Exhibit 99.1

Corporate Presentation January 2022 www.RockwellMed.com ROCKWELL MEDICAL, INC. TRANSFORMING IRON DEFICIENCY AND ANEMIA MANAGEMENT (NASDAQ: RMTI)

FORWARD - LOOKING STATEMENTS 2 Certain statements in this presentation may constitute "forward - looking statements" within the meaning of federal securities laws, including, but not limited to, Rockwell Medical’s intention to develop FPC for new indications, and maintain concentrate sales . Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," “can,” "could," “would,” “develop,” "plan," "potential," "predict," "forecast," "project," "plan", "intend" or the negatives of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward - looking statements . While Rockwell Medical believes these forward - looking statements are reasonable, undue reliance should not be placed on any such forward - looking statements, which are based on information available to us on the date of this presentation . These forward - looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Rockwell Medical’s SEC filings), many of which are beyond our control and subject to change . Actual results could be materially different . Risks and uncertainties include : the impact of COVID - 19 pandemic (including, applicable federal, state and local orders) on business, clinical development plans and operating results, including our supply chain and dialysis concentrates business ; the challenges inherent in new product development and other indications and therapeutic areas for our products ; the likelihood of success and timing of our international development and commercialization plans, including regulatory filings and clinical trials ; the success and our commercialization of Triferic domestically and internationally ; the expected number of annualized treatments for Triferic Dialysate and Triferic AVNU ; the risk that regulatory authorities delay or fail to approve FPC for new indications ; the risk that market opportunities are smaller than estimated ; the risk that Rockwell Medical is not able to seek reimbursement for FPC for new indications ; the risk that FPC is unsafe for new indications ; the risk that clinical study designs, timing and costs are different than estimated ; expected financial performance, including cash flows, revenues, growth, margins, funding, liquidity and capital resources ; and those risks more fully discussed in the “Risk Factors” section of our Quarterly Report on Form 10 - Q for the quarter ended September 30 , 2021 and of our Annual Report on Form 10 - K for the year ended December 31 , 2020 , as such description may be amended or updated in any future reports we file with the SEC . Rockwell Medical expressly disclaims any obligation to update our forward - looking statements, except as may be required by law .
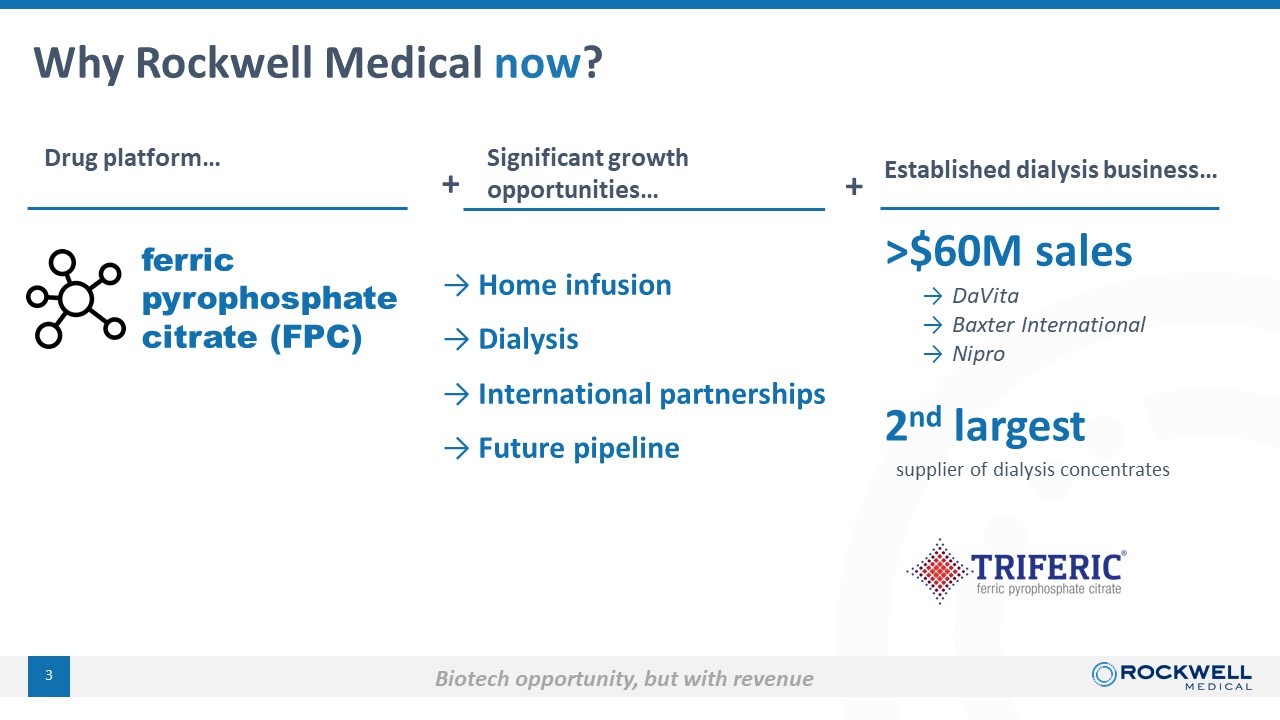
Why Rockwell Medical now ? 3 Drug platform… ferric pyrophosphate citrate (FPC) Significant growth opportunities… Established dialysis business… 2 nd largest supplier of dialysis concentrates → Home infusion → Dialysis → International partnerships → Future pipeline >$60M sales Biotech opportunity, but with revenue + + → DaVita → Baxter International → Nipro
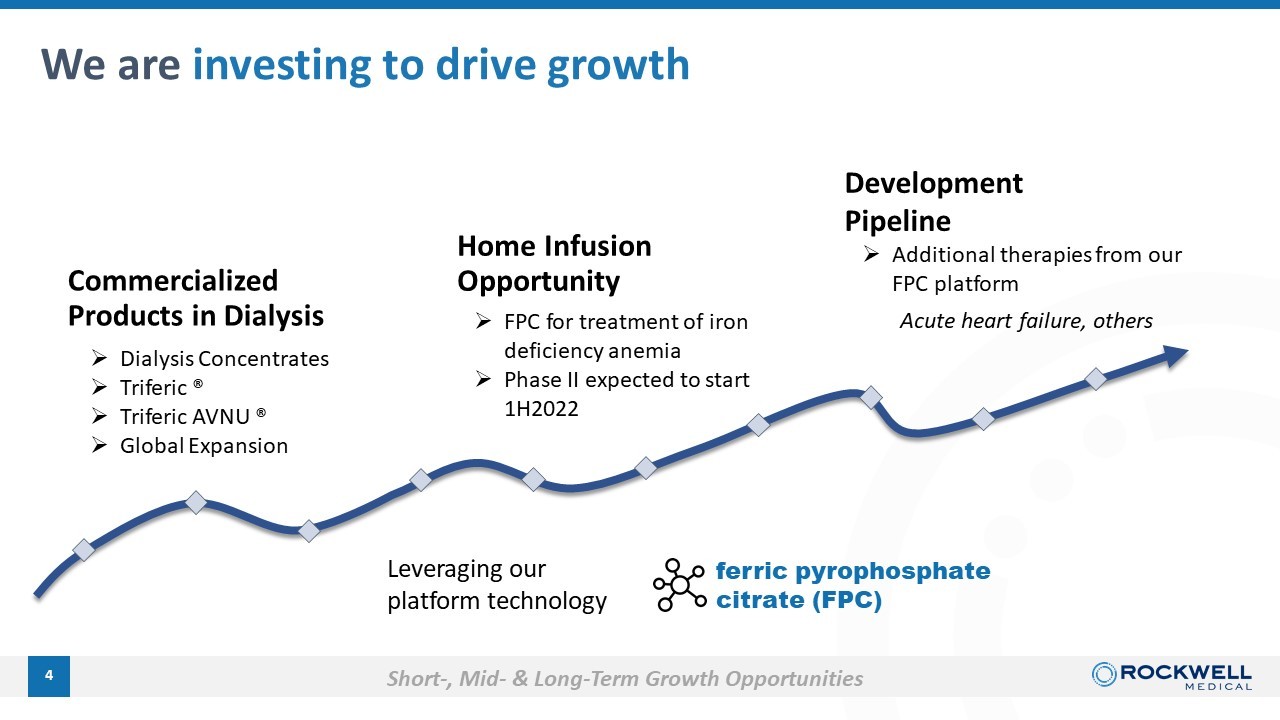
4 We are investing to drive growth Commercialized Products in Dialysis » Dialysis Concentrates » Triferic ® » Triferic AVNU ® » Global Expansion Home Infusion Opportunity » FPC for treatment of iron deficiency anemia » Phase II expected to start 1H2022 Development Pipeline » Additional therapies from our FPC platform Acute heart failure, others ferric pyrophosphate citrate (FPC) Leveraging our platform technology Short - , Mid - & Long - Term Growth Opportunities

PLATFORM TECHNOLOGY Ferric Pyrophosphate Citrate (FPC)
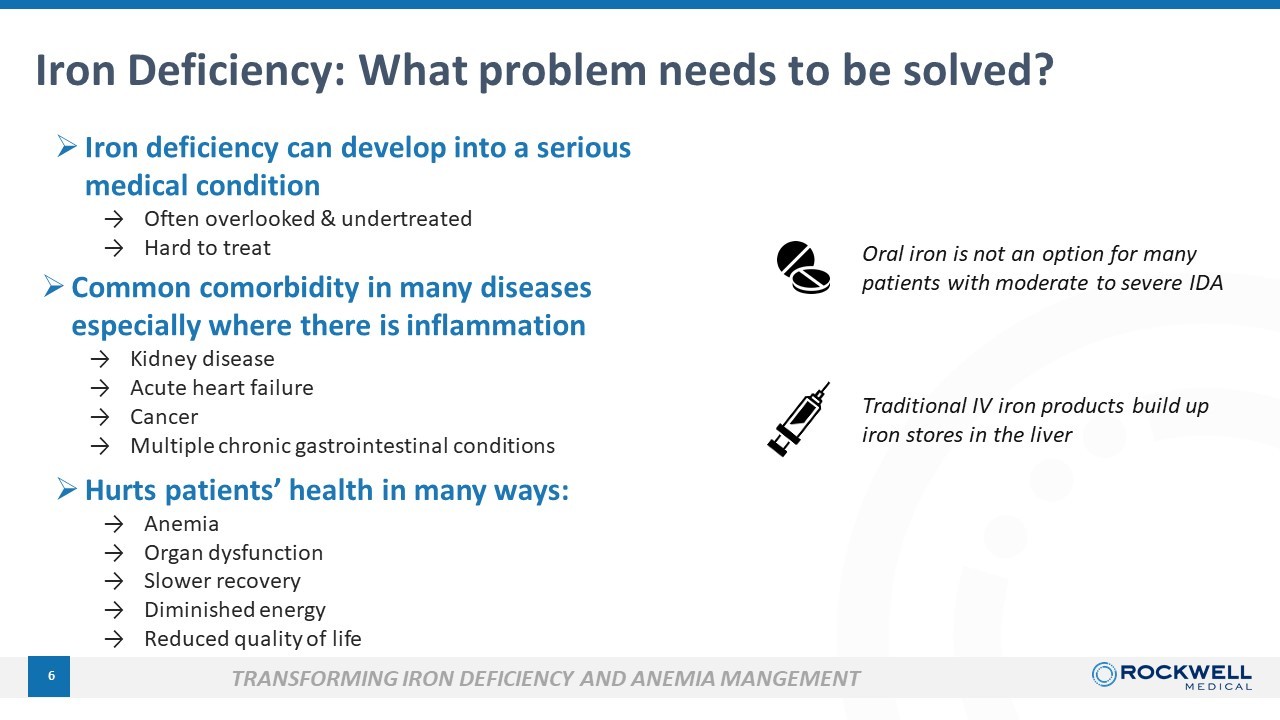
6 Iron Deficiency: What problem needs to be s olved ? TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT » Iron deficiency can develop into a serious medical condition → Often overlooked & undertreated → Hard to treat » Common comorbidity in many diseases especially where there is inflammation → Kidney disease → Acute heart failure → Cancer → Multiple chronic gastrointestinal conditions » Hurts patients’ health in many ways: → Anemia → Organ dysfunction → Slower recovery → Diminished energy → Reduced quality of life Traditional IV iron products build up iron stores in the liver Oral iron is not an option for many patients with moderate to severe IDA
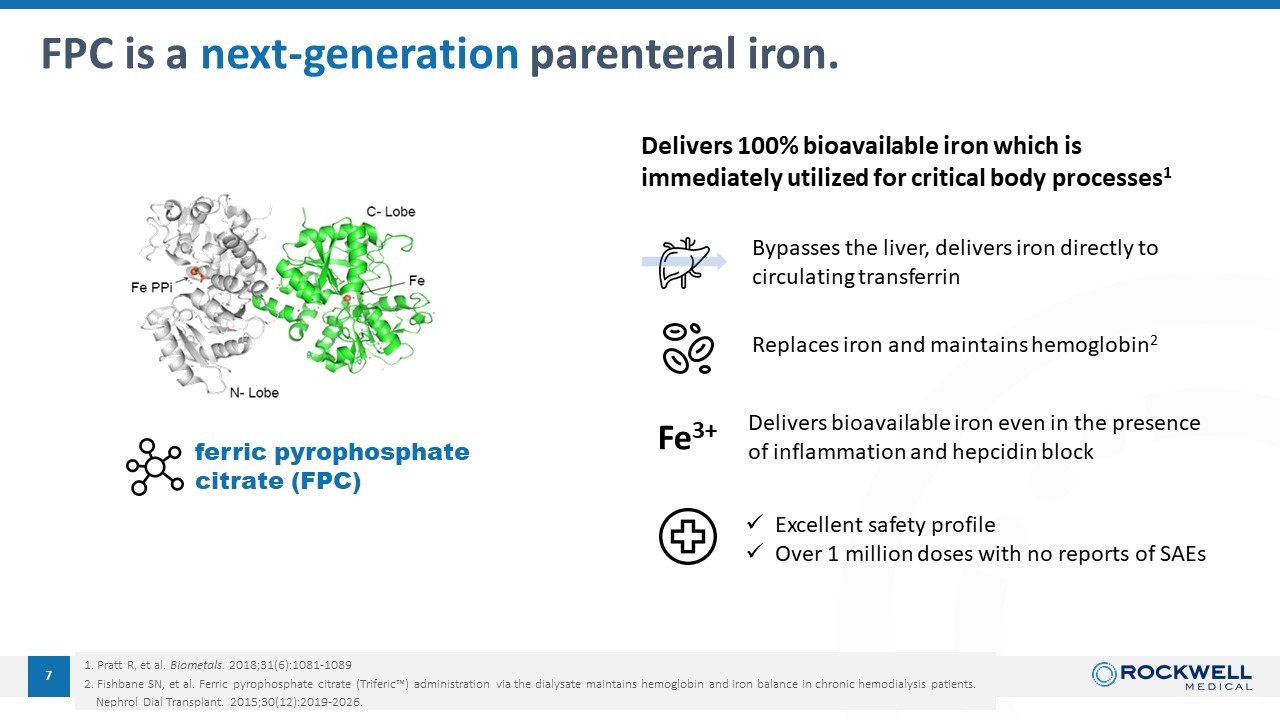
1. Pratt R, et al. Biometals . 2018;31(6):1081 - 1089 2. Fishbane SN, et al. Ferric pyrophosphate citrate (Triferic Ρ ) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015;30(12):2019 - 2026. 7 FPC is a next - generation parenteral iron. ferric pyrophosphate citrate (FPC) Delivers 100% bioavailable iron which is immediately utilized for critical body processes 1 Bypasses the liver, delivers iron directly to circulating transferrin Delivers bioavailable iron even in the presence of inflammation and hepcidin block x Excellent safety profile x Over 1 million doses with n o reports of SAEs Replaces iron and maintains hemoglobin 2 Fe 3+

HOME INFUSION OPPORTUNITY Newly formulated FPC products Novel presentations 505(b)(1) FDA approvals Unique J - codes Differentiated pricing 8

9 Home infusion is rapidly growing and is an opportunity for FPC . Iron Deficiency Anemia is a common comorbidity . • Large and growing market opportunity • Efficacy and safety already established Presents a significant opportunity for FPC . What is “Home I nfusion” therapy? It is… provision of IV treatments at home. Home infusion therapies allow patients with diseases requiring regular infusions of IV medications to be treated in the comfort of their own homes and avoid costly and inconvenient visits to hospitals or outpatient infusion clinics. It is not dialysis. Management of iron deficiency anemia is a broken process. An IV iron product suitable for home infusion is a significant unmet medical need.
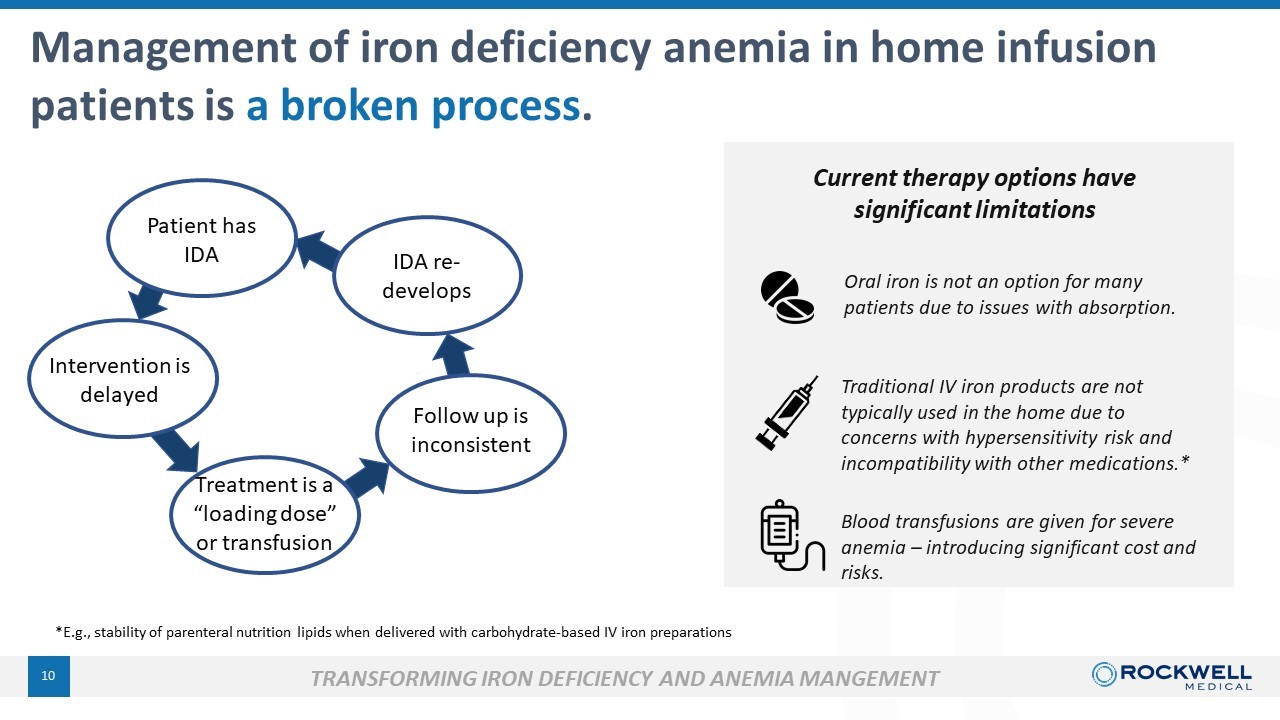
10 *E.g., stability of parenteral nutrition lipids when delivered with carbohydrate - based IV iron preparations Management of iron deficiency anemia in home infusion patients is a broken process . Traditional IV iron products are not typically used in the home due to concerns with hypersensitivity risk and incompatibility with other medications.* Oral iron is not an option for many patients due to issues with absorption. Patient has IDA Intervention is delayed Treatment is a “loading dose” or transfusion Follow up is inconsistent IDA re - develops Current therapy options have significant limitations Blood transfusions are given for severe anemia – introducing significant cost and risks. TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT
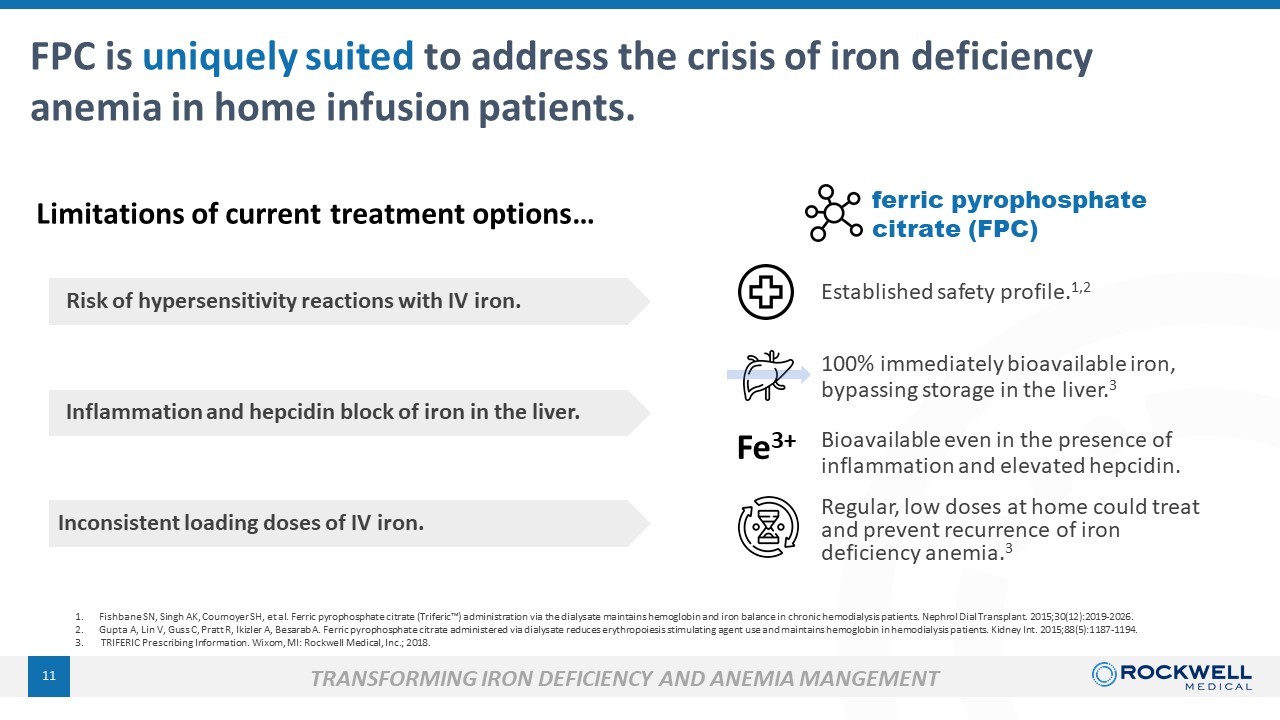
11 Bioavailable even in the presence of inflammation and elevated hepcidin. Regular, low doses at home could treat and prevent recurrence of iron deficiency anemia. 3 1. Fishbane SN, Singh AK, Cournoyer SH, et al. Ferric pyrophosphate citrate (Triferic Ρ ) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Trans pla nt. 2015;30(12):2019 - 2026. 2. Gupta A, Lin V, Guss C, Pratt R, Ikizler A, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis stimulating agent use and maintains hemoglo bi n in hemodialysis patients. Kidney Int. 2015;88(5):1187 - 1194. 3. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. Established safety profile. 1,2 FPC is uniquely suited to address the crisis of iron deficiency anemia in home infusion patients. Limitations of current treatment options… Fe 3+ Risk of hypersensitivity reactions with IV iron. Inflammation and hepcidin block of iron in the liver. Inconsistent loading doses of IV iron. ferric pyrophosphate citrate (FPC) TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT 100% immediately bioavailable iron, bypassing storage in the liver. 3
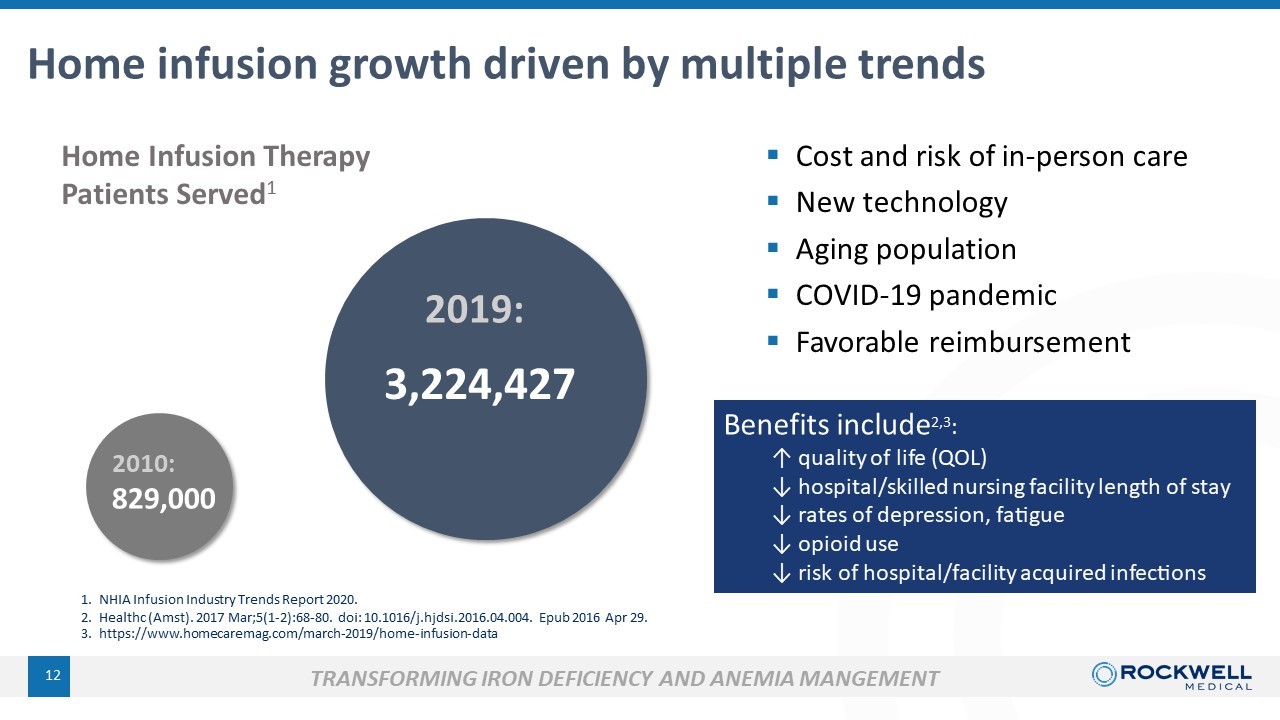
1. NHIA Infusion Industry Trends Report 2020. 12 2010: 829,000 2019: 3,224,427 Home Infusion Therapy Patients Served 1 Home infusion growth driven by multiple trends ▪ Cost and risk of in - person care ▪ New technology ▪ Aging population ▪ COVID - 19 pandemic ▪ Favorable reimbursement TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT Benefits include 2 ,3 : ↑ quality of life ( QOL) ↓ hospital/skilled nursing facility length of stay ↓ rates of depression, fatigue ↓ opioid use ↓ risk of hospital/facility acquired infections 2. Healthc ( Amst ). 2017 Mar;5(1 - 2):68 - 80. doi : 10.1016/j.hjdsi.2016.04.004. Epub 2016 Apr 29. 3. https://www.homecaremag.com/march - 2019/home - infusion - data
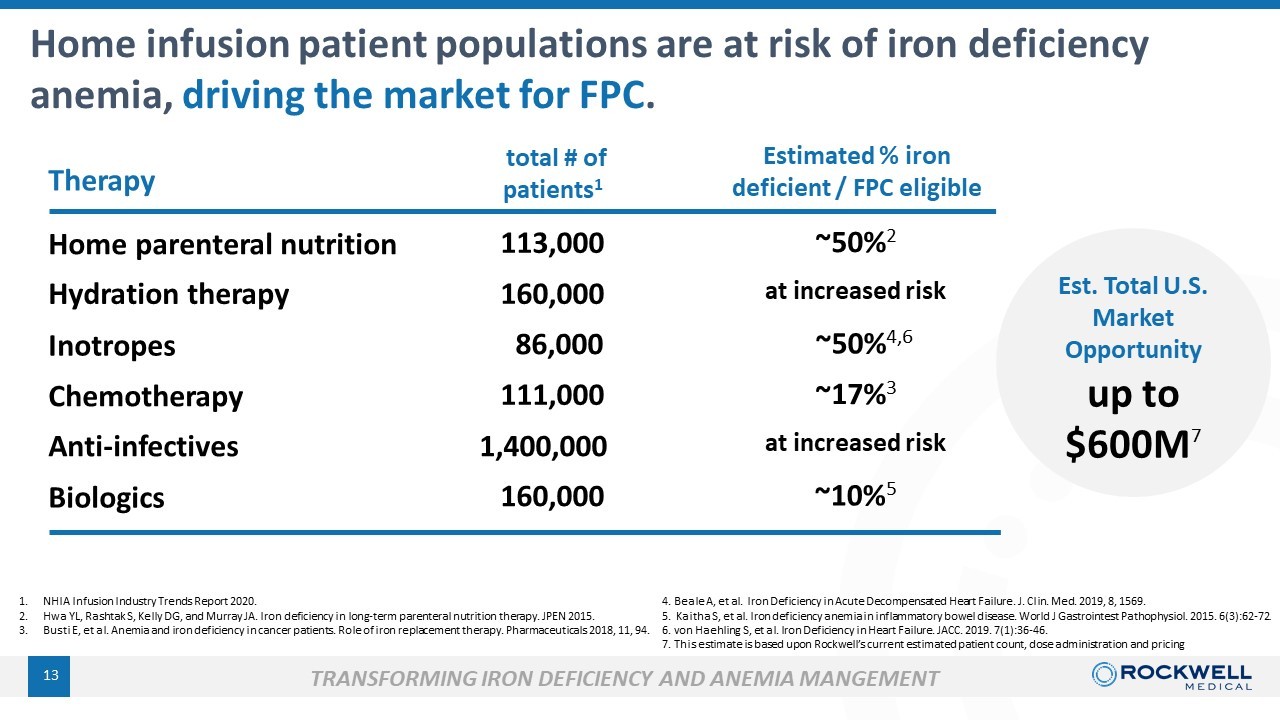
1. NHIA Infusion Industry Trends Report 2020. 2. Hwa YL, Rashtak S, Kelly DG, and Murray JA. Iron deficiency in long - term parenteral nutrition therapy. JPEN 2015. 3. Busti E, et al. Anemia and iron deficiency in cancer patients. Role of iron replacement therapy. Pharmaceuticals 2018, 11, 94. Home parenteral nutrition Hydration therapy Biologics Inotropes 4. Beale A, et al. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569. 5. Kaitha S, et al. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015. 6(3):62 - 72 . 6. von Haehling S, et al. Iron Deficiency in Heart Failure. JACC. 2019. 7(1):36 - 46 . 7. This estimate is based upon Rockwell’s current estimated patient count, dose administration and pricing 13 Home infusion patient populations are at risk of iron deficiency anemia, driving the market for FPC . Therapy total # of patients 1 Estimated % iron deficient / FPC eligible 111,000 160,000 86,000 160,000 ~17% 3 at increased risk ~50% 2 at increased risk TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT Chemotherapy 113,000 ~50% 4,6 up to $600M 7 Anti - infectives 1,400,000 ~10% 5 Est . T otal U.S. Market Opportunity

DIALYSIS OPPORTUNITIES
15 Dialysis Concentrates Opportunities for Growth We have a strong presence in the hemodialysis market. TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT
All hemodialysis patients at risk for iron deficiency anemia Anemia is challenging to manage with the current approach Triferic delivers 100% bioavailable iron, stabilizing hemoglobin 1,2,3 Conveniently delivered via IV * or dialysate 2 Demonstrated to reduce overall cost of anemia drugs 4 16 Full Prescribing Information available at www.Triferic.com 1. Fishbane SN, et al. Ferric pyrophosphate citrate administration via the dialysate maintains Hb and iron in chronic HD patients. Nephr ol Dial Trans. 2015;30(12):2019 - 2026. 2. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. 3. Gupta A, et al. Ferric pyrophosphate citrate administered via dialysate reduces ESA use and maintains hemoglobin in hemod ial ysis patients. Kidney Int. 2015;88(5):1187 - 1194. Triferic ® is ferric pyrophosphate citrate marketed in the U.S. for treatment of IDA in hemodialysis patients. 4. Dellafera L, et al. Institutional Usage of Ferric Pyrophosphate Citrate in Reducing Erythropoiesis - Stimulating Agents. Critical Care Medi cine. Jan 2021:49(1).551.
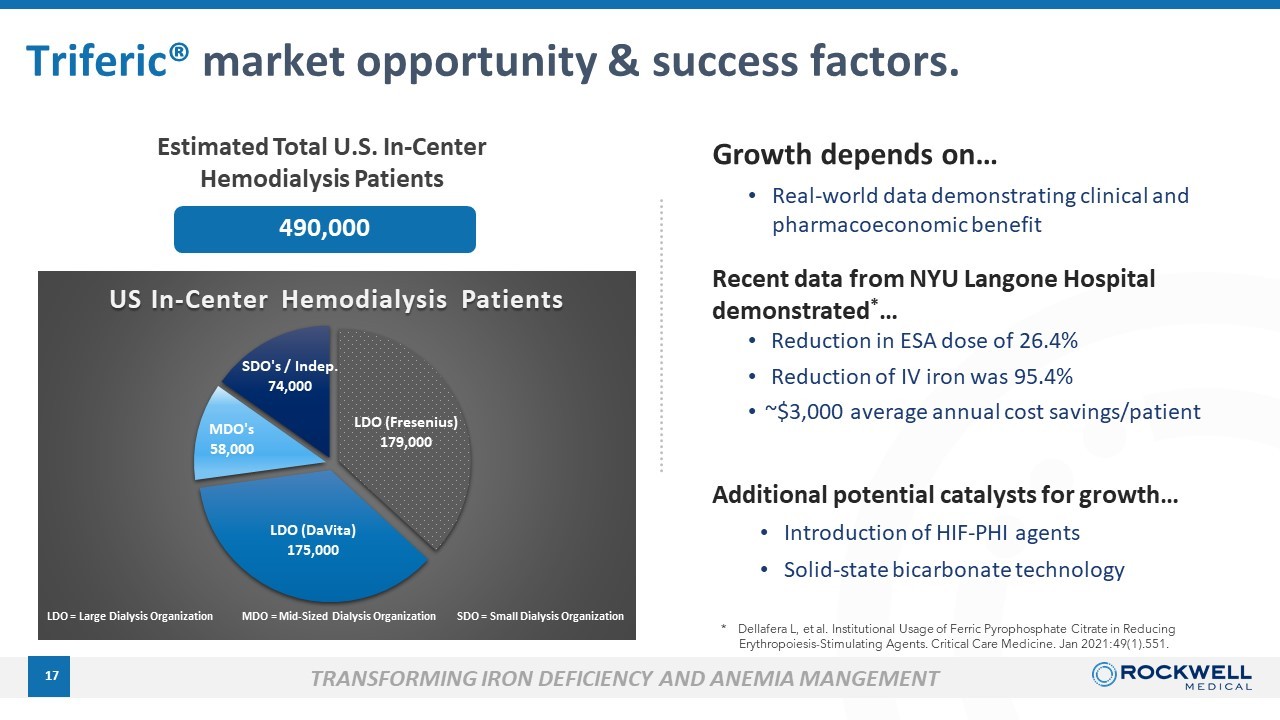
17 LDO (DaVita) 175k Patients 36% 3 MDOs 58k Patients 10% 490,000 Estimated Total U.S. In - Center Hemodialysis Patients → Current target market is 27% or 132,000 patients Triferic® market opportunity & success factors . TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT Growth depends on… • Real - world data demonstrating clinical and pharmacoeconomic benefit * Dellafera L, et al. Institutional Usage of Ferric Pyrophosphate Citrate in Reducing Erythropoiesis - Stimulating Agents. Critical Care Medicine. Jan 2021:49(1).551. Additional potential catalysts for growth… • Introduction of HIF - PHI agents • Solid - state bicarbonate technology Recent data from NYU Langone Hospital demonstrated * … • Reduction in ESA dose of 26.4% • Reduction of IV iron was 95.4% • ~$3,000 average annual cost savings/patient LDO (Fresenius) 179,000 LDO (DaVita) 175,000 MDO's 58,000 SDO's / Indep. 74,000 US In - Center Hemodialysis Patients LDO = Large Dialysis Organization MDO = Mid - Sized Dialysis Organization SDO = Small Dialysis Organization

PIPELINE DEVELOPMENT: ACUTE HEART FAILURE Newly formulated FPC products Novel presentations 505(b)(1) FDA approvals Unique J - codes Differentiated pricing

Potential for FPC in treating hospitalized acute heart failure patients. □ Why acute heart failure? • > 1 million patients annually • Iron deficiency in 50 - 70% • IV iron improves cardiac energetics in CHF • Iron mobilization limited by inflammation • FPC uniquely suited for acutes - immediately bioavailable iron □ Meeting with FDA expected in 1H 2022 to review proposed clinical development program • Phase 2 study to determine whether FPC provides a sufficient influx of iron to the cardiac tissue to improve myocardial energetics and associated outcomes 19 In the pipeline for FPC : T reatment of acute heart failure . TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT

UPCOMING MILESTONES
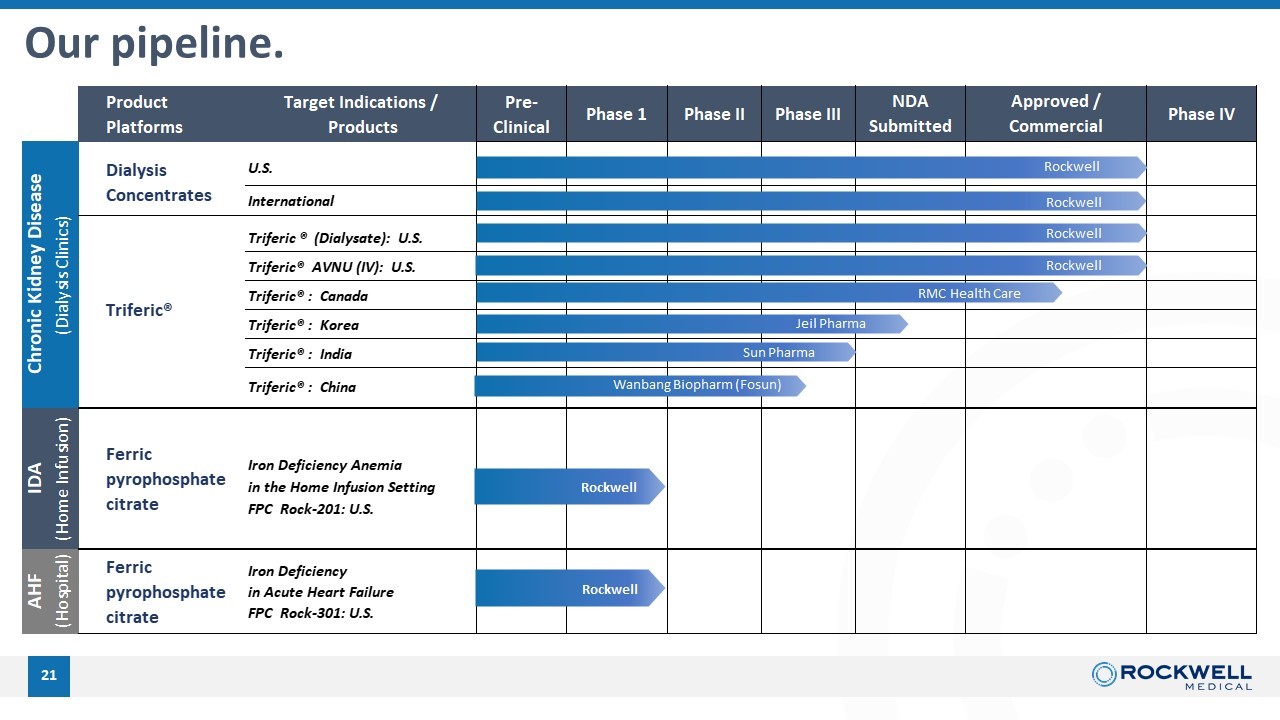
21 Our pipeline. Product Platforms Target Indications / Products Pre- Clinical U.S. International Triferic® (Dialysate): U.S. Triferic® AVNU (IV): U.S. Triferic® : Canada Triferic® : Korea Triferic® : India Triferic® : China Iron Deficiency Anemia in the Home Infusion Setting FPC Rock-201: U.S. Phase IV NDA Submitted Approved / Commercial Phase 1 Phase II Phase III Chronic Kidney Disease (Dialysis Clinics) IDA (Home Infusion) Dialysis Concentrates Triferic® Iron Deficiency in Acute Heart Failure FPC Rock-301: U.S. AHF (Hospital) Ferric pyrophosphate citrate Ferric pyrophosphate citrate RMC Health Care Jeil Pharma Sun Pharma Wanbang Biopharm (Fosun) Rockwell Rockwell Rockwell Rockwell Rockwell Rockwell

Proven track record and expertise in biopharmaceutical commercialization and clinical development 22 Management Team Marc Hoffman, M.D. Chief Medical Officer Years: 30+ Our experienced management team. TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT Mike DeYoung VP Operations Years: 25 + Russell Ellison, M.D. Chief Executive Officer Years: 35+ Russell Skibsted Chief Financial Officer Years: 25+ Ray Pratt, M.D. Chief Development Officer Years: 25+ Tim Chole Senior VP Sales and Marketing Years: 20+
Rockwell Medical is a commercial stage biopharmaceutical company investing in growth 23 x Proprietary Platform Technology Applicable To Many Disease States x Significant Growth Opportunities in new indications » Home Infusion » Pipeline: Acute Heart Failure x Building on the Success of a Significant Dialysis Business ($60m revenue) x Multiple Upcoming Milestones x New Management Team With Experience & Skills to Deliver Transforming iron deficiency and anemia management. TRANSFORMING IRON DEFICIENCY AND ANEMIA MANGEMENT

THANK YOU























