UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Consent Statement Pursuant to Section 14(a) of the Securities
and Exchange Act of 1934 (Amendment No. )
Filed by the Registranto
Filed by a Party other than the Registrantþ
Check the appropriate box:
| |
| o | Preliminary Proxy Statement |
| |
| o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| o | Definitive Proxy Statement |
| |
| þ | Definitive Additional Materials |
| |
| o | Soliciting Material Pursuant to sec. 240.14a-12 |
NEOPHARM, INC.
(Name of Registrant as Specified in its Charter)
JOHN N. KAPOOR, Ph.D.
(Name of Person(s) Filing Consent Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| |
| o | Fee computed on table below per Exchange ActRules 14a-6(i)(l) and 0-11 |
| |
| (1) | Title of each class of securities to which transaction applies: |
| |
| (2) | Aggregate number of securities to which transaction applies: |
| |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| (4) | Proposed maximum aggregate value of transaction: |
| |
| o | Fee paid previously with preliminary materials. |
| |
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| |
| (1) | Amount Previously Paid: |
| |
| (2) | Form, Schedule or Registration Statement No.: |

| Institutional Shareholder Services, Inc. John N. Kapoor, Ph.D. October 5, 2004 |

| Safe-Harbor Statement Various statements made in this presentation about future expectations, plans and prospects for the Company constitute forward-looking statements for purposes of the Safe-Harbor provisions under The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including those discussed in the Risk Factors section of the Company's most recent Annual Report on Form 10-K and in the Company's prospectus supplement and accompanying prospectus which are on file with the Securities and Exchange Commission. |

| NeoPharm, Inc. A New ACTIVE Board Is Required NOW to Restore Stockholder Value |

| Objectives: Focus on IL-13 PE38QQR PRECISE trial and get approval for commercialization Restructure Company operations to cut the burn rate from $55-58 million (Company's August 6, 2004 press release) to approximately $30 million annually Streamline Company's clinical programs to make them more efficient and cost effective Eliminate the need to rely on the public equity market to fund Company operations, which would result in significant dilution |

| The Issues: Collapsing market price Do-nothing, insular board Lack of financial discipline No plan to restore stockholder value |
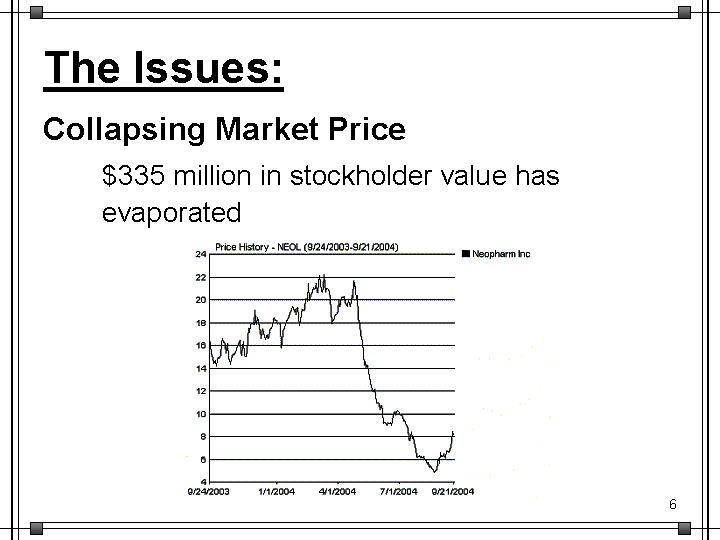
| The Issues: Collapsing Market Price $335 million in stockholder value has evaporated |

| The Issues: Do-Nothing, Insular Board Concerns expressed about excessive burn rate for over a year. Lack of discipline on budget due to gamble on arbitration with Pharmacia, which resulted in no award (May 3, 2004). Management continues "business as usual" approach, with no challenge from the board to cut expenses. Management and board counts on raising money to fund excessive burn rate. Dr. Kapoor recommends road map to reduce expenses (May 4, 2004) - board takes no action |

| The Issues: Do-Nothing, Insular Board Board complacent during massive stock price decline Stock declined from $16.43 (May 4) to as low as $4.85 (August 24) Until filing of consent solicitation, this board took no action to reduce burn rate Board fails to understand urgency-currently "planning to" cut expenses to $43-47 million next year. Why not now? |

| The Issues: Do-Nothing, Insular Board Other board members hand-picked by former CEO James Hussey Messrs. Rogan, Flaum and Safavi were previous business associates of Hussey Mr. Hanson was CEO of Option Care, a company where Dr. Kapoor serves as Chairman Mr. Hanson was dismissed from this position for poor performance |

| The Issues: Lack of Financial Discipline Cost structure inflated compared to other development stage companies No focus on objectives and goals Inadequate budgeting Unable to adjust to business developments, such as the Pharmacia setback Unacceptable dilution inevitable if Company continues on present course |

| The Issues: No Plan to Restore Stockholder Value Dr. Kapoor has been sole voice for change on the NeoPharm board No reforms have been considered until consent solicitation filing Other board members never challenged management No plan given as to how they will cut burn rate No guidance as to what products they will discontinue Leaves no choice but to pursue consent solicitation to restore stockholder value |

| The Solution: Install active board dedicated to implementing reform Reduce burn rate to approximately $30 million per annum Focus resources on development and approval for marketing of IL-13 PE38QQR Streamline all other development projects |

| The Solution: Install active board dedicated to implementing reform Appoint a highly qualified, independent and knowledgeable board who will be responsive to stockholder concerns Dr. Bernard Fox (Immunology) Mr. Ron Eidell (Financial) Mr. Brian Tambi (Operations/Development) Dr. Kapoor will gladly step down as Chairman at next annual meeting (2005), or even earlier, if board determines in Company's best interest Expand board by next annual meeting with additional highly qualified, independent board members New active board will: Challenge management to restore stockholder value Discharge fiduciary obligations to NeoPharm and all stockholders |

| The Solution: Cut Burn Rate to Approximately $30 million per annum Experience in restructuring: First Horizon Pharmaceutical (FHRX) Option Care, Inc. (OPTN) Familiar with biotech development-stage companies' cost structure: Introgen Therapeutics, Inc. (INGN) Experience in licensing and development: Pentamadine Adenocard Liposomol Ampho B Adenoscan Marinol |

| The Solution: Cut Burn Rate to Approximately $30 million per annum Thorough Due Diligence Process The due diligence consisted of discussions with consultants (including those used by the Company), similar companies in biotechnology and Dr. Kapoor's knowledge of NeoPharm Key elements of cost model (see Appendix A for details): Cost per patient in PRECISE trials Per patient cost for completing Phase I and Phase II Total number of patients needed to complete Phase I and Phase II G&A, R&D and Clinical costs to support the development activity at NeoPharm Headcount reduction |

| The Solution: Focus Resources on IL-13 PE38QQR Development and Approval Only continue PRECISE trials which are needed for approval Stop trials on initially diagnosed GBM patients to save money and avoid distraction Stop pediatric and European GBM trials Prioritize test centers in three categories (A,B,C) based on patient availability and focus on A&B centers Generate daily reports on patient recruitment to make it the focus of the entire Company Work with insurance companies and Medicare to get partial reimbursement, thereby reducing cost of trials |

| The Solution: Streamline all other Development Projects LE-SN38 Complicated protocol caused Phase I trials to last 4 years, draining Company's financial resources Simplify Phase II protocol as suggested by oncology consultants to expedite progress and reduce expenditures LEP-ETU Use 505(b)(2) approach as suggested by oncology consultants |

| The Solution: Streamline all other Development Projects LErafAON Outsource completion of Phase I/Phase II NeoPhectin Development is complete. Find partners to further its potential for success It is critical to complete Phase II before these products can be licensed to recognize full value |
| Conclusion: NeoPharm needs a new active board that will implement the reforms necessary to set it on a course for the sustainable future Dr. Kapoor pushed for these reforms, but was silenced by the current board-left with no other choice but to pursue this consent solicitation The current board sat idly by through a massive decline in stockholder value We believe the choice is clear Please consent on the WHITE card today |

| Appendix A In arriving at the cash burn rate goal of $30 million per year, Dr. Kapoor was not primarily focused on examining the Company's current expenses. This is because management and the other board members have never been able to explain why NeoPharm is spending money at the current rate. Dr. Kapoor believes the Company's business objectives and results of operations as a whole must be reexamined and its cost structure overhauled. He developed a zero-based budgeting approach to arrive at an expense level he believes is appropriate based on the Company's situation and the status of its products. Dr. Kapoor began with the belief that the only trials which the Company currently should pursue are the Phase III PRECISE trials for IL 13-PE38QQR and the three trials involving the Company's liposomal products (LErafAON, LEP-ETU and SN-38). The PRECISE Phase III trials for IL 13-PE38QQR are expected to be completed in approximately 1 1/2 years (50 patients enrolled with an additional 250 needed), the LErafAON Phase I and Phase II trials in approximately 2 years, the LEP-ETU trials, assuming a 505(b)(2) filing is used, in approximately 3 1/2 years and the SN-38 Phase II trials in approximately 1 1/2 years. Dr. Kapoor believes that in order to determine the appropriate level of expenditures for the Company, the starting point should be an assessment of the necessary clinical trials and their likely costs. |

| Appendix A (continued) Based on Dr. Kapoor's experience with clinical trials of other drugs and his knowledge of pharmaceutical company cost structures, particularly his experience as the Chairman of the Board of Introgen Therapeutics, Inc., a publicly-traded biopharmaceutical company which has an active clinical trials program and is focused, like NeoPharm, on the development and commercialization of cancer drugs, and his knowledge of NeoPharm's key products, Dr. Kapoor believes that the Company should be able to conduct: The remaining PRECISE trials at a cost of approximately $13,750,000 ($55,000 approximate cost per patient times 250 patients remaining); The LErafAON Phase I trials at a cost of approximately $375,000 ($15,000 approximate cost per patient times 25 patients); The LErafAON Phase II trials at a cost of approximately $1,500,000 ($25,000 approximate cost per patient times 60 patients); The LEP-ETU trials at a cost of approximately $7,500,000 assuming the project is pursued with a 505(b)(2) filing ($25,000 approximate cost per patient times approximately 300 patients); and The SN-38 Phase II trials at a cost of approximately $1,700,000 ($25,000 approximate cost per patient times 60 patients for a total of $1,500,000, plus a Pharmakenetic cost of $200,000). |
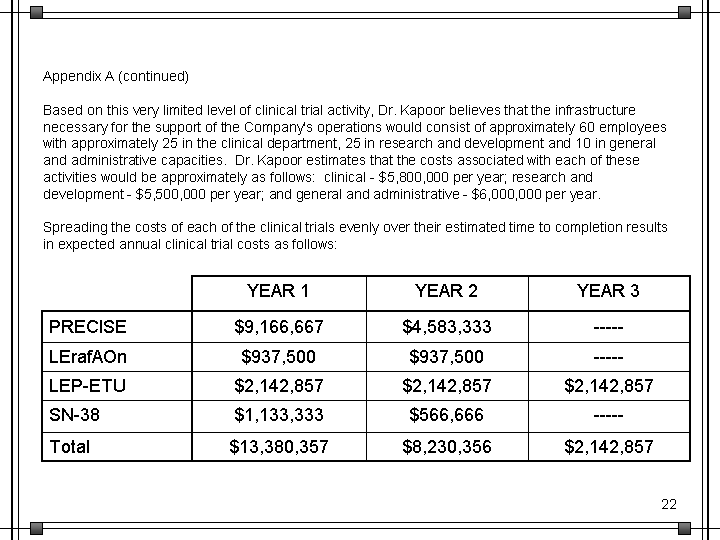
| Appendix A (continued) Based on this very limited level of clinical trial activity, Dr. Kapoor believes that the infrastructure necessary for the support of the Company's operations would consist of approximately 60 employees with approximately 25 in the clinical department, 25 in research and development and 10 in general and administrative capacities. Dr. Kapoor estimates that the costs associated with each of these activities would be approximately as follows: clinical - $5,800,000 per year; research and development - $5,500,000 per year; and general and administrative - $6,000,000 per year. Spreading the costs of each of the clinical trials evenly over their estimated time to completion results in expected annual clinical trial costs as follows: YEAR 1 YEAR 2 YEAR 3 PRECISE $9,166,667 $4,583,333 ----- LErafAOn $937,500 $937,500 ----- LEP-ETU $2,142,857 $2,142,857 $2,142,857 SN-38 $1,133,333 $566,666 ----- Total $13,380,357 $8,230,356 $2,142,857 |
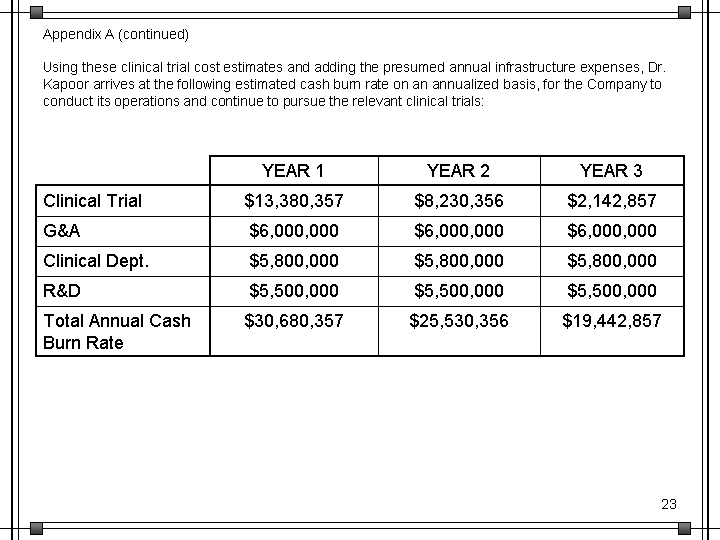
| Appendix A (continued) Using these clinical trial cost estimates and adding the presumed annual infrastructure expenses, Dr. Kapoor arrives at the following estimated cash burn rate on an annualized basis, for the Company to conduct its operations and continue to pursue the relevant clinical trials: YEAR 1 YEAR 2 YEAR 3 Clinical Trial $13,380,357 $8,230,356 $2,142,857 G&A $6,000,000 $6,000,000 $6,000,000 Clinical Dept. $5,800,000 $5,800,000 $5,800,000 R&D $5,500,000 $5,500,000 $5,500,000 Total Annual Cash Burn Rate $30,680,357 $25,530,356 $19,442,857 |

| Appendix B Memorandum of May 4, 2004 To: Mr. Rick Hanson From: Dr. John N. Kapoor ___________________________________ Rick, Just to reiterate my conversation with you, for consideration at the Independent Board conference call today. I recommend the following strategy: The company's burn rate should be cut back to approximately $35 million per year from the current $60 million. This will give us two plus yearly worth of cash. Each clinical program will be thoroughly studied and streamlined. The IL-13 project in Phase III will be the highest priority and no cuts will be made. I strongly believe we can streamline and cut the expenditures in all projects without sacrificing the time-line. Each group, with significant expenditure, will be challenged to cut the burn rate to meet the objective of $35 million per year. Key areas are: R&D clinical - Jeff Sherman R&D Pre-clinical - Irman Ahmed G&A - Larry Kenyon I also recommend the change in Jim's position be made NOW rather than waiting for the recruitment of 2nd in command, which could take 3-6 months. I, as chairman, will take the lead position to implement all the strategic changes and will work closely with the Governance Committee. No changes will be made without the Governance Committee's agreeing with the decisions. John N. Kapoor, Ph.D. Chairman |























