Exhibit 99.1
Exhibit 99.1Rockwell Medical Announces Second Quarter 2022 Results
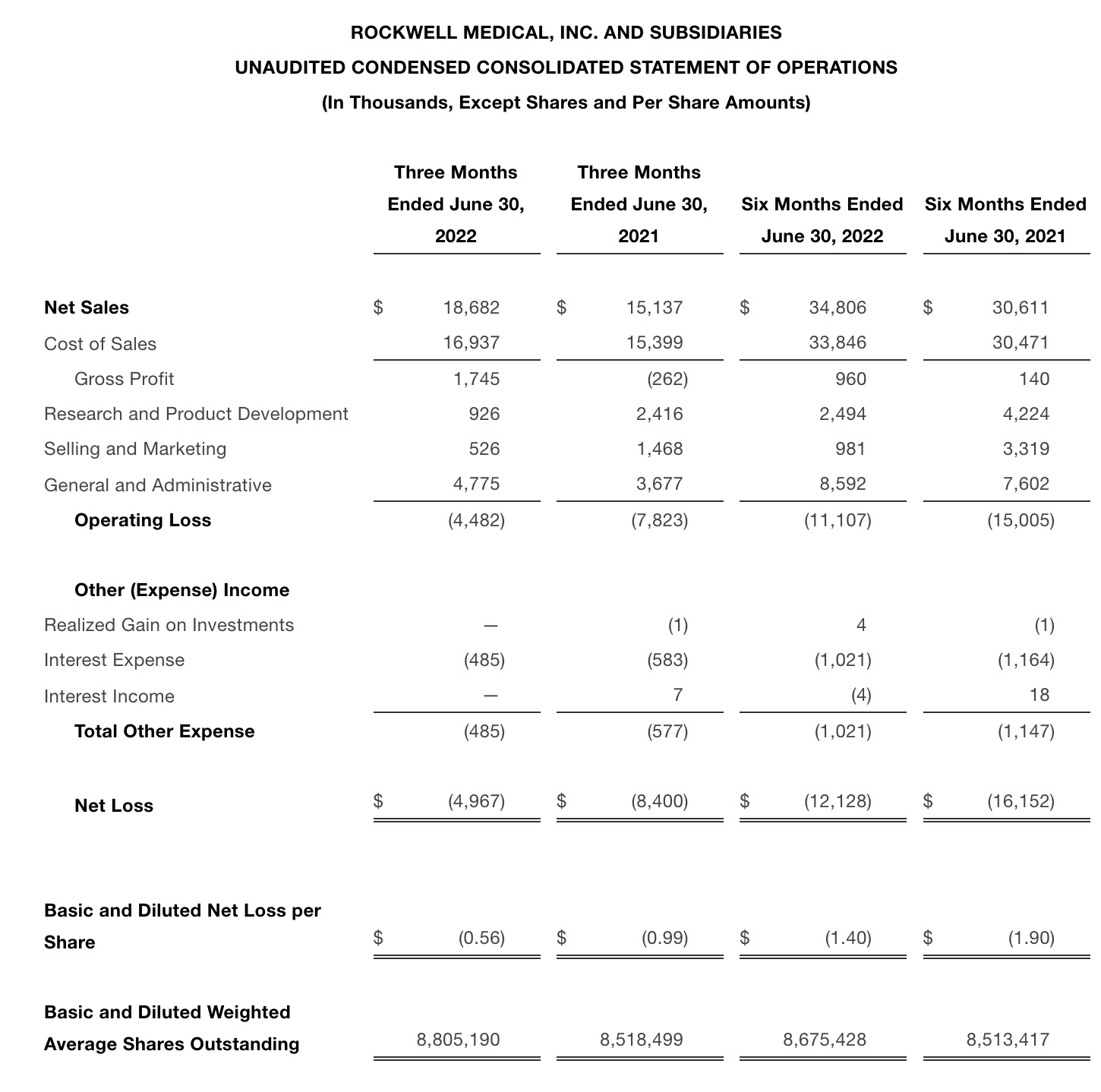
•Revenue was $18.7 million for the second quarter 2022, a 16% increase over the first quarter 2022, representing the highest quarterly revenue to-date for Rockwell
•Gross profit was $1.7 million in the second quarter 2022 compared to gross loss of $786,000 in the first quarter 2022
•Cash used in operating activities was $5.8 million for the second quarter 2022, a 40% decrease over the first quarter of 2022
•Completed $30 million financing and stabilized the business
Wixom, Michigan, August 15, 2022 – Rockwell Medical, Inc. (Nasdaq: RMTI), a commercial healthcare company focused on providing life-sustaining products for patients suffering from blood disorders and diseases associated with the kidney, today announced its operational and financial updates for the second quarter ended June 30, 2022.
SECOND QUARTER 2022 FINANCIAL HIGHLIGHTS
•Rockwell raised $30 million during the second quarter 2022, including a $15 million investment in the Company’s convertible preferred stock by DaVita, Inc.
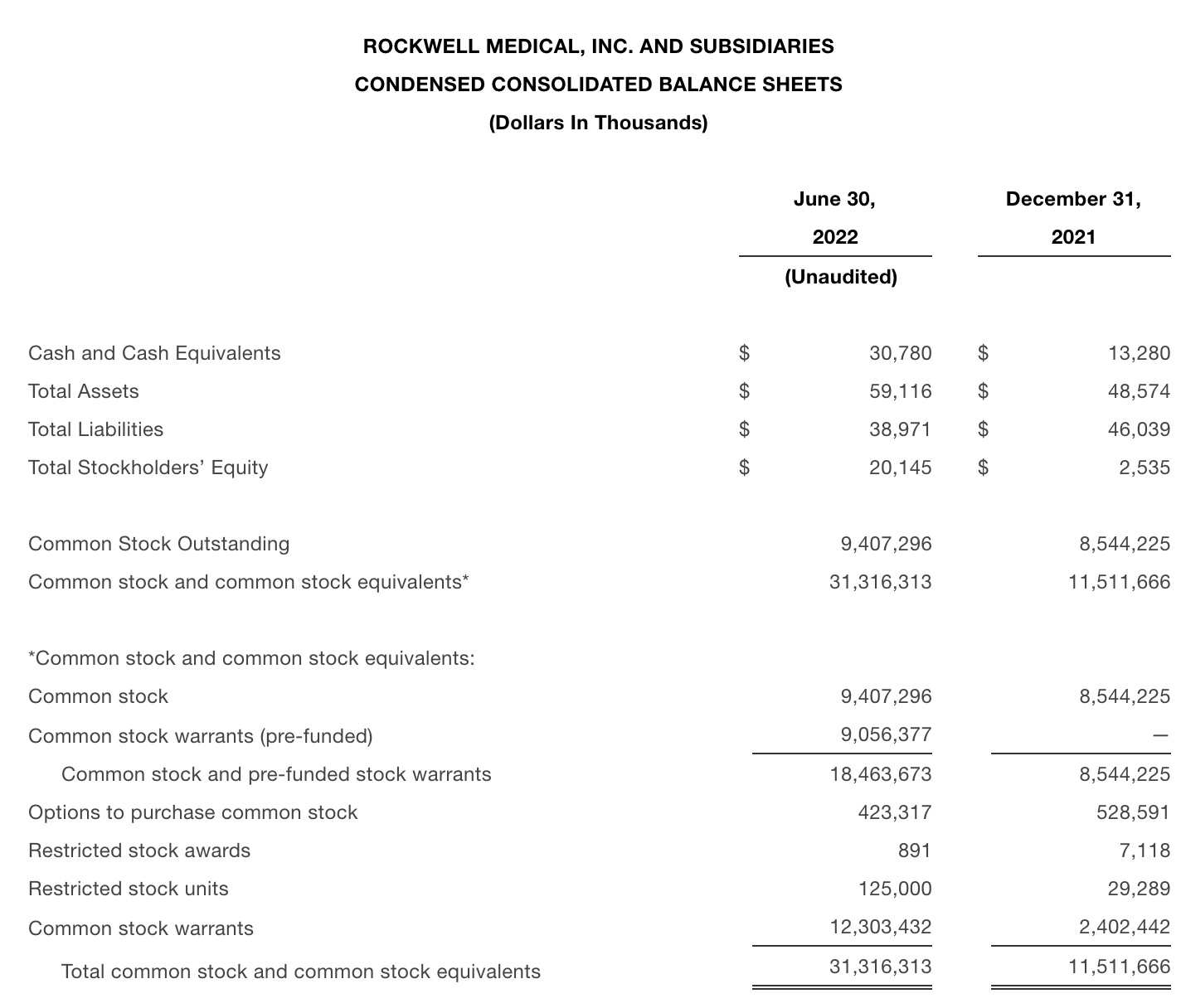
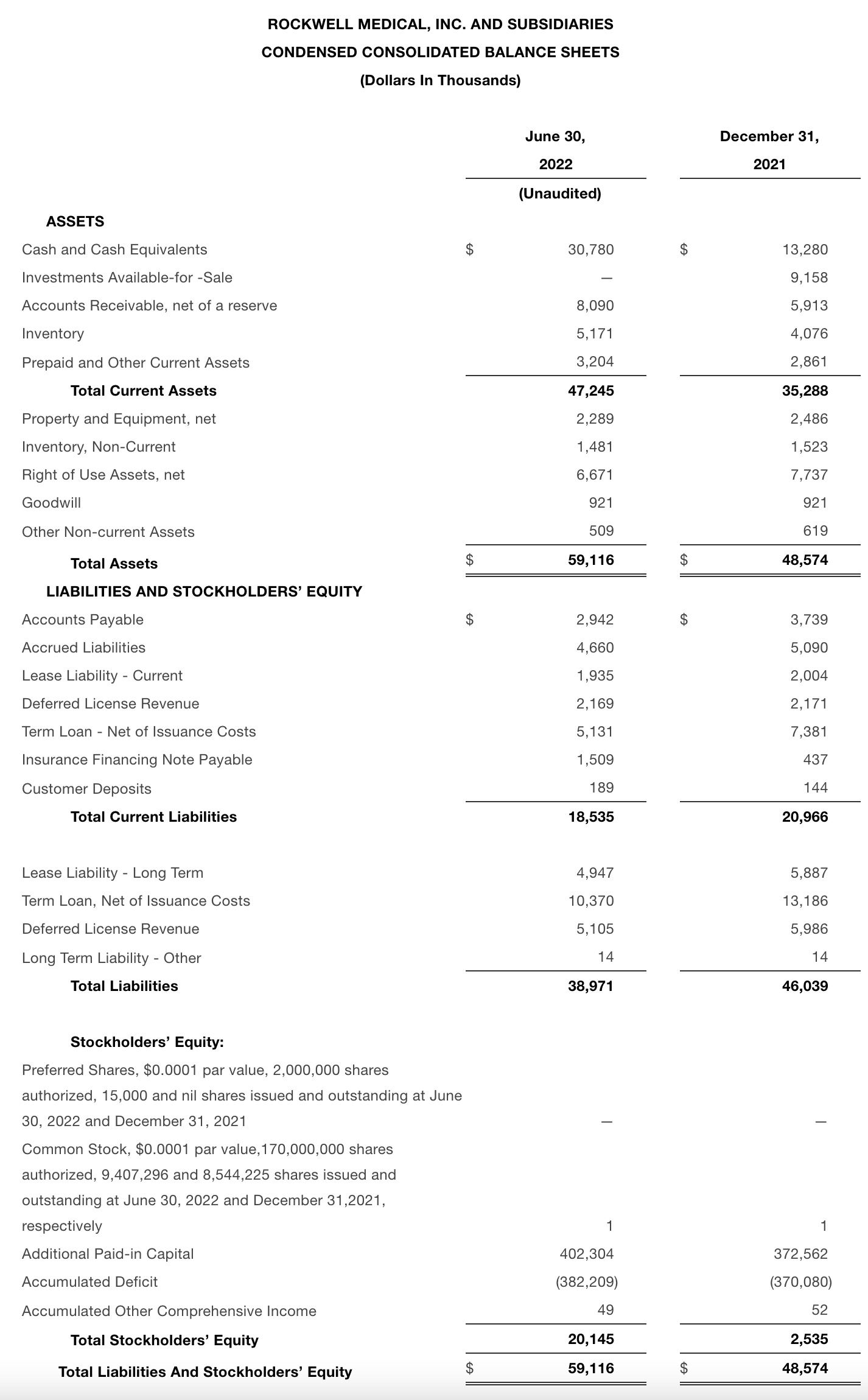
•As of June 30, 2022, Rockwell had cash and cash equivalents of $30.8 million and 9,407,296 shares outstanding and 9,056,377 pre-funded warrants. In addition, the Company lowered its outstanding debt from $20.6 million at December 31, 2021 to $16.5 million at June 30, 2022.
•Revenue for the three months ended June 30, 2022 was $18.7 million. This represents a 16% increase quarter-over-quarter compared to $16.1 million for the three months ended March 31, 2022, and a 24% increase year-over-year compared to $15.1 million for the three months ended June 30, 2021.
•For the second quarter of 2022, gross profit was $1.7 million. This is compared to a gross loss of $0.8 million for the first quarter of 2022 and a gross loss of $0.3 million for the second quarter of 2021.
•Cash used in operating activities for the three months ended June 30, 2022 was $5.8 million, compared to $9.8 million for the three months ended March 31, 2022, representing a 40% decrease quarter-over-quarter.
•Rockwell effected a 1-for-11 reverse split of the Company’s issued and outstanding common stock on May 13, 2022.
•Rockwell regained compliance with Nasdaq listing requirements on May 31, 2022.
SECOND QUARTER 2022 OPERATING HIGHLIGHTS
•Rockwell appointed Mark Strobeck, Ph.D. as President and Chief Executive Officer.
•Rockwell expanded its hemodialysis concentrates partnership with DaVita, one of the leading providers of kidney care services in the United States.
 Exhibit 99.1
Exhibit 99.1•Rockwell has begun to right-size its business to improve the financial performance of its concentrates business and other business lines.
•Rockwell’s partner in South Korea, Jeil Pharmaceutical, commercially launched TRIFERIC® in South Korea subsequent to the second quarter 2022. Rockwell signed an exclusive license agreement with Jeil in September 2020 under which Jeil will be the exclusive development and commercialization partner for TRIFERIC® in South Korea. In consideration for the license, Rockwell received an upfront fee and will be eligible for milestone payments and royalties on net sales.
•Rockwell’s partner in China, Wanbang Biopharmaceuticals, a subsidiary of Shanghai Fosun Pharmaceutical, enrolled the final patient six months ahead of schedule, totaling 442 patients, in its pivotal Phase 3 clinical trial for TRIFERIC®.
•Rockwell has begun to address requests from the U.S. Food and Drug Administration for additional microbial and stability data for Rockwell’s FPC formulation for treatment of patients in the Home Infusion setting.
“In the second quarter, we took a number of important steps to stabilize our business, and are now in a better position to unlock the value that has been created by the Company,” said Mark Strobeck, Ph.D., President and CEO at Rockwell Medical. “Our improved relationships with our partners and our improved financial position afford us the ability to deliver more life-sustaining products to patients suffering from blood disorders and diseases associated with the kidney longer-term. Going forward, Rockwell’s focus will be to drive profitability for our overall business, to continually assess our strategic priorities and capital structure to manage cash and place Rockwell on firmer financial footing; and to develop our FPC programs to key value milestones consistent with a prudent capital deployment strategy. We expect to have more to say about these changes in the coming months,” concluded Dr. Strobeck.
CONFERENCE CALL AND WEBCAST DETAILS
Please call 10 minutes prior to the call to register.
Date: Monday, August 15, 2022
Time: 8:00am ET
Webcast: www.RockwellMed.com/Results
Live Number: (888) 660-6347 // (International) 1 (929) 201-6594
Replay Number: (800) 770-2030 // (International) 1 (647) 362-9199
Access Code: 4944610
Speakers: Mark Strobeck, Ph.D., President and Chief Executive Officer; and Russell Skibsted, Chief Financial Officer and Chief Business Officer
Format: Discussion of second quarter 2022 operational and financial results followed by Q&A.
 Exhibit 99.1
Exhibit 99.1
A replay will be available via the replay number and webcast through September 14, 2022.
About Rockwell Medical
Rockwell Medical is a commercial healthcare company focused on providing life-sustaining products for patients suffering from blood disorders and diseases associated with the kidney. Rockwell is the second largest supplier of acid and bicarbonate concentrates for dialysis patients in the United States. The Company is developing and commercializing a next-generation, proprietary parenteral iron technology platform, Ferric Pyrophosphate Citrate (“FPC”), which has the potential to transform treatment options for iron deficiency in multiple disease states, reduce healthcare costs and improve patients' lives. Rockwell has two FDA-approved therapies indicated for patients undergoing hemodialysis, which are the first two products developed from the FPC platform. Rockwell is also advancing its FPC platform by developing FPC for the treatment of iron deficiency anemia in patients outside of dialysis, who are receiving medications in the home infusion setting. For more information, visit www.RockwellMed.com.
Forward-Looking Statements
Certain statements in this press release may constitute "forward-looking statements" within the meaning of the federal securities laws. Words such as, "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "could," "can," "would," "develop," "plan," "potential," "predict," "forecast," "project," "intend," "look forward to," "remain confident" or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward looking statements. There can be no assurance that Rockwell Medical will be able to maintain timing for planned clinical trials and regulatory filings, achieve planned cost savings to operate its concentrates business profitability, or that Rockwell Medical will be able to satisfy the funding conditions for the second tranche of the DaVita investment. While Rockwell Medical believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Rockwell Medical's SEC filings), many of which are beyond our control and subject to change. Actual results could be materially different. Risks and uncertainties include, but are not limited to those risks more fully discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the year ended December 31, 2021, as such description may be amended or updated in any future reports we file with the SEC. Rockwell Medical expressly disclaims any obligation to update our forward-looking statements, except as may be required by law.
IR CONTACT:
Heather R. Hunter
(248) 432-1362
IR@RockwellMed.com
Financial Tables Follow
###
 Exhibit 99.1
Exhibit 99.1
 Exhibit 99.1
Exhibit 99.1
 Exhibit 99.1
Exhibit 99.1 Exhibit 99.1
Exhibit 99.1