
Unlocking protein production with translational read-through for rare genetic diseases FY2017 Webcast & Conference Call March 20, 2018 Exhibit 99.2

Certain statements included in this presentation are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These include statements of management’s intentions, belief, plans and future expectations and, therefore, you are cautioned not to place undue reliance on them. Such forward-looking statements involve risks and uncertainties and actual results could differ materially from any forward-looking statements expressed or implied herein. The risks and uncertainties that could result in actual results to differ materially from those forward-looking statements expressed or implied herein include, but are not limited to: the Company's ability to continue as a going concern; the ability of the Company to consummate additional financings; the development of the Company's technology; the approval of the Company's patent applications; the Company's ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company's research and development programs and collaborations; the success of the Company's license agreements; the timing and success of the Company's preliminary studies, preclinical research, clinical trials and related regulatory filings; if approved, the acceptance by the market of the Company's products; and the continued quotation of the Company's common stock on the over-the-counter securities market, as well as other factors expressed from time to time in the Company’s 10-K, 10-Qs and other filings with the SEC. The forward-looking statements contained herein are made only as of the date of this presentation, and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstances. Forward-Looking Statements
Key positive organoid data in Cystic Fibrosis Heterozygous and homozygous CFTR mutations Key positive model data in Cystinosis Reduction of kidney cystine levels On track for completion of Phase 1 studies SAD completed MAD enrolling Initiation of Phase 2 studies in Cystic Fibrosis and Cystinosis (4 Q) Participation at Key Scientific Conferences Eloxx to nominate second novel molecule for development in rare/ultra-rare orphan disease Eloxx Pharmaceuticals Highlights

The Promise of Read-Through >1,800 Genetic diseases involve nonsense mutations In every genetic disease a subset of patients have nonsense mutations that impair the production of essential proteins Translational read through is directed at restoring the production of full length proteins by overcoming the premature stop codon and nonsense mediated decay Cystic Fibrosis Cystinosis MPS I Syndrome Rett Syndrome Duchenne Muscular Dystrophy Built upon a molecular scaffold with a defined ribosomal effect Active at all three premature stop codons Potential to achieve clinically meaningful restoration of functional essential protein Advances in our understanding of translational read-through has enabled design of novel small molecules
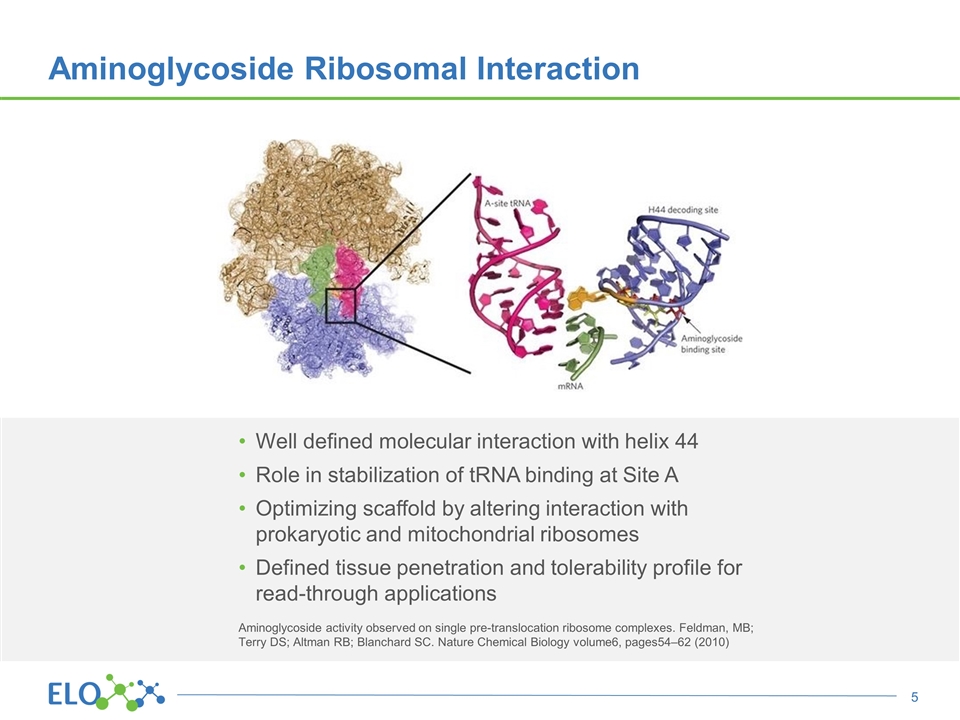
Aminoglycoside Ribosomal Interaction Well defined molecular interaction with helix 44 Role in stabilization of tRNA binding at Site A Optimizing scaffold by altering interaction with prokaryotic and mitochondrial ribosomes Defined tissue penetration and tolerability profile for read-through applications Aminoglycoside activity observed on single pre-translocation ribosome complexes. Feldman, MB; Terry DS; Altman RB; Blanchard SC. Nature Chemical Biology volume6, pages54–62 (2010)

CLINICALTRIALS.GOV Identifier: NCT03292302 A Phase 1a, Randomized, Double-blinded, Placebo-Controlled, Single Dose Escalation Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of ELX-02 in Healthy Adult Volunteers CLINICALTRIALS.GOV Identifier: NCT03309605 A Phase 1, Randomized, Double-Blinded, Placebo-Controlled, Third Party Open, Multiple Dose Escalation, Single Center Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Subcutaneously Administered ELX-02 in Independent Consecutive Cohorts of Healthy Subjects Planned Enrollment: 45 ELX-02 Clinical Development – Phase 1 Studies TO DATE: No SAE Observed No renal or otoacoustic SAE Generally well tolerated COMPLETED ONGOING
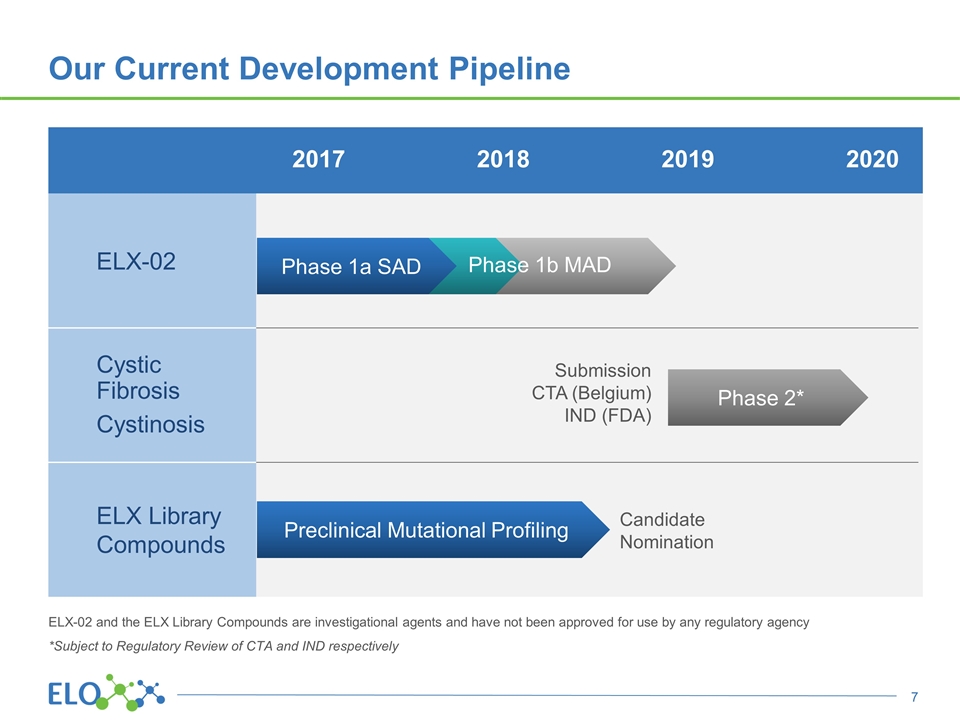
ELX-02 Cystic Fibrosis Cystinosis ELX Library Compounds Our Current Development Pipeline Phase 1a SAD Phase 1b MAD Submission CTA (Belgium) IND (FDA) Phase 2* Preclinical Mutational Profiling Candidate Nomination ELX-02 and the ELX Library Compounds are investigational agents and have not been approved for use by any regulatory agency *Subject to Regulatory Review of CTA and IND respectively 2017 2018 2019 2020
Most prevalent genetic disease in the western world CF is the most common fatal inherited disease in Caucasians Caused by mutations in transmembrane conductance regulator (CFTR) Chloride channel Mutations lead to dysregulation in multiple organ systems Current standard of care based on molecular chaperones for trafficking and conformation Target Class II – Class V CFTR Defects No currently approved drugs for Class I CFTR Defects Currently available data for our investigational drug, ELX-02, suggests the potential for: Active for both homozygous and heterozygous Class I nonsense mutations Increase translational read-through Improve chloride currents in HBEs and organoids Demonstrate synergy with correctors and potentiators in heterozygous population Cystic Fibrosis Development Program Zoltan Bozoky et al. PNAS 2013;110:47:E4427-E4436
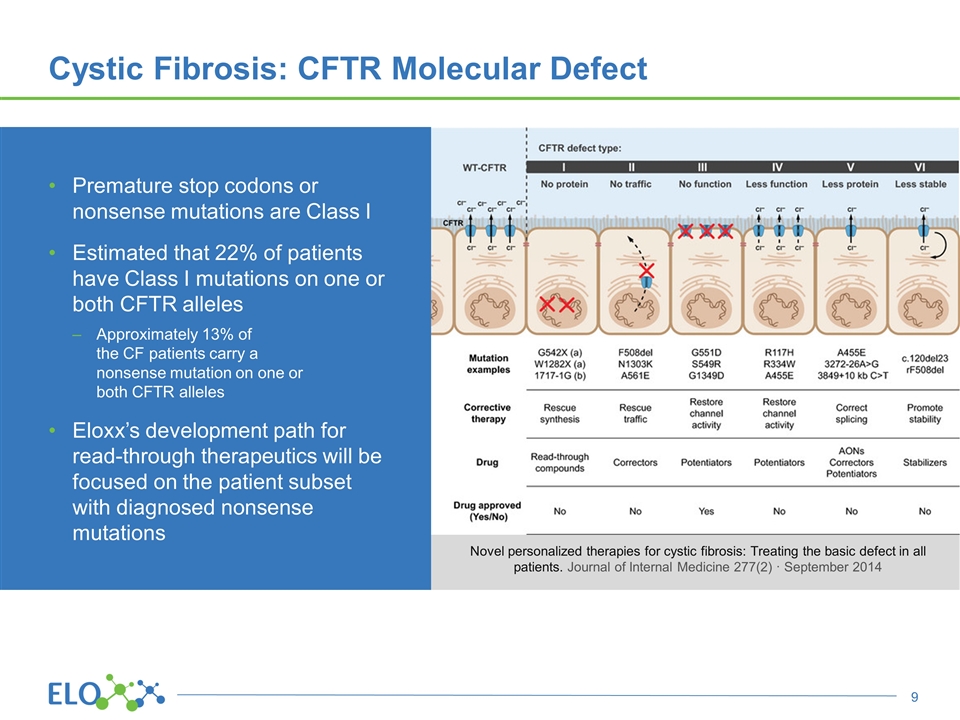
Premature stop codons or nonsense mutations are Class I Estimated that 22% of patients have Class I mutations on one or both CFTR alleles Approximately 13% of the CF patients carry a nonsense mutation on one or both CFTR alleles Eloxx’s development path for read-through therapeutics will be focused on the patient subset with diagnosed nonsense mutations Cystic Fibrosis: CFTR Molecular Defect Novel personalized therapies for cystic fibrosis: Treating the basic defect in all patients. Journal of Internal Medicine 277(2) · September 2014
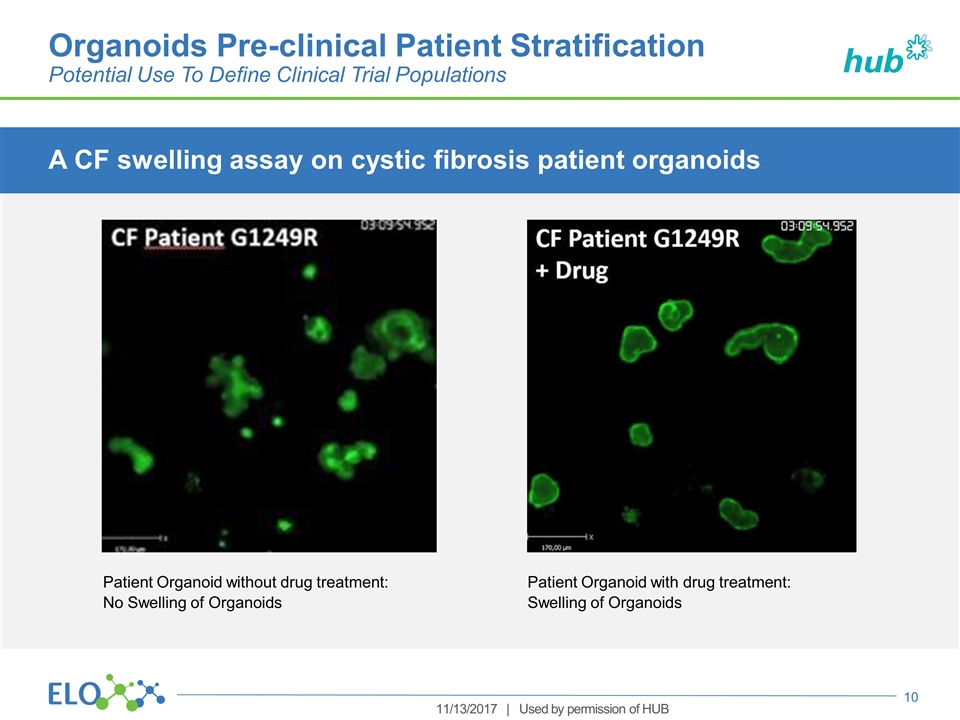
A CF swelling assay on cystic fibrosis patient organoids Organoids Pre-clinical Patient Stratification Potential Use To Define Clinical Trial Populations Patient Organoid without drug treatment: No Swelling of Organoids Patient Organoid with drug treatment: Swelling of Organoids 11/13/2017 | Used by permission of HUB
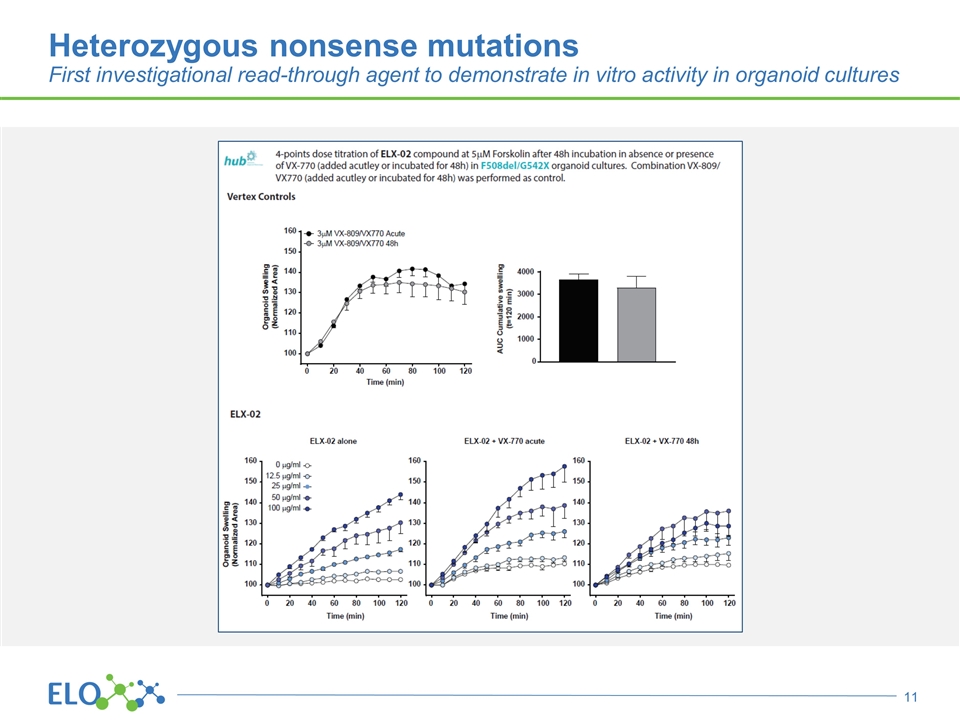
Heterozygous nonsense mutations First investigational read-through agent to demonstrate in vitro activity in organoid cultures
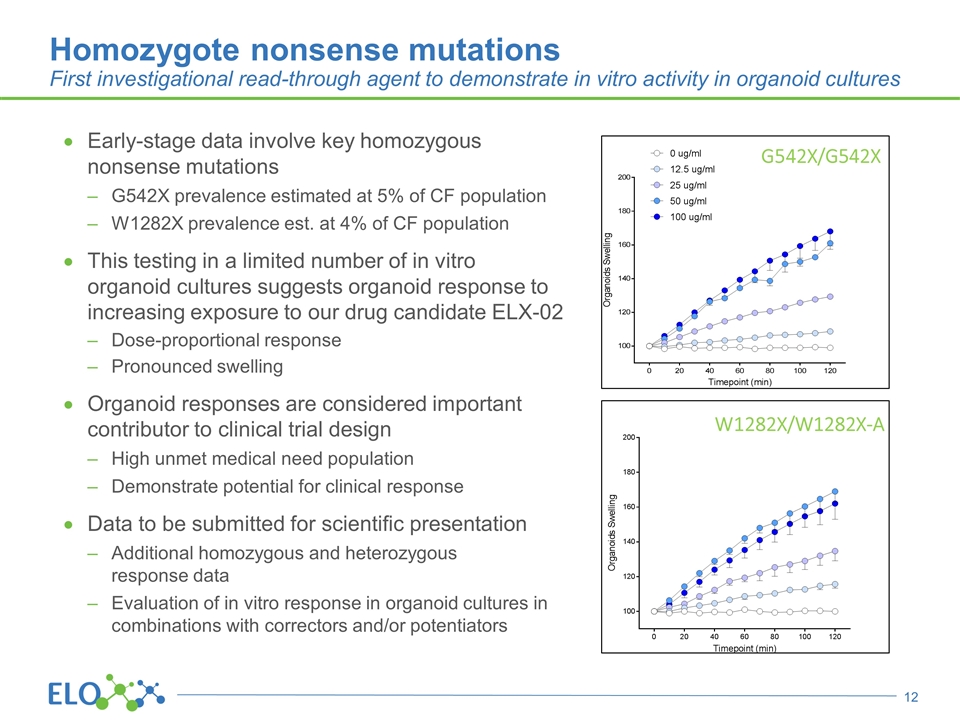
Homozygote nonsense mutations First investigational read-through agent to demonstrate in vitro activity in organoid cultures G542X/G542X W1282X/W1282X-A Early-stage data involve key homozygous nonsense mutations G542X prevalence estimated at 5% of CF population W1282X prevalence est. at 4% of CF population This testing in a limited number of in vitro organoid cultures suggests organoid response to increasing exposure to our drug candidate ELX-02 Dose-proportional response Pronounced swelling Organoid responses are considered important contributor to clinical trial design High unmet medical need population Demonstrate potential for clinical response Data to be submitted for scientific presentation Additional homozygous and heterozygous response data Evaluation of in vitro response in organoid cultures in combinations with correctors and/or potentiators
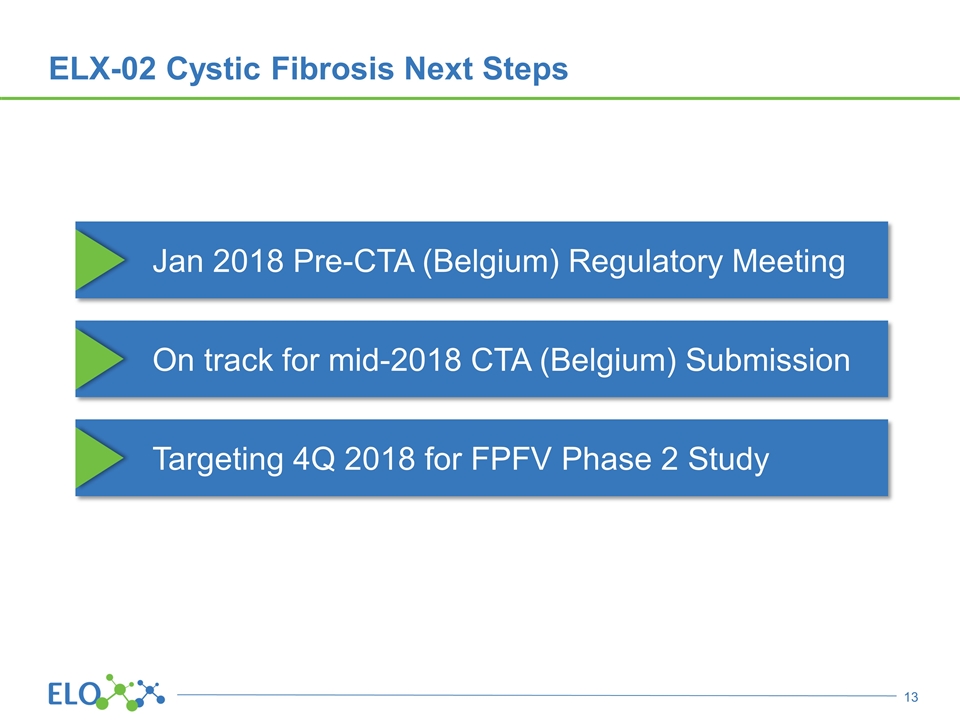
ELX-02 Cystic Fibrosis Next Steps Jan 2018 Pre-CTA (Belgium) Regulatory Meeting On track for mid-2018 CTA (Belgium) Submission Targeting 4Q 2018 for FPFV Phase 2 Study
Ultra-rare lysosomal storage disease Caused by mutations in cystinosin (CTNS) Cysteine efflux channel Cystine lysosomal accumulation causes manifestations of disease The current standard of care, Cysteamine acts within the lysosome to convert cystine into forms which can exit the lysosome via cysteine transport pathways. W138X most common nonsense mutation is estimated to represent 1/3 of patient population Currently available data on our investigational drug candidate, ELX-02, suggest the potential to: Increase translational read-through Reduce NMD Restore CTNS mRNA to near normal levels Lower cystine accumulation in vitro and in vivo Cystinosis Development Program
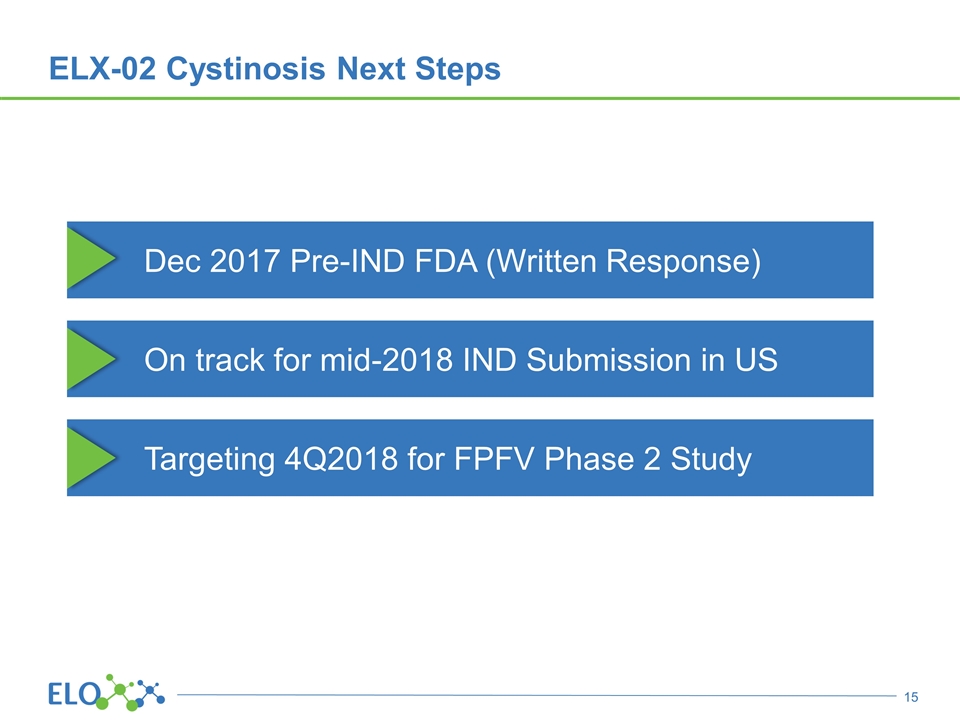
On track for mid-2018 IND Submission in US ELX-02 Cystinosis Next Steps Dec 2017 Pre-IND FDA (Written Response) Targeting 4Q2018 for FPFV Phase 2 Study
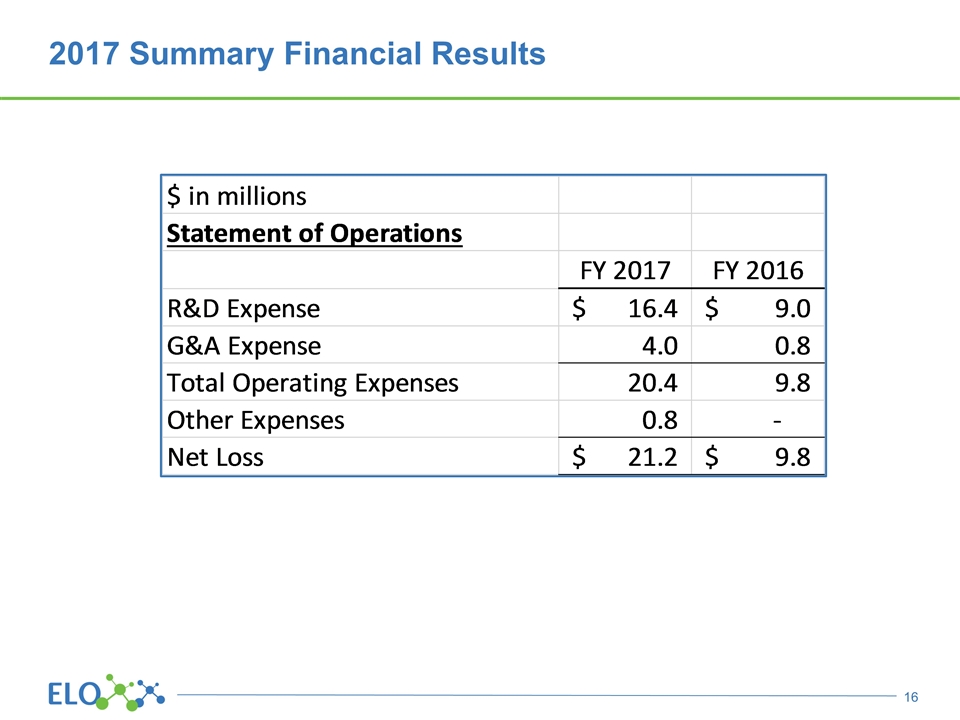
2017 Summary Financial Results

Financial Summary $24 million cash as of December 31, 2017 No debt Funded through at least the end of the first quarter 2019 Shares outstanding totals 27.5 million Traded OTC: ELOX
Key positive organoid data in Cystic Fibrosis Heterozygous and homozygous CFTR mutations Key positive model data in Cystinosis Reduction of kidney cystine levels On track for completion of Phase 1 studies SAD completed MAD enrolling Initiation of Phase 2 studies in Cystic Fibrosis and Cystinosis (4 Q) Participation at Key Scientific Conferences Eloxx to nominate second novel molecule for development in rare/ultra-rare orphan disease Eloxx Pharmaceuticals Highlights

Thank you. FY2017 Webcast & Conference Call March 20, 2018


















