Exhibit 99.1
Investor Presentation March 2023 Last modified March 9, 2023

Disclaimers Certain statements in this presentation are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are provided under the protection of the safe harbor for forward-looking statements provided by that Act. Forward-looking statements are based on current expectations of future events and often can be identified by words such as “allow,” “anticipate,” “believe,” “continue,” “estimate,” “expect,” “future,” “intend,” “may,” “plan,” “potential,” “target,” or other words of similar meaning or the use of future dates. Examples of forward-looking statements include benefits of the combined entities, future determinations of the characteristics of drug candidates and their effectiveness, publication of results, other trial activities and the timing of the same, and expected financial or operating results. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially and adversely from the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: (i) our ability to obtain additional funding to complete a randomized clinical trial; (ii) progress and success of our clinical development program; (iii) the impact of the current COVID-19 pandemic on our ability to conduct our clinical trials; (iv) our ability to demonstrate the safety and effectiveness of our product candidates: ivospemin (SBP-101) and eflornithine (CPP-1X) (v) our reliance on a third party for the execution of the registration trial for our product candidate Flynpovi; (vi) our ability to obtain regulatory approvals for our product candidates, ivospemin and eflornithine in the United States, the European Union or other international markets; (vii) the market acceptance and level of future sales of our product candidates, ivospemin and eflornithine; (viii) the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidates, ivospemin and eflornithine; (ix) the rate of progress in establishing reimbursement arrangements with third-party payors; (x) the effect of competing technological and market developments; (xi) the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; (xii) our ability to maintain the listing of our common stock on a national securities exchange, and (xiii) such other factors as discussed in Part I, Item 1A under the caption “Risk Factors” in our most recent Annual Report on Form 10-K, any additional risks presented in our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Any forward-looking statement made by us in this presentation is based on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement or reasons why actual results would differ from those anticipated in any such forward- looking statement, whether written or oral, whether as a result of new information, future developments or otherwise. This presentation includes information about investigational agents. The efficacy and safety of such investigational agents have not yet been established. Drug development is uncertain and investigative agents may be terminated along the development process. All trademarks, company and product names or logos are the property of their respective owners.
Combined Company Presents A Unique Opportunity: Multi-Targeted Approach to Reset Dysregulated Biology Scientific focus on polyamines, key regulators of normal biology altered in many disease states 1 Multiple near term inflection points PP acquisition completed in 2022 to create complementary, multiproduct, multi-indication portfolio2 Late-stage programs are orphan oncology related: pancreatic cancer and FAP (familial adenomatous polyposis) Combined pipeline spans from pre-clinical to Phase III registration programs 3 Approximately a $5 Billion aggregate market potential across lead indications 4 Commercial partner for Phase III FAP program: fully funded registration program in the US Company retains ROW rights5 Strategic synergies and partnerships (NCI, SWOG, COG, JDRF, JHU SOM) Combined clinical program pipeline to create significant shareholder value NCI-National Cancer Institute; SWOG-Southwest Oncology Group; COG-Children’s Oncology Group; JDRF – Juvenile Diabetes Research Foundation; JHU SOM-Johns Hopkins University School of Medicine

Highly Experienced Management Team with Proven Track Record Proven orphan and oncology drug discovery, development and commercialization expertise Dr. Jennifer Simpson Chief Executive Officer 15+ Years Sue Horvath Chief Financial Officer 15+ Years Rachel Bragg VP, Clinical Development 14+ Years Dr. Elizabeth Bruckheimer VP, Chief Scientific Officer 13+ Years Dr. Ashok Chavan VP, CMC, QA, Supply Chain 15+ Years Team collectively has 10+ FDA approvals and decades of Pharma experience positioning Panbela to execute on the clinical development programs
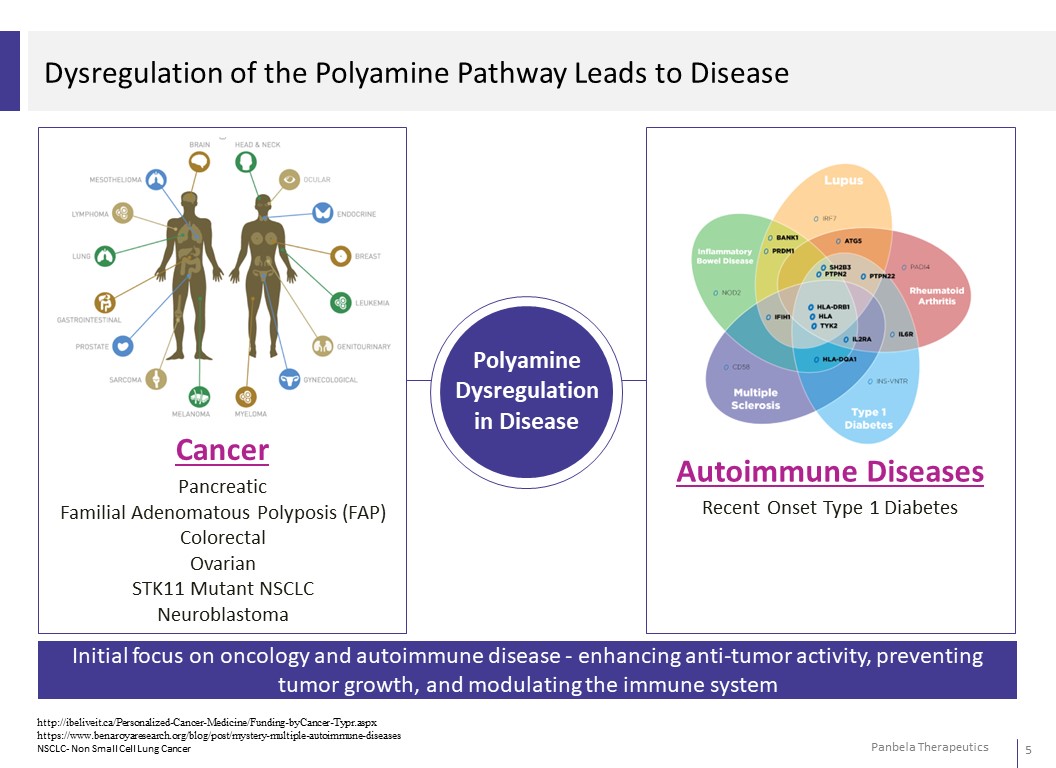
Dysregulation of the Polyamine Pathway Leads to Disease Initial focus on oncology and autoimmune disease -enhancing anti-tumor activity, preventing tumor growth, and modulating the immune system Cancer Pancreatic Familial Adenomatous Polyposis (FAP) Colorectal Ovarian STK11 Mutant NSCLC Neuroblastoma Autoimmune Diseases Recent Onset Type 1 Diabetes Polyamine Dysregulation in Disease http://ibeliveit.ca/Personalized-Cancer-Medicine/Funding-byCancer-Typr.aspx https://www.benaroyaresearch.org/blog/post/mystery-multiple-autoimmune-diseases NSCLC- Non Small Cell Lung Cancer

Preclinical IND Ready Phase I Phase II Phase III Milestones PDA (First Line Metastatic) PDA Neoadjuvant Ovarian Familial Adenomatous Polyposis (FAP) (Partnered6i.n6US; CPP Pursuing EU/Asia Approval) Colon Cancer Risk Reduction (NCI Fund via Partnership with SWOG) Phase III Ongoing; Interim Analysis Q1 2024 Phase II Ready – Open 1H 2023 Phase I Ready – Open Early 2023 Fully funded by licensing partner FPI – 2H 2023 Futility Analysis – 1H 2023 Relapsed Refractory Neuroblastoma (Chemo + Unituxin) with COG/NCI NSCLC (STK11 Mut) with Keytruda PD1 Inhibitor-nonresponsive Cancers Phase I NSCLC Ready FPI – 1H 2023 Phase II NSCLC FPI – 2H 2023 Early Onset Type 1 Diabetes Phase II Ready FPI 1H 2023 Indiana University / JDRF / Panbela Collaboration Announcement – 1H 2023 Publication of Phase I Results – 1H 2023 PDA – Pancreatic Ductal Adenocarcinoma; FPI-First Patient In Panbela Therapeutics
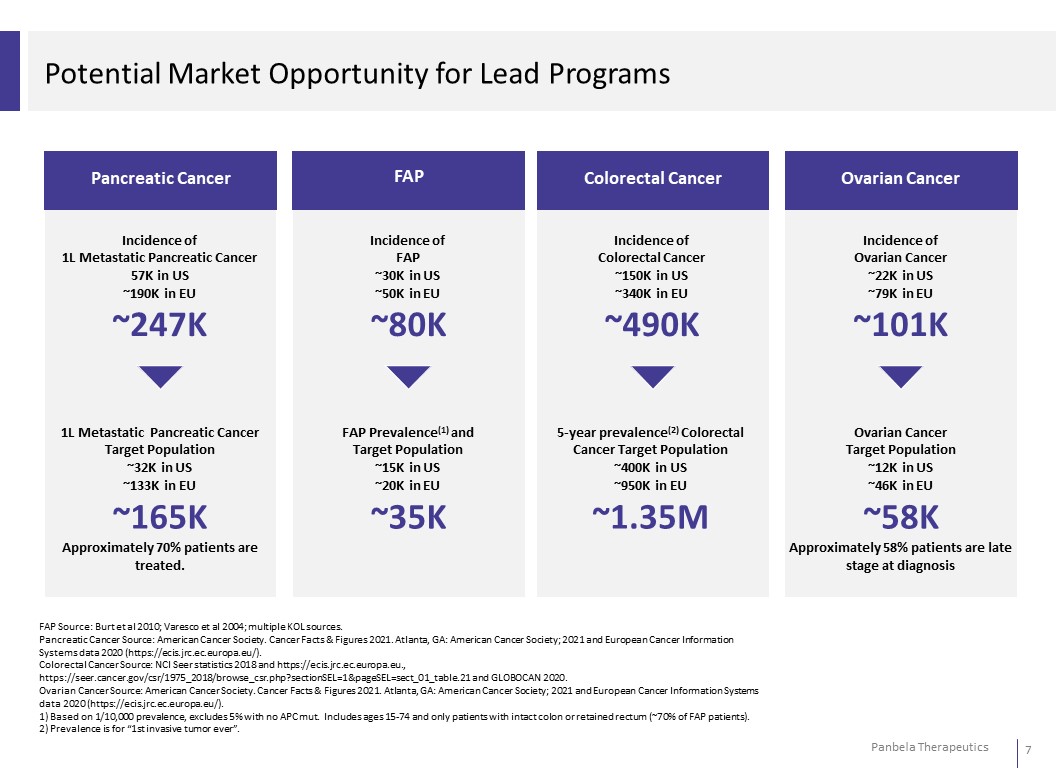
Potential Market Opportunity for Lead Programs Pancreatic Cancer FAP Colorectal Cancer Ovarian Cancer Incidence of 1L Metastatic Pancreatic Cancer 57K in US ~190K in EU ~247K Incidence of FAP ~30K in US ~50K in EU ~80K Incidence of Colorectal Cancer ~150K in US ~340K in EU ~490K Incidence of Ovarian Cancer ~22K in US ~79K in EU ~101K 1L Metastatic Pancreatic Cancer Target Population ~32K in US ~133K in EU ~165K Approximately 70% patients are treated. FAP Prevalence(1) and Target Population ~15K in US ~20K in EU ~35K 5-year prevalence(2) Colorectal Cancer Target Population ~400K in US ~950K in EU ~1.35M Ovarian Cancer Target Population ~12K in US ~46K in EU ~58K Approximately 58% patients are late stage at diagnosis FAP Source: Burt et al 2010; Varesco et al 2004; multiple KOL sources. Pancreatic Cancer Source: American Cancer Society. Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021 and European Cancer Information Systems data 2020 (https://ecis.jrc.ec.europa.eu/). Colorectal Cancer Source: NCI Seer statistics 2018 and https://ecis.jrc.ec.europa.eu., https://seer.cancer.gov/csr/1975_2018/browse_csr.php?sectionSEL=1&pageSEL=sect_01_table.21 and GLOBOCAN 2020. Ovarian Cancer Source: American Cancer Society. Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021 and European Cancer Information Systems data 2020 (https://ecis.jrc.ec.europa.eu/). 1) Based on 1/10,000 prevalence, excludes 5% with no APC mut. Includes ages 15-74 and only patients with intact colon or retained rectum (~70% of FAP patients). 2) Prevalence is for “1st invasive tumor ever”.
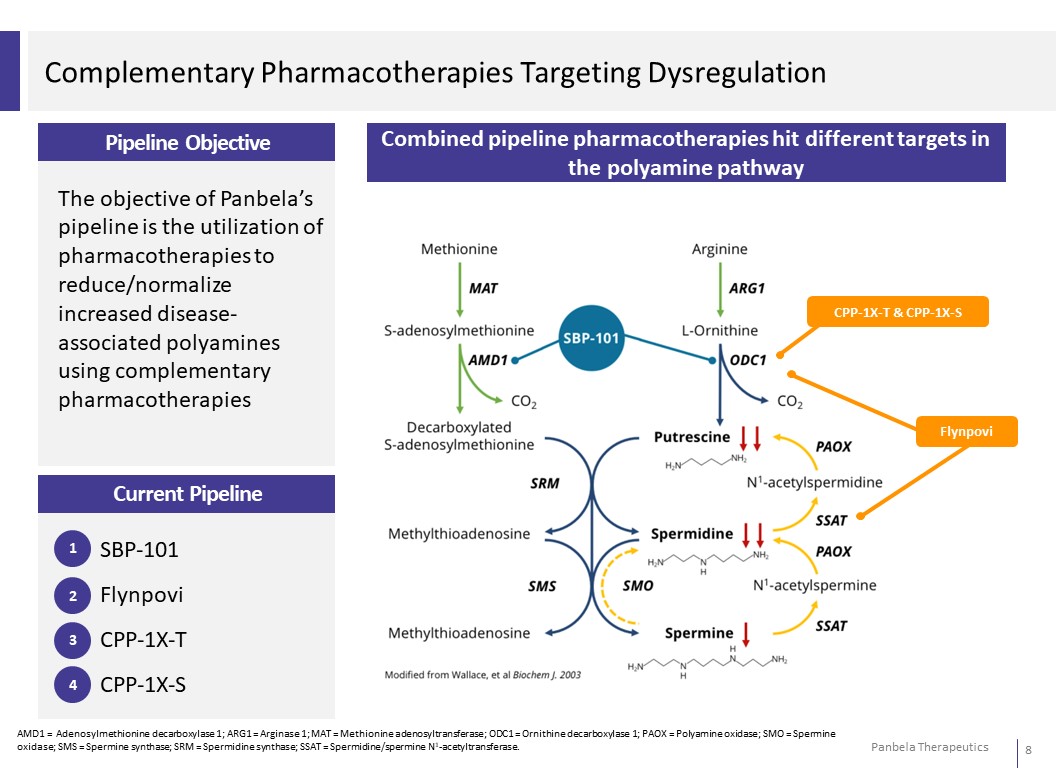
Complementary Pharmacotherapies Targeting Dysregulation Pipeline Objective The objective of Panbela’s pipeline is the utilization of pharmacotherapies to reduce/normalize increased disease- associated polyamines using complementary pharmacotherapies Combined pipeline pharmacotherapies hit different targets in the polyamine pathway CPP-1X-T & CPP-1X-S Flynpovi Current Pipeline 1 SBP-101 2 Flynpovi 3 CPP-1X-T 4 CPP-1X-S AMD1 = Adenosylmethionine decarboxylase 1; ARG1 = Arginase 1; MAT = Methionine adenosyltransferase; ODC1 = Ornithine decarboxylase 1; PAOX = Polyamine oxidase; SMO = Spermine oxidase; SMS = Spermine synthase; SRM = Spermidine synthase; SSAT = Spermidine/spermine N1-acetyltransferase.

SBP-101
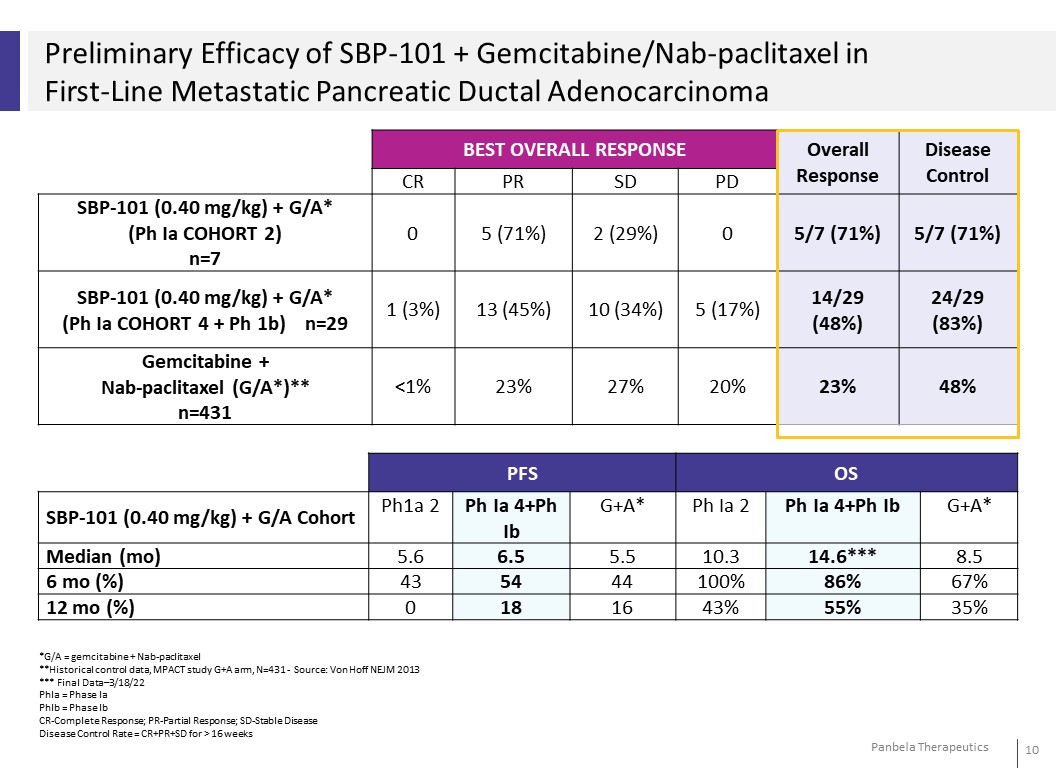
Preliminary Efficacy of SBP-101 + Gemcitabine/Nab-paclitaxel in First-Line Metastatic Pancreatic Ductal Adenocarcinoma BEST OVERALL RESPONSE Overall Response Disease Control CR PR SD PD SBP-101 (0.40 mg/kg) + G/A* (Ph Ia COHORT 2) n=7 0 5 (71%) 2 (29%) 0 5/7 (71%) 5/7 (71%) SBP-101 (0.40 mg/kg) + G/A* (Ph Ia COHORT 4 + Ph 1b) n=29 1 (3%) 13 (45%) 10 (34%) 5 (17%) 14/29 (48%) 24/29 (83%) Gemcitabine + Nab-paclitaxel (G/A*)** n=431 <1% 23% 27% 20% 23% 48% PFS OS SBP-101 (0.40 mg/kg) + G/A Cohort Ph1a 2 Ph Ia 4+Ph Ib G+A* Ph Ia 2 Ph Ia 4+Ph Ib G+A* Median (mo) 5.6 6.5 5.5 10.3 14.6*** 8.5 6 mo (%) 43 54 44 100% 86% 67% 12 mo (%) 0 18 16 43% 55% 35% *G/A = gemcitabine + Nab-paclitaxel **Historical control data, MPACT study G+A arm, N=431 - Source: Von Hoff NEJM 2013 *** Final Data–3/18/22 PhIa = Phase Ia PhIb = Phase Ib CR-Complete Response; PR-Partial Response; SD-Stable Disease Disease Control Rate = CR+PR+SD for > 16 weeks
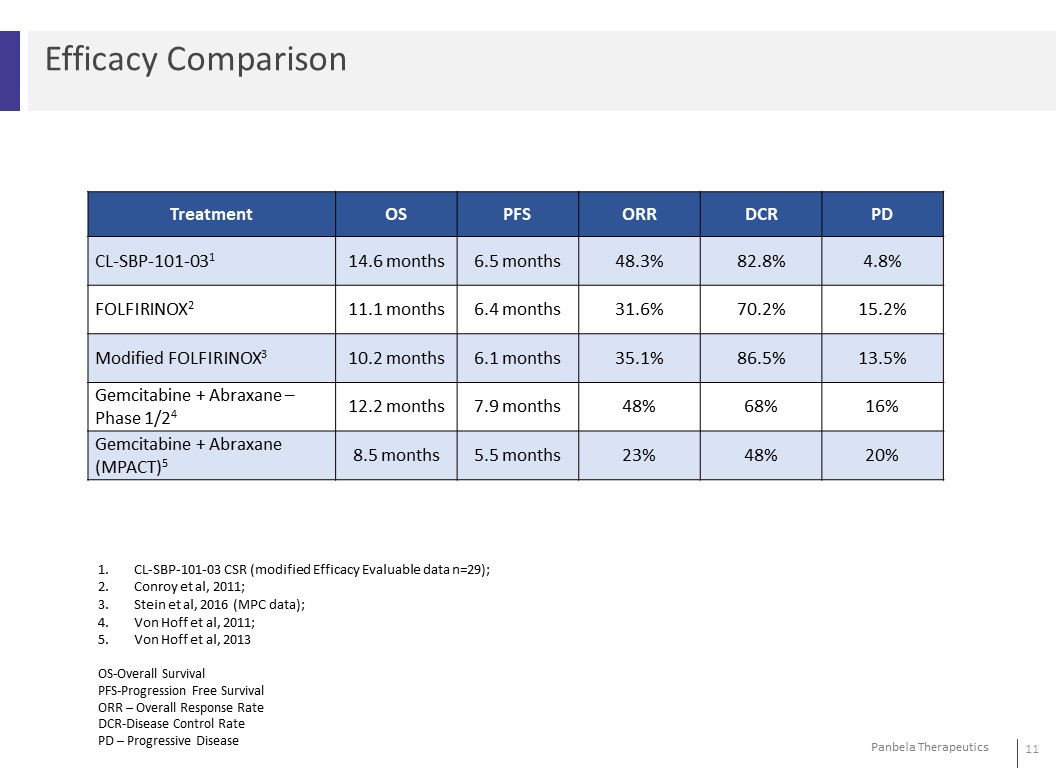
Efficacy ComparisonTreatment OS PFS ORR DCR PDCL-SBP-101-031 14.6 months 6.5 months 48.3% 82.8% 4.8%FOLFIRINOX2 11.1 months 6.4 months 31.6% 70.2% 15.2%Modified FOLFIRINOX3 10.2 months 6.1 months 35.1% 86.5% 13.5%Gemcitabine + Abraxane – Phase 1/24 12.2 months 7.9 months 48% 68% 16%Gemcitabine + Abraxane(MPACT)5 8.5 months 5.5 months 23% 48% 20% 1. CL-SBP-101-03 CSR (modified Efficacy Evaluable data n=29); 2. Conroy et al, 2011; 3. Stein et al, 2016 (MPC data); 4. Von Hoff et al, 2011; 5. Von Hoff et al, 2013 OS-Overall Survival PFS-Progression Free Survival ORR – Overall Response Rate DCR-Disease Control Rate PD – Progressive Disease
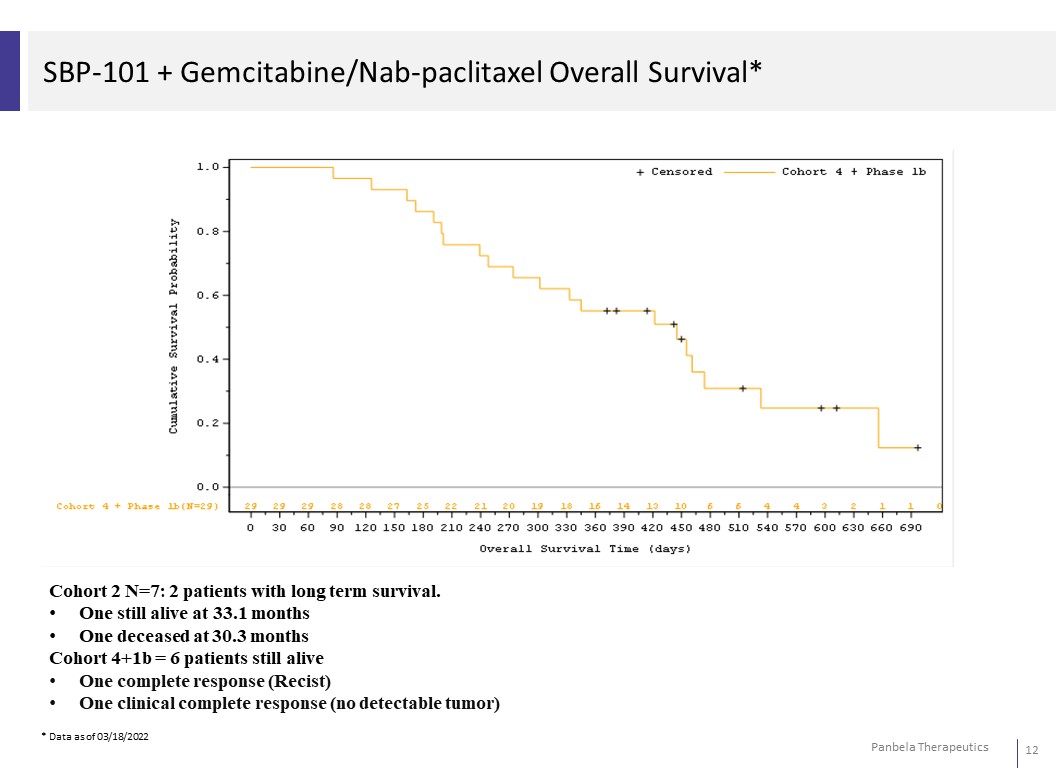
SBP-101 + Gemcitabine/Nab-paclitaxel Overall Survival* Cohort 2 N=7: 2 patients with long term survival. • One still alive at 33.1 months • One deceased at 30.3 months Cohort 4+1b = 6 patients still alive • One complete response (Recist) • One clinical complete response (no detectable tumor) * Data as of 03/18/2022
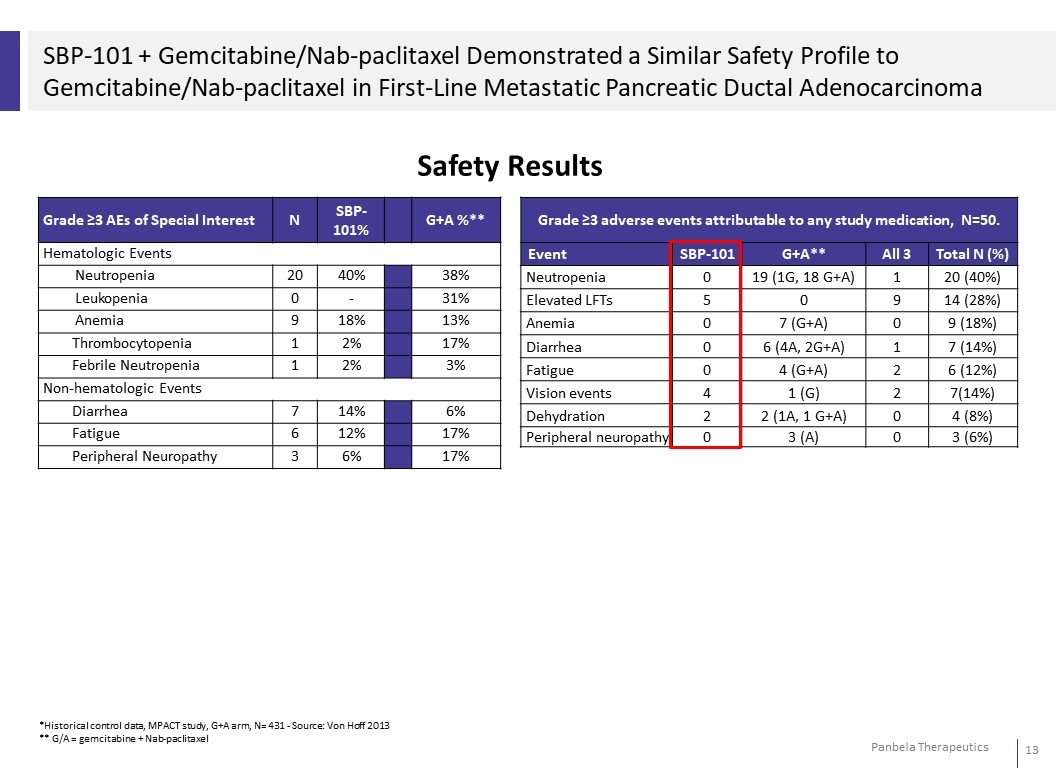
SBP-101 + Gemcitabine/Nab-paclitaxel Demonstrated a Similar Safety Profile to Gemcitabine/Nab-paclitaxel in First-Line Metastatic Pancreatic Ductal Adenocarcinoma Safety Results Grade ≥3 AEs of Special Interest N SBP- 101% G+A %** Hematologic Events Neutropenia 20 40% 38% Leukopenia 0 - 31% Anemia 9 18% 13% Thrombocytopenia 1 2% 17% Febrile Neutropenia 1 2% 3% Non-hematologic Events Diarrhea 7 14% 6% Fatigue 6 12% 17% Peripheral Neuropathy 3 6% 17%
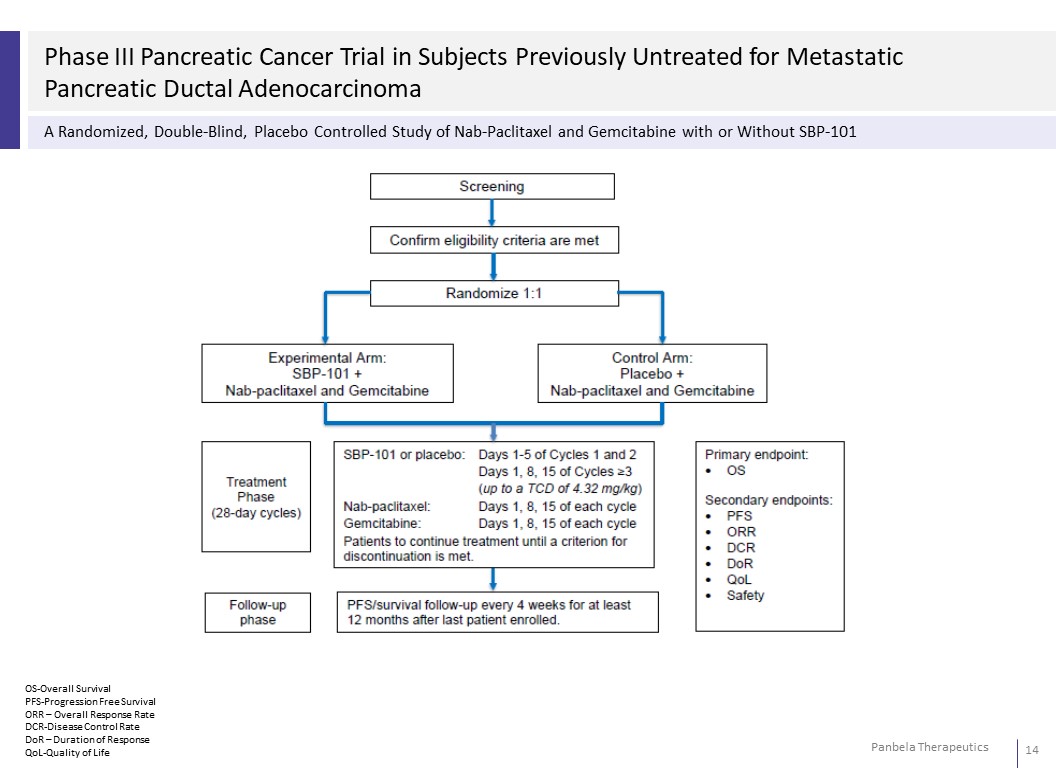
Phase III Pancreatic Cancer Trial in Subjects Previously Untreated for Metastatic Pancreatic Ductal Adenocarcinoma A Randomized, Double-Blind, Placebo Controlled Study of Nab-Paclitaxel and Gemcitabine with or Without SBP-101 OS-Overall Survival PFS-Progression Free Survival ORR – Overall Response Rate DCR-Disease Control Rate DoR – Duration of Response QoL-Quality of Life
FLYNPOVI: A combination of CPP-1X and Sulindac
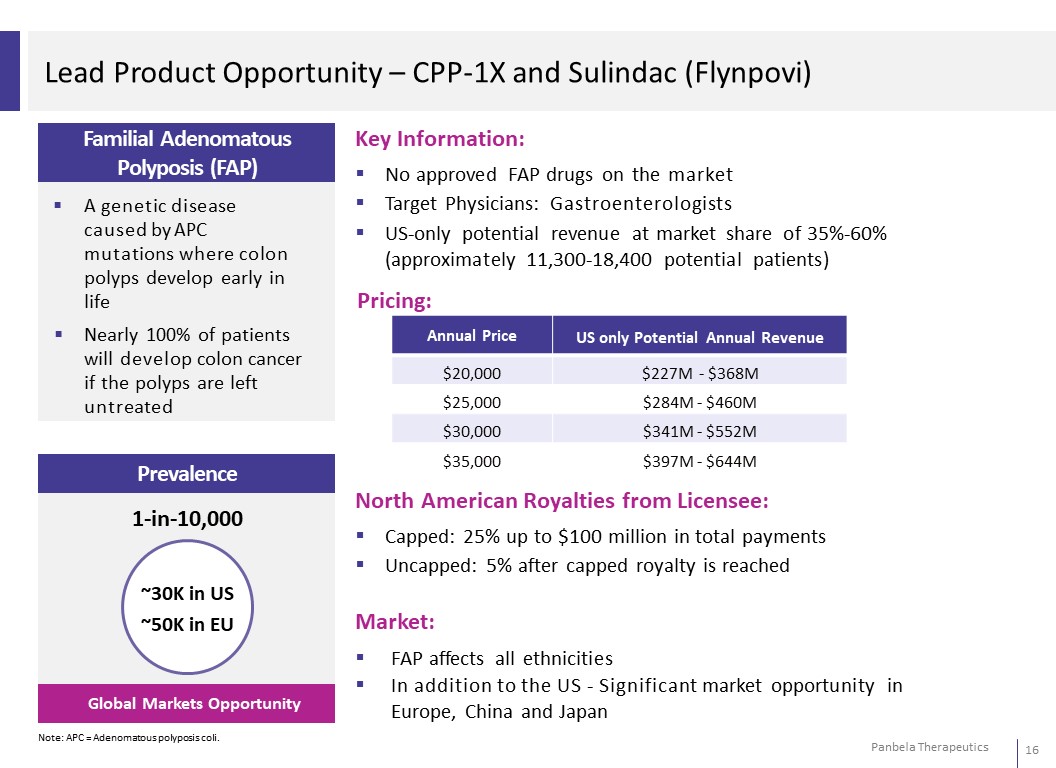
Lead Product Opportunity – CPP-1X and Sulindac (Flynpovi) Familial Adenomatous Polyposis (FAP) A genetic disease caused by APC mutations where colon polyps develop early in life Nearly 100% of patients will develop colon cancer if the polyps are left untreated Prevalence 1-in-10,000 ~30K in US ~50K in EU Global Markets Opportunity Note: APC = Adenomatous polyposis coli. Key Information: No approved FAP drugs on the market Target Physicians: Gastroenterologists US-only potential revenue at market share of 35%-60% (approximately 11,300-18,400 potential patients) Pricing: Annual Price US only Potential Annual Revenue $20,000 $227M - $368M $25,000 $284M - $460M $30,000 $341M - $552M $35,000 $397M - $644M North American Royalties from Licensee: Capped: 25% up to $100 million in total payments Uncapped: 5% after capped royalty is reached Market: FAP affects all ethnicities In addition to the US - Significant market opportunity in Europe, China and Japan
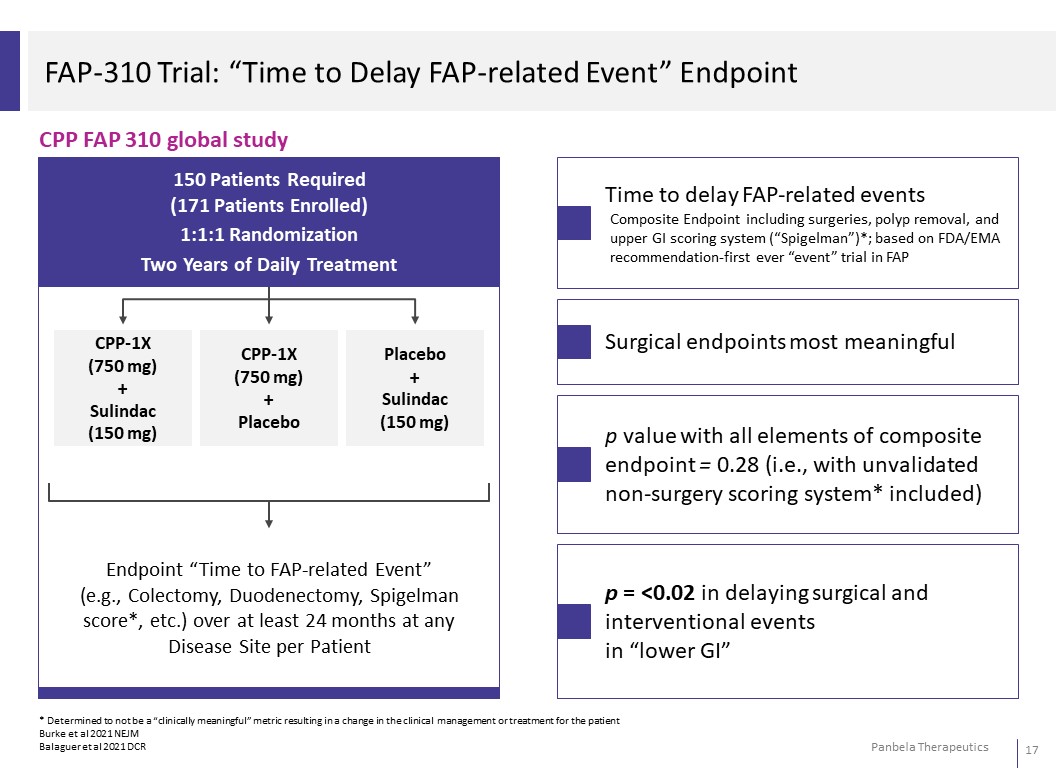
FAP-310 Trial: “Time to Delay FAP-related Event” Endpoint CPP FAP 310 global study Time to delay FAP-related events Composite Endpoint including surgeries, polyp removal, and upper GI scoring system (“Spigelman”)*; based on FDA/EMA recommendation-first ever “event” trial inFAP Surgical endpoints most meaningful p value with all elements of composite endpoint = 0.28 (i.e., with unvalidated non-surgery scoring system* included) p = <0.02 in delaying surgical and interventional events in “lower GI” * Determined to not be a “clinically meaningful” metric resulting in a change in the clinical management or treatment for the patient Burke et al 2021 NEJM Balaguer et al 2021 DCR
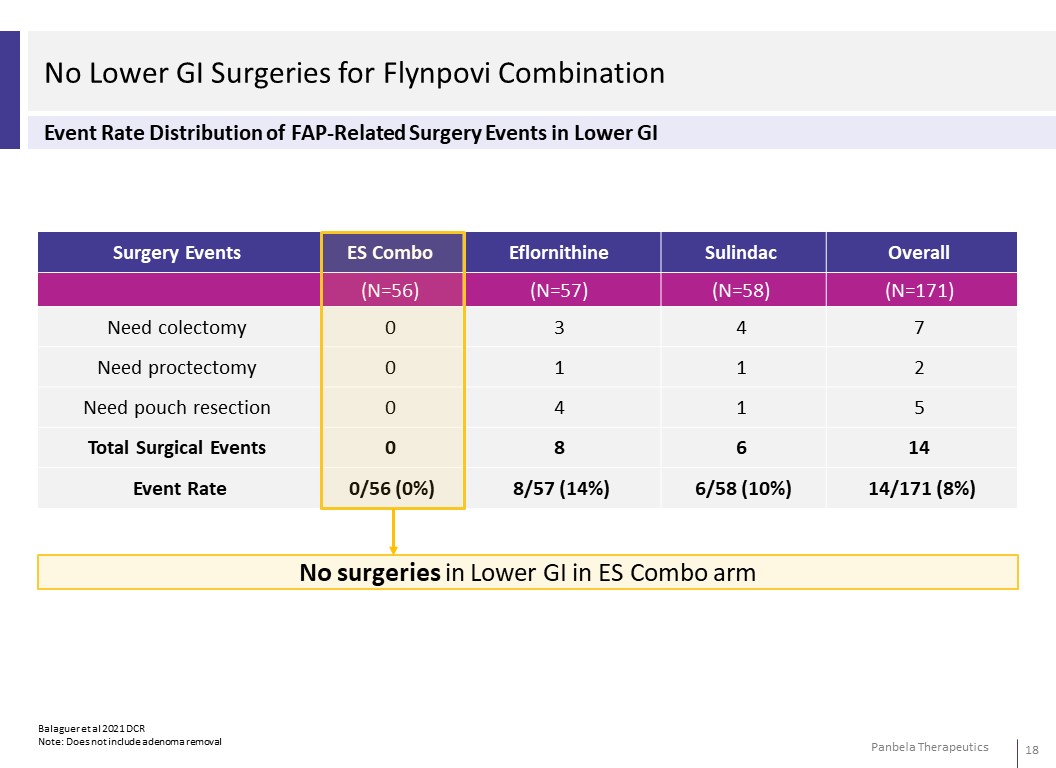
No Lower GI Surgeries for Flynpovi Combination Event Rate Distribution of FAP-Related Surgery Events in Lower GI Surgery Events Eflornithine Sulindac Overall (N=57) (N=58) (N=171)Need colectomy 3 4 7 Need proctectomy 1 1 2 Need pouch resection 4 1 5 Total Surgical Events 8 6 14 Event Rate 8/57 (14%) 6/58 (10%) 14/171 (8%) No surgeries in Lower GI in ES Combo arm
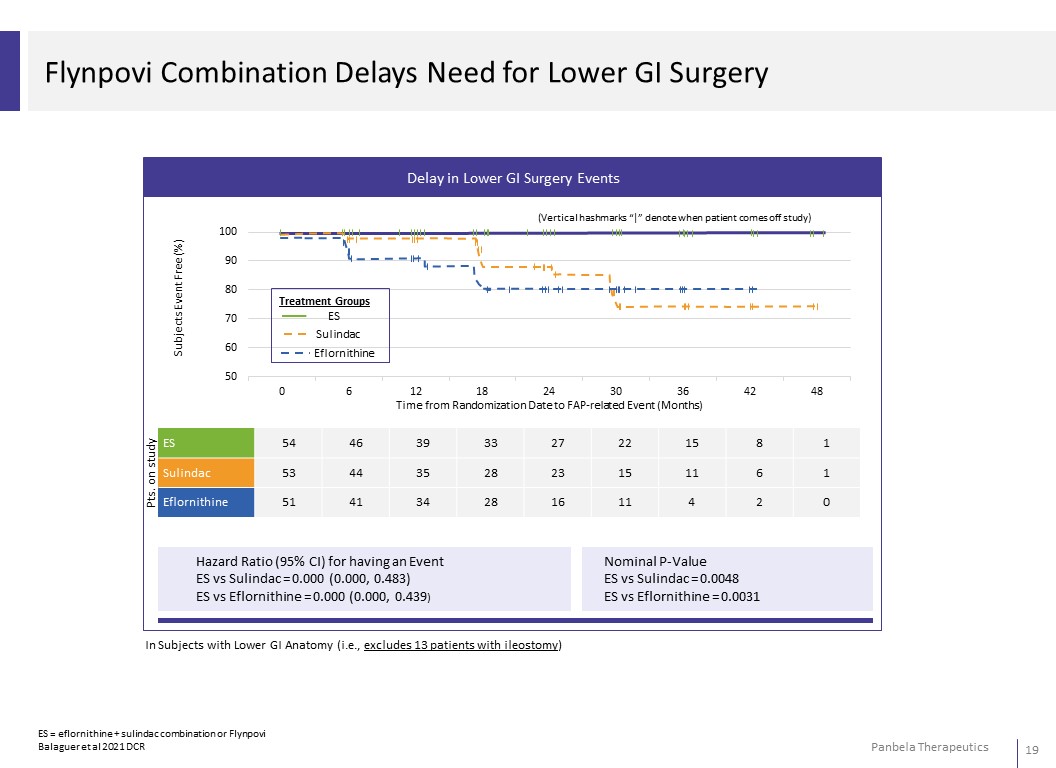
Delay in Lower GI Surgery Events 100 (Vertical hashmarks “|” denote when patient comes off study) 90 80 Treatment Groups 70 ES Sulindac 60 Eflornithine 50 0 6 12 18 24 30 36 42 48 Time from Randomization Date to FAP-related Event (Months) ES 54 46 39 33 27 22 15 8 1 Sulindac 53 44 35 28 23 15 11 6 1 Eflornithine 51 41 34 28 16 11 4 2 0 Hazard Ratio (95% CI) for having an Event ES vs Sulindac = 0.000 (0.000, 0.483) ES vs Eflornithine = 0.000 (0.000, 0.439) Nominal P-Value ES vs Sulindac = 0.0048 ES vs Eflornithine = 0.0031 In Subjects with Lower GI Anatomy (i.e., excludes 13 patients with ileostomy) ES = eflornithine + sulindac combination or Flynpovi Balaguer et al 2021 DCR
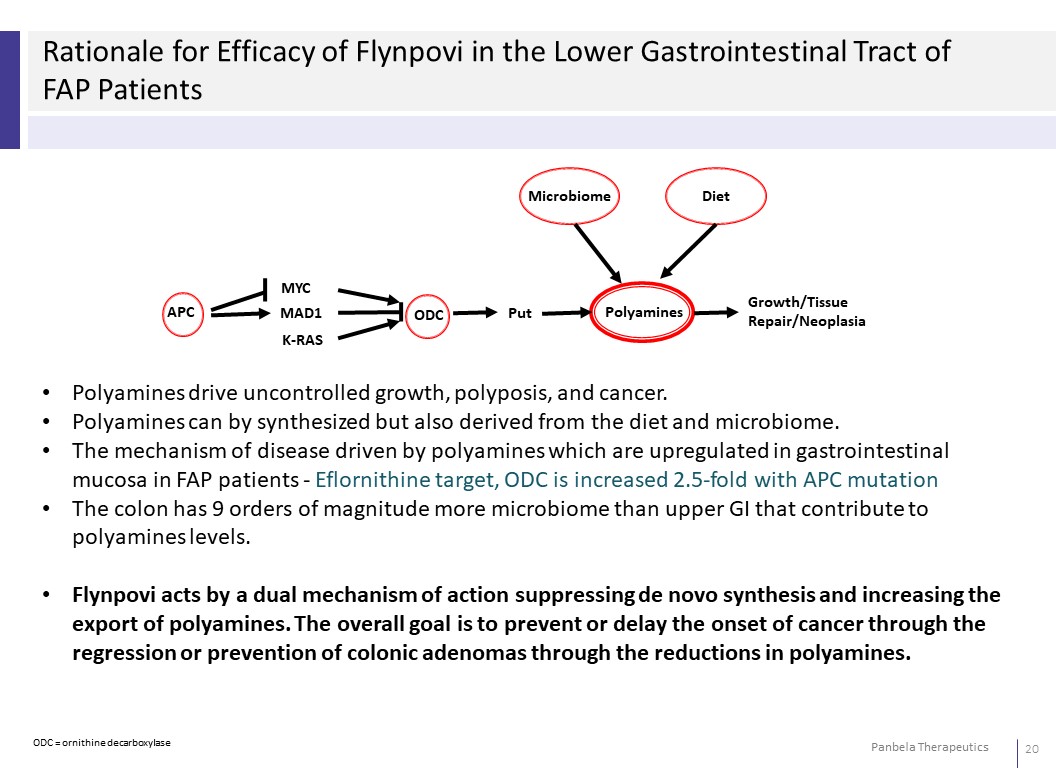
Rationale for Efficacy of Flynpovi in the Lower Gastrointestinal Tract of FAP Patients Microbiome Diet APC MYC MAD1 K-RAS ODC Put Polyamines Growth/Tissue Repair/Neoplasia • Polyamines drive uncontrolled growth, polyposis, and cancer. • Polyamines can by synthesized but also derived from the diet and microbiome. • The mechanism of disease driven by polyamines which are upregulated in gastrointestinal mucosa in FAP patients - Eflornithine target, ODC is increased 2.5-fold with APC mutation • The colon has 9 orders of magnitude more microbiome than upper GI that contribute to polyamines levels. • Flynpovi acts by a dual mechanism of action suppressing de novo synthesis and increasing the export of polyamines. The overall goal is to prevent or delay the onset of cancer through the regression or prevention of colonic adenomas through the reductions in polyamines.
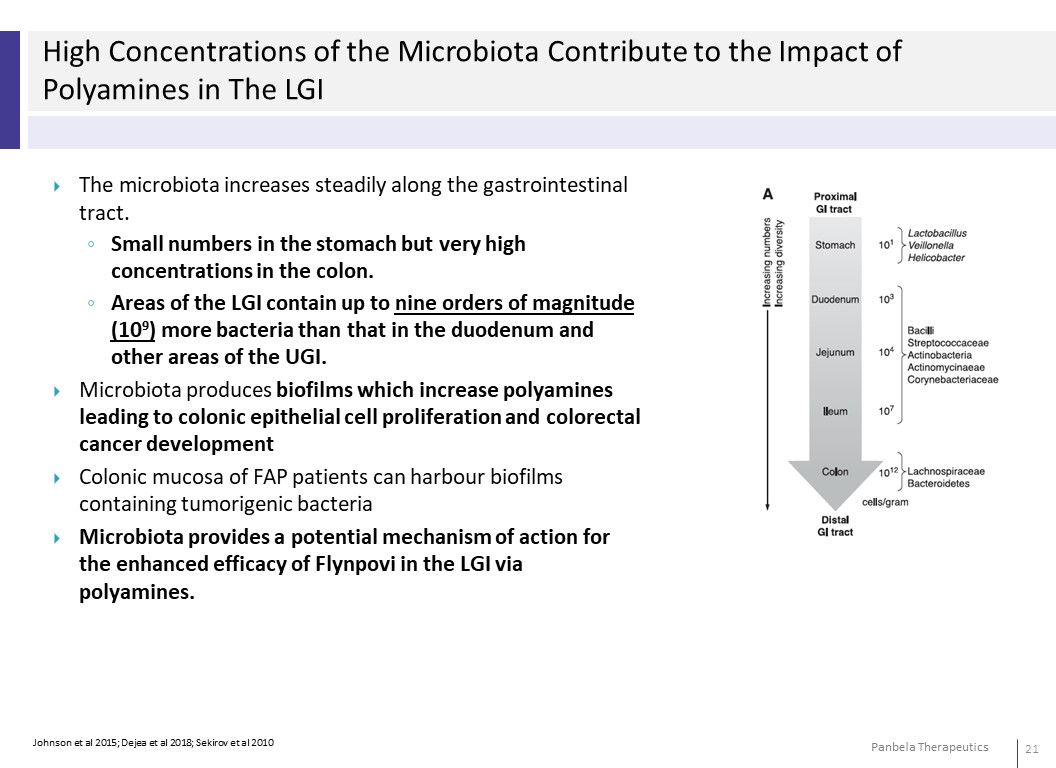
High Concentrations of the Microbiota Contribute to the Impact of Polyamines in The LGI The microbiota increases steadily along the gastrointestinal tract. ◦ Small numbers in the stomach but very high concentrations in the colon. ◦ Areas of the LGI contain up to nine orders of magnitude (109) more bacteria than that in the duodenum and other areas of the UGI. Microbiota produces biofilms which increase polyamines leading to colonic epithelial cell proliferation and colorectal cancer development Colonic mucosa of FAP patients can harbour biofilms containing tumorigenic bacteria Microbiota provides a potential mechanism of action for the enhanced efficacy of Flynpovi in the LGI via polyamines.
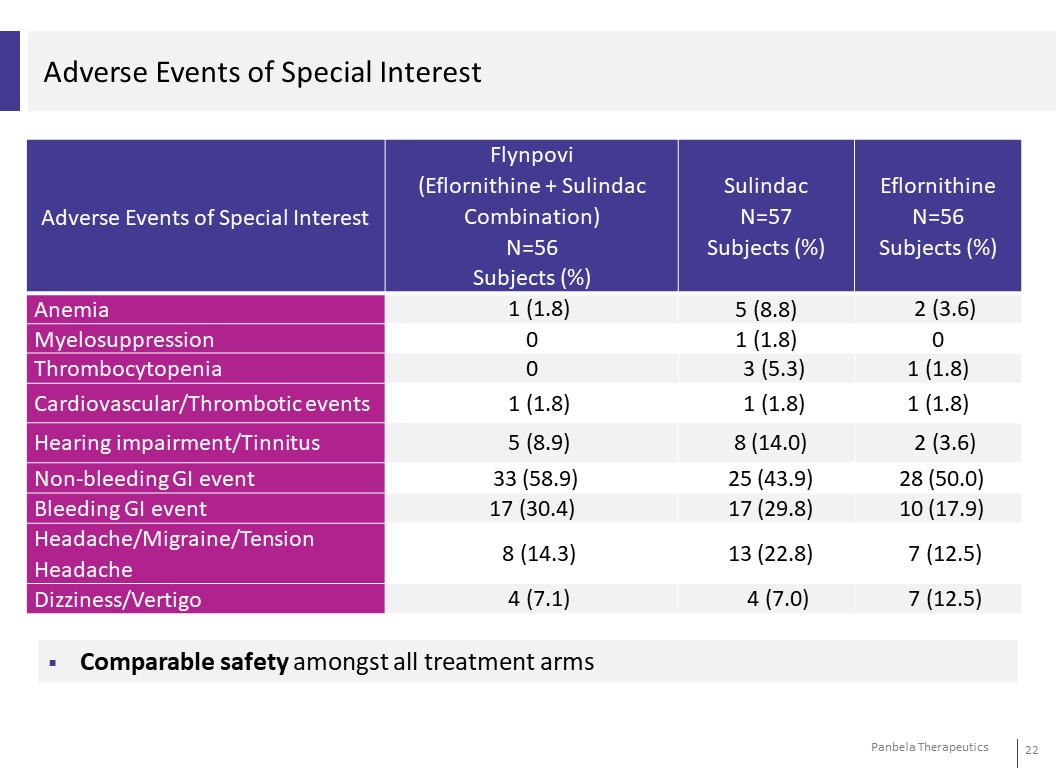
Adverse Events of Special Interest Adverse Events of Special Interest Flynpovi (Eflornithine + Sulindac Combination) N=56 Subjects (%) Sulindac Eflornithine N=57 N=56 Subjects (%) Subjects (%) Anemia 1 (1.8) 5 (8.8) 2 (3.6) Myelosuppression 0 1 (1.8) 0 Thrombocytopenia 0 3 (5.3) 1 (1.8) Cardiovascular/Thrombotic events 1 (1.8) 1 (1.8) 1 (1.8) Hearing impairment/Tinnitus 5 (8.9) 8 (14.0) 2 (3.6) Non-bleeding GI event 33 (58.9) 25 (43.9) 28 (50.0) Bleeding GI event 17 (30.4) 17 (29.8) 10 (17.9) Headache/Migraine/Tension Headache 8 (14.3) 13 (22.8) 7 (12.5) Dizziness/Vertigo 4 (7.1) 4 (7.0) 7 (12.5) Comparable safety amongst all treatment arms

Basis for FDA Complete Response Letter Clinical Single pivotal study efficacy was based on exploratory post-hoc analysis using a modified primary endpoint in a subgroup of the intent-to treat population o Asked to perform a new study focused on FAP patients with an intact lower- GI tract which showed statistically significant effect in post-hoc analysis of FAP310 trial Quality Proposed dissolution condition for testing of Eflornithine is different from the FDA Guidance for Industry: Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Revised dissolution specifications Limited discriminating ability of the dissolution method for compression force and no discriminating ability for the magnesium stearate or sulindac particle size distribution o Dissolution method being revised to address concerns

Moving Ahead: New Phase III Trial North America Licensee funding next trial FDA and EMA focus on new Phase III trial: Lower GI focus (LGI), informed by positive data from FAP-310 and regulatory feedback Reaffirm current clarity & acceptable aspects of CMC, clin pharm, and non- clinical package Initiate new LGI focused pivotal trial FDA: Meeting to ensure alignment for new trial (via Licensee): completed 2H22 Initiate new trial: 2H23 EMA: Select National Competent Authority (NCA) engagement on new trial and clear registration path (Sweden/Netherlands based on CHMP feedback) Follow up SAWP engagement with revised approached, informed by CHMP and NCA interactions

Summary of Approach to Approval Strong Results in the Lower GI Focus on Lower GI: statistically significant p-values and hazard ratios for clinically meaningful surgical endpoints—emphasized by regulators—in Phase III pivotal trial Expected safety profile over 2-4 years of daily dosing in Phase III pivotal trial Totality of the evidence includes mechanistic, preclinical, and clinical supportive data No Other Approved Therapy for this Orphan Disease
Polyamines & Immune Dysregulation
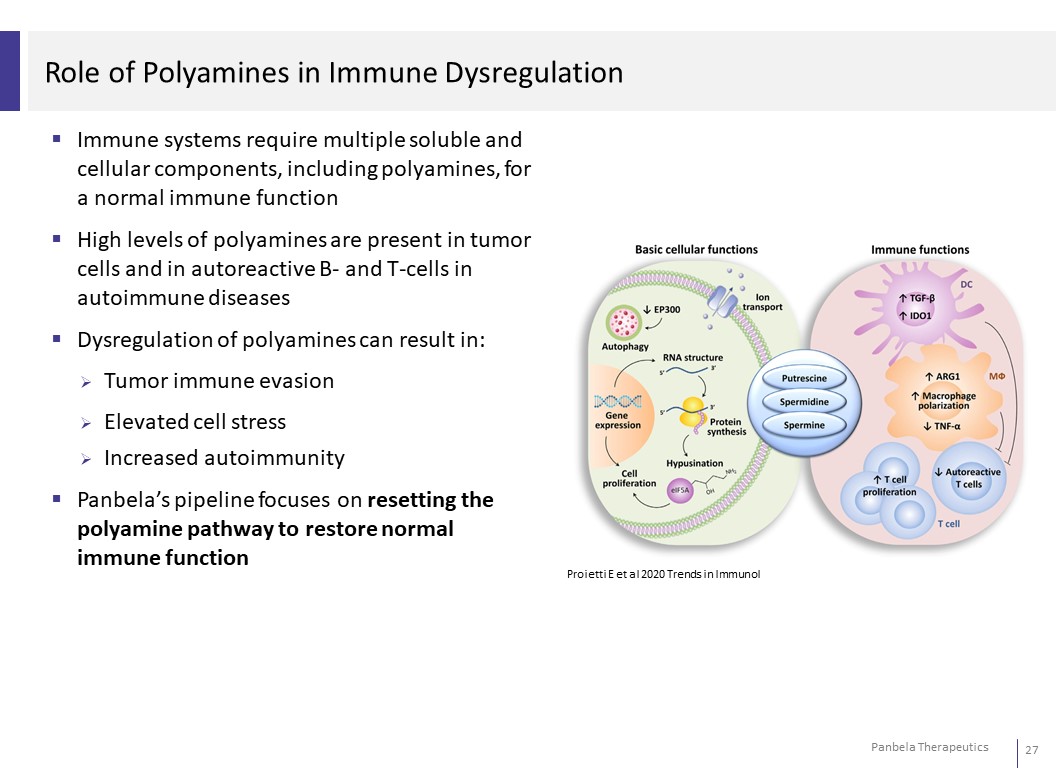
Role of Polyamines in Immune Dysregulation Immune systems require multiple soluble and cellular components, including polyamines, for a normal immune function High levels of polyamines are present in tumor cells and in autoreactive B- and T-cells in autoimmune diseases Dysregulation of polyamines can result in: Tumor immune evasion Elevated cell stress Increased autoimmunity Panbela’s pipeline focuses on resetting the polyamine pathway to restore normal immune function Proietti E et al 2020 Trends in Immunol
Role of Polyamines in Diffuse Large B Cell Lymphoma (DLBCL) MYC overexpression in relapsed/refractory DLBCL prior to CAR-T cell therapy negatively associated with durable response to CAR-T cell therapy. Polyamine metabolism upregulation through oncogenic MYC is a common metabolic irregularity in aggressive cancers, including lymphomas. Myeloid-derived suppressor cells (MDSCs) and higher IL-6 cytokine levels associated with reduced CAR-T cell expansion/poor anti-lymphoma responses after anti-CD19 CAR-T cell therapy. Polyamines have been reported to increase MDSCs and lead to an immunosuppresive tumor microenvironment. Polyamines have been shown to be important for effector function of several immune cell types including T cells. Circulating acetylated polyamine levels may function as a predictor of therapeutic outcome to CAR-T cell therapy. This suggests a possible strategy to target polyamine metabolism to augment the efficacy of CAR-T cell therapy. Fahrmann JF et al 2022 Cell Reports Medicine
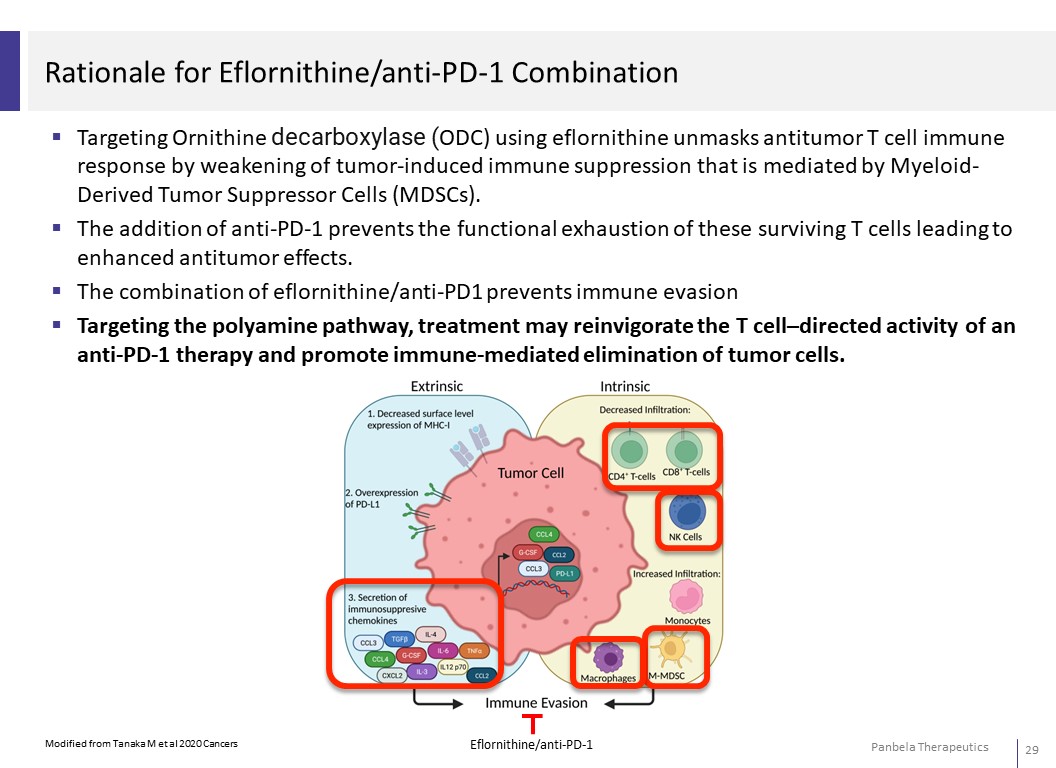
Rationale for Eflornithine/anti-PD-1 Combination Targeting Ornithine decarboxylase (ODC) using eflornithine unmasks antitumor T cell immune response by weakening of tumor-induced immune suppression that is mediated by Myeloid- Derived Tumor Suppressor Cells (MDSCs). The addition of anti-PD-1 prevents the functional exhaustion of these surviving T cells leading to enhanced antitumor effects. The combination of eflornithine/anti-PD1 prevents immune evasion Targeting the polyamine pathway, treatment may reinvigorate the T cell–directed activity of an anti-PD-1 therapy and promote immune-mediated elimination of tumor cells.
CPP-1X-S
Rationale for CPP-1X-S (Eflornithine Sachets) in STK11 Mutant NSCLC In preclinical tumor models, eflornithine treatment improves anti-PD-1 efficacy by: Increasing tumor-specific cytotoxic T-cell populations Increasing expression of PD-1 on tumor-associated CD8+ T cells ~10% of NSCLC cancers have STK11 mutations STK11 mutant NSCLC tumors have: Reduced cytotoxic T-cells infiltrates Upregulation of the arginine pathway which utilizes ODC to increase levels of the polyamine putrescine Respond poorly to immune checkpoint inhibitor therapy Cancer Genomic Atlas Research 2014 Nature Skoulidis F et al 2018 Cancer Discov Skoulidis F et al 2019 JCO Dryia P et al 2021 J Immunother Alexander Et et al 2020 Mol Cancer Ther
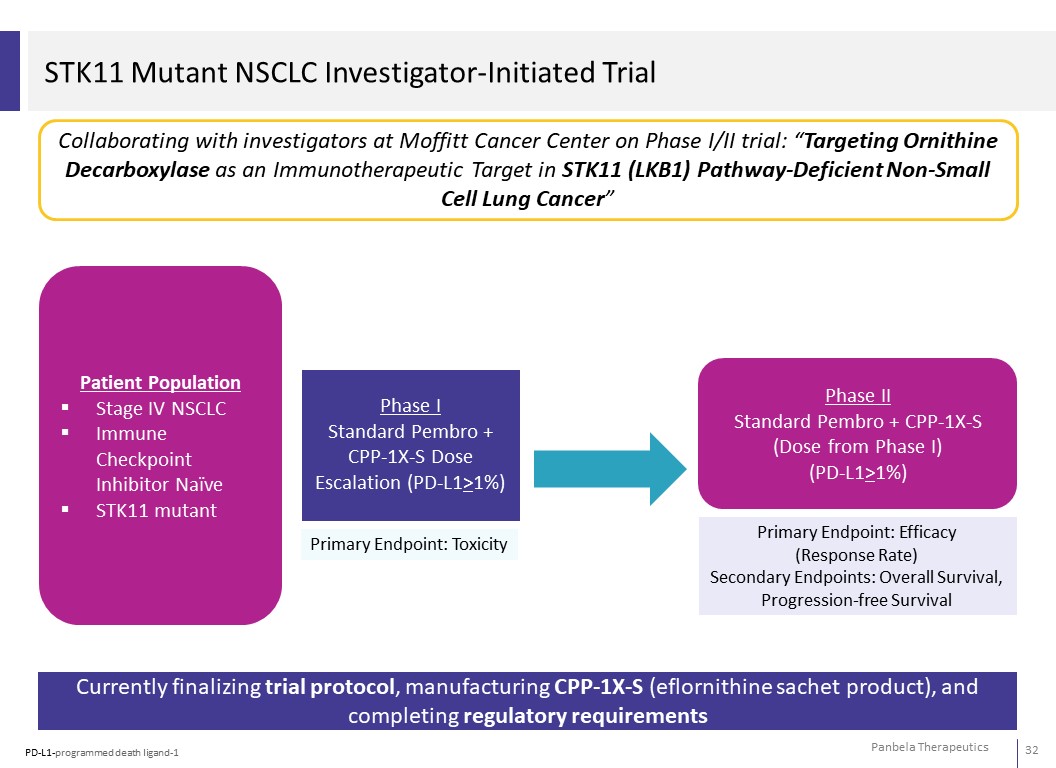
STK11 Mutant NSCLC Investigator-Initiated Trial Collaborating with investigators at Moffitt Cancer Center on Phase I/II trial: “Targeting Ornithine Decarboxylase as an Immunotherapeutic Target in STK11 (LKB1) Pathway-Deficient Non-Small Cell Lung Cancer” Patient Population Stage IV NSCLC Immune Checkpoint Inhibitor Naïve STK11 mutant Phase I Standard Pembro + CPP-1X-S Dose Escalation (PD-L1>1%) Primary Endpoint: Toxicity Phase II Standard Pembro + CPP-1X-S (Dose from Phase I) (PD-L1>1%) Primary Endpoint: Efficacy (Response Rate) Secondary Endpoints: Overall Survival, Progression-free Survival Currently finalizing trial protocol, manufacturing CPP-1X-S (eflornithine sachet product), and completing regulatory requirements PD-L1-programmed death ligand-1
Business Overview
Barriers to Entry – Flynpovi/CPP-1X Orphan status (7 years exclusivity US; 10 years exclusivity EU) for CPP-1X ◦ Granted in US and EU for FAP ◦ Granted in US and EU for Neuroblastoma ◦ Granted in US for Gastric cancer and Pancreatic cancer Pharmaceutical Composition Patent ◦ Fixed dose combination eflornithine + sulindac ◦ Broadly nationalized with potential protection through 2037 Method of Use Patents/Patent Applications ◦ Fixed dose combination of eflornithine + sulindac and eflornithine single agent ◦ “Theranostic” patents will afford protection to 2034 (“theranostic”= drug + diagnostic to guide therapy) ◦ Use in Neuroblastoma protection through 2035 ◦ Use for Treating FAP with potential protection through 2040 ◦ Use in Treating Recent Onset Type 1 Diabetes with potential protection through 2041 ◦ New patents under consideration

Barriers to Entry – SBP-101 Orphan status (7 for years exclusivity US; 10 years exclusivity EU) for SPB-101 ◦ Granted in US and EU for pancreatic cancer METHODS FOR PRODUCING (6S,15S)-3,8,13,18-TETRAAZAICOSANE-6,15-DIOL ◦ PCT application filed January 29, 2019 ◦ Granted in United States, India ◦ Pending in United States (CON), Europe, Australia, Canada, China and Japan ◦ 20-year expiration date is January 29, 2039 DOSING REGIMENS AND METHODS FOR TREATING CANCER ◦ PCT application filed January 20, 2021 ◦ Pending in United States, Australia, Canada and Japan ◦ Filing in progress in Europe (due August 20, 2022) ◦ 20-year expiration date is January 20, 2041

Summary of Milestones 1H 2023 Open Phase I Ovarian Cancer Trial Open- Phase I NSCLC Trial Open- Neoadjuvant Pancreatic Cancer Trial Open – Phase II Type I onset Diabetes Trial Futility Analysis Phase III Colon Cancer Risk Reduction Trial (PACES) Publication of Phase I Type I early onset Diabetes Data Publication of Final Phase I Metastatic Pancreatic Trial Data Gastric Cancer Prevention Phase II Results 2H 2023 Open FAP Registration Trial (funded by Licensee) Phase I NSCLC Data Open Phase II NSCLC Trial 1H 2024 Overall Survival Interim Analysis Phase III ASPIRE Trial ASCO-American Society of Clinical Oncology GI-Gastrointestinal mPC-Metastatic Pancreatic Cancer FPI-First Patient In FAP-Familial Adenomatous Polyposis NSCLC – Non Small Cell Lung Cancer
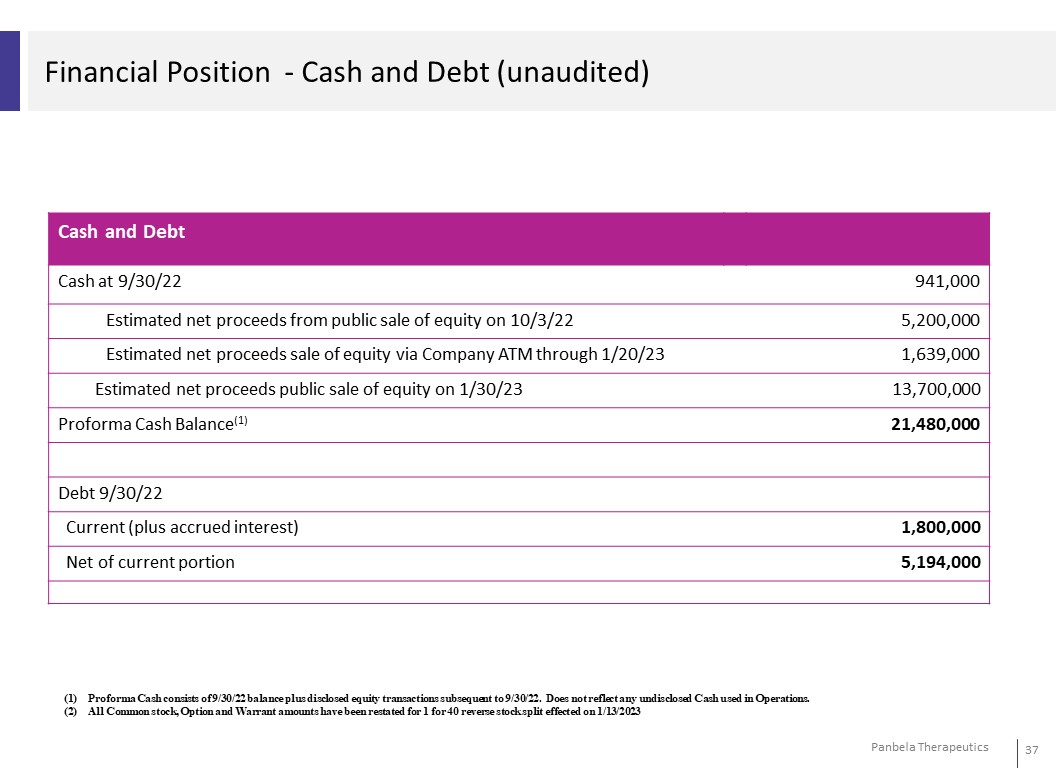
Financial Position - Cash and Debt (unaudited) Cash and Debt Cash at 9/30/22 941,000 Estimated net proceeds from public sale of equity on 10/3/22 5,200,000 Estimated net proceeds sale of equity via Company ATM through 1/20/23 1,639,000 Estimated net proceeds public sale of equity on 1/30/23 13,700,000 Proforma Cash Balance(1) 21,480,000 Debt 9/30/22 Current (plus accrued interest) 1,800,000 Net of current portion 5,194,000 (1) Proforma Cash consists of 9/30/22 balance plus disclosed equity transactions subsequent to 9/30/22. Does not reflect any undisclosed Cash used in Operations. (2) All Common stock, Option and Warrant amounts have been restated for 1 for 40 reverse stock split effected on 1/13/2023
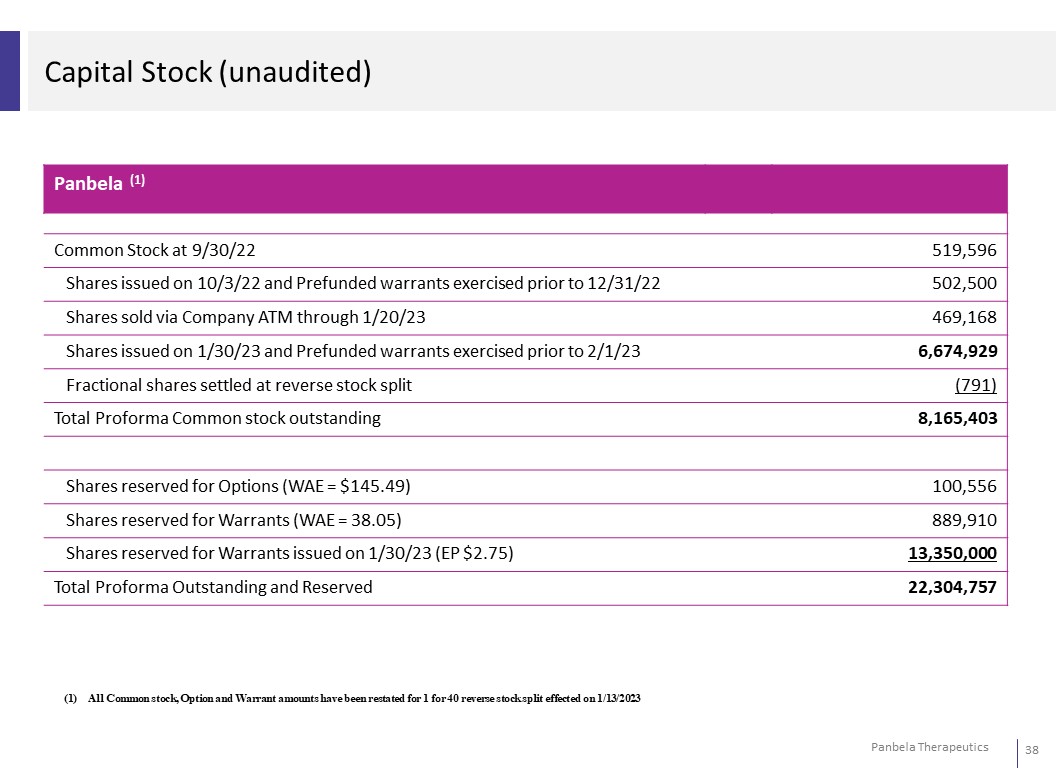
Capital Stock (unaudited) Panbela (1) Common Stock at 9/30/22 519,596 Shares issued on 10/3/22 and Prefunded warrants exercised prior to 12/31/22 502,500 Shares sold via Company ATM through 1/20/23 469,168 Shares issued on 1/30/23 and Prefunded warrants exercised prior to 2/1/23 6,674,929 Fractional shares settled at reverse stock split (791) Total Proforma Common stock outstanding 8,165,403 Shares reserved for Options (WAE = $145.49) 100,556 Shares reserved for Warrants (WAE = 38.05) 889,910 Shares reserved for Warrants issued on 1/30/23 (EP $2.75) 13,350,000 Total Proforma Outstanding and Reserved 22,304,757 (1) All Common stock, Option and Warrant amounts have been restated for 1 for 40 reverse stock split effected on 1/13/2023
Summary: Unique Scientific Approach: Resetting dysregulation via polyamine pathway Broad, late-stage de-risked pipeline opportunities Multiple partnerships supporting development programs Significant market potential Multiple data readouts expected Experienced management team