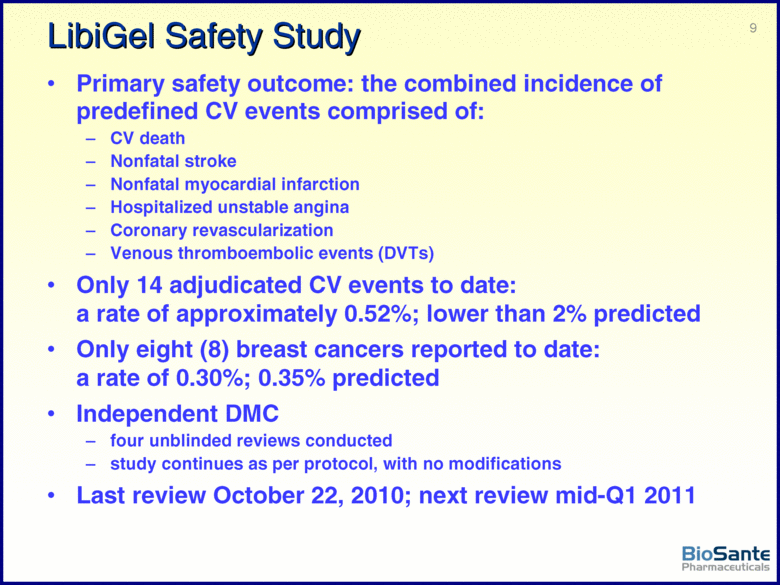
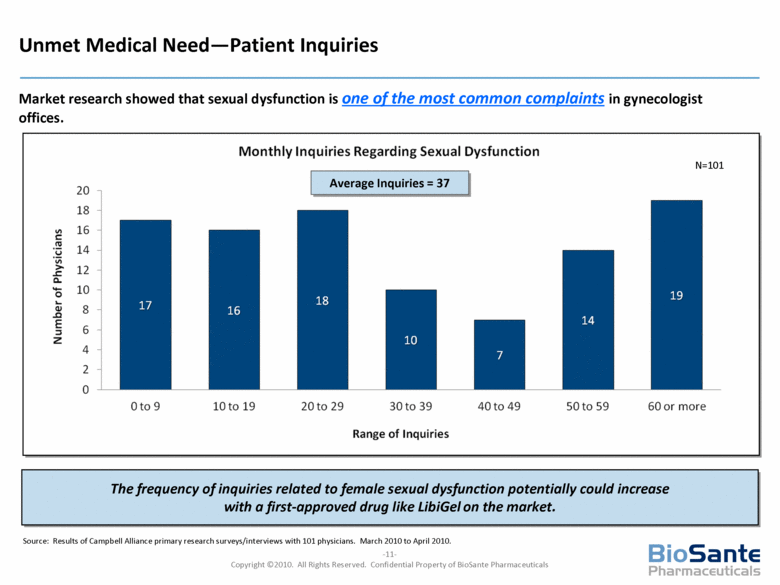
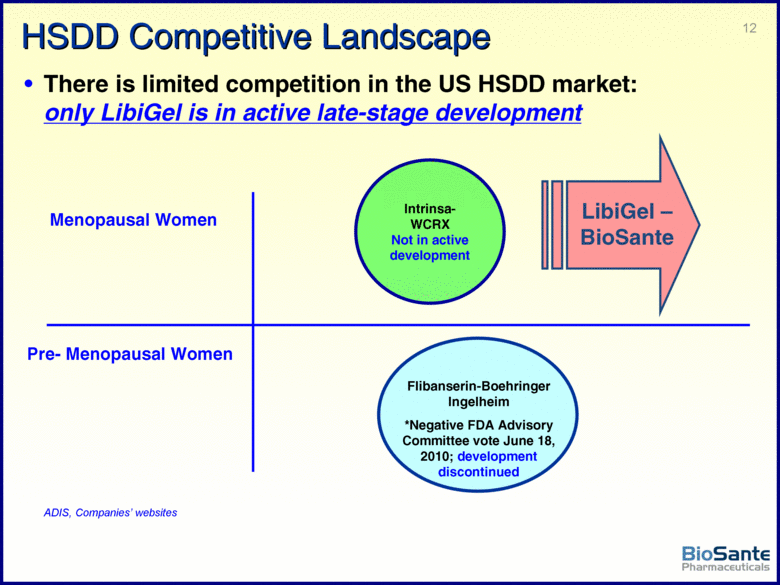
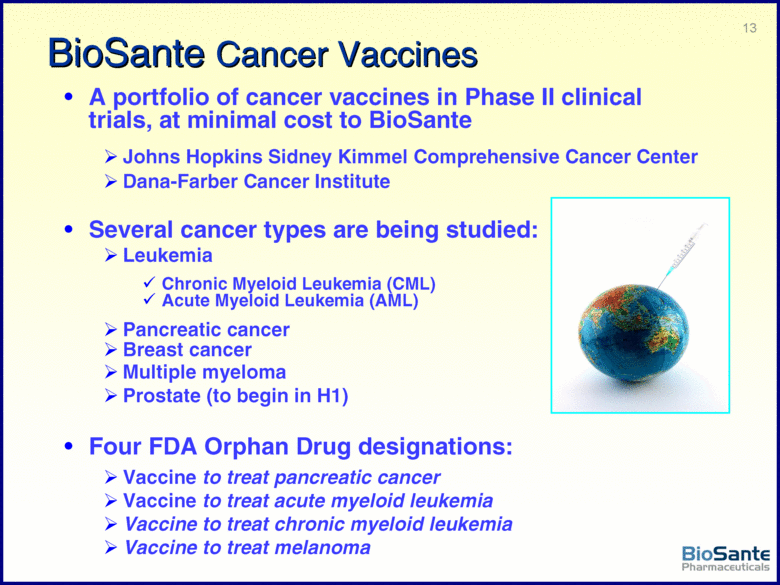
| This presentation contains forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include statements about BioSante’s plans, objectives, expectations and intentions with respect to future operations and products, future market acceptance, size and potential of LibiGel and other statements identified by words such as “will,” “potential,” “could,” “would,” “can,” “believe,” “intends,” “continue,” “plans,” “expects,” “anticipates,” “estimates,” “may,” other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause BioSante’s actual results to be materially different than those expressed in or implied by BioSante’s forward-looking statements. For BioSante, particular uncertainties and risks include, among others, the difficulty of developing pharmaceutical products, obtaining regulatory and other approvals and achieving market acceptance; the marketing success of BioSante’s licensees or sublicensees; the success of clinical testing; and BioSante’s need for and ability to obtain additional financing. More detailed information on these and additional factors that could affect BioSante’s actual results are described in BioSante’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q. All forward-looking statements in this presentation speak only as of the date of this presentation. BioSante undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. |