QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No. 5)
| Filed by the Registrant | | ý |
| Filed by a Party other than the Registrant | | o |
| Check the appropriate box: |
| o | | Preliminary proxy statement |
| o | | Confidential, for the Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ý | | Definitive proxy statement |
| o | | Definitive additional materials |
| o | | Soliciting Material Pursuant to Rule 240.14a-12 |
WORLD HEART CORPORATION
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ý | | No fee required. |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | | 1) | | Title of each class of securities to which transaction applies: |
|
|
|
|
|
| | | 2) | | Aggregate number of securities to which transaction applies: |
|
|
|
|
|
| | | 3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined) : |
|
|
|
|
|
| | | 4) | | Proposed maximum aggregate value of transaction: |
|
|
|
|
|
| | | 5) | | Total fee paid: |
|
|
|
|
|
| o | | Fee paid previously with preliminary materials. |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | | 1) | | Amount previously paid: |
|
|
|
|
|
| | | 2) | | Form, Schedule or Registration Statement No.: |
|
|
|
|
|
| | | 3) | | Filing party: |
|
|
|
|
|
| | | 4) | | Date filed: |
|
|
|
|
|
WORLD HEART CORPORATION
1 Laser Street, Ottawa, Ontario, Canada
K2E 7V1
NOTICE OF ANNUAL AND SPECIAL MEETING OF SHAREHOLDERS
NOTICE IS HEREBY GIVEN that the Annual and Special Meeting of the Shareholders of World Heart Corporation (WorldHeart) will be held at the Fairmont Royal York Hotel, 100 Front Street West Toronto, Ontario, Canada M5J 1E3 in the Algonquin Room on Monday, July 18, 2005 at 10:00 a.m. for the following purposes:
- 1.
- to receive and to consider the audited consolidated financial statements of WorldHeart for the year ended December 31, 2004, that have been prepared in accordance with generally accepted accounting principles in Canada and the United States, together with the report of the auditors thereon;
- 2.
- to elect directors;
- 3.
- to appoint auditors and to authorize the directors to fix their remuneration;
- 4.
- to consider and, if thought advisable, pass a resolution (the MedQuest Resolution) to approve the issuance of WorldHeart common shares to MedQuest Products, Inc. (MedQuest) in connection with the acquisition by WorldHeart of the business of MedQuest, conditional upon the approval of items 5 and 6 set out in this Notice;
- 5.
- to consider and, if thought advisable, pass a resolution (the Maverick Resolution) to approve the private placement of WorldHeart common shares to Maverick Venture Management, LLC (Maverick), conditional upon the approval of items 4 and 6 set out in this Notice;
- 6.
- to consider and, if thought advisable, pass a resolution approving the reduction of the exercise price of the warrants issued by WorldHeart in September 2004, from $1.55 per common share to $1.00 per common share, for a period of 14 days following the approval of such resolution (the Warrant Amendment Resolution);
- 7.
- to consider and, if thought advisable, to pass a resolution (ESOP Resolution #1) approving an amendment to the World Heart Corporation Employee Stock Option Plan (the Plan) increasing the maximum number of common shares that may be issued under the Plan;
- 8.
- to consider and, if thought advisable, to pass a resolution (ESOP Resolution #2) approving an amendment to the Plan removing the restriction that the aggregate number of shares issued to any person under the Plan be limited to 5% of the issued and outstanding common shares.
- 9.
- to consider and, if thought advisable, to pass a resolution (the Option Amendment Resolution) amending certain options granted to directors and executive officers of WorldHeart, conditional on approval of item 7 set out in this Notice;
- 10.
- to consider and, if thought advisable, to pass a special resolution authorizing the continuance of WorldHeart under theCanada Business Corporations Act (the Continuance Resolution); and
- 11.
- to transact such other business as may properly come before the meeting or any adjournment thereof.
This Notice is accompanied by a Form of Proxy and a Management Information Circular. The holders of common shares of record on June 10, 2005 will be entitled to receive notice of the Annual and Special Meeting of Shareholders.
DATED at Ottawa, Canada, this 13th day of June, 2005.
BY ORDER OF THE BOARD OF DIRECTORS

C. Ian Ross,Chairman
If you are unable to attend the meeting in person, you are invited to complete, sign and return the accompanying Form of Proxy in the envelope provided and the Form of Proxy must be deposited not later than 5:00 p.m. (Ottawa time) on Friday, July 15, 2005 with CIBC Mellon Trust Company, Attention: Proxy Department, 200 Queen's Quay East, Unit #6, Toronto, Ontario, M5A 4K9.
WORLD HEART CORPORATION
MANAGEMENT INFORMATION CIRCULAR
June 13, 2005
SUMMARY
�� The following is a summary of the information provided in this circular and should be read together with the more detailed information and financial data and statements contained elsewhere in this circular.
| MedQuest Products, Inc. | | MedQuest is a development stage medical devices company focused on the development of a non-pulsatile continuous blood flow, or rotary, ventricular assist device. Currently, most heart assist devices intended for chronic use are pulsatile, mimicking the natural heart's pumping action. Continuous blood flow ventricular assist devices (VADs) are expected to be smaller, less complex and valveless, have lower energy needs and be potentially less costly than other VADs. MedQuest has conducted animal trials with respect to its rotary VAD, which incorporates advanced magnetic levitation technology. Human trials of this VAD are expected to commence in 2005. See "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest — MedQuest Business" starting on page 69. |
| Agreement with MedQuest | | We have entered into an agreement, as amended by amendment no. 1, (the MedQuest Agreement), pursuant to which we will: |
| | | • | acquire all of the assets and business of MedQuest (the MedQuest Acquisition); |
| | | • | assume substantially all of MedQuest's contracts and licenses; |
| | | • | assume responsibility for certain liabilities of MedQuest, including amounts outstanding to its majority shareholder, Maverick Venture Management, LLC (Maverick), for loans to fund continuing operations between the date of signing the MedQuest Agreement and completion of the MedQuest Acquisition; |
| | | • | issue 9,300,000 common shares to MedQuest, valued at $1.35 per common share; and |
| | | • | pay $10,000 in cash. |
| | | See "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest — MedQuest Agreement" starting on page 72. |
| Maverick | | Maverick holds approximately 82% of the outstanding common stock of MedQuest and 100% of the outstanding preferred stock of MedQuest. Maverick is a limited liability company controlled by Kevin Compton. |
| Agreement with Maverick | | Concurrently with the MedQuest Agreement, we have entered into an agreement, as amended by amendment no. 1, (the Maverick Agreement) with MedQuest's majority shareholder, Maverick, pursuant to which, we will: |
| | | • | issue 8,888,889 common shares to Maverick at a price of $1.35 per common share for total gross proceeds of $12,000,000; and |
| | | • | pay $3,500,000 owed to Maverick by MedQuest for loans to fund the continuing operations of MedQuest between the date of signing the MedQuest Agreement and closing of the MedQuest Acquisition. |
1
|
|
|
|
|
|
See "MATTERS TO BE ACTED UPON — Maverick Resolution — Private Placement to Maverick — Maverick Agreement" starting on page 78. |
Reasons for seeking approval from shareholders |
|
Under the terms of the MedQuest Agreement and the Maverick Agreement, we will, subject to the approval of our shareholders and the satisfaction of certain conditions, issue an aggregate of 18,188,889 common shares. The rules of the Nasdaq National Market (NASDAQ) and the Toronto Stock Exchange (TSX) require us to seek shareholder approval whenever we propose to issue shares in such an amount that the recipient of the shares will have enough voting power to materially affect the control of WorldHeart. Because of Maverick's substantial equity interest in MedQuest, the stock exchange rules state that shares issued to MedQuest and to Maverick must be aggregated in determining whether control of WorldHeart is materially affected by the proposed transactions. The acquisition of MedQuest and concurrent private placement to Maverick will result in MedQuest and Maverick holding in aggregate approximately one third of the outstanding common shares of WorldHeart assuming the proposed conversion of the debentures and exercise of the warrants. See "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest — Regulatory Approvals" starting on page 66 and " — Maverick Resolution — Private Placement to Maverick — Regulatory Approvals" starting on page 78. |
Recommendation of the Board |
|
The board of directors considered various positive and negative factors in assessing the MedQuest Acquisition and concurrent private placement. These factors are described in detail in the section "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest" and " — Maverick Resolution — Private Placement to Maverick". After careful consideration, the board of directors concluded that the MedQuest Acquisition and private placement to Maverick are in the best interests of WorldHeart. |
|
|
The board of directors recommends that shareholdersvote for the resolution approving the issuance of common shares in connection with the MedQuest Acquisition and the Maverick private placement. |
Effects on Shareholders |
|
As a result of the number of common shares being issued pursuant to the MedQuest Agreement and the Maverick Agreement there will be significant dilution of existing shareholders' equity interests in WorldHeart. |
|
|
Assuming the issuance of 9,300,000 common shares to MedQuest and without regard to the other issuances proposed in this circular, the voting power of each common share would be reduced by 35.2%. Assuming the issuance of 8,888,889 shares to Maverick and without regard to the other issuances proposed in this circular, the voting power of each common share would be reduced by 34.2%. The combined effect of the issuances to MedQuest and Maverick without regard to the other issuances proposed in this circular is that the voting power of each common share will be reduced by 51.6%. |
As a condition to the closing of the MedQuest Acquisition and Maverick private placement, the holders of debentures convertible into, and warrants to purchase, WorldHeart common shares issued in September 2004
2
must convert their debentures and exercise their warrants, if shareholders approve the amendment to the warrant exercise price of $1.00 per common share at this Annual and Special Meeting. Under their terms, the debentures are convertible and the warrants are exercisable at the option of the holder at any time up to September 15, 2009. As an inducement for the exercise of the warrants we have agreed to amend the warrants, and to make a tender offer to exchange amended warrants, subject to shareholder approval, to reduce the exercise price from $1.55 per common share to $1.00 per common share for a period of up to 14 days following approval by our shareholders. See "MATTERS TO BE ACTED UPON — Warrant Amendment Resolution — Amendment to September 2004 Warrants" starting on page 84.
Assuming the conversion of all debentures and exercise of all warrants, and without regard to the other issuances proposed in this circular, the voting power of each common share will be reduced by 53.85%. The combined effect of the conversion of all debentures and exercise of all warrants, and the issuances to MedQuest and Maverick, is that the voting power of each common share will be reduced by 69.06%.
The board of directors believes the amendment to the exercise price of the warrants is in the best interests of WorldHeart and recommends that shareholdersvote for the warrant amendment resolution.
WorldHeart is dependent on its ability to attract and retain qualified scientific, technical and key management personnel. In accordance with the revised WorldHeart strategic plan adopted by the board of directors in August 2004 and a revised approach to compensation of its employees, including its executive officers and key employees. The board of directors determined that at present, cash incentives should be reduced and the long term equity component of the compensation of WorldHeart's executive officers and key employees should be increased whereby on an aggregate basis executive officers and key employees should be entitled to hold up to 10% of the common shares of WorldHeart on a fully diluted basis. The board of directors determined that such a long term equity component would create greater incentive for the WorldHeart executive officers and key employees.
In order to achieve the objectives of the revised approach to compensation, WorldHeart needs to amend its employee stock option plan, as amended and restated on January 31, 2004 (the Plan), to increase the maximum number of common shares which may be issued under the Plan from 1,501,857 common shares to 9,772,505 common shares, and to remove from the Plan the restriction that the aggregate number of shares issued to any person under the Plan be limited to 5% of the issued and outstanding common shares, from time to time. See "MATTERS TO BE ACTED UPON — ESOP Resolution #1 and ESOP Resolution #2 — Amendments to the World Heart Corporation Employee Stock Option Plan" starting on page 85.
In the event that none of the issuances proposed in this circular are approved and the number of common shares available under the Plan is increased, the Plan will constitute 18% of the issued and outstanding common shares and 12% of the fully diluted common shares.
The board of directors believes the amendments to the Plan are in the best interests of WorldHeart and recommends that shareholdersvote for the Plan amendment resolutions.
In addition to the amendments to the Plan, our shareholders are also being asked to approve certain amendments to options held by executive officers and directors of WorldHeart. On September 23, 2004, the executive officers and directors of WorldHeart agreed to forfeit conditionally certain options held by them, none of which had an exercise price that was at or below the then-current market price of our common shares, and also received a new grant of options conditional on shareholder approval. For stock exchange purposes, the forfeiture and new grant of options are regarded as amendments to the existing options held by the executive officers and directors and are treated as having the effect of repricing the forfeited options, and because these individuals are insiders of WorldHeart, approval of our disinterested shareholders is required in respect of the amendments to these options. For a complete list of the forfeited options and grant of new options, see "MATTERS TO BE ACTED UPON — Option Amendment Resolution — Amendment to Certain Options Held by Executive Officers and Directors" starting at page 89.
3
The board of directors believes the amendments to certain options held by executive officers and directors are in the best interests of WorldHeart and recommends that shareholdersvote for the option amendment resolution.
We intend to file articles of continuance pursuant to Section 187 of theCanada Business Corporations Act (the CBCA) to continue WorldHeart under the provisions of the CBCA from theBusiness Corporations Act (Ontario) as if the company had been incorporated under the CBCA. Management and the board of directors believe that the continuance of WorldHeart under the CBCA is appropriate to permit increased non-Canadian participation on the board of directors given the international scope of WorldHeart's business. Continuance under the CBCA will permit WorldHeart to take advantage of recent amendments to the CBCA which modernize corporate law procedures and requirements, and will help facilitate the planned movement of its operations to the United States. See "MATTERS TO BE ACTED UPON — Continuance Resolution — Continuance under the Canada Business Corporations Act" starting on page 90.
The board of directors believes the continuance of WorldHeart under the CBCA is in the best interests of WorldHeart and recommends that shareholdersvote for the resolution authorizing the continuance.
Business of WorldHeart
WorldHeart is a global medical devices company focused on the development, production, sales and support of VADs. VADs in general are mechanical assist devices that can provide an effective treatment for end-stage heart failure by supplementing the circulatory function of the heart by re-routing blood flow through a mechanical pump. The short supply of donor organs makes it impossible for medical professionals to keep pace with the number of patients requiring heart transplants each year. Heart-assist devices such as WorldHeart's products provide end-stage heart failure patients with the opportunity to live more normal and active lives during bridge-to-transplant and destination therapy. There have been more than 10,000 VADs, produced by a number of suppliers, used clinically throughout the world over the past 10 years. See "BUSINESS OF WORLD HEART CORPORATION" starting on page 19.
Risk Factors
You should be aware of the following risks relating to WorldHeart's business and operations as well as to the combined operations of WorldHeart and MedQuest, if the MedQuest Acquisition is approved by our shareholders:
- •
- We have had substantial losses and limited revenues earned since incorporation.
- •
- We will require significant capital investment to bring future products and product enhancements to market.
- •
- We may be unable to obtain regulatory approvals.
- •
- We are dependent on a limited number of products.
- •
- Market acceptance of our technologies and products is uncertain.
- •
- We face significant competition and technological obsolescence of our products.
- •
- There are limitations on third-party reimbursement for the cost of implanting our devices.
- •
- We control intellectual property and if we cannot protect our intellectual property, our business could be adversely affected.
- •
- We are exposed to product liability claims because of the nature of our business.
- •
- We face risks associated with our manufacturing operations, risks resulting from dependence on third-party manufacturers, and risks related to dependence on sole suppliers.
- •
- We are dependent on key personnel.
- •
- The price of our shares is highly volatile.
4
- •
- We incur risks associated with acquisitions.
In addition to the above risk factors, you should also be aware of the following risks associated with the particular matters being put before you at this Annual and Special Meeting:
- •
- The availability of additional common shares on the completion of the transactions could depress the price of our common shares.
- •
- The acquisition of MedQuest may stimulate competition and we may not be able to compete successfully.
- •
- The integration of the WorldHeart and MedQuest businesses may be costly and we may fail to successfully effect the integration.
See "RISK FACTORS" starting on page 51.
Summary Financial Information
| | Three Months Ended March 31,
| |
| |
| |
| |
| |
| |
|---|
| | Year Ended December 31,
| |
|---|
U.S. GAAP
| |
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
| |
|---|
| Net revenue | | $ | 3,416,988 | | $ | 9,575,761 | | $ | 6,755,807 | | $ | 6,436,696 | | $ | 5,329,357 | | $ | 3,147,950 | |
| Net loss for the period | | | (3,942,396 | ) | | (26,141,798 | ) | | (14,629,054 | ) | | (24,552,673 | ) | | (35,012,010 | ) | | (26,210,754 | ) |
| Net loss applicable to common shareholders | | | (3,942,396 | ) | | (26,141,798 | ) | | (18,168,304 | ) | | (29,010,753 | ) | | (39,578,696 | ) | | (28,284,926 | ) |
| Basic and diluted loss per common share | | | (0.24 | ) | | (1.70 | ) | | (2.90 | ) | | (11.42 | ) | | (18.39 | ) | | (13.31 | ) |
| | As at March 31,
| | As at December 31,
|
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Total assets | | $ | 38,443,395 | | $ | 42,258,430 | | $ | 50,180,069 | | $ | 27,739,896 | | $ | 46,060,330 | | $ | 72,138,615 |
| Long term liabilities | | | 7,482,670 | | | 8,193,508 | | | — | | | 2,167,860 | | | 2,187,246 | | | 3,529,947 |
| | Three Months
Ended
March 31,
| |
| |
| |
| |
| |
| |
|---|
| | Year Ended December 31,
| |
|---|
Canadian GAAP
| |
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
| |
|---|
| Net revenue | | $ | 3,416,988 | | $ | 9,575,761 | | $ | 6,755,807 | | $ | 6,436,696 | | $ | 5,329,357 | | $ | 3,147,950 | |
| Net loss for the period | | | (4,547,982 | ) | | (22,806,805 | ) | | (23,547,313 | ) | | (31,690,859 | ) | | (42,405,977 | ) | | (20,462,528 | ) |
| Net loss applicable to common shareholders | | | (4,547,982 | ) | | (22,806,805 | ) | | (25,471,708 | ) | | (31,690,859 | ) | | (42,405,977 | ) | | (20,462,528 | ) |
| Basic and diluted loss per common share | | | (0.27 | ) | | (1.48 | ) | | (4.07 | ) | | (12.48 | ) | | (19.70 | ) | | (9.63 | ) |
| | As at
March 31,
| | As at December 31,
|
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Total assets | | $ | 38,443,395 | | $ | 42,258,430 | | $ | 50,180,069 | | $ | 28,553,444 | | $ | 48,896,985 | | $ | 77,309,002 |
| Long term liabilities | | | 2,806,699 | | | 2,547,027 | | | — | | | 46,114,470 | | | 41,278,601 | | | 40,191,228 |
Complete audited consolidated financial statements for WorldHeart under U.S. GAAP and Canadian GAAP can be found in Appendices B-1 and B-2, respectively and interim unaudited consolidated financial statements for three months ended March 31, 2005 for WorldHeart under U.S. GAAP can be found in Appendix B-3.
5
Selected Historical Financial Data of MedQuest Products, Inc.
| | Nine Months Ended March 31,
| |
| |
| |
| |
| |
| |
|---|
| | Year Ended June 30,
| |
|---|
U.S. GAAP
| |
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
| |
|---|
| Net revenue | | $ | 101,840 | | $ | 99,389 | | $ | — | | $ | 304,149 | | $ | — | | $ | — | |
| Net loss for the period | | | (3,077,030 | ) | | (2,232,937 | ) | | (3,777,799 | ) | | (4,650,433 | ) | | (1,875,889 | ) | | (40,104 | ) |
| | As at
March 31,
| | As at June 30,
|
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | | $ | 1,717,199 | | $ | 4,131,038 | | $ | 409,074 |
| Long term liabilities | | | 110,114 | | | 111,193 | | | 157,948 | | | 221,240 | | | 46,799 | | | 3,900 |
| | Nine Months Ended
March 31,
| |
| |
| |
|---|
| | Year Ended June 30,
| |
|---|
Canadian GAAP
| |
|---|
| | 2005
| | 2004
| | 2003
| |
|---|
| | (Unaudited)
| |
| |
| |
|---|
| Net revenue | | $ | 101,840 | | $ | 99,389 | | $ | — | |
| Net loss for the period | | | (3,207,773 | ) | | (2,573,702 | ) | | (4,238,997 | ) |
| | As at
March 31,
| | As at June 30,
|
|---|
| | 2005
| | 2004
| | 2003
|
|---|
| | (Unaudited)
| |
| |
|
|---|
| Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 |
| Long term liabilities | | | 110,114 | | | 111,193 | | | 157,948 |
Complete audited financial statements for MedQuest can be found in Appendix D.
| | U.S. GAAP
Three Months Ended
March 31, 2005
| | U.S. GAAP
Year Ended
December 31, 2004
| | Canadian GAAP
Year Ended
December 31, 2004
| |
|---|
| Net revenue | | $ | 3,416,988 | | $ | 9,575,761 | | $ | 9,575,761 | |
| Net loss for the year | | | (4,797,650 | ) | | (28,626,183 | ) | | (26,514,976 | ) |
| Loss applicable to common shareholders | | | (4,797,650 | ) | | (28,626,183 | ) | | (26,514,976 | ) |
| Basic and diluted loss per common share | | | (0.09 | ) | | (0.52 | ) | | (0.48 | ) |
| | U.S. GAAP
As at
March 31, 2005
| | Canadian GAAP
As at
December 31, 2004
|
|---|
| Total assets | | $ | 61,506,468 | | $ | 77,012,518 |
| Long term obligations | | | 118,944 | | | 126,482 |
| Book value per common share | | | 0.92 | | | 1.24 |
Complete pro forma combined condensed financial statements can be found in Appendix F.
6
TABLE OF CONTENTS
| | Page
|
|---|
| SUMMARY | | 1 |
| | MedQuest Acquisition and Maverick Private Placement | | 1 |
| | Amendment to September 2004 Warrants | | 2 |
| | Amendments to the World Heart Corporation Employee Stock Option Plan | | 3 |
| | Amendments to Certain Options Held by Executive Officers and Directors | | 3 |
| | Continuance under the CBCA | | 4 |
| Business of WorldHeart | | 4 |
| Risk Factors | | 4 |
| Summary Financial Information | | 5 |
| | Selected Historical Financial Data of World Heart Corporation | | 5 |
| | Selected Historical Financial Data of MedQuest Products, Inc. | | 6 |
| | Selected Unaudited Pro Forma Combined Condensed Financial Information | | 6 |
| SOLICITATION OF PROXIES BY MANAGEMENT | | 9 |
| APPOINTMENT OF PROXY HOLDERS AND REVOCATION OF PROXIES | | 9 |
| VOTING OF PROXIES | | 9 |
| INFORMATION FOR BENEFICIAL SHAREHOLDERS | | 10 |
| VOTING SHARES, RECORD DATE AND PRINCIPAL HOLDERS | | 11 |
| SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS | | 14 |
| CORPORATE STRUCTURE | | 15 |
| Name, Address, and Incorporation | | 15 |
| Intercorporate Relationships | | 15 |
| DEVELOPMENT OF THE BUSINESS | | 15 |
| Three Year History and Development of WorldHeart | | 15 |
| Significant Acquisitions | | 18 |
| BUSINESS OF WORLD HEART CORPORATION | | 19 |
| Business Overview and Our Products | | 19 |
| | The Novacor LVAS | | 19 |
| | Our Next-Generation VAD Platform | | 19 |
| Third-party Reimbursement for VADs | | 20 |
| Application of VADs in Patient Care | | 20 |
| | Current Treatment Methods for End-Stage Heart Failure | | 20 |
| | Advantages of VADs | | 21 |
| Marketing, Training and Distribution Strategy | | 22 |
| Sources and Availability of Raw Materials | | 22 |
| Intellectual Property | | 23 |
| Product Liability Insurance | | 23 |
| Competition | | 23 |
| | Overview | | 23 |
| | Future Competition | | 24 |
| Regulatory Matters | | 25 |
| | Overview | | 25 |
| | United States Regulation | | 25 |
| | Canadian Regulation | | 25 |
| | Regulatory Requirements in Other Countries | | 25 |
| | Other Regulatory Requirements | | 26 |
| Our Employees | | 26 |
| Our Facilities | | 26 |
| Annual and Quarterly Information | | 26 |
| | Selected Historical Financial Data of World Heart Corporation | | 26 |
| | Selected Historical Financial Data of MedQuest Products, Inc. | | 28 |
| MANAGEMENT'S DISCUSSION & ANALYSIS | | 30 |
| SELECTED PRO FORMA INFORMATION | | 30 |
| Selected Unaudited Pro Forma Consolidated Financial Information | | 30 |
| SHARE CAPITAL AND PRIOR SALES | | 31 |
| | Common Shares | | 31 |
| | Preferred Shares | | 32 |
| | Dividends | | 33 |
| | Options to Purchase Shares | | 33 |
| CORPORATE GOVERNANCE | | 34 |
| Information about the Board of Directors and its Committees | | 34 |
| | Audit Committee | | 34 |
| | Compensation Committee | | 34 |
| | Corporate Governance and Nominating Committee | | 35 |
| | Strategic Planning Committee | | 35 |
| Toronto Stock Exchange Corporate Governance Practices | | 35 |
| Report of the Audit Committee | | 39 |
| Communication with Shareholders | | 40 |
| Shareholder Proposals | | 40 |
| MEETINGS OF DIRECTORS | | 40 |
| Board of Directors and Committee Meetings Held | | 41 |
| COMPENSATION OF DIRECTORS | | 41 |
| DIRECTORS' AND OFFICERS' INDEMNIFICATION | | 42 |
| EXECUTIVE OFFICERS | | 42 |
| EXECUTIVE COMPENSATION | | 44 |
| OPTION GRANTS IN LAST FISCAL YEAR | | 45 |
| LONG TERM INCENTIVE PLAN AWARDS IN LAST FISCAL YEAR | | 46 |
| AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR AND OPTIONS/SAR VALUES | | 46 |
| EMPLOYMENT AGREEMENTS | | 46 |
| EQUITY COMPENSATION PLAN INFORMATION | | 47 |
| LOANS TO DIRECTORS AND OFFICERS | | 48 |
| REPORT ON EXECUTIVE COMPENSATION AND COMPOSITION OF THE COMPENSATION COMMITTEE | | 48 |
| PERFORMANCE GRAPH | | 50 |
| | Stock Exchange Price | | 50 |
| RISK FACTORS | | 51 |
| Risk Factors Relating to our Business | | 51 |
| Risk Factors Related to the MedQuest Acquisition, the Maverick Private Placement and the Warrant Exercise & Debenture Conversion | | 57 |
| MATERIAL CONTRACTS | | 58 |
| MATTERS TO BE ACTED UPON | | 61 |
| Audited Consolidated Financial Statements | | 61 |
| Election of Directors | | 61 |
| | Recommendation of WorldHeart's Board of Directors | | 65 |
| Appointment of Auditors | | 65 |
| | Audit Service Fees | | 65 |
| | | |
7
| | Audit-Related Service Fees | | 66 |
| | Tax Service Fees | | 66 |
| | Other Service Fees | | 66 |
| | Recommendation of WorldHeart's Board of Directors | | 66 |
| MedQuest Resolution — Acquisition of MedQuest | | 66 |
| | Regulatory Approvals | | 66 |
| | Background and Reasons for the MedQuest Acquisition | | 67 |
| | MedQuest Business | | 71 |
| | MedQuest Agreement | | 74 |
| | Related Agreements | | 79 |
| | General Effect on Existing Shareholders | | 79 |
| | Recommendations of WorldHeart's Board of Directors | | 80 |
| Maverick Resolution — Private Placement to Maverick | | 80 |
| | Regulatory Approvals | | 80 |
| | Background and Reasons for the Maverick Private Placement | | 81 |
| | Maverick Agreement | | 82 |
| | Related Agreements | | 85 |
| | General Effect on Existing Shareholders | | 85 |
| | Recommendations of WorldHeart's Board of Directors | | 85 |
| Warrant Amendment Resolution — Amendment to September 2004 Warrants | | 85 |
| | Recommendation of WorldHeart's Board of Directors | | 87 |
| ESOP Resolution #1 and ESOP Resolution #2 — Amendments to the World Heart Corporation Employee Stock Option Plan | | 87 |
| | Recommendations of WorldHeart's Board of Directors | | 91 |
| Option Amendment Resolution — Amendment to Certain Options Held by Executive Officers and Directors | | 91 |
| | Recommendations of WorldHeart's Board of Directors | | 92 |
| Continuance Resolution — Continuance under the Canada Business Corporations Act | | 92 |
| | Required Shareholder Approval and Conditions | | 92 |
| | Reasons for the Proposed Continuance | | 93 |
| | CBCA Versus OBCA | | 94 |
| | Articles of Continuance | | 95 |
| | Dissent Rights | | 95 |
| | Recommendation of WorldHeart's Board of Directors | | 95 |
| MATERIAL TAX CONSIDERATIONS | | 95 |
| OTHER MATTERS | | 95 |
| TRANSFER AGENT | | 96 |
| EXPERTS | | 96 |
| ADDITIONAL INFORMATION | | 96 |
| APPROVAL OF THE BOARD OF DIRECTORS | | 97 |
| CONSENT OF INDEPENDENT ACCOUNTANTS | | 98 |
| AUDITORS' CONSENT | | 99 |
| CONSENT OF INDEPENDENT ACCOUNTANTS | | 100 |
| APPENDIX A-1 MEDQUEST RESOLUTION | | A-1-1 |
| APPENDIX A-2 MAVERICK RESOLUTION | | A-2-1 |
| APPENDIX A-3 WARRANT AMENDMENT RESOLUTION | | A-3-1 |
| APPENDIX A-4 ESOP RESOLUTION #1 | | A-4-1 |
| APPENDIX A-5 ESOP RESOLUTION #2 | | A-5-1 |
| APPENDIX A-6 OPTION AMENDMENT RESOLUTION | | A-6-1 |
| APPENDIX A-7 CONTINUANCE RESOLUTION | | A-7-1 |
| APPENDIX B-1 WORLD HEART CORPORATION US GAAP FINANCIAL ANNUAL STATEMENTS | | B-1-1 |
| APPENDIX B-2 WORLD HEART CORPORATION CANADIAN GAAP ANNUAL FINANCIAL STATEMENTS | | B-2-1 |
| APPENDIX B-3 WORLD HEART CORPORATION US GAAP QUARTERLY FINANCIALS FOR THE PERIOD ENDED MARCH 31, 2005 | | B-3-1 |
| APPENDIX C-1 WORLD HEART CORPORATION US GAAP MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | C-1-1 |
| APPENDIX C-2 WORLD HEART CORPORATION CANADIAN GAAP MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | C-2-1 |
| APPENDIX C-3 WORLD HEART CORPORATION MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS FOR THE PERIOD ENDED MARCH 31, 2005 | | C-3-1 |
| APPENDIX D MEDQUEST PRODUCTS, INC. FINANCIAL STATEMENTS | | D-1 |
| APPENDIX E MEDQUEST PRODUCTS, INC. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | E-1 |
| APPENDIX F PRO FORMA FINANCIAL STATEMENTS | | F-1 |
| APPENDIX G MEDQUEST AGREEMENT | | G-1 |
| APPENDIX H MAVERICK AGREEMENT | | H-1 |
| APPENDIX I FORM OF CONVERSION AND EXERCISE AGREEMENT | | I-1 |
| APPENDIX J RIGHTS OF DISSENTING SHAREHOLDERS | | J-1 |
| APPENDIX K — LIST OF 2004 WARRANTHOLDERS | | K-1 |
8
SOLICITATION OF PROXIES BY MANAGEMENT
This circular is furnished in connection with thesolicitation by the management of World Heart Corporation (WorldHeart) of proxies to be used at the Annual and Special Meeting of Shareholders of WorldHeart to be held on Monday, July 18, 2005 (the Annual and Special Meeting) at the Fairmont Royal York Hotel, 100 Front Street West, Toronto, Ontario, Canada M5J 1E3 in the Algonquin Room at 10:00 a.m. and at any adjournment thereof for the purposes set forth in the accompanying Notice of Annual and Special Meeting of Shareholders. While management intends to solicit most proxies by mail, some proxies may be solicited by telephone or other personal contact by directors or officers of WorldHeart. The cost of such solicitation will be borne by WorldHeart. The information provided herein is given as of June 10, 2005, unless otherwise specified.
We express all dollar amounts in this circular in United States dollars, except where we indicate otherwise. References to "$" are to United States dollars and references to "Cdn$" are to Canadian dollars. On June 10, 2005, the noon exchange rate of Canadian currency in exchange for United States currency, as reported by the Bank of Canada, was Cdn$1.00 = $0.8004.
This circular is being sent to both registered and non-registered owners of common shares of WorldHeart. If you are a non-registered owner, and WorldHeart or its agent has sent these materials directly to you, your name and address and information about your holdings of common shares, have been obtained in accordance with applicable securities regulatory requirements from the intermediary holding common shares on your behalf.
APPOINTMENT OF PROXY HOLDERS AND REVOCATION OF PROXIES
The persons named in the accompanying form of proxy are directors and/or officers of WorldHeart.A registered shareholder has the right to appoint a person, who need not be a shareholder of WorldHeart, other than the persons designated in the accompanying form of proxy, to attend and act on behalf of the shareholder at the meeting. To exercise this right, a shareholder may either insert such other person's name in the blank space provided in the accompanying form of proxy or complete another appropriate form of proxy.
To be valid, a proxy must be dated and signed by the shareholder or the shareholder's attorney authorized in writing or, if the shareholder is a corporation, by a duly authorized officer or attorney. The proxy, to be acted upon, must be deposited with WorldHeart, c/o its registrar and transfer agent, CIBC Mellon Trust Company, Attention: Proxy Department, 200 Queen's Quay East, Unit #6, Toronto, Ontario, M5A 4K9 by the close of business on the last business day prior to the date on which the meeting or any adjournment thereof is held, or with the Chair of the meeting on the day of the Annual and Special Meeting or any adjournment thereof.
A shareholder who has given a proxy may revoke it (a) by depositing an instrument in writing (including another proxy) executed by the shareholder or by the shareholder's attorney authorized in writing, either (i) with the Secretary at the registered office of WorldHeart, 1 Laser Street, Ottawa, Ontario, Canada K2E 7V1, at any time up to and including the last business day prior to the day of the Annual and Special Meeting or any adjournment thereof at which the proxy is to be used, or (ii) with the Chair of the meeting on the day of the meeting at any time before it is exercised on any particular matter, or (b) by attending the Annual and Special Meeting in person and personally voting the shares represented by the proxy prior to the exercise thereof, or (c) in any other manner permitted by law.
VOTING OF PROXIES
The directors and/or officers whose names are printed on the accompanying form of proxy will, on a show of hands or any ballot that may be called for, vote or withhold from voting the shares in respect of which they are appointed in accordance with the direction of the shareholder appointing them. If no choice is specified by the shareholder, the shares will be voted FOR the election of the management nominees for directors, the appointment of auditors, the MedQuest Resolution, the Maverick Resolution, the Warrant Amendment Resolution, ESOP Resolution #1 and ESOP Resolution #2, the Option Amendment Resolution and the Continuance Resolution, each as defined in, and on the terms disclosed in, this circular. Proxies indicating a vote FOR or AGAINST a particular proposal will not be voted on any motion to adjourn or postpone the consideration of that proposal.
The enclosed form of proxy confers discretionary authority upon the persons named therein with respect to any other matter which may properly come before the meeting. As of the date of this circular, management is not aware of any other matter proposed or likely to come before the meeting. However, if any other matter properly comes before the meeting, it is the intention of the persons named in the enclosed form of proxy to vote on such other business in accordance with their judgement.
9
Voting at the Annual and Special Meeting will be by show of hands, except where a ballot is demanded by a shareholder or proxyholder entitled to vote at the Annual and Special Meeting or the chair of the meeting believes that the number of votes against a resolution is greater than 5% of all votes to be cast at the meeting in respect of the resolution. A shareholder or proxyholder may demand a ballot either before or after any vote by show of hands. In determining whether the requisite majority has been achieved to approve any proposal put before shareholders at the Annual and Special Meeting, only votes that have been cast FOR or AGAINST the proposal will be counted. Thus, if approval of a proposal requires a majority of the votes cast at the meeting, the proposal will be approved if the number of votes FOR is greater than the number of votes AGAINST. If approval is required from a certain percentage of the votes cast at the meeting, the proposal will be approved if the votes FOR as a percentage of the total sum of the votes FOR and AGAINST meets or exceeds the required percentage.
If an executed proxy is returned and the shareholder has specifically abstained from voting on any matter, the shares represented by such proxy will be considered present at the Annual and Special Meeting for purposes of determining a quorum but will not be considered to have been cast for purposes of calculating the vote on such matter and thus will have no effect on the outcome of the proposal.
If an executed proxy is returned by a broker, bank or other agent holding shares in street name that indicates that the broker does not have discretionary authority as to certain shares to vote on a proposal (broker non-votes), such shares will be considered present at the Annual and Special Meeting for purposes of determining a quorum but will not be considered to be entitled to vote on and thus will have no effect on the outcome of such proposal.
INFORMATION FOR BENEFICIAL SHAREHOLDERS
The information set forth in this section may be of importance to many shareholders, as a substantial number of shareholders do not hold the common shares of WorldHeart in their own name (beneficial shareholders). In certain cases, a shareholder's shares may be registered in the name of a third party, such as a broker, securities dealer, trust company, bank or other similar intermediary. Only proxies deposited by shareholders who appear on the records maintained by WorldHeart's registrar and transfer agent as registered holders of common shares of WorldHeart will be recognized and acted upon at the Annual and Special Meeting. If the common shares are listed in an account statement provided to a beneficial shareholder by a broker, the common shares are likely not to be registered in the shareholder's name. The common shares are likely to be registered under the name of the shareholder's broker or an agent of the broker. A significant number of shares are registered under the name of CEDE & Co. (the registration name for the Depository Trust Company which acts as nominee for many United States brokerage firms) or CDS & Co. (the registration name for The Canadian Depositary for Securities, which acts as nominee for many Canadian brokerage firms). Common shares held by brokers (or their agents or nominees) on behalf of the broker's client can only be voted (for or against resolutions) at the direction of the beneficial holder except for "routine matters" such as the election of directors and appointment of auditors.
Existing regulatory policy requires brokers and intermediaries to seek voting instructions from beneficial shareholders in advance of shareholders meetings. The various brokers and other intermediaries have their own mailing procedures and provide their own return instructions to clients, which should be carefully followed by beneficial shareholders in order to ensure that their common shares are voted at the Annual and Special Meeting. The voting instruction form supplied to a beneficial shareholder by its broker (or the agent of the broker) is substantially similar to the form of proxy provided directly to the registered shareholders of WorldHeart. However, its purpose is limited to instructing the registered shareholder (i.e. the broker or agent of the broker) how to vote on behalf of the beneficial shareholder. A significant number of brokers now delegate the responsibility for obtaining instructions from clients to ADP Investor Communications (ADP) in Canada. ADP typically prepares a machine-readable voting instruction form, mails those forms to beneficial shareholders and asks beneficial shareholders to return the forms to ADP, or otherwise communicate voting instructions to ADP (by way of the Internet or telephone, for example). The voting instruction form will name the same persons as the proxy to represent the beneficial shareholder at the Annual and Special Meeting. A beneficial shareholder has the right to appoint a person, including the beneficial shareholder, other than the persons designated in the voting instruction form, to represent the beneficial shareholder at the Annual and Special Meeting. To exercise this right, the beneficial shareholder should insert the name of the desired representative in the blank space provided in the voting instruction form. ADP then tabulates the results of all instructions received and provides appropriate instruction respecting the voting of the common shares to be represented at the meeting.A beneficial
10
shareholder who receives an ADP voting instruction form cannot use the form to vote the common shares directly at the Annual and Special Meeting. The voting instruction forms must be returned to ADP (or instructions respecting the voting of the common shares must otherwise be communicated to ADP) in advance of the Annual and Special Meeting in order to have the common shares voted. If you have any questions respecting the voting of the common shares held through a broker or intermediary, please contact the broker or intermediary for assistance.
VOTING SHARES, RECORD DATE AND PRINCIPAL HOLDERS
As of June 10, 2005, there were issued and outstanding 17,101,400 common shares of WorldHeart. The board of directors has fixed the close of business on June 10, 2005 as the record date for the purposes of determining the shareholders entitled to receive notice of the Annual and Special Meeting (the Record Date). Each registered common shareholder is entitled to one vote for each common share held that is shown as registered in such holder's name on the list of shareholders prepared as of the close of business on the Record Date.
In the event of any transfer of shares by any shareholder after the Record Date, the transferee is entitled to vote those shares if the transferee produces properly endorsed share certificates or otherwise establishes that the transferee owns the shares, and requests the Secretary of WorldHeart to include the transferee's name on the shareholders' list not later than ten days before the meeting.
To the knowledge of the directors and officers of WorldHeart, as of June 10, 2005, the persons who beneficially own or exercise control or direction over common shares carrying more than 5% of the voting rights attached to all the common shares of WorldHeart entitled to be voted at the meeting and the shareholdings of the executive officers and directors of WorldHeart, are as follows:
Name and Address of Shareholder
| | Title of
Class
| | Amount and Nature of
Beneficial Ownership
| | Percentage of Class
|
|---|
Austin W. Marxe and David M. Greenhouse
153 East 53rd Street
New York, NY 10022 | | Common Shares | | 12,064,250 | (1) | 45.16% |
Edwards Lifesciences Corporation
One Edwards Way
Irvine, California 92614 | | Common Shares | | 2,437,634 | (2) | 7.47% |
Zesiger Capital Group LLC
320 Park Avenue, 30th Floor
New York, NY 10022 | | Common Shares | | 3,478,666 | (3) | 18.02% |
Jal S. Jassawalla
34 East Altarinda Drive
Orinda, CA 94563 | | Common Shares | | 10,000 | (4) | 0.06% |
D. Mark Goudie
4 Woodrow Avenue
Stittsville, ON K2S 1G9 | | Common Shares | | 45,035 | (5) | 0.26% |
Piet Jansen
A.J. Ernststraat 893
1081 HL Amsterdam
Netherlands | | Common Shares | | 0 | (6) | 0% |
John Marinchak
1720 Magnolia Way
Walnut Creek, CA 94595 | | Common Shares | | 0 | (7) | 0% |
Phillip Miller
968 Regal Road
Berkeley, CA 94708 | | Common Shares | | 5,000 | (8) | 0.03% |
Richard Juelis
349 Oyster Point Blvd. Ste 200
S San Francisco, CA 94080 | | Common Shares | | 0 | (9) | 0% |
| | | | | | | |
11
C. Ian Ross
140 Pioneer Lane, RR # 3
Collingwood, ON L9Y 3Z2 | | Common Shares | | 865 | (10) | less than 0.01% |
John F. Carlson
2 Raccoon Road
St. Paul, MN 55127-2118 | | Common Shares | | 0 | (11) | 0% |
William C. (Bill) Garriock
1 St. Ives Crescent
Toronto, Ontario M4N 3B3 | | Common Shares | | 0 | (12) | 0% |
Robert J. Majteles
31 Sea View Avenue
Piedmont, CA 94611 | | Common Shares | | 0 | (13) | 0% |
Michael A. Roth and Brian J. Stark
3600 South Lake Drive
St. Francis, WI 53235 | | Common Shares | | 4,314,875 | (14) | 20.15% |
Reid S. Walker, G. Stacy Smith
and Patrick P. Walker
c/o Walker Smith
300 Crescent Court, Suite 1111
Dallas, TX 75201 | |
Common Shares | |
3,206,525 |
(15) |
16.36% |
Bristol Investment Fund Ltd.
c/o Bristol Capital Advisors, LLC
10990 Wilshire Boulevard,
Suite 1410
Los Angeles, CA 90024 | | Common Shares | | 1,606,743 | (16) | 8.9% |
Sherfam Inc.
150 Signet Drive
Weston, Ontario M9L 1T9 | | Common Shares | | 1,409,113 | (17) | 7.61% |
Federated Investors, Inc.
Federated Investors Tower,
Pittsburgh, PA 15222-3779 | | Common Shares | | 1,410,138 | (18) | 7.62% |
MedCap Partners
500 Third Street, Suite 535
San Francisco, CA 94107 | | Common Shares | | 921,658 | (19) | 5.11% |
Sceptre Investment Counsel Limited
26 Wellington St. E., 12th Floor
Toronto, Ontario M5E 1W4 | | Common Shares | | 1,232,856 | (20) | 6.72% |
FCP OP MEDICAL BioHe@lth Trends
4, rue Jean Monnet
Luxembourg
L-2180 | | Common Shares | | 960,000 | (21) | 5.32% |
John Vajda
330 N. Mathilda Ave. Ste. 407
Sunnyvale, CA 94085 | | Common Shares | | 0 | (22) | 0% |
| Executive Officers and Directors of WorldHeart as a group (11) | | Common Shares | | 60,900 | | 0.24% |
- (1)
- Special Situations Fund III, L.P. owns 1,421,381 common shares, Special Situations Cayman Fund, L.P. owns 331,420 common shares and Special Situations Private Equity Fund, L.P. owns 698,546 common shares (collectively, the Special Situation Funds). These funds also hold debentures convertible into 4,000,000 common shares and warrants to purchase 5,612,902 common shares, of which warrants
12
to acquire 1,612,902 common shares have an exercise price of Cdn$7.42 expiring September 23, 2008 and warrants to acquire 4,000,000 common shares have an exercise price of $1.55 expiring September 15, 2009. MGP Advisors Limited (MGP) is the general partner of Special Situations Fund III, L.P. AWM Investment Company, Inc. (AWM) is the general partner of MGP and the general partner of and investment advisor to Special Situations Cayman Fund, L.P. MG Advisors, L.L.C. (MG) is the general partner of and investment advisor to Special Situations Private Equity Fund, L.P. Austin W. Marxe and David M. Greenhouse are the principal owners of MGP, AWM and MG and are principally responsible for the selection, acquisition and disposition of the portfolio securities by each investment adviser on behalf of its fund.
- (2)
- Edwards Lifesciences Corporation, a publicly traded company, indirectly through Edwards Lifesciences LLC (Edwards) and Edwards Lifesciences (U.S.) Inc. owns 1,269,063 common shares and warrants to acquire 1,168,571 common shares.
- (3)
- Zesiger Capital Group LLC (Zesiger) is an investment adviser registered under the U.S. Investment Advisers Act of 1940. The beneficial owners of Zesiger are Albert Leroy Zesiger, Barrie Ramsay Zesiger, John Jude Kayola, James Francis Cleary, Donald Lee Devivo and Robert Kennedy Winters. Zesiger exercises control or direction, on behalf of accounts fully managed by it, over 1,276,964 common shares, 3% unsecured debentures in the aggregate principal amount of $800,000 convertible into 640,000 common shares, and warrants to purchase an additional 1,561,702 common shares. The common shares held by Zesiger are held on behalf of accounts over which Zesiger has discretionary authority. Pursuant to its investment management agreements with its clients, Zesiger has full discretion to acquire, hold and dispose of all of the securities referred to above. Zesiger also has the right to exercise the voting rights associated with the securities with respect to accounts holding 821,710 common shares. The following are the persons who beneficially own the shares held by Zesiger Capital Group LLC: City of Milford Pension and Retirement Fund; National Federation of Independent Business; National Federation of Independent Business Pension Trust; Norwalk Employees Pension Plan; City of Stamford Firemen's Pension Fund; Asphalt Green, Inc.; Asphalt Green Defined Contribution Plan; William B. Lazar; Lazar Foundation; Helen Hunt (includes common shares held by Helen Hunt Alternatives Fund, of which Helen Hunt is the President); Barrie Ramsay Zesiger; Alexa Zesiger Carver; David Zesiger; HBL Charitable Unitrust; Jeanne L. Morency; Psychology Associates; Peter Looram; Meehan Foundation; Miriam F. Meehan Trust; Domenic J. Mizio; Morgan Trust Co. of the Bahamas Ltd. as Trustee U/A/D 11/30/93; John Rowan; Susan Iris Halpern; Theeuwes Family Trust, Felix Theeuwes Trustee; Robert K. Winters; Francois deMenil; and Allan B. and Joanne K. Vidinsky 1993 Trust.
- (4)
- Mr. Jassawalla holds options for 1,788,929 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (5)
- Mr. Goudie holds options for 80,000 common shares, all of which are subject to shareholder approval. Of those, options for 40,000 common shares are exercisable within 60 days.
- (6)
- Mr. Jansen holds options for 325,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (7)
- Mr. Marinchak holds options for 260,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (8)
- Mr. Miller holds options for 325,270 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (9)
- Mr. Juelis holds options for 275,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (10)
- Mr. Ross holds options for 130,365 common shares, all of which are subject to shareholder approval. Of those, options for 365 common shares are exercisable within 60 days.
- (11)
- Mr. Carlson holds options for 130,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (12)
- Mr. Garriock holds options for 130,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (13)
- Mr. Majteles holds options for 130,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
- (14)
- Michael Roth and Brian Stark currently beneficially own an aggregate of 4,314,875 common shares, including 1,600,000 common shares issuable pursuant to the conversion of debentures and 2,291,244 common shares issuable pursuant to the exercise of warrants. All of the foregoing represents securities held directly by SF Capital Partners Ltd. ("SF Capital"). Michael Roth and Brian Stark are the managing members of Stark Offshore Management, LLC ("Stark Offshore"), which acts as investment manager and has sole power to direct the management of SF Capital. Through Stark Offshore, they possess voting and dispositive power over all of the foregoing shares.
- (15)
- Reid S. Walker, G. Stacy Smith and Patrick P. Walker beneficially own debentures convertible into 896,431 common shares and warrants to acquire 1,600,000 common shares. The common shares are owned by (i) WS Capital, L.L.C., a Texas limited liability company ("WS Capital"), for the account of (1) Walker Smith Capital, L.P., a Texas limited partnership ("WSC"), (2) Walker Smith Capital (Q.P.), L.P., a Texas limited partnership ("WSCQP"), and (3) Walker Smith International Fund, Ltd., a British Virgin Islands exempted company ("WS International"), and (ii) WSV Management, L.L.C., a Texas limited liability company ("WSV"), for the account of (1) WS Opportunity Fund, L.P., a Texas limited partnership ("WSO"), (2) WS Opportunity Fund (Q.P.), L.P., a Texas limited partnership ("WSOQP"), and (3) WS Opportunity Fund International, Ltd., a Cayman Islands exempted company ("WSO International"). WS Capital is the general partner of WS Capital Management, L.P., a Texas limited partnership ("WSC Management"). WSC Management is the general partner of WSC and WSCQP and the agent and attorney-in-fact for WS International. WSV is the general partner of WS Ventures Management, L.P., a Texas limited partnership ("WSVM"). WSVM is the general partner of WSO and
13
WSOQP and the agent and attorney-in-fact for WSO International. Reid S. Walker and G. Stacy Smith are principals of WS Capital and WSV, and Patrick P. Walker is a principal of WSV.
- (16)
- Bristol Investment Fund Ltd. owns debentures convertible into 160,000 common shares and warrants to acquire 800,000 common shares. Paul Kessler, as manager of Bristol Capital Advisors, LLC, the investment manager to Bristol Investment Fund, Ltd., has voting and investment control over the securities held by Bristol Investment Fund, Ltd.
- (17)
- Sherfam Inc. owns 416,257 common shares, debentures convertible into 330,000 common shares and warrants to acquire 662,856 common shares. Bernard C. Sherman has sole voting and dispositive control of The Bernard Sherman 2000 Trust and owns 99% of the outstanding capital stock of Sherman Holdings Inc., which collectively hold 99% of the outstanding common and preferred shares of Shermco Inc., which in turn owns all of the outstanding capital stock of Sherfam Inc.
- (18)
- Federated Investors, Inc. owns 820,257 common shares and warrants to acquire 1,410,138 common shares. Federated Investors, Inc. is the parent holding company of Federated Investment Management Company, Federated Investment Counseling, and Federated Global Investment Management Corp. (the Investment Advisers), which act as investment advisers to registered investment companies and separate accounts that own shares of common stock in WorldHeart. All of the outstanding voting stock of Federated Investors, Inc. is held in the Voting Shares Irrevocable Trust for which John F. Donahue, Rhodora J. Donahue and J. Christopher Donahue act as trustees.
- (19)
- MedCap Partners owns 460,829 common shares and warrants to acquire 460,829 common shares. MedCap Management & Research LLC (MMR) as general partner and investment manager of MedCap Partners, and C. Fred Toney as managing member of MMR, may be deemed to own beneficially the securities owned by MedCap Partners in that they may be deemed to have the power to direct the voting or disposition of the securities.
- (20)
- Sceptre Investment Counsel Limited, a publicly traded mutual fund company, owns warrants to acquire 1,232,856 common shares.
- (21)
- FCP OP MEDICAL BioHe@lth Trends, a publicly traded investment fund, owns debentures convertible into 480,000 common shares and warrants to acquire 480,000 common shares.
- (22)
- Mr. Vajda holds options for 250,000 common shares, all of which are subject to shareholder approval. Of those, no options are exercisable within 60 days.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This circular includes "forward-looking statements" within the meaning of Section 27A of the United States Securities Act of 1933 and Section 21E of the United States Securities Exchange Act of 1934. The forward-looking statements contain information that is generally stated to be anticipated, expected or projected by WorldHeart, and involves known and unknown risks, uncertainties and other factors that may cause the actual results and performance of WorldHeart to be materially different from any future results and performance expressed or implied by such forward-looking information. Potential risks and uncertainties include, without limitation, the uncertainties inherent in the development of a new product for use in the human body, our potential need for significant additional funding, our need for acceptance from third-party payers, extensive government regulation of our products, and rapid developments in technology, including developments by competitors. Important factors that could cause our actual results to differ materially from those expressed or implied by such forward-looking statements include:
- •
- our ability to successfully complete pre-clinical and clinical development of our products;
- •
- our ability to successfully integrate technologies or companies that we may acquire, from time to time, into our operations;
- •
- our ability to obtain and enforce in a timely manner patent and other intellectual property protection for our technology and products;
- •
- our ability to avoid, either by product design, licensing arrangement or otherwise, infringement of third parties' intellectual property;
- •
- decisions, and the timing of decisions made by health regulatory agencies regarding approval of our products;
- •
- our ability to complete and maintain corporate alliances relating to the development and commercialization of our technology and products;
- •
- the competitive environment and impact of technological change;
- •
- the continued availability of capital to finance our activities in the event that profitability is not achieved; and
- •
- other factors we discuss in this circular under the heading "RISK FACTORS."
14
We undertake no obligation to update publicly or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
CORPORATE STRUCTURE
Name, Address, and Incorporation
We were incorporated by articles of incorporation under the laws of the Province of Ontario on April 1, 1996. Our registered office is located at 1 Laser Street, Ottawa, Ontario, Canada, K2E 7V1 and our telephone number is (613) 226-4278. Our head office is located at 7799 Pardee Lane, Oakland, California, USA, 94621 and we have an office at Cereslaan 34, 5384 VT, Heesch, Netherlands.
Our articles of incorporation were amended on June 22, 2000 to create a first series of 1,374,750 preferred shares designated as Cumulative Redeemable Convertible Preferred Shares, Series A (the Series A Shares), in connection with the acquisition by WorldHeart of the Novacor division (Novacor) of Edwards Lifesciences LLC (Edwards), a subsidiary of Edwards Lifesciences Corporation. On November 26, 2003, our articles were amended to amend the rights and privileges of the Series A Shares in connection with the conversion by Edwards Lifesciences (U.S) Inc., a subsidiary of Edwards Lifesciences Corporation, of its Series A Shares as part of our financing transaction completed in September 2003. Our articles were amended again on December 1, 2003, to effect a one common share for seven common shares share consolidation.
Intercorporate Relationships
World Heart Inc. is our wholly-owned subsidiary, incorporated under the laws of the State of Delaware on May 22, 2000. World Heart Inc. acquired the assets and liabilities of the Novacor division of Edwards in June 2000 and is responsible for the manufacturing and primary sales, marketing and support of the Novacor® left ventricular assist system (Novacor LVAS) as well as next-generation product development.
World Heart B.V. is our wholly-owned subsidiary, incorporated under the laws of the Netherlands on March 5, 2004, through which we carry on sales, sales support and distribution in Europe.
2007262 Ontario Inc. (2007262), is an associated research and development company of WorldHeart, incorporated under the laws of the Province of Ontario on November 29, 2001 to carry out specified research and development for us. WorldHeart and New Generation Biotech (Equity) Fund Inc. (NewGen), an Ontario labour-sponsored venture capital corporation, each hold 100 common shares of 2007262. WorldHeart holds all the issued and outstanding non-voting Series 1 preferred shares and non-voting Series 2 preferred shares of 2007262.
WorldHeart has entered into an asset purchase agreement, as amended by amendment no. 1, (the MedQuest Agreement) with MedQuest Products, Inc. (MedQuest) to acquire the assets of MedQuest and not the issued and outstanding shares of MedQuest, as more fully described in this circular. Therefore, if the acquisition is approved and completed, the assets of MedQuest will form a part of WorldHeart's assets and no new subsidiary will be created.
DEVELOPMENT OF THE BUSINESS
Three Year History and Development of WorldHeart
WorldHeart is a global medical devices company focused on the development, production, sales and support of ventricular assist devices (VADs). VADs are mechanical assist devices that supplement the circulatory function of the heart by re-routing blood flow through a mechanical pump. VADs are used for treatment of patients with end-stage heart failure (i.e. patients whose hearts are irreversibly damaged and which cannot be treated effectively by medical or surgical means other than a transplant). Bridge-to-transplant therapy involves implanting a VAD in a transplant-eligible patient to maintain or improve the patient's health until a donor heart becomes available. Destination therapy is the implanting of a VAD to provide long-term support for a patient not currently eligible for a natural heart transplant.
Our current business is based on two generations of implantable, pulsatile VADs, the current generation commercially available Novacor LVAS, and an in-development, next-generation Novacor II. A decision was made by management in August 2002, to consolidate WorldHeart's next-generation pulsatile VAD programs. At that time, development on the HeartSaverVAD, our initial next generation product, was effectively terminated in order to focus a more concentrated development effort on a new design of our next generation product, the Novacor II. This delayed our product development by about two years. As described in this circular, upon
15
completion of the MedQuest Acquisition we will be acquiring a next-generation, non-pulsatile, magnetically levitated, centrifugal, rotary VAD. These devices are also intended for use in both bridge-to-transplant therapy and destination therapy.
Following the acquisition of the Novacor division from Edwards in June 2000, we have focused our operations on:
- •
- the sales, support, production and enhancement of the Novacor LVAS; and
- •
- the development and commercialization of Novacor II, an optimized product based on patented technologies and experience gained from the development and commercialization of the Novacor LVAS and experience with other VAD development programs.
The Novacor LVAS and Novacor II are described in more detail in "BUSINESS OF WORLD HEART CORPORATION" starting on page 19.
Since January 1, 2002 a number of significant events have occurred indicating the increasing acceptance by the medical community of VAD-based therapy for treatment of end-stage heart failure that we expect will positively impact WorldHeart's business as well as all competitive VAD suppliers. We believe these events signal a future expansion in the use of VADs.
Significant industry developments include:
- •
- A decision in November 2002 by the United States Food and Drug Administration (FDA) to extend the use of VAD therapy to heart failure patients as destination therapy, which validates the use of VADs as a long-term treatment for late stage heart failure; and
- •
- Two significant increases in reimbursement for VADs in the United States (October 2003 and October 2004) which have increased average reimbursements for VAD implantation by over 70% to between $130,000 and $140,000.
A number of significant developments related specifically to WorldHeart's Novacor LVAS products have also occurred:
- •
- Approval was given by the FDA in the United States (January 2003) and by Health Canada (March 2003) of our expanded polytetrafluoroethylene (ePTFE) inflow conduit as a component of the Novacor LVAS product. Results in over 250 implanted patients to date indicate a reduction in stroke rate.
- •
- Approval was given by the FDA (January 2004) and Health Canada (September 2003) of product enhancements to the Novacor LVAS aimed at improving the recipient's quality of life — quieter pump operation, new battery packs that are 40% lighter and operate for 60% longer, and a smaller, quieter battery charging system. These enhancements have been commercially available in Europe since June 2002.
- •
- Approval was given in Japan for use (August 2001), importation (January 2002) and reimbursement (effective April 2004) of the Novacor LVAS.
- •
- Conditional approval was given by the FDA on July 21, 2004 of a Premarket Approval (PMA) Supplement application for a revision to the labeling of the Novacor LVAS to reflect the current device reliability data available to patients and physicians. The revised labeling indicates the demonstrated performance longevity of the Novacor LVAS, which encompasses implant duration from zero to more than 36 months. The revised labeling, which is based upon statistical analysis of 1,077 device implants, shows the chance for reoperation to either fix or replace the Novacor LVAS is 1.6% between zero and 6 months; 2.1% between 6 and 12 months; 11% between 12 and 24 months; and 16% between 24 and 36 months.
- •
- Unconditional approval was given by the FDA on July 30, 2004 of our Investigational Device Exemption Supplement for the RELIANT (RandomizedEvaluation of the NovacorLVASinaNon-Transplant Population) clinical trial (the RELIANT Trial). The RELIANT Trial will be conducted in up to 40 clinical centers and will enroll up to 390 patients. The data from the RELIANT Trial will support a PMA Supplement that will request a revision to the indication for use of the Novacor LVAS to include end-stage heart failure patients that are ineligible for heart transplantation. The RELIANT Trial randomizes patients who enter the RELIANT Trial to receive either a Novacor LVAS or a Thoratec Corporation (Thoratec) HeartMate ® XVE LVAS, (the HeartMate XVE), on a 2:1 ratio. Costs of implants, including device costs, for both the Novacor LVAS and HeartMate XVE are eligible for reimbursement by Centers
16
In 2003, we rationalized and reallocated our workforce to focus on sales, production, and support of the Novacor LVAS, on a basis consistent with the development plans and strategy for WorldHeart's next-generation VAD. We are continuing our rationalization and expect to consolidate certain of our operations with those of MedQuest if the acquisition of MedQuest is completed.
Effective January 1, 2004, we assumed full sales and support responsibility for the Novacor LVAS worldwide with the exception of Japan. Edwards was previously our exclusive distributor for the Novacor LVAS in all countries other than the United States where we sell directly. We continue to distribute the Novacor LVAS through Edwards in Japan and through other distributors in selected markets.
In September 2003, we completed a significant equity financing and restructuring. This financing and restructuring is described in the U.S. GAAP audited consolidated financial statements which are attached to this circular as Appendix B-1 and in the Canadian GAAP audited consolidated financial statements which are attached to this circular as Appendix B-2. In summary, the financing and restructuring included:
- •
- A Cdn$63,500,000 private placement of equity through the issuance of units, each unit was comprised of one common share and one warrant exercisable for one common share at an exercise price of Cdn$7.42. The warrants are callable by WorldHeart for cash should our common shares trade at or above Cdn$17.50 for 20 consecutive trading days. However, no more than 20% of the total warrants issued may be called in any three month period;
- •
- An exchange of the preferred shares of World Heart Inc. held by Edwards in the amount of $58 million plus accrued dividends for 711,589 common shares of WorldHeart;
- •
- The conversion of the Series A Shares of WorldHeart held by Edwards Lifesciences (U.S.) Inc. at a value of $20 million plus accrued dividends into 500,000 common shares of WorldHeart and 1,000,000 warrants, with each warrant exercisable for one common share of WorldHeart at an exercise price of Cdn$7.49 per common share; and
- •
- A consolidation of WorldHeart's common shares on the basis of one post-consolidation common share for seven pre-consolidation common shares.
On March 18, 2004, our common shares, which had been delisted from the NASDAQ National Market (NASDAQ) on October 15, 2002, were re-listed on NASDAQ under the symbol WHRT.
On April 22, 2004, with the announcement of our first quarter results, we commenced reporting our results in United States dollars.
On July 12, 2004, we announced that our first quarter results would be restated, and on August 12, 2004, we released our restated first quarter results. Restated revenues for the first quarter decreased by $1.3 million to $2.2 million and shipments of the Novacor LVAS kits decreased by 25 kits to a total of 26 kits. The restatement resulted partly in respect of a sale during the first quarter of 20 kits to a world-leading center, which has implanted more than 100 Novacor LVAS kits. It became apparent that there was the expectation on the part of the buyer that it would receive a limited right of return for certain Novacor LVAS kits that it purchased in the event that they were not implanted prior to reaching their sterilization expiry dates. As a result, we determined that the appropriate accounting treatment was to exclude revenue from this sale in accordance with our revenue recognition criteria. The effect of these restatements on the net loss was an increase of approximately $585,000 to a net loss of $4.1 million and an increase in the net loss per common share by four cents to a net loss per common share of 27 cents.
On July 28, 2004, we announced certain management changes including the appointment of Mr. Jal S. Jassawalla as President and Chief Executive Officer replacing Mr. Roderick M. Bryden who resigned from those positions and from the board of directors of WorldHeart. Mr. Griffin's employment was terminated as part of a number of management changes. Dr. Piet Jansen was appointed Managing Director, Europe and continued as Chief Medical Officer for WorldHeart. Mr. John Marinchak was appointed Vice President, Marketing and Sales. Also Mr. Robert W. Corson was appointed Vice President, Manufacturing replacing Mr. Robert Griffin who left WorldHeart in July 2004. Mr. Bryden resigned from WorldHeart at that time because the board and Mr. Bryden determined that new leadership was required for WorldHeart to seize new opportunities and to continue to build WorldHeart's leadership position in the heart assist business.
17
On August 12, 2004, Mr. Mark Goudie, Vice President, Finance and Chief Financial Officer was appointed to the board of directors to fill the vacancy created by the resignation of Mr. Bryden.
On August 25, 2004, we announced the consolidation of our operations from Ottawa, Ontario to Oakland, California to be substantially completed over a period of nine months.
On September 16, 2004, we completed the sale of $13,318,750 aggregate principal amount of convertible debentures and warrants. The debentures are convertible, at the option of the holder, into common shares at a conversion rate of $1.25 per common share until September 15, 2009. The purchasers of the convertible debentures were also issued a total of 10,655,000 warrants to purchase common shares at an exercise price of $1.55 per common share exercisable until September 15, 2009.
On October 1, 2004, Mr. Phillip J. Miller was appointed Vice President, Research and Development.
On March 22, 2005, WorldHeart announced that it had ceased to be a foreign private issuer as defined in the United States Securities Exchange Act of 1934 because, as a result of the consolidation of WorldHeart's operations to Oakland, California, the mind and management of WorldHeart is located in California. In addition, as part of the consolidation of WorldHeart's operations WorldHeart announced that Mark Goudie would resign as Vice President, Finance and Chief Financial Officer and Robert Corson will resign as Vice President, Manufacturing in the second quarter of 2005. On May 17, 2005, Mr. Goudie resigned as Vice President, Finance and Chief Financial Officer. Mr. Goudie will provide consulting and advisory services to WorldHeart until September 2005. Both Mr. Goudie and Mr. Corson, having decided not to relocate to Oakland, California for personal reasons, are remaining in Ontario, Canada to pursue other opportunities. On June 2, 2005, Richard Juelis was appointed Vice President, Finance and Chief Financial Officer. On June 9, 2005, WorldHeart appointed John J. Vajda as Vice President, Manufacturing.
Significant Acquisitions
WorldHeart did not complete any significant acquisitions or dispositions in the most recent fiscal year ended December 31, 2004.
On January 31, 2005, we entered into the MedQuest Agreement under which we agreed to purchase all of the assets of MedQuest, subject to certain conditions including shareholder approval (the MedQuest Acquisition).
Also, on January 31, 2005, we entered into a purchase agreement, as amended by amendment no. 1, (the Maverick Agreement) with Maverick Venture Management, LLC (Maverick) pursuant to which Maverick has agreed to purchase in a private placement approximately 8.9 million common shares of WorldHeart at $1.35 per common share for an aggregate purchase price of $12 million. The closing of the private placement is contingent on the closing of the transactions contemplated in the MedQuest Agreement.
Pursuant to the MedQuest Agreement, WorldHeart will acquire all the assets of MedQuest, subject to certain approvals (including approval by our shareholders and MedQuest's stockholders), consents and conditions. MedQuest, based in Salt Lake City, Utah, USA is in the final development stages of its HeartQuest™ VAD, a non-pulsatile rotary flow blood pump (the MedQuest rotary VAD). WorldHeart currently offers the pulsatile Novacor LVAS and is in development of the Novacor II. The acquisition of MedQuest will add an advanced rotary pump to our VAD platform.
The private placement and the exercise of warrants for approximately $10.7 million will raise additional equity of approximately $23 million, a portion of which will be used to pay $3.5 million owed to Maverick by MedQuest in connection with the funding of ongoing operations of MedQuest until the closing of the MedQuest Acquisition. Other than liabilities incurred in the normal course for day-to-day operations, WorldHeart is not assuming any other liabilities from MedQuest. Pursuant to a loan agreement dated January 31, 2005, Maverick agreed to lend up to $3.5 million, without interest, to MedQuest, to be advanced in instalments, to fund ongoing operations until the closing of the MedQuest Acquisition, at which time the full amount of the loan then outstanding would become due and payable. In addition, the conversion of outstanding debentures issued in September 2004 will result in the conversion to equity of approximately $13.3 million of our outstanding debt. Subject to shareholder approval, the exercise price of the warrants will be reduced from $1.55 to $1.00 per common share in return for all warrant holders' acceptance of a tender offer of amended warrants and their agreement to exercise the warrants early and convert their debentures (the Warrant Exercise & Debenture Conversion). The conversion price of the debentures will remain at $1.25 per common share.
18
Upon completion of the acquisition, WorldHeart will retain a substantial number of MedQuest's employees at the facility in Salt Lake City, Utah, which will serve as the development and manufacturing site for our rotary pump products. WorldHeart expects that upon completion of the MedQuest Acquisition to continue operations at the facility in Salt Lake City, Utah but to consolidate certain functions such as finance, human resources, marketing and clinical support at the head offices in Oakland, California. WorldHeart expects that this will result in efficiencies in operations and cost savings on a going forward basis.
Maverick is a majority shareholder of MedQuest, holding approximately 82% of the outstanding common stock and 100% of the preferred stock of MedQuest. At the closing of the transactions, Maverick and the shareholders of MedQuest will collectively hold approximately 33% of the common shares of WorldHeart. Maverick will be given the right to nominate up to two designees to WorldHeart's board of directors so long as it holds or controls at least 25% of our issued and outstanding common shares. If Maverick holds or controls less than 25% but at least 15% of our issued and outstanding common shares, Maverick will have the right to nominate one designee to WorldHeart's board of directors.
BUSINESS OF WORLD HEART CORPORATION
Business Overview and Our Products
Our current business is based on the Novacor LVAS and Novacor II. We will also acquire the MedQuest rotary VAD through our purchase of MedQuest.
The Novacor LVAS has been in clinical use for more than 20 years and has been implanted in approximately 1,600 patients worldwide. The Novacor LVAS is an electromagnetically-driven pump, about the size of a human heart, that provides circulatory support for patients with life-threatening heart failure by taking over part or all of the workload of the left ventricle of the heart. It is implanted within the abdominal wall and mimics the natural heart by creating a pulsing flow of blood. The natural heart continues to deliver what it can, and may recover partially while assisted by the Novacor LVAS. The Novacor LVAS is completely self-regulating, responding instantaneously to the recipient's changing heartbeat and circulatory demands.
The experience in long-term support continues to grow. The majority of recipients have had a support duration of less than one year. However, over 139 recipients have been supported for more than one year, 35 of whom have lived with the device for more than two years, 14 for more than three years, three for more than four years and one for more than six years. No patient deaths have been attributed to the Novacor LVAS failure. A single patient who was transplant-ineligible at the time of initial implant of the Novacor LVAS in 1998 received continuous circulatory support for more than six years from the Novacor LVAS, including a scheduled replacement of the initial Novacor LVAS after four years and this patient subsequently received a natural heart.
The Novacor LVAS is commercially available as a bridge-to-transplant in Europe, the United States and Canada. In Europe, it is also available with an unrestricted indication for destination therapy. In Japan, the Novacor LVAS is the only approved implantable VAD for use by cardiac patients at risk of imminent death from non-reversible left ventricular failure for which there is no alternative but a heart transplant.
In the United States, WorldHeart commenced the RELIANT Trial in the second quarter of 2004.
Our next-generation pulsatile VAD, Novacor II, is currently under development and is expected to result in a fully implantable, pulsatile VAD designed specifically for destination therapy patients. Novacor II is being developed to be approximately 50% of the size of the Novacor LVAS, with no mechanical bearings. For the fully implantable configuration, its dual chamber design eliminates the need for volume compensation and the need for external venting, and incorporates remote power and monitoring (i.e. across-the-skin power and data transfer) and an implanted controller/battery. These features are expected to provide recipients with an enhanced quality of life by allowing freedom of movement and minimal limitations to their regular activities. It is magnetically-driven, allowing for simple operation with no wearing elements or precision components. Initially, the Novacor II will be available with a percutaneous lead to provide clinicians and patients with the simplest possible implant configuration.
We expect that the Novacor II will begin animal trials in 2005, begin initial clinical trials in 2007 and receive commercial approvals in Europe in 2008 and in the United States in 2010.
19
Through the acquisition of the assets of MedQuest we will obtain the MedQuest rotary VAD, an advanced, next-generation rotary blood pump intended for destination therapy. Unlike the initial generation of rotary pumps with blood-lubricated bearings, the MedQuest rotary VAD is a compact, magnetically levitated, centrifugal pump with an impeller that is completely magnetically levitated. Full magnetic levitation eliminates wear mechanisms within the pump and provides for greater clearances for more optimized blood flow around the impeller, while eliminating dependence on the patient's blood for suspension. The MedQuest rotary VAD's levitation technology employs a unique combination of passive and single-axis active control, resulting in a system of optimal simplicity.
The MedQuest rotary VAD is now at an advanced stage of development and is expected to commence an initial feasibility clinical trial in 2005, and is expected to receive commercial approvals in Europe in 2007 and in the United States in 2009.
Third-party Reimbursement for VADs
The United States and Canada currently provide for public reimbursement of VADs used as a bridge-to- transplant. In addition, certain private insurance carriers in the United States provide reimbursement for VAD use. On October 1, 2003, a National Coverage Decision by the CMS in the United States extended reimbursement to VADs approved by the FDA for destination therapy. On October 1, 2004, CMS implemented a previously announced increase in payments for implantation of all VADs, representing a 30% to 40% increase in reimbursements for centers implanting VADs and resulting in average reimbursement rates in the United States of $130,000 to $140,000.
Several countries in Europe provide reimbursement for VADs. Reimbursements, however, vary between countries and budget constraints, to date, have limited reimbursements.
Also, effective April 1, 2004, reimbursement in Japan for implantable VADs was approved.
Application of VADs in Patient Care
The search for an effective treatment for end-stage heart failure is ongoing. Therapies such as medication and transplantation have significant limitations, and alternative emerging technologies, such as xenografts (animal organs or tissue transplanted into humans, with rejection being an even greater challenge), cardiomyoplasty (the use of another muscle wrapped around the heart and electrically stimulated to augment heart function), genetic tissue engineering, multiple site pacing (pacing wires placed on two to three separate ventricular sites), passive constraint devices and implantable artificial hearts (total artificial hearts and ventricular assist devices, pulsatile and non-pulsatile), are being investigated. The following are treatment methods currently being employed for advanced heart failure:
Medication. Pharmaceutical drugs are the first line of defence against heart failure; however, in spite of many advances, drug therapies continue to be able to provide only very limited benefit particularly in advanced heart failure patients. Drug therapies usually do not treat the underlying disorder and, thus, can only slow the disease's progression. Moreover, a significant number of heart failure patients may be resistant to treatment with drug therapies and often such therapies have adverse side-effects, some of which can be quite serious for certain patients.
Heart Transplantation. Heart transplantation is currently the intervention of choice for some patients with end-stage heart failure. Patients receiving heart transplants have a 71% five-year survival rate (source: American Heart Association (AHA), 2004 update). However, the availability of donor organs as well as other major limitations, described below, has limited the number of transplants worldwide to about 4,000 per year. In the United States, the number of heart transplants is just over 2,000 annually (2,057 in 2003; 2,154 in 2002; 2,202 in 2001; 2,199 in 2000; 2,184 in 1999; 2,340 in 1998), according to the AHA, despite the fact that the AHA estimates that each year at least 40,000 Americans under age 65 would benefit from heart transplants. Another negative feature of transplants is the need for immuno-suppressive medication to prevent rejection of the implanted organ, which can make patients vulnerable to other diseases and which may have other adverse side effects. In addition, heart
20
transplantation procedures are rarely used in emergency situations because of the typically long waiting time required to obtain a suitable donor organ. Furthermore, the transplanted natural heart can itself develop complications. Finally, the costs of heart transplants are reported to be up to $250,000 for the first year.
Artificial Heart Technology. Both VADs and total artificial hearts (each a form of mechanical circulatory support) have been used clinically and have been demonstrated to be viable treatments for end-stage heart failure. These devices have already saved thousands of lives during temporary use as a bridge-to-transplant while the recipients await a heart transplant and recently have been used as a bridge-to-recovery or as an alternative to transplantation. The devices currently on the market are designed for implantation in the abdomen or thorax or placement outside the body with connections to the heart, requiring perforation of the diaphragm and/or skin tissue. The external devices typically require at least two perforations of the protective skin and tissue, and connection to a console, which restricts patient mobility. Implantable devices typically require a percutaneous (through-the-skin) lead for power, data transfer and venting.
Advantages of VADs
VADs that are either externally placed or abdominally implanted have been demonstrated as being effective in supporting blood circulation in patients with a failing heart. More than 10,000 patients have been supported by pulsatile VADs that are in the market.
The following advantages over other treatments generally apply to VADs that are currently approved and in use, including the Novacor LVAS. We expect that these advantages will also apply to our Novacor II and the MedQuest rotary VAD that we will acquire:
Unlimited Supply. As a manufactured device, VADs are available as and when needed, including on an emergency basis, to treat advanced heart failure patients.
Potential Reduced Hospitalization. Unlike transplant patients, VAD patients go to surgery without a protracted wait for a donor organ, and in the case of implantable VADs patients are generally able to leave the hospital after a relatively short recovery period, thus potentially reducing health care costs.
Potential Improved Patient Health. After VAD implantation, blood circulation is improved throughout the body and most patients experience improved levels of health as shown in a number of clinical studies, including those for the Novacor LVAS.
Potential Reduction in Medication Use. Unlike transplants, VADs typically do not cause rejection responses and, as a result, VAD patients typically do not need the administration of immuno-suppressive medication. Patients are accordingly not subject to the risks and costs associated with long-term administration of these medications.
Potential Natural Heart Recovery. Unlike total artificial heart systems, VADs leave the natural heart intact and assist it when it is unable to provide sufficient cardiac function to maintain life. VADs have, in some cases, permitted recovery of natural heart function to the point where the device was eventually removed.
21
Marketing, Training and Distribution Strategy
Our principal markets are currently North America and Europe. Revenue for each geographic market for fiscal 2004 and 2003 was as follows:
| | Revenue
|
|---|
| | 2004
| | 2003
|
|---|
| United States | | $ | 5,343,725 | | $ | 4,129,322 |
| Europe(1) | | | 3,033,294 | | | 1,862,335 |
| Canada(1) | | | 510,672 | | | 245,424 |
| Japan | | | 688,070 | | | 538,726 |
| | |
| |
|
| | | $ | 9,575,761 | | $ | 6,775,807 |
| | |
| |
|
- (1)
- Until December 31, 2003, sales in Europe and Canada were made through our distributor Edwards.
With the acquisition of the Novacor division of Edwards in June 2000, we immediately gained access to many key medical centres involved in cardiac transplantation in North America, Europe and Japan. The Novacor LVAS has been available for implant in approximately 100 centres in these geographic markets. We sell directly within the United States. Prior to December 31, 2003, outside the United States, the Novacor LVAS was represented by Edwards. On January 1, 2004, we assumed full sales and support responsibility for the Novacor LVAS worldwide with the exception of Japan. WorldHeart continues to distribute its products through Edwards in Japan and through other distributors in selected markets.
The United States and European-based operations of our Novacor business have experienced clinical and technical personnel who support active implant centres and their leading clinicians.
Product improvements to the Novacor LVAS, which were introduced in Europe during 2002, have now also been introduced in the United States and Canada. The RELIANT Trial in the United States, the data from which is expected to support a PMA Supplement for the approval of the Novacor LVAS for destination therapy, is discussed above in "DEVELOPMENT OF THE BUSINESS — Three Year History and Development of WorldHeart". Our United States sales force has also been expanded in order to increase our sales in the United States.
Sources and Availability of Raw Materials
In 2004, we experienced difficulties in obtaining certain externally supplied inputs to the Novacor LVAS and related equipment. This was mainly the result of a program to reduce manufacturing costs associated with the Novacor LVAS and the commencement of the RELIANT Trial.
During 2004 we continued our initiative to reduce manufacturing costs associated with the Novacor LVAS. An element of this program was to find alternative sources of high-quality inputs at improved pricing. Changes in suppliers and the tightly controlled quality procedures and qualification process resulted in temporary interruptions in the supply of components, most notably with respect to armatures.
In addition, during 2004 the RELIANT Trial commenced which will include the participation of up to 40 centers. Approximately 30 of these will be new centers that need to be equipped with start-up equipment that is used during the implant procedure and patient take-home equipment. We had largely and recently been utilizing start-up equipment that we had in inventory and which became insufficient as a result of an expected significant growth in the customer bases primarily associated with the RELIANT Trial. As we re-commenced manufacturing of this complicated and largely customized start-up equipment we found that, in certain cases, previous suppliers of components had to be re-acquainted with our requirements or were simply not able to supply adequate components, and new suppliers had to be identified and qualified. The most common items affected were power supplies, capacitors, converters and transformers.
These initiatives have continued into 2005. Although we continue to experience occasional difficulties with certain suppliers unable to consistently meet our precision quality requirements, we believe that we have
22
identified a vendor base that is fully capable of meeting our demanding quality standards and supplying the quantities of input that we will require in a timely manner.
Intellectual Property
We have been granted sixteen United States patents for the Novacor LVAS and its associated subsystems. A subset of these patents has also been filed and granted in the major European countries, in Canada and in Japan.
WorldHeart has nine material patents registered in the United States. The six patents related to the Novacor LVAS have expiry dates ranging from November 20, 2006 to January 13, 2018. The one issued patent related to the Novacor II has an expiry date of April 2, 2019. The patents related to the calormetical determination of the battery charge state and the biventricular circulatory support system expire on April 3, 2010 and April 18, 2006, respectively. Several of these patents are also registered in other jurisdictions around the world including Canada, various countries in the European Union and Japan. In all instances except one, the expiry dates in the jurisdictions outside the United States are beyond those in the United States.
To date, we have been granted one patent for the Novacor II in the United States, and have applied for a second patent, and a European patent has been granted covering the United Kingdom, France, Germany, Italy, Switzerland and Liechtenstein. We have applied for patents for Novacor II in Canada and in Japan. The Transcutaneous Energy Transfer technology licensed to WorldHeart from the Ottawa Heart Institute intended to be incorporated in Novacor II has been patented in the United States, Canada and the United Kingdom.
We generally enter into confidentiality or license agreements with our employees, consultants and vendors, and generally control access to and distribution of information related to our technology and products, documentation and other proprietary information.
We do not expect any material impact on our business as a result of the expiry of patents in coming years. Generally, advances in technology limit the commercial life of ventricular assist devices to a period shorter than the period of patent protection. We anticipate that by the time our existing patents expire we will have moved to more advanced technology. We expect a series of patent applications to be filed and approved over the years as our designs evolve and are refined such that as the technology advances so will the period of patent protection for our key products. Moreover, our technology involves a significant amount of proprietary confidential information and trade secrets that will not be affected by the expiry of patents.
Product Liability Insurance
We maintain product liability insurance in the amount of $10,000,000 per occurrence and to a maximum of $10,000,000.
Competition
In addition to competing with other less-invasive therapies for heart failure, the Novacor LVAS and next-generation VADs will compete with existing VADs and VADs under development, sold by a number of existing and emerging companies. Competition from medical device companies and medical device subsidiaries of healthcare companies is intense and may increase. Many of our competitors may have substantially greater financial, technical, manufacturing, distribution and marketing resources.
At present, only two companies in North America have developed implantable VADs approved for commercial sale: WorldHeart and Thoratec. Thoratec has two pulsatile, left ventricular assist device models of its HeartMate that have been approved in the United States for commercial sale. One is pneumatically driven (Heartmate IP LVAS), and the other is electrically driven (HeartMate XVE). The HeartMate XVE and the Novacor LVAS have been approved for commercial sale for bridge-to-transplant indications in the United States and without restriction for use for heart-failure patients in Europe. Each of these VADs is implanted in the abdomen and has a percutaneous lead that combines the power wires and vent.
The HeartMate XVE received approval for destination therapy in the United States in April 2003. WorldHeart commenced the RELIANT Trial in the second quarter of 2004, in which the Novacor LVAS is being
23
randomized to the HeartMate XVE which is the control arm. It is expected that data from this trial will support a PMA Supplement which will be submitted to the FDA for the use of the Novacor LVAS for destination therapy.
Thoratec, and other companies such as Abiomed, Inc. (Abiomed), have VADs that are designed for temporary use but are not implanted in the body. Their pump is external and attached to the natural heart via connecting tubes running through the recipient's skin and tissue. Abiomed's VAD is approved by the FDA for in-hospital use only. In 2001, Thoratec received approval from the FDA for a portable console driver for its VAD that can enable recipients to leave the hospital.
To date, the only VADs that have been approved in the United States for commercial sale are not totally implantable or remotely powered, monitored and controlled, and, with the exception of the Novacor LVAS and HeartMate XVE, are primarily for acute (short-term) use or for bridge-to-transplant applications.
In Europe and certain other countries outside North America, Berlin Heart Institute and Medos Medizentechnik AG provide external VADs comparable to those manufactured and distributed by Thoratec.
Currently, most heart assist devices intended for chronic use are pulsatile, mimicking the natural heart's pumping action. Non-pulsatile, or continuous blood flow VADs, are under development and have been tested in animals and in human trials as a bridge-to-transplant or bridge-to-recovery. At the present time, the scientific community has varying opinions as to whether or not continuous flow VADs will be viable for long-term implantation in humans. Continuous flow VADs are expected to be smaller, less complex and valveless, to have lower energy needs and to be potentially less costly. There is a lack of long-term clinical data on non-pulsatile pump reliability and on human physiological and haematological responses, including the determination of the human body's requirement for a pulsatile blood flow for long-term health.
We believe that effective treatment of advanced heart failure will require both pulsatile and rotary pumps to treat the full spectrum of clinical needs of end and late-stage heart failure patients. We believe that pulsatile devices are best suited for end-stage patients with poor ventricular contractility, who require full support (or, functional replacement); while rotary devices are better suited for late-stage patients, with some left ventricular contractility, who require only partial support (or, assist).
We believe that the only fully implantable, pulsatile VADs under development are the Arrow LionHeart™ VAD and our next-generation VAD, Novacor II.
There are a large number of non-pulsatile, or rotary flow, VADs in varying stages of development. One such device, which has been undergoing United States clinical trials (as a bridge-to-transplant and for destination therapy) and which is approved for use in Europe, is the MicroMed DeBakey® VAD being developed by MicroMed Technology, Inc. This device provides continuous left ventricular assist via a small rotary pump that is implanted in the abdomen and is powered via wires that perforate the skin, connected to an external controller and rechargeable battery pack. The Jarvik 2000 Flowmaker®, developed by Jarvik Heart, Inc., is a similar device at a comparable state of development.
We believe that the MedQuest rotary VAD under development, which we will acquire upon completion of the MedQuest Acquisition, is a technologically advanced, next generation rotary pump. Its magnetically-levitated rotor results in a pump with no moving parts subject to wear, resulting in a small device expected to provide multi-year support over a wide range of flows.
Combining MedQuest's rotary VAD with WorldHeart's clinical and commercialization infrastructure and our current and next-generation, long-term, pulsatile Novacor systems will be critical in expanding our activities in the destination therapy market. If the MedQuest Acquisition is completed, WorldHeart will be the only
24
company having small, silent, next-generation pulsatile and rotary systems under development, as well as having a current-generation pulsatile device in the market today.
Regulatory Matters
Most countries, including the United States, Canada and countries in Europe, require regulatory approval prior to the commercial distribution of medical devices. In particular, implantable medical devices generally are subject to rigorous clinical testing as a condition of approval by the FDA and by similar authorities in Canada, in Europe and in other countries. The approval process for our Novacor II and MedQuest's rotary VAD will be expensive and time consuming.
In the United States, the FDA regulates the manufacture, distribution, labeling and promotion of medical devices pursuant to the United States Federal Food, Drug and Cosmetic Act (FDC Act) and regulations under the Act. The Novacor LVAS, Novacor II and MedQuest's rotary VAD devices are regulated as Class III medical devices. Human clinical trials are conducted pursuant to an Investigational Design Exemption (IDE), the results of which must demonstrate, to the satisfaction of the FDA, the safety and efficacy of the system.
Before commercial distribution of our devices is permitted in the United States, an application for Premarket Approval must be approved by the FDA.
In addition, any medical device distributed in the United States is subject to pervasive and continuing regulation by the FDA. Products must be manufactured in registered establishments and must be manufactured in accordance with the Quality System Regulation. Labeling and promotional activities are subject to scrutiny by the FDA and, in certain instances, by the Federal Trade Commission. Failure to comply with these requirements could result in enforcement action, including seizure, injunction, prosecution, civil penalties, recall and suspension of FDA approval.
The sale and advertising of medical devices in Canada are governed by the Food and Drugs Act (Canada) through the Medical Devices Regulations, administered by the Medical Devices Bureau of Health Canada (MDB). The current Medical Devices Regulations are undergoing revisions that may align the Canadian regulatory process with those of Canada's international trading partners. We believe that international harmonization of the regulatory process will be more likely to accelerate than slow the approval process as it relates to the Novacor II and the MedQuest rotary VAD.
The Novacor II and the MedQuest rotary VAD are expected to be classified as Class IV medical devices under the Medical Devices Regulations, requiring WorldHeart to apply for authorization from the MDB to conduct investigational testing on human subjects in Canada. At the conclusion of the human clinical trials, we will apply for a medical device license that will allow for general marketing of the device.
It is also our intention to market the Novacor II and the MedQuest rotary VAD in European and other countries. We will be required to meet the applicable medical devices standards in each such country or region. Although harmonization has been under negotiation for some time among various countries, the approval process varies from country to country and approval in one country does not necessarily result in approval in another.
We intend to apply for various applicable certifications under the International Standards Organization (ISO), a worldwide federation of national bodies, founded in Geneva, Switzerland in 1946. ISO has over 92 member countries, including the United States and Canada. ISO standards are integrated requirements which, when implemented, form the foundation and framework for an effective quality management system. These standards were developed and published by the ISO. ISO certification is widely regarded as essential to enter Western European markets. All companies are required to obtain ISO certification and the "CE" mark, an international symbol of quality and compliance, in order to market medical devices in Europe. ISO 13485 certification is the most stringent standard in the ISO series and covers design, production, installation and servicing of products. WorldHeart received ISO 13485 certification in 2003. WorldHeart has received "CE" mark certification for the Novacor LVAS, with applicable European medical device directives.
25
We are also subject to various Canadian and/or United States federal, provincial, state and local laws and regulations relating to such matters as safe working conditions, laboratory and manufacturing practices, and the use, handling and disposal of hazardous or potentially hazardous substances used in connection with WorldHeart's research, development and production work. The manufacture of biomaterials is also subject to compliance with various federal environmental regulations and/or those of various provincial, state and local agencies. Although we believe that we are in compliance with these laws and regulations in all material respects, there can be no assurance that we will not be required to incur significant cost to comply with environmental and health and safety regulations in the future.
Our Employees
At June 10, 2005, we employed 108 full-time staff and consultants. Of these employees, three were employed in Ottawa, Ontario, 98 in Oakland, California, and 7 in Heesch, Netherlands. Approximately 68% of our employees were involved with research, development, manufacturing, quality, and clinical affairs, 12% were in sales and clinical support, and 20% were in finance, administration, and regulatory affairs. In January 2004, we assumed the sales and sales support employees in the Netherlands' office who previously were employed by Edwards.
The completion of the MedQuest Acquisition will result in the addition of approximately 24 employees at a facility located in Salt Lake City, Utah.
We currently maintain compensation, benefits, equity participation and work environment policies intended to assist in attracting and retaining qualified personnel.
We believe that the success of our business will depend, to a significant extent, on our ability to attract and retain such personnel. We have access to highly-skilled labour pools in Oakland where there are well-developed technology industries.
None of our employees is subject to a collective bargaining agreement nor have we experienced any work stoppages. We believe that our relations with our employees are good.
Our Facilities
Our registered office is located in Ottawa, Ontario. Our facilities in Ottawa comprise 22,755 square feet of manufacturing and office space with a lease that expires on December 31, 2006. We have negotiated an assignment of the office space for July 1, 2005 and we will maintain only a sales function in Canada. As part of the consolidation of our operations which commenced in August 2004, we have moved our head office to Oakland, California where we have two buildings and approximately 40,000 square feet of manufacturing and office space. This space was obtained as part of the Novacor acquisition and continues to be the sole manufacturing facility for the Novacor LVAS. The Oakland lease expires on April 30, 2007. Our office in Heesch, Netherlands consists of approximately 2,500 square feet under a lease expiring December 31, 2007. Upon completion of the acquisition of MedQuest, we will also have facilities in Salt Lake City, Utah totalling approximately 24,000 square feet.
We are not aware of any environmental issues that may affect the use of our properties.
Annual and Quarterly Information
The following tables present selected historical consolidated financial data of WorldHeart under accounting principles generally accepted in the United States ("U.S. GAAP") and Canada ("Canadian GAAP"). The selected balance sheet data as at December 31, 2003 and 2004 and March 31, 2005 and the selected income data for the historical years ended December 31, 2002, 2003 and 2004 and the three month period ended March 31, 2005 have been derived from WorldHeart's audited consolidated financial statements attached as Appendices B-1 and B-2 to this circular and WorldHeart's interim unaudited consolidated financial statements for the three months ended March 31, 2005 attached as Appendix B-3 to this circular. The differences between accounting principles generally accepted in Canada and the United States as they relate to WorldHeart are set out in Note 22 to the U.S. GAAP audited consolidated financial statements in Appendix B-1 and Note 6 to the interim unaudited consolidated financial statements in Appendix B-3. The selected balance sheet data as at December 31, 2000, 2001 and 2002 and income data for the historical years ended December 31, 2000 and 2001 have been derived from Canadian GAAP audited consolidated financial statements which were reported on in Canadian dollars, that are not included in this
26
circular but which may be obtained in the manner described under the heading "ADDITIONAL INFORMATION" at page 94 of this circular.
When you read this selected historical consolidated financial data, it is important that you read the historical consolidated financial statements and related notes in our annual, quarterly and current reports filed with the SEC in the United States and provincial securities regulatory agencies in Canada, as well as the section of our annual, quarterly and current reports titled "Management's Discussion and Analysis of Financial Condition and Results of Operations".
Summary of Annual Results — U.S. GAAP
| | Year Ended December 31,
| |
|---|
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| Net revenue | | $ | 9,575,761 | | $ | 6,755,807 | | $ | 6,436,696 | | $ | 5,329,357 | | $ | 3,147,950 | |
| Net loss for the year | | | (26,141,798 | ) | | (14,629,054 | ) | | (24,552,673 | ) | | (35,012,010 | ) | | (26,210,754 | ) |
| Net loss applicable to common shareholders | | | (26,141,798 | ) | | (18,168,304 | ) | | (29,010,753 | ) | | (39,578,696 | ) | | (28,284,926 | ) |
| Basic and diluted loss per common share | | | (1.70 | ) | | (2.90 | ) | | (11.42 | ) | | (18.39 | ) | | (13.31 | ) |
| | As at December 31,
|
|---|
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| Total assets | | $ | 42,258,430 | | $ | 50,180,069 | | $ | 27,739,896 | | $ | 46,060,330 | | $ | 72,138,615 |
| Long term liabilities | | | 8,193,508 | | | — | | | 2,167,860 | | | 2,187,246 | | | 3,529,947 |
Summary of Annual Results — Canadian GAAP
| | Year Ended December 31,
| |
|---|
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| Net revenue | | $ | 9,575,761 | | $ | 6,755,807 | | $ | 6,436,696 | | $ | 5,329,357 | | $ | 3,147,950 | |
| Net loss for the year | | | (22,806,805 | ) | | (23,547,313 | ) | | (31,690,859 | ) | | (42,405,977 | ) | | (20,462,528 | ) |
| Net loss applicable to common shareholders | | | (22,806,805 | ) | | (25,471,708 | ) | | (31,690,859 | ) | | (42,405,977 | ) | | (20,462,528 | ) |
| Basic and diluted loss per common share | | | (1.48 | ) | | (4.07 | ) | | (12.48 | ) | | (19.70 | ) | | (9.63 | ) |
| | As at December 31,
|
|---|
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| Total assets | | $ | 42,258,430 | | $ | 50,180,069 | | $ | 28,553,444 | | $ | 48,896,985 | | $ | 77,309,002 |
| Long term liabilities | | | 2,547,027 | | | — | | | 46,114,470 | | | 41,278,601 | | | 40,191,228 |
Summary of Quarterly Unaudited Results — U.S. GAAP
| | Three Months Ended
| |
|---|
| | March 31,
2005
| |
|---|
| Net revenue | | $ | 3,416,988 | |
| Net loss for the period | | | (3,942,396 | ) |
| Net loss applicable to common shareholders | | | (3,942,396 | ) |
| Basic and diluted loss per common share | | | (0.24 | ) |
| | Three Months Ended
| |
|---|
| | December 31,
2004
| | September 30,
2004
| | June 30,
2004
| | March 31,
2004
| |
|---|
| Net revenue | | $ | 2,986,980 | | $ | 2,250,050 | | $ | 2,146,388 | | $ | 2,192,343 | |
| Net loss for the period | | | (6,293,510 | ) | | (10,935,893 | ) | | (5,402,792 | ) | | (3,509,603 | ) |
| Net loss applicable to common shareholders | | | (6,293,510 | ) | | (10,935,893 | ) | | (5,402,792 | ) | | (3,509,603 | ) |
| Basic and diluted loss per common share | | | (0.41 | ) | | (0.71 | ) | | (0.35 | ) | | (0.23 | ) |
27
| | Three Months Ended
| |
|---|
| | December 31,
2003
| | September 30,
2003
| | June 30,
2003
| | March 31,
2003
| |
|---|
| Net revenue | | $ | 559,704 | | $ | 2,023,170 | | $ | 1,944,702 | | $ | 2,248,231 | |
| Net loss for the period | | | (4,846,928 | ) | | (8,125,804 | ) | | (95,642 | ) | | (1,560,680 | ) |
| Net loss applicable to common shareholders | | | (6,864,152 | ) | | (9,222,682 | ) | | (308,212 | ) | | (1,773,258 | ) |
| Basic and diluted loss per common share | | | (0.47 | ) | | (2.24 | ) | | (0.10 | ) | | (0.60 | ) |
Summary of Quarterly Unaudited Results — Canadian GAAP
| | Three Months Ended
| |
|---|
| | March 31,
2005
| |
|---|
| Net revenue | | $ | 3,416,988 | |
| Net loss for the period | | | (4,547,982 | ) |
| Net loss applicable to common shareholders | | | (4,547,982 | ) |
| Basic and diluted loss per common share | | | (0.27 | ) |
| | Three Months Ended
| |
|---|
| | December 31,
2004
| | September 30,
2004
| | June 30,
2004
| | March 31,
2004
| |
|---|
| Net revenue | | $ | 2,986,980 | | $ | 2,250,050 | | $ | 2,146,388 | | $ | 2,192,343 | |
| Net loss for the period | | | (6,352,323 | ) | | (6,324,604 | ) | | (6,002,772 | ) | | (4,127,106 | ) |
| Net loss applicable to common shareholders | | | (6,352,323 | ) | | (6,324,604 | ) | | (6,002,772 | ) | | (4,127,106 | ) |
| Basic and diluted loss per common share | | | (0.41 | ) | | (0.41 | ) | | (0.39 | ) | | (0.27 | ) |
| | Three Months Ended
| |
|---|
| | December 31,
2003
| | September 30,
2003
| | June 30,
2003
| | March 31,
2003
| |
|---|
| Net revenue | | $ | 559,704 | | $ | 2,023,170 | | $ | 1,944,702 | | $ | 2,248,231 | |
| Net loss for the period | | | (5,170,572 | ) | | (9,358,182 | ) | | (2,896,337 | ) | | (6,122,222 | ) |
| Net loss applicable to common shareholders | | | (7,094,967 | ) | | (9,358,182 | ) | | (2,896,337 | ) | | (6,122,222 | ) |
| Basic and diluted loss per common share | | | (0.48 | ) | | (2.27 | ) | | (0.92 | ) | | (2.07 | ) |
Selected Historical Financial Data of MedQuest Products, Inc.
The following tables present selected historical financial data of the MedQuest business under accounting principles generally accepted in the United States and Canada. The selected balance sheet data and the selected income data as at and for the years ended June 30, 2003 and 2004 have been derived from MedQuest audited financial statements which are attached to this circular as Appendix D. The selected balance sheet data and the selected income data as at and for the nine months ended March 31, 2004 and 2005 have been derived from MedQuest financial statements included in Appendix D and are unaudited. In the opinion of MedQuest management, all interim data includes necessary adjustments for a fair statement of the results for the interim periods. The differences between accounting principles generally accepted in Canada and the United States as they relate to MedQuest are set out in Note 14 to MedQuest's audited financial statements. The selected balance sheet data and income data as at and for the historical years ended June 30, 2001, 2002 and 2003 have been derived from audited financial statements that are not included in this circular. MedQuest Canadian GAAP financial data is unavailable on a quarterly basis and for annual periods prior to 2003.
When you read this selected historical financial data, it is important that you read the historical consolidated financial statements and related notes in the audited financial statements (see Appendix D) and the report titled "Management's Discussion and Analysis of Financial Condition and Results of Operations" (see Appendix E).
28
Summary of Annual Results and Interim Period to March 31, 2005 — U.S. GAAP
| | Nine Months Ended March 31,
| | Year Ended June 30,
| |
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
| |
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
| |
|---|
| Net revenue | | $ | 101,840 | | $ | 99,389 | | $ | — | | $ | 304,149 | | $ | — | | $ | — | |
| Net loss for the period | | | (3,077,030 | ) | | (2,232,937 | ) | | (3,777,799 | ) | | (4,650,433 | ) | | (1,875,889 | ) | | (40,104 | ) |
| | As at March 31,
| | As at June 30,
|
|---|
| | 2005
| | 2004
| | 2003
| | 2002
| | 2001
| | 2000
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | | $ | 1,717,199 | | $ | 4,131,038 | | $ | 409,074 |
| Long term liabilities | | | 110,114 | | | 111,193 | | | 157,948 | | | 221,240 | | | 46,799 | | | 3,900 |
Summary of Annual Results and Interim Period to March 31, 2005 — Canadian GAAP
| | Nine Months Ended March 31,
| | Year Ended June 30,
| |
| |
| |
|
|---|
| | 2005
| | 2004
| | 2003
| |
| |
| |
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Net revenue | | $ | 101,840 | | $ | 99,389 | | $ | — | | | | | | |
| Net loss for the period | | | (3,207,773 | ) | | (2,573,702 | ) | | (4,238,997 | ) | | | | | |
| | As at March 31,
| | As at June 30,
| |
| |
| |
|
|---|
| | 2005
| | 2004
| | 2003
| |
| |
| |
|
|---|
| | (Unaudited)
| |
| |
| |
| |
| |
|
|---|
| Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | | | | | | |
| Long term liabilities | | | 110,114 | | | 111,193 | | | 157,948 | | | | | | |
Summary of Quarterly Unaudited Results — U.S. GAAP
| | Three Months Ended
| |
|---|
| | March 31,
2005
| |
|---|
| Net revenue | | $ | 61,619 | |
| Gross margin | | | 61,619 | |
| Net loss | | | (1,214,100 | ) |
| | Three Months Ended
| |
|---|
| | March 31,
2004
| | June 30,
2004
| | September 30,
2004
| | December 31,
2004
| |
|---|
| Net revenue | | $ | 63,625 | | $ | 35,764 | | $ | — | | $ | 40,221 | |
| Gross margin | | | 63,625 | | | 35,764 | | | — | | | 40,221 | |
| Net loss | | | (221,411 | ) | | (794,745 | ) | | (658,386 | ) | | (1,204,544 | ) |
| | Three Months Ended
| |
|---|
| | March 31,
2003
| | June 30,
2003
| | September 30,
2003
| | December 31,
2003
| |
|---|
| Net revenue | | $ | — | | $ | — | | $ | — | | $ | — | |
| Gross margin | | | — | | | — | | | — | | | — | |
| Net loss | | | (921,947 | ) | | (812,309 | ) | | (677,639 | ) | | (539,141 | ) |
29
| | Three Months Ended
| |
|---|
| | September 30,
2002
| | December 31,
2002
| |
|---|
| Net revenue | | $ | — | | $ | — | |
| Gross margin | | | — | | | — | |
| Net loss | | | (903,004 | ) | | (1,140,539 | ) |
MANAGEMENT'S DISCUSSION & ANALYSIS
WorldHeart's management's discussion and analysis of financial condition and results of operations for the past two years are attached hereto as Appendices C-1 and C-2 and WorldHeart's management's discussion and analysis of financial condition and results of operations for the three months ended March 31, 2005 is attached hereto as Appendix C-3.
MedQuest's management's discussion and analysis of financial condition and results of operations for the past two years and the interim nine months to March 31, 2005 are attached hereto as Appendix E.
SELECTED PRO FORMA INFORMATION
Selected Unaudited Pro Forma Consolidated Financial Information
On January 31, 2005, WorldHeart entered into a definitive agreement to acquire all of the assets of MedQuest for 9,300,000 common shares of WorldHeart, subject to certain consents, conditions and shareholder approval. In addition, Maverick has agreed to fund MedQuest operations until the closing of the transaction at which point WorldHeart will assume the resulting net liability. In connection with the acquisition of MedQuest, WorldHeart has entered into a private placement agreement with Maverick. At the closing of the acquisition, Maverick will purchase 8,888,889 common shares of WorldHeart at a purchase price of $1.35 per common share. See "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest" at page 66 and "— Maverick Resolution — Private Placement to Maverick" at page 78.
In addition, as a condition of this transaction, investors that participated in WorldHeart's September 2004 convertible debenture transaction have agreed to convert any remaining debentures at their stated conversion price of $1.25 per common share into common shares. The accrued interest will be converted into common shares at a conversion price equal to the weighted average trading price on NASDAQ for the five days preceding the date of conversion. In addition, the holders of warrants issued in September 2004 may exercise their warrants into 10,655,000 common shares of WorldHeart at an adjusted exercise price of $1.00 per common share. The warrants are currently exercisable until September 2009 at a price of $1.55 per common share. WorldHeart is seeking shareholder approval for a $0.55 reduction in the warrant exercise price. See "MATTERS TO BE ACTED UPON — Warrant Amendment Resolution — Amendment to September 2004 Warrants" at page 84.
The following table presents selected unaudited pro forma consolidated financial data of WorldHeart and the MedQuest business for the year ended December 31, 2004 and as at and for the three months ended March 31, 2005. The data has been prepared giving effect to the probable acquisition under the purchase method of accounting as if it occurred prior to January 1, 2004 for the pro forma consolidated, condensed statement of operations and as at December 31, 2004 for the consolidated, condensed proforma balance sheet under both U.S. GAAP and Canadian GAAP, using a measurement date for the acquisition of January 31, 2005. This information should be read in conjunction with the unaudited pro forma combined condensed financial statements and related notes attached as Appendix F to this circular. The selected unaudited pro forma combined condensed financial data is presented for illustrative purposes only and is not necessarily indicative of the operating results or financial position that would have been achieved had the acquisition been consummated as of dates indicated or that may be achieved in the future.
The selected unaudited pro forma combined condensed financial information should be read in conjunction with the audited consolidated financial statements and related notes of WorldHeart and MedQuest attached to
30
this circular as Appendices B-1, B-2 and D and the interim unaudited consolidated financial statements of WorldHeart attached to this circular as Appendix B-3.
| | Pro-forma
U.S. GAAP
Three Months Ended
March 31, 2005
| | Pro-forma
U.S. GAAP
Year Ended
December 31, 2004
| | Pro-forma
Canadian GAAP
Year Ended
December 31, 2004
| |
|---|
| | (Unaudited)
| | (Unaudited)
| | (Unaudited)
| |
|---|
| Net revenue | | $ | 3,416,988 | | $ | 9,575,761 | | $ | 9,575,761 | |
| Net loss for the period | | | (4,797,650 | ) | | (28,626,183 | ) | | (26,514,976 | ) |
| Loss applicable to common shareholders | | | (4,797,650 | ) | | (28,626,183 | ) | | (26,514,976 | ) |
| Basic and diluted loss per common share | | | (0.09 | ) | | (0.52 | ) | | (0.48 | ) |
| | Pro-forma
As at
March 31, 2005
| | Pro-forma
As at
December 31, 2004
|
|---|
| | (Unaudited)
| | (Unaudited)
|
|---|
| Total assets | | $ | 61,506,468 | | $ | 77,012,518 |
| Long term obligations | | | 118,944 | | | 126,482 |
| Book value per common share | | | 0.92 | | | 1.24 |
SHARE CAPITAL AND PRIOR SALES
The authorized share capital of WorldHeart consists of an unlimited number of common shares without par value and an unlimited number of preferred shares without par value, issuable in series. As of May 20, 2005, there are 17,101,400 common shares outstanding.
The common shares entitle the holders thereof to one vote per share at meetings of the shareholders of WorldHeart, except meetings at which only the holders of another class or series of shares are entitled to vote and, subject to the prior rights of holders of any preferred shares, to receive any dividends declared by the board of directors and to receive the property of WorldHeart upon liquidation, dissolution or winding up. All outstanding common shares are fully paid and non-assessable.
On November 25, 2003, the shareholders of WorldHeart approved an amendment to the Articles of Incorporation of WorldHeart to consolidate our common shares on the basis of one new common share for seven existing common shares. On December 1, 2003, WorldHeart filed articles of amendment effecting the share consolidation. The numbers presented in this section are presented with the numbers of common shares and common share prices adjusted retroactively to reflect the consolidation of common shares on a one-for-seven basis.
Common shares issued during the past three fiscal years and the current fiscal year to date are described below:
2002
On December 19, 2001, we completed a private placement of 432,429 special warrants at a per unit price of Cdn$38.50, for total net proceeds of Cdn$14,886,649 after deducting issue costs of Cdn$1,761,851. Each special warrant was convertible into one common share and one warrant to purchase a common share for a period of two years at a price of Cdn$42.00 per common share. In January 2002, the special warrants were converted into an equivalent number of common shares and warrants.
2003
In January 2003, we completed a private placement of units for total net proceeds of Cdn$2,615,272 after deducting issue costs of Cdn$384,728. A total of 334,821 common shares and warrants to purchase 334,821 common shares for a period of five years at a price of Cdn$11.20 per common share were issued as result of this transaction.
On January 31, 2003, and pursuant to the terms of an agreement dated December 19, 2001 between us and 2007262 Ontario Inc., New Generation Biotech (Equity) Fund Inc. exchanged Series 1 preferred shares of 2007262 Ontario Inc. for 91,000 common shares of WorldHeart and 91,000 warrants of WorldHeart at an exercise price of
31
Cdn$42.07 per common share expiring January 24, 2004. Net proceeds to us totaled Cdn$3,420,016 after deducting issue costs of Cdn$83,485.
On April 2, 2003, we issued 142,857 common shares at a price of Cdn$11.20 per common share for net proceeds of Cdn$1,568,242 after deducting transaction costs of Cdn$31,758, pursuant to the exercise of previously issued warrants with an expiry date of January 2, 2008.
On September 23, 2003, we completed a private placement of units, for total net proceeds of Cdn$56,800,000 after deducting issue costs of Cdn$6,692,980. A total of 10,671,338 common shares and warrants to acquire an equivalent number of shares were issued as a result of this transaction.
On September 22, 2003, we issued 711,589 common shares in exchange for 4,981,128 Series A cumulative participating preferred shares of World Heart Inc. held by Edwards.
On November 27, 2003, we issued 500,000 common shares and 1,000,000 warrants, exercisable at Cdn$8.05 per share expiring November 27, 2008 to Edwards Lifesciences (U.S.) Inc. on conversion of 1,374,750 Series A Shares of WorldHeart.
On December 12, 2003, we issued 5,000 common shares on the exercise of warrants at a price of Cdn$8.40 per common share, for total gross proceeds of Cdn$42,000.
2004
On March 3, 2004, we issued 250,000 common shares on the exercise of warrants at a price of Cdn$8.05 per common share, for total gross proceeds of Cdn$2,012,500.
On September 15, 2004, we issued 470,833 common shares as agent's commission relating to the sale of convertible debentures and warrants.
2005
From January 20, 2005 to June 10, 2005, we issued a total of 1,356,836 common shares upon the conversion of an aggregate of $1,581,779 principal amount of debentures and accrued interest thereon.
The board of directors has the authority to issue an unlimited number of preferred shares, issuable in series, and to determine prior to any such issuance the price, rights, preferences, privileges and restrictions, including voting rights, of each series of those shares without any further vote or action by the shareholders. Preferred shares may, at the discretion of the board of directors, be entitled to preference over the common shares and any other shares ranking junior to the preferred shares with respect to the payment of dividends and distribution of assets in the event of liquidation, dissolution or winding up. If any cumulative dividends or amounts payable on return of capital are not paid in full, preferred shares of all issued series would participate rateably in accordance with the amounts that would be payable on such shares if all such dividends were declared and paid in full or the sums which would be payable on such shares on the return of capital if all amounts so payable were paid in full, as the case may be. As of April 28, 2005, there is no series of preferred shares authorized and available to be issued.
On May 24, 2000, WorldHeart and Edwards entered into an exchange agreement pursuant to which Edwards received the right for one year following June 30, 2002 to require WorldHeart to exchange 4,981,128 Series A cumulative participating preferred shares of World Heart Inc. issued to it on June 30, 2000 for an equal number of WorldHeart pre-consolidation common shares. On May 8, 2002, WorldHeart and Edwards amended the exchange agreement to extend the period of time within which Edwards could require the exchange of shares to two years and to extend the commencement date of the exchange window to June 30, 2003. As consideration for the extension of this period, Edwards agreed that the dividend payable on its preferred shares of World Heart Inc. would be decreased by one percent effective July 1, 2002.
On June 30, 2000, we issued to Edwards Lifesciences (U.S.) Inc. for $20.0 million Series A Shares convertible at Edwards Lifesciences (U.S.) Inc.'s option after June 30, 2006 into 1,374,570 pre-consolidation common shares (representing a per share pre-consolidation conversion price of $14.55). These preferred shares were non-voting except that Edwards could nominate one director of WorldHeart. These shares were callable for cash at the face amount plus accumulated but unpaid dividends at our option at any time up to June 30, 2007, at which time they were to be mandatorily redeemable for the face amount of $20 million plus accumulated dividends. Dividends accumulated at 5% per year for the first three years and 10% per year for years four through seven.
On September 22, 2003, WorldHeart and Edwards amended the exchange agreement to shorten the commencement date of the exchange window by one year and Edwards exchanged 4,981,128 Series A cumulative participating preferred shares of World Heart Inc. for 711,589 common shares on that date.
32
On September 22, 2003, we entered into a conversion agreement with Edwards Lifesciences (U.S.) Inc. that provided that, subject to shareholder approval, WorldHeart would acquire for cancellation the 1,374,570 Series A Shares of WorldHeart held by Edwards Lifesciences (U.S.) Inc. by issuing to Edwards Lifesciences (U.S.) Inc. upon the conversion of those shares, 500,000 common shares of WorldHeart and 1,000,000 warrants, each exercisable for one common share of WorldHeart at a price of Cdn$8.05 per share, which was adjusted to Cdn$7.49 at the time of the September financing.
On November 25, 2003, WorldHeart's shareholders approved an amendment to the articles of incorporation of WorldHeart to permit the conversion of the 1,374,570 Series A Shares of WorldHeart held by Edwards Lifesciences (U.S.) Inc. into 500,000 common shares and warrants to acquire 1,000,000 common shares as described above, expiring November 27, 2008. Further to the approval of the shareholders, on November 26, 2003, WorldHeart filed articles of amendment to amend the rights and privileges of the Series A Shares and on November 27, 2003, Edwards Lifesciences (U.S.) Inc. converted its 1,374,570 Series A shares into 500,000 common shares and the warrants to acquire 1,000,000 common shares as described above.
To date, we have not paid any dividends on our common shares. Our policy at the present time is to retain earnings for corporate purposes. The payment of dividends in the future will depend on the earnings and financial condition of WorldHeart and on such other factors as the board of directors may consider appropriate.
The following table sets out information as to options to purchase securities of WorldHeart that are held as of June 10, 2005:
Options to Purchase Securities as of June 10, 2005
|
|---|
Number of
Option Holders
| | Designation
and Number
of Securities
Under Option
| | Purchase Price
of Securities (or formula for calculating purchase price)
| | Expiration Date
| | Market Value of Securities Under Option as at Date of Grant
(if ascertainable)
| | Current Market Value of Securities Under Option
(if ascertainable)
|
|---|
| Aggregate past and present executive officers — 16 | | 3,514,733 | (1) | $1.12 to $49.70 | | November 1, 2005 to
January 31, 2014 | | $ | 6,404,082 | | $ | 4,006,796 |
| Aggregate past and present non-executive directors — 10 | | 608,951 | (1) | $1.12 to $29.05 | | March 31, 2006 to January 31, 2014 | | $ | 839,538 | | $ | 694,204 |
| Aggregate past and present employees not counted above — 328 | | 1,463,096 | (1) | $1.02 to $105.43 | | April 1, 2005 to January 31, 2014 | | $ | 6,019,865 | | $ | 1,667,929 |
Aggregate
consultants — 48 | | 88,556 | (1) | $1.12 to $88.55 | | July 14, 2005 to January 31, 2014 | | $ | 521,990 | | $ | 100,954 |
Aggregate future employees and consultants conditional on the closing of the MedQuest
Acquisition — 25(2) | | 1,309,500 | (1) | $1.12 | | January 31, 2012 to January 31, 2014 | | $ | 1,938,060 | | $ | 1,492,830 |
- (1)
- Includes options granted to employees, consultants, executive officers and directors that are subject to shareholder approval at this Annual and Special Meeting.
- (2)
- Grants of options to employees and consultants of MedQuest are conditional on the closing of the MedQuest Acquisition and such individuals commencing employment or consulting arrangements with WorldHeart.
33
CORPORATE GOVERNANCE
Information about the Board of Directors and its Committees
The board of directors of WorldHeart currently has four committees: the Compensation Committee; the Audit Committee; the Corporate Governance and Nominating Committee and the Strategic Planning Committee. Each of these committees has a charter which sets out its governance practices. The charters provide the board of directors with the necessary authority and practices to review and evaluate WorldHeart's business operations and make decisions that are independent of WorldHeart's management. The charters establish the practices and guidelines that the committees of the board of directors will follow with respect to board and committee composition, meetings of the committees, involvement of senior management, chief executive officer and management team evaluation, succession planning and director and management compensation.
The committee charters are reviewed periodically and updated as necessary to reflect changes in regulatory requirements and evolving oversight practices.
The Audit Committee consists of John F. Carlson (Chair), C. Ian Ross and William C. Garriock. The Compensation Committee consists of Robert J. Majteles (Chair), C. Ian Ross and William C. Garriock. The Corporate Governance and Nominating Committee consists of C. Ian Ross (Chair), John F. Carlson and William Garriock. The Strategic Planning Committee consists of William C. Garriock (Chair) and all of the other directors and Jal S. Jassawalla, President and Chief Executive Officer. With the exception of the Strategic Planning Committee, each of these committees is comprised solely of outside, unrelated and independent directors as determined in accordance with stock exchange and U.S. and Canadian regulatory requirements.
The Audit Committee assists the board of directors in its oversight of the quality and integrity of the accounting, auditing and reporting practices of WorldHeart. The Audit Committee's role includes overseeing WorldHeart's internal accounting and auditing processes and discussing with WorldHeart's management the processes to manage the business, financial risk and compliance with applicable legal, ethical and regulatory requirements. The Committee is responsible for reviewing all financial continuous disclosure filings of WorldHeart including press releases with respect to earnings and related matters. The Audit Committee is responsible for the appointment, compensation, retention, and oversight of the independent auditor engaged to prepare and issue audit reports on the financial statements of WorldHeart. The Committee also approves all non-audit expenditures. The Audit Committee relies on the expertise and knowledge of management and the independent auditor in carrying out its oversight responsibilities. The board of directors has determined that each member of the Audit Committee has sufficient knowledge in financial and auditing matters to serve on the Audit Committee. In addition, the board of directors has determined that John F. Carlson is an "audit committee financial expert" as defined by the SEC rules. The specific responsibilities and functions of the Audit Committee are delineated in the Amended and Restated Audit Committee Charter, a copy of which has been filed with securities regulatory authorities.
The principal responsibilities of the Compensation Committee are: (i) to review, approve and recommend to the board of directors the annual goals and objectives of the president and chief executive officer of WorldHeart and to evaluate performance against those goals and objectives; (ii) to approve and recommend the compensation of the president and chief executive officer; (iii) to oversee the performance evaluation of WorldHeart's other executive officers and approve and recommend their compensation; (iv) to oversee and advise the board of directors on the adoption of policies that govern WorldHeart's compensation programs; (v) to oversee the administration of WorldHeart's equity-based compensation and other benefit plans, and (vi) to approve grants of equity compensation awards under WorldHeart's employee stock option plan. The Compensation Committee's role includes producing the report on executive compensation required to be included in the circular. The specific responsibilities and functions of the Compensation Committee are delineated in the Amended and Restated Compensation Committee Charter.
34
The principal responsibilities of the Corporate Governance and Nominating Committee are: (i) to determine the slate of director nominees for election to the WorldHeart board of directors; (ii) to identify and recommend candidates to fill vacancies that occur between annual shareholder meetings; (iii) to review the composition of the committees of the board of directors; and (iv) to monitor compliance with, review and recommend changes to WorldHeart's compliance with corporate governance regulatory requirements including issues of significance to WorldHeart and its stakeholders. The Corporate Governance and Nominating Committee reviews all continuous disclosure documents that are filed by WorldHeart. The Corporate Governance and Nominating Committee annually reviews with the board of directors the applicable skills and characteristics required of board of director nominees in the context of current board of director composition and WorldHeart's current circumstances. The Committee considers the qualifications, background and experience of each of the directors. The specific responsibilities and functions of the Corporate Governance and Nominating Committee are delineated in the Corporate Governance and Nominating Committee Charter.
The Committee will consider shareholder recommendations for candidates for the board. The name of any recommended candidate for director, together with a brief biographical sketch, a document indicating the candidate's willingness to serve, if elected, and evidence of the nominating shareholder's ownership of WorldHeart common shares should be sent to the attention of the Corporate Governance and Nominating Committee.
The Strategic Planning Committee's overall responsibility is to assist the board of directors in its long range financial and strategic planning efforts. The primary responsibilities of the Strategic Planning Committee are (i) to make choices on strategic direction of WorldHeart; (ii) to focus the financial resources of WorldHeart on program initiatives which have the greatest opportunity for technical, commercial and financial success based on the markets and the factors required to succeed in those markets; and (iii) to identify the resources required to ensure a successful implementation of WorldHeart's strategic plan. The specific responsibilities and functions of the Strategic Planning Committee are delineated in the Strategic Planning Committee Charter.
Toronto Stock Exchange Corporate Governance Practices
The board of directors and management of WorldHeart consider good corporate governance to be essential for the effective and efficient operation of WorldHeart. The TSX has issued a series of guidelines for effective corporate governance and has adopted as a listing requirement the disclosure by each listed corporation of its approach to corporate governance with reference to the guidelines. These guidelines are expected to be replaced by a policy and a disclosure rule in all Canadian provinces, published for comment in October 2004 by the Canadian Securities Administrators. Each of the TSX's current guidelines is presented below in bold with WorldHeart's applicable corporate governance practices disclosed thereafter.
- 1.
- The board of directors of every corporation should explicitly assume responsibility for the stewardship of the corporation and, as part of the overall stewardship responsibility, should assume responsibility for the following matters:
The board of directors, either directly or through its committees, supervises the management and business affairs of WorldHeart. It has the statutory authority and obligation to protect and enhance the assets of WorldHeart in the interests of the shareholders. The board of directors met 16 times during the year ended December 31, 2004.
- a)
- adoption of a strategic planning process;
The Strategic Planning Committee of the board of directors was created on December 3, 2003. All directors of WorldHeart and the President and Chief Executive Officer are members of the Committee. The mandate of the Strategic Planning Committee, as more specifically set out in its Charter, is to review the long-range financial and strategic planning efforts of WorldHeart as well as to focus on strategic direction, development and successful implementation of WorldHeart's strategic plan. The Committee held its first meeting in May 2004.
35
The board of directors revised the 2004 strategic plan for WorldHeart in August 2004 after the appointment of Jal S. Jassawalla as President and Chief Executive Officer of WorldHeart. In December 2004, the board of directors adopted a new strategic plan which included the possible acquisition of MedQuest and the transformation of WorldHeart into a platform company. As part of the integration of MedQuest, assuming approval by the shareholders and completion of all the conditions to closing, the Strategic Planning Committee will meet following closing to discuss the overall implementation of the strategic plan with the inclusion of the MedQuest operations.
- b)
- the identification of the principal risks of the corporation's business and ensuring the implementation of appropriate systems to manage these risks;
The board of directors in its deliberations considers the principal risks of WorldHeart's business and receives reports from management on WorldHeart's assessment and management of those risks. Management and the board of directors are in the process of evaluating our current risk environment and, where necessary, implementing new systems to manage the risks of WorldHeart. In addition, management and the Audit Committee are in the process of evaluating internal control environment and associated risks. This work is expected to continue through 2005 and into 2006. Management has advised the board of directors that enhancements will be made to certain systems or new systems will be introduced in response to any control weaknesses that are identified to ensure that WorldHeart adequately manages key risks and complies with all regulatory requirements in Canada and the United States.
- c)
- succession planning, including appointing, training and monitoring senior management;
The Compensation Committee has responsibility for succession planning and appointing and monitoring senior management. WorldHeart uses a performance management system to monitor performance of senior management and the Compensation Committee has instituted a process to review those metrics on a quarterly basis against WorldHeart's overall objectives. The board of directors encourages senior management to participate in appropriate professional and personal development activities, courses and programs and supports management's commitment to the training and development of all employees. The Strategic Planning Committee also considers aspects of succession planning.
In 2004, the Compensation Committee oversaw several changes in the WorldHeart senior management team including the appointment of Jal S. Jassawalla as the President and Chief Executive Officer of WorldHeart. As part of the Compensation Committee's mandate, the Committee has assisted, and continues to assist, the WorldHeart management team in finding suitable replacements for senior management positions which have arisen or are arising as a result of the consolidation of WorldHeart's operations to Oakland, California. WorldHeart has hired a Director of Human Resources and has been interviewing possible candidates for the positions of Vice President, Manufacturing and Vice President, Finance and Chief Financial Officer.
- d)
- a communications policy for the corporation; and
WorldHeart has maintained a Communications Policy since 1996 that includes a series of requirements to ensure effective, accurate, regular and timely communications between WorldHeart, its shareholders, potential investors and the general public. The Audit Committee reviews all press releases and information with respect to financial information before such information is released.
WorldHeart reviews this Communications Policy annually to ensure it is current in all respects. Under this Communications Policy, the principal spokesperson for WorldHeart is the President and Chief Executive Officer.
- e)
- the integrity of the corporation's internal control and management information systems.
The Audit Committee is responsible for assessing and reporting to the board of directors on the integrity of WorldHeart's internal control and management information systems. The Audit Committee is responsible for reviewing the financial statements of WorldHeart and such other
36
- 2.
- The board of directors of every corporation should be constituted with a majority of individuals who qualify as "unrelated" directors (free from conflicting interests).
The board of directors currently consists of five directors, including four unrelated directors within the meaning of the TSX guidelines. Each is independent from management and is free from any interest and any business or other relationship, other than interests and relationships arising from shareholding, which could interfere, or could reasonably be perceived to interfere, materially with the director's ability to act with a view to the best interests of WorldHeart. The four unrelated directors are:
- •
- C. Ian Ross
- •
- John F. Carlson
- •
- William C. Garriock
- •
- Robert J. Majteles
- (1)
- Mr. Roderick M. Bryden, former President and Chief Executive Officer of WorldHeart, was a member of the board of directors prior to his resignation on July 28, 2004. Mr. Goudie was appointed to the board of directors on August 12, 2004. Mr. Goudie resigned as Vice President, Finance and Chief Financial Officer effective May 17, 2005.
All five members of the current board of directors will stand for election at the Annual and Special Meeting. However, pursuant to the Maverick Agreement and as a result of the consolidation of the operations of WorldHeart in Oakland, California and the proposed continuance of WorldHeart to the CBCA, it is contemplated that Mark Goudie will resign from the board of directors and a nominee of Maverick will be appointed to the board of directors upon completion of the MedQuest Acquisition and the Maverick private placement. The Corporate Governance and Nominating Committee is also reviewing at this time a possible increase in the number of directors from five to seven, to accommodate the appointments of Jal S. Jassawalla, President and Chief Executive Officer, as a director, and the second nominee of Maverick under the Maverick Agreement.
- 3.
- Disclose for each director whether he or she is unrelated and how that conclusion was reached.
The board of directors conducted a review of each director based on the criteria of the TSX, NASDAQ and applicable securities regulatory authorities with respect to independence. Apart from the Vice President, Finance and Chief Financial Officer, all directors are unrelated to WorldHeart and to each other. None of the non-management directors provides services, directly or indirectly, to WorldHeart other than services as a director.
- 4.
- The board of directors of every corporation should appoint a committee of directors composed exclusively of outside, i.e., non-management, directors, a majority of whom are unrelated directors, with the responsibility for proposing to the full board new nominees to the board and for assessing directors on an ongoing basis.
The Corporate Governance and Nominating Committee is composed solely of unrelated directors and is responsible for this mandate in accordance with the Committee's charter.
Pursuant to the terms of the financing completed in September 2003, Special Situations Funds has the right to nominate two persons for election or appointment to the board of directors of WorldHeart provided Special Situations Funds and the other U.S. investors who participated in the financing in September 2003 collectively maintain ownership of 25% of the registrable securities purchased in the financing, namely, common shares and common shares issuable upon the exercise of the warrants. The registrable securities cease to be such when they are sold or when they become eligible for sale pursuant to Rule 144(k) under the United States Securities Act of 1933. In addition, under the Maverick Agreement, Maverick would be
37
entitled to nominate two persons for election or appointment to the board of directors of WorldHeart provided Maverick maintains ownership of a certain percentage of shares of WorldHeart.
As noted in the response to Item 2 above, WorldHeart is contemplating certain changes to its board of directors during 2005. The Corporate Governance and Nominating Committee will have the responsibility of assessing the proposed Maverick nominees to ensure their suitability and skills as part of the board of directors.
- 5.
- Every board of directors should implement a process to be carried out by the nominating committee or other appropriate committee for assessing the effectiveness of the board as a whole, the committees of the board and the contribution of individual directors.
The Corporate Governance and Nominating Committee is responsible for implementing this process and making these assessments in accordance with the Committee's charter and other procedures that may be established by the Committee from time to time.
- 6.
- Every corporation, as an integral element of the process for appointing new directors, should provide an orientation and education program for new recruits to the board.
The Corporate Governance and Nominating Committee continues to develop an educational package for new directors and is considering additional orientation and educational programs for directors.
- 7.
- Every board of directors should examine its size and, with a view to determining the impact of the number upon effectiveness, undertake where appropriate, a program to reduce the number of directors to a number which facilitates more effective decision-making.
The board of directors is in the process of evaluating the appropriate number of directors for WorldHeart given the operations of the business and contractual obligations with certain shareholders. Given certain Canadian residency requirements under theBusiness Corporations Act (Ontario) and theCanada Business Corporations Act, in the event the shareholders approve the Continuance Resolution at this Annual and Special Meeting, the board of directors is contemplating the appointment of Mr. Jassawalla, President and Chief Executive Officer, to the board of directors and, therefore, the board of directors may seek to increase the number of directors to seven, as noted in Item 2 above.
In the event that the board of directors determines that the size of the board of directors should be increased to more than five persons, pursuant to the terms of the financing completed in September 2003, Special Situations Funds would have the right to designate a number of nominees representing at least 40% of the members of the board of directors provided certain criteria are met. In addition, as described above, pursuant to the Maverick Agreement, Maverick will have the right to nominate two nominees to the board of directors upon completion of the MedQuest Acquisition and the Maverick private placement.
- 8.
- The board of directors should review the adequacy and form of the compensation of directors and ensure the compensation realistically reflects the responsibilities and risk involved in being an effective director.
The Compensation Committee periodically reviews the adequacy and form of compensation of directors taking into account the responsibilities and risks involved in being a director.
- 9.
- Committees of the board of directors should generally be composed of outside directors, a majority of whom are unrelated directors, although some board committees, such as the executive committee, may include one or more inside directors.
The board of directors currently has four committees: the Compensation Committee; the Audit Committee; the Corporate Governance and Nominating Committee; and the Strategic Planning Committee.
With the exception of the Strategic Planning Committee, each of these committees is comprised solely of outside and unrelated directors. The Strategic Planning Committee includes D. Mark Goudie, a related director, who is Vice President, Finance and Chief Financial Officer.
38
- 10.
- Every board of directors should expressly assume responsibility for, or assign to a committee of directors the general responsibility for, developing the corporation's approach to governance issues. This committee would, amongst other things, be responsible for the corporation's response to these governance guidelines.
The Corporate Governance and Nominating Committee has responsibility for these matters in accordance with the Committee's charter.
- 11.
- The board of directors, together with the CEO, should develop position descriptions for the board and for the CEO, involving the definition of the limits to management's responsibilities. In addition, the board should approve or develop the corporate objectives, which the CEO is responsible for meeting.
The Compensation Committee reviews the Chief Executive Officer's responsibilities and corporate objectives annually and the Committee has implemented a process of receiving quarterly updates to understand progress toward those objectives. The Committee discusses and develops these objectives in conjunction with the President and Chief Executive Officer and makes recommendations to the board of directors in respect of these objectives.
- 12.
- Every board of directors should have in place appropriate structures and procedures to ensure that the board can function independently of management.
The Board has functioned, and is of the opinion that it can continue to function, independently as required. The Chair is not a member of management. The non-management directors meet separately on a quarterly basis at a minimum and on such other occasions as the Board deems appropriate, and where appropriate, exclude the Vice President, Finance and Chief Financial Officer from deliberations in the course of any of these meetings. The Chair is also a member of each of the Committees.
- 13.
- The audit committee of every board of directors should be composed only of outside directors. The roles and responsibilities of the audit committee should be specifically defined so as to provide appropriate guidance to audit committee members as to their duties.
The Audit Committee adopted an amended and restated charter on December 16, 2003 as filed with securities regulatory authorities in Canada and the United States that specifically sets out its duties including responsibility for overseeing management reporting with respect to internal controls. All members of the Audit Committee are non-management, unrelated and independent directors.
- 14.
- The board of directors should implement a system, which enables an individual director to engage an outside advisor at the expense of the corporation in appropriate circumstances. The engagement of the outside advisor should be subject to the approval of an appropriate committee of the board.
Within the Charters of each of the four Committees of the board of directors, provision has been made to allow the non-management directors to seek professional or other independent advice, at the expense of WorldHeart, when necessary.
Report of the Audit Committee
The Audit Committee represents and assists the board of directors in its oversight of the integrity of WorldHeart's financial reporting; the independence, qualifications, and performance of WorldHeart's independent auditors; and compliance with legal and regulatory requirements. The Audit Committee consists of the members listed below, and each is an independent director under NASDAQ listing requirements and TSX requirements and, in accordance with United States and Canadian securities regulatory requirements. John Carlson, Chair of the Audit Committee, qualifies as an "audit committee financial expert" within the meaning of that term as defined by the SEC pursuant to Section 407 of the Sarbanes-Oxley Act of 2002. The Audit Committee has a written charter describing its functions which was amended and restated and approved by the board of directors in December 2003.
WorldHeart's management is responsible for preparing WorldHeart's financial statements and the overall reporting process, including WorldHeart's system of internal controls. The Audit Committee is directly responsible for the compensation, appointment and oversight of WorldHeart's independent accounting firm, PricewaterhouseCoopers LLP. The auditors report directly to the Audit Committee and are responsible for
39
auditing the financial statements and expressing an opinion on the conformity of the audited financial statements with generally accepted accounting principles. The Audit Committee also meets privately in separate sessions periodically with management and the independent auditors.
In this context, the Audit Committee has held discussions with management and the independent auditors. Management represented to the Audit Committee that WorldHeart's audited consolidated financial statements included in this circular were prepared in accordance with generally accepted accounting principles in Canada and the United States, and the Audit Committee has reviewed and discussed the audited consolidated financial statements with management and the independent auditors.
The Audit Committee has discussed with the independent auditors the matters required to be discussed under generally accepted auditing standards and the independent auditors have discussed with the Audit Committee their independence and confirmed their independence in writing.
Based on the considerations above, the Audit Committee recommended to the board of directors and the board of directors has approved, the inclusion of the U.S. GAAP and Canadian GAAP audited consolidated financial statements in this circular. The Audit Committee has recommended PricewaterhouseCoopers LLP as WorldHeart's independent auditors for fiscal 2005. Audit and any permitted non-audit services provided to WorldHeart by PricewaterhouseCoopers LLP are pre-approved by the Audit Committee.
Audit Committee:
John Carlson, Chair
William Garriock
C. Ian Ross
Communication with Shareholders
Shareholders may contact an individual director, the board of directors as a group or a specific committee of the board of directors, including the non-management directors as a group, by the following means:
Mail: World Heart Corporation
7799 Pardee Lane
Oakland California, USA 94621
Email:investors@worldheart.com
Each communication should specify the applicable addressee or addressees to be contacted as well as the general topic of communication. WorldHeart will initially receive and process the communications before forwarding them to the addressee. WorldHeart generally will not forward to the directors a shareholder communication that it determines to be primarily commercial in nature or related to an improper or irrelevant topic, or that requests general information about WorldHeart.
Shareholder Proposals
Under WorldHeart's governing statute, any shareholder entitled to vote at a meeting is entitled to submit a proposal for consideration at any meeting of shareholders. For the next annual meeting of WorldHeart, shareholders must submit any proposals that they wish to have considered by April 28, 2006. For any other meeting of shareholders of Worldheart, shareholders must submit any proposals that they wish to have considered at the meeting at least 60 days before the meeting, after which date the proposal will be considered to be untimely.
MEETINGS OF DIRECTORS
The information set out below reflects the meetings held by the board of directors and its committees during the year ended December 31, 2004.
40
Board of Directors and Committee Meetings Held
| Board of Directors | | 16 |
| Audit Committee | | 9 |
| Compensation Committee | | 7 |
| Corporate Governance and Nominating Committee | | 5 |
| Strategic Planning Committee | | 1 |
All meetings were attended by 100% of the directors except for two meetings of the board of directors and one audit committee meeting that were not attended by one director.
COMPENSATION OF DIRECTORS
The board of directors currently consists of five directors. Non-management directors of WorldHeart may receive compensation by way of fees and stock options pursuant to the Plan for serving on, and attending meetings of, the board of directors and its constituent committees.
An annual grant of options for 14,285 common shares was made to each non-management director for participation on the board of directors and on committees in December 2003. Those options vested as to one third on each of the first, second and third anniversary of the date of grant. In March 2004, the board of directors reviewed the compensation of directors and as a result of the review determined that going forward the initial grant of options for new non-management directors would be increased to 15,000 common shares and would vest as to one quarter at the end of each quarter over a 12 month period.
In September 2004, as a result of the revised strategic plan of WorldHeart including the change in the executive officers of WorldHeart, the board of directors implemented a revised approach to compensation for its management and key employees to be weighted more heavily toward long term equity based compensation. As part of the implementation of this revised approach, the Compensation Committee also considered increasing the long term equity based compensation component of the non-management director remuneration. In September 2004, the board of directors increased the maximum number of shares under the Plan from 1,501,857 common shares to 5,189,672 common shares, subject to regulatory and shareholder approval. At this time, each of the existing non-management directors of WorldHeart agreed to forfeit conditionally those options which each director held. Each of the directors received a new option grant of options for 80,000 common shares which vest as to one third on each of the first, second and third anniversary of the date of grant and are conditional on shareholder approval. The forfeiture of these options and grant of new options as more fully described under the heading "MATTERS TO BE ACTED UPON — Option Amendment Resolution — Amendments to Certain Options Held by Executive Officers and Directors", is regarded by regulatory authorities as repricing of the forfeited options and is subject to shareholder approval being sought at this Annual and Special Meeting.
In January 2005, as a result of the proposed MedQuest Acquisition, Maverick private placement and Warrant Exercise & Debenture Conversion, the board of directors increased the maximum number of common shares available under the Plan to 9,772,505 common shares, subject to shareholder approval being sought at this Annual and Special Meeting. Each of the non-management directors of WorldHeart received a new option grant of 50,000 common shares which vest as to one third on each of the first, second and third anniversary of the date of grant.
The Compensation Committee also determined that newly appointed directors would each receive an initial grant of 120,000 common shares which would vest as to one third on each of the first, second and third anniversary of the date of grant. The Compensation Committee will consider on an annual basis whether there will be an additional grants of options to non-management directors.
In March 2004, the Compensation Committee revised the fees for directors. Non-management directors of WorldHeart receive an annual cash fee of Cdn$11,000. The Chair of the board of directors receives an additional cash fee of Cdn$37,000, the Chair of the Audit Committee receives an additional cash fee of Cdn$19,000, and the Chairs of each of the other committees receive an additional cash fee of Cdn$14,000. An additional Cdn$1,000 per diem fee is paid for meetings attended in person and a Cdn$500 per diem fee is paid for meetings attended by telephone, with a daily maximum of Cdn$1,000. Non-management directors continue to be entitled to reimbursed for travel and other expenses incurred in attending meetings of the board of directors or of a committee.
41
For the year ended December 31, 2004, the following option grants were made to non-management directors, subject to regulatory and shareholder approval:
Director
| | Option Grant
| | Date of Grant
| | Exercise Price
|
|---|
| John F. Carlson(1) | | 80,000 | | September 23, 2004 | | $1.12 |
| William C. Garriock(2) | | 80,000 | | September 23, 2004 | | Cdn$1.43 |
| Robert J. Majteles(3) | | 80,000 | | September 23, 2004 | | $1.12 |
| C. Ian Ross(4) | | 80,000 | | September 23, 2004 | | Cdn$1.43 |
- (1)
- On September 23, 2004, Mr. Carlson conditionally forfeited options for 14,285 common shares granted on November 5, 2003 at an exercise price of Cdn$10.15 per share. On January 31, 2005, subject to regulatory and shareholder approval, Mr. Carlson received a further grant of options for 50,000 common shares at an exercise price of $1.48 per share.
- (2)
- On September 23, 2004, Mr. Garriock conditionally forfeited options for 14,285 common shares granted on December 16, 2003 at an exercise price of Cdn$10.28 per share. On January 31, 2005, subject to regulatory and shareholder approval, Mr. Garriock received a further grant of options for 50,000 common shares at an exercise price of Cdn$1.84 per share.
- (3)
- On September 23, 2004, Mr. Majteles conditionally forfeited options for 14,285 common shares granted on October 21, 2003 at an exercise price of Cdn$10.29 per share. On January 31, 2005, subject to regulatory and shareholder approval, Mr. Majteles received a further grant of options for 50,000 common shares at an exercise price of $1.48 per share.
- (4)
- On September 23, 2004, Mr. Ross conditionally forfeited options for 18,141 common shares, of which options for 14,285 common shares were granted on October 21, 2003 at an exercise price of Cdn$10.29 per share, options for 1,714 common shares were granted on January 4, 2002 at an exercise price of Cdn$46.41 and options for 2,142 common shares were granted on March 3, 2003 at an exercise price of Cdn$13.93. On January 31, 2005, subject to regulatory and shareholder approval, Mr. Ross received a further grant of options for 50,000 common shares at an exercise price of Cdn$1.84 per share.
DIRECTORS' AND OFFICERS' INDEMNIFICATION
WorldHeart maintains directors' and officers' liability insurance in the aggregate amount of $15,000,000. The aggregate annual premium in 2004 for such insurance was $522,000. The by-laws of WorldHeart provide that WorldHeart shall indemnify a director or officer of WorldHeart against liability incurred in such capacity to the extent permitted or required by theBusiness Corporations Act (Ontario). To the extent WorldHeart is required to indemnify the directors or officers pursuant to the by-laws, the insurance policy provides that WorldHeart is liable for the initial $200,000 in respect of each securities claim and the initial $75,000 with respect to each other claim.
EXECUTIVE OFFICERS
Name
| | Age
| | Office(s)
|
|---|
| Jal S. Jassawalla | | 59 | | Mr. Jassawalla was appointed President and Chief Executive Officer as of July 28, 2004. From June 2003 to July 28, 2004, Mr. Jassawalla was Executive Vice President and Chief Technical Officer of WorldHeart and was responsible for research and development, clinical affairs, and clinical and technical support and quality. Mr. Jassawalla was a co-founder of Novacor Medical Corporation in 1979 and became an employee of WorldHeart when WorldHeart acquired the Novacor division of Edwards in June 2000. |
| | | | | |
42
D. Mark Goudie |
|
39 |
|
Mr. Goudie joined WorldHeart in April 2002 as Director of Finance. On October 1, 2003, Mr Goudie became Vice President, Finance and Chief Financial Officer. Prior to joining WorldHeart, Mr. Goudie was Vice-President of Finance of the Ottawa Senators Hockey Club Corporation and Palladium Corporation from June 1996 to January 1998 and Chief Financial Officer of both corporations from January 1998 to April 2000 and from April 2001 to April 2002. From April 2000 to April 2001, Mr. Goudie served as Chief Financial Officer of Rebel.com Inc. Mr. Goudie is a Chartered Accountant who started his career with PricewaterhouseCoopers LLP in the auditing, accounting and tax group before joining the management consulting practice in their business control group. Mr. Goudie resigned on May 17, 2005 but will provide consulting services to WorldHeart until September 2005. |
Piet Jansen |
|
45 |
|
Mr. Jansen joined WorldHeart in February 2004 as Chief Medical Officer and was appointed Managing Director, Europe, as of July 28, 2004. Mr. Jansen continues to serve as Chief Medical Officer as well. Prior to joining WorldHeart, Mr. Jansen was the Vice-President, Clinical Programs at Orqis International GmbH from 2003 to February 2004 and at Jarvik Heart Inc. as Vice President, Clinical Affairs from 2001 to 2003. From 1997 to 2001, Mr. Jansen worked for the Novacor Division for Edwards Lifesciences LLC, formerly Baxter Corporation. |
John Marinchak |
|
43 |
|
In October 2003, Mr. Marinchak joined WorldHeart as Director of Sales, North America. On July 28, 2004, Mr. Marinchak was appointed Vice President, Marketing and Sales. Prior to working for WorldHeart, Mr. Marinchak was Director Sales at Cardica from May 2003. From 1999 to May 2003, Mr. Marinchak was the Western Area Sales and International Sales Manager with Thoratec Corporation. |
Phillip Miller |
|
56 |
|
Mr. Miller became an employee of WorldHeart when WorldHeart acquired the Novacor division of Edwards in June 2000. Mr. Miller was the Director of Biomedical Engineering and was appointed Vice President, Research and Development, on November 2, 2004. Prior to the acquisition of the Novacor division in June 2000, Mr. Miller was Director, Biomedical Engineering at Edwards Lifesciences LLC, formerly Baxter Corporation, commencing in 1973. |
Robert Corson |
|
58 |
|
Mr. Corson rejoined WorldHeart in July 2004 as Vice President, Manufacturing. Mr. Corson was previously employed by WorldHeart as Executive Vice President, Operations responsible for research and development, quality control and manufacturing operations from 1997 to 2000. From 2000 to 2004, Mr. Corson, through his management consulting company, provided various executive management and operations management services to several private companies. |
43
EXECUTIVE COMPENSATION
The following table sets forth the summary information concerning the compensation paid or earned during the three most recently completed financial years by WorldHeart's Chief Executive Officer and the four highest paid executive officers, who were serving as executive officers at December 31, 2004 (the Named Executive Officers). The table also includes information for Roderick M. Bryden, who would have been a Named Executive Officer except that he was not serving as officer at year-end.
| | Annual Compensation
| | Long Term Compensation
| |
|---|
Name and Principal Position
| | Year
| | Salary
($)
| | Bonus
($)
| | Other Annual Compensation
($)
| | Securities Underlying Options/SARs
(#)
| | All Other Compensation
($)
| |
|---|
Jal S. Jassawalla(1)
President and Chief Executive Officer | | 2004
2003
2002 | | $267,000
$227,000
$227,000 | | Nil
$ 64,381
$ 27,000 |
(13)
| Nil
Nil
Nil | | 787,225
104,558
4,542 | (7)
| Nil
$ 24,000
$ 24,000 | (14)
(14)
(14) |
D. Mark Goudie(2)
Vice President Finance and Chief Financial Officer | | 2004
2003
2002 | | Cdn$202,209
Cdn$144,900
Cdn$ 95,000 | | Nil
Cdn$ 95,300
Cdn$ 11,500 |
(13)
| Nil
Nil
Nil | | 40,000
37,395
2,475 | (8)
| Cdn$209,667
Nil
Nil | (19)
|
Piet Jansen(3)
Managing Director Europe and Chief Medical Officer | | 2004
2003
2002 | | € 153,750
Nil
Nil | | Nil
Nil
Nil | | Nil
Nil
Nil | | 261,500
Nil
Nil | (9)
| Nil
Nil
Nil | |
John Marinchak(4)
Vice President
Marketing and Sales | | 2004
2003
2002 | | $142,096
$ 21,635
Nil | | Nil
Nil
Nil | | $ 6,600
$ 1,142
Nil | (17)
(17)
| 160,000
18,256
Nil | (10)
| $157,291
$ 22,500
Nil | (16)
(16)
|
Phillip Miller(5)
Vice President
Research and Development | | 2004
2003
2002 | | $141,414
$130,305
$124,100 | | Nil
$ 23,455
$ 14,631 | | Nil
Nil
Nil | | 180,000
5,036
1,076 | (11)
| $ 7,280
$ 4,689
$ 4,267 | (18)
(18)
(18) |
Roderick M. Bryden(6)
former President and Chief Executive Officer | | 2004
2003
2002 | | Cdn$175,000
Cdn$250,000
Cdn$250,000 | | Cdn$ 45,000
Cdn$225,625
Cdn$ 40,500 | (20)
(13)
| Cdn$25,772
Cdn$41,640
Cdn$41,640 | | Nil
464,101
4,164 | (12)
| Cdn$823,475
Cdn$162,225
Cdn$133,605 | (15)
|
- (1)
- Mr. Jassawalla became President and Chief Executive Officer of WorldHeart on July 28, 2004. Previously, Mr. Jassawalla was the Executive Vice-President and Chief Technical Officer.
- (2)
- Mr. Goudie became the Chief Financial Officer of WorldHeart on October 1, 2003. Mr. Goudie commenced employment with WorldHeart on April 1, 2002 as Director of Finance. Mr. Goudie resigned as Vice President, Finance and Chief Financial Officer of WorldHeart on May 17, 2005.
- (3)
- Dr. Jansen became Managing Director, Europe on July 28, 2004 and continued as Chief Medical Officer. Dr. Jansen joined WorldHeart in February 2004 as Chief Medical Officer.
- (4)
- Mr. Marinchak became Vice President, Marketing and Sales of WorldHeart on July 28, 2004. Mr. Marinchak joined WorldHeart in October 2003 as Director of Sales North America.
- (5)
- Mr. Miller became Vice President, Research and Development on November 2, 2004. Previously Mr. Miller was Director Biomedical Engineering.
- (6)
- Mr. Bryden resigned as President and Chief Executive Officer of WorldHeart on July 28, 2004. Mr. Bryden's services had been provided to WorldHeart since April 1, 1996 by SC Stormont Corporation, a corporation controlled by Mr. Bryden.
- (7)
- Options for 787,225 common shares were granted to Mr. Jassawalla on September 23, 2004 and options for 105,397 common shares were forfeited by Mr. Jassawalla, subject to shareholder approval. On January 31, 2005, options for 1,000,000 common shares were granted to Mr. Jassawalla, subject to shareholder approval. (See "MATTERS TO BE ACTED UPON — Amendments to WorldHeart's Employee Stock Option Plan" and — "Amendments to Certain Options Held by Executive Officers and Directors.")
- (8)
- Options for 40,000 common shares were granted to Mr. Goudie, subject to meeting certain milestones, on September 23, 2004 and options for 39,820 common shares were forfeited by Mr. Goudie, subject to shareholder approval. On January 31, 2005, options for 40,000 common shares were granted to Mr. Goudie, subject to shareholder approval. (See "MATTERS TO BE ACTED UPON — Amendments to WorldHeart's Employee Stock Option Plan" and — "Amendments to Certain Options Held by Executive Officers and Directors.")
44
- (9)
- Options for 240,000 common shares were granted to Mr. Jansen on September 23, 2004 and options for 21,500 common shares were forfeited by Mr. Jansen, subject to shareholder approval. On January 31, 2005, options for 40,000 common shares were granted to Mr. Jansen, subject to shareholder approval. (See "MATTERS TO BE ACTED UPON — Amendments to WorldHeart's Employee Stock Option Plan" and — "Amendments to Certain Options Held by Executive Officers and Directors.")
- (10)
- Options for 160,000 common shares were granted to Mr. Marinchak on September 23, 2004 and options for 18,256 common shares were forfeited by Mr. Marinchak, subject to shareholder approval. On January 31, 2005, options for 100,000 common shares were granted to Mr. Marinchak, subject to shareholder approval. (See "MATTERS TO BE ACTED UPON — Amendments to WorldHeart's Employee Stock Option Plan" and — "Amendments to Certain Options Held by Executive Officers and Directors.")
- (11)
- Options for 180,000 common shares were granted to Mr. Miller on September 23, 2004 and options for 5,716 common shares were forfeited by Mr. Miller, subject to shareholder approval. On January 31, 2005, options for 145,000 common shares were granted to Mr. Miller, subject to shareholder approval. (See "MATTERS TO BE ACTED UPON — Amendments to WorldHeart's Employee Stock Option Plan" and — "Amendments to Certain Options Held by Executive Officers and Directors.")
- (12)
- Mr. Bryden retained options for 86,422 common shares under the terms of his severance arrangements.
- (13)
- Includes a special transaction bonus related to the completion of the financing in September 2003 in the following amounts: Mr. Bryden Cdn$160,000, Mr. Goudie Cdn$73,700; and Mr. Jassawalla $15,301.
- (14)
- In each of 2003 and 2002, WorldHeart paid a travel allowance to Mr. Jassawalla of $24,000. No travel allowances were paid to Mr. Jassawalla in 2004.
- (15)
- During 2004, WorldHeart paid insurance premiums of Cdn$105,795 relating to Cdn$10 million of life insurance coverage, of which certain named family members of Mr. Bryden are the beneficiaries. The policy was cancelled effective August 2004. The balance of Cdn$717,680 represents notice, severance, benefits and vacation lump sum payment to Mr. Bryden.
- (16)
- These amounts represent sales commissions earned by Mr. Marinchak.
- (17)
- These amounts represent the car allowances received by Mr. Marinchak.
- (18)
- These amounts represent royalties on the sales of the Novacor LVAS pursuant to a royalty agreement between Mr. Miller and WorldHeart dated April 22, 1998.
- (19)
- Amount represents a payment for notice, severance and vacation lump sum to be paid in 2005 as part of Mr. Goudie's termination arrangements pursuant to Mr. Goudie's agreement with WorldHeart effective October 1, 2003.
- (20)
- In connection with Mr. Bryden's employment contract he was paid a quarterly retention bonus.
OPTION GRANTS IN LAST FISCAL YEAR
The following table provides information with respect to stock option grants by WorldHeart to the Named Executive Officers for the year ended December 31, 2004.
| |
| |
| |
| |
| | Potential Realizable Value at Assumed Annual Rates of Stock Price Appreciation for Option Term
|
|---|
Named
Executive Officers
| | Common Shares Under Options Granted
(#)
| | As a Percentage of Total Options Granted to Employees in Financial Year
(%)
| | Exercise of Base Price
($/Common Share)
| |
|
|---|
| | Expiration Date
| | 5%
| | 10%
|
|---|
| Jal S. Jassawalla(1) | | 787,225 | | 31.3% | | $1.12 | | Sep 22, 2013 | | $396,882 | | $793,764 |
| D. Mark Goudie(2) | | 40,000 | | 1.6% | | Cdn$1.43 | | Sep 22, 2013 | | Cdn$ 25,748 | | Cdn$ 51,496 |
| Piet Jansen | | 7,166
7,166
7,168
240,000 | | 0.3%
0.3%
0.3%
9.5% | | $7.03
$7.03
$7.03
$1.12 | | Dec 31, 2008
Dec 31, 2009
Dec 31, 2010
Sep 22, 2013 | | $ 12,387
$ 14,906
$ 17,430
$120,997 | | $ 24,774
$ 29,812
$ 34,860
$241,994 |
| John Marinchak | | 160,000 | | 6.4% | | $1.12 | | Sep 22, 2013 | | $ 80,665 | | $161,329 |
| Phillip Miller | | 180,000 | | 7.2% | | $1.12 | | Sep 22, 2013 | | $ 90,748 | | $181,495 |
| Roderick M. Bryden(3) | | Nil | | Nil | | N/A | | | | Nil | | Nil |
- (1)
- Mr. Jassawalla became President and Chief Executive Officer of WorldHeart on July 28, 2004.
- (2)
- Mr. Goudie resigned as Vice President, Finance and Chief Financial Officer of WorldHeart on May 17, 2005.
- (3)
- Mr. Bryden resigned as President and Chief Executive Officer of WorldHeart on July 28, 2004.
45
The aggregate number of options granted to all Named Executive Officers during the year ended December 31, 2004 was 1,428,725 common shares.
LONG TERM INCENTIVE PLAN AWARDS IN LAST FISCAL YEAR
Named Executive Officers
| | Common Shares
Under Options
Granted
(#)
| | Expiration Date
| | Estimated Future Payouts
Under non-stock price
based plans
|
|---|
| Jal S. Jassawalla(1) | | 787,225 | | Sep 22, 2013 | | — |
| D. Mark Goudie(2) | | 40,000 | | Sep 22, 2013 | | — |
| Piet Jansen | | 7,166
7,166
7,168
240,000 | | Dec 31, 2008
Dec 31, 2009
Dec 31, 2010
Sep 22, 2013 | | —
—
—
— |
| John Marinchak | | 160,000 | | Sep 22, 2013 | | — |
| Phillip Miller | | 180,000 | | Sep 22, 2013 | | — |
| Roderick M. Bryden(3) | | Nil | | | | — |
- (1)
- Mr. Jassawalla became President and Chief Executive Officer of WorldHeart on July 28, 2004.
- (2)
- Mr. Goudie resigned as Vice President, Finance and Chief Financial Officer of WorldHeart on May 17, 2005.
- (3)
- Mr. Bryden resigned as President and Chief Executive Officer of WorldHeart on July 28, 2004.
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR AND OPTIONS/SAR VALUES
Named Executive Officers
| | Shares Acquired on Exercise
(#)
| | Value Realized
($)
| | Number of Securities Underlying Unexercised Options/SARs at December 31, 2004
Exercisable/Unexercisable
| | Value of Unexercised In-the-Money Options/SARs at December 31, 2004
Exercisable/Unexercisable
|
|---|
| Jal S. Jassawalla | | — | | — | | 570/788,359 | | $0/$1,157,221 |
| D. Mark Goudie | | — | | — | | 0/40,000 | | $0/$58,800 |
| Piet Jansen | | — | | — | | 0/240,000 | | $0/$352,800 |
| John Marinchak | | — | | — | | 0/160,000 | | $0/$235,200 |
| Phillip Miller | | — | | — | | 90/180,180 | | $0/$264,600 |
| Roderick M. Bryden | | — | | — | | 86,422/0 | | $0/$0 |
The Named Executive Officers exercised no options during the year ended December 31, 2004.
EMPLOYMENT AGREEMENTS
WorldHeart entered into an employment arrangement with Mr. Jal S. Jassawalla dated June 23, 2000, which arrangement was updated as of July 28, 2004 and further amended as of October 25, 2004. As of July 28, 2004, Mr. Jassawalla is entitled to receive a base salary of $267,000 per annum. Mr. Jassawalla was granted 787,255 options with an exercise price of $1.12, vesting as to one third on each of September 23, 2005, 2006 and 2007, which will all expire on September 22, 2013, and is also eligible to receive grants of options pursuant to the Plan at the discretion of the board. Mr. Jassawalla is entitled to a severance payment of 104 weeks upon termination by WorldHeart pursuant to the terms and conditions of World Heart Inc. severance pay plan.
Mr. Mark Goudie entered into an employment agreement effective October 1, 2003. This agreement provided for certain termination payments in the event that the position of Chief Financial Officer was re-located from Ottawa, Ontario. As part of the decision to consolidate in August 2004 of the operations of WorldHeart to Oakland California, WorldHeart entered into a letter agreement with Mr. Goudie dated November 5, 2004, as amended, which provided for the ongoing services of Mr. Goudie, defined the terms under which termination payments would be made and required Mr. Goudie to provide 60 days written notice to
46
WorldHeart prior to a voluntary termination of employment by him. Mr. Goudie provided notice of termination in March 2005. Mr. Goudie is entitled to receive a termination payment of Cdn$180,000 less statutory deductions. Such termination payments commenced in January 2005 and also included payment for accrued and unused vacation to December 31, 2004. All remaining payments will be paid by March 31, 2005. Mr. Goudie was granted 40,000 options on September 23, 2004 at a price of C$1.43, vesting as to 100% on the termination date of employment with WorldHeart, subject to regulatory approval and achievement of certain objectives prior to the termination date. All vested options will continue for a period of three years from the vesting date.
WorldHeart entered into an employment arrangement with Mr. Phil Miller dated June 23, 2000, which arrangement was updated as of October 12, 2004. As of October 2004, Mr. Miller is entitled to receive a base salary of $160,000 per annum. Mr. Miller was conditionally granted 180,000 options with an exercise price of $1.12, vesting as to one third on each of September 23, 2005, 2006 and 2007, which will all expire on September 22, 2013, and is also eligible to receive grants of options pursuant to the Plan at the discretion of the board. Under the October 12, 2004 arrangement Mr. Miller retained royalty rights pursuant to a royalty agreement between Mr. Miller and WorldHeart dated April 22, 1988 entitling him to certain insignificant payments on the sale of Novacor LVAS kits.
Dr. Piet Jansen entered into an employment arrangement with WorldHeart on December 9, 2003, which arrangement was updated as of October 12, 2004. As of October 2004, Dr. Jansen is entitled to receive a base salary of €170,000 per annum. Dr. Jansen was conditionally granted 240,000 options with an exercise price of $1.12, vesting as to one third on each of September 23, 2005, 2006 and 2007, which will all expire on September 22, 2013, and is also eligible to receive grants of options pursuant to the Plan at the discretion of the board.
WorldHeart entered into an employment arrangement with Mr. John Marinchak dated October 6, 2003, which arrangement was updated as of October 12, 2004. As of October 2004, Mr. Marinchak is entitled to receive a base salary of $160,000 per annum. Mr. Marinchak is also entitled to receive commissions based on sales of Novacor LVAS kits equal to $1,000 per kit, the formula for which commissions may be adjusted from time to time. Mr. Marinchak was conditionally granted 160,000 options with an exercise price of $1.12, vesting as to one third on each of September 23, 2005, 2006 and 2007, which will all expire on September 22, 2013, and is also eligible to receive grants of options pursuant to the Plan at the discretion of the board.
WorldHeart entered into an agreement with SC Stormont Corporation and Mr. Roderick M. Bryden on July 28, 2004 in respect of Mr. Bryden's departure from WorldHeart. Mr. Bryden was paid a settlement amount, in two payments which have been paid in full, of Cdn$717,680, less any required withholdings, representing notice, severance and vacation pay. Mr. Bryden received an accelerated vesting of 7,615 options and the extension of the expiry date with respect to 86,422 options under the agreement.
EQUITY COMPENSATION PLAN INFORMATION
WorldHeart adopted the Plan on December 6, 1996 and amended and restated the Plan on March 6, 1997, October 27, 1997, October 27, 1998, February 23, 1999, May 15, 2000, April 26, 2001, May 1, 2002, October 16, 2003, March 9, 2004, September 23, 2004 and on January 31, 2005. The Plan is intended to encourage ownership in WorldHeart's shares by employees, officers, directors and consultants of WorldHeart. The Plan is administered by the Compensation Committee. The maximum number of common shares which may be reserved and set aside under options to eligible persons pursuant to the Plan is 1,501,857 common shares, and subject to shareholder approval being sought at this Annual and Special Meeting will be increased to 9,772,505 common shares. Subject to shareholder approval being sought at this Annual and Special Meeting, the maximum number of common shares reserved for issuance to any one person under the Plan has been amended by the board of directors to remove the limitation of 5% of the issued and outstanding common shares from time to time. Pursuant to the Plan, the option exercise price for all options is determined by the Compensation Committee based on the closing price of the common shares on the TSX on the date immediately prior to the date of grant. Unless otherwise determined by the Compensation Committee, options will vest in equal amounts on each of the first, second and third anniversaries of the date of grant and must be exercised within a four year
47
period from the last date of vesting. Some options previously granted under the Plan vest over a five year period and must be exercised within a four year period from the date of vesting. As at December 31, 2004;
Plan Category
| | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights
(a)
| | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights
(b)
| | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a))
(c)
| |
|---|
| Equity compensation plans approved by security holders | | 3,172,771 | (1) | $ | 3.81 | | 1,950,275 | (2) |
| | |
| |
| |
| |
| Total | | 3,172,771 | | $ | 3.81 | | 1,950,275 | |
| | |
| |
| |
| |
- (1)
- Options for 2,383,725 common shares were granted on September 23, 2004 subject to shareholder approval of an increase in common shares available under the Plan. The September 23, 2004 grant to executive officers and directors is conditional on the forfeiture of options for 251,685 common shares already issued and outstanding under the Plan. These forfeited options continue to be included in the number of options outstanding.
- (2)
- Options for 3,418,750 common shares were granted on January 31, 2005 subject to shareholder approval of an increase in the number of common shares available under the Plan to 9,772,505 common shares. If approved, the number of securities to be issued upon exercise of outstanding options, warrants and rights would be 6,591,521 common shares; the weighted-average exercise price of the outstanding options, warrants and rights would be $2.42; and the number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) would be 3,114,358 common shares.
LOANS TO DIRECTORS AND OFFICERS
None of the directors or officers of WorldHeart, or any associate of the directors or officers of WorldHeart, has been or is indebted to WorldHeart.
REPORT ON EXECUTIVE COMPENSATION AND COMPOSITION OF THE COMPENSATION COMMITTEE
The Compensation Committee reviews and makes recommendations to the board of directors with respect to the overall approach to compensation for WorldHeart and its employees and specifically with respect to the executive officers of WorldHeart, including the President and Chief Executive Officer, reviews remuneration of directors of WorldHeart, and administers the Plan. The Compensation Committee comprised of Robert J. Majteles (Chair), C. Ian Ross and William Garriock, met seven times in 2004.
WorldHeart's executive compensation plan is based on a pay for performance philosophy. However, there has been a temporary shift in the approach to compensation away from cash incentives, except for sales commissions, to a compensation scheme more heavily weighted in favor of long term equity incentives in the form of stock options. The Compensation Committee believes that long term equity incentives are an important component of compensation to motivate executive officers and the employees and more closely align these individuals with the interest of shareholders.
Initially, from January 2004, the compensation for each of the Named Executive Officers and key employees as a group consisted of four elements: a fixed base salary, a variable incentive component, a longer term incentive in the form of stock options, and benefits. In addition, the compensation program for Mr. Marinchak included a sales-based commission component.
As part of the revised strategic plan of WorldHeart adopted by the board of directors in August 2004 there was a shift in the approach to compensation for the executive officers and key employees. Long term equity compensation in the form of stock options and a fixed moderate base salary, measured against similar businesses in the market, were determined to be the key components of compensation as well as benefits. The board of directors, using such reports as the 2003 Medical Device Industry Compensation Benchmark Survey (MEDIC) and 6th Edition (2003) TechEdge Compensation Survey (TechEdge), determined that executive officers and key
48
employees should, as a group, have an increased ownership stake in WorldHeart of approximately 10% of the common shares on a fully diluted basis. Cash bonuses and cash incentives, except for the commission-based cash component for the sales team, were eliminated for the present time. The option grants to executive officers and key employees were provided on a one time basis, to vest over a three year period, but with the possibility of additional grants to individuals by the board of directors from time to time depending on certain performance milestones and other events.
Base salaries for all employees, including executives, use WorldHeart's estimated market value of each position based on the 50th percentile for market compensation data obtained from industry salary surveys, including the 2003 MEDIC report for US-based companies and the TechEdge report for the Canadian hi-tech marketplace. Base salaries are then set at between plus or minus 20 percent of the estimated market value. Increases or decreases in salary levels, effective January 1 each year, are proposed to the Compensation Committee for review and a recommendation is subsequently made to the board of directors for approval.
Prior to August 2004, the executive officers' participation in the annual bonus and incentive based stock plans was based on recommendations by the Compensation Committee at the commencement of each fiscal year and subject to actual performance against documented corporate and individual goals and objectives. At the conclusion of the year, the Compensation Committee would review the actual performance of both WorldHeart and individual executive officers against the predetermined goals and objectives to arrive at a recommended bonus and option grant considered as earned during the year. In August 2004, the Compensation Committee adjusted this approach to compensation by eliminating annual cash bonuses and performance options and moving to a compensation philosophy based on a moderate base salary and long term equity compensation. Each executive officer and key employee received an option grant in September 2004 to allow the executive officers and key employees to receive, as a group, a targeted ownership of 10% of the common shares of WorldHeart on a fully diluted basis. To maintain the 10% targeted executive officer and key employee ownership of the common shares of WorldHeart on a fully diluted basis, additional options were granted to executive officers and key employees in January 2005 due to proposed share issuances pursuant to the MedQuest Acquisition and private placement to Maverick. The Compensation Committee will consider from time to time if there should be periodic grants of options to key employees and executive officers.
Chief Executive Officer's Compensation
When Mr. Jassawalla became President and Chief Executive Officer, the Compensation Committee reviewed Mr. Jassawalla's existing compensation in light of his new position and responsibility and adjusted it based on the market information of comparable companies. Also the Compensation Committee adjusted the compensation to reflect a revised compensation model based primarily on a moderate base salary and long term equity compensation. The Compensation Committee determined that the base salary would be fixed at $267,000 and that comparable chief executive officers received long term equity compensation ranging from three to five percent of the fully diluted issued and outstanding common shares of the company. The Compensation Committee determined to issue Mr. Jassawalla approximately 1.7 million options or approximately 3% of the issued and outstanding common shares of WorldHeart after giving effect to the MedQuest Acquisition and Maverick private placement and the Warrant Exercise & Debenture Conversion. A portion of such options was granted to Mr. Jassawalla in September 2004 with an additional portion in January 2005 based on performance and the proposed transactions. These option grants are subject to shareholder approval of the amendments to the Plan and the amendments to certain options held by executive officers and directors.
This report on executive compensation is submitted by the directors of the Compensation Committee:
49
PERFORMANCE GRAPH
The following graph compares the percentage change since December 31, 1999 in the per share value of WorldHeart's common shares with the percentage change since December 31, 1999 in the value of the S&P/Nasdaq Composite Index and the S&P/Nasdaq Healthcare Index. Our common shares began trading on the NASDAQ in August 1998 but were delisted on October 15, 2002 and were quoted on the NASDAQ OTC Bulletin Board from October 15, 2002 until March 17, 2004. Our common shares were readmitted to trading on NASDAQ on March 18, 2004.
Performance Graph
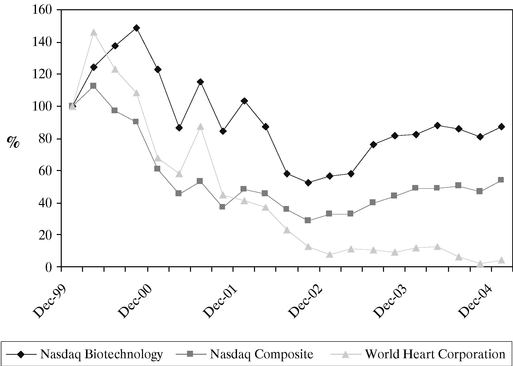
Our common shares trade on the NASDAQ under the symbol WHRT and on the TSX under the symbol WHT. Our common shares began trading on the NASDAQ commencing in August 1998 but were delisted on October 15, 2002 and were quoted on the NASDAQ OTC Bulletin Board from October 15, 2002 until March 17, 2004. Our common shares were readmitted to trading on NASDAQ on March 18, 2004. On December 1, 2003, WorldHeart amended its articles to effect a one common share for seven common shares consolidation and began trading on a post-consolidated basis on December 4, 2003.
The following table sets forth the high and low bid prices for our common shares as reported on the NASDAQ OTC Bulletin Board for each of the quarters from the quarter ended March 31, 2003 through March 17, 2004.
| | Common Shares
| |
|
|---|
Quarter Ended
| | Volume Traded
|
|---|
| | High
| | Low
|
|---|
| March 31, 2003 | | $ | 12.25 | | $ | 5.25 | | 67,700 |
| June 30, 2003 | | $ | 8.05 | | $ | 4.55 | | 43,500 |
| September 30, 2003 | | $ | 12.60 | | $ | 4.55 | | 129,300 |
| December 31, 2003 | | $ | 9.50 | | $ | 6.23 | | 380,900 |
| January 1, 2004 — March 17, 2004 | | $ | 8.40 | | $ | 6.15 | | 2,315,900 |
50
The following table sets forth the high and low sales prices for our common shares as reported on the NASDAQ for each of the quarters from the quarter ended March 31, 2004 through June 10, 2005.
| | Common Shares
| |
|
|---|
Quarter Ended
| | Volume Traded
|
|---|
| | High
| | Low
|
|---|
| March 31, 2004 (March 18, 2004 — March 31, 2004) | | $ | 9.00 | | $ | 7.68 | | 405,600 |
| June 30, 2004 | | $ | 8.50 | | $ | 3.70 | | 3,407,300 |
| September 30, 2004 | | $ | 5.10 | | $ | 1.05 | | 7,183,600 |
| December 31, 2004 | | $ | 2.68 | | $ | 0.97 | | 12,907,600 |
| March 31, 2005 | | $ | 2.62 | | $ | 1.12 | | 7,775,600 |
Month Ended
|
|
|
|
|
|
|
|---|
| April 1, 2005 to April 30, 2005 | | $ | 1.30 | | $ | 0.90 | | 661,500 |
| May 1, 2005 to May 31, 2005 | | $ | 1.24 | | $ | 0.94 | | 747,900 |
| June 1, 2005 to June 10, 2005 | | $ | 1.23 | | $ | 0.98 | | 211,700 |
The following table sets forth the high and low sales prices for our common shares as reported on the TSX for each of the quarters from the quarter ended March 31, 2003 through June 10, 2005.
| | Common Shares
| |
|
|---|
Quarter Ended
| | Volume Traded
|
|---|
| | High
| | Low
|
|---|
| March 31, 2003 | | Cdn$18.90 | | Cdn$8.47 | | 1,869,200 |
| June 30, 2003 | | Cdn$12.04 | | Cdn$8.40 | | 535,600 |
| September 30, 2003 | | Cdn$12.74 | | Cdn$6.79 | | 1,034,600 |
| December 31, 2003 | | Cdn$12.53 | | Cdn$8.05 | | 1,212,200 |
| March 31, 2004 | | Cdn$11.40 | | Cdn$8.40 | | 1,972,600 |
| June 30, 2004 | | Cdn$10.85 | | Cdn$5.06 | | 1,879,600 |
| September 30, 2004 | | Cdn$ 6.48 | | Cdn$1.33 | | 4,611,000 |
| December 31, 2004 | | Cdn$ 3.20 | | Cdn$1.16 | | 3,484,800 |
| March 31, 2005 | | Cdn$ 3.00 | | Cdn$1.33 | | 2,124,300 |
Month Ended
|
|
|
|
|
|
|
|---|
| April 1, 2005 to April 30, 2005 | | Cdn$1.60 | | Cdn$1.21 | | 217,000 |
| May 1, 2005 to May 31, 2005 | | Cdn$1.59 | | Cdn$1.18 | | 333,400 |
| June 1, 2005 to June 10, 2005 | | Cdn$1.54 | | Cdn$1.24 | | 37,700 |
RISK FACTORS
You should carefully consider the following risk factors related to our business and the MedQuest Acquisition, the Maverick private placement and the Warrant Exercise & Debenture Conversion, in evaluating whether to approve the proposal related to these transactions. The risk factors include risks related to the combined businesses of WorldHeart and MedQuest. Additional risks and uncertainties not presently known to us or that we currently consider not material may also impair our business, financial condition and results of operations. If any of the events described below actually occurs, our business, financial condition or results of operations could be materially adversely affected.
Risk Factors Relating to our Business
We have had substantial losses and limited revenues earned since incorporation.
Since its founding in 1996, WorldHeart has invested approximately $200 million in internally developed and acquired technologies. These form the basis for the business of WorldHeart today. Research and development expenses have been reduced in the past two years and are expected to be increased in relation to the completion and regulatory approval of products currently under development. In addition, overall expense levels have been
51
reduced during the past two years, while marketing and sales expenses have increased. If the MedQuest Acquisition is completed, there will be an increase in our research and development and other expenses.
WorldHeart generated revenues of $9,785,761 from commercial sales of the Novacor LVAS for the year ended December 31, 2004 ($6,775,807 for the year ended December 31, 2003). We believe that we will be able to generate increased revenues on the basis of the current product and regulatory approvals that have been granted to date. However, there can be no assurance that we will be able to generate the level of revenues required to achieve profitability and to fund research and development activities.
We anticipate the closing of the MedQuest Acquisition in the second quarter of 2005. Since its founding in 1993, MedQuest has generated losses from operations and currently does not have a commercially available revenue-generating product. For the six months ended December 31, 2004, MedQuest had a net loss of $1.9 million ($2.2 million for the year ended June 30, 2004). If the MedQuest Acquisition is completed, we are committed to making additional significant expenditures for the MedQuest rotary VAD project in fiscal 2005 and subsequent fiscal years, which will likely result in losses from these activities in future periods. These expenditures include costs associated with performing pre-clinical and clinical trials for the MedQuest rotary VAD, continuing research and development, seeking regulatory approvals and, if we receive these approvals, commencing commercial manufacturing, sales and marketing of the MedQuest rotary VAD.
We will require significant capital investment to bring future products and product enhancements to market.
Our investment of capital could be significant. Developing our technology, future products and continued product enhancements, including those of the MedQuest rotary VAD technology that we are acquiring, require a commitment of substantial funds to conduct the costly and time-consuming research and clinical trials necessary for such development. If adequate funds are not available when required, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs or obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies, potential products or products that we would otherwise seek to develop or commercialize ourselves. The inability to obtain additional financing when needed would likely have a material adverse effect on our business, financial condition and results of operations.
We may be unable to obtain regulatory approvals.
Most countries, including the United States, Canada and countries in Europe, require regulatory approval prior to the commercial distribution of medical devices. In particular, implanted medical devices generally are subject to rigorous clinical testing as a condition of approval by the FDA and by similar authorities in Canada, and in European and other countries. The approval process is expensive and time consuming. Non-compliance with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, refusal of the government to grant marketing approval for devices, withdrawal of marketing approvals and criminal prosecution. The inability to obtain the appropriate regulatory approvals for our products in the United States, Canada and the rest of the world could have a material adverse effect on our business, financial condition and results of operations.
In the United States, WorldHeart commenced its RELIANT Trial in the second quarter of 2004 where the Novacor LVAS is being randomized to the HeartMate XVE, which is the control arm. It is expected that data from this trial will support a PMA Supplement, which will be submitted to the FDA for the use of the Novacor LVAS for destination therapy. However, there can be no assurance that the FDA will act quickly or favourably in the review of this application to market.
The Novacor II is expected to begin animal trials in 2005, commence initial clinical trials in 2007 and receive commercial approvals in Europe in 2008 and in the United States in 2010. In addition, the MedQuest rotary VAD is expected to commence an initial feasibility clinical trial in 2005 and is expected to receive commercial approvals in Europe in 2007 and in the United States in 2009.
There can be no assurance that the FDA, Health Canada or any other regulatory authority will act favorably or quickly in its review of our applications, if and when made, and significant difficulties and costs may be
52
encountered by us to obtain such approvals that could delay or preclude us from selling our next-generation products in the United States, Europe, Canada and elsewhere. Failure to receive, or delays in receiving, such approvals, including the need for extended clinical trials or additional data as a prerequisite to approval, limitations on the intended use of our next-generation products, the restriction, suspension or revocation of any approvals obtained or any failure to comply with approvals obtained could have a material adverse effect on our business, financial condition and results of operations.
We are dependent on a limited number of products.
Our future financial performance is expected to depend on the successful sales and marketing of the Novacor LVAS, the development, introduction, customer acceptance and successful sales and marketing of WorldHeart's Novacor II and, if the MedQuest Acquisition is completed, of a rotary VAD. To date, all of our revenues have resulted from sales of the Novacor LVAS and related technologies. The MedQuest rotary VAD is now at an advanced stage of development and is expected to commence initial feasibility clinical trial in 2005, and is expected to receive commercial approvals in Europe in 2007 and in the United States in 2009. Our Novacor II is expected to begin animal trials in 2005, commence initial clinical trials in 2007 and receive commercial approvals in Europe in 2008 and in the United States in 2010. There is presently limited pre-clinical data relating to our Novacor II and no applications for any clinical trials or regulatory approvals have been made. Prior to any commercial use, the technologies relating to the Novacor II and to the MedQuest rotary VAD will require pre-clinical and extensive clinical testing, as well as regulatory approvals.
Our future financial performance will depend, in significant part, on successful development, introduction and customer acceptance of our current generation Novacor LVAS and future Novacor II and MedQuest rotary VAD products. We currently have no clinical data relating to our products under development. Prior to any commercial use, our products currently under development will require significant additional capital for research and development efforts, extensive pre-clinical and clinical testing and regulatory approval.
New product development is highly uncertain and unanticipated developments, clinical and regulatory delays, adverse or unexpected side effects or inadequate therapeutic efficacy could delay or prevent the successful commercialization of the Novacor II and of the MedQuest rotary VAD. There can be no assurance that we will not experience difficulties that could delay or prevent the commercialization of the Novacor II or of the MedQuest rotary VAD. Any significant delays in, or premature termination of, clinical trials of our products under development could have a material adverse effect on our business, financial condition and results of operations.
Market acceptance of our technologies and products is uncertain.
The Novacor LVAS and our next-generation Novacor II and MedQuest rotary VAD represent ventricular assist technologies that must compete with other products as well as with other therapies for heart failure, such as medication, transplants, cardiomyoplasty and total artificial heart devices.
Failure of our products and their underlying technologies to achieve significant market acceptance could have a material adverse effect on our business, financial condition and results of operations.
We face significant competition and technological obsolescence of our products.
In addition to competing with other less-invasive therapies for heart failure, including drugs and pacing, our products will compete with ventricular assist technology being developed and sold by a number of other companies. Competition from device companies and medical device subsidiaries of healthcare and pharmaceutical companies is intense and expected to increase.
Some of our competitors have financial, technical, manufacturing, distribution and marketing resources substantially greater than ours. Third parties may succeed in developing or marketing technologies and products which are more effective than those developed or marketed by us or that could render our technology and products non-competitive or obsolete, or we may not be able to keep pace with technological developments, all of which could have a material adverse effect on our business, financial condition and results of operations.
53
There are limitations on third-party reimbursement for the cost of implanting our devices.
Individual patients will seldom be able to pay directly for the costs of implanting our devices. Successful commercialization of our products will depend in large part upon the availability of adequate reimbursement for the treatment and medical costs associated with the products from third-party payers, including governmental and private health insurers and managed care organizations. Consequently, we expect that our products will typically be purchased by healthcare providers, clinics, hospitals and other users who will bill various third-party payers, such as government programs and private insurance plans, for the healthcare services provided to their patients.
The coverage and the level of payment provided by third-party payers in the United States and other countries vary according to a number of factors, including the medical procedure, third-party payer, location and cost. In the United States, many private payers follow the recommendations of the CMS, which establishes guidelines for the governmental coverage of procedures, services and medical equipment.
There can be no assurance with respect to any markets in which we seek to distribute our products that third-party coverage and reimbursement will be adequate, that current levels of reimbursement will not be decreased in the future or that future legislation, regulation or reimbursement policies of third-party payers will not otherwise adversely affect the demand for our products or our ability to sell our products on a profitable basis, particularly if the installed cost of our systems and devices should be more expensive than competing products or procedures. The unavailability of third-party payer coverage or the inadequacy of reimbursement could have a material adverse effect on our business, financial condition and results of operations.
We control intellectual property and if we cannot protect our intellectual property, our business could be adversely affected.
Our intellectual property rights and those we will acquire from MedQuest are and will continue to be a critical component of our success. The loss of critical licenses, patent or trade secret protection for technologies or know-how relating to our current product and our products in development could adversely affect our business prospects. Our business will also depend in part on our ability to defend our existing and future licenses, patents and rights and conduct our business activities free of infringement claims by third parties. We intend to seek additional patents, but our pending and future patent applications may not be approved, may not give us a competitive advantage and could be challenged by others. Patent proceedings in the United States and in other countries may be expensive and time consuming. In addition, patents issued by foreign countries may afford less protection than is available under United States patent law, and may not adequately protect our proprietary information. Our competitors may independently develop proprietary non-infringing technologies and processes that are substantially equivalent to ours, or design around our patents. Companies in the medical device industry typically obtain patents and frequently engage in substantial intellectual property litigation. Our products and technologies could infringe on the rights of others. If a third party successfully asserts a claim for infringement against us, we may be liable for substantial damages, we may be unable to sell products using that technology or we may have to seek a license or redesign the related product. These alternatives may be uneconomical or impossible. Patent litigation could be costly, result in product development delays, and divert the efforts and attention of management from our business.
The validity of any of our critical licenses or patents may be challenged by others, and we could encounter legal and financial difficulties in enforcing our licenses and patent rights against alleged infringers. In addition, others could develop technologies or obtain patents, which would render our patents and patent rights obsolete. Although we do not believe patents are the sole determinant in the commercial success of our products, the loss of a significant percentage of our licenses or patents could have a material adverse effect on our business, financial condition and results of operations.
Claims by competitors and other third parties that our products allegedly infringe the patent rights of others could have a material adverse effect on our business. The medical device industry is characterized by frequent and substantial intellectual property litigation. The cardiovascular device market is characterized by extensive patent and other intellectual property claims. Intellectual property litigation is complex and expensive and the outcome of this litigation is difficult to predict. Any future litigation, regardless of outcome, could result in
54
substantial expense and significant diversion of the efforts of WorldHeart's technical and management personnel. An adverse determination in any such proceeding could subject us to significant liabilities or require us to seek additional licenses from third parties or pay royalties that may be substantial. Furthermore, we cannot assure that necessary licenses would be available on satisfactory terms, or at all. Accordingly, an adverse determination in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing or selling certain of our products, any of which could have a material adverse effect on our business, financial condition and results of operations.
We are exposed to product liability claims.
Our business exposes us to an inherent risk of potential product liability claims related to the manufacturing, marketing and sale of the Novacor LVAS, the Novacor II and, if the MedQuest Acquisition is completed, the MedQuest rotary VAD, by device recipients in whom the devices are implanted or by their families. Claims of this nature, if successful, could result in substantial damage awards to the claimants, which may exceed the limits of any applicable insurance coverage held by WorldHeart. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations. Claims against WorldHeart, regardless of their merit or potential outcome, could also have a material adverse effect on our ability to obtain physician endorsement of our products or to expand our business, which could have a material adverse effect on our business, financial condition and results of operations.
We face risks associated with our manufacturing operations, risks resulting from dependence on third-party manufacturers, and risks related to dependence on sole suppliers.
The manufacture of our products and MedQuest products is a complex operation involving a number of separate processes and components. Material costs are high and certain of the manufacturing processes involved are labor-intensive. Achieving continued cost reductions will depend upon our ability to reduce material costs and reduce the time required to complete certain manufacturing processes. The conduct of manufacturing operations is subject to numerous risks, including reliance on third-party manufacturers, unanticipated technological problems and delays. WorldHeart, or any entity manufacturing products or components on our behalf, may not be able to comply with applicable governmental regulations or satisfy regulatory inspections in connection with the manufacture of our products. Failure or delay by WorldHeart or any third-party manufacturer of our products or product components to comply with applicable regulations or to satisfy regulatory inspections could have a material adverse effect on our business, financial condition and results of operations.
We are currently dependent on single-source third-party manufacturers for several of the components used in the Novacor LVAS. We do not have agreements with many of such single-source manufacturers and purchase these components pursuant to purchase orders placed from time to time in the ordinary course of business. We are substantially dependent on the ability of these manufacturers to provide adequate inventories of these components on a timely basis and on favourable terms. These manufacturers also produce components for certain of our competitors, as well as other large customers, and there can be no assurance that such manufacturers will have sufficient production capacity to satisfy our inventory or scheduling requirements during any period of sustained demand, or that we will not be subject to the risk of price fluctuations and periodic delays. Although we believe that our relationships with our manufacturers are satisfactory and that alternative sources for the components we currently purchase from single-source suppliers are currently available, the loss of the services of such manufacturers or substantial price increases imposed by such manufacturers, in the absence of readily available alternative sources of supply, would have a material adverse effect on us. Failure or delay by such manufacturers in supplying components to us on favourable terms could also adversely affect our operating margins and our ability to develop and deliver our products on a timely and competitive basis which could have a material adverse effect on our business, financial condition and results of operations.
55
We are dependent on key personnel.
As a result of the specialized scientific nature of our business, we are dependent on our ability to attract and retain qualified scientific, technical and key management personnel. We face intense competition for such persons and we may not be able to attract or retain such individuals.
On August 25, 2004, we announced plans to consolidate our operations from Ottawa, Ontario to Oakland, California over a period of nine months and, as a result, employees previously based in Ottawa have been terminated. The failure to manage successfully the geographic consolidation and the integration of new employees could have a material adverse effect on our business, financial condition and results of operations.
In the past MedQuest has depended heavily on the contributions of its President and Chief Executive Officer, certain directors, and several other principal members of its technical, sales and support, regulatory and clinical, operating and administrative management and staff, many of whom would be difficult to replace. It is anticipated that all key MedQuest employees will become employees of WorldHeart upon completion of the MedQuest Acquisition. However, there is no assurance that we will be able to retain these individuals.
The price of our shares is highly volatile.
As a one-product, small capitalization biotechnology company, the price of our common shares has been, and is likely to continue to be, highly volatile. Future announcements concerning us or our competitors, quarterly variations in operating results, introduction of new products, delays in the introduction of new products or changes in product pricing policies by us or our competitors, acquisition or loss of significant customers, partners, distributors and suppliers, changes in earnings' estimates by analysts, regulatory developments, or fluctuations in the economy or general market conditions, among other factors, could cause the market price of our common shares to fluctuate substantially. There can be no assurance that the market price of our common shares will not decline below its current price or that it will not experience significant fluctuations in the future, including fluctuations that are unrelated to our performance.
The stock market in recent years has experienced extreme price and volume fluctuations that have particularly affected the market prices of many smaller companies. The trading price of our common shares is expected to be subject to significant fluctuations in response to variations in quarterly operating results, announcements of innovations by us or our competitors, general conditions in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions, may have a material adverse effect on the market price of our common shares.
We incur risks associated with acquisitions.
We evaluate, and following the MedQuest Acquisition will continue to evaluate, potential acquisitions of complementary businesses, products and technologies. Such acquisitions could subject us to numerous risks, including risks associated with the integration into WorldHeart of new employees and technology. Moreover, the negotiation and completion of such transactions involves the diversion of resources from our core business operations. In addition, acquisitions could result in immediate and substantial dilution to our existing shareholders, large one-time write-offs or the creation of goodwill or other intangible assets. The failure to successfully evaluate, negotiate, effect and integrate an acquisition transaction could have a material adverse effect on our business, financial condition and results of operations.
We incur risks associated with changes in currency valuations.
WorldHeart's business is conducted in multiple currencies and WorldHeart may be subject to foreign exchange losses.
WorldHeart's functional and reporting currency is the United States dollar. However, certain assets, liabilities, revenues and expenses are dominated and occur in foreign currencies including the Euro and Canadian dollar. There can be no assurances that WorldHeart will not suffer losses associated with fluctuations of the United States dollar compared to these foreign currencies.
56
Risk Factors Related to the MedQuest Acquisition, the Maverick Private Placement and the Warrant Exercise & Debenture Conversion
The availability of additional common shares upon the completion of the MedQuest Acquisition, the Maverick private placement and the Warrant Exercise & Debenture Conversion could depress the price of our common shares.
Immediately following the completion of the MedQuest Acquisition, the Maverick private placement and the Warrant Exercise & Debenture Conversion a significant number of additional WorldHeart common shares will be available for trading in the public market. The additional shares in the market may cause the price of our common shares to decline. Also, if our shareholders sell substantial numbers of our common shares in the public market following completion of the transactions, the market price of our common shares could fall. These sales might also make it more difficult for us to sell equity or equity related securities at a time and price that we would deem appropriate. All of the common shares of WorldHeart issued to MedQuest pursuant to the MedQuest Acquisition and to Maverick pursuant to the private placement will be registered shortly following completion of those transactions, and following registration will become freely tradable without restrictions or further registration. Immediately upon completion of the Warrant Exercise & Debenture Conversion all of the WorldHeart common shares issued pursuant to that transaction, will become freely tradable without restrictions or further registration, in each case, under the Securities Act of 1933, as amended, unless the shares are held by an "affiliate" of WorldHeart, as that term is defined under the Securities Act of 1933, as amended.
The MedQuest Acquisition may stimulate competition and we may not be able to compete successfully.
The MedQuest Acquisition may cause our competitors to enter business combinations or accelerate product development. These and other competitive practices could create more powerful or aggressive competitors. There is a risk that we will not be able to compete successfully as future markets evolve. Increased competitive pressure could lead to lower sales and prices of our products, and this could harm our business, results of operations and financial condition.
The integration of the WorldHeart and MedQuest businesses may be costly and we may fail to successfully effect the integration.
Our ability to realize some of the anticipated benefits of the MedQuest Acquisition will depend in part on our ability to integrate MedQuest's operations into our current operations in a timely and efficient manner. The integration process will require significant efforts from each of our management and the management of MedQuest. The MedQuest rotary VAD development will continue to be conducted from the current MedQuest facilities in Salt Lake City, Utah. The integration process may distract management's attention from the day-to-day business of the combined company. If we are unable to successfully integrate the operations of MedQuest or if this integration process is delayed or costs of integration are more than expected, our business, operating results and financial condition may be negatively impacted.
The concentration of our capital stock ownership following the completion of the MedQuest Acquisition, private placement to Maverick and Warrant Exercise & Debenture Conversion will limit your ability to influence corporate matters.
As of June 10, 2005, one shareholder exercised control over approximately 14.33% of our issued common shares. Upon completion of the MedQuest Acquisition and the private placement to Maverick and the related conversion of debentures and exercise of warrants, Maverick, directly and indirectly, will control approximately 33% of our issued common shares. Maverick is a limited liability company controlled by Kevin Compton. As such, Maverick will exercise significant influence over all matters requiring shareholder approval, including the election of directors and significant corporate acquisitions such as a merger or other sale of our company or its assets for the foreseeable future. This concentrated control will limit your ability to influence corporate matters, and, as a result, we may take actions that our shareholders do not view as beneficial. As a result, the market price of our common shares could be adversely affected.
57
MATERIAL CONTRACTS
On June 30, 2000, we entered into a distribution agreement with Edwards whereby Edwards would be the sole distributor, except in the United States, of our heart assist and heart replacement products for a period of five years commencing July 1, 2000. As a result of the distribution agreement, WorldHeart was obligated to pay Edwards a minimum annual fee of $2 million less the actual gross margin for the duration of the agreement. On June 30, 2000, we also entered into a supply agreement with Edwards whereby Edwards would be the sole supplier of certain components of our heart assist products for a period of five years.
On December 31, 2003, WorldHeart amended its distribution agreement with Edwards. Since WorldHeart's acquisition of Novacor in 2000, Edwards had been responsible for worldwide distribution of the Novacor LVAS and associated hardware with the exception of the United States where WorldHeart sells directly. In an effort to have greater control over its sales, distribution and customer support WorldHeart entered into the amendment in order to take direct responsibility for worldwide distribution of the Novacor LVAS and related products worldwide with the exception of Japan, where Edwards will continue to be the exclusive distributor for WorldHeart. In conjunction with this arrangement, WorldHeart and Edwards entered into a transition services agreement on December 31, 2003, under which for a period of three months Edwards would assist WorldHeart until WorldHeart's European operations were fully functional. WorldHeart also entered into an interim distribution agreement with a Netherlands company, referred to in this circular as the interim European distributor, which commenced on December 31, 2003 and terminated on June 30, 2004. The interim European distributor provided WorldHeart with distribution capabilities in Europe until WorldHeart's European distribution infrastructure was in place and operational. In order to allow the interim European distributor to service the European market without interruption, the interim European distributor purchased all of Edwards' Novacor LVAS kits and related equipment inventory as at December 31, 2003 which totalled approximately €2.0 million and which could be resold only to customers of WorldHeart, at WorldHeart-negotiated terms. WorldHeart, upon request from the interim European distributor, was required to purchase any unsold inventory that was acquired from Edwards. In 2003, WorldHeart repurchased a total of $1,551,324 in inventory from the interim European distributor.
On May 24, 2000, WorldHeart, Edwards, Edwards Novacor LLC and World Heart Inc. entered into a contribution agreement pursuant to which WorldHeart acquired the assets and liabilities of Novacor from Edwards through World Heart Inc. Pursuant to that agreement, on June 30, 2000, we issued 1,374,570 Series A Shares to Edwards Lifesciences (U.S.) Inc. for $20 million that were convertible at Edward's option after June 30, 2006 into 196,367 post-consolidation common shares (representing a per share conversion price of $101.85), plus additional common shares for the accumulated but unpaid dividends to the date of conversion, without payment of additional consideration. These preferred shares were non-voting. Edwards could nominate one director of WorldHeart pursuant to a shareholders agreement. These shares were callable for cash at the face amount plus accumulated but unpaid dividends at our option at any time up to June 30, 2007, at which time they were mandatorily redeemable for the face amount of $20.0 million plus accumulated dividends. Dividends accumulated at 5% per year for the first three years and 10% per year for years four through seven.
On September 22, 2003, we entered into a conversion agreement with Edwards Lifesciences (U.S.) Inc. which provided that, subject to shareholder approval, WorldHeart would acquire for cancellation the 1,374,570 Series A Shares of WorldHeart held by Edwards Lifesciences (U.S.) Inc. by issuing to Edwards Lifesciences (U.S.) Inc. upon the conversion of those shares, 500,000 common shares of WorldHeart and 1,000,000 warrants, each exercisable for one common share of WorldHeart. On November 25, 2003, WorldHeart's shareholders approved an amendment to the articles of incorporation of WorldHeart to permit the conversion of 1,374,570 Series A Shares of WorldHeart into 500,000 common shares and warrants to acquire 1,000,000 common shares at an exercise price of Cdn$8.05 per common share, expiring November 27, 2008. The exercise price was subsequently adjusted to Cdn$7.49 as a result of the financing in September 2004. On November 26, 2003, WorldHeart filed articles of amendment to amend the terms of the Series A Shares and on November 27, 2003, Edwards Lifesciences (U.S.) Inc. converted its 1,374,570 Series A shares into 500,000 common shares and warrants to acquire 1,000,000 common shares.
On May 24, 2000, in addition to the agreement to acquire Novacor assets, WorldHeart and Edwards also entered into an exchange agreement pursuant to which Edwards received the right for one year following the
58
second anniversary of the issuance to it of 4,981,128 Series A cumulative participating preferred shares of World Heart Inc. to require us to exchange those shares for an equal number of WorldHeart common shares. On May 8, 2002, WorldHeart and Edwards amended the exchange agreement and the contribution agreement to extend the period of time within which Edwards could require the exchange of shares to two years and to extend the commencement date of the exchange window by one year. As consideration for the extension of this period, Edwards agreed that the dividend payable on its preferred shares would be decreased by one percent effective July 1, 2002. On September 22, 2003, WorldHeart and Edwards amended the exchange agreement to shorten the commencement date of the exchange window by one year and Edwards exchanged the 4,981,128 Series A cumulative participating preferred shares of World Heart Inc. for 711,589 common shares.
In November 2001, WorldHeart was approved for a grant from Technology Partners Canada (TPC) pursuant to a TPC agreement with the Minister of Industry for Canada. The amount received pursuant to this grant was Cdn$9.98 million in connection with the prototype development and clinical trials of HeartSaver, WorldHeart's initial proposed VAD. These costs are subject to audit by Industry Canada. WorldHeart was initially required to pay TPC a royalty equal to 1% of gross revenues from the first version of HeartSaver for a period of six years from commencement of commercial sales. As part of the agreement, TPC received warrants for 92,857 common shares of WorldHeart, exercisable until December 2006 at an exercise price of Cdn$46.27 per share. On May 21, 2003, the agreement was amended in order to allow changes in the HeartSaver development program to be considered eligible costs. TPC's royalty was also amended to 0.65% of consolidated gross business revenues for a period commencing in January 2004 and ending in January 2012. In the event that cumulative royalty payments have not reached Cdn$26.4 million by this date, the royalty period will continue for a further three years or until the cumulative royalties reach Cdn$26.4 million, whichever comes first.
In connection with the formation of 2007262 Ontario Inc., on December 19, 2001, we entered into an asset transfer agreement with 2007262 Ontario Inc. pursuant to which WorldHeart sold to 2007262 Ontario Inc. certain technology in exchange for 100,000 Series 2 preferred shares of 2007262 Ontario Inc. and a promissory note in the amount of Cdn$2,000,000. The promissory note was repaid to WorldHeart from the proceeds of Series 1 preferred shares of 2007262 Ontario Inc., subscribed for by New Generation Biotech (Equity) Inc. or NewGen for a subscription price of Cdn$3,503,500. The balance of the Series 1 preferred share proceeds were used to improve and enhance the technology transferred by WorldHeart. On January 31, 2003, and pursuant to the terms of the agreement, NewGen redeemed the Series 1 preferred shares for 91,000 common shares and 91,000 warrants. Each warrant was exercisable into one common share of WorldHeart at a price of Cdn$42.07 at any time up to January 24, 2004; however, these warrants expired unexercised.
In separate transactions on January 3, 2003 and January 8, 2003, we completed private placements of units for gross proceeds of Cdn$3,000,000 (issue costs were Cdn$384,728). The units comprise a total of 334,821 common shares and warrants to acquire 334,821 common shares at an exercise price of Cdn$11.20 per share for a period of five years. In association with the transaction we also granted 33,482 broker warrants, to the placement agent. Each broker warrant is exercisable at a price of Cdn$11.20 per share into one common share and one compensation warrant at any time prior to December 31, 2004. Each compensation warrant is exercisable into one common share of WorldHeart at a price of Cdn$11.20 per common share at any time prior to December 31, 2007.
On January 28, 2003, we entered into a senior loan agreement for loans totaling Cdn$7,000,000 and a subordinated loan agreement for loans totaling Cdn$3,000,000 and an intercreditor agreement. The loans were originally scheduled to mature on July 31, 2003 and bear interest rates of 18% per annum, payable monthly in arrears. The loans were to be repaid in full on the earlier of the maturity date, and the completion of a replacement financing. These loans were amended to extend the maturity date from July 31, 2003 to August 15, 2003 and then further extended to September 23, 2003. The loans were repaid on September 23, 2003. We paid the lenders a fee of 4% and issued them a total of 428,571 warrants, with each warrant exercisable into one common share of WorldHeart for a period of five years at a price of Cdn$11.20 per share. As collateral for the loans, we entered into general security agreements granting a security interest in all of our assets. Upon repayment of the loans, the security agreements were discharged.
On April 2, 2003, we issued 142,857 common shares, at a price of Cdn$11.20 per share for gross proceeds of Cdn$1,600,000 pursuant to the exercise of previously issued warrants with an expiry date of January 2, 2008. As
59
compensation for the early exercise of the warrants an additional 251,428 warrants were issued. These warrants are exercisable into common shares of WorldHeart at Cdn$11.20 per share until April 2, 2008.
On July 23, 2003, we entered into a Note Purchase Facility Agreement with Export Development Canada to assist us in our export marketing activities by permitting customers outside of Canada to pay for purchases by issuing a note payable at an agreed future date, with Export Development Canada purchasing the note from us. The facility is for a maximum of $4,000,000 and will carry an interest rate as set for each note purchase by Export Development Canada, payable semi-annually in arrears.
On July 30, 2003, we issued Senior Subordinated convertible debentures in the principal amount of Cdn$1,200,000, guaranteed by World Heart Inc. The debentures had a maturity date of October 29, 2003 and were convertible at the holder's option into common shares of WorldHeart for Cdn$7.00 per share. The debentures were callable by us at any time after September 15, 2003, upon payment of the outstanding principal amount plus accrued interest, subject to the prior right of the holders to convert. The debentures had an interest rate of 14% per annum, and, in connection with the sale, warrants to purchase 171,428 common shares at an exercise price of Cdn$8.40 per share for a period of three years were issued. As security for the debentures, we entered into a general security agreement granting a security interest in all of our assets. The debentures were repaid, in full, on September 23, 2003 and the security agreements were discharged.
On September 4, 2003, we entered into a Bridge Loan Agreement for a loan of Cdn$1,000,000. The loan had an interest rate of 14% per annum, payable monthly in arrears and was repayable, in full, at the earlier of the completion of a financing event and October 22, 2003. The loan also carried a fee of 4%, payable at the time of repayment, and in connection with the loan we issued 118,571 warrants with each warrant exercisable into one common share of WorldHeart at a price of Cdn$8.40 per share, until October 16, 2006. This loan was repaid, in full, on September 23, 2003.
On September 10, 2003, we entered into the Novacor Liquidating Trust Buyout Agreement which provided WorldHeart with a right to repurchase, upon the payment of $1,500,000, the Novacor Liquidating Trust's entitlement to royalties on revenues generated from the sale of the Novacor LVAS and equipment. On September 22, 2003 we exercised our repurchase right.
On September 23, 2003, we completed a private placement of units in Canada and the United States for gross proceeds of Cdn$63,492,980 (issue costs were Cdn$6,692,980). In total, the units comprise 10,671,351 common shares and warrants to purchase 10,671,351 common shares of WorldHeart at an exercise price of Cdn$8.05 per common share (subject to adjustment as provided by the terms of the warrant and warrant indenture) until September 22, 2008. The exercise price was subsequently adjusted to Cdn$7.42 as a result of the financing in September 2004. In association with the transaction we also granted agents' warrants to the agents exercisable for 1,004,536 common shares at a price of Cdn$8.05 per common share for a period of five years. As part of the private placement, WorldHeart entered into, on September 23, 2003, a registration rights agreement with the purchasers of units in the United States in which we agreed to register the common shares and the common shares that are issuable upon exercise of the warrants held by them. On October 22, 2003, we filed a registration statement on Form F-3 (File No. 333-109876) with the SEC, which the SEC declared effective on October 30, 2003. On December 23, 2003 we filed a registration statement on Form F-3 (File No. 333-111512) with the SEC, which the SEC declared effective on February 4, 2004 to register the common shares and the common shares issuable upon the exercise of the warrants held by the Canadian purchasers in the private placement completed in September 2003. We also agreed to use commercially reasonable efforts to cause that registration statement to remain continuously effective until the earlier of (i) such date as all of the securities that can be sold under the prospectus which forms a part of that registration statement by the selling security-holders have been sold, and (ii) such time as the securities covered by the prospectus which forms a part of that registration statement are no longer subject to restrictions on resale as provided in Rule 144(k) under the Securities Act of 1933. We agreed, in the United States purchase agreement, that we would prepare and submit to Nasdaq an application to list our common shares on NASDAQ. On March 18, 2004, our common shares were relisted on NASDAQ.
We also entered into a warrant indenture with CIBC Mellon Trust Company, as warrant agent, which sets forth the terms and conditions of the warrants issued to the purchasers of units in Canada and the United States and an agency agreement with the Canadian agent, Research Capital Corporation, which set forth certain representations and warranties and terms and conditions with respect to the issue of units to purchasers in Canada.
60
On July 28, 2004, we entered into a severance agreement with Roderick M. Bryden, former President and Chief Executive Officer of WorldHeart. Pursuant to the severance agreement, Mr. Bryden was paid a settlement amount, in two payments which have been paid in full, of Cdn$717,680, less any required withholdings, representing notice, severance and vacation pay.
On September 16, 2004, we completed the sale of $13,318,750 aggregate principal amount of convertible debentures and warrants. The convertible debentures are convertible, at the option of the holder, into common shares at a conversion rate of $1.25 per common share until September 15, 2009. The purchasers of the convertible debentures were also issued a total of 10,655,000 warrants to purchase common shares at an exercise price of $1.55 per common share until September 15, 2009. The convertible debentures earn interest at the rate of 3% per annum, non-compounding. The interest accrues until maturity, and the aggregate amount of interest is convertible into common shares based on the market price of the common shares at the time of conversion or maturity. As part of the sale of the convertible debentures and warrants, a total of 193,800 agent's warrants were granted to the Canadian agent who participated in the private placement of the convertible debentures and warrants. Each agent's warrant is exercisable for one common share at an exercise price of $1.55 per common share until September 15, 2009. Also as part of the sale of the convertible debentures and warrants, a total of 470,833 common shares were issued to the United States agent who participated in the private placement of the convertible debentures and warrants. As part of the private placement, WorldHeart entered into, on September 15, 2004, a registration rights agreement with the debenture holders in which we agreed to register the common shares issuable upon the conversion of the debentures and accrued interest and the exercise of the warrants held by them. On October 14, 2004, we filed a registration statement on Form F-3 (File No. 333-119750) with the SEC which the SEC declared effective on November 2, 2004. We also agreed to use commercially reasonable efforts to cause that registration statement to remain continuously effective until the earlier of (i) such date as all of the securities that can be sold under the prospectus which forms a part of that registration statement by the selling security-holders have been sold, and (ii) such time as the securities covered by the prospectus which forms a part of that registration statement are no longer subject to restrictions on resale as provided in Rule 144(k) under the Securities Act of 1933. See "MATTERS TO BE ACTED UPON — Warrant Amendment Resolution — Amendment to September 2004 Warrants" at page 84.
MATTERS TO BE ACTED UPON
Audited Consolidated Financial Statements
WorldHeart's U.S. GAAP audited consolidated financial statements for the year ended December 31, 2004 and Canadian GAAP audited consolidated financial statements for the year ended December 31, 2004, including the reports of the auditors, will be placed before the shareholders at the Annual and Special Meeting for the purposes of discussion or comment by the shareholders. The audited consolidated financial statements are not approved by a resolution by the shareholders. The U.S. GAAP audited consolidated financial statements and Canadian GAAP audited consolidated financial statements are attached as Appendices B-1 and B-2, respectively, to this circular.
Election of Directors
The Articles of Incorporation of WorldHeart provide for a board of directors of not less than one and not more than ten directors to be elected annually. The number of directors is currently fixed at five. Accordingly, WorldHeart's management proposes to nominate five persons to be elected at the Annual and Special Meeting. Each nominee for election as a director is currently a director of WorldHeart.
On September 23, 2003, WorldHeart completed a private placement transaction in Canada and the United States of units, each unit consisting of one common share and one warrant exercisable for one common share (the Financing). Pursuant to the purchase agreement dated as of September 22, 2003 (the Purchase Agreement) between WorldHeart and the purchasers of units in the United States, Special Situations Funds on behalf of the United States investors who participated in the financing, has the right to nominate two persons for election or appointment to the board of directors of WorldHeart, provided Special Situations Funds and the other United States investors maintain ownership of 25% of the registrable securities purchased in the financing, namely, common shares and common shares issuable upon the exercise of the warrants. The registrable
61
securities cease to be such when they are sold or become eligible for sale pursuant to Rule 144(k) under the Securities Act of 1933. Robert J. Majteles2 and John F. Carlson are the nominees of Special Situation Funds.
- 2
- Mr. Majteles is the managing member of Treehouse Capital, LLC (Treehouse), an investment firm. Special Situations Fund III, L.P., Special Situations Cayman Fund, L.P., Special Situations Private Equity Fund, L.P. and Special Situations Fund, L.P. have entered into an agreement with Mr. Majteles and Treehouse pursuant to which Treehouse, through Mr. Majteles, provides certain management and financial advisory services for the funds on request. If Mr. Majteles's services are requested by the funds with respect to a particular portfolio investment, Treehouse is entitled to 10% of the funds' net gain (as defined) or net loss (as defined) on the investment during the term of the agreement, offset by certain fees that may be paid by the portfolio company to Treehouse or Mr. Majteles directly. Under the agreement, Mr. Majteles is required to act independently of the funds in discharging his fiduciary duties to shareholders of any company for which he serves as a member of the board of directors and also is obligated not to disclose to the funds or use for his own benefit any confidential information he obtains in connection with his service for a particular portfolio company. Mr. Majteles does not have or share voting or dispositive power over any securities held by the funds. Mr. Majteles has agreed to serve as a director of WorldHeart pursuant to this agreement.
Pursuant to the agency agreement dated September 22, 2003 between WorldHeart and Research Capital Corporation (Research), as agent for the Canadian purchasers of units, Research had the right to nominate one person for election or appointment to the board of directors. William Garriock was its nominee. Despite the right to a nominee being terminated, Mr. Garriock continues as an independent director of WorldHeart and will stand for re-election at this Annual and Special Meeting.
The following table lists certain information concerning each of the persons proposed to be nominated for election as a director, the date on which he first became a director of WorldHeart, his principal occupation, business or employment during the last five years, the number of shares and options held, and all other positions with WorldHeart, if any:
|
Name
| | Age
| | Director Since
| | Position with WorldHeart
| | Number of
Common Shares
and Options Held
|
|---|
|
C. Ian Ross(1)(2)(3)(4)
Collingwood, Ontario,
Canada | | 63 | | February 22, 2000 | | Director of WorldHeart, Chair of the Board of Directors and Chair of the Corporate Governance and Nominating Committee. | | 500 shares
130,365 options(7) |
| From July 1999 to September 2003, Mr. Ross was Senior Director, Administration in the Dean's Office at the Richard Ivey School of Business, University of Western Ontario, with responsibility for managing the support functions for the business school. Mr. Ross joined the business school in 1997 as Executive in Residence for the business school's Institute for Entrepreneurship, Innovation and Growth. |
| Since September 2003, Mr. Ross has been a professional company director. Mr. Ross serves as a director of Ontario Power Generation, which is a wholly-owned business corporation, charged with managing and operating Crown provincial electricity generation assets, GrowthWorks Canadian Fund (Chair) and GrowthWorks Opportunity Fund (Chair), both labour-sponsored venture capital corporations, PetValu Canada Inc. (Chair), a specialty retailer of pet food and pet supplies in Canada and the United States, Comcare Health Services, a community-based company providing health care solutions and Praeda Management Systems Inc., a management systems development company providing software to police and the courts. |
|
62
John F. Carlson(1)(3)(4)
St. Paul, Minnesota,
United States | | 66 | | December 3, 2003 | | Director of WorldHeart and Chair of the Audit Committee | | 130,000 options(8) |
| Mr. John F. Carlson is the former Chair and Chief Executive Officer of Cray Research, Inc., a supercomputer manufacturer and a predecessor to the business of Cray Inc., and held those positions from 1992 to 1995. Mr. Carlson joined Cray Research, Inc. in 1976 and held several positions at Cray Research, Inc., including as Chief Financial Officer. He received his CPA certificate in 1966. |
| Since 2000, Mr. Carlson has been a professional company director. Mr. Carlson serves as a director on a number of private and public company boards including BioNumerik Pharmaceuticals Inc. since June 1995. BioNumerik Pharmaceuticals Inc. is a pharmaceutical company focused on the design and development of small molecule drugs for treating patients with cancer. Mr. Carlson is Chair and a director of each of BioEnergy, Inc. and Excorp Medical, Inc. BioEnergy, Inc. is a life sciences company utilizing proprietary platform technologies centered on cellular energy metabolism to improve cardio-vascular health and tissue function. Excorp Medical, Inc. is developing a bio-artificial liver system intended for use in hospitals, almost exclusively in intensive care units. Mr. Carlson is also a director of Superconductor Technologies Inc., a wireless infrastructure company. |
|
William C. (Bill) Garriock(1)(2)(3)(4)(5)
Toronto, Ontario,
Canada | | 66 | | December 3, 2003 | | Director of WorldHeart and Chair of the Strategic Planning Committee | | 130,000 options(9) |
| Mr. Garriock is a professional company director and the retired Chair (a position held from 2000 to 2003) and former President (a position held from 1994 to 1999) of MDS SCIEX, the analytical instrument division of MDS Inc. Mr. Garriock was also the Executive-at-Large for MDS Inc, a health and life sciences company from 2000 to 2003. From 1993 to 1994, he was Vice President and Managing Partner (Pharmaceuticals) of MDS Health Ventures Inc. following 18 years as President and CEO of Miles Canada Inc. (now Bayer Canada Inc.), a pharmaceutical, diagnostics and consumer products company. |
| He serves as Chair or director of a number of public and private companies, including Nexia Biotechnologies Inc., a company that develops and manufactures complex recombinant proteins in the form of biomaterials with industrial and medical applications and biopharmaceutical products, Aegera Therapeutics Inc., a company developing new therapeutics to enhance existing cancer treatment paradigms, GSW Inc., a leading North American manufacturer of consumer durable products, and Cipher Pharmaceuticals Inc., a pharmaceutical development company. |
|
Robert J. Majteles(2)(4)
Piedmont, California, United States | | 40 | | September 23, 2003 | | Director of WorldHeart and Chair of the Compensation Committee | | 130,000 options(10) |
| Mr. Majteles founded Treehouse Capital, LLC (Treehouse), a firm focused on making venture-style investments in micro-cap public technology companies in 2002. Mr. Majteles has been a Chief Executive Officer of three different high tech companies from 1992 to 2002. Prior to leading these companies, Mr. Majteles was a merchant banker with Investment Advisers, Inc. and a mergers and acquisitions attorney at Skadden, Arps, Slate, Meagher & Flom LLP. |
| Currently, Mr. Majteles is on the board of directors of Adept Technology, Inc., a leading manufacturer of flexible automation for the semiconductor, life sciences, electronics and automotive industries, Vertical Communications, Inc., a leading provider of software-based telephone systems, Unify Corporation, a provider of software production solutions that help companies deliver robust and reliable web and business applications, and BigFix, Inc., a developer of software for automating hardware and software maintenance. In addition, Mr. Majteles is a lecturer at the Lester Center for Entrepreneurship, Haas School of Business, University of California, Berkeley and is a Member of the Board of Trustees of the Head-Royce School, Oakland, California. |
|
63
| D. Mark Goudie(6) | | 39 | | August 12, 2004 | | Director and Consultant | | 35 shares
80,000 options(11) |
| Mr. Goudie joined WorldHeart in April 2002 as Director of finance, and on October 1, 2003, Mr. Goudie was appointed Vice President, Finance and Chief Financial Officer. Mr. Goudie resigned from these positions on May 17, 2005. Mr. Goudie will continue to act as a consultant to WorldHeart until September 2005. In May 2005, Mr. Goudie was appointed Chief Financial Officer of Mxi Technologies Inc. Prior to joining WorldHeart, Mr. Goudie was Vice-President of Finance of the Ottawa Senators Hockey Club Corporation and Palladium Corporation from June 1996 to January 1998 and Chief Financial Officer of both corporations from January 1998 to April 2000 and from April 2001 to April 2002. From April 2000 to April 2001, Mr. Goudie served as Chief Financial Officer of Rebel.com Inc. Prior to June 1996, Mr. Goudie was the Vice President of Finance and Administration, CFO of a network services and manufacturing company and the controller of a publicly traded networking equipment manufacturer. |
| Mr. Goudie is a Chartered Accountant who started his career with PricewaterhouseCoopers LLP in the auditing, accounting and tax group before joining the management consulting practice in their business control group. Mr. Goudie holds a Bachelor of Commerce degree from Carleton University in Ottawa, Ontario and is a member of the Ottawa Hospital Foundation board of directors and finance committee. |
|
- (1)
- Member of the Audit Committee.
- (2)
- Member of the Compensation Committee.
- (3)
- Member of the Corporate Governance and Nominating Committee.
- (4)
- Member of the Strategic Planning Committee.
- (5)
- In April 2001, Mr. Garriock assumed the role of Director, Chairman and acting President of Ultravision Inc. to assist in the turnaround of the company. He ceased to be acting President shortly thereafter and resigned as Chairman and Director in July 2002. Ultravision subsequently filed a notice of intent to make a proposal under the Bankruptcy and Insolvency Act (Canada)(BIA) in July 2002 and made an assignment in bankruptcy under the BIA in September 2002.
- (6)
- In July 2001, Rebel.com Inc. was placed in receivership and in November 2001 an order was made to declare it bankrupt. During 2003, Ottawa Senators Hockey Club Corporation was declared to be a debtor company to which theCompanies Creditors Arrangements Act (Canada) applied. During 2003, an interim receiver was appointed for Palladium Corporation pursuant to the BIA.
- (7)
- 365 options for common shares currently exercisable or exercisable within 60 days by Mr. Ross. All of the options held by Mr. Ross are subject to shareholder approval.
- (8)
- No options for common shares are currently exercisable or exercisable within 60 days by Mr. Carlson. All of the options held by Mr. Carlson are subject to shareholder approval.
- (9)
- No options for common shares are currently exercisable or exercisable within 60 days by Mr. Garriock. All of the options held by Mr. Garriock are subject to shareholder approval.
- (10)
- No options for common shares are currently exercisable or exercisable within 60 days by Mr. Majteles. All of the options held by Mr. Majteles are subject to shareholder approval.
- (11)
- 40,000 options for common shares are currently exercisable or exercisable within 60 days by Mr. Goudie. All of the options held by Mr. Goudie are subject to shareholder approval.
On March 22, 2005, WorldHeart announced that Mr. Goudie would be resigning from WorldHeart as Vice President, Finance and Chief Financial Officer in the second quarter of 2005. On May 17, 2005, Mr. Goudie resigned as an officer of WorldHeart but will continue for a period as a director of WorldHeart. Mr. Goudie may be replaced by a nominee of Maverick, if the MedQuest Acquisition is completed and WorldHeart becomes a CBCA corporation. Mr. Goudie will continue in a consulting and advisory capacity until September 2005.
64
AFTER CAREFUL CONSIDERATION, THE CORPORATE GOVERNANCE AND NOMINATING COMMITTEE AND THE BOARD OF DIRECTORS DETERMINED THE ELECTION OF THE FIVE NOMINEES FOR DIRECTORS TO BE IN THE BEST INTERESTS OF WORLDHEART'S SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE SLATE OF NOMINEES FOR DIRECTOR AND RECOMMENDS APPROVAL BY WORLDHEART'S SHAREHOLDERS.
Proxies in the accompanying form appointing the directors and/or officers named therein to act will (unless otherwise instructed) be voted in favour of the election of the five nominees for directors listed above. The five nominees receiving the highest number of affirmative votes of the shares present or represented and entitled to vote shall be elected directors. Votes withheld from any director are counted only for the purposes of determining the absence or presence of quorum for the transaction of business. Management does not anticipate that any of the nominees for election as directors will be unable to serve as a director, but if that should occur for any reason prior to the meeting, the persons named in the enclosed form of proxy reserve the right to vote for another nominee at their discretion. Each of the nominees listed above has consented to be named in this circular, and if elected, to serve as a director. Each director elected will hold office until the next annual meeting of shareholders or until a successor is elected or appointed, unless the office is vacated earlier.
Appointment of Auditors
Each of the Audit Committee and the board of directors of WorldHeart recommends that the shareholders vote for the appointment of PricewaterhouseCoopers LLP, Chartered Accountants, 99 Bank Street, Ottawa, Ontario, K1P 1K6 as auditors of WorldHeart to hold office until the next annual meeting of shareholders at a remuneration to be fixed by the board of directors. PricewaterhouseCoopers LLP have been auditors of WorldHeart since April 1, 1996. The remuneration of the auditors would have to be fixed by the shareholders if the shareholders do not authorize the directors to fix the remuneration of the auditors. The auditors' remuneration is determined by the Audit Committee and board by reviewing the auditors' letter of engagement with respect to the services to be offered by the auditors to WorldHeart. Periodically management also looks at the audit fees reported by comparable companies in their public filings to determine costs in the competitive market place.
A representative from PricewaterhouseCoopers LLP expects to be present at the Annual and Special Meeting. Should PricewaterhouseCoopers LLP desire to make any statements at the Annual and Special Meeting, PricewaterhouseCoopers LLP will have the opportunity to do so. The representative will be available to respond to appropriate questions from shareholders.
For the years ended December 31, 2004 and 2003, the fees paid by WorldHeart for audit work and other services performed by PricewaterhouseCoopers LLP were as follows:
| | Year ended December 31,
| |
|---|
| | 2004
| | 2003
| |
|---|
| Audit Services | | Cdn$ | 230,000 | (1) | Cdn$ | 214,000 | (1) |
| Audit-Related Services | | | — | | | — | |
| Tax Services | | Cdn$ | 90,000 | | Cdn$ | 60,000 | |
| Other Services | | | — | | | — | |
| | |
| |
| |
| Totals | | Cdn$ | 320,000 | | Cdn$ | 274,000 | |
| | |
| |
| |
- (1)
- Included in the Audit Services are fees of Cdn$50,000 (Cdn$35,000 in 2003) related to consultations and review of documents related to capital transactions for WorldHeart.
Audit service fees were paid for professional services rendered by the auditors for (i) the audit of the annual consolidated financial statements including the services provided in connection with statutory and regulatory filings, (ii) reviews of the unaudited interim financial statements, and (iii) consultations related to capital
65
transactions, including comfort letters, consents and assistance with and review of documents filed with securities regulatory authorities.
No audit-related service fees were paid.
Tax service fees are for all services performed by the auditor's tax personnel excluding audit and audit — related fees and include tax compliance, tax planning and tax advice.
No other service fees were paid.
Pursuant to WorldHeart's amended and restated audit committee charter adopted by the board of directors on December 16, 2003, the Audit Committee approves in advance all auditing services of the external auditors and related fees and terms and all non-audit service mandates including related fees and terms, to the extent permitted by applicable laws, regulations and policies. The Audit Committee may delegate to one or more members of the Audit Committee the authority to pre-approve non-audit services to be provided by the external auditors provided that any such approvals made by the designated individuals will be reported to the full Audit Committee at its next scheduled meeting.
AFTER CAREFUL CONSIDERATION, THE AUDIT COMMITTEE AND THE BOARD OF DIRECTORS DETERMINED THE APPOINTMENT OF PRICEWATERHOUSECOOPERS LLP AS WORLDHEART'S AUDITOR TO BE IN THE BEST INTERESTS OF WORLDHEART'S SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS AND THE AUDIT COMMITTEE UNANIMOUSLY APPROVED THE APPOINTMENT AND RECOMMEND APPROVAL BY WORLDHEART'S SHAREHOLDERS.
MedQuest Resolution — Acquisition of MedQuest
This section of the circular describes material aspects of the proposed acquisition of MedQuest and also describes certain aspects of the concurrent private placement to Maverick. See "MATTERS TO BE ACTED UPON — Maverick Resolution — Private Placement to Maverick". Under the terms of the MedQuest Agreement attached as Appendix G to this circular, WorldHeart will, subject to WorldHeart shareholder approval, issue an aggregate of 9,300,000 common shares of WorldHeart. While WorldHeart believes that the description covers the material terms of the acquisition, this summary may not contain all of the information that is important to you.You should read this entire document and the other documents referred to in this circular carefully for a more complete understanding of the transactions.
Under the rules of the TSX, WorldHeart must obtain the approval of its shareholders whenever it proposes to issue shares in such an amount that the recipient of the shares will have enough voting power to materially affect the control of the corporation. WorldHeart's common shares are also listed on NASDAQ and are subject to the NASDAQ Marketplace Rules. NASDAQ Rule 4350 requires shareholder approval prior to the sale, issuance or potential issuance of 20% or more of the outstanding voting shares or securities convertible into voting shares before the issuance, in one transaction or a series of related transactions other than a public offering, at less than the greater of the book or market value of the stock. The MedQuest Resolution has been put forward, among other reasons discussed in this proposal, in order to comply with the application of these NASDAQ rules. Because of Maverick's substantial equity interest in MedQuest, the NASDAQ and TSX rules require that the shares issued to MedQuest and to Maverick in respect of the Maverick private placement, be aggregated in determining whether control of WorldHeart is materially affected by the proposed transactions. The acquisition of MedQuest and concurrent private placement to Maverick will result in MedQuest and
66
Maverick holding in aggregate approximately one third of the outstanding common shares of WorldHeart, after taking into account the conversion of debentures and exercise of warrants. See "MATTERS TO BE ACTED UPON — Warrant Amendment Resolution — Amendment to September 2004 Warrants". As such, the proposed transactions will result in a material change in the control of WorldHeart and must be approved by the shareholders before they can be completed.
WorldHeart and MedQuest are both engaged in the business of developing and commercializing mechanical circulatory support devices for the treatment of heart failure patients. The executives of WorldHeart and MedQuest have been acquainted with each other, over many years, from their interactions at meetings of professional societies such as the International Society of Heart and Lung Transplantation (ISHLT) and the American Society of Artificial Internal Organs (ASAIO).
At the Society of Thoracic Surgeons Meeting in Louisville, (August 27- 29, 2004), Mr. Jal S. Jassawalla, WorldHeart's President and CEO initiated a brief discussion with Mr. Pratap Khanwilkar, President and CEO of MedQuest, about exploring alternatives for a strategic transaction between the two companies. Mr. Khanwilkar expressed interest in looking at a possible alliance.
Following the meeting in Louisville, Mr. Jassawalla and Mr. Khanwilkar had a follow up phone conversation and agreed to proceed with their discussions and signed a Mutual Confidentiality Disclosure agreement in early September 2004. Thereafter, the two companies exchanged preliminary information about each other's business.
On September 27, 2004, representatives from WorldHeart, including Mr. Jassawalla, met in Salt Lake City at MedQuest's facility with MedQuest personnel, including Mr. Khanwilkar. MedQuest representatives provided a product and business overview, which was followed by a facility tour and a product demonstration. WorldHeart representatives also provided a business overview.
On October 18, 2004, representatives from MedQuest, including Mr. Khanwilkar, visited WorldHeart's facility in Oakland, California. WorldHeart representatives conducted a plant tour and a demonstration of its products, followed by a discussion of its infrastructure and capabilities.
At a meeting of the board of directors of WorldHeart held on October 26, 2004, management presented a strategic vision for WorldHeart which included an evolution from a single product company to one that would be able to offer alternative mechanical assist treatments for the late-stage congestive heart failure market. This would likely occur through the acquisition of a complementary rotary flow device or technology. Management expressed its opinion that the MedQuest Acquisition was the best transaction available to realize this goal. The nature of MedQuest's technology was a primary consideration in the selection of MedQuest. MedQuest's technology is based on magnetically levitated pumps where the rotors are suspended by a magnetic field and do not rely on the blood as a lubricant. Other types of rotary pumps utilize small bearings lubricated by the circulation of the blood, which is potentially stressful to the cellular elements in the blood. It was management's view, and also the general direction in which rotary pumps have evolved over time, that centrifugal pumps with full magnetic levitation represent the best technology for rotary blood pumps. Having focused on the technology that management was interested in acquiring, potential acquisitions were limited: the DuraHeart (Terumo Corporation), the HeartMate III (Thoratec) and the HeartQuest (MedQuest). Terumo Corporation, a large company relative to WorldHeart, is making a substantial investment in the DuraHeart design and it was the judgment of management that the probability of acquiring it was very low. Thoratec is also a large company relative to WorldHeart, is WorldHeart's direct competitor and is committed to the HeartMate III device, making its availability highly unlikely. In contrast, MedQuest is a small development-stage company. In management's judgment its technology represents the state-of-the art in centrifugal pumps with full magnetic levitation and there are good business reasons why the acquisition of that technology is in the best interest of the shareholders of both companies. MedQuest currently does not have the full infrastructure needed to commercialize the HeartQuest, whereas WorldHeart has the infrastructure to do so and will benefit from economies of scale in the process. Consequently, it was management's judgment that from both a technology and availability perspective, an acquisition of MedQuest was the appropriate choice. Management described the discussions it had pursued
67
to date with MedQuest and the board instructed management to pursue further discussions and to explore ways to implement the proposed strategy.
On November 19, 2004, Mr. Jassawalla met with Mr. Khanwilkar and Mr. Kevin Compton, a principal of Maverick and director of MedQuest, at WorldHeart's Oakland office. The meeting was focused on the potential benefits of an acquisition and the creation of a platform company with both pulsatile and rotary systems for circulatory support. On November 23, 2004, Mr. Jassawalla and Mr. Robert J. Majteles met with Mr. Khanwilkar and Mr. Compton, at WorldHeart's Oakland office to further discuss the possibility of the acquisition of MedQuest's assets by WorldHeart.
After management's discussions with all the members of the board WorldHeart entered into a non-binding term sheet ("Term Sheet") with MedQuest with a view to continuing and completing its due diligence process. The Term Sheet was entered into on December 6, 2004. The material terms of the Term Sheet were:
- •
- WorldHeart would acquire all of the assets of MedQuest for consideration to be paid in shares of WorldHeart;
- •
- Maverick would make an investment in common shares of WorldHeart of $12,000,000 for the purpose of funding the MedQuest rotary VAD through the commencement of clinical trials;
- •
- WorldHeart would enter into employment or consulting agreements with key executives and employees of MedQuest as WorldHeart might deem necessary;
- •
- WorldHeart would negotiate with the holders of approximately 10.7 million warrants issued by WorldHeart in September 2004 with a view to obtaining their agreement to exercise the warrants;
- •
- WorldHeart would negotiate with the holders of debentures convertible into approximately 10.7 million common shares, issued by WorldHeart in September 2004, with a view to obtaining their agreement to convert the debentures; and
- •
- WorldHeart and MedQuest would enter into a definitive purchase agreement by January 15, 2005.
In deciding on the consideration to be paid for the MedQuest technology, taking into account the funding from Maverick to take the MedQuest rotary VAD through to clinical trials, management determined that shares equivalent to 33% of the total number of shares that would be outstanding after the MedQuest Acquisition, Maverick private placement, conversion of debentures and exercise of warrants was appropriate. In reaching this decision, management assessed the increased market WorldHeart expects to be able to serve and the anticipated contribution to WorldHeart's total revenues once the MedQuest rotary VAD has reached commercialization. The division of that amount between MedQuest and Maverick was subject to a determination of the appropriate share price to be used, which the parties ultimately determined through negotiations to be $1.35 per share, as described under "Maverick Resolution — Private Placement to Maverick — Maverick Agreement — Consideration".
On December 14, 2004 management presented to the board the Term Sheet and described the potential opportunity in the form of an acquisition of MedQuest, based on preliminary discussions that WorldHeart had participated in with MedQuest. During that meeting the board was presented with the range of treatment options for congestive heart failure available on that date as well as the current and next-generation procedures and products that were available and under development. The board reviewed a competitive matrix that evaluated management's view of the MedQuest technology against current and under-development VADs, reviewed the valuation of comparable companies with rotary VAD development programs and reviewed a possible transaction structure that would include the acquisition of the MedQuest technology contemporaneously with a private placement with Maverick and conversion of our convertible debentures and exercise of the warrants that were issued with the convertible debentures. The board ratified the execution of the Term Sheet and authorized management to work to a definitive agreement.
Following the Term Sheet, as amended January 14, 2005 (the only change in which was to give the parties until January 25, 2005 to sign a definitive purchase agreement), and up until the execution of definitive agreements with MedQuest, the board met on each of January 14, January 24 and January 31, 2005 in order to receive reports and updates from management on the due diligence results as well as the evolution of the
68
transaction negotiations and structure and to deliberate on the positive and negative elements of the possible transactions. These management reports included additional in-depth reviews of rotary flow device challenges and benefits (including a review, as requested by the board, by an independent surgeon who is also a member of WorldHeart advisory committee), the merits and the potentially complementary fit of a rotary flow device with our current and next generation pulsatile device technology, and the intellectual property and employee base of MedQuest. Management also provided an assessment of the impact of the transaction as it pertained to dilution of current shareholders' interests and the growth potential of the combined entity on a going forward basis.
On January 31, 2005 the parties signed a definitive agreement for WorldHeart to acquire all the assets of MedQuest, subject to certain approvals, consents and conditions.
The WorldHeart board of directors believe that the acquisition and private placement are in the best interest of WorldHeart and its shareholders. The decision of the board of directors was based on several potential benefits of the acquisition and private placement that the board of directors believes will contribute to WorldHeart's success. These potential benefits include:
- •
- the ability to address a broader section of the late-stage heart failure market with the MedQuest rotary VAD for patients with less severe symptoms and a pulsatile VAD for patients with more severe symptoms;
- •
- a corresponding investment in WorldHeart by an experienced investor which will allow for an acceleration in the Novacor II development program; and
- •
- the ability to become a multiple product company in the treatment of late-stage congestive heart failure.
The WorldHeart board of directors reviewed a number of factors in evaluating the acquisition, including but not limited to the following:
- •
- the overall market for heart failure treatments including the late-stage heart failure market;
- •
- the MedQuest technology on a competitive basis compared to existing late-stage heart failure treatments that are both being marketed and, to the best of our knowledge, under development;
- •
- historical information concerning WorldHeart's and MedQuest's respective business focus, financial performance and condition, operations, technology and management;
- •
- WorldHeart management's view of the financial condition, results of operations and business of WorldHeart and MedQuest before and after giving effect to the acquisition, and the determination by the WorldHeart board of directors of the effect on WorldHeart shareholder value;
- •
- current financial market conditions and historical stock market prices, volatility and trading information;
- •
- the consideration WorldHeart will pay in the acquisition in light of valuations of comparable companies;
- •
- the period of time during which MedQuest will be unable to dispose of the common shares to be issued to it, as agreed in the MedQuest Agreement;
- •
- the terms of the MedQuest Agreement;
- •
- the impact of the acquisition on WorldHeart's customers and employees;
- •
- results of the due diligence investigation conducted by WorldHeart's management, accountants and legal counsel; and
- •
- the expectation that the acquisition will be accounted for as a purchase for accounting purposes.
In considering the value of the transaction to WorldHeart, the board reiterated its belief that acquiring a rotary VAD and becoming a multiple product company is strategically important for WorldHeart, and that MedQuest's rotary VAD employs a more advanced technology as compared to other potentially available target companies. The board then considered whether the consideration being paid was reasonable in light of valuations of comparable companies.
69
In particular, the board looked at Ventracor Limited and HeartWare Limited, both of which are single-product companies developing rotary VADs which have not obtained any regulatory approvals. These companies were the most comparable to MedQuest in terms of their technology and regulatory approval status, and their valuations were considered to be good benchmarks for the MedQuest Acquisition. HeartWare Limited is at a similar stage of development as MedQuest and Ventracor Limited is slightly farther ahead, having proceeded to clinical trials. Both of those companies have centrifugal pumps that rely on blood lubricated bearings, whereas MedQuest's technology involves a centrifugal pump with magnetic levitation that eliminates the limitations of blood lubricated bearings.
The board considered including the Terumo DuraHeart and the Thoratec HeartMate III in its analyses because they are centrifugal pumps with magnetic levitation, but the board determined that they could not easily be used as valuation benchmarks. Both pumps are under development in relatively large companies with large portfolios of products, including other types of products that are unrelated to mechanical circulatory support. The board was therefore unable to separate the value of the DuraHeart and HeartMate III pumps from the value of their respective companies.
Ventracor Limited is an Australian pubic company with approximately 193.37 million shares outstanding. During the period from September 1, 2004 to December 31, 2004, its average closing price per share was Aus$1.58. Its average market capitalization during the period was approximately Aus$305,000,000, the equivalent of approximately $229,000,000. In December 2004, shares of HeartWare were offered in an initial public offering on the Australian Stock Exchange, at an offer price of Aus$0.50, with an expected capitalization of Aus$74,000,000, the equivalent of approximately $55,000,000. The HeartWare initial public offering closed in January 2005 at the market capitalization expected in the prospectus. WorldHeart's average trading price per common share during the period from September 1, 2004 to December 31, 2004 was $1.27. With approximately 15.74 million shares outstanding, WorldHeart's average market capitalization during this period was approximately $20,000,000.
Based on the valuations of the selected comparable companies in the range of $55,000,000 to $229,000,000, and the fact that WorldHeart will receive the MedQuest technology plus $12,000,000 to continue its development, the board judged the consideration of a post-transaction combined ownership by Maverick and the shareholders of MedQuest of 33% of WorldHeart to be reasonable. The board came to its conclusion on the valuation through a review of the quality and complimentary nature of the technology of MedQuest as compared to the comparable companies assessed by the board and the relatively low valuation of MedQuest in comparison to the comparable companies that the board examined in conjunction with this transaction. The board did not consider any other qualitative analysis because of the limited number of companies with complimentary technology to WorldHeart which would be relevant to WorldHeart for the purposes of building a platform company. The board did not seek a valuation from the third party investment banker as management was in a stronger position to evaluate the technology of MedQuest and also asked one of the WorldHeart's clinical advisory committee to review the technology as well as an independent verification.
During the course of its deliberations concerning the acquisition and the private placement, the WorldHeart board of directors also identified and considered a variety of potentially negative factors that could materialize as a result of the transactions, including but not limited to the following:
- •
- the risk that the potential benefits sought in the acquisition might not be fully realized;
- •
- the possibility that the acquisition might not be completed, even if approved by the WorldHeart shareholders;
- •
- the effect of the public announcement of the acquisition on WorldHeart's business and MedQuest's business, including the effect on employees and customers; and
- •
- the costs and risks associated with obtaining the necessary approvals required to complete the acquisition.
The WorldHeart board of directors concluded that these factors were outweighed by the potential benefits to be gained by the acquisition. In view of the wide variety of factors, both positive and negative, considered by the WorldHeart board of directors, the directors did not find it practical to, and did not, quantify or otherwise assign relative weights to the specific factors discussed above.
70
History and Development of MedQuest
MedQuest is a development stage medical device company focused on the development of rotary VADs. VADs in general are mechanical assist devices that supplement the circulatory function of the heart by re-routing blood flow through a mechanical pump. VADs are used for treatment of patients with end-stage heart failure (i.e. patients whose hearts are irreversibly damaged and which cannot be treated by medical or surgical means other than a transplant).
Since inception in 1993, MedQuest's goal has been to develop and commercialize a family of blood pump products, providing the most comprehensive treatment for heart failure by meeting the needs of more than 90% of the patient population eligible for a long-term, implantable, mechanical blood pump. In its early years, MedQuest boot-strapped its operations, having been successful at its contract cardiovascular R&D business, its physiological cardiovascular simulator business, and its coatings development business. All these other businesses have now been terminated with two product applications being out-licensed.
Since 2000, MedQuest's complete focus has been to successfully complete the development of, clinically evaluate, and commercialize the HeartQuest VAD, which is expected to address the needs of 75% of this patient market for long-term, implantable support. Once this is achieved, MedQuest may commercialize an add-on device that will allow long-term biventricular support to the other 15% of the eligible patients, and a scaled-down device for short-term use in newborns born with congenital heart defects.
MedQuest has focused its present operations on the development of the HeartQuest VAD product, a product based on patented technologies.
Since 2000, a number of significant developments related specifically to MedQuest's HeartQuest VAD have occurred:
- •
- Funding of more than $15 million has been obtained and applied to the HeartQuest VAD. A portion of this funding was obtained as part of a $4.2 million, 4-year grant from the United States National Institutes of Health, the only implantable adult left-ventricular assist device so awarded under the Bioengineering Research Partnership program. The remaining funding was obtained as an equity investment and debt that has since been converted to equity. The debt incurred since January 31, 2005 will be assumed by WorldHeart upon completion of the MedQuest Acquisition.
- •
- The clinical HeartQuest VAD pump and accompanying components for initial clinical use have been designed with the help of an expanded development team that included Dr. James Antaki, a well-known blood pump designer, Dr. Brad Paden, a magnetic levitation designer, and his company Magnetic Moments, as well as Mr. Gordon Jacobs, a system engineer who was the lead engineer for the Jarvik 2000 — Flowmaker rotary blood pump.
- •
- This clinical HeartQuest VAD has evolved quickly through only three iterations as compared to fifty or so design iterations typically undergone by other blood pump companies. This fast development cycle was enabled by a proprietary software package that was developed exclusively by MedQuest for use in designing a clinically feasible VAD. The first pump ever designed with this computer-model based approach was successfully assembled and tested in animals for more than 100 days, with total time from concept to animal trial start being nine months.
- •
- As of May 1, 2005, the HeartQuest VAD has been successfully implanted for chronic use in 26 calves for more than 2.5 cumulative years, with the longest duration experiment being 5 months. In these experiments, the HeartQuest VAD has demonstrated its capacity to safely pump an adequate amount of blood without damaging the blood or heart.
- •
- Since 2001, the HeartQuest VAD's fit has been successfully evaluated in several end-stage heart failure patients, including a 15-year old patient.
- •
- In 2003, MedQuest moved into its present integrated facilities, where it is installing its pilot manufacturing assembly line and associated equipment.
71
- •
- In March 2003, based on the adult HeartQuest VAD, a pediatric VAD was conceived. This VAD was one of five nationwide to receive a 5-year, $4.2 million NIH grant. The PediaFlow™ VAD is still in the research phase.
- •
- In 2004, the clinical HeartQuest VAD was configured for its initial clinical use, including its accessories such as power supplies and batteries.
- •
- As of May 2005, Ethics Committee Approval has been obtained from both prospective clinical centers for the HeartQuest VAD clinical feasibility trials outside the United States.
Since 2000, MedQuest has faced the following significant challenges:
- •
- In December 2000, MedQuest obtained an equity investment of $5 million from an affiliate of Maverick. The funding allowed MedQuest to continue operations through mid-2002. From mid-2002 until November 2003, MedQuest obtained necessary funding from bridge loans provided by Maverick. By November 2003, MedQuest had depleted the capital provided by the bridge loans. Despite extensive efforts by management, MedQuest was unsuccessful in closing additional financing transactions, including a proposed $20 million investment from an institutional venture capital firm. MedQuest could not meet its payroll obligations from mid-November 2003 until April 2004. In April 2004, MedQuest re-aligned its plans to focus on a less investment-intensive, faster-to-clinical introduction milestone for the HeartQuest VAD which contemplated an initial clinical study by year-end 2005. At such time, Maverick agreed to provide additional funding which has allowed MedQuest to continue its operations and development activities.
- •
- Due to limited funding described above, MedQuest was forced to lay-off approximately 30% of its workforce in November 2001. During 2003 and 2004, MedQuest lost additional employees. Notwithstanding the additional funding obtained from Maverick in April 2004, MedQuest has been challenged in attracting, hiring, and retaining qualified technical staff primarily due to the financial risk posed by the necessity of raising additional funding.
- •
- Since April 2004, significant delays have been caused in the schedule for feasibility clinical trials by the redesign of, and subsequent long lead time to manufacture pump parts needed for bench and animal performance evaluation and pre-clinical tests.
Significant Acquisitions
MedQuest did not complete any significant acquisitions or dispositions in the most recent fiscal year ended June 30, 2004, or in any subsequent financial quarter, other than the contemplated sale to WorldHeart.
MedQuest's Products
The only product MedQuest currently has in development is the HeartQuest VAD. The HeartQuest VAD is a small, rotary pump, now at an advanced stage of development and in preclinical animal and bench testing with an initial feasibility clinical trial expected to be initiated by the end of 2005.
The HeartQuest VAD is an advanced, next generation, rotary blood pump intended for destination therapy. Unlike the previous generation of rotary pumps with blood-lubricated bearings, it is a compact, centrifugal pump with an impeller which is completely magnetically levitated. Full magnetic levitation eliminates wear mechanisms within the pump and provides for greater clearances for more optimized blood flow around the impeller, while eliminating dependence on the patient's blood for suspension. HeartQuest's levitation technology employs a unique combination of passive and single-axis active control, resulting in a system of optimal simplicity.
Marketing, Training and Distribution Strategy
MedQuest's principal markets are anticipated to be North America and Europe.
MedQuest's present staff has extensive medical device development and commercialization experience, having successfully commercialized more than 160 medical devices during their collective historical employment with various other organizations. Senior personnel at MedQuest and at its development partners have
72
contributed to the development, clinical use and commercialization of several of the blood pumps clinically available today. Assuming the consummation of the MedQuest Acquisition, MedQuest anticipates almost doubling its staff to accelerate development and manufacturing towards destination therapy use in the next year. Upon completion of the MedQuest Acquisition, WorldHeart will employ a substantial portion of the current staff employed by MedQuest.
MedQuest plans to utilize experienced clinical and technical personnel to support implant centres and their leading clinicians. MedQuest plans to distribute products directly and through distributors in selected markets.
Sources and Availability of Raw Materials
Since 2004, MedQuest has experienced moderate difficulty in obtaining high-quality and critical tolerance machined titanium components in a timely manner from its suppliers. MedQuest continues to work with its suppliers in an attempt to ensure an adequate flow of quality raw materials and subassemblies which will allow it to meet the pre-clinical and clinical needs for the HeartQuest VAD pump. Otherwise, MedQuest has not had any other major difficulty in obtaining quality raw materials or quality subassemblies. MedQuest does not anticipate that the prices of raw materials will be subject to substantial volatility.
Intellectual Property
MedQuest and other parties from whom MedQuest holds licenses have been granted 12 United States patents for the HeartQuest VAD and its associated subsystems, which will expire over the period from 2016 to 2021. A subset of these patents has also been filed in key European Union countries, Australia, Canada, and Japan. In addition, MedQuest has six patents pending relating to various aspects of the HeartQuest VAD or its associated subsystems.
MedQuest has entered into various license agreements with university research foundations and other organizations pursuant to which MedQuest has obtained licenses to use and commercialize certain technologies and inventions embodied in the HeartQuest VAD. All of the license agreements grant exclusive rights to MedQuest, except for one agreement that relates to the physiological control of rotary blood pumps. Each license agreement may be terminated by the licensor if MedQuest fails to reach certain milestones or if other conditions are not satisfied. Subject to these termination provisions, each license agreement continues until the expiration of the last-to-expire patent resulting from the license agreement. Pursuant to the terms of the license agreements, MedQuest is obligated to pay certain one-time and continuing license fees until sales of the HeartQuest VAD commence. MedQuest estimates that it will incur total license fees in the amount of $300,000 in 2005, $50,000 in 2006 and $75,000 in 2007. MedQuest further estimates that sales of the HeartQuest VAD will commence in 2008, at which time the license fees will be replaced with ongoing royalties in an aggregate amount of up to 1.1% of net sales. In addition, MedQuest has agreed to pay a royalty in the amount of 3.15% of net sales to a prior development partner. MedQuest may incur additional licensing fees and royalty obligations in connection with future development and commercialization of the HeartQuest VAD.
MedQuest generally enters into confidentiality or license agreements with its employees, consultants and vendors, and generally controls access to and distribution of information related to its technology and products, documentation and other proprietary information.
Product Liability Insurance
Before MedQuest commences clinical trials it anticipates obtaining a limited amount of product liability insurance to provide coverage against potential liabilities.
Competition
There are a large number of non-pulsatile VADs in varying stages of development. One such device, which has been undergoing United States clinical trials as a bridge-to-transplant and which is approved for use in Europe, is the MicroMed DeBakey VAD being developed by MicroMed Technology, Inc. This device provides non-pulsatile left ventricular assist via a small rotary pump that is implanted in the abdomen and is powered via wires that perforate the skin, connected to an external controller and rechargeable battery pack. A pivotal trial
73
for this device to be evaluated for destination therapy in the United States started in 2004. Also, in February 2005, the HeartMate II VAD from Thoratec started its United States pivotal trial for destination therapy. The Jarvik 2000 VAD remains in clinical trials in Europe and in the United States for a bridge-to-transplant.
The Arrow CorAide VAD, Ventracor VentrAssist VAD, Terumo DuraHeart and BerlinHeart Incor I have started clinical trials in Europe.
It is anticipated that by 2006, the HeartQuest's other competition (the Thoratec HeartMate III, and the HeartWare VAD) will be in European clinical trials.
MedQuest believes that compared to the HeartQuest VAD, the major third generation blood pumps in development and/or clinical use are limited by larger device size, higher complexity in hardware and software that leads to lower durability, and lesser applicability to patient needs.
MedQuest's Employees
At April 28, 2005, MedQuest employed 25 full-time staff and consultants. Approximately 85% of the employees are involved with research, development and clinical affairs, and 15% are in finance, administration, and regulatory affairs.
MedQuest currently maintains compensation, benefits, equity participation and work environment policies intended to assist in attracting and retaining qualified personnel.
MedQuest believes that the success of its business will depend, to a significant extent, on its ability to attract and retain such personnel. MedQuest has access to highly-skilled labour pools in Salt Lake City, Utah where there is a well-developed medical device industry.
None of its employees is subject to a collective bargaining agreement nor has MedQuest experienced any work stoppages. MedQuest believes that its relations with its employees are good.
MedQuest's Facilities
MedQuest's registered office is located at 4750 Wiley Post Way, Suite 120, Salt Lake City, Utah, 84116, USA. The facilities in Salt Lake City comprise 24,044 square feet of manufacturing and office space with a lease that expires on January 31, 2008. MedQuest is not aware of any environmental issues that may affect the use of its property.
The following is a brief summary of the material provisions of the MedQuest Agreement and amendment no. 1 to the MedQuest Agreement, copies of which are attached as Appendix G to this circular. While we believe that the summary covers the material terms of the MedQuest Agreement, this summary may not contain all of the information that is important to you. We urge all WorldHeart shareholders to read the MedQuest Agreement in its entirety for a more complete description of the terms and conditions of the acquisition and related matters.
General
Following the approval of the issuance of common shares of WorldHeart to MedQuest by the shareholders of WorldHeart as contemplated by the MedQuest Agreement, and the satisfaction or waiver of the other conditions to the acquisition:
- •
- WorldHeart will acquire of all the assets of MedQuest;
- •
- WorldHeart will assume substantially all of MedQuest's contracts and licenses; and
- •
- WorldHeart will assume responsibility for certain liabilities of MedQuest, including amounts outstanding to Maverick for loans to fund continuing operations between the date of signing the MedQuest Agreement and completion of the acquisition.
74
If all conditions to the acquisition are satisfied or waived, the acquisition will become effective on or about the date that is 14 days after the WorldHeart shareholders approve the issuance of WorldHeart shares in connection with the acquisition or such later date as the parties may agree.
Consideration
Upon the completion of the acquisition of the MedQuest business in accordance with the MedQuest Agreement, WorldHeart will have issued 9,300,000 common shares to MedQuest and will have paid $10,000 in cash to MedQuest. In addition, WorldHeart will have assumed normal course operating liabilities of MedQuest and a $3.5 million liability associated with the Maverick loans to MedQuest.
Representations and Warranties
In the MedQuest Agreement, WorldHeart and MedQuest have made a number of representations and warranties. These representations and warranties relate to, among other things:
- •
- their organization, existence, corporate good standing, corporate power and similar corporate matters;
- •
- their authorization, execution, delivery, performance and the enforceability of the MedQuest Agreement and related matters;
- •
- required governmental and third-party consents;
- •
- the absence of material adverse changes, as specified in the MedQuest Agreement;
- •
- tax matters;
- •
- litigation;
- •
- legal compliance;
- •
- permits; and
- •
- brokers' and related fees.
MedQuest has also made representations and warranties as to:
- •
- the ownership of all outstanding shares of MedQuest;
- •
- the authorization, execution, delivery and performance of the MedQuest Agreement and related matters by MedQuest;
- •
- financial statements of MedQuest;
- •
- the absence of undisclosed liabilities;
- •
- the ownership and condition of assets being transferred under the terms of the MedQuest Agreement;
- •
- real property leases;
- •
- intellectual property;
- •
- inventory;
- •
- material contracts;
- •
- accounts receivable;
- •
- insurance policies;
- •
- warranties;
- •
- employees and employee benefit plans;
- •
- environmental matters;
- •
- customers and suppliers;
- •
- business relationships with affiliates;
75
- •
- restrictions on business activities; and
- •
- banking facilities.
WorldHeart has also made representation and warranties as to:
- •
- its capitalization;
- •
- the required vote of its shareholders; and
- •
- documents and reports filed with the SEC and financial statements of WorldHeart included in those documents.
Covenants
Each of WorldHeart and MedQuest has agreed to use its reasonable best efforts to take all actions and to do all things necessary, proper or advisable to consummate the transactions contemplated by the MedQuest Agreement.
Governmental and Third-Party Notices and Consents. Each of WorldHeart and MedQuest has agreed to use its reasonable best efforts to obtain, at their own expense, all waivers, approvals, or consents from third parties and give notice to all third parties which are necessary to be obtained by them to consummate the acquisition.
Each of WorldHeart and MedQuest has agreed, at its own expense, to use their reasonable best efforts to obtain all waivers, permits, consents, approvals and other authorizations from governmental entities and to effect all registrations, filings and notices with or to governmental entities which are required to consummate the acquisition and to otherwise comply with all applicable laws and regulations in connection with the consummation of the acquisition.
Circular and Shareholders Meeting. WorldHeart agreed to prepare and file a proxy circular, and any amendments to such circular, with applicable securities regulatory authorities in Canada and the United States.
WorldHeart has further agreed to take all actions in accordance with applicable law and its articles of organization and by-laws to promptly and duly call, give notice of and convene a meeting of the WorldHeart shareholders for the purpose of approving the issuance of 9,300,000 common shares of WorldHeart to MedQuest in connection with the acquisition and the concurrent issuance of 8,888,889 common shares of WorldHeart to Maverick in connection with the private placement.
Operations of the MedQuest Business Prior to Acquisition. Except as contemplated by the MedQuest Agreement, MedQuest has agreed that it will conduct operations relating to the MedQuest business in the ordinary course of business and in compliance with applicable laws.
MedQuest has agreed to use its commercially reasonable efforts to preserve substantially intact in all material respects its present business organization, to maintain its existence in good standing, to keep available the services of its key officers and employees, to maintain insurance on its tangible assets and businesses in such amount and against such risks and losses as are currently in effect, to preserve its relationships with suppliers and others having significant business dealings with it and to comply in all material respects with all applicable laws.
MedQuest has further agreed to amend its articles immediately after the closing of the acquisition to change its corporate name to one that does not include any trade mark or trade name included in the acquired assets and to cancel all registrations of business names included in the acquired assets.
Access to Information. MedQuest has agreed to provide WorldHeart with access to all premises, properties, personnel, environmental and financial records, contracts and other documents of MedQuest as is reasonably appropriate.
76
Conditions to Obligations to Effect the Acquisition
The obligation of WorldHeart to complete the acquisition is subject to the satisfaction or waiver of a number of conditions, including the following:
- •
- receipt of all approvals and consents as are required to permit the change of ownership of the acquired assets;
- •
- receipt of all consents with respect to contracts to be assigned to WorldHeart upon the closing of the acquisition, which, if not obtained, would reasonably be expected to have a material adverse effect on WorldHeart's ability to carry on the MedQuest business;
- •
- the representations and warranties of MedQuest in the MedQuest Agreement being true and correct as of the date of the closing of the acquisition;
- •
- MedQuest shall have performed or complied in all material respects with its agreements and covenants required to be performed or complied with under the MedQuest Agreement as of or prior to closing the acquisition;
- •
- no material adverse affect on the business, financial condition or results of operation of the MedQuest business shall have occurred;
- •
- MedQuest shall have delivered to WorldHeart a certificate stating that the specified conditions of the MedQuest Agreement have been satisfied;
- •
- WorldHeart shall have received other customary certificates and instruments as it reasonably requests in connection with the closing of the acquisition;
- •
- each of WorldHeart and MedQuest shall have obtained the approval of its respective shareholders;
- •
- WorldHeart shall have obtained all necessary regulatory approvals to complete the acquisition and the concurrent private placement; and
- •
- MedQuest will have negotiated modified terms in respect of the license agreements and subcontracting arrangements identified by the WorldHeart, to the satisfaction of WorldHeart, acting reasonably.
In addition, the obligation of MedQuest to effect the acquisition is subject to the satisfaction or waiver of a number of conditions, including the following:
- •
- the representations and warranties of WorldHeart in the MedQuest Agreement being true and correct as of the date of the closing of the acquisition;
- •
- WorldHeart shall have performed or complied with in all material respects its agreements and covenants required to be performed or complied with under the MedQuest Agreement as of or prior to closing the acquisition;
- •
- no material adverse effect on the business, financial condition and results of operations of WorldHeart shall have occurred;
- •
- prior to the closing of the acquisition, the holders of WorldHeart's convertible debentures will have converted the convertible debentures into common shares of WorldHeart;
- •
- prior to the closing of the acquisition, the holders of warrants to acquire common shares of WorldHeart, issued in September 2004, will have exercised the warrants for common shares of WorldHeart;
- •
- each of WorldHeart and MedQuest shall have obtained the approval of its respective shareholders;
- •
- WorldHeart, MedQuest and Maverick will have entered into the Registration Rights Agreement;
- •
- WorldHeart shall have delivered to MedQuest a certificate stating that the specified conditions of the MedQuest Agreement have been satisfied;
- •
- MedQuest shall have received other certificates and instruments as it reasonably requests in connection with the closing of the acquisition;
77
- •
- the common shares of WorldHeart issued to MedQuest shall have been authorized for quotation on NASDAQ; and
- •
- WorldHeart shall have obtained all necessary regulatory approvals to complete the acquisition and concurrent private placement.
MedQuest is close to finalizing its shareholder information to be mailed to MedQuest shareholders for approval of the MedQuest Acquisition. MedQuest will mail this information to its shareholders shortly after this circular is mailed to WorldHeart shareholders.
NASDAQ and the TSX have been notified of the transactions and the TSX has conditionally approved the transactions subject to shareholder approval and filing of certain information with the TSX on the closing of the transaction.
Amendments to license agreements to be assigned to WorldHeart from MedQuest are currently under negotiation with various third parties whose consents are required.
Post-Closing Covenants
MedQuest has agreed that from and after the time of closing of the acquisition it will refrain from disclosing information of a confidential nature or not generally known to the public concerning the MedQuest business.
WorldHeart has agreed that from and after the time of closing of the acquisition it will:
- •
- for a period of six years, allow MedQuest to have reasonable access to financial statements, records of past sales, customer lists, supplier lists, payroll records, inventory data, inventory master records and accounts receivable data for the limited purpose of concluding MedQuest's involvement with the operations of the MedQuest business as conducted by MedQuest prior to the closing of the acquisition;
- •
- employ designated employees of MedQuest, on terms and conditions which are generally consistent with their respective current employment terms and length of service with MedQuest;
- •
- provide service credit under all WorldHeart benefit plans for all designated employees of MedQuest for service accrued by these employees as of the closing of the acquisition; and
- •
- with respect to all of the designated employees of the MedQuest business, recognize up to an equal number of accrued days or weeks of vacation as such designated employee receives in his or her offer of employment from WorldHeart, as may be accrued and unused by such designated employee through the date of closing of the acquisition.
Indemnification
The MedQuest Agreement provides that MedQuest will indemnify WorldHeart for, among other things, any and all damages, subject to the limitations described below, that WorldHeart or an affiliate of WorldHeart may suffer as a result of:
- •
- a misrepresentation or breach of a warranty of MedQuest contained in the MedQuest Agreement; and
- •
- any failure to perform any covenant or agreement of MedQuest contained in the MedQuest Agreement.
MedQuest's representations and warranties contained in the MedQuest Agreement continue in effect for one year from the closing of the acquisition.
The acquisition agreement provides that WorldHeart will indemnify MedQuest for any and all damages, subject to the limitations described below, that MedQuest or an affiliate of MedQuest may suffer as a result of:
- •
- a misrepresentation or breach of warranty of WorldHeart contained in the MedQuest Agreement; and
- •
- any failure to perform any covenant or agreement of WorldHeart contained in the MedQuest Agreement.
WorldHeart' representations and warranties contained in the MedQuest Agreement continue in effect for one year from the closing of the acquisition.
78
MedQuest will only be liable for any damages once the aggregate amount of damages exceeds $50,000 and then only to the extent that such damages exceed such amount, and in any event, only to a maximum of the fair value of the shares held in escrow for such purpose, other than, in either case, any claim attributable to the lack of ownership of or title to the assets acquired under the MedQuest Agreement or any losses suffered by WorldHeart as a result of a breach of the MedQuest Agreement by MedQuest. Pursuant to the terms of the escrow, 930,000 common shares issued to MedQuest pursuant to the MedQuest Agreement will be held in escrow until the date that is one year after the closing date of the MedQuest Acquisition. MedQuest may not dispose of these shares until they are released from escrow.
WorldHeart will only be liable for any damages once the aggregate amount of damages exceeds $50,000 and then only to the extent that such damages exceed such amount, and in any event, only to a maximum of $2 million, other than, in either case, any claim for losses suffered by MedQuest as a result of a breach of the MedQuest Agreement by WorldHeart.
Termination
The MedQuest Agreement may be terminated at any time prior to the closing of the acquisition, by:
- •
- mutual written consent; or
- •
- either WorldHeart or MedQuest giving written notice to the other party if the closing has not occurred on or before May 23, 2005 or such later date in the event of regulatory delays, by reason of the failure of any condition precedent to the terminating party's closing of the acquisition, unless the failure results primarily from a breach by the terminating party of any representation, warranty or covenant contained in the MedQuest Agreement or results from delays caused by regulatory intervention.
Amendment and Waiver
Generally, WorldHeart and MedQuest may amend the MedQuest Agreement at any time prior to the closing of the acquisition. Amendments must be in writing and signed by all parties and waivers must be in writing and signed by the waiving party.
Employment and Consulting Agreements
In connection with the acquisition, WorldHeart entered into an employment agreement with each of Pratap Khanwilkar, President of MedQuest, who will be appointed as the Vice President, Rotary Systems and Business Development with WorldHeart upon the completion of the MedQuest Acquisition and Gordon Jacobs, Chief Biomedical Scientist and Director of Pre-Clinical Studies of MedQuest who will be appointed as Chief Biomedical Scientist and Director, Preclinical Studies, Rotary Systems of WorldHeart upon the completion of the MedQuest Acquisition. The terms of these agreements are similar to agreements WorldHeart has with existing executive officers in comparable roles. The employment agreements take effect upon the closing of the acquisition; however, in the event of termination of the MedQuest Agreement or in the event the acquisition does not close the employment agreements shall automatically terminate. Mr. Khanwilkar will receive an annual salary of $165,000 and options for 425,000 common shares, subject to shareholder approval of an increase in the number of options under the Plan. Mr Jacobs will receive an annual salary of $110,000 and options for 100,000 common shares, subject to shareholder approval.
In addition, upon closing WorldHeart will enter into a consulting agreement with Dr. James Long, a director of MedQuest, which provides for a cash fee and options. The consulting agreement will provide that Dr. Long may not provide services to any competitor of WorldHeart without the consent of WorldHeart's President and Chief Executive Officer. Mr. Long will receive options for 500,000 common shares and will receive compensation of $10,500 per month.
The private placement to MedQuest will result in an additional 9,300,000 common shares of WorldHeart being issued. This will have a significant dilutive effect on existing shareholders. As an illustration, currently
79
10,000 common shares of WorldHeart represents 0.000585% of the issued and outstanding common shares. After the MedQuest Acquisition and without giving effect to any other issuances, 10,000 common shares will represent 0.000379% of the issued and outstanding common shares.
Shareholders are therefore being asked to consider and, if thought advisable, to approve the MedQuest Resolution, a copy of which is attached to this circular as Appendix A-1. Approval of the MedQuest Resolution is required from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THE ISSUANCE OF WORLDHEART COMMON SHARES IN CONNECTION WITH THE MEDQUEST ACQUISITION TO BE IN WORLDHEART SHAREHOLDERS' BEST INTERESTS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE ISSUANCE OF THE WORLDHEART COMMON SHARES AND RECOMMENDS APPROVAL OF THE ISSUANCE OF WORLDHEART COMMON SHARES BY WORLDHEART'S SHAREHOLDERS PURSUANT TO THE MEDQUEST ACQUISITION.
Maverick Resolution — Private Placement to Maverick
This section of the circular described material aspects of the Maverick private placement as well as certain aspects of the MedQuest Acquisition. See "MATTERS TO BE ACTED UPON — MedQuest Resolution — Acquisition of MedQuest". Under the terms of the Maverick Agreement attached as Appendix H to this circular, WorldHeart will, subject to WorldHeart shareholder approval, issue an aggregate of 8,888,889 common shares of WorldHeart. While WorldHeart believes that the description covers the material terms of the acquisition, this summary may not contain all of the information that is important to you.You should read this entire document and the other documents referred to in this circular carefully for a more complete understanding of the transaction.
Regulatory Approvals
Under the rules of the TSX, WorldHeart must obtain the approval of its shareholders whenever it proposes to issue shares in such an amount that the recipient of the shares will have enough voting power to materially affect the control of the corporation. WorldHeart's common shares are also listed on NASDAQ and are subject to the NASDAQ Marketplace Rules. NASDAQ Rule 4350 requires shareholder approval prior to the sale, issuance or potential issuance of 20% or more of the outstanding voting shares or securities convertible into voting shares before the issuance, in one transaction or a series of transactions other than a public offering, at less than the greater of the book or market value of the stock. Pursuant to the Maverick Agreement we intend to issue 8,888,889��common shares to Maverick at a price of $1.35 per share which represents a price per share that is below the market value of our shares on the date we entered into the agreement. On January 31, 2005, the price per share on NASDAQ was $1.58 and the price per share on the Toronto Stock Exchange was Cdn.$1.83. NASDAQ Rule 4350 also requires shareholder approval in connection with an issuance of securities that could result in a change of control of an issuer. While the rule currently does not specifically define when a change in control of an issuer may be deemed to occur, NASDAQ suggests in its guidance that a change of control would occur, subject to certain limited exceptions, if after a transaction, a person or an entity will hold 20% or more of the issuer's then outstanding capital stock. The Maverick Resolution has been put forward, among other reasons discussed in this proposal, in order to comply with the application of these NASDAQ rules. Because of Maverick's substantial equity interest in MedQuest, the NASDAQ and TSX rules require the share issuance to Maverick be aggregated with the issuance to MedQuest in respect of the MedQuest Acquisition in determining that the control of WorldHeart is materially affected by the proposed transactions. The Maverick private placement and the concurrent acquisition of MedQuest will result in Maverick and MedQuest holding in aggregate approximately one third of the outstanding common shares of WorldHeart, after taking into account the conversion of the debentures and the exercise of the warrants. See "MATTERS TO BE ACTED UPON — Warrant Amendment Resolution — Amendment to September 2004 Warrants".
Maverick holds approximately 82% of the outstanding common stock of MedQuest and 100% of the outstanding preferred stock of MedQuest. Maverick is a limited liability company controlled by Kevin Compton.
80
Background and Reasons for the Maverick Private Placement
During the preliminary discussions with MedQuest from August through November. See "MATTERS TO BE ACTED UPON — MedQuest Acquisition — Acquisition of MedQuest", WorldHeart understands that MedQuest was discussing these developments with its board of directors and its principal shareholder, Maverick.
In November, as discussions were progressing, Mr. Kevin Compton, a principal of Maverick and a director of MedQuest, together with Mr. Khanwilkar, President and CEO of MedQuest, met with Mr. Jassawalla, and subsequently, Mr. Majteles, director of WorldHeart, to discuss the benefits of the MedQuest acquisition and the creation of a platform company as well. As part of these discussions, WorldHeart had reviewed the financial position of MedQuest and its financial requirements in more depth and so there was further discussion with Messrs. Compton and Khanwilkar about a possible private placement of WorldHeart common shares to assist in the funding of the MedQuest research and the overall platform company on a going forward basis.
Further discussions were had with Mr. Compton to better determine an appropriate level of investment as well as a possible discount to the market price of the common shares of WorldHeart. As part of this discussion, Mr. Compton also indicated that it would be necessary to eliminate some of the perceived overhang on the common shares of WorldHeart by requiring the warrantholders from the September 2004 private placement to exercise their warrants and for the debentureholders from the same private placement to convert their debentures.
On December 14, 2004 at a meeting of the board of directors, management presented to the board the Term Sheet related to the MedQuest Acquisition and also the related Maverick private placement which management explained would happen contemporaneously with the acquisition of MedQuest as well as the conversion of the debentures and the exercise of the warrants that were issued with the convertible debentures. At that meeting of the board, WorldHeart's board ratified the execution of the Term Sheet signed on December 6, 2004, and later amended on January 14, 2005, and authorized management to continue to pursue negotiations with Maverick as to the final terms of the private placement.
The WorldHeart board met on each of January 14, January 24 and January 31, 2005, to discuss the matters related to the Maverick private placement including the proposed amount of the private placement, the repayment of certain loans to Maverick as part of the ongoing funding of MedQuest during the signing and closing of the MedQuest Acquisition, and the price per share for the private placement. During this period, Mr. Jassawalla and Mr. Compton had several discussions about the terms of the private placement.
On January 31, 2005, the parties signed a definitive agreement for a private placement of 8,888,889 common shares of WorldHeart to Maverick for an aggregate purchase price of $12 million or $1.35 per share, subject to certain approvals, consents and conditions and contingent on the closing of the MedQuest Acquisition.
The WorldHeart board of directors believe that the private placement is in the best interest of WorldHeart and its shareholders. The WorldHeart board considered a number of factors in evaluating the proposed private placement, including but not limited to:
- •
- current financial market conditions and historical stock market prices, volatility and trading information;
- •
- WorldHeart's management's view of the financial condition, results of operations and business of WorldHeart before and after giving effect to the private placement; and the determination by the WorldHeart board of directors of the effect on WorldHeart shareholder value;
- •
- the discount to market as at the date of approval of the transaction in the price of the common shares WorldHeart will issue to Maverick pursuant to the private placement;
- •
- the period of time during which Maverick will be unable to dispose of the common shares to be issued to them, as agreed in the Maverick Agreement;
- •
- the terms of the Maverick Agreement.
The decision of the board of directors was based on several potential benefits of the private placement that the board of directors believes will contribute to WorldHeart's success.
81
The potential benefits of the private placement include but are not limited to:
- •
- an investment in WorldHeart by an experienced investor to allow for future growth of the WorldHeart strategy of a platform company; and
- •
- potential participation by Maverick's designees on the WorldHeart board of directors;
The potential negative factors that could materialize as a result of the private placement include but are not limited to:
- •
- the possibility that the private placement may not be completed, even if approved by the WorldHeart shareholders; and
- •
- the effect of the public announcement of the private placement on WorldHeart's business and share price.
The WorldHeart board of directors concluded that the potential benefits outweigh the potential negative consequences. The WorldHeart board of directors did not find it practical to, and did not, quantify or otherwise assign relative weights to the specific factors discussed above.
General
Following the approval of the issuance of common shares of WorldHeart to Maverick by the shareholders of WorldHeart as contemplated by the Maverick Agreement and amendment no. 1 to the Maverick Agreement, and the satisfaction or waiver of the other conditions to the private placement:
- •
- WorldHeart will issue 8,888,889 common shares to Maverick;
- •
- Maverick will pay $12 million to WorldHeart pursuant to the private placement; and
- •
- the board of directors of WorldHeart will take the necessary steps to appoint two nominees of Maverick to the board of directors.
If all conditions to the private placement are satisfied or waived, the private placement will become effective on the date of the closing of the acquisition of MedQuest by WorldHeart, or such other date as the parties may agree.
Consideration
Upon satisfaction or waiver of the conditions contemplated by the Maverick Agreement, WorldHeart will issue 8,888,889 common shares to Maverick at a price of $1.35 per share for total gross proceeds of $12 million. Pursuant to the MedQuest Agreement, WorldHeart will have assumed certain liabilities of MedQuest including $3.5 million owed to Maverick in connection with the ongoing operations of the MedQuest business and will repay that amount to Maverick upon the closing of the acquisition. As a result, WorldHeart will receive net proceeds of $8.5 million in cash pursuant to the private placement.
The amount of the investment by Maverick was negotiated between us and Maverick. The amount of $12 million was negotiated as an amount that was intended to provide sufficient incremental liquidity to continue to fund MedQuest's rotary pump development program for a minimum of one year and through the commencement of clinical trials. Based on the closing January 31, 2005 price (NASDAQ) of US$1.58 the price of $1.35 represented a discount of approximately 14% which the parties negotiated on an arm's length basis taking into account the other proposed transactions, the average trading price of WorldHeart's common shares over the preceding four months and the various Canadian and US legend periods.
Because of the time delay between the signing and closing of the MedQuest Acquisition and the Maverick private placement, we agreed that it would be in our best interests if MedQuest had access to immediate funding to continue its development program up to the closing of the MedQuest Acquisition. To accommodate this, and to maintain the $12 million limit on Maverick's funding commitment, we agreed that if Maverick made a loan to MedQuest to fund continuing operations we would assume the loan on closing. The $12 million was pro rated over the projected period of three to four months between signing and closing to reach the $3.5 million amount
82
of the loan. We will acquire any unused portion of the funds advanced pursuant to the loan upon closing of the MedQuest Acquisition, subject to $325,000 which will remain in MedQuest to pay certain normal course liabilities not being assumed pursuant to the MedQuest Agreement.
Representations and Warranties
In the Maverick Agreement, WorldHeart and Maverick have made a number of representations and warranties. These representations and warranties relate to, among other things:
- •
- their organization, existence, corporate good standing, corporate power and similar corporate matters;
- •
- their authorization, execution, delivery, performance and the enforceability of the Maverick Agreement and related matters; and
- •
- the absence of brokers' and related fees.
Maverick has also made representations and warranties as to:
- •
- the shares to be acquired by Maverick being acquired for investment purposes;
- •
- its knowledge and experience in financial and business matters such that it is capable of evaluating the merits and risks of the investment contemplated by the private placement;
- •
- access to information regarding WorldHeart;
- •
- its status as an accredited investor; and
- •
- its ownership of outstanding shares of MedQuest.
WorldHeart has also made representation and warranties as to:
- •
- its capitalization;
- •
- the absence of conflicts, required governmental and third-party consents;
- •
- the required vote of its shareholders;
- •
- board of directors approval;
- •
- the absence of material adverse changes, as specified in the Maverick Agreement;
- •
- valid authorization and issuance of the common shares to be issued to Maverick;
- •
- tax matters;
- •
- environmental matters;
- •
- tangible and intellectual property;
- •
- litigation;
- •
- legal compliance;
- •
- permits; and
- •
- documents and reports filed with the SEC and financial statements of WorldHeart included in those documents.
Covenants
Access to Information. WorldHeart agreed to provide Maverick, upon reasonable request, with access to information relating to WorldHeart and its subsidiaries, other than material nonpublic information.
Operations of WorldHeart. Until WorldHeart has satisfied its obligations under a registration rights agreement to be entered into at closing between WorldHeart, Maverick and MedQuest (the Registration Rights Agreement), WorldHeart has agreed that it will conduct operations in the ordinary course of business and in compliance with applicable laws, and will not take any action, enter into any agreement or make any commitment that would conflict with the Maverick Agreement.
83
Listing of Shares. WorldHeart has agreed to take all necessary action, at its own expense, to cause the common shares issued pursuant to the private placement to be listed for trading on the TSX and NASDAQ.
Director Designee. WorldHeart has agreed that as long as Maverick holds at least 25% of WorldHeart's outstanding common shares, Maverick will have the right to nominate two directors for election to WorldHeart's board of directors, and if Maverick holds less than 25% but not less than 15% of WorldHeart's common shares, Maverick will have the right to nominate one director to WorldHeart's board of directors.
MedQuest Shareholder Vote. Maverick has agreed to vote all of its shares of MedQuest in favour of the sale of MedQuest's assets to WorldHeart on any resolution put before MedQuest shareholders.
Conditions to Obligations to Effect the Private Placement
The obligation of WorldHeart to complete the private placement is subject to the satisfaction or waiver of a number of conditions, including the following:
- •
- execution by Maverick of the Registration Rights Agreement;
- •
- the representations and warranties of Maverick being true and correct as of the date of the closing of the private placement; and
- •
- receipt by WorldHeart from Maverick of the purchase price for the common shares.
In addition, the obligation of Maverick to effect the private placement is subject to the satisfaction or waiver of a number of conditions, including the following:
- •
- the representations and warranties of WorldHeart in the Maverick Agreement being true and correct as of the date of the closing of the private placement;
- •
- WorldHeart shall have performed or complied with in all material respects its agreements and covenants required to be performed or complied with under the Maverick Agreement as of or prior to closing the private placement;
- •
- WorldHeart and MedQuest shall have completed the acquisition;
- •
- the approval of the private placement by WorldHeart's shareholders;
- •
- WorldHeart shall have delivered to Maverick a certificate stating that the specified conditions of the Maverick Agreement have been satisfied;
- •
- Maverick shall have received other certificates and instruments as it reasonably requests in connection with the closing of the private placement, including opinions from WorldHeart's counsel;
- •
- WorldHeart shall have executed and delivered the Registration Rights Agreement;
- •
- the absence of any legal impediment to the closing of the private placement;
- •
- there shall not have been any suspension of public trading of WorldHeart's common shares imposed by the TSX, NASDAQ, the SEC or any other regulatory body; and
- •
- WorldHeart shall have obtained all necessary regulatory approvals to complete the private placement including the approval of the TSX and NASDAQ.
Indemnification
The Maverick Agreement provides that WorldHeart will indemnify Maverick for, among other things, any and all damages, subject to the limitations described below, that Maverick or an affiliate of Maverick may suffer as a result of a breach of the Maverick Agreement by WorldHeart.
WorldHeart's representations and warranties contained in the Maverick Agreement continue in effect for one year from the closing of the private placement. In general, WorldHeart's covenants and agreements shall survive for one year, subject to the following exceptions:
- •
- WorldHeart's covenants with respect to Maverick's right to nominate board members shall continue for so long as Maverick satisfies the share ownership thresholds;
84
- •
- WorldHeart will fulfil its covenants regarding compliance with laws, absence of conflicting agreements and listing of the common shares until the termination of WorldHeart's obligations under the Registration Rights Agreement, unless earlier agreed by WorldHeart and Maverick.
Amendment and Waiver
Generally, WorldHeart and Maverick may amend the Maverick Agreement at any time prior to the closing of the acquisition. Amendments must be in writing and signed by all parties and waivers must be in writing and signed by the waiving party.
Registration Rights Agreement
In connection with the acquisition and private placement, WorldHeart will enter into the Registration Rights Agreement with Maverick and MedQuest. The Registration Rights Agreement will provide that within 120 days following the closing of the private placement, WorldHeart shall prepare and file with the SEC a registration statement on Form S-2 permitting the resale of the common shares to be issued pursuant to the acquisition and the private placement. WorldHeart will also agree to indemnify and hold harmless Maverick and MedQuest against any losses arising from:
The private placement to Maverick will result in an additional 8,888,889 common shares of WorldHeart being issued. This will have a significant dilutive effect on existing shareholders. As an illustration, currently 10,000 common shares of WorldHeart represents 0.000585% of the issued and outstanding common shares. After the private placement to Maverick, and without giving effect to any other issuances, 10,000 common shares will represent only 0.000385% of the issued and outstanding common shares. In addition, upon completion of the transactions and subject to certain conditions, Maverick will be entitled to designate two nominees for election to the board of directors of WorldHeart.
Shareholders are therefore being asked to consider and, if thought advisable, to approve the Maverick Resolution, a copy of which are attached to this circular as Appendix A-2. Approval of the Maverick Resolution is required from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THE ISSUANCE OF WORLDHEART COMMON SHARES IN CONNECTION WITH THE MAVERICK PRIVATE PLACEMENT TO BE IN WORLDHEART SHAREHOLDERS' BEST INTERESTS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE ISSUANCE OF WORLDHEART COMMON SHARES AND RECOMMENDS APPROVAL OF THE ISSUANCE OF WORLDHEART COMMON SHARES BY WORLDHEART'S SHAREHOLDERS PURSUANT TO THE MAVERICK PRIVATE PLACEMENT.
Warrant Amendment Resolution — Amendment to September 2004 Warrants
WorldHeart issued an aggregate principal amount of $13,318,750 convertible debentures and 10,655,000 common share purchase warrants, at a rate of one warrant for each $1.25 principal amount of debentures on September 15, 2004 as part of a private placement in the United States and Canada to raise additional operating funds for WorldHeart. As a condition to the closing of the acquisition of the assets of MedQuest and the private placement of common shares with Maverick, the holders of debentures convertible into, and warrants to purchase, WorldHeart common shares, issued in September 2004, must convert their
85
debentures and exercise their warrants. During the negotiations of the transactions between MedQuest, Maverick and WorldHeart, Maverick agreed to complete a private placement with WorldHeart contingent on two requirements, namely, the completion of the acquisition of the asset of MedQuest by WorldHeart and the agreement of the debentureholders and warrantholders who invested in September 2004, to convert their debentures and to exercise their warrants if they accept a tender offer for amended warrants. The conversion of the debentures would result in WorldHeart ceasing to have any debt instruments outstanding and the exercise of the warrants would result in additional funds being injected into WorldHeart and assist in improving the capital structure of WorldHeart to remove some of the overhang in the market for the warrants. Under their terms, the debentures are convertible and the warrants are exercisable at the option of the holder at any time up to September 15, 2009.
The board considered the advice of management that given that the warrants did not expire for five years and the exercise price was close to the trading price at the time of the negotiation of the Maverick Agreement, WorldHeart would have to propose some incentive for the warrantholders to agree to exercise their warrants. The board of directors determined that a reduction in the exercise price was an appropriate incentive and was preferable to offering further warrants or other forms of stock incentive, particularly in light of the advice of management that removal of the overhang created by the warrants would improve WorldHeart's capital structure. The board instructed management to negotiate an appropriate reduction with the warrantholders and after extensive negotiations management was able to obtain the agreement of all warrantholders under which we will make a tender offer to exchange warrants permitting holders to exercise their amended warrants in connection with the closing the MedQuest Acquisition at an exercise price reduced to $1.00.
Consequently, WorldHeart entered into an agreement with each holder of debentures and warrants (the form of which is attached as Appendix I) requiring the holder to convert the debentures, and exercise the warrants upon amendment of the exercise price of the warrants. Under the agreement, the debentures will be converted into common shares and the warrants would be exercised to purchase common shares upon approval by shareholders of the amendment to the exercise price of the warrants. We subsequently released the warrant holders from this agreement and we will make a tender offer to the warrant holders to exchange warrants for amended warrants at a reduced exercise price and the amended warrants will be exercised to purchase common shares upon approval by shareholders of the amendment to the exercise price of the warrants. Under the rules of the TSX, WorldHeart must seek approval of the TSX and of its shareholders, if the warrant being amended is held, directly or indirectly, by an insider or if the proposed exercise price, in our case, $1.00, is less than the market price for the common shares which is determined on the date of the amending agreements with the warrantholders, which was $1.58. Therefore, before the amendment can become effective WorldHeart is required to obtain the approval of a majority of its shareholders other than shares held directly or indirectly by an interested party, namely, Austin Marxe and David Greenhouse who are interested parties as defined under the TSX rules because their beneficial ownership of the outstanding common shares of WorldHeart is more than 10% (see "VOTING SHARES, RECORD DATE AND PRINCIPAL HOLDERS" starting at page 10). Appendix K to this circular sets out a table of who owns the securities that will be affected by this resolution and the number of securities each such security holder will receive as a result of the conversion and exercise. All of the debentureholders and warrantholders are at arm's length to WorldHeart. Each of Zesiger Capital Group LLC, SF Capital Partners Ltd., Reid S. Walker, G. Tracy Smith and Patrick P. Walker, Bristol Investment Fund Ltd., Sherform Inc. and FCP OP Medical BioHe@lth Trends and Austin Marxe and David Greenhouse, the debentureholders and warrantholders listed above hold over 5% of the voting rights attached to the common shares of WorldHeart assuming conversion of their debentures, warrants and other securities held by each respective entity.
Shareholders are therefore being asked to consider and, if thought advisable, to approve the Warrant Amendment Resolution, a copy of which is attached to this circular as Appendix A-3. Approval of the Warrant Amendment Resolution is required from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy, other than the votes attaching to common shares beneficially owned by Austin Marxe and David Greenhouse and their respective affiliates and associates. Accordingly, the votes attached to 2,451,347 common shares will not be entitled to be cast in connection with the Warrant Amendment Resolution.
The exercise of the warrants will result in an increase of $10,655,000 in WorldHeart's cash balances. The conversion of the debentures will result in the elimination of WorldHeart's obligation to pay up to $1,745,893.35
86
in total future interest and to repay the remaining $11,639,289 of principal amount of the debentures (of the original $13,318,750 principal amount of debentures issued $1,679,461 worth of debentures has already been converted into common shares). The conversion of all of the remaining debentures will result in the issuance by WorldHeart of approximately 9,311,500 common shares, plus an additional number of common shares for the accrued interest on the debentures to the date of conversion.
The conversion of the remaining debentures and exercise of all of the warrants will result in approximately 19,966,500 additional common shares of WorldHeart being issued. This will have a significant dilutive effect on existing shareholders. As a result of the conversion of the debentures and exercise of warrants the voting power of each existing common share will be reduced by 53.85% without giving effect to the other issuances contemplated in this circular.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THE AMENDMENT TO THE EXERCISE PRICE OF THE WORLDHEART WARRANTS ISSUED TO INVESTORS IN SEPTEMBER 2004 TO BE IN THE BEST INTERESTS OF WORLDHEART SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE AMENDMENT TO THE EXERCISE PRICE OF THE WORLDHEART WARRANTS ISSUED TO INVESTORS IN SEPTEMBER 2004 AND RECOMMENDS APPROVAL OF THE WARRANT AMENDMENT RESOLUTION BY WORLDHEART'S SHAREHOLDERS.
ESOP Resolution #1 and ESOP Resolution #2 — Amendments to the World Heart Corporation Employee Stock Option Plan
WorldHeart is dependent on its ability to attract and retain qualified scientific, technical and key management personnel. Employees, consultants, officers and directors are eligible to receive grants of option under WorldHeart's employee stock option plan (the Plan). In a competitive job environment, management and the board of directors has determined that compensation arrangements should include, where appropriate, stock options and, in September 2004, the board of directors determined that executive officers and key employees of WorldHeart should be granted a greater percentage of potential ownership in WorldHeart to create greater incentive for the WorldHeart management team. The board of directors determined that management should on an aggregate basis be entitled to hold up to 10% of the common shares of WorldHeart on a fully diluted basis.
Management reviewed various studies in the marketplace, including the 2003 MEDIC report for companies based in the United States and the TechEdge report for the Canadian technology market, and several other less comprehensive equity compensation surveys for publicly and privately held companies all of which were compiled by independent research firms. Management created an overall matrix of the levels of cash and equity compensation received by executive officers of similarly situated companies and presented the overall findings and ranges to the Compensation Committee. The Compensation Committee, based on the market information available, subsequently recommended to the board that the market data supported a compensation model that encourages participation by the employees, executive officers and consultants in the ownership of WorldHeart and incentivizes the key employees through various forms of compensation plans, in amounts between 10% and 15% of the issued and outstanding common shares of WorldHeart. In some instances, the market data indicated that companies have opted to have compensation plans that exceeded 15% of the issued and outstanding common shares of the company. Based on the matrix of compensation information and the various ranges and also given WorldHeart's general movement away from cash bonuses and incentives, the board determined it was appropriate for the number of options reserved under the Plan to be approximately 12% of the common shares issued and outstanding after giving effect to the issuances contemplated in this circular and on a fully diluted basis, or 9,772,505 common shares, being the middle of the range of between 10% and 15% for similarly situated companies.
The Compensation Committee determined by reviewing comparable statistics for similar companies that key employees, including the executive officers, receive a significant portion of overall ownership of the company pursuant to compensation plans. Based on a review of the current employment base at WorldHeart and the market data, the Compensation Committee determined that equity ownership of executive officers of WorldHeart should be set at approximately 10% of the issued and outstanding common shares. This would result in an appropriate level of ownership for key employees similar to comparable companies and would create
87
the appropriate incentives for key personnel during this period of WorldHeart's growth. The allocation of the options to the key employees, was determined by the Compensation Committee in discussions with the Chief Executive Officer and was based on the individual's position within WorldHeart, seniority, and cash compensation including sales commissions for sales executives. The board also determined that the new Chief Executive Officer of WorldHeart, should be entitled to receive approximately 3% of the 10% allocated for key employees as being comparable to similar situated companies where the range was between 2% and 5% of the issued and outstanding shares of the company. The board considered it to be appropriate to commence Mr. Jassawalla's option entitlement at the lower end of the range because he was new to the position. In addition, approximately 7% is allocated to the executive officers as a group, both current executive officers and expected additional executive officers. The specific allocation depends on the individual's position, seniority and salary.
The board believes that the MedQuest Acquisition, Maverick private placement and conversion of the debentures and exercise of the warrants will be completed because the shareholders of WorldHeart will recognize the benefits of creating a platform strategy for the company by acquiring the MedQuest technology and therefore, believes that the increase in the number of options under the Plan is appropriate. In the event that these transactions do not occur, this will result in options for common shares representing 57% of the issued and outstanding common shares. This would be a significantly higher percentage than the board believes is optimal but the board recognizes that WorldHeart would contemplate other transactions which would likely result in additional common shares of WorldHeart being issued and therefore this percentage would decrease to an acceptable percentage in keeping with similarly situated companies.
The Plan provides that options vest as to one third on each of the first, second and third anniversary of the date of grant and expire on the fifth, sixth and seventh anniversary of the date of grant, respectively. Options are priced at the closing price on the TSX on the trading date immediately prior to the date of grant.
The following is a brief summary of all material United States Federal income tax consequences, under the United States Internal Revenue Code of 1986, as amended, as in effect on the date of this summary, applicable to WorldHeart and recipients of stock options under the Plan. This summary is not intended to be exhaustive of all potential consequences under all tax legislation, and, among other things, does not describe state, local or non-United States tax consequences, or the effect of gift, estate or inheritance taxes. References to "WorldHeart" in this summary of tax consequences mean WorldHeart or any subsidiary of WorldHeart that employs or receives the services of a recipient of an option under the Plan.
The grant of stock options under the Plan will not result in taxable income to the recipient of the option or an income tax deduction for WorldHeart. However, the transfer of common shares to an option holder upon exercise of his or her option may or may not give rise to taxable income to the option holder and a tax deduction for WorldHeart depending upon whether the option is an incentive stock option or a non-qualified stock option.
The exercise of a non-qualified stock option by an option holder generally results in immediate recognition of ordinary income by the option holder and a corresponding tax deduction for WorldHeart in the amount by which the fair market value of the common shares purchased, on the date of exercise, exceeds the aggregate option exercise price paid. Any appreciation or depreciation in the fair market value of those shares after the exercise date of the option will generally result in a capital gain or loss to the option holder at the time he or she disposes of those shares.
In general, the exercise of an incentive stock option is exempt from income tax, although not from the alternative minimum tax, and does not result in a tax deduction for WorldHeart at any time unless the option holder disposes of the shares purchased within two years from the date the incentive stock option was granted or one year from the date of exercise of the option (known as a "disqualifying disposition"). If these holding period requirements under the Internal Revenue Code are satisfied, and if the option holder has been an employee of WorldHeart at all times from the grant date of the incentive stock option to the day three months before exercise of the option, or twelve months in the case of termination of employment due to disability, then the option holder will recognize any gain or loss upon disposition of those shares as a capital gain or loss. However, if the option holder makes a disqualifying disposition of any of those shares, he or she will generally be obligated to report as ordinary income for the year in which that disqualifying disposition occurs the excess, with certain adjustments, of the fair market value of the shares disposed of, on the date the incentive stock option was
88
exercised, over the option exercise price paid for those shares. WorldHeart would be entitled to a tax deduction equal to that amount of ordinary income reported by the option holder. Any additional gain realized by the option holder on the disqualifying disposition would be a capital gain. If the total amount realized in a disqualifying disposition is less than the option exercise price of the incentive stock option, the difference would be a capital loss for the option holder.
Under section 162(m) of the Internal Revenue Code, WorldHeart may be limited as to Federal income tax deductions to the extent that total annual compensation in excess of $1 million is paid to WorldHeart's President and Chief Executive Officer or any one of WorldHeart's other four highest paid executive officers who are employed by WorldHeart on the last day of WorldHeart's taxable year. Compensation resulting from options under the Plan may be subject to the deduction limitations imposed by section 162(m) of the Internal Revenue Code.
Under certain circumstances, the accelerated vesting, exercise or payment of options under the Plan in connection with a "change of control" of WorldHeart might be deemed an "excess parachute payment" for purposes of the golden parachute payment provisions of section 280G of the Internal Revenue Code. To the extent it is so considered, the participant holding that option would be subject to an excise tax equal to 20% of the amount of the excess parachute payment, and WorldHeart would be denied a tax deduction for the excess parachute payment.
The holders of common shares will therefore be asked to consider and, if thought advisable, to approve amendments to the Plan, (i) to increase the maximum number of common shares which may be issued under the Plan, as amended and restated on January 31, 2005, from 1,501,857 shares to 9,772,505 shares (ESOP Resolution #1), and (ii) to remove from the Plan the restriction that the aggregate number of shares issued to any person under the Plan be limited to 5% of the issued and outstanding WorldHeart common shares (ESOP Resolution #2).
Currently, the maximum number of common shares which may be issued under the Plan is 1,501,857 common shares. In September 2004, as part of the board of directors revised approach to compensation, there was a movement away from cash bonuses and incentives for management and employees until such time as WorldHeart has sufficient cash resources to reconsider such compensation components. The board of directors determined to move to a compensation scheme that would put a greater emphasis on long term stock incentives such as stock options.
On September 23, 2004, the Board passed a resolution increasing the maximum number of common shares under the Plan from 1,501,857 common shares to 5,189,672 common shares, subject to regulatory approval and shareholder approval. On January 31, 2005, in light of the proposed MedQuest Acquisition, Maverick Agreement and conversion of debentures and exercise of warrants the board of directors resolved to increase the maximum number of common shares to 9,772,505 common shares under the Plan, subject to regulatory and shareholder approval. The number was arrived at by calculating current option grants and by aggregating allocated amounts for current and future employees determined by management to be appropriate for particular positions or employee subgroups. Assuming that the transactions contemplated herein occur, this will represent approximately 18% of the issued and outstanding common shares, after the completion of the transactions contemplated herein and approximately 12% of the common shares on a fully diluted basis, as of May 20, 2005.
On January 31, 2005, the board of directors also determined that the Plan should remove the restriction providing that the aggregate number of options reserved for issuance to any one person shall not exceed 5% of the issued and outstanding common shares from time to time. The board of directors determined, given market comparisons and its revised approach to compensation, that there may be individuals in management who may from time to time be entitled to in excess of the 5% of the issued and outstanding common shares.
89
On September 23, 2004 and January 31, 2005, the board of directors approved the issuance of option grants to employees, consultants, executive officers and directors of WorldHeart, subject to the shareholders approving ESOP Resolution #1 and ESOP Resolution #2 and in respect of certain executive officers and directors subject to shareholder approval of the Option Amendment Resolution at this Annual and Special Meeting. The board of directors approved the issuances on September 23, 2004 to be a one time grant for a period of three years, subject to additional issuances from time to time in the discretion of the board of directors. The option issuances approved on January 31, 2005, were to realign the number of options held by the executive officers, directors and key employees in light of the dilution contemplated by the transactions contemplated herein.
As of June 10, 2005, and assuming approval of ESOP Resolution #1, ESOP Resolution #2 and the Option Amendment Resolution, there are available 2,549,358 common shares for the grant of options under the Plan to employees, officers, directors and consultants. Options for 6,367,475 common shares have been granted to employees, consultants, officers and directors, subject to shareholder approval and options for 251,685 of common shares have been forfeited, subject to shareholder approval.
As of June 10, 2005, the following options for common shares were held by the executive officers individually and as a group, non executive directors as a group and non-executive officers as a group:
EMPLOYEE STOCK OPTION BENEFITS
| | World Heart Corporation Employee
Stock Option Plan
|
|---|
Name and Position
| | Number of Common Shares(1)
| | Dollar Value of Options Held
|
|---|
Jal S. Jassawalla,
President and Chief Executive Officer | | 1,788,929 | | $ | 2,039,379 |
Richard Juelis,
Vice President, Finance and Chief Financial Officer | | 275,000 | | $ | 313,500 |
Piet Jansen,
Managing Director, Europe and Chief Medical Officer | | 325,000 | | $ | 370,500 |
John Marinchak,
Vice President, Marketing and Sales | | 260,000 | | $ | 296,400 |
Phillip Miller,
Vice President, Research and Development | | 325,270 | | $ | 370,808 |
John Vajda,
Vice President, Manufacturing | | 250,000 | | $ | 285,000 |
| Executive Group | | 3,224,199 | | $ | 3,675,587 |
| Non-Executive Director Group | | 600,365 | | $ | 684,416 |
| Non-Executive Officer Employee Group | | 1,397,654 | | $ | 1,593,326 |
- (1)
- All of the options held by the executive officers and 600,000 options held by non-executive directors are subject to shareholder approval.
Shareholders are therefore being asked to consider and, if thought advisable, to approve ESOP Resolution #1 and ESOP Resolution #2, copies of which are attached to this circular as Appendix A-4 and Appendix A-5, respectively. Approval of ESOP Resolution #1 will be sought from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy. Approval of ESOP Resolution #2 is required from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy, other than the votes attaching to common shares beneficially owned by directors and officers of WorldHeart and their respective associates. Accordingly, the votes attached to 20,535 common shares will not be entitled to be cast in connection with ESOP Resolution #2.
90
WorldHeart's common shares are listed on the TSX and NASDAQ and are subject to the TSX rules and NASDAQ Marketplace Rules. NASDAQ Rule 4350 and the TSX rules require shareholder approval when a plan or other equity compensation arrangement is established or materially amended. ESOP Resolution #1 and ESOP Resolution #2 have been put forward, among other reasons discussed in this proposal, in order to comply with the application of the TSX and NASDAQ rules.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THE AMENDMENTS TO THE WORLDHEART EMPLOYEE STOCK OPTION PLAN TO BE IN THE BEST INTERESTS OF THE WORLDHEART SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE AMENDMENTS TO THE WORLDHEART EMPLOYEE STOCK OPTION PLAN AND RECOMMENDS APPROVAL OF ESOP RESOLUTION #1 AND ESOP RESOLUTION #2 BY WORLDHEART'S SHAREHOLDERS.
Option Amendment Resolution — Amendment to Certain Options Held by Executive Officers and Directors
Pursuant to the revised approach to compensation of executive officers and key employees, more fully described under the heading "MATTERS TO BE ACTED UPON — ESOP Resolution #1 and ESOP Resolution #2 — Amendments to the World Heart Corporation Employee Stock Option Plan", the board of directors determined that there would be greater emphasis on long term share compensation for all employees including the executive officers and key employees and a movement away from cash incentives, except with respect to sales commissions.
In September 2004, upon reviewing comparable companies within the industry as described in the MEDIC report and TechEdge report as well as other similar data, the board of directors determined to make certain grants of options to employees, executive officers and directors of WorldHeart. At that time, certain executive officers and directors agreed to forfeit options as set forth below, conditional on receipt of certain new options which grant of the new options is subject to shareholder approval as described under the heading "MATTERS TO BE ACTED UPON — ESOP Resolution #1 and ESOP Resolution #2 — Amendment to the World Heart Corporation Employee Stock Option Plan." The directors and executive officers of WorldHeart agreed to forfeit the options so that those options would be returned to the pool and each individual would commence on a common basis and with more equitable terms. The board determined that this would assist in creating incentive for the executive officers of WorldHeart given salary constraints and a move away from cash incentives. At the time of the forfeiture, none of the options held by the executive officers or directors had an exercise price that was at or below the then current market price of our common shares. There is no direct correlation between the number of options forfeited and the number of new options granted to each director and executive officer but the nature of these actions is considered to be a repricing of the options received by these executive officers and directors and, therefore, because these individuals are insiders of WorldHeart, the options granted to these individuals are subject to shareholder approval.
91
The following table sets forth the number of options forfeited by the executive officers and directors and the new options granted to these individuals in September 2004:
Name
| | Forfeited Options
| | Exercise Price
| | Expiry Date
| | New Options Granted
| | Exercise Price
| | Expiry Date
|
|---|
| Executives | | | | | | | | | | | | |
| Jal S. Jassawalla | | 105,397 | | $7.77-$29.05 | | Jan 4, 2007 to
Dec 31, 2010 | | 787,225 | | $1.12 | | Sept 22, 2013 |
| John Marinchak | | 18,256 | | $7.21-$7.83 | | Oct 26, 2008
to Dec 31, 2010 | | 160,000 | | $1.12 | | Sept 22, 2013 |
| Piet Jansen | | 21,500 | | $7.03 | | Dec 31, 2008
to Dec 31, 2010 | | 240,000 | | $1.12 | | Sept 22, 2013 |
| Phillip Miller | | 5,716 | | $7.77-$29.05 | | Jan 4, 2007
to Dec 31, 2010 | | 180,000 | | $1.12 | | Sept 22, 2013 |
| | |
| | | | | |
| | | | |
| | | 150,869 | | | | | | 1,367,225 | | | | |
| | |
| | | | | |
| | | | |
Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
| C. Ian Ross | | 18,141 | | Cdn$10.29-$46.41 | | March 31, 2006
to Dec 31, 2010 | | 80,000 | | Cdn$1.43 | | Sept 22, 2013 |
| Robert J. Majteles | | 14,285 | | Cdn$10.29 | | Dec 31, 2008 to
Dec 31, 2010 | | 80,000 | | Cdn$1.43 | | Sept 22, 2013 |
| John F. Carlson | | 14,285 | | Cdn$10.15 | | Dec 31, 2008
to Dec 31, 2010 | | 80,000 | | Cdn$1.43 | | Sept 22, 2013 |
| William C. Garriock | | 14,285 | | Cdn$10.28 | | Dec 31, 2008
to Dec 31, 2010 | | 80,000 | | Cdn$1.43 | | Sept 22, 2013 |
| D. Mark Goudie | | 39,820 | | Cdn$8.54-$37.45 | | April 1, 2007
to Dec 31, 2010 | | 40,000 | | Cdn$1.43 | | Sept 22, 2013 |
| | |
| | | | | |
| | | | |
| | | 100,816 | | | | | | 360,000 | | | | |
| | |
| | | | | |
| | | | |
| Total | | 251,685 | | | | | | 1,727,225 | | | | |
| | |
| | | | | |
| | | | |
Shareholders are therefore being asked to consider and, if thought advisable, to approve the Option Amendment Resolution, a copy of which is attached to this circular as Appendix A-6. Approval of the Option Amendment Resolution is required from a majority of the votes cast at the Annual and Special Meeting, in person or by proxy, other than the votes attaching to common shares beneficially owned by directors and executive officers of WorldHeart and their respective associates. Accordingly, the votes attached to 20,535 common shares will not be entitled to be cast in connection with Option Amendment Resolution.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THE OPTION AMENDMENTS FOR THE EXECUTIVE OFFICERS AND DIRECTORS TO BE IN THE BEST INTERESTS OF THE WORLDHEART SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE OPTION AMENDMENTS FOR CERTAIN EXECUTIVE OFFICERS AND DIRECTORS AND RECOMMENDS APPROVAL OF THE OPTION AMENDMENT RESOLUTION BY WORLDHEART'S SHAREHOLDERS.
Continuance Resolution — Continuance under the Canada Business Corporations Act
At the meeting, shareholders will be asked to consider, and if thought advisable to pass, the Continuance Resolution substantially in the form set out in Appendix A-7 attached to this circular, to continue WorldHeart from theBusiness Corporations Act (Ontario) (the OBCA), which currently governs its affairs, to theCanada Business Corporations Act (the CBCA), a process referred to in this circular as the Continuance.
92
The effect of a continuance is that WorldHeart becomes a corporation to which the CBCA applies as if it had been incorporated under the CBCA and to which the OBCA no longer applies.
If the Continuance Resolution is approved at the meeting, subject to the discretion of the board of directors to decide otherwise, WorldHeart will seek approval of the Director under the OBCA to apply to the Director under the CBCA for the Continuance, as required by Section 181 of the OBCA. To approve the Continuance, the Director under the OBCA must be satisfied that with the Continuance no creditors or shareholders will be adversely affected.
WorldHeart intends to file articles of continuance pursuant to Section 187 of the CBCA to continue WorldHeart under the provisions of the CBCA as soon as practicable after the meeting. Subject to appropriate shareholder approval and such filings, the Continuance will be effective on the date of the certificate of continuance, which is issued by the Director under the CBCA upon receipt of articles of continuance pursuant to Subsection 187(4) of the CBCA. The OBCA will cease to apply to WorldHeart on the date of the certificate of continuance. WorldHeart will file a copy of the certificate and articles of continuance with the Director under the OBCA within 60 days after the date of the issuance of the certificate of continuance.
Notwithstanding the approval of the Continuance by a special resolution of the shareholders, the board of directors of WorldHeart may, without further approval of the shareholders, abandon the application for Continuance at any time prior to the issue of a certificate of continuance by the Director under the CBCA.
Continuance under the CBCA does not create a new legal entity and will not prejudice or affect the continuity of WorldHeart. The Continuance will not result in any change in the business of WorldHeart.
The persons who constitute WorldHeart's board of directors will continue to be those persons elected by the shareholders at the Annual and Special Meeting. The officers of WorldHeart will continue to be those persons appointed by the board of directors.
Under the CBCA, upon the Continuance, the rights are preserved in respect of in: (i) the ownership of corporate property of WorldHeart; (ii) liability for obligations of WorldHeart; (iii) the existence of a cause of action, claim or liability to prosecution of WorldHeart; (iv) enforcement against WorldHeart of any civil, criminal or administrative proceedings pending; and (v) the enforceability of any conviction or judgment against or in favour of WorldHeart. Furthermore, any shares issued before the Continuance are deemed to have been issued in compliance with the CBCA and with the articles of continuance. Continuance does not deprive a holder of common shares of any right or privilege, or relieve a holder of common shares of any liability in respect of an issued share.
Management believes that the Continuance of WorldHeart under the CBCA is appropriate given the international scope of WorldHeart's business and the previously announced intention to consolidate WorldHeart's business. Continuance under the CBCA will permit WorldHeart to take advantage of recent amendments to the CBCA which modernize corporate law procedures and requirements, and will help facilitate the planned movement of operations to the United States by allowing WorldHeart to recruit additional non-Canadian directors to its board. In addition, the CBCA permits broader indemnification of directors necessary to attract additional high calibre directors to WorldHeart.
Management is of the view that the CBCA is consistent with corporate legislation in most other Canadian jurisdictions and will provide shareholders with substantially the same rights that are available to shareholders under the OBCA, including rights of dissent, and rights to bring derivative actions and oppression actions. The CBCA amendments relating to residency requirements of directors (described below) will provide WorldHeart with greater flexibility in the composition of the board by removing a constraint on the pool of experienced and qualified individuals who are able to serve as directors of WorldHeart.
93
The OBCA and the CBCA are similar in many respects, although there are a number of notable differences in respect of corporate law matters. The following is a brief summary of the material differences between the OBCA and the CBCA. This summary is not intended to be exhaustive and shareholders should consult their legal advisors regarding implications of the Continuance which may be of particular importance to them.
Voting Rights Where Shares Transferred After Record Date
Under the OBCA, where a shareholder transfers shares after the record date for a meeting, the transferee is entitled to vote these shares at the meeting if the transferee establishes ownership and demands, not later than ten days before the meeting, that his/her name be included in the list of shareholders entitled to vote at the meeting. Under the CBCA transferees of shares after a record date are not entitled to vote the transferred shares at the meeting.
Board of Directors
Under the OBCA, a majority of a corporation's directors, and a majority of the members of any committee of directors, must be resident Canadians. The CBCA requires that only 25% of the directors be resident Canadians (unless WorldHeart has fewer than four directors in which case at least one must be a Canadian resident), and imposes no residency requirements on committees.
Shareholder Proposals
Both the OBCA and the CBCA provide for shareholder proposals. Under the OBCA, any shareholder entitled to vote at a meeting of shareholders may submit a proposal. Under the CBCA, a registered or beneficial owner of shares entitled to be voted at a meeting may submit a proposal, although the registered or beneficial shareholder must either (i) have owned for six months not less than 1% of the total number of voting shares or voting shares with a fair market value of at least Cdn$2,000; or (ii) have the support of persons who have owned for six months not less than 1% of the total number of voting shares or voting shares with a fair market value of at least Cdn$2,000.
Although the CBCA sets out certain restrictions on which shareholders can bring forward a shareholder proposal at a meeting of shareholders, the tests set forth under the CBCA are not onerous but provide for at least a minimum level of commitment and participation by a shareholder. This provides all shareholders with a minimum level of protection that the shareholder proposals that are put forward are less likely to be frivolous in nature and merit the attention and resources of WorldHeart and its shareholders for consideration at a meeting of shareholders.
Director Indemnification
In comparison to the OBCA, the CBCA permits a company to indemnify its directors and officers in a slightly broader range of proceedings, including investigative and other proceedings. Unlike the OBCA, the CBCA permits a corporation to advance funds to a director or officer to cover the costs and expenses of a proceeding.
Financial Assistance
The OBCA requires disclosure of financial assistance given by a corporation in connection with the purchase of shares of the company or its affiliates, to shareholders, directors and their associates of the corporation and its affiliates. The CBCA has no such requirement.
Rights of Dissent
Under both the OBCA and the CBCA, shareholders have substantially the same rights of dissent, described in detail below in the section entitled Dissent Rights, if WorldHeart resolves to effect certain fundamental changes.
94
WorldHeart is currently an Ontario corporation which has articles of incorporation which set out the name, the authorized capital, the classes of shares, any maximum number of shares that may be issued, the rights, privileges, restrictions and conditions attaching to shares, any restriction on the right to transfer shares, the maximum or minimum number of directors and any restrictions on the business of WorldHeart. Upon the Continuance taking effect, the articles of continuance filed under the CBCA will replace the articles of incorporation filed under the OBCA.
The rights, privileges, restrictions and conditions that are presently applicable to the common shares are substantially the same as the rights, privileges, restrictions and conditions that will attach to such common shares after the Continuance and are set out in the articles of continuance.
Under the provisions of Section 185 of the OBCA, a registered shareholder of WorldHeart is entitled to send a written objection to the Continuance Resolution. In addition to any other right a shareholder may have, when the action authorized by the Continuance Resolution becomes effective, a registered shareholder who complies with the dissent procedure under Section 185 of the OBCA is entitled to be paid the fair value of his or her shares in respect of which he or she dissents, determined as at the close of business on the day before the Continuance Resolution is adopted.
The full text of the dissent procedures provided by Section 185 of the OBCA is set out at Appendix J. Shareholders who may wish to dissent should read Appendix J carefully and in its entirety.
Persons who are beneficial owners of shares registered in the name of a broker, custodian, nominee, other intermediary or in some other name who wish to dissent, should be aware that only the registered owner of such securities is entitled to dissent.
A shareholder is not entitled to dissent if such shareholder votes any of the shares beneficially held by him in favour of the Continuance Resolution. The execution or exercise of a proxy does not constitute a written objection for the purposes of Section 185 of the OBCA.
Failure to adhere strictly to the requirements of Section 185 of the OBCA and the time frames specified therein may result in the loss or unavailability of rights under that Section.
In order to become effective, the Continuance Resolution must be approved by a vote of not less than two-thirds of the votes cast by shareholders at the meeting, present in person or by proxy.
AFTER CAREFUL CONSIDERATION, THE WORLDHEART BOARD OF DIRECTORS HAS DETERMINED THAT THE CONTINUANCE IS IN THE BEST INTERESTS OF THE WORLDHEART SHAREHOLDERS. WORLDHEART'S BOARD OF DIRECTORS UNANIMOUSLY APPROVED THE CONTINUANCE AND RECOMMENDS APPROVAL OF THE CONTINUANCE RESOLUTION BY WORLDHEART'S SHAREHOLDERS.
MATERIAL TAX CONSIDERATIONS
There will be no income tax consequences to the holders of WorldHeart common shares as a result of the completion of any of the MedQuest Acquisition and Maverick private placement. There will be no income tax consequences to the securityholders of WorldHeart as a result of the Continuance being completed.
OTHER MATTERS
The management of WorldHeart knows of no amendment or variation of the matters referred to in the Notice of Annual and Special Meeting and of no other business to be brought before the meeting. However, if any amendment, variation or other business is properly brought before the meeting, the accompanying Form of Proxy confers discretionary authority on the persons named therein to vote on any amendment or variation of
95
the matters referred to in the Notice of Annual and Special Meeting, or such other business, in accordance with their best judgment.
TRANSFER AGENT
WorldHeart's registrar and transfer agent is CIBC Mellon Trust Company, 200 Queen's Quay East, Unit #6, Toronto, Ontario, M5A 4K9. The register of common shareholders of WorldHeart is maintained in Toronto by the transfer agent and registrar. WorldHeart's co-transfer agent is Mellon Investor Services LLC, 85 Challenger Road, Ridgefield Park, NJ 07660.
EXPERTS
The U.S. GAAP and Canadian GAAP audited consolidated financial statements of WorldHeart as of December 31, 2004 and December 31, 2003, and the consolidated statements of loss, shareholders' equity (deficiency) and cash flows for the years ended December 31, 2004, 2003 and 2002, are included as Appendix B-1 and Appendix B-2 to this circular in reliance upon the report of PricewaterhouseCoopers LLP, independent auditors, as indicated in their reports with respect thereto, and included herein in reliance on the authority of that firm as experts in accounting and auditing in giving those reports.
The audited financial statements of MedQuest as of June 30, 2004 and June 30, 2003, and the statements of loss, shareholders' equity (deficiency) and cash flows for the years ended June 30, 2004 and 2003, are included as Appendix D to this circular in reliance upon the report of PricewaterhouseCoopers LLP, independent auditors as indicated in their reports with respect thereto and included herein in reliance on the authority of the firm as experts in accounting and auditing in giving those reports.
WorldHeart's lawyers own less than .001% of the issued and outstanding common shares of WorldHeart.
ADDITIONAL INFORMATION
Additional information relating to WorldHeart is available on the System for Electronic Document Analysis and Retrieval at www.sedar.com. Shareholders may request copies of the corporation's financial statements and management's discussion and analysis by writing to the Director of Finance of WorldHeart at 7799 Pardee Lane, Oakland, California, USA 94621 or by calling (510) 563-5000 or obtain copies on WorldHeart's website www.worldheart.com. Financial information is provided in WorldHeart's audited consolidated financial statements and management's discussion and analysis for the year ended December 31, 2004.
Prior to March 21, 2005, WorldHeart was subject to the informational requirements of the Securities Exchange Act of 1934 (the Exchange Act) applicable to foreign private issuers and fulfilled the obligations of these requirements by filing reports with the SEC. As a foreign private issuer, WorldHeart was exempt from the rules under the Exchange Act relating to the furnishing and content of proxy statements, and its officers, directors and principal shareholders were exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, WorldHeart was not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, WorldHeart filed with the SEC, within six months after the end of each fiscal year, an annual report on Form 20-F containing financial statements examined and reported on, with an opinion expressed, by an independent public accounting firm. In addition, WorldHeart furnished the following types of information to the SEC under cover of Form 6-K:
- •
- material information WorldHeart otherwise made publicly available in reports it filed with regulatory authorities in Canada;
- •
- material information that WorldHeart filed with the Toronto Stock Exchange; and
- •
- material information WorldHeart distributed to its shareholders.
As of March 21, 2005, the board of directors determined that WorldHeart ceased to be a foreign private issuer and since that date has complied with the filing and reporting obligations under the Exchange Act.
96
You may read this circular and any document WorldHeart files with the Securities and Exchange Commission, without charge at the Securities and Exchange Commission's public reference room at 450 Fifth Street, N.W., Washington, D.C. 20549 and at the Commission's regional office at Citicorp Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the Securities and Exchange Commission at 450 Fifth Street, N.W., Washington, D.C. 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the public reference room. Documents are also available through the web site maintained by the SEC at www.sec.gov.
APPROVAL OF THE BOARD OF DIRECTORS
The contents and the sending of this circular have been approved by the board of directors of WorldHeart.
DATED at Ottawa, Ontario, Canada, this 13th day of June, 2005.

C. Ian Ross,Chairman
97
CONSENT OF INDEPENDENT ACCOUNTANTS
We hereby consent to the use in this Management Information Circular of World Heart Corporation of both of our reports dated March 11, 2005 relating to the financial statements of World Heart Corporation, which appear in such Management Information Circular. We also consent to the reference to us under the heading "Experts" in such Management Information Circular.
/s/PRICEWATERHOUSECOOPERS LLP
Ottawa, Canada
June 13, 2005.
98
AUDITORS' CONSENT
We have read the Management Information Circular of World Heart Corporation (the Company) dated June 13, 2005 relating to the issue of common shares of the Company. We have complied with Canadian generally accepted standards for an auditor's involvement with offering documents.
We consent to the use in the above-mentioned Management Information Circular of our reports to the shareholders of the Company on the consolidated balance sheets of the Company as at December 31, 2004 and 2003, and the consolidated statements of operations, equity and cash flows for each of the years in the three period ended December 31, 2004. Our reports are dated March 11, 2005.
/s/PRICEWATERHOUSECOOPERS LLP
Ottawa, Canada
June 13, 2005.
99
CONSENT OF INDEPENDENT ACCOUNTANTS
We agree to the inclusion in this Management Information Circular of World Heart Corporation of our report dated January 11, 2005, except for notes 14 and 15 as to which the date is May 23, 2005 relating to our audit of the financial statements of MedQuest Products, Inc. We also agree to the reference to us under the heading "Experts" in such Management Information Circular.
/s/PRICEWATERHOUSECOOPERS LLP
Salt Lake City, Utah
June 13, 2005.
100
APPENDIX A-1
MEDQUEST RESOLUTION
PURCHASE OF ASSETS FROM MEDQUEST PRODUCTS, INC.
RESOLVED that:
- 1.
- the Corporation is hereby authorized to issue 9,300,000 common shares of the Corporation to MedQuest Products, Inc. ("MedQuest"), at a price of $1.35 per common share, in connection with the purchase of all of the assets and the assumption of certain liabilities of MedQuest, on the terms and conditions set out in the asset purchase agreement between the Corporation and MedQuest, dated January 31, 2005, as amended, all as more fully described in the management information circular delivered to shareholders in connection with the meeting; and
- 2.
- any director or officer of the Corporation is authorized and directed to execute and deliver or cause to be executed and delivered all such documents and instruments and to take or cause to be taken all such other actions as such director or officer may determine to be necessary or desirable to carry out the intent of the foregoing special resolutions, such determination to be conclusively evidenced by the execution and delivery of such documents and instruments and the taking of such actions.
A-1-1
APPENDIX A-2
MAVERICK RESOLUTION
PRIVATE PLACEMENT TO MAVERICK VENTURE MANAGEMENT, LLC
RESOLVED that
- 1.
- the Corporation is hereby authorized to issue 8,888,889 common shares of the Corporation by way of a private placement, at a price of $1.35 per common share, to Maverick Venture Management, LLC ("Maverick"), the principal shareholder of MedQuest, on the terms and conditions set out in the purchase agreement between the Corporation and Maverick, dated January 31, 2005, as amended, all as more fully described in the management information circular delivered to shareholders in connection with the meeting; and
- 2.
- any director or officer of the Corporation is authorized and directed to execute and deliver or cause to be executed and delivered all such documents and instruments and to take or cause to be taken all such other actions as such director or officer may determine to be necessary or desirable to carry out the intent of the foregoing special resolutions, such determination to be conclusively evidenced by the execution and delivery of such documents and instruments and the taking of such actions.
A-2-1
APPENDIX A-3
WARRANT AMENDMENT RESOLUTION
REDUCTION OF WARRANT EXERCISE PRICE
RESOLVED that:
- 1.
- the reduction of the exercise price of the warrants issued by the Corporation as of September 15, 2004 (the "Warrants"), from $1.55 per common share to $1.00 per common share, for a period of 14 days following the approval of this resolution, is approved;
- 2.
- the exercise price of the Warrants is hereby amended without further action by the Corporation and no new certificates need be issued in respect of the Warrants; and
- 3.
- upon the expiry of the 14 day period following the approval of this resolution, the exercise price of any unexercised Warrants shall revert to $1.55 per common share without further action by the Corporation.
A-3-1
APPENDIX A-4
ESOP RESOLUTION #1
AMENDMENT TO THE WORLD HEART CORPORATION EMPLOYEE STOCK OPTION PLAN
RESOLVED that the number of common shares of the Corporation reserved for issuance under the World Heart Corporation Employee Stock Option Plan be increased from 1,501,857 common shares to 9,772,505 common shares.
A-4-1
APPENDIX A-5
ESOP RESOLUTION #2
AMENDMENT TO THE WORLD HEART CORPORATION EMPLOYEE STOCK OPTION PLAN
RESOLVED that Section 4 of the World Heart Corporation Employee Stock Option Plan be amended to remove the restriction providing that the aggregate number of options reserved for issuance to any one person shall not exceed five percent (5%) of the issued and outstanding common shares from time to time.
A-5-1
APPENDIX A-6
OPTION AMENDMENT RESOLUTION
AMENDMENT OF CERTAIN OPTIONS HELD BY EXECUTIVE OFFICERS AND DIRECTORS
RESOLVED that the grants of options to the executive officers and directors of the Corporation and the forfeiture of options by the executive officers and directors of the Corporation set forth below are approved:
Name
| | Options Granted
| | Exercise Price
| | Expiry Date
| | Options Forfeited
|
|---|
| Jal S. Jassawalla | | 787,225 | | $1.12 | | Sept. 22, 2013 | | 105,397 |
| John Marinchak | | 160,000 | | $1.12 | | Sept. 22, 2013 | | 18,256 |
| Piet Jansen | | 240,000 | | $1.12 | | Sept. 22, 2013 | | 21,500 |
| D. Mark Goudie | | 40,000 | | Cdn$1.43 | | Sept. 22, 2013 | | 39,820 |
| Phillip Miller | | 180,000 | | $1.12 | | Sept. 22, 2013 | | 5,716 |
| C. Ian Ross | | 80,000 | | Cdn$1.43 | | Sept. 22, 2013 | | 18,141 |
| Robert J. Majteles | | 80,000 | | Cdn$1.43 | | Sept. 22, 2013 | | 14,285 |
| John F. Carlson | | 80,000 | | Cdn$1.43 | | Sept. 22, 2013 | | 14,285 |
| William C. Garriock | | 80,000 | | Cdn$1.43 | | Sept. 22, 2013 | | 14,285 |
A-6-1
APPENDIX A-7
CONTINUANCE RESOLUTION
CONTINUANCE UNDER THE CANADA BUSINESS CORPORATIONS ACT
RESOLVED that:
- 1.
- the Corporation is authorized to apply to the Director appointed under theCanada Business Corporations Act (the "CBCA") pursuant to section 187 of the CBCA for a certificate of continuance continuing the Corporation as if it had been incorporated thereunder (the "Continuance");
- 2.
- the Corporation is authorized to apply to the Director appointed under theBusiness Corporations Act (Ontario) (the "OBCA") for authorization to permit the Continuance;
- 3.
- notwithstanding that this special resolution shall have been duly passed by the shareholders of the Corporation, the directors of the Corporation are authorized, in their sole discretion, to abandon the application for a certificate of continuance under the CBCA at any time prior to the issue thereof without further approval of the shareholders of the Corporation;
- 4.
- any director or officer of the Corporation is authorized and directed to execute and deliver or cause to be executed and delivered articles of continuance; and
- 5.
- any director or officer of the Corporation is authorized and directed to execute and deliver or cause to be executed and delivered all such documents and instruments and to take or cause to be taken all such other actions as such director or officer may determine to be necessary or desirable to carry out the intent of this special resolution, such determination to be conclusively evidenced by the execution and delivery of such documents and instruments and the taking of such actions.
A-7-1
APPENDIX B-1
WORLD HEART CORPORATION
Audited Financial Statements
For the years ended December 31, 2004 and 2003
B-1-1
MANAGEMENT'S STATEMENT OF RESPONSIBILITY
Management is responsible for the preparation of the consolidated financial statements and all other information in the annual report. The financial statements have been prepared in accordance with generally accepted accounting principles in the United States ("U.S. GAAP") and reflect management's best estimates and judgments. The financial information presented elsewhere in the annual report is consistent with the consolidated financial statements.
Management has developed and maintains a system of internal controls to provide reasonable assurance that all assets are safeguarded and to facilitate the preparation of relevant, reliable and timely financial information. Consistent with the concept of reasonable assurance, the Corporation recognizes that the relative cost of maintaining these controls should not exceed their expected benefits.
The Audit Committee, which is comprised of independent directors, reviews the consolidated financial statements, considers the report of the external auditors, assesses the adequacy of the Corporation's internal controls, and recommends to the Board of Directors the independent auditors for appointment by the shareholders. The consolidated financial statements were reviewed by the Audit Committee and approved by the Board of Directors.
The consolidated financial statements were audited by PricewaterhouseCoopers LLP, the external auditors, in accordance with generally accepted auditing standards on behalf of the shareholders.
| Original signed by: | | Original signed by: |
| JAL. S. JASSAWALLA | | D. MARK GOUDIE |
| President | | Chief Financial Officer |
B-1-2
AUDITORS' REPORT TO THE SHAREHOLDERS OF WORLD HEART CORPORATION
We have audited the consolidated balance sheets of World Heart Corporation as at December 31, 2004 and 2003, and the consolidated statements of operations, shareholders' equity (deficiency) and cash flow for the years ended December 31, 2004, 2003 and 2002. These financial statements are the responsibility of the Corporation's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with generally accepted auditing standards in Canada and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Corporation as at December 31, 2004 and 2003 and the results of its operations and its cash flows for the years ended December 31, 2004, 2003 and 2002 in accordance with generally accepted accounting principles in the United States.
On March 11, 2005,we reported separately to the shareholders of the Corporation on the consolidated financial statements for the same period, prepared in accordance with accounting principles generally accepted in Canada.
| Ottawa, Canada | | PRICEWATERHOUSECOOPERS LLP |
| March 11, 2005 | | Chartered Accountants |
B-1-3
COMMENTS BY AUDITORS FOR U.S. READERS ON CANADA-U.S. REPORTING DIFFERENCES
In the United States, reporting standards for auditors require the addition of an explanatory paragraph (following the opinion paragraph) when the financial statements are affected by conditions and events that cast substantial doubt on the Corporation's ability to continue as a going concern, such as those described in Note 1 to the consolidated financial statements. Although we conducted our audits in accordance with generally accepted auditing standards in Canada and the standards of the Public Company Accounting Oversight Board (United States), our report to the shareholders dated March 11, 2005 is expressed in accordance with Canadian reporting standards which do not permit a reference to such conditions and events in the auditors' report when these are adequately disclosed in the financial statements
| Ottawa, Canada | | PRICEWATERHOUSECOOPERS LLP |
| March 11, 2005 | | Chartered Accountants |
B-1-4
WORLD HEART CORPORATION
CONSOLIDATED BALANCE SHEETS
(United States Dollars)
| | December 31, 2004
| | December 31, 2003
| |
|---|
| ASSETS | | | | | | | |
Current assets |
|
|
|
|
|
|
|
| | Cash and cash equivalents (note 4) | | $ | 3,818,767 | | $ | 6,337,677 | |
| | Short-term investments (note 4) | | | 4,999,034 | | | 11,720,510 | |
| | Trade and other receivables | | | 4,238,049 | | | 3,894,911 | |
| | Prepaid expenses | | | 575,261 | | | 614,222 | |
| | Inventory (note 5) | | | 8,112,525 | | | 5,902,866 | |
| | |
| |
| |
| | | | 21,743,636 | | | 28,470,186 | |
| Long-term receivable | | | 318,553 | | | — | |
| Cash pledged as collateral for lease (note 17 (a)) | | | 750,000 | | | 527,997 | |
| Capital assets (note 6) | | | 2,011,586 | | | 3,041,657 | |
| Goodwill | | | 17,179,643 | | | 17,179,643 | |
| Intangible assets (note 7) | | | 255,012 | | | 879,118 | |
| Other assets | | | — | | | 81,468 | |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
| | Accounts payable and accrued liabilities | | $ | 3,854,833 | | $ | 8,214,090 | |
| | Taxes payable (note 9) | | | 832,000 | | | — | |
| | Accrued compensation | | | 1,343,364 | | | 1,748,364 | |
| | Deferred revenue | | | 1,607,622 | | | 176,573 | |
| | |
| |
| |
| | | | 7,637,819 | | | 10,139,027 | |
| Convertible debentures (note 10) | | | 8,177,140 | | | — | |
| Long-term obligation | | | 16,368 | | | — | |
| | |
| |
| |
| | | | 15,831,327 | | | 10,139,027 | |
| | |
| |
| |
Commitments and contingencies (note 17) |
|
|
|
|
|
|
|
Shareholders' equity |
|
|
|
|
|
|
|
| | Common shares | | | 214,616,061 | | | 212,457,492 | |
| | | Issued and outstanding — 15,744,522 common shares (December 31, 2003 — 15,023,689) (note 12) | | | | | | | |
| | Additional paid-in capital | | | 11,451,267 | | | 1,081,977 | |
| | Cumulative other comprehensive income | | | (6,285,577 | ) | | (6,285,577 | ) |
| | Accumulated deficit | | | (193,354,648 | ) | | (167,212,850 | ) |
| | |
| |
| |
| | | | 26,427,103 | | | 40,041,042 | |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
Signed on behalf of the Board of Directors
Original signed by John F. Carlson and C. Ian Ross |
Director |
|
Director |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-1-5
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(United States Dollars)
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Revenue | | $ | 9,575,761 | | $ | 6,775,807 | | $ | 6,436,696 | |
| Cost of goods sold | | | (7,680,384 | ) | | (4,968,898 | ) | | (6,284,996 | ) |
| | |
| |
| |
| |
| Gross margin | | | 1,895,377 | | | 1,806,909 | | | 151,700 | |
| | |
| |
| |
| |
Expenses |
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | | (13,363,796 | ) | | (7,263,759 | ) | | (6,686,437 | ) |
| | Research and development (note 13) | | | (5,838,754 | ) | | (9,604,080 | ) | | (15,931,912 | ) |
| | Restructuring costs (note 14) | | | (1,787,129 | ) | | (3,356,487 | ) | | — | |
| | Amortization of intangibles | | | (515,012 | ) | | (1,938,595 | ) | | (2,603,311 | ) |
| | |
| |
| |
| |
| | | | (21,504,691 | ) | | (22,162,921 | ) | | (25,221,660 | ) |
| | |
| |
| |
| |
| Loss before the undernoted | | | (19,609,314 | ) | | (20,356,012 | ) | | (25,069,960 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
|
|
|
| | Foreign exchange gain (loss) | | | (308,338 | ) | | 10,055,129 | | | 509,208 | |
| | Investment income | | | 99,427 | | | 105,596 | | | 117,589 | |
| | Loss on disposal of capital assets | | | (46,431 | ) | | (27,147 | ) | | (32,087 | ) |
| | Interest expense and financing costs | | | (6,277,142 | ) | | (4,406,620 | ) | | (77,423 | ) |
| | |
| |
| |
| |
| Net loss for the year | | | (26,141,798 | ) | | (14,629,054 | ) | | (24,552,673 | ) |
| Accretion on preferred shares | | | — | | | (1,614,855 | ) | | (4,458,080 | ) |
| Inducement related to conversion of preferred shares | | | — | | | (1,924,395 | ) | | — | |
| | |
| |
| |
| |
| Net loss applicable to common shareholders | | $ | (26,141,798 | ) | $ | (18,168,304 | ) | $ | (29,010,753 | ) |
| | |
| |
| |
| |
| Weighted average number of common shares outstanding | | | 15,373,689 | | | 6,259,757 | | | 2,539,912 | |
| | |
| |
| |
| |
| Basic and diluted loss per common share | | $ | (1.70 | ) | $ | (2.90 | ) | $ | (11.42 | ) |
| | |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-1-6
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (Deficiency)
(United States Dollars)
| |
| |
| | Special Warrants, Warrants, Options and Additional Paid-in Capital
| |
| |
| |
| |
|---|
| | Common Stock
| |
| |
| |
| |
|---|
| | Cumulative Other Comprehensive Income
| | Accumulated Deficit
| | Shareholders' Equity (Deficiency)
| |
|---|
| | Number
| | Amount
| |
|---|
| Balance as at December 31, 2001 | | 2,134,656 | | $ | 83,661,951 | | $ | 9,453,641 | | $ | (51,579 | ) | $ | (122,631,352 | ) | $ | (29,567,339 | ) |
| Special warrants converted into common shares and warrants | | 432,428 | | | 9,839,811 | | | (9,453,641 | ) | | — | | | — | | | 386,170 | |
| Warrants issued in connection with government grant (note 17 (c)) | | — | | | — | | | 808,011 | | | — | | | — | | | 808,011 | |
| Stock options issued for services | | — | | | — | | | 115,287 | | | — | | | — | | | 115,287 | |
| Warrants issued for services and in connection with short-term loan | | — | | | — | | | 208,419 | | | — | | | — | | | 208,419 | |
| Expired, cancelled and forfeited options and warrants | | — | | | — | | | — | | | — | | | — | | | — | |
| Accretion on preferred shares | | — | | | — | | | — | | | — | | | (4,458,080 | ) | | (4,458,080 | ) |
| Reduction in APIC caused by accretion on preferred shares | | — | | | — | | | (1,131,717 | ) | | — | | | 1,131,717 | | | — | |
| Cumulative translation adjustment (note 3 (c)) | | — | | | — | | | — | | | (527,138 | ) | | — | | | (527,138 | ) |
| Net loss for the year ended December 31, 2002 | | — | | | — | | | — | | | — | | | (24,552,673 | ) | | (24,552,673 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance as at December 31, 2002 | | 2,567,084 | | | 93,501,762 | | | — | | | (578,717 | ) | | (150,510,388 | ) | | (57,587,343 | ) |
Common shares issued through private placement and public offering |
|
11,006,159 |
|
|
43,692,929 |
|
|
— |
|
|
— |
|
|
— |
|
|
43,692,929 |
|
| Common shares and warrants granted upon the conversion of warrants and rights | | 238,857 | | | 3,211,698 | | | — | | | — | | | — | | | 3,211,698 | |
| Warrants issued in connection with debentures and loans | | — | | | — | | | 2,354,370 | | | — | | | — | | | 2,354,370 | |
| Common shares and warrants issued upon conversion of preferred shares | | 1,211,589 | | | 72,051,103 | | | — | | | — | | | (1,924,395 | ) | | 70,126,708 | |
| Stock options issued for services | | — | | | — | | | 193,449 | | | — | | | — | | | 193,449 | |
| Expired, cancelled and forfeited options and warrants | | — | | | — | | | — | | | — | | | — | | | — | |
| Accretion on preferred shares | | — | | | — | | | — | | | — | | | (1,614,855 | ) | | (1,614,855 | ) |
| Reduction in APIC caused by accretion on preferred shares | | — | | | — | | | (1,465,842 | ) | | — | | | 1,465,842 | | | — | |
| Cumulative translation adjustment (note 3 (c)) | | — | | | — | | | — | | | (5,706,860 | ) | | — | | | (5,706,860 | ) |
| Net loss for the year ended December 31, 2003 | | — | | | — | | | — | | | — | | | (14,629,054 | ) | | (14,629,054 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance as at December 31, 2003 | | 15,023,689 | | | 212,457,492 | | | 1,081,977 | | | (6,285,577 | ) | | (167,212,850 | ) | | 40,041,042 | |
Common shares and warrants issued in connection with convertible debentures |
|
470,833 |
|
|
659,166 |
|
|
4,548,920 |
|
|
— |
|
|
— |
|
|
5,208,086 |
|
| Value of debentures' beneficial conversion feature | | — | | | — | | | 5,821,370 | | | — | | | — | | | 5,821,370 | |
| Common shares issued upon exercise of warrants | | 250,000 | | | 1,499,403 | | | — | | | — | | | — | | | 1,499,403 | |
| Warrants repurchased | | — | | | — | | | (1,000 | ) | | — | | | — | | | (1,000 | ) |
| Stock options issued for services | | — | | | — | | | — | | | — | | | — | | | — | |
| Expired, cancelled and forfeited options and warrants | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the year ended December 31, 2004 | | — | | | — | | | — | | | — | | | (26,141,798 | ) | | (26,141,798 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance as at December 31, 2004 | | 15,744,522 | | $ | 214,616,061 | | $ | 11,451,267 | | $ | (6,258,577 | ) | $ | (193,354,648 | ) | $ | 26,427,103 | |
| | |
| |
| |
| |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-1-7
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOW
(United States Dollars)
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| CASH FLOWS FROM (USED IN) | | | | | | | | | | |
Operating activities |
|
|
|
|
|
|
|
|
|
|
| | Net loss for the period | | $ | (26,141,798 | ) | $ | (14,629,054 | ) | $ | (24,552,673 | ) |
| | Items not involving cash — | | | | | | | | | | |
| | | Amortization | | | 1,390,508 | | | 3,302,044 | | | 3,628,188 | |
| | | Loss on disposal of capital assets | | | 46,431 | | | 27,147 | | | 32,087 | |
| | | Write down of capital and intangible assets | | | 547,366 | | | — | | | — | |
| | | Issuance of options and warrants for services and financing costs | | | 81,466 | | | 2,704,247 | | | 258,704 | |
| | | Interest on debt | | | 6,266,027 | | | — | | | — | |
| | | Unrealized foreign exchange loss (gain) | | | 7,956 | | | (9,728,237 | ) | | (566,322 | ) |
| | | Gain on settlement of obligation | | | — | | | (1,694,300 | ) | | — | |
| | Change in operating components of working capital (note 18) | | | (5,132,726 | ) | | (4,939,234 | ) | | 7,421,170 | |
| | |
| |
| |
| |
| | | | (22,934,770 | ) | | (24,957,387 | ) | | (13,778,846 | ) |
| | |
| |
| |
| |
| Investing activities | | | | | | | | | | |
| | Purchase of short-term investments | | | (4,999,035 | ) | | (10,847,245 | ) | | — | |
| | Redemption of short-term investments | | | 11,504,032 | | | — | | | 4,382,422 | |
| | Purchase of capital assets | | | (363,224 | ) | | (150,135 | ) | | (388,611 | ) |
| | Cash pledged as collateral for lease | | | (222,003 | ) | | 217,178 | | | (753,532 | ) |
| | |
| |
| |
| |
| | | | 5,919,770 | | | (10,780,202 | ) | | 3,240,279 | |
| | |
| |
| |
| |
| Financing activities | | | | | | | | | | |
| | Capital lease repayments | | | — | | | (40,460 | ) | | (101,627 | ) |
| | Short-term loan proceeds | | | — | | | — | | | 1,288,494 | |
| | Repayment of short-term loan and accumulated interest | | | — | | | (1,308,558 | ) | | — | |
| | Senior and subordinated loan proceeds | | | — | | | 6,542,790 | | | — | |
| | Repayment of senior and subordinated loan and accumulated interest | | | — | | | (7,367,568 | ) | | — | |
| | Convertible debenture proceeds | | | 13,318,750 | | | 856,837 | | | — | |
| | Repayment of convertible debenture and accumulated interest | | | — | | | (884,108 | ) | | — | |
| | Bridge loan proceeds | | | — | | | 767,771 | | | — | |
| | Repayment of bridge loan and accumulated interest | | | — | | | (775,068 | ) | | — | |
| | Issuance of common shares through private placement | | | — | | | 48,453,282 | | | — | |
| | Funds received on the exercise of warrants | | | 1,499,403 | | | 1,120,555 | | | — | |
| | Payment of expenses relating to financing | | | (378,162 | ) | | (379,646 | ) | | (26,672 | ) |
| | Repurchase of warrants | | | (1,000 | ) | | — | | | — | |
| | Payment of expenses on the exercise of warrants | | | — | | | (33,618 | ) | | — | |
| | Payment of expenses related to the issuance of common shares | | | — | | | (4,760,352 | ) | | — | |
| | |
| |
| |
| |
| | | | 14,438,991 | | | 42,191,857 | | | 1,160,195 | |
| | |
| |
| |
| |
| Effect of exchange rate changes on cash and cash equivalents | | | 57,099 | | | (273,905 | ) | | (100,724 | ) |
| | |
| |
| |
| |
| Change in cash and cash equivalents for the period | | | (2,518,910 | ) | | 6,180,363 | | | (9,479,096 | ) |
| Cash and cash equivalents, beginning of the period | | | 6,337,677 | | | 157,314 | | | 9,636,410 | |
| | |
| |
| |
| |
| Cash and cash equivalents, end of the period | | $ | 3,818,767 | | $ | 6,337,677 | | $ | 157,314 | |
| | |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-1-8
WORLD HEART CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. GOING CONCERN ASSUMPTION
These consolidated financial statements have been prepared on the basis of accounting principles applicable to a going concern, which assume that World Heart Corporation (the "Corporation" or "WorldHeart") will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations.
The use of these principles may not be appropriate because, as at December 31, 2004, there was substantial doubt that the Corporation would be able to continue as a going concern. The Corporation's ability to continue as a going concern is dependent upon its ability to obtain additional financing and become commercially successful.
Subsequent to year end, on January 31, 2005, the Corporation announced a definitive agreement to concurrently: acquire all the assets of MedQuest Products, Inc. ("MedQuest") for 9,300,000 common shares; issue approximately 8,900,000 common shares, through a private placement, for gross proceeds of $12,000,000; convert all of its outstanding convertible debentures; and issue 10,655,000 common shares upon the exercise of 10,655,000 warrants for gross proceeds of $10,655,000. This transaction is further described in Note 20. There can be no assurance that the Corporation will be successful in completing this transaction and therefore sustain operations for the foreseeable future.
These consolidated financial statements do not reflect adjustments to the carrying values of assets and liabilities, the reported revenues and expenses, and the balance sheet classifications used that would be necessary if the going concern assumption were not appropriate. Such adjustments could be material.
2. NATURE OF OPERATIONS
WorldHeart is a medical devices company with facilities in Ottawa, Ontario, Canada; Oakland, California, USA; and Heesch, Netherlands. WorldHeart is currently focused on the development, commercialization and manufacturing of pulsatile ventricular assist devices ("VADs") which are intended for patients with end-stage heart failure. WorldHeart currently produces and sells the Novacor® LVAS (Left Ventricular Assist System) and is developing a pulsatile next generation VAD.
3. SIGNIFICANT ACCOUNTING POLICIES
These consolidated financial statements have been prepared by management in accordance with accounting principles generally accepted in the United States ("U.S. GAAP"), and include all assets, liabilities, revenues and expenses of the Corporation and its wholly owned subsidiaries; World Heart Inc. and World Heart B.V., as well as its equity investment in 2007262 Ontario Inc. (see Note 12). All material intercompany transactions and balances have been eliminated. These principles also conform in all material respects with Canadian generally accepted accounting principles ("Canadian GAAP") except as described in Note 22. The consolidated financial statements are expressed in U.S. dollars as described in Note 3 (c).
- (b)
- Use of estimates
Effective January 1, 2004, the functional currency of the Corporation changed from the Canadian dollar to the U.S. dollar. In general, this change resulted from the Corporation's restructuring and issuance of common shares and warrants during the year ended December 31, 2003 along with a gradual increase in the overall proportion of business activities conducted in U.S. dollars. Concurrent with this change in functional currency, the Corporation adopted the U.S. dollar as its reporting currency.
The change was effected for prior periods as follows: assets and liabilities have been translated at the spot rate on that date; income and expense items for those periods have been translated at the average rate for each period; and equity transactions have been translated at historic rates. The resulting net translation adjustment has been posted to the cumulative other comprehensive income account.
- (d)
- Cash equivalents and short-term investments
Cash equivalents and short-term investments include debt instruments of commercial enterprises, financial institutions and government entities. The Corporation has established guidelines relative to credit ratings, diversification and maturities that are intended to mitigate risk and provide safety and liquidity. Cash equivalents include highly liquid and highly rated investments with
B-1-9
| Furniture and fixtures | | 20% declining balance |
| Computer equipment and software | | 30% declining balance |
| Manufacturing and research equipment | | 30% declining balance |
| Leasehold improvements | | Straight-line over the lease term |
Goodwill is not amortized but is tested for impairment on at least an annual basis as required under Statement of Financial Accounting Standard No. 142 ("SFAS No. 142"). Intangible assets with a definite life, consisting of purchased technology, patents, trademarks and other identified rights, are amortized over their legal or estimated useful lives, whichever is shorter, which generally ranges from 3 to 5 years.
The Corporation reviews the carrying amounts of intangible assets with a definite life whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Such events or circumstances might include a significant decline in market share, a significant decline in profits, changes in technology, significant litigation or other items.
In evaluating whether there is an impairment of goodwill, management compares the carrying amount of goodwill with its fair value as required by SFAS No. 142. Because WorldHeart operates in one business segment and all of its goodwill was derived from the purchase of that same business, we are able to determine the value of the reporting unit containing the goodwill by using the trading value of the outstanding common shares and the number of outstanding shares at year-end. We determined the fair value of the goodwill by subtracting the fair value of all of the Corporation's assets and liabilities, excluding goodwill, from the value of the reporting unit. An impairment is recognized to the extent that the fair value of the goodwill is less than its carrying amount.
- (h)
- Income taxes
Income taxes are provided for using the asset and liability method whereby deferred tax assets and liabilities are recognized using current tax rates on the difference between the financial statement carrying amounts and the respective tax basis of the assets and liabilities. The Corporation provides a valuation allowance on deferred tax assets when it is more likely than not that such assets will not be realized.
- (i)
- Common shares
Common shares are recorded as the net proceeds received on issuance after deducting all share issue costs.
- (j)
- Revenue recognition
Revenue from product sales is recognized when all of the following criteria are met; persuasive evidence of an agreement exists; delivery has occurred or services have been rendered; the price is fixed and determinable; and collection is reasonably assured. The Corporation derives revenue from the sale of implant kits and peripheral equipment and training for implant centres.
Revenue from the sale of implant kits and peripheral equipment is generally recognized when such products are shipped. Where the terms of sale provide customers with rights of return or other guarantees, or where collection is uncertain, revenue is deferred until such time as the rights of return or guarantee expire or, where collection is uncertain, payment is received.
Training revenue is recognized once the training is provided.
B-1-10
Sales to new implant centers typically include implant kits, related peripheral equipment and training. For these multiple element sales, the Corporation allocates the total contract value to the elements based on their respective fair values. Revenue is recognized from each element in accordance with the criteria set out above. Revenue is deferred for any undelivered element when the undelivered element is not considered essential to the functionality of the delivered elements. If undelivered elements are essential to the functionality of the delivered elements, the entire contract value is deferred. For most customers, the training component is not considered essential to the use of or the functionality of the implant kits, as the customers already possess sufficient expertise and experience to perform implants.
The Corporation's implantable products and related peripheral equipment are covered by limited warranties. Warranty costs, which are based on historical experience, are estimated and recorded when the related revenue is recognized. Actual warranty costs are charged against the warranty provision as incurred.
- (k)
- Stock-based compensation
The Corporation has a stock option plan as described in Note 12. The Corporation accounts for its stock option plan in accordance with the Financial Accounting Standards Board's ("FASB") Statement of Financial Accounting Standards ("SFAS") No. 123, "Accounting for Stock Based Compensation", and Accounting Principles Board ("APB") Opinion No. 25, "Accounting for Stock Issued to Employees". No compensation expense is recognized when shares are issued at prevailing market prices or options are issued to employees with exercise prices at the grant date equal to prevailing market prices. Any consideration paid by employees on the exercise of stock options or purchase of capital stock is credited to share capital. The pro-forma fair value expense of options issued by the Corporation is disclosed in Note 12.
Stock options that are re-priced by the Corporation are accounted for using variable accounting under FASB Interpretation ("FIN") No. 44, "Accounting for Certain Transactions Involving Stock Compensation", whereby any positive difference between the prevailing market price and the exercise price is recognized as an expense over the vesting period of the option.
Stock options issued in lieu of cash to non-employees for services performed are recorded at the fair value of the options at the time they are issued and are expensed as service is provided.
- (l)
- Research and development costs
Government assistance is recognized when the expenditures that qualify for assistance are made and the Corporation has complied with the conditions for the receipt of government assistance. Government assistance is applied to reduce the carrying value of any assets acquired or to reduce eligible expenses incurred. A liability to repay government assistance, if any, is recorded in the period when the conditions arise that cause the assistance to become repayable. Goverment assistance recognized to date consists of government programs that provide refundable credits for qualifying scientific research and development expenditures such as the Ontario Business Research Institute tax credit program described in Note 13, the Technology Partnerships Canada program described in Note 17(c) and other similar regimes that the Corporation claims under from time to time.
- (n)
- Foreign currency translation
The consolidated financial statements of the Corporation are presented in U.S. dollars. The financial statements of the Corporation and certain of its subsidiaries whose functional currency is not the U.S. dollar are translated into U.S. dollar as follows: monetary assets and liabilities are translated at exchange rates prevailing at the balance sheet date; non-monetary items and any related amortization of such items are translated at the rates of exchange in effect when the assets were acquired or obligations incurred; and all other income and expense items are translated at average exchange rates prevailing during the year. Exchange gains and losses are included in net loss for the year. Unrealized translation gains and losses on the Corporation's net investment in these operations are accumulated as a component of other comprehensive income (loss).
B-1-11
4. CASH, CASH EQUIVALENTS AND SHORT-TERM INVESTMENTS
| | 2004
| | 2003
|
|---|
| | Cash and Cash Equivalents
| | Short-Term Investments
| | Cash and Cash Equivalents
| | Short-Term Investments
|
|---|
| Cash | | $ | 1,518,767 | | $ | — | | $ | 5,102,947 | | $ | — |
| Guaranteed investment certificates | | | 2,300,000 | | | — | | | — | | | — |
| Asset backed notes held with financial institutions | | | — | | | — | | | 1,234,730 | | | — |
| Corporate securities | | | — | | | 4,999,034 | | | — | | | 11,720,510 |
| | |
| |
| |
| |
|
| | | $ | 3,818,767 | | $ | 4,999,034 | | $ | 6,337,677 | | $ | 11,720,510 |
| | |
| |
| |
| |
|
The corporate securities held at December 31, 2004 were purchased on December 21, 2004, earn interest at rates ranging from 2.36% to 4.5% and have maturity dates ranging from June 1, 2005 to March 16, 2006. The securities are classified as available for sale.
5. INVENTORY
| | 2004
| | 2003
|
|---|
| Raw materials | | $ | 2,337,702 | | $ | 1,076,551 |
| Work in progress | | | 3,014,302 | | | 1,764,925 |
| Finished goods | | | 2,760,521 | | | 3,061,390 |
| | |
| |
|
| | | $ | 8,112,525 | | $ | 5,902,866 |
| | |
| |
|
Included in finished goods inventory is $409,360 (2003 — $Nil) delivered to customers and for which title has transferred to the customer. These transfers are treated as consignment sales and revenue is deferred until the Corporation determines that persuasive evidence of an agreement exists; the price is fixed and determinable; and collection is reasonably assured.
6. CAPITAL ASSETS
| | 2004
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book Value
|
|---|
| Furniture and fixtures | | $ | 443,349 | | $ | 334,184 | | $ | 109,165 |
| Computer equipment and software | | | 1,465,905 | | | 1,061,608 | | | 404,297 |
| Manufacturing and research equipment | | | 4,996,274 | | | 3,498,150 | | | 1,498,124 |
| Leasehold improvements | | | 507,755 | | | 507,755 | | | — |
| | |
| |
| |
|
| | | $ | 7,413,283 | | $ | 5,401,697 | | $ | 2,011,586 |
| | |
| |
| |
|
| | 2003
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book Value
|
|---|
| Furniture and fixtures | | $ | 407,318 | | $ | 248,922 | | $ | 158,396 |
| Computer equipment and software | | | 1,581,625 | | | 975,081 | | | 606,544 |
| Manufacturing and research equipment | | | 5,458,867 | | | 3,182,150 | | | 2,276,717 |
| Leasehold improvements | | | 507,755 | | | 507,755 | | | — |
| | |
| |
| |
|
| | | $ | 7,955,565 | | $ | 4,913,908 | | $ | 3,041,657 |
| | |
| |
| |
|
B-1-12
7. INTANGIBLE ASSETS
The cost and accumulated amortization of the Corporation's intangible assets as of December 31, 2004 and December 31, 2003 are as follows:
| | 2004
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book Value
|
|---|
| Purchased technology | | $ | 6,191,698 | | $ | 5,936,686 | | $ | 255,012 |
| Other intangible assets | | | 4,371,573 | | | 4,371,573 | | | — |
| | |
| |
| |
|
| | | $ | 10,563,271 | | $ | 10,308,259 | | $ | 255,012 |
| | |
| |
| |
|
| | 2003
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book Value
|
|---|
| Purchased technology | | $ | 6,191,698 | | $ | 5,426,633 | | $ | 765,065 |
| Other intangible assets | | | 4,371,573 | | | 4,257,520 | | | 114,053 |
| | |
| |
| |
|
| | | $ | 10,563,271 | | $ | 9,684,153 | | $ | 879,118 |
| | |
| |
| |
|
8. SHORT-TERM LOANS
On December 18, 2002, the Corporation entered into a $1,288,494 non-revolving term loan with a maturity date of June 18, 2003 and interest at the rate of 2% per month. The loan was collateralized by a general security agreement covering the Corporation's right, title and interest in all of its property and assets and an assignment of all patents, trademarks and licence agreements of the Corporation. On January 28, 2003, prior to the maturity date of the loan, the Corporation repaid the loan in full and the security was discharged.
On January 28, 2003, the Corporation entered into a senior loan agreement totaling $4,579,953 and a subordinated loan agreement totaling $1,962,837 ("Loans"). The Loans originally matured on July 31, 2003 and with an interest rate of 18% per annum payable monthly plus a 4% lenders fee. As collateral for the Loans, the Corporation entered into general security agreements over all of its assets. The Loans were amended to extend the maturity date to September 23, 2003 at which point they were repaid in full. Fees relating to loan extensions on the senior and subordinated loans amounted to $336,782.
On July 30, 2003, the Corporation issued senior subordinated convertible debentures for gross proceeds of $856,837 with a maturity date of October 22, 2003, and an interest rate of 14%. The subordinated convertible debentures were repaid in full on September 23, 2003.
On September 4, 2003 the Corporation entered into a bridge loan agreement totaling $767,771 with a maturity date of the earlier of the completion of a private placement and October 22, 2003, with an interest rate of 14% per annum payable monthly, plus a lenders fee of 4%. The bridge loan was repaid in full on September 23, 2003.
9. TAXES PAYABLE
The Corporation has accrued $832,000 related to a sales tax assessment received from the Province of Ontario during the year. The amount is included in research and development expenses as the assessment relates to taxes assessed on certain of those expenses incurred in the prior years. Until the assessment has been paid, the Ministry of Finance for Ontario has placed a lien for the value of the assessed amount on the assets of the Corporation.
10. CONVERTIBLE DEBENTURES
On September 15, 2004, the Corporation issued unsecured convertible debentures ("Debentures") and detachable warrants ("Warrants") for gross proceeds of $13,318,750. The Debentures, which become due on September 15, 2009, are convertible at any time at the holders' option into common shares of the Corporation at a price of $1.25 per share and accumulate non-compounding interest at an annual rate of 3%. Interest is convertible at any time into common shares of the Corporation at a price based on the five day weighted average trading price immediately prior to conversion. The Corporation can redeem the Debentures when the trading price of the Corporation's common shares is greater than $3.00 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Debentures in any 90-day period. In addition, Warrants to purchase 10,655,000 common shares of the Corporation at an exercise price of $1.55 per share and exercisable for a period of five years were issued to the Debenture holders. The Corporation may call the
B-1-13
Warrants when the trading price of the Corporation's common shares is greater than $4.50 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Warrants in any 90-day period.
The Corporation issued to its agents 470,833 common shares of the Corporation with a value of $659,166 and granted agent's warrants exercisable for 193,800 common shares of the Corporation with a value of $179,759 having the same terms as the Warrants.
The proceeds of the issue have been split between the Debentures and the Warrants based on their relative fair values. As a result of allocating some of the value of the proceeds to the Warrants, the Debentures contain a beneficial conversion feature equal to the intrinsic value of the Debentures on the date of issuance. The respective fair values of the components of the Debentures at the time of issue and as at December 31, 2004 are as follows:
| | September 15, 2004
| | December 31, 2004
|
|---|
| Convertible debentures | | $ | 1,911,128 | | $ | 8,177,140 |
| Warrants | | | 4,369,170 | | | 4,369,170 |
| Beneficial conversion feature | | | 5,821,370 | | | — |
| | |
| |
|
| | | $ | 12,101,668 | | $ | 12,546,310 |
| | |
| |
|
On September 16, 2004, the earliest possible exercise date, the full value of the beneficial conversion feature was charged to interest expense. Interest is accrued at the effective rate of the Debentures up to a maturity value of $15,316,563 on September 15, 2009. As a result, an additional $444,642 of interest was accrued to December 31, 2004.
As a condition of the MedQuest acquisition described in Note 20, the holders of the Debentures have unanimously agreed to convert the Debentures and accrued interest into common shares of the Corporation
11. PREFERRED SHARES
On September 22, 2003, Edwards exchanged all of the Series A redeemable, cumulative, participating preferred shares of World Heart Inc. for 711,589 common shares.
On November 27, 2003, Edwards Lifesciences (U.S.) Inc. converted all of the Series A cumulative, redeemable, convertible preferred shares into 500,000 common shares and warrants to acquire 1,000,000 common shares at $5.93 per share, expiring November 27, 2008. A fair value of $6,015,680 was attributed to the additional common shares and warrants of which $4,091,286 was applied to reduce the outstanding liability for accrued dividends and the remaining $1,924,394 was charged to accumulated deficit.
12. SHAREHOLDERS' EQUITY
Common shares
- (a)
- Authorized
Authorized common shares of the Corporation consist of an unlimited number of shares with no par value. On November 25, 2003, the Corporation's shareholders approved a one-for-seven consolidation of the Corporation's common shares and common share equivalents, and on December 1, 2003 WorldHeart filed Articles of Amendment effecting the share consolidation. All references to common share, option, warrant and per common share amounts for all periods presented have been retroactively restated to reflect the share consolidation.
Employee Stock Option Plan
The Corporation has an employee stock option plan ("ESOP"). The maximum number of shares that may be reserved and set aside under options to eligible persons pursuant to the ESOP may not exceed 1,501,857 common shares (2003 — 1,501,857). As at December 31, 2004, options for 789,046 common shares have been granted. In addition on September 23, 2004, the Corporation granted, subject to shareholder approval of an increase in the number of options available under the plan, options for
B-1-14
2,383,725 common shares to employees, officers, directors and consultants. Approval will be sought at the next annual meeting of shareholders. In addition, the grant is conditional on the forfeiture by the employees, officers, directors and consultants of 251,685 options for common shares already issued and outstanding under the plan. The maximum number of common shares at any time available for issuance under the ESOP, or pursuant to other outstanding options, to any one person is 5% of the common shares then issued and outstanding. A committee appointed by the Board of Directors administers the ESOP. The option exercise price for all options issued under the ESOP is based on the fair market value of the common shares on the date of grant priced on the close of market in the Toronto Stock Exchange ("TSX") immediately prior to the date of grant. The options generally vest annually in equal portions over either a five-year period or three-year period and must be exercised within a four-year period from each date of vesting.
On March 5, 2003, the Board of Directors approved a proposal to offer employees, officers, directors and consultants of the Corporation the opportunity to exchange options ("Option Exchange") held by them and granted prior to 2003 ("Existing Options"), for a reduced number of options ("New Options") at a price that more closely reflected the then current trading price. Approval of the shareholders was required with respect to option exchanges for persons considered to be insiders of the Corporation including the Corporation's executive officers and members of the Board of Directors. Shareholders approved the insiders' exchange on June 16, 2003. The Toronto Stock Exchange approved the Option Exchange on June 20, 2003. In total, participants under the ESOP chose to exchange a total of 121,218 Existing Options for 23,660 New Options. These new options are accounted for using variable accounting. No expense has been recognized in these consolidated financial statements.
Rights related to 2007262 Ontario Inc.
On December 19, 2001, the Corporation incorporated 2007262 Ontario Inc. ("2007262") to carry out specified research and development. The Corporation and New Generation Biotech (Equity) Fund Inc. ("NewGen"), an Ontario labour sponsored venture capital corporation, subscribed for an equal number of common shares of 2007262. Additionally, NewGen subscribed for 91,000 Series 1 preferred shares ("Series 1 Shares") of 2007262 for net proceeds of $2,157,330 after deducting expenses of the placement of $83,484. The Corporation sold to 2007262 certain technology in exchange for 14,285 Series 2 preferred shares ("Series 2 Shares") of 2007262 and a promissory note in the amount of $1,261,591.
The promissory note was repaid to the Corporation from the proceeds of the Series 1 preferred shares and the balance of the Series 1 preferred share proceeds was used to improve and enhance the technology transferred by the Corporation.
On January 31, 2003, NewGen redeemed the Series 1 preferred shares for 91,000 common shares of the Corporation and 91,000 common share purchase warrants ("Purchase Warrants") of the Corporation. Each Purchase Warrant was exercisable into one common share of WorldHeart at a price of $26.25 at any time up to January 24, 2004 but expired unexercised as at that date.
2007262 has been accounted for as a research and development arrangement. The Corporation recorded the NewGen funding as a liability and the amounts expended by 2007262 from the NewGen funding as research and development expenses in the period that they occurred. Expenses incurred by 2007262 in the year were $230,071 (2003 — $608,970; 2002 — $951,274).
B-1-15
Stock Option and Warrant Activity
The following table summarizes the number of options and warrants outstanding and the weighted average exercise price:
| | Employees
| | Non-Employees
| |
| |
|---|
| | Options
#
| | Weighted average exercise price
$
| | Options
#
| | Weighted average exercise price
$
| | Warrants
#
| | Weighted average exercise price
$
| | Total
#
| |
|---|
| Outstanding at Dec. 31, 2001 | | 177,113 | | 56.47 | | 6,078 | | 53.16 | | 151,066 | | 36.02 | | 334,257 | |
| Granted | | 132,801 | | 27.82 | | 4,826 | | 29.05 | | 490,641 | | 24.92 | | 628,268 | |
| Exercised | | — | | — | | — | | — | | (22,499 | ) | 26.72 | | (22,499 | ) |
| Expired | | (3,518 | ) | 45.93 | | — | | — | | (12,143 | ) | 97.04 | | (15,661 | ) |
| Forfeited | | (45,099 | ) | 38.87 | | (1,221 | ) | 42.56 | | — | | — | | (46,320 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2002 | | 261,297 | | 45.09 | | 9,683 | | 42.49 | | 607,065 | | 26.18 | | 878,045 | |
| Granted | | 1,079,538 | | 8.07 | | 34,350 | | 8.25 | | 14,138,457 | | 6.18 | | 15,252,345 | |
| Exercised | | — | | — | | — | | — | | (147,857 | ) | 7.12 | | (147,857 | ) |
| Cancelled | | (118,108 | ) | 53.77 | | (3,110 | ) | 46.99 | | — | | — | | (121,218 | ) |
| Expired | | (2,758 | ) | 39.51 | | — | | — | | (5,000 | ) | 57.75 | | (7,758 | ) |
| Forfeited | | (75,621 | ) | 19.36 | | (1,258 | ) | 9.41 | | — | | — | | (76,879 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2003 | | 1,144,348 | | 10.98 | | 39,665 | | 13.54 | | 14,592,665 | | 6.99 | | 15,776,678 | |
| Granted | | 127,852 | | 6.46 | | 3,095 | | 7.77 | | 10,923,566 | | 1.58 | | 11,054,513 | |
| Exercised | | — | | — | | — | | — | | (250,000 | ) | 5.93 | | (250,000 | ) |
| Repurchased | | — | | — | | — | | — | | (300,000 | ) | 5.93 | | (300,000 | ) |
| Expired | | (7,166 | ) | 52.46 | | — | | — | | (541,998 | ) | 26.84 | | (549,164 | ) |
| Forfeited | | (514,165 | ) | 8.01 | | (4,583 | ) | 8.48 | | — | | — | | (518,748 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2004 | | 750,869 | | 11.85 | | 38,177 | | 13.68 | | 24,424,233 | | 4.15 | | 25,213,279 | |
| | |
| |
| |
| |
| |
| |
| |
| |
B-1-16
| | Options
| |
|
|---|
| | Employees
| | Non-Employees
| | Warrants
|
|---|
| Weighted average exercise price of exercisable options and warrants: | | | | | | | | | |
| | December 31, 2002 | | $ | 52.24 | | $ | 51.84 | | $ | 27.59 |
| | December 31, 2003 | | | 45.67 | | | 50.55 | | | 7.03 |
| | December 31, 2004 | | | 18.09 | | | 18.34 | | | 4.15 |
| Number of exercisable options and warrants: | | | | | | | | | |
| | December 31, 2002 | | | 87,916 | | | 3,372 | | | 607,065 |
| | December 31, 2003 | | | 66,283 | | | 4,036 | | | 14,592,665 |
| | December 31, 2004 | | | 287,102 | | | 17,867 | | | 24,424,233 |
| Range of exercise prices of all options and warrants at December 31, 2004: | | | | | | | | | |
| | From | | $ | 1.02 | | $ | 5.31 | | $ | 1.55 |
| | To | | | 105.43 | | | 59.95 | | | 29.05 |
| Range of expiry dates of all options and warrants at December 31, 2004: | | | | | | | | | |
| | From | | | Jan. 5, 2005 | | | Jul. 14, 2005 | | | Dec. 19, 2005 |
| | To | | | Dec. 7, 2011 | | | Dec. 31, 2010 | | | Sep. 14, 2009 |
The following table summarizes information about the outstanding options and warrants as at December 31, 2004:
Range of exercise price
| | Number outstanding
| | Weighted average exercise price
| | Weighted average remaining life in years
|
|---|
| $ 1.02 to 2.50 | | 10,868,424 | | $ | 1.55 | | 4.7 |
| 2.51 to 5.00 | | 1,484 | | | 4.08 | | 5.5 |
| 5.01 to 7.50 | | 13,258,314 | | | 6.01 | | 3.7 |
| 7.51 to 10.00 | | 864,269 | | | 8.00 | | 4.4 |
| 10.01 to 20.00 | | 7,838 | | | 14.68 | | 1.3 |
| 20.01 to 40.00 | | 170,095 | | | 28.72 | | 2.1 |
| 40.01 to 105.43 | | 42,855 | | | 56.44 | | 1.6 |
| | |
| |
| |
|
| | | 25,213,279 | | $ | 4.40 | | 4.1 |
| | |
| |
| |
|
On September 23, 2004, the Corporation conditionally granted, subject to shareholder approval of an increase in the number of options available under the plan, options for 2,383,725 common shares to employees, officers, directors, and consultants. These conditional grants are not included in the number of options and warrants outstanding of 25,213,279. In addition, the grant is conditional on the forfeiture by the employees, officers, directors and consultants of 251,685 options for common shares already issued and outstanding under the plan. Options were granted as follows: 215,000 to Canadian based individuals with an exercise price of Cdn $1.43 and 2,168,725 to individuals based outside of Canada with an exercise price of $1.12. Options vest one third on each of the first, second and third anniversaries of the date of grant and expire on September 22, 2013.
The Corporation accounts for its stock option plan in accordance with the provisions of SFAS No. 123, which requires companies to recognize as expense, over the vesting period, the fair value of all stock based awards on the date of grant or to continue to apply the provisions of APB Opinion No. 25 and provide pro forma net income and pro forma income per share disclosures as if the fair value based method defined in SFAS No. 123 had been applied to stock-based awards. The Corporation follows this alternative method. The estimated share-based compensation costs based on stock options granted to directors and employees and the pro forma net loss and loss per share are as follows:
| | 2004
| | 2003
| | 2002
| |
|---|
| Net loss applicable to common shareholders | | $ | (26,141,798 | ) | $ | (18,168,304 | ) | $ | (29,010,753 | ) |
| Share-based compensation costs | | | (2,134,707 | ) | | (1,579,378 | ) | | (1,653,660 | ) |
| | |
| |
| |
| |
| Adjusted pro forma net loss | | $ | (28,276,505 | ) | $ | (19,747,682 | ) | $ | (30,664,413 | ) |
| | |
| |
| |
| |
| Reported basic and diluted loss per share | | $ | (1.70 | ) | $ | (2.90 | ) | $ | (11.42 | ) |
| Share-based compensation costs per share | | | (0.14 | ) | | (0.25 | ) | | (0.65 | ) |
| | |
| |
| |
| |
| Pro forma basic loss per share | | $ | (1.84 | ) | $ | (3.15 | ) | $ | (12.07 | ) |
| | |
| |
| |
| |
B-1-17
The share-based compensation costs and the adjusted pro forma net loss do not include changes related to the options to purchase 2,383,725 common shares that were conditionally granted on September 23, 2004. The weighted average fair value of the options issued during the year ended December 31, 2004 was $4.39 (2003 — $5.48; 2002 — $18.83). The fair values of options granted are determined using the Black-Scholes model. For 2004, 2003 and 2002, the following weighted average assumptions were utilized:
| | 2004
| | 2003
| | 2002
|
|---|
| Expected option life, in years | | 6 | | 6 | | 6 |
| Volatility | | 73% | | 75% | | 75% |
| Risk free interest rate | | 4.00% | | 3.65% | | 3.65% |
| Dividend yield | | Nil | | Nil | | Nil |
13. GOVERNMENT ASSISTANCE
During the year ended December 31, 2001, the Corporation accrued a tax credit receivable related to the Ontario Business Research Institute tax credit ("OBRITC") program, in the amount of $2,304,492 that was associated with research payments made to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation from 1997 to 2000. Following the completion of the provincial Ministry of Finance's audit and proposed amendments to the OBRITC legislation, the Corporation received payment of $597,538 related to this claim during the year ended December 31, 2003. The full amount of the claim is under dispute and, due to the uncertainty of the ultimate resolution of the claim, the Corporation recorded a provision for the remainder of $1,706,954 in 2003. This provision was charged to research and development expense.
14. RESTRUCTURING
On August 25, 2004, the Corporation approved a plan to consolidate its Ottawa, Ontario operations into its facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve the Corporation's operational efficiency and effectiveness. The Corporation expects the restructuring to be completed by the end of June 2005 and expects to incur total restructuring charges of $2,611,764 comprising employee severance costs of $1,509,192; contract settlement costs of $719,894; write down of capital assets to net realizable value of $338,595; and other costs of $44,083. The amounts accrued and expensed to date are as follows:
| | Costs accrued during the year
| | Costs paid or settled in the year
| | Balance in accrued liabilities as at December 31
|
|---|
| Employee severance costs | | $ | 1,396,037 | | $ | 795,923 | | $ | 600,114 |
| Contract settlement costs | | | 57,653 | | | — | | | 57,653 |
| Write down of capital assets | | | 338,595 | | | 338,595 | | | — |
| Other costs | | | 22,509 | | | 8,624 | | | 13,885 |
| | |
| |
| |
|
| | | $ | 1,814,794 | | $ | 1,143,142 | | $ | 671,652 |
| | |
| |
| |
|
During the year ended December 31, 2003, the Corporation restructured its worldwide distribution to increase emphasis on direct sales and marketing and refocused its next-generation VAD development programs. Associated restructuring costs incurred for 2003 totaled $3,494,676. Employee severance and termination costs of $27,665 were reversed in the year ended December 31, 2004. The Corporation does not anticipate incurring any additional costs relating to the 2003 restructuring activities.
B-1-18
15. LOSS PER COMMON SHARE
For all of the years presented, diluted loss per share equals basic loss per share due to the anti-dilutive effect of convertible preferred shares, stock options and warrants. These instruments could potentially dilute basic earnings per share in the future by being converted into common shares:
| | Number of common shares to be issued on exercise or conversion
|
|---|
| | 2004
| | 2003
| | 2002
|
|---|
| Convertible preferred shares | | — | | — | | 932,502 |
| Employee and non-employee stock options | | 789,046 | | 1,184,013 | | 270,980 |
| Warrants | | 24,424,233 | | 14,592,665 | | 840,139 |
| Convertible debentures | | 10,655,000 | | — | | — |
| Stock options subject to shareholder approval | | 2,383,725 | | — | | — |
| | |
| |
| |
|
| Total potentially dilutive instruments | | 38,252,004 | | 15,776,678 | | 2,043,621 |
| | |
| |
| |
|
16. INCOME TAXES
The Corporation operates in several tax jurisdictions. Its income is subject to varying rates of tax and losses incurred in one jurisdiction cannot be used to offset income taxes payable in another. A reconciliation of the combined Canadian federal and provincial income tax rate with the Corporation's effective tax rate is as follows:
| | 2004
| | 2003
| | 2002
| |
|---|
| Canadian loss | | $ | (15,789,595 | ) | $ | (10,114,669 | ) | $ | (7,922,019 | ) |
| United States' loss | | | (9,052,555 | ) | | (4,185,394 | ) | | (16,630,654 | ) |
| European loss | | | (1,299,648 | ) | | (328,991 | ) | | — | |
| | |
| |
| |
| |
| Loss before income taxes | | $ | (26,141,798 | ) | $ | (14,629,054 | ) | $ | (24,552,673 | ) |
| | |
| |
| |
| |
| Expected statutory rate | | | 36.12% | | | 36.62% | | | 38.62% | |
| Expected recovery of income tax | | $ | (9,440,000 | ) | $ | (5,360,000 | ) | $ | (9,490,000 | ) |
| Effect of foreign tax rate differences | | | (510,000 | ) | | (350,000 | ) | | (490,000 | ) |
| Permanent differences | | | 2,316,000 | | | (1,860,000 | ) | | (40,000 | ) |
| Change in valuation allowance | | | 7,930,000 | | | 12,360,000 | | | 8,240,000 | |
| Effect of changes in SR&ED carryforwards | | | 2,256,000 | | | 760,000 | | | 800,000 | |
| Effect of tax rate changes | | | 268,000 | | | 270,000 | | | 1,360,000 | |
| Effect of exchange rate differences | | | (2,820,000 | ) | | (5,820,000 | ) | | (380,000 | ) |
| | |
| |
| |
| |
| Recovery of income taxes | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
The Canadian statutory income tax rate of 36.12% is comprised of federal income tax at approximately 22.12% and provincial income tax at approximately 14.00%. For the years ended December 31, 2003 and 2002, the permanent differences relate primarily to imputed interest expense on the Preferred Shares and goodwill amortization.
B-1-19
The primary temporary differences affecting deferred taxes and their approximate effects are as follows:
| | 2004
| | 2003
| |
|---|
| Deferred tax assets: | | | | | | | |
| | SR&ED expenditures | | $ | 10,280,000 | | $ | 9,390,000 | |
| | Net operating losses | | | 37,750,000 | | | 32,190,000 | |
| | Investment tax credits | | | 5,540,000 | | | 4,660,000 | |
| | Share issue costs | | | 4,790,000 | | | 4,500,000 | |
| | Asset basis differences | | | 1,500,000 | | | 1,320,000 | |
| | |
| |
| |
| | | | 59,860,000 | | | 52,060,000 | |
| | Less: valuation allowance | | | (59,570,000 | ) | | (51,630,000 | ) |
| | |
| |
| |
| | | | 290,000 | | | 430,000 | |
Deferred tax liabilities: |
|
|
|
|
|
|
|
| | Asset basis differences | | | (290,000 | ) | | (430,000 | ) |
| | |
| |
| |
| Net deferred income tax liability | | $ | — | | $ | — | |
| | |
| |
| |
As at December 31, 2004 the Corporation has unclaimed Scientific Research and Experimental Development ("SR&ED") expenditures, income tax loss carryforwards and investment tax credits. The unclaimed amounts and their expiry dates are as follows:
| | 2004
| | 2003
|
|---|
| SR&ED expenditures — carried forward without expiry | | $ | 28,500,000 | | $ | 25,610,000 |
| Income tax loss carryforwards: | | | | | | |
| | Federal (Canada) (expire 2005-2011) | | | 57,054,000 | | | 49,210,000 |
| | Provincial (expire 2005-2011) | | | 55,900,000 | | | 52,290,000 |
| | United States (expire 2010-2020) | | | 49,100,000 | | | 46,430,000 |
| | Europe | | | 1,400,000 | | | 140,000 |
| Investment tax credits (expire 2006-2014) | | | 7,100,000 | | | 6,170,000 |
17. COMMITMENTS AND CONTINGENCIES
| Year ended December 31, | | 2005 | | $ | 1,440,597 |
| | | 2006 | | | 1,407,680 |
| | | 2007 | | | 354,770 |
| | | 2008 | | | 37,476 |
| | | 2009 | | | 2,197 |
Total rent expense for the years ended December 31, 2004, 2003 and 2002 was $1,026,315, $1,114,717, and $1,092,705 respectively. During 2002, the Corporation pledged cash in the amount of $750,000 as collateral for obligations under a premises lease for the Oakland operation. During 2003, $222,003 was drawn against the cash amount pledged to cover certain rent obligations and in 2004 the Corporation restored the balance to the original pledged amount of $750,000.
- (b)
- Novacor LVAS Royalties
The Corporation is committed, under the Novacor LVAS royalty agreement to royalty payments to certain employees. Royalties are payable on annual consolidated gross revenues at a rate of 0.808% up to a cumulative maximum of $3,232,000. Royalty payments to date total $1,027,184; included in accounts payable and accrued liabilities at December 31, 2004 is $95,646 (2003 — $37,478).
- (c)
- Technology Partnerships Canada Contribution Agreement
During 2002, the Corporation entered into a shared funding program with Technology Partnerships Canada ("TPC") under which the Canadian government shares costs of certain research and development activities. The Corporation claimed funding in the amount of approximately $6,624,000. As at December 31, 2004, $203,250 (2003 — $193,648) is included in accounts receivable. Effective January 1, 2004, repayment is in the form of royalties payable on annual consolidated gross revenues at a rate of 0.65%
B-1-20
for a nine-year period ended December 31, 2012. If during this period royalty payments reach the maximum of $20,250,000 no further repayments will be required. If the royalty payments do not exceed $20,250,000 during this period they will continue until 2015 or until the maximum is reached, whichever comes first. During the year ended December 31, 2004, the Corporation has accrued royalties owing of approximately $69,000.
In connection with the agreement, the Corporation granted TPC 92,857 warrants to purchase an equivalent number of common shares of the Corporation, exercisable until December 4, 2006 at an exercise price of $29.05 per share.
- (d)
- Research Agreement
Effective April 1, 1996, the Corporation entered into a research agreement with the Cardiovascular Devices Division ("CVD") of the Ottawa Heart Institute Research Corporation ("Research Agreement") under which the Corporation agreed to fund a substantial portion of CVD's remaining research efforts relating to artificial heart technology. The Corporation acquired joint ownership with CVD of the technology arising from CVD's research under the Research Agreement and an exclusive twenty-five year license to market the product and certain other related technologies for an initial license fee of $147,544 and royalties of 7%. The Corporation is no longer focused on developing or commercializing this technology and as a result is not expected to make any royalty payments to CVD.
The Corporation's research funding to CVD under the Research Agreement was $13,400,000 for the period April 1, 1996 to December 31, 2004. The Corporation does not anticipate any future payments under the Research Agreement, as it is not pursuing development of the CVD artificial heart technology.
The Corporation also agreed with CVD to fund approximately Cdn $150,000 per year for the period from July 1, 1996 to June 30, 2006 for a research chair in medical devices at the University of Ottawa Heart Institute.
CVD was considered a related party by virtue of the fact that the Chairman and Chief Scientific Officer of the Corporation prior to September 23, 2003 was also the Director of CVD. In September 2003, that individual resigned as Chairman and Chief Scientific Officer and as a result CVD is no longer considered a related party.
Prior to September 23, 2003, the Corporation incurred $159,200 (2002 — $499,480) for research and development fees to CVD under the Research Agreement described in Note 17 (a). In addition, the Corporation incurred $95,200 (2002 — $107,030) to CVD relating to the research chair under the Research Agreement.
Prior to September 23, 2003, the Corporation incurred salaries of $372,323 (2002 — $476,213) relating to employees that have been seconded by the Corporation to CVD. These expenditures were recovered by the Corporation from CVD.
18. NET CHANGE IN OPERATING COMPONENTS OF WORKING CAPITAL AND SUPPLEMENTAL CASH FLOW DISCLOSURE
| | 2004
| | 2003
| | 2002
|
|---|
| Accounts and other receivables | | $ | (475,399 | ) | $ | (334,286 | ) | $ | 2,017,870 |
| Prepaid expenses | | | 52,000 | | | (275,978 | ) | | 224,705 |
| Inventory | | | (2,176,785 | ) | | (1,812,506 | ) | | 1,148,080 |
| Accounts payable and accrued liabilities | | | (2,172,728 | ) | | (2,502,353 | ) | | 3,418,486 |
| Accrued compensation | | | (359,814 | ) | | (14,111 | ) | | 612,029 |
| | |
| |
| |
|
| | | $ | (5,132,726 | ) | $ | (4,939,234 | ) | $ | 7,421,170 |
| | |
| |
| |
|
Interest and financing costs paid in the year ended December 31, 2004 totalled $nil (2003 — $1,235,367; 2002 — $nil).
19. SEGMENTED INFORMATION
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the Corporation's chief decision maker in deciding how to allocate resources and assess performance. The Corporation's chief decision maker is the Chief Executive Officer.
On September 23, 2003, the Corporation realigned its business operations to focus on commercial operations and to reduce research and development costs. Research and development efforts were refocused to concentrate on developing future products that will evolve from current commercialized technologies and products into next generation technologies and marketable products. Accordingly, the Corporation is no longer organized by multiple operating segments for the purpose of making operating decisions or assessing performance and, as a result, the Corporation operates in one reportable segment.
B-1-21
The following geographic area data includes revenue based on product shipment destination and long-lived assets based on physical location. The Corporation has locations in Canada, the United States and Europe:
| | 2004
| | 2003
| | 2002
|
|---|
| | Revenue
| | Long-lived assets
| | Revenue
| | Long-lived assets
| | Revenue
| | Long-lived assets
|
|---|
| United States | | $ | 5,343,725 | | $ | 16,038,366 | | $ | 4,129,322 | | $ | 17,023,263 | | $ | 3,452,779 | | $ | 18,248,248 |
| Europe | | | 3,033,294 | | | 515,011 | | | 1,862,335 | | | 611,979 | | | 2,187,115 | | | — |
| Canada | | | 510,672 | | | 2,892,864 | | | 245,424 | | | 3,465,176 | | | 599,450 | | | 3,136,947 |
| Japan | | | 688,070 | | | — | | | 538,726 | | | — | | | 616,114 | | | — |
| Less: Edwards fee | | | — | | | — | | | — | | | — | | | (418,762 | ) | | — |
| | |
| |
| |
| |
| |
| |
|
| | | $ | 9,575,761 | | $ | 19,446,241 | | $ | 6,775,807 | | $ | 21,100,418 | | $ | 6,436,696 | | $ | 21,385,195 |
| | |
| |
| |
| |
| |
| |
|
At December 31, 2004 and 2003 accounts receivable from Edwards comprised 8% and 24%, respectively, of accounts receivable and one other (2003 — one other) account receivable amounted to greater than 10% of the total respective accounts receivable balances. During 2004, Edwards accounted for 7% of revenue and one other customer accounted for 10% of revenue. During 2003, Edwards accounted for 38% of revenue and two other customers accounted for 23% of revenue. During 2002, Edwards accounted for 50% of revenue and no other customer accounted for more than 10% of revenue.
In conjunction with Edwards' investment in the Corporation and the Corporation's acquisition of the Novacor division from Edwards, the Corporation entered into a distribution agreement (the "Distribution Agreement") whereby Edwards was the sole distributor, except in the United States, of the Corporation's heart assist products for a period of five years commencing July 1, 2000. On December 31, 2003, the Distribution Agreement was amended to reflect Edwards as sole distributor in Japan only. Also on December 31, 2003, the Corporation entered into a transition agreement to acquire certain inventory and capital assets from Edwards. For the year ended December 31, 2004, purchases from Edwards for components were $818,383 (2003 — $674,328; 2002 — $601,580). Edwards agreed to provide administrative services relating to distribution activities on an interim basis. Other purchases from Edwards for research and development materials and support and other services amounted to $180,723 (2003 — $897,121; 2002 — $975,017).
20. SUBSEQUENT EVENTS
On January 31, 2005, the Corporation entered into an agreement to acquire all of the assets of MedQuest for 9,300,000 common shares of the Corporation, subject to certain consents, conditions and shareholder approval. In connection with the acquisition of MedQuest, the Corporation has also entered into a private placement agreement with Maverick Venture Management, LLC ("Maverick"). At the closing of the acquisition, Maverick will purchase 8,888,889 common shares of the Corporation at a purchase price of $1.35 per share.
In addition, as a condition of this transaction, investors that participated in the Corporation's September 2004 convertible debenture transaction (Note 10) have unanimously agreed, subject to shareholder approval of a reduction in the Warrant exercise price from $1.55 to $1.00 per share, to convert their debentures at their stated conversion price of $1.25 per common share into approximately 10,655,000 common shares. The accrued interest will be converted into common shares at a conversion price equal to the market prices at the date of conversion. Subsequent to December 31, 2004, approximately $1.5 million of the debt and approximately $11,300 accrued interest has been converted into approximately 1.4 million common shares of the Corporation. The convertible debenture holders have also agreed to exercise the corresponding warrants into 10,655,000 common shares of the Corporation at an adjusted exercise price of $1.00 per common. The Warrants are currently exercisable until September 2009 at a price of $1.55 per common share. The Company will seek shareholder approval for a $0.55 reduction in the warrant exercise price.
On January 31, 2005, the Corporation conditionally granted, 3,418,750 options to employees, officers, directors and consultants subject to shareholder approval of an increase in the number of options available under the plan; 1,309,500 of these options are reserved for new directors, officers and employees. The options have an exercise price of $1.48, vest one third on each of the first, second and third anniversaries of the date of grant and expire six years after each vesting date.
21. FINANCIAL INSTRUMENTS
Financial instruments recognized in the balance sheet at December 31, 2004 consist of cash and cash equivalents, short-term investments, accounts and other receivables, accounts payable and certain accrued liabilities and convertible debentures. The Corporation does not hold or issue financial instruments for trading purposes.
The Corporation invests the majority of its excess cash in high-grade instruments and diversifies the concentration of cash among different financial institutions.
B-1-22
The Corporation believes that the carrying values of its financial instruments approximate their fair values because of their short terms to maturity, except for the long-term receivable and the convertible debentures. The long-term receivable is valued at the present value of future cash flows at a rate of 4%. The convertible debentures with a face value of $13,318,750 are presented in their debt and equity components measured at their relative fair values at the time of issue (see Note 10). The current fair value of the convertible debentures is not readily determinable.
- (b)
- Interest rate risk
The Corporation is subject to interest rate risks from time to time because of the term to maturity of its cash equivalents and short-term investments.
- (c)
- Foreign exchange risk
The Corporation transacts mainly in U.S. dollars but also in Canadian dollars and Euros. While the Corporation derives revenues in both Euros and Canadian dollars, it currently has net cash outflows in those currencies. The Corporation keeps cash balances on hand in those currencies that it estimates it requires to cover net cash outflow for the next quarter on a rolling basis.
- (d)
- Credit risk
Financial instruments that potentially subject the Corporation to a concentration of credit risk consist of cash, cash equivalents, short-term investments and accounts receivable. The Corporation has established guidelines for cash, cash equivalents and short-term investments relative to credit ratings, diversification and maturities that are intended to maintain safety and liquidity. The Corporation has a limited number of customers, all of which operate in the health-care industry. As at December 31, 2004, approximately 8% (2002 — 24%; 2002 — 68%) of the accounts receivable balance was due from Edwards. The Corporation performs ongoing credit evaluations of its customers' financial condition and generally requires no collateral from its customers. The Corporation maintains an allowance for doubtful accounts receivable of $146,307 (2003 — $41,782) based upon the expected collectibility of accounts receivable.
22. CANADIAN ACCOUNTING PRINCIPLES
The consolidated financial statements have been prepared in accordance with U.S. GAAP. These principles differ, as they affect the Corporation, for the years ended December 31, 2004, 2003 and 2002 in the following material respects from Canadian GAAP. There are no differences in reported cash flows for the years presented.
B-1-23
- (a)
- Balance sheets — Canadian GAAP
| | 2004
| | 2003
| |
|---|
| ASSETS | | | | | | | |
| Current assets | | $ | 21,743,636 | | $ | 28,470,186 | |
| | Long-term receivable | | | 318,553 | | | — | |
| | Cash pledged as collateral for lease | | | 750,000 | | | 527,997 | |
| | Capital assets | | | 2,011,586 | | | 3,041,657 | |
| | Goodwill and intangible assets | | | 17,434,655 | | | 18,058,761 | |
| | Other assets | | | — | | | 81,468 | |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
| Current liabilities | | $ | 7,637,819 | | $ | 10,139,027 | |
| | Convertible debentures(1) | | | 2,530,659 | | | — | |
| | Long-term obligation | | | 16,368 | | | — | |
| | |
| |
| |
| | | | 10,184,846 | | | 10,139,027 | |
| | |
| |
| |
Shareholders' equity (deficiency) |
|
|
|
|
|
|
|
| | Common shares(2)(3) | | | 187,027,058 | | | 184,868,489 | |
| | Contributed surplus(1)(3) | | | 22,871,780 | | | 4,893,750 | |
| | Cumulative translation adjustment | | | (6,490,184 | ) | | (6,490,184 | ) |
| | Accumulated deficit(1)(2)(3)(4) | | | (171,335,070 | ) | | (143,231,013 | ) |
| | |
| |
| |
| | | | 32,073,584 | | | (40,041,042 | ) |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
- (b)
- Statements of loss — Canadian GAAP
| | 2004
| | 2003
| | 2002
| |
|---|
| Net loss applicable to common shareholders in accordance with U.S. GAAP | | $ | (26,141,798 | ) | $ | (18,168,304 | ) | $ | (29,010,753 | ) |
| | Accretion on preferred shares | | | — | | | 1,614,855 | | | 4,458,080 | |
| | Inducement related to conversion of preferred shares | | | — | | | 1,924,395 | | | — | |
| Net loss in accordance with U.S. GAAP | | | (26,141,798 | ) | | (14,629,054 | ) | | (24,552,673 | ) |
| Adjustments to reconcile to Canadian GAAP: | | | | | | | | | | |
| | Non-cash share based compensation costs(6) | | | (2,134,707 | ) | | — | | | — | |
| | Interest and accretion on convertible debentures(1) | | | 5,469,700 | | | — | | | — | |
| | Amortization of purchased in-process research and development(5) | | | — | | | (916,180 | ) | | (2,060,095 | ) |
| | Interest on preferred shares(3) | | | — | | | (4,322,110 | ) | | (4,872,441 | ) |
| | Foreign exchange translation on shares(3) | | | — | | | (1,985,669 | ) | | (205,650 | ) |
| | Gain on settlement of obligation under research and development arrangement(4) | | | — | | | (1,694,300 | ) | | — | |
| | |
| |
| |
| |
| Net loss in accordance with Canadian GAAP | | | (22,806,805 | ) | | (23,547,313 | ) | | (31,690,859 | ) |
| | Inducement related to conversion of preferred shares | | | — | | | (1,924,395 | ) | | — | |
| | |
| |
| |
| |
| Net loss applicable to common shareholders in accordance with Canadian GAAP | | $ | (22,806,805 | ) | $ | (25,471,708 | ) | $ | (31,690,859 | ) |
| | |
| |
| |
| |
| Weighted average number of common shares outstanding | | | 15,373,689 | | | 6,259,757 | | | 2,539,912 | |
| | |
| |
| |
| |
| Basic and diluted loss per common share | | $ | (1.48 | ) | $ | (4.07 | ) | $ | (12.48 | ) |
| | |
| |
| |
| |
- (c)
- Footnotes
- (1)
- Under U.S. GAAP the proceeds of the issue on the convertible debentures were split between the convertible debentures and the related warrants for common shares based on their relative fair values. As a result of allocating some of the proceeds to the warrants, the convertible debentures contain a beneficial conversion feature equal to the intrinsic value of the convertible debentures on the date of issuance. The value of the warrants and the beneficial conversion feature were recorded in additional paid-in capital. On September 16, 2004, the earliest possible exercise date, the full value of the beneficial conversion
B-1-24
| | September 15, 2004
| | December 31, 2004
|
|---|
| Convertible debentures | | $ | 1,734,350 | | $ | 2,530,659 |
| Warrants | | | 4,504,127 | | | 4,504,127 |
| Conversion feature | | | 5,863,191 | | | 5,863,191 |
| | |
| |
|
| | | $ | 12,101,668 | | $ | 12,897,977 |
| | |
| |
|
23. NEW ACCOUNTING PRONOUNCEMENTS
In June 2001, the FASB issued SFAS No. 148,which amended the transitional provisions of SFAS No. 123 for companies electing to recognize employee stock-based compensation using the fair value based method. The provisions of FASB No. 148 will be effective for years beginning on or after January 1, 2006. In addition, during 2004, the FASB issued SFAS No. 123R, which also amended the provisions of SFAS No. 123. The provisions of FASB No. 123R will be effective for years beginning on or after January 1, 2006. The Corporation plans on adopting these new pronouncements in 2006, the impact of which is not known at this time.
In January 2005, the Canadian Institute of Chartered Accountants ("CICA") released Section 1531, "Comprehensive Income"("Section 1531"), which requires financial instruments to be measured at fair value. The provisions of Section 1531 are effective for years starting on or after October 31, 2006. The Corporation plans on adopting this new pronouncement in 2007, the impact of which is not known at this time.
B-1-25
APPENDIX B-2
WORLD HEART CORPORATION
Audited Financial Statements
For the years ended December 31, 2004 and 2003
B-2-1
MANAGEMENT'S STATEMENT OF RESPONSIBILITY
Management is responsible for the preparation of the consolidated financial statements and all other information in the annual report. The financial statements have been prepared in accordance with Canadian generally accepted accounting principles ("Canadian GAAP") and reflect management's best estimates and judgments. The financial information presented elsewhere in the annual report is consistent with the consolidated financial statements.
Management has developed and maintains a system of internal controls to provide reasonable assurance that all assets are safeguarded and to facilitate the preparation of relevant, reliable and timely financial information. Consistent with the concept of reasonable assurance, the Corporation recognizes that the relative cost of maintaining these controls should not exceed their expected benefits.
The Audit Committee, which is comprised of independent directors, reviews the consolidated financial statements, considers the report of the external auditors, assesses the adequacy of the Corporation's internal controls, and recommends to the Board of Directors the independent auditors for appointment by the shareholders. The consolidated financial statements were reviewed by the Audit Committee and approved by the Board of Directors.
The consolidated financial statements were audited by PricewaterhouseCoopers LLP, the external auditors, in accordance with generally accepted auditing standards on behalf of the shareholders.
| Original signed by: | | Original signed by: |
| JAL. S. JASSAWALLA | | D. MARK GOUDIE |
| President | | Chief Financial Officer |
AUDITORS' REPORT TO THE SHAREHOLDERS OF WORLD HEART CORPORATION
We have audited the consolidated balance sheets of World Heart Corporation as at December 31, 2004 and 2003, and the consolidated statements of operations, shareholders' equity (deficiency) and cash flow for the years ended December 31, 2004, 2003 and 2002. These financial statements are the responsibility of the Corporation's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with generally accepted auditing standards in Canada and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Corporation as at December 31, 2004 and 2003 and the results of its operations and its cash flows for the years ended December 31, 2004, 2003 and 2002 in accordance with Canadian generally accepted accounting principles.
On March 11, 2005, we reported separately to the shareholders of the Corporation on the consolidated financial statements for the same period, prepared in accordance with accounting principles generally accepted in the United States.
Ottawa, Canada |
|
PRICEWATERHOUSECOOPERS LLP |
| March 11, 2005 | | Chartered Accountants |
B-2-2
WORLD HEART CORPORATION
CONSOLIDATED BALANCE SHEETS
(United States Dollars)
| | December 31, 2004
| | December 31, 2003
| |
|---|
| ASSETS | | | | | | | |
Current assets |
|
|
|
|
|
|
|
| | Cash and cash equivalents (note 4) | | $ | 3,818,767 | | $ | 6,337,677 | |
| | Short-term investments (note 4) | | | 4,999,034 | | | 11,720,510 | |
| | Trade and other receivables | | | 4,238,049 | | | 3,894,911 | |
| | Prepaid expenses | | | 575,261 | | | 614,222 | |
| | Inventory (note 5) | | | 8,112,525 | | | 5,902,866 | |
| | |
| |
| |
| | | | 21,743,636 | | | 28,470,186 | |
| Long-term receivable | | | 318,553 | | | — | |
| Cash pledged as collateral for lease (note 17 (a)) | | | 750,000 | | | 527,997 | |
| Capital assets (note 6) | | | 2,011,586 | | | 3,041,657 | |
| Goodwill | | | 17,179,643 | | | 17,179,643 | |
| Intangible assets (note 7) | | | 255,012 | | | 879,118 | |
| Other assets | | | — | | | 81,468 | |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
| | Accounts payable and accrued liabilities | | $ | 3,854,833 | | $ | 8,214,090 | |
| | Taxes payable (note 9) | | | 832,000 | | | — | |
| | Accrued compensation | | | 1,343,364 | | | 1,748,364 | |
| | Deferred revenue | | | 1,607,622 | | | 176,573 | |
| | |
| |
| |
| | | | 7,637,819 | | | 10,139,027 | |
| Convertible debentures (note 10) | | | 2,530,659 | | | — | |
| Long-term obligation | | | 16,368 | | | — | |
| | |
| |
| |
| | | | 10,184,846 | | | 10,139,027 | |
| | |
| |
| |
Commitments and contingencies (note 17) |
|
|
|
|
|
|
|
Shareholders' equity |
|
|
|
|
|
|
|
| | Common shares | | | 187,027,058 | | | 184,868,489 | |
| | | Issued and outstanding — 15,744,522 common shares
(December 31, 2003 — 15,023,689) (note 12) | | | | | | | |
| | Contributed surplus | | | 22,871,780 | | | 4,893,750 | |
| | Cumulative translation adjustment | | | (6,490,184 | ) | | (6,490,184 | ) |
| | Accumulated deficit | | | (171,335,070 | ) | | (143,231,013 | ) |
| | |
| |
| |
| | | | 32,073,584 | | | 40,041,042 | |
| | |
| |
| |
| | | $ | 42,258,430 | | $ | 50,180,069 | |
| | |
| |
| |
Signed on behalf of the Board of Directors
Original signed by John F. Carlson and C. Ian Ross |
Director |
|
Director |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-2-3
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(United States Dollars)
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Revenue | | $ | 9,575,761 | | $ | 6,775,807 | | $ | 6,436,696 | |
| Cost of goods sold | | | (7,680,384 | ) | | (4,968,898 | ) | | (6,284,996 | ) |
| | |
| |
| |
| |
| Gross margin | | | 1,895,377 | | | 1,806,909 | | | 151,700 | |
| | |
| |
| |
| |
Expenses |
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | | (13,363,796 | ) | | (7,263,759 | ) | | (6,686,437 | ) |
| | Research and development (note 13) | | | (5,838,754 | ) | | (11,298,380 | ) | | (15,931,912 | ) |
| | Share-based compensation costs | | | (2,134,707 | ) | | — | | | — | |
| | Restructuring costs (note 14) | | | (1,787,129 | ) | | (3,356,487 | ) | | — | |
| | Amortization of intangibles | | | (515,012 | ) | | (2,854,774 | ) | | (4,663,406 | ) |
| | |
| |
| |
| |
| | | | (23,639,398 | ) | | (24,773,400 | ) | | (27,281,755 | ) |
| | |
| |
| |
| |
| Loss before the undernoted | | | (21,744,021 | ) | | (22,966,491 | ) | | (27,130,055 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
|
|
|
| | Foreign exchange gain (loss) | | | (308,338 | ) | | 8,069,459 | | | 303,558 | |
| | Investment income | | | 99,427 | | | 105,596 | | | 117,589 | |
| | Loss on disposal of capital assets | | | (46,431 | ) | | (27,147 | ) | | (32,087 | ) |
| | Interest expense and financing costs | | | (807,442 | ) | | (8,728,730 | ) | | (4,949,864 | ) |
| | |
| |
| |
| |
| Net loss for the year | | | (22,806,805 | ) | | (23,547,313 | ) | | (31,690,859 | ) |
| | |
| |
| |
| |
| Inducement related to conversion of preferred shares | | | — | | | (1,924,395 | ) | | — | |
| | |
| |
| |
| |
| Net loss applicable to common shareholders | | $ | (22,806,805 | ) | $ | (25,471,708 | ) | $ | (31,690,859 | ) |
| | |
| |
| |
| |
| Weighted average number of common shares outstanding | | | 15,373,689 | | | 6,259,757 | | | 2,539,912 | |
| | |
| |
| |
| |
| Basic and diluted loss per common share | | $ | (1.48 | ) | $ | (4.07 | ) | $ | (12.48 | ) |
| | |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-2-4
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (Deficiency)
(United States Dollars)
| |
| |
| | Special Warrants, Warrants, Options and Contributed Surplus
| |
| |
| |
| |
|---|
| | Common Stock
| |
| |
| |
| |
|---|
| | Cumulative Translation Adjustment
| | Accumulated Deficit
| | Shareholders' Equity (Deficiency)
| |
|---|
| | Number
| | Amount
| |
|---|
| Balance as at December 31, 2001 | | 2,134,656 | | $ | 50,794,769 | | $ | 38,182,878 | | $ | (2,609,064 | ) | $ | (86,068,446 | ) | $ | 300,137 | |
| Special warrants converted into common shares and warrants | | 432,428 | | | 9,839,811 | | | (9,839,811 | ) | | — | | | — | | | — | |
| Warrants issued in connection with government grant (note 17 (c)) | | — | | | — | | | 808,011 | | | — | | | — | | | 808,011 | |
| Stock options issued for services | | — | | | — | | | 115,287 | | | — | | | — | | | 115,287 | |
| Warrants issued for services and in connection with short-term loan | | — | | | — | | | 208,419 | | | — | | | — | | | 208,419 | |
| Expired, cancelled and forfeited options and warrants | | ��� | | | — | | | — | | | — | | | — | | | — | |
| Cumulative translation adjustment (note 3 (c)) | | — | | | — | | | — | | | 159,364 | | | — | | | 159,364 | |
| Net loss for the year ended December 31, 2002 | | — | | | — | | | — | | | — | | | (31,690,859 | ) | | (31,690,859 | ) |
| | |
| |
| |
| |
| |
| |
| |
Balance as at December 31, 2002 |
|
2,567,084 |
|
|
60,634,580 |
|
|
29,474,784 |
|
|
(2,449,700 |
) |
|
(117,759,305 |
) |
|
(30,099,641 |
) |
| Common shares issued through private placement and public offering | | 11,006,159 | | | 43,692,929 | | | — | | | — | | | — | | | 43,692,929 | |
| Common shares and warrants granted upon the conversion of warrants and rights | | 238,857 | | | 3,211,698 | | | (2,171,852 | ) | | — | | | — | | | 1,039,846 | |
| Warrants issued in connection with debentures and loans | | — | | | — | | | 2,354,370 | | | — | | | — | | | 2,354,370 | |
| Common shares and warrants issued upon conversion of preferred shares | | 1,211,589 | | | 77,329,282 | | | (24,957,001 | ) | | — | | | (1,924,395 | ) | | 50,447,886 | |
| Stock options issued for services | | — | | | — | | | 193,449 | | | — | | | — | | | 193,449 | |
| Expired, cancelled and forfeited options and warrants | | — | | | — | | | — | | | — | | | — | | | — | |
| Cumulative translation adjustment (note 3 (c)) | | — | | | — | | | — | | | (4,040,484 | ) | | — | | | (4,040,484 | ) |
| Net loss for the year ended December 31, 2003 | | — | | | — | | | — | | | — | | | (23,547,313 | ) | | (23,547,313 | ) |
| | |
| |
| |
| |
| |
| |
| |
Balance as at December 31, 2003 |
|
15,023,689 |
|
|
184,868,489 |
|
|
4,893,750 |
|
|
(6,490,184 |
) |
|
(143,231,013 |
) |
|
40,041,042 |
|
| Share-based compensation adjustment (note 12) | | — | | | — | | | 5,297,252 | | | — | | | (5,297,252 | ) | | — | |
| Share-based compensation expenses | | — | | | — | | | 2,134,707 | | | — | | | — | | | 2,134,707 | |
| Common shares and warrants issued in connection with convertible debentures | | 470,833 | | | 659,166 | | | 4,683,880 | | | — | | | — | | | 5,343,046 | |
| Value of debentures' beneficial conversion feature | | — | | | — | | | 5,863,191 | | | — | | | — | | | 5,863,191 | |
| Common shares issued upon exercise of warrants | | 250,000 | | | 1,499,403 | | | — | | | — | | | — | | | 1,499,403 | |
| Warrants repurchased | | — | | | — | | | (1,000 | ) | | — | | | — | | | (1,000 | ) |
| Stock options issued for services | | — | | | — | | | — | | | — | | | — | | | — | |
| Expired, cancelled and forfeited options and warrants | | — | | | — | | | — | | | — | | | — | | | — | |
| Net loss for the year ended December 31, 2004 | | — | | | — | | | — | | | — | | | (22,806,805 | ) | | (22,806,805 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Balance as at December 31, 2004 | | 15,744,522 | | $ | 187,027,058 | | $ | 22,871,780 | | $ | (6,490,184 | ) | $ | (171,335,070 | ) | $ | 32,073,584 | |
| | |
| |
| |
| |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-2-5
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOW
(United States Dollars)
| | Year ended
December 31,
2004
| | Year ended
December 31,
2003
| | Year ended
December 31,
2002
| |
|---|
| CASH FLOWS FROM (USED IN) | | | | | | | | | | |
Operating activities |
|
|
|
|
|
|
|
|
|
|
| | Net loss for the period | | $ | (22,806,805 | ) | $ | (23,547,313 | ) | $ | (31,690,859 | ) |
| | Items not involving cash — | | | | | | | | | | |
| | | Amortization | | | 1,390,508 | | | 4,218,223 | | | 5,688,284 | |
| | | Loss on disposal of capital assets | | | 46,431 | | | 27,147 | | | 32,087 | |
| | | Write down of capital and intangible assets | | | 547,366 | | | — | | | — | |
| | | Issuance of options and warrants for services and financing costs | | | 81,466 | | | 2,704,247 | | | 258,704 | |
| | | Share-based compensation | | | 2,134,707 | | | — | | | — | |
| | | Interest on preferred shares and debt | | | 796,327 | | | 4,322,110 | | | 4,872,441 | |
| | | Unrealized foreign exchange loss (gain) | | | 7,956 | | | (7,742,567 | ) | | (360,673 | ) |
| | Change in operating components of working capital (note 18) | | | (5,132,726 | ) | | (4,939,234 | ) | | 7,421,170 | |
| | |
| |
| |
| |
| | | | (22,934,770 | ) | | (24,957,387 | ) | | (13,778,846 | ) |
| | |
| |
| |
| |
Investing activities |
|
|
|
|
|
|
|
|
|
|
| | Purchase of short-term investments | | | (4,999,035 | ) | | (10,847,245 | ) | | — | |
| | Redemption of short-term investments | | | 11,504,032 | | | — | | | 4,382,422 | |
| | Purchase of capital assets | | | (363,224 | ) | | (150,135 | ) | | (388,611 | ) |
| | Cash pledged as collateral for lease | | | (222,003 | ) | | 217,178 | | | (753,532 | ) |
| | |
| |
| |
| |
| | | | 5,919,770 | | | (10,780,202 | ) | | 3,240,279 | |
| | |
| |
| |
| |
Financing activities |
|
|
|
|
|
|
|
|
|
|
| | Capital lease repayments | | | — | | | (40,460 | ) | | (101,627 | ) |
| | Short-term loan proceeds | | | — | | | — | | | 1,288,494 | |
| | Repayment of short-term loan and accumulated interest | | | — | | | (1,308,558 | ) | | — | |
| | Senior and subordinated loan proceeds | | | — | | | 6,542,790 | | | — | |
| | Repayment of senior and subordinated loan and accumulated interest | | | — | | | (7,367,568 | ) | | — | |
| | Convertible debenture proceeds | | | 13,318,750 | | | 856,837 | | | — | |
| | Repayment of convertible debenture and accumulated interest | | | — | | | (884,108 | ) | | — | |
| | Bridge loan proceeds | | | — | | | 767,771 | | | — | |
| | Repayment of bridge loan and accumulated interest | | | — | | | (775,068 | ) | | — | |
| | Issuance of common shares through private placement | | | — | | | 48,453,282 | | | — | |
| | Funds received on the exercise of warrants | | | 1,499,403 | | | 1,120,555 | | | — | |
| | Payment of expenses relating to financing | | | (378,162 | ) | | (379,646 | ) | | (26,672 | ) |
| | Repurchase of warrants | | | (1,000 | ) | | — | | | — | |
| | Payment of expenses on the exercise of warrants | | | — | | | (33,618 | ) | | — | |
| | Payment of expenses related to the issuance of common shares | | | — | | | (4,760,352 | ) | | — | |
| | |
| |
| |
| |
| | | | 14,438,991 | | | 42,191,857 | | | 1,160,195 | |
| | |
| |
| |
| |
| Effect of exchange rate changes on cash and cash equivalents | | | 57,099 | | | (273,905 | ) | | (100,724 | ) |
| | |
| |
| |
| |
| Change in cash and cash equivalents for the period | | | (2,518,910 | ) | | 6,180,363 | | | (9,479,096 | ) |
| Cash and cash equivalents, beginning of the period | | | 6,337,677 | | | 157,314 | | | 9,636,410 | |
| | |
| |
| |
| |
| Cash and cash equivalents, end of the period | | $ | 3,818,767 | | $ | 6,337,677 | | $ | 157,314 | |
| | |
| |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-2-6
WORLD HEART CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. GOING CONCERN ASSUMPTION
These consolidated financial statements have been prepared on the basis of accounting principles applicable to a going concern, which assume that World Heart Corporation (the "Corporation" or "WorldHeart") will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations.
The use of these principles may not be appropriate because, as at December 31, 2004, there was substantial doubt that the Corporation would be able to continue as a going concern. The Corporation's ability to continue as a going concern is dependent upon its ability to obtain additional financing and become commercially successful.
Subsequent to year end, on January 31, 2005, the Corporation announced a definitive agreement to concurrently: acquire all the assets of MedQuest Products, Inc. ("MedQuest") for 9,300,000 common shares; issue approximately 8,900,000 common shares, through a private placement, for gross proceeds of $12,000,000; convert all of its outstanding convertible debentures; and issue 10,655,000 common shares upon the exercise of 10,655,000 warrants for gross proceeds of $10,655,000. This transaction is further described in Note 20. There can be no assurance that the Corporation will be successful in completing this transaction and therefore sustain operations for the foreseeable future.
These consolidated financial statements do not reflect adjustments to the carrying values of assets and liabilities, the reported revenues and expenses, and the balance sheet classifications used that would be necessary if the going concern assumption were not appropriate. Such adjustments could be material.
2. NATURE OF OPERATIONS
WorldHeart is a medical devices company with facilities in Ottawa, Ontario, Canada; Oakland, California, USA; and Heesch, Netherlands. WorldHeart is currently focused on the development, commercialization and manufacturing of pulsatile ventricular assist devices ("VADs") which are intended for patients with end-stage heart failure. WorldHeart currently produces and sells the Novacor® LVAS (Left Ventricular Assist System) and is developing a pulsatile next generation VAD.
3. SIGNIFICANT ACCOUNTING POLICIES
- (a)
- Basis of presentation
These consolidated financial statements have been prepared by management in accordance with Canadian generally accepted accounting principles ("Canadian GAAP"), and include all assets, liabilities, revenues and expenses of the Corporation and its wholly owned subsidiaries; World Heart Inc. and World Heart B.V., as well as its equity investment in 2007262 Ontario Inc. (see Note 12). All material intercompany transactions and balances have been eliminated. The consolidated financial statements are expressed in U.S. dollars as described in Note 3 (c).
- (b)
- Use of estimates
The preparation of these consolidated financial statements in conformity with Canadian GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from these estimates.
- (c)
- Change in functional and reporting currency
Effective January 1, 2004, the functional currency of the Corporation changed from the Canadian dollar to the U.S. dollar. In general, this change resulted from the Corporation's restructuring and issuance of common shares and warrants during the year ended December 31, 2003 along with a gradual increase in the overall proportion of business activities conducted in U.S. dollars. Concurrent with this change in functional currency, the Corporation adopted the U.S. dollar as its reporting currency.
The change was effected for prior periods as follows: assets and liabilities have been translated at the spot rate on that date; income and expense items for those periods have been translated at the average rate for each period; and equity transactions have been translated at historic rates. The resulting net translation adjustment has been posted to the cumulative translation adjustment account.
- (d)
- Cash equivalents and short-term investments
Cash equivalents and short-term investments include debt instruments of commercial enterprises, financial institutions and government entities. The Corporation has established guidelines relative to credit ratings, diversification and maturities that are intended to mitigate risk and provide safety and liquidity. Cash equivalents include highly liquid and highly rated investments with maturity periods of three months or less when purchased. Short-term investments include those investments with maturities in excess of three months but less than one year.
B-2-7
Investment tax credits, which are earned as a result of qualifying research and development expenditures, are recognized when the expenditures are made and their realization is reasonably assured and are applied to reduce related costs and expenses in the year.
- (g)
- Capital assets
Capital assets are recorded at cost. Amortization is calculated using the following rates and bases:
| Furniture and fixtures | | 20% declining balance |
| Computer equipment and software | | 30% declining balance |
| Manufacturing and research equipment | | 30% declining balance |
| Leasehold improvements | | Straight-line over the lease term |
Goodwill is not amortized but is tested for impairment on at least an annual basis. Intangible assets with a definite life, consisting of purchased technology, patents, trademarks and other identified rights, are amortized over their legal or estimated useful lives, whichever is shorter, which generally ranges from 3 to 5 years.
The Corporation reviews the carrying amounts of intangible assets with a definite life whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Such events or circumstances might include a significant decline in market share, a significant decline in profits, changes in technology, significant litigation or other items.
In evaluating whether there is an impairment of goodwill, management compares the carrying amount of goodwill with its fair value. Because WorldHeart operates in one business segment and all of its goodwill was derived from the purchase of that same business, we are able to determine the value of the reporting unit containing the goodwill by using the trading value of the outstanding common shares and the number of outstanding shares at year-end. We determined the fair value of the goodwill by subtracting the fair value all of the Corporation's assets and liabilities, excluding goodwill, from the value of the reporting unit. An impairment is recognized to the extent that the fair value of the goodwill is less than its carrying amount.
- (i)
- Income taxes
Revenue from product sales is recognized when all of the following criteria are met; persuasive evidence of an agreement exists; delivery has occurred or services have been rendered; the price is fixed and determinable; and collection is reasonably assured. The Corporation derives revenue from the sale of implant kits and peripheral equipment and training for implant centres.
Revenue from the sale of implant kits and peripheral equipment is generally recognized when such products are shipped. Where the terms of sale provide customers with rights of return or other guarantees, or where collection is uncertain, revenue is deferred until such time as the rights of return or guarantee expire or, where collection was uncertain, payment is received.
Training revenue is recognized once the training is provided.
Sales to new implant centers typically include implant kits, related peripheral equipment and training. For these multiple element sales, the Corporation allocates the total contract value to the elements based on their respective fair values. Revenue is recognized
B-2-8
from each element in accordance with the criteria set out above. Revenue is deferred for any undelivered element when the undelivered element is not considered essential to the functionality of the delivered elements. If undelivered elements are essential to the functionality of the delivered elements, the entire contract value is deferred. For most customers, the training component is not considered essential to the use of or the functionality of the implant kits, as the customers already possess sufficient expertise and experience to perform implants.
The Corporation's implantable products and related peripheral equipment are covered by limited warranties. Warranty costs, which are based on historical experience, are estimated and recorded when the related revenue is recognized. Actual warranty costs are charged against the warranty provision as incurred.
- (l)
- Stock-based compensation
The Corporation has a stock option plan as described in Note 12. The Corporation accounts for its stock option plan in accordance with the Canadian Institute of Chartered Accountants' ("CICA") Accounting Standards Board Handbook Section 3870, "Stock-based Compensation and Other Stock-based Payments" ("CICA 3870"), which requires companies to account for employee stock options using the fair value based method. Under the fair value based method, compensation cost is measured at fair value at the date of grant and is expensed over the awards vesting period. Any consideration paid by employees on the exercise of stock options or purchase of capital stock is credited to share capital. The pro-forma fair value expense of options issued by the Corporation is disclosed in Note 12.
Stock options issued in lieu of cash to non-employees for services performed are recorded at the fair value of the options at the time they are issued and are expensed as service is provided.
- (m)
- Research and development costs
Research costs, including research performed under contract by third parties, are expensed as incurred. Development costs are also generally expensed as incurred unless such costs meet the criteria necessary for deferral and amortization. To qualify for deferral, the costs must relate to a technically feasible, identifiable product that the Corporation intends to produce and market, there must be a clearly defined market for the product and the Corporation must have the resources, or access to the resources, necessary to complete the development. The Corporation has not deferred any such development costs to date.
- (n)
- Government assistance
Government assistance is recognized when the expenditures that qualify for assistance are made and the Corporation has complied with the conditions for the receipt of government assistance. Government assistance is applied to reduce the carrying value of any assets acquired or to reduce eligible expenses incurred. A liability to repay government assistance, if any, is recorded in the period when the conditions arise that cause the assistance to become repayable. Goverment assistance recognized to date consists of government programs that provide refundable credits for qualifying scientific research and development expenditures such as the Ontario Business Research Institute tax credit program described in Note 13, the Technology Partnerships Canada program described in Note 17(c) and other similar regimes that the Corporation claims under from time to time.
- (o)
- Foreign currency translation
4. CASH, CASH EQUIVALENTS AND SHORT-TERM INVESTMENTS
| | 2004
| | 2003
|
|---|
| | Cash and Cash Equivalents
| | Short-term Investments
| | Cash and Cash Equivalents
| | Short-term Investments
|
|---|
| Cash | | $ | 1,518,767 | | $ | — | | $ | 5,102,947 | | $ | — |
| Guaranteed investment certificates | | | 2,300,000 | | | — | | | — | | | — |
| Asset backed notes held with financial institutions | | | — | | | — | | | 1,234,730 | | | — |
| Corporate securities | | | — | | | 4,999,034 | | | — | | | 11,720,510 |
| | |
| |
| |
| |
|
| | | $ | 3,818,767 | | $ | 4,999,034 | | $ | 6,337,677 | | $ | 11,720,510 |
| | |
| |
| |
| |
|
B-2-9
The corporate securities earn interest at rates ranging from 2.36% to 4.5% and have maturity dates ranging from June 1, 2005 to March 16, 2006.
5. INVENTORY
| | 2004
| | 2003
|
|---|
| Raw materials | | $ | 2,337,702 | | $ | 1,076,551 |
| Work in progress | | | 3,014,302 | | | 1,764,925 |
| Finished goods | | | 2,760,521 | | | 3,061,390 |
| | |
| |
|
| | | $ | 8,112,525 | | $ | 5,902,866 |
| | |
| |
|
Included in finished goods inventory is $409,360 (2003 — $Nil) delivered to customers and for which title has transferred to the customer. These transfers are treated as consignment sales and revenue is deferred until the Corporation determines that persuasive evidence of an agreement exists; the price is fixed and determinable; and collection is reasonably assured.
6. CAPITAL ASSETS
| | 2004
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book
Value
|
|---|
| Furniture and fixtures | | $ | 443,349 | | $ | 334,184 | | $ | 109,165 |
| Computer equipment and software | | | 1,465,905 | | | 1,061,608 | | | 404,297 |
| Manufacturing and research equipment | | | 4,996,274 | | | 3,498,150 | | | 1,498,124 |
| Leasehold improvements | | | 507,755 | | | 507,755 | | | — |
| | |
| |
| |
|
| | | $ | 7,413,283 | | $ | 5,401,697 | | $ | 2,011,586 |
| | |
| |
| |
|
| | 2003
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book
Value
|
|---|
| Furniture and fixtures | | $ | 407,318 | | $ | 248,922 | | $ | 158,396 |
| Computer equipment and software | | | 1,581,625 | | | 975,081 | | | 606,544 |
| Manufacturing and research equipment | | | 5,458,867 | | | 3,182,150 | | | 2,276,717 |
| Leasehold improvements | | | 507,755 | | | 507,755 | | | — |
| | |
| |
| |
|
| | | $ | 7,955,565 | | $ | 4,913,908 | | $ | 3,041,657 |
| | |
| |
| |
|
7. INTANGIBLE ASSETS
The cost and accumulated amortization of the Corporation's intangible assets as of December 31, 2004 and December 31, 2003 are as follows:
| | 2004
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book
Value
|
|---|
| Purchased technology | | $ | 11,599,555 | | $ | 11,344,543 | | $ | 255,012 |
| Other intangible assets | | | 4,371,573 | | | 4,371,573 | | | — |
| | |
| |
| |
|
| | | $ | 15,971,128 | | $ | 15,716,116 | | $ | 255,012 |
| | |
| |
| |
|
B-2-10
| | 2003
|
|---|
| | Cost
| | Accumulated Amortization
| | Net Book
Value
|
|---|
| Purchased technology | | $ | 11,599,555 | | $ | 10,834,490 | | $ | 765,065 |
| Other intangible assets | | | 4,371,573 | | | 4,257,520 | | | 114,053 |
| | |
| |
| |
|
| | | $ | 15,971,128 | | $ | 15,092,010 | | $ | 879,118 |
| | |
| |
| |
|
8. SHORT-TERM LOANS
On December 18, 2002, the Corporation entered into a $1,288,494 non-revolving term loan with a maturity date of June 18, 2003 and interest at the rate of 2% per month. The loan was collateralized by a general security agreement covering the Corporation's right, title and interest in all of its property and assets and an assignment of all patents, trademarks and licence agreements of the Corporation. On January 28, 2003, prior to the maturity date of the loan, the Corporation repaid the loan in full and the security was discharged.
On January 28, 2003, the Corporation entered into a senior loan agreement totaling $4,579,953 and a subordinated loan agreement totaling $1,962,837 ("Loans"). The Loans originally matured on July 31, 2003 and with an interest rate of 18% per annum payable monthly plus a 4% lenders fee. As collateral for the Loans, the Corporation entered into general security agreements over all of its assets. The Loans were amended to extend the maturity date to September 23, 2003 at which point they were repaid in full. Fees relating to loan extensions on the senior and subordinated loans amounted to $336,782.
On July 30, 2003, the Corporation issued senior subordinated convertible debentures for gross proceeds of $856,837 with a maturity date of October 22, 2003, and an interest rate of 14%. The subordinated convertible debentures were repaid in full on September 23, 2003.
On September 4, 2003 the Corporation entered into a bridge loan agreement totaling $767,771 with a maturity date of the earlier of the completion of a private placement and October 22, 2003, and with an interest rate of 14% per annum payable monthly, plus a lenders fee of 4%. The bridge loan was repaid in full on September 23, 2003.
9. TAXES PAYABLE
The Corporation has accrued $832,000 related to a sales tax assessment received from the Province of Ontario during the year. The amount is included in research and development expenses as the assessment relates to taxes assessed on certain of those expenses incurred in the prior years. Until the assessment has been paid, the Ministry of Finance for Ontario has placed a lien for the value of the assessed amount on the assets of the Corporation.
10. CONVERTIBLE DEBENTURES
On September 15, 2004, the Corporation issued unsecured convertible debentures ("Debentures") and warrants ("Warrants") for gross proceeds of $13,318,750. The Debentures, which become due on September 15, 2009, are convertible at any time at the holders' option into common shares of the Corporation at a price of $1.25 per share and accumulate non-compounding interest at an annual rate of 3%. Interest is convertible at any time into common shares of the Corporation at a price based on the five day weighted average trading price immediately prior to conversion. The Corporation can redeem the Debentures when the trading price of the Corporation's common shares is greater than $3.00 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Debentures in any 90-day period. In addition, Warrants to purchase 10,655,000 common shares of the Corporation at an exercise price of $1.55 per share and exercisable for a period of five years were issued to the Debenture holders. The Corporation may call the Warrants when the trading price of the Corporation's common shares is greater than $4.50 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Warrants in any 90-day period.
The Corporation issued to its agents 470,833 common shares of the Corporation with a value of $659,166 and granted agent's warrants exercisable for 193,800 common shares of the Corporation with a value of $179,759 having the same terms as the Warrants.
The Debentures and Warrants have been accounted for in accordance with their substance and are presented in the financial statements in their debt and equity components, measured at their respective fair values at the time of issue. The debt component has been calculated as the present value of future cash flows. The equity component is shown as contributed surplus and consists of the value of the Warrants and the conversion feature component of the Debentures.
B-2-11
The respective fair values of the components of the Debentures at the time of issue and as at December 31, 2004 are as follows:
| | September 15,
2004
| | December 31,
2004
|
|---|
| Convertible debentures | | $ | 1,734,350 | | $ | 2,530,659 |
| Warrants | | | 4,504,127 | | | 4,504,127 |
| Conversion feature | | | 5,863,191 | | | 5,863,191 |
| | |
| |
|
| | | $ | 12,101,668 | | $ | 12,897,977 |
| | |
| |
|
Interest is accrued at the effective rate of the Debentures up to a maturity value of $15,316,563 on September 15, 2009. Interest of $796,309 was accrued to December 31, 2004.
As a condition of the MedQuest acquisition described in Note 20, the holders of the Debentures have unanimously agreed to convert the Debentures and accrued interest into common shares of the Corporation.
11. PREFERRED SHARES
On September 22, 2003, Edwards exchanged all of the Series A redeemable, cumulative, participating preferred shares of World Heart Inc. for 711,589 common shares.
On November 27, 2003, Edwards Lifesciences (U.S.) Inc. converted all of the Series A cumulative, redeemable, convertible preferred shares into 500,000 common shares and warrants to acquire 1,000,000 common shares at $5.93 per share, expiring November 27, 2008. A fair value of $6,015,680 was attributed to the additional common shares and warrants of which $4,091,286 was applied to reduce the outstanding liability for accrued dividends and the remaining $1,924,394 was charged to accumulated deficit.
12. SHAREHOLDERS' EQUITY
Common shares
- (a)
- Authorized
Authorized common shares of the Corporation consist of an unlimited number of shares with no par value.
On November 25, 2003, the Corporation's shareholders approved a one-for-seven consolidation of the Corporation's common shares and common share equivalents, and on December 1, 2003 WorldHeart filed Articles of Amendment effecting the share consolidation. All references to common share, option, warrant and per common share amounts for all periods presented have been retroactively restated to reflect the share consolidation.
Employee Stock Option Plan
The Corporation has an employee stock option plan ("ESOP"). The maximum number of shares that may be reserved and set aside under options to eligible persons pursuant to the ESOP may not exceed 1,501,857 common shares (2003 — 1,501,857). As at December 31, 2004, options for 789,046 common shares have been granted. In addition on September 23, 2004, the Corporation granted, subject to shareholder approval of an increase in the number of options available under the plan, options for 2,383,725 common shares to employees, officers, directors and consultants. Approval will be sought at the next annual meeting of shareholders. In addition, the grant is conditional on the forfeiture by the employees, officers, directors and consultants of 251,685 options for common shares already issued and outstanding under the plan. The maximum number of common shares at any time available for issuance under the ESOP, or pursuant to other outstanding options, to any one person is 5% of the common shares then issued and outstanding. A committee appointed by the Board of Directors administers the ESOP. The option exercise price for all options issued under the ESOP is based on the fair market value of the common shares on the date of grant priced on the close of market in the Toronto Stock Exchange ("TSX") immediately prior to the date of grant. The options generally vest annually in equal portions over either a five-year period or three-year period and must be exercised within a four-year period from each date of vesting.
B-2-12
On March 5, 2003, the Board of Directors approved a proposal to offer employees, officers, directors and consultants of the Corporation the opportunity to exchange options ("Option Exchange") held by them and granted prior to 2003 ("Existing Options"), for a reduced number of options ("New Options") at a price that more closely reflected the then current trading price. Approval of the shareholders was required with respect to option exchanges for persons considered to be insiders of the Corporation including the Corporation's executive officers and members of the Board of Directors. Shareholders approved the insiders' exchange on June 16, 2003. The Toronto Stock Exchange approved the Option Exchange on June 20, 2003. In total, participants under the ESOP chose to exchange a total of 121,218 Existing Options for 23,660 New Options.
Rights related to 2007262 Ontario Inc.
On December 19, 2001, the Corporation incorporated 2007262 Ontario Inc. ("2007262") to carry out specified research and development. The Corporation and New Generation Biotech (Equity) Fund Inc. ("NewGen"), an Ontario labour sponsored venture capital corporation, subscribed for an equal number of common shares of 2007262. Additionally, NewGen subscribed for 91,000 Series 1 preferred shares ("Series 1 Shares") of 2007262 for net proceeds of $2,157,330 after deducting expenses of the placement of $83,484. The Corporation sold to 2007262 certain technology in exchange for 14,285 Series 2 preferred shares ("Series 2 Shares") of 2007262 and a promissory note in the amount of $1,261,591.
The promissory note was repaid to the Corporation from the proceeds of the Series 1 preferred shares and the balance of the Series 1 preferred share proceeds was used to improve and enhance the technology transferred by the Corporation.
On January 31, 2003, NewGen redeemed the Series 1 preferred shares for 91,000 common shares of the Corporation and 91,000 common share purchase warrants ("Purchase Warrants") of the Corporation. Each Purchase Warrant was exercisable into one common share of WorldHeart at a price of $26.25 at any time up to January 24, 2004 but expired unexercised as at that date.
2007262 has been accounted for as a research and development arrangement. The Corporation recorded the NewGen funding as contributed surplus and the amounts expended by 2007262 from the NewGen funding as research and development expenses in the period that they occurred. Expenses incurred by 2007262 in the year were $230,071 (2003 — $608,970; 2002 — $951,274).
Stock Option and Warrant Activity
The following table summarizes the number of options and warrants outstanding and the weighted average exercise price:
| | Employees
| | Non-Employees
| |
| |
| |
| |
|---|
| | Options
#
| | Weighted average exercise price
$
| | Options
#
| | Weighted average exercise price
$
| | Warrants
#
| | Weighted average exercise price
$
| | Total
#
| |
|---|
| Outstanding at Dec. 31, 2001 | | 177,113 | | 56.47 | | 6,078 | | 53.16 | | 151,066 | | 36.02 | | 334,257 | |
| Granted | | 132,801 | | 27.82 | | 4,826 | | 29.05 | | 490,641 | | 24.92 | | 628,268 | |
| Exercised | | — | | — | | — | | — | | (22,499 | ) | 26.72 | | (22,499 | ) |
| Expired | | (3,518 | ) | 45.93 | | — | | — | | (12,143 | ) | 97.04 | | (15,661 | ) |
| Forfeited | | (45,099 | ) | 38.87 | | (1,221 | ) | 42.56 | | — | | — | | (46,320 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2002 | | 261,297 | | 45.09 | | 9,683 | | 42.49 | | 607,065 | | 26.18 | | 878,045 | |
| Granted | | 1,079,538 | | 8.07 | | 34,350 | | 8.25 | | 14,138,457 | | 6.18 | | 15,252,345 | |
| Exercised | | — | | — | | — | | — | | (147,857 | ) | 7.12 | | (147,857 | ) |
| Cancelled | | (118,108 | ) | 53.77 | | (3,110 | ) | 46.99 | | — | | — | | (121,218 | ) |
| Expired | | (2,758 | ) | 39.51 | | — | | — | | (5,000 | ) | 57.75 | | (7,758 | ) |
| Forfeited | | (75,621 | ) | 19.36 | | (1,258 | ) | 9.41 | | — | | — | | (76,879 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2003 | | 1,144,348 | | 10.98 | | 39,665 | | 13.54 | | 14,592,665 | | 6.99 | | 15,776,678 | |
| Granted | | 127,852 | | 6.46 | | 3,095 | | 7.77 | | 10,923,566 | | 1.58 | | 11,054,513 | |
| Exercised | | — | | — | | — | | — | | (250,000 | ) | 5.93 | | (250,000 | ) |
| Repurchased | | — | | — | | — | | — | | (300,000 | ) | 5.93 | | (300,000 | ) |
| Expired | | (7,166 | ) | 52.46 | | — | | — | | (541,998 | ) | 26.84 | | (549,164 | ) |
| Forfeited | | (514,165 | ) | 8.01 | | (4,583 | ) | 8.48 | | — | | — | | (518,748 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Outstanding at Dec. 31, 2004 | | 750,869 | | 11.85 | | 38,177 | | 13.68 | | 24,424,233 | | 4.15 | | 25,213,279 | |
| | |
| |
| |
| |
| |
| |
| |
| |
B-2-13
| | Options
| |
|
|---|
| | Employees
| | Non-Employees
| | Warrants
|
|---|
| Weighted average exercise price of exercisable options and warrants: | | | | | | | | | |
| | December 31, 2002 | | $ | 52.24 | | $ | 51.84 | | $ | 27.59 |
| | December 31, 2003 | | | 45.67 | | | 50.55 | | | 7.03 |
| | December 31, 2004 | | | 18.09 | | | 18.34 | | | 4.15 |
| Number of exercisable options and warrants: | | | | | | | | | |
| | December 31, 2002 | | | 87,916 | | | 3,372 | | | 607,065 |
| | December 31, 2003 | | | 66,283 | | | 4,036 | | | 14,592,665 |
| | December 31, 2004 | | | 287,102 | | | 17,867 | | | 24,424,233 |
| Range of exercise prices of all options and warrants at December 31, 2004: | | | | | | | | | |
| | From | | $ | 1.02 | | $ | 5.31 | | $ | 1.55 |
| | To | | | 105.43 | | | 59.95 | | | 29.05 |
| Range of expiry dates of all options and warrants at December 31, 2004: | | | | | | | | | |
| | From | | | Jan. 5, 2005 | | | Jul. 14, 2005 | | | Dec. 19, 2005 |
| | To | | | Dec. 7, 2011 | | | Dec. 31, 2010 | | | Sep. 14, 2009 |
The following table summarizes information about the outstanding options and warrants as at December 31, 2004:
Range of exercise price
| | Number outstanding
| | Weighted average exercise price
| | Weighted average remaining life in years
|
|---|
| $ 1.02 to 2.50 | | 10,868,424 | | $ | 1.55 | | 4.7 |
| 2.51 to 5.00 | | 1,484 | | | 4.08 | | 5.5 |
| 5.01 to 7.50 | | 13,258,314 | | | 6.01 | | 3.7 |
| 7.51 to 10.00 | | 864,269 | | | 8.00 | | 4.4 |
| 10.01 to 20.00 | | 7,838 | | | 14.68 | | 1.3 |
| 20.01 to 40.00 | | 170,095 | | | 28.72 | | 2.1 |
| 40.01 to 105.43 | | 42,855 | | | 56.44 | | 1.6 |
| | |
| |
| |
|
| | | 25,213,279 | | $ | 4.40 | | 4.1 |
| | |
| |
| |
|
On September 23, 2004, the Corporation conditionally granted, subject to shareholder approval of an increase in the number of options available under the plan, options for 2,383,725 common shares to employees, officers, directors, and consultants. These conditional grants are not included in the number of options and warrants outstanding of 25,213,279. In addition, the grant is conditional on the forfeiture by the employees, officers, directors and consultants of 251,685 options for common shares already issued and outstanding under the plan. Options were granted as follows: 215,000 to Canadian based individuals with an exercise price of Cdn $1.43 and 2,168,725 to individuals based outside of Canada with an exercise price of $1.12. Options vest one third on each of the first, second and third anniversaries of the date of grant and expire on September 22, 2013.
The Corporation accounts for its stock option plan in accordance with the provisions of CICA 3870, which requires companies to account for stock options using the fair value based method. Under the fair value based method, compensation cost is measured at fair value at the date of grant and is expensed over the award's vesting period.
In accordance with one of the transitional options permitted under CICA 3870, the Corporation has determined the value of previously issued options using the fair value based method and has adjusted the opening contributed surplus and accumulated deficit at January 1, 2004 for the amounts determined under that method that were previously not expensed. The effect of the retroactive adjustment at January 1, 2004 increased contributed surplus and accumulated deficit by $5,297,252. The non-cash share-based compensation costs for the year ended December 31, 2004 do not include charges related to the options to purchase 2,383,725 common shares that were conditionally granted on September 23, 2004.
Also under these new recommendations, where the fair value based method of accounting has not been used to account for employee stock options, companies are required to disclose pro forma net income and pro forma earnings per share, as if the fair value based
B-2-14
method of accounting had been used to account for these stock based awards. The estimated share-based compensation costs based on stock options granted to directors and employees and the pro forma net loss and loss per share are as follows:
| | 2003
| | 2002
| |
|---|
| Net loss applicable to common shareholders | | $ | (25,471,707 | ) | $ | (31,690,859 | ) |
| Share-based compensation costs | | | (1,579,378 | ) | | (1,653,660 | ) |
| | |
| |
| |
| Adjusted pro forma net loss | | $ | (27,051,085 | ) | $ | (33,344,519 | ) |
| | |
| |
| |
| Reported basic and diluted loss per share | | $ | (4.07 | ) | $ | (12.48 | ) |
| Share-based compensation costs per share | | | (0.25 | ) | | (0.65 | ) |
| | |
| |
| |
| Pro forma basic loss per share | | $ | (4.32 | ) | $ | (13.13 | ) |
| | |
| |
| |
The weighted average fair value of the options issued during the year ended December 31, 2004 was $4.39 (2003 — $5.48; 2002 — $18.83). The fair values of options granted are determined using the Black-Scholes model. For 2004, 2003 and 2002, the following weighted average assumptions were utilized:
| | 2004
| | 2003
| | 2002
|
|---|
| Expected option life, in years | | 6 | | 6 | | 6 |
| Volatility | | 73% | | 75% | | 75% |
| Risk free interest rate | | 4.00% | | 3.65% | | 3.65% |
| Dividend yield | | Nil | | Nil | | Nil |
13. GOVERNMENT ASSISTANCE
During the year ended December 31, 2001, the Corporation accrued a tax credit receivable related to the Ontario Business Research Institute tax credit ("OBRITC") program, in the amount of $2,304,492 that was associated with research payments made to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation from 1997 to 2000. Following the completion of the provincial Ministry of Finance's audit and proposed amendments to the OBRITC legislation, the Corporation received payment of $597,538 related to this claim during the year ended December 31, 2003. The full amount of the claim is under dispute and, due to the uncertainty of the ultimate resolution of the claim, the Corporation recorded a provision for the remainder of $1,706,954 in 2003. This provision was charged to research and development expense.
14. RESTRUCTURING
On August 25, 2004, the Corporation approved a plan to consolidate its Ottawa, Ontario operations into its facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve the Corporation's operational efficiency and effectiveness. The Corporation expects the restructuring to be completed by the end of June 2005 and expects to incur total restructuring charges of $2,611,764 comprising employee severance costs of $1,509,192; contract settlement costs of $719,894; write down of capital assets to net realizable value of $338,595; and other costs of $44,083. The amounts accrued and expensed to date are as follows:
| | Costs accrued during the year
| | Costs paid or settled in the year
| | Balance in accrued liabilities as at December 31
|
|---|
| Employee severance costs | | $ | 1,396,037 | | $ | 795,923 | | $ | 600,114 |
| Contract settlement costs | | | 57,653 | | | — | | | 57,653 |
| Write down of capital assets | | | 338,595 | | | 338,595 | | | — |
| Other costs | | | 22,509 | | | 8,624 | | | 13,885 |
| | |
| |
| |
|
| | | $ | 1,814,794 | | $ | 1,143,142 | | $ | 671,652 |
| | |
| |
| |
|
During the year ended December 31, 2003, the Corporation restructured its worldwide distribution to increase emphasis on direct sales and marketing and refocused its next-generation VAD development programs. Associated restructuring costs incurred for 2003 totaled
B-2-15
$3,494,676. Employee severance and termination costs of $27,665 were reversed in the year ended December 31, 2004. The Corporation does not anticipate incurring any additional costs relating to the 2003 restructuring activities.
15. LOSS PER COMMON SHARE
For all of the years presented, diluted loss per share equals basic loss per share due to the anti-dilutive effect of convertible preferred shares, stock options and warrants. These instruments could potentially dilute basic earnings per share in the future by being converted into common shares:
| | Number of common shares to be issued on
exercise or conversion
|
|---|
| | 2004
| | 2003
| | 2002
|
|---|
| Convertible preferred shares | | — | | — | | 932,502 |
| Employee and non-employee stock options | | 789,046 | | 1,184,013 | | 270,980 |
| Warrants | | 24,424,233 | | 14,592,665 | | 840,139 |
| Convertible debentures | | 10,655,000 | | — | | — |
| Stock options subject to shareholder approval | | 2,383,725 | | — | | — |
| | |
| |
| |
|
| Total potentially dilutive instruments | | 38,252,004 | | 15,776,678 | | 2,043,621 |
| | |
| |
| |
|
16. INCOME TAXES
The Corporation operates in several tax jurisdictions. Its income is subject to varying rates of tax and losses incurred in one jurisdiction cannot be used to offset income taxes payable in another. A reconciliation of the combined Canadian federal and provincial income tax rate with the Corporation's effective tax rate is as follows:
| | 2004
| | 2003
| | 2002
| |
|---|
| Canadian loss | | $ | (12,454,602 | ) | $ | (16,921,757 | ) | $ | (9,701,130 | ) |
| United States' loss | | | (9,052,555 | ) | | (6,296,565 | ) | | (21,989,729 | ) |
| European loss | | | (1,299,648 | ) | | (328,991 | ) | | — | |
| | |
| |
| |
| |
| Loss before income taxes | | $ | (22,806,805 | ) | $ | (23,547,313 | ) | $ | (31,690,859 | ) |
| | |
| |
| |
| |
| Expected statutory rate | | | 36.12% | | | 36.62% | | | 38.62% | |
| Expected recovery of income tax | | $ | (8,240,000 | ) | $ | (8,630,000 | ) | $ | (12,240,000 | ) |
| Effect of foreign tax rate differences | | | (510,000 | ) | | (340,000 | ) | | (420,000 | ) |
| Permanent differences | | | 1,111,000 | | | 1,080,000 | | | 1,920,000 | |
| Change in valuation allowance | | | 7,940,000 | | | 12,680,000 | | | 8,960,000 | |
| Effect of changes in SR&ED carryforwards | | | 2,251,000 | | | 760,000 | | | 800,000 | |
| Effect of tax rate changes | | | 268,000 | | | 270,000 | | | 1,360,000 | |
| Effect of exchange rate differences | | | (2,820,000 | ) | | (5,820,000 | ) | | (380,000 | ) |
| | |
| |
| |
| |
| Recovery of income taxes | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
The Canadian statutory income tax rate of 36.12% is comprised of federal income tax at approximately 22.12% and provincial income tax at approximately 14.00%. For the years ended December 31, 2003 and 2002, the permanent differences relate primarily to imputed interest expense on the Preferred Shares and goodwill amortization.
B-2-16
The primary temporary differences affecting future taxes and their approximate effects are as follows:
| | 2004
| | 2003
| |
|---|
| Future tax assets: | | | | | | | |
| | SR&ED expenditures | | $ | 10,280,000 | | $ | 9,390,000 | |
| | Net operating losses | | | 37,750,000 | | | 32,190,000 | |
| | Investment tax credits | | | 5,540,000 | | | 4,660,000 | |
| | Share issue costs | | | 4,790,000 | | | 4,500,000 | |
| | Asset basis differences | | | 1,500,000 | | | 1,320,000 | |
| | |
| |
| |
| | | | 59,860,000 | | | 52,060,000 | |
| | Less: valuation allowance | | | (59,570,000 | ) | | (51,630,000 | ) |
| | |
| |
| |
| | | | 290,000 | | | 430,000 | |
Future tax liabilities: |
|
|
|
|
|
|
|
| | Asset basis differences | | | (290,000 | ) | | (430,000 | ) |
| | |
| |
| |
| Net future income tax liability | | $ | — | | $ | — | |
| | |
| |
| |
As at December 31, 2004 the Corporation has unclaimed Scientific Research and Experimental Development ("SR&ED") expenditures, income tax loss carryforwards and investment tax credits.
The unclaimed amounts and their expiry dates are as follows:
| | 2004
| | 2003
|
|---|
| SR&ED expenditures — carried forward without expiry | | $ | 28,500,000 | | $ | 25,610,000 |
| Income tax loss carryforwards: | | | | | | |
| | Federal (Canada) (expire 2005-2011) | | | 57,082,000 | | | 49,210,000 |
| | Provincial (expire 2005-2011) | | | 56,000,000 | | | 52,290,000 |
| | United States (expire 2010-2020) | | | 49,100,000 | | | 46,430,000 |
| | Europe | | | 1,400,000 | | | 140,000 |
| Investment tax credits (expire 2006-2014) | | | 7,100,000 | | | 6,170,000 |
17. COMMITMENTS AND CONTINGENCIES
| Year ended December 31, | | 2005 | | $ | 1,440,597 |
| | | 2006 | | | 1,407,680 |
| | | 2007 | | | 354,770 |
| | | 2008 | | | 37,476 |
| | | 2009 | | | 2,197 |
Total rent expense for the years ended December 31, 2004, 2003 and 2002 was $1,026,315, $1,114,717, and $1,092,705 respectively. During 2002, the Corporation pledged cash in the amount of $750,000 as collateral for obligations under a premises lease for the Oakland operation. During 2003, $222,003 was drawn against the cash amount pledged to cover certain rent obligations and in 2004 the Corporation restored the balance to the original pledged amount of $750,000.
- (b)
- Novacor LVAS Royalties
The Corporation is committed, under the Novacor LVAS royalty agreement to royalty payments to certain employees. Royalties are payable on annual consolidated gross revenues at a rate of 0.808% up to a cumulative maximum of $3,232,000. Royalty payments to date total $1,027,184; included in accounts payable and accrued liabilities at December 31, 2004 is $95,646 (2003 — $37,478).
B-2-17
- (c)
- Technology Partnerships Canada Contribution Agreement
During 2002, the Corporation entered into a shared funding program with Technology Partnerships Canada ("TPC") under which the Canadian government shares costs of certain research and development activities. The Corporation claimed funding in the amount of approximately $6,624,000. As at December 31, 2004, $203,250 (2003 — $193,648) is included in accounts receivable. Effective January 1, 2004, repayment is in the form of royalties payable on annual consolidated gross revenues at a rate of 0.65% for a nine-year period ended December 31, 2012. If during this period royalty payments reach the maximum of $20,250,000 no further repayments will be required. If the royalty payments do not exceed $20,250,000 during this period they will continue until 2015 or until the maximum is reached, whichever comes first. During the year ended December 31, 2004, the Corporation has accrued royalties owing of approximately $69,000.
In connection with the agreement, the Corporation granted TPC 92,857 warrants to purchase an equivalent number of common shares of the Corporation, exercisable until December 4, 2006 at an exercise price of $29.05 per share.
- (d)
- Research Agreement
Effective April 1, 1996, the Corporation entered into a research agreement with the Cardiovascular Devices Division ("CVD") of the Ottawa Heart Institute Research Corporation ("Research Agreement") under which the Corporation agreed to fund a substantial portion of CVD's remaining research efforts relating to artificial heart technology. The Corporation acquired joint ownership with CVD of the technology arising from CVD's research under the Research Agreement and an exclusive twenty-five year license to market the product and certain other related technologies for an initial license fee of $147,544 and royalties of 7%. The Corporation is no longer focused on developing or commercializing this technology and as a result is not expected to make any royalty payments to CVD.
The Corporation's research funding to CVD under the Research Agreement was $13,400,000 for the period April 1, 1996 to December 31, 2004. The Corporation does not anticipate any future payments under the Research Agreement, as it is not pursuing development of the CVD artificial heart technology.
The Corporation also agreed with CVD to fund Cdn $150,000 per year for the period from July 1, 1996 to June 30, 2006 for a research chair in medical devices at the University of Ottawa Heart Institute.
CVD was considered a related party by virtue of the fact that the Chairman and Chief Scientific Officer of the Corporation prior to September 23, 2003 was also the Director of CVD. In September 2003, that individual resigned as Chairman and Chief Scientific Officer and as a result CVD is no longer considered a related party.
Prior to September 23, 2003, the Corporation incurred $159,200 (2002 — $499,480) for research and development fees to CVD under the Research Agreement described in Note 17 (a). In addition, the Corporation incurred $95,200 (2002 — $107,030) to CVD relating to the research chair under the Research Agreement.
Prior to September 23, 2003, the Corporation incurred salaries of $372,323 (2002 — $476,213) relating to employees that have been seconded by the Corporation to CVD. These expenditures were recovered by the Corporation from CVD.
18. NET CHANGE IN OPERATING COMPONENTS OF WORKING CAPITAL AND SUPPLEMENTAL CASH FLOW DISCLOSURE
| | 2004
| | 2003
| | 2002
|
|---|
| Accounts and other receivables | | $ | (475,399 | ) | $ | (334,286 | ) | $ | 2,017,870 |
| Prepaid expenses | | | 52,000 | | | (275,978 | ) | | 224,705 |
| Inventory | | | (2,176,785 | ) | | (1,812,506 | ) | | 1,148,080 |
| Accounts payable and accrued liabilities | | | (2,172,728 | ) | | (2,502,353 | ) | | 3,418,486 |
| Accrued compensation | | | (359,814 | ) | | (14,111 | ) | | 612,029 |
| | |
| |
| |
|
| | | $ | (5,132,726 | ) | $ | (4,939,234 | ) | $ | 7,421,170 |
| | |
| |
| |
|
Interest and financing costs paid in the year ended December 31, 2004 totalled $nil (2003-$1,235,367; 2002-$nil).
B-2-18
19. SEGMENTED INFORMATION
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the Corporation's chief decision maker in deciding how to allocate resources and assess performance. The Corporation's chief decision maker is the Chief Executive Officer.
On September 23, 2003, the Corporation realigned its business operations to focus on commercial operations and to reduce research and development costs. Research and development efforts were refocused to concentrate on developing future products that will evolve from current commercialized technologies and products into next generation technologies and marketable products. Accordingly, the Corporation is no longer organized by multiple operating segments for the purpose of making operating decisions or assessing performance and, as a result, the Corporation operates in one reportable segment.
The following geographic area data includes revenue based on product shipment destination and long-lived assets based on physical location. The Corporation has locations in Canada, the United States and Europe:
| | 2004
| | 2003
| | 2002
|
|---|
| | Revenue
| | Long-lived assets
| | Revenue
| | Long-lived assets
| | Revenue
| | Long-lived assets
|
|---|
| United States | | $ | 5,343,725 | | $ | 16,038,366 | | $ | 4,129,322 | | $ | 17,023,264 | | $ | 3,452,779 | | $ | 18,248,248 |
| Europe | | | 3,033,294 | | | 515,011 | | | 1,862,335 | | | 611,979 | | | 2,187,115 | | | — |
| Canada | | | 510,672 | | | 2,892,864 | | | 245,424 | | | 3,465,176 | | | 599,450 | | | 3,136,947 |
| Japan | | | 688,070 | | | — | | | 538,726 | | | — | | | 616,114 | | | — |
| Less: Edwards fee | | | — | | | — | | | — | | | — | | | (418,762 | ) | | — |
| | |
| |
| |
| |
| |
| |
|
| | | $ | 9,575,761 | | $ | 19,446,241 | | $ | 6,775,807 | | $ | 21,100,419 | | $ | 6,436,696 | | $ | 21,385,195 |
| | |
| |
| |
| |
| |
| |
|
At December 31, 2004 and 2003 accounts receivable from Edwards comprised 8% and 24%, respectively, of accounts receivable and one other (2003 — one other) account receivable amounted to greater than 10% of the total respective accounts receivable balances. During 2004, Edwards accounted for 7% of revenue and one other customer accounted for 10% of revenue. During 2003, Edwards accounted for 38% of revenue and two other customers accounted for 23% of revenue. During 2002, Edwards accounted for 50% of revenue and no other customer accounted for more than 10% of revenue.
In conjunction with Edwards' investment in the Corporation and the Corporation's acquisition of the Novacor division from Edwards, the Corporation entered into a distribution agreement (the "Distribution Agreement") whereby Edwards was the sole distributor, except in the United States, of the Corporation's heart assist products for a period of five years commencing July 1, 2000. On December 31, 2003, the Distribution Agreement was amended to reflect Edwards as sole distributor in Japan only. Also on December 31, 2003, the Corporation entered into a transition agreement to acquire certain inventory and capital assets from Edwards. For the year ended December 31, 2004, purchases from Edwards for components were $818,383 (2003 — $674,328; 2002 — $601,580). Edwards agreed to provide administrative services relating to distribution activities on an interim basis. Other purchases from Edwards for research and development materials and support and other services amounted to $180,723 (2003 — $897,121; 2002 — $975,017).
20. SUBSEQUENT EVENTS
On January 31, 2005, the Corporation entered into an agreement to acquire all of the assets of MedQuest for 9,300,000 common shares of the Corporation, subject to certain consents, conditions and shareholder approval. In connection with the acquisition of MedQuest, the Corporation has also entered into a private placement agreement with Maverick Venture Management, LLC ("Maverick"). At the closing of the acquisition, Maverick will purchase 8,888,889 common shares of the Corporation at a purchase price of $1.35 per share.
In addition, as a condition of this transaction, investors that participated in the Corporation's September 2004 convertible debenture transaction (Note 10) have unanimously agreed, subject to shareholder approval of a reduction in the Warrant exercise price from $1.55 to $1.00 per share, to convert their debentures at their stated conversion price of $1.25 per common share into approximately 10,655,000 common shares. The accrued interest will be converted into common shares at a conversion price equal to the market prices at the date of conversion. Subsequent to December 31, 2004, approximately $1.5 million of the debt and approximately $11,300 accrued interest has been converted into approximately 1.4 million common shares of the Corporation. The convertible debenture holders have also agreed to exercise the corresponding warrants into 10,655,000 common shares of the Corporation at an adjusted exercise price of $1.00 per common. The Warrants are currently exercisable until September 2009 at a price of $1.55 per common share. The Company will seek shareholder approval for a $0.55 reduction in the warrant exercise price.
On January 31, 2005, the Corporation conditionally granted, 3,418,750 options to employees, officers, directors and consultants subject to shareholder approval of an increase in the number of options available under the plan; 1,309,500 of these options are reserved for
B-2-19
new directors, officers and employees. The options have an exercise price of $1.48, vest one third on each of the first, second and third anniversaries of the date of grant and expire six years after each vesting date.
21. FINANCIAL INSTRUMENTS
Financial instruments recognized in the balance sheet at December 31, 2004 consist of cash and cash equivalents, short-term investments, accounts and other receivables, accounts payable and certain accrued liabilities and convertible debentures. The Corporation does not hold or issue financial instruments for trading purposes.
The Corporation invests the majority of its excess cash in high-grade instruments and diversifies the concentration of cash among different financial institutions.
- (a)
- Fair value
The Corporation believes that the carrying values of its financial instruments approximate their fair values because of their short terms to maturity, except for the long-term receivable and the convertible debentures. The long-term receivable is valued at the present value of future cash flows at a rate of 4%. The convertible debentures with a face value of $13,318,750 are presented in their debt and equity components measured at their relative fair values at the time of issue (see Note 10). The current fair value of the convertible debentures is not readily determinable.
- (b)
- Interest rate risk
The Corporation is subject to interest rate risks from time to time because of the term to maturity of its cash equivalents and short-term investments.
- (c)
- Foreign exchange risk
The Corporation transacts mainly in U.S. dollars but also in Canadian dollars and Euros. While the Corporation derives revenues in both Euros and Canadian dollars, it currently has net cash outflows in those currencies. The Corporation keeps cash balances on hand in those currencies that it estimates it requires to cover net cash outflow for the next quarter on a rolling basis.
- (d)
- Credit risk
Financial instruments that potentially subject the Corporation to a concentration of credit risk consist of cash, cash equivalents, short-term investments and accounts receivable. The Corporation has established guidelines for cash, cash equivalents and short-term investments relative to credit ratings, diversification and maturities that are intended to maintain safety and liquidity. The Corporation has a limited number of customers, all of which operate in the health-care industry. As at December 31, 2004, approximately 8% (2002 — 24%; 2002 — 68%) of the accounts receivable balance was due from Edwards. The Corporation performs ongoing credit evaluations of its customers' financial condition and generally requires no collateral from its customers. The Corporation maintains an allowance for doubtful accounts receivable of $146,307 (2003 — $41,782) based upon the expected collectibility of accounts receivable.
B-2-20
APPENDIX B-3
WORLD HEART CORPORATION
CONSOLIDATED BALANCE SHEETS
(United States Dollars)
| | March 31,
2005
| | December 31,
2004
| |
|---|
| | (unaudited)
| |
| |
|---|
| ASSETS | | | | | | | |
Current assets |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 4,536,861 | | $ | 3,818,767 | |
| | Short-term investments | | | — | | | 4,999,034 | |
| | Trade and other receivables | | | 4,137,000 | | | 4,238,049 | |
| | Prepaid expenses | | | 991,467 | | | 575,261 | |
| | Deferred transaction and financing costs | | | 330,247 | | | — | |
| | Inventory | | | 8,453,189 | | | 8,112,525 | |
| | |
| |
| |
| | | | 18,448,764 | | | 21,743,636 | |
| Long-term receivable | | | — | | | 318,553 | |
| Cash pledged as collateral for lease | | | 750,000 | | | 750,000 | |
| Capital assets | | | 1,937,490 | | | 2,011,586 | |
| Goodwill | | | 17,179,643 | | | 17,179,643 | |
| Intangible assets | | | 127,498 | | | 255,012 | |
| | |
| |
| |
| | | $ | 38,443,395 | | $ | 42,258,430 | |
| | |
| |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
| | Accounts payable and accrued liabilities | | $ | 4,234,969 | | $ | 3,854,833 | |
| | Taxes payable | | | 744,000 | | | 832,000 | |
| | Accrued compensation | | | 744,249 | | | 1,343,364 | |
| | Deferred revenue | | | 1,702,163 | | | 1,607,622 | |
| | |
| |
| |
| | | | 7,425,381 | | | 7,637,819 | |
| Convertible debentures (note 3) | | | 7,473,840 | | | 8,177,140 | |
| Long-term obligation | | | 8,830 | | | 16,368 | |
| | |
| |
| |
| | | | 14,908,051 | | | 15,831,327 | |
| | |
| |
| |
Shareholders' equity |
|
|
|
|
|
|
|
| | Common shares | | | 215,740,523 | | | 214,616,061 | |
| | | Issued and outstanding — 17,101,359 common shares
(December 31, 2004 — 15,744,522 common shares) | | | | | | | |
| | Additional paid-in capital | | | 11,377,442 | | | 11,451,267 | |
| | Cumulative other comprehensive income | | | (6,285,577 | ) | | (6,285,577 | ) |
| | Accumulated deficit | | | (197,297,044 | ) | | (193,354,648 | ) |
| | |
| |
| |
| | | | 23,535,344 | | | 26,427,103 | |
| | |
| |
| |
| | | $ | 38,443,395 | | $ | 42,258,430 | |
| | |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-3-1
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND ACCUMULATED DEFICIT
(United States Dollars)
| | Three months ended
| |
|---|
| | March 31,
2005
| | March 31,
2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| Revenue | | $ | 3,416,988 | | $ | 2,192,343 | |
| Cost of goods sold | | | (2,111,702 | ) | | (1,178,397 | ) |
| | |
| |
| |
| Gross margin | | | 1,305,286 | | | 1,013,946 | |
| | |
| |
| |
Expenses |
|
|
|
|
|
|
|
| | Selling, general and administrative | | | (3,445,829 | ) | | (3,035,445 | ) |
| | Research and development | | | (1,187,558 | ) | | (1,345,568 | ) |
| | Restructuring costs (note 5) | | | (129,498 | ) | | — | |
| | Amortization of intangibles | | | (127,514 | ) | | (129,166 | ) |
| | |
| |
| |
| | | | (4,890,399 | ) | | (4,510,179 | ) |
| | |
| |
| |
| Operating loss before the undernoted | | | (3,585,113 | ) | | (3,496,233 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
| | Foreign exchange loss | | | (24,372 | ) | | (100,467 | ) |
| | Investment income | | | 20,026 | | | 90,797 | |
| | Interest expense and financing costs | | | (352,937 | ) | | (3,704 | ) |
| | |
| |
| |
| Net loss for the period | | | (3,942,396 | ) | | (3,509,607 | ) |
| Accumulated deficit, beginning of the period | | | (193,354,648 | ) | | (167,212,850 | ) |
| | |
| |
| |
| Accumulated deficit, end of the period | | $ | (197,297,044 | ) | $ | (170,722,457 | ) |
| | |
| |
| |
| Weighted average number of common shares outstanding | | | 16,556,950 | | | 15,117,096 | |
| | |
| |
| |
| Basic and diluted loss per common share | | $ | (0.24 | ) | $ | (0.23 | ) |
| | |
| |
| |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-3-2
WORLD HEART CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOW
(United States Dollars)
| | Three months ended
| |
|---|
| | March 31,
2005
| | March 31,
2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| CASH FLOWS FROM (USED IN) | | | | | | | |
Operating activities |
|
|
|
|
|
|
|
| | Net loss for the period | | $ | (3,942,396 | ) | $ | (3,509,607 | ) |
| | Items not involving cash — | | | | | | | |
| | | Amortization | | | 294,522 | | | 325,840 | |
| | | Issuance of options and warrants for services and financing costs | | | — | | | 8,201 | |
| | | Interest and accretion on convertible debentures | | | 347,322 | | | — | |
| | | Unrealized foreign exchange loss | | | 97,547 | | | 123,345 | |
| | Change in operating components of working capital | | | (971,549 | ) | | (4,189,108 | ) |
| | |
| |
| |
| | | | (4,174,554 | ) | | (7,241,329 | ) |
| | |
| |
| |
Investing activities |
|
|
|
|
|
|
|
| | Redemption of short-term investments | | | 4,999,034 | | | 11,504,032 | |
| | Purchase of capital assets | | | (92,912 | ) | | (34,251 | ) |
| | Cash pledged as collateral for lease | | | — | | | (222,003 | ) |
| | |
| |
| |
| | | | 4,906,122 | | | 11,247,778 | |
| | |
| |
| |
Financing activities |
|
|
|
|
|
|
|
| | Common shares issued through exercise of warrants | | | — | | | 1,499,404 | |
| | |
| |
| |
| | | | — | | | 1,499,404 | |
| | |
| |
| |
| Effect of exchange rate changes on cash and cash equivalents | | | (13,474 | ) | | 27,929 | |
| | |
| |
| |
| Change in cash and cash equivalents for the period | | | 718,094 | | | 5,533,782 | |
| Cash and cash equivalents, beginning of the period | | | 3,818,767 | | | 6,337,677 | |
| | |
| |
| |
| Cash and cash equivalents, end of the period | | $ | 4,536,861 | | $ | 11,871,459 | |
| | |
| |
| |
Supplementary Cash Flow Information
| | Three months ended
|
|---|
| | March 31,
2005
| | March 31,
2004
|
|---|
| | (unaudited)
| | (unaudited)
|
|---|
| Convertible debentures settled with the issuance of common shares | | $ | 1,679,461 | | $ | — |
| Accrued interest on convertible debentures settled with the issuance of common shares | | $ | 19,977 | | $ | — |
(The accompanying notes are an integral part of these consolidated financial statements.)
B-3-3
WORLD HEART CORPORATION
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
1. GOING CONCERN ASSUMPTION
These consolidated financial statements have been prepared on the basis of accounting principles applicable to a going concern, which assume that World Heart Corporation (the "Corporation" or "WorldHeart") will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations.
The use of these principles may not be appropriate because, as at March 31, 2005, there was substantial doubt that the Corporation would be able to continue as a going concern. The Corporation's ability to continue as a going concern is dependent upon its ability to obtain additional financing and become commercially successful.
On January 31, 2005, the Corporation announced a definitive agreement to concurrently: acquire all the assets of MedQuest Products, Inc. ("MedQuest") for 9,300,000 common shares; issue 8,888,889 common shares, through a private placement, for gross proceeds of $12,000,000; convert all of its outstanding convertible debentures; and issue 10,655,000 common shares upon the exercise of 10,655,000 warrants for gross proceeds of $10,655,000. During the quarter, $1,679,461 of the convertible debentures and $19,977 of accrued interest were converted into 1,356,837 common shares of the Corporation (see Note 3). There can be no assurance that the Corporation will be successful in completing this transaction and therefore sustain operations for the foreseeable future.
These financial statements do not reflect adjustments to the carrying values of assets and liabilities, the reported revenues and expenses, and the balance sheet classifications used that would be necessary if the going concern assumption were not appropriate. Such adjustments could be material.
2. SIGNIFICANT ACCOUNTING POLICIES
The accompanying interim unaudited consolidated financial statements have been prepared by management in accordance with accounting principles generally accepted in the United States ("GAAP"), and include all assets, liabilities, revenues and expenses of the Corporation and its wholly owned subsidiaries; World Heart Inc. and World Heart B.V. All material intercompany transactions and balances have been eliminated. These interim unaudited consolidated financial statements follow the same accounting policies and methods of their application as the Corporation's audited consolidated financial statements for the year ended December 31, 2004. These interim unaudited consolidated financial statements do not conform in all respects to the requirements of GAAP for annual financial statements. These condensed notes to the interim unaudited consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes for the year ended December 31, 2004.
The preparation of these unaudited consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the unaudited consolidated financial statements and the accompanying notes. Actual results could differ from these estimates and the operating results for the interim periods presented are not necessarily indicative of the results expected for the full year. In the opinion of management, these unaudited consolidated financial statements reflect all normal and recurring adjustments considered necessary to state fairly the results for the periods presented.
3. CONVERTIBLE DEBENTURES
On September 15, 2004, the Corporation issued unsecured convertible debentures ("Debentures") and detachable warrants ("Warrants") for gross proceeds of $13,318,750 and net proceeds of $12,101,668 after share issue costs of $1,217,082. The Debentures, which become due on September 15, 2009, are convertible at any time at the holders' option into common shares of the Corporation at a price of $1.25 per share and accumulate non-compounding interest at an annual rate of 3%. Interest is convertible at any time into common shares of the Corporation at a price based on the five day weighted average trading price immediately prior to conversion. The Corporation can redeem the Debentures when the trading price of the Corporation's common shares is greater than $3.00 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Debentures in any 90-day period. In addition, Warrants to purchase 10,655,000 common shares of the Corporation at an exercise price of $1.55 per share and exercisable for a period of five years were issued to the Debenture holders. The Corporation may call the Warrants when the trading price of the Corporation's common shares is greater than $4.50 for 20 consecutive trading days, subject to a maximum of 20% of the initially issued Warrants in any 90-day period.
B-3-4
The proceeds of the issue were split between the Debentures, the Warrants and the beneficial conversion feature included in the Debentures based on their relative fair values. The respective fair values of the components of the Debentures as at the time of issue, as at December 31, 2004 and as at March 31, 2005 are as follows:
| | September 15,
2004
| | December 31,
2004
| | March 31,
2005
|
|---|
| |
| |
| | (unaudited)
|
|---|
| Convertible debentures | | $ | 1,911,128 | | $ | 8,177,140 | | $ | 7,473,840 |
| Warrants | | | 4,369,170 | | | 4,369,170 | | | 4,369,170 |
| Beneficial conversion feature | | | 5,821,370 | | | — | | | — |
| | |
| |
| |
|
| | | $ | 12,101,668 | | $ | 12,546,310 | | $ | 11,843,010 |
| | |
| |
| |
|
On September 16, 2004, the earliest possible conversion date, the full value of the beneficial conversion feature was charged to interest expense. Interest and accretion is accrued at the effective rate of the Debentures up to its original maturity value of $15,316,563 on September 15, 2009. As a result, $347,322 of interest and accretion was charged during the three months ended March 31, 2005.
As a condition of the MedQuest transaction, investors that participated in the Corporation's September 2004 convertible debenture transaction have unanimously agreed, subject to shareholder approval of a reduction in the Warrant exercise price from $1.55 to $1.00 per share, to convert their Debentures at their stated conversion price of $1.25 per common share into approximately 10,655,000 common shares. The accrued interest will be converted into common shares at a conversion price equal to the market prices at the date of conversion. The Debenture holders have also unanimously agreed, subject to shareholder approval of the reduction in the Warrant exercise price, to exercise the corresponding Warrants into 10,655,000 common shares of the Corporation at an exercise price of $1.00 per share. The Warrants are currently exercisable until September 2009 at a price of $1.55 per common share. The Corporation is seeking shareholder approval for the $0.55 reduction in the Warrant exercise price.
4. STOCK-BASED COMPENSATION
The Corporation accounts for its stock option plan in accordance with the Financial Accounting Standards Board's ("FASB") Statement of Financial Accounting Standards ("SFAS") No. 123, "Accounting for Stock Based Compensation", which requires companies to recognize as expense, over the vesting period, the fair value of all stock based awards on the date of grant or to continue to apply the provisions of Accounting Principles Board ("APB") Opinion No. 25, "Accounting for Stock Issued to Employees" and provide pro forma net income and pro forma income per share disclosures as if the fair value based method defined in SFAS No. 123 had been applied to stock-based awards. The Corporation follows this alternative method.
The estimated share-based compensation costs based on stock options granted to directors and employees and the pro forma net loss and loss per share are as follows:
| | Three months ended
March 31, 2005
| | Three months ended
March 31, 2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| Net loss for the period | | $ | (3,942,396 | ) | $ | (3,509,607 | ) |
| Share-based compensation costs | | | (331,720 | ) | | (617,503 | ) |
| | |
| |
| |
| Adjusted pro forma net loss | | $ | (4,274,116 | ) | $ | (4,127,110 | ) |
| | |
| |
| |
| Reported basic and diluted loss per common share | | $ | (0.24 | ) | $ | (0.23 | ) |
| Share-based compensation costs per common share | | | (0.02 | ) | | (0.04 | ) |
| | |
| |
| |
| Pro forma basic and diluted loss per common share | | $ | (0.26 | ) | $ | (0.27 | ) |
| | |
| |
| |
On September 23, 2004, the Corporation conditionally granted 2,383,725 options to purchase common shares of the Corporation, to employees, officers, directors, and consultants, subject to shareholder approval of an increase in the number of options available under the employee stock option plan. These options have an exercise price of $1.12 per share, except for 215,000 options which have an exercise price of Cdn $1.43 per share. The options vest one third on each of the first, second and third anniversaries of the date of grant and expire on September 22, 2013. In addition, the Corporation conditionally granted on January 31, 2005, 3,418,750 options to purchase common shares of the Corporation, to employees, officers, directors and consultants, subject to shareholder approval of an increase in the number of options available under the employee stock option plan; 1,309,500 of these options are reserved for new officers and employees. These options have an exercise price of $1.48 per share, vest one third on each of the first, second and third
B-3-5
anniversaries of the date of grant and expire six years after each vesting date. The share-based compensation costs and the adjusted pro forma net loss above do not include the charges related to the options conditionally granted by the Corporation. These options will be measured and the resulting compensation cost will be recorded after shareholder approval.
The Corporation did not have any new option issuances requiring measurement during the three months ended March 31, 2005. The weighted average fair value of the options issued during the three months ended March 31, 2004 was $5.09. The fair values of options granted are determined using the Black-Scholes model. For the three months ended March 31, 2004, the following weighted average assumptions were utilized:
| | Three months ended March 31, 2004
|
|---|
| Expected option life, in years | | 6 |
| Volatility | | 73% |
| Risk free interest rate | | 4.00% |
| Dividend yield | | Nil |
5. RESTRUCTURING
On August 25, 2004, the Corporation approved a plan to consolidate its Ottawa, Ontario operations into its facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve the Corporation's operational efficiency and effectiveness. The Corporation expects the restructuring to be completed by the end of June 2005 and expects to incur total restructuring charges of $2,661,002 comprised of employee severance costs of $1,535,510; contract settlement costs of $723,685; write down of capital assets to net realizable value of $335,049; and other costs of $66,758.
The amounts accrued and expensed to date are as follows:
| | Employee severance
| | Contract settlement
| | Write down
| | Other
| | Total
| |
|---|
| Costs accrued during 2004 | | $ | 1,396,037 | | $ | 57,653 | | $ | 338,595 | | $ | 22,509 | | $ | 1,814,794 | |
| Costs paid or settled in 2004 | | | (795,923 | ) | | — | | | (338,595 | ) | | (8,624 | ) | | (1,143,142 | ) |
| | |
| |
| |
| |
| |
| |
| Balance in accrued liabilities as at December 31, 2004 | | | 600,114 | | | 57,653 | | | — | | | 13,885 | | | 671,652 | |
| Costs accrued during the period | | | 110,539 | | | — | | | (3,546 | ) | | 22,505 | | | 129,498 | |
| Costs paid or settled in the period | | | (674,414 | ) | | — | | | 3,546 | | | (20,518 | ) | | (691,386 | ) |
| | |
| |
| |
| |
| |
| |
| Balance in accrued liabilities as at March 31, 2005 | | $ | 36,239 | | $ | 57,653 | | $ | — | | $ | 15,872 | | $ | 109,764 | |
| | |
| |
| |
| |
| |
| |
6. CANADIAN ACCOUNTING PRINCIPLES
The interim unaudited consolidated financial statements have been prepared in accordance with U.S. GAAP. These principles differ, as they affect the Corporation, for the three month period ended March 31, 2005 and for the balance sheets as at March 31, 2005 and December 31, 2004, in the following material respects from Canadian GAAP. There are no differences in reported cash flows for the periods presented.
B-3-6
- (a)
- Balance sheets — Canadian GAAP
| | March 31,
2005
| | December 31,
2004
| |
|---|
| | (unaudited)
| |
| |
|---|
| ASSETS | | | | | | | |
| Current assets | | $ | 18,448,764 | | $ | 21,743,636 | |
| | Long-term receivable | | | — | | | 318,553 | |
| | Cash pledged as collateral for lease | | | 750,000 | | | 750,000 | |
| | Capital assets | | | 1,937,490 | | | 2,011,586 | |
| | Goodwill and intangible assets | | | 17,307,141 | | | 17,434,655 | |
| | |
| |
| |
| | | $ | 38,443,395 | | $ | 42,258,430 | |
| | |
| |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
| Current liabilities | | $ | 7,425,381 | | $ | 7,637,819 | |
| | Convertible debentures(1) | | | 2,797,869 | | | 2,530,659 | |
| | Long-term obligation | | | 8,830 | | | 16,368 | |
| | |
| |
| |
| | | | 10,232,080 | | | 10,184,846 | |
| | |
| |
| |
Shareholders' equity (deficiency) |
|
|
|
|
|
|
|
| | Common shares(2) | | | 188,194,744 | | | 187,027,058 | |
| | Contributed surplus(1) | | | 22,389,807 | | | 22,871,780 | |
| | Cumulative translation adjustment | | | (6,490,184 | ) | | (6,490,184 | ) |
| | Accumulated deficit(1)(2) | | | (175,883,052 | ) | | (171,335,070 | ) |
| | |
| |
| |
| | | | 28,211,315 | | | 32,073,584 | |
| | |
| |
| |
| | | $ | 38,443,395 | | $ | 42,258,430 | |
| | |
| |
| |
- (b)
- Statements of loss — Canadian GAAP
| | March 31,
2005
| | March 31,
2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| Net loss for the period in accordance with U.S. GAAP | | $ | (3,942,396 | ) | $ | (3,509,607 | ) |
| Adjustments to reconcile to Canadian GAAP: | | | | | | | |
| | Non-cash share based compensation costs(3) | | | (331,720 | ) | | (617,503 | ) |
| | Interest and accretion on convertible debentures(1) | | | (273,866 | ) | | — | |
| | |
| |
| |
| Net loss for the period in accordance with Canadian GAAP | | | (4,547,982 | ) | | (4,127,110 | ) |
| | |
| |
| |
| Weighted average number of common shares outstanding | | | 16,556,950 | | | 15,117,096 | |
| | |
| |
| |
| Basic and diluted loss per common share | | $ | (0.27 | ) | $ | (0.27 | ) |
| | |
| |
| |
- (c)
- Footnotes
- (1)
- Under U.S. GAAP the proceeds of the issue on the convertible debentures were split between the convertible debentures, the warrants for common shares, and the beneficial conversion feature based on their relative fair values. The value of the warrants and the beneficial conversion feature were recorded in additional paid-in capital. On September 16, 2004, the earliest possible exercise date, the full value of the beneficial conversion feature was charged to interest expense. Under Canadian
B-3-7
| | September 15,
2004
| | December 31,
2004
| | March 31,
2005
|
|---|
| |
| |
| | (unaudited)
|
|---|
| Convertible debentures | | $ | 1,734,350 | | $ | 2,530,677 | | $ | 2,797,869 |
| Warrants | | | 4,504,127 | | | 4,504,127 | | | 4,504,127 |
| Conversion feature | | | 5,863,191 | | | 5,863,191 | | | 5,049,501 |
| | |
| |
| |
|
| | | $ | 12,101,668 | | $ | 12,897,995 | | $ | 12,351,497 |
| | |
| |
| |
|
The value allocated to the debentures will accrete to the cash value at maturity at the effective interest rate. Due to the conversion of debentures and the related accrued interest during the three months ended March 31, 2005, under U.S. GAAP $1,050,622 was transferred from convertible debentures and $73,825 was transferred from additional paid-in capital to common shares. Under Canadian GAAP, the amount transferred from convertible debentures was $353,996 and from contributed capital was $813,693.
- (2)
- The difference between the issue price and initial public offering (IPO) price of shares issued within a one-year period prior to the IPO is generally accounted for as an expense and charged against earnings for the period with a corresponding and equal amount recorded as paid-in capital. There is no corresponding requirement under Canadian GAAP. The difference of $32,867,182 related to the Corporation's IPO in 1996, increases the common shares and accumulated deficit reported under U.S. GAAP, with no difference reported in total shareholders' equity.
- (3)
- Under U.S. GAAP, the Corporation accounts for its stock option plan in accordance with APB Opinion No. 25 and provides pro forma net income and pro forma income per share disclosure as if the fair value based method as defined in SFAS No. 123 had been applied to stock-based awards. Under Canadian GAAP, the Corporation accounts for its stock option plan in accordance with CICA 3870 which requires entities to account for employee stock options using the fair value based method, where compensation cost is measured at fair value at the date of grant and is expensed over the stock-based awards vesting period.
7. NEW ACCOUNTING PRONOUNCEMENTS
In June 2001, the FASB issued SFAS No. 148, which amended the transitional provisions of SFAS No. 123 for companies electing to recognize employee stock-based compensation using the fair value based method. The provisions of FASB No. 148 will be effective for years beginning on or after January 1, 2006. In addition, during 2004, the FASB issued SFAS No. 123R, which also amended the provisions of SFAS No. 123. The provisions of FASB No. 123R will be effective for years beginning on or after January 1, 2006. The Corporation plans on adopting these new pronouncements in 2006.
B-3-8
APPENDIX C-1
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
INTRODUCTION
World Heart Corporation and its subsidiaries are collectively referred to as "WorldHeart". The following Management Discussion and Analysis of Financial Condition and Results of Operations ("MD&A") was prepared by management as of March 21, 2005, was approved by the Board of Directors and discusses material changes in WorldHeart's financial condition and results of operations and cash flows for the years ended December 31, 2004, 2003 and 2002. Such discussion and comments on the liquidity and capital resources should be read in conjunction with the information contained in the accompanying audited consolidated financial statements prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP"). In this discussion, all amounts are in United States dollars ("U.S. dollars") unless otherwise stated.
The discussion and comments contained hereunder include both historical information and forward-looking information. The forward-looking information, which generally is information stated to be anticipated, expected, or projected by management, involves known and unknown risks, uncertainties and other factors that may cause the actual results and performance to be materially different from any future results and performance expressed or implied by such forward-looking information. Potential risks and uncertainties include, without limitation, the uncertainties inherent in the development of new products for use in the human body, WorldHeart's need for significant additional funding, successful integration of acquisitions, WorldHeart's need to establish reimbursement mechanisms and product acceptance from third-party payers, extensive government regulation of our products, rapid developments in technology, including developments by competitors, potential lack of anticipated synergies and efficiencies of scale related to the MedQuest Products, Inc. ("MedQuest") acquisition, and potential difficulties in the integration of the MedQuest operations.
OVERVIEW
WorldHeart is a global medical device company currently focused on the development and commercialization of implantable pulsatile ventricular assist devices ("VADs"), which are mechanical pumps that allow for the restoration of normal blood circulation to patients suffering from late-stage congestive heart failure. WorldHeart has facilities in Ottawa, Ontario, Canada, Oakland, California, United States and Heesch, Netherlands. WorldHeart currently derives substantially all of its revenue from its Novacor® LVAS (left ventricular assist system) and related peripheral equipment.
WorldHeart manufactures and distributes the Novacor LVAS. The Corporation sells its Novacor products directly to medical clinics and hospitals in the United States, most of Europe and Canada and through a distributor in Japan and certain other countries.
The Novacor LVAS is commercially approved as a bridge to transplantation device in the United States and Canada. In Europe, the Novacor LVAS has unrestricted approval for use as an alternative to transplantation, a bridge to transplantation, and to support patients who may have an ability to recover the use of their natural heart. In Japan, the device is commercially approved for use in cardiac patients at risk of imminent death from non-reversible left ventricular failure for which there is no alternative except heart transplantation.
WorldHeart has commenced a long-term use (Destination Therapy) trial in the United States called RELIANT (RandomizedEvaluation of the NovacorLVASinaNon-Transplant Population). Data from the RELIANT Trial is expected to support a Pre-market Approval Supplement that will request approval for the use of the Novacor LVAS by non-transplant eligible patients (Destination Therapy) in the United States.
WorldHeart is in the process of developing a next-generation implantable pulsatile VAD and, subject to certain approvals, has recently agreed to acquire, from MedQuest, the technology associated with a magnetically levitated centrifugal rotary VAD. Development work on the Novacor II miniature pulsatile VAD will continue with initial clinical trials in 2007, and initial commercial approvals in Europe in 2008 and in the United States in 2010.
C-1-1
SELECTED FINANCIAL INFORMATION
All financial information is prepared in accordance with U.S. GAAP and is stated in U.S. dollars. Effective January 1, 2004, our functional currency was deemed to have changed from the Canadian dollar to the U.S. dollar. In general, this change resulted from the restructuring and issuance of common shares and warrants that occurred in the year ended December 31, 2003, along with a gradual increase in the overall proportion of business activities conducted in U.S. dollars and with the expectation that this trend will continue. Concurrent with this change in functional currency, we adopted the U.S. dollar as our reporting currency.
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Net revenue | | $ | 9,575,761 | | $ | 6,775,807 | | $ | 6,436,696 | |
| Net loss for the year | | | (26,141,798 | ) | | (14,629,054 | ) | | (24,552,673 | ) |
| Net loss applicable to common shareholders | | | (26,141,798 | ) | | (18,168,304 | ) | | (29,010,753 | ) |
| Basic and diluted loss per share | | | (1.70 | ) | | (2.90 | ) | | (11.42 | ) |
| Total assets | | | 42,258,430 | | | 50,180,069 | | | 27,739,896 | |
| Long term liabilities | | $ | 8,193,508 | | $ | — | | $ | 2,167,860 | |
SUMMARY OF QUARTERLY RESULTS
| | Three Months Ended
| |
|---|
| | December 31, 2004
| | September 30, 2004
| | June 30,
2004
| | March 31, 2004
| |
|---|
| Net revenue | | $ | 2,986,980 | | $ | 2,250,050 | | $ | 2,146,388 | | $ | 2,192,343 | |
| Net loss | | | (6,293,510 | ) | | (10,935,893 | ) | | (5,402,792 | ) | | (3,509,603 | ) |
| Net loss applicable to common shareholders | | | (6,293,510 | ) | | (10,935,893 | ) | | (5,402,792 | ) | | (3,509,603 | ) |
| Basic and diluted loss per common share | | $ | (0.41 | ) | $ | (0.71 | ) | $ | (0.35 | ) | $ | (0.23 | ) |
| | Three Months Ended
| |
|---|
| | December 31, 2003
| | September 30, 2003
| | June 30,
2003
| | March 31, 2003
| |
|---|
| Net revenue | | $ | 559,704 | | $ | 2,023,170 | | $ | 1,944,702 | | $ | 2,248,231 | |
| Net loss | | | (4,846,928 | ) | | (8,125,804 | ) | | (95,642 | ) | | (1,560,680 | ) |
| Net loss applicable to common shareholders | | | (6,864,152 | ) | | (9,222,682 | ) | | (308,212 | ) | | (1,773,258 | ) |
| Basic and diluted loss per common share | | $ | (0.47 | ) | $ | (2.24 | ) | $ | (0.10 | ) | $ | (0.60 | ) |
C-1-2
RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2004 COMPARED TO THE YEAR ENDED DECEMBER 31, 2003
Consolidated results of operations
| | Year ended December 31, 2004
| | As a % of net revenue
| | December 31, 2003
| | As a % of net revenue
|
|---|
| Revenue | | | | | | | | | | |
| | Gross revenues | | $ | 9,575,761 | | | | $ | 7,883,203 | | |
| | Less: returns | | | — | | | | | (1,107,396 | ) | |
| | |
| | | |
| | |
| | Net revenue | | | 9,575,761 | | 100% | | | 6,775,807 | | 100% |
| Cost of goods sold | | | (7,680,384 | ) | 80% | | | (4,968,898 | ) | 73% |
| | |
| | | |
| | |
| Gross margin | | | 1,895,377 | | 20% | | | 1,806,909 | | 27% |
| Selling, general and administrative | | | (13,363,796 | ) | | | | (7,263,759 | ) | |
| Research and development | | | (5,838,754 | ) | | | | (9,604,080 | ) | |
| Restructuring costs | | | (1,787,129 | ) | | | | (3,356,487 | ) | |
| Amortization of intangibles | | | (515,012 | ) | | | | (1,938,595 | ) | |
| Foreign exchange gain (loss) | | | (308,338 | ) | | | | 10,055,129 | | |
| Investment income | | | 99,427 | | | | | 105,596 | | |
| Loss on disposal of capital assets | | | (46,431 | ) | | | | (27,147 | ) | |
| Interest and financing expenses | | | (6,277,142 | ) | | | | (4,406,620 | ) | |
| | |
| | | |
| | |
| Net loss | | $ | (26,141,798 | ) | | | $ | (14,629,054 | ) | |
| | |
| | | |
| | |
Revenue. The sale of Novacor LVAS implant kits and related peripheral equipment and services accounts for substantially all of WorldHeart's revenues. WorldHeart primarily sells its products directly, except for certain countries, including Japan, where we sell through distributors. Prior to January 1, 2004, WorldHeart sold its products directly in the United States and to our distributor, Edwards Lifesciences LLC ("Edwards"), outside the United States.
The composition of revenue is as follows:
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| |
|---|
| | $
| | Number of Units
| | $
| | Number of Units
| |
|---|
| Gross implant revenue / kits | | 7,055,643 | | 113 | | 5,996,738 | | 118 | |
| Peripherals and other | | 2,520,118 | | | | 1,886,465 | | | |
| | |
| |
| |
| |
| |
| Gross revenue before returns | | 9,575,761 | | 113 | | 7,883,203 | | 118 | |
| Re-purchased Edwards inventory: | | | | | | | | | |
| | — Implant kits | | — | | — | | (602,339 | ) | (20 | ) |
| | — Peripherals and other | | — | | | | (505,057 | ) | | |
| | |
| |
| |
| |
| |
| Net revenue / kits | | 9,575,761 | | 113 | | 6,775,807 | | 98 | |
| | |
| |
| |
| |
| |
Net revenue for the year ended December 31, 2004 increased to approximately $9.6 million representing an increase of 41% over the year ended December 31, 2003. Implant kit net revenue in 2003 includes the return of 20 units, which WorldHeart re-acquired in connection with the amendments to the distribution agreement with Edwards in December 2003. Net implant kits recognized as revenue in 2004 increased by 15% compared to 2003.
Peripherals and other revenues, including Novacor LVAS hardware and peripheral sales and services for the year ended December 31, 2004, were approximately $2.5 million, which represents an increase of 82% over other revenue of approximately $1.4 million (gross revenue of approximately $1.9 million less returns of approximately $505,000) recorded in the year ended December 31, 2003. We anticipate that the proportionate sales may shift
C-1-3
from hardware, peripheral and service sales to implant kit sales as centres enrolled in our RELIANT Trial increase implant volume under the program.
Gross revenue increased to approximately $9.6 million representing an increase of 21% over the year ended December 31, 2003. Novacor LVAS implant kit gross revenue increased to approximately $7.1 million for the year ended December 31, 2004 compared to approximately $6.0 million for the year ended December 31, 2003 representing an increase of 18%. The gross number of implant kits recognized as revenue in the year ended December 31, 2004 was 113 compared to 118 in the year ended December 31, 2003. The revenue per kit increased substantially for sales outside of the United States and Japan due primarily to the shift to selling direct to medical centers rather than through a distributor.
An additional 17 implant kits were shipped and invoiced in the year ended December 31, 2004, however, these kits were deferred as revenue at the time of shipment as the transactions did not meet our revenue recognition criteria. These kits remain in deferred revenue at December 31, 2004 representing approximately $1,020,000 and will be recognized as the earnings process is completed in future periods.
WorldHeart sold 59 implant kits in the United States in 2004 compared to 51 implant kits in 2003. In Europe, Canada and the rest-of-world, excluding Japan, WorldHeart sold 44 implant kits in 2004 compared to 44 in 2003 (64 kits less the return of 20 kits). Japanese sales totaled ten implant kits in 2004 compared to three in 2003.
During the second quarter of 2004, WorldHeart announced its RELIANT Trial and implantation commenced under the RELIANT Trial in the third quarter of 2004. The RELIANT Trial is a randomized 40 center equivalency trial where patients will receive a Novacor LVAS or Thoratec Corporation's Heartmate® XVE LVAS on a 2:1 ratio and where Heartmate is the control arm. At December 31, 2004, WorldHeart has enrolled nine (9) centers under the RELIANT Trial with five (5) implants.
Cost of goods sold. For the year ended December 31, 2004, the overall cost of goods sold increased to 80% of revenue as compared to 73% for the year ended December 31, 2003.
Despite continued efforts to improve per unit manufacturing costs, we continued to experience significant production inefficiencies during 2004 caused by supply shortages due to quality control and production volumes well below capacity. This resulted in higher Novacor LVAS per unit production costs. We expect the cost per unit to decrease as we continue to address the supply issues and increase production.
Gross margin for the year ended December 31, 2004 decreased to 20% of revenues from 27% of revenues for the year ended December 31, 2003. This decrease was due to production inefficiencies, which were partially offset by a shift to direct selling. In addition, certain low margin sales were made to new centers for the initial equipment investment needed to begin a Novacor LVAS implant program at the center. Improvement in gross margin percentage is expected as discounts offered to new centers decrease and as costs per unit decrease from contract manufacturing of some components and production volume increases.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of payroll and related expenses for executives, sales, marketing, accounting and administrative personnel, professional fees, communication expenses, promotional activities, costs associated with meeting multi-jurisdictional regulatory requirements, insurance premiums, and occupancy and other general corporate expenses.
The composition of selling, general and administrative expenses is as follows:
| | Year ended December 31, 2004
| | Year ended December 31, 2003
|
|---|
| Selling | | $ | 7,369,558 | | $ | 4,518,270 |
| General and administrative | | | 5,994,238 | | | 2,745,489 |
| | |
| |
|
| | | $ | 13,363,796 | | $ | 7,263,759 |
| | |
| |
|
C-1-4
Selling, general and administrative expenses for the year ended December 31, 2004 increased by approximately $6.1 million or 84% from the same period in 2003. Selling expenses increased during 2004, as compared to 2003, as WorldHeart assumed responsibility for its direct sales in Europe and increased sales and marketing program expenditures in the United States. The comparative sales expenses for the year ended December 31, 2003 were constrained as a result of our financial position at that time. General and administrative expenses have also increased with the expanded infrastructure required to support a broadened sales responsibility and due to increases in communication expenses, professional fees, insurance costs and multi-jurisdictional regulatory costs. Costs related to the European distribution operations and the related support are fully reflected in the results for the year ended December 31, 2004. In the third quarter of 2004, WorldHeart commenced the consolidation of its North American operations. This is anticipated to result in a decrease in general and administrative expenses in 2005.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing, and configuration of equipment expenses, trial expenses, and regulatory affairs expenses.
Research and development expenses for the year ended December 31, 2004 decreased by approximately $3.8 million or 39% compared to year ended December 31, 2003. Gross research and development expenditures excluding the impact of government programs decreased by 42% to approximately $6.0 million in 2004 compared to approximately $10.3 million in 2003.
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| |
|---|
| Gross research and development expenses | | $ | 5,975,344 | | $ | 10,299,324 | |
| Investment tax credits | | | (136,590 | ) | | (695,244 | ) |
| | |
| |
| |
| Net research and development expenses | | $ | 5,838,754 | | $ | 9,604,080 | |
| | |
| |
| |
Lower research and development costs for year ended December 31, 2004 compared to the same period in 2003 reflect expense reductions associated with the merged next-generation VAD development program and reduced pre-clinical and clinical trial expenses. The cost reduction programs were completed in fiscal year 2003 and the expense reductions were realized in 2004. Development work on the next-generation pulsatile VAD will continue with initial clinical trials expected to begin in 2007.
Investment tax credits for the year ended December 31, 2004 totaled approximately $137,000 and relate to a Canadian provincial government investment tax credit program. A credit of approximately $2.3 million was recorded against research and development expense for the year ended December 31, 2003 relating to a Canadian federal tax credit program. However, in the same period a provision of approximately $1.7 million was recorded against research and development expense relating to a provincial tax credit program claim that was recorded in a prior period but was, and continues to be, under dispute. The dispute relates to a tax credit recorded as receivable by WorldHeart in the year ended December 31, 2001 related to the Ontario Business Research Institute tax credit ("OBRITC") program in the amount of $2,304,492 that was associated with research payments made by WorldHeart to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation from 1997 to 2000. Following the completion of a provincial audit of the claim and proposed amendments by the province to the OBRITC legislation, WorldHeart received payment of $597,538 of this claim during the year ended December 31, 2003. The Ministry has advised WorldHeart that it believes that additional amounts claimed are ineligible and that it would not be making any further payments. We are disputing the Ministry's decision, but due to the uncertainty of the ultimate resolution of the claim, we recorded a provision for the remaining receivable of $1,706,954 in 2003. WorldHeart continues to review various tax credit programs but only records a credit when collection of the claim is reasonably assured.
Restructuring costs. On August 25, 2004, we approved a plan to consolidate the Ottawa, Ontario operations into our facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve operational efficiency and effectiveness.
C-1-5
WorldHeart expects the restructuring to be completed by the end of June 2005 and expects to incur total restructuring charges of approximately $2.6 million comprised as follows:
| | 2004
|
|---|
| | Total estimated restructuring costs
| | Actual costs incurred to December 31
| | Costs paid or settled in the year
| | Balance in accrued liabilities as at December 31
|
|---|
| Employee severance costs | | $ | 1,509,192 | | $ | 1,396,037 | | $ | 795,923 | | $ | 600,114 |
| Contract settlement costs | | | 719,894 | | | 57,653 | | | — | | | 57,653 |
| Write down of capital assets | | | 338,595 | | | 338,595 | | | 338,595 | | | — |
| Other costs | | | 44,083 | | | 22,509 | | | 8,624 | | | 13,885 |
| | |
| |
| |
| |
|
| | | $ | 2,611,764 | | $ | 1,814,794 | | $ | 1,143,142 | | $ | 671,652 |
| | |
| |
| |
| |
|
During the year ended December 31, 2004, actual restructuring costs of approximately $1.8 million have been recorded. Employee severance costs represent the amount employees have earned to December 31, 2004. Contract settlement costs are recorded when notice is provided that the contract is being terminated and WorldHeart ceases to use the right conveyed under the contract. The capital assets were written down to their net realizable value when the decision was made to consolidate the North American operations and it was determined that the carrying value of the capital assets was impaired. Other restructuring costs are recognized when the services are actually received.
During the year ended December 31, 2003, WorldHeart realigned its business operations to focus on Novacor LVAS commercial operations and to reduce research and development costs by merging our three next-generation VAD programs. Total restructuring costs for the year ended December 31, 2003 amounted
to approximately $3.4 million. The restructuring costs related to employee severance and termination
costs were approximately $1.6 million; costs related to the buyout of a Novacor LVAS royalty agreement were approximately $1.0 million; costs related to the excess of inventory purchase cost over the carrying value of the inventory re-purchased from Edwards were approximately $770,000; and other miscellaneous costs were approximately $13,000. The royalty buy-out eliminated royalty payment obligations going forward of 4% of Novacor LVAS sales. Approximately $2.2 million of the 2003 restructuring costs were paid in 2003 with approximately $1.2 million being paid in 2004. The difference of approximately $28,000 was adjusted in 2004 to reflect revised estimates for employee severance and termination costs.
Amortization of intangibles. Amortization of intangibles for the year ended December 31, 2004 was approximately $515,000 compared to approximately $1.9 million for the same period in 2003. The majority of the intangible assets arose from the purchase of the Novacor business in June 2000. Most of these assets have now reached the end of their original estimated useful life and have been fully amortized. As at December 31, 2004, intangible assets totaling approximately $255,000 remain unamortized.
Foreign exchange gains and losses. During the year ended December 31, 2004, a foreign exchange loss of approximately $308,000 was recorded compared to a gain of approximately $10.0 million for the year ended December 31, 2003. The 2004 foreign exchange loss relates to the effect of a strengthening U.S. dollar compared to the Canadian dollar during the first half of the year on net monetary assets, predominately cash and cash equivalents and short-term investments, less current liabilities, denominated in Canadian dollars. The majority of the 2003 foreign exchange gain can be attributed to the impact of a strengthening Canadian dollar on WorldHeart's U.S. dollar denominated preferred shares. From WorldHeart's inception through the end of 2003, its functional currency was the Canadian dollar.
Investment income. Investment income primarily represents interest earned on cash equivalents and short-term investments. During the year ended December 31, 2004, investment income of approximately $99,000 was recorded compared to income of approximately $106,000 for the year ended December 31, 2003. The decrease is due to having, on average over the course of 2004, less surplus cash to invest as compared to 2003.
Interest expense and financing costs. During the year ended December 31, 2004, interest and financing costs of approximately $6.3 million were recorded compared to costs of approximately $4.4 million during the year
C-1-6
ended December 31, 2003. Interest expense and financing costs in 2004 consisted mainly of the non-cash charge of the beneficial conversion feature of the convertible debentures issued on September 15, 2004, totaling approximately $5.8 million and the corresponding interest related to the convertible debentures. These non-cash interest charges will continue to accrete ratably on the convertible debentures until their conversion or maturity. It is anticipated that all outstanding debentures will be converted in accordance with the terms of the MedQuest acquisition. Interest expense and financing costs for the year ended December 31, 2003 consisted of interest on loan obligations that were outstanding during the year as well as the costs of entering into the loan obligations. In September 2003, the loans were repaid in full.
RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2003 COMPARED TO THE YEAR ENDED DECEMBER 31, 2002
Consolidated results of operations
| | Year ended December 31, 2003
| | As a % of net revenue
| | Year ended December 31, 2002
| | As a % of net revenue
|
|---|
| Revenue | | | | | | | | | | |
| | Gross revenues | | $ | 7,883,203 | | | | $ | 6,855,458 | | |
| | Less: Sales returns | | | (1,107,396 | ) | | | | — | | |
| | Edwards guarantee fee | | | — | | | | | (418,762 | ) | |
| | |
| | | |
| | |
| | Net revenue | | | 6,775,807 | | 100% | | | 6,436,696 | | 100% |
| Cost of goods sold | | | (4,968,898 | ) | 73% | | | (6,284,996 | ) | 98% |
| | |
| |
| |
| |
|
| Gross margin | | | 1,806,909 | | 27% | | | 151,700 | | 2% |
Selling, general and administrative |
|
|
(7,263,759 |
) |
|
|
|
(6,686,437 |
) |
|
| Research and development | | | (9,604,080 | ) | | | | (15,931,912 | ) | |
| Restructuring costs | | | (3,356,487 | ) | | | | — | | |
| Amortization of intangibles | | | (1,938,595 | ) | | | | (2,603,311 | ) | |
| Foreign exchange gain | | | 10,055,129 | | | | | 509,208 | | |
| Investment income | | | 105,596 | | | | | 117,589 | | |
| Loss on disposal of capital assets | | | (27,147 | ) | | | | (32,087 | ) | |
| Interest and financing expenses | | | (4,406,620 | ) | | | | (77,423 | ) | |
| | |
| | | |
| | |
| Net loss | | $ | (14,629,054 | ) | | | $ | (24,552,673 | ) | |
| | |
| | | |
| | |
Revenue. The sale of Novacor LVAS Implant Kits and related peripheral equipment and services accounts for substantially all of WorldHeart's revenues. For the years presented above, WorldHeart sold its products directly in the United States and to its distributor, Edwards, outside the United States.
The composition of revenue is as follows:
| | Year ended December 31, 2003
| | Year ended December 31, 2002
|
|---|
| | $
| | Number of Units
| | $
| | Number of Units
|
|---|
| Gross implant revenue / kits | | 5,996,738 | | 118 | | 5,243,915 | | 104 |
| Peripherals and other | | 1,886,465 | | | | 1,611,543 | | |
| | |
| |
| |
| |
|
| Gross revenue before returns and fees | | 7,883,203 | | 118 | | 6,855,458 | | 104 |
| Re-purchased Edwards inventory: | | | | | | | | |
| — Implant kits | | (602,339 | ) | (20 | ) | — | | — |
| — Peripherals and other | | (505,057 | ) | | | — | | |
| Edwards guarantee fee | | — | | | | (418,762 | ) | |
| | |
| |
| |
| |
|
| Net revenue / kits | | 6,775,807 | | 98 | | 6,436,696 | | 104 |
| | |
| |
| |
| |
|
C-1-7
Gross revenues from sales of the Novacor LVAS and related equipment (before returns and the Edwards guarantee) increased 15% from approximately $6.9 million in the year ended December 31, 2002 to approximately $7.9 million in the year ended December 31, 2003. At December 31, 2003, WorldHeart purchased Novacor inventory and other assets from Edwards as part of the amendments to the distribution agreement. The purchase of the inventory was accounted for as a sales return which reduced 2003 revenue by approximately $1.1 million
In 2003 the gross number of kits shipped for revenue increased by 13% to 118 kits compared to 104 kits in 2002. The net sale of implant kits decreased to 98 kits in 2003 from 104 kits in 2002 due to 20 kits that were treated as returns when WorldHeart re-acquired inventory associated with the amendments to the distribution agreement with Edwards in December 2003.
In the United States, where WorldHeart sold directly, selling prices were significantly higher than non-U.S. sales where we sold to our distributor Edwards. WorldHeart sold 51 implant kits in the United States in 2003 compared to 39 implant kits in 2002. On December 31, 2003, WorldHeart amended its distribution agreement with Edwards in order to sell directly in the rest of the world with the exception of Japan where Edwards will remain the sole distributor. For future periods, selling prices outside of the United States, except Japan, will increase to reflect end-user pricing. In Europe, Canada and the rest-of-world, excluding Japan, WorldHeart sold 64 implant kits in 2003 compared to 57 in 2002. Japanese sales totaled three (3) implant kits in 2003 compared to eight (8) in 2002.
In its distribution agreement with Edwards, WorldHeart was required to pay a minimum gross margin guarantee in the event that Edwards' gross margin generated on the sales of WorldHeart products were below $2 million annually. The guarantee shortfall was accounted for as a reduction of revenues. Edwards' sales of WorldHeart's products were sufficient during 2003 to meet the minimum guarantee requirement; therefore, no guarantee payment was required. The guarantee was terminated effective January 1, 2004 as part of the amendment to the Edwards distribution agreement.
Cost of goods sold. Cost of goods sold decreased as a percentage of net revenue from 98% in 2002 to 73% in 2003.
The decrease as a percent of revenue is partially the result of a higher proportion of product being sold in the U.S. where higher margins are earned as compared to Europe and the rest of the world in 2003 versus 2002, the commencement of a cost reduction program for cost of sales in the fourth quarter of 2003, as well as managed reductions in indirect manufacturing support costs and direct manufacturing overheads.
Correspondingly, gross margins in 2003 increased to 27% of revenues from 2% of net revenues in 2002.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of payroll and related expenses for executives, sales, marketing, accounting and administrative personnel, professional fees, communication expenses, promotional activities, costs associated with meeting multi-jurisdictional regulatory requirements, insurance premiums, occupancy and other general corporate expenses.
The composition of selling, general and administrative expenses is as follows:
| | Year ended December 31, 2003
| | Year ended December 31, 2002
|
|---|
| Selling | | $ | 4,518,270 | | $ | 4,194,405 |
| General and administrative | | | 2,745,489 | | | 2,492,032 |
| | |
| |
|
| | | $ | 7,263,759 | | $ | 6,686,437 |
| | |
| |
|
Selling, general and administrative expenses for year ended December 31, 2003 increased by approximately $577,000 or 9% from the same period in 2002. Actual expenses remained fairly constant year over year, however, due to the average exchange rates applied upon the change in our reporting currency, the reported U.S. dollar values show an increase in expenses. The average exchange rate for 2003 increased approximately 12% over the average exchange rate for 2002.
C-1-8
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing, and configuration of equipment, trial expenses, regulatory affairs and quality control with respect to prototype development.
Gross research and development expenses, excluding the impact of government assistance, decreased by approximately $8.5 million or 45% to approximately $10.3 million in 2003 as compared to approximately $18.8 million in 2002. Net research and development costs for 2003 were approximately $6.3 million or 40%, lower than 2002.
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Gross research and development expenses | | $ | 10,299,324 | | $ | 18,776,949 | |
| Investment tax credits | | | (695,244 | ) | | (2,845,037 | ) |
| | |
| |
| |
| Net research and development expenses | | $ | 9,604,080 | | $ | 15,931,912 | |
| | |
| |
| |
Lower research and development costs in 2003 compared to 2002 are due to year-over-year expense reductions associated with the merged next-generation VAD development programs and reduced pre-clinical and clinical trial activity compared to 2002. WorldHeart continued to reduce expenditures predominantly due to a consolidation of our next generation VAD development programs that were commenced late in 2002, which resulted in a decelerated investment in research and development throughout 2003.
WorldHeart continued implants under the feasibility phase of the Novacor LVAS clinical trial (INTrEPID) throughout all of 2002 and into the first quarter of 2003. In addition, the next generation pre-clinical trials were terminated in the third quarter of 2002 and there was no next generation trial activity in 2003.
During 2002, the consolidated next-generation VAD program and change in the scope necessitated an amendment to the federal government Technology Partners Canada ("TPC") contribution agreement. On May 21, 2003, TPC approved this amendment allowing WorldHeart to claim eligible expenditures for reimbursement against the funding available under the $6.6 million contribution agreement. As part of the amendment to the agreement, the royalty rate payable to TPC was reduced to 0.65% and is to be calculated on the consolidated revenues of WorldHeart commencing January 1, 2004.
WorldHeart had previously recorded as a credit against research and development expense a tax credit receivable in the amount of approximately $2.3 million related to the provincial Ontario Business Research Institute tax credit ("OBRITC") program. The claim was associated with research payments made to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation ("CVD") from 1997 to 2000. Following the completion of the provincial Ministry of Finance's audit of the claim and proposed amendments to the OBRITC legislation, a payment of approximately $598,000 was received during the year ended December 31, 2003. Subsequent to receipt of this payment, WorldHeart was advised by the Ministry that it was seeking repayment of the amount received and would not be making further payments. We are disputing the Ministry's decision, but due to the uncertainty of the ultimate resolution of the claim, we recorded a provisions for the remaining receivable of $1,706,954 in 2003.
Restructuring costs. On September 23, 2003, WorldHeart realigned its business operations to focus on commercial operations and to reduce research and development costs related to our next generation VAD.
WorldHeart restructured its worldwide distribution agreement with Edwards to allow for better control over direct sales, marketing and distribution for all territories, with the exception of Japan. WorldHeart also purchased certain inventory and capital assets from Edwards in Europe and commenced direct sales activities effective January 1, 2004. Edwards agreed to provide certain transitional services until WorldHeart established the appropriate infrastructure to support direct sales in Europe. Early in 2004, World Heart B.V., a wholly owned subsidiary, was incorporated with a head office in Heesch, Netherlands, and the European infrastructure was established in-house in the first half of 2004.
We also refocused our efforts to reduce manufacturing costs associated with the production of the Novacor LVAS. We continue to evolve our processes to move towards more cost-effective manufacturing processes
C-1-9
expected to lead to reduced costs for inventory and cost of goods sold. We also reduced the rate of investment associated with the development of our next-generation VAD.
As part of these initiatives 37 positions were eliminated, of which 24 related to research and development, nine related to general and administration and four related to manufacturing.
Total restructuring costs incurred for the year ended December 31, 2003 were approximately $3.4 million and related to the following:
- •
- employee severance and termination costs of approximately $1.6 million;
- •
- costs related to the excess of inventory purchase cost over carrying value of the inventory re-purchased from Edwards amounting to approximately $770,000;
- •
- costs related to the buyout of a Novacor LVAS royalty agreement of approximately $1.0 million; and,
- •
- other miscellaneous costs amounting to approximately $13,000.
As at December 31, 2003, approximately $2.2 million of the total restructuring costs had been paid with the remainder of approximately $1.2 million included in accounts payable and accrued liabilities all of which except approximately $28,000 was paid in the year ended December 31, 2004. The $28,000 was determined to be an overestimate of accrued salaries and was credited to the 2004 expense.
Amortization of intangibles. Amortization of intangibles for 2003 amounted to approximately $1.9 million compared to approximately $2.6 million for 2002. The majority of the intangible assets arose from the purchase of the Novacor business in June 2000. The decrease in amortization is due to the majority of these assets reaching the end of the original estimated useful life during the year. As at December 31, 2003, intangible assets of approximately $879,000 remain unamortized
Foreign exchange gains. A foreign exchange gain of approximately $10.0 million was recorded in 2003 compared to a gain of approximately $509,000 in 2002. Certain balance sheet items are denominated in Canadian dollars. Foreign exchange gains and losses arose when the value of the U.S. dollar changed relative to the value of the Canadian dollar. The majority of the foreign exchange gain can be attributed to the impact of a strengthening Canadian dollar on WorldHeart's U.S. dollar denominated preferred shares. From WorldHeart's inception through the end of 2003, our functional currency was the Canadian dollar.
Investment income. Investment income primarily represents interest earned on cash equivalents and short-term investments. Investment income decreased to approximately $106,000 in 2003 from approximately $118,000 in 2002. The decrease relates to having a larger sum of excess cash for a shorter period of time in 2003. WorldHeart generated substantially all of its investment income in the fourth quarter of 2003 on excess funds received upon completion of the private placement late in the third quarter of 2003.
Interest expense and financing costs. Interest expense and financing costs consist mainly the costs associated with debt financing transactions entered into during the first nine months of 2003.
Interest and financing expenses in 2003 totaled approximately $4.4 million compared to approximately $77,000 in 2002. The interest, fees and other costs were associated with loans entered into in 2003 to allow WorldHeart to meet its cash requirements until an equity transaction could be completed.
C-1-10
LIQUIDITY AND CAPITAL RESOURCES
Historically, WorldHeart has funded losses from operations through the sale of equity and issuance of debt instruments. Combined with revenues, these funds have provided us with the resources to operate our business, attract and retain key personnel, fund our research and development program and clinical trials, apply and obtain the necessary regulatory approvals and develop our technology and products.
Liquidity. At December 31, 2004, WorldHeart had cash, cash equivalents and short-term investments of approximately $8.8 million and net working capital of approximately $14.1 million compared to cash, cash equivalents and short-term investments of approximately $18.1 million and net working capital of approximately $18.3 million at December 31, 2003.
Cash, cash equivalents and short-term investments decreased in 2004 by approximately $9.3 million. This primarily related to cash used in investing activities of approximately $585,000 for the purchase of capital assets and cash pledged as collateral for a lease; an operating cash net use of approximately $22.9 million including a net pay down of approximately $3.9 million of accounts payable and accrued compensation and an increase in inventory of approximately $2.2 million; offset by the net proceeds received on the issuance of convertible debentures and warrants for approximately $12.9 million; and approximately $1.5 million received on the issuance of common shares through the exercise of warrants.
During the year, WorldHeart completed a convertible debenture and warrant issuance for gross proceeds of approximately $13.3 million.
Subsequent to year end, on January 31, 2005, WorldHeart announced that it had entered in to a definitive agreement to acquire MedQuest, subject to certain approvals, consents, conditions and the approval of the shareholders. MedQuest, based in Salt Lake City, Utah, is in the final development stages of its HeartQuest™ ventricular assist device, a magnetically levitated centrifugal rotary blood pump. As separate components of that transaction, WorldHeart also announced that it had definitive agreements to issue through a private placement, 8.9 million common shares, at $1.35 per share, for gross proceeds of $12 million and raise approximately $10.7 million through the exercise of 10.7 million existing warrants at an exercise price of $1.00 per warrant. These transactions are expected to be completed contemporaneously with the MedQuest acquisition in the second quarter of 2005. Upon completion of the private placement and warrant exercise it is anticipated that WorldHeart will receive approximately $22.7 million of cash and extinguish all of its convertible debentures providing adequate resources to fund operations into 2006 when we may be required to obtain additional funding to sustain operations.
Our long-term working capital and capital requirements will depend upon numerous factors, including the following: our continued focus on improving commercial operations and viability through the expanded sales of Novacor LVAS; increasing revenues through higher average selling prices and enrollment in the RELIANT Trial (Randomized Evaluation of the Novacor LVAS in a Non-Transplant Population); decreasing production costs through a manufacturing cost reduction program; the rate of investment in our next-generation technologies, including our under-development pulsatile VAD and the rotary VAD expected to be acquired in the second quarter of 2005; the costs associated with clinical trials and the approval process for the next-generation technologies; and our general efforts to improve operational efficiency and effectiveness.
OUTLOOK
External environment. The market for effective pharmacological, electro-physiological or device treatments for late-stage heart failure continues to grow, and despite significant progress on many fronts, no competitive breakthroughs have been announced or are believed to be imminent. The number of diagnosed heart failure patients is increasing as a result of both population demographic trends, and also as a response to the increasing number of survivors from sudden cardiac events, many of whom subsequently develop congestive heart failure. We believe the potential market for our heart assist devices, with their current regulatory approvals, continues to be at least 100,000 patients per annum in North America, Europe, Japan and certain other countries.
There is growing evidence of increasing clinical acceptance of the use of mechanical circulatory assist therapies in the treatment of late stage heart failure, and on two separate occasions in March 2003 and
C-1-11
May 2004, the Centers for Medicare and Medicaid Services in the United States announced the extension of reimbursement coverage for both bridge and destination therapy VAD use. These extensions have resulted in increased payments for procedures using such devices. These developments are expected to continue to accelerate the use of ventricular assist devices both in the United States and elsewhere. Also, effective April 1, 2004, reimbursement in Japan for implantable VADs was approved. Novacor LVAS is the only approved device of its kind in Japan.
In the United States, WorldHeart commenced a randomized equivalency trial called RELIANT in the second quarter of 2004. In this trial the Novacor LVAS is being randomized to the Thoratec HeartMate® XVE LVAS, which will be the control arm. RELIANT is a randomized 40 center equivalency trial where patients will receive a Novacor LVAS or Thoratec Corporation's Heartmate®XVE LVAS on a 2:1 ratio. It is expected that data from this trial will support a Premarket Approval Supplement, which will be submitted to the U.S. Food and Drug Administration for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy).
We continue to believe that the long-term use or Destination Therapy market will evolve rapidly when devices are accepted as reliable and durable. A wider adoption of VADs as a Destination Therapy will likely occur with the next-generation VADs and we believe that late-stage heart failure will be best served by a combination of pulsatile and rotary flow VADs.
Internal environment. We expects to continue to incur net operating losses on a quarterly basis, through 2006, assuming the completion of the MedQuest acquisition, which is expected to be completed in the second quarter of 2005. We believe that our commercial operations associated with Novacor LVAS will continue to improve. We expect to continue to increase revenues as a result of higher Novacor LVAS Implant Kit sales, particularly in the United States. Enrollment in the RELIANT Trial commenced in the second quarter of 2004 and implantation under the RELIANT Trial commenced in the third quarter of 2004 and is expected to continue through 2005 and 2006. We also expect gross margin contribution to increase through a combination of higher volumes which should lead to lower per unit manufacturing costs through more efficient and optimal manufacturing levels and continuation of our manufacturing cost reduction program.
In addition, in the third quarter of 2004, a plan was put in place to consolidate the Ottawa operations into our Oakland operations to further improve operating cost structure, increase efficiency and improve effectiveness.
WorldHeart expects to complete the acquisition of MedQuest in the second quarter of 2005 and commence an initial feasibility clinical trial of its magnetically levitated centrifugal rotary VAD by the beginning of 2006. Initial commercial approvals for the VAD are expected to occur in Europe 2007 with approvals for the VAD in the United States two years later.
Development work on the Novacor II pulsatile VAD will continue with initial clinical trials in 2007, and initial commercial approvals in Europe in 2008 and in the United States in 2010.
Commitments
(a) Contractual Obligations
The following table sets forth WorldHeart's contractual obligations, excluding MedQuest commitments, as of December 31, 2004:
| | Total
| | Less than 1 Year
| | 1-3 Years
| | 3-5 Years
|
|---|
| Operating lease obligations | | $ | 3,242,720 | | $ | 1,440,597 | | $ | 1,762,450 | | $ | 39,673 |
| Long-term debt obligations | | | 15,316,563 | | | — | | | — | | | 15,316,563 |
| Capital lease obligations | | | — | | | — | | | — | | | — |
| Purchase obligations | | | — | | | — | | | — | | | — |
| Other long-term liabilities reflected on the balance sheet under GAAP | | $ | 16,368 | | $ | — | | $ | 16,368 | | $ | — |
C-1-12
(b) Operating Leases
During 2003, $222,003 was drawn against the cash amount pledged as collateral for obligations under a premises lease for the Oakland, California operations. In 2004, the balance was restored to the original pledged amount of $750,000. The cash remains pledged against a letter of credit issued by WorldHeart in support of its obligations and is not available for general operations.
(c) Novacor LVAS Royalties
WorldHeart is committed, under the Novacor LVAS royalty agreement to royalty payments to certain employees. Royalties are payable on annual consolidated gross revenues at a rate of 0.808% up to a maximum of $3,232,000. Royalty payments to date total $1,027,184, included in accounts payable and accrued liabilities at December 31, 2004 is $95,646 (December 31, 2003 — $37,478).
(d) Technology Partnerships Canada Contribution Agreement
During 2002, WorldHeart entered into a shared funding program with Technology Partnerships Canada ("TPC") under which the Canadian government shares costs of certain research and development activities. Funding in the amount of approximately $6,624,000 was claimed. Effective January 1, 2004, repayment is in the form of royalties payable on annual consolidated gross revenues at a rate of 0.65% for a 9 year period ended December 31, 2012. If during this period royalty payments reach the maximum of $20,250,000 no further repayments will be required. If the royalty payments do not exceed $20,250,000 during this period they will continue until 2015 or until the maximum is reached.
CAPITAL EXPENDITURES
| | 2004
| | 2003
| | 2002
|
|---|
| Capital Expenditures | | $ | 363,224 | | $ | 150,135 | | $ | 388,611 |
| | |
| |
| |
|
Capital expenditures for 2004 decreased substantially from 2003 as WorldHeart reduced costs and expenditures. WorldHeart anticipates that capital expenditures for 2005 will be similar to 2004.
At December 31, 2004, WorldHeart occupied three locations. The Ottawa location comprises 22,755 square feet of manufacturing and office space with a lease that expires on December 31, 2006. The Ottawa location is currently being shut down as part of the restructuring plan approved in August 2004. The second location is in Oakland with two buildings consisting of approximately 40,000 square feet of manufacturing and office space. The Oakland leases were renewed in 2002 for a five-year term expiring on April 30, 2007. The third location is in Heesch, Netherlands with approximately 2,500 square feet of warehouse and office space. The Heesch lease was signed in 2004 for a three-year term expiring on December 31, 2007.
MARKET RISK
WorldHeart is subject to investment risk on investments that it makes with excess cash.
Investment risk is mitigated by close adherence to an established investment policy, which has been approved by the Board of Directors. The policy sets liquidity criteria, and counter party risk diversification criteria and restricts investments to investment grade quality instruments of AA or better or R1 medium or better in the case of commercial paper. There exists modest income exposure to a decline in interest rates. This is not significant due to the short terms to maturity of the instruments held and the already low current interest rates.
WorldHeart has assets and liabilities denominated in foreign currencies, including the Canadian dollar and the Euro. Occasionally, WorldHeart enters into foreign exchange contracts in order to match its foreign exchange requirements. At December 31, 2004, there were no outstanding foreign exchange forward contracts. WorldHeart generally attempts to match its cash balances to its expected net cash outflow for a particular currency during the year.
C-1-13
CRITICAL ACCOUNTING ESTIMATES
We have identified the following policies as critical to our business and the understanding of our results of operations. For a more detailed discussion on the application of these and other accounting policies, see the notes to the audited consolidated financial statements.
Revenue recognition
Revenue from product sales is recognized when all of the following criteria are met; persuasive evidence of an agreement exists; delivery has occurred or services have been rendered; the price is fixed and determinable; and collection is reasonably assured. The Corporation derives revenue from the sale of implant kits and peripheral equipment and training for implant centres.
Revenue from the sale of implant kits and peripheral equipment is generally recognized when such products are shipped. Where the terms of sale provide customers with rights of return or other guarantees, or where collection is uncertain, revenue is deferred until such time as the rights of return or guarantee expire or, where collection is uncertain, payment is received.
Training revenue is recognized once the training is provided.
Sales to new implant centers typically include implant kits, related peripheral equipment and training. For these multiple element sales, the Corporation allocates the total contract value to the elements based on their respective fair values. Revenue is recognized from each element in accordance with the criteria set out above. Revenue is deferred for any undelivered element when the undelivered element is not considered essential to the functionality of the delivered elements. If undelivered elements are essential to the functionality of the delivered elements, the entire contract value is deferred. For most customers, the training component is not considered essential to the use of or the functionality of the implant kits, as the customers already possess sufficient expertise and experience to perform implants.
The Corporation's implantable products and related peripheral equipment are covered by limited warranties. Warranty costs, which are based on historical experience, are estimated and recorded when the related revenue is recognized. Actual warranty costs are charged against the warranty provision as incurred.
Inventory valuation
WorldHeart will write-down from time to time certain inventory that it considers obsolete or excess based on its expected demand. To the extent inventory movement or product demand is not as anticipated, the write-down may be higher or lower than the usage that is ultimately experienced. Once written down, the inventory is not written back up.
Valuation of goodwill
In accordance with SFAS No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets", WorldHeart reviews the carrying value of long-lived assets including goodwill whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Such events or circumstances might include a significant decline in market share, a significant decline in profits, changes in technology, significant litigation or other items.
In evaluating whether there is an impairment of goodwill, management compares the carrying amount of goodwill with its fair value as required by SFAS No. 142. Because WorldHeart operates in one business segment and all of its goodwill was derived from the purchase of that same business, we are able to determine the value of the reporting unit containing the goodwill by using the trading value of the outstanding common shares and the number of outstanding shares at year-end. We determined the fair value of the goodwill by subtracting the fair value of all of the Corporation's assets and liabilities, excluding goodwill, from the value of the reporting unit. An impairment is recognized to the extent that the fair value of the Goodwill is less than its carrying amount. The enterprise value determined on December 31, 2004 was sufficient to support the carrying value of goodwill.
C-1-14
In accordance with SFAS No. 142, "Goodwill and Other Intangible Assets", WorldHeart completes on an annual basis an impairment test of goodwill. Upon completion of the impairment test as of the end of the fiscal 2004, it was determined that goodwill is not impaired.
Restructuring
In August of 2004, a restructuring plan was implemented to consolidate the North American operations, which included a reduction in workforce, lease and contract termination costs, a write down of capital assets to their net realizable value and other costs was implemented. At each reporting date, WorldHeart evaluates the accruals, which are based on estimates, to ensure that they are still appropriate. Included in accruals as of December 31, 2003, were approximately $600,000 related to employee severance costs, approximately $58,000 related to contract termination costs and approximately $14,000 related to other costs. In certain instances, it may be determined that these accruals are no longer required because of efficiencies in carrying out the restructuring plan. In these cases, WorldHeart would reverse any related accrual to income when it is determined it is no longer required. Alternatively, in certain circumstances, it may be determined that certain accruals are insufficient as new events occur or as additional information is obtained. In these cases, WorldHeart would increase the applicable existing accrual with the offset recorded as a restructuring charge.
OTHER FACTORS
Current Product. WorldHeart's near-term commercial operations are exclusively focused on its Novacor LVAS Implant Kit and related equipment and support. Commercial success will therefore be dependant on WorldHearts ability to continue to increase Novacor LVAS sales. Novacor LVAS is approved for use by eligible heart failure patients without limitation, in Europe and Japan and as a bridge-to-transplant device in the United States and Canada. WorldHeart has commenced an equivalency clinical trial in the second quarter of 2004 in the United States called RELIANT in which patients will be randomized to receive either the Novacor LVAS or the Thoratec HeartMate® XVE LVAS. It is expected that data from this clinical trial will support a Premarket Approval Supplement, which will be submitted to the U.S. Food and Drug Administration, for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy). Implants under this trial are eligible for reimbursement by the Centers for Medicare and Medicaid Services ("CMS") and certain private insurers. We believe that approval of the Novacor LVAS for long-term or destination therapy use in the United States would increase the market for the Novacor LVAS substantially.
Pulsatile VADs. WorldHeart has several competitors with commercially approved pulsatile VADs having pumps that are externally located or abdominally implanted. The devices which are developed by the Corporation's competitors are now primarily used as bridges to transplant, although one product (Thoratec HeartMate® XVE) was approved in 2002 for a destination therapy indication for use in the United States. Some of WorldHeart's existing known competitors have significantly greater financial, production and marketing resources. There can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
WorldHeart believes that our pulsatile next-generation implantable VAD is the only pulsatile device that is currently at an advanced stage of development. During 2002 management merged the three next-generation VAD development programs into one VAD program. WorldHeart expects clinical trials for our next-generation implantable pulsatile VAD will commence in 2007. However, there can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
Non-pulsatile VADs. As discussed above, subsequent to year-end WorldHeart entered into a definitive agreement to purchase the assets of MedQuest including its rotary flow, centrifugal, magnetically levitated next generation VAD technology. WorldHeart expects clinical trials for our next-generation implantable non-pulsatile VAD will commence in early 2006. However, there can be no assurance that the development program, including pre-clinical and human clinical trials, will be successful or that the necessary regulatory approvals will be received.
C-1-15
Research and development by WorldHeart's competitors is proceeding for several next-generation non-pulsatile continuous flow assist devices. Certain of these devices have received CE Mark in Europe and are in clinical trials in the United States and Europe. It has not been determined whether these non-pulsatile devices will be suitable for long-term use.
Novacor LVAS Sales. Sales of the Novacor LVAS contribute to overhead and other indirect costs of producing the Novacor LVAS product. To date, however, at current volumes the Novacor commercial operations have contributed only modest gross margin or have resulted in gross margin deficits. WorldHeart expects to continue to increase gross margin through increased sales and reduced manufacturing costs, however, to the extent that it is unable to do so, there is risk that WorldHeart could be adversely affected and the Novacor operations could continue to consume cash resources.
ACCOUNTING POLICIES
In previous years WorldHeart presented its consolidated financial statements in accordance with Canadian GAAP. However, WorldHeart changed its presentation to U.S. GAAP at December 31, 2004. Significant differences between Canadian GAAP and U.S. GAAP are presented in Note 22 to the audited consolidated financial statements.
OUTSTANDING SHARE DATA
The outstanding share data as at December 31, 2004, is a follows:
| | Number outstanding
|
|---|
| Common shares | | 15,744,522 |
| Options | | 3,172,771 |
| Warrants | | 24,424,233 |
| Convertible debentures | | 10,655,000 |
| Interest on convertible debentures | | 1,997,813 |
Included in options above are 2,383,725 options subject to shareholder approval of an increase in the number of options available under the employee stock option plan.
A conversion rate of $1.00 per common share was assumed for the interest on the convertible debentures. Interest accrues on the face value of the convertible debentures at an annual non-compounding rate of 3%.
NEW ACCOUNTING PRONOUNCEMENTS
In June 2001, the FASB issued SFAS No. 148, which amended the transitional provisions of SFAS No. 123 for companies electing to recognize employee stock-based compensation using the fair value based method. The provisions of FASB No. 148 will be effective for years beginning on or after January 1, 2006. In addition, during 2004, the FASB issued SFAS No. 123R, which also amended the provisions of SFAS No. 123. The provisions of FASB No. 123R will be effective for years beginning on or after January 1, 2006. The Corporation plans on adopting these new pronouncements in 2006, the impact of which is not known at this time.
In January 2005, the Canadian Institute of Chartered Accountants ("CICA") released Section 1531, "Comprehensive Income" ("Section 1531"), which requires financial instruments to be measured at fair value. The provisions of Section 1531 are effective for years starting on or after October 31, 2006. The Corporation plans on adopting this new pronouncement in 2007, the impact of which is not known at this time.
C-1-16
APPENDIX C-2
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
INTRODUCTION
World Heart Corporation and its subsidiaries are collectively referred to as "WorldHeart". The following Management Discussion and Analysis of Financial Condition and Results of Operations ("MD&A") was prepared by management as of March 21, 2005, was approved by the Board of Directors and discusses material changes in WorldHeart's financial condition and results of operations and cash flows for the years ended December 31, 2004, 2003 and 2002. Such discussion and comments on the liquidity and capital resources should be read in conjunction with the information contained in the accompanying audited consolidated financial statements prepared in accordance with Canadian generally accepted accounting principles ("Canadian GAAP"). In this discussion, all amounts are in United States dollars ("U.S. dollars") unless otherwise stated.
The discussion and comments contained hereunder include both historical information and forward-looking information. The forward-looking information, which generally is information stated to be anticipated, expected, or projected by management, involves known and unknown risks, uncertainties and other factors that may cause the actual results and performance to be materially different from any future results and performance expressed or implied by such forward-looking information. Potential risks and uncertainties include, without limitation, the uncertainties inherent in the development of new products for use in the human body, WorldHeart's need for significant additional funding, successful integration of acquisitions, WorldHeart's need to establish reimbursement mechanisms and product acceptance from third-party payers, extensive government regulation of our products, rapid developments in technology, including developments by competitors, potential lack of anticipated synergies and efficiencies of scale related to the MedQuest Products, Inc. ("MedQuest") acquisition, and potential difficulties in the integration of the MedQuest operations.
OVERVIEW
WorldHeart is a global medical device company currently focused on the development and commercialization of implantable pulsatile ventricular assist devices ("VADs"), which are mechanical pumps that allow for the restoration of normal blood circulation to patients suffering from late-stage congestive heart failure. WorldHeartand has facilities in Ottawa, Ontario, Canada, Oakland, California, United States and Heesch, Netherlands. WorldHeart currently derives substantially all of its revenue from its Novacor® LVAS (left ventricular assist system) and related peripheral equipment.
WorldHeart manufactures and distributes the Novacor LVAS. The Corporation sells its Novacor products directly to medical clinics and hospitals in the United States, most of Europe and Canada and through a distributor in Japan and certain other countries.
The Novacor LVAS is commercially approved as a bridge to transplantation device in the United States and Canada. In Europe, the Novacor LVAS has unrestricted approval for use as an alternative to transplantation, a bridge to transplantation, and to support patients who may have an ability to recover the use of their natural heart. In Japan, the device is commercially approved for use in cardiac patients at risk of imminent death from non-reversible left ventricular failure for which there is no alternative except heart transplantation.
WorldHeart has commenced a long-term use (Destination Therapy) trial in the United States called RELIANT (RandomizedEvaluation of the NovacorLVASinaNon-Transplant Population). Data from the RELIANT Trial is expected to support a Pre-market Approval Supplement that will request approval for the use of the Novacor LVAS by non-transplant eligible patients (Destination Therapy) in the United States.
WorldHeart is in the process of developing a next-generation implantable pulsatile VAD and, subject to certain approvals, has recently agreed to acquire, from MedQuest, the technology associated with a magnetically levitated centrifugal rotary VAD. Development work on the Novacor II miniature pulsatile VAD will continue with initial clinical trials in 2007, and initial commercial approvals in Europe in 2008 and in the United States in 2010.
C-2-1
SELECTED FINANCIAL INFORMATION
All financial information is prepared in accordance with Canadian GAAP and is stated in U.S. dollars. Effective January 1, 2004, our functional currency was deemed to have changed from the Canadian dollar to the U.S. dollar. In general, this change resulted from the restructuring and issuance of common shares and warrants that occurred in the year ended December 31, 2003, along with a gradual increase in the overall proportion of business activities conducted in U.S. dollars and with the expectation that this trend will continue. Concurrent with this change in functional currency, we adopted the U.S. dollar as our reporting currency.
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Net revenue | | $ | 9,575,761 | | $ | 6,775,807 | | $ | 6,436,696 | |
| Net loss for the year | | | (22,806,805 | ) | | (23,547,313 | ) | | (31,690,859 | ) |
| Net loss applicable to common shareholders | | | (22,806,805 | ) | | (25,471,708 | ) | | (31,690,859 | ) |
| Basic and diluted loss per share | | | (1.48 | ) | | (4.07 | ) | | (12.48 | ) |
| Total assets | | | 42,258,430 | | | 50,180,069 | | | 28,553,444 | |
| Long term liabilities | | $ | 2,547,027 | | $ | — | | $ | 46,114,470 | |
SUMMARY OF QUARTERLY RESULTS
| | Three Months Ended
| |
|---|
| | December 31, 2004
| | September 30, 2004
| | June 30,
2004
| | March 31,
2004
| |
|---|
| Net revenue | | $ | 2,986,980 | | $ | 2,250,050 | | $ | 2,146,388 | | $ | 2,192,343 | |
| Net loss | | | (6,352,323 | ) | | (6,324,604 | ) | | (6,002,772 | ) | | (4,127,106 | ) |
| Net loss applicable to common shareholders | | | (6,352,323 | ) | | (6,324,604 | ) | | (6,002,772 | ) | | (4,127,106 | ) |
| Basic and diluted loss per common share | | $ | (0.41 | ) | $ | (0.41 | ) | $ | (0.39 | ) | $ | (0.27 | ) |
| | Three Months Ended
| |
|---|
| | December 31, 2003
| | September 30, 2003
| | June 30,
2003
| | March 31,
2003
| |
|---|
| Net revenue | | $ | 559,704 | | $ | 2,023,170 | | $ | 1,944,702 | | $ | 2,248,231 | |
| Net loss | | | (5,170,572 | ) | | (9,358,182 | ) | | (2,896,337 | ) | | (6,122,222 | ) |
| Net loss applicable to common shareholders | | | (7,094,967 | ) | | (9,358,182 | ) | | (2,896,337 | ) | | (6,122,222 | ) |
| Basic and diluted loss per common share | | $ | (0.48 | ) | $ | (2.27 | ) | $ | (0.92 | ) | $ | (2.07 | ) |
C-2-2
RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2004 COMPARED TO THE YEAR ENDED DECEMBER 31, 2003
Consolidated results of operations
| | Year ended December 31, 2004
| | As a % of net revenue
| | Year ended December 31, 2003
| | As a % of net revenue
|
|---|
| Revenue | | | | | | | | | | |
| | Gross revenues | | $ | 9,575,761 | | | | $ | 7,883,203 | | |
| | Less: returns | | | — | | | | | (1,107,396 | ) | |
| | |
| | | |
| | |
| | Net revenue | | | 9,575,761 | | 100% | | | 6,775,807 | | 100% |
| Cost of goods sold | | | (7,680,384 | ) | 80% | | | (4,968,898 | ) | 73% |
| | |
| |
| |
| |
|
| Gross margin | | | 1,895,377 | | 20% | | | 1,806,909 | | 27% |
Selling, general and administrative |
|
|
(13,363,796 |
) |
|
|
|
(7,263,759 |
) |
|
| Research and development | | | (5,838,754 | ) | | | | (11,298,380 | ) | |
| Share-based compensation costs | | | (2,134,707 | ) | | | | — | | |
| Restructuring costs | | | (1,787,129 | ) | | | | (3,356,487 | ) | |
| Amortization of intangibles | | | (515,012 | ) | | | | (2,854,774 | ) | |
| Foreign exchange gain (loss) | | | (308,338 | ) | | | | 8,069,459 | | |
| Investment income | | | 99,427 | | | | | 105,596 | | |
| Loss on disposal of capital assets | | | (46,431 | ) | | | | (27,147 | ) | |
| Interest and financing expenses | | | (807,442 | ) | | | | (8,728,730 | ) | |
| | |
| | | |
| | |
| Net loss | | $ | (22,806,805 | ) | | | $ | (23,547,313 | ) | |
| | |
| | | |
| | |
Revenue. The sale of Novacor LVAS implant kits and related peripheral equipment and services accounts for substantially all of WorldHeart's revenues. WorldHeart primarily sells its products directly, except for certain countries, including Japan, where we sell through distributors. Prior to January 1, 2004, WorldHeart sold its products directly in the United States and to our distributor, Edwards Lifesciences LLC ("Edwards"), outside the United States.
The composition of revenue is as follows:
| | $
| | Year ended December 31, 2004
| | $
| | Year ended December 31, 2003
| |
|---|
| Gross implant revenue / kits | | 7,055,643 | | 113 | | 5,996,738 | | 118 | |
| Peripherals and other | | 2,520,118 | | | | 1,886,465 | | | |
| | |
| |
| |
| |
| |
| Gross revenue before returns | | 9,575,761 | | 113 | | 7,883,203 | | 118 | |
| Re-purchased Edwards inventory: | | | | | | | | | |
| — Implant kits | | — | | — | | (602,339 | ) | (20 | ) |
| — Peripherals and other | | — | | | | (505,057 | ) | | |
| | |
| |
| |
| |
| |
| Net revenue / kits | | 9,575,761 | | 113 | | 6,775,807 | | 98 | |
| | |
| |
| |
| |
| |
Net revenue for the year ended December 31, 2004 increased to approximately $9.6 million representing an increase of 41% over the year ended December 31, 2003. Implant kit net revenue in 2003 includes the return of 20 units, which WorldHeart re-acquired in connection with the amendments to the distribution agreement with Edwards in December 2003. Net implant kits recognized as revenue in 2004 increased by 15% compared to 2003.
C-2-3
Peripherals and other revenues, including Novacor LVAS hardware and peripheral sales and services for the year ended December 31, 2004, were approximately $2.5 million, which represents an increase of 82% over other revenue of approximately $1.4 million (gross revenue of approximately $1.9 million less returns of approximately $505,000) recorded in the year ended December 31, 2003. We anticipate that the proportionate sales may shift from hardware, peripheral and service sales to implant kit sales as centres enrolled in our RELIANT Trial increase implant volume under the program.
Gross revenue increased to approximately $9.6 million representing an increase of 21% over the year ended December 31, 2003. Novacor LVAS implant kit gross revenue increased to approximately $7.0 million for the year ended December 31, 2004 compared to approximately $6.0 million for the year ended December 31, 2003 representing an increase of 18%. The gross number of implant kits recognized as revenue in the year ended December 31, 2004 was 113 compared to 118 in the year ended December 31, 2003. The revenue per kit increased substantially for sales outside of the United States and Japan due primarily to the shift to selling direct to medical centers rather than through a distributor.
An additional 17 implant kits were shipped and invoiced in the year ended December 31, 2004, however, these kits were deferred as revenue at the time of shipment as the transactions did not meet our revenue recognition criteria. These kits remain in deferred revenue at December 31, 2004 representing approximately $1,020,000 and will be recognized as the earnings process is completed in future periods.
WorldHeart sold 59 implant kits in the United States in 2004 compared to 51 implant kits in 2003. In Europe, Canada and the rest-of-world, excluding Japan, WorldHeart sold 44 implant kits in 2004 compared to 44 in 2003 (64 kits less the return of 20 kits). Japanese sales totaled ten implant kits in 2004 compared to three in 2003.
During the second quarter of 2004, WorldHeart announced its RELIANT Trial and implantation commenced under the RELIANT Trial in the third quarter of 2004. The RELIANT Trial is a randomized 40 center equivalency trial where patients will receive a Novacor LVAS or Thoratec Corporation's Heartmate® XVE LVAS on a 2:1 ratio and where Heartmate is the control arm. At December 31, 2004, WorldHeart has enrolled nine (9) centers under the RELIANT Trial with five (5) implants.
Cost of goods sold. For the year ended December 31, 2004, the overall cost of goods sold increased to 80% of revenue as compared to 73% for the year ended December 31, 2003.
Despite continued efforts to improve per unit manufacturing costs, we continued to experience significant production inefficiencies during 2004 caused by supply shortages due to quality control and production volumes well below capacity. This resulted in higher Novacor LVAS per unit production costs. We expect the cost per unit to decrease as we continue to address the supply issues and increases production.
Gross margin for the year ended December 31, 2004 decreased to 20% of revenues from 27% of revenues for the year ended December 31, 2003. This decrease was due to production inefficiencies which were partially offset by a shift to direct selling. In addition, certain low margin sales were made to new centers for the initial equipment investment needed to begin a Novacor LVAS implant program at the center. Improvement in gross margin percentage is expected as discounts offered to new centers decrease and as costs per unit decrease from contract manufacturing of some components and production volume increases.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of payroll and related expenses for executives, sales, marketing, accounting and administrative personnel, professional fees, communication expenses, promotional activities, costs associated with meeting multi-jurisdictional regulatory requirements, insurance premiums, and occupancy and other general corporate expenses.
C-2-4
The composition of selling, general and administrative expenses is as follows:
| | Year ended December 31, 2004
| | Year ended December 31, 2003
|
|---|
| Selling | | $ | 7,369,558 | | $ | 4,518,270 |
| General and administrative | | | 5,994,238 | | | 2,745,489 |
| | |
| |
|
| | | $ | 13,363,796 | | $ | 7,263,759 |
| | |
| |
|
Selling, general and administrative expenses for the year ended December 31, 2004 increased by approximately $6.1 million or 84% from the same period in 2003. Selling expenses increased during 2004, as compared to 2003, as WorldHeart assumed responsibility for its direct sales in Europe and increased sales and marketing program expenditures in the United States. The comparative sales expenses for the year ended December 31, 2003 were constrained as a result of our financial position at that time. General and administrative expenses have also increased with the expanded infrastructure required to support a broadened sales responsibility and due to increases in communication expenses, professional fees, insurance costs and multi-jurisdictional regulatory costs. Costs related to the European distribution operations and the related support are fully reflected in the results for the year ended December 31, 2004. In the third quarter of 2004, WorldHeart commenced the consolidation of its North American operations. This is anticipated to result in a decrease in general and administrative expenses in 2005.
Share-based compensation costs. WorldHeart adopted the provisions of CICA Handbook Section 3870 — "Stock-based Compensation and Other Stock-based Payments" ("CICA 3870") retroactively without restatement of prior periods. CICA 3870 requires entities to account for employee stock options using the fair value based method. Under the fair value based method, compensation cost is measured at fair value at the date of grant and is expensed over the award's vesting period. The share-based compensation cost recorded for the year ended December 31, 2004 was approximately $2.1 million. The comparative cost of approximately $1.6 million for the year ended December 31, 2003 is not shown in the financial statements. The increase in costs resulted from the grant of a large number of stock options in the fourth quarter of 2003. The pro forma effect of these costs on net loss and basic loss per share is set out below:
| | Year ended December 31, 2003
| |
|---|
| Net loss attributable to common shareholders | | $ | (25,471,708 | ) |
| Share-based compensation costs | | | (1,579,378 | ) |
| | |
| |
| Adjusted net loss | | $ | (27,051,086 | ) |
| | |
| |
| Reported loss per share | | $ | (4.07 | ) |
| Share-based compensation costs per common share | | | (0.25 | ) |
| | |
| |
| Pro forma basic loss per common share | | $ | (4.32 | ) |
| | |
| |
Share-based compensation costs are expected to increase upon approval, of an increase in the number of options available under the employee stock option plan, by the shareholders for the approximately 2.4 million options granted on September 23, 2004, and the approximately 3.4 million options granted, subsequent to year end, on January 31, 2005.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing, and configuration of equipment expenses, trial expenses, and regulatory affairs expenses.
Research and development expenses for the year ended December 31, 2004 decreased by approximately $5.4 million or 48% compared to year ended December 31, 2003. Gross research and development expenditures
C-2-5
excluding the impact of government programs decreased by 50% to approximately $6.0 million in 2004 compared to approximately $11.9 million in 2003.
| | Year ended December 31, 2004
| | Year ended December 31, 2003
| |
|---|
| Gross research and development expenses | | $ | 5,975,344 | | $ | 11,993,624 | |
| Investment tax credits | | | (136,590 | ) | | (695,244 | ) |
| | |
| |
| |
| Net research and development expenses | | $ | 5,838,754 | | $ | 11,298,380 | |
| | |
| |
| |
Lower research and development costs for year ended December 31, 2004 compared to the same period in 2003 reflect expense reductions associated with the merged next-generation VAD development program and reduced pre-clinical and clinical trial expenses. The cost reduction programs were completed in fiscal year 2003 and the expense reductions were realized in 2004. Development work on the next-generation pulsatile VAD will continue with initial clinical trials expected to begin in 2007.
Investment tax credits for the year ended December 31, 2004 totaled approximately $137,000 and relate to a Canadian provincial government investment tax credit program. A credit of approximately $2.3 million was recorded against research and development expense for the year ended December 31, 2003 relating to a Canadian federal tax credit program. However, in the same period a provision of approximately $1.7 million was recorded against research and development expense relating to a provincial tax credit program claim that was recorded in a prior period but was, and continues to be, under dispute. The dispute relates to a tax credit recorded as receivable by WorldHeart in the year ended December 31, 2001 related to the Ontario Business Research Institute tax credit ("OBRITC") program in the amount of $2,304,492 that was associated with research payments made by WorldHeart to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation from 1997 to 2000. Following the completion of a provincial audit of the claim and proposed amendments by the province to the OBRITC legislation, WorldHeart received payment of $597,538 of this claim during the year ended December 31, 2003. The Ministry has advised WorldHeart that it believes that additional amounts claimed are ineligible and that it would not be making any further payments. We are disputing the Ministry's decision, but due to the uncertainty of the ultimate resolution of the claim, we recorded a provisions for the remaining receivable of $1,706,954 in 2003. WorldHeart continues to review various tax credit programs but only records a credit when collection of the claim is reasonably assured.
Restructuring costs. On August 25, 2004, we approved a plan to consolidate the Ottawa, Ontario operations into our facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve operational efficiency and effectiveness.
WorldHeart expects the restructuring to be completed by the end of June 2005 and expects to incur total restructuring charges of approximately $2.6 million comprised as follows:
| | 2004
|
|---|
| | Total estimated restructuring costs
| | Actual costs incurred to December 31
| | Costs paid or settled in the year
| | Balance in accrued liabilities as at December 31
|
|---|
| Employee severance costs | | $ | 1,509,192 | | $ | 1,396,037 | | $ | 795,923 | | $ | 600,114 |
| Contract settlement costs | | | 719,894 | | | 57,653 | | | — | | | 57,653 |
| Write down of capital assets | | | 338,595 | | | 338,595 | | | 338,595 | | | — |
| Other costs | | | 44,083 | | | 22,509 | | | 8,624 | | | 13,885 |
| | |
| |
| |
| |
|
| | | $ | 2,611,764 | | $ | 1,814,794 | | $ | 1,143,142 | | $ | 671,652 |
| | |
| |
| |
| |
|
During the year ended December 31, 2004, actual restructuring costs of approximately $1.8 million have been recorded. Employee severance costs represent the amount employees have earned to December 31, 2004. Contract settlement costs are recorded when notice is provided that the contract is being terminated and
C-2-6
WorldHeart ceases to use the right conveyed under the contract. The capital assets were written down to their net realizable value when the decision was made to consolidate the North American operations and it was determined that the carrying value of the capital assets was impaired. Other restructuring costs are recognized when the services are actually received.
During the year ended December 31, 2003, WorldHeart realigned its business operations to focus on Novacor LVAS commercial operations and to reduce research and development costs by merging our three next-generation VAD programs. Total restructuring costs for the year ended December 31, 2003 amounted to approximately $3.4 million. The restructuring costs related to employee severance and termination costs were approximately $1.6 million; costs related to the buyout of a Novacor LVAS royalty agreement were approximately $1.0 million; costs related to the excess of inventory purchase cost over the carrying value of the inventory re-purchased from Edwards were approximately $770,000; and other miscellaneous costs were approximately $13,000. The royalty buy-out eliminated royalty payment obligations going forward of 4% of Novacor LVAS sales. Approximately $2.2 million of the 2003 restructuring costs were paid in 2003 with approximately $1.2 million being paid in 2004. The difference of approximately $28,000 was adjusted in 2004 to reflect revised estimates for employee severance and termination costs.
Amortization of intangibles. Amortization of intangibles for the year ended December 31, 2004 was approximately $515,000 compared to approximately $2.8 million for the same period in 2003. The majority of the intangible assets arose from the purchase of the Novacor business in June 2000. Most of these assets have now reached the end of their original estimated useful life and have been fully amortized. As at December 31, 2004, intangible assets totaling approximately $255,000 remain unamortized.
Foreign exchange gains and losses. During the year ended December 31, 2004, a foreign exchange loss of approximately $308,000 was recorded compared to a gain of approximately $8.0 million for the year ended December 31, 2003. The 2004 foreign exchange loss relates to the effect of a strengthening U.S. dollar compared to the Canadian dollar during the first half of the year on net monetary assets, predominately cash and cash equivalents and short-term investments, less current liabilities, denominated in Canadian dollars. The majority of the 2003 foreign exchange gain can be attributed to the impact of a strengthening Canadian dollar on WorldHeart's U.S. dollar denominated preferred shares. From WorldHeart's inception through the end of 2003, our functional currency was the Canadian dollar.
Investment income. Investment income primarily represents interest earned on cash equivalents and short-term investments. During the year ended December 31, 2004, investment income of approximately $99,000 was recorded compared to income of approximately $105,000 for the year ended December 31, 2003. The decrease is due to having, on average over the course of 2004, less surplus cash to invest as compared to 2003.
Interest expense and financing costs. During the year ended December 31, 2004, interest and financing costs of approximately $807,000 were recorded compared to costs of approximately $8.7 million during the year ended December 31, 2003. Interest expense and financing costs in 2004 consisted mainly of the interest and accretion related to the convertible debentures issued on September 15, 2004. These interest charges will continue to accrete ratably on the convertible debentures until their conversion or maturity. It is anticipated that all outstanding debentures will be converted in accordance with the terms of the MedQuest acquisition. Interest expense and financing costs for the year ended December 31, 2003 consisted of interest on redeemable preferred shares and loan obligations that were outstanding during the year as well as the costs of entering into the loan obligations. In late 2003, these preferred shares were converted to common shares and warrants and the loans were repaid in full.
C-2-7
RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2003 COMPARED TO THE YEAR ENDED DECEMBER 31, 2002
Consolidated results of operations
| | Year ended December 31, 2003
| | As a % of net revenue
| | Year ended December 31, 2002
| | As a % of net revenue
|
|---|
| Revenue | | | | | | | | | | |
| | Gross revenues | | $ | 7,883,203 | | | | $ | 6,855,458 | | |
| | Less: Sales returns | | | (1,107,396 | ) | | | | — | | |
| | | Edwards guarantee fee | | | — | | | | | (418,762 | ) | |
| | |
| |
| |
| |
|
| | Net revenue | | | 6,775,807 | | 100% | | | 6,436,696 | | 100% |
| Cost of goods sold | | | (4,968,898 | ) | 73% | | | (6,284,996 | ) | 98% |
| | |
| |
| |
| |
|
| Gross margin | | | 1,806,909 | | 27% | | | 151,700 | | 2% |
| Selling, general and administrative | | | (7,263,759 | ) | | | | (6,686,437 | ) | |
| Research and development | | | (11,298,380 | ) | | | | (15,931,912 | ) | |
| Restructuring costs | | | (3,356,487 | ) | | | | — | | |
| Amortization of intangibles | | | (2,854,774 | ) | | | | (4,663,406 | ) | |
| Foreign exchange gain (loss) | | | 8,069,459 | | | | | 303,558 | | |
| Investment income | | | 105,596 | | | | | 117,589 | | |
| Loss on disposal of capital assets | | | (27,147 | ) | | | | (32,087 | ) | |
| Interest and financing expenses | | | (8,728,730 | ) | | | | (4,949,864 | ) | |
| | |
| | | |
| | |
| Net loss | | $ | (23,547,313 | ) | | | $ | (31,690,859 | ) | |
| | |
| | | |
| | |
Revenue. The sale of Novacor LVAS Implant Kits and related peripheral equipment and services accounts for substantially all of WorldHeart's revenues. For the years presented above, WorldHeart sold its products directly in the United States and to its distributor, Edwards, outside the United States.
The composition of revenue is as follows:
| | Year ended December 31, 2003
| | Year ended December 31, 2002
|
|---|
| | $
| | # of Units
| | $
| | # of Units
|
|---|
| Gross implant revenue / kits | | 5,996,738 | | 118 | | 5,243,915 | | 104 |
| Peripherals and other | | 1,886,465 | | | | 1,611,543 | | |
| | |
| |
| |
| |
|
| Gross revenue before returns and fees | | 7,883,203 | | 118 | | 6,855,458 | | 104 |
| Re-purchased Edwards inventory: | | | | | | | | |
| | — Implant kits | | (602,339 | ) | (20 | ) | — | | — |
| | — Peripherals and other | | (505,057 | ) | | | — | | |
| Edwards guarantee fee | | — | | | | (418,762 | ) | |
| | |
| |
| |
| |
|
| Net revenue / kits | | 6,775,807 | | 98 | | 6,436,696 | | 104 |
| | |
| |
| |
| |
|
Gross revenues from sales of the Novacor LVAS and related equipment (before returns and the Edwards guarantee) increased 15% from approximately $6.9 million in the year ended December 31, 2002 to approximately $7.9 million in the year ended December 31, 2003. At December 31, 2003, WorldHeart purchased Novacor inventory and other assets from Edwards as part of the amendments to the distribution agreement. The purchase of the inventory was accounted for as a sales return which reduced 2003 revenue by approximately $1.1 million.
C-2-8
In 2003 the gross number of kits shipped for revenue increased by 13% to 118 kits compared to 104 kits in 2002. The net sale of implant kits decreased to 98 kits in 2003 from 104 kits in 2002 due to 20 kits that were treated as returns when WorldHeart re-acquired inventory associated with the amendments to the distribution agreement with Edwards in December 2003.
In the United States, where WorldHeart sold directly, selling prices were significantly higher than non-U.S. sales where we sold to our distributor Edwards. WorldHeart sold 51 implant kits in the United States in 2003 compared to 39 implant kits in 2002. On December 31, 2003, WorldHeart amended its distribution agreement with Edwards in order to sell directly in the rest of the world with the exception of Japan where Edwards will remain the sole distributor. For future periods, selling prices outside of the United States, except Japan, will increase to reflect end-user pricing. In Europe, Canada and the rest-of-world, excluding Japan, WorldHeart sold 64 implant kits in 2003 compared to 57 in 2002. Japanese sales totaled three (3) implant kits in 2003 compared to eight (8) in 2002.
In our distribution agreement with Edwards, WorldHeart was required to pay a minimum gross margin guarantee in the event that Edwards' gross margin generated on the sales of WorldHeart products were below $2 million annually. The guarantee shortfall was accounted for as a reduction of revenues. Edwards' sales of WorldHeart's products were sufficient during 2003 to meet the minimum guarantee requirement; therefore, no guarantee payment was required. The guarantee was terminated effective January 1, 2004 as part of the amendment to the Edwards distribution agreement.
Cost of goods sold. Cost of goods sold decreased as a percentage of net revenue from 98% in 2002 to 73% in 2003.
The decrease as a percent of revenue is partially the result of a higher proportion of product being sold in the U.S. where higher margins are earned as compared to Europe and the rest of the world in 2003 versus 2002, the commencement of a cost reduction program for cost of sales in the fourth quarter of 2003, as well as managed reductions in indirect manufacturing support costs and direct manufacturing overheads.
Correspondingly, gross margins in 2003 increased to 27% of revenues from 2% of net revenues in 2002.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of payroll and related expenses for executives, sales, marketing, accounting and administrative personnel, professional fees, communication expenses, promotional activities, costs associated with meeting multi-jurisdictional regulatory requirements, insurance premiums, occupancy and other general corporate expenses.
The composition of selling, general and administrative expenses is as follows:
| | Year ended December 31, 2003
| | Year ended December 31, 2002
|
|---|
| Selling | | $ | 4,518,270 | | $ | 4,194,405 |
| General and administrative | | | 2,745,489 | | | 2,492,032 |
| | |
| |
|
| | | $ | 7,263,759 | | $ | 6,686,437 |
| | |
| |
|
Selling, general and administrative expenses for year ended December 31, 2003 increased by approximately $577,000 or 9% from the same period in 2002. Actual expenses remained fairly constant year over year, however, due to the average exchange rates applied upon the change in our reporting currency, the reported U.S. dollar values show an increase in expenses. The average exchange rate for 2003 increased approximately 12% over the average exchange rate for 2002.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing, and configuration of equipment, trial expenses, regulatory affairs and quality control with respect to prototype development.
Gross research and development expenses, excluding the impact of government assistance, decreased by approximately $6.8 million or 36% to approximately $11.9 million in 2003 as compared to approximately
C-2-9
$18.8 million in 2002. Net research and development costs for 2003 were approximately $11.3 million or 29%, lower than 2002.
| | Year ended December 31, 2003
| | Year ended December 31, 2002
| |
|---|
| Gross research and development expenses | | $ | 11,993,624 | | $ | 18,776,949 | |
| Investment tax credits | | | (695,244 | ) | | (2,845,037 | ) |
| | |
| |
| |
| Net research and development expenses | | $ | 11,298,380 | | $ | 15,931,912 | |
| | |
| |
| |
Lower research and development costs in 2003 compared to 2002 are due to year-over-year expense reductions associated with the merged next-generation VAD development programs and reduced pre-clinical and clinical trial activity compared to 2002. WorldHeart continued to reduce expenditures predominantly due to a consolidation of our next generation VAD development programs that were commenced late in 2002, which resulted in a decelerated investment in research and development throughout 2003.
WorldHeart continued implants under the feasibility phase of the Novacor LVAS clinical trial (INTrEPID) throughout all of 2002 and into the first quarter of 2003. In addition, the next generation pre-clinical trials were terminated in the third quarter of 2002 and there was no next generation trial activity in 2003.
During 2002, the consolidated next-generation VAD program and change in the scope necessitated an amendment to the federal government Technology Partners Canada ("TPC") contribution agreement. On May 21, 2003, TPC approved this amendment allowing WorldHeart to claim eligible expenditures for reimbursement against the funding available under the $6.6 million contribution agreement. As part of the amendment to the agreement, the royalty rate payable to TPC was reduced to 0.65% and is to be calculated on the consolidated revenues of WorldHeart commencing January 1, 2004.
WorldHeart had previously recorded as a credit against research and development expense a tax credit receivable in the amount of approximately $2.3 million related to the provincial Ontario Business Research Institute tax credit ("OBRITC") program. The claim was associated with research payments made to the Cardiovascular Devices Division of the Ottawa Heart Institute Research Corporation ("CVD") from 1997 to 2000. Following the completion of the provincial Ministry of Finance's audit and proposed amendments to the OBRITC legislation, a payment of approximately $598,000 was received during the year ended December 31, 2003. Subsequent to receipt of this payment, WorldHeart was advised by the Ministry that it was seeking repayment of the amount received and would not be making further payments. We are disputing the Ministry's decision, but due to the uncertainty of the ultimate resolution of the claim, we recorded a provisions for the remaining receivable of $1,706,954 in 2003.
Restructuring costs. On September 23, 2003, WorldHeart realigned its business operations to focus on commercial operations and to reduce research and development costs related to our next generation VAD.
WorldHeart restructured its worldwide distribution agreement with Edwards to allow for better control over direct sales, marketing and distribution for all territories, with the exception of Japan. WorldHeart also purchased certain inventory and capital assets from Edwards in Europe and commenced direct sales activities effective January 1, 2004. Edwards agreed to provide certain transitional services until WorldHeart established the appropriate infrastructure to support direct sales in Europe. Early in 2004, World Heart B.V., a wholly-owned subsidiary, was incorporated with a head office in Heesch, Netherlands, and the European infrastructure was established in-house in the first half of 2004.
We also refocused our efforts to reduce manufacturing costs associated with the production of the Novacor LVAS. We continue to evolve our processes to move towards more cost-effective manufacturing processes expected to lead to reduced costs for inventory and cost of goods sold. We also reduced the rate of investment associated with the development of our next-generation VAD.
As part of these initiatives 37 positions were eliminated, of which 24 related to research and development, nine related to general and administration and four related to manufacturing.
C-2-10
Total restructuring costs incurred for the year ended December 31, 2003 were approximately $3.4 million and related to the following:
- •
- employee severance and termination costs of approximately $1.6 million;
- •
- costs related to the excess of inventory purchase cost over carrying value of the inventory re-purchased from Edwards amounting to approximately $770,000;
- •
- costs related to the buyout of a Novacor LVAS royalty agreement of approximately $1.0 million; and,
- •
- other miscellaneous costs amounting to approximately $13,000.
As at December 31, 2003, approximately $2.2 million of the total restructuring costs had been paid with the remainder of approximately $1.2 million included in accounts payable and accrued liabilities all of which except approximately $28,000 was paid in the year ended December 31, 2004. The $28,000 was determined to be an overestimate of accrued salaries and was credited to the 2004 expense.
Amortization of intangibles. Amortization of intangibles for 2003 amounted to approximately $2.8 million compared to approximately $4.7 million for 2002. The majority of the intangible assets arose from the purchase of the Novacor business in June 2000. The decrease in amortization is due to the majority of these assets reaching the end of the original estimated useful life during the year. As at December 31, 2003, intangible assets of approximately $879,000 remain unamortized
Foreign exchange gains and losses. A foreign exchange gain of approximately $8 million was recorded in 2003 compared to a gain of approximately $303,000 in 2002. Certain balance sheet items are denominated in Canadian dollars. Foreign exchange gains and losses arose when the value of the U.S. dollar changed relative to the value of the Canadian dollar. The majority of the 2003 foreign exchange gain can be attributed to the impact of a strengthening Canadian dollar on WorldHeart's U.S. dollar denominated preferred shares. From WorldHeart's inception through the end of 2003, our functional currency was the Canadian dollar.
Investment income. Investment income primarily represents interest earned on cash equivalents and short-term investments. Investment income decreased to approximately $106,000 in 2003 from approximately $118,000 in 2002. The decrease relates to having a larger sum of excess cash for a shorter period of time in 2003. WorldHeart generated substantially all of its investment income in the fourth quarter of 2003 on excess funds received upon completion of the private placement late in the third quarter of 2003.
Interest expense and financing costs. Interest expense and financing costs consist of non-cash interest on preferred shares and the costs associated with debt financing transactions entered into during the first nine months of 2003.
The preferred shares were accounted for in accordance with their substance and were presented in the financial statements according to their debt and equity components measured at their respective fair values at the time of issue. The debt components were calculated as the present value of the interest payments discounted at 12%, approximating the interest rate that would have been applicable to non-convertible debt at the time the preferred shares were issued. Interest expense was determined on the debt component as the amount necessary to increase the debt component to its face amount plus accumulated dividends at maturity. Total non-cash interest expense on the preferred shares for 2003 was approximately $4.3 million compared to approximately $4.8 million in 2002. During the third quarter of 2003, $58.0 million of Series A redeemable, cumulative, participating preferred shares plus accrued dividends were exchanged by Edwards for 711,589 post-consolidation common shares of WorldHeart. In addition, during 2003, Edwards Lifesciences (U.S.) Inc. exchanged $20.0 million of redeemable, convertible preferred shares plus accrued dividends for 500,000 post-consolidation common shares of WorldHeart and 1,000,000 post-consolidation warrants.
Other interest and financing expenses in 2003 totaled approximately $4.4 million compared to approximately $77,000 in 2002. The interest, fees and other costs were associated with loans entered into in 2003 to allow WorldHeart to meet its cash requirements until an equity transaction could be completed.
C-2-11
LIQUIDITY AND CAPITAL RESOURCES
Historically, WorldHeart has funded losses from operations through the sale of equity and issuance of debt instruments. Combined with revenues, these funds have provided us with the resources to operate our business, attract and retain key personnel, fund our research and development program and clinical trials, apply and obtain the necessary regulatory approvals and develop our technology and products.
Liquidity. At December 31, 2004, WorldHeart had cash, cash equivalents and short-term investments of approximately $8.8 million and net working capital of approximately $14.1 million compared to cash, cash equivalents and short-term investments of approximately $18.1 million and net working capital of approximately $18.3 million at December 31, 2003.
Cash, cash equivalents and short-term investments decreased in 2004 by approximately $9.3 million. This primarily related to cash used in investing activities of approximately $585,000 for the purchase of capital assets and cash pledged as collateral for a lease; an operating cash net use of approximately $22.9 million including a net pay down of approximately $3.9 million of accounts payable and accrued compensation and an increase in inventory of approximately $2.2 million; offset by the net proceeds received on the issuance of convertible debentures and warrants for approximately $12.9 million; and approximately $1.5 million received on the issuance of common shares through the exercise of warrants.
During the year, WorldHeart completed a convertible debenture and warrant issuance for gross proceeds of approximately $13.3 million.
Subsequent to year end, on January 31, 2005, WorldHeart announced that it had entered in to a definitive agreement to acquire MedQuest, subject to certain approvals, consents, conditions and the approval of the shareholders. MedQuest, based in Salt Lake City, Utah, is in the final development stages of its HeartQuest™ ventricular assist device, a magnetically levitated centrifugal rotary blood pump. As separate components of that transaction, WorldHeart also announced that it had definitive agreements to issue through a private placement, 8.9 million common shares, at $1.35 per share, for gross proceeds of $12 million and raise approximately $10.7 million through the exercise of 10.7 million existing warrants at an exercise price of $1.00 per warrant. These transactions are expected to be completed contemporaneously with the MedQuest acquisition in the second quarter of 2005. Upon completion of the private placement and warrant exercise it is anticipated that WorldHeart will receive approximately $22.7 million of cash and extinguish all of its convertible debentures providing adequate resources to fund operations into 2006 when we may be required to obtain additional funding to sustain operations.
Our long-term working capital and capital requirements will depend upon numerous factors, including the following: our continued focus on improving commercial operations and viability through the expanded sales of Novacor LVAS; increasing revenues through higher average selling prices and enrollment in the RELIANT Trial (RandomizedEvaluation of theNovacorLVASinaNon-Transplant Population); decreasing production costs through a manufacturing cost reduction program; the rate of investment in our next-generation technologies, including our under-development pulsatile VAD and the rotary VAD expected to be acquired in the second quarter of 2005; the costs associated with clinical trials and the approval process for the next-generation technologies; and our general efforts to improve operational efficiency and effectiveness.
OUTLOOK
External environment. The market for effective pharmacological, electro-physiological or device treatments for late-stage heart failure continues to grow, and despite significant progress on many fronts, no competitive breakthroughs have been announced or are believed to be imminent. The number of diagnosed heart failure patients is increasing as a result of both population demographic trends, and also as a response to the increasing number of survivors from sudden cardiac events, many of whom subsequently develop congestive heart failure. We believe the potential market for our heart assist devices, with their current regulatory approvals, continues to be at least 100,000 patients per annum in North America, Europe, Japan and certain other countries.
There is growing evidence of increasing clinical acceptance of the use of mechanical circulatory assist therapies in the treatment of late stage heart failure, and on two separate occasions in March 2003 and May 2004, the Centers for Medicare and Medicaid Services in the United States announced the extension of
C-2-12
reimbursement coverage for both bridge and destination therapy VAD use. These extensions have resulted in increased payments for procedures using such devices. These developments are expected to continue to accelerate the use of ventricular assist devices both in the United States and elsewhere. Also, effective April 1, 2004, reimbursement in Japan for implantable VADs was approved. Novacor LVAS is the only approved device of its kind in Japan.
In the United States, WorldHeart commenced a randomized equivalency trial called RELIANT in the second quarter of 2004. In this trial the Novacor LVAS is being randomized to the Thoratec HeartMate® XVE LVAS, which will be the control arm. RELIANT is a randomized 40 center equivalency trial where patients will receive a Novacor LVAS or Thoratec Corporation's Heartmate®XVE LVAS on a 2:1 ratio. It is expected that data from this trial will support a Premarket Approval Supplement, which will be submitted to the U.S. Food and Drug Administration for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy).
We continue to believe that the long-term use or Destination Therapy market will evolve rapidly when devices are accepted as reliable and durable. A wider adoption of VADs as a Destination Therapy will likely occur with the next-generation VADs and we believe that late-stage heart failure will be best served by a combination of pulsatile and rotary flow VADs.
Internal environment. We expect to incur net operating losses on a quarterly basis, through 2006, assuming the completion of the MedQuest acquisition, which is expected to be completed in the second quarter of 2005. We believe that our commercial operations associated with Novacor LVAS will continue to improve. We expect to continue to increase revenues as a result of higher Novacor LVAS Implant Kit sales, particularly in the United States. Enrollment in the RELIANT Trial commenced in the second quarter of 2004 and implantation under the RELIANT Trial commenced in the third quarter of 2004 and is expected to continue through 2005 and 2006. We also expect gross margin contribution to increase through a combination of higher volumes which should lead to lower per unit manufacturing costs through more efficient and optimal manufacturing levels and continuation of our manufacturing cost reduction program.
In addition, in the third quarter of 2004, a plan was put in place to consolidate the Ottawa operations into our Oakland operations to further improve operating cost structure, increase efficiency and improve effectiveness.
WorldHeart expects to complete the acquisition of MedQuest in the second quarter of 2005 and commence an initial feasibility clinical trial of its magnetically levitated centrifugal rotary VAD by the beginning of 2006. Initial commercial approvals for the VAD are expected to occur in Europe 2007 with approvals for the VAD in the United States two years later.
Development work on the Novacor II pulsatile VAD will continue with initial clinical trials in 2007, and initial commercial approvals in Europe in 2008 and in the United States in 2010.
Commitments
- (a)
- Contractual Obligations
The following table sets forth WorldHeart's contractual obligations, excluding MedQuest commitments, as of December 31, 2004:
| | Total
| | Less than 1 Year
| | 1-3 Years
| | 3-5 Years
|
|---|
| Operating lease obligations | | $ | 3,242,720 | | $ | 1,440,597 | | $ | 1,762,450 | | $ | 39,673 |
| Long-term debt obligations | | | 15,316,563 | | | — | | | — | | | 15,316,563 |
| Capital lease obligations | | | — | | | — | | | — | | | — |
| Purchase obligations | | | — | | | — | | | — | | | — |
| Other long-term liabilities reflected on the balance sheet under GAAP | | $ | 16,368 | | $ | — | | $ | 16,368 | | $ | — |
C-2-13
- (b)
- Operating Leases
During 2003, $222,003 was drawn against the cash amount pledged as collateral for obligations under a premises lease for the Oakland, California operations. In 2004, the balance was restored to the original pledged amount of $750,000. The cash remains pledged against a letter of credit issued by WorldHeart in support of its obligations and is not available for general operations.
- (c)
- Novacor LVAS Royalties
WorldHeart is committed, under the Novacor LVAS royalty agreement to royalty payments to certain employees. Royalties are payable on annual consolidated gross revenues at a rate of 0.808% up to a maximum of $3,232,000. Royalty payments to date total $1,027,184, included in accounts payable and accrued liabilities at December 31, 2004 is $95,646 (December 31, 2003 — $37,478).
- (d)
- Technology Partnerships Canada Contribution Agreement
During 2002, WorldHeart entered into a shared funding program with Technology Partnerships Canada ("TPC") under which the Canadian government shares costs of certain research and development activities. Funding in the amount of approximately $6,624,000 was claimed. Effective January 1, 2004, repayment is in the form of royalties payable on annual consolidated gross revenues at a rate of 0.65% for a 9 year period ended December 31, 2012. If during this period royalty payments reach the maximum of $20,250,000 no further repayments will be required. If the royalty payments do not exceed $20,250,000 during this period they will continue until 2015 or until the maximum is reached.
CAPITAL EXPENDITURES
| | 2004
| | 2003
| | 2002
|
|---|
| Capital Expenditures | | $ | 363,224 | | $ | 150,135 | | $ | 388,611 |
| | |
| |
| |
|
Capital expenditures for 2004 decreased substantially from 2003 as WorldHeart reduced costs and expenditures. WorldHeart anticipates that capital expenditures for 2005 will be similar to 2004.
At December 31, 2004, WorldHeart occupied three locations. The Ottawa location comprises 22,755 square feet of manufacturing and office space with a lease that expires on December 31, 2006. The Ottawa location is currently being shut down as part of the restructuring plan approved in August 2004. The second location is in Oakland with two buildings consisting of approximately 40,000 square feet of manufacturing and office space. The Oakland leases were renewed in 2002 for a five-year term expiring on April 30, 2007. The third location is in Heesch, Netherlands with approximately 2,500 square feet of warehouse and office space. The Heesch lease was signed in 2004 for a three-year term expiring on December 31, 2007.
MARKET RISK
WorldHeart is subject to investment risk on investments that it makes with excess cash.
Investment risk is mitigated by close adherence to an established investment policy, which has been approved by the Board of Directors. The policy sets liquidity criteria, and counter party risk diversification criteria and restricts investments to investment grade quality instruments of AA or better or R1 medium or better in the case of commercial paper. There exists modest income exposure to a decline in interest rates. This is not significant due to the short terms to maturity of the instruments held and the already low current interest rates.
WorldHeart has assets and liabilities denominated in foreign currencies, including the Canadian dollar and the Euro. Occasionally, WorldHeart enters into foreign exchange contracts in order to match its foreign exchange requirements. At December 31, 2004, there were no outstanding foreign exchange forward contracts. WorldHeart generally attempts to match its cash balances to its expected net cash outflow for a particular currency during the year.
C-2-14
CRITICAL ACCOUNTING ESTIMATES
We have identified the following policies as critical to our business and the understanding of our results of operations. For a more detailed discussion on the application of these and other accounting policies, see the notes to the audited consolidated financial statements.
Revenue recognition
Revenue from product sales is recognized when all of the following criteria are met; persuasive evidence of an agreement exists; delivery has occurred or services have been rendered; the price is fixed and determinable; and collection is reasonably assured. The Corporation derives revenue from the sale of implant kits and peripheral equipment and training for implant centres.
Revenue from the sale of implant kits and peripheral equipment is generally recognized when such products are shipped. Where the terms of sale provide customers with rights of return or other guarantees, or where collection is uncertain, revenue is deferred until such time as the rights of return or guarantee expire or, where collection is uncertain, payment is received.
Training revenue is recognized once the training is provided.
Sales to new implant centers typically include implant kits, related peripheral equipment and training. For these multiple element sales, the Corporation allocates the total contract value to the elements based on their respective fair values. Revenue is recognized from each element in accordance with the criteria set out above. Revenue is deferred for any undelivered element when the undelivered element is not considered essential to the functionality of the delivered elements. If undelivered elements are essential to the functionality of the delivered elements, the entire contract value is deferred. For most customers, the training component is not considered essential to the use of or the functionality of the implant kits, as the customers already possess sufficient expertise and experience to perform implants.
The Corporation's implantable products and related peripheral equipment are covered by limited warranties. Warranty costs, which are based on historical experience, are estimated and recorded when the related revenue is recognized. Actual warranty costs are charged against the warranty provision as incurred.
Inventory valuation
WorldHeart will write-down from time to time certain inventory that it considers obsolete or excess based on its expected demand. To the extent inventory movement or product demand is not as anticipated, the write-down may be higher or lower than the usage that is ultimately experienced. Once written down, the inventory is not written back up.
Valuation of goodwill
In accordance with Canadian Institute of Chartered Accountants Handbook Section 3062 ("CICA 3062"), "Goodwill and Other Intangibles", WorldHeart reviews the carrying value of long-lived assets including goodwill whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Such events or circumstances might include a significant decline in market share, a significant decline in profits, changes in technology, significant litigation or other items.
In evaluating whether there is an impairment of goodwill, management compares the carrying amount of goodwill with its fair value. Because WorldHeart operates in one business segment and all of its goodwill was derived from the purchase of that same business, we are able to determine the value of the reporting unit containing the goodwill by using the trading value of the outstanding common shares and the number of outstanding shares at year-end. We determined the fair value of the goodwill by subtracting the fair value of all of the Corporation's assets and liabilities, excluding goodwill, from the value of the reporting unit. An impairment is recognized to the extent that the fair value of the goodwill is less than its carrying amount. The enterprise value determined on December 31, 2004 was sufficient to support the carrying value of goodwill.
C-2-15
In accordance with CICA 3062, WorldHeart completes on an annual basis an impairment test of goodwill. Upon completion of the impairment test as of the end of the fiscal 2004, it was determined that goodwill is not impaired.
Restructuring
In August of 2004, a restructuring plan was implemented to consolidate the North American operations, which included a reduction in workforce, lease and contract termination costs, a write down of capital assets to their net realizable value and other costs. At each reporting date, WorldHeart evaluates the accruals, which are based on estimates, to ensure that they are still appropriate. Included in accruals as of December 31, 2003, were approximately $600,000 related to employee severance costs, approximately $58,000 related to contract termination costs and approximately $14,000 related to other costs. In certain instances, it may be determined that these accruals are no longer required because of efficiencies in carrying out the restructuring plan. In these cases, WorldHeart would reverse any related accrual to income when it is determined it is no longer required. Alternatively, in certain circumstances, it may be determined that certain accruals are insufficient as new events occur or as additional information is obtained. In these cases, WorldHeart would increase the applicable existing accrual with the offset recorded as a restructuring charge.
OTHER FACTORS
Current Product. WorldHeart's near-term commercial operations are exclusively focused on its Novacor LVAS Implant Kit and related equipment and support. Commercial success will therefore be dependant on WorldHearts ability to continue to increase Novacor LVAS sales. Novacor LVAS is approved for use by eligible heart failure patients without limitation, in Europe and Japan and as a bridge-to-transplant device in the United States and Canada. WorldHeart has commenced an equivalency clinical trial in the second quarter of 2004 in the United States called RELIANT in which patients will be randomized to receive either the Novacor LVAS or the Thoratec HeartMate® XVE LVAS. It is expected that data from this clinical trial will support a Premarket Approval Supplement, which will be submitted to the U.S. Food and Drug Administration, for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy). Implants under this trial are eligible for reimbursement by the Centers for Medicare and Medicaid Services ("CMS") and certain private insurers. We believe that approval of the Novacor LVAS for long-term or destination therapy use in the United States would increase the market for the Novacor LVAS substantially.
Pulsatile VADs. WorldHeart has several competitors with commercially approved pulsatile VADs having pumps that are externally located or abdominally implanted. The devices which are developed by the Corporation's competitors are now primarily used as bridges to transplant, although one product (Thoratec HeartMate® XVE) was approved in 2002 for a destination therapy indication for use in the United States. Some of WorldHeart's existing known competitors have significantly greater financial, production and marketing resources. There can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
WorldHeart believes that our pulsatile next-generation implantable VAD is the only pulsatile device that is currently at an advanced stage of development. During 2002 management merged the three next-generation VAD development programs into one VAD program. WorldHeart expects clinical trials for our next-generation implantable pulsatile VAD will commence in 2007. However, there can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
Non-pulsatile VADs. As discussed above, subsequent to year-end WorldHeart entered into a definitive agreement to purchase the assets of MedQuest including its rotary flow, centrifugal, magnetically levitated next generation VAD technology. WorldHeart expects clinical trials for our next-generation implantable non-pulsatile VAD will commence in early 2006. However, there can be no assurance that the development program, including pre-clinical and human clinical trials, will be successful or that the necessary regulatory approvals will be received.
C-2-16
Research and development by WorldHeart's competitors is proceeding for several next-generation non-pulsatile continuous flow assist devices. Certain of these devices have received CE Mark in Europe and are in clinical trials in the United States and Europe. It has not been determined whether these non-pulsatile devices will be suitable for long-term use.
Novacor LVAS Sales. Sales of the Novacor LVAS contribute to overhead and other indirect costs of producing the Novacor LVAS product. To date, however, at current volumes the Novacor commercial operations have contributed only modest gross margin or have resulted in gross margin deficits. WorldHeart expects to continue to increase gross margin through increased sales and reduced manufacturing costs, however, to the extent that it is unable to do so, there is risk that WorldHeart could be adversely affected and the Novacor operations could continue to consume cash resources.
OUTSTANDING SHARE DATA
The outstanding share data as at December 31, 2004, is a follows:
| | Number outstanding
|
|---|
| Common shares | | 15,744,522 |
| Options | | 3,172,771 |
| Warrants | | 24,424,233 |
| Convertible debentures | | 10,655,000 |
| Interest on convertible debentures | | 1,997,813 |
Included in options above are 2,383,725 options subject to shareholder approval of an increase in the number of options available under the employee stock option plan.
A conversion rate of $1.00 per common share was assumed for the interest on the convertible debentures. Interest accrues on the face value of the convertible debentures at an annual non-compounding rate of 3%.
NEW ACCOUNTING PRONOUNCEMENTS
In January 2005, the Canadian Institute of Chartered Accountants ("CICA") released Section 1531, "Comprehensive Income"("Section 1531"), which requires financial instruments to be measured at fair value. The provisions of Section 1531 are effective for years starting on or after October 31, 2006. The Corporation plans on adopting this new pronouncement in 2007, the impact of which is not known at this time.
C-2-17
APPENDIX C-3
MANAGEMENT'S DISCUSSION AND ANALYSIS
INTRODUCTION
World Heart Corporation and its subsidiaries are collectively referred to as "WorldHeart". The following Management Discussion and Analysis of Financial Condition and Results of Operations ("MD&A") was prepared by management as of April 26, 2005, was approved by the Board of Directors and is an update of our discussion and analysis for the year ended December 31, 2004. This MD&A discusses material changes in WorldHeart's financial condition and results of operations and cash flows for the three months ended March 31, 2005. Such discussion and comments on the liquidity and capital resources should be read in conjunction with the information contained in WorldHeart's audited consolidated financial statements for the year ended December 31, 2004, prepared in accordance with accounting principles generally accepted in the United States ("GAAP") included in our Annual Report on Form 10 – KSB for the fiscal year ended December 31, 2004. Significant differences between Canadian GAAP and U.S. GAAP are presented in Note 6 to the unaudited consolidated financial statements for the three months ended March 31, 2005. In this discussion, all amounts are in United States dollars ("U.S. dollars") unless otherwise stated.
The discussion and comments contained hereunder include both historical information and forward-looking information. The forward-looking information, which generally is information stated to be anticipated, expected, or projected by management, involves known and unknown risks, uncertainties and other factors that may cause the actual results and performance to be materially different from any future results and performance expressed or implied by such forward-looking information. Potential risks and uncertainties include, without limitation, the uncertainties inherent in the development of new products for use in the human body, WorldHeart's need for significant additional funding, successful integration of acquisitions, WorldHeart's need to establish reimbursement mechanisms and product acceptance from third-party payers, extensive government regulation of our products, rapid developments in technology, including developments by competitors, potential lack of anticipated synergies and efficiencies of scale related to the MedQuest Products, Inc. ("MedQuest") acquisition, and potential difficulties in the integration of the MedQuest operations.
OVERVIEW
WorldHeart is a global medical device company currently focused on the development and commercialization of implantable pulsatile ventricular assist devices ("VADs"), which are mechanical pumps that allow for the restoration of normal blood circulation to patients suffering from late-stage congestive heart failure. WorldHeart has facilities in Oakland, California, United States, Ottawa, Ontario, Canada, and Heesch, Netherlands. On August 25, 2004, we approved a plan to consolidate the Ottawa operations into our facility in Oakland. WorldHeart currently derives substantially all of its revenue from its Novacor® LVAS (left ventricular assist system) and related peripheral equipment.
WorldHeart manufactures and distributes the Novacor LVAS. The Corporation sells its Novacor products directly to medical clinics and hospitals in the United States, most of Europe and Canada and through a distributor in Japan and certain other countries.
The Novacor LVAS is commercially approved as a bridge to transplantation device in the United States and Canada. In Europe, the Novacor LVAS has unrestricted approval for use as an alternative to transplantation, a bridge to transplantation, and to support patients who may have an ability to recover the use of their natural heart. In Japan, the device is commercially approved for use in cardiac patients at risk of imminent death from non-reversible left ventricular failure for which there is no alternative except heart transplantation.
WorldHeart has commenced a long-term use (Destination Therapy) trial in the United States called RELIANT (RandomizedEvaluation of the NovacorLVAS in aNon-TransplantPopulation). Data from the RELIANT Trial is expected to support a Pre-market Approval Supplement that will request approval for the use of the Novacor LVAS by non-transplant eligible, or Destination Therapy. patients in the United States.
WorldHeart is in the process of developing a next-generation implantable pulsatile VAD and, subject to certain approvals, has recently agreed to acquire, from MedQuest, the technology associated with a magnetically
C-3-1
levitated centrifugal rotary VAD. Development work on the Novacor II small pulsatile VAD will continue with initial clinical trials in 2007, and initial commercial approvals in Europe in 2008 and in the United States in 2010.
SUMMARY OF QUARTERLY RESULTS
| | Three Months Ended
| |
|---|
| | March 31,
2005
| | December 31,
2004
| | September 30,
2004
| | June 30,
2004
| |
|---|
| Net revenue | | $ | 3,416,988 | | $ | 2,986,980 | | $ | 2,250,050 | | $ | 2,146,388 | |
| Net loss | | | (3,942,396 | ) | | (5,608,818 | ) | | (11,620,584 | ) | | (5,402,789 | ) |
| Net loss applicable to common shareholders | | | (3,942,396 | ) | | (6,293,510 | ) | | (10,935,893 | ) | | (5,402,792 | ) |
| Basic and diluted loss per share | | $ | (0.24 | ) | $ | (0.41 | ) | $ | (0.71 | ) | $ | (0.35 | ) |
| | Three Months Ended
| |
|---|
| | March 31,
2004
| | December 31,
2003
| | September 30,
2003
| | June 30,
2003
| |
|---|
| Net revenue | | $ | 2,192,343 | | $ | 559,704 | | $ | 2,023,170 | | $ | 1,944,702 | |
| Net loss | | | (3,509,607 | ) | | (4,846,928 | ) | | (8,125,804 | ) | | (95,642 | ) |
| Net loss applicable to common shareholders | | | (3,509,607 | ) | | (6,864,152 | ) | | (9,222,682 | ) | | (308,212 | ) |
| Basic and diluted loss per share | | $ | (0.23 | ) | $ | (0.47 | ) | $ | (2.24 | ) | $ | (0.10 | ) |
RESULTS OF OPERATIONS FOR THE THREE MONTH PERIOD ENDED MARCH 31, 2005 COMPARED TO THE THREE MONTH PERIOD ENDED MARCH 31, 2004
Consolidated results of operations
| | Three months ended March 31, 2005
| | As a % of revenue
| | Three months ended March 31, 2004
| | As a % of revenue
|
|---|
| Revenue | | $ | 3,416,988 | | 100% | | $ | 2,192,343 | | 100% |
| Cost of goods sold | | | (2,111,702 | ) | 62% | | | (1,178,397 | ) | 54% |
| | |
| |
| |
| |
|
| Gross margin | | | 1,305,286 | | 38% | | | 1,013,946 | | 46% |
| Selling, general and administrative | | | (3,445,829 | ) | | | | (3,035,445 | ) | |
| Research and development | | | (1,187,558 | ) | | | | (1,345,568 | ) | |
| Restructuring costs | | | (129,498 | ) | | | | — | | |
| Amortization of intangibles | | | (127,514 | ) | | | | (129,166 | ) | |
| Foreign exchange loss | | | (24,372 | ) | | | | (100,467 | ) | |
| Investment income | | | 20,026 | | | | | 90,797 | | |
| Interest and financing expenses | | | (352,937 | ) | | | | (3,704 | ) | |
| | |
| | | |
| | |
| Net loss | | $ | (3,942,396 | ) | | | $ | (3,509,607 | ) | |
| | |
| | | |
| | |
Revenue. The sale of Novacor LVAS implant kits ("implant kits") and related peripheral equipment and services accounts for substantially all of WorldHeart's revenues. WorldHeart primarily sells its products directly, except for certain countries, including Japan, where we sell through distributors.
The composition of revenue is as follows:
| | Three months ended March 31, 2005
| | Three months ended March 31, 2004
|
|---|
| | $
| | Number of Units
| | $
| | Number of Units
|
|---|
| Implant kits | | 2,146,326 | | 32 | | 1,729,266 | | 26 |
| Peripherals and other | | 1,270,662 | | | | 463,077 | | |
| | |
| | | |
| | |
| Revenue | | 3,416,988 | | | | 2,192,343 | | |
| | |
| | | |
| | |
C-3-2
Revenue for the first quarter of 2005 increased to approximately $3.4 million representing an increase of 56% over the same quarter in 2004. Novacor LVAS implant kit revenue increased to approximately $2.1 million for the quarter ended March 31, 2005 compared to approximately $1.7 million for the quarter ended March 31, 2004, representing an increase of 24%. Implant kits recognized as revenue in the first quarter of 2005 totaled 32 compared to 26 in the first quarter of 2004. The revenue per implant kit increased during the first quarter of 2005. In the first quarter of 2004 there was a multiple implant kit transaction with a discounted price per implant kit. We expect the average revenue per implant kit to continue to increase as centers become enabled with the required peripheral equipment and increase implant volume under the RELIANT Trial.
An additional five implant kits were shipped and invoiced in the first quarter of 2005; however, revenue relating to these implant kits was deferred at the time of shipment as the transactions did not meet our revenue recognition criteria. As at December 31, 2004, 17 implant kits had been shipped and invoiced but had been deferred as revenue at the time of shipment as the transactions also did not meet our revenue recognition criteria. Revenue was recognized on four of these implant kits during the first quarter of 2005, leaving 18 implant kits in deferred revenue at March 31, 2005. This represents approximately $1.1 million and will be recognized as the earnings process is completed in future periods. The remaining balance in deferred revenue of approximately $564,000 mainly relates to undelivered components of multiple element transactions, which will be recognized when delivered.
Peripherals and other revenues, including Novacor LVAS hardware and peripheral sales and services for the quarter ended March 31, 2005, were approximately $1.3 million, which represents an increase of 174% over peripherals and other revenue of approximately $460,000 recorded in the quarter ended March 31, 2004. We anticipate that the proportionate sales may shift from hardware, peripheral and service sales to implant kit sales as centers enrolled in our RELIANT Trial increase implant volume under the RELIANT Trial. As at March 31, 2005, there were 10 centers and nine patients enrolled in the RELIANT Trial.
Cost of goods sold. For the first quarter of 2005, the overall cost of goods sold increased to 62% of revenue as compared to 54% for the first quarter of 2004.
Despite continued efforts to improve per unit manufacturing costs, we continued to experience significant production inefficiencies during the first quarter of 2005 caused by supply shortages associated with the acceptance process for components being manufactured by new suppliers and production volumes well below capacity. This resulted in higher Novacor LVAS per unit production costs. We expect the cost per unit to decrease as we continue to address the supply issues and increase production.
Gross Margin. Gross margin for the three months ended March 31, 2005 decreased to 38% of revenues from 46% of revenues for the three months ended March 31, 2004. This decrease was due in part to production inefficiencies. In addition, during the first quarter of 2005, certain lower margin sales were made to new centers for the initial equipment investment needed to begin a Novacor LVAS implant program at those centers. Improvement in gross margin percentage is expected as costs per unit decrease from contract manufacturing of some components and production volume increases. In addition, we expect the effects of higher incentives on start-up equipment initially offered to certain new centers to decrease as the centers shift to purchasing implant kits in response to usage.
Selling, general and administrative. Selling, general and administrative expenses consist primarily of payroll and related expenses for executives, sales, marketing, accounting and administrative personnel, professional fees, communication expenses, promotional activities, costs associated with meeting multi-jurisdictional regulatory requirements, insurance premiums, and occupancy and other general corporate expenses.
The composition of selling, general and administrative expenses is as follows:
| | Three months ended March 31, 2005
| | Three months ended March 31, 2004
|
|---|
| Selling | | $ | 1,768,114 | | $ | 1,196,555 |
| General and administrative | | | 1,677,715 | | | 1,838,890 |
| | |
| |
|
| | | $ | 3,445,829 | | $ | 3,035,445 |
| | |
| |
|
C-3-3
Selling, general and administrative expenses for the three months ended March 31, 2005 increased by approximately $410,000 or 14% over the same period in 2004. Selling expenses increased in the first quarter of 2005, as compared to the first quarter of 2004, as we increased sales personnel and our sales and marketing program initiatives in the United States and Europe. General and administrative expenses have decreased in the first quarter of 2005 compared to the first quarter of 2004 as we have started to experience the costs savings associated with the consolidation of our North American operations.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing, and configuration of equipment expenses, trial expenses, and regulatory affairs expenses.
Research and development expenses for the three months ended March 31, 2005 decreased by approximately $158,000 or 12% compared to the three months ended March 31, 2004. Gross research and development expenditures excluding the impact of government programs decreased by 20% to approximately $1.2 million in first quarter of 2005 compared to approximately $1.5 million in the first quarter of 2004.
Lower research and development costs for the first quarter of 2005 compared to the same period in 2004 reflect the reduction in expenses due to the consolidation of the North American operations. Development work on the next-generation pulsatile VAD will continue with initial clinical trials expected to begin in 2007.
Investment tax credits for the three months ended March 31, 2004 totalled approximately $137,000 and relate to Canadian provincial government assistance received. We continue to review various tax credit programs but only record a credit when collection of the claim is reasonably assured.
Restructuring costs. On August 25, 2004, we approved a plan to consolidate the Ottawa, Ontario operations into our facilities in Oakland, California where the Novacor LVAS is manufactured. The consolidation of the North American operations into one location is expected to reduce ongoing business expenses and improve operational efficiency and effectiveness.
We expect the restructuring to be completed by the end of June 2005 and expect to incur total restructuring charges of approximately $2.7 million comprised of employee severance costs of approximately $1.6 million; contract settlement costs of approximately $724,000; write down of capital assets to net realizable value of approximately $335,000; and other costs of approximately $67,000. As at March 31, 2005, we revised our estimate of total restructuring costs to reflect additional employee severances of approximately $26,000, changes in our estimates that resulted in an increase in contract settlement costs of approximately $3,800 and other restructuring costs of approximately $23,000 and the receipt of proceeds from the disposition of capital assets of approximately $4,000.
Restructuring costs accrued and expensed to date are as follows:
| | Employee severance
| | Contract settlement
| | Write down
| | Other
| | Total
| |
|---|
| Costs accrued during 2004 | | $ | 1,396,037 | | $ | 57,653 | | $ | 338,595 | | $ | 22,509 | | $ | 1,814,794 | |
| Costs paid or settled in 2004 | | | (795,923 | ) | | — | | | (338,595 | ) | | (8,624 | ) | | (1,143,142 | ) |
| | |
| |
| |
| |
| |
| |
| Balance in accrued liabilities as at December 31, 2004 | | | 600,114 | | | 57,653 | | | — | | | 13,885 | | | 671,652 | |
| Costs accrued during the period | | | 110,539 | | | — | | | (3,546 | ) | | 22,505 | | | 129,498 | |
| Costs paid or settled in the period | | | (674,414 | ) | | — | | | 3,546 | | | (20,518 | ) | | (691,386 | ) |
| | |
| |
| |
| |
| |
| |
| Balance in accrued liabilities as at March 31, 2005 | | $ | 36,239 | | $ | 57,653 | | $ | — | | $ | 15,872 | | $ | 109,764 | |
| | |
| |
| |
| |
| |
| |
During the quarter ended March 31, 2005, actual restructuring costs of approximately $129,000 have been recorded. Employee severance costs that have been recorded represent the amounts employees have earned to March 31, 2005. Contract settlement costs are recorded when notice is provided that the contract is being terminated and WorldHeart ceases to use the right conveyed under the contract. We expect to provide these notices in the second quarter of 2005 with a cease use date of June 30, 2005. The capital assets were written
C-3-4
down to their net realizable value when the decision was made to consolidate the North American operations and it was determined that the carrying value of the capital assets was impaired. Other restructuring costs are recognized when the services are actually received.
Amortization of intangibles. Amortization of intangibles for the first quarter of 2005 was approximately $127,000 compared to approximately $129,000 for the same period in 2004. The majority of the intangible assets arose from the purchase of the Novacor business in June 2000. Most of these assets have now reached the end of their original estimated useful life and have been fully amortized. As at March 31, 2005, intangible assets totaling approximately $127,000 remain unamortized.
Interest expense and financing costs. During the first quarter of 2005, interest and financing costs of approximately $353,000 were recorded compared to costs of approximately $4,000 during the first quarter of 2004. Interest expense and financing costs in the three month period ended March 31, 2005, consisted mainly of the interest and accretion related to the convertible debentures. These non-cash interest charges will continue to accrete ratably on the convertible debentures until their conversion or maturity. It is anticipated that all outstanding debentures will be converted in accordance with the terms of the MedQuest acquisition.
LIQUIDITY AND CAPITAL RESOURCES
Historically, WorldHeart has funded losses from operations through the sale of equity and issuance of debt instruments. Combined with revenues, these funds have provided us with the resources to operate our business, attract and retain key personnel, fund our research and development program and clinical trials, apply and obtain the necessary regulatory approvals and develop our technology and products.
Liquidity. At March 31, 2005, WorldHeart had cash and cash equivalents of approximately $4.5 million and net working capital of approximately $11.0 million compared to cash, cash equivalents and short-term investments of approximately $8.8 million and net working capital of approximately $14.1 million at December 31, 2004.
Cash, cash equivalents and short-term investments decreased in the first quarter of 2005 by approximately $4.3 million. This primarily related to cash used to fund operating losses of approximately $4.2 million including a net pay down of approximately $307,000 of accounts payable and accrued compensation and an increase in inventory of approximately $341,000 and cash used in investing activities of approximately $93,000 for the purchase of capital assets
On January 31, 2005, WorldHeart announced that it had entered in to a definitive agreement to acquire the assets of MedQuest, subject to certain approvals, consents, conditions and the approval of the shareholders. MedQuest, based in Salt Lake City, Utah, is in the final development stages of its HeartQuest™ ventricular assist device, a magnetically levitated centrifugal rotary blood pump. As separate components of that transaction, WorldHeart also announced that it had entered into definitive agreements to issue through a private placement, approximately 8.9 million common shares, at $1.35 per share, for gross proceeds of $12 million and raise approximately $10.7 million through the exercise of 10.7 million existing warrants at an exercise price of $1.00 per share, assuming shareholder approval of a reduction in the Warrant exercise price from $1.55 to $1.00 per share. These transactions are expected to be completed contemporaneously with the MedQuest acquisition in the second quarter of 2005. Upon completion of the private placement and warrant exercise it is anticipated that WorldHeart will receive approximately $22.7 million of cash and extinguish all of its convertible debentures providing adequate resources to fund operations into 2006 when we may be required to obtain additional funding to sustain operations.
Our long-term working capital and capital requirements will depend upon numerous factors, including the following: our continued focus on improving commercial operations and viability through the expanded sales of Novacor LVAS; increasing revenues through higher average selling prices and enrollment in the RELIANT Trial; decreasing production costs through a manufacturing cost reduction program; the rate of investment in our next-generation technologies, including our under-development pulsatile VAD and the rotary VAD expected to be acquired in the second quarter of 2005; the costs associated with clinical trials and the approval process for the next-generation technologies; and our general efforts to improve operational efficiency and effectiveness.
C-3-5
OUTLOOK
External environment. The market for effective pharmacological, electro-physiological or device treatments for late-stage heart failure continues to grow, and despite significant progress on many fronts, no competitive breakthroughs have been announced or are believed to be imminent. The number of diagnosed heart failure patients is increasing as a result of both population demographic trends, and also as a response to the increasing number of survivors from sudden cardiac events, many of whom subsequently develop congestive heart failure. We believe the potential market for our heart assist devices, with their current regulatory approvals, continues to be at least 100,000 patients per annum in North America, Europe, Japan and certain other countries.
There is growing evidence of increasing clinical acceptance of the use of mechanical circulatory assist therapies in the treatment of late stage heart failure, and on two separate occasions in March 2003 and May 2004, the Centers for Medicare and Medicaid Services in the United States announced the extension of reimbursement coverage for both bridge and destination therapy VAD use. These extensions have resulted in increased payments for procedures using such devices. These developments are expected to continue to accelerate the use of ventricular assist devices both in the United States and elsewhere. Also, effective April 1, 2004, reimbursement in Japan for implantable VADs was approved. Novacor LVAS is the only approved device of its kind in Japan.
In the United States, WorldHeart commenced a randomized equivalency trial called RELIANT in the second quarter of 2004. In this trial the Novacor LVAS is being randomized to the Thoratec HeartMate® XVE LVAS, which will be the control arm. RELIANT is a randomized 40 center equivalency trial where patients will receive a Novacor LVAS or Thoratec Corporation's Heartmate®XVE LVAS on a 2:1 ratio. It is expected that data from this trial will support a Premarket Approval Supplement, which will be submitted to the United States Food and Drug Administration for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy).
We continue to believe that the long-term use or Destination Therapy market will evolve rapidly when devices are accepted as reliable and durable. A wider adoption of VADs as a Destination Therapy will likely occur with the next-generation VADs and we believe that late-stage heart failure will be best served by a combination of pulsatile and rotary flow VADs.
Internal environment. We expect to continue to incur net operating losses on a quarterly basis, through 2006, assuming the completion of the MedQuest acquisition in the second quarter of 2005. We believe that our commercial operations associated with Novacor LVAS will continue to improve. We expect to continue to increase revenues as a result of higher Novacor LVAS Implant Kit sales, particularly in the United States. Enrollment in the RELIANT Trial commenced in the second quarter of 2004 and implantation under the RELIANT Trial commenced in the third quarter of 2004 and is expected to continue through 2005 and 2006. We also expect gross margin contribution to increase through a combination of higher volumes, which should lead to lower per unit manufacturing costs through more efficient and optimal manufacturing levels and continuation of our manufacturing cost reduction program.
In addition, in the third quarter of 2004, a plan was put in place to consolidate the Ottawa operations into our Oakland operations to further improve operating cost structure, increase efficiency and improve effectiveness.
We expect to complete the acquisition of MedQuest in the second quarter of 2005 and commence an initial feasibility clinical trial of its magnetically levitated centrifugal rotary VAD by the beginning of 2006. Initial commercial approvals for the MedQuest rotary VAD are expected to occur in Europe in 2007 with approvals for the MedQuest rotary VAD in the United States two years later.
Development work on the Novacor II pulsatile VAD will continue with anticipated initial clinical trials in 2007, and anticipated initial commercial approvals in Europe in 2008 and in the United States in 2010.
OTHER FACTORS
Current Product. WorldHeart's near-term commercial operations are exclusively focused on its Novacor LVAS Implant Kit and related equipment and support. Commercial success will therefore be dependant on our
C-3-6
ability to continue to increase Novacor LVAS sales. Novacor LVAS is approved for use by eligible heart failure patients without limitation, in Europe and Japan and as a bridge-to-transplant device in the United States and Canada. WorldHeart has commenced an equivalency clinical trial in the second quarter of 2004 in the United States called RELIANT in which patients will be randomized to receive either the Novacor LVAS or the Thoratec HeartMate® XVE LVAS. It is expected that data from this clinical trial will support a Premarket Approval Supplement, which will be submitted to the United States Food and Drug Administration, for the use of the Novacor LVAS by non-transplant eligible patients (i.e. destination therapy). Implants under this trial are eligible for reimbursement by the Centers for Medicare and Medicaid Services ("CMS") and certain private insurers. We believe that approval of the Novacor LVAS for long-term or destination therapy use in the United States would increase the market for the Novacor LVAS substantially.
Pulsatile VADs. WorldHeart has several competitors with commercially approved pulsatile VADs having pumps that are externally located or abdominally implanted. The devices which are developed by the Corporation's competitors are now primarily used as bridges to transplant, although one product (Thoratec HeartMate® XVE) was approved in 2002 for a destination therapy indication for use in the United States. Some of WorldHeart's existing known competitors have significantly greater financial, production and marketing resources. There can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
WorldHeart believes that our pulsatile next-generation implantable VAD is the only pulsatile device that is currently at an advanced stage of development. During 2002 management merged the three next-generation VAD development programs into one VAD program. We expect clinical trials for our next-generation implantable pulsatile VAD will commence in 2007. However, there can be no assurance that the development program, including pre-clinical and human clinical trials will be successful or that the necessary regulatory approvals will be received.
Non-pulsatile VADs. As discussed above, subsequent to year-end WorldHeart entered into a definitive agreement to purchase the assets of MedQuest including its rotary flow, centrifugal, magnetically levitated next generation VAD technology. We expect clinical trials for our next-generation implantable non-pulsatile VAD will commence in early 2006. However, there can be no assurance that the development program, including pre-clinical and human clinical trials, will be successful or that the necessary regulatory approvals will be received.
Research and development by WorldHeart's competitors is proceeding for several next-generation non-pulsatile continuous flow assist devices. Certain of these devices have received CE Mark in Europe and are in clinical trials in the United States and Europe. It has not been determined whether these non-pulsatile devices will be suitable for long-term use.
Novacor LVAS Sales. Sales of the Novacor LVAS contribute to overhead and other indirect costs of producing the Novacor LVAS product. To date, however, at current volumes the Novacor commercial operations have contributed only modest gross margin or have resulted in gross margin deficits. We expect to continue to increase gross margin through increased sales and reduced manufacturing costs, however, to the extent that we are unable to do so, there is risk that we could be adversely affected and the Novacor operations could continue to consume cash resources.
NEW ACCOUNTING PRONOUNCEMENTS
In June 2001, the FASB issued SFAS No. 148,which amended the transitional provisions of SFAS No. 123 for companies electing to recognize employee stock-based compensation using the fair value based method. The provisions of FASB No. 148 will be effective for years beginning on or after January 1, 2006. In addition, during 2004, the FASB issued SFAS No. 123R, which also amended the provisions of SFAS No. 123. The provisions of FASB No. 123R will be effective for years beginning on or after January 1, 2006. The Corporation plans on adopting these new pronouncements in 2006.
C-3-7
APPENDIX D
MedQuest Products, Inc.
(A Development Stage Enterprise)
Financial Statements as of June 30, 2004 and 2003,
and for the years then ended, and
for the period from inception
(December 16, 1993) to June 30, 2004,
And
Financial Statements as of March 31, 2005
and for the nine months ended
March 31, 2005 and 2004 (unaudited)
D-1
CONTENTS
| | Page
|
|---|
| REPORT OF INDEPENDENT AUDITORS | | D-3 |
BALANCE SHEETS |
|
D-4 |
STATEMENTS OF OPERATIONS |
|
D-5 |
STATEMENT OF STOCKHOLDERS' DEFICIT |
|
D-7 |
STATEMENTS OF CASH FLOWS |
|
D-8 |
NOTES TO FINANCIAL STATEMENTS |
|
D-10 |
D-2
REPORT OF INDEPENDENT AUDITORS
To the Board of Directors and Stockholders
of MedQuest Products, Inc.
In our opinion, the accompanying balance sheets and the related statements of operations, of stockholders' deficit and of cash flows present fairly, in all material respects, the financial position of MedQuest Products, Inc. (a development stage enterprise) (the "Company") at June 30, 2004 and 2003, and the results of its operations and its cash flows for the years then ended and, cumulatively, for the period from inception (December 16, 1993) to June 30, 2004 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States of America, which require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has a working capital deficit as of June 30, 2004 and has sustained recurring net losses and negative cash flows from operations since inception. These matters raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
[/s/ PricewaterhouseCoopers LLP]
Salt Lake City, Utah
January 11, 2005, except for notes 14 and 15
as to which the date is May 23, 2005
D-3
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
BALANCE SHEETS
| | March 31, 2005
| | June 30, 2004
| | June 30, 2003
| |
|---|
| | (unaudited)
| |
| |
| |
|---|
| Assets | | | | | | | | | | |
| Current assets: | | | | | | | | | | |
| Cash and cash equivalents | | $ | 412,456 | | $ | 890,452 | | $ | 62,326 | |
| Accounts receivable, net | | | 58,110 | | | 35,764 | | | — | |
| Other current assets | | | 19,685 | | | 22,682 | | | 23,778 | |
| | |
| |
| |
| |
| | | Total current assets | | | 490,251 | | | 948,898 | | | 86,104 | |
Property and equipment, net |
|
|
204,570 |
|
|
95,724 |
|
|
179,799 |
|
| Purchased intangibles, net | | | 336,097 | | | 357,796 | | | 386,728 | |
| | |
| |
| |
| |
| | | Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | |
| | |
| |
| |
| |
Liabilities and Stockholders' Deficit |
|
|
|
|
|
|
|
|
|
|
| Current liabilities: | | | | | | | | | | |
| Accounts payable | | $ | 165,608 | | $ | 168,623 | | $ | 224,622 | |
| Accrued liabilities | | | 243,478 | | | 792,975 | | | 443,919 | |
| Notes payable | | | 1,000,000 | | | 6,750,000 | | | 4,050,000 | |
| License fees payable, current | | | 28,496 | | | 46,319 | | | 43,493 | |
| Capital lease obligations, current | | | 434 | | | 18,260 | | | 20,152 | |
| | |
| |
| |
| |
| | | Total current liabilities | | | 1,438,016 | | | 7,776,177 | | | 4,782,186 | |
License fees payable, non-current |
|
|
110,114 |
|
|
110,114 |
|
|
138,609 |
|
| Capital lease obligations, non-current | | | — | | | 1,079 | | | 19,339 | |
| | |
| |
| |
| |
| | | Total liabilities | | | 1,548,130 | | | 7,887,370 | | | 4,940,134 | |
| | |
| |
| |
| |
| Commitments (Note 9) | | | | | | | | | | |
Stockholders' deficit: |
|
|
|
|
|
|
|
|
|
|
| | Preferred stock, $. 001 par value, 25,000,000 shares authorized; 6,675,782 shares issued and outstanding at March 31, 2005 (unaudited); 105,179 shares issued and outstanding at June 30, 2004 and 2003 (Liquidation preference of $1.36 per share at March 31, 2005 — Unaudited) | | | 6,676 | | | 105 | | | 105 | |
| | Common stock, $. 001 par value, 40,000,000 shares authorized; 127,588 shares issued and outstanding at March 31, 2005 (unaudited); 22,409 shares issued and outstanding at June 30, 2004 and 2003 | | | 127 | | | 22 | | | 22 | |
| Additional paid-in capital | | | 14,996,956 | | | 5,958,862 | | | 5,958,862 | |
| Deficit accumulated during the development stage | | | (15,520,971 | ) | | (12,443,941 | ) | | (10,211,004 | ) |
| Deferred compensation from issuance of stock options | | | — | | | — | | | (35,488 | ) |
| | |
| |
| |
| |
| | | Total stockholders' deficit | | | (517,212 | ) | | (6,484,952 | ) | | (4,287,503 | ) |
| | |
| |
| |
| |
| | | Total liabilities and stockholders' deficit | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | |
| | |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
D-4
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
STATEMENTS OF OPERATIONS
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| | Period From Inception (December 16, 1993) to June 30, 2004
| |
|---|
| Revenues: | | | | | | | | | | |
| | Research funding and other revenues | | $ | — | | $ | — | | $ | 6,759,964 | |
| | Governmental grants | | | 99,389 | | | — | | | 1,585,244 | |
| | |
| |
| |
| |
| | | Total revenues | | | 99,389 | | | — | | | 8,345,208 | |
| | |
| |
| |
| |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
| | Research and development | | | 1,006,482 | | | 1,828,991 | | | 13,472,468 | |
| | Depreciation and amortization | | | 136,676 | | | 151,087 | | | 674,370 | |
| | General and administrative | | | 845,552 | | | 1,562,618 | | | 6,225,853 | |
| | |
| |
| |
| |
| | | Total operating expenses | | | 1,988,710 | | | 3,542,696 | | | 20,372,691 | |
| | |
| |
| |
| |
| Loss from operations | | | (1,889,321 | ) | | (3,542,696 | ) | | (12,027,483 | ) |
| | |
| |
| |
| |
Interest income |
|
|
1,260 |
|
|
4,902 |
|
|
180,123 |
|
| Interest expense | | | (344,776 | ) | | (237,838 | ) | | (623,395 | ) |
| | |
| |
| |
| |
| Loss before income taxes | | | (2,232,837 | ) | | (3,775,632 | ) | | (12,470,755 | ) |
Income tax benefit (expense) |
|
|
(100 |
) |
|
(2,167 |
) |
|
26,814 |
|
| | |
| |
| |
| |
| Net loss | | $ | (2,232,937 | ) | $ | (3,777,799 | ) | $ | (12,443,941 | ) |
| | |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
D-5
| | Nine Months Ended March 31, 2005
| | Nine Months Ended March 31, 2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| Revenues: | | | | | | | |
| | Governmental grants | | $ | 101,840 | | $ | 63,625 | |
| | |
| |
| |
Operating expenses: |
|
|
|
|
|
|
|
| | Research and development | | | 2,168,460 | | | 594,347 | |
| | Depreciation and amortization | | | 83,593 | | | 107,100 | |
| | General and administrative | | | 1,184,603 | | | 533,741 | |
| | |
| |
| |
| | | Total operating expenses | | | 3,436,656 | | | 1,235,188 | |
| | |
| |
| |
| Loss from operations | | | (3,334,816 | ) | | (1,171,563 | ) |
Interest income |
|
|
6,065 |
|
|
327 |
|
| Interest expense | | | (12,150 | ) | | (266,955 | ) |
| Gains on forgiveness of accrued interest | | | 263,871 | | | — | |
| | |
| |
| |
| Net loss | | $ | (3,077,030 | ) | $ | (1,438,191 | ) |
| | |
| |
| |
The accompanying notes are an integral part of these financial statements.
D-6
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
STATEMENT OF STOCKHOLDERS' DEFICIT
Period from Inception (December 16, 1993) to March 31, 2005
| | Preferred Stock
| | Common Stock
| |
| |
| |
| |
| |
|---|
| |
| | Deficit Accumulated During the Development Stage
| | Deferred Compensation from Issuance of Stock Options
| |
| |
|---|
| | Additional Paid-in Capital
| | Total Stockholders' Equity (Deficit)
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| |
|---|
| Balance at inception (December 16, 1993) | | — | | $ | — | | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| Issuance of preferred stock on December 6, 2000 at $47.539 per share | | 105,179 | | | 105 | | — | | | — | | | 4,999,895 | | | — | | | — | | | 5,000,000 | |
| Issuance of common stock upon the exercise of stock options | | — | | | — | | 2,609 | | | 2 | | | 5,217 | | | — | | | — | | | 5,219 | |
| Issuance of common stock for services on December 16, 1993, at $0.125 per share | | | | | | | 19,800 | | | 20 | | | 2,455 | | | | | | | | | 2,475 | |
| Deferred compensation from issuance of stock options | | — | | | — | | — | | | — | | | 995,059 | | | — | | | (995,059 | ) | | — | |
| Amortization of deferred compensation from issuance of stock options | | — | | | — | | — | | | — | | | — | | | — | | | 863,385 | | | 863,385 | |
| Accumulated deficit from inception (December 16, 1993) to June 30, 2002 | | — | | | — | | — | | | — | | | — | | | (6,433,205 | ) | | — | | | (6,433,205 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2002 | | 105,179 | | | 105 | | 22,409 | | | 22 | | | 6,002,626 | | | (6,433,205 | ) | | (131,674 | ) | | (562,126 | ) |
| Adjustment for forfeiture of unvested stock options | | — | | | — | | — | | | — | | | (68,288 | ) | | — | | | 68,288 | | | — | |
| Issuance of stock options for services on February 1, 2003, at $15.00 per option | | — | | | — | | — | | | — | | | 24,524 | | | — | | | — | | | 24,524 | |
| Amortization of deferred compensation from issuance of stock options | | — | | | — | | — | | | — | | | — | | | — | | | 27,898 | | | 27,898 | |
| Net loss | | | | | | | | | | | | | | | | (3,777,799 | ) | | | | | (3,777,799 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2003 | | 105,179 | | | 105 | | 22,409 | | | 22 | | | 5,958,862 | | | (10,211,004 | ) | | (35,488 | ) | | (4,287,503 | ) |
| Amortization of deferred compensation from issuance of stock options | | — | | | — | | — | | | — | | | — | | | — | | | 35,488 | | | 35,488 | |
| Net loss | | — | | | — | | — | | | — | | | — | | | (2,232,937 | ) | | — | | | (2,232,937 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at June 30, 2004 | | 105,179 | | | 105 | | 22,409 | | | 22 | | | 5,958,862 | | | (12,443,941 | ) | | — | | | (6,484,952 | ) |
| Issuance of common stock upon conversion of preferred stock on September 10, 2004 (unaudited) | | (105,179 | ) | | (105 | ) | 105,179 | | | 105 | | | — | | | — | | | — | | | — | |
| Issuance of preferred stock upon conversion of notes payable and accrued interest on September 10, 2004 (unaudited) | | 5,937,701 | | | 5,938 | | — | | | — | | | 8,038,832 | | | — | | | — | | | 8,044,770 | |
| Issuance of preferred stock on September 30, 2004, at $1.36 per share (unaudited) | | 738,081 | | | 738 | | — | | | — | | | 999,262 | | | — | | | — | | | 1,000,000 | |
| Net loss (unaudited) | | — | | | — | | — | | | — | | | — | | | (3,077,030 | ) | | — | | | (3,077,030 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2005 (unaudited) | | 6,675,782 | | $ | 6,676 | | 127,588 | | $ | 127 | | $ | 14,996,956 | | $ | (15,520,971 | ) | $ | — | | $ | (517,212 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these financial statements.
D-7
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CASH FLOWS
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| | Period From Inception (December 16, 1993) to June 30, 2004
| |
|---|
| Cash flows from operating activities: | | | | | | | | | | |
| | Net loss | | $ | (2,232,937 | ) | $ | (3,777,799 | ) | $ | (12,443,941 | ) |
| | Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | |
| | | Depreciation and amortization | | | 136,676 | | | 151,087 | | | 674,370 | |
| | | Common stock and stock options issued for services | | | — | | | 24,524 | | | 26,999 | |
| | | Amortization of deferred compensation from issuance of stock options | | | 35,488 | | | 27,898 | | | 926,771 | |
| | | Loss on disposal of purchased intangible | | | — | | | 2,000 | | | 2,000 | |
| | | Changes in current assets and liabilities: | | | | | | | | | | |
| | | | Accounts receivable, net | | | (35,764 | ) | | — | | | (35,764 | ) |
| | | | Other current assets | | | 1,096 | | | 16,422 | | | (22,682 | ) |
| | | | Accounts payable and accrued liabilities | | | 293,057 | | | 209,114 | | | 961,598 | |
| | |
| |
| |
| |
| | | | | Net cash used in operating activities | | | (1,802,384 | ) | | (3,346,754 | ) | | (9,910,649 | ) |
| | |
| |
| |
| |
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
| | Capital expenditures | | | (23,669 | ) | | (29,255 | ) | | (517,699 | ) |
| | Purchased intangibles | | | — | | | — | | | (175,000 | ) |
| | |
| |
| |
| |
| | | | | Net cash used in investing activities | | | (23,669 | ) | | (29,255 | ) | | (692,699 | ) |
| | |
| |
| |
| |
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
| | Principal payments on license fees payable | | | (25,669 | ) | | (77,039 | ) | | (162,478 | ) |
| | Principle payments on capital lease obligations | | | (20,152 | ) | | (21,266 | ) | | (98,941 | ) |
| | Proceeds from notes payable | | | 2,700,000 | | | 2,550,000 | | | 6,750,000 | |
| | Proceeds from issuance of preferred stock | | | — | | | — | | | 5,000,000 | |
| | Proceeds from exercise of common stock options | | | — | | | — | | | 5,219 | |
| | |
| |
| |
| |
| | | | | Net cash provided by financing activities | | | 2,654,179 | | | 2,451,695 | | | 11,493,800 | |
| | |
| |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | 828,126 | | | (924,314 | ) | | 890,452 | |
| Cash and cash equivalents at beginning of period | | | 62,326 | | | 986,640 | | | — | |
| | |
| |
| |
| |
| Cash and cash equivalents at end of period | | $ | 890,452 | | $ | 62,326 | | $ | 890,452 | |
| | |
| |
| |
| |
Supplemental cash flow information: |
|
|
|
|
|
|
|
|
|
|
| | Cash paid for interest | | $ | 14,508 | | $ | 26,965 | | $ | 66,379 | |
Supplemental noncash investing and financing activity: |
|
|
|
|
|
|
|
|
|
|
| | Licenses purchased with license fee payable | | $ | — | | $ | — | | $ | 318,911 | |
| | Property and equipment acquired under capital leases | | $ | — | | $ | — | | $ | 118,281 | |
The accompanying notes are an integral part of these financial statements.
D-8
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CASH FLOWS
| | Nine Months Ended March 31,
2005
| | Nine Months Ended March 31,
2004
| |
|---|
| | (unaudited)
| | (unaudited)
| |
|---|
| Cash flows from operating activities: | | | | | | | |
| | Net loss | | $ | (3,077,030 | ) | $ | (1,438,191 | ) |
| | Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | |
| | | Gain on forgiveness of accrued interest | | | (263,871 | ) | | — | |
| | | Depreciation and amortization | | | 83,593 | | | 107,100 | |
| | | Amortization of deferred compensation from issuance of stock options | | | — | | | 26,616 | |
| | | Changes in current assets and liabilities: | | | | | | | |
| | | | Accounts receivable, net | | | (22,346 | ) | | — | |
| | | | Other current assets | | | 2,997 | | | 2,131 | |
| | | | Accounts payable and accrued liabilities | | | 6,129 | | | 548,893 | |
| | |
| |
| |
| | | | | Net cash used in operating activities | | | (3,270,528 | ) | | (753,451 | ) |
| | |
| |
| |
Cash flows from investing activities: |
|
|
|
|
|
|
|
| | Capital expenditures | | | (170,740 | ) | | (11,692 | ) |
| | |
| |
| |
| | | | | Net cash used in investing activities | | | (170,640 | ) | | (11,692 | ) |
| | |
| |
| |
Cash flows from financing activities: |
|
|
|
|
|
|
|
| | Principal payments on license fees payable | | | (17,823 | ) | | (22,594 | ) |
| | Principal payments on capital lease obligations | | | (18,905 | ) | | (15,640 | ) |
| | Proceeds from notes payable | | | 2,000,000 | | | 1,700,000 | |
| | Proceeds from issuance of preferred stock | | | 1,000,000 | | | — | |
| | |
| |
| |
| | | | | Net cash provided by financing activities | | | 2,963,292 | | | 1,661,766 | |
| | |
| |
| |
| Net (decrease) increase in cash and cash equivalents | | | (477,996 | ) | | 896,623 | |
| Cash and cash equivalents at beginning of period | | | 890,452 | | | 62,326 | |
| | |
| |
| |
| Cash and cash equivalents at end of period | | $ | 412,456 | | $ | 958,949 | |
| | |
| |
| |
Supplemental cash flow information: |
|
|
|
|
|
|
|
| | Cash paid for interest | | $ | 1,613 | | $ | 24,251 | |
Supplemental noncash investing and financing activity: |
|
|
|
|
|
|
|
| | Conversion of notes payable to preferred stock | | $ | 7,750,000 | | $ | — | |
| | Conversion of accrued interest to preferred stock | | $ | 294,770 | | $ | — | |
The accompanying notes are an integral part of these financial statements.
D-9
MEDQUEST PRODUCTS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS
1. THE COMPANY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The Company
Since its inception on December 16, 1993, MedQuest Products, Inc. (the "Company") has been a development stage company engaged primarily in the research and development of a series of implantable blood pumps which will require several years of research and various levels of regulatory approval before they are marketed commercially. The Company's blood pumps incorporate proprietary technology and are designed to be used for long-term recovery of the natural heart and as an alternative to a heart transplant.
Interim Condensed Financial Statements
In the opinion of management, the accompanying unaudited financial statements include all necessary adjustments to present fairly the financial position of the Company as of March 31, 2005, and the results of its operations and its cash flows for the nine months ended March 31, 2005 and 2004, in conformity with accounting principles generally accepted in the United States of America for interim financial information applied on a consistent basis. The results for the nine months ended March 31, 2005 are not necessarily indicative of the results to be expected for the full fiscal year.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been omitted from the unaudited financial statements.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Revenue recognition
The Company recognizes revenues from research and grant funding upon receipt and when the Company is in compliance with the requirements of the funding, if any.
Governmental grants include amounts received indirectly from the U.S. National Institute of Health ("NIH") as part of a $4,200,000 grant administered through another entity. The NIH retains certain rights to any technology developed as a result of the grant.
Cash and cash equivalents
The Company considers all highly liquid investments with an initial maturity of three months or less to be cash equivalents. The Company deposits cash with high credit quality financial institutions located in Utah. Deposits may at times exceed federally insured levels.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current year's presentation. Such reclassifications had no effect on total assets, total liabilities or net loss.
Research and development costs
Research and development costs are expensed as incurred. Research and development activities include the design of new products and product enhancements and are performed by both internal and external resources.
Income taxes
Deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. If appropriate, deferred tax assets are reduced by a valuation allowance which reflects expectations of the extent to which such assets may not be realized.
D-10
Property and equipment
Property and equipment are recorded at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the shorter of the estimated useful lives of the assets, or the lease term of assets under capital leases. The estimated useful lives of assets range between three and five years. Normal maintenance and repairs are expensed as incurred.
The cost and related accumulated depreciation of assets sold or otherwise disposed of are removed from the accounts and any gain or loss is reflected in the statement of operations.
Purchased intangibles
Purchased intangibles consist of licenses for certain blood pump related technologies. Amortization is computed using the straight-line method over the estimated 17 year useful life of the underlying patents associated with the licenses.
Long-lived assets
The Company periodically evaluates the recoverability of its long-lived assets in accordance with Statement of Financial Accounting Standards ("SFAS") No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets." SFAS No. 144 requires recognition of an impairment loss if the carrying amount of a long-lived asset is not recoverable from its undiscounted cash flows.
Stock-based compensation
The Company accounts for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees," and its related interpretations and complies with the disclosure requirements of SFAS No. 123, "Accounting for Stock-Based Compensation" and SFAS No. 148, "Accounting for Stock Based Compensation — Transition and Disclosure," an amendment to SFAS No. 123.
The Company accounts for stock issued to non-employees in accordance with the provisions of SFAS No. 123 and Emerging Issues Task Force Issue No. 96-18, "Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods, or Services."
Had compensation expense for the Company's stock-based compensation plan been determined based on the fair value at the grant dates for the awards under a method prescribed by SFAS No. 123, the Company's net loss would have been increased to the pro forma amounts indicated below:
| |
| |
| | Year Ended
| |
|---|
| | Nine Months Ended March 31, 2005
| | Nine Months Ended March 31, 2004
| | June 30, 2004
| | June 30, 2003
| |
|---|
| | (unaudited)
| | (unaudited)
| |
| |
| |
|---|
| Net loss: | | | | | | | | | | | | | |
| Net loss as reported | | $ | (3,077,030 | ) | $ | (1,438,191 | ) | $ | (2,232,937 | ) | $ | (3,777,799 | ) |
| Add: Stock-based employee compensation expense included in reported net loss | | | — | | | 26,616 | | | 35,488 | | | 52,422 | |
| Less: Total stock-based employee compensation expense determined under the fair value based method for all awards, net of related tax effects | | | (130,743 | ) | | (75,200 | ) | | (100,266 | ) | | (97,080 | ) |
| | |
| |
| |
| |
| |
| Pro forma net loss | | $ | (3,207,773 | ) | $ | (1,486,775 | ) | $ | (2,297,715 | ) | $ | (3,822,457 | ) |
| | |
| |
| |
| |
| |
New accounting pronouncements
Financial Accounting Standards Board ("FASB") Interpretation No. 46 "Consolidation of Variable Interest Entities, an interpretation of ARB No. 15" ("FIN 46") which was issued in January 2003, was further revised in December 2003 by FIN 46R. FIN 46, as amended provides guidance on the identification and consolidation of entities (termed "variable interest entities") for which control is achieved by means other than through voting rights. FIN 46R is effective immediately for variable interest entities created after December 31, 2003. For variable interest entities created before December 31, 2003, FIN 46R is effective for the Company for reporting periods
D-11
beginning after December 15, 2004. The adoption of this standard is not expected to have a material impact on the Company's results of operations or financial position.
In May 2003, the FASB issued SFAS No. 150 "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity" ("SFAS 150"). SFAS 150 clarifies the accounting for certain financial instruments with characteristics of both liabilities and equity and requires that those instruments be classified as liabilities in the statement of financial position. Previously, many of these financial instruments were classified as equity. SFAS 150 was originally effective for all financial instruments entered into or modified after May 31, 2003, and was otherwise effective at the beginning of the first interim period after June 15, 2003. On November 5, 2003, the FASB deferred the effective date of SFAS 150 for certain financial instruments. For instruments that are mandatorily redeemable on fixed dates, the classification, measurement and disclosure provisions of SFAS 150 shall be effective for the Company for fiscal periods beginning after December 15, 2004. For all other financial instruments that are mandatorily redeemable, the classification, measurement and disclosure provisions of SFAS 150 are deferred indefinitely pending further action by the FASB. The adoption of SFAS 150 is not expected to have a significant impact on the Company's results of operations, financial position or cash flows.
In December 2004, the FASB issued a revision to SFAS No. 123, "Accounting for Stock-Based Compensation" SFAS No. 123-R, "Share-Based Payment." SFAS 123-R focuses primarily on transactions in which an entity exchanges its equity instruments for employee services and generally establishes standards for the accounting for transactions in which an entity obtains goods or services in share-based payment transactions. The provisions of SFAS 123-R are effective for the Company for the first fiscal year beginning after December 15, 2005.
2. BASIS OF PRESENTATION
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The Company had a working capital deficit of $6,827,279 as of June 30, 2004. In addition, the Company has sustained recurring net losses and negative cash flows from operations since inception, all of which raise substantial doubt about the Company's ability to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company believes that these conditions have resulted from the inherent risks associated with early stage or start-up companies. Such risks include, but are not limited to, the ability to (a) generate sales, (b) attract additional capital in order to finance growth, and (c) successfully compete with other companies having financial, production and marketing resources significantly greater than those of the Company.
The sale of the Company's assets to World Heart Corporation ("World Heart") contemplated by the Asset Purchase Agreement executed on January 31, 2005 (Note 15) is expected to provide the resources necessary for the Company to continue its operations for at least the next twelve months as the Company would drastically reduce its operations through the implementation of a plan of liquidation and dissolution. As part of the Asset Purchase Agreement, the Company would retain a portion of its cash as set out in the Asset Purchase Agreement and would have the ability to sell the stock received from World Heart from the transaction if necessary. In the event the sale does not close, the Company has a funding commitment for $3.5 million from a shareholder to cover costs while the Company seeks other sources of liquidity (Note 10). In the past, the Company has been able to obtain funding necessary for its operations through the issuance of common stock and preferred stock and short-term loans from investors. If sufficient financing is not obtained, the Company may be forced to reduce the scope of its operations, reduce its headcount or otherwise decrease its development efforts. There can be no assurance that sufficient funds will be obtained to sustain the current scope of operations.
D-12
3. BALANCE SHEET COMPONENTS
| | March 31, 2005
| | June 30, 2004
| | June 30, 2003
|
|---|
| | (unaudited)
| |
| |
|
|---|
| Accounts receivable, net: | | | | | | | | | |
| | Accounts receivable | | $ | 58,110 | | $ | 35,764 | | $ | — |
| | Less: Allowance for doubtful accounts | | | — | | | — | | | — |
| | |
| |
| |
|
| | | $ | 58,110 | | $ | 35,764 | | $ | — |
| | |
| |
| |
|
| | Estimated Useful Lives
| | March 31, 2005
| | June 30, 2004
| | June 30, 2003
| |
|---|
| |
| | (unaudited)
| |
| |
| |
|---|
| Property and equipment, net: | | | | | | | | | | | | |
| Office furniture and equipment | | 3-5 | | $ | 253,672 | | $ | 157,686 | | $ | 157,686 | |
| Laboratory equipment | | 3 | | | 357,862 | | | 215,012 | | | 191,343 | |
| Assets under capital leases | | 3 | | | 13,667 | | | 84,487 | | | 84,487 | |
| | | | |
| |
| |
| |
| | | | | | 625,201 | | | 457,185 | | | 433,516 | |
| Less: Accumulated depreciation | | | | | (420,631 | ) | | (361,461 | ) | | (253,717 | ) |
| | | | |
| |
| |
| |
| | | | | $ | 204,570 | | $ | 95,724 | | $ | 179,799 | |
| | | | |
| |
| |
| |
Accumulated depreciation of assets under capital leases totaled $13,300, $68,676 and $50,118 at March 31, 2005 (unaudited), June 30, 2004 and 2003, respectively. Depreciation expense for the nine month periods ended March 31, 2005 (unaudited) and March 31, 2004 (unaudited) and years ended June 30, 2004 and 2003 totaled $61,894, $85,401, $107,744 and $116,251, respectively.
4. INTANGIBLE ASSETS
| |
| | June 30,
| |
|
|---|
| | March 31, 2005
| | Weighted Average Amortization Period (Years)
|
|---|
| | 2004
| | 2003
|
|---|
| | (unaudited)
| |
| |
| |
|
|---|
| Purchased license agreements | | $ | 491,911 | | $ | 491,911 | | $ | 491,911 | | 17 |
| Less accumulated amortization | | | (155,814 | ) | | (134,115 | ) | | (105,183 | ) | |
| | |
| |
| |
| | |
| | | $ | 336,097 | | $ | 357,796 | | $ | 386,728 | | |
| | |
| |
| |
| | |
Amortization expense for the nine month periods ended March 31, 2005 (unaudited) and March 31, 2004 (unaudited) and years ended June 30, 2004 and 2003 totaled $21,699, $21,699, $28,932 and $34,836, respectively. The Company's future amortization of intangible assets is expected to be as follows:
| Year ending June 30, | | | |
| | 2005 | | $ | 28,936 |
| | 2006 | | | 28,936 |
| | 2007 | | | 28,936 |
| | 2008 | | | 28,936 |
| | 2009 | | | 28,936 |
| Thereafter | | | 213,116 |
| | |
|
| | | $ | 357,796 |
| | |
|
D-13
5. ACCRUED LIABILITIES
| |
| | June 30,
|
|---|
| | March 31, 2005
|
|---|
| | 2004
| | 2003
|
|---|
| | (unaudited)
| |
| |
|
|---|
| Accrued compensation | | $ | 124,433 | | $ | 160,301 | | $ | 161,954 |
| Accrued interest | | | 10,537 | | | 558,641 | | | 230,965 |
| Accrued professional fees | | | 94,146 | | | 71,500 | | | 35,000 |
| Other liabilities | | | 14,362 | | | 2,533 | | | 16,000 |
| | |
| |
| |
|
| | | $ | 243,478 | | $ | 792,975 | | $ | 443,919 |
| | |
| |
| |
|
6. INCOME TAXES
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| |
|---|
| Current: | | | | | | | |
| | U.S. federal | | $ | — | | $ | — | |
| | State and local | | | (100 | ) | | (2,167 | ) |
| | |
| |
| |
| | | | (100 | ) | | (2,167 | ) |
| | |
| |
| |
Deferred: |
|
|
|
|
|
|
|
| | U.S. federal | | | — | | | — | |
| | State and local | | | — | | | — | |
| | |
| |
| |
| | | $ | (100 | ) | $ | (2,167 | ) |
| | |
| |
| |
| | June 30,
2004
| | June 30,
2003
| |
|---|
| Deferred tax assets: | | | | | | | |
| | Asset reserves and accrued expenses | | $ | 27,242 | | $ | 29,024 | |
| | Net operating loss carryforwards | | | 4,282,361 | | | 3,480,094 | |
| | Deferred compensation from issuance of stock options | | | 82,088 | | | 68,851 | |
| | Property and equipment | | | 14,444 | | | — | |
| | |
| |
| |
| | | | 4,406,135 | | | 3,577,969 | |
| | |
| |
| |
Deferred tax liabilities: |
|
|
|
|
|
|
|
| | Property and equipment | | | — | | | (217 | ) |
| | Purchased intangibles | | | (3,833 | ) | | (2,393 | ) |
| | |
| |
| |
| | | | (3,833 | ) | | (2,610 | ) |
| | |
| |
| |
| Net deferred tax asset before valuation allowance | | | 4,402,302 | | | 3,575,359 | |
| Valuation allowance | | | (4,402,302 | ) | | (3,575,359 | ) |
| | |
| |
| |
| Net deferred tax asset | | $ | — | | $ | — | |
| | |
| |
| |
D-14
Income tax expense for the years ended June 30, 2004 and 2003 is different from the benefit computed by applying the statutory federal tax rate of 34% to the loss before income taxes as follows:
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| |
|---|
| Benefit at statutory rate | | $ | 759,165 | | $ | 1,283,715 | |
| State income taxes | | | 73,657 | | | 124,101 | |
| Option compensation | | | — | | | (3,825 | ) |
| Other | | | (5,979 | ) | | 44,814 | |
| Change in valuation allowance | | | (826,943 | ) | | (1,450,972 | ) |
| | |
| |
| |
| | Income tax expense | | $ | (100 | ) | $ | (2,167 | ) |
| | |
| |
| |
SFAS No. 109 requires that a valuation allowance be provided if it is more likely than not that some portion or all of a deferred tax asset will not be realized. The Company's ability to realize the benefit of its deferred tax asset will depend on the generation of future taxable income. Because the Company's intended future operations for several years will be focused on product development and not operational profitability, the Company believes that a full valuation allowance should be provided.
As of June 30, 2004, the Company had federal and state net operating loss carryforwards totaling $11,480,859. These net operating loss carryforwards will expire between 2021 and 2024, if not utilized. As required by Internal Revenue Code Section 382, the Company's utilization of $649,606 of its available net operating loss carryforwards against future taxable income is subject to an annual limitation of $10,672 because of the change in ownership that resulted from the issuance of preferred stock in 2000.
7. NOTES PAYABLE
On April 22, 2002, the Company obtained a $1.5 million note payable from an investor ("the Lender"). This note carried an interest rate of 7% and was due in full on April 22, 2003. In the event the Company consummates a subsequent round of equity financing, the Lender shall participate in such financing by converting all of the outstanding principal and interest under the note into equity securities of the Company at the applicable rate of the issuance.
During the period from September 1, 2002 through September 10, 2004, the Company obtained $6,250,000 in additional notes from the Lender (see Note 8). These notes carry interest rates ranging from 2.26% to 7% and have principal amounts and maturity dates as shown in the table below. In the event the Company consummates a subsequent round of equity financing, the Lender shall participate in such financing by converting all of the outstanding principal and interest under the additional notes into equity securities of the Company at the applicable rate of the issuance.
Amount of Note
| | Maturity Date
| |
|
|---|
| $1,350,000 | | September 1, 2003 | | |
| $1,000,000 | | December 16, 2003 | | |
| $ 200,000 | | June 2, 2004 | | |
| $ 200,000 | | July 2, 2004 | | |
| $ 250,000 | | August 13, 2004 | | |
| $ 250,000 | | October 23, 2004 | | |
| $1,000,000 | | March 25, 2005 | | |
| $1,000,000 | | May 28, 2005 | | |
| $1,000,000 | | July 30, 2005 | | |
In January 2005, the Company obtained a loan of $1.0 million from the Lender. As part of the Company's agreement to sell all of its assets to World Heart, World Heart will assume the liability for the loan. In the event the agreement to sell its assets to World Heart is not consummated, the loan is immediately converted to equity securities of the Company at $1.35 per share.
8. DEBT RESTRUCTURING
On July 29, 2004, the Lender retroactively lowered the interest rates on the $7.75 million of outstanding notes. As a result, the Company recognized a gain on the forgiveness of accrued interest of $263,871.
As of September 10, 2004, all notes and remaining accrued interest were converted to a new class of Series A preferred stock at $1.36 per share, the price per share received from the issuance of new Series A preferred stock for cash on September 30, 2004. The terms and conditions of this new Series A preferred stock are described in Note 10.
D-15
9. COMMITMENTS
Leases
The Company leases office space and equipment under noncancelable operating and capital leases with various expiration dates through January 2008. Rent expense for the nine month periods ended March 31, 2005 (unaudited) and March 31, 2004 (unaudited) and years ended June 30, 2004 and 2003 was $110,056, $109,753, $146,325 and $134,364, respectively.
Future minimum lease payments under noncancelable operating and capital leases are as follows:
Year Ended June 30,
| | Capital Leases
| | Operating Leases
|
|---|
| 2005 | | $ | 19,520 | | $ | 109,754 |
| 2006 | | | 1,090 | | | 106,465 |
| 2007 | | | — | | | 108,595 |
| 2008 | | | — | | | 36,436 |
| | |
| |
|
| Total minimum lease payments | | | 20,610 | | $ | 361,250 |
| | | | | |
|
| Less: Amount representing interest | | | (1,271 | ) | | |
| | |
| | | |
| | | | 19,339 | | | |
| Less: Current portion | | | (18,260 | ) | | |
| | |
| | | |
| Capital lease obligations, non-current | | $ | 1,079 | | | |
| | |
| | | |
License and Royalty Agreements
Effective March 31, 1999, the Company entered into two license agreements with two research foundations. The license agreements required initial fees of $75,000 and $100,000, respectively. These fees are reflected as purchased intangibles on the Company's balance sheet. One of the agreements also requires a royalty payment of between 0.25% and 0.5% of the net sales associated with the technology. Through March 31, 2005 (unaudited), there have been no royalty payments required under this agreement.
Additionally, effective February 22, 2000, the Company entered into an agreement to outsource a development project to a limited liability company. Under this agreement, the Company agreed to reimburse the company for all development costs incurred in addition to paying future royalty payments of 0.5% of net sales. For the years ended June 30, 2004 and 2003, the Company paid $15,260 and $572,991, respectively, for development costs in connection with this agreement, but did not incur any obligation for royalty payments.
Effective May 22, 2001, the Company entered into a royalty agreement with a limited liability company and a research foundation. The agreement transferred all previously licensed technology to the Company in exchange for a royalty payment of 3.15% of the net sales associated with the technology. Through March 31, 2005 (unaudited), there have been no royalty payments required under this agreement.
Effective February 13, 2002, the Company entered into a license agreement with a research foundation. The license agreement requires scheduled payments through June 2007, the present value of which discounted at 8% was $318,911. The balance of the license fee payable at June 30, 2004 and 2003 totaled $156,433 and $182,102, respectively. The $318,911 is reflected as purchased intangibles on the Company's balance sheet. The agreement requires a royalty payment of 0.1% of the net sales associated with the technology. Through March 31, 2005 (unaudited), there have been no royalty payments required under this agreement.
10. CONVERTIBLE PREFERRED STOCK
The Company sold 105,179 shares of Series A preferred stock on December 6, 2000 for $5,000,000. The rights, privileges and preferences of the Series A preferred stock are as follows:
Dividends
The holders of Series A preferred stock shall be entitled to receive non-cumulative dividends in an amount equal to that paid on the outstanding shares of common stock, payable when and if, declared by the Board of Directors. As of March 31, 2005, no dividends had been declared or paid.
D-16
Conversion
Each share of Series A preferred stock may be converted into shares of common stock on a one-for-one basis, subject to adjustments under specific circumstances. Conversion is either (i) at the option of the preferred stockholder or (ii) upon the occurrence of a liquidity event, as defined. The Company has reserved shares of common stock for the conversion of all outstanding shares of preferred stock.
On September 10, 2004, the stockholders approved a recapitalization of the Company. As part of the recapitalization, the 105,179 shares of Series A preferred stock were converted into 105,179 shares of common stock, a new class of Series A preferred stock was created, and the Company issued 5,937,701 shares of new Series A preferred stock in exchange for conversion of $7.75 million principal amount of outstanding notes plus accrued interest. (See Note 8).
The Lender also committed to invest $4 million in the Company in exchange for 2,952,326 shares of the new Series A preferred Stock. On September 30, 2004, the Lender invested $1 million of the $4 million commitment. In asociation with the acquisition transaction (see Note 15), the remaining $3 million equity commitment was converted into a commitment to provide up to $3.5 million (see Note 2) in debt funding.
Voting Rights
The holders of Series A preferred stock and the holders of common stock shall vote together and not as a separate class. As of June 30, 2004, the Series A votes were. 0314 per Series A share which is the equivalent of 3,300 common stock votes. The ratio is subject to adjustment based on certain events. As of September 10, 2004, the new Series A votes were one (1) per each new Series A share.
Liquidation
In the event of any liquidation, dissolution or winding up of the Company, either voluntary or involuntary, holders of new Series A preferred stock are entitled to receive in preference over common stockholders, an amount of $1.36 per share, plus any declared but unpaid dividends. Upon completion of the distribution to the new Series A preferred stockholders, the remaining assets of the Company will be distributed ratably to both the common stockholders and the new Series A preferred stockholders.
11. STOCK OPTION PLAN
In November 1999, the Company adopted the MedQuest 1999 Stock Option Plan (the "Plan"). Options granted under the Plan may be qualified or nonqualified stock options and may be granted to Company employees, directors and/or consultants. Options under the Plan may be granted for periods of up to ten years and at prices determined by the Board of Directors. Vesting terms are also based upon the determination of the Board of Directors.
| | Options
| | Weighted Average Exercise Price
|
|---|
| Outstanding at June 30, 2002 | | 662,733 | | $ | 2.80 |
| | Options granted | | 45,280 | | | 15.00 |
| | Options cancelled | | (8,700 | ) | | 1.65 |
| | |
| | | |
| Options outstanding at June 30, 2003 | | 699,313 | | | 3.30 |
| | Options granted | | — | | | — |
| | Options cancelled | | (17,023 | ) | | 6.60 |
| | |
| | | |
| Options outstanding at June 30, 2004 | | 682,290 | | | 3.30 |
| | Options granted (unaudited) | | 1,930,101 | | | 0.90 |
| | Options cancelled (unaudited) | | — | | | — |
| | |
| | | |
| Options outstanding at March 31, 2005 (unaudited) | | 2,612,391 | | $ | 1.59 |
| | |
| |
|
| Options exercisable at March 31, 2005 (unaudited) | | 1,730,289 | | $ | 1.76 |
| | |
| |
|
D-17
Options Outstanding at June 30, 2004
| | Options Exercisable at
June 30, 2004
|
|---|
Range of Exercise Prices
| | Options
| | Weighted Average Remaining Life
| | Weighted Average Exercise Price
| | Options
| | Weighted Average Exercise Price
|
|---|
| $1.00 | | 527,585 | | 5.42 yrs | | $ | 1.00 | | 527,585 | | $ | 1.00 |
| $2.00-2.20 | | 34,155 | | 6.25 yrs | | $ | 2.00 | | 34,155 | | $ | 2.00 |
| $10.00 | | 3,500 | | 7 yrs | | $ | 10.00 | | 3,500 | | $ | 10.00 |
| $15.00-16.50 | | 117,050 | | 8.02 yrs | | $ | 15.10 | | 70,322 | | $ | 15.10 |
| | |
| | | | | | |
| | | |
| | | 682,290 | | | | | | | 635,562 | | | |
| | |
| | | | | | |
| | | |
The Company calculated the fair value of each option grant on the date of grant using the Black-Scholes option pricing method with the following assumptions, for the year ended June 30, 2004 and 2003 and the nine month period ended March 31, 2005 (unaudited):
| |
| | Year
Ended
June 30,
|
|---|
| | Nine Months Ended
March 31, 2005
|
|---|
| | 2004
| | 2003
|
|---|
| | (unaudited)
| |
| |
|
|---|
| Risk-free interest rate | | 3.35% | | 3.35% | | 2.65% |
| Expected life (years) | | 5 | | 5 | | 5 |
| Expected volatility | | 0% | | 0% | | 0% |
| Expected dividend yield | | 0% | | 0% | | 0% |
The weighted average fair value of options granted during the year ended June 30, 2003 and the period ended March 31, 2005 (unaudited) were $1.86 and $0.11, respectively. No options were granted during the year ended June 30, 2004.
2004 Stock Option Plan
In September 2004, the Company adopted the MedQuest 2004 Stock Option Plan (the "Plan"). Options granted under the Plan may be qualified or nonqualified stock options and may be granted to Company employees, directors and/or consultants.
The 1,815,000 options available for grant under the 2004 Stock Option Plan may be granted for periods of up to ten years and at prices determined by the Board of Directors. Vesting terms are also based upon the determination of the Board of Directors.
12. REVERSE STOCK SPLIT
On July 29, 2004, the Company's Board of Directors approved a proposal to amend the Company's certificate of incorporation to effect a reverse stock split. On September 10, 2004, the stockholders approved a 1-for-10 reverse split. All share amount and per share data reflected in these financial statements are shown after giving retroactive effect to the 1-for-10 reverse stock split.
13. RESTRICTED STOCK PLAN
In September 2004, the Company adopted the MedQuest 2004 Restricted Stock Plan (the "Restricted Stock Plan"). Stock granted under the Restricted Stock Plan may be granted to Company employees, directors and/or consultants.
The 2,370,000 shares available for grant under the 2004 Restricted Stock Plan may be granted for periods of up to ten years and at prices determined by the Board of Directors. Vesting terms are also based upon the determination of the Board of Directors.
14. CANADIAN ACCOUNTING PRINCIPLES
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP"). These principles differ, as they affect the Company, for the nine months ended March 31, 2005 and for the years ended June 30, 2004 and 2003 in the following material respects from Canadian generally accepted accounting principles ("Canadian GAAP"). There are no differences in reported cash flows for the periods presented.
D-18
| |
| | June 30,
| |
|---|
| | March 31, 2005
| |
|---|
| | 2004
| | 2003
| |
|---|
| | (unaudited)
| |
| |
| |
|---|
| Total assets | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | |
| | |
| |
| |
| |
| Accounts payable | | | 165,608 | | | 168,623 | | | 224,622 | |
| Accrued liabilities | | | 243,478 | | | 1,526,334 | | | 901,292 | |
| Notes payable | | | 1,000,000 | | | 5,702,160 | | | 3,466,800 | |
| Other current liabilities | | | 28,930 | | | 64,579 | | | 63,645 | |
| | |
| |
| |
| |
| Total current liabilities | | | 1,438,016 | | | 7,461,696 | | | 4,656,359 | |
| | |
| |
| |
| |
| Long-term liabilities | | | 110,114 | | | 111,193 | | | 157,948 | |
| | |
| |
| |
| |
| Total liabilities | | | 1,548,130 | | | 7,572,889 | | | 4,814,307 | |
| Preferred stock | | | 6,676 | | | 105 | | | 105 | |
| Common stock | | | 127 | | | 22 | | | 22 | |
| Additional paid-in capital | | | 16,398,952 | | | 6,496,755 | | | 6,396,489 | |
| Equity component of notes payable | | | 0 | | | 1,047,840 | | | 583,200 | |
| Accumulated deficit | | | (16,922,967 | ) | | (13,715,194 | ) | | (11,141,492 | ) |
| | |
| |
| |
| |
| Total stockholders equity (deficit) | | | (517,212 | ) | | (6,170,471 | ) | | (4,161,676 | ) |
| | |
| |
| |
| |
| Total liabilities and stockholders' equity (deficit) | | $ | 1,030,918 | | $ | 1,402,418 | | $ | 652,631 | |
| | |
| |
| |
| |
- (b)
- Statements of operations
| |
| | Years ended June 30,
| |
|---|
| | Nine months ended
March 31,
2005
| |
|---|
| | 2004
| | 2003
| |
|---|
| | (unaudited)
| |
| |
| |
|---|
| Net loss in accordance with U.S. GAAP | | $ | 3,077,030 | | $ | 2,232,937 | | $ | 3,777,799 | |
| Interest charges on convertible notes (1) | | | 0 | | | 275,987 | | | 416,540 | |
| Stock based compensation included in net loss (2) | | | 0 | | | (35,488 | ) | | (52,422 | ) |
| Stock-based compensation (2) | | | 130,743 | | | 100,266 | | | 97,080 | |
| | |
| |
| |
| |
| Net loss in accordance with Canadian GAAP | | $ | 3,207,773 | | $ | 2,573,702 | | $ | 4,238,997 | |
| | |
| |
| |
| |
- (c)
- Footnotes
- 1.
- Convertible notes payable
Under Canadian GAAP, the Company's convertible notes must be accounted for as compound financial instruments and bifurcated into their separate components: (i) notes payable and (ii) the option to acquire shares. The proceeds from the issue of convertible notes are split between the debt and equity components based on their relative fair values at the time of issue. Under U.S. GAAP, these instruments are not split, but are reflected as a debt instrument.
The Company has allocated the proceeds from the notes between debt and equity components to give an effective interest rate on the liability component of the convertible notes of approximately 25%, being management's estimate of the Company's fair value borrowing rate. The residual value, representing the value of the conversion feature of the notes, has been recorded as a separate component of stockholders' equity. The amortization of the option to convert the notes is reflected as additional
D-19
| |
| | Years ended June 30,
| |
|---|
Notes issued during
| | Nine months ended
March 31, 2005
| |
|---|
| | 2004
| | 2003
| |
|---|
| | (unaudited)
| |
| |
| |
|---|
| Face value of the convertible notes | | $ | 1,000,000 | | $ | 2,700,000 | | $ | 2,550,000 | |
| Equity component | | | — | | | (464,640 | ) | | (367,200 | ) |
| | |
| |
| |
| |
| Debt component | | $ | 1,000,000 | | $ | 2,235,360 | | $ | 2,182,800 | |
| | |
| |
| |
| |
The entire value of the outstanding notes and accrued interest was transferred to Series A preferred stock and additional-paid-in-capital on September 10, 2004 when the notes were converted to equity as described in Note 8 as part of the Company's debt restructuring.
The loan obtained by the Company in January 2005 does not meet the criteria for bifurcation, as such, has been recorded as a liability.
- 2.
- Stock-based compensation expense
Canadian GAAP requires that the fair value of vested employee and director stock options be expensed in the statement of operations. Under U.S. GAAP the fair value of employee and director stock options are not expensed in the statement of operations and are only disclosed in the footnotes to the financial statements. The details of the fair value calculations of stock options can be found in Note 11 in these financial statements.
15. SUBSEQUENT EVENT — SALE OF ALL ASSETS
On January 31, 2005, the Company announced that it had signed a definitive Asset Purchase Agreement to sell all of its assets to World Heart for 9.3 million common shares of World Heart, subject to certain consents, conditions and shareholder approval.
16. SUBSEQUENT EVENTS (UNAUDITED)
Subsequent to March 31, 2005, the Company obtained an additional loan of $2.5 million from the Lender. As part of the Company's agreement to sell all of its assets to World Heart, World Heart will assume the liability for the loan.
D-20
APPENDIX E
MEDQUEST PRODUCTS, INC.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
INTRODUCTION
The following discussion was prepared by management on May 23, 2005, was approved by the Board of Directors of the Company and explains material changes in financial condition and results of operations of MedQuest Products, Inc. (a development stage enterprise) ("MedQuest" or "Company") for the year ended June 30, 2004 with comparisons to the year ended June 30, 2003 and for the nine month period ended March 31, 2005 with comparisons to the nine month period ended March 31, 2004. Such discussion and comments on the liquidity and capital resources of the Company should be read in conjunction with the information contained in the financial statements and related notes to the financial statements of the Company. All amounts presented are in US dollars.
The discussion and comments contained hereunder include both historical information and forward-looking information. The forward-looking information, which generally is information stated to be anticipated, expected, or projected by the Company, involves known and unknown risks, uncertainties and other factors that may cause the actual results and performance of the Company to be materially different from any future results and performance expressed or implied by such forward-looking information. Potential risks and uncertainties include, without limitation, the uncertainties inherent in the development of new products for use in the human body, the Company's need for additional funding, the Company's need to establish reimbursement mechanisms and product acceptance from third-party payers, extensive government regulation of the Company's products, and rapid developments in technology, including developments by competitors.
OVERVIEW
MedQuest is a development stage company that was established in 1993 and is primarily engaged in research and development of its HeartQuest™ Ventricular Assist Device ("HeartQuest™ VAD"), which is an advanced second-generation magnetically levitated centrifugal rotary blood pump. MedQuest intends to commercialize the HeartQuest™ VAD primarily as an alternative to heart transplant ("Destination Therapy").
To date, MedQuest has funded its operations through the private sale of equity and debt securities, non-equity contributions from NIH and private foundation grants, and early profitable contract research and development (R&D). During the period from inception through March 2005, MedQuest raised $13.75 million in net proceeds from private equity financing.
RECENT EVENTS
On September 10, 2004, the stockholders of MedQuest approved a recapitalization of the Company. As part of the recapitalization, all original Series A preferred shares were converted into common shares and the Company effected a 10:1 reverse stock split. In addition, Maverick Venture Management, LLC ("Maverick") converted $7,750,000 of outstanding notes plus $294,770 of accrued interest into 5,937,701 shares of a new Series A preferred class. In connection with the recapitalization, Maverick agreed to provide $4.0 million of equity funding to MedQuest. In October 2004, $1.0 million of the $4.0 million was provided to the Company in exchange for 738,081 shares of new Series A preferred stock.
The rights, privileges and preferences of the new Series A preferred stock, which are similar to old Series A preferred stock, are as follows:
Dividends
The holders of Series A preferred stock shall be entitled to receive non-cumulative dividends in an amount equal to that paid on the outstanding shares of common stock, payable when and if, declared by the Board of Directors. As of March 31, 2005, no dividends had been declared or paid.
E-1
Conversion
Each share of Series A preferred stock may be converted into shares of common stock on a one-for-one basis, subject to adjustments under specific circumstances. Conversion is either (i) at the option of the preferred stockholder or (ii) upon the occurrence of a liquidity event, as defined. The Company has reserved shares of common stock for the conversion of all outstanding shares of preferred stock.
Voting Rights
The holders of each share of new Series A preferred stock shall have the right to one vote for each share of Common Stock into which each share of new Series A preferred could then be converted with full voting rights and powers equal to the voting rights and powers of the holders of Common stock. The holders are entitled to notice of any stockholders' meeting in accordance with the bylaws of the Company and are entitled to vote with the holders of the Common Stock, with respect to any matter upon which holders of Common Stock have the right to vote.
Liquidation
In the event of any liquidation or winding up of the Company, the holders of the new Series A preferred shall be entitled to receive in preference to the holders of the Common Stock a per share amount equal to the original purchase price plus any declared but unpaid dividends. After the payment of the liquidation preference to the holders of the new Series A preferred stock, the remaining assets shall be distributed ratably to the holders of the Common Stock and the holders of the new Series A preferred stock (on an as converted basis). A merger, acquisition, sale of voting control or sale of substantially all of the assets shall be deemed to be a liquidation.
On January 31, 2005, MedQuest signed a definitive asset purchase agreement to sell all of the Company's assets to World Heart Corporation ("WorldHeart"), subject to certain approvals, consents, and conditions. It is expected that the approvals will be obtained in order for WorldHeart and MedQuest to close this transaction in June 2005. In connection with this transaction, MedQuest and Maverick agreed that, in lieu of the remaining $3.0 million commitment of equity funding entered into at the time of the recapitalization in September 2004, Maverick would advance up to $3.5 million in loans to fund MedQuest operations until the closing of the WorldHeart acquisition. On January 31, 2005, Maverick advanced $1.0 million of such loans to MedQuest. In April 2005, Maverick advanced the remaining $2.5 million to MedQuest. All loans provided by Maverick to MedQuest will be repaid to Maverick by WorldHeart at the time of closing as part of the asset purchase agreement.
SELECTED FINANCIAL INFORMATION
All financial information is prepared in accordance with accounting principles generally accepted in the United States (GAAP) and stated in US dollars.
Critical Accounting Policies
Revenue recognition
The Company recognizes revenues from research and grant funding upon receipt and when the Company is in compliance with the requirements of the funding, if any.
Governmental grants include amounts received indirectly from the U.S. National Institute of Health ("NIH") as part of a $4,200,000 grant administered through another entity. The NIH retains certain rights to any technology developed as a result of the grant.
Research and development costs
Research and development costs are expensed as incurred. Research and development activities include the design of new products and product enhancements and are performed by both internal and external resources.
E-2
Stock-based compensation
The Company accounts for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board Opinion ("APB") No. 25, "Accounting for Stock Issued to Employees," and its related interpretations and complies with the disclosure requirements of Statement of Financial Standards No. 123, "Accounting for Stock-Based Compensation" and SFAS No. 148, "Accounting for Stock Based Compensation — Transition and Disclosure," an amendment to SFAS No. 123.
The Company accounts for stock issued to non-employees in accordance with the provisions of SFAS No. 123 and Emerging Issues Task Force Issue No. 96-18, "Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods, or Services."
Financial Information
All references to shares and options for all periods presented have been retroactively restated to reflect the aforementioned one-for-ten reverse stock split.
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| | Nine Months Ended March 31, 2005
| | Nine Months Ended March 31, 2004
| |
|---|
| |
| |
| | (unaudited)
| | (unaudited)
| |
|---|
| Net revenue | | $ | 99,389 | | $ | — | | $ | 101,840 | | $ | 63,625 | |
| Net loss for the period | | | (2,232,937 | ) | | (3,777,799 | ) | | (3,077,030 | ) | | (1,438,191 | ) |
| Total assets | | | 1,402,418 | | | 652,631 | | | 1,030,918 | | | 1,440,023 | |
| Long term liabilities | | | 111,193 | | | 157,948 | | | 110,114 | | | 157,948 | |
RESULTS OF OPERATIONS
Consolidated results of operations
| | Year Ended June 30, 2004
| | Year Ended June 30, 2003
| | Percentage Change Increase/(Decrease)
|
|---|
| Revenue | | | | | | | | |
| Governmental grants | | $ | 99,389 | | $ | — | | — |
Operating Expenses |
|
|
|
|
|
|
|
|
| Research and development | | | 1,006,482 | | | 1,828,991 | | (45.0)% |
| Depreciation and amortization | | | 136,676 | | | 151,087 | | (9.5)% |
| General and administrative | | | 845,552 | | | 1,562,618 | | (45.9)% |
| Interest and other (income)/expense | | | 343,616 | | | 235,103 | | 46.2% |
| | |
| |
| |
|
| Net loss | | $ | (2,232,937 | ) | $ | (3,777,799 | ) | (40.9)% |
| | |
| |
| |
|
Revenues. For the years presented above, MedQuest's sole source of revenues was from governmental research and grant funding for work performed on specific VAD related research projects. The Company searches for and reviews various grant funding opportunities on an ongoing basis. Grant funding is recognized as it is earned through the performance of research work in accordance with funding contracts. There was no grant revenue in 2003, but awards granted in December 2003 and March 2004 resulted in revenues of $99,389 for the year ended June 2004.
Operating Expenses.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing and configuration of equipment, animal trial expenses, regulatory affairs and quality control with respect to prototype. Expenses for the year ended June 30, 2004 decreased by $822,509 or 45.0% from the same period in 2003 due to severe cash constraints. In late 2003, the Company had anticipated closing a round of funding from several venture capital groups, which ultimately failed. During the period from November 2003 through March 2004, MedQuest was forced to significantly
E-3
reduce spending and limit its operations. In April 2004, a commitment for $7,000,000 of funding was obtained and by June 2004 normal operations were resumed.
Depreciation and Amortization. Depreciation expense for the years ended June 30, 2004 and June 30, 2003 totaled $107,744 and $116,251, respectively. As of June 30, 2004, the Company had net property and equipment of $95,724. Amortization of intangibles for the years ended June 30, 2004 and 2003 totaled $28,932 and $34,836, respectively. The majority of the intangible assets arose from the purchase of licenses in 1999 and 2002 from research foundations and are being amortized over 17 years from the time of purchase. As of June 30, 2004, intangible assets of $357,796 remain unamortized.
General and administrative. General and administrative expenses consist primarily of payroll and related expenses for executives, accounting and administrative personnel, professional fees, communications, insurance, occupancy and other general corporate expenses. Expenses for the year ended June 30, 2004 decreased by $717,066 or 45.9% from the same period in 2003 due to severe cash constraints and the Company's limited operations in late 2003 and early 2004, as noted above.
Interest and other income/expense. Interest and other income/expense consists primarily of interest incurred on notes payable to the Company's majority shareholder. The balance of notes payable increased from $1,500,000 at July 1, 2002 to $4,050,00 at June 30, 2003 and further increased to $6,750,000 at June 30, 2004. The notes payable carried interest at 7% and were converted into preferred shares subsequent to June 30, 2004 (see Liquidity for further details). Total interest expense for the years ended June 30, 2004 and 2003 was $344,776 and $237,838, respectively.
RESULTS OF OPERATIONS
Consolidated results of operations (Unaudited)
| | Nine Months Ended March 31, 2005
| | Nine Months Ended March 31, 2004
| | Percentage Change Increase/(Decrease)
|
|---|
| Revenue | | | | | | | | |
| Governmental grants | | $ | 101,840 | | $ | 63,625 | | 60.1 % |
Operating Expenses |
|
|
|
|
|
|
|
|
| Research and development | | | 2,168,460 | | | 594,347 | | 264.8 % |
| Depreciation and amortization | | | 83,593 | | | 107,100 | | (21.9)% |
| General and administrative | | | 1,184,603 | | | 533,741 | | 121.9 % |
| Interest and other (income)/expense | | | (257,786 | ) | | 266,628 | | (196.7)% |
| | |
| |
| |
|
| Net loss | | $ | (3,077,030 | ) | $ | (1,438,191 | ) | 114.0 % |
| | |
| |
| |
|
Revenues. For each of the nine months presented above, MedQuest's sole source of revenues was from research and grant funding for work performed on specific VAD related research projects. The Company searches for and reviews various grant funding opportunities on an ongoing basis. Grant funding is recognized as it is earned through the performance of research work in accordance with funding contracts. Awards granted in December 2003 and March 2004 resulted in revenues of $101,840 and $63,625 for the nine months ended March 31, 2005 and March 31, 2004, respectively.
Operating Expenses.
Research and development. Research and development expenses consist principally of payroll and related expenses for development staff, prototype manufacturing, testing and configuration of equipment, animal trial expenses, regulatory affairs and quality control with respect to prototype. Expenses for the nine months ended March 31, 2005 increased by $1,574,113 or 264.8% from the same period in 2004. During the nine months ended March 31, 2005, significant purchases were made for materials and equipment, animal trials resumed, and staffing increased to 25 full-time equivalents ("FTEs"). The Company ramped up manufacturing and purchases
E-4
of materials and supplies for the nine months ended March 31, 2005 which totaled $829,009 compared to $2,997 for the same period in 2004.
Depreciation and Amortization. Depreciation expense for the nine months ended March 31, 2005 and March 31, 2004 totaled $61,894 and $85,401, respectively. As of March 31, 2005, the Company had net property and equipment of $204,570. Amortization of intangibles for the nine months ended March 31, 2005 and 2004 totaled $21,699 and $21,699, respectively. The majority of the intangible assets arose from the purchase of licenses in 1999 and 2002 from research foundations and are being amortized over 17 years from the time of purchase. As of March 31, 2005, intangible assets of $336,097 remained unamortized.
General and administrative. General and administrative expenses consist primarily of payroll and related expenses for executives, accounting and administrative personnel, professional fees, communications, insurance, occupancy and other general corporate expenses. Expenses for the nine months ended March 31, 2005 increased by $650,862 or 121.9% from the same period in 2004. In June 2004, the Company resumed normal operations.
Interest and other income/expense. Interest and other income/expense consists primarily of interest incurred on notes payable to the Company's majority shareholder. On July 29, 2004, the interest rate on the $6,750,000 of outstanding notes was retroactively lowered from 7.0% to between 1.81% and 3.21%, resulting in a gain on forgiveness of accrued interest in the amount of $263,871. The Company obtained an additional $1,000,000 on July 30, 2004 in a convertible bridge loan carrying a 2.26% interest rate. The notes payable were converted into preferred shares on September 10, 2004. On January 31, 2005, the Company obtained a loan of $1,000,000 from the Company's majority shareholder. The Company recorded interest expense of $12,150 for the nine months ended March 31, 2005 compared to interest expense of $266,955 for the nine months ended March 31, 2004.
SUMMARY OF QUARTERLY RESULTS
| | Three Months Ended
| |
|---|
| | March 31, 2004
| | June 30, 2004
| | September 30, 2004
| | December 31, 2004
| | March 31, 2005
| |
|---|
| Net revenue | | $ | 63,625 | | $ | 35,764 | | $ | — | | $ | 40,221 | | $ | 61,619 | |
| Net loss | | | (221,411 | ) | | (794,745 | ) | | (658,386 | ) | | (1,204,544 | ) | | (1,214,100 | ) |
| | Three Months Ended
| |
| |
|---|
| | March 31, 2003
| | June 30, 2003
| | September 30, 2003
| | December 31, 2003
| |
| |
|---|
| Net revenue | | $ | — | | $ | — | | $ | — | | $ | — | | | |
| Net loss | | | (921,947 | ) | | (812,309 | ) | | (677,639 | ) | | (539,141 | ) | | |
| |
| |
| | Three Months Ended
| |
| |
|---|
| |
| |
| | September 30, 2002
| | December 31, 2002
| |
| |
|---|
| Net revenue | | $ | — | | $ | — | | | |
| Net loss | | | (903,004 | ) | | (1,140,539 | ) | | |
Net loss for the quarters in 2004 and 2005 generally increased as the Company increased its spending following additional commitments for funding.
LIQUIDITY AND CAPITAL RESOURCES
At March 31, 2005, the Company had cash and cash equivalents of $412,456 and a working capital deficit of $947,765 compared to cash and cash equivalents of $890,452 and a working capital deficit of $6,827,279 at June 30, 2004. As of June 30, 2004, the Company had a balance of $6,750,000 in notes payable, which was cancelled in exchange for shares of a new Series A preferred class with the recapitalization in September 2004. The recapitalization also contemplated additional equity funding from Maverick, the Company's principal shareholder. Pursuant to the proposed acquisition of MedQuest by WorldHeart, Maverick agreed to finance up
E-5
to $3.5 million in loans to fund operations of MedQuest until the closing of the acquisition. On January 31, 2005, Maverick advanced $1.0 million of such loans to MedQuest. In April 2005, Maverick advanced the remaining $2.5 million to MedQuest. If the acquisition by WorldHeart is not consummated, all such loans will convert to shares of new Series A preferred stock at a conversion price of $1.36.
Cash decreased by $477,996 during the nine month period ended March 31, 2005. This decrease in cash was primarily due to cash used in operating activities of $3,270,528 and investing activities of $170,740. This decrease in cash was offset by net cash provided by financing activities of $2,963,272.
Net cash used in operating activities increased to $3,270,528 for the nine months ended March 31, 2005 compared to $753,451 for same period during 2004. This was primarily due to the increased net loss in the nine month period ended March 31, 2005 and the decrease in cash provided from working capital changes.
Net cash used in investing activities during the nine months ended March 31, 2005 was $170,740. This cash was used to purchase research and development and manufacturing equipment and leasehold improvements.
Net cash provided by financing activities during the nine months ended March 31, 2005 was primarily in three $1 million increments: one increment was received as a note payable, one as the first issuance of preferred stock which followed the recapitalization of the Company, and the other as a loan.
The Company's independent auditors, in their report dated January 11, 2005 (except for notes 14 and 15 as to which the date is May 23, 2005) with respect to the financial statements of the Company as of June 30, 2004 and 2003, and for the years then ended, and for the period from inception (December 16, 1993) to June 30, 2004, included a "going concern" qualification. As discussed in note 2 to the financial statements, the Company had a working capital deficit as of June 30, 2004 and has sustained recurring net losses and negative cash flows from operations since inception. These matters raise substantial doubt about the Company's ability to continue as a going concern.
The WorldHeart acquisition is expected to provide the resources necessary for the Company to continue for at least the next twelve months. Upon closing of the acquisition, WorldHeart will assume substantially all of MedQuest's liabilities and issue a total of 9,300,000 shares of WorldHeart common stock to MedQuest, subject to the terms of the asset purchase agreement. Following closing, MedQuest will implement a plan of liquidation and dissolution and would have the opportunity to sell the WorldHeart stock if necessary.
In the event the WorldHeart acquisition does not close, the $3.5 million in loans advanced by Maverick in January 2005 and April 2005 will be converted into shares of new Series A preferred stock and will be available to fund continuing operations. If the WorldHeart acquisition is not consummated, MedQuest will seek additional sources of funding and significantly reduce its operations. The Company believes it could continue limited operations with its existing capital until December 2005.
In the past, the Company has been able to obtain funding necessary for its operations from various sources, including the issuance of common and preferred stock and short-term loans from investors. If sufficient financing is not obtained, the Company may be forced to reduce the scope of its operations, reduce its headcount or otherwise decrease its development efforts. There can be no assurance that sufficient funds will be obtained to sustain the current scope of operations on a "stand alone" basis or to finance future activities of the Company.
On January 31, 2005, MedQuest signed a definitive agreement to sell all of the Company's assets and business to WorldHeart, as discussed above. Management believes that the likelihood of success with respect to the Company's business will be greatly enhanced by the capital resources, efficiencies of scale, and platform synergies that WorldHeart has to offer.
OUTLOOK
External environment
The market for effective pharmacological, electro-physiological or device treatments for late-stage heart failure continues to grow, and despite significant progress on many fronts, no competitive breakthroughs have been announced, or are believed to be imminent. The number of diagnosed heart failure patients is increasing as
E-6
a result of both population demographic trends, and also as a response to the increasing number of survivors from sudden cardiac events, many of whom subsequently develop congestive heart failure. The Company believes the potential market for its heart assist device to be at least 100,000 patients each year in North America, Europe, Japan and certain other countries.
There is growing evidence of increasing clinical acceptance of the use of circulatory assist therapies in the treatment of late stage heart failure and effective as of October 1, 2003, the Centers for Medicare and Medicaid Services in the U.S. issued a National Coverage Decision announcing the extension of reimbursement coverage for destination therapy LVAD use. These developments are expected to accelerate the use of ventricular assist devices both in the U.S. and elsewhere.
Internal environment
The Company expects to commence sales of the HeartQuest VAD in 2008. Even after revenues begin to be generated, the Company plans that losses from operations will continue until 2010, at which time the Company expects earnings and positive cash flow from operations. The foregoing expectations and plans are subject to various risks and uncertainties, as identified under the heading "Introduction" above, including the risk of not obtaining sufficient capital to fund the Company's planned operations. The Company may from time to time apply for additional NIH grants and pursue other sources of financing.
Total operating expenses are expected to increase in 2005 and beyond, primarily due to the incremental expenses related to the Company's planned clinical studies and increased headcount. MedQuest anticipates it will be commencing its first-in-human feasibility trial outside the United States in the second half of 2005. Due to the planned increase in operations, the projected headcount is expected to grow from 25 as of March 2005 to 44 as of December 2005.
Commitments
The Company leases office space and equipment under noncancelable operating and capital leases with various expiration dates through January 2008. As at June 30, 2004, the Company's obligations and commitments related to operating and capital leases are as follows:
Year Ended June 30,
| | Capital Leases
| | Operating Leases
|
|---|
| 2005 | | $ | 19,520 | | $ | 109,754 |
| 2006 | | | 1,090 | | | 106,465 |
| 2007 | | | — | | | 108,595 |
| 2008 | | | — | | | 36,436 |
CAPITAL EXPENDITURES
| | Nine Months Ended
March 31,
|
|---|
| | 2005
| | 2004
|
|---|
| Capital Expenditures | | $ | 170,740 | | — |
| | |
| |
|
Capital expenditures for the nine months ended March 31, 2005 totaled $170,740 of which $55,675 was for machinery and equipment, and leasehold improvements related to the Company's start-up of its manufacturing facilities. Capital expenditures for the nine months ended March 31, 2004 were $0 as the Company reduced expenditures due to a lack of funding.
The Company anticipates that capital expenditures for 2005 will be approximately $865,000, the majority of which will be for the purchase of manufacturing equipment.
At March 31, 2005, MedQuest occupied 24,044 square feet of leased manufacturing and office space in Salt Lake City, Utah. The lease was signed in February of 2003 for a five-year term expiring on January 31, 2008 with an option to renew.
E-7
APPENDIX F
On January 31, 2005, WorldHeart entered into a definitive agreement to acquire substantially all of the assets of MedQuest Products, Inc. ("MedQuest") for 9,300,000 common shares of WorldHeart, subject to certain consents, conditions and shareholder approval (the "MedQuest Transaction"). In connection with the acquisition of MedQuest, WorldHeart has entered into a private placement agreement with Maverick Venture Management, LLC, ("Maverick") who is a majority shareholder of MedQuest. At the closing of the acquisition, Maverick will purchase 8,888,889 common shares of the Corporation at a purchase price of $1.35 per share. In addition, Maverick has agreed to fund MedQuest operations until the closing of the transaction at which point WorldHeart will assume the resulting net liability.
We will launch a tender offer for the debentures issued in September 2004 to have the debentureholders agree to convert their debentures at the stated conversion price of $1.25 per common share into common shares within 14 days after the date on which the shareholders of WorldHeart approve the MedQuest Transaction. The accrued interest will be converted into common shares at a conversion price equal to the weighted average trading price on NASDAQ for the 5 days preceding the date of conversion. In addition, we will launch a tender offer for the warrants issued in September 2004 to have the warrantholders agree to exercise their warrants into 10,655,000 common shares of the Corporation at an adjusted exercise price of $1.00 per common share within 14 days after the date on which the shareholders of WorldHeart approve the MedQuest Transaction. The Warrants are currently exercisable until September 2009 at a price of $1.55 per common share. WorldHeart will seek shareholder approval for a $0.55 reduction in the warrant exercise price.
The following unaudited pro forma combined condensed financial statements and related notes are presented to give effect to the probable acquisition by WorldHeart of MedQuest assets and represent the combined company's unaudited pro forma combined condensed statements of operations for the year ended December 31, 2004 and the three months ended March 31, 2005 and the combined condensed balance sheets as at December 31, 2004 and March 31, 2005. The MedQuest statements of operations have been updated to present the twelve month period ended December 31, 2004 by adding the unaudited six month period ending December 31, 2004 and deducting the unaudited six month period ending December 31, 2003 from the twelve month period ending June 30, 2004 presented in its audited financial statements presented in Appendix D to this Circular. The data has been prepared giving effect to the probable acquisition under the purchase method of accounting as if it occurred prior to January 1, 2004 for the pro forma statements of operations and as at March 31, 2005 for the pro forma consolidated condensed balance sheet under both U.S. and Canadian GAAP using a measurement date for the acquisition of January 31, 2005. The unaudited pro forma combined condensed financial data is presented for illustrative purposes only and is not necessarily indicative of the operating results or financial position that would have been achieved had the acquisition been consummated as of dates indicated or that may be achieved in the future. Certain MedQuest financial statement items have been reclassified to conform with WorldHeart accounting policies.
The unaudited pro forma combined condensed financial information should be read in conjunction with the audited consolidated financial statements and related notes of WorldHeart and MedQuest included elsewhere in this proxy statement.
F-1
WORLD HEART CORPORATION
U.S. GAAP UNAUDITED PRO FORMA COMBINED CONDENSED STATEMENTS OF OPERATIONS
For the fiscal year ended December 31, 2004
| | World Heart
Corporation
| | MedQuest
Products, Inc.
| | Pro Forma Adjustments
| | Pro Forma
World Heart
Corporation
| |
|---|
| |
| |
| | (1)(c)
| | (2)
| | (3)
| | (6)
| |
| |
|---|
| Revenue | | 9,575,761 | | 139,610 | | | | (139,610 | ) | | | | | 9,575,761 | |
| Cost of Sales | | (7,680,384 | ) | — | | | | | | | | | | (7,680,384 | ) |
| | |
| |
| | | | | | | | | |
| |
| Gross Margin | | 1,895,377 | | 139,610 | | | | | | | | | | 1,895,377 | |
| | |
| |
| | | | | | | | | |
| |
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | (13,363,796 | ) | (1,065,550 | ) | | | | | | | | | (14,429,346 | ) |
| | Research and development | | (5,838,754 | ) | (1,991,050 | ) | | | 139,610 | | | | | | (7,690,194 | ) |
| | Non-cash share based compensation | | — | | (17,744 | ) | | | | | | | | | (17,744 | ) |
| | Restructuring costs | | (1,787,129 | ) | — | | | | | | | | | | (1,787,129 | ) |
| | Amortization of intangibles | | (515,012 | ) | (28,932 | ) | 28,932 | | | | | | | | (515,012 | ) |
| | |
| |
| | | | | | | | | |
| |
| | | (21,504,691 | ) | (3,103,276 | ) | | | | | | | | | (24,439,425 | ) |
| | |
| |
| | | | | | | | | |
| |
| Loss before the undernoted | | (19,609,314 | ) | (2,963,666 | ) | | | | | | | | | (22,544,048 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Foreign exchange loss | | (308,338 | ) | — | | | | | | | | | | (308,338 | ) |
| | Investment income | | 99,427 | | 5,727 | | | | | | | | | | 105,154 | |
| | Loss on disposal of capital assets | | (46,431 | ) | — | | | | | | | | | | (46,431 | ) |
| | Gain on forgiveness of accrued interest | | — | | 263,871 | | | | | | (263,871 | ) | | | — | |
| | Interest expense and financing costs | | (6,277,142 | ) | (184,996 | ) | | | | | 184,996 | | 444,642 | | (5,832,500 | ) |
| | |
| |
| | | | | | | | | |
| |
| Loss before income taxes | | (26,141,798 | ) | (2,879,064 | ) | | | | | | | | | (28,626,163 | ) |
| Income tax (benefit) expense | | — | | (20 | ) | | | | | | | | | (20 | ) |
| | |
| |
| | | | | | | | | |
| |
| Net Loss for the year | | (26,141,798 | ) | (2,879,084 | ) | | | | | | | | | (28,626,183 | ) |
| | |
| |
| | | | | | | | | |
| |
Weighted average number of common shares outstanding |
|
15,373,689 |
|
|
|
|
|
|
|
|
|
|
|
54,960,078 |
(7) |
| Basic and diluted loss per common share | | (1.70 | ) | | | | | | | | | | | (0.52 | ) |
F-2
WORLD HEART CORPORATION
UNAUDITED PRO FORMA COMBINED CONDENSED STATEMENTS OF OPERATIONS
For the three months ended March 31, 2005
| | World Heart
Corporation
| | MedQuest
Products, Inc.
| | Pro Forma Adjustments
| | Pro Forma
World Heart
Corporation
| |
|---|
| |
| |
| | (1)(c)
| | (2)
| | (3)
| | (6)
| |
| |
|---|
| Revenue | | 3,416,988 | | 61,619 | | | | (61,619 | ) | | | | | 3,416,988 | |
| Cost of Sales | | (2,111,702 | ) | — | | | | | | | | | | (2,111,702 | ) |
| | |
| |
| | | | | | | | | |
| |
| Gross Margin | | 1,305,286 | | 61,619 | | | | | | | | | | 1,305,286 | |
| | |
| |
| | | | | | | | | |
| |
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | (3,445,829 | ) | (476,785 | ) | | | | | | �� | | | (3,922,614 | ) |
| | Research and development | | (1,187,558 | ) | (788,730 | ) | | | 61,619 | | | | | | (1,914,669 | ) |
| | Non-cash share based compensation | | — | | — | | | | | | | | | | — | |
| | Restructuring costs | | (129,498 | ) | — | | | | | | | | | | (129,498 | ) |
| | Amortization of intangibles | | (127,514 | ) | (7,233 | ) | 7,233 | | | | | | | | (127,514 | ) |
| | |
| |
| | | | | | | | | |
| |
| | | (4,890,399 | ) | (1,272,748 | ) | | | | | | | | | (6,094,295 | ) |
| | |
| |
| | | | | | | | | |
| |
| Loss before the undernoted | | (3,585,113 | ) | (1,211,129 | ) | | | | | | | | | (4,789,009 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Foreign exchange gain (loss) | | (24,372 | ) | | | | | | | | | | | (24,372 | ) |
| | Investment income | | 20,026 | | 1,320 | | | | | | | | | | 21,346 | |
| | Loss on disposal of capital assets | | — | | | | | | | | | | | | — | |
| | Interest expense and financing costs | | (352,937 | ) | (4,291 | ) | | | | | 4,291 | | 347,322 | | (5,615 | ) |
| | |
| |
| | | | | | | | | |
| |
| Loss before income taxes | | (3,942,396 | ) | (1,214,100 | ) | | | | | | | | | (4,797,650 | ) |
| Income tax (benefit) expense | | — | | — | | | | | | | | | | — | |
| | |
| |
| | | | | | | | | |
| |
| Net Loss for the year | | (3,942,396 | ) | (1,214,100 | ) | | | | | | | | | (4,797,650 | ) |
| | |
| |
| | | | | | | | | |
| |
Weighted average number of common shares outstanding |
|
16,556,950 |
|
|
|
|
|
|
|
|
|
|
|
56,143,339 |
(7) |
| Basic and diluted loss per common share | | (0.24 | ) | | | | | | | | | | | (0.09 | ) |
F-3
WORLD HEART CORPORATION
UNAUDITED PRO FORMA COMBINED CONDENSED BALANCE SHEET
As at March 31, 2005
| | World Heart
Corporation
| | MedQuest
Products, Inc.
| | Pro Forma Adjustments
| | Pro Forma
World Heart
Corporation
| |
|---|
| |
| |
| | (1)(a)
| | (1)(b)
| | (4)
| | (5)
| | (6)
| |
| |
|---|
| Balance Sheet | | | | | | | | | | | | | | | | | |
| ASSETS | | | | | | | | | | | | | | | | | |
| | Current assets | | | | | | | | | | | | | | | | | |
| | | Cash and cash
equivalents | | 4,536,861 | | 412,456 | | | | (325,000 | ) | 12,000,000 | | 10,655,000 | | | | 27,279,317 | |
| | | Short-term investments | | — | | — | | | | | | | | | | | | — | |
| | | Trade and other receivables | | 4,137,000 | | 58,110 | | | | | | | | | | | | 4,195,110 | |
| | | Prepaid expenses | | 991,467 | | 19,685 | | | | | | | | | | | | 1,011,152 | |
| | | Deferred transaction and financing costs | | 330,247 | | | | | | | | | | | | | | 330,247 | |
| | | Inventory | | 8,453,189 | | — | | | | | | | | | | | | 8,453,189 | |
| | |
| |
| | | | | | | | | | | |
| |
| | | 18,448,764 | | 490,251 | | | | | | | | | | | | 41,269,015 | |
| | Long-term receivable | | — | | — | | | | | | | | | | | | — | |
| | Cash pledged as collateral for lease | | 750,000 | | — | | | | | | | | | | | | 750,000 | |
| | Capital assets | | 1,937,490 | | 204,570 | | | | 38,252 | | | | | | | | 2,180,312 | |
| | Goodwill | | 17,179,643 | | — | | | | | | | | | | | | 17,179,643 | |
| | Intangible assets | | 127,498 | | 336,097 | | | | (336,097 | ) | | | | | | | 127,498 | |
| | |
| |
| | | | | | | | | | | |
| |
| | | 38,443,395 | | 1,030,918 | | | | | | | | | | | | 61,506,468 | |
| | |
| |
| | | | | | | | | | | |
| |
LIABILITIES AND SHAREHOLDERS EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Current liabilities | | | | | | | | | | | | | | | | | |
| | | Accounts payable and accrued liabilities | | 4,234,969 | | 284,653 | | | | 417,000 | | 100,000 | | | | | | 5,036,622 | |
| | | Taxes payable | | 744,000 | | — | | | | | | | | | | | | 744,000 | |
| | | Accrued compensation | | 744,249 | | 124,433 | | | | | | | | | | | | 868,682 | |
| | | Capital lease obligation | | — | | 434 | | | | | | | | | | | | 434 | |
| | | License fee payable | | — | | 28,496 | | | | | | | | | | | | 28,496 | |
| | | Deferred revenue | | 1,702,163 | | — | | | | | | | | | | | | 1,702,163 | |
| | | Notes payable | | — | | 1,000,000 | | | | | | | | | | | | 1,000,000 | |
| | |
| |
| | | | | | | | | | | |
| |
| | | 7,425,381 | | 1,438,016 | | | | | | | | | | | | 9,380,397 | |
| | Convertible debentures | | 7,473,840 | | — | | | | | | | | | | (7,473,840 | ) | — | |
| | License fee payable | | — | | 110,114 | | | | | | | | | | | | 110,114 | |
| | Capital lease obligation | | — | | — | | | | | | | | | | | | — | |
| | Long-term obligation | | 8,830 | | — | | | | | | | | | | | | 8,830 | |
| | |
| |
| | | | | | | | | | | |
| |
| | | 14,908,051 | | 1,548,130 | | | | | | | | | | | | 9,499,341 | |
| | |
| |
| | | | | | | | | | | |
| |
| | Shareholders equity | | | | | | | | | | | | | | | | | |
| | | Common shares | | 215,740,523 | | 127 | | (127 | ) | 12,555,000 | | 11,900,000 | | 20,884,420 | | 13,257,846 | | 274,337,789 | |
| | | Preferred shares | | — | | 6,676 | | (6,676 | ) | | | | | | | | | — | |
| | | APIC | | 11,377,442 | | 14,996,956 | | (14,996,956 | ) | | | | | (4,369,170 | ) | (5,784,006 | ) | 1,224,266 | |
| | | CTA | | (6,285,577 | ) | — | | | | | | | | | | | | (6,285,577 | ) |
| | | Accumulated deficit | | (197,297,044 | ) | (15,520,971 | ) | 15,520,971 | | (14,112,057 | ) | | | (5,860,250 | ) | | | (217,269,351 | ) |
| | |
| |
| | | | | | | | | | | |
| |
| | | 23,535,344 | | (517,212 | ) | | | | | | | | | | | 52,007,127 | |
| | |
| |
| | | | | | | | | | | |
| |
| | | 38,443,395 | | 1,030,918 | | | | | | | | | | | | 61,506,468 | |
| | |
| |
| | | | | | | | | | | |
| |
| | Other Data: | | | | | | | | | | | | | | | | | |
| | | Shares outstanding | | 17,101,359 | | | | | | | | | | | | | | 56,687,748 | (8) |
| | | Book value per share | | 1.38 | (9) | | | | | | | | | | | | | 0.92 | (9) |
F-4
NOTES TO THE U.S. GAAP UNAUDITED PRO FORMA
COMBINED CONDENSED FINANCIAL STATEMENTS
- (1)
- The probable acquisition has been treated as a purchase of MedQuest assets for the purposes of presenting these pro forma combined condensed financial statements. WorldHeart will acquire all of the assets, except for cash of $325,000 and assume all of the operating liabilities and notes payable, except for income and other tax liabilities relating to the ongoing MedQuest Inc. entity. Accordingly, the MedQuest equity has been removed and the assets and liabilities have been adjusted to estimated fair values for the purposes of preparing the U.S. GAAP pro forma combined condensed balance sheets. The estimated purchase price of $14,520,130 consists of the following components:
| | March 31,
2005
|
|---|
| Share Consideration | | $ | 12,555,000 |
| MedQuest Liabilities assumed | | | 1,548,130 |
| Acquisition costs | | | 417,000 |
| | |
|
| | | $ | 14,520,130 |
| | |
|
The allocation of the estimated purchase price to the assets and the in-process research and development is:
| | March 31,
2005
|
|---|
| Current assets | | $ | 165,251 |
| Capital Assets | | | 242,822 |
| IPR&D | | | 14,112,057 |
| | |
|
| | | $ | 14,520,130 |
| | |
|
We believe that all intangible assets acquired from MedQuest relate to one project, the research and development of the HeartQuest Ventricular Assist Device and the related peripheral devices collectively referred to herein as the HeartQuest Ventricular Assist System ("HeartQuest VAS"), that will potentially lead to revenues and cash flows from the commercialization of the HeartQuest VAS. While we may monitor and assess the research and development progress on various HeartQuest programs, we believe that they all ultimately relate to the same project and a single stream of expenses and cash flows and in the future, if development is successful, revenues. Because the HeartQuest VAS project has realized no revenue to date and has no identifiable date for commercialization, we have treated the probable transaction as a purchase of assets as opposed to the purchase of a business. In addition, we believe that all of the continuing efforts related to MedQuest will be related to research and development until it becomes clear that an identifiable market exists for the HeartQuest VAS. The intangible assets to be acquired from MedQuest have been preliminarily identified as follows:
| | March 31,
2005
|
|---|
| Patented and other technology | | $ | 12,857,972 |
| Workforce in place | | | 741,959 |
| Internally developed software | | | 512,126 |
| | |
|
| Total intangible assets | | $ | 14,112,057 |
| | |
|
Because these assets are intended for use in the continuing research and development of the HeartQuest VAS with no alternative future use identified, we have determined preliminarily that these items will be charged to research and development expenses when they are acquired. The acquired in-process research and development (IPR&D) has been charged to accumulated deficit in the U.S. GAAP pro forma combined condensed balance sheets. It has not been reflected in the U.S GAAP pro forma combined condensed statements of operations.
We used an income approach in valuing the acquired IPR&D. The income approach reflects the present value of the operating cash flows generated by the IPR&D after taking into account the cost to complete the project, the relative risk of the project, the contribution of other assets and a 41% after-tax discount rate was applied to the in-process product cash flows, to reflect the time value of invested capital. We expect to begin deriving revenue from the MedQuest LVAS in 2007 and incur costs of approximately $23 million until that point.
The projected revenue, expenses and cash flows used in the income approach are based upon probable revenues, expenses and cash flows likely to be generated upon completion of the IPR&D and the beginning of commercial sales, as estimated by MedQuest management. The projections assume that the HeartQuest™ VAD project will be successful and that the product development and commercialization meet management's current time schedule. The revenue, expenses and cash flows related to the IPR&D is projected
F-5
over a ten year period. This forecast period has been selected after discussing with management the significant functionality that is expected to occur within the project that has been identified. It is also expected that the technology associated with the acquired IPR&D will contribute to future product development.
A 41% after-tax discount rate was applied to the in-process product cash flows after adjusting for the stage of completion of the project. The discount rate applied to the IPR&D cash flows reflects the risk of an investment in the business. Included in this overall risk rate are technological and market risks as well as the time value of money. Technological risk refers to the risk that the product under development will not perform as expected or will require considerably more development effort than expected. Market risk refers to the risk that the product will not receive required market approval from the major markets such as the United States, Europe and Japan or generally not meet with acceptance from the market. As a result of both factors, the cash flows derived from the product may be variable and may have material adverse effects on the future financial condition and results of operation of WorldHeart after the merger.
The forecast data employed in the analyses is based upon product level forecast information obtained by MedQuest from numerous internal and external sources. WorldHeart management has reviewed and challenged the forecast data and related assumptions and has used the information in calculating IPR&D. The forecast data and assumptions are inherently uncertain and unpredictable. However, based upon the information available at this time, WorldHeart believes the forecast data and assumptions to be reasonable. These assumptions may be incomplete or inaccurate, and no assurance can be given that unanticipated events and circumstances will not occur. Accordingly, actual results may vary from the forecasted results. Any such variance may result in a material adverse effect on the future financial condition and results of operations of WorldHeart after the merger.
In determining the allocation of purchase price to the IPR&D, the concept of alternative future use was specifically considered for the project under development. The acquired IPR&D consists of MedQuest's completed work on the HeartQuest VAD program at the time of acquisition. There are no identified alternative uses for the in-process programs if the programs fail in clinical trials or are otherwise deemed unfeasible. The development effort for the acquired IPR&D does not possess an alternative future use for WorldHeart after the merger as defined by generally accepted accounting principles.
We valued the assembled workforce in place using the replacement cost approach. Under this approach, the workforce is valued by calculating the costs avoided by WorldHeart through obtaining a pre-existing, trained and fully efficient team rather than incurring the costs to assemble and train this workforce. The savings realized include avoided recruiting costs and avoided loss of productivity.
We valued the internally developed system simulation software technology using the cost approach. This approach is based on the current cost to recreate or duplicate the assets less an appropriate allowance for obsolescence from all causes — physical, functional and economic. The fair value was determined by multiplying the replacement cost new value by a 100% completion premium and reducing it by a 50% obsolescence factor.
The pro forma adjustments recorded that relate to the acquisition are further detailed as follows:
- (a)
- An adjustment to eliminate the balances from MedQuest shareholder's equity.
- (b)
- An adjustment to record the issuance of 9,300,000 common shares at $1.35 per share for a total increase in common shares of $12,555,000, decrease cash by $325,000 for the amount to be retained by MedQuest, increase liabilities by $417,000 to record estimated acquisition costs, reduce the carrying value of MedQuest intangible assets to zero and increase the accumulated deficit for intangible assets acquired for use in continuing research and development.
- (c)
- An adjustment to eliminate the amortization of MedQuest intangibles.
- (2)
- The grant revenue recorded by MedQuest has been reclassified to offset research and development expenditures to be consistent with WorldHeart's accounting policies.
- (3)
- Interest expense and gain on forgiveness of interest accrued on the MedQuest Notes Payable has been eliminated to reflect the assumption that the Notes would have been converted to equity prior to the transaction.
- (4)
- As a condition of the transaction, the MedQuest majority shareholder will purchase 8,888,889 common shares at $1.35 per share for gross proceeds of $12,000,000. The gross proceeds have been offset by estimated issue costs of $100,000.
- (5)
- As a condition of the transaction, certain warrant holders will exercise their warrants at a price of $1 per share providing gross proceeds of $10,655,000. The change in fair value attributed to the decrease in the exercise price of the warrants from $1.55 to $1.00, of $5,860,250 has been recorded as an increase in accumulated deficit and an increase in the carrying value of the warrants included in APIC. The value attributed to the increase in the carrying value of the warrants will be charged to income available to common shareholders at the completion of the probable acquisition expected to occur during the three month period ending September 30, 2005. Share Capital will increase by $20,884,420 representing the gross proceeds received from the warrant exercise plus the entire value of the warrants included in APIC.
- (6)
- The convertible debentures will convert the original principal at a conversion price of $1.25 and the accrued interest of approximately $140,000 will be converted at the prevailing market share price which is $1.60. The conversion will eliminate all of the convertible debentures and decrease APIC by $5,784,006, the amount allocated to the beneficial conversion feature. Interest and accretion accrued
F-6
on the convertible debentures has been eliminated from the pro forma combined condensed statements of operation totalling $444,642 for the year ended December 31, 2004 and $347,322 for the three months ended March 31, 2005.
- (7)
- The pro forma net loss per common share is computed by dividing the pro forma net loss by the pro forma weighted average number of shares outstanding, assuming WorldHeart and MedQuest had merged on January 1, 2004. The pro forma weighted average number of shares outstanding is calculated as follows:
| | Year Ended
December 31, 2004
| | Three Months Ended
March 31, 2005
|
|---|
| WorldHeart weighted average common shares | | 15,373,689 | | 16,556,950 |
| Common shares issued for MedQuest assets | | 9,300,000 | | 9,300,000 |
| Common shares issued to MedQuest majority shareholder | | 8,888,889 | | 8,888,889 |
| Common shares issued upon exercise of 10,655,000 warrants | | 10,655,000 | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures principal | | 10,655,000 | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures interest | | 87,500 | | 87,500 |
| | |
| |
|
| | | 54,960,078 | | 56,143,339 |
| | |
| |
|
- (8)
- The adjustment to March 31, 2005 WorldHeart common shares is determined as follows:
| | As At
March 31, 2005
|
|---|
| WorldHeart common shares outstanding | | 17,101,359 |
| Common shares issued for MedQuest assets | | 9,300,000 |
| Common shares issued to MedQuest majority shareholder | | 8,888,889 |
| Common shares issued upon exercise of 10,655,000 warrants | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures principal | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures interest | | 87,500 |
| | |
|
| | | 56,687,748 |
| | |
|
- (9)
- Net book value per share is determined by dividing shareholders equity by the number of shares outstanding at the period end.
F-7
WORLD HEART CORPORATION
CANADIAN GAAP UNAUDITED PRO FORMA
COMBINED CONDENSED STATEMENTS OF OPERATIONS
For the fiscal year ended December 31, 2004
| | World Heart
Corporation
| | MedQuest
Products, Inc.
| | Pro Forma Adjustments
| | Pro Forma
World Heart
Corporation
| |
|---|
| |
| |
| | (1)(c)
| | (2)
| | (3)
| | (6)
| |
| |
|---|
| Revenue | | 9,575,761 | | 139,610 | | | | (139,610 | ) | | | | | 9,575,761 | |
| Cost of Sales | | (7,680,384 | ) | — | | | | | | | | | | (7,680,384 | ) |
| | |
| |
| | | | | | | | | |
| |
| Gross Margin | | 1,895,377 | | 139,610 | | | | | | | | | | 1,895,377 | |
| | |
| |
| | | | | | | | | |
| |
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | (13,363,796 | ) | (1,065,550 | ) | | | | | | | | | (14,429,346 | ) |
| | Research and development | | (5,838,754 | ) | (1,991,050 | ) | | | 139,610 | | | | | | (7,690,194 | ) |
| | Non-cash share based compensation | | (2,134,707 | ) | (185,387 | ) | | | | | | | | | (2,320,094 | ) |
| | Restructuring costs | | (1,787,129 | ) | — | | | | | | | | | | (1,787,129 | ) |
| | Amortization of Intangibles | | (515,012 | ) | (28,932 | ) | (1,378,878 | ) | | | | | | | (1,922,822 | ) |
| | |
| |
| | | | | | | | | |
| |
| | | (23,639,398 | ) | (3,270,919 | ) | | | | | | | | | (28,149,585 | ) |
| | |
| |
| | | | | | | | | |
| |
| Loss before the undernoted | | (21,744,021 | ) | (3,131,309 | ) | | | | | | | | | (26,254,208 | ) |
Other income (expenses) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Foreign exchange loss | | (308,338 | ) | — | | | | | | | | | | (308,338 | ) |
| | Investment income | | 99,427 | | 5,727 | | | | | | | | | | 105,154 | |
| | Loss on disposal of capital assets | | (46,431 | ) | — | | | | | | | | | | (46,431 | ) |
| | Gain on forgiveness of accrued interest | | — | | 263,871 | | | | | | (263,871 | ) | | | — | |
| | Interest expense and financing costs | | (807,442 | ) | (417,089 | ) | | | | | 417,089 | | 796,309 | | (11,133 | ) |
| | |
| |
| | | | | | | | | |
| |
Loss before income taxes |
|
(22,806,805 |
) |
(3,278,800 |
) |
|
|
|
|
|
|
|
|
(26,514,956 |
) |
| Income tax (benefit) expense | | — | | (20 | ) | | | | | | | | | (20 | ) |
| | |
| |
| | | | | | | | | |
| |
| Net Loss for the year | | (22,806,805 | ) | (3,278,820 | ) | | | | | | | | | (26,514,976 | ) |
| | |
| |
| | | | | | | | | |
| |
Weighted average number of common shares outstanding |
|
15,373,689 |
|
|
|
|
|
|
|
|
|
|
|
54,960,078 |
(7) |
Basic and diluted loss per common
share | | (1.48 | ) | | | | | | | | | | | (0.48 | ) |
F-8
WORLD HEART CORPORATION
CANADIAN GAAP UNAUDITED PRO FORMA COMBINED CONDENSED BALANCE SHEET
As at December 31, 2004
| | World Heart
Corporation
| | MedQuest
Products, Inc.
| | Pro Forma Adjustments
| | Pro Forma
World Heart
Corporation
| |
|---|
| |
| |
| | (1)(a)
| | (1)(b)
| | (1)(c)
| | (4)
| | (5)
| | (6)
| |
| |
|---|
| Balance Sheet | | | | | | | | | | | | | | | | | | | |
| ASSETS | | | | | | | | | | | | | | | | | | | |
| | Current assets | | | | | | | | | | | | | | | | | | | |
| | | Cash and cash equivalents | | 3,818,767 | | 618,899 | | | | (325,000 | ) | | | 12,000,000 | | 10,655,000 | | | | 26,767,666 | |
| | | Short-term investments | | 4,999,034 | | — | | | | | | | | | | | | | | 4,999,034 | |
| | | Trade and other receivables | | 4,238,049 | | 12,663 | | | | | | | | | | | | | | 4,250,712 | |
| | | Prepaid expenses | | 575,261 | | 19,803 | | | | | | | | | | | | | | 595,064 | |
| | | Inventory | | 8,112,525 | | — | | | | | | | | | | | | | | 8,112,525 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 21,743,636 | | 651,365 | | | | | | | | | | | | | | 44,725,001 | |
| | Long-term receivable | | 318,553 | | — | | | | | | | | | | | | | | 318,553 | |
| | Cash pledged as collateral for lease | | 750,000 | | — | | | | | | | | | | | | | | 750,000 | |
| | Capital assets | | 2,011,586 | | 208,159 | | | | 14,309 | | | | | | | | | | 2,234,054 | |
| | Goodwill | | 17,179,643 | | — | | | | | | | | | | | | | | 17,179,643 | |
| | Intangible assets | | 255,012 | | 343,330 | | | | 12,585,803 | | (1,378,878 | ) | | | | | | | 11,805,267 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 42,258,430 | | 1,202,854 | | | | | | | | | | | | | | 77,012,518 | |
| | |
| |
| | | | | | | | | | | | | |
| |
LIABILITIES AND SHAREHOLDERS EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Current liabilities | | | | | | | | | | | | | | | | | | | |
| | | Accounts payable and accrued liabilities | | 3,854,833 | | 197,050 | | | | 417,000 | | | | 100,000 | | | | | | 4,568,883 | |
| | | Taxes payable | | 832,000 | | — | | | | | | | | | | | | | | 832,000 | |
| | | Accrued compensation | | 1,343,364 | | 136,784 | | | | | | | | | | | | | | 1,480,148 | |
| | | Capital lease obligation | | — | | 9,891 | | | | | | | | | | | | | | 9,891 | |
| | | License fee payable | | — | | 52,127 | | | | | | | | | | | | | | 52,127 | |
| | | Deferred revenue | | 1,607,622 | | — | | | | | | | | | | | | | | 1,607,622 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 7,637,819 | | 395,852 | | | | | | | | | | | | | | 8,550,671 | |
| | Convertible debentures | | 2,530,659 | | — | | | | | | | | | | | | (2,530,659 | ) | — | |
| | License fee payable | | — | | 110,114 | | | | | | | | | | | | | | 110,114 | |
| | Long-term obligation | | 16,368 | | — | | | | | | | | | | | | | | 16,368 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 10,184,846 | | 505,966 | | | | | | | | | | | | | | 8,677,153 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| |
Shareholders equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | Common shares | | 187,027,058 | | 128 | | (128 | ) | 12,555,000 | | | | 11,900,000 | | 21,019,377 | | 7,982,030 | | 240,483,465 | |
| | | Preferred shares | | — | | 6,676 | | (6,676 | ) | | | | | | | | | | | — | |
| | | Contributed surplus | | 22,871,780 | | 16,490,303 | | (16,490,303 | ) | | | | | | | (4,504,127 | ) | (5,451,371 | ) | 12,916,282 | |
| | | CTA | | (6,490,184 | ) | — | | | | | | | | | | | | | | (6,490,184 | ) |
| | | Accumulated deficit | | (171,335,070 | ) | (15,800,219 | ) | 15,800,219 | | | | (1,378,878 | ) | | | (5,860,250 | ) | | | (178,574,198 | ) |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 32,073,584 | | 696,888 | | | | | | | | | | | | | | 68,335,365 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | | 42,258,430 | | 1,202,854 | | | | | | | | | | | | | | 77,012,518 | |
| | |
| |
| | | | | | | | | | | | | |
| |
| | Other Data: | | | | | | | | | | | | | | | | | | | |
| | | Shares outstanding | | 15,744,522 | | | | | | | | | | | | | | | | 55,330,911 | (8) |
| | | Book value per share | | 2.04 | (9) | | | | | | | | | | | | | | | 1.24 | (9) |
F-9
NOTES TO THE CANADIAN GAAP UNAUDITED PRO FORMA
COMBINED CONDENSED FINANCIAL STATEMENTS
- (1)
- The probable acquisition has been treated as a purchase of MedQuest assets for the purposes of presenting these pro forma combined condensed financial statements. WorldHeart will acquire all of the assets, except for cash of $325,000 and assume all of the operating liabilities and notes payable, except for income and other tax liabilities relating to the ongoing MedQuest Inc. entity. Accordingly, the MedQuest equity has been removed and the assets and liabilities have been adjusted to estimated fair values for the purposes of preparing the Canadian GAAP pro forma combined condensed balance sheets. The estimated purchase price of $13,477,966 consists of the following components:
| | December 31,
2004
|
|---|
| Share Consideration | | $ | 12,555,000 |
| MedQuest Liabilities Assumed | | | 505,966 |
| Acquisition Costs | | | 417,000 |
| | |
|
| | | $ | 13,477,966 |
| | |
|
| | December 31,
2004
|
|---|
| Current Assets | | $ | 326,365 |
| Capital Assets | | | 222,468 |
| Acquired Intangible Assets | | | 12,929,133 |
| | |
|
| | | $ | 13,477,966 |
| | |
|
Acquired intangible assets are amortized over a weighted average estimated useful life of 9.3 years.
The pro forma adjustments recorded that relate to the acquisition are further detailed as follows:
- (a)
- An adjustment to eliminate the balances from MedQuest shareholder's equity.
- (b)
- An adjustment to record the issuance of 9,300,000 common shares at $1.35 per share for a total increase in common shares of $12,555,000, decrease cash by $325,000 for the amount to be retained by MedQuest, increase liabilities by $417,000 to record estimated acquisition costs, reduce the carrying value of MedQuest intangible assets by $343,330 and increase intangible assets by $12,929,133 for assets acquired.
- (c)
- An adjustment of $1,378,878 to eliminate the amortization of legacy MedQuest intangibles of $28,932 and amortize the intangible assets arising from the acquisition of $1,407,810.
- (2)
- The grant revenue recorded by MedQuest has been reclassified to offset research and development expenditures to be consistent with WorldHeart's accounting policies.
- (3)
- Interest expense and gain on forgiveness of interest accrued on the MedQuest Notes Payable has been eliminated to reflect the assumption that the Notes would have been converted to equity prior to the transaction.
- (4)
- As a condition of the transaction, the MedQuest majority shareholder will purchase 8,888,889 common shares at $1.35 per share for gross proceeds of $12,000,000. The gross proceeds have been offset by estimated issue costs of $100,000.
- (5)
- As a condition of the transaction, certain warrant holders will exercise their warrants at a price of $1 per share providing gross proceeds of $10,655,000. The change in fair value attributed to the decrease in the exercise price of the warrants from $1.55 to $1.00, or concession to exercise the warrants, of $5,860,250 has been recorded as an increase in accumulated deficit and an increase in the carrying value of the warrants included in contributed surplus. Share Capital will increase by $21,019,377 representing the gross proceeds received from the warrant exercise plus the entire value of the warrants included in contributed surplus.
- (6)
- The convertible debentures will convert the original principal at a conversion price of $1.25 and the accrued interest of approximately $140,000 will be converted at the prevailing market share price which is $1.60. The conversion will eliminate all of the convertible debentures and decrease contributed surplus by $5,451,371, the amount allocated to equity. Interest accrued on the convertible debentures has been eliminated from the pro forma combined condensed statements of operation totalling $796,309.
F-10
- (7)
- The pro forma net loss per common share is computed by dividing the pro forma net loss by the pro forma weighted average number of shares outstanding, assuming WorldHeart and MedQuest had merged on January 1, 2004. The pro forma weighted average number of shares outstanding for the year ended December 31, 2004 is calculated as follows:
| | December 31,
2004
|
|---|
| WorldHeart weighted average common shares | | 15,373,689 |
| Common shares issued for MedQuest assets | | 9,300,000 |
| Common shares issued to MedQuest majority shareholder | | 8,888,889 |
| Common shares issued upon exercise of 10,655,000 warrants | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures principal | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures interest | | 87,500 |
| | |
|
| | | 54,960,078 |
| | |
|
- (8)
- The adjustment to December 31, 2004 WorldHeart common shares is determined as follows:
| | December 31,
2004
|
|---|
| WorldHeart common shares outstanding | | 15,744,522 |
| Common shares issued for MedQuest assets | | 9,300,000 |
| Common shares issued to MedQuest majority shareholder | | 8,888,889 |
| Common shares issued upon exercise of 10,655,000 warrants | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures principal | | 10,655,000 |
| Common shares issued upon conversion of convertible debentures interest | | 87,500 |
| | |
|
| | | 55,330,911 |
| | |
|
- (9)
- Net book value per share is determined by dividing December 31, 2004 shareholders equity by the number of shares outstanding at December 31, 2004.
F-11
APPENDIX G
ASSET PURCHASE AGREEMENT
BETWEEN
WORLD HEART CORPORATION
AND
MEDQUEST PRODUCTS, INC.
MADE AS OF
JANUARY 31, 2005
G-1
TABLE OF CONTENTS
| |
| | Page
|
|---|
| ARTICLE 1 — INTERPRETATION | | G-4 |
| 1.01 | | Definitions | | G-4 |
| 1.02 | | Headings | | G-7 |
| 1.03 | | Extended Meanings | | G-7 |
| 1.04 | | Statutory References | | G-7 |
| 1.05 | | Currency | | G-7 |
| 1.06 | | Schedules | | G-8 |
| 1.07 | | Knowledge | | G-9 |
ARTICLE 2 — SALE AND PURCHASE |
|
G-9 |
| 2.01 | | Assets to be Sold and Purchased | | G-9 |
| 2.02 | | Purchase Price | | G-10 |
| 2.03 | | Property Taxes | | G-10 |
| 2.04 | | Assumption of Obligations and Liabilities | | G-11 |
| 2.05 | | Assumption of Existing Obligations and Liabilities | | G-11 |
| 2.06 | | Obligations and Liabilities Not Assumed | | G-11 |
| 2.07 | | Non-Assignable Contracts and Commitments | | G-11 |
ARTICLE 3 — REPRESENTATIONS AND WARRANTIES |
|
G-11 |
| 3.01 | | Vendor's Representations and Warranties | | G-11 |
| 3.02 | | Survival of Vendor's Representations, Warranties and Covenants | | G-20 |
| 3.03 | | Purchaser's Representations and Warranties | | G-20 |
| 3.04 | | Survival of Purchaser's Representations, Warranties and Covenants | | G-22 |
ARTICLE 4 — COVENANTS |
|
G-22 |
| 4.01 | | Covenants of the Vendor | | G-22 |
| 4.02 | | Covenants of the Purchaser | | G-24 |
| 4.03 | | Employees | | G-24 |
| 4.04 | | Cooperation on Tax Matters | | G-25 |
| 4.05 | | Tax Controversies | | G-25 |
| 4.06 | | Amended Tax Returns | | G-26 |
ARTICLE 5 — CONDITIONS |
|
G-26 |
| 5.01 | | Conditions for the Benefit of the Purchaser | | G-26 |
| 5.02 | | Conditions for the Benefit of the Vendor | | G-28 |
ARTICLE 6 — CLOSING ARRANGEMENTS |
|
G-28 |
| 6.01 | | Closing | | G-28 |
| 6.02 | | Examination of Records and Assets | | G-29 |
| 6.03 | | Transfer of Possession | | G-29 |
| 6.04 | | Risk of Loss | | G-30 |
| | | | | |
G-2
ARTICLE 7 — GENERAL |
|
G-30 |
| 7.01 | | Indemnification | | G-30 |
| 7.02 | | Further Assurances | | G-30 |
| 7.03 | | Time of the Essence | | G-30 |
| 7.04 | | Fees and Commissions | | G-30 |
| 7.05 | | Public Announcements | | G-31 |
| 7.06 | | Benefit of the Agreement | | G-31 |
| 7.07 | | Entire Agreement | | G-31 |
| 7.08 | | Amendments and Waivers | | G-31 |
| 7.09 | | Assignment | | G-31 |
| 7.10 | | Notices | | G-31 |
| 7.11 | | Remedies Cumulative | | G-32 |
| 7.12 | | No Third Party Beneficiaries | | G-32 |
| 7.13 | | Governing Law; Consent to Jurisdiction; WAIVER OF JURY TRIAL | | G-32 |
| 7.14 | | Counterparts | | G-33 |
| 7.15 | | Facsimiles | | G-33 |
G-3
ASSET PURCHASE AGREEMENT
THIS AGREEMENT is made as of January 31, 2005
BETWEEN
— and —
MedQuest Products, Inc., a corporation incorporated under the laws of the State of Utah (the "Vendor"),
WHEREAS the Vendor carries on the primary business of research and development and manufacturing related to a series of rotary blood pumps;
AND WHEREAS the Vendor desires to sell and the Purchaser desires to purchase all of the assets of the Vendor pertaining to its blood pump business and its coating and blade business upon and subject to the terms and conditions set out in this Agreement;
NOW THEREFORE, in consideration of the covenants and agreements herein contained, the parties agree as follows:
ARTICLE 1 — INTERPRETATION
1.01 Definitions
In this Agreement, unless something in the subject matter or context is inconsistent therewith:
"Affiliate" has the meaning attributed thereto in theBusiness Corporations Act (Ontario).
"Agreement" means this agreement, including its recitals and schedules, as amended from time to time.
"Applicable Law" means
- (i)
- any applicable domestic or foreign law including any statute, subordinate legislation or treaty, and
- (ii)
- any applicable guideline, directive, rule, standard, requirement, policy, order, judgment, injunction, award or decree of a Governmental Authority having the force of law.
"Assets" means the assets and undertaking referred to or described in Section 2.01.
"Audited Balance Sheet" means the balance sheet of the Vendor as at the Audited Balance Sheet Date.
"Audited Balance Sheet Date" means June 30, 2004.
"Audited Financial Statements" has the meaning set out in Section 3.01(k).
"Balance Sheet Date" means December 31, 2004.
"Benefit Plans" has the meaning set out in Section 3.01(yy).
"Business Day" means a day other than a Saturday, Sunday or statutory holiday in Ottawa, Ontario or Oakland, California or Salt Lake City, Utah.
"Canadian GAAP" means the generally accepted accounting principles from time to time approved by the Canadian Institute of Chartered Accountants, consistently applied, as in effect from time to time.
"Cash Amounts" has the meaning set out in Section 2.06(2).
"Claims" means all losses, damages, expenses, liabilities (whether accrued, actual, contingent, latent or otherwise), claims and demands of whatever nature or kind including all legal fees and costs on a solicitor and client basis.
G-4
"Closing Date" means the later of (i) May 9, 2005, and (ii) a date to be determined by adding that number of days beyond May 9, 2005 that the Time of Closing has been extended due to (A) a regulatory authority in Canada or the United States reviewing, or taking a similar action, with respect to the Purchaser's proxy materials or such other materials as may be required to be submitted to such regulatory authority, (B) procedural delays as a result of such regulatory authority's review, such as the requirement to republish and extend the date on which the Purchaser's meeting of shareholders can occur, and (C) the time required by the Purchaser to respond to any such regulatory authority's requests or inquiries, provided that the Purchaser shall use reasonable commercial efforts to respond to any such requests or inquiries as quickly as possible under the circumstances, or such earlier or later date as may be agreed in writing between the Vendor and the Purchaser.
"Code" means the United States Internal Revenue Code of 1986, as amended.
"Common Shares" means the common shares of the Purchaser.
"Compensation Policies" has the meaning set out in Section 3.01(zz).
"Convertible Debentureholders" means the holders of convertible debentures of the Purchaser issued on September 15, 2004 in an aggregate principal amount of $13,318,750.
"Designated Employees" has the meaning set out in Section 4.03.
"Effective Date" means January 31, 2005.
"Environmental Law" means any Applicable Law relating to the environment including those pertaining to
- (i)
- reporting, licensing, permitting, investigating, remediating and cleaning up in connection with any presence or Release, or the threat of the same, of Hazardous Substances, and
- (ii)
- the manufacture, processing, distribution, use, treatment, storage, disposal, transport, handling and the like of Hazardous Substances, including those pertaining to occupational health and safety.
"Escrow Agent" means CIBC Mellon Trust Company.
"Escrow Agreement" means the escrow agreement to be entered into between the Purchaser, the Vendor and the Escrow Agent.
"Escrowed Shares" means 930,000 Common Shares registered in the name of the Vendor being a partial payment of the Purchase Price to be held by the Escrow Agent pursuant to the Escrow Agreement.
"ERISA" means the United States Employee Retirement Income Security Act of 1974, as amended.
"Governmental Authority" means any domestic or foreign legislative, executive, judicial or administrative body or person having or purporting to have jurisdiction in the relevant circumstances.
"Governmental Body" means any governmental authority, or any university or related organization, or any other entity funded by a governmental authority.
"Hazardous Substance" means any substance or material that is prohibited, controlled or regulated by any Governmental Authority pursuant to Environmental Laws.
"Indemnitee" has the meaning set out in Section 7.01.
"Indemnitor" has the meaning set out in Section 7.01.
"Intellectual Property" means intellectual property of whatever nature and kind including all domestic and foreign trade-marks, business names, trade names, domain names, trading styles, patents, trade secrets, Software, industrial designs and copyrights, whether registered or unregistered, and all applications for registration thereof, and inventions, formulae, recipes, product formulations, processes and processing methods, technology and techniques, know-how and manuals.
"Interim Period" means the period between the close of business on the Effective Date and the Time of Closing.
"Inventories" means all materials or inventories purchased for use in the Purchased Business.
G-5
"Leased Premises" means all leasehold property and interests therein described in Schedule 2.01(b) attached hereto including all rights of way, licences or rights of occupation, easements or other similar rights of the Purchased Business in connection with such leasehold property.
"Licensed Intellectual Property" has the meaning set out in Section 2.01(m).
"Maverick" means Maverick Venture Management, LLC.
"Maverick Loan" has the meaning set out in Section 4.01(7).
"NASDAQ" means the means the Nasdaq National Market or the Nasdaq Small Cap Market.
"Owned Intellectual Property" has the meaning set out in Section 2.01(l).
"Permits" means all permits, consents, waivers, licences, certificates, approvals, authorizations, registrations, franchises, rights, privileges and exemptions, or any item with a similar effect, issued or granted by any person.
"Proceeds" has the meaning set out in Section 6.04.
"Purchase Agreement" means the purchase agreement entered into between the Purchaser and Maverick dated as of the date hereof.
"Purchase Price" has the meaning set out in Section 2.02.
"Purchased Business" means the business of rotary blood pumps and coating and blades at present and heretofore carried on by the Vendor.
"Release" means any release or discharge of any Hazardous Substance including any discharge, spray, injection, inoculation, abandonment, deposit, spillage, leakage, seepage, pouring, emission, emptying, throwing, dumping, placing, exhausting, escape, leach, migration, dispersal, dispensing or disposal.
"SEC" means the United States Securities and Exchange Commission.
"Software" means all software relating to the Purchased Business including all versions thereof, and all related documentation, manuals, source code and object code, program files, data files, computer related data, field and data definitions and relationships, data definition specifications, data models, program and system logic, interfaces, program modules, routines, sub-routines, algorithms, program architecture, design concepts, system designs, program structure, sequence and organization, screen displays and report layouts, and all other material related to such software.
"Subsidiary" has the meaning attributed thereto in theBusiness Corporations Act (Ontario).
"Tax Returns" has the meaning set out in Section 3.01(uuu).
"Taxes" mean all taxes, assessments, charges, duties, fees, levies or other governmental charges, including, without limitation, all federal, state, provincial, local, foreign and other income, franchise, profits, gross receipts, capital gains, capital stock, transfer, property, sales, use, value-added, occupation, property, excise, severance, windfall profits, stamp, license, payroll, social security, withholding and other taxes, assessments, charges, duties, fees, levies or other governmental charges of any kind whatsoever (whether payable directly or by withholding and whether or not requiring the filing of a Tax Return), all estimated taxes, deficiency assessments, additions to tax, penalties and interest and shall include any liability for such amounts as a result either of being a member of a combined, consolidated, unitary or affiliated group or of a contractual obligation to indemnify any person or other entity.
"Time of Closing" means 10:00 a.m. (Ottawa Time) on the Closing Date.
"Third Party Programs" has the meaning set out in Section 3.01(kk).
"Transfer Taxes" has the meaning set out in Section 4.02(3).
"TSX" mean the Toronto Stock Exchange.
"US GAAP" means the generally accepted accounting principles of the United States consistently applied, as in effect from time to time.
G-6
"Warrantholders" means the holders of warrants of the Purchaser issued on September 15, 2004 exercisable in aggregate for 10,655,000 Common Shares.
"WorldHeart Financial Statements" has the meaning set out in Section 3.03(i).
"WorldHeart Reports" has the meaning set out in Section 3.03(h).
1.02 Headings
The division of this Agreement into Articles and Sections and the insertion of a table of contents and headings are for convenience of reference only and do not affect the construction or interpretation of this Agreement. The terms "hereof", "hereunder" and similar expressions refer to this Agreement and not to any particular Article, Section or other portion hereof. Unless something in the subject matter or context is inconsistent therewith, references herein to Articles, Sections and Schedules are to Articles and Sections of and Schedules to this Agreement.
1.03 Extended Meanings
In this Agreement words importing the singular number only include the plural andvice versa, words importing any gender include all genders and words importing persons include individuals, corporations, limited and unlimited liability companies, general and limited partnerships, associations, trusts, unincorporated organizations, joint ventures and Governmental Authorities. The term "including" means "including without limiting the generality of the foregoing".
1.04 Statutory References
In this Agreement, unless something in the subject matter or context is inconsistent therewith or unless otherwise herein provided, a reference to any statute is to that statute as now enacted or as the same may from time to time be amended, re-enacted or replaced and includes any regulations made thereunder.
1.05 Currency
All references to currency herein are to lawful money of the United States unless otherwise specified.
G-7
1.06 Schedules
The following are the Schedules to this Agreement:
|
|---|
Schedule Heading
| | Schedule Reference
|
|---|
|
| Leased Premises | | 2.01(b) |
|
| Tangible Assets | | 2.01(e) |
|
| Leases of Machinery and Equipment | | 2.01(f) |
|
| Franchise, License and Management Agreements | | 2.01(j) |
|
| Additional Contracts and Commitments | | 2.01(j)(ii) |
|
| Licenses, Registrations and Permits | | 2.01(k) |
|
| Owned Intellectual Property | | 2.01(l) |
|
| Licensed Intellectual Property | | 2.01(m) |
|
| Liabilities Prior to Closing | | 2.05 |
|
| Cash Amounts | | 2.06(2) |
|
| Rights to Acquire Stock in Vendor | | 3.01(h) |
|
| Audited Financial Statements of Vendor | | 3.01(k) |
|
| Unaudited Financial Statements of Vendor | | 3.01(l) |
|
| Encumbrances | | 3.01(r) |
|
| Defaults | | 3.01(w) |
|
| Real Property Leases | | 3.01(y) |
|
| Ownership Interests | | 3.01(z) |
|
| Royalties and License Fees | | 3.01(ff) |
|
| Consents to License and Sub-license | | 3.01(gg) |
|
| Third Party Programs | | 3.01(kk) |
|
| Distributors, Sales Agents and Representatives | | 3.01(mm) |
|
| Licences, Maintenance or Support Agreements, Development Contracts | | 3.01(nn) |
|
| Governmental Body Funding | | 3.01(oo) |
|
| Employment and Consulting Contracts | | 3.01(ss) |
|
| Employee Information | | 3.01(tt) |
|
| Benefit Plans | | 3.01(yy) |
|
| Compensation Policies | | 3.01(zz) |
|
| Insurance Policies | | 3.01(eeee) |
|
| Rights to Acquire Stock in Purchaser | | 3.03(f) |
|
| Designated Employees | | 4.03 |
|
| Form of General Conveyance Agreement | | 5.01(1)(l) |
|
| Distribution of Purchase Price by Vendor | | 5.01(1)(m) |
|
| Consents | | 5.01(1)(n) |
|
| Form of Consulting Agreement | | 5.01(1)(r) |
|
G-8
1.07 Knowledge
For purposes of the representations and warranties made by the Vendor in Section 3.01, the Vendor will be deemed to have "Knowledge" of a particular fact or other matter if any of Pratap Khanwilkar, Barbara Madsen and Gill Bearnson (i) has, or at any time had, actual awareness of that fact or matter, or (ii) would reasonably be expected to discover or otherwise become aware of that fact or matter by virtue of that individual's position, duties or responsibilities with the Vendor.
For purposes of the representations and warranties made by the Purchaser in Section 3.03, the Purchaser will be deemed to have "Knowledge" of a particular fact or other matter if any of Jal Jassawalla and Mark Goudie (i) has, or at any time had, actual awareness of that fact or matter, or (ii) would reasonably be expected to discover or otherwise become aware of that fact or matter by virtue of that individual's position, duties or responsibilities with the Purchaser.
ARTICLE 2 — SALE AND PURCHASE
2.01 Assets to be Sold and Purchased
Upon and subject to the terms and conditions hereof, the Vendor will sell to the Purchaser and the Purchaser will purchase from the Vendor, as of and with effect from the opening of business on the Closing Date, all of the right, title, benefit and interest of the Vendor in and to the following assets:
- (a)
- cash less any Cash Amounts described in Section 2.06;
- (b)
- the Leased Premises described in Schedule 2.01(b);
- (c)
- all structures, erections, improvements, appurtenances and fixtures situate on or forming part of the Leased Premises other than the fixed machinery and fixed equipment referred to in Section 2.01(d);
- (d)
- all fixed machinery and fixed equipment situate on or forming part of the Leased Premises;
- (e)
- all other machinery and equipment and all vehicles, tools, spare parts, handling equipment, furniture, furnishings, computer hardware and peripheral equipment, supplies and accessories of the Purchased Business, including the machinery and equipment described in Schedule 2.01(e);
- (f)
- all leases of machinery and equipment in which the Vendor is lessee relating to the Purchased Business described in Schedule 2.01(f);
- (g)
- all Inventories;
- (h)
- all new and unused production, shipping and packaging supplies of the Purchased Business;
- (i)
- all the accounts receivable of the Purchased Business;
- (j)
- all franchise, licence or management agreements described in Schedule 2.01(j) and all other contracts or commitments relating to the Purchased Business including,
- (i)
- all forward commitments to the Vendor for supplies or materials entered into in the usual and ordinary course of the Purchased Business for use in the Purchased Business whether or not there are any written contracts with respect thereto; and
- (ii)
- the further contracts and commitments described in Schedule 2.01(j)(ii);
- (k)
- all licences, registrations and permits required to carry on the Purchased Business in its usual and ordinary course including the licences, registrations and permits listed or described in Schedule 2.01(k);
- (l)
- all Intellectual Property owned by the Vendor and belonging to or used in the Purchased Business (the "Owned Intellectual Property"), including the Intellectual Property listed in Schedule 2.01(l);
- (m)
- all Intellectual Property not owned by the Vendor but belonging to or used in the Purchased Business (the "Licensed Intellectual Property"), including the right to use the Intellectual Property listed in Schedule 2.01(m);
G-9
- (n)
- the goodwill of the Purchased Business, including
- (i)
- the exclusive right to the Purchaser to represent itself as carrying on the Purchased Business in continuation of and in succession to the Vendor and the right to use any words indicating that the Purchased Business is so carried on, including the use if otherwise permitted by law of the corporate name "MedQuest", and the domain name "www.medquest-inc.com", and
- (ii)
- all records of sales, customer lists and supplier lists of or used in connection with the Purchased Business;
- (o)
- all securities held by the Vendor in any corporation or other entity;
- (p)
- all pre-paid expenses and deposits relating to the Purchased Business including all pre-paid Taxes and water rates, all pre-paid purchases of gas, oil and hydro, all pre-paid lease payments and all items referred to in Section 4.03(2);
- (q)
- all plans and specifications in the Vendor's possession or under its control relating to the structures, erections, improvements, appurtenances and fixtures situate on or forming part of the Leased Premises including all such electrical, mechanical and structural drawings related thereto as are in the possession or under the control of the Vendor; and
- (r)
- all personnel records, inspection records and other records, books, documents and data bases recorded or stored by means of any device, including in electronic form, relating to the Purchased Business, the Assets and those employees who are, pursuant to the provisions of this Agreement, to be employed by the Purchaser as are in the possession or under the control of the Vendor.
2.02 Purchase Price
(1) The purchase price to the Vendor for the Assets (the "Purchase Price") shall consist of (i) the issuance by the Purchaser to the Vendor of 9,300,000 Common Shares at a price per share of $1.35 per share, (ii) a cash amount of $10,000, and (iii) the assumption of the liabilities assumed under Sections 2.04 and 2.05 at the Closing Date.
(2) At the Time of Closing, the Purchaser will pay:
- (a)
- a partial payment of the Purchase Price, by issuing to the Vendor 8,370,000 Common Shares evidenced by a certificate in the name of the Vendor, such certificate bearing such legends as are required under applicable securities laws;
- (b)
- partial payment of the Purchase Price by issuing the Escrowed Shares in the name of the Vendor and providing the Escrowed Shares to the Escrow Agent on the Closing Date, evidenced by a certificate in the name of the Vendor, such certificate bearing such legends as are required under applicable securities laws; and
- (c)
- $10,000 by the wire transfer at the Time of Closing of immediately available funds to an account specified by the Vendor.
(3) On the Closing Date, the Vendor agrees to deposit in escrow the securities constituting the Escrowed Shares to be held by the Escrow Agent pursuant to the Escrow Agreement.
2.03 Property Taxes
All property Taxes imposed on or with respect to the purchased assets for the tax year that includes the Closing Date will be prorated between the Vendor and the Purchaser as of the Closing Date. The Vendor will be liable for the portion of such Taxes based on the number of days in the year occurring prior to the Closing Date, and the Purchaser will be liable for the portion of such Taxes based on the number of days in the year occurring on and after the Closing Date. For any year in which an apportionment is required, the Purchaser will file all required Tax Returns incident to these Taxes assessed for the year in which the Closing Date occurs that are not paid by the Vendor as of the Closing Date and the Vendor shall pay to the Purchaser the Taxes for which the Vendor is liable under the preceding sentence no later than 15 days prior to the due date of such Taxes.
G-10
2.04 Assumption of Obligations and Liabilities
The Purchaser will assume, fulfil and perform the obligations and liabilities of the Vendor accruing after the close of business on the day before the Closing Date under the contracts and other commitments of the Vendor relating to the Purchased Business specifically described in Schedules 2.01(j), 2.01(j)(ii), 2.01(m), 3.01(y) and 3.01(nn).
2.05 Assumption of Existing Obligations and Liabilities
The Purchaser will assume, fulfil and perform the obligations and liabilities of the Vendor accruing before the close of business on the day before the Closing Date under the contracts and other commitments of the Vendor relating to the Purchased Business specifically described in Schedule 2.05.
2.06 Obligations and Liabilities Not Assumed
(1) Except as provided in this Agreement, the Purchaser does not assume and will not be liable for any obligations or liabilities of the Vendor whatsoever including any Taxes under theIncome Tax Act (Canada) ("Tax Act") or the United StatesInternal Revenue Code ("Code") or any other Taxes whatsoever that may be or become payable by the Vendor including any income or corporation Taxes resulting from or arising as a consequence of the sale by the Vendor to the Purchaser of the Assets hereunder.
(2) The Purchaser will permit the Vendor to retain cash in an amount of $325,000 and such other amounts as the Purchaser may determine in its sole discretion at the Time of Closing ("Cash Amounts") for payment of certain obligations not assumed by the Purchaser and identified by the Vendor and the Purchaser on Schedule 2.06(2) on or prior to the day immediately preceding the Closing Date.
2.07 Non-Assignable Contracts and Commitments
(1) The Vendor will use reasonable efforts (other than the payment of money or assumption of obligations) to obtain any third party consents or waivers necessary to permit the assignment to, and assumption by, the Purchaser of all the contracts and other commitments to be assigned to and assumed by the Purchaser pursuant to this Agreement.
(2) Nothing in this Agreement will constitute an agreement to assign or an attempted assignment of any contract or other commitment for which any requisite consent or waiver to the assignment thereof has not been obtained. Subject to the provisions of Section 5.01(1), to the extent permitted by Applicable Law, if any requisite consent or waiver has not been obtained on or prior to Closing, the applicable contract or other commitment will be held by the Vendor in trust for the benefit of the Purchaser and the Purchaser will perform the obligations of the Vendor thereunder and be entitled to receive all money becoming due and payable under and other benefits derived from the contract or other commitment immediately after receipt by the Vendor.
ARTICLE 3 — REPRESENTATIONS AND WARRANTIES
3.01 Vendor's Representations and Warranties
The Vendor represents and warrants to the Purchaser that:
Corporate
- (a)
- The Vendor is a corporation duly incorporated, organized and subsisting under the laws of the State of Utah with the corporate power to own its assets and to carry on its business and has made all necessary filings under all applicable corporate, securities and taxation laws or any other laws to which the Vendor is subject.
- (b)
- The Vendor has the power, authority and right to enter into and deliver this Agreement and to transfer the legal and beneficial title and ownership of the Assets to the Purchaser free and clear of all liens, charges, encumbrances and any other rights of others.
- (c)
- This Agreement constitutes a valid and legally binding obligation of the Vendor, enforceable against the Vendor in accordance with its terms subject to applicable bankruptcy, insolvency, reorganization
G-11
G-12
- (iv)
- present fairly all of the assets and liabilities of the Vendor as at the Audited Balance Sheet Date including all contingent liabilities of the Vendor as at the Audited Balance Sheet Date.
- (l)
- The unaudited financial statements of the Vendor for the period ended on the Balance Sheet Date, consisting of the balance sheet, statements of income, retained earnings and changes in financial position for the period ended on the Balance Sheet Date (collectively, the "Unaudited Financial Statements"), a copy of which is attached hereto as Schedule 3.01(l):
- (i)
- are in accordance with the books and accounts of the Vendor as at the Balance Sheet Date;
- (ii)
- are true and correct and present fairly the financial position of the Vendor as at the Balance Sheet Date;
- (iii)
- have been prepared in accordance with US GAAP consistently applied;
- (iv)
- present fairly all the assets and liabilities of the Vendor as at the Balance Sheet Date including all contingent liabilities of the Vendor as at the Balance Sheet Date.
- (m)
- Since the Balance Sheet Date the Purchased Business has been carried on in its usual and ordinary course and the Vendor has not entered into any transaction or incurred any liabilities out of the usual and ordinary course of the Purchased Business.
- (n)
- Since the Balance Sheet Date there has been no change in the affairs, business, prospects, operations or condition of the Purchased Business, financial or otherwise, whether arising as a result of any legislative or regulatory change, revocation of any licence or right to do business, fire, explosion, accident, casualty, labour dispute, flood, drought, riot, storm, condemnation, act of God, public force or otherwise, except changes occurring in the usual and ordinary course of business that have not adversely affected the affairs, business, prospects, operations or condition of the Purchased Business, financial or otherwise.
- (o)
- No current or former director, officer, shareholder or employee of the Vendor or any person not dealing at arm's length within the meaning of the Code with any such person or with the Vendor is indebted to the Vendor.
- (p)
- The Vendor maintains a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management's general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with US GAAP and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management's general or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. Since the Audited Balance Sheet Date, there have been no significant changes in the Vendor's internal controls over financial reporting or, to the Vendor's Knowledge, in other factors that would, individually or in the aggregate, reasonably be expected to result in a material adverse effect on the affairs, business, prospects, operations or condition of the Purchased Business, financial or otherwise.
- (q)
- Neither the Vendor, nor, to the Vendor's Knowledge, any director, officer, employee, auditor, accountant or representative of the Vendor, has received or otherwise had or obtained knowledge of any written or material unwritten complaint, allegation, assertion or claim regarding the accounting or auditing practices, procedures, methodologies or methods of the Vendor or its internal accounting controls, including any complaint, allegation, assertion or claim of any type that the Vendor has engaged in improper or illegal accounting or auditing practices and no attorney representing the Vendor, whether or not employed by the Vendor, has reported evidence of a material violation of securities laws, breach of fiduciary duty or similar violation by the Vendor or any of its officers, directors, employees or agents to the Vendor or the board of directors or any committee thereof or to any director or officer of the Vendor. There have been no internal investigations regarding accounting or revenue recognition discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, or the Vendor's board of directors or any committee thereof.
G-13
Intellectual Property
- (bb)
- The Vendor has the exclusive right to use the Owned Intellectual Property except to the extent the Vendor has licensed, or otherwise has granted any right (whether or not currently exercisable) or interest in, the Owned Intellectual Property to others, which licences or other grants are listed in Schedule 2.01(l).
- (cc)
- The Vendor has the exclusive right to use the Licensed Intellectual Property except to the extent the rights are identified in Schedule 2.01(m) as being non-exclusive.
- (dd)
- The Owned Intellectual Property is in good standing and has been duly registered or patented or applications to register or patent the same have been filed in all appropriate offices to preserve the rights therein and of the Vendor thereto. Schedule 2.01(l) accurately identifies and describes each item of Owned Intellectual Property in which the Vendor has an ownership interest of any nature (whether
G-14
Employees
- (rr)
- The Vendor is not a party to or bound by any contract or commitment to pay any management fee pertaining to the Purchased Business.
G-15
- (ss)
- Except as set forth in Schedule 3.01(ss), the Vendor does not have any written or oral employment contract or consulting contract relating to the Purchased Business with any person whomsoever.
- (tt)
- Schedule 3.01(tt) sets out:
- (i)
- the names of all employees or consultants of the Purchased Business;
- (ii)
- their annual salary or remuneration;
- (iii)
- their job title;
- (iv)
- their total length of employment including any prior employment as disclosed in the Vendor's records that would affect calculation of years of service for purposes of benefit entitlement (including statutory notice or statutory severance pay) or pension entitlement;
- (v)
- the length of any consulting contract;
- (vi)
- whether any such employees are on any approved or statutory leave of absence and, if so, the reason for the absence; and
- (vii)
- other terms and conditions of their employment (other than Benefit Plans and Compensation Policies).
- (uu)
- The Vendor is not bound by or a party to any collective bargaining agreement relating to the Purchased Business.
- (vv)
- There are no actual, threatened or pending organizing activities of any trade union, council of trade unions, employee bargaining agency or affiliated bargaining agent or any actual, threatened or pending unfair labour practice complaints, strikes, work stoppages, picketing, lock-outs, hand-billings, boycotts, slowdowns, arbitrations, grievances, complaints, charges or similar labour related disputes or proceedings pertaining to the Purchased Business, and there have not been any such activities or disputes or proceedings within the last year.
- (ww)
- All vacation pay for employees of the Purchased Business is properly reflected and accrued in the books and accounts of the Vendor.
- (xx)
- Since the Balance Sheet Date, there have been no changes in the terms and conditions of employment of any employees of the Purchased Business, including their salaries, remuneration and any other payments to them, and there have been no changes in any remuneration payable or benefits provided to any officer, director, consultant, independent or dependent contractor or agent of the Purchased Business, and the Vendor has not agreed or otherwise become committed to change any of the foregoing since that date.
Benefits Plans
- (yy)
- Schedule 3.01(yy) contains a list of every benefit and employment plan, program, agreement or arrangement (whether written or unwritten and whether or not subject to ERISA) maintained, contributed to, or provided by the Vendor thereof for the benefit of any of its employees or dependents or independent contractors of the Vendor or their respective dependants or beneficiaries (the "Benefit Plans") including all bonus, deferred compensation, incentive compensation, share purchase, share option, stock appreciation, phantom stock, savings, profit sharing, severance or termination pay, health or other medical, life, disability or other insurance (whether insured or self-insured), supplementary unemployment benefit, pension, retirement and supplementary retirement plans, programs, agreements and arrangements.
- (zz)
- Schedule 3.01(zz) contains a list of all written or unwritten compensation policies and practices of the Vendor ("Compensation Policies") applicable to employees and dependents and independent contractors of the Vendor.
G-16
- (aaa)
- The Vendor has delivered to the Purchaser true, complete and up-to-date copies of all Benefit Plans and Compensation Policies and all amendments thereto together with all summary descriptions of the Benefit Plans and Compensation Policies provided to past or present participants therein.
- (bbb)
- No fact, condition or circumstance exists that would materially affect the information contained in the documents provided pursuant to Section 3.01(yy) and, in particular, no promises or commitments have been made by the Vendor to amend any Benefit Plan or Compensation Policy.
- (ccc)
- Neither the Vendor nor any of its Affiliates (nor any employer (whether or not incorporated) that would be treated together with the Vendor or any such Affiliate as a single employer within the meaning of Section 414 of the Code has ever maintained or contributed to, or had any obligation to contribute to (or borne any liability with respect to) any "employee pension benefit plan," within the meaning of Section 3(2) of ERISA, that is a "multiemployer plan," within the meaning of Section 3(37) of ERISA, or subject to Section 412 of the Code, or Section 302 or Title IV of ERISA.
- (ddd)
- Each Benefit Plan intended to be qualified under Section 401(a) of the Code, has, as currently in effect, been determined to be so qualified by the United States Internal Revenue Service, and since the date of each such determination, no event has occurred and no condition or circumstance has existed that resulted or is likely to result in the revocation of any such determination.
- (eee)
- The Vendor has not incurred, and no event has occurred and no condition or circumstance exists that could result, directly or indirectly, in, any unsatisfied liability (including, without limitation, any indirect, contingent or secondary liability) of the Vendor under Title IV of ERISA or Section 412 of the Code or Section 302 of ERISA arising in connection with any employee pension benefit plan covered or previously covered by Title IV of ERISA or such sections of the Code or ERISA.
- (fff)
- The Vendor has complied in all respects with the applicable requirements of Part 6 of Subtitle B of Title I of ERISA and Section 4980B of the Code ("COBRA"), and will comply with all COBRA obligations arising in connection with the transactions contemplated hereby, and the Vendor is not subject to any liability as a result of any failure to administer or operate any "group health plan" (as defined in COBRA) in compliance with COBRA.
- (ggg)
- The execution of this Agreement and the consummation of the transactions contemplated hereby, do not constitute a triggering event under any Benefit Plan or Compensation Policy or other policy, arrangement, statement, commitment or agreement, whether or not legally enforceable, which (either alone or upon the occurrence of any additional or subsequent event) will or may result in any payment (whether of severance pay or otherwise), vesting or increase in benefits to any present or former employee or director of the Vendor.
Leased Premises
- (hhh)
- The operations of the Purchased Business from the Leased Premises are not subject to any restriction or limitation that would materially adversely affect the Purchased Business and are not in contravention of any law or regulation or of any decree or order of any court or other body having jurisdiction.
- (iii)
- The Vendor has not received notice of any assessment or any capital charges or levies assessed or proposed to be assessed against any of the Assets by a Governmental Authority or that any Governmental Authority intends to require the Vendor to pay for any future roads, utilities or services relating to the Leased Premises.
- (jjj)
- All improvements (including all plant, buildings, structures, erections, appurtenances and fixtures) situate on or forming part of the Leased Premises were completed in a good and competent manner and in all material respects in accordance with the requirements of all applicable Governmental Authorities and all such improvements are free of material defect.
G-17
- (kkk)
- The Leased Premises are serviced by all private and public utility services that are necessary for the operations of the Purchased Business on the Leased Premises.
Environmental
- (lll)
- The Purchased Business, as carried on by the Vendor, and the Assets are in compliance in all material respects with all Environmental Laws and there are no facts relating to the past or present operation of the Purchased Business that could give rise to a notice of non-compliance with any Environmental Law, or any liability under Environmental Laws.
- (mmm)
- All environmental Permits used in or required to carry on the Purchased Business in its usual and ordinary course are in full force and effect and are transferable to the Purchaser on Closing.
- (nnn)
- The Vendor has not used any of the facilities or Leased Premises pertaining to the Purchased Business, or permitted them to be used, to generate, manufacture, refine, treat, transport, store, handle, dispose, transfer, produce or process Hazardous Substances except in compliance in all material respects with all Environmental Laws. None of the Leased Premises has been used for or been designated as a waste disposal site.
- (ooo)
- To the Vendor's Knowledge, there are no pending changes to Environmental Laws that would render illegal, or materially restrict, the operation of the Purchased Business in its usual and ordinary course.
- (ppp)
- The Vendor has not been convicted of an offence or been subjected to any judgment, injunction or other proceeding or been fined or otherwise sentenced for non-compliance with any Environmental Laws, and it has not settled any prosecution or other proceeding short of conviction in connection therewith, in relation to the Purchased Business.
- (qqq)
- The Vendor has not caused or permitted the Release of any Hazardous Substance at, on or under the Leased Premises, or the Release of any Hazardous Substance off-site of the Leased Premises in relation to the Purchased Business, except in compliance in all material respects with Environmental Laws.
- (rrr)
- The Vendor has not breached any obligation to report a Release to any person imposed by any Environmental Law.
Taxes
- (sss)
- The Purchaser is acquiring the ownership, possession or use under this Agreement of all or substantially all of the property that can reasonably be regarded as being necessary for the Purchaser to be capable of carrying on the Purchased Business as a business within the meaning of section 167 of theExcise Tax Act (Canada).
- (ttt)
- There are no liens for Taxes upon the Assets, except for statutory liens for current Taxes not yet due.
- (uuu)
- The Vendor has timely filed or caused to be timely filed with the appropriate taxing authorities all tax returns, statements, forms and reports (including elections, declarations, disclosures, schedules, estimates and information Tax Returns) for Taxes ("Tax Returns") that are required to be filed with respect to the Purchased Business and such Tax Returns have been true, correct and complete in all material respects.
- (vvv)
- All Taxes due with respect to the Purchased Business for all taxable years or other taxable periods that end on or before the Closing Date and, with respect to any taxable year or other taxable period beginning on or before and ending after the Closing Date, the portion of such taxable year or period ending on and including the Closing Date ("Pre-Closing Period") have been paid or accrued and fully provided for in accordance with US GAAP on the Balance Sheet.
- (www)
- The Vendor has not been the subject of an audit or other examination of Taxes by the tax authorities of any nation, state or locality with respect to the Purchased Business; (ii) no such audit
G-18
General
- (zzz)
- There are no actions, suits or proceedings (whether or not purportedly on behalf of the Vendor):
- (i)
- pending or threatened against or adversely affecting, or which could adversely affect, the Purchased Business or the Assets, or
- (ii)
- before or by any Governmental Authority.
- (aaaa)
- The Vendor is not conducting the Purchased Business in any jurisdiction other than the State of Utah.
- (bbbb)
- The Vendor has no clinical sites and has the use of only one animal testing facility located on the premises of the University of Utah.
- (cccc)
- The Vendor is conducting the Purchased Business in material compliance with all Applicable Laws, is not in breach of any such Applicable Laws and is duly licensed, registered or qualified in the State of Utah and all municipalities thereof in which the Vendor carries on the Purchased Business to enable the Purchased Business to be carried on as now conducted and its assets to be owned, leased and operated, and all such licences, registrations and qualifications are valid and subsisting and in good standing and none of the same contains any term, provision, condition or limitation which has or may have a material adverse effect on the operation of the Purchased Business or which may be affected by the completion of the transactions contemplated hereby.
- (dddd)
- Schedule 2.01(k) is a true and complete list of all licences, registrations and permits necessary or required to enable the Purchased Business to be carried on as now conducted and its assets to be owned, leased and operated and all such licences, registrations and permits are transferable by the Vendor to the Purchaser on the Closing Date.
- (eeee)
- Attached as Schedule 3.01(eeee) is a true and complete list of all insurance policies maintained by the Vendor that also specifies the insurer, the amount of the coverage, the type of insurance, the policy number and any pending claims thereunder.
- (ffff)
- The Vendor has not received (i) any notice of cancellation of any policy described in Schedule 3.01(eeee) or refusal of coverage thereunder, (ii) any notice that any issuer of such policy has filed for protection under applicable bankruptcy laws or is otherwise in the process of liquidating or has been liquidated, or (iii) any other indication that such policies are no longer in full force or effect or that the issuer of any such policy is no longer willing or able to perform its obligations hereunder.
- (gggg)
- Except for matters reflected and reserved against in the Balance Sheet, the Vendor has not incurred any liabilities or obligations (whether absolute, accrued, contingent, fixed or otherwise, or whether due or to become due) of any nature that would be required by US GAAP to be reflected on a balance sheet of the Vendor (including the notes thereto).
- (hhhh)
- Neither the Vendor nor to the best of the Vendor's Knowledge, any Affiliate of the Vendor beneficially owns any Common Shares.
G-19
3.02 Survival of Vendor's Representations, Warranties and Covenants
(1) The representations and warranties of the Vendor set forth in Section 3.01 will survive the completion of the sale and purchase of the Assets herein provided for and, notwithstanding such completion, will continue in full force and effect for the benefit of the Purchaser for a period of one year from the Closing Date.
(2) The covenants of the Vendor set forth in this Agreement (other than the covenant set forth in Section 4.01(2) with respect to representations and warranties being true at the Time of Closing) will survive the completion of the sale and purchase of the Assets herein provided for and, notwithstanding such completion, will continue in full force and effect for the benefit of the Purchaser in accordance with the terms of this Agreement.
3.03 Purchaser's Representations and Warranties
The Purchaser represents and warrants to the Vendor that:
Corporate
- (a)
- The Purchaser is a corporation duly incorporated, organized and subsisting under the laws of the Province of Ontario with the corporate power to own its assets and to carry on its business and has made all necessary material filings under all applicable corporate, securities and taxation laws or any other laws to which the Purchaser is subject.
- (b)
- The Purchaser has the power, authority and right to enter into and deliver this Agreement and to transfer the legal and beneficial title and ownership of the Shares to the Vendor free and clear of all liens, charges, encumbrances and any other rights of others.
- (c)
- This Agreement constitutes a valid and legally binding obligation of the Purchaser, enforceable against the Purchaser in accordance with its terms subject to applicable bankruptcy, insolvency, reorganization and other laws of general application limiting the enforcement of creditors' rights generally and to the fact that specific performance is an equitable remedy available only in the discretion of the court.
- (d)
- Neither the entering into nor the delivery of this Agreement nor the completion of the transactions contemplated hereby by the Purchaser will result in the violation of:
- (i)
- any of the provisions of the incorporation documents or by-laws of the Purchaser;
- (ii)
- any material agreement or other instrument to which the Purchaser is a party or by which the Purchaser is bound; or
- (iii)
- any Applicable Law.
- (e)
- The authorized capital of the Purchaser consists of unlimited common shares and unlimited preferred shares issuable in series of which 16,430,470 Common Shares and no preferred shares are issued and outstanding as of the date hereof. Upon completion of the Closing, the Common Shares issued to the Vendor, including the Escrowed Shares, will have been duly authorized and be validly issued and outstanding, as fully paid and non-assessable shares in the capital of the Purchaser.
- (f)
- Except as provided in this Agreement or as set forth in Schedule 3.03(f), (i) no subscription, warrant, option, convertible security or other right (contingent or otherwise) to purchase or acquire any shares of the capital stock of the Purchaser or the Purchaser Subsidiaries are authorized or outstanding, (ii) neither the Purchaser nor the Purchaser Subsidiaries have any obligation (contingent or otherwise) to issue any subscription, warrant, option (except option grants to Purchaser's employees and consultants under the Purchaser's employee stock option plan in the ordinary course), convertible security or other such right to issue or distribute to holders of any shares of its capital stock any evidence of indebtedness or assets of the Purchaser or any subsidiary of the Purchaser.
- (g)
- On or prior to the Effective Date, the execution, delivery and performance of this Agreement by the Purchaser and the consummation by the Purchaser of the transactions contemplated hereby have been duly and validly approved by the board of directors of the Purchaser.
G-20
Financial
- (h)
- The Purchaser has made available to the Vendor prior to the execution of this Agreement a true and complete copy of each form, report, schedule, registration statement, definitive proxy statement and other document (together with all amendments thereof and supplements thereto) filed by the Purchaser or any of its Subsidiaries with Canadian securities regulatory authorities and the SEC, the TSX and NASDAQ since January 1, 2003 (as such documents have since the time of their filing been amended or supplemented, the "WorldHeart Reports"), which are all the documents (other than preliminary material) that the Purchaser and its Subsidiaries were required to file with the SEC, Canadian securities regulatory authorities, the TSX and NASDAQ since such date. As of their respective dates, the WorldHeart Reports (i) complied as to form in all material respects with the requirements of the United States Securities Act of 1933, the United States Securities Exchange Act of 1934 or Canadian securities laws, the TSX and NASDAQ, and (ii) did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading.
- (i)
- The audited consolidated financial statements and unaudited interim consolidated financial statements (including, in each case, the notes, if any, thereto) included in the WorldHeart Reports (the "WorldHeart Financial Statements") complied as to form in all material respects with the published rules and regulations of the Canadian securities regulatory authorities with respect thereto, were prepared in accordance with Canadian GAAP applied on a consistent basis during the periods involved (except as may be indicated therein or in the notes thereto and except with respect to unaudited statements as permitted by Canadian securities laws) and fairly present (subject, in the case of the unaudited interim financial statements, to the absence of certain footnotes and to normal, recurring year-end audit adjustments and to the absence of complete notes (which are not expected to be, individually or in the aggregate, materially adverse to the Purchaser and its Subsidiaries taken as a whole)) the consolidated financial position of the Purchaser and its consolidated Subsidiaries as at the respective dates thereof and the consolidated results of their operations and cash flows for the respective periods then ended. Each Subsidiary of the Purchaser is treated as a consolidated subsidiary of the Purchaser in the WorldHeart Financial Statements for all periods covered thereby.
- (j)
- Since September 30, 2004, there has been no change in the affairs, business, prospects, operations or condition of the Purchaser's business, financial or otherwise, whether arising as a result of any legislative or regulatory change, revocation of any licence or right to do business, fire, explosion, accident, casualty, labour dispute, flood, drought, riot, storm, condemnation, act of God, public force or otherwise, except changes occurring in the usual and ordinary course of business that have not adversely affected the affairs, business, prospects, operations or condition of the Purchaser's business, financial or otherwise.
G-21
- (n)
- To the Purchaser's Knowledge, the Intellectual Property owned by the Purchaser is not invalid or unenforceable. To the Purchaser's Knowledge, no infringement, misuse or misappropriation of the Intellectual Property owned by the Purchaser has occurred.
General
- (o)
- There are no material actions, suits or proceedings (whether or not purportedly on behalf of the Purchaser):
- (i)
- pending or threatened against or adversely affecting, or which could adversely affect, the business of the Purchaser, or
- (ii)
- before or by any Governmental Authority.
- (p)
- The Purchaser is conducting its business in material compliance with all Applicable Laws, is not in breach of any such Applicable Laws and is duly licensed, registered or qualified in the Province of Ontario and State of California and all municipalities thereof in which the Purchaser carries on the business to enable its business to be carried on an its assets to be owned, leased and operated, and all such licences, registrations and qualifications are valid and subsisting and in good standing and none of the same contains any term, provision, condition or limitation which has or may have a material adverse effect on the operation of the business or which may be affected by the completion of the transactions contemplated hereby.
3.04 Survival of Purchaser's Representations, Warranties and Covenants
(1) The representations and warranties of the Purchaser set forth in Section 3.03 will survive the completion of the sale and purchase of the Assets herein provided for and, notwithstanding such completion, will continue in full force and effect for the benefit of the Vendor for a period of one year from the Closing Date.
(2) The covenants of the Purchaser set forth in this Agreement (other than the covenant set forth in Section 4.02(2) with respect to representations and warranties being true at the Time of Closing) will survive the completion of the sale and purchase of the Assets herein provided for and, notwithstanding such completion, will continue in full force and effect for the benefit of the Vendor in accordance with the terms of this Agreement.
ARTICLE 4 — COVENANTS
4.01 Covenants of the Vendor
(1) In addition to any other provision for indemnification by the Vendor contained in this Agreement, the Vendor will indemnify and save harmless the Purchaser and the directors, officers, employees and agents of the Purchaser from and against:
- (a)
- (i) all Claims directly or indirectly relating to the Purchased Business and accruing up to the close of business on the day before the Closing Date and
- (ii)
- all other Claims directly or indirectly relating to the Vendor including Claims relating to income, sales, excise or other Taxes; and
- (iii)
- all Claims relating to the Taxes imposed with respect to the Purchased Business arising prior to the Closing Date;
except, in either case, any Claim for which indemnification is provided under Section 4.01(1)(b) or (c) and those Claims with respect to which the Purchaser is liable under this Agreement including those accruing under the contracts and other commitments referred to in Section 2.05 and those for Taxes, duties and other charges referred to in Section 4.02(3);
- (b)
- all Claims directly or indirectly arising in connection with the use of the Inventories by any person or the purchase of any of the Inventories by any third party except for any Claims directly or indirectly resulting from the negligence of the Purchaser; and
G-22
- (c)
- all Claims incurred by the Purchaser directly or indirectly resulting from any breach of any covenant of the Vendor contained in this Agreement or, from any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.01.
The Vendor appoints the Purchaser as the trustee for the Purchaser's directors, officers, employees and agents of the covenant of the Vendor with respect to such persons and the Purchaser accepts such appointment.
(2) The Vendor will ensure that the representations and warranties of the Vendor set out in Section 3.01 over which the Vendor has reasonable control are true and correct at the Time of Closing and that the conditions of closing for the benefit of the Purchaser set out in Section 5.01(1) over which the Vendor has reasonable control have been performed or complied with by the Time of Closing.
(3) The Vendor immediately after the Closing Date will file
- (a)
- articles of amendment changing its corporate name to one that does not include any trade mark or trade name included in the Assets, and
- (b)
- cancellations with all applicable Governmental Authorities of the registrations of all business names included in the Assets.
(4) Notwithstanding any of the other provisions of this Agreement, the Vendor will not be liable to the Purchaser or to the directors, officers, employees or agents of the Purchaser in respect of:
- (a)
- any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.01 after one year after the Closing Date;
- (b)
- any matter from and against which the Purchaser and the directors, officers, employees and agents of the Purchaser are indemnified pursuant to Sections 4.01(1)(a) and (b) after one year after the Closing Date; or
- (c)
- any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.01 or resulting from any matter from and against which the Purchaser and the directors, officers, employees and agents of the Purchaser are indemnified hereunder
- (i)
- unless and until the aggregate of all such Claims exceeds $50,000, and then only to the extent that such aggregate exceeds such amount, or
- (ii)
- in excess of the fair market value of the Escrowed Shares determined at the time of the final determination or settlement of the Claim,
other than, in either case, any Claim attributable to the lack of ownership of, or title to, the Assets and any Claim referred to in Section 4.01(1)(c) relating to any breach of any covenant of the Vendor contained in this Agreement.
(5) Except to the event that the Purchaser otherwise previously consents in writing, until the Closing Date, the Vendor will use commercially reasonable efforts to preserve substantially intact in all material respects its present business organization, to maintain its existence in good standing, to keep available the services of its key officers and employees, to maintain insurance on its tangible assets and businesses in such amount and against such risks and losses as are currently in effect, to preserve its relationships with suppliers and others having significant business dealings with it and to comply in all material respects with all Applicable Laws.
(6) The Vendor will not issue any additional shares of common stock or preferred stock prior to the Closing Date except upon the exercise of previously issued options to employees and consultants of the Vendor. The Vendor will inform the Purchaser in writing of any exercise of options and issuance of common shares.
(7) The Vendor agrees to enter into a loan arrangement or similar arrangement with Maverick between the Effective Date and the Closing Date for purposes of funding the ongoing operations of the Vendor ("Maverick Loan").
G-23
4.02 Covenants of the Purchaser
(1) In addition to any other provision for indemnification by the Purchaser contained in this Agreement, the Purchaser will indemnify and save harmless the Vendor and the directors, officers, employees and agents of the Vendor from and against all Claims incurred by the Vendor directly or indirectly resulting from any breach of any covenant of the Purchaser contained in this Agreement or from any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.03.
The Purchaser appoints the Vendor as the trustee for the Vendor's directors, officers, employees and agents of the covenant of the Purchaser with respect to such persons and the Vendor accepts such appointment.
(2) The Purchaser will ensure that the representations and warranties of the Purchaser set out in Section 3.03 over which the Purchaser has reasonable control are true and correct at the Time of Closing and that the conditions of closing for the benefit of the Vendor set out in Section 5.02(1) over which the Purchaser has reasonable control have been performed or complied with by the Time of Closing.
(3) The Purchaser will be liable for and will pay, or will cause to be paid, all transfer, land transfer, value added,ad-valorem, excise, sales, use, consumption, goods or services, harmonized sales, retail sales, social services, or other similar Taxes or duties (collectively, "Transfer Taxes") payable under any Applicable Law on or with respect to the sale and purchase of the Assets under this Agreement. The Purchaser will prepare and file any affidavits or returns required in connection with the foregoing at its own cost and expense. To the extent that any Transfer Taxes are required to be paid by or are imposed upon the Vendor, the Purchaser will reimburse, or will cause to be reimbursed, to the Vendor such Taxes within five Business Days of payment of such Taxes by the Vendor. All amounts payable by the Purchaser to the Vendor hereunder do not include Transfer Taxes.
(4) Notwithstanding any of the other provisions of this Agreement, the Purchaser will not be liable to the Vendor or to the directors, officers, employees or agents of the Vendor in respect of
- (a)
- any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.03 after one year after the Closing Date, or
- (b)
- any inaccuracy or misrepresentation in any representation or warranty set forth in Section 3.03 or resulting from any matter from and against which the Vendor and the directors, officers, employees and agents of the Vendor are indemnified hereunder
- (i)
- unless and until the aggregate of all such Claims exceeds $50,000, and then only to the extent that such aggregate exceeds such amount, or
- (ii)
- in excess of $2,000,000 in the aggregate,
4.03 Employees
(1) On or before the Closing Date, the Purchaser shall, in its sole discretion, offer "at will" employment to those employees of the Vendor appearing on Schedule 4.03 (the "Designated Employees") on terms and conditions which are generally consistent with their respective employment terms and length of service with the Vendor (other than any equity-based compensation) as set out on Schedule 3.01(tt) upon which such Designated Employees are employed by the Vendor immediately prior to the Closing Date unless otherwise agreed to in writing by such Designated Employee and the Purchaser. Such offer of employment shall be effective as of the Closing Date; provided, however, that, with respect to any Designated Employee who is absent from work on the Closing Date due to illness, injury, leave of absence, short-term disability or long-term disability, such offer of employment shall be effective when such employee is fit to return to work and reports for work with the Purchaser. The Designated Employees will receive full service credit for all years of employment with the Vendor for purposes of eligibility to participate in Purchaser's benefit plans. The Designated Employees will be entitled to carry over up to an equal number of days or weeks of vacation, if so accrued by such Designated Employee, as such Designated Employee receives in his or her offer of employment from the Purchaser ("Carry Forward"). The Carry Forward provision will only apply to those Designated Employees who provide notice in writing to the Vendor at least five business days prior to the Closing Date; otherwise such accrued vacation amounts shall be paid out in full to the Designated Employee.
G-24
(2) Subject to the provisions of Section 4.03(1), the Vendor will continue to be responsible for and will discharge all obligations and liabilities for wages, severance pay, termination pay, notice of termination of employment or pay in lieu of such notice, damages for wrongful dismissal or other employee benefits or claims (including vacation pay accrued up to the close of business on the date immediately preceding the Closing Date) in respect of all employees of the Vendor in the Purchased Business on the close of business on the Closing Date or otherwise relating to or as a result of employment by or termination of employment from the Vendor, or as a result of, or in connection with, the consummation of the transactions contemplated hereby. The Purchaser assumes and will discharge all such obligations and liabilities accruing with respect to service as an employee of the Purchaser after the close of business on the day immediately preceding the Closing Date in respect of all former employees of the Vendor in the Purchased Business employed by the Purchaser; provided, however, that the parties acknowledge and agree that the transactions provided for in this Agreement may result in obligations on the part of the Vender and one or more of the Benefit Plans to comply with the health care continuation requirements of COBRA (as defined in Section 3.01(fff)) or state law, as applicable, and that the Purchaser and the Purchaser's benefit plans shall have no responsibility for compliance with such health care continuation requirements (i) for qualified beneficiaries who previously elected to receive continuation coverage under the Benefit Plans or who between the date of this Agreement and the Closing Date elect to receive continuation coverage, or (ii) with respect to those employees or former employees of the Vendor who may become eligible to receive such continuation coverage on or prior to the Closing or in connection with the transactions contemplated by this Agreement.
(3) Notwithstanding any other provision of this Agreement, the Vendor will be responsible for satisfaction of, and will indemnify and save harmless the Purchaser from and against, all Claims with respect to any employee employed or formerly employed in the Purchased Business, whether or not he or she is to be or is employed by the Purchaser pursuant to Section 4.03(1), but who is absent from work on the Closing Date due to illness, injury, leave of absence, short term disability or long term disability, until such employee is fit to return to work and reports for work with the Purchaser.
(4) (i) The Purchaser shall not be obligated to assume, continue or maintain any of the Benefit Plans or Compensation Policies; (ii) no assets or liabilities of the Benefit Plans or Compensation Policies shall be transferred to, or assumed by, the Purchaser or the Purchaser's benefit plans; and (iii) the Vendor shall be solely responsible for funding and/or paying any benefits under any of the Benefit Plans and the Compensation Policies.
(5) Nothing in this Agreement, express or implied, shall: (i) confer upon any employee of the Vendor, or any representative of any such employee, any rights or remedies, including any right to employment or continued employment for any period or terms of employment, of any nature whatsoever, or (ii) be interpreted to prevent or restrict the Purchaser or its Subsidiaries from modifying or terminating the employment or terms of employment of any former employee of the Vendor employed by the Purchaser, including the amendment or termination of any employee benefit or compensation plan, program or arrangement, after the Closing Date.
(6) The Vendor shall make available to the Purchaser such personnel and similar information as the Purchaser may request with respect to any former employee of the Vendor employed by the Purchaser, including compensation and employment records.
4.04 Cooperation on Tax Matters
The Vendor and the Purchaser will furnish or cause to be furnished to each other, each at its own expense, as promptly as practicable, such information and assistance, and provide additional information and explanations of any material provided, relating to the Assets as is reasonably necessary for the filing of any Tax Returns, for the preparation of any audit, and for the prosecution or defence of any claim, suit or proceeding relating to any adjustment or proposed adjustment with respect to Taxes.
4.05 Tax Controversies
(1) The Purchaser shall promptly notify Vendor upon receipt by the Purchaser or any affiliate of the Purchaser of written notice of any inquiries, claims, assessments, audits or similar events with respect to Taxes relating to the Purchased Business relating to a taxable period ending on or prior to the Closing Date for which the Vendor may be liable under this Agreement (any such inquiry, claim, assessment, audit or similar event, a
G-25
"Tax Matter"). The Vendor, or the Vendor's representative, at its sole expense, shall have the authority to control the defense, compromise or other resolution of any Tax Matter, including responding to inquiries, contesting, defending against and resolving any assessment for additional Taxes or notice of Tax deficiency or other adjustment of Taxes of, or relating to, a Tax Matter;provided,however, that neither the Vendor nor any of its affiliates shall enter into any settlement of or otherwise compromise any Tax Matter that adversely affects or may adversely affect the Tax liability with respect to the Purchased Business for any period (or portion thereof) ending after the Closing Date without the prior written consent of the Purchaser, which consent shall not be unreasonably withheld or delayed. The Vendor or the Vendor's Representative shall keep the Purchaser fully and timely informed with respect to the commencement, status and nature of any Tax Matter. The Vendor shall, in good faith, allow Purchaser, to make comments to the Vendor or the Vendor's Representative, regarding the conduct of or positions taken in any such proceeding.
(2) Except as otherwise provided in Section 4.05(1) above, the Purchaser shall have the sole right to control any audit or examination by any taxing authority, initiate any claim for refund or amend any Tax Return, and contest, resolve and defend against any assessment for additional Taxes, notice of Tax deficiency or other adjustment of Taxes of, or relating to the Purchased Business for all taxable periods;provided,however, that the Purchaser shall not, and shall cause its affiliates not to, enter into any settlement of any contest or otherwise compromise any issue with respect to the portion of a taxable period that straddles the Closing Date ending on the Closing Date without the prior written consent of the Vendor, which consent shall not be unreasonably withheld or delayed.
4.06 Amended Tax Returns
Neither the Vendor nor any of its affiliates will file or cause to be filed any amended Tax Returns or claims for refund with respect to the Purchased Business without the prior written consent the Purchaser, which consent shall not be unreasonably withheld or delayed.
ARTICLE 5 — CONDITIONS
5.01 Conditions for the Benefit of the Purchaser
(1) The sale by the Vendor and the purchase by the Purchaser of the Assets is subject to the following conditions, which are for the exclusive benefit of the Purchaser and which are to be performed or complied with at or prior to the Time of Closing:
- (a)
- the representations and warranties of the Vendor set forth in Section 3.01 will be true and correct at the Time of Closing with the same force and effect as if made at and as of such time;
- (b)
- the Vendor will have performed or complied with all of the terms, covenants and conditions of this Agreement to be performed or complied with by the Vendor at or prior to the Time of Closing;
- (c)
- the Purchaser will be furnished with such certificates or other instruments (including instruments of conveyance with respect to the Assets) of the Vendor or of officers of the Vendor as the Purchaser or the Purchaser's counsel may reasonably think necessary in order to establish that the terms, covenants and conditions contained in this Agreement to have been performed or complied with by the Vendor at or prior to the Time of Closing have been performed or complied with and that the representations and warranties of the Vendor herein given are true and correct at the Time of Closing;
- (d)
- there will have been obtained from all appropriate federal, provincial, municipal or other governmental or administrative bodies such other approvals or consents as are required to permit the change of ownership of the Assets contemplated hereby and to permit the Purchased Business to be carried on by the Purchaser as now conducted;
- (e)
- no action or proceeding in Canada or the United States will be pending or threatened by any person, government, governmental authority, regulatory body or agency to enjoin, restrict or prohibit
- (i)
- the sale and purchase of the Assets contemplated hereby or
- (ii)
- the right of the Purchaser to conduct the Purchased Business;
G-26
- (f)
- no material damage by fire or other hazard to the Assets will have occurred from the date hereof to the Time of Closing;
- (g)
- the Purchaser and the Vendor will each have obtained all necessary approvals of their respective shareholders with respect to this Agreement and the transactions contemplated herein;
- (h)
- the Purchaser will have obtained all necessary regulatory approvals including the approval of the TSX and NASDAQ with respect to the Agreement and the transactions contemplated herein and the filing of any notices under theInvestment Canada Act (Canada), if required;
- (i)
- prior to or concurrent with the Time of Closing, Maverick will have completed the private placement contemplated in the Purchase Agreement;
- (j)
- the Vendor will have entered into an Escrow Agreement in a form acceptable to the Vendor and Purchaser, each acting reasonably;
- (k)
- the Designated Employees will have signed employment contracts with the Purchaser prior to the Closing Date;
- (l)
- the Vendor will have executed a general conveyance agreement substantially in the form attached hereto as Schedule 5.01(1)(l);
- (m)
- the Vendor will have executed a letter in favour of the Purchaser with respect to the distribution of the Common Shares forming part of the Purchase Price to the Vendor's shareholders, substantially in the form attached hereto as Schedule 5.01(1)(m);
- (n)
- all consents identified in Schedule 5.01(1)(n) will have been obtained and shall be in full force and effect except those consents which individually or in the aggregate would not be expected to have a material adverse effect on the Purchaser's ability to conduct the Purchased Business immediately after the Closing Date;
- (o)
- the Vendor will have negotiated modified terms in respect of the Vendor's license agreements and/or subcontracting arrangements identified by the Purchaser, to the satisfaction of the Purchaser, acting reasonably;
- (p)
- all necessary steps and proceedings will have been taken to permit the Assets to be duly and regularly transferred to and registered in the name of the Purchaser;
- (q)
- the employment agreement entered into as of the date hereof between each of Pratap Khanwilkar and Gordon Jacobs and the Purchaser shall continue to be in full force and effect as of the Closing Date;
- (r)
- a consulting agreement between Dr. James Long and the Purchaser shall be executed prior to the Closing Date, substantially in the form attached as Schedule 5.01(r);
- (s)
- the Cash Amounts on Schedule 2.06(2) will have been agreed by the Vendor and the Purchaser; and
- (t)
- the form and legality of all matters incidental to the sale by the Vendor and the purchase by the Purchaser of the Assets will be subject to the approval of the Purchaser's counsel.
(2) In case any term or covenant of the Vendor or condition to be performed or complied with for the benefit of the Purchaser at or prior to the Time of Closing has not been performed or complied with at or prior to the Time of Closing, the Purchaser, without limiting any other right that the Purchaser has, may at its sole option either:
- (a)
- rescind this Agreement by notice to the Vendor, and in such event the Purchaser will be released from all obligations hereunder, or
- (b)
- waive compliance with any such term, covenant or condition in whole or in part on such terms as may be agreed upon without prejudice to any of its rights of rescission in the event of non-performance of any other term, covenant or condition in whole or in part;
and, if the Purchaser rescinds this Agreement pursuant to Section 5.01(2)(a), the Vendor will also be released from all obligations hereunder unless the term, covenant or condition for which the Purchaser has rescinded this Agreement was one that the Vendor had covenanted, pursuant to Section 4.01(2), to ensure had been performed
G-27
or complied with, in which event the Vendor will be liable to the Purchaser for any Claims incurred by the Purchaser directly or indirectly as a result of such breach.
5.02 Conditions for the Benefit of the Vendor
(1) The sale by the Vendor and the purchase by the Purchaser of the Assets is subject to the following conditions, which are for the exclusive benefit of the Vendor and which are to be performed or complied with at or prior to the Time of Closing:
- (a)
- the representations and warranties of the Purchaser set forth in Section 3.03 will be true and correct at the Time of Closing with the same force and effect as if made at and as of such time;
- (b)
- the Purchaser will have performed or complied with all of the terms, covenants and conditions of this Agreement to be performed or complied with by the Purchaser at or prior to the Time of Closing;
- (c)
- prior to the Time of Closing, the Convertible Debentureholders will have converted the convertible debentures which are held by them;
- (d)
- prior to the Time of Closing, the Warrantholders will have converted the warrants which are held by them;
- (e)
- the Purchaser will have entered into the Escrow Agreement in a form acceptable to the Vendor and the Purchaser, each acting reasonably;
- (f)
- the Purchaser and the Vendor each will have obtained all necessary approvals of their respective shareholders in respect of this Agreement and the transactions contemplated herein;
- (g)
- the Purchaser will have obtained all necessary regulatory approvals including the approval of the TSX and NASDAQ with respect to the Agreement and the transactions contemplated herein;
- (h)
- the Cash Amounts on Schedule 2.06(2) will have been agreed by the Vendor and the Purchaser; and
- (i)
- the Vendor will be furnished with such certificates or other instruments of the Purchaser or of officers of the Purchaser as the Vendor or the Vendor's counsel may reasonably think necessary in order to establish that the terms, covenants and conditions contained in this Agreement to have been performed or complied with by the Purchaser at or prior to the Time of Closing have been performed or complied with and that the representations and warranties of the Purchaser herein given are true and correct at the Time of Closing.
(2) In case any term or covenant of the Purchaser or condition to be performed or complied with for the benefit of the Vendor at or prior to the Time of Closing has not been performed or complied with at or prior to the Time of Closing, the Vendor, without limiting any other right that the Vendor has, may at its sole option either:
- (a)
- rescind this Agreement by notice to the Purchaser, and in such event the Vendor will be released from all obligations hereunder, or
- (b)
- waive compliance with any such term, covenant or condition in whole or in part on such terms as may be agreed upon without prejudice to any of its rights of rescission in the event of non-performance of any other term, covenant or condition in whole or in part
and, if the Vendor rescinds this Agreement pursuant to Section 5.02(2)(a), the Purchaser will also be released from all obligations hereunder unless the term, covenant or condition for which the Vendor has rescinded this Agreement was one that the Purchaser had covenanted, pursuant to Section 4.02(2), to ensure had been performed or complied with, in which event the Purchaser will be liable to the Vendor for any Claims incurred by the Vendor directly or indirectly as a result of such breach.
ARTICLE 6 — CLOSING ARRANGEMENTS
6.01 Closing
The sale and purchase of the Assets will be completed at the Time of Closing at the offices of McCarthy Tétrault LLP, 40 Elgin Street, Suite 1400, Ottawa, Ontario K1P 5K6 or such other location(s) as the parties may determine.
G-28
6.02 Examination of Records and Assets
(1) The Vendor will forthwith make available to the Purchaser and its authorized representatives all data bases recorded or stored by means of any device, including in electronic form, title documents, abstracts of title, deeds, surveys, leases, certificates of trade marks and copyrights, contracts and commitments in its possession or under its control relating to any of the Assets or the Purchased Business. The Vendor will forthwith make available to the Purchaser and its authorized representatives for examination all books of account and accounting records relating to the Purchased Business. The Vendor will, if reasonably requested, provide copies, at the cost of the Purchaser, of the following records maintained in connection with the Purchased Business: financial statements, records of past sales, customer lists, supplier lists, payroll records, inventory data, inventory master records and accounts receivable data. The Vendor will give the Purchaser and its authorized representatives every reasonable opportunity to have access to and to inspect the Assets. The exercise of any rights of access or inspection by or on behalf of the Purchaser under this Section 6.02(1) will not affect or mitigate the covenants, representations and warranties of the Vendor in this Agreement which will continue in full force and effect.
(2) At the Time of Closing the Vendor will deliver to the Purchaser all of the documents referred to in Section 6.02(1). The Purchaser will preserve the documents so delivered for a period of six years from the Closing Date, or for such other period as is required by any Applicable Law, and will permit the Vendor and its authorized representatives reasonable access thereto in connection with the affairs of the Vendor, but the Purchaser will not be responsible or liable to the Vendor for or as a result of any loss or destruction of or damage to any such documents.
(3) Both prior to the Closing Date and, if the sale and purchase of the Assets hereunder fails to occur for whatever reason, thereafter the Purchaser will not disclose to anyone or use for its own or for any purpose other than the purpose contemplated by this Agreement any confidential information concerning the Vendor or the Purchased Business obtained by the Purchaser pursuant hereto, will hold all such information in the strictest confidence and, if the sale and purchase of the Assets hereunder fails to occur for whatever reason, will return all documents, records and all other information or data relating to the Vendor or to the Purchased Business which the Purchaser obtained pursuant to this Agreement.
(4) From and after the Closing Date the Vendor will not disclose to anyone or use for any purpose any confidential information concerning the Purchased Business purchased by the Purchaser pursuant to this Agreement and will hold all such information in the strictest confidence.
6.03 Transfer of Possession
(1) During the Interim Period the Purchased Business will be managed and operated by the Vendor in the usual and ordinary course of business for the account of the Purchaser and the Purchaser will be entitled to inspect all operations of the Purchased Business during the Interim Period. Any monies received by the Vendor from the Maverick Loan will be used solely for such purposes. All acts and proceedings taken by the Vendor in the management and operation of the Purchased Business from the date hereof involving a commitment in excess of $30,000 and/or any payment in excess of $30,000, including for greater certainty any arrangements or agreements with respect to the Maverick Loan, made by the Vendor during the Interim Period, will be subject to the prior approval of the Purchaser, which approval will not be unreasonably withheld. The Purchaser will be entitled to the income and profits in connection with the Purchased Business during the Interim Period and the Vendor will account to the Purchaser for all receipts, money, profits, benefits and advantages derived by or accruing to the Vendor from the Purchased Business during the Interim Period.
(2) If the sale and purchase of the Assets provided for herein is not completed and closed in accordance with the terms and conditions hereof, then the Vendor will be under no obligation to account to the Purchaser for the receipts, money, profits, benefits and advantages derived by or accruing to the Vendor from the Purchased Business during the Interim Period as referred to in Section 6.03(1) and the Purchaser will be released from its obligation to assume, fulfil and perform the obligations and liabilities of the Vendor accruing after the close of business on the day before the Effective Date pursuant to Section 2.04.
G-29
6.04 Risk of Loss
(1) Until the Time of Closing the Assets will remain at the risk of the Vendor. The Vendor will maintain all risk insurance in respect of loss or damage to or any other casualty in respect of the Assets which provides for loss settlement on a replacement cost basis if the Assets are repaired or replaced and on an actual cash value basis if the Assets are not repaired or replaced. The Vendor will also maintain all risk business interruption insurance with respect to any such loss, damage or other casualty. In the event of any loss, damage or claim in respect of any risk for which insurance is to be carried as aforesaid arising before the Time of Closing the Purchaser, as an additional condition of closing, will be entitled to be satisfied that the insurers have accepted the claim of the Vendor for payment in accordance with the terms of the policies. If any destruction or damage occurs to the Assets on or before the Time of Closing or if any or all of the Assets are appropriated, expropriated or seized by governmental or other lawful authority on or before the Time of Closing, the Vendor will forthwith give notice thereof to the Purchaser and the Purchaser will have the option, exercisable by notice to the Vendor on or before the Time of Closing
- (a)
- to reduce the Purchase Price by an amount equal to the proceeds of insurance (and, if any such policy provided for a deductible amount, by an amount equal to such deductible amount) or compensation for destruction or damage or appropriation, expropriation or seizure and business interruption with respect thereto (in this Section 6.04 referred to as the "Proceeds"), and to complete the purchase or
- (b)
- to complete the purchase without reduction of the Purchase Price, in which event all Proceeds will be payable to the Purchaser and all right and claim of the Vendor to any such amounts not paid by the Closing Date will be assigned to the Purchaser.
(2) If the Purchaser elects to reduce the Purchase Price pursuant to Section 6.04(1)(a), the Vendor and the Purchaser will at the Time of Closing determine the amount of the reduction to the extent that it is then determinable and will undertake to adjust such amount after the Closing Date, if necessary.
ARTICLE 7 — GENERAL
7.01 Indemnification
Except as provided in Section 4.05, the obligations of the Vendor and the Purchaser under this Agreement to indemnify and save harmless the other and its directors, officers, employees and agents are, in the case of any Claim by a third party, conditional upon the party that is otherwise entitled to be indemnified (the "Indemnitee") giving prompt notice to the other (the "Indemnitor") of such Claim and permitting the Indemnitor at its expense to participate in all negotiations relating thereto, to assume the defence of any action or proceeding relating thereto and to determine (with the Indemnitee, acting reasonably) whether any settlement should be made with respect thereto; provided that if, in the sole opinion of the Indemnitee, the interests of the Indemnitee are different from those of the Indemnitor in connection with such Claim, the Indemnitee will have the right, at the Indemnitor's expense, to defend its own interests provided that any settlement of such Claim is on terms and conditions approved by the Indemnitor, acting reasonably. If the Indemnitor does not defend any Claim, the Indemnitee will have the right to do so on its own behalf and on behalf of the Indemnitor at the expense of the Indemnitor.
7.02 Further Assurances
Each of the Vendor and the Purchaser will from time to time execute and deliver all such further documents and instruments and do all acts and things as the other party may, either before or after the Closing Date, reasonably require to effectively carry out or better evidence or perfect the full intent and meaning of this Agreement.
7.03 Time of the Essence
Time is of the essence of this Agreement.
7.04 Fees and Commissions
Each of the Vendor and the Purchaser will pay its respective legal and accounting costs and expenses incurred in connection with the preparation, execution and delivery of this Agreement and all documents and
G-30
instruments executed pursuant to this Agreement and any other costs and expenses whatsoever and howsoever incurred and will indemnify and save harmless the other from and against any Claim for any broker's, finder's or placement fee or commission alleged to have been incurred as a result of any action by it in connection with the transactions under this Agreement.
7.05 Public Announcements
Except as required by law, no public announcement or press release concerning the sale and purchase of the Assets may be made by the Vendor or the Purchaser without the prior consent and joint approval of the Vendor and the Purchaser.
7.06 Benefit of the Agreement
This Agreement will enure to the benefit of and be binding upon the respective successors and permitted assigns of the parties hereto.
7.07 Entire Agreement
This Agreement constitutes the entire agreement between the parties hereto with respect to the subject matter hereof and cancels and supersedes any prior understandings and agreements between the parties hereto with respect thereto. There are no representations, warranties, terms, conditions, undertakings or collateral agreements, express, implied or statutory, between the parties other than as expressly set forth in this Agreement.
7.08 Amendments and Waivers
No amendment to this Agreement will be valid or binding unless set forth in writing and duly executed by both of the parties hereto. No waiver of any breach of any provision of this Agreement will be effective or binding unless made in writing and signed by the party purporting to give the same and, unless otherwise provided, will be limited to the specific breach waived.
7.09 Assignment
This Agreement may not be assigned by the Vendor without the written consent of the Purchaser but may be assigned by the Purchaser without the consent of the Vendor to an Affiliate of the Purchaser, as determined by the provisions of theBusiness Corporations Act (Ontario), provided that such Affiliate enters into a written agreement with the Vendor to be bound by the provisions of this Agreement in all respects and to the same extent as the Purchaser is bound and provided that the Purchaser will continue to be bound by all the obligations hereunder as if such assignment had not occurred and perform such obligations to the extent that such Affiliate fails to do so.
7.10 Notices
Any demand, notice or other communication to be given in connection with this Agreement must be given in writing and will be given by personal delivery, by registered mail or by electronic means of communication addressed to the recipient as follows:
To the Vendor:
MedQuest Products, Inc.
4750 Wiley Post Way, Suite 120
Salt Lake City, Utah, 84116
Fax No.: 801-355-7622
Attention: President
G-31
with a copy to:
Holland & Hart LLP
60 East South Temple, Suite 2000
Salt Lake City, Utah 84111
Fax No.: 801-364-9124
Attention: Gregory E. Lindley
To the Purchaser:
World Heart Corporation
7799 Pardee Lane
Oakland, California, 94621
Fax No.: 510-563-4800
Attention: President
with a copy to:
McCarthy Tétrault LLP
40 Elgin Street, Suite 1400
Ottawa, Ontario, K1P 5K6
Fax No: 613-563-9386
Attention: Virginia K. Schweitzer
or to such other street address, individual or electronic communication number or address as may be designated by notice given by either party to the other. Any demand, notice or other communication given by personal delivery will be conclusively deemed to have been given on the day of actual delivery thereof and, if given by registered mail, on the third Business Day following the deposit thereof in the mail and, if given by electronic communication, on the day of transmittal thereof if given during the normal business hours of the recipient and on the Business Day during which such normal business hours next occur if not given during such hours on any day. If the party giving any demand, notice or other communication knows or ought reasonably to know of any difficulties with the postal system that might affect the delivery of mail, any such demand, notice or other communication may not be mailed but must be given by personal delivery or by electronic communication.
7.11 Remedies Cumulative
The right and remedies of the parties under this Agreement are cumulative and are in addition to, and not in substitution for, any other rights and remedies available at law or in equity or otherwise. No single or partial exercise by a party of any right or remedy precludes or otherwise affects the exercise of any other right or remedy to which that party may be entitled.
7.12 No Third Party Beneficiaries
Except as provided in Sections 4.01(1), 4.02(1), 7.01 and 7.06, this Agreement is solely for the benefit of
- (a)
- the Vendor and its successors and permitted assigns, with respect to the obligations of the Purchaser under this Agreement, and
- (b)
- the Purchaser, and its successors and permitted assigns, with respect to the obligations of the Vendor under this Agreement
and this Agreement will not be deemed to confer upon or give to any other person any remedy, claim, liability, reimbursement, cause of action or other right.
7.13 Governing Law; Consent to Jurisdiction; WAIVER OF JURY TRIAL
This Agreement shall be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof. The parties irrevocably submit to the
G-32
non-exclusive jurisdiction of the courts of the State of New York located in New York County and the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgement relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on the parties anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. The parties irrevocably consent to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. The parties irrevocably waive any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.THE PARTIES WAIVE ANY RIGHT TO REQUEST A TRIAL BY JURY IN ANY LITIGATION WITH RESPECT TO THIS AGREEMENT AND REPRESENTS THAT COUNSEL HAS BEEN CONSULTED SPECIFICALLY AS TO THIS WAIVER.
7.14 Counterparts
This Agreement may be executed in any number of counterparts, each of which will be deemed to be an original and all of which taken together will be deemed to constitute one and the same instrument.
7.15 Facsimiles
Delivery of an executed signature page to this Agreement by any party by electronic transmission will be as effective as delivery of a manually executed copy of this Agreement by such party.
IN WITNESS WHEREOF the parties have executed this Agreement.
| | | WORLD HEART CORPORATION | | |
| | | Per: | /s/ JAL S. JASSAWALLA
Jal S. Jassawalla
President and Chief Executive Officer | | |
| | | | | | |
| | | MEDQUEST PRODUCTS, INC. | | |
| | | Per: | /s/ PRATAP KHANWILKAR
Pratap Khanwilkar
President and Chief Executive Officer | | c/s |
G-33
AMENDMENT NO. 1 TO THE ASSET PURCHASE AGREEMENT
B E T W E E N
World Heart Corporation, a corporation incorporated under the laws of the Province of Ontario (the "Purchaser"),
— and —
MedQuest Products, Inc., a corporation incorporated under the laws of the State of Utah (the "Vendor"),
WHEREAS the parties hereto are parties to an asset purchase agreement (the "Existing Agreement"), dated as of January 31, 2005;
AND WHEREAS the parties wish to amend the terms of the Existing Agreement to change the definition of the Closing Date;
NOW THEREFORE, in consideration of the covenants and agreements herein contained, the parties agree as follows:
ARTICLE 1 — INTERPRETATION
1.01 Definitions
Unless something in the subject matter or context is inconsistent therewith, all terms defined in the Existing Agreement and not otherwise defined herein will have the same meanings as in the Existing Agreement and all conventions of interpretation that are established in the Existing Agreement will have the same effect herein as in the Existing Agreement.
1.02 Headings
The division of this Amendment into Articles and Sections and the insertion of headings are for convenience of reference only and do not affect the construction or interpretation of this Amendment. The terms "hereof", "hereunder" and similar expressions refer to this Amendment and not to any particular Article, Section or other portion hereof. Unless something in the subject matter or context is inconsistent therewith, references herein to Articles, Sections and Schedules are to Articles and Sections of and Schedules to this Amendment.
1.03 Extended Meanings
In this Amendment words importing the singular number only include the plural andvice versa, words importing any gender include all genders and words importing persons include individuals, corporations, limited and unlimited liability companies, general and limited partnerships, associations, trusts, unincorporated organizations, joint ventures and governmental authorities. The term "including" means "including without limiting the generality of the foregoing".
ARTICLE 2 — AMENDMENTS
2.01 Closing Date
The Existing Agreement is hereby amended by deleting all references to "May 9, 2005" in the definition of "Closing Date" and replacing them with "May 23, 2005".
ARTICLE 3 — GENERAL
3.01 Confirmation
Except as hereinbefore provided, the parties hereto confirm the terms and conditions of the Existing Agreement as heretofore amended and acknowledge that the Existing Agreement is in full force and effect. If
G-34
any term of the Existing Agreement is inconsistent with the terms of this Amendment the terms of this Amendment will prevail.
3.02 Benefit of the Amendment
This Amendment will enure to the benefit of and be binding upon the Purchaser and the Vendor and their respective successors and permitted assigns.
3.03 Governing Law
This Amendment will be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof.
3.04 Counterparts
This Amendment may be executed in counterparts, each of which will be deemed to be an original and both of which taken together will be deemed to constitute one and the same instrument.
3.05 Electronic Transmission
Delivery of an executed signature page to this Amendment by any party by electronic transmission will be as effective as delivery of a manually executed copy of the Amendment by such party.
IN WITNESS WHEREOF the parties have executed this Amendment.
| | | WORLD HEART CORPORATION | | |
|
|
Per: |
/s/ JAL. S. JASSAWALLA |
|
|
| | | |
| | |
|
|
MEDQUEST PRODUCTS, INC. |
|
|
|
|
Per: |
/s/ PRATAP KHANWILKAR |
|
|
| | | |
| | |
G-35
APPENDIX H
PURCHASE AGREEMENT
THIS PURCHASE AGREEMENT ("Agreement") is made and entered into as of the 31st day of January, 2005 by and among World Heart Corporation, a corporation incorporated under the laws of the Province of Ontario, Canada (the "Company"), and Maverick Venture Management, LLC, a limited liability corporation incorporated under the laws of Nevada (the "Investor").
Recitals
A. The Company and the Investor are executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by the provisions of Regulation D ("Regulation D"), as promulgated by the U.S. Securities and Exchange Commission (the "SEC") under the Securities Act of 1933, as amended; and
B. The Investor wishes to purchase from the Company, and the Company wishes to sell and issue to the Investor, upon the terms and conditions stated in this Agreement, 8,888,889 common shares of the Company, at a price of US$1.35 per share for an aggregate purchase price of $12,000,000; and
C. Contemporaneous with the sale of the Common Stock the parties hereto will execute and deliver a Registration Rights Agreement, in the form attached hereto as Exhibit B (the "Registration Rights Agreement"), pursuant to which the Company will agree to provide certain registration rights under the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder, and applicable state securities laws.
In consideration of the mutual promises made herein and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
1. Definitions. In addition to those terms defined above and elsewhere in this Agreement, for the purposes of this Agreement, the following terms shall have the meanings here set forth:
"Affiliate" means, with respect to any Person, any other Person which directly or indirectly Controls, is controlled by, or is under common control with, such Person.
"Business Day" means a day, other than a Saturday or Sunday, on which banks in New York City, New York, Ottawa, Ontario and Salt Lake City, Utah are open for the general transaction of business.
"Common Stock" means the common shares of the Company, and any securities into which the common shares may be reclassified.
"Company's Knowledge" means the actual knowledge of the named officers of the Company as set forth in Schedule 1.1.
"Confidential Information" means trade secrets, confidential information and know-how (including but not limited to ideas, formulae, compositions, processes, procedures and techniques, research and development information, computer program code, performance specifications, support documentation, drawings, specifications, designs, business and marketing plans, and customer and supplier lists and related information).
"Control" means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
"Dollars" or "$" means United States dollars.
"Intellectual Property" means all of the following: (i) patents, patent applications, patent disclosures and inventions (whether or not patentable and whether or not reduced to practice); (ii) trademarks, service marks, trade dress, trade names, corporate names, logos, slogans and Internet domain names, together with all goodwill associated with each of the foregoing; (iii) copyrights and copyrightable works; (iv) registrations, applications
H-1
and renewals for any of the foregoing; and (v) proprietary computer software (including but not limited to data, data bases and documentation).
"Material Adverse Effect" means a material adverse effect on (i) the assets, liabilities, results of operations, condition (financial or otherwise), business, or prospects of the Company and its Subsidiaries taken as a whole, or (ii) the ability of the Company to perform its obligations under the Transaction Documents.
"Maverick Shares" has the meaning set forth in Section 5.11.
"MedQuest" means MedQuest Products, Inc.
"MedQuest Purchase Agreement" means the asset purchase agreement between the Company and MedQuest dated as of January 31, 2005.
"NASDAQ" means the NASDAQ Stock Market, its successors and assigns.
"Person" means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
"Purchase Price" means $12,000,000.
"SEC Filings" has the meaning set forth in Section 4.6.
"Shares" means the shares of Common Stock being purchased by the Investor hereunder.
"Subsidiary" has the meaning set forth in Section 4.1.
"Transaction Documents" means this Agreement and the Registration Rights Agreement.
"TSX" means the Toronto Stock Exchange and its successors and assigns.
"1933 Act" means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
"1934 Act" means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
2. Purchase and Sale of the Shares. Subject to the terms and conditions of this Agreement, on the Closing Date, the Investor shall purchase, and the Company shall sell and issue to the Investor, the Shares in exchange for the Purchase Price as specified in Section 3 below.
3. Closing. Upon confirmation that the other conditions to closing specified herein have been satisfied, the Company shall deliver to Holland & Hart LLP, in trust, a certificate or certificates, registered in the name of the Investor representing the Shares, to be held for release to the Investor only upon payment of the Purchase Price to the Company. Upon receipt by Holland & Hart LLP of the certificate, the Investor shall promptly cause a wire transfer in same day funds to be sent to the account of the Company as instructed in writing by the Company, in an amount representing the Purchase Price. On the date (the "Closing Date") the Company receives such funds, the certificates evidencing the Shares shall be released to the Investor (the "Closing"). The purchase and sale of the Shares shall take place at the offices of McCarthy Tétrault LLP, 40 Elgin Street, Suite 1400, Ottawa, Ontario, K1P 5K6, or at such other location and on such other date as the Company and the Investor shall mutually agree.
4. Representations and Warranties of the Company. The Company hereby represents and warrants to the Investor that, except as set forth in the schedules delivered herewith (collectively, the "Disclosure Schedules"):
4.1 Organization, Good Standing and Qualification. Each of the Company and its Subsidiaries is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation and has all requisite corporate power and authority to carry on its business as now conducted and to own its properties. Each of the Company and its Subsidiaries is duly qualified to do business as a foreign corporation and is in good standing in each jurisdiction in which the conduct of its business or its ownership or leasing of property makes such qualification or leasing necessary unless the failure to so qualify has not and
H-2
could not reasonably be expected to have a Material Adverse Effect. The Company's subsidiaries are reflected on Schedule 4.1 hereto (the "Subsidiaries").
4.2 Authorization. The Company has full power and authority and has taken all requisite action on the part of the Company, its officers and directors necessary for (i) the authorization, execution and delivery of the Transaction Documents, (ii) authorization of the performance of all obligations of the Company hereunder or thereunder, and (iii) the authorization, issuance (or reservation for issuance) and delivery of the Shares. The Transaction Documents constitute the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with their terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability, relating to or affecting creditors' rights generally.
4.3 Capitalization. Schedule 4.3 sets forth (a) the authorized capital stock of the Company on the date hereof; (b) the number of shares of capital stock issued and outstanding; (c) the number of shares of capital stock issuable pursuant to the Company's stock option plans; and (d) the number of shares of capital stock issuable and reserved for issuance pursuant to securities (other than the Shares) exercisable for, or convertible into or exchangeable for any shares of capital stock of the Company. All of the issued and outstanding shares of the Company's capital stock have been duly authorized and validly issued and are fully paid, nonassessable and free of pre-emptive rights and were issued in full compliance with applicable law and any rights of third parties. All of the issued and outstanding shares of capital stock of each Subsidiary have been duly authorized and validly issued and are fully paid, nonassessable and free of pre-emptive rights, were issued in full compliance with applicable law and any rights of third parties and are owned by the Company, beneficially and of record, subject to no lien, encumbrance or other adverse claim. No Person is entitled to pre-emptive or similar statutory or contractual rights with respect to any securities of the Company. Except as described on Schedule 4.3, there are no outstanding warrants, options, convertible securities or other rights, agreements or arrangements of any character under which the Company or any of its Subsidiaries is or may be obligated to issue any equity securities of any kind and except as contemplated by this Agreement, neither the Company nor any of its Subsidiaries is currently in negotiations for the issuance of any equity securities of any kind. Except as described on Schedule 4.3 and except for the Registration Rights Agreement, there are no voting agreements, buy-sell agreements, option or right of first purchase agreements or other agreements of any kind among the Company and any of the securityholders of the Company relating to the securities of the Company held by them. Except as described on Schedule 4.3, the Company has not granted any Person the right to require the Company to register any securities of the Company under the 1933 Act, whether on a demand basis or in connection with the registration of securities of the Company for its own account or for the account of any other Person.
Schedule 4.3 sets forth a true and complete table setting forth the pro forma capitalization of the Company on a fully diluted basis giving effect to (i) the issuance of the Shares, (ii) any adjustments in other securities resulting from the issuance of the Shares, and (iii) the exercise or conversion of all outstanding securities. Except as described on Schedule 4.3, the issuance and sale of the Shares hereunder will not obligate the Company to issue shares of Common Stock or other securities to any other Person (other than the Investor) and will not result in the adjustment of the exercise, conversion, exchange or reset price of any outstanding security.
The Company does not have outstanding shareholder purchase rights or any similar arrangement in effect giving any Person the right to purchase any equity interest in the Company upon the occurrence of certain events except those contemplated in the MedQuest Purchase Agreement.
4.4 Valid Issuance. The Shares have been duly and validly authorized and, when issued and paid for pursuant to this Agreement, will be validly issued, fully paid and nonassessable, and shall be free and clear of all encumbrances and restrictions, except for restrictions on transfer set forth in the Transaction Documents or imposed by applicable securities laws.
4.5 Consents. The execution, delivery and performance by the Company of the Transaction Documents and the offer, issuance and sale of the Shares require no consent of, action by or in respect of, or filing with, any Person, governmental body, agency, or official other than (i) approvals required to be obtained by the TSX and NASDAQ, all of which shall be obtained and shall be in full force and effect prior to the Closing, (ii) approval by shareholders of the Company, (iii) completion of the transactions contemplated by the MedQuest Purchase Agreement simultaneously with the sale of the Shares, and (iv) filings that have been made pursuant to
H-3
applicable securities laws and post-sale filings pursuant to applicable state, federal and provincial securities laws which the Company undertakes to file within the applicable time periods. The Company has taken all action necessary to exempt (i) the issuance and sale of the Shares, and (ii) the other transactions contemplated by the Transaction Documents from the provisions of any anti-takeover, business combination or control share law or statute binding on the Company or to which the Company or any of its assets and properties may be subject or any provision of the Company's Articles of Incorporation, By-laws or any shareholder rights agreement that is or could become applicable to the Investor as a result of the transactions contemplated hereby, including without limitation, the issuance of the Shares and the ownership, disposition or voting of the Shares by the Investor or the exercise of any right granted to the Investor pursuant to this Agreement or the other Transaction Documents.
4.6 Delivery of SEC Filings; Business. The Company has provided the Investor with copies of the Company's most recent Annual Report on Form 40-F for the fiscal year ended December 31, 2003 (the "40-F"), and all other reports filed by the Company pursuant to the 1934 Act since the filing of the 40-F and prior to the date hereof (collectively, the "SEC Filings"). The SEC Filings are the only filings required of the Company pursuant to the 1934 Act for such period. The Company and its Subsidiaries are engaged only in the business described in the SEC Filings and as of their respective dates the SEC Filings contain a complete and accurate description in all material respects of the business of the Company and its Subsidiaries, taken as a whole.
4.7 Use of Proceeds. The proceeds of the sale of the Shares hereunder shall be used by the Company for working capital and general corporate purposes.
4.8 No Material Adverse Change. Since December 31, 2003, except as identified and described in the SEC Filings or as described on Schedule 4.8, there has not been:
(i) any change in the consolidated assets, liabilities, financial condition or operating results of the Company from that reflected in the financial statements included in the 40-F, except for changes in the ordinary course of business which have not and could not reasonably be expected to have a Material Adverse Effect, individually or in the aggregate;
(ii) any declaration or payment of any dividend, or any authorization or payment of any distribution, on any of the capital stock of the Company, or any redemption or repurchase of any securities of the Company;
(iii) any material damage, destruction or loss, whether or not covered by insurance to any assets or properties of the Company or its Subsidiaries;
(iv) any waiver, not in the ordinary course of business, by the Company or any Subsidiary of a material right or of a material debt owed to it;
(v) any satisfaction or discharge of any lien, claim or encumbrance or payment of any obligation by the Company or a Subsidiary, except in the ordinary course of business and which is not material to the assets, properties, financial condition, operating results or business of the Company and its Subsidiaries taken as a whole (as such business is presently conducted and as it is proposed to be conducted);
(vi) any change or amendment to the Company's Articles of Incorporation or by-laws, or material change to any material contract or arrangement by which the Company or any Subsidiary is bound or to which any of their respective assets or properties is subject;
(vii) any material labor difficulties or labor union organizing activities with respect to employees of the Company or any Subsidiary;
(viii) any transaction entered into by the Company or a Subsidiary other than in the ordinary course of business;
(ix) the loss of the services of any key employee, or material change in the composition or duties of the senior management of the Company or any Subsidiary;
(x) the loss or threatened loss of any customer which has had or could reasonably be expected to have a Material Adverse Effect; or
H-4
(xi) any other event or condition of any character that has had or could reasonably be expected to have a Material Adverse Effect.
4.9 SEC Filings. At the time of filing thereof, the SEC Filings complied as to form in all material respects with the requirements of the 1934 Act and did not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading.
4.10 No Conflict, Breach, Violation or Default. The execution, delivery and, subject to receipt of approval by the shareholders of the Company, performance of the Transaction Documents by the Company and the issuance and sale of the Shares will not conflict with or result in a breach or violation of any of the terms and provisions of, or constitute a default under (i) the Company's Articles of Incorporation or the Company's Bylaws, both as in effect on the date hereof (copies of which have been provided to the Investor before the date hereof), or (ii)(a) any statute, rule, regulation or order of any governmental agency or body or any court, domestic or foreign, having jurisdiction over the Company, any Subsidiary or any of their respective assets or properties, or (b) any agreement or instrument to which the Company or any Subsidiary is a party or by which the Company or a Subsidiary is bound or to which any of their respective assets or properties is subject.
4.11 Tax Matters. The Company and each Subsidiary has timely prepared and filed all material tax returns required to have been filed by the Company or such Subsidiary with all appropriate governmental agencies and timely paid all taxes shown thereon or otherwise owed by it. The charges, accruals and reserves on the books of the Company in respect of taxes for all fiscal periods are adequate in all material respects, and there are no material unpaid assessments against the Company or any Subsidiary nor, to the Company's Knowledge, any basis for the assessment of any additional taxes, penalties or interest for any fiscal period or audits by any federal, state, local or provincial taxing authority except for any assessment which is not material to the Company and its Subsidiaries, taken as a whole. All material taxes and other assessments and levies that the Company or any Subsidiary is required to withhold or to collect for payment have been duly withheld and collected and paid to the proper governmental entity or third party when due. There are no tax liens or claims pending or, to the Company's Knowledge, threatened against the Company or any Subsidiary or any of their respective assets or property. Except as described on Schedule 4.11, there are no outstanding tax sharing agreements or other such arrangements between the Company and any Subsidiary or other corporation or entity.
4.12 Title to Properties. Except as disclosed in the SEC Filings or as described on Schedule 4.12, the Company and each Subsidiary has good and marketable title to all real properties and all other properties and assets owned by it, in each case free from liens, encumbrances and defects that would materially affect the value thereof or materially interfere with the use made or currently planned to be made thereof by them; and except as disclosed in the SEC Filings, the Company and each Subsidiary holds any leased real or personal property under valid and enforceable leases with no exceptions that would materially interfere with the use made or currently planned to be made thereof by them.
4.13 Certificates, Authorities and Permits. The Company and each Subsidiary possess adequate certificates, authorities or permits issued by appropriate governmental agencies or bodies necessary to conduct the business now operated by it, and neither the Company nor any Subsidiary has received any notice of proceedings relating to the revocation or modification of any such certificate, authority or permit that, if determined adversely to the Company or such Subsidiary, could reasonably be expected to have a Material Adverse Effect, individually or in the aggregate.
4.14 No Labor Disputes. No material labor dispute with the employees of the Company or any Subsidiary exists or, to the Company's Knowledge, is imminent.
4.15 Intellectual Property.
(a) All Intellectual Property of the Company and its Subsidiaries is currently in compliance with all legal requirements (including timely filings, proofs and payments of fees) and is valid and enforceable. To the Company's Knowledge, no Intellectual Property of the Company or its Subsidiaries which is material to the conduct of Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted has been or is now involved in any cancellation, dispute or litigation, and, to the
H-5
Company's Knowledge, no such action is threatened. To the Company's Knowledge, no material patent of the Company or its Subsidiaries has been or is now involved in any interference, reissue, re-examination or opposition proceeding.
(b) All of the licenses and sublicenses and consent, royalty or other agreements concerning Intellectual Property which are material to the conduct of the Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted to which the Company or any Subsidiary is a party or by which any of their assets are bound (other than generally commercially available, non-custom, off-the-shelf software application programs having a retail acquisition price of less than $10,000 per license) (collectively, "License Agreements") are valid and binding obligations of the Company or its Subsidiaries that are parties thereto and, to the Company's Knowledge, the other parties thereto, enforceable in accordance with their terms, except to the extent that enforcement thereof may be limited by bankruptcy, insolvency, reorganization, moratorium, fraudulent conveyance or other similar laws affecting the enforcement of creditors' rights generally, and there exists no event or condition which will result in a material violation or breach of or constitute (with or without due notice or lapse of time or both) a default by the Company or any of its Subsidiaries under any such License Agreement.
(c) The Company and its Subsidiaries own or have the valid right to use all of the Intellectual Property that is material to the conduct of the Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted and for the ownership, maintenance and operation of the Company's and its Subsidiaries' properties and assets, free and clear of all liens, encumbrances, adverse claims or obligations to license all such owned Intellectual Property and Confidential Information, other than licenses entered into in the ordinary course of the Company's and its Subsidiaries' businesses. The Company and its Subsidiaries have a valid and enforceable right to use all third party Intellectual Property and Confidential Information used or held for use in the respective businesses of the Company and its Subsidiaries.
(d) To the Company's Knowledge, the conduct of the Company's and its Subsidiaries' businesses as currently conducted does not infringe or otherwise impair or conflict with (collectively, "Infringe") any Intellectual Property rights of any third party or any confidentiality obligation owed to a third party, and the Intellectual Property and Confidential Information of the Company and its Subsidiaries which are material to the conduct of Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted are not being Infringed by any third party. There is no litigation or order pending or outstanding or, to the Company's Knowledge, threatened or imminent, that seeks to limit or challenge or that concerns the ownership, use, validity or enforceability of any Intellectual Property or Confidential Information of the Company and its Subsidiaries and the Company's and its Subsidiaries' use of any Intellectual Property or Confidential Information owned by a third party, and, to the Company's Knowledge, there is no valid basis for the same.
(e) The consummation of the transactions contemplated hereby will not result in the alteration, loss, impairment of or restriction on the Company's or any of its Subsidiaries' ownership or right to use any of the Intellectual Property or Confidential Information which is material to the conduct of the Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted.
(f) The Company and its Subsidiaries have taken reasonable steps to protect the Company's and its Subsidiaries' rights in their Intellectual Property and Confidential Information. Each employee, consultant and contractor who has had access to Confidential Information which is material to the conduct of the Company's and each of its Subsidiaries' respective businesses as currently conducted or as currently proposed to be conducted has either (i) executed an agreement to maintain the confidentiality of such Confidential Information; (ii) executed appropriate agreements that are substantially consistent with the Company's standard forms thereof or (iii) undertaken similar safeguards to protect and preserve the confidentiality of all Confidential Information. Except under confidentiality obligations, to the Company's Knowledge there has been no material disclosure of any of the Company's or its Subsidiaries' Confidential Information to any third party.
4.16 Environmental Matters. Neither the Company nor any Subsidiary is in violation of any statute, rule, regulation, decision or order of any governmental agency or body or any court, domestic or foreign, relating to the use, disposal or release of hazardous or toxic substances or relating to the protection or restoration of the environment or human exposure to hazardous or toxic substances (collectively, "Environmental Laws"), owns or
H-6
operates any real property contaminated with any substance that is subject to any Environmental Laws, is liable for any off-site disposal or contamination pursuant to any Environmental Laws, and is subject to any claim relating to any Environmental Laws, which violation, contamination, liability or claim has had or could reasonably be expected to have a Material Adverse Effect, individually or in the aggregate; and to the Company's Knowledge there is no pending or threatened investigation that might lead to such a claim.
4.17 Litigation. Except as described on Schedule 4.17, there are no pending actions, suits or proceedings against or affecting the Company, its Subsidiaries or any of its or their properties; and to the Company's Knowledge, no such actions, suits or proceedings are threatened or contemplated.
4.18 Financial Statements. The financial statements included in each SEC Filing present fairly, in all material respects, the consolidated financial position of the Company as of the dates shown and its consolidated results of operations and cash flows for the periods shown, and such financial statements have been prepared in conformity with Canadian generally accepted accounting principles applied on a consistent basis (except as may be disclosed therein or in the notes thereto). Except as set forth in the financial statements of the Company included in the SEC Filings filed prior to the date hereof or as described on Schedule 4.18, neither the Company nor any of its Subsidiaries has incurred any liabilities, contingent or otherwise, except those incurred in the ordinary course of business, consistent (as to amount and nature) with past practices since the date of such financial statements, none of which, individually or in the aggregate, have had or could reasonably be expected to have a Material Adverse Effect.
4.19 Insurance Coverage. The Company and each Subsidiary maintains in full force and effect insurance coverage that is customary for comparably situated companies for the business being conducted and properties owned or leased by the Company and each Subsidiary, and the Company reasonably believes such insurance coverage to be adequate against all liabilities, claims and risks against which it is customary for comparably situated companies to insure, other than as described in Schedule 4.19.
4.20 Brokers and Finders. No Person will have, as a result of the transactions contemplated by this Agreement, any valid right, interest or claim against or upon the Company, any Subsidiary or the Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company.
4.21 No Directed Selling Efforts or General Solicitation. Neither the Company nor any Person acting on its behalf has conducted any general solicitation or general advertising (as those terms are used in Regulation D) under the 1933 Act in connection with the offer or sale of any of the Shares.
4.22 No Integrated Offering. Neither the Company nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales of any Company security or solicited any offers to buy any security, under circumstances that would adversely affect reliance by the Company on Section 4(2) of the 1933 Act for the exemption from registration for the transactions contemplated hereby or would require registration of the Shares under the 1933 Act.
4.23 Private Placement. The offer and sale of the Shares to the Investor as contemplated hereby is exempt from the registration requirements of the 1933 Act.
5. Representations and Warranties of the Investor. The Investor hereby represents and warrants to the Company that:
5.1 Organization and Existence. The Investor is a validly existing limited liability company and has all requisite limited liability company power and authority to invest in the Shares pursuant to this Agreement.
5.2 Authorization. The execution, delivery and performance by the Investor of the Transaction Documents to which the Investor is a party have been duly authorized and will each constitute the valid and legally binding obligation of the Investor, enforceable against the Investor in accordance with their respective terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability, relating to or affecting creditors' rights generally.
5.3 Purchase Entirely for Own Account. The Shares to be received by the Investor hereunder will be acquired for the Investor's own account, not as nominee or agent, and not with a view to the resale or
H-7
distribution of any part thereof in violation of the 1933 Act, and the Investor has no present intention of selling, granting any participation in, or otherwise distributing the same in violation of the 1933 Act. The Investor is not a registered broker dealer or an entity engaged in the business of being a broker dealer.
5.4 Investment Experience. The Investor acknowledges that it can bear the economic risk and complete loss of its investment in the Shares and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment contemplated hereby.
5.5 Disclosure of Information. The Investor has had an opportunity to receive all additional information related to the Company requested by it and to ask questions of and receive answers from the Company regarding the Company, its business and the terms and conditions of the offering of the Shares. The Investor acknowledges receipt of copies of the SEC Filings. Neither such inquiries nor any other due diligence investigation conducted by the Investor shall modify, amend or affect the Investor's right to rely on the Company's representations and warranties contained in this Agreement.
5.6 Restricted Shares. The Investor understands that the Shares are characterized as "restricted securities" under the U.S. federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such securities may be resold without registration under the 1933 Act only in certain limited circumstances.
5.7 Legends. It is understood that, except as provided below, certificates evidencing such Shares may bear the following or any similar legend:
(a) THE SECURITIES REPRESENTED HEREBY HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE "U.S. SECURITIES ACT") OR THE SECURITIES LAWS OF ANY STATE OF THE UNITED STATES. THE HOLDER HEREOF, BY PURCHASING SUCH SECURITIES, AGREES FOR THE BENEFIT OF THE COMPANY THAT SUCH SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) OUTSIDE THE UNITED STATES IN ACCORDANCE WITH RULE 903 or 904 OF REGULATION S UNDER THE U.S. SECURITIES ACT, IF APPLICABLE (C) INSIDE THE UNITED STATES (1) PURSUANT TO THE EXEMPTION FROM THE REGISTRATION REQUIREMENTS UNDER THE U.S. SECURITIES ACT PROVIDED BY RULE 144 THEREUNDER, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAW OR (2) IN A TRANSACTION THAT DOES NOT OTHERWISE REQUIRE REGISTRATION UNDER THE U.S. SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAW, PROVIDED THE HOLDER HAS FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OF RECOGNIZED STANDING TO THAT EFFECT IN FORM AND SUBSTANCE, REASONABLY SATISFACTORY TO THE COMPANY,OR (3) PURSUANT TO A REGISTRATION STATEMENT PURSUANT TO THE U.S. SECURITIES ACT.
(b) THE HOLDER, BY ITS ACCEPTANCE OF THIS SECURITY, REPRESENTS, ACKNOWLEDGES, AND AGREES THAT IT WILL NOT AND WILL NOT BE ENTITLED TO, DIRECTLY OR INDIRECTLY, SELL OR TRANSFER THE SECURITIES INTO CANADA OR TO RESIDENTS OF CANADA, EXCEPT IN COMPLIANCE WITH APPLICABLE CANADIAN SECURITIES LAWS. NO SALE OR TRANSFER INTO CANADA OR TO A CANADIAN RESIDENT WILL BE REGISTERED BY THE COMPANY'S TRANSFER AGENT AND ANY ATTEMPT TO EFFECT SUCH A TRANSFER IS INVALID UNLESS MADE IN COMPLIANCE WITH THE ABOVE-NOTED RESTRICTIONS. UNLESS PERMITTED UNDER CANADIAN SECURITIES LEGISLATION, THE HOLDER OF THE SECURITIES SHALL NOT TRADE THE SECURITIES BEFORE[FOUR MONTHS PLUS ONE DAY FROM THE DATE OF ISSUANCE].
(c) THE COMMON SHARES REPRESENTED BY THIS CERTIFICATE ARE LISTED ON THE TORONTO STOCK EXCHANGE; HOWEVER, THE SAID SECURITIES CAN NOT BE TRADED THROUGH THE FACILITIES OF SUCH EXCHANGE SINCE THEY ARE NOT FREELY TRANSFERABLE, AND CONSEQUENTLY ANY CERTIFICATE REPRESENTING SUCH SECURITIES IS NOT "GOOD DELIVERY" IN SETTLEMENT OF TRANSACTIONS ON THE TORONTO STOCK EXCHANGE.
H-8
If required by the authorities of any state in connection with the issuance of sale of the Shares, the legend required by such state authority.
Upon the earlier of (i) registration for resale pursuant to the Registration Rights Agreement and receipt by the Company of the Investor's written confirmation that such Shares will not be disposed of except in compliance with the prospectus delivery requirements of the 1933 Act or (ii) Rule 144(k) becoming available, the Company shall, upon the Investor's written request, promptly cause certificates evidencing the Shares to be replaced with certificates which do not bear the restrictive legend set forth in paragraph (a) above. From and after the anniversary of four months and one day of the Closing, the Company shall, upon the Investor's written request, promptly cause certificates evidencing the Shares to be replaced with certificates which do not bear the restrictive legend set forth in paragraph (b) above.
5.8 Accredited Investor. The Investor is an accredited investor as defined in Rule 501(a) of Regulation D, as amended, under the 1933 Act.
5.9 No General Solicitation. The Investor did not learn of the investment in the Shares as a result of any public advertising or general solicitation.
5.10 Brokers and Finders. No Person will have, as a result of the transactions contemplated by this Agreement, any valid right, interest or claim against or upon the Company, any Subsidiary or the Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Investor.
5.11 Ownership of Stock of MedQuest Products, Inc. The Investor is the registered and beneficial owner of 105,179 shares of common stock of MedQuest representing 82.43% of the issued and outstanding common shares of MedQuest and 6,675,782 shares of preferred stock of MedQuest representing 100% of the issued and outstanding preferred shares of MedQuest and, together, 99.67% of the issued and outstanding voting shares of MedQuest (collectively, the "Maverick Shares").
5.12 No Company Representations and Warranties with respect to MedQuest. The Investor acknowledges that the Company will acquire all of the assets of MedQuest pursuant to the MedQuest Purchase Agreement, a copy of which has been delivered to the Investor; and the Investor acknowledges that none of the representations and warranties and covenants of the Company herein shall include any representations, warranty or covenant with respect to the assets, business or any other matter related to MedQuest.
6. Conditions to Closing.
6.1 Conditions to the Investor's Obligations. The obligation of the Investor to purchase the Shares at the Closing is subject to the fulfillment to the Investor's satisfaction, on or prior to the Closing Date, of the following conditions, any of which may be waived by the Investor agreeing hereunder to purchase a majority of the Shares (the "Investor"):
(a) The representations and warranties made by the Company in Section 4 hereof qualified as to materiality shall be true and correct at all times prior to and on the Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date, and, the representations and warranties made by the Company in Section 4 hereof not qualified as to materiality shall be true and correct in all material respects at all times prior to and on the Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all material respects as of such earlier date. The Company shall have performed in all material respects all obligations and conditions herein required to be performed or observed by it on or prior to the Closing Date.
(b) The Company shall have obtained in a timely fashion any and all consents, permits, approvals, registrations and waivers (including, without limitation, any required approval of the TSX and NASDAQ) necessary or appropriate for consummation of the purchase and sale of the Shares and the consummation of the other transactions contemplated hereby, all of which shall be in full force and effect.
(c) The Company shall have obtained all necessary shareholder approvals.
(d) The Company shall have executed and delivered the Registration Rights Agreement.
H-9
(e) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated hereby or in the other Transaction Documents.
(f) The Company shall have delivered a Certificate, executed on behalf of the Company by its Chief Executive Officer or its Chief Financial Officer, dated as of the Closing Date, certifying to the fulfilment of the conditions specified in subsections (a), (b) and (c) of this Section 6.1.
(g) The Company shall have delivered a Certificate, executed on behalf of the Company by its Secretary, dated as of the Closing Date, certifying the resolutions adopted by the Board of Directors of the Company approving the transactions contemplated by this Agreement and the other Transaction Documents and the issuance of the Shares, certifying the current versions of the Articles of Incorporation and Bylaws of the Company and certifying as to the signatures and authority of persons signing the Transaction Documents and related documents on behalf of the Company.
(h) The Investor shall have received opinions from White & Case LLP and/or McCarthy Tétrault LLP, the Company's counsel, dated as of the Closing Date, in form and substance reasonably acceptable to the Investor and addressing such legal matters as the Investor may reasonably request.
(i) No stop order or suspension of trading shall have been imposed by the TSX and NASDAQ, the SEC or any other governmental regulatory body with respect to public trading in the Common Stock.
(j) The Company shall have simultaneously completed the transaction contemplated in the MedQuest Purchase Agreement substantially on the terms and conditions set forth therein.
6.2 Conditions to Obligations of the Company. The Company's obligation to sell and issue the Shares at the Closing is subject to the fulfillment to the satisfaction of the Company on or prior to the Closing Date of the following conditions, any of which may be waived by the Company:
(a) The representations and warranties made by the Investor in Section 5 hereof, other than the representations and warranties contained in Sections 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9 and 5.11 (the "Investment Representations"), shall be true and correct in all material respects when made, and shall be true and correct in all material respects on the Closing Date with the same force and effect as if they had been made on and as of said date. The Investment Representations shall be true and correct in all respects when made, and shall be true and correct in all respects on the Closing Date with the same force and effect as if they had been made on and as of said date. The Investor shall have performed in all material respects all obligations and conditions herein required to be performed or observed by them on or prior to the Closing Date.
(b) The Investor shall have executed and delivered the Registration Rights Agreement.
(c) The Investor shall have delivered the Purchase Price to the Company.
6.3 Termination of Obligations to Effect Closing; Effects.
(a) The obligations of the Company, on the one hand, and the Investor, on the other hand, to effect the Closing shall terminate as follows:
(i) Upon the mutual written consent of the Company and the Investor;
(ii) By the Company if any of the conditions set forth in Section 6.2 shall have become incapable of fulfillment, and shall not have been waived by the Company;
(iii) By the Investor if any of the conditions set forth in Section 6.1 shall have become incapable of fulfillment, and shall not have been waived by the Investor; or
(iv) By the Investor if the Closing has not occurred by the later of (i) May 9, 2005, and (ii) a date to be determined by adding that number of days beyond May 9, 2005 that the Closing has been extended due to (A) a regulatory authority in Canada or the United States reviewing, or taking a similar action, with respect to the Company's proxy materials or such other materials as may be required to be submitted to such regulatory authority, (B) procedural delays as a result of such
H-10
regulatory authority's review, such as the requirement to republish and extend the date on which the Company's meeting of shareholders can occur, and (C) the time required by the Company to respond to any such regulatory authority's requests or inquiries, provided that the Company shall use reasonable commercial efforts to respond to any such requests or inquiries as quickly as possible under the circumstances;
provided, however, that, except in the case of clause (i) above, the party seeking to terminate its obligation to effect the Closing shall not then be in breach of any of its representations, warranties, covenants or agreements contained in this Agreement or the other Transaction Documents if such breach has resulted in the circumstances giving rise to such party's seeking to terminate its obligation to effect the Closing.
(b) In the event of termination by the Company or the Investor of their obligations to effect the Closing pursuant to this Section 6.3, written notice thereof shall forthwith be given to the other parties hereto and the obligation of all parties to effect the Closing shall be terminated, without further action by any party. Nothing in this Section 6.3 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents.
7. Covenants and Agreements of the Company.
7.1 Reports. The Company will furnish to such Investor and/or its assignees such information relating to the Company and its Subsidiaries as from time to time may reasonably be requested by such Investor and/or their assignees; provided, however, that the Company shall not disclose material nonpublic information to the Investor, or to advisors to or representatives of the Investor, unless prior to disclosure of such information the Company identifies such information as being material nonpublic information and provides the Investor, such advisors and representatives with the opportunity to accept or refuse to accept such material nonpublic information for review and the Investor or its advisors or representatives wishing to obtain such information, enter into an appropriate confidentiality agreement with the Company with respect thereto.
7.2 No Conflicting Agreements. The Company will not take any action, enter into any agreement or make any commitment that would conflict or interfere in any material respect with the obligations to the Investor under the Transaction Documents.
7.3 Insurance. The Company shall not materially reduce the insurance coverages described in Section 4.19.
7.4 Compliance with Laws. The Company will comply in all material respects with all applicable laws, rules, regulations, orders and decrees of all governmental authorities.
7.5 Listing of Shares and Related Matters. Promptly following the date hereof, the Company shall take all necessary action to cause the Shares to be listed, subject to issuance, on the TSX and NASDAQ no later than the Closing Date. Further, if the Company applies to have its Common Stock or other securities traded on any other principal stock exchange or market, it shall include in such application the Shares and will take such other action as is necessary to cause such Common Stock to be so listed. The Company will use commercially reasonable efforts to continue the listing and trading of its Common Stock on NASDAQ and, in accordance, therewith, will use commercially reasonable efforts to comply in all respects with the Company's reporting, filing and other obligations under the bylaws or rules of such market or exchange, as applicable.
7.6 Termination of Covenants. The provisions of Sections 7.2 through 7.5 shall terminate and be of no further force and effect upon the earlier of (i) the mutual consent of the Company and the Investor, or (ii) the date on which the Company's obligations under the Registration Rights Agreement to register or maintain the effectiveness of any registration covering the Registrable Shares (as such term is defined in the Registration Rights Agreement) shall terminate.
7.7 Director Designee.
(a) So long as the Investor holds at least 25% of the issued and outstanding Common Stock, the Investor shall have the right to nominate two persons for election to the board of directors of the Company (the "Investor
H-11
Designees") and the number of directors shall not exceed seven; and so long as the Investor holds at least 15% of the issued and outstanding Common Stock but less than 25% of the issued and outstanding Common Stock, the Investor shall have the right to designate one Investor Designee. The Company shall use its best efforts to cause the Investor Designees to be elected to the Company's board of directors. The Investor shall have the right to remove or replace any Investor Designees by giving notice to such Investor Designee and the Company. The Company shall use its best efforts to effect the removal or replacement of such Investor Designee.
(b) Subject to any limitations imposed by applicable law, the Investor Designee shall be entitled to the same perquisites, including stock options, reimbursement of expenses and other similar rights in connection with such person's membership on the Board of Directors of the Company, as every other non-executive member of the Board of Directors of the Company.
8. Covenants of the Investor
8.1 MedQuest Purchase Agreement. The Investor will vote all of its shares of common stock of MedQuest and all of its shares of preferred stock of MedQuest, including the Maverick Shares and any shares of common stock and preferred stock of MedQuest acquired by the Investor after the date hereof and prior to Closing, in favor of a resolution to be submitted by the board of directors of MedQuest to a meeting of the stockholders of MedQuest or a resolution of the MedQuest stockholders in writing, to approve the transactions contemplated in the MedQuest Purchase Agreement.
9. Survival and Indemnification.
9.1 Survival. All representations, warranties, covenants and agreements contained in this Agreement shall be deemed to be representations, warranties, covenants and agreements as of the date hereof and shall survive the execution and delivery of this Agreement for a period of one year from the date of this Agreement; provided, however, that the provisions contained in Section 7 hereof shall survive in accordance therewith.
9.2 Indemnification. The Company agrees to indemnify and hold harmless the Investor and its Affiliates and their respective directors, officers, employees and agents from and against any and all losses, claims, damages, liabilities and expenses (including without limitation reasonable attorney fees and disbursements and other expenses incurred in connection with investigating, preparing or defending any action, claim or proceeding, pending or threatened and the costs of enforcement hereof) (collectively, "Losses") to which such Person may become subject as a result of any breach of representation, warranty, covenant or agreement made by or to be performed on the part of the Company under the Transaction Documents, and will reimburse any such Person for all such amounts as they are incurred by such Person.
9.3 Conduct of Indemnification Proceedings. Promptly after receipt by any Person (the "Indemnified Person") of notice of any demand, claim or circumstances which would or might give rise to a claim or the commencement of any action, proceeding or investigation in respect of which indemnity may be sought pursuant to Section 9.2, such Indemnified Person shall promptly notify the Company in writing and the Company shall assume the defense thereof, including the employment of counsel reasonably satisfactory to such Indemnified Person, and shall assume the payment of all fees and expenses; provided, however, that the failure of any Indemnified Person so to notify the Company shall not relieve the Company of its obligations hereunder except to the extent that the Company is materially prejudiced by such failure to notify. In any such proceeding, any Indemnified Person shall have the right to retain its own counsel, but the fees and expenses of such counsel shall be at the expense of such Indemnified Person unless: (i) the Company and the Indemnified Person shall have mutually agreed to the retention of such counsel; or (ii) in the reasonable judgment of counsel to such Indemnified Person representation of both parties by the same counsel would be inappropriate due to actual or potential differing interests between them. The Company shall not be liable for any settlement of any proceeding effected without its written consent, which consent shall not be unreasonably withheld, but if settled with such consent, or if there be a final judgment for the plaintiff, the Company shall indemnify and hold harmless such Indemnified Person from and against any loss or liability (to the extent stated above) by reason of such settlement or judgment. Without the prior written consent of the Indemnified Person, which consent shall not be unreasonably withheld, the Company shall not effect any settlement of any pending or threatened proceeding in respect of which any Indemnified Person is or could have been a party and indemnity could have been sought
H-12
hereunder by such Indemnified Party, unless such settlement includes an unconditional release of such Indemnified Person from all liability arising out of such proceeding.
10. Miscellaneous.
10.1 Successors and Assigns. This Agreement may not be assigned by a party hereto without the prior written consent of the Company or the Investor, as applicable, provided, however, that the Investor may assign its rights and delegate its duties hereunder in whole or in part to an Affiliate without the prior written consent of the Company, after notice duly given by the Investor to the Company, provided, that no such assignment or obligation shall affect the obligations of the Investor hereunder. The provisions of this Agreement shall inure to the benefit of and be binding upon the respective permitted successors and assigns of the parties. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
10.2 Counterparts; Faxes. This Agreement may be executed in two counterparts, each of which shall be deemed an original, but both of which together shall constitute one and the same instrument. This Agreement may also be executed via facsimile, which shall be deemed an original.
10.3 Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
10.4 Notices. Unless otherwise provided, any notice required or permitted under this Agreement shall be given in writing and shall be deemed effectively given as hereinafter described (i) if given by personal delivery, then such notice shall be deemed given upon such delivery, (ii) if given by telecopier, then such notice shall be deemed given upon receipt of confirmation of complete transmittal, (iii) if given by mail, then such notice shall be deemed given upon the earlier of (A) receipt of such notice by the recipient or (B) three days after such notice is deposited in first class mail, postage prepaid, and (iv) if given by an internationally recognized overnight air courier, then such notice shall be deemed given one day after delivery to such carrier. All notices shall be addressed to the party to be notified at the address as follows, or at such other address as such party may designate by ten days' advance written notice to the other party:
If to the Company:
World Heart Corporation
7799 Pardee Lane
Oakland, California 94621
Attention: Chief Financial Officer
Fax: (510) 563-4800
With a copy to:
McCarthy Tétrault LLP
Suite 1400, 40 Elgin Street
Ottawa, Ontario
K1P 5K6
Attention: Virginia K. Schweitzer
Fax: (613) 563-9386
If to the Investor:
Maverick Venture Management, LLC
14950 Gypsy Hill Road
Saratoga, California 95070
Attention: Kevin R. Compton
Fax: (650) 233-0300
H-13
and with a copy to:
Holland & Hart LLP
60 East South Temple, Suite 2000
Salt Lake City, Utah 84111
Attention: Gregory E. Lindley
Fax: (801) 364-9124
to the addresses set forth on the signature pages hereto.
10.5 Expenses. The parties hereto shall pay their own costs and expenses in connection herewith. In the event that legal proceedings are commenced by any party to this Agreement against another party to this Agreement in connection with this Agreement or the other Transaction Documents, the party which does not prevail in such proceedings shall severally, but not jointly, pay the reasonable attorneys' fees and other reasonable out-of-pocket costs and expenses incurred by the prevailing party in such proceedings.
10.6 Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Investor. Any amendment or waiver effected in accordance with this paragraph shall be binding upon the holder of any Shares purchased under this Agreement at the time outstanding, and the Company.
10.7 Publicity. No public release or announcement concerning the transactions contemplated hereby shall be issued by the Company or the Investor without the prior consent of the other party (which consent shall not be unreasonably withheld), except as such release or announcement may be required by law or the applicable rules or regulations of any securities exchange or securities market, in which case the Company or the Investor, as the case may be, shall allow the Company, as applicable, to the extent reasonably practicable in the circumstances, reasonable time to comment on such release or announcement in advance of such issuance.
10.8 Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the maximum extent permitted by applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the parties hereby waive any provision of law which renders any provision hereof prohibited or unenforceable in any respect.
10.9 Entire Agreement. This Agreement, including the Exhibits and the Disclosure Schedules, and the other Transaction Documents constitute the entire agreement among the parties hereof with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, both oral and written, between the parties with respect to the subject matter hereof and thereof.
10.10 Further Assurances. The parties shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the agreements herein contained.
10.11 Governing Law; Consent to Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof. Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the courts of the State of New York located in New York County and the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
H-14
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| The Company: | | WORLD HEART CORPORATION |
| | | By: | /s/ JAL S. JASSAWALLA
Name: Jal S. Jassawalla
Title: President and Chief Executive Officer |
| | | | |
| The Investor: | | MAVERICK VENTURE MANAGEMENT, LLC |
| | | By: | /s/ KEVIN COMPTON
Name: Kevin Compton
Title: Manager |
H-15
AMENDMENT NO. 1 TO THE PURCHASE AGREEMENT
B E T W E E N
World Heart Corporation, a corporation incorporated under the laws of the Province of Ontario (the "Purchaser"),
— and —
Maverick Venture Management, LLC, a corporation incorporated under the laws of the State of Nevada (the "Vendor"),
WHEREAS the parties hereto are parties to a purchase agreement (the "Existing Agreement"), dated as of January 31, 2005;
AND WHEREAS the parties wish to amend the terms of the Existing Agreement to change the definition of the Closing Date;
NOW THEREFORE, in consideration of the covenants and agreements herein contained, the parties agree as follows:
ARTICLE 1 — INTERPRETATION
1.01 Definitions
Unless something in the subject matter or context is inconsistent therewith, all terms defined in the Existing Agreement and not otherwise defined herein will have the same meanings as in the Existing Agreement and all conventions of interpretation that are established in the Existing Agreement will have the same effect herein as in the Existing Agreement.
1.02 Headings
The division of this Amendment into Articles and Sections and the insertion of headings are for convenience of reference only and do not affect the construction or interpretation of this Amendment. The terms "hereof", "hereunder" and similar expressions refer to this Amendment and not to any particular Article, Section or other portion hereof. Unless something in the subject matter or context is inconsistent therewith, references herein to Articles, Sections and Schedules are to Articles and Sections of and Schedules to this Amendment.
1.03 Extended Meanings
In this Amendment words importing the singular number only include the plural andvice versa, words importing any gender include all genders and words importing persons include individuals, corporations, limited and unlimited liability companies, general and limited partnerships, associations, trusts, unincorporated organizations, joint ventures and governmental authorities. The term "including" means "including without limiting the generality of the foregoing".
ARTICLE 2 — AMENDMENTS
2.01 Closing Date
The Existing Agreement is hereby amended by deleting all references to "May 9, 2005" in Section 6.3(a)(iv) of the Existing Agreement and replacing them with "May 23, 2005".
H-16
ARTICLE 3 — GENERAL
3.01 Confirmation
Except as hereinbefore provided, the parties hereto confirm the terms and conditions of the Existing Agreement as heretofore amended and acknowledge that the Existing Agreement is in full force and effect. If any term of the Existing Agreement is inconsistent with the terms of this Amendment the terms of this Amendment will prevail.
3.02 Benefit of the Amendment
This Amendment will enure to the benefit of and be binding upon the Purchaser and the Vendor and their respective successors and permitted assigns.
3.03 Governing Law
This Amendment will be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof.
3.04 Counterparts
This Amendment may be executed in counterparts, each of which will be deemed to be an original and both of which taken together will be deemed to constitute one and the same instrument.
3.05 Electronic Transmission
Delivery of an executed signature page to this Amendment by any party by electronic transmission will be as effective as delivery of a manually executed copy of the Amendment by such party.
IN WITNESS WHEREOF the parties have executed this Amendment.
| | | WORLD HEART CORPORATION |
|
|
Per: |
/s/ JAL. S. JASSAWALLA
|
| | | | |
| | | MAVERICK VENTURE MANAGEMENT, LLC |
|
|
Per: |
/s/ KEVIN COMPTON
|
H-17
APPENDIX I
CONVERSION AND EXERCISE AGREEMENT
This CONVERSION AND EXERCISE AGREEMENT is made as of this day of , 2005, by and between World Heart Corporation ("WorldHeart") and the security holder identified below.
WHEREAS the security holder purchased from WorldHeart an unsecured convertible debenture (the "Debenture"), dated as of September 15, 2004, convertible into common shares of WorldHeart ("Common Shares") at a conversion price of US$1.25 per Common Share;
AND WHEREAS in connection with the purchase of the Debenture the security holder received warrants to purchase Common Shares of WorldHeart (the "Warrants") in an amount equal to one warrant for each US$1.25 in principal amount of Debenture, with an exercise price of US$1.55 per Common Share;
AND WHEREAS WorldHeart desires that the security holder convert the Debenture and exercise the Warrants;
AND WHEREAS the security holder is prepared to convert the Debenture and exercise the Warrants on the terms and subject to the conditions set out below;
NOW THEREFORE, in consideration of the covenants and agreements herein contained and other good and valuable consideration (the receipt and sufficiency of which are hereby acknowledged) the parties hereto agree as follows:
- 1.
- The security holder will, subject to the conditions set out below, convert the Debenture as to the full principal amount thereof and as to all interest accrued to the date of conversion, on or before the date (the "Final Date") which is a date no later than 14 days after the date on which the shareholders of WorldHeart approve the acquisition by WorldHeart of either all of the assets or all of the outstanding shares of MedQuest Products, Inc. and the simultaneous investment, directly or indirectly, by Maverick Venture Management, LLC of US$12,000,000 in WorldHeart (together, the "MedQuest Transaction").
- 2.
- The security holder will, subject to the conditions set out below, exercise all of the Warrants on or before the Final Date.
- 3.
- WorldHeart will, at the time it seeks approval from its shareholders for the MedQuest Transaction, also seek approval from its shareholders to amend the exercise price of the Warrants in accordance with paragraph 4 hereof (the "Amendment"), and the security holder will not be obligated to convert the Debenture or exercise any Warrants unless the shareholders of WorldHeart approve the Amendment.
- 4.
- The Amendment will provide that during the period of time commencing on the date the Amendment is approved by shareholders of WorldHeart and ending on the Final Date the exercise price of the Warrants will be amended from US$1.55 per Common Share to US$1.00 per Common Share.
- 5.
- The conversion of the Debenture, including interest accrued thereon to the date of conversion, and the issuance of Common Shares therefor will be done in accordance with the terms of the Debenture.
- 6.
- The exercise of the Warrants and the issuance of Common Shares therefor will be done in accordance with the terms of the Warrants and the warrant indenture dated as of September 15, 2004 governing the Warrants (the "Indenture"), as amended by the Amendment.
- 7.
- The security holder acknowledges that the Corporation now possesses and may hereafter possess certain non-public information concerning the Corporation and its affiliates beyond the transactions contemplated in this Agreement that may or may not be independently known to security holder (the "Corporation Non-Public Information") which may constitute material information with respect to the foregoing. The security holder agrees to terms and conditions of this Agreement notwithstanding that it is aware that the Corporation Non-Public Information exists and that the Corporation has not disclosed any Corporation Non-Public Information to it. The security holder acknowledges that it is a sophisticated seller and is acting independently with respect to the purchase and sale of securities such as the Debentures and Warrants and that the Corporation has no obligations to the security holder to disclose such Corporation Non-Public
I-1
Information and no fiduciary obligations to the security holder. Additionally, the security holder acknowledges that it has adequate information concerning the transactions contemplated in this Agreement, and the business and financial condition of the Corporation and its affiliates, to make an informed decision regarding the entering into of this Agreement, and has independently and without reliance upon the Corporation and without solicitation from the Corporation, and based upon such information as the security holder has deemed appropriate, made its own analysis and decision to enter into this Agreement with the Corporation.
- 8.
- The security holder, pursuant to section 8.1 of the Indenture, hereby consents to the Amendment.
IN WITNESS WHEREOF the parties have executed this Agreement.
|
|
as security holder |
|
|
|
|
Per: |
|
|
|
| | | |
Name:
Title: | | |
|
|
|
|
|
c/s |
|
|
|
|
|
|
| | | |
Name:
Title: | | |
|
|
WORLD HEART CORPORATION |
|
|
|
|
Per: |
|
|
|
| | | |
Name:
Title: | | |
I-2
APPENDIX J
RIGHTS OF DISSENTING SHAREHOLDERS
SECTION 185 OF THEBUSINESS CORPORATIONS ACT (ONTARIO)
Rights of Dissenting Shareholders
185.(1) Subject to subsection (3) and to sections 186 and 248, if a corporation resolves to,
- •
- amend its articles under section 168 to add, remove or change restrictions on the issue, transfer or ownership of shares of a class or series of the shares of the corporation;
- •
- amend its articles under section 168 to add, remove or change any restriction upon the business or businesses that the corporation may carry on or upon the powers that the corporation may exercise;
- •
- amalgamate with another corporation under sections 175 and 176;
- •
- be continued under the laws of another jurisdiction under section 181; or
- •
- sell, lease or exchange all or substantially all its property under subsection 184 (3),
- •
- a holder of shares of any class or series entitled to vote on the resolution may dissent.
(2) If a corporation resolves to amend its articles in a manner referred to in subsection 170 (1), a holder of shares of any class or series entitled to vote on the amendment under section 168 or 170 may dissent, except in respect of an amendment referred to in,
- (a)
- clause 170 (1) (a), (b) or (e) where the articles provide that the holders of shares of such class or series are not entitled to dissent; or
- (b)
- subsection 170 (5) or (6).
(3) A shareholder of a corporation incorporated before the 29th day of July, 1983 is not entitled to dissent under this section in respect of an amendment of the articles of the corporation to the extent that the amendment,
- (a)
- amends the express terms of any provision of the articles of the corporation to conform to the terms of the provision as deemed to be amended by section 277; or
- (b)
- deletes from the articles of the corporation all of the objects of the corporation set out in its articles, provided that the deletion is made by the 29th day of July, 1986.
(4) In addition to any other right the shareholder may have, but subject to subsection (30), a shareholder who complies with this section is entitled, when the action approved by the resolution from which the shareholder dissents becomes effective, to be paid by the corporation the fair value of the shares held by the shareholder in respect of which the shareholder dissents, determined as of the close of business on the day before the resolution was adopted.
(5) A dissenting shareholder may only claim under this section with respect to all the shares of a class held by the dissenting shareholder on behalf of any one beneficial owner and registered in the name of the dissenting shareholder.
(6) A dissenting shareholder shall send to the corporation, at or before any meeting of shareholders at which a resolution referred to in subsection (1) or (2) is to be voted on, a written objection to the resolution, unless the corporation did not give notice to the shareholder of the purpose of the meeting or of the shareholder's right to dissent.
(7) The execution or exercise of a proxy does not constitute a written objection for purposes of subsection (6).
(8) The corporation shall, within ten days after the shareholders adopt the resolution, send to each shareholder who has filed the objection referred to in subsection (6) notice that the resolution has been adopted,
J-1
but such notice is not required to be sent to any shareholder who voted for the resolution or who has withdrawn the objection.
(9) A notice sent under subsection (8) shall set out the rights of the dissenting shareholder and the procedures to be followed to exercise those rights.
(10) A dissenting shareholder entitled to receive notice under subsection (8) shall, within twenty days after receiving such notice, or, if the shareholder does not receive such notice, within twenty days after learning that the resolution has been adopted, send to the corporation a written notice containing,
- (a)
- the shareholder's name and address;
- (b)
- the number and class of shares in respect of which the shareholder dissents; and
- (c)
- a demand for payment of the fair value of such shares.
(11) Not later than the thirtieth day after the sending of a notice under subsection (10), a dissenting shareholder shall send the certificates representing the shares in respect of which the shareholder dissents to the corporation or its transfer agent.
(12) A dissenting shareholder who fails to comply with subsections (6), (10) and (11) has no right to make a claim under this section.
(13) A corporation or its transfer agent shall endorse on any share certificate received under subsection (11) a notice that the holder is a dissenting shareholder under this section and shall return forthwith the share certificates to the dissenting shareholder.
(14) On sending a notice under subsection (10), a dissenting shareholder ceases to have any rights as a shareholder other than the right to be paid the fair value of the shares as determined under this section except where,
- (a)
- the dissenting shareholder withdraws notice before the corporation makes an offer under subsection (15);
- (b)
- the corporation fails to make an offer in accordance with subsection (15) and the dissenting shareholder withdraws notice; or
- (c)
- the directors revoke a resolution to amend the articles under subsection 168 (3), terminate an amalgamation agreement under subsection 176 (5) or an application for continuance under subsection 181 (5), or abandon a sale, lease or exchange under subsection 184 (8),
in which case the dissenting shareholder's rights are reinstated as of the date the dissenting shareholder sent the notice referred to in subsection (10), and the dissenting shareholder is entitled, upon presentation and surrender to the corporation or its transfer agent of any certificate representing the shares that has been endorsed in accordance with subsection (13), to be issued a new certificate representing the same number of shares as the certificate so presented, without payment of any fee.
(15) A corporation shall, not later than seven days after the later of the day on which the action approved by the resolution is effective or the day the corporation received the notice referred to in subsection (10), send to each dissenting shareholder who has sent such notice,
- (a)
- a written offer to pay for the dissenting shareholder's shares in an amount considered by the directors of the corporation to be the fair value thereof, accompanied by a statement showing how the fair value was determined; or
- (b)
- if subsection (30) applies, a notification that it is unable lawfully to pay dissenting shareholders for their shares.
(16) Every offer made under subsection (15) for shares of the same class or series shall be on the same terms.
J-2
(17) Subject to subsection (30), a corporation shall pay for the shares of a dissenting shareholder within ten days after an offer made under subsection (15) has been accepted, but any such offer lapses if the corporation does not receive an acceptance thereof within thirty days after the offer has been made.
(18) Where a corporation fails to make an offer under subsection (15) or if a dissenting shareholder fails to accept an offer, the corporation may, within fifty days after the action approved by the resolution is effective or within such further period as the court may allow, apply to the court to fix a fair value for the shares of any dissenting shareholder.
(19) If a corporation fails to apply to the court under subsection (18), a dissenting shareholder may apply to the court for the same purpose within a further period of twenty days or within such further period as the court may allow.
(20) A dissenting shareholder is not required to give security for costs in an application made under subsection (18) or (19).
(21) If a corporation fails to comply with subsection (15), then the costs of a shareholder application under subsection (19) are to be borne by the corporation unless the court otherwise orders.
(22) Before making application to the court under subsection (18) or not later than seven days after receiving notice of an application to the court under subsection (19), as the case may be, a corporation shall give notice to each dissenting shareholder who, at the date upon which the notice is given,
- (a)
- has sent to the corporation the notice referred to in subsection (10); and
- (b)
- has not accepted an offer made by the corporation under subsection (15), if such an offer was made,
of the date, place and consequences of the application and of the dissenting shareholder's right to appear and be heard in person or by counsel, and a similar notice shall be given to each dissenting shareholder who, after the date of such first mentioned notice and before termination of the proceedings commenced by the application, satisfies the conditions set out in clauses (a) and (b) within three days after the dissenting shareholder satisfies such conditions.
(23) All dissenting shareholders who satisfy the conditions set out in clauses (22) (a) and (b) shall be deemed to be joined as parties to an application under subsection (18) or (19) on the later of the date upon which the application is brought and the date upon which they satisfy the conditions, and shall be bound by the decision rendered by the court in the proceedings commenced by the application.
(24) Upon an application to the court under subsection (18) or (19), the court may determine whether any other person is a dissenting shareholder who should be joined as a party, and the court shall fix a fair value for the shares of all dissenting shareholders.
(25) The court may in its discretion appoint one or more appraisers to assist the court to fix a fair value for the shares of the dissenting shareholders.
(26) The final order of the court in the proceedings commenced by an application under subsection (18) or (19) shall be rendered against the corporation and in favour of each dissenting shareholder who, whether before or after the date of the order, complies with the conditions set out in clauses (22) (a) and (b).
(27) The court may in its discretion allow a reasonable rate of interest on the amount payable to each dissenting shareholder from the date the action approved by the resolution is effective until the date of payment.
(28) Where subsection (30) applies, the corporation shall, within ten days after the pronouncement of an order under subsection (26), notify each dissenting shareholder that it is unable lawfully to pay dissenting shareholders for their shares.
(29) Where subsection (30) applies, a dissenting shareholder, by written notice sent to the corporation within thirty days after receiving a notice under subsection (28), may,
- (a)
- withdraw a notice of dissent, in which case the corporation is deemed to consent to the withdrawal and the shareholder's full rights are reinstated; or
J-3
- (b)
- retain a status as a claimant against the corporation, to be paid as soon as the corporation is lawfully able to do so or, in a liquidation, to be ranked subordinate to the rights of creditors of the corporation but in priority to its shareholders.
(30) A corporation shall not make a payment to a dissenting shareholder under this section if there are reasonable grounds for believing that,
- (a)
- the corporation is or, after the payment, would be unable to pay its liabilities as they become due; or
- (b)
- the realizable value of the corporation's assets would thereby be less than the aggregate of its liabilities.
(31) Upon application by a corporation that proposes to take any of the actions referred to in subsection (1) or (2), the court may, if satisfied that the proposed action is not in all the circumstances one that should give rise to the rights arising under subsection (4), by order declare that those rights will not arise upon the taking of the proposed action, and the order may be subject to compliance upon such terms and conditions as the court thinks fit and, if the corporation is an offering corporation, notice of any such application and a copy of any order made by the court upon such application shall be served upon the Commission.
(32) The Commission may appoint counsel to assist the court upon the hearing of an application under subsection (31), if the corporation is an offering corporation.
J-4
APPENDIX K
List of 2004 Warrantholders
|
|---|
Name
| | Common Shares
Underlying Conversion
of convertible debentures
| | Common Shares
Underlying Conversion
of Accruing Interest on convertible debentures
| | Common Shares
Underlying Exercise
of Warrants Issued in Connection with the convertible debentures
|
|---|
|
Bristol Investment Fund Ltd.
10990 Wilshire Blvd Suite 1410
Los Angeles, CA 90024 | | 800,000 | | 150,000 | | 800,000 |
|
Clarion Capital Corporation
1801 East Ninth
Cleveland, Ohio
44114 | | 160,000 | | 30,000 | | 160,000 |
|
Morton A. Cohen Revocable Living Trust
1801 East 9th Str
Suite 1120
Cleveland, Ohio
44114 | | 40,000 | | 7,500 | | 40,000 |
|
Special Situations Fund III L.P.(1)
153E 53rd Str
55th floor
New York, NY
10022 | | 2,400,000 | | 450,000 | | 2,400,000 |
|
Special Situations Cayman Fund L.P.
153E 53rd Str
55th floor
New York, NY
10022 | | 800,000 | | 150,000 | | 800,000 |
|
Special Situations Private Equity Fund L.P.
153E 53rd Str
55th floor
New York, NY
10022 | | 800,000 | | 150,000 | | 800,000 |
|
SF Capital Partners Ltd.
3600 South Lake Drive
St. Francis, WI
53235 | | 1,600,000 | | 300,000 | | 1,600,000 |
|
City of Milford Pension and Retirement Fund
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 156,000 | | 29,250 | | 156,000 |
|
National Federation of Independent Business Corporate Account
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 38,400 | | 7,200 | | 38,400 |
|
Norwalk Employees Pension Plan
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 84,000 | | 15,750 | | 84,000 |
|
City of Stamford Firemen's Pension Fund
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 76,000 | | 14,250 | | 76,000 |
|
Asphalt Green Inc.(2)
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 16,000 | | 3,000 | | 16,000 |
|
K-1
|
|---|
Name
| | Common Shares
Underlying Conversion
of convertible debentures
| | Common Shares
Underlying Conversion
of Accruing Interest on convertible debentures
| | Common Shares
Underlying Exercise
of Warrants Issued in Connection with the convertible debentures
|
|---|
|
William B. Lazar
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 10,400 | | 1,950 | | 10,400 |
|
Lazar Foundation
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 16,000 | | 3,000 | | 16,000 |
|
Helen Hunt(3)
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 16,000 | | 3,000 | | 16,000 |
|
Barrie Ramsay Zesiger
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 24,000 | | 4,500 | | 24,000 |
|
Alexa Zesiger Carver
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 8,000 | | 1,500 | | 8,000 |
|
David Zesiger
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,000 | | 2,250 | | 12,000 |
|
HBL Charitable Unitrust
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,000 | | 2,250 | | 12,000 |
|
Jeanne L. Morency
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 10,400 | | 1,950 | | 10,400 |
|
Psychology Associates
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 4,800 | | 900 | | 4,800 |
|
Peter Looram
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 7,200 | | 1,350 | | 7,200 |
|
Meehan Foundation
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,800 | | 2,400 | | 12,800 |
|
Domenic J. Mizio
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 21,600 | | 4,050 | | 21,600 |
|
| Morgan Trust Co. of the Bahamas Ltd as Trustee U/A/D 11/30/93 | | 46,400 | | 8,700 | | 46,400 |
|
John Rowan
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 2,400 | | 450 | | 2,400 |
|
K-2
|
|---|
Name
| | Common Shares
Underlying Conversion
of convertible debentures
| | Common Shares
Underlying Conversion
of Accruing Interest on convertible debentures
| | Common Shares
Underlying Exercise
of Warrants Issued in Connection with the convertible debentures
|
|---|
|
Susan Iris Halpern
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 28,000 | | 5,250 | | 28,000 |
|
Theeuwes Family Trust, Felix Theeuwes Trustee
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,000 | | 2,250 | | 12,000 |
|
Robert K. Winters
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 800 | | 150 | | 800 |
|
Francois deMenil
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,800 | | 2,400 | | 12,800 |
|
Allan B. and Joanne K. Vidinsky 1993 Trust
c/o Zesiger Capital Group LLC
320 Park Ave
New York, NY 10022 | | 12,000 | | 2,250 | | 12,000 |
|
Walker Smith International Fund, Ltd
300 Crescent Court Suite 880
Dallas, TX 75201 | | 497,120 | | 93,210 | | 497,120 |
|
Walker Smith Capital L.P.
300 Crescent Court Suite 880
Dallas, TX 75201 | | 79,360 | | 14,880 | | 79,360 |
|
Walker Smith Capital (Q.P.) L.P.
300 Crescent Court Suite 880
Dallas, TX 75201 | | 423,520 | | 79,410 | | 423,520 |
|
WS Opportunity Fund 75201International, Ltd.
300 Crescent Court Suite 880
Dallas, TX | | 226,640 | | 42,495 | | 226,640 |
|
WS Opportunity Fund L.P.
300 Crescent Court Suite 880
Dallas, TX | | 167,280 | | 31,365 | | 167,280 |
|
WS Opportunity Fund (Q.P.) L.P.
300 Crescent Court Suite 880
Dallas, TX | | 206,080 | | 38,640 | | 206,080 |
|
Strauss Partners L.P.
605 3rd Ave
New York, NY
10158-3698 | | 120,000 | | 22,500 | | 120,000 |
|
Strauss-GEPT Partners
605 3rd Ave
New York, NY
10158-3698 | | 80,000 | | 15,000 | | 80,000 |
|
YMG Private Wealth Opportunities Fund
c/o Royal Bank of Canada
200 Bay St South
Tower S-L Level
Toronto, ON M5J 2J5 | | 250,000 | | 46,875 | | 250,000 |
|
K-3
|
|---|
Name
| | Common Shares
Underlying Conversion
of convertible debentures
| | Common Shares
Underlying Conversion
of Accruing Interest on convertible debentures
| | Common Shares
Underlying Exercise
of Warrants Issued in Connection with the convertible debentures
|
|---|
|
YMG Capital Management Inc.
c/o Investor Co.
77 Bloor Street W.
2nd Floor
Toronto, ON M4Y 2T1 | | 30,000 | | 5,625 | | 30,000 |
|
YMG Capital Management Inc.
c/o Investor Co.
77 Bloor Street W.
2nd Floor
Toronto, ON M4Y 2T1 | | 300,000 | | 56,250 | | 300,000 |
|
Rise J. Glasenberg
c/o Investor Co. IFT #6N0674E
77 Bloor Street W
2nd Floor
Toronto, On
M4Y 2T1 | | 55,000 | | 10,313 | | 55,000 |
|
Kinetic Capital "TNT" Limited Partnership
1460-777 Atornely Street
Vancouver, BC
V6Z 1S4 | | 400,000 | | 75,000 | | 400,000 |
|
Hauck and Aufhauser Luxembourg
Deputy Treasurer
23 Avenue dela Liberte
Luxembourg L-1931
Luxembourg | | 100,000 | | 18,750 | | 100,000 |
|
| Oppemheim Prumerica Asset Management on behalf of FCP OP MEDICAL BioHe@lth-Trends | | 480,000 | | 90,000 | | 480,000 |
|
Rikare Management Inc.
1 Queen Street East, Suite 2300
Toronto, ON
M5C 2W5 | | 30,000 | | 5,625 | | 30,000 |
|
| | | 10,655,000 | | 1,997,813 | | 10,655,000 |
|
- (1)
- MGP Advisors Limited ("MGP") is the general partner of Special Situations Fund III, L.P. AWM Investment Company, Inc. ("AWM") is the general partner of MGP and the general partner of and investment adviser to the Special Situations Cayman Fund, L.P. MG Advisers, L.L.C. ("MG") is the general partner of and investment adviser to the Special Situations Private Equity Fund, L.P. Austin W. Marxe and David M. Greenhouse are the principal owners of MGP, AWM and MG and are principally responsible for the selection, acquisition and disposition of the portfolio securities by each investment adviser on behalf of its fund.
- (2)
- Includes 6,000 common shares held by the Asphalt Green Defined Contribution Plan.
- (3)
- Includes 17,000 shares held by the Helen Hunt Alternatives Fund, of which Helen Hunt is the President.
K-4
The following table sets out the names of the person or persons holding voting or dispositive power over the common shares and warrants, if different from the beneficial holder:
|
|---|
Holder of Common Shares or Warrants
| | Person or Persons Holding Voting or Dispositive Power
over the Common Shares and Warrants
|
|---|
|
| Bristol Investment Fund Ltd. | | Paul Kessler, as the manager of Bristol Capital Advisors, LLC, investment manager to Bristol Investment Fund Ltd. |
|
Clarion Capital Corporation
Clarion Offshore Fund
Clarion Partners, L.P.
Dynamic Equity Hedge Fund | | Morton A. Cohen, Chairman |
|
| Morton A. Cohen Revocable Living Trust | | Morton A. Cohen as Trustee |
|
Special Situations Fund III L.P.(4)
Special Situations Cayman Fund, L.P.
Special Situations Private Equity Fund, L.P. | | Austin Marxe and David Greenhouse as Executive Officers of the Investment Advisers to the Funds |
|
| SF Capital Partners Ltd. | | Michael A. Roth and Brian J. Stark |
|
| Lazar Foundation | | William B. Lazar as Trustee |
|
| Walker Smith International Fund, Ltd., Walker Smith Capital L.P., and Walker Smith Capital (QP) L.P. | | Patrick P. Walker, Reid S. Walker, and G. Stacy Smith |
|
| WS Opportunity Fund International, Ltd., WS Opportunity Fund L.P., and WS Opportunity Fund (QP) L.P. | | G. Stacy Smith and Reid S. Walker |
|
| Strauss Partners, L.P. and Strauss-GEPT Partners | | Melville Strauss, Managing Principal |
|
| YMG Private Wealth Opportunities Fund and YMG Capital Management Inc. | | Neil Nisker, President, YMG Private Wealth Management |
|
| Sherfam Inc. | | Barry Sherman, President |
|
| Kinetic Capital "TNT" Limited Partnership | | Frank Barker or Dallas Ross as General Partners |
|
| Hauck and Aufhauser Luxembourg | | Christian Henkgen and Mario Warmy as Officers |
|
| Oppenheim Prumerica Asset Management S.à.r.l on behalf of FCP OP MEDICAL BioHe@lth-Trends | | Heinz Heisterkamp as Managing Director and Julia Brauckmann as Senior Associate of Oppenheim Prumerica Asset Management S.à.r.l. |
|
- (4)
- Mr. Robert Matjeles and Mr. John F. Carlson, members of our board of directors, are nominees of the Special Situations Funds.
K-5
APPENDIX L

PROXY
ANNUAL AND SPECIAL MEETING OF SHAREHOLDERS
JULY 18, 2005
This proxy is solicited on behalf of the management of World Heart Corporation (the Corporation). The undersigned hereby appoints C. Ian Ross, Chair of the Corporation or failing him, Jal S. Jassawalla, President Chief Executive Officer of the Corporation or, instead of either of them, .............................................................. .............................................. as nominee for the undersigned, with the power to appoint their substitute and hereby authorizes them to represent and vote as designated below all the Common Shares of the Corporation held of record by the undersigned at theAnnual and Special Meeting of Shareholders to be held on Monday, July 18, 2005, or at any adjournment or adjournments thereof in the same manner, to the same extent and with the same powers as if the undersigned was personally present at the said meeting or any adjournment thereof.
Shareholders have the right to appoint a person (who need not be a shareholder) to attend and act for them and on their behalf other than the nominees designated above and may exercise such right by inserting the name of their nominee in the blank space provided for that purpose.
- 1.
- To elect directors – for nominees listed (mark either (a) or (b)) (Instructions:To withhold authority to vote for any individual nominee strike a line through the nominee's name in the list below):
- 2.
- To appoint auditors and to authorize the directors to fix their remuneration (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 3.
- Approval of the MedQuest Resolution approving the issuance of 9,300,000 common shares of the Corporation to MedQuest Products, Inc., conditional on the approval of the Maverick Resolution and Warrant Amendment Resolution (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 4.
- Approval of the Maverick Resolution approving the issuance of 8,888,889 common shares of the Corporation to Maverick Venture Management LLC, conditional on the approval of the MedQuest Resolution and Warrant Amendment Resolution (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 5.
- Approval of the Warrant Amendment Resolution approving an amendment to the exercise price of warrants issued by the Corporation in September, 2004 (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
L-1
- 6.
- Approval of ESOP Resolution #1 approving an increase in the number of common shares reserved for issuance under the World Heart Corporation Employee Stock Option Plan (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 7.
- Approval of ESOP Resolution #2 approving the removal of the restriction World Heart Corporation Employee Stock Option Plan prohibiting aggregate grants to individuals of more than 5% of the issued and outstanding (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 8.
- Approval of the Option Amendment Resolution approving the amendment of certain options granted by the Corporation to executive officers and directors, conditional on the approval of ESOP Resolution #1 (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
- 9.
- Approval of the Continuance Resolution as a special resolution, approving the continuance of the Corporation under theCanada Business Corporations Act (mark either (a), (b) or (c)):
- (a)
- o FOR
- (b)
- o AGAINST
- (c)
- o WITHHOLD FROM VOTING
In addition, the undersigned appoints such person as proxy to vote and act as aforesaid on all other matters that may properly come before the meeting.
This proxy when properly executed will be voted in the manner directed herein by the undersigned shareholder. If no choice is indicated in respect of any matter in the space provided above, the common shares represented by this proxy will be voted FOR such matter.
This proxy form should be read in conjunction with the accompanying Notice of Annual and Special Meeting and Management Information Circular.
| | | | | |
Name of Shareholder (Please Print) | | | | |
| | | | | |
| | | | | |
| | | | | |
Signature | | | |
Dated |
This proxy must be delivered no later than 5 p.m. on Friday, July 15, 2005 to CIBC Mellon Trust Company, Attention: Proxy Department, in the envelope provided for this purpose, or hand delivered to CIBC Mellon Trust Company at 200 Queen's Quay East, Unit 6, Toronto, Ontario, M5A 4K9.
If this proxy is not dated in the space provided, it will be deemed to bear the date on which it is mailed to you by management.
Please sign exactly as your shares are registered. When the shares are held by joint tenants, both must sign. When signing as attorney, executor, administrator, trustee or guardian, please give full title as such. If partnership, please sign in partnership name by an authorized person. If the proxy is being given by a corporation, the proxy must be signed under seal by the corporation's duly authorized officers or, if not signed under seal, the office held by each person signing on behalf of the corporation must be indicated. The name of the corporation must be written-in above the signature of the officers.
L-2
QuickLinks
WORLD HEART CORPORATION 1 Laser Street, Ottawa, Ontario, Canada K2E 7V1NOTICE OF ANNUAL AND SPECIAL MEETING OF SHAREHOLDERSWORLD HEART CORPORATION MANAGEMENT INFORMATION CIRCULAR June 13, 2005Performance GraphEMPLOYEE STOCK OPTION BENEFITSCONSENT OF INDEPENDENT ACCOUNTANTSAUDITORS' CONSENTCONSENT OF INDEPENDENT ACCOUNTANTSAPPENDIX A-1 MEDQUEST RESOLUTIONAPPENDIX A-2 MAVERICK RESOLUTIONAPPENDIX A-3 WARRANT AMENDMENT RESOLUTIONAPPENDIX A-4 ESOP RESOLUTION #1APPENDIX A-5 ESOP RESOLUTION #2APPENDIX A-6 OPTION AMENDMENT RESOLUTIONAPPENDIX A-7 CONTINUANCE RESOLUTIONMANAGEMENT'S STATEMENT OF RESPONSIBILITYAUDITORS' REPORT TO THE SHAREHOLDERS OF WORLD HEART CORPORATIONCOMMENTS BY AUDITORS FOR U.S. READERS ON CANADA-U.S. REPORTING DIFFERENCESWORLD HEART CORPORATION CONSOLIDATED BALANCE SHEETS (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF OPERATIONS (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (Deficiency) (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF CASH FLOW (United States Dollars)WORLD HEART CORPORATION NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTSMANAGEMENT'S STATEMENT OF RESPONSIBILITYAUDITORS' REPORT TO THE SHAREHOLDERS OF WORLD HEART CORPORATIONWORLD HEART CORPORATION CONSOLIDATED BALANCE SHEETS (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF OPERATIONS (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (Deficiency) (United States Dollars)CONSOLIDATED STATEMENTS OF CASH FLOWAPPENDIX B-3 WORLD HEART CORPORATION CONSOLIDATED BALANCE SHEETS (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF OPERATIONS AND ACCUMULATED DEFICIT (United States Dollars)WORLD HEART CORPORATION CONSOLIDATED STATEMENTS OF CASH FLOW (United States Dollars)APPENDIX C-1 MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSAPPENDIX C-2 MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSAPPENDIX C-3 MANAGEMENT'S DISCUSSION AND ANALYSISAPPENDIX DCONTENTSREPORT OF INDEPENDENT AUDITORSMEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) BALANCE SHEETSMEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) STATEMENTS OF OPERATIONSMEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) STATEMENT OF STOCKHOLDERS' DEFICIT Period from Inception (December 16, 1993) to March 31, 2005MEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) STATEMENTS OF CASH FLOWSMEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) STATEMENTS OF CASH FLOWSMEDQUEST PRODUCTS, INC. (A Development Stage Enterprise) NOTES TO FINANCIAL STATEMENTSAPPENDIX E MEDQUEST PRODUCTS, INC. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSAPPENDIX FWORLD HEART CORPORATION U.S. GAAP UNAUDITED PRO FORMA COMBINED CONDENSED STATEMENTS OF OPERATIONS For the fiscal year ended December 31, 2004WORLD HEART CORPORATION UNAUDITED PRO FORMA COMBINED CONDENSED STATEMENTS OF OPERATIONS For the three months ended March 31, 2005WORLD HEART CORPORATION UNAUDITED PRO FORMA COMBINED CONDENSED BALANCE SHEET As at March 31, 2005NOTES TO THE U.S. GAAP UNAUDITED PRO FORMA COMBINED CONDENSED FINANCIAL STATEMENTSWORLD HEART CORPORATION CANADIAN GAAP UNAUDITED PRO FORMA COMBINED CONDENSED STATEMENTS OF OPERATIONS For the fiscal year ended December 31, 2004WORLD HEART CORPORATION CANADIAN GAAP UNAUDITED PRO FORMA COMBINED CONDENSED BALANCE SHEET As at December 31, 2004NOTES TO THE CANADIAN GAAP UNAUDITED PRO FORMA COMBINED CONDENSED FINANCIAL STATEMENTSAPPENDIX GTABLE OF CONTENTSASSET PURCHASE AGREEMENTARTICLE 1 — INTERPRETATIONARTICLE 2 — SALE AND PURCHASEARTICLE 3 — REPRESENTATIONS AND WARRANTIESARTICLE 4 — COVENANTSARTICLE 5 — CONDITIONSARTICLE 6 — CLOSING ARRANGEMENTSARTICLE 7 — GENERALAMENDMENT NO. 1 TO THE ASSET PURCHASE AGREEMENTARTICLE 1 — INTERPRETATIONARTICLE 2 — AMENDMENTSARTICLE 3 — GENERALAPPENDIX HPURCHASE AGREEMENTRecitalsAMENDMENT NO. 1 TO THE PURCHASE AGREEMENTARTICLE 1 — INTERPRETATIONARTICLE 2 — AMENDMENTSARTICLE 3 — GENERALCONVERSION AND EXERCISE AGREEMENTAPPENDIX J RIGHTS OF DISSENTING SHAREHOLDERSAPPENDIX KList of 2004 WarrantholdersAPPENDIX L

