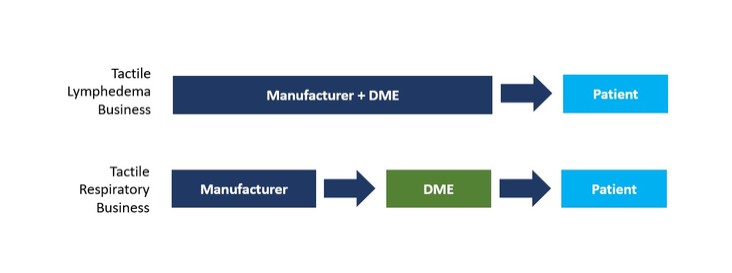
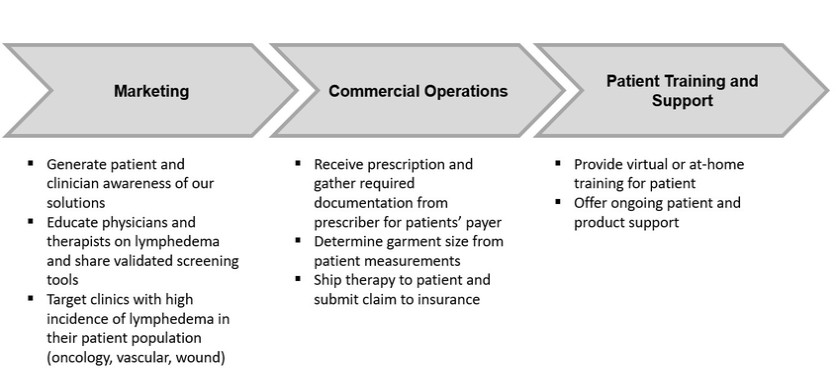
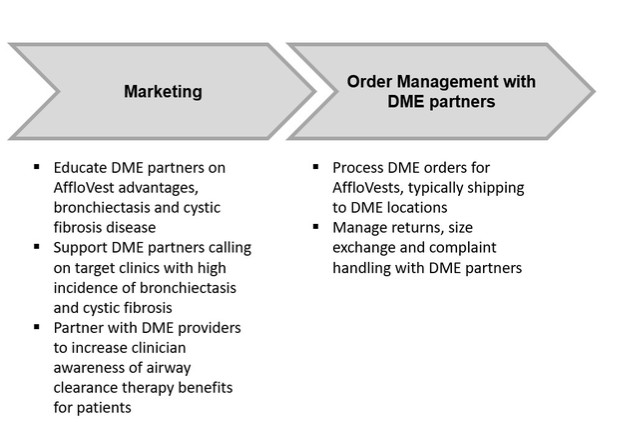
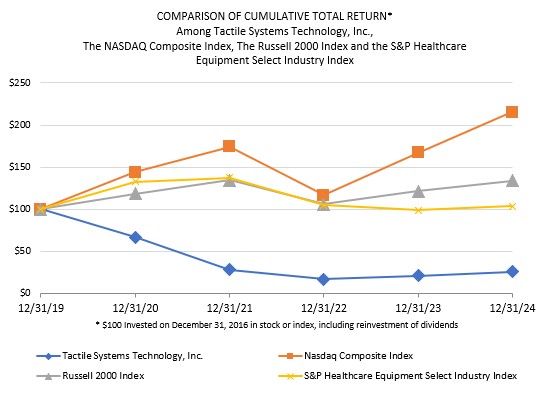
or business from paying, offering, promising or authorizing the provision of money (such as a bribe or kickback) or anything else of value (such as an improper gift, hospitality, or favor), directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision in order to assist the individual or business in obtaining, retaining, or directing business or other advantages (such as favorable regulatory rulings). In addition to the FCPA, there are other federal and state anti-corruption laws to which we may be subject, including, the U.S. domestic bribery statute contained in 18 USC § 201 (which prohibits bribing U.S. government officials) and the U.S. Travel Act (which in some instances addresses private-sector or commercial bribery both within and outside the United States). Also, a number of other countries have their own domestic and international anti-corruption laws, such as the UK Bribery Act 2010.
We could be held liable under the FCPA and other anti-corruption laws for the illegal activities of our employees, representatives, contractors, collaborators, agents, subsidiaries, or affiliates, even if we did not explicitly authorize such activity. Although we will seek to comply with anti-corruption laws, there can be no assurance that all of our employees, representatives, contractors, collaborators, agents, subsidiaries or affiliates will comply with these laws at all times. Violation of these laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and/or debarment from contracting with certain governments or other persons, importation restriction, the loss of export privileges, reputational harm, adverse media coverage and other collateral consequences. In addition, our directors, officers, employees, and other representatives who engage in violations of the FCPA and certain other anti-corruption statutes may face imprisonment, fines and penalties.
State and federal transparency/reporting requirements. As part of the Patient Protection and Affordable Care Act, or ACA, the Federal government has created a transparency program known as Open Payments (the Physician Payments Sunshine Act) which requires applicable manufacturers of drugs, devices, biologicals and medical supplies to report annually to the CMS, an agency within the U.S. Department of Health and Human Services, or HHS, information related to payments and other transfers of value provided to physicians and teaching hospitals (“covered recipients”) and certain ownership and investment interests held by physicians and their immediate family members. Beginning in 2021, tracking and reporting was expanded to include additional covered recipients, namely physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists and certified nurse-midwives. Failure to submit timely, accurate and complete information may result in significant civil monetary penalties for "knowing failures to report." Certain states have their own versions of the Physician Payments Sunshine Act, and may also require implementation of commercial compliance programs and compliance with the device industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, and/or prohibition and tracking and reporting of gifts, compensation and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
The laws described above impact the kinds of financial arrangements we may have with hospitals, healthcare professionals or other potential purchasers of our products. If our operations are found to be in violation of any of the laws or regulations described above or others that apply to us, we may be subject to penalties, including potentially significant criminal, civil and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations.
HIPAA. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as covered entities. The standards promulgated under HIPAA's regulations include those that:
| ● | restrict the use and disclosure of individually identifiable health information, or "protected health information"; |
| ● | establish standards for common electronic healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures; |