
Senesco Technologies, Inc. Changing Cancer Therapy Annual Meeting 2014 February 11, 2014

Certain statements included in this presentation are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Actual results could differ materially from such statements expressed or implied herein as a result of a variety of factors, including, but not limited to : the Company’s ability to recruit patients for its clinical trial, the ability of the Company to consummate additional financings ; the development of the Company’s gene technology ; the approval of the Company’s patent applications ; the successful implementation of the Company’s research and development programs and collaborations ; the success of the Company's license agreements ; the acceptance by the market of the Company’s products ; the timing and success of the Company’s preliminary studies, preclinical research and clinical trials ; competition and the timing of projects and trends in future operating performance, the Company’s ability to comply with the continued listing standards of the NYSE/MKT, as well as other factors expressed from time to time in the Company’s periodic filings with the Securities and Exchange Commission (the "SEC") . As a result, this press release should be read in conjunction with the Company’s periodic filings with the SEC . The forward - looking statements contained herein are made only as of the date of this press release, and the Company undertakes no obligation to publicly update such forward - looking statements to reflect subsequent events or circumstances . . 2 Safe Harbor Statement

Senesco’s Gene Regulation Platform Technology » C linical stage biotech company specializing in cancer » P roprietary gene regulation technology eliminates cancerous cells and protects healthy cells from premature death » The company is running a Phase 1b/2a trial with a product that treats B - cell cancers (which include multiple myeloma and non - Hodgkins B - cell lymphomas ) » Trial sites include the Mayo Clinic and the Fred Hutchinson Cancer Research Center

Senesco’s Platform A novel therapeutic approach based on gene regulation 4
Senesco’s Platform Targets B - cell Cancers » Multiple myeloma is an incurable cancer of plasma cells – “B - cell” cancer » Diffuse large B - cell lymphoma (DLBCL) is the most commonly diagnosed lymphoma » Mantle cell lymphoma (MCL) is an aggressive tumor with poor outcomes 5 All orphan drug indications

Large Market & Unmet Medical N eed » Multiple myeloma market projected at $6B by 2018 • Revlimid ( lenalidomide ) and Velcade ( bortezomib ) are most important approved drugs » B - cell cancers together are well in excess of $10B
Turning on a Normal Process » Lysine Protein – need to turn up ▪ Activates programmed cell death ( apoptosis) » Hypusine Protein – need to turn down ▪ Stimulates cell growth » Senesco’s drug candidate SNS01 - T reprograms the cells to recognize the death message by modulating the levels of both proteins 7
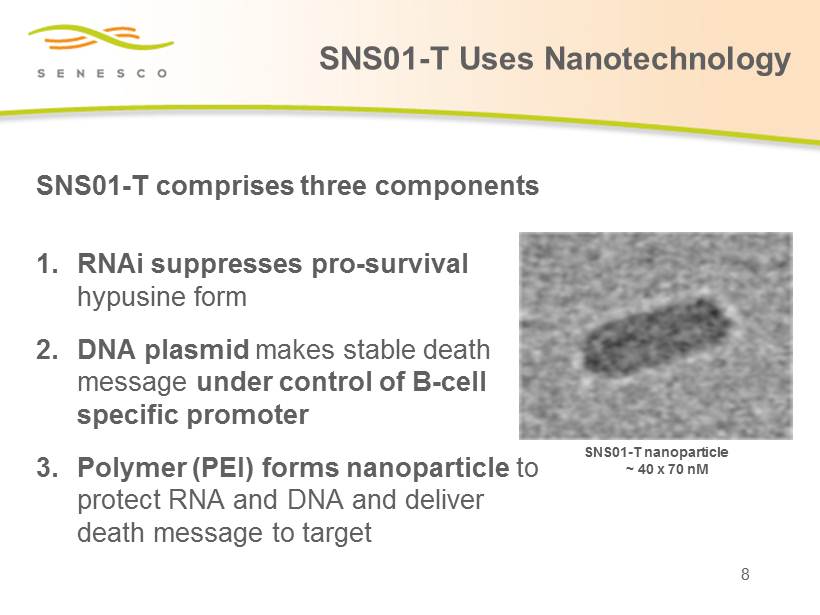
SNS01 - T comprises three components 1. RNAi suppresses pro - survival hypusine form 2. DNA plasmid makes stable death message under control of B - cell specific promoter 3. Polymer (PEI) forms nanoparticle to protect RNA and DNA and deliver death message to target SNS01 - T nanoparticle ~ 40 x 70 nM 8 SNS01 - T Uses Nanotechnology

Clinical Trial Status
First In - human Phase 1b/2a Study Design » Relapsed or refractory MM, MCL or DLBCL » Open - label dose escalation » Four dose cohorts – 0.0125, 0.05, 0.2 and 0.375 mg/kg » Twice weekly I.V. infusion for 6 consecutive weeks » Data have been monitored, study ongoing Primary objectives » Evaluate the safety and tolerability of multiple escalating doses of SNS01 - T Secondary objectives » Pharmacokinetics » Tumor response markers • M protein, % plasma cells » Time to relapse or progression
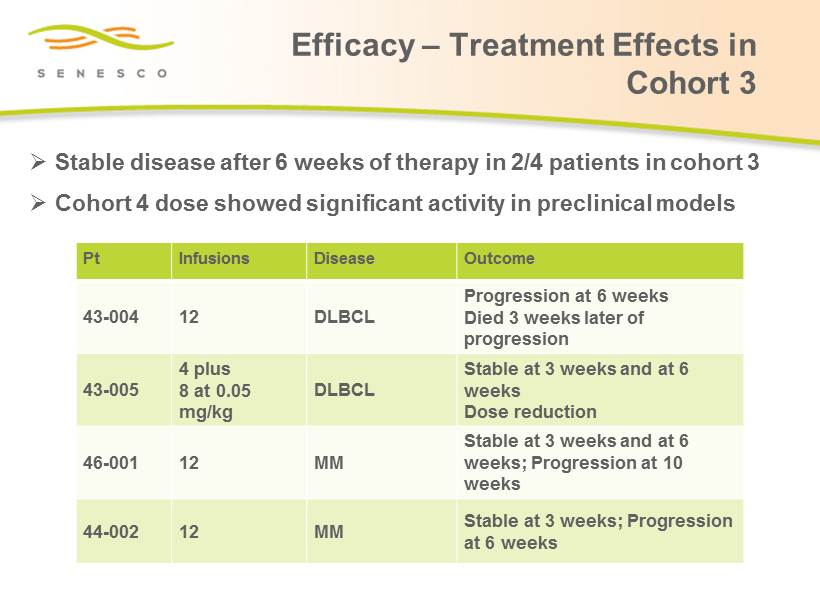
Efficacy – Treatment Effects in Cohort 3 Pt Infusions Disease Outcome 43 - 004 12 DLBCL Progression at 6 weeks Died 3 weeks later of progression 43 - 005 4 plus 8 at 0.05 mg/kg DLBCL Stable at 3 weeks and at 6 weeks Dose reduction 46 - 001 12 MM Stable at 3 weeks and at 6 weeks; Progression at 10 weeks 44 - 002 12 MM Stable at 3 weeks; Progression at 6 weeks » Stable disease after 6 weeks of therapy in 2/4 patients in cohort 3 » Cohort 4 dose showed significant activity in preclinical models
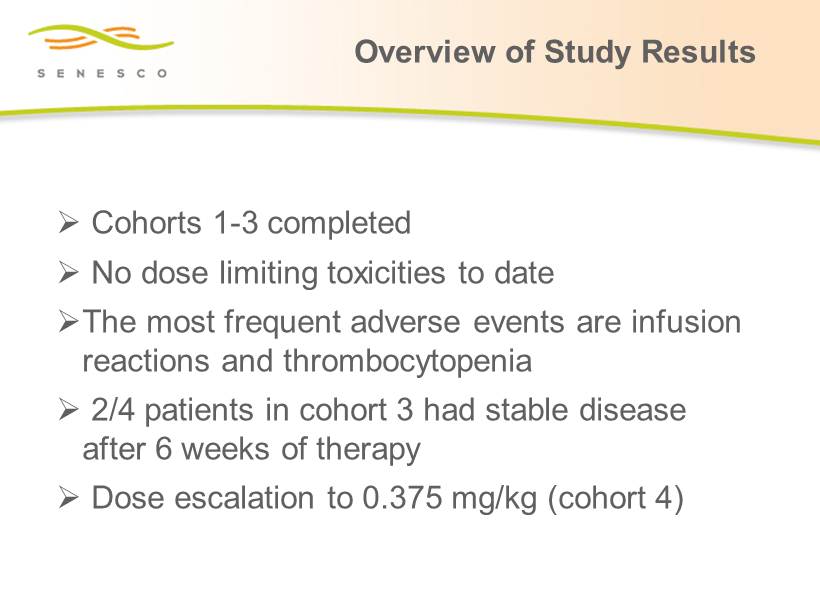
Overview of Study Results » Cohorts 1 - 3 completed » No dose limiting toxicities to date » The most frequent adverse events are infusion reactions and thrombocytopenia » 2/4 patients in cohort 3 had stable disease after 6 weeks of therapy » Dose escalation to 0.375 mg/kg (cohort 4)

Clinical Development Plans 13 ▪ Topline results of Phase 1b/2a in 1H - 2014 ▪ Phase 2 initiation planned for 2H - 2014 • Focus on multiple myeloma, mantle and diffuse large B - cell lymphomas
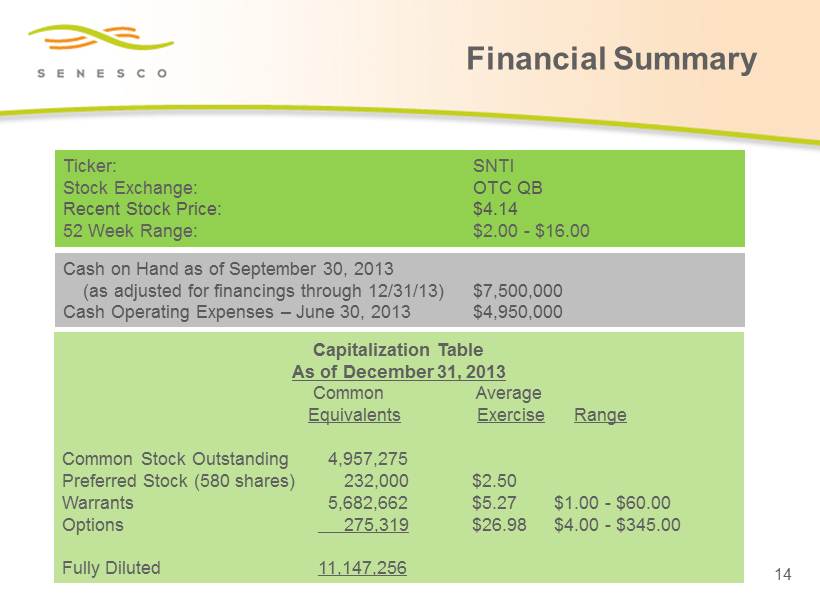
Ticker: SNTI Stock Exchange: OTC QB Recent Stock Price: $4.14 52 Week Range: $2.00 - $16.00 Financial Summary 14 Capitalization Table As of December 31, 2013 Common Average Equivalents Exercise Range Common Stock Outstanding 4,957,275 Preferred Stock (580 shares) 232,000 $2.50 Warrants 5,682,662 $5.27 $1.00 - $60.00 Options 275,319 $26.98 $4.00 - $345.00 Fully Diluted 11,147,256 Cash on Hand as of September 30, 2013 (as adjusted for financings through 12/31/13) $7,500,000 Cash Operating Expenses – June 30, 2013 $4,950,000

» Large product opportunity in hematology » Near - term clinical trial results from Phase 1b/2a » Multiple additional applications in solid tumors » New investor Dr. Phillip Frost, Chairman of Teva » Upcoming acquisition of Fabrus 15 Summary

Fabrus Annual Meeting 2014

Fabrus History » Fabrus was founded in 2007 by Vaughn Smider , M.D., Ph.D. (Stanford), in Pfizer incubator in La Jolla, CA. » OPKO Health invested in Fabrus in 2010 as part of its series A financing. » In June 2013 Vaughn Smider was a co - author on the Scripps Institute’s publication of the crystal structure of bovine antibodies. » Fabrus acquired the chimerasome assets of Chimeros in January 2014 . 17
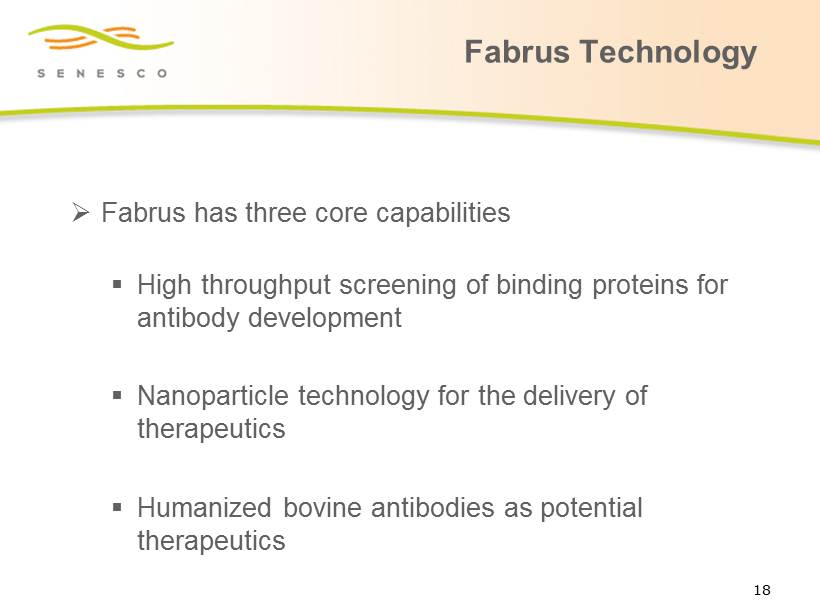
Fabrus Technology » Fabrus has three core capabilities ▪ High throughput screening of binding proteins for antibody development ▪ Nanoparticle technology for the delivery of therapeutics ▪ H umanized bovine antibodies as potential therapeutics 18
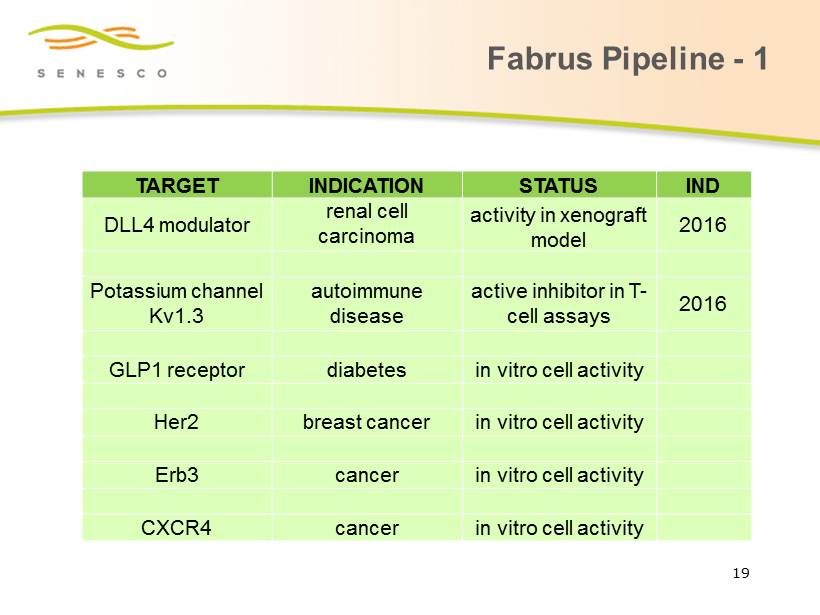
Fabrus Pipeline - 1 19 TARGET INDICATION STATUS IND DLL4 modulator renal cell carcinoma activity in xenograft model 2016 Potassium channel Kv1.3 autoimmune disease active inhibitor in T - cell assays 2016 GLP1 receptor diabetes in vitro cell activity Her2 breast cancer in vitro cell activity Erb3 cancer in vitro cell activity CXCR4 cancer in vitro cell activity

Fabrus Pipeline - 2 » Delta - Like Ligand 4 ( DLL4) Modulator ▪ Anti - angiogenic antibody inhibits blood vessel growth and renal cell tumor growth in mouse xenograft model » Next Steps ▪ Process development ▪ Animal efficacy 20
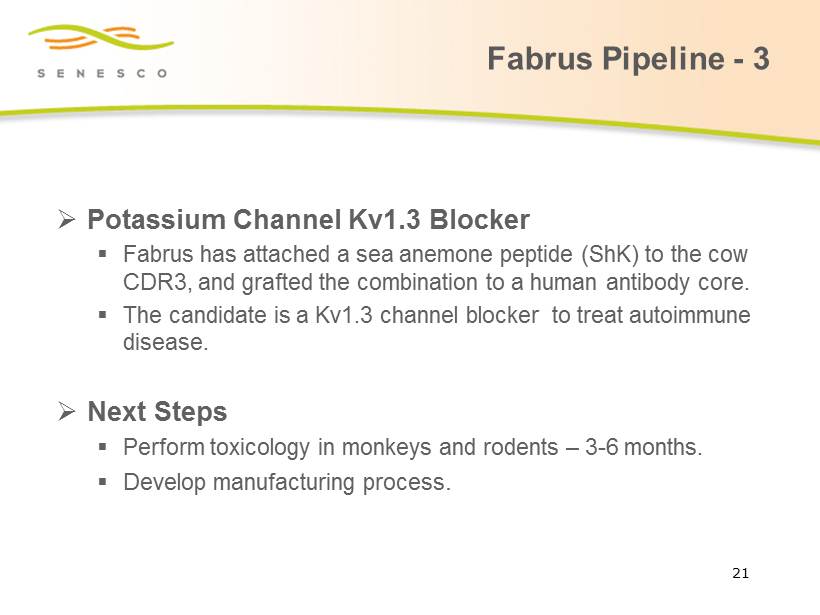
Fabrus Pipeline - 3 » Potassium Channel Kv1.3 Blocker ▪ Fabrus has attached a sea anemone peptide ( ShK ) to the cow CDR3, and grafted the combination to a human antibody core. ▪ The candidate is a Kv1.3 channel blocker to treat autoimmune disease. » Next Steps ▪ Perform toxicology in monkeys and rodents – 3 - 6 months. ▪ Develop manufacturing process . 21

Fabrus Deal Terms Transaction is designed to be a 50/50 merger . Senesco will issue shares of common stock and warrants equal to the amount of Senesco common stock and warrants outstanding at time of closing .
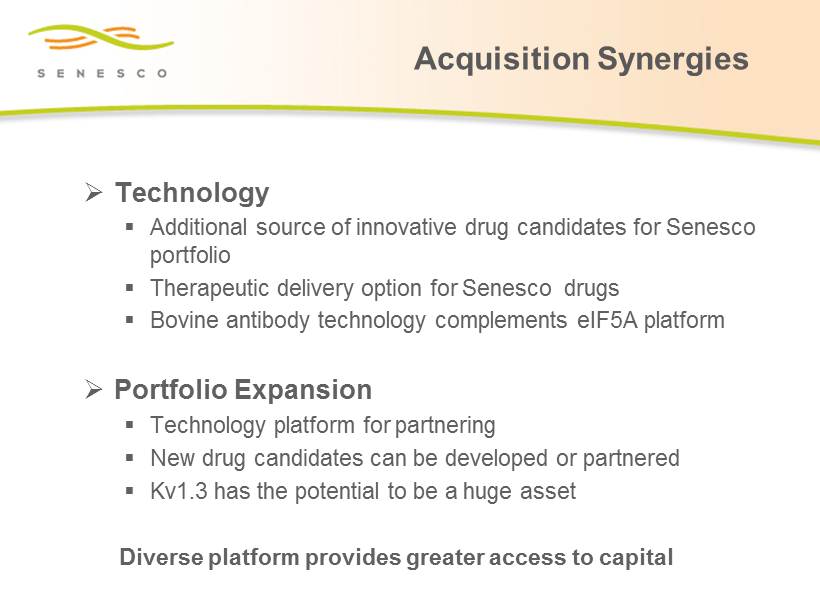
Acquisition Synergies » Technology ▪ Additional source of innovative drug candidates for Senesco portfolio ▪ Therapeutic delivery option for Senesco drugs ▪ B ovine antibody technology complements eIF5A platform » Portfolio Expansion ▪ Technology platform for partnering ▪ New drug candidates can be developed or partnered ▪ Kv1.3 has the potential to be a huge asset Diverse platform provides greater access to capital






















