UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| | | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 0-26483
VaxGen, Inc.
(Exact name of registrant as specified in its charter)
| | | |
| Delaware | | 94-3236309 |
| (State or other jurisdiction of | | (I.R.S. Employer Identification No.) |
| incorporation or organization) | | |
379 Oyster Point Boulevard, Suite 10
South San Francisco, California 94080
(Address of principal executive offices, including zip code)
(650) 624-1000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock,
$0.01 par value per share
Title of Class
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of the Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o
(Do not check if a smaller reporting company) | Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No þ
The aggregate market value of the registrant’s stock held by non-affiliates of the registrant on June 30, 2009 was $11.7 million. For purposes of this disclosure, shares of common stock held by entities and individuals who own 5% or more of the outstanding common stock and shares of common stock held by each officer and director have been excluded in that such persons may be deemed to be “affiliates” as that term is defined under the Rules and Regulations of the Securities Exchange Act of 1934. This determination of affiliate status is not necessarily conclusive.
The number of shares outstanding of the registrant’s Common Stock, par value $0.01 per share, as of March 25, 2010 was 33,106,523.
PART I
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or Securities Act, and within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, or Exchange Act, which are subject to the “safe harbor” created by those sections. In particular, statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are contained or incorporated by reference in this report. We have based these forward-looking statements on our current expectations about future events. While we believe these expectations are reasonable, such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. Our actual results may differ materially from those suggested by these forward-looking statements for various reasons, including those discussed in Item 1A. — Risk Factors herein. Given these risks and uncertainties, you are cautioned not to place undue reliance on such forward-looking statements. The forward-looking statements included in this report are made only as of the date hereof. We do not undertake and specifically decline any obligation to update any such statements or to publicly announce the results of any revisions to any such statements to reflect future events or developments. When used in this report, unless otherwise indicated, the “Company,” “we,” “our” and “us” refers to VaxGen, Inc., or VaxGen.
Item 1. Business
VaxGen is a biopharmaceutical company based in South San Francisco, California. The Company owns a state-of-the-art biopharmaceutical manufacturing facility with a 1,000-liter bioreactor that can be used to make cell culture or microbial biologic products. This facility is located within leased premises. The Company has ended all product development activities and sold or otherwise terminated its drug development programs. The Company is seeking to maximize the value of its remaining assets through a strategic transaction or series of strategic transactions.
VaxGen was incorporated on November 27, 1995. From 1995 through 2004, VaxGen was developing vaccine candidates to prevent or reduce HIV infection. These HIV vaccine candidates failed to succeed in two phase three studies, and in 2004 VaxGen licensed its HIV vaccine candidates to Global Solution for Infectious Disease. VaxGen retains certain rights to commercialize these vaccine candidates in the event that they are successfully licensed in the future.
During 2002 through 2006, VaxGen developed vaccines against inhalation anthrax and smallpox for the purpose of biodefense. In December 2006, the Department of Health and Human Services, or HHS, terminated its contract with VaxGen related to the development and delivery of a next-generation anthrax vaccine. Following the HHS decision, VaxGen ceased actively developing its anthrax vaccine, scaled back its biodefense activities and began pursuing strategic and other alternatives.
Through March 31, 2007, VaxGen’s principal source of revenue was the U.S. government, principally the National Institutes of Health, or NIH, and related entities. From April 2007 to April 2008, VaxGen’s principal source of revenue was from services provided to Celltrion, Inc., or Celltrion, a company developing and operating a mammalian cell culture biomanufacturing facility in the Republic of Korea.
We discontinued clinical development of our anthrax vaccine candidate, rPA102, after HHS terminated our SNS Contract in December 2006. In May, 2008, the Company completed the sale of all assets and rights related to its recombinant protective antigen (rPA) anthrax vaccine product candidate and related technology to Emergent BioSolutions, Inc., or Emergent. Under the terms of the transaction, Emergent paid VaxGen $2.0 million upon execution of the definitive agreement, an additional $1.0 million milestone payment during the third quarter of 2008, and may be obligated to pay VaxGen up to an additional $7.0 million in milestone payments, plus specified percentages of future net sales for 12.5 years beginning from the first commercial sale. The Company recorded $3.0 million as other income during the year ended December 31, 2008 for the sale of the Anthrax Program. In December 2009, HHS withdrew the pending Request for Proposal, (RFP) for the procurement of a second generation anthrax vaccine, such as rPA102, delaying indefinitely the potential commercialization and associated payments under this asset sale agreement.
In addition, in June 2007 we terminated our contract with the Chemo-Sero-Therapeutic Research Institute of Japan, or Kaketsuken, to develop a smallpox vaccine. We had previously devoted substantially all of our research, development and clinical efforts and financial resources toward the development of rPA102, and we have no product candidates in clinical or preclinical development. In connection with the termination of our clinical development of rPA102, we announced restructuring activities, including significant workforce reductions, and as a result have no remaining internal capability to discover or develop product candidates. We had research and development costs of zero, $1.4 million and $19.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
In November 2007, the Company and two of its wholly-owned subsidiaries entered into an Agreement and Plan of Merger, as amended in December 2007 and February 2008, or Merger Agreement, with Raven biotechnologies, inc., or Raven. Raven was a private, development stage biopharmaceutical company focused on the discovery, development and commercialization of monoclonal antibody-based products for the treatment of cancer.
3
In connection with the Raven Merger Agreement, the Company also entered into a Bridge Loan with Raven, which provided for the Company to lend Raven up to $6 million in cash in the aggregate, beginning December 1, 2007. In March 2008, due to a lack of stockholder support for the transaction, VaxGen and Raven agreed to terminate the Merger Agreement and amend the terms of VaxGen’s Bridge Loan to Raven. The balance on the Bridge Loan was paid in full in 2008. The Company recorded in general and administrative expenses, $2.3 million of costs, primarily professional fees related to the proposed merger with Raven during the year ended December 31, 2008.
In April 2008, the Company announced that it was restructuring to reduce operating expenses following the termination of the proposed merger with Raven by decreasing its workforce of twenty-two employees by approximately 75 percent. During August 2008, the Company further reduced its remaining workforce by 25 percent. The Company incurred restructuring costs of approximately $1.3 million for one-time termination costs during the year ended December 31, 2008. (See Note 4 to the Consolidated Financial Statements included herein.)
In October 2009, the Company and OXiGENE, Inc., or OXiGENE, entered into a definitive merger agreement pursuant to which a wholly-owned subsidiary of OXiGENE would merge with VaxGen, such that VaxGen would become a wholly-owned subsidiary of OXiGENE, and in exchange VaxGen’s stockholders would receive common stock of OXiGENE. OXiGENE is a publicly-traded development-stage biopharmaceutical company. On February 3, 2010, the Company’s stockholders failed to approve the merger. VaxGen recorded $1.2 million of costs, primarily professional fees, related to the proposed merger with OXiGENE during the year ended December 31, 2009.
As a result of the termination of the proposed merger with OXiGENE, the Company is considering various alternate strategic transactions to return value to its stockholders. If the Company is unable to identify and complete an alternate strategic transaction, the Company will liquidate. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Celltrion
Celltrion is an FDA licensed biologics manufacturing company incorporated on February 26, 2002. Since that date, its principal activities have consisted of design, construction and operation of a manufacturing facility in Incheon, Republic of Korea, and partially funding the construction of our U.S. biopharmaceutical manufacturing facility, as well as raising capital and recruiting scientific and management personnel.
As a part of the initial capitalization of Celltrion and as part of the Celltrion joint venture agreement we signed with various Korean entities, including Nexol Co., Ltd., or Nexol, we made an in-kind contribution to Celltrion of the license and sub-license of certain cell-culture technology used for the manufacture of pharmaceutical products. We received 7.8 million shares of Celltrion’s common stock for this contribution, representing approximately 48% of the then-outstanding shares. In September 2005, we entered into agreements to sell 1.2 million of our shares in Celltrion to a group of Korean investors and raised $15.1 million in gross proceeds. Nexol purchased 250,000 of these shares.
During June and December 2006, we received aggregate gross proceeds of $130.3 million from the sale of substantially all of our Celltrion common stock to Nexol and affiliates of Nexol. As a result, we were no longer entitled to hold two seats on Celltrion’s Board of Directors or appoint a Representative Director. Accordingly, we no longer had the ability to exercise significant influence over operating and financial policies of Celltrion, and beginning July 1, 2006, we accounted for our investment in Celltrion under the cost method. At December 31, 2007, we held a nominal ownership interest in Celltrion. During 2008, a public market developed for Celltrion common stock in the Republic of Korea. During 2009 we sold our remaining investment in Celltrion common stock. This transaction resulted in a realized gain of $357,000.
Manufacturing Facility
We lease a 65,000 square-foot facility in South San Francisco, California of which approximately 15,000 square feet is dedicated to biologics manufacturing. The remainder of this facility contains laboratories, office and expansion space. We lease this facility from a third party, and we are the sole occupant. The facility was designed for the flexible manufacture of biopharmaceutical products including those grown in bacteria, as well as products produced in mammalian cell culture, such as monoclonal antibody products. Following the termination of the proposed merger with Raven, we determined that it was not economical to retain the facility and began exploring alternatives, including the sale of the equipment and assignment or sub-lease of the property.
Employees
As of December 31, 2009, we had three employees. None of our employees is subject to a collective bargaining agreement and we believe that our relations with our employees are good. Our finance and accounting group engages consultants providing service equivalent to approximately one full time employee. We engage additional consultants as necessary.
4
Segment Information
We historically have operated in one business segment, the development of vaccines that immunize against infectious disease. All of our operating assets are located in the United States of America. Substantially all of our revenue has been derived from federal government contracts and grants, primarily from HHS, NIH and related entities.
Other Information
We were incorporated in Delaware in November 1995. Our principal executive office is located at 379 Oyster Point Boulevard, Suite 10, South San Francisco, California 94080, and our telephone number is (650) 624-1000. Our website is http://www.vaxgen.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and any amendments to these reports filed or furnished pursuant to Section 13(a) of the Exchange Act are available, free of charge, on our Internet website as soon as reasonably practicable after we electronically file such materials with, or furnish them to, the SEC.
Our code of ethics for the employees, officers and directors of the Company is available, free of charge, on our website, http://www.vaxgen.com, or by written request to VaxGen’s Corporate Secretary, 379 Oyster Point Boulevard, Suite 10, South San Francisco, California 94080. The SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at http://www.sec.gov. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Our website address is included in this document as an inactive textual reference only and the information contained on our website is not incorporated into this Annual Report on Form 10-K.
Item 1A. Risk Factors
You should carefully consider the following risk factors as well as other information in our filings under the Exchange Act before making any investment decisions regarding our common stock. The risks and uncertainties described herein are not the only ones we face. Additional risks and uncertainties that we do not know or that we currently deem immaterial may also impair our business, financial condition, operating results and prospects. If events corresponding to any of these risks actually occur, they could materially adversely affect our business, financial condition, operating results or prospects. In that case, the trading price of our common stock could decline. When used in this Annual Report, unless otherwise indicated, “we,” “our” and “us” refers to VaxGen.
We do not currently have capabilities to develop products or offer any services; the continuation of our business as a going concern is wholly dependent on our ability to identify and successfully complete a strategic transaction, and/or the sale of the Company’s assets which we may be unable to accomplish.
We discontinued clinical development of our anthrax vaccine candidate, rPA102, after HHS terminated our Strategic National Stockpile (SNS) Contract in December 2006. In addition, in June 2007 we terminated our contract with the Chemo-Sero-Therapeutic Research Institute of Japan, or Kaketsuken, to develop a smallpox vaccine. We had previously devoted substantially all of our research, development and clinical efforts and financial resources toward the development of rPA102, and we have no product candidates in clinical or preclinical development. In connection with the termination of our clinical development of rPA102, we announced restructuring activities, including significant workforce reductions, and as a result have no remaining internal capability to discover or develop product candidates. Following the termination of our SNS Contract, we evaluated strategic alternatives and retained a financial advisor. As a result of this process, we entered into the Merger Agreement with Raven on November 12, 2007, which was subsequently terminated by mutual agreement on March 28, 2008 due to a lack of stockholder support, and entered into a merger agreement with OXiGENE in October 2009, which was not approved by our stockholders.
We cannot predict whether we will be able to identify alternate strategic transactions which will either provide us with a product pipeline or return value to our stockholders on a timely basis or at all. We also cannot predict whether any such transaction would be consummated on favorable terms, and anticipate that such transaction may require us to incur significant additional costs. We are unable to predict if the Company will be able to sell its remaining assets (principally, its manufacturing facility and equipment) or if such a sale can be consummated on favorable terms. We are also unable to predict if the Company will be able to assign, sub-lease or terminate the lease on the property containing its manufacturing facility, or if such actions can be consummated on favorable terms. If we are unable to identify and complete an alternate strategic transaction, our business will be liquidated.
We may use some or all of our remaining resources, including available cash, while we seek to identify a strategic transaction; we may fail to identify an appropriate transaction; our stockholders may vote against a proposed transaction; and even if a strategic transaction is completed, it may be unsuccessful in creating value for stockholders.
5
As a result of the termination of our SNS Contract, we have been evaluating strategic alternatives since January 2007. In November 2007 we entered into a merger agreement with Raven, which was terminated in March 2008 due to a lack of stockholder support. The process of identifying, negotiating and seeking stockholder approval to the proposed Raven merger was time consuming and expensive. For example, we recorded $2.3 million of costs, primarily professional fees, related to the proposed merger with Raven, during the year ended December 31, 2008. In October 2009 we entered into a merger agreement with OXiGENE, and in February 2010 our stockholders failed to approve the merger. The process of identifying, negotiating and seeking stockholder approval to the proposed OXiGENE merger was time consuming and expensive, and we recorded $1.2 million of costs, primarily professional fees, related to the proposed merger with OXiGENE, during the year ended December 31, 2009.
We expect the process of identifying potential alternate strategic transactions will similarly be time-consuming and expensive, regardless of whether we are successful. We cannot predict whether we will be able to identify alternate strategic transactions which will either provide us with a pipeline or return value to our stockholders on a timely basis or at all. We also cannot predict whether any such transaction, once identified, would be approved by our stockholders or consummated on favorable terms. Significant ownership of our common stock is concentrated among several large stockholders and those stockholders may vote against a transaction, even if our Board of Directors and management view the transaction as beneficial. We may use a portion or all of our remaining resources seeking to identify and complete a strategic transaction, but ultimately be unable to do so. Even if completed, such a transaction may not provide us with a pipeline or return value to stockholders, and either outcome could cause our stockholders to lose some or all of their investment in our common stock.
We may be unable to satisfactorily settle our lease obligation or sublease our manufacturing facility.
We lease a 65,000 square-foot facility in South San Francisco, California of which approximately 15,000 square feet is dedicated to biologics manufacturing. The remainder of this facility contains laboratories, office and expansion space. We are the sole occupant. Following the termination of our proposed merger with Raven, we determined that it was not economical to retain the facility and began exploring alternatives, including the sale of the equipment and assignment or sub-lease of the property. To date, we have been unable to assign or sub-let this property. The lease on this property terminates in December 2016, and our remaining lease commitment, as of December 31, 2009, was $17.6 million. Continued failure or inability to assign or reach an agreement to terminate the lease or execute a sublease on terms favorable to us may adversely affect our ability to complete a strategic transaction due to the ongoing lease obligation that an acquirer may be required to assume, or may significantly limit proceeds available for distribution in a liquidation.
We may need to raise additional capital to support our operations and in order to continue as a going concern if we successfully complete a strategic transaction.
We believe that our existing cash, cash equivalents and investment securities as of December 31, 2009 will be sufficient to meet our projected operating requirements through at least December 31, 2010 and we are exploring strategic alternatives. Our restructuring measures implemented to date and any future transactions may disappoint investors and further depress the price of our common stock and the value of an investment in our common stock, thereby limiting our ability to raise additional funds or consummate a strategic transaction.
We will require substantial funds to conduct development activities if we acquire additional products or companies or consummate a strategic transaction. Our ability to conduct the required development activities related to any new product candidates will be significantly limited if we are unable to obtain the necessary capital. We may seek to raise additional funds through the sale of equity or debt to meet our working capital and capital expenditure needs. We do not know, however, whether additional financing will be available when needed, or whether it will be available on favorable terms or at all. Failure to obtain adequate financing also may adversely affect our ability to operate as a going concern.
As a result of the reductions in our workforce that we announced throughout 2007 and 2008, we may not be successful in retaining key employees and in attracting qualified new employees as required in the future. If we are unable to retain our management or to attract additional qualified personnel, our ability to rebuild our business will be seriously jeopardized.
Several times during 2007 and 2008 we implemented restructurings resulting in the reduction of our workforce. As of December 31, 2009, we had only three employees. Competition among biotechnology companies for qualified employees is intense, and the ability to retain and attract qualified individuals will be critical to our success if we rebuild our business. Our ability to recruit new employees may be diminished as a result of the restructurings we have implemented. If we rebuild our business and need to recruit qualified personnel, including scientific staff and scientific advisors, we may be unable to attract or retain key personnel on acceptable terms, if at all.
6
We have only a limited operating history and we expect to continue to generate operating losses.
To date, we have engaged primarily in research, development and clinical testing. Since our inception in 1995, our operations have not been profitable, and we cannot be certain that we will ever achieve or sustain operating profitability. At December 31, 2009, we had an accumulated deficit of $273.4 million. Developing any future product candidates will require significant additional research and development, including non-clinical testing and clinical trials, as well as regulatory approval. We expect these activities, together with our general and administrative expenses, to result in operating losses for the foreseeable future.
Natural disasters, including earthquakes, may damage our facilities.
Our corporate and manufacturing facilities are primarily located in California. Our facilities in California are in close proximity to known earthquake fault zones. As a result, our corporate, research and manufacturing facilities are susceptible to damage from earthquakes and other natural disasters, such as fires, floods and similar events. Although we maintain general business insurance against fires and some general business interruptions, there can be no assurance that the scope or amount of coverage will be adequate in any particular case.
We may become subject to product liability claims, which could result in damages that exceed our insurance coverage.
We face an inherent risk of exposure to product liability suits in connection with product candidates previously tested in human clinical trials. We may become subject to a product liability suit if any product candidate we tested causes injury, or if individuals subsequently become infected or otherwise suffer adverse effects from our product candidates. If a product liability claim is brought against us, the cost of defending the claim could be significant and any adverse determination could result in liabilities in excess of our insurance coverage. We maintained product liability insurance, including clinical trial liability, in the amount of $10.0 million for our programs up until March 2007, when our programs were suspended. We have an extended reporting period for claims that may arise under this coverage. We cannot be certain that additional insurance coverage, if required, could be obtained on acceptable terms, if at all.
We may be subject to claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
As is commonplace in the biotechnology industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We are not currently listed on a national exchange and there can be no assurance we will ever be listed.
As a result of our failure to make timely filings of financial statements, we were delisted from NASDAQ, and our common stock is not currently listed on any national stock exchange. We have completed all delinquent filings with the SEC pursuant to Sections 13 and 15(d) of the Exchange Act, but we have not yet applied for our common stock to be listed on a national exchange. We do not know when, if ever, this will be completed, and thus, whether our common stock will ever be listed. In addition, we cannot be certain that NASDAQ will approve our stock for relisting or that any other exchange will approve our stock for listing. In order to be eligible for relisting or listing, we must meet NASDAQ’s or another exchange’s initial listing criteria, including a minimum per share price. Our common stock is quoted on the OTC Bulletin Board under the symbol VXGN.OB.
Our stockholders could experience substantial dilution as a result of the issuance of additional shares of common or preferred stock.
Our Board of Directors has the authority to establish the designation of almost 20,000,000 shares of preferred stock that are convertible into common stock without any action by our stockholders, and to fix the rights, preferences, privileges and restrictions, including voting rights, of such shares. In February 2006, we raised net proceeds of $25.2 million through a private placement of 3.5 million shares of common stock at $7.70 per share to a group of accredited institutional investors. We also issued to the investors five-year warrants initially exercisable to purchase 698,637 shares of common stock at an exercise price of $9.24 per share. Because we did not file all of our delinquent periodic reports with the SEC by January 31, 2007, the warrants became exercisable for an additional 698,630 shares of common stock, at a price of $9.24 per share. If we complete a strategic transaction, we may raise additional funds through public or private offerings of our preferred stock or our common stock, or through issuance of debt securities that are convertible into shares of our common stock. The issuance of additional shares of our common stock, or conversion of preferred stock or debt securities into shares of common stock, would further dilute the percentage ownership of our stockholders.
7
Shares of our common stock eligible for future sale may adversely affect the market for our common stock.
Shares of our common stock issued in a private placement in February 2006 were registered for resale under a registration statement, which was declared effective by the SEC in February 2008. In addition, pursuant to Rule 144, generally, a non-affiliated stockholder who has satisfied a six-month holding period may, under certain circumstances, sell restricted securities without any limitation. Certain stockholders who purchased shares of our common stock in our November 2004 and February 2006 private placements are eligible to conduct sales under Rule 144. Any substantial sale of our common stock under effective resale registration statement or pursuant to Rule 144 or pursuant to any resale prospectus may have a material adverse effect on the market price of our securities.
Our stock price is likely to be volatile.
Currently, our common stock is quoted on the OTC Bulletin Board. Stocks traded OTC typically are subject to greater volatility than stocks traded on stock exchanges, such as the NASDAQ Global Market or the NASDAQ Capital Market, due to the fact that OTC trading volumes are generally significantly less than those on stock exchanges. This lower volume may allow a relatively few number of stock trades to greatly affect the stock price. The trading price of our common stock has been and is likely to continue to be extremely volatile. For example, between August 9, 2004 and December 31, 2009, the closing price of our common stock has ranged from a high of $18.55 per share to a low of $0.33 per share.
Our stock price could continue to be subject to wide fluctuations in response to a variety of factors, including:
| | • | | timing and consistency of filing financial statements; |
| |
| | • | | changes in financial estimates by securities analysts and our failure to meet or exceed such estimates; |
| |
| | • | | rumors about our business prospects or product development efforts; |
| |
| | • | | issuances of debt or equity securities; |
| |
| | • | | issuances of securities or the expectation of the issuance of securities as part of a merger or other strategic transaction; |
| |
| | • | | actual or expected sales by our stockholders of substantial amounts of our common stock, including shares issued upon exercise of outstanding options and warrants; |
| |
| | • | | developments in or the outcome of litigation against us; and |
| |
| | • | | other events or factors, many of which are beyond our control. |
In addition, the stock market in general and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of actual operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against companies. If we face securities litigation in the future, even if it is without merit or unsuccessful, it would result in substantial costs and a diversion of management attention and resources, which could have a material adverse effect on our business.
We have no history of paying dividends on our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We plan to retain any future earnings to finance our growth. If we decide to pay dividends to the holders of our common stock, such dividends may not be paid on a timely basis.
Our charter documents and Delaware law may discourage an acquisition of VaxGen.
Provisions of our certificate of incorporation, bylaws, and Delaware law could make it more difficult for a third party to acquire us, even if doing so would be beneficial to our stockholders. We may issue shares of preferred stock in the future without stockholder approval and upon such terms as our Board of Directors may determine. Our issuance of this preferred stock could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from acquiring, a majority of our outstanding stock. Our charter and bylaws also provide that special stockholders meetings may be called only by our Chairman of the Board of Directors, by our Secretary at the written request of the Chairman or by our Board of Directors, with the result that any third-party takeover not supported by the Board of Directors could be subject to significant delays and difficulties.
8
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We lease approximately 65,000 square feet of laboratory, office and manufacturing space in South San Francisco, California. Our South San Francisco lease terminates in December 2016; however, we have options to renew it for two additional five-year periods. We are evaluating our future use of these facilities.
Item 3. Legal Proceedings
Beginning on October 23, 2009, several putative stockholder class action lawsuits were filed against the Company, members of its Board of Directors, OXiGENE and OXiGENE Merger Sub, Inc. in the Superior Court of California, County of San Mateo in connection with the proposed merger with OXiGENE. The complaints, styled respectivelyJensen v. Panek et al., Case No. CIV 488075;Ming v. VaxGen, Inc. et al., Case No. CIV 489164; andHawes v. VaxGen, Inc. et al., Case No. CIV 489313, allege, among other things, that the members of the Company’s Board of Directors violated their fiduciary duties by failing to maximize value for the Company’s stockholders when negotiating and entering into the merger agreement with OXiGENE. The complaints also allege that the Company and OXiGENE aided and abetted those purported breaches. The plaintiffs seek, among other things, to enjoin the acquisition of the Company by OXiGENE or, in the alternative, to rescind the acquisition should it occur before the lawsuit is resolved. On February 3, 2010, the Company held a special meeting of stockholders at which the proposed merger failed to receive sufficient votes to be approved. Accordingly, the plaintiffs have informed the Company that they expect to dismiss the action without prejudice in the near future. Since we do not believe that a significant adverse result in this litigation is probable and since the amount of potential damages in the event of an adverse result is not reasonably estimable, no expense has been recorded with respect to the contingent liability associated with this matter.
On or about July 7, 2009, the Company filed an action in California Superior Court for San Mateo County against Firstenberg Machinery Company alleging claims for breach of contract and common count arising out of Firstenberg Machinery Company’s failure to remit to the Company the proceeds from Firstenberg Machinery Company’s sale on consignment of certain equipment, machinery and other property of the Company pursuant to a Sales Representative Agreement between the parties. The complaint seeks compensatory damages of at least $77,800. On November 24, 2009, Firstenberg Machinery Company filed an answer to the complaint, denying the Company’s allegations, and a cross-complaint against the Company, alleging claims for breach of the Sales Representative Agreement, breach of the implied covenant of good faith and fair dealing, unfair business practices, negligent misrepresentation and promissory estoppel. The cross-complaint seeks unspecified damages in excess of $231,000. The Company believes that it has valid defenses to the claims alleged in the cross-complaint and intends to vigorously defend against them.
Item 4. Removed and Reserved
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Price Range of Common Stock
Our common stock is quoted on the OTC Bulletin Board under the symbol VXGN.OB.
The following table lists quarterly information on the high and low closing sales prices for our common stock for the periods indicated below. These prices do not include retail markups, markdowns or commissions.
| | | | | | | | | |
| | | High | | | Low | |
| | | | | | | | | |
| Fiscal 2009: | | | | | | | | |
| Fourth Quarter | | $ | 0.73 | | | $ | 0.44 | |
| Third Quarter | | $ | 0.74 | | | $ | 0.45 | |
| Second Quarter | | $ | 0.52 | | | $ | 0.42 | |
| First Quarter | | $ | 0.49 | | | $ | 0.34 | |
| |
| Fiscal 2008: | | | | | | | | |
| Fourth Quarter | | $ | 0.55 | | | $ | 0.33 | |
| Third Quarter | | $ | 0.72 | | | $ | 0.50 | |
| Second Quarter | | $ | 0.68 | | | $ | 0.43 | |
| First Quarter | | $ | 0.70 | | | $ | 0.42 | |
As of March 25, 2010, there were 350 holders of record of our common stock.
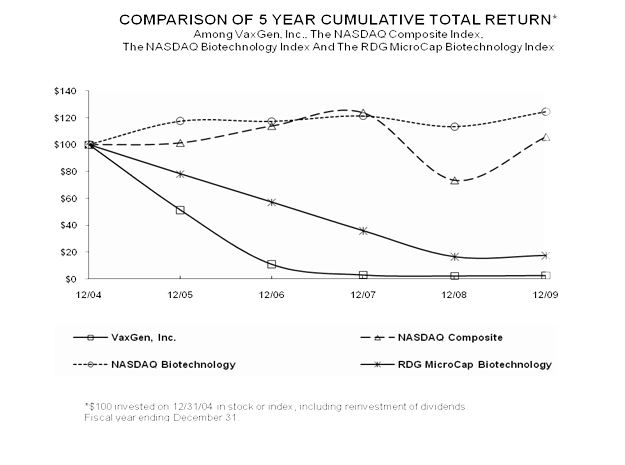
Performance Measurement Comparison (1)
The following graph shows a comparison of total stockholder return for holders of the Common Stock from December 31, 2004 through December 31, 2009 compared with the NASDAQ Composite Index, the NASDAQ Biotechnology Index and the RDG MicroCap Biotechnology Index© . The RDG MicroCap Biotechnology Index© is composed of U.S. publicly traded biotechnology companies with a market capitalization of up to $300 million. This graph is presented pursuant to SEC rules. The Company believes that, while total stockholder return can be an important indicator of corporate performance, the stock prices of biopharmaceutical stocks like that of the Company are subject to a number of market-related factors other than company performance, such as competitive announcements, mergers and acquisitions in the industry, the general state of the economy, and
9
the performance of other biopharmaceutical stocks. As a result of the Company’s inability to timely file financial statements, the Company was delisted effective August 9, 2004 from NASDAQ. Since that time, the Company’s common stock has traded OTC and was quoted on the Pink Sheets under the symbol VXGN.PK until March 12, 2008, when it began to be quoted on the OTC Bulletin Board under the symbol VXGN.OB. All values assume reinvestment of the full amount of all dividends and are calculated as of December 31 of each year:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Cumulative Total Return | |
| | | 12/04 | | | 12/05 | | | 12/06 | | | 12/07 | | | 12/08 | | | 12/09 | |
| VaxGen, Inc. | | | 100.00 | | | | 51.47 | | | | 11.18 | | | | 3.24 | | | | 2.53 | | | | 2.88 | |
| NASDAQ Composite | | | 100.00 | | | | 101.41 | | | | 114.05 | | | | 123.94 | | | | 73.43 | | | | 105.89 | |
| NASDAQ Biotechnology | | | 100.00 | | | | 117.54 | | | | 117.37 | | | | 121.37 | | | | 113.41 | | | | 124.58 | |
| RDG MicroCap Biotechnology | | | 100.00 | | | | 78.32 | | | | 57.31 | | | | 36.04 | | | | 16.68 | | | | 17.65 | |
| | | |
| (1) | | This Section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of the Company under the 1933 Act or the 1934 Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
Dividend Policy
We have not declared or paid any cash dividends on our common stock since inception. We currently intend to retain all of our cash and any future earnings to finance the growth and development of our business and therefore we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant.
Issuer Purchases of Equity Securities
None.
10
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with, and are qualified by reference to, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our Consolidated Financial Statements and the related Notes to those Consolidated Financial Statements, included elsewhere in this Annual Report.
Statement of Operations Data:
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| | | (in thousands, except per share amounts) | |
| | | | | | | | | | | | | | | (A) | | | (B) | |
| Revenues: | | | | | | | | | | | | | | | | | | | | |
| Research contracts and grants | | $ | — | | | $ | — | | | $ | 4,098 | | | $ | 13,205 | | | $ | 29,073 | |
| Other services | | | — | | | | 293 | | | | 913 | | | | — | | | | — | |
| Related party services | | | — | | | | — | | | | — | | | | 1,631 | | | | 866 | |
| | | | | | | | | | | | | | | | |
| Total revenues | | | — | | | | 293 | | | | 5,011 | | | | 14,836 | | | | 29,939 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | — | | | | 1,387 | | | | 19,653 | | | | 49,001 | | | | 64,230 | |
| General and administrative | | | 7,280 | | | | 12,700 | | | | 20,437 | | | | 27,683 | | | | 32,905 | |
| Impairment of/loss on assets held for sale | | | 306 | | | | 8,848 | | | | — | | | | — | | | | — | |
| Impairment of property and equipment | | | — | | | | — | | | | 10,681 | | | | — | | | | — | |
| Restructuring | | | — | | | | 1,314 | | | | 5,374 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| Total operating expenses | | | 7,586 | | | | 24,249 | | | | 56,145 | | | | 76,684 | | | | 97,135 | |
| | | | | | | | | | | | | | | | |
| Loss from operations | | | (7,586 | ) | | | (23,956 | ) | | | (51,134 | ) | | | (61,848 | ) | | | (67,196 | ) |
| | | | | | | | | | | | | | | | |
| Other income (expense) | | | | | | | | | | | | | | | | | | | | |
| Interest expense | | | (132 | ) | | | (1,901 | ) | | | (2,447 | ) | | | (2,470 | ) | | | (2,360 | ) |
| Interest and other income | | | 289 | | | | 1,839 | | | | 4,681 | | | | 2,239 | | | | 967 | |
| Valuation adjustments | | | — | | | | 3,500 | | | | 4,720 | | | | (5,295 | ) | | | (1,129 | ) |
| Realized gain on sale of available for sale investments | | | 357 | | | | — | | | | — | | | | — | | | | — | |
| Gain on convertible debt repurchase | | | | | | | 4,915 | | | | — | | | | — | | | | — | |
| Gain on sale of Anthrax Program | | | — | | | | 3,000 | | | | — | | | | — | | | | — | |
| Equity in loss of affiliate | | | — | | | | — | | | | — | | | | (5,290 | ) | | | (2,370 | ) |
| Gain on foreign currency transactions | | | — | | | | — | | | | — | | | | 7,454 | | | | 825 | |
| Gain on sale of investment in affiliate | | | — | | | | — | | | | — | | | | 104,012 | | | | 11,196 | |
| | | | | | | | | | | | | | | | |
| Total other income (expense), net | | | 514 | | | | 11,353 | | | | 6,954 | | | | 100,650 | | | | 7,129 | |
| | | | | | | | | | | | | | | | |
| Income (loss) before taxes | | | (7,072 | ) | | | (12,603 | ) | | | (44,180 | ) | | | 38,802 | | | | (60,067 | ) |
| Provision for (benefit of) income taxes | | | (864 | ) | | | (40 | ) | | | — | | | | 1,210 | | | | — | |
| | | | | | | | | | | | | | | | |
| Net income (loss) | | | (6,208 | ) | | | (12,563 | ) | | | (44,180 | ) | | | 37,592 | | | | (60,067 | ) |
| Net loss attributable to noncontrolling interests | | | — | | | | — | | | | — | | | | — | | | | 4,109 | |
| | | | | | | | | | | | | | | | |
| Net income (loss) attributable to VaxGen | | $ | (6,208 | ) | | $ | (12,563 | ) | | $ | (44,180 | ) | | $ | 37,592 | | | $ | (55,958 | ) |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) per share, basic and diluted | | $ | (0.19 | ) | | $ | (0.38 | ) | | $ | (1.33 | ) | | $ | 1.15 | | | $ | (1.89 | ) |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Shares used in computing net income (loss) per share: | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 33,107 | | | | 33,107 | | | | 33,107 | | | | 32,723 | | | | 29,599 | |
| | | | | | | | | | | | | | | | |
| Diluted | | | 33,107 | | | | 33,107 | | | | 33,107 | | | | 32,797 | | | | 29,599 | |
| | | | | | | | | | | | | | | | |
11
| | | | | | | | | | | | | | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| | | (in thousands) | |
| | | | | | | | | | | | | | | (A) | | | (B) | |
| | | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents and investment securities | | $ | 32,339 | | | $ | 38,540 | | | $ | 71,615 | | | $ | 97,743 | | | $ | 17,026 | |
| Working capital | | | 32,589 | | | | 39,334 | | | | 65,004 | | | | 89,761 | | | | 1,638 | |
| Property and equipment | | | — | | | | — | | | | 10,806 | | | | 28,417 | | | | 32,275 | |
| Investment in affiliate | | | — | | | | — | | | | — | | | | — | | | | 17,761 | |
| Total assets | | | 35,142 | | | | 42,097 | | | | 89,965 | | | | 142,060 | | | | 81,833 | |
| Derivative liabilities | | | — | | | | — | | | | 3,500 | | | | 8,220 | | | | 2,925 | |
| Convertible senior subordinated notes | | | — | | | | — | | | | 30,679 | | | | 30,321 | | | | 29,965 | |
| Non-current obligations | | | — | | | | — | | | | — | | | | — | | | | — | |
| Non-controlling interest in variable interest entity | | | — | | | | — | | | | — | | | | — | | | | — | |
| Total stockholders’ equity | | | 29,978 | | | | 36,219 | | | | 47,039 | | | | 89,061 | | | | 24,145 | |
| | | |
| (A) | | Reflects adoption of the fair value recognition provisions of ASC 718,Share-Based Payment. Refer to Note 2 in the accompanying Consolidated Financial Statements for further discussion. Also reflects accounting for our investment in Celltrion under the equity method for the six months ended June 30, 2006 and under the cost method for the six months ended December 31, 2006. |
| |
| (B) | | Reflects the consolidation of Celltrion from January 1, 2005 through June 30, 2005 and the deconsolidation of Celltrion effective July 1, 2005. |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
OVERVIEW
VaxGen is a biopharmaceutical company based in South San Francisco, California. The Company owns a state-of-the-art biopharmaceutical manufacturing facility with a 1,000-liter bioreactor that can be used to make cell culture or microbial biologic products. This facility is located within leased premises. The Company has ended all product development activities and sold or otherwise terminated its drug development programs. The Company is seeking to maximize the value of its remaining assets through a strategic transaction or series of strategic transactions.
VaxGen was incorporated on November 27, 1995. From 1995 through 2004, VaxGen was developing vaccine candidates to prevent or reduce HIV infection. These HIV vaccine candidates failed to succeed in two phase three studies, and in 2004 VaxGen licensed its HIV vaccine candidates to Global Solution for Infectious Disease. VaxGen retains certain rights to commercialize these vaccine candidates in the event that they are successfully licensed in the future.
During 2002 through 2006, VaxGen developed vaccines against inhalation anthrax and smallpox for the purpose of biodefense. In December 2006, the Department of Health and Human Services, or HHS, terminated its contract with VaxGen related to the development and delivery of a next-generation anthrax vaccine. Following the HHS decision, VaxGen ceased actively developing its anthrax vaccine, scaled back its biodefense activities and began pursuing strategic and other alternatives.
Through March 31, 2007, VaxGen’s principal source of revenue was the U.S. government, principally the National Institutes of Health, or NIH, and related entities. From April 2007 to April 2008, VaxGen’s principal source of revenue was from services provided to Celltrion, Inc., or Celltrion, a company developing and operating a mammalian cell culture biomanufacturing facility in the Republic of Korea.
We discontinued clinical development of our anthrax vaccine candidate, rPA102, after HHS terminated our SNS Contract in December 2006. In May, 2008, the Company completed the sale of all assets and rights related to its recombinant protective antigen (rPA) anthrax vaccine product candidate and related technology to Emergent BioSolutions, Inc., or Emergent. Under the terms of the transaction, Emergent paid VaxGen $2.0 million upon execution of the definitive agreement, an additional $1.0 million milestone payment during the third quarter of 2008, and may be obligated to pay VaxGen up to an additional $7.0 million in milestone payments, plus specified percentages of future net sales for 12.5 years beginning from the first commercial sale. The Company recorded $3.0 million as other income during the year ended December 31, 2008 for the sale of the Anthrax Program. In December 2009, HHS withdrew the pending Request for Proposal, (RFP) for the procurement of a second generation anthrax vaccine, such as rPA102, delaying indefinitely the potential commercialization and associated payments under this asset sale agreement.
12
In addition, in June 2007 we terminated our contract with the Chemo-Sero-Therapeutic Research Institute of Japan, or Kaketsuken, to develop a smallpox vaccine. We had previously devoted substantially all of our research, development and clinical efforts and financial resources toward the development of rPA102, and we have no product candidates in clinical or preclinical development. In connection with the termination of our clinical development of rPA102, we announced restructuring activities, including significant workforce reductions, and as a result have no remaining internal capability to discover or develop product candidates. We had research and development costs of zero, $1.4 million and $19.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
In November 2007, the Company and two of its wholly-owned subsidiaries entered into an Agreement and Plan of Merger, as amended in December 2007 and February 2008, or Merger Agreement, with Raven biotechnologies, inc., or Raven. Raven was a private, development stage biopharmaceutical company focused on the discovery, development and commercialization of monoclonal antibody-based products for the treatment of cancer.
In connection with the Raven Merger Agreement, the Company also entered into a Bridge Loan with Raven, which provided for the Company to lend Raven up to $6 million in cash in the aggregate, beginning December 1, 2007. In March 2008, due to a lack of stockholder support for the transaction, VaxGen and Raven agreed to terminate the Merger Agreement and amend the terms of VaxGen’s Bridge Loan to Raven. The balance on the Bridge Loan was paid in full in 2008. The Company recorded in general and administrative expenses, $2.3 million of costs, primarily professional fees related to the proposed merger with Raven during the year ended December 31, 2008.
In April 2008, the Company announced that it was restructuring to reduce operating expenses following the termination of the proposed merger with Raven by decreasing its workforce of twenty-two employees by approximately 75 percent. During August 2008, the Company further reduced its remaining workforce by 25 percent. The Company incurred restructuring costs of approximately $1.3 million for one-time termination costs during the year ended December 31, 2008. (See Note 4 to the financial statements included herein.)
In October 2009, the Company and OXiGENE, Inc., or OXiGENE, entered into a definitive merger agreement pursuant to which a wholly-owned subsidiary of OXiGENE would merge with VaxGen, such that VaxGen would become a wholly-owned subsidiary of OXiGENE, and in exchange VaxGen’s stockholders would receive common stock of OXiGENE. OXiGENE is a publicly-traded development-stage biopharmaceutical company. On February 3, 2010, the Company’s stockholders failed to approve the merger. VaxGen recorded $1.2 million of costs, primarily professional fees, related to the proposed merger with OXiGENE during the year ended December 31, 2009.
As a result of the termination of the proposed merger with OXiGENE, the Company is considering various alternate strategic transactions to return value to its stockholders. If the Company is unable to identify and complete an alternate strategic transaction, the Company will liquidate. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Celltrion
Celltrion is an FDA licensed biologics manufacturing company incorporated on February 26, 2002. Since that date, its principal activities have consisted of design, construction and operation of a manufacturing facility in Incheon, Republic of Korea, and partially funding the construction of our U.S. biopharmaceutical manufacturing facility, as well as raising capital and recruiting scientific and management personnel.
As a part of the initial capitalization of Celltrion and as part of the Celltrion joint venture agreement we signed with various Korean entities, including Nexol Co., Ltd., or Nexol, we made an in-kind contribution to Celltrion of the license and sub-license of certain cell-culture technology used for the manufacture of pharmaceutical products. We received 7.8 million shares of Celltrion’s common stock for this contribution, representing approximately 48% of the then-outstanding shares. In September 2005, we entered into agreements to sell 1.2 million of our shares in Celltrion to a group of Korean investors and raised $15.1 million in gross proceeds. Nexol purchased 250,000 of these shares.
During June and December 2006, we received aggregate gross proceeds of $130.3 million from the sale of substantially all of our Celltrion common stock to Nexol and affiliates of Nexol. As a result, we were no longer entitled to hold two seats on Celltrion’s Board of Directors or appoint a Representative Director. Accordingly, we no longer had the ability to exercise significant influence over operating and financial policies of Celltrion, and beginning July 1, 2006, we accounted for our investment in Celltrion under the cost method. At December 31, 2007, we held a nominal ownership interest in Celltrion. During 2008, a public market developed for Celltrion common stock in the Republic of Korea. During 2009 we sold our remaining investment in Celltrion common stock. This transaction resulted in a realized gain of $357,000.
Significant Debt Transactions
In April 2005, we raised aggregate net proceeds of $29.7 million through a private placement of $31.5 million of Notes, due April 1, 2010. During 2008 we repurchased the $31.5 million of Notes for $25.4 million resulting in a gain from Note repurchase of $4.9 million, net of $0.6 million of unamortized discount and $0.6 million of deferred financing costs.
13
Significant Equity Transaction
In February 2006, we raised net proceeds of $25.2 million through a private placement of 3.5 million shares of common stock at $7.70 per share to a group of accredited institutional investors. We also issued to the investors five-year warrants initially exercisable to purchase 698,637 shares of common stock at an exercise price of $9.24 per share. Because we did not file all of our delinquent periodic reports with the SEC by January 31, 2007, the warrants became exercisable for an additional 698,630 shares of common stock, at a price of $9.24 per share.
Subsequent Events
In October 2009, VaxGen and OXiGENE entered into a definitive merger agreement pursuant to which a wholly-owned subsidiary of OXiGENE would merge with VaxGen, such that VaxGen would become a wholly-owned subsidiary of OXIGENE, and in exchange VaxGen’s stockholders would receive common stock of OXiGENE. On February 3, 2010, the Company’s stockholders failed to approve the merger.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our significant accounting policies are described in Note 2 to the Consolidated Financial Statements included herein. Our discussion and analysis of our operating results and financial condition are based upon our Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of the financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances; we review our estimates on an ongoing basis. While we believe our estimates, judgments and assumptions are reasonable, the inherent nature of estimates is that actual results will likely be different from the estimates made. We believe that our critical accounting estimates have the following attributes: (1) we are required to make judgments and assumptions about matters that are uncertain at the time of the estimate; (2) our use of reasonably different assumptions would change our estimates and (3) changes in estimates could have a material effect on our financial condition or results of operations. Our estimates, particularly estimates of the fair value of the embedded derivatives associated with our Notes have changed significantly from period to period. We expect that our estimates will continue to fluctuate in future periods. Application of the following critical accounting policies and estimates requires us to exercise judgments that affect our financial statements.
Revenue Recognition
Substantially all of our revenues to date relate to cost-plus-fixed-fee contractual arrangements with agencies of the U.S. government. Revenue is recognized as work is performed, based on allowable actual costs incurred. We generally issue invoices on a monthly basis. Under cost-plus-fixed-fee contracts, we are reimbursed for allowable costs and receive a fixed fee, which is negotiated and specified in the contract. Revenues for the fixed fee portion are recognized when milestones are achieved and accepted by the customer. Contract costs include direct and indirect research and development costs and allowable indirect general and administrative expenses.
U.S. government contracts and subcontracts are subject to annual audit, various profit and cost controls and standard provisions for termination at the convenience of the U.S. government. In April 2007, our direct and indirect contract costs were settled with the U.S. government and NIAID agreed to pay us $11.0 million.
For non-government arrangements, the Company recognizes revenues in accordance with FASB Accounting Standards Codification (“ASC”), ASC 605,Revenue Recognition, as amended and interpreted. In such instances, revenues are recognized when there is persuasive evidence of an arrangement, delivery has occurred or services have been performed, the selling price to the buyer is fixed or determinable and collectability is reasonably assured.
Property and Equipment
We estimate that the undiscounted future cash flows expected to result from the use of property and equipment compare it to the current carrying value of these assets. Any adverse change in the estimate of these undiscounted future cash flows could necessitate an impairment charge that would adversely affect operating results if the fair market value of the assets is less than the net book value. We estimate useful lives for our assets based on historical experience, estimates of assets’ commercial lives, and the likelihood of technological obsolescence. Should the actual useful life of a class of assets differ from the estimated useful life, we would record an impairment charge. We review useful lives and obsolescence and we assess commercial viability of these assets periodically.
For the year ended December 31, 2007, we recorded an impairment charge of $10.7 million triggered by the Merger Agreement with Raven signed in November 2007 as well as an October 2007 lease amendment. The impairment recognized the write-down of the manufacturing facility, equipment, certain software and leasehold improvements. The Company estimated the fair market value of the impaired assets related to the Merger Agreement based on estimated realizable value upon sale of the facility determined by discussions with sales agents and efforts to date in the marketing of the facility; discussions with resellers relating to the manufacturing equipment.
14
During 2008 the Company committed to a plan to sell the equipment and leasehold improvements related to its California manufacturing facility. These assets met the criteria for, and have been classified as “held for sale” in accordance with ASC 360-10-35-15,Impairment of Long-Lived Assets.
Assets Held for Sale
We use the criteria in ASC 360-10-35-15,Impairment of Long-Lived Assets, to determine when an asset is classified as “held for sale.” Upon classification as “held for sale,” the long-lived asset or asset group is measured at the lower of its carrying amount or fair value less cost to sell, depreciation is ceased and the asset or asset group is separately presented on the Consolidated Balance Sheets. When an asset or asset group meets the ASC 360-10-35-15 criteria for classification as “held for sale” within the Consolidated Balance Sheets, we do not retrospectively adjust prior period balance sheets to conform to current year presentation.
During 2008, we committed to a plan to sell our equipment and leasehold improvements related to our California manufacturing facility. These assets met the criteria for, and were classified as “held for sale” in accordance with ASC 360-10-35-15.
Valuation of Derivative Instruments
We valued certain embedded features issued in connection with the financing of our Notes in 2005 as a derivative liability. This methodology allows flexibility in incorporating various assumptions such as probabilities of certain triggering events. The valuations are based on the information available as of the various valuation dates. Factors affecting the amount of this liability include the market value of our common stock, the estimated volatility of our common stock, our market capitalization, the risk-free interest rate and other assumptions such as the probability of a change in control event. Of these valuation parameters, management’s assessment of the probability of a change in control is the most subjective and also has the greatest influence on fair value. At December 31, 2008, due to our repurchase of the entire $31.5 million of Notes, the derivative liability was reduced to zero.
Stock-Based Compensation Expense
We account for stock-based compensation using the fair value recognition provisions of ASC 718,Share-Based Payment,which was adopted using the modified-prospective transition method effective January 1, 2006. Determining the appropriate fair value model and calculating the fair value of stock-based payment awards require the input of various highly-subjective assumptions, including the expected life of the stock-based payment awards, our stock price volatility and the expected forfeiture rate of our options. Management determined the expected stock price volatility assumption based upon our historical volatility. We believe this method of computing volatility is more reflective and a better indicator of the expected future volatility, than using an average of a comparable market index or of a comparable company in the same industry. The expected term of options granted was derived from the short-cut method. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The assumptions used in calculating the fair value of stock-based payment awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future. In addition, we are required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. If our actual forfeiture rate is materially different from our estimate, the stock-based compensation expense could be significantly different from what we have recorded in the current period. See Notes 2 and 10 to the Consolidated Financial Statements for more information regarding stock-based compensation.
Income Taxes
Effective January 1, 2007, we adopted the provisions of the ASC 740,Accounting for Uncertainty in Income Taxes.See Note 11 to the Consolidated Financial Statements for more information regarding our income tax policies. We have filed tax returns with positions that may be challenged by the tax authorities. These positions relate to, among others, deductibility of certain expenses, expenses included in our research and development tax credit computations, as well as other matters. Although the outcome of tax audit is uncertain, in management’s opinion, adequate provisions for income taxes have been made for potential liabilities resulting from such matters. We regularly assess the tax positions for such matters and include reserves for those differences in position. The reserves are utilized or reversed once the statute of limitations has expired and/or at the conclusion of the tax examination. We believe that the ultimate outcome of these matters will not have a material impact on our financial position, financial operations or liquidity.
15
RESULTS OF OPERATIONS
Comparison of Years Ended December 31, 2009, 2008 and 2007
Revenue
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Research contracts and grants | | $ | — | | | $ | — | | | $ | 4,098 | | | | * | | | | -100 | % |
| Other services | | | — | | | | 293 | | | | 913 | | | | -100 | % | | | -68 | % |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Total revenues | | $ | — | | | $ | 293 | | | $ | 5,011 | | | | -100 | % | | | -94 | % |
| | | | | | | | | | | | | | | | | | |
| | | |
| * | | Calculation not meaningful |
Revenues for 2008 were from service revenues earned as part of a consulting services agreement with Celltrion to provide technical assistance related to the design, engineering and start-up of Celltrion’s manufacturing facility. Revenue for 2007 was primarily driven by expensed activity reimbursed by the U.S. government. Research contracts and grants revenue in 2007 primarily related to the reimbursement of restructuring costs resulting from the termination of our SNS Contract in December 2006. Research contracts and grants revenue in 2006 primarily related to work performed under our Anthrax contracts.
Revenues earned in one period are not indicative of revenues to be earned in future periods. We do not expect any revenues in 2010.
Research and development expenses
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Research and development expenses | | $ | — | | | $ | 1,387 | | | $ | 19,653 | | | | -100 | % | | | -93 | % |
Research expenses include costs associated with research and testing of our product candidates prior to reaching the development stage and include the costs of internal personnel, outside contractors, allocated overhead and laboratory supplies. Product development expenses include costs of preclinical development and conducting clinical trials, costs of internal personnel, drug supply costs, research fees charged by outside contractors, allocated overhead and co-development costs. Since inception, our research and development activities have been concentrated upon the development, manufacture and commercialization of biologic products for the prevention and treatment of human infectious disease. In 2008 and 2007, these costs primarily related to maintaining our manufacturing facility and prior research findings in support of our evaluation of various strategic transactions.
We ceased research and development activities during the first quarter of 2008 and therefore no research and development expenses were incurred during 2009.
The decrease in research and development expenses in 2008 compared to 2007 was primarily due to:
| | • | | Labor and related expenses, which decreased by $5.8 million primarily due to no research and development expenses incurred after the first quarter of 2008 and decreased headcount following multiple reductions in force during 2007 and 2008; and |
| |
| | • | | Facilities and overhead costs, which decreased by $12.4 million due to reduced operations and consolidation of facilities during the first quarter of 2008 and no research and development expenses incurred after the first quarter of 2008. |
We expect to incur no research and development expenses during 2010.
General and administrative expenses
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| General and administrative expenses | | $ | 7,280 | | | $ | 12,700 | | | $ | 20,437 | | | | -43 | % | | | -38 | % |
General and administrative expenses consist primarily of compensation costs, occupancy costs including depreciation expense, fees for accounting, legal and other professional services and other general corporate expenses.
16
The decrease in general and administrative expenses of $5.4 million for 2009 over 2008 was primarily due to lower labor and benefit costs from reduced headcount in 2009 resulting from the 2008 reductions in force ($2.2 million) and lower outside labor costs ($2.3 million). The 2008 outside labor expenses included costs incurred in connection with the proposed merger with Raven which was partially offset by $1.2 million of outside labor expenses incurred in connection with the proposed merger with OXiGENE.
The decrease in general and administrative expenses in 2008 compared to 2007 was primarily due to:
| | • | | Labor and benefits, which decreased by $2.4 million primarily associated with the 2007 and 2008 reductions in force; and |
| |
| | • | | Consultant and outside labor costs, which decreased by $4.5 million due to non-recurring costs incurred during 2007 to file multiple SEC filings. |
We expect quarterly general and administrative expenses to decrease during 2010 due to the termination of our proposed merger agreement with OxiGene.
Impairment of/loss on Assets Held for Sale
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Impairment of/loss on assets held for sale | | $ | 306 | | | $ | 8,848 | | | $ | — | | | | -97 | % | | | * | |
| | | |
| * | | Calculation not meaningful |
Based on the lack of success in finding a buyer for our facility and the expectation of a need to dismantle to sell, we performed impairment assessments of our manufacturing facilities at 349 Oyster Point Blvd., South San Francisco, during 2008. Based on these assessments, we estimated that the fair market values of these assets were less than the carrying values of these assets by $8.8 million, which was recorded as an impairment of assets held for sale in the Statement of Operations for the year ended December 31, 2008. The impairment includes all leasehold improvements relating to the facility of approximately $6.5 million, as these items are not expected to have any future economic benefit to us. We used the market approach to determine fair market value of its assets held for sale.
During the year, the Company sold assets held for sale of $131,000, recorded impairment charges of/loss on assets held for sale of $306,000, resulting in assets held for sale of $356,000 at December 31, 2009.
Impairment of Property and Equipment
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Impairment of property and equipment | | $ | — | | | $ | — | | | $ | 10,681 | | | | * | | | | -100 | % |
| | | |
| * | | Calculation not meaningful |
We record impairments of property and equipment when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. The October 2007 amendment to our facility lease agreement and the November 2007 announcement of a proposed merger with Raven acted as triggers for an assessment of an impairment of our equipment and leasehold improvements in our California manufacturing and office facilities, and based upon the impairment test performed, we noted a decrease in the associated estimated future undiscounted cash flows. At December 31, 2007, we estimated that the fair market value of these assets was less than the current carrying value of these assets by $10.7 million.
Restructuring
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Restructuring expense | | $ | — | | | $ | 1,314 | | | $ | 5,374 | | | | -100 | % | | | -76 | % |
17
During 2008 we reduced our workforce to reduce operating costs. We incurred restructuring costs associated with this plan of $1.3 million for employee termination benefits. In January 2007, we restructured operations to significantly reduce operating costs. We incurred restructuring costs associated with this plan of $2.6 million for employee termination benefits, $0.1 million related to the acceleration of stock options and $1.0 million of costs associated with the consolidation of our facilities in California. The majority of these costs were recovered from the U.S. government as part of the April 2007 settlement agreement. In May and September 2007, we further reduced our workforce to decrease operating costs. Restructuring costs of $1.7 million relating to the May and September 2007 workforce reductions included employee termination and benefit costs.
Other income (expense)
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Interest expense | | $ | (132 | ) | | $ | (1,901 | ) | | $ | (2,447 | ) | | | -93 | % | | | -22 | % |
| Interest income | | | 242 | | | | 1,815 | | | | 4,595 | | | | -87 | % | | | -61 | % |
| Valuation adjustments | | | — | | | | 3,500 | | | | 4,720 | | | | -100 | % | | | -26 | % |
| Realized gain on sale of available for sale investments | | | 357 | | | | — | | | | — | | | | * | | | | * | |
| Gain on convertible debt repurchase | | | — | | | | 4,915 | | | | — | | | | -100 | % | | | * | |
| Gain on sale of Anthrax Program | | | — | | | | 3,000 | | | | — | | | | -100 | % | | | * | |
| Other | | | 47 | | | | 24 | | | | 86 | | | | 96 | % | | | -72 | % |
| | | | | | | | | | | | | | | | | | |
| Other income, net | | $ | 514 | | | $ | 11,353 | | | $ | 6,954 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | |
| * | | Calculation not meaningful |
The decrease in other income in 2009 from 2008 was primarily due to:
| | • | | Gain on the sale of our Anthrax Program of $3.0 million in 2008; |
| |
| | • | | Gain of $4.9 million on the repurchase of $31.5 million principal amount of our Notes at a discount in 2008; |
| |
| | • | | Valuation gain of $3.5 million in 2008 reflects the decrease in the fair value on mark-to-market adjustments related to the valuation of our outstanding derivatives on our 5 1/2% Convertible Senior Subordinated Notes, due April 1, 2010, or Notes, due to the repurchase of $31.5 million principal amount of the Notes; |
| |
| | • | | Decreased interest income due to lower interest rates and lower overall cash, cash equivalents and investment balances primarily due to our repurchase of Convertible Notes during 2008; and |
| |
| | • | | A partial offset of decreased in interest expense in 2009, following the repurchase of our Convertible Notes, during 2008. |
The increase in other income in 2008 from 2007 was primarily due to:
| | • | | Valuation gain of $3.5 million in 2008 reflecting the decrease in the fair value on mark-to-market adjustments related to the repurchase of $31.5 million principal amount of the Notes; |
| |
| | • | | Gain of $4.9 million on the repurchase of $31.5 million principal amount of our Notes at a discount; and |
| |
| | • | | Gain on the sale of our Anthrax Program of $3.0 million; |
We anticipate future investment income will fluctuate and will be primarily driven by our future cash, cash equivalent and investment balances.
Income taxes
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Annual Percent Change | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009/2008 | | | 2008/2007 | |
| | | (in thousands) | | | | | | | |
| Income tax benefit | | $ | (864 | ) | | $ | (40 | ) | | $ | — | | | | 2060 | % | | | * | |
18
| | | |
| * | | Calculation not meaningful |
We had an $864,000 income tax benefit for 2009 compared to an income tax benefit of $40,000 in 2008. The $864,000 income tax benefit was due to the Company carrying back certain net operating losses due to a one-time tax law change in the fourth quarter of 2009 and the release of a reserve for an uncertain tax position. We did not have income tax expense for 2009 due to the Company’s net operating loss carryforward position.
LIQUIDITY AND CAPITAL RESOURCES
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | (in thousands) | |
As of December 31: | | | | | | | | | | | | |
| Cash, cash equivalents and investment securities | | $ | 32,339 | | | $ | 38,540 | | | $ | 71,615 | |
| Working capital | | | 32,589 | | | | 39,334 | | | | 65,004 | |
| | | | | | | | | | | | | |
Year ended December 31: | | | | | | | | | | | | |
| Cash provided by (used in) | | | | | | | | | | | | |
| Operating activities | | $ | (6,465 | ) | | $ | (10,038 | ) | | $ | (25,166 | ) |
| Investing activities | | | 1,195 | | | | 5,330 | | | | 16,817 | |
| Financing activities | | | — | | | | (25,400 | ) | | | — | |
Our primary capital requirements for the year ended December 31, 2009 were the result of operating losses and expenses incurred for the proposed OxiGene merger and related strategic asset disposals. Through December 31, 2009, we financed our operations primarily through sales of our common stock, the issuance of Series A Preferred Stock, the issuance of convertible debt, sales of our Celltrion common stock as well as through revenues from research contracts and grants. Our future capital requirements will depend upon our ability to identify and exploit business development opportunities including actively pursuing avenues to enhance stockholder value through a strategic transaction.
Cash Flows from Operating Activities
Cash used by operating activities was $6.5 million during 2009, primarily due to net loss of $6.2 million offset by a decrease of prepaids and other current assets of $0.6 million.
Cash used by operating activities was $10.0 million during 2008, primarily due to net loss of $12.6 million and non-cash items such as valuation adjustments of $3.5 million and gain on convertible debt repurchase of $4.9 million, partially offset by impairment of assets held for sale of $8.8 million.
Cash used by operating activities was $25.2 million during 2007, primarily due to net loss of $44.2 million and non-cash items such as valuation adjustments of $4.7 million, partially offset by depreciation of $6.8 million, decreased receivables of $7.7 million due to the timing billings sent to the U.S. government and impairment of property and equipment of $10.7 million.
Cash Flows from Investing Activities
Cash provided by investing activities was $1.2 million in 2009, principally reflecting the net proceeds of investments of $0.7 million and proceeds from the sale of Celltrion common stock of $0.4 million.
Cash provided by investing activities was $5.3 million in 2008, principally reflecting the net proceeds of investments of $3.4 million and net loan repayments from Raven of $1.3 million.
Cash provided by investing activities was $16.8 million in 2007 consisted primarily of the net of activities relating to the purchase and sale of investment securities of $19.0 million partially offset by expenses related to our proposed merger with Raven of $1.8 million.
Cash Flows from Financing Activities
Cash used by financing activities was $25.4 million in 2008 reflecting the repurchase of $31.5 million principal amount of Notes.
Capital Expenditures
Capital expenditures for 2007 consisted of a nominal amount of equipment associated with maintaining our California facilities.
Capital expenditures are not expected to be significant in 2010.
Adequacy of cash resources to meet future needs
19
We believe that our existing cash, cash equivalents and investment securities will be sufficient to cover our working capital needs and commitments through at least December 31, 2010. Our future capital requirements will depend on our ability to identify and complete additional business opportunities. We are considering various strategic transactions to return value to our stockholders. If we are unable to identify and complete an alternate strategic transaction, we will liquidate.
Contractual Obligations
The following summarizes our contractual obligations as of December 31, 2009 for minimum lease payments related to facility leases and equipment under non-cancelable operation leases (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Less than | | | 1-3 | | | 3-5 | | | More than | |
| | | Total | | | 1 year | | | Years | | | Years | | | 5 years | |
| Operating Lease Obligations | | $ | 17,756 | | | $ | 2,474 | | | $ | 4,800 | | | $ | 5,086 | | | $ | 5,396 | |
At December 31, 2009, the Company had a liability for unrecognized tax benefits totaling $1.4 million. Due to the uncertainties related to these tax matters, we are unable to reasonably estimate when a cash settlement with a taxing authority will occur.
Off-Balance Sheet Arrangements
As of December 31, 2009, we had no off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Recent Accounting Pronouncements
Adopted
On July 1, 2009, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles,” also known as FASB Accounting Standards Codification (“ASC”) 105-10, “Generally Accepted Accounting Principles” (“ASC 105-10”) (the “Codification”). ASC 105-10 establishes the exclusive authoritative reference for U.S. GAAP for use in financial statements, except for SEC rules and interpretive releases, which are also authoritative GAAP for SEC registrants. The Codification will supersede all existing non- SEC accounting and reporting standards. We have included the references to the Codification, as appropriate, in these Consolidated Financial Statements.
Issued but not yet adopted
In October 2009, the FASB issued Accounting Standards Update, 2009-13, Revenue Recognition (Topic 605):Multiple Deliverable Revenue Arrangements — A Consensus of the FASB Emerging Issues Task Force.”This update provides application guidance on whether multiple deliverables exist, how the deliverables should be separated and how the consideration should be allocated to one or more units of accounting. This update establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence, if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific or third-party evidence is available. The Company will be required to apply this guidance prospectively for revenue arrangements entered into or materially modified after January 1, 2011; however, earlier application is permitted. The Company has not determined the impact that this update may have on its financial statements.
In June 2009, the FASB issued guidance related to accounting for transfers of financial assets. This guidance improves the information that a reporting entity provides in its financial reports about a transfer of financial assets; the effects of a transfer on its financial position, financial performance and cash flows; and a continuing interest in transferred financial assets. In addition, this guidance amends various ASC concepts with respect to accounting for transfers and servicing of financial assets and extinguishments of liabilities, including removing the concept of qualified special purpose entities. This guidance must be applied to transfers occurring on or after the effective date. The Company will adopt this guidance in its first annual and interim reporting periods beginning after November 15, 2009. The Company has not determined the impact that this guidance may have on its financial statements.
In June 2009, the FASB issued guidance which amends certain ASC concepts related to consolidation of variable interest entities. Among other accounting and disclosure requirements, this guidance replaces the quantitative-based risks and rewards calculation for determining which enterprise has a controlling financial interest in a variable interest entity with an approach focused on identifying which enterprise has the power to direct the activities of a variable interest entity and the obligation to absorb losses of the entity or the right to receive benefits from the entity. The Company will adopt this guidance in its first annual and interim reporting periods beginning after November 15, 2009. The Company has not determined the impact that this guidance may have on its financial statements.
20
In January 2010, the FASB issued updated guidance related to fair value measurements and disclosures, which requires a reporting entity to disclose separately the amounts of significant transfers in and out of Level 1and Level 2 fair value measurements and to describe the reasons for the transfers. In addition, in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3, a reporting entity should disclose separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than one net number). The updated guidance also requires that an entity should provide fair value measurement disclosures for each class of assets and liabilities and disclosures about the valuation techniques and inputs used to measure fair value for both recurring and non-recurring fair value measurements for Level 2 and Level 3 fair value measurements. The updated guidance is effective for interim or annual financial reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward activity in Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010 and for interim periods within those fiscal years. We do not expect adoption of the updated guidance to have a material impact on our consolidated results of operations or financial condition.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not applicable because we are a smaller reporting company.
Item 8. Financial Statements and Supplementary Data
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To Board of Directors and Stockholders
of VaxGen, Inc.
In our opinion, the accompanying Consolidated Financial Statements listed in the index appearing under Item 15(a)(1), present fairly, in all material respects, the financial position of VaxGen, Inc. and its subsidiaries (the “Company”) at December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009 are in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related Consolidated Financial Statements. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 1, the Company’s management is considering various alternative strategic transactions to return value to its shareholders, which if unsuccessful will result in the liquidation of the Company. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
San Jose, California
March 29, 2010
21
VaxGen, Inc.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | | | | | | | |
ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 29,348 | | | $ | 34,618 | |
| Investment securities | | | 2,991 | | | | 3,922 | |
| Prepaid expenses and other current assets | | | 681 | | | | 792 | |
| Assets held for sale | | | 356 | | | | 783 | |
| | | | | | | |
| Total current assets | | | 33,376 | | | | 40,115 | |
| Restricted cash | | | 1,400 | | | | 1,556 | |
| Other assets | | | 366 | | | | 426 | |
| | | | | | | |
| Total assets | | $ | 35,142 | | | $ | 42,097 | |
| | | | | | | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 100 | | | $ | 75 | |
| Accrued and other current liabilities | | | 687 | | | | 706 | |
| | | | | | | |
| Total current liabilities | | | 787 | | | | 781 | |
| Deferred rent and other liabilities | | | 4,377 | | | | 5,097 | |
| | | | | | | |
| Total liabilities | | | 5,164 | | | | 5,878 | |
| | | | | | | |
| | | | | | | | | |
| Commitments and Contingencies (Note 12) | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $0.01 par value, 19,979,500 shares authorized; none issued or outstanding | | | — | | | | — | |
| Common stock, $0.01 par value, 65,000,000 shares authorized; 33,106,523 shares issued and outstanding at December 31, 2009 and 2008 | | | 331 | | | | 331 | |
| Additional paid-in capital | | | 303,095 | | | | 302,856 | |
| Accumulated deficit | | | (273,449 | ) | | | (267,241 | ) |
| Accumulated other comprehensive income | | | 1 | | | | 273 | |
| | | | | | | |
| Total stockholders’ equity | | | 29,978 | | | | 36,219 | |
| | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 35,142 | | | $ | 42,097 | |
| | | | | | | |
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
22
VaxGen, Inc.
CONSOLIDATED STATEMENT OF OPERATION
(in thousands, except per share data)
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | |
| Research contracts and grants | | $ | — | | | $ | — | | | $ | 4,098 | |
| Other services | | | — | | | | 293 | | | | 913 | |
| | | | | | | | | | |
| Total revenues | | | — | | | | 293 | | | | 5,011 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Research and development | | | — | | | | 1,387 | | | | 19,653 | |
| General and administrative | | | 7,280 | | | | 12,700 | | | | 20,437 | |
| Impairment of/loss on assets held for sale | | | 306 | | | | 8,848 | | | | — | |
| Impairment of property and equipment | | | — | | | | — | | | | 10,681 | |
| Restructuring | | | — | | | | 1,314 | | | | 5,374 | |
| | | | | | | | | | |
| Total operating expenses | | | 7,586 | | | | 24,249 | | | | 56,145 | |
| | | | | | | | | | |
| Loss from operations | | | (7,586 | ) | | | (23,956 | ) | | | (51,134 | ) |
| | | | | | | | | | |
| Other income (expense) | | | | | | | | | | | | |
| Interest expense | | | (132 | ) | | | (1,901 | ) | | | (2,447 | ) |
| Interest income | | | 242 | | | | 1,815 | | | | 4,595 | |
| Valuation adjustments | | | — | | | | 3,500 | | | | 4,720 | |
| Realized gain on sale of available for sale investments | | | 357 | | | | — | | | | — | |
| Gain on convertible debt repurchase | | | — | | | | 4,915 | | | | — | |
| Gain on sale of Anthrax Program | | | — | | | | 3,000 | | | | — | |
| Other | | | 47 | | | | 24 | | | | 86 | |
| | | | | | | | | | |
| Total other income, net | | | 514 | | | | 11,353 | | | | 6,954 | |
| | | | | | | | | | |
| Loss before taxes | | | (7,072 | ) | | | (12,603 | ) | | | (44,180 | ) |
| Income tax benefit | | | (864 | ) | | | (40 | ) | | | — | |
| | | | | | | | | | |
| Net loss | | $ | (6,208 | ) | | $ | (12,563 | ) | | $ | (44,180 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Net loss per share, basic and diluted | | $ | (0.19 | ) | | $ | (0.38 | ) | | $ | (1.33 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Shares used in computing net loss per share: | | | | | | | | | | | | |
| Basic | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | |
| Diluted | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | |
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
23
VaxGen, Inc.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE LOSS
(in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | | | | | | | | | Additional | | | | | | | Other | | | Total | |
| | | Common Stock | | | Paid-in | | | Accumulated | | | Comprehensive | | | Stockholders’ | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Income | | | Equity | |
| Balance at December 31, 2006 | | | 33,106,523 | | | $ | 331 | | | $ | 299,226 | | | $ | (210,498 | ) | | $ | 2 | | | $ | 89,061 | |
| | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (44,180 | ) | | | — | | | | (44,180 | ) |
| Change in net unrealized gain on securities | | | — | | | | — | | | | — | | | | — | | | | 6 | | | | 6 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (44,174 | ) |
| Stock-based compensation | | | — | | | | — | | | | 2,152 | | | | — | | | | — | | | | 2,152 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2007 | | | 33,106,523 | | | | 331 | | | | 301,378 | | | | (254,678 | ) | | | 8 | | | | 47,039 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (12,563 | ) | | | — | | | | (12,563 | ) |
| Change in net unrealized gain on securities | | | — | | | | — | | | | — | | | | — | | | | 265 | | | | 265 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (12,298 | ) |
| Stock-based compensation | | | — | | | | — | | | | 1,478 | | | | — | | | | — | | | | 1,478 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2008 | | | 33,106,523 | | | | 331 | | | | 302,856 | | | | (267,241 | ) | | | 273 | | | | 36,219 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (6,208 | ) | | | — | | | | (6,208 | ) |
| Change in net unrealized gain on securities | | | — | | | | — | | | | — | | | | — | | | | (272 | ) | | | (272 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (6,480 | ) |
| Stock-based compensation | | | — | | | | — | | | | 239 | | | | — | | | | — | | | | 239 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2009 | | | 33,106,523 | | | $ | 331 | | | $ | 303,095 | | | $ | (273,449 | ) | | $ | 1 | | | $ | 29,978 | |
| | | | | | | | | | | | | | | | | | | |
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
24
VaxGen, Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | | | | |
| Cash flows from operating activities: | | | | | | | | | | | | |
| Net loss | | $ | (6,208 | ) | | $ | (12,563 | ) | | $ | (44,180 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | — | | | | 587 | | | | 6,757 | |
| Impairment of/loss on assets held for sale | | | 306 | | | | 8,848 | | | | — | |
| Impairment of property and equipment | | | — | | | | — | | | | 10,681 | |
| Valuation adjustments | | | — | | | | (3,500 | ) | | | (4,720 | ) |
| Gain on sale of Celltrion common stock | | | (357 | ) | | | — | | | | — | |
| Gain on convertible debt repurchase | | | — | | | | (4,915 | ) | | | — | |
| Stock-based compensation | | | 239 | | | | 1,478 | | | | 2,152 | |
| Amortization of premiums and discounts on investment securities | | | — | | | | (178 | ) | | | (1,210 | ) |
| Non-cash interest expense | | | 132 | | | | 904 | | | | 713 | |
| Raven merger costs previously capitalized | | | — | | | | 1,932 | | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts Receivable | | | — | | | | 199 | | | | 7,708 | |
| Prepaid expenses and other current assets | | | 112 | | | | 374 | | | | 1,417 | |
| Reserve on other assets | | | 97 | | | | — | | | | — | |
| Accounts payable | | | 25 | | | | (2,076 | ) | | | (3,932 | ) |
| Accrued and other current liabilities | | | (19 | ) | | | (1,620 | ) | | | (692 | ) |
| Other | | | (792 | ) | | | 492 | | | | 140 | |
| | | | | | | | | | |
| Net cash used in operating activities | | | (6,465 | ) | | | (10,038 | ) | | | (25,166 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Purchase of property and equipment | | | — | | | | — | | | | (19 | ) |
| Proceeds (fees) from sale of investment in affiliate | | | — | | | | — | | | | (2,441 | ) |
| Proceeds from sale of assets held for sale | | | 23 | | | | 588 | | | | — | |
| Proceeds from sale and maturity of investment securities | | | 11,627 | | | | 23,900 | | | | 69,116 | |
| Purchase of investment securities | | | (10,968 | ) | | | (20,490 | ) | | | (50,121 | ) |
| Proceeds from sale of Celltrion common stock | | | 357 | | | | — | | | | — | |
| Loan to Raven biotechnologies, inc. | | | — | | | | (4,668 | ) | | | — | |
| Repayment of loan by Raven biotechnologies, inc. | | | — | | | | 6,000 | | | | — | |
| Change in restricted cash | | | 156 | | | | — | | | | 1,339 | |
| Expenses related to proposed merger and bridge loan | | | — | | | | — | | | | (1,848 | ) |
| Other | | | — | | | | — | | | | 791 | |
| | | | | | | | | | |
| Net cash provided by investing activities | | | 1,195 | | | | 5,330 | | | | 16,817 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Repurchase of senior subordinated convertible notes | | | — | | | | (25,400 | ) | | | — | |
| | | | | | | | | | |
| Net cash used in financing activities | | | — | | | | (25,400 | ) | | | — | |
| | | | | | | | | | |
| Net decrease in cash and cash equivalents | | | (5,270 | ) | | | (30,108 | ) | | | (8,349 | ) |
| Cash and cash equivalents, beginning of period | | | 34,618 | | | | 64,726 | | | | 73,075 | |
| | | | | | | | | | |
| Cash and cash equivalents, end of period | | $ | 29,348 | | | $ | 34,618 | | | $ | 64,726 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
| Interest paid | | $ | — | | | $ | 1,408 | | | $ | 1,733 | |
| Income taxes paid | | | — | | | | — | | | | 1,150 | |
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
25
VaxGen, Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization
Nature of Business Activities
VaxGen is a biopharmaceutical company based in South San Francisco, California. The Company owns a state-of-the-art biopharmaceutical manufacturing facility with a 1,000-liter bioreactor that can be used to make cell culture or microbial biologic products. This facility is located within leased premises. The Company has ended all product development activities and sold or otherwise terminated its drug development programs. The Company is seeking to maximize the value of its remaining assets through a strategic transaction or series of strategic transactions.
VaxGen was incorporated on November 27, 1995. From 1995 through 2004, VaxGen was developing vaccine candidates to prevent or reduce HIV infection. These HIV vaccine candidates failed to succeed in two phase three studies, and in 2004 VaxGen licensed its HIV vaccine candidates to Global Solution for Infectious Disease. VaxGen retains certain rights to commercialize these vaccine candidates in the event that they are successfully licensed in the future.
During 2002 through 2006, VaxGen developed vaccines against inhalation anthrax and smallpox for the purpose of biodefense. In December 2006, the Department of Health and Human Services, or HHS, terminated its contract with VaxGen related to the development and delivery of a next-generation anthrax vaccine. Following the HHS decision, VaxGen ceased actively developing its anthrax vaccine, scaled back its biodefense activities and began pursuing strategic and other alternatives.
Through March 31, 2007, VaxGen’s principal source of revenue was the U.S. government, principally the National Institutes of Health, or NIH, and related entities. From April 2007 to April 2008, VaxGen’s principal source of revenue was from services provided to Celltrion, Inc., or Celltrion, a company developing and operating a mammalian cell culture biomanufacturing facility in the Republic of Korea.
The Company discontinued clinical development of the Company’s anthrax vaccine candidate, rPA102, after HHS terminated the Company’s SNS Contract in December 2006. In May, 2008, the Company completed the sale of all assets and rights related to its recombinant protective antigen (rPA) anthrax vaccine product candidate and related technology to Emergent BioSolutions, Inc., or Emergent. Under the terms of the transaction, Emergent paid VaxGen $2.0 million upon execution of the definitive agreement, an additional $1.0 million milestone payment during the third quarter of 2008, and may be obligated to pay VaxGen up to an additional $7.0 million in milestone payments, plus specified percentages of future net sales for 12.5 years beginning from the first commercial sale. The Company recorded $3.0 million as other income during the year ended December 31, 2008 for the sale of the Anthrax Program. In December 2009, HHS withdrew the pending Request for Proposal, (RFP) for the procurement of a second generation anthrax vaccine, such as rPA102, delaying indefinitely the potential commercialization and associated payments under this asset sale agreement.
In addition, in June 2007 the Company terminated the contract with the Chemo-Sero-Therapeutic Research Institute of Japan, or Kaketsuken, to develop a smallpox vaccine. The Company had previously devoted substantially all research, development and clinical efforts and financial resources toward the development of rPA102, and the Company has no product candidates in clinical or preclinical development. In connection with the termination of the Company’s clinical development of rPA102, the Company announced restructuring activities, including significant workforce reductions, and as a result the Company has no remaining internal capability to discover or develop product candidates. The Company had research and development costs of zero, $1.4 million and $19.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
In November 2007, the Company and two of its wholly-owned subsidiaries entered into an Agreement and Plan of Merger, as amended in December 2007 and February 2008, or Merger Agreement, with Raven biotechnologies, inc., or Raven. Raven was a private, development stage biopharmaceutical company focused on the discovery, development and commercialization of monoclonal antibody-based products for the treatment of cancer.
In connection with the Raven Merger Agreement, the Company also entered into a Bridge Loan with Raven, which provided for the Company to lend Raven up to $6 million in cash in the aggregate, beginning December 1, 2007. In March 2008, due to a lack of stockholder support for the transaction, VaxGen and Raven agreed to terminate the merger agreement and amend the terms of VaxGen’s Bridge Loan to Raven. The balance on the Bridge Loan was paid in full in 2008. The Company recorded in general and administrative expenses, $2.3 million of costs, primarily professional fees related to the proposed merger with Raven during the year ended December 31, 2008.
In April 2008, the Company announced that it was restructuring to reduce operating expenses following the termination of the proposed merger with Raven by decreasing its workforce of twenty-two employees by approximately 75 percent. During August 2008, the Company further reduced its remaining workforce by 25 percent. The Company incurred restructuring costs of approximately $1.3 million for one-time termination costs during the year ended December 31, 2008. (See Note 4 to the financial statements included herein.)
26
In October 2009, the Company and OXiGENE, Inc., or OXiGENE, entered into a definitive merger agreement pursuant to which a wholly-owned subsidiary of OXiGENE would merge with VaxGen, such that VaxGen would become a wholly-owned subsidiary of OXiGENE, and in exchange VaxGen’s stockholders would receive common stock of OXiGENE. OXiGENE is a publicly-traded development-stage biopharmaceutical company. On February 3, 2010, the Company’s stockholders failed to approve the merger. VaxGen recorded $1.2 million of costs, primarily professional fees, related to the proposed merger with OXiGENE during the year ended December 31, 2009.
As a result of the termination of the proposed merger with OXiGENE, the Company is considering various alternate strategic transactions to return value to its stockholders. If the Company is unable to identify and complete an alternate strategic transaction, the Company will liquidate. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of Consolidated Financial Statements in conformity with generally accepted accounting principles, or GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, which include, among others, those related to long-lived assets, property and equipment, embedded derivative liability, stock-based compensation, income taxes and other contingencies. The estimates are based on historical experience and on various other assumptions that appear to be reasonable under the circumstances, the results of which form the basis for making judgments about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Cash Equivalents
All short-term investments with an original maturity at date of purchase of less than three months are considered to be cash equivalents.
Investment Securities
The Company determines the appropriate classification of its investments in debt and equity securities at the time of purchase and re-evaluates classifications at each balance sheet date. If declines in value are deemed other-than-temporary, a charge is made to net income (loss) for the period. At December 31, 2009 and 2008, investment securities consisted of corporate obligations with an original maturity date at purchase greater than three months. These securities were classified as “available-for-sale” securities. Available-for-sale securities are carried at fair value, with unrealized gains and losses reported as a component of other comprehensive income (loss). All investment securities are held for use in current operations and are classified in current assets. Realized gains and losses on sales of investment securities are determined on the specific identification method and are included in investment income.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents, investment securities and restricted cash. VaxGen’s cash and cash equivalents are deposited in demand and money market accounts at two financial institutions. The Company’s balances are in excess of federal depository insurance limitations. The Company has not experienced any losses on its deposits of cash and cash equivalents.
Assets Held for Sale
The Company considers an asset held for sale when all of the following criteria per FASB Accounting Standards Codification (“ASC”) ASC 360-10-35-15,Impairment of Long-Lived Assets, are met:
| | a) | | Management commits to a plan to sell the asset; |
| |
| | b) | | The asset is available for immediate sale in its present condition; |
| |
| | c) | | An active marketing plan to sell the asset has been initiated at a reasonable price; |
| |
| | d) | | The sale of the asset is probable within one year; and, |
| |
| | e) | | It is unlikely that significant changes to the plan to sell the asset will be made. |
27
Upon designation of a property as an asset held for sale and in accordance with the provisions of ASC 360-10-35-15, the Company records the carrying value of the property at the lower of its carrying value or its estimated fair market value, less estimated selling costs, and the Company ceases depreciation of the asset.
All losses and gains on assets sold and held for sale (including any related impairment charges) are included in “loss from operations” in the Consolidated Statement of Operation. All assets held for sale and the liabilities related to these assets are separately disclosed in the Consolidated Balance Sheet. The amount the Company will ultimately realize could differ from the amount recorded in the financial statements.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. Equipment, consisting of manufacturing machinery, laboratory equipment, computers and other office furniture and equipment, is depreciated using the straight-line method over the assets’ estimated useful lives of three to six years. Software is depreciated using the straight-line method over the assets’ estimated useful life of five years. Leasehold improvements and capital lease assets are amortized using the straight-line method over the shorter of the assets’ estimated useful lives or the remaining term of the lease. Significant additions and improvements that materially increase values, change capacities or extend useful lives are capitalized, while repairs and maintenance costs are charged to expenses as incurred. Property and equipment purchased for specific research and development projects with no alternative uses are expensed. When assets are retired or otherwise disposed of, the cost and accumulated depreciation or amortization are removed from the accounts and any resulting gain or loss is reflected in operations in the period recognized.
The Company evaluates the carrying value of property and equipment in accordance with the provisions of ASC 360-10-35-15 whenever events or circumstances indicate that the carrying amount of an asset may not be fully recoverable. Events and circumstances that would trigger an impairment analysis include a significant decrease in the market value of an asset; a significant, other-than-temporary change in the manner or extent that an asset is used, including a decision to abandon acquired products, services or technologies; a significant adverse change in operations or business climate affecting the asset; and historical operating or cash flow losses expected to continue for the foreseeable future associated with the asset. When such an event occurs, management determines whether there has been impairment by comparing the undiscounted future net cash flows to the related asset’s carrying value. If an asset is considered impaired, the asset is written down to fair value. Fair value is determined based either on estimated realizable value, discounted cash flows or appraised values, depending on the nature of the asset. All long-lived assets held for sale are reported at fair market value, less expected selling costs.
Clinical Trial Accruals
The Company accrues and expenses costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines its estimates through discussion with internal clinical personnel and outside service providers as to progress or stage of completion of trials or services and the agreed upon fee to be paid for such services. These estimates may or may not match the actual services performed by the organizations as determined by patient enrollment levels and related activities. The Company monitors patient enrollment levels and related activities to the extent possible; however, if the Company underestimated activity levels associated with various studies at a given point in time, the Company could record significant research and development expenses in future periods. No clinical trials activity occurred during 2009.
Revenue Recognition
Substantially all of the Company’s revenues relate to written contractual arrangements with agencies of the U.S. government or entities associated with the U.S. government. Cost-reimbursable contracts with the U.S. government are accounted for in accordance with ASC 605,Revenue Recognition, as amended and interpreted. The fees under U.S. government contracts may be increased or decreased in accordance with cost or performance incentive provisions which measure actual performance against established targets or other criteria. Such incentive fee awards or penalties are included in revenues at the time the amounts can be determined reasonably.
For non-government arrangements, the Company recognizes revenues in accordance with ASC 605,Revenue Recognition, as amended and interpreted. In such instances, revenues are recognized when there is persuasive evidence of an arrangement, delivery has occurred or services have been performed, the selling price to the buyer is fixed or determinable and collectability is reasonably assured.
No revenue was recorded during 2009.
28
Research and Development Costs
Research and development costs are charged to expense as incurred. Such costs include salaries, benefits and other costs associated with internal personnel, contractor fees and laboratory supplies as well as preclinical development costs, clinical trial and related clinical manufacturing costs, facilities and overhead costs and research and certain clinical trial activities conducted by various third parties, including contract research organizations, which provide contractually defined administration and management services.
No research and development expenses were incurred during 2009.
Income Taxes
Deferred income taxes are provided based on the estimated future tax effects of temporary differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates that are expected to apply to taxable income in the years in which those temporary differences are expected to be recovered. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is established to reduce deferred tax assets to the amount that is more likely than not to be realized.
Effective January 1, 2007, the Company adopted ASC 740,Accounting for Uncertainty in Income Taxes.The Company regularly assesses its tax positions and includes reserves for differences in position in accordance with ASC 740. The reserves are utilized or reversed once the statute of limitations has expired and/or at the conclusion of a tax examination or upon settlement.
Valuation of Derivative Instruments
Prior to the October 2008 repurchase, the Company valued certain embedded features it issued in connection with the financing of Convertible Senior Subordinated Notes, or Notes, in 2005 as a derivative liability under ASC 815,Derivatives. The Company estimated the fair value of its derivative liability each quarter using the Monte Carlo Simulation methodology. Factors affecting the amount of the derivative liability include the market value of our common stock, the estimated volatility of our common stock, our market capitalization, the risk-free interest rate and other assumptions such as the probability of a change in control event. The embedded derivative liability did not qualify for hedge accounting under ASC 815-15,Embedded Derivatives, and therefore, changes in value were recorded as non-cash valuation adjustments within other income (expense) in the Company’s Consolidated Statement of Operation. These changes in the carrying value of the derivative are recorded in the Company’s Consolidated Financial Statements.
Fair Value of Financial Instruments
The Company values its financial instruments according to the provisions of ASC 820 —Fair Value Measurements and Disclosures, with respect to its financial assets and liabilities only. ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. ASC 820 establishes a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| | • | | Level 1 — Quoted prices in active markets for identical assets or liabilities. |
| |
| | • | | Level 2 — Inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data. |
| |
| | • | | Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs. |
Our cash equivalents and investments are classified within Level 1 or Level 2 of the fair value hierarchy because they are valued using quoted market prices in active markets, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. The types of instruments valued based on quoted market prices in active markets include most U.S. government and agency securities, sovereign government obligations, and money market securities. Such instruments are generally classified within Level 1 of the fair value hierarchy.
The types of instruments valued based on other observable inputs include investment-grade corporate bonds, mortgage-backed and asset-backed products, state, municipal and provincial obligations. Such instruments are generally classified within Level 2 of the fair value hierarchy.
29
Stock-Based Compensation
The Company accounts for stock-based compensation using the fair value recognition provisions of ASC 718,Share-Based Payment,which was adopted using the modified-prospective transition method effective January 1, 2006. Determining the appropriate fair value model and calculating the fair value of stock-based payment awards require the input of various highly-subjective assumptions, including the expected life of the stock-based payment awards, our stock price volatility and the expected forfeiture rate of our options. The Company determined the expected stock price volatility assumption based upon our historical volatility. The Company believes this method of computing volatility is more reflective and a better indicator of the expected future volatility, than using an average of a comparable market index or of a comparable company in the same industry. The expected term of options granted was derived from the short-cut method. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The assumptions used in calculating the fair value of stock-based payment awards represent the Company’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future. In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. If our actual forfeiture rate is materially different from our estimate, the stock-based compensation expense could be significantly different from what we have recorded in the current period.
See Note 10, Stock Options and Warrants, for more information regarding stock-based compensation.
Adopted
On July 1, 2009, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles,” also known as FASB Accounting Standards Codification (“ASC”) 105-10, “Generally Accepted Accounting Principles” (“ASC 105-10”) (the “Codification”). ASC 105-10 establishes the exclusive authoritative reference for U.S. GAAP for use in financial statements, except for SEC rules and interpretive releases, which are also authoritative GAAP for SEC registrants. The Codification supersedes all existing non-SEC accounting and reporting standards. We have included the references to the Codification, as appropriate, in these Consolidated Financial Statements.
Issued but not yet adopted
In June 2009, the FASB issued guidance related to accounting for transfers of financial assets. This guidance improves the information that a reporting entity provides in its financial reports about a transfer of financial assets; the effects of a transfer on its financial position, financial performance and cash flows; and a continuing interest in transferred financial assets. In addition, this guidance amends various ASC concepts with respect to accounting for transfers and servicing of financial assets and extinguishments of liabilities, including removing the concept of qualified special purpose entities. This guidance must be applied to transfers occurring on or after the effective date. The Company will adopt this guidance in its first annual and interim reporting periods beginning after November 15, 2009. The Company has not determined the impact that this guidance may have on its financial statements.
In June 2009, the FASB issued guidance which amends certain ASC concepts related to consolidation of variable interest entities. Among other accounting and disclosure requirements, this guidance replaces the quantitative-based risks and rewards calculation for determining which enterprise has a controlling financial interest in a variable interest entity with an approach focused on identifying which enterprise has the power to direct the activities of a variable interest entity and the obligation to absorb losses of the entity or the right to receive benefits from the entity. The Company will adopt this guidance in its first annual and interim reporting periods beginning after November 15, 2009. The Company has not determined the impact that this guidance may have on its financial statements.
In January 2010, the FASB issued updated guidance related to fair value measurements and disclosures, which requires a reporting entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfers. In addition, in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3, a reporting entity should disclose separately information about purchases, sales, issuances, and settlements (that is, on a gross basis rather than one net number). The updated guidance also requires that an entity should provide fair value measurement disclosures for each class of assets and liabilities and disclosures about the valuation techniques and inputs used to measure fair value for both recurring and non-recurring fair value measurements for Level 2 and Level 3 fair value measurements. The updated guidance is effective for interim or annual financial reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward activity in Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010 and for interim periods within those fiscal years. We do not expect adoption of the updated guidance to have a material impact on our consolidated results of operations or financial condition.
30
3. Net Income (Loss) per Share
Basic net income (loss) per share, or EPS, is computed as net income (loss) divided by the weighted average number of common shares outstanding for the period. Diluted net income (loss) per share is computed as net income (loss) divided by the weighted average number of common shares and dilutive potential common shares outstanding for the period. Potential common shares include stock options under employee stock option plans, Convertible Senior Subordinated Notes and warrants using the treasury stock method. The following table summarizes the computation for basic and diluted income (loss) per share (in thousands, except per share data):
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | | | | |
| Net loss (Numerator) | | $ | (6,208 | ) | | $ | (12,563 | ) | | $ | (44,180 | ) |
| | | | | | | | | | |
| Weighted average shares (Denominator): | | | | | | | | | | | | |
| Weighted average shares used for basic EPS | | | | | | | | | | | | |
| Effect of potentially dilutive common shares | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| Adjusted weighted average shares for diluted EPS | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Basic net loss per share | | $ | (0.19 | ) | | $ | (0.38 | ) | | $ | (1.33 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Diluted net loss per share | | $ | (0.19 | ) | | $ | (0.38 | ) | | $ | (1.33 | ) |
| | | | | | | | | | |
The following is a summary of potentially dilutive common shares that were excluded from the computation of diluted net income (loss) per common share for the periods presented because including them would have had an antidilutive effect (in thousands):
| | | | | | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Options to purchase common stock | | | 1,783 | | | | 2,188 | | | | 5,350 | |
| Warrants to purchase common stock | | | 2,098 | | | | 2,317 | | | | 2,317 | |
| Convertible Senior Subordinated Notes | | | — | | | | — | | | | 2,134 | |
| | | | | | | | | | |
| Total | | | 3,881 | | | | 4,505 | | | | 9,801 | |
| | | | | | | | | | |
4. Balance Sheet Components
Investment Securities
The following is a summary of available-for-sale investment securities at December 31 (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Amortized | | | Gross
Unrealized | | | Gross
Unrealized | | | Fair Market | |
| | | Cost | | | Gains | | | Losses | | | Value | |
2009: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 2,991 | | | $ | — | | | $ | — | | | $ | 2,991 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
2008: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 3,649 | | | $ | — | | | $ | — | | | $ | 3,649 | |
| Common shares | | | — | | | | 273 | | | | — | | | | 273 | |
| | | | | | | | | | | | | |
| | | $ | 3,649 | | | $ | 273 | | | $ | — | | | $ | 3,922 | |
| | | | | | | | | | | | | |
Investments at December 31, 2009 and December 31, 2008 include $3.0 and $3.6 million, respectively, of certificates of deposit with contractual maturities of less than one year. At December 31, 2007, we held a nominal ownership interest in Celltrion, a company developing and operating a mammalian cell culture biomanufacturing facility in the Republic of Korea. During 2008, a public market developed for Celltrion common stock in the Republic of Korea. Based on the market price of Celltrion common stock, the value of our Celltrion investment was $0.3 million at December 31, 2008. During 2009 we sold the Celltrion common stock which resulted in a gain of $357,000.
31
Investment income for the years ended December 31, 2009, 2008 and 2007 of $0.6 million, $1.8 million and $4.7 million, respectively, includes interest income and amortization and accretion of discounts and premiums, as well as realized gains and losses.
Assets held for sale
In March 2008, the Company committed to a plan to sell the equipment related to its California manufacturing facility. These assets have met the criteria for, and have been classified as “held for sale” in accordance with ASC Topic 360. The Company uses the market approach to determine fair market value of its assets held for sale. Based on the lack of success in finding a buyer for its facility and the expectation of a need to dismantle to sell, the Company performed an impairment assessment of the facility during the year ended December 31, 2008. At December 31, 2008, the Company estimated that the fair market values of these assets were less than the carrying values of these assets by $8.8 million, which was recorded as an impairment of assets held for sale in the Statement of Operations for the year ended December 31, 2008. The impairment includes all leasehold improvements relating to the facility of $6.5 million, as these items will have no future economic benefit.
Based on impairment assessments performed throughout 2009 of the facility, the Company recorded an additional impairment of assets held for sale in the Statement of Operations for the quarter and the year ended December 31, 2009 of $59,000 and $290,000, respectively. Assets held for sale at December 31, 2009 of $0.4 million consist primarily of equipment, furniture and fixtures.
Total assets held for sale are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Fair Value Measurements Using | | | | |
| | | | | | | Quoted | | | | | | | | | | |
| | | | | | | Prices in | | | | | | | | | | |
| | | | | | | Active | | | Significant | | | | | | | |
| | | Year | | | Markets for | | | Other | | | Significant | | | | |
| | | Ended | | | Identical | | | Observable | | | Unobservable | | | Total | |
| | | December | | | Assets | | | Inputs | | | Inputs | | | Gains | |
| Description | | 31, 2009 | | | (Level 1) | | | (Level 2) | | | (Level 3) | | | (Losses) | |
| | | | | | | | | | | | | | | | | | | | | |
| Assets held for sale | | $ | 356 | | | $ | — | | | $ | — | | | $ | 356 | | | $ | (290 | ) |
The Company’s measurement of the assets held for sale fair value incorporated significant unobservable inputs as a result of a lack of any available observable market information to determine the fair value. The Company calculated the fair value of assets held for sale using a market value technique that relies on Level 3 inputs, including quoted prices for similar assets. During the year, the Company sold assets held for sale of $131,000, recorded impairment charges / loss of $306,000, resulting in assets held for sale of $356,000 at December 31, 2009.
Property and Equipment
Depreciation and amortization expense was $0.6 million for the year ended December 31, 2008. During 2008 the Company committed to a plan to sell the equipment and leasehold improvements related to its California manufacturing facility. These assets met the criteria for, and have been classified as “held for sale” in accordance with ASC Topic 360.
The Company records impairments of property and equipment when events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. In October 2007, the Company amended its lease agreement and agreed to relinquish occupancy of one of its two buildings subject to the lease, effective March 1, 2008. Accordingly, the useful life of the leasehold improvements in this building was revised to end on that date. Upon completion of an impairment analysis as of December 31, 2007, the Company determined that the estimated future undiscounted cash flows of these leasehold improvements were less than their carrying value. As of December 31, 2007, the Company estimated that the fair market value of these leasehold improvements was less than the carrying value by $0.4 million, which was recorded as an impairment of property and equipment in the Statement of Operations for the year ended December 31, 2007.
The November 2007 announcement of a proposed merger with Raven acted as a trigger for an assessment of an impairment of the Company’s equipment, software and leasehold improvements related to its California manufacturing facility, and based upon the impairment test performed, the Company noted a decrease in its estimated future undiscounted cash flows to below the carrying value of these assets. At December 31, 2007, the Company estimated that the fair market values of these assets were less than the carrying values of these assets by $10.3 million, which was recorded as an impairment of property and equipment in the Statement of Operations for the year ended December 31, 2007. The Company estimated the fair market value of these assets based on estimated realizable value upon sale of the facility determined by discussions with sales agents and efforts to date in the marketing of the facility; discussions with resellers relating to the manufacturing equipment.
32
Restricted Cash
At December 31, 2009 and 2008, VaxGen had restricted cash of $1.4 million and $1.6 million, respectively, as compensating balances to support outstanding letters of credit. The letters of credit relate to the Company’s facilities leases and are renewed annually.
Other Non-current Assets
At December 31, 2009 and 2008, other assets included $0.4 million and $0.4 million for a long-term prepaid commission expense associated with the Company lease.
Accrued and Other Current Liabilities
The Company’s accrued and other current liabilities consist of the following (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Employee benefits and severance | | $ | 82 | | | $ | 154 | |
| Legal and professional fees | | | 163 | | | | 167 | |
| Deferred Rent | | | 423 | | | | 318 | |
| Other | | | 19 | | | | 67 | |
| | | | | | | |
| Total accrued and other current liabilities | | $ | 687 | | | $ | 706 | |
| | | | | | | |
Restructurings
In January 2007, the Company restructured operations to significantly reduce operating costs and announced it was actively pursuing avenues to enhance stockholder value through a strategic transaction and/or the sale of its assets. The Company incurred restructuring costs associated with this plan of $2.6 million for employee termination benefits, $0.1 million related to the acceleration of stock options and $1.0 million of costs associated with the consolidation of its facilities in California. The majority of these costs were recovered from the U.S. government as part of the April 2007 settlement agreement. In May and September 2007, the Company further reduced its workforce to decrease operating costs. Restructuring costs of $1.7 million relating to the May and September 2007 workforce reductions included employee termination and benefit costs. Severance, benefit and other costs associated with restructuring activities were recorded in accordance with ASC 420-10,Costs Associated with Exit or Disposal.
In April 2008, the Company announced that it was restructuring to reduce operating expenses following the termination of the proposed merger with Raven by decreasing its workforce of twenty-two employees by approximately 75 percent. During August 2008, the Company further reduced its workforce by 25 percent. The Company incurred restructuring costs of approximately $1.3 million for one-time termination costs during 2008. As part of restructuring, the vesting of stock options held by the former Vice President, Corporate and Business Development and former Chief Financial Officer were accelerated. This resulted in the Company recording an additional $1.1 million of stock based compensation in general and administrative expense during 2008.
All restructuring costs were paid by December 31, 2008 as follows (in thousands):
| | | | | | | | | | | | | |
| | | | | | | Consolidation | | | | |
| | | | | | | of Excess | | | | |
| | | Workforce | | | Facilities and | | | | |
| | | Reduction | | | Other | | | Total | |
| Restructuring Costs | | | | | | | | | | | | |
| Costs incurred in 2007 | | $ | 4,368 | | | $ | 1,006 | | | $ | 5,374 | |
| Amounts paid in 2007 | | | (4,068 | ) | | | (1,006 | ) | | | (5,074 | ) |
| | | | | | | | | | |
| Balance accrued as of December 31, 2007 | | | 300 | | | | — | | | | 300 | |
| Costs incurred in 2008 | | | 1,314 | | | | — | | | | 1,314 | |
| Amounts paid in 2008 | | | (1,614 | ) | | | — | | | | (1,614 | ) |
| | | | | | | | | | |
| Balance accrued as of December 31, 2008 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | |
33
Accumulated Other Comprehensive Loss
Comprehensive loss combines net loss and other comprehensive income (loss). Other comprehensive loss represents unrealized gains or losses on investment securities that are reported as a component of stockholders’ equity in the Consolidated Balance Sheet.
5. Sale of Anthrax Program
On May 2, 2008, the Company completed the sale of all assets and rights related to its recombinant protective antigen (rPA) anthrax vaccine product candidate and related technology to Emergent BioSolutions, Inc., or Emergent. Under the terms of the transaction, Emergent paid VaxGen $2.0 million upon execution of the definitive agreement, an additional $1.0 million milestone payment during the third quarter of 2008, and may be obligated to pay VaxGen up to an additional $7.0 million in milestone payments, plus specified percentages of future net sales for 12.5 years beginning from the first commercial sale. The Company recorded $3.0 million as other income during the year ended December 31, 2008 for the sale of the Anthrax Program. No milestones were reached during 2009 and accordingly no income was recorded during 2009.
6. Contracts
In April 2007, the Company entered into a settlement agreement with U.S. Department of Health and Human Services, or HHS . In accordance with the agreement, the parties terminated the remaining cost-plus contract related to the development and delivery of a next-generation anthrax vaccine through a separate contract modification. As part of the settlement agreement, NIAID paid the Company $11.0 million. The settlement agreement also released both parties of all liabilities associated with the Company’s three anthrax government contracts: the 2002 Anthrax Contract, the 2003 Anthrax Contract and the SNS Contract. As part of the settlement agreement, the parties converted the termination of the SNS Contract to a termination for convenience and also terminated the 2003 Anthrax Contract under a bilateral contract modification for the convenience of the government on a no-cost basis, effective April 3, 2007.
In June 2007, the Company and the Chemo-Sero-Therapeutic Research Institute of Japan, or Kaketsuken, terminated by mutual consent their agreement to co-develop a next-generation, attenuated smallpox vaccine, LC16m8, for use in the United States and elsewhere. Under the terms of the termination agreement, VaxGen transferred to Kaketsuken or its designee all reports, data and materials and all intellectual property rights that relate to conducting non-clinical and clinical development.
7. Convertible Senior Subordinated Notes
In April 2005, the Company raised aggregate net proceeds of $29.7 million through a private placement of $31.5 million of Notes, due April 1, 2010. The Notes had the following terms:
| | • | | semi-annual payments of interest in cash at a rate of 5 1/2%, due April 1 and October 1; |
| |
| | • | | convert, at the option of the holder, into the Company’s common stock at an initial conversion price of $14.76 per share subject to adjustment; |
| |
| | • | | provisional redemption at the Company’s option for a redemption price of 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest, plus an interest make-whole payment, under certain circumstances, including among others, that the closing price of the Company’s common stock has exceeded $22.14 per share, subject to adjustment, for at least 20 trading days within a period of 30 consecutive trading days; |
| |
| | • | | constitute the Company’s Senior Subordinated obligations; and |
| |
| | • | | if a change in control occurs, as defined in the indenture, on or prior to the stated maturity of the Notes, under certain circumstances the holders of the Notes may require the Company to repurchase the Notes and pay a make-whole premium to the holders of the Notes. If the stock price on the effective date of redemption is less than $12.30 per share, no make-whole premium will be paid. If the holders request such a repurchase, the Company or the successor entity, may choose to pay in cash, common stock or a combination of cash and common stock. This feature constitutes a put-option derivative liability. |
The Company initially valued the derivative liability associated with the Notes at $1.8 million. This amount was accounted for as a reduction in the initial carrying value of the Notes and an increase to current liabilities. This discount to the Notes was being accreted over five years using the effective interest method. Interest expense for the years ended December 31, 2008 and, 2007 reflects non-cash accretion charges of $0.2 million and $0.4 million, respectively, related to this discount. For the years ended December 31, 2008 and 2007, valuation adjustments expense (income) of ($3.5) million and ($4.7) million, respectively, represented the increase (decrease) in the fair value of the derivative liability.
The Company incurred financing expenses associated with the Notes of $1.8 million. This amount was accounted for as an increase to non-current other assets. The financing expenses were being amortized over five years using the straight-line method. Interest expense for the years ended December 31, 2008 and 2007 reflects non-cash amortization charges related to these expenses of $0.2 million and $0.3 million, respectively.
34
Interest expense on the Notes for the years ended December 31, 2008 and 2007 was $1.0 million and $1.7 million, respectively.
During 2008, the Company repurchased the entire $31.5 million of the Notes for $25.4 million resulting in a net gain of $4.9 million, net of $0.6 million of unamortized discount and $0.6 million of deferred financing costs.
Valuation of Notes and Note Derivatives
The Notes and related derivatives have been valued using the Monte Carlo fair value methodology. The Company completed the valuation of the put option upon a change in control. The Monte Carlo fair value methodology allows flexibility in incorporating various assumptions such as probabilities of a change in control. The valuations are based on the information available as of the various valuation dates.
The inputs for valuation analysis include the market value of the Company’s common stock, the estimated volatility of the Company’s common stock, the Company’s market capitalization, the conversion rate of the Notes, the risk-free interest rate as well as the credit spread, which represents the Company’s risk premium over the risk-free interest rate.
The key inputs for the valuation analysis were as follows:
| | | | | |
| | | December 31, | |
| | | 2007 | |
| Volatility | | | 109 | % |
| Risk free interest rate | | | 3.1 | % |
| Credit spread | | | 22.9 | % |
| Probability of change in control | | | 40 | % |
The derivative liability was reduced to zero at December 31, 2008 resulting from the repurchase of the entire outstanding $31.5 million of Convertible Notes.
8. Celltrion
In June 2005, Celltrion entered into several agreements to manufacture biologic products being developed by Bristol-Myers Squibb Company, or BMS. VaxGen and Celltrion entered into a Technical Support and Services Sub-Agreement, or Sub-Agreement, effective in June 2005. The Sub-Agreement provides for VaxGen to assist Celltrion with services required under Celltrion’s agreement with BMS. Under the Sub-Agreement, VaxGen was paid for out-of-pocket expenses and services rendered. VaxGen recognized $0.9 million of services revenue under this agreement during the year ended December 31, 2007, as Celltrion is no longer considered to be a related party due to the sale of substantially all of the Company’s investment in Celltrion in 2006.
In September 2006, the Revised JVA was terminated and the Korean investors entered into a Celltrion shareholders’ agreement. In November 2006, Celltrion’s stockholders approved the appointment of their non-VaxGen Co-CEO as the sole CEO of Celltrion. At December 31, 2007, VaxGen held a nominal ownership interest in Celltrion.
In February 2006, Celltrion and VaxGen entered into an agreement whereby Celltrion agreed to use its best efforts to timely prepare annual and quarterly financial statements in accordance with U.S. GAAP. Under the agreement, VaxGen agreed to reimburse Celltrion for all invoiced costs of the independent accountants relating to the preparation of U.S. GAAP financial statements as well as all invoiced costs of audits and reviews performed by another independent registered public accounting firm. In addition, VaxGen compensated Celltrion for the cost of internal resources utilized in support of these activities at a rate of 190% of the employee’s hourly wage; such internal costs not to exceed the U.S. dollar equivalent of 300 million Korean Won (equivalent to $0.3 million at the exchange rate on February 28, 2006) per year and subject to VaxGen’s approval. During the year ended December 31, 2007 VaxGen incurred expenses of $1.2 million for internal and external fees and services relating to the preparation, review and audit of Celltrion financial statements in accordance with U.S. GAAP. No expenses were incurred during the years ended December 31, 2009 and 2008 since Celltrion is no longer a related party.
9. Employee Benefit Plans
401(k) Plan
At the beginning of 2008 the Company had a 401(k) Retirement Plan, as amended, or 401(k) Plan, which covered substantially all full-time employees of VaxGen. Under the 401(k) Plan, VaxGen matched a portion of employee contributions with Company common stock or cash. In 2008 and 2007, VaxGen matched employee contributions under the 401(k) Plan with cash and recorded expense related to 401(k) matching of $48,000 and $0.3 million, respectively. In July 2008, the Company terminated the 401(k) Plan.
35
10. Stock Options and Warrants
(a) Stock Options
1996 Stock Option Plan
The 1996 Stock Option Plan, or Plan, initially had 4,750,000 shares of common stock authorized for issuance and a provision that automatically increased this number by 3.5% of the issued and outstanding common stock on the last trading day of the December immediately preceding each fiscal year through January 2007. Options granted under the Plan may be designated as qualified or nonqualified at the discretion of the Compensation Committee of the Board of Directors. Generally, shares issuable upon exercise of options vest ratably over four years, beginning one year from the date of grant; however, options can vest upon grant. All options expire no later than 10 years from the date of grant. Qualified stock options are exercisable at not less than the fair market value of the stock at the date of grant and nonqualified stock options are exercisable at prices determined at the discretion of the Board of Directors, but not less than 85% of the fair market value of the stock at the date of grant.
1998 Director Stock Option Plan
The 1998 Director Stock Option Plan, or Director Plan, for non-employee directors has 300,000 shares of common stock authorized for issuance. Under the Director Plan, new non-employee directors will receive an initial option grant to acquire 20,000 shares at the fair market value of VaxGen’s common stock on the grant date. Initial option grants shall vest over three years, beginning one year from the date of grant, subject to certain meeting attendance requirements. In addition, non-employee directors who have served on the Board of Directors for at least six months shall receive annual option grants of 10,000 shares on the date of each annual stockholders’ meeting. The exercise price of each annual option grant under the Director Plan is to be the fair market value of VaxGen’s common stock on the grant date. Each annual option grant fully vests on the grant date. All options expire no later than 10 years from the date of grant. The Company suspended the grant of any options to non-employee directors of the Company under the Director Plan in 2005.
The following is a summary of VaxGen’s stock option activity (including the inducement stock options) and related information for the years ended December 31:
| | | | | | | | | |
| | | | | | | Weighted | |
| | | | | | | Average | |
| | | Number of | | | Exercise | |
| | | Shares | | | Price | |
| Outstanding at January 1, 2007 | | | 4,441,425 | | | $ | 10.47 | |
| Options granted | | | 2,033,675 | | | | 2.22 | |
| Options exercised | | | — | | | | — | |
| Options cancelled/forfeited/expired | | | (1,125,132 | ) | | | 8.23 | |
| | | | | | | | |
| Outstanding at December 31, 2007 | | | 5,349,968 | | | $ | 7.80 | |
| Options granted | | | 95,000 | | | | 0.68 | |
| Options exercised | | | — | | | | — | |
| Options cancelled/forfeited/expired | | | (3,225,825 | ) | | | 7.10 | |
| | | | | | | | |
| Outstanding at December 31, 2008 | | | 2,219,143 | | | $ | 8.53 | |
| Options granted | | | — | | | | — | |
| Options exercised | | | — | | | | — | |
| Options cancelled/forfeited/expired | | | (436,232 | ) | | | 10.64 | |
| | | | | | | | |
| Outstanding at December 31, 2009 | | | 1,782,911 | | | $ | 8.02 | |
| | | | | | | |
| Exercisable at December 31, 2009 | | | 1,610,721 | | | $ | 8.68 | |
| | | | | | | |
| The following table summarizes information about VaxGen’s stock options granted during the years ended December 31: |
| | | | | | | | | | | | | | | | | |
| | | 2008 | | | 2007 | |
| | | | | | | Weighted | | | | | | | Weighted | |
| | | Weighted | | | Average | | | Weighted | | | Average | |
| Exercise Price on grant | | Average | | | Exercise | | | Average Fair | | | Exercise | |
| date | | Fair Value | | | Price | | | Value | | | Price | |
| Equals market price | | $ | 0.55 | | | $ | 0.68 | | | $ | 1.67 | | | $ | 2.22 | |
| Is below market price | | | — | | | | — | | | | — | | | | — | |
36
Detailed information on VaxGen’s options outstanding at December 31, 2009 by price range is set forth below:
| | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | | Weighted | | | | | | | | | | | |
| | | | | | | Average | | | | | | | | | | | |
| | | | | | | Remaining | | | Weighted | | | | | | Weighted | |
| | | | | | | Contractual | | | Average | | | | | | Average | |
| | | Number of | | | Term | | | Exercise | | | Number of | | | Exercise | |
| Range of Exercise Price | | Shares | | | (Years) | | | Price | | | Shares | | | Price | |
| $0.6800 to $0.6800 | | | 75,625 | | | | 8.44 | | | $ | 0.68 | | | | 34,999 | | | $ | 0.68 | |
| $0.6801 to $2.2300 | | | 622,500 | | | | 7.12 | | | | 2.23 | | | | 490,936 | | | | 2.23 | |
| $2.2301 to $5.7400 | | | 345,000 | | | | 3.08 | | | | 5.49 | | | | 345,000 | | | | 5.49 | |
| $5.7401 to $13.5000 | | | 157,946 | | | | 2.15 | | | | 10.12 | | | | 157,946 | | | | 10.12 | |
| $13.5001 to $14.9000 | | | 420,000 | | | | 1.75 | | | | 14.87 | | | | 420,000 | | | | 14.87 | |
| $14.9001 to $21.2500 | | | 161,840 | | | | 1.56 | | | | 19.28 | | | | 161,840 | | | | 19.28 | |
| | | | | | | | | | | | | | | | | | | |
| $0.6800 to $21.2500 | | | 1,782,911 | | | | 4.18 | | | $ | 8.02 | | | | 1,610,721 | | | $ | 8.68 | |
| | | | | | | | | | | | | | | | | | | |
The aggregate intrinsic value of outstanding options and exercisable options was zero at December 31, 2009 because none of the options were in-the-money. The aggregate intrinsic value represents the total pretax intrinsic value (the difference between the Company’s closing stock price on December 31, 2009 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2009. This amount changes based upon the fair market value of the Company’s common stock. As of December 31, 2009, exercisable options had a remaining weighted average contractual life of 3.8 years. As of December 31, 2009, there was approximately $0.2 million of total unrecognized stock-based compensation before estimated forfeitures related to unvested arrangements granted under the Company’s equity-based incentive plans. As of that date, this cost was expected to be recognized over a weighted average period of 1.4 years. The total intrinsic value of options exercised was zero during the three years ended December 31, 2009 determined as of the date of exercise. The total fair value of shares vested during the year ended December 31, 2009 was $0.2 million.
Non-cash Compensation Expense
Non-cash compensation expense for the years ended December 31, 2009, 2008 and 2007 was $0.2 million, $1.5 million and $2.2 million, respectively. Such expense was primarily related to fair-value compensation related to stock options and compensation related to the extension of the exercise period of terminated employees’ vested options due to the lack of current financial statements and failure to file periodic reports. In 2005, the Board of Directors authorized a modification to options held by employees who left the Company such that the ability to exercise their options was extended from 90 days following termination to 90 days following listing on a national securities exchange or the date on which the Company is able to issue new registered shares of common stock. In February 2006, in connection with the adoption by the Internal Revenue Service of certain provisions of Section 409(A) of the Code related to deferred compensation, upon the agreement of affected stock option holders, the Company further modified the period of exercisability from 90 days following listing on a national securities exchange to 30 days following listing on a national securities exchange or the date on which the Company is able to issue new registered shares of common stock. The Company issues new shares upon the exercise of options. The Company recorded a non-cash expense for these modifications of $0.2 million during the year ended December 31, 2007. The charge related to modifications for the year ended December 31, 2008 and 2009 was immaterial.
(b) Impact of Adoption of ASC 718
In January 2006, the Company adopted the fair value recognition provisions of ASC 718, which requires the recognition of the fair value of stock-based compensation expense for all stock-based payment awards, including grants of stock options, made to the Company’s employees and directors. Under the fair value recognition provisions of ASC 718, stock-based compensation cost is estimated at the grant date based on the fair value of the awards ultimately expected to vest and is recognized as expense ratably over the requisite service period of the award. The Company uses the Black-Scholes valuation model to estimate the fair value of its stock-based awards utilizing various assumptions with respect to stock price volatility, forfeiture rates and expected life. If any of the assumptions used in the Black-Scholes model change significantly, stock-based compensation expense may differ materially in the future from that recorded in the current period.
The Company uses various subjective assumptions, including the following:
| | • | | Expected Volatility: The expected stock price volatility is based upon the Company’s historical volatility. The Company believes this method of computing volatility is more reflective and a better indicator of the expected future volatility, than using an average of a comparable market index or of a comparable company in the same industry. |
| |
| | • | | Expected Average Life: The expected average life of the ten-year contractual term options granted in 2009 of 6.0 years is based upon application of the simplified method as promulgated in ASC 718. |
37
| | • | | Risk-Free Interest Rate: The risk-free rate for the expected term of stock options is based upon the rates for U.S. Treasury Bonds with terms equal to the options’ expected term in effect at the time of grant. |
| |
| | • | | Expected Dividend: The Company has not paid and does not anticipate paying any dividends in the near future. |
| |
| | • | | Estimated Pre-vesting Forfeitures: When estimating forfeitures, the Company considers voluntary termination behaviors as well as trends of actual forfeitures of vested stock options. |
The Company used the following assumptions for the year ended December 31:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| Risk-free interest rate | | | 2.8 | % | | | 4.7 - 4.8 | % |
| Expected average life | | 6.0 years | | 5.8 years |
| Volatility | | | 102 | % | | | 99 - 103 | % |
During periods following the adoption of ASC 718, the Company recorded stock-based compensation expense for awards granted prior to, but not yet vested as of December 31, 2005, using the fair value method required for pro forma disclosure under ASC 718 in effect for expense recognition purposes, adjusted for estimated forfeitures. The adoption of ASC 718 had no effect on the Company’s Statement of Cash Flows.
The impact on consolidated results of operations of recording stock-based compensation was as follows (in thousands, except per share data):
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Research and development | | $ | — | | | $ | 42 | | | $ | 991 | |
| General and administrative | | | 239 | | | | 1,436 | | | | 1,161 | |
| |
| | | | | | | | | | |
| Effect on net income | | $ | (239 | ) | | $ | (1,478 | ) | | $ | (2,152 | ) |
| | | | | | | | | | |
| |
| Effect on net income per share, basic | | $ | (0.01 | ) | | $ | (0.04 | ) | | $ | (0.06 | ) |
| Effect on net income per share, diluted | | $ | (0.01 | ) | | $ | (0.04 | ) | | $ | (0.06 | ) |
(c) Common Stock Warrants
In connection with the February 2006 common stock financing, the Company issued to the accredited institutional investors five-year warrants initially exercisable to purchase 698,637 shares of common stock at an exercise price of $9.24 per share. Because the Company did not file all of its delinquent periodic reports with the SEC by January 31, 2007, the warrants became exercisable for an additional 698,630 shares of common stock, at a price of $9.24 per share.
On September 21, 2004, the Company completed transactions in which warrants that were issued in 2001 in connection with its Series A Preferred Stock financing, or Series A Warrants, were surrendered in exchange for two new series of warrants, or Exchange Warrants. The Company issued to the holders of the Series A Warrants, Exchange Warrants to purchase a total of 1,146,388 shares of common stock, exercisable until September 21, 2005, at an exercise price of $0.01 per share, or $0.01 Warrants, and Exchange Warrants to purchase a total of 655,078 shares of common stock, exercisable until September 21, 2007, at an exercise price of $16.00 per share, or $16.00 Warrants. In connection with the exchange, the agreements governing the Series A Warrants were terminated. In 2004, 716,494 shares of common stock were issued upon the exercise of some of the $0.01 Warrants. In January 2005, 429,640 shares of common stock were issued as a result of the net exercise of the remaining $0.01 Warrants. In December 2006, the Company entered into an addendum, or Addendum, with the holders of the $16.00 Warrants under which the term of these warrants was extended by three additional years. The $16.00 Warrants, as amended, will expire September 21, 2010 instead of September 21, 2007. No other terms of the $16.00 Warrants were amended. In connection with entering into the Addendum, the Company received releases from the holders of the $16.00 Warrants regarding potential claims related to these warrants.
38
The following common stock warrants were outstanding at December 31, 2009:
| | | | | | | | | | | | | |
| | | Exercise | | | | | | | |
| | | Price Per | | | Term | | | | |
| Issue Date | | Share | | | (in years) | | | Shares | |
| February 2006 | | $ | 9.24 | | | | 5 | | | | 1,397,267 | |
| Exchange Warrants, September 2004, as amended in December 2006 | | | 16.00 | | | | 6 | | | | 655,078 | |
| January 2000 | | | 11.50 | | | | 10 | | | | 27,196 | |
| 2001 | | | 20.25 | | | | 10 | | | | 18,000 | |
| | | | | | | | | | | | |
| |
| Weighted average/Total | | | 11.47 | | | | | | | | 2,097,541 | |
| | | | | | | | | | | | |
11. Income Taxes
Based on the weight of available evidence, including cumulative losses since inception and expected future losses, the Company has determined that it is more likely than not that the deferred tax asset amount will not be realized and, therefore, a valuation allowance has been provided on net deferred tax assets.
Effective January 1, 2007, we adopted the provisions of the ASC 740,Accounting for Uncertainty in Income Taxes, which requires that the Company recognize the financial statement effects of a tax position when it becomes more likely than not, based upon the technical merits, that the position will be sustained upon examination. The gross amount of unrecognized tax benefits as of December 31, 2008 was $1.9 million. As a result of the change on the measurement of the tax position, the unrecognized tax benefits decreased to $1.4 million in 2009, which if realized, $0.2 million will affect the effective tax rate and $1.2 million will not due to a valuation allowance.
The following table reflects the changes in the gross unrecognized tax benefits during the years ended December 31 (in thousands):
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Unrecognized tax benefits balance at January 1 | | $ | 1,866 | | | $ | 2,702 | | | $ | 1,210 | |
| Gross increase for tax positions of prior years | | | 106 | | | | — | | | | 1,492 | |
| Gross decrease for tax positions of prior years | | | (556 | ) | | | (836 | ) | | | — | |
| | | | | | | | | | |
| Unrecognized tax benefits balance at December 31, | | $ | 1,416 | | | $ | 1,866 | | | $ | 2,702 | |
| | | | | | | | | | |
The Company does not expect any material changes in the next 12 months in unrecognized tax benefits. The Company recognizes interest and/or penalties related to uncertain tax positions. To the extent accrued interest and penalties do not ultimately become payable, amounts accrued will be reduced and reflected in the period that such determination is made. The amount of interest and penalties accrued for ASC 740 as interest expense, as of December 31, 2009 and 2008, was $0.6 million and $0.5 million, respectively, which is consistent with the Company’s policy.
Under the provisions of Section 382 and 383 of the Internal Revenue Code, substantial changes in the Company’s ownership may limit the amount of net operating loss carryforwards and research and development credits that can be utilized in the future to offset taxable income.
The Company files U.S. federal and California state tax returns. The Company is currently not subject to any income tax examinations. All prior years remain open for examination.
At December 31, 2009, VaxGen had U.S. federal and California net operating loss carryforwards of $178.6 million and $123.0 million, respectively. Of the deferred net operating loss carryforwards, $4.2 million would be recognized directly to additional paid in capital when realized because they relate to expenses generated from stock option awards. The U.S. federal net operating loss carryforwards expire between 2019 and 2029 and the California net operating loss carryforwards expire between 2012 and 2029. Additionally, VaxGen has federal research credits of $4.6 million, which expire between 2016 and 2026 and $4.6 million for California state tax which does not expire. The $0.9 million income tax benefit was due to the recording of a net operating loss carryback and the release of the ASC 740 reserve.
39
The tax provision is composed as follows (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Current Tax Expense: | | | | | | | | |
| Federal | | $ | (989 | ) | | $ | (40 | ) |
| State | | | 125 | | | | — | |
| | | | | | | |
| Total current tax expense | | | (864 | ) | | | (40 | ) |
| |
| Deferred Tax Expense | | | | | | | | |
| Federal | | | (548 | ) | | | (4,255 | ) |
| State | | | 3,524 | | | | (911 | ) |
| Change in valuation allowance | | | (2,977 | ) | | | 5,166 | |
| | | | | | | |
| Total deferred tax expense | | | (1 | ) | | | — | |
| Net deferred tax assets | | $ | (865 | ) | | $ | (40 | ) |
| | | | | | | |
The tax effects of temporary differences and carryforwards that give rise to deferred tax assets are as follows (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Deferred tax assets: | | | | | | | | |
| Net operating loss carryforwards | | $ | 63,689 | | | $ | 62,796 | |
| Research and other credit carryforwards | | | 8,074 | | | | 8,074 | |
| Deferred research expenses | | | 1,581 | | | | 1,969 | |
| Depreciation | | | 3,201 | | | | 6,570 | |
| Accrued liabilities | | | 1,475 | | | | 1,588 | |
| Other | | | 3,524 | | | | 3,524 | |
| | | | | | | |
| Total gross deferred tax assets | | | 81,544 | | | | 84,521 | |
| Less: Valuation allowance | | | (81,544 | ) | | | (84,521 | ) |
| | | | | | | |
| Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | |
The differences between the U.S. statutory tax rate and the Company’s effective tax rate are as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Statutory rate | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
| State taxes | | | 5.8 | % | | | 5.7 | % | | | 3.4 | % |
| Adjustments to prior year matters | | | -82.6 | % | | | — | | | | — | |
| Tax Refund | | | 6.2 | % | | | — | | | | — | |
| Adjustments for uncertain tax provisions | | | 6.5 | % | | | — | | | | — | |
| Other | | | -0.3 | % | | | 1.6 | % | | | -0.6 | % |
| Change in valuation allowance | | | 42.9 | % | | | -41.0 | % | | | -36.8 | % |
| | | | | | | | | | |
| Effective tax rate | | | 12.5 | % | | | 0.3 | % | | | 0.0 | % |
| | | | | | | | | | |
12. Commitments and Contingencies
Leases
VaxGen leases office facilities under a non-cancelable operating lease in South San Francisco, California, which expires in 2016.
In April 2005, VaxGen entered into an amended lease agreement, or Lease Amendment I, to replace two previous leases, including a lease for 20,000 square feet of laboratories and office space and a sublease for 50,000 square feet of manufacturing, laboratories and office space. It also provides an additional 35,000 square feet of new space. Lease Amendment I secured space to support the production of its recombinant anthrax vaccine candidate as well as its other programs. Lease Amendment I terminates in December 2016; however, VaxGen has options to renew the lease for two additional five-year periods. In connection with Lease Amendment I, an amended letter of credit in the amount of $2.4 million was issued to the lessor. The amended letter of credit is collateralized by a certificate of deposit held by the bank that issued the letter of credit. In addition, under Lease Amendment I the Company received $2.2 million in reimbursements for the costs of certain tenant improvements.
40
In October 2007, the Company again amended its lease agreement, or Lease Amendment II. Lease Amendment II calls for the Company to relinquish occupancy of one of its two buildings subject to the lease, effective March 1, 2008. The Company paid a surrender fee to the landlord of $0.1 million. Under Lease Amendment II, the amount of the $2.4 million letter of credit delivered by the Company in favor of the landlord was reduced by $1.0 million, with further reductions over the remaining term of the lease upon the achievement of financial benchmarks by the Company. The savings account which collateralizes this reduced letter of credit is included in restricted cash in the Consolidated Balance Sheet as of December 31, 2009. VaxGen has been unable to negotiate a termination of this lease with the landlord for acceptable terms, and is seeking to identify a sub-tenant(s) for the facility.
VaxGen also has a lease for 6,000 square feet of warehouse and office space in South San Francisco, which expires on September 30, 2011. In addition, VaxGen has one operating lease of office equipment.
Future minimum annual payments under all non-cancelable operating leases as of December 31, 2009 are as follows (in thousands):
| | | | | |
| 2010 | | $ | 2,474 | |
| 2011 | | | 2,367 | |
| 2012 | | | 2,433 | |
| 2013 | | | 2,505 | |
| 2014 | | | 2,581 | |
| 2015 and beyond | | | 5,396 | |
| | | | |
| Total | | $ | 17,756 | |
| | | | |
Rent expense for the years ended December 31, 2009, 2008 and 2007 was $2.1 million, $2.3 million and $3.3 million, respectively. The Company recognizes rent expense on a straight-line basis over the expected lease term.
Included in deferred rent and other liabilities at December 31, 2009 and 2008 is $3.8 million and $4.1 million of deferred rent associated with Lease Amendment I and II. As a result of the timing of cash flows under the amended lease, this balance will be amortized through 2016.
Litigation
On or about July 7, 2009, VaxGen filed an action in California Superior Court for San Mateo County against Firstenberg Machinery Company alleging claims for breach of contract and common count arising out of Firstenberg Machinery Company’s failure to remit to VaxGen the proceeds from Firstenberg Machinery Company’s sale on consignment of certain equipment, machinery and other property of VaxGen pursuant to a Sales Representative Agreement between the parties. The complaint seeks compensatory damages of at least $77,800. On November 24, 2009, Firstenberg Machinery Company filed an answer to the complaint, denying VaxGen’s allegations, and a cross-complaint against VaxGen, alleging claims for breach of the Sales Representative Agreement, breach of the implied covenant of good faith and fair dealing, unfair business practices, negligent misrepresentation and promissory estoppel. The cross-complaint seeks unspecified damages in excess of $231,000. VaxGen believes that it has valid defenses to the claims alleged in the cross-complaint and intends to vigorously defend against them.
Contingencies
If VaxGen’s President’s employment with VaxGen is terminated without cause, or the President resigns for good reason, as defined in his employment agreement, the President would be entitled to receive as severance a lump sum payment equal to 99% of 12 months of his base salary ($0.2 million) and all of his outstanding unvested stock options would be accelerated and become immediately exercisable.
The Company is also subject to a wide variety of laws and regulations. Certain claims, suits and complaints in the ordinary course of business are pending or may arise. While there can be no assurance as to the ultimate outcome of any litigation involving the Company, the Company does not believe any pending legal proceeding will result in a judgment or settlement that would have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows.
13. Fair Value Measurements
The Company’s cash equivalents and investments are classified within Level 1 or Level 2 of the fair value hierarchy because they are valued using quoted market prices in active markets, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. The types of instruments valued based on quoted market prices in active markets include common shares and money market securities. Such instruments are generally classified within Level 1 of the fair value hierarchy.
The types of instruments valued based on other observable inputs include certificates of deposit. Such instruments are generally classified within Level 2 of the fair value hierarchy.
41
The Company valued certain embedded features issued in connection with the financing of the Convertible Notes in 2005 as a derivative liability. The Company estimated the fair value of the derivative liability each quarter using the Monte Carlo Simulation methodology. This methodology allows flexibility in incorporating various assumptions such as probabilities of certain triggering events. The valuations are based on the information available as of the various valuation dates. Factors affecting the amount of this liability include the market value of our common stock, the estimated volatility of our common stock, our market capitalization, the risk-free interest rate and other assumptions such as the probability of a change in control event. Of these valuation parameters, management’s assessment of the probability of a change in control is the most subjective and also has the greatest influence on fair value. Changes in value were recorded as non-cash valuation adjustments within other income (expense) in our Consolidated Statement of Operation. Prior to the repurchase of the Convertible Notes in 2008 and the corresponding elimination of the derivative liability, the derivative was classified within Level 3 of the fair value hierarchy.
As December 31, 2009, the fair value hierarchy of the Company’s marketable securities at fair value are summarized below (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets: | | | | | | | | | | | | | | | | |
| Cash equivalents: | | | | | | | | | | | | | | | | |
| Money market funds | | $ | 2,103 | | | $ | — | | | $ | — | | | $ | 2,103 | |
| Investment securities: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | | | | | | 2,991 | | | | | | | | 2,991 | |
| Restricted cash: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | | | | | | 1,400 | | | | | | | | 1,400 | |
| | | | | | | | | | | | | |
| Total Financial Assets | | $ | 2,103 | | | $ | 4,391 | | | $ | — | | | $ | 6,494 | |
| | | | | | | | | | | | | |
14. Quarterly Financial Data (Unaudited)
The tables below present selected quarterly financial data (in thousands, except per share amounts).
42
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, 2009 | |
| | | First | | | Second | | | Third | | | Fourth | |
| | | Quarter | | | Quarter | | | Quarter | | | Quarter | |
| Net revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Total operating expenses | | | (2,228 | ) | | | (1,551 | ) | | | (1,937 | ) | | | (1,854 | ) |
| Other income (expense), net | | | 482 | | | | 43 | | | | 36 | | | | (63 | ) |
| Income tax benefit | | | — | | | | — | | | | — | | | | (864 | ) |
| Net loss | | | (1,746 | ) | | | (1,508 | ) | | | (1,901 | ) | | | (1,053 | ) |
| Net loss per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.05 | ) | | $ | (0.05 | ) | | $ | (0.06 | ) | | $ | (0.03 | ) |
| | | | | | | | | | | | | |
| Diluted | | $ | (0.05 | ) | | $ | (0.05 | ) | | $ | (0.06 | ) | | $ | (0.03 | ) |
| | | | | | | | | | | | | |
| Weighted average shares used in computing per share calculations: | | | | | | | | | | | | | | | | |
| Basic | | | 33,107 | | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | | | | |
| Diluted | | | 33,107 | | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, 2008 | |
| | | First | | | Second | | | Third | | | Fourth | |
| | | Quarter | | | Quarter | | | Quarter | | | Quarter | |
| Net revenue | | $ | 278 | | | $ | 15 | | | $ | — | | | $ | — | |
| Total operating expenses | | | (6,787 | ) | | | (13,028 | ) | | | (2,275 | ) | | | (2,159 | ) |
| Other income (expense), net | | | (962 | ) | | | 2,589 | | | | 7,791 | | | | 1,935 | |
| Income tax benefit | | | — | | | | — | | | | — | | | | (40 | ) |
| Net income (loss) | | | (7,471 | ) | | | (10,424 | ) | | | 5,516 | | | | (184 | ) |
| Net income (loss) per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.23 | ) | | $ | (0.31 | ) | | $ | 0.17 | | | $ | (0.01 | ) |
| | | | | | | | | | | | | |
| Diluted | | $ | (0.23 | ) | | $ | (0.31 | ) | | $ | (0.03 | ) | | $ | (0.07 | ) |
| | | | | | | | | | | | | |
| Weighted average shares used in computing per share calculations: | | | | | | | | | | | | | | | | |
| Basic | | | 33,107 | | | | 33,107 | | | | 33,107 | | | | 33,107 | |
| | | | | | | | | | | | | |
| Diluted | | | 33,107 | | | | 33,107 | | | | 33,831 | | | | 33,232 | |
| | | | | | | | | | | | | |
15. Subsequent Events
In October 2009, VaxGen and OXiGENE entered into a definitive merger agreement pursuant to which OXiGENE would acquire VaxGen in exchange for common stock of OXiGENE. Upon closing of the transaction, VaxGen would become a wholly-owned subsidiary of OXiGENE. On February 4, 2010, the Company’s shareholders failed to approve the merger.
Beginning on October 23, 2009, several putative stockholder class action lawsuits were filed against the Company, members of its Board of Directors, OXiGENE and OXiGENE Merger Sub, Inc. in the Superior Court of California, County of San Mateo in connection with the proposed merger with OXiGENE. The complaints, styled respectivelyJensen v. Panek et al., Case No. CIV 488075;Ming v. VaxGen, Inc. et al., Case No. CIV 489164; andHawes v. VaxGen, Inc. et al., Case No. CIV 489313, allege, among other things, that the members of the Company’s Board of Directors violated their fiduciary duties by failing to maximize value for the Company’s stockholders when negotiating and entering into the merger agreement with OXiGENE. The complaints also allege that the Company and OXiGENE aided and abetted those purported breaches. The plaintiffs seek, among other things, to enjoin the acquisition of the Company by OXiGENE or, in the alternative, to rescind the acquisition should it occur before the lawsuit is resolved. On February 3, 2010, the Company held a special meeting of stockholders at which the proposed merger failed to receive sufficient votes to be approved. Accordingly, the plaintiffs have informed the Company that they expect to dismiss the action without prejudice in the near future. Since the Company does not believe that a significant adverse result in this litigation is probable and since the amount of potential damages in the event of an adverse result is not reasonably estimable, no expense has been recorded with respect to the contingent liability associated with this matter.
In connection with the preparation of the Consolidated Financial Statements, the Company evaluated events subsequent to the balance sheet date of December 31, 2009 through the financial statement issuance date and determined that all material transactions have been recorded and disclosed properly.
| | |
| Item 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
43
| | |
| Item 9A. | | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
As of the end of the year covered by this Annual Report, management performed, with the participation of our Principal Executive and Financial Officer, or PEO, an evaluation of the effectiveness of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act of 1934, as amended, or Exchange Act. Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our PEO, to allow timely decisions regarding required disclosures. Based on this evaluation, our PEO has concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2009.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles in the United States, or GAAP. A company’s internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the Consolidated Financial Statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projection of any evaluation of effectiveness to future periods is subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management, with the participation of our PEO, assessed the effectiveness of our internal control over financial reporting as of December 31, 2009. Management’s assessment of internal control over financial reporting was conducted using the criteria inInternal Control—Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. Management concluded that, as of December 31, 2009, the Company’s internal control over financial reporting was effective.
This Annual Report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| | |
| Item 9B. | | Other Information |
None.
44
PART III
| | |
| Item 10. | | Directors, Executive Officers and Corporate Governance |
Executive Officers
The executive officers of the Company and their respective ages and positions as of December 31, 2009 are as follows:
| | | | | | | | | |
| Name of Executive Officer | | Age | | Position |
| |
| James P. Panek | | | 56 | | | President and Director |
There is no family relationship between the executive officer or directors.
James P. Panek
Mr. Panek has served as our President since January 2007 and was named Principal Financial Officer in May 2008. He previously served as our Chief Executive Officer from January 2007 to January 2009, Executive Vice President since September 2006 and Senior Vice President, Manufacturing Operations, since February 2002. From 1982 to 2001, Mr. Panek served in various capacities with Genentech, including Senior Vice President, Product Operations, and Vice President, Manufacturing, Engineering and Facilities, where he led the development of the world’s largest biotechnology manufacturing facility and was responsible for all operations involved in supplying products for preclinical, clinical, and commercial use. Mr. Panek led the development of manufacturing facilities that enabled FDA approval and launch of recombinant products to treat pediatric growth hormone deficiency (Nutropin Depot ® and Protropin ®), heart attack (TNKase™), non-Hodgkin’s lymphoma (Rituxan ®) and breast cancer (Herceptin ®). Mr. Panek was also responsible for the purification of Genentech’s human pharmaceuticals for clinical and commercial use, and led the successful start-up and licensure of operations for purification of Activase ®, the first large-scale cell culture product approved by the FDA. Prior to joining Genentech, Mr. Panek spent six years with Eli Lilly in a variety of engineering and development positions. Mr. Panek received a B.S. and an M.S. in chemical engineering from the University of Michigan.
Directors
The members of the Board of Directors of the Company as of December 31, 2009 are as follows:
| | | | | | | | | | | |
| | | | | | | | | Director |
| Name of Director | | Age | | Principal Occupation | | Since |
| | | | | | | | | | | |
| James P. Panek | | | 56 | | | President of VaxGen | | | 2007 | |
| | | | | | | | | | | |
| Franklin M. Berger, CFA | | | 59 | | | Independent Biotechnology Analyst. Former Managing Director, Equity Research and Senior Biotechnology Analyst, J.P. Morgan | | | 2003 | |
| | | | | | | | | | | |
| Lori F. Rafield, Ph.D. | | | 54 | | | Consultant to both the biotechnology and device industries | | | 2008 | |
| | | | | | | | | | | |
| Kevin L. Reilly | | | 66 | | | Former President, Wyeth Vaccines and Nutrition | | | 2005 | |
James P. Panek
See Mr. Panek’s biography under “Executive Officers” above.
Franklin M. Berger, CFA
Mr. Berger has served as a director since November 2003 and is the Chairman of the Audit Committee. Since 2003, Mr. Berger has served as an independent biotechnology analyst. From 1998 to 2003, Mr. Berger was a Managing Director, Equity Research and Senior Biotechnology Analyst for J. P. Morgan Securities, Inc. From 1997 to 1998, he served as a Director, Equity Research and Senior Biotechnology Analyst for Salomon Smith Barney. From 1991 to 1997, he served as a Managing Director, Research and Biotechnology Analyst for Josephthal & Co. Mr. Berger serves on the Board of Directors of Thallion Pharmaceuticals, Inc., Seattle Genetics, Inc., and Isotechnika and is on the audit committees of Seattle Genetics, Inc. and Isotechnika. Mr. Berger received a B.A. in International Relations and an M.A. in International Economics from Johns Hopkins University and an M.B.A. from Harvard University.
45
Lori F. Rafield, Ph.D.
Dr. Rafield has served as a director since May 2008. Since 2005, Dr. Rafield has served as a consultant to both the biotechnology and device industries working with entrepreneurs to create and finance companies with both institutional and strategic partners. Previously, from 1998 to 2005, Dr. Rafield was Managing Director at Apax Partners (formerly Patricof & Co. Ventures), where she was the head of healthcare and responsible for developing a diversified healthcare investment strategy for a $250MM healthcare portfolio within a $1 billion fund. While at Apax, she was predominantly focused on the creation of therapeutic product companies from pharmaceutical assets and resources which led to investments in Zymogenetics (Novo Nordisk), Affymax (GSK) and Aerovance (Bayer). Prior to that, from 1995 to 1997, she was an investment Principal at Robertson Stephens Early Stage Venture Fund and an Affiliate at Institutional Venture Partners from 1991 to 1995. Dr. Rafield held scientific research and management positions at Somatix Therapy Corp. from 1989 to 1991 and at Integrated Genetics, Inc. from 1986 to 1989 where she directed molecular biology efforts in the development and expression of recombinant proteins. She received a Ph.D. in 1981 in microbiology at University of Virginia Medical School, and was a Postdoctoral Fellow at Harvard Medical School.
Kevin L. Reilly
Mr. Reilly has served as a director since July 2005 and as Chairman of the Board of Directors since May 19, 2008. Mr. Reilly is the Chairman of Compensation Committee. Since 2002, Mr. Reilly has served as a consultant and advisor to the biotechnology industry. From 1984 through 2002, he served at Wyeth Inc. in a variety of capacities including as the Chairman and President of Wyeth-Ayerst’s Canadian operations, Area Vice President for Wyeth’s Pacific Canada Group, Group Vice President of the Pacific Rim Group, President of Wyeth Nutritionals International and most recently, as the President of Wyeth Vaccines and Nutrition. Under his leadership, he directed the accelerated growth of Wyeth’s worldwide vaccine and nutritional business. From 1973 to 1984, Mr. Reilly served as Senior Vice President for Connaught Laboratories with primary responsibilities of export operations and strategic development. He is also a Trustee of the Board for the Sabin Vaccine Institute. Mr. Reilly received his M.B.A. from York University in Toronto. He is also a graduate of the Advanced Management Program at the Harvard Business School.
Director Qualifications
The Board believes that the following attributes are key to ensuring the continued vitality of the Board and excellence in the execution of its duties: experience as a leader of a business, firm or institution; mature and practical judgment; the ability to comprehend and analyze complex matters; effective interpersonal and communication skills; and strong character and integrity. Each of the Company’s directors has these attributes.
The VaxGen Board is composed of a diverse group of leaders in their respective fields. Further, the Company’s directors also have other experience that makes them valuable members, such as prior research experience, business expertise, and outside board experience that provides insight into issues faced by companies.
The Committee and the Board believe that the above-mentioned attributes, along with the leadership skills and other experiences of its Board members described in the table below, provide the Company with the perspectives and judgment necessary to guide the Company’s strategies and monitor their execution.
| • | | Global business experience in various capacities at Wyeth Inc. including most recently, as the President of Wyeth Vaccines and Nutrition. |
| |
| • | | Industry experience as a consultant and advisor to the biotechnology industry since 2002. |
| |
| • | | Experience as a Trustee of the Board for the Sabin Vaccine Institute. |
| • | | Financial analysis and research experience as a Managing Director, Equity Analyst and Senior Biotechnology Analyst at J.P. Morgan Securities and other financial firms. |
| |
| • | | Outside board experience as a director of Thallion Pharmaceuticals, Inc. Seattle Genetics, Inc. and Isotechnika. |
| |
| • | | Audit committee experience as a member of the audit committees of Seattle Genetics, Inc. and Isotechnika. |
| • | | Entrepreneurial and finance experience as Managing Director at Apax Partners and investment Principal at Robertson Stephens Early Stage Venture Fund. |
| |
| • | | Business experience as a consultant to the biotech and device industries since 2005. |
| |
| • | | Research and management experience at Somatic Therapy Corp and Integrated Genetics, Inc. |
| • | | Senior management and business experience — served as Co-CEO and Chairman of Celltrion, VaxGen’s Korean joint venture and Senior Vice President, Product Operations at Genentech. |
| |
| • | | Biopharmaceutical development and manufacturing experience in various capacities with Genentech including Senior Vice President, Product Operations, and Vice President, Manufacturing, Engineering and Facilities. |
| |
| • | | Engineering, construction and facilities experience — Mr. Panek led the development of the world’s largest biotechnology manufacturing facility. |
Board of Directors meetings and committees
As required under NASDAQ listing standards, the Company’s independent directors meet in regularly scheduled executive sessions at which only independent directors are present. Persons interested in communicating with the independent directors regarding their concerns or issues may address correspondence to a particular director, or to the independent directors generally, in care of the Secretary, VaxGen, Inc. at 379 Oyster Point Boulevard, Suite 10, South San Francisco, California 94080. If no particular director is named, letters will be forwarded, depending on the subject matter, to the Chair of the Audit, Compensation, Nominating and Governance Committee, or Strategic Transactions Committee.
The Board of Directors met twenty-five times during the fiscal year ended December 31, 2009. All directors attended at least 75% of the aggregate telephonic and in-person meetings of the Board of Directors and, with the exception of Mr. DeStefano’s attendance at meetings held by the Nominating and Governance Committee, 75% of the meetings held by all committees on which they served, held during the period for which they were directors or committee members, respectively.
The Board of Directors has four standing committees: the Audit Committee, the Compensation Committee, the Nominating and Governance Committee, and the Strategic Transactions Committee. The following table provides membership and meeting information for fiscal year 2009 for each of the committees of the Board of Directors:
| | | | | | | | | | | | | | | | | |
| | | | | | | Strategic | | | | | | | | |
| | | | | | | Transactions | | | | | | | Nominating & | |
| Name | | Audit | | | (1) | | | Compensation | | | Governance | |
| Franklin M. Berger, CFA | | | X | * | | | | | | | X | | | | X | |
| Paul DeStefano (2) | | | X | | | | X | | | | | | | | X | |
| James P. Panek | | | | | | | X | | | | | | | | | |
| Lori F. Rafield, PhD. | | | | | | | X | * | | | X | * | | | | |
| Kevin L. Reilly | | | X | | | | | | | | X | | | | X | |
| |
| Total meetings in fiscal year | | | 4 | | | | * | * | | | 2 | | | | 0 | |
| | | |
| * | | Committee Chairperson |
| |
| ** | | The Strategic Transactions Committee met informally on an ad hoc basis during 2009. |
| |
| (1) | | The Strategic Transactions Committee disbanded in September 2009. |
| |
| (2) | | Effective December 1, 2009, Mr. DeStefano resigned from the Board of Directors and the committees thereof to which he then-served as a member. |
46
Below is a description of each committee of the Board of Directors. Each committee has the authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. Except as specifically described below, the Board of Directors has determined that each member of each committee meets the applicable rules and regulations regarding “independence” and that each member is free of any relationship that would interfere with his or her individual exercise of independent judgment with regard to the Company.
audit committee The Audit Committee oversees the Company’s corporate accounting and financial reporting process. For this purpose, the Audit Committee performs several functions. The Audit Committee evaluates the performance of and assesses the qualifications of the independent auditors; determines and approves the engagement of the independent auditors; determines whether to retain or terminate the existing independent auditors or to appoint and engage new independent auditors; reviews and approves the retention of the independent auditors to perform any proposed permissible non-audit services; confers with management; establishes procedures, as required under applicable law, for the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters and the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters; evaluates the cooperation received by the independent auditors during their audit examination; monitors the rotation of the partners of the independent auditors on the Company’s audit engagement team; reviews the financial statements to be included in the Company’s Annual Report on Form 10-K; discusses with management and the independent auditors the results of the annual audit of the financial statements and the timely reviews of quarterly financial information; considers and adopts, if appropriate, a policy regarding employment of individuals formerly employed by the independent auditors; reviews and discusses with management and the independent auditors disclosures under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Company’s periodic reports filed with the Securities and Exchange Commission; reviews with management and independent auditors earnings press releases and financial information; reviews with management and the independent auditors the Company’s guidelines and policies regarding risk assessment and risk management, and steps taken by management to monitor and control risk exposure; reviews with management and the independent auditors any management or internal control letter issued or proposed to be issued by the independent auditors, as well as management’s response or any other written communication between the independent auditors and management; reviews with the independent auditors communications between representatives of such auditors and such auditors’ national office, regarding accounting or auditing issues; reviews with management and the independent auditors material conflicts or disagreements between management and the independent auditors regarding financial reporting, accounting practices or policies, and proposed resolutions of such conflicts or disagreements; considers and reviews with management correspondence with regulators or governmental agencies or published reports which raise material issues regarding the Company’s financial statements; reviews with counsel, management and the independent auditors significant regulatory, legal or accounting initiatives which impact the Company’s financial statements; reviews management’s efforts to ensure adherence to applicable laws, rules and the Company’s code of Conduct; investigates any matter brought to its attention if necessary or appropriate; prepares the report required by the SEC to be included in the Proxy Statement; annually reviews and reassesses the adequacy of the charter of the Audit Committee; and reports to the Board of Directors material issues that arise related to the quality or integrity of the Company’s financial statements, the performance of the independent auditors or other matters it deems appropriate and conducts an annual evaluation of its performance.
Two directors comprise the current Audit Committee: Messrs. Berger and Reilly. The Audit Committee met four times during the fiscal year ended December 31, 2009. The Audit Committee has adopted a written charter that is available to stockholders on the Company’s website at http://www.vaxgen.com; however, information found on our website is not incorporated by reference into this Annual Report. The Board of Directors annually reviews the NASDAQ listing standards definition of independence for Audit Committee members and has determined that all members of the Company’s Audit Committee are independent (as independence is currently defined in Rules 5605(c)(2)(A)(i) and (ii) of the NASDAQ listing standards). The Board of Directors has determined that Mr. Berger qualifies as an “audit committee financial expert,” as defined in applicable Securities and Exchange Commission, or SEC, rules. The Board of Directors made a qualitative assessment of Mr. Berger’s level of knowledge and experience based on a number of factors, including his formal education and experience as a research analyst employed by brokerage firms.
compensation committee The Compensation Committee reviews and approves the overall compensation strategy and oversees the Company’s compensation policies, plans and programs, and reviews and determines the compensation to be paid to the Company’s executive officers and directors. In addition, the Compensation Committee prepares and reviews a report included in the Company’s annual proxy statement in accordance with the SEC’s rules and regulations. The Compensation Committee reviews and approves corporate performance goals and objectives relevant to the compensation of the Company’s Chief Executive Officer; reviews and approves the compensation and other terms of employment of the Company’s Chief Executive Officer; reviews and approves changes in director and committee member compensation and director retirement policies; administers the Company’s stock option plan, 401(k) plan and other similar programs; reviews the individual and corporate performance goals and objectives of the Company’s executive officers, as defined in Section 16 of the Exchange Act, and determines and approves the compensation and other terms of employment for such executive officers; reviews, discusses and assesses its own performance at least annually; and periodically reviews and assesses the adequacy of the charter of the Compensation Committee and proposes changes to the Board of Directors.
47
The Compensation Committee also reviews with management the Company’s Compensation Discussion and Analysis and to consider whether to recommend that it be included in proxy statements and other filings. Three directors comprise the current Compensation Committee: Messrs. Reilly and Berger and Dr. Rafield. All members of the Company’s Compensation Committee are independent (as independence is currently defined in Rule 5605(a)(2) of the NASDAQ listing standards). The Compensation Committee met two (2) times during the fiscal year ended December 31, 2009. The Compensation Committee has adopted a written charter that is available to stockholders on the Company’s website at www.vaxgen.com; however, information found on our website is not incorporated by reference into this Annual Report.
nominating and governance committee The Nominating and Governance Committee is responsible for identifying, reviewing and evaluating candidates to serve as directors of the Company (consistent with criteria approved by the Board of Directors); selecting and recommending to the Board of Directors candidates for election to the Board of Directors; reviewing and making recommendations to the Board of Directors regarding the membership of the committees of the Board of Directors; reviewing and investigating conduct alleged to be in violation of the Company’s Code of Business Conduct and Ethics; reviewing and recommending changes to the Company’s Bylaws; reviewing and assessing the Company’s processes and procedures of providing information to the Board of Directors and its Committees; developing a set of corporate governance principles for the Company; reviewing, discussing and assessing performance of the Board of Directors and its Committees; annually recommending to the Board of Directors Chairmanship and membership of each committee; reviewing with the President plans for succession to the office of the Company’s President and making recommendations to the Board of Directors with respect to selection of an appropriate successor; and annually reviewing, discussing and assessing its own performance and periodically reviewing and assessing the adequacy of the charter of the Nominating Committee. The Nominating and Corporate Governance Committee has adopted a written charter that is available to stockholders on the Company’s website at http://www.vaxgen.com; however, information found on our website is not incorporated by reference into this Annual Report. Two directors comprise the current Nominating Committee: Messrs. Berger, and Reilly. All members of the Nominating Committee are independent (as independence is currently defined in Rule 5605(a)(2) of the NASDAQ listing standards). The Nominating Committee did not meet during the fiscal year ended December 31, 2009.
The Nominating Committee believes that candidates for director should have certain minimum qualifications, including being able to read and understand basic financial statements and having the highest personal integrity and ethics. The Nominating Committee also intends to consider such factors as possessing relevant expertise upon which to be able to offer advice and guidance to management, having sufficient time to devote to the affairs of the Company, demonstrated excellence in his or her field, possessing relevant experience in and knowledge of the biotechnology industry, having the ability to exercise sound business judgment and having the commitment to rigorously represent the long-term interests of the Company’s stockholders. However, the Nominating Committee reserves the right to modify these qualifications from time to time. The Nominating Committee uses its network of contacts to compile a list of potential candidates, but may also engage, if it deems appropriate, a professional search firm. The Nominating Committee conducts any appropriate and necessary inquiries into the backgrounds and qualifications of possible candidates after considering the function and needs of the Board of Directors. The Nominating Committee meets to discuss and consider such candidates’ qualifications and then selects a nominee for recommendation to the Board of Directors by majority vote. To date, the Nominating Committee has not paid a fee to any third party to assist in the process of identifying or evaluating director candidates. The Nominating Committee will consider director candidates recommended by stockholders. The Nominating Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether the candidate was recommended by a stockholder or not. Stockholders who wish to recommend individuals for consideration by the Nominating Committee to become nominees for election to the Board of Directors may do so by delivering a written recommendation to the Nominating and Governance Committee at the following address: 379 Oyster Point Boulevard, Suite 10, South San Francisco, California, 94080, at least 120 days prior to the anniversary date of the mailing of the Company’s proxy statement for the last Annual Meeting of Stockholders or, if the date of the Annual Meeting of Stockholders has changed by more than 30 days from the prior year, then within a reasonable amount of time. Submissions must include the full name of the proposed nominee, a description of the proposed nominee’s business experience for at least the previous five years, complete biographical information, a description of the proposed nominee’s qualifications as a director and a representation that the nominating stockholder is a beneficial or record owner of the Company’s stock. Any such submission must be accompanied by the written consent of the proposed nominee to be named as a nominee and to serve as a director if elected.
strategic transactions committee
The Strategic Transactions Committee is responsible for identifying, reviewing and evaluating potential strategic transactions and alternatives thereto, in cooperation and consultation with the Company’s advisors, and making recommendations to the Board of Directors with regard to strategic transactions. Other than Mr. Panek, all members of the Strategic Transactions Committee are independent (as independence is currently defined in Rule 5605(a)(2) of the NASDAQ listing standards). Three directors comprised the Strategic Transactions Committee: Dr. Rafield and Messrs. DeStefano and Panek. The Strategic Transaction Committee met informally on an ad hoc basis during 2009 and was disbanded in September 2009.
48
Code of Ethics
The Company has adopted the VaxGen, Inc. Code of Business Conduct and Ethics, as revised, or Code, consolidating and restating the formerly separate Code of Business Conduct, Code of Ethics for Chief Executive Officer and Senior Financial Officers, and “Whistle-Blowing” and Complaint Policy. This Code applies to all officers, directors and employees. The Code is available on our website at http://www.vaxgen.com; however, information found on our website is not incorporated by reference into this Annual Report. If the Company makes any substantive amendments to the Code or grants any waiver from a provision of the Code to any executive officer or director, the Company intends to promptly disclose the nature of the amendment or waiver on its website.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, or 1934 Act, requires the Company’s directors and executive officers, and persons who own more than ten percent of a registered class of the Company’s equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on a review of the copies of such reports furnished to the Company and written representations that no other reports were required, during the fiscal year ended December 31, 2009, its officers, directors and greater than ten percent beneficial owners complied with all applicable Section 16(a) filing requirements.
Item 11. Executive Compensation
COMPENSATION DISCUSSION AND ANALYSIS
Overview
The Company’s executive compensation program is intended to align executive goals and rewards with the Company and stockholder goals and progress. This description of compensation policies and practices applies to the Company’s President and former Chief Executive Officer referred to as the Named Executive Officer or NEO.
Role of our Compensation Committee
The Compensation Committee acts on behalf of the Board of Directors in fulfilling the Board of Directors’ responsibilities to oversee the Company’s compensation policies, plans and programs, and to review and determine the compensation to be paid to the Company’s executive officers and directors; compensation includes salary, bonuses, perquisites, equity incentives, severance arrangements, retirement benefits and other related benefits and benefit plans. The Compensation Committee is composed entirely of non-employee directors.
Historically, the Compensation Committee has evaluated corporate performance objectives and made or proposed adjustments to annual compensation and determined bonus and equity awards at one or more meetings held during the first quarter of the year or at the end of the preceding year. However, the Compensation Committee also considers matters related to individual compensation, such as compensation for new executive hires, as well as high-level strategic issues, such as the efficacy of the Company’s compensation strategy, potential modifications to that strategy and new trends, plans or approaches to compensation, at various meetings throughout the year. Generally, the Compensation Committee’s process comprises two related elements: the evaluation of performance objectives and the determination of compensation levels. In the case of the President, the evaluation of his performance is conducted by the Compensation Committee, which proposes to the Board of Directors adjustments to his compensation as well as awards to be granted.
Compensation Program Objectives
The Company’s executive compensation program is designed to achieve the following objectives:
| | • | | attract and retain talented and experienced executives in an extremely competitive labor market of biotechnology companies located in Northern California; |
| |
| | • | | motivate and reward key contributors whose knowledge; |
| |
| | • | | provide a compensation package that includes performance-based rewards and aligns rewards with accomplishment of objectives; |
| |
| | • | | provide performance-based rewards for the accomplishment of planned Company’s and/or individual’s achievement of goals; |
49
| | • | | recognizing the contributions each executive makes to the Company’s progress and achievement of corporate goals; and |
| |
| | • | | foster teamwork and a shared commitment among executives to overall corporate progress by aligning the Company’s and their individual goals. |
Components of the Executive Compensation Program
For 2009, the principal components of the Company’s executive compensation program consisted of:
| | • | | base salary; |
| |
| | • | | equity incentives in the form of stock options; |
| |
| | • | | severance protection; and |
| |
| | • | | other components of executive compensation. |
Base Salary. Base salary is intended to enable the Company to attract and retain executives with greater than average experience and skills, when compared to comparable biotechnology companies. For each executive position, the Company sets as its target base compensation between the 50th and 75th percentile of compensation compared to peer company data for benchmarked, comparable positions.
Stock Options. The Company grants stock options to its executives, as well as its employees, to provide long-term incentives that align the interests of its employees with the achievement of the Company’s long-term development programs and the interests of our stockholders over the long term. Given the time periods involved in biopharmaceutical development, the Company believes that these long-term incentives are critical to the Company’s success. The fair market value of our grants of equity awards is generally the closing price of our common stock on the effective date of approval of the grant by the Board of Directors or Compensation Committee.
Severance Protection. The Company may make Termination and Change in Control payments to certain of its executive officers under certain circumstances. The Company determined that peer companies commonly offered comparable benefits. Given the risks associated with the biopharmaceutical industry and the increasing frequency of acquisitions in the industry, the Compensation Committee continues to believe that severance protection is necessary to attract and retain qualified executives. These potential benefits are more fully described below in the Potential Payments Upon Termination or Change in Control table.
Other Components of Executive Compensation Program. The remaining components of the Company’s executive compensation program, like its broader employee compensation programs, are intended to make the Company’s overall compensation program competitive with those of its peer companies and include health insurance and life and disability insurance plans which are available to all Company employees.
The Company utilizes short-term compensation, including base salary, to recognize the experience, skills, knowledge and responsibilities required of each named executive officer, to meet competitive market conditions, and to motivate and reward key executives to perform. In addition, equity incentives, through the grant of stock options, are designed to directly align interests of the executive officers with the interests of the stockholders over the long term and encourage the growth of stockholder value through upside potential. The Company addressed this through maintenance of equity ownership levels for the Principal Executive Officer consistent with market comparisons.
Competitive Market Review
Historically, the Compensation Committee reviews executive compensation of the Named Executive Officers with those reported for peer companies in the Northern California biotechnology industry to ensure that total compensation (base salary, annual bonus targets and stock ownership) is market competitive, based on business and individual performance, as well as fair, based on internal equity in pay practices.
In 2009, the Compensation Committee did not use any formal benchmarking data or surveys to establish compensation levels, and instead generally relied upon publicly-available information regarding compensation levels of similar biotechnology and pharmaceutical companies in Northern California as well as its own general business knowledge to design compensation packages that it believes are competitive and provide appropriate reward opportunities for achieving high levels of performance, compared to those similar organizations in the marketplace.
50
Performance and Compensation Process
At the beginning of each year, the Board of Directors in consultation with the Principal Executive Officer establishes corporate goals that it believes are the most significant objectives for the Company in the upcoming year and that are critical to the success of the Company in the short and long term. These corporate goals normally include departmental, functional goals as well as project-based, cross-functional goals. These corporate goals typically include associated timelines and are normally reviewed and may be updated or adjusted by the Board of Directors in consultation with the Principal Executive Officer at mid- year, if determined appropriate. In 2009, the corporate goals primarily revolved around an anticipated strategic transaction, divestment of the Company’s manufacturing facility, and conservation of cash. The Company does not disclose the specific target levels for its performance goals as they contain competitively sensitive information and are not material to an understanding of compensation awards to the Named Executive Officers.
James P. Panek, President
Actions for 2008
| | • | | Annual Performance Bonus. In 2009, the Board of Directors awarded Mr. Panek a cash bonus of $58,500 related to 2008 performance, representing 15% of his 2008 base salary. |
Actions for 2009
| | • | | Amended and Restated Employment Agreement. In 2009, the Board of Directors approved an amendment and restatement of Mr. Panek’s executive employment agreement, or the Panek Agreement. In connection with the Panek Agreement, Mr. Panek agreed to resign as Chief Executive Officer and reduce his full-time schedule to a part-time schedule of at least 25 hours per week. The Panek Agreement provided for a one-time cash payment in 2009 by the Company to Mr. Panek in the amount of $193,050. Under the Panek Agreement, Mr. Panek will receive a reduced annual base salary of $195,000, equivalent to 50% of his previous base salary. |
Equity Grant Practices
Our equity grant date practices require that stock options and other equity compensation have prices determined based on the fair market value on the date of grant. The fair market value of our grants of equity awards is the closing price of our common stock on the effective date of approval of the grant by the Board of Directors or Compensation Committee. In February 2006, the Board of Directors authorized a modification to options held by employees who left the Company such that they would be permitted to exercise their options up to 90 days after the date the Company becomes listed on a national stock exchange or the date on which the Company is able to issue new registered shares of common stock. The Company issues new shares upon the exercise of options. Such modification also applies to the executive officers of the Company.
Executive Retention Program
In February 2007, the Board of Directors, at the recommendation of its Compensation Committee, adopted an executive retention program. The Board of Directors determined that it was imperative to retain the Company’s current executives to negotiate and execute any potential transaction that was in the best interests of stockholders. The retention program consists of a special stock option award and conditional cash payments.
Special retention stock option awards were granted to the Company’s current executives with a vesting commencement date of February 12, 2007 with an exercise price of $2.23, the closing market price of the Company’s common stock on that date. Each option award vests monthly on a pro-rata basis over a 48-month period in accordance with the Company’s normal option vesting policy.
The respective awards are as shown below:
| | | | | |
| | | Number | |
| | | of | |
| Name | | Shares | |
| James P. Panek | | | 200,000 | |
The cash retention bonus payments under the retention program comprises two parts, each dependent upon the executive remaining a regular full-time employee of the Company in good standing at the time the condition is met. In addition, the second of the two retention bonus payments of the same amount would be made only to the extent its amount exceeds the intrinsic value of the then exercisable special retention stock options awarded under this program. The first payments under this program were made in July 2007 and the second and final payments in the amounts shown were made in 2008.
51
The total amount of the retention bonuses are shown below:
| | | | | |
| | | Retention | |
| | | Bonus | |
| Name | | Payments | |
| James P. Panek | | $ | 156,000 | |
Employment Agreements
In January 2009, the Company entered into an Amended and Restated Executive Employment Agreement with Mr. Panek, or the Panek Agreement, which superseded and replaced his Amended Agreement. Under the Panek Agreement, in contrast to his Amended Agreement, Mr. Panek is not eligible for the Annual Performance Bonus and the Change of Control Bonus. Consistent with his Amended Agreement, if Mr. Panek’s employment with VaxGen is terminated without cause, or if he resigns for Good Reason, as defined in the Panek Agreement, Mr. Panek would be entitled to receive as severance a lump sum payment of $193,050, less standard withholdings and deductions, and all of Mr. Panek’s outstanding unvested stock options or other equity awards would be accelerated and become immediately exercisable. In consideration of Mr. Panek’s agreement to forego certain severance benefits of $386,100 provided for under his Amended Agreement as a result of his reduction in time commitment to VaxGen and commensurate reduction in base salary effective February 2009, the Panek Agreement provided for a one-time cash payment to Mr. Panek in the amount of $193,050 which was paid during 2009.
Defined Contribution Plans
Through the first half of 2008, the Company had a 401(k) Retirement Plan, as amended, or 401(k) Plan, which covered substantially all full-time employees, including executive officers of the Company. The 401(k) Plan permitted eligible employees to defer a percentage of their annual compensation, subject to certain limitations imposed by the Code. The Company had the option to match a portion of employee contributions with Company common stock or cash. In 2008, all of the NEOs participated in the 401(k) Plan. In July 2008, the Company terminated the 401(k) Plan to reduce the financial and administrative impacts on the Company.
Other Elements of Compensation
Health Insurance
We provide comprehensive health insurance benefits for all our eligible employees and their eligible dependents, including executive officers. Upon termination, all eligible employees, including executive officers, are eligible for continuation coverage in accordance with federal COBRA law or applicable state law, at their own expense.
Life and Disability Insurance
We provide life and disability insurance for our employees including executive officers. We do not maintain key person insurance on any of our executive officers.
Perquisites
From time to time, the Board of Directors may grant perquisites to certain executive officers; however, no perquisites have been granted to any named executive officer in 2009 that aggregated $10,000 or more.
The following table shows compensation awarded to, paid to or earned by, the Company’s President and former Chief Executive Officer, referred to as the Named Executive Officer or NEO, at December 31, 2009:
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Non-Equity | | | | | | | |
| | | | | | | | | | | | | | | Incentive | | | All Other | | | | |
| | | | | | | | | | | Option | | | Plan | | | Annual | | | | |
| Name and Principal | | | | | | Salary | | | Award | | | Compensation | | | Compensation | | | Total | |
| Position | | Year | | | ($) | | | ($)(1)) | | | ($) | | | ($) | | | ($) | |
| James P. Panek | | | 2009 | | | | 211,250 | | | | 184,073 | | | | — | | | | 193,050 | (4) | | | 588,373 | |
| President | | | 2008 | | | | 390,000 | | | | 209,782 | | | | 58,500 | (2) | | | 9,100 | (5) | | | 667,382 | |
| | | | 2007 | | | | 390,000 | | | | 147,681 | | | | 230,500 | (3) | | | 9,000 | (5) | | | 777,181 | |
| | | |
| (1) | | This relates to the compensation cost we recognized in 2009, 2008 and 2007 on stock options granted in 2008 and 2007 and in prior years. Please see Note 2, Summary of Significant Accounting Policies and Note 10, Stock Options and Warrants in the Notes to Consolidated Financial Statements in this Annual Report for our accounting policy regarding FAS 123R and our valuation of option awards, respectively, in accordance with FAS 123R. At December 31, 2009, the market price of the Company’s common stock was less than the exercise price of all outstanding options. |
52
| | | |
| (2) | | Represents an annual performance bonus for 2008 that was paid in 2009. |
| |
| (3) | | Represents a $78,000 retention bonus paid in 2007, a $78,000 retention bonus paid in 2008, a $55,000 annual performance bonus for 2005 that was paid in 2007, and a $19,500 annual performance bonus for 2007 that was paid in 2008. |
| |
| (4) | | Represents a lump-sum payment under the 2009 Amended and Restated Employment Agreement. |
| |
| (5) | | Includes 401(k) Plan Company matching contributions |
No grants of plan-based awards to the NEOs occurred for the year ended December 31, 2009. No NEOs exercised any option during the year ended December 31, 2009. No NEOs acquired or vested in a stock award during the year ended December 31, 2009.
The following table provides certain information concerning each unexercised stock option held by the NEOs as of December 31, 2009.
OUTSTANDING EQUITY AWARDS AT DECEMBER 31, 2009
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Number of | | | Number of | | | | | | | |
| | | | | | | Securities | | | Securities | | | | | | | |
| | | | | | | Underlying | | | Underlying | | | | | | | |
| | | | | | | Unexercised | | | Unexercised | | | Option | | | | |
| | | | | | | Options | | | Options | | | Exercise | | | Option | |
| Name and Principal | | Grant | | | (#) | | | (#) | | | Price | | | Expiration | |
| Position | | Date | | | Exercisable | | | Unexercisable | | | ($) | | | Date | |
| James P. Panek | | | 2/7/2007 | | | | 432,498 | | | | 107,502 | | | | 2.23 | | | | 2/12/2017 | (1) |
| President | | | | | | | | | | | | | | | | | | | | |
| | | |
| (1) | | Each option award vests monthly on a pro-rata basis over a 48-month period following the vesting commencement date of February 12, 2007, in accordance with the Company’s normal option vesting policy. |
Termination or Change in Control
Pursuant to the Amended and Restated Employment Agreement in effect on December 31, 2009, Mr. Panek’s employment relationship is at-will. As such, Mr. Panek’s employment and/or the Amended Agreement may be terminated with or without cause and with or without advance notice, at any time by either the executive or by VaxGen. If Mr. Panek’s employment with VaxGen is terminated without cause or the executive resigns for Good Reason, the executive would be entitled to receive as severance a lump sum payment of $193,050, less standard withholdings and deductions, and all of the executive’s outstanding unvested stock options or other equity awards would be accelerated and become immediately exercisable.
Pursuant to the Severance Benefit Plan eligible officers receive: (a) salary continuation equal to two months of the employees’ base salary; and (b) full payment of the officers’ COBRA premiums for two months following termination, in addition to any severance benefits such officers are entitled to pursuant to other agreements they have entered into with the Company. Upon termination, Mr. Pfeffer and Mr. Whitehead received under the Severance Benefit plan, $50,000 and $49,674, respectively. These amounts are included in the Actual Payments Upon Termination table below.
53
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
AT DECEMBER 31, 2009
| | | | | | | | | | | | | |
| | | | | | | | | | | Termination | |
| | | | | | | | | | | without Cause | |
| | | | | | | | | | | by the | |
| | | | | | | Termination | | | Company or | |
| | | | | | | without Cause | | | Good Reason | |
| | | | | | | by the | | | Resignation (in | |
| | | | | | | Company or | | | connection with | |
| | | | | | | Good Reason | | | a Change in | |
| | | | | | | Resignation | | | Control) | |
| Name and Principal Position | | | | | | ($)(1) | | | ($)(1) | |
| James P. Panek, President | | | | | | | | | | | | |
| Base salary | | | (2 | ) | | | 193,050 | | | | 193,050 | |
| Vesting acceleration | | | (3 | ) | | | 181,355 | | | | 181,355 | |
| | | | | | | | | | | |
| | | | | | | | 374,405 | | | | 374,405 | |
| | | | | | | | | | | |
| | | |
| (1) | | Amounts shown for potential payments assume the triggering event took place on the last business day of VaxGen’s last completed fiscal year, December 31, 2009. On that date, the closing price of the Company’s common stock was $0.49. |
| |
| (2) | | Severance pay equal to 50 percent of base salary. |
| |
| (3) | | Compensation charge to the Company resulting from the accelerated vesting of unvested stock options. |
Compensation of Directors
Each member of the Board of Directors who is not the chairperson receives a $25,000 annual retainer, and the chairperson of the Board of Directors receives $40,000 annual retainer. Each member, who is not the chairperson, of the Audit Committee, Compensation Committee and Nominating and Governance Committee receives an additional $7,500, $5,000 and $2,500 annual retainer, respectively.
With respect to the Strategic Transactions Committee, Dr. Rafield was entitled to receive $32,000 per month, Mr. DeStefano was entitled to receive $27,000 per month and Mr. Panek was not and is not entitled to any compensation. The members of the Board of Directors are also eligible for reimbursement for their expenses incurred in attending meetings of the Board of Directors in accordance with Company policy. The Strategic Transactions Committee was disbanded in September 2009.
Each non-employee director of the Company is also eligible to receive stock option grants under the 1998 Director Stock Option Plan, or Director Plan. Only non-employee directors of the Company are eligible to receive options under the Director Plan. Options granted under the Director Plan are intended by the Company not to qualify as incentive stock options under the Internal Revenue Code. In 2008, the four non-employee directors received annual grants of 17,500 options. These options vest monthly over four years from the grant date. For the year ended December 31, 2009, no options had been exercised under the Director Plan.
The following table shows certain information with respect to the compensation of all non-employee directors of the Company for the year ended December 31, 2009:
DIRECTOR COMPENSATION FOR FISCAL YEAR 2009
| | | | | | | | | | | | | |
| | | Fees Earned | | | | | | | |
| | | or Paid in | | | Option | | | | |
| | | Cash | | | Awards | | | Total | |
| Name of Director | | ($) | | | ($)(1)(2) | | | ($) | |
| Franklin M. Berger, CFA | | | 45,625 | | | | 17,425 | | | | 63,050 | |
| Paul DeStefano (3) | | | 249,542 | | | | 3,920 | | | | 253,462 | |
| Lori F. Rafield, Ph.D. | | | 372,500 | (4) | | | 3,920 | | | | 376,420 | |
| Kevin Reilly | | | 60,625 | | | | 22,831 | | | | 83,456 | |
| | | |
| (1) | | This relates to the compensation cost we recognized in 2009 on stock options granted in 2009 and prior years. Please see Note 2, Summary of Significant Accounting Policies and Note 11, Stock Options and Warrants, elsewhere in this Annual Report for our accounting policy regarding ASC 718 and our valuation of option awards, respectively, in accordance with FAS 123R. |
54
| | | |
| (2) | | Includes the following stock options outstanding at December 31, 2009: Mr. Berger — 82,500 shares; Mr. DeStefano — 10,625 shares; Dr. Rafield — 30,000 shares; Mr. Reilly — 65,000 shares. |
| |
| (3) | | Effective December 1, 2009, Mr. DeStefano resigned from the Board of Directors. |
| |
| (4) | | Includes $84,000 paid to Dr. Rafield for consulting services provided to VaxGen during 2009. |
Compensation Committee Interlocks and Insider Participation
Messrs. Berger, Reilly and Dr. Rafield served as members of our Compensation Committee during 2009. None of the members of our Compensation Committee was, at any time since our formation, an officer or employee of VaxGen. None of our executive officers serves as a member of the Board of Directors or Compensation Committee of any entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
COMPENSATION COMMITTEE REPORT
Our Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with management and, based on such review and discussions, our Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
COMPENSATION COMMITTEE
Franklin M. Berger
Lori F. Rafield, Chairperson
Kevin L. Reilly
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth certain information regarding the ownership of the Company’s common stock as of February 26, 2010 by: (i) each director; (ii) the Named Executive Officers; (iii) all executive officers and directors of the Company as a group; and (iv) all those known by the Company to be beneficial owners of more than five percent of its common stock. Unless otherwise noted, the address for the person or entity listed in the table is c/o VaxGen, Inc., 379 Oyster Point Blvd., Suite 10, South San Francisco, California, 94080:
Beneficial Ownership (1)
| | | | | | | | | |
| | | Number of | | | Percent of | |
| Beneficial Owner | | Shares | | | Total | |
| Entities affiliated with Gruber & McBaine Capital Management, LLC, 50 Osgood Place, Penthouse, San Francisco, CA, 94133 (2) | | | 4,438,150 | | | | 13.3 | |
| Entities affiliated with BIP GP LLC, 29 Commonwealth Avenue, 10th Floor, Boston, MA, 02116 (3) | | | 3,029,926 | | | | 9.1 | |
| Entities affiliated with ROI Capital Management, Inc., 300 Drakes Landing Road, Suite 175, GreenBrae, CA 94904 (4) | | | 2,729,610 | | | | 8.2 | |
| | | | | | | | | |
Directors and Executive Officers | | | | | | | | |
| Franklin M. Berger, Director (5) | | | 65,611 | | | | * | |
| James P. Panek, President, Principal Executive and Financial Officer and Director (5) | | | 483,354 | | | | 1.4 | |
| Lori Rafield, Director (5) | | | 13,681 | | | | * | |
| Kevin Reilly, Director (5) | | | 45,477 | | | | * | |
| | | | | | | | | |
| All executive officers and directors as a group (4 persons) (5) | | | 608,123 | | | | 1.8 | |
| | | |
| * | | Less than one percent |
| |
| (1) | | This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13D and 13G filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 33,106,523 shares outstanding on February 26, 2010 adjusted as required by rules promulgated by the SEC. |
55
| | | |
| (2) | | Gruber & McBaine Capital Management, LLC (“GMCM”) is a registered investment advisor whose clients have the right to receive or the power to direct the receipt of dividends from, or the proceeds from the sale of the Company’s stock. Jon D. Gruber and J. Patterson McBaine are the Managers, controlling persons and portfolio managers of GMCM. No individual client’s holding of the Company’s stock is more than five percent of the outstanding stock of the Company. |
| |
| (3) | | The shares may be deemed beneficially owned by (i) a private investment partnership of which BIP GP LLC is the sole general partner and Weiss Asset Management L.P. (“Weiss Asset Management”) is the sole investment manager and (ii) a private investment corporation for which Weiss Asset Management is the sole investment manager. Andrew Weiss is the Managing Member of BIP GP LLC and the Managing Member of the general partner of Weiss Asset Management, as such, the shares may be deemed beneficially owned by Mr. Weiss. Mr. Weiss disclaims beneficial ownership of the shares except to the extent of his pecuniary interest therein. |
| |
| (4) | | ROI Capital Management, Inc. (“ROI”) is a registered investment advisor whose clients have the right to receive or the power to direct the receipt of dividends from, or the proceeds from the sale of the Company’s stock. Mitchell J. Soboleski and Mark T. Boyer are the controlling persons of ROI. By reason of the provisions of Rule 13d-3 of the Securities Exchange Act of 1934, each of ROI, Mr. Soboleski and Mr. Boyer may be deemed to own beneficially 2,729,610 shares of the Company’s stock. |
| |
| (5) | | Includes options under the Company’s stock option plans potentially exercisable within 60 days of February 26, 2009 for the following number of shares: Mr. Berger — 65,611; Mr. Panek — 464,176; Dr. Rafield — 13,681; Mr. Reilly — 45,477; and all current executive officers and directors as a group — 588,945 options. |
Securities Authorized for Issuance under Equity Compensation Plans
The table below sets forth certain information with respect to our common stock which is authorized for issuance under our equity compensation plans, principally stock option plans as of December 31, 2009.
| | | | | | | | | | | | | |
| | | | | | | | | | | Number of | |
| | | | | | | | | | | securities | |
| | | Number of | | | | | | | remaining | |
| | | securities to | | | | | | | available for | |
| | | be | | | | | | | issuance under | |
| | | issued upon | | | Weighted- | | | equity | |
| | | exercise of | | | average exercise | | | compensation | |
| | | outstanding | | | price of | | | plans | |
| | | options, | | | outstanding | | | (excluding | |
| | | warrants | | | options, | | | securities | |
| | | and | | | warrants and | | | reflected in | |
| | | rights | | | rights | | | column (a)) | |
| Plan Category | | (a) | | | (b) | | | (c) | |
| Equity compensation plans approved by security holders | | | 1,382,911 | | | $ | 6.03 | | | | 7,440,490 | |
| | | | | | | | | | | | | |
| Equity compensation plans not approved by security holders (1) | | | 400,000 | | | $ | 14.90 | | | | 150,000 | |
| | | | | | | | | | | |
Total | | | 1,782,911 | | | $ | 8.02 | | | | 7,590,490 | |
| | | | | | | | | | | |
| | | |
| (1) | | In September 2001, VaxGen granted employment inducement stock options for 400,000 shares of VaxGen common stock outside the Plan to a newly hired executive with an exercise price of $14.90 per share. In October 2004, VaxGen granted stock options for 120,000 shares and 30,000 shares outside the approved plans to a new executive and director with an exercise price of $11.40 per share and $12.27 per share, respectively. These options were granted without stockholder approval, but pursuant to NASDAQ Marketplace Rules then applicable to VaxGen on terms that are substantially in accordance with VaxGen’s standard stock option terms. As of December 31, 2009, none of these stock options have been exercised or repurchased; however, 67,500 of these options were canceled during 2006 upon the termination of the executive to whom the options had been granted. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
The Company did not enter into any related person transactions in which certain of the Company’s executive officers, directors or greater than five percent stockholders or any members of the immediate family of any of the foregoing had or have a direct or indirect material interest. All future related party transactions, including any loans from the Company to its employees, officers, directors, principal stockholders or affiliates, will be approved by a majority of the Board of Directors, including a majority of the independent and disinterested members of the Board of Directors or, if required by law, a majority of disinterested stockholders, and will be on terms no less favorable to the Company than could be obtained from unaffiliated third parties. In addition, our Code encourages employees to discuss any potential related-party transaction with our compliance officer and officers and directors to seek authorization from the Audit Committee.
56
Related-Person Transactions Policy
The Company has a written Related-Person Transactions Policy to establish the procedures for the identification, review, consideration and approval or ratification of transactions involving the Company and any Related Person (as defined below) by the Audit Committee of the Board of Directors or by such other committee of the Board of Directors as appropriate. This policy reinforces the Company’s Code in which these matters are addressed generally. The Nominating and Governance Committee is responsible for establishing this policy and, from time to time, will review and recommend to the Board of Directors any amendments to this policy.
Under the policy, a Related Person means:
| | • | | person who is, or at any time since the beginning of the Company’s last fiscal year, was, a director or executive officer of the Company or a nominee to become a director of the Company; |
| |
| | • | | security holder known by the Company to be the beneficial owner of more than 5% of any class of the Company’s voting securities, or a Significant Stockholder; |
| |
| | • | | immediate family member of any of the foregoing, which means any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law of such person, and any person (other than a tenant or employee) sharing the household of such person; and |
| |
| | • | | firm, corporation or other entity in which any of the foregoing persons is an executive, partner or principal or similar control position or in which such person has a 5% or greater beneficial ownership interest, or an Affiliate. |
A Related-Person Transaction is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which the Company and any Related Person are, were or will be participants in which the amount involved exceeds $120,000. Transactions involving compensation for services provided to the Company as an employee, consultant or director shall not be considered Related-Person Transactions under this policy.
Each director and executive officer shall identify, and the Company shall request each Significant Stockholder to identify, any Related-Person Transaction involving such director or executive officer or his or her Affiliates and immediate family members and seek approval from the Committee pursuant to this policy before he or she or, with respect to immediate family members, any of their Affiliates, may engage in the transaction.
Any proposed transaction that has been identified as a Related Person Transaction may be consummated or materially amended only after approval by the Audit Committee in accordance with the provisions of this policy. If for reasons of conflict of interest or other reasons, it is inappropriate for the Audit Committee to review the transaction, after taking into account possible recusals by Audit Committee members, the Related-Person Transaction shall be approved by another independent body of the Board of Directors. The approving body shall be referred to in this policy as the Committee.
The Committee shall approve only those Related-Person Transactions that, in light of known circumstances, are in, or are not inconsistent with, the best interests of the Company and its stockholders, as the Committee determines in the good faith exercise of its discretion.
The Company has entered into indemnity agreements with each of its executive officers and directors which provide, among other things, that the Company will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgments, fines and settlements he or she may be required to pay in actions or proceedings which he or she is or may be made a party by reason of his or her position as a director, officer or other agent of the Company, and otherwise to the fullest extent permitted under Delaware law and the Company’s Bylaws.
Independence of Board of Directors
While the Company is not currently listed on a national securities exchange, it has used the listing standards of NASDAQ to evaluate the independence of its Board of Directors and the committees thereof. The NASDAQ listing standards require that a majority of the members of a listed company’s Board of Directors qualify as independent, as affirmatively determined by the Board of Directors. The Board of Directors consults with the Company’s counsel to ensure that the Board of Directors’ determinations are consistent with all relevant securities and other laws and regulations regarding the definition of independent, including those set forth in pertinent listing standards of NASDAQ, as in effect from time to time.
57
Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his or her family members, and the Company, its senior management and its independent registered public accounting firm, the Board of Directors affirmatively has determined that all of the Company’s directors are independent directors within the meaning of the applicable NASDAQ listing standards, except for Mr. Panek, the President of the Company.
Item 14. Principal Accountant Fees and Services
The following table represents aggregate fees billed to the Company for work performed during the years ended December 31, 2009 and 2008, by PricewaterhouseCoopers LLP, or PwC, the Company’s Independent Registered Public Accounting Firm during those years, respectively (in thousands):
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Audit fees (1) | | $ | 501 | | | $ | 666 | |
| Audit-related fees | | | — | | | | — | |
| Tax fees | | | — | | | | — | |
| All other fees | | | — | | | | — | |
| | | | | | | |
| Total Fees | | $ | 501 | | | $ | 666 | |
| | | | | | | |
| | | |
| (1) | | Includes fees for services that normally would be provided by an independent registered public accounting firm in connection with regulatory filings. Also includes fees necessary to perform an audit or review in accordance with auditing standards generally accepted in the United States of America and fees for services that generally only the independent registered public accounting firm can reasonably provide. |
Company policy requires the Audit Committee to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm. Management is responsible for reporting to the Audit Committee on a quarterly basis regarding expenditures for non-audit services made in accordance with this pre-approval. All audit and non-audit services were pre-approved in accordance with Company policy for the years ended December 31, 2009 and 2008.
PART IV
Item 15. Exhibits and Financial Statement Schedule
(a) (1) Financial Statements
| | | | | |
| | | Page | |
VaxGen, Inc. | | | | |
| | | | | |
| | | 21 | |
| | | | | |
| | | 22 | |
| | | | | |
| | | 23 | |
| | | | | |
| | | 24 | |
| | | | | |
| | | 25 | |
| | | | | |
| | | 26 | |
| | | | | |
(a) (2) Financial Statement Schedule
58
SCHEDULE II
VaxGen, Inc.
VALUATION AND QUALIFYING ACCOUNTS
(in thousands)
| | | | | | | | | | | | | | | | | |
| | | Balance at | | | | | | | | | | | Balance at | |
| | | Beginning of | | | | | | | | | | | End of | |
| | | Year | | | Additions | | | Deductions | | | Year | |
Tax valuation allowance Year ended | | | | | | | | | | | | | | | | |
| 2009 | | $ | 84,521 | | | $ | — | | | $ | (2,977 | ) | | $ | 81,544 | |
| 2008 | | $ | 79,355 | | | $ | 5,166 | | | $ | — | | | $ | 84,521 | |
| 2007 | | $ | 70,652 | | | $ | 8,703 | | | $ | — | | | $ | 79,355 | |
(a)(3) Exhibits (Note: Management contracts and compensatory plans or arrangements are identified with a “+” in the following list.)
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 2.1 | | | Agreement and Plan of Merger, dated November 12, 2007, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 11-13-07 | | | 2.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 2.2 | | | Amendment No. 1 to Agreement and Plan of Merger, dated December 20, 2007, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | S-4 | | 333-148312 | | 12-24-07 | | | 2.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 2.3 | | | Amendment No. 2 to Agreement and Plan of Merger, dated February 6, 2008, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | S-4/A | | 333-148312 | | 02-06-08 | | | 2.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.1 | | | Amended and Restated Certificate of Incorporation. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.2 | | | Amendment to the Amended and Restated Certificate of Incorporation. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.4 | | | Amended and Restated Bylaws, dated as of May 18, 2007. | | 8-K | | 000-26483 | | 05-23-07 | | | 3.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.5 | | | Amendment to the Amended and Restated Certificate of Incorporation, dated as of August 10, 2005. | | 10-Q | | 0-26483 | | 05-31-07 | | | 3.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.1 | | | Reference is made to Exhibits 3.1, 3.2, 3.4 and 3.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | 4.2 | | | Certificate of Designations, Rights and Preferences of Series A 6% Cumulative Convertible Preferred Stock. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.2 | | | |
59
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | �� | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 4.3 | | | Securities Purchase Agreement by and among Registrant and Certain Stockholders. | | 8-K | | 000-26483 | | 5-24-01 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.4 | | | Registration Rights Agreement by and among Registrant and Certain Stockholders. | | 8-K | | 000-26483 | | 5-24-01 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.5 | | | Form of Common Stock Purchase Warrant. | | 8-K | | 000-26483 | | 5-24-01 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.6 | | | Specimen Stock Certificate for Common Stock of Registrant. | | S-1 | | 333-78065 | | 6-11-99 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.1 | | | Registration Rights Agreement between VaxGen and Genentech, dated as of May 5, 1997. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.2 | | | Form of 1996 Registration Rights Agreement between VaxGen and certain stockholders. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.3 | | | Form of 1998 Registration Rights Agreement between VaxGen and certain stockholders. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.5 | | | 1998 Director Stock Option Plan. + | | S-1 | | 333-78065 | | 5-7-99 | | | 10.5 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.6 | | | Form of stock option agreement. + | | S-1 | | 333-78065 | | 5-7-99 | | | 10.6 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.7 | | | Form of common stock warrant. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.7 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.14 | | | Lease Agreement between VaxGen and Oyster Point Tech Center LLC, dated October 26, 1998. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.18 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.24 | | | Joint Venture Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.24 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.25 | | | Land Purchase and Sale Agreement between VaxGen and Incheon Metropolitan City, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.25 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.26 | | | Contribution Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.26 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.31 | | | Assignment Agreement between VaxGen and Celltrion, Inc., dated March 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.31 | | | |
60
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.35 | | | License Agreement between VaxGen and Celltrion, dated as of March 25, 2002. | | 10-Q | | 000-26483 | | 5-15-02 | | | 10.35 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.36 | | | Sub-License Agreement between VaxGen and Celltrion, dated as of March 25, 2002. | | 10-Q | | 000-26483 | | 5-15-02 | | | 10.36 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.39 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-25494, dated September 30, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.39 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.40 | | | Amendment of contract between VaxGen and the National Institutes of Health, under Contract No. N01-AI-95373, dated September 30, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.40 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.41 | | | Employment Agreement between VaxGen and Piers C. Whitehead, dated as of July 1, 2002. + | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.41 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.42 | | | Joint Venture Agreement between VaxGen and Celltrion, Inc., dated as of June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.42 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.43 | | | License Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.43 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.44 | | | Sub-License Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.44 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.45 | | | Consulting Services Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.45 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.46 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-95373, dated July 9, 1999. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.46 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.47 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-30053, dated September 30, 2003. | | 10-Q | | 000-26483 | | 11-19-03 | | | 10.47 | | | |
61
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.48 | | | 2001 Employee Stock
Purchase Plan + | | S-8 | | 333-10811 | | 08-21-03 | | | 99.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.49 | | | License Agreement between VaxGen and U.S. Army Medical Research Institute of Infectious Diseases, dated as of October 7, 2003. | | 8-K | | 000-26483 | | 12-02-03 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.51 | | | Partnership Agreement between VaxGen, Inc. and the Chemo-Sero-Therapeutic Research Institute. | | 10-K | | 000-26483 | | 03-30-04 | | | 10.51 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.58 | | | Amendment, dated July 14, 2004, to Joint Venture Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-Q | | 000-26483 | | 02-07-07 | | | 10.58 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.59 | | | Warrant Exchange Agreement, by and between VaxGen and CD Investment Partners, Ltd, dated September 21, 2004. | | 8-K | | 000-26483 | | 9-24-04 | | | 10.52 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.60 | | | Warrant Exchange Agreement by and between VaxGen and Societe Generale, Kepler Capital, LLC, Cheyenne LLC and Prism Capital 5, L.P., dated September 21, 2004. | | 8-K | | 000-26483 | | 9-24-04 | | | 10.53 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.63 | | | Stock Option Agreement between VaxGen and Myron Levine, dated October 21, 2004. + | | 8-K | | 000-26483 | | 10-27-04 | | | 10.58 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.64 | | | Supply Contract No. HHS0100200300001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc. dated November 4, 2004.* | | 8-K/A | | 000-26483 | | 11-17-04 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.65 | | | Agreement by and between VaxGen and Celltrion pursuant to which VaxGen acquired all of Celltrion’s interest in VaxGen-Celltrion, Inc., dated December 30, 2004 | | 10-K | | 0-26483 | | 02-07-07 | | | 10.65 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.66 | | | Revised Joint Venture Agreement between VaxGen and certain investors, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.66 | | | |
| �� | | | | | | | | | | | | | | | | |
| | 10.67 | | | Termination Agreement between VaxGen and Celltrion, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.67 | | | |
62
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.68 | | | Surrender Agreement between VaxGen and certain investors, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.68 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.69 | | | Technical Support and Services Agreement between VaxGen and Celltrion, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.69 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.70 | | | Form of Stock Purchase Agreement between VaxGen and certain institutional investors, entered into November 19, 2004. | | 8-K | | 000-26483 | | 11-24-04 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.71 | | | Form of 5 1 / 2 % Convertible Senior Subordinated Note due 2010. | | 8-K | | 0-26483 | | 4-11-05 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.72 | | | Indenture, dated April 5, 2005, between VaxGen and U.S. Bank National Association, as trustee. | | 8-K | | 0-26483 | | 4-11-05 | | | 4.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.73 | | | Form of Purchase Agreement between VaxGen and the purchasers of the 5 1 / 2 % Convertible Senior Subordinated Notes due 2010. | | 8-K | | 0-26483 | | 4-11-05 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.74 | | | Fifth Amendment to the Lease Agreement by and between VaxGen, Inc. and Oyster Point Tech Center LLC, dated April 14, 2005. | | 8-K | | 0-26483 | | 4-21-05 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.76 | | | Stock Purchase Agreement, dated September 15, 2005, by and between VaxGen and Nexol Co., Ltd. | | 8-K | | 0-26483 | | 10-31-05 | | | 10.59 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.80 | | | Contract No. HHSO100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc. dated November 4, 2004.* | | 8-K | | 0-26483 | | 1-12-06 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.81 | | | Form of Stock and Warrant Purchase Agreement entered into by and between VaxGen, Inc. and certain accredited investors, dated February 8, 2006. | | 8-K | | 0-26483 | | 2-15-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.82 | | | Form of Warrant issued by VaxGen, Inc. pursuant to the Stock and Warrant Purchase Agreements entered into by and between VaxGen, Inc. and certain accredited investors, dated February 8, 2006. | | 8-K | | 0-26483 | | 2-15-06 | | | 10.2 | | | |
63
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.83 | | | Executive Employment Agreement between Matthew J. Pfeffer and VaxGen, Inc., dated March 28, 2006. + | | 8-K | | 0-26483 | | 4-05-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.84 | | | Modification #01 to Contract No. HHS0100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc., dated May 5, 2006.* | | 8-K | | 0-26483 | | 5-11-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.85 | | | Share Purchase Agreement, between Nexol Co., Ltd., Nexol Biotech Co., Ltd., Nexol Venture Capital Co., Ltd. and VaxGen, Inc., dated June 28, 2006. | | 8-K | | 0-26483 | | 7-06-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.86 | | | Form of Amended and Restated Executive Employment Agreement. + | | 8-K | | 0-26483 | | 10-26-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.87 | | | Form of Indemnity Agreement. + | | 8-K | | 0-26483 | | 10-26-06 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.90 | | | Modification #04 to Contract No. HHS0100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc., dated December 19, 2006. | | 8-K | | 0-26483 | | 12-20-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.91 | | | Termination Letter — Contract No. HHS01002005000001C (11/4/04) “Acquisition of rPA Anthrax Vaccine for the Strategic National Stockpile” | | 8-K | | 0-26483 | | 12-20-06 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.93 | | | Form of Addendum to Warrant to Purchase Shares of Common Stock, at an exercise price of $16.00 per share, dated December 22, 2006. | | 8-K | | 0-26483 | | 12-27-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.94 | | | Bonus, Stock Option Awards, Stock Option Exchange Program, and Retention Awards of Named Executive Officers.+ | | 8-K | | 0-26843 | | 02-13-07 | | | | | | |
64
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.95 | | | Resignation Agreement between VaxGen, Inc. and Lance K. Gordon, Ph.D., dated February 6, 2007. + | | 10-Q | | 0-26843 | | 09-20-07 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.96 | | | Settlement Agreement by and between VaxGen, Inc., the Department of Health and Human Services and the National Institute of Allergy and Infectious Diseases, dated April 3, 2007.* | | 8-K | | 000-26843 | | 04-05-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.97 | | | Severance Benefit Plan, dated August 1, 2007. | | 8-K | | 000-26483 | | 08-07-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.98 | | | Sixth Amendment to Lease Agreement by and between VaxGen, Inc. and Oyster Point Tech Center LLC, dated October 11, 2007. | | 8-K | | 0-26483 | | 10-17-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.99 | | | Loan Agreement, dated November 12, 2007, between VaxGen. Inc. and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 11-13-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.100 | | | Note Repurchase Agreement, dated February 4, 2006, by and among VaxGen, Inc., Xmark Opportunity Fund, L.P. and Xmark Opportunity Fund, Ltd. | | 8-K | | 000-26483 | | 2-8-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.101 | | | Termination of Merger Agreement, Acknowledgment and Amendment to Loan Agreement and Secured Promissory Note, dated March 28, 2008, by and among VaxGen, Inc. and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 3-28-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.102 | | | Form of Amendment to Executive Employment Agreement | | 8-K | | 000-26483 | | 4-1-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.103 | | | Separation Agreement between VaxGen, Inc. and Matthew J. Pfeffer, dated April 7, 2008 | | 10-Q | | 000-26483 | | 5-14-2008 | | | 10.103 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.104 | | | VaxGen, Inc. Amended and
Restated 1996 Stock Option Plan. + | | 10-Q | | 000-26483 | | 11-10-2008 | | | 10.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.105 | | | Note Repurchase Agreement, dated July 1, 2008, by and between VaxGen, Inc. and Alexandra Global Master Fund Ltd. | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.106 | | | Amendment No. 1 to Note Repurchase Agreement, dated July 1, 2008 by and between Alexandra Global Master Fund Ltd. and VaxGen, Inc. | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.2 | | | |
65
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Incorporated by | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.107 | | | Note Repurchase Agreement, dated July 3, 2008, by and between VaxGen, Inc. and Whitebox Convertible Arbitrage Partners, LP | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.108 | | | Note Repurchase Agreement, dated July 3, 2008, by and between VaxGen, Inc. and Guggenheim Portfolio Company XXXI, LLC | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.109 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and QVT Fund LP. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.110 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and Quintessence Fund L.P. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.111 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and Deutsche Bank AG London. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.112 | | | Note Repurchase Agreement, dated October 8, 2008, by and between VaxGen, Inc. and Quattro Fund, Ltd. | | 8-K | | 000-26483 | | 10-10-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.113 | | | Note Repurchase Agreement, dated October 27, 2008, by and between VaxGen, Inc. and Drawbridge Special Opportunities Fund LP. | | 8-K | | 000-26483 | | 10-30-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.114 | | | Amended and Restated Executive Employment Agreement for James P. Panek, effective February 1, 2009. | | 8-K | | 000-26483 | | 1-29-2009 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 14.1 | | | VaxGen, Inc. Code of Business Conduct and Ethics | | 8-K | | 0-26483 | | 4-5-06 | | | 14.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 21.1 | | | Subsidiaries | | 10-K | | 0-26483 | | 8-30-07 | | | 21.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 24.1 | | | Power of Attorney (included in the signature pages hereto) | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | 31.1 | | | Certification required by Rule
13a-14(a) or Rule 15d-14(a) | | | | | | | | | | | | X |
| | | | | | | | | | | | | | | | | |
| | 32.1 | | | Certification required by Rule
13a-14(a) or Rule 15d-14(a) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350) | | | | | | | | | | | | X |
| | | |
| * | | Confidential treatment requested. The redacted portions have been separately filed with the SEC as required by Rule 406 of Regulation C. |
66
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| | VaxGen, Inc.
(Registrant)
| |
| | By: | /s/ James P. Panek | |
| | | James P. Panek | |
| March 29, 2010 | | President
(Principal Executive, Financial and Accounting officer) | |
| |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James P. Panek as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that said attorney-in-fact and agent or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | |
| By: | | /s/ James P. Panek | | March 29, 2010 |
| | | James P. Panek | | |
| | | President and Director | | |
| | | (Principal Executive, Financial and Accounting Officer) | | |
| | | | | |
| By: | | /s/ Franklin M. Berger | | March 29, 2010 |
| | | Franklin M. Berger | | |
| | | Director | | |
| | | | | |
| By: | | /s/ Lori F. Rafield | | March 29, 2010 |
| | | Lori F. Rafield. PhD | | |
| | | Director | | |
| | | | | |
| By: | | /s/ Kevin L. Reilly | | March 29, 2010 |
| | | Kevin L. Reilly | | |
| | | Director | | |
67
EXHIBIT INDEX
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 2.1 | | | Agreement and Plan of Merger, dated November 12, 2007, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 11-13-07 | | | 2.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 2.2 | | | Amendment No. 1 to Agreement and Plan of Merger, dated December 20, 2007, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | S-4 | | 333-148312 | | 12-24-07 | | | 2.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 2.3 | | | Amendment No. 2 to Agreement and Plan of Merger, dated February 6, 2008, by and among VaxGen, Inc., TLW Merger Sub, Inc., TLW, LLC, and Raven biotechnologies, inc. | | S-4/A | | 333-148312 | | 02-06-08 | | | 2.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.1 | | | Amended and Restated Certificate of Incorporation. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.2 | | | Amendment to the Amended and Restated Certificate of Incorporation. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.4 | | | Amended and Restated Bylaws, dated as of May 18, 2007. | | 8-K | | 000-26483 | | 05-23-07 | | | 3.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 3.5 | | | Amendment to the Amended and Restated Certificate of Incorporation, dated as of August 10, 2005. | | 10-Q | | 0-26483 | | 05-31-07 | | | 3.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.1 | | | Reference is made to Exhibits 3.1, 3.2, 3.4 and 3.5 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | 4.2 | | | Certificate of Designations, Rights and Preferences of Series A 6% Cumulative Convertible Preferred Stock. | | S-8 | | 333-84922 | | 3-26-02 | | | 4.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.3 | | | Securities Purchase Agreement by and among Registrant and Certain Stockholders. | | 8-K | | 000-26483 | | 5-24-01 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.4 | | | Registration Rights Agreement by and among Registrant and Certain Stockholders. | | 8-K | | 000-26483 | | 5-24-01 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.5 | | | Form of Common Stock Purchase Warrant. | | 8-K | | 000-26483 | | 5-24-01 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 4.6 | | | Specimen Stock Certificate for Common Stock of Registrant. | | S-1 | | 333-78065 | | 6-11-99 | | | 4.1 | | | |
68
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.1 | | | Registration Rights Agreement between VaxGen and Genentech, dated as of May 5, 1997. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.2 | | | Form of 1996 Registration Rights Agreement between VaxGen and certain stockholders. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.3 | | | Form of 1998 Registration Rights Agreement between VaxGen and certain stockholders. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.5 | | | 1998 Director Stock Option Plan. + | | S-1 | | 333-78065 | | 5-7-99 | | | 10.5 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.6 | | | Form of stock option agreement. + | | S-1 | | 333-78065 | | 5-7-99 | | | 10.6 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.7 | | | Form of common stock warrant. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.7 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.14 | | | Lease Agreement between VaxGen and Oyster Point Tech Center LLC, dated October 26, 1998. | | S-1 | | 333-78065 | | 5-7-99 | | | 10.18 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.24 | | | Joint Venture Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.24 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.25 | | | Land Purchase and Sale Agreement between VaxGen and Incheon Metropolitan City, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.25 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.26 | | | Contribution Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.26 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.31 | | | Assignment Agreement between VaxGen and Celltrion, Inc., dated March 25, 2002. | | 10-K | | 000-26483 | | 4-01-02 | | | 10.31 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.35 | | | License Agreement between VaxGen and Celltrion, dated as of March 25, 2002. | | 10-Q | | 000-26483 | | 5-15-02 | | | 10.35 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.36 | | | Sub-License Agreement between VaxGen and Celltrion, dated as of March 25, 2002. | | 10-Q | | 000-26483 | | 5-15-02 | | | 10.36 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.39 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-25494, dated September 30, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.39 | | | |
69
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.40 | | | Amendment of contract between VaxGen and the National Institutes of Health, under Contract No. N01-AI-95373, dated September 30, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.40 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.41 | | | Employment Agreement between VaxGen and Piers C. Whitehead, dated as of July 1, 2002. + | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.41 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.42 | | | Joint Venture Agreement between VaxGen and Celltrion, Inc., dated as of June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.42 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.43 | | | License Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.43 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.44 | | | Sub-License Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.44 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.45 | | | Consulting Services Agreement between VaxGen and VaxGen-Celltrion, Inc., dated June 7, 2002. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.45 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.46 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-95373, dated July 9, 1999. | | 10-Q | | 000-26483 | | 11-14-02 | | | 10.46 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.47 | | | Contract between VaxGen and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. N01-AI-30053, dated September 30, 2003. | | 10-Q | | 000-26483 | | 11-19-03 | | | 10.47 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.48 | | | 2001 Employee Stock
Purchase Plan + | | S-8 | | 333-10811 | | 08-21-03 | | | 99.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.49 | | | License Agreement between VaxGen and U.S. Army Medical Research Institute of Infectious Diseases, dated as of October 7, 2003. | | 8-K | | 000-26483 | | 12-02-03 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.51 | | | Partnership Agreement between VaxGen, Inc. and the Chemo-Sero-Therapeutic Research Institute. | | 10-K | | 000-26483 | | 03-30-04 | | | 10.51 | | | |
70
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.58 | | | Amendment, dated July 14, 2004, to Joint Venture Agreement between VaxGen and certain investors, dated February 25, 2002. | | 10-Q | | 000-26483 | | 02-07-07 | | | 10.58 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.59 | | | Warrant Exchange Agreement, by and between VaxGen and CD Investment Partners, Ltd, dated September 21, 2004. | | 8-K | | 000-26483 | | 9-24-04 | | | 10.52 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.60 | | | Warrant Exchange Agreement by and between VaxGen and Societe Generale, Kepler Capital, LLC, Cheyenne LLC and Prism Capital 5, L.P., dated September 21, 2004. | | 8-K | | 000-26483 | | 9-24-04 | | | 10.53 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.63 | | | Stock Option Agreement between VaxGen and Myron Levine, dated October 21, 2004. + | | 8-K | | 000-26483 | | 10-27-04 | | | 10.58 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.64 | | | Supply Contract No. HHS0100200300001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc. dated November 4, 2004.* | | 8-K/A | | 000-26483 | | 11-17-04 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.65 | | | Agreement by and between VaxGen and Celltrion pursuant to which VaxGen acquired all of Celltrion’s interest in VaxGen-Celltrion, Inc., dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.65 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.66 | | | Revised Joint Venture Agreement between VaxGen and certain investors, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.66 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.67 | | | Termination Agreement between VaxGen and Celltrion, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.67 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.68 | | | Surrender Agreement between VaxGen and certain investors, dated December 30, 2004 | | 10-K | | 0-26483 | | 02-07-07 | | | 10.68 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.69 | | | Technical Support and Services Agreement between VaxGen and Celltrion, dated December 30, 2004. | | 10-K | | 0-26483 | | 02-07-07 | | | 10.69 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.70 | | | Form of Stock Purchase Agreement between VaxGen and certain institutional investors, entered into November 19, 2004. | | 8-K | | 000-26483 | | 11-24-04 | | | 4.1 | | | |
71
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.71 | | | Form of 5 1 / 2% Convertible Senior Subordinated Note due 2010. | | 8-K | | 0-26483 | | 4-11-05 | | | 4.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.72 | | | Indenture, dated April 5, 2005, between VaxGen and U.S. Bank National Association, as trustee. | | 8-K | | 0-26483 | | 4-11-05 | | | 4.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.73 | | | Form of Purchase Agreement between VaxGen and the purchasers of the 5 1 / 2% Convertible Senior Subordinated Notes due 2010. | | 8-K | | 0-26483 | | 4-11-05 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.74 | | | Fifth Amendment to the Lease Agreement by and between VaxGen, Inc. and Oyster Point Tech Center LLC, dated April 14, 2005. | | 8-K | | 0-26483 | | 4-21-05 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.76 | | | Stock Purchase Agreement, dated September 15, 2005, by and between VaxGen and Nexol Co., Ltd. | | 8-K | | 0-26483 | | 10-31-05 | | | 10.59 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.80 | | | Contract No. HHSO100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc. dated November 4, 2004.* | | 8-K | | 0-26483 | | 1-12-06 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.81 | | | Form of Stock and Warrant Purchase Agreement entered into by and between VaxGen, Inc. and certain accredited investors, dated February 8, 2006. | | 8-K | | 0-26483 | | 2-15-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.82 | | | Form of Warrant issued by VaxGen, Inc. pursuant to the Stock and Warrant Purchase Agreements entered into by and between VaxGen, Inc. and certain accredited investors, dated February 8, 2006. | | 8-K | | 0-26483 | | 2-15-06 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.83 | | | Executive Employment Agreement between Matthew J. Pfeffer and VaxGen, Inc., dated March 28, 2006. + | | 8-K | | 0-26483 | | 4-05-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.84 | | | Modification #01 to Contract No. HHS0100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc., dated May 5, 2006.* | | 8-K | | 0-26483 | | 5-11-06 | | | 10.1 | | | |
72
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.85 | | | Share Purchase Agreement, between Nexol Co., Ltd., Nexol Biotech Co., Ltd., Nexol Venture Capital Co., Ltd. and VaxGen, Inc., dated June 28, 2006. | | 8-K | | 0-26483 | | 7-06-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.86 | | | Form of Amended and Restated Executive Employment Agreement. + | | 8-K | | 0-26483 | | 10-26-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.87 | | | Form of Indemnity Agreement. + | | 8-K | | 0-26483 | | 10-26-06 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.90 | | | Modification #04 to Contract No. HHS0100200500001C, between the Department of Health and Human Services, Office of Research and Development Coordination and Office of Public Health Emergency Preparedness and VaxGen, Inc., dated December 19, 2006. | | 8-K | | 0-26483 | | 12-20-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.91 | | | Termination Letter — Contract No. HHS01002005000001C (11/4/04) “Acquisition of rPA Anthrax Vaccine for the Strategic National Stockpile” | | 8-K | | 0-26483 | | 12-20-06 | | | 99.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.93 | | | Form of Addendum to Warrant to Purchase Shares of Common Stock, at an exercise price of $16.00 per share, dated December 22, 2006. | | 8-K | | 0-26483 | | 12-27-06 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.94 | | | Bonus, Stock Option Awards, Stock Option Exchange Program, and Retention Awards of Named Executive Officers.+ | | 8-K | | 0-26843 | | 02-13-07 | | | | | | |
| | | | | | | | | | | | | | | | | |
| | 10.95 | | | Resignation Agreement between VaxGen, Inc. and Lance K. Gordon, Ph.D., dated February 6, 2007. + | | 10-Q | | 0-26843 | | 09-20-07 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.96 | | | Settlement Agreement by and between VaxGen, Inc., the Department of Health and Human Services and the National Institute of Allergy and Infectious Diseases, dated April 3, 2007.* | | 8-K | | 000-26843 | | 04-05-07 | | | 10.1 | | | |
73
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.97 | | | Severance Benefit Plan, dated August 1, 2007. | | 8-K | | 000-26483 | | 08-07-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.98 | | | Sixth Amendment to Lease Agreement by and between VaxGen, Inc. and Oyster Point Tech Center LLC, dated October 11, 2007. | | 8-K | | 0-26483 | | 10-17-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.99 | | | Loan Agreement, dated November 12, 2007, between VaxGen. Inc. and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 11-13-07 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.100 | | | Note Repurchase Agreement, dated February 4, 2006, by and among VaxGen, Inc., Xmark Opportunity Fund, L.P. and Xmark Opportunity Fund, Ltd. | | 8-K | | 000-26483 | | 2-8-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.101 | | | Termination of Merger Agreement, Acknowledgment and Amendment to Loan Agreement and Secured Promissory Note, dated March 28, 2008, by and among VaxGen, Inc. and Raven biotechnologies, inc. | | 8-K | | 000-26483 | | 3-28-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.102 | | | Form of Amendment to Executive Employment Agreement. | | 8-K | | 000-26483 | | 4-1-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.103 | | | Separation Agreement between VaxGen, Inc. and Matthew J. Pfeffer, dated April 7, 2008 | | 10-Q | | 000-26483 | | 5-14-2008 | | | 10.103 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.104 | | | VaxGen, Inc. Amended and Restated 1996 Stock OptionPlan. + | | 10-Q | | 000-26483 | | 11-10-2008 | | | 10.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.105 | | | Note Repurchase Agreement, dated July 1, 2008, by and between VaxGen, Inc. and Alexandra Global Master Fund Ltd. | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.106 | | | Amendment No. 1 to Note Repurchase Agreement, dated July 1, 2008 by and between Alexandra Global Master Fund Ltd. and VaxGen, Inc. | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.107 | | | Note Repurchase Agreement, dated July 3, 2008, by and between VaxGen, Inc. and Whitebox Convertible Arbitrage Partners, LP | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.3 | | | |
74
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Reference | | |
| Exhibit | | | | | | | | Filing | | Exhibit | | Filed |
| No. | | Exhibit | | Form | | File No. | | Date | | No. | | Herewith |
| | 10.108 | | | Note Repurchase Agreement, dated July 3, 2008, by and between VaxGen, Inc. and Guggenheim Portfolio Company XXXI, LLC | | 8-K | | 000-26483 | | 7-8-2008 | | | 10.4 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.109 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and QVT Fund LP. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.110 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and Quintessence Fund L.P. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.2 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.111 | | | Note Repurchase Agreement, dated July 22, 2008, by and between VaxGen, Inc. and Deutsche Bank AG London. | | 8-K | | 000-26483 | | 7-24-2008 | | | 10.3 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.112 | | | Note Repurchase Agreement, dated October 8, 2008, by and between VaxGen, Inc. and Quattro Fund, Ltd. | | 8-K | | 000-26483 | | 10-10-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.113 | | | Note Repurchase Agreement, dated October 27, 2008, by and between VaxGen, Inc. and Drawbridge Special Opportunities Fund LP. | | 8-K | | 000-26483 | | 10-30-2008 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 10.114 | | | Amended and Restated Executive Employment Agreement for James P. Panek, effective February 1, 2009. | | 8-K | | 000-26483 | | 1-29-2009 | | | 10.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 14.1 | | | VaxGen, Inc. Code of Business Conduct and Ethics | | 8-K | | 0-26483 | | 4-5-06 | | | 14.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 21.1 | | | Subsidiaries | | 10-K | | 0-26483 | | 8-30-07 | | | 21.1 | | | |
| | | | | | | | | | | | | | | | | |
| | 24.1 | | | Power of Attorney (included in the signature pages hereto) | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | 31.1 | | | Certification required by Rule 13a-14(a) or Rule 15d-14(a) | | | | | | | | | | | | X |
| | | | | | | | | | | | | | | | | |
| | 32.1 | | | Certification required by Rule 13a-14(a) or Rule 15d-14(a) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350) | | | | | | | | | | | | X |
| | | |
| * | | Confidential treatment requested. The redacted portions have been separately filed with the SEC as required by Rule 406 of Regulation C. |
75