UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| | x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013
OR
| | o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _______ to ________
Commission file number: 0-27605
VASCULAR SOLUTIONS, INC.
(Exact name of registrant as specified in its charter)
| Minnesota | | 41-1859679 |
| (State or other jurisdiction of incorporation or organization) | | (IRS Employer Identification No.) |
6464 Sycamore Court North
Minneapolis, Minnesota 55369
(Address of principal executive offices, including zip code)
(763) 656-4300
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | | Name of each exchange on which registered: |
| Common Stock, par value $.01 per share | | The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (section 229.406 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated, or a smaller reporting company. See the definitions of “large accelerated filer,” accelerated filer,” and smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer x | Non-accelerated filer o | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold on June 28, 2013 was $233,096,719.
As of January 31, 2014, the number of shares outstanding of the registrant’s common stock was 17,015,776.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for its 2013 Annual Meeting of Shareholders to be held on April 30, 2014 are incorporated by reference in Part III of this Annual Report on Form 10-K.
PART I
Overview
Vascular Solutions, Inc. (we, us or Vascular) is focused on bringing clinically advanced solutions to interventional cardiologists, interventional radiologists, electrophysiologists, and vein practices worldwide. As a vertically-integrated medical device company, we generate ideas, create new minimally invasive medical devices, and then deliver these products and related services to physicians through our direct domestic sales force and our international distribution network. The innovative products and services we offer are divided into three categories:
| · | Catheter products consist principally of catheters used in minimally invasive medical procedures for the diagnosis or treatment of vascular conditions, such as the GuideLiner® catheter used to access discrete regions of the coronary anatomy and the Pronto® extraction catheters used in treating acute myocardial infarction. This category also includes products used in connection with gaining percutaneous access to the vasculature to perform minimally invasive procedures, such as micro-introducer kits. |
| · | Hemostat products consist principally of blood clotting products, such as the D-Stat® Dry hemostat, a topical thrombin-based pad with a bandage used to control surface bleeding, and the D-Stat Flowable, a thick yet flowable thrombin-based mixture for preventing bleeding in subcutaneous pockets. This category also includes our line of devices used in radial artery procedures, such as our Accumed™ wrist positioning splints and Vasc™ Band inflatable compression bands. |
| · | Vein products and services consist principally of the Vari-Lase® endovenous laser, a laser console and procedure kit used for the treatment of varicose veins, and a reprocessing service for the ClosureFAST® radiofrequency vein ablation catheter. |
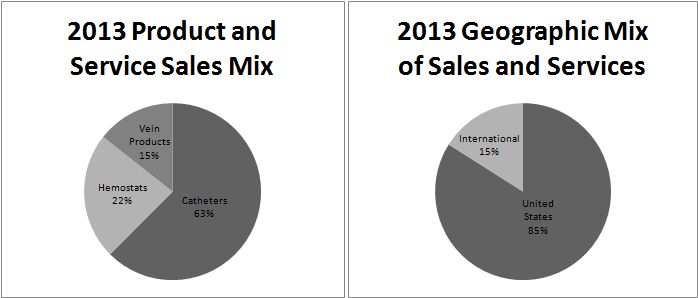
During 2013, 63% of our product and service sales were derived from catheter products, 22% from hemostat products, and 15% from vein products and services. Approximately 85% of our 2013 product and service sales were generated in the United States through our direct sales force and approximately 15% of our sales were to our network of independent distributors outside the United States.
History
We were incorporated in the state of Minnesota in December 1996, and began operations in February 1997. In 2000, we received FDA clearance for our first product, the Duett™ sealing device, which was used to seal the puncture site following catheterization procedures. In 2001, we made the strategic decision to develop additional products and to de-emphasize our Duett product. We have grown from net revenue of $6.2 million in 2000 solely from sales of our Duett product to net revenue of $110.5 million in 2013 from sales of over 80 products we have developed, licensed, or acquired since 2002. This increase in revenue represents a compound annual growth rate of 25% and is driven by our commitment to the development and launch of multiple new devices to diagnose and treat vascular conditions.
Interventional Cardiology and Interventional Radiology Industry Background
According to data from the United States Centers for Disease Control and Prevention (CDC), an estimated 80 million Americans have one or more types of cardiovascular disease—diseases of the heart and blood vessels. The CDC data indicate that cardiovascular disease is the number one cause of death in the United States and is replacing infectious disease as the world’s pre-eminent health risk. Advances in medicine have enabled physicians to perform an increasing number of diagnostic and therapeutic treatments of cardiovascular disease using minimally invasive methods, such as catheters placed inside the arteries, instead of highly invasive open surgery.
Cardiologists and radiologists use diagnostic procedures, such as angiography, to confirm, and interventional procedures, such as angioplasty and stenting, to treat, diseases of the coronary and peripheral arteries. Each angiographic procedure using a catheter requires a puncture in an artery, usually the femoral artery in the groin area and sometimes the radial artery in the wrist, to gain access for the catheter. The catheter then is deployed through an introducer sheath into the vessel to be diagnosed or treated. Upon completion of the procedure and removal of the catheter, the physician must seal this puncture in the artery and the tissue tract that leads from the skin surface to the artery to stop bleeding. Based on industry statistics, we estimate that cardiologists and radiologists performed over nine million diagnostic and interventional catheterization procedures worldwide in 2013.
During the past few years, the number of catheterization procedures performed worldwide has been declining gradually due to a number of factors – among them, the effects of weak economies on overall health care utilization rates, efforts by third-party payers to lower costs associated with medical procedures, investigations by government agencies into potential over-utilization of procedures, the implementation by hospitals of policies designed to reduce the incidence of unnecessary procedures in the wake of these outside investigations, and new diagnostic imaging and functional assessment modalities that more effectively screen patients to determine the need for treatment. Although worldwide demographic factors, including the growing incidence of cardiovascular disease, seem to favor long-term growth in the number of interventional procedures, we believe that the recent structural pressures on utilization rates are likely to continue and to result in relatively flat catheterization volumes for the foreseeable future. Based on our analysis of the publicly-available sales figures from several companies that participate in the cardiovascular device sector, we estimate the worldwide market for interventional medical devices used in cardiovascular procedures in 2013 exceeded $12 billion.
The interventional medical device industry is characterized by intense competition, cost-containment efforts by hospitals and other providers, rapidly-evolving technology, and a high degree of government regulation. To grow our business, we have focused on continually developing and commercializing new products and services. Looking ahead, we expect our business may be impacted by the following trends and opportunities:
| · | The future regulatory approval of newly-developed products. Any new product that we develop must be approved by the Food and Drug Administration (FDA) in the United States and by similar regulatory bodies in other countries before they can be sold. The requirements for obtaining product approval have undergone change, and the FDA frequently implements changes to the product approval process. We monitor the changing regulatory landscape and modify our regulatory submissions as necessary to obtain product approvals. |
| · | Successfully integrating acquired products and services into our existing operations. The acquisition of products and services complementary to our existing product portfolio and customer call points provides an additional business opportunity, but is dependent on the successful integration of the acquired products into our existing business structure. |
| o | In September 2013, we made an initial payment to Northeast Scientific, Inc. (NES) for the acquisition of exclusive eight-year rights to sell reprocessing services in the U.S. for an existing commercial medical device, pending FDA approval of the reprocessing procedure, which NES is pursuing in 2014. |
| o | In December 2012, we acquired the Teirstein EdgeTM device organizer and the AngioAssistTM docking station assets from Shepherd Scientific, Inc. (Shepherd Scientific). Prior to the acquisition, we had been distributing these products under an agreement with Shepherd Scientific. |
| o | In August 2012, we acquired the Venture® wire control catheter (Venture) assets from St. Jude Medical, Cardiology Division, Inc. (St. Jude Medical) and during 2013 fully integrated this product into our operations. |
| o | In June 2012, we acquired the Accumed wrist positioning splint assets from Accumed Systems, Inc. (Accumed) and have integrated this product into our operations. |
| o | In January 2012, we acquired the rights, patents and intellectual property relating to our Pronto catheter from Dr. Pedro Silva and his affiliates. |
| o | In December 2011, we acquired exclusive five-year rights to sell reprocessing services for the ClosureFAST catheter in the United States from Northeast Scientific, Inc. |
| o | In January 2011, we acquired the Guardian® hemostasis valve assets from Zerusa Limited (Zerusa) and have established a subsidiary in the Republic of Ireland to continue the manufacturing of these products. |
For additional information on these acquisitions, see Note 15 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013.
| · | Managing intellectual property. The interventional medical device industry is characterized by numerous patent filings and litigation claims made to protect new and evolving product ideas. To maximize the profitability of new product ideas, we seek patent protection for those product design and method concepts which we believe have the potential to provide substantial product revenue. On October 14, 2013 we reached an agreement with Terumo Corporation and Terumo Medical Corporation (Terumo) to settle a patent and trademark infringement lawsuit brought by Terumo against us related to a prior version of our Vasc Band radial compression device in exchange for a one-time payment to Terumo in the amount of $812,500. On May 16, 2013, we filed a patent and copyright infringement complaint in the U.S. District Court for the District of Minnesota against Boston Scientific Corporation alleging that it is infringing three of our United States patents related to our GuideLiner guide extension product. Boston Scientific filed a counterclaim alleging that our GuideLiner catheter infringes a Boston Scientific patent that has recently expired. The Boston Scientific patent litigation is ongoing. Managing intellectual property assets and claims is a significant challenge for our business. |
Products and Services
Our product and service offerings are divided into three categories: catheter products, hemostat products, and vein products and services. The competitive advantages of our products and services are enhanced by the experience of our direct U.S. sales employees and international independent distributors, the experience of our management team, and our dedication to bringing clinically unique solutions to the markets we serve worldwide.
For information about our revenue, profits and total assets, see our Consolidated Financial Statements included in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013.
Catheter Products
Catheter products represent the largest of our three product categories. In 2013, worldwide sales of our catheter products totaled $69.9 million, an increase of 14% from the 2012 level of $61.3 million. Catheter products represented approximately 63% of our total product and service sales during 2013. Our catheter products consist of a variety of devices used to gain access, diagnose, and treat vascular conditions during minimally invasive catheterization procedures.
Our largest selling catheter products during 2013 are listed in the table below.
| Product | Market Introduction | 2013 Sales ($MM) | Estimated Market Potential ($MM) | Description |
| GuideLiner | 2009 | $20.8 | >$50 | Guide extension catheter used for deep-seating, back-out support, and optimal stenting in challenging interventions |
| Pronto | 2003 | $20.3 | $100 | Manual aspiration catheter for the removal of fresh, soft thrombus and emboli |
| Micro-Introducers | 2003 | $8.9 | $75 | Access kit products used to gain percutaneous access to the vasculture for performing arterial and venous catheterization procedures |
Langston® | 2004 | $5.8 | >$10 | Dual lumen catheter used to measure intravascular pressure gradients, primarily for the diagnosis of aortic valve stenosis |
SmartNeedle® | 2010 | $3.8 | >$5 | Doppler-guided needle device designed to provide auditory ultrasound-guided access to arteries and veins during catheterization procedures |
| Venture | 2013 | <$3 | >$5 | Deflectable tip catheter used for steering guidewires in challenging interventions, acquired from St. Jude Medical in 2012 |
Guardian | 2007 | <$3 | $100 | Hemostat valve used in catheterization procedures to allow placement of multiple devices simultaneously in the artery while minimizing blood loss |
| SuperCross™ | 2011 | <$3 | >$20 | Microcatheters for support and delivery of guidewires during challenging coronary and peripheral catheterization procedures |
| Snares | 2008 | <$3 | > $40 | Interventional snares used to retrieve or manipulate objects in the cardiovascular system |
Minnie® | 2009 | <$3 | $30 | Support catheter used to facilitate placement and exchange of guidewires and other interventional devices. |
Our highest selling catheter product is the GuideLiner guide extension device. We received CE Mark and launched GuideLiner in Europe in October 2009 and we received 510(k) clearance and launched GuideLiner in the U.S. in November 2009. With the introduction of the GuideLiner, Vascular Solutions pioneered the interventional technique of rapid exchange guide extension. The GuideLiner catheter is a unique coaxial “mother and child” guide extension with rapid exchange convenience that enables deep seating, guide back-up support, and selective intubation in challenging coronary interventions. In December 2011, we launched the second generation of our GuideLiner catheter with increased flexibility, a smaller size version, and a longer extension. In August 2013, we launched the third generation version, GuideLiner V3, which features a polymer “half-pipe” collar to enhance stent and wire deployment. In December 2013, we received Shonin approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) to sell GuideLiner in Japan. We also received Japanese reimbursement designation, effective January 1, 2014. With the addition of the Japanese market, we believe the worldwide market opportunity for GuideLiner is in excess of $50 million per year.
Our Pronto catheters consist of a catheter with a proprietary distal tip and large extraction lumen that can be delivered into arteries to mechanically remove blood clots using simple vacuum suction. The original Pronto extraction catheter was developed by Dr. Pedro Silva of Milan, Italy, who exclusively licensed the design to us in 2002. We received CE mark approval and commenced international sales of the Pronto in August 2003, and received FDA clearance in December 2003 and commenced sales in the United States in early 2004. In the fourth quarter of 2005, we launched the third generation design of the Pronto, named the Pronto V3. The V3 version of the Pronto resulted in a substantial increase in Pronto sales in 2006. In January 2011, we commenced the launch of the Pronto V4, which utilizes an embedded longitudinal wire for maximum extraction with enhanced deliverability and kink resistance. On January 6, 2012, we purchased the intellectual property relating to the Pronto extraction catheter from Dr. Silva and his affiliates in exchange for $3.25 million, which thereby eliminated royalty payments on sales of Pronto catheters occurring after December 31, 2011. We believe that the market size for the removal of soft thrombus is approximately $100 million per year worldwide.
In addition to the Pronto V3 and V4, we have developed and launched five additional versions of extraction catheters - the Pronto-Short, Pronto 035, Pronto LP, QXT® and XL™. The Pronto-Short is a shorter and larger version, designed for use in clotted dialysis grafts that was launched in August 2005. The Pronto 035 is a much larger version, designed for use in large vessels that was launched in August 2007. The Pronto LP is a low profile version, designed for use in smaller vessels that was launched in January 2008. The QXT is a low-cost version, designed to be sold in certain international markets that was launched in March 2008. The XL extraction catheter is a larger version with either a straight or pigtail tip, designed for use in larger vessels that was launched in January 2012.
In July 2003, we launched a variety of access kit products used to gain percutaneous access to the vasculature for performing arterial and venous catheterization procedures. These products include a full line of micro-introducer kits and a variety of specialty guidewires.
At the end of the third quarter of 2004, we received regulatory clearance in the United States for the Langston dual lumen pigtail catheter. The Langston catheter is used for the measurement of intravascular pressure gradients, primarily measured to diagnose aortic valve stenosis. In March 2011, we launched the second version of our Langston catheter with improved pressure measurement and reduced kinking. We believe our Langston catheter is the only dual lumen pigtail catheter on the U.S. market that is designed specifically for this clinical purpose. We believe the U.S. market opportunity for the Langston catheter product line is greater than $10 million per year.
In April 2010, we acquired the SmartNeedle and pdACCESS Doppler guided needle products from Escalon Vascular Access, Inc. The SmartNeedle and pdACCESS products consist of a hand-held monitor and one-time use needles designed to provide auditory ultrasound guided access to arteries and veins during catheterization procedures.
In August 2012, we acquired the Venture catheter from St. Jude Medical. After integrating manufacturing operations into our own facilities, we re-launched Venture in the U.S. in April 2013 and in international markets in August 2013. There are three versions of the Venture catheter: rapid exchange (Rx), over-the-wire (OTW), and coronary sinus (CS). The Venture catheters are indicated for directing, steering, controlling, and supporting a guidewire to access discrete regions of the coronary and peripheral vasculature. The device features a tip that deflects up to 90 degrees for precise direction of 0.014” guidewires around tortuous anatomy.
During 2007, we entered into an agreement with Zerusa Limited (Zerusa) to act as the exclusive U.S. distributor of Zerusa’s Guardian hemostatic valve. The Guardian hemostatic valve is a valve used in catheterization procedures to allow the placement of multiple devices simultaneously in the artery with a unique push-button operation that is designed to minimize blood loss. In November 2009, we began distribution of a second generation version called the Guardian II hemostatic valve. In January 2011, we acquired substantially all of the assets of Zerusa, including the Guardian hemostasis valves and established a wholly-owned subsidiary in the Republic of Ireland to continue the manufacture of the Guardian product.
In January 2011, we launched the SuperCross microcatheters. The SuperCross microcatheters are used in conjunction with steerable guidewires to access discrete regions of the coronary and peripheral vasculature and are sold in multiple versions. Straight tip versions are designed for superior wire tracking, while angled tip versions in 45, 90, and 120 degree pre-formed tips are designed to direct wire placement in bifurcated vessels. In December 2013, we received Shonin approval from the MHLW to market our SuperCross FT, the flexible tip version, in Japan. SuperCross FT also received Japanese reimbursement designation, effective January 1, 2014.
During 2008, we entered into an agreement with Radius Medical, Ltd. to distribute its Micro Elite™ and Expro Elite™ Snares within the United States. The Elite snares feature a highly torqueable shaft design for control and maneuverability when accessing distal targets. In October 2010, we acquired the entire snare and retrieval product line from Radius. We believe the market opportunity for snares within interventional cardiology and radiology is over $40 million per year.
In January 2009, we launched the Minnie support catheter. The Minnie support catheter is designed to provide guidewire support and exchange during complex interventions. We believe the current market size for support catheters is approximately $30 million per year.
Hemostat Products
Our second product category, hemostat products, generated 2013 sales of $23.8 million, a 5% increase from the 2012 level of $22.7 million. Hemostat products represented 22% of our total product and service sales during 2013.
Our hemostat products primarily utilize thrombin, a powerful bovine-derived blood clotting protein, to deliver a rapid seal of bleeding with a variety of shelf-stable product configurations. Internally, we have development a proprietary manufacturing process to terminally sterilize our thrombin-based hemostats, which has resulted in our ability to create unique advantages in storage, shipping, preparation and application of our hemostat products. With the recent rapid adoption of radial artery catheterizations in the U.S., we have added a major emphasis on products for use in radial procedures in our hemostat products category.
Our five largest selling hemostat products during 2013 are listed in the table below.
| Product | Market Introduction | 2013 Sales ($MM) | Estimated Market Potential ($MM) | Description |
D-Stat Dry, Thrombix® | 2003 | $13.1 | $50 | Thrombin-based hemostat bandages used in conjunction with manual compression to control bleeding at the site of femoral artery punctures created for interventional access |
| D-Stat Flowable | 2002 | $5.7 | $10 | Flowable mixture of collagen, thrombin, and diluent used to control bleeding in the pectoral pockets created in pacemaker and implantable cardioverter defibrillator (ICD) implants |
| Vasc Band radial hemostat | 2013 | <$3 | $50 | Compression device using an inflatable balloon to assist with bleeding control following a catheterization or other puncture into a blood vessel in the patient’s arm |
| D-Stat Radial | 2004 | <$3 | $25 | Wrist band that combines a thrombin pad with a compression collar to control bleeding at the radial artery access site created for interventional access |
| Accumed wrist positioning splint | 2012 | <$3 | $15 | Splint that positions and stabilizes the hand, wrist, and forearm for arterial puncture and support during radial artery catheterizations |
Our most popular hemostat product is the D-Stat Dry hemostat bandage. In September 2003, we received regulatory clearance and commenced sales of our D-Stat Dry hemostat bandage in the United States and international markets. The D-Stat Dry hemostat bandage is a version of our proprietary blood clotting substance that is lyophilized (freeze-dried) into a gauze pad and combined with an adhesive bandage for application. The D-Stat Dry is used as an adjunct to manual compression for managing bleeding after catheterization procedures.
The traditional method for sealing the puncture site after catheterization procedures has been a manual process whereby a healthcare professional applies direct pressure to the puncture site, sometimes using a sand bag or a large C-clamp, for 20 minutes to an hour in order to form a blood clot. The healthcare professional then monitors the patient, who must remain immobile in order to prevent dislodging of the clot, for an additional two to 24 hours. Patients subjected to manual compression generally experience significant pain and discomfort during compression of the puncture site and during the period in which they are required to be immobile. Many patients report that this pain is the most uncomfortable aspect of the catheterization procedure. In addition, patients can develop a substantial coagulated mass of blood, or hematoma, around the puncture site. Finally, the need for healthcare personnel to provide compression and the use of hospital beds during the recovery period results in substantial costs to the institution, which, under virtually all current healthcare payment systems, are not separately reimbursed.
Until 1996, manual compression was used following virtually all catheterization procedures. In late 1995, the first vascular sealing device which did not rely on compression was introduced in the United States. In addition to invasive (below the skin surface) sealing devices, starting in 2000, non-invasive “patches” began to be used as an assist to manual compression following catheterizations. Non-invasive patches are used by physicians who (principally due to cost, complexity or risk of complications) do not wish to use invasive sealing devices, and for those patients who are contra-indicated for an invasive sealing device. Based on the number of catheterization procedures performed annually by cardiologists and radiologists, industry sources report that the total market opportunity for vascular sealing devices (invasive and non-invasive) is more than $1 billion annually.
We completed a 376-patient; five center randomized clinical study that demonstrated a 50% reduction in the median time-to-hemostasis when using the D-Stat Dry bandage compared to simple manual compression. In the third quarter of 2006, we received FDA clearance of our claim that the D-Stat Dry reduces the time-to-hemostasis in diagnostic catheterizations. In the first quarter of 2008, we received FDA clearance and began selling two new versions of the original D-Stat Dry bandage. The first new version, the D-Stat Dry Clear hemostatic bandage, is packaged with a clear bandage which allows for better visibility of the site while the bandage is in place. The second new version, Thrombix, uses a lower cost manufacturing process which offers price flexibility within the product line. In the first quarter of 2009, we received FDA clearance and began selling a new version of the original D-Stat Dry bandage. This new product, the D-Stat Dry Wrap hemostatic bandage, contains a pre-cut specifically designed for the control of bleeding around indwelling lines. In May 2011, we received FDA clearance and began selling a new version of our D-Stat Dry bandages with silver impregnated into the gauze pad to help prevent the colonization of microorganisms on the pad. We believe that the market for hemostat pads following catheterization procedures has grown substantially since the first competitive patch was introduced in 2000, with a market size of approximately $50 million.
The market for hemostat patches used to stop bleeding of femoral artery punctures is mature, with multiple competitors and ongoing pricing pressures. In addition, two trends are negatively affecting the demand for our D-Stat and Thrombix patches: the recent slow-down in growth of catheterization procedures in general and the movement toward greater reliance on using the radial artery in the arm, rather than the femoral artery in the leg, as the access site for diagnostic and interventional procedures.
Radial artery access, otherwise known as transradial access, is undergoing rapid adoption in the U.S. According to industry data, the proportion of U.S. procedures performed using radial access grew from just 1.2% during the first quarter of 2007 to 16.1% by the third quarter of 2012. The level of penetration for radial artery access in the U.S. is still far behind most other parts of the world – such as Canada at 50%, Japan at 60%, and the average for European countries at around 35% of catheterization procedures, according to industry data. Based on projected continued growth in radial access, we believe the number of radial artery access procedures performed in the U.S. will grow to approximately one million by the end of 2016.
While the movement to radial access has presented obstacles to expansion of our femoral hemostat patch business, the trend has created opportunities for growth through our new hemostat products. For example, our D-Stat Radial hemostat band is a version of the D-Stat Dry that includes a compression band to allow it to be applied over the radial artery in the wrist. The D-Stat Radial is the first device for radial artery hemostasis that contains an active blood clotting agent together with a compression collar for this purpose. We received regulatory clearance for the D-Stat Radial hemostat band in September 2003, and made manufacturing improvements to the product before launching it in the United States in early 2004. In December 2009, we made further manufacturing improvements and launched a new version called the D-Stat Rad-Band® in the United States.
During 2012, we expanded our strategic focus on launching hemostat products that cater to the significant growth opportunity in the radial access market. In May of 2013, we launched the first version of the Vasc Band radial compression device under an exclusive U.S. distribution agreement with Lepu Medical Technology (Beijing) Co., Ltd. The Vasc Band is placed around the patient’s wrist after a radial catheterization procedure and uses an inflatable compression band to assist hemostasis. In June 2012, we acquired the Accumed wrist positioning splint device from Accumed Systems, Inc. The Accumed wrist positioning split consists of a molded plastic brace that simplifies access to the radial artery by holding the hand, wrist, and forearm in an appropriate, comfortable position. The device is also designed to facilitate placement of a hemostatic band to control bleeding after the procedure.
Our D-Stat Flowable hemostat, which we began selling worldwide in February 2002, is a thick yet flowable mixture of collagen, thrombin and diluent that can be delivered topically and into voids and open spaces to control active bleeding. The D-Stat Flowable hemostat can be used in a wide variety of procedures as an adjunct to hemostasis. In December 2006, we received FDA approval of our premarket approval (PMA) supplement for the use of D-Stat Flowable in the prepectoral pockets created in pacemaker and implantable cardioverter defibrillator (ICD) implants. Our PMA supplement was supported by the results of our 269-patient “Pocket Protector” clinical study that demonstrated a 48% reduction in the incidence of clinically relevant hematomas through the use of D-Stat Flowable compared to the standard of care. We estimate that the U.S. market opportunity for this prepectoral pocket indication is greater than 100,000 procedures or $10 million annually.
Vein Products and Services
Our third business category, vein products and services, generated $16.5 million in revenues during 2013, an increase of 17% from the 2012 level of $14.1 million. Vein products represented approximately 15% of our total product and service sales during 2013.
Our two largest selling vein products and services during 2013 are listed in the table below.
| Product/Service | Market Introduction | 2013 Revenue ($MM) | Estimated Market Potential ($MM) | Description |
| ClosureFAST reprocessing service | 2012 | $8.2 | >$20 | Reprocessing offered in the U.S. in collaboration with Northeast Scientific, Inc. for Covidien’s ClosureFAST radiofrequency ablation catheters |
| Vari-Lase procedure kits, laser consoles and procedure packs | 2003 | $7.4 | >$200 | Procedure kits consist of laser fibers and other components used in endovenous laser ablation procedures (e.g., access needles, and guidewires). Laser consoles consist of an 810 nm wavelength, 15 watt laser used to perform endovenous laser ablation procedures. Procedure packs are sterile bundles of access components used in venous procedures, such as syringes, needles, scalpels; gowns and dressings |
Our vein products are generally used in the treatment of reflux of the great saphenous vein, commonly referred to as varicose veins. More than one million people in the United States seek treatment each year for varicose veins. Left untreated, varicose veins can result in serious clinical consequences, including limited mobility and venous stasis ulcers. Historically, an invasive surgical procedure known as vein stripping was the only treatment for severe varicose veins. While vein stripping is still performed, since 2002 a non-surgical procedure using energy (laser or radiofrequency) to treat and close the diseased vein has become a preferred alternative. We believe the current market size for treating varicose veins using endovenous therapy is greater than $200 million per year.
On December 22, 2011, we entered into an exclusive five-year license agreement with NES, under which we sell a reprocessing service to customers utilizing the ClosureFAST radiofrequency catheter in the treatment of varicose veins in the United States. The ClosureFAST catheter is owned and marketed by VNUS Medical Technologies, Inc., a subsidiary of Covidien, and our reprocessing service is not licensed by or associated with Covidien. Under the reprocessing service provided by NES, the customer sends its used ClosureFAST catheters to NES, where they are inspected, cleaned, tested, repackaged, resterilized, and shipped back to the customer. Customers benefit from a reprocessing service by significantly reducing their costs and cutting down on medical waste. Since the reprocessing service was launched in January of 2012 through the end of 2013, more than 40,000 ClosureFAST catheters have been successfully reprocessed by NES and sent to customers of the service. We have reprocessed catheters for more than 500 accounts and approximately 65% of these customers were entirely new accounts for Vascular Solutions. We believe the U.S. market opportunity for reprocessing the ClosureFAST catheter is greater than $20 million per year.
In addition to this reprocessing service, we have leveraged our expanded vein products customer base by selling ancillary products used in vein procedures. In September 2013, we launched our VSI 0.025” Guidewire which can be used during radiofrequency ablation procedures to facilitate placement of the ClosureFAST catheter into desired treatment locations. We also launched a 0.018” guidewire designed for venous access in vein ablation procedures. We now offer a full range of accessory products for the vein ablation procedures, including 7F introducer sheath systems, an 18-gauge ecogenic needle for percutaneous entry, syringe kits and infiltration pumps for administration of local anesthesia, and a broad line of procedure packs.
Our Vari-Lase endovenous laser products consist of a laser console, procedure kits, procedure packs and accessories used in the treatment of varicose veins. The first product we launched in our vein product line was our Vari-Lase procedure kit in July 2003 in the United States. Our Vari-Lase procedure kit is custom-designed for the endovenous procedure. In December 2003, we received FDA clearance for our Vari-Lase laser console, which is manufactured to our specifications by MedArt, a Denmark-based medical laser manufacturer. Since 2004, we have continued our expansion by adding several accessory items to our vein product line. In April 2007, we launched the Vari-Lase Bright Tip™ fiber which utilizes a ceramic sleeve to the distal tip of the laser fiber to provide improved ultrasound visibility and prevent contact between the energy-transmitting fiber tip and the vein wall during the application of laser energy. In January 2010, we launched a new 15 Watt version of our Vari-Lase laser console.
The amount of total revenue contributed by each of our product lines and by geographic areas for the last three fiscal years is set forth in Notes 2 and 11 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013.
Agreements with King Pharmaceuticals, Inc.
In January 2007, we entered into a License Agreement with King Pharmaceuticals, Inc. (King), which is now a subsidiary of Pfizer, Inc. Under the terms of the License Agreement, we granted to King an exclusive, royalty-free, fully-paid up, perpetual, worldwide right and license to all of our patents and know-how relating to the development, manufacture, use, sale, importation or other exploitation of our Thrombi-Pad® trauma bandage, Thrombi-Gel® hemostats, Thrombi-Paste™ hemostat (collectively, the “Products”) and all future medical devices having application in the Field (as defined below) and intended to produce hemostasis by accelerating the clotting process of blood (a “hemostat device”). The “Field” is defined as all applications of hemostat devices in all areas other than catheterization laboratories (cardiac and peripheral), electrophysiology laboratories and holding and recovery rooms for such laboratories. Upon execution of the License Agreement, King paid us a one-time payment of $6.0 million. No other payments are due from King to us under the License Agreement. The term of the License Agreement commenced on January 9, 2007 and continues until the later of the expiration of each licensed patent or King’s relinquishment of its license rights under the licensed know-how.
Also in January 2007, we entered into a Thrombin-JMI® Supply Agreement with King. Under the terms of the Thrombin-JMI Supply Agreement, King agreed to manufacture and supply thrombin to us on a non-exclusive basis. King agreed to supply us with such quantity of thrombin as we may order for use in devices not intended for sale by King in the Field at a fixed price throughout the term of the Thrombin-JMI Supply Agreement as adjusted for inflation, variations in potency and other factors. King also agreed to provide thrombin to us under the Thrombin-JMI Supply Agreement at no cost for incorporation into Products and hemostat devices intended for sale in the Field by King. The Thrombin-JMI Supply Agreement has an initial term of 10 years, followed by successive automatic one-year extensions, subject to termination by the parties under certain circumstances, including (1) termination by King without cause any time after the fifth anniversary of its execution upon five years prior written notice to us and (2) termination by us without cause any time after the fifth anniversary of its execution upon five years prior written notice to King, provided that a Device Supply Agreement we entered into with King in January 2007 has expired on its terms or the parties have agreed to terminate it.
Business Strategy
Our primary objective is to establish ourselves as a leading supplier of clinically superior medical devices and services for substantial, unique opportunities within interventional medicine. The key steps in achieving our primary objective are the following:
| Ÿ | Maintain and Improve our Clinically-Oriented Direct Sales Force in the United States. During 2000 we commenced sales of our products in the United States through a direct sales force of clinically trained account managers who sell and train interventional cardiologists, radiologists and catheterization laboratory personnel on the use of our products. As our product lines have increased, we have increased the size of our sales force to 91 at the end of 2013, which provides substantially complete geographic coverage of the United States. |
| Ÿ | Expand our Existing Products to Our Existing Market. Since the beginning of 2003, we have launched multiple new products and services in the United States through our direct sales force to our existing markets. Pursuing this multiple product and services strategy has generated material sales growth, and we believe that each of our current product lines has the potential to generate continued sales growth during 2014 and beyond. |
| Ÿ | Develop New Devices and Services to be Sold Through our Direct Sales Force to our Existing Customers. Since the beginning of 2003, we have created and developed multiple new products that are sold to our existing customers. We intend to continue to leverage our direct sales force by creating, developing and launching additional new products and services to the interventional physician. |
| Ÿ | Acquire Additional Products or Services to be Sold Through our Direct Sales Force to our Existing Customers. We intend to continue to leverage our direct sales force by bringing additional products and services to the interventional physician through acquisitions. Over the past three years, we have acquired products and services from Zerusa, NES, Accumed, St. Jude Medical, and Shepherd Scientific (see Note 15 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013). We expect to continue to acquire complementary products and services and to enter into distribution agreements for the distribution of other companies’ products through our direct U.S. sales force. |
| Ÿ | Explore Corporate Relationships to Augment our Direct Sales Force. In markets for our products beyond the interventional physician (such as occurred with our Thrombi-Gel, Thrombi-Paste and Thrombi-Pad products) and in other situations where synergistic sales can result, we intend to enter into corporate relationships to broaden our products’ reach and increase our revenues without distracting our direct sales force. |
Sales, Marketing and Distribution
In 2000, we commenced sales of our first product, the Duett sealing device, in the United States through our direct sales organization. As of December 31, 2013, our U.S. direct sales force consisted of 91 employees, all of whom sell our entire line of products and services. We believe that the majority of interventional catheterization procedures in the United States are performed in high volume catheterization laboratories, and that these institutions can be served by our focused direct sales force.
Our international sales and marketing strategy has been to sell to interventional cardiologists and interventional radiologists through established independent distributors in major international markets, subject to required regulatory approvals. We currently have independent distributors that cover 49 countries outside the U.S. We have entered into multi-year written distribution agreements with each of our independent distributors, and we ship our products to these distributors upon receipt of purchase orders. Each of our independent distributors has the exclusive right to sell our products within a defined territory. These distributors also market other medical products, although they have agreed not to sell directly competitive products. Our independent distributors purchase our products from us at a discount from list price and resell the device to hospitals and clinics. Sales to international distributors are denominated in United States dollars, with the exception of sales from our subsidiary in Ireland and sales to our German distributor, which are denominated in Euros. The end-user price is determined by the distributor and varies from country to country.
As part of our sales strategy, our direct U.S. sales force and international independent distributors are clinically trained to be able to train physicians and other healthcare personnel on the use of our products and services. We believe that effective training is a key factor in encouraging physicians to use interventional medical devices and services. We have created, and will continue to work to improve, an in-the-field training program for the use of all of our products and services. We also develop and maintain close working relationships with our customers to continue to receive input concerning our product and service development plans.
We are focused on building market awareness and acceptance of our products and services. Our marketing department provides a wide range of programs, materials and events that support our sales force. These include product and service training, conference and trade show appearances and sales literature and promotional materials.
New Product and Service Development
Our growth depends in large part on the continuous introduction of new and innovative products and services, together with ongoing enhancements to our existing products and services, through internal product development, technology licensing and strategic alliances. We recognize the importance of, and intend to continue to make investments in, research and development. We incurred expenses of $13,191,000 in 2013, $11,870,000 in 2012, and $10,240,000 in 2011 for research and development activities, which constituted 12%, 12%, and 11%, respectively, of net sales. R&D activities include research, product development and intellectual property development. We expect that our R&D expenditures will be approximately 11 to 12% of net sales in 2014.
Our research and product development group works closely with our sales force to incorporate customer feedback into our development and design process. We believe that we have a reputation within interventional cardiology and interventional radiology as a good partner for product and service development because of our tradition of close physician collaboration, dedicated market focus, responsiveness and execution capabilities for product and service development and commercialization.
To further leverage our efficiencies, our research and development group continues to develop in-house capabilities to manufacture some of the components currently produced by outside vendors.
We expect our research and development activities to continue to expand to include evaluation of new concepts, products and services for the interventional cardiology and interventional radiology field. We believe that there are many potential new interventional products and services that would fit within the development, clinical, manufacturing and distribution network we have created for our existing products and services.
Manufacturing
We manufacture our products in our facilities located in the suburbs of Minneapolis, Minnesota and in the country of Ireland. The manufacturing and packaging processes generally occur under a controlled clean room environment. Our quality system, manufacturing facilities and processes have been certified to be compliant with the European Medical Device Directive 93/42/EEC, ISO 13485:2012, the Canadian Medical Device Regulations SOR/98-282, and they comply with FDA Quality System Regulations.
We purchase components from various suppliers and rely on single sources for several parts of our products. We purchase our thrombin (a component in all of the D-Stat products) for use in manufacturing products sold in the U.S. under the Thrombin-JMI® Supply Agreement with King. To date, we have not experienced any significant adverse effects resulting from shortages of components.
The manufacture and sale of our products entails significant risk of product liability claims. Although we have product liability insurance coverage in an amount which we consider reasonable, it may not be adequate to cover potential claims. Any product liability claims asserted against us could result in costly litigation, reduced sales and significant liabilities and divert the attention of our technical and management personnel away from the development and marketing of our products for significant periods of time.
Competition
We encounter significant competition across our product lines and in each market in which our products are sold. These markets are characterized by rapid change resulting from technological advances and scientific discoveries. We face competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products.
Our primary competitors include: Medtronic, Boston Scientific, Covidien, Merit Medical, Marine Polymer Technologies, Cook Medical, Spectranetics, AngioDynamics and Terumo.
Many of our competitors have substantially greater financial, technological, research and development, regulatory, marketing, sales and personnel resources than we do. Competitors may also have greater experience in developing products, obtaining regulatory approvals, and manufacturing and marketing such products. Additionally, competitors may obtain patent protection or regulatory approval or clearance, or achieve product commercialization before us, any of which could materially adversely affect us. We compete on the basis of our clinically differentiated products and services and focused opportunities within this interventional medical device and service market.
In each of our product and service areas, we believe that several other companies are developing new devices and services. The medical device industry is characterized by rapid and significant technological changes as well as the frequent emergence of new technologies. There are likely to be research and development projects related to these market areas of which we are currently unaware. A new technology, product or service may emerge that results in a reduced need for our products and services or results in a product or service that renders our product or service noncompetitive.
Regulatory Requirements
United States
Our products and services are regulated in the United States as medical devices by the FDA under the Federal Food, Drug and Cosmetic Act. The FDA classifies medical devices and services into one of three classes based upon controls the FDA considers necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls such as labeling, adherence to good manufacturing practices and maintenance of product complaint records, and are usually exempt from premarket notification requirements. Class II devices are subject to the same general controls and also are subject to special controls such as performance standards and FDA guidelines, require premarket notification (510k clearance) prior to commercialization, and may also require clinical testing prior to clearance. Class III devices are subject to the highest level of controls because they are used in life-sustaining or life-supporting implantable devices. Class III devices require rigorous clinical testing prior to their approval and generally require a premarket approval (PMA) or supplement application prior to commercialization.
If a medical device manufacturer can establish that a device is “substantially equivalent” to a legally marketed Class I or Class II device, or to an unclassified device, or to a Class III device for which the FDA has not called for PMAs, the manufacturer may seek clearance from the FDA to market the device by filing a 510(k) premarket notification. The 510(k) notification must be supported by appropriate data establishing the claim of substantial equivalence to the satisfaction of the FDA. Following submission of the 510(k) notification, the manufacturer may not place the device into commercial distribution in the United States until a clearance letter is issued by the FDA.
Manufacturers must file an investigational device exemption (IDE) application if human clinical studies of a device are required and if the FDA considers experimental use of the device to represent significant risk to the patient. The IDE application must be supported by data, typically including the results of animal and mechanical testing of the device. If the IDE application is approved by the FDA, human clinical studies may begin at a specific number of investigational sites with a maximum number of patients, as approved by the FDA. The clinical studies must be conducted under the review of an independent institutional review board to ensure the protection of patient rights.
Generally, upon completion of human clinical studies, a manufacturer seeks approval of a Class III medical device from the FDA by submitting a PMA application. A PMA application must be supported by extensive data, including the results of the clinical studies, as well as literature to establish the safety and effectiveness of the device.
Our D-Stat Flowable is classified as both a Class III and Class II device based on the three distinct indications for use that have been assigned to this product.
Our remaining products generally are classified as Class II products and therefore require FDA clearance of a 510(k) notification prior to being sold in the United States. Each of our Class II product lines was initially subject to a 510(k) notification and determined to be “substantially equivalent” to a legally marketed predicate device by the FDA, thereby allowing commercial marketing in the United States. In some instances, we are able to launch a modified product without filing a 510(k) with the FDA.
We also are subject to FDA regulations concerning manufacturing processes and reporting obligations. These regulations require that manufacturing operations be performed according to FDA standards and in accordance with documentation, control and testing standards. We are subject to inspection by the FDA on an on-going basis. We are required to provide information to the FDA on adverse events and maintain a document and record keeping system in accordance with FDA guidelines. The advertising of our products is subject to both FDA and Federal Trade Commission jurisdiction. If the FDA believes that we are not in compliance with any aspect of the law, it can institute proceedings to detain or seize products, issue a recall, stop future violations and assess civil and criminal penalties against us, our officers and our employees.
International
The European Union has adopted rules which require that medical products receive the right to affix the CE mark, an international symbol of adherence to quality standards and compliance with applicable European medical device directives. As part of CE mark certification, manufacturers are required to comply with certain quality systems standards. We received CE mark certification for our first product and certification of our quality system in July 1998, and we have subsequently received CE mark certification for other products we distribute in the European Union.
Our hemostatic products contain bovine-derived thrombin and are subject to additional regulatory review within the European Union to minimize the risk of exposure to viral and Bovine Spongiform Encephalopathy (BSE) pathogens. The regulations in this area continue to evolve and our products may be subject to additional regulatory scrutiny in the future.
International sales of our products are subject to the regulatory requirements of each country in which we sell. These requirements vary from country to country, but generally are less stringent than those in the United States. Regulatory approvals are obtained, usually by our distributors, where required to sell our products in those countries.
Third Party Reimbursement
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, principally the Centers for Medicare and Medicaid Services or CMS and private health insurance plans, to reimburse all or part of the cost of catherization procedures. We believe that in the current United States reimbursement system, all of our products are subject to reimbursement rules depending on the specific medical procedure in which they are utilized.
Market acceptance of our products in international markets is dependent in part upon the availability of reimbursement from healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country. The main types of healthcare payment systems in international markets are government-sponsored healthcare and private insurance. Countries with government-sponsored healthcare, such as the United Kingdom, have a centralized, nationalized healthcare system. New devices are brought into the system through negotiations between departments at individual hospitals at the time of budgeting. In most foreign countries, there are also private insurance systems that may offer payments for alternative therapies.
Intellectual Property: Patents, Trade Secrets, and Trademarks
We file patent applications to protect technological innovations related to new medical devices and improvements on existing medical devices that are significant to the development of our business, and use trade secrets and trademarks to protect other areas of our business. We currently have 22 U.S. patents issued and 9 additional U.S. patent applications pending relating to our GuideLiner catheter, Langston dual lumen catheter, Venture catheter and multiple products in development. We also have obtained issued international patents and are currently pursuing additional international patent applications. Our 22 issued U.S. patents have expiration dates ranging from May 2018 to July 2031.
The interventional medical device market is characterized by frequent and substantial intellectual property litigation. Intellectual property litigation in recent years has proven to be complex and expensive, and the outcome of such litigation is difficult to predict. The interpretation of patents, for example, involves complex and evolving legal and factual questions.
Currently, we are involved in patent litigation with Boston Scientific Corporation (Boston Scientific). On May 16, 2013, we filed a patent infringement complaint in the United States District Court for the District of Minnesota against Boston Scientific alleging that Boston Scientific has infringed three U.S. patents we own concerning rapid exchange guide extension technology by manufacturing and selling its Guidezilla™ guide extension catheter. On July 11, 2013, Boston Scientific filed its answer and counterclaim, alleging that our patents are invalid, that the Guidezilla catheter does not infringe our patents, and that our past manufacture and sale of the GuideLiner catheter violates a U.S. patent owned by Boston Scientific that has recently expired (see Item 3 – Legal Proceedings of Part I of this Annual Report on Form 10-K for the year ended December 31, 2013).
We may become the subject of additional intellectual property litigation in the future related to our products or services. Our defense of any intellectual property litigation claims, regardless of the merits of the complaint, could divert the attention of our technical and/or management personnel away from the development and marketing of our products and services for significant periods of time. The costs incurred to defend these claims could be substantial and adversely affect us, even if we are ultimately successful.
We rely on trade secret protection for certain aspects of our technology. We typically require our employees, consultants and vendors for major components to execute confidentiality agreements upon their commencing services with us or before we disclose confidential information to them. These agreements generally provide that all confidential information developed, or otherwise made known to the other party, during the course of that party’s relationship with us is to be kept confidential and not disclosed to third parties, except in special circumstances. The agreements with our employees also provide that all inventions conceived or developed in the course of providing services to us shall be our exclusive property.
We register trademarks for certain products in the United States and use unregistered trademarks for other products. United States trademark registrations are generally for a term of 10 years, renewable every 10 years as long as the trademark is used in the regular course of trade.
The registered trademark “ClosureFAST®” is owned by Covidien LP Limited Partnership.
Employees
As of December 31, 2013, we had 406 full-time employees. Of these employees, 145 were in manufacturing activities, 133 were in sales and marketing activities, 53 were in regulatory, quality assurance and clinical research activities, 47 were in research and development activities, and 28 were in general and administrative functions. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements. We believe our employee relations are good. We are an Equal Opportunity Employer.
Executive Officers of the Registrant
Our executive and other officers as of January 31, 2014 are as follows:
Name | Age | Position |
| Howard Root | 53 | Chief Executive Officer and Director |
| James Hennen | 41 | Chief Financial Officer, Senior Vice President of Finance and Corporate Secretary |
| Charmaine Sutton | 54 | Senior Vice President of Operations |
| William Rutstein | 61 | Senior Vice President of Worldwide Sales |
| Jonathan Hammond | 46 | Vice President of Manufacturing Engineering |
| Brett Demchuk | 50 | Vice President of Product Quality |
| Susan Christian | 45 | Vice President of Sales Operations |
| Carrie Powers | 39 | Vice President of Marketing |
| Phillip Nalbone | 52 | Vice President of Corporate Development |
| Gordon Weber | 50 | General Counsel |
Howard Root has served as Chief Executive Officer and a member of our Board of Directors since he co-founded Vascular Solutions in February 1997. From 1990 to 1995, Mr. Root was employed by ATS Medical, Inc., a mechanical heart valve company, most recently as Vice President and General Counsel. Prior to joining ATS Medical, Mr. Root practiced corporate law, specializing in representing emerging growth companies, at the law firm of Dorsey & Whitney LLP for over five years.
James Hennen has served as our Chief Financial Officer since January 2004. Mr. Hennen served as our Controller & Director of Finance from February 2002 through December 2003. Prior to joining us, Mr. Hennen served in various accounting positions, most recently as International Controller with WAM!NET, Inc., a globally networked information technology company for media transfer, where he worked from December 1997 through February 2002. From October 1995 through December 1997, Mr. Hennen was an auditor for Ernst & Young, LLP. Mr. Hennen is a Certified Public Accountant (inactive).
Charmaine Sutton has served as our Senior Vice President of Operations since March 2010. Ms. Sutton previously served on our Board of Directors from July 2007 to March 2010. Ms. Sutton is an expert in regulatory strategies for gaining market authorization of Class II and III devices and diagnostics, and in the development, implementation, troubleshooting and improvement of ISO 13485 and FDA QSR quality systems. From 1991 to 2011, Ms. Sutton was principal consultant and co-founder of The Tamarack Group, an association of consultants assisting developers and manufacturers of medical devices, diagnostics, pharmaceuticals, biologics and combination products with regulatory and quality system activities. Prior to co-founding The Tamarack Group, Ms. Sutton held Director and VP level Engineering, Regulatory, Quality and Clinical positions in start-up companies, and was a research scientist in the laser fusion program at Lawrence Livermore National Laboratory. From 2006 to 2011 Ms. Sutton was an adjunct instructor for the Regulatory Affairs and Services graduate program at St. Cloud State University.
Bill Rutstein has served as our Senior Vice President of Worldwide Sales since July 2010. Mr. Rutstein previously served as our Vice President of International Sales starting in October 2008, Senior Director of International Sales starting in January 2008, and Director of International Sales upon joining Vascular Solutions in August 1999. Prior to joining us, Mr. Rutstein was the Business Unit Director for the cardiosurgery division of Minntech Corporation, a medical device company, from April 1997 to July 1999. From November 1988 to March 1997, Mr. Rutstein worked for Daig Corporation (a St. Jude Medical Company), a medical device company specializing in cardiology and electrophysiology catheters, where he served as Regional Sales Manager, National Sales Manager, OEM Sales Manager and International Sales Manager.
Jonathan Hammond has served as our Vice President of Manufacturing Engineering since January 2010. Mr. Hammond previously served as our Director of Process Development from January 2008 to December 2009, our Process Development Manager from January 2007 to December 2007, and our Senior Process Development Engineer from the time he joined us in July of 2005 until December 2006. Prior to joining us, Mr. Hammond served as Senior Manufacturing Engineer with Enpath Medical, a leading supplier of venous vessel introducers, where he worked from November 2002 through June of 2005. From March 1993 through October 2002, Mr. Hammond served in various engineering and technical product management roles for MICROVENA Corporation (ev3).
Brett Demchuk has served as our Vice President of Product Quality since July 2007. Prior to joining us, Mr. Demchuk worked at ATS Medical, Inc. where he was Senior Director of Operations from 1998 to July 2007 and Quality Manager from 1992 to 1998. Prior to ATS Medical, Mr. Demchuk held quality assurance engineering positions at Orthomet and GV Medical.
Susan Christian has served as our Vice President of Sales Operations since October 2008. Ms. Christian previously served as our Senior Director of Sales Operations and Director of Sales Administration upon joining the company in September 2006. Prior to joining us, Ms. Christian served as the Senior Vice President of Finance & Operations of Tad Ware & Company, Inc., a marketing communications agency, where she worked from April 1992 to September 2006. From August 1990 through March 1992, Ms. Christian was a Tax Accountant for Arthur Andersen & Co. Ms. Christian is a Certified Public Accountant (inactive).
Carrie Powers has served as our Vice President of Marketing since July 2009. Ms. Powers previously served as our Senior Director of Product Management and Training from July 2008 to June 2009, Director of Training from March 2007 to July 2008, Product Manager for the Hemostasis Product Line from July 2006 to March 2007 and began her employment with us as an Associate Product Manager for the Hemostasis Product Line from January 2006 to July 2006. Prior to joining us, Ms. Powers was employed by St. Mary’s Hospital in Madison, Wisconsin from 2002 to 2006, most recently as a Registered Nurse in the Interventional Cardiac Catheterization Lab.
Phillip Nalbone has served as our Vice President of Corporate Development since September 2011. Prior to joining us, Mr. Nalbone spent nearly 20 years as a medical devices analyst at various investment firms, including Hambrecht & Quist, Volpe Brown Whelan & Co., Solomon Smith Barney, RBC Capital Markets, and Wedbush Securities.
Gordon Weber has served as our General Counsel since February 2013. Prior to joining us, Mr. Weber served as Associate General Counsel – Commercial for Smiths Medical, a global provider of medical devices for the hospital, emergency, home and specialist environments, from October 2010 to February 2013. Before joining Smiths Medical, Mr. Weber practiced law for 13 years, most recently as a Partner in the Corporate group, with the law firm currently known as Faegre Baker Daniels LLP. Mr. Weber began his career with the accounting firm currently known as KPMG.
Available Information
We make available free of charge on or through our internet website at www.vasc.com our Annual Reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, required Interactive Data files and amendments to these reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission.
ITEM 1A. RISK FACTORS
The risks and uncertainties described below are not the only ones facing our company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of the following risks occur, our business, financial condition or results of operations could be seriously harmed.
We will not be successful if the interventional medical device community does not adopt our new products or services.
We have launched over 80 new products or services since 2003. Our success will depend on the continued launch of new products and services and the medical community’s acceptance of our new products and services. We cannot predict how quickly, if at all, the medical community will accept our new products and services, or, if accepted, the continuation or extent of their use. Our potential customers must:
| · | believe that our products or services offer benefits compared to the methodologies and/or devices that they are currently using; |
| · | use our products or services and obtain acceptable clinical outcomes; |
| · | believe that our products or services are worth the price that they will be asked to pay; and |
| · | be willing to commit the time and resources required to change their current methodology. |
Because we are often selling a new technology, we have limited ability to predict the level of growth or timing of sales of these products or services. If we encounter difficulties in growing our sales of our new medical devices or services, our business will be seriously harmed.
We are involved in, and may face additional, intellectual property litigation, which could prevent us from manufacturing and selling our products or services or result in us incurring substantial costs and liabilities.
The interventional medical device industry is characterized by numerous patent filings. As a result, participants in the industry frequently experience substantial intellectual property litigation.
Some companies in the interventional medical device industry have employed intellectual property litigation in an attempt to gain a competitive advantage. In addition, non-practicing patent assertion entities have accumulated patent rights related to the medical device industry and are asserting them against operating companies in an attempt to collect settlements or licensing fees. Intellectual property litigation has proven to be very complex, and the outcome of such litigation is difficult to predict. While we do not believe that any of our products or services infringe any existing patent or other intellectual property right, we have been, and are, involved in substantial intellectual property litigation and expect to continue to become subject to intellectual property claims with respect to our new or existing products or services.
On February 14, 2013, Terumo Corporation and Terumo Medical Corporation (Terumo) filed a complaint for patent and trademark infringement against us and Lepu Medical Technology (Beijing) Co. in the United States District Court for the District of New Jersey. The complaint alleged that a prior version of the Vasc Band radial compression device manufactured by Lepu Medical Technology infringed a Terumo patent. On October 11, 2013, we reached an agreement with Terumo to settle the litigation. Under the settlement, we made a one-time payment to Terumo in the amount of $812,500 and Terumo agreed that the current design of our Vasc Band product does not infringe any claim of any issued Terumo patent and agreed that it will not sue the Company on any future issued patent concerning the current design of the Vasc Band product.
On May 16, 2013, we filed a patent and copyright infringement complaint in the U.S. District Court for the District of Minnesota against Boston Scientific Corporation alleging that it is infringing three of our United States patents by manufacturing and selling its Guidezilla guide extension catheter. Boston Scientific filed a counterclaim alleging that our GuideLiner catheter infringes a Boston Scientific patent that has recently expired. We cannot be sure of the outcome of our claim or the counterclaim. If we prevail in our claim, we cannot be sure whether any remedy will adequately protect our intellectual property. If Boston Scientific prevails in its counterclaim, we may be required to pay damages.
An adverse determination in any intellectual property litigation or interference proceedings against us could prohibit us from selling a product or service, subject us to significant immediate payments to third parties and require us to seek licenses from third parties. The costs associated with these license arrangements may be substantial and could include substantial up-front payments and ongoing royalties. Furthermore, the necessary licenses may not be available to us on satisfactory terms, if at all. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling a product or service.
Our involvement in intellectual property claims, whether ongoing or filed in the future and regardless of the merits of any asserted claim against us, could divert the attention of our technical and management personnel away from the development and marketing of our products and services for significant periods of time. Furthermore, the penalties involved with an adverse outcome may be severe, and the costs incurred related to defending such claims could have a material adverse effect on our results of operations or financial condition, even if we ultimately prevail in them.
The medical device industry is the subject of numerous governmental investigations into marketing and other business practices, and we currently are the subject of a pending government criminal investigation. A governmental criminal investigation could result in the commencement of litigation, indictment of the company, substantial fines, penalties and administrative remedies, including the potential disqualification from participation in government health care programs including Medicare and Medicaid. An adverse determination in the current government criminal investigation or any future civil or criminal litigation could have a material adverse effect on our ability to continue to operate our business as currently conducted and negatively impact our financial results and stock price.
The products and business activities of medical device companies are subject to rigorous regulation by the FDA and other federal, state and international governmental authorities under statutes and regulations governing health care fraud. The U.S. Attorney’s Offices have increased their scrutiny over the medical device industry in recent years. The U.S. Congress, Department of Justice, Office of Inspector General of the Department of Health and Human Services, and Department of Defense have all issued subpoenas and other requests for information to conduct investigations of, and commenced civil and criminal litigation against, medical device manufacturers, primarily related to financial arrangements with health care providers, regulatory compliance and product promotional practices.
During 2012, the U.S. Attorney’s Office for the Western District of Texas intervened in a qui tam civil lawsuit initiated against us involving allegations of off-label promotion of our Vari-Lase Short Kit endovenous laser product (see Item 3 – Legal Proceedings: Governmental Proceedings of Part I of this Annual Report on Form 10-K for the year ended December 31, 2013). Subsequently, we learned that the U.S. Attorney’s Office has commenced a criminal investigation of the same matter. On January 22, 2014, we agreed with the U.S. Attorney’s Office to settle the civil lawsuit. The terms of the settlement are that we will make a payment of $520,000, we will make no admission of fault or liability, and the U.S. Attorney’s Office will dismiss the civil lawsuit with prejudice and release all civil claims that were, or could have been, brought against us in the civil lawsuit. Settlement of the civil lawsuit will have no effect upon the criminal investigation, which we expect will continue.
We cannot predict when the criminal investigation will be resolved, the outcome of the investigation, its impact on us, or whether other federal or state legal or regulatory authorities will initiate additional related or unrelated investigations or lawsuits against us. The outcome of a criminal investigation could include substantial fines, penalties and administrative remedies such as a deferred prosecution agreement or corporate integrity agreement, or the indictment of the company or its officers. Upon conviction of a felony under statutes related to health care fraud, the company would become automatically excluded from participation in government health care programs including Medicare and Medicaid, which would substantially adversely affect our ability to continue to conduct our business. The cost of defense of any potential criminal litigation could be material and could divert the attention of management from the day-to-day operations of our business. These potential consequences of the current investigation, as well as consequences of any future governmental investigation or lawsuit of any related or unrelated matter, could have a material adverse effect on our business, results of operations and stock price.
Our future operating results are difficult to predict and may vary significantly from quarter to quarter, which may adversely affect the price of our common stock.
The ongoing introduction of new products and services that affect our overall product mix make the prediction of future operating results difficult. You should not rely on our past revenue growth as any indication of future growth rates or operating results. The price of our common stock will likely fall in the event that our operating results do not meet the expectations of analysts and investors. Comparisons of our operating results between quarters are an unreliable indication of our future performance because they are likely to vary significantly based on many factors, including:
| · | the level of sales of our products and services in our markets; |
| · | our ability to introduce new products or services and enhancements in a timely manner; |
| · | the demand for, and acceptance of, our products and services; |
| · | the success of our competition and the introduction of alternative products or services; |
| · | our ability to command favorable pricing for our products and services; |
| · | the growth of the market for our products and services; |
| · | the expansion and rate of success of our direct sales force in the United States and our independent distributors internationally; |
| · | actions relating to ongoing FDA compliance; |
| · | the effects of intellectual property disputes; |
| · | the effects of government investigations and litigation; |
| · | the size and timing of orders from independent distributors or customers; |
| · | the attraction and retention of key personnel, particularly in sales and marketing, regulatory, manufacturing and research and development; |
| · | unanticipated delays or an inability to control costs; |
| · | general economic conditions, as well as those specific to our customers and markets; and |
| · | seasonal fluctuations in revenue due to the elective nature of some procedures. |
We may face product liability claims that could result in costly litigation and significant liabilities.
The manufacture and sale of medical products entails significant risk of product liability claims. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, cause us to incur significant liabilities and divert our management’s time, attention and resources. We cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all.
The market for interventional medical devices and services is highly competitive and will likely become more competitive, and our competitors may be able to respond more quickly to new or emerging technologies and changes in customer requirements that may render our products or services obsolete.
The existing market for interventional medical devices and services is intensely competitive. We expect competition to increase further as companies develop new products and services or modify their existing products and services to compete directly with ours. Each of our products and services encounters competition from several medical device companies, including Medtronic Inc., Boston Scientific Corporation and Covidien plc. Each of these companies has:
| · | better name recognition; |
| · | greater sales, marketing and distribution capabilities; |
| · | significantly greater financial resources; |
| · | larger research and development staffs and facilities; and |
| · | existing relationships with some of our potential customers. |
We may not be able to effectively compete with these companies. In addition, broad product lines may allow our competitors to negotiate exclusive, long-term supply contracts and offer comprehensive pricing for their products or services. Broader product lines may also provide our competitors with a significant advantage in marketing competing products or services to group purchasing organizations and other managed care organizations that are increasingly seeking to reduce costs through centralized purchasing. Greater financial resources and product development capabilities may allow our competitors to respond more quickly to new or emerging technologies and changes in customer requirements that may render our products or services obsolete.
We rely on continued development and improvement of our products and service, which if not successful, may have an adverse effect on our financial condition and results of operations.
We are continually engaged in developing new products and services and improving our existing products and services. New or improved products and services represent a significant component of our sales growth. We dedicate significant financial and managerial resources to product and services development and improvement. We may not achieve our development objectives within our schedule and budget, or at all, due to technical or operational challenges. Failure to achieve our development objectives on schedule may increase our development expenses and adversely impact our revenues. If we do achieve our development objectives, we may not be able to obtain regulatory approval for new or improved products or services, and we may not be successful in marketing and selling such products or services. Failure to obtain regulatory approval or successfully market and sell new or improved products and services could adversely impact our revenues and results of operations.
Constraints or interruption in production from any of our key suppliers may have a material adverse effect on our business, financial condition and results of operations.
In the interest of operational efficiency and due to quality considerations, we obtain some of the components for the products that we manufacture from a sole source. If the availability of components from a sole source of supply is constrained or interrupted, there is no assurance that we could find an alternative source of supply quickly and cost effectively, if at all. In addition, due to the stringent regulations and requirements of the FDA and equivalent regulatory entities regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources for some components or materials. Unavailability of components could have a material adverse effect on our ability to manufacture and sell products and our results of operations.
We also distribute finished products that are available only from their manufacturer. In addition, the reprocessing services that we offer are performed by a single provider. Operational, quality or regulatory issues the manufacturer of the products we distribute, or the provider of our reprocessing services, could constrain or interrupt the availability of those products or services. Any constrain or interruption in supply of finished products that we distribute, or the reprocessing services that we offer, could have a material adverse effect on our ability to sell products and services to customers, our financial condition and our results of operations.
Our international sales are subject to a number of risks that could seriously harm our ability to successfully commercialize our products and services in any international market.
Our international sales are subject to several risks, including:
| · | the ability of our independent distributors to sell our products and services; |
| · | the impact of recessions in economies outside the United States; |
| · | greater difficulty in collecting accounts receivable and longer collection periods; |
| · | unexpected changes in regulatory requirements, tariffs or other trade barriers; |
| · | weaker intellectual property rights protection in some countries; |
| · | potentially adverse tax consequences; and |
| · | political and economic instability. |
The occurrence of any of these events could seriously harm our future international sales and our ability to successfully commercialize our products and services in any international market.
Our business and services and results of operations may be seriously harmed by changes in third-party reimbursement policies.
We could be seriously harmed by changes in reimbursement policies of governmental or private healthcare payors, particularly to the extent any changes affect reimbursement for catheterization procedures in which our products or services are used. Failure by physicians, hospitals and other users of our products or services to obtain sufficient reimbursement from healthcare payors for procedures in which our products or services are used, or adverse changes in governmental and private third-party payors’ policies toward reimbursement for such procedures, would seriously harm our business.
In the United States, healthcare providers, including hospitals and clinics that purchase medical devices or services such as our products and services, generally rely on third-party payors, principally federal Medicare, state Medicaid and private health insurance plans, to reimburse all or part of the cost of catheterization procedures. Any changes in this reimbursement system could seriously harm our business.
In international markets, acceptance of our products and services is dependent in part upon the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country. Our failure to receive international reimbursement approvals could have a negative impact on market acceptance of our products and services in the markets in which these approvals are sought.
Our products and services and our manufacturing activities are subject to extensive governmental regulation that could prevent us from selling our products or services in the United States or introducing new and improved products or services.
Our products and services and our manufacturing activities are subject to extensive regulation by a number of governmental agencies, including the FDA and comparable international agencies. We are required to:
| · | obtain the clearance of the FDA and international agencies before we can market and sell our products and services; |
| · | satisfy these agencies’ content requirements for all of our labeling, sales and promotional materials; and |
| · | undergo rigorous inspections by these agencies. |
Compliance with the regulations of these agencies may delay or prevent us from introducing any new model of our existing products or other new products or services. Furthermore, we may be subject to sanctions, including temporary or permanent suspension of operations, product recalls and marketing restrictions if we fail to comply with the laws and regulations pertaining to our business.
We are also required to demonstrate compliance with the FDA’s quality system regulations. The FDA enforces its quality system regulations through pre-approval and periodic post-approval inspections. These regulations relate to product testing, vendor qualification, design control and quality assurance, as well as the maintenance of records and documentation. If we are unable to conform to these regulations, the FDA may take actions which could seriously harm our business. In addition, government regulation may be established that could prevent, delay, modify or rescind regulatory clearance or approval of our products or services.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our offices in the United States are located in three buildings totaling approximately 177,000 square feet of space in two suburbs of Minneapolis, Minnesota (Maple Grove and Plymouth). We currently lease two of the buildings totaling approximately 106,000 square feet of space. These leased facilities include approximately 33,000 square feet used for manufacturing activities and approximately 5,000 square feet used for research and laboratory activities, with the remainder used for warehouse and general office space. On October 15, 2010, we amended and replaced both lease agreements with a consolidated lease agreement to add an additional 12,000 square feet, with additional renewal options.
In November 2012, we purchased a three-story office building located adjacent to our primary leased manufacturing facility in Maple Grove, Minnesota. We occupy approximately 23,000 square feet of the building as general office space and continue to lease the remaining 47,000 square feet of the building to the previous tenants.
Our offices in Ireland are located in one building totaling 1,150 square feet of leased space in Galway. This facility includes approximately 450 square feet used for research and laboratory activities, with the remainder used for warehouse and general office space. This lease is set to expire in July 2014, however it does contain extension options.
ITEM 3. LEGAL PROCEEDINGS
Boston Scientific Corporation Litigation
On May 16, 2013, we filed a patent infringement complaint in the United States District Court for the District of Minnesota against Boston Scientific Corporation (Boston Scientific). The complaint alleges that Boston Scientific has infringed three of our patents concerning rapid exchange guide extension technology by manufacturing and selling its Guidezilla guide extension catheter, which received FDA 510(k) clearance in March 2013. We are seeking an injunction against Boston Scientific prohibiting the manufacture and sale of its Guidezilla catheter, as well as damages for lost profits and legal costs. On June 10, 2013, we filed a motion for preliminary injunction asking the court to enjoin Boston Scientific’s manufacture and sale of the Guidezilla catheter during the pendency of the litigation. On July 11, 2013, Boston Scientific filed its answer and counterclaim, alleging our patents are invalid, that the Guidezilla catheter does not infringe, and that the Company’s manufacture and sale of its GuideLiner catheter violates a U.S. patent owned by Boston Scientific that has recently expired. The counterclaim seeks unspecified damages and payment of Boston Scientific’s attorney’s fees, expenses and costs. On December 9, 2013, the District Court granted our motion for a preliminary injunction prohibiting Boston Scientific from making, using, offering for sale or selling its Guidezilla catheter during the pendency of the parties’ on-going patent infringement litigation. On December 12, 2013, the District Court stayed the preliminary injunction to permit it to consider whether to permit Boston Scientific to move for reconsideration of the preliminary injunction. On December 23, 2013, the District Court denied Boston Scientific’s request for permission to file a motion to reconsider the preliminary injunction and lifted the stay on the preliminary injunction effective January 13, 2014. On December 27, 2013, Boston Scientific filed a motion with the United States Court of Appeals for the Federal Circuit for a stay, pending appeal, of the District Court’s preliminary injunction order and an interim stay pending the Federal Circuit’s consideration of the motion. On January 8, 2014, the Federal Circuit issued an order denying Boston Scientific’s motion for an interim stay of the preliminary injunction. On January 13, 2014, the preliminary injunction became effective prohibiting Boston Scientific from making, using, offering for sale or selling the Guidezilla guide extension catheter in the United States during the pendency of the patent litigation, currently scheduled to be ready for trial on or after March 2015. On January 17, 2014, the Federal Circuit issued an order denying Boston Scientific’s motion for a stay of the preliminary injunction pending appeal. The preliminary injunction order remains subject to an appeal by Boston Scientific before the Federal Circuit.
Terumo Medical Corporation Litigation
On February 14, 2013, Terumo Corporation and Terumo Medical Corporation (Terumo) filed a complaint for patent and trademark infringement against Vascular Solutions, Inc. and Lepu Medical Technology (Beijing) Co. in the United States District Court for the District of New Jersey. The complaint alleged that a prior version of the Vasc Band radial compression device manufactured by Lepu Medical Technology infringed a Terumo patent, U.S. Patent No. 7,498,477 (the ‘477 Patent). On October 11, 2013, we reached an agreement with Terumo to settle the litigation, whereby we made a one-time payment to Terumo in the amount of $812,500 and Terumo agreed that the design of the current Vasc Band product does not infringe any claim of any issued Terumo patent and that Terumo will not sue us on any future issued patent concerning that design of the Vasc Band. The settlement payment was made on December 13, 2013.
Governmental Proceedings
On June 28, 2011, we received a subpoena from the U.S. Attorney’s Office for the Western District of Texas under the Health Insurance Portability & Accountability Act of 1996 (HIPAA) requesting the production of documents related to Vari-Lase products, and in particular the use of the Vari-Lase Short Kit for the treatment of perforator veins. Subsequently, we learned that the U.S. Attorney’s Office has commenced a criminal investigation of the same matter. The Vari-Lase Short Kit has been sold under a 510(k) clearance for the treatment of incompetence and reflux of superficial veins in the lower extremity since 2007 with total U.S. sales through December 31, 2013 of approximately $479,000 (0.1% of our total U.S. sales for such period) and has not been the subject of any reported serious adverse clinical event. On August 14, 2012, the United States District Court for the Western District of Texas unsealed a qui tam complaint that had been filed on November 19, 2010 by Desalle Bui, a former sales employee, which is the basis for the U.S. Attorney’s civil investigation, to which the federal government, after three extensions of time, elected to intervene. The complaint contains allegations of off-label promotion of Vari-Lase products for the treatment of perforator veins, re-use of single-use Vari-Lase products and kickbacks to physicians, resulting in alleged damages to the government of approximately $20 million. An amended complaint limited to allegations of off-label promotion of the Vari-Lase Short Kit resulting in an unspecified amount of damages and penalties was filed by the U.S. Attorney’s Office in December 2012. On January 22, 2014, we agreed with the U.S. Attorney’s Office to settle the civil lawsuit. The terms of the settlement are that we will make a payment of $520,000, we will make no admission of fault or liability, and the U.S. Attorney’s Office will dismiss the civil lawsuit with prejudice and release all civil claims that were, or could have been, brought against us in the civil lawsuit. Settlement of the civil lawsuit will have no effect upon the criminal investigation, which we expect will continue.
From time to time we are involved in legal proceedings arising in the normal course of our business. As of the date of this report we are not a party to any legal proceedings not described in this section in which an adverse outcome would reasonably be expected to have a material adverse effect on our results of operations or financial condition.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is traded on the NASDAQ Global Select Market under the symbol “VASC”. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported by the NASDAQ Global Select Market.
| | High | | | Low | |
| 2013 | | | | | | |
| First Quarter | | $ | 16.99 | | | $ | 14.03 | |
| Second Quarter | | | 17.18 | | | | 13.92 | |
| Third Quarter | | | 17.37 | | | | 14.42 | |
| Fourth Quarter | | | 23.40 | | | | 16.02 | |
| | | | | | | | |
| 2012 | | | | | | | | |
| First Quarter | | $ | 11.49 | | | $ | 10.20 | |
| Second Quarter | | | 12.73 | | | | 10.09 | |
| Third Quarter | | | 14.84 | | | | 12.49 | |
| Fourth Quarter | | | 15.83 | | | | 13.00 | |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Holders
As of December 31, 2013, we had 247 shareholders of record. Such number of record holders does not reflect shareholders who beneficially own common stock held in nominee or street name.
Dividends
We have paid no cash dividends on our common stock, and do not intend to pay cash dividends on our common stock in the future.
Performance Graph
The following graph shows a comparison of cumulative total returns for our common stock, the NASDAQ U.S. Benchmark TR Index, the NASDAQ Stock Market Index (U.S.), the NASDAQ OMX Medical Supplies Index and the NASDAQ Medical Industry Index (Medical Devices, Instruments and Supplies), assuming the investment of $100 in our common stock and each index on December 31, 2008 and the reinvestment of dividends, if any. NASDAQ OMX, which supplies us with total return data for the comparative indexes, is replacing values prepared by the Center for Research in Security Prices with its own global indexes. The NASDAQ U.S. Benchmark TR Index replaces the NASDAQ Stock Market Index (U.S.). The NASDAQ OMX Medical Supplies Index replaces the NASDAQ Medical Industry Index (Medical Devices, Instruments and Supplies) and includes only companies that produce medical supplies, as we do. The NASDAQ Medical Industry Index (Medical Devices, Instruments and Supplies) included companies that produce medical supplies and companies that product medical equipment.
ITEM 6. SELECTED FINANCIAL DATA
The following selected financial data as of December 31, 2013 and 2012 and for the three years ended December 31, 2013, 2012 and 2011 are derived from, and should be read together with, our consolidated financial statements included elsewhere in this Form 10-K. The following selected financial data as of December 31, 2011, 2010 and 2009 and for the fiscal years ended December 31, 2010 and 2009 are derived from consolidated financial statements not included herein. The information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” the Consolidated Financial Statements and Notes thereto and other financial information included elsewhere in this Form 10-K.
| | Year Ended December 31, |
| | 2013 | | | 2012 | | | 2011 | | | 2010 | | | 2009 |
| | (in thousands, except per share amounts) |
| Statements of Operations Data: | | | | | | | | | | | | | | |
| Net revenue: | | | | | | | | | | | | | | |
| Product revenue | | $ | 110,197 | | | $ | 98,036 | | | $ | 86,589 | | | $ | 77,419 | | | $ | 66,726 | |
| License and collaboration revenue | | | 301 | | | | 350 | | | | 3,367 | | | | 1,024 | | | | 1,701 | |
| Total net revenue | | | 110,498 | | | | 98,386 | | | | 89,956 | | | | 78,443 | | | | 68,427 | |
| | | | | | | | | | | | | | | | | | | | |
| Product costs and operating expenses: | | | | | | | | | | | | | | | | | | | | |
| Cost of goods sold | | | 36,156 | | | | 32,561 | | | | 29,844 | | | | 26,465 | | | | 22,917 | |
| Collaboration expenses | | | 60 | | | | - | | | | - | | | | 175 | | | | 850 | |
| Research and development | | | 13,191 | | | | 11,870 | | | | 10,240 | | | | 9,524 | | | | 7,847 | |
| Clinical and regulatory | | | 4,408 | | | | 4,358 | | | | 4,332 | | | | 3,551 | | | | 2,886 | |
| Sales and marketing | | | 27,372 | | | | 25,777 | | | | 24,126 | | | | 23,188 | | | | 21,206 | |
| General and administrative | | | 8,991 | | | | 6,522 | | | | 4,997 | | | | 5,183 | | | | 4,555 | |
| Litigation | | | 1,332 | | | | - | | | | - | | | | (3,529 | ) | | | - | |
| Medical device excise tax | | | 1,319 | | | | - | | | | - | | | | - | | | | - | |
| Amortization of purchased technology and intangibles | | | 1,572 | | | | 1,393 | | | | 831 | | | | 304 | | | | - | |
| Total product costs and operating expenses | | | 94,401 | | | | 82,481 | | | | 74,370 | | | | 64,861 | | | | 60,261 | |
| Operating earnings | | | 16,097 | | | | 15,905 | | | | 15,586 | | | | 13,582 | | | | 8,166 | |
| | | | | | | | | | | | | | | | | | | | |
| Other earnings (expenses): | | | | | | | | | | | | | | | | | | | | |
| Interest earnings | | | - | | | | - | | | | 16 | | | | 38 | | | | 48 | |
| Interest expense | | | (13 | ) | | | (13 | ) | | | (13 | ) | | | (20 | ) | | | (38 | ) |
| Foreign exchange gain (loss) | | | (1 | ) | | | - | | | | 110 | | | | (42 | ) | | | (10 | ) |
| | | | | | | | | | | | | | | | | | | | |
| Earnings before income taxes | | | 16,083 | | | | 15,892 | | | | 15,699 | | | | 13,558 | | | | 8,166 | |
| | | | | | | | | | | | | | | | | | | | |
| Income tax benefit (expense) | | | (4,941 | ) | | | (5,983 | ) | | | (5,960 | ) | | | 7,819 | | | | (2,788 | ) |
| Net earnings | | $ | 11,142 | | | $ | 9,909 | | | $ | 9,739 | | | $ | 21,377 | | | $ | 5,378 | |
| Net earnings per common share – Basic | | $ | 0.68 | | | $ | 0.62 | | | $ | 0.59 | | | $ | 1.30 | | | $ | 0.34 | |
| Net earnings per common share – Diluted | | $ | 0.65 | | | $ | 0.60 | | | $ | 0.57 | | | $ | 1.26 | | | $ | 0.33 | |
| Weighted average number of basic common shares outstanding | | | 16,412 | | | | 16,004 | | | | 16,638 | | | | 16,478 | | | | 16,047 | |
| Weighted average number of diluted common shares | | | 17,025 | | | | 16,456 | | | | 17,184 | | | | 17,008 | | | | 16,475 | |
| As of December 31, | |
| 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| (in thousands) | |
| Balance Sheet Data: | | | | | | | | | | |
| Cash, cash equivalents and available- for-sale securities | | $ | 30,785 | | | $ | 11,554 | | | $ | 13,726 | | | $ | 17,360 | | | $ | 17,794 | |
| Working capital | | | 56,355 | | | | 38,016 | | | | 38,650 | | | | 38,927 | | | | 35,145 | |
| Total assets | | | 108,141 | | | | 88,002 | | | | 76,483 | | | | 78,457 | | | | 51,755 | |
| Total shareholders’ equity | | | 96,350 | | | | 76,867 | | | | 66,706 | | | | 64,103 | | | | 40,399 | |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our Consolidated Financial Statements and Notes thereto, and the other financial information included elsewhere in this Form 10-K. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains descriptions of our expectations regarding future trends affecting our business. These forward-looking statements and other forward-looking statements made elsewhere in this document that are not strictly historical fact are made in reliance upon safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based on management’s current expectations as of the date of this report but involve risks, uncertainties and other factors which may cause actual results to differ materially from those contemplated by such forward looking statements. Item 1A of Part I of this Annual Report on Form 10-K for the year ended December 31, 2013, sets forth certain factors we believe could cause actual results to differ materially from those contemplated by the forward-looking statements. We do not intend to update any of these forward-looking statements after the date of this Form 10-K to conform them to actual results.
Overview
We are a medical device company focused on bringing clinically advanced solutions to interventional cardiologists and interventional radiologists. As a vertically-integrated medical device company, we generate ideas, create new minimally invasive medical devices, and then deliver those products and related services to physicians through our direct domestic sales force and international distribution network. We continue to develop new products and services and new applications for our existing products.
During the past few years, the number of catheterization procedures performed worldwide has been declining gradually due to a number of factors – among them, the effects of weak economies on overall health care utilization rates, efforts by third-party payers to lower costs associated with medical procedures, investigations by government agencies into potential over-utilization of procedures, the implementation by hospitals of policies designed to reduce the incidence of unnecessary procedures in the wake of these outside investigations, and new diagnostic imaging and functional assessment modalities that more effectively screen patients to determine the need for treatment. Although worldwide demographic factors, including the growing incidence of obesity, diabetes, and cardiovascular disease, seem to favor long-term growth in the number of interventional procedures, we believe these recent pressures on utilization rates are likely to result in relatively flat catheterization volumes for the foreseeable future. We intend to remain competitive in this market through the continued introduction of new products and services. We expect to originate these new products and services primarily through our internal research and development and clinical efforts, but we may supplement them with targeted acquisitions or other external collaborations. Additionally, our growth has been, and will continue to be, impacted by our expansion and penetration into new geographic markets, the expansion and penetration of our direct sales organization in existing geographic markets, and our continuing focus on increasing the efficiency of our existing direct sales organization.
Our product portfolio includes a broad spectrum of over 80 products consisting of over 600 stock keeping units (SKUs) covering a wide array of blood clotting devices, extraction catheters, access catheters, guide catheters, micro-introducer kits, guidewires, snare and retrieval devices, a reprocessing service for radiofrequency catheters and endovenous laser and procedure kits for the treatment of varicose veins. Our management, including our chief executive officer who is our chief operating decision maker, report and manage our operations in three main categories based on similarities in the products or services sold. We have corporate infrastructure and direct sales capabilities in the United States and have established distribution relationships in most major international markets. In order to drive sales growth, we have invested not only in the expansion of our global distribution system, but also new product development and clinical trials to obtain regulatory approvals. A significant portion of our net revenue historically has been, and we expect to continue to be, attributable to new and enhanced products and services. We expect to continue to further validate the clinical and competitive benefits of our technology platforms to drive utilization of our current products and the development of new and enhanced products and services.
Results of Operations
The following table sets forth, for the periods indicated, certain items from our statements of operations expressed as a percentage of net sales:
| | Year Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Net revenue: | | | | | | | | | |
| Product revenue | | | 100 | % | | | 100 | % | | | 96 | % |
| License and collaboration revenue | | | - | | | | - | | | | 4 | % |
| Total net revenue | | | 100 | % | | | 100 | % | | | 100 | % |
| | | | | | | | | | | | |
| Product costs and operating expenses: | | | | | | | | | | | | |
| Cost of goods sold | | | 33 | % | | | 33 | % | | | 33 | % |
| Collaboration expenses | | | - | | | | - | | | | - | |
| Research and development | | | 12 | % | | | 12 | % | | | 11 | % |
| Clinical and regulatory | | | 4 | % | | | 5 | % | | | 5 | % |
| Sales and marketing | | | 25 | % | | | 26 | % | | | 27 | % |
| General and administrative | | | 8 | % | | | 7 | % | | | 5 | % |
| Litigation | | | 1 | % | | | - | | | | - | |
| Medical device excise tax | | | 1 | % | | | - | | | | - | |
| Amortization of purchased technology and intangibles | | | 1 | % | | | 1 | % | | | 1 | % |
| Total product costs and operating expenses | | | 85 | % | | | 84 | % | | | 82 | % |
Operating earnings | | | 15 | % | | | 16 | % | | | 18 | % |
| | | | | | | | | | | | |
| Interest earnings/expense and foreign exchange loss, net | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
| Earnings before income taxes | | | 15 | % | | | 16 | % | | | 18 | % |
| | | | | | | | | | | | |
| Income tax benefit (expense) | | | (5 | %) | | | (6 | %) | | | (7 | %) |
| Net earnings | | | 10 | % | | | 10 | % | | | 11 | % |
Our primary products and related services fall into three categories. The following table sets forth, for the periods indicated, net revenue by product category along with the change from the previous year:
| | For Years Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| | Net Revenue | | | Percent Change | | | Net Revenue | | | Percent Change | | | Net Revenue | | | Percent Change | |
| | | | | | | | | | | | | | | | | | |
| Catheter products | | $ | 69,936,000 | | | | 14 | % | | $ | 61,262,000 | | | | 16 | % | | $ | 53,040,000 | | | | 27 | % |
| Hemostat products | | | 23,752,000 | | | | 5 | % | | | 22,676,000 | | | | (2 | %) | | | 23,065,000 | | | | (6 | %) |
| Vein products | | | 16,509,000 | | | | 17 | % | | | 14,098,000 | | | | 34 | % | | | 10,484,000 | | | | (4 | %) |
| Total product revenue | | | 110,197,000 | | | | 12 | % | | | 98,036,000 | | | | 13 | % | | | 86,589,000 | | | | 12 | % |
| License & collaboration | | | 301,000 | | | | (14 | %) | | | 350,000 | | | | (90 | %) | | | 3,367,000 | | | | 229 | % |
| Total net revenue | | $ | 110,498,000 | | | | 12 | % | | $ | 98,386,000 | | | | 9 | % | | $ | 89,956,000 | | | | 15 | % |
Year ended December 31, 2013 compared to the years ended December 31, 2012 and December 31, 2011.
Net revenue increased 12% to $110,498,000 for the year ended December 31, 2013 from $98,386,000 for the year ended December 31, 2012. This increase in revenue is comprised of the following components:
| | % Change | |
| Volume of existing product and service revenue | | | 10 | % |
| New product or service introductions, which consist of any product or service that had no revenue in the comparable period in 2012 | | | 2 | % |
| Product and service pricing | | | - | % |
| | | 12 | % |
Approximately 85% of our net revenue was earned in the United States and 15% of our net revenue was earned in international markets for the year ended December 31, 2013.
Net revenue increased 9% to $98,386,000 for the year ended December 31, 2012 from $89,956,000 for the year ended December 31, 2011. This increase in revenue is comprised of the following components:
| | % Change | |
| Volume of existing product and service revenue | | | 9 | % |
| New product or service introductions, which consist of any product or service that had no revenue in the comparable period in 2011 | | | 6 | % |
| Product and service pricing | | | (3 | %) |
| Decline in licensing revenue | | | (3 | %) |
| | | 9 | % |
Approximately 84% of our net revenue was earned in the United States and 16% of our net revenue was earned in international markets for the year ended December 31, 2012.
We recognized $301,000 of licensing and collaboration revenue during the year ended December 31, 2013, compared to $350,000 during the year ended December 31, 2012 and $3,367,000 during the year ended December 31, 2011. On July 6, 2011, King notified us that it was terminating the development of the Thrombi-Paste products and terminating efforts to obtain the surgical indication for the Thrombi-Gel products under our Device Supply Agreement. As a result of King’s decision, we recognized revenue of $2,762,000 in the third quarter of 2011, which represented the remaining deferred license revenue originally allocated to the Thrombi-Paste products and the surgical indication of the Thrombi-Gel under the Device Supply Agreement. Collaboration revenue of $60,000 recognized in the year ended December 31, 2013 resulted from an agreement we entered into with a pharmaceutical company to develop a new hemostatic device. License and collaboration revenue is expected to be approximately $500,000 in 2014.
Gross margin across all product lines remained constant at 67% for the year ended December 31, 2013, compared to 67% for the year ended December 31, 2012 and 66% for the year ended December 31, 2011. The increase in gross margin for the year ended December 31, 2012 compared to 2011 primarily resulted from our acquisition of the intellectual property related to our Pronto extraction catheters in January 2012, which eliminated the royalties paid on sales of the product after December 31, 2011 (see Note 15 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013). We expect product gross margins to be in the range of 67% to 68% in 2014, subject to variations in our selling mix between United States and international markets and between our lower margin products such as the Vari-Lase products and our higher margin products such as the D-Stat Dry and GuideLiner products.
Collaboration expense was $60,000 for the year ended December 31, 2013, compared to $-0- for the years ended December 31, 2012 and December 31, 2011. Collaboration expense was primarily the result of an agreement we entered into to develop a new hemostatic device with a pharmaceutical company. We expect collaboration expenses to be approximately $200,000 in 2014.
Research and development expense for the year ended December 31, 2013 totaled $13,191,000, or 12% of revenue, compared to $11,870,000, or 12% of revenue for the year ended December 31, 2012 and $10,240,000, or 11% of revenue for the year ended December 31, 2011. Research and development expenses have increased on a dollar basis, but remaining flat on a percentage basis, as we have hired additional employees to improve the through-put of our new product development projects. We expect our continuing research and development expense to be approximately 11% to 12% of revenue in 2014 as we continue to pursue additional new products and move our longer term development projects forward.
Clinical and regulatory expense for the year ended December 31, 2013 totaled $4,408,000, or 4% of revenue, compared to $4,358,000, or 5% of revenue for the year ended December 31, 2012 and $4,332,000, or 5% of revenue for the year ended December 31, 2011. Clinical and regulatory expenses have remained relatively constant on a dollar basis and have declined as a percentage of revenue compared to the years ended December 31, 2012 and December 31, 2011. We expect clinical and regulatory expense to be approximately 4% of revenue in 2014.
Sales and marketing expense for the year ended December 31, 2013 totaled $27,372,000, or 25% of revenue, compared to $25,777,000, or 26% of revenue for the year ended December 31, 2012 and $24,126,000, or 27% of revenue for the year ended December 31, 2011. The decline in sales and marketing expense as a percentage of revenue primarily resulted from maintaining our U.S. direct sales force at between 88 and 92 full-time employees while continuing to grow revenue. We expect to maintain the same approximate size of our direct sales force during 2014. As a result, we expect our sales and marketing expense will continue to decline as a percentage of revenue to approximately 24% of revenue by the end of 2014.
General and administrative expense for the year ended December 31, 2013 totaled $8,991,000, or 8% of revenue, compared to $6,522,000, or 7% of revenue for the year ended December 31, 2012 and $4,997,000, or 5% of revenue for the year ended December 31, 2011. General and administrative expense increased in 2013 on a dollar and percentage basis compared to the year ended December 31, 2012, as we incurred an additional $1,071,000 of legal expenses compared to 2012, primarily related to the patent infringement lawsuit filed against Boston Scientific in May 2013, the patent and trademark infringement lawsuit that was commenced by Terumo Corporation in February 2013 and the U.S. Attorney’s litigation and investigation that commenced in June 2011 (see Note 14 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013). General and administrative expense increased in 2012 on a dollar and percentage basis compared to the year ended December 31, 2011 due to increases in our staffing and an additional $204,000 of legal expenses compared to 2011 related to the U.S. Attorney’s litigation and investigation that commenced in June 2011. In accordance with accounting rules (Accounting Standards Codification “ASC” 805, Business Combinations), on September 30, 2011 we reduced the amount of the earn-out liability related to our acquisition of the snare and retrieval products from Radius Medical by $586,000, which reduced general and administrative expenses by a corresponding amount. During 2012 and 2013 we further reduced the Radius earn-out liability by $232,000 and $79,000, respectively. We expect general and administrative expense to be approximately 7.0% to 7.5% of revenue during 2014.
Litigation expense was $1,332,000 for the year ended December 31, 2013, compared to $-0- for both years ended December 31, 2012 and December 31, 2011. Litigation expense consisted of $812,500 incurred in the third quarter of 2013 and paid on December 13, 2013 in connection with the settlement of a patent and trademark infringement lawsuit brought by Terumo Corporation (see Item 3 – Legal Proceedings of Part I of this Annual Report on Form 10-K for the year ended December 31, 2013) and $520,000 incurred in the fourth quarter of 2013 and to be paid in 2014 related to our entering into an agreement on January 22, 2014 with the U.S. Attorney’s Office to settle a civil lawsuit related to our Vari-Lase Short Kit product (see Item 3 – Legal Proceedings of Part I of this Annual Report on Form 10-K for the year ended December 31, 2013).
Medical device excise taxes for the year ended December 31, 2013 totaled $1,319,000, or 1.2% of revenue, compared to $-0- for both years ended December 31, 2012 and December 31, 2011. An excise tax on the sale of medical devices in the U.S. became effective on January 1, 2013, and therefore no corresponding medical device excise taxes were reported in 2012 and 2011. The statutory rate of the medical device excise tax is 2.3% of revenues on initial sales of finished medical products sold in the United States. The tax does not apply to service revenues. The Company’s effective rate for this tax is less than the statutory rate due to international sales, service revenues and sales of purchased finished goods (where the original seller is responsible for paying the tax). We expect our medical device excise taxes to be approximately 1.2% of revenue in 2014.
Amortization of purchased technology and other intangibles was $1,572,000 for the year ended December 31, 2013, compared to $1,393,000 for the year ended December 31, 2012, and $831,000 for the year ended December 31, 2011. The amortization resulted from our product acquisitions. As part of these asset purchases and licensing agreements, we allocated $16,000,000 to purchased technology and other intangibles that are being amortized over a period of 9 to 11 years (see Notes 3 and 15 to the Consolidated Financial Statements included in Item 8 of Part II of this Annual Report on 10-K for the year ended December 31, 2013).
We recorded income tax expense of $4,941,000 for the year ended December 31, 2013, compared to an income tax expense of $5,983,000 for the year ended December 31, 2012 and $5,960,000 for the year ended December 31, 2011. This represents an income tax rate of 31% for the year ended December 31, 2013, and 38% for the years ended December 31, 2012 and December 31, 2011. The reduced effective tax rate of 31% for the year ended December 31, 2013 was due primarily to the impact of $1.3 million of stock option exercise differences, an $849,000 domestic manufacturing deduction which had been limited in prior years by alternative minimum taxes, recognition of $300,000 of research and development credits in the first quarter of 2013 that had been deferred due to congressional delay at the end of 2012, and the recognition of $53,000 of refundable Ireland research and development credits in the second quarter of 2013. We expect our income tax rate will be between 34% and 36% in 2014.
As of December 31, 2013, we had a total of $7.7 million of deferred tax assets on our balance sheet, consisting of $6.0 million of current deferred tax assets and $1.7 million of long term deferred tax assets. In addition, at December 31, 2013, we had the following tax benefits not recorded on our balance sheet:
| · | Approximately $900,000 of federal and state research and development tax credit carryforwards which began to expire in the year 2013. We have recorded an allowance of approximately $100,000 against this amount, reflecting the amount of these research and development tax credit carryforwards expected to expire prior to being utilized. |
| · | Approximately $40,000 of Irish foreign tax loss carryforwards, which do not expire. |
We project our income tax expense to be between $7.5 million and $8.0 million in 2014. Of this total, we expect to be able to utilize $3.5 million of deferred tax assets to offset our tax payments, resulting in our use of between $4.0 million and $4.5 million in cash to pay state and federal taxes in 2014.
Liquidity and Capital Resources
Our cash and cash equivalents totaled $30,785,000 at December 31, 2013, compared to $11,554,000 in cash and cash equivalents at December 31, 2012, an increase of $19,231,000. The majority of our cash is maintained in our operating accounts. A portion of our cash equivalents are invested in a money market fund invested in high quality, short-term money market instruments denominated in U.S. dollars such as debt instruments guaranteed by the governments of the United States, Western Europe, Australia, Japan and Canada, high quality corporate issuers and bank obligations. The money market fund’s assets are rated in the highest short-term category by nationally recognized rating agencies, such as Moody’s or Standard & Poor’s.
Cash provided by operations. We generated $19,386,000 of cash from operations during the year ended December 31, 2013, primarily resulting from our net earnings of $11,142,000, non-cash depreciation and amortization expense of $4,433,000, non-cash stock-based compensation of $3,343,000 and non-cash taxes of $3,138,000. Cash from operations increased by $485,000 due to an increase in accrued expenses. Cash from operations decreased $1,148,000 due to an increase in accounts receivable and inventory for the year ended December 31, 2013. The increases in inventory and accounts receivable were consistent with our growing business and expectations and at a lower rate than our revenue growth.
Cash used for investing activities. We used $5,032,000 of cash in investing activities during the year ended December 31, 2013, consisting of $4,234,000 for capital expenditures relating to building improvements, the purchase of manufacturing equipment, as well as the purchase of additional research and development equipment. We also used $500,000 to acquire exclusive U.S. rights from Northeast Scientific for a reprocessing service for an existing commercial medical device, pending regulatory approval of the reprocessing procedure, which Northeast Scientific is pursuing and $320,000 to acquire a product that we were previously distributing.
Cash used for financing activities. We generated $4,849,000 of cash in financing activities during the year ended December 31, 2013. This amount consisted of the receipt of $2,784,000 upon the exercise of outstanding stock options, and the receipt of $1,192,000 in exchange for the sale of common stock pursuant to our Employee Stock Purchase Plan. These amounts were partially reduced by $1,211,000 of cash used to repurchase 75,402 shares of our common stock that vested under outstanding restricted stock awards to satisfy income tax withholding obligations.
The following table summarizes our contractual cash commitments as of December 31, 2013:
| | Payments Due by Period | |
Contractual Obligations | | Total | | | Less than 1 year | | | 1 - 3 years | | | 3 - 5 years | | | More than 5 years | |
| Facility operating leases | | $ | 1,590,000 | | | $ | 908,000 | | | $ | 682,000 | | | | - | | | | - | |
| Product license rights | | | 400,000 | | | | 400,000 | | | | - | | | | - | | | | - | |
| Total | | $ | 1,990,000 | | | $ | 1,308,000 | | | $ | 682,000 | | | $ | - | | | $ | - | |
We do not have any other significant cash commitments related to supply agreements, nor do we have any significant commitments for capital expenditures.
Off-balance sheet arrangements. We do not have any off-balance sheet arrangements, as defined by the rules and regulations of the Securities and Exchange Commission, that have or are reasonably likely to have a material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources. As a result, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these arrangements.
We currently anticipate that we will experience positive cash flow from our normal operating activities for the foreseeable future. We currently believe that our working capital of $56.4 million at December 31, 2013 will be sufficient to meet all of our operating and capital requirements for the foreseeable future. However, our actual liquidity and capital requirements will depend upon numerous unpredictable factors, including the amount of revenues from sales of our existing and new products; the cost of maintaining, enforcing and defending patents and other intellectual property rights; competing technological and market developments; developments related to regulatory and third party reimbursement matters; and other factors.
Critical Accounting Policies
Management’s Discussion and Analysis of Financial Condition and Results of Operations addresses our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our consolidated financial statements requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an on-going basis, we evaluate these estimates and judgments. We base our estimates and judgments on historical experience and on various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our accounting policies are described in Note 2 to the Consolidated Financial Statements included in Item 8 of Part II of this Annual Report on 10-K for the year ended December 31, 2013. We set forth below those material accounting policies that we believe are the most critical to an investor’s understanding of our financial results and condition and that require complex management judgment.
Inventory
We state our inventory at the lower of cost (weighted average first-in, first-out method) or market. The estimated value of excess, obsolete and slow-moving inventory as well as inventory with a carrying value in excess of its net realizable value is established by us on a quarterly basis through review of inventory on hand and assessment of future demand, anticipated release of new products into the market, historical experience and product expiration. Our stated value of inventory could be materially different if demand for our products decreased because of competitive conditions or market acceptance, or if products become obsolete because of advancements in the industry. We have approximately $347,000 of thrombin in inventory at December 31, 2013, which we expect to use in our hemostat products sold in international markets. We received regulatory approval in February 2008 allowing us to use this thrombin in our international hemostat products. In the fourth quarter of 2013 and in the fourth quarter of 2008, we wrote off $65,000 and $670,000 of this thrombin, which we expect will expire before we are able to use it. We will continue to review our thrombin needs and we will write off any amounts we anticipate will not be used.
Revenue Recognition
We recognize revenue in accordance with generally accepted accounting principles as outlined in ASC 605-10-S99, Revenue Recognition, which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence of an arrangement exists; (ii) the price is fixed or determinable; (iii) collectability is reasonably assured; and (iv) product delivery has occurred or services have been rendered. We recognize revenue as products are shipped based on FOB shipping point terms when title passes to customers. We negotiate credit terms on a customer-by-customer basis and products are shipped at an agreed upon price. All product returns must be pre-approved and, if approved, customers are subject to a 20% restocking charge.
We generate revenue from license agreements and research collaborations and recognize these revenues when earned. In accordance with ASC 605, for deliverables which contain multiple deliverables, we separate the deliverables into separate accounting units if they meet the following criteria: (i) the delivered items have a stand-alone value to the customer; (ii) the fair value of any undelivered items can be reliably determined; and (iii) if the arrangement includes a general right of return, delivery of the undelivered items is probable and substantially controlled by the seller. Deliverables that do not meet these criteria are combined with one or more other deliverables into one accounting unit. Revenue from each accounting unit is recognized based on the applicable accounting literature, primarily ASC 605-10-S99.
We also generate revenue from the ClosureFAST catheter reprocessing service rights acquired from NES in December 2011. In accordance with ASC 605-45, the reprocessing revenue is reported as our revenue and the amount paid to NES is reported as our cost of sales (see Note 2 to our Consolidated Financial Statements in Item 8 of Part II of this Annual Report on Form 10-K for the year ended December 31, 2013).
On January 9, 2007, we entered into three separate agreements with King, consisting of a License Agreement, a Device Supply Agreement and a Thrombin-JMI Supply Agreement. We licensed the exclusive rights to our products Thrombi-Pad, Thrombi-Gel and Thrombi-Paste to King for $7 million. As part of the Device Supply Agreement, we agreed to complete the development and conduct clinical studies for Thrombi-Gel and Thrombi-Paste, with the costs related to these activities to be paid by King. We have recognized collaboration revenue on this development agreement as it was earned under the agreements with King. On July 6, 2011, King notified us that it was terminating the development of the Thrombi-Paste products and terminating efforts to obtain the surgical indication for the Thrombi-Gel products. As a result of King making this decision not to proceed, we recognized revenue of $2,762,000 in the third quarter of 2011, which represented the remaining deferred license revenue originally allocated to the Thrombi-Paste products and the surgical indication of the Thrombi-Gel products as part of the King agreements. We are amortizing the remaining license fee on a straight-line basis over 10 years. We continue to manufacture and sell the licensed products to King under the Device Supply Agreement.
In addition, we have reviewed the provisions of ASC 808, Collaborative Arrangements, and believe the adoption of this ASC will have no impact on the amounts recorded under these agreements.
We analyze the rate of historical returns when evaluating the adequacy of the allowance for sales returns, which is included with the allowance for doubtful accounts on our balance sheet. At December 31, 2013, this reserve was $50,000 compared to $55,000 at December 31, 2012. If the historical data we use to calculate these estimates does not properly reflect future returns, revenue could be overstated.
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. This allowance is regularly evaluated by us for adequacy by taking into consideration factors such as past experience, credit quality of the customer base, age of the receivable balances, both individually and in the aggregate, and current economic conditions that may affect a customer’s ability to pay. At December 31, 2013, this reserve was $150,000 compared to $130,000 at December 31, 2012. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
Income Taxes
The carrying value of our net deferred tax assets assumes that we will be able to generate sufficient taxable earnings in the United States based on estimates and assumptions. We record a valuation allowance to reduce the carrying value of our net deferred tax asset to the amount that is more likely than not to be realized. For the year ended December 31, 2013, we recorded a $882,000 uncertain tax position reserve related to our net deferred tax assets of $7.7 million as a result of our adoption of ASC 740, Income Taxes. At December 31, 2013, we have accrued $-0- for the payment of tax related interest and there was no tax interest or penalties recognized in the statements of operations. We continue to assess the potential realization of our deferred tax assets on an annual basis, or on an interim basis if circumstances warrant.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivables. We maintain our accounts for cash and cash equivalents principally at one major bank and one investment firm in the United States. We have a formal written investment policy that restricts the placement of investments to issuers evaluated as creditworthy. We have not experienced any losses on our deposits of our cash and cash equivalents.
With respect to accounts receivable, we perform credit evaluations of our customers and do not require collateral. There have been no material losses on accounts receivables.
In the United States, we sell our products directly to hospitals and clinics. In international markets, we sell our products to independent distributors who, in turn, sell to medical clinics. We sell our product in these countries through independent distributors denominated in United States dollars, with the exception of sales from our subsidiary in Ireland and sales to our distributor in Germany, where sales are denominated in Euros.
We distribute certain products on behalf of certain U.S. and international manufacturers. We pay for all distributed products in United States dollars.
We do not believe our operations are currently subject to significant market risks for interest rates, foreign currency exchange rates, commodity prices or other relevant market price risks of a material nature. A change of 0.1 in the Euro exchange rate would result in an increase or decrease of approximately $17,000 in the amount of United States dollars we receive in payment on accounts receivable from our German distributor Nicolai, GmbH and $7,000 on accounts receivable due our subsidiary in Ireland. Under our current policies, we do not use foreign currency derivative instruments to manage exposure to fluctuations in the Euro exchange rate.
We currently have no indebtedness and allowed our previous line of credit to expire on December 31, 2013. As such, we have no exposure to interest rate changes on indebtedness. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. However, we are being exposed to declines in the interest rates paid on deposited funds. A 0.1% decline in the current market interest rates paid on deposits would result in interest earnings being reduced by approximately $31,000 on an annual basis.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Consolidated Financial Statements and Notes thereto required pursuant to this Item begin on page 48 of this Annual Report on Form 10-K for the year ended December 31, 2013.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were effective.
Changes in Internal Controls.
During the fiscal quarter ended December 31, 2013, there has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2013.
Attestation Report of Independent Registered Public Accounting Firm.
Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm, has issued an attestation report on our internal control over financial reporting as of December 31, 2013. The attestation report of Baker Tilly Virchow Krause, LLP on our internal control over financial reporting as of December 31, 2013 is included on page 47 and incorporated by reference herein.
ITEM 9B. OTHER INFORMATION
None
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPRATE GOVERNANCE
Incorporated herein by reference to the Sections under the headings “Proposal 1: Election of Directors,” “Corporate Governance - Committees of the Board of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2013.
See the section under the heading “Executive Officers of the Registrant” in Item 1 of Part I herein for information regarding our executive officers.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers (including our chief executive officer, chief financial officer, chief accounting officer, and any person performing similar functions) and employees. We have posted our Code of Ethics in the “Corporate Governance” section of our website, http://www.vasc.com.
ITEM 11. EXECUTIVE COMPENSATION
Incorporated herein by reference to the Sections under the headings “Director Compensation” and “Executive Compensation” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2013.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Incorporated herein by reference to the Section under the heading “Security Ownership of Certain Beneficial Owners and Management” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2013.
Equity Compensation Plans
The following table sets forth the securities authorized to be issued under our current equity compensation plans as of December 31, 2013:
Plan category | | Number of securities to be issued upon exercise of outstanding options and rights | | | Weighted-average exercise price of outstanding options and rights | | | Number of securities remaining available for future issuance under equity compensation plans (excluding outstanding options and rights) | |
| Equity compensation plans approved by security holders | | | 788,000 | | | $ | 11.52 | | | | 1,986,000 (1 | ) (2) |
| Equity compensation plans not approved by security holders | | None | | | None | | | None | |
| Total | | | 788,000 | | | $ | 11.52 | | | | 1,986,000 | |
| (1) | Includes 1,021,000 shares reserved and available for issuance under our Stock Option and Stock Award Plan. The shares available for issuance under our Stock Option and Stock Award Plan automatically increases on an annual basis through 2016, by the lesser of: |
| · | 5% of the common-equivalent shares outstanding at the end of our prior fiscal year; or |
| · | a smaller amount determined by our Board of Directors or the committee administering the plan. |
| (2) | Includes 965,000 shares reserved and available for issuance under our Employee Stock Purchase Plan. The shares available for issuance under our Employee Stock Purchase Plan automatically increases on an annual basis through 2020, by the lesser of: |
| · | 2% of the common-equivalent shares outstanding at the end of our prior fiscal year; or |
| · | a smaller amount determined by our Board of Directors or the committee administering the plan. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Incorporated herein by reference to the Sections under the headings “Related Person Transaction Policy and Related Person Transactions,” “Proposal 1: Election of Directors,” and “Corporate Governance – Committees of the Board of Directors” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2013.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Incorporated herein by reference to the Section under the heading “Additional Information about our Independent Registered Public Accounting Firm” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2013.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Documents filed as part of this Report.
(1) The following financial statements are filed herewith in Item 8 in Part II of this Annual Report on Form 10-K for the year ended December 31, 2013.
| (i) | Report of Independent Registered Public Accounting Firm |
| (ii) | Consolidated Balance Sheets |
| (iii) | Consolidated Statements of Earnings |
| (iv) | Consolidated Statements of Comprehensive Earnings |
| (v) | Consolidated Statements of Changes in Shareholders’ Equity |
| (vi) | Consolidated Statements of Cash Flows |
| (vii) | Notes to Consolidated Financial Statements |
(2) Financial Statement Schedule
Schedule II – Valuation and Qualifying Accounts. Such schedule should be read in conjunction with the consolidated financial statements. All other supplemental schedules are omitted because of the absence of conditions under which they are required.
(3) Exhibits
Exhibit Number | | Description |
| 3.1 | | Amended and Restated Articles of Incorporation of Vascular Solutions, Inc. (incorporated by reference to Exhibit 3.1 to Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2000 (File No. 0-27605)). |
| 3.2 | | Amended and Restated Bylaws of Vascular Solutions, Inc. (incorporated by reference to Exhibit 3.1 of Vascular Solutions’ Form 8-K dated October 19, 2007 (File No. 0-27605)). |
| 4.1 | | Specimen of Common Stock certificate (incorporated by reference to Exhibit 4.1 of Vascular Solutions’ Registration Statement on Form S-1 (File No. 333-84089)). |
| 10.1 | | Lease Agreement dated August 30, 2002 by and between First Industrial, L.P. as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2002 (File No. 0-27605)). |
| 10.2 | | Fifth Amendment to Lease Agreement, dated November 12, 2007, by and between IRET - Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 99.1 of Vascular Solutions’ Form 8-K dated November 14, 2007 (File No. 0-27605)). |
| 10.3 | | Lease Agreement dated December 28, 2006 by and between IRET - Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 10.4 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006 (File No. 0-27605)). |
| 10.4 | | First Amendment to Lease Agreement, dated November 12, 2007, by and between IRET – Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 99.2 of Vascular Solutions’ Form 8-K dated November 14, 2007 (File No. 0-27605)). |
| 10.5 | | Second Amendment to Lease Agreement, dated October 23, 2010, by and between IRET – Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated October 23, 2010 (File No. 0-27605)). |
| 10.6** | | Form of Employment Agreement by and between Vascular Solutions, Inc. and each of its executive officers (incorporated by reference to Exhibit 10.5 of Vascular Solutions’ Form 10-Q for the quarter ended March 31, 2004 (File No. 0-27605)). |
| 10.7** | | Form of Incentive Stock Option Agreement (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated September 22, 2004 (File No. 0-27605)). |
| 10.8** | | Form of Nonqualified Stock Option Agreement (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 8-K dated September 22, 2004 (File No. 0-27605)). |
| 10.9** | | Form of Board of Directors Stock Option Agreement, as amended December 9, 2005 (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 8-K dated December 9, 2005 (File No. 0-27605)). |
| 10.10** | | Form of Restricted Stock Award Agreement (incorporated by reference to Exhibit 10.3 of Vascular Solutions’ Form 8-K dated December 9, 2005 (File No. 0-27605)). |
| 10.11** | | Amended and restated Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated April 22, 2010 (File No. 0-27605)). |
| 10.12 | | License agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.22 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006 (File No. 0-27605)). |
| 10.13*** | | Device Supply agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.23 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006 (File No. 0-27605)). |
| 10.14*** | | Thrombin-JMI® Supply Agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.24 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006 (File No. 0-27605)). |
| 10.15*** | | Settlement Agreement dated June 2, 2008 among VNUS Medical Technologies, Inc. (acquired by Covidien), AngioDynamics, Inc. and Vascular Solutions, Inc. (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 10-Q for the quarter ended June 30, 2008 (File No. 0-27605)). |
| 10.16 | | Asset Purchase Agreement dated August 16, 2012 by and between Vascular Solutions, Inc. and St. Jude Medical, Cardiology Division, Inc. (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2012 (File No. 0-27605)). |
| 10.17** | | Chairman of the Board Agreement dated as of May 1, 2011 (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated April 22, 2011 (File No. 0-27605)). |
| 10.18** | | Amendment No. 1 dated April 22, 2011, to the Vascular Solutions, Inc. Stock Option and Stock Award Plan (as amended January 25, 2006) (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 8-K dated April 22, 2011 (File No. 0-27605)). |
| 10.19** | | Chairman of the Board Stock Option Agreement dated April 22, 2011 (incorporated by reference to Exhibit 10.3 of Vascular Form 8-K dated April 22, 2011 (File No. 0-27605)). |
| 10.20** | | Vascular Solutions, Inc. Amended and Restated Stock Option and Stock Award Plan, as amended through July 27, 2012 (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2012 (File No. 0-27605)). |
| 10.21** | | Vascular Solutions, Inc. Restricted Stock Award Program for Non-Employee Directors (incorporated by reference to Exhibit 10.3 of Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2012 (File No. 0-27605)). |
| 10.22** | | Form of Restricted Stock Award Agreement for Non-Employee Directors (incorporated by reference to Exhibit 10.4 of Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2012 (File No. 0-27605)). |
| 10.23** | | Employment Agreement by and between Vascular Solutions, Inc. and its Chief Executive Officer dated as of January 27, 2012 (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 10-Q for the quarter ended March 31, 2012 (File No. 0-27605)). |
| 10.24** | | Chief Executive Officer Stock Option Agreement dated as of January 27, 2012 (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 10-Q for the quarter ended March 31, 2012 (File No. 0-27605)). |
| 10.25 | | Real Estate Purchase and Sale Agreement dated October 22, 2012 by and between Vascular Solutions, Inc. and Dayhu Investments U.S. Corporation (incorporated by reference to Exhibit 10.41 of Vascular Solutions’ Form 10-K for the year ended December 31, 2012 (File No. 0-27605)). |
| | Form of Executive Compensation Recoupment Agreement (filled herewith). |
| | Consent of Baker Tilly Virchow Krause, LLP. |
| 24.1 | | Power of Attorney (included on signature page). |
| | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 101.INS* | | XBRL Instance Document |
| 101.SCH* | | XBRL Taxonomy Extension Schema |
| 101.CAL* | | XBRL Taxonomy Extension Calculation Linkbase |
| 101.DEF* | | XBRL Taxonomy Extension Definition Linkbase |
| 101.LAB* | | XBRL Taxonomy Extension Label Linkbase |
| 101.PRE* | | XBRL Taxonomy Extension Presentation Linkbase |
* Filed herewith.
**Management contract or compensatory plan or arrangement required to be filed as an Exhibit to this Form 10-K.
*** Pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, confidential portions of these exhibits have been deleted and filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on the 4th day of February 2014.
| VASCULAR SOLUTIONS, INC. |
| | |
| By: | /s/ Howard Root | |
| | Howard Root |
| | Chief Executive Officer and Director |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Howard Root, James Hennen and Gordon Weber (with full power to act alone), as his true and lawful attorneys-in-fact and agents, with full powers of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to the Annual Report on Form 10-K of Vascular Solutions, Inc. for the year ended December 31, 2013, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed on the 4th day of February 2014, by the following persons in the capacities indicated.
Signature | | Title |
| | |
| /s/ Howard Root | | Chief Executive Officer and Director |
| Howard Root | | (principal executive officer) |
| | |
| /s/ James Hennen | | Senior Vice President, Finance and Chief Financial Officer |
| James Hennen | | (principal financial officer) |
| | |
| /s/ Timothy Slayton | | Controller |
| Timothy Slayton | | (principal accounting officer) |
| | |
| /s/ Richard Nigon | | Director |
| Richard Nigon | | |
| | |
| /s/ Richard Kramp | | Director |
| Richard Kramp | | |
| | |
| /s/ Paul O’Connell | | Director |
| Paul O’Connell | | |
| | |
| /s/ John Erb | | Director |
| John Erb | | |
| | |
| /s/ Jorge Saucedo | | Director |
| Jorge Saucedo | | |
| | |
| /s/ Martin Emerson | | Director |
| Martin Emerson | | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders, Audit Committee and Board of Directors
Vascular Solutions, Inc.
Minneapolis, MN
We have audited the accompanying consolidated balance sheets of Vascular Solutions, Inc. as of December 31, 2013 and 2012, and the related consolidated statements of earnings, comprehensive earnings, changes in shareholders' equity and cash flows for the years ended December 31, 2013, 2012 and 2011. We also have audited Vascular Solutions, Inc.'s internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control ‑ Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). These consolidated financial statements are the responsibility of the company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management as well as evaluating the overall consolidated financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Vascular Solutions, Inc. as of December 31, 2013 and 2012 and the results of their operations and cash flows for the years ended December 31, 2013, 2012 and 2011, in conformity with U.S. generally accepted accounting principles. Also in our opinion, Vascular Solutions, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control ‑ Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
/s/ Baker Tilly Virchow Krause, LLP
Minneapolis, Minnesota
February 4, 2014
Vascular Solutions, Inc.
Consolidated Balance Sheets
| | December 31, | |
| | 2013 | | | 2012 | |
| Assets | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 30,785,000 | | | $ | 11,554,000 | |
| Accounts receivable, net of reserves of $200,000 and $185,000 at December 31, 2013 and 2012, respectively | | | 14,481,000 | | | | 13,780,000 | |
| Inventories | | | 14,002,000 | | | | 13,737,000 | |
| Prepaid expenses and other | | | 2,472,000 | | | | 2,670,000 | |
| Current portion of deferred tax assets | | | 6,000,000 | | | | 6,800,000 | |
| Total current assets | | | 67,740,000 | | | | 48,541,000 | |
| | | | | | | | |
| Property, plant and equipment, net | | | 16,187,000 | | | | 14,756,000 | |
| Goodwill | | | 10,532,000 | | | | 10,387,000 | |
| Intangible assets, net | | | 11,943,000 | | | | 12,325,000 | |
| Deferred tax assets, net of current portion and liabilities | | | 1,739,000 | | | | 1,993,000 | |
| Total assets | | $ | 108,141,000 | | | $ | 88,002,000 | |
| | | | | | | | |
| Liabilities and shareholders’ equity | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 3,762,000 | | | $ | 3,842,000 | |
| Accrued compensation | | | 4,365,000 | | | | 4,123,000 | |
| Accrued expenses | | | 2,467,000 | | | | 1,927,000 | |
| Accrued royalties | | | 235,000 | | | | 288,000 | |
| Current portion of deferred revenue and contingent consideration | | | 556,000 | | | | 345,000 | |
| Total current liabilities | | | 11,385,000 | | | | 10,525,000 | |
| | | | | | | | |
| Long-term deferred revenue and contingent consideration, net of current portion | | | 406,000 | | | | 610,000 | |
| | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | |
| Shareholders’ equity: | | | | | | | | |
| Common stock, $0.01 par value: | | | | | | | | |
| Authorized shares – 40,000,000 | | | | | | | | |
| Issued and outstanding shares – 16,964,953 – 2013;16,378,923 – 2012 | | | 170,000 | | | | 164,000 | |
| Additional paid-in capital | | | 92,346,000 | | | | 84,160,000 | |
| Other | | | (1,000 | ) | | | (150,000 | ) |
| Accumulated earnings (deficit) | | | 3,835,000 | | | | (7,307,000 | ) |
| Total shareholders’ equity | | | 96,350,000 | | | | 76,867,000 | |
| Total liabilities and shareholders’ equity | | $ | 108,141,000 | | | $ | 88,002,000 | |
See accompanying notes.
Vascular Solutions, Inc.
Consolidated Statements of Earnings
| | Year Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Net revenue: | | | | | | | | | |
| Product revenue | | $ | 110,197,000 | | | $ | 98,036,000 | | | $ | 86,589,000 | |
| License and collaboration revenue | | | 301,000 | | | | 350,000 | | | | 3,367,000 | |
| Total revenue | | | 110,498,000 | | | | 98,386,000 | | | | 89,956,000 | |
| | | | | | | | | | | | |
| Product costs and operating expenses: | | | | | | | | | | | | |
| Cost of goods sold | | | 36,156,000 | | | | 32,561,000 | | | | 29,844,000 | |
| Collaboration expenses | | | 60,000 | | | | – | | | | – | |
| Research and development | | | 13,191,000 | | | | 11,870,000 | | | | 10,240,000 | |
| Clinical and regulatory | | | 4,408,000 | | | | 4,358,000 | | | | 4,332,000 | |
| Sales and marketing | | | 27,372,000 | | | | 25,777,000 | | | | 24,126,000 | |
| General and administrative | | | 8,991,000 | | | | 6,522,000 | | | | 4,997,000 | |
| Litigation | | | 1,332,000 | | | | – | | | | – | |
| Medical device excise taxes | | | 1,319,000 | | | | – | | | | – | |
| Amortization of purchased technology and intangibles | | | 1,572,000 | | | | 1,393,000 | | | | 831,000 | |
| Total product costs and operating expenses | | | 94,401,000 | | | | 82,481,000 | | | | 74,370,000 | |
| | | | | | | | | | | | |
| Operating earnings | | | 16,097,000 | | | | 15,905,000 | | | | 15,586,000 | |
| | | | | | | | | | | | |
| Other earnings (expenses): | | | | | | | | | | | | |
| Interest earnings | | | – | | | | – | | | | 16,000 | |
| Interest expense | | | (13,000 | ) | | | (13,000 | ) | | | (13,000 | ) |
| Foreign exchange gain (loss) | | | (1,000 | ) | | | – | | | | 110,000 | |
| | | | | | | | | | | | |
| Earnings before income taxes | | | 16,083,000 | | | | 15,892,000 | | | | 15,699,000 | |
| | | | | | | | | | | | |
| Income tax expense | | | (4,941,000 | ) | | | (5,983,000 | ) | | | (5,960,000 | ) |
| Net earnings | | $ | 11,142,000 | | | $ | 9,909,000 | | | $ | 9,739,000 | |
| | | | | | | | | | | | |
| Basic net earnings per common share | | $ | 0.68 | | | $ | 0.62 | | | $ | 0.59 | |
| Diluted net earnings per common share | | $ | 0.65 | | | $ | 0.60 | | | $ | 0.57 | |
| Shares used in computing basic net earnings per common share | | | 16,412,322 | | | | 16,003,932 | | | | 16,638,078 | |
| Shares used in computing diluted net earnings per common share | | | 17,025,090 | | | | 16,455,729 | | | | 17,183,579 | |
See accompanying notes.
Vascular Solutions, Inc.
Consolidated Statements of Comprehensive Earnings
| | Year Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Net earnings | | $ | 11,142,000 | | | $ | 9,909,000 | | | $ | 9,739,000 | |
| | | | | | | | | | | | |
| Other comprehensive earnings (loss), net of tax of $0: | | | | | | | | | | | | |
| Foreign currency translation adjustments | | | 149,000 | | | | 54,000 | | | | (288,000 | ) |
Comprehensive earnings | | $ | 11,291,000 | | | $ | 9,963,000 | | | $ | 9,451,000 | |
See accompanying notes.
Vascular Solutions, Inc.
Consolidated Statements of Changes in Shareholders’ Equity
| | Common Stock | | | Additional | | | | | | Accumulated | | | | |
| | Shares | | | Amount | | | Paid-In Capital | | | Other | | | Earnings (Deficit) | | | Total | |
| Balance at December 31, 2010 | | | 16,889,360 | | | $ | 169,000 | | | $ | 90,805,000 | | | $ | 84,000 | | | $ | (26,955,000 | ) | | $ | 64,103,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 87,190 | | | | 1,000 | | | | 348,000 | | | | – | | | | – | | | | 349,000 | |
| Issuance of common stock under the Employee Stock Purchase Plan | | | 115,511 | | | | 1,000 | | | | 949,000 | | | | – | | | | – | | | | 950,000 | |
| Stock-based compensation | | | 236,252 | | | | 3,000 | | | | 2,247,000 | | | | – | | | | – | | | | 2,250,000 | |
| Repurchase and cancellation of common stock upon the vesting of restricted shares | | | (47,207 | ) | | | (1,000 | ) | | | (513,000 | ) | | | – | | | | – | | | | (514,000 | ) |
| Repurchase of common stock under stock repurchase agreement | | | (902,901 | ) | | | (9,000 | ) | | | (9,874,000 | ) | | | – | | | | – | | | | (9,883,000 | ) |
| Comprehensive earnings: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | – | | | | – | | | | – | | | | – | | | | 9,739,000 | | | | 9,739,000 | |
| Translation adjustment | | | – | | | | – | | | | – | | | | (288,000 | ) | | | – | | | | (288,000 | ) |
| Total comprehensive earnings | | | | | | | | | | | | | | | | | | | | | | | 9,451,000 | |
| Balance at December 31, 2011 | | | 16,378,205 | | | $ | 164,000 | | | $ | 83,962,000 | | | $ | (204,000 | ) | | $ | (17,216,000 | ) | | $ | 66,706,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 157,590 | | | | 2,000 | | | | 487,000 | | | | – | | | | – | | | | 489,000 | |
| Issuance of common stock under the Employee Stock Purchase Plan | | | 114,060 | | | | 1,000 | | | | 1,003,000 | | | | – | | | | – | | | | 1,004,000 | |
| Stock-based compensation | | | 213,309 | | | | 2,000 | | | | 2,981,000 | | | | – | | | | – | | | | 2,983,000 | |
| Repurchase and cancellation of common stock upon the vesting of restricted shares | | | (59,106 | ) | | | (1,000 | ) | | | (651,000 | ) | | | – | | | | – | | | | (652,000 | ) |
| Repurchase of common stock under stock repurchase agreement | | | (425,135 | ) | | | (4,000 | ) | | | (4,757,000 | ) | | | – | | | | – | | | | (4,761,000 | ) |
| Excess tax benefit from stock-based compensation | | | – | | | | – | | | | 1,135,000 | | | | – | | | | – | | | | 1,135,000 | |
| Comprehensive earnings: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | – | | | | – | | | | – | | | | – | | | | 9,909,000 | | | | 9,909,000 | |
| Translation adjustment | | | – | | | | – | | | | – | | | | 54,000 | | | | – | | | | 54,000 | |
| Total comprehensive earnings | | | | | | | | | | | | | | | | | | | | | | | 9,963,000 | |
| Balance at December 31, 2012 | | | 16,378,923 | | | $ | 164,000 | | | $ | 84,160,000 | | | $ | (150,000 | ) | | $ | (7,307,000 | ) | | $ | 76,867,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 354,300 | | | | 4,000 | | | | 2,780,000 | | | | – | | | | – | | | | 2,784,000 | |
| Issuance of common stock under the Employee Stock Purchase Plan | | | 124,098 | | | | 1,000 | | | | 1,191,000 | | | | – | | | | – | | | | 1,192,000 | |
| Stock-based compensation | | | 183,034 | | | | 2,000 | | | | 3,341,000 | | | | – | | | | – | | | | 3,343,000 | |
| Cancellation of common stock upon the vesting of restricted shares | | | (75,402 | ) | | | (1,000 | ) | | | (1,210,000 | ) | | | – | | | | – | | | | (1,211,000 | ) |
| Excess tax benefit from stock-based compensation | | | – | | | | – | | | | 2,084,000 | | | | – | | | | – | | | | 2,084,000 | |
| Comprehensive earnings: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net earnings | | | – | | | | – | | | | – | | | | – | | | | 11,142,000 | | | | 11,142,000 | |
| Translation adjustment | | | – | | | | – | | | | – | | | | 149,000 | | | | – | | | | 149,000 | |
| Total comprehensive earnings | | | | | | | | | | | | | | | | | | | | | | | 11,291,000 | |
| Balance at December 31, 2013 | | | 16,964,953 | | | $ | 170,000 | | | $ | 92,346,000 | | | $ | (1,000 | ) | | $ | 3,835,000 | | | $ | 96,350,000 | |
See accompanying notes.
Vascular Solutions, Inc.
Consolidated Statements of Cash Flows
| | Year Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| Operating activities | | | | | | | | | |
| Net earnings | | $ | 11,142,000 | | | $ | 9,909,000 | | | $ | 9,739,000 | |
| Adjustments to reconcile net earnings to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation | | | 2,861,000 | | | | 2,158,000 | | | | 2,115,000 | |
| Amortization | | | 1,572,000 | | | | 1,393,000 | | | | 831,000 | |
| Stock-based compensation | | | 3,343,000 | | | | 2,983,000 | | | | 2,250,000 | |
| Deferred taxes, net | | | 3,138,000 | | | | 5,287,000 | | | | 5,445,000 | |
| Excess tax benefit from stock-based compensation | | | (2,084,000 | ) | | | (1,135,000 | ) | | | – | |
| Loss (gain) on disposal of fixed assets | | | (17,000 | ) | | | 6,000 | | | | 36,000 | |
| Change in fair value of contingent consideration | | | (79,000 | ) | | | (232,000 | ) | | | (586,000 | ) |
| Change in accounts receivable allowance | | | 15,000 | | | | 35,000 | | | | (10,000 | ) |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | (713,000 | ) | | | (2,084,000 | ) | | | (670,000 | ) |
| Inventories | | | (435,000 | ) | | | 1,032,000 | | | | (2,179,000 | ) |
| Prepaid expenses and other | | | 158,000 | | | | (977,000 | ) | | | 29,000 | |
| Accounts payable | | | (86,000 | ) | | | 995,000 | | | | 194,000 | |
| Accrued compensation and expenses | | | 485,000 | | | | 192,000 | | | | 739,000 | |
| Amortization of deferred license fees and other deferred revenue | | | 86,000 | | | | (351,000 | ) | | | (3,353,000 | ) |
| Net cash provided by operating activities | | | 19,386,000 | | | | 19,211,000 | | | | 14,580,000 | |
| | | | | | | | | | | | |
| Investing activities | | | | | | | | | | | | |
| Purchase of property and equipment, net | | | (4,234,000 | ) | | | (3,145,000 | ) | | | (2,389,000 | ) |
| Purchase of building and land | | | – | | | | (7,986,000 | ) | | | – | |
| Proceeds from the sale of equipment | | | 22,000 | | | | 10,000 | | | | – | |
| Cash paid for acquisitions | | | (820,000 | ) | | | (7,500,000 | ) | | | (6,621,000 | ) |
| Net cash used in investing activities | | | (5,032,000 | ) | | | (18,621,000 | ) | | | (9,010,000 | ) |
| | | | | | | | | | | | |
| Financing activities | | | | | | | | | | | | |
| Net proceeds from the exercise of stock options | | | 2,784,000 | | | | 489,000 | | | | 349,000 | |
| Net proceeds from the sale of common stock, employee stock purchase plan | | | 1,192,000 | | | | 1,004,000 | | | | 950,000 | |
| Excess tax benefit from stock-based compensation | | | 2,084,000 | | | | 1,135,000 | | | | – | |
| Repurchase of common shares | | | (1,211,000 | ) | | | (5,413,000 | ) | | | (10,397,000 | ) |
| Net cash provided by (used in) financing activities | | | 4,849,000 | | | | (2,785,000 | ) | | | (9,098,000 | ) |
| Effect of exchange rate changes on cash and cash equivalents | | | 28,000 | | | | 23,000 | | | | (106,000 | ) |
| Increase (decrease) in cash and cash equivalents | | | 19,231,000 | | | | (2,172,000 | ) | | | (3,634,000 | ) |
| Cash and cash equivalents at beginning of year | | | 11,554,000 | | | | 13,726,000 | | | | 17,360,000 | |
| Cash and cash equivalents at end of year | | $ | 30,785,000 | | | $ | 11,554,000 | | | $ | 13,726,000 | |
| | | | | | | | | | | | |
| Supplemental disclosure of cash flow | | | | | | | | | | | | |
| Cash paid for interest | | $ | 13,000 | | | $ | 13,000 | | | $ | 13,000 | |
| Cash paid for federal and state income taxes | | $ | 1,673,000 | | | $ | 902,000 | | | $ | 535,000 | |
See accompanying notes
1. Description of Business
Vascular Solutions, Inc. (the Company) is a medical device company focused on bringing clinically advanced solutions to interventional cardiologists and interventional radiologists. The Company has three product categories as follows:
| · | Catheter products consist principally of catheters used in minimally invasive medical procedures for the diagnosis or treatment of vascular conditions, such as the GuideLiner® catheter used to access discrete regions of the coronary anatomy and the Pronto® extraction catheters used in treating acute myocardial infarction. This category also includes products used in connection with gaining percutaneous access to the vasculature to perform minimally invasive procedures, such as micro-introducer kits. |
| · | Hemostat products consist principally of blood clotting products, such as the D-Stat® Dry hemostat, a topical thrombin-based pad with a bandage used to control surface bleeding, and the D-Stat Flowable, a thick yet flowable thrombin-based mixture for preventing bleeding in subcutaneous pockets. This category also includes our line of devices used in radial artery procedures, such as our Accumed™ wrist positioning splints and Vasc™ Band inflatable compression bands. |
| · | Vein products and services consist principally of the Vari-Lase® endovenous laser, a laser console and procedure kit used for the treatment of varicose veins, and a reprocessing service for the ClosureFAST® radiofrequency vein ablation catheter. |
As a vertically-integrated medical device company, the Company generates ideas and creates new minimally invasive devices or services and then delivers these products and services to the physicians through a direct domestic sales force and an international distribution network. The Company was incorporated in the state of Minnesota in December 1996 and began operations in February 1997.
2. Summary of Significant Accounting Policies
Basis of Consolidation
The consolidated financial statements include the accounts of Vascular Solutions, Inc. and its wholly owned subsidiary Vascular Solutions Zerusa Limited, after elimination of intercompany accounts and transactions.
Segment Reporting
A business segment is a distinguishable component of an enterprise that is engaged in providing an individual product or service or a group of related products or services and that is subject to risks and returns that are different from those of other business segments. The Company’s segments have similar economic characteristics and are similar in the nature of the products sold, type of customers, methods used to distribute the Company’s products and regulatory environment. Management believes that the Company meets the criteria for aggregating its operating segments into a single reporting segment.
2. Summary of Significant Accounting Policies (Continued)
The Company uses three product categories for reporting revenue. The following table sets forth, for the periods indicated, net revenue by product category along with the percent change from the previous year:
| | For Years Ended December 31, | |
| | 2013 | | | 2012 | | | 2011 | |
| | Net Revenue | | | Percent Change | | | Net Revenue | | | Percent Change | | | Net Revenue | | | Percent Change | |
| Catheter products | | $ | 69,936,000 | | | | 14 | % | | $ | 61,262,000 | | | | 16 | % | | $ | 53,040,000 | | | | 27 | % |
| Hemostat products | | | 23,752,000 | | | | 5 | % | | | 22,676,000 | | | | (2 | %) | | | 23,065,000 | | | | (6 | %) |
| Vein products | | | 16,509,000 | | | | 17 | % | | | 14,098,000 | | | | 34 | % | | | 10,484,000 | | | | (4 | %) |
| Total product revenue | | | 110,197,000 | | | | 12 | % | | | 98,036,000 | | | | 13 | % | | | 86,589,000 | | | | 12 | % |
| License & Collaboration | | | 301,000 | | | | (14 | %) | | | 350,000 | | | | (90 | %) | | | 3,367,000 | | | | 229 | % |
| Total Net Revenue | | $ | 110,498,000 | | | | 12 | % | | $ | 98,386,000 | | | | 9 | % | | $ | 89,956,000 | | | | 15 | % |
Foreign Currency Translation and Transactions
The functional currency of the company's foreign operations is the applicable local currency. The functional currency is translated into U.S. dollars for balance sheet accounts using current exchange rates in effect as of the balance sheet date and for revenue and expense accounts using a weighted-average exchange rate during the fiscal year. The translation adjustments are deferred as a component of other comprehensive income within the consolidated statements of comprehensive income and the consolidated statements of shareholders' equity. Gains or losses resulting from transactions denominated in foreign currencies are included in other income, net in the consolidated statements of earnings.
The Company sells products to an international distributor in Germany at prices denominated in Euros. As a result, the Company is exposed to foreign exchange movements during the time between the shipment of the product and payment. The Company currently has terms of net 60 days with this distributor under the agreement providing for payment in Euros.
Comprehensive Earnings
The components of comprehensive earnings are net earnings and the effects of foreign currency translation adjustments. The accumulated other comprehensive earnings for the foreign currency translation adjustment at December 31, 2013 and 2012 was ($1,000) and ($150,000), respectively.
Fair Value of Financial Instruments
The carrying amount for cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximates fair value due to the immediate or short-term maturity of these financial instruments.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of deferred tax assets and liabilities, as well as other amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
2. Summary of Significant Accounting Policies (Continued)
Cash and Cash Equivalents
The Company classifies all highly liquid investments with initial maturities of three months at the date of purchase or less as cash equivalents. Cash equivalents consist of cash and money market funds and are stated at cost, which approximates market value. The Company deposits its cash in high quality financial institutions. The balances, at times, may exceed federally insured limits.
Credit Risk and Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. This allowance is regularly evaluated by the Company for adequacy by taking into consideration factors such as past experience, credit quality of the customer base, age of the receivable balances, both individually and in the aggregate, and current economic conditions that may affect a customer’s ability to pay. Accounts receivable over 60 days past due are considered past due. The Company does not accrue interest on past due accounts receivable. Receivables are written off only after all collection attempts have failed and are based on individual credit evaluation and the specific circumstances of the customer. At December 31, 2013 and 2012, the allowance for doubtful accounts was $150,000 and $130,000, respectively.
All product returns must be pre-approved and, if approved, customers are subject to a 20% restocking charge. The Company analyzes the rate of historical returns when evaluating the adequacy of the allowance for sales returns, which is included with the allowance for doubtful accounts on its balance sheet. At December 31, 2013 and 2012, the sales and return allowance was $50,000 and $55,000, respectively.
Accounts receivable are shown net of the combined total of the allowance for doubtful accounts and allowance for sales returns of $200,000 and $185,000 at December 31, 2013 and 2012, respectively.
Inventories
Inventories are stated at the lower of cost (weighted average first-in, first-out method) or market. Appropriate consideration is given to deterioration, obsolescence and other factors in evaluating net realizable value. Inventories are comprised of the following at December 31:
| | 2013 | | | 2012 | |
| Raw materials | | $ | 6,386,000 | | | $ | 6,674,000 | |
| Work-in-process | | | 926,000 | | | | 780,000 | |
| Finished goods | | | 6,690,000 | | | | 6,283,000 | |
| | $ | 14,002,000 | | | $ | 13,737,000 | |
2. Summary of Significant Accounting Policies (Continued)
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Depreciation is provided on a straight-line basis over the estimated useful lives of the assets as follows:
| Manufacturing equipment | 1 to 8 years |
| Office and computer equipment | 1 to 5 years |
| Furniture and fixtures | 3 to 8 years |
| Leasehold improvements | Shorter of useful life or remaining term of the lease |
| Research and development equipment | 3 to 7 years |
| Building | 30 years |
Impairment of Long-Lived Assets
The Company will record impairment losses on long-lived assets when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. The amount of impairment loss recorded will be measured as the amount by which the carrying value of the assets exceeds the fair value of the assets. To date, the Company has determined that no impairment of long-lived assets exists.
Revenue Recognition
In the United States the Company sells its products and services directly to hospitals and clinics. Revenue is recognized in accordance with generally accepted accounting principles as outlined in Accounting Standards Codification (“ASC”) 605-10-S99, Revenue Recognition, which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence of an arrangement exists; (ii) the price is fixed or determinable; (iii) collectability is reasonably assured; and (iv) product delivery has occurred or services have been rendered. The Company recognizes revenue as products are shipped based on FOB shipping point terms when title passes to customers. The Company negotiates credit terms on a customer-by-customer basis and products are shipped at an agreed-upon price.
In all international markets, the Company sells its products to international distributors which subsequently resell the products to hospitals and clinics. The Company has agreements with each of its distributors which provide that title and risk of loss pass to the distributor upon shipment of the products to the distributor. The Company warrants that its products are free from manufacturing defects at the time of shipment to the distributor. Revenue is recognized upon shipment of products to distributors following the receipt and acceptance of a distributor’s purchase order. Allowances are provided for estimated returns and costs at the time of shipment. Sales and use taxes are reported on a net basis, excluding them from revenue.
The Company’s revenues from license agreements and research collaborations are recognized when earned (see Note 14). In accordance with ASC 605, for deliverables which contain multiple deliverables, the Company separates the deliverables into separate accounting units if they meet the following criteria: (i) the delivered items have a stand-alone value to the customer; (ii) the fair value of any undelivered items can be reliably determined; and (iii) if the arrangement includes a general right of return, delivery of the undelivered items is probable and substantially controlled by the seller. Deliverables that do not meet these criteria are combined with one or more other deliverables into one accounting unit. Revenue from each accounting unit is recognized based on the applicable accounting literature, primarily ASC 605.
2. Summary of Significant Accounting Policies (Continued)
The Company currently has a license agreement with King Pharmaceuticals, Inc. (King), now a subsidiary of Pfizer, Inc., under which the Company licensed the exclusive rights of Thrombi-Pad®, Thrombi-Gel® and Thrombi-Paste products to King in exchange for a license fee. The Company is amortizing the license fees on a straight-line basis over the projected 10 year economic life of the products. The Company determines the economic life of the products under its license agreements by evaluating similar products the Company has launched or other similar products in the medical industry. In addition, the Company had a five-year license agreement with Nicolai, GmbH in which the Company was amortizing the license fee on a straight-line basis over the five-year life of the agreement. This agreement was fully amortized during 2013.
As part of the agreements with King, the Company agreed to complete the development and conduct clinical studies for the Thrombi-Gel and Thrombi-Paste products, with the costs related to the clinical studies paid by King. The Company was recognizing the collaboration revenue on this development agreement as it is earned in accordance with ASC 605. On July 6, 2011, King notified the Company that King was terminating the development of the Thrombi-Paste products and terminating efforts to obtain the surgical indication for the Thrombi-Gel products (see Note 14).
Starting in January 2012, the Company began to generate revenue from selling a reprocessing service for ClosureFAST radiofrequency catheters. In accordance with ASC 605-45, the Company recognizes this revenue gross, with the amount paid to the supplier of the reprocessing service reflected as cost of sales.
In addition, the Company has reviewed the provisions of ASC 808, Collaborative Arrangements, and the adoption of this ASC has had no impact on the amounts recorded under these agreements.
Shipping and Handling Costs
In accordance with the ASC 605-45-45, the Company includes shipping and handling revenues in net sales and shipping and handling costs in cost of goods sold.
Research and Development Costs
All research and development costs are charged to operations as incurred.
Warranty Costs
Certain of the Company’s products are covered by warranties against defects in material and workmanship for periods of up to 24 months. The Company records a liability for warranty claims at the time of sale. The amount of the liability is based on the amount the Company is charged from its original equipment manufacturer to cover the warranty period. The original equipment manufacturers include a one year warranty with each product sold to the Company. The Company records a liability for the uncovered warranty period offered to a customer, provided the warranty period offered exceeds the initial one year warranty period covered by the original equipment manufacturers. Each of the Company’s manufacturer’s warranties cover the first year of service and as a result the Company’s exposure to uncovered warranty periods was minimal at December 31, 2013.
2. Summary of Significant Accounting Policies (Continued)
Warranty provisions and claims for the years ended December 31, 2013, 2012 and 2011, were as follows:
| | 2013 | | | 2012 | | | 2011 | |
| Beginning balance | | $ | 29,000 | | | $ | 23,000 | | | $ | 13,000 | |
| Warranty provisions | | | 69,000 | | | | 57,000 | | | | 79,000 | |
| Warranty claims | | | (75,000 | ) | | | (51,000 | ) | | | (69,000 | ) |
| Ending balance | | $ | 23,000 | | | $ | 29,000 | | | $ | 23,000 | |
Advertising Costs
The Company follows the policy of charging production costs of advertising to expense as incurred. Advertising expense was $119,000, $103,000 and $71,000 for the years ended December 31, 2013, 2012 and 2011, respectively.
Stock-Based Compensation
The Company has various types of stock-based compensation plans. These plans are administered by the Compensation Committee of the Board of Directors, which selects persons to receive awards and determines the number of shares subject to each award and the terms, conditions, performance measures and other provisions of the award. Refer to Notes 8 and 9 for additional information related to these stock-based compensation plans.
The following amounts have been recognized as stock-based compensation expense in the Consolidated Statements of Operations:
| | 2013 | | | 2012 | | | 2011 | |
| Stock-based compensation included in: | | | | | | | | | |
| Cost of goods sold | | $ | 196,000 | | | $ | 94,000 | | | $ | 86,000 | |
| Research and development | | | 373,000 | | | | 389,000 | | | | 250,000 | |
| Clinical and regulatory | | | 322,000 | | | | 313,000 | | | | 239,000 | |
| Sales and marketing | | | 858,000 | | | | 941,000 | | | | 825,000 | |
| General and administrative | | | 1,594,000 | | | | 1,246,000 | | | | 850,000 | |
| | $ | 3,343,000 | | | $ | 2,983,000 | | | $ | 2,250,000 | |
The Company uses the Black-Scholes option-pricing model to estimate fair value of stock-based awards with the following weighted average assumptions:
| | 2013 | | | 2012 | | | 2011 | |
| Stock Options and Awards: | | | | | | | | | |
| Expected life (years) | | | 3.60 | | | | 6.50 | | | | 5.50 | |
| Expected volatility | | | 39 | % | | | 48 | % | | | 49 | % |
| Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
| Risk-free interest rate | | | 0.86 | % | | | 1.21 | % | | | 2.12 | % |
| | | | | | | | | | | | |
| Employee Stock Purchase Plan: | | | | | | | | | | | | |
| Expected life (years) | | | 2.0 | | | | 2.0 | | | | 2.0 | |
| Expected volatility | | | 29 | % | | | 32 | % | | | 34 | % |
| Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
| Risk-free interest rate | | | 0.32 | % | | | 0.28 | % | | | 0.47 | % |
2. Summary of Significant Accounting Policies (Continued)
Restricted stock awards fair value is calculated as the market price on the date of grant for the years ended December 31, 2013, 2012 and 2011 and the fair value is amortized on a straight line basis over the requisite service period of four years for employee awards and one year for board of director awards. The weighted average fair value of restricted stock awards granted during 2013, 2012 and 2011 was $16.31, $11.10 and $10.77, respectively.
The weighted average fair value of stock options granted with an exercise price equal to the deemed stock price on the date of grant during 2012 and 2011 was $5.41 and $4.72, respectively. There were no stock option grants during 2013. The weighted average fair value of stock options granted with an exercise price greater than the deemed stock price on the date of grant during 2012 was $5.11.
The Company calculates expected volatility for stock options and awards using historical volatility. The starting point for the historical period used is based on a material change in the Company’s operations that occurred in the third quarter of 2003. The Company uses a 10% forfeiture rate for key employees and a 15% forfeiture rate for non-key employees for stock options and awards. The Company calculates expected volatility for employee stock purchase plan shares using historical volatility over a two-year period. A two-year period is used to coincide with the maximum two-year offering period under the employee stock purchase plan. The risk-free rates for the expected terms of the stock options and awards and the employee stock purchase plan is based on the U.S. Treasury yield curve in effect at the time of grant.
Income Taxes
Income taxes are accounted for under the liability method. Deferred income taxes are provided for temporary differences between the financial reporting and the tax bases of assets and liabilities. Deferred tax assets are reduced by a valuation allowance to the extent that realization of the related deferred tax asset is not assured. If the Company determines in the future that it is more likely than not that the Company will realize all or a portion of the deferred tax assets, the Company will adjust the valuation allowance in the period the determination is made (see Note 7).
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more-likely-than-not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance sheet along with any associated interest and penalties that would be payable to the taxing authorities upon examination.
The Company has recorded ASC 740, Income Taxes, reserves of $1,268,000 and $1,074,000 at December 31, 2013 and 2012. The impact of tax related interest and penalties is recorded as a component of income tax expense. At December 31, 2013, the Company has recorded $-0- for the payment of tax related interest and there were no tax penalties or interest recognized in the consolidated statements of earnings.
2. Summary of Significant Accounting Policies (Continued)
Net Earnings Per Common Share
In accordance with ASC 260, Earnings Per Share, basic net earnings per common share is computed by dividing net earnings by the weighted average common shares outstanding during the periods presented. Diluted net earnings per common share is computed by dividing net earnings by the weighted average common and potential dilutive common shares outstanding computed in accordance with the treasury stock method.
The number of shares used in earnings per share computations is as follows for the years ended December 31:
| | | 2013 | | | 2012 | | | 2011 | |
| Weighted average common shares outstanding— basic | | | 16,412,322 | | | | 16,003,932 | | | | 16,638,078 | |
| Effect of dilutive securities | | | 612,768 | | | | 451,797 | | | | 545,501 | |
| Weighted average common shares outstanding— diluted | | | 17,025,090 | | | | 16,455,729 | | | | 17,183,579 | |
The effect of dilutive securities in the above table excludes -0-, 270,000 and 70,000 of options for which the exercise price was higher than the average market price for the years ended December 31, 2013, 2012 and 2011, respectively.
Goodwill and Other Intangible Assets
Goodwill is reviewed for impairment annually on December 31st or more frequently if changes in circumstances or the occurrence of events suggest impairment exists using a two-step process. In step 0, the Company can elect to perform an optional quantitative analysis and based on the results, skip the remaining two steps. Consistent with prior years, in the current year the Company chose to skip step 0 and perform a quantitative analysis in Step 1. In the step 1, the fair value of each reporting unit is compared to its carrying value, including goodwill. If the fair value exceeds the carrying value, no further work is required and no impairment loss is recognized. If the carrying value exceeds the fair value, the goodwill of the reporting unit is potentially impaired and the Company would then complete step 2 in order to measure the impairment loss. In step 2, the Company would calculate the implied fair value of goodwill by deducting the fair value of all tangible and intangible net assets (including unrecognized intangible assets) of the reporting unit from the fair value of the reporting unit. If the implied fair value of goodwill is less than the carrying value of goodwill, the Company would recognize an impairment loss, in the period identified, equal to the difference. The Company has concluded that no impairment of goodwill existed as of December 31, 2013.
Other intangible assets consist of purchased technology, trademark/tradenames, developed technology, customer relationships and licenses. The Company reviewed intangible assets for impairment as changes in circumstances or the occurrence of events suggested the remaining value was not recoverable.
Amortization on the intangibles is provided on a straight-line basis over the estimated useful lives of the assets as follows:
| Trademark/tradename | 10 to 11 years |
| Purchased and developed technology | 9 to 11 years |
| Customer relationships | 9 years |
| Licenses | 5 to 10 years |
2. Summary of Significant Accounting Policies (Continued)
Leases and Deferred Rent
On November 30, 2012, the Company closed on the purchase of an office building located next to the building which previously housed the Company’s principal executive offices. The Company occupies approximately 23,000 square feet of the building and leases the remaining 47,000 square feet of the building to third-party tenants.
In addition, during the year ended December 31, 2013, the Company also leased additional office and manufacturing space. Leases are accounted for under the provisions of ASC 840, Leases, which requires that leases be evaluated and classified as operating or capital leases for financial reporting purposes. As of December 31, 2013, all of the Company’s leases were accounted for as operating leases. For leases that contain rent escalations, the Company records the total rent payable during the lease term on a straight-line basis over the term of the lease and records the difference between the rents paid and the straight-line rent as a deferred rent. For any lease incentives the Company receives for items such as leasehold improvements, the Company records a deferred credit for the amount of the lease incentive and amortizes it over the lease term, which may or may not equal the amortization period of the leasehold improvements in accordance with ASC 840-20.
3. Goodwill and Other Intangible Assets
The Company acquired trademark/tradename, developed technology and customer relationships most recently from the following (see Note 15):
| · | Zerusa Limited in January 2011, |
| · | Accumed Systems, Inc. (Accumed) in June 2012, |
| · | St. Jude Medical in August 2012, and |
| · | Shepherd Scientific, Inc. (Shepherd Scientific) in December 2012 |
The Company is amortizing these intangibles over their useful lives of 9 to 11 years. The goodwill acquired in the same transactions will not be amortized. In December 2011, the Company acquired the exclusive right to sell reprocessing services for ClosureFAST radiofrequency catheters in the U.S. and is amortizing the cost over the five year term of the license (see Note 15). In January 2012, the Company acquired the rights, patents and intellectual property relating to a two-lumen catheter for distal protection and material extraction used in the Company’s Pronto catheters (see Note 15). In September 2013, the Company entered into a services agreement with Northeast Scientific, Inc. to obtain FDA approval for reprocessing another medical device (see Note 15). Amortization expense was $1,572,000, $1,393,000 and $831,000 for the years ended December 31, 2013, 2012 and 2011, respectively.
Balances of acquired intangible assets as of December 31, 2013 were as follows:
| | Carrying Amount | | | Accumulated Amortization | | | Net | |
| Amortizing intangibles: | | | | | | | | | |
| Purchased and developed technology | | $ | 8,140,000 | | | $ | 2,360,000 | | | $ | 5,780,000 | |
| Purchased licenses | | | 5,050,000 | | | | 1,010,000 | | | | 4,040,000 | |
| Trademark / tradename | | | 2,210,000 | | | | 592,000 | | | | 1,618,000 | |
| Customer relationships | | | 640,000 | | | | 133,000 | | | | 507,000 | |
| Foreign currency translation adjustments | | | 16,000 | | | | 18,000 | | | | (2,000 | ) |
| | $ | 16,056,000 | | | $ | 4,113,000 | | | $ | 11,943,000 | |
3. Goodwill and Other Intangible Assets (Continued)
Balances of acquired intangible assets as of December 31, 2012 were as follows:
| | Carrying Amount | | | Accumulated Amortization | | | Net | |
| Amortizing intangibles: | | | | | | | | | |
| Purchased and developed technology | | $ | 7,960,000 | | | $ | 1,555,000 | | | $ | 6,405,000 | |
| Purchased licenses | | | 4,150,000 | | | | 505,000 | | | | 3,645,000 | |
| Trademark / tradename | | | 2,170,000 | | | | 390,000 | | | | 1,780,000 | |
| Customer relationships | | | 620,000 | | | | 74,000 | | | | 546,000 | |
| Foreign currency translation adjustments | | | (50,000 | ) | | | 1,000 | | | | (51,000 | ) |
| | $ | 14,850,000 | | | $ | 2,525,000 | | | $ | 12,325,000 | |
Based on the intangibles assets as of December 31, 2013, future amortization expense was as follows:
| 2014 | | $ | 1,734,000 | |
| 2015 | | | 1,824,000 | |
| 2016 | | | 1,817,000 | |
| 2017 | | | 1,644,000 | |
| 2018 | | | 1,644,000 | |
| Thereafter | | | 3,280,000 | |
| | $ | 11,943,000 | |
The following table provides a summary of additions and disposals of goodwill for each reporting period:
| Balance at December 31, 2011 | | $ | 8,117,000 | |
| Acquisition of Accumed wrist positioning splint | | | 562,000 | |
| Acquisition of St. Jude Medical’s Venture wire control catheter | | | 1,444,000 | |
| Acquisition of Shepherd Scientific’s AngioAssist & Teirstein Edge | | | 210,000 | |
| Foreign currency translation adjustments | | | 54,000 | |
| Balance at December 31, 2012 | | $ | 10,387,000 | |
| Acquisition of Disposable Vein Hook | | | 59,000 | |
| Foreign currency translation adjustments | | | 86,000 | |
| Balance at December 31, 2013 | | $ | 10,532,000 | |
4. Property, Plant and Equipment
Property, plant and equipment consist of the following at December 31:
| | 2013 | | | 2012 | |
| Plant and equipment: | | | | | | |
| Manufacturing equipment | | $ | 12,019,000 | | | $ | 10,174,000 | |
| Buildings | | | 6,407,000 | | | | 6,407,000 | |
| Office and computer equipment | | | 2,798,000 | | | | 2,265,000 | |
| Research and development equipment | | | 2,567,000 | | | | 1,707,000 | |
| Leasehold improvements | | | 2,489,000 | | | | 2,269,000 | |
| Furniture and fixtures | | | 1,039,000 | | | | 782,000 | |
| Building improvements | | | 665,000 | | | | – | |
| Construction-in-process | | | 658,000 | | | | 977,000 | |
| | | 28,642,000 | | | | 24,581,000 | |
| Less accumulated depreciation and amortization | | | (14,055,000 | ) | | | (11,425,000 | ) |
| Net plant and equipment | | | 14,587,000 | | | | 13,156,000 | |
| Land | | | 1,600,000 | | | | 1,600,000 | |
| Net property, plant and equipment | | $ | 16,187,000 | | | $ | 14,756,000 | |
5. Lines of Credit
As of December 31, 2013, the Company had a secured asset-based revolving credit agreement with U.S. Bank National Association dated December 21, 2009 (as amended on December 6, 2012; December 20, 2011 and December 20, 2010). The revolving credit agreement was a one-year, $10,000,000 facility with availability based primarily on eligible customer receivables, inventory and property and equipment.
The revolving credit agreement required a quarterly payment based on an annual fee of 0.125% of the average unused portion of the committed revolving line as determined by the bank and reviewed by management.
The revolving credit agreement included one covenant that the Company cannot have a maximum cash flow leverage ratio greater than 2.5 to 1. The calculation of this covenant is determined by multiplying annual lease expense times six and adding any loans, then dividing this amount by the sum of earnings before interest, taxes, depreciation, amortization and annual operating lease payments. The covenant is computed quarterly based on a rolling 12-month period. The Company was in compliance with the covenant as of December 31, 2013.
As of and through-out the year ended December 31, 2013, the Company had no outstanding balance against the revolving credit agreement. The revolving credit agreement expired on December 31, 2013 and has not been renewed.
6. Leases
The Company leases two buildings in Minnesota totaling approximately 106,000 square-feet under an operating lease. The lease continues to remain in effect through September 2015 with an option to renew. The Company leases one building in Ireland totaling approximately 1,150 square feet. This lease is scheduled to expire in July 2014, however it does contain extension provisions. Rent expense related to the operating leases was approximately $1,350,000, $1,273,000 and $1,297,000 for the years ended December 31, 2013, 2012, and 2011, respectively.
6. Leases (Continued)
Future minimum lease commitments under the operating leases as of December 31, 2013 were as follows:
| 2014 | | $ | 908,000 | |
| 2015 | | | 682,000 | |
| 2016 | | | – | |
| 2017 | | | – | |
| 2018 | | | – | |
| Thereafter | | | – | |
| | $ | 1,590,000 | |
7. Income Taxes
At December 31, 2013, the Company had recorded the following components of deferred taxes with a finite life:
| | 2013 | | Expiration Date |
| Federal and Minnesota research and development tax credit carryforwards | | $ | 4,766,000 | | Beginning in 2014 |
| Foreign research and development tax credit carryforwards | | | 93,000 | | Do not expire |
| Foreign operations operating loss carryforwards | | | 643,000 | | Do not expire |
The Company has recorded an allowance of approximately $144,000 relating to those research and development tax credit carryforwards expected to expire prior to utilization.
The Company is subject to income tax in numerous jurisdictions and at various rates and the use of estimates is required in determining the provision for income taxes. For the year ended December 31, 2013, the Company recorded tax expense of $4,941,000 on earnings before tax of $16,083,000 resulting in an effective income tax rate of 31%. For the year ended December 31, 2013, income before taxes relating to U.S. operations was $16,080,000, while the loss from foreign operations was $108,000. For the year ended December 31, 2012, the Company recorded tax expense of $5,983,000 on earnings before tax of $15,892,000 resulting in an effective income tax rate of 38%. For the year ended December 31, 2012, income before taxes relating to U.S. operations was $15,594,000, while income before tax from foreign operations was $298,000.
The Company is subject to income tax examinations in the U.S. Federal jurisdiction, as well as in the Republic of Ireland and various state jurisdictions. U.S. Federal tax years that remain open to examination at December 31, 2013 are 2010 through 2013 and state tax years that remain open to examination at December 31, 2013 are 2009 through 2013.
7. Income Taxes (Continued)
A reconciliation of the beginning and ending amount of unrecognized tax benefit is as follows:
| Balance at December 31, 2011 | | $ | 1,020,000 | |
| Increases as a result of tax positions taken during a prior period | | | – | |
| Increases as a result of tax positions taken during the current period | | | 54,000 | |
| Reductions as a result of lapse of the applicable statute of limitations | | | – | |
| Decreases relating to settlements with taxing authorities | | | – | |
| Balance at December 31, 2012 | | $ | 1,074,000 | |
| Increases as a result of tax positions taken during a prior period | | | – | |
| Increases as a result of tax positions taken during the current period | | | 223,000 | |
| Reductions as a result of lapse of the applicable statute of limitations | | | (29,000 | ) |
| Decreases relating to settlements with taxing authorities | | | – | |
| Balance at December 31, 2013 | | $ | 1,268,000 | |
The components of the Company’s deferred tax assets and liabilities as of December 31, 2013 and 2012 are as follows:
| | 2013 | | | 2012 | |
| Deferred tax assets: | | | | | | |
| Tax credit carryforwards | | $ | 4,766,000 | | | $ | 5,610,000 | |
| Stock-based compensation | | | 1,151,000 | | | | 1,514,000 | |
| Net operating loss carryforwards | | | – | | | | 1,288,000 | |
| Federal and state AMT credits | | | 985,000 | | | | 996,000 | |
| Inventory reserve | | | 609,000 | | | | 557,000 | |
| Depreciation and amortization | | | 624,000 | | | | 520,000 | |
| Accrued compensation | | | 578,000 | | | | 487,000 | |
| Deferred revenue | | | 229,000 | | | | 320,000 | |
| Legal settlement | | | 196,000 | | | | – | |
| Foreign operations net operating loss carryforwards | | | 96,000 | | | | 47,000 | |
| Other | | | 115,000 | | | | 130,000 | |
| Gross deferred tax assets | | | 9,349,000 | | | | 11,469,000 | |
| Deferred tax liability relating to amortization | | | (584,000 | ) | | | (336,000 | ) |
| Net deferred taxes assets before reserve for uncertain tax positions and valuation allowances | | | 8,765,000 | | | | 11,133,000 | |
| Reserve for uncertain tax positions | | | (882,000 | ) | | | (906,000 | ) |
| Less valuation allowances | | | (144,000 | ) | | | (1,434,000 | ) |
| Net deferred tax asset | | $ | 7,739,000 | | | $ | 8,793,000 | |
| | | | | | | | |
| | 2013 | | | 2012 | |
| Deferred taxes recorded on the balance sheet: | | | | | | | | |
| Net deferred tax assets – current | | $ | 6,000,000 | | | $ | 6,800,000 | |
| Net deferred tax assets – long-term | | | 1,739,000 | | | | 1,993,000 | |
| Net deferred tax assets | | $ | 7,739,000 | | | $ | 8,793,000 | |
7. Income Taxes (Continued)
The Company regularly assesses the likelihood that the deferred tax assets will be recovered from future taxable earnings. At December 31, 2013 and 2012, the valuation allowance was $144,000 and $1,434,000, respectively. The increase (decrease) in the valuation allowance was ($1,290,000), ($683,000) and $185,000 for the years ended December 31, 2013, 2012 and 2011, respectively. For the years ended December 31, 2013 and 2012, the Company recorded stock option and employee stock purchase plan tax deductions of $2,116,000 and $1,181,000, respectively. For the year ended December 31, 2013 and 2012, the Company recorded stock option and employee stock purchase plan tax benefits against “additional paid-in capital” and reduced taxes payable by a corresponding amount of $2,084,000 and $1,135,000, respectively.
Reconciliation of the statutory federal income tax rate to the Company’s effective tax rate is as follows:
| | | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Tax at statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
| State income taxes, net of federal benefit | | | 4.4 | | | | 6.2 | | | | 5.8 | |
| Permanent differences | | | (0.8 | ) | | | 1.9 | | | | 1.2 | |
| Change in valuation reserve | | | 0.2 | | | | 0.2 | | | | (1.1 | ) |
| R&D credits generated | | | (4.2 | ) | | | (1.1 | ) | | | (3.1 | ) |
| Reserve for uncertain tax positions | | | 0.9 | | | | 0.1 | | | | 0.6 | |
| Foreign operations tax rate difference | | | (0.5 | ) | | | (0.7 | ) | | | – | |
| Disallowed federal R&D credits at 12.31.12 | | | (1.9 | ) | | | – | | | | – | |
| Other adjustments | | | (1.0 | ) | | | (3.0 | ) | | | 0.6 | |
| Effective income tax rate | | | 31.1 | % | | | 37.6 | % | | | 38.0 | % |
| | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Current taxes | | | 26.8 | % | | | 12.8 | % | | | 3.8 | % |
| Deferred taxes | | | 6.6 | | | | 26.1 | | | | 34.7 | |
| Benefit from foreign operation tax rates | | | (0.5 | ) | | | (0.7 | ) | | | – | |
| Benefit from domestic manufacturers deduction | | | (1.8 | ) | | | (0.6 | ) | | | (0.5 | ) |
| Effective income tax rate | | | 31.1 | % | | | 37.6 | % | | | 38.0 | % |
8. Stock Options and Restricted Shares
Stock Option and Stock Award Plan
The Company has a stock option and stock award plan (the Stock Option Plan) which provides for the granting of stock options, restricted shares and stock appreciation rights to employees, directors, and consultants. Incentive stock options may be granted only to employees of the Company. Options which do not qualify as incentive stock options and awards of restricted shares may be granted to both employees and to non-employee directors and consultants. As of December 31, 2013, the Company had reserved 5,500,000 shares of common stock under the Stock Option Plan. Under the Stock Option Plan, stock options must be granted at an exercise price not less than the fair market value of the Company’s common stock on the grant date. Vesting requirements of all awards under this plan are time based and vary by individual grant. The options expire on the date determined by the Board of Directors but may not extend more than 10 years from the grant date. The incentive stock options generally become exercisable over a four-year period and the nonqualified stock options generally become exercisable over a one-year period. Vested and unexercised options are canceled three-months after termination,
8. Stock Options and Restricted Shares (Continued)
and unvested awards are canceled on the date of termination of employment and become available under the Stock Option Plan for future grants.
Prior to 2013 the Company granted annual stock options to its directors under the Stock Option Plan. The ten-year options issued to the Company’s directors vest over a one-year period based on the continuation of service as a director of the Company. The Company used a 0% forfeiture rate for all director options granted.
In January 2012, the Company granted stock options to the chief executive officer under the Stock Option Plan. The ten-year options issued to the Company’s chief executive officer vest over five successive one-year periods based on the continuation of service with the Company and contain a $1 per share escalation in the exercise price each year. The Company used a 0% forfeiture rate for this option grant.
Option activity is summarized as follows:
| | Shares Available for Grant (exclusive of restricted | | | Plan Options | | | Exercise | | | Weighted Average Exercise | | | Aggregate Intrinsic | |
| | shares issued) | | | Outstanding | | | Price | | | Price | | | Value | |
| | | | | | | | | | | | | | | |
| Balance at December 31, 2010 | | | 3,878,000 | | | | 837,000 | | | $ | 0.78–$10.98 | | | $ | 6.62 | | | | | |
| Shares reserved | | | 500,000 | | | | – | | | | – | | | | – | | | | | |
| Granted | | | (70,000 | ) | | | 70,000 | | | | 11.72 | | | | 11.72 | | | | | |
| Exercised | | | – | | | | (87,000 | ) | | | 0.84– 9.46 | | | | 4.00 | | | | | |
| Forfeited | | | – | | | | – | | | | – | | | | – | | | | | |
| Expired | | | 19,000 | | | | (19,000 | ) | | | 0.84– 9.46 | | | | 5.45 | | | | | |
| Balance at December 31, 2011 | | | 4,327,000 | | | | 801,000 | | | $ | 0.78–$11.72 | | | $ | 7.38 | | | | | |
| Shares reserved | | | (1,900,000 | ) | | | – | | | | – | | | | – | | | | | |
| Granted | | | (500,000 | ) | | | 500,000 | | | | 11.03– 15.03 | | | | 12.89 | | | | | |
| Exercised | | | – | | | | (158,000 | ) | | | 0.84– 10.28 | | | | 3.10 | | | | | |
| Forfeited | | | – | | | | – | | | | – | | | | – | | | | | |
| Expired | | | – | | | | – | | | | – | | | | – | | | | | |
| Balance at December 31, 2012 | | | 1,927,000 | | | | 1,143,000 | | | $ | 0.78–$15.03 | | | $ | 10.38 | | | | | |
| Shares reserved | | | 500,000 | | | | – | | | | – | | | | – | | | | | |
| Granted | | | – | | | | – | | | | – | | | | – | | | | | |
| Exercised | | | – | | | | (354,000 | ) | | | 0.78– 11.72 | | | | 7.86 | | | | | |
| Forfeited | | | – | | | | – | | | | – | | | | – | | | | | |
| Expired | | | 1,000 | | | | (1,000 | ) | | | 0.84– 5.74 | | | | 5.38 | | | | | |
| Balance at December 31, 2013 | | | 2,428,000 | | | | 788,000 | | | $ | 6.36–$15.03 | | | $ | 11.52 | | | $ | 9,164,000 | |
| Exercisable at December 31, 2013 | | | | | | | 421,000 | | | | | | | $ | 9.80 | | | $ | 5,625,000 | |
The number of common shares available for the grant of future stock awards is limited to 1,021,000 common shares. The shares available for grant number disclosed in the table above does not include 1,407,000 common shares issued in the form of restricted shares.
The weighted average remaining contractual term of options exercisable at December 31, 2013, was 4.8 years. The total intrinsic value of options exercised during fiscal 2013, 2012 and 2011, was $3,057,000, $1,464,000 and $677,000, respectively.
8. Stock Options and Restricted Shares (Continued)
The following table summarizes information about stock options outstanding at December 31, 2013:
| | | Options Outstanding | | | Options Exercisable | |
Range of Exercise Prices | | | Outstanding as of December 31, 2013 | | | Weighted Average Remaining Contractual Life | | | Weighted Average Exercise Price | | | Exercisable as of December 31, 2013 | | | Weighted Average Exercise Price | |
| | | | | | | | | | | | | | | | |
| $ 6.36–$ 7.88 | | | | 76,000 | | | | 3.9 | | | $ | 6.83 | | | | 76,000 | | | $ | 6.83 | |
| 7.89– 9.46 | | | | 115,000 | | | | 1.5 | | | | 9.44 | | | | 115,000 | | | | 9.44 | |
| 9.47– 10.98 | | | | 67,000 | | | | 4.2 | | | | 10.08 | | | | 67,000 | | | | 10.08 | |
| 10.99– 12.03 | | | | 260,000 | | | | 8.0 | | | | 11.58 | | | | 163,000 | | | | 11.32 | |
| 12.04– 13.03 | | | | 90,000 | | | | 8.1 | | | | 13.03 | | | | – | | | | – | |
| 13.04– 15.03 | | | | 180,000 | | | | 8.1 | | | | 14.53 | | | | – | | | | – | |
| | | | | 788,000 | | | | 6.3 | | | $ | 11.52 | | | | 421,000 | | | $ | 9.80 | |
As of December 31, 2013, there was $1,287,000 of total unrecognized compensation costs related to the outstanding stock options, which is expected to be recognized over a weighted average period of 3.08 years.
The holder of a restricted share award is generally entitled at all times on and after the date of issuance of the restricted shares to exercise the rights of a shareholder of the Company, including the right to vote the shares and the right to receive dividends on the shares. These shareholders do not have the ability to sell, transfer or otherwise encumber the restricted share awards until they fully vest. During 2013, 2012 and 2011, the Company granted restricted shares to directors and employees under the Stock Option Plan. The restricted shares granted to employees of the Company vest over a four-year period based on the continuation of employment. The restricted shares granted to the Company’s director’s vest over a one-year period based on the continuation of service as a director.
Restricted share activity is summarized as follows:
| | Shares | | | Weighted Average Grant Date Fair | |
| | Outstanding | | | Value | |
| | | | | | |
| Balance at December 31, 2010 | | | 463,000 | | | $ | 8.49 | |
| Granted | | | 274,000 | | | | 10.77 | |
| Vested | | | (132,000 | ) | | | 8.72 | |
| Forfeited | | | (38,000 | ) | | | 10.05 | |
| Balance at December 31, 2011 | | | 567,000 | | | $ | 9.43 | |
| Granted | | | 245,000 | | | | 11.10 | |
| Vested | | | (167,000 | ) | | | 8.12 | |
| Forfeited | | | (32,000 | ) | | | 10.67 | |
| Balance at December 31, 2012 | | | 613,000 | | | $ | 10.39 | |
| Granted | | | 199,000 | | | | 16.31 | |
| Vested | | | (208,000 | ) | | | 9.96 | |
| Forfeited | | | (16,000 | ) | | | 11.22 | |
| Balance at December 31, 2013 | | | 588,000 | | | $ | 12.52 | |
As of December 31, 2013, there was $2,010,000 of total unrecognized compensation costs related to the outstanding restricted shares, which is expected to be recognized over a weighted average period of 1.01 years.
8. Stock Options and Restricted Shares (Continued)
The Company estimates the forfeiture rate for restricted stock using 0% for directors, 10% for key employees and 15% for non-key employees.
9. Employee Stock Purchase Plan
The Company has an Employee Stock Purchase Plan (the Purchase Plan) under which 2,700,000 shares of common stock have been reserved for issuance. Eligible employees may contribute 1% to 10% of their compensation to purchase shares of the Company’s common stock at a discount of 15% of the market value at certain plan-defined dates, up to a maximum of 2,000 shares per purchasing period. The Purchase Plan terminates in July 2020. In fiscal 2013, 2012 and 2011, 124,100 shares, 114,100 shares, and 115,500 shares, respectively, were issued under the Purchase Plan. At December 31, 2013, 965,000 shares were available for issuance under the Purchase Plan.
As of December 31, 2013, there was $340,000 of total unrecognized compensation costs related to the Purchase Plan, which is expected to be recognized over a weighted average period of 0.80 years.
10. Employee Retirement Savings Plan
The Company has an employee 401(k) retirement savings plan (the Plan). The Plan provides eligible employees with an opportunity to make tax-deferred contributions into a long-term investment and savings program. All employees over the age of 21 are eligible to participate in the Plan beginning with the first quarterly open enrollment date following start of employment. The Plan allows eligible employees to contribute up to 50% of their annual compensation, subject to a maximum limit determined by the Internal Revenue Service, with the Company contributing an amount equal to 25% of the first 5% contributed to the Plan. The Company recorded an expense of $243,000, $204,000 and $194,000 for contributions to the Plan for the years ended December 31, 2013, 2012, and 2011, respectively.
11. Concentrations of Credit and Other Risks
In the United States the Company sells its products directly to hospitals and clinics. In all international markets, the Company sells its products to distributors who, in turn, sell to hospitals and clinics. Loss, termination, or ineffectiveness of distributors to effectively promote the Company’s product could have a material adverse effect on the Company’s financial condition and results of operations.
No customer represented more than 10% of total revenue for any year ended December 31, 2013, 2012 and 2011.
The Company performs credit evaluations of its customers and does not require collateral to extend credit to an account. No customer represented more than 10% of gross accounts receivable at December 31, 2013 and 2012. There have been no material losses on customer receivables.
Product revenue by geographic destination as a percentage of total product revenues were as follows for the years ended December 31:
| | 2013 | | | 2012 | | | 2011 | |
| | | | | | | | | |
| Domestic | | | 85 | % | | | 84 | % | | | 84 | % |
| Foreign | | | 15 | | | | 16 | | | | 16 | |
12. Related Party Activity
During the years ended December 31, 2013, 2012 and 2011, the Company sold $361,000, $451,000 and $504,000, respectively, of product to a company of which a board member of the Company is an officer. As of December 31, 2013 and 2012, the Company had an accounts receivable balance due of $14,000 and $39,000, respectively, from this related party. In addition, the Company purchases product from this related party and during the years ended December 31, 2013, 2012 and 2011 the Company purchased $10,000, $10,000 and $15,000, respectively, of product from this related party. As of December 31, 2013 and 2012, the Company had an accounts payable balance due of $-0- to this related party.
13. Dependence on Key Suppliers
King Pharmaceuticals
The Company purchases certain key components from single-source suppliers. Any significant component delay or interruption could require the Company to qualify new sources of supply, if available, and could have a material adverse effect on the Company’s financial condition and results of operations. The Company purchases its requirements for thrombin (a component in the Hemostat products) under a Thrombin-JMI Supply Agreement entered into with King Pharmaceuticals, Inc. (King) on January 9, 2007. Under the terms of the Thrombin-JMI Supply Agreement, King agrees to manufacture and supply thrombin to the Company on a non-exclusive basis. The Thrombin-JMI Supply Agreement does not contain any minimum purchase requirements. King agrees to supply the Company with such quantity of thrombin as the Company may order at a fixed price throughout the term of the Thrombin-JMI Supply Agreement as adjusted for inflation, variations in potency and other factors. The Thrombin-JMI Supply Agreement has an initial term of 10 years, followed by successive automatic one-year extensions, subject to termination by the parties under certain circumstances, including: (i) termination by King without cause any time after the fifth anniversary of the date of the Thrombin-JMI Supply Agreement upon five years prior written notice to the Company, and (ii) termination by the Company without cause any time after the fifth anniversary of the date of the Thrombin-JMI Supply Agreement upon five years prior written notice to King provided that the Device Supply Agreement, which the Company also entered into with King on January 9, 2007, has expired on its terms or the parties have agreed to terminate it.
14. Commitments and Contingencies
All legal cost related to litigation are charged to operations as incurred, except settlements which are expensed when a claim is probable and estimable.
Boston Scientific Corporate Litigation
On May 16, 2013, the Company filed a patent infringement complaint in the United States District Court for the District of Minnesota against Boston Scientific Corporation (Boston Scientific). The complaint alleges that Boston Scientific has infringed three of the Company’s patents concerning rapid exchange guide extension technology by manufacturing and selling its Guidezilla™ guide extension catheter, which received FDA 510(k) clearance in March 2013. The Company is seeking an injunction against Boston Scientific prohibiting the manufacture and sale of its Guidezilla catheter, as well as damages for lost profits and legal costs. On June 10, 2013, the Company filed a motion for preliminary injunction asking the court to enjoin Boston Scientific’s manufacture and sale of the Guidezilla catheter during the pendency of the litigation. On July 11, 2013, Boston Scientific filed its answer and counterclaim, alleging the Company’s patents are invalid, that the Guidezilla catheter does not infringe, and that the Company’s manufacture and sale of its GuideLiner catheter violates a U.S. patent owned by Boston Scientific that has recently expired. The counterclaim seeks unspecified damages and payment of Boston Scientific’s attorney’s fees, expenses and costs. On December 9, 2013, the District Court granted the Company’s motion for a preliminary injunction prohibiting Boston Scientific from making, using,
14. Commitments and Contingencies (Continued)
offering for sale or selling its Guidezilla catheter during the pendency of the parties’ on-going patent infringement litigation. On December 12, 2013, the District Court stayed the preliminary injunction to permit it to consider whether to permit Boston Scientific to move for reconsideration of the preliminary injunction. On December 23, 2013, the District Court denied Boston Scientific’s request for permission to file a motion to reconsider the preliminary injunction and lifted the stay on the preliminary injunction effective January 13, 2014. On December 27, 2013, Boston Scientific filed a motion with the United States Court of Appeals for the Federal Circuit for a stay, pending appeal, of the District Court’s preliminary injunction order and an interim stay pending the Federal Circuit’s consideration of the motion. On January 8, 2014, the Federal Circuit issued an order denying Boston Scientific’s motion for an interim stay of the preliminary injunction. On January 13, 2014, the preliminary injunction became effective prohibiting Boston Scientific from making, using, offering for sale or selling the Guidezilla guide extension catheter in the United States during the pendency of the patent litigation, currently scheduled to be ready for trial on or after March 2015. On January 17, 2014, the Federal Circuit issued an order denying Boston Scientific’s motion for a stay of the preliminary injunction pending appeal. The preliminary injunction order remains subject to an appeal by Boston Scientific before the Federal Circuit.
Governmental Proceedings
On June 28, 2011, the Company received a subpoena from the U.S. Attorney’s Office for the Western District of Texas under the Health Insurance Portability & Accountability Act of 1996 (HIPAA) requesting the production of documents related to Vari-Lase products, and in particular the use of the Vari-Lase Short Kit for the treatment of perforator veins. Subsequently, the Company learned that the U.S. Attorney’s Office has commenced a criminal investigation of the same matter. The Vari-Lase Short Kit has been sold under a 510(k) clearance for the treatment of incompetence and reflux of superficial veins in the lower extremity since 2007 with total U.S. sales through December 31, 2013 of approximately $479,000 (0.1% of the Company’s total U.S. sales for such period) and has not been the subject of any reported serious adverse clinical event. On August 14, 2012, the United States District Court for the Western District of Texas unsealed a qui tam complaint that had been filed on November 19, 2010 by Desalle Bui, a former sales employee of the Company, which is the basis for the U.S. Attorney’s civil investigation, to which the federal government, after three extensions of time, elected to intervene. The complaint contains allegations of off-label promotion of Vari-Lase products for the treatment of perforator veins, re-use of single-use Vari-Lase products and kickbacks to physicians, resulting in alleged damages to the government of approximately $20 million. An amended complaint limited to allegations of off-label promotion of the Vari-Lase Short Kit resulting in an unspecified amount of damages and penalties was filed by the U.S. Attorney’s Office in December 2012. On January 22, 2014, the Company agreed with the U.S. Attorney’s Office to settle the civil lawsuit. The terms of the settlement are that the Company will make a payment of $520,000, the Company will make no admission of fault or liability, and the U.S. Attorney’s Office will dismiss the civil lawsuit with prejudice and release all civil claims that were, or could have been, brought against the Company in the civil lawsuit. Settlement of the civil lawsuit will have no effect upon the criminal investigation, which the Company expects will continue.
From time to time, the Company is involved in additional legal proceedings arising in the normal course of business. As of the date of this report the Company is not a party to any legal proceeding not described in this section in which an adverse outcome would reasonably be expected to have a material adverse effect on the Company’s results of operations or financial condition.
14. Commitments and Contingencies (Continued)
King Agreements
On January 9, 2007, the Company entered into a License Agreement and a Device Supply Agreement with King (see Note 13). King was acquired by Pfizer, Inc. on February 28, 2011. Under the License Agreement, the Company licensed the exclusive rights to the Company’s products Thrombi-Pad, Thrombi-Gel and Thrombi-Paste to King in exchange for a one-time license fee. Under the Device Supply Agreement, the Company agreed to manufacture the licensed products for sale to King in exchange for an initial payment. The unamortized license fee was $610,000, $815,000 and $1,019,000 at December 31, 2013, 2012 and 2011, respectively. Amortization of the deferred revenue will be $204,000 per year for the remainder of the 10-year license period. The amortization of license fee was $205,000, $204,000, and $3,222,000 for the years ended December 31, 2013, 2012 and 2011, respectively.
Nicolai, GmbH Agreement
Effective April 1, 2008, the Company entered into a five-year distribution agreement with Nicolai GmbH. In connection with this distribution agreement, the Company received 500,000 Euros from Nicolai GmbH, which was deferred and was being recognized ratably over the five-year term of the distribution agreement.
The agreement included provisions requiring the Company to pay Nicolai GmbH specific amounts if the Company terminated the distribution agreement prior to the end of the five-year term. The Company did not terminate the distribution agreement prior to the termination date. The unamortized license fee was $-0-, $36,000 and $182,000 at December 31, 2013, 2012 and 2011, respectively. The amortization of license fee was $36,000, $146,000 and $145,000 for the years ended December 31, 2013, 2012 and 2011, respectively.
15. Business Combinations and Asset Acquisitions
During the first quarter of fiscal year 2010, the Company adopted ASC 805, Business Combinations, related to business combinations. This authoritative guidance establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interests in the acquiree and the goodwill acquired. The underlying purchase method of accounting for acquisitions was retained, but the new guidance incorporates a number of changes. These changes include the capitalization of purchased in-process research and development, expensing of acquisition related costs and the recognition of contingent purchase price consideration at fair value at the acquisition date.
When the Company acquires another company or a group of assets, the purchase price is allocated, as applicable, among in-process research and development, other identifiable intangible assets, net tangible assets and the remainder, if any, is recognized as goodwill, as required by U.S. GAAP. Goodwill represents the excess of the aggregate purchase price over the fair value of net assets of acquired businesses, which is not amortized in accordance with ASC 350, Intangibles-Goodwill and Other. The values assigned to other identifiable intangible assets are based on valuations as determined by the Company or independent third party appraisers. The techniques used by these appraisers include estimating the future cash flows of each project or technology and discounting the net cash flows back to their present values utilizing an appropriate risk-adjusted rate of return (discount rate). The discount rate used is determined at the time of the acquisition in accordance with accepted valuation methods.
15. Business Combinations and Asset Acquisitions (Continued)
Shepherd Scientific, Inc.
On December 21, 2012, the Company acquired the assets relating to the Teirstein Edge™ device organizer and AngioAssist™ docking station from Shepherd Scientific, Inc. (Shepherd Scientific). Under the terms of the agreement, the Company agreed to pay Shepherd Scientific a total of $500,000, which was paid on December 21, 2012 at closing. The Teirstein Edge assists in the organization of guidewires and catheters during interventional procedures, while the AngioAssist facilitates the introduction of guidewires into catheters and atherectomy burrs.
The Company accounted for the transaction as a business combination in the fourth quarter of 2012. In accordance with ASC 805, the purchase price is being allocated based on estimates of the fair value of assets acquired, as no liabilities were assumed.
The purchase price was allocated as follows:
| Purchased technology | | $ | 170,000 | |
| Inventory and equipment | | | 70,000 | |
| Other intangibles | | | 50,000 | |
| Goodwill | | | 210,000 | |
| | $ | 500,000 | |
Amortizable Acquired Assets
Purchased technology. Purchased technology consists of $170,000 of developed technology acquired. The technology was valued using the income method utilizing a discounted cash flow model. The Company is amortizing the technology assets on a straight line basis over their estimated useful life of nine years.
Other intangibles. Other intangibles consist of $30,000 representing trademarks and trade names relating to the Teirstein Edge and AngioAssist products and $20,000 representing customer relationships. The customer relationships relate to the ability to sell existing and future services to existing customers of Shepherd Scientific. The fair value of trademarks and trade names and customer relationships has been estimated using the income method utilizing a discounted cash flow model. The Company is amortizing the trademark and trade name intangible assets on a straight line basis over their estimated useful life of approximately ten years. The customer relationship intangible assets are being amortized on a straight line basis over nine years.
The weighted average amortization period for total amortizable intangible assets acquired in connection with the acquisition of the Teirstein Edge and AngioAssist products is nine years.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of the net tangible and intangible assets acquired. The establishment of goodwill was primarily due to the expected revenue growth that is attributable to increased market penetration from future customers. All of the goodwill is expected to be deductible for income tax purposes.
With the exception of sales to one customer, the Company was the sole U.S. distributor of Teirstein Edge and AngioAssist products prior to the acquisition. The Company has seen sales increase slightly during 2013, but expects to realize additional revenue and improved margins in the coming years as all remaining older inventory has been sold.
15. Business Combinations and Asset Acquisitions (Continued)
St. Jude Medical, Cardiology Division, Inc.
On August 16, 2012, the Company acquired the assets related to the Venture wire control catheter from St. Jude Medical, Cardiology Division, Inc. (“St. Jude Medical”). Under the terms of the agreement, the Company agreed to pay St. Jude Medical a total of $3,000,000, consisting of $2,250,000 paid in cash at August 16, 2012 and $750,000 paid in cash on April 17, 2013, upon the successful completion of the transfer of the manufacturing processes from St. Jude Medical to the Company. The Venture wire control catheter is used as a deflectable tip catheter for steering a 0.014 inch guidewire via the arterial system to the coronary or peripheral vasculature. This acquisition provides the Company with additional products that are sold directly into the Company’s existing customer base to generate incremental revenue.
The Company accounted for the transaction as a business combination in the third quarter of 2012. In accordance with ASC 805, the purchase price is being allocated based on estimates of the fair value of assets acquired, as no liabilities were assumed.
The purchase price was allocated as follows:
| Purchased technology | | $ | 850,000 | |
| Other intangibles | | | 500,000 | |
| Inventory and equipment | | | 189,000 | |
| Goodwill | | | 1,461,000 | |
| | $ | 3,000,000 | |
Amortizable Acquired Assets
Purchased technology. Purchased technology consists of $850,000 of developed technology acquired. The technology was valued using the income method utilizing a discounted cash flow model. The Company started amortizing the technology assets on a straight line basis over their estimated useful life of nine years once the Company started selling the products in the second quarter of 2013.
Other intangibles. Other intangibles consist of $220,000 representing trademarks and trade names relating to the Venture wire control catheter products and $280,000 representing customer relationships. The customer relationships relate to the ability to sell existing and future products and services to existing customers of St. Jude Medical. The fair value of trademarks and trade names and customer relationships has been estimated using the income method utilizing a discounted cash flow model. The Company started amortizing the trademark and trade name intangible assets on a straight line basis over their estimated useful life of approximately ten years once the Company started selling the products in the second quarter of 2013. The customer relationship intangible assets are being amortized on a straight line basis over nine years.
The weighted average amortization period for total amortizable intangible assets acquired in connection with the acquisition of the Venture wire control catheter products is nine years.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of the net tangible and intangible assets acquired. The establishment of goodwill was primarily due to the expected revenue growth associated with this new product for the Company and possibility of selling additional products to these new customers. All of the goodwill is expected to be deductible for income tax purposes.
15. Business Combinations and Asset Acquisitions (Continued)
The Company has recognized additional revenue of $1,573,000 and net earnings of approximately $1,021,000 relating to the sale of Venture wire control catheters through December 31, 2013. The amount of additional revenue and net earnings is expected to increase in coming years now that the transfer of the manufacturing processes from St. Jude Medical to the Company is complete.
Accumed Systems, Inc.
On June 11, 2012, the Company acquired the assets related to the Accumed wrist positioning splint business from Accumed Systems, Inc. (“Accumed”). Under the terms of the agreement, the Company paid Accumed a total of $1,500,000 at closing and no additional payments are required to be made. The Accumed wrist positioning splint product consists of a plastic molded brace that simplifies arterial access by holding the wrist and forearm in an appropriate, comfortable position. This acquisition provides the Company with an additional product that is sold directly into the Company’s existing customer base to generate incremental revenue.
The Company accounted for the transaction as a business combination in the second quarter of 2012. In accordance with ASC 805, the purchase price is being allocated based on estimates of the fair value of assets acquired, as no liabilities were assumed.
The purchase price was allocated as follows:
| Purchased technology | | $ | 740,000 | |
| Other intangibles | | | 190,000 | |
| Inventory and equipment | | | 8,000 | |
| Goodwill | | | 562,000 | |
| | $ | 1,500,000 | |
Amortizable Acquired Assets
Purchased technology. Purchased technology consists of $740,000 of developed technology acquired. The technology was valued using the income method utilizing a discounted cash flow model. The Company is amortizing the technology assets on a straight line basis over their estimated useful life of nine years.
Other intangibles. Other intangibles consist of $100,000 representing trademarks and trade names relating to the Accumed wrist positioning splint products and $90,000 representing customer relationships. The customer relationships relate to the ability to sell existing and future products and services to existing customers of Accumed. The fair value of trademarks and trade names and customer relationships has been estimated using the income method utilizing a discounted cash flow model. The Company is amortizing the trademark and trade name intangible assets on a straight line basis over their estimated useful life of approximately ten years. The customer relationship intangible assets are being amortized on a straight line basis over nine years.
The weighted average amortization period for total amortizable intangible assets acquired in connection with the acquisition of the Accumed wrist positioning splint products is nine years.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of the net tangible and intangible assets acquired. The establishment of goodwill was primarily due to the expected revenue growth that is attributable to increased market penetration from future customers. All of the goodwill is expected to be deductible for income tax purposes.
15. Business Combinations and Asset Acquisitions (Continued)
The Company has recognized additional revenue of $1,211,000 and net earnings of approximately $497,000 relating to the sales of the Accumed wrist positioning splint products from June 11, 2012 through December 31, 2013.
Dr. Pedro Silva and Affiliates
On January 6, 2012, the Company entered into an agreement with Dr. Pedro Silva and his affiliates, whereby the Company paid $3,250,000 for the rights, patents and intellectual property relating to a two-lumen catheter for distal protection and material extraction used in the Company’s Pronto catheters. Upon payment, the existing License Agreement between N.G.C. Medical S.p.A. and the Company has been deemed paid-in-full, and no future royalties will be owed on any sale of a Pronto catheter after December 31, 2011.
The Company has accounted for the transaction as a non-business license acquisition in the first quarter of 2012. In accordance with ASC 805, the purchase price was assigned to a license intangible asset equivalent to the cash amount paid on January 6, 2012, and no goodwill was recognized. The Company is amortizing the license intangible on a straight-line basis over a period of ten years.
Northeast Scientific
On September 17, 2013, the Company entered into an eight-year reprocessing services agreement with Northeast Scientific, Inc. (NES), a FDA-registered reprocessor of medical devices, whereby the Company paid NES a non-refundable amount of $500,000 for the exclusive right to offer NES’ reprocessing services in the United States for a commercial medical device. NES is pursuing FDA approval for its reprocessing services for the device. In the event FDA approval is obtained prior to July 1, 2014, the Company is required under the terms of the agreement to pay NES a non-refundable amount $500,000. If FDA approval is obtained on/or after July 1, 2014, the Company is required to pay NES a non-refundable amount of $400,000. The agreement has an annual minimum unit termination clause, allowing NES to terminate the agreement if the Company does not meet certain annual minimums.
The Company has accounted for the transaction as a non-business asset acquisition in the third quarter of 2013. In accordance with ASC 805, the purchase price of $900,000 is being assigned to an intangible and no goodwill was recognized. The Company recorded a $400,000 accrual in the third quarter of 2013 in addition to the $500,000 payment, as the Company believes the FDA approval will occur after July 1, 2014. The Company will begin amortizing the intangible asset on a per unit basis over the remaining term of the agreement once the Company begins to send units to NES to be reprocessed.
On December 22, 2011, the Company entered into a license agreement with NES, a FDA-registered reprocessor of medical devices, whereby the Company acquired the exclusive rights to NES’ reprocessing services for the ClosureFAST radiofrequency catheter in the United States for a term of five years. The ClosureFAST catheter is owned and marketed by VNUS Medical Technologies, Inc., a subsidiary of Covidien, and is used in the treatment of varicose veins. Under the reprocessing service, the customer sends its used ClosureFAST catheters to NES, where they are inspected, cleaned, tested, repackaged, resterilized and shipped back to the customer. In exchange for the exclusive rights, the Company paid a total of $900,000 to NES and a former third party distributor on December 22, 2011.
The Company has accounted for the transaction as a non-business asset acquisition in the fourth quarter of 2011. In accordance with ASC 805, the purchase price is being assigned to an intangible and no goodwill was recognized. The Company is amortizing the license intangible on a straight-line basis over the five-year term of the agreement.
15. Business Combinations and Asset Acquisitions (Continued)
Zerusa Limited
On January 27, 2011, the Company entered into an asset purchase agreement of substantially all the assets of Zerusa Limited (“Zerusa”), a Galway, Ireland based medical device company engaged in the manufacture and distribution of the Guardian® hemostasis valves. Under the terms of the agreement the Company paid Zerusa a total of 3,121,000 Euros ($4,272,000), consisting of 2,850,000 Euros ($3,882,000) paid in cash at January 27, 2011 and 271,000 Euros ($390,000) which was paid on September 2, 2011. The final payment amount was subject to adjustment based upon the value of inventory transferred. The Guardian hemostasis valves are designed to maintain hemostasis during interventional catheterization procedures through a novel sealing system which allows simple introductions and removal of interventional devices while providing the option to lock guidewires in place.
The Company accounted for the transaction as a business combination in the first quarter of 2011. In accordance with ASC 805, the purchase price is being allocated based on estimates of the fair value of assets acquired, as no liabilities were assumed.
The purchase price was allocated as follows:
| Purchased technology | | $ | 1,000,000 | |
| Other intangibles | | | 800,000 | |
| Inventory and equipment | | | 48,000 | |
| Goodwill | | | 2,424,000 | |
| | $ | 4,272,000 | |
Amortizable Acquired Assets
Purchased technology. Purchased technology consists of $1,000,000 of developed technology acquired. The technology was valued using the income method utilizing a discounted cash flow model. The Company is amortizing the technology assets on a straight line basis over their estimated useful lives of 11 years.
Other intangibles. Other intangibles consist of $800,000 representing trademarks and trade names relating to the Guardian hemostasis valve products. The fair value of trademarks and trade names has been estimated using the income method utilizing a discounted cash flow model. The Company is amortizing the trademark and trade name intangible assets on a straight line basis over their estimated useful life of approximately 11 years.
The weighted average amortization period for total amortizable intangible assets acquired in connection with the acquisition of the Guardian hemostasis valve products is 11 years.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of the net tangible and intangible assets acquired. The establishment of goodwill was primarily due to the expected revenue growth that is attributable to increased market penetration from future customers. All of the goodwill is expected to be deductible for income tax purposes.
15. Business Combinations and Asset Acquisitions (Continued)
Unaudited Supplemental Pro Forma Financial Information
The following unaudited supplemental pro forma information combines the Company’s results with those of Shepherd Scientific, St. Jude, Accumed and Zerusa as if the acquisitions had occurred at the beginning of each of the periods presented. This unaudited pro forma information is not intended to represent or be indicative of the Company’s consolidated results of operations or financial condition that would have been reported for the periods presented had the acquisition been completed at the beginning of each of the periods presented, and should not be taken as indicative of the Company’s future consolidated results of operations or financial condition.
| | Years Ended December 31, | |
| | 2013 | | | 2012 | |
| | | | | | |
| Revenue | | $ | 110,498,000 | | | $ | 99,051,000 | |
| Net earnings | | | 11,142,000 | | | | 9,779,000 | |
| Net earnings per share | | | | | | | | |
| Basic | | $ | 0.68 | | | $ | 0.61 | |
| Diluted | | $ | 0.65 | | | $ | 0.59 | |
Certain pro forma adjustments have been made to reflect the impact of the purchase transaction, primarily consisting of amortization of intangible assets with determinable lives and income taxes to reflect the Company’s effective tax rate for the periods presented.
16. Quarterly Financial Data (Unaudited, in Thousands, Except per Share Data)
2013 | | Fourth Quarter | | | Third Quarter | | | Second Quarter | | | First Quarter | |
| Net revenue: | | | | | | | | | | | | |
| Product | | $ | 29,000 | | | $ | 27,926 | | | $ | 27,294 | | | $ | 25,977 | |
| License | | | 72 | | | | 83 | | | | 59 | | | | 87 | |
| Total net revenue | | | 29,072 | | | | 28,009 | | | | 27,353 | | | | 26,064 | |
| | | | | | | | | | | | | | | | |
| Selected costs and expenses: | | | | | | | | | | | | | | | | |
| Costs of goods sold | | | 9,724 | | | | 9,197 | | | | 8,538 | | | | 8,697 | |
| | | | | | | | | | | | | | | | |
| Operating earnings | | | 4,731 | | | | 4,020 | | | | 4,442 | | | | 2,904 | |
| | | | | | | | | | | | | | | | |
| Net earnings | | | 3,537 | | | | 2,672 | | | | 2,808 | | | | 2,125 | |
| | | | | | | | | | | | | | | | |
| Basic net earnings per share | | $ | 0.21 | | | $ | 0.16 | | | $ | 0.17 | | | $ | 0.13 | |
| Diluted net earnings per share | | $ | 0.20 | | | $ | 0.16 | | | $ | 0.17 | | | $ | 0.13 | |
16. Quarterly Financial Data (Unaudited, in Thousands, Except per Share Data) (Continued)
2012 | | Fourth Quarter | | | Third Quarter | | | Second Quarter | | | First Quarter | |
| Net revenue: | | | | | | | | | | | | |
| Product | | $ | 25,215 | | | $ | 24,465 | | | $ | 24,650 | | | $ | 23,706 | |
| License | | | 89 | | | | 87 | | | | 87 | | | | 87 | |
| Total net revenue | | | 25,304 | | | | 24,552 | | | | 24,737 | | | | 23,793 | |
| | | | | | | | | | | | | | | | |
| Selected costs and expenses: | | | | | | | | | | | | | | | | |
| Costs of goods sold | | | 8,309 | | | | 8,183 | | | | 8,231 | | | | 7,838 | |
| | | | | | | | | | | | | | | | |
| Operating earnings | | | 4,688 | | | | 4,150 | | | | 3,940 | | | | 3,127 | |
| | | | | | | | | | | | | | | | |
| Net earnings | | | 3,051 | | | | 2,565 | | | | 2,384 | | | | 1,909 | |
| | | | | | | | | | | | | | | | |
| Basic net earnings per share | | $ | 0.19 | | | $ | 0.16 | | | $ | 0.15 | | | $ | 0.12 | |
| Diluted net earnings per share | | $ | 0.18 | | | $ | 0.16 | | | $ | 0.15 | | | $ | 0.12 | |
17. Subsequent Events
The Company has evaluated subsequent events occurring after the date of the consolidated financial statements for events requiring recording or disclosure in the financial statements.
Vascular Solutions, Inc.
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
Description | | Balance at Beginning of Year | | | Additions Charged to Costs and Expenses | | | Less Deductions | | | Balance at End of Year | |
| | | | | | | | | | | | |
| YEAR ENDED DECEMBER 31, 2013: | | | | | | | | | | | | |
| Sales return allowance | | $ | 55,000 | | | $ | 20,000 | | | $ | (25,000 | ) | | $ | 50,000 | |
| Allowance for doubtful accounts | | | 130,000 | | | | 90,000 | | | | (70,000 | ) | | | 150,000 | |
| Total | | $ | 185,000 | | | $ | 110,000 | | | $ | (95,000 | ) | | $ | 200,000 | |
| | | | | | | | | | | | | | | | |
| YEAR ENDED DECEMBER 31, 2012: | | | | | | | | | | | | | | | | |
| Sales return allowance | | $ | 30,000 | | | $ | 45,000 | | | $ | (20,000 | ) | | $ | 55,000 | |
| Allowance for doubtful accounts | | | 120,000 | | | | 75,000 | | | | (65,000 | ) | | | 130,000 | |
| Total | | $ | 150,000 | | | $ | 120,000 | | | $ | (85,000 | ) | | $ | 185,000 | |
| | | | | | | | | | | | | | | | |
| YEAR ENDED DECEMBER 31, 2011: | | | | | | | | | | | | | | | | |
| Sales return allowance | | $ | 45,000 | | | $ | 30,000 | | | $ | (45,000 | ) | | $ | 30,000 | |
| Allowance for doubtful accounts | | | 115,000 | | | | 87,000 | | | | (82,000 | ) | | | 120,000 | |
| Total | | $ | 160,000 | | | $ | 117,000 | | | $ | (127,000 | ) | | $ | 150,000 | |