Materials necessary to manufacture our product candidates currently under development may not be available on commercially reasonable terms, or at all, which may delay our development and commercialization of these drugs.
Some of the materials necessary for the manufacture of our product candidates currently under development may, from time to time, be available either in limited quantities, or from a limited number of manufacturers, or both. We and/or our collaborators need to obtain these materials for our clinical trials and, potentially, for commercial distribution when and if we obtain marketing approval for these compounds. Suppliers may not sell us these materials at the time we need them or on commercially reasonable terms. If we and/or our collaborators are unable to obtain the materials needed for the conduct of our clinical trials, product testing and potential regulatory approval could be delayed, adversely impacting our ability to develop the product candidates. If it becomes necessary to change suppliers for any of these materials or if any of our suppliers experience a shutdown or disruption in the facilities used to produce these materials, due to technical, regulatory or other problems, it could significantly hinder or prevent manufacture of our drug candidates and any resulting products.
RISKS RELATED TO COMPETITION
The drug research and development industry is highly competitive, and we compete with some companies that have a broader range of capabilities and better access to resources than we do.
The pharmaceutical and biotechnology industries are characterized by rapid and continuous technological innovation. We compete with companies worldwide that are engaged in the research and discovery, licensing, development and commercialization of drug candidates, including, in the area of small molecule anti-cancer therapeutics, biotechnology companies such as Amgen, Inc., Ariad Pharmaceuticals, Inc., Astellas Pharma, Inc., Array BioPharma Inc., Astex Therapeutics, Cell Therapeutics, Inc., Curis, Inc., Cytokinetics, Inc., Exelixis, Inc., Evotec AG, GlaxoSmithKline, Gilead, FORMA Therapeutics, Incyte Corporation, Infinity Pharmaceuticals, Inc., Novartis, Pfizer, Roche and many others.
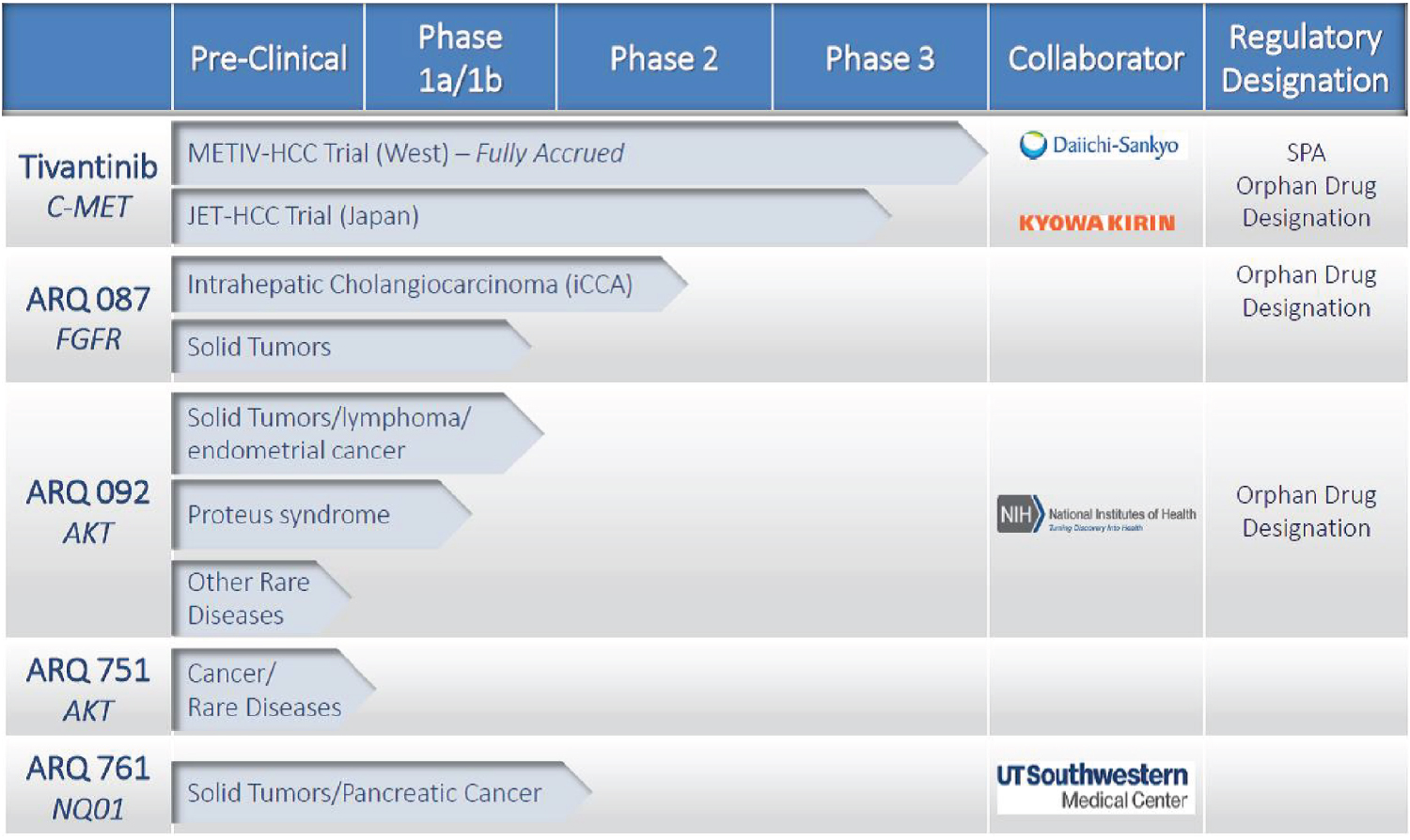
In addition, with respect to tivantinib, we are aware of a number of companies that are or may be pursuing a number of different approaches to MET inhibition, including Amgen Inc., AstraZeneca/Hutchison MediPharma, AVEO Pharmaceuticals, Inc., Bristol-Myers Squibb Company, Exelixis, Inc., Incyte, Gilead, GlaxoSmithKline, Johnson & Johnson, Merck, Pfizer, Roche, Takeda and others. With respect to HCC, our lead indication, we are aware of a number of companies with products under development, including Abbott, Bayer, Bristol-Myers Squibb, Celgene, Dainippon Sumitomo, Eisai Co., Eli Lilly, Incyte, Merck, Novartis, Polaris Group, Roche, Servier, and 4SC AG. There can be no assurance that our competitors will not develop more effective or more affordable products or technology or achieve earlier product development and commercialization than ArQule, thus rendering our technologies and/or products obsolete, uncompetitive or uneconomical.
With respect to ARQ 087, we are aware of a number of companies that are or may be pursuing a number of different approaches to FGFR inhibition, including Novartis, Astra Zeneca, Bayer, Debiopharm, Boeheringer Ingelheim, Eisai, Incyte, Johnson & Johnson, Clovis, Ariad, Pfizer, BioClin and Five Prime. With respect to iCCA, our lead indication for ARQ 087, we are aware of a number of companies with products under development, including Novartis, Concordia Healthcare, Agios, Bristol Meyers Squibb, Bayer, Dainippon Sumitomo, Exelexis, Oncotherapy, Spectrum, Delcath and Cellact Pharma Gmbh.
Regarding ARQ 092, we are aware of a number of companies that are or may be pursuing a number of different approaches to AKT inhibition, including Merck, Astra Zeneca, Bayer, Norvartis, Eli Lilly, Rexahn. Moreover, numerous companies have pursued and are pursuing inhibitors of PI3K and mTOR, two kinases in the PI3K-AKT-mTOR pathway, including Idelalisib, an approved PI3K inhibitor, and Everolimus, Temsirolimus and Rapamycin, approved mTOR inhibitors.
Even if we are successful in bringing products to market, we face substantial competitive challenges in effectively marketing and distributing our products. Companies and research institutions, including large pharmaceutical companies with much greater financial resources and more experience in developing products, conducting clinical trials, obtaining FDA and foreign regulatory approvals and bringing new