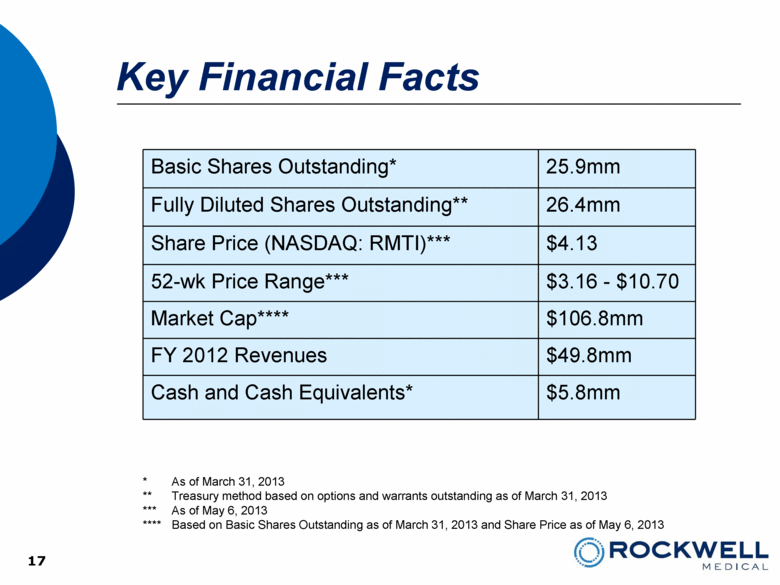
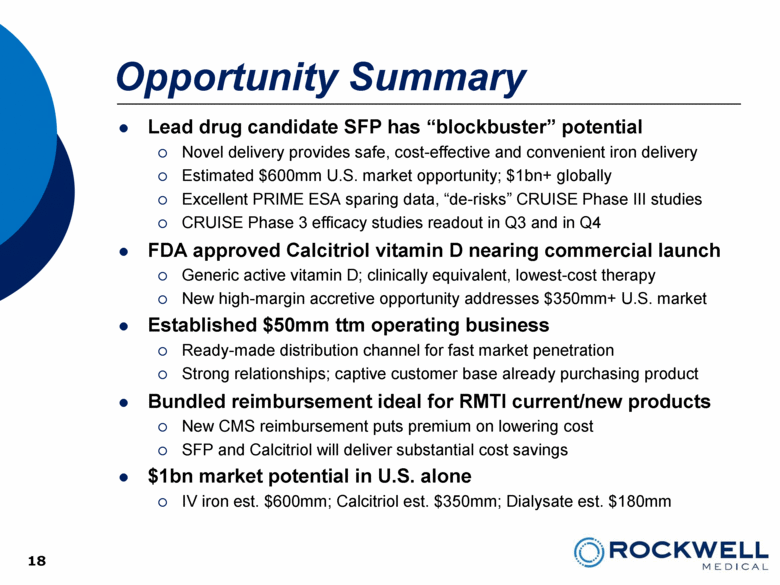
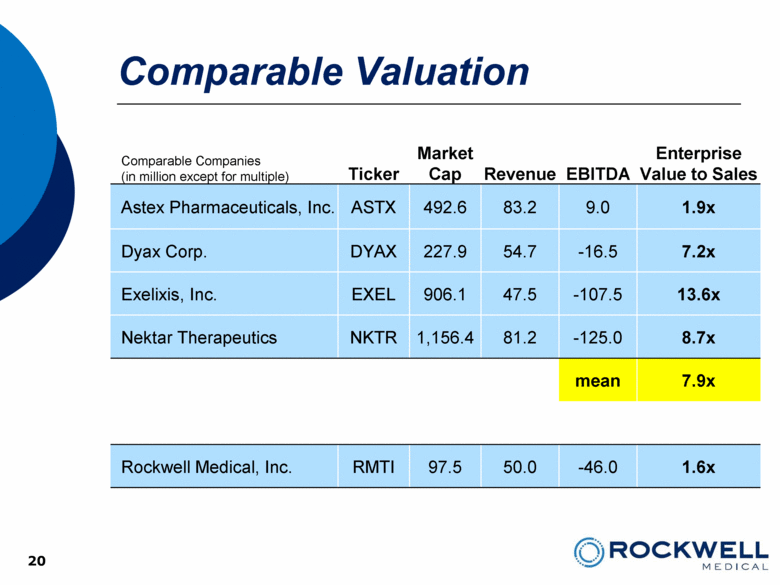
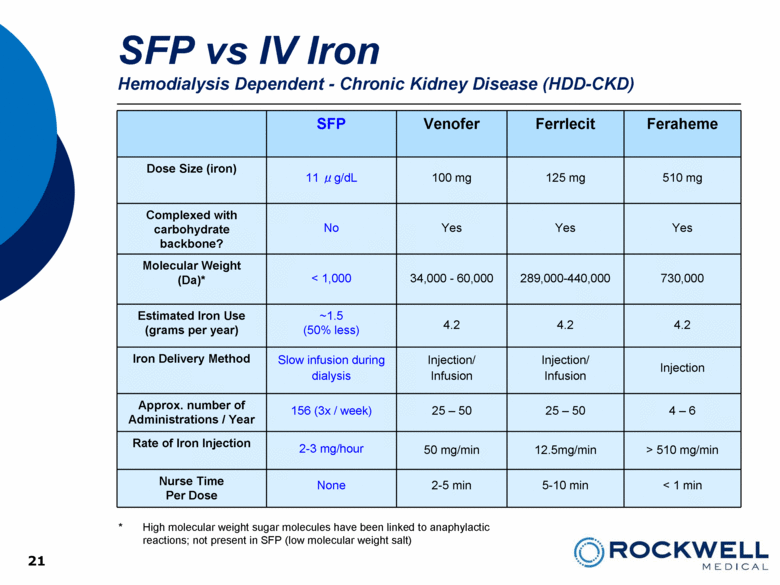
| Safe Harbor Statement This presentation contains forward-looking statements. All statements, other than statements of historical facts, including, among others, statements regarding the Company’s future plans, products (investigational or otherwise), financial position, business strategy, projected levels of growth, projected costs and projected financing needs, projected roll-out or approval dates, are forward-looking statements. Those statements include statements regarding the intent, belief or current expectations of Rockwell Medical and members of the Company’s management team, as well as the assumptions on which such statements are based, and generally are identified by the use of words such as “may,” “will,“ “seeks,” “anticipates,” “forecast,” “upcoming,” “believes,” “estimates,” “expects,” “plans,” “intends,” “should”, “potential” or similar expressions. Forward looking statements are not guarantees of future performance and involve risks and uncertainties, including those set forth in the Company’s annual and quarterly reports filed with the SEC. Actual results may differ materially from those contemplated by such forward-looking statements. The Company believes these forward-looking statements are reasonable. However, undue reliance should not be placed on any forward-looking statements, which are based on current expectations. All written and oral forward-looking statements attributable to the Company or persons acting on its behalf are qualified in their entirety by these cautionary statements. Further, forward-looking statements speak only as of the date they are made, and the Company undertakes no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to future operating results over time unless required by law. 2 |