill. SFAS 142 will require that goodwill and certain intangibles no longer be amortized, but instead tested for impairment at least annually. SFAS 142 is required to be applied starting with fiscal years beginning after December 15, 2001, with early application permitted in certain circumstances. We will adopt SFAS 141 and SFAS 142 in fiscal 2002. The balance of goodwill is approximately $25,888 at December 31, 2001. We do not expect any impairment of goodwill upon adoption. Goodwill will not be amortized beginning on January 1, 2002. Goodwill amortization expenses were $3,967 for the year ended December 31, 2001.
In June 2001, the FASB issued Statement of Financial Accounting Standards No. 143 ("SFAS 143"), "Accounting for Asset Retirement Obligations," which is effective January 1, 2003. SFAS 143 addresses the financial accounting and reporting for obligations and retirement costs related to the retirement of tangible long-lived assets. We do not expect that the adoption of SFAS 143 will have a significant impact on our financial statements.
In August 2001, the FASB issued Statement of Financial Accounting Standards No. 144 ("SFAS 144"), "Accounting for the Impairment or Disposal of Long-Lived Assets," which is effective January 1,
45
2002. SFAS 144 supersedes FASB Statement No 121, "Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of," and the accounting and reporting provisions relating to the disposal of long-lived assets. We do not expect that the adoption of SFAS 144 will have a significant impact on our financial statements.
3. RELATED PARTIES
We have entered into a number of license, research and development agreements (the "Agreements") with corporate collaborators. Two agreements were entered into with Solvay Pharmaceuticals B.V. ("Solvay") and Wyeth Pharmaceuticals ("Wyeth"). Revenue related to these Agreements is included in compound development revenue-related parties. Solvay and Wyeth are related parties as they each have a representative on our Board of Directors. Subsequent to year end, in agreement with Wyeth, we discontinued our collaboration.
4. CASH EQUIVALENTS AND MARKETABLE SECURITIES
The following is a summary of the fair market value of available-for-sale marketable securities we held at December 31, 2000 and 2001:
| |
| | DECEMBER 31,
|
|---|
| | MATURITY
| | 2000
| | 2001
|
|---|
| U.S. Government obligations | | Within 1 year | | $ | 6,760 | | $ | 5,000 |
| Corporate bonds | | Within 18 months | | | 17,180 | | | 49,742 |
| | | | |
| |
|
| | | | | $ | 23,940 | | $ | 54,742 |
| | | | |
| |
|
At December 31, 2000 and 2001, marketable securities are carried at fair market value. The vast majority of our marketable securities are classified as current at December 31, 2000 and 2001 as the funds are highly liquid and are available to meet working capital needs and to fund current operations. We had approximately $3,672 in long-term marketable securities at December 31, 2001. The gross unrealized gains on marketable securities for the years ended December 31, 2000 and 2001 were $29 and $78, respectively.
5. PROPERTY AND EQUIPMENT
Property and equipment consist of the following:
| |
| | DECEMBER 31,
|
|---|
| | USEFUL LIFE
ESTIMATED
(YEARS)
|
|---|
| | 2000
| | 2001
|
|---|
| Land | | — | | $ | — | | $ | 6,487 |
| Buildings | | 30 | | | — | | | 14,230 |
| Machinery and equipment | | 5 | | | 17,185 | | | 20,954 |
| Leasehold improvements | | 3-20 | | | 26,785 | | | 26,752 |
| Furniture and fixtures | | 7 | | | 1,653 | | | 1,701 |
| Computer equipment | | 3 | | | 9,097 | | | 11,324 |
| Construction-in-progress | | — | | | 3,087 | | | 1,225 |
| | | | |
| |
|
| | | | | | 57,807 | | | 82,673 |
| Less-accumulated depreciation and amortization | | | | | 24,108 | | | 28,655 |
| | | | |
| |
|
| | | | | $ | 33,699 | | $ | 54,018 |
| | | | |
| |
|
Assets held under capital leases at December 31, 2000 consisted of $1,900 of machinery and equipment, $1,785 of leasehold improvements, $703 in computer equipment and $107 of furniture and fixtures.
46
Accumulated amortization of these assets totaled $4,495 at December 31, 2000. For the years ended December 31, 1999 and 2000, amortization expense related to assets held under capital lease obligations was $889 and $316 respectively. We retired these assets on January 1, 2001. Total depreciation and amortization expense for the years ended December 31, 1999, 2000 and 2001 was $7,690, $7,350 and $8,971, respectively.
6. ACQUISITION OF CAMITRO CORPORATION
On January 29, 2001, we acquired Camitro Corporation, a privately held predictive modeling company based in Menlo Park, California in a transaction accounted for as a purchase business combination. Pursuant to the terms of the merger agreement, we issued approximately 3.4 million shares of our common stock and $1,733 in cash in exchange for all of Camitro's outstanding shares and the assumption of all of Camitro's outstanding stock options and warrants. The merger transaction was valued at $84,268 based on our share price on the measurement date for the merger. The results of operations of Camitro, the estimated fair value of the assets acquired and liabilities assumed are included in our financial statements from the date of acquisition.
The purchase price was allocated to the identifiable tangible and intangible assets acquired and liabilities assumed based on our estimates of fair value at the acquisition date. The purchase price exceeded the amounts allocated to the identifiable tangible and intangible assets acquired and liabilities assumed by $29,699. This excess was classified as goodwill, which was being amortized on a straight-line basis over its estimated useful life of seven years through December 2001. Under SFAS 142, our goodwill will no longer be amortized, but instead testing annually for impairment.
The following table shows the allocation of the purchase price for the acquisition of Camitro:
BALANCE SHEET CATEGORY
| | VALUE ASSIGNED TO
ASSETS & LIABILITIES
ACQUIRED
| |
|---|
| Current assets | | $ | 980 | |
| Property, plant and equipment, net | | | 1,195 | |
| Intangible assets: | | | | |
| | Acquired core technology | | | 23,600 | |
| | Assembled workforce | | | 200 | |
| | In-process R & D | | | 18,000 | |
| | Deferred compensation | | | 12,552 | |
| | Goodwill | | | 29,699 | |
| Other assets | | | 244 | |
| Short term liabilities | | | (1,442 | ) |
| Long term liabilities | | | (760 | ) |
| | |
| |
| | | $ | 84,268 | |
| | |
| |
Approximately $18,000 of the purchase price represents the estimated fair value of the purchased in-process research and development ("IPR&D") that had not yet reached technological feasibility and had no alternative future use. Accordingly, this amount was immediately expensed in the consolidated statement of operations upon the acquisition date. The value assigned to IPR&D technology was comprised of the initial suite of ADMET (absorption, distribution, metabolism, elimination, toxicity) models and the upgrade suite of ADME models. The valuation of the IPR&D was determined using the discounted cash flow method. Revenue and expense projections, as well as technology assumptions, were prepared through 2008 based on information provided by Camitro's management. Revenue projections for each in-process development project were identified as follows: (1) revenue derived from products relying on core technology, and (2) revenue derived from projects relying on a new IPR&D project. The projected cash flows, adjusted based on probability of success, were discounted
47
using a 50% rate for core technology and a 60% rate for in-process technology. The fair value of IPR&D was determined separately from all other acquired assets using the income approach. Management is responsible for the assumptions used to determine the estimated fair value of the IPR&D.
As we seek to change the nature of the drug discovery process, we will continue to improve the predictive modeling and other aspects of our integrated technology platform. In March 2002, we signed a technology access agreement with Pharmacia Corporation granting Pharmacia non-exclusive access to its proprietary ADMET simulation technology, the foundation of which is the computational predictive models developed by Camitro. According to the agreement, Pharmacia will have access to ArQule's technology for the purposes of generating ADMET predictions about the nature of Pharmacia's compounds. The agreement allows specified access for one year, with an option for renewal.
The following unaudited pro forma financial summary is presented as if the operations of ArQule and Camitro were combined as of January 1, 2000 and January 1, 2001, respectively. The unaudited pro forma combined results are not necessarily indicative of the actual results that would have occurred had the acquisition been consummated on those dates, or of the future operations of the combined entities. The following pro forma financial summary does not include the charge for in process Research and Development of $18.0 million as this is a material non-recurring charge.
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 2000
| | 2001
| |
|---|
| Revenue | | $ | 50,666 | | $ | 58,396 | |
| Net loss | | | (15,842 | ) | | (23,849 | ) |
| Basic and diluted net loss per share | | | (0.93 | ) | | (1.20 | ) |
7. INTANGIBLES AND OTHER ASSETS
Intangibles and other assets include the following:
| | DECEMBER 31,
|
|---|
| | 2000
| | 2001
|
|---|
| Goodwill | | $ | — | | $ | 25,890 |
| Core technology | | | — | | | 20,510 |
| | |
| |
|
| | | Total intangibles | | $ | — | | $ | 46,400 |
| | |
| |
|
| Other assets include: | | | | | | |
| | Investment in unconsolidated affiliates | | $ | — | | $ | 5,000 |
| | Security deposits | | | 1,750 | | | 338 |
| | Other | | | — | | | 60 |
| | |
| |
|
| | | | Total other assets | | $ | 1,750 | | $ | 5,398 |
| | |
| |
|
In connection with our acquisition of Camitro, we recognized amortization expense in 2001 of $4,013 and $3,091 for goodwill and core technology, respectively.
In July 2001, we purchased approximately 1.8 million preferred shares of a privately owned proteomics company for $5,000. This represented an approximately 8% ownership interest. We are accounting for this under the cost method as we do not exert significant influence in the Company. This investment is included in other assets on the Consolidated Balance Sheet.
48
8. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses include the following:
| | DECEMBER 31,
|
|---|
| | 2000
| | 2001
|
|---|
| Accounts payable | | $ | 1,867 | | $ | 2,041 |
| Accrued payroll | | | 1,647 | | | 2,627 |
| Accrued professional fees | | | 182 | | | 552 |
| Accrued lease termination | | | 150 | | | 800 |
| Other accrued expenses | | | 325 | | | 1,495 |
| | |
| |
|
| | | $ | 4,171 | | $ | 7,515 |
| | |
| |
|
Included in other accrued expenses in 2001 is $758 reserved for future fixed asset purchases in connection with our Pfizer collaboration.
9. DEBT
In March 1999, we entered into a term loan agreement with Fleet National Bank ("Fleet"). The terms of this agreement allow for borrowings up to a maximum of $15,000 based on 80% of qualifying property and equipment purchases, provided that we comply with certain covenants, including the maintenance of specified financial ratios. Borrowings under this facility are classified as either "Tranche A" (term loans entered into before June 30, 1999) or "Tranche B" (term loans entered into between July 1, 1999 and June 30, 2000). Principal amounts due are payable in 16 equal quarterly installments beginning on September 30, 1999 and September 30, 2000 for "Tranche A" and "Tranche B" borrowings, respectively. Interest payments are made monthly in arrears beginning on the first day of the month following commencement of this agreement and accrue at one month LIBOR plus 1.75%. We entered into an interest rate swap agreement with Fleet primarily to reduce the impact of changes in interest rates on our term loan agreement. At December 31, 2001, we had two interest rate swaps with notional amounts of $2,325 and $4,875 respectively. Under these agreements, we will pay Fleet at rates of 7.94% and 8.99%, respectively. Settlement accounting is used for these interest rate swaps which expire on June 2003 and June 2004, respectively. The impact on our financial position and results of operations from likely changes in interest rates is not material, as our hedging of transactions is limited to these specific liabilities. In March 2001, we amended and extended our term loan agreement with Fleet National Bank to borrow an additional $16,000 in order to finance our facility and land purchase ("Tranche C"). Principal amounts are payable in 16 equal quarterly installments beginning on April 1, 2001. Interest payments are made monthly in arrears beginning on the first day of the month following commencement of the amended agreement. Interest accrues at the rate of one month LIBOR plus 1.75%. On December 31, 2001, our interest rate on Tranche C was 3.68%. As of December 31, 2001, we had total outstanding balances of $2,325, $4,875 and $12,000, respectively, on our "Tranche A", "Tranche B" and "Tranche C" loans. This facility is collateralized by all of our property and equipment.
Our principal amounts due under the term loan agreement are as follows:
YEAR ENDING
DECEMBER 31,
| | TRANCHE A
| | TRANCHE B
| | TRANCHE C
|
|---|
| 2002 | | | 1,550 | | | 1,950 | | | 4,000 |
| 2003 | | | 775 | | | 1,950 | | | 4,000 |
| 2004 | | | — | | | 975 | | | 4,000 |
| | |
| |
| |
|
| | Total payments due | | $ | 2,325 | | $ | 4,875 | | $ | 12,000 |
| | |
| |
| |
|
49
10. STOCKHOLDERS' EQUITY
We are authorized to issue up to one million shares of preferred stock. As of December 31, 2001 and 2000 there were no outstanding shares of preferred stock. Our Board of Directors will determine the terms of the preferred stock if and when the shares are issued.
Our amended Certificate of Incorporation authorizes the issuance of up to 50 million shares of $0.01 par value common stock.
On November 15, 2000, we completed a follow-on offering of 3,358,000 shares of common stock at $22.50 per share, which included the underwriters' exercise of their over-allotment of 438,000 shares of common stock on November 21, 2000. We realized total net proceeds of approximately $70,691.
At December 31, 2001, we have 5,122,423 common shares reserved for purchase of common stock under the Employee Stock Purchase Plan and for the exercise of common stock options pursuant to the Equity Incentive Plan, the Directors Plan and the options acquired from Camitro's Stock Plan.
As part of the exchange of all of Camitro's outstanding shares, stock options and warrants on January 29, 2001 for ArQule's, we acquired Restricted Common Stock that was still subject to vesting at the time of the merger. This Restricted Stock is subject to stock repurchase agreements whereby we have the right to repurchase any unvested shares in the event of termination of employment with the company, at which time we will immediately cancel all such forfeited shares. Shares subject to this agreement vest over four year periods. As of December 31, 2001, the number of unvested common shares is 138,169.
11. STOCK OPTION PLANS
During 2001, our stockholders approved an amendment to the 1994 Amended and Restated Equity Incentive Plan (the "Equity Incentive Plan") increasing the number of shares of common stock available for awards under the Equity Incentive Plan to 6,700,000. All shares are awarded at the discretion of our Board of Directors in a variety of stock-based forms including stock options and restricted stock. Pursuant to the Equity Incentive Plan, incentive stock options may not be granted at less than the fair market value of our common stock at the date of the grant, and the option term may not exceed ten years. For holders of 10% or more of our voting stock, options may not be granted at less than 110% of the fair market value of the common stock at the date of the grant, and the option term may not exceed five years. Stock appreciation rights granted in tandem with an option shall have an exercise price not less than the exercise price of the related option. As of December 31, 2001, no stock appreciation rights have been issued. In September 2001, a sub-plan, known as the "2001 Inland Revenue Approved Sub-Plan" was added to the 1994 Amended and Restated Equity Incentive Plan and was approved by our Board of Directors. This plan grants Approved stock options to the employees of the United Kingdom from the Equity Incentive Plan available awards pool. During 2001, 60,381 Approved stock options were granted. At December 31, 2001, there were 1,571,402 shares available for future grant under the Equity Incentive Plan.
Subject to the restrictions above, the Board of Directors is authorized to designate the options, awards, and purchases under the Equity Incentive Plan, the number of shares covered by each option, award and purchase, and the related terms, exercise dates, prices and methods of payment.
50
On January 29, 2001, we assumed Camitro Corporation's outstanding options under the Camitro Stock Plan. Each Camitro Stock Option assumed by ArQule will continue to have the same terms and conditions, including vesting schedule, set forth in the Camitro Stock Plan, except for the number of whole shares which have been converted from 1,055,500 to 292,157 based upon the Option Conversion Factor defined in the Merger Agreement. As of December 31, 2001, 262,513 ArQule options were outstanding from the acquired Camitro Stock Plan. There are no longer any shares available for future grant under this Plan.
During 2001, the 1996 Director Stock Option Plan (the "Director Plan") for non-employee directors had 190,500 shares of common stock available for awards. Under this plan, eligible directors are automatically granted once a year, at our annual meeting of stockholders, options to purchase 3,500 shares of common stock, which are exercisable on the date of grant. Upon initial election of an eligible director, options to purchase 7,500 shares of common stock will be granted which will become exercisable in three equal annual installments commencing on the date of our next annual stockholders' meeting held after the date of grant. All options granted pursuant to the Director Plan have a term of ten years with exercise prices equal to fair market value on the date of grant. Through December 31, 2001, options to purchase 151,500 shares of common stock have been granted under this plan of which 139,000 shares are currently exercisable. As of December 31, 2001, 39,000 shares are available for future grant.
During 2000 and 2001, we issued 25,500 and 10,000 options, respectively, to certain members of our Scientific Advisory Board (SAB) under the Equity Incentive Plan. In 1999, 18,000 shares were cancelled. Compensation expense in 2000 and 2001 was $660 and $129, respectively. Also during 2000 and 2001, compensation expenses of $220 and $69 respectively was recorded for employees who received non-qualified stock options at below fair market value on the date of grant. The remaining employee stock compensation of $112 as of December 31, 2001 is being amortized as compensation expense over the vesting period of the options.
Compensation expense for 2001 related to the acquired Camitro stock options that were unvested at the time of acquisition was $6,751. The remaining deferred compensation related to the stock options of Camitro of $4,397 as of December 31, 2001 is being amortized as compensation expense over the vesting period of the options and shares.
We apply APB No. 25 and related interpretations in accounting for employee grants under the Equity Incentive Plan. Had compensation cost been determined based on the estimated, value of options at the grant date consistent with the provisions of SFAS No. 123, our pro forma net loss, pro forma basic net loss per share and diluted net loss per share would have been as follows:
| | DECEMBER 31,
| |
|---|
| | 1999
| | 2000
| | 2001
| |
|---|
| Pro forma net loss | | $ | (21,746 | ) | $ | (3,321 | ) | $ | (46,848 | ) |
| Pro forma basic and diluted net loss per share | | $ | (1.73 | ) | $ | (0.24 | ) | $ | (2.35 | ) |
For the purposes of pro forma disclosure, the estimated value of each employee and non-employee option grant was calculated on the date of grant using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, option-pricing models require the use of highly subjective assumptions, including the expected stock price volatility. Because our employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective assumptions can materially affect the fair value estimates, in management's opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock-based compensation. The model was calculated using the following weighted-average assumptions: no dividend yield for all years; 75% volatility for 1999, 95% for 2000
51
and 2001; risk-free interest rates of 5.46% in 1999, 6.0% in 2000 and 4.24% in 2001; and expected lives of 4 years in 1999, 2000 and 2001 for options granted.
Option activity under the Plans for the three years ended December 31, 2001 was as follows:
STOCK OPTIONS
| | NUMBER
OF SHARES
| | WEIGHTED
AVERAGE
EXERCISE
PRICE
|
|---|
| Outstanding at December 31, 1998 | | 2,338,586 | | $ | 6.68 |
| Granted | | 1,050,755 | | | 4.65 |
| Assumed in acquisition | | 71,552 | | | .21 |
| Exercised | | (536,473 | ) | | 1.67 |
| Cancelled | | (243,125 | ) | | 7.36 |
| | |
| | | |
| Outstanding at December 31, 1999 | | 2,681,295 | | | 6.83 |
| Granted | | 597,658 | | | 18.05 |
| Assumed in acquisition | | 69,887 | | | .55 |
| Exercised | | (758,403 | ) | | 7.39 |
| Cancelled | | (183,362 | ) | | 8.10 |
| | |
| | | |
| Outstanding as of December 31, 2000 | | 2,407,075 | | | 9.50 |
| Granted | | 1,112,875 | | | 13.83 |
| Assumed in acquisition | | 150,718 | | | 1.27 |
| Exercised | | (122,112 | ) | | 6.16 |
| Cancelled | | (151,279 | ) | | 12.11 |
| Outstanding as of December 31, 2001 | | 3,397,277 | | | 10.18 |
| | |
| | | |
| Exercisable at December 31, 2001 | | 1,475,816 | | | 9.30 |
| | |
| | | |
| Weighted average estimated value of options granted during the year ended December 31, 2001 | | | | | 10.01 |
The following table summarizes information about options outstanding at December 31, 2001:
| | OPTIONS OUTSTANDING
| | OPTIONS EXERCISABLE
|
|---|
RANGE OF EXERCISE PRICES
| | NUMBER
OUTSTANDING AT
DECEMBER 31,
2001
| | WEIGHTED
AVERAGE
REMAINING
CONTRACTUAL
LIFE
| | WEIGHTED
AVERAGE
EXERCISE
PRICE
| | EXERCISABLE
AS OF
DECEMBER 31,
2001
| | WEIGHTED
AVERAGE
EXERCISE
PRICE
|
|---|
| $0.0000—2.8000 | | 295,013 | | 7.6 | | $ | 0.88 | | 93,437 | | $ | 0.55 |
| 2.8001—5.6000 | | 1,144,735 | | 7.0 | | | 4.71 | | 700,861 | | | 4.74 |
| 5.6001—8.4000 | | 289,874 | | 7.4 | | | 7.14 | | 149,999 | | | 6.45 |
| 8.4001—11.2000 | | 611,455 | | 8.9 | | | 10.11 | | 52,500 | | | 10.56 |
| 11.2001—14.0000 | | 61,000 | | 9.1 | | | 12.24 | | 7,500 | | | 12.00 |
| 14.0001—16.8000 | | 51,850 | | 6.5 | | | 15.19 | | 40,600 | | | 15.34 |
| 16.8001—19.6000 | | 535,100 | | 7.9 | | | 18.05 | | 328,275 | | | 17.94 |
| 19.6001—22.4000 | | 208,500 | | 8.2 | | | 20.06 | | 64,833 | | | 20.14 |
| 22.4001—25.2000 | | 59,000 | | 8.0 | | | 23.07 | | 27,874 | | | 23.01 |
| 25.2001—28.0000 | | 140,750 | | 9.0 | | | 28.00 | | 9,937 | | | 28.00 |
| | |
| | | | | | |
| | | |
| | | 3,397,277 | | 7.8 | | $ | 10.18 | | 1,475,816 | | $ | 9.30 |
| | |
| | | | | | |
| | | |
In 1996, the stockholders adopted the 1996 Employee Stock Purchase Plan (the "Purchase Plan"). This plan enables eligible employees to exercise rights to purchase our common stock at 85% of the fair market value of the stock on the date the right was granted or the date the right is exercised, whichever is lower. Rights to purchase shares under the Purchase Plan are granted by the Board of
52
Directors. The rights are exercisable during a period determined by the Board of Directors; however, in no event will the period be longer than twenty-seven months. The Purchase Plan is available to substantially all employees, subject to certain limitations. At December 31, 2001, 405,261 shares have been purchased pursuant to the Purchase Plan. In May 1999 the Board of Directors approved an increase from 120,000 shares reserved to 420,000 shares reserved of common stock for purchase under the Purchase Plan. This increase was approved at the May 2000 annual meeting of stockholders. In May 2001 annual meeting, the shareholders approved an additional 100,000 shares of common stock was reserved under the Purchase Plan. As of December 31, 2001, there were 114,739 shares available for future sale under the Employee Stock Purchase Plan.
12. Income Taxes
The current and deferred tax expenses for the years ended December 31, 1999, 2000 and 2001 are as follows:
| | 1999
| | 2000
| | 2001
|
|---|
| Current: | | | | | | | | | |
| | Federal | | $ | — | | $ | — | | $ | — |
| | State | | | — | | | 1 | | | 2 |
| | Foreign (U.K.) | | | — | | | — | | | 32 |
| | |
| |
| |
|
| | | $ | — | | $ | 1 | | $ | 34 |
| | |
| |
| |
|
| | 1999
| | 2000
| | 2001
|
|---|
| Deferred: | | | | | | | | | |
| | Federal | | $ | — | | $ | (140 | ) | $ | — |
| | State | | | — | | | 211 | | | — |
| | Foreign (U.K.) | | | — | | | — | | | — |
| | |
| |
| |
|
| | | | — | | | 71 | | | — |
| Valuation allowance | | | — | | | (71 | ) | | — |
| | |
| |
| |
|
| | | $ | — | | $ | — | | $ | — |
| | |
| |
| |
|
The following is a reconciliation between the U.S. federal statutory rate and the effective rate for the years ended December 31, 1999, 2000 and 2001, respectively:
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 1999
| | 2000
| | 2001
| |
|---|
| Income tax benefit (expense) at statutory rate | | $ | 6,102 | | $ | (1,311 | ) | $ | 13,949 | |
| State tax benefit (expense), net of Federal tax benefit (expense) | | | 1,076 | | | (390 | ) | | 1,073 | |
| Permanent items | | | — | | | (3 | ) | | (6,130 | ) |
| Losses and credits without current tax benefit | | | (7,170 | ) | | — | | | — | |
| Effect of change in valuation allowance | | | — | | | (71 | ) | | (4,070 | ) |
| Credits | | | — | | | 1,774 | | | 1,456 | |
| Other | | | (8 | ) | | — | | | (6,312 | ) |
| | |
| |
| |
| |
| | Tax expense | | $ | — | | $ | (1 | ) | $ | (34 | ) |
| | |
| |
| |
| |
53
The deferred tax consequences of temporary differences in reporting items for financial statement and income tax purposes are recognized, if appropriate. Realization of the future tax benefits related to the deferred tax assets is dependent on many factors, including our ability to generate taxable income within the net operating loss carryforward period. Management has considered these factors in reaching its conclusion as to the valuation allowance for financial reporting purposes. The income tax effect of temporary differences comprising the deferred tax assets and deferred tax liabilities on the accompanying balance sheets is a result of the following:
| | YEAR ENDED DECEMBER 31,
| |
|---|
| | 1999
| | 2000
| | 2001
| |
|---|
| | Preoperating costs capitalized for tax purposes | | $ | 189 | | $ | 164 | | $ | 233 | |
| | Net operating loss carryforwards | | | 6,671 | | | 11,417 | | | 20,037 | |
| | Tax credit carryforwards | | | 3,773 | | | 6,790 | | | 8,179 | |
| | Equity based compensation | | | 486 | | | 830 | | | 3,599 | |
| | Book depreciation in excess of tax | | | 1,361 | | | 411 | | | (481 | ) |
| | Reserves and accruals | | | 329 | | | 138 | | | 888 | |
| | Deferred revenue | | | 6,362 | | | 4,523 | | | 4,434 | |
| | Other | | | 18 | | | 6 | | | 27 | |
| | |
| |
| |
| |
| | | $ | 19,189 | | $ | 24,279 | | $ | 36,916 | |
| Deferred tax liabilities: | | | | | | | | | | |
| Intangible asset | | $ | — | | $ | — | | $ | (8,170 | ) |
| Valuation allowance | | | (19,189 | ) | | (24,279 | ) | | (28,746 | ) |
| | |
| |
| |
| |
| | Net deferred tax assets | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
Of the $28,746 valuation allowance at December 31, 2001, $5,247 relating to deductions for nonqualified stock options will be credited to paid-in capital, if realized.
As of December 31, 2001, we had federal net operating loss ("NOL") and research & development credit carryforwards of approximately $52,714 and $5,016, respectively, which can be used to offset future federal income tax liabilities and expire at various dates through 2021. As required by Statement of Accounting Standards No. 109, we evaluated positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of net operating loss and research & development credit carryforwards. We have determined that it is more likely than not that we will not recognize the benefits of federal and state deferred tax assets and, as a result, a valuation allowance of approximately $28,746 has been established at December 31, 2001.
Ownership changes, as defined in the Internal Revenue Code, may have limited the amount of net operating loss carryforwards that can be utilized annually to offset future taxable income. Subsequent ownership changes could further affect the limitation in future years.
54
13. COLLABORATIONS
The following table summarizes our collaborations with pharmaceutical companies:
COMPANY
| | PRODUCTS/SERVICES PROVIDED
|
|---|
| Pfizer Inc. | | Technology transfer of AMAP Chemistry Operating System, Design Tools, Lead Generation Capabilities and Custom Array libraries |
| Bayer AG | | Custom Array libraries |
| Wyeth Pharmaceuticals | | Mapping Array and Directed Array libraries |
| Solvay Pharmaceuticals B.V. | | Mapping Array, Compass Array and Directed Array Libraries and a non-exclusive license to our AMAP Chemistry Operating System |
| G.D. Searle, a division of | | |
| | Pharmacia Corp. | | Mapping Array, Compass Array and Directed Array libraries and lead optimization services |
| Sankyo Company, Ltd. | | Mapping Array, Compass Array and Directed Array libraries |
| Johnson & Johnson, Inc. | | Mapping Array libraries and Compass Array libraries |
| GlaxoSmithKline | | Compass Array libraries and lead optimization services |
| Abbott Laboratories | | Mapping Array and Directed Array libraries |
| Roche Bioscience | | Directed Array libraries |
In July 1999, we entered into a four and one half year technology acquisition agreement with Pfizer Inc. We manage and staff a dedicated facility containing an AMAP Chemistry Operating System for Pfizer in Medford, Massachusetts. The facility produces Custom Array libraries exclusively for Pfizer. Pfizer owns all rights in compounds produced at this facility. In addition, we have trained Pfizer staff to use our AMAP Chemistry Operating System. On December 31, 2003, Pfizer will receive a non-exclusive license to the AMAP Chemistry Operating System. In December 2001, we entered into a new seven year agreement with Pfizer which superceded our July 1999 agreement. In addition to continuing to provide custom array libraries for Pfizer, we will also transfer our library design tools on a non-exclusive basis to Pfizer. Pfizer has also committed to perform one lead optimization program with ArQule. We may earn up to $345,000 over the length of the contract, depending on the achievement of certain delivery milestones. Pfizer has committed to maintain certain cash payments resulting from our prior collaboration as it relates to the transfer of our AMAP chemistry operation system. As of March 1, 2002, we have received $80,549 under these agreements. Pfizer has also agreed to make equity investments totaling $18,000 depending on the achievement of certain delivery milestones. Pfizer made a $10,000 equity investment in December 2001. Pfizer may terminate the new agreement after four years for any reason. Pfizer will pay no milestones or royalties to us on compounds that they develop and market.
In October 1999, we entered into a three-year collaboration with Bayer AG to produce Custom Array libraries. In October 2001, we extended the production period until June 30, 2003. Bayer will own all rights in compounds for an initial period, after which we will co-own rights in compounds that Bayer has not claimed in a patent application. We received a $3,000 upfront payment and will receive up to an additional $27,000 during the term of the agreement for delivery and success fees. As of March 1, 2002, we have received $17,541 under this agreement. Bayer will pay no milestones or royalties to us on compounds that they develop and market.
55
In July 1997, we entered into a four and one half year agreement with Wyeth Pharmaceuticals ("Wyeth"). Under this agreement, Wyeth subscribed to our Mapping Array and Directed Array Programs. We discontinued our Mapping Array program as of 2002, and as a consequence and in agreement with Wyeth, we did not renew our collaboration. Wyeth made a $2,000 equity investment in ArQule in June 1998. As of March 1, 2002, we have received all $26,134 that we are due under this agreement. Wyeth has agreed to pay us development milestones and royalties from the sales of products resulting from compounds we shipped during the collaboration. To date, we have not received any milestone or royalty payments.
In November 1995, we entered into a five-year agreement with Solvay Duphar B.V. Under this agreement, Solvay subscribed to our Mapping Array and Directed Array programs and received a non-exclusive license to our AMAP Chemistry Operating System. This agreement was superseded by an amended and restated agreement with Solvay Pharmaceuticals B.V., which became effective on January 1, 2001. The amended agreement extends the collaboration through December 31, 2003. Under the amended agreement, Solvay receives our Compass Array libraries and continues to access our Mapping Array libraries and Directed Array programs. We discontinued our Mapping Array program as of 2002 as planned. The remainder of the contract was not affected. As of March 1, 2002, we have received $19,877 under these agreements. Solvay has also agreed to make additional payments if we achieve certain development milestones and to pay royalties on sales of any drugs that result from the relationship. To date, we have not received any milestone or royalty payments. In connection with the original collaboration, signed in November 1995, an affiliate of Solvay, Physica B.V., made a $7,000 equity investment in ArQule.
We entered into a five-year collaboration with Monsanto Company (now Pharmacia Corporation) in December 1996. Under this agreement, we provided Monsanto with access to our Mapping and Directed Array programs for use in the development of agrochemicals. In January 2000, we expanded this collaboration to cover life science applications, including pharmaceutical use by Monsanto's G.D. Searle division, and extended the term until December 2002. We also agreed to provide Monsanto with Compass Array and Mapping Array libraries through 2001 and Compass Array libraries only through December 2002. We also converted the Monsanto agrochemical Directed Array Program into a credit for pharmaceutical lead optimization services. Pharmacia is committed to make payments totaling $13,000 under this agreement. In addition, Monsanto has agreed to pay us development milestones and royalties from the sales of products resulting from the collaboration. In July 1998, we received a milestone payment for a Mapping Array compound selected by Monsanto for entry into field trials. On June 30, 2000, in connection with the merger between Monsanto and Pharmacia, we replaced our existing collaboration agreement with a new collaboration agreement with G.D. Searle & Co., a division of Pharmacia. The financial terms of the new agreement are substantially the same as the prior agreement. However, we expanded the scope of the agreement to enable Pharmacia and its affiliates to screen our compounds, which may result in milestone and royalty payments in the future. To date, we have received $12,443 under this agreement. In March 2002, we entered into a one year technical access agreement with Pharmacia which granted them non-exclusive access to our proprietary ADMET simulation technology.
In November 1997, we entered into a three-year agreement with Sankyo Company, Ltd. to discover and optimize drug candidates. Under the terms of the agreement, Sankyo received a subscription to
56
our Mapping Array program to discover new lead compounds. Sankyo also committed to a minimum number of Directed Array Programs during the term of the agreement. In April 2001, we extended our agreement with Sankyo through 2004 to include the Compass Array libraries in addition to continuing to use our Directed Array program. The total value of the extended agreement is up to $14,800 in committed payments. To date, we have received $11,080 under both agreements. Sankyo has also agreed to pay us developmental milestones and royalties resulting from sales of any products resulting from this collaboration. To date, we have not received any milestone or royalty payments under this agreement.
In December 1998, we entered into a three-year collaboration with R.W. Johnson Pharmaceutical Research Institute, a division of Johnson & Johnson, Inc., in which R.W. Johnson subscribed to our Mapping Array program. We discontinued our Mapping Array program as of 2002, and, as a consequence and in agreement with R.W. Johnson, we did not renew our collaboration. As of March 1,2002, we have received all $8,592 that we are due under this agreement. In addition, R.W. Johnson has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. To date, we have not received any milestone or royalty payments.
In November 2000, we entered into a five-year collaboration and license agreement with SmithKline Beecham Corporation (now GlaxoSmithKline). Under the terms of the agreement, GlaxoSmithKline receives access to our Compass Array libraries and Mapping Array libraries for screening primarily in the anti-infective field. In addition, we have initiated the first of potentially two drug discovery programs based on a lead compound discovered in a GlaxoSmithKline compound library. GlaxoSmithKline receives all rights in compounds developed in these drug discovery programs. As of March 1, 2002, we have received $1,194 under this collaboration. GlaxoSmithKline may terminate the agreement before the end of the five-year term. GlaxoSmithKline has agreed to pay us development milestones and royalties on sales of products resulting from the collaboration. To date, we have not received any milestone or royalty payments.
In June 1995, we entered into an agreement with Abbott Laboratories. Under this agreement Abbott subscribed to our Mapping Array and Directed Array programs. This collaboration was extended on two occasions and ended successfully in March 1999. Abbott has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. We have not received any milestone or royalty payments from Abbott.
In September 1996, we entered into an agreement with Roche Bioscience. Under this agreement, we synthesized Directed Array compounds. Our obligations under this agreement ended in March 1999. Roche Bioscience has agreed to pay us developmental milestones and royalties from sales of any products resulting from this collaboration. In May 1999, we received a milestone payment from Roche Bioscience for a Directed Array compound that was chosen for Investigational New Drug application, or IND, enabling toxicology studies.
Biotechnology Collaborations
In the past, we have entered into a number of collaborations with biotechnology companies, primarily providing them with access to our Mapping Array libraries. Going forward, we intend to enter
57
into only a select number of focused biotechnology collaborations with companies that can contribute significant numbers of important targets, generally around a specific target class or therapeutic area. These collaborations should pool the necessary resources from each company to conduct a drug discovery program aimed at delivering at least one IND candidate per collaboration.
Genome Therapeutics Corporation. On October 17, 2000, we entered into a collaborative drug discovery agreement with Genome Therapeutics Corporation to discover and develop anti-infective drug candidates. Under the agreement, we will use our Parallel Track Drug Discovery program to screen and optimize compounds against a significant number of proprietary validated anti-infective targets which Genome Therapeutics has derived from its PathoGenome(TM) Database. We will share equally in all downstream value created by the collaboration, including future milestone, royalty and upfront payments resulting from the outlicensing of clinical candidates or later stage compounds derived from the collaboration.
ACADIA Pharmaceuticals. On December 18, 2000, ArQule and ACADIA Pharmaceuticals entered into a drug discovery collaboration. Under the agreement, ACADIA offers its functional genomics platform and ArQule contributes its Parallel Track™ Drug Discovery program to discover novel small molecule drug candidates directed at individual G-protein coupled receptor (GPCR) targets. We will share intellectual property resulting from the collaboration, and equally contribute to at least one joint drug discovery program. We will share any revenues resulting from the commercialization of joint drug discovery programs. In addition to these joint drug discovery programs, each of us will receive exclusive rights to certain compounds that we have decided not to develop in a joint drug discovery program, subject to a royalty payment to the other party. On April 7, 1998, we entered into a material transfer and screening agreement with ACADIA. Under this agreement, we provided to ACADIA access to certain ArQule compound arrays for screening against their target collection. On May 10, 2000, we entered into a compound license agreement with ACADIA. Under this agreement, we granted to ACADIA an exclusive license to certain of our compounds having activity against certain of their targets, in return for milestone payments and royalties.
14. COMMITMENTS
We lease facilities and equipment under noncancelable operating leases. The future minimum lease commitments under these leases are as follows:
YEAR ENDING
DECEMBER 31,
| | OPERATING
LEASES
|
|---|
| 2002 | | | 2,148 |
| 2003 | | | 1,724 |
| 2004 | | | 1,596 |
| 2005 | | | 1,492 |
| 2006 | | | 786 |
| | |
|
| | Total minimum lease payments | | $ | 7,746 |
| | |
|
Rent expense under noncancelable operating leases was approximately $1,420, $2,228 and $1,863 for the years ended December 31, 1999, 2000 and 2001, respectively.
Our research facilities also include approximately 56,000 square feet of laboratory and office space in Medford, Massachusetts, the majority of which is dedicated to the Pfizer collaboration. We lease these facilities under two lease agreements, one of which expires on July 30, 2005 and one of which expires on July 30, 2006. We sublease these facilities pursuant to three sublease agreements.
58
On August 1, 2001, Cummings Properties, LLC ("Cummings") significantly raised our rent on the lease that expires July 30, 2006. We believe this increase to be in excess of market rates and, as a result, we are disputing the increase. We have accrued the difference between our estimate of market rate rent and amounts due to us under the subleases for the leased space we do not occupy. While we are currently paying the rental rate proposed by our landlord, we are currently disputing the amount of the rent increase with the landlord. If the amount of rent we must pay is in excess of our current estimate of market rates, we will need to recognize additional expense.
On January 16, 2001, we brought a complaint for declaratory relief and damages against Cummings Properties, LLC ("Cummings") arising out of Cummings' attempts to avoid its contractual obligations under our two Medford, Massachusetts leases entered into between our company and Cummings and Cummings' attempts to increase our lease rates to what we believe is a higher amount than is due under the lease agreement. We seek recovery of the funds that we have already paid under protest, without waiver of any rights, to Cummings in response to Cummings' threats to accelerate rental payments and commence eviction proceedings against us.
In connection with our acquisition of Camitro Corporation on January 29, 2001, we assumed Camitro's existing lease for approximately 24,958 square feet of office space in Menlo Park, California, which will expire on September 30, 2002. Prior to expiration, we have the option to renew this lease for a term of 18 months. Our lease payments are $46,282 per month.
In January 2001, Camitro Corporation, through its wholly-owned subsidiary Camitro UK, Ltd. leased approximately 10,000 square feet of office and laboratory space in Cambridge, England for 15,416 British Pounds per month (approximately $21,891 per month as of March 1, 2002). We lease this facility under an agreement which expires in December 2005. In March 2001, we executed a two year sublease with a third party for approximately 4,000 square feet of the premises for 7,333 British Pounds per month (approximately $10,413 per month as of March 1, 2002). This sublease extends until March 2003.
15. CONCENTRATION OF CREDIT RISK
Revenues from five of our customers accounted for 30%, 20%, 16%, 13% and 12% of total revenues during 1999. Revenues from five of our customers accounted for 60%, 12%, 11%, 7% and 4% of total revenues during 2000. Revenues from five of our customers accounted for 62%, 16%, 6%, 4% and 4% of total revenues during 2001. Four of our customers accounted for 42%, 32%, 12%, and 11% of our accounts receivable balance at December 31, 2000. Three of our customers accounted for 33%, 30% and 16% of our accounts receivable balance at December 31, 2001. We do not require collateral on accounts receivable balances.
59
16. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
| | First
Quarter
| | Second
Quarter
| | Third
Quarter
| | Fourth
Quarter
| |
|---|
| 2001 | | | | | | | | | | | | | |
| Net revenues | | $ | 13,941 | | $ | 13,672 | | $ | 14,334 | | $ | 16,449 | |
| Gross profit | | | 7,410 | | | 6,991 | | | 6,568 | | | 7,986 | |
| Net loss | | | (20,256 | ) | | (6,121 | ) | | (7,539 | ) | | (7,111 | ) |
| Net loss per share, (diluted) | | $ | (1.07 | ) | $ | (0.31 | ) | $ | (0.37 | ) | $ | (0.35 | ) |
| 2000 | | | | | | | | | | | | | |
| Net revenues | | $ | 10,388 | | $ | 12,084 | | $ | 13,785 | | $ | 14,039 | |
| Gross profit | | | 4,982 | | | 6,767 | | | 8,744 | | | 8,460 | |
| Net income (loss) | | | (1,342 | ) | | 609 | | | 2,978 | | | 1,610 | |
| Net income (loss) per share, (diluted) | | $ | (0.10 | ) | $ | 0.04 | | $ | 0.20 | | $ | 0.10 | |
| 1999 | | | | | | | | | | | | | |
| Net revenues | | $ | 4,012 | | $ | 4,591 | | $ | 4,860 | | $ | 5,119 | |
| Gross profit | | | 338 | | | 672 | | | 768 | | | (653 | ) |
| Net loss | | | (3,966 | ) | | (3,594 | ) | | (3,657 | ) | | (6,216 | ) |
| Net loss per share, (diluted) | | $ | (0.32 | ) | $ | (0.29 | ) | $ | (0.29 | ) | $ | (0.49 | ) |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
None.
60
PART III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT
The response to this item is contained in part under the caption "Executive Officers of the Registrant" in Part I, Item 1A of this Annual Report of Form 8-K and the remainder is incorporated by reference into this Annual Report on Form 10-K from the discussion responsive thereto under the caption "Election of Directors" in the our Proxy Statement relating to our 2001 Annual Meeting of Stockholders scheduled for May 16, 2002.
ITEM 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference into this Annual Report of Form 10-K from the discussion responsive thereto under the caution "Executive Compensation" in our Proxy Statement relating to our 2001 Annual Meeting of Stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The response to this item is incorporated by reference into this Annual Report on Form 10-K from the discussion responsive thereto under the caption "Share Ownership" in our Proxy Statement relating to our 2001 Annual Meeting of Stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
See "Compensation Committee Interlocks and Insider Participation" under the caption "Executive Compensation" in Part III, Item 11 of this Annual Report on Form 10-K.
PART IV
ITEM 14. EXHIBITS, FINANCIAL STATEMENT SCHEDULE AND REPORTS ON FORM 8-K
| |
|
|---|
| (a) 1. | | FINANCIAL STATEMENTS
The financial statements are listed under Item 8 of this report. |
| 2. | | Financial Statement Schedules
The financial statement schedules listed under Item 8 of this report are omitted because they are not applicable or required information and are shown in the financial statements of the footnotes thereto. |
| (b) | | REPORTS ON FORM 8-K DURING FOURTH QUARTER OF 2001
On December 21, 2001, we filed on Form 8-K to disclose the expanded strategic alliance between ArQule, Inc. and Pfizer Inc. |
| (c) | | EXHIBITS |
EXHIBIT
NO.
| | DESCRIPTION
|
|---|
| 2.1 | | Agreement and Plan of Merger among the Company, Camitro Acquisition Corporation, Camitro Corporation, and certain stockholders of Camitro Corporation dated as of January 16, 2001. Previously filed as Exhibit 2.1 to the Company's Current Report on Form 8-K (File No. 000-21429). Filed with the Commission on February 1, 2001 and incorporated herein by reference. |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Company. Filed as Exhibit 3.1 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
61
| 3.2 | | Amended and Restated By-laws of the Company. Filed as Exhibit 3.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 4.1 | | Specimen Common Stock Certificate. Filed as Exhibit 4.1 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.1* | | Amended and Restated 1994 Equity Incentive Plan, as amended through May 17, 2001. Filed as Exhibit 99.1 to the Company's Registration Statement on Form S-8 filed on August 21, 2001 (File No. 333-68058) and incorporated herein by reference. |
| 10.2* | | Amended and Restated 1996 Employee Stock Purchase Plan. Filed as Exhibit 99.1 to the Company's Registration Statement on Form S-8 filed on August 21, 2001 (File No. 333-68058) and incorporated herein by reference. |
| 10.3* | | Amended and Restated 1996 Director Stock Option Plan. Filed As Appendix C to the Company's Proxy Statement on Schedule 14A filed with the Commission on April 14, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.4 | | Form of Indemnification Agreement between the Company and its directors. Such agreements are materially different only as to the signing directors and the dates of execution. Filed as Exhibit 10.4 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.5 | | Lease Agreement, dated July 27, 1995, between the Company and Cummings Properties Management, Inc. as amended. Filed as Exhibit 10.7 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.6+ | | Research, Development and License Agreement between the Company and Solvay Duphar B.V. dated November 2, 1995. Filed as Exhibit 10.14 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.6.1+ | | Amended and Restated Research, Development and License Agreement between Solvay Pharmaceuticals B.V. and the Company, dated as of January 1, 2001. Filed as exhibit 10.1 to the Company's Quarterly Report of Form 10-Q for the quarter ended March 31, 2001 (File No. 000-21429) and incorporated herein by reference. |
| 10.7+ | | Research & Development and License Agreement between the Company and Abbott Laboratories dated June 15, 1995, as amended. Filed as Exhibit 10.15 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.8* | | Adoption Agreement for Fidelity Management and Research Company (the Company's 401(k) plan). Filed as Exhibit 10.18 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.9+ | | Research and License Agreement between the Company and Roche Bioscience dated September 13, 1996. Filed as Exhibit 10.19 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.10+ | | Array Delivery and Testing Agreement between the Company and Monsanto Company dated as of December 23, 1996. Filed as Exhibit 10.20 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
| 10.11+ | | Amendment No. 2 to Research & Development License Agreement between the Company and Abbott Laboratories dated as of December 24, 1996. Filed as Exhibit 10.21 to the Company's incorporated herein by reference. |
62
| 10.12 | | Lease Agreement, dated December 20, 1996 between the Company and Cummings Property Management, Inc. Filed as Exhibit 10.22 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
| 10.13+ | | Research and License Agreement between the Company and American Home Products Corporation acting through its Wyeth-Ayerst Research Division dated July 3, 1997. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 1997 (File No. 000-21249) and incorporated herein by reference. |
| 10.14+ | | Second Amendment to Option Agreement and Research and Development Agreement between the Company and Amersham Pharmacia Biotech AB dated September 23, 1996. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1997 (File No. 000-21429) and incorporated herein by reference. |
| 10.15+ | | Third Amendment to Option Agreement and Research and Development Agreement between the Company and Amersham Pharmacia Biotech AB dated June 24, 1997. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1997 (File No. 000-21429) and incorporated herein by reference. |
| 10.16+ | | Research and Development Agreement between the Company and Sankyo Co., Ltd. dated November 1, 1997. Filed as Exhibit 10.29 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1997, filed with the Commission on March 17, 1998 (File No. 000-21429) and incorporated herein by reference. |
| 10.16.1+ | | Amended and Restated Research and Development Agreement between the Company and Sankyo Co., Ltd., dated as of April 2, 2001. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2001 (File No. 000-21429) and incorporated herein by reference. |
| 10.17+ | | Amendment No. 3 to Research & Development and License Agreement between the Company and Abbott Laboratories dated December 23, 1997. Filed as Exhibit 10.30 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1997 filed with the Commission on March 17, 1998 (File No. 000-21249) and incorporated herein by reference. |
| 10.18+ | | Research Collaboration and License Agreement between the Company and Amersham Pharmacia Biotech AB dated August 13, 1998. Filed as Exhibit 10.1 to the Company's Registration Statement on Form S-3 (File No. 333-62203) and incorporated herein by reference. |
| 10.19+ | | Commercialization Agreement between the Company and Amersham Pharmacia Biotech AB dated August 13, 1998. Filed as Exhibit 10.2 to the Company's Registration Statement on Form S-3 (File No. 333-62203) and incorporated herein by reference. |
| 10.20+ | | Amendment No. 1 to Research and License Agreement between the Company and Roche Bioscience, a division of Syntex, Inc., dated as of September 30, 1998. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1998 (File No. 000-21429) and incorporated herein by reference. |
| 10.21 | | Lease by and between MetroNorth Corporate Center LLC and the Company dated as of May 29, 1998. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1998 (File No. 000-21429) and incorporated herein by reference. |
63
| 10.22+ | | Compound Supply and License Agreement between the Company and R.W. Johnson Pharmaceutical Research Institute, a Division of Ortho-McNeil Pharmaceutical, Inc., dated as of December 15, 1998. Filed as Exhibit 10.36 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1999, filed with the Commissioner on March 29, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.23+ | | Employment agreement between Dr. Stephen A. Hill and the Company dated as of December 8, 1998, as amended. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.24 | | Term loan agreement between Fleet National Bank and the Company, dated as of March 18, 1999. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the Quarter ended March 31, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.25* | | Technology Acquisition Agreement between Pfizer Inc and the Company, dated as of July 19, 1999. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter Ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.26+ | | Sublease between Pfizer Inc. and the Company, dated July 16, 1999. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.27+ | | Research Cooperation Agreement between Bayer AG and the Company, dated October 1, 1999. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter Ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.28* | | Employment Agreement with Philippe Bey, dated July 21, 1999. Filed as Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.29+ | | Amended and Restated Array Delivery and Testing Agreement between the Company and Monsanto Company dated as of January 11, 2000. Filed as Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the Commission on March 15, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.30+ | | Array Delivery and Testing Agreement between the Company and G.D. Searle & Co. dated June 20, 2000. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.31+ | | Termination Agreement between the Company and Pharmacia Corporation dated June 30, 2000. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.32+ | | Technology Transfer and License Agreement between the Company and Amersham Pharmacia Biotech A.B. dated July 10, 2000. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the Quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
64
| 10.33* | | Employment agreement between the Company and Harold E. Selick Dated as of January 29, 2001. Filed as Exhibit 10.33 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.34 | | Lease between Camitro Corporation and WVP Income Plus 3 dated February 4, 1999, as amended. Filed as Exhibit 10.34 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.35+ | | Compound Discovery Collaboration Agreement between the Company and Genome Therapeutics Corporation dated October 17, 2000. Filed as Exhibit 10.1 to the Company's Registration Statement on Form S-3 filed With the Commission on October 20, 2000 (File No. 333-48358) and incorporated herein by reference. |
| 10.36+ | | Collaboration and License Agreement between the Company and SmithKline Beecham Corporation dated November 27, 2000. Filed as Exhibit 10.36 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.37+ | | Compound Discovery Collaboration Agreement between the Company and ACADIA Pharmaceuticals, Inc. dated December 18, 2000. Filed as Exhibit 10.37 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.38+ | | Amendment No. 1 to the Compound Supply and License Agreement between the Company and R.W. Johnson Pharmaceutical Research Institute, a division of Ortho-McNeil Pharmaceutical, Inc. dated as of August 14, 2000. Filed as Exhibit 99.1 to the Company's Current Report on Form 8-K (File No. 000-21429) filed with the Commission on October 17, 2000 and incorporated herein by reference. |
| 10.39+ | | Collaboration Agreement between Pfizer Inc and the Company, dated as of December 19, 2001. Filed herewith. |
| 21.1 | | Subsidiaries of the Company. Filed herewith. |
- *
- Indicates a management contract or compensatory plan.
- +
- Certain confidential material contained in the document has been omitted and filed separately, with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended or Rule 24b-2 of the Securities and Exchange Act of 1934, as amended.
65
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Woburn, Commonwealth of Massachusetts, on March 27, 2002.
| | | ARQULE, INC. |
|
|
By: |
/s/ STEPHEN A. HILL
Stephen A. Hill
President and Chief Executive Officer |
SIGNATURE
| | TITLE
| | DATE
|
|---|
|
|
|
|
|
/s/ STEPHEN A. HILL
Stephen A. Hill | | President, Chief Executive Officer and Director (Principal Executive Officer) | | March 27, 2002 |
/s/ DAVID C. HASTINGS
David C. Hastings |
|
Vice President, Chief Financial Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer) |
|
March 27, 2002 |
/s/ LAURA AVAKIAN
Laura Avakian |
|
Director |
|
March 27, 2002 |
/s/ TIMOTHY C. BARABE
Timothy C. Barabe |
|
Director |
|
March 27, 2002 |
/s/ WERNER CAUTREELS
Werner Cautreels |
|
Director |
|
March 27, 2002 |
/s/ ARIEL ELIA
Ariel Elia |
|
Director, Chairman of the Board |
|
March 27, 2002 |
/s/ TUAN HA-NGOC
Tuan Ha-Ngoc |
|
Director |
|
March 27, 2002 |
/s/ MICHAEL ROSENBLATT
Michael Rosenblatt |
|
Director |
|
March 27, 2002 |
66
EXHIBIT INDEX
EXHIBIT
NO.
| | DESCRIPTION
|
|---|
| 2.1 | | Agreement and Plan of Merger among the Company, Camitro Acquisition Corporation, Camitro Corporation, and certain stockholders of Camitro Corporation dated as of January 16, 2001. Previously filed as Exhibit 2.1 to the Company's Current Report on Form 8-K (File No. 000-21429). Filed with the Commission on February 1, 2001 and incorporated herein by reference. |
| 3.1 | | Amended and Restated Certificate of Incorporation of the Company. Filed as Exhibit 3.1 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
| 3.2 | | Amended and Restated By-laws of the Company. Filed as Exhibit 3.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 4.1 | | Specimen Common Stock Certificate. Filed as Exhibit 4.1 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.1* | | Amended and Restated 1994 Equity Incentive Plan, as amended through May 17, 2001. Filed as Exhibit 99.1 to the Company's Registration Statement on Form S-8 filed on August 21, 2001 (File No. 333-68058) and incorporated herein by reference. |
| 10.2* | | Amended and Restated 1996 Employee Stock Purchase Plan. Filed as Exhibit 99.1 to the Company's Registration Statement on Form S-8 filed on August 21, 2001 (File No. 333-68058) and incorporated herein by reference. |
| 10.3* | | Amended and Restated 1996 Director Stock Option Plan. Filed As Appendix C to the Company's Proxy Statement on Schedule 14A filed with the Commission on April 14, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.4 | | Form of Indemnification Agreement between the Company and its directors. Such agreements are materially different only as to the signing directors and the dates of execution. Filed as Exhibit 10.4 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.5 | | Lease Agreement, dated July 27, 1995, between the Company and Cummings Properties Management, Inc. as amended. Filed as Exhibit 10.7 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.6+ | | Research, Development and License Agreement between the Company and Solvay Duphar B.V. dated November 2, 1995. Filed as Exhibit 10.14 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.6.1+ | | Amended and Restated Research, Development and License Agreement between Solvay Pharmaceuticals B.V. and the Company, dated as of January 1, 2001. Filed as exhibit 10.1 to the Company's Quarterly Report of Form 10-Q for the quarter ended March 31, 2001 (File No. 000-21429) and incorporated herein by reference. |
| 10.7+ | | Research & Development and License Agreement between the Company and Abbott Laboratories dated June 15, 1995, as amended. Filed as Exhibit 10.15 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.8* | | Adoption Agreement for Fidelity Management and Research Company (the Company's 401(k) plan). Filed as Exhibit 10.18 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.9+ | | Research and License Agreement between the Company and Roche Bioscience dated September 13, 1996. Filed as Exhibit 10.19 to the Company's Registration Statement on Form S-1 (File No. 333-11105) and incorporated herein by reference. |
| 10.10+ | | Array Delivery and Testing Agreement between the Company and Monsanto Company dated as of December 23, 1996. Filed as Exhibit 10.20 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
| 10.11+ | | Amendment No. 2 to Research & Development License Agreement between the Company and Abbott Laboratories dated as of December 24, 1996. Filed as Exhibit 10.21 to the Company's incorporated herein by reference. |
| 10.12 | | Lease Agreement, dated December 20, 1996 between the Company and Cummings Property Management, Inc. Filed as Exhibit 10.22 to the Company's Registration Statement on Form S-1 (File No. 333-22945) and incorporated herein by reference. |
| 10.13+ | | Research and License Agreement between the Company and American Home Products Corporation acting through its Wyeth-Ayerst Research Division dated July 3, 1997. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 1997 (File No. 000-21249) and incorporated herein by reference. |
| 10.14+ | | Second Amendment to Option Agreement and Research and Development Agreement between the Company and Amersham Pharmacia Biotech AB dated September 23, 1996. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1997 (File No. 000-21429) and incorporated herein by reference. |
| 10.15+ | | Third Amendment to Option Agreement and Research and Development Agreement between the Company and Amersham Pharmacia Biotech AB dated June 24, 1997. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1997 (File No. 000-21429) and incorporated herein by reference. |
| 10.16+ | | Research and Development Agreement between the Company and Sankyo Co., Ltd. dated November 1, 1997. Filed as Exhibit 10.29 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1997, filed with the Commission on March 17, 1998 (File No. 000-21429) and incorporated herein by reference. |
| 10.16.1+ | | Amended and Restated Research and Development Agreement between the Company and Sankyo Co., Ltd., dated as of April 2, 2001. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2001 (File No. 000-21429) and incorporated herein by reference. |
| 10.17+ | | Amendment No. 3 to Research & Development and License Agreement between the Company and Abbott Laboratories dated December 23, 1997. Filed as Exhibit 10.30 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1997 filed with the Commission on March 17, 1998 (File No. 000-21249) and incorporated herein by reference. |
| 10.18+ | | Research Collaboration and License Agreement between the Company and Amersham Pharmacia Biotech AB dated August 13, 1998. Filed as Exhibit 10.1 to the Company's Registration Statement on Form S-3 (File No. 333-62203) and incorporated herein by reference. |
| 10.19+ | | Commercialization Agreement between the Company and Amersham Pharmacia Biotech AB dated August 13, 1998. Filed as Exhibit 10.2 to the Company's Registration Statement on Form S-3 (File No. 333-62203) and incorporated herein by reference. |
| 10.20+ | | Amendment No. 1 to Research and License Agreement between the Company and Roche Bioscience, a division of Syntex, Inc., dated as of September 30, 1998. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1998 (File No. 000-21429) and incorporated herein by reference. |
| 10.21 | | Lease by and between MetroNorth Corporate Center LLC and the Company dated as of May 29, 1998. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1998 (File No. 000-21429) and incorporated herein by reference. |
| 10.22+ | | Compound Supply and License Agreement between the Company and R.W. Johnson Pharmaceutical Research Institute, a Division of Ortho-McNeil Pharmaceutical, Inc., dated as of December 15, 1998. Filed as Exhibit 10.36 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1999, filed with the Commissioner on March 29, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.23+ | | Employment agreement between Dr. Stephen A. Hill and the Company dated as of December 8, 1998, as amended. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.24 | | Term loan agreement between Fleet National Bank and the Company, dated as of March 18, 1999. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the Quarter ended March 31, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.25* | | Technology Acquisition Agreement between Pfizer Inc and the Company, dated as of July 19, 1999. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter Ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.26+ | | Sublease between Pfizer Inc. and the Company, dated July 16, 1999. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.27+ | | Research Cooperation Agreement between Bayer AG and the Company, dated October 1, 1999. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter Ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.28* | | Employment Agreement with Philippe Bey, dated July 21, 1999. Filed as Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 1999 (File No. 000-21429) and incorporated herein by reference. |
| 10.29+ | | Amended and Restated Array Delivery and Testing Agreement between the Company and Monsanto Company dated as of January 11, 2000. Filed as Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the Commission on March 15, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.30+ | | Array Delivery and Testing Agreement between the Company and G.D. Searle & Co. dated June 20, 2000. Filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.31+ | | Termination Agreement between the Company and Pharmacia Corporation dated June 30, 2000. Filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.32+ | | Technology Transfer and License Agreement between the Company and Amersham Pharmacia Biotech A.B. dated July 10, 2000. Filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the Quarter ended June 30, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.33* | | Employment agreement between the Company and Harold E. Selick Dated as of January 29, 2001. Filed as Exhibit 10.33 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.34 | | Lease between Camitro Corporation and WVP Income Plus 3 dated February 4, 1999, as amended. Filed as Exhibit 10.34 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.35+ | | Compound Discovery Collaboration Agreement between the Company and Genome Therapeutics Corporation dated October 17, 2000. Filed as Exhibit 10.1 to the Company's Registration Statement on Form S-3 filed With the Commission on October 20, 2000 (File No. 333-48358) and incorporated herein by reference. |
| 10.36+ | | Collaboration and License Agreement between the Company and SmithKline Beecham Corporation dated November 27, 2000. Filed as Exhibit 10.36 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.37+ | | Compound Discovery Collaboration Agreement between the Company and ACADIA Pharmaceuticals, Inc. dated December 18, 2000. Filed as Exhibit 10.37 to the Company's Annual Report on Form 10-K for the year ended December 31, 2000 (File No. 000-21429) and incorporated herein by reference. |
| 10.38+ | | Amendment No. 1 to the Compound Supply and License Agreement between the Company and R.W. Johnson Pharmaceutical Research Institute, a division of Ortho-McNeil Pharmaceutical, Inc. dated as of August 14, 2000. Filed as Exhibit 99.1 to the Company's Current Report on Form 8-K (File No. 000-21429) filed with the Commission on October 17, 2000 and incorporated herein by reference. |
| 10.39+ | | Collaboration Agreement between Pfizer Inc and the Company, dated as of December 19, 2001. Filed herewith. |
| 21.1 | | Subsidiaries of the Company. Filed herewith. |
- *
- Indicates a management contract or compensatory plan.
- +
- Certain confidential material contained in the document has been omitted and filed separately, with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended or Rule 24b-2 of the Securities and Exchange Act of 1934, as amended.
QuickLinks
DOCUMENTS INCORPORATED BY REFERENCEPART IPART IIINDEX TO CONSOLIDATED FINANCIAL STATEMENTSREPORT OF INDEPENDENT ACCOUNTANTSARQULE, INC.CONSOLIDATED BALANCE SHEETARQULE, INC.CONSOLIDATED STATEMENT OF OPERATIONSARQULE, INC.CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITYARQULE, INC.CONSOLIDATED STATEMENT OF CASH FLOWSARQULE, INC.NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (IN THOUSANDS, EXCEPT SHARE AND PER SHARE DATA)PART IIIPART IVSIGNATURESEXHIBIT INDEX

