
Analyst & Investor Presentation June 2018 Exhibit 99.3

Forward Looking Statement This presentation contains "forward-looking" statements, including statements regarding the conduct of clinical trials of SD-101, including results from the Phase 1b/2 trial, planned optimal dosage for the Phase 3 trial, and potential value of SD-101 across multiple tumor types. Actual results may differ materially from those set forth in this presentation due to the risks and uncertainties inherent in our business, including whether we can timely provide adequate clinical supplies; initiation, enrollment and completion of clinical trials of SD-101; the results of clinical trials and the impact of those results on the initiation or continuation of subsequent trials and issues arising in the regulatory process; the ability to successfully develop and commercialize SD-101; and whether or not Dynavax and parties with whom we are collaborating may reach any future agreement on further studies or a more extensive collaboration beyond the clinical trials contemplated under the existing agreements, as well as other risks detailed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and in Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, as well as discussions of potential risks, uncertainties and other important factors in our other filings with the U.S. Securities and Exchange Commission. We undertake no obligation to revise or update information herein to reflect events or circumstances in the future, even if new information becomes available. Information on Dynavax's website at www.dynavax.com is not incorporated by reference in our current periodic reports with the SEC.

Presenters COMPANY Eddie Gray – Chief Executive Officer Robert L. Coffman, PhD – Chief Scientific Officer Rob Janssen, MD – Chief Medical Officer Jean Chang – VP, Cancer Strategy and Business Development Michael Ostrach – Chief Financial Officer (available for Q&A) Erick Gamelin – VP, Clinical Development – Oncology (available for Q&A) GUEST PRESENTER Antoni Ribas, MD, PhD Professor of Medicine, Surgery, Molecular and Medical Pharmacology, Director, Tumor Immunology Program, Jonsson Comprehensive Cancer Center (JCCC); Director, Parker Institute for Cancer Immunotherapy (PICI) Center at UCLA Chair, Melanoma Committee at SWOG

Agenda TLR9 Agonists for Cancer Immunotherapy – Mechanisms Robert L. Coffman Resistance to PD-1 Blockade Due to Lack of Pre-existing Antitumor immunity Toni Ribas Clinical Development – Ph1b/2 Melanoma Data and Additional Programs Rob Janssen Maximizing Value of Dynavax’s TLR9 Agonist Portfolio in the Evolving Treatment Landscape Jean Chang Conclusion Eddie Gray 2 3 4 5 1
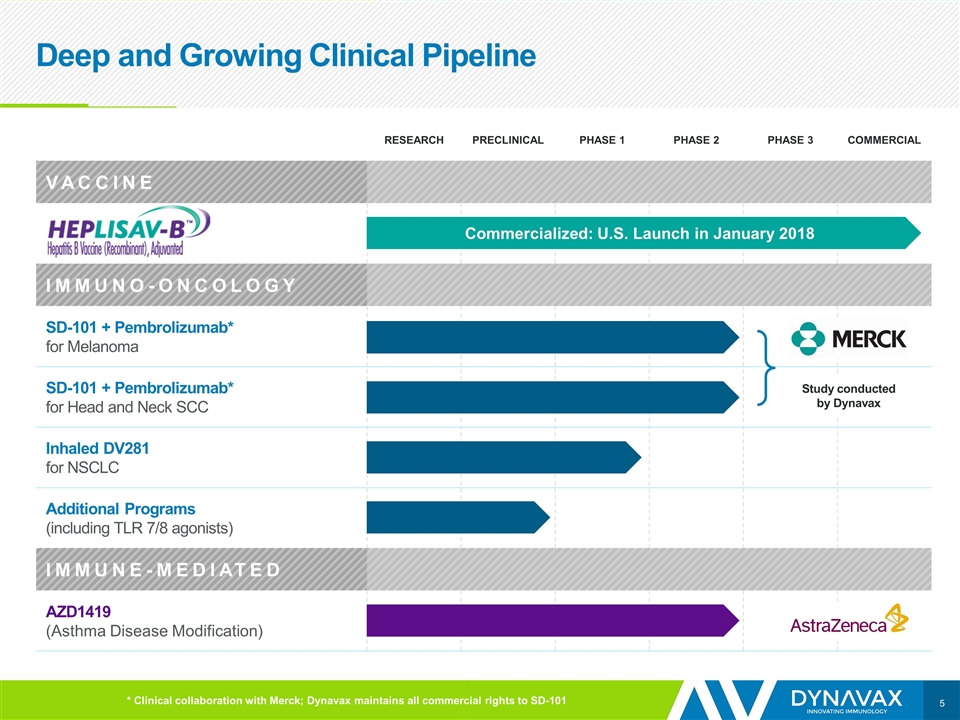
Deep and Growing Clinical Pipeline RESEARCH PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL VACCINE IMMUNO-ONCOLOGY SD-101 + Pembrolizumab* for Melanoma SD-101 + Pembrolizumab* for Head and Neck SCC Inhaled DV281 for NSCLC Additional Programs (including TLR 7/8 agonists) IMMUNE-MEDIATED AZD1419 (Asthma Disease Modification) Commercialized: U.S. Launch in January 2018 * Clinical collaboration with Merck; Dynavax maintains all commercial rights to SD-101 Study conducted by Dynavax

Robert L. Coffman, PhD
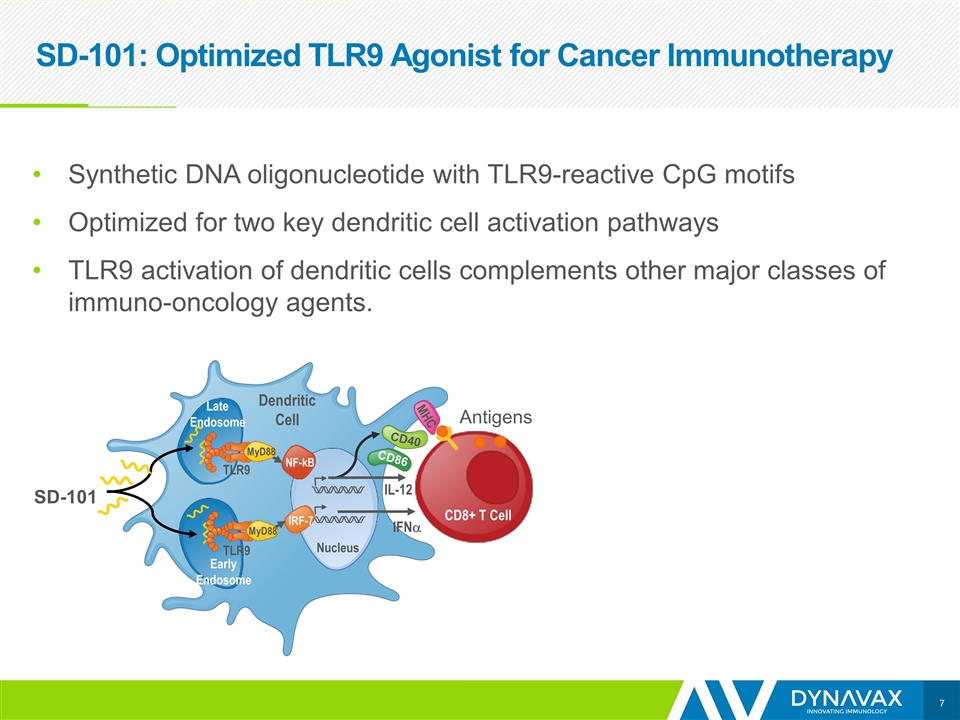
SD-101: Optimized TLR9 Agonist for Cancer Immunotherapy SD-101 Synthetic DNA oligonucleotide with TLR9-reactive CpG motifs Optimized for two key dendritic cell activation pathways TLR9 activation of dendritic cells complements other major classes of immuno-oncology agents. NF-kB IRF-7 Nucleus IFNa IL-12 Dendritic Cell Late Endosome Early Endosome MyD88 MyD88 TLR9 TLR9 CD40 CD86 MHC CD8+ T Cell Antigens
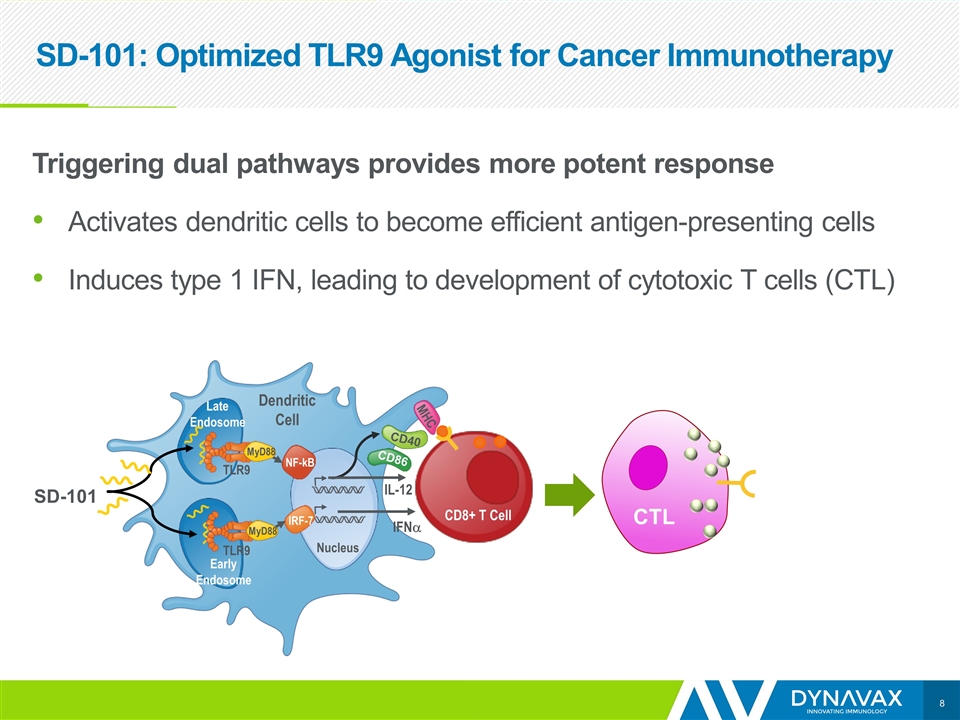
SD-101: Optimized TLR9 Agonist for Cancer Immunotherapy Triggering dual pathways provides more potent response Activates dendritic cells to become efficient antigen-presenting cells Induces type 1 IFN, leading to development of cytotoxic T cells (CTL) SD-101 CTL NF-kB IRF-7 Nucleus IFNa IL-12 Dendritic Cell Late Endosome Early Endosome MyD88 MyD88 TLR9 TLR9 CD40 CD86 MHC CD8+ T Cell
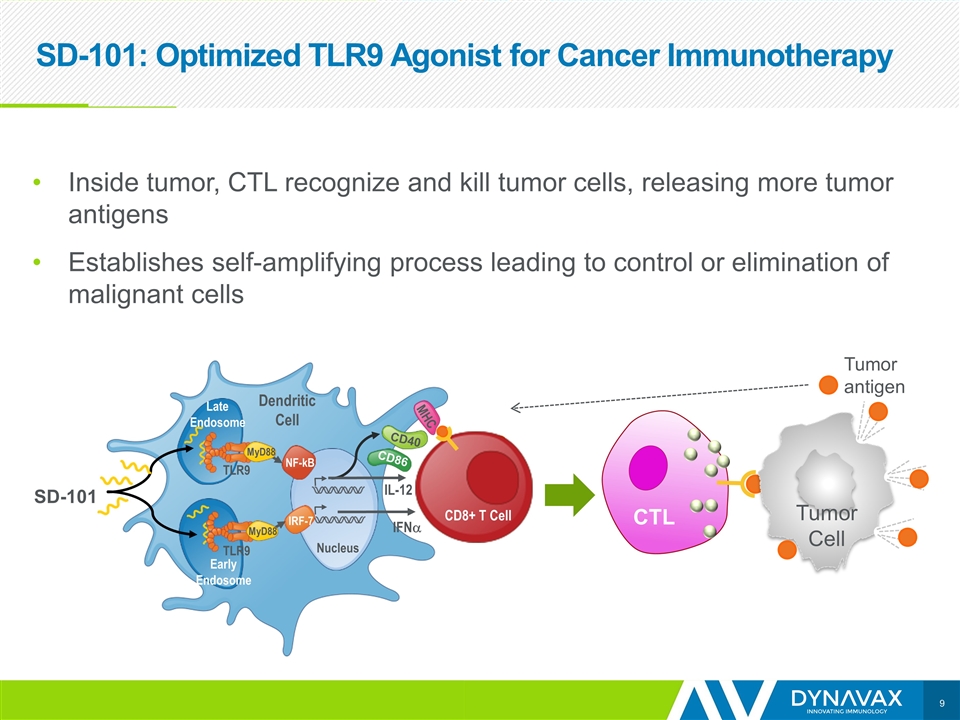
SD-101: Optimized TLR9 Agonist for Cancer Immunotherapy Inside tumor, CTL recognize and kill tumor cells, releasing more tumor antigens Establishes self-amplifying process leading to control or elimination of malignant cells SD-101 CTL NF-kB IRF-7 Nucleus IFNa IL-12 Dendritic Cell Late Endosome Early Endosome MyD88 MyD88 TLR9 TLR9 CD40 CD86 MHC CD8+ T Cell Tumor Cell Tumor antigen
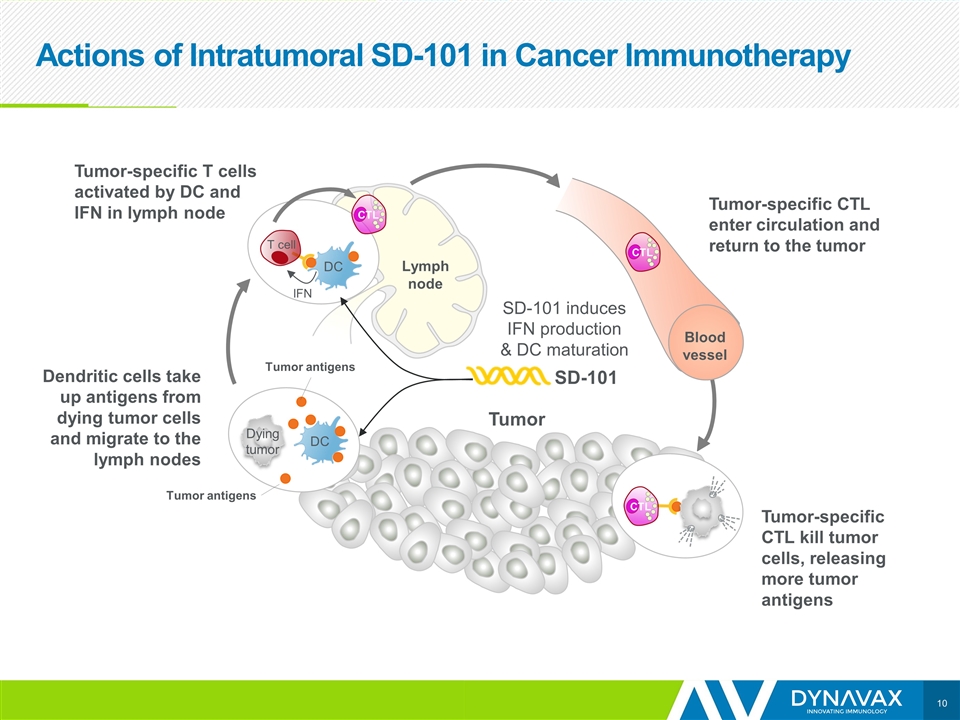
Actions of Intratumoral SD-101 in Cancer Immunotherapy Tumor IFN DC T cell Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes SD-101 Tumor antigens Lymph node DC Dying tumor Tumor-specific T cells activated by DC and IFN in lymph node Blood vessel CTL CTL CTL SD-101 induces IFN production & DC maturation Tumor-specific CTL kill tumor cells, releasing more tumor antigens Tumor Tumor-specific CTL enter circulation and return to the tumor Tumor antigens
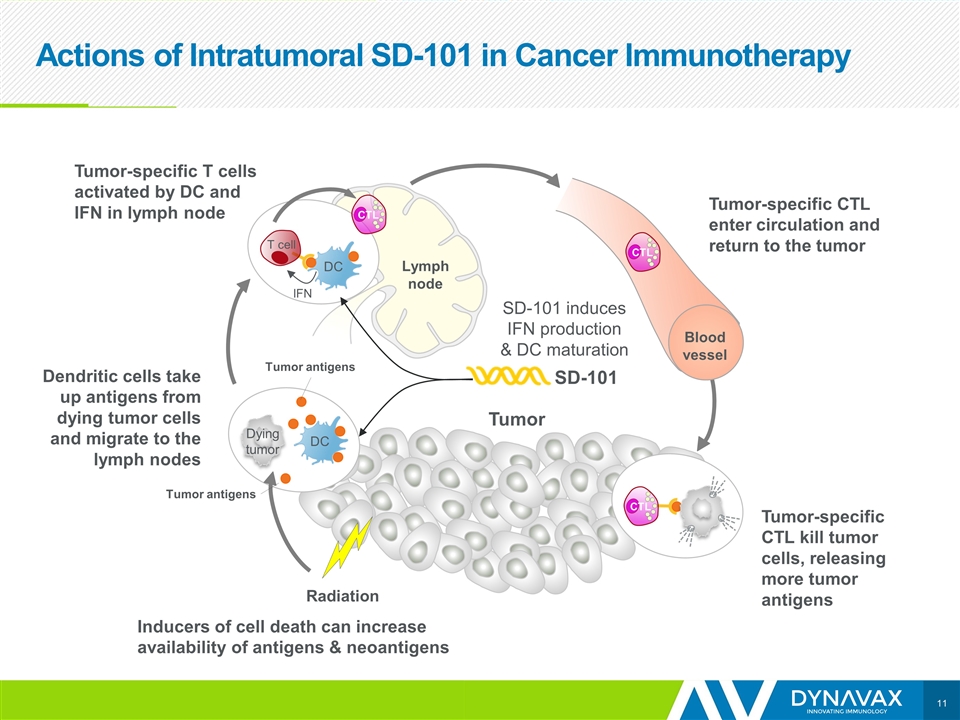
Actions of Intratumoral SD-101 in Cancer Immunotherapy Tumor IFN DC T cell Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes SD-101 Lymph node DC Dying tumor Tumor-specific T cells activated by DC and IFN in lymph node Blood vessel CTL CTL CTL SD-101 induces IFN production & DC maturation Tumor-specific CTL kill tumor cells, releasing more tumor antigens Tumor Tumor-specific CTL enter circulation and return to the tumor Radiation Inducers of cell death can increase availability of antigens & neoantigens Tumor antigens Tumor antigens
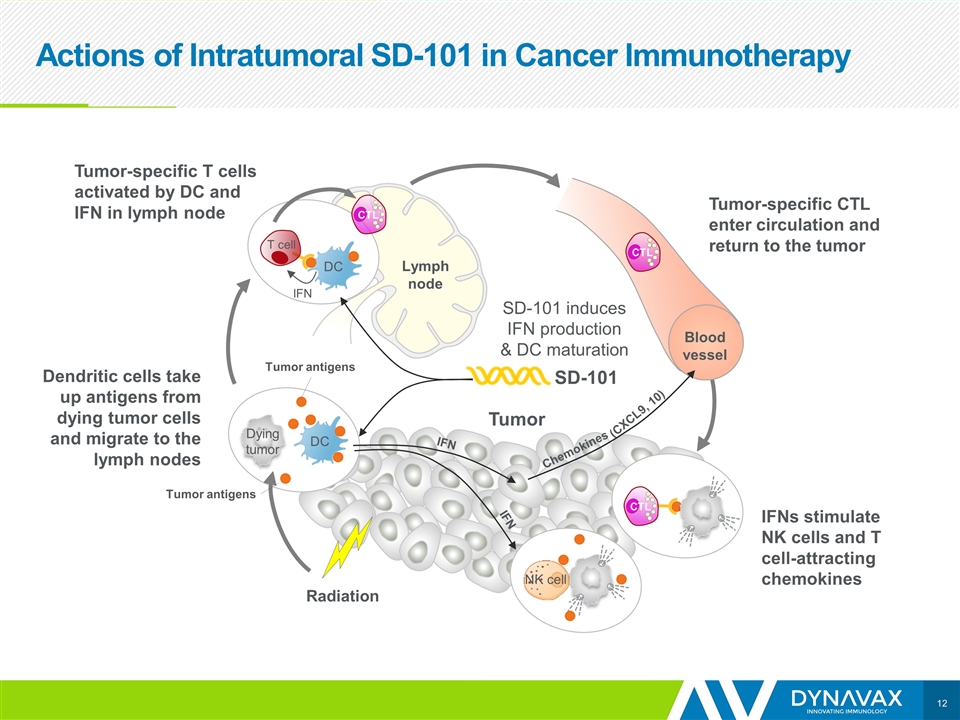
Actions of Intratumoral SD-101 in Cancer Immunotherapy Tumor IFN DC T cell Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes SD-101 Lymph node DC Dying tumor Tumor-specific T cells activated by DC and IFN in lymph node Blood vessel CTL CTL CTL SD-101 induces IFN production & DC maturation IFNs stimulate NK cells and T cell-attracting chemokines Tumor Tumor-specific CTL enter circulation and return to the tumor Radiation IFN IFN Chemokines (CXCL9, 10) NK cell Tumor antigens Tumor antigens
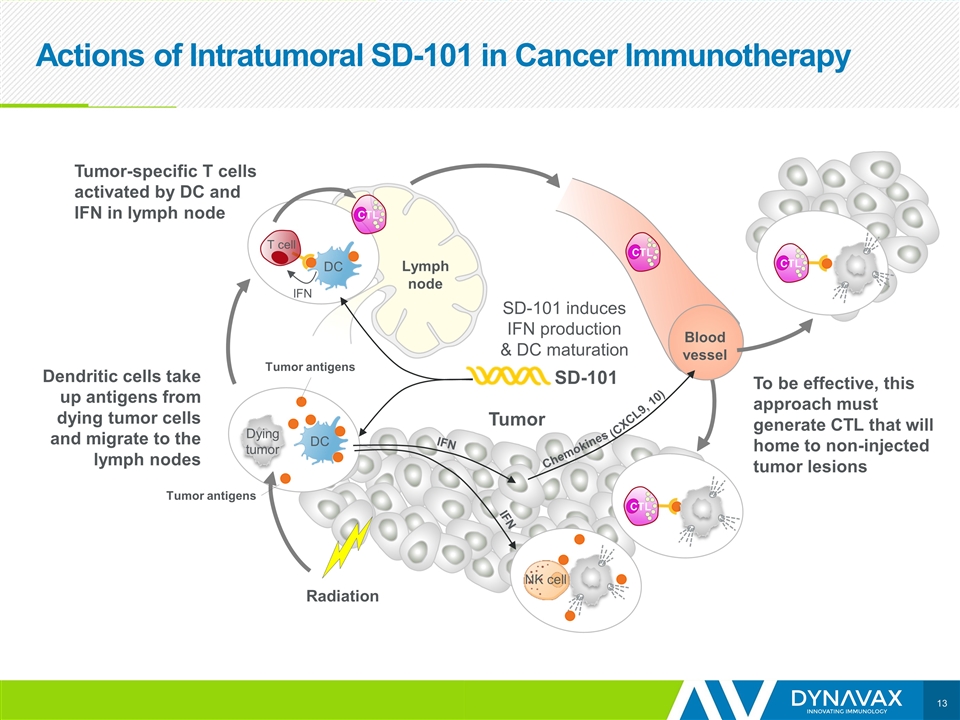
Actions of Intratumoral SD-101 in Cancer Immunotherapy Tumor IFN DC T cell Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes SD-101 Lymph node DC Dying tumor Tumor-specific T cells activated by DC and IFN in lymph node Blood vessel CTL CTL CTL SD-101 induces IFN production & DC maturation To be effective, this approach must generate CTL that will home to non-injected tumor lesions Tumor Radiation IFN IFN Chemokines (CXCL9, 10) NK cell CTL Tumor antigens Tumor antigens
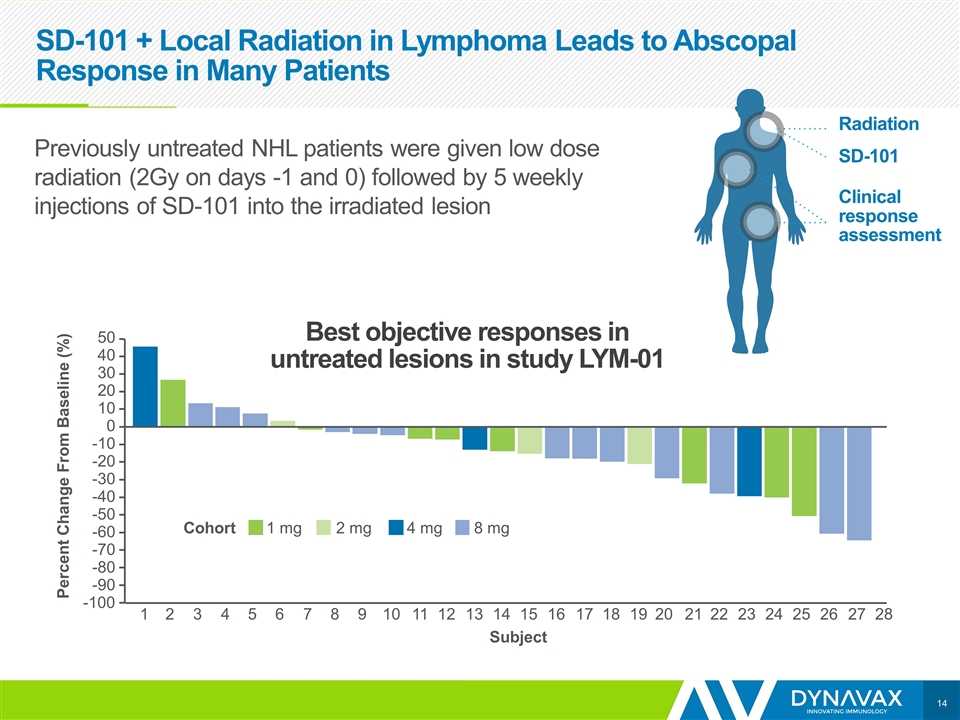
SD-101 + Local Radiation in Lymphoma Leads to Abscopal Response in Many Patients 1 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 2 3 4 5 6 7 8 9 10 11 12 13 14 Subject 15 16 17 18 19 20 21 22 23 24 25 26 27 28 1 mg 2 mg 4 mg 8 mg Cohort Percent Change From Baseline (%) Best objective responses in untreated lesions in study LYM-01 Radiation SD-101 Clinical response assessment Previously untreated NHL patients were given low dose radiation (2Gy on days -1 and 0) followed by 5 weekly injections of SD-101 into the irradiated lesion
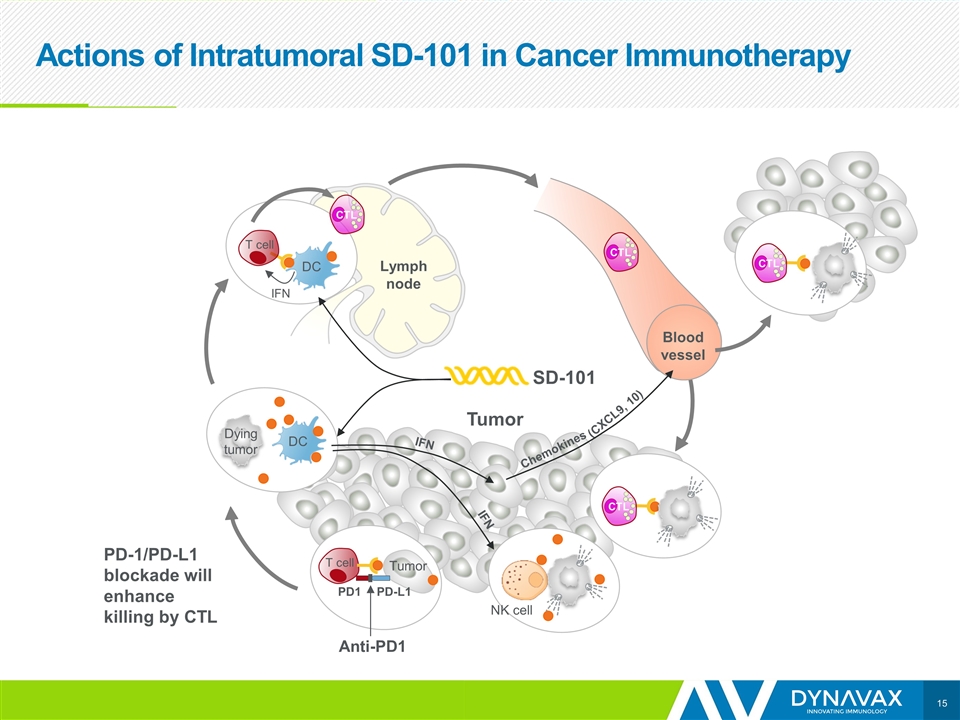
Actions of Intratumoral SD-101 in Cancer Immunotherapy Tumor IFN DC T cell SD-101 Lymph node DC Dying tumor Blood vessel CTL CTL CTL Tumor IFN IFN Chemokines (CXCL9, 10) NK cell CTL T cell PD-L1 PD1 Anti-PD1 Tumor PD-1/PD-L1 blockade will enhance killing by CTL
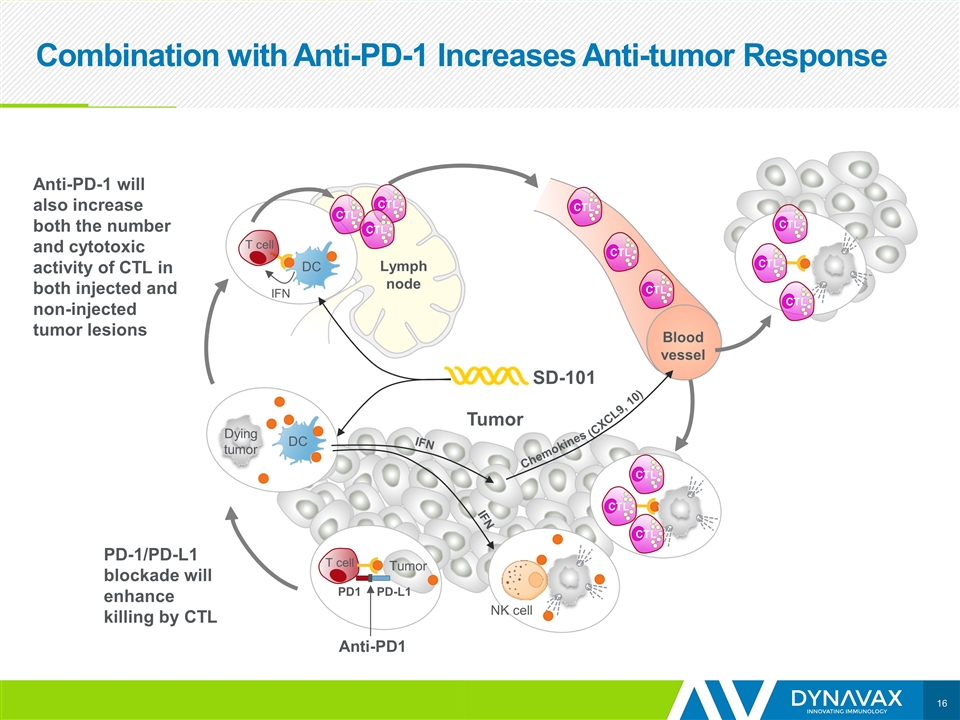
Combination with Anti-PD-1 Increases Anti-tumor Response Tumor IFN DC T cell SD-101 Lymph node DC Dying tumor Blood vessel CTL CTL CTL Tumor IFN IFN Chemokines (CXCL9, 10) NK cell CTL T cell PD-L1 PD1 Anti-PD1 Tumor PD-1/PD-L1 blockade will enhance killing by CTL Anti-PD-1 will also increase both the number and cytotoxic activity of CTL in both injected and non-injected tumor lesions CTL CTL CTL CTL CTL CTL CTL CTL
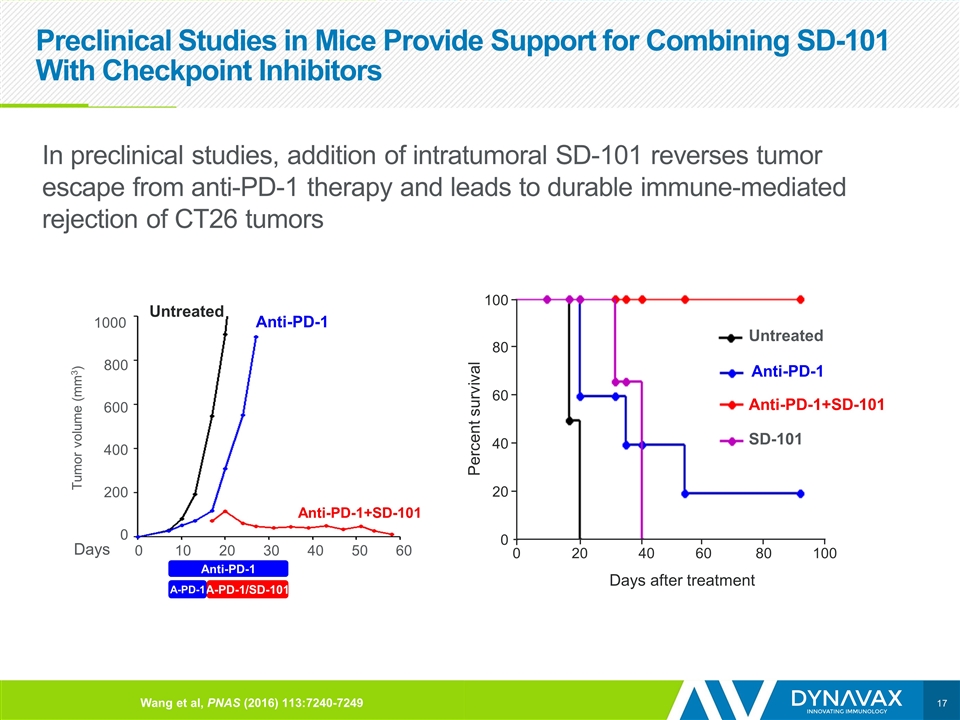
Preclinical Studies in Mice Provide Support for Combining SD-101 With Checkpoint Inhibitors In preclinical studies, addition of intratumoral SD-101 reverses tumor escape from anti-PD-1 therapy and leads to durable immune-mediated rejection of CT26 tumors Untreated Anti-PD-1+SD-101 Anti-PD-1 Untreated Days Tumor volume (mm3) 1000 800 600 400 200 0 0 10 20 30 40 50 60 100 80 60 40 20 0 100 80 60 40 20 0 Percent survival Days after treatment SD-101 Anti-PD-1+SD-101 Untreated Anti-PD-1 Anti-PD-1 A-PD-1/SD-101 A-PD-1 Wang et al, PNAS (2016) 113:7240-7249
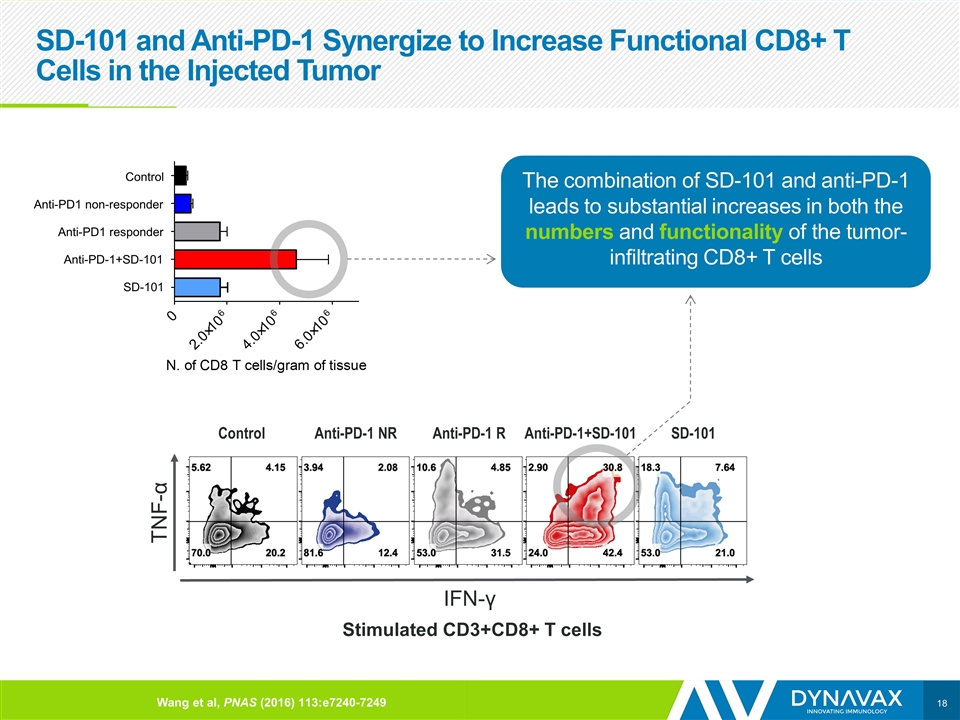
SD-101 and Anti-PD-1 Synergize to Increase Functional CD8+ T Cells in the Injected Tumor Stimulated CD3+CD8+ T cells IFN-γ TNF-α The combination of SD-101 and anti-PD-1 leads to substantial increases in both the numbers and functionality of the tumor-infiltrating CD8+ T cells Control Anti-PD1 non-responder Anti-PD1 responder Anti-PD-1+SD-101 SD-101 Control Anti-PD-1 NR SD-101 Anti-PD-1 R Wang et al, PNAS (2016) 113:e7240-7249 Anti-PD-1+SD-101
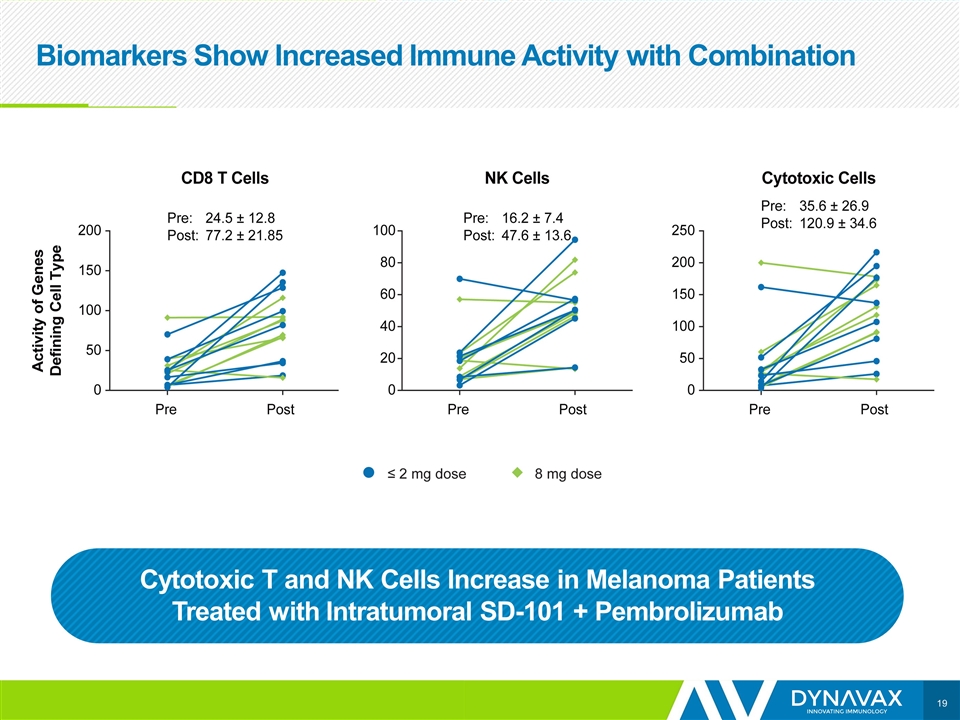
Biomarkers Show Increased Immune Activity with Combination Cytotoxic T and NK Cells Increase in Melanoma Patients Treated with Intratumoral SD-101 + Pembrolizumab

Toni Ribas, MD, PhD

Resistance to PD-1 blockade due to lack of pre-existing antitumor immunity Antoni Ribas, M.D., Ph.D. Professor of Medicine, Surgery, Molecular and Medical Pharmacology Director, Tumor Immunology Program, Jonsson Comprehensive Cancer Center (JCCC) Director, Parker Institute for Cancer Immunotherapy (PICI) Center at UCLA University of California Los Angeles (UCLA) Chair, Melanoma Committee at SWOG
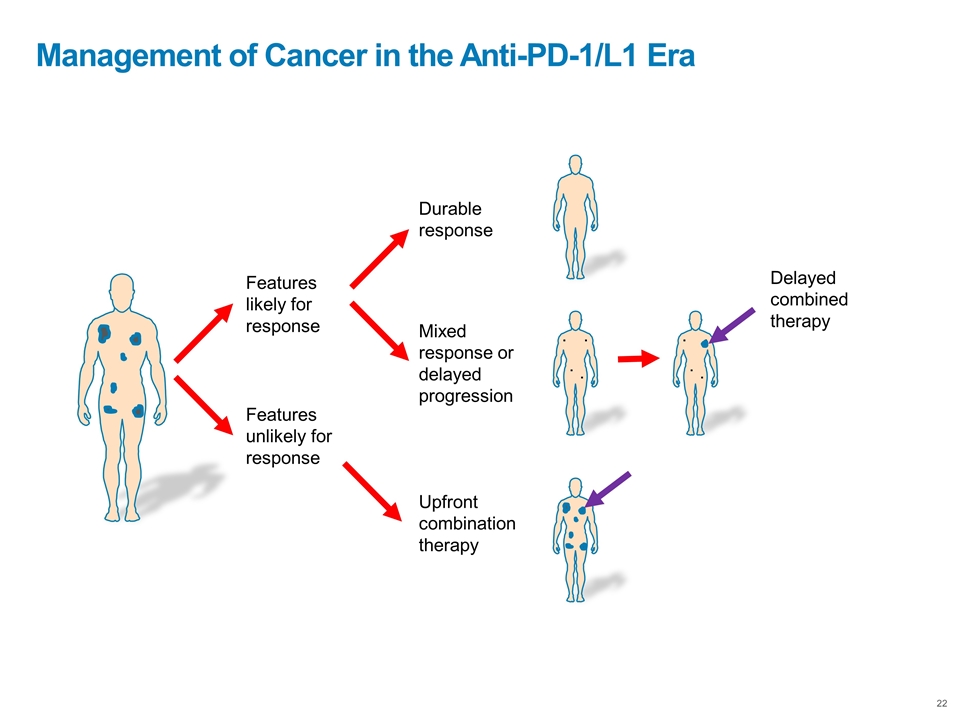
Management of Cancer in the Anti-PD-1/L1 Era Features likely for response Features unlikely for response Upfront combination therapy Durable response Mixed response or delayed progression . . . . . . . . Delayed combined therapy
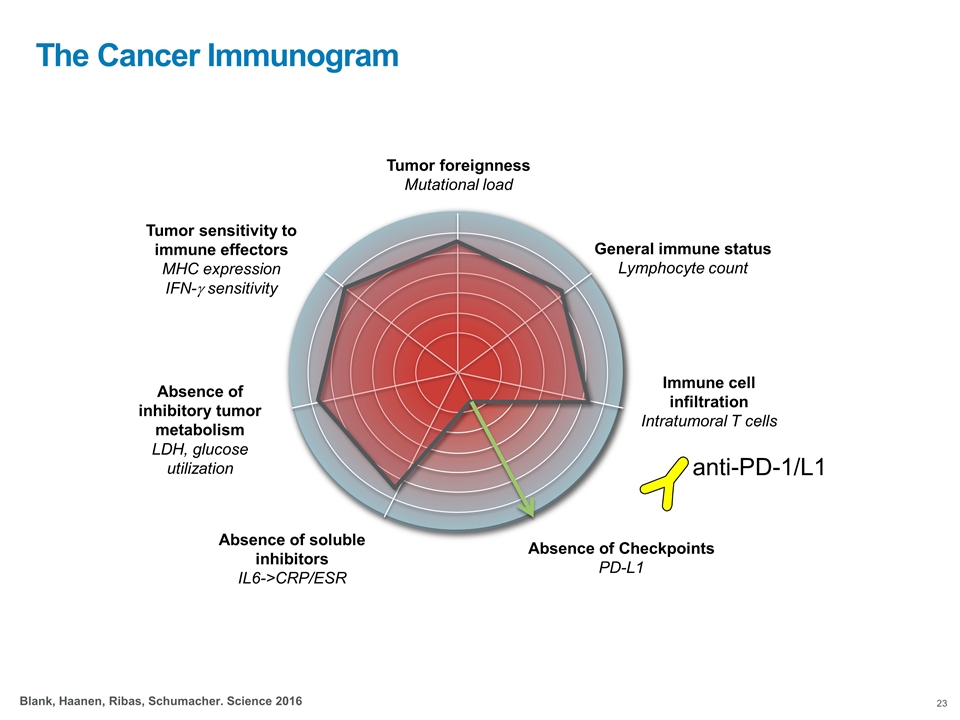
Tumor foreignness Mutational load General immune status Lymphocyte count Immune cell infiltration Intratumoral T cells Absence of Checkpoints PD-L1 Absence of soluble inhibitors IL6->CRP/ESR Tumor sensitivity to immune effectors MHC expression IFN-g sensitivity Absence of inhibitory tumor metabolism LDH, glucose utilization Blank, Haanen, Ribas, Schumacher. Science 2016 The Cancer Immunogram anti-PD-1/L1
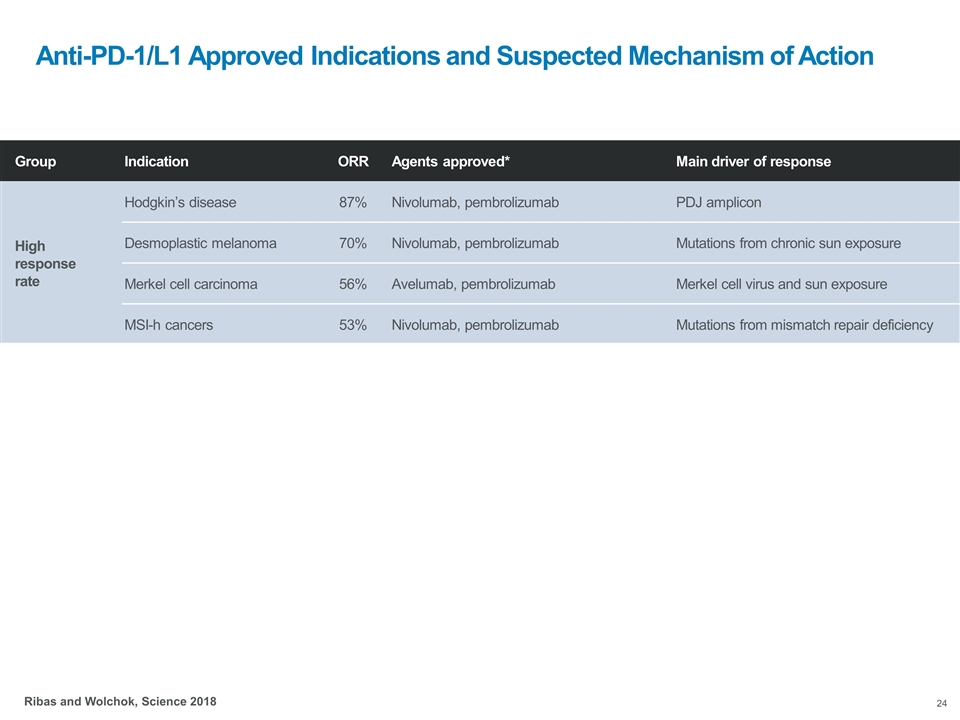
Anti-PD-1/L1 Approved Indications and Suspected Mechanism of Action Group Indication ORR Agents approved* Main driver of response High response rate Hodgkin’s disease 87% Nivolumab, pembrolizumab PDJ amplicon Desmoplastic melanoma 70% Nivolumab, pembrolizumab Mutations from chronic sun exposure Merkel cell carcinoma 56% Avelumab, pembrolizumab Merkel cell virus and sun exposure MSI-h cancers 53% Nivolumab, pembrolizumab Mutations from mismatch repair deficiency Intermediate response rate Skin melanoma 35-40% Nivolumab, pembrolizumab Mutations from intermittent sun exposure Lung cancer 20% Atezolizumab, nivolumab, pembrolizumab Mutations from cigarette smoking Head and neck cancers 15% Nivolumab, pembrolizumab Mutations from cigarette smoking Gastro-esophageal cancer 15% Pembrolizumab Mutations from cigarette smoking Bladder/urinary tract cancers 15% Atezolizumab, avelumab, durvalumab, nivolumab, pembrolizumab Mutations from cigarette smoking Renal cell carcinoma 25% Nivolumab, pembrolizumab Insertion/deletions (indels) Hepatocellular carcinoma 20% Nivolumab Hepatitis virus Ribas and Wolchok, Science 2018
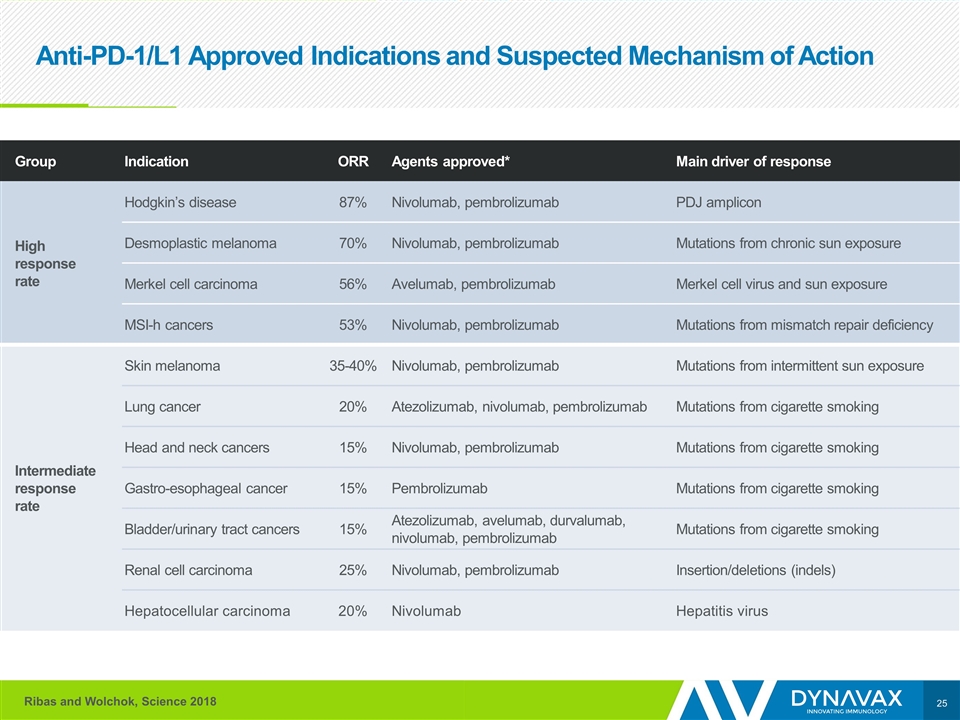
Anti-PD-1/L1 Approved Indications and Suspected Mechanism of Action Group Indication ORR Agents approved* Main driver of response High response rate Hodgkin’s disease 87% Nivolumab, pembrolizumab PDJ amplicon Desmoplastic melanoma 70% Nivolumab, pembrolizumab Mutations from chronic sun exposure Merkel cell carcinoma 56% Avelumab, pembrolizumab Merkel cell virus and sun exposure MSI-h cancers 53% Nivolumab, pembrolizumab Mutations from mismatch repair deficiency Intermediate response rate Skin melanoma 35-40% Nivolumab, pembrolizumab Mutations from intermittent sun exposure Lung cancer 20% Atezolizumab, nivolumab, pembrolizumab Mutations from cigarette smoking Head and neck cancers 15% Nivolumab, pembrolizumab Mutations from cigarette smoking Gastro-esophageal cancer 15% Pembrolizumab Mutations from cigarette smoking Bladder/urinary tract cancers 15% Atezolizumab, avelumab, durvalumab, nivolumab, pembrolizumab Mutations from cigarette smoking Renal cell carcinoma 25% Nivolumab, pembrolizumab Insertion/deletions (indels) Hepatocellular carcinoma 20% Nivolumab Hepatitis virus Ribas and Wolchok, Science 2018
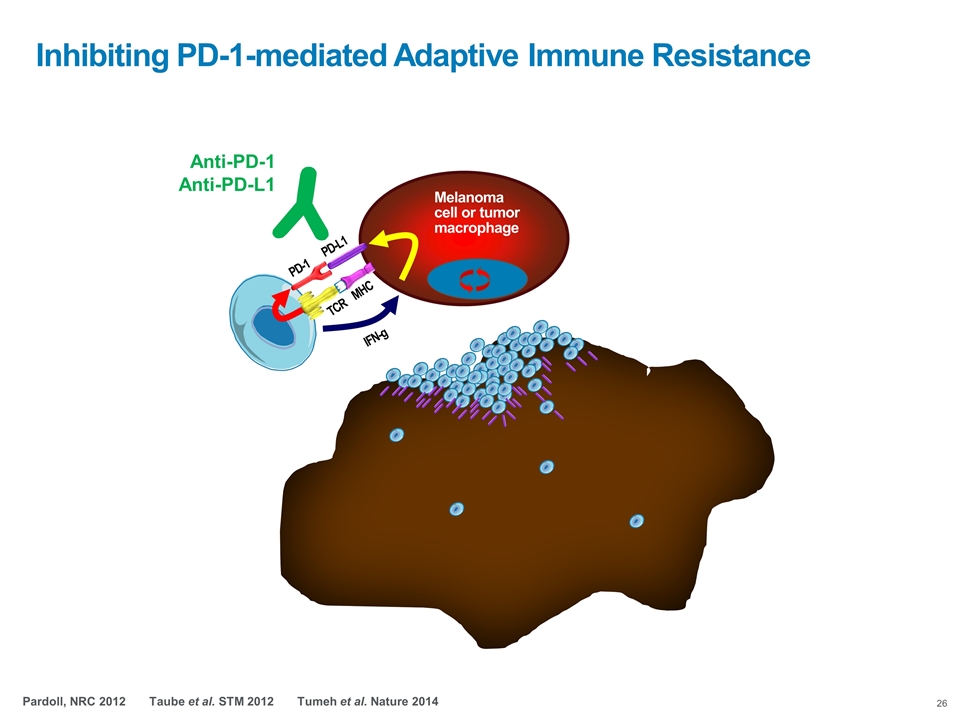
Inhibiting PD-1-mediated Adaptive Immune Resistance TCR MHC Melanoma cell or tumor macrophage PD-1 PD-L1 IFN-g Anti-PD-1 Anti-PD-L1 Pardoll, NRC 2012 Taube et al. STM 2012 Tumeh et al. Nature 2014
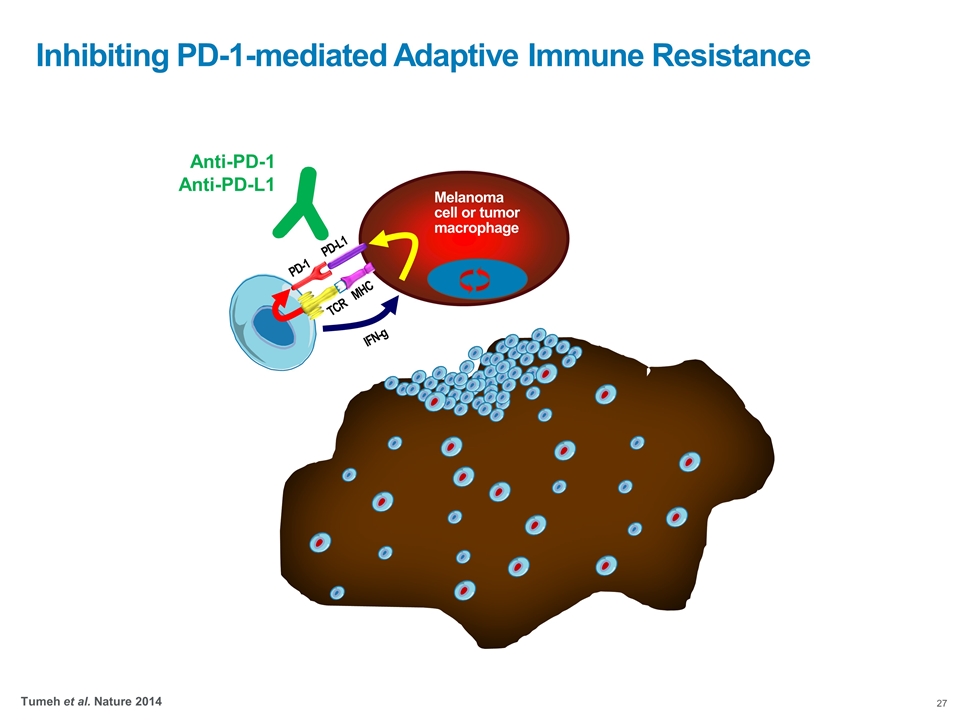
Inhibiting PD-1-mediated Adaptive Immune Resistance Tumeh et al. Nature 2014 TCR MHC Melanoma cell or tumor macrophage PD-1 PD-L1 IFN-g Anti-PD-1 Anti-PD-L1
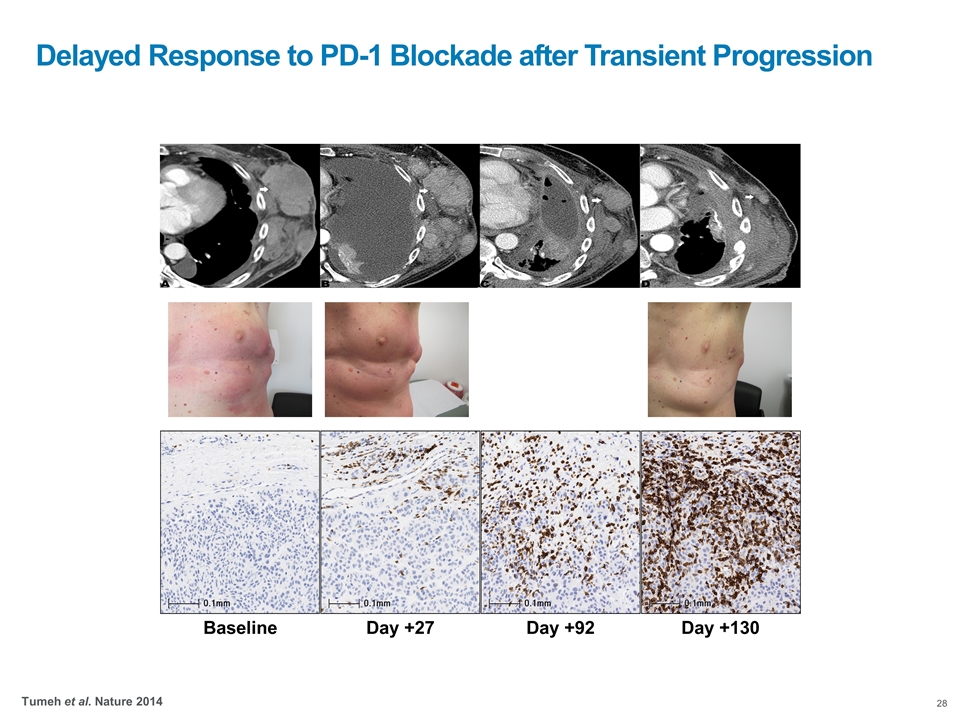
Baseline Day +27 Day +92 Day +130 Delayed Response to PD-1 Blockade after Transient Progression Tumeh et al. Nature 2014
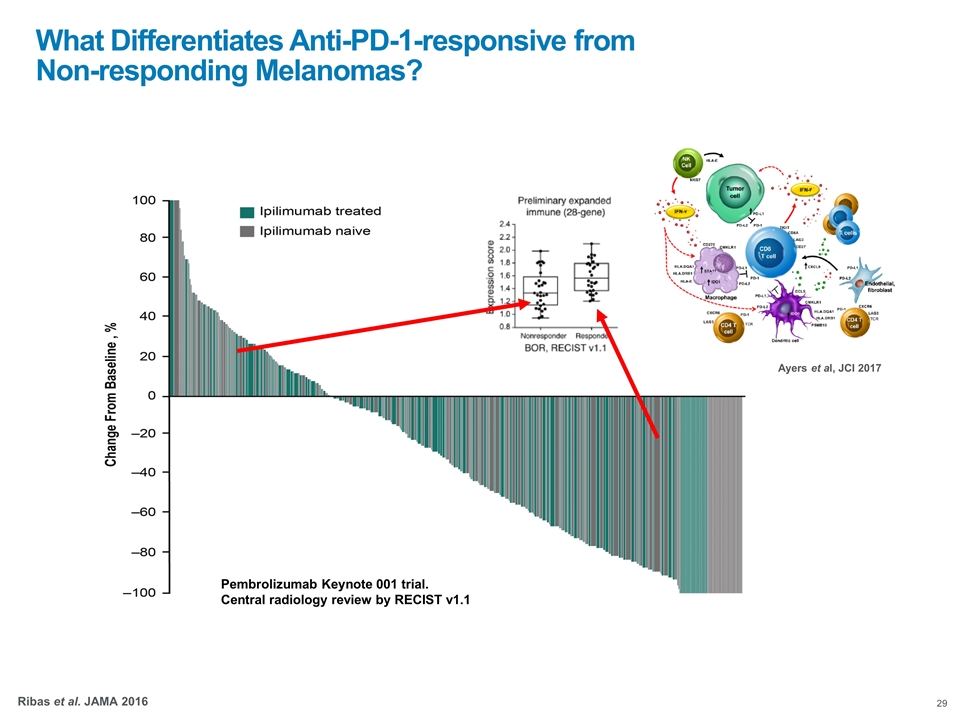
Pembrolizumab Keynote 001 trial. Central radiology review by RECIST v1.1 What Differentiates Anti-PD-1-responsive from Non-responding Melanomas? Ribas et al. JAMA 2016 Ayers et al, JCI 2017
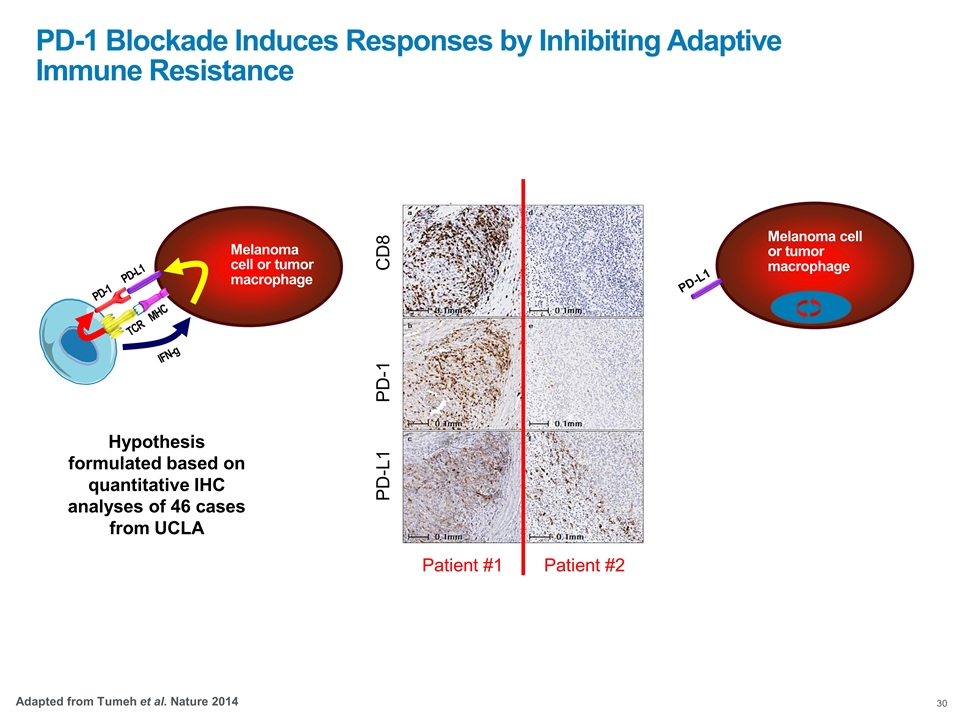
Melanoma cell or tumor macrophage PD-L1 Hypothesis formulated based on quantitative IHC analyses of 46 cases from UCLA Response Progression CD8 PD-1 PD-L1 Patient #1 Patient #2 PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance Adapted from Tumeh et al. Nature 2014 TCR MHC Melanoma cell or tumor macrophage PD-1 PD-L1 IFN-g
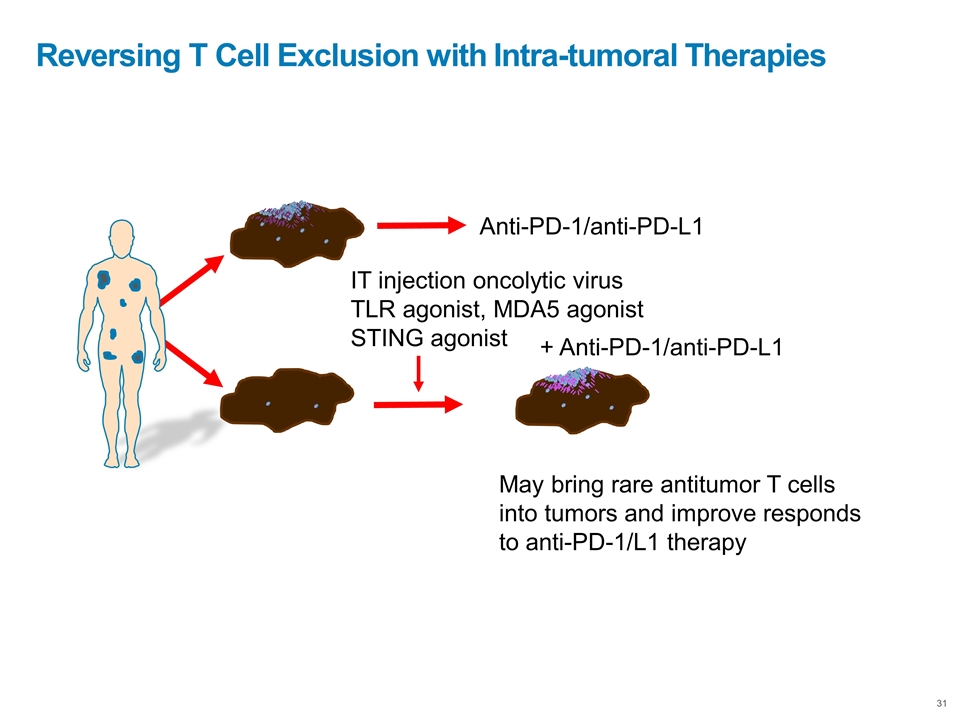
Reversing T Cell Exclusion with Intra-tumoral Therapies Anti-PD-1/anti-PD-L1 IT injection oncolytic virus TLR agonist, MDA5 agonist STING agonist May bring rare antitumor T cells into tumors and improve responds to anti-PD-1/L1 therapy + Anti-PD-1/anti-PD-L1
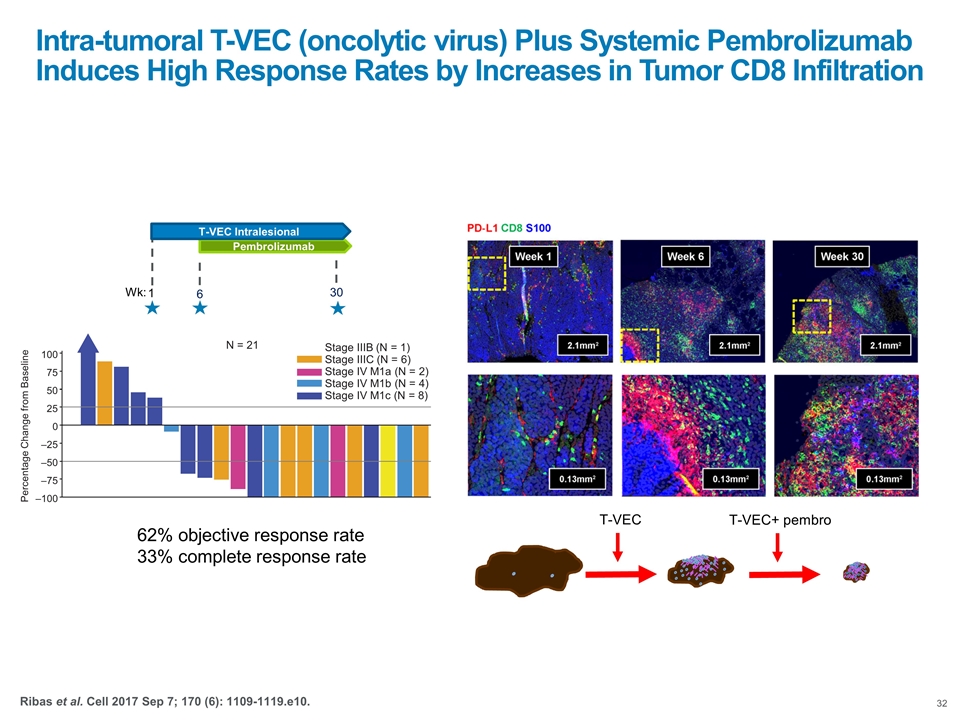
Intra-tumoral T-VEC (oncolytic virus) Plus Systemic Pembrolizumab Induces High Response Rates by Increases in Tumor CD8 Infiltration T-VEC T-VEC+ pembro –100 –75 –50 –25 0 25 50 75 100 Stage IV M1c (N = 8) Stage IV M1b (N = 4) Stage IV M1a (N = 2) Stage IIIC (N = 6) Stage IIIB (N = 1) N = 21 Percentage Change from Baseline Pembrolizumab 6 T-VEC Intralesional 1 30 Wk: 62% objective response rate 33% complete response rate Ribas et al. Cell 2017 Sep 7; 170 (6): 1109-1119.e10.
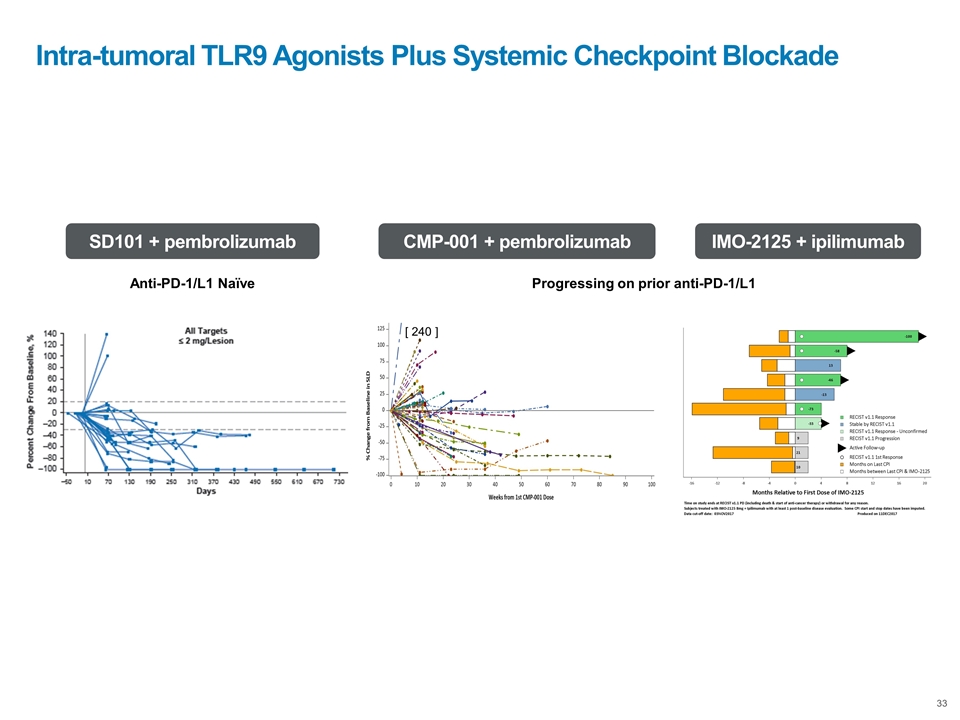
Intra-tumoral TLR9 Agonists Plus Systemic Checkpoint Blockade Anti-PD-1/L1 Naïve SD101 + pembrolizumab Progressing on prior anti-PD-1/L1 CMP-001 + pembrolizumab IMO-2125 + ipilimumab [ 240 ]

Conclusions Inhibiting adaptive immune resistance is the mechanistic basis of the antitumor activity of PD-1 blockade therapies Patients without a pre-existing tumor antigen-specific T cell infiltrate inducing reactive PD-L1 expression are unlikely to respond to PD-1 blockade therapy Inducing intratumoral infiltration by antigen-specific T cells is likely to potentiate the antitumor activity of PD-1 blockade therapy

Rob Janssen, MD
SYNERGY-001 (MEL-01) Phase 1b/2 study of the combination of SD-101 and pembrolizumab in advanced melanoma and head and neck squamous cell cancer OBJECTIVES: Evaluate the safety of the combination Evaluate the preliminary efficacy of the combination Establish the dose of SD-101 for the Phase 3 trial (2 mg vs. 8 mg)

Understanding Dose Selection SD-101 Mechanism of Action is to stimulate the immune system to respond to specific antigens Efficacy may be most informative – Looking for “sweet-spot”/the Goldilocks dose. Not too hot, not too cold! Blood PK not informative – Primary mechanism is local in the tumor and draining lymph nodes Safety may not be informative – Benign profile may not distinguish between doses—no MTD No biomarkers of CpG-mediated anti-tumor effects established – Immune stimuli generally stimulate dose-dependent feedback mechanisms complicating interpretation of markers of stimulation Seeking balance to achieve most potent immune response
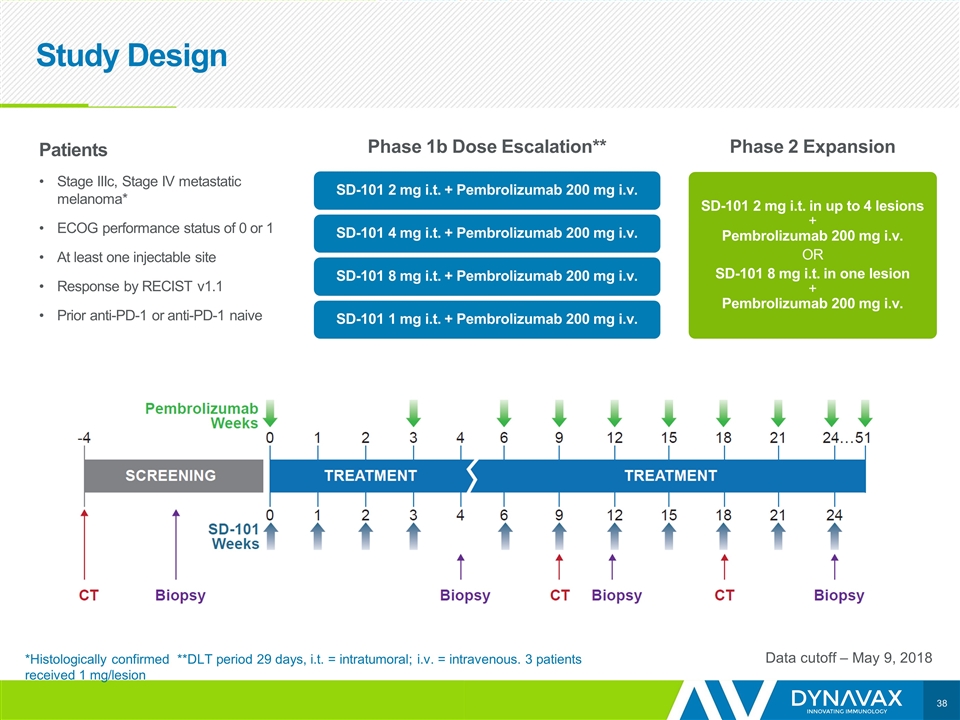
Study Design Data cutoff – May 9, 2018 Patients Stage IIIc, Stage IV metastatic melanoma* ECOG performance status of 0 or 1 At least one injectable site Response by RECIST v1.1 Prior anti-PD-1 or anti-PD-1 naive Phase 1b Dose Escalation** Phase 2 Expansion SD-101 2 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 4 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 8 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 1 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 2 mg i.t. in up to 4 lesions + Pembrolizumab 200 mg i.v. OR SD-101 8 mg i.t. in one lesion + Pembrolizumab 200 mg i.v. *Histologically confirmed **DLT period 29 days, i.t. = intratumoral; i.v. = intravenous. 3 patients received 1 mg/lesion
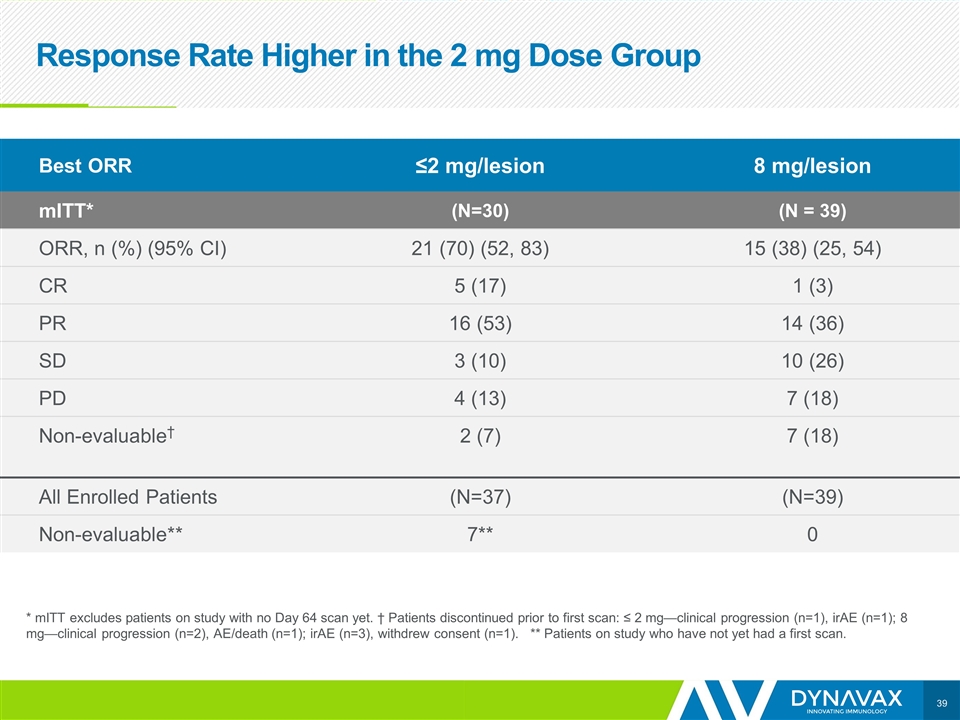
Response Rate Higher in the 2 mg Dose Group * mITT excludes patients on study with no Day 64 scan yet. † Patients discontinued prior to first scan: ≤ 2 mg—clinical progression (n=1), irAE (n=1); 8 mg—clinical progression (n=2), AE/death (n=1); irAE (n=3), withdrew consent (n=1). ** Patients on study who have not yet had a first scan. Best ORR ≤2 mg/lesion 8 mg/lesion mITT* (N=30) (N = 39) ORR, n (%) (95% CI) 21 (70) (52, 83) 15 (38) (25, 54) CR 5 (17) 1 (3) PR 16 (53) 14 (36) SD 3 (10) 10 (26) PD 4 (13) 7 (18) Non-evaluable† 2 (7) 7 (18) All Enrolled Patients (N=37) (N=39) Non-evaluable** 7** 0
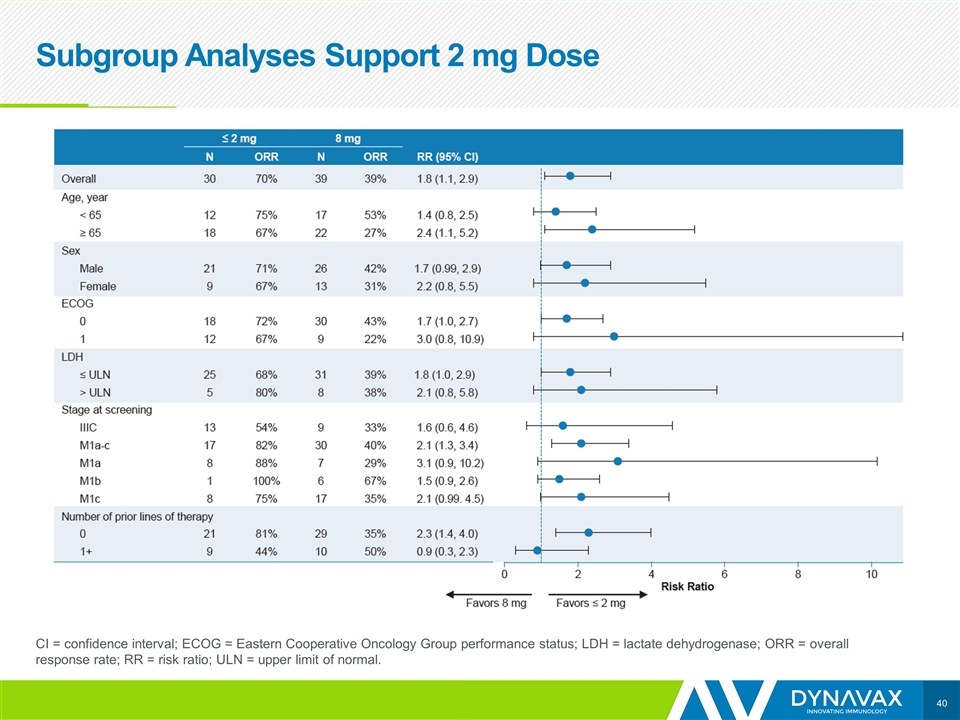
Subgroup Analyses Support 2 mg Dose CI = confidence interval; ECOG = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; ORR = overall response rate; RR = risk ratio; ULN = upper limit of normal.
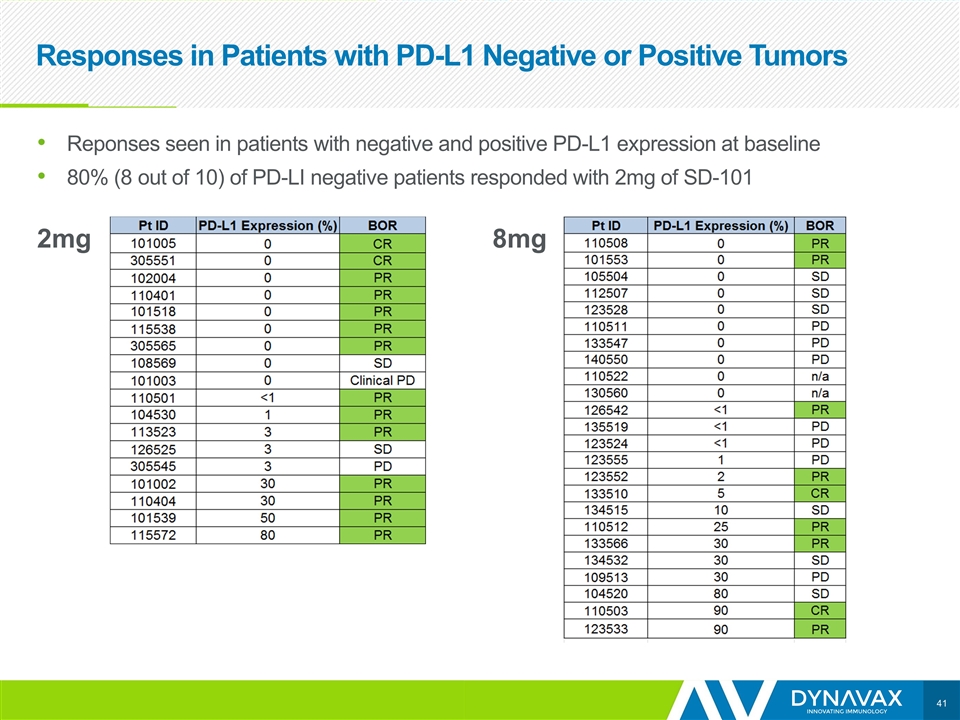
2mg 8mg Responses in Patients with PD-L1 Negative or Positive Tumors Reponses seen in patients with negative and positive PD-L1 expression at baseline 80% (8 out of 10) of PD-LI negative patients responded with 2mg of SD-101
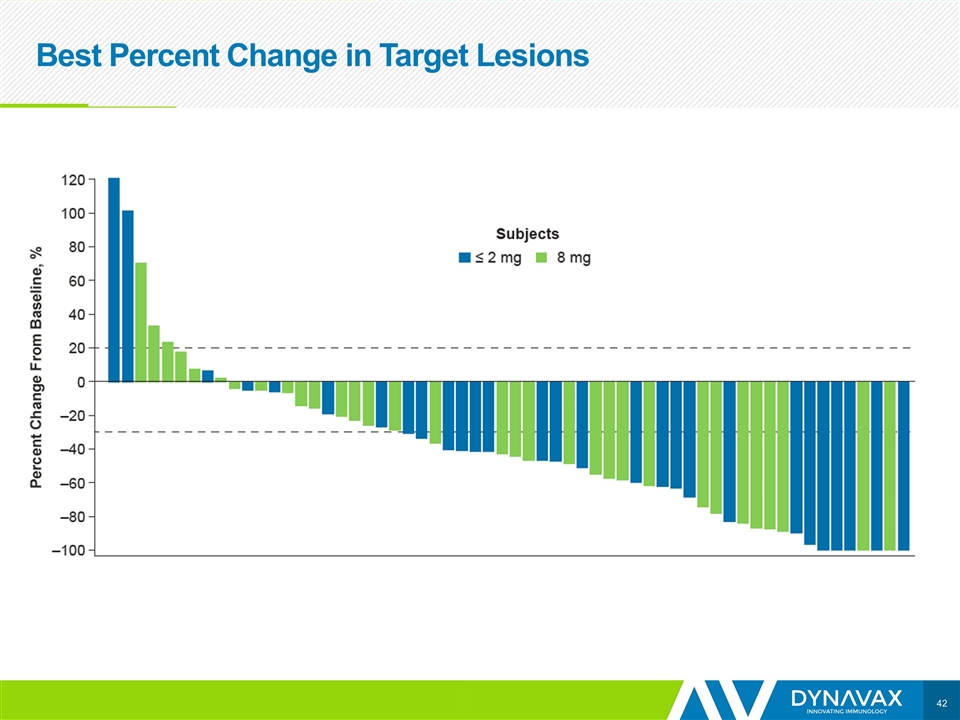
Best Percent Change in Target Lesions
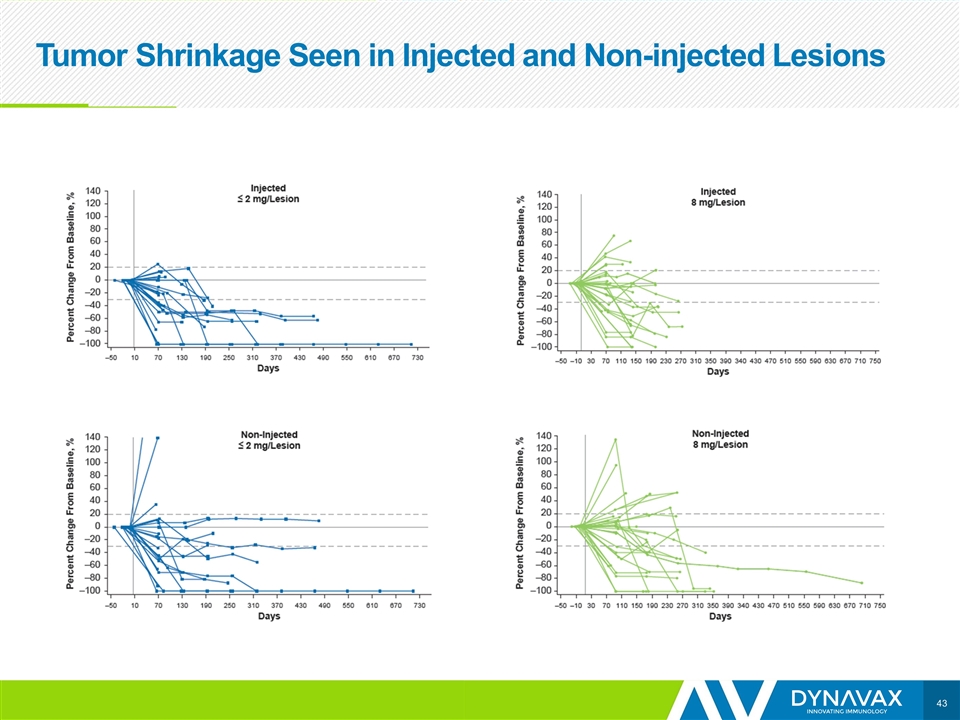
Tumor Shrinkage Seen in Injected and Non-injected Lesions
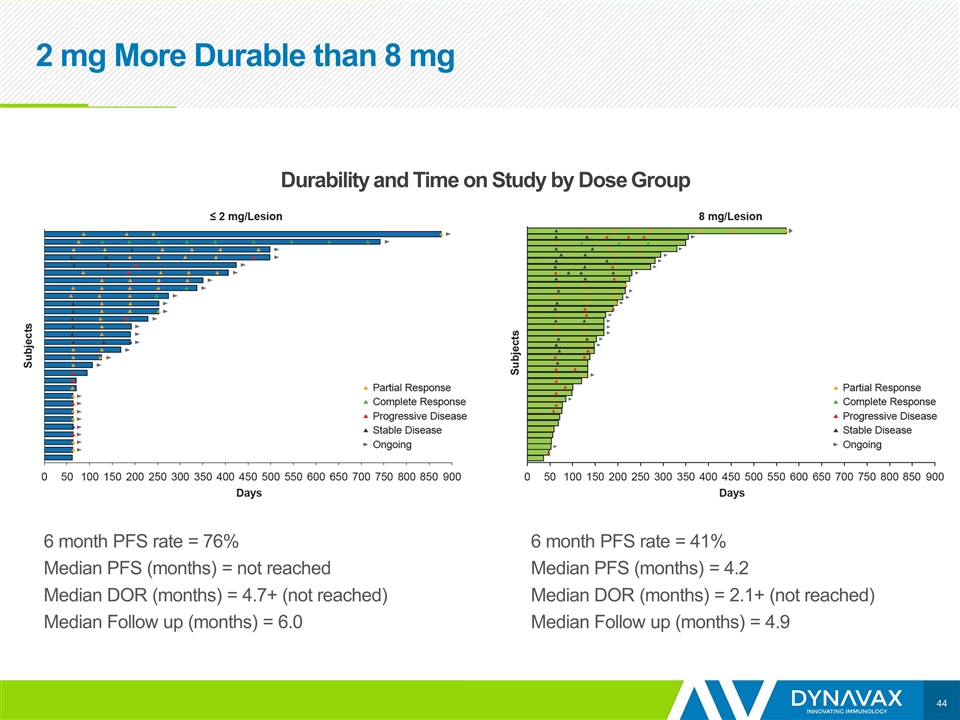
6 month PFS rate = 76% Median PFS (months) = not reached Median DOR (months) = 4.7+ (not reached) Median Follow up (months) = 6.0 6 month PFS rate = 41% Median PFS (months) = 4.2 Median DOR (months) = 2.1+ (not reached) Median Follow up (months) = 4.9 Durability and Time on Study by Dose Group 2 mg More Durable than 8 mg
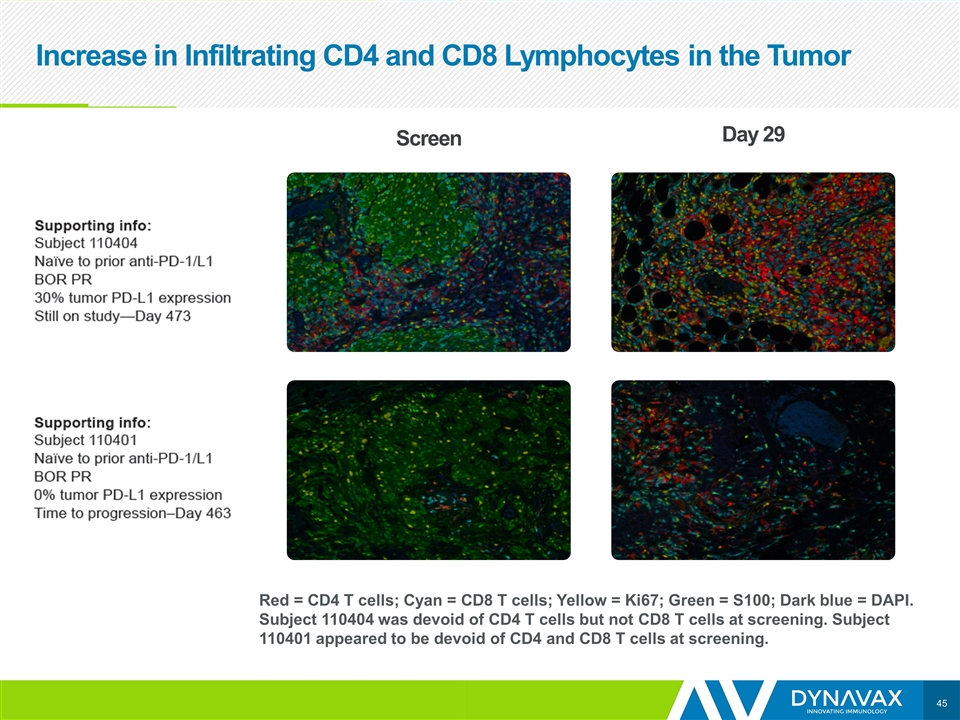
Increase in Infiltrating CD4 and CD8 Lymphocytes in the Tumor Red = CD4 T cells; Cyan = CD8 T cells; Yellow = Ki67; Green = S100; Dark blue = DAPI. Subject 110404 was devoid of CD4 T cells but not CD8 T cells at screening. Subject 110401 appeared to be devoid of CD4 and CD8 T cells at screening. Screen Day 29
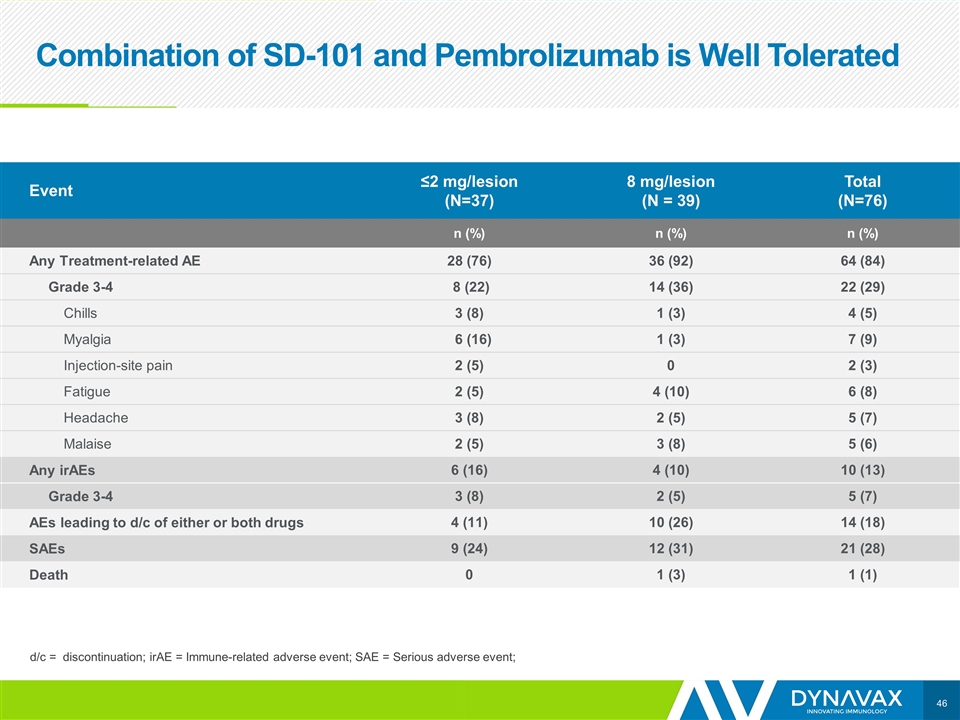
Combination of SD-101 and Pembrolizumab is Well Tolerated Event ≤2 mg/lesion (N=37) 8 mg/lesion (N = 39) Total (N=76) n (%) n (%) n (%) Any Treatment-related AE 28 (76) 36 (92) 64 (84) Grade 3-4 8 (22) 14 (36) 22 (29) Chills 3 (8) 1 (3) 4 (5) Myalgia 6 (16) 1 (3) 7 (9) Injection-site pain 2 (5) 0 2 (3) Fatigue 2 (5) 4 (10) 6 (8) Headache 3 (8) 2 (5) 5 (7) Malaise 2 (5) 3 (8) 5 (6) Any irAEs 6 (16) 4 (10) 10 (13) Grade 3-4 3 (8) 2 (5) 5 (7) AEs leading to d/c of either or both drugs 4 (11) 10 (26) 14 (18) SAEs 9 (24) 12 (31) 21 (28) Death 0 1 (3) 1 (1) d/c = discontinuation; irAE = Immune-related adverse event; SAE = Serious adverse event;
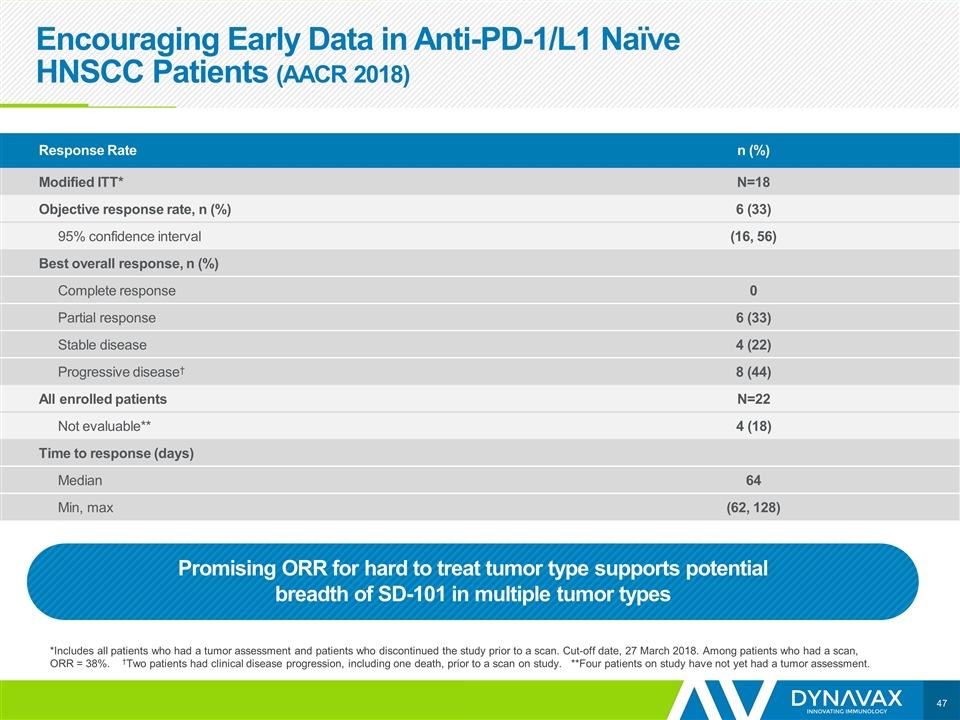
Encouraging Early Data in Anti-PD-1/L1 Naïve HNSCC Patients (AACR 2018) *Includes all patients who had a tumor assessment and patients who discontinued the study prior to a scan. Cut-off date, 27 March 2018. Among patients who had a scan, ORR = 38%. †Two patients had clinical disease progression, including one death, prior to a scan on study. **Four patients on study have not yet had a tumor assessment. Response Rate n (%) Modified ITT* N=18 Objective response rate, n (%) 6 (33) 95% confidence interval (16, 56) Best overall response, n (%) Complete response 0 Partial response 6 (33) Stable disease 4 (22) Progressive disease† 8 (44) All enrolled patients N=22 Not evaluable** 4 (18) Time to response (days) Median 64 Min, max (62, 128) Promising ORR for hard to treat tumor type supports potential breadth of SD-101 in multiple tumor types
SYNERGY-001 Conclusions Efficacy - addition of SD-101 to pembrolizumab appears to improve pembrolizumab responses ≤ 2 mg of SD-101 per lesion induced a higher and more durable response rate than pembrolizumab with 8 mg of SD-101 ≤ 2 mg dose better than 8 mg dose in all disease stages Tumor shrinkage in injected and non-injected lesions including lung and liver Responses in patients with negative or positive baseline PD-L1 expression Safety Transient, mild-moderate flu like symptoms No increase in frequency or severity of irAEs No treatment-related, unexpected safety event
Proceeding to Phase 3 Continue to enroll patients in Phase 2 to receive 2 mg SD-101: Melanoma: anti-PD-1/L1 experienced HNSCC: anti-PD-1/L1 naïve and experienced Proposed Phase 3 study design: Randomized, double-blind, placebo controlled 2 mg of SD-101 in 1 to 4 lesions Unresectable or metastatic melanoma in patients who have not received anti-PD-1/L1 therapy Endpoints Primary — PFS Secondary — OS, ORR, safety Approximately 600 patients

Jean Chang
Evolution of Cancer Therapy Landscape Combinations Checkpoint IO Targeted Chemotherapy Kills all rapidly dividing cells Toxicities Response rates limited Specific genes or proteins in cancer cells Rapid responses Better tolerated Responses not durable Activate immune system against the tumor by inhibiting tumor’s suppressive mechanisms Long lasting responses in multiple tumor types Minority of patients respond Hit multiple pathways Improve response rates Durable, longer lasting responses Expand treatment options for refractory and resistant patients
A Range of Combinations Will Emerge Over Time Immune system is intricate Multiple and redundant feedback mechanisms Mechanisms maintain immune system balance Patients are heterogeneous Prior therapies Tumor microenvironment Tumor mutational burden
Four Classes of Agents Appear Particularly Well Suited to Complement PD-1 Blockade Increasing appreciation in IO field that stimulation of innate immune system is required to optimally exploit T cell activation Other agents that inhibit or block a checkpoint Activate or expand T cells Agents that alter the TME Innate immune activators TLR9 agonists SD-101, DV281
TLR9 Agonists + Anti-PD1: A Promising Combination Characteristics of a successful combination treatment Robust response Durable disease control Not reliant on predictive biomarkers Minimal additive toxicity burden for patients Implications Earlier use may maximize outcomes, e.g. neoadjuvant setting Basis of triplet combinations going forward Opportunity for other modes of local delivery DV281 inhaled for lung
SD-101+ Anti-PD1: Development in Melanoma Establish SD-101 + pembrolizumab as core combination Advance SD-101+ pembrolizumab combination into earlier treatment settings Unresectable or metastatic melanoma naïve population Phase 3 SD-101 + pembrolizumab Unresectable or metastatic melanoma refractory/resistant to anti-PD-1 Phase 2 SD-101+pembrolizumab Neoadjuvant in melanoma
SD-101 + Anti-PD1: Leveraging Shift in Melanoma Treatment towards Neoadjuvant Setting Checkpoint blockade, anti-PD1 in particular, is moving into the neoadjuvant setting (earlier is better) Pembrolizumab is showing early efficacy SD-101 fits well within this shift TLR9 agonists can improve responses to PD-1 blockade; opportunity to expand upon this Intratumoral injection leverages primary tumor as an antigen source for expansion and activation of T cells; SD-101 orchestrates and primes T cell response Potential benefits Greater fitness of host immunity Significantly higher proportion of patients amenable to intratumoral injection
TLR9 Agonists + Anti-PD1: Application to Additional Indications in Neoadjuvant Setting Criteria for cancer selection Stage at diagnosis Amenable to series of injections or inhalation Evidence of anti-PD1 activity Potential to raise the bar for outcomes Clinical response Pathologic complete/major response Breast cancer SD-101 Lung cancer DV281
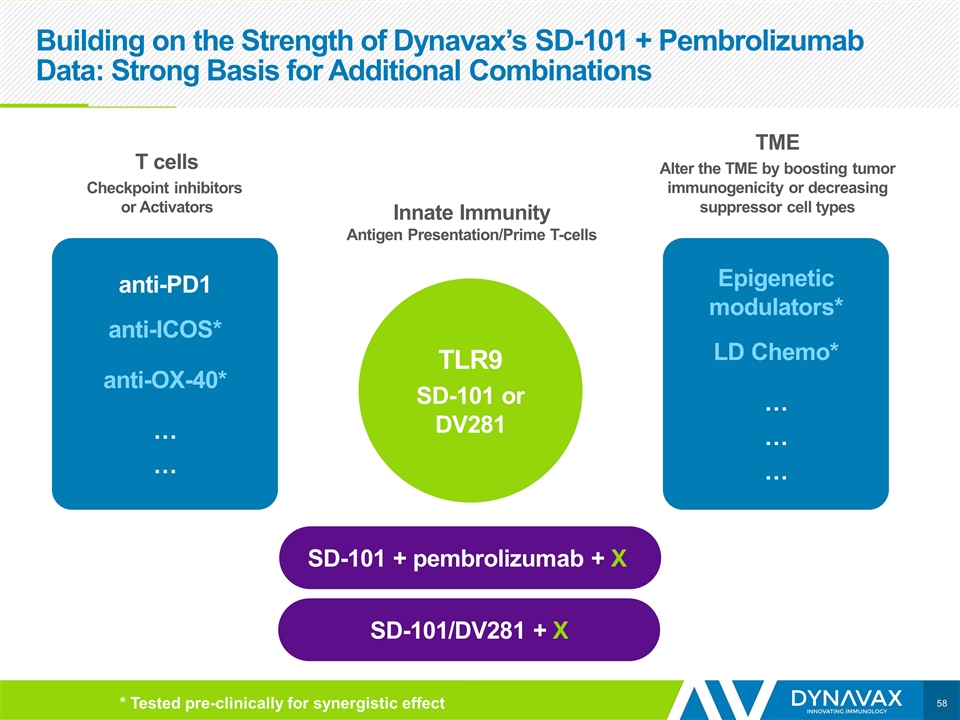
Building on the Strength of Dynavax’s SD-101 + Pembrolizumab Data: Strong Basis for Additional Combinations T cells Checkpoint inhibitors or Activators anti-PD1 anti-ICOS* anti-OX-40* … … Epigenetic modulators* LD Chemo* … … … TLR9 SD-101 or DV281 Innate Immunity Antigen Presentation/Prime T-cells TME Alter the TME by boosting tumor immunogenicity or decreasing suppressor cell types SD-101 + pembrolizumab + X SD-101/DV281 + X * Tested pre-clinically for synergistic effect
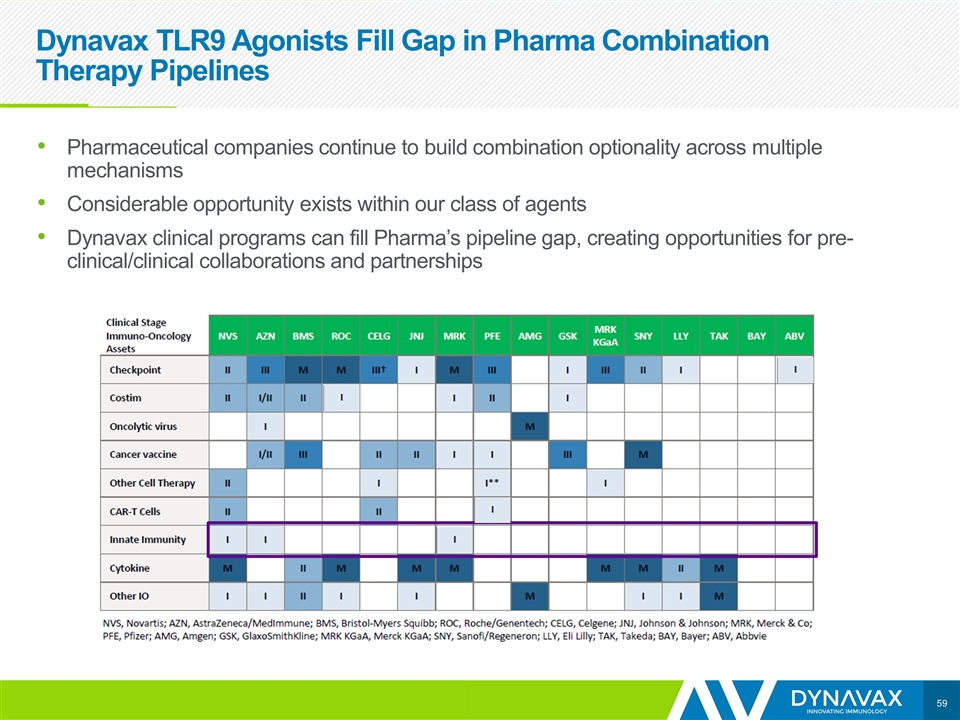
Dynavax TLR9 Agonists Fill Gap in Pharma Combination Therapy Pipelines Pharmaceutical companies continue to build combination optionality across multiple mechanisms Considerable opportunity exists within our class of agents Dynavax clinical programs can fill Pharma’s pipeline gap, creating opportunities for pre-clinical/clinical collaborations and partnerships

Use Partnering to Make TLR9 Agonists Broadly Available and Integral to Combination Therapy Goals for collaborations and partnerships Maximize breadth across tumor types Maximize combination use Doublets or triplets with anti-PD1 treatments Doublets with other T cell agonists, particularly in tumors not responsive to anti-PD1 Triplets with T cell agonists and TME targeting agents
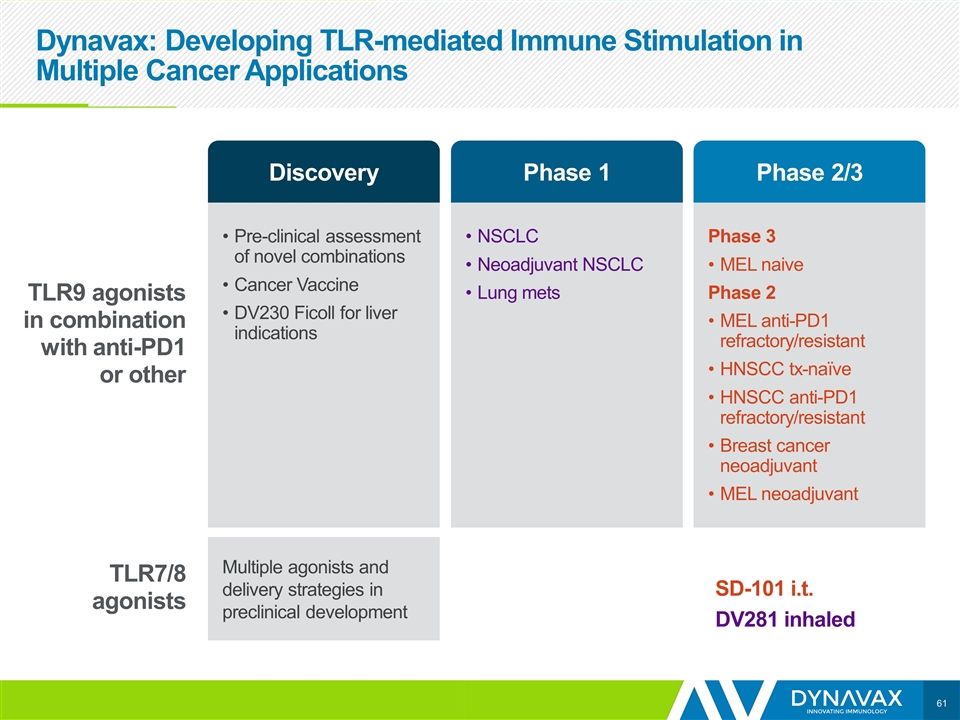
Dynavax: Developing TLR-mediated Immune Stimulation in Multiple Cancer Applications Pre-clinical assessment of novel combinations Cancer Vaccine DV230 Ficoll for liver indications NSCLC Neoadjuvant NSCLC Lung mets Phase 3 MEL naive Phase 2 MEL anti-PD1 refractory/resistant HNSCC tx-naïve HNSCC anti-PD1 refractory/resistant Breast cancer neoadjuvant MEL neoadjuvant Multiple agonists and delivery strategies in preclinical development TLR9 agonists in combination with anti-PD1 or other TLR7/8 agonists SD-101 i.t. DV281 inhaled Discovery Phase 1 Phase 2/3

Eddie Gray

Concluding Remarks TLR9 agonist technology has demonstrated encouraging potential in immuno-oncology as a combination agent Growing immuno-oncology database validates our ongoing studies and expansion of our program Positioning ourselves to take advantage of anti-PD-1 therapy shift into neoadjuvant setting Upcoming catalysts in 2H18 consist of new data in melanoma and HNSCC, safety data in DV281 (lung), and initiation of Phase 3 in advanced melanoma Business development efforts focused on partnerships/collaborations to maximize use of SD-101 in tumor types and in combinations






























































