
© 2024 ANI Pharmaceuticals, Inc. 1 Acquisition of Alimera Sciences, Inc. June 24, 2024

© 2024 ANI Pharmaceuticals, Inc. 2 Disclaimer This presentation contains not only historical information, but also forward-looking statements made pursuant to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements represent the Company’s and Alimera’s expectations or beliefs concerning future events, including the timing of the transaction and other information relating to the proposed transaction including statements regarding the benefits of proposed transaction (including future non-GAAP performance), and the anticipated timing of the proposed transaction. These forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “continue,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “shall,” “would” other words of similar meaning, derivations of such words and the use of future dates. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: (i) the risk that the proposed transaction may not be completed in a timely manner or at all, (ii) the failure to satisfy the conditions to the consummation of the proposed transaction, (iii) the occurrence of any event, change or other circumstance that could give rise to the delay or termination of the proposed transaction, (iv) the inability to complete the proposed transaction due to the failure of a party or parties to satisfy conditions to completion of the proposed transaction, including the receipt on a timely basis or at all of any required regulatory clearances and receipt by Alimera of stockholder approval, (v) the failure of the contemplated debt financing or any alternative financing to be obtained on a timely basis or at all, (vi) the effect of the announcement or pendency of the proposed transaction on the Company’s and/or Alimera’s business relationships, operating results, and business generally, (vii) risks that the proposed transaction may disrupt current plans and operations of the Company and/or Alimera and potential difficulties of Alimera in retaining employees as a result of the proposed transaction, (viii) the outcome of any legal proceedings that may be instituted in connection with the proposed transaction, (ix) volatility in the price of the Company’s and/or Alimera’s stock, including as a result of the proposed transaction, (x) changes in competitive and regulated industries in which the Company operates, variations in operating performance across competitors, changes in laws and regulations affecting the Company’s business and changes in the combined capital structure, (xi) the ability to implement business plans, forecasts, and other expectations after the completion of the proposed transaction, and identify and realize additional opportunities and, in particular, failure to achieve anticipated synergies, (xii) costs and regulatory requirements relating to contract manufacturing arrangements, (xiii) delays or failure in obtaining product approvals from the FDA, (xiv) general business and economic conditions, (xv) market trends for the Company’s and/or Alimera’s products, including but not limited to, ILUVIEN®, YUTIQ® and Cortrophin Gel, and the ability to achieve anticipated sales for such products, (xvi) regulatory environment and changes, (xvii) regulatory and other approvals relating to product development and manufacturing, and (xviii) costs related to the proposed transaction and the failure to realize anticipated benefits of the proposed transactions or to realize estimated pro forma results and underlying assumptions. This presentation refers to financial measures that are not in accordance with U.S. generally accepted accounting principles (“GAAP”). Because the non-GAAP financial measures are not calculated in accordance with GAAP, they should not be considered superior to or as a substitute for the related financial measures that are prepared in accordance with GAAP and are not intended to be considered in isolation and may not be the same as or comparable to similarly titled measures presented by other companies due to possible differences in method and in the items being adjusted. A reconciliation of the forward-looking non-GAAP measures presented in this communication is not provided due to the inherent difficulty in forecasting and quantifying items that are necessary for such reconciliation. In addition, the Company believes such a reconciliation would imply a degree of precision and certainty that could be confusing to investors. The variability of the specified items may have a significant and unpredictable impact on future financial performance. The financial guidance is subject to risks and uncertainties applicable to all forward-looking statements as described elsewhere in this communication.

© 2024 ANI Pharmaceuticals, Inc. 3 Additional Information More detailed information on these and additional factors that could affect the Company’s actual results are described in the Company’s filings with the Securities and Exchange Commission (SEC), including its most recent annual report on Form 10-K and quarterly reports on Form 10-Q, as well as other filings with the SEC. All forward-looking statements in this news release speak only as of the date of this news release and are based on the Company’s current beliefs, assumptions, and expectations. The Company undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. In connection with the proposed transaction, Alimera intends to file a preliminary and definitive proxy statement. The definitive proxy statement and proxy card will be delivered to Alimera’s stockholders in advance of the special meeting relating to the proposed acquisition. Each of the Company and Alimera also plan to file other relevant materials with the SEC in connection with the proposed transaction. INVESTORS IN AND SECURITY HOLDERS OF ALIMERA ARE URGED TO READ THE DEFINITIVE PROXY IN ITS ENTIRETY WHEN IT BECOMES AVAILABLE, AS WELL AS ANY OTHER RELEVANT DOCUMENTS THAT ARE FILED OR FURNISHED OR WILL BE FILED OR WILL BE FURNISHED BY EACH OF THE COMPANY AND ALIMERA WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THESE DOCUMENTS, CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION. Materials filed by the Company and Alimera can be obtained free of charge at the SEC’s website, www.sec.gov. In addition, materials filed by the Company can be obtained free of charge at the Company’s website, www.anipharmaceuticals.com, and materials filed by Alimera can be obtained free of charge at Alimera’s website, www.alimerasciences.com.

© 2024 ANI Pharmaceuticals, Inc. 4 Nikhil Lalwani President and Chief Executive Officer Chris Mutz Head of Rare Disease Steve Carey Chief Financial Officer Speakers

© 2024 ANI Pharmaceuticals, Inc. 5 Nikhil Lalwani (President and Chief Executive Officer) Overview & Strategic Rationale Chris Mutz (Head of Rare Disease) Commercial Overview Stephen Carey (Chief Financial Officer) Financial Overview & Transaction Details Q&A Nikhil Lalwani (President and Chief Executive Officer) Stephen Carey (Chief Financial Officer) Chris Mutz (Head of Rare Disease) 1 2 3 4 Agenda

© 2024 ANI Pharmaceuticals, Inc. 6 Nikhil Lalwani (President and Chief Executive Officer) Overview & Strategic Rationale Chris Mutz (Head of Rare Disease) Commercial Overview Stephen Carey (Chief Financial Officer) Financial Overview & Transaction Details Q&A 1 2 3 4 Agenda Nikhil Lalwani (President and Chief Executive Officer) Stephen Carey (Chief Financial Officer) Chris Mutz (Head of Rare Disease)

© 2024 ANI Pharmaceuticals, Inc. 7 Deal snapshot Substantial value creation for shareholders of both companies Key Deal Terms $5.50 per share in cash (~75% premium to June 21 closing price) CVR of up to $0.50 per share Transaction value of $381M Closing expected in late Q3 2024, subject to customary closing conditions, including receipt of required regulatory approvals and approval by Alimera’s shareholders Company Overview Markets two novel differentiated treatments for ophthalmological indications: diabetic macular edema and chronic non-infectious uveitis – posterior segment ~160 global employees 2024 revenue guidance of approximately $105M Key Products:
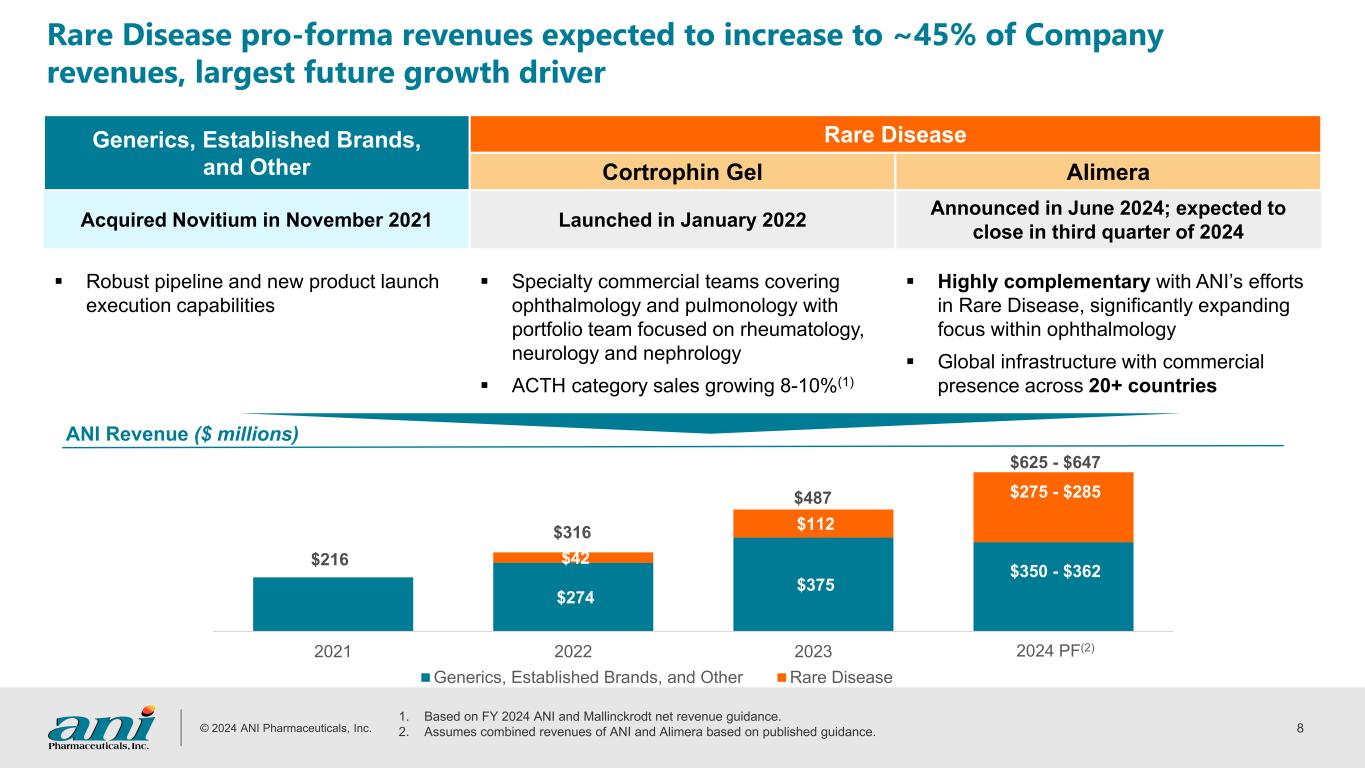
© 2024 ANI Pharmaceuticals, Inc. 8 Rare Disease pro-forma revenues expected to increase to ~45% of Company revenues, largest future growth driver Generics, Established Brands, and Other Rare Disease Cortrophin Gel Alimera Acquired Novitium in November 2021 Launched in January 2022 Announced in June 2024; expected to close in third quarter of 2024 Robust pipeline and new product launch execution capabilities Specialty commercial teams covering ophthalmology and pulmonology with portfolio team focused on rheumatology, neurology and nephrology ACTH category sales growing 8-10%(1) Highly complementary with ANI’s efforts in Rare Disease, significantly expanding focus within ophthalmology Global infrastructure with commercial presence across 20+ countries ANI Revenue ($ millions) $216 $274 $375 $42 $112 2021 2022 2023 Generics, Established Brands, and Other Rare Disease $316 $487 $275 - $285 $625 - $647 $350 - $362 1. Based on FY 2024 ANI and Mallinckrodt net revenue guidance. 2. Assumes combined revenues of ANI and Alimera based on published guidance. 2024 PF(2)

© 2024 ANI Pharmaceuticals, Inc. 9 Further strengthens ANI’s Rare Disease business as largest driver of future growth Expected to add ~$105M pro forma revenues making Rare Disease ~45% of Company revenues Combination enhances an attractive Rare Disease growth platform serving patients across therapeutic areas Increased geographic diversification with Alimera’s established ex-US footprint, including direct operations in Europe Adds two durable commercial assets with significant growth potential ILUVIEN and YUTIQ are durable assets with high barriers to genericization which the Company believes have a clear role to treat patients in need of other therapeutic options Long-term clinical studies, real-world use, and ongoing trials provide a strong foundation for ILUVIEN and YUTIQ Significant growth potential expected to be further unlocked through commercial synergies and execution Expands foothold in ophthalmology and accelerates growth of Cortrophin Gel in this key therapeutic area Combined nationwide ophthalmology salesforce planned to be ~45 dedicated to Cortrophin, ILUVIEN, and YUTIQ Expands reach to over 3,600 ophthalmologists, with over ~50% overlap between high potential prescribers of Cortrophin and ILUVIEN/YUTIQ Potential for substantial shareholder value creation Expected high single-digit to low double-digit accretion in 2025 adjusted non-GAAP EPS and substantially accretive thereafter Anticipated additional $35-$38 million in 2025 adjusted non-GAAP EBITDA inclusive of approximately $10 million in identified cost synergies; incremental EBITDA contribution expected from accelerated growth of Cortrophin Gel within ophthalmology Anticipated 3.2x(1) pro-forma leverage upon close; expect to significantly de-lever organically in 2025 Transaction to expand ANI’s Rare Disease business with potential for substantial shareholder value creation 1 2 3 4 1. Calculated based upon pro forma LTM EBITDA and net debt.

© 2024 ANI Pharmaceuticals, Inc. 10 Transaction aligned with M&A strategy Expands Scope and Scale of Rare Disease Business Priority Therapeutic Area Assets with Growth & Durability ● Ophthalmology as a percentage of total ACTH prescribers has almost doubled to more than 10% over four years(1) ● Double-digit growth assets ● Patent protection ● High barriers to genericization 1. Per Veeva Compass claims dataset for Acthar + Cortrophin internal prescribing data.

© 2024 ANI Pharmaceuticals, Inc. 11 Potential for meaningful expansion in Rare Disease and total Company revenues Total Company Revenues ($ millions)(1) Rare Disease Revenues ($ millions)(1) 2021 2022 2023 2024E $216 $316 $487 $520-$542 $625-$647 ANI Pharmaceutical Alimera Sciences 2022 2023 2024E 1. ANIP 2024 estimates reflect 2024 guidance, initially provided on February 29, 2024 and reiterated on May 10, 2024; ALIM 2024 estimate reflects 2024 guidance provided on March 7, 2024 and reiterated on May 14, 2024. $42 $112 $170-$180 $275-$285 CORTROPHIN GEL ILUVIEN + YUTIQ

© 2024 ANI Pharmaceuticals, Inc. 12 ANI’s Rare Disease business focuses on patients who are not well served by other therapies • Idiopathic Nephrotic Syndrome • Lupus Nephritis • Systemic Dermatomyositis • Sarcoidosis • Keratitis • Chronic Non-Infectious Uveitis Posterior Segment • Rheumatoid Arthritis • Multiple Sclerosis • Systemic Lupus Erythematosus • Psoriatic Arthritis • Ankylosing Spondylitis • Acute Gouty Arthritis • Diabetic Macular Edema • Non-Infectious Uveitis * Based on US FDA considered definition of rare disease - disorders affecting <200 000 persons, translating to a prevalence of 58.5 per 100 000 at current time Underserved patients; high prevalence disease Rare disease* Select Indications

© 2024 ANI Pharmaceuticals, Inc. 13 Chronic Non-Infectious Uveitis ● Disease state: Chronic non-infectious uveitis affecting the posterior segment (NIU-PS) is inflammation of the eye that can lead to pain, visual impairment and vision loss ● Over 500,000 patients in U.S., many of working age, with non-infectious uveitis ● Classified by onset, duration and etiology Diabetic Macular Edema ● Disease state: DME, a chronic disease that is the leading cause of vision loss in diabetic patients ● >4% of diabetic patients develop clinically significant macular edema ● Causes blurred vision in the early stage and may cause cumulative damage over the long term ILUVIEN and YUTIQ: intravitreal implants designed to deliver continuous low dose treatment US Ex-US

© 2024 ANI Pharmaceuticals, Inc. 14 Nikhil Lalwani (President and Chief Executive Officer) Overview & Strategic Rationale Chris Mutz (Head of Rare Disease) Commercial Overview Stephen Carey (Chief Financial Officer) Financial Overview & Transaction Details Q&A 1 2 3 4 Agenda Nikhil Lalwani (President and Chief Executive Officer) Stephen Carey (Chief Financial Officer) Chris Mutz (Head of Rare Disease)

© 2024 ANI Pharmaceuticals, Inc. 15 ANI Rare Disease would combine three commercial products with growth and durability US Indications Diabetic macular edema (DME) Non-infectious uveitis affecting the posterior segment (NIU-PS) Severe acute and chronic allergic and inflammatory conditions affecting the eye and its adnexa (ophthalmology only) Ex-US Indications DME and NIU-PS Middle East and 17 European countries -- -- US Approval Date September 2014 October 2018 Alimera acquired from Eyepoint in May 2023 November 2021 sNDA 2023 Sales(1) ~$59 million ~$36 million ~$112 million (all indications) (1) Pro forma results for YUTIQ, including Eyepoint results prior to May 2023 acquisition by Alimera.

© 2024 ANI Pharmaceuticals, Inc. 16 Combined sales team expected to accelerate growth across the ophthalmology business Combined efforts expected to expand the ability to drive appropriate utilization of all three products for patients in need Deployed a targeted ophthalmology-focused salesforce in Q1 2024 Recently expanded US commercial team by ~20% to 35 field reps Significant overlap between ILUVIEN/YUTIQ and Cortrophin targeted ophthalmologists >50% overlap among those with the highest prescribing potential Expanded team increases reach to ~3,600 ophthalmologists Identifying patients with unmet needs Complementary patient support capabilities focused on ensuring patients have access to therapyPlanned combined team of ~45 ophthalmology specialists
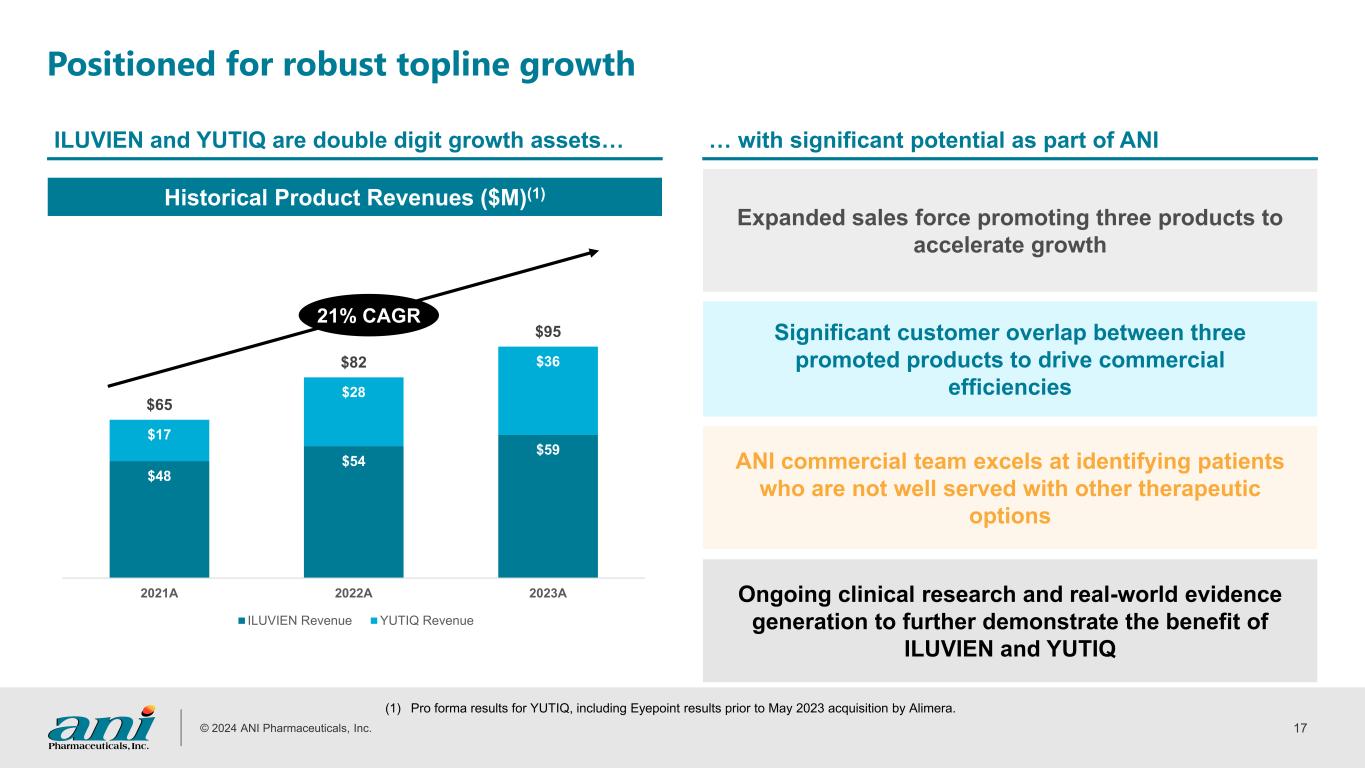
© 2024 ANI Pharmaceuticals, Inc. 17 11 Positioned for robust topline growth ILUVIEN and YUTIQ are double digit growth assets… … with significant potential as part of ANI Expanded sales force promoting three products to accelerate growth ANI commercial team excels at identifying patients who are not well served with other therapeutic options Ongoing clinical research and real-world evidence generation to further demonstrate the benefit of ILUVIEN and YUTIQ Significant customer overlap between three promoted products to drive commercial efficiencies $48 $54 $59 $17 $28 $36 $65 $82 $95 2021A 2022A 2023A ILUVIEN Revenue YUTIQ Revenue Historical Product Revenues ($M)(1) (1) 21% CAGR (1) Pro forma results for YUTIQ, including Eyepoint results prior to May 2023 acquisition by Alimera.

© 2024 ANI Pharmaceuticals, Inc. 18 The most underserved patient group within DME represents more than 50,000 patients in the US alone DME epidemiology model flow – inputs informed by ANI’s market research Diagnosed DME population: ~3% = ~900,000 patients Treated DME population: ~50% = ~450,000 Patients Patients receiving 2+ anti-VEGFs: 57% = ~260,000 patients Suboptimal response to anti-VEGFs: 29% = ~75,000 patients Positive steroid trial (i.e., low IOP risk): ~70% = ~53,000 pts Source: Ophthalmologists survey, n = 64 >50,000 patients in the US are not well served by anti-VEGF therapy Significant room for ILUVIEN growth - <5,000 patient starts annually for DME in the US

© 2024 ANI Pharmaceuticals, Inc. 19 110 patient eyes enrolled to receive YUTIQ Recruitment completed in January 2024 Topline data readout expected 2H 2024 Largest head-to-head (306 patients) comparison of any corticosteroid therapy and anti-VEGF therapy in the treatment of newly diagnosed patients suffering from DME Topline data expected Q1 2025 Long-term clinical studies, real-world use, and ongoing trials provide a strong foundation for ILUVIEN and YUTIQ Body of clinical data expected to continue to grow Synchronicity Study

© 2024 ANI Pharmaceuticals, Inc. 20 17 Establishes global commercial footprint for ANI’s Rare Disease business unit Direct commercial operations in • United States • Germany • United Kingdom • Portugal • Ireland High-quality partnerships throughout Europe, the Middle East, and Asia Alimera generates ~30% of revenue ex-US ($24M in 2023, 20% YoY growth)

© 2024 ANI Pharmaceuticals, Inc. 21 Overview & Strategic Rationale Chris Mutz (Head of Rare Disease) Commercial Overview Stephen Carey (Chief Financial Officer) Financial Overview & Transaction Details Q&A 1 2 3 4 Agenda Nikhil Lalwani (President and Chief Executive Officer) Stephen Carey (Chief Financial Officer) Chris Mutz (Head of Rare Disease) Nikhil Lalwani (President and Chief Executive Officer)

© 2024 ANI Pharmaceuticals, Inc. 22 Transaction overview Consideration ANI to acquire all outstanding shares of Alimera for up-front consideration of approximately $381 million comprised of: • $5.50 per share in cash (~$320M) • Pay-off of $72.5M of Alimera debt net of estimated Alimera cash at time of close (~$11M) Non-tradable CVR for up to $0.50 per share, based on achieving certain levels of net revenue • Up to $0.25 per share upon net revenues of between $140M to $150M in 2026 • Up to $0.25 per share upon net revenues of between $160M to $175M in 2027 Financing Expected to fund using a combination of cash on hand and incremental debt ANI has obtained $280M of committed financing from J.P. Morgan and Blackstone • To be completed within the bounds of our existing credit agreement Pro forma net leverage of ~3.2x(1) at time of close • Enhanced cash flow generation from growth and synergies to drive rapid deleveraging Pro Forma Results Expect high single-digit to low double-digit accretion in adjusted non-GAAP EPS in 2025(2) and substantially accretive thereafter Deal expected to add $35-$38 million in adjusted non-GAAP EBITDA in 2025 inclusive of ~$10M of identified cost synergies; with incremental EBITDA contribution expected from accelerated growth of Cortrophin Gel within ophthalmology Timing Transaction is expected to close in late Q3 2024, subject to customary closing conditions, including receipt of required regulatory approvals and approval by Alimera’s shareholders 1. Calculated based upon pro forma LTM EBITDA and net debt. 2. Adjusted EPS accretion based on estimated pro forma 2025 adjusted EPS vs. FactSet consensus adjusted EPS for ANIP as of 6/21/24.

© 2024 ANI Pharmaceuticals, Inc. 23 Overview & Strategic Rationale Chris Mutz (Head of Rare Disease) Commercial Overview Stephen Carey (Chief Financial Officer) Financial Overview & Transaction Details Q&A 1 2 3 4 Agenda Nikhil Lalwani (President and Chief Executive Officer) Stephen Carey (Chief Financial Officer) Chris Mutz (Head of Rare Disease) Nikhil Lalwani (President and Chief Executive Officer)

© 2024 ANI Pharmaceuticals, Inc. 24 Acquisition of Alimera Sciences, Inc. June 24, 2024