Exhibit 99.2

RARE Thinking for RARE Solutions Topline ELX - 02 Combination Phase 2 Cystic Fibrosis (CF) Results September 14, 2022

/ 2 Forward - looking statements This press release contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 199 5. All statements other than statements of present and historical facts contained in this press release, including without limitatio n, the expected timing of trials of our product candidates and the potential of our product candidate to treat nonsense mutations ar e forward - looking statements. Forward - looking statements can be identified by the words “aim,” “may,” “will,” “would,” “should,” “expect,” “explore,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “pre dic t,” “potential,” “seeks,” or “continue” or the negative of these terms similar expressions, although not all forward - looking statements contain t hese words. Forward - looking statements are based on management's current plans, estimates, assumptions and projections based on information currently available to us. Forward - looking statements are subject to known and unknown risks, uncertainties and assumptions, and actual results or outcomes may differ materially from those expressed or implied in the forward - looking stateme nts due to various important factors, including, but not limited to: our ability to progress any product candidates in preclinica l o r clinical trials; the uncertainty of clinical trial results and the fact that positive results from preclinical studies are not always ind icative of positive clinical results; the scope, rate and progress of our preclinical studies and clinical trials and other research and de velopment activities; the competition for patient enrollment from drug candidates in development; the impact of the global COVID - 19 pandem ic on our clinical trials, operations, vendors, suppliers, and employees; our ability to obtain the capital necessary to fund ou r o perations; the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; our abilit y t o obtain financial in the future through product licensing, public or private equity or debt financing or otherwise; general business con ditions, regulatory environment, competition and market for our products; and business ability and judgment of personnel, and the avai lab ility of qualified personnel and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10 - Q for the quarter ended June 30, 2022, as any such factors may be updated from time to time in our other filings with the SEC, accessible on the SEC’s website at www.sec.gov and the “Financials & Filings” page of our website at https://investors.eloxxpharma.com/financials - filings . All forward - looking statements speak only as of the date of this press release and, except as required by applicable law, we hav e no obligation to update or revise any forward - looking statements contained herein, whether as a result of any new information, futu re events, changed circumstances or otherwise.
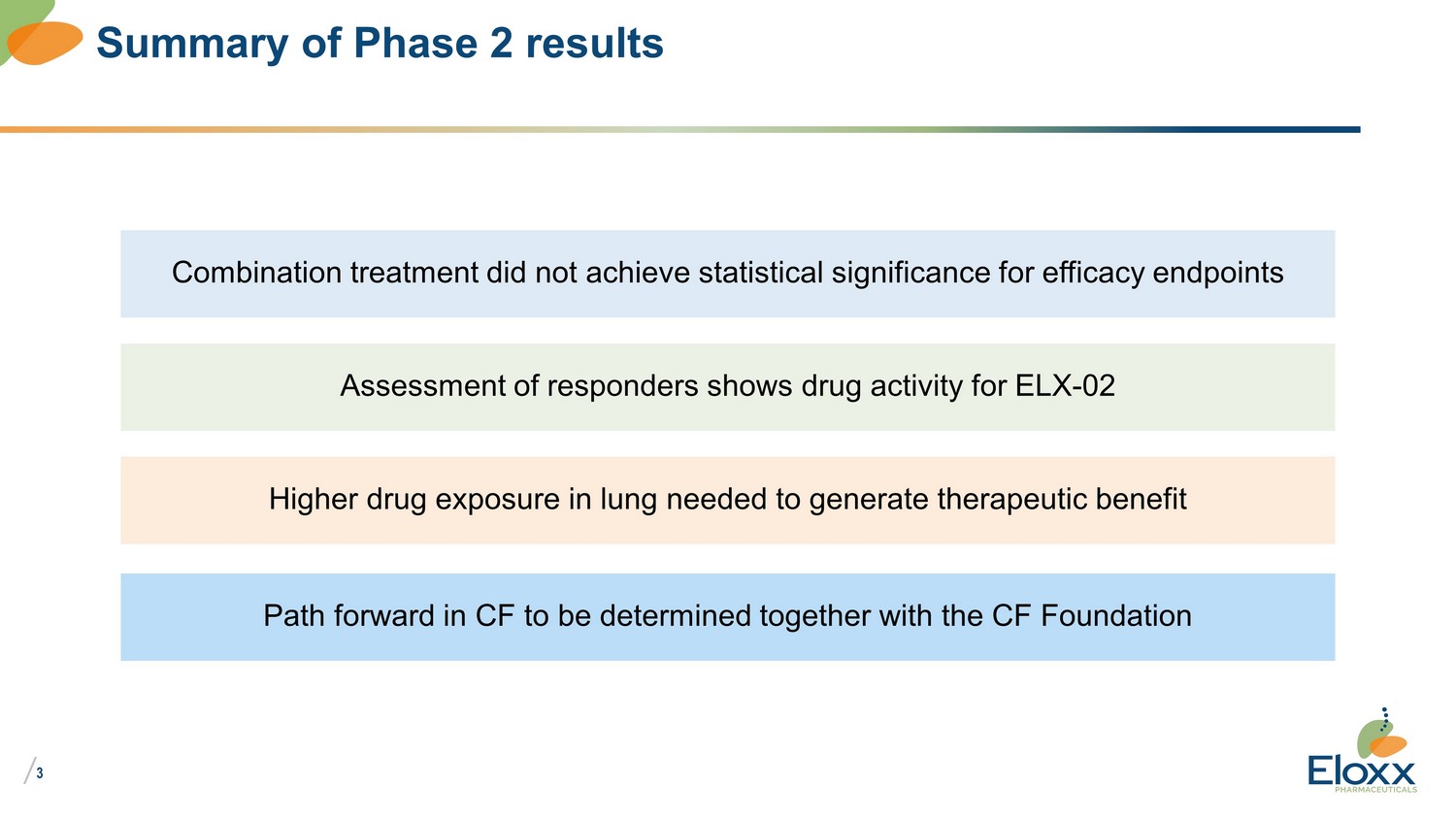
/ 3 Summary of Phase 2 results Combination treatment did not achieve statistical significance for efficacy endpoints Assessment of responders shows drug activity for ELX - 02 Higher drug exposure in lung needed to generate therapeutic benefit Path forward in CF to be determined together with the CF Foundation
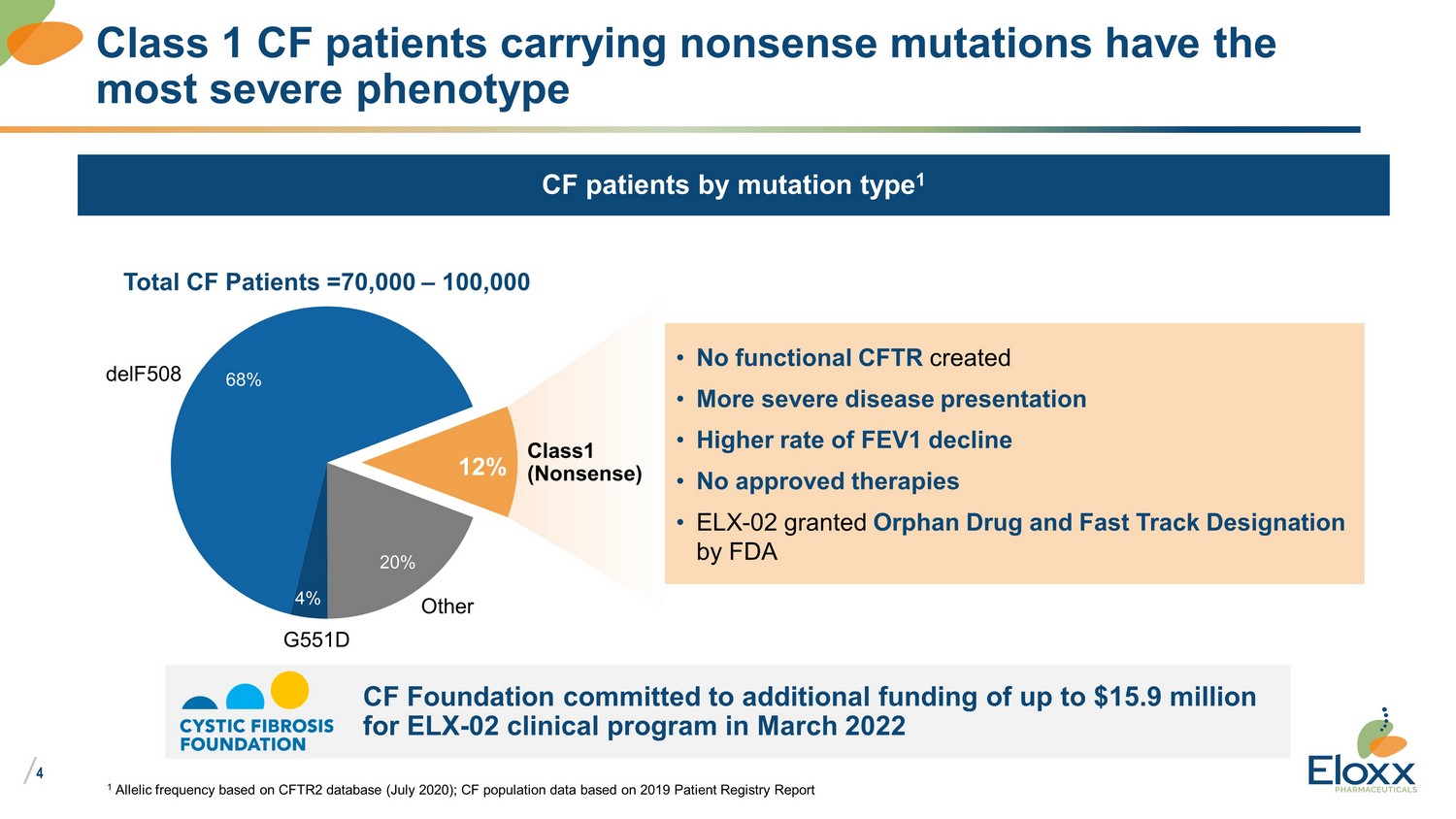
/ 4 Class 1 CF patients carrying nonsense mutations have the most severe phenotype CF patients by mutation type 1 • No functional CFTR created • More severe disease presentation • Higher rate of FEV1 decline • No approved therapies • ELX - 02 granted Orphan Drug and Fast Track Designation by FDA Total CF Patients =70,000 – 100,000 12% 20% 4% 68% Other G551D delF508 Class1 (Nonsense) CF Foundation committed to additional funding of up to $15.9 million for ELX - 02 clinical program in March 2022 1 Allelic frequency based on CFTR2 database (July 2020); CF population data based on 2019 Patient Registry Report
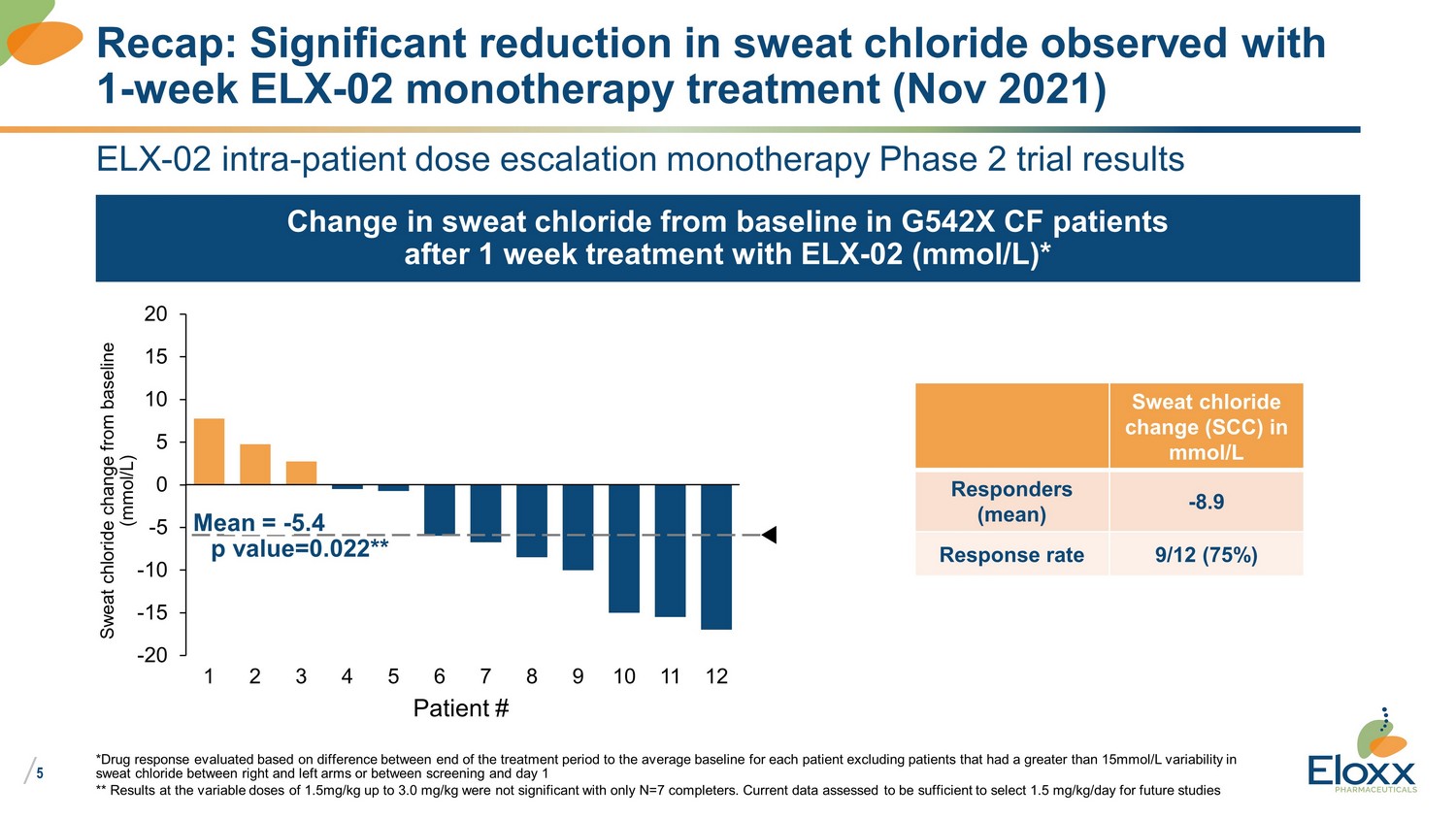
/ 5 ELX - 02 intra - patient dose escalation monotherapy Phase 2 trial results Change in sweat chloride from baseline in G542X CF patients after 1 week treatment with ELX - 02 (mmol/L)* *Drug response evaluated based on difference between end of the treatment period to the average baseline for each patient exc lud ing patients that had a greater than 15mmol/L variability in sweat chloride between right and left arms or between screening and day 1 ** Results at the variable doses of 1.5mg/kg up to 3.0 mg/kg were not significant with only N=7 completers. Current data asse sse d to be sufficient to select 1.5 mg/kg/day for future studies Recap: Significant reduction in sweat chloride observed with 1 - week ELX - 02 monotherapy treatment (Nov 2021) p value=0.022** -20 -15 -10 -5 0 5 10 15 20 4 9 1 3 2 7 6 5 8 10 12 11 Mean = - 5.4 Patient # Sweat chloride change from baseline (mmol/L) Sweat chloride change (SCC) in mmol/L Responders (mean) - 8.9 Response rate 9/12 (75%)
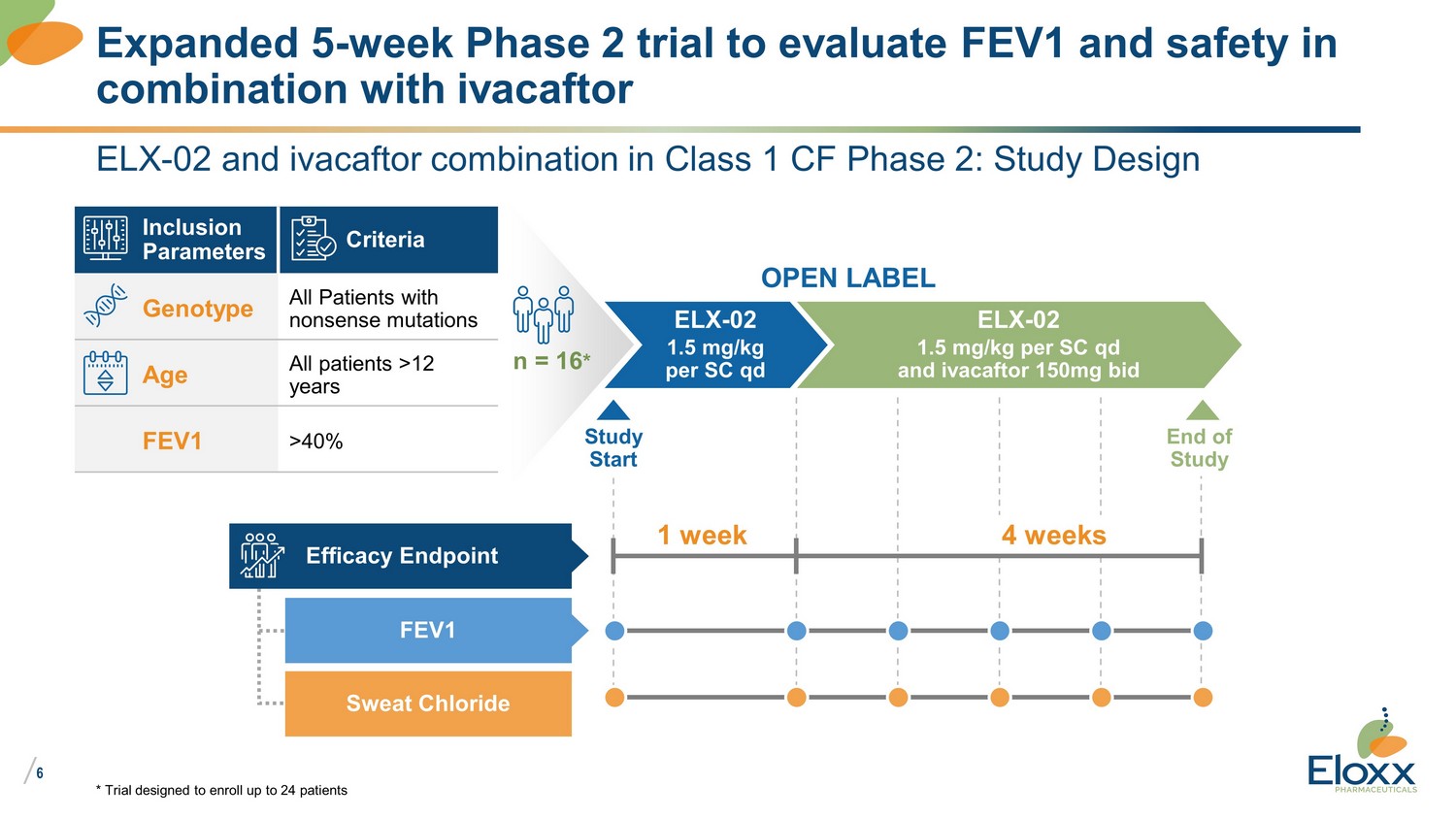
/ 6 ELX - 02 and ivacaftor combination in Class 1 CF Phase 2: Study Design Expanded 5 - week Phase 2 trial to evaluate FEV1 and safety in combination with ivacaftor Inclusion Parameters Criteria Genotype All Patients with nonsense mutations Age All patients >12 years FEV1 >40% n = 16 * ELX - 02 1.5 mg/kg per SC qd ELX - 02 1.5 mg/kg per SC qd and ivacaftor 150mg bid OPEN LABEL End of Study Efficacy Endpoint FEV1 Sweat Chloride 1 week Study Start 4 weeks * Trial designed to enroll up to 24 patients

/ 7 Cumulative safety experience across all Phase 2 patients * Patient had an undisclosed history of tinnitus No systemic safety signals observed for ELX - 02 No ELX - 02 related serious adverse events (SAEs) ELX - 02 was well tolerated at 1.5 mg/kg dose across Phase 2 patients (n=31) – Combination therapy at 1.5 mg/kg showed drug related discontinuations • 2 patients discontinued due to injection site reactions (mild to moderate) • 1 patient withdrew from trial due to injection burden prior to dosing • 1 patient with tinnitus*
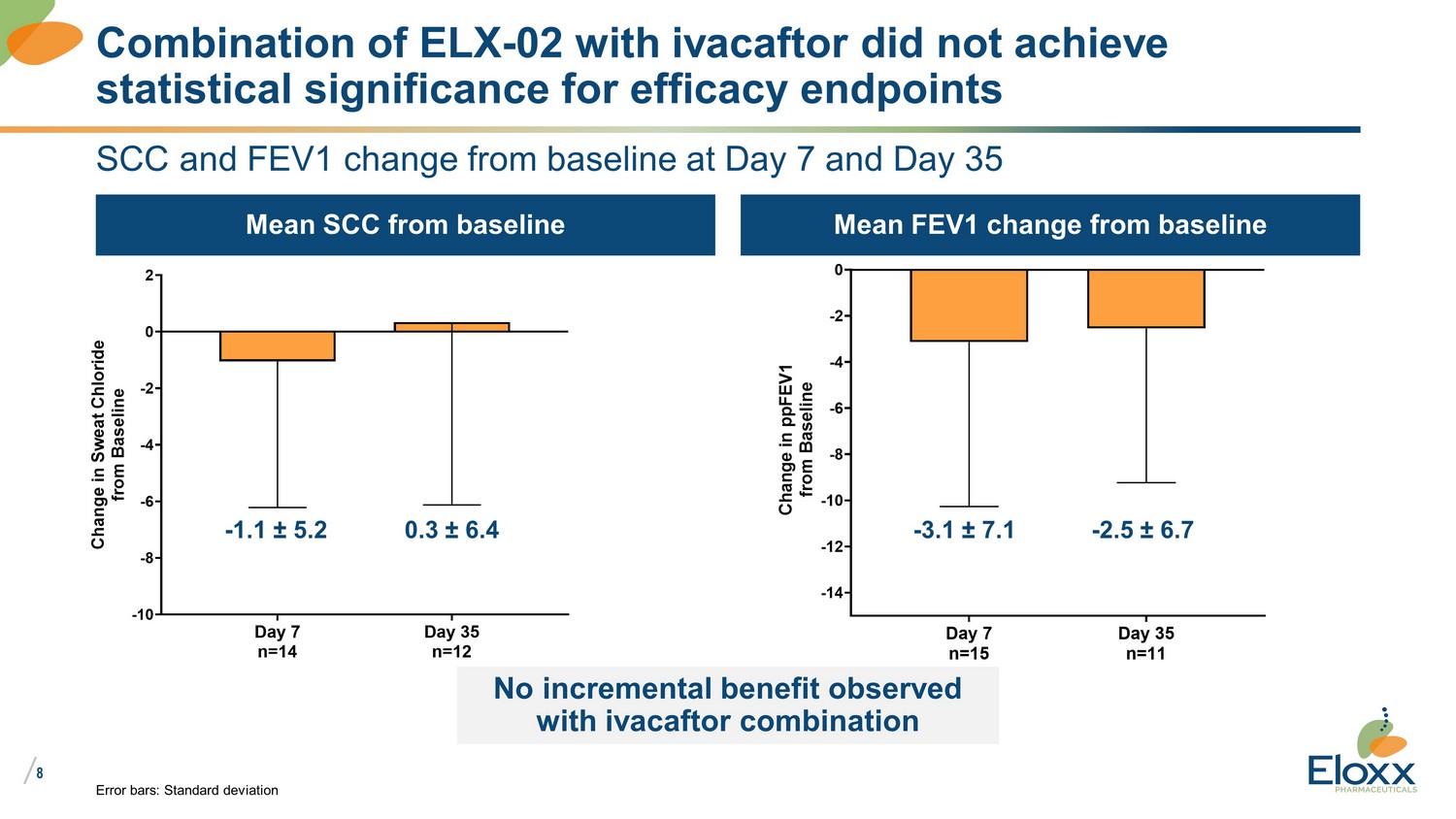
/ 8 SCC and FEV1 change from baseline at Day 7 and Day 35 Mean SCC from baseline Mean FEV1 change from baseline Error bars: Standard deviation Combination of ELX - 02 with ivacaftor did not achieve statistical significance for efficacy endpoints Day 7 n=14 Day 35 n=12 -10 -8 -6 -4 -2 0 2 C h a n g e i n S w e a t C h l o r i d e f r o m B a s e l i n e Day 7 n=15 Day 35 n=11 -14 -12 -10 -8 -6 -4 -2 0 C h a n g e i n p p F E V 1 f r o m B a s e l i n e - 1.1 ± 5.2 0.3 ± 6.4 - 3.1 ± 7.1 - 2.5 ± 6.7 No incremental benefit observed with ivacaftor combination
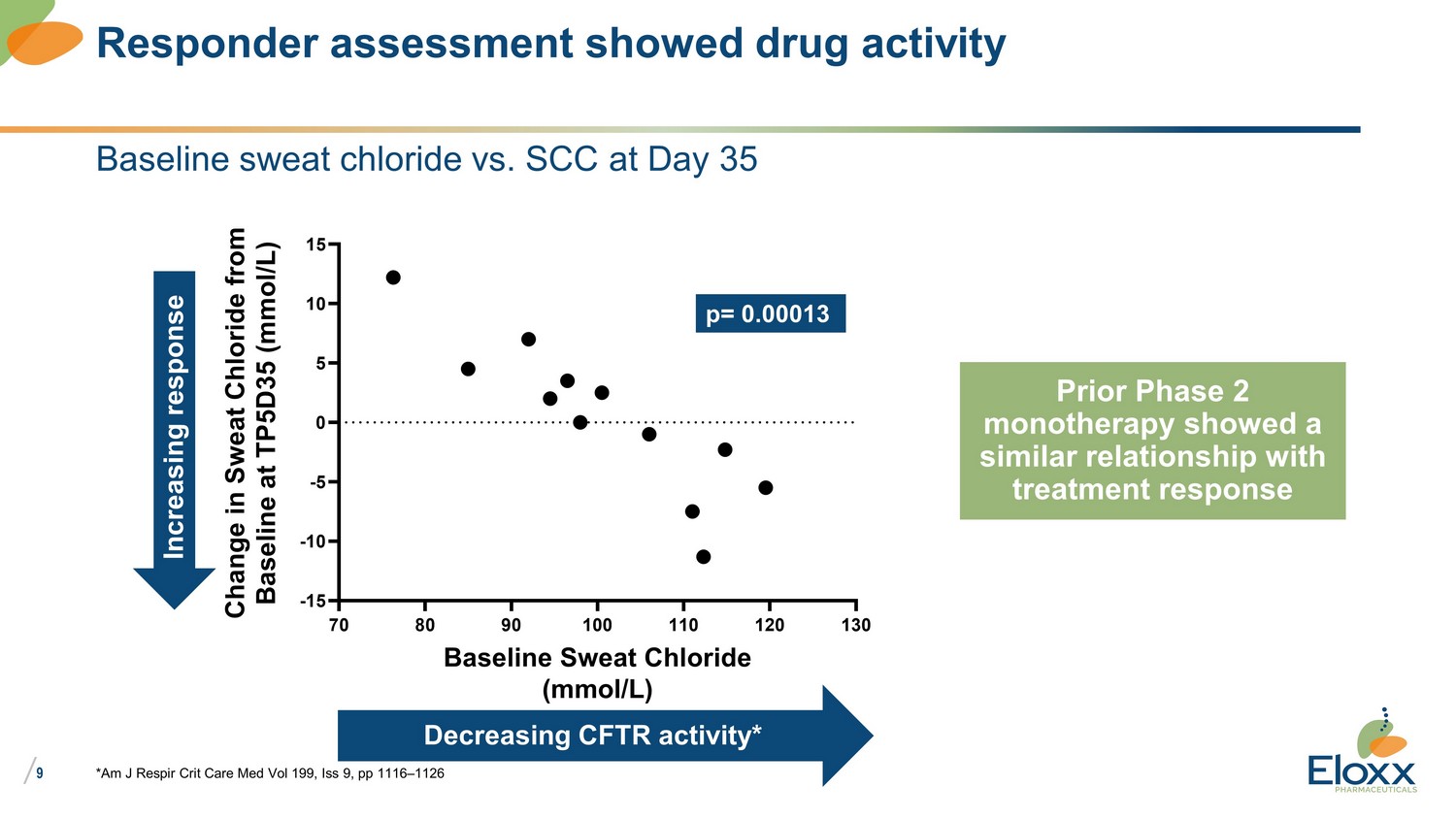
/ 9 *Am J Respir Crit Care Med Vol 199, Iss 9, pp 1116 – 1126 Responder assessment showed drug activity 70 80 90 100 110 120 130 -15 -10 -5 0 5 10 15 Baseline Sweat Chloride (mmol/L) C h a n g e i n S w e a t C h l o r i d e f r o m B a s e l i n e a t T P 5 D 3 5 ( m m o l / L ) p= 0.00013 Decreasing CFTR activity* Increasing response Baseline sweat chloride vs. SCC at Day 35 Prior Phase 2 monotherapy showed a similar relationship with treatment response
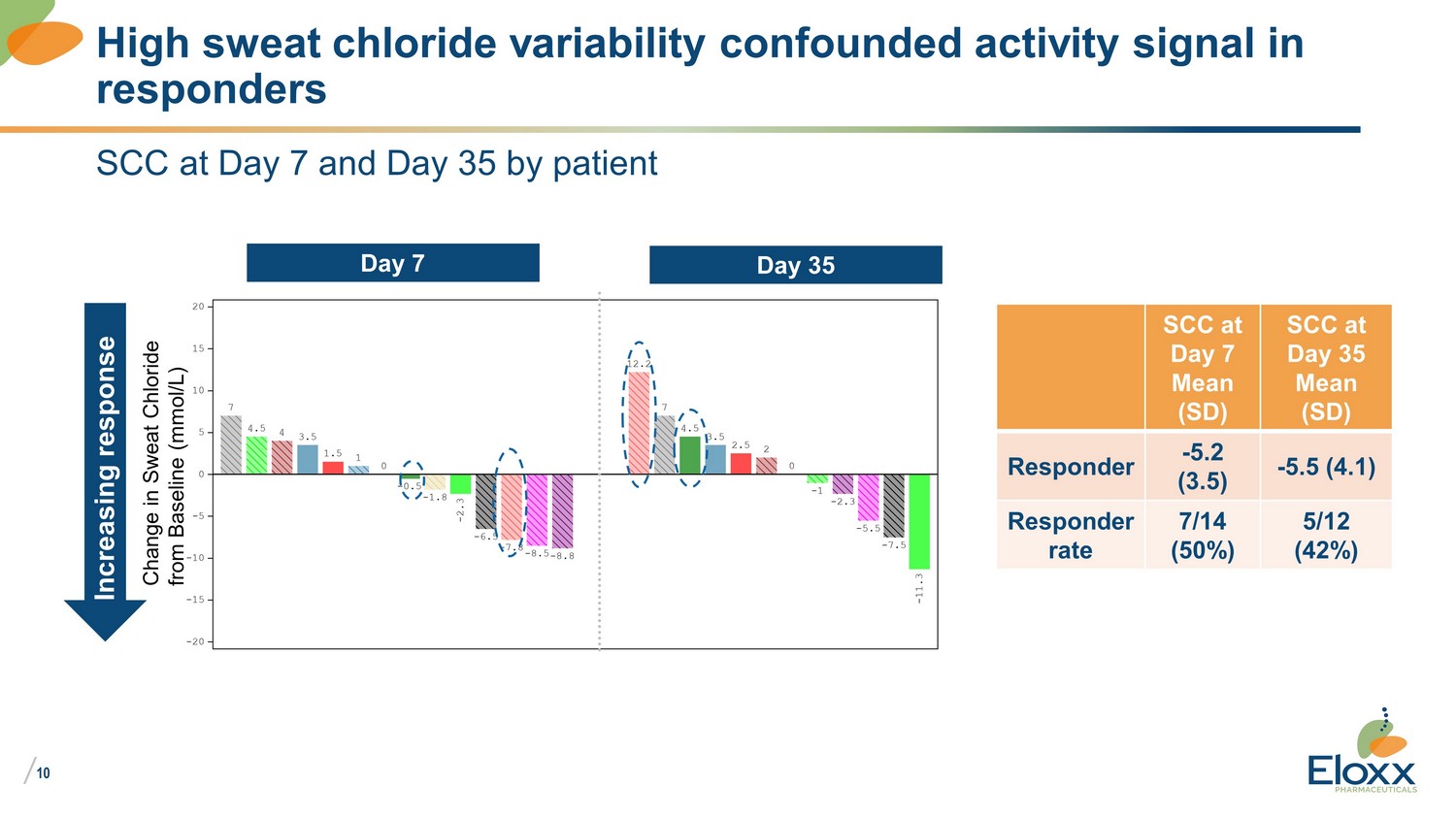
/ 10 SCC at Day 7 and Day 35 by patient High sweat chloride variability confounded activity signal in responders Day 7 Day 35 Increasing response Change in Sweat Chloride from Baseline (mmol/L) SCC at Day 7 Mean (SD) SCC at Day 35 Mean (SD) Responder - 5.2 (3.5) - 5.5 (4.1) Responder rate 7/14 (50%) 5/12 (42%)
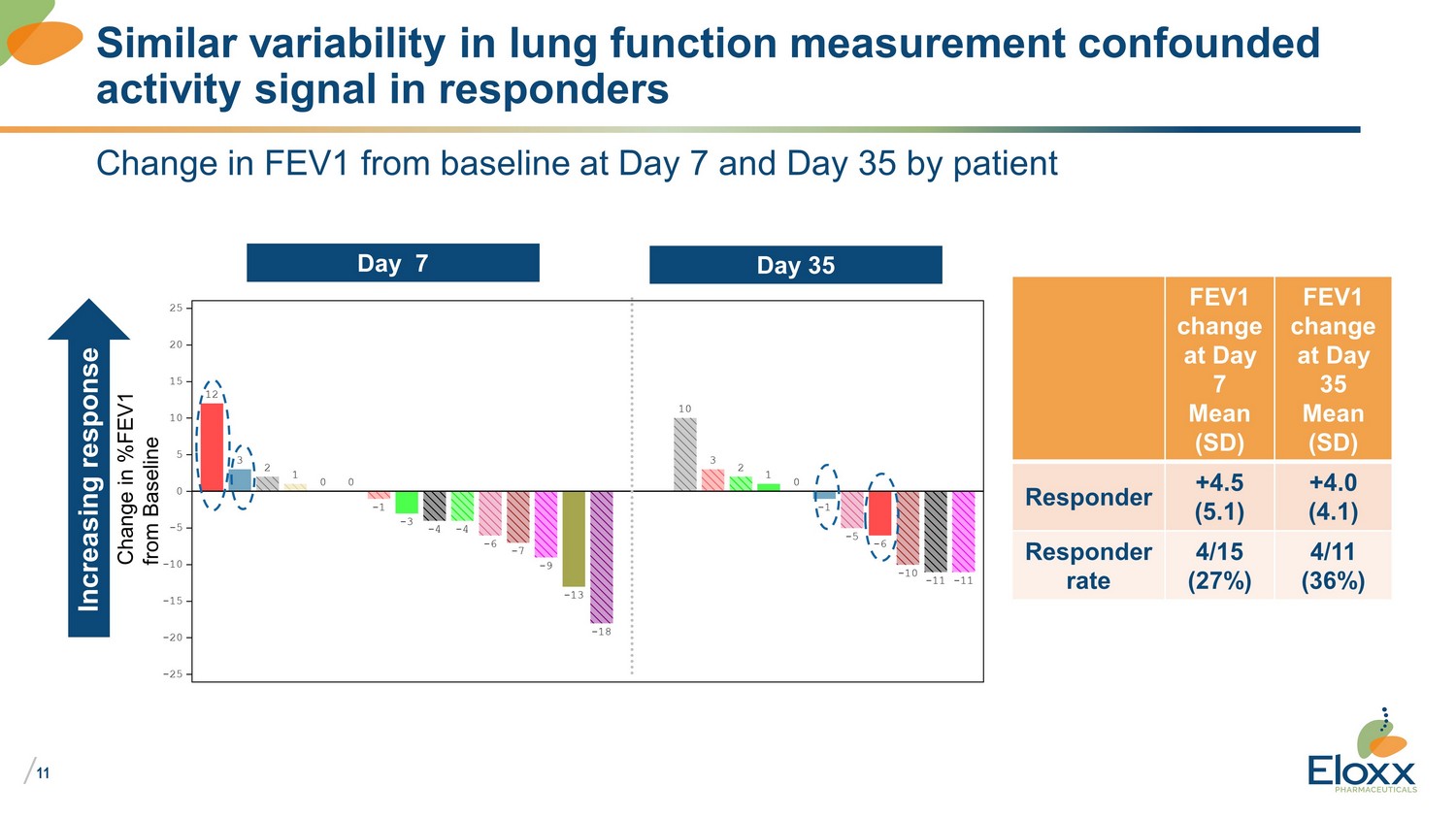
/ 11 Change in FEV1 from baseline at Day 7 and Day 35 by patient Similar variability in lung function measurement confounded activity signal in responders Day 7 Day 35 FEV1 change at Day 7 Mean (SD) FEV1 change at Day 35 Mean (SD) Responder +4.5 (5.1) +4.0 (4.1) Responder rate 4/15 (27%) 4/11 (36%) Increasing response Change in %FEV1 from Baseline
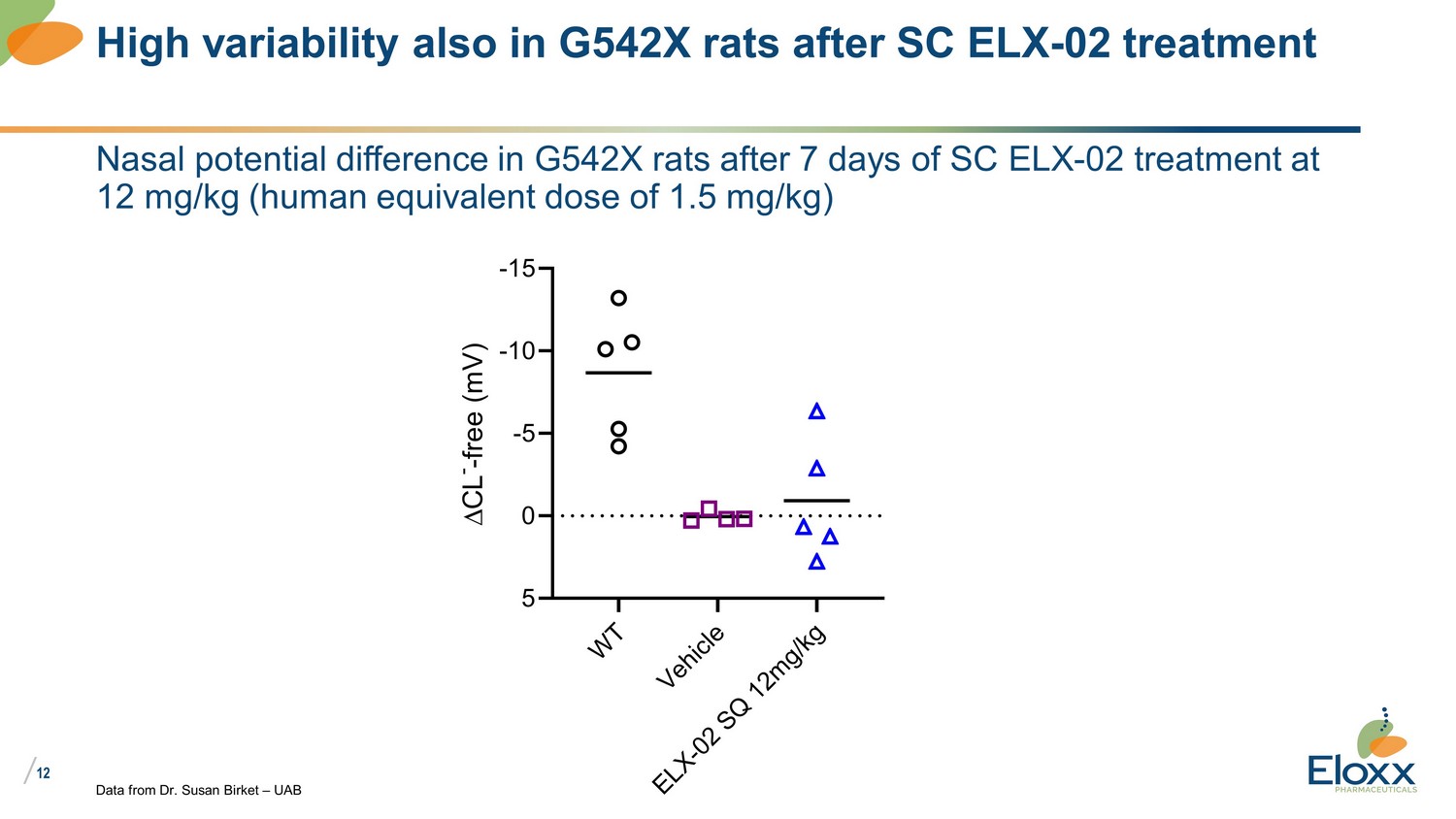
/ 12 Nasal potential difference in G542X rats after 7 days of SC ELX - 02 treatment at 12 mg/kg (human equivalent dose of 1.5 mg/kg) Data from Dr. Susan Birket – UAB High variability also in G542X rats after SC ELX - 02 treatment W T V e h i c l e E L X - 0 2 S Q 1 2 m g / k g -15 -10 -5 0 5 C L - - f r e e ( m V )
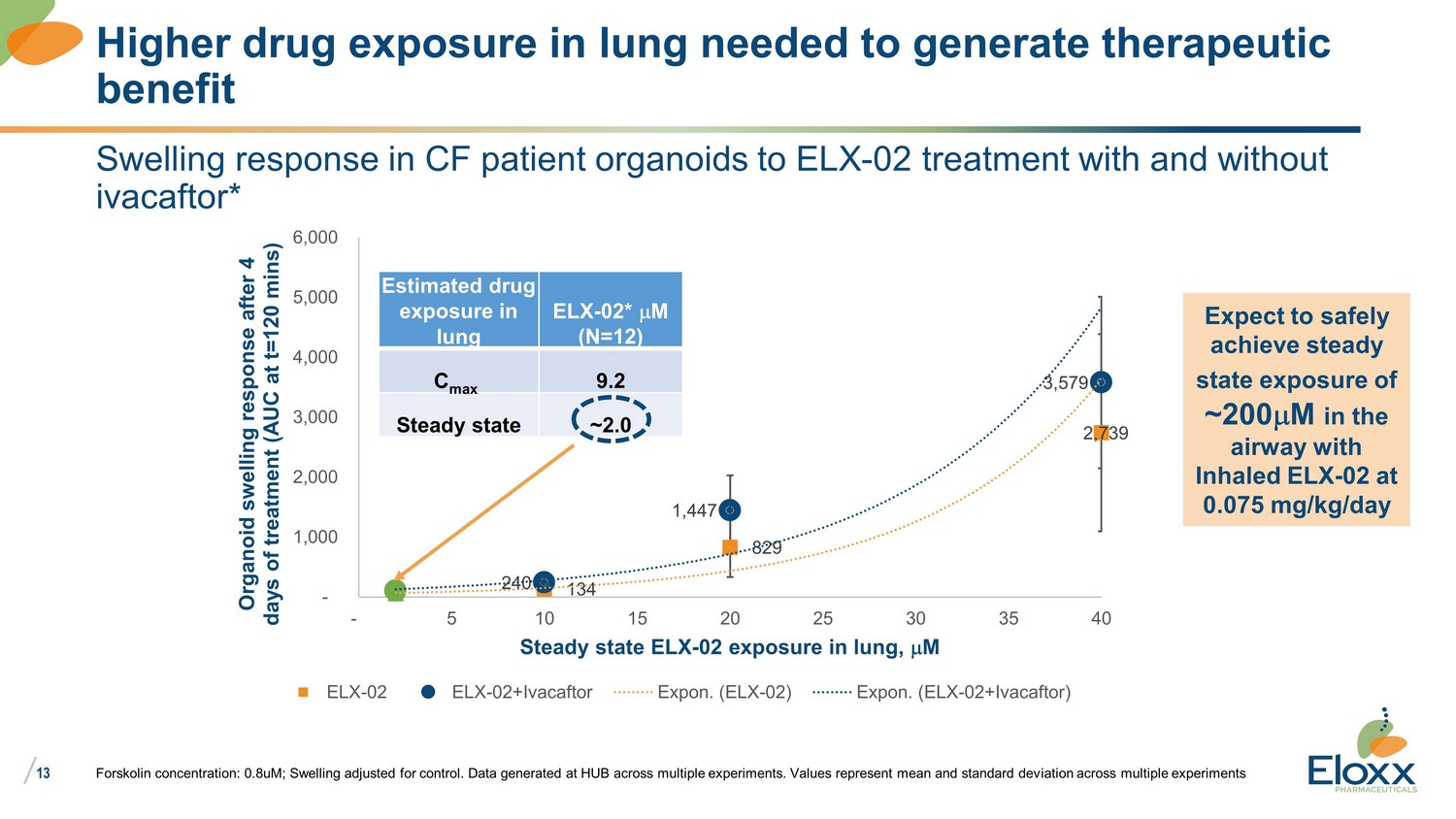
/ 13 Swelling response in CF patient organoids to ELX - 02 treatment with and without ivacaftor* 134 2,739 829 240 3,579 1,447 - 1,000 2,000 3,000 4,000 5,000 6,000 - 5 10 15 20 25 30 35 40 Organoid swelling response after 4 days of treatment (AUC at t=120 mins) Steady state ELX - 02 exposure in lung, m M ELX-02 ELX-02+Ivacaftor Expon. (ELX-02) Expon. (ELX-02+Ivacaftor) Forskolin concentration: 0.8uM; Swelling adjusted for control. Data generated at HUB across multiple experiments. Values repr ese nt mean and standard deviation across multiple experiments Higher drug exposure in lung needed to generate therapeutic benefit Estimated drug exposure in lung ELX - 02* m M (N=12) C max 9.2 Steady state ~2.0 Expect to safely achieve steady state exposure of ~200 m M in the airway with Inhaled ELX - 02 at 0.075 mg/kg/day
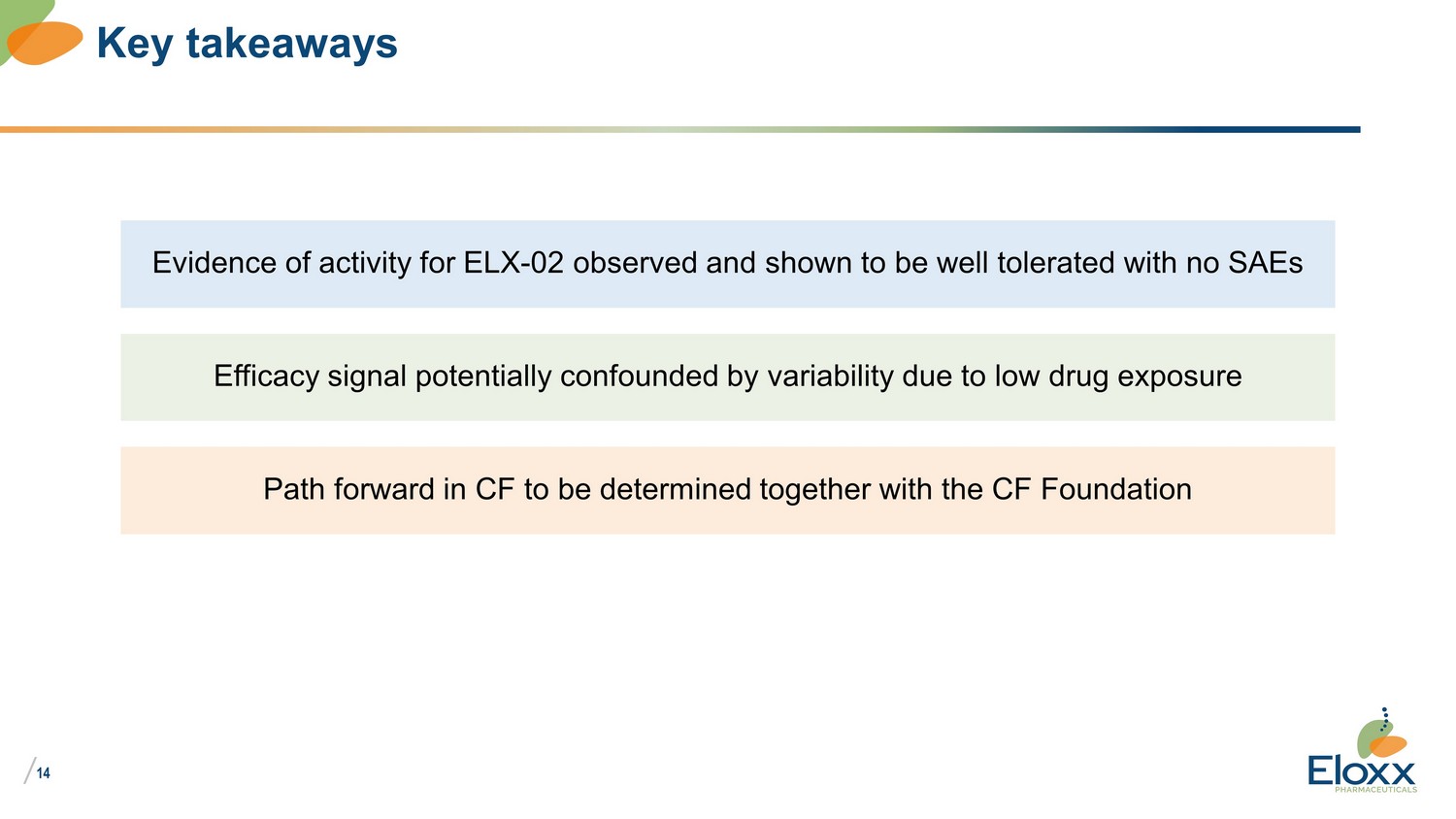
/ 14 Key takeaways Evidence of activity for ELX - 02 observed and shown to be well tolerated with no SAEs Efficacy signal potentially confounded by variability due to low drug exposure Path forward in CF to be determined together with the CF Foundation
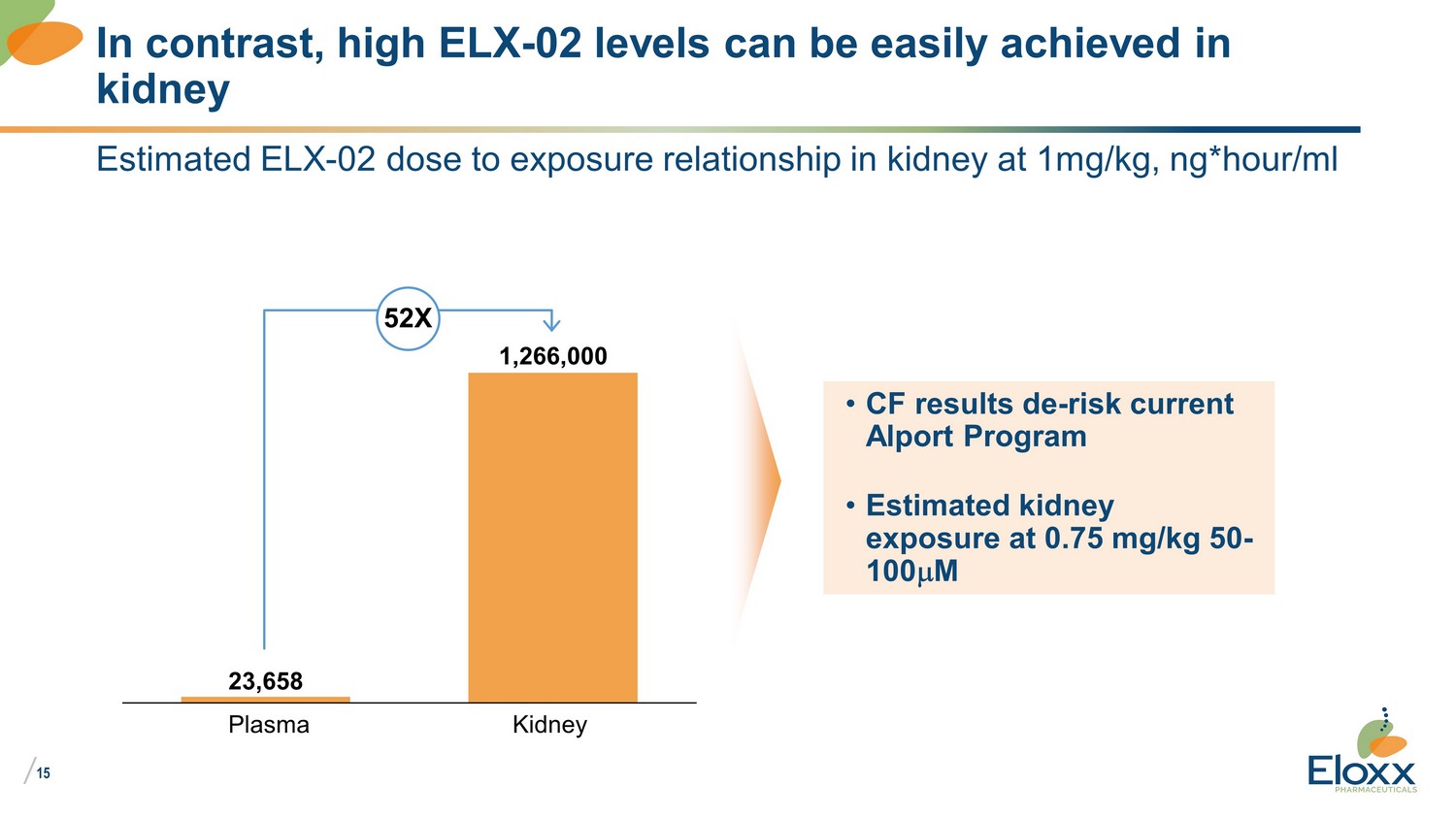
/ 15 Estimated ELX - 02 dose to exposure relationship in kidney at 1mg/kg, ng*hour/ml In contrast, high ELX - 02 levels can be easily achieved in kidney 23,658 1,266,000 Plasma Kidney • CF results de - risk current Alport Program • Estimated kidney exposure at 0.75 mg/kg 50 - 100 m M 52X
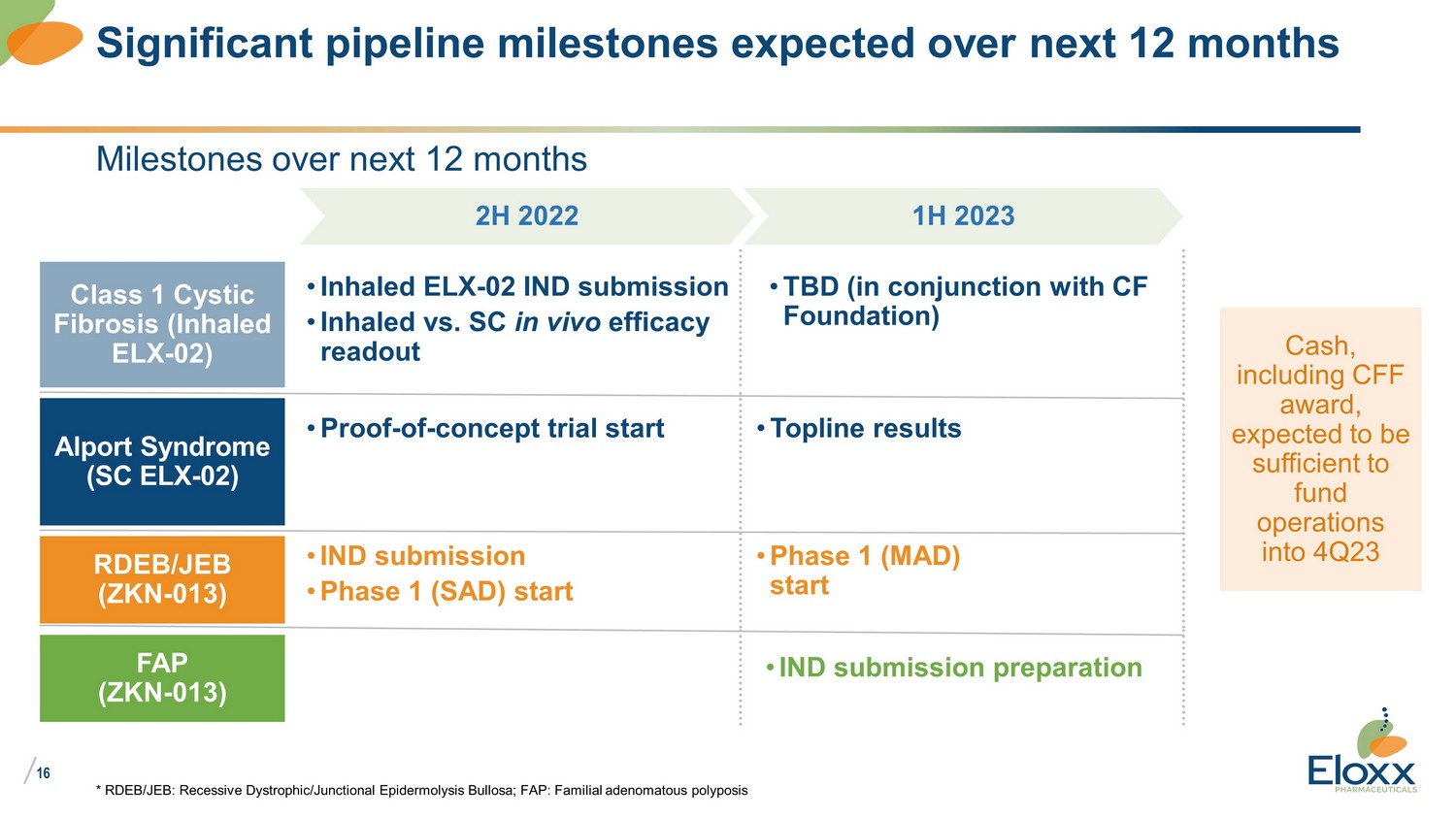
/ 16 Milestones over next 12 months * RDEB/JEB: Recessive Dystrophic/Junctional Epidermolysis Bullosa; FAP: Familial adenomatous polyposis Significant pipeline milestones expected over next 12 months Class 1 Cystic Fibrosis (Inhaled ELX - 02) • Inhaled ELX - 02 IND submission • Inhaled vs. SC in vivo efficacy readout 2H 2022 1H 2023 Alport Syndrome (SC ELX - 02) • Proof - of - concept trial start • Topline results RDEB/JEB (ZKN - 013) • IND submission • Phase 1 (SAD) start • Phase 1 (MAD) start FAP (ZKN - 013) Cash, including CFF award, expected to be sufficient to fund operations into 4Q23 • IND submission preparation • TBD (in conjunction with CF Foundation)

/ 17 Questions? Answers.

/ 18

















