Exhibit 99.1

Copyright (c) 2015 Simulations Plus, Inc. Simulations Plus, Inc. (NASDAQ:SLP) Annual Shareholders’ Meeting February 24, 2015

Copyright (c) 2015 Simulations Plus, Inc. • Welcome and Introductions • Discussion of Voting Issues • Chairman’s Remarks • Financial presentation • Products and Services • Questions & Answers • Adjournment of Official Meeting Agenda

Copyright (c) 2015 Simulations Plus, Inc. 3 With the exception of historical information, the matters discussed in this presentation are forward - looking statements that involve a number of risks and uncertainties . The actual results of the Company could differ significantly from those statements . Factors that could cause or contribute to such differences include, but are not limited to : continuing demand for the Company’s products, competitive factors, the Company’s ability to finance future growth, the Company’s ability to produce and market new products in a timely fashion, the Company’s ability to continue to attract and retain skilled personnel, and the Company’s ability to sustain or improve current levels of productivity . Further information on the Company’s risk factors is contained in the Company’s quarterly and annual reports and filed with the Securities and Exchange Commission . Safe Harbor Statement

Copyright (c) 2015 Simulations Plus, Inc. Management & Directors • Board of Directors: – David Z. D’Argenio, Ph.D., Director • Professor, Biomedical Engineering, USC • Director, Co - Director, Biological Simulation Resource Center, USC since 1985 – Thaddeus H. “Ted” Grasela, Ph.D., Director and President • Founder of Cognigen Corporation – John K. Paglia Ph.D., CFA, CPA , Director • Associate Dean; Associate Professor of Finance, Pepperdine University – David Ralph, Ph.D., Director • Chair, Department of Economics, Law & Marketing, Pepperdine University – Walter S. Woltosz, M.S., M.A.S., Chairman & CEO. • Co - founder, Words+, Inc. and Simulations Plus, Inc. • Senior Management Team – John R. Kneisel, CPA, Chief Financial Officer – John DiBella, M.S., Vice President, Marketing, and Sales – Michael B. Bolger, Ph.D., Chief Scientist and Director of Simulation Sciences – Robert Clark, Ph.D., Director, Cheminformatics Sciences

Copyright (c) 2015 Simulations Plus, Inc. • Registered Independent Auditors • Rose, Snyder & Jacobs, Encino, CA • Tax Specialists • Rose, Snyder & Jacobs, Encino, CA • Legal Counsel • Procopio, Cory, Hargreaves & Savitch LLP Outside Counsels

Copyright (c) 2015 Simulations Plus, Inc. 6 • Through this morning 89.73% of outstanding share votes have been cast • Proposal 1: Election of Directors ‒ Result: All elected with 99+% voting for • Proposal 2: Ratify Rose, Snyder & Jacobs CPA’s as Independent Registered Public Accounts ‒ Result: Ratified with 99+% voting to ratify VOTING ISSUES/PROXY PROPOSALS 6

Copyright (c) 2015 Simulations Plus, Inc. 7 • Major software provider for pharmaceutical research and development • Consulting services for problem drugs and formulations • Expertise and software tools span from earliest drug discovery through clinical trials and beyond patent life to supporting generic companies • New applications being explored in aerospace and general healthcare • Acquisition of Cognigen Corp. in September 2014 more than doubled workforce, expected to add approximately $5 million to revenues in FY15 – first combined fiscal quarter was completed on November 30 • Consolidated revenues up 54% over last year’s first fiscal quarter; gross profit increased 41% • Currently distributing dividend of $0.20/year per share ($0.05/quarter) , subject to board approval each quarter Simulations Plus, Inc. Overview

Copyright (c) 2015 Simulations Plus, Inc. 8 • Year ended August 31, 2014 vs 2013 • Fiscal Quarter Ended November 30, 2014 vs 2013 • Fiscal Quarter Ended November 30, 2014 by division(Simulations Plus/Cognigen) • Financial Charts FINANCIAL PRESENTATION

Copyright (c) 2015 Simulations Plus, Inc. • FY14 compared to FY13: – Sales increased by $1.4 million or 13.8% to $11.5 million from $10.1 million to set a new fiscal year record – Cost of sales as a percentage of sales decreased 1.3% or $ 17,000 to 14.2% from 16.3% in FY13 • Decrease was attributable to a reduction in royalty expenses attributable to the buyout of the TSRL agreement – Gross profit increased 16.7% to $9.8 million from $8.4 million in FY13 – Gross margin increased to 85.8% from 83.7% – SG&A increased 25.1% to $4.4 million from $ 3.5 million • As a percent of revenues, SG&A increase to 38.7% from 35.2% • Due to increases in marketing, travel, one - time consulting and professional fees, sales commissions, and salaries and wages. • Professional fees associated with the Cognigen acquisition accounted for almost 75% of the 3.4% increase SLP FY14 Highlights Fiscal year ended 8/31/14: 9

Copyright (c) 2015 Simulations Plus, Inc. • FY14 compared to FY13 ( con’t ) – R&D expense increased 18.7% to $953,000 from $802,000 the prior year • Increase was due to an increase in life science staffing and included R&D expenditures for the COX2 /COX1 NCE project of approximately $50,000 – Net Income increased by $139,000 or 4.8% to $3.0 million in FY14 from $ 2.9 million in FY13 – Diluted earnings per share was $0.1844, an increase of $0.0075 from $0.1769 in FY13 – Cash dividends totaling $0.19/share were distributed during FY14 SLP FY14 Highlights Fiscal year ended 8/31/14: 10
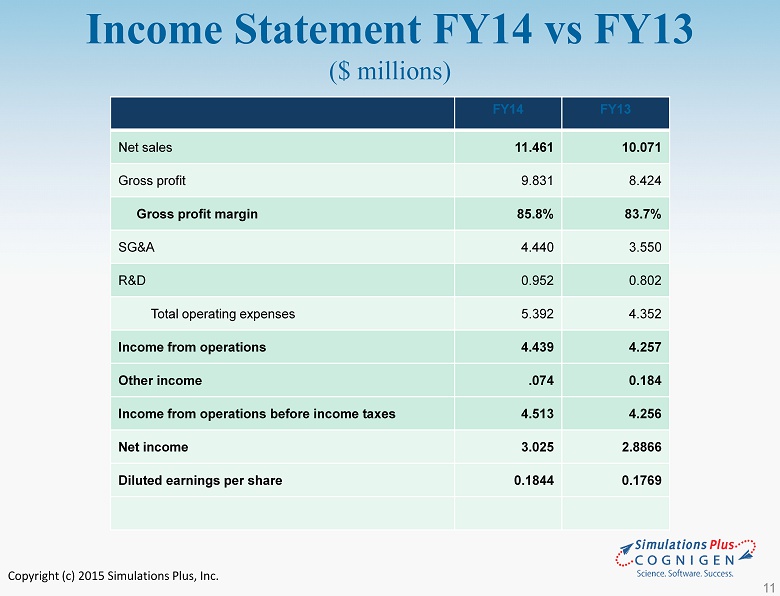
Copyright (c) 2015 Simulations Plus, Inc. Income Statement FY14 vs FY13 ($ millions) FY14 FY13 Net sales 11.461 10.071 Gross profit 9.831 8.424 Gross profit margin 85.8% 83.7% SG&A 4.440 3.550 R&D 0.952 0.802 Total operating expenses 5.392 4.352 Income from operations 4.439 4.257 Other income .074 0.184 Income from operations before income taxes 4.513 4.256 Net income 3.025 2.8866 Diluted earnings per share 0.1844 0.1769 11
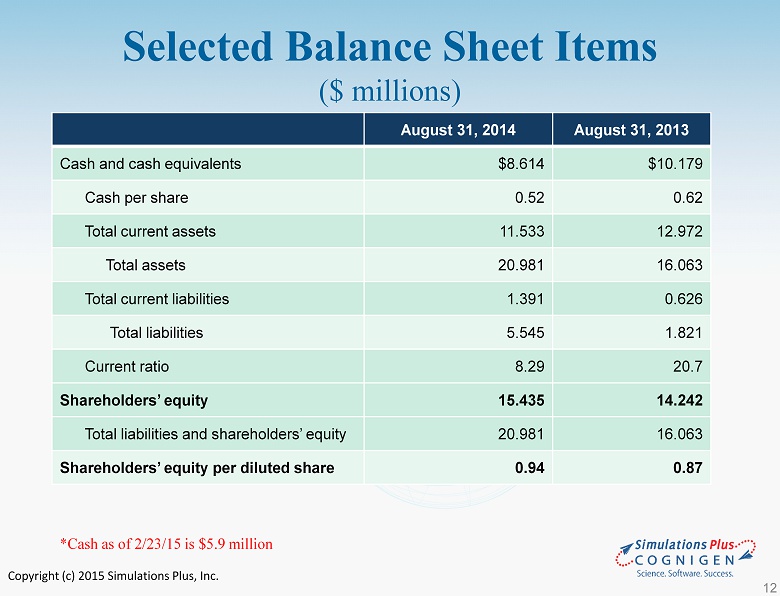
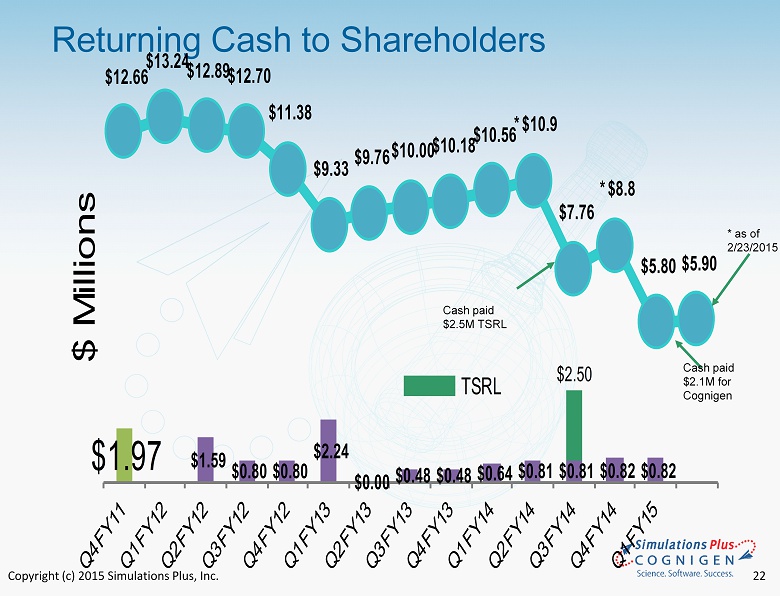
Copyright (c) 2015 Simulations Plus, Inc. Selected Balance Sheet Items ($ millions) August 31, 2014 August 31, 2013 Cash and cash equivalents $8.614 $10.179 Cash per share 0.52 0.62 Total current assets 11.533 12.972 Total assets 20.981 16.063 Total current liabilities 1.391 0.626 Total liabilities 5.545 1.821 Current ratio 8.29 20.7 Shareholders’ equity 15.435 14.242 Total liabilities and shareholders’ equity 20.981 16.063 Shareholders’ equity per diluted share 0.94 0.87 *Cash as of 2/23/15 is $5.9 million 12

Copyright (c) 2015 Simulations Plus, Inc. 13 • FQY15.1 - Revenues and earnings continued seven - year - plus profitable trend - Cognigen acquisition increases revenues, expands offerings into clinical pharmacology - Earnings for quarter lower due to one - time charges associated with the Cognigen acquisition • Continuing to grow - 54.7% revenue growth seen in first combined fiscal quarter - 35 new staff added with acquisition of Cognigen • Strong cash position – returning cash to shareholders - Cash dividends totaling approximately $9 million have been distributed, yet cash position remains strong, c urrently $5.9 million at 2/23/15 • Continued Aggressive marketing and sales activities - Intensive conference/trade show/workshop schedule - Workshops scheduled for North America, Europe, and Asia in 2015 - Expanded Cognigen marketing and sales activities Summary FQY 15.1
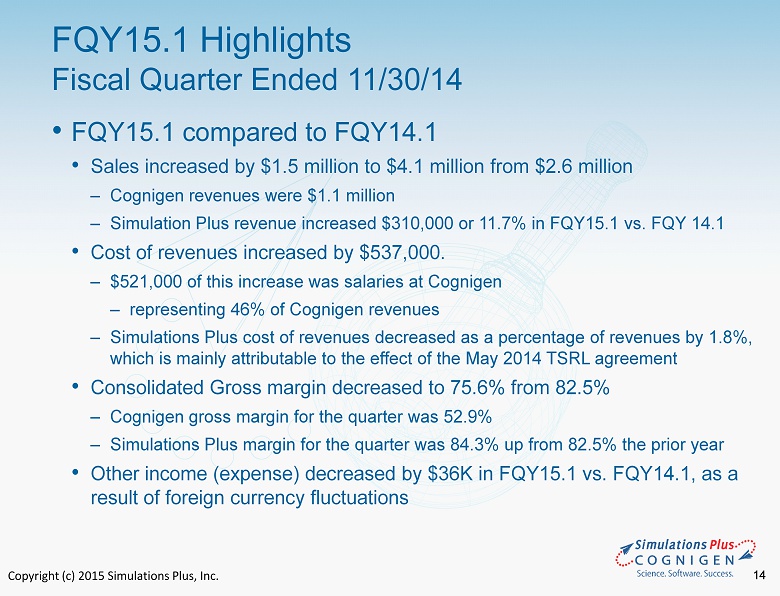
Copyright (c) 2015 Simulations Plus, Inc. 14 • FQY15.1 compared to FQY14.1 • Sales increased by $1.5 million to $4.1 million from $ 2.6 million ‒ Cognigen revenues were $1.1 million ‒ Simulation Plus revenue increased $310,000 or 11.7% in FQY15.1 vs. FQY 14.1 • Cost of revenues increased by $537,000. ‒ $521,000 of this increase was salaries at Cognigen ‒ representing 46% of Cognigen revenues ‒ Simulations Plus cost of revenues decreased as a percentage of revenues by 1.8 %, which is mainly attributable to the effect of the May 2014 TSRL agreement • Consolidated Gross margin decreased to 75.6% from 82.5% ‒ Cognigen gross margin for the quarter was 52.9% ‒ Simulations Plus margin for the quarter was 84.3% up from 82.5% the prior year • Other income ( expense) decreased by $36K in FQY15.1 vs. FQY14.1, as a result of foreign currency fluctuations FQY15.1 Highlights Fiscal Quarter Ended 11/30/14
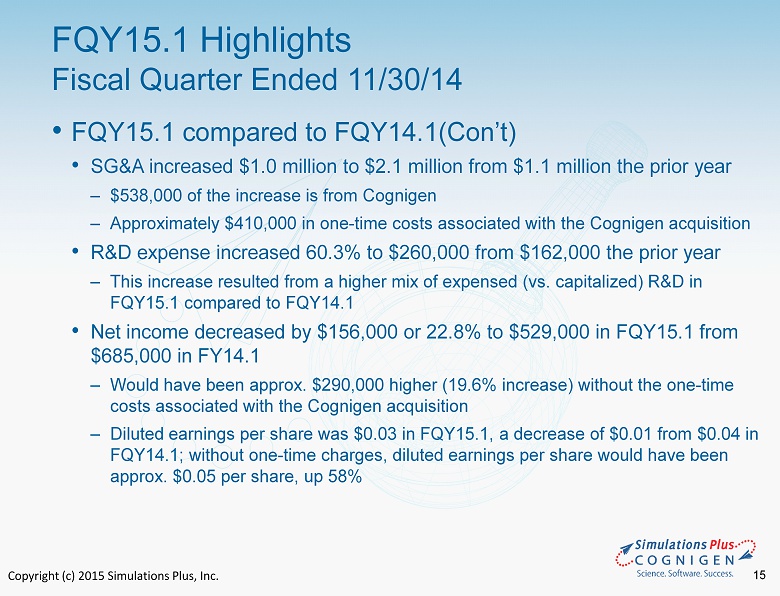
Copyright (c) 2015 Simulations Plus, Inc. 15 • FQY15.1 compared to FQY14.1(Con’t) • SG&A increased $1.0 million to $2.1 million from $1.1 million the prior year ‒ $538,000 of the increase is from Cognigen ‒ Approximately $410,000 in one - time costs associated with the Cognigen acquisition • R&D expense increased 60.3% to $260,000 from $162,000 the prior year ‒ This increase resulted from a higher mix of expensed (vs. capitalized) R &D in FQY15.1 compared to FQY14.1 • Net income decreased by $156,000 or 22.8% to $529,000 in FQY15.1 from $685,000 in FY14.1 ‒ Would have been approx. $290,000 higher (19.6% increase) without the one - time costs associated with the Cognigen acquisition ‒ Diluted earnings per share was $0.03 in FQY15.1, a decrease of $0.01 from $0.04 in FQY14.1; without one - time charges, diluted earnings per share would have been approx. $0.05 per share, up 58% FQY15.1 Highlights Fiscal Quarter Ended 11/30/14

Copyright (c) 2015 Simulations Plus, Inc. 16 Income Statement FQY15.1 versus QY14.1 ($ millions) FQY15.1 FQY14.1 Net sales 4.086 2.641 Gross profit 3.088 2.193 Gross profit margin 75.6% 83.0% SG&A 2.079 1.071 R&D 0.260 0.162 Total operating expenses 2.339 1.233 Income from operations 0.749 0.959 Other income(expense) (.003) 0.033 Income from operations before income taxes 0.746 0.992 Net income 0.529 0.685 Diluted earnings per share 0.031 0.043 Note : Cognigen merger became effective 9/2/2014; November 30, 2014 presents consolidated results, November 30, 2013 shows Simulations Plus results.

Copyright (c) 2015 Simulations Plus, Inc. 17 Income Statement FQY15.1 by division ($ millions) SLP Cognigen Eliminations FQY15.1 Net sales 2.951 1.135 0.00 4.086 Gross profit 2.488 0.600 0.00 3.088 Gross profit margin 84.3% 52.9% 0.00 75.6% SG&A 1.541 0.537 0.00 2.079 R&D 0.260 0.000 0.00 0.260 Total operating expenses 1.801 0.537 0.00 2.339 Income from operations 0.686 0.063 0.00 0.749 Other income(expense) (.003) .000 0.00 (.003) Income from operations before income taxes 0.683 0.063 0.00 0.746 Net income 0.489 0.040 0.00 0.529
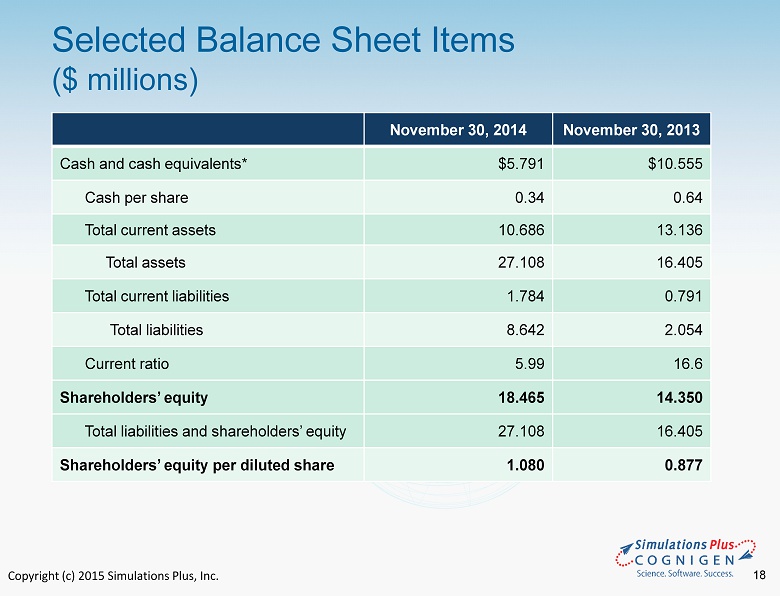
Copyright (c) 2015 Simulations Plus, Inc. 18 Selected Balance Sheet Items ($ millions) November 30, 2014 November 30, 2013 Cash and cash equivale nts* $5.791 $10.555 Cash per share 0.34 0.64 Total current assets 10.686 13.136 Total assets 27.108 16.405 Total current liabilities 1.784 0.791 Total liabilities 8.642 2.054 Current ratio 5.99 16.6 Shareholders’ equity 18.465 14.350 Total liabilities and shareholders’ equity 27.108 16.405 Shareholders’ equity per diluted share 1.080 0.877
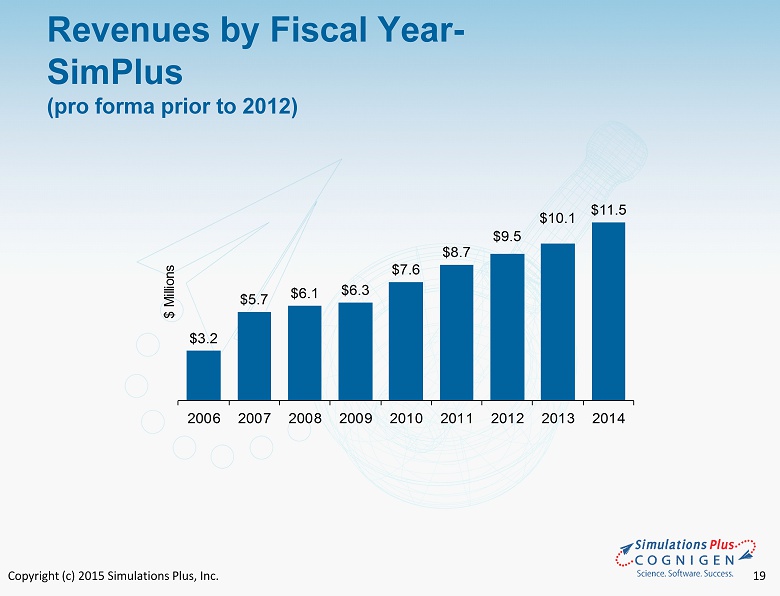
Copyright (c) 2015 Simulations Plus, Inc. 19 $3.2 $5.7 $6.1 $6.3 $7.6 $8.7 $9.5 $10.1 $11.5 2006 2007 2008 2009 2010 2011 2012 2013 2014 $ Millions Revenues by Fiscal Year - SimPlus (pro forma prior to 2012)
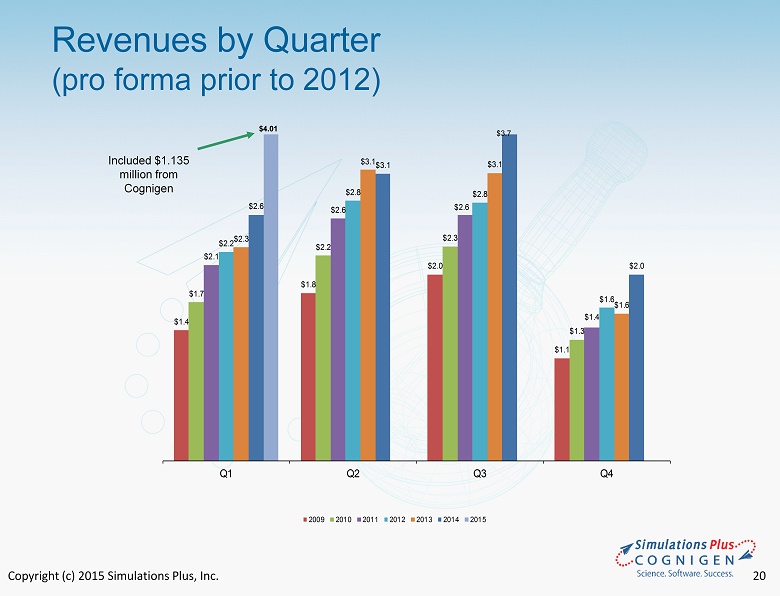
Copyright (c) 2015 Simulations Plus, Inc. 20 Revenues by Quarter (pro forma prior to 2012) $1.4 $1.8 $2.0 $1.1 $1.7 $2.2 $2.3 $1.3 $2.1 $2.6 $2.6 $1.4 $2.2 $2.8 $2.8 $1.6 $2.3 $3.1 $3.1 $1.6 $2.6 $3.1 $2.0 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015 $3.7 $4.01 Included $1.135 million from Cognigen
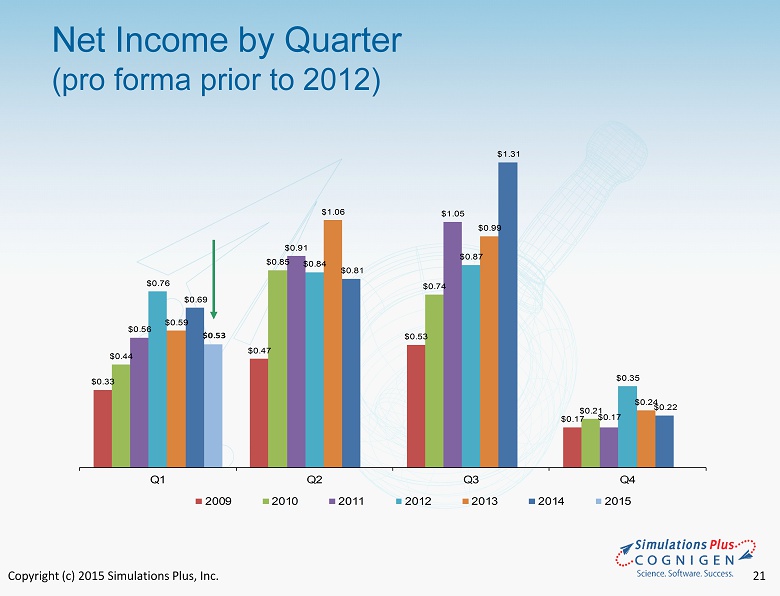
Copyright (c) 2015 Simulations Plus, Inc. 21 Net Income by Quarter (pro forma prior to 2012) $ 0.33 $ 0.47 $ 0.53 $ 0.17 $ 0.44 $ 0.85 $ 0.74 $ 0.21 $ 0.56 $ 0.91 $ 1.05 $ 0.17 $ 0.76 $ 0.84 $ 0.87 $ 0.35 $ 0.59 $ 1.06 $ 0.99 $ 0.24 $ 0.69 $ 0.81 $ 1.31 $ 0.22 $ 0.53 Q1 Q2 Q3 Q4 2009 2010 2011 2012 2013 2014 2015

Copyright (c) 2015 Simulations Plus, Inc. 23 Products & Services
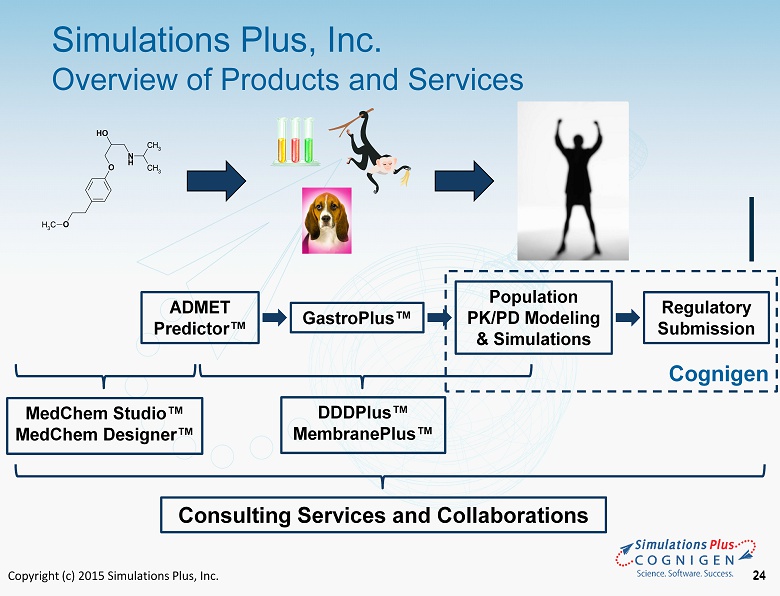
Copyright (c) 2015 Simulations Plus, Inc. 24 Simulations Plus, Inc. Overview of Products and Services 24 N H O OH O CH 3 CH 3 CH 3 ADMET Predictor ™ GastroPlus ™ Population PK/PD Modeling & Simulations Regulatory Submission MedChem Studio ™ MedChem Designer™ DDDPlus ™ MembranePlus ™ Consulting Services and Collaborations Cognigen
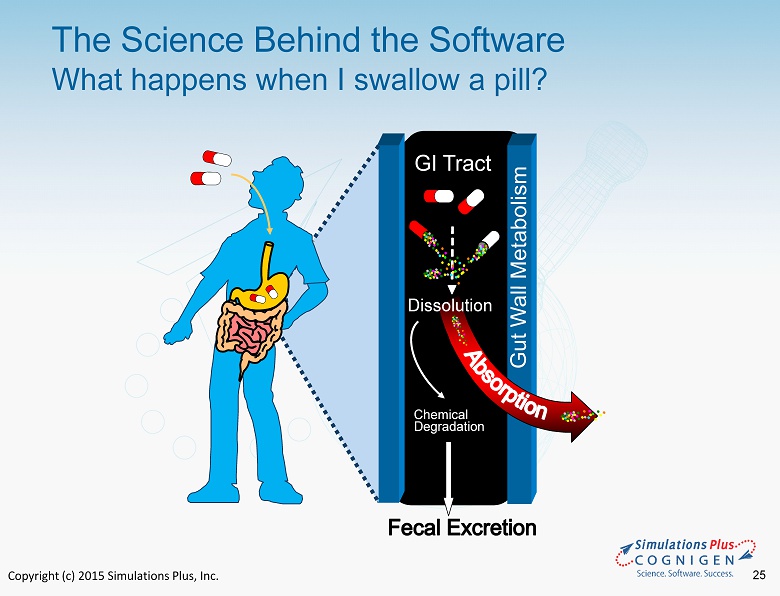
Copyright (c) 2015 Simulations Plus, Inc. 25 The Science Behind the Software What happens when I swallow a pill? GI Tract Chemical Degradation Dissolution

Copyright (c) 2015 Simulations Plus, Inc. 26 The Science Behind the Software What happens after the medicine is absorbed?
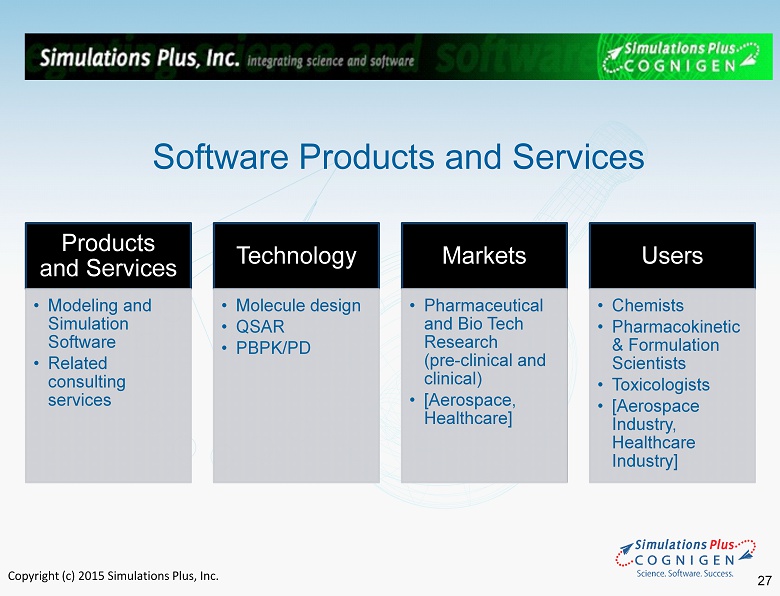
Copyright (c) 2015 Simulations Plus, Inc. Products and Services • Modeling and Simulation Software • Related consulting services Technology • Molecule design • QSAR • PBPK/PD Markets • Pharmaceutical and Bio Tech Research (pre - clinical and clinical) • [Aerospace, Healthcare ] Users • Chemists • Pharmacokinetic & Formulation Scientists • Toxicologists • [Aerospace Industry, Healthcare Industry] Software Products and Services 27

Copyright (c) 2015 Simulations Plus, Inc. Simulations Plus Software Product News • Version 8.6 was released in August 2014; Version 9.0 slated for March 2015 release • Populations simulations linked with Drug - Drug Interaction (DDI) predictions • New animal PBPK models • Tighter integration with ADMET Predictor™ models – now create PBPK models from structure • Coming soon: PBPK modeling for biologics, dermal and oral cavity delivery models • Version 7.1 released in July 2014; Version 7.2 slated for March 2015 release • Significantly enhanced pKa model developed in collaboration with Bayer HealthCare • All property models retrained, leading to more accurate predictions • New transporter and toxicity models • Significantly improved and expanded metabolism predictions • Version 4.0 & MedChem Designer 3.0 released in May 2014 • New Optical Structure Recognition (OSR) feature • New ultra - fast screening methods for large molecular libraries • New 64 - bit versions offered for screening very large libraries • Version 4.5 slated for release in June 2015 • Virtual Trials added to show expected variances in experiments • Coming soon: integration of ADMET Predictor models to predict dissolution inputs from structure • Coming soon: upgraded handling of complex dosage forms/formulations • Version 1.0 released in November 2014 • Explores mechanisms of permeability through cell layers • Optimize and design the in vitro experimental setup to properly capture absorption kinetics • Integration with GastroPlus to accurately predict IVIVE for absorption processes • First license sale in Q2, ~10 evaluations ongoing Copyright (c) 2015 Simulations Plus, Inc.
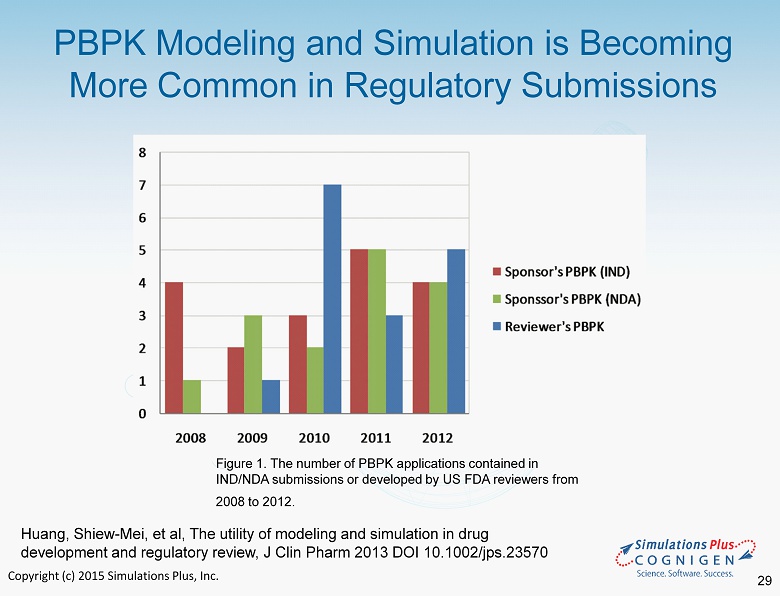
Copyright (c) 2015 Simulations Plus, Inc. PBPK Modeling and Simulation is Becoming More Common in Regulatory Submissions Figure 1. The number of PBPK applications contained in IND/NDA submissions or developed by US FDA reviewers from 2008 to 2012. Huang, Shiew - Mei, et al, The utility of modeling and simulation in drug development and regulatory review, J Clin Pharm 2013 DOI 10.1002/jps.23570 29
Copyright (c) 2015 Simulations Plus, Inc. 30 Application of artificial neural network ensemble (ANNE) technology used to build predictive models from complex input data for various industries • ADMET Modeler ™ (embedded in ADMET Predictor ™) • Predicts large number of important drug properties without the need to synthesize and test molecules • AEROModeler™ (under development) • Predicts aerodynamic force coefficients for missiles at arbitrary Mach number and angle of attack - much faster than conventional methods • MRIModeler ™ ( under development ) • Analyzes magnetic resonance imaging (MRI) data to classify patients as healthy or likely to experience various disease states Modeler ™ Overview
Copyright (c) 2015 Simulations Plus, Inc. 31 • FDA Center for Food Safety and Applied Nutrition (modeling of toxicity endpoints) • FDA Office of Testing and Research (mechanistic absorption and IVIVCs) • NIEHS National Toxicology Program (screening of the Tox21 database using QSAR and PBPK modeling) • FDA Office of Generic Drugs (ocular absorption) • Recently awarded $200,000/year contract for up to 3 years • Numerous client - funded projects to enhance software platform for specialty purposes Strong Relationships with Regulators (and Pharma Companies) Driving Applications

Copyright (c) 2015 Simulations Plus, Inc. 32 • Provides modeling & simulation support for Pharma and Biotech companies • Primary focus on pharmacokinetic, biomarker, and outcome data from preclinical and clinical studies • 30 - 40 drugs per year; >100 different compounds over the last 5 years • Track record of successful regulatory submissions utilizing pharmacometric modeling & simulation • Well - established quality management system and successful client audit record • KIWI ™ - proprietary software provides access to validated private cloud for model development • Significant recent investments in IT infrastructure: upgraded computer systems , implemented automated offsite backup storage , and installed green technology to reduce computer cooling costs Cognigen Division - Overview
Copyright (c) 2015 Simulations Plus, Inc. 33 SLP – Customers Highlights • FY14 Renewal rates (in line with historical performance): • Accounts = 91% • Fees = 96% • Over 600 license “units” in FY14: • 18% increase vs. FY13 • Over 200 organizations licensing the technology, including 19 of the top 20 pharmaceutical companies

Copyright (c) 2015 Simulations Plus, Inc. 34 • Of 2,500 companies registered with FDA, both foreign and domestic, we have ~10% penetration with Simulations Plus products • http://www.fda.gov/Drugs/NewsEvents/ucm339912.htm • Continued enhancement of current software products to expand application space • Dermal and improved oral cavity dosing modules in GastroPlus™ • Addition of biologics (antibodies) to GastroPlus • Addition of large animals to GastroPlus • MembranePlus™ • New software product to simulate in vitro permeability experiments released in 1Q15; Webinar held December 2, 2014 – nearly 100 attendees Simulations Plus, Inc. Additional Growth Opportunities

Copyright (c) 2015 Simulations Plus, Inc. 35 • Conferences/scientific meetings continue to be primary source of leads • In 4Q14, we presented at our first aerospace conference • In 1Q15 we presented at our first MRI conference • Trainings and workshops • Strategic digital marketing initiatives • Active updates of LinkedIn, Facebook, and Twitter accounts help with outreach programs • Fundamental industry shift continues • Added 75 new customers globally during FY14 (includes new companies, as well as new departments within existing large customers) • A dded 23 new customers in 1QFY15 Simulations Plus, Inc. Marketing and Sales Program

Copyright (c) 2015 Simulations Plus, Inc. 36 Summary • For FY2014: - Financial performance continues our 7 - year profitable trend - Cognigen acquisition one - time charges = investment in the future - Buyout of TSRL agreement reduces expenses • Continuing to Expand our team : - 3 new employees added to scientific team at Simulations Plus - Cognigen acquisition doubled the employee count of the consolidated entity • Expanding Sales Team and Activities - Greater staff time spent on marketing and sales activities - Expanded training workshops offered around the globe • Simulations Plus is globally recognized as a leader - Continued outstanding reputation for scientific expertise and innovation - Continued outstanding reputation for strong customer support • Strong cash position continues - Cash dividends of $0.19/share were distributed in FY 2014 - Cash used for TSRL agreement buyout represents investment to improve earnings - Cash used for Cognigen acquisition represents strategic investment for growth





































