Use these links to rapidly review the document
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ý | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES AND EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2007 |
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to |
Commission File Number: 001-32715
INTERLEUKIN GENETICS, INC.
(Name of Registrant in its Charter)
Delaware
(State or other jurisdiction of
incorporation or organization) | | 94-3123681
(I.R.S. Employer
Identification No.) |
135 Beaver Street, Waltham, MA
(Address of principal executive offices) |
|
02452
(Zip Code) |
Registrant's Telephone Number:(781) 398-0700 |
Securities registered pursuant to Section 12(b) of the Exchange Act: |
Common Stock, $0.001 par value
per share |
|
American Stock Exchange
and
Boston Stock Exchange |
Securities registered pursuant to Section 12(g) of the Exchange Act: |
Common Stock, $0.001 par value per share |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YESo NOý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. YESo NOý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YESý NOo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained in this form and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-Ký.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | | Accelerated filer o | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YESo NOý
The aggregate market value of the registrant's voting and non-voting common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold as of the last business day of the registrant's most recently completed second fiscal quarter was $45,183,049.
As of March 27, 2008 there were 30,832,102 shares of the registrant's Common Stock and 5,000,000 shares of the registrant's Series A Preferred Stock, issued and outstanding.
Documents Incorporated By Reference
Portions of the registrant's Definitive Proxy Statement for the 2008 Annual Meeting of Shareholders to be held on or about June 12, 2008, are incorporated by reference in Part III hereof.
INTERLEUKIN GENETICS, INC.
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2007
Table of Contents
| PART I |
Item 1. |
|
Business |
|
3 |
Item 1A. |
|
Risk Factors |
|
20 |
Item 1B. |
|
Unresolved Staff Comments |
|
28 |
Item 2. |
|
Properties |
|
28 |
Item 3. |
|
Legal Proceedings |
|
28 |
Item 4. |
|
Submission of Matters to a Vote of Security Holders |
|
29 |
PART II |
Item 5. |
|
Market for Registrant's Common Equity and Related Stockholder Matters |
|
30 |
Item 6. |
|
Selected Consolidated Financial Data |
|
31 |
Item 7. |
|
Management's Discussion and Analysis of Financial Condition and Results of Operations |
|
32 |
Item 7A. |
|
Quantitative and Qualitative Disclosure about Market Risk |
|
40 |
Item 8. |
|
Financial Statements and Supplementary Data |
|
40 |
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
71 |
Item 9A. |
|
Controls and Procedures |
|
71 |
Item 9A(T). |
|
Controls and Procedures |
|
73 |
Item 9B. |
|
Other Information |
|
73 |
PART III |
Item 10. |
|
Directors, Executive Officers and Corporate Governance |
|
74 |
Item 11. |
|
Executive Compensation |
|
74 |
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management |
|
74 |
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
74 |
Item 14. |
|
Principal Accountant Fees and Services |
|
74 |
PART IV |
Item 15. |
|
Exhibits and Financial Statement Schedules |
|
75 |
2
PART I
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K and, in particular, the description of our Business set forth in Item 1, the Risk Factors set forth in Item 1A and Management's Discussion and Analysis of Financial Condition and Results of Operations set forth in Item 7, and the documents incorporated by reference into this report contain or incorporate certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Statements contained in this report that are not statements of historical fact may be deemed to be forward-looking statements. Words or phrases such as "may," "will," "could," "should," "potential," "continue," "expect," "intend," "plan," "estimate," "anticipate," "believe," "project," "likely," "outlook," or similar words or expressions or the negatives of such words or expressions are intended to identify forward-looking statements. We base these statements on our beliefs as well as assumptions we made using information currently available to us. Such statements are subject to risks, uncertainties and assumptions, including those identified in Item 1A "Risk Factors" and elsewhere in this report, as well as other matters not yet known to us or not currently considered material by us. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those anticipated, estimated or projected. Given these risks and uncertainties, prospective investors are cautioned not to place undue reliance on such forward-looking statements. Forward-looking statements do not guarantee future performance and should not be considered as statements of fact. All information set forth in this Form 10-K is as of the date of filing this Form 10-K and should not be relied upon as representing our estimate as of any subsequent date. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so to reflect actual results, changes in assumptions or changes in other factors affecting such forward-looking statements.
Item 1. Business
Overview
Interleukin Genetics, Inc. is a genetics-focused personalized health company that develops preventive consumer products and genetic tests for sale to the emerging personalized health market. Our vision is to build a leading personalized health and wellness company using the science of applied genetics to empower consumers to personalize their health. We currently have two primary segments to our business. The first is a personalized health segment primarily focused on researching and developing genetic tests that leverage and target the role that genetically determined variations in the inflammatory response have on health and disease. Revenue is being generated from our Clinical Laboratory Improvement Act of 1988 (CLIA) certified lab and from sponsored research from partners (including Alticor, a related party see Note 4 to our consolidated financial statements, Item 8.). Our second segment, comprising the Interleukin brands consumer products business, focuses on developing, selling and marketing nutritional supplements and products into retail consumer channels. These two segments contribute toward our overall mission of developing tests and products that can help individuals improve and maintain their health through preventive measures. We plan to pursue improving personalized healthcare for patients by:
- •
- developing genetic risk assessment tests for use in multiple indications, countries and demographics;
- •
- processing genetic risk assessment tests in our CLIA-certified lab or in labs of sublicensees;
- •
- developing or acquiring nutritional products distributed in mass retail consumer channels, and expanding their distribution to multiple consumer channels; and
3
- •
- conducting research and development of personalized preventive and therapeutic botanicals based on individuals' genetic information.
We believe that by identifying individuals whose risk for certain chronic diseases is potentially increased due to variants in one or more genes and combining this knowledge with personalized interventions, we can help individuals improve their health outcomes. We have patents covering the influence of certain gene variations on risk for a number of common chronic diseases and conditions.
We believe that one of the great challenges confronting medicine today is to understand why some people are more prone than others to developing serious chronic diseases and why some people respond to treatments for those diseases differently than others. Until doctors are able to understand the underlying causes of such variability, the practice of medicine will remain largely constrained to the current approach of prescribing therapies based on broad recommendations in which genetically different individuals receive the same treatment.
Until recently, scientific study of chronic diseases has largely focused on identifying factors that "cause" a disease. Common examples of such factors include high levels of cholesterol in the case of heart disease, bacteria in the case of periodontal disease and reduced estrogen levels in the case of osteoporosis. However, the mere presence of these initiating factors does not necessarily mean a person will develop a disease. Common diseases arise in part as a result of how our bodies respond to various environmental factors.
In March 2003, we entered into a broad strategic alliance with Alticor. Alticor has a long history of manufacturing and distributing nutritional supplements and skin care products to a worldwide market.
In August 2006, we acquired the assets and business of the Alan James Group, which develops, markets and sells nutritional products into retail consumer channels. As part of the acquisition, we acquired a portfolio of branded nutritional supplements, some of which are leaders in market share, that are distributed at leading discount retailers, drugstores, grocery stores and warehouse clubs in the United States. The Alan James Group maintains an efficient operational infrastructure, highlighted by relationships with key manufacturing, logistics, and technology partners, and an experienced marketing and product development team, who are based in Boca Raton, Florida. Future expansion in the areas of product and channel development offers us opportunities to expand and enhance its product portfolio.
The Role of Inflammation in our Genetic Test Products
One of the many benefits from the sequencing of the human genome is a new understanding of the role of genetic variations, such as single nucleotide polymorphisms (SNP) and haplotypes. Once used as a tool to help scientists decipher the human genome, SNP and haplotype analysis now is an important tool used to study the relevance of genetic variations to human health. A common SNP may cause a gene to make a different amount of a protein or to make a variant protein, both of which may lead to a discernible physiological impact. We have focused on the SNP variations associated with inflammation and have over the years conducted clinical studies involving over 20,000 individuals. During the last decade we have worked with the University of Sheffield in the United Kingdom, to identify several SNPs that influence the body's inflammatory response. We have concluded our research collaboration with the University of Sheffield, but the ten-year collaboration helped us generate several patents and the principal investigator, Dr. Gordon Duff, continues to serve as a member of our scientific advisory board. In addition, we have conducted clinical studies throughout the world to make these research findings clinically useful. Some of our clinical research collaborations include studies at the Mayo Clinic; Brigham & Women's Hospital (Harvard Medical College); University of California at San Francisco; University of California at San Diego; New York University Medical Center; University
4
of Sheffield, UK; Yonsei University Medical Center, Seoul Korea; Tongji Medical College, Wuhan China; and Tuft's University Medical Center.
Inflammation is one of the body's most ancient protective mechanisms. Over the last dozen years, understanding of the role of inflammation in several diseases has increased. It is now accepted that many chronic diseases begin with a challenge to the tissues of the body and that the inflammatory response system of an individual mediates the clinical manifestation of the disease. The following diagram reflects some of the diseases that are thought to be significantly influenced by inflammation. It is now thought that SNP variations in the genes that influence the inflammatory process can have an important impact on a person's risk/trajectory of a disease.
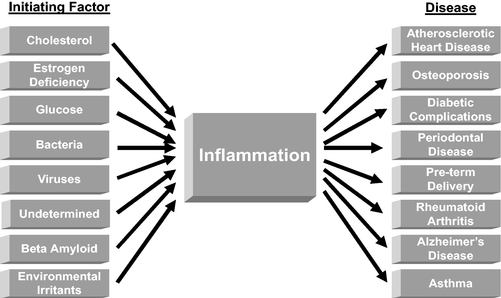
Inflammation is the first organized response to any injurious challenge to the body, such as a bacterial infection. It is a well-defined process that involves the migration and activation of leukocytes from the blood to the site of challenge. The objective of inflammation is to localize and destroy the deleterious agent. If the deleterious agent cannot be cleared, the inflammation becomes chronic.
There are classic inflammatory diseases, such as rheumatoid arthritis, but in recent years inflammation has been found to affect several other major diseases. For example, it is now known that chronic inflammation can influence the process that leads to acute heart attacks. If an individual has a strong inflammatory response, he or she may be more successful in clearing a bacterial infection than an individual with a less robust response. However, an individual with a strong response may actually be at increased risk for a more severe course in one or more of the chronic diseases of mid to later life, such as cardiovascular disease, osteoporosis, and Alzheimer's disease.
Historical Development
In the early 1990s, as we were beginning to focus on the importance of interleukin-1 (IL-1), Dr. Gordon Duff in the United Kingdom identified the first SNPs in the IL-1 and tumor necrosis factor alpha (TNFa) genes, and he and other investigators demonstrated that individuals with some of those variations produced higher levels of IL-1 and TNFa proteins. In 1993, we initiated research collaborations with Dr. Duff, and in 1994, we initiated a collaboration with the University of Sheffield to investigate and patent the clinical use of variations in the genes that control inflammation. We have concluded our research collaboration with the University of Sheffield, but the ten-year collaboration
5
helped us generate several patents and the principal investigator, Dr. Duff, continues to serve as a member of our scientific advisory board.
Studies by us and others have now shown that individuals who have certain IL-1 gene variations or patterns of variations tend to have increased levels of IL-1 proteins and also tend to have increased levels of other inflammatory mediators that are produced downstream of IL-1 proteins.
Individuals with another specific genotype pattern tend to have lower levels of inflammatory mediators. It is also important to note that the IL-1 gene variations on which we are focused are highly prevalent in the population, with 8-10% of the Caucasian population being homozygous (having two copies) for the less frequent variant and an estimated 30% of the Caucasian population having one copy of the gene variant. Also, up to 59% of the Caucasian population will test positive for some of the various IL-1 high-risk patterns.
Our Approach to Test Development
Our intellectual property is focused on the discoveries that link genetic variations in key inflammation genes to risk for disease. We have concentrated our efforts on variations in the genes for IL-1, since the IL-1 gene appears to be one of the strongest control points for the development and severity of inflammation. We have patents issued on single SNPs and SNP patterns in the IL-1 gene cluster as they relate to use for identifying individuals on a rapid path to chronic disease complications and use for guiding selection of preventive and therapeutic agents. Groups of IL-1 SNPs are often inherited together as patterns called haplotypes. We have a U.S. patent issued on haplotypes in the IL-1 gene cluster and their biological and clinical significance. We believe these patents are controlling relative to IL-1 SNP and haplotype patterns that would be used for genetic risk assessment tests.
Multiple genes and complex gene interactions with environmental factors determine the risk for the common diseases for which we are developing tests. We will develop a test based on our proprietary genetic factors if: a) clinical studies show that their effect has a critical and unique influence on the clinical expression of disease, or b) our genetic factors guide the development or use of preventive or therapeutic agents that modulate the specific actions of those genetic factors. In the former application, the risk effects of our genetic factors must be sufficiently powerful such that these genetic factors cannot be excluded from a test panel without substantially reducing the practical clinical usefulness of the test. For example, in patients with a history of heart disease, higher levels of inflammation (as measured by C-reactive protein) are as predictive of future heart attacks as higher levels of LDL cholesterol. We believe that our proprietary genetic variations identify healthy individuals who have a lifelong tendency to experience elevated inflammation and therefore to have higher risk for heart disease.
There are gene families that influence other non-inflammatory biological mechanisms involved in cardiovascular disease such as the genetic factors involved in cholesterol metabolism. For each targeted clinical disease area that meets our criteria, we are developing, or plan to develop, proprietary risk assessment tests that are anchored by our intellectual property plus additional candidate genes that have been validated and shown to be of value in assessing risk. Other genes to be added to a test panel may be in-licensed or may be available from the public domain. Since knowledge about the genes involved in health risks will continue to evolve over many years, we may introduce test panels that initially have our proprietary genetic factors with successive versions of additional genes. The heart health risk assessment panel introduced in the Alticor channel in 2006 involves three SNPs in two genes covered by our intellectual property. The osteoporosis risk assessment panel we are developing includes multiple SNPs covered by our intellectual property plus additional in-licensed genes that have been validated as risk factors for osteoporosis.
In the past few years, genome-wide association (GWA) studies have become possible as one approach to identify the association of many genes with specific health risks. These studies are now
6
practical due to the commercial availability of genome-wide array technologies. Most of the GWA studies are being conducted through government-funded consortia. We have access to GWA technologies and expertise through some of our collaborators. In diseases/conditions for which GWA technologies are being used in large government-funded studies, we may in-license or access publicly available SNPs for our panels. In diseases/conditions for which other GWA studies are not available, we may choose to employ GWA technologies either internally or through external collaborations to add value to our test panels. All of these technologies are dependent on high quality clinical databases, which we are collecting throughout the world for selected health risks. The use of GWA approaches to health risks is new, and data coming out of the first studies may take many years to validate.
In the past few years, the use of haplotypes has become a standard approach to genetic risk assessment for complex diseases. Haplotypes are blocks of SNPs that are inherited together from one parent and in some cases the specific block of SNPs has functional significance beyond the biological functions attributable to the individual SNPs. As recently reported studies support, the same SNP may have very different effects on gene function in different individuals depending on the haplotype context. We believe that we have expertise, experience and intellectual property related to the use of haplotypes in assessing genetic risk for complex diseases. We have recently reported that the same SNP may have very different effects on gene function in different individuals depending on the haplotype context.
We have in-licensed international rights to the use of gene variations, or genotypes, that regulate one important mechanism involved in fat metabolism. U.S. patents have been filed to cover the use of these genetic factors. When an individual consumes more calories than he or she burns, the excess energy is stored in fat cells as lipid droplets. One of the key chemicals that regulates the mobilization of fat from the lipid droplet to be burned as energy is called perilipin. Investigators at Tufts University Medical School and Tufts Human Nutrition and Research Center have identified variations in the perilipin gene that appear to regulate fat metabolism and body weight. Studies have been completed on several hundred individuals showing that women with one specific perilipin genotype weigh an average of 22 pounds more than women with another perilipin genotype. Seven clinical studies were published from 2004 through 2007 on the influence of perilipin genotypes on weight and related biological parameters. This research was conducted under the direction of Dr. Jose Ordovas, an international expert on the genetics of cardiovascular disease and on the interactions of genetics and nutrition. We have licensed rights to the use of this genetic test for weight management and for the use of this genetic information to develop nutritional products to facilitate weight management in individuals who have certain perilipin gene variations. Our collaborators have completed research which indicates that the perilipin genetic variations could be used for the medical guidance of weight management. We plan to conduct a significant amount of additional research which may lead to the development of genetic tests for this use.
In some cases, we have and may continue to develop genetic test panels that have limited or no exclusive intellectual property but meet specific needs of Alticor, our distribution partner. The general nutrition panel launched in the U.S. and under development internationally, as described below, is an example of such a test panel.
Business Strategy
Our strategy is to develop products and perform services and commercialize such products and services through strategic alliances. In the near term, we plan to build out the direct-to-consumer marketing strategy and launch new products in new channels, including in-licensing products. Our genetic testing business will continue to explore high potential markets such as weight management, osteoporosis, and arthritis. Our research and development initiatives will continue to enhance our intellectual property position, ensure commercial and technical success of our products, and develop formulary solutions that enable our partners to offer prevention and intervention therapeutics in a consumer and/or medical segment.
7
In March 2003, we entered into a broad strategic alliance with Alticor to develop and market novel genetic risk assessment tests and nutritional and skin care products. The alliance utilizes our intellectual property and expertise in genetics to develop risk assessment tests and aids Alticor in its effort to develop personalized consumer products. The alliance has included loans, equity investments, multi-year research and development agreements, the deferment of outstanding loan repayment and the refinancing of bridge financing obligations. A licensing agreement includes sales of selected genetic tests to Alticor for distribution within their channel. In addition, we receive minimal royalties on marketed Alticor nutritional products that are linked to the genetic tests.
We expect that this alliance will open our products and services to our partner's proven marketing and distribution channels (including in Asia). We believe that Alticor shares our belief that the future of personalized nutritional supplements and skin care will be based on an individual's genetic makeup. This alliance is currently focused on developing genetic risk assessment tests to determine a genetic profile of an individual and developing nutritional supplements and skin care products that will benefit individuals of that genetic profile. Our activities in the skin care field are in the planning stage.
8
Product Development Focus
We expect our revenue model to consist of: 1) fees for processing genetic risk assessment tests; 2) royalties or profit sharing from sales of genetic test products developed with and marketed by a partner; 3) fees for contract research; 4) sales of consumer products, including those acquired in our August 2006 acquisition of the business and assets of the Alan James Group; and 5) royalties from sales of supplement products licensed to commercial partners.
Products Available for Sale
Gensona Genetic Tests
We have research agreements with Alticor to develop certain genetic tests, which Alticor will market to consumers through its channels under Alticor's GENSONA® brand. In 2006, we provided two genetic risk assessment tests through Alticor. The GENSONA® Heart Health Genetic Test uses SNP testing of two genes to identify persons who may have an over-expression of inflammation and therefore may be at increased risk for cardiovascular disease. The GENSONA® General Nutrition Genetic Test identifies SNPs of potential importance in two genes that affect vitamin B metabolism and four genes involved in responding to oxidative stress. The GENSONA® tests are marketed solely through the Alticor business channel.
Nutritional Supplements
We currently market and sell a line of branded nutritional supplements, most of which have been in the market for longer than 10 years, to major discount retailers, drugstores, grocery stores and warehouse clubs in the United States. Our portfolio includes items in multiple segments, including Energy, Memory, Leg Vein Health, and Heart Health. Recognizable brand names include Ginsana®, Ginkoba®, Venastat®, Ginsana Gold Multiplex®, Optiform SAM-e® and Cransana®, which are company-owned, and Kyolic®, which is marketed under a distribution agreement with Wakunaga. In addition, we market a line of private label skincare products on an exclusive basis to General Nutrition Centers (GNC).
We maintain an efficient operational infrastructure, in which we leverage strategic relationships with contract manufacturers, logistics companies and technology partners, enabling us to source and distribute products on a competitive basis, and maintain excellent service levels with our customers. We also maintain relationships with key sales brokers, who in conjunction with our sales and support personnel, can effectively manage the product related and promotional initiatives needed to support and grow the business.
Our objectives are to increase sales, improve our operating efficiencies, and enhance our position in the market through the following key initiatives:
- •
- Increase Sales of our Existing Nutritional Supplement Products We expect to maintain and strengthen our supplement sales through a combination of targeted promotional activities at both the store and consumer levels. Specifically, we use print advertising in selected periodicals that effectively reach our target consumer, to emphasize product attributes and benefits. In addition, by using in store promotional activities, such as temporary price reductions, to coincide with our marketing initiatives, we can further enhance the value proposition offered to our consumers, stimulating both repeat purchase, and attracting new customers into our franchise.
- •
- Introduce New Products Given the dynamic nature of demand for consumer products, and the continued emphasis on vitamins, minerals, and nutritional supplements in the context of promoting health and well being, we actively seek opportunities to introduce innovative products, that have unique attributes and can establish a competitive position in the marketplace. We
9
PST® Genetic Tests
We currently out-license sales and marketing of our Periodontal Susceptibility Test (PST®) to national and international commercial partners. PST® is a genetic test that analyzes two IL-1 genes for variations that identify an individual's predisposition for over-expression of inflammation and risk for periodontal disease. We expect to review a number of business development strategies in 2008 to increase sales of PST® tests.
Product Development
Our current plan is to develop products in three categories:
Our genetic test development efforts are focused on the following programs:
- •
- IL-1 Cardiovascular Genetic Test — Asian populations and validation studies in Caucasians
- •
- Weight Management Genetic Test — North America and International populations
- •
- Obesity (from Perilipin) Genetic Test — Caucasian populations
- •
- Gastric Cancer and Atrophic Gastritis Genetic Test — In Asian populations including Japan
- •
- Osteoporosis Genetic Test — North America and International populations
- •
- Osteoarthritis Genetic Test — North America populations
10
IL-1 Cardiovascular Genetic Test
In the last decade, studies in men and women have shown that inflammation is an important risk factor for cardiovascular disease. Cardiovascular disease is the leading cause of death in North America. Recent scientific discoveries indicate that some of the risk for cardiovascular disease, including heart attacks, is due to variations in the genes that we inherit. Just as with conventional cardiovascular risk factors such as high cholesterol, smoking and diabetes, the presence of one or more of these DNA variations does not mean that an individual will develop cardiovascular disease. However, using knowledge about genetic risk factors to make informed choices about diet and lifestyle may reduce the risk of developing cardiovascular disease in the future.
Coronary artery disease (CAD) is a disease in which plaque builds up inside the coronary arteries. These arteries supply your heart muscle with oxygen-rich blood. Plaque is made up of fat, cholesterol, and other substances found in the blood. When plaque builds up in the arteries, the condition is called atherosclerosis. Over time, CAD can weaken the heart muscle and lead to heart failure and arrhythmias. CAD is the most common type of heart disease and it is the leading cause of death in the United States for both men and women. Lifestyle changes, medicines, and/or medical procedures can effectively prevent or treat CAD in most people. Over 105 million American adults have total blood cholesterol values of 200 mg/dL and higher, and 36.6 million American adults have levels of 240 or above. Doctors consider total cholesterol levels of 240 mg/dL or greater high in adults and levels from 200 to 239 mg/dL borderline-high.
Recent studies have shown that excess inflammation is as powerful a predictor of heart attacks as high LDL cholesterol levels. Our heart health genetic test analyzes two IL-1 genes for variations that identify an individual's predisposition for over-expression of inflammation and which may cause an increased risk for cardiovascular disease. This test is not intended to and does not diagnose an existing disease but rather is intended for healthy individuals to help assess their risk for future disease. The IL-1 cardiovascular genetic test is based on data from genetic association studies obtained through collaborations with experts in cardiovascular disease at leading academic institutions. This genetic test provides risk information independent of traditional risk factors, including family history, hypertension and smoking, in assessing risk for heart disease. This test panel was introduced in the Alticor North American channel in the first quarter of 2006. To date, we have determined that the high-risk patterns are commonly found in all major ethnic populations and thus far have been demonstrated to correlate strongly to disease in Caucasian populations. Other population studies are planned or in process. We have genetic association studies on cardiovascular disease in progress in Korea and China to determine how the risk assessment test will translate into other ethnic groups in specific environments.
In March 2007, results of a genetic variation study on CAD that we conducted in collaboration with the Mayo Clinic and investigators at the University of California at San Diego were presented at the American Cardiology Conference by Drs. Joseph Witztum and Sotiros Tsimikas. The results showed that in Caucasian patients with identified genetic variations as measured by the IL-1 genetic test there was a significantly increased risk of acute myocardial infarction (MI) or CAD, which is considered to be at least a fifty percent blockage of at least one vessel. Increasing levels of oxidized low-density lipoprotein (oxLDL) or lipoprotein a (Lp (a)) showed increasing risk. In patients without this genetic pattern risk remained constant over all oxLDL and Lp (a) levels. In patients within the top quartile of oxLDL the odds ratio for risk of CAD was 6.92 (p<0.00001). Confirmatory studies and studies in other ethnic populations are planned. We expect to complete these validation studies for risk of CAD in Caucasian populations in 2008.
General Nutrition Genetic Test
To function properly, cells depend on the action of a vast number of genes. Our general nutrition genetic test analyzes variations in several genes that influence how the body uses certain vitamins and
11
micronutrients. The test identifies individuals who may have altered B vitamin dependent metabolism or reduced response to oxidative stress. It analyzes two genes important to B vitamin utilization and four genes that are important in managing oxidative stress. This test, which is not proprietary, may be able to identify individuals who may benefit from particular nutritional supplements, and who may be at increased likelihood for health complications. This test is not intended to and does not diagnose a specific disease or assess a specific health condition. It is intended to provide information to individuals who are interested in knowledge that may help them make choices about the consumption of certain vitamins and anti-oxidants.
- •
- B Vitamin Genes: The genes analyzed related to B vitamin metabolism are 5-10-methylenetetrahydrofolate reductase (MTHFR) gene and the transcobalamin 2 (TCN2) gene. The variant of the MTHFR gene that was tested has been associated with less efficient activity of certain enzymes that depend on B vitamins for optimal function. The variant of the TCN2 gene that was tested has been associated with affecting the body's need for vitamin B-12 and how effectively it reaches cells.
- •
- Oxidative Stress Genes: The genes analyzed related to oxidative stress are manganese superoxide dismutase 2 (SOD2) gene, glutathione s-transferase M1 (GSTM1) gene, paroxanase 1 (PON1) gene, and x-ray repair cross complementing (XRCC1) gene. In some studies, individuals with these genetic variations have a different response to oxidative stress. Knowing genetic variations associated with nutrient and vitamin metabolism may help guide decisions about use of vitamins and anti-oxidants.
Weight Management Genetic Test — North America and International
According to the 1999-2003 National Health and Nutrition Examination Survey, an estimated 65% of adults in the U.S. are overweight (Body Mass Index >25). Overweight and obese (Body Mass Index >30) individuals are at increased risk for many diseases including heart disease, type II diabetes, and some types of cancer. Our objective is to develop a test that offers information about how specific individuals gain and maintain weight. We have developed the basic elements of a genetic test panel that identifies genetically-determined metabolic differences that may contribute to weight management. This test panel will guide nutritional and exercise choices to enhance an individual's efforts to maintain a desirable weight.
Obesity Genetic Test — North America
Obesity is the second leading cause of preventable death in the U.S. Approximately, 127 million adults in the U.S. are overweight, 60 million are obese (Body Mass Index >30) and 9 million are extremely obese (BMI >40). 65% of the people over age 20 are overweight and 30.5% are obese. The estimated annual costs of the overweight and obese population in the U.S. are $123 billion. This includes direct costs related to health care for prevention, diagnostic and treatment services of an estimated $64 billion. Indirect costs related to obesity include, among others, the value of wages lost by people unable to work because of obesity-driven illness or disability, as well as the value of future earning lost by premature death. Genetic factors play a significant role in the predisposition of individuals to developing obesity and potentially, to the successful outcome of current therapies. Obesity is a complex disease and can be attributed to both monogenic (involving a single gene) and polygenic (involving multiple genes) causes. There have been numerous reports on the association between genes and obesity phenotype, however, very few have been associated with resistance to weight loss on a low calorie diet. The perilipin gene is, therefore, unique in that specific polymorphisms in this gene have been associated with resistance to weight loss on calorie-restricted diets. Perilipin is a phosphoprotein that is found in the periphery of lipid droplets within adipocytes and plays a key role in regulating the storage and release of triglycerides. Evidence that perilipin is a candidate gene in the pathogenesis in polygenic obesity comes from both animal and clinical research. The perilipin knockout
12
mouse is lean and demonstrates resistance to the adipogenic effects of a high-fat diet. In humans, the perilipin gene is located on chromosome 15q26 which is a linkage locus for diabetes, hypertriglyceridemia and obesity. Perilipin polymorphisms have been found to be associated with anthropometric measures, risks of obesity and resistance to weight loss. Our program in obesity seeks to confirm and extend previous studies to provide information about the inherited tendencies of individuals' to dietary energy restriction and other metabolic risk parameters. Treatment or dietary strategies could be tailored to individuals, if predictive information about the response to these strategies were available.
Gastric Cancer Genetic Test — International
Gastric cancer (GC) continues to be the second most deadly cancer worldwide. It is most prevalent in East Asia, including Japan. In the high-risk geographies it is the number one or two cause of cancer deaths. In 2002, new cases of gastric cancer were estimated at 934 thousand worldwide compared to 1.2 million new cases of breast cancer. Five-year survival rates from GC are approximately 20%. The U.S. gastric cancer rate per 100,000 adult males is 8.4, compared to 62.1 in Japan, and 41.4 in China.Helicobacter pylori (HP) the primary risk factor for GC, infects approximately 70-80% of the Japanese population over age 50. Despite increased efforts at early screening, the incidence remains unacceptably high. Though there is some indication that eradication of HP with antibiotics may decrease the incidence of GC, such measures are not practical in parts of Asia where the infection rate is as high as 80% of the adult population. Screening for GC is laborious, inconvenient, and expensive. There is a need to stratify GC risk among HP positive patients, so informed decisions can be made regarding HP eradication and GC screening frequency and intensity. While lifestyle issues such as diet and cigarette smoking are environmental risk factors for GC, inflammation is a major GC risk factor. IL-1 genetic variations have been shown to be a major determinant of precancerous changes (atrophic gastritis) and GC in Caucasian populations. In the Japanese population, IL-1 genetic variations are associated with atrophic gastritis but their association with GC is less certain. Our program in gastric cancer genetics seeks to identify the key genetic determinants of GC to identify individuals at high risk for developing atrophic gastritis and GC, enabling physicians to target these patients for intensive HP eradication and/or GC screening.
Osteoporosis Genetic Test — North America and International
Osteoporosis, the most common age-related bone disease, results in a decrease in the strength of the bone that leaves the affected individual more susceptible to fractures. According to the National Institute of Health, 10 million Americans suffer from the disease and another 34 million have low bone mass, placing them at increased risk for the disease. Although osteoporosis occurs in both men and women, it begins earlier and progresses more rapidly in women after menopause. The consequences of osteoporosis can be both physical and financial. Hip and vertebral fractures, which are commonly associated with osteoporosis, have a profound impact on quality of life. We have conducted research projects with major osteoporosis centers. Results of these studies have indicated that a number of small variations in the IL-1 gene cluster, referred to as polymorphisms, are associated with a more rapid rate of bone loss and an increased risk of vertebral fracture in post-menopausal Caucasian women. A genetic risk assessment test could identify women at elevated risk for developing osteoporosis-related vertebral fracture comparatively early in the course of the disease and allow these women and their physicians to pursue risk reduction practices. This would enable nutritional or therapeutic intervention or recommendations for changes in lifestyle or diet at an early stage, so that bone loss and fractures are minimized or prevented.
We are developing an osteoporosis risk assessment test that combines the IL-1 SNPs with SNPs in other genes known to be associated with bone loss to form a test panel. This test panel has been evaluated in one of the largest clinical databases of fractures caused by osteoporosis, the Study of
13
Osteoporotic Fractures (SOF), directed out of the University of California at San Francisco. The IL-1 SNPs are proprietary to us, and other genes in the panel are either public domain or will be in-licensed as needed. Efforts to develop the osteoporosis risk assessment test and the marketing have been driven in part by our research agreement with Alticor. We have completed a genetic association study on bone changes related to osteoporosis in Japan and have studies on osteoporosis in progress in Korea to determine how the risk assessment test will translate into other ethnic groups in specific environments.
Osteoarthritis Genetic Test
Osteoarthritis (OA) is the most common adult joint disease, increasing in frequency and severity in all aging populations. The estimated U.S. prevalence is 20-40 million patients or 5 times that of rheumatoid arthritis. OA involvement of the hand, knee, hip and spine is common, with total knee replacements numbering over 250,000/yr and total hip replacements numbering over 150,000/yr in the U.S. alone. OA may involve a single joint or multiple joints in the same individual, with current therapy focused on pain relief, as there is no FDA-approved therapy that arrests or reverses the joint deterioration. The etiology of OA is multifactorial involving both mechanical and biochemical factors. OA progression is associated with accelerated cartilage degradation leading to joint space narrowing (JSN), painful joint disruption, and functional compromise. The pattern of manifestation of OA in many ways mimics that of osteoporosis in that it is more common in women than in men, and it appears to be related to postmenopausal changes with hormone replacement therapy suppressing cartilage degradation. OA disease progression is characterized by a proinflammatory gene expression pattern in cartilage and in joint synovium, with a reactive increase in bone density in the subchondral bone. Large amounts of data provide support for a central role of IL-1 in the pathogenesis of OA including animal susceptibility models, models of IL-1-targeted therapy, genetic association studies, and elevated IL-1 gene expression in patients with generalized OA. Genetic variations in the IL-1 gene cluster have been previously determined to be associated with multiple clinical phenotypes in OA. Our OA program plans to investigate if IL-1 gene variation together with several other inflammatory gene variations is associated with the occurrence of multijoint OA for the development of a genetic risk assessment test.
Research with Alticor
On February 28, 2008, we announced a new research collaboration with Access Business Group International LLC (ABG), a subsidiary of Alticor Inc. The research agreement encompasses four primary areas; osteoporosis, cardiovascular disease, nutrigenomics, and dermagenomics. We will be conducting various clinical studies, which will be fully funded by Alticor. Studies will look to correlate SNP gene variations to the risk of osteoporosis or cardiovascular disease in Asian populations. Other studies will seek to identify genetic factors that influence athletic performance and skin appearance (e.g., wrinkles, elasticity, aging) for the purpose of developing products to enhance healthy aging. Under the terms of the agreement, ABG will pay us $1.2 million during 2008 for this contract research. In addition, we will recognize approximately $800 thousand of deferred receipts, which were unused from prior research agreements with Alticor.
Skin care products comprise several different treatments to manage the appearance of the skin. The worldwide skin care products market is expected to reach more than $7 billion by 2010. Anti-aging products are expected to retain double-digit growth rates in the next several years, while sales of moisturizers and cleansers are also expected to experience good growth. Worldwide sales of products in this category were $5.8 billion in 2006.
Retail sales of sports nutritional products are expected to exceed $12.7 billion by 2011. Posting a 23% growth rate between 2005 and 2006 from $4.5 billion to $5.5 billion, the market sector is being driven by the continued trend for health and wellness and balanced eating amongst serious athletes and
14
the baby boomer generation. Sports beverages are said to be leading the sector, followed by bars, gels and supplements.
Laboratory Testing Procedure
To conduct a genetic risk assessment test, the consumer collects cells from inside the cheek on a brush and submits it by mail to our laboratory. Samples are only processed with a requisition signed from a physician. Our clinical laboratory then performs the test following our specific protocol and informs the consumer and, depending on the regulations in the particular state or (in Canada) province, his designated health care provider, of the results.
During 2004, we completed the construction of our genetic testing laboratory (for which we obtained registration under CLIA in 2005) to process the test samples. The regulatory requirements associated with a clinical laboratory are addressed under the section titled "Government Regulation." In early 2007 we obtained a clinical laboratory permit from the State of New York for our IL-1 Cardiovascular Genetic Test.
Marketing and Distribution Strategy
We market and distribute our genetic tests under the GENSONA® brand through our strategic partnership with Alticor. We market and distribute our nutritional products through major retailers. We market and distribute our PST® tests through sales and marketing partners directly to dentists and periodontists.
We intend to develop tests for partners in the pharmaceutical, biotechnology and other industries. Once tests are developed and launched, reimbursement may come from various sources including insurance, partners or directly from consumers. Under our distribution agreement with Alticor, Alticor pays us directly for the processed tests. If in the future we develop products that are sold through the medical channel, our ability to successfully commercialize these products may depend on obtaining adequate reimbursement from third-party payers.
Partnerships with Academic Researchers
We have (or have had) research collaborations at the University of Sheffield (UK), Tufts University, New York University, Harvard University, the Mayo Clinic, California Pacific Medical Center, Boston University, the University of Arkansas, Tongji Medical College (China) and Yonsei University (Korea). Through these collaborations, we have been able to take advantage of research conducted by these third parties in connection with the development of our genetic risk assessment tests and other possible products.
Intellectual Property
Our intellectual property and proprietary technology are subject to numerous risks, which we discuss in the section entitled "Risk Factors" of this report. Our commercial success may depend at least in part on our ability to obtain appropriate patent protection on our therapeutic and diagnostic products and methods. We currently own rights in twenty issued U.S. patents, which have expiration dates between 2015 and 2020, and have twenty-one additional U.S. patent applications pending, which are based on novel genes or novel associations between particular gene sequences and certain inflammatory diseases, and disorders. Of the twenty issued U.S. patents, sixteen relate to genetic tests for periodontal disease, osteoporosis, asthma, coronary artery disease, sepsis and other diseases associated with IL-1 inflammatory haplotypes.
We have been granted a number of corresponding foreign patents and have a number of foreign counterparts of our U.S. patents and patent applications pending.
15
In addition, through our Alan James Group subsidiary, which we acquired in August 2006, we own a portfolio of nutritional products brands, including Ginkoba®, Ginsana®, and Venastat®. We have received trademark protection for PST®, our periodontal genetic risk assessment test.
Competition
The competition in the field of personalized health is defined, but the markets and customer base are not well established. There are a number of companies involved in identifying and commercializing genetic markers. The companies differ in product end points and target customers. The companies in the industry break down into four sectors, including, 1) predictive medicine companies, 2) SNP discovery companies, 3) personalized health companies, and 4) technology platform companies.
Our potential competitors in the United States and abroad are numerous and include, among others, major pharmaceutical and diagnostic companies, specialized biotechnology firms, universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we have, which may allow these competitors to discover important genes or successfully commercialize these discoveries before us. If we do not discover genes that are linked to a health risk, characterize their functions, develop genetic tests and related information services based on such discoveries, obtain regulatory and other approvals, and launch these services or products before competitors, we could be adversely affected. Additionally, some of our competitors receive data and funding from government agencies. To the extent our competitors receive data and funding from those agencies at no cost to them, they may have a competitive advantage over us.
In the case of newly introduced products requiring "change of behavior" (such as genetic risk assessment tests), the presence of multiple competitors may accelerate market acceptance and penetration through increasing awareness. Moreover, two different genetic risk assessment tests for the same disease may in fact test or measure different components, and thus, actually be complementary when given in parallel as an overall assessment of risk, rather than being competitive with each other.
Furthermore, the primary focus of most companies in the field is performing gene-identification research for pharmaceutical companies for therapeutic purposes, with genetic risk assessment testing being a secondary goal. In contrast, our primary business focus is developing and commercializing genetic risk assessment tests for health risks and forward-integrating these tests with additional products and services.
The business of manufacturing, distributing and marketing nutritional supplements is highly competitive. Many of our competitors are substantially larger and have greater financial resources with which to manufacture and market their products. The barriers to competition are low in the nutritional products markets because the products are generally not protected by patents. In particular, the retail segment is highly competitive. In many cases, competitors are able to offer price incentives for retail purchasers and establish frequent buyer programs for consumers. Some retail competitors also manufacture their own products and therefore they have the ability and financial incentive to promote sales of their own products. Our ability to remain competitive depends on the successful introduction and addition of new offerings to our product line. We will also continue to focus on increased sales and marketing of our current products.
Government Regulation
The genetic risk assessment tests that we are developing and our current and future nutritional supplements will be subject to regulation by governmental entities.
16
Genetic Testing
CLIA
CLIA provides for the regulation of clinical laboratories by the United States Department of Health and Human Services. CLIA requires the certification of clinical laboratories that perform tests on human specimens and imposes specific conditions for certification. CLIA is intended to ensure the accuracy, reliability and timeliness of patient test results performed in clinical laboratories in the United States by mandating specific standards in the areas of, among other things, personnel qualification, administration participation in proficiency testing, patient test management, quality control, quality assurance and inspections. CLIA contains guidelines for the qualification, responsibilities, training, working conditions and oversight of clinical laboratory employees. In addition, specific standards are imposed for each type of test that is performed in a laboratory. The categorization of commercially marketedin vitro diagnostic tests marketed under CLIA is the responsibility of the FDA. The FDA will assign commercially marketed test systems into one of three CLIA regulatory categories based on their potential risk to human health. Tests will be designated as waived, of moderate complexity or of high complexity. CLIA and the regulations promulgated there under are enforced through quality inspections of test methods, equipment, instrumentation, materials and supplies on a periodic basis. Our commercial laboratory is CLIA-certified for high complexity tests, such as genetic tests.
Other Laboratory Regulations
CLIA does not preempt state laws that are more stringent than federal law. Some states independently regulate clinical laboratories and impose standards and requirements in addition to or more stringent than the CLIA regulations. Moreover, some states impose regulations on out-of-state laboratories that conduct tests on their residents. Finally, some foreign jurisdictions may also impose regulations on how we process tests for their residents. We are required to comply with all applicable laboratory regulations.
Food and Drug Administration
The FDA regulates the sale and distribution of medical devices, including in vitro diagnostic test kits, in interstate commerce. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA and its intended use. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls, including labeling, pre-market notification and adherence to FDA's quality system regulations, which are device-specific good manufacturing practices. Class II devices are subject to general controls and special controls, including performance standards and post-market surveillance. Class III devices are subject to most of the previously identified requirements and to pre-market approval. Most in vitro diagnostic kits are regulated as Class I or II devices. Entities that fail to comply with FDA requirements may be subject to enforcement actions, such as recalls, detentions, orders to cease manufacturing and restrictions on labeling and promotion or approval or clearance of new products. In addition, those entities may be subject to criminal and civil penalties.
The FDA presently requires clearance or approval of most diagnostic test kits that are sold to labs, hospitals and doctors, because those kits are considered to be medical devices. However, diagnostic tests that are developed and performed by a CLIA-certified reference laboratory, also known as "home-brew," "in-house" or "laboratory-developed" tests, generally have been considered clinical laboratory services.
17
The FDA has consistently claimed that it has the regulatory authority to regulate laboratory-developed tests that are validated by the developing laboratory. However, it has generally exercised enforcement discretion in not otherwise regulating most tests developed by CLIA-certified laboratories. Recently, the FDA indicated that it was reviewing the regulatory requirements that will apply to laboratory-developed tests, and in September 2006, it published a draft guidance document, which it revised in July 2007, or the Draft Guidance, that may be relevant to tests developed by us. The Draft Guidance describes the FDA's current thinking about potential regulation ofIn Vitro Diagnostic Multivariate Index Assays, or IVDMIAs, and the revision provided additional examples of the types that would be subject to the Draft Guidance. An IVDMIA is defined by the FDA as a device that combines the values of multiple variables using an interpretation function to yield a single patient-specific result intended for use in the diagnosis of a disease or other condition or is used in the cure, mitigation, treatment, or prevention of disease, and provides a result whose derivation is non-transparent and cannot be independently derived or verified by the end user. The FDA has indicated that it believes that most IVDMIAs will be either Class II or III devices.
The first version of the Draft Guidance and related discussions about IVDMIAs have attracted the attention of the U.S. Congress and in March 2007, the Laboratory Test Improvement Act was introduced in the U.S. Senate. The Bill, if enacted into law, would mandate that all providers of laboratory-developed tests provide evidence to the FDA that verifies the analytical validity of such tests. It would also require the development of a mechanism for the enhanced reimbursement of cleared and approved in vitro diagnostic products and laboratory-developed tests. The Bill was referred to committee and no further action has been taken as of the date of this annual report.
Although we are not currently offering or developing IVDMIAs, the FDA's interest in or actual regulation of laboratory-developed tests or increased regulation of the medical devices used in laboratory-developed testing could lead to periodic inquiry letters from the FDA and increased costs and delays in introducing new tests, including genetic tests. It is possible that a changing regulatory climate could someday require regulatory clearance or approval prior to the launch of genetic risk assessment tests, which could have a material adverse effect on our business.
HIPAA Compliance and Privacy Protection
The Health Insurance Portability and Accountability Act of 1996 (HIPAA) established for the first time comprehensive federal protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations ("Covered Entities"): health plans, health care clearing houses, and health care providers who conduct certain health care transactions electronically. Covered Entities must have in place administrative, physical and technical standards to guard against the misuse of individually identifiable health information. Additionally, some state laws impose privacy protections more stringent than those of HIPAA. There are also international privacy laws, such as the European Data Directive, that impose restrictions on the access, use, and disclosure of health information. Any of these laws may impact our business. We are not currently a Covered Entity subject to the HIPAA privacy and security standard. It is possible that in the future we will become a Covered Entity (for example if any of the tests that we perform become reimbursable by insurers). Regardless of our own Covered Entity status, HIPAA may apply to our customers.
Dietary Supplements
The manufacturing, processing, formulation, packaging, labeling and advertising of our nutritional products are subject to regulation by a number of federal agencies, including the FDA and the Federal Trade Commission (FTC). Our activities are also regulated by various state and local agencies where our products are sold.
18
FDA
The FDA is primarily responsible for the regulation of the manufacturing, labeling and sale of our nutritional products as "dietary supplements." The Dietary Supplement Health and Education Act of 1994 (DSHEA) amended the Federal Food, Drug and Cosmetic Act by defining dietary supplements, which include vitamins, minerals, nutritional supplements and herbs, and by providing a regulatory framework to ensure safe, quality dietary supplements and the dissemination of accurate information about such products. Dietary supplements are regulated as foods under DSHEA and the FDA is generally prohibited from regulating the active ingredients in dietary supplements as food additives, or as drugs unless product claims trigger drug status. Generally, dietary ingredients not used in dietary supplements marketed before October 15, 1994, the date of DSHEA's enactment, require pre-market submission to the FDA of evidence of a history of their safe use, or other evidence establishing that they are reasonably expected to be safe. There can be no assurance that the FDA will accept the evidence of safety for any new dietary ingredient that we may decide to use. FDA's refusal to accept such evidence could result in regulation of such dietary ingredients as food additives, requiring FDA approval based on newly conducted, costly safety testing.
DSHEA provides for specific nutritional labeling requirements for dietary supplements and permits substantiated, truthful and non-misleading statements of nutritional support to be made in labeling, such as statements describing general well being from consumption of a dietary ingredient or the role of a nutrient or dietary ingredient in affecting or maintaining structure or function of the body. There can be no assurance that the FDA will not consider particular labeling statements used by us to be drug claims rather than acceptable statements of nutritional support, necessitating the preparation and submission by us of a costly new drug application. It is also possible that the FDA could allege false statements were submitted to it if structure/function claim notifications were either non-existent or so lacking in scientific support as to be plainly false.
In addition, the DSHEA authorizes the FDA to promulgate current good manufacturing practices (cGMPs) specific to the manufacture of dietary supplements, to be modeled after food cGMPs. We currently use a third-party manufacturer for our dietary supplement products, which manufacturer must comply with food cGMPs.
Dietary supplements are also subject to the Nutrition, Labeling and Education Act (NLEA), which regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in a product. NLEA prohibits the use of any health claim for dietary supplements unless the health claim is supported by significant scientific agreement and is pre-approved by the FDA.
In certain markets, including the United States, claims made with respect to dietary supplements may change the regulatory status of our products. For example, in the United States, the FDA could possibly take the position that claims made for some of our products make those products new drugs requiring approval or compliance with a published FDA over the counter (OTC) monograph. If the FDA were to assert that our product claims cause them to be considered new drugs or fall within the scope of OTC regulations, we would be required to, file a new drug application, comply with the applicable monographs, or change the claims made in connection with those products.
The FTC regulates the marketing practices and advertising of all our products. In recent years, the FTC instituted enforcement actions against several dietary supplement companies for false and misleading marketing practices and advertising of certain products. These enforcement actions have resulted in consent decrees and monetary payments by the companies involved. Although the FTC has never threatened an enforcement action against us for the advertising of our products, there can be no assurance that the FTC will not question the advertising for our products in the future.
We believe that we are currently in compliance with all applicable government regulations. We cannot predict what new legislation or regulations governing our operations will be enacted by
19
legislative bodies or promulgated by agencies that regulate its activities or what changes in interpretations of existing regulations may be adopted by the FDA or the FTC.
Other Information
Our executive offices are located at 135 Beaver Street, Waltham, Massachusetts 02452, and our telephone number is (781) 398-0700. We were incorporated in Texas in 1986 and we re-incorporated in Delaware in March 2000. We maintain a website at www.ilgenetics.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to such reports are available to you free of charge through the Investor Relations Section of our website as soon as practicable after such materials have been electronically filed with, or furnished to, the Securities and Exchange Commission. The information contained on our website is not incorporated by reference into this Form 10-K. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
Item 1A. Risk Factors
The market for personalized health generally and genetic risk assessment tests in particular is unproven.
Competition in the field of personalized health is defined, but the markets and customer base are not well established. Adoption of technologies in this emerging field requires substantial market development. Although our primary customer, Alticor, has begun to develop the direct-to-consumer marketing personalized health, the overall market is unproven and there can be no assurance that other channels for marketing our products can be developed. As a result, there can be no assurance that our products will be successful upon launch or that they can be sold at sufficient volumes to make them profitable. If our potential customers do not accept our products, or take a longer time to accept them than we anticipate, it will reduce our anticipated sales, resulting in additional losses to us.
The market for genetic risk assessment tests, as part of the field of personalized health, is at an early stage of development and may not continue to grow. The scientific community, including us, has only a limited understanding of the role of genes in predicting disease. When we identify a gene or genetic marker that may influence risk for disease, we conduct clinical trials to confirm the initial scientific discovery and to establish the scientific discovery's clinical utility in the marketplace. The results of these clinical trials could limit or delay our ability to bring a test to market, reduce a test's acceptance by our potential customers or cause us to cancel the program, any of which would limit or delay sales and cause additional losses to us. The marketplace may never accept our products, and we may never be able to sell our products at a profit. Further, we may not complete development of or commercialize our other genetic risk assessment tests.
The success of our genetic risk assessment tests will depend upon their acceptance as being medically useful and cost-effective by patients, physicians and other members of the medical community, as well as third-party payers, such as insurance companies and the government. Our efforts to commercialize our intellectual property have had little success outside of the Alticor channel to date. We can only achieve broad market acceptance with substantial education about the benefits and limitations of genetic risk assessment tests.
Technological changes may cause our tests to become obsolete.
We have to date focused our efforts on genetic tests based on a small number of candidate genes. It is now possible to use array technology to conduct whole genome association studies for risk assessment, which may make our technologies obsolete. To date, our tests have been developed on behalf of, and marketed to, our primary customer, Alticor. In order to develop new customers and markets for our genetic risk assessment tests, we will be required to invest substantial additional capital and other resources into further developing these tests.
20
We currently have only one customer for our genetic risk assessment tests and it is our largest shareholder and the sole distributor for our tests.
To date, we have had only one customer for our genetic risk assessment tests, Alticor, which is also our largest stockholder and the sole distributor for our tests. We have limited experience and capabilities with respect to distributing, marketing and selling genetic risk assessment tests. We have relied and plan to continue to rely significantly on our sales, marketing and distribution arrangements with Alticor, which seek to leverage Alticor's established marketing and distribution channels. If Alticor does not successfully market our products, sales will decrease and our losses will increase. We may attempt to negotiate marketing and distribution agreements with third parties, although there can be no assurances we will be able to do so. As a result of our dependence upon Alticor, in some cases, we have and may continue to dedicate our resources toward the development of genetic test panels that have limited or no exclusive intellectual property benefit to us, but meet specific needs of Alticor.
Our consumer products business is heavily concentrated in one customer.
We currently market and sell our line of branded nutritional supplements to major discount retailers, drugstores, grocery stores and warehouse clubs in the United States, but 47% of our revenues in this business segment is derived from sales to one customer. In addition, we market a line of private label skincare products on an exclusive basis to GNC. Since this business is dependent upon consumers, we rely on one customer to a large extent to assist us in our promotional and advertising materials, as well as placement of our products in its stores. We are challenged constantly to develop innovative ways to maintain the interest and attention of our existing consumers and attract new consumers, thereby enhancing our largest customer's interest in marketing and distributing our products. Further, we are reliant upon contract manufacturers, logistics companies and technology partners to source and distribute these nutritional products. In each case, problems with these third parties could result in manufacturing, logistical, sourcing and distribution problems that could have a material adverse effect on our consumer products business.
The profitability of our consumer products businesses may suffer if we are unable to establish and maintain close working relationships with our customers.
For the year ended December 31, 2007, approximately 71% of our revenues were derived from our consumer products business, which consists of developing, marketing and selling nutritional supplements and products into retail consumer channels. This business relies to a great extent on close working relationships with our customers, rather than long-term exclusive contractual arrangements. Customer concentration in this business is relatively high and one customer accounted for approximately 47% of our revenues in that business. In addition, customers of our branded and private label consumer products, generally large food, drug and mass retailers, purchase those products through purchase orders only and are not obligated to make future purchases. We therefore rely on our ability to deliver quality products on time in order to retain existing and generate new customers. If we fail to meet our customers' needs or expectations, whether due to manufacturing issues that affect quality or capacity issues that result in late shipments, we will harm our reputation and customer relationships and likely lose customers. Additionally, if we are unable to maintain close working relationships with our customers, sales of all of our products and our ability to successfully launch new products could suffer. The loss of a major customer and the failure to generate new accounts could significantly reduce our revenues or prevent us from achieving projected growth.
We have a history of operating losses and expect these losses to continue in the future.
We have experienced significant operating losses since our inception and expect these losses to continue for some time. We incurred losses from operations of $6.1 million in 2005, $6.5 million in 2006 and $5.9 million in 2007. As of December 31, 2007, our accumulated deficit was $74.4 million.
21
Our losses result primarily from research and development, selling, general and administrative expenses and amortization of intangible assets. Although we have recently begun to generate revenues from sales of our genetic risk assessment tests and nutritional products, these may not be sufficient to result in net income in the foreseeable future. We will need to generate significant revenue to continue our research and development programs and achieve profitability. We cannot predict when, if ever, we will achieve profitability.
We are subject to government regulation, which may significantly increase our costs and delay introduction of future products.
Changes in existing regulations at either the state or federal level could require advance regulatory approval of genetic risk assessment tests, resulting in a substantial curtailment or even prohibition of our activities without regulatory approval. If our genetic tests ever require regulatory approval, on either a state or federal level, then the costs of introduction may increase or marketing and sales of products may be significantly delayed. We currently are performing the testing procedure in our own genetic testing laboratory, which currently is certified under CLIA, administered by the Health Care Financing Administration. We anticipate there will also be additional state and local regulations governing the operation of this laboratory. An inability to maintain our CLIA certification or any applicable state or local certification would reduce our anticipated revenue and increase our net losses. In September 2006, the FDA issued draft guidelines pursuant to which it would require pre-market review of certain types of genetic tests. Although we do not think that our current genetic tests and those in development are covered by the draft guidelines, the FDA is currently evaluating and could assert pre-market review of all types of genetic tests.
An inability to manage our growth or successfully integrate acquired businesses could adversely affect our business.
Our business is in a period of growth, with total revenues increasing from $23 thousand in 2005 to $9.7 million in 2007 largely due to a significant acquisition that we made in August 2007, as well as revenue from Alticor, a related party. We may make more acquisitions in the future. The successful integration of acquired businesses requires a significant effort and expense across all operational areas, including sales and marketing, research and development, manufacturing, finance and administration and information technologies. Our future operating results will depend on the ability of our management to continue to implement and improve our research, product development, manufacturing, sales and marketing and customer support programs, enhance our operational and financial control systems, expand, train and manage our employee base, integrate acquired businesses, and effectively address new issues related to our growth as they arise. There can be no assurance that we will be able to manage our recent or any future expansion or acquisition successfully, and any inability to do so could have a material adverse effect on our results of operations.
Intangible assets that we have recorded in connection with our acquisition of the Alan James Group could become impaired, requiring us to take significant charges against earnings.
In connection with the accounting for our acquisition of the Alan James Group, we have recorded a significant amount of intangible assets. Under current accounting guidelines, we must assess, at least annually and potentially more frequently, whether the value of intangible assets has been impaired. Any reduction or impairment of the value of intangible assets will result in a charge against earnings, which could materially adversely affect our reported results of operations in future periods.
22
If we deliver products with defects, our credibility may be harmed, market acceptance of our products may decrease and we may be exposed to liability in excess of our product liability insurance coverage.
The manufacturing and marketing of consumer, professional diagnostic and nutritional products involve an inherent risk of product liability claims and associated negative publicity. In addition, product development and production of our products are extremely complex and could expose our products to defects. Any defects could harm our credibility and decrease market acceptance of our products. In particular, our marketing of nutritional products may cause us to be subject to various product liability claims, including, among others, claims that the nutritional products have inadequate warnings concerning side effects and interactions with other substances.
We currently maintain product liability insurance, but it is often difficult to obtain, expensive and may not be available in the future on economically acceptable terms. In addition, potential product liability claims may exceed the amount of our insurance coverage or may be excluded from coverage under the terms of our policy. We may become subject to product liability claims that, even if they are without merit, could result in significant legal defense costs to us. If we are held liable for claims for which we are not indemnified or for damages exceeding the limits of our insurance coverage, those claims could materially damage our business and our financial condition. Any product liability claim against us or resulting recall of our products could create significant negative publicity.
The sales of branded nutritional supplements we acquired in 2006 have been flat to slightly declining due to trends in the various segments in which we compete (as the overall category consumption has declined year over year). This trend could continue and we may experience continued flatness or declines of those products.
In 2007, the aggregate sales of our brand name nutritional products, including among others Ginsana®, Ginkoba®, and Venastat® demonstrated a slight decline from the prior year. We believe that these products are performing consistently within these segments; however, some of the segments in which we compete have shown a decline year over year. We are subject to future distribution review and possibly loss (just like any other product carried by retailers) as retailers conduct their annual category reviews. We face competition with private label offerings as well as other branded product introductions, while our opportunities for new distribution on the existing product lines are limited. As a result, we do not expect sales growth of our existing nutritional products and may even experience declines in the future.
Failure to appropriately respond to changing consumer preferences and demand for new products could significantly harm our customer relationships and product sales.
Our nutritional products business is particularly subject to changing consumer trends and preferences. Our continued success depends in part on our ability to anticipate and respond to these changes, and we may not be able to respond to these changes in a timely or commercially appropriate manner. Our failure to accurately predict these trends could negatively impact consumer opinion of us as a source for the latest products, which could harm our customer relationships and cause losses to our market share. The success of our new product offerings depends upon a number of factors, including our ability to:
- •
- accurately anticipate customer needs;
- •
- innovate and develop new products;
- •
- successfully commercialize new products in a timely manner;
- •
- price our products competitively;
- •
- manufacture and deliver our products in sufficient volumes and in a timely manner; and
23
- •
- differentiate our product offerings from those of our competitors.
If we do not introduce new products or make enhancements to our current products to meet the changing needs of our customers in a timely manner, some of our products could become obsolete, which could have a material adverse effect on our revenues and operating results.
Sales of our specific nutritional supplements could be negatively impacted by media attention or other news developments that challenge the safety and effectiveness of those specific nutritional products.
Most growth in the nutritional products industry is attributed to new products that tend to generate greater attention in the marketplace than do older products. Positive media attention resulting from new scientific studies or announcements can spur rapid growth in individual segments of the market, and also impact individual brands. Conversely, news that challenges individual segments or products can have a negative impact on the industry overall as well as on sales of the challenged segments or products. Many of our nutritional products serve well-established market segments and, absent unforeseen new developments or trends, are not expected to benefit from rapid growth and may, in fact, suffer flat or declining sales as they mature.
Period-to-period comparisons of our operating results may not be meaningful due to acquisitions.
We completed the acquisition of the business of the Alan James Group in 2006, which makes it difficult to analyze our pre-acquisition and post-acquisition results of operations and to compare them from period to period. Period-to-period comparisons of our results of operations may not be meaningful due to this acquisition and possible future acquisitions and are not indications of our future performance. Any future acquisitions will also make our results difficult to compare from period to period in the future.
If we fail to obtain additional capital, or obtain it on unfavorable terms, then we may have to end our research and development programs and other operations.
We expect that our current and anticipated financial resources are adequate to maintain our current and planned operations for at least the next twelve months. If we are not generating sufficient cash or cannot raise additional capital prior to that date, we will be unable to fund our business operations and will be required to seek other strategic alternatives.
Our future capital needs depend on many factors. We may need capital for the commercial launch of additional genetic tests, continued research and development efforts, obtaining and protecting patents and administrative expenses. Additional financing may not be available when needed, or, if available, it may not be available on favorable terms. If we cannot obtain additional funding on acceptable terms when needed, we may have to discontinue operations, or, at a minimum, curtail one or more of our research and development programs.
If we are unsuccessful in establishing additional strategic alliances, our ability to develop and market products and services may be damaged.
Entering into strategic alliances, in addition to our relationship with Alticor, for the development and commercialization of products and services based on our discoveries is an important element of our business strategy. We anticipate entering into additional collaborative arrangements with Alticor. In addition, we may enter into strategic arrangements with other parties in the future. We face significant competition in seeking appropriate collaborators. If we fail to maintain our existing alliance with Alticor or establish additional strategic alliances or other alternative arrangements, then our ability to develop and market products and services will be damaged. In addition, the terms of any future strategic alliances may be unfavorable to us or these strategic alliances may be unsuccessful.
24
If we fail to obtain patent protection for our products and preserve our trade secrets, then competitors may develop competing products and services, which will likely decrease our sales and market share.
Our success will partly depend on our ability to obtain patent protection, in the United States and in other countries, for our products and services. In addition, our success will also depend upon our ability to preserve our trade secrets and to operate without infringing upon the proprietary rights of third parties.
We own rights in twenty issued U.S. patents and have a number of additional U.S. patent applications pending. We have also been granted a number of corresponding foreign patents and have a number of foreign counterparts of our U.S. patents and patent applications pending. Our patent positions, and those of other pharmaceutical and biotechnology companies, are generally uncertain and involve complex legal, scientific and factual questions. Our ability to develop and commercialize products and services depends on our ability to:
- •
- obtain patents;
- •
- obtain licenses to the proprietary rights of others;
- •
- prevent others from infringing on our proprietary rights; and
- •
- protect trade secrets.
Our pending patent applications may not result in issued patents and any issued patents may never afford meaningful protection for our technology or products or provide us with a competitive advantage. If the patents are not issued to us, we can only rely on common law trademark rights to protect these trademarks and our trade dress. Additionally, in general nutritional products are not patentable, instead we must rely on trademark rights to protect our products. Common law trademark rights do not provide the same level of protection as afforded by a United States federal registration of a trademark. Also, common law trademark rights are limited to the geographic area in which the trademark is actually used. Further, others may develop competing products, which avoid legally infringing upon, or conflicting with, our patents. There is no assurance that another company will not replicate one or more of our products, and this may harm our ability to do business. In addition, competitors may challenge any patents issued to us, and these patents may subsequently be narrowed, invalidated or circumvented.
We also rely on trade secrets and proprietary know-how that we seek to protect, in part, with confidentiality agreements. The third parties we contract with may breach these agreements, and we may not have adequate remedies for any breach. If they do not protect our rights, third parties could use our technology, and our ability to compete in the market would be reduced. We also realize that our trade secrets may become known through other means not currently foreseen by us. Our competitors may discover or independently develop our trade secrets.
Third parties may own or control patents or patent applications and require us to seek licenses, which could increase our costs or prevent us from developing or marketing our products or services.
We may not have rights under patents or patent applications that are related to our current or proposed products. Third parties may own or control these patents and patent applications in the United States and abroad. Therefore, in some cases, to develop or sell any proposed products or services, with patent rights controlled by third parties, our collaborators or we may seek, or may be required to seek, licenses under third-party patents and patent applications. If this occurs, we may have to pay license fees or royalties or both to the licensor. If licenses are not available to us on acceptable terms, our collaborators or we may be prohibited from developing or selling our products or services.
If third parties believe our products or services infringe upon their patents, they could bring legal proceedings against us seeking damages or seeking to enjoin us from testing, manufacturing or
25
marketing our products or services. Any litigation could result in substantial expenses to us and significant diversion of attention by our technical and management personnel. Even if we prevail, the time, cost and diversion of resources of patent litigation would likely damage our business. If the other parties in any patent litigation brought against us are successful, in addition to any liability for damages, we may have to cease the infringing activity or obtain a costly license.
We could become subject to intense competition from other companies, which may damage our business.
Our industry is highly competitive. Our potential competitors in the United States and abroad are numerous and include, among others, major pharmaceutical and diagnostic companies, consumer products companies, specialized biotechnology firms, universities and other research institutions. Many of our competitors have considerably greater financial, technical, marketing and other resources than we do. Furthermore, many of these competitors are more experienced than we are in discovering, commercializing and marketing products. These greater resources may allow our competitors to discover important genes or genetic markers before we do. If we do not discover genes that are linked to a health risk, characterize their functions, develop genetic tests and related information services based on such discoveries, obtain regulatory and other approvals and launch these services, or products before our competitors, then our ability to generate sales and revenue will be reduced or eliminated, and could make our products obsolete. We expect competition to intensify in our industry as technical advances are made and become more widely known.
Ethical, legal and social issues related to genetic testing may reduce demand for our products.
Genetic testing has raised issues regarding the appropriate utilization and the confidentiality of information provided by genetic testing. Genetic tests for assessing a person's likelihood of developing a chronic disease have focused public attention on the need to protect the privacy of genetic information. For example, concerns have been expressed that insurance carriers and employers may use these tests to discriminate on the basis of genetic information, resulting in barriers to the acceptance of genetic tests by consumers. This could lead to governmental authorities prohibiting genetic testing or calling for limits on or regulating the use of genetic testing, particularly for diseases for which there is no known cure. Any of these scenarios would decrease demand for our products and result in substantial losses.
Our dependence on key executives and scientists could adversely impact the development and management of our business.
Our success substantially depends on the ability, experience and performance of our senior management and other key personnel. If we lose one or more of the members of our senior management or other key employees, it could damage our development programs and our business. In addition, our success depends on our ability to continue to hire, train, retain and motivate skilled managerial and scientific personnel. The pool of personnel with the skill that we require is limited. Competition to hire from this limited pool is intense. We compete with numerous pharmaceutical and healthcare companies, as well as universities and nonprofit research organizations in the highly competitive Boston, Massachusetts business area. Our current senior management team is employed by us under agreements that may be terminated by them for any reason upon adequate notice. There can be no assurances, therefore, that we will be able to retain our senior executives or replace them, if necessary. We do not maintain key man life insurance on any of our personnel.
If Alticor enters a business in competition with ours, certain of our directors might have a conflict of interest.
In conjunction with our strategic alliance with Alticor, we have agreed to certain terms for allocating opportunities as permitted under Section 122(17) of the Delaware General Corporation Law.
26
This agreement, as set forth in the Purchase Agreement, regulates and defines the conduct of certain of our affairs as they may involve Alticor as our majority stockholder and its affiliates, and our powers, rights, duties and liabilities and those of our officers and directors in connection with corporate opportunities.
Except under certain circumstances, Alticor and its affiliates have the right to engage in the same or similar activities or lines of business or have an interest in the same classes or categories of corporate opportunities as we do. If Alticor or one of our directors appointed by Alticor, and its affiliates acquire knowledge of a potential transaction or matter that may be a corporate opportunity for both Alticor and its affiliates and us, to the fullest extent permitted by law, Alticor and its affiliates will not have a duty to inform us about the corporate opportunity or be liable to us or to you for breach of any fiduciary duty as a stockholder of ours for not informing us of the corporate opportunity, keeping it for its own account, or referring it to another person.
Additionally, except under limited circumstances, if an officer or employee of Alticor who is also one of our directors is offered a corporate opportunity, such opportunity shall not belong to us. In addition, we agreed that such director will have satisfied his duties to us and not be liable to us or to you in connection with such opportunity.
The terms of this agreement will terminate on the date that no person who is a director, officer or employee of ours is also a director, officer, or employee of Alticor or its affiliates.
We may be prohibited from fully using our net operating loss carryforwards, which could affect our financial performance.
As a result of the losses incurred since inception, we have not recorded a federal income tax provision and have recorded a valuation allowance against all future tax benefits. As of December 31, 2007, we had gross net operating loss and research tax credit carryforwards of approximately $53.6 million and $1.07 million respectively, for federal income tax purposes, expiring in varying amounts through the year 2027. As of December 31, 2007, we had gross net operating loss and research tax credit carryforwards of approximately $21.3 million and $570 thousand, respectively, for state income tax purposes, expiring in varying amounts through the year 2012. Our ability to use these net operating loss and credit carryforwards is subject to restrictions contained in the Internal Revenue Code which provide for limitations on our utilization of our net operating loss and credit carryforwards following a greater than 50% ownership change during the prescribed testing period. We have experienced two such ownership changes. One change arose in March 2003 and the other was in June 1999. As a result, our net operating loss carryforwards that relate to periods prior to March 2003 and June 1999 are limited in utilization. The annual limitation may result in the expiration of the carryforwards prior to utilization. In addition, in order to realize the future tax benefits of our net operating loss and tax credit carryforwards, we must generate taxable income, of which there is no assurance.
Our Series A Preferred Stock has certain rights that are senior to common stockholder rights and this may reduce the value of our common stock.
Our Series A Preferred Stock, which was issued to Alticor in March 2003, accrues dividends at the rate of 8% of the original purchase price per year, payable only when, as and if declared by the Board of Directors and are non-cumulative. If we declare a distribution, with certain exceptions, payable in securities of other persons, evidences of indebtedness issued by us or other persons, assets (excluding cash dividends) or options or rights to purchase any such securities or evidences of indebtedness, then, in each such case the holders of the Series A Preferred Stock shall be entitled to a proportionate share of any such distribution as though the holders of the Series A Preferred Stock were the holders of the number of shares of our common stock into which their respective shares of Series A Preferred Stock
27
are convertible as of the record date fixed for the determination of the holders of our common stock entitled to receive such distribution. As of December 31, 2007, our Series A Preferred Stock was convertible into 28,160,200 shares of our common stock, which is subject to standard antidilution protections as well as adjustments in the event we issue any shares of capital stock for a price lower than the conversion price of the Series A Preferred Stock.
In the event of any liquidation, dissolution or winding up of our company, whether voluntary or involuntary, the holders of Series A Preferred Stock shall be entitled to receive, prior and in preference to any distribution of any of our assets or surplus funds to the holders of our common stock by reason of their ownership thereof, the amount of two times the then-effective purchase price per share, as adjusted for any stock dividends, combinations or splits with respect to such shares, plus all declared but unpaid dividends on such share for each share of Series A Preferred Stock then held by them. After receiving this amount, the holders of Series A Preferred Stock shall participate on an as-converted basis with the holders of common stock in any of our remaining assets.
Because a single stockholder has a controlling percentage of our voting power, other stockholders' voting power is limited.
As of December 31, 2007, Alticor was our largest stockholder and owned, or had rights to acquire, approximately 52.1% of our outstanding common stock. Accordingly, this stockholder may be able to determine the outcome of stockholder votes, including votes concerning the election of directors, the adoption or amendment of provisions in our Certificate of Incorporation or By-Laws and the approval of certain mergers and other significant corporate transactions, including a sale of substantially all of our assets. This stockholder may make decisions that are adverse to other stockholders' interests. This ownership concentration may also adversely affect the market price of our common stock. Four of our five directors are individuals chosen by this single stockholder and this stockholder has the right to choose an additional director. These directors might pursue policies in the interest of this single stockholder to the detriment of our other stockholders.
We do not expect to pay dividends for the foreseeable future and you should not expect to receive any funds without selling your shares of common stock, which you may only be able to do at a loss.
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain any earnings for use in the operation and expansion of our business and do not anticipate paying any cash dividends on our common stock in the foreseeable future. Therefore, you should not expect to receive any funds without selling your shares, which you may only be able to do at a loss.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our offices and laboratories are located at 135 Beaver Street, Waltham, Massachusetts 02452. In February 2004, we entered into a new lease expanding this space to approximately 19,000 square feet and extended the term of the lease through March 2009. As part of our acquisition of the Alan James Group assets in August 2006, we also acquired a lease for 4,156 square feet of office space in Boca Raton, Florida. In July 2007, we extended this lease through June 2009.
Item 3. Legal Proceedings
On March 25, 2008, we entered into an agreement with David A. Finkelstein, Timothy J. Richerson, Alan James Group, LLC, AJG-NB, LLC, AJG-BI, LLC and AJG-GNC, LLC, who we refer to collectively as the Claimants, pursuant to which the Claimants agreed to release us from any further
28
obligations under the Asset Purchase Agreement we entered into with the Claimants on August 17, 2006 or related to the employment of Messrs. Richerson and Finkelstein by us, and we agreed to release the Claimants from certain obligations under the Asset Purchase Agreement or related to our employment of Messrs. Richerson and Finkelstein. Pursuant to the agreement, we agreed to pay the Claimants an aggregate of $1.2 million. As of June 30, 2007 we had accrued an aggregate expense of $600,000 in connection with the departures of Messrs. Richerson and Finkelstein. We also agreed to limit the duration of non-competition restrictions applicable to Richerson to July 31, 2009 and Finkelstein to July 2, 2009. The Claimants agreed that no further amounts are or will become due under the Purchase Agreement (including its earn-out provisions), their Employment Agreements or other related documents. Under applicable law the Claimants are entitled to a seven-day right to rescind this agreement. This period expires on April 2, 2008.
Item 4. Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the fourth quarter of the year ended December 31, 2007.
29
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock currently trades under the symbol "ILI" on the American Stock Exchange and the Boston Stock Exchange. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock, as reported by the American Stock Exchange.
| | High
| | Low
|
|---|
| 2007: | | | | | | |
| | First Quarter | | $ | 5.75 | | $ | 4.12 |
| | Second Quarter | | $ | 4.41 | | $ | 1.77 |
| | Third Quarter | | $ | 1.98 | | $ | 1.00 |
| | Fourth Quarter | | $ | 2.25 | | $ | 1.02 |
|
|
High
|
|
Low
|
|---|
| 2006: | | | | | | |
| | First Quarter | | $ | 9.23 | | $ | 4.60 |
| | Second Quarter | | $ | 8.14 | | $ | 4.85 |
| | Third Quarter | | $ | 6.90 | | $ | 4.80 |
| | Fourth Quarter | | $ | 6.95 | | $ | 5.50 |
Stockholders
As of March 14, 2008, there were approximately 120 stockholders of record and according to our best estimate, approximately 3,500 beneficial owners of our common stock.
Dividends
We have not declared any dividends to date and do not plan to declare any dividends on our common stock in the foreseeable future.
Sale of Unregistered Securities
None that have not been previously reported.
Issuer Purchases of Equity Securities
None.
30
Item 6. Selected Consolidated Financial Data
The following table sets forth selected consolidated financial data as of and for each of the five years ended December 31, 2007. The selected consolidated financial data as of and for each of the five years ended December 31, 2007 has been derived from our audited consolidated financial statements. This data should be read in conjunction with our audited consolidated financial statements and related notes that are included elsewhere in this Annual Report on Form 10-K and with "Management's Discussion and Analysis of Financial Condition and Results of Operations" included in Item 7 below.
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| Statement of Operations Data: | | | | | | | | | | | | | | | | |
| Revenue | | $ | 9,700,493 | | $ | 4,731,026 | | $ | 22,877 | | $ | 34,671 | | $ | 54,103 | |
| | |
| |
| |
| |
| |
| |
| Gross profit | | | 5,001,047 | | | 1,888,429 | | | 22,877 | | | 34,320 | | | 33,447 | |
| | |
| |
| |
| |
| |
| |
| Operating Expenses: | | | | | | | | | | | | | | | | |
| | Research and development | | | 2,928,249 | | | 3,262,349 | | | 3,127,086 | | | 4,078,316 | | | 3,457,861 | |
| | Selling, general and administrative | | | 6,367,973 | | | 4,506,799 | | | 2,916,858 | | | 2,636,045 | | | 2,436,801 | |
| | Amortization of intangible assets | | | 1,651,244 | | | 646,065 | | | 36,921 | | | 21,992 | | | 6,418 | |
| | |
| |
| |
| |
| |
| |
| | Total operating expenses | | | 10,947,466 | | | 8,415,213 | | | 6,080,865 | | | 6,736,353 | | | 5,901,080 | |
| | |
| |
| |
| |
| |
| |
| Loss from operations | | | (5,946,419 | ) | | (6,526,784 | ) | | (6,057,988 | ) | | (6,702,033 | ) | | (5,867,633 | ) |
| | |
| |
| |
| |
| |
| |
| Net loss | | $ | (6,218,785 | ) | $ | (6,946,756 | ) | $ | (6,570,824 | ) | $ | (7,246,202 | ) | $ | (6,558,914 | ) |
| | |
| |
| |
| |
| |
| |
| Accretion of convertible preferred stock discount | | | — | | | — | | | — | | | — | | | (8,094,727 | ) |
| | |
| |
| |
| |
| |
| |
| Net loss attributable to common stockholders | | $ | (6,218,785 | ) | $ | (6,946,756 | ) | $ | (6,570,824 | ) | $ | (7,246,202 | ) | $ | (14,653,641 | ) |
| | |
| |
| |
| |
| |
| |
| Basic and diluted net loss per common share | | $ | (0.22 | ) | $ | (0.27 | ) | $ | (0.28 | ) | $ | (0.31 | ) | $ | (0.63 | ) |
| | |
| |
| |
| |
| |
| |
| | |
| |
| |
| |
| |
| |
| | As of December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| | 2004
| | 2003
| |
|---|
| Selected Balance Sheet Data: | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 7,646,468 | | $ | 10,082,919 | | $ | 3,415,174 | | $ | 4,528,425 | | $ | 4,759,453 | |
| Working capital | | $ | 3,849,973 | | $ | 5,602,760 | | $ | 574,914 | | $ | 3,276,072 | | $ | (4,216,466 | ) |
| Total assets | | $ | 16,385,949 | | $ | 22,630,285 | | $ | 4,970,075 | | $ | 6,185,501 | | $ | 5,340,604 | |
| Long term debt and capital lease obligations, less current portion | | | — | | | — | | $ | 1,671,588 | | $ | 1,212,691 | | $ | 765,129 | |
| Stockholders' equity (deficit) | | [$ | 10,192,414 | ] | $ | 13,785,931 | | $ | 283,745 | | $ | 3,527,507 | | $ | (3,912,371 | ) |
31
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read in conjunction with our "Selected Consolidated Financial Data" and the audited Consolidated Financial Statements and the notes thereto included elsewhere in this document.
General Overview and Trends
We are a genetics-focused personalized health company that develops preventive consumer products and genetic tests for sale to the emerging personalized health market. Our vision is to build a leading personalized health and wellness company using the science of applied genetics to empower consumers to personalize their health. We currently have two primary business segments that include:
- •
- Personalized health segment (PHS) — this segment researches and develops genetic tests that leverage and target the role that genetically determined variations in the inflammatory response have on health and disease
- •
- Consumer products segment (CPS) — comprising the Alan James Group (AJG) business, which we purchased on August 17, 2006, this segment is focused on developing, selling and marketing nutritional supplements and products into retail consumer channels.
These two segments contribute toward our overall mission of developing tests and products that can help individuals improve and maintain their health through preventive measures. We plan to pursue this by:
- •
- developing genetic risk assessment tests for use in multiple indications, countries and various demographics in our PHS;
- •
- processing genetic risk assessment tests in our Clinical Laboratory Improvement Act of 1988 (CLIA) certified lab or in those of sublicensees in our PHS;
- •
- developing and acquiring nutritional products to be distributed in multiple consumer channels in our CPS; and
- •
- conducting research and development of personalized preventive and therapeutic botanicals based on individuals' genetic information in our CPS.
The consumer products segment sells branded nutritional products, including Ginsana®, Ginkoba®, and Venastat® through the nation's largest food, drug and mass retailers. The addition of AJG added substantial revenues to our business and in the year ended December 31, 2007, AJG represented over 70% of our consolidated revenues. Customer concentration in our CPS segment is high and our largest customer accounted for approximately 47% of revenues in that segment. Also in 2006, sales of our personalized health products (PHS) began under marketing and other business arrangements with Alticor. For 2007, Alticor represents a significant customer representing virtually all of our PHS revenues and over 29% of consolidated revenues.
In 2006, the addition of AJG also added to the selling general and administrative costs necessary to run a consumer products business and it added substantial amortization of intangible assets acquired in the purchase. In the year ended December 31, 2007, amortization of intangible assets was approximately $1.7 million compared to less than $54 thousand in the year prior to the acquisition. Such amortization expense will continue in 2008 and beyond as described in Note 6 to the consolidated financial statements.
We have traditionally spent approximately $3 to 4 million annually on research and development. We currently anticipate that range of spending to continue into 2008. Our current development programs focus on heart disease, osteoporosis, osteoarthritis, gastric cancer, skin appearance, sports nutrition and weight management genetic risk assessment tests as well as new proprietary supplements
32
for distribution through the CPS. We expect that these programs will also lead to the personalized selection of nutritional and therapeutic products, and provide consumers and healthcare professionals with better preventive product alternatives.
In March 2003, we entered into a research agreement with Alticor to develop genetic tests and software to assess personalized risk and develop and use screening technologies to validate the effectiveness of the nutrigenomic consumables Alticor is developing. In March 2005 and in March 2007, we entered into new agreements with Alticor to continue the research being performed. In June 2004, we entered into another research agreement with Alticor to conduct research into the development of a test to identify individuals with specific genetic variations that affect how people gain and maintain weight. This project was completed during 2006. In June 2006, we entered into another research agreement with Alticor to perform association studies on composite genotypes to skin inflammatory response. As of December 31, 2007, the research agreements described above have been completed. See financial statement footnote 4 for a discussion of our strategic alliance with Alticor.
On February 25, 2008, we entered into a new research agreement with Access Business Group International LLC (ABG), a subsidiary of Alticor Inc. The research agreement encompasses four primary areas: osteoporosis, cardiovascular disease, nutrigenomics, and dermagenomics. We will be conducting various clinical studies, which shall be fully funded by Alticor.
Some of these studies aim to correlate SNP gene variations to the risk of osteoporosis or cardiovascular disease in Asian populations. Other studies conducted in North American populations will seek to identify genetic factors that influence athletic performance (nutrigenomics) and skin health, such as wrinkles, elasticity, aging (dermagenomics), for the purpose of developing products to enhance healthy aging. Under the terms of the agreement, ABG will pay us $1.2 million during 2008 for the research. In addition, we will recognize approximately $800 thousand of deferred receipts which were unused from prior research agreements with Alticor.
In the CPS segment, the nutritional products and supplement industry is characterized by rapid and frequent changes in demand for products and new product introductions. The success of new product offerings depends upon a number of factors, including:
accurately anticipating customer needs;
innovating and developing new products;
successfully commercializing new products in a timely manner;
pricing our products competitively;
manufacturing and delivering our products in sufficient volumes and in a timely manner; and
differentiating our product offerings from those of our competitors.
In 2007, the aggregate sales of our brand name nutritional products, including Ginkoba®, Ginsana®, and Venastat® demonstrated a slight decline from the prior year which we believe is consistent with a general decline in this segment of the industry year over year. We face competition with private label offerings as well as other branded product introductions. Further, our opportunities for new distribution on the existing product lines are limited. As a result, we do not expect sales growth of our existing nutritional products and may even experience declines in the future. We believe that, our growth will be more dependent on our ability to adapt to changing consumer trends with the introduction of new products or improvements to existing products.
In our PHS, the competition is defined, but the markets and customer base are not well established. Adoption of such technologies by consumers requires substantial market development. We have placed a significant focus of this effort in our relationship with our primary customer, Alticor, a significant direct marketing company. Alticor has begun to develop the direct-to-consumer market,
33
however, the overall market is unproven and our challenge in 2008 and beyond will be to work to develop this market. Since Alticor has not previously sold a product similar to the genetic risk assessment tests, we cannot predict any fluctuations we may experience in our test revenues or whether revenues derived from Alticor related to the heart health and general nutrition genetic tests will be sustained in future periods.
Liquidity and Capital Resources
As of December 31, 2007, we had cash and cash equivalents of $7.6 million and borrowings available under our credit facilities of $14.3 million, expiring on August 17, 2008. Net cash used in operating activities was $2.5 million and $3.4 million during the years ended December 31, 2007 and 2006, respectively.
Net cash used in investing activities was $314 thousand and $8.0 million during the years ended December 31, 2007 and 2006, respectively. In August 2006, we acquired the assets and business of the Alan James Group as described above. We paid initial consideration at the closing consisting of approximately $7.0 million in cash and the obligation to place in escrow $250 thousand and 88,055 shares of Common Stock. Capital additions and increases in other assets were $252 thousand and $332 thousand for the year ended December 31, 2007 and 2006, respectively.
Cash provided by financing activities was $417 thousand for the year ended December 31, 2007 compared to $18.1 million for the year ended December 31, 2006. During 2007, we received $417 thousand from the exercise of stock options, stock purchases through the employee stock purchase plan and from our rights offering completed in January 2007. In August 2006, we issued and sold to Alticor 2,750,037 shares of Common Stock for an aggregate purchase price of $15,615,537, or $5.6783 per share. In addition, during 2006, we received $1.5 million of research funding from our strategic alliance with Alticor, $1.1 million from the exercise of stock options and warrants and $44 thousand from stock purchases through the employee stock purchase plan. These amounts were offset by $3 thousand of payments of our capital lease obligations. We currently do not have any commitments for any material capital expenditures.
A summary of our contractual obligations as of December 31, 2007 is included in the table below:
| | Payments Due By Period
|
|---|
Contractual Obligations
| | Total
| | Less than
1 Year
| | 1-3 Years
| | 3-5 Years
| | More than
5 Years
|
|---|
| Long-Term Debt Obligations | | $ | 595,336 | | $ | 595,336 | | $ | — | | $ | — | | $ | — |
| Operating Lease Obligations | | | 766,027 | | | 572,495 | | | 193,532 | | | — | | | — |
| | |
| |
| |
| |
| |
|
| TOTAL | | $ | 1,361,363 | | $ | 1,167,831 | | $ | 193,532 | | $ | — | | $ | — |
| | |
| |
| |
| |
| |
|
Based on our current operating and capital expenditure forecasts, we believe that the combination of funds currently available and our available lines of credit will be adequate to finance our ongoing operations for at least the next twelve months.
There is adequate capital to meet our current needs per our overall strategic plan. Going forward there will be investment in new product development for genetic testing and AJG products, improving our distribution channels, strategic marketing of tests, which may require raising additional capital.
Comparison of Year Ended December 31, 2007 to Year Ended December 31, 2006
Revenue for the year ended December 31, 2007 was $9.7 million compared to $4.7 million for the year ended December 31, 2006, an increase of $5.0 million or 105%. The increase of $4.8 million was due largely to inclusion of the consumer products segment for the full year 2007 whereas in 2006 that
34
segment was only included since its acquisition on August 17, 2006. The PHS resulted in an increase of $147 thousand in 2007 over 2006. Revenue from our segments consisted of the following:
| | Year Ended
December 31,
| |
| |
| |
|---|
| |
| | % Change
| |
|---|
| | 2007
| | 2006
| | $ Change
| |
|---|
| Personalized health: | | | | | | | | | | | | |
| | Genetic Testing | | $ | 779,238 | | $ | 2,660,535 | | $ | (1,881,297 | ) | (71 | )% |
| | Contract research and development | | | 2,028,031 | | | — | | | 2,028,031 | | — | |
| | Other | | | 20,015 | | | 20,227 | | | (212 | ) | (1 | )% |
| | |
| |
| |
| |
| |
| | | Segment total | | | 2,827,284 | | | 2,680,762 | | | 146,522 | | 5 | % |
| | |
| |
| |
| |
| |
| Consumer products | | | 6,873,209 | | | 2,050,264 | | | 4,822,945 | | 235 | % |
| | |
| |
| |
| |
| |
| | | Total | | $ | 9,700,493 | | $ | 4,731,026 | | $ | 4,969,467 | | 105 | % |
| | |
| |
| |
| |
| |
We have two significant customers. In our PHS, our significant customer, Alticor, which is the Company's majority shareholder, represented approximately 99% of the revenues of that segment and in our CPS segment, our significant customer represented approximately 47% of the revenues of that segment. Together, these two customers account for approximately 60% of revenues.
Gross profit was approximately $5.0 million, or 52% of revenue, for the year ended December 31, 2007. Gross profit from the PHS was approximately $1.9 million, or 65% of its revenue compared to approximately $1.5 million and 55% in the year ended December 31, 2006. Gross profit from the CPS was approximately $3.3 million, or 47% of its revenue compared to approximately $420,000 and 21% in the period from acquisition through December 31, 2006.
Research and development expenses were approximately $2.9 million for the year ended December 31, 2007 compared to approximately $3.3 million for the year ended December 31, 2006, a decrease of $334 thousand or 10%. Funded research and development expenses were approximately $1.5 million for the year ended December 31, 2007 compared to approximately $1.7 million for the year ended December 31, 2006, a decrease of approximately $182 thousand or 11%. Between March 2003 and March 2007, we entered into various research agreements with Alticor as described above in "General Overview and Trends."
Selling, general and administrative expenses were approximately $6.4 million for year ended December 31, 2007 compared to approximately $4.5 million for the year ended December 31, 2006, an increase of approximately $1.9 million or 41%. Approximately $1.3 million of this increase results from the inclusion of our CPS for the full year 2007 and only since its acquisition in August 2006 in the prior year.
Amortization of intangible assets was approximately $1.7 million for year ended December 31, 2007 compared to approximately $646 thousand during the prior year. This increase was primarily attributable to amortization expense associated with acquisition-related intangible assets for the full year in 2007 and only since acquisition in August 2006, in the year ended December 31, 2006.
Other expense, net, decreased by approximately $156 thousand to approximately $256 thousand principally as a result of increased interest income.
Revenue, gross profit, operating and other expenses contributed to a net loss of approximately $6.2 million, or $(0.22) per share, for the year ended December 31, 2007 compared to a loss of approximately $6.9 million, or $(0.27) per share for 2006.
35
Comparison of Year Ended December 31, 2006 to Year Ended December 31, 2005
Revenue for the year ended December 31, 2006 was approximately $4.7 million compared to approximately $23 thousand for the year ended December 31, 2005, an increase of approximately $4.7 million. The largest increase was due to revenues of approximately $2.7 million from our PHS, including approximately $2.6 million from Alticor for the heart health genetic test and the general nutrition genetic test, both of which were launched by Alticor during the first quarter of 2006. Additionally, approximately $2.1 million was generated from our CPS since its acquisition on August 17, 2006.
Gross profit was approximately $1.9 million, or 40% of revenue, for the year ended December 31, 2006. Gross profit from personalized health testing, including fixed overhead costs associated with laboratory operations, was approximately $1.5 million, or 55% of revenue. Gross profit from consumer products revenue was approximately $420 thousand for the period from August 17, 2006 (the date of our acquisition of that business) to December 31, 2006, or approximately 21% of revenue. This lower than normal gross profit was primarily due to the sale of inventory revalued at the date of acquisition and sold through to customers during the period.
Selling, general and administrative expenses were approximately $4.5 million for year ended December 31, 2006 compared to approximately $2.9 million for the year ended December 31, 2005, an increase of approximately $1.6 million or 55%. This increase was largely attributable to costs of approximately $893 thousand incurred by the consumer products group since its acquisition August 17, 2006 and the recording of approximately $709 thousand of stock-based compensation expense for year ended December 31, 2006 as a result of adopting SFAS No. 123R. In addition, selling, general and administrative expenses for the year ended December 31, 2006 includes approximately $193 thousand of costs associated with the termination of employees as a result of cost cutting efforts. This increase in costs in 2006 was partially offset by non-recurring professional fees incurred in 2005 associated with the implementation of Sarbanes-Oxley Section 404.
Amortization of intangible assets was approximately $646 thousand for year ended December 31, 2006 compared to approximately $37 thousand during the prior year. This increase was primarily attributable to amortization expense associated with the August 2006 acquisition of the consumer products segment and their related intangible assets.
Other expense, net, decreased by approximately $100 thousand primarily as a result of an increase in net interest income (net of interest expense) of approximately that amount.
Revenue, gross profit, operating and other expenses for the year ended December 31, 2006 contributed to a net loss of $6.9 million, or $(0.27) per share, compared to a loss of $6.6 million, or $(0.28) per share for 2005.
Critical Accounting Policies and Estimates
Critical accounting policies and estimates are defined as those that are reflective of significant judgments and uncertainties, and could potentially result in materially different results under different assumptions and conditions. We believe that our most critical accounting policies and estimates upon which our financial condition depends, and which involve the most complex or subjective decisions or assessments are the following:
Strategic alliance with Alticor:
We account for our strategic alliance with Alticor in accordance with Emerging Issues Task Force (EITF) No. 01-1, Accounting for Convertible Instruments Granted or Issued to a Nonemployee for Goods or Services or a Combination of Goods or Services and Cash (EITF No. 01-1). Under EITF No. 01-1, the proceeds received from Alticor in connection with the March 5, 2003 transaction must
36
first be allocated to the fair value of the convertible instruments issued. As of March 5, 2003, the fair value of the convertible instruments issued was $23.7 million; therefore proceeds received from Alticor in connection with the March 5, 2003 transaction, up to $23.7 million, have been recorded as equity.
Revenue Recognition:
Revenue from genetic testing services is recognized when there is persuasive evidence of an arrangement, service has been rendered, the sales price is determinable and collectibility is reasonably assured. Service is deemed to be rendered when the results have been reported to the individual who ordered the test.
Revenue from product sales is recognized when there is persuasive evidence of an arrangement, delivery has occurred and title and risk of loss have transferred to the customer, the sales price is determinable and collectibility is reasonably assured. We have no consignment sales. Product revenue is reduced for allowances and adjustments, including returns, discontinued items, discounts, trade promotions and slotting fees.
Revenue from contract research and development is recognized over the term of the contract as we perform our obligations under the contract.
Allowance for Sales Returns:
Our recognition of revenue from sales to retailers is impacted by giving them rights to return damaged and outdated products as well as the fact that as a practical business matter, our sales force, along with our customers, is constantly working to ensure profitability of our products within retailers by rotating slow moving items out of stores and replacing those products with what we and the retailer expect will be more profitable, faster selling items. For product sales, we believe we can reasonably and reliably estimate future returns, we recognize revenue at the time of sale. For product sales which we cannot estimate future returns, particularly new products, we defer revenue recognition until the return privilege has substantially expired or the amount of future returns can be reasonably estimated. An adverse change in any of these factors may result in the need for additional sales returns.
We analyze sales returns in accordance with Statement of Financial Accounting Standards (SFAS) No. 48,Revenue Recognition When Right of Return Exists. We are able to make reasonable and reliable estimates based on history. We also monitor the buying patterns of the end-users of our products based on sales data received. We review our estimated product returns based on expected data communicated by our customers. We also monitor the levels of inventory at our largest customers to avoid excessive customer stocking of merchandise. We believe we have sufficient interaction and knowledge of our customers and of the industry trends and conditions to adjust the accrual for returns when necessary. We believe that this analysis creates appropriate estimates of expected future returns. There is no guarantee that future returns will not increase to, or exceed, the levels experienced in the past. Furthermore, the possibility exists that should we lose a major account, we may agree to accept a substantial amount of returns.
Trade Promotions:
We use objective procedures for estimating our allowance for trade promotions. The allowance for trade promotions offered to customers is based on contracted terms or other arrangements agreed in advance.
Inventory:
We value our inventory at the lower of cost or market. We monitor our inventory and analyze it on a regular basis. Cycle counts are taken periodically to verify inventory levels. In addition, we analyze
37
the movement of items within our inventory in an effort to determine the likelihood that inventory will be sold or used before expiration dates are reached. We provide an allowance against that portion of inventory that we believe is unlikely to be sold or used before expiration dates are reached. An adverse change in any of these factors may result in the need for additional inventory allowance.
Stock-based compensation:
We account for our stock-based compensation expense in accordance with SFAS No. 123 (Revised 2004),Share-Based Payment (SFAS No. 123R) using the modified prospective basis. SFAS No. 123R addresses all forms of share-based payment (SBP) awards, including shares issued under employee stock purchase plans, stock options, restricted stock and stock appreciation rights. SFAS No. 123R requires us to expense SBP awards with compensation cost for SBP transactions measured at fair value. SFAS No. 123R applies to new equity awards and to equity awards modified, repurchased or canceled after the effective date. Additionally, compensation cost for the portion of awards for which the requisite service has not been rendered that are outstanding as of the effective date shall be recognized as the requisite service is rendered on or after the effective date. The compensation cost for that portion of awards shall be based on the grant-date fair value of those awards as calculated from the pro forma disclosures under SFAS No. 123. Additionally, common stock purchased pursuant to our employee stock purchase plan will be expensed based upon the fair market value in excess of purchase price.
Intangible Assets:
Purchase accounting requires extensive use of accounting estimates and judgments to allocate the purchase price to the fair market value of the assets purchased and liabilities assumed. We have accounted for our acquisitions using the purchase method of accounting. Values were assigned to intangible assets based on third-party independent valuations, as well as management's forecasts and projections that include assumptions related to future revenue and cash flows generated from the acquired assets.
Income taxes:
The preparation of our consolidated financial statements requires us to estimate our income taxes in each of the jurisdictions in which we operate, including those outside the United States, which may be subject to certain risks that ordinarily would not be expected in the United States. The income tax accounting process involves estimating our actual current exposure together with assessing temporary differences resulting from differing treatment of items, such as deferred revenue, for tax and accounting purposes. These differences result in the recognition of deferred tax assets and liabilities. We must then record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized.
Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against deferred tax assets. We have recorded a valuation allowance against our deferred tax assets of $21.2 million as of December 31, 2007, due to uncertainties related to our ability to utilize these assets. The valuation allowance is based on our estimates of taxable income by jurisdiction in which we operate and the period over which our deferred tax assets will be recoverable. In the event that actual results differ from these estimates or we adjust these estimates in future periods, we may need to adjust our valuation allowance which could materially impact our financial position and results of operations.
In January 2007, we adopted FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes (an interpretation of FASB Statement No. 109) (FIN 48). FIN 48 prescribes how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that a
38
company has taken or expects to take on a tax return. At December 31, 2007, we reviewed all material tax positions for all years open to statute and for all tax jurisdictions open to statute to determine whether it was more likely than not that the positions taken would be sustained based upon the technical merits of those positions. Based on our analysis, we concluded that a tax reserve for uncertain tax positions does not need to be established as a result of our adoption of FIN 48.
Contingencies:
Estimated losses from contingencies are accrued by management based upon the likelihood of a loss and the ability to reasonably estimate the amount of the loss. Estimating potential losses, or even a range of losses, is difficult and involves a great deal of judgment. Management relies primarily on assessments made by its external legal counsel to make our determination as to whether a loss contingency arising from litigation should be recorded or disclosed. Should the resolution of a contingency result in a loss that we did not accrue because management did not believe a loss was probable or capable of being reasonably estimated, then this loss would result in a charge to income in the period the contingency was resolved.
Recent Accounting Pronouncements:
In September 2006, the Financial Accounting Standards Board (FASB) issued SFAS No. 157,Fair Value Measurements, SFAS No. 157 was issued to provide consistency and comparability in determining fair value measurements and to provide for expanded disclosures about fair measurements. The definition about of fair value maintains the exchange price notion in earlier definitions of fair value but focuses on the exit price of the asset or liability. The exit price is the price that would be received to sell the asset or paid to transfer the liability adjusted for certain inherent risks and restrictions. Expanded disclosures are also required about the use of fair value to measure assets and liabilities. The effective date is for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We have not yet determined the impact, if any, of adopting this statement on its financial position, results of operations and cash flows.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities — Including an amendment of FASB Statement No. 115, which is effective for fiscal years beginning after November 15, 2007. The statement permits entities to choose to measure many financial instruments and certain other items at fair value. We have not yet determined the impact, if any, of adopting this statement on its financial position, results of operations and cash flows.
In July 2007, the Emerging Issues Task Force (EITF) issued EITF 07-3, "Accounting for Nonrefundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities" (EITF 07-3). EITF 07-3 clarifies the accounting for nonrefundable advance payments for goods or services that will be used or rendered for research and development activities. EITF 07-3 states that such payments should be capitalized and recognized as an expense as the goods are delivered or the related services are performed. If an entity does not expect the goods to be delivered or the services rendered, the capitalized advance payment should be charged to expense. EITF 07-3 is effective for fiscal years beginning after December 15, 2007. We are currently evaluating the effect of EITF 07-3 on our financial statements but do not expect the adoption of EITF 07-3 to have a material effect on our financial position or the results of our operations.
In December 2007, the FASB issued Statement No. 141R, "Business Combinations," which establishes principles for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired and liabilities assumed in a business combination, recognizes and measures the goodwill acquired in a business combination, and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of a business combination.
39
We are required to apply this Statement prospectively to business combinations for which the acquisition date is on or after January 1, 2009. Earlier application is not permitted.
In December 2007, the FASB ratified a consensus opinion reached by the EITF on EITF Issue 07-1, "Accounting for Collaborative Arrangements" (EITF 07-1). The guidance in EITF 07-1 defines collaborative arrangements and establishes presentation and disclosure requirements for transactions within a collaborative arrangement (both with third parties and between participants in the arrangement). The consensus in EITF 07-1 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2008. The consensus requires retrospective application to all collaborative arrangements existing as of the effective date, unless retrospective application is impracticable. The impracticability evaluation and exception should be performed on an arrangement-by-arrangement basis. We are evaluating the impact of EITF 07-1 will have on its financial statements. We currently do not believe that the adoption of EITF 07-1 will have a significant effect on the financial statements.
In December 2007, the SEC staff issued Staff Accounting Bulletin (SAB) 110, "Share-Based Payment" (SAB 110) which amends SAB 107, "Share-Based Payment", to permit public companies, under certain circumstances, to use the simplified method in SAB 107 for employee option grants after December 31, 2007. Use of the simplified method after December 2007 is permitted only for companies whose historical data about their employees' exercise behavior does not provide a reasonable basis for estimating the expected term of the options.
We currently use the simplified method to estimate the expected term for employee option grants, as adequate historical experience is not available to provide a reasonable estimate. SAB 110 is effective for employee options granted after December 31, 2007. We intend to adopt SAB 110 effective January 1, 2008 and continue applying the simplified method until enough historical experience is readily available to provide a reasonable estimate of the expected term for employee option grants.
Item 7A. Quantitative and Qualitative Disclosure about Market Risk
As of December 31, 2007, the only financial instruments we carried were cash and cash equivalents. We believe the market risk arising from holding these financial instruments is immaterial.
Some of our sales and some of our costs occur outside the United States and are transacted in foreign currencies. Accordingly, we are subject to exposure from adverse movements in foreign currency exchange rates. At this time we do not believe this risk is material and we do not currently use derivative financial instruments to manage foreign currency fluctuation risk. However, if foreign sales increase and the risk of foreign currency exchange rate fluctuation increases, we may in the future consider utilizing derivative instruments to mitigate these risks.
Item 8. Financial Statements and Supplementary Data
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Reports of Independent Registered Public Accounting Firm | | 41 |
| Consolidated Balance Sheets | | 42 |
| Consolidated Statements of Operations | | 43 |
| Consolidated Statements of Stockholders' Equity | | 44 |
| Consolidated Statements of Cash Flows | | 45 |
| Notes to Consolidated Financial Statements | | 46 |
Note: See Item 15 for Financial Statement Schedule.
40
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and
Shareholders of Interleukin Genetics, Inc.
We have audited the accompanying consolidated balance sheets of Interleukin Genetics, Inc. and subsidiaries (a Delaware corporation) (the "Company") as of December 31, 2007 and 2006, and the related consolidated statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2007. Our audits of the basic financial statements included the financial statement schedule listed in the index appearing under Item 15(a)(1). These consolidated financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Interleukin Genetics, Inc. and subsidiaries as of December 31, 2007 and 2006, and the results of their operations and their cash flows for the each of the three years in the period ended December 31, 2007, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Notes 3 and 11 to the consolidated financial statements, the Company changed its method of accounting for Share-Based Payments to conform to Statement of Financial Accounting Standards No. 123(R) as of January 1, 2006.
| |
|
|---|
| /s/ GRANT THORNTON LLP | | |
Boston, Massachusetts
March 31, 2008 |
|
|
41
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| ASSETS | | | | | | | |
Current assets: |
|
|
|
|
|
|
|
| | Cash and cash equivalents | | $ | 7,646,468 | | $ | 10,082,919 | |
| | Accounts receivable from related party | | | 48,147 | | | 199,395 | |
| | Trade accounts receivable, net of allowances for doubtful accounts of $6,696 and $28,000 in 2007 and 2006, respectively | | | 942,115 | | | 769,053 | |
| | Inventory | | | 999,392 | | | 1,504,154 | |
| | Deferred tax asset | | | 41,000 | | | — | |
| | Prepaid expenses and other current assets | | | 335,386 | | | 435,592 | |
| | |
| |
| |
| | | Total current assets | | | 10,012,508 | | | 12,991,113 | |
Fixed assets, net |
|
|
578,706 |
|
|
875,934 |
|
Intangible assets, net |
|
|
5,741,402 |
|
|
8,726,820 |
|
Other assets |
|
|
53,333 |
|
|
36,418 |
|
| | |
| |
| |
| | | Total assets | | $ | 16,385,949 | | $ | 22,630,285 | |
| | |
| |
| |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
Current liabilities: |
|
|
|
|
|
|
|
| | Accounts payable | | $ | 836,071 | | $ | 948,421 | |
| | Accrued expenses | | | 1,948,364 | | | 2,119,729 | |
| | Deferred receipts | | | 1,458,208 | | | 1,277,132 | |
| | State taxes payable | | | 32,500 | | | — | |
| | Commitments for funded research and development projects | | | 92,056 | | | 165,556 | |
| | Due to seller | | | 1,200,000 | | | 744,053 | |
| | Current portion of convertible debt, net of discount of $0 and $461,874 in 2007 and 2006, respectively | | | 595,336 | | | 2,133,462 | |
| | |
| |
| |
| | | Total current liabilities | | | 6,162,535 | | | 7,388,353 | |
Contingent acquisition consideration |
|
|
— |
|
|
1,449,001 |
|
| Deferred tax liability | | | 31,000 | | | 7,000 | |
| | |
| |
| |
| | | Total liabilities | | | 6,193,535 | | | 8,844,354 | |
Stockholders' equity: |
|
|
|
|
|
|
|
| | Convertible preferred stock, $0.001 par value — 6,000,000 shares authorized; 5,000,000 shares of Series A issued and outstanding at December 31, 2007 and 2006; aggregate liquidation preference of $18,000,000 at December 31, 2007 and 2006 | | | 5,000 | | | 5,000 | |
| | Common stock, $0.001 par value — 75,000,000 shares authorized; 30,832,102 and 27,406,984 shares issued and outstanding at December 31, 2007 and 2006, respectively | | | 30,832 | | | 27,407 | |
| | Additional paid-in capital | | | 84,517,903 | | | 81,896,060 | |
| | Accumulated deficit | | | (74,361,321 | ) | | (68,142,536 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 10,192,414 | | | 13,785,931 | |
| | |
| |
| |
| | | Total liabilities and stockholders' equity | | $ | 16,385,949 | | $ | 22,630,285 | |
| | |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
42
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| | For the Years Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Revenue: | | | | | | | | | | |
| | Revenue from related party | | $ | 2,792,683 | | $ | 2,652,198 | | $ | — | |
| | Revenue from others | | | 6,907,810 | | | 2,078,828 | | | 22,877 | |
| | |
| |
| |
| |
| | Total revenue | | | 9,700,493 | | | 4,731,026 | | | 22,877 | |
Cost of revenue |
|
|
4,699,446 |
|
|
2,842,597 |
|
|
— |
|
| | |
| |
| |
| |
| | Gross profit | | | 5,001,047 | | | 1,888,429 | | | 22,877 | |
| | |
| |
| |
| |
| Operating Expenses: | | | | | | | | | | |
| | Research and development | | | 2,928,249 | | | 3,262,349 | | | 3,127,086 | |
| | Selling, general and administrative | | | 6,367,973 | | | 4,506,799 | | | 2,916,858 | |
| | Amortization of intangible assets | | | 1,651,244 | | | 646,065 | | | 36,921 | |
| | |
| |
| |
| |
| | | Total operating expenses | | | 10,947,466 | | | 8,415,213 | | | 6,080,865 | |
| | |
| |
| |
| |
| Loss from operations | | | (5,946,419 | ) | | (6,526,784 | ) | | (6,057,988 | ) |
| | |
| |
| |
| |
| Other income (expense): | | | | | | | | | | |
| | Interest income | | | 437,017 | | | 283,191 | | | 131,656 | |
| | Interest expense | | | (236,932 | ) | | (234,289 | ) | | (182,617 | ) |
| | Other expense | | | 533 | | | — | | | — | |
| | Amortization of note discount | | | (457,484 | ) | | (461,874 | ) | | (461,875 | ) |
| | |
| |
| |
| |
| | | Total other expense, net | | | (256,866 | ) | | (412,972 | ) | | (512,836 | ) |
| | |
| |
| |
| |
| Net loss before income taxes | | | (6,203,285 | ) | | (6,939,756 | ) | | (6,570,824 | ) |
Provision for income taxes |
|
|
(15,500 |
) |
|
(7,000 |
) |
|
— |
|
| | |
| |
| |
| |
| Net loss | | $ | (6,218,785 | ) | $ | (6,946,756 | ) | $ | (6,570,824 | ) |
| | |
| |
| |
| |
| Basic and diluted net loss per common share | | $ | (0.22 | ) | $ | (0.27 | ) | $ | (0.28 | ) |
| | |
| |
| |
| |
| Weighted average common shares outstanding | | | 27,723,754 | | | 25,340,107 | | | 23,702,967 | |
| | |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
43
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
For the Years Ended December 31, 2007, 2006 and 2005
| | Convertible Preferred Stock
| | Common Stock
| |
| |
| |
| |
|---|
| | Shares
| | $0.001
par value
| | Shares
| | $0.001
par value
| | Additional
Paid-in
Capital
| | Accumulated
Deficit
| | Total
| |
|---|
| Balance as of December 31, 2004 | | 5,000,000 | | $ | 5,000 | | 23,594,337 | | $ | 23,595 | | $ | 58,123,868 | | $ | (54,624,956 | ) | $ | 3,527,507 | |
| Net loss | | — | | | — | | — | | | — | | | — | | | (6,570,824 | ) | | (6,570,824 | ) |
| Investment by Alticor: | | | | | | | | | | | | | | | | | | | | |
| | Research funding | | — | | | — | | — | | | — | | | 2,517,474 | | | — | | | 2,517,474 | |
| | Other | | — | | | — | | — | | | — | | | 196,000 | | | — | | | 196,000 | |
| Common stock issued: | | | | | | | | | | | | | | | | | | | | |
| | Exercise of stock options | | — | | | — | | 320,342 | | | 320 | | | 577,710 | | | — | | | 578,030 | |
| | Employee stock purchase plan | | — | | | — | | 12,647 | | | 12 | | | 35,546 | | | — | | | 35,558 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance as of December 31, 2005 | | 5,000,000 | | | 5,000 | | 23,927,326 | | | 23,927 | | | 61,450,598 | | | (61,195,780 | ) | | 283,745 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss | | — | | | — | | — | | | — | | | — | | | (6,946,756 | ) | | (6,946,756 | ) |
| Investment by Alticor: | | | | | | | | | | | | | | | | | | | | |
| | Research funding | | — | | | — | | — | | | — | | | 1,451,978 | | | — | | | 1,451,978 | |
| | Other | | — | | | — | | — | | | — | | | 1,274,210 | | | — | | | 1,274,210 | |
| | Private placement, net of issuance costs of $150,063 | | — | | | — | | 2,750,037 | | | 2,750 | | | 15,462,724 | | | — | | | 15,465,474 | |
| Common stock issued: | | | | | | | | | | | | | | | | | | | | |
| | Exercise of stock warrants | | — | | | — | | 125,000 | | | 125 | | | 312,375 | | | — | | | 312,500 | |
| | Exercise of stock options | | — | | | — | | 539,050 | | | 539 | | | 830,194 | | | — | | | 830,733 | |
| | Employee stock purchase plan | | — | | | — | | 9,074 | | | 9 | | | 44,350 | | | — | | | 44,359 | |
| | Restricted stock awards | | — | | | — | | 28,497 | | | 29 | | | (29 | ) | | — | | | — | |
| | Common stock awards | | — | | | — | | 28,000 | | | 28 | | | (28 | ) | | — | | | — | |
| Stock-based compensation expense | | — | | | — | | — | | | — | | | 1,069,688 | | | — | | | 1,069,688 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance as of December 31, 2006 | | 5,000,000 | | | 5,000 | | 27,406,984 | | | 27,407 | | | 81,896,060 | | | (68,142,536 | ) | | 13,785,931 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss | | — | | | — | | — | | | — | | | — | | | (6,218,785 | ) | | (6,218,785 | ) |
| Common stock issued: | | | | | | | | | | | | | | | | | | | | |
| | Exercise of stock options | | — | | | — | | 194,917 | | | 195 | | | 347,217 | | | — | | | 347,412 | |
| | Employee stock purchase plan | | — | | | — | | 7,702 | | | 8 | | | 16,713 | | | — | | | 16,721 | |
| | Restricted stock awards | | — | | | — | | 12,500 | | | 12 | | | (12 | ) | | — | | | — | |
| | Common stock awards | | — | | | — | | 7,000 | | | 7 | | | (7 | ) | | — | | | — | |
| | Conversion of long-term debt to equity | | — | | | — | | 3,190,987 | | | 3,191 | | | 2,032,098 | | | — | | | 2,035,289 | |
| | Rights offering, net of issuance costs | | — | | | — | | 12,012 | | | 12 | | | 52,670 | | | — | | | 52,682 | |
| Stock-based compensation expense | | — | | | — | | — | | | — | | | 173,164 | | | — | | | 173,164 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance as of December 31, 2007 | | 5,000,000 | | $ | 5,000 | | 30,832,102 | | $ | 30,832 | | $ | 84,517,903 | | $ | (74,361,321 | ) | $ | 10,192.414 | |
| | |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
44
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | For the Years Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | |
| | Net loss | | $ | (6,218,785 | ) | $ | (6,946,756 | ) | $ | (6,570,824 | ) |
| | Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | |
| | | Depreciation and amortization | | | 1,983,326 | | | 983,400 | | | 340,237 | |
| | | Amortization of note discount | | | 457,484 | | | 461,874 | | | 461,875 | |
| | | Interest capitalized in debt to equity conversion | | | 39,679 | | | | | | | |
| | | Stock-based compensation expense | | | 173,164 | | | 1,069,688 | | | — | |
| | | Loss on disposal of fixed asset | | | 3,968 | | | — | | | — | |
| | | Changes in operating assets and liabilities, net of acquired assets and liabilities: | | | | | | | | | | |
| | | | Accounts receivable, net | | | (21,814 | ) | | 511,667 | | | 9,853 | |
| | | | Inventory | | | 504,762 | | | 495,846 | | | — | |
| | | | Prepaid expenses and other current assets | | | 100,206 | | | (152,777 | ) | | 8,615 | |
| | | | Accounts payable | | | (112,350 | ) | | 313,472 | | | 75,694 | |
| | | | Accrued expenses | | | (171,365 | ) | | (456,499 | ) | | (394,395 | ) |
| | | | Deferred receipts | | | 181,076 | | | 428,684 | | | (1,990,000 | ) |
| | | | Due to seller | | | 600,000 | | | — | | | — | |
| | | | Commitments for funded research and development projects | | | (73,500 | ) | | (152,463 | ) | | (90,525 | ) |
| | | | Deferred tax provision | | | 15,500 | | | 7,000 | | | — | |
| | |
| |
| |
| |
| | Net cash used in operating activities | | | (2,538,649 | ) | | (3,436,864 | ) | | (4,169,470 | ) |
| | |
| |
| |
| |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | |
| | Capital additions | | | (38,822 | ) | | (146,297 | ) | | (118,057 | ) |
| | Increase in other assets | | | (213,116 | ) | | (185,712 | ) | | (138,473 | ) |
| | Acquisition of the assets and business of the Alan James Group, LLC, including transaction costs paid of $634,192 | | | (62,679 | ) | | (7,665,449 | ) | | — | |
| | |
| |
| |
| |
| | Net cash used in investing activities | | | (314,617 | ) | | (7,997,458 | ) | | (256,530 | ) |
| | |
| |
| |
| |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | |
| | Proceeds from investment by Alticor, net of issuance costs | | | — | | | 16,917,452 | | | 2,713,474 | |
| | Proceeds from exercises of rights offering, stock warrants, options and employee stock purchase plan | | | 416,815 | | | 1,187,592 | | | 613,588 | |
| | Principal payments of capital lease obligations | | | — | | | (2,977 | ) | | (14,313 | ) |
| | |
| |
| |
| |
| | Net cash provided by financing activities | | | 416,815 | | | 18,102,067 | | | 3,312,749 | |
| | |
| |
| |
| |
| Net (decrease) increase in cash and cash equivalents | | | (2,436,451 | ) | | 6,667,745 | | | (1,113,251 | ) |
| Cash and cash equivalents, beginning of year | | | 10,082,919 | | | 3,415,174 | | | 4,528,425 | |
| | |
| |
| |
| |
| Cash and cash equivalents, end of year | | $ | 7,646,468 | | $ | 10,082,919 | | $ | 3,415,174 | |
| | |
| |
| |
| |
| Supplemental disclosures of cash flow information: | | | | | | | | | | |
| Non-cash investing and financing activities: | | | | | | | | | | |
| | Deferred receipt reclassified to equity | | $ | — | | $ | 1,274,210 | | $ | — | |
| Interest paid: | | | | | | | | | | |
| | Cash paid for interest | | $ | 197,628 | | $ | 234,289 | | $ | 182,617 | |
| | |
| |
| |
| |
Non-cash activities:
On December 17, 2007, pursuant to the terms of certain promissory notes, Pyxis Innovations, Inc., an affiliate of Alticor, converted indebtedness due on December 31, 2007, representing an aggregate principal amount of $2 million and accrued interest of $39,679, into 3,190,987 shares of the Company's common stock.
On March 25, 2008, the Company agreed to pay a total of $1.2 million to former officers of the Alan James Group under the settlement agreement. The $1,200,000 due to sellers is recorded as a current liability at December 31, 2007. The Company applied $600,000 of the settlement cost against the previously accrued separation expense that was recorded on June 30, 2007 and the remaining $600,000 was applied against the $2,130,374 aggregate total of contingent liabilities and amounts due under escrow recorded as part of the original acquisition. The remaining contingent liabilities and amounts due under escrow balance of $1,530,374 was eliminated as no longer due and applied as a reduction in the balances on a prorata basis of the intangibles assets recorded as part of the original acquisition, including the effect of term reduction on the non-compete agreements.
The accompanying notes are an integral part of these consolidated financial statements.
45
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1—Company Overview
Interleukin Genetics, Inc. and subsidiaries (Interleukin or the Company) is a company focused on developing, acquiring, and commercializing personalized health products that can help individuals improve and maintain their health through preventive measures. It uses functional genomics to help in the development of risk assessment tests based on the genetic variations in people. The Company also develops and markets nutritional products. Interleukin has commercialized genetic tests for periodontal disease risk assessment, cardiovascular risk assessment, and general nutrition assessment. In addition, through its Alan James Group subsidiary which it acquired in August 2006, Interleukin sells its nutritional product brands, including Ginsana®, Ginkoba®, and Venastat®, through the nation's largest food, drug and mass retailers. The Company's current development programs focus on osteoporosis and weight management genetic risk assessment tests.
The Company was incorporated in Texas in 1986 and re-incorporated in Delaware in March 2000.
The Company has experienced operating losses since its inception through December 31, 2007. During the last three years such losses totaled $19.7 million contributing to an accumulated deficit of $74.4 million. Based on the current operating and capital expenditure forecasts, the Company believes that the combination of funds currently available, funds to be generated from operations and the available lines of credit will be adequate to finance their ongoing operations for at least the next twelve months.
Note 2—Acquisition
In August 2006, the Company acquired the assets and business of the Alan James Group, LLC (the Alan James Group). The acquired business primarily develops, markets and sells nutritional products and engages in related activities. The combination is intended to create a diversified, fully integrated provider of products and services in the consumer and professional healthcare marketplace. Interleukin and the Alan James Group have complementary capabilities in genetic testing services and preventive healthcare products distribution. By combining these capabilities, the Company will be positioned to expand its science-based solutions portfolio, commercialize its products and services and offer a broad selection of innovative, preventive and personalized products to its customers. The initial purchase price consisted of the payment of $7,031,257 in cash and the obligation to place in escrow $250,000 and 88,055 shares of the Company's Common Stock valued at $500,000, or $5.6873 per share (based on the volume-weighted average closing stock price for the 20 consecutive trading days ending August 15, 2006). The $250,000 and 88,055 common stock shares that were to have been placed in escrow was settled as part of the agreement dated March 25, 2008. The acquisition was accounted for as a purchase in accordance with Statement of Financial Accounting Standards (SFAS) No. 141,Business Combinations (SFAS No. 141). Accordingly, the consolidated financial statements include the results of the acquired company's operations since the acquisition date, August 17, 2006.
The purchase price was allocated to the tangible and intangible assets acquired and liabilities assumed based on their estimated fair values at the date of acquisition. The estimated fair value of the assets acquired and liabilities assumed exceeded the initial payments by approximately $2.2 million resulting in negative goodwill. Pursuant to SFAS No. 141, the Company recorded as a liability, contingent consideration up to the amount of negative goodwill. If and when contingent payments become due, the Company will apply the contingent payments against the liability. Contingent payments in excess of $2.2 million, was to be recorded as goodwill.
On March 25, 2008, pursuant to the terms of a settlement agreement between the Company and former officers of the Alan James Group including the acquisition of the assets and business of the
46
Alan James Group, the Company agreed to pay a total of $1,200,000. The $1,200,000 due to sellers is recorded as a current liability at December 31, 2007. The Company applied $600,000 of the settlement cost against the previously accrued separation expense that was recorded on June 30, 2007 and the remaining $600,000 was applied against the $2,130,374 aggregate total of contingent liabilities and amounts due under escrow recorded as part of the original acquisition. The remaining contingent liabilities and amounts due under escrow balance of $1,530,374 was eliminated as no longer due and applied as a reduction in the balances on a prorata basis of the intangibles assets recorded as part of the original acquisition, including the effect of term reduction on the non-compete agreements.
The components of the purchase price allocation are as follows:
| Purchase Price: | | | | |
| Cash | | $ | 7,031,257 | |
| Transaction costs | | | 632,260 | |
| | |
| |
| | | $ | 7,663,517 | |
| | |
| |
| Allocation: | | | | |
| Accounts Receivable | | $ | 1,479,837 | |
| Inventory | | | 2,000,000 | |
| Other current assets | | | 108,611 | |
| Property and equipment | | | 110,144 | |
| Acquired intangible assets | | | 8,800,000 | |
| Accounts payable and accrued expenses | | | (2,618,334 | ) |
| Contingent acquisition costs | | | (2,216,741 | ) |
| | |
| |
| | | $ | 7,663,517 | |
| | |
| |
Acquired intangible assets were valued based upon third-party independent valuations and studies performed by the Company in August 2006 and consist of the following (see Note 6):
Identified Intangible Assets
| | Estimated
Fair Value
| | Estimated
Remaining
Useful Life
|
|---|
| Retailer Relationships | | $ | 5,200,000 | | 5 years |
| Indefinite Lived Trademarks | | | 1,000,000 | | N/A |
| Definite Lived Trademarks | | | 1,100,000 | | 5 years |
| Non-Compete Agreements | | | 200,000 | | 4 years |
| OTCeutical Formulations | | | 1,300,000 | | 5 years |
| | |
| | |
| Total Fair Value of Intangible Assets | | $ | 8,800,000 | | |
| | |
| | |
For tax purposes, the fair value of the non-current tangible and intangible assets will be reduced pro rata to the extent of the contingent liability with a resultant reduction in amortization for tax purposes. If, and when, the contingent liability is paid, the tax basis of the non-current tangible assets will be increased pro rata in the amount of the contingent payment up to the non-current assets fair value at the date of acquisition. The unamortized tax basis will be amortized over the assets remaining useful life.
47
Had the acquisition of the Alan James Group been completed at the beginning of 2005, the Company's pro forma results would have been as follows:
| | For the Years Ended December 31,
| |
|---|
| | 2006
| | 2005
| |
|---|
| Revenue | | $ | 9,740,883 | | $ | 9,417,076 | |
| Net loss | | $ | (8,691,470 | ) | $ | (7,420,641 | ) |
| | |
| |
| |
| Basic and diluted net loss per common share | | $ | (0.32 | ) | $ | (0.28 | ) |
| | |
| |
| |
Note 3—Summary of Significant Accounting Policies
The consolidated financial statements include the accounts of Interleukin Genetics, Inc., and its wholly owned subsidiaries, Interleukin Genetics Laboratory Services, Inc. and AJG Brands, Inc. doing business as the Alan James Group. All intercompany accounts and transactions have been eliminated. Results of AJG Brands, Inc. are included in operations since August 17, 2006, the date of acquisition.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenue and expenses during the reported periods. Actual results could differ from those estimates. The Company's most critical accounting policies are in areas of its strategic alliance with Alticor, revenue recognition, allowance for sales returns, trade promotions, accounts receivable, inventory, stock-based compensation, income taxes, long-lived assets, beneficial conversion feature of convertible instruments, and below market interest rate on debt. These critical accounting policies are more fully discussed in these notes to the consolidated financial statements.
In a private placement on March 5, 2003, the Company entered into a Stock Purchase Agreement with Alticor, pursuant to which Alticor purchased from the Company 5,000,000 shares of the Company's Series A Preferred Stock, $0.001 per share, for $7,000,000 in cash and $2,000,000 in cash to be paid, if at all, upon the Company reaching a milestone pursuant to the terms of the Stock Purchase Agreement (see Note 4). The Series A Preferred Stock issued in the private placement was initially convertible into 28,157,683 shares of the Company's Common Stock at the purchaser's discretion. Pursuant to the terms of the Stock Purchase Agreement, Alticor also agreed to refinance, in the form of convertible debt, certain of the Company's indebtedness in the form of previously issued promissory notes that were held by Alticor and certain individuals. This amounted to $2,595,336 in debt refinanced and was initially convertible into 5,219,903 shares of the Company's Common Stock. Concurrent with the closing of the Stock Purchase Agreement, the Company entered into a research agreement with Alticor that would provide additional funding of $5,000,000 to be paid quarterly over a two-year period.
In accordance with Emerging Issues Task Force (EITF) No. 01-1, the terms of both the agreement for goods or services provided and the convertible instruments should be evaluated to determine whether their separately stated pricing is equal to the fair value of the goods or services provided and the convertible instruments. If that is not the case, the terms of the respective transactions should be adjusted. The convertible instruments should be recognized at fair value with a corresponding increase or decrease in the sales price of the goods or services.
48
On March 5, 2003, the Company was obligated to issue up to 33,377,586 shares of its common stock underlying the convertible preferred stock and the convertible debt issued. Based on the last reported trade price of $0.71 per common share of the Company's common stock on March 5, 2003, the convertible instruments had a fair value of $23,698,086 on the date of issuance. Based on the fair value of the convertible instruments and the guidance provided by EITF 01-1, the Company recognized the fair value of the convertible instruments, to the extent of proceeds received, with a corresponding decrease to the sales price of the goods and services provided. At March 5, 2003, the Company treated the $5,000,000 committed research funding as an equity investment rather than revenue and any costs of performing the research services under the agreement were classified as research and development expenses. Any subsequent proceeds that the Company received from Alticor that were linked to the March 2003 transaction, were considered equity rather than revenue to the extent of the fair value of the convertible instruments at March 5, 2003. In June 2004, the Company entered into another research agreement with Alticor for potential funding up to $2,200,000 and in March 2005, the Company entered into two more agreements to provide additional funding of $5,057,651 over two years beginning April 1, 2005 (see Note 4). In addition, since March 5, 2003, the Company received various purchase orders from Alticor valued at $501,800 to conduct genotyping test for research purposes. These purchase orders, together with the research agreements entered into in June 2004 and March 2005, were deemed to be linked to the March 2003 transaction, and, accordingly, were treated as equity rather than revenue. As of December 31, 2007, proceeds received from Alticor, which were recorded as consideration for the fair value of the convertible instruments issued in March 2003, amounted to $23,698,086.
In March 2007, the Company entered into an agreement, effective January 1, 2007, to expand the research being performed under its current agreements with Alticor through 2007. The Company recorded the funds received associated with this agreement as revenue in accordance with its revenue recognition policy.
Revenue from genetic testing services is recognized when there is persuasive evidence of an arrangement, service has been rendered, the sales price is determinable and collectibility is reasonably assured. Service is deemed to be rendered when the results have been reported to the individual who ordered the test. To the extent that tests have been prepaid but results have not yet been reported, recognition of all related revenue is deferred. As of December 31, 2007 and 2006, deferred receipts includes $12,250 and $0, respectively, for tests that have been prepaid but results have not yet been reported.
Revenue from product sales is recognized when there is persuasive evidence of an arrangement, delivery has occurred and title and risk of loss have transferred to the customer, the sales price is determinable and collectibility is reasonably assured. The Company has no consignment sales. Product revenue is reduced for allowances and adjustments, including returns, discontinued items, discounts, trade promotions and slotting fees.
Revenue from contract research and development is recognized over the term of the contract as the Company performs its obligations under that contract (including revenue from Alticor, a related party).
The Company's recognition of revenue from sales to retailers is impacted by giving them rights to return damaged and outdated products as well as the fact that as a practical business matter, its sales force, along with its customers, is constantly working to ensure profitability of its products within retailers by rotating slow moving items out of stores and replacing those products with what the
49
Company and the retailer expect will be more profitable, faster selling items. For product sales the Company believes it can reasonably and reliably estimate future returns, it recognizes revenue at the time of sale. For product sales which it cannot estimate future returns, particularly new products, the Company defers revenue recognition until the return privilege has substantially expired or the amount of future returns can be reasonably estimated. As of December 31, 2007 and 2006, the Company has deferred $93,080 and $59,949, respectively, of revenue for sales in which it cannot reasonably and reliably estimate future returns.
The Company analyzes sales returns in accordance with SFAS No. 48,Revenue Recognition When Right of Return Exists. The Company is able to make reasonable and reliable estimates based on its history. The Company also monitors the buying patterns of the end-users of its products based on sales data received. The Company reviews its estimated product returns based on expected data communicated by its customers. The Company also monitors the levels of inventory at its largest customers to avoid excessive customer stocking of merchandise. The Company believes it has sufficient interaction and knowledge of its customers and of the industry trends and conditions to adjust the accrual for returns when necessary. The Company believes that this analysis creates appropriate estimates of expected future returns. The possibility exists that should the Company lose a major account, it may agree to accept a substantial amount of returns.
The Company uses objective procedures for estimating its allowance for trade promotions. The allowance for trade promotions offered to customers is based on contracted terms or other arrangements agreed in advance.
Trade accounts receivable are stated at their estimated net realizable value, which is generally the invoiced amount less any estimated discount related to payment terms. The Company offers its customers a 2% cash discount if payment is made within 30 days of invoice date, however, most customers take the discount regardless of when payment occurs. As of December 31, 2007 and 2006, the Company has reduced trade accounts receivable by $17,851 and $9,327, respectively, for anticipated discounts taken. A provision is made for estimated bad debts based on management's estimate of the amount of possible credit losses in the Company's existing accounts receivable. As of December 31, 2007 and 2006, the Company has provided an allowance for uncollectible accounts of $6,696 and $28,000, respectively.
Inventory is stated at the lower of cost or market. Cost is determined using the specific identification method. Management periodically evaluates inventory to identify items, that are slow moving or have excess quantities. Management also considers whether certain items are carried at values, that exceed the ultimate sales price less selling costs. Where such items are identified, management adjusts the carrying value to lower of cost or market.
Inventory on hand primarily consisted of the following at December 31, 2007 and 2006:
| | 2007
| | 2006
|
|---|
| Raw materials | | $ | 93,022 | | $ | 17,375 |
| Finished goods | | | 906,370 | | | 1,486,779 |
| | |
| |
|
| | Total | | $ | 999,392 | | $ | 1,504,154 |
| | |
| |
|
50
The Company accounts for its stock-based compensation expense in accordance with SFAS No. 123 (Revised 2004),Share-Based Payment (SFAS No. 123R) using the modified prospective basis. SFAS No. 123R addresses all forms of share-based payment (SBP) awards, including shares issued under employee stock purchase plans, stock options, restricted stock and stock appreciation rights. SFAS No. 123R requires the Company to expense SBP awards with compensation cost for SBP transactions measured at fair value. SFAS No. 123R applies to new equity awards and to equity awards modified, repurchased or canceled after the effective date, January 1, 2006. Additionally, compensation cost for the portion of awards for which the requisite service has not been rendered that are outstanding as of the effective date shall be recognized as the requisite service is rendered on or after the effective date. The compensation cost for that portion of awards shall be based on the grant-date fair value of those awards as calculated from the pro forma disclosures under SFAS No. 123. Additionally, the Company records an expense for the amount that the fair market value exceeds the purchase cost for common stock purchased pursuant to its employee stock purchase plan.
The preparation of its consolidated financial statements requires the Company to estimate its income taxes in each of the jurisdictions in which it operates, including those outside the United States, which may be subject to certain risks that ordinarily would not be expected in the United States. The Company accounts for income taxes in accordance with SFAS No. 109,Accounting for Income Taxes, which requires the recognition of taxes payable or refundable for the current year and deferred tax liabilities and assets for the future tax consequences of events that have been recognized in the financial statements or tax returns. The measurement of current and deferred tax liabilities and assets is based on provisions of the enacted tax law; the effects of future changes in tax laws or rates are not anticipated. The Company records a valuation allowance to reduce its deferred tax assets to the amount that is more likely than not to be realized.
Significant management judgment is required in determining the Company's provision for income taxes, its deferred tax assets and liabilities and any valuation allowance recorded against deferred tax assets. The Company has recorded a full valuation allowance against its deferred tax assets of $21.2 million as of December 31, 2007, due to uncertainties related to its ability to utilize these assets. The valuation allowance is based on management's estimates of taxable income by jurisdiction in which the Company operates and the period over which the deferred tax assets will be recoverable. In the event that actual results differ from these estimates or management adjusts these estimates in future periods, the Company may need to adjust its valuation allowance, which could materially impact its financial position and results of operations.
In January 2007, the Company adopted FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes (an interpretation of FASB Statement No. 109) (FIN 48). FIN 48 prescribes how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that a company has taken or expects to take on a tax return. At December 31, 2007, the Company reviewed all material tax positions for all years open to statute and for all tax jurisdictions open to statute to determine whether it was more likely than not that the positions taken would be sustained based upon the technical merits of those positions. The implementation of FIN 48 had no impact on the Company's financial statements.
Research and development costs are expensed as incurred.
51
The Company applies SFAS No. 128,Earnings per Share, which establishes standards for computing and presenting earnings per share. Basic and diluted net loss per share was determined by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share is the same as basic net loss per share for all the periods presented, as the effect of the potential common stock equivalents is anti-dilutive due to the loss in each period. Potential common stock equivalents excluded from the calculation of diluted net loss per share consists of stock options, warrants, convertible preferred stock and convertible debt as described in the table below:
| | 2007
| | 2006
| | 2005
|
|---|
| Options outstanding | | 1,366,406 | | 1,893,015 | | 2,477,815 |
| Warrants outstanding | | 400,000 | | 400,000 | | 525,000 |
| Convertible preferred stock | | 28,160,200 | | 28,160,200 | | 28,160,200 |
| Convertible debt | | 931,377 | | 4,060,288 | | 4,060,288 |
| | |
| |
| |
|
| Total | | 30,857,983 | | 34,513,503 | | 35,223,303 |
| | |
| |
| |
|
Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. During the years ended December 31, 2007, 2006 and 2005, there were no items other than net loss included in the comprehensive loss.
The Company, using available market information, has determined the estimated fair values of financial instruments. The stated values of cash and cash equivalents, accounts receivable and accounts payable approximate fair value due to the short-term nature of these instruments. The carrying amounts of the Company's capital lease obligations also approximate fair value. The carrying amounts of borrowings under short-term agreements approximate their fair value as the rates applicable to the financial instruments reflect changes in overall market interest rates.
Cash equivalents consist of money market funds at a financial institution. These funds are not federally insured.
Fixed assets are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization are provided using the straight-line method over estimated useful lives of three to five years. Leasehold improvements are amortized over the estimated useful life of the asset, or the remaining term of the lease, whichever is shorter.
The Company applies the provisions of SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets (SFAS No. 144). SFAS No. 144 requires that the Company evaluate its long-lived assets for impairment whenever events or changes in circumstances indicate that carrying amounts of such assets may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted net cash flows expected to
52
be generated by the asset. Any write-downs, based on fair value, are to be treated as permanent reductions in the carrying amount of the assets. The Company believes that no impairment exists related to the Company's long-lived assets at December 31, 2007.
Purchase accounting requires extensive use of accounting estimates and judgments to allocate the purchase price to the fair market value of the assets purchased and liabilities assumed. The Company accounted for its acquisitions using the purchase method of accounting. Values were assigned to goodwill and intangible assets based on third-party independent valuations, as well as management's forecasts and projections that include assumptions related to future revenue and cash flows generated from the acquired assets.
The Company applies the provisions of SFAS No. 142,Goodwill and Other Intangible Assets. SFAS No. 142 requires impairment tests be periodically repeated and on an interim basis, if certain conditions exist, with impaired assets written down to fair value. An analysis performed by management on December 31, 2007, determined that the indefinite lived trademarks had a current fair market value of $764,000. Management adjusted the book value of the indefinite lived trademarks to reflect this $236,000 impairment in value. See Note 6 for adjustments of intangible assets per settlement dated March 25, 2008.
Based on EITF No. 00-27,Application of Issue No. 98-5 to Certain Convertible Instruments (EITF No. 00-27), which provides guidance on the calculation of a beneficial conversion feature of a convertible instrument, the Company has determined that the convertible debt issued on March 5, 2003 contained a beneficial conversion feature.
Based on the effective conversion price of the convertible debt of $0.2875 and the market value per share of $0.71 at March 5, 2003, the intrinsic value was calculated to be $2,205,522; however in accordance with EITF No. 00-27, the amount of the discount allocated to the beneficial conversion feature is limited to the amount of the proceeds allocated to the instrument. The beneficial conversion feature resulted in a discount of the convertible debt of $1,500,609 at March 5, 2003. The amount of the discount allocated to the beneficial conversion feature of the convertible debt is amortized from the date of issuance to the earlier of the maturity or conversion date. Therefore, the Company charged $310,471 for each of the years ended December 31, 2007, 2006 and 2005 to amortization of note discount. As of December 31, 2007, there is no remaining unamortized discount.
The convertible debt has a stated interest rate of prime plus 1%. However, the promissory notes, refinanced with the convertible debt, originally had a stated interest rate of 15%. Therefore, the Company determined the fair value of the convertible debt, using an interest rate comparable to that of the refinanced promissory notes, at $1,863,553. The resulting discount of $731,783 is amortized from the date of issuance to the earlier of maturity or conversion date. Therefore, the Company charged $147,013 for the year ended December 31, 2007and $151,403 for the years ended December 31, 2006 and 2005 to amortization of note discount. As of December 31, 2007, there is no remaining unamortized discount.
53
In September 2006, the Financial Accounting Standards Board (FASB) issued SFAS No. 157,Fair Value Measurements. SFAS No. 157 was issued to provide consistency and comparability in determining fair value measurements and to provide for expanded disclosures about fair value measurements. The definition of fair value maintains the exchange price notion in earlier definitions of fair value but focuses on the exit price of the asset or liability. The exit price is the price that would be received to sell the asset or paid to transfer the liability adjusted for certain inherent risks and restrictions. Expanded disclosures are also required about the use of fair value to measure assets and liabilities. The effective date is for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The Company has not yet determined the impact, if any, of adopting this statement on its financial position, results of operations and cash flows.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities-Including an amendment of FASB Statement No. 115, which is effective for fiscal years beginning after November 15, 2007. The statement permits entities to choose to measure many financial instruments and certain other items at fair value. The Company has not yet determined the impact, if any, of adopting this statement on its financial position, results of operations and cash flows.
In July 2007, the Emerging Issues Task Force (EITF) issued EITF 07-3, "Accounting for Nonrefundable Advance Payments for Goods or Services to be Used in Future Research and Development Activities" (EITF 07-3). EITF 07-3 clarifies the accounting for nonrefundable advance payments for goods or services that will be used or rendered for research and development activities. EITF 07-3 states that such payments should be capitalized and recognized as an expense as the goods are delivered or the related services are performed. If an entity does not expect the goods to be delivered or the services rendered, the capitalized advance payment should be charged to expense. EITF 07-3 is effective for fiscal years beginning after December 15, 2007. The Company is currently evaluating the effect of EITF 07-3 on its financial statements but does not expect the adoption of EITF 07-3 to have a material effect on the Company's financial position or results of operations.
In December 2007, the FASB issued Statement No. 141R, "Business Combinations," which establishes principles for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired and liabilities assumed in a business combination, recognizes and measures the goodwill acquired in a business combination, and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of a business combination. The Company is required to apply this Statement prospectively to business combinations for which the acquisition date is on or after January 1, 2009. Earlier application is not permitted.
In December 2007, the FASB ratified a consensus opinion reached by the EITF on EITF Issue 07-1, "Accounting for Collaborative Arrangements (EITF 07-1). The guidance in EITF 07-1 defines collaborative arrangements and establishes presentation and disclosure requirements for transactions within a collaborative arrangement (both with third parties and between participants in the arrangement).
The consensus in EITF 07-1 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2008. The consensus requires retrospective application to all collaborative arrangements existing as of the effective date, unless retrospective application is impracticable. The impracticability evaluation and exception should be performed on an arrangement-by-arrangement basis.
The Company intends to adopt EITF 07-1 effective January 1, 2009 and retrospectively apply the requirements of this consensus to its collaborative arrangements in existence on that date.
54
The Company is evaluating the impact of EITF 07-1 will have on its financial statements. The Company currently does not believe that the adoption of EITF 07-1 will have a significant effect on its financial statements.
In December 2007, the SEC staff issued Staff Accounting Bulletin (SAB) 110, "Share-Based Payment" (SAB 110) which amends SAB 107, "Share-Based Payment", to permit public companies, under certain circumstances, to use the simplified method in SAB 107 for employee option grants after December 31, 2007. Use of the simplified method after December 2007 is permitted only for companies whose historical data about their employees' exercise behavior does not provide a reasonable basis for estimating the expected term of the options.
The Company currently uses the simplified method to estimate the expected term for employee option grants as adequate historical experience is not available to provide a reasonable estimate. SAB 110 is effective for employee options granted after December 31, 2007. The Company intends to adopt SAB 110 effective January 1, 2008 and continue applying the simplified method until enough historical experience is readily available to provide a reasonable estimate of the expected term for employee option grants.
Note 4—Strategic Alliance with Alticor Inc.
�� On March 5, 2003, the Company entered into a broad strategic alliance with several affiliates of the Alticor family of companies to develop and market novel nutritional and skin care products. The alliance utilizes Interleukin Genetics' intellectual property and expertise in genomics to develop risk assessment tests and to aid Alticor in its efforts to develop personalized consumer products.
The alliance initially included an equity investment, a multi-year research and development agreement, a licensing agreement with royalties on marketed products, the deferment of outstanding loan repayment and the refinancing of bridge financing obligations. The major elements of the initial alliance were:
- •
- The purchase by Alticor of $7,000,000 of equity in the form of 5 million shares of Series A Preferred Stock for $1.40 per share. These were convertible into 28,157,683 shares of common stock at a stated conversion price equal to $0.2486 per share. On March 11, 2004, upon achievement of a defined milestone, Alticor contributed an additional $2,000,000 to the Company for a total equity funding of $9,000,000 and a new stated conversion price of $0.3196 per share, or 28,160,200 shares of common stock.
- •
- The right of the Series A holders to nominate and elect four directors to a five person board.
- •
- A research and development agreement (Research Agreement I) providing the Company with funding of $5,000,000, payable over the twenty-four month period from April 2003 through March 2005, to conduct certain research projects with a royalty on resulting products.
- •
- Credit facilities in favor of the Company, as described further in Note 8, as follows:
- •
- $1,500,000 working capital credit line to initiate selected research agreements with third party entities approved by the board of directors of the Company;
- •
- $2,000,000 refinancing of notes previously held by Alticor, extending the maturity date and reducing the interest rate; and
- •
- $595,336 refinancing on July 1, 2003 of bridge financing notes previously held by third parties, extending the maturity date and reducing the interest rate.
As of December 31, 2007, there was $595,336 outstanding under the terms of these credit facilities.
55
On June 17, 2004, the Company entered into another research agreement (Research Agreement II), valued at $2,200,000, as amended, with Alticor to conduct research into the development of a test to identify individuals with specific genetic variations that affect how people gain and maintain weight. During the first phase of the agreement, the Company received $1,380,000 in research funding over a period of six months beginning on July 1, 2004. If Alticor determines, in its sole discretion, that it has reasonable likelihood of commercializing weight management nutritional products, the Company will be eligible to receive, during the second phase of the agreement, an additional $820,000 in funding over a six-month period. No funding related to this agreement was received during the years ended December 31, 2007 and 2006 and the Company is not anticipating any additional funding under this agreement.
On March 5, 2005, the Company entered into an agreement with Alticor to expand the research being performed under Research Agreement I (Research Agreement III) to provide additional funding of $2,716,151 over the two years beginning April 1, 2005. Also on March 5, 2005, the Company entered into an additional research agreement (Research Agreement IV) with Alticor for exploratory research valued at $2,341,500 over a two-year period commencing April 1, 2005. These research agreements provided the Company with a total of $5,000,000 during the two years ending March 2007. The Company received $2,540,161 and $2,517,474 in funding related to these agreements during the year ended December 31, 2006 and 2005, respectively, and is not anticipating any additional funding under these agreements. As of December 31, 2006, $1,123,183 of this amount was received in advance of performing the related activity and is included in deferred receipts on the accompanying consolidated balance sheet.
Also on April 18, 2005, Alticor paid the Company $2,000,000 as a non-refundable advance payment for genetic risk assessment tests to be processed under the terms of the Distribution Agreement, which expired on March 22, 2006. On February 23, 2006, the Company entered into two new purchase agreements with Alticor. The two new purchase agreements cover two genetic health assessment tests that Interleukin Genetics developed on behalf of Alticor. These are: 1) the heart health genetic test, which analyzes DNA variations in the Interleukin-1A and Interleukin-1B genes to identify whether an individual may have a predisposition for chronically elevated measures of inflammation and an increased risk for heart disease; and 2) the general nutrition genetic test, which analyzes DNA variations in two genes that affect Vitamin B metabolism and four genes that are involved in responding to oxidative stress. The purchase agreement for the heart health genetic test provides for sales of these tests to Alticor through March 2008. Both parties agreed that $600,000 of the $2,000,000 prepayment received pursuant to the Distribution Agreement would be applied to purchases made under the purchase agreement for the heart health genetic tests from March 23, 2006 through December 31, 2006 to the extent tests are processed. Of the remaining $1,400,000 prepayment, $125,790 was recognized as revenue for tests processed during the remaining term of the Distribution Agreement and the balance of $1,274,210 has been reclassified from deferred receipts to equity. The general nutrition genetic test purchase agreement term is through January 2008.
On June 30, 2006, the Company entered into an agreement with Alticor to perform association studies on composite genotypes to skin inflammatory response. The agreement provided $94,000 of funding, all of which was received in 2006. As of December 31, 2007, $94,000 was included in deferred receipts on the accompanying consolidated balance sheets.
On August 17, 2006, Alticor purchased from the Company an aggregate of 2,750,037 shares of Common Stock for an aggregate purchase price of $15,615,537, or $5.6783 per share (based on the volume-weighted average closing stock price for the 20 consecutive trading days ending August 15, 2006). In addition, Alticor also agreed to extend to the Company a credit line of $14,384,463 of working capital borrowings at any time until August 17, 2008 (See Note 8). The Company incurred $83,707 of issuance costs associated with this private placement. As a condition of the financing, the Company initiated a rights offering of 2,533,234 shares of its Common Stock to existing stockholders
56
(other than Alticor) at a per share price of $5.6783. The costs, incurred as a result of the rights offering were $66,356 and these costs have been netted against the proceeds received from the financing.
On March 29, 2007, the Company entered into an agreement, effective January 1, 2007, to expand the research being performed under its current agreements with Alticor through 2007. The Company received $2,000,000 during 2007 under the research agreement, on a time and material basis.
On December 17, 2007, pursuant to the terms of certain promissory notes, Pyxis Innovations, Inc., an affiliate of Alticor, converted indebtedness due on December 31, 2007, representing an aggregate principal amount of $2,000,000 and accrued interest of $39,679, into 3,190,987 shares of the Company's common stock.
Note 5—Fixed Assets
The fixed assets' useful lives and balances at December 31, 2007 and 2006 consisted of the following:
| | Useful Life
| | 2007
| | 2006
| |
|---|
| Computer software, computer equipment and office equipment | | 3 years | | $ | 212,539 | | $ | 181,487 | |
| R&D lab equipment | | 5 years | | | 270,649 | | | 375,351 | |
| Genetic testing lab and equipment | | 5 years | | | 964,308 | | | 892,001 | |
| Furniture and fixtures | | 5 years | | | 101,716 | | | 101,716 | |
| Leasehold improvements | | 5 years | | | 265,563 | | | 265,563 | |
| Equipment under capital leases | | 3 to 5 years | | | 63,390 | | | 63,390 | |
| | | | |
| |
| |
| | | | | | 1,878,165 | | | 1,879,508 | |
| Less — Accumulated depreciation and amortization | | | | | (1,299,458 | ) | | (1,003,574 | ) |
| | | | |
| |
| |
| | Total | | | | $ | 578,707 | | $ | 875,934 | |
| | | | |
| |
| |
Depreciation and amortization expense of these fixed assets was $332,082, $337,336, and $303,316 for the years ended December 31, 2007, 2006 and 2005, respectively.
Note 6—Intangible Assets
The intangible assets' useful lives and balances at December 31, 2007 and 2006 consisted of the following:
| | Useful Life
| | 2007
| | 2006
| |
|---|
| Amortizing intangible assets: | | | | | | | | | |
| | Retailer relationships | | 5 years | | $ | 4,348,586 | | $ | 5,200,000 | |
| | Trademarks | | 5 years | | | 919,893 | | | 1,100,000 | |
| | OTCeutical formulations | | 5 years | | | 1,087,146 | | | 1,300,000 | |
| | Non-compete agreements | | 3 years | | | 150,000 | | | 200,000 | |
| | Other | | 10 years | | | 834,425 | | | 638,216 | |
| Non-amortizing intangible assets: | | | | | | | | | |
| | Trademarks | | Indefinite | | | 764,000 | | | 1,000,000 | |
| | | | |
| |
| |
| | | | | | 8,104,050 | | | 9,438,216 | |
| Less — Accumulated amortization | | | | | (2,362,648 | ) | | (711,396 | ) |
| | | | |
| |
| |
| | Net | | | | $ | 5,741,402 | | $ | 8,726,820 | |
| | | | |
| |
| |
57
On March 25, 2008, The Company entered into an agreement with David A. Finkelstein and Timothy J. Richerson regarding the acquisition of the assets and business of the Alan James Group. Under the agreement, the Claimants agreed to release the Company from any further obligations under the Asset Purchase Agreement, relating to the acquisition of the assets and business of the Alan James Group on August 17, 2006. The Claimants agreed that no further amounts are or will become due under the Purchase Agreement (including its earn-out provisions), their Employment Agreements or other related documents (see Note 17).
If the amount initially recognized as if it was a liability exceeds the fair value of the consideration issued or issuable, that excess shall be allocated as a pro rata reduction of the amounts assigned to assets acquired in accordance with SFAS No. 141. The intangible balances as of December 31, 2007 reflect the resolution of the contingency resulting from the acquisition of the assets and business of the Alan James Group.
On March 25, 2008, pursuant to the terms of a settlement agreement between the Company and former officers of the Alan James Group including the acquisition of the assets and business of the Alan James Group, the Company agreed to pay a total of $1,200,000. The $1,200,000 due to sellers is recorded as a current liability at December 31, 2007. The Company applied $600,000 of the settlement cost against the previously accrued separation expense that was recorded on June 30, 2007 and the remaining $600,000 was applied against the $2,130,374 aggregate total of contingent liabilities and amounts due under escrow recorded as part of the original acquisition. The remaining contingent liabilities and amounts due under escrow balance of $1,530,374 was eliminated as no longer due and applied as a reduction in the balances on a prorata basis of the intangibles assets recorded as part of the original acquisition, including the effect of term reduction on the non-compete agreements.
Amortization expense of these intangible assets was $1,651,242, $646,065 and $36,921 for the years ended December 31, 2007, 2006 and 2005, respectively. Expected amortization expense over the next four years is as follows:
Year Ending December 31,
| |
|
|---|
| 2008 | | $ | 986,568 |
| 2009 | | | 967,818 |
| 2010 | | | 936,568 |
| 2011 | | | 616,644 |
| | |
|
| | | $ | 3,507,598 |
| | |
|
Note 7—Accrued Expenses
Accrued expenses consist of the following:
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Payroll and vacation | | $ | 345,040 | | $ | 403,195 |
| Research | | | 21,876 | | | 21,873 |
| Accrued returns | | | 908,309 | | | 1,420,265 |
| Accrued trade promotions | | | 422,465 | | | 131,327 |
| Other | | | 250,674 | | | 143,069 |
| | |
| |
|
| | Total | | $ | 1,948,364 | | $ | 2,119,729 |
| | |
| |
|
In November 2006, the Company involuntarily terminated the employment of five individuals. In connection with these terminations, the Company offered continued separation pay based either on (i) a contractually negotiated period of time or (ii) for a period of time based on years of service the
58
individual had accumulated as of the date of separation. Accordingly, the Company recorded a liability for the estimated separation pay in the amount of $234,060 at the time of termination for the year ended December 31, 2006. As of December 31, 2006, the Company had paid $234,060 of the separation pay.
As of December 31, 2007 and 2006, accrued returns include $104,720 and $781,903, respectively, of estimated future returns of OTCeutical products that were shipped prior to the Company's acquisition of the Alan James Group business.
Note 8—Debt
On March 5, 2003 as part of its strategic alliance with Alticor Inc., the Company was granted credit facilities as follows:
- •
- $1,500,000 working capital credit line to initiate selected research agreements with third party entities approved by the board of directors of the Company;
- •
- $2,000,000 refinancing of notes previously held by Alticor, extending the maturity date and reducing the interest rate; and
- •
- $595,336 refinancing on July 1, 2003 of bridge financing notes previously held by third parties, extending the maturity date and reducing the interest rate.
On February 23, 2006, these credit facilities with Alticor were amended to provide the Company with access to an additional $2,000,000 of working capital borrowing at any time prior to April 1, 2007. Any amounts borrowed will bear interest at prime plus 1%, require quarterly interest payments and be due five years from the date of borrowing issuance. In addition, the restrictions on the existing $1,500,000 line of credit were removed so that it can be used for general working capital purposes. These credit facilities expired unused on April 1, 2007.
On August 17, 2006, these credit facilities with Alticor were further amended to provide the Company with access to an additional $14,400,000 of working capital borrowings at any time prior to August 17, 2008. Any amounts borrowed will bear interest at prime plus 1%, require quarterly interest payments and be due on August 16, 2011. The principal amount of any borrowing under this credit facility is convertible at Alticor's election into a maximum of 2,533,234 shares of Common Stock, reflecting a conversion price of $5.6783 per share. As a condition of this financing, the Company initiated a rights offering of 2,533,234 shares of its Common Stock to existing stockholders (other than Alticor) at a per share price of $5.6783. Any proceeds received from the rights offering will reduce the availability under the credit facility (see Note 4). As a result of the rights offering, the availability under the credit facility has been reduced by $68,208, leaving approximately $14.3 million available. No amounts are outstanding under these credit facilities as of December 31, 2007.
On December 17, 2007, pursuant to the terms of the notes, an affiliate of Alticor converted the indebtedness due on December 31, 2007, representing an aggregate principal amount of $2,000,000 and accrued interest of $39,679, into 3,190,987 shares of the Company's common stock. The credit facilities will mature in June 2008, bear interest at 1% over the prime rate (7.5% at December 31, 2007), are collateralized by a security interest in the Company's intellectual property (except intellectual property related to periodontal disease and sepsis), and are convertible at the election of Alticor into 4,060,288 shares of common stock, as adjusted, at a stated conversion price equal to $0.6392 per share. At December 31, 2007 and 2006, there was $595,336 and $2,595,336, outstanding under the terms of these credit facilities, gross of unamortized discount of $0 and $461,874, respectively.
59
Note 9—Commitments and Contingencies
The Company leases its offices and laboratory space under non-cancelable operating leases expiring at various dates through June 2009. The Company also leases certain office equipment under lease obligations, all of which are classified as operating leases. Future minimum lease commitments under lease agreements with initial or remaining terms of one year or more at December 31, 2007, are as follows:
Year Ending December 31,
| |
|
|---|
| 2008 | | $ | 572,495 |
| 2009 | | | 180,497 |
| 2010 | | | 7,590 |
| 2011 | | | 5,445 |
| | |
|
| | | $ | 766,027 |
| | |
|
Rent expense was $599,285, $497,457 and $434,677 for the years ended December 31, 2007, 2006 and 2005, respectively.
In connection with the research agreement with Alticor dated March 5, 2003, the Company is obligated to purchase two clinical databases. As of June 30, 2004, the Company determined that this obligation met the criteria for accrual of SFAS No. 5,Accounting for Contingencies, and estimated the cost of these two databases at $450,000. Accordingly, the Company recorded a liability and charged research and development expenses of $450,000 at that time. As of December 31, 2007 and 2006, the Company had cumulative expenditures of $357,944 and $284,444, respectively, associated with the acquisition of these databases. The Company believes that the acquisition of the databases will not exceed the amount that the Company has estimated, however actual amounts could differ.
In connection with the research agreement with Alticor dated March 5, 2005, the Company entered into a sponsored research agreement with Yonsei University to conduct a clinical study. The sponsored research agreement is for an amount of $499,882 and is payable upon achievement of certain milestones. As of December 31, 2007, Yonsei University had achieved milestones valued at $183,000. The remaining commitment on this agreement is $316,882. When, and if, Yonsei University completes the other milestones associated with this sponsored research agreement, the Company will record these costs as research and development expenses.
In connection with both the research agreement with Alticor dated March 5, 2005 and March 29, 2007, the Company entered into a sponsored research agreement with SOGO Clinical Pharmacology Co., LTD (SOGO) to conduct a clinical study. The sponsored research agreement is for an amount of ¥26,346,600, or approximately $224,000 (based on the exchange rate on March 30, 2007 of 117.56 ¥ to 1 US$) and is payable upon achievement of certain milestones. As of December 31, 2007, SOGO had achieved milestones valued at ¥26,346,600 or $232,131 based on actual payment in US dollars.
The Company has no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on its financial condition, results of operations and cash flows.
60
The Company has entered into employment agreements with certain key employees of the Company. These agreements expire at various dates through January 22, 2010. As of December 31, 2007, the remaining commitments under these agreements, based on continued employment, was as follows:
Year Ending December 31,
| | Base
Salary
| | Car
Allowance
| | Stock
Award
(# of shares)
|
|---|
| 2008 | | $ | 836,667 | | $ | 14,400 | | 112,500 |
| 2009 | | | 471,250 | | | 3,600 | | 112,500 |
| 2010 | | | 28,333 | | | | | |
| | |
| |
| |
|
| | | $ | 1,336,250 | | $ | 18,000 | | 225,000 |
| | |
| |
| |
|
Note 10—Capital Stock
At December 31, 2007, the Company had authorized 6,000,000 shares of $0.001 par value Series A Preferred Stock, of which 5,000,000 were issued and outstanding. At December 31, 2007, the Company had authorized 100,000,000 shares of $0.001 par value common stock of which 67,263,672 shares were outstanding or reserved for issuance. Of those, 30,832,102 shares were outstanding; 28,160,200 shares were reserved for the conversion of Series A Preferred to common stock; 931,377 shares were reserved for the conversion of approximately $600,000 of debt; 3,984,840 shares were reserved for the exercise of authorized and outstanding stock options; 400,000 shares were reserved for the exercise of outstanding warrants to purchase common stock; 437,763 shares were reserved for the exercise of rights held under the Employee Stock Purchase Plan; 2,521,222 shares were reserved for the issuance upon the conversion of convertible notes.
On March 5, 2003, the Company entered into a Stock Purchase Agreement with Alticor, pursuant to which Alticor purchased from the Company 5,000,000 shares of Series A Preferred Stock for $7,000,000 in cash on that date, and an additional $2,000,000 in cash that was paid, as a result of the Company achieving a certain milestone, on March 11, 2004.
The Series A Preferred Stock accrues dividends at the rate of 8% of the original purchase price per year, payable only when, as and if declared by the Board of Directors and are non-cumulative. To date, no dividends have been declared on these shares. If the Company declares a distribution, with certain exceptions, payable in securities of other persons, evidences of indebtedness issued by the Company or other persons, assets (excluding cash dividends) or options or rights to purchase any such securities or evidences of indebtedness, then, in each such case the holders of the Series A Preferred Stock shall be entitled to a proportionate share of any such distribution as though the holders of the Series A Preferred Stock were the holders of the number of shares of our Common Stock into which their respective shares of Series A Preferred Stock are convertible as of the record date fixed for the determination of the holders of our Common Stock entitled to receive such distribution.
In the event of any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, the holders of the Series A Preferred Stock shall be entitled to receive, prior and in preference to any distribution of any of the Company's assets or surplus funds to the holders of its Common Stock by reason of their ownership thereof, the amount of two times the then-effective purchase price per share, as adjusted for any stock dividends, combinations or splits with respect to such shares, plus all declared but unpaid dividends on such share for each share of Series A Preferred
61
Stock then held by them. The liquidation preference at December 31, 2007 was $18,000,000. After receiving this amount, the holders of the Series A Preferred Stock shall participate on an as-converted basis with the holders of common stock in any of the remaining assets.
Each share of Series A Preferred Stock is convertible at any time at the option of the holder into a number of shares of the Company's common stock determined by dividing the then-effective purchase price ($1.80, and subject to further adjustment) by the conversion price in effect on the date the certificate is surrendered for conversion. As of December 31, 2007, the Series A Preferred Stock was convertible into 28,160,200 shares of Common Stock reflecting a current conversion price of $0.3196 per share.
Each holder of Series A Preferred Stock is entitled to vote its shares of Series A Preferred Stock on an as-converted basis with the holders of Common Stock as a single class on all matters submitted to a vote of the stockholders, except as otherwise required by applicable law. This means that each share of Series A Preferred Stock will be entitled to a number of votes equal to the number of shares of Common Stock into which it is convertible on the applicable record date.
Note 11—Stock-Based Compensation Arrangements
Stock-based compensation arrangements consisted of the following as of December 31, 2007: three share-based compensation plans, restricted stock awards; an employee stock purchase plan; and employee compensation agreements. Total compensation cost that has been charged against income for stock-based compensation arrangements is as follows:
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Stock option grants beginning of period | | $ | 158,676 | | $ | 623,967 |
| Stock-based arrangements during the period: | | | | | | |
| | Stock option grants | | | 1,460 | | | — |
| | Restricted stock issued | | | 10,073 | | | 206,200 |
| | Unrestricted stock issued: | | | | | | |
| | | Employee stock purchase plan | | | 2,955 | | | 9,875 |
| | | Employment Agreements | | | — | | | 229,646 |
| | |
| |
|
| | | $ | 173,164 | | $ | 1,069,688 |
| | |
| |
|
Stock option grants prior to January 1, 2006
The weighted-average grant-date fair value of employee stock options granted prior to January 1, 2006 was $2.65 per share, as determined using the Black-Scholes option-pricing model. No employee stock options were granted during the year ended December 31, 2006. For purposes of determining the stock-based compensation expense for grant awards issued prior to January 1, 2006 and for pro forma disclosure required by SFAS 123, the Black-Scholes option pricing model was used with the following weighted-average assumptions:
| | 2005
|
|---|
| Risk-free interest rate | | 5.00% |
| Expected life | | 7 years |
| Expected volatility | | 70% |
Using these assumptions, the weighted average grant date fair value of options granted in 2005 was $2.29.
62
In accordance with the modified prospective transition method, the Company's Consolidated Financial Statements for prior periods have not been restated to reflect, and do not include, the impact of SFAS No. 123R. Had compensation cost for the Company's employee stock awards been determined consistent with SFAS No. 123, the Company's net loss applicable to common stock and net loss per share would have been as follows:
| | Year Ended
December 31, 2005
| |
|---|
| Net loss applicable to common stockholders: | | | | |
| | As reported | | $ | (6,570,824 | ) |
| | Stock-based employee compensation | | | (724,779 | ) |
| | |
| |
| | Pro forma | | $ | (7,295,603 | ) |
| | |
| |
| Basic and diluted net loss per common share: | | | | |
| | As reported | | $ | (0.28 | ) |
| | Pro forma | | $ | (0.31 | ) |
Stock option grants after January 1, 2006
During 2007, there were 26,000 stock options granted to employees. No employee stock options were granted during the year ended December 31, 2006. For purposes of determining the stock-based compensation expense for grant awards issued after January 1, 2006, the Black-Scholes option-pricing model was used with the following weighted-average assumptions:
| | 2007
|
|---|
| Risk-free interest rate | | 4.6% |
| Expected life | | 7 years |
| Expected volatility | | 83% |
Using these assumptions, the weighted average grant date fair value of options granted in 2007 was $0.90.
During the years ended December 31, 2007 and 2005, the Company did not grant any restricted stock awards. During the year ended December 31, 2006, the Company granted 33,385 restricted stock awards to employees, of which 28,497 lapsed and 4,888 had been canceled as of December 31, 2006. Holders of these awards participate fully in the rewards of stock ownership of the Company, including voting and dividend rights. The employees are not required to pay any consideration to the Company for these restricted stock awards. The recognition of compensation expense for these types of awards did not change as a result of adopting SFAS No. 123R on January 1, 2006. The Company measured the fair value of the shares based on the last reported price at which the Company's common stock traded on the date of the grant and compensation cost is recognized over the remaining service period.
63
As December 31, 2007 and 2005 there were no restricted shares outstanding, granted, lapsed or canceled. The following table details restricted stock activity for the year ended December 31, 2006:
| | 2006
|
|---|
| | Number of
Shares
| | Weighted Avg
Grant Date
Fair Value
|
|---|
| Outstanding, beginning of year | | — | | | — |
| | Granted | | 33,385 | | $ | 6.85 |
| | Lapsed | | (28,497 | ) | | 6.83 |
| | Canceled | | (4,888 | ) | | 6.94 |
| | |
| |
|
| Outstanding, end of year | | — | | $ | — |
| | |
| |
|
Purchases made under the Company's Employee Stock Purchase Plan are now deemed to be compensatory under SFAS No. 123R because employees may purchase stock at a price equal to 85% of the fair market value of the Company's common stock on either the first day or the last day of a calendar quarter, whichever is lower. During the year ended December 31, 2007 and 2006, employees purchased 7,702 and 9,074 shares, respectively, of common stock at a weighted-average purchase price of $2.17 and $4.89, while the weighted-average fair market value was $2.55 and $5.98 per share, resulting in compensation expense of $2,955 and $9,875.
During the year ended December 31, 2006, the Company entered into employment agreements with certain key employees of the Company. These agreements provide for the issuance of up to 72,500 shares of the Company's common stock at various dates through 2009 assuming continued employment with the Company. The employees are not required to pay any consideration to the Company for these stock awards. As of December 31, 2007 and 2006, 28,000 shares, respectively, of the Company's common stock have been issued pursuant to these agreements. The recognition of compensation expense for these types of awards did not change as a result of adopting SFAS No. 123R on January 1, 2006. The Company measures the fair value of the shares, prior to issuance, based on the last reported price at which the Company's common stock traded for the reporting period and compensation cost is recognized ratably over the employment period required to earn the stock award. At time of issuance, the Company will measure the fair value of the shares based on the last reported price at which the Company's common stock traded on the date of the issuance and will record a cumulative adjustment, if any.
On July 8, 2007, two of these key employees resigned effective as of July 2007. Accordingly, 50,000 shares of the Company's common stock for these stock awards have been canceled.
A summary of stock compensation cost included in the statement of operations for the year ended December 31, 2007 is as follows:
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Cost of revenue | | $ | 25,961 | | $ | 35,604 |
| Research and development expenses | | | 114,546 | | | 325,377 |
| Selling, general and administrative expenses | | | 32,657 | | | 708,707 |
| | |
| |
|
| | Total | | $ | 173,164 | | $ | 1,069,688 |
| | |
| |
|
64
In June 1996, the Company's shareholders approved the adoption of the 1996 Equity Incentive Plan (the 1996 Plan). The 1996 Plan provides for the award of nonqualified and incentive stock options, restricted stock and stock bonuses to employees, directors, officers and consultants of the Company. A total of 1,300,000 shares of the Company's common stock had been reserved for award under the 1996 Plan of which 369,839 remained unissued at December 31, 2007. This plan has been terminated with respect to new grants.
In June 2000, the Company's shareholders approved the adoption of the Interleukin Genetics, Inc. 2000 Employee Stock Compensation Plan (the 2000 Plan). The 2000 Plan provides for the award of nonqualified and incentive stock options, restricted stock, and stock awards to employees, directors, officers, and consultants of the Company. A total of 2,000,000 shares of the Company's common stock have been reserved for award under the 2000 Plan of which 450,782 were available for future issuance at December 31, 2007.
In June 2004, the Company's shareholders approved the adoption of the Interleukin Genetics, Inc. 2004 Employee Stock Compensation Plan (the 2004 Plan). The 2004 Plan provides for the award of nonqualified and incentive stock options, restricted stock, and stock awards to employees, directors, officers, and consultants of the Company. A total of 2,000,000 shares of the Company's common stock have been reserved for award under the 2004 Plan of which 1,797,813 were available for future issuance at December 31, 2007.
Nonqualified and incentive stock options with a life of 10 years are granted at exercise prices equal to the fair market value of the common stock on the date of grant. Options generally vest over a period of three to five years.
A summary of the status of the Company's stock options, issued under the 1996, 2000 and 2004 Plans and outside of these plans, at December 31, 2007, 2006 and 2005, and changes during these years is presented in the tables below:
The following table details all stock option activity for the years ended December 31, 2007, 2006 and 2005:
| | 2007
| | 2006
| | 2005
|
|---|
| | Shares
| | Weighted Avg
Exercise
Price
| | Shares
| | Weighted Avg
Exercise
Price
| | Shares
| | Weighted Avg
Exercise
Price
|
|---|
| Outstanding, beginning of year | | 1,893,015 | | $ | 2.99 | | 2,477,815 | | $ | 2.69 | | 2,985,474 | | $ | 2.81 |
| | Granted | | 26,000 | | | 1.21 | | — | | | — | | 291,500 | | | 3.25 |
| | Exercised | | (194,917 | ) | | 1.78 | | (539,050 | ) | | 1.54 | | (320,342 | ) | | 1.80 |
| | Canceled | | (282,567 | ) | | 3.19 | | (43,750 | ) | | 4.19 | | (418,817 | ) | | 4.30 |
| | Expired | | (75,125 | ) | | 2.58 | | (2,000 | ) | | 2.85 | | (60,000 | ) | | 4.47 |
| | |
| |
| |
| |
| |
| |
|
| Outstanding, end of year | | 1,366,406 | | $ | 3.11 | | 1,893,015 | | $ | 2.99 | | 2,477,815 | | $ | 2.69 |
| | |
| |
| |
| |
| |
| |
|
| Exercisable, end of year | | 1,313,906 | | $ | 3.13 | | 1,646,015 | | $ | 2.94 | | 1,895,815 | | $ | 2.36 |
| | |
| |
| |
| |
| |
| |
|
65
The following table details further information regarding stock options outstanding and exercisable at December 31, 2007:
| | Stock Options Outstanding
| | Stock Options Exercisable
|
|---|
Range of Exercise Price:
| | Shares
| | Weighted Avg
remaining
contractual life
(years)
| | Weighted Avg
Exercise
Price
| | Shares
| | Weighted Avg
Exercise
Price
|
|---|
| $0.50–$0.91 | | 45,750 | | 3.34 | | $ | 0.85 | | 45,750 | | $ | 0.85 |
| $1.10–$1.39 | | 176,000 | | 4.22 | | | 1.22 | | 150,000 | | | 1.22 |
| $1.50–$1.79 | | 37,156 | | 4.56 | | | 1.64 | | 37,156 | | | 1.64 |
| $2.13–$2.40 | | 14,500 | | 3.77 | | | 2.23 | | 14,500 | | | 2.23 |
| $2.50–$2.88 | | 598,000 | | 1.77 | | | 2.83 | | 598,000 | | | 2.83 |
| $3.50–$3.71 | | 89,800 | | 6.81 | | | 3.65 | | 79,800 | | | 3.64 |
| $4.10–$4.20 | | 43,200 | | 6.74 | | | 4.13 | | 26,700 | | | 4.14 |
| $4.70–$4.75 | | 362,000 | | 5.95 | | | 4.70 | | 362,000 | | | 4.70 |
| | |
| |
| |
| |
| |
|
| $0.50–$4.75 | | 1,366,406 | | 3.83 | | $ | 3.11 | | 1,313,906 | | $ | 3.13 |
| | |
| |
| |
| |
| |
|
| Aggregate intrinsic value | | 11,081 | | | | | | | 11,081 | | | |
| | |
| | | | | | |
| | | |
The aggregate intrinsic value in the preceding table represents the total pre-tax intrinsic value, based on the last reported price at which the Company's common stock traded on December 31, 2007, the last trading day of fiscal 2007, of $1.09, which would have been received by the option holders had they exercised their options as of that date.
The following table summarizes the status of the Company's non-vested options for the years ended December 31, 2007, 2006 and 2005:
| | 2007
| | 2006
| | 2005
|
|---|
| | Shares
| | Weighted Avg
Exercise
Price
| | Shares
| | Weighted Avg
Exercise
Price
| | Shares
| | Weighted Avg
Exercise
Price
|
|---|
| Non-vested options, beginning of year | | 247,000 | | $ | 3.29 | | 582,000 | | $ | 3.78 | | 642,009 | | $ | 4.16 |
| | Granted | | 26,000 | | | 1,21 | | — | | | — | | 291,500 | | | 3.25 |
| | Vested | | (30,750 | ) | | 3.63 | | (291,250 | ) | | 4.14 | | (196,692 | ) | | 4.01 |
| | Forfeited | | (189,750 | ) | | 3.14 | | (43,750 | ) | | 4.19 | | (154,817 | ) | | 4.14 |
| | |
| |
| |
| |
| |
| |
|
| Non-vested options, end of year | | 52,500 | | $ | 2.60 | | 247,000 | | $ | 3.29 | | 582,000 | | $ | 3.78 |
| | |
| |
| |
| |
| |
| |
|
As of December 31, 2007 and 2006, there was approximately $70,000 and $500,000, respectively, of total unrecognized cost related to non-vested share-based compensation arrangements granted under the Company's stock plans. That cost is expected to be recognized over a weighted average period of approximately 2 years. Options to purchase 194,917 and 539,050 shares were exercised during the year ended December 31, 2007 and 2006; these options had an intrinsic value of approximately $464 thousand and $2.4 million on their date of exercise, respectively. The fair value of stock options that vested during the year ended December 31, 2007 and 2006 was approximately $83 thousand and $0.9 million, respectively.
Note 12—Employee Benefit Plan
In 1998, the Company adopted a profit sharing plan covering substantially all of its employees. Under the profit sharing plan, the Company may, at the discretion of the Board of Directors,
66
contribute a portion of the Company's current or accumulated earnings. In September 1998, the Company amended and restated the profit sharing plan to include provisions for Section 401(k) of the Internal Revenue Code, which allowed for pre-tax employee contributions to the plan. Under the amended and restated plan, the Company may, at the discretion of the Board of Directors, match a portion of the participant contributions. The Company currently contributes 15% of any amount employees contribute, up to a maximum of $1,000 per participant per calendar year. Company contributions, if any, are credited to the participants' accounts and vest over a period of four years based on the participants' initial service date with the Company. During the years ended December 31, 2007, 2006 and 2005, $10,199, $14,273 and $10,884 was contributed to the plan, respectively.
Note 13—Income Taxes
For the years ended December 31, 2007, 2006 and 2005, the provision for income taxes was $15,500, $7,000 and $0, respectively. The Company's federal statutory income tax rate for 2007 and 2006 was 34%. The Company used a blended federal and state income tax rate of 40% for 2007 and 2006. Interleukin Genetics, Inc. has incurred losses from operations but has not recorded an income tax benefit for 2007 or 2006, as the Company has recorded a valuation allowance against its net operating losses and other net deferred tax assets due to uncertainties related to the realizability of these tax assets. AJG Brands, Inc. had taxable income for 2007 and a loss for 2006. AJG Brands, Inc. recorded a state income tax benefit for 2007 due to utilization of the net operating loss generated in 2006, against which a valuation allowance was recorded, and from expectations concerning realizability of deferred tax assets. The state tax benefit recorded by AJG Brands, Inc. is a result of filing in separate entity filing jurisdictions.
Deferred tax assets and liabilities are determined based on the difference between financial statement and tax bases using enacted federal and state tax rates in effect for the year in which the differences are expected to reverse. As of December 31, 2007 and 2006, the approximate income tax effect of the Company's deferred tax assets (liabilities) consisted of the following:
| | 2007
| | 2006
| |
|---|
| Deferred tax asset: | | | | | | | |
| | Tax effect of: | | | | | | | |
| | | Net operating loss carryforwards | | $ | 18,527,000 | | $ | 17,498,000 | |
| | | Accrued expenses | | | 491,000 | | | 352,000 | |
| | | Amortization of definite lived intangible assets | | | 731,000 | | | 179,000 | |
| | | Non-qualified stock option compensation | | | 186,000 | | | 204,000 | |
| | | Depreciation | | | 63,000 | | | 47,000 | |
| | | Other | | | 96,000 | | | 21,000 | |
| | | Patents | | | (256,000 | ) | | (207,000 | ) |
| | Research tax credit carryforwards | | | 1,445,000 | | | 1,270,000 | |
| | |
| |
| |
| | | Total deferred tax assets (liabilities) | | | 21,283,000 | | | 19,364,000 | |
| | Valuation allowance | | | (21,242,000 | ) | | (19,364,000 | ) |
| | |
| |
| |
| | Net deferred tax assets | | | 41,000 | | | -0- | |
Deferred tax liability: |
|
|
|
|
|
|
|
| | | Amortization of indefinite lived intangible assets | | | (31,000 | ) | | (7,000 | ) |
| | |
| |
| |
| | Net deferred tax assets (liabilities) | | $ | 10,000 | | $ | (7,000 | ) |
| | |
| |
| |
A portion of the funds received from Alticor and its subsidiaries for research and other agreements are reflected as equity in the financial statements but are reported as revenue for tax purposes and included in the calculation of the net operating loss carryforward for the year ended
67
December 31, 2006 in the amount of $2,700,000. None of the funds received from Alticor and its subsidiaries were reflected as equity in 2007.
As of December 31, 2007, the Company had gross net operating loss (NOL) and research tax credit carryforwards of approximately $53,600,000 and $1,070,000, respectively, for federal income tax purposes, expiring in varying amounts through the year 2027. Of the $53,600,000 NOL loss carryforward, $2.5 million relates to stock-based compensation and has not been reflected in the deferred taxes and when the benefit of these losses, if any, is realized, it would result in a credit to additional paid in capital as a component of stockholder's equity.
As of December 31, 2007, the Company had gross NOL and research tax credits carryforwards of approximately $21,300,000 and $484,000 for state income tax purposes, expiring in varying amounts through the year 2012. Of the $21.3 million net operating loss carryforward, $2,500,000 relates to stock-based compensation and has not been reflected in the deferred inventory and when the benefit of these losses, if any, is realized, it would result in a credit to additional paid in capital as a component of stockholder's equity.
The Company's ability to use its NOL and tax credit carryforwards to reduce future taxes is subject to the restrictions provided by Section 382 of the Internal Revenue Code of 1986. These restrictions provide for limitations on the Company's utilization of its NOL and tax credit carryforwards following a greater than 50% ownership change during the prescribed testing period. On March 5, 2003, the Company had such a change. As a result, all of the Company's NOL carryforwards as of that date are limited in utilization. The annual limitation may result in the expiration of certain of the carryforwards prior to utilization.
The provision for income taxes differs from the federal statutory rate due to the following:
| | Years Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Tax at statutory rate | | (34.0 | )% | (34.0 | )% | (34.0 | )% |
| State taxes, net of federal benefit | | (6.1 | ) | (6.1 | ) | (6.1 | ) |
| Research payments | | — | | 8.4 | | 15.7 | |
| SFAS No. 123 expense | | 0.8 | | 4.0 | | — | |
| Forfeited prepayment of genetic test services | | — | | 7.4 | | — | |
| Other | | 2.7 | | 1.3 | | 2.3 | |
| Change in valuation allowance | | 36.8 | | 19.1 | | 22.1 | |
| | |
| |
| |
| |
| Effective tax rate | | 0.2 | % | 0.1 | % | 0.0 | % |
| | |
| |
| |
| |
Note 14—Segment Information
The Company follows SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information (SFAS No. 131), which establishes standards for reporting information about operating segments in annual and interim financial statements, and requires that companies report financial and descriptive information about its reportable segments based on a management approach. SFAS No. 131 also establishes standards for related disclosures about products and services, geographic areas and major customers. As a result of the acquisition of the assets and business of the Alan James Group in August 2006, the Company has two reportable segments: Personalized Health and Consumer Products.
Interleukin Genetics, Inc. develops genetic tests and performs testing services that can help individuals improve and maintain their health through preventive measures. AJG Brands, Inc., doing business as the Alan James Group, develops, markets and sells nutritional products and related activities. The Company's principal operations and markets are located in the United States.
68
The accounting policies of each of the segments are the same as those described in the summary of significant accounting policies. The Company evaluates performance based on revenue and earnings before interest, taxes, depreciation and amortization (EBITDA). Common costs not directly attributable to a segment are included in the Personalized Health segment. These costs include corporate costs such as legal, audit, tax and other professional fees.
The following is a summary of the Company's operations by operating segment:
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Personalized Health: | | | | | | | | | | |
| | Revenue | | $ | 2,827,284 | | $ | 2,680,762 | | $ | 22,877 | |
| | |
| |
| |
| |
| | Net loss, before interest, taxes, depreciation and amortization of $640,017, $791,860 and $853,073 for the years ended December 31, 2007, 2006 and 2005, respectively | | $ | (4,754,531 | ) | $ | (5,090,996 | ) | $ | (5,717,751 | ) |
| | |
| |
| |
| |
| Consumer Products:* | | | | | | | | | | |
| | Revenue | | $ | 6,873,209 | | $ | 2,050,264 | | | — | |
| | |
| |
| |
| |
| | Net loss, before interest, taxes, depreciation and amortization of $1,616,706 and $611,512 for the year ended December 31, 2007 and 2006, respectively | | $ | 791,970 | | $ | (452,388 | ) | | — | |
Consolidated: |
|
|
|
|
|
|
|
|
|
|
| | Total revenue | | $ | 9,700,493 | | $ | 4,731,026 | | $ | 22,877 | |
| | |
| |
| |
| |
| | EBITDA | | $ | (3,962,561 | ) | $ | (5,543,384 | ) | $ | (5,717,751 | ) |
| | Interest, net | | | 200,085 | | | 48,902 | | | (50,961 | ) |
| | Provision for income taxes | | | (15,500 | ) | | (7,000 | ) | | — | |
| | Depreciation | | | (332,083 | ) | | (337,335 | ) | | (303,316 | ) |
| | Amortization | | | (2,108,726 | ) | | (1,107,939 | ) | | (498,796 | ) |
| | |
| |
| |
| |
| | Net loss | | $ | (6,218,785 | ) | $ | (6,946,756 | ) | $ | (6,570,824 | ) |
| | |
| |
| |
| |
- *
- since August 17, 2006 — date of acquisition
The Company has no operations outside of the United States. For the years ended December 31, 2007, 2006 and 2005, the Company had minimal royalty income derived from distributors outside the United States, minimal expenses derived from research partners outside the United States and minimal assets outside of the United States. The Company does not believe this risk is material and does not use derivative financial instruments to manage foreign currency fluctuation risk.
69
Note 15—Selected Quarterly Financial Data (Unaudited)
The following are selected quarterly financial data for the years ended December 31, 2007 and 2006:
| | Quarter Ended
| |
|---|
| | March 31,
2007
| | June 30,
2007
| | September 30,
2007
| | December 31,
2007
| |
|---|
| Revenue | | $ | 2,419,277 | | $ | 2,408,469 | | $ | 2,561,285 | | $ | 2,311,462 | |
| Gross profit | | $ | 1,172,917 | | $ | 1,259,389 | | $ | 1,353,563 | | $ | 1,215,178 | |
| Loss from operations | | $ | (1,607,978 | ) | $ | (1,900,480 | ) | $ | (1,009,119 | ) | $ | (1,428,847 | ) |
| Net loss | | $ | (1,667,909 | ) | $ | (1,964,689 | ) | $ | (1,081,659 | ) | $ | (1,500,532 | ) |
| Basic and diluted net loss per common share | | $ | (0.06 | ) | $ | (0.07 | ) | $ | (0.04 | ) | $ | (0.05 | ) |
| | Quarter Ended
| |
|---|
| | March 31,
2006
| | June 30,
2006
| | September 30,
2006
| | December 31,
2006
| |
|---|
| Revenue | | $ | 232,234 | | $ | 1,344,680 | | $ | 1,316,938 | | $ | 1,837,164 | |
| Gross profit | | $ | 34,582 | | $ | 936,398 | | $ | 327,994 | | $ | (589,455 | ) |
| Loss from operations | | $ | (1,459,624 | ) | $ | (784,817 | ) | $ | (1,735,322 | ) | $ | (2,547,021 | ) |
| Net loss | | $ | (1,589,624 | ) | $ | (923,182 | ) | $ | (1,833,649 | ) | $ | (2,600,447 | ) |
| Basic and diluted net loss per common share | | $ | (0.07 | ) | $ | (0.04 | ) | $ | (0.07 | ) | $ | (0.11 | ) |
Note 16—Industry Risk and Concentration
The Company develops genetic risk assessment tests under contract, performs research for its own benefit and provides research services to a collaborative partner. As of December 31, 2007, the Company has commercially introduced three genetic tests, two of which are sold exclusively through its strategic partner — Alticor, and is in various stages of development for several others. Commercial success of the Company's genetic risk assessment tests will depend on their acceptance as scientifically credible and cost-effective by consumers and the marketing success of its collaborative partner.
Research in the field of disease predisposing genes and genetic markers is intense and highly competitive. The Company has many competitors in the United States and abroad that have considerably greater financial, technical, marketing, and other resources available. If the Company does not discover disease predisposing genes or genetic markers and develop risk assessment tests and launch such services or products before its competitors, then the potential for significant revenues may be reduced or eliminated.
The market for health supplement products is competitive and other companies sell products similar to those sold by the Company. The Company's sales and margins may be influenced by competitor actions or other factors, such as the cost of product, contract terms and general market conditions.
For the year ended December 31, 2007, approximately 47% of the consumer products revenue was from a single customer. As of December 31, 2007, approximately 35.5% of the trade accounts receivable was from that same customer.
During 2007, the majority of the Company's consumer products were sourced from three suppliers. The Company pays a contracted rate per completed unit for each product. The suppliers are responsible for procuring raw materials and packaging finished products. If the Company is unable to maintain the relationship with these suppliers, it will need to find an alternative.
70
Note 17—Subsequent Event
On March 25, 2008, The Company entered into an agreement with David A. Finkelstein, Timothy J. Richerson, Alan James Group, LLC, AJG-NB, LLC, AJG-BI, LLC and AJG-GNC, LLC, collectively the "Claimants", pursuant to which the Claimants agreed to release the Company from any further obligations under the Asset Purchase Agreement, relating to the acquisition of the assets and business of the Alan James Group, the Company entered into with the Claimants on August 17, 2006 and related to the employment of Messrs. Richerson and Finkelstein by the Company, and the Company agreed to release the Claimants from certain obligations under the Asset Purchase Agreement and related to the employment of Messrs. Richerson and Finkelstein. Pursuant to the agreement, the Company agreed to pay the Claimants an aggregate of $1,200,000. As of June 30, 2007 the Company had accrued an aggregate expense of $600,000 in connection with the departures of Messrs. Richerson and Finkelstein, and the remaining $600,000 was applied against the $2,130,374 aggregate total of contingent liabilities and amounts due under escrow recorded as part of the original acquisition. The remaining contingent liabilities and amounts due under escrow balance of $1,530,374 was eliminated as no longer due and applied as a reduction in the balances on a prorata basis of the intangibles assets recorded as part of the original acquisition. The Company agreed to limit the duration of non-competition restrictions applicable to Richerson to July 31, 2009 and Finkelstein to July 2, 2009. The Claimants agreed that no further amounts are or will become due under the Purchase Agreement (including its earn-out provisions), their Employment Agreements or other related documents. Under applicable law the Claimants are entitled to a seven-day right to rescind this agreement. This period expires on April 2, 2008.
Effective as of January 22, 2008, the Company entered into a two-year employment agreement with Lewis H. Bender for the position of Chief Executive Officer that provides for automatic annual renewal terms. The agreement also provides that Mr. Bender will serve as a member of the Company's Board of Directors for as long as he serves as the Company's Chief Executive Officer and the Company expects to add Mr. Bender to its Board of Directors prior to the Board's next regularly scheduled meeting. The agreement further provides for a minimum annual base salary of $340,000, a sign-on bonus of up to $35,000 payable over the first six months of employment and annual, discretionary bonuses of up to 50% of his base salary based upon the Company's financial performance. In addition, the agreement provides for the reimbursement of Mr. Bender's relocation and living expenses for the first twelve months of employment. Upon hire, Mr. Bender was also granted an option to purchase 500,000 shares of the Company's common stock at an exercise price equal to the closing price as reported on the American Stock Exchange on the effective date of the agreement, which option shall vest in equal annual installments on the option grant date and February 1 of each of the years 2009, 2011, 2012 and 2013.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company's senior management is responsible for establishing and maintaining a system of disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the "Exchange Act") designed to ensure that the information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports
71
that it files or submits under the Exchange Act is accumulated and communicated to the issuer's management, including its principal executive officer or officers and principal financial officer or officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
The Company has evaluated the effectiveness of the design and operation of its disclosure controls and procedures under the supervision of and with the participation of management, including the Chief Executive Officer who is also deemed to be our Principal Financial and Accounting Officer as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer has concluded that our disclosure controls and procedures are effective.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles in the United States. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 2007.
Changes in Internal Control and Financial Reporting.
As of December 31, 2006, management and our independent accountants identified, as a material weakness in our internal control over financial reporting, the fact that we did not perform sufficient analysis on our historical sales return data by customer to appropriately document our basis for estimating future returns on a timely basis. In addition, we did not obtain information from our customers regarding the levels of inventory subject to rights of return on a timely basis. This limited our ability to reasonably and reliably estimate future returns on a timely basis. These issues were limited to our Alan James Group, which we acquired in August 2006.
As of December 31, 2007, the Company has remediated the identified material weaknesses by implementing, since December 31, 2006, improvements to its process of estimating product returns, including: (a) tracking actual returns data by customer on a monthly basis and (b) obtaining and maintaining information from our major customers regarding levels of inventory subject to returns on a monthly and quarterly basis.
The Alan James Group, which we acquired on August 17, 2006, has been included in our evaluation of internal controls over financial reporting for the period covered in this report. No other change in internal control over financial reporting occurred during the quarter ended December 31, 2007 that has materially affected, or is reasonably likely to materially affect, such internal control over financial reporting.
There are inherent limitations in any system of internal control. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that its objectives are met. Further, the design of a control system must consider that resources are not unlimited and the
72
benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgment in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls.
Item 9A(T). Controls and Procedures
This Annual Report on Form 10-K does not include an attestation report of the Company's registered public accounting firm regarding the Company's internal control over financial reporting. Management's report on internal control over financing reporting was not subject to attestation by the Company's registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide management's report on internal control over financial reporting without an accompanying attestation report by the Company's registered public accounting firm in this Annual Report on Form 10-K.
Item 9B. Other Information
On March 25, 2008 the Compensation Committee of our Board of Directors approved a one-time, completion-of-service, discretionary bonus of $30 thousand for Thomas R. Curran, Jr. in recognition of Mr. Curran's performance as our Interim Chief Executive Officer from July 2, 2007 to January 22, 2008.
73
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information responsive to this item is incorporated by reference from the relevant discussions in our Proxy Statement for the 2008 Annual Meeting of Stockholders under the captions "Management," "Compliance with Section 16(a) of the Securities Exchange Act of 1934," "Code of Conduct and Ethics," and "Corporate Governance Matters."
Item 11. Executive Compensation
Information responsive to this item is incorporated by reference from the relevant discussions in our Proxy Statement for the 2008 Annual Meeting of Stockholders under the captions "Executive Compensation," "Compensation Committee Interlocks and Insider Participation," and "Compensation Committee Report."
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information responsive to this item is incorporated by reference from the relevant discussions in our Proxy Statement for the 2008 Annual Meeting of Stockholders under the captions "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information."
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information responsive to this item is incorporated by reference from the relevant discussions in our Proxy Statement for the 2008 Annual Meeting of Stockholders under the captions "Certain Relationships and Related Transactions" and "Corporate Governance Matters — Director Independence."
Item 14. Principal Accountant Fees and Services
Information responsive to this item is incorporated by reference from the relevant discussions in our Proxy Statement for the 2008 Annual Meeting of Stockholders under the captions "Ratify Appointment of Independent Public Accountants."
74
PART IV
Item 15. Exhibits and Financial Statement Schedules
- (a)
- Documents Filed as Part of this Report
(1) Financial Statement Schedules:
Schedule II: Valuation and Qualifying Accounts for the Years Ended December 31, 2005, 2006 and 2007
All other information required by this item is not applicable or has been included in the consolidated financial statements and related notes thereto.
INTERLEUKIN GENETICS, INC. AND SUBSIDIARIES
SCHEDULE II —VALUATION AND QUALIFYING ACCOUNTS
YEARS ENDED DECEMBER 31, 2005, 2006 AND 2007
Description
| | Balance at
Beginning
of Year
| | Assumed
in the
Acquisition
| | Charges to
Cost and
Expense
| | Deductions
| | Balance at
End of Year
|
|---|
| Allowance for Doubtful Accounts: | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2005 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| | Year Ended December 31, 2006 | | $ | — | | $ | — | | $ | 28,000 | | $ | — | | $ | 28,000 |
| | Year Ended December 31, 2007 | | $ | 28,000 | | $ | — | | $ | — | | $ | (21,304 | ) | $ | 6,696 |
Allowance for Sales Returns: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Year Ended December 31, 2005 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| | Year Ended December 31, 2006 | | $ | — | | $ | 1,833,960 | | $ | 324,035 | | $ | (737,730 | ) | $ | 1,420,265 |
| | Year Ended December 31, 2007 | | $ | 1,420,265 | | $ | — | | $ | 1,365,616 | | $ | (1,877,574 | ) | $ | 908,307 |
Allowance for Trade Promotions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Year Ended December 31, 2005 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — |
| | Year Ended December 31, 2006 | | $ | — | | $ | 303,366 | | $ | 261,491 | | $ | (433,530 | ) | $ | 131,327 |
| | Year Ended December 31, 2007 | | $ | 131,327 | | $ | — | | $ | 845,404 | | $ | (554,266 | ) | $ | 422,465 |
(2) Exhibits:
The exhibits listed below are filed as part of or incorporated by reference into this Annual Report. Where certain exhibits are incorporated by reference from a previous filing, the exhibit numbers and previous filings are identified in parentheses.
Exhibit No.
| | Identification of Exhibit
|
|---|
| 3.1 | | Articles of Incorporation of the Company, as amended (incorporated herein by reference to Exhibit 3.1 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 3.2 | | Bylaws of the Company, as adopted on June 5, 2000 (incorporated herein by reference to Exhibit 3.2 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 3.3 | | Certificate of Designations, Preferences and Rights of Series A Preferred Stock (incorporated herein by reference to Exhibit 3.1 of the Company's Current Report filed on Form 8-K on March 5, 2003) |
| 3.4 | | Certificate of Amendment to Certificate of Incorporation, as filed with the Delaware Secretary of State on August 5, 2003 (incorporated herein by reference to Exhibit 3.1 of the Company's Quarterly Report on Form 10-Q filed on November 12, 2003) |
75
| 3.5 | | Certificate of Amendment to Certificate of Incorporation, as filed with the Delaware Secretary of State on June 21, 2007 (incorporated herein by reference to Exhibit 3.1 of the Company's Quarterly Report on Form 10-Q filed on August 9, 2007) |
| 4.1 | | Form of Stock Certificate representing Common Stock, $0.001 par value, of the Company (incorporated herein by reference to Exhibit 4.1 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 10.1@ | | Interleukin Genetics, Inc. 1996 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.17 of the Company's Registration Statement No. 333-37441 on Form SB-2 filed October 8, 1997) |
| 10.2@ | | Amendment to the Interleukin Genetics, Inc. 1996 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.18 of the Company's Registration Statement No. 333 — 37441 on Form SB-2 filed October 8, 1997) |
| 10.3@ | | Form of Stock Option Agreement (incorporated herein by reference to Exhibit 10.19 of the Company's Registration Statement No. 333-37441 on Form SB-2 filed October 8, 1997) |
| 10.4@ | | Stock Option Exercise Agreement (incorporated herein by reference to Exhibit 10.20 of the Company's Registration Statement No. 333-37441 on Form SB-2 filed October 8, 1997) |
| 10.5@ | | Non-Qualified Stock Option Agreement dated June 1, 1999, between the Company and Philip R. Reilly (incorporated herein by reference to Exhibit 10.2 of the Company's Quarterly Report on Form 10-QSB filed August 16, 1999) |
| 10.6@ | | Non-Qualified Stock Option Agreement dated November 30, 1999 between the Company and Philip R. Reilly (incorporated herein by reference to Exhibit 4.5 of the Company's Registration Statement No. 333-32538 on Form S-8 filed March 15, 2000) |
| 10.7@ | | 2000 Employee Stock Compensation Plan for the Company (incorporated herein by reference to Exhibit 10.3 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 10.8@ | | Form of Nonqualified Stock Option Grant (incorporated herein by reference to Exhibit 10.4 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 10.9@ | | Form of Incentive Stock Option Grant (incorporated herein by reference to Exhibit 10.5 of the Company's Quarterly Report on Form 10-Q filed August 14, 2000) |
| 10.10 | | Note Purchase Agreement between the Company and Pyxis Innovations Inc. dated October 22, 2002 (incorporated herein by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K filed on October 28, 2002) |
| 10.11 | | Security Agreement between the Company and Pyxis Innovations Inc. dated October 22, 2002 (incorporated herein by reference to Exhibit 10.2 of the Company's Current Report on Form 8-K filed on October 28, 2002) |
| 10.12 | | Form of Common Stock Purchase Warrant (incorporated herein by reference to Exhibit 10.3 of the Company's Quarterly Report on Form 10-Q filed on November 7, 2002) |
| 10.13 | | Registration Rights Agreement dated August 9, 2002 (incorporated herein by reference to Exhibit 10.4 of the Company's Quarterly Report on Form 10-Q filed on November 7, 2002) |
| 10.14 | | Stock Purchase Agreement between the Company and Pyxis Innovations Inc. dated March 5, 2003 (incorporated herein by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
76
| 10.15 | | Amendment No. 3 to Note Purchase Agreement between the Company and Pyxis Innovations Inc., dated March 5, 2003 (incorporated herein by reference to Exhibit 10.2 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.16 | | Amendment No. 2 to the Security Agreement between the Company and Pyxis Innovations Inc., dated March 5, 2003 (incorporated herein by reference to Exhibit 10.3 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.17 | | Form of Amended and Restated Promissory Note (incorporated herein by reference to Exhibit 10.4 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.18 | | Amendment No. 2 to Note Purchase Agreement between the Company and Pyxis Innovations Inc. (incorporated herein by reference to Exhibit 10.5 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.19+ | | Exclusive License Agreement between the Company and Access Business Group dated March 5, 2003 (incorporated herein by reference to Exhibit 10.7 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.20 | | Registration Rights Agreement between the Company and Pyxis Innovations Inc. dated March 5, 2003 (incorporated herein by reference to Exhibit 10.8 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.21@ | | Form of Director's Indemnity Agreement dated March 5, 2003 (incorporated herein by reference to Exhibit 10.13 of the Company's Current Report on Form 8-K filed on March 5, 2003) |
| 10.22 | | Commercial Lease Agreement between the Company and Clematis LLC dated February 13, 2004 (incorporated herein by reference to Exhibit 10.44 of the Company's Annual Report on Form 10-K filed on March 29, 2004) |
| 10.23+ | | Distribution Agreement with the Company and Access Business Group International LLC, dated February 26, 2004 (incorporated herein by reference to Exhibit 10.45 of the Company's Annual Report on Form 10-K filed on March 29, 2004) |
| 10.24 | | Interleukin Genetics, Inc. 2004 Employee, Director and Consultant Stock Plan (incorporated by reference to Exhibit 99.1 of the Company's Registration Statement No. 333-118551 on Form S-8 filed on August 25, 2004) |
| 10.25+ | | Research Agreement by and between the Company and Access Business Group LLC dated March 29, 2007 (incorporated by reference to Exhibit 10.1 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2007) |
| 10.26 | | First Amendment to Distribution Agreement with the Company and Access Business Group International LLC, dated February 28, 2005 (incorporated by reference to Exhibit 10.40 of the Company's Annual Report on Form 10-K filed on April 26, 2005) |
| 10.27 | | Amendment No. 4 to Note Purchase Agreement between the Company and Pyxis Innovations Inc. dated March 3, 2003 (incorporated by reference to Exhibit 10.1 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2006) |
| 10.28 | | Purchase Agreement between the Company and Access Business Group LLC dated February 23, 2006 effective as of January 31, 2006 (incorporated by reference to Exhibit 10.2 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2006) |
| 10.29 | | Purchase Agreement between the Company and Access Business Group LLC dated February 23, 2006 effective as of March 23, 2006 (incorporated by reference to Exhibit 10.3 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2006) |
77
| 10.30@ | | Employment Agreement dated March 31, 2006 between the Company and Kenneth S. Kornman (incorporated by reference to Exhibit 10.6 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2006) |
| 10.31@ | | Employment Agreement dated March 31, 2006 between the Company and Philip R. Reilly (incorporated by reference to Exhibit 10.6 of the Company's Quarterly Report on Form 10-Q filed on May 10, 2006) |
| 10.32 | | Second Amendment to Stock Purchase Agreement between the Company and Pyxis Innovations Inc. dated February 28, 2005 (incorporated by reference to Exhibit 10.41 of the Company's Annual Report on Form 10-K filed on April 26, 2005) |
| 10.33 | | Asset Purchase Agreement By and Among Alan James Group, LLC, AJG-NB, LLC, AJG-BI Brands, LLC, AJG-GNC, LLC, The Owners of Each of the Foregoing, AJG Brands Inc. and the Company dated August 17, 2006 (incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 10.35 | | Stock Purchase Agreement Between the Company and Pyxis Innovations Inc. dated August 17, 2006 (incorporated by reference to Exhibit 10.2 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 10.36 | | Amendment No. 5 to Note Purchase Agreement between the Company and Pyxis Innovations Inc. dated March 3, 2003 (incorporated by reference to Exhibit 10.3 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 10.37 | | Form of Promissory Note (incorporated by reference to Exhibit 10.4 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 10.38@ | | Employment Agreement dated August 17, 2006 between the Company and Timothy J. Richerson (incorporated by reference to Exhibit 10.5 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 10.39@ | | Employment Agreement dated August 17, 2006 between the Company and David A. Finkelstein (incorporated by reference to Exhibit 10.6 of the Company's Current Report on Form 8-K/A filed on October 31, 2006) |
| 21.1* | | Subsidiaries of the Company |
| 23.1* | | Consent of Grant Thornton LLP |
| 31.1* | | Certification of Chief Executive Officer pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| 31.2* | | Certification of Principal Financial Officer pursuant to Section 302 of Sarbanes-Oxley Act of 2002 |
| 32.1* | | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 2002 |
- *
- Filed herewith.
- +
- The Securities and Exchange Commission with respect to certain portions of this exhibit has previously granted confidential treatment. Omitted portions have been filed separately with the Securities and Exchange Commission.
- ++
- Confidential treatment requested as to certain portions of the document, which portions have been omitted and filed separately with the Securities and Exchange Commission.
- @
- Management contract or compensatory plan, contract or arrangement.
78
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | INTERLEUKIN GENETICS, INC. |
|
|
By: |
/s/ LEWIS H. BENDER
Lewis H. Bender
Chief Executive Officer |
Date: March 31, 2008
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated below.
Signatures
| | Title
| | Date Signed
|
|---|
|
|
|
|
|
/s/ LEWIS H. BENDER
Lewis H. Bender | | Chief Executive Officer
(Principal Executive Officer and deemed Principal Financial and Accounting Officer) | | March 31, 2008 |
/s/ KENNETH S. KORNMAN
Kenneth S. Kornman |
|
President, Chief Scientific Officer and Director |
|
March 31, 2008 |
/s/ JAMES WEAVER
James Weaver |
|
Chairman of the Board of Directors |
|
March 31, 2008 |
/s/ DIANNE E. BENNETT
Dianne E. Bennett |
|
Director |
|
March 31, 2008 |
/s/ GEORGE CALVERT
George Calvert |
|
Director |
|
March 31, 2008 |
/s/ THOMAS R. CURRAN JR.
Thomas R. Curran Jr. |
|
Director |
|
March 31, 2008 |
79
