Exhibit 99.1

Investor Presentation
August 3, 2011
Forward Looking Statements
2
Statements contained in this presentation, including those pertaining to clinical research, commercialization plans, and
intellectual property protection, other than statements of historical fact, are forward-looking statements subject to a number
of uncertainties that could cause actual results to differ materially from statements made. These statements, including their
underlying assumptions, are subject to risks and uncertainties and are not guarantees of future performance. Results may
differ due to various factors, such as our ability to obtain additional financing to support our clinical programs and operations;
costs and delays associated with research and development, manufacturing, pre-clinical testing and clinical trials for our
products and next-generation candidates, such as the MiFlow™ VAD and the PediaFlow® VAD; our dependence on a limited
number of products; our ability to manufacture, sell and market our products; decision, and the timing of decisions made by
health regulatory agencies regarding approval of our products; competition from other products and therapies for heart
failure; continued slower than anticipated destination therapy adoption rate for VADs; limitations on third-party
reimbursements; our ability to obtain and enforce in a timely manner patent and other intellectual property protection for
our technology and products; our ability to avoid, either by product design, licensing arrangement or otherwise, infringement
of third parties’ intellectual property; our ability to enter into corporate alliances or other strategic relationships relating to
the development and commercialization of our technology and products; loss of commercial market share to competitors due
to our financial condition, timing of restarting or completing our clinical studies, and future product development by
competitors; our ability to remain listed on the NASDAQ Capital Market; as well as other risks and uncertainties set forth
under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2010.
Additionally, while statistics and data included in this presentation are offered in good faith and believed to be accurate and
current, the Company makes no representations, warranties or guarantees, express or implied, respecting the accuracy or
completeness thereof.
The statements presented in this presentation speak only as of today’s date. Please note that except as required by
applicable law, we undertake no obligation to revise or update publicly any forward-looking statements for any reason.
Presenters/Management Team
3
Name
Prior Experience
Years Experience
Alex Martin
Chief Executive Officer
Edwards Lifesciences: President, North America
Johnson & Johnson (Cordis): SVP, International
29
Morgan Brown
EVP & Chief Financial Officer
Lifetree Clinical Research: SVP & CFO
NPS Pharmaceuticals: VP Finance & Treasurer
KPMG: Senior Audit Manager
18
WorldHeart Highlights
Two advanced Ventricular Assist Device programs
MiFlowTM VAD – Fully magnetically levitated miniature rotary blood pump;
designed to provide long-term circulatory support for earlier-stage heart failure
with minimally invasive implant
PediaFlow® – Fully magnetically levitated miniature rotary blood pump for
children and infant population
High complication rates are associated with current VAD
technologies
MiFlow and PediaFlow designed to address significant issues of
current devices including stroke, bleeding and device failures
Encouraging data on vWF preservation with magnetic levitation pumps
4
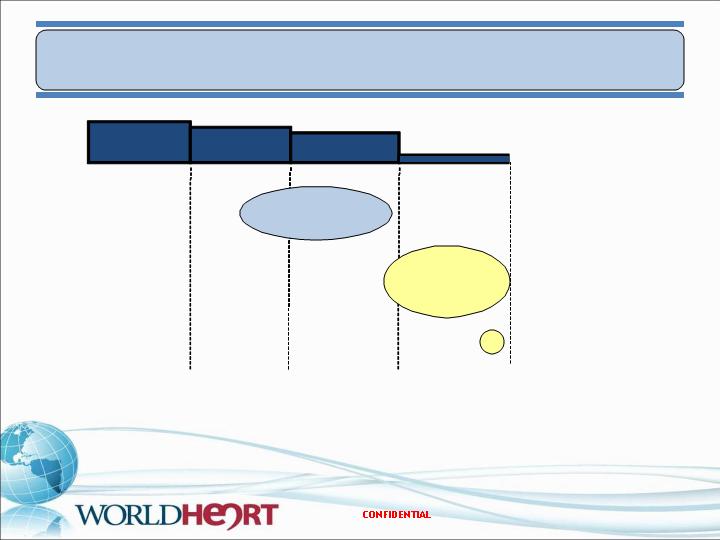
Heart Failure Device Market Segments
American College of Cardiology Consensus Report on MCS 2000 for class size.
5
Class I
(1.7M)
Class II
(1.6M)
Class III
(1.4M)
Class IV
(0.36M)
Resynchronization
Therapy
Cardiac
100,000 Pts
$9 Billion
Potential
Market
BTT
LVAD Destination
Therapy (DT)
4,000 Pts
$360 Million
Market Needs
Better Clinical Outcomes
Stroke, bleeding, infections and device failures
Less invasive implantation
Smaller device
Non-sternotomy implant
Minimal by-pass time
Improved user convenience and device interface
Easier to use
Remote monitoring / diagnostic capabilities
Smaller peripherals
Fully implantable system (Longer-term view)
6
Current Technologies Have Not Effectively
Addressed Complication Rates
7
1 – FDA Summary of Safety and Effectiveness Data, 2008.
2 – Slaughter MS, et al. Advanced Heart Failure Treated with Continuous –Flow Left Ventricular Assist Device. NEJM 2009.
3- Aaronson, Late Breaking Clinical Trial , AHA November 2010 Presentation.
Complication rates of existing devices need significant improvement
Bleeding rates, infection, and thrombus/stroke
Complication Rates
Heartmate II
(BTT)1
Heartmate II (DT)2
HeartWare (BTT)3
HeartWare (BTT) (Per
Patient Year) 3
Generation
2nd
2nd
3rd
3rd
Survival
80% (6 mo.)
68% (1 yr.)
92% (6 mo.)
91%
Disabling stroke
10%
18%
10%
16%
Surgery due to bleeding
29%
30%
15%
27%
Surgical repair or
replacement
5%
9%
5%
7%
Infection
29%
35%
17%
32%
Approved
2008
2010
CE Mark
CE Mark
Recent Article on Acquired von Willebrand
Syndrome
8
Acquired von Willebrand Syndrome in Patients with an Axial Flow Left Ventricular
Assist Device
Anna L. Meyer, Doris Malehsa, Christoph Bara, Ulrich Budde, Mark S. Slaughter, Axel
Haverich and Martin Strueber
Circ Heart Fail published online Aug 25, 2010;
DOI: 10.1161/CIRCHEARTFAILURE.109.877597
Circulation: Heart Failure is published by the American Heart Association. 7272 Greenville Avenue, Dallas,
TX 72514
Copyright © 2010 American Heart Association. All rights reserved. Print ISSN: 1941-3289. Online ISSN:
1941-3297
“…future efforts must address the challenges of long term
support including the impact of the devices on inflammation,
platelet function and the coagulation system.”
Recent Article on Acquired von Willebrand
Syndrome – cont.
9
European Journal of Cardio-thoracic Surgery 35 (2009) 1091-1093
Case Report
Acquired von Willebrand syndrome after exchange of the HeartMate XVE to
the HeartMate II ventricular assist device
Doris Malehsa, Anna L. Meyer, Christoph Bara, Martin Strüber
Department of Cardiothoracic, Transplant and Vascular Surgery, Hanover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany
Received 7 October 2008; received and revised form 29 January 2009; accepted 29 January 2009; Available online 20 March 2009
“…design of rotary blood pumps should not only focus on
mechanical stability and hemolysis, but also on other blood
components like vWF and platelets.”
Levacor® - Preliminary Performance Evaluation
In Independent Investigator Biomarker Study
The blood handling characteristics of Levacor® demonstrate reduced impact on
von Willebrand Factor (“VWF”)
VWF is a blood glycoprotein involved in hemostasis
10
Preliminary Results of Independent Investigator Biomarker Study 1
PRE
ON LVAD
ON VAD
ON LVAD
ON LVAD
POST
Pre-op
14 days
30 days
60 days
90 days
30 days Post-TX
Levacor
100%
(6/6)
100%
(6/6)
100%
(6/6)
100%
(6/6)
100%
(5/5) 3
100%
(1/1)
CF VAD
100%
(10/10)
0%
(0/9) 2
0%
(0/10)
0%
(0/10)
0%
(0/10)
100%
(2/2)
P-value*
1
<0.001
<0.001
<0.001
<0.001
1 - Results from independent investigator initiated study by Dr. James W. Long, M.D., Ph.D, Director of Advanced Cardiac Care at INTEGRIS
Baptist Medical Center and a consultant to WorldHeart.
2 - Failed to draw blood sample on one patient.
3 - Only 5 of 6 subjects arrived at 90 day mark as of November 30, 2010.
Parameters Evaluated: Preservation of vWF Multimers (eletrophoresis), vWF Functionality (vWF:Ag) and vWF Collagen Binding (vWF:CB)

Proprietary Product Design
2nd Generation
3rd Generation
4th Generation
Axial Flow Pumps
(~10,000 rpm)
Jarvik (Jarvik Heart)
HeartMate II (Thoratec)
Centrifugal Flow Pump
(2 – 3,000 rpm)
HVAD (HeartWare)
DuraHeart (Terumo)*
Next Gen Devices
MVAD (Heartware)
Heartmate III (Thoratec)
Heartmate X (Thoratec)
DuraHeart II (Terumo)
11
5th Generation
Miniature Maglev
Pump
(10-14,000 rpm)
MiFlow
* Uses mechanical motor bearings
MiFlow™ is designed to be the smallest, bearingless, fully maglev VAD
with shortest blood flow pathway which reduces blood damaging shear
forces. Maglev should enable improved blood handling and significantly
improves durability.
MiFlow is designed for minimally invasive non-sternotomy
with minimal by-pass time
Proprietary maglev technology, utilized across the product portfolio,
has demonstrated improved hemostatic characteristics (minimizing risk
of bleeding incidents by preservation of von Willebrand Factor)
With the capability of employing a wide range of flow rates, magnetic
levitation is designed to enable physicians to wean patients off the
device and rebuild heart strength
WorldHeart Proprietary Product Design

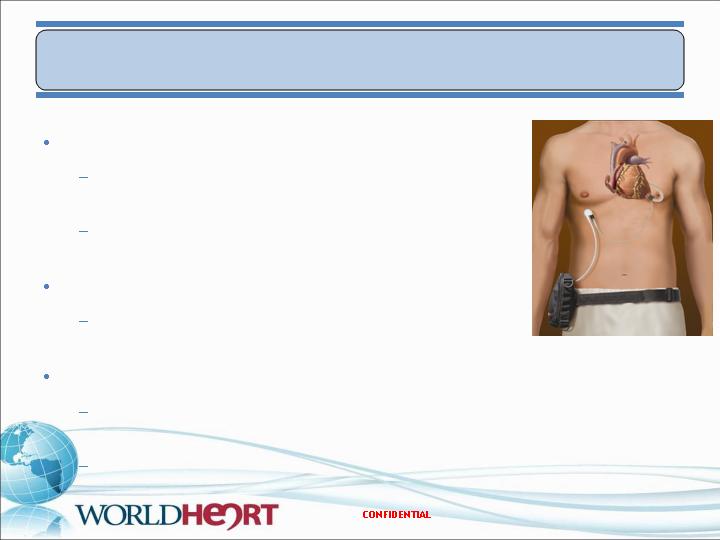
MiFlow™ Overview
12
MiFlow VAD is Designed with Following Key Features:
Fully magnetically levitated rotor
Miniaturized profile (approximately the size of a AA battery)
Wide operating range (2 L/min to 6 L/min)
Pump integrated within inflow cannula tip
Minimal blood / device surface interface
Innovative peripherals
Maglev clinical experience demonstrates preservation of von
Willebrand factor
Designed to address a broad range of patient populations
Key Features
13
Fully magnetically levitated rotor:
Designed for optimized flow path (wide clearances and reduced shear
rates)
Designed to improved blood handling (bleeding, pump thrombosis and
strokes)
Designed to preserve von Willebrand factor
Miniaturized Profile
Designed to allow non-sternotomy placement that will provide less
invasive surgery, faster recovery, reduced clinical complications and
shorter hospital stay
Designed to allow for wider range of patient anatomy
Wide operating range (2 L/min to 6 L/min)
Majority of applicable Class IV heart failure patients
100% of applicable Class III heart failure patients
Key Features
14
Pump integrated within inflow cannula tip
Designed to significantly reduce inflow path
surface area
Designed to improve device placement and
orientation
Minimal blood / device surface interface
Designed to minimize foreign body blood interaction
and inflammatory response
Innovative peripherals
Safety (modular percutaneous lead, integrated back-up
battery in controller)
Patient Comfort (controller and battery ~ 2 lbs total)

PediaFlow VAD (Basis for MiFlow VAD)
Small size
Full magnetic levitation
Simplest (single axis)
Bearingless
No wear mechanisms
Simple blood path
(no secondary flow path)
Inflow
Outflow
Rotor
Stator
blades
Impellor
blades
15
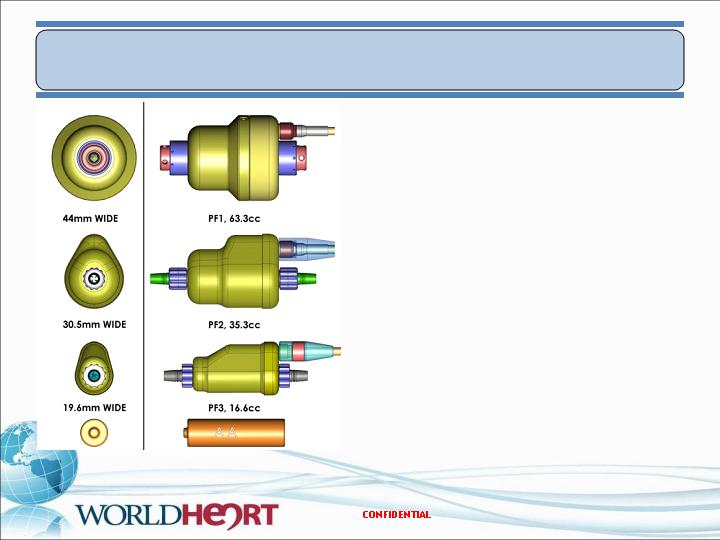
PediaFlow Prototype Development
PF1 – Proof of principle
Implants to 17 days
PF2 – Hemodynamic optimization
Implants to 70 days
PF3 – Miniaturized pump
Implant of 72 days
16

In Vivo Results – PF1
Post-explant device analysis
First chronic animal study – 6 Days
No thrombus deposition on pump surfaces
Impeller Blades
Stator vanes
Pump Housing
17
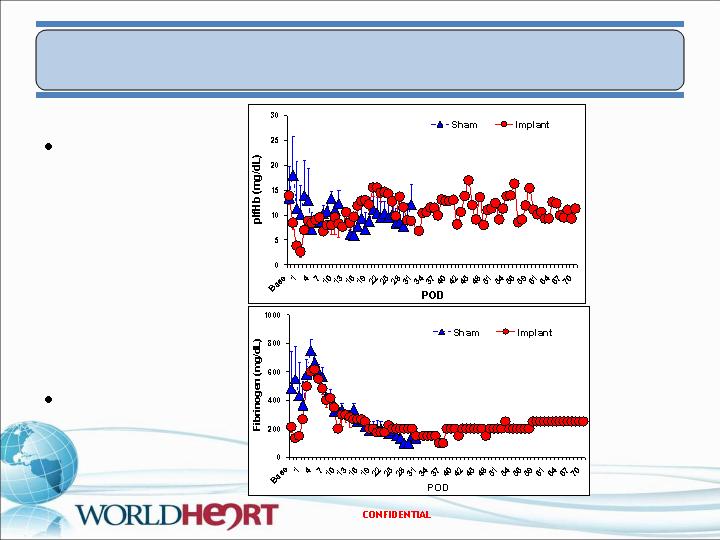
In Vivo Results – PF2
Hemolysis
Fibrinogen
18
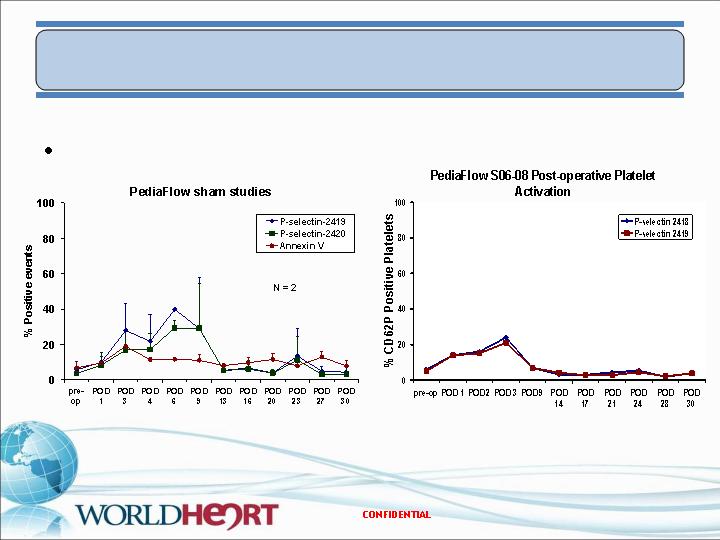
In Vivo Results – PF2
Platelet Activation
Pump implant
Sham surgery
19
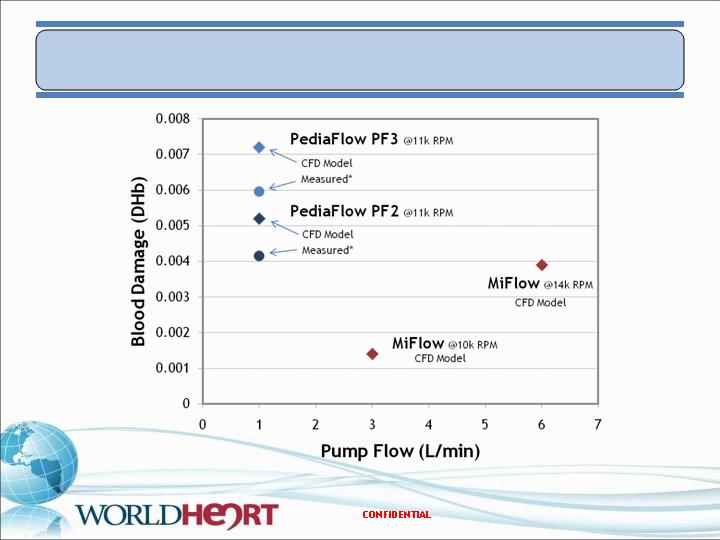
PediaFlow/MiFlow – Blood Damage Modeling
20
*Scaled from NIH measurements
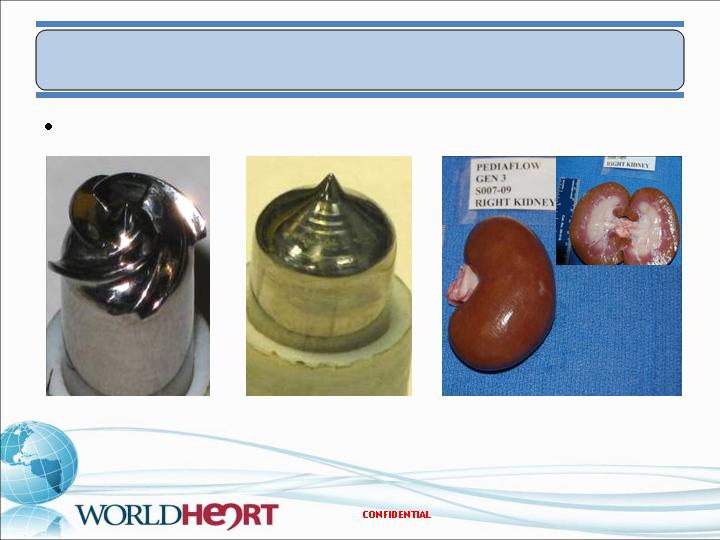
In Vivo Results – PF3
Post-explant device analysis
Implant duration – 72 Days
No deposition on pump surfaces; kidneys free of infarcts
21

Blood Path Comparison
VAD
Blood-Contacting
Area (cm2 )
Priming Volume
(ml)
Annular Gap
Cross-section
(mm2 )
Levacor
268
20.7
--
MiFlow
36
3.4
79
PediaFlow PF3
26
2.3
45
22
MiFlow and
PediaFlow PF4 rotors
(SLA components for
in vitro testing)
- Blood paths for PediaFlow and MiFlow are similar
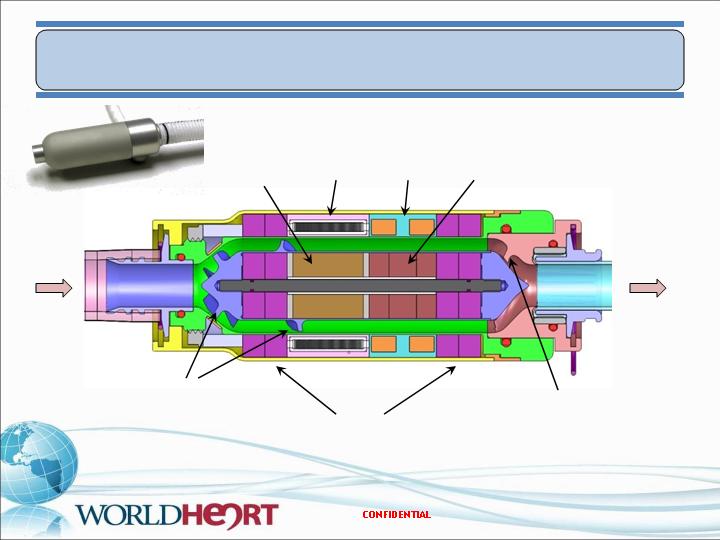
MiFlow Miniaturized VAD - Pump Cross-Section
23
Inflow
Outflow
Stator
blades
Permanent magnet
radial support
Motor
stator
Voice
coil
Motor
magnets
Axial
magnets
Impellor
blades
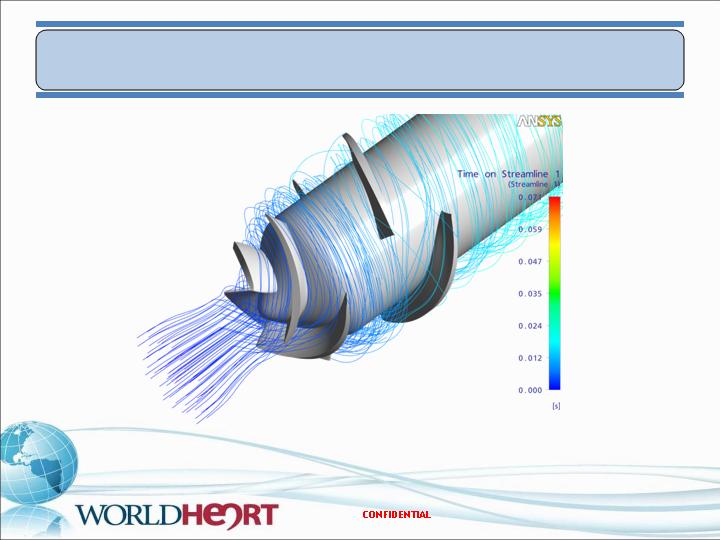
Streamlines
Miniaturized VAD – CFD Evaluation
24
Q=5.0L/min, 14K rpm
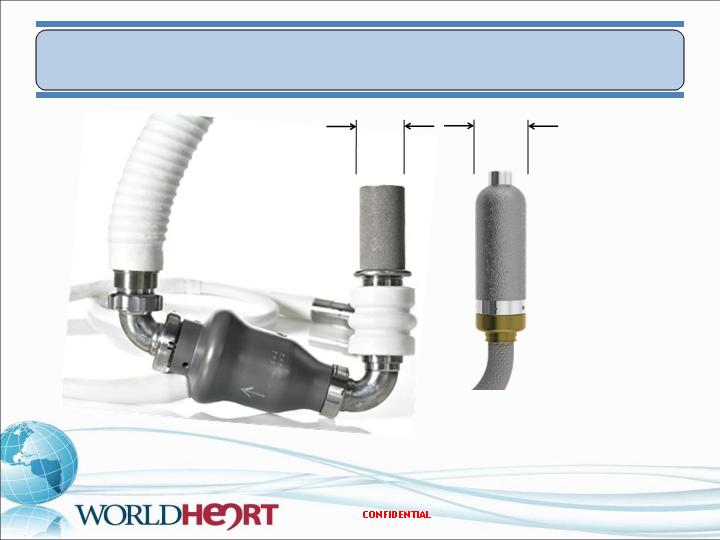
MiFlow – Size Comparison
25
HeartMate II
MiFlow
19 mm
21 mm
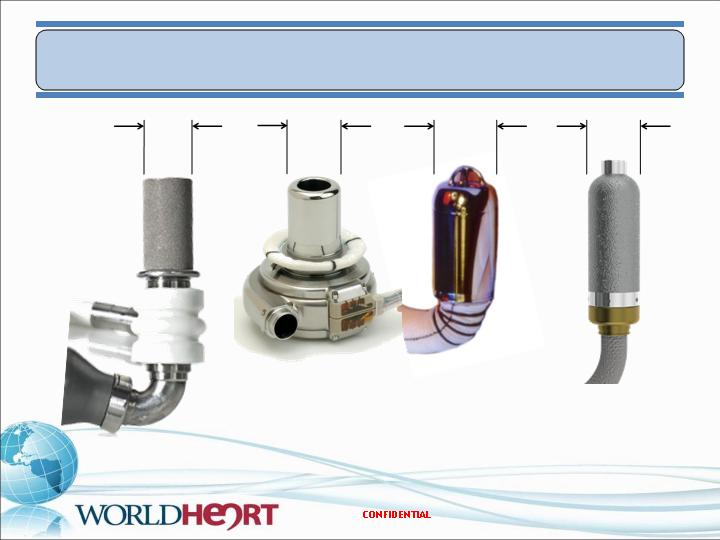
MiFlow – Size Comparison
26
19 mm
21 mm
21 mm
25 mm
HeartMate II
HVAD
Jarvik 2000
MiFlow
63 cc, 281 gms
50 cc, 145 gms
25 cc, 90 gms
20 cc, 65 gms
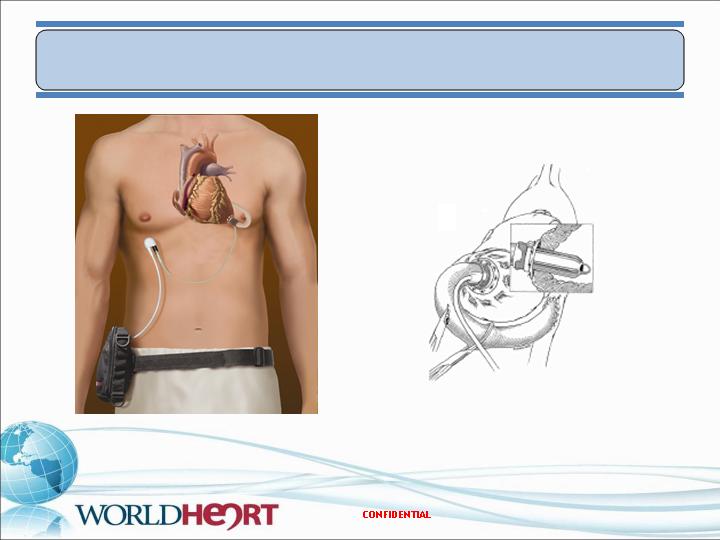
Minaturized VAD Configuration – w/ perc lead
27
Outflow to descending aorta
Experience with Jarvik 20001,2
validates this anatomic placement
1- Frazier OH. Implantation of the Jarvik 2000 LVAD without use of CPB. Ann Thor Surg 2003:75:1028-30
2-Selzman HC. Off-Pump Insertion of the Jarvik 2000 LVAD. CTS Net Feb 21, 2008

Development Timeline – MiFlow VAD
28
Development costs estimated to $35M (perc lead configuration)
28
Financial Summary
Cash Balance (3/31/11)
$17.7M
Monthly Cash Burn
$1.2M-$1.3M
52 Week Stock Price (High and
Low) *
$3.26-$.45
Shares Outstanding
26.7M
Options Outstanding
2.1M
Warrants Outstanding
13.3M
29
* As of 8/1/11
Strong Intellectual Property Protection
30
Strong IP position for single-active axis Maglev rotary pump
IP focused on latest-generation technologies
(bearingless, magnetically levitated/driven)
Rotary pumps (Levacor, PediaFlow, MiFlow) –
15 issued patents (13 owned or exclusively licensed,
2 non-exclusively licensed) and 5 pending
Transcutaneous Energy Transfer (TET) System –
1 issued patent (exclusively licensed)
WorldHeart Highlights
Two advanced Ventricular Assist Device programs
MiFlowTM VAD – Fully magnetically levitated miniature rotary blood pump;
designed to provide long-term circulatory support for earlier-stage heart failure
with minimally invasive implant
PediaFlow® – Fully magnetically levitated miniature rotary blood pump for
children and infant population
High complication rates are associated with current VAD
technologies
MiFlow and PediaFlow designed to address significant issues of
current devices including stroke, bleeding and device failures
Encouraging data on vWF preservation with magnetic levitation pumps
31
End
32































