Exhibit 99.2
| [LOGO] |
| To the extent any statements made in this presentation deal with information that is not historical, these are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about BioSante’s plans, objectives, expectations and intentions with respect to future operations and products and other statements identified by words such as “will,” “potential,” “could,” “can,” “believe,” ”intends,” “continue,” “plans,” “expects,” “estimates” or comparable terminology. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause BioSante’s actual results to be materially different than those expressed in or implied by BioSante’s forward-looking statements. For BioSante, particular uncertainties and risks include, among others, risks associated with BioSante’s proposed merger with Cell Genesys, Inc., including the failure of the BioSante or Cell Genesys stockholders to approve the proposed merger or the failure of either party to meet any of the other conditions to the closing of the transaction; the failure to realize the anticipated benefits from the merger or delay in realization thereof; operating costs and business disruption following the merger; general business and economic conditions; BioSante’s need for and ability to obtain additional financing; the difficulty of developing pharmaceutical products, obtaining regulatory and other approvals and achieving market acceptance; the marketing success of BioSante’s licensees or sublicensees and the success of clinical testing. More detailed information on these and additional factors that could affect BioSante’s actual results are described in BioSante’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q. All forward-looking statements in this presentation speak only as of the date of this presentation. BioSante undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. |
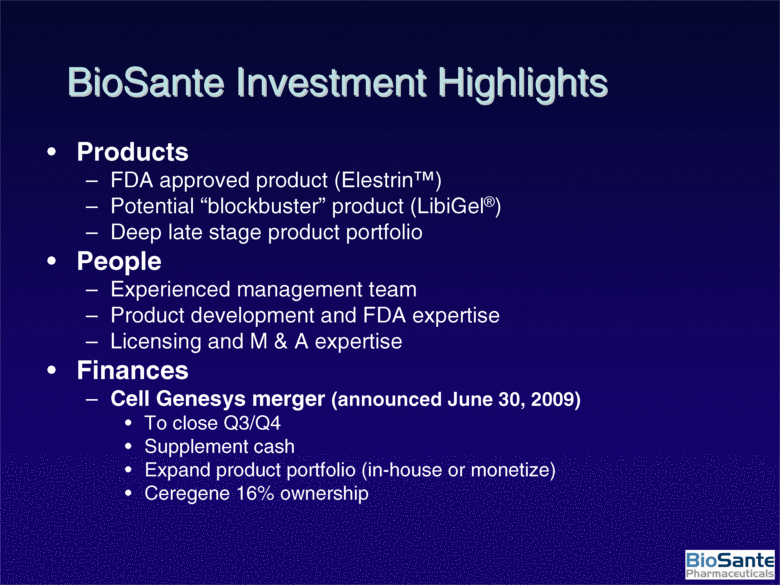
| BioSante Investment Highlights Products FDA approved product (Elestrin™) Potential “blockbuster” product (LibiGel®) Deep late stage product portfolio People Experienced management team Product development and FDA expertise Licensing and M & A expertise Finances Cell Genesys merger (announced June 30, 2009) To close Q3/Q4 Supplement cash Expand product portfolio (in-house or monetize) Ceregene 16% ownership |
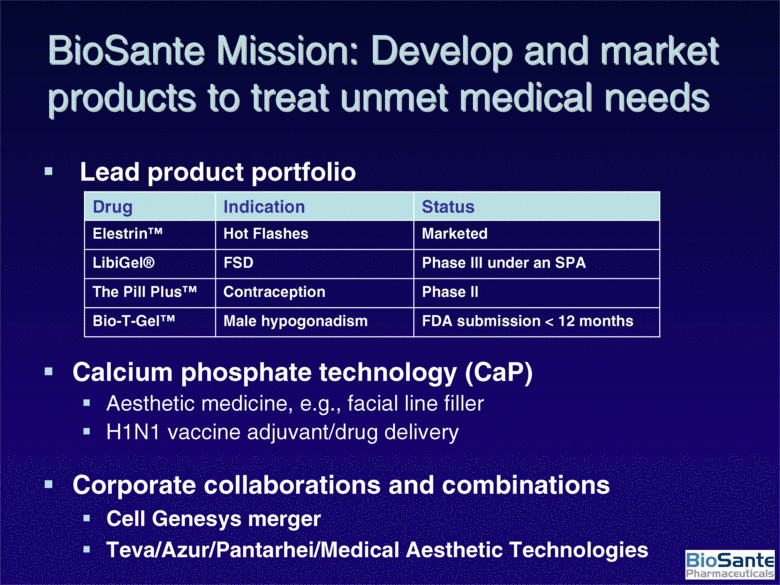
| BioSante Mission: Develop and market products to treat unmet medical needs Lead product portfolio Calcium phosphate technology (CaP) Aesthetic medicine, e.g., facial line filler H1N1 vaccine adjuvant/drug delivery FDA submission < 12 months Male hypogonadism Bio-T-Gel™ Phase II Contraception The Pill Plus™ Phase III under an SPA FSD LibiGel® Marketed Hot Flashes Elestrin™ Status Indication Drug Corporate collaborations and combinations Cell Genesys merger Teva/Azur/Pantarhei/Medical Aesthetic Technologies |
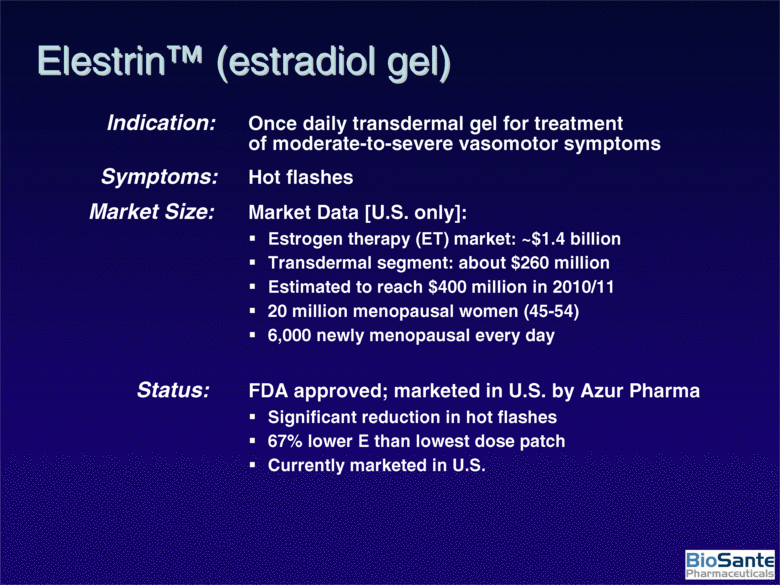
| Indication: Once daily transdermal gel for treatment of moderate-to-severe vasomotor symptoms Symptoms: Hot flashes Market Size: Market Data [U.S. only]: Estrogen therapy (ET) market: ~$1.4 billion Transdermal segment: about $260 million Estimated to reach $400 million in 2010/11 20 million menopausal women (45-54) 6,000 newly menopausal every day Status: FDA approved; marketed in U.S. by Azur Pharma Significant reduction in hot flashes 67% lower E than lowest dose patch Currently marketed in U.S. Elestrin™ (estradiol gel) |
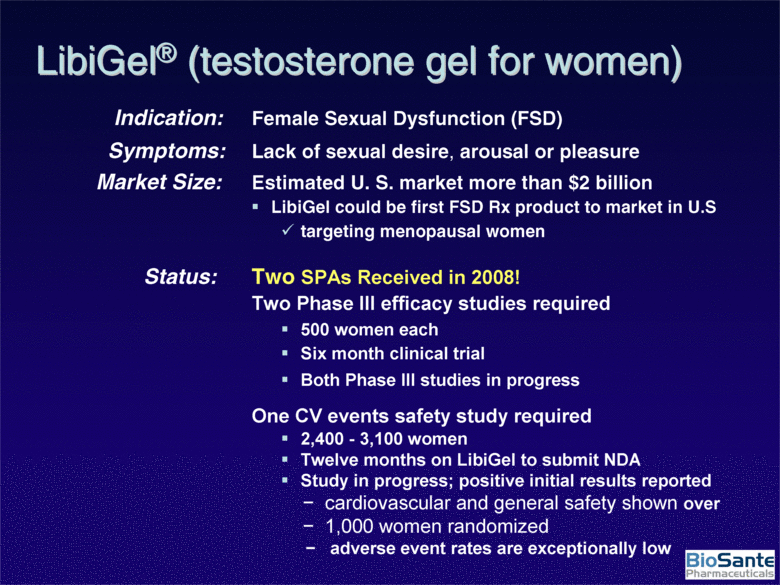
| Indication: Female Sexual Dysfunction (FSD) Symptoms: Lack of sexual desire, arousal or pleasure Market Size: Estimated U. S. market more than $2 billion LibiGel could be first FSD Rx product to market in U.S targeting menopausal women Status: Two SPAs Received in 2008! Two Phase III efficacy studies required 500 women each Six month clinical trial Both Phase III studies in progress One CV events safety study required 2,400 - 3,100 women Twelve months on LibiGel to submit NDA Study in progress; positive initial results reported - cardiovascular and general safety shown over - 1,000 women randomized - adverse event rates are exceptionally low LibiGel® (testosterone gel for women) |
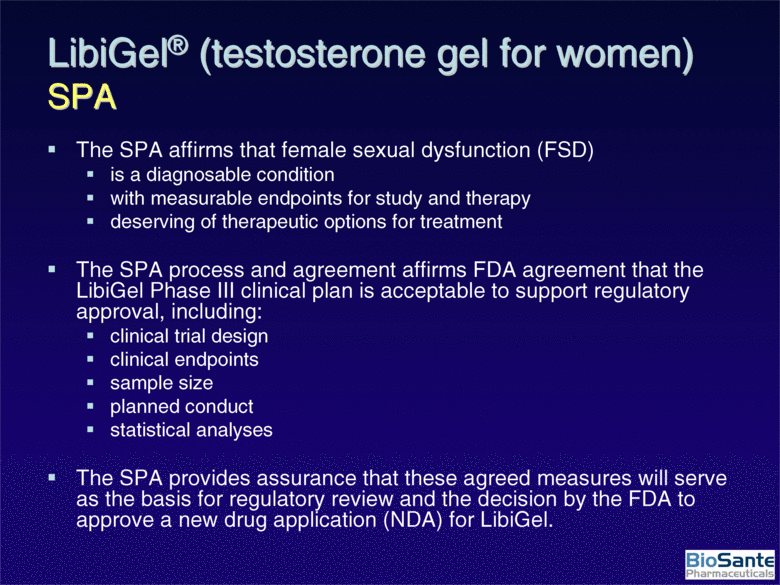
| LibiGel® (testosterone gel for women) SPA The SPA affirms that female sexual dysfunction (FSD) is a diagnosable condition with measurable endpoints for study and therapy deserving of therapeutic options for treatment The SPA process and agreement affirms FDA agreement that the LibiGel Phase III clinical plan is acceptable to support regulatory approval, including: clinical trial design clinical endpoints sample size planned conduct statistical analyses The SPA provides assurance that these agreed measures will serve as the basis for regulatory review and the decision by the FDA to approve a new drug application (NDA) for LibiGel. |
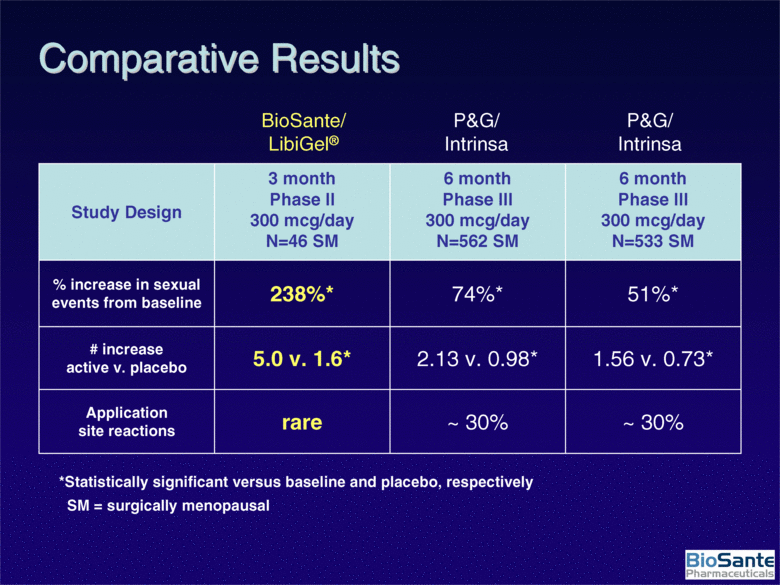
| ~ 30% ~ 30% rare Application site reactions 1.56 v. 0.73* 2.13 v. 0.98* 5.0 v. 1.6* # increase active v. placebo 51%* 74%* 238%* % increase in sexual events from baseline 6 month Phase III 300 mcg/day N=533 SM 6 month Phase III 300 mcg/day N=562 SM 3 month Phase II 300 mcg/day N=46 SM Study Design BioSante/LibiGel® P&G/Intrinsa P&G/Intrinsa *Statistically significant versus baseline and placebo, respectively SM = surgically menopausal Comparative Results |
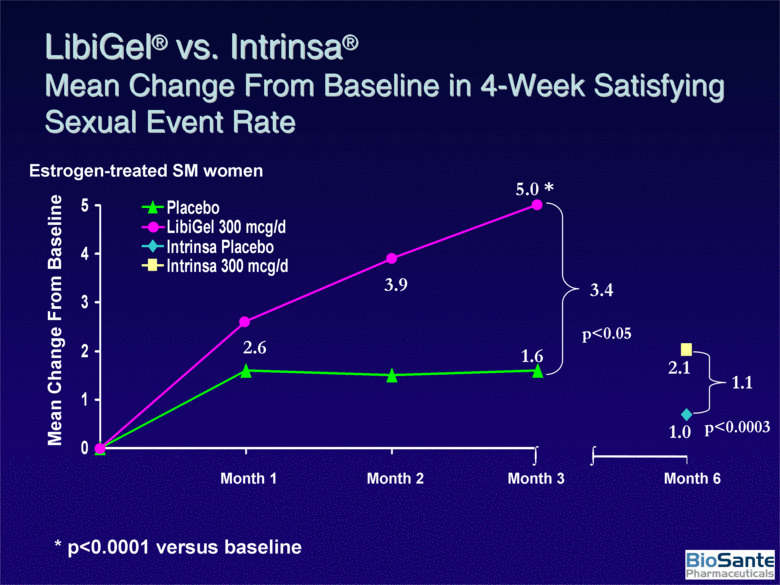
| Mean Change From Baseline p<0.05 3.4 * p<0.0001 versus baseline 5.0 * 1.6 3.9 2.6 Estrogen-treated SM women Month 1 Month 2 Month 3 Month 6 2.1 1.0 1.1 p<0.0003 LibiGel® vs. Intrinsa® Mean Change From Baseline in 4-Week Satisfying Sexual Event Rate 0 1 2 3 4 5 Placebo LibiGel 300 mcg/d Intrinsa Placebo Intrinsa 300 mcg/d |
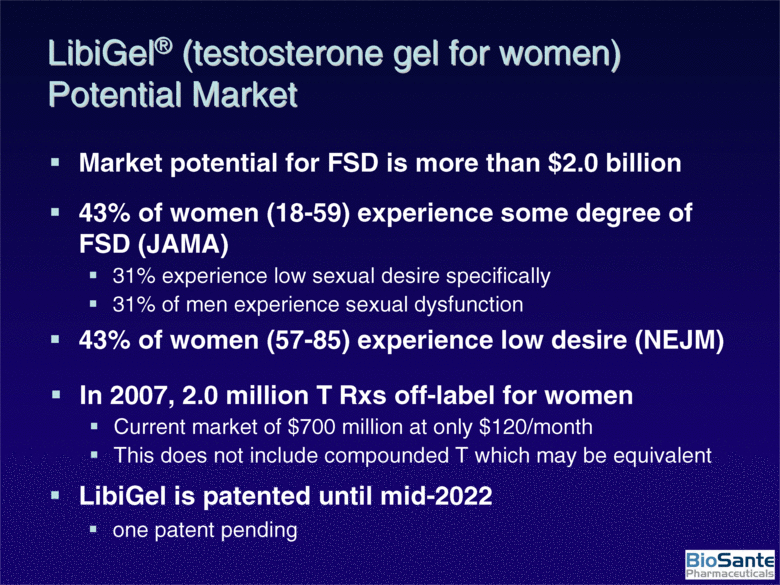
| LibiGel® (testosterone gel for women) Potential Market Market potential for FSD is more than $2.0 billion 43% of women (18-59) experience some degree of FSD (JAMA) 31% experience low sexual desire specifically 31% of men experience sexual dysfunction 43% of women (57-85) experience low desire (NEJM) In 2007, 2.0 million T Rxs off-label for women Current market of $700 million at only $120/month This does not include compounded T which may be equivalent LibiGel is patented until mid-2022 one patent pending |
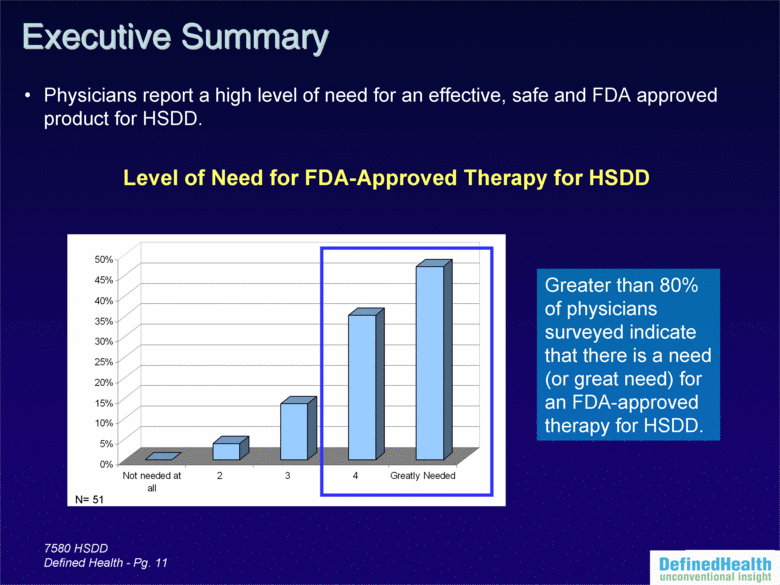
| 7580 HSDD Defined Health - Pg. 11 Greater than 80% of physicians surveyed indicate that there is a need (or great need) for an FDA-approved therapy for HSDD. Executive Summary Physicians report a high level of need for an effective, safe and FDA approved product for HSDD. N= 51 Level of Need for FDA-Approved Therapy for HSDD 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Not needed at all 2 3 4 Greatly Needed |
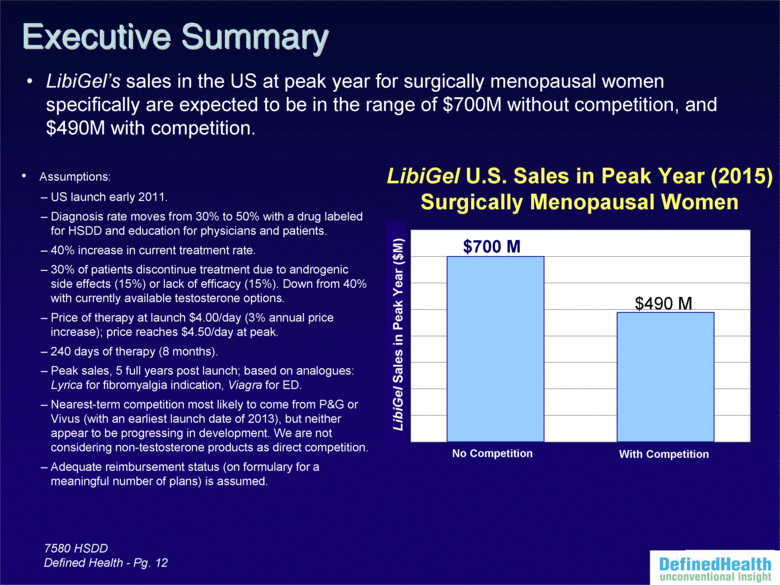
| 7580 HSDD Defined Health - Pg. 12 Assumptions: US launch early 2011. Diagnosis rate moves from 30% to 50% with a drug labeled for HSDD and education for physicians and patients. 40% increase in current treatment rate. 30% of patients discontinue treatment due to androgenic side effects (15%) or lack of efficacy (15%). Down from 40% with currently available testosterone options. Price of therapy at launch $4.00/day (3% annual price increase); price reaches $4.50/day at peak. 240 days of therapy (8 months). Peak sales, 5 full years post launch; based on analogues: Lyrica for fibromyalgia indication, Viagra for ED. Nearest-term competition most likely to come from P&G or Vivus (with an earliest launch date of 2013), but neither appear to be progressing in development. We are not considering non-testosterone products as direct competition. Adequate reimbursement status (on formulary for a meaningful number of plans) is assumed. No Competition With Competition LibiGel U.S. Sales in Peak Year (2015) Surgically Menopausal Women $700 M $490 M LibiGel’s sales in the US at peak year for surgically menopausal women specifically are expected to be in the range of $700M without competition, and $490M with competition. Executive Summary LibiGel Sales in Peak Year ($M) |
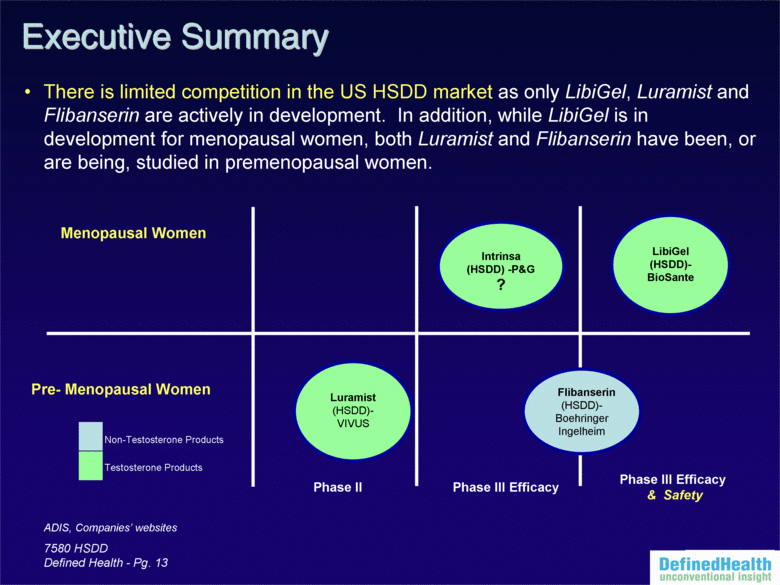
| 7580 HSDD Defined Health - Pg. 13 ADIS, Companies’ websites Phase II Phase III Efficacy Flibanserin (HSDD)-Boehringer Ingelheim Luramist (HSDD)-VIVUS Testosterone Products Non-Testosterone Products Pre-Menopausal Women Menopausal Women There is limited competition in the US HSDD market as only LibiGel, Luramist and Flibanserin are actively in development. In addition, while LibiGel is in development for menopausal women, both Luramist and Flibanserin have been, or are being, studied in premenopausal women. Executive Summary LibiGel (HSDD)-BioSante Phase III Efficacy & Safety Intrinsa (HSDD) -P&G? |
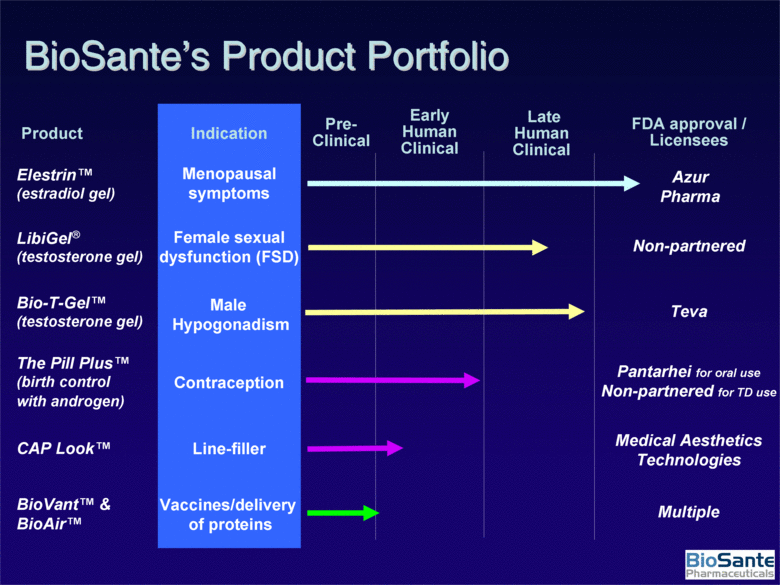
| CAP Look™ LibiGel® (testosterone gel) Female sexual dysfunction (FSD) The Pill Plus™ (birth control with androgen) Male Hypogonadism Bio-T-Gel™ (testosterone gel) Contraception Elestrin™ (estradiol gel) Early Human Clinical Late Human Clinical FDA approval / Licensees Menopausal symptoms Line-filler BioVant™ & BioAir™ Vaccines/delivery of proteins Pre-Clinical Product Indication BioSante’s Product Portfolio Azur Pharma Non-partnered Teva Pantarhei for oral use Non-partnered for TD use Medical Aesthetics Technologies Multiple |
| BioSante Pharmaceuticals, Inc. Corporate Summary |
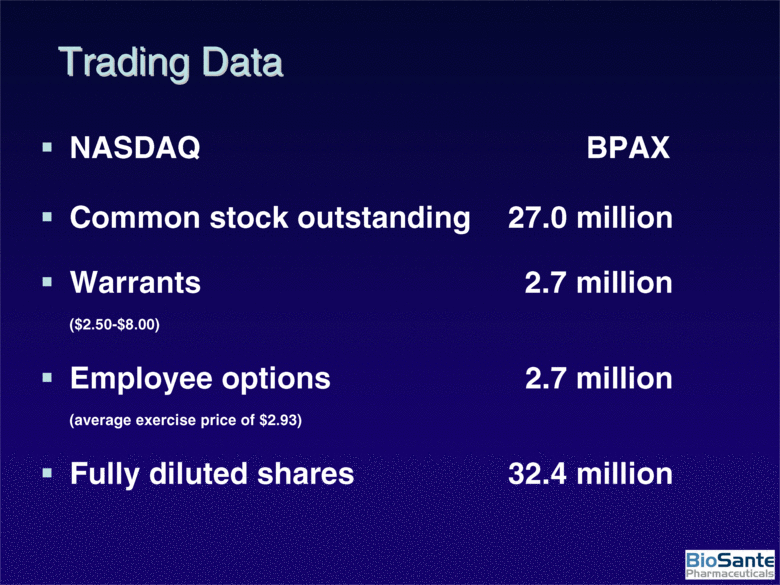
| NASDAQ BPAX Common stock outstanding 27.0 million Warrants 2.7 million ($2.50-$8.00) Employee options 2.7 million (average exercise price of $2.93) Fully diluted shares 32.4 million Trading Data |
| Cash at 6/30/09 Approximately $6.0 million Burn rate Approximately $1.2 million/month Financial Highlights |
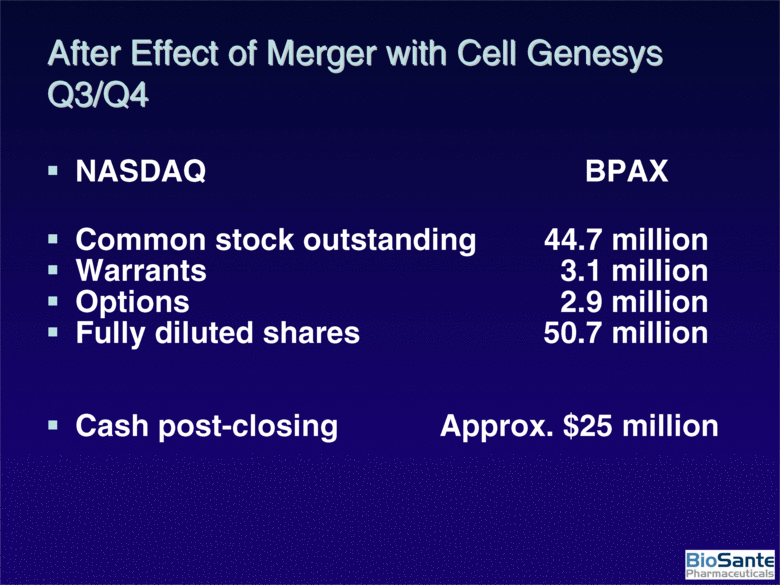
| After Effect of Merger with Cell Genesys Q3/Q4 NASDAQ BPAX Common stock outstanding 44.7 million Warrants 3.1 million Options 2.9 million Fully diluted shares 50.7 million Cash post-closing Approx. $25 million |
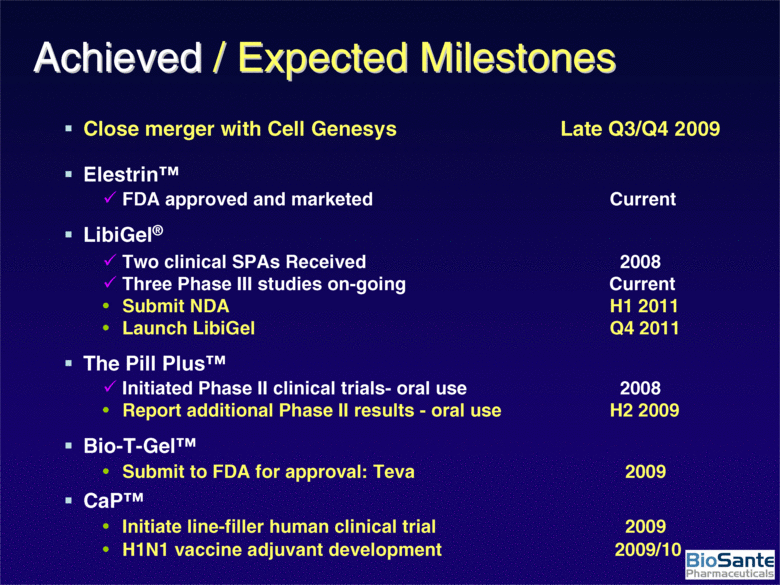
| Achieved / Expected Milestones Close merger with Cell Genesys Late Q3/Q4 2009 Elestrin™ FDA approved and marketed Current LibiGel® Two clinical SPAs Received 2008 Three Phase III studies on-going Current Submit NDA H1 2011 Launch LibiGel Q4 2011 The Pill Plus™ Initiated Phase II clinical trials- oral use 2008 Report additional Phase II results - oral use H2 2009 Bio-T-Gel™ Submit to FDA for approval: Teva 2009 CaP™ Initiate line-filler human clinical trial 2009 H1N1 vaccine adjuvant development 2009/10 |
| [LOGO] |