Exhibit 99.2

A Specialty Pharmaceutical Company NASDAQ: ANIP GENERIC AND BRANDED PRESCRIPTION DRUG PRODUCTS Corporate Presentation April 2015

Forward - Looking Statements To the extent any statements made in this presentation deal with information that is not historical, these are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about price increases, the Company’s future operations, products financial position, operating results and prospects , the Company’s pipeline or potential markets therefore, and other statements that are not historical in nature, particularly those that utilize terminology such as “anticipates,” “will,” “expects,” “plans,” “potential,” “future,” “believes,” “intends,” “continue,” other words of similar meaning, derivations of such words and the use of future dates. Uncertainties and risks may cause the Company’s actual results to be materially different than those expressed in or implied by such forward - looking statements. Uncertainties and risks include, but are not limited to, the risk that the Company may face with respect to importing raw materials; increased competition; delays or failure in obtaining product approval from the U.S. Food and Drug Administration; general business and economic conditions; market trends; products development; regulatory and other approvals and marketing. More detailed information on these and additional factors that could affect the Company’s actual results are described in the Company’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10 - K and quarterly reports on Form 10 - Q, as well as its proxy statement. All forward - looking statements in this presentation speak only as of the date of this presentation and are based on the Company’s current beliefs, assumptions, and expectations. The Company undertakes no obligation to update or revise any forward - looking statement, whether as a result of new information, future events or otherwise. 2

3 ANI Mission Statement ANI Pharmaceuticals is an integrated specialty pharmaceutical company developing, manufacturing and marketing branded and generic prescription pharmaceuticals. ANI’s mission is to develop, manufacture, and market niche generic pharmaceuticals, focusing on opportunities in pain management (narcotics), anti - cancer (oncolytics), women’s health (hormones and steroids), and complex formulations including extended release and combination products.

ANI Overview – Positioned for Growth ANI Today ▪ Experienced management team ▪ Current business – For the year ended December 31, 2014: $56.0 million total net revenues □ $46.9 million ANI Rx product revenues □ $9.1 million c ontract manufacturing/services revenues □ Growth of 86% year/year ▪ Guidance for 2015 (1) – Net revenues of $80 million to $88 million – Adjusted non - GAAP EBITDA of $48.8 to $53.1 million – Adjusted non - GAAP diluted earnings per share of $2.48 to $2.72 ▪ 46 products in development; total current market $3.0 billion (2) ▪ $169 million in cash for accretive acquisitions (1) February 19, 2015 press release (2) Based on Company estimates, and recent IMS and NSP Audit data 4

5 ANI History and Highlights 2010 Management implemented strategy to focus on ANI - labeled Rx products 2011 Expanded marketed Rx portfolio to seven products 2013 Completed merger with BioSante Pharmaceuticals and obtains NASDAQ Global Market listing (NASDAQ: ANIP), June 2013 2013 Announced agreement to acquire 31 previously marketed generic products from Teva for $12.5 million and a percentage of future gross profits, December 2013 2014 Closed public offering of common shares netting $46.8 million, March 2014 2014 Acquired Vancocin ® and related assets for $11 million, August 2014 2014 Closed public offering of $143.8 million of convertible debt with simultaneous bond hedge and warrant transactions, December 2014 2014 Acquired Lithobid ® for $12 million, July 2014 2014 Launched Methazolamide Tablets, first product from portfolio of approved generic products acquired from Teva, November 2014 2015 Acquired approved ANDA for Flecainide tablets from Teva , March 2015

6 Sales and Marketing / Financial Overview
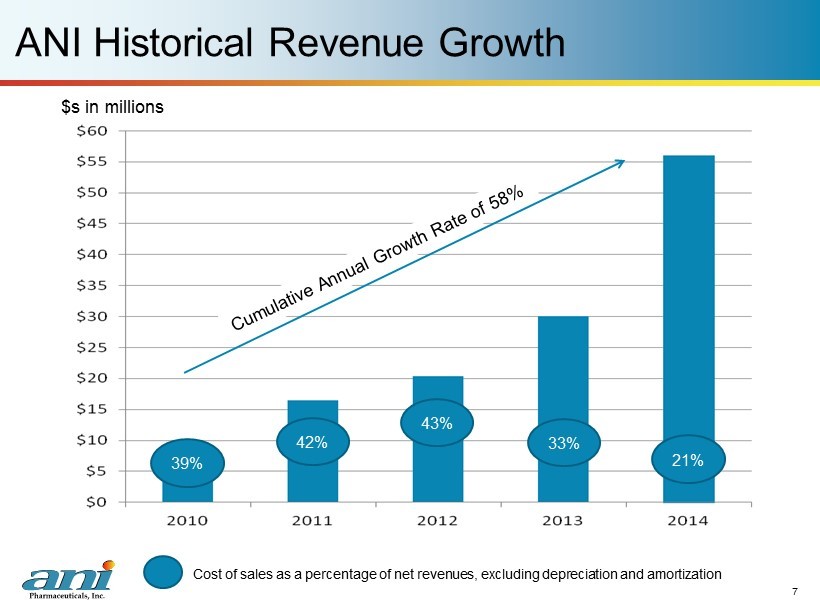
ANI Historical Revenue Growth 7 $s in millions 39% C ost of sales as a percentage of net revenues, excluding depreciation and amortization 42% 43% 33% 21%
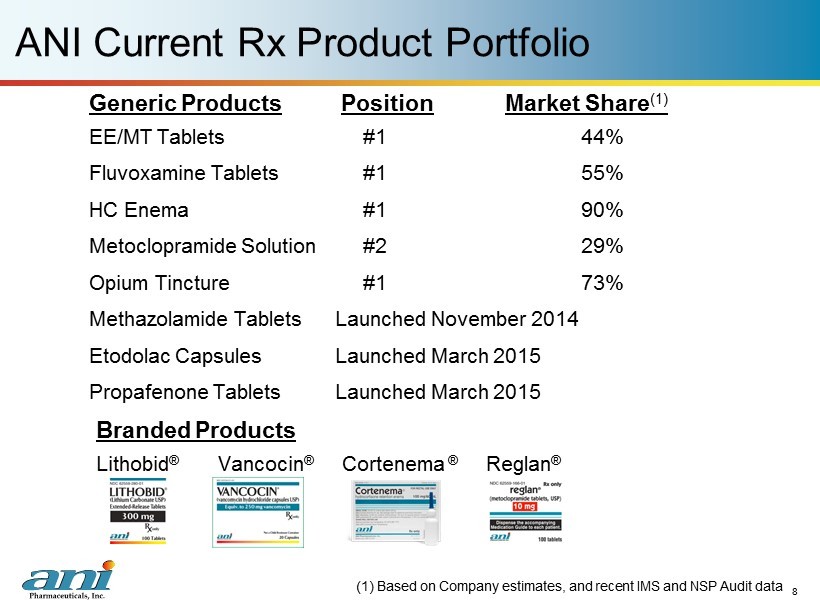
ANI Current Rx Product Portfolio Generic Products Position Market Share (1) EE/MT Tablets #1 44% Fluvoxamine Tablets #1 55% HC Enema #1 90% Metoclopramide Solution #2 29% Opium Tincture #1 73% Methazolamide Tablets Launched November 2014 Etodolac Capsules Launched March 2015 Propafenone Tablets Launched March 2015 8 Branded Products Lithobid ® Vancocin ® C ortenema ® Reglan ® (1) Based on Company estimates, and recent IMS and NSP Audit data

ANI Contract Manufacturing Revenues 9 Current Business ▪ $9.1 million in contract manufacturing and services revenues during the year ended December 31, 2014 ▪ Four customers – Eight products and nineteen SKUs Future Opportunities ▪ Potential future royalty: Teva’s testosterone gel
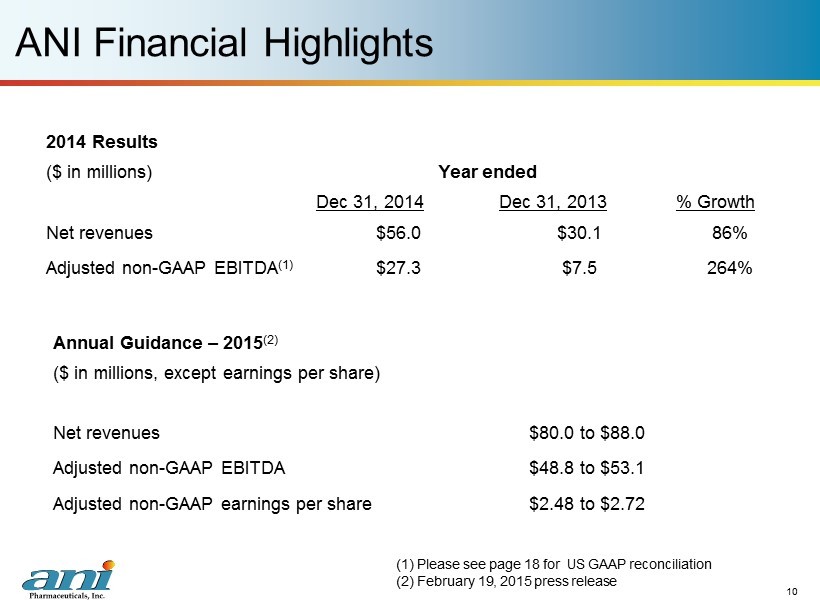
ANI Financial Highlights 10 2014 Results ($ in millions) Year ended Dec 31, 2014 Dec 31, 2013 % Growth Net revenues $56.0 $30.1 86% Adjusted non - GAAP EBITDA (1) $27.3 $7.5 264% (1) Please see page 18 for US GAAP reconciliation (2) February 19, 2015 press release Annual Guidance – 2015 (2) ($ in millions, except earnings per share) Net revenues $80.0 to $88.0 Adjusted non - GAAP EBITDA $48.8 to $53.1 Adjusted non - GAAP earnings per share $2.48 to $2.72

11 Product Development / Business Development Overview
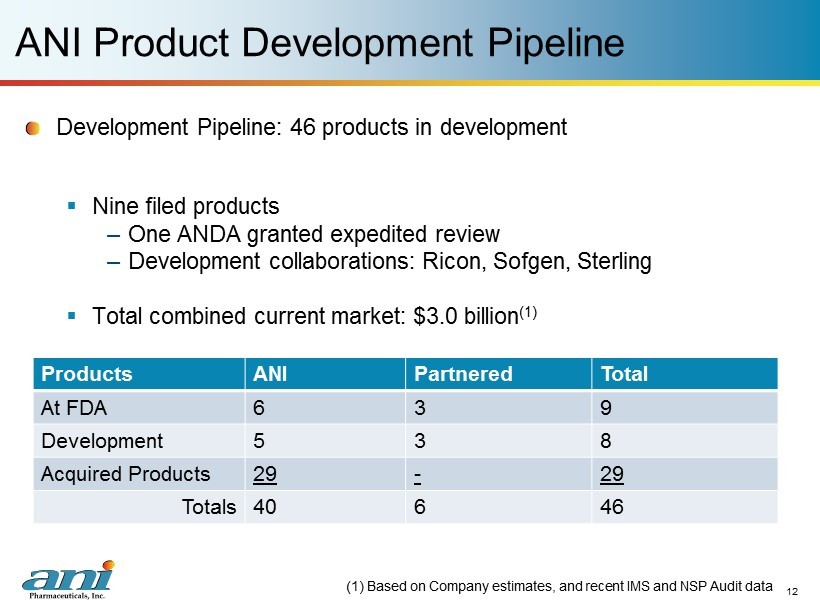
12 ANI Product Development Pipeline Development Pipeline: 46 p roducts in development ▪ Nine filed products – One ANDA granted expedited review – Development collaborations: Ricon, Sofgen, Sterling ▪ Total combined current market: $3.0 billion (1) (1) Based on Company estimates, and recent IMS and NSP Audit data Products ANI Partnered Total At FDA 6 3 9 Development 5 3 8 Acquired Products 29 - 29 Totals 40 6 46

13 ANI Business Development Highlights Acquired approved ANDA for Flecainide tablets from Teva , March 2015 Acquired Vancocin ® and related assets, August 2014 Acquired Lithobid ® , July 2014 Acquired 31 generic products, December 2013 Product development partnership with Sofgen, August 2013; expanded, April 2014 Product development partnership with Ricon, June 2011 Acquired Reglan ® tablets, June 2011 Business Development Focus In - licensing/acquisitions/alliances for development stage ANDAs, revenue generating products Enhancing generic product pipeline through development partnerships Company acquisitions

14 Manufacturing Overview

15 ANI Manufacturing – Main Street Facility Location: Baudette , Minnesota ▪ 52,000 square feet of manufacturing , packaging, and warehouse facilities ▪ Rx solutions , suspensions , topicals , tablets , and capsules ▪ DEA - licensed for Schedule II controlled substances ▪ 17,000 square feet of laboratory space for product development and analytical testing

16 ANI Manufacturing – IDC Road Facility Location: Baudette, Minnesota ▪ Fully - contained h igh potency facility with capabilities to manufacture h ormone , steroid , and oncolytic products ▪ 47,000 square feet of manufacturing and packaging, and warehouse facilities ▪ 100 nano - gram per eight - hour weighted average maximum exposure limit to ensure employee safety ▪ DEA Schedule IIIN capability
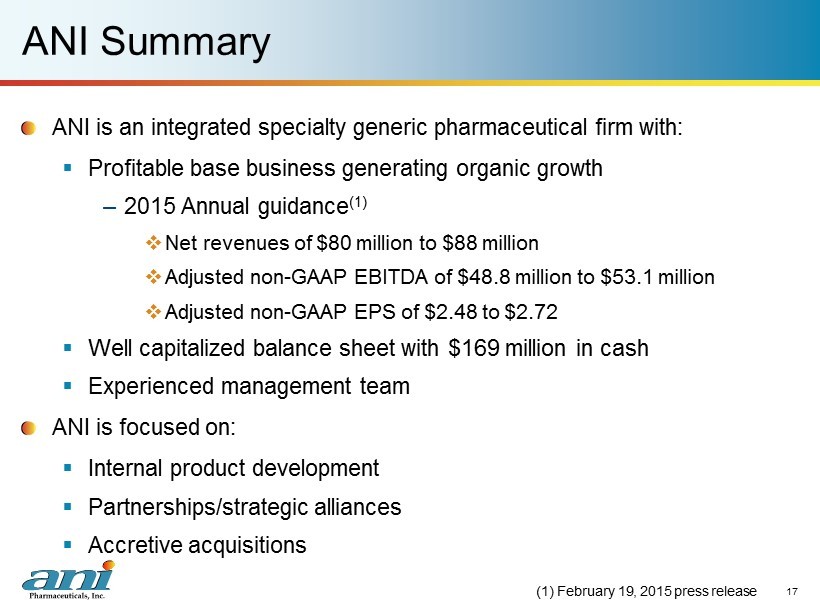
ANI Summary ANI is an integrated specialty generic pharmaceutical firm with: ▪ Profitable base business generating organic growth – 2015 Annual guidance (1) □ Net revenues of $80 million to $88 million □ Adjusted non - GAAP EBITDA of $48.8 million to $53.1 million □ Adjusted non - GAAP EPS of $2.48 to $2.72 ▪ Well capitalized balance sheet with $169 million in cash ▪ Experienced management team ANI is focused on: ▪ Internal product development ▪ Partnerships/strategic alliances ▪ Accretive acquisitions 17 (1) February 19, 2015 press release
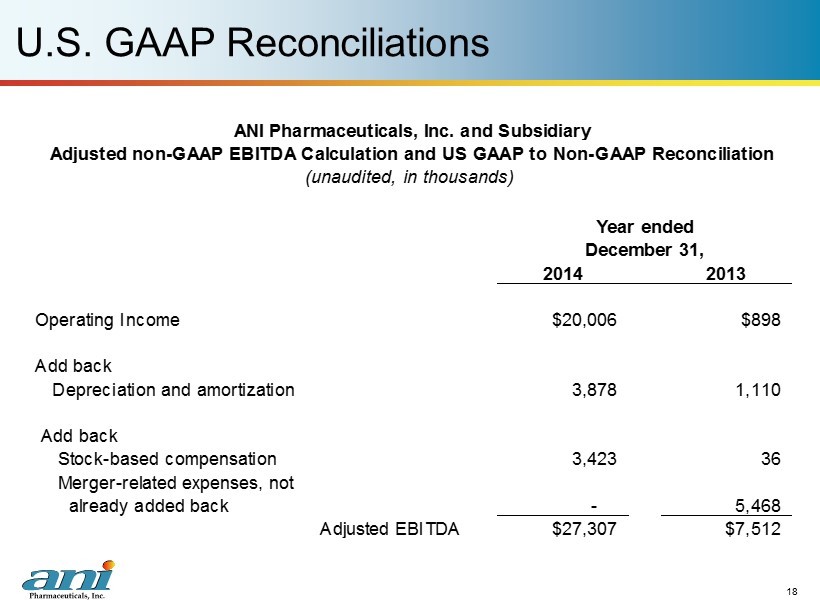
U.S. GAAP Reconciliations 18 2014 2013 Operating Income $20,006 $898 Add back Depreciation and amortization 3,878 1,110 Add back Stock-based compensation 3,423 36 Merger-related expenses, not already added back Adjusted EBITDA $27,307 $7,512 5,468 - ANI Pharmaceuticals, Inc. and Subsidiary Adjusted non-GAAP EBITDA Calculation and US GAAP to Non-GAAP Reconciliation (unaudited, in thousands) Year ended December 31,

















