- SLP Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Simulations Plus (SLP) 8-KOther Events
Filed: 20 Nov 12, 12:00am
Exhibit 99.1

Simulations Plus, Inc. (NASDAQ:SLP) Fourth Quarter and Fiscal Year 2012 Conference Call and Webinar November 19, 2012

With the exception of historical information, the matters discussed in this presentation are forward looking statements that involve a number of risks and uncertainties. The actual results of the Company could differ significantly from those statements. Factors that could cause or contribute to such differences include, but are not limited to: continuing demand for the Company’s products, competitive factors, the Company’s ability to finance future growth, the Company’s ability to produce and market new products in a timely fashion, the Company’s ability to continue to attract and retain skilled personnel, and the Company’s ability to sustain or improve current levels of productivity. Further information on the Company’s risk factors is contained in the Company’s quarterly and annual reports and filed with the Securities and Exchange Commission. Safe Harbor Statement
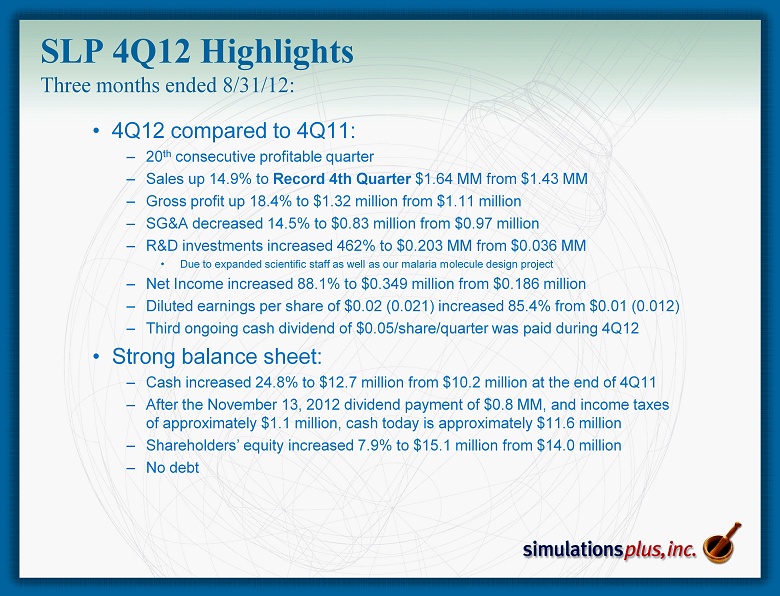
• 4 Q12 compared to 4Q11: – 20 th consecutive profitable quarter – Sales up 14.9% to Record 4th Quarter $1.64 MM from $1.43 MM – Gross profit up 18.4% to $1.32 million from $1.11 million – SG&A decreased 14.5% to $0.83 million from $0.97 million – R&D investments increased 462% to $0.203 MM from $0.036 MM • Due to expanded scientific staff as well as our malaria molecule design project – Net Income increased 88.1% to $0.349 million from $0.186 million – Diluted earnings per share of $0.02 (0.021) increased 85.4% from $0.01 (0.012) – Third ongoing cash dividend of $0.05/share/quarter was paid during 4Q12 • Strong balance sheet: – Cash increased 24.8% to $12.7 million from $10.2 million at the end of 4Q11 – After the November 13, 2012 dividend payment of $0.8 MM, and income taxes of approximately $1.1 million, cash today is approximately $11.6 million – Shareholders’ equity increased 7.9% to $15.1 million from $14.0 million – No debt SLP 4Q12 Highlights Three months ended 8/31/12:
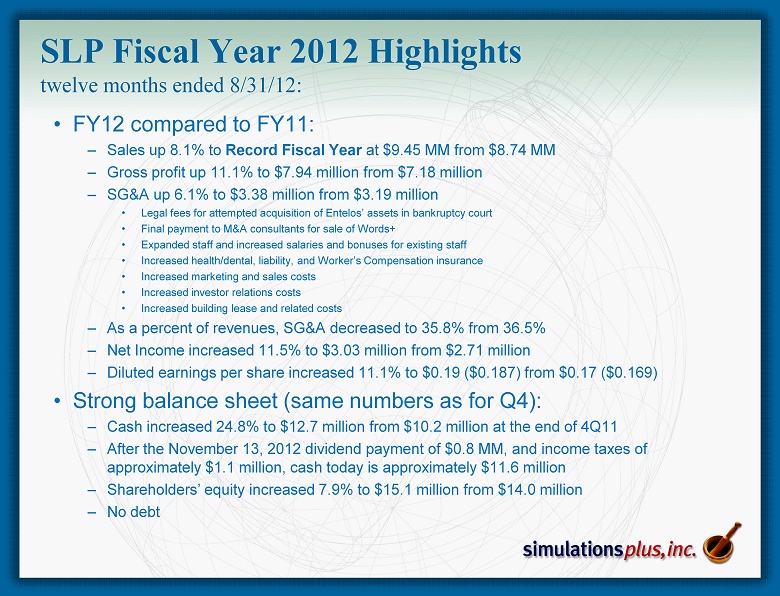
• FY12 compared to FY11: – Sales up 8.1% to Record Fiscal Year at $9.45 MM from $8.74 MM – Gross profit up 11.1% to $7.94 million from $7.18 million – SG&A up 6.1% to $3.38 million from $3.19 million • Legal fees for attempted acquisition of Entelos’ assets in bankruptcy court • Final payment to M&A consultants for sale of Words+ • Expanded staff and increased salaries and bonuses for existing staff • Increased health/dental, liability, and Worker’s Compensation insurance • Increased marketing and sales costs • Increased investor relations costs • Increased building lease and related costs – As a percent of revenues, SG&A decreased to 35.8% from 36.5% – Net Income increased 11.5% to $3.03 million from $2.71 million – Diluted earnings per share increased 11.1% to $0.19 ($0.187) from $0.17 ($0.169) • Strong balance sheet (same numbers as for Q4): – Cash increased 24.8% to $12.7 million from $ 10.2 million at the end of 4Q11 – After the November 13, 2012 dividend payment of $0.8 MM, and income taxes of approximately $ 1.1 million, cash today is approximately $11.6 million – Shareholders’ equity increased 7.9% to $15.1 million from $14.0 million – No debt SLP Fiscal Year 2012 Highlights twelve months ended 8/31/12:
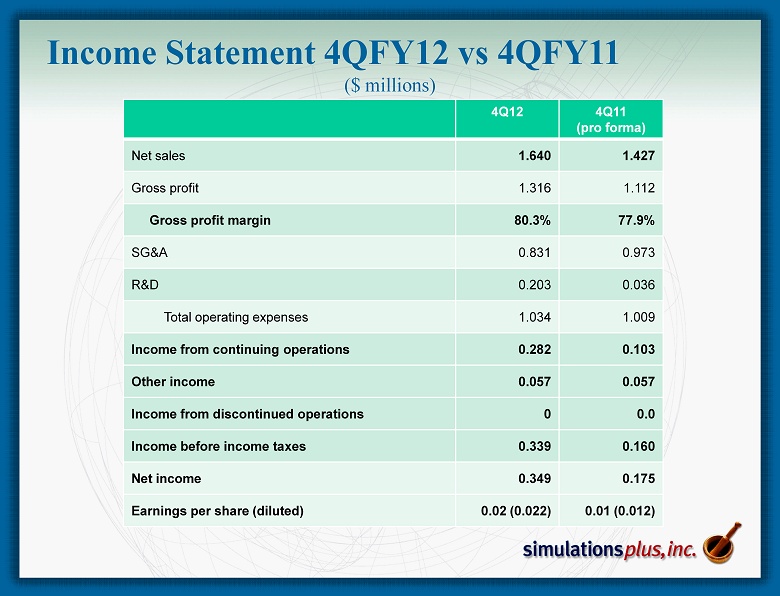
Income Statement 4 QFY12 vs 4QFY11 ($ millions) 4Q12 4Q11 (pro forma) Net sales 1.640 1.427 Gross profit 1.316 1.112 Gross profit margin 80.3% 77.9% SG&A 0.831 0.973 R&D 0.203 0.036 Total operating expenses 1.034 1.009 Income from continuing operations 0.282 0.103 Other income 0.057 0.057 Income from discontinued operations 0 0.0 Income before income taxes 0.339 0.160 Net income 0.349 0.175 Earnings per share (diluted) 0.02 (0.022) 0.01 (0.012)
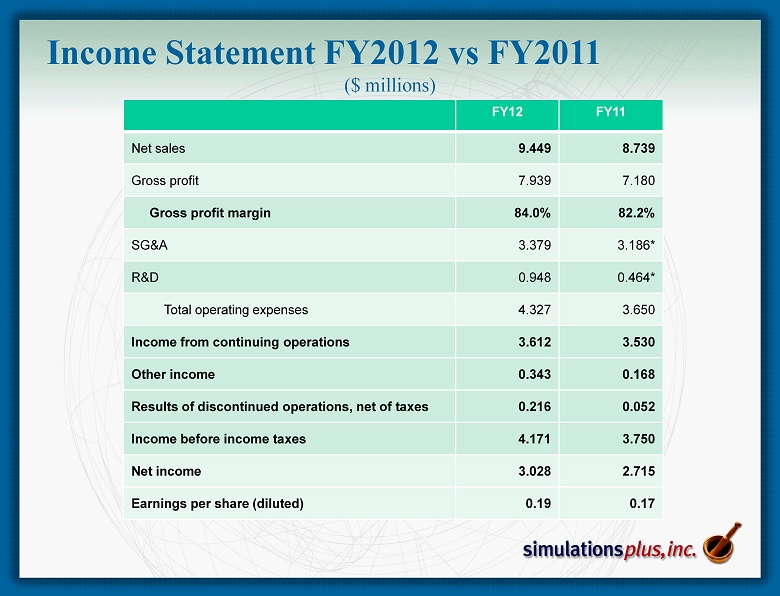
Income Statement FY2012 vs FY2011 ($ millions) FY12 FY11 Net sales 9.449 8.739 Gross profit 7.939 7.180 Gross profit margin 84.0% 82.2% SG&A 3.379 3.186* R&D 0.948 0.464* Total operating expenses 4.327 3.650 Income from continuing operations 3.612 3.530 Other income 0.343 0.168 Results of discontinued operations, net of taxes 0.216 0.052 Income before income taxes 4.171 3.750 Net income 3.028 2.715 Earnings per share (diluted) 0.19 0.17
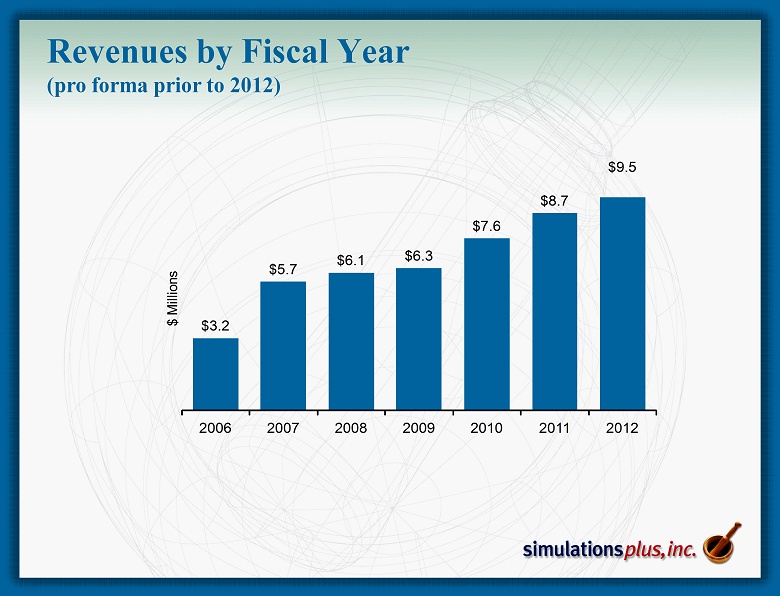
$3.2 $5.7 $6.1 $6.3 $7.6 $8.7 $9.5 2006 2007 2008 2009 2010 2011 2012 $ Millions Revenues by Fiscal Year (pro forma prior to 2012)
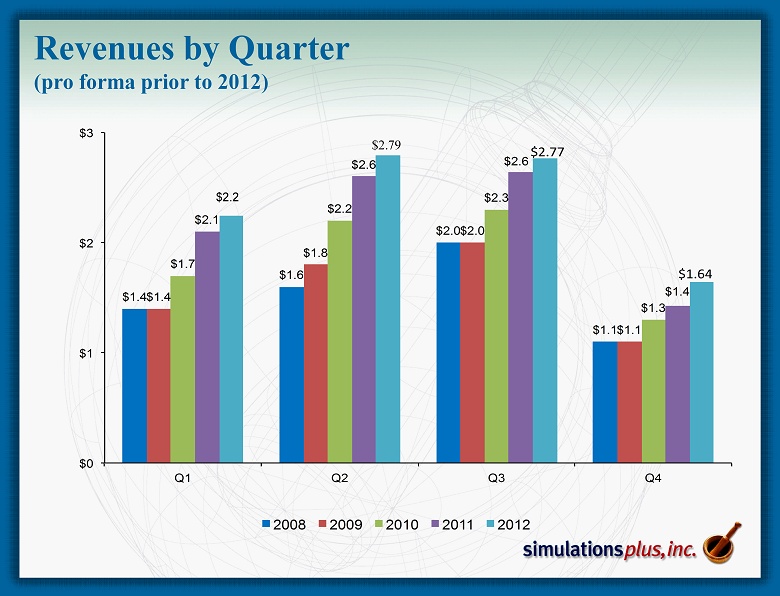
Revenues by Quarter (pro forma prior to 2012) $1.4 $1.6 $2.0 $1.1 $1.4 $1.8 $2.0 $1.1 $1.7 $2.2 $2.3 $1.3 $2.1 $2.6 $2.6 $1.4 $0 $1 $2 $3 Q1 Q2 Q3 Q4 2008 2009 2010 2011 2012 $2.77 $2.77 $2.77 $2.77 $1.64 $2.2 $2.79
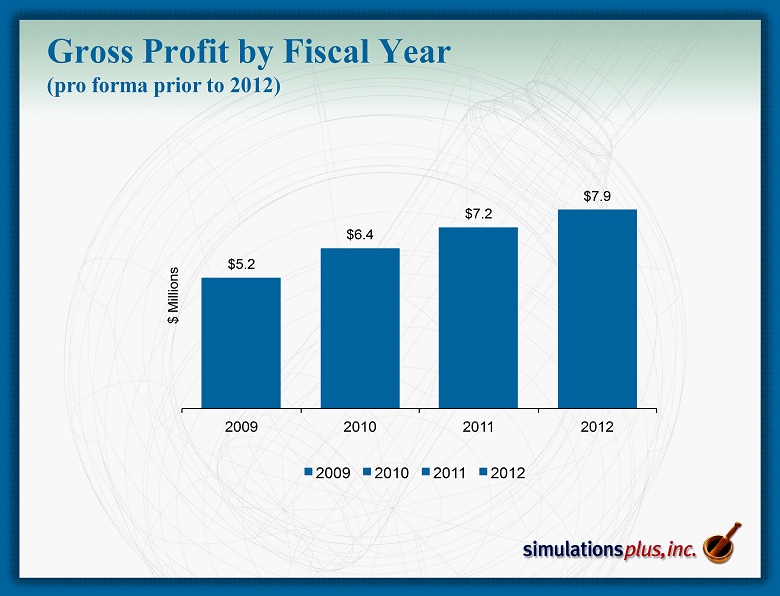
$5.2 $6.4 $7.2 $7.9 2009 2010 2011 2012 $ M i l l i o n s 2009 2010 2011 2012 Gross Profit by Fiscal Year (pro forma prior to 2012)
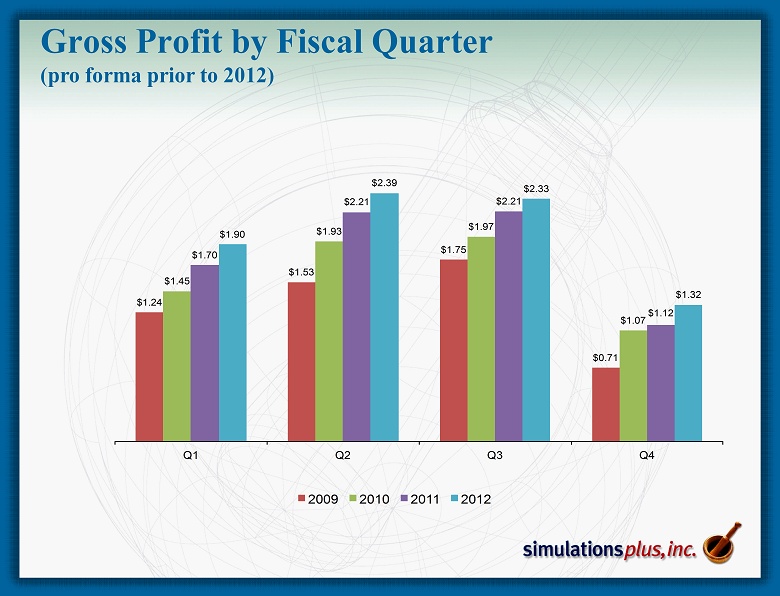
Gross Profit by Fiscal Quarter (pro forma prior to 2012) $1.24 $1.53 $1.75 $0.71 $1.45 $1.93 $1.97 $1.07 $1.70 $2.21 $2.21 $1.12 $1.90 $2.39 $2.33 $1.32 Q1 Q2 Q3 Q4 2009 2010 2011 2012
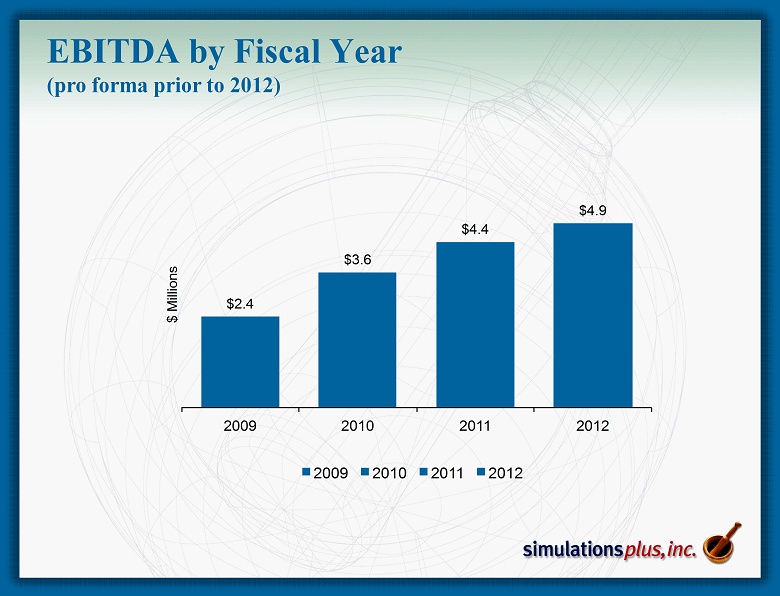
$2.4 $3.6 $4.4 $4.9 2009 2010 2011 2012 $ M i l l i o n s 2009 2010 2011 2012 EBITDA by Fiscal Year (pro forma prior to 2012)
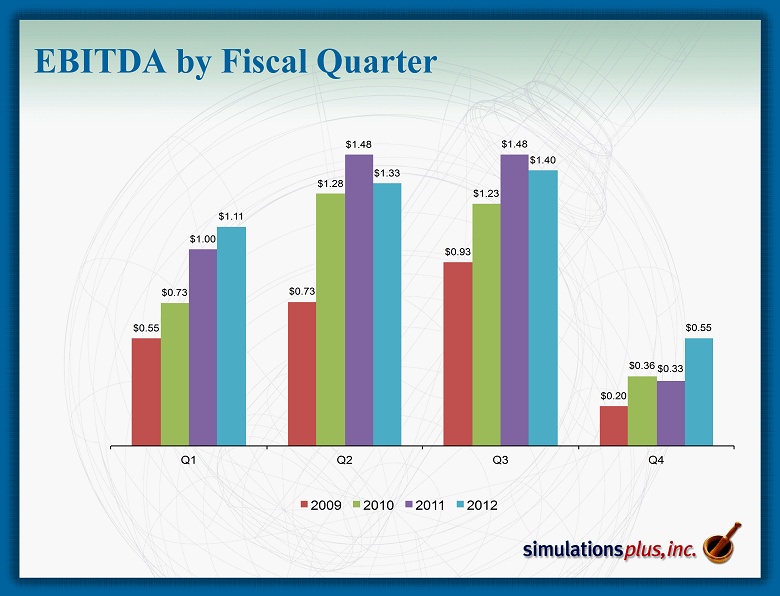
EBITDA by Fiscal Quarter $0.55 $0.73 $0.93 $0.20 $0.73 $1.28 $1.23 $0.36 $1.00 $1.48 $1.48 $0.33 $1.11 $1.33 $1.40 $0.55 Q1 Q2 Q3 Q4 2009 2010 2011 2012
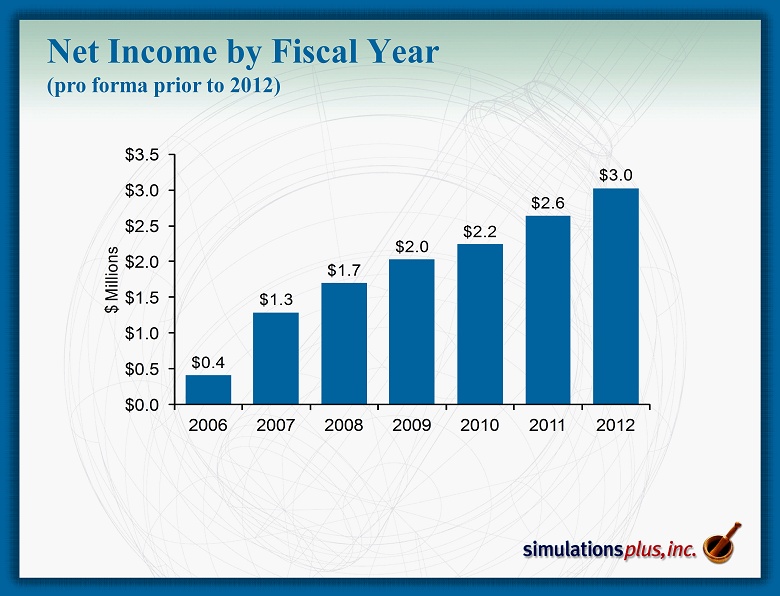
$0.4 $1.3 $1.7 $2.0 $2.2 $2.6 $3.0 $0.0 $0.5 $1.0 $1.5 $2.0 $2.5 $3.0 $3.5 2006 2007 2008 2009 2010 2011 2012 $ Millions Net Income by Fiscal Year (pro forma prior to 2012)
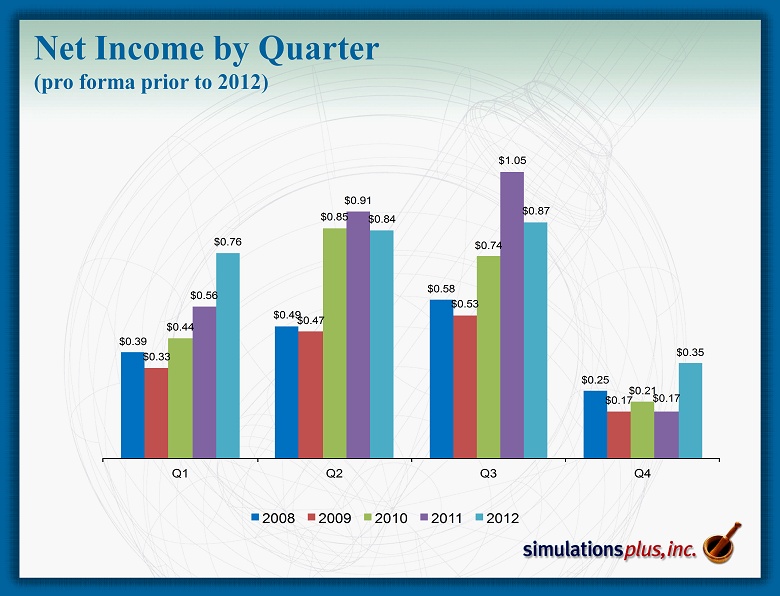
Net Income by Quarter (pro forma prior to 2012) $0.39 $0.49 $0.58 $0.25 $0.33 $0.47 $0.53 $0.17 $0.44 $0.85 $0.74 $0.21 $0.56 $0.91 $1.05 $0.17 $0.76 $0.84 $0.87 $0.35 Q1 Q2 Q3 Q4 2008 2009 2010 2011 2012
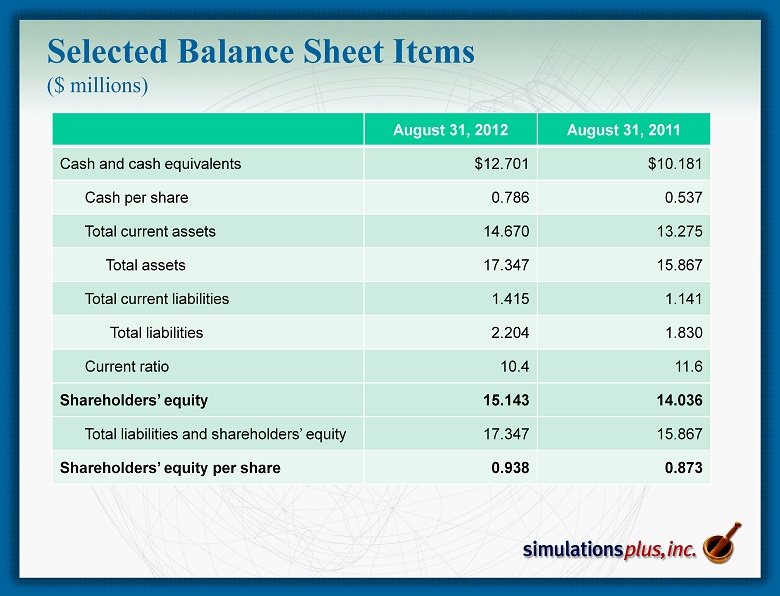
Selected Balance Sheet Items ($ millions) August 31, 2012 August 31, 2011 Cash and cash equivalents $12.701 $10.181 Cash per share 0.786 0.537 Total current assets 14.670 13.275 Total assets 17.347 15.867 Total current liabilities 1.415 1.141 Total liabilities 2.204 1.830 Current ratio 10.4 11.6 Shareholders’ equity 15.143 14.036 Total liabilities and shareholders’ equity 17.347 15.867 Shareholders’ equity per share 0.938 0.873
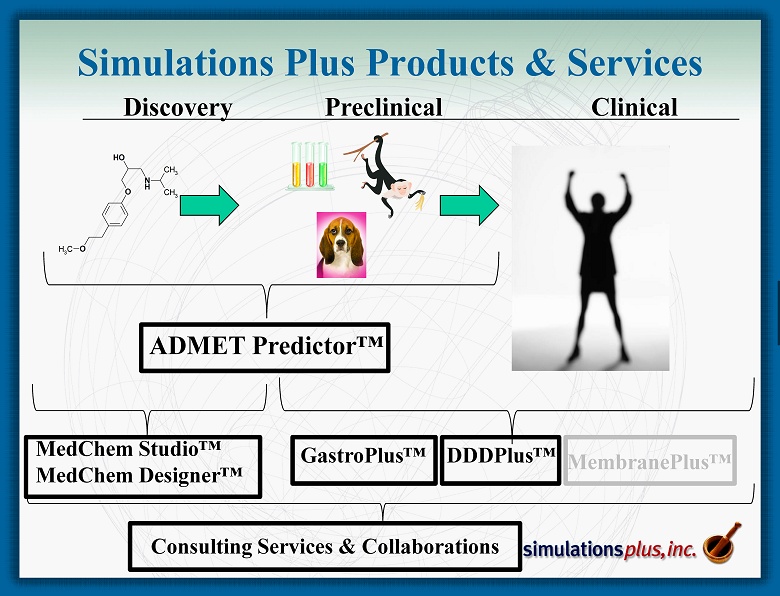
N H O OH O CH 3 CH 3 CH 3 Discovery Preclinical Clinical MedChem Studio ™ MedChem Designer™ GastroPlus™ DDDPlus ™ ADMET Predictor™ Simulations Plus Products & Services Consulting Services & Collaborations MembranePlus ™

• Simulations Plus continues to increase its technological advantage Recent Enhancements • Version 8.0 released May 2012 • Expanded drug - drug interaction to include transporters and induction • Expanded PDPlus ™ pharmacodynamics modeling module • Expanded ocular delivery model • Expanded nasal/pulmonary delivery model • Version 6.0 released May 2012 • Prediction of sites of metabolism now available • Best - in - class pKa models improved and display enhanced • Retrained models with new atomic level descriptors – our already top - ranked property predictions were further improved • Version 3.0 & MedChem Designer 2.0 released May 2012 • Integration of new MedChem Designer 2.0 software • MedChem Designer downloads/activations over 3000 • Significant improvements in processing speed • Prediction of metabolite structures to be available soon • Version 4.0 released in June 2011 • Virtual Trials added to show expected variances in experiments • FDA added more licenses during 3 rd quarter • Customer base continues to grow

Marketing and Sales Program • Added Cheminformatics salesperson July 2012 to increase sales of ADMET Design Suite™ (MedChem Studio™, MedChem Designer™, and ADMET Predictor™) • Conferences/Scientific Meetings continue to be primary source of leads • During Q4 we did 14 meetings in the U.S., Europe, and Asia • Total of 44 posters/presentations, and >25 peer - reviewed client publications during FY2012 • Training Workshops – Basic GastroPlus and Cheminformatics training workshops added to our Advanced GastroPlus™ training workshops • New Cheminformatics training workshop to be held in February • Strategic Digital Marketing Initiatives • Collaborations/Consulting/Grants – Progressing on our 5 - year collaboration with the FDA Center for Food Safety and Applied Nutrition to build many toxicity models with ADMET Predictor/Modeler™ for food additives and contaminants – Consulting studies continue – provides exposure of software to new groups – New funded collaboration announced for enhancement of the GastroPlus oral cavity dosing model – New funded collaboration announced for enhancement of the GastroPlus pulmonary dosing model • We believe fundamental industry shift continues – Software tools are constantly gaining wider acceptance and applications are growing – 10 new customers during 4QFY12 (includes new companies as well as new departments within existing large customers ), and 43 new in all of FY2012

FDA Food Safety Research Collaboration • Completed the first year of a 5 - year renewable Research Collaboration Agreement (RCA) with Center for Food Safety and Applied Nutrition (CFSAN) to provide model - building capabilities for a large number (>70,000) of substances that can be in foods as additives or contaminants, only a small fraction of which have been testes for various toxicities. • Models will then be used to predict the likely toxicity of the molecules that were not tested to identify likely problems • Requested code modifications from the FDA became a part of ADMET Predictor 6.0, which was released in May. • The first new toxicity model for ADMET Predictor coming from this collaboration was released with Version 6.0. This is a model for predicting rodent carcinogenicity. Others are in progress.

NCE (New Chemical Entity) Project • Using a public database courtesy of GlaxoSmithKline, and applying MedChem Studio/MedChem Designer/ADMET Predictor, we designed a number of lead candidates to inhibit the malaria parasite and qualified them for acceptable properties using only ADMET Predictor predictions. • Seven molecules were received from synthesis and were tested against the malaria parasite. All seven showed inhibition of the growth of the parasite , with two active at a nanomolar level against both wild - type and drug - resistant strains of the parasite . • Note that last week according to Reuters, GlaxoSmithKline Plc reported disappointing results for their malaria vaccine for infants in Africa’s largest ever clinical trial of over 6,500 babies aged 6 - 12 weeks. It was effective for about 30% of babies. Reuter noted further that GSK plans to push ahead with vaccine development. Note that our molecules are not intended as vaccines, but as therapeutic drugs. • We have initiated communications with outside organizations that are known to fund developments for malaria and other diseases to determine whether there is interest in funding further work by Simulations Plus using the methods we have demonstrated.

Summary • For 4 QFY12 and FY2012: - Financial performance continues our 5 - year profitable trend - Sale of Words+ has simplified and focused the business - margins increased, reporting and auditing much simpler • Continuing to Expand our Life Sciences team: - Two new Ph.D.s started in August, interviews continuing - Promoting development of new products and services (e.g., MembranePlus ) - Strengthens and supports our marketing and sales efforts • Expanding Sales Team and Activities - New field sales manager for cheminformatics products and services started in July - Greater staff time spent on marketing and sales activities - New training workshop for chemistry tools to be held in Boston in February • Simulations Plus is globally recognized as a leader - Outstanding reputation for scientific expertise and innovation - Strong customer support • Strong cash position and no debt - Four quarterly cash dividends of $0.05/share/quarter have now been paid for a total of just under $3.2 million, yet cash grew by over $2.5 million during FY2012 .

Q&A