UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
| o | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE COMMISSION |
| |
| | | OR |
| |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
| | | For the fiscal year ended: December 31, 2002 |
| |
| | | OR |
| |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission for file number 1-14710
Amersham plc
(Exact Name of Registrant as Specified in its Charter)
England
(Jurisdiction of Incorporation or Organization)
Amersham Place, Little Chalfont, Buckinghamshire, England HP7 9NA
(Address of Principal Executive Offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | | | | |
| | | Name of each |
| Title of each class | | exchange on which registered |
| |
|
Ordinary Shares of 5p each | | New York Stock Exchange |
American Depositary Shares, each representing five Ordinary Shares of 5p each | | New York Stock Exchange |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to section 15(d) of the Act:
None
The number of outstanding shares of each class of stock of Amersham plc as of December 31, 2002 was:
| | | | | |
| Title of Class | | Number of Shares |
| |
|
| Ordinary Shares of 5p each | | | 701,812,083 | |
| American Depositary Shares, each representing five Ordinary Shares of 5p each | | | 2,298,410 | |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports, and (2) has been subject to such filing requirements for the past 90 days.
Yesþ Noo
Indicate by check mark which financial statement item the registrant has elected to follow:
Item 17o Item 18þ
TABLE OF CONTENTS
TABLE OF CONTENTS
| | | | | | | | | | | |
| | | | | | | | | Page |
| | | | | | | | |
|
| | | | | Definitions and Usage | | | 2 | |
| | | | | | | PART I | | | | |
| Item 1 | | Identity of Directors, Senior Management and Advisers | | | 4 | |
| Item 2 | | Offer Statistics and Expected Timetable | | | 4 | |
| Item 3 | | Key Information | | | 4 | |
| Item 4 | | Information on the Company | | | 17 | |
| Item 5 | | Operating and Financial Review and Prospects | | | 62 | |
| Item 6 | | Directors, Senior Management and Employees | | | 92 | |
| Item 7 | | Major Shareholders and Related Party Transactions | | | 107 | |
| Item 8 | | Financial Information | | | 108 | |
| Item 9 | | The Offer and Listing | | | 109 | |
| Item 10 | | Additional Information | | | 113 | |
| Item 11 | | Quantitative and Qualitative Disclosures About Market Risk | | | 116 | |
| Item 12 | | Description of Securities Other than Equity Securities | | | 117 | |
| | | | | | | PART II | | | | |
| Item 13 | | Defaults, Dividend Arrears and Delinquencies | | | 118 | |
| Item 14 | | Material Modifications to the Rights of Security Holders and Use of Proceeds | | | 118 | |
| Item 15 | | Controls and Procedures | | | 118 | |
| Item 16 | | Reserved | | | 118 | |
| | | | | | | PART III | | | | |
| Item 17 | | Financial Statements | | | 119 | |
| Item 18 | | Consolidated Financial Statements | | | 119 | |
| Item 19 | | Exhibits | | | 119 | |
| | | | | Signature | | | | |
| | | | | Certifications | | | | |
DEFINITIONS AND USAGE
Definitions
The following definitions apply throughout this document unless the context requires otherwise:
| | | | | |
| ADR | | - - | | American Depositary Receipt, evidencing American Depositary Shares |
| | | | | |
| ADS | | - - | | American Depositary Share, each representing five Amersham plc Ordinary Shares |
| | | | | |
| Act | | - - | | the UK Companies Act 1985 (as amended) |
| | | | | |
| Amersham International | | - - | | Amersham International plc, a former name of Amersham plc (see also Nycomed Amersham plc) which was changed in October 1997 |
| | | | | |
| Amersham | | - - | | Amersham plc and its consolidated subsidiaries |
| | | | | |
| Amersham Biosciences or the Bioscience business (formerly Amersham Pharmacia Biotech or APBiotech) | | - - | | Amersham Biosciences Limited, a 100% subsidiary of Amersham plc since March 21, 2002 |
| | | | | |
| Amersham Health or the Health business (formerly Nycomed Amersham Imaging or Imaging) | | - - | | Amersham’s medical imaging business |
| | | | | |
| Board | | - - | | the Board of Directors of the Company |
| | | | | |
| Combined Code | | - - | | London Stock Exchange Principles of Good Governance and the provisions of the code of Best Practice issued on June 25, 1998 |
| | | | | |
| Company | | - - | | Amersham plc |
| | | | | |
| EBITDA | | | | earnings before interest, taxation, depreciation and amortization |
| | | | | |
| FDA | | - - | | Food and Drug Administration, a governmental agency in the US |
| | | | | |
| Group | | - - | | Amersham plc and its consolidated subsidiaries (used interchangeably with Amersham including Amersham International and Nycomed Amersham plc) |
| | | | | |
| Noon Buying Rate | | - - | | the noon buying rate in the City of New York for cable transfers in foreign currencies as specified for custom purposes by the Federal Reserve Bank of New York |
| | | | | |
| NMP | | - - | | Nihon Medi-Physics Company Ltd., the issued share capital of which is 50% owned by Amersham plc |
| | | | | |
| Nycomed | | - - | | Nycomed ASA and its consolidated subsidiaries |
| | | | | |
| Nycomed Amersham plc | | - - | | former name of Amersham plc which was changed in July 2001 |
2
| | | | | |
| Ordinary Shares | | - - | | ordinary shares of 5p each in the capital of Amersham plc |
| | | | | |
| Nycomed Pharma or Pharma | | - - | | Amersham’s former therapeutic business |
| | | | | |
Pharmacia & Upjohn Inc
or Pharmacia & Upjohn | | - - | | references to Pharmacia & Upjohn now relate to Pharmacia Corporation, the entity’s name having changed |
| | | | | |
| R&D | | - - | | research and development |
| | | | | |
| UK GAAP | | - - | | UK generally accepted accounting principles |
| | | | | |
| US GAAP | | - - | | US generally accepted accounting principles |
| | | | | |
| VPS | | - - | | Verdipapirsentralen, the Norwegian paperless securities
registration settlement system |
3
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable
ITEM 3. KEY INFORMATION
A. Selected financial data
The results of Amersham plc are presented below for the year to December 31, 2002 together with comparative data for prior periods.
The following selected financial data for the five years ended December 31, 2002, 2001, 2000, 1999 and 1998 and at December 31, 2002, 2001, 2000, 1999, and 1998 should be read in conjunction with the audited UK GAAP Consolidated Financial Statements for the Company included in Item 18 “Consolidated Financial Statements”.
The Consolidated Financial Statements are prepared in accordance with UK GAAP, which differs in certain respects from US GAAP. Certain significant differences between UK GAAP and US GAAP are discussed in Note 38 of Item 18 “Consolidated Financial Statements”, which includes reconciliations of certain amounts calculated in accordance with UK GAAP to US GAAP.
Presentation changes
Certain changes have been made to the presentation of the consolidated financial information to show as discontinued operations the Industrial Quality and Safety Assurance (QSA) business, which we sold on February 27, 1998, and the Nycomed Pharma business, which we sold on May 14, 1999. Where appropriate, comparative figures have been restated so that they are presented on a consistent basis.
Following the introduction of Financial Reporting Standard (FRS) 18 ‘Accounting Policies’ in December 2000, the Group has changed its revenue recognition accounting policy in 2001 with respect to technology transfer agreements. During 2002, we implemented FRS 19, ‘Deferred Taxation’ and now deferred tax liabilities will be recognized for most types of timing differences regardless of whether they are anticipated to reverse in the foreseeable future. Deferred tax assets will be recognized to the extent that it is more likely than not they will be recoverable. The figures for 1998, 1999, 2000 and 2001 have correspondingly been restated.
The consolidated results for the year ended December 31, 2002 and the financial position as at December 31, 2002 shown below in US dollars, have been translated at the year end rate of £1=$1.61.
4
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12 months to December 31 |
| | |
|
| | | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | | 2002 |
| | | (restated) | | (restated) | | (restated) | | (restated) | | | | (unaudited) |
| | | (£m) | | (£m) | | (£m) | | (£m) | | (£m) | | ($m) |
| | |
| |
| |
| |
| |
| |
|
CONSOLIDATED PROFIT AND LOSS DATA | | | | | | | | | | | | | | | | | | | | | | | | |
Amounts in accordance with UK GAAP: | | | | | | | | | | | | | | | | | | | | | | | | |
Turnover(1) | | | 1,320.6 | | | | 1,291.6 | | | | 1,282.8 | | | | 1,515.2 | | | | 1,537.7 | | | | 2,475.7 | |
| Cost of sales | | | (467.0 | ) | | | (471.0 | ) | | | (431.0 | ) | | | (500.1 | ) | | | (481.0 | ) | | | (774.4 | ) |
Gross profit(2) | | | 853.6 | | | | 820.6 | | | | 851.8 | | | | 1,015.1 | | | | 1,056.7 | | | | 1,701.3 | |
Operating profit of continuing operations(1)(2) | | | 221.6 | | | | 250.0 | | | | 241.2 | | | | 292.5 | | | | 309.8 | | | | 498.8 | |
Operating profit from discontinued operations(2) | | | 37.7 | | | | 10.3 | | | | — | | | | — | | | | — | | | | — | |
Exceptional items(3) | | | (35.1 | ) | | | (55.2 | ) | | | (27.0 | ) | | | 46.6 | | | | — | | | | — | |
| Goodwill amortization | | | (2.4 | ) | | | (8.9 | ) | | | (10.7 | ) | | | (11.7 | ) | | | (36.6 | ) | | | (58.9 | ) |
Amounts written off investments(3) | | | — | | | | — | | | | — | | | | (4.4 | ) | | | (2.4 | ) | | | (3.9 | ) |
| Profit before interest and taxation | | | 221.8 | | | | 196.2 | | | | 203.5 | | | | 323.0 | | | | 270.8 | | | | 436.0 | |
| Interest | | | (33.3 | ) | | | (27.1 | ) | | | (10.0 | ) | | | (8.7 | ) | | | (7.1 | ) | | | (11.4 | ) |
| Taxation | | | (75.2 | ) | | | (70.9 | ) | | | (79.9 | ) | | | (96.5 | ) | | | (94.3 | ) | | | (151.8 | ) |
Exceptional item – taxation(3) | | | — | | | | — | | | | — | | | | — | | | | 9.2 | | | | 14.8 | |
| Minority Interest | | | (13.9 | ) | | | (10.3 | ) | | | (6.6 | ) | | | (7.1 | ) | | | 0.1 | | | | 0.1 | |
| Profit attributable to shareholders from continuing operations | | | 84.8 | | | | 83.0 | | | | 107.0 | | | | 210.7 | | | | 178.7 | | | | 287.7 | |
| Profit attributable to shareholders | | | 99.4 | | | | 87.9 | | | | 107.0 | | | | 210.7 | | | | 178.7 | | | | 287.7 | |
Average number of shares(4) | | | 627.5m | | | | 630.5m | | | | 630.9m | | | | 634.4m | | | | 684.7m | | | | 684.7m | |
Earnings per share(4) | | | 15.8p | | | | 13.9p | | | | 17.0p | | | | 33.2p | | | | 26.1p | | | | 42.0c | |
Diluted earnings per share(4) | | | 15.7p | | | | 13.8p | | | | 16.8p | | | | 33.0p | | | | 25.9p | | | | 41.7c | |
Dividends per share(4) | | | 5.30p | | | | 5.85p | | | | 6.40p | | | | 7.10p | | | | 7.80p | | | | 12.6c | |
Amounts in accordance with US GAAP: | | | | | | | | | | | | | | | | | | | | | | | | |
(Loss)/income from continuing operations(5) | | | (3.8 | ) | | | 14.6 | | | | 64.6 | | | | 124.4 | | | | 160.5 | | | | 258.4 | |
| Net (loss)/income | | | (3.8 | ) | | | 14.6 | | | | 64.6 | | | | 124.4 | | | | 160.5 | | | | 258.4 | |
Average number of shares(4) | | | 627.5m | | | | 630.5m | | | | 630.9m | | | | 634.4m | | | | 684.7m | | | | 684.7m | |
Net (loss)/income per share, basic(4)(6) | | | (0.6)p | | | | 2.3p | | | | 10.2p | | | | 19.6p | | | | 23.4p | | | | 37.7c | |
Net (loss)/income per share, diluted(4)(6) | | | (0.6)p | | | | 2.3p | | | | 10.1p | | | | 19.5p | | | | 23.3p | | | | 37.5c | |
The consolidated profit and loss statements have been restated for each of the years ended December 31, 1998, 1999, 2000 and 2001 following the implementation of FRS 19 as explained in Note 37 of Item 18 “Consolidated Financial Statements”.
| 1. | | Figures for the years ended December 31, 1998, 1999 and 2000 have been restated following the introduction of FRS 18. |
| |
| 2. | | Figures for the year ended December 31, 1998 have been restated following the implementation of FRS 12 and to include the results of Nycomed Pharma as a discontinued operation. |
| |
| 3. | | 12 months to December 31, 1998
The 12 months to December 31, 1998 includes £29.8 million of integration costs related to the Pharmacia Biotech and Nycomed mergers, £3.6 million of integration costs related to the acquisition of Molecular Dynamics and £1.7 million of Year 2000 costs. |
| |
| | | 12 months to December 31, 1999
The 12 months to December 31, 1999 include £36.9 million of integration costs related to the Pharmacia Biotech and Nycomed mergers, £4.1 million of integration costs related to Molecular Dynamics, £4.1 million of Year 2000 costs, £4.4 million of write down of investments and intangible assets, a £17.2 million loss on disposal of fixed assets from continuing operations and £11.5 million profit on disposal of Nycomed Pharma. |
| |
| | | 12 months to December 31, 2000
The 12 months to December 31, 2000 includes £9.7 million of costs associated with the disposal of Nycomed Pharma, £10.9 million relating to the rationalization of radiopharmaceutical production in Nycomed Amersham Imaging and £6.4 million relating to the proposed partial flotation of Amersham Biosciences. |
| |
| | | 12 months to December 31, 2001
The 12 months to December 31, 2001 include £3.4 million of costs relating to the proposed partial flotation of Amersham Biosciences, £5.8 million related to the costs of exiting the disease profiling project, £4.4 million of write down of investments and intangible assets, and £8.5 million for the costs of exiting the Harwell site as well as income of £9.0 million relating to the transfer of pension assets from former pension plans. In addition, in September 2001, the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4 million and realized £50.6 million for the outstanding Nycomed Pharma loan notes held by the Group. The Group has recorded a profit on the transaction of £55.3 million after charging costs associated with the disposal, including costs incurred in transferring the Nycomed name to Nycomed Pharma |
5
| | | 12 months to December 31, 2002
As a result of the formation of a consolidated tax group in the US on July 31, 2002, the Group has recognized a deferred taxation asset of £9.2 million primarily related to the future utilization of tax losses arising from prior periods. |
| |
| 4. | | Adjusted for the stock split and bonus issue approved by shareholders on May 15, 1998. Details of the diluted number of shares are found within Note 10 of Item 18 “Consolidated Financial Statements”. |
| |
| 5. | | Includes the results of Nycomed Pharma as a continued operation until September 2001 when we sold the remaining 29% investment. Under US GAAP, the results of Nycomed Pharma are not classified as a discontinued operation in the period to this date (see Note 38 of Item 18 “Consolidated Financial Statements”). |
| |
| 6. | | Calculations of basic and diluted earnings per share under US GAAP for the five years ended December 31, 2002, 2001, 2000, 1999 and 1998 are set out in Note 39 of Item 18 “Consolidated Financial Statements”. |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | At December 31 |
| | |
|
| | | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | | 2002 |
| | | (restated) | | (restated) | | (restated) | | (restated) | | | | (unaudited)) |
| | | (£m) | | (£m) | | (£m) | | (£m) | | (£m) | | ($m) |
| | |
| |
| |
| |
| |
| |
|
CONSOLIDATED BALANCE SHEET DATA | | | | | | | | | | | | | | | | | | | | | | | | |
Amounts in accordance with UK GAAP: | | | | | | | | | | | | | | | | | | | | | | | | |
| Total current assets | | | 788.2 | | | | 708.6 | | | | 654.3 | | | | 753.1 | | | | 673.3 | | | | 1,084.0 | |
| Total assets | | | 1,539.4 | | | | 1,386.1 | | | | 1,431.0 | | | | 1,541.0 | | | | 2,164.7 | | | | 3,485.2 | |
| Current liabilities | | | (389.8 | ) | | | (348.6 | ) | | | (388.1 | ) | | | (483.3 | ) | | | (526.8 | ) | | | (848.1 | ) |
| Non-current liabilities (including grants) | | | (671.9 | ) | | | (412.9 | ) | | | (373.7 | ) | | | (158.0 | ) | | | (207.5 | ) | | | (334.1 | ) |
| Provisions | | | (213.5 | ) | | | (199.3 | ) | | | (190.6 | ) | | | (240.8 | ) | | | (253.5 | ) | | | (408.1 | ) |
| Equity shareholders’ funds | | | 215.1 | | | | 363.7 | | | | 416.9 | | | | 596.3 | | | | 1,172.0 | | | | 1,886.9 | |
| Minority interests | | | 49.1 | | | | 61.6 | | | | 61.7 | | | | 62.6 | | | | 4.9 | | | | 7.9 | |
Amounts in accordance with US GAAP: | | | | | | | | | | | | | | | | | | | | | | | | |
| Goodwill and identifiable intangible assets | | | 1,696.5 | | | | 1,442.3 | | | | 1,316.4 | | | | 1,175.2 | | | | 1,738.4 | | | | 2,798.9 | |
| Total assets | | | 3,162.2 | | | | 2,762.2 | | | | 2,690.6 | | | | 2,561.6 | | | | 3,184.5 | | | | 5,127.0 | |
| Minority interest | | | 155.1 | | | | 158.0 | | | | (146.9 | ) | | | (138.2 | ) | | | (4.9 | ) | | | (7.9 | ) |
| Shareholders’ equity | | | 1,618.4 | | | | 1,547.5 | | | | 1,549.7 | | | | 1,627.0 | | | | 2,140.2 | | | | 3,445.7 | |
The consolidated balance sheets have been restated following the implementation of FRS 19 as explained in Note 37 of Item 18 “Consolidated Financial Statements”. Also, the consolidated balance sheets at December 31, 1998, 1999 and 2000 had previously been restated for the introduction of FRS 18 “Accounting Policies” as explained in Note 38 of Item 18 “Consolidated Financial Statements” contained in the Annual Report on Form 20-F filed with the Securities and Exchange Commission on June 26, 2002. Further, the consolidated balance sheet at December 31, 1998 had previously been restated for the introduction of FRS 12 (Provisions, contingent liabilities and contingent assets), as explained in note 3 of Item 18 “Consolidated Financial Statements” contained in the Annual Report on Form 20-F filed with the SEC in June 2000.
Dividends
The Company has paid dividends on its Ordinary Shares in respect of each fiscal year since 1982. If a dividend is declared with respect to shares of any class, the same dividend must be declared with respect to all shares of that class.
The following table sets forth the aggregate amounts of dividends (net of tax credits) paid in respect of each fiscal period indicated and translated, solely for convenience, into US dollars per share at the Noon Buying Rate on each of the respective payment dates for such dividends:
| | | | | | | | | | | | | |
| | | Pence per | | Translated into US | | Translated into US |
| | | share | | dollars per share | | dollars per ADS |
| Period ended | | Total | | Total | | Total |
| |
| |
| |
|
December 31, 1998(1) | | | 5.30 | | | | 0.08818 | | | | 0.4409 | |
| December 31, 1999 | | | 5.85 | | | | 0.09448 | | | | 0.4724 | |
| December 31, 2000 | | | 6.40 | | | | 0.09536 | | | | 0.4768 | |
| December 31, 2001 | | | 7.10 | | | | 0.10295 | | | | 0.5148 | |
December 31, 2002(2) | | | 7.80 | | | | 0.12558 | | | | 0.6279 | |
| (1) | | Following the share split and bonus issue approved by shareholders on May 15, 1998. |
| |
| (2) | | Includes proposed final dividend of 5.15 pence per share ($0.082915) |
6
Following the ratification of the new US/UK Double Tax Treaty (the New Treaty) on 31 March 2003, a holder of Ordinary Shares or ADRs who is a resident of the United States for the purposes of the New Treaty and who receives a dividend on or after 1 May 2003 will no longer be entitled to receive a payment in respect of a UK tax credit, unless certain elections are made (see “Item 10 — E. Taxation — Taxation of dividends”).
The Company expects to continue to pay dividends. The Company is not currently subject to any laws or agreements that restrict materially its ability to pay dividends. The declaration and payment of any future dividends depends upon our income, availability of sufficient distributable profit, financial condition and cash flow, as well as other factors affecting the Company.
Exchange Rates
The following table sets forth, for the periods and dates indicated, certain information concerning the exchange rate for pounds sterling expressed in US dollars per pound sterling, for cable transfers in foreign currencies as certified by the Federal Reserve Bank of New York based on the Noon Buying Rate in the City of New York.
No representation is made that amounts in pounds sterling have been, could have been or could be converted into US dollars at that rate or at any other rate.
| | | | | | | | | | | | | | | | | |
| Year ended December 31 | | Period End(1) | | Average(2) | | High(3) | | Low(3) |
| |
| |
| |
| |
|
| | | US Dollars per £1 |
| 1998 | | | 1.66 | | | | 1.66 | | | | 1.70 | | | | 1.63 | |
| 1999 | | | 1.62 | | | | 1.62 | | | | 1.65 | | | | 1.58 | |
| 2000 | | | 1.49 | | | | 1.51 | | | | 1.62 | | | | 1.42 | |
| 2001 | | | 1.45 | | | | 1.44 | | | | 1.47 | | | | 1.40 | |
| 2002 | | | 1.61 | | | | 1.51 | | | | 1.61 | | | | 1.40 | |
| (1) | | The period end rate at December 31 |
| |
| (2) | | The average of the rates on the last day of each month during the calendar year. |
| |
| (3) | | The high and low rates during the 12-month period ended December 31. |
The following table sets forth, for the periods and dates indicated, certain information concerning the exchange rate for pounds sterling, expressed in US dollars per pound sterling, for the high and low exchange rates each month, for the previous six completed months.
| | | | | | | | | | | | | |
| | | Period End(1) | | High(2) | | Low(2) |
| | |
| |
| |
|
| | | US Dollars per £1 |
| November 2002 | | | 1.55 | | | | 1.60 | | | | 1.54 | |
| December 2002 | | | 1.61 | | | | 1.61 | | | | 1.54 | |
| January 2003 | | | 1.65 | | | | 1.66 | | | | 1.59 | |
| February 2003 | | | 1.58 | | | | 1.65 | | | | 1.57 | |
| March 2003 | | | 1.58 | | | | 1.62 | | | | 1.55 | |
| April 2003 | | | 1.60 | | | | 1.60 | | | | 1.55 | |
| (1) | | The period end rate as per each calendar month end. |
| |
| (2) | | The high and low rates during the calendar month. |
The Noon Buying Rate on April 16, 2003 was $1.57 = £1.
B. Capitalization and indebtedness
Not applicable
C. Reasons for the offer and use of proceeds
Not applicable
7
D. Risk factors
Described below are some of the risks affecting our business generally and our two principal business segments. These are not intended to be a fully comprehensive list but rather a concise listing of those major risks which are considered to be material to Amersham plc. In addition, profits are an economic reward for taking risk and other, different risks may rise in profile in the future. Any of the following risks may materially and adversely affect our business, financial condition or results of operations.
1. General risks affecting Amersham
Foreign exchange fluctuations may adversely affect our reported earnings and the value of our assets.
We record our transactions and prepare our financial statements in pounds sterling, but a significant portion of our earnings and expenditures are in other currencies. Changes in exchange rates between the pound sterling and these other currencies can result in increases or decreases in our costs and revenues. Fluctuations in exchange rates between the pound sterling and other currencies may also affect the book value of our assets outside the UK and the amount of shareholders’ equity. We seek to minimize our currency exposure by engaging in hedging of transactional exposures, where we deem it appropriate. To mitigate some of these risks, we have hedged certain assets and liabilities held in foreign currency and anticipated future foreign currency cash flows for 2003. We cannot predict, however, all changes in currency and interest rates, inflation or other factors which could affect our international businesses.
Our operations must comply with environmental statutes and regulations, and any failure to comply could result in extensive costs which would harm our business.
The manufacture of some of our products involves the use, transportation, storage and disposal of hazardous or toxic materials and is subject to various environmental protection and occupational health and safety laws and regulations in the countries in which we operate. This has exposed us in the past, and could expose us in the future, to risks of accidental contamination and events of non-compliance with environmental laws which could result in regulatory enforcement or personal injury and property damage claims or could lead to a shutdown of some of our operations, which could have an adverse effect on our business and results of operations. We currently incur costs to comply with environmental laws and regulations and these costs may become more significant.
The environmental laws of many jurisdictions impose actual and potential obligations on us to remediate contaminated sites. These obligations may relate to sites:
| • | | that we currently own or operate; |
| |
| • | | that we formerly owned or operated; or |
| |
| • | | where waste from our operations was disposed. |
These environmental remediation obligations could reduce our operating results. In particular, our accruals for these obligations may be insufficient if the assumptions underlying the accruals prove incorrect or if we are held responsible for additional, currently undiscovered contamination.
Stricter environmental, safety and health laws and enforcement policies could result in substantial costs and liabilities to us, and could subject our handling, manufacture, use, reuse or disposal of substances or pollutants to more rigorous scrutiny than is currently the case. Consequently, compliance with these laws could result in capital expenditures as well as other costs and liabilities, thereby harming our business and operating results.
If our business continuity plans are inadequate, a major production or supply chain interruption could result in a loss of customers and market share.
Recent world events have made it apparent that the possibility exists for major events to occur that could disrupt or interrupt production or delivery of goods and services to customers and / or
8
from vendors. In addition, our business is dependent on key facilities and employees for production, which could be significantly disrupted by a catastrophic event. In addition, we are dependent upon some sole supply arrangements. We currently have business continuity plans in place to react to these types of events. However, it is possible that these plans are inadequate to fully insulate our business from their impact. Therefore, we may be exposed to the loss of sales, market share and customers if our business continuity plans are unable to address a particular production or supply chain interruption.
If we pursue acquisitions in the future, we may absorb significant resources or be unsuccessful.
As part of the strategy of our business, we may pursue acquisitions, investments and/or other alliances. Acquisitions may involve significant cash expenditures, debt incurrence, additional operating losses and expenses that could have a material adverse effect on the operating results of the business.
It may be difficult for us to complete such transactions quickly and to integrate acquired businesses efficiently into the current business. Future acquisitions could expose us to unforeseen liabilities and result in significant charges relating to intangible assets. Sizable acquisitions may also divert senior management from focusing on our existing businesses.
In addition, if any transaction involves customer bases or businesses located outside the United Kingdom, the transaction may be further complicated by the need to consider differing tax structures, the effects of currency fluctuations on the reported results of the business and compliance with foreign laws and regulations. The failure to properly identify and address these and other risks could limit our ability to realize the anticipated benefits of an acquisition.
If our businesses cannot attract, employ and retain the highly skilled staff necessary to develop, market and support our technologically advanced products, we may be unable to generate revenue growth and maintain market share.
Technologies in our industry have undergone, and are expected to continue to undergo, rapid and significant change. Highly skilled staff are integral to developing, marketing and supporting new products that will meet or exceed the expectations of the marketplace and achieve market acceptance. Without these highly skilled staff, our businesses may be unable to maintain or grow market share, which could result in lower than expected revenues and earnings.
2. Amersham Health
Risks relating to our Health business
Our Health business faces competition from new products and from generic products.
All products within the patented portfolio of the Health business face competition from competitors’ proprietary products. This competition may increase as new products enter the market. The business also faces competition from generic products after patents on its products, or the patents on a competitor’s products, expire. Loss of patent protection may lead to loss of sales in the products’ markets and could affect future income. Patent protection is not available in major markets for a number of the Health business’s leading products, notably Omnipaque™ and OncoSeed™, both of which already face generic competition.
As new competitive products enter the market, they may be more effective or more effectively marketed and sold than Amersham Health’s products. If our Health business fails to maintain its competitive position, this could have a material adverse effect on the segment’s results. Information on patents held and their expiry dates is provided within Item 4 “Information on the Company”.
9
Research and development efforts in our Health business may not succeed or our competitors may develop more effective or successful products.
In order to remain competitive, we commit substantial resources each year to research and development (R&D) through both our own dedicated resources and various collaborations with third parties. Amersham Health’s ongoing investment in new product launches and R&D for future products could produce higher costs without a proportional increase in revenues.
In the pharmaceutical diagnostics business, the R&D process can take up to 12 years from discovery to commercial product launch. This process is conducted in various stages, and during each stage there is a substantial risk that we will not achieve our goals and accordingly may abandon a product in which we have invested substantial amounts. If Amersham Health fails to continue developing commercially successful products, or if competitors develop more effective products or a greater number of successful new products, this could have a material adverse effect on the results of the business.
Amersham Health operates in a highly competitive industry.
The principal competitors of our Health business are major international corporations with substantial resources for R&D, production and marketing. In addition, a significant proportion of Amersham Health’s US business is through contracts with large Group Purchasing Organizations (GPOs). Periodically these contracts come up for renewal and/or are subject to competitive tender. In addition, the size of certain hospital accounts means that these are individually significant to our business. Loss of a significant GPO or significant hospital contract to a competitor would have an adverse effect on the income stream of the business, which would adversely affect our results.
Product liability claims could adversely affect the business results of Amersham Health.
Product liability is a potential risk for the business. Substantial damages awards have been made in some jurisdictions against healthcare companies based upon claims for injuries allegedly caused by the use of their products. Although we mitigate an amount of this risk through insurance, there can be no assurance that a future product liability claim or series of claims brought against us would not have an adverse effect on our Health business or the results of operations.
Our Health business depends on the ongoing availability of materials and assets to continue manufacturing our products and an interruption to this availability could adversely affect our business and results.
The products marketed, distributed and sold by our Health business are either manufactured at our dedicated manufacturing facilities, through toll manufacturing arrangements, or through supply agreements with third parties. In as much as many of its products are chemically based and are the result of technically complex manufacturing processes, we can provide no assurances that supply will not be interrupted due to a number of different factors and causes, including the possibility of regulatory intervention.
Risks relating to the industry in which Amersham Health operates
There is a possibility that products, waste material or related medical equipment owned or supplied by the Company could be subject to malevolent misuse.
Although Amersham Health’s nuclear interests are mainly in short lived isotopes for medical uses, there is a possibility that products, waste material or related medical equipment owned or supplied by the Company could be subject to malevolent misuse. Such misuse of our products or of another company’s similar products, waste or equipment could result in restrictions being placed upon Amersham’s products or sources of supply during production, storage, transportation, distribution to customers or subsequent usage. Such restrictions could have a material impact on the business’ sales which could materially impact its financial results.
10
Price controls can limit revenues and adversely affect the results of our Health business.
In addition to normal price competition in the market place, the prices of our Health business’s products are affected by price controls imposed by governments and healthcare providers in many countries. Price controls operate differently in different countries and can cause wide variations in prices between markets. Currency fluctuations can aggravate these differences. The existence of price controls can limit the revenues earned from our Health products and may have an adverse effect on our business and results of operations.
There is the potential in any country to which Amersham Health sells that a government could use its purchasing power to demand price reductions or discounts from suppliers thereby reducing revenue earning opportunities for suppliers. Many governments have implemented or are introducing healthcare reforms in an attempt to curb increasing healthcare costs. In some markets, governmental price-cut rounds can occur regularly. In response to rising healthcare costs, many governments and private medical care providers, such as Health Maintenance Organizations (HMOs) have instituted reimbursement schemes that favor the substitution of generic products for more expensive brand-names. There can be no guarantee that current price levels will remain at today’s level in any market the business serves.
Product regulation may adversely affect the ability of Amersham Health to bring new products to market or to continue to supply existing products to the market.
We, like our competitors, are subject to strict government controls on the development, manufacture, labeling, distribution and marketing of products. We must obtain and maintain regulatory approval for our products from regulatory agencies before products may be sold in a particular jurisdiction. The submission of an application to a regulatory authority does not guarantee that a license to market the product will be granted. Each authority may impose its own requirements and delay or refuse to grant approval, even though a product has been approved in another country.
In the principal markets of our Health business, the approval process for a new product is complex, lengthy and expensive. The time taken to obtain approval varies by country but generally takes from six months to several years from the date of application. Regulatory delays, the inability to successfully complete clinical trials, claims and concerns about safety and efficacy, new discoveries, patents and products of competitors and related patent disputes and claims about adverse side effects are only a few of the factors that could adversely affect the realization of product registration. These factors increase both the cost and the risk that Amersham Health will not succeed in selling new products successfully.
In addition, like our competitors, our pharmaceutical and medical device production activities are required to meet the standards of Good Manufacturing Practice (GMP) specified by the national regulatory authorities of those countries into which our products are supplied. Failure to maintain compliance may result in expensive remedial activity, substantial fines, the detention of imports to markets or withdrawal of the approval of a product New Drug Application or Marketing Authorization.
3. Amersham Biosciences
Risks related to our Biosciences business
If our Biosciences business does not keep up with rapid technological change or continue to introduce new products, we may be unable to maintain market share or to recover investments we make in our technologies.
Technologies in the life sciences industry have undergone, and are expected to continue to undergo, rapid and significant change. Amersham Biosciences may not be able to keep pace with the rapid rate of change and introduce new products that will adequately meet the requirements of the marketplace or achieve market acceptance. If we fail to introduce new and innovative products, we could lose market share to our competitors and experience a reduction in our growth rate and damage to our reputation and business.
11
The future success of our Biosciences business will depend in large part on our ability to maintain a competitive position with respect to these technologies. We believe that successful new product introductions provide a significant competitive advantage because customers make an investment of time in selecting and learning to use a new product, and are reluctant to switch to a competing product after making their initial selection. However, our Biosciences business or others may make rapid technological developments, which could result in our technologies, products or services becoming obsolete before we are able to recover the expenses incurred to develop them.
If our Biosciences business cannot enter into new strategic alliances or licensing agreements or maintain the ones that we have, we may be unable to develop and commercialize our technologies into new products and services or continue to commercialize existing products or services.
Our strategy of providing products to researchers working on a variety of life sciences projects requires us to continually develop a wide spectrum of products. To generate broad product lines, it is advantageous to license technologies from others and to enter into strategic alliances in addition to our own product development efforts. As a result, the ability of our Biosciences business to obtain new technologies from third parties, is, and will continue to be for the foreseeable future, critical to our ability to develop and offer new products to further our business. Under our current strategy, and for the foreseeable future, we will remain dependent on licensing agreements and strategic alliances to further our business.
Amersham Biosciences may be unable to maintain or expand existing strategic alliances or establish additional alliances or licensing arrangements necessary to continue to develop and commercialize products, and any of those arrangements may not be on terms favorable to the business. In addition, current or any future arrangements may be unsuccessful. If our Biosciences business is unable to obtain or maintain any third party license required to sell or develop our products or product enhancements, we may choose to obtain substitute technology either through licensing from another third party or by developing the necessary technology ourselves. Any substitute technology may be of lower quality or may involve increased cost, either of which could adversely affect the ability of our Biosciences business to provide our products competitively and harm our business.
Amersham Biosciences also depends on collaborators for the development and manufacture of complex instrument systems and chemicals and other materials that are used in laboratory experiments and for development of software packages for analyzing data produced by these instrument systems. We cannot control the amount and timing of resources our collaborators devote to our products. We may not be able to enter into or satisfactorily retain these research, development and manufacturing collaborations and licensing agreements, which could reduce our growth and harm our competitive position. For a discussion of existing collaborations you should read Item 4 “Information on the Company — Business overview — Amersham Biosciences”.
If our Biosciences business does not successfully distinguish our products and services from those of our competitors, we may be unable to compete successfully to generate revenue growth.
Competition in the life sciences industry is intense, and we expect it to increase. Rapid technological change and frequent new product introductions characterize the markets served by the segment. We compete with a variety of businesses that offer or are developing products and services that are substantially similar to our products and services. Many of the businesses competing with Amersham Biosciences have significant financial and other resources to invest in new technologies, substantial intellectual property portfolios, substantial experience in new product development, regulatory expertise, manufacturing capabilities and the distribution channels to deliver products to customers. An inability to continue developing products, which distinguish us from our primary competitors, could limit our ability to generate revenue growth.
12
If Biosciences manufacturing were disrupted, we may be unable to supply products to our customers and achieve expected revenues.
Amersham Biosciences has an established global manufacturing base with six primary facilities in Piscataway, New Jersey, San Francisco and Sunnyvale, California in the US, Uppsala and Umea in Sweden, and Cardiff in the United Kingdom. If any of these facilities, particularly the Uppsala chemical production site, were disrupted, the Biosciences business may not be able to deliver expected revenues or earnings. Although Amersham Biosciences has implemented safety measures and prepared contingency plans to mitigate this risk, it is uncertain whether these steps will successfully address the risks that will arise if manufacturing is disrupted.
Operating results may fluctuate from period to period.
Our Biosciences business’ operating results may vary from period to period due to numerous factors, many of which are outside our control, including the number, timing and market acceptance of new products the business or our competitors introduce. In addition, the business is subject to seasonal fluctuations due to calendar year budgeting for large capital items by customers, resulting generally in lower levels of sales in the first and third quarters of each year and higher levels of sales in the second and fourth quarters. Factors that may cause the business’ results to vary by period include:
| • | | the volume and timing of orders for its products and services; |
| |
| • | | changes in the mix of its products and services; |
| |
| • | | the number, timing and significance of new products and services introduced by its competitors; |
| |
| • | | the ability of the business to develop, market and introduce new and enhanced products and services on a timely basis; |
| |
| • | | changes in the cost, quality and availability of reagents and components required to manufacture or use its products; |
| |
| • | | the timing and costs of any acquisitions of businesses or technologies; |
| |
| • | | the extent, nature and speed of restructuring may differ from that forecast, yielding different levels of cost and or savings to those expected; |
| |
| • | | the availability of commercial and government funding to customers of Amersham Biosciences’ products and services; |
| |
| • | | reduced capital investment by significant pharmaceutical companies for extended periods subsequent to mergers. |
R&D costs associated with Amersham Biosciences’ technologies, products and services, as well as personnel costs, marketing programs and overhead account for a substantial portion of its operating expenses. These expenses cannot be adjusted quickly in the short term. If revenues of the business decline or do not grow as anticipated, Amersham Biosciences may not be able to reduce its operating expenses accordingly. Failure to achieve anticipated levels of revenue could therefore significantly harm our operating results for a particular period.
Risks related to the Life Sciences industry
Use of genomics and proteomics information to develop or commercialize products is unproven.
The development of new drugs and the diagnosis of disease based on genomic or proteomic information is unproven. Few therapeutic or diagnostic products based on genomic or proteomic
13
discoveries have been developed and commercialized. If our customers are unsuccessful in developing and commercializing products based on our products or services, our customers and we may be unable to generate sufficient revenues and our Biosciences business may suffer as a result. The development of genomics or proteomics-based products is subject to risks of failure, in that such products:
| • | | may be found to be toxic; |
| |
| • | | may be ineffective; |
| |
| • | | may fail to receive regulatory approvals; |
| |
| • | | may fail to be developed prior to the successful marketing of similar products by competitors; or |
| |
| • | | may infringe on proprietary rights of third parties. |
If ethical and other concerns surrounding the use of genetic information become widespread, there may be less demand for some of the key products of Amersham Biosciences.
Genetic testing has raised ethical issues regarding confidentiality and the appropriate use of the resulting information. For these reasons, governmental authorities may call for limits on, or regulation of, the use of genetic testing, or they may prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. The development of products based on the use of genetic information represents a significant market opportunity for our discovery systems business. Any of the scenarios mentioned above could reduce the potential market for our products.
Reductions in R&D budgets and capital spending policies of our customers may reduce our Biosciences business’ sales. Reductions in government funding of Biosciences research may also reduce demand for Amersham Biosciences’ products.
A significant portion of our Biosciences products, such as our DNA sequencing systems or our bioprocess systems are capital purchases for our customers, which include pharmaceutical, biotechnology and agrochemical companies, research institutes, universities and medical research centers. Fluctuations in the R&D budgets or capital spending policies of these organizations could have a significant effect on the demand for our Biosciences products. R&D budgets and capital spending policies fluctuate due to changes in available resources, spending priorities and institutional budgetary policies. Any significant decrease in life sciences R&D expenditures by our customers could seriously damage our business.
A significant portion of Amersham Biosciences’ sales are to researchers, universities, government laboratories and private foundations whose funding is dependent upon both the level and timing of funding from government sources. As a result, the timing and amount of revenues from these sources may vary significantly due to factors that can be difficult to foresee. Although the level of research funding has increased during the past several years, grants have, in the past, been frozen for extended periods or have otherwise become unavailable to various institutions without advance notice. Government funding of R&D is subject to the political process, which is inherently unpredictable. Also, government proposals aiming to reduce or eliminate budgetary deficits have sometimes included reduced allocations to the U.S. National Institute of Health (“NIH”) and other government agencies that fund R&D activities. If researchers were not able to obtain, for any extended period, government funding necessary to purchase our products or if there is a decrease in overall research funding, it could reduce the sales to this segment and damage our business.
14
Risks related to intellectual property held by Amersham Biosciences
The scope of our issued patents may not provide us with adequate protection of our intellectual property, which would harm our competitive position. The measures that we rely upon in addition to patents to protect our intellectual property may not be adequate to protect our products and services and could affect our Biosciences business’ ability to compete.
Any issued patents that cover Amersham Biosciences proprietary technologies may not provide us with substantial protection or be commercially beneficial to the business. The issuance of a patent is not conclusive as to its validity or its enforceability. The US federal courts or equivalent national courts or patent offices elsewhere may invalidate these patents or find them unenforceable. Competitors may also be able to design around our patents. If we are unable to protect our patented technologies, we may not be able to commercialize our technologies, products or services and our competitors could commercialize our technologies.
Our Biosciences business also relies on a combination of trade secrets, copyrights and trademarks, non-disclosure agreements and other contractual provisions and technical measures to protect our intellectual property rights. While we generally require employees, collaborators, consultants and other third parties to enter into confidentiality agreements where appropriate, it is not always possible to enforce these arrangements.
Monitoring the unauthorized use of our technology is difficult, and the steps we have taken may not prevent unauthorized use of our technology. The disclosure or misappropriation of our intellectual property for any of the above reasons could harm our ability to protect our rights and our competitive position.
We may become involved in disputes regarding our patents and other intellectual property rights, which could result in the forfeiture of these rights, expose the business to significant liability and divert management’s focus.
In order to protect or enforce our patent rights, our Biosciences business may need to initiate patent litigation against third parties. In addition, we have been and may in the future be sued by third parties alleging that we are infringing their intellectual property rights. These lawsuits are expensive, take significant time and divert management’s focus from other business concerns. These lawsuits could result in the invalidation or limitation of the scope of Amersham Biosciences’ patents, forfeiture of the rights associated with these patents or an injunction preventing Amersham Biosciences from selling any allegedly infringing product. In addition, Amersham Biosciences may not prevail or a court may find damages or award other remedies in favor of the opposing party in any of these suits. During the course of these suits, there may be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. Securities analysts or investors may perceive these announcements to be negative, which could cause the market price of the Group’s stock to decline.
Many of Amersham Biosciences’ products are based on complex, rapidly developing technologies. Although Amersham Biosciences tries to identify all relevant third party patents, these products could be developed by the business without knowledge of published or unpublished patent applications that cover some aspect of these technologies. The life sciences industry has experienced intensive enforcement of intellectual property rights by litigation and licensing. If we are found to be infringing the intellectual property of others, we could be required to stop the infringing activity, or we may be required to design around or license the intellectual property in question. If we are unable to obtain a required license on acceptable terms, or are unable to design around any third party patent, we may be unable to sell some of our products and services, which could result in reduced revenue.
4. Forward Looking Information
The Private Securities Litigation Reform Act of 1995 provides for a safe harbor for forward-looking statements. This Annual Report contains words such as “believe”, “expect” and “anticipate” and
15
similar expressions, that identify forward-looking statements, which reflect the Group’s views about future events and financial performance. Such forward-looking statements relate to, among other things, the outlook of Amersham plc, including in particular, the expected growth of certain of the Group’s products or the markets for those products, the impact of currency movements during 2003 and the Group’s expectations regarding the development of its business, particularly its biosciences business and the fields of genomics and proteomics. Such statements include but are not limited to statements under “Item 3. Key Information — D. Risk Factors”, “Item 5. Operating and Financial Review and Prospects” and “Item 11. Quantitative and Qualitative Disclosures about Market Risk”. Actual results could differ materially from those projected in such forward-looking statements as a result of various factors that may be beyond the control of the Group.
The following important factors could cause actual results to differ materially from those projected or implied in any forward-looking statements:
| i. | | Changes in demand for the products of Amersham plc worldwide or in the markets for those products as well as changes in management’s expectation of the development of new markets and the timing of completion of various trials leading to the introduction of new products; |
| |
| ii. | | Changes in the cost or supply of raw materials, changes in interest rates and the impact of competition; and |
| |
| iii. | | Price controls and price reductions, fluctuations in exchange rates for foreign currencies, changes in governmental regulation, and the risk of loss of patents or trademarks. |
Accordingly readers are cautioned not to place undue reliance on these forward-looking statements. In any event, these statements speak only as of the date on which they are made, and the Company does not undertake any obligation to update or revise any of them, whether as a result of new information, future events or otherwise.
16
ITEM 4. INFORMATION ON THE COMPANY
A. History and development of the company
Amersham plc is a public company with limited liability organized under the laws of and registered in England and Wales. Amersham plc and its subsidiary undertakings constitute an international group (the “Group”) engaged in the R&D, production, sale, distribution and licensing of medical imaging contrast media and radiopharmaceuticals through its Amersham Health business. The Group is also engaged in R&D, manufacture and sale of specialized products and services for life sciences research, and the manufacture of biopharmaceuticals through its Amersham Biosciences business.
Background
Amersham International was incorporated in the UK on February 16, 1971. Following privatization in 1982, Amersham International successfully developed its healthcare and life sciences business by means of organic growth and by a series of strategic acquisitions and alliances. Amersham International became a world leader in the field of nuclear medicine with a range of therapeutic and diagnostic products, and was at the forefront of the development of innovative radioactive and non-radioactive products for use in life sciences research.
During 1997, Amersham International entered into two mergers in order to enhance its position in the fields ofin vivodiagnostic imaging and life sciences.
On August 5, 1997, Amersham International and Pharmacia Biotech, the Sweden-based biotechnology supply business of Pharmacia Corporation, transferred their life sciences businesses to a new company, Amersham Pharmacia Biotech Limited. In 2001, the name of Amersham Pharmacia Biotech was changed to Amersham Biosciences. At December 31, 2001, Amersham Biosciences was owned 55% by Amersham plc and 45% by Pharmacia Corporation. On March 21, 2002, the Company purchased the 45% ownership stake in Amersham Biosciences held by Pharmacia Corporation, thereby taking 100% ownership of Amersham Biosciences. The cash consideration for the purchase was £704 million and was financed by the issue of new share capital plus the use of existing cash resources and drawings under Amersham’s committed bank facilities.
On October 21, 1997, Amersham International merged with Nycomed ASA. The healthcare business of Amersham International and the medical imaging business of Nycomed were consolidated into the Group’s imaging business. Following this merger, the Company changed its name to Nycomed Amersham plc on October 22, 1997.
On July 12, 2001, the Company changed its name from Nycomed Amersham plc to Amersham plc. On October 15, 2001 the Company announced its two business groupings were changing their names to Amersham Biosciences and Amersham Health.
Recent Developments
On February 26, 2003, the Group announced a restructuring of its discovery systems business area. The restructuring program, which is intended to deliver a more efficient manufacturing cost base and focus R&D on fewer sites, will result in the net loss of approximately 400 jobs. The Group will incur one-off costs in the range of £45-50 million, which are expected to result in savings running at the rate of £30-35 million per annum by the end of 2004. The restructuring plans are expected to bring benefits in 2003 moving the business to profitability during 2004.
On April 22, 2003 we announced an intended merger of our brachytherapy business with the urology business of Galil Medical to create a stand-alone marketing company. The combined sales of both businesses would have been approximately £57 million ($90 million) in 2002. The new company, which is 75% owned by Amersham, will be accounted for as a joint venture.
17
On April 30, 2003 we reported sales of £381 million for the first three months of 2003 (2002: £381 million), up 6% at constant exchange and excluding discontinued products. We confirmed that our outlook for the year is unchanged and that the restructuring of our discovery systems business area is on track to deliver profitability during 2004.
The full text of our press release can be found on our internet site www.amersham.com
Registered Address
The registered address of the Company is:
Amersham plc
Amersham Place
Little Chalfont
Buckinghamshire
HP7 9NA
England
Tel: +44 1494 544000
Internet address: www.amersham.com
None of the information contained in our website is incorporated in this Annual Report.
Acquisitions and Dispositions
As part of its strategy to expand its operations, the Group has made the following significant business acquisitions since January 1, 1993:
| | | | | | | | | |
| | | Principal Market | | Date Acquisition | | Purchase Price* |
| Acquired Company | | Areas | | Completed | | (In £millions) |
| |
| |
| |
|
| United States Biochemical Corporation | | US | | April 1993 | | | 43.9 | |
| Nihon Medi-Physics (NMP) | | Japan | | December 1994,
October 1996 | | | 110.3 | ** |
| Pharmacia Biotech AB | | Europe/US/Japan | | August 1997 | | | 39.9 | *** |
| Nycomed ASA | | North Europe/Europe/US/Japan | | October 1997 | | | 1,595.7 | |
| Molecular Dynamics Inc. | | US | | September 1998 | | | 125.1 | |
| AG Technology Corp and InnovaSep Technology Corporation | | US | | January 2002 | | | 31 to 42 | **** |
Pharmacia Biosystems AB/Amersham
Biosciences Limited | | Europe/US/Japan | | March 2002 | | | 704.1 | ***** |
| Cimarron Software, Inc. | | US | | May 2002 | | | 4.0 | ****** |
| CodeLink™ | | US | | July 2002 | | | 14.0 | |
| * | | Represents the purchase price of equity. |
| |
| ** | | Represents 50% ownership in NMP. |
| |
| *** | | Represents acquired assets. |
| |
| **** | | For more detail on how the purchase price will be calculated, see Note 24(b) of Item 18 “Consolidated Financial Statements”. |
| |
| ***** | | Represents the purchase of the remaining 45% of Amersham Biosciences from Pharmacia Corporation. |
| |
| ****** | | Represents the acquisition of a further 16% holding, taking the Group’s total holding to 35%. The Group has a controlling stake as a result of a shareholders’ agreement. Since May 10, 2002, the Group had subscribed for and was allotted additional share capital, taking its holding at December 31, 2002 to 44%. See Note 24(b) to Item 18 “Consolidated Financial Statements”. |
18
United States Biochemical Corporation
In April 1993, the Company acquired 100% of the shares of United States Biochemical Corporation (“USB”), based in Ohio, US. The company was a major supplier of reagents, biochemicals, intermediates and related services to the North American life sciences industry and strengthened Amersham’s position as one of the leading suppliers of products in this area.
Nihon Medi-Physics
In December 1994, the Company acquired a 20% stake in Nihon Medi-Physics Company Ltd (“NMP”). On April 24, 1996, the Company entered into an agreement to increase its shareholding in NMP from 20% to 50%. Pursuant to this agreement, the Company’s Japanese healthcare business merged with NMP on August 31, 1996, increasing the Company’s interest to 33.95%. The transaction was completed on October 18, 1996 when the Company increased its interest in NMP to 50%.
Pharmacia Biotech AB
On August 5, 1997, Amersham International and Pharmacia Biotech, the Sweden-based biotechnology supply business of Pharmacia Corporation, transferred their life sciences businesses to a new company, Amersham Pharmacia Biotech Limited. In 2001, the name of Amersham Pharmacia Biotech was changed to Amersham Biosciences. At December 31, 2001, Amersham Biosciences was owned 55% by the Company and 45% by Pharmacia Corporation.
On March 21, 2002, the Company purchased the 45% ownership stake in Amersham Biosciences held by Pharmacia Corporation through the acquisition of Pharmacia Biosystems AB which held 39% and the direct acquisition of the remaining 6% thereby taking 100% ownership of Amersham Biosciences.
Nycomed ASA
On October 21, 1997, Amersham International merged with Nycomed ASA. The healthcare business of Amersham International and the medical imaging business of Nycomed were consolidated into the Group’s imaging business. The purchase consideration was satisfied by the issue of 37.1 million 25p ordinary shares and 25.4 million 25p non-voting shares at £24.2 and £23.4 per share respectively, £78.5m in cash under the Mandatory Cash offer and future cash consideration of £23.9m in respect of the compulsory purchase which was fully paid by December 31, 1999.
Molecular Dynamics Inc
On September 11, 1998, Amersham Pharmacia Biotech acquired, following a cash tender offer, the 91% of Molecular Dynamics shares it did not already own. The net cash paid during the year in respect of this acquisition was £125.1 million. The acquisition further strengthened Amersham Pharmacia Biotech’s competitive position in the market for DNA sequencing, genomic analysis and fluorescent imaging.
AG Technology Corp and InnovaSep Technology Corporation
On January 31, 2002, Amersham Biosciences broadened its bioprocess capabilities through the acquisition of 100% of the issued share capital of two US companies in the membrane separations field, AG Technology and InnovaSep Technology, both based near Boston, Massachusetts.
Cimarron Software, Inc
On May 10, 2002, Amersham Biosciences acquired a further 16% holding in Cimarron Software, Inc for £4 million, taking the Group’s total holding at acquisition to 35%. Since May 10, 2002, Cimarron Software, Inc has been consolidated as a subsidiary undertaking as the Group controls the company through a shareholders’ agreement from that date. Cimarron Software, Inc is an information company located in Salt Lake City, Utah, which supplies software for life sciences research. Since May 10, 2002, the Group has subscribed for and was allotted additional share capital, taking its holding at December 31, 2002 to 44%. The Group has obligations to subscribe for a further 7% of share capital over the next three years, and has options to acquire the remaining 49% of the company for a maximum consideration of $33 million, the total consideration payable being contingent on certain specified performance conditions.
19
CodeLink™
On July 11, 2002, Amersham Biosciences acquired the net assets of the CodeLink™ pre-arrayed slides business in Tempe, Arizona from Motorola Life Sciences for £14 million. Arrays are chips, or small slides, onto which genes and proteins are placed. They are used to study how genes or proteins are “expressed” — switched on or switched off — in a particular disease state, so that researchers may understand the relationship between gene or protein function to disease and thus design better therapeutic drugs and diagnostic tests. The CodeLink™ platform is based on a unique, patented manufacturing process that produces high-quality arrays.
Dispositions
QSA
On February 20, 1998, the Company signed an agreement for the sale of its Industrial Quality and Safety Assurance Business (QSA) to AEA Technology plc with transfer of assets and payments phased over the next four years. We completed the sale on February 27, 1998. The Group incurred a loss on the disposal of QSA of £22.2 million which includes provision for operating losses to the date of disposal, decommissioning of the remaining plant and buildings and other costs which were recorded in the results for the nine months ended December 31, 1997.
Nycomed Pharma
In May 1999, the Group sold 71% of its Nycomed Pharma business to a new company formed by a private equity group. The Group recorded a pre tax gain of £11.5 million in the year ended December 31, 1999 after adding back attributable goodwill previously written off to reserves. The tax charge on the disposal amounted to £9.5 million. Further costs of £9.7 million with a related tax credit of £1.1 million, associated with exiting from the Pharma business, were accounted for in the year ended December 31, 2000 giving a total loss associated with the disposal of £6.6 million after tax in 1999 and 2000. In September 2001, the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4 million and realized £50.6 million for the outstanding Nycomed Pharma loan notes held by the Group. The Group recorded a profit on the transaction of £55.3 million after charging costs associated with the disposal, including the costs incurred in transferring the Nycomed name to Nycomed Pharma.
The Group did not account for its remaining 29% equity interest in Nycomed Pharma as an associated undertaking under UK GAAP as Nycomed Pharma was controlled by its majority shareholder and, in the opinion of the directors, the Group did not exercise significant influence over its operations. The results of Nycomed Pharma were therefore included as a discontinued operation for all years presented within the financial statements. Under US GAAP, the investment was accounted for using the equity method. The reconciliation of significant differences between UK GAAP and US GAAP is set out in Note 38 of Item 18 “Consolidated Financial Statements”.
Other Developments
In February 2002, Amersham Biosciences announced a court mediated settlement with Applied Biosystems Group which includes a cross-licensing agreement covering all patents involved in the litigation, and a co-development arrangement for the joint development, supply and commercialization of certain new DNA analysis technologies.
On May 8, 2002, the Company announced a further stage of development with the expansion of Imanet™ through the formation in Sweden of a company called Uppsala Research Imaging Solutions AB (URIS). URIS is a joint venture owned 75% by Amersham Health and 25% by Uppsala University Holdings (UUH) and is formed out of the Uppsala PET (Positron-Emission Tomography) Centre.
On February 27, 2003 the Company expanded Imanet™ further through the formation in Finland of a company called Turku Research Imaging Solutions OY (TRIS). TRIS will conduct business through the Turku PET Centre.
20
B. Business Overview
The Business of Amersham plc
Amersham plc is currently organized into two principal lines of business: the medical imaging business (“Amersham Health”) and the biosciences business (“Amersham Biosciences”).
Amersham Health is a world leader in the development, manufacture and supply of innovative products for the diagnosis and treatment of disease. Medical imaging products are used when information is needed about parts of the body, such as organs, tissues and blood vessels, which are not easily distinguishable from the normal body background.
Amersham Biosciences is a world-leading provider of biotechnology systems and solutions for research into genes and proteins, the discovery and development of drugs, and the manufacture of biopharmaceuticals.
Regulation
General
The international pharmaceutical industry is one of the world’s most highly regulated industries. Regulatory controls are a major determinant of whether a substance can be developed into a marketable product and how long such development takes.
In common with other pharmaceutical companies, the Group is subject to strict controls on the manufacture, distribution and marketing of its products. Further controls exist over the clinical trial phases of the development of a product. Of particular importance is the requirement in most countries to obtain and maintain registration or marketing authorization for a product from the relevant regulatory authority to enable it to be marketed in those countries. Pharmaceutical product registration or licensing is principally concerned with the safety, efficacy and quality of new medicines. Before such a product is approved for marketing, it must undergo exhaustive and lengthy clinical trials. It takes many years from the start of development of a new pharmaceutical compound to the submission of an application for registration. The application will include specific details of the plant and procedures involved in production. The time taken from submission of such application to launch of the product is typically one to two years. Europe, the US, Australia, Canada and Japan have very high standards of regulatory appraisal and consequently, in most cases, a lengthy approval process. The trend in recent years has been towards greater regulation and higher standards, but also towards international harmonization of documentation requirements for product registration.
After a product has been approved by the regulatory authorities and has been launched, it is a condition of the product approval that all aspects relating to its safety, efficacy and quality must be kept under review. Depending on the jurisdiction, fines or other penalties may be imposed for failure to adhere to the conditions of product licenses and, in extreme cases, the product license can be revoked. During a product’s development and following its launch, the product may be the subject of third party studies and reports that evaluate or comment upon its efficacy and relative benefit, alone and in combination with other products. These studies and reports, even if not widely accepted by the scientific community, may influence the acceptance of the product in the market.
During the life of a product, strict procedures must be in place to monitor, evaluate and report any potential adverse reactions. Where adverse reactions occur or it is judged that they may occur, changes may be required to prescribing advice and to the product licenses. In extreme cases, the product may need to be withdrawn.
Internal monitoring procedures are also maintained in relation to registered products to ensure that quality is assured and that operations are conducted in line with approved processes. During the life of a product, improvements and modifications to manufacturing processes may be made either directly by the manufacturer or, as necessary, with the approval of the relevant regulator. Approval is also required for any changes to product formulation, packaging or labeling. In
21
addition, manufacturing plants and processes are subject to periodic external inspection by the regulators as part of their monitoring procedures to ensure that manufacturers are complying with prescribed standards of operation.
Amersham’s pharmaceutical and medical device production activities are required to meet the standards of Good Manufacturing Practice (GMP) specified by the national regulatory authorities of those countries into which its products are supplied.
The principles of GMP are laid out in regulations — principally the Code of Federal Regulations of the US Food and Drug Administration and the EC Guide to Good Manufacturing Practice for Medicinal Products — which cover manufacturing and control practices, the design of premises, process validation, training, storage and distribution, and the systems of working and documentary practices by which they are managed.
Compliance with current interpretations of these principles is enforced by periodic inspection by authorized inspectors from major national regulatory authorities or by the sharing of reports generated under the Pharmaceutical Inspection Convention (PIC) scheme, or in the case of medical devices by audits performed by Notified Bodies such as the British Standards Institute (BSI) or Lloyds Register Quality Assurance (LRQA).
Within Europe, the process for approval of marketing authorizations is now uniform across the European Union member states due to regulations which came into force on January 1, 1995. These regulations make provision for and require the use of one of two procedures when applying for marketing approval throughout the community. The centralized procedure uses the European Medicines Evaluation Agency along with the Committee for Proprietary Medicinal Products to oversee assessment of applications, and results in the issuance of a single authorization for the entire European Union. The mutual recognition (or decentralized) procedure provides for issuance of a national marketing authorization in one member state and then requires mutual recognition by other European Union national authorities.
In the US, the pharmaceutical industry is subject to regulation by national, state and local agencies and to the regulations of the FDA, which administers requirements covering the testing, approval, manufacturing, distribution and marketing of drugs and reviews the quality, safety and efficacy of drugs marketed in the US. With respect to many new products, the effect of the FDA drug approval process has been to increase substantially the amount of time and money necessary to develop and market such products in the US. The FDA Modernization and Accountability Act, which was enacted on November 21, 1997 and reauthorized on October 1, 2002, has helped to reduce review times for new drugs. In the US, generic substitution statutes which permit, or in some instances require, the dispensing pharmacist to substitute a different manufacturer’s version of a drug for the one prescribed have been enacted by virtually all states. The Group is registered with the FDA as a producer and distributor of drugs and of medical devices. Pursuant to such registration, the Group’s products that are exported to, or manufactured in, the US are listed with the FDA by the Group or its licensees. In addition, the Group or its licensees file applicable pre-market application and notifications pursuant to the Federal Food, Drug and Cosmetic Act. The Group believes that it is in compliance with applicable FDA regulations.
In the UK the Group’s nuclear activities are regulated by the Nuclear Installations Inspectorate (NII), part of the Health & Safety Executive (HSE). Sites are issued with a Nuclear Site License by the NII and Certificates of Authorisation from the Environment Agency (EA), part of the Department of Environment, Food and Rural Affairs (DEFRA). These Authorisations regulate gaseous, liquid and solid discharges. The Sites are also covered by the 1999 Ionising Radiations Regulations. Sites are regularly inspected by both NII and EA appointed Site Inspectors.
In the US, the possession, use and distribution of reactor byproduct radioactive material is regulated by the Nuclear Regulatory Commission (NRC). In states that have entered into agreement with the NRC to regulate byproduct material, these so-called Agreement States have jurisdiction. Naturally occurring and accelerator produced materials are regulated by individual states, more than half of which are Agreement States. Regulation is administered through regulations and licenses issued by both the NRC and these states. Regulatory compliance is monitored and enforced by inspection and investigation as a result of reported incidents. The U.S.
22
Environmental Protection Agency (EPA) also has regulations concerning release of radioactive materials. Transportation of radioactive material is regulated by the US Department of Transportation (DOT).
In Germany, Amersham Buchler has a handling and transport license for radioactive materials issued under the Radiological Protection Ordinance.
The closure of any nuclear licensed site in the UK would require surrender of the license and full decommissioning of the site to the satisfaction of both the EA and the NII.
Pharmaceuticals Pricing
General
The overall cost of providing healthcare services has been and will continue to be subject to review by governmental and legislative bodies around the world. Prices for products are sometimes subject to direct price controls and drug reimbursement programs which have varying price control mechanisms. Governments may influence the price of pharmaceutical products through their control of national health care organizations which may bear a large part of the cost of supplying such products to consumers. In the US, Japan, Germany and certain other countries, pressure can be exerted on prices by government-funded or private medical care plans. The Group expects that governments and other bodies will increasingly seek to control costs and that the pharmaceutical industry will continue to be affected by pressure to contain health care expenditures.
In addition to the normal competitive forces that affect the level of prices, a further constraint exists in most countries in which the Group sells its products through the presence of price controls on pharmaceuticals. These controls arise because governmental agencies in a particular country are the principal purchasers of the product. Price control mechanisms operate differently from country to country and can result in large differentials in prices between markets. The effects of the inflexibility of such mechanisms may be aggravated by currency fluctuations. These price differentials are sometimes exploited by traders (parallel importers) who purchase branded products in low-priced markets and export to high-priced markets.
Price controls can be exercised in a number of different ways. In many countries, the prices of individual products are controlled by governments. In addition, in certain countries, manufacturers must file separate price applications to qualify for reimbursement by the national health insurance system. Under reference price systems, which are now in effect in a number of European countries including Germany, the Netherlands, Norway, Sweden and Denmark, governments will only reimburse patients’ drug costs on the basis of a reference price which corresponds roughly to the price of the cheapest medicine in each drug category. Products whose price exceeds the reference price will thus be more expensive for the patient to purchase. An EU directive, which became effective on January 1, 1990, also sets forth certain rules concerning the governmental operation of price controls and reimbursement applications.
In the US, there are currently no government price controls over private sector purchases; however, in 1990, federal legislation was enacted which requires drug manufacturers to pay prescribed rebates to the states on certain drugs in order for such drugs to be eligible for Medicaid reimbursement. Since the enactment of such federal legislation, several states have enacted similar legislation in respect of state entitlement programs (i.e. requiring rebates in exchange for eligibility for reimbursement by such programs).
The Japanese government sets the prices at which pharmaceuticals are reimbursed by all insurance carriers. Every other year a revision of these prices is carried out; however, not every drug class or brand within a class is affected by these revisions. For the past decade these revisions have resulted in significant overall price decreases for existing products. The average price revision for all pharmaceutical products in the Japanese market was 9.7% effective on April 1, 1998, 7% effective on April 1, 2000 and 6.3% effective on April 1, 2002.
23
Environmental and Other Regulatory Matters
General
The Amersham Group is subject to various environmental protection and occupational safety and health laws and regulations in the countries in which it operates. In addition, in its current operations and over the years, the Group has handled, manufactured, used and disposed, and will continue to deal in or otherwise handle, manufacture, use and dispose of materials and wastes classified as hazardous or toxic by one or more regulatory agencies.
The Group has developed a worldwide program of corporate environmental and safety policies. These policies are designed to ensure that environmental and occupational safety and health protection are key business objectives at all locations, and they detail the purpose, scope and responsibilities of environmental and occupational safety and health concern throughout the Group’s worldwide operations. Continuing improvements are being made in the reduction of waste and levels of discharges and emissions from its manufacturing processes, including radioactive waste.
Significant capital expenditures, as well as operating costs, have been incurred to comply with the laws and regulations governing the protection of the environment, occupational safety and health, and the handling of hazardous materials. There are inherent risks in handling hazardous or toxic materials and wastes, and the Group incurs costs to comply with the environmental regulations applicable to its operations. Amersham plc has no reason to believe that current and expected expenditures and risks occasioned by these circumstances are likely to have a material adverse effect on its financial condition; however, no assurance can be given that a change in circumstances may not result in such a material adverse effect. The Group’s manufacturing operations are all subject to these environmental laws and regulations. Although the Group continues to make capital expenditures for environmental protection, it does not anticipate being obliged as a result of such laws and regulations to incur costs which would have a material impact upon the Group’s financial condition or results of operations.
Radioactive waste
Radioactive waste is inevitably generated in the manufacture of radioactive materials, and the Group’s policy, which is managed by an executive committee, is to minimize the generation of such waste and, to the extent possible, dispose of it in a timely fashion through safe and authorized routes of disposal. The Group’s radioactive manufacturing processes result in radioactive solid waste by-products that can be placed pursuant to the UK’s Radioactive Waste Regulations into three categories as follows:
| • | | Very Low Level Waste (VLLW) is waste that represents a low enough concentration of radioactivity/volume to require no controls on disposal to landfill; |
| |
| • | | Low Level Waste (LLW) is waste arising from materials used as part of the manufacturing process, which have become contaminated. It is defined as beta gamma waste of less than 12 GBq per tonne and alpha waste of less than 4 GBq per tonne or less for fissile material. Generally, this material can be characterized as small items of laboratory equipment, packaging, glassware and other consumables used within a laboratory. All LLW is consigned to the national above-surface repository at Drigg that is managed by British Nuclear Fuels plc (BNFL) on behalf of the UK Government; |
| |
| • | | Intermediate Level Waste (ILW) is any material above the activity limits specified as LLW that is not heat producing. ILW can range from extremely small, low activity items, which result in a high specific activity such as smoke detector foils, to heavily contaminated enclosures, or items specifically designed to transport or contain higher levels of activity. |
Disposal routes currently exist for VLLW and LLW and, dependant on the physical and chemical attributes of the waste, for many types of ILW where the specific activity is within a factor of five of that described as LLW using e.g. incineration.
24
The remaining ILW, depending on half-life, may be stored to decay to either LLW or VLLW at which point it will be disposed of accordingly. The Group stores both its ILW for decay and its residual ILW (that which will remain ILW within a reasonable period of institutional supervision, generally taken to be 50 years) in safe conditions on operational sites. This is pending the availability of a national facility, whereupon risk and title will be transferred to the appropriate government funded organization. With respect to radioactive materials generated or used by the US facilities, such materials, in line with specific conditions of acceptance relating to activity, physical and chemical properties, are disposed of in the US, reused or recycled. If no disposal or recycling/reuse route exists, in the last instance, radioactive materials generated or used by the US facilities are returned to the country of origin in line with any international agreements that may be in place.
The costs and liabilities of environmental and other regulatory matters
At December 31, 2002, the Group’s provision for costs associated with the eventual decommissioning of facilities and the eventual disposal of intermediate level waste (ILW) was £65.5 million. The amounts charged to profit and loss in respect of decommissioning and effluent costs for the year to December 31, 2002 were £5.1 million. The Group’s accounting policy with respect to radioactive decommissioning costs and costs associated with radioactive waste arising from operations (effluent costs) are set out in Note 1 of Item 18 “Consolidated Financial Statements”.
The Group presently has decommissioning plans for the Harwell facility and portions of the Amersham and Braunschweig manufacturing facilities. The Harwell facility handled radioactive materials up until 1997 and appropriate measures are being taken to comply with applicable regulations governing closure of the property, which is expected to be completed by 2005. Idle portions of the manufacturing facilities at Amersham and Braunschweig will also be decommissioned, with the work expected to be completed by 2010.
Provisions for decommissioning and effluent costs relate to the costs, determined on a going concern basis, associated with decommissioning radioactive facilities and with the eventual disposal of the Group’s ILW which arises from operations. Decommissioning costs will be incurred after the facilities are withdrawn from use in radioactive operations and extend out to 2036. Total decommissioning and effluent costs to be incurred in the next 10 years amount to approximately £37.4 million. The provisions are based on the latest technical assessment of the process and methods likely to be used, and are stated at current prices and discounted using a real discount rate of 4%.
25
Segmental information
The following table sets forth the Group’s turnover by major business sector and geographic area, and their percentage contribution to the Group’s total turnover, for the three years ended December 31, 2002, 2001 and 2000.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | | | | | | | |
| | | December 31, 2000 | | December 31, 2001 | | 12 months to |
| | | (restated) | | (restated) | | December 31, 2002 |
| | |
| |
| |
|
| | | £m | | % | | £m | | % | | £m | | % |
| | |
| |
| |
| |
| |
| |
|
Turnover by major business sector | | | | | | | | | | | | | | | | | | | | | | | | |
Amersham Health(1) | | | 773.9 | | | | 56.2 | | | | 922.0 | | | | 57.5 | | | | 947.7 | | | | 58.6 | |
| Amersham Biosciences | | | 603.7 | | | | 43.8 | | | | 680.5 | | | | 42.5 | | | | 670.5 | | | | 41.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | | 1,377.6 | | | | 100.0 | | | | 1,602.5 | | | | 100.0 | | | | 1,618.2 | | | | 100.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Turnover by geographic area(2) | | | | | | | | | | | | | | | | | | | | | | | | |
| Europe | | | 766.5 | | | | 55.6 | | | | 722.1 | | | | 45.1 | | | | 903.9 | | | | 55.9 | |
| North America | | | 743.4 | | | | 54.0 | | | | 853.7 | | | | 53.3 | | | | 871.8 | | | | 53.9 | |
| Asia Pacific and Rest of World | | | 271.9 | | | | 19.7 | | | | 286.3 | | | | 17.9 | | | | 277.0 | | | | 17.1 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 1,781.8 | | | | 129.3 | | | | 1,862.1 | | | | 116.3 | | | | 2,052.7 | | | | 126.9 | |
| Less intersegment sales | | | (404.2 | ) | | | (29.3 | ) | | | (259.6 | ) | | | (16.3 | ) | | | (434.5 | ) | | | (26.9 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | | 1,377.6 | | | | 100.0 | | | | 1,602.5 | | | | 100.0 | | | | 1,618.2 | | | | 100.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (1) | | Included within Amersham Health is the Group’s share of NMP sales for each of the three years to December 31. |
| |
| (2) | | Based on region of origin. |
1. Amersham Health
Business Summary
Note: Market numbers within the text represent internal company estimates unless stated otherwise.
Amersham Health is a market-leading, global business specializing in the field ofin vivodiagnostic products for the early and accurate detection of disease. Today our products are primarily used to aid in the diagnosis of anatomical and functional abnormalities of soft tissue within the body. Increasingly, however, Amersham is developing products that detect changes in cells at the molecular level. Such changes usually occur well before symptoms or functional changes become apparent.
We focus on the management of heart disease, circulatory disease and stroke; degeneration of the brain such as seen in Alzheimer’s disease and Parkinson’s disease; lung disease and a range of cancers. In addition, we are a major provider of therapy products to treat prostate and thyroid cancer.
As the cost of healthcare continues to rise and populations age, governments and healthcare providers increasingly recognize the value of investment in early and accurate diagnosis, in order to most effectively manage therapeutic intervention and its associated costs. This, together with increased capability and numbers of scanning machines, greater incidence of disease and improved medical practices (which rely on the identification of the underlying cause of disease), continues to drive growth and demand for our products.
26
Product portfolio and market share
Before patients can be treated, they must first be diagnosed. Medical imaging products play a very significant role in making the invisiblevisible, enabling healthcare professionals to expand and improve their abilities to detect and then define the extent of disease, so they can better treat disease and improve quality of life.
Medical imaging has evolved beyond recognition since the first use of X-rays to visualize bones in the living body over a century ago. Today, the ability to scan the entire body in a matter of seconds and identify disease-induced abnormalities with accuracy is taken for granted. Medical imaging is now established as an important part of modern healthcare, demonstrated by the fact that in 2002 over 800 million medical imaging procedures were performed worldwide. Of these, approximately 120 million scans were ‘enhanced’ or made possible with medical diagnostic products, generating revenues of around £2.8 billion. The relevance of imaging to the practice of modern medicine is expected to continue to increase in future years.
One of the most significant reasons for the increasing relevance of imaging is the greater average longevity of the global population and the rise in prevalence of age-related diseases such as cardiovascular disease, cancer and neurological disease (e.g. Alzheimer’s, Parkinson’s disease and stroke). Imaging is particularly well suited to assist in the diagnosis and management of such diseases and indeed, statistics show that people over the age of 45 require on average three to four times as many diagnostic investigations as those under 45. Age-related diseases are among the most common in the industrialized nations and represent a cost burden to the healthcare system. Accordingly, there is a health benefit to develop earlier and more reliable diagnostic tests to assist in the treatment and prevention of these diseases.
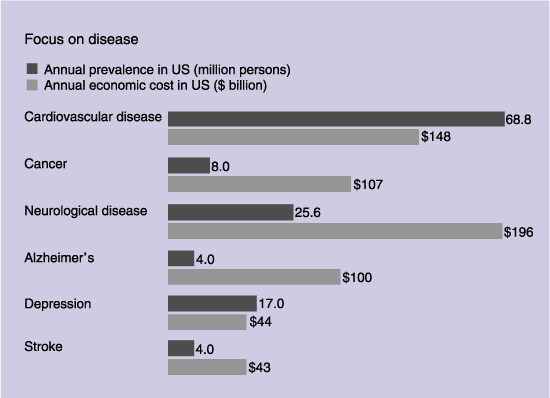
Source: Pharmaceutical Research and Manufacturers of America, 2000Through access to the internet, patients are also becoming better informed about their medical conditions and the management of those conditions. Accordingly, physicians will have to incorporate each patient into the clinical decision making process which increasingly involves the selection of appropriate imaging techniques.
27
Global medical diagnostic market
Amersham Health, Bracco, Tyco, Schering and Bristol-Myers Squibb (BMS), together with their respective licensees, are the major players in the medical diagnostics market...
Diagnosis plays an increasingly important part in modern healthcare, particularly in growing disease areas such as cardiology, neurology and oncology. By providing structural and functional diagnostic information about the body, medical imaging reduces healthcare cost and improves quality of life.
There are four main medical imaging modalities — X-ray (including computer tomography, CT), magnetic resonance imaging (MRI), radiopharmaceutical imaging, and ultrasound. Radiopharmaceutical imaging always requires a diagnostic product, while the other three modalities may or may not use a diagnostic product, depending on the procedure. Each modality is rapidly evolving to meet the growing demands of modern healthcare requirements. Overall, the emphasis in technology advancement is to improve the speed, efficiency and functionality of instrumentation, lower its cost and hence, increase accessibility. Improving the speed will allow for increased numbers of procedures per unit, keeping pace with the rising demand.
The selection of imaging modality is based on a variety of factors including the amount and type of information which can be obtained regarding the suspected disease or condition, how quickly and conveniently the information can be obtained, the side effects, image quality and cost effectiveness.
X-ray/CT medical diagnostics
| | | |
| Total scans | | approx. 630 million |
| Enhanced scans | | approx. 75 million |
| Market size | | approx £1.4 billion |
X-ray is the most frequently performed imaging procedure covering all body areas and is often the entry point for the diagnostic work-up. X-ray computed tomography, or CT scanning, has had the greatest impact on the medical diagnostics market. CT scanning allows cross-sectional imaging of the body with exquisite depiction of anatomic detail, and is finding growing use in coronary angiography and interventional procedures. Approximately 40% of CT scans currently involve the use of medical diagnostic products.
The development of multi-slice CT machines, which are capable of performing sub-second imaging of millimeter-thin slices of body tissues, has opened up a host of new applications such as blood vessel imaging and blood clot imaging, many of which require large volumes of medical diagnostic contrast media.
MRI medical diagnostics
| | | |
| Total scans | | approx. 40 million |
| Enhanced scans | | approx. 10 million |
| Market size | | over £320 million |
| No. MR scanners | | over 15,000 |
Magnetic resonance imaging (MRI) is often the method of choice for imaging the central nervous system, particularly for detecting cancers in the brain and abnormalities in the spine. More recently, MRI has found use in imaging the blood vessels and depicting brain regions affected by stroke. Roughly 25% of the MRI scans use a medical diagnostic product with every segment of contrast-enhanced MRI experiencing growth, and we expect this trend to continue.
New MRI contrast media are being developed for vascular imaging, cardiac imaging, visualization of air spaces in the lungs, and for targeting specific tissues such as lymph nodes and blood clots. The ability to identify high-risk patients in the foreseeable future, along with the need for accurate anatomical and functional information, are expected to drive the market for these new products.
28
Radiopharmaceutical medical diagnostics
| | | |
| Enhanced scans | | approx. 28 million |
| Market size | | over £1.1 billion |
| No. cameras | | over 15,000 |
Radiopharmaceutical imaging provides metabolic and functional information about diseases such as stroke, dementia, coronary artery disease and cancer that complements anatomical imaging such as CT or MRI. Radiopharmaceutical imaging always requires the use of a radiolabeled medical diagnostic product, which binds to and accumulates in the cells or tissues being studied, and the image is obtained with the aid of a gamma camera.
Recent innovations in radiopharmaceutical imaging enable the visualization of precise cellular activities and subtle changes in organ function, such as changes in brain function due to Parkinsonism.
The latest advance in radiopharmaceutical imaging is positron-emission tomography (PET). PET, an advanced molecular imaging technique, can provide images of the entire body and visualizes biochemical events at the cellular level. It is an extremely sensitive technique for the early detection of tumors and metastases, and PET procedures are now reimbursed by Medicare in the US for seven types of cancer. The clinical application of PET technology is a rapidly growing emerging market, with around 300,000 procedures performed in the US in 2002. Continued advances in instrumentation and improvements in PET chemistry could expand its use into other significant markets, e.g. the management of neurological conditions such as Alzheimer’s disease.
Another significant development is the advent of compound modality instruments combining high-resolution CT images of the anatomy with low-resolution functional (PET or single photon emission computed tomography (SPECT)) images during parts of the same imaging procedure. These enable more accurate localization of disease and assist the planning of surgical interventions and radiotherapy. Such developments in so-called “fusion imaging” are expected to fuel the growth of both CT and PET imaging.
Ultrasound medical diagnostics
| | | |
| Total scans | | approx. 140 million |
| Enhanced scans | | approx. 0.5 million |
| Market size | | over £10 million |
| No. instruments | | over 150,000 |
Ultrasound is used extensively by both hospital and office-based cardiologists to evaluate patients with known or suspected coronary artery disease. The vast majority of cardiac patients are screened by ultrasound, and in 2002 over 25 million ultrasound scans of the heart were performed throughout the world. However, results are often equivocal with patients frequently referred on to more reliable and expensive techniques (e.g. CT and MRI).
Only a fraction of the ultrasound scans currently use a medical diagnostic product, the primary use being to improve the assessment of heart wall motion through endocardial border delineation, or visualization of the surface of the heart muscle. The true utility of ultrasound in cardiology could be realized with the visualization of myocardial perfusion. This will require advances in instrumentation along with advance ultrasound diagnostic products, in order to obtain a reliable and robust imaging procedure. In radiology, contrast-enhanced ultrasound has the potential to differentiate between benign and malignant tumors in various tissues. Developments such as these would expand the ultrasound medical diagnostics market.
Radiotherapy market
The most significant therapeutic market for radioisotopes is prostate brachytherapy, a minimally invasive outpatient procedure in which radioactive iodine or palladium seeds are implanted within the prostate gland to irradiate the tumor. The mounting clinical evidence supporting the efficacy of this treatment was further underpinned in 2002 through two important studies, one relating to the treatment of high-risk patients with palladium-103 seeds in combination with external beam
29
radiation, and the other demonstrating the benefits of Amersham’s RAPID Strand™ iodine-125 delivery system.
In the US, around 25% of men presenting with early stage prostate cancer are treated with brachytherapy seed implants. Slower market growth and increased competition, with the number of competitors rising from two in 1998 to 14 in 2002, have resulted in significant pricing and market share pressure. In 2002, the US Medicare reimbursement system set a fixed cost for brachytherapy procedures and we expect further changes which may intensify price pressures to take effect in 2003.
Brachytherapy is gaining acceptance in Europe, and while only a small percentage of early stage prostate cancer patients currently receive seed implants, the market has substantial development potential. See “Recent Developments” in Item 4 where we announce the merger of our brachytherapy business with the urology business of Galil Medical to create a stand-alone marketing company.
Radiolabeled monoclonal antibodies are being developed for the treatment of several types of cancer. The antibodies target specific tumor cells and then attach to them, allowing the radioactive component to destroy tumor cells while sparing the surrounding healthy cells. 2002 saw the first of these compounds licensed by the FDA for the treatment of non-Hodgkins lymphoma patients who have failed to respond to previous therapies.
Other radiotherapy products include strontium-89, which can target growing bone cancers and help to alleviate pain, and iodine-131 for the treatment of thyroid tumors.
Business Environment
The importance of early and accurate diagnosis of disease as a critical first step to effective treatment has been understood by physicians for centuries. Growing awareness of this need amongst patients, technological advances in the use of electronic scanning forin vivoimaging and the aging of populations in North America, Europe and Japan, are stimulating a significant increase in the use of diagnostic techniques.
Our extensive distribution network of Amersham owned companies around the world, and multiple distribution arrangements into other countries, mean that we are well placed to serve this growing market. Worldwide spending on healthcare is expected to continue to increase over coming years. Growing realization by governments and healthcare providers, who fund the majority of the healthcare cost, that targeted treatments at well diagnosed disease can show cost efficiency over more generalized treatment is also contributing to growth in the diagnostic sector.
In the radiotherapy market, the widespread prevalence of prostate cancer amongst men over 50 is becoming widely known and the need for early intervention is increasingly understood. Effective, proven alternatives to radical surgery are becoming accepted in most markets as a legitimate form of disease management in this category.
Products
X-ray Imaging
In 1961, our Health business introduced our first internally developed ionic X-ray contrast product. The key breakthrough for the contrast media market and for Amersham came in 1982 with Amersham’s introduction of Omnipaque™, a highly stable non-ionic contrast product with safety and tolerance for a wide range of diagnostic applications. An ionic substance is one which dissociates into electronically charged particles when placed in solution, while conversely, a non-ionic substance remains electrically neutral.
Second generation, non-ionic products have fewer adverse effects and, due to reduced patient movement during the procedure, better imaging capabilities than ionic products. The non-ionic contrast market is expected to continue to grow, as a result of an increase in the number of diagnostic procedures driven by population growth, increasing scanner installations, changes in
30
medical practice including the need for good diagnostic information prior to the use of new therapeutic products.
Omnipaque™is Amersham Health’s largest selling imaging product with sales for 2002 of £222 million, up 6% on 2001 at constant exchange rates. It is also the world’s largest sellingin vivodiagnostic imaging product. The active substance in Omnipaque™is iohexol, which is the product of several complex chemical production steps. In addition, Amersham Health has licensed this product to Daiichi and Schering to whom Amersham sells bulk iohexol for manufacture into finished Omnipaque for selling into Japan and parts of Europe.
In 1996, Amersham Health launched a third generation non-ionic X-ray contrast product, Visipaque™,which has the same isomolality (concentration) as human blood. This isomolality and its non-ionic structure means it offers a greater degree of safety and comfort to patients in certain procedures. In 2003, new clinical data have been released indicating that Visipaque™ may offer better utility and renal safety in select high risk patients. Worldwide sales of Visipaque™in 2002 grew by 18% at constant exchange rates to £91 million compared to 2001.
Magnetic Resonance Imaging (MRI)
Omniscan™is Amersham Health’s leading MRI product and is a non-ionic gadolinium-based contrast medium. The benefits of non-ionic over ionic products have been less significant in MRI than in X-ray imaging; however, the non-ionic nature of Omniscan™makes it a potentially safer and more effective contrast medium, particularly in procedures requiring rapid injection of high doses such as MR angiography and whole body imaging. Omniscan™was developed initially for CNS (central nervous system) indications, but new indications are being approved in an increasing number of countries, including whole body and pediatric indications. Amersham Health has approval for body indications in 18 countries in Europe, and in the US, Canada, parts of Latin America and Japan. The introduction of pre-filled syringes has made application of the product easier for those administering the product and safer for patients. Sales of Omniscan™in 2002 were £96 million, up 17% on 2001 at constant exchange rates.
Radiopharmaceutical Imaging
Myoview™is Amersham Health’s advanced technetium99m labeled heart imaging product. It is approved in all major markets for the diagnosis and localization of myocardial ischemia and / or infarction. Myoview™offers a more convenient preparation and faster imaging time than any other technetium heart imaging product and is continuing to increase its market share. Sales of Myoview™ in 2002 were £133 million, up 26% on 2001 at constant exchange rates.
Amersham Health also produces Ceretec™for the imaging of blood flow in the brain and for localizing areas of infection, and an extensive range of generic nuclear imaging products. In 2002 sales of Ceretec™ amounted to £21 million.
In September 2000, Amersham Health launched DaTSCAN™, a new diagnostic product for Parkinson’s disease, in Europe. This represents an important step in Amersham’s evolution towards the commercialization of disease-specific molecular diagnostic products. Approximately 1 million people across Europe suffer from Parkinson’s disease, with many new cases arising each year. This debilitating neurological disorder occurs due to a significant decrease in a key chemical transmitter in the brain, which commences several years before symptoms are visible. DaTSCAN™specifically identifies the loss of the key chemical transmitter and hence can determine the patient’s clinical outcome. For the first time, patients and physicians are able to objectively define the presence of Parkinson’s disease, distinct from Essential Tremor which has similar symptoms but a very different treatment regime, minimizing the potential for misdiagnosis. Sales of DaTSCAN™ in 2002 were £3 million.
Ultrasound
In January 2002, Amersham Health gained exclusive rights to Optison™, the first of a new class of ultrasound products, which shows a robust capability to image the endocardial borders of the heart. These rights include the markets of North America, Europe, Latin America and much of Asia, excluding Japan. This key entry into the emerging ultrasound market has established
31
Amersham in a leading market position in a short period of time. Amersham has established a specialized Optison™ sales team whose objective is to educate the medical community on the advantages of this new diagnostic tool.
Sonazoid™
In the US and Europe, myocardial perfusion has been the primary target market for the development of Sonazoid™, a third generation ultrasound product. The use of ultrasound for this indication has been limited by the slow development of sufficiently robust imaging procedures which replaces advanced ultrasound equipment. Therefore, although Sonazoid™ has shown promising safety data, a decision has been taken to suspend work in the US and Europe and to continue to focus on Optison™, which is marketed for improved visualization of the surfaces of the heart muscle. In Japan, the development of Sonazoid™ with Daiichi continues. The first use of Sonazoid in Japan will be established in the liver disease market.
Radiotherapy
Amersham Health’s leading radiotherapy products are OncoSeed™and the proprietary RAPID Strand™, which are surgically implanted in the prostate gland for the treatment of early stage prostate cancer. Usually conducted as an outpatient procedure, treatment with these seed implants has demonstrated comparable efficacy to prostatectomy and external beam radiation therapy, with lower complications of impotency and incontinence in certain patient groups. We have maintained our market leadership in this area through a time of greatly increased competition, with the only iodine product on the market with more than 10 year patient survival data. In November 2000, we added the leading palladium brachytherapy seed in the US, TheraSeed™, to our portfolio via a distribution agreement with Theragenics. In June 2001, our Health business introduced EchoSeed™, a unique seed implant with enhanced visualization under ultrasound. EchoSeed™’s patented groove design, in combination with conventional ultrasound imaging, allows the physician to see more seeds during the implant procedure, thus facilitating optimal placement in the prostate. Sales of all seeds in 2002 were £50 million, down 10% from 2001, at constant exchange rates. Amersham Health also manufactures and sells Metastron™, which provides pain relief for cancer patients with bone metastases.
Production
Production facilities and sources
Our Health business synthesizes bulk material (iohexol, iodixanol, gadodiamide, iopentol) for X-ray and MRI contrast media at our production facilities at Lindesnes, Norway.
The finished X-ray and MRI products are formulated and packaged at its facilities in Oslo, Norway in Cork, Ireland and in Shanghai, China. Production also occurs under contract manufacture agreements with third parties.
Daiichi Pharmaceutical Co., Ltd. (“Daiichi”) is licensed to process iohexol into Omnipaque™and gadodiamide into Omniscan™in Japan. Schering AG (“Schering”) is licensed to process iohexol into Omnipaque™for sale in certain European countries.
In 2001, we expanded primary production facilities at our Lindesnes plant in Norway as worldwide demand for X-ray contrast media continues to grow. In June 2002, we approved a new £47 million investment at Lindesnes to source increased volumes of a significant intermediate for contrast media production, to be completed in 2005. Secondary manufacturing capacity was also increased with the opening in September 2002 of an extension to our secondary manufacturing facility in Cork, Ireland.
We produce Myoview™and Ceretec™at our facility in Gloucester, UK, for worldwide distribution. Our production facility in Oslo was approved for manufacturing of Myoview™as a second supplier in May 2002.
Our primary manufacturing plant and distribution center for nuclear imaging products in the US, and our only production site for RAPID Strand™, is located in Arlington Heights, Illinois, near
32
O’Hare Airport, which permits rapid distribution throughout the entire US marketplace. Product manufacturing also takes place in South Plainfield, New Jersey. Utilizing three cyclotrons in the US, it produces numerous radiopharmaceuticals for the North American market.
We produce OncoSeed™,Metastron™and technetium generators and other radioisotopes used in imaging at our manufacturing site in Amersham, UK. DaTSCAN™, another radiopharmaceutical product, is produced in our production unit in Eindhoven, Netherlands. Production facilities are currently been upgraded to secure future increase in supply.
At the end of 2000, we announced our intention to close our nuclear manufacturing facility in Saluggia, Italy. This closure is expected to occur during 2003.
Raw Materials
Radioactive isotopes used in the manufacture of certain nuclear imaging products are particularly essential to the operation of Amersham Health’s business. In addition, the manufacturing processes of X-ray imaging products require significant quantities of iodine. Amersham Health utilizes suppliers based in Chile and Japan to ensure the supply of this essential raw material. Our manufacturing operations require a wide variety of raw materials, electronic and mechanical components, chemical and biochemical materials, and other supplies, some of which are occasionally found to be in short supply. We have multiple commercial sources for most components and supplies but are dependent on single sources for a limited number of such items, in particular certain radioactive isotopes.
Marketing and Distribution
X-ray Imaging
Our Health business markets and distributes our X-ray imaging products through licensees, subsidiaries, distributors and local agents.
We utilize our own subsidiaries to market Omnipaque™(iohexol) in most European countries, the US and Canada. We use Sanofi Synthelabo and Inaltec as distributors in Central and South America. In some large markets in Europe and in Japan, we license major companies to market Omnipaque™. We sell iohexol in bulk to our licensees. In addition, we receive royalty payments based on the licensees’ final sales of Omnipaque™.
Amersham Health has granted Schering AG, a German pharmaceutical company, a non-exclusive license to formulate and sell Omnipaque™in certain countries, including Germany. The license agreement with Schering does not extend to future X-ray imaging products of the Group.
Our Health business sells iohexol to Daiichi, a major Japanese pharmaceutical company. We have a license agreement for Omnipaque™pursuant to which Daiichi has the rights to formulate and market Omnipaque™contrast media in Japan. In addition, Daiichi has a separate R&D agreement with us for contrast media products (excluding nuclear medicine and magnetic resonance imaging products), which gives Daiichi an option to acquire sole rights to produce and sell such new products in Japan.
Visipaque™is marketed by Amersham Health in all major European countries. Visipaque™was launched in the US in 1996 and is marketed by Amersham Health’s US subsidiary. Visipaque™ is marketed in Japan by Daiichi.
In the US, Amersham Health markets directly to end-users. We distribute both directly to end-users and through wholesalers. We continue to be the market leader in the US for X-ray contrast media.
In October 1998, we renewed a five-year agreement with Novation, the supply company of two healthcare alliances comprising hospitals and community health centers covering a third of the US market. The agreement enables us to supply Novation customers with X-ray and MRI contrast media products. In December 2000, this agreement was extended for two years through March 2005.
33
We invest in end-user support programs, such as advanced educational programs in radiology, scientific research and publications. Our Health business is also involved in the development of new packaging systems for simpler and safer administration of contrast media to the patient, for example pre-filled syringes, see “Products — MRI”.
Magnetic Resonance Imaging
Our Health business currently markets Omniscan™in several countries through our own distribution network. Omniscan™ was launched in Japan in June 1996 through our licensee, Daiichi Pharmaceutical.
Under a licensing agreement with Schering, the parties have granted each other a non-exclusive worldwide license to develop, produce and market existing and future MRI contrast media which are covered by either party’s patents. Schering also has the right to co-market certain future MRI contrast media developed by us. The agreement provides for the payment of certain financial consideration by the Group to Schering.
Ultrasound
In May 2000, Amersham Health and Mallinckrodt announced a major intellectual property settlement giving Amersham Health a $10 million upfront license fee and joint access to Mallinckrodt’s Optison™ ultrasound contrast agent. We also agreed to collaborate on further joint development and commercialization of Mallinckrodt’s Optison™ and of our Sonazoid™. In 2001, we agreed with Tyco Inc, the new owners of Mallinckrodt, to terminate this agreement and from January 2002, we obtained full rights to revenues from Optison™ worldwide outside Japan and took on the sole responsibility for and costs of further ultrasound developments and marketing and distributing this product.
Daiichi has the option to market the Group’s new ultrasound imaging products, excluding Optison™, in Japan. Daiichi exercised its option to market the Group’s ultrasound contrast medium Sonazoid™in Japan in March 1998.
Radiopharmaceutical Imaging
The principal customers for Amersham Health’s radiopharmaceutical imaging products are nuclear medicine doctors and their related support staff. In addition, promotional activity is aimed at doctors who refer their patients for nuclear medicine procedures. We have a substantial sales and marketing organization which is able to maintain regular contact with all significant nuclear medicine centers in all major markets.
We believe that we have the broadest commercial and manufacturing infrastructure within the nuclear medicine industry. A major presence in the principal regions of North America, major western European countries and Japan is supported with active sales organizations in each region. Strategically, we have positioned ourselves as a provider of a broad range of nuclear medicine products in each region.
The nature of Amersham Health’s product portfolio places significant demands on our infrastructure for rapid distribution of radioisotopes from our manufacturing centers and radiopharmacies to customers.
The market place in the US represents the single largest market for radiopharmaceuticals. With three major competitors in this market, distribution is an important competitive factor. In contrast to the European market, most radiopharmaceuticals in the US are channeled through commercial radiopharmacies. Amersham Health’s 30 radiopharmacies are aligned with 35 facilities owned and operated by Mallinckrodt Medical Inc. together with 122 independently operated pharmacies to provide distribution of its products through a national network of 187 radiopharmacies.
In January 1999 Amersham Health was awarded a new three year contact to provide Novation customers with a full range of nuclear imaging products. This agreement was also extended, through December 2003.
34
In April 1997, Amersham Health and Bracco entered into a patent licensing agreement and an option agreement enabling us to develop, make and sell Myoview™ until December 31, 1999, with an extension under the contract manufacture option to June 28, 2002, the expiration date of the Bracco patent. Under this agreement, Amersham Health was required to pay a royalty on gross sales of Myoview™ in the US to Bracco. This royalty agreement ceased on June 28, 2002 following which no royalty became payable. Amersham continues to manufacture certain products for Bracco under the contract manufacture option element of the agreement.
Through NMP, our joint venture with Sumitomo Chemical Company Ltd., we have become the leading supplier of nuclear healthcare products in Japan. NMP supplies a wide range of radiopharmaceutical products and dosage forms throughout Japan. As is the case in other regions of the world, the most important single application for radiopharmaceuticals in Japan has been the nuclear cardiology imaging segment. With these products, including Thallium 201 and Myoview™, our Health business is able to meet a wide range of customer demands for heart imaging. Production in Japan also includes radioisotope product forms specific to the Japanese market. Distribution in Japan is directed from two manufacturing facilities, one near Tokyo and one in the Osaka region.
Together with NMP, we have achieved a leadership position in the radiopharmaceutical segment of the nuclear medicine market through a combination of organic business growth, acquisition, alliances and joint ventures.
Therapy
In May 2002, Amersham Health was awarded the Novation contract for brachytherapy seeds to add to our portfolio of contracts with group purchasing organizations. The Novation award was a three year sole-source agreement to provide its customers with a full line of brachytherapy products including OncoSeed™, RAPID Strand™, EchoSeed™ and TheraSeed™.
Competition
We operate in a highly competitive market place against other international pharmaceutical companies and specialists in the manufacture and distribution of contrast products. Due to the high cost of developing new products and of maintaining distribution channels internationally, the industry has rationalized to a core group of 5 major companies. Ownership changes in recent years (e.g. Tyco’s acquisition of Mallinckrodt and Bristol Myers Squibb’s acquisition of Dupont’s pharmaceutical and diagnostics business) have assisted an industry drive to improve profitability after a history of price discounting in the 1990s. As a major player in the industry, we maintain our drive to maximize revenues through product differentiation, line extensions, market leadership and premium services.
The US patent for Omnipaque™expired on December 31, 1999 and Omnipaque™has been exposed to generic competition in the US market since that time. However, sales of the product continue to grow due to market demand.
The MRI market continues to be very competitive with a few companies actively developing new MRI contrast products. While the ultrasound contrast media market is still in development, several companies in addition to Amersham Health are actively developing ultrasound contrast products.
The nuclear imaging market also continues to be highly competitive, characterized by the application of advanced technology. There are numerous companies which specialize in, and a number of larger companies which devote a significant portion of their resources to the development, manufacture, and sale of products which compete with those manufactured or sold by us. Many of our competitors are well-known manufacturers with a high degree of technical proficiency. In addition, competition is intensified by the ever-changing nature of the technologies in the industries in which we are engaged. The markets for our products are characterized by specialized manufacturers, which often have strength in narrow segments of these markets. While the absence of reliable statistics makes it difficult to determine precisely our relative market position in our industry segments, Amersham Health believes we are one of the principal manufacturers in our fields, marketing a broad line of nuclear imaging and radiotherapy products.
35
Distribution channels are a means of competitive advantage in the radiopharmaceutical market. With respect to our nuclear imaging and radiotherapy products, the requirements for local language labeling, when combined with relatively small production volumes and short half-life isotopes, support Amersham Health’s strategy of multiple production sites, which management believes, gives us a considerable competitive advantage. Although different market dynamics exist in Europe compared to North America, a small number of city or regional radiopharmacies serving several hospitals are beginning to be established in Europe.
The US market for brachytherapy seeds in prostate cancer became increasingly competitive during the year, with generic producers continuing to erode prices in both Palladium and Iodine segments. In addition, new presentations such as pre-loaded needles and stranded products were added to the portfolio of several competitors.
Medicare reimbursement changes in early 2002 were introduced and will be followed by further adjustments in 2003. The effect of these changes has been to increase pricing awareness amongst our customers and further pressurize the competitive pricing environment. Despite these difficulties we have maintained our leadership position by continuing to promote the clinical benefits of our key brands.
Research & Development
General
We are committed to the ongoing search for new products and have a track record in bringing them successfully to market. However, R&D in the pharmaceutical industry is both expensive and prolonged; it also entails considerable uncertainty. The process of developing novel therapeutic drugs and contrast products typically takes years from discovery through testing and registration to initial product launch, although this process varies considerably from case to case and country to country.
All potential pharmaceutical products go through two major stages of development before a product registration can be granted: pre-clinical and clinical. In pre-clinical testing, potential compounds are tested comprehensively to determine whether they are safe to administer to humans. This stage of development involves investigating the effects of the compound on various bodily functions as well as studying its absorption, distribution, metabolism and excretion in selected species.
Clinical trials and full product development will often be carried out on a joint basis in more than one country to optimize the rate of commercialization globally. Clinical development of a potential pharmaceutical product falls into the following three phases:
| | | | | |
| Phase | | Description | | Purpose |
| |
| |
|
| I | | The first trials of a new drug in humans. The drug is given for a short period to a small number of healthy volunteers or patients suffering from the disease for which the drug is intended. | | To gain preliminary elucidation of the human pharmacological properties and safety of the drug/imaging agent. |
| | | | | |
| II | | Pilot studies, usually open trials on a small number of patients | | To determine if the drug/imaging agent has the intended effect, and to approximate the therapeutic dose range. |
| | | | | |
| III | | Expanded trials | | To determine the efficacy of the drug/imaging agent and the pattern and frequency of the side effects, as well as to compare the drug with established methods of treatment or standard treatments or diagnosis. |
36
R&D activities in medical imaging are conducted principally in Oslo, Norway; Amersham, UK; Princeton and Durham, US; Munich, Germany; Malmo, Sweden and by NMP (50% owned) and Daiichi (technical and commercial relationship) in Japan. Daiichi and NMP perform additional clinical trials necessary to develop products for the Japanese market.
In the year ended December 31, 2002, expenditure on imaging and radiotherapeutic R&D (excluding that undertaken by NMP) amounted to £95.1 million, 10% of Health turnover.
The following table summarizes the status of Amersham Health’s key products either (i) under development, (ii) seeking new medical indications or (iii) recently marketed in certain territories:
| | | | | | | | | | | |
| | | | | Application | | Development Status |
| | | | | (not all imaging | |
|
| Product name | | Technology | | products) | | US | | Japan | | Europe |
| |
| |
| |
| |
| |
|
| DaTSCAN™ | | Nuclear imaging | | Dopamine transporter imaging for Parkinson’s disease | | — | | Phase II | | Launched Sept 2000 |
| | | | | | | | | | | |
| Omniscan™ | | MRI | | MR Angiography
Cardiac perfusion | | Both in Phase IIB | | — | | Cardiac perfusion
in Phase IIB |
| | | | | | | | | | | |
| Myoview™ | | Nuclear imaging | | Cardiac perfusion
imaging under
pharmacological
stress | | Approved Nov 2001 | | *** | | — |
| | | | | | | | | | | |
| Myoview™ | | Nuclear imaging | | Detection of suspected breast tumors following mammography | | — | | — | | Approved in UK July 2001. Registration in rest of Europe ongoing |
| | | | | | | | | | | |
| Myoview™ | | Nuclear imaging | | Detection and monitoring of left ventricular function | | Approved Feb 2003 | | **** | | |
| | | | | | | | | | | |
| NeoTect™* | | Nuclear imaging | | Lung cancer | | Marketed — rights transferred back to Schering in 2002 | | — | | Approved December
2000 |
| | | | | | | | | | | |
| Sonazoid™ | | Ultrasound | | Heart perfusion | | Phase II completed. Entry into Phase III suspended | | — | | Phase II completed. Entry into Phase III suspended |
| | | | | | | | | | | |
| Sonazoid™ | | Ultrasound | | Radiology (liver) | | Phase II completed April 2002. Further development terminated for commercial reasons | | **
Phase III | | Phase II completed April 2002. Further development terminated for commercial reasons. |
| | | | | | | | | | | |
| Clariscan™ | | MRI | | Cardiac function/perfusion,
coronary
angiography,
angiogenesis, tumor
imaging | | Terminated Jan 2002 | | — | | Terminated Jan 2002 |
37
| | | | | | | | | | | |
| | | | | Application | | Development Status |
| | | | | (not all imaging | |
|
| Product name | | Technology | | products) | | US | | Japan | | Europe |
| |
| |
| |
| |
| |
|
| Metastron™ | | Radiotherapy | | Metastatic bone
pain relief | | Marketed | | Phase III | | Marketed in most
countries |
| | | | | | | | | | | |
| Helispin™ | | MRI | | Functional lung
imaging | | Phase II | | — | | Phase II |
| | | | | | | | | | | |
| NC100668 | | Nuclear Imaging | | Deep Vein
Thrombosis/Pulmonary
Embolism | | Phase II | | — | | Phase II |
| | | | | | | | | | | |
| FDG | | PET imaging | | Oncology, cardiology and neurology imaging | | Technology
assessment ongoing | | Phase III | | Technology
assessment ongoing |
| | | | | | | | | | | |
| Bexxar™***** | | Radio-immunotherapy | | Treatment of Non-Hodgkin’s Lymphoma | | — | | — | | Phase III |
| * | | Trademark owned by Schering AG and licensed exclusively to Amersham |
| |
| ** | | Marketing partner in Japan is Daiichi Pharmaceutical |
| |
| *** | | Pharmacological Stress agents are not approved in Japan |
| |
| **** | | Myoview™license in Japan is of a broad scope, which can allow assessment of cardiac function |
| |
| ***** | | Trademark owned by Coulter Pharmaceutical, Inc.. |
Research & Development partnerships
Amersham Health looks forward to contributing to the advancement of medicine. Our research efforts and the collaborations we are forging are designed to bring us closer to achieving this goal, and to help us develop powerful new products.
Bexxar™ (tositumomab and Iodine I131 tositumomab)
In October 2001, we announced an agreement to develop and market a novel radioimmunotherapy in Europe for the treatment of Non-Hodgkins Lymphoma (NHL). This agreement is with the Corixa Corporation (Corixa) and covers the product which is known in the US as Bexxar™. Under the terms of the agreement, Corixa and Amersham Health cooperate with the aim of registering the product in Europe for the treatment of certain types of NHL, a cancer of the white blood cells. Corixa is responsible for generation of clinical trial data to support registration in Europe while Amersham Health is responsible for manufacture and sale of radiolabeled antibody in the territory. There are currently over 40,000 patients newly diagnosed with NHL in the five largest European countries every year.
General Electric Medical Systems
In November 2001, we announced a collaborative agreement with General Electric Medical Systems (GEMS) in the field of production of imaging agents for Positron Emission Tomography (PET). PET is a rapidly growing nuclear imaging modality which is currently used primarily in the diagnosis of cancer and monitoring of response to cancer therapies. PET is reimbursed by insurance companies for a number of indications in cancer in the US. PET is also used in and reimbursed by insurance companies for the diagnosis of certain types of heart problems. In the research setting PET has shown great promise in the diagnosis of a number of neurological diseases although currently no PET technique is reimbursed by insurance companies for such indications in either the US or Europe.
38
Currently the manufacture of the radioactive substances used in PET imaging is a relatively complex process. The complexity of the manufacturing process and lack of GMP may impede the development of new, patent protected PET ligands in the future. The collaboration between GEMS and Amersham Health is designed to greatly simplify and improve the manufacture of a wide range of PET imaging agents and thus to facilitate the overall growth of PET as a clinical imaging modality and to give both GEMS and Amersham Health a strong basis from which to compete in this market. In this collaboration, GEMS will develop a mechanized process which manufactures PET agents, probably to fully certified GMP standards, using proprietary chemistry belonging both to GEMS and Amersham Health. In turn, Amersham Health will invent and own important new chemistries for use in this automated system and will develop new PET imaging agents for sale. The first two agents to be produced on this new system are anticipated to be fluorodeoxyglucose, FDG, and Fluoro-DOPA (F-DOPA). FDG is the most widely used PET imaging agent and is currently used primarily in the diagnosis and monitoring of cancer. The use of FDG is reimbursed by insurance companies in the US. F-DOPA may be used in the diagnosis and monitoring of neurological diseases such as Parkinson’s disease. It is anticipated, but not guaranteed, that other PET imaging agents will be developed that utilize this novel system.
Imanet™
In early 2001, we established Imanet™, an Imaging Research network designed to exploit our capability in spin signal technology (SST) and Positron Emission Tomography (PET). Spin signal technology is an enhanced form of magnetic resonance imaging (MRI), based on bombarding inert gases such as helium and xenon with high power lasers. These capabilities were increased by our 75% holding in Imaging Research Solutions Ltd (IRSL) which owns assets acquired from the UK Medical Research Council (MRC) in February 2001. Imanet™ is pursuing collaboration agreements with leading pharmaceutical companies who are seeking to license SST and PET products for use in their R&D activities. In early 2002 the first significant collaboration with Pfizer was announced. This collaboration provides a basis for Amersham Health to access new molecules that have the potential to be developed intoin vivoimaging agents from Pfizer’s extensive library of novel chemical compounds while Pfizer benefits by using Amersham’s PET technology to make faster decisions on its potential therapeutic development candidates.
On May 8, 2002, the Company announced a further stage of development with the expansion of Imanet™ through the formation in Sweden of Uppsala Research Imaging Solutions AB (URIS). URIS is a joint venture owned 75% by Amersham Health and 25% by Uppsala University Holdings (UUH) and is formed out of the Uppsala PET (Positron-Emission Tomography) Centre, which has a worldwide reputation for its academic and commercial excellence in PET.
On February 27, 2003, the Company announced a third partnership that further strengthens our Imanet™ PET network. By establishing a new PET imaging business within the facilities of and in collaboration with the Turku PET Center in Finland, Amersham now is able to bring the expertise of this world-class PET center into the overall Imanet™ network. The addition of the Turku PET Center now allows Amersham an expanded capacity and capability to partner with pharmaceutical firms to assist them in developing their therapeutic molecules through the use of PET.
39
Patents and Trademarks
We have obtained patents in many countries covering significant products developed through our R&D activities. Patent protection is available in the US, Western Europe, Japan and most other developed countries for new products, as well as for pharmaceutical formulations and manufacturing processes.
We believe that we continue to have patent protection for our important existing products in our major markets and, in addition, have obtained patents, or anticipate that patents will be granted, for a majority of new compounds and compositions which are under development.
Patent applications will normally be filed to protect a compound or composition at an early stage in the development of diagnostic and therapeutic products. The life of a patent is typically 20 years from the time of filing. With effect from June 8, 1995, the US Patent Law (which had previously been a notable exception to this norm) was amended such that the term of a US patent will also be 20 years from the time of filing, (US patents in force on June 8, 1995 will have a term which is the greater of 20 years from filing or 17 years from grant). By the time a new drug is launched in the market, it may have a significantly shorter patent protection due to the length of the R&D period and the time taken to obtain the necessary marketing approvals from national regulatory authorities. In major markets, such as the European Economic Area (EEA) (which includes the EU member countries, plus Norway, Iceland and Liechtenstein), Japan and the US, an extension to the term of protection may be granted for medical products; thereby taking some account of the marketing delay arising from compliance with regulatory requirements.
Within the EEA a Supplementary Protection Certificate (“SPC”) may be granted for a period of up to 5 years to ensure an effective term of protection of up to 15 years based on the first product authorization within the EEA. In the US and Japan the patent term is extended based on the time elapsed during the product registration procedure. The maximum extension is five years, and in the US the extension given is typically 2-3 years. Amersham Health will generally file applications for SPCs and extensions whenever possible.
In addition, we have obtained, or intend where possible to apply for, patents relating to all new technologies and compounds, which we are developing for our businesses. Finally, with Amersham Health’s deepening disease expertise, we anticipate filing patents for diagnostic “methods of use” that encompass methods and processes for the diagnosis and monitoring of disease.
Amersham Health seeks to obtain trademark protection for its products in its major markets. Trademark protection in some countries depends upon whether the trademark has remained in use. In other countries, trademark protection continues as long as the mark is registered subject to cancellation. A trademark generally assumes increasing importance when the patent on a product expires. We protect our trademarks vigorously against infringements.
40
The following table sets forth the patents and patent expiration dates on significant medical imaging products held by Amersham Health in its major markets:
| | | | | | | | | | | | | | | | | | | | | |
| | | Expiration date |
| | |
|
| | | United | | | | | | | | | | Great | | | | |
| Products | | States | | Japan | | Germany | | Britain | | France |
| |
| |
| |
| |
| |
|
Visipaque™ | | | | | | | | | | | | | | | | | | | | |
| (a) Chemical substance (iodixanol) | | | 2011 | | | | 2008 | | | | 2008 | | | | 2008 | | | | 2008 | |
| (b) Formulation | | | 2011 | | | | 2011 | | | | 2011 | | | | 2011 | | | | 2011 | |
Omniscan™ | | | | | | | | | | | | | | | | | | | | |
| (a) Chemical substance (gadodiamide) | | | 2007 | @ | | | 2005 | | | | 2007 | @ | | | 2007 | @ | | | 2007 | @ |
| (b) Pharmaceutical formulation | | | — | | | | 2009 | | | | 2009 | | | | 2009 | | | | 2009 | |
Ceretec™ | | | | | | | | | | | | | | | | | | | | |
| (a) Propylene amine oxime Tc complexes | | | 2003 | | | | 2004 | | | | 2004 | | | | 2004 | | | | 2004 | |
| (b) Chemical structure | | | 2006 | | | | 2006 | | | | 2006 | | | | 2006 | | | | 2006 | |
| (c) Cell labeling use | | | — | | | | 2007 | | | | 2007 | | | | 2007 | | | | 2007 | |
Myoview™ | | | | | | | | | | | | | | | | | | | | |
| (a) Tc complex patent | | | — | | | | 2008 | | | | 2008 | | | | 2008 | | | | 2008 | |
| (b) Tetrofosmin specific | | | 2010 | (1) | | | 2009 | | | | 2009 | | | | 2009 | | | | 2009 | |
| * | | Term of protection extended from 2003 to 2008 in Germany, Great Britain, France and Japan |
| |
| @ | | Term of protection extended from 2004 to 2007 in the United States and from 2005 to 2007 in France and Germany |
| |
| (i) | | Includes 16-month patent term extension (US only). |
2. Amersham Biosciences
Note: Market numbers within text represent internal company estimates unless stated otherwise.
On March 21, 2002, the Company purchased the 45% ownership stake in Amersham Biosciences held by Pharmacia Corporation, thereby taking 100% ownership of Amersham Biosciences.
Business summary and environment
Amersham Biosciences is a leading global provider of systems (instruments, reagents, consumables and software) and services used in gene and protein research, drug discovery and development, and biopharmaceutical manufacturing.
Amersham Biosciences offers a comprehensive product range that spans the entire drug discovery and development process. We have a proven track record of providing high quality technology to our customers, who number approximately 50,000 in 100 countries. Amersham Biosciences has a strong, global customer franchise, with pharmaceutical, biotechnology and agrochemical companies, research institutes, universities and medical research centers. Our customers include all of the top 20 pharmaceutical companies. Amersham Biosciences has a strong track record in innovation and R&D, spending a substantial percentage of our annual revenues on these activities. Our long-standing commitment to R&D provides our customers with a broad range of innovative technologies and products, including products for gene analysis, protein analysis, drug screening, toxicology studies, clinical drug development and drug manufacturing.
Many of Amersham Biosciences products form the basis of what we call integrated systems. These are whole systems that enable our customers to efficiently meet their research and manufacturing objectives. These systems consist of equipment or instruments and reagents optimized for use with instruments and software to analyze and link the research results.
41
The Amersham Biosciences business includes two principal business streams, discovery systems and protein separations that share activities in R&D, sales and marketing, distribution and manufacturing. Effective January 1, 2002, Amersham Biosciences reorganized its business streams, creating discovery systems consisting of the former drug discovery, laboratory products, and service, agency and other business streams, and maintaining the protein separations business stream. Segmental reporting has been restated as appropriate for periods prior to January 1, 2002.
Discovery systems consists of products and services in Genomics, Proteomics, Bioassays and Informatics, while protein separations consists of products and services in bioprocess and laboratory separations. The revenues from these business streams are further discussed in Item 5 “Operating and Financial Review and Prospects”, within the Amersham Biosciences segment.
The following is a brief background of the life sciences market and an overview of the specific markets within which Amersham Biosciences competes.
Fifty years have passed since the discovery of the double helix structure of DNA heralded a new era of medicine. At the beginning of the 21st century, catalyzed by the completion of the human genome, life science continues to be seen as a major driver towards improved healthcare for all. Having the map of the human genome enabled life scientists to move into a new era of understanding biological processes at the cellular level. This new precision in biology is expected to lead to a fundamental shift in the practice of medicine, enabling delivery of the goal of personalized medicine by facilitating better understanding, profiling, prediction and treatment of disease. In addition to technological advances, our knowledge-based society feeds a more informed and responsive public who continue to expect availability of cost-effective healthcare to improve their lives.
Research work in academia is critical for understanding the mechanisms of disease and underpinning improved healthcare; this has been reflected by generally stable sector funding during 2002. Government and charity spending to support the academic segment continued to be strong with double-digit growth in 2001. In the US, for example, the NIH budget increased by 14 per cent to approximately $23 billion in 2002, but it is anticipated that growth in funding in 2003 will be decreased.
Capital funding of start-up biotechnology companies has been under significant pressure in 2002 and the first quarter of 2003, and sources of funding are now shifting from the public capital markets to a combination of the venture capital community and other private sources. Following the sequencing of the human genome, the market is uncertain about where the next frontier will be found. Companies are now turning towards functional biology, where the interest is in deciphering what the genes and their protein products actually do. In addition companies that previously commercialized software and genomic data have developed new business strategies, moving from discovery companies to therapeutic developers, e.g. Celera’s acquisition of Axys Pharmaceuticals and Millennium’s acquisition of COR Therapeutics. Consolidation is likely to continue, frequently in association with large pharmaceutical companies through marketing alliances, to create powerful biotechnology players.
Despite the availability of far more potential targets than previously, there has been a reduction in the number of innovative drugs being registered by pharmaceutical companies worldwide. Escalating R&D costs are reducing productivity and placing a greater importance on steps to limit the possibility of failure in the later stages of development. By better understanding the function and variation of genes and proteins in disease and health, pharmaceutical companies will increase their opportunity of identifying disease-relevant targets leading to better-targeted molecular drugs. In an attempt to maximize returns, companies have refocused some of their resources to later stage development to support life-cycle management. These companies also faced additional pressures such as market pricing and increased generic competition. In 2002 these significant issues were coupled with a backdrop of global recessionary pressure, increased focus on corporate governance and contraction of capital budgets.
Notwithstanding the global market challenges of 2002, dynamism and change remain the hallmarks of the biosciences market.
42
Overview of selected market segments for Amersham Biosciences
The market in which Amersham Biosciences competes has a worldwide value of approximately £5 billion. We have set out selected application segments in the table below. Customers include R&D groups within academic and clinical research laboratories, pharmaceutical companies, biotech companies and biopharmaceutical manufacturing groups.
| | | | | | | |
| Selected application segment | | Market size (£m) | | Principal competitors |
| |
| |
|
| Bioprocess (industrial protein separations) | | | 590 | | | Millipore, Merck KgaA, Tosoh
Bioscience |
| | | | | | | |
| Laboratory protein separations | | | 345 | | | Bio-Rad, Applied Biosystems |
| | | | | | | |
| Genomics – sequencing | | | 500 | | | Applied Biosystems |
| | | | | | | |
| Genomics – gene expression | | | 400 | | | Affymetrix, Agilent |
| | | | | | | |
| Proteomics | | | 740 | | | Applied Biosystems, Bio-Rad, Waters |
| | | | | | | |
| High throughput drug screening | | | 1200 | | | Cellomics, Molecular Devices,
Perkin-Elmer |
Key market drivers are the sourcing and allocation of funds in these customer segments and the number of biopharmaceuticals in clinical trials. Total worldwide pharmaceutical/biotech R&D expenditure increased by approximately nine per cent in 2001; growth slowed to an estimated six per cent for leading companies in 2002.
2002 company estimate.
The biosciences market is fuelled by integrated systems, instruments, consumables and informatics, to support customers to achieve their goals faster and more efficiently.
Increasingly, sophisticated platforms such as biochips are being applied across genomics and are starting to enter proteomics. These technologies generate large amounts of data in a much shorter time than previously achieved, and have high potential in general research as well as in other applications such asin vitroclinical diagnostics.
Biologically based drugs (e.g. insulin, monoclonal antibodies, vaccines, DNA medicines) continue to grow in importance. Nine out of the 26 drug approvals by the FDA during 2002 were biologics.
43
Protein separations
Bioprocess
The number of biopharmaceutical candidates in clinical trials and gaining regulatory approval has continued to increase. There are now over 100 such biopharmaceuticals from companies such as Amgen, Eli Lilly, Johnson & Johnson and NovoNordisk. The key purification steps in the production of these drugs require systems, membranes and chromatography media designed and manufactured to the highest standards.
Biotechnology drug candidates in clinical trials comprise a wide range of compounds including anti-sense DNA, monoclonal antibodies, DNA-based gene therapies, growth factors, interferon and vaccines. The growth rates in this area are expected to be maintained or to rise in the future as an increasing number of novel proteins, peptides and anti-sense drugs are brought to market as therapeutic products. There are currently more than 500 biopharmaceuticals in phase I, II and III clinical trials worldwide. The market growth for bioprocess products will be underpinned by the increasing propensity of pharmaceutical companies to look for manufacturing technologies that further improve overall production economy. Because of the relatively large doses required in their use, the increased number of monoclonal antibodies in later stage development has triggered investment in manufacturing capacity to meet the expected demand.
Growth in number of licensed biopharmaceuticals
Laboratory separations
Chromatography is one of the core technologies used in protein analysis, and, although affected by the slowdown in capital spending on tools, the longer term growth in this market is being fuelled by the overall market expansion in proteomics. Laboratory chromatography techniques are also used in method development and scale-up for biopharmaceutical manufacturing.
44
Discovery systems
Genomics
The genomics market is heterogeneous and is composed of sub-segments such as gene sequencing, gene expression and genetic variation, which are growing at different rates.
Sequencing efforts are now increasingly focused on exploration of genetic differences in the four nucleotide bases within, and between, a variety of species. Technologies are being applied to improving sample preparation prior to sequencing as well as reducing sample volumes, providing greater ease of use and increasing overall efficiencies.
Measuring and monitoring the level to which different genes are expressed is increasingly performed using microarray platforms with pre-arrayed nucleic acids (biochips) or with technologies to allow researchers to prepare their own arrays.
Tailoring of drug treatments is an important goal of personalized medicine. Analysis of single nucleotide polymorphisms (SNPs) should facilitate a better understanding of individual susceptibility to disease and response to drug treatment. The market is currently very small, but with technological advances it could show dramatic growth.
Proteomics
Proteomics — studies to discover the identity, function and interaction of proteins in living organisms — is currently experiencing good growth. Proteins are responsible for cellular structure, metabolism and function including signaling and growth, and are therefore the targets of most drug discovery efforts.
In contrast to genomics, the ‘industrialization’ of proteomics is in its infancy. Unlike DNA, proteins cannot be readily amplified to aid detection and analysis. Also, in contrast to the 35,000 to 45,000 human genes, there may be up to two million different types of proteins, and these have complex differences in their three dimensional structure which affect their function.
Current trends include the drive towards integrated approaches to provide increased sample throughput, sensitivity and accuracy. Technologies such as microarrays are just starting to be applied for protein analysis, but are considerably more complex than their DNA counterparts.
Bioassays
Researchers use bioassays to measure and quantify the biological processes involved in cellular activities, metabolism and disease. Bioassays are involved at almost every stage of the preclinical drug discovery R&D process. Validation of target molecules, primary screening of possible drug candidates, secondary screening to select lead candidates and finally, testing of the lead candidates to validate their metabolic/toxicological properties are among the hurdles that must be passed before a potential drug candidate can move into clinical trials.
The success of primary screening approaches has now led to development of the market for ‘high information’ secondary-screening formats. Researchers are now starting to use such formats to examine how modification of selected genes within cells is translated into physiological changes.
Informatics
The integration of the vast amounts of data generated from increasingly large numbers of sample studies and multiple technology platforms represents one of the key challenges for life science research in the 21st century. While major corporations such as IBM are becoming active in this segment, current market needs are focused on laboratory information management systems (LIMS) which integrate platforms, sample and reagent logistics and enable production research by handling data from sample to end result. The LIMS market is predicted to grow to over £0.5 billion by 2007. (Source: Frost and Sullivan).
Amersham Biosciences’ Business Strategy and Competitive Strengths
Amersham Biosciences’ vision is to enable molecular medicine in which the genetic basis for disease will be understood, leading to earlier and more accurate diagnosis of disease, and more effective treatment. Our strategy is to build on our position as a leading provider of biotechnology
45
systems to enable the molecular medicine revolution. Our discovery systems and protein separations streams operate in markets representing significant opportunities for growth. Our systems, products and services are critical components of research into genes and proteins, the discovery and development of drugs, and the manufacture of biopharmaceuticals. We believe that we are well positioned with both our commercial and academic customers as a result of the following set of competitive strengths:
| • | | The breadth of Amersham Biosciences’ technologies and our comprehensive product lines span many segments of the drug discovery, development and manufacturing processes.
Amersham Biosciences offers well-recognized branded products such as our MegaBACE™ DNA sequencing system, our LEADseeker™ drug screening system and our Sepharose™ separations media used in biopharmaceutical manufacturing. |
| |
| • | | Our commitment to R&D, our long-standing reputation for scientific excellence and our development of innovative products.
Amersham Biosciences has a strong record in developing, and continuing to develop, state-of-the-art technologies, both internally and in partnership. We expect this commitment to R&D will result in new product introductions in 2003. Our strong intellectual property portfolio, which is a vital asset, supports our R&D efforts. |
| |
| • | | Amersham Biosciences’ global market franchise with strong customer relationships.
Amersham Biosciences global strengths in sales, operations, and R&D enables us to offer our customers a complete set of products to meet their needs. We have technology and commercial partnerships with many of the top 50 pharmaceutical companies. We believe that our bioprocess products are used in the production of almost all licensed biopharmaceuticals. |
| |
| • | | Strong management team.
Amersham Biosciences has a strong management team with an average of over 15 years of experience in the life sciences industry. |
| |
| • | | The partnership approach.
Amersham Biosciences has a history of strategic developments through acquisitions, investments and research alliances, as well as through strategic partnerships with our customers. These alliances have resulted in some of our key products and have provided us with early access to new technologies. |
Specific plans for each principal business stream are as follows:
| • | | Discovery systems.
Amersham Biosciences plans to capitalize on the continuing growth in the drug discovery market by commercializing innovative technologies to develop systems to enable gene and protein studies and drug screening. We have demonstrated this by launching leading, innovative systems in each of these areas and will continue their development with extensions into second or third generation systems supported by informatics. We are focusing our portfolio to capitalize on the market changes towards gene expression and proteomics. This business stream is expected to benefit from expansion in informatics, which Amersham Biosciences plans to develop in the two areas of laboratory workflow software and bioinformatics to take advantage of the increased need for data management, data integration and further analysis. This will include the study of data across experimental domains, for example using data for gene expression and protein expression in the same study. Data mining tools are required for this purpose. Some but not all of these products will be developed in Amersham Biosciences laboratories, while others will be licensed-in through a focused licensing strategy. |
| |
| • | | Protein separations.
We are positioning ourselves to capitalize on the expanding pipeline of biopharmaceutical products and grow our protein separations business through the introduction of new products and technologies, including membrane filtration technology acquired in January 2002. |
46
Amersham Biosciences Products
Discovery systems
Amersham Biosciences has a leading position in the market for systems that enable faster, more efficient drug discovery. This business stream covers the following areas: genomics, proteomics, bioassays and informatics.
Genomics
Interpreting the DNA sequence can lead to the identification of the proteins derived from it. In addition, comparison of DNA sequences between normal and affected individuals across a disease population provides valuable information on the genetic changes giving rise to a disease, thereby facilitating the development of improved diagnostic and therapeutic techniques and the development of drugs targeted toward an individual’s specific genotype.
Amersham Biosciences provides a comprehensive range of instruments, reagents and software sold individually or as integrated systems to enable genetic analysis. Some of the products, in particular MegaBACE™ high throughput sequencing systems, have been critical to the sequencing of the human genome. Microarray products are crucial for comparative gene expression analysis that will occupy scientists for many years to come.
The principal genomics products are summarized below:
| | | | | |
| Product Group | | Type of product | | Primary area of use |
| |
| |
|
| Products being marketed | | | | |
| | | | | |
| MegaBACE™ 4000 DNA Analysis System | | 384 capillary DNA sequencer | | Automated DNA sequencing with ultra high throughput |
| | | | | |
| MegaBACE™ 1000 DNA Analysis System | | 96 capillary DNA sequencer | | Automated DNA sequencing & genotyping |
| | | | | |
| MegaBACE™ 500 | | Flexible throughput capillary array genetic analysis | | Automated DNA sequencing & genotyping |
| | | | | |
| Lucidea™ Microarray System | | DNA microarray spotter, scanner and slide processor | | Differential gene expression |
| | | | | |
| Thermo Sequenase™ | | DNA polymerase which is stable at high temperatures for use in sequencing reactions | | Automated DNA sequencing &
genotyping |
| | | | | |
| DYEnamic™ Energy Transfer primers and terminators | | Fluorescently labeled primers and terminators for DNA sequencing giving highly sensitive detection | | Automated DNA
sequencing |
| | | | | |
| TempliPhi™ | | Template preparation using novel
enzyme | | Preparation of template for DNA sequencing |
| | | | | |
| SNuPe™ reagent | | High throughput, single nucleotide
Primer extensions for MegaBACE™ | | Genotyping, SNP validation and scoring |
| | | | | |
| CodeLink™ array technology | | Pre-arrayed slides | | Gene expression, SNP validation and analysis |
| | | | | |
| Genetic Profiler 2.0 Analysis software | | Genotyping software | | Genotyping on the MegaBACE™ DNA Analysis System |
| | | | | |
| Ready-To-Go™ | | Reagents and kits | | Molecular biology research |
| | | | | |
| Typhoon™ | | Multi-modal detection imager | | Laboratory detection applications |
| | | | | |
| Products in development | | | | |
| | | | | |
| CodeLink™ array technology | | Pre-arrayed slides with new content | | Gene expression, SNP validation and analysis |
| | | | | |
| GenomiPhi™ DNA Amplification kit | | Template amplification/purification | | Whole genome amplification from cells and blood for genetic analysis |
47
The MegaBACE™ 4000 DNA sequencer is an automated, fluorescent DNA sequencing system that consists of hardware, software and reagents designed for industrial-scale DNA analysis. It uses 384 capillaries, each about the size of a human hair, filled with a gel which acts as a separating filter during electrophoresis, the separation of particles using an electric field. Operating in parallel, the capillaries separate, detect and analyze fluorescently labeled DNA fragments. The MegaBACE™ system automates gel preparation, sample injection, DNA separation and data analysis, allowing significant productivity gains compared to traditional slab gel systems. MegaBACE™ also provides customers with enhanced processing speed. MegaBACE™ has played a significant role in several public and private sector genome sequencing operations, and we have manufactured over 1,000 systems as of December 31, 2002. The MegaBACE™ systems generate recurring revenues through sales of reagents and other consumables that are used with the system.
In addition to DNA sequencing, the MegaBACE™ platform can support a range of drug discovery applications, including microsatellite genotyping, or identifying the genes possessed by individuals, and the study of inheritance patterns and variations in single base pairs of DNA. In 1999, Amersham Biosciences launched a second application on the MegaBACE™ 1000 platform allowing researchers to detect small differences in similar DNA samples. This application, known as the MegaBACE™ 1000 Genotyper, uses the same hardware as the MegaBACE™ sequencing system, but employs different software and chemistries.
The MegaBACE™ brand was further extended to the wider sequencing market, by introducing a flexible concept with the MegaBACE™ 500 system. The MegaBACE™ 500 enables users to move between 32, 48 and 96 capillaries on the MegaBACE™ platform, meeting the lower throughput needs of many researchers at sequencing laboratories.
Microarrays are small arrays of biological material on an inert substance, typically genes on a glass slide that can be used to test for functional criteria. They are powerful tools for scientists to monitor and analyze the expression levels of thousands of genes in a single experiment. Amersham Biosciences microarray systems use dyes and scanners and large sets of DNA, which spot onto a glass surface to enable researchers to study how genes are expressed.
In July 2002, Amersham Biosciences acquired the CodeLink™ pre-arrayed slides business in Tempe, Arizona from Motorola Life Sciences. The acquisition moved Amersham Biosciences into the pre-arrayed (ready to use) chip market, building on its well established business of spotters and scanners for researchers to make their own arrays. Arrays are chips, or small slides, onto which genes and proteins are placed. They are used to study how genes or proteins are “expressed” — switched on or switched off — in a particular disease state, so that researchers may understand the relationship between gene or protein function to disease and thus design better therapeutic drugs and diagnostic tests. CodeLink™ has a unique, patented manufacturing process that produces arrays of high quality, sensitivity and reproducibility, and with more useable data points than any other pre-arrayed slide currently in the market. There are currently five CodeLink™ products on the market, with several more in development including higher density chips with 20,000 DNA spots per slide. CodeLink™ has been undergoing comparison tests in customer laboratories and feedback to date has been positive.
Proteomics
Interest in proteomics research is growing rapidly as pharmaceutical and biotechnology companies seek to understand the role of proteins in diseases, and to generate new ideas for drugs or interventions in cellular processes. The complexity of this task and its potential in the drug discovery and development process creates an opportunity to develop high throughput systems for protein analysis, involving the next generation of two dimensional gel electrophoresis, mass spectrometry and liquid chromatography technology. Two dimensional gel electrophoresis is a technique for the separation of proteins by electrical charge and by size. Mass spectrometry is a technique for identifying chemicals, including proteins, by their molecular weight. Liquid chromatography is the separation of proteins and peptides according to their physical properties. Amersham Biosciences currently markets products using differential gel electrophoresis, mass spectrometry and liquid chromatography techniques.
48
Under the brand name Ettan™, Amersham Biosciences has built on its experience in protein analysis, separation and purification to develop high throughput analysis systems for proteomic research, and is well positioned to take advantage of the proteomic market opportunity. Over the next few years, Amersham Biosciences expects to launch a series of integrated protein analysis systems, also under the Ettan™ brand, based on linking two dimensional gel electrophoresis, imaging instruments, mass spectrometry and liquid chromatography. The Ettan™ MALDI ToF Pro mass spectrometer, related CAF chemistry kits for sequencing, Ettan™ 2D DIGE (two dimensional differential imaging gel electrophoresis), and the Ettan™ Spot Handling WorkStation were launched in 2002.
The principal proteomics products are summarized below.
| | | | | |
| Product Group | | Type of product | | Primary area of use |
| |
| |
|
| Products being marketed | | | | |
| | | | | |
| Ettan™ 2D DIGE or Two Dimensional Differential Imaging Gel Electrophoresis system | | Fluorescent dyes for labeling proteins, imaging instruments and software | | Protein expression analysis, which is the process of detecting differences in protein populations found in normal and diseased cells |
| | | | | |
| Ettan™ Dalt Six and Twelve | | Gel electrophoresis systems for the 2nd dimension | | Protein expression analysis |
| | | | | |
| IPGPhor™ | | Gel electrophoresis system for the 1st dimension | | Protein expression analysis |
| | | | | |
| ImageMaster™ and DeCyder™ | | Imaging analysis software | | Protein expression analysis |
| | | | | |
| GST recombinant fusion products | | Vectors and reagents to facilitate the production of recombinant proteins | | Protein identification and characterization, which is the process of determining the structure and function of a protein |
| | | | | |
| Hoefer™ | | Electrophoresis and blotting instruments | | Detection and analysis of proteins |
| | | | | |
| Ettan™ Spot Picker | | Accurate automatic spot picking | | Protein expression analysis and identification and characterization |
| | | | | |
| Ettan™ MALDI ToF Pro | | Mass spectrometry | | Protein identification and characterization |
| | | | | |
| Ettan™ Spot Handling Work Station | | Integrated spot picker, digester and spotter | | Protein expression analysis and identification and characterization |
| | | | | |
| Ettan™ ESI ToF LC-MS | | Liquid chromatography-mass spectrometry integrated system | | Protein identification and characterization |
| | | | | |
| Products in development | | | | |
| | | | | |
| Ettan™ 2D DIGE extensions (new dyes, improved software, low cost image analysis system) | | Fluorescent dyes for labeling proteins, imaging instruments and software | | Protein expression analysis |
| | | | | |
| Multidimensional chromatography system MDLC | | Chromatography system for separation of proteins | | Protein identification and characterization |
| | | | | |
| P4-Parallel Purification Protein Platform | | High throughput protein purification platform | | Protein amplification and purification used to isolate protein samples for research |
49
Bioassays
Scientists are discovering many new drug targets as a result of genomic and proteomic research efforts. These discoveries, coupled with the greater availability of large libraries of possible drug compounds, have resulted in demand from the pharmaceutical industry for higher throughput technology for drug screening to alleviate this major bottleneck in the drug discovery process. In addition, pharmaceutical companies are also seeking more sophisticated technology to enable them to understand the metabolism and toxicology of drug candidates earlier in the drug development process in order to eliminate drug candidates with high toxicity and reduce the need for expensive animal testing and clinical trials. Drugs with high toxicity are unsuitable for use in treating disease.
Once a target has been identified, many candidate chemicals are tested rapidly in a process termed “high throughput screening” in order to identify lead candidates which interact with the target. These lead candidates are then investigated intensively with respect to both their biochemical and chemical properties in order to optimize the lead candidates, through secondary drug screening, to a stage where they may perform adequately as a novel medicine for the treatment of disease. Typically, researchers will screen between 100,000 and 2,000,000 candidates to identify the handful that may constitute lead candidates. These studies require the sensitive analysis of complex reactions involving a number of biochemicals, proteins and nucleic acids. The assays used require advanced technology for both labeling and detection of biomolecules and researchers must be able to perform the assays quickly.
Amersham Biosciences established an early market leadership position in high throughput screening through the introduction of our Scintillation Proximity Assay in the early 1990s, sold through technology access contracts. Building on this success, Amersham Biosciences introduced the innovative LEADseeker™ homogeneous imaging system in 1998 and has continued to develop the capabilities of this system culminating in the launch of the LEADseeker™ multimodality imaging system in 2002. This system can be used for detection of radiometric proximity assays, fluorescence intensity, fluorescence polarization, time-resolved fluorescence and luminescence. Using charged coupled device detection, the system can read any plate format sensitively and rapidly. Fluorescence assays can be read in as little as 10s which, combined with a flexible and rapid plate delivery mechanism, means throughput of over 100,000 compounds per hour. The open automation architecture (hardware and software) is compatible with most currently available automation solutions, giving a choice of set up.
The acquisition of Praelux in February 2000 provided Amersham Biosciences with access to advanced cell imaging technology, which permits development of systems for cell-based secondary assays for cellular screening and target validation. The INCell Analyzer system enables real time tracking and measurement of the movement of molecules within a cell. This significant advance over existing technology uniquely combines high throughput and high information content. In addition to the INCell instrument, Amersham Biosciences also provides customized assays for specific customers and has developed novel biologies and reagents that enable the profiling of hit compounds from screening so as to increase the productivity of the drug development process
Amersham Biosciences is also a major provider of services for the custom radioactive labeling of potential drug compounds for absorption, distribution, metabolism and excretion studies.
The principal bioassays products are summarized below:
| | | | | |
| Product group | | Type of product | | Primary area of use |
| |
| |
|
| Products being marketed | | | | |
| | | | | |
| Scintillation Proximity Assay, or SPA | | Beads which emit light when a radioactive compound is bound close to the particle surface | | High throughput screening |
| | | | | |
| Cyanine Dye labels | | Fluorescent dyes optimized for labeling of proteins and nucleic acids | | Assay technology for screening and development assays |
| | | | | |
| Custom labeling | | Custom synthesis of molecules with radio or stable isotope labels | | Toxicology, metabolism and distribution studies |
50
| | | | | |
| Product group | | Type of product | | Primary area of use |
| |
| |
|
| Green Fluorescent Proteins | | Licenses for research and screening applications | | Cell-based assays |
| | | | | |
| INCell Analyzer | | Advanced imaging device and biological assay reagents | | Cell-based system for cellular screening and target validation in drug discovery process |
| | | | | |
| LEADseeker™ Multimodality Imaging System | | Advanced system addressing all major modalities for screening assays | | Very high throughput screening |
Informatics
Informatics is a broad term referring to software, which enables researchers to capture and transform massive amounts of data from different systems into organized databases that researchers can analyze. As high throughput tools for drug discovery become established, Amersham Biosciences expects that the major bottleneck will no longer be hardware, but the ability of the customer to manage raw data generated from laboratories and to transform that data into an organized body of information that can facilitate the drug discovery process. The market environment is now set to change as customers adopt bioinformatics products (a subset of informatics products) to capitalize on the ability of these high-end systems to generate data. Customers are also recognizing the power of integrating their data across disciplines, other institutions and steps in the drug discovery process, which has created a market for analytical software packages and expert knowledge systems. Amersham Biosciences is introducing informatics software in order to support its integrated systems and customers’ laboratory workflow systems. These systems are designed to:
| • | | design, automate and acquire data, such as gene sequences, from biological experiments; |
| |
| • | | analyze and interpret experiment results; |
| |
| • | | enable the transfer of refined data into drug discovery databases; and |
| |
| • | | extract data from databases and analyze it further in order to compare results from one experimental process to another to gain greater understanding of biological systems. |
Amersham Biosciences expects these systems will further the goal of enabling molecular medicine by speeding up the drug discovery process through more efficient data handling.
In 1999, Amersham Biosciences began collaborating with Cimarron Software, Inc. (Cimarron), an informatics company located in Salt Lake City, Utah which supplies software for life sciences research, to develop a number of laboratory workflow software packages. In May 2002, Amersham Biosciences acquired a controlling stake in Cimarron, pursuant to its 35% share holding (44% as of December 31, 2002) and a shareholders’ undertaking, providing Amersham Biosciences with control over completion of these packages. Although these packages integrate across Amersham Biosciences discovery systems technology platforms, including MegaBACE™, Lucidea™ microarray systems and Ettan™, they are based on open architecture, thus allowing these packages to be connected to other software packages. Amersham Biosciences launched the first informatics software products developed jointly with Cimarron in September 2002.
The principal informatics products are summarized below:
| | | | | |
| Product group | | Type of product | | Primary area of use |
| |
| |
|
| Products being marketed | | | | |
| | | | | |
| Sequencing LWS 1.0 | | High throughput DNA sequencing laboratory work flow system | | Automated DNA sequencing |
| | | | | |
| Genotyping LWS 1.0 | | High throughput DNA genotyping laboratory work flow system | | Automated DNA genotyping |
| | | | | |
| Gene Expression LWS 1.0 | | High throughput gene expression laboratory work flow system | | Data acquisition for gene expression experiments |
| | | | | |
| Products in development | | | | |
| | | | | |
| Ettan WS LWS 1.0 | | High throughput laboratory work flow system for use with Ettan Work Station | | Protein expression analysis |
51
| | | | | |
| Product group | | Type of product | | Primary area of use |
| |
| |
|
| Gene expression LWS 1.2 | | Updated system for gene expression to include new workflows and instruments | | Data acquisition for gene expression experiments, including integration of CodeLink™ |
| | | | | |
| Genotyping LWS 1.2 | | Updated system for genotyping | | Automated DNA genotyping |
| | | | | |
| Proteomics LWS 1.2 | | Updated system for proteomics to include new features | | Expands use of proteomics instruments e.g. MALDI systems |
Protein separations
Amersham Biosciences’ protein separations business is built on extensive experience in liquid chromatography and understanding of proteins. The business is organized into two parts — bioprocess and laboratory separations. Bioprocess is the production of systems for the purification and production of biopharmaceuticals on a large scale, while laboratory separations, is the production of smaller, laboratory-scale systems for the purification and separation of proteins.
Bioprocess
Amersham Biosciences’ bioprocess business helps customers manufacture biomolecular based medicines. We provide biopharmaceutical manufacturers with scaleable and integrated technologies for purifying biopharmaceuticals, and services that offer reduced process development time and cost efficient production of biopharmaceutical medicines, such as hormones, enzymes, blood clotting factors, vaccines, antibodies, growth factors and antisense nucleic acid sequences.
Biopharmaceuticals are drugs based on active biomolecules, such as proteins, peptides or DNA. Having Amersham Biosciences’ products designed-in to the biopharmaceutical purification process is the key to sustainable revenues in the biopharmaceutical manufacturing process. Design-in is facilitated through biotechnology and pharmaceutical companies using Amersham Biosciences’ purification products successfully during their development phases, which are designed to be readily scaled-up, validated and registered in full-scale manufacturing. Once the FDA or other regulatory body approves a manufacturing process, changing this process is time consuming and expensive for the manufacturer. Amersham Biosciences’ chromatography media are used in the manufacture of over 90% of approved biopharmaceuticals and are involved in the majority of biopharmaceuticals that are currently in clinical trials.
Apart from demonstrated scalability, Amersham Biosciences believes its bioprocess business has a competitive advantage based on:
| • | | the reputation built in the purification field since 1959; |
| |
| • | | consistent quality supported by ISO 9001 certification; |
| |
| • | | a large range of media, allowing choice, and in some cases, custom design; |
| |
| • | | product lines with well-known brands, recognized by customers and regulatory organizations; |
| |
| • | | the manufacturing capacity to ensure reliable supply of chromatography media; |
| |
| • | | a complete solution, including equipment and regulatory support; |
| |
| • | | documentation to support validation and registration; and |
| |
| • | | a dedicated and experienced global sales force. |
A key strength of Amersham Biosciences’ bioprocess business is reliability and a proven record of results. One of its leading products, Sephadex™, which brought a new level of speed and simplicity to purification techniques, was introduced over 40 years ago, and is still a leading brand in separations media today. Sephadex™ has had an important role in many scientific discoveries and has been instrumental in Nobel Prize winning research. It is also widely used in industry, for example, in the production of highly purified gamma globulin used to treat a variety of diseases including Acquired Immune Deficiency Syndrome. Amersham Biosciences also produces a widely used purification media based on Sepharose™ originally introduced in the 1980s and other
52
products based on Sepharose™ for the purification of active proteins, as well as system and equipment components used in these processes. These products are able to address the challenges brought by new types of source materials and new types of biomolecules, such as those being tested for vaccines and antisense strategies.
Biotechnology innovation continues to drive the bioprocess business. Amersham Biosciences is a leading supplier of manufacturing systems and reagents for industrial DNA synthesis used by the molecular diagnostic industry and the emerging antisense industry. Antisense drugs involve the use of nucleic acids that bind to other target nucleic acids producing a therapeutic effect. These drugs are also known as oligonucleotide pharmaceuticals because of their use of short DNA sequences, or oligonucleotides. Amersham Biosciences’ leading patented product, OligoProcess™ enabled the antisense industry to manufacture the first antisense drugs at a commercially viable cost and scale.
Amersham Biosciences’ reputation, product offerings and support services in the bioprocess business create strong customer relationships. For example, Fast Trak™ services are provided to aid customers in complying with FDA requirements by providing validation support and regulatory support information. Fast Trak™ also provides comprehensive training programs for people working in biopharmaceutical manufacturing and development.
Amersham Biosciences continues to explore opportunities for the expansion of the bioprocess business. In August 2001, Amersham Biosciences acquired Dan-Process A/S, a Danish company, specializing in high-pressure chromatography instruments for the purification of biomolecules such as peptides. The acquisition strengthened the separations portfolio by enhancing the ability to offer a complete systems solution — with both media and instruments — to biopharmaceutical companies. In addition, in January 2002, two companies in the membrane separations field, AG Technology and InnovaSep Technology were acquired. Membrane separations or filtration is a natural extension of the chromatography business, in which Amersham Biosciences is a market leader, and these acquisitions give immediate access to the growing membrane separations market.
Laboratory separations
In laboratory separations, Amersham Biosciences provides systems for the purification of proteins, peptides and oligonucleotides. Customers include pharmaceutical and biotechnology companies and academic and research institutes. The product range covers chromatography systems and chromatography media. Amersham Biosciences currently has over 20,000 liquid chromatography, or LC, systems installed world-wide. The ÄKTA™ product range is the most widely used chromatography system in the industry. Amersham Biosciences continues to expand the ÄKTA™ platform, and during 2002 introduced several upgrades and new products, including the ÄKTApilot™ and several new media products.
Amersham Biosciences’ laboratory separations business is linked to the bioprocess business through the drug development design-in process. Researchers in industry use Amersham Biosciences laboratory separations systems to develop small-scale processes for the purification of biopharmaceuticals, which they later scale-up using Amersham Biosciences bioprocess products.
The principal bioprocess and laboratory separations products are summarized below.
| | | | | |
| Product group | | Type of product | | Primary areas of use |
| |
| |
|
| Products being marketed | | | | |
| | | | | |
| Sephadex™, Sepharose™, Source™ | | Chromatographic separation materials | | Purification of active proteins used in biopharmaceuticals |
| | | | | |
| Streamline™ | | Expanded bed chromatography system | | Primary chromatography step used for purification of active proteins used |
| | | | | in |
| | | | | biopharmaceuticals |
| | | | | |
| Cytodex™ | | Micro carrier for cell culture | | Research and vaccine production |
53
| | | | | |
| Product group | | Type of product | | Primary areas of use |
| |
| |
|
| Ficoll™, Percoll™ | | Media for cell separation | | Research applications,in vivotreatment |
| | | | | |
| Chromaflow™ Columns BioprocessSystems | | Production scale chromatography columns and systems | | Purification of active proteins used in biopharmaceuticals |
| | | | | |
| Mabselect™ | | Separations media | | Purification of monoclonal antibodies |
| | | | | |
| HiTrap™ | | Laboratory scale prepacked chromatography column | | Laboratory scale purification of biological molecules |
| | | | | |
| OligoPilot™, OligoProcess™ | | Systems for oligonucleotide synthesis | | Synthesis of oligonucleotides for production of pharmaceuticals and diagnostic probes |
| | | | | |
| Primer Support™ | | Solid phase support beads for oligonucleotides synthesis | | Synthesis of oligonucleotides for production of pharmaceuticals and diagnostic probes |
| | | | | |
| ÄKTAFPLC™ | | Chromatography systems for preparative purification of biomolecules | | Research and pharmaceutical methods development |
| | | | | |
| ÄKTApilot™ | | Laboratory chromatography system | | Small production scale protein purification under good manufacturing practices |
| | | | | |
| High flow Protein A | | High productivity affinity medium | | Antibody purification |
| | | | | |
| Primer Support 200 | | Solid phase support beads | | Synthesis of longer diagnostic probes |
| | | | | |
| Products in development | | | | |
| | | | | |
| Streamline™ reactor/expanded bed absorption media | | Equipment and medium for expanded bed absorption | | System for initial capture of biopharmaceuticals from production medium |
| | | | | |
| New ion exchange media, Niex | | New chromatography media | | Novel ion exchange media with improved productivity |
| | | | | |
| Bioselect | | Customized affinity ligands | | Biopharmaceutical manufacturing |
| | | | | |
| Viral Inactine™ | | Viral inactivation chemical | | Purification of plasma and proteins produced from cell culture, virus clearance in biopharmaceutical manufacturing |
| | | | | |
| ÄKTA™ 3D | | High throughput protein purification | | Protein purification, structural genomics |
54
Production
Manufacturing
Amersham Biosciences has an established global manufacturing base with six primary facilities in Piscataway, New Jersey, San Francisco and Sunnyvale, California in the US, Uppsala and Umea in Sweden, and Cardiff in the United Kingdom. Amersham Biosciences believes it has sufficient manufacturing capacity to meet commercial demand for its products for the foreseeable future. We manage non-radiochemical reagents operations at the Cardiff site, and the manufacture of radiochemical reagents used in radiolabeling assays and kits in line with the terms of the Amersham Biosciences nuclear license.
Amersham Biosciences’ skilled manufacturing staff, of over 1,000 employees, has technological expertise, significant interaction with R&D teams, direct customer contact and flexible skills. The manufacturing facilities are co-located with R&D and focus on the same technologies.
The primary manufacturing facilities optimize material flow and personnel movement with integrated manufacturing and quality control operations. We design access and safety features to meet federal, state and local health ordinances. Except for Piscataway, all Amersham Biosciences manufacturing facilities are accredited to ISO 9001 international standards.
We rely upon outside vendors to manufacture a number of components of instruments and some reagents, which are provided in systems. Amersham Biosciences regularly audits these vendors.
Amersham Biosciences is implementing a resource planning system to manage and control material and product inventories. This system encompasses product costing, materials procurement, production planning and scheduling, inventory tracking and control and batch records, with links to document control for all manufacturing, quality assurance and regulatory compliance procedures.
Raw Materials
Amersham Biosciences’ manufacturing operations require a wide variety of raw materials, electronic and mechanical components, chemical and biochemical materials, and other supplies some of which are occasionally in short supply. Dual sourcing is available for most components and supplies, but where Amersham Biosciences is dependent on single sources for a limited number of these items it normally secures long-term supply contracts.
Strategic Alliances
An element of Amersham Biosciences strategy is to seek strategic alliances with major academic research centers and biotechnology and pharmaceutical companies to access technology. This strategy enables acquisition of new technologies and maximization of R&D expenditures.
The table below lists some of Amersham Biosciences’ major strategic partners.
| | | | | |
| Strategic Partner | | Technology | | Product |
| |
| |
|
| Affibody Technology Sweden AB | | Ligands | | Ligands for protein purification |
| | | | | |
| Analytica of Branford, Inc. | | Mass spectrometry | | Ettan™ range |
| | | | | |
| Aurora Biosciences Corp. | | Green Fluorescent Proteins | | Right to sell licenses to use reagents in biological research and drug discovery |
| | | | | |
| Beckman Coulter Inc. | | Sequencing technology | | Separation matrices for capillary electrophoresis |
| | | | | |
| BioImage AS | | Cell-based assays | | LEADseeker™ cellular analysis system |
| | | | | |
| Dyax Corp. | | Ligands | | Peptide ligands for protein purification |
55
| | | | | |
| Strategic Partner | | Technology | | Product |
| |
| |
|
| Gyros AB | | CD-based miniaturization | | Technology for various miniaturized drug discovery products |
| | | | | |
| Molecular Staging, Inc. | | Template isolation & amplification | | TempliPhi range of products |
| | | | | |
| Nonlinear Dynamics Ltd | | Software for automated image analysis | | Ettan™ ImageMaster and Progenesis |
| | | | | |
| Proteometric LLC | | Software for mass spectroscopy | | Ettan™ range |
| | | | | |
| Scientific Analysis Instruments Ltd. | | Mass spectroscopy | | Ettan™ range |
| | | | | |
| Sloan Kettering Institute | | Functional Proteomics | | InCell Assays |
| | | | | |
| Thermo Electron Corporation | | Mass Spectrometry | | Mass Spectrometry |
| | | | | |
| Uppsala University, Sweden | | Surface interaction biotechnology | | Technology development for separation resins |
| | | | | |
| V. I. Technologies, Inc. | | Bioprocess | | Virus inactivation compounds |
Amersham Biosciences does not consider any of the agreements set forth in the table above material to its business. These agreements involve additional milestone or other payments (including minimum royalty payments) that in aggregate do not exceed £30 million, the majority of which are expected to be made within the next five years.
Amersham Biosciences reviews potential acquisition, investment and divestment opportunities in each of its business streams. Except as otherwise disclosed in this form, Amersham Biosciences currently does not have any material commitments or agreements for any acquisition or investments. An acquisition or investment may be structured as an acquisition, joint venture, or licensing arrangements. Amersham Biosciences may make an acquisition or investment if it believes the transaction will enhance the portfolio of offered products, particularly where the transaction relates to the identification and commercialization of innovative new technologies.
Marketing and Distribution
Marketing
Amersham Biosciences has an established worldwide sales and marketing network, with products sold in approximately 100 countries, including an integrated sales and marketing organization of approximately 1,600 employees serving the two principal business segments, with direct sales offices in 31 countries. The sales and marketing force is divided into four regional organizations: North America, Europe, Japan, and Rest of the World. The broad customer base and integrated product offerings allow the sales and marketing force to generate sales across business segments. We have also established direct marketing through catalog and brochure mailing, telemarketing and web-based marketing channels. Amersham Biosciences also distributes products provided by external suppliers.
e-business
Given the large product range and number of customers in the discovery systems business, Amersham Biosciences is pursuing a strategy to market products through the Internet. The Internet is expected to provide efficiency gains in the management of the market for recurring purchases that do not require extensive sales support. The improved efficiency in order processing and invoicing offered by Internet marketing channels will be complemented by the development of further e-business services that will offer technical support to customers. Amersham Biosciences is investing in new systems and procedures to improve the existing web presence to enable simpler on-line purchasing and provide technical information.
In 1999, Amersham Biosciences began developing systems to sell products through our electronic purchasing channels. The Company’s e-commerce website has been operating in the US since February 2000. e-commerce sales represented approximately 2% of Amersham Biosciences’ net sales in 2002.
56
Distribution
Amersham Biosciences has a worldwide distribution network consisting of two central warehouses and two regional warehouses employing over 100 people.
Research and Development
The key objectives of Amersham Biosciences’ R&D strategy are to develop systems to enable customers to understand the gene-function relationship and the molecular basis of diseases, to increase the efficiency and decrease the costs of drug discovery and enable the manufacture of biopharmaceutical products. R&D spending decisions are based on a technology’s commercial viability and its ability to meet return on investment criteria.
The following is a list of key systems and products, which Amersham Biosciences has developed internally over the last three years:
| • | | MegaBACE™ sequencer and genotyper; |
| |
| • | | Lucidea™ microarray systems; |
| |
| • | | Ettan™ series; |
| |
| • | | Two dimensional gel electrophoresis system; |
| |
| • | | LEADseeker™; |
| |
| • | | Typhoon™; |
| |
| • | | Ready-To-Run™; |
| |
| • | | Source™; |
| |
| • | | STREAMLINE™; |
| |
| • | | ÄKTAFPLC™; |
| |
| • | | ÄKTAprime™; |
| |
| • | | TempliPhi™; |
| |
| • | | INCell Analyzer, |
| |
| • | | AKTApilot, |
| |
| • | | DYEnamic™, |
| |
| • | | ET terminators. |
Amersham Biosciences employed in 2002 approximately 770 scientists in R&D at the following facilities and specializing in the following scientific areas:
| • | | Amersham, United Kingdom — Molecular sciences and proteomics developments; |
| |
| • | | Cardiff, United Kingdom — Cellular sciences and bioassays development; |
| |
| • | | Piscataway, New Jersey, US — Genomic sciences and development, notably DNA chemistry and enzymology; |
| |
| • | | Sunnyvale, California, US — Instrumentation sciences and genomics development including imaging and sequencing systems; |
| |
| • | | San Francisco, California, US — Proteomics development, notably 2-D systems; |
| |
| • | | Tokyo, Japan — Material sciences; |
| |
| • | | Uppsala, Sweden — Proteomics and protein separation sciences and development. |
Amersham Biosciences divides its R&D process into three stages: the research stage, the development stage and commercial product support. The research stage demonstrates technology feasibility, generates intellectual property and develops new systems or processes, which form the basis for new technology platforms. The development stage is commercially driven and involves the development of novel or next generation products. Commercial product support involves demonstration experiments for potential customers, providing technology training and consulting and collaborating with customers’ laboratories both before sales and after product launch.
R&D processes are managed at different levels to enable better decisions on resource allocation and prioritization. The development function reports through a formal structure to Business area executive teams to monitor milestones and resources, to obtain approval to commence projects and to recommend ending a project if it is not meeting its objectives. Amersham Biosciences’ executive management is responsible for setting R&D strategy, defining licensing activities,
57
realizing operating efficiencies and setting priorities. Eminent life scientists on the Scientific Advisory Board evaluate the R&D portfolio and advise on trends and related matters.
In 2002, Amersham Biosciences spent £88.0 million on R&D, or 13% of turnover. In 2001, £86.4 million was spent on R&D, or 13% of turnover, and in 2000, £71.5 million was spent on R&D, or 12% of turnover. No R&D expenditures were sponsored by customers.
Intellectual Property
Amersham Biosciences considers the protection of its proprietary technologies and products to be important to the success of its business. The Company relies on a combination of patents, licenses and trademarks to establish and protect proprietary rights to technologies and products. Patents have been obtained in many countries covering significant products developed through R&D activities. Amersham Biosciences currently owns 234 issued patents in the US and 424 issued patents in other major industrialized nations, and has 901 patent applications pending.
Amersham Biosciences believes that it continues to have patent protection for most important existing products in major markets, and in addition, has obtained patents, or anticipates that the appropriate regulatory bodies will grant patents for a majority of the new products and technologies being developed. Amersham Biosciences intends to continue to file patent applications as new products and technologies are developed.
U.S. patents normally have a term of 17 years from the date of issue for patents issued from applications filed prior to June 8, 1995 and 20 years from the date of filing of the application in the case of patents issued from applications filed on or after June 8, 1995. Patents in most other countries have a term of 20 years from the date of filing the patent application.
Amersham Biosciences is party to various exclusive and non-exclusive licensing agreements with third parties that grant it rights to use key components within Amersham Biosciences’ technologies. Amersham Biosciences believes the licensing agreements listed below are important to its business. Each of these agreements terminates upon the expiration of the related patents.
| • | | University of California — exclusive license to patents relating to MegaBACE™ sequencing systems and energy transfer dyes; |
| |
| • | | Harvard University — exclusive license to patents relating to Thermo Sequenase™ DNA Polymerase; and |
| |
| • | | California Institute of Technology — non-exclusive license to patents relating to fluorescent sequencing technology. |
| |
| • | | Carnegie Mellon University — exclusive license to patents relating to fluorescent cyanine dyes. |
Amersham Biosciences relies in part on trade secret protection of its intellectual property and by entering into confidentiality agreements with third parties, employees and consultants. Employees and consultants also sign agreements to assign to Amersham Biosciences their interests in patents and copyrights arising from their work for the Company. Employees also agree not to use confidential information after their employment. However, these agreements can be breached and an adequate remedy may not be available. Also, a third party may learn trade secrets through means other than by breach of confidentiality agreements, or competitors could independently develop them.
Seasonality
Certain of Amersham Biosciences subsidiaries are subject to seasonal trends within the year. The fourth quarter is heavily affected by the annual purchasing patterns of its customers.
Backlog
Amersham Biosciences’ backlog at December 31, 2002 approximated £38.6 million. Amersham Biosciences does not consider backlog to be a key measure of future sales. We anticipate that all products included in backlog will be delivered before the end of 2003. It is our policy to include in backlog only purchase orders or production releases that have firm delivery dates within one year. However, not all recorded backlog may result in actual sales because of cancellation of orders or other factors.
58
C. Organizational Structure
The following information relating to subsidiary, joint venture and associated undertakings is given on those entities that, in the opinion of the Directors, materially affected the profits or assets of the Group.
The primary activity of the majority of the undertakings shown below is the development and manufacture and/or sale of specialized products for research-based biotechnology supply and the diagnosis and treatment of disease. Subsidiary, joint venture, and associated undertakings are listed under the relevant business operation. Where the relevant company serves more than one business it is listed under the dominant business operation.
With the exception of Amersham Buchler GmbH & Co KG and Amersham Biosciences Limited, the Company’s holdings in the following undertakings are represented by ordinary shares. Amersham Buchler GmbH & Co KG is a partnership in which the Group’s interest is in limited liability form. The company holds ordinary and non-voting preference shares in Amersham Biosciences Limited in the proportion stated below.
All of the companies listed below operate principally in their country of incorporation or registration and have coterminous year-ends.
| | | | | | | | | | | | | |
| | | | | | | Proportion of nominal value of
issued shares held |
| | | | | | |
|
| | | Country of | | by the | | by subsidiary |
| | | incorporation or | | Company | | undertakings |
| | | registration | | % | | % |
| | |
| |
| |
|
Holding companies: | | | | | | | | | | | | |
| Amersham Health Norge AS | | Norway | | | — | | | | 100 | |
| Acam Overseas Holdings Limited | | UK | | | — | | | | 100 | |
| Acam Holdings (UK) Limited | | UK | | | 100 | | | | — | |
| Amersham Benelux BV | | Netherlands | | | — | | | | 100 | |
| Amersham Biosystems AB | | Sweden | | | | | | | 100 | |
| |
Amersham Biosciences | | | | | | | | | | | | |
| Amersham Biosciences Limited | | UK | | | 61 | | | | 39 | |
| Amersham Biosciences Corp | | US | | | — | | | | 100 | |
| Amersham Biosciences UK Limited | | UK | | | — | | | | 100 | |
| Amersham Biosciences Europe GmbH | | Germany | | | — | | | | 100 | |
| Amersham Biosciences AB | | Sweden | | | — | | | | 100 | |
| Amersham Biosciences (SV) Corp | | US | | | — | | | | 100 | |
| Amersham Biosciences KK | | Japan | | | — | | | | 100 | |
| Amersham Biosciences Limited | | Hong Kong | | | — | | | | 100 | |
| AG Technology Corp | | US | | | | | | | 100 | |
| InnovaSep Technology Corporation | | US | | | | | | | 100 | |
| |
Amersham Health | | | | | | | | | | | | |
| Amersham Buchler GmbH & Co KG | | Germany | | | — | | | | 60 | |
| Amersham Health AS | | Norway | | | — | | | | 100 | |
| Amersham Health | | Ireland | | | — | | | | 100 | |
| Amersham Health Inc | | US | | | — | | | | 100 | |
| Amersham Health Pte Ltd | | Singapore | | | — | | | | 100 | |
| Nycomed Amersham Sorin Srl | | Italy | | | 100 | | | | — | |
| Amersham Health Limited | | China | | | — | | | | 80 | |
| Amersham Health Holdings Inc | | US | | | — | | | | 100 | |
Joint venture: | | | | | | | | | | | | |
Amersham Health | | | | | | | | | | | | |
| Nihon Medi-Physics (NMP)* | | Japan | | | 29 | | | | 21 | |
| * | | The investment in NMP relates to ordinary shares. The shares carry standard pre-emption rights |
59
D. Property, Plant and Equipment
The net book value of the Group’s properties amounted to £205.9 million at December 31, 2002.
Amersham’s principal executive offices are located in Buckinghamshire, England. Listed below are the location of the Group’s principal facilities, together with summary details of the form of title, approximate floor space of the buildings and the principal activities conducted at such facilities. The Group considers all the facilities listed below to be reasonably appropriate for the purposes for which they are used, including manufacturing, R&D, and administrative purposes. All properties are maintained in good working order and, in the case of those used for manufacturing purposes, are substantially utilized on the basis of at least one shift.
| | | | | | | | | |
| Location | | Principal Activities | | Square meters | | (L) Leased
(O) Owned |
| |
| |
| |
|
| Canada, Quebec | | Offices & distribution | | | 1,376 | | | L |
| | | | | | | | | |
| China, Shanghai | | Manufacturing | | | 9,100 | | | O |
| | | | | | | | | |
England, Buckinghamshire
(The Grove Centre) | | R&D/manufacturing— all divisions | | | 30,371 | | | O |
| | | | | | | | | |
England, Buckinghamshire
(Pollards Wood) | | Executive office | | | 4,475 | | | O |
| | | | | | | | | |
England, Buckinghamshire,
(Amersham Place) | | Executive office | | | 4,903 | | | L |
| | | | | | | | | |
| England, Gloucester | | Offices, production, warehousing | | | 3,570 | | | L |
| | | | | | | | | |
| Germany, Braunschweig | | Manufacturing/Commercial | | | 5,908 2,370 | | | O
L |
| | | | | | | | | |
| Germany, Freiburg | | Executive office | | | 1,912 2,377 | | | L
O |
| | | | | | | | | |
| Hong Kong | | Commercial/manufacturing | | | 2,094 | | | L |
| | | | | | | | | |
| Ireland, Cork | | Production, warehousing and offices | | | 16,350 | | | O |
| | | | | | | | | |
| Italy, Saluggia | | Manufacturing/R&D/
Commercial | | | 4,500 | | | L |
| | | | | | | | | |
Japan, Chiba(1) | | Manufacturing/R&D | | | 12973 | | | L |
| | | | | | | | | |
Japan, Hyogo (Nishinomiya
main office)(1) | | Executive office | | | 1,812 | | | L |
| | | | | | | | | |
Japan, Hyogo(1) | | Manufacturing | | | 11,039 | | | O |
| | | | | | | | | |
Japan, Tokyo(1) | | Executive office | | | 2,543 | | | L |
| | | | | | | | | |
| Japan, Tokyo | | Commercial/R&D | | | 3,700 | | | L |
| | | | | | | | | |
| Norway, Lindesnes | | Production, warehousing and offices | | | 27,000 | | | O |
| | | | | | | | | |
| Norway, Oslo | | Offices, R&D, productions and warehousing | | | 46,777 | | | O |
| | | | | | | | | |
| Sweden, Staffanstorp | | Commercial/manufacturing | | | 3,800 | | | L |
| | | | | | | | | |
| Sweden, Umea | | Commercial/manufacturing | | | 18,100 | | | O |
| | | | | | | | | |
| Sweden, Uppsala | | Executive office/ manufacturing/R&D | | | 105,000 | | | O |
| | | | | | | | | |
| US, Arlington Heights, Illinois | | Offices and manufacturing | | | 16,000 | | | O |
| | | | | | | | | |
| US, Chandler, Arizona | | Manufacturing & R&D | | | 7,804 | | | L |
| | | | | | | | | |
| US, Lawrenceville, New Jersey | | R&D offices | | | 1,500 | | | L |
| | | | | | | | | |
| US, Piscataway, New Jersey | | Commercial/R&D/ Manufacturing | | | 25,000 | | | O |
| | | | | | | | | |
| US, Princeton, New Jersey | | Offices | | | 10,219 | | | L |
| | | | | | | | | |
| US, San Francisco | | Offices | | | 4,467 | | | O |
| | | | | | | | | |
| US, South Plainfield, New Jersey | | Manufacturing | | | 2,090 | | | O |
| | | | | | | | | |
| US, Sunnyvale, California (two sites) | | Commercial/R&D/ Manufacturing | | | 10,078 | | | L |
| | | | | | | | | |
| US, Westborough, Massachusetts | | Manufacturing | | | 9,290 | | | L |
| | | | | | | | | |
| Wales, Cardiff (The Maynard Centre) | | R&D and manufacturing | | | 32,963 | | | O |
60
Plans for expansion
Amersham Health has recently received approval from the Group to extend a project to expand production capacity at the Lindesnes plant in Norway. This project is planned to run until approximately 2005. In addition, Amersham Health is currently carrying out an IT upgrade project due to be completed in the UK at the end of 2003. Amersham Biosciences is in the process of refurbishing its Pollards Wood site in the UK which is planned to be completed by May 2003.
61
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
Introduction
The following discussion and analysis is based on and should be read in conjunction with the Consolidated Financial Statements and Notes thereto included in Item 18 “Consolidated Financial Statements”. The Consolidated Financial Statements have been prepared in accordance with UK GAAP. The principal differences between UK GAAP and US GAAP as they relate to the Group are described in Note 38 of Item 18 “Consolidated Financial Statements”. Reconciliation of the significant differences between UK GAAP and US GAAP are also set forth in Note 38 of Item 18 “Consolidated Financial Statements”.
Basis of preparation
Summary
The discussion and analysis of the Consolidated Financial Statements is set out as follows and provides comparisons of statutory reported data for the following:
| • | | Results of operations for the 12 months to December 31, 2002 compared with the 12 months to December 31, 2001; |
| |
| • | | Results of operations for the 12 months to December 31, 2001 compared with the 12 months to December 31, 2000; |
| |
| • | | Liquidity and capital resources. |
Commentary on the Company’s R&D policies and intellectual property is included in Item 4 “Information on the Company”, and within the discussion of the results of operations for each year and in “Trend Information” below. Commentary on significant trends in production, turnover and inventory is included within the discussion of the results of operations for each year below and in “Trend Information” below. Associated risks are discussed in Item 3. “Key Information” — D. “Risk Factors”
Overview and general matters
Amersham plc is currently organized into two principal lines of business: the medical imaging business (“Amersham Health”) and the biosciences business (“Amersham Biosciences”).
Amersham Health
Amersham Health is a world leader in the development, manufacture and supply of innovative products for the diagnosis and treatment of disease. Medical imaging products are used when information is needed about parts of the body, such as organs, tissues and blood vessels, which are not easily distinguishable from the normal body background.
Amersham Health focuses on the management of heart and circulatory disease, diseases of the brain such as stroke, Alzheimer’s Disease and Parkinson’s Disease, lung disease, and various cancers. Advances in scanning technologies and procedures, and greater usage of such diagnostic procedures, are driving demand for its products, along with the ageing population with its higher incidence of such diseases.
Amersham Health is actively managing and developing its portfolio of diagnostic and therapeutic products, which are focused in the areas of cardiology, neurology, oncology and pulmonology.
As well as discussing revenues from these disease areas in the Operating and Financial Review, Amersham Health further presents revenues from patented and unpatented products, revenues by modality and also by their regional performance.
62
Amersham Biosciences
Amersham Biosciences is a world-leading provider of biotechnology systems and solutions for research into genes and proteins, the discovery and development of drugs, and the manufacture of biopharmaceuticals.
Effective January 1, 2002, Amersham Biosciences reorganized its business streams, creating discovery systems consisting of the former drug discovery, laboratory products, and service, agency and other business streams, and maintaining the protein separations business stream. Segmental reporting has been restated as appropriate for periods prior to January 1, 2002.
Through the two business areas, discovery systems and protein separations, we bridge the gap between life sciences and healthcare. In discovery systems, we provide high throughput systems to help researchers in industry and academia understand the genetic and molecular basis of disease and speed up their drug development. In protein separations, we provide research and manufacturing technologies that enable pharmaceutical and biotech companies to bring new biological drugs to the market. Together, these activities cover the gene to drug spectrum.
Presentation of Information
We continue to assess the performance of the business on a like for like comparable basis which excludes the impact of exceptional items, goodwill amortization, foreign exchange rate fluctuations and disposed businesses. Accordingly, in addition to quoting the absolute growth rates, we also quote a like for like comparable growth rate excluding the impact of foreign exchange rate fluctuations and, as appropriate, any other applicable items (ie exceptional items, goodwill amortization and disposed businesses). Where “other items” are not listed, they had no impact on the growth rate. The impact of foreign exchange rate fluctuations is excluded by translating the results of comparable periods using a common set of exchange rates.
Exchange Rate Fluctuations
The consolidated results of operations of Amersham plc are presented in pounds sterling. The Group operates in currencies other than pounds sterling, in particular the Norwegian kroner, Swedish krona, euro, US dollar and the Japanese yen. As a result, fluctuations in these currencies compared to the pound sterling are significant to Amersham plc and its shareholders because, among other things, they affect the translation of the results of the non-UK operations into pounds sterling, for the purposes of Amersham’s consolidated financial statements. Movements in the exchange rates used to translate such foreign currencies into pounds sterling may have a significant impact on Amersham’s reported results of operations from year to year.
The principal financial exposure is to movements in foreign currency. These comprise transaction exposures arising from currency cash flows and translation exposures arising from the conversion of the results of foreign operations into sterling.
The Group follows policies to reduce the impact of these currency fluctuations through hedging. Anticipated transaction exposures are hedged on a rolling six-month basis. In addition where sterling has deviated significantly from purchasing power parity rates, longer-term forward cover is undertaken. Profits earned overseas are not hedged. Treasury activities are coordinated and managed by the Group treasury department in accordance with policies and procedures approved by the Board. The department operates under close management supervision and is subject to internal audit. It does not operate as a profit center and no transactions of a speculative nature are undertaken.
63
The key exchange rates for the Group in 2002, compared to 2001 and 2000, were as follows:
| | | | | | | | | | | | | |
| | | Average rate | | Average rate | | Average rate |
| Currency | | to sterling in 2000 | | to sterling in 2001 | | to sterling in 2002 |
| |
| |
| |
|
| Dollar | | | 1.51 | | | | 1.44 | | | | 1.51 | |
| Japanese yen | | | 164.00 | | | | 183.00 | | | | 188.00 | |
| Euro | | | 1.65 | | | | 1.61 | | | | 1.59 | |
| Swedish krona | | | 13.96 | | | | 14.99 | | | | 14.57 | |
| Norwegian kroner | | | 13.36 | | | | 12.97 | | | | 11.99 | |
The impact of exchange rates on profit before tax in the year ended December 31, 2002 was neutral, after hedging, when compared to the year ended December 31, 2001, as a result of the benefits from translating profits earned in the strengthening Scandinavian currencies offsetting the impact of weakening sales in Japanese Yen and US Dollars.
In 2001, profit before tax was impacted by a favorable exchange effect of £26 million after hedging, when compared to the year ended December 31, 2000, as a result of the strengthening US dollar and the weakening of the Scandinavian currencies against sterling.
Dividends of Amersham, when declared, are paid in pounds sterling, with Norwegian holders (holding their Amersham Ordinary Shares through the VPS, the Norwegian securities registry) receiving dividends in Norwegian kroner at the exchange rate applicable on the dividend record date. US holders of American Depository Receipts (ADRs) receive dividends in US dollars at the exchange rate applicable on the dividend record date. If all other factors remain constant, an increase or decrease in the value of the US dollar or Norwegian kroner against the pound sterling would respectively decrease or increase the US dollar or Norwegian kroner value of dividends declared and paid by Amersham in pounds sterling. Each of the relevant rates of exchange has been volatile in the recent past, and no assurance can be given that they will not be volatile in the future. Persons who wish to convert dividends or other distributions declared and paid in pounds sterling into US dollars or Norwegian kroner may be required to pay a transaction cost.
Effect of Inflation
Inflation is not considered to have a material effect on the results of the Group.
Critical accounting policies
Our financial statements are based on the selection and application of significant accounting policies, some of which require management to make estimates and assumptions. These policies are in accord with all applicable United Kingdom accounting standards. The following policies are believed to involve a higher degree of judgment and complexity than other accounting policies, all of which are described in Note 1 of Item 18 “Consolidated Financial Statements”:
Investments — trade investments
The Group’s accounting policy is to carry trade investments at cost less any provision for impairment, restated to year end exchange rates if the underlying investment is denominated in foreign currency. During 2002, a charge of £2.4 million was made against the profit and loss account to reflect a reduction in the carrying value of listed investments following a material reduction in their market value. The market value of listed investments at December 31, 2002 was £3.6 million and the carrying value after provisions for impairment was £5.5 million. In applying its accounting policies, the Group has made an assessment as to the long term value of the listed investments which assumes that the market value will recover to at least meet their carrying value. Should the value of the investments not recover and the market value decline further, then it is possible that further provisions will be necessary. For US GAAP reporting purposes, the listed trade investments are carried at market value with the change in market value reflected in Other Comprehensive Income. In the event a decline in market value is not considered temporary, the loss will be taken as a charge to US GAAP current earnings.
64
Goodwill
From January 1, 1998, the Company’s accounting policy has been to capitalize goodwill arising on acquisitions and to amortize it over its estimated useful economic life when the goodwill is considered to have a limited useful economic life in accordance with FRS 10 ‘Goodwill and Intangible Assets’. Goodwill is amortized on a straight-line basis at rates currently from 5% to 14% per annum. In accordance with UK reporting standards, capitalized goodwill is reviewed for evidence of impairment at the end of the first full financial year following the initial date of recognition and, from then on, if management considers that events or changes in circumstance indicate that the amortized carrying value of the goodwill is not recoverable. In accordance with FRS 10, the Company will be required during 2003 to review goodwill arising on acquisitions it has made in 2002, amounting to £742.8 million and which are identified in Note 24 of Item 18 “Consolidated Financial Statements”. When goodwill is considered to have a useful economic life, impairment testing under UK GAAP allows only the cash generation during the useful economic life to be included. Under US reporting standards, with the introduction of Statement of Financial Accounting Standards (SFAS) 142 for fiscal years starting after December 15, 2001, goodwill is assumed to be a non-wasting asset, having an indefinite useful life and will be tested at least annually for impairment. As goodwill is assumed to be non-wasting, the inclusion of a perpetuity value is allowed in impairment testing.
Management does not believe that under either UK or US GAAP there is any impairment in the carrying value of existing goodwill, based on its view of the business and the expected likely cash flows that will be derived from the businesses to which the goodwill relates. However, changes in market conditions or business performance could cause fluctuations in the estimates of future cash flows of the businesses to which goodwill relates and give rise to a charge for impairment under both UK and US GAAP. This could be material to both the balance sheet and profit and loss account of a particular year. At December 31, 2002, under US GAAP, the carrying value of goodwill was £1,336 million and under SFAS 142 there was no impairment. Under UK GAAP the carrying value of goodwill at December 31, 2002 was £704.2 million with £36.6 million being amortized in the profit and loss account for the year. The significant difference in the level of goodwill under UK GAAP and US GAAP is due to the fact that the change in accounting policy to capitalize goodwill, as a result of the introduction under UK GAAP of FRS 10, did not need to be applied retrospectively to goodwill written off against shareholders’ funds in previous years.
Provisions for radioactive decommissioning costs
The Group recognizes in full, under both UK and US GAAP, a provision (discounted) for the cost of radioactive decommissioning when the relevant fixed asset becomes operational and an equivalent asset is recognized. The cost of radioactive decommissioning is determined on a going concern basis on the basis that the manufacture or handling of radioactive products continues at the relevant plant or facility on which the asset is operated for the foreseeable future. In the unlikely event that the Company is required to totally discontinue activities at one of its nuclear operating sites, the full costs of exiting and decommissioning would be higher. The relevant decommissioning asset is capitalized in the balance sheet in association with the fixed asset to which it relates, and is amortized over the life of the asset. The unwinding of the discount on the decommissioning provision is charged in the profit and loss account as part of the interest charge.
The cost of decommissioning is estimated based on the latest technical assessments of the process and methods likely to be used in carrying out the decommissioning and the expected date of decommissioning the relevant facility. For those sites where the assets are currently being used and are active, these assumptions are reviewed regularly by management and the provision is adjusted to reflect changes in assumption over the life of the assets. While these assumptions reflect a high degree of uncertainty because they are looking at facts and circumstances well into the future, they are unlikely to result in a material change to either the balance sheet provision or profit and loss account charge. However, when a decision is taken to exit a site, a more detailed cost estimate is prepared and this is reviewed on an on-going basis as the work progresses. Because of the technical uncertainties involved in carrying out the decommissioning work, it is possible that this review could result in a significant change in the overall decommissioning cost which will be reflected as an immediate charge to the profit and loss account. In the unlikely event that the Company is required to totally discontinue activities at one of its nuclear operating sites the full costs of exiting and decommissioning would be higher than provided for.
65
Effluent costs associated with radioactive waste arising from operations for which an authorized disposal route is available, principally low level and very low level waste, are written off in the year in which they are incurred. Provision is also made as incurred for the eventual disposal costs of the Group’s intermediate level waste, which arises from its operations. As with decommissioning costs, these provisions are based on the latest technical assessment of the process and methods likely to be used to dispose of this waste and on industry estimates of the likely costs of the necessary disposal facilities under the relevant government’s preferred disposal route. While these assumptions reflect a high degree of uncertainty because they are looking at facts and circumstances well into the future, they are unlikely to result in a material change to either the balance sheet provision or profit and loss account charge.
At December 31, 2002, the Group’s provision for costs associated with the eventual decommissioning of facilities and the eventual disposal of intermediate level waste was £65.5 million. The amounts charged to profit and loss in respect of decommissioning and effluent costs for the year to December 31, 2002 were £5.1 million. Total decommissioning and effluent costs to be incurred in the next ten years amount to approximately £37.4 million. The provisions are based on the latest technical assessment of the process and methods likely to be used stated at current prices and are discounted using a real discount rate of 4%.
Pensions
The Group currently operates both defined benefit and defined contribution pension schemes under arrangements that have been separately established by each company in the Group. Costs are charged as incurred for defined contribution schemes. However, for defined benefit schemes, the Group’s policy is to account for pension costs by charging the expected cost of providing pensions over the period during which the Group benefits from the employee’s service. The effects of variations from regular cost are spread over the expected remaining service lives of members of the scheme. This approach is in accord with the current UK Statement of Standard Accounting Practice (SSAP) 24 ‘Accounting for Pension Costs’. SSAP 24 requires the selection of a number of actuarial assumptions including the expected long-term return on assets of the pension fund. Actuarial assumptions reflect the best estimate of outcomes, which carry significant uncertainty. These assumptions are reviewed regularly with the fund actuaries and may be adjusted periodically based on current conditions. The focus of the Group’s policy is on achieving a relatively stable charge to the profit and loss account to cover the Group’s long-term future pension liability based on current actuarial assumptions. Therefore changes in assumption are unlikely to result in a material change to the balance sheet or profit and loss account charge in a particular year. However, SSAP 24 does not require us to reflect either the fair, or market, value of the fund’s assets or liabilities.
The Group accounts for pension costs under SFAS 87 for US GAAP based on a comparison of the projected benefit obligation with the market value of the underlying plan assets. Principally, as a result of this difference in methodology, the Group’s US GAAP pension cost is more sensitive to changing economic conditions.
A new UK accounting standard for pensions, FRS 17 ‘Retirement Benefits’, was originally planned for implementation in 2003 but has been deferred by the UK standard setters until 2005. FRS 17, similar to SFAS 87, approaches pensions accounting from a balance sheet perspective with the aim of ensuring that financial statements reflect the fair, or market, value of pension assets and liabilities. Gains and losses will be reflected as incurred rather than being spread over future accounting periods. An actuarial review of the impact of moving to a fair, or market, value for the pension fund assets and liabilities under FRS 17 indicates that the provision for pension costs would be increased by £127 million on the Group’s balance sheet as at December 31, 2002, whilst the profit and loss account charge is broadly the same. As required under the disclosure requirements of FRS 17, this is detailed within Note 30 of Item 18 “Consolidated Financial Statements”.
Deferred taxation
The Group provides for deferred taxation under FRS 19 ‘Deferred Taxation’. This requires liabilities to be recognized for most types of timing differences regardless of whether they are anticipated to reverse in the foreseeable future. Deferred tax assets are only recognized to the extent it is more likely than not that they will be recoverable. The recoverability of such deferred tax assets is based upon likely business performance, normally the business’s budget or strategic
66
plan and tax planning opportunities available to the Group. In 2002, we recognized a deferred tax asset of £9.2 million, relating to the utilization of prior period tax losses following the formation of a consolidated tax group in the US.
At December 31, 2002, the Group provided for a deferred taxation liability of £61.5 million based on the current accounting policy and deferred tax assets of £32.9 million. The 2001 year end accounts have now been restated to reflect the introduction of FRS 19 and a deferred tax liability of £41.9 million has been recorded in the December 31, 2001 balance sheet together with deferred tax assets of £30 million.
Under US GAAP, the Group provides for deferred taxation on a full liability basis and a valuation allowance is established when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Contingent liabilities
The Group is involved in various disputes, and has a number of unresolved issues, of a nature considered typical for its business, including those relating to product liability, waste management and infringements of intellectual property rights, validity of patents and taxation. Although there can be no assurance regarding the outcome of any of these disputes, the Group believes that on the basis of current available information they will not have a material adverse impact on the Group’s financial position.
Revenue recognition
Revenues are recognized once the realization of the consideration is reasonably assured and the provision of the goods or services is substantially complete with respect to the delivery of the specific product or performance of the related service. This will normally be the case when there are no material uncertainties or performance duties outstanding which could prevent the Company from enforcing the sales’ transaction and collectibility is reasonably assured. Product sales are normally recognized only when the goods are shipped to customers in accordance with the terms of the contract with the customer. Service revenues are recognized when the service is performed and there are no significant uncertainties regarding the revenue to be received. The revenue generated from technology transfer agreements is recognized evenly over the life of the agreement in order to provide greater comparability between different arrangements.
Under US GAAP, more prescriptive criteria are applied to assess whether the culmination of the earnings process has occurred and as disclosed in Note 38 of Item 18 “Consolidated Financial Statements”, the Group complies with Staff Accounting Bulletin 101.
Inventory valuations
Inventories are stated at the lower of cost and net realizable value under both UK and US GAAP. Provision is made for obsolescent and slow moving stocks and for radioactive decay. The value of any provision for obsolescence or slow moving inventory will consider management’s best estimate of future sales. Such forward looking estimates will carry uncertainty and so as projections change to reflect actual trading performance, the level of provision may also vary.
Receivable provisions
Provisions under both UK and US GAAP against doubtful receivables are made taking into consideration the local market situation and past experience of our dealings with the customer. It is possible that recoverability of a receivable may change due to circumstances not currently foreseen by management.
Initial Adoption of New Accounting Standards
During 2002, we implemented FRS 19 ‘Deferred Taxation’, and have included the further relevant disclosure requirements of FRS 17 ‘Retirement Benefits’.
Prior to implementing FRS 19, provision was made for deferred taxation, using the liability method, on all material timing differences to the extent that it was probable that a liability or asset would crystallize. Following the implementation of FRS 19, liabilities will be recognized for most types of timing differences regardless of whether they are anticipated to reverse in the foreseeable future. Deferred tax assets will be recognized to the extent that it is more likely than not they will
67
be recoverable. The introduction of FRS 19 has not had any significant impact on the Group’s balance sheet. We have however restated the effective tax charge for 2001 to take account of the new accounting standard, increasing last year’s effective tax rate from 33.1% to 34.7% and reducing 2001 earnings per share before exceptional and goodwill amortization from 27.2p to 26.6p.
FRS 17 will change the way that companies account for pensions and other retirement benefits. During 2002 the introduction of the standard was deferred until accounting periods ending after January 1, 2005. However, the transition disclosure period has been extended until this time and so there are further new disclosures required for the first time in 2002, which are contained in Note 30 of Item 18 “Consolidated Financial Statements”. In common with many of our peer companies, the fall in equity markets has had a significant impact on our net liability under FRS 17, which has increased from £24 million to £127 million taking into account provisions we have on our balance sheet for pensions, primarily for unfunded plans in territories where it is not normal practice to have funded arrangements. This level of net liability, while significant, can be managed comfortably within the liquidity of the Group going forward. Had we adopted FRS 17 in 2002, then the charge would have been broadly the same as under the current accounting standard SSAP 24. It should also be noted that the net liability required to be disclosed under FRS 17 is not necessarily the basis on which the funding decisions of the pension plans within the Group are made. In particular, the triennial valuation of the Company’s UK plan will be made in 2003 and we expect the outcome of the valuation to be adopted in 2003.
Under US GAAP, following the introduction of SFAS 142, goodwill and intangible assets with indefinite useful lives are not amortized, but reviewed annually for impairment. The 2002 amortization charge on such goodwill recognized for UK GAAP has been reversed for US GAAP purposes as disclosed in Note 38 of Item 18 “Consolidated Financial Statements”.
Recently Issued Accounting Standards
UK GAAP
Retirement Benefits
In November 2000, the Accounting Standards Board, (ASB), issued FRS 17Retirement Benefits. The standard will have a significant impact on the way that companies account for pensions and other post retirement benefits in the future. Although the standard does not become mandatory until accounting periods beginning on or after January 1, 2005, it sets down transitional arrangements which require footnote disclosures in the interim period up to full adoption. Transitional disclosures required under the standard are given in note 30 to the accounts.
US GAAP
Asset Retirement Obligations
In June 2001, the FASB published SFAS 143,Accounting for Asset Retirement Obligations. The standard codifies the accounting for any obligations arising from asset retirements, requiring the fair value of a liability for obligations to be recognized in the period it is incurred. Upon initial recognition of such a liability, entities should capitalize the cost by recognizing an increase in the carrying value of the related tangible fixed asset. The statement is effective for financial years beginning after June 15, 2002.
Costs Associated with Exit or Disposal Activities
In June 2002, the FASB published SFAS 146,Accounting for Costs Associated with Exit or Disposal Activitieswhich addresses financial accounting and reporting for costs associated with exit or disposal activities and nullifies Emerging Issues Task Force (EITF) Issue no 94-3, Liability Recognition for Certain Employee Termination Benefits and other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring). The statement requires that a liability for a cost associated with an exit or disposal activity be recognized when the liability is incurred and can be measured at fair value. The provisions of this statement are effective prospectively for exit or disposal activities initiated after December 31, 2002.
68
Disclosure Requirements for Guarantees
In November 2002, the FASB published Interpretation No 45, (FIN 45),Guarantors Accounting and Disclosure Requirements for Guarantors, Including Indirect Guarantees of the Indebtedness of Others. FIN 45 elaborates on the existing disclosure requirements for most guarantees, including loan guarantees such as standby letters of credit. FIN 45 will be effective on a prospective basis to guarantees issued or modified after December 31, 2002.
Consolidation of Variable Interest Entities
In January 2003, the FASB published Interpretation No. 46, (FIN 46)Consolidation of Variable Interest Entities.Under FIN 46, certain entities known as “Variable Interest Entities” (VIE) must be consolidated by the “primary beneficiary” of the entity. The primary beneficiary is generally defined as having the majority of the risks and rewards arising from the VIE. For VIE’s in which a significant (but not majority) variable interest is held, certain disclosures are required. The measurement principles of this interpretation will be effective for the Group’s December 31, 2003 financial statements.
Financial Instruments with Characteristics of both Liabilities and Equity
In May 2003, the FASB published SFAS 150,Accounting For Certain Financial Instruments with Characteristics of both Liabilities and Equity.The standard establishes how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity and requires that an issuer classify a financial instrument that is within its scope as a liability or an asset in some circumstances. SFAS 150 will be effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003.
The Group is still evaluating the impact of SFAS 143, SFAS 146, FIN 45, FIN 46 and SFAS 150.
Events impacting comparability
Set out below are events, which impact the comparability of reported financial information between the periods presented. Further discussion of acquisitions and dispositions can be found in Item 4 “Information on the Company.”
Acquisitions
On March 21, 2002, the Group completed the purchase of the 45% minority interest holding in Amersham Biosciences that it did not already own. The purchase consideration was £704.1 million of cash, plus costs associated with the purchase of £4.5 million. The purchase was financed by a placing of 57.5 million new Ordinary Shares on March 18, 2002, which generated £397.0 million after costs of issue, together with the use of existing cash resources and drawings under the Group’s committed bank facilities. The transaction was accounted for using purchase accounting. Goodwill of £692.3 million arose on the transaction, and will be amortized over 20 years from the date of the purchase. The 20 year useful life reflects the continuing investment in the development of the business. Amersham Biosciences was already consolidated with the rest of the Group as a result of our holding of the majority 55% stake, and so its profits and cash flows are already included in the consolidated profit and loss account and cash flow statement of the Group for 2000,2001 and 2002.
On January 31, 2002, the Group acquired 100% of the issued share capital of two filtration companies, AG Technology Corp and InnovaSep Technology Corporation. The fair value of the total consideration is £38.1 million, of which £11.8 million is deferred consideration and £6.7 million is the Group’s best estimate of deferred contingent consideration. The deferred contingent consideration is dependent on the acquired companies reaching technological milestones and on performance conditions. The ultimate amount of deferred and contingent consideration payable is expected to be in the range of $15-$33 million. Goodwill arising of £36.9 million is being amortized over ten years.
On May 10, 2002, the Group acquired a further 16% holding in Cimarron Software, Inc, taking the Group’s total holding at acquisition to 35%. Cimarron Software, Inc is being consolidated as a subsidiary undertaking as the Group controls the company through a shareholders’ agreement. Goodwill arising of £6.3 million is being amortized over seven years. Prior to May 10, 2002 Cimarron Software, Inc was accounted for as a trade investment.
69
Since May 10, 2002, the Group has subscribed for and was allotted additional share capital in Cimarron Software Inc, taking its holding at December 31, 2002 to 44%. Goodwill recognized on the post-acquisition allotments of share capital amounts to £1.7 million. The Group has obligations to subscribe for a further 7% of share capital over the next three years, and has options to acquire the remaining 49% of the company for a maximum consideration of $33 million, the total consideration payable being contingent on certain specified performance conditions.
On July 11, 2002, the Group acquired the net assets of the CodeLink™ pre-arrayed slides business from Motorola Life Sciences for £14 million. Goodwill arising of £6.8 million is being amortized over ten years.
The Group’s estimated useful life for these acquisitions reflects the period over which the Group expects to receive benefits based on acquired patents and technology and will thus be different in each acquisition.
Other events impacting comparability of Group operating profit
In December 2000, Amersham Health recorded a charge of £10.9 million for the costs of rationalizing our radiopharmaceutical production capacity at specific sites in the US and Europe. The costs of the rationalization comprise severance and other cash costs of £8.4 million and non-cash write off of £2.5 million. No further costs associated with the rationalization are expected.
In 2000 and 2001, costs of £6.4 million and £3.4 million respectively were incurred in connection with the proposed partial flotation of Amersham Biosciences. The costs relate primarily to adviser fees in preparing the prospectus for the flotation and other related costs.
In July 2000, the Group sold its business Nycomed Amersham Medical Systems SA (NAMS), in Paris, France, to Cathnet Sciences Holding A/S for a total consideration of £10.0 million. After taking into account costs of disposal, no gain or loss was made on the sale of the facility. Consideration which remained unpaid at the end of 2002, of an immaterial amount, was provided for in the 2002 accounts.
In 2001, the Company received £22.5 million related to a transfer of assets from former pension plans. Of this amount £13.5 million has been applied against ongoing liabilities primarily for employees who have transferred into Amersham pension schemes and the balance of £9.0 million has been recognized as exceptional income, where the Company has no further liability.
Also in 2001, costs of £8.5 million have been accrued in relation to the decision to terminate the Group’s lease at the Harwell site following a cessation of the Group’s manufacturing activities at the site. The costs relate primarily to asset write offs and decommissioning costs.
In 2002, we recognized as an exceptional item a deferred tax asset of £9.2 million, relating to the utilization of prior period tax losses following the formation of a consolidated tax group in the US.
Loss on disposal of discontinued operations and fixed assets
In May 1999, the Group sold 71% of its Nycomed Pharma business to a new company formed by a private equity group. The Group recorded a pre-tax gain of £11.5 million in the year ended December 31, 1999 after adding back attributable goodwill previously written off to reserves. The tax charge on the disposal amounted to £9.5 million. Further costs of £9.7 million with a related tax credit of £1.1 million, associated with exiting from the Pharma business, were accounted for in the year ended December 31, 2000 giving a total loss associated with the disposal of £6.6 million after tax in 1999 and 2000. In September 2001, the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4 million and realized £50.6 million for the outstanding Nycomed Pharma loan notes held by the Group. The Group has recorded a profit on the transaction of £55.3 million after charging costs associated with the disposal, including the costs incurred in transferring the Nycomed name to Nycomed Pharma. Subsequent to the transaction the Company changed its name from Nycomed Amersham plc to Amersham plc. The shares in Nycomed Pharma were owned by a legal entity in Holland and under Dutch tax law, the gain on their disposal is not taxable.
The Group did not account for its remaining 29% equity interest in Nycomed Pharma as an associated undertaking under UK GAAP as the company was controlled by its majority
70
shareholder and, in the opinion of the directors, the Group did not exercise significant influence over its operations. The results of Nycomed Pharma were therefore included as a discontinued operation for all years presented within the financial statements. Under US GAAP, the investment was accounted for using the equity method. The reconciliation of significant differences between UK GAAP and US GAAP is set out in Note 38 of Item 18 “Consolidated Financial Statements”.
Cash costs of £4.1 million and non-cash write offs of £1.7 million were incurred in the second half of 2001 in concluding research on, and winding up the operation of, an early stage investment in disease profiling. An investment of £4.0 million was made in the research project in the first half of 2001 and £3.7 million in the year ended December 31, 2000 which has been charged to operating activities. No further costs will be incurred in relation to this project.
A. Operating Results
The following table sets forth certain items from the Group’s Consolidated Profit and Loss Account for the periods indicated, and also expressed as a percentage of turnover:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31 |
| | |
|
| | | 2000 | | 2001 | | 2002 |
| | |
| |
| |
|
| | | (in £millions, except percentage data) |
| | | (Restated) | | (Restated) | | | | | | | | |
Turnover including turnover of joint venture | | | 1,377.6 | | | | 100.0 | % | | | 1,602.5 | | | | 100.0 | % | | | 1,618.2 | | | | 100.0 | % |
| Less share of joint venture turnover | | | (94.8 | ) | | | (6.9 | %) | | | (87.3 | ) | | | (5.5 | %) | | | (80.5 | ) | | | (5.0 | %) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Group turnover | | | 1,282.8 | | | | 93.1 | % | | | 1,515.2 | | | | 94.5 | % | | | 1,537.7 | | | | 95.0 | % |
| Cost of sales | | | (441.9 | ) | | | (32.1 | %) | | | (499.6 | ) | | | (31.2 | %) | | | (481.0 | ) | | | (29.7 | %) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Gross profit | | | 840.9 | | | | 61.0 | % | | | 1,015.6 | | | | 63.3 | % | | | 1,056.7 | | | | 65.3 | % |
| Distribution, selling and administration costs | | | (518.2 | ) | | | (37.6 | %) | | | (595.8 | ) | | | (37.2 | %) | | | (622.5 | ) | | | (38.5 | %) |
| R&D expenses | | | (149.0 | ) | | | (10.8 | %) | | | (173.8 | ) | | | (10.8 | %) | | | (184.2 | ) | | | (11.3 | %) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Group operating profit | | | 173.7 | | | | 12.6 | % | | | 246.0 | | | | 15.3 | % | | | 250.0 | | | | 15.5 | % |
| Share of operating profits of joint venture and associates | | | 29.8 | | | | 2.2 | % | | | 26.1 | | | | 1.6 | % | | | 23.2 | | | | 1.4 | % |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total operating profit | | | 203.5 | | | | 14.8 | % | | | 272.1 | | | | 16.9 | % | | | 273.2 | | | | 16.9 | % |
| Net interest payable | | | (10.0 | ) | | | (0.8 | %) | | | (8.7 | ) | | | (0.5 | %) | | | (7.1 | ) | | | (0.4 | %) |
| Amounts written off investments | | | — | | | | — | | | | (4.4 | ) | | | (0.3 | %) | | | (2.4 | ) | | | (0.2 | %) |
| Profit on sale of fixed asset investment | | | — | | | | — | | | | 55.3 | | | | 3.5 | % | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Profit before taxation | | | 193.5 | | | | 14.0 | % | | | 314.3 | | | | 19.6 | % | | | 263.7 | | | | 16.3 | % |
| Tax on profit on ordinary activities | | | (79.9 | ) | | | (5.8 | %) | | | (96.5 | ) | | | (6.0 | %) | | | (85.1 | ) | | | (5.3 | %) |
| (Profit)/loss attributable to minority interests | | | (6.6 | ) | | | (0.5 | %) | | | (7.1 | ) | | | (0.5 | %) | | | 0.1 | | | | 0.0 | % |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Profit attributable to shareholders | | | 107.0 | | | | 7.8 | % | | | 210.7 | | | | 13.1 | % | | | 178.7 | | | | 11.0 | % |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Figures for the years ending December 31, 2000 and 2001 have been restated following the introduction of FRS19, as explained in Note 37 of Item 18 “Consolidated Financial Statements”.
71
Financial year ended December 31, 2002 compared with the financial year ended December 31, 2001
Turnover
Turnover increased by £15.7 million, or 1%, to £1,618.2 million in 2002, compared to £1,602.5 million in 2001. Excluding sales attributable to disposed businesses in Amersham Biosciences (which contributed a 1% increase in sales growth) and at constant exchange rates, turnover increased by 7% in 2002. Overall sales growth in sterling was adversely impacted by 5% by the weakening of the dollar and yen against sterling during the year.
Excluding sales attributable to the 50% share of NMP, our joint venture in Japan with Sumitomo Chemical Co Ltd., Group turnover increased by £22.5 million, or 1%, to £1,537.7 million in 2002 compared to £1,515.2 million in 2001.
There was growth in turnover across all regions with the exception of Amersham Health bulk sales in Japan, reflecting the continuing difficult trading conditions in this market due to the general economic climate and a reduction in in-market prices as a result of the Japanese government’s biennial pharmaceutical price review.
Turnover of our business segments for the following periods is stated below:
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2001 | | 2002 |
| | |
| |
|
| | | (in millions) |
Turnover | | | £ | | | | £ | |
| Amersham Health(1) | | | 922.0 | | | | 947.7 | |
| Amersham Biosciences | | | 680.5 | | | | 670.5 | |
| | | |
| | | |
| |
| | | | 1,602.5 | | | | 1,618.2 | |
| | | |
| | | |
| |
| (1) | | Included within Amersham Health is the Group’s share of NMP joint venture turnover of £80.5 million (2001 — £87.3 million) |
Amersham Health
Turnover increased by £25.7 million, or 3%, to £947.7 million in 2002, compared to £922.0 million in 2001. At constant exchange rates, turnover increased by 8% in 2002.
Our patented medical diagnostic products maintained the growth trends of earlier years, growing by £54.5 million, or 18%, to £353.5 million in 2002, compared to £299.0 million in 2001. At constant exchange rates, this represents growth of 22%. Our cash generative unpatented product sales fell by £2.5 million, or 0.6%, from £363.3 million in 2001 to £360.8 million in 2002. At constant exchange rates, this represents growth of 5%. There were a number of products that were previously classified within Therapy but were moved to the unpatented portfolio during 2002. Hence, the 2001 unpatented sales, which were previously disclosed as £353.0 million, have been restated accordingly. In the second half of 2002, patented product sales exceeded sales of unpatented products for the first time.
The following table shows turnover within the Health segment split by modality. 2001 figures have been reclassified in line with changes to modalities during 2002.
| | | | | | | | | | |
| | | | Year ended December 31, |
| | | |
|
| | | | 2001 | | 2002 |
| | | |
| |
|
| | | | (in millions) |
Net turnover – Amersham Health | | | £ | | | | £ | |
| X-Ray: North America | | | 195.6 | | | | 199.8 | |
| | Rest of world | | | 128.5 | | | | 135.3 | |
| | | |
| | | |
| |
| Total X-Ray | | | 324.1 | | | | 335.1 | |
| | | |
| | | |
| |
72
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2001 | | 2002 |
| | |
| |
|
| | | (in millions) |
Net turnover – Amersham Health | | | £ | | | | £ | |
| Radiopharmaceutical imaging | | | 245.0 | | | | 269.1 | |
| MRI | | | 85.8 | | | | 96.9 | |
| Bulk sales and royalties | | | 90.9 | | | | 74.7 | |
| Therapy | | | 71.3 | | | | 61.5 | |
| NMP (50% joint venture) | | | 87.3 | | | | 80.5 | |
| Other | | | 17.6 | | | | 29.9 | |
| | | |
| | | |
| |
| | | | 922.0 | | | | 947.7 | |
| | | |
| | | |
| |
In X-ray diagnostics, turnover in 2002 increased by £11.0 million, or 3%, to £335.1 million in 2002, from £324.1 million in 2001. At constant exchange rates, sales of X-ray diagnostics in 2002 increased by 8% compared to 2001. The increase in turnover of X-ray diagnostics was mainly due to increased turnover of Omnipaque™ and Visipaque™, which increased in the year by 6% and 18% respectively at constant exchange rates. The X-ray product Omnipaque™ was launched over 20 years ago and is still the best sellingin vivodiagnostic product on the market.
The radiopharmaceuticals diagnostic segment grew by £24.1 million, or 10%, to £269.1 million in 2002 from £245.0 million in 2001. At constant exchange rates, this represents an increase of 14%. Products within this category include Myoview which grew by £22.6 million, or 20%, to £132.9 million in 2002. This represents an increase of 26% at constant exchange rates. Sales of our innovative molecular diagnostic DaTSCAN™ have grown every year since its launch in Europe in 2000.
Magnetic resonance imaging (MRI) sales grew by £11.1 million, or 13%, to £96.9 million in 2002 from £85.8 million in 2001. This represents an increase of 17% at constant exchange rates. Omniscan™, our leading MRI product, is used in neurology to detect stroke, brain tumors and other brain abnormalities. Omniscan continues to be a market leader in this area and is the number one non-ionic MRI contrast medium sold throughout the world, achieving sales of £96.1 million in 2002.
Bulk sales and royalties reduced by £16.2 million, or 18%, to £74.7 million in 2002 compared to £90.9 million in 2001. This represents a decrease of 14% at constant exchange rates. The majority of these sales are in Japan and were impacted negatively by a government price reduction which took effect in spring 2002.
Therapy products suffered price and volume reductions for OncoSeed™ in the US with total sales of therapy products down £9.8 million, or 14%, from £71.3 million in 2001 to £61.5 million in 2002. This represents a decrease of 10% at constant exchange rates. The full portfolio of brachytherapy seeds for prostate cancer treatment achieved sales of £50.5 million in 2002, a decrease of £7.7 million, or 13%, from sales of £58.2 million in 2001. This represents a decrease of 10% at constant exchange rates. Continued increases in demand for Rapid Strand™ (up 27%, or 30% at constant exchange rates), mainly as a result of new seed implant centre openings, helped to partially offset the OncoSeed™ decline.
NMP, our joint venture with Sumitomo Chemical Company Limited in Japan, produced turnover of £80.5 million in 2002, compared to £87.3 million in 2001, a decrease of £6.8 million or 8%. At constant exchange rates, this represented a decrease of less than 1%.
In its first full year under Amersham’s ownership, Optison™ achieved satisfactory results with worldwide sales of £9 million in the new, yet emerging, ultrasound contrast media market. In North America, we have built a specialized sales force for developing this new market through focused education and by working with key opinion leaders. EU marketing authorization for Optison™ was granted in June 2002.
Amersham Biosciences
Sales decreased by £10 million, or 1%, to £670.5 million in 2002 compared to £680.5 million in 2001. Excluding sales from disposed businesses and at constant exchange rates, this represents
73
an increase in sales of 6%. The disposed businesses had a 2% benefit to sales growth whilst the impact of exchange was 5% adverse. The decrease in turnover compared to 2001 was caused by the effect of exchange rates and a reduction in discovery systems product sales where customers, in particularly biotechnology companies, were impacted by the weakened economy and equity market conditions largely offset by growth in protein separations turnover globally.
In each geographic region protein separations growth was able to offset the reduction in turnover in discovery systems. The following table sets forth the change in turnover by geographic region in 2002 compared to 2001 after adjusting for the effect of exchange rate fluctuations:
| | | | | |
| Geographic Region | | % increase / (decrease) |
| |
|
| North America | | | 3 | % |
| Europe | | | 8 | % |
| Japan | | | 11 | % |
| Asia Pacific | | | (2 | %) |
| Rest of World | | | (5 | %) |
| Company Overall | | | 6 | % |
The following table shows turnover within the Amersham Biosciences segment split into individual business streams. Effective January 1, 2002, Amersham Biosciences reorganized its business streams, creating discovery systems consisting of the former drug discovery, laboratory products, and service, agency and other business streams, and maintaining the protein separations business stream. Segmental reporting has been restated as appropriate for periods prior to January 1, 2002.
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2001 | | 2002 |
| | |
| |
|
| | | (in £ millions) |
Turnover | | | | | | | | |
| Discovery systems | | | 412.8 | | | | 394.2 | |
| Protein separations | | | 267.7 | | | | 276.3 | |
| | | |
| | | |
| |
| | | | 680.5 | | | | 670.5 | |
| | | |
| | | |
| |
In the discovery systems stream, turnover decreased to £394.2 million in 2002, down £18.6 million, or 5%, from £412.8 million in 2001. This represents a decrease of 1% at constant exchange rates. Sales were impacted by market conditions, notably the slowdown in pharmaceutical company spending on capital equipment. Instrument sales, representing about 25% of discovery systems sales, were down significantly compared with 2001. However, customer spending on consumables, reagents and software remained good.
In genomics, we are the market leader in enzymology and our sales of reagents and consumables not only reduce our exposure to the market slowdown in instrumentation sales, but also underpin our vision of developing technologies to enable personalized medicine. TempliPhi™, the DNA template preparation kit, continued to make good progress. All of the five major public genome centers now use TempliPhi, with three of them — the Joint Genome Institute, the Whitehead Institute, and Baylor College of Medicine — using it in daily sequence production. It is also seeing increasing acceptance in Japan. New products based on this technology are in development, and the first of these, GenomiPhi™, will be launched in 2003 for use in whole genome amplification.
The proteomics product area also saw sales growth in reagents and consumables. Our market-leading 2D DIGE technology, which allows the protein content of several samples to be simultaneously analyzed and compared, continued to grow well and development work is ongoing to expand further the range of DIGE reagents and software. During 2002, we launched our new MALDI mass spectrometer, the Ettan™ MALDI TOF Pro for protein characterization, as part of the complete Ettan range of systems for stand alone or integrated protein analysis.
Researchers use bioassays products to quantify the biological processes involved in cellular activity, metabolism and disease. Pharmaceutical customers use our products to identify drug targets and develop potential new drugs, eliminating poor drug candidates and those with side
74
effects as early as possible, thus reducing the time and cost spent in drug development. We launched two new instruments in 2002. The latest generation LEADseeker™ was launched in September 2002. The INCell Analyzer high throughput system, which enables researchers to study the effects of a potential new drug in living cells in real time, continued to be well received by customers, and a number of systems were sold in the last quarter of the year. We also saw particularly good growth in custom-labeled products used in the drug development process, as pharmaceutical companies began to outsource more of this work.
Turnover in protein separations increased by £8.6 million, or 3%, to £276.3 million compared to £267.7 million in 2001. At constant exchange rates, this represents an increase of 15%, driven by growth in bioprocess sales and including first-time benefit from filtration or membrane separation products. As anticipated, our sales accelerated through the year, with the fourth quarter being particularly strong as biotech and pharmaceutical companies purchased manufacturing instruments and media for new production plants and for new drugs going into clinical trials. We also saw good replenishment sales of media for existing biopharmaceuticals.
In addition, two further areas contributed to the growth for industrial-scale bioprocess systems in 2002. First, the market for products used in the manufacture of DNA-based pharmaceuticals began to grow rapidly, driving sales of our OligoProcess™ systems. Second, the membrane separation businesses, acquired in January, delivered good sales as biopharmaceutical customers took advantage of the broader product offering. Membrane separation has been successfully integrated into Amersham Biosciences and a new site in Massachusetts, US, will be completed in the first half of 2003.
The laboratory separations area saw lower instrument sales, following the exceptionally high growth in 2001, and as a result of more cautious spending by pharmaceutical companies. Media sales continued to be good, and a new instrument, ÄKTA™ Pilot, launched in December for the production of clinical grade proteins on the benchtop, has been well received by customers.
Gross profit
Gross profit increased by £41.1 million, or 4%, to £1,056.7 million in 2002 compared to £1,015.6 million in 2001. Gross profit as a percentage of turnover increased to 65.3% in 2002 compared to 63.3% in 2001. There were no exceptional items in 2002. In 2001, exceptional items amounted to a credit of £0.5 million relating to the net of a £8.5 million decommissioning provision and a £9.0 million pension refund.
The increase in profitability of continuing operations was mainly due to increased revenue from a better product mix including strong growth in high margin patented products across both businesses, partially offset by reduced sales of bulk products in Japan in Amersham Health.
Distribution, selling and administrative costs
Total Group distribution, selling and administration costs increased by £26.7 million, or 4%, to £622.5 million in 2002 compared to £595.8 million in 2001. As a percentage of turnover this represents an increase from 37.2% in 2001 to 38.5% in 2002. Goodwill amortization, which increased by £24.9 million from £11.7 million in 2001 to £36.6 million in 2002, is charged to distribution, selling and administration costs.
Included in distribution, selling and administration costs in 2001 were exceptional expenses of £9.2 million. There are no exceptional costs in 2002. In 2001, these related to costs incurred in the exit of the disease profiling project and costs relating to the proposed partial flotation of Amersham Biosciences. See ‘Events impacting comparability’.
Excluding exceptional items, costs from continuing operations increased by £35.9 million, or 6%, from £586.6 million in 2001 to £622.5 million in 2002. As a percentage of turnover however, this represents an increase of 1.9% from 36.6% to 38.5%.
Costs grew with inflation and in Amersham Health higher costs were incurred due to the addition of five new radiopharmacies in North America and the full year costs of building a sales team for the promotion of the new ultrasound product Optison™.
75
R&D expenses
R&D costs for the year ended December 31, 2002 were £184.2 million, an increase of £10.4 million or 6% compared to 2001. Amersham Health R&D costs increased by £12.1 million, or 15%, to £95.1 million in 2002 compared to £83.0 million in 2001. Costs related to Imanet™ (which are covered by revenues from pharmaceutical companies for scanning services) accounted for £3 million of this increase. This represents 10% of Amersham Health’s turnover in 2002 compared with 9% in 2001. R&D expenditure in Amersham Biosciences increased by £1.6 million, or 2%, from £86.4 million in 2001 to £88.0 million in 2002. This represents 13% of Amersham Biosciences’ turnover in 2002, unchanged as a percentage of turnover from 2001. R&D expenditure for the Corporate and Other segment was £1.1 million, reduced from the £4.4 million of exceptional costs recorded in 2001 relating to the disease profiling project which was discontinued during that year.
Amersham Health
Cardiology
Cardiovascular disease continues to be one of the most rapidly expanding diseases globally and a major cause of death in North America and Europe. Amersham offers a broad range of products across the range of modalities to enable physicians to understand the health of a patient’s heart.
Myoview™ is our leading radiopharmaceutical diagnostic for visualizing the supply of blood to the heart. In 2001, this product was approved for use with pharmacological stress agents. In 2002 we completed a phase III trial with Myoview for a new cardiac indication, left ventricular function, and US regulatory approval was received in February 2003.
The safety profile of our third generation X-ray diagnostic, Visipaque™, was further strengthened through a landmark study carried out by the Karolinska Institute in Stockholm, Sweden in 2002 and published in the prestigious New England Journal of Medicine in February 2003. The NEPHRIC study showed that Visipaque™ significantly reduces the relative risk of developing contrast media-induced impairment of renal function, a serious clinical problem in some patient groups.
We successfully completed phase II trials to extend the use of our MRI product Omniscan™ in cardiac perfusion and for monitoring blood flow through the renal arteries. Phase III studies in the US and Europe are being planned and should commence towards the end of 2003.
In the US and Europe, myocardial perfusion has been the primary target market for the development of Sonazoid™, a third generation ultrasound product. The use of ultrasound for this indication has been limited by the slow development of sufficiently robust imaging procedures. Therefore, although Sonazoid™ has shown promising safety data, a decision has been taken to suspend work in the US and Europe and to continue to focus on Optison™, our ultrasound product which is marketed for improved visualization of the surfaces of the heart muscle. In Japan, the development of Sonazoid™ by our partner, Daiichi Pharmaceutical Co. Ltd., continues. The first use of Sonazoid™ imaging in Japan will be established in the liver disease market.
Neurology
DaTSCAN™, a molecular diagnostic, is Amersham’s newest entrant to the field of neuro-imaging. It is the only registered product enabling physicians to objectively identify Parkinson’s disease through cellular change in the brain, and to distinguish this from a similar disease, Essential Tremor. DaTSCAN™ is also being developed for the differentiation of Lewy body from other forms of dementia. Based on supporting proof-of-concept data from external studies, a revised development program has been initiated to greatly reduce time-to-market and we will proceed directly to phase III studies of DaTSCAN™ for this new indication.
A technetium-based product, Trodat, also for the diagnosis of Parkinson’s disease, is being developed for the US market and all pre-clinical milestones have been met to date.
76
Cancer
Myoview™, our radiopharmaceutical diagnostic, obtained approval for a new indication in Europe for use in breast tumor imaging in 2002. Breast cancer is one of the most common cancers in women, and while advances have been made in breast cancer treatment, the success of these treatments is highly dependent upon early and accurate diagnosis of the functional status of the disease. Such diagnosis can now be achieved with the use of Myoview.
The new chemical entity NC100692 for the imaging of angiogenesis (blood supply to a tumor) and other potential indications has progressed well in its pre-clinical safety, pharmacology and formulation stages.
Pulmonology
Lung disorders involve a range of problems, from chronic conditions such as asthma to sudden death due to pulmonary embolism. We are beginning to see promising results from our Thrombus agent, NC100668, which moved into phase II trials in April. This radiopharmaceutical agent has the potential to identify patients with pulmonary embolism earlier than other diagnostic techniques. We also continue to develop the technology relating to Spin Signal™, using Helispin™ to enable early detection of lung disease through high-resolution ventilation imaging of gases using MRI.
Developments in PET imaging
The use of positron emission tomography (PET) for the early detection of tumors is becoming widely accepted, using fluorodeoxyglucose (FDG) as the imaging agent. Progress is being made in our collaboration with General Electric Medical Systems on the development of a solid-phase chemistry delivery system for use in an FDG “cassette”. Through this collaboration, we will be able to provide hospitals and clinics with PET imaging products such as FDG, with consistently high quality. We are also working to develop new PET diagnostics for neurological indications.
NMP, our joint venture with Sumitomo Chemical Company Limited in Japan, is planning to invest £68 million in manufacturing facilities to deliver PET diagnostic products to key medical centers in Japan. NMP aims to construct six new manufacturing facilities to supplement its three existing sites. Under current regulations NMP expects to gain a six-year exclusivity period in the Japanese market for its first PET diagnostic product.
We continue to expand our Imanet™ network of imaging research centers, which began in 2001 with the formation of a joint venture with the Medical Research Council in the UK. In 2002 we added a second Imanet™ centre in conjunction with the University of Uppsala, Sweden. In February 2003, we established a new centre in Finland, TRIS (Turku Research Imaging Solutions Oy), which will work in partnership with Turku PET Centre. Imanet™ provides diagnostic services, including PET, to assist the pharmaceutical industry in monitoring the effectiveness of new drugs during the development stage. This helps us identify new diagnostic molecules for use in very specific therapeutic applications and in January 2002 we announced a major research collaboration with Pfizer to achieve this. With contractual work for the majority of the world’s top pharmaceutical companies in addition to the strategic collaboration with Pfizer, Imanet™ is progressing well financially as well as scientifically.
Amersham Biosciences
Amersham Biosciences has now completed a sustained period of building investment in R&D, particularly within the discovery systems business stream, creating a broad portfolio from which to launch new products.
Discovery systems R&D expenditure decreased to £66.5 million in 2002 compared to £69.4 million in 2001, a decrease of £2.9 million or 4%. The minor reduction was primarily due to genomics eliminating development expenditure as certain significant instruments were completed and launched in 2001, like the MegaBACE™ 4000. Products completing development and launched in 2002 include, in proteomics, two new high throughput systems to add to the existing Ettan range, Ettan™ 2D DIGE and the Ettan™ MALDI ToF Pro.
77
Protein separations R&D expenditure increased to £21.5 million in 2002 compared to £17.0 million in 2001. The 2002 expenditure increased as expected to encompass filtration technology through recent investments and due to the start up of new bulk resin development projects.
Group operating profit
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2001 | | 2002 |
| | |
| |
|
| | | (in £ millions) |
Operating profit | | | | | | | | |
| Total continuing operating profit before non-recurring items | | | 280.8 | | | | 273.2 | |
| Exceptional items | | | (8.7 | ) | | | — | |
| | | |
| | | |
| |
| | | | 272.1 | | | | 273.2 | |
| | | |
| | | |
| |
Group operating profit increased by £1.1 million to £273.2 million in 2002, and as a percentage of sales remains unchanged from 2001 at 16.9%. At constant exchange rates, however, operating profit fell by 3% as a result of a £24.9 million increase in goodwill amortization following the purchase of the 45% minority interest in Amersham Biosciences.
Group operating profit before exceptional items and goodwill amortization increased by £17.3 million or 11% from £292.5 million in 2001 to £309.8 million in 2002. Exceptional items were nil and a charge of £8.7 million in 2002 and 2001 respectively and goodwill amortization was a charge of £36.6 million and £11.7 million respectively. There was a 1% adverse impact on Group operating profit due to exchange rate fluctuations in 2002. Excluding the impact of exceptional items, goodwill amortization and at constant exchange rates, operating profit in 2002 increased by 7%.
See “Events impacting comparability” for further details of the exceptional items in 2001.
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2001 | | 2002 |
| | |
| |
|
| | | (in £millions) |
Operating profit | | | | | | | | |
| Amersham Health | | | 231.0 | | | | 254.7 | |
| Amersham Biosciences | | | 59.5 | | | | 39.6 | |
| Corporate and Other | | | (18.4 | ) | | | (21.1 | ) |
| | | |
| | | |
| |
| | | | 272.1 | | | | 273.2 | |
| | | |
| | | |
| |
Operating profit of the Amersham Health segment increased by £23.7 million, or 10%, to £254.7 million in 2002 from £231.0 million in 2001. The operating margin for the segment was 27% in 2002 compared to 25% in 2001. In 2002 and 2001 respectively, goodwill amortization of £0.9 million and £1.4 million and exceptional items of nil and £8.5 million were attributable to the segmental operating profit. Excluding goodwill amortization and exceptional items, operating profit increased by 6% from £240.9 million in 2001 to £255.6 million in 2002, representing an increase of 9% at constant exchange rates with a 3% adverse impact caused by exchange rate fluctuations. Trading margins were strong throughout with the benefits of product mix and manufacturing efficiencies.
Operating profit of the Amersham Biosciences segment fell by £19.9 million, or 33%, from £59.5 million in 2001 to £39.6 million in 2002. Operating margins fell from 9% in 2001 to 6% in 2002. In 2002 and 2001 respectively, goodwill amortization of £35.7 million and £10.3 million and exceptional items of nil and £2.6 million were attributable to the segmental operating profit. The increase in goodwill amortization primarily related to the acquisition of the minority holding in Amersham Biosciences in March 2002.
Excluding goodwill amortization and exceptional items, operating profit for the segment increased to £75.3 million in 2002 compared to £72.4 million in 2001 and represents a flat profit at constant exchange rates and with an adverse impact of exchange rate fluctuations of less than 0.5%. The
78
CodeLink™ and Cimarron acquisitions had a dilutive impact which if excluded would see operating profit excluding goodwill amortization and exceptional items, and at constant exchange rates increase to 9% from the flat growth noted above. This is as a result of a good operating margin in protein separations and the settlement in February 2002 of the Applera patent litigation.
Within Amersham Biosciences, operating profit before exceptional items and goodwill amortization of protein separations grew by £11.5 million, or 12%, to £107.5 million in 2002, compared to £96 million in 2001. This was driven by the 15% increase in turnover as discussed above. In discovery systems, the operating loss before exceptional items and goodwill amortization was £32.2 million in 2002 compared to £23.6 million in 2001. The increase in operating loss was due to negative market conditions, notably the slowdown in pharmaceutical company spending on capital equipment.
Profit on sale of fixed asset investment
In September 2001, the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4 million and received £50.6 million in respect of the outstanding Nycomed Pharma loan notes held by the Group. The Group has recorded a profit on the transaction of £55.3 million after charging costs associated with the disposal, including the costs incurred in transferring the Nycomed name to Nycomed Pharma.
Amounts written off investments
We have taken a charge in 2002 of £2.4 million against the carrying value of listed investments following a reduction in their equity value in 2002.
Net interest payable
Interest costs of £7.1 million in 2002 were £1.6 million lower than in 2001 as our net debt was held in currencies with lower interest rates compared to 2001.
Tax on profit on ordinary activities
In 2002, the taxation charge was £85.1 million compared to a charge of £96.5 million in 2001, representing an effective tax rate of 32.3% in 2002 compared to 30.7% in 2001.
The taxation charge before exceptional items and goodwill amortization was £94.3 million in 2002 compared to a charge of £97.0 million in 2001. The effective tax rate before exceptional items and goodwill amortization of 31.4% in 2002 was down from 34.7% in 2001. The 3.3% reduction in effective tax rate before exceptional items and goodwill amortization reflects the tax benefits derived following the purchase of the minority stake in Amersham Biosciences and the favorable settlement of a number of prior year items, which contributed approximately 1.1% to the reduction.
Following the introduction of FRS 19 ‘Deferred Taxation’ during 2002, the 2001 tax charge was restated to £96.5 million, being previously £92.0 million, increasing the effective tax rate in 2001 by 1.4% to 30.7%. This was primarily as a result of the treatment of tax deductible goodwill required by FRS 19. The 2001 taxation charge before exceptional items and goodwill amortization was restated to £97.0 million from £92.5 million, increasing the effective tax rate before exceptional items and goodwill amortization by 1.6% to 34.7%. The impact of the introduction of this new accounting standard is disclosed in Note 37 of Item 18 “Consolidated Financial Statements”.
79
Profit attributable to shareholders
Profit and losses attributable to minority interest relates primarily to the 45% interest that Pharmacia Corporation held in Amersham Biosciences. Profit attributable to shareholders after charging exceptional items and goodwill amortization was £178.7 million in 2002 and £210.7 million in 2001 (as restated to reflect the introduction of FRS 19 ‘Deferred Taxation’).
80
Financial year ended December 31, 2001 compared with the financial year ended December 31, 2000
Turnover
Turnover increased by £224.9 million, or 16%, to £1,602.5 million in 2001, compared to £1,377.6 million in 2000. This represents an increase of 13% at constant exchange rates. For Amersham Health this calculation excludes turnover of £6.8 million relating to the Amersham Medical Systems (NAMS) business, which was sold in 2000, and £2.2 million relating to the discontinued NMPin vitrobusiness. Overall, exchange rates had a favorable impact of £55.0 million or 3% on sales in 2001.
Excluding sales attributable to the 50% share of NMP, our joint venture with Sumitomo Chemical Co Ltd. in Japan, Group turnover was £1,515.2 million in 2001, an increase of £232.4 million or 18%, on turnover of £1,282.8 million in 2000.
There was strong growth in turnover across all regions with the exception of Amersham Health bulk sales in Japan, reflecting the difficult trading conditions in this market due to the general economic situation and the pharmaceutical pricing environment.
Turnover of our business segments for the following periods is stated below:
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2000 | | 2001 |
| | |
| |
|
| | | (in £millions) |
Turnover | | | | | | | | |
Amersham Health(1) | | | 773.9 | | | | 922.0 | |
| Amersham Biosciences | | | 603.7 | | | | 680.5 | |
| | | |
| | | |
| |
| | | | 1,377.6 | | | | 1,602.5 | |
| | | |
| | | |
| |
| (1) | | Included within Health is the Group’s share of NMP joint venture turnover of £87.3 million (2000 — £94.8 million) |
Amersham Health
Turnover increased by £148.1 million, or 19%, to £922.0 million in 2001 compared to £773.9 million in 2000. Excluding the loss of third party turnover following the disposals of the NAMS business in 2000 and the discontinued NMPin vitrobusiness and at constant exchange rates, turnover in 2001 increased by 13% compared to 2000.
Our patented medical diagnostic products maintained the growth trends of earlier years, growing by £74.3 million, or 33%, from £224.7 million in 2000 to £299.0 million in 2001. At constant exchange rates, this represents a growth of 25%. Our cash generative, unpatented products grew by £52.9 million, or 18%, from £300.1 million to £353.0 million. At constant exchange rates, this represents a growth rate of 10%.
The following table shows turnover within the Health segment split by modality. 2000 figures have been reclassified in line with changes during 2001.
81
| | | | | | | | | | |
| | | | Year ended December 31, |
| | | |
|
| | | | 2000 | | 2001 |
| | | |
| |
|
| | | | (in £millions) |
Net turnover – Amersham Health | | | | | | | | |
| X-Ray: North America | | | 166.6 | | | | 195.6 | |
| | Rest of world | | | 99.8 | | | | 128.5 | |
| | | |
| | | |
| |
| Total X-Ray | | | 266.4 | | | | 324.1 | |
| Radiopharmaceutical Imaging | | | 191.0 | | | | 240.7 | |
| MRI | | | 66.4 | | | | 85.8 | |
| Bulk sales and royalties | | | 68.1 | | | | 90.9 | |
| Therapy | | | 65.2 | | | | 78.0 | |
| NMP (50% Joint Venture) | | | 94.8 | | | | 87.3 | |
| Other | | | 22.0 | | | | 15.2 | |
| | | |
| | | |
| |
| | | | 773.9 | | | | 922.0 | |
| | | |
| | | |
| |
In X-ray diagnostics, turnover in 2001 was £324.1 million, up 22% on 2000, mainly due to turnover of Omnipaque™ and Visipaque™, which increased in the year by 21% and 36% respectively. At constant exchange rates, this represents an increase over 2000 in total sales of X-ray diagnostics of 14%.
Sales in magnetic resonance imaging (MRI) grew by £19.4 million, or 29%, to £85.8 million in 2001. At constant exchange rates, this represents an increase in sales of 19%. Omniscan™, our leading MRI product, is used in neurology for detecting cancers of the brain and stroke.
The radiopharmaceuticals diagnostic segment grew by £49.7 million or 26% to £240.7 million in 2001 from £191.0 million in 2000. At constant exchange rates, this represents an increase in sales of 17%. Products within this category include Myoview which grew by £29.8 million to £110.3 million in 2001. Sales of our molecular diagnostic DaTSCAN™ made good progress since its launch in Europe in 2000.
Bulk sales and royalties increased by £22.8 million, or 33%, to £90.9 million in 2001 compared to £68.1 million in 2000. The majority of these sales were in Japan. At constant exchange rates, this represents an increase in sales of 9%.
Therapy products returned to growth with sales up £12.8 million, or 20%, to £78.0 million in 2001. At constant exchange rates, this represents an increase of 9%. The full portfolio of brachytherapy seeds for prostate cancer treatment achieved sales of £58.8 million in 2001, up 23% on 2000 sales. This growth was driven by four new sole source contracts with US group purchasing organizations in the first half of the year and by the addition of the echogenic EchoSeed™ and the palladium product TheraSeed™ to the portfolio.
NMP, our joint venture with Sumitomo Chemical Company Ltd in Japan, gave rise to turnover of £87.3 million in 2001, compared to £94.8 million in 2000, a decrease of £7.5 million or 8%. During 2000, thein vitrobusiness of NMP was discontinued which had a 6% impact on the sales growth. Excluding discontinued NMP operations and at constant exchange rates (with exchange having an adverse 2% impact on sales growth), sales of NMP business remained flat compared to 2000.
Other turnover decreased by £6.8 million, or 30%, to £15.2 million in 2001 compared to £22.0 million in 2000, largely as a result of the loss in third party sales following the closure of our NAMS business in France.
Amersham Biosciences
Turnover increased by £76.8 million, or 13%, to £680.5 million in 2001 compared to £603.7 million in 2000. The 2000 figure was restated to take account of a change in the Group’s revenue recognition policies following the adoption of FRS 18. At constant exchange rates, this represents an increase in sales of 12%.
82
Throughout the year, the breadth of Amersham Biosciences’ innovative product range and our geographical reach helped to underpin continued good growth overall. At constant rates, growth in Japan was up 11% on 2000; in Europe, up 8% on 2000; in Asia Pacific, up 36% on 2000, and in Latin America, up 20% on 2000. North American turnover improved after a slow third quarter of 2000 giving good annual growth in 2001 of 11%.
The following table shows turnover within the Amersham Biosciences segment split into individual business streams. Effective January 1, 2002, Amersham Biosciences reorganized its business streams, creating discovery systems consisting of the former drug discovery, laboratory products, and service, agency and other business streams, and maintaining the protein separations business stream. Segmental reporting has been restated as appropriate for periods prior to January 1, 2002.
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2000 | | 2001 |
| | |
| |
|
| | | (in £millions) |
Turnover | | | | | | | | |
| Discovery systems | | | 382.0 | | | | 412.8 | |
| Protein separations | | | 221.7 | | | | 267.7 | |
| | | |
| | | |
| |
| | | | 603.7 | | | | 680.5 | |
| | | |
| | | |
| |
In the discovery systems stream, turnover increased to £412.8 million in 2001, up £30.8 million, or 8%, from £382.0 million in 2000. At constant exchange rates, this represents an increase in sales of 9% compared to 2000. Within genomics the launch of new technologies, together with good sales in Asia Pacific and Latin America, helped to offset the impact of the slowdown in the US and European sequencing markets following the very high growth in 2000 as scientists rushed to complete the draft human genome. Strong growth was seen in sales of proteomics systems in 2001, driven by well-established market leaders such as the 2D-electrophoresis systems for protein expression analysis including 2D-DIGE. During the year, Amersham Biosciences added two new high throughput systems to the Ettan™ range of protein systems, the Ettan™ LCMS (liquid chromatography mass spectrometer) for protein identification, and the Ettan™ Spot Handling Workstation, enabling researchers to integrate multiple steps in their protein studies.
Sales in protein separations increased by £46.0 million, or 21%, to £267.7 million compared to £221.7 million in 2000. At constant exchange rates, this represents an increase in sales of 18% compared to 2000. In laboratory separations, Amersham Biosciences sold over 2,500 ÄKTA™ benchtop protein purification systems. Several new products were launched in 2001, including next generation Unicorn™ software used with our chromatography systems and Primer Support™ 200, a media for large scale oligonucleotide synthesis. Good progress was made in the ongoing development of new bioprocess media including next generation Sepharose™ and Streamline™.
Gross profit
Gross profit increased by £174.7 million, or 21%, to £1,015.6 million in 2001 compared to £840.9 million in 2000. Gross profit as a percentage of turnover increased to 63.3% in 2001 compared to 61.0% in 2000. In 2001, exceptional items amounted to a credit of £0.5 million relating to the net of a £8.5 million decommissioning provision and a £9.0 million pension refund. In 2000 exceptional items related to £10.9 million of costs associated with manufacturing rationalization in the Health segment. Excluding these one-off items, gross profit from continuing operations increased by £163.3 million, or 19%.
The increase in profitability of continuing operations was mainly due to increased revenue from a better product mix including strong growth in high margin patented products, partially offset by reduced sales of bulk products in Japan.
Distribution, selling and administrative costs
Total Group distribution, selling and administration costs increased by £77.6 million, or 15%, to £595.8 million in 2001 compared to £518.2 million in 2000. As a percentage of turnover, this represents a decrease from 37.6% in 2000 to 37.2% in 2001.
83
Included in distribution, selling and administration costs in 2001 and 2000 were exceptional expenses of £9.2 million and £16.1 million respectively. In 2001, these related to costs incurred in the exit of the disease profiling project and costs relating to the proposed partial flotation of Amersham Biosciences. In 2000 these consisted of costs incurred in connection with the disposal of Nycomed Pharma and the proposed partial flotation of Amersham Biosciences. See “Events impacting comparability”.
Excluding exceptional items, costs from continuing operations increased by £84.5 million, or 17%, from £502.1 million in 2000 to £586.6 million in 2001. As a percentage of turnover, however, this represents an increase of only 0.2% from 36.4% to 36.6%.
Higher costs were incurred in Amersham Health due to commissioning expenses for the new plant in Lindesnes, Norway and the costs of building a sales team for the promotion of the new ultrasound product Optison™. Within Amersham Biosciences in both 2000 and 2001 legal costs were incurred in the defense of the Group’s intellectual property in patent suits against Applied Biosystems Group. The court mediated settlement with Applied Biosystems Group was announced in February 2002 and includes a cross-licensing agreement covering all patents involved in the litigation, and a co-development arrangement for the joint development, supply and commercialization of certain new DNA analysis technologies.
R&D expenses
R&D costs for the year ended December 31, 2001 were £173.8 million, an increase of £24.8 million, or 17%, on 2000. R&D expenditure in Amersham Biosciences increased by £14.9 million, or 21%, from £71.5 million in 2000 to £86.4 million in 2001. This represents 13% of Amersham Biosciences’ turnover in 2001 compared with 12% in 2000. Amersham Health’s R&D expenditure increased by £9.1 million, or 12%, to £83.0 million in 2001 compared to £73.9 million in 2000. This represents 9% of Amersham Health’s turnover in 2001 compared with 10% in 2000. Within the Corporate and Other segment exceptional costs of £3.6 and £4.4 million were incurred in 2000 and 2001 relating to the disease profiling project which was discontinued during 2001.
Amersham Health
Cardiology
Amersham Health made good progress in 2001 on its ultrasound product Sonazoid™, which was developed for the diagnosis of both heart and liver disease. In cardiology, Sonazoid™ entered phase IIB dose ranging studies to assess blood flow to the heart muscle following successful completion of phase IIA efficacy studies. For the liver indication, phase IIB clinical trials on Sonazoid™ were completed in Japan and phase III trials commenced in 2001. The prevalence of primary liver cancer in East Asia is seven times higher than in the rest of the world. In the US and Europe, phase IIB trials for the liver indication were also completed in 2001, but, following independent evaluation, phase III trials for using the product outside Japan which were planned for the second half of 2002 were suspended.
Phase IIB clinical trials were under way to extend the uses of its MRI product Omniscan™ in diseases of the cardiac and vascular systems and were concluded in the first half of 2002.
Our Health business pipeline aims to further expand physician knowledge. For example, phase 1 clinical trials began in November 2001 on NC 100668, a technetium-based molecular diagnostic for the detection of thrombus formation. This product will target the important pulmonary embolism and deep vein thrombosis markets.
Neurology
In the US, Amersham Health entered phase 1 clinical studies with Trodat, for the diagnosis of Parkinson’s Disease in 2001. In July 2001, Amersham Health entered into an agreement with Neurochem to collaborate on a product for the diagnosis of Alzheimer’s Disease. The goal for this innovative diagnostic will be the early identification of amyloid plaques in the brains of Alzheimer’s patients, even before the symptoms of the disease are observed.
Spin Signal
Amersham Health is developing a technology known as Spin Signal™, which for the first time allows high-resolution, high speed imaging of gases using MRI.
84
Spin Signal is anticipated to have a wide range of applicability within both medical diagnostics and in life sciences. Amersham Health’s first target area is in high-resolution ventilation imaging of the lungs. Our clinical program is targeted at early detection, staging and monitoring of lung diseases and our first product, Helispin™, is in phase II.
R&D Partnerships
In August 2001, Amersham Health entered into a research agreement with Affitech AS, in Oslo, Norway, for the discovery of human antibodies for diagnostic applications. Affitech will combine its proprietary antibody library technology and its automated affinity maturation process to generate human antibodies to develop and test Amersham Health’s novel concepts for molecular diagnosis.
In November 2001, Amersham Health signed a broad collaborative research agreement with GE Medical Systems to accelerate the development of new positron emission tomography (PET) based molecular diagnostic imaging technologies and systems. The first products to be developed will use a novel PET synthesis system to produce a new generation of high pharmaceutical quality, targeted molecular radiopharmaceuticals, FDG and F-DOPA, to aid the diagnosis and evaluation of patients suffering from cancer and Parkinson’s disease.
Through our Imanet™ initiative, we are working with leading pharmaceutical companies to identify the role that PET can play in the creation of novel diagnostic products that can complement new therapeutic drugs. In January 2002, Amersham Health announced a major research collaboration in this area with Pfizer Inc.
Through collaborations such as these, Amersham Health is making a firm commitment to become a global leader in the PET radiopharmaceutical market.
Amersham Biosciences
Amersham Biosciences completed in 2001 a sustained period of building investment in R&D, particularly within the discovery systems business stream, creating a broad portfolio from which to launch new products.
Discovery systems R&D expenditure increased to £69.4 million in 2001 compared to £53.2 million in 2000, an increase of £16.2 million, or 30%. Split evenly across the year, this was incurred in order to bring a range of new platforms to market in 2001 particularly within the areas of Genomics and Proteomics. Products launched in 2001 in genomics included our 384-capillary MegaBACE 4000, TempliPhi, a new DNA template presentation kit, and, in Proteomics, two new high throughput systems to add to the existing Ettan range.
Protein separations R&D expenditure decreased to £17.0 million in 2001 compared to £18.3 million in 2000.
Group operating profit
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2000 | | 2001 |
| | |
| |
|
| | | (in £millions) |
| | | Restated | | | | |
Operating Profit | | | | | | | | |
| Total continuing operating profit before non-recurring items | | | 230.5 | | | | 280.8 | |
| Exceptional items | | | (27.0 | ) | | | (8.7 | ) |
| | | |
| | | |
| |
| | | | 203.5 | | | | 272.1 | |
| | | |
| | | |
| |
Group operating profit increased by £68.6 million, or 34%, from £203.5 million in 2000 to £272.1 million in 2001. As a percentage of sales this represents an increase of 2.2% from 14.8% to 17.0%.
Excluding the impact of exchange, operating profit before exceptional items increased by 8%. Exceptional items amounted to £8.7 million in 2001 and £27.0 million in 2000. See “Events impacting comparability”.
85
| | | | | | | | | |
| | | Year ended December 31, |
| | |
|
| | | 2000 | | 2001 |
| | |
| |
|
| | | (in £millions) |
| | | Restated | | | | |
Operating profit | | | | | | | | |
| Amersham Health | | | 182.0 | | | | 231.0 | |
| Amersham Biosciences | | | 50.8 | | | | 59.5 | |
| Corporate and Other | | | (29.3 | ) | | | (18.4 | ) |
| | | |
| | | |
| |
| | | | 203.5 | | | | 272.1 | |
| | | |
| | | |
| |
Operating profit of the Amersham Health segment increased by £49.0 million in 2001, giving an operating margin of 25% for the segment compared to 24% in 2000. In 2000 and 2001 respectively, goodwill amortization of £1.7 million and £1.4 million and exceptional items of £10.9 million and £8.5 million were attributable to the operating profit of Amersham Health. Excluding goodwill amortization and exceptional items, Amersham Health increased operating profit from £194.6 million in 2000 to £240.9 million in 2001 representing an increase of 10% at constant exchange rates. Exchange rate fluctuations in 2001 had an adverse impact on actual growth rates of 13%. Trading margins were strong throughout as the benefits of product mix and exchange offset the costs of commissioning the Norway plant and building the Optison™ sales force.
Operating profit of the Amersham Biosciences segment increased by £8.7 million, or 17%, from £50.8 million in 2000 to £59.5 million in 2001. Operating margins increased to 9% in 2001 from 8% in 2000. In 2000 and 2001 respectively, goodwill amortization of £9.0 million and £10.3 million and exceptional items of £3.5 million and £2.6 million were attributable to the operating profit of Amersham Biosciences. Excluding goodwill amortization and exceptional items, operating profit in 2001 was £72.4 million compared to £63.3 million in 2000 representing an increase of 6% at constant exchange rates. Exchange rate fluctuations in 2001 had an adverse impact on actual growth rates of 8%.
In protein separations, operating profit before exceptional items and goodwill amortization grew by £28.3 million, or 42%, to £96.0 million in 2001 compared to £67.4 million in 2000 due to strong growth in turnover and continuing margin improvement. In discovery systems, investment in R&D and in defending our intellectual property and a change in product mix, resulted in an operating loss before exceptional items and amortization of £36.5 million in 2001 compared to £16.6 million in 2000.
Goodwill amortization was £11.7 million compared to £10.7 million in 2000. The increase in amortization primarily related to the acquisition of Dan-Process A/S in August 2001, plus a full year of amortization for Praelux Inc., which was acquired in mid February 2000.
Profit on sale of fixed asset investment
In September 2001, the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4 million and realized £50.6 million for the outstanding Nycomed Pharma loan notes held by the Group. The Group has recorded a profit on the transaction of £55.3 million after charging costs associated with the disposal, including the costs incurred in transferring the Nycomed name to Nycomed Pharma.
Amounts written off investments
We have also taken a charge of £4.4 million against the carrying value of listed investments following a material reduction in their equity value in 2001.
Net interest payable
Interest costs in 2001 were £8.7 million compared to £10.0 million charged in 2000. Strong cash flow and the additional reduction in net debt following the sale of the trade investment in Nycomed Pharma in September 2001 resulted in the reduction in net interest expense by £1.3 million.
86
Tax on profit on ordinary activities
In 2001, the taxation charge before exceptional items and goodwill amortization was £97.0 million compared to a charge of £82.3 million in 2000. This represents an effective tax rate of 34.7% in 2001 and 35.6% in 2000 before exceptional items and goodwill amortization. The rate was lower principally as a result of the favorable settlement of a number of outstanding tax items relating to earlier years.
Profit attributable to shareholders
Profit attributable to minority interest relates primarily to the 45% interest that Pharmacia Corporation held in Amersham Biosciences. Profit attributable to shareholders after charging exceptional items and goodwill amortization was £210.7 million in 2001 and £107.0 million in 2000.
87
B. Liquidity and Capital Resources
Cash flow
Net cash flow from operating activities was £345.5 million in 2002, £354.3 million in 2001 and £224.7 million in 2000. Before exceptional cash items consisting of an inflow of £12.0 million in 2001 and an outflow of £14.8 million in 2000 and integration costs of £15.8 million in 2000, net cash inflow from operating activities before exceptional items and integration costs was £345.5 million in 2002, £342.3 million in 2001 and £255.3 million in 2000. The increase in net cash flow in 2002 compared to 2001 of £3.2 million was driven by growth in operating profit before charging amortization and depreciation offset by additional working capital through the building of inventories in Amersham Health. The increase in net cash flow in 2001 compared to 2000 of £87.0 million was driven by operating profit growth and reduced working capital with a significant increase in payables.
Capital expenditure in 2002 was £143.4 million. Capital expenditure was increased in 2002 with a large investment to expand manufacturing capacity and support our strategy of packaging differentiation in Amersham Health. £3.4 million was spent in 2002 on trade investments and £4.8 million on intangible fixed assets. Capital expenditure in 2001 was £91.6 million, down from £123.5 million in 2000. This was due to the completion of the US manufacturing and research expansion plan at the Piscataway site as well as IT projects in 2000. During 2001, £4.9 million was spent on trade investments and £10.2 million on intangible fixed assets. £123.0 million was raised from the disposal of the investment in Nycomed Pharma and a further £22.1 million from the sale of fixed assets including £21.7 million for the sale of the Torsten Almen Research Center. Capital expenditure in 2000 was £123.5 million. During 2000, £10.5 million was spent on trade investments, and £16.5 million was used to acquire shares of Amersham plc to fulfill obligations of share options granted to employees.
Net cash outflow of £749.2 million in 2002 related to acquisitions and disposals. This was primarily associated with the acquisition of the 45% minority in Amersham Biosciences (£708.6 million including associated costs), two filtration companies, AG Technology Corp and InnovaSep Technology Corporation, and the net assets of the CodeLink™ pre-arrayed slides business from Motorola Life Sciences. Net cash outflow of £3.9 million in 2001 related to acquisitions and disposals. This was primarily associated with the acquisition of Dan-Process A/S. Net cash outflow of £4.9 million in 2000 related to acquisitions and disposals. This was mainly in connection with the acquisition of Praelux Inc and Imaging Research Inc, offset by the sale of Nycomed Amersham Medical Systems SA.
Net debt increased to £182.3 million at December 31, 2002 due to a net cash outflow of £249.1 million offset by favorable currency movements of £92.2 million during 2002. In February 2002, the Group repaid out of its cash revenues $20 million aggregate principal amount on the series A of its fixed rate US private placement unsecured loan notes. At December 31, 2001, net debt was £25.4 million having decreased from £323.5 million at December 31, 2000 due to a net cash inflow of £304.5 million offset by adverse currency movements of £6.4 million.
Capital resource requirements
At December 31, 2002, shareholders’ equity was £1,172.0 million, an increase of £575.7 million in 2002 primarily due to £121.4 million of retained earnings, £47.8 million due to foreign currency translation of overseas net assets and £407.7 million of new share capital and share premium. At December 31, 2001, shareholders’ equity was £596.3 million, as restated for the introduction of FRS 19 as outlined in Note 37 of Item 18 “Consolidated Financial Statements”. This is an increase of £179.4 million in 2001 primarily due to £165.5 million of retained earnings and £25.2 million of new share capital and share premium. At December 31, 2000, shareholders’ equity was £416.9 million, as restated for the introduction of FRS 19 as outlined in Note 37 of Item 18 “Consolidated Financial Statements”, an increase of £53.2 million in 2000 primarily due to £66.6 million of retained earnings, offset by £18.9 million of foreign exchange losses arising on the retranslation of subsidiaries’ balance sheets into sterling.
88
The Group is of the opinion that, taking into account the $750 million Facility committed to February 2007, the Group has sufficient working capital to cover its requirements at least until December 31, 2003. Total unused lines of credit at December 31, 2002 for cash advances under the $750 million facility amounted to approximately £404.3 million.
Financial covenants contained in the $750 million Facility impose certain limitations on Amersham. The $750 million Facility requires Amersham to cover interest by at least three times EBITDA and restrict net debt to less than 3.5 times EBITDA. This is tested semi-annually for the previous rolling twelve month accounting period. EBITDA is defined as consolidated profit before interest and tax adding back exceptional items, minority interests, depreciation and amortization. Additional covenants customary for such an agreement also apply. Such covenants may have the effect of restricting Amersham’s ability to incur certain indebtedness or interest costs or engage in certain other activities. A breach of one of the financial covenants may result in any outstanding drawing under the facility becoming due and payable immediately and the total facility may be cancelled. Further, if the amounts outstanding under the facility are greater than £30 million and are declared due and payable and the company is unable to make those repayments, then the outstanding amounts under the US private placement unsecured loan notes may also become immediately due and payable. The Facility does not restrict the Company’s ability to pay dividends.
The $750 million Facility will expire on February 20, 2007. In order to repay amounts outstanding under the Facility, Amersham may be required to refinance such borrowings under other credit facilities, or by the issuance of indebtedness in the private or public markets. The means by which the principal due at maturity will be repaid will depend on market conditions at the time of any such refinancing.
As at December 31, 2002, the Group had fixed rate debt obligations of three series of US private placement unsecured loan notes with the following interest rates and maturities:
| | | | | | | | | | | | | |
| Series | | Amount | | Interest Rate | | Maturity |
| |
| |
| |
|
| Series B | | $15 million | | | 8.79 | % | | February 2005 |
| Series C | | $163 million | | | 8.77 | % | | November 2004 |
| Series D | | $15 million | | | 8.97 | % | | November 2009 |
The Series A notes were repaid in February 2002.
The private placements carry a number of financial covenants. The Group is required to maintain a consolidated adjusted net worth balance of greater than £500 million. For the purposes of this calculation, the Group is permitted to reinstate 50% of the Nycomed goodwill merger reserve written off to reserves in October 1997.
The maximum permitted gearing for Amersham under the covenants is 125% of consolidated adjusted net worth. The underlying priority debt and the secured loans are limited to 20% of the amount determined as the consolidated adjusted net worth of the Group (as defined above). The limit of interest cover is four times consolidated profit before interest and tax.
A private placement covenant limits the Group asset disposals to 15% of gross assets (£395.8 million at December 31, 2002) in any 365 day period. If this limit is exceeded, Amersham has 12 months to offer to prepay the private placement notes, or to demonstrate to the note holders that the proceeds have been reinvested in the business.
The Board believes that the Company is in full compliance with the covenants contained in the $750 million Facility and the private placement unsecured loan notes and that they do not presently impose undue restrictions on its activities.
An analysis of the financial liabilities and assets of the Group are given in Note 20 of Item 18 “Consolidated Financial Statements”.
89
In addition to the financial liabilities disclosed above, the Group has granted put options over shares in Corixa Corporation, Cimarron Software Inc, Imaging Research Inc. Imaging Research Solutions Ltd and Uppsala Research Imaging Solutions AB and holds call options over shares in Cimarron Software Inc., Imaging Research Inc, Imaging Research Solutions Ltd, Uppsala Research Imaging Solutions AB and Analytica of Branford which meet the definition of financial instruments. The maximum potential aggregate cash consideration under these contracts is $71.5 million and £7.2 million. These options expire by 2006.
Going concern
After making enquiries the Directors have reasonable expectation that the Company and the Group have adequate resources to continue in operational existence for the foreseeable future, and that it is therefore appropriate to adopt the going concern basis in preparing its financial statements. In forming this view the Directors have reviewed the Annual Profit Plan for the year ending 31 December 2003 and strategic plan projections for subsequent years. The Directors have satisfied themselves that the Group is in a sound financial position and that sufficient borrowing facilities will be available to meet the Group’s foreseeable cash requirements.
Capital Expenditure
Committed capital expenditure at December 31, 2002 amounted to £41.3 million. The purpose of these commitments is modification and extension of manufacturing and administrative facilities at the Group’s existing sites. This expenditure will be funded through working capital and headroom under the Group’s existing bank credit facilities. This is disclosed in Note 28 of Item 18 “Consolidated Financial Statements”.
Treasury Policy
Our treasury activities are coordinated and managed by the Group treasury department in accordance with policies approved by the Board. These policies include the management of investments and counterparty risk, interest rate risk and the hedging of currency risk are reviewed regularly and have not changed significantly over the past year. Compliance with these policies is tested on a regular basis. Treasury transactions are undertaken only with a small number of financially strong counterparties under a mandate approved by the Board. The majority of the Group’s external debt has been lent to Amersham plc. This is then lent to other Group companies as required. Surplus cash generated is used to repay short term floating rate debt at the earliest opportunity. Short term surpluses are invested by Group Treasury in US dollars, euros and sterling.
Environmental and Regulatory Matters
Item 4, “Information on the Company” provides a description of environmental and other regulatory matters related to the Group including a description of the costs and liabilities associated with these matters.
Contractual Obligations
The following table shows the contractual obligations and commitments of Amersham plc as at December 31, 2002:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Payments due by period |
| | | | | | |
|
| | | | | | | Less | | | | | | | | | | | | |
| | | | | | | than 1 | | 1 - 2 | | 2 - 5 | | After 5 |
| | | Total | | year | | years | | years | | years |
| Contractual Obligations | | £m | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
| |
|
| Long term debt | | | 234.3 | | | | 44.3 | | | | 105.9 | | | | 75.0 | | | | 9.1 | |
| Capital lease obligations | | | 2.8 | | | | 0.3 | | | | 0.3 | | | | 0.6 | | | | 1.6 | |
| Operating leases | | | 89.2 | | | | 29.3 | | | | 25.0 | | | | 23.2 | | | | 11.7 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 326.3 | | | | 73.9 | | | | 131.2 | | | | 98.8 | | | | 22.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
For more information, please see Notes 18, 19, 20 and 29 of Item 18 “Consolidated Financial Statements”.
90
The above table shows the ageing of long term debt as disclosed in Notes 18, 19 and 20 of Item 18 “Consolidated Financial Statements”, being the aggregate amount of outstanding bank loans and overdrafts, unsecured loan notes and a short term loan from the joint venture in NMP.
Of the operating lease commitments, £54.9 million relates to leases over land and buildings and £34.7 million over plant and machinery, fixtures and fittings, and motor vehicles.
| | | | | | | | | | | | | | | | | |
| | | | | | | Amount of Commitment |
| | | | | | | Expiration per Period |
| | | | | | |
|
| | | | | | | Less than 1 | | | | | | Over 5 |
| | | Total | | year | | 1 - 5 years | | years |
| Contractual Obligations | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
|
| Lines of credit | | | 465.8 | | | | — | | | | 465.8 | | | | — | |
| Guarantees | | | 8.2 | | | | 8.2 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 474.0 | | | | 8.2 | | | | 465.8 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
The line of credit shown above represents the $750 million facility described above.
The largest guarantee reported within the £8.2 million total in the table above is a £7.9 million counter-indemnity granted to a New York bank in relation to their Irrevocable Letter of Credit to the Illinois Department of Nuclear Safety on behalf of Medi-Physics, Inc.
C. Research and Development, Patents and Licenses
For information on our research and development and intellectual property, see Item 4 “Information on the Company”, and for associated risks, see Item 3. Key Information — D. “Risk Factors”.
D. Trend information
Going forward, Amersham is well placed to achieve further good growth in sales and profit in 2003. The following guidance is given before the impact of foreign exchange:
| | • | | Amersham Health is expected to continue the good growth in medical diagnostics excluding Japan, and operating margins are anticipated to be at similar levels to those achieved in 2002. Amersham Health sales in Japan are expected to decline at a lower rate than in 2002. |
| |
| | • | | Protein separations is expected to see good growth with sales growth again expected to be stronger in the second half of the year. Increased investment in R&D will bring operating margins down slightly. |
| |
| | • | | Discovery systems is expected to see good sales growth in reagents, consumables and software. Visibility for capital expenditure on instrumentation remains low. The restructuring plans are expected to bring benefits in 2003 moving the business to profitability during 2004. |
E. Off Balance sheet items
Other than operating leases disclosed in Note 29 of Item 18 “Consolidated Financial Statements”, we do not engage in off-balance sheet financing activities, and do not have any special purpose entities.
91
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
As at April 16, 2003 the Board comprised five Executive Directors, eight independent Non-Executive Directors and an employee representative Non-Executive Director. Throughout the reported period, the offices of Chairman and Chief Executive have been held separately. The Chairman, Mr R D Lapthorne, is a Non-Executive Director. The following are the Directors and Executive Officers of the Group:
| | | | | | | | | |
| Name | | Age | | Position Details | | Date appointed |
| |
| |
| |
|
Mr Richard D
Lapthorne, CBE, BCom,
FCMA, FCCA, FCT, CBIM | | | 59 | | | Independent Non Executive Chairman
Chairman of the Board in 1996 and again since 1999, Deputy Chairman from 1997 to 1999 and a board member since joining Amersham International plc as a Non-Executive Director in 1988. Mr Lapthorne will be retiring from the Board after the Annual General Meeting on May 7, 2003.
Recruited as Finance Director of British Aerospace plc in July 1992, becoming Vice Chairman in April 1998. Retired from British Aerospace in September 1999.
Also serves as Non-Executive Chairman of Cable and Wireless plc from January 10, 2003, Morse plc, Avecia Ltd, Tunstall Holdings Limited, TI Automotive Limited and a Non-Executive Director of Oasis International Leasing in Abu Dhabi.*†# | | December 1988 |
| | | | | | | | | |
| Mr Johan Fr. Odfjell, MBA , Siv. Øk | | | 54 | | | Independent Non-Executive Deputy Chairman
Deputy Chairman of the Board since 1999. From 1997 to 1999 served as Chairman of the Board, and since 1996 as a Non-Executive board member of Nycomed ASA.
Served as Chief Executive of Vesta Insurance Group from 1986 to 1994. From 1994 has combined his own consultancy and investment firm with several Non-Executive board positions. Also Chairman of Orkla ASA, Star Shipping A/S and Nera ASA. Co-Chairman of Det Norske Veritas; and a board member of Bergesen ASA. *†# | | October 1997 |
| | | | | | | | | |
| Sir William M Castell, BA, FCA | | | 55 | | | Chief Executive (Executive Director and officer)
Chief Executive since 1989, when he joined Amersham International plc.
Previously Commercial Director Wellcome plc 1987-1989, following a career in Wellcome spanning over twenty years.
Also serves as Chairman of The Prince’s Trust and Council Member of the Medical Research Council, and is on the External Advisory Board for the Life Sciences Institute at Michigan. Knighted in June 2000 for services to the life sciences industry. | | November 1989 |
| | | | | | | | | |
| Mr Giles F B Kerr, BA, ACA | | | 43 | | | Finance Director (Executive Director and officer)
Appointed Finance Director in 1997. Joined Amersham International plc as Group Financial Controller 1991 to 1994, Commercial Controller 1994 to 1996, Company Secretary and Director of Corporate Development 1996 to 1997.
Worked for Arthur Andersen & Co from 1981, in positions including Audit and Accounting Manager and Partner, Corporate Finance and Investigations | | November 1997 |
| | | | | | | | | |
| Dr Andrew Carr, Ph.D | | | 43 | | | President of Amersham Biosciences (Executive Director and officer)
Joined the Company in 1987 as Senior Development Scientist and has held a number of senior management positions in research and development, manufacturing, and sales and marketing. Appointed President of Amersham Biosciences in 2000 and joined the Board in May 2002. | | May 2002 |
92
| | | | | | | | | |
| Name | | Age | | Position Details | | Date appointed |
| |
| |
| |
|
| Mr Peter Loescher, MBA | | | 45 | | | President of Amersham Health (Executive Director and officer)
Joined the Company in December 2002. Previously with Hoechst AG and its successor company Aventis working in a variety of roles in Germany, Japan, Spain, UK, and US. From 1999 to 2002 served as Chairman and Chief Executive Officer of Aventis Pharma in Japan, having previously served as Chief Executive Officer of Hoechst Marion Roussell Limited in the UK from 1997. | | December 2002 |
| | | | | | | | | |
| Mr George W Battersby, BSc | | | 56 | | | Group Human Resources Director (Executive Director and officer)
Joined the Company in 2000 as Group Human Resources Director. Previously with Laporte plc from 1996 to 2000 as Group Human Resources Director, and Fisons plc from 1985 to 1996 as Group Human Resources Director.
Earlier career included sales and marketing, consulting and human resources appointments in companies including International Computers Limited and Air Products and Chemicals Inc. | | September 2000 |
| | | | | | | | | |
| Mr Donald H Brydon, OBE, BSc | | | 57 | | | Independent Non-Executive Director (Chairman designate)
A Non-Executive Director since 1997 and will succeed Richard Lapthorne as Chairman following the Annual General Meeting in May 2003.
Joined AXA Group in 1997 after a 20-year career with the Barclays Group. Now serving as Chairman of AXA Investment Managers, and a Non-Executive Director of Allied Domecq and Chairman of the Financial Services Practitioner Panel. *†# | | June 1997 |
| | | | | | | | | |
Mr John H Johansen, MSc
| | | 56 | | | Non-Executive Director (Employee representative)
A Non-Executive Director since 1997, having served on the Hafslund Nycomed ASA Board since 1994 as an elected employee representative.
Joined the Norwegian organization in 1980 as a Research Scientist, peptide chemistry, holding management positions in chemistry research and development. A Senior Research Scientist in Amersham Health from 1991 to the present. Appointed a member of the Board of Playboard Magazine AS in 2001. | | October 1997
|
| | | | | | | | | |
| Dr John S Patterson FRCP | | | 55 | | | Independent Non-Executive Director
A Non-Executive Director since 2001.
Appointed Vice President for Clinical Research and Medical Affairs of ICI Pharmaceuticals for the US in 1988 after a long career with the company. In 1990 joined its Board as Medical Director. Appointed Executive Vice President Product Strategy and Licensing for AstraZeneca in 1999 and currently serves as Chair on its Portfolio Management and Licensing Executive sub-committees. Also serves as a Director of the British Pharmaceuticals Group and is President of the Association of the British Pharmaceutical Industry. *†# | | January 2001 |
| | | | | | | | | |
| Sir Keith Peters MB BCh, FRCP, FRCPath, FRS | | | 64 | | | Independent Non-Executive Director A Non-Executive Director since 2000. Also Chairman of the Amersham plc Science Advisory Board and a member of the Amersham plc Portfolio Committee.
Became Regius Professor of Physic at the University of Cambridge in 1987. President, Academy of Medical Sciences; Honorary Consultant Physician at Addenbroke’s NHS Trust. Governing Trustee, The Nuffield Trust; Trustee, Strangeways Research Laboratory. Member of Council of Heads of Medical Schools and Dean of UK Faculties of Medicine; member of Scientific Advisory Boards of the Gairdner Foundation, Merck Institute, Merck Sharp & Dohme Research Laboratories Neuroscience Research Centre and Cambridge Antibody Technology. | | August 2000 |
93
| | | | | | | | | |
| Name | | Age | | Position Details | | Date appointed |
| |
| |
| |
|
| Mr Jacques F Rejeange, MBA | | | 63 | | | Independent Non-Executive Director
A Non-Executive Director since 1997, having been a Non-Executive board member of Hafslund Nycomed ASA since 1994.
From 1966 to 1992 was employed by the Sandoz Group, rising from Management Trainee to President and Chief Executive of Sandoz Pharmaceuticals Inc in the US.
Became President and Chief Executive of Sterling Winthrop Inc in 1994 and in 1998 until June 2002 was Chief Executive of NMT Management AG. Also a Non-Executive Director of Antares Inc and Pozen Inc, both in the US. *†# | | October 1997 |
| | | | | | | | | |
| Prof Erik Thorsby, MD | | | 64 | | | Independent Non-Executive Director
A Non-Executive Director since 1997, having earlier that year joined the Nycomed ASA Board as a Non-Executive member.
Held a number of positions in medical institutions, international scientific societies and committees and in 1983 was appointed Professor of Medicine at the University of Oslo. Currently Head of the Institute of Immunology at The National Hospital and University of Oslo. Also serves as President of the European Federation for Immunogenetics and is Chairman of the Research Council at The National Hospital. *† | | October 1997 |
| | | | | | | | | |
| Professor Mathias Uhlen | | | 48 | | | Independent Non-Executive Director
Appointed Non-Executive Director in May 2002, having served as a Non-Executive Director of Amersham Biosciences since 1997 and a member of the Amersham plc Portfolio Committee.
Professor of Biotechnology at the Royal Institute of Technology (KTH) Stockholm since 1988. Serves on the Boards of Magnetic Biosolutions AB, Prevas AB, Personal Chemistry AB, Skanditec AB, Affibody AB, Pyrosequencing AB, Biovitrum AB and KTH Holding AB. *† | | May 2002 |
| | | | | | | | | |
| Mr Robert E B Allnutt, LLB | | | 48 | | | Company Secretary (Officer)
Appointed Company Secretary in December 1997. | | December 1997 |
| * | | Members of the Remuneration Committee, which is chaired by Mr R D Lapthorne. |
| |
| # | | Members of the Audit Committee, which is chaired by Mr D H Brydon. |
| |
| † | | Members of the Nomination Committee, which is chaired by Mr J Fr. Odfjell |
There is no family relationship between any Director or officer and any other Director or officer.
B. Compensation
Remuneration policy for Executive Directors
In determining the remuneration policy for Executive Directors, the Remuneration Committee has considered a number of factors including:
| • | | the importance of attracting, retaining and motivating management of the appropriate caliber to further the success of the business; |
| |
| • | | the linking of reward to both individual and business performance; and |
| |
| • | | ensuring that the interests of the Directors are aligned with those of the shareholders. |
To this end, the Remuneration Committee seeks to pay Executive Directors base salaries at a median level and incentives (in the form of bonuses and long term option arrangements) at an upper quartile level when compared to compensation levels and packages in other companies of comparable size and complexity, and also in companies in the same business sector. The total
94
package is aimed to lie between the median and upper quartile. For 2003, the Committee aims to target 50% of total package in the form of fixed remuneration (base salary and benefits) and 50% performance-related remuneration (target annual bonus and Black Scholes value of annual share option awards). In 2002, the ratio was 55% fixed and 45% performance-related remuneration.
In 2002, the Remuneration Committee considered a valuation of all elements of Executive Directors’ remuneration to ensure that it was aware of the total remuneration within the Company and its selected comparators. This valuation approach included the use of the Black-Scholes option valuation technique for assessing the value of long term incentives. The same approach will be used in 2003.
In establishing this policy, the Committee has appointed independent consultants, New Bridge Street Consultants who provide advice on remuneration and share plans both for Executive Directors and the wider executive population. The Committee has also appointed Watson Wyatt who provide advice on pensions both for Executive Directors and advice to the Company as a whole. Neither New Bridge Street Consultants nor Watson Wyatt provide any other services to the Company. In addition, the Committee is advised on reward strategy by Mr Malcolm Saffin, Vice President of Compensation and Benefits for Amersham.
The Remuneration Committee believes that the policy adopted in its remuneration of executive directors and senior managers has contributed to the sustained financial success and long term growth of the Company. This policy has enabled the Company both to attract and keep a high caliber management team — essential for a well run and growing business. The strong shareholder returns achieved by the Company are a testament to this policy, which reflects best market practice. This policy will continue to be reviewed in the light of changes in market practice and legislation, which impact upon the Company.
The current elements of the remuneration packages can be summarized as follows:
Base salary and benefits
Base salaries for Executive Directors are reviewed by the Committee, normally annually, having regard to competitive market practice and individual performance for the financial year.
The general benefits provided to the Executive Directors are a fully expensed car, pension, life, disability and health insurance and where appropriate relocation expenses.
During the year, Peter Loescher joined the Company from Japan. The cost of his relocation, his housing and his children’s education are being met by the Company in line with the Company’s expatriate policy. In addition, he received a signing on bonus of euros 500,000 (£316,455) and the Company has agreed to compensate Mr Loescher for the loss of the bonus he would have received from his previous company had he not joined Amersham. Full details are given in Note 3 on page 98. All amounts are disclosed in the Directors’ Remuneration Table on page 98.
Annual performance-related bonus
The annual performance-related bonus is dependent upon a number of factors. The level of bonus is payable on a sliding scale between 0% and 100% of the base salary. Bonus payments totaling £1,135,930 (2001 — £1,125,420) have been achieved, based on the financial performance measures attained for 2002, namely sales and operating profit, cash flow, growth in earnings per share and performance against a number of personal objectives for each Executive Director.
In respect of the year ended December 31, 2003, it is proposed to pay a bonus on a sliding scale of 0% to 150% of base salary. The actual payment will be based on the same financial performance measures as used in 2002. Two-thirds of the bonus shall be paid in cash and one-third in the form of restricted shares, which will vest 50% on the second anniversary of the bonus payment date and 50% on the third anniversary of the bonus payment date.
95
In conjunction with the increased annual bonus opportunity, the Company has introduced Share Ownership guidelines, which will require the Executive Directors to hold shares equivalent in value to two times their base salary. This holding must be acquired within five years.
Share Option Plans
Details of Directors’ share ownership in the Company and details of stock options granted to the Directors for the year are disclosed in Item 6 E. “Share Ownership”.
Pensions
Sir William Castell is entitled to a total target pension upon retirement at age 60 of 60% of the average of the last three years’ basic salary. The pension payable by the Company will be this target pension, less pension benefits earned in previous employments. This pension will also include the pension which could be notionally secured by Sir William Castell from past remuneration supplements.
Approximately two-thirds of the total target pension is being funded, through a combination of approved and unapproved arrangements. With effect from December 2002, this proportion will be increased over time with the objective that at retirement, the whole of the target pension will be funded. Details of the accrual of his pension are provided below.
Mr G W Battersby, Mr G F B Kerr and Mr P Loescher accrue pension benefits through a combination of approved defined benefit arrangements and unapproved defined contribution arrangements. Dr J M Padfield also accrued pension benefits through a combination of approved defined benefit arrangements and unapproved defined contribution arrangements up to his retirement on December 31, 2002. Details are shown below of the defined benefit accrual. The contribution to the Funded Unapproved Retirement Benefits Scheme (“FURBS”) element is identified in the column headed “pension contributions” in the table on page 98. Dr A Carr accrues a defined benefit pension through both UK and US pension arrangements.
In previous years’ accounts, disclosures of the accrual of defined benefits have been made under the requirements of the Stock Exchange Listing Rules. These Rules are still in place, but it is now also necessary to make disclosure in accordance with the Directors’ Remuneration Report Regulations 2002. The information below sets out the disclosures under the two sets of requirements.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Increase in |
| | | | | | | | | | | | | | | Increase in | | | | | | | | | | | | | | transfer value |
| | | | | | | Accrued | | Increase in | | accrued pension | | Transfer value of | | Transfer value of | | Director’s | | over the year, |
| | | | | | | Pension | | accrued pension | | during the year | | accrued pension | | accrued pension | | contribution | | net of director’s |
| | | | | | | at 31.12.02 | | during the year | | (net of inflation) | | at 31.12.02 | | at 31.12.01 | | during the year | | contribution |
| Name | | Age | | £ pa | | £ pa | | £ pa | | £ | | £ | | £ | | £ |
| |
| |
| |
| |
| |
| |
| |
| |
|
| W M Castell | | | 55 | | | | 202,987 | | | | 44,887 | | | | 42,199 | | | | 2,747,681 | | | | 2,272,171 | | | | — | | | | 475,510 | |
| G W Battersby | | | 56 | | | | 3,764 | | | | 1,662 | | | | 1,626 | | | | 54,093 | | | | 31,523 | | | | 4,838 | | | | 17,732 | |
| A Carr | | | 43 | | | | 89,233 | | | | 15,189 | | | | 14,093 | | | | 479,131 | | | | 554,573 | | | | — | | | | (75,442 | ) |
| G F B Kerr | | | 43 | | | | 26,233 | | | | 5,212 | | | | 4,855 | | | | 189,651 | | | | 186,208 | | | | 4,838 | | | | (1,395 | ) |
| P Loescher | | | 45 | | | | 275 | | | | 275 | | | | 275 | | | | 2,081 | | | | — | | | | 405 | | | | 1,676 | |
| J M Padfield | | | 55 | | | | 5,377 | | | | 1,700 | | | | 1,637 | | | | 74,857 | | | | 54,060 | | | | 4,838 | | | | 15,959 | |
Notes:
| (1) | | The accrued pensions are the amounts which would be paid if the director left service at the relevant date, but ignoring any vesting periods. |
| |
| (2) | | The transfer value represents the lump sum capital value of the director’s pension benefits as at the year end, calculated using assumptions certified by our actuary in accordance with actuarial guidance note GN11. These assumptions include a link to current stock market levels for younger members and bond markets for older members. Stock markets have fallen during the year and the transfer values for younger directors have fallen accordingly. |
96
Further information about the Directors’ pension benefits is given below.
| | | | | | | | | | | |
| | | Normal Retirement | | | Early Retirement | | | |
| Name | | Age | | Terms | | Dependant's Pension | | Pension Increases |
| |
| |
| |
| |
|
| W M Castell | | | 60 | | | Reduced by 4% for each year earlier than 60 | | 66% of Member’s Pension | | RPI up to 5% |
| | | | | | | | | | | |
| G W Battersby | | | 63 | | | Unreduced from age 60 and between age 50 and 60 reduced by 4% each year earlier than 60 (pro-rata for months) | | 50% of Member’s Pension | | RPI up to 7.5% plus 50% of RPI in excess subject to maximum of 8.75% |
| | | | | | | | | | | |
| A Carr | | | US: 65 | | | Unreduced from age 62 and between age 55 and 62 reduced by 4% each year earlier than 62 | | Any dependant’s pension is provided by reducing the Director’s own pension by an actuarially equivalent amount | | Nil |
| | | | | | | | | | | |
| A Carr | | | UK: 63 | | | Unreduced from age 60 and between age 50 and 60 reduced by 4% each year earlier than 60 (pro-rata for months) | | 50% of Member’s Pension | | RPI up to 7.5% plus 50% of RPI in excess subject to maximum of 8.75% |
| | | | | | | | | | | |
| G F B Kerr | | | 63 | | | Unreduced from age 60 and between age 50 and 60 reduced by 4% each year earlier than 60 (pro-rata for months) | | 66% of Member’s Pension | | RPI up to 7.5% plus 50% of RPI in excess subject to maximum of 8.75% |
| | | | | | | | | | | |
| P Loescher | | | 63 | | | Unreduced from age 60 and between age 50 and 60 reduced by 4% each year earlier than 60 (pro-rata for months) | | 50% of Member’s Pension | | RPI up to 7.5% plus 50% of RPI in excess subject to maximum of 8.75% |
| | | | | | | | | | | |
| J M Padfield | | | 63 | | | Unreduced from age 60 and between age 50 and 60 reduced by 4% each year earlier than 60 (pro-rata for months) | | 50% of Member’s Pension | | RPI up to 7.5% plus 50% of RPI in excess subject to maximum of 8.75% |
97
Directors’ remuneration
The remuneration of each Director for the period ended December 31, 2002 is analyzed as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Total | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | remuneration | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | for 12 months to | | | | | | | | | | Total | | Total |
| | | | | | | | | | | | | | | Dec 31, 2002 | | | | | | | | | | remuneration | | remuneration |
| | | Salary | | | | | | | | | | before pension | | Pension | | Compensation for | | for 12 months to | | for 12 months to |
| | | and fees | | Benefits1 | | Bonus | | contributions | | contributions | | loss of office | | Dec 31, 2002 | | Dec 31, 2001 |
| | | £ | | £ | | £ | | £ | | £ | | £ | | £ | | £ |
| | |
| |
| |
| |
| |
| |
| |
| |
|
Executive Directors | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
W M Castell
(Chief Executive) | | | 575,000 | | | | 88,395 | | | | 397,200 | | | | 1,060,595 | | | | — | | | | — | | | | 1,060,595 | | | | 963,485 | |
| G W Battersby | | | 266,250 | | | | 32,659 | | | | 182,050 | | | | 480,959 | | | | 85,747 | | | | — | | | | 566,706 | | | | 589,030 | |
A Carr2 (appointed 9 May 2002) | | | 261,558 | | | | 23,275 | | | | 91,600 | | | | 376,433 | | | | 2,349 | | | | — | | | | 378,782 | | | | — | |
| G F B Kerr | | | 302,500 | | | | 93,717 | | | | 198,710 | | | | 594,927 | | | | 10,056 | | | | — | | | | 604,983 | | | | 557,739 | |
P Loescher3 (appointed December 1, 2002) | | | 353,955 | | | | 104,855 | | | | — | | | | 458,810 | | | | — | | | | — | | | | 458,810 | | | | — | |
J M Padfield4 | | | 386,364 | | | | 46,925 | | | | 266,370 | | | | 699,659 | | | | 129,518 | | | | 841,035 | | | | 1,670,212 | | | | 788,173 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total – Executive | | | 2,145,627 | | | | 389,826 | | | | 1,135,930 | | | | 3,671,383 | | | | 227,670 | | | | 841,035 | | | | 4,740,088 | | | | 2,898,427 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Non-Executive
Directors | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
R D Lapthorne
(Chairman) | | | 155,000 | | | | — | | | | — | | | | 155,000 | | | | — | | | | — | | | | 155,000 | | | | 120,000 | |
| J Fr Odfjell | | | 66,353 | | | | — | | | | — | | | | 66,353 | | | | — | | | | — | | | | 66,353 | | | | 60,000 | |
| D H Brydon | | | 41,667 | | | | — | | | | — | | | | 41,667 | | | | — | | | | — | | | | 41,667 | | | | 30,000 | |
J H Johansen5 | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | 30,000 | |
| J Patterson | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | 29,348 | |
K Peters6 | | | 71,483 | | | | — | | | | — | | | | 71,483 | | | | — | | | | — | | | | 71,483 | | | | 67,500 | |
| J F Rejeange | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | 30,000 | |
| E Thorsby | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | — | | | | — | | | | 35,833 | | | | 30,000 | |
M Uhlen7 (appointed 9 May 2002) | | | 25,141 | | | | — | | | | — | | | | 25,141 | | | | — | | | | — | | | | 25,141 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total – Non-Executive | | | 502,976 | | | | — | | | | — | | | | 502,976 | | | | — | | | | — | | | | 502,976 | | | | 396,848 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Past Directors | | | | | | | | | | | | | | | | | | | — | | | | — | | | | | | | | | |
M J Crumpton8 | | | 61,500 | | | | 10,304 | | | | — | | | | 71,804 | | | | — | | | | — | | | | 71,804 | | | | 90,823 | |
T V Jacobsen9 | | | — | | | | 77 | | | | — | | | | 77 | | | | — | | | | — | | | | 77 | | | | 136,866 | |
R E Long10 | | | 343,763 | | | | 15,934 | | | | — | | | | 359,697 | | | | — | | | | — | | | | 359,697 | | | | 275,754 | |
| T F W McKillop | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,500 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total – Past Directors | | | 405,263 | | | | 26,315 | | | | — | | | | 431,578 | | | | — | | | | — | | | | 431,578 | | | | 505,943 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total | | | 3,053,866 | | | | 416,141 | | | | 1,135,930 | | | | 4,605,937 | | | | 227,670 | | | | 841,035 | | | | 5,674,642 | | | | 3,801,218 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| 1 | | The figures shown for benefits include amounts payable in respect of private medical insurance, permanent health insurance, the provision of company cars and fuel and amounts received in cash for pensions. |
| |
| 2 | | In addition to the figures shown in the table above in respect of Dr A Carr, he received $196,153.83 (£130,769.22) in respect of his employment as President of Amersham Biosciences prior to his appointment to the Board on May 9, 2002 (2001:$879,229 (£582,271)). |
| |
| 3 | | Mr P Loescher received a signing-on bonus of euros 500,000 (£316,455). This amount is included in the figures shown in the table above. The figure for Mr P Loescher’s benefits include the costs of his relocation from Japan and the costs for his housing and children’s education. In addition, the Company has agreed to compensate Mr Loescher for the loss of the bonus he would have received from his previous company had he not joined Amersham. This amounts to euros 325,000 and is not included in the table above. |
| |
| 4 | | Dr J M Padfield received £841,035 in respect of his contractual entitlements following his retirement on December 31, 2002. This amount is included in the figures shown in the table above and explained in detail on page 99. |
| |
| 5 | | In addition to the fees shown in the table above Mr J H Johansen received NOK 511,923 (£45,973) in salary and NOK 18,161 (£1,631) in benefits as an employee. |
98
| 6 | | Professor Sir Keith Peters received fees of £35,650 in respect of his services on the Science Advisory Board. This amount is included in the figures shown in the table above. |
| |
| 7 | | In addition to the figures shown in the table above in respect of Prof M Uhlen, he received £7,141 in respect of his services as a Non-Executive director of Amersham Biosciences Limited before his appointment to the Board on May 9, 2002. In addition 1,200,000 SEK (£86,894) was paid to MU Bioteknik AB, a company of which Mr Uhlen is the sole director and sole shareholder in respect of royalty payments on patent rights. Payments have been made for the past seven years. They are not regarded as material. The Board accordingly regards Prof Uhlen as independent for corporate governance purposes. |
| |
| 8 | | Mr Crumpton received consultancy fees during the year of £45,000 and £16,500 in respect of his services on the Science Advisory Board. In addition, he received £10,304 in benefits. |
| |
| 9 | | Mr Jacobsen resigned as a director of the Company on June 16, 1999. During the year he received NOK 859 (£77) in benefits. |
| |
| 10 | | Mr R E Long resigned as a director of the Company on March 23, 2001. During the year, Mr R E Long has provided consultancy services to the Company for which he has received £80,592 as fees and £15,934 in benefits. In addition, £263,171 has been paid in consultancy fees to Scicona Limited, a company of which he is a director. These figures are disclosed in the above table. |
C. Board practices
Service Contracts
Each of the Executive Directors are employed on a rolling contract subject to one year’s notice if given by the Company and six months notice if given by the Executive Director. This notice period was reduced in 2002 from two years and new service contracts have been entered into as follows:
| | | | | |
| Director | | Employing Company | | Date of Contract |
| |
| |
|
| G W Battersby | | Amersham plc | | March 1, 2002 |
| A Carr | | Amersham Biosciences UK Limited | | December 16, 2002 |
| W M Castell | | Amersham plc | | March 1, 2002 |
| G F B Kerr | | Amersham plc | | March 1, 2002 |
| P Loescher | | Amersham plc | | December 1, 2002 |
The Committee believes that in order to attract Executive Directors of the right caliber and to compete for talent with our competitors, it is necessary to offer service contracts with notice periods of one year, which explicitly set out the provisions relating to termination of the contract.
Under the terms of the Executive Directors’ service contracts, on termination of employment by the Company, except in the case of dismissal for cause, the Company shall make a payment to the Executive Director equal to 95% of:
| a. | | the Executive’s basic salary for the period of notice, |
| |
| b. | | the cost of provision of pension and benefits for the notice period and |
| |
| c. | | the bonus applicable for the notice period, based on the actual bonus paid in the previous three years. |
Such payment shall be reduced proportionately in the event that the Executive works part of his period of notice. When the Company has given notice of termination, such payment shall be made within three months of the date of notice. Any payment shall be entirely discretionary when the Executive has given notice of termination. In the event that the employment is terminated for any other reason, such payment shall be made forthwith. There are no special provisions in the event of a change of control of the Company except for Mr Battersby. In the event of a change of control of the Company, which leads to Mr Battersby leaving the Company within a period of 12 months following such change of control, the pension element of the Agreed Payment detailed above may be enhanced by up to five years’ contributions. This enhancement shall reduce progressively to zero by 2008 and was agreed at the time of his recruitment.
On December 31, 2002, Dr J M Padfield retired from the Board in order to facilitate succession planning. He received a payment of £841,035 in respect of his contractual entitlements, comprising 95% of the total of his salary (£390,000), bonus (£300,300), pension benefits (£156,000) and other benefits (£39,000). These figures are included in the remuneration table and notes on page 98.
99
Non-Executive Directors do not have service contracts but are employed under letters of appointment for an initial period of three years. The remuneration of the Non-Executive Directors is reviewed by the Chief Executive who makes recommendations to the Board. The Board determines the remuneration of the Non-Executive Directors within the limits set out in the Articles of Association. The responsibilities of the role and the level of fees paid in UK organizations of a similar size and complexity to Amersham are considered in setting remuneration policy for Non-Executive Directors. Non-Executive Directors are appointed for an initial period of three years, subject to renewal by agreement. Details of when each Director was appointed are shown in Item 6A Directors and Senior Management. There are no compensation provisions for early termination of non Executive Director appointments.
Mr Johansen, an employee representative also has a service contract terminable by either party on three months’ notice.
The Board
The Board comprises five Executive Directors and nine Non-Executive Directors. Throughout the financial year, the offices of Chairman and Chief Executive have been held separately. The Chairman, Mr R D Lapthorne, is an independent Non-Executive Director, as deemed by the Board, who will be retiring from the Board at the Annual General Meeting on May 7, 2003. The role of Chairman will then be held by Mr D H Brydon, who is also deemed an independent Non-Executive Director.
The Deputy Chairman, Mr J Fr Odfjell has been identified as the senior independent Non-Executive Director.
Biographies of the Board members appear in Item 6 A. Directors and Senior Management which also show the membership of the Nomination, Remuneration and Audit Committees.
Apart from John Johansen, who is an employee, all the Non-Executive Directors were independent of management and free from any business or other relationship which could materially interfere with the exercise of independent judgment. All Directors have access to the Company Secretary.
The Board has a schedule of matters reserved to it for decision and the requirement for Board approval on these matters is communicated widely throughout the Group. To enable the Board to function effectively and allow Directors to discharge their responsibilities, full and timely access is given to all relevant information and appropriate resources to discharge their responsibilities.
Newly appointed Directors are given training appropriate to the level of their previous experience. All are apprised of their roles and duties as Directors of a public company.
The Board is responsible for the overall direction, strategy, performance and management of the Group. Authority for implementing the Board’s policies is delegated to the Chief Executive within certain limits authorized by the Board.
The Nomination Committee
The Nomination Committee met six times during the year to review the composition of, and succession to, the Board and make recommendations to the Board on the appointment of Non-Executive and Executive Directors. The Committee comprises seven of the independent Non-Executive Directors and is chaired by Mr J Fr Odfjell, the Deputy Chairman of the Board. The Nomination Committee reviews the composition of the Board in light of corporate governance regulation and best practice both current and proposed.
Members of the Committee abstain when matters affecting their own appointments are discussed. The Committee has available to it the services of external advisers as it deems necessary and at the Company’s expense. The Chief Executive attends the meetings of the Nomination Committee.
100
The Remuneration Committee
The Remuneration Committee met four times during the year. The Remuneration Committee is responsible for determining the remuneration policy and the terms and conditions of service of the Executive Directors. The Remuneration Committee also determines Group policy relating to share option plans and the level at which share options are granted to senior executives within the Group. The Committee has available to it the services of independent advisers. The Committee comprises six of the independent Non-Executive Directors including the Chairman and Deputy Chairman of the Board and is chaired by Mr R D Lapthorne, the Chairman of the Board. Following the retirement of Mr R D Lapthorne at the Annual General Meeting held on May 7, 2003, the Remuneration Committee will be chaired by Dr J S Patterson. The Chief Executive and the Human Resources Director attend the meetings of the Committee to discuss the performance of the other Executive Directors and to make proposals as necessary but they are not present when their own remuneration is being considered.
The Audit Committee
The Audit Committee met four times during the year. The Audit Committee monitors and reviews the internal controls and accounts of the Group. It also considers the Group’s compliance with the Combined Code. The Committee is chaired by Mr D H Brydon. The Committee comprises four further independent Non-Executive Directors: Mr R D Lapthorne, Mr J Fr Odfjell, Dr J S Patterson and Mr J F Rejeange. The Committee can request the external auditors, who are normally present, Executive Directors and other officers of the Group to attend its meetings. The Audit Committee receives regular reports from the Risk and Operational Review department on all aspects of the Group’s system of internal control. The Audit Committee recommends the appointment of the external auditors, reviews the external audit fee and audit plan and pre-approves all non-audit work in respect of the external auditors prior to commitment.
Disclosure Committee
As recommended in the provisions of the US Sarbanes-Oxley Act, a Disclosure Committee, chaired by Mr G F B Kerr (CFO) and comprising relevant senior managers has been established to ensure a robust verification and disclosure process throughout the Group. This will enable the Chief Executive and the Finance Director (CFO) to make the required certifications. The Disclosure Committee reports directly into the Chief Executive.
Appointments to the Board
Any Director appointed during the year is required, under the provisions of the Company’s Articles of Association, to retire and seek re-appointment by shareholders at the next Annual General Meeting. The Articles also require that one third of the Directors retire by rotation each year and seek re-appointment at the Annual General Meeting. The Directors required to retire will be those in office longest since their previous appointment or re-appointment and this will usually mean that each Director retires at least every three years. The Board has resolved that each Director will retire at least every three years, even if this is not strictly required by the application of the provisions of the Articles of Association.
D. Employees
The following summarizes the weighted average number of employees for the last three years, by type of activity:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| Type of Activity | | December 31, 2000 | | December 31, 2001 | | December 31, 2002 |
| |
| |
| |
|
| Production and development | | | 4,201 | | | | 4,587 | | | | 4,962 | |
| Distribution and selling | | | 2,834 | | | | 3,140 | | | | 3,262 | |
| Administration | | | 1,720 | | | | 1,792 | | | | 1,827 | |
| | | |
| | | |
| | | |
| |
| Total | | | 8,755 | | | | 9,519 | | | | 10,051 | |
| | | |
| | | |
| | | |
| |
Many of the Group’s employees are represented by Trade Unions. The Group considers its employee relations to be good and has not experienced any material work stoppages in recent years.
101
E. Share Ownership
Directors’ interests in the Company
The Directors’ and their families’ beneficial interests in the share capital of the Company are shown below:
| | | | | | | | | |
| | | | | | | As at Dec 31, 2001 |
| | | Dec 31, 2002 | | or at date of appointment |
| | | Ordinary 5p Shares | | Ordinary 5p Shares |
| | |
| |
|
| G W Battersby | | | 25,000 | | | | 25,000 | |
| D H Brydon | | | 2,585 | | | | 2,585 | |
| A Carr | | | 1,958 | | | | 1,566 | |
| W M Castell | | | 243,570 | | | | 243,570 | |
| J H Johansen | | | 11,610 | | | | 11,610 | |
| G F B Kerr | | | 10,000 | | | | 10,000 | |
| R D Lapthorne | | | 105,170 | | | | 105,170 | |
| P Loescher | | | — | | | | — | |
| J Fr Odfjell | | | 53,150 | | | | 53,150 | |
| J M Padfield | | | 3,564 | | | | 3,564 | |
| J Patterson | | | 2,923 | | | | 2,923 | |
| K Peters | | | 3,111 | | | | — | |
| J F Rejeange | | | — | | | | — | |
| E Thorsby | | | 17,800 | | | | 17,800 | |
| M Uhlen | | | — | | | | — | |
| | | |
| | | |
| |
| | | | 480,441 | | | | 476,938 | |
| | | |
| | | |
| |
Details of interests in conditional awards under the LTIP are detailed on page 105 of this Report. Each Director is deemed to be interested in all of the Ordinary Shares held by the Amersham ESOP Trust which at December 31, 2002, amounted to 545,282 Ordinary 5p shares (January 1, 2002, 550,287 Ordinary Shares). Each Executive Director is deemed to be interested in all of the shares held by Amersham Trustees Limited (the trustee of a UK Inland Revenue tax approved QUEST) which amounted to 770,057 Ordinary Shares at December 31, 2002 (January 1, 2002, 1,607,294 Ordinary Shares).
On March 13, 2003, W M Castell and G F B Kerr acquired 27,041 and 15,939 shares respectively on the exercise of awards made to them under the terms of the LTIP.
Share Option Schemes
Tax approved and unapproved Executive Share Option Schemes are available to Executive Directors and senior managers.
Options granted to Executive Directors prior to 2001 under the terms of the 1993 Executive Share Option Scheme are subject to the attainment of growth in the Company’s earnings per share of at least 6% more than the increase in the Retail Prices Index over any three consecutive financial years prior to the exercise of the option.
Options granted to Executive Directors from 2001 under the terms of the 2001 Executive Share Option Scheme are not normally exercisable until the third anniversary of the date of grant and to the extent that the performance conditions specified prior to the grant of the option have been satisfied. For options granted to Executive Directors in 2002 (which are detailed in the Interests in Share Options Table on page 104), 50% of each option grant will vest if the Company’s normalized earnings per share growth (as determined by the Remuneration Committee) matches or exceeds the growth in the Retail Prices Index plus 3% per annum. The entire option grant will vest if the Company’s normalized earnings per share growth matches or exceeds the growth in the Retail Prices Index plus 5% per annum. There is proportionate vesting between 3% and 5%. Performance is always measured from the end of the financial year prior to the grant of the option. 25% of the options may vest after one year with a further 25% after two years and a further 50%
102
after three years. To the extent that these targets are not achieved by those times, the performance period will be extended, one financial year at a time. To the extent that the performance conditions have not been met by the fifth anniversary of the grant, the option lapses. Option grants made to Dr Carr in 2001 and 2002 are not subject to performance conditions as he was not an Executive Director at the date of grant. Option grants made to all Executive Directors in 2003 are subject to the same performance conditions as applied to Executive Director option grants in 2001 and 2002.
Although performance conditions are not a common feature of option plans operated by our international competitors, we recognize that as a company with our primary listing in the UK, it is appropriate that options granted to our Executive Directors are subject to performance conditions. The performance conditions we have chosen mean that options will only vest when there has been a correspondingly good return for our shareholders. More demanding performance conditions would mean that the Black Scholes valuation of share options awarded would be lower and therefore in order to maintain the same Black Scholes value of award, higher option grants would need to be given.
Executive Directors are also entitled to participate in the UK Inland Revenue approved Sharesave (SAYE) share option scheme, which is available to all UK employees. The scheme is subject to a cumulative maximum investment of £250 per month for each individual. The share option runs for three, five or seven years. At the end of the chosen period, the shares may be purchased by the employee at a 20 per cent discount to the share price at the start of the period.
The beneficial interests of the Executive Directors in share options are shown on page 104.
103
Interests in share options
The interests of the Directors in Amersham plc share options held under the Executive Share Option Scheme (ESOS) and the Sharesave Scheme (SAYE) are set out below. These options are exercisable from November 1996 to September 2012 and are held under the Company’s plans referred to in Note 25 of Item 18 “Consolidated Financial Statements”. No options lapsed during the year.
Option Interests1
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | At Dec 31, 2001 or date | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | of appointment | | Granted during the year | | Exercised during the year | | At Dec 31, 2002 |
| | | | | | |
| |
| |
| |
|
| | | | | | | | | | | Weighted | | | | | | Weighted | | | | | | Weighted | | Market | | | | | | Weighted |
| | | | | | | Number | | average | | Number of | | average | | Number | | average | | price on | | Number of | | average |
| | | | | | | ordinary | | subscription | | ordinary | | subscription | | ordinary | | subscription | | date of | | ordinary | | subscription |
| | | | | | | shares | | price | | shares | | price | | shares | | price | | exercise | | shares | | price |
| | | | | | |
| |
| |
| |
| |
| |
| |
| |
| |
|
| G W Battersby | | ESOS 1993 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 128,618 | b | | | £6.22 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 128,618 | | | | £6.22 | |
| | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 85,716 | b | | | £5.60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 85,716 | | | | £5.60 | |
| | | | | | | | — | | | | — | | | | 67,136 | b | | | £7.15 | | | | — | | | | — | | | | — | | | | 67,136 | | | | £7.15 | |
| | | SAYE | | | 3,402 | a | | | £4.96 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,402 | | | | £4.96 | |
| |
| A Carr | | ESOS 1993 | | | 83,654 | a | | | £4.43 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 83,654 | | | | £4.43 | |
| | | | | | | | — | b | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 289,948 | b | | | £5.60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 289,948 | | | | £5.60 | |
| | | | | | | | — | | | | — | | | | 102,756 | b | | | £7.15 | | | | — | | | | — | | | | — | | | | 102,756 | | | | £7.15 | |
| | | SAYE | | | 3,045 | a | | | £4.47 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,045 | | | | £4.47 | |
| | | | | | | | 392 | a | | | £2.46 | | | | — | | | | — | | | | 392 | | | | £2.46 | | | | £5.52 | | | | — | | | | — | |
| | | Options for all | | | 1,000 | b | | | £6.35 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,000 | | | | £6.35 | |
| |
| W M Castell | | ESOS 1993 | | | 116,988 | a | | | £4.82 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 116,988 | | | | £4.82 | |
| | | | | | | | 186,934 | b | | | £6.23 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 186,934 | | | | £6.23 | |
| | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 178,572 | b | | | £5.60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 178,572 | | | | £5.60 | |
| | | | | | | | — | | | | — | | | | 139,864 | b | | | £7.15 | | | | — | | | | — | | | | — | | | | 139,864 | | | | £7.15 | |
| | | SAYE | | | 2,679 | a | | | £3.30 | | | | 1,001 | | | | £4.49 | | | | — | | | | — | | | | — | | | | 3,680 | | | | £3.62 | |
| |
| G F B Kerr | | ESOS 1993 | | | 244,918 | a | | | £4.18 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 244,918 | | | | £4.18 | |
| | | | | | | | 37,913 | b | | | £6.23 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 37,913 | | | | £6.23 | |
| | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 100,000 | a | | | £5.60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 100,000 | | | | £5.60 | |
| | | | | | | | — | | | | — | | | | 78,324 | b | | | £7.15 | | | | — | | | | — | | | | — | | | | 78,324 | | | | £7.15 | |
| | | SAYE | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| |
| P Loescher | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | — | | | | — | | | | 309,280 | b | | | £5.82 | | | | — | | | | — | | | | — | | | | 309,280 | | | | £5.82 | |
| |
| J M Padfield | | ESOS 1993 | | | 349,662 | a | | | £4.29 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 349,662 | | | | £4.29 | |
| | | | | | | | 57,785 | b | | | £6.23 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 57,785 | | | | £6.23 | |
| | | ESOS 2001 | | | — | a | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | 121,432 | b | | | £5.60 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 121,432 | | | | £5.60 | |
| | | | | | | | — | | | | — | | | | 95,108 | b | | | £7.15 | | | | — | | | | — | | | | — | | | | 95,108 | | | | £7.15 | |
| | | SAYE | | | 5,568 | a | | | £3.30 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,568 | | | | £3.30 | |
| 1 | | Options granted under ESOS and ESOS 2001 are exercisable between 3 and 10 years from the date of grant and options granted under SAYE are exercisable, 3, 5 or 7 years from the date of grant. These options are subject to the performance conditions explained on pages 102 to 103. |
| |
| | | Options granted where the market share price on December 31, 2002 (£5.56) was above the grant price are marked with ana. |
| |
| | | Options granted where the market share price on December 31, 2002 (£5.56) is below the grant prices are marked with ab. |
104
On March 3, 2003, additional grants of options were made to the undernoted Directors of the company under the terms of the Executive Share Option Scheme 2001.
| | | | | | | | | |
| | | Number of options granted | | Subscription price |
| | |
| |
|
| G W Battersby | | | 125,572 | | | | £4.38 | |
| A Carr | | | 171,236 | | | | £4.38 | |
| W M Castell | | | 273,976 | | | | £4.38 | |
| G F B Kerr | | | 141,536 | | | | £4.38 | |
| P Loescher | | | 205,480 | | | | £4.38 | |
No other Directors have been granted options over the shares of the Company or any other Group companies.
Restricted Shares
On December 4, 2002, 77,320 restricted shares were awarded to P Loescher on his joining the Company. 50% of these shares vest on December 4, 2004 and the remaining 50% of the shares on December 4, 2005, subject to his still being an employee of the Company.
The share price on the date of the award was £5.69. The shares for the award to P Loescher will be supplied by the Amersham ESOP Trust. The Remuneration Committee concluded that such awards were necessary to recruit P Loescher and therefore the committee made such an award relying on the exemption in chapter 13.13A of the UKLA Listing Rules. In the event that P Loescher ceases to be employed by the Company, his awards will lapse, unless the Remuneration Committee determine otherwise, except in the circumstances of his death, when his personal representatives may exercise his awards. The Remuneration Committee will not make amendments to the arrangements established for P Loescher that are to his advantage, without seeking the consent of shareholders. No benefits under the arrangements are pensionable.
Long term incentive plan
In previous years, the Company has operated a long term incentive plan (the Plan or LTIP) which was intended to provide a long term incentive to Executives and to further align their interests with those of the shareholders.
Following the introduction of the new Executive Share Option Scheme in 2001, it is not proposed to make any further awards under the Plan. No awards were made under this Plan in 2002.
The following grants of awards of ordinary shares each have been made to Executive Directors under the Plan:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | Number of | | | | | | | | |
| | | | | | | | | | | Market value | | | | | | Market value | | awards | | | | | | Market value |
| | | Number of | | Date | | on date | | Date | | on vesting | | vesting during | | Date | | on exercise |
| | | awards1 | | granted | | of grant | | vested2,3 | | date | | the year4 | | exercised | | date |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| G W Battersby | | Nil | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| A Carr | | | 8,381 | | | 31-Mar-00 | | | £5.1544 | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| W M Castell | | | 27,041 | | | 17-Jun-99 | | | £4.8075 | | | 17-Jun-02 | | | £5.80 | | | | 24,336 | | | | N/A | | | | N/A | |
| | | | 29,878 | | | 31-Mar-00 | | | £5.1544 | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| G F B Kerr | | | 15,939 | | | 22-Apr-98 | | | £4.2656 | | | 22-Apr-01 | | | £4.77 | | | Nil | | | N/A | | | | N/A | |
| | | | 15,809 | | | 17-Jun-99 | | | £4.8075 | | | 17-Jun-02 | | | £5.80 | | | | 14,228 | | | | N/A | | | | N/A | |
| | | | 17,461 | | | 31-Mar-00 | | | £5.1544 | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| P Loescher | | Nil | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| J M Padfield | | | 23,281 | | | 31-Mar-00 | | | £5.1544 | | | | N/A | | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| | |
| |
| |
| |
| |
| |
| |
| |
|
105
Under the Plan, awards of Ordinary Shares have been made to participants in the Plan by the trustees of the Amersham plc ESOP Trust, a trust established by the Company and operated by an independent firm of trustees.
| 1 | | No awards were made in 2002 |
| |
| 2 | | Awards vest 3 years from the date of grant, subject to performance conditions. The receipt of Ordinary Shares by the participants depends upon the performance of the Company over a three year period, which is measured by the growth in the total shareholder return of the Company relative to the FTSE 100 index of companies. |
| |
| 3 | | Awards will only vest if the Company’s Total Shareholder Return would place it in at least 60th position amongst the FTSE 100 companies ranked by Total Shareholder Return and the maximum number of Ordinary Shares will vest only if the performance would rank it at 20th place or better. |
| |
| 4 | | The awards made in 1999 vested as to 90% in 2002 as the Company was ranked 25th out of the 100 companies in its comparator group. |
On March 13, 2003, W M Castell and G F B Kerr exercised their awards made in June 1999 and April 1998 in respect of 27,041 and 15,939 shares respectively.
The Company’s register of Directors’ interests, which is open for inspection, contains full details of Directors’ share interests, options to subscribe for shares and awards of restricted shares.
106
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
Amersham is not, to its knowledge, directly or indirectly owned or controlled by another corporation or by any government, and there are no arrangements known to the Company where the operation of which may, at a subsequent date, result in a change of control of the Company.
As of April 16, 2003, two registered shareholders hold more than 5% of the Company’s outstanding shares, one of which is HSBC Global Custody Nominee Limited, the Authorized Depositary of the VPS system (through which all holdings on the Norwegian sub-register are held). To the Company’s knowledge, no other person owns more than 5% of the Company’s outstanding shares.
| | | | | | | |
| | | Number of | | % of |
| Parties | | Ordinary 5p Shares | | Total Issued Share |
| Interested | | Outstanding | | Capital |
| |
| |
|
HSBC Global Custody
Nominee Limited
A/C 866616 | | 101,200,609 ordinary 5p shares | | | 14.41 | % |
Wellington
Management Company | | 36,009,900 ordinary 5p shares
90,402 ADRs | | | 5.19 | % |
Under the UK Companies Act, holders of voting securities of a listed UK company must notify the company of the level of their holding whenever it reaches, exceeds or falls below specified thresholds. These thresholds are 3% (beneficial) and 10% (non-beneficial) and every one percent movement in the number of shares held which have voting rights. The following table sets forth, as of April 16, 2003, the number of ordinary shares held by holders of more than 3% and their percentage ownership which have been notified to the Company in accordance with the provisions noted above.
| | | | | | | | | |
| | | Number of shares | | % |
| | |
| |
|
| Legal and General Investment | | | 21,453,876 | | | | 3.06 | |
| Folketrygdfondet | | | 21,620,300 | | | | 3.08 | |
| Lazard Asset Management | | | 21,658,981 | | | | 3.09 | |
| Fidelity Investments | | | 23,269,146 | | | | 3.31 | |
| Franklin Resources Inc. and its affiliates | | | 34,629,982 | | | | 4.93 | |
The Company’s major shareholders do not benefit from different voting rights from other shareholders in the same class.
The proportion of each class of the Company’s securities held in the US and the number of record holders in the US are given under Item 9, “The Offer and Listing”.
B. Related Party Transactions
Related party transactions have been disclosed in Note 35 of Item 18 “Consolidated Financial Statements”. Apart from the transactions disclosed within this Note there were no other material items requiring disclosure.
107
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
Financial Statements
See Item 18 “Consolidated Financial Statements”.
Export Sales
Total sales from continuing operations in 2002 were £1,618 million including a £81 million share of joint venture sales of NMP. Exports from the United Kingdom amounted to £203 million, representing 13% of total sales from continuing operations.
Legal Proceedings
The Company is involved in various disputes of a nature considered typical for its business, including those relating to product liability and infringements of intellectual property rights and validity of patents. Although there can be no assurance regarding the outcome of any of the disputes referred to in this section, the Company believes that they will not have a material adverse effect on the Company’s financial condition or results of operations.
Dividends
See Item 3 “Key Information” for the Company’s policy on dividends and historical information on aggregate dividends paid in each fiscal period.
B. Significant Changes
On February 26, 2003, the Group announced a restructuring of its discovery systems business area. The restructuring program, which is intended to deliver a more efficient manufacturing cost base and focus R&D on fewer sites, will result in the net loss of approximately 400 jobs. The Group will incur one-off costs in the range of £45-50 million, which are expected to result in savings running at the rate of
£30-35 million per annum by the end of 2004. The restructuring plans are expected to bring benefits in 2003 moving the business to profitability during 2004.
On April 22, 2003 we announced an intended merger of our brachytherapy business with the urology business of Galil Medical to create a stand-alone marketing company. The combined sales of both businesses would have been approximately £57 million ($90 million) in 2002. The new company, which is 75% owned by Amersham, will be accounted for as a joint venture.
On April 30, 2003 we reported sales of £381 million for the first three months of 2003 (2002: £381 million), up 6% at constant exchange and excluding discontinued business. We confirmed that our outlook for the year is unchanged and that the restructuring of our discovery systems business area is on track to deliver profitability during 2004.
The full text of our press release can be found on our internet site www.amersham.com
108
ITEM 9. THE OFFER AND LISTING
Market Value Data
The London Stock Exchange (“LSE”) is the principal trading market for the Company’s Ordinary Shares, nominal value of 5p each. As of June 10, 1998 the Non-voting Shares, nominal value 25p each, (together with the Company’s Ordinary Shares nominal value of 25p each) were converted into Ordinary Shares with a nominal value of 5p and are no longer listed on the LSE. The Company’s Ordinary Shares are also listed on the Oslo Stock Exchange and on the New York Stock Exchange (“NYSE”) in the form of American Depositary Shares (“ADSs”), each representing five Ordinary Shares. The ADSs are evidenced by American Depositary Receipts (“ADRs”) issued by Citibank N.A. as the depositary.
As of April 16, 2003 there were 13,432 holders of record of 702,084,809 Ordinary Shares of which 62 (0.46%) had registered addresses in the US and held a total of 151,563 (0.022%) Ordinary Shares. Because certain of the Ordinary Shares and ADSs are held by brokers or other nominees, the number of holders of record or registered holders in the US is not representative of the number of beneficial holders or of the residence of beneficial holders.
109
London Stock Exchange
The table below sets out, for the quarters indicated the reported high and low middle-market closing quotations (in pence) of Amersham’s Ordinary Shares (after adjusting for the share split in June 1998) on the LSE as reported in The Daily Official List. The middle-market closing quotation of a security is the standard method of describing the price of a security listed on the LSE, and is the mean of (i) the highest price offered by any registered market maker to purchase the security (the bid price); and (ii) the lowest price required by any registered market maker to sell the security (the offer price), as at the close of business on a given day.
| | | | | | | | | | | | | |
| | | | | | | Ordinary Shares* |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | (p) | | (p) |
| | 2003 | | | Quarter ended June 30, 2003 (through April 16, 2003) | | | 434.50 | | | | 411.00 | |
| | | | | Quarter ended March 31, 2003 | | | 561.50 | | | | 363.00 | |
| | 2002 | | | Quarter ended December 31, 2002 | | | 599.00 | | | | 507.50 | |
| | | | | Quarter ended September 30, 2002 | | | 598.50 | | | | 455.50 | |
| | | | | Quarter ended June 30, 2002 | | | 768.50 | | | | 558.00 | |
| | | | | Quarter ended March 31, 2002 | | | 768.50 | | | | 638.00 | |
| | 2001 | �� | | Quarter ended December 31, 2001 | | | 673.50 | | | | 560.00 | |
| | | | | Quarter ended September 30, 2001 | | | 635.00 | | | | 502.00 | |
| | | | | Quarter ended June 30, 2001 | | | 580.00 | | | | 460.00 | |
| | | | | Quarter ended March 31, 2001 | | | 600.00 | | | | 428.50 | |
| | 2000 | | | Quarter ended December 31, 2000 | | | 671.00 | | | | 512.00 | |
| | | | | Quarter ended September 30, 2000 | | | 700.00 | | | | 583.00 | |
| | | | | Quarter ended June 30, 2000 | | | 602.50 | | | | 471.00 | |
| | | | | Quarter ended March 31, 2000 | | | 732.00 | | | | 361.25 | |
| | 1999 | | | Quarter ended December 31, 1999 | | | 421.25 | | | | 350.00 | |
| | | | | Quarter ended September 30, 1999 | | | 485.00 | | | | 356.00 | |
| | | | | Quarter ended June 30, 1999 | | | 540.00 | | | | 421.00 | |
| | | | | Quarter ended March 31, 1999 | | | 540.00 | | | | 378.00 | |
| | 1998 | | | Quarter ended December 31, 1998 | | | 443.50 | | | | 332.00 | |
| | | | | Quarter ended September 30, 1998 | | | 461.00 | | | | 340.00 | |
| | | | | Quarter ended June 30, 1998 | | | 450.00 | | | | 355.00 | |
| | | | | Quarter ended March 31, 1998 | | | 490.14 | | | | 410.44 | |
| | 1997 | | | Quarter ended December 31, 1997 | | | 474.47 | | | | 389.75 | |
| | | | | Quarter ended September 30, 1997 | | | 419.73 | | | | 318.67 | |
| | | | | Quarter ended June 30, 1997 | | | 310.73 | | | | 234.53 | |
| | | | | Quarter ended March 31, 1997 | | | 310.73 | | | | 235.11 | |
| | | | | | | | | | | | | |
| | | | | | | Ordinary Shares |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | (p) | | (p) |
| | 2003 | | | Month ended April 30, 2003 (through April 16) | | | 434.50 | | | | 411.00 | |
| | | | | Month ended March 31, 2003 | | | 430.50 | | | | 363.00 | |
| | | | | Month ended February 28, 2003 | | | 498.00 | | | | 424.00 | |
| | | | | Month ended January 31, 2003 | | | 561.50 | | | | 459.00 | |
| * | | The market prices of the Ordinary shares in issue prior to June 10, 1998 have been adjusted to take account of the sub-division of the Ordinary 25p shares into Ordinary 5p shares and the 3.4% bonus issue on June 10, 1998. |
110
Oslo Stock Exchange
The table below sets out, for the period following the date of the merger, i.e. from October 22, 1997, the reported high and low middle-market quotations in Norwegian kroner (NOK) of Amersham’s Ordinary Shares on The Oslo Stock Exchange as reported on the Norwegian Official List.
| | | | | | | | | | | | | |
| | | | | | | Ordinary Shares * |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | (NOK) | | (NOK) |
| | 2003 | | | Quarter ended June 30, 2003 (through April 16, 2003) | | | 51.00 | | | | 47.50 | |
| | | | | Quarter ended March 31, 2003 | | | 63.00 | | | | 43.00 | |
| | 2002 | | | Quarter ended December 31, 2002 | | | 68.00 | | | | 59.00 | |
| | | | | Quarter ended September 30, 2002 | | | 70.00 | | | | 51.00 | |
| | | | | Quarter ended June 30, 2002 | | | 95.50 | | | | 64.00 | |
| | | | | Quarter ended March 31, 2002 | | | 93.50 | | | | 90.00 | |
| | 2001 | | | Quarter ended December 31, 2001 | | | 86.00 | | | | 75.50 | |
| | | | | Quarter ended September 30, 2001 | | | 81.50 | | | | 64.00 | |
| | | | | Quarter ended June 30, 2001 | | | 75.00 | | | | 60.00 | |
| | | | | Quarter ended March 31, 2001 | | | 76.00 | | | | 55.50 | |
| | 2000 | | | Quarter ended December 31, 2000 | | | 88.50 | | | | 65.00 | |
| | | | | Quarter ended September 30, 2000 | | | 95.00 | | | | 77.50 | |
| | | | | Quarter ended June 30, 2000 | | | 80.50 | | | | 63.00 | |
| | | | | Quarter ended March 31, 2000 | | | 97.25 | | | | 48.30 | |
| | 1999 | | | Quarter ended December 31, 1999 | | | 53.75 | | | | 45.35 | |
| | | | | Quarter ended September 30, 1999 | | | 53.75 | | | | 45.70 | |
| | | | | Quarter ended June 30, 1999 | | | 66.75 | | | | 54.00 | |
| | | | | Quarter ended March 31,1999 | | | 67.00 | | | | 47.00 | |
| | 1998 | | | Quarter ended December 31, 1998 | | | 54.00 | | | | 39.00 | |
| | | | | Quarter ended September 30, 1998 | | | 58.00 | | | | 42.00 | |
| | | | | Quarter ended June 30, 1998 | | | 58.00 | | | | 44.00 | |
| | | | | Quarter ended March 31, 1998 | | | 65.00 | | | | 49.00 | |
| | 1997 | | | Period from October 22, 1997 through December 31, 1997 | | | 54.00 | | | | 53.00 | |
| | | | | | | | | | | | | |
| | | | | | | Ordinary Shares |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | (NOK) | | (NOK) |
| | 2003 | | | Month ended April 30, 2003 (through April 16) | | | 51.00 | | | | 47.50 | |
| | | | | Month ended March 31, 2003 | | | 50.50 | | | | 43.00 | |
| | | | | Month ended February 28, 2003 | | | 57.00 | | | | 47.00 | |
| | | | | Month ended January 31, 2003 | | | 63.50 | | | | 52.00 | |
| | 2002 | | | Month ended December 31, 2002 | | | 68.00 | | | | 63.50 | |
| | | | | Month ended November 30, 2002 | | | 68.00 | | | | 60.50 | |
| * | | The market prices of the ordinary shares in issue dated prior to June 10, 1998 have been adjusted to take account of the sub-division of the ordinary 25p shares into ordinary 5p shares and the 3.4% bonus issue on June 10, 1998. |
111
New York Stock Exchange
The table below sets out, for the period following the date of the merger, i.e. from October 22, 1997, the reported sales prices (in US dollars) of Amersham’s ADSs on The New York Stock Exchange as reported on the NYSE composite tape.
| | | | | | | | | | | | | |
| | | | | | | ADSs |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | ($) | | ($) |
| | 2003 | | | Quarter ended June 30, 2003 (through April 16, 2003) | | | 33.9000 | | | | 32.5100 | |
| | | | | Quarter ended March 31, 2003 | | | 39.7000 | | | | 32.5500 | |
| | 2002 | | | Quarter ended December 31, 2002 | | | 45.0500 | | | | 44.4000 | |
| | | | | Quarter ended September 30, 2002 | | | 44.0600 | | | | 42.0100 | |
| | | | | Quarter ended June 30, 2002 | | | 48.2100 | | | | 44.3600 | |
| | | | | Quarter ended March 31, 2002 | | | 54.5000 | | | | 44.1000 | |
| | 2001 | | | Quarter ended December 31, 2001 | | | 47.8100 | | | | 41.6500 | |
| | | | | Quarter ended September 30, 2001 | | | 45.7500 | | | | 36.0000 | |
| | | | | Quarter ended June 30, 2001 | | | 41.4000 | | | | 36.3000 | |
| | | | | Quarter ended March 31, 2001 | | | 42.4000 | | | | 34.6000 | |
| | 2000 | | | Quarter ended December 31, 2000 | | | 45.3750 | | | | 40.7500 | |
| | | | | Quarter ended September 30, 2000 | | | 48.5000 | | | | 45.0625 | |
| | | | | Quarter ended June 30, 2000 | | | 45.0000 | | | | 38.0000 | |
| | | | | Quarter ended March 31, 2000 | �� | | 58.8750 | | | | 29.7500 | |
| | 1999 | | | Quarter ended December 31, 1999 | | | 33.2500 | | | | 29.4375 | |
| | | | | Quarter ended September 30, 1999 | | | 34.0000 | | | | 30.5000 | |
| | | | | Quarter ended June 30, 1999 | | | 42.6250 | | | | 34.7500 | |
| | | | | Quarter ended March 31, 1999 | | | 40.7500 | | | | 31.0000 | |
| | 1998 | | | Quarter ended December 31, 1998 | | | 36.0000 | | | | 27.8100 | |
| | | | | Quarter ended September 30, 1998 | | | 37.0000 | | | | 29.6300 | |
| | | | | Quarter ended June 30, 1998 | | | 37.8600 | | | | 31.0400 | |
| | | | | Quarter ended March 31, 1998 | | | 41.5000 | | | | 33.8700 | |
| | 1997 | | | Period from October 22, 1997 through December 31, 1997 | | | 40.3700 | | | | 34.7500 | |
| | | | | | | | | | | | | |
| | | | | | | ADSs |
| | | | | | |
|
| | | | | | | High | | Low |
| | | | | | |
| |
|
| | | | | | | ($) | | ($) |
| | 2003 | | | Month ended April 30, 2003 (through April 16) | | | 33.9000 | | | | 32.5100 | |
| | | | | Month ended March 31, 2003 | | | 34.0900 | | | | 29.8500 | |
| | | | | Month ended February 28, 2003 | | | 40.1000 | | | | 34.2500 | |
| | | | | Month ended January 31, 2003 | | | 44.7900 | | | | 37.8900 | |
| | 2002 | | | Month ended December 31, 2002 | | | 45.6000 | | | | 43.2400 | |
| | | | | Month ended November 30, 2002 | | | 46.1500 | | | | 43.9000 | |
The implementation of the Sub-division and the Enfranchisement proposals in full in June 1998 resulted in the following:
| • | | each A ADS and each B ADS converted into the right to receive five new Ordinary Shares, nominal value 5p and five New Non-voting Shares, nominal value 5p, respectively; |
| |
| • | | each holder of B ADSs became entitled to receive one New ADS for each B ADS surrendered. Each A ADS represent one New ADS; |
| |
| • | | a holder of 1,000 A ADSs on the Record Date of June 5, 1998 received a bonus issue of 34 New ADSs. |
112
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
The description of the material rights of holders of the Company’s ordinary shares under the provisions of the Company’s memorandum and articles of association and English law is incorporated by reference to the Annual Report on Form 20-F filed by Amersham on June 26, 2002, since when there have been no changes.
C. Material Contracts
There are no material contracts outside of the Company’s ordinary course of business.
D. Exchange Controls
There are currently no US, UK or Norwegian decrees or regulations restricting the import or export of capital or affecting the remittance of dividends or other payments to the holders of the Amersham shares who are non-residents of the UK.
E. Taxation
US and UK Tax Considerations of Ownership and Disposition of Amersham Shares or Amersham ADSs as evidenced by Amersham ADRs.
The following is a summary of certain United Kingdom taxation and United States federal income tax considerations that may be relevant to a US holder of Amersham shares (Shares) or Amersham ADSs (ADSs) as evidenced by Amersham ADRs (ADRs). This summary only applies to a shareholder that holds Shares or ADSs as capital assets, is a citizen or resident of the United States or a domestic corporation or that is otherwise subject to United States federal income taxation on a net income basis in respect of the Shares or ADSs, and is not resident in the United Kingdom for UK tax purposes and does not hold shares for the purposes of a trade, profession or vocation that is carried on in the United Kingdom through a branch or agency.
Shareholders who are subject to special rules or who are in any doubt about their taxation position should consult their own professional advisors.
The new UK/US Double Tax Treaty (the New Treaty) was signed on 24 July 2001, was ratified on 31 March 2003 and came into force from that date. The statements regarding the United Kingdom and United States tax laws and practices set out below are based on those laws and practices in force on the date of this report.
For the purposes of the New Treaty and for the purposes of the US Internal Revenue Code 1986, as amended (the Code), US holders of ADSs will generally be treated as the owners of the Shares underlying those ADSs.
113
Taxation of Dividends
For US federal income tax purposes, distributions paid with respect to Shares or ADSs (other than certain distributions of capital stock of the Company or rights to subscribe for shares of capital stock of the Company) will be treated as a dividend to the extent paid out of current or accumulated earnings and profits (as determined under US federal income tax principles). Such dividends will not be eligible for the “dividends received deduction” generally allowed to corporations under the Code. To the extent that a distribution exceeds the Company’s current and accumulated earnings and profits, it will be treated as a non taxable return of capital to the extent of the US holder’s tax basis in the ordinary shares or ADSs, and thereafter as capital gain. The amount of the distribution will equal the US dollar value of the pounds sterling received, calculated by reference to the exchange rate in effect on the date such distribution is received (which for holders of ADSs, will be the date such distribution is received by the Depository), whether or not the Depository or the US holder in fact converts any pounds sterling received into US dollars at that time. Any gains or losses resulting from the conversion of pounds sterling into US dollars will be treated as ordinary income or loss, as the case may be, of the US holder and will be US source.
Under the New Treaty, US holders will not be eligible to claim foreign tax credits in respect of dividends paid on or after May 1, 2003. However, it may be possible for US holders to elect to have the provisions of the previous Double Tax Treaty (in effect before the New Treaty entered into force) applied in their entirety for a period of 12 months from the date on which the New Treaty otherwise would have effect. In such a case where the previous Double Tax Treaty is applied, US holders may be eligible, subject to generally applicable limitations, to receive a special US foreign tax credit equal to one-ninth of the amount of cash dividends they receive on the ADSs so long as they make an election to include in their income, as an additional notional dividend, an amount equal to the tax credit. The US Treasury has expressed concerns that parties to whom ADSs are released may be taking actions that are inconsistent with the claiming of foreign tax credits for US holders of ADSs. Accordingly, the analysis of the creditability of UK taxes could be affected by future actions that may be taken by the US Treasury. Instead of claiming a credit, US holders applying the previous Double Tax Treaty may, at their election, deduct one-ninth of the amount of cash dividends they receive on the ADSs. Each US holder’s own tax position will determine whether effective use can be made of special US foreign tax credits against the US tax liability and US holders should consult their own professional advisers in this respect.
Taxation of Capital Gains
Generally, US holders will not be subject to UK capital gains tax, but will be subject to US tax on capital gains realized on the sale or other disposal of Shares or ADSs.
A US holder will, upon the sale, exchange or redemption of a Share or ADS, generally recognize the capital gain or loss for US federal income tax purposes in an amount equal to the difference between the US dollar value of the amount realized (excluding in the case of a redemption any amount treated as a dividend for US federal income tax purposes) and the US holder’s tax basis (determined in US dollars) in the Share or ADS.
Stamp Duty & Stamp Duty Reserve Tax
United Kingdom stamp duty or, as the case may be, stamp duty reserve tax will, subject to certain exemptions, be payable on any issue or transfer of Shares to the ADR custodian or depository at a rate of 1.5 per cent of their price (if issued) the amount of any consideration provided (if transferred on sale), or their value (if transferred for no consideration).
No United Kingdom stamp duty should be payable on the transfer of an ADS provided that the ADR and any separate instrument of transfer is executed and remains at all times outside the United Kingdom. Any stamp duty on the transfer of the ADS would be payable at a rate of 0.5 per cent of the consideration for the transfer. Any sale of the underlying shares would result in a liability to UK stamp duty at a rate of 0.5 per cent. There is a minimum charge of £5 where a stamp duty liability arises.
114
Stamp duty reserve tax at a rate of 0.5% of the consideration would be payable on any transfer of any Shares or any interest therein unless an instrument transferring the Shares is executed and stamped within six years of the transfer, in which event any stamp duty reserve tax paid will be refundable and any stamp duty reserve tax not paid will cease to be due. No stamp duty reserve tax would be payable on the transfer of an ADS evidenced by an ADR.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
The Company’s UK statutory books are on display at its registered address. See Item 4 “Information on the Company” for the Company’s registered address.
I. Subsidiary Information
Information concerning material subsidiaries of Amersham plc can be found within Item 4.C “Organizational Structure” and within Note 31 of Item 18 “Consolidated Financial Statements”.
115
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market rate sensitive instruments and risk management
The following discussion about the Group’s risk management activities includes “forward-looking statements” which are based on current expectations regarding important risk factors. Actual results may differ materially from those projected in the statements.
The analysis below presents the sensitivity of the market value of the derivative and financial instruments held by the Group at December 31, 2002, to changes in interest rates and foreign exchange rates. The Group uses interest rate swaps, forward foreign exchange contracts and, sometimes, currency options to manage these primary market exposures associated with underlying assets, liabilities, and transactions. The Group uses these instruments to reduce risk by essentially creating offsetting market exposures. The Group does not hold or issue financial instruments for trading purposes. For further discussion of the Group’s accounting policies for financial instruments and further disclosures relating to financial instruments see Notes 1 and 20 of Item 18 “Consolidated Financial Statements”.
In the normal course of business, the Group also faces risks that are non-financial or non-quantifiable. Such risks principally include country risk, credit risk and legal risk, and are not included in the following analysis.
Interest rate risk management and sensitivity analysis
The Group’s interest charge is exposed to movements in interest rates as well as debt levels. The Group maintains a policy of holding between 30% and 70% of debt at fixed rates. The Group uses currency and interest rate swaps to manage its interest rate exposures on debt and cash positions. The interest rate profile of the Group is shown in Note 20 of Item 18 “Consolidated Financial Statements”. Under an interest swap, the Group agrees with another party to exchange, at specified intervals, the difference between fixed rate and floating rate interest amounts based on an agreed upon notional principal amount, without the trade of underlying notional principal amounts. Under a currency swap the principal amounts are also exchanged at the start and end of the agreement period. The net payments or receipts from interest rate swaps are recorded as part of interest expense.
At December 31, 2002, the effect of the Group’s interest rate swap agreements was to convert a portion (£31.1 million) of the outstanding fixed rate debt to floating rate debt for a period of time.
For floating rate debt, interest rate changes generally do not affect the fair market value but do impact future earnings and cash flows, assuming other factors are held constant. Conversely, for fixed rate debt, interest rate changes affect the fair market value but do not impact earnings or cash flows.
The Group’s net debt position as at December 31, 2002, after interest rate swaps included a net liability exposure of £94.2 million (2001 net asset — £91.8 million, 2000 net liability — £189.6 million) to floating interest rates. Based on the Group’s 2002 year end level and composition of net debt, holding other variables (such as foreign exchange rates) constant, an increase in average interest rates of 1% per annum, would result in a decrease in future earnings, before tax, of £0.9 million (in 2001, an increase in £0.9 million; in 2000, a decrease of £1.9 million) per annum, and a decrease in the fair market value of the fixed rate debt of £3.4 million (2001 — £4.2 million; 2000 — £5.5 million).
Foreign exchange risk management and sensitivity analysis
The Group’s principal financial exposures are to movements in currency exchange rates. These comprise transaction exposures arising from currency cash flows and translation exposures arising from the conversion of the results of foreign subsidiaries. Transaction exposures are hedged on a rolling six month basis, primarily by forward foreign exchange transactions. In
116
addition, where sterling has deviated significantly from its long term purchasing power parity rates, strategic hedging is partly undertaken for anticipated net sales for periods of up to three years. Translation exposures on the net monetary assets of foreign subsidiaries are hedged partly by borrowings in those currencies and partly by financial instruments including forward foreign exchange contracts.
As at December 31, 2002, the Group had outstanding foreign exchange contracts with a fair market value of £29.6 million (2001 — £9.6 million; 2000 — £25.0 million). With all other variables held constant (such as interest rates), a 10% appreciation of sterling would result in a decrease in the fair market value of foreign exchange contracts of £15.9 million (2001 decrease, £19.0 million; 2000 increase, £2.1 million).
In addition to these foreign exchange contracts, the Group is exposed to foreign exchange movements on its net debt position. With all other variables held constant, a 10% appreciation of sterling would result in a decrease in the carrying value of foreign denominated debt and currency swaps of £10.6 million (2001 — £1.9 million; 2000 — £29.7 million). Further information can be found in Note 20(d) of Item 18 “Consolidated Financial Statements”.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
117
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARS AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
ITEM 15. CONTROLS AND PROCEDURES
Evaluation of disclosure controls and procedures
The Chief Executive and the Finance Director, after evaluating the effectiveness of the Company’s “disclosure controls and procedures” (as defined in Exchange Act Rules 13a-14(c) and 15-d-14(c)) as of a date (the “Evaluation Date”) within 90 days of the filing date of this Annual Report, have concluded that as of the Evaluation Date, the Company’s disclosure controls and procedures were adequate and designed to ensure that material information relating to the Company and its consolidated subsidiaries would be made known to them by others within those entities.
Changes in internal controls
There were no significant changes in the Company’s internal controls or, to the knowledge of the Chief Executive or Finance Director, in other factors that could significantly affect the Company’s internal controls subsequent to the Evaluation Date.
ITEM 16. [Reserved]
118
PART III
ITEM 17. FINANCIAL STATEMENTS
The registrant has responded to Item 18 in lieu of responding to this item.
ITEM 18. CONSOLIDATED FINANCIAL STATEMENTS
Reference is made to pages F-1 through F- 74.
The following financial statements are filed as part of this Form 20-F:
| | | | | |
| | | Page |
| | |
|
| Report of Independent Accountants | | | F-2 | |
| Consolidated Financial Statements | | | | |
| Consolidated Profit and Loss Account | | | F-3 | |
| Consolidated Statement of Total Recognized Gains and Losses | | | F-4 | |
| Consolidated Balance Sheet | | | F-5 | |
| Consolidated Cash Flow Statement | | | F-6 | |
| Notes to the Consolidated Financial Statements | | | F-7 | |
ITEM 19. EXHIBITS
The following exhibits are filed as part of this Form 20-F:
| Exhibit 4.1. | | Employment Agreement between Amersham plc and Peter Hans Loescher dated September 17, 2002. |
| |
| Exhibit 4.1a. | | Letter dated December 4, 2002 confirming effective date of Peter Hans Loescher service contract. |
| |
| Exhibit 4.2. | | Letter dated December 4, 2002 from Amersham Biosciences UK Limited to Dr Andrew Carr amending the Employment Agreement between the two parties dated May 1, 2001. |
| |
| Exhibit 4.2a. | | Letter dated September 9, 2002 from Amersham plc to Dr Andrew Carr regarding his UK pension arrangements. |
| |
| Exhibit 4.3. | | Letter dated January 27, 2003 from Amersham plc to Dr Andrew Carr setting out the basis on which he will provide services as a Director of Amersham plc. |
| |
| Exhibit 4.4. | | Letter dated February 20, 2003 from Amersham plc to W M Castell advising of increase in salary. |
| |
| Exhibit 4.5. | | Letter dated February 20, 2003 from Amersham plc to G W Battersby advising of increase in salary. |
| |
| Exhibit 4.6. | | Letter dated February 20, 2003 from Amersham plc to A Carr advising of increase in salary. |
| |
| Exhibit 4.7. | | Letter dated February 20, 2003 from Amersham plc to G F B Kerr advising of increase in salary. |
| |
| Exhibit 4.8. | | Letter dated February 20, 2003 from Amersham plc to P Loescher advising of increase in salary. |
| |
| Exhibit 12.1. | | Certification required by Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| Exhibit 12.2. | | Consent of Independent Accountants. |
119
SIGNATURE
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | | | |
| | | AMERSHAM PLC |
| | | | | |
| | | By: | | |
| | | | |
/s/ Giles F B Kerr
Finance Director |
Date: 23 May, 2003
CERTIFICATIONS
I, Sir William Castell, Chief Executive of Amersham plc, certify that:
1. I have reviewed this Annual Report on Form 20-F of Amersham plc;
2. Based on my knowledge, this Annual Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Annual Report;
3. Based on my knowledge, the financial statements, and other financial information included in this Annual Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Annual Report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
| | (a) | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this Annual Report is being prepared; |
| |
| | (b) | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this Annual Report (the “Evaluation Date”); and |
| |
| | (c) | presented in this Annual Report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s Board of directors (or persons performing the equivalent function):
| | (a) | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
6. The registrant’s other certifying officer and I have indicated in this Annual Report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
Date: 23 May, 2003
| |
/s/ Sir William Castell
Chief Executive, Amersham plc |
I, Giles F B Kerr, Finance Director of Amersham plc, certify that:
1. I have reviewed this Annual Report on Form 20-F of Amersham plc;
2. Based on my knowledge, this Annual Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Annual Report;
3. Based on my knowledge, the financial statements, and other financial information included in this Annual Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this Annual Report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
| | (a) | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this Annual Report is being prepared; |
| |
| | (b) | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this Annual Report (the “Evaluation Date”); and |
| |
| | (c) | presented in this Annual Report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s Board of directors (or persons performing the equivalent function):
| | (a) | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
6. The registrant’s other certifying officer and I have indicated in this Annual Report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
Date: 23 May, 2003
| |
/s/ Giles F B Kerr
Finance Director, Amersham plc |
AMERSHAM PLC AND SUBSIDIARIES
STATEMENT OF DIRECTORS’ RESPONSIBILITIES
The following statements, which should be read in conjunction with the Report of Independent Accountants set out on page F-2 and are an excerpt from the December 31, 2002 Annual Report of Amersham plc dated February 26, 2003, are made with a view to distinguishing for shareholders the respective responsibilities of the Directors and of the Auditors in relation to the consolidated financial statements.
The Directors are required by the Companies Act 1985 to prepare consolidated financial statements for each financial year which give a true and fair view of the state of affairs of the Company (Amersham plc) and the Group (the Company and its subsidiary undertakings) as at the end of the financial period and of the profit or loss of the Group for that period. It is also the Directors’ responsibility to keep proper accounting records and to take reasonable steps to safeguard the assets of the Group and to prevent and detect fraud and other irregularities.
The Directors confirm that suitable accounting policies, consistently applied and supported by reasonable and prudent judgements and estimates, have been used in the preparation of the financial statements and that applicable accounting standards have been followed.
CORPORATE GOVERNANCE
The Board is committed to high standards of corporate governance throughout the Group.
The Board of Directors has overall responsibility for the Group’s system of internal control. The Group’s system of internal control is designed to provide reasonable, but not absolute, assurance that assets are safeguarded, transactions authorized and properly recorded and that material errors or irregularities are either prevented or would be detected within a timely period.
The full Board meets regularly and has put in place an organization structure with clearly defined lines of responsibility and delegations of authority. There are established procedures for,inter alia, capital expenditure approval and Treasury Management. Other written internal control procedures have been prepared and approved by business and subsidiary management, and by the Board where appropriate. There is a comprehensive budgeting system with an annual budget approved by the Board. Monthly results are reported against budget and revised forecasts for the year are prepared regularly.
The internal control system is monitored and supported by an internal audit function that operates on a global basis, and reports to management and the Audit Committee on the Group’s operations. The Audit Committee receives reports from internal and external auditors on a regular basis and has reviewed the operation and effectiveness of the Group’s system of internal control for the period.
GOING CONCERN
After making inquiries the Directors have reasonable expectation that the Company and the Group have adequate resources to continue in operational existence for the foreseeable future, and that it is therefore appropriate to adopt the going concern basis in preparing the financial statements. In forming this view the Directors have reviewed the Annual Profit Plan for the year ended December 31, 2003 and longer-range cash flow projections. The Directors have satisfied themselves that the Group is in a sound financial position and that sufficient borrowing facilities will be available to meet the Group’s foreseeable cash requirements.
F-1
AMERSHAM PLC AND SUBSIDIARIES
REPORT OF INDEPENDENT ACCOUNTANTS
To the Board of Directors and the Shareholders of Amersham plc
In our opinion, the accompanying consolidated balance sheets and the related consolidated profit and loss accounts, cash flows and total recognized gains and losses present fairly, in all material respects, the financial position of Amersham plc and its subsidiaries (together, the ‘Group’) at December 31, 2002 and December 31, 2001, and the results of their operations and their cash flows for each of the three periods, the year ended December 31, 2002, the year ended December 31, 2001 and the year ended December 31, 2000, in conformity with generally accepted accounting principles in the United Kingdom. These financial statements are the responsibility of the Company’s management; our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States. These standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for the opinion expressed above.
Accounting principles generally accepted in the United Kingdom vary in certain respects from accounting principles generally accepted in the United States. The application of the latter would have affected the determination of consolidated profit, as restated, expressed in sterling for the year ended December 31, 2002, December 31, 2001 and December 31, 2000 and the determination of consolidated shareholders’ equity, as restated, also expressed in sterling at December 31, 2002 and December 31, 2001 to the extent summarized in Note 38 to the consolidated financial statements.
As discussed in Notes 1 and 37 to the consolidated financial statements, following the introduction of UK Financial Reporting Standard 19, ‘Deferred Taxation’ in 2002, the Group changed its accounting policy with respect to deferred taxation. This change has been accounted for by restating comparative information at December 31, 2001 and 2000, and for the years then ended.
/s/ PricewaterhouseCoopers LLP
Chartered Accountants and Registered Auditors
London, England
February 26, 2003
F-2
AMERSHAM PLC AND SUBSIDIARIES
CONSOLIDATED PROFIT AND LOSS ACCOUNT
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | 12 months to | | 12 months to | | | | | | | | | |
| | | | | | | Dec 31, 00 | | Dec 31, 01 | | 12 months to Dec 31, 02 |
| | | | | | | (restated)* | | (restated)* | | | | | | (unaudited) |
| | | Notes | | £m | | £m | | £m | | $m** |
| | |
| |
| |
| |
| |
|
Sales including share of joint venture | | | 2 | | | | 1,377.6 | | | | 1,602.5 | | | | 1,618.2 | | | | 2,605.3 | |
| Less share of joint venture sales | | | | | | | (94.8 | ) | | | (87.3 | ) | | | (80.5 | ) | | | (129.6 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Group turnover | | | | | | | 1,282.8 | | | | 1,515.2 | | | | 1,537.7 | | | | 2,475.7 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Group operating profit before exceptional items and goodwill amortization | | | | | | | 211.4 | | | | 266.4 | | | | 286.6 | | | | 461.4 | |
| Exceptional items charged to operating profit | | | 3 | | | | (27.0 | ) | | | (8.7 | ) | | | — | | | | — | |
| Goodwill amortization | | | | | | | (10.7 | ) | | | (11.7 | ) | | | (36.6 | ) | | | (58.9 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Group operating profit | | | | | | | 173.7 | | | | 246.0 | | | | 250.0 | | | | 402.5 | |
| Share of operating profit of joint venture and associates | | | | | | | 29.8 | | | | 26.1 | | | | 23.2 | | | | 37.4 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Total operating profit | | | 2,4 | | | | 203.5 | | | | 272.1 | | | | 273.2 | | | | 439.9 | |
| Profit on disposal of fixed asset investment | | | 3 | | | | — | | | | 55.3 | | | | — | | | | — | |
| Amounts written off investments | | | | | | | — | | | | (4.4 | ) | | | (2.4 | ) | | | (3.9 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Profit before interest and taxation | | | | | | | 203.5 | | | | 323.0 | | | | 270.8 | | | | 436.0 | |
| Net interest payable | | | 7 | | | | (10.0 | ) | | | (8.7 | ) | | | (7.1 | ) | | | (11.4 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Profit on ordinary activities before taxation | | | | | | | 193.5 | | | | 314.3 | | | | 263.7 | | | | 424.6 | |
| Tax on profit on ordinary activities | | | 8 | | | | (79.9 | ) | | | (96.5 | ) | | | (85.1 | ) | | | (137.0 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Profit on ordinary activities after taxation | | | | | | | 113.6 | | | | 217.8 | | | | 178.6 | | | | 287.6 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| (Profit)/loss attributable to equity minority interests | | | | | | | (3.7 | ) | | | (4.2 | ) | | | 0.7 | | | | 1.1 | |
| Profit attributable to non-equity minority interests | | | | | | | (2.9 | ) | | | (2.9 | ) | | | (0.6 | ) | | | (0.9 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
(Profit)/loss attributable to minority interests | | | | | | | (6.6 | ) | | | (7.1 | ) | | | 0.1 | | | | 0.2 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Profit for the financial year attributable to shareholders | | | | | | | 107.0 | | | | 210.7 | | | | 178.7 | | | | 287.8 | |
| Dividends paid and proposed | | | 9 | | | | (40.4 | ) | | | (45.2 | ) | | | (57.3 | ) | | | (92.3 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Retained profit for the financial year | | | | | | | 66.6 | | | | 165.5 | | | | 121.4 | | | | 195.5 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Earnings per ordinary share | | | 10 | | | | | | | | | | | | | | | | | |
| - basic | | | | | | | 17.0 | p | | | 33.2 | p | | | 26.1 | p | | | 42.0 | c |
| - before exceptional items and goodwill amortization | | | | | | | 21.9 | p | | | 26.6 | p | | | 29.9 | p | | | 48.1 | c | |
| - average number of shares | | | | | | | 630.9 | m | | | 634.4 | m | | | 684.7 | m | | | 684.7 | m |
Diluted earnings per ordinary share | | | | | | | | | | | | | | | | | | | | |
| - after exceptional items and goodwill amortization | | | | | | | 16.8 | p | | | 33.0 | p | | | 25.9 | p | | | 41.7 | c |
Dividends per ordinary share | | | 9 | | | | 6.4 | p | | | 7.1 | p | | | 7.8 | p | | | 12.6 | c |
| | | All results are derived from continuing operations. |
| |
| | | The Group’s share of turnover from its joint venture in NMP is £80.5m, (2001 — £87.3m, 2000 — £94.8m). The Group’s share of gross profit is £29.3m, (2001 — £32.6m, 2000 — £36.0m), and the Group’s share of profit from continuing operations before exceptional items is £23.2m, (2001 — £26.1m, 2000 — £29.8m). The Group’s share of net income is £6.5m, (2001 — £8.4m, 2000 — £11.6m). |
| |
| | * | Figures for the 12 months to December 31, 2000 and the 12 months to December 31, 2001 and have been restated following the introduction of FRS19 as explained in note 37. |
| |
| | | See ** on the following page |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
AMERSHAM PLC AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF TOTAL RECOGNIZED GAINS AND LOSSES
| | | | | | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | (restated) | | | | | | (unaudited) |
| | | £m | | £m | | £m | | $m** |
| | |
| |
| |
| |
|
Profit for the period attributable to shareholders | | | 107.0 | | | | 210.7 | | | | 178.7 | | | | 287.8 | |
| Tax (charge)/credit on foreign currency hedge loan | | | 3.5 | | | | 0.3 | | | | (5.4 | ) | | | (8.7 | ) |
| Foreign currency translation of net investment in subsidiaries | | | (22.3 | ) | | | (4.0 | ) | | | 53.4 | | | | 86.0 | |
| Foreign currency translation of net investment in joint venture | | | (0.1 | ) | | | (0.2 | ) | | | (0.2 | ) | | | (0.3 | ) |
| | | |
| | | |
| | | |
| | | |
| |
Total recognized gains for the period | | | 88.1 | | | | 206.8 | | | | 226.5 | | | | 364.8 | |
| | | |
| | | |
| | | |
| | | |
| |
| Prior year adjustment | | | | | | | | | | | 4.4 | | | | 7.0 | |
| | | | | | | | | | | |
| | | |
| |
Total gains recognized since last Annual Report | | | | | | | | | | | 230.9 | | | | 371.8 | |
| | | | | | | | | | | |
| | | |
| |
| ** | | For illustrative purposes, the consolidated results for the year ended December 31, 2002 shown above in U.S. dollars, which are solely for the convenience of the reader, have been translated at the December 31, 2002 noon buying rate of £1 = $1.61. Such translations should not be construed as representations that the pound sterling amounts represent, have been or could be converted into US dollars at that or any rate. No adjustments have been made to restate the financial statements to comply with U.S. generally accepted accounting principles (US GAAP). A summary of the significant adjustments that would be required to restate net income for the periods ended December 31, 2000, 2001, and 2002 in accordance with US GAAP is presented in note 38. |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
AMERSHAM PLC AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEET
| | | | | | | | | | | | | | | | | | |
| | | | | | | | At Dec 31, 01 | | At Dec 31, 02 |
| | | | | | | | (restated) | | | | | | (unaudited) |
| | | | Notes | | £m | | £m | | $m* |
| | | |
| |
| |
| |
|
Fixed assets | | | | | | | | | | | | | | | | |
| Intangible assets | | | 11 | | | | 152.7 | | | | 751.2 | | | | 1,209.4 | |
| Tangible assets | | | 12 | | | | 533.1 | | | | 640.8 | | | | 1,031.7 | |
| Investments | | | | | | | | | | | | | | | | |
| | Investment in joint venture: | | | | | | | | | | | | | | | | |
| | - Share of gross assets | | | | | | | 93.7 | | | | 105.2 | | | | 169.4 | |
| | - Share of gross liabilities | | | | | | | (26.6 | ) | | | (32.2 | ) | | | (51.9 | ) |
| | | | | | | |
| | | |
| | | |
| |
| | - Share of net assets | | | 13 | | | | 67.1 | | | | 73.0 | | | | 117.5 | |
| | Investments in associates and other investments | | | 13 | | | | 35.0 | | | | 26.4 | | | | 42.5 | |
| | | | | | | |
| | | |
| | | |
| |
| | | | 13 | | | | 102.1 | | | | 99.4 | | | | 160.0 | |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | 787.9 | | | | 1,491.4 | | | | 2,401.1 | |
| | | | | | | |
| | | |
| �� | | |
| |
Current assets | | | | | | | | | | | | | | | | |
| Stocks | | | 14 | | | | 180.6 | | | | 208.0 | | | | 334.9 | |
| Debtors - amounts due within one year | | | 15 | | | | 360.8 | | | | 349.2 | | | | 562.2 | |
| Debtors - amounts due after one year | | | 16 | | | | 55.4 | | | | 61.3 | | | | 98.7 | |
| Short term deposits and investments | | | 17 | | | | 99.4 | | | | 22.1 | | | | 35.6 | |
| Cash at bank and in hand | | | | | | | 56.9 | | | | 32.7 | | | | 52.6 | |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | 753.1 | | | | 673.3 | | | | 1,084.0 | |
| | | | | | | |
| | | |
| | | |
| |
Creditors – amounts due within one year | | | | | | | | | | | | | | | | |
| Loans | | | 18 | | | | (30.8 | ) | | | (44.6 | ) | | | (71.8 | ) |
| Other creditors | | | 18 | | | | (452.5 | ) | | | (482.2 | ) | | | (776.3 | ) |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | (483.3 | ) | | | 526.8 | | | | (848.1 | ) |
| | | | | | | |
| | | |
| | | |
| |
Net current assets | | | | | | | 269.8 | | | | 146.5 | | | | 235.9 | |
| | | | | | | |
| | | |
| | | |
| |
Total assets less current liabilities | | | | | | | 1,057.7 | | | | 1,637.9 | | | | 2,637.0 | |
| | | | | | | |
| | | |
| | | |
| |
Creditors – amounts due after one year | | | | | | | | | | | | | | | | |
| Loans | | | 19 | | | | (148.0 | ) | | | (192.5 | ) | | | (310.0 | ) |
| Other creditors | | | 19 | | | | (6.4 | ) | | | (11.5 | ) | | | (18.5 | ) |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | (154.4 | ) | | | (204.0 | ) | | | (328.5 | ) |
| | | | | | | |
| | | |
| | | |
| |
Provisions for liabilities and charges | | | 22 | | | | (240.8 | ) | | | (253.5 | ) | | | (408.1 | ) |
| | | | | | | |
| | | |
| | | |
| |
Accruals and deferred income | | | | | | | (3.6 | ) | | | (3.5 | ) | | | (5.6 | ) |
| | | | | | | |
| | | |
| | | |
| |
Total net assets | | | | | | | 658.9 | | | | 1,176.9 | | | | 1,894.8 | |
| | | | | | | |
| | | |
| | | |
| |
Equity capital and reserves | | | | | | | | | | | | | | | | |
| Share capital | | | 25 | | | | 32.1 | | | | 35.1 | | | | 56.5 | |
| Share premium account | | | 26 | | | | 64.3 | | | | 469.0 | | | | 755.1 | |
| Other reserves | | | 26 | | | | 94.0 | | | | 94.0 | | | | 151.3 | |
| Profit and loss account | | | 26 | | | | 405.9 | | | | 573.9 | | | | 924.0 | |
| | | | | | | |
| | | |
| | | |
| |
Equity shareholders’ funds | | | | | | | 596.3 | | | | 1,172.0 | | | | 1,886.9 | |
| | | | | | | |
| | | |
| | | |
| |
Minority interests | | | | | | | | | | | | | | | | |
| | Equity | | | | | | | 20.0 | | | | 4.9 | | | | 7.9 | |
| | Non-equity | | | | | | | 42.6 | | | | — | | | | — | |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | 62.6 | | | | 4.9 | | | | 7.9 | |
| | | | | | | |
| | | |
| | | |
| |
| | | | | | | | 658.9 | | | | 1,176.9 | | | | 1,894.8 | |
| | | | | | | |
| | | |
| | | |
| |
| * | | For illustrative purposes, the consolidated balance sheet at December 31, 2002 shown above in U.S. dollars, which are solely for the convenience of the reader, have been translated at the December 31, 2002 noon buying rate of £1= $1.61. Such translations should not be construed as representations that the pound sterling amounts represent, have been or could be converted into US dollars at that or any rate. No adjustments have been made to restate the financial statements to comply with U.S. generally accepted accounting principles (US GAAP). A summary of the significant adjustments that would be required to restate equity shareholders’ funds at December 31, 2001 and 2002 in accordance with US GAAP is presented in note 38. |
| |
| | | Included within the Group’s share of the net assets of NMP are current assets of £65.2m, (2001 — £54.1m), current liabilities of £13.1m, (2001 — £13.7m), non-current assets of £40.0m, (2001 — £39.6m), non-current liabilities of £19.1m, (2001 — £12.9m). |
The accompanying notes are an integral part of these consolidated financial statements
F-5
AMERSHAM PLC AND SUBSIDIARIES
CONSOLIDATED CASH FLOW STATEMENT
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 12 months to |
| | | | | | | | | | | | | | | | Dec 31, 02 |
| | | | | | | 12 months to | | 12 months to | |
|
| | | | | | | Dec 31, 00 | | Dec 31, 01 | | | | | | (unaudited) |
| | | Notes | | £m | | £m | | £m | | $m** |
| | | | | | |
| |
| |
| |
|
Net cash flow from operating activities before exceptional items | | | 32 | | | | 255.3 | | | | 342.3 | | | | 345.5 | | | | 556.3 | |
| Integration costs | | | | | | | (15.8 | ) | | | — | | | | — | | | | — | |
| Other exceptional items | | | | | | | (14.8 | ) | | | 12.0 | | | | — | | | | — | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Net cash inflow from operating activities | | | 32 | | | | 224.7 | | | | 354.3 | | | | 345.5 | | | | 556.3 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Dividend received from joint venture | | | | | | | 3.9 | | | | 4.8 | | | | 5.4 | | | | 8.7 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Returns on investments and servicing of finance | | | | | | | | | | | | | | | | | | | | |
| Interest paid | | | | | | | (30.5 | ) | | | (19.7 | ) | | | (9.2 | ) | | | (14.8 | ) |
| Interest received | | | | | | | 13.5 | | | | 10.3 | | | | 4.9 | | | | 7.9 | |
| Dividends paid by subsidiary undertakings to minority interests | | | | | | | (1.4 | ) | | | (1.7 | ) | | | (1.7 | ) | | | (2.7 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | (18.4 | ) | | | (11.1 | ) | | | (6.0 | ) | | | (9.6 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Taxation | | | | | | | | | | | | | | | | | | | | |
| UK corporation tax (paid)/received | | | | | | | (2.7 | ) | | | 4.5 | | | | (2.1 | ) | | | (3.4 | ) |
| Overseas tax paid | | | | | | | (13.1 | ) | | | (52.1 | ) | | | (55.1 | ) | | | (88.7 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | (15.8 | ) | | | (47.6 | ) | | | (57.2 | ) | | | (92.1 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Capital expenditure and financial investment | | | | | | | | | | | | | | | | | | | | |
| Purchases of tangible fixed assets | | | | | | | (123.5 | ) | | | (91.6 | ) | | | (143.4 | ) | | | (230.9 | ) |
| Purchases of intangible fixed assets | | | | | | | (4.5 | ) | | | (10.2 | ) | | | (4.8 | ) | | | (7.7 | ) |
| Sales of tangible fixed assets | | | | | | | 0.3 | | | | 22.1 | | | | 1.3 | | | | 2.1 | |
| Sales of investments | | | | | | | 4.2 | | | | — | | | | 1.4 | | | | 2.2 | |
| Purchases of trade investments | | | | | | | (10.5 | ) | | | (4.9 | ) | | | (3.4 | ) | | | (5.5 | ) |
| Purchase of own shares | | | | | | | (16.5 | ) | | | — | | | | — | | | | — | |
| Cash received on disposal of investment in Nycomed Pharma | | | | | | | — | | | | 123.0 | | | | — | | | | — | |
| Costs associated with the disposal of investment in Nycomed Pharma | | | | | | | — | | | | (4.9 | ) | | | (3.5 | ) | | | (5.6 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | (150.5 | ) | | | 33.5 | | | | (152.4 | ) | | | (245.4 | ) |
| | | | | | | |
| �� | | |
| | | |
| | | |
| |
Acquisitions and disposals | | | | | | | | | | | | | | | | | | | | |
| Purchase of 45% minority in Amersham Biosciences | | | 24 | | | | — | | | | — | | | | (704.1 | ) | | | (1,133.6 | ) |
| Cost associated with purchase of 45% minority in Amersham Biosciences | | | 24 | | | | — | | | | — | | | | (4.5 | ) | | | (7.2 | ) |
| Other acquisitions | | | | | | | (4.9 | ) | | | (3.9 | ) | | | (40.6 | ) | | | (65.4 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | (4.9 | ) | | | (3.9 | ) | | | (749.2 | ) | | | (1,206.2 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Equity dividends paid | | | | | | | (38.2 | ) | | | (41.7 | ) | | | (51.6 | ) | | | (83.1 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Net cash flow before use of liquid resources* and financing | | | | | | | 0.8 | | | | 288.3 | | | | (665.5 | ) | | | (1,071.4 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Management of liquid resources* | | | 34 | | | | 64.1 | | | | (91.4 | ) | | | 74.5 | | | | 119.9 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Financing | | | | | | | | | | | | | | | | | | | | |
| Issue of share capital | | | | | | | 6.2 | | | | 16.2 | | | | 413.2 | | | | 665.3 | |
| Costs associated with issue of share capital | | | | | | | — | | | | — | | | | (5.5 | ) | | | (8.9 | ) |
| Loans and finance leases | | | 34 | | | | (70.3 | ) | | | (205.3 | ) | | | 161.9 | | | | 260.7 | |
| Repayment of long term loan | | | 34 | | | | — | | | | — | | | | (13.6 | ) | | | (21.9 | ) |
| Capital contribution by minority interest in Amersham Biosciences | | | | | | | — | | | | — | | | | 8.7 | | | | 14.0 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | (64.1 | ) | | | (189.1 | ) | | | 564.7 | | | | 909.2 | |
| | | | | | | |
| | | |
| | | |
| | | |
| |
Cash flow in the year | | | 34 | | | | 0.8 | | | | 7.8 | | | | (26.3 | ) | | | (42.3 | ) |
| | | | | | | |
| | | |
| | | |
| | | |
| |
| * | | Liquid resources are defined as short term deposits with banks and current asset investments in bonds and equities. These are shown in the consolidated balance sheet as short term deposits £22.1m (2001 — £96.5m, 2000 — £2.5m) and interest bearing and other investments £nil (2001 — £nil, 2000 — £2.2m). |
| |
| ** | | For illustrative purposes, the consolidated cash flows for the year ended December 31, 2002 shown above in U.S. dollars, which are solely for the convenience of the reader, have been translated at the December 31, 2002 noon buying rate of £1= $1.61. Such translations should not be construed as representations that the pound sterling amounts represent, have been or could be converted into US dollars at that or any rate. No adjustments have been made to restate the financial statements to comply with U.S. generally accepted accounting principles (US GAAP). Summary statements of cash flows for the 12 month periods ended December 31, 2000, 2001 and 2002 presented in accordance with US GAAP are presented in note 38. |
The accompanying notes are an integral part of these consolidated financial statements
F-6
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Nature of business and organization
Amersham plc (the “Company”) and its subsidiary undertakings (together, the “Group’’) are engaged in the worldwide development, manufacture and sale of specialized products for research-based biotechnology supply and for the diagnosis and treatment of disease. In September 2001 the Group sold its remaining trade investment in Nycomed Pharma and subsequent to the transaction the Company changed its name from Nycomed Amersham plc to Amersham plc. Details of the transaction are included in note 3. In March 2002 the Group purchased the 45% minority interest in Amersham Biosciences through the acquisition of Pharmacia Biosystems AB which held 39% and the direct acquisition of the remaining 6% thereby taking 100% ownership of Amersham Biosciences. Details of the transaction are included in note 24.
1. ACCOUNTING POLICIES
The main Group accounting policies are set out below. The accounts are prepared in accordance with the historical cost convention, and all applicable United Kingdom accounting standards. As the profit and loss account is already at historical cost, no separate note of historical cost profit and loss account is included as allowed by Financial Reporting Standard (FRS) 3 “Reporting Financial Performance”.
Changes in presentation of financial information
Where necessary prior year information has been reclassified to ensure comparability.
New accounting policies
The following new accounting standard, issued by the UK Accounting Standards Board, has been adopted this year:
Financial Reporting Standard (FRS) 19 Deferred Taxation
The change to existing accounting policies arising from the adoption of FRS 19 is described in note 37 below.
Basis of consolidation
The consolidated accounts include the accounts of the Company and all its subsidiary undertakings made up to December 31, 2002, December 31, 2001 and December 31, 2000. The results of subsidiaries sold or acquired are included in the consolidated profit and loss account up to, or from, the date control passes. Intra-Group sales and profits are eliminated fully on consolidation.
On acquisition of a subsidiary, all of the subsidiary’s assets and liabilities that exist at the date of acquisition are recorded at their fair values reflecting their condition at that date. All changes to those assets and liabilities, and the resulting gains and losses that arise after the Group gains control of the subsidiary, are dealt with in the post acquisition profit and loss account.
Foreign currencies
The results of the overseas subsidiary, joint venture and associated undertakings are translated at the average exchange rates of the period during which they arise. Overseas subsidiary undertakings’ assets and liabilities and the net investments in joint venture and associated undertakings are translated at the year end exchange rates. Differences arising on translation together with differences on foreign currency liabilities of the Group which match investments are dealt with through reserves.
F-7
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Derivatives and other financial instruments
The Group enters into derivative instruments primarily to manage exposures to fluctuations in interest rates and foreign currency exchange rates. Interest differentials under currency and interest rate swap instruments and forward rate agreements (FRAs) are recognized on an accruals basis.
It is the Group’s policy, where appropriate, to protect the sterling value of its estimated future foreign currency receivables through the use of forward contracts or purchased currency options. Net revenues and related net receivables covered by forward exchange contracts or options are translated into sterling at contract rates. No account is taken of the potential but unrealized profits or losses on open forward exchange contracts or options which are intended as a hedge against future transactions; such profits and losses are accounted for so as to match the exchange differences arising on the underlying currency transactions.
Amersham plc currently uses four types of derivative instruments as part of an overall risk management strategy. The Company does not enter into derivative instruments for trading purposes. The accounting treatment depends upon the risk that is hedged.
Interest rate swaps are used to change the duration of the underlying debt. Interest differentials of the swap are accrued to the profit and loss account on a time-apportioned basis. If the underlying debt were to be repaid, the swap would be either closed out or reversed with the allied cost or benefit attributed to and accounted for in the same manner as the unexpected event causing the debt to be repaid.
Interest on currency swaps is treated as above. Currency revaluations of the outstanding principal to the period end rates would be transferred to reserves if the swap were specifically hedging Group net assets; otherwise, revaluations pass through the profit and loss account. If the underlying asset or liability being hedged was sold or repaid, the cost or benefit of selling or reversing the currency swap would be attributed to and accounted for in the same manner as the underlying event.
Forward foreign exchange contracts are used to hedge equity investments abroad. Normally the Group borrows in currencies to hedge net equity investments abroad. Where the need for hedges exceeds the need for debt, short-term foreign exchange contracts are entered into and designated as equity investment hedges. The forward premium or discount in the contract is accrued to interest payable on a time-apportioned basis. The revaluation of the outstanding currency flows is taken to reserves. If the underlying investments were to be sold, such that the hedge was no longer required, the cost of reversing the hedge would be attributed to and accounted for in the same manner as the disposal of the underlying assets.
Forward foreign exchange contracts used to hedge forecast trading flows of the Group are accounted for through the profit and loss account either upon maturity of the contract or deferred and recognized in operating profit when the hedged transaction has itself been reflected in the Group’s financial statements. The forward premium or discount for these contracts is not accounted for as interest but as part of the hedge rate achieved. If underlying forecast flows do not materialize as envisaged, the hedges would either be reversed or swapped forward to a future financing period. The adjusting hedges would still be accounted for through the profit and loss account on a realized basis.
Forward foreign exchange contracts are used to hedge Group monetary assets and liabilities. The forward premium or discount in the contract is recognized on an accruals basis through interest. The unrealized profit or losses on these open forward exchange contracts is taken to the profit and loss account each month as are the related hedged monetary assets or liabilities.
Currency options are sometimes purchased to hedge forecast trading flows. These are accounted for in the same manner as the forward foreign exchange contracts above except that the premium paid is deferred to the point of exercise or lapse of the option.
F-8
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Research and development expenditure
Research and development expenditure is written off as it is incurred.
Fixed assets
Fixed assets are stated at cost less depreciation and provision for impairment, where appropriate. Cost includes appropriate labor and overheads associated with own manufactured assets. It is not the policy of the Group to capitalize interest associated with the funding of capital projects. Directly acquired intangible assets, comprising patents, patent applications, trademarks and know-how, are stated at cost less amortization. Intangible assets, other than goodwill, acquired in a business combination which are not separable, identifiable and measurable are not capitalized. When fixed assets are retired or sold, their costs and related accumulated depreciation are written off and the resulting gain or loss is included in the profit and loss account. Repair and maintenance costs are expensed as incurred.
Goodwill arising on consolidation represents the excess of the fair value of the consideration given over the fair value of the identifiable net assets acquired. Goodwill arising on the acquisition of subsidiary undertakings, joint venture and associated undertakings prior to December 31, 1997 was written off immediately against reserves. Goodwill arising on acquisitions after January 1, 1998 is capitalized and amortized over its estimated useful economic life where the goodwill is regarded as having a limited useful economic life. In accordance with the requirement of FRS 10, “Goodwill and intangible assets”, capitalized goodwill is reviewed for evidence of impairment at the end of the first full financial year following the initial date of recognition.
Depreciation and amortization are calculated as follows:
| | | |
| Intangible assets | | Amortized on a straight-line basis at rates currently from 5% to 36% p.a. |
| | | |
| Goodwill | | Amortized on a straight-line basis at rates currently from 5% to 14% p.a. |
| | | |
| Tangible assets | | Depreciation is calculated by the straight-line method at the following annual rates: |
| | | |
| Land | | Nil |
| | | |
| Buildings | | 2.5% |
| | | |
| Plant and machinery, fixtures, fittings, tools and equipment | | At varying rates calculated to write-off the assets over their expected useful lives. A substantial part of the Group’s plant represents production support facilities depreciated at the same rate, 2.5% p.a., as the buildings in which they are contained. Most of the other assets are depreciated at rates currently from 9% to 12% p.a. |
| | | |
| Assets under construction | | Nil |
Investments
(a) Trade investments
Trade investments are valued at cost, less any provision for impairment, restated to year end exchange rates if the underlying investment is denominated in foreign currency.
(b) Investments in joint ventures and associated undertakings
A joint venture is an undertaking which the Group controls jointly with one or more other venturers.
An associated undertaking is one in which the Group has a long term participating interest, usually from 20% to 50%, and over which it exercises significant influence.
The Group’s share of the profits less losses of joint ventures and associated undertakings is included in the consolidated profit and loss account and its interest in their net assets is included in investments in the consolidated balance sheet.
(c) Company investments
Investments in subsidiary and other undertakings are held at original cost less any provision for impairment, except where they are foreign currency investments hedged in whole or in part by related foreign currency borrowings or other hedging instruments. Hedged investments are held at cost less provision for impairment, where appropriate, but are retranslated for foreign exchange movements to the extent that they are hedged.
F-9
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Where the amount of a foreign currency hedge is in excess of cost, the investment is stated at the underlying foreign currency net asset value.
Turnover
Turnover consists of royalties and sales of goods and services at invoiced values net of discounts, returns and value added tax. Sales between Group companies are excluded.
Revenues are recognized once the realization of the consideration is reasonably assured and the provision of goods and services is substantially complete with respect to the delivery of the specific product or performance of the related service. This will normally be the case when there are no material uncertainties or performance duties outstanding which could prevent the Group from enforcing the sales transaction and collectability is reasonably assured.
The Group recognizes revenue principally on five types of transactions, namely sales of consumable products, sales of instruments, agreements which provide access to or license of certain of the Group’s proprietary technology and know-how, contracts for ongoing service and support of instruments sold to customers, and royalty income on third party use of the Group’s patents.
Revenues on sales of instruments in the United States and on sales of consumable products in all markets are recognized upon transfer of title and risk of loss to the customer, which is generally upon delivery to the carrier. In markets outside of the United States, sales of instruments are made with a reservation of title until payment in full by the customer for the equipment. For these sales, revenue is recognized upon transfer of risk of loss to the customer, which is generally upon delivery to the carrier, as the reservation of title is solely for credit protection purposes.
Revenues earned under agreements which provide access to or license the Group’s proprietary technology and know-how are recognized on a straight line basis over the life of the agreement based on the total fees received under the agreement over the period the access to the technology and know-how is provided.
Revenues earned under contracts for ongoing service are recognized pro-rata over the term of the service agreements.
Revenues earned from royalties on patents are recognized pro-rata over the life of the royalty agreement.
Stocks
Stocks are stated at the lower of cost and net realizable value. Cost is determined on a first in, first out basis and includes all direct expenditure and, in the case of manufactured items, production overheads based on the normal level of business activity. Net realizable value is the price at which the stocks can be realized in the normal course of business after allowing for the costs of realization and, where appropriate, the costs of conversion. Provision is made for obsolescent and slow moving stocks and for radioactive decay.
Effluent costs
The costs associated with radioactive waste arising from operations for which an authorized disposal route is available, principally low level and very low level waste, are written off in the year in which they are incurred. Provision is also made as incurred for the eventual disposal costs of the Group’s intermediate level waste which arises from its operations. These provisions are based on the latest technical assessment of the process and methods likely to be used to dispose of this waste and on industry estimates of the likely costs of the necessary disposal facilities under the relevant Government’s preferred disposal route. They are stated in the balance sheet at current price levels, with the restatement of provisions made in prior years to reflect current price levels included as a charge in the profit and loss account for the year.
Radioactive decommissioning costs
The Group recognizes in full a provision, (discounted), for the cost of radioactive decommissioning when the relevant fixed asset becomes operational and an equivalent asset is recognized. The cost of radioactive decommissioning is determined on a going concern basis on the basis that the manufacture or handling of radioactive products continues at the relevant plant or facility on which the asset is operated for the foreseeable future. In the unlikely event that the Company is required to totally discontinue activities at one of its nuclear
F-10
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
operating sites, the full costs of exiting and decommissioning would be higher. The relevant decommissioning asset is capitalized in the balance sheet in association with the fixed asset to which it relates, and is amortized over the life of the asset. The unwinding of the discount on the decommissioning provision is charged in the profit and loss account as part of the interest charge.
Government grants
Investment grants have been treated as deferred income and are released to the profit and loss account at the same rate as the depreciation charge on the assets to which they relate. Revenue grants are matched with the related expenditure.
Taxation
Taxation charged against profits is calculated at the appropriate local rate of tax for each Group company. Except for timing differences relating to revaluations, rolled over taxable gains and unremitted income, provision is made for all timing differences that would give rise to a deferred tax liability. Deferred tax assets are recognized to the extent that it is more likely than not they will be recoverable.
Pensions
The Group operates defined benefit and defined contribution pension schemes under arrangements, which have been separately established by each Group company. In respect of the defined benefit schemes, actuarial valuations are made regularly and the contributions payable are adjusted as appropriate. Pension costs are accounted for on the basis of charging the expected cost of providing pensions over the period during which the Group benefits from the employees’ services. The effects of variations from regular cost are spread over the expected remaining service lives of members of the scheme. In respect of defined contribution pension schemes, costs are charged as incurred.
Leases
Rentals payable under operating leases are charged over the lease term on a straight-line basis or on the basis of actual rentals payable where this fairly reflects usage. Rentals receivable are treated as income in the periods to which they relate.
Where assets are financed by leasing arrangements that give rights approximating to ownership (finance leases), the assets are treated as if they had been purchased outright and the corresponding liability to the leasing company is included as an obligation under finance leases.
F-11
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
2. SEGMENTAL ANALYSIS
Segmental analysis by business sector
In 1998, the Group adopted SFAS No. 131 ‘Disclosures about Segments of an Enterprise and Related Information’. SFAS 131 supersedes SFAS 14 replacing the ‘industry segment’ approach with the ‘management approach’. The management approach designates the internal organization that is used by management for making operating decisions and assessing performance as the source of the Group’s reportable segments.
The Group’s operations are managed through Amersham Health (development, manufacture and supply ofin-vivodiagnostic imaging agents and radiotherapy products) and Amersham Biosciences (development, manufacture and sale of specialized products and services for life science research and manufacture of biopharmaceuticals). Inter-segment sales are small. The Group evaluates the performance of its segments and allocates resources to them based on profit before interest and tax.
The sales, profit before interest and tax both before and after exceptional items and goodwill amortization, and net assets of the various activities of the Group are set out below.
Amersham plc incurs costs for services which are common to all divisions. These shared service costs are allocated on the most appropriate basis for the category of cost.
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
(a) Sales | | | | | | | | | | | | |
| Amersham Health | | | 773.9 | | | | 922.0 | | | | 947.7 | |
| Amersham Biosciences | | | 603.7 | | | | 680.5 | | | | 670.5 | |
| | | |
| | | |
| | | |
| |
| | | | 1,377.6 | | | | 1,602.5 | | | | 1,618.2 | |
| | | |
| | | |
| | | |
| |
Included within Amersham Health is the Group’s share of the joint venture Nihon Medi-Physics Company Ltd (NMP) sales for the year of £80.5m (2001 — £87.3m, 2000 — £94.8m).
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| (b) Total operating profit before exceptional items and goodwill amortization | | | | | | | | | | | | |
| Amersham Health | | | 194.6 | | | | 240.9 | | | | 255.6 | |
| Amersham Biosciences | | | 63.3 | | | | 72.4 | | | | 75.3 | |
| Corporate and other | | | (16.7 | ) | | | (20.8 | ) | | | (21.1 | ) |
| | | |
| | | |
| | | |
| |
| | | | 241.2 | | | | 292.5 | | | | 309.8 | |
| | | |
| | | |
| | | |
| |
F-12
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table shows total operating profit after exceptional items and goodwill amortization. The exceptional items charged in 2002 relate £nil (2001 — £8.5m, 2000 — £10.9m) to Amersham Health, £nil (2001 — £2.6m, 2000 — £3.5m) to Amersham Biosciences and £nil (2001 — £2.4 income, 2000 — £12.6m charge) to Corporate and other. Goodwill charged in 2002 relates £0.9m, (2001 — £1.4m, 2000 — £1.7m), to Amersham Health, and £35.7m, (2001 — £10.3m, 2000 — £9.0m) to Amersham Biosciences.
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Total operating profit after exceptional items and goodwill amortization | | | | | | | | | | | | |
| Amersham Health | | | 182.0 | | | | 231.0 | | | | 254.7 | |
| Amersham Biosciences | | | 50.8 | | | | 59.5 | | | | 39.6 | |
| Corporate and other | | | (29.3 | ) | | | (18.4 | ) | | | (21.1 | ) |
| | | |
| | | |
| | | |
| |
| | | | 203.5 | | | | 272.1 | | | | 273.2 | |
| | | |
| | | |
| | | |
| |
Included within Amersham Health is the Group’s share of the NMP joint venture total operating profit after exceptional items and goodwill amortization for the year of £23.2m, (2001 — £26.1m, 2000 — £29.8m).
| | | | | | | | | |
| | | Dec 31, 01 | | | | |
| | | (restated) | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
(c) Net assets | | | | | | | | |
| Amersham Health | | | 451.5 | | | | 559.9 | |
| Amersham Biosciences | | | 413.2 | | | | 1,032.6 | |
| Corporate and other | | | (20.2 | ) | | | (33.7 | ) |
| | | |
| | | |
| |
| Net operating assets | | | 844.5 | | | | 1,558.8 | |
Unallocated net liabilities | | | | | | | | |
| Current and deferred taxation | | | (129.9 | ) | | | (163.6 | ) |
| Dividends payable | | | (30.3 | ) | | | (36.0 | ) |
| Net debt | | | (25.4 | ) | | | (182.3 | ) |
| | | |
| | | |
| |
| | | | 658.9 | | | | 1,176.9 | |
| | | |
| | | |
| |
The net operating assets of Amersham Biosciences include goodwill at a net book value of £630.6m at December 31, 2002 arising from the purchase of the 45% minority interest in Amersham Biosciences.
Included within the net operating assets of Amersham Health is the Group’s share of the NMP joint venture assets of £73.0m, (2001 — £67.1m).
F-13
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Segmental analysis by geographical sector
The figures set out below for each geographical area show the sales, total operating profit both before and after exceptional items and goodwill amortization, net assets and long lived assets of the subsidiary undertakings located in that area. Export sales and related profits are included in the area from which those sales are made. Transfers between Group companies are charged at commercial rates.
| | | | | | | | | | | | | | | | | |
| | | | | | | 12 months to | | 12 months to | | 12 months to |
| | | | | | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| By origin | | £m | | £m | | £m |
| |
| |
| |
|
(a) Sales | | | | | | | | | | | | |
| Europe | | | 766.5 | | | | 722.1 | | | | 903.9 | |
| North America | | | 743.4 | | | | 853.7 | | | | 871.8 | |
| Japan | | | 206.8 | | | | 202.1 | | | | 187.8 | |
| Asia Pacific | | | 58.2 | | | | 77.5 | | | | 84.0 | |
| Rest of the World | | | 6.9 | | | | 6.7 | | | | 5.2 | |
| | | | | | | |
| | | |
| | | |
| |
| | | | 1,781.8 | | | | 1,862.1 | | | | 2,052.7 | |
| Less inter-segment sales | | | (404.2 | ) | | | (259.6 | ) | | | (434.5 | ) |
| | | | | | | |
| | | |
| | | |
| |
| | | | 1,377.6 | | | | 1,602.5 | | | | 1,618.2 | |
| | | | | | | |
| | | |
| | | |
| |
Included within Japan is the Group’s share of NMP joint venture sales during the year of £80.5m (2001 — £87.3m, 2000 — £94.8m).
Sales from the UK to external customers totaled £91.7m (2001 — £85.1m, 2000 — £81.5m).
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| (b) Total operating profit before exceptional items and goodwill amortization | | | | | | | | | | | | |
| Europe | | | 146.9 | | | | 193.0 | | | | 219.3 | |
| North America | | | 74.5 | | | | 89.7 | | | | 80.3 | |
| Japan | | | 34.6 | | | | 30.4 | | | | 31.9 | |
| Asia Pacific | | | 1.8 | | | | (0.3 | ) | | | (0.9 | ) |
| Rest of the World | | | 0.1 | | | | 0.5 | | | | 0.3 | |
| Corporate | | | (16.7 | ) | | | (20.8 | ) | | | (21.1 | ) |
| | | |
| | | |
| | | |
| |
| | | | 241.2 | | | | 292.5 | | | | 309.8 | |
| | | |
| | | |
| | | |
| |
The following table shows total operating profit after exceptional items and goodwill amortization. The exceptional items charged in 2002 relate £nil (2001 - - £10.0m, 2000 — £11.8m) to Europe, £nil (2001 — £1.1m, 2000 — £2.6m) to North America and £nil (2001 — £2.4 income, 2000 — £12.6m charge) to Corporate and Other.
F-14
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Total operating profit after exceptional items and goodwill amortization | | | | | | | | | | | | |
| Europe | | | 135.1 | | | | 182.7 | | | | 210.4 | |
| North America | | | 61.2 | | | | 77.2 | | | | 57.6 | |
| Japan | | | 34.6 | | | | 30.4 | | | | 26.9 | |
| Asia Pacific | | | 1.8 | | | | (0.3 | ) | | | (0.9 | ) |
| Rest of the World | | | 0.1 | | | | 0.5 | | | | 0.3 | |
| Corporate | | | (29.3 | ) | | | (18.4 | ) | | | (21.1 | ) |
| | | |
| | | |
| | | |
| |
| | | | 203.5 | | | | 272.1 | | | | 273.2 | |
| | | |
| | | |
| | | |
| |
Included within Japan is the Group’s share of NMP joint venture profit before interest and tax for the year of £23.2m (2001 — £26.1m, 2000 — £29.8m).
| | | | | | | | | | |
| | | | 12 months to | | | | |
| | | | Dec 31, 01 | | 12 months to |
| | | | (restated) | | Dec 31, 02 |
| | | | £m | | £m |
| | | |
| |
|
(c) Net assets | | | | | | | | |
| Europe | | | 383.1 | | | | 709.3 | |
| North America | | | 322.4 | | | | 603.0 | |
| Japan | | | 107.8 | | | | 228.9 | |
| Asia Pacific | | | 49.8 | | | | 49.5 | |
| Rest of the World | | | 1.6 | | | | 1.8 | |
| Corporate | | | (20.2 | ) | | | (33.7 | ) |
| | | |
| | | |
| |
| Net operating assets | | | 844.5 | | | | 1,558.8 | |
Unallocated net liabilities | | | | | | | | |
| Current and deferred taxation | | | (129.9 | ) | | | (163.6 | ) |
| Dividends payable | | | (30.3 | ) | | | (36.0 | ) |
| Net debt | | | (25.4 | ) | | | (182.3 | ) |
| | | |
| | | |
| |
| | | | 658.9 | | | | 1,176.9 | |
| | | |
| | | |
| |
Net operating assets include goodwill at a net book value of £630.6m at December 31, 2002 arising from the purchase of the 45% minority interest in Amersham Biosciences. The goodwill is allocated to Europe, (£207.1m), North America (£304.1m) and Japan (£119.4m) based on expected revenues arising in these territories.
Included within the net operating assets of Japan is the Group’s share of NMP joint venture assets of £73.0m, (2001 — £67.1m).
F-15
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| (d) Long lived assets | | | | | | | | |
| Europe | | | 441.4 | | | | 764.9 | |
| North America | | | 257.7 | | | | 521.2 | |
| Japan | | | 77.7 | | | | 202.3 | |
| Asia Pacific | | | 26.1 | | | | 21.7 | |
| Rest of World | | | 0.6 | | | | 0.5 | |
| Corporate assets | | | 9.8 | | | | 9.1 | |
| | | |
| | | |
| |
| | | | 813.3 | | | | 1,519.7 | |
| Unallocated assets — Deferred Taxation | | | — | | | | 32.9 | |
| | | |
| | | |
| |
| Total long lived assets | | | 813.3 | | | | 1,552.6 | |
| | | |
| | | |
| |
Included within Europe are long lived assets in the UK of £212.2m (2001 — £166.0m)
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| (e) Sales — by destination | | | | | | | | | | | | |
| Europe | | | 346.6 | | | | 392.0 | | | | 427.8 | |
| North America | | | 647.2 | | | | 767.7 | | | | 798.5 | |
| Japan | | | 279.5 | | | | 290.7 | | | | 264.1 | |
| Asia Pacific | | | 58.1 | | | | 80.4 | | | | 80.6 | |
| Rest of the World | | | 46.2 | | | | 71.7 | | | | 47.2 | |
| | | |
| | | |
| | | |
| |
| | | | 1,377.6 | | | | 1,602.5 | | | | 1,618.2 | |
| | | |
| | | |
| | | |
| |
Included within Japan is the Group’s share of NMP joint venture sales during the year of £80.5m (2001 — £87.3m, 2000 — £94.8m).
F-16
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
3. EXCEPTIONAL ITEMS
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Costs associated with the disposal of Nycomed Pharma | | | (9.7 | ) | | | — | | | | — | |
| Costs of manufacturing rationalization in Amersham Health | | | (10.9 | ) | | | — | | | | — | |
| Costs of the proposed partial flotation of Amersham Biosciences | | | (6.4 | ) | | | (3.4 | ) | | | — | |
| Transfer of pension assets | | | — | | | | 9.0 | | | | — | |
| Costs of exiting the disease profiling project | | | — | | | | (5.8 | ) | | | — | |
| Costs of exiting Harwell site | | | — | | | | (8.5 | ) | | | — | |
| | | |
| | | |
| | | |
| |
Charged to operating profit | | | (27.0 | ) | | | (8.7 | ) | | | — | |
| Profit on disposal of 29% of Nycomed Pharma | | | — | | | | 55.3 | | | | — | |
| | | |
| | | |
| | | |
| |
Profit on disposal of fixed asset investment | | | — | | | | 55.3 | | | | — | |
| | | |
| | | |
| | | |
| |
Tax credit relating to Group reorganization | | | — | | | | — | | | | 9.2 | |
| | | |
| | | |
| | | |
| |
Total exceptional items | | | (27.0 | ) | | | 46.6 | | | | 9.2 | |
| | | |
| | | |
| | | |
| |
| Tax credit/(charge) related to exceptional items | | | | | | | | | | | | |
| - tax on exceptional items charged to operating profit | | | 2.4 | | | | (2.2 | ) | | | — | |
| - tax on disposal of fixed assets – continuing operations | | | — | | | | 2.7 | | | | — | |
| | | |
| | | |
| | | |
| |
| | | | 2.4 | | | | 0.5 | | | | — | |
| | | |
| | | |
| | | |
| |
The minority interest impact is £nil (2001 — £2.0m, 2000 — £nil), relating to the exceptional items charged to operating profit.
(a) Tax credit relating to Group reorganization
As a result of the formation of a consolidated tax group in the US on 31 July 2002, the Group has recognized a deferred taxation asset of £9.2m primarily related to the future utilization of tax losses arising from prior periods.
(b) Proposed partial flotation of Amersham Biosciences
Costs of £nil (2001 — £3.4m, 2000 — £6.4m) were incurred in relation to the proposed partial flotation of Amersham Biosciences. The costs related primarily to adviser fees and other related costs in preparing the prospectus for flotation and in relocating the business to the US.
(c) Transfer of pension assets
In 2001 the Company received £22.5m related to a transfer of assets from former pension plans. Of this amount, £13.5m has been applied against ongoing liabilities primarily for employees who have transferred into Amersham pension schemes and the balance of £9.0m has been recognized as exceptional income, where the Company has no further liability.
(d) Costs of exiting the disease profiling project
Cash costs of £4.1m and non-cash write offs of £1.7m have been incurred in the second half of 2001 in concluding research on, and winding up the operation of, an early stage investment in disease profiling. An investment of £4.0m was made in the research project in the first half of 2001 and £3.7m in the year ended December 31, 2000, which was charged to operating activities.
(e) Cost of exiting the Harwell site
In 2001, costs of £8.5m have been accrued in relation to the decision to terminate the Group’s lease at the Harwell site following a complete cessation of the Group’s activities at this site. The costs relate primarily to asset write offs and decommissioning costs.
(f) Nycomed Pharma
The sale of Nycomed Pharma to a new company formed for the purpose of the transaction was completed on May 14, 1999. The Group recorded a pre tax gain of £11.5m in the year ended December 31, 1999 after adding back attributable goodwill previously written off to reserves. The tax charge on the disposal amounted
F-17
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
to £9.5m. Further costs of £9.7m, with a related tax credit of £1.1m, associated with exiting from the Pharma business, have been accounted for in the year ended December 31, 2000 giving a total loss associated with the disposal of £6.6m after tax in 1999 and 2000. In September 2001 the Group sold its remaining investment in Nycomed Pharma, which consisted of shares and warrants, for £72.4m and realized £50.6m for the outstanding Nycomed Pharma loan notes held by the Group. The Group has recorded a profit on the transaction of £55.3m after charging costs associated with the disposal, including the costs incurred in transferring the Nycomed name to Nycomed Pharma. Subsequent to the transaction the Company changed its name from Nycomed Amersham plc to Amersham plc. The shares in Nycomed Pharma were owned by a legal entity in Holland and under Dutch tax law, the gain on their disposal is not taxable.
(g) Manufacturing rationalization in Amersham Health
In 2000, Amersham Health recorded a charge of £10.9m for the costs of rationalizing its radiopharmaceutical production capacity at specific sites in the US and Europe. The costs of the rationalization comprise severance and other cash costs of £8.4m and non-cash write offs of £2.5m. No further costs associated with the rationalization are expected.
4. TOTAL OPERATING PROFIT
| | | | | | | | | | | | | |
| | | Before exceptional | | | | | | | | |
| | | items | | Exceptional items | | Total |
| Total operating profit 2002 | | £m | | £m | | £m |
| |
| |
| |
|
| Turnover | | | 1,537.7 | | | | — | | | | 1,537.7 | |
| Cost of sales | | | (481.0 | ) | | | — | | | | (481.0 | ) |
| | | |
| | | |
| | | |
| |
Gross profit | | | 1,056.7 | | | | — | | | | 1,056.7 | |
| Distribution and selling costs | | | (419.7 | ) | | | — | | | | (419.7 | ) |
| Administrative expenses | | | (387.0 | ) | | | — | | | | (387.0 | ) |
| | | |
| | | |
| | | |
| |
Group operating profit | | | 250.0 | | | | — | | | | 250.0 | |
| Share of operating profits of joint venture and associates | | | 23.2 | | | | — | | | | 23.2 | |
| | | |
| | | |
| | | |
| |
Total operating profit | | | 273.2 | | | | — | | | | 273.2 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | |
| | | Before exceptional | | | | | | | | |
| | | items | | Exceptional items | | Total |
| Total operating profit 2001 | | £m | | £m | | £m |
| |
| |
| |
|
| Turnover | | | 1,515.2 | | | | — | | | | 1,515.2 | |
| Cost of sales | | | (500.1 | ) | | | 0.5 | | | | (499.6 | ) |
| | | |
| | | |
| | | |
| |
Gross profit | | | 1,015.1 | | | | 0.5 | | | | 1,015.6 | |
| Distribution and selling costs | | | (401.8 | ) | | | — | | | | (401.8 | ) |
| Administrative expenses | | | (358.6 | ) | | | (9.2 | ) | | | (367.8 | ) |
| | | |
| | | |
| | | |
| |
Group operating profit | | | 254.7 | | | | (8.7 | ) | | | 246.0 | |
| Share of operating profits of joint venture and associates | | | 26.1 | | | | — | | | | 26.1 | |
| | | |
| | | |
| | | |
| |
Total operating profit | | | 280.8 | | | | (8.7 | ) | | | 272.1 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | |
| | | Before exceptional | | | | | | | | |
| | | items | | Exceptional items | | Total |
| Total operating profit 2000 | | £m | | £m | | £m |
| |
| |
| |
|
| Turnover | | | 1,282.8 | | | | — | | | | 1,282.8 | |
| Cost of sales | | | (431.0 | ) | | | (10.9 | ) | | | (441.9 | ) |
| | | |
| | | |
| | | |
| |
Gross profit | | | 851.8 | | | | (10.9 | ) | | | 840.9 | |
| Distribution and selling costs | | | (365.9 | ) | | | (3.4 | ) | | | (369.3 | ) |
| Administrative expenses | | | (285.2 | ) | | | (12.7 | ) | | | (297.9 | ) |
| | | |
| | | |
| | | |
| |
Group operating profit | | | 200.7 | | | | (27.0 | ) | | | 173.7 | |
| Share of operating profits of joint venture and associates | | | 29.8 | | | | — | | | | 29.8 | |
| | | |
| | | |
| | | |
| |
Total operating profit | | | 230.5 | | | | (27.0 | ) | | | 203.5 | |
| | | |
| | | |
| | | |
| |
F-18
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Total operating profit is arrived at after charging: | | | | | | | | | | | | |
| Remuneration of the Group’s auditors | | | | | | | | | | | | |
| - Audit fees | | | 1.2 | | | | 1.3 | | | | 1.6 | |
| - Other services to the Group | - in the UK | | | 0.4 | | | | 0.2 | | | | 0.1 | |
| | - in rest of world (primarily related to taxation) | | | 1.4 | | | | 0.2 | | | | 0.4 | |
| Operating lease rentals | | | | | | | | | | | | |
| - Hire of plant and machinery | | | 12.2 | | | | 13.9 | | | | 14.5 | |
| - Hire of land and buildings and other fixed assets | | | 11.5 | | | | 14.3 | | | | 15.1 | |
| Amortization of government grants | | | (0.3 | ) | | | (0.2 | ) | | | (0.2 | ) |
| Depreciation of tangible fixed assets | | | | | | | | | | | | |
| - Owned | | | 50.0 | | | | 61.1 | | | | 72.0 | |
| - Held under finance leases | | | 0.5 | | | | 0.4 | | | | 0.3 | |
| Amortization of intangible fixed assets | | | | | | | | | | | | |
| - Goodwill | | | 10.7 | | | | 11.7 | | | | 36.6 | |
| - Other intangibles | | | 10.7 | | | | 6.8 | | | | 7.7 | |
| Loss on disposal of tangible fixed assets | | | 1.1 | | | | 0.3 | | | | 1.5 | |
| | | |
| | | |
| | | |
| |
Research and development costs included in administrative expenses of £184.2m (2001 — £173.8m, 2000 — £149.0m), relate to Amersham Health £95.1m (2001 — £83.0m, 2000 — £73.9m), Amersham Biosciences £88.0m (2001 — £86.4m, 2000 — £71.5m), and Corporate and other £1.1m (2001 — £4.4m, 2000 — £3.6m).
Goodwill amortization charged to administrative expenses in 2002 is £36.6m (2001 — £11.7m, 2000 — £10.7m).
5. DIRECTORS’ EMOLUMENTS
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Basic salary | | | 1.3 | | | | 1.4 | | | | 2.2 | |
| Fees | | | 0.4 | | | | 0.4 | | | | 0.5 | |
| Benefits | | | 0.2 | | | | 0.3 | | | | 0.4 | |
| Annual bonuses | | | 0.9 | | | | 1.1 | | | | 1.2 | |
| Payments of pension contributions | | | 0.1 | | | | 0.2 | | | | 0.2 | |
| Compensation for loss of office | | | — | | | | — | | | | 0.8 | |
| Payments to past directors | | | 0.2 | | | | 0.4 | | | | 0.4 | |
| | | |
| | | |
| | | |
| |
| | | | 3.1 | | | | 3.8 | | | | 5.7 | |
| Gains made on exercise of share options and long term incentive shares | | | 0.4 | | | | 1.4 | | | | — | |
| | | |
| | | |
| | | |
| |
| | | | 3.5 | | | | 5.2 | | | | 5.7 | |
| | | |
| | | |
| | | |
| |
Detailed disclosures of Directors’ individual remuneration, pension entitlements, share options and long term incentives are given within Item 6 “Directors, Senior Management and Employees”.
F-19
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
6. EMPLOYEE INFORMATION
The weighted average number of persons (including Executive Directors) employed by the Group during the year is analyzed below by type of work:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | Number | | Number | | Number |
| | |
| |
| |
|
| Production and development | | | 4,201 | | | | 4,587 | | | | 4,962 | |
| Distribution and selling | | | 2,834 | | | | 3,140 | | | | 3,262 | |
| Administration | | | 1,720 | | | | 1,792 | | | | 1,827 | |
| | | |
| | | |
| | | |
| |
| | | | 8,755 | | | | 9,519 | | | | 10,051 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Group employment costs — all employees including Executive Directors | | | | | | | | | | | | |
| Aggregate gross wages and salaries | | | 301.1 | | | | 337.3 | | | | 376.2 | |
| Employers National Insurance contributions or foreign equivalents | | | 43.2 | | | | 55.3 | | | | 72.5 | |
| Employer’s pension costs under Group pension schemes | | | 17.9 | | | | 20.9 | | | | 27.1 | |
| | | |
| | | |
| | | |
| |
| Total direct costs of employment | | | 362.2 | | | | 413.5 | | | | 475.8 | |
| | | |
| | | |
| | | |
| |
7. NET INTEREST PAYABLE
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Interest payable and similar charges | | | | | | | | | | | | |
| On bank loans and overdrafts | | | (9.7 | ) | | | (3.5 | ) | | | (1.9 | ) |
| On other loans | | | (9.5 | ) | | | (13.3 | ) | | | (6.9 | ) |
| Unwinding of discount on decommissioning provision | | | (2.2 | ) | | | (2.1 | ) | | | (2.8 | ) |
| | | |
| | | |
| | | |
| |
| | | | (21.4 | ) | | | (18.9 | ) | | | (11.6 | ) |
Interest receivable and similar income | | | | | | | | | | | | |
| Interest on short term deposits and interest bearing investments | | | 11.4 | | | | 10.2 | | | | 4.5 | |
| | | |
| | | |
| | | |
| |
| Net interest payable | | | (10.0 | ) | | | (8.7 | ) | | | (7.1 | ) |
| | | |
| | | |
| | | |
| |
Included within interest payable and similar charges, relating to the unwind of the decommissioning provision, is £0.6m (2001 — £0.6m, 2000 — £0.6m), attributable to the Group’s joint venture in NMP.
F-20
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
8. TAX ON PROFIT ON ORDINARY ACTIVITIES
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | (restated) | | |
| | | £m | | £m | | £m |
| | |
| |
| |
|
UK corporation tax at 30% (2001 — 30%, 2000 — 30%) | | | | | | | | | | | | |
| Current | | | 11.9 | | | | 23.0 | | | | 4.0 | |
| Double tax relief | | | (11.1 | ) | | | (15.4 | ) | | | (8.2 | ) |
| | | |
| | | |
| | | |
| |
| | | | 0.8 | | | | 7.6 | | | | (4.2 | ) |
| Overseas taxation | | | 62.1 | | | | 69.3 | | | | 83.9 | |
| Over-provision in respect of prior years | | | (4.9 | ) | | | (0.6 | ) | | | (18.6 | ) |
| Share of joint venture and associates | | | 13.3 | | | | 11.7 | | | | 10.1 | |
| | | |
| | | |
| | | |
| |
| Total current tax | | | 71.3 | | | | 88.0 | | | | 71.2 | |
| | | |
| | | |
| | | |
| |
Deferred tax | | | | | | | | | | | | |
| Origination and reversal of timing differences | | | 8.8 | | | | 13.8 | | | | 7.1 | |
| Prior year deferred tax movement | | | 2.2 | | | | (4.8 | ) | | | 16.0 | |
| | | |
| | | |
| | | |
| |
| | | | 11.0 | | | | 9.0 | | | | 23.1 | |
| | | |
| | | |
| | | |
| |
| Tax before exceptional items | | | 82.3 | | | | 97.0 | | | | 94.3 | |
| Exceptional items | | | (2.4 | ) | | | (0.5 | ) | | | (9.2 | ) |
| | | |
| | | |
| | | |
| |
| | | | 79.9 | | | | 96.5 | | | | 85.1 | |
| | | |
| | | |
| | | |
| |
The Group’s tax charge for the 12 months ended December 31, 2002 has benefited from a net over-provision in respect of prior years of £2.6m (net movement between current and deferred tax). This is principally as a result of the favorable settlement of earlier years’ taxation issues. Furthermore, as a result of the purchase of the 45% minority interest in Amersham Biosciences in the 12 months ended December 31, 2002 and the subsequent formation of a US consolidated tax group, it has been possible to recognize certain tax losses in the US. The deferred tax benefit arising has been treated as an exceptional item.
Profit on ordinary activities before taxes as shown in the consolidated profit and loss account is analyzed over its component parts as follows:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| United Kingdom | | | 10.3 | | | | 12.8 | | | | 13.5 | |
| Overseas | | | 183.2 | | | | 301.5 | | | | 250.2 | |
| | | |
| | | |
| | | |
| |
| | | | 193.5 | | | | 314.3 | | | | 263.7 | |
| | | |
| | | |
| | | |
| |
At December 31, 2002, certain worldwide subsidiaries had tax loss carry-forwards, the combined tax effect of which is approximately £25.7m. These carry forward amounts expire as follows:
| | | | | |
| Year | | £m |
| |
|
| 2003 | | | — | |
| 2004 | | | — | |
| 2005 | | | 0.5 | |
| 2006 | | | 1.2 | |
| 2007 | | | 0.3 | |
| 2008 and thereafter | | | 23.7 | |
| | | |
| |
| Total | | | 25.7 | |
| | | |
| |
F-21
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Reconciliation of current tax charge to statuto rate on profits
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | (restated) | | |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Profit on ordinary activities before tax before exceptional items | | | 220.5 | | | | 267.7 | | | | 263.7 | |
| Profit on ordinary activities multiplied by the standard rate of corporation tax in the UK of 30% (2001 — 30%, 2000 — 30%) | | | 66.2 | | | | 80.3 | | | | 79.1 | |
Effects of: | | | | | | | | | | | | |
| Adjustments in respect of prior period | | | (4.9 | ) | | | (0.6 | ) | | | (18.6 | ) |
| Adjustments in respect of foreign tax rates | | | 3.5 | | | | 4.6 | | | | 7.4 | |
Expenses not deductible for tax purposes: | | | | | | | | | | | | |
| Non tax deductible goodwill | | | 3.8 | | | | 5.1 | | | | 11.7 | |
| Other | | | 2.3 | | | | 0.8 | | | | 1.7 | |
Timing differences: | | | | | | | | | | | | |
| Capital allowances in excess of depreciation | | | (0.2 | ) | | | (0.8 | ) | | | (8.0 | ) |
| Tax deductible goodwill | | | (3.8 | ) | | | (3.8 | ) | | | (3.8 | ) |
| Unrealized stock profit | | | 6.7 | | | | (0.1 | ) | | | 4.8 | |
| Research and development credits | | | (5.0 | ) | | | (3.9 | ) | | | (3.9 | ) |
| Unutilized tax losses | | | 2.7 | | | | 6.4 | | | | 0.8 | |
| | | |
| | | |
| | | |
| |
| | | | 71.3 | | | | 88.0 | | | | 71.2 | |
| Exceptional items | | | (2.4 | ) | | | (0.5 | ) | | | — | |
| | | |
| | | |
| | | |
| |
| | | | 68.9 | | | | 87.5 | | | | 71.2 | |
| | | |
| | | |
| | | |
| |
9. DIVIDENDS PAID AND PROPOSED
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Paid — interim dividend of 2.65p per share
(2001 — 2.35p; 2000 — 2.15p) | | | 13.5 | | | | 14.9 | | | | 21.3 | |
Proposed — final dividend of 5.15p per share
(2001 — 4.75p; 2000 — 4.25p) | | | 26.9 | | | | 30.3 | | | | 36.0 | |
| | | |
| | | |
| | | |
| |
| | | | 40.4 | | | | 45.2 | | | | 57.3 | |
| | | |
| | | |
| | | |
| |
Dividends paid of £21.3m for the 12 months to December 31, 2002, contains £2.9m relating to the final 2001 dividend paid to holders of the 57.5m new ordinary shares which were issued by means of a placing on March 18, 2002.
A final dividend of 5.15p per share is proposed, bringing the total dividend for the year to 7.8p per share.
F-22
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
10. EARNINGS PER ORDINARY SHARE
Basic earnings per ordinary share is calculated by dividing the earnings attributable to ordinary shareholders by the weighted average number of ordinary shares in issue in the year, excluding those held by employee share trusts. For diluted earnings per ordinary share, the weighted average number of ordinary shares is adjusted to assume conversion of all potentially dilutive ordinary shares.
The calculation of basic and diluted earnings per ordinary share and earnings per ordinary share before exceptional items and goodwill amortization is based on the following attributable profit and weighted average number of shares.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | (restated) | | |
| | |
| |
| |
|
| | | £m | | p | | £m | | p | | £m | | p |
| | |
| |
| |
| |
| |
| |
|
Attributable profit | | | | | | | | | | | | | | | | | | | | | | | | |
| Profit attributable to shareholders — basic | | | 107.0 | | | | 17.0 | | | | 210.7 | | | | 33.2 | | | | 178.7 | | | | 26.1 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Add back the following items: | | | | | | | | | | | | | | | | | | | | | | | | |
| Exceptional items | | | 27.0 | | | | 4.3 | | | | (46.6 | ) | | | (7.3 | ) | | | — | | | | — | |
| Goodwill amortization | | | 10.7 | | | | 1.7 | | | | 11.7 | | | | 1.9 | | | | 36.6 | | | | 5.3 | |
Adjust for related amounts of: | | | | | | | | | | | | | | | | | | | | | | | | |
| Taxation | | | (2.4 | ) | | | (0.4 | ) | | | (0.5 | ) | | | (0.1 | ) | | | (9.2 | ) | | | (1.3 | ) |
| Minority interest | | | (4.2 | ) | | | (0.7 | ) | | | (6.7 | ) | | | (1.1 | ) | | | (1.3 | ) | | | (0.2 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Profit attributable to shareholders before exceptional items and goodwill amortization | | | 138.1 | | | | 21.9 | | | | 168.6 | | | | 26.6 | | | | 204.8 | | | | 29.9 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
|
Average number of shares | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 630.9m | | | | | | | | 634.4m | | | | | | | | 684.7m | | | | | |
| Dilution effect of outstanding share options | | | 7.4m | | | | | | | | 4.3m | | | | | | | | 4.0m | | | | | |
| | | |
| | | | | | | |
| | | | | | | |
| | | | | |
| Diluted | | | 638.3m | | | | | | | | 638.7m | | | | | | | | 688.7m | | | | | |
| | | |
| | | | | | | |
| | | | | | | |
| | | | |
Earnings per ordinary share | | | | | | | | | | | | | | | | | | | | | | | | |
| - Basic | | | | | | | 17.0p | | | | | | | | 33.2p | | | | | | | | 26.1p | |
| - Before exceptional items and goodwill amortization | | | | | | | 21.9p | | | | | | | | 26.6p | | | | | | | | 29.9p | |
| - Diluted (after exceptional items and goodwill amortization) | | | | | | | 16.8p | | | | | | | | 33.0p | | | | | | | | 25.9p | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Earnings per ordinary share before exceptional items and goodwill amortization are presented in order to show the impact on earnings of non-recurring items and goodwill.
F-23
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
11. INTANGIBLE FIXED ASSETS
| | | | | | | | | | | | | |
| | | Goodwill | | Other Intangibles | | Total |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Cost | | | | | | | | | | | | |
| At December 31, 2000 | | | 137.2 | | | | 61.2 | | | | 198.4 | |
| Foreign currency translation | | | 3.2 | | | | 0.1 | | | | 3.3 | |
| Additions | | | 6.5 | | | | 12.0 | | | | 18.5 | |
| Disposals | | | — | | | | (0.3 | ) | | | (0.3 | ) |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 146.9 | | | | 73.0 | | | | 219.9 | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | (54.6 | ) | | | 11.5 | | | | (43.1 | ) |
| Acquisitions of subsidiaries | | | — | | | | 3.9 | | | | 3.9 | |
| Goodwill subsumed on purchase of 45% minority interest in Amersham Biosciences | | | (79.3 | ) | | | — | | | | (79.3 | ) |
| Additions | | | 744.5 | | | | 6.4 | | | | 750.9 | |
| Disposals | | | — | | | | (6.3 | ) | | | (6.3 | ) |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 757.5 | | | | 88.5 | | | | 846.0 | |
| | | |
| | | |
| | | |
| |
Amortization | | | | | | | | | | | | |
| At December 31, 2000 | | | 23.8 | | | | 25.1 | | | | 48.9 | |
| Foreign currency translation | | | (0.2 | ) | | | — | | | | (0.2 | ) |
| Disposals | | | — | | | | — | | | | — | |
| Charge for the year | | | 11.7 | | | | 6.8 | | | | 18.5 | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 35.3 | | | | 31.9 | | | | 67.2 | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | (3.2 | ) | | | 6.4 | | | | 3.2 | |
| Disposals | | | — | | | | (4.5 | ) | | | (4.5 | ) |
| Amortization subsumed on purchase of 45% minority interest in Amersham Biosciences | | | (15.4 | ) | | | — | | | | (15.4 | ) |
| Charge for the year | | | 36.6 | | | | 7.7 | | | | 44.3 | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 53.3 | | | | 41.5 | | | | 94.8 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | |
Net book value | | | | | | | | | | | | |
At December 31, 2002 | | | 704.2 | | | | 47.0 | | | | 751.2 | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 111.6 | | | | 41.1 | | | | 152.7 | |
| | | |
| | | |
| | | |
| |
Additions to goodwill during the year to December 31, 2002 amounted to £744.5m; £692.3m arose on the purchase of the 45% minority interest in Amersham Biosciences, (including 45% of Amersham Biosciences goodwill of £63.9m), and £52.2m arose on other acquisitions see note 24.
Additions to goodwill during the year to December 31, 2001 primarily arose on the acquisition of Dan-Process A/S.
Additions to other intangibles of £6.4m (2001 — £12.0m) comprise a number of patents acquired and license agreements entered into by the Group.
F-24
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
12. TANGIBLE FIXED ASSETS
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Plant | | Fixtures, | | Assets | | | | |
| | | Land and | | and | | fittings tools | | under | | | | |
| | | buildings | | machinery | | and equipment | | construction | | Total |
| | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
|
Cost | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2000 | | | 249.8 | | | | 232.2 | | | | 122.9 | | | | 72.5 | | | | 677.4 | |
| Foreign currency translation | | | (1.0 | ) | | | (2.1 | ) | | | (1.7 | ) | | | 0.1 | | | | (4.7 | ) |
| Transfers | | | 9.9 | | | | 21.6 | | | | 16.3 | | | | (47.8 | ) | | | — | |
| Additions | | | 9.3 | | | | 22.2 | | | | 30.3 | | | | 32.7 | | | | 94.5 | |
| Disposals | | | (8.2 | ) | | | (22.6 | ) | | | (17.7 | ) | | | (0.4 | ) | | | (48.9 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 259.8 | | | | 251.3 | | | | 150.1 | | | | 57.1 | | | | 718.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | 9.7 | | | | 14.3 | | | | 0.7 | | | | 3.2 | | | | 27.9 | |
| Adjustment relating to fair value | | | 10.2 | | | | 3.2 | | | | — | | | | — | | | | 13.4 | |
| Acquisition of subsidiaries | | | — | | | | 3.4 | | | | 2.8 | | | | — | | | | 6.2 | |
| Transfers | | | 12.1 | | | | 18.2 | | | | 2.2 | | | | (32.5 | ) | | | — | |
| Transfers to current assets | | | — | | | | — | | | | (2.4 | ) | | | — | | | | (2.4 | ) |
| Additions | | | 12.7 | | | | 17.2 | | | | 28.1 | | | | 93.3 | | | | 151.3 | |
| Disposals | | | (1.2 | ) | | | (13.7 | ) | | | (13.3 | ) | | | — | | | | (28.2 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 303.3 | | | | 293.9 | | | | 168.2 | | | | 121.1 | | | | 886.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
Depreciation | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2000 | | | 47.0 | | | | 75.5 | | | | 31.4 | | | | — | | | | 153.9 | |
| Foreign currency translation | | | (2.7 | ) | | | (1.5 | ) | | | (1.1 | ) | | | — | | | | (5.3 | ) |
| Disposals | | | (2.2 | ) | | | (9.8 | ) | | | (12.9 | ) | | | — | | | | (24.9 | ) |
| Charge for the year | | | 11.8 | | | | 22.1 | | | | 27.6 | | | | — | | | | 61.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 53.9 | | | | 86.3 | | | | 45.0 | | | | — | | | | 185.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | 3.9 | | | | 8.2 | | | | 1.9 | | | | — | | | | 14.0 | |
| Transfers | | | — | | | | 0.7 | | | | (0.7 | ) | | | — | | | | — | |
| Transfers to current assets | | | — | | | | — | | | | (1.5 | ) | | | — | | | | (1.5 | ) |
| Disposals | | | (1.7 | ) | | | (10.9 | ) | | | (11.7 | ) | | | — | | | | (24.3 | ) |
| Charge for the year | | | 12.4 | | | | 27.2 | | | | 32.7 | | | | — | | | | 72.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 68.5 | | | | 111.5 | | | | 65.7 | | | | — | | | | 245.7 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
Net book value | | | | | | | | | | | | | | | | | | | | |
At December 31, 2002 | | | 234.8 | | | | 182.4 | | | | 102.5 | | | | 121.1 | | | | 640.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 205.9 | | | | 165.0 | | | | 105.1 | | | | 57.1 | | | | 533.1 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The net book value of the Group’s freehold land and buildings is £232.6m (2001 — £203.6m). Included in the cost of land and buildings is £3.2m (2001 — £3.0m), plant and machinery £nil, (2001 — £nil), and £0.8m (2001 — £0.8m) in fixtures, fittings, tools and equipment, in respect of assets held under finance leases for the Group. Depreciation on assets held under finance leases amounted to £0.3m (2001 — £0.4m). Accumulated depreciation on assets held under finance leases amounted to £1.4m in 2002 (2001 — £1.8m).
The £0.9m (2001 — £nil) net book value transferred to current assets relates to demonstration equipment reclassified to within finished goods stock.
F-25
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
13. FIXED ASSET INVESTMENTS
| | | | | | | | | | | | | | | | | | | | | |
| | | Investment in | | Joint venture | | Associates | | Trade | | Total |
| | | own shares | | | | | | investments | | |
| | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
|
Cost or valuation | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2000 | | | 18.6 | | | | 66.1 | | | | 3.8 | | | | 18.4 | | | | 106.9 | |
| Foreign currency translation | | | 0.8 | | | | (7.4 | ) | | | (0.1 | ) | | | (0.3 | ) | | | (7.0 | ) |
| Transfers to employees | | | (3.0 | ) | | | — | | | | — | | | | — | | | | (3.0 | ) |
| Additions | | | 4.9 | | | | — | | | | — | | | | 7.3 | | | | 12.2 | |
| Disposals | | | — | | | | — | | | | — | | | | (6.0 | ) | | | (6.0 | ) |
| Share of retained profit | | | — | | | | 8.4 | | | | — | | | | — | | | | 8.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 21.3 | | | | 67.1 | | | | 3.7 | | | | 19.4 | | | | 111.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | (0.9 | ) | | | (0.6 | ) | | | — | | | | (0.9 | ) | | | (2.4 | ) |
| Transfers to employees | | | (3.9 | ) | | | — | | | | — | | | | — | | | | (3.9 | ) |
| Fair value adjustments relating to the purchase of Amersham Biosciences | | | — | | | | — | | | | — | | | | (1.2 | ) | | | (1.2 | ) |
| Transfer to subsidiary | | | — | | | | — | | | | — | | | | (2.6 | ) | | | (2.6 | ) |
| Additions | | | 1.8 | | | | — | | | | — | | | | 3.4 | | | | 5.2 | |
| Disposals | | | — | | | | — | | | | — | | | | (5.3 | ) | | | (5.3 | ) |
| Share of retained profit | | | — | | | | 6.5 | | | | — | | | | — | | | | 6.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 18.3 | | | | 73.0 | | | | 3.7 | | | | 12.8 | | | | 107.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
Provision | | | | | | | | | | | | | | | | | | | | |
| At December 31, 2000 | | | 0.9 | | | | — | | | | 1.5 | | | | 0.8 | | | | 3.2 | |
| Charge for the year | | | 0.6 | | | | — | | | | 1.2 | | | | 4.4 | | | | 6.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 1.5 | | | | — | | | | 2.7 | | | | 5.2 | | | | 9.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Foreign currency translation | | | — | | | | — | | | | — | | | | (0.2 | ) | | | (0.2 | ) |
| Disposals | | | — | | | | — | | | | — | | | | (3.2 | ) | | | (3.2 | ) |
| Charge for the year | | | 0.4 | | | | — | | | | 1.0 | | | | 1.0 | | | | 2.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 1.9 | | | | — | | | | 3.7 | | | | 2.8 | | | | 8.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Net book value | | | | | | | | | | | | | | | | | | | | |
At December 31, 2002 | | | 16.4 | | | | 73.0 | | | | — | | | | 10.0 | | | | 99.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 19.8 | | | | 67.1 | | | | 1.0 | | | | 14.2 | | | | 102.1 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Investment in own shares
Investment in own shares is held through a Qualifying Employee Share Ownership Trust (QUEST), through the Amersham ESOP Trust (A ESOP), and by the Amersham US Stock Option Plan Trust 2000.
Shares held by the QUEST are issued by the Company and held on trust for employees. The shares are transferred to employees on the exercise of options under an authorized Save As You Earn Scheme (Sharesave)
Shares held by A ESOP and the Amersham US Stock Option Plan Trust 2000 are purchased in the market using funds advanced by the Company and are held on trust for the employees eligible under the Company’s long term incentive plan, cash options under the Amersham Executive Share Option Scheme 1993 and options under the Amersham US Stock Option Plan 2000.
The cost of the funds advanced to A ESOP that relate to the Company’s long term incentive plans (LTIP) is amortized in the profit and loss account over the three-year life of the scheme.
770,057 shares are held by the QUEST, with a market value at December 31, 2002 of £4.3m and a nominal value of £0.04m.
F-26
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
545,282 shares are held by the A ESOP, with a market valuation at December 31, 2002 of £3.0m and a nominal value of £0.03m.
2,251,315 shares are held by the Amersham US Stock Option Plan Trust 2000 with a market valuation at December 31, 2002 of £12.5m and a nominal value of £0.1m.
Dividends have been partially waived for all schemes. 100% of the shares held by the AESOP and the Amersham US Stock Option Plan Trust 2000 are under option to employees. None of the QUEST shares are under option to employees.
Transfer to subsidiary
The £2.6m transfer to subsidiary relates to the existing trade investment in Cimarron Software Inc, which became a subsidiary undertaking in the year.
Market value of listed investments
The market value of listed investments at December 31, 2002 is £3.6m (2001 — £4.9m) and the carrying value after provisions is £5.5m (2001 — £6.3m).
14. STOCKS
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Raw materials and consumables | | | 46.5 | | | | 53.0 | |
| Work in progress | | | 45.5 | | | | 56.7 | |
| Finished goods and goods for resale | | | 88.6 | | | | 98.3 | |
| | | |
| | | |
| |
| | | | 180.6 | | | | 208.0 | |
| | | |
| | | |
| |
The replacement cost of stocks is not materially different from original cost.
15. DEBTORS — AMOUNTS DUE WITHIN ONE YEAR
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | |
| | | £m | | £m |
| | |
| |
|
| Trade debtors | | | 298.2 | | | | 301.7 | |
| Amounts owed by joint venture and associates | | | 1.3 | | | | 1.0 | |
| Other debtors | | | 34.7 | | | | 21.2 | |
| Prepayments and accrued income | | | 26.6 | | | | 25.3 | |
| | | |
| | | |
| |
| | | | 360.8 | | | | 349.2 | |
| | | |
| | | |
| |
Trade debtors are shown net of the provision for doubtful debts of £7.4m (2001 — £8.1m).
F-27
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
16. DEBTORS — AMOUNTS DUE AFTER ONE YEAR
| | | | | | | | | |
| | | Dec 31, 01 | | |
| | | (restated) | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Other debtors | | | 16.7 | | | | 17.3 | |
| Prepayments and accrued income | | | 8.7 | | | | 11.1 | |
| Deferred taxation assets | | | 30.0 | | | | 32.9 | |
| | | |
| | | |
| |
| | | | 55.4 | | | | 61.3 | |
| | | |
| | | |
| |
17. SHORT TERM DEPOSITS AND INTEREST BEARING INVESTMENTS
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Short term deposits included in net debt (see note 33) | | | 96.5 | | | | 22.1 | |
| Loan notes | | | 2.9 | | | | — | |
| | | |
| | | |
| |
| | | | 99.4 | | | | 22.1 | |
| | | |
| | | |
| |
18. CREDITORS — AMOUNTS DUE WITHIN ONE YEAR
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
Loans | | | | | | | | |
| Short term loan from joint venture and associates | | | — | | | | 26.2 | |
| Bank loans and overdrafts | | | 14.8 | | | | 16.2 | |
| Finance lease obligations | | | 0.3 | | | | 0.3 | |
| Unsecured loan notes | | | 15.7 | | | | 1.9 | |
| | | |
| | | |
| |
| | | | 30.8 | | | | 44.6 | |
| | | |
| | | |
| |
Other creditors | | | | | | | | |
| Trade creditors | | | 86.4 | | | | 71.2 | |
| Amounts owed to joint venture and associates | | | 0.1 | | | | 0.1 | |
| United Kingdom corporation tax | | | 9.9 | | | | 6.1 | |
| Overseas subsidiary taxation | | | 108.1 | | | | 128.9 | |
| Other taxes and social security | | | 19.7 | | | | 27.0 | |
| Other creditors | | | 42.9 | | | | 63.0 | |
| Accruals and deferred income | | | 155.1 | | | | 149.9 | |
| Dividends payable | | | 30.3 | | | | 36.0 | |
| | | |
| | | |
| |
| | | | 452.5 | | | | 482.2 | |
| | | |
| | | |
| |
| | | | 483.3 | | | | 526.8 | |
| | | |
| | | |
| |
F-28
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
19. CREDITORS — AMOUNTS DUE AFTER ONE YEAR
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Bank loans and overdrafts | | | 8.7 | | | | 68.5 | |
| Finance lease obligations | | | 2.7 | | | | 2.5 | |
| Unsecured loan notes | | | 136.6 | | | | 121.5 | |
| | | |
| | | |
| |
| | | | 148.0 | | | | 192.5 | |
| Other creditors | | | 6.4 | | | | 11.5 | |
| | | |
| | | |
| |
| | | | 154.4 | | | | 204.0 | |
| | | |
| | | |
| |
| Repayments are due as follows: | | | | | | | | |
| Between one and two years | | | 7.9 | | | | 108.6 | |
| Between two and three years | | | 118.6 | | | | 16.8 | |
| Between three and four years | | | 14.6 | | | | 0.9 | |
| Between four and five years | | | 1.1 | | | | 66.6 | |
| In five years or more | | | 12.2 | | | | 11.1 | |
| | | |
| | | |
| |
| | | | 154.4 | | | | 204.0 | |
| | | |
| | | |
| |
The Company has US$193.0m (2001 — US$213.0m) equivalent to £123.4m (2001 — £152.3m) of private placement unsecured loan notes (see note 20). The carrying value of these notes includes a fair value adjustment of £3.5m (2001 — £5.4m). The fair value adjustment arose on the acquisition of the notes at the time of the merger with Nycomed. Of the carrying value, £1.9m (2001 — £15.7m) is reported in amounts due within one year.
20. DERIVATIVES AND OTHER FINANCIAL INSTRUMENTS
A discussion of the Group’s objectives and policies with regard to the use of financial instruments is found in the Treasury Policy and Liquidity section of Item 5 “Operating and Financial Review and Prospects”. Where permitted by FRS13, disclosures in this note exclude short term debtors and creditors.
(a) Currency and interest rate composition of financial liabilities of the Group
The following table analyses the currency and interest rate composition of the Group’s financial liabilities at December 31 after taking into account interest rate and currency swaps. The financial liabilities comprise bank loans and overdrafts plus any long term contractual obligations to deliver cash or other financial assets to third parties.
F-29
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | | | | | | | | | Non- | | | | | | | | | | | | | | Non- | | | | |
| | | Fixed | | Floating | | interest | | | | | | Fixed | | Floating | | interest | | | | |
| | | rate | | rate | | bearing | | Total | | rate | | rate | | bearing | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Sterling | | | 0.3 | | | | 15.6 | | | | 0.1 | | | | 16.0 | | | | 42.6 | | | | 4.2 | | | | — | | | | 46.8 | |
| US dollars | | | 99.2 | | | | 77.7 | | | | 5.1 | | | | 182.0 | | | | 135.5 | | | | 17.8 | | | | 5.2 | | | | 158.5 | |
| Euro | | | 0.8 | | | | 2.6 | | | | 0.2 | | | | 3.6 | | | | — | | | | 2.6 | | | | 0.2 | | | | 2.8 | |
| Danish krone | | | 1.4 | | | | 0.3 | | | | 0.8 | | | | 2.5 | | | | 3.6 | | | | 0.1 | | | | 0.8 | | | | 4.5 | |
| Norwegian kroner | | | 0.4 | | | | — | | | | 2.8 | | | | 3.2 | | | | 0.5 | | | | 0.4 | | | | — | | | | 0.9 | |
| Swedish krona | | | — | | | | 0.3 | | | | — | | | | 0.3 | | | | — | | | | — | | | | — | | | | — | |
| Other currencies | | | 13.3 | | | | 0.2 | | | | — | | | | 13.5 | | | | 11.3 | | | | 0.8 | | | | 0.2 | | | | 12.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 115.4 | | | | 96.7 | | | | 9.0 | | | | 221.1 | | | | 193.5 | | | | 25.9 | | | | 6.4 | | | | 225.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Within financial liabilities of £221.1m (2001 — £225.8), £210.9m (2001 - £178.8m) is included within net debt, £nil (2001 — £42.6m) represents the minority interest share of non-equity redeemable cumulative preference shares of Amersham Biosciences, and £10.2m (2001 — £4.4m) represents other financial liabilities. In addition a short term loan from the Group’s joint venture of £26.2m (2001 — £nil) is included within the analysis of net debt.
The average interest rates for the financial liabilities of the Group at December 31, together with the periods for which the rates were fixed, are set out in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
|
| | | | | | | Average years to | | | | | | Average years to |
| | | Average interest | | | | maturity of | | | | | | maturity of |
| | | rate of fixed rate | | Average years to | | non-interest | | Average interest | | Average years to | | non-interest |
| | | liabilities | | maturity of fixed | | bearing financial | | rate of fixed rate | | maturity of fixed | | bearing financial |
| | | % | | rate liabilities | | liabilities | | % | | rate liabilities | | liabilities |
| | |
| |
| |
| |
| |
| |
|
| Sterling | | | 5.0 | | | | 0.0 | | | | 1.5 | | | | 8.0 | | | | n/a | | | | — | |
| US dollars | | | 8.6 | | | | 2.4 | | | | 0.0 | | | | 7.3 | | | | 3.2 | | | | 0.0 | |
| Euro | | | 3.4 | | | | 6.4 | | | | 0.5 | | | | — | | | | — | | | | 1.3 | |
| Danish krone | | | 6.0 | | | | 1.2 | | | | 2.7 | | | | 6.0 | | | | 0.5 | | | | 3.7 | |
| Norwegian kroner | | | 3.9 | | | | 1.5 | | | | 2.4 | | | | 4.3 | | | | 1.1 | | | | — | |
| Other currencies | | | 5.5 | | | | 1.2 | | | | — | | | | 5.9 | | | | 2.3 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 4.2 | | | | 1.5 | | | | 0.9 | | | | 7.3 | | | | 2.4 | | | | 0.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Financial liabilities are analysed as:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | | | | | | | | | Non- | | | | | | | | | | | | | | Non- | | | | |
| | | Fixed | | Floating | | interest | | | | | | Fixed | | Floating | | interest | | | | |
| | | rate | | rate | | bearing | | Total | | rate | | rate | | bearing | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Bank loans and overdrafts | | | 15.3 | | | | 62.8 | | | | 6.6 | | | | 84.7 | | | | 12.2 | | | | 5.7 | | | | 5.6 | | | | 23.5 | |
| Finance lease obligations | | | — | | | | 2.8 | | | | — | | | | 2.8 | | | | — | | | | 3.0 | | | | — | | | | 3.0 | |
| Unsecured loan notes | | | 123.4 | | | | — | | | | — | | | | 123.4 | | | | 152.3 | | | | — | | | | — | | | | 152.3 | |
| Currency and interest rate swaps | | | (31.1 | ) | | | 31.1 | | | | — | | | | — | | | | (17.2 | ) | | | 17.2 | | | | — | | | | — | |
| Other financial liabilities | | | 7.8 | | | | — | | | | 2.4 | | | | 10.2 | | | | 3.6 | | | | — | | | | 0.8 | | | | 4.4 | |
| Non-equity minority interest | | | — | | | | — | | | | — | | | | — | | | | 42.6 | | | | — | | | | — | | | | 42.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 115.4 | | | | 96.7 | | | | 9.0 | | | | 221.1 | | | | 193.5 | | | | 25.9 | | | | 6.4 | | | | 225.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Floating rate interest on bank loans and overdrafts is based on local bank base rates, and the floating rate interest on swaps is based on LIBOR.
The Group’s principal fixed rate debt obligations are three series of US private placement unsecured loan notes with the following rates and maturities:
F-30
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | |
| | | Amount | | Interest Rate | | |
| Series | | $ m | | % | | Maturity |
| |
| |
| |
|
| Series B | | | 15 | | | | 8.79 | | | February 2005 |
| Series C | | | 163 | | | | 8.77 | | | November 2004 |
| Series D | | | 15 | | | | 8.97 | | | November 2009 |
The carrying value of Series C and D notes includes a fair value adjustment of £3.5m (2001 — £5.4m), which arose on the acquisition of the debt instruments at the time of the merger with Nycomed. The interest rate of US Dollar fixed rate liabilities is adjusted for the partial unwinding of this fair value adjustment during the periods reported.
(b) Currency and interest rate composition of financial assets of the group
The following tables analyse the currency and interest rate composition of the Group’s financial assets at 31 December. The financial assets comprise cash at bank, short term deposits, trade investments and interest bearing investments plus any long term contractual rights to receive cash or other financial assets from third parties.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | Non interest bearing | | Non interest bearing |
| | |
| |
|
| | | | | | | | | | | Equity | | | | | | | | | | | | | | | | | | Equity | | | | | | | | |
| | | Fixed | | Floating | | invest- | | | | | | | | | | Fixed | | Floating | | invest- | | | | | | | | |
| | | rate | | rate | | ments | | Other | | Total | | rate | | Rate | | ments | | Other | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
| Sterling | | | 0.2 | | | | 1.9 | | | | 0.1 | | | | 17.5 | | | | 19.7 | | | | 0.5 | | | | 3.4 | | | | — | | | | 2.3 | | | | 6.2 | |
| US dollars | | | 0.8 | | | | 7.9 | | | | 8.0 | | | | 7.4 | | | | 24.1 | | | | 0.9 | | | | 71.5 | | | | 11.8 | | | | 13.7 | | | | 97.9 | |
| Japanese yen | | | — | | | | 0.1 | | | | — | | | | 0.1 | | | | 0.2 | | | | — | | | | 7.5 | | | | — | | | | — | | | | 7.5 | |
| Euro | | | 0.6 | | | | 4.9 | | | | — | | | | 2.0 | | | | 7.5 | | | | 3.3 | | | | 19.8 | | | | — | | | | 2.4 | | | | 25.5 | |
| Norwegian kroner | | | 0.1 | | | | 6.5 | | | | — | | | | 0.1 | | | | 6.7 | | | | 18.9 | | | | 6.3 | | | | — | | | | — | | | | 25.2 | |
| Danish krone | | | — | | | | 0.5 | | | | — | | | | — | | | | 0.5 | | | | 0.7 | | | | 0.2 | | | | — | | | | — | | | | 0.9 | |
| Swedish krona | | | 0.1 | | | | 1.6 | | | | — | | | | 0.3 | | | | 2.0 | | | | — | | | | 0.7 | | | | 1.0 | | | | 0.8 | | | | 2.5 | |
| Other currencies | | | 0.7 | | | | 5.3 | | | | — | | | | 1.3 | | | | 7.3 | | | | 0.6 | | | | 8.3 | | | | — | | | | 0.9 | | | | 9.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 2.5 | | | | 28.7 | | | | 8.1 | | | | 28.7 | | | | 68.0 | | | | 24.9 | | | | 117.7 | | | | 12.8 | | | | 20.1 | | | | 175.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Within financial assets of £68.0m (2001 — £175.5m), £54.8m (2001 — £153.4m) is included within net debt and £13.2m (2001 — £22.1m), represents other financial assets.
The interest rates on the majority of floating rate financial assets are based on local market floating rates.
The average interest rates for the financial assets of the group at December 31, together with the period for which the rates were fixed, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
|
| | | | | | | Average years to | | | | | | Average years to |
| | | Average interest | | | | maturity of | | | | | | maturity of |
| | | rate of fixed rate | | Average years to | | non-interest | | Average interest | | Average years to | | non-interest |
| | | assets | | maturity of fixed | | bearing financial | | rate of fixed rate | | maturity of fixed | | bearing financial |
| | | % | | rate assets | | Assets | | % | | rate assets | | assets |
| | |
| |
| |
| |
| |
| |
|
| Sterling | | | 3.7 | | | | 0.0 | | | | 0.0 | | | | 3.7 | | | | 0.1 | | | | 0.1 | |
| US dollars | | | 3.9 | | | | 0.3 | | | | 0.4 | | | | 8.2 | | | | 1.8 | | | | 0.6 | |
| Japanese yen | | | — | | | | — | | | | 0.0 | | | | — | | | | — | | | | — | |
| Euro | | | 2.0 | | | | 0.0 | | | | 0.2 | | | | 6.8 | | | | 4.3 | | | | 0.0 | |
| Norwegian kroner | | | 2.8 | | | | 0.3 | | | | 0.8 | | | | 6.7 | | | | 0.1 | | | | — | |
| Danish krone | | | — | | | | — | | | | — | | | | 1.9 | | | | 8.9 | | | | 0.0 | |
| Swedish krona | | | 0.2 | | | | 0.0 | | | | 0.0 | | | | — | | | | — | | | | — | |
| Other currencies | | | 3.8 | | | | 0.0 | | | | 0.0 | | | | 1.8 | | | | 0.1 | | | | 0.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 3.3 | | | | 0.1 | | | | 0.1 | | | | 6.4 | | | | 1.0 | | | | 0.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
F-31
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Financial assets are analysed as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | | | | | | | | | Non- | | | | | | | | | | | | | | Non- | | | | |
| | | Fixed | | Floating | | interest | | | | | | Fixed | | Floating | | interest | | | | |
| | | rate | | rate | | bearing | | Total | | rate | | rate | | bearing | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Cash at bank and in hand | | | 1.7 | | | | 24.3 | | | | 6.7 | | | | 32.7 | | | | 1.2 | | | | 43.3 | | | | 12.4 | | | | 56.9 | |
| Short term deposits | | | 0.4 | | | | 4.4 | | | | 17.3 | | | | 22.1 | | | | 20.0 | | | | 74.4 | | | | 2.1 | | | | 96.5 | |
| Interest bearing investments | | | — | | | | — | | | | — | | | | — | | | | 2.9 | | | | — | | | | — | | | | 2.9 | |
| Equity investments | | | — | | | | — | | | | 8.1 | | | | 8.1 | | | | — | | | | — | | | | 12.8 | | | | 12.8 | |
| Other financial assets | | | 0.4 | | | | — | | | | 4.7 | | | | 5.1 | | | | 0.8 | | | | — | | | | 5.6 | | | | 6.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 2.5 | | | | 28.7 | | | | 36.8 | | | | 68.0 | | | | 24.9 | | | | 117.7 | | | | 32.9 | | | | 175.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
In addition to the financial liabilities and financial assets disclosed above, the Group has granted put options over shares in Corixa Corporation, Cimarron Software Inc, Imaging Research Inc. Imaging Research Solutions Ltd and Uppsala Research Imaging Solutions AB and holds call options over shares in Cimarron Software Inc., Imaging Research Inc, Imaging Research Solutions Ltd, Uppsala Research Imaging Solutions AB and Analytica of Branford which meet the definition of financial instruments. The maximum potential aggregate cash consideration under these contracts is $71.5 million and £7.2 million. These options expire by 2006.
(c) Maturity of financial liabilities
The maturity of the Group’s financial liabilities at December 31 is analysed as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Non-equity | | | | |
| | | Bank loans and | | Finance lease | | Unsecured | | Other financial | | minority | | |
| | | overdrafts | | obligations | | loan notes | | liabilities | | interest | | Total |
| December 31, 2002 | | £m | | £m | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
| |
| |
|
Analysis by year of repayment | | | | | | | | | | | | | | | | | | | | | | | | |
| Due within one year or on demand | | | 16.2 | | | | 0.3 | | | | 1.9 | | | | 1.5 | | | | — | | | | 19.9 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Between one and two years | | | 3.0 | | | | 0.3 | | | | 102.9 | | | | 1.5 | | | | — | | | | 107.7 | |
| Between two and five years | | | 65.2 | | | | 0.6 | | | | 9.8 | | | | 7.2 | | | | — | | | | 82.8 | |
| In five years or more | | | 0.3 | | | | 1.6 | | | | 8.8 | | | | — | | | | — | | | | 10.7 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Due after more than one year | | | 68.5 | | | | 2.5 | | | | 121.5 | | | | 8.7 | | | | — | | | | 201.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total repayments | | | 84.7 | | | | 2.8 | | | | 123.4 | | | | 10.2 | | | | — | | | | 221.1 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Non-equity | | | | |
| | | Bank loans and | | Finance lease | | Unsecured | | Other financial | | minority | | |
| | | overdrafts | | obligations | | loan notes | | liabilities | | interest | | Total |
| December 31, 2001 | | £m | | £m | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
| |
| |
|
| Analysis by year of repayment | | | | | | | | | | | | | | | | | | | | | | | | |
| Due within one year or on demand | | | 14.8 | | | | 0.3 | | | | 15.7 | | | | 2.2 | | | | — | | | | 33.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Between one and two years | | | 2.0 | | | | 0.3 | | | | 2.0 | | | | 0.7 | | | | — | | | | 5.0 | |
| Between two and five years | | | 6.7 | | | | 0.7 | | | | 124.7 | | | | 1.5 | | | | — | | | | 133.6 | |
| In five years or more | | | — | | | | 1.7 | | | | 9.9 | | | | — | | | | 42.6 | | | | 54.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Due after more than one year | | | 8.7 | | | | 2.7 | | | | 136.6 | | | | 2.2 | | | | 42.6 | | | | 192.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total repayments | | | 23.5 | | | | 3.0 | | | | 152.3 | | | | 4.4 | | | | 42.6 | | | | 225.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The Group has undrawn committed borrowing facilities available to it at December 31 as follows:
| | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | | £m | | £m |
| | |
| |
|
| Expiring within one year | | | — | | | | 430.0 | |
| Expiring between four years and five years | | | 404.3 | | | | — | |
| | | |
| | | |
| |
| | | | 404.3 | | | | 430.0 | |
| | | |
| | | |
| |
(d) Currency risk exposure
The following table shows the currency exposure of the net monetary assets and (liabilities) of Group companies at December 31.
F-32
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The exposures arise where monetary assets and liabilities are denominated in currencies other than the functional currency of the operating company in which they are held. The gains or losses on these monetary assets and liabilities are recorded in the profit and loss account.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Japanese | | | | | | Norwegian | | Swedish | | | | | | | | |
| | | Sterling | | US dollars | | yen | | Euro | | kroner | | krona | | Other | | Total |
| December 31, 2002 | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
| |
| |
| |
| |
|
Functional currency of operating company | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sterling | | | — | | | | 8.9 | | | | 1.8 | | | | (1.8 | ) | | | 7.6 | | | | (0.6 | ) | | | (3.2 | ) | | | 12.7 | |
| US dollars | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (0.1 | ) | | | (0.1 | ) |
| Japanese yen | | | (0.3 | ) | | | 1.6 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1.3 | |
| Euro | | | 0.9 | | | | 5.6 | | | | — | | | | — | | | | (1.2 | ) | | | — | | | | 0.3 | | | | 5.6 | |
| Norwegian kroner | | | 7.5 | | | | 1.8 | | | | (16.1 | ) | | | (6.5 | ) | | | — | | | | (0.1 | ) | | | (9.4 | ) | | | (22.8 | ) |
| Swedish krona | | | (3.8 | ) | | | (9.8 | ) | | | (1.8 | ) | | | 7.4 | | | | — | | | | — | | | | 0.2 | | | | (7.8 | ) |
| Other currencies | | | (0.1 | ) | | | (13.5 | ) | | | — | | | | (0.7 | ) | | | (5.0 | ) | | | (0.1 | ) | | | — | | | | (19.4 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 4.2 | | | | (5.4 | ) | | | (16.1 | ) | | | (1.6 | ) | | | 1.4 | | | | (0.8 | ) | | | (12.2 | ) | | | (30.5 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Japanese | | | | | | Norwegian | | Swedish | | | | | | | | |
| | | Sterling | | US dollars | | yen | | Euro | | kroner | | krona | | Other | | Total |
| December 31, 2001 | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| |
| |
| |
| |
| |
| |
| |
| |
|
| Functional currency of operating company | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sterling | | | — | | | | 15.3 | | | | (0.9 | ) | | | (11.9 | ) | | | 7.0 | | | | 9.7 | | | | (3.0 | ) | | | 16.2 | |
| US dollars | | | 3.4 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1.6 | | | | 5.0 | |
| Japanese yen | | | — | | | | (1.7 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1.7 | ) |
| Euro | | | (0.4 | ) | | | 3.7 | | | | — | | | | — | | | | 0.1 | | | | 0.3 | | | | 0.1 | | | | 3.8 | |
| Norwegian kroner | | | 3.0 | | | | (5.5 | ) | | | 2.7 | | | | 4.5 | | | | — | | | | (0.1 | ) | | | 0.4 | | | | 5.0 | |
| Swedish krona | | | 4.0 | | | | (11.6 | ) | | | 3.9 | | | | 3.3 | | | | (0.3 | ) | | | — | | | | (1.8 | ) | | | (2.5 | ) |
| Other currencies | | | — | | | | (3.2 | ) | | | — | | | | — | | | | 4.0 | | | | — | | | | — | | | | 0.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 10.0 | | | | (3.0 | ) | | | 5.7 | | | | (4.1 | ) | | | 10.8 | | | | 9.9 | | | | (2.7 | ) | | | 26.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
(e) Fair values and disclosures
The estimated book values and fair values of the Group’s financial instruments at December 31 are set out below:
Primary financial instruments held or issued to finance the Group’s operations
| | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | Book value | | Fair value | | Book value | | Fair value |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
Cash at bank1 | | | 32.7 | | | | 32.7 | | | | 56.9 | | | | 56.9 | |
Short term deposits1 | | | 22.1 | | | | 22.1 | | | | 96.5 | | | | 96.5 | |
Interest bearing investments1 | | | — | | | | — | | | | 2.9 | | | | 2.9 | |
Equity investments2 | | | 8.1 | | | | 6.2 | | | | 12.8 | | | | 11.4 | |
Other financial assets3 | | | 5.1 | | | | 5.1 | | | | 6.4 | | | | 6.6 | |
Bank loans and overdrafts1 | | | (84.7 | ) | | | (84.7 | ) | | | (23.5 | ) | | | (23.5 | ) |
Non-equity minority interest4 | | | — | | | | — | | | | (42.6 | ) | | | (42.6 | ) |
Finance lease obligations1 | | | (2.8 | ) | | | (2.8 | ) | | | (3.0 | ) | | | (3.0 | ) |
Other financial liabilities5 | | | (10.2 | ) | | | (10.2 | ) | | | (4.4 | ) | | | (4.4 | ) |
Unsecured loan notes6 | | | (123.4 | ) | | | (140.0 | ) | | | (152.3 | ) | | | (171.0 | ) |
| 1 | | The fair values of cash, short term deposits, interest bearing investments (excluding subordinated loan notes) and overdrafts approximate to carrying values due to the short maturity period of these financial assets. The difference between carrying value and fair value is also considered to be immaterial for floating rate bank loans and finance lease obligations. Interest bearing investments included subordinated loan notes that were valued by discounting the associated future cash flows to December 31 2001. The difference between carrying value and fair value is considered immaterial. |
| |
| 2 | | Equity investments represent equity holdings in trade investments. The fair value of listed investments is equal to the year end market price. |
F-33
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| 3 | | Other financial assets include investments in unit trusts and capital funds that are valued at market value. |
| |
| 4 | | The non-equity minority interest represents 45% of the 8% non-equity redeemable cumulative preference shares of Amersham Biosciences, which was extinguished in 2002 on the purchase of the 45% minority in Amersham Biosciences. |
| |
| 5 | | Other financial liabilities largely comprise discounted deferred consideration on acquisitions, for which book value is not materially different to fair value. |
| |
| 6 | | The book value of the unsecured loan notes includes a fair value adjustment of £3.5m (2001 — £5.4m), that arose as a result of the acquisition of the notes at the time of the merger with Nycomed. The fair value quoted at December 31 is based on the present value of future cash flows of the notes. |
Derivative financial instruments held to manage interest rate and currency exposures7
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Assets | | Liabilities | | Assets | | Liabilities |
| | | Dec 31, 2002 | | Dec 31, 2002 | | Dec 31, 2001 | | Dec 31, 2001 |
| | |
| |
| |
| |
|
| | | Notional | | Fair | | Notional | | Fair | | Notional | | Fair | | Notional | | Fair |
| | | principal | | value | | principal | | value | | principal | | value | | principal | | value |
| | | £m | | £m | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Interest rate swaps | | | 31.1 | | | | — | | | | (31.1 | ) | | | 3.2 | | | | 51.7 | | | | 3.2 | | | | (34.5 | ) | | | (1.3 | ) |
| Forward foreign exchange contracts | | | 923.4 | | | | 26.5 | | | | (890.6 | ) | | | 3.1 | | | | 900.6 | | | | 17.7 | | | | (873.1 | ) | | | (8.1 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total for financial assets | | | 954.5 | | | | 26.5 | | | | | | | | | | | | 952.3 | | | | 20.9 | | | | | | | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total for financial liabilities | | | | | | | | | | | (921.7 | ) | | | 6.3 | | | | | | | | | | | | (907.6 | ) | | | (9.4 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| 7 | | Interest rate swaps and forward foreign exchange contracts are stated at current market values. |
(f) Hedges
As explained in Item 5 “Operating and Financial Review and Prospects” on pages 62 — 90, the Group’s policy is to hedge exposures to interest rate risk and currency exposures using a variety of derivative financial instruments. Gains and losses on these instruments are not recognized until the exposure that is being hedged is itself recognized.
Gains or losses on the fair value of financial instruments are classified as unrecognized where they have not yet been recorded in the financial statements. Where gains or losses have crystallized, but are carried forward in the balance sheet pending their recognition in the profit and loss account in a future period, they are treated as deferred.
F-34
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table analyses the movement in unrecognized and deferred gains or losses on hedge instruments during the year to December 31, 2002.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 |
| | |
|
| | | Unrecognized | | Deferred |
| | |
| |
|
| | | | | | | | | | | Net | | | | | | | | | | Net |
| | | | | | | | | | | gains/ | | | | | | | | | | gains/ |
| | | Gains | | Losses | | (losses) | | Gains | | Losses | | (losses) |
| | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
|
Unrecognized gains/(losses) on hedges | | | | | | | | | | | | | | | | | | | | | | | | |
At January 1, 2002 | | | 13.8 | | | | (8.3 | ) | | | 5.5 | | | | 21.9 | | | | (10.8 | ) | | | 11.1 | |
| Gains/(losses) arising in previous years that were recognized in 2002 | | | (12.0 | ) | | | 8.3 | | | | (3.7 | ) | | | (12.7 | ) | | | 6.0 | | | | (6.7 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Gains/(losses) arising before January 1, 2003 that were not recognized in 2002 | | | 1.8 | | | | — | | | | 1.8 | | | | 9.2 | | | | (4.8 | ) | | | 4.4 | |
| Gains/(losses) arising in 2002 that were not recognized in 2002 | | | 17.5 | | | | (1.0 | ) | | | 16.5 | | | | 14.2 | | | | 1.0 | | | | 15.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Unrecognized gains/(losses) on hedges at December 31, 2002 | | | 19.3 | | | | (1.0 | ) | | | 18.3 | | | | 23.4 | | | | (3.8 | ) | | | 19.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Of which | | | | | | | | | | | | | | | | | | | | | | | | |
| Gains/(losses) expected to be recognized in 2003 | | | 17.9 | | | | (1.0 | ) | | | 16.9 | | | | 15.5 | | | | (3.8 | ) | | | 11.7 | |
| Gains/(losses) expected to be recognized in 2004 or later | | | 1.4 | | | | — | | | | 1.4 | | | | 7.9 | | | | — | | | | 7.9 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
As at December 31, the Company has outstanding foreign exchange contracts with commercial banks, as set out in the following table
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | Principal | | Principal | | | | | | Principal | | Principal | | | | |
| | | receivable | | payable | | Total | | receivable | | payable | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
|
| Held to manage assets and liabilities | | | 525.4 | | | | (507.9 | ) | | | 17.5 | | | | 425.8 | | | | (416.9 | ) | | | 8.9 | |
| Held to manage transaction exposures | | | 398.0 | | | | (382.7 | ) | | | 15.3 | | | | 474.8 | | | | (456.2 | ) | | | 18.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 923.4 | | | | (890.6 | ) | | | 32.8 | | | | 900.6 | | | | (873.1 | ) | | | 27.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The contracts held to manage assets and liabilities at 31 December can be analysed as follows
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | |
| |
|
| | | Principal | | Principal | | | | | | Principal | | Principal | | | | |
| | | receivable | | payable | | Total | | receivable | | payable | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
|
| US Dollars | | | 43.5 | | | | (250.1 | ) | | | (206.6 | ) | | | 41.5 | | | | (197.8 | ) | | | (156.3 | ) |
| Japanese yen | | | 2.9 | | | | (79.2 | ) | | | (76.3 | ) | | | 5.2 | | | | (99.9 | ) | | | (94.7 | ) |
| Sterling | | | 58.2 | | | | (110.1 | ) | | | (51.9 | ) | | | 52.3 | | | | (35.7 | ) | | | 16.6 | |
| Euros | | | 36.0 | | | | (15.1 | ) | | | 20.9 | | | | 99.7 | | | | (44.4 | ) | | | 55.3 | |
| Norwegian kroner | | | 372.6 | | | | (11.9 | ) | | | 360.7 | | | | 154.3 | | | | (15.3 | ) | | | 139.0 | |
| Other currencies | | | 12.2 | | | | (41.5 | ) | | | (29.3 | ) | | | 72.1 | | | | (23.8 | ) | | | 48.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 525.4 | | | | (507.9 | ) | | | 17.5 | | | | 425.1 | | | | (416.9 | ) | | | 8.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
F-35
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
21 FINANCIAL COMMITMENTS
The Group has commitments to make research and development milestone payments and payments under license agreements to third parties subject to achievement of agreed stages of product development. At December 31, 2002 the maximum amounts payable under these agreements was £10.2m (2001 — £24.2m).
22. PROVISIONS FOR LIABILITIES AND CHARGES
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Decomm- | | | | | | | | | | | | | | | | |
| | | | | | | issioning | | Pensions | | | | | | | | | | | | |
| | | Deferred | | and effluent | | and similar | | Disposal | | Other | | | | |
| | | tax | | costs | | obligations | | costs | | provisions | | Total |
| | | £m | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
| |
|
| At December 31, 2000 as previously stated | | | 20.6 | | | | 53.7 | | | | 81.9 | | | | 23.4 | | | | 23.3 | | | | 202.9 | |
| Foreign currency translation | | | (0.8 | ) | | | (0.1 | ) | | | (1.6 | ) | | | 0.1 | | | | — | | | | (2.4 | ) |
| Reclassification | | | — | | | | — | | | | (3.1 | ) | | | — | | | | 3.1 | | | | — | |
| Deferred tax asset in debtors at January 1, 2001 | | | (4.6 | ) | | | — | | | | — | | | | — | | | | — | | | | (4.6 | ) |
| Charged to profit and loss | | | 4.5 | | | | 12.4 | | | | 13.0 | | | | 8.8 | | | | 6.4 | | | | 45.1 | |
| Utilized during the year | | | — | | | | (2.8 | ) | | | (9.0 | ) | | | (12.6 | ) | | | (5.0 | ) | | | (29.4 | ) |
| Transfer of assets | | | — | | | | — | | | | 7.0 | | | | — | | | | — | | | | 7.0 | |
| Net transfer of deferred tax assets | | | 1.4 | | | | — | | | | — | | | | — | | | | — | | | | 1.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 | | | 21.1 | | | | 63.2 | | | | 88.2 | | | | 19.7 | | | | 27.8 | | | | 220.0 | |
| Prior year adjustment | | | 20.8 | | | | — | | | | — | | | | — | | | | — | | | | 20.8 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 as restated | | | 41.9 | | | | 63.2 | | | | 88.2 | | | | 19.7 | | | | 27.8 | | | | 240.8 | |
| Foreign currency translation | | | (0.5 | ) | | | 1.1 | | | | 0.2 | | | | (1.0 | ) | | | 0.2 | | | | — | |
| Transfer of deferred tax assets | | | 2.9 | | | | — | | | | — | | | | — | | | | — | | | | 2.9 | |
| Transfer to current tax creditor | | | (2.1 | ) | | | — | | | | — | | | | — | | | | — | | | | (2.1 | ) |
| Adjustment relating to fair value | | | — | | | | — | | | | (0.3 | ) | | | — | | | | — | | | | (0.3 | ) |
| Charge to profit and loss | | | 23.1 | | | | 5.1 | | | | 14.7 | | | | 0.2 | | | | 2.3 | | | | 45.4 | |
| Charge to statement of total recognized gains and losses | | | 5.4 | | | | — | | | | — | | | | — | | | | — | | | | 5.4 | |
| Utilized during the year | | | — | | | | (3.9 | ) | | | (11.3 | ) | | | (9.3 | ) | | | (4.9 | ) | | | (29.4 | ) |
| Exceptional item | | | (9.2 | ) | | | — | | | | — | | | | — | | | | — | | | | (9.2 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2002 | | | 61.5 | | | | 65.5 | | | | 91.5 | | | | 9.6 | | | | 25.4 | | | | 253.5 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
A further analysis of the deferred taxation provision is given in note 23. The prior year adjustment relates to the introduction of FRS19, “Deferred Taxation”, as explained in note 37.
Provisions for decommissioning and effluent costs relate to the costs determined on a going concern basis, associated with decommissioning radioactive facilities and with the eventual disposal of the Group’s intermediate level waste which arises from operations. Decommissioning costs will be incurred after the facilities are withdrawn from use in radioactive operations and extend out to 2036. Total decommissioning and effluent costs to be incurred in the next 10 years amount to approximately £37.4m. The provisions are based on the latest technical assessment of the process and methods likely to be used, and are stated at current prices and discounted using a real discount rate of 4%.
The provisions for pensions and similar obligations primarily relate to pension schemes, details of which are given in note 30. A transfer of assets was received from former pension plans during the 12 months ended December 31, 2001. The proportion of the transfer relating to current employees of the Group is included in provisions and is being spread over the remaining service life of the employees.
The £9.6m provision for disposal costs related to sale of businesses comprises £0.8m for the disposal of the Tomojet business in 2002, £0.8m for the closure of the disease profiling project in 2001, £7.3m for the disposal
F-36
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
of Nycomed Pharma, £0.3m for the sale of Puerto Rico Plant in 1998, and £0.4m for the sale of Puridec arising in 1996. These provisions are for legal or constructive obligations arising from the sale of businesses and are expected to be incurred within the next five years.
Other provisions are principally insurance claims, warranties and costs associated with the rationalisation of radiopharmaceutical production within Amersham Health. The provision at December 31, 2002 of £25.4m (2001 — £27.4m) is made up of £8.0m (2001 — £5.5m) relating to insurance reserves, £2.4m (2001 — £3.0m) relating to warranties, £3.8m (2001 — £4.5m) relating to radiopharmaceutical rationalisation costs and £11.2m (2001 — £14.8m) of legal and other provisions.
23. DEFERRED TAXATION
| | | | | | | | | | | | | |
| | | | | | | Dec 31, 2001 | | | | |
| | | | | (restated) | | Dec 31, 2002 |
| | | Note | | £m | | £m |
| | |
| |
| |
|
| Provision for deferred tax comprises: | | | | | | | | | | | | |
| Accelerated capital allowances | | | | | | | 10.7 | | | | 22.4 | |
| Tax deductible goodwill | | | | | | | 25.2 | | | | 26.2 | |
| Capital gains held over | | | | | | | 2.2 | | | | 6.6 | |
| Unrealized stock profit | | | | | | | (18.1 | ) | | | (22.2 | ) |
| Other short term timing differences | | | | | | | (8.1 | ) | | | (4.4 | ) |
| | | | | | | |
| | | |
| |
| | | | | | | | 11.9 | | | | 28.6 | |
| Transfer to deferred taxation assets | | | 16 | | | | 30.0 | | | | 32.9 | |
| | | | | | | |
| | | |
| |
| | | | 22 | | | | 41.9 | | | | 61.5 | |
| | | | | | | |
| | | |
| |
The Group has a potential deferred tax asset of £28.8m in the US Biosciences business of which £9.2m has been recognized, as an exceptional item, based on future financial projections which forecast future taxable profits in excess of those arising from the reversal of deferred tax liabilities. The remaining £19.6m is not recognized as there is insufficient evidence, at this time, that the asset will be recoverable in the foreseeable future.
In addition, the Group has further unrecognized deferred tax assets of £13.5m arising in other territories. These relate primarily to tax losses in those territories. There is insufficient evidence, at this time, that these assets will be recoverable. The unrecognized assets will be recoverable when sufficient evidence becomes available to support that recoverability.
Deferred tax is measured on a non-discounted basis at the tax rates that are expected to apply in the periods in which timing differences reverse, based on tax rates and laws substantively enacted at the balance sheet date.
24. ACQUISITIONS
During the year the Group made acquisitions with a total consideration of £767.9m, of which £708.6m related to the purchase of the 45% minority interest in Amersham Biosciences from Pharmacia Corp.
(a) Purchase of 45% minority interest holding in Amersham Biosciences
On March 21, 2002 the Group completed the purchase of the 45% minority interest holding in Amersham Biosciences that it did not already own.
The purchase consideration was £704.1m of cash, plus costs associated with the purchase of £4.5m. The purchase was financed by a placing of 57.5m new ordinary shares on March 18, 2002, which generated £397.0m after costs of issue, together with the use of existing cash resources and drawings under the Group’s committed bank facilities.
F-37
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The transaction was accounted for using acquisition accounting.
Goodwill of £692.3m arose on the transaction, and will be amortized over 20 years from the date of the purchase. The 20 year useful life reflects the continuing investment in the development of the business.
The values ascribed to the net assets acquired in Amersham Biosciences, and details of the fair value adjustments, which are provisional, are set out below:
| | | | | | | | | | | | | | | | | |
| | | Book value | | | | | | | | | | Fair value |
| | | At Mar 21, 2002 | | Revaluation | | Other | | At Mar 21, 2002 |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
| Tangible fixed assets (including investments) | | | 205.0 | | | | 27.2 | | | | — | | | | 232.2 | |
| Intangible fixed assets | | | 146.9 | | | | — | | | | (142.0 | ) | | | 4.9 | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 351.9 | | | | 27.2 | | | | (142.0 | ) | | | 237.1 | |
| Current assets (including intra-group deposits of £60m) | | | 353.4 | | | | — | | | | — | | | | 353.4 | |
| Current liabilities (including intra-group loans of £339m) | | | (482.1 | ) | | | — | | | | — | | | | (482.1 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| Net current liabilities | | | (128.7 | ) | | | — | | | | — | | | | (128.7 | ) |
| Total assets less current liabilities | | | 223.2 | | | | 27.2 | | | | (142.0 | ) | | | 108.4 | |
| Non-current liabilities | | | (25.2 | ) | | | — | | | | — | | | | (25.2 | ) |
| Provisions for liabilities and charges | | | (47.6 | ) | | | 0.6 | | | | — | | | | (47.0 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 150.4 | | | | 27.8 | | | | (142.0 | ) | | | 36.2 | |
| | | |
| | | |
| | | |
| | | |
| |
| Net assets acquired (45%) | | | | | | | | | | | | | | | 16.3 | |
| Cost of acquisition — cash consideration | | | | | | | | | | | | | | | 704.1 | |
| Acquisition expenses — professional fees and tax costs | | | | | | | | | | | | | | | 4.5 | |
| | | | | | | | | | | | | | | |
| |
| Goodwill arising on acquisition | | | | | | | | | | | | | | | 692.3 | |
| | | | | | | | | | | | | | | |
| |
The other adjustment relates to goodwill in the Amersham Biosciences balance sheet eliminated on the purchase of the minority interest. No accounting policy alignment adjustments are required as Amersham Biosciences’ accounting policies are consistent with those of the Amersham Group. Tangible fixed assets have been revalued to their fair value and listed investments have been written down to their market value. Adjustments within provisions for liabilities and charges relate to the funded status of pensions, primarily unrecognized actuarial gains. A 45% share of the revaluation and other adjustment has been recognized in the Group accounts.
As the purchase of Amersham Biosciences is an increase in stake, the profits and cash flows of Amersham Biosciences are already contained within the consolidated profit and loss account and cash flow statement of the Amersham Group for the current and prior year.
The minority interest for the period to March 21, 2002 was £0.8m credit, represented by a £0.6m charge for non-equity minority interests and £1.4m credit for equity minority interests.
The purchase of the 45% minority interest was partly achieved by the Group acquiring a 100% interest in Pharmacia Biosystems AB, (now known as Amersham Biosystems AB), a former trading company in the Pharmacia Corporation group. At the date of the purchase this company had no assets or liabilities, apart from the investment in Amersham Biosciences. Full warranties and indemnities were received from Pharmacia Corporation in respect of this company.
(b) Other Acquisitions
On January 31, 2002, the Group acquired 100% of the issued share capital of two filtration companies, AG Technology Corp and InnovaSep Technology Corp. The fair value of the total consideration is £38.1m, of which £11.8m is deferred consideration and £6.7m is the Group’s best estimate of deferred contingent consideration. The deferred contingent consideration is dependent on the acquired companies reaching technological milestones and on performance conditions. The ultimate amount of deferred and contingent consideration payable is expected to be in the range of $15-33m. Goodwill arising of £36.9m is being amortized over ten years.
F-38
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
On April 29, 2002, the Group acquired 100% of the issued share capital of Synthia Lab Systems (Sweden) AB. Goodwill arising of £0.5m is being amortized over seven years.
On May 10, 2002, the Group acquired a further 16% holding in Cimarron Software Inc, taking the Group’s total holding at acquisition to 35%. Cimarron Software Inc is being consolidated as a subsidiary undertaking as the group controls the company through a shareholders’ agreement. Goodwill arising of £6.3m is being amortized over seven years.
Since May 10, 2002, the Group has subscribed for additional allotted share capital in Cimarron Software Inc, taking its holding at the year end to 44%. Goodwill recognized on the post-acquisition allotments of share capital amounts to £1.7m. The Group has obligations to subscribe for a further 7% of share capital over the next three years, and has options to acquire the remaining 49% of the company for a maximum consideration of $33m, the total consideration payable being contingent on performance conditions.
On July 11, 2002, the Group acquired the net assets of Motorola’s Codelink Pre-Arrayed Slide business. Goodwill arising of £6.8m is being amortized over ten years.
All transactions were accounted for using acquisition accounting. The Group’s estimated useful life for these acquisitions reflects the period over which the Group expects to receive benefit based on acquired patents and technology.
Set out below is a summary of the net assets acquired and provisional fair value adjustments:
| | | | | | | | | | | | | |
| | | Book value | | Revaluation | | Fair value |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Fixed assets | | | 6.2 | | | | 3.9 | | | | 10.1 | |
| Net current assets | | | 1.8 | | | | (2.8 | ) | | | (1.0 | ) |
| | | |
| | | |
| | | |
| |
| Net assets | | | 8.0 | | | | 1.1 | | | | 9.1 | |
| | | |
| | | |
| | | |
| |
| Net assets acquired | | | | | | | | | | | 8.8 | |
| | | | | | | | | | | | | |
| Cost of acquisition: | | | | | | | | | | | | |
| Cash consideration | | | | | | | | | | | 38.2 | |
| Deferred consideration | | | | | | | | | | | 18.5 | |
| Existing trade investment | | | | | | | | | | | 2.6 | |
| | | | | | | | | | | |
| |
| | | | | | | | | | | | 59.3 | |
| | | | | | | | | | | |
| |
| Goodwill arising on acquisition | | | | | | | | | | | 50.5 | |
| | | | | | | | | | | |
| |
Adjustments to fixed assets relate to recognition of intangible assets relating to patented technology acquired and the adjustment to net current assets relates to the recognition of liabilities assumed on acquisition. No accounting policy alignment adjustments have been recorded as the acquired entities accounting policies are consistent with those of the Amersham Group.
As discussed above, goodwill of £1.7m arose on the acquisition of additional holdings in Cimarron Software Inc. Total goodwill arising on other acquisitions during the year was £52.2m.
Included within cash paid for acquisitions during the year of £40.6m in the consolidated cash flow statement is £2.4m for milestone payments relating to prior year acquisitions.
F-39
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
25 CALLED UP SHARE CAPITAL
| | | | | | | | | |
| | | Ordinary 5p shares |
| | |
|
| | | 5p | | 5p |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | m | | m |
| | |
| |
|
Authorized: | | | | | | | | |
| Value | | | £45.0 | | | | £45.0 | |
| Number | | | 900.0 | | | | 900.0 | |
| | | |
| | | |
| |
Issued, called up and fully paid: | | | | | | | | |
| Value | | | £32.1 | | | | £35.1 | |
| Number | | | 641.7 | | | | 701.8 | |
| | | |
| | | |
| |
By ordinary resolution passed on May 15, 1998 each 25p ordinary share and each 25p non-voting share (whether issued or unissued) was subdivided into five 5p ordinary shares and five 5p non-voting shares respectively with effect from June 10, 1998. Pursuant to the enfranchisement of the non-voting shares, holders of existing ordinary 25p shares were allotted new ordinary 5p shares in the proportion of 170 new ordinary 5p shares for every 1,000 existing ordinary shares of 25p each held on June 5 (i.e. a 3.4% bonus issue to the holders of the 25p ordinary shares). Each 5p non-voting share was converted into one 5p ordinary share with effect from June 10, 1998. As a result, the authorized share capital of the Company is £45 million divided into 900 million ordinary shares of 5p each.
During the 12 months ended December 31, 2002 a total of 60,101,551 ordinary shares of 5p each (nominal value £3m) were issued for an aggregate net cash consideration of £407.7m.
Of these, 57,500,000 shares were issued on March 18, 2002, in connection with the placing of shares on March 12, 2002, to finance the purchase of the outstanding shares in Amersham Biosciences Limited for cash consideration of £397.0m, net of issue costs of £5.5m.
The shares were issued at a price of £7.00 per share, and the market price on the March 12, 2002 was £7.365 per share. The 57,500,000 shares were allotted, as follows:
| • | | 50,000,000 placing shares to persons nominated by Morgan Stanley Securities Limited and Hoare Govett Limited; |
| |
| • | | 7,500,000 shares issued to Morgan Stanley Securities Limited, pursuant to the exercise of an over-allotment option. |
The 50,000,000 placing shares were ultimately allotted to institutional investors.
A further 438,890 shares were issued to the Qualifying Employee Share Ownership Trust (QUEST) for an aggregate net cash consideration of £2.5m. The remaining 2,162,661 ordinary shares of 5p each were issued in connection with employee share option schemes for an aggregate net cash consideration of £8.2m.
F-40
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Share option schemes
The following options remained exercisable at the year end.
| | | | | | | | | | | | | | | | | |
| | | | | | | Ordinary | | Ordinary | | No of shares |
| | | | | | | 5p shares | | 5p shares | | exercisable |
| Option price | | Options exercisable | | Dec 31, 01 | | Dec 31, 02 | | 2003 |
| |
| |
| |
| |
|
| Save As You Earn Scheme |
| | £1.38 | | | February 2000 or February 2002 | | | 170,812 | | | | — | | | | — | |
| | £1.39 | | | September 2000 or September 2002 | | | 204,865 | | | | 3,304 | | | | 3,304 | |
| | £1.54 | | | September 2001 or September 2003 | | | 262,363 | | | | 215,058 | | | | 215,058 | |
| | £2.46 | | | September 2002 or September 2004 | | | 1,137,938 | | | | 185,302 | | | | 27,243 | |
| | £3.00 | | | December 2003 or December 2005 | | | 1,551,095 | | | | 1,463,643 | | | | 1,218,791 | |
| | £3.30 | | | December 2004 or December 2006 | | | 898,809 | | | | 855,627 | | | | — | |
| | £4.96 | | | December 2005 or December 2007 | | | 975,260 | | | | 910,294 | | | | — | |
| | £4.80 | | | December 2004,December 2006 | | | 808,109 | | | | 770,955 | | | | — | |
| | | | | or December 2008 | | | | | | | | | | | | |
| | £4.49 | | | December 2005, December 2007 | | | — | | | | 1,077,315 | | | | — | |
| | | | | or December 2009 | | | | | | | | | | | | |
| | | | | | | |
| | | |
| | | |
|
| Total | | | | | 6,009,251 | | | | 5,481,498 | | | | 1,464,396 | |
| | | | | | | |
| | | |
| | | |
|
F-41
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | | | | |
| Option Price | | Options exercisable | | Ordinary 5p shares | | Ordinary 5p shares | | No of shares |
| | | | | Dec 31, 01 | | Dec 31, 02 | | exercisable 2003 |
| |
| |
| |
| |
|
| Executive Share Option Scheme 1993 | | | | | | | | | | | | | | |
| | £1.13 | | | November 1995 to November 2002 | | | 18,250 | | | | — | | | | — | |
| | £1.49 | | | June 1996 to June 2003 | | | 20,731 | | | | 20,731 | | | | 20,731 | |
| | £1.83 | | | November 1996 to November 2003 | | | 15,663 | | | | 3,979 | | | | 3,979 | |
| | £1.87 | | | July 1997 to July 2004 | | | 29,261 | | | | 29,261 | | | | 29,261 | |
| | £1.72 | | | November 1997 to November 2004 | | | 91,250 | | | | 17,061 | | | | 17,061 | |
| | £1.74 | | | June 1998 to June 2005 | | | 59,741 | | | | 59,741 | | | | 59,741 | |
| | £1.69 | | | November 1998 to November 2005 | | | 103,692 | | | | 77,157 | | | | 77,157 | |
| | £1.89 | | | July 1999 to July 2006 | | | 68,809 | | | | 68,809 | | | | 68,809 | |
| | £3.42 | | | July 2000 to July 2007 | | | 305,493 | | | | 163,374 | | | | 163,374 | |
| | £3.92 | | | December 2000 to November 2007 | | | 130,112 | | | | 130,112 | | | | 130,112 | |
| | £3.80 | | | May 2001 to April 2008 | | | 793,940 | | | | 529,569 | | | | 529,569 | |
| | £3.56 | | | September 2001 to September 2008 | | | 571,685 | | | | 241,519 | | | | 241,519 | |
| | £4.38 | | | May 2002 to May 2009 | | | 1,615,781 | | | | 1,252,833 | | | | 1,252,833 | |
| | £4.66 | | | June 2002 to June 2009 | | | 63,523 | | | | — | | | | — | |
| | £3.74 | | | August 2002 to August 2009 | | | 913,689 | | | | 696,789 | | | | 696,789 | |
| | £4.11 | | | September 2002 to September 2009 | | | 291,970 | | | | 291,970 | | | | 291,970 | |
| | £4.12 | | | September 2002 to September 2009 | | | 71,750 | | | | 55,750 | | | | 55,750 | |
| | £5.20 | | | March 2003 to February 2010 | | | 2,045,799 | | | | 1,902,573 | | | | 1,902,573 | |
| | £6.19 | | | April 2003 to April 2010 | | | 13,942 | | | | 13,942 | | | | 13,942 | |
| | £6.23 | | | August 2003 to August 2010 | | | 453,153 | | | | 453,153 | | | | 453,153 | |
| | £6.22 | | | September 2003 to September 2010 | | | 128,618 | | | | 128,618 | | | | 128,618 | |
| | | | | | | |
| | | |
| | | |
|
| Total | | | | | 7,806,852 | | | | 6,136,941 | | | | 6,136,941 | |
| | | | | | | |
| | | |
| | | |
|
Nycomed Share
Option Plan |
| $ | 4.02 | | | July 1997 to April 2002 | | | 83,910 | | | | — | | | | — | |
| | | | | | | |
| | | |
| | | |
|
| Total | | | | | 83,910 | | | | — | | | | — | |
| | | | | | | |
| | | |
| | | |
|
| Options awarded under the Nycomed Share Option Plan are denominated in US dollars. |
| |
| Amersham US Stock Option Plan 2000 |
| | £6.19 | | | March 2001 to March 2010 | | | 2,334,832 | | | | 1,908,914 | | | | 477,229 | |
| | £6.19 | | | June 2001 to June 2010 | | | 6,500 | | | | 5,500 | | | | 1,375 | |
| | £6.23 | | | August 2001 to August 2010 | | | 53,056 | | | | 50,193 | | | | 12,548 | |
| | £6.31 | | | October 2001 to October 2010 | | | 1,500 | | | | 1,500 | | | | 375 | |
| | £5.45 | | | December 2001 to December 2010 | | | 9,000 | | | | 5,000 | | | | 1,250 | |
| | | | | | | |
| | | |
| | | |
|
| Total | | | | | 2,404,888 | | | | 1,971,107 | | | | 492,777 | |
| | | | | | | |
| | | |
| | | |
|
Amersham US Stock Option Plan options are subject to phased vesting of 25% per year from date of grant.
F-42
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | | | | |
| Option Price | | | | Ordinary | | Ordinary | | | | |
| Executive Share Option | | | | 5p shares | | 5p shares | | No of shares |
| Scheme 2001 | | Options exercisable | | Dec 31, 01 | | Dec 31, 02 | | exercisable 2003 |
| |
| |
| |
| |
|
| | £5.60 | | | May 2002 to May 2011 | | | 6,324,060 | | | | 5,968,731 | | | | 1,310,496 | |
| | £5.61 | | | May 2002 to May 2011 | | | 10,076 | | | | 10,076 | | | | 2,519 | |
| | £5.57 | | | June 2002 to May 2011 | | | 102,336 | | | | 102,336 | | | | 25,584 | |
| | £6.09 | | | August 2002 to August 2011 | | | 287,460 | | | | 287,460 | | | | 71,865 | |
| | £6.06 | | | September 2002 to September 2011 | | | 20,272 | | | | 20,272 | | | | 5,068 | |
| | £5.95 | | | September 2002 to September 2011 | | | 9,988 | | | | 9,988 | | | | 2,497 | |
| | £7.15 | | | March 2003 to March 2012 | | | — | | | | 7,159,214 | | | | 1,669,007 | |
| | £7.28 | | | March 2003 to March 2012 | | | — | | | | 38,864 | | | | 9,716 | |
| | £7.63 | | | April 2003 to April 2012 | | | — | | | | 13,376 | | | | 3,344 | |
| | £5.58 | | | August 2003 to August 2012 | | | — | | | | 864,500 | | | | 216,125 | |
| | £5.68 | | | September 2003 to September 2012 | | | — | | | | 23,768 | | | | 5,942 | |
| | £5.82 | | | December 2003 to December 2012 | | | — | | | | 309,280 | | | | — | |
| | | | | | | |
| | | |
| | | |
|
| | | | | | | | 6,754,192 | | | | 14,807,865 | | | | 3,322,163 | |
| | | | | | | |
| | | |
| | | |
|
2001 options are subject to 25% vesting from date of grant, except Directors’ options which usually vest three years after date of grant. The exercise of these options is subject to performance conditions.
Options for All
| | | | | | | | | | | | | | | | | |
| | £6.35 | | | October 2004 to October 2007 | | | 9,150,000 | | | | 8,581,352 | | | | — | |
26 SHARE PREMIUM ACCOUNT AND RESERVES
| | | | | | | | | | | | | | | | | |
| | | Share | | Profit | | | | | | | | |
| | | premium | | and loss | | Merger | | Other |
| | | account | | account | | reserves | | reserves |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
| At December 31, 2000 | | | 39.4 | | | | 243.2 | | | | 73.0 | | | | 21.0 | |
| Prior year adjustment | | | — | | | | 8.5 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2000 as restated | | | 39.4 | | | | 251.7 | | | | 73.0 | | | | 21.0 | |
| Foreign currency translation | | | — | | | | (3.9 | ) | | | — | | | | — | |
| Shares issued in the year | | | 24.9 | | | | — | | | | — | | | | — | |
| Contribution relating to Qualifying Employee Share Ownership Trust (QUEST) | | | — | | | | (7.4 | ) | | | — | | | | — | |
| Retained profit for the year | | | — | | | | 165.5 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| At December 31, 2001 as restated | | | 64.3 | | | | 405.9 | | | | 73.0 | | | | 21.0 | |
| Foreign currency translation | | | — | | | | 47.8 | | | | — | | | | — | |
| Issue costs | | | (5.5 | ) | | | — | | | | — | | | | — | |
| Shares issued in the year | | | 410.2 | | | | — | | | | — | | | | | |
| Contribution to Qualifying Employees Share Ownership Trust (QUEST) | | | — | | | | (1.2 | ) | | | — | | | | — | |
| Retained profit for the year | | | — | | | | 121.4 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
At December 31, 2002 | | | 469.0 | | | | 573.9 | | | | 73.0 | | | | 21.0 | |
| | | |
| | | |
| | | |
| | | |
| |
The amounts included in other reserves relate to foreign subsidiary undertakings and represent amounts which cannot be distributed under local company law, and negative goodwill arising on the acquisition of Pharmacia Biotech.
F-43
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The cumulative value of goodwill written off amounts to £1,439.9m (2001 — £1,439.9m, 2000 — £1,439.9m).
The Company’s profit attributable to shareholders is £72.8m (2001 — £66.0m, 2000 — £31.6m).
In accordance with the Group’s accounting policy, an exchange gain of £46.4m (2001 — £6.4m, 2000 — £9.7m loss) on the foreign currency loan hedging Group investments has been matched with the exchange loss on those investments in reserves. A related tax charge of £5.4m (2001 — £0.3m credit, 2000 — £3.5m credit) has also been offset in reserves.
At December 31, 2002, the cumulative translation adjustments to equity were £23.8m loss (2001 — £71.6m loss, 2000 — £67.7m loss).
27 RECONCILIATION OF MOVEMENTS IN EQUITY SHAREHOLDERS’ FUNDS
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | (restated) | | (restated) | | |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Profit for the year | | | 107.0 | | | | 210.7 | | | | 178.7 | |
| Dividends | | | (40.4 | ) | | | (45.2 | ) | | | (57.3 | ) |
| | | |
| | | |
| | | |
|
Retained earnings | | | 66.6 | | | | 165.5 | | | | 121.4 | |
| Foreign currency translation | | | (18.9 | ) | | | (3.9 | ) | | | 47.8 | |
| New share capital and premium | | | 6.2 | | | | 25.2 | | | | 413.2 | |
| Costs associated with share issue | | | — | | | | — | | | | (5.5 | ) |
| Loss related to Qualifying Employee Share Ownership Trust (QUEST) | | | (0.7 | ) | | | (7.4 | ) | | | (1.2 | ) |
| | | |
| | | |
| | | |
|
Net increase in equity shareholders’ funds | | | 53.2 | | | | 179.4 | | | | 575.7 | |
| | | |
| | | |
| | | |
|
Equity shareholders’ funds at the start of the year as previously reported | | | 351.4 | | | | 408.4 | | | | 591.9 | |
| Prior year adjustments | | | | | | | | | | | | |
| - deferred taxation | | | 12.3 | | | | 8.5 | | | | 4.4 | |
| | | |
| | | |
| | | |
|
Restated | | | 363.7 | | | | 416.9 | | | | 596.3 | |
| | | |
| | | |
| | | |
|
| Equity shareholders’ funds at the end of the year | | | 416.9 | | | | 596.3 | | | | 1,172.0 | |
| | | |
| | | |
| | | |
| |
28 CAPITAL COMMITMENTS
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Expenditure contracted | | | 22.7 | | | | 41.3 |
| |
F-44
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
29 OPERATING AND FINANCE LEASE COMMITMENTS
Operating leases
Payments which the Group is committed to make during the following year against operating leases are as follows:
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Land and buildings: | | | | | | | | |
| Expiring within one year | | | 1.9 | | | | 1.3 | |
| Expiring between two and five years | | | 7.6 | | | | 10.3 | |
| Expiring after more than five years | | | 4.3 | | | | 3.1 | |
| | | |
| | | |
| |
| | | | 13.8 | | | | 14.7 | |
| | | |
| | | |
| |
| Other leases: | | | | | | | | |
| Expiring within one year | | | 1.8 | | | | 1.4 | |
| Expiring between two and five years | | | 13.8 | | | | 12.9 | |
| Expiring after more than five years | | | — | | | | 0.3 | |
| | | |
| | | |
| |
| | | | 15.6 | | | | 14.6 | |
| | | |
| | | |
| |
Finance lease commitments
The finance lease obligations to which the Group is committed are as follows:
| | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m |
| | |
| |
|
| Payable within one year | | | 0.4 | | | | 0.5 | |
| Payable between two and five years | | | 1.5 | | | | 1.4 | |
| Payable after 5 years | | | 2.1 | | | | 1.9 | |
| | | |
| | | |
| |
| | | | 4.0 | | | | 3.8 | |
| Less finance charges allocated to future periods | | | (1.0 | ) | | | (1.0 | ) |
| | | |
| | | |
| |
| | | | 3.0 | | | | 2.8 | |
| | | |
| | | |
| |
30 PENSIONS
The Company participates in a variety of pension arrangements on behalf of its UK employees. The principal scheme is the Amersham Pension Scheme, which is a defined benefit arrangement, the assets of which are held in a separate trustee administered fund.
An actuarial valuation of this scheme as at March 31, 2000 was made by Watson Wyatt, an independent firm of actuaries, using the projected unit method. The main assumptions adopted in that valuation for the purposes of the UK Statement of Standard Accounting Practice SSAP 24, “Accounting for Pension Costs”, were that over the long term the rate of return on investments would be 6.9% pa, the increase in pensionable pay 5% pa and pensions in payment 3% pa. On the basis of these assumptions the market value of the scheme’s assets as at 31 March 2000 represented 112% of the value of the benefits to members at that date including allowance for future salary and pension increases. The market value of the scheme’s assets on March 31, 2000 was £209m.
The pension cost in the Company’s financial statements and the associated amounts of prepayment are set out below:
F-45
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | |
| | | Dec 31, 2001 | | Dec 31, 2002 |
| | | £m | | £m |
| | |
| |
|
| Prepayment at start of year | | | 6.8 | | | | 8.7 | |
| Regular cost | | | (9.0 | ) | | | (10.3 | ) |
| Variation from regular cost | | | 1.6 | | | | 1.6 | |
| Contributions paid | | | 9.3 | | | | 10.7 | |
| | | |
| | | |
| |
| Prepayment at end of year | | | 8.7 | | | | 10.7 | |
| | | |
| | | |
| |
Overseas schemes
The Group operates a number of pension schemes overseas, which have been developed in line with, and are funded under, local practices. The principal overseas schemes are in the US, Norway and Sweden and are mainly of the defined benefit type funded via separately administered trusts or guaranteed by insurance policies. The scheme in Sweden is an unfunded arrangement and there is an unfunded arrangement in Norway in addition to the funded scheme. These are provided for in the Group financial statements and secured on the ongoing business.
Watson Wyatt have prepared pension costs using actuarial valuations carried out in accordance with local requirements, for the purposes of Group reporting under SSAP 24. A surplus or deficit is amortized over the average remaining service lives of the relevant employees using the straight-line method. The review was made as at January 1, 2002, using the projected unit method and using the following principal assumptions.
| | | | | | | | | | | | | |
| | | US | | Norway | | Sweden |
| | | % | | % | | % |
| | |
| |
| |
|
| Return on investment | | | 7.5 | | | | 6.5 | | | | 7.0 | |
| Increase in pensionable salary | | | 4.5 | | | | 3.5 | | | | 4.0 | |
| Increase in pensions in payment | | | — | | | | 2.0 | | | | 2.5 | |
The pension cost in the Group’s financial statements and associated amounts of provision are set out below, in aggregate, for these principal overseas funded and unfunded schemes.
| | | | | | | | | | | | | | | | | |
| | | Dec 31, 2001 | | Dec 31, 2002 |
| | |
| |
|
| | | Funded | | Unfunded | | Funded | | Unfunded |
| | | Schemes | | Schemes | | Schemes | | Schemes |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
| Provision at start of year | | | 18.8 | | | | 41.5 | | | | 18.6 | | | | 43.2 | |
| Foreign currency translation | | | 0.5 | | | | (1.6 | ) | | | (1.6 | ) | | | 2.3 | |
| Pension cost | | | 5.3 | | | | 4.0 | | | | 7.2 | | | | 4.6 | |
| Contributions/pensions paid | | | (6.0 | ) | | | (0.7 | ) | | | (8.2 | ) | | | (1.3 | ) |
| | | |
| | | |
| | | |
| | | |
| |
Provision at end of year | | | 18.6 | | | | 43.2 | | | | 16.0 | | | | 48.8 | |
| | | |
| | | |
| | | |
| | | |
| |
On the basis of the above assumptions the actuarial value of the scheme assets for the principal overseas funded schemes was £76.0m (2001 — £76.5m) and represents 80% (2001 — 87%) of the actuarial value of benefits to members as at January 1, 2002, including allowance for future salary and pension increases.
In addition to the schemes included in the above analysis the Group also operates defined benefit schemes in other territories, principally Japan and Germany.
FRS 17
In November 2000, the Accounting Standards Board published FRS 17 ‘Retirement Benefits’ to replace the existing standard SSAP 24 ‘Accounting for Pension costs’. Although the new standard does not become mandatory until accounting periods beginning on or after January 1, 2005, it sets down transitional arrangements which require footnote disclosures in the interim period up to full adoption.
The most recent actuarial valuations have been updated by Watson Wyatt to take account of the requirements of FRS 17 in order to assess the liabilities of the schemes at December 31, 2002. For the principal overseas schemes this latest valuation was at December 31, 2001 including both the US and Norway and at December 31, 1999 for Sweden. Scheme assets are stated at their market value at December 31, 2002.
F-46
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The future agreed contribution rate for the UK scheme is currently 15.2%. This is subject to review during 2003. For the overseas schemes the contribution rate will be assessed on a year on year basis. All of the Group pension schemes are open as at December 31, 2002.
Main assumptions for FRS 17 purposes
| | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other |
| December 31, 2002 | | %pa | | % pa | | % pa | | % pa |
| |
| |
| |
| |
|
| Rate of increase in salaries | | | 4.25 | | | | 4.25 | | | | 3.50 | | | | 3.75 | |
| Rate of increase in pensions in payment | | | 2.25 | | | | 0.00 | | | | 2.00 | | | | 2.00 | |
| Discount rate | | | 5.50 | | | | 6.75 | | | | 6.50 | | | | 5.50 | |
| Inflation | | | 2.25 | | | | 3.00 | | | | 2.50 | | | | 2.50 | |
| | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other |
| December 31, 2001 | | %pa | | % pa | | % pa | | % pa |
| |
| |
| |
| |
|
| Rate of increase in salaries | | | 4.25 | | | | 4.25 | | | | 3.50 | | | | 3.25 | |
| Rate of increase in pensions in payment | | | 2.25 | | | | 0.00 | | | | 2.00 | | | | 2.25 | |
| Discount rate | | | 5.75 | | | | 7.25 | | | | 7.00 | | | | 5.00 | |
| Inflation | | | 2.25 | | | | 3.00 | | | | 2.50 | | | | 2.50 | |
The market value of assets in the schemes at December 31, 2002 and the long term expected rates of return at that date were:
| | | | | | | | | | | | | | | | | |
| December 31, 2002 | | Equities | | Bonds | | Other | | Total |
| |
| |
| |
| |
|
Long term rate of return expected at December 31, 2002 (% pa) | | | | | | | | | | | | | | | | |
| UK | | | 8.50 | | | | 5.00 | | | | 6.00 | | | | — | |
| US | | | 8.75 | | | | 6.25 | | | | 4.50 | | | | — | |
| Norway | | | 8.75 | | | | 6.00 | | | | 5.00 | | | | — | |
| Other | | | 5.50 | | | | 2.50 | | | | 2.00 | | | | — | |
Value at December 31, 2002 (£m) | | | | | | | | | | | | | | | | |
| UK | | | 127.3 | | | | 34.5 | | | | 8.4 | | | | 170.2 | |
| US | | | 29.4 | | | | 16.4 | | | | 0.7 | | | | 46.5 | |
| Norway | | | 2.9 | | | | 16.7 | | | | 9.0 | | | | 28.6 | |
| Other | | | 4.9 | | | | 3.4 | | | | 0.7 | | | | 9.0 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | | 164.5 | | | | 71.0 | | | | 18.8 | | | | 254.3 | |
| | | |
| | | |
| | | |
| | | |
| |
F-47
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The market value of assets in the schemes at December 31, 2001 and the long term expected rates of return at that date were:
| | | | | | | | | | | | | | | | | |
| December 31, 2001 | | Equities | | Bonds | | Other | | Total |
| |
| |
| |
| |
|
Long term rate of return expected at December 31, 2001 (% pa) | | | | | | | | | | | | | | | | |
| UK | | | 8.00 | | | | 5.50 | | | | 5.00 | | | | — | |
| US | | | 8.75 | | | | 7.00 | | | | 4.50 | | | | — | |
| Norway | | | 8.25 | | | | 6.75 | | | | 6.00 | | | | — | |
| Other | | | 6.25 | | | | 3.00 | | | | 2.00 | | | | — | |
Value at December 31, 2001 (£m) | | | | | | | | | | | | | | | | |
| UK | | | 136.5 | | | | 26.7 | | | | 27.4 | | | | 190.6 | |
| US | | | 32.0 | | | | 21.7 | | | | 0.7 | | | | 54.4 | |
| Norway | | | 4.8 | | | | 15.2 | | | | 2.7 | | | | 22.7 | |
| Other | | | 4.4 | | | | 3.2 | | | | 0.7 | | | | 8.3 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | | 177.7 | | | | 66.8 | | | | 31.5 | | | | 276.0 | |
| | | |
| | | |
| | | |
| | | |
| |
The following amounts at December 31, 2002 were measured in accordance with the requirements of FRS17:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
|
| Total market value of assets | | | 170.2 | | | | 46.5 | | | | 28.6 | | | | 9.0 | | | | 254.3 | |
| Present value of scheme liabilities | | | (268.3 | ) | | | (102.2 | ) | | | (33.8 | ) | | | (58.3 | ) | | | (462.6 | ) |
| Deficit in the scheme | | | (98.1 | ) | | | (55.7 | ) | | | (5.2 | ) | | | (49.3 | ) | | | (208.3 | ) |
| Related deferred tax asset | | | 7.5 | | | | 22.3 | | | | 1.4 | | | | 15.4 | | | | 46.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Net pension liabilities | | | (90.6 | ) | | | (33.4 | ) | | | (3.8 | ) | | | (33.9 | ) | | | (161.7 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The following amounts at December 31, 2001 were measured in accordance with the requirements of FRS17:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | | £m | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
| |
|
| Total market value of assets | | | 190.6 | | | | 54.4 | | | | 22.7 | | | | 8.3 | | | | 276.0 | |
| Present value of scheme liabilities | | | (212.4 | ) | | | (92.2 | ) | | | (26.3 | ) | | | (48.7 | ) | | | (379.6 | ) |
| Deficit in the scheme | | | (21.8 | ) | | | (37.8 | ) | | | (3.6 | ) | | | (40.4 | ) | | | (103.6 | ) |
| Related deferred tax asset | | | — | | | | 15.1 | | | | 1.0 | | | | 12.5 | | | | 28.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Net pension liabilities | | | (21.8 | ) | | | (22.7 | ) | | | (2.6 | ) | | | (27.9 | ) | | | (75.0 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
F-48
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
If the above amounts had been recognized in the financial statements, the Group’s net assets and reserves at December 31, 2002 would be as follows:
| | | | | | | | | |
| | | Dec 31, 2002 | | Dec 31, 2001 |
| | | | | Restated |
| | | £m | | £m |
| | |
| |
|
Net assets | | | | | | | | |
| Net assets | | | 1,176.9 | | | | 658.9 | |
| Add back SSAP 24 net pension liability and prepayments | | | 80.8 | | | | 79.5 | |
| Less effect of NMP joint venture scheme | | | (0.2 | ) | | | (0.1 | ) |
| Less FRS 17 net pension liability, including NMP | | | (208.3 | ) | | | (103.6 | ) |
| Net effect on related deferred tax asset | | | 19.6 | | | | 2.2 | |
| Impact on minority interest | | | — | | | | (0.2 | ) |
| | | |
| | | |
| |
| Net assets including FRS 17 pension liability | | | 1,068.8 | | | | 636.7 | |
| | | |
| | | |
| |
Reserves | | | | | | | | |
| Reserves | | | 1,136.9 | | | | 564.2 | |
| Add back SSAP 24 net pension liability and prepayments | | | 80.8 | | | | 79.5 | |
| Less effect of NMP joint venture scheme | | | (0.2 | ) | | | (0.1 | ) |
| Less FRS 17 net pension liability, including NMP | | | (208.3 | ) | | | (103.6 | ) |
| Net effect on related deferred tax asset | | | 19.6 | | | | 2.2 | |
| Impact on minority interest | | | — | | | | (0.2 | ) |
| | | |
| | | |
| |
| Reserves including FRS 17 pension liability | | | 1,028.8 | | | | 542.0 | |
| | | |
| | | |
| |
F-49
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The amounts charged to operating profit under FRS17 would have been:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m |
| Current service cost | | | 10.7 | | | | 4.8 | | | | 1.6 | | | | 2.9 | | | | 20.0 | |
| Past service cost | | | — | | | | 0.3 | | | | — | | | | — | | | | 0.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total operating charge | | | 10.7 | | | | 5.1 | | | | 1.6 | | | | 2.9 | | | | 20.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The amounts charged to financing under FRS17 would have been:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m |
| Expected return on pension scheme assets | | | (14.1 | ) | | | (4.3 | ) | | | (1.8 | ) | | | (0.4 | ) | | | (20.6 | ) |
| Interest on pension scheme liabilities | | | 12.1 | | | | 6.3 | | | | 2.0 | | | | 2.8 | | | | 23.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total financing charge/(income) | | | (2.0 | ) | | | 2.0 | | | | 0.2 | | | | 2.4 | | | | 2.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
The total pension charge for the Group, including NMP, is £23.8m. Excluding items outside the scope of SSAP 24 of £0.6m, the charge for the year is £23.1m. The effect on the charge of adopting FRS 17 would therefore be a decrease of £0.2m.
Analysis of the amount that would have been included within the Statement of Total Recognized Gains and Losses under FRS17:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m |
| Actual return less expected return on pension scheme assets | | | (44.8 | ) | | | (10.1 | ) | | | (1.4 | ) | | | (1.4 | ) | | | (57.7 | ) |
| Experience gains/(losses) arising on the scheme liabilities | | | 4.2 | | | | (2.7 | ) | | | 2.3 | | | | 1.3 | | | | 5.1 | |
Changes in assumptions underlying the present value of scheme liabilities1 | | | (37.7 | ) | | | (8.8 | ) | | | (2.9 | ) | | | (3.1 | ) | | | (52.5 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Actuarial loss recognized in group statement of total recognized gains and losses | | | (78.3 | ) | | | (21.6 | ) | | | (2.0 | ) | | | (3.2 | ) | | | (105.1 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
1 Includes the impact of adopting Urgent Issues Task Force (UITF) No 35 ‘Death-in-service and incapacity benefits’.
The analysis of experience gains and losses, recognized in the statement of total recognized gains and losses, is as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | |
| |
| |
| |
| |
|
| Actual return less expected return on pension scheme assets (£m) | | | (44.8 | ) | | | (10.1 | ) | | | (1.4 | ) | | | (1.4 | ) | | | (57.7 | ) |
| Percentage of scheme assets at end of year | | | 26 | % | | | 22 | % | | | 5 | % | | | 16 | % | | | 23 | % |
| Experience gains/(losses) arising on the scheme liabilities (£m) | | | 4.2 | | | | (2.7 | ) | | | 2.3 | | | | 1.3 | | | | 5.1 | |
| Percentage of scheme liabilities at end of year | | | 2 | % | | | (3 | %) | | | 7 | % | | | 2 | % | | | 1 | % |
| Total actuarial loss recognized in statement of total recognized gains and losses (£m) | | | (78.3 | ) | | | (21.6 | ) | | | (2.0 | ) | | | (3.2 | ) | | | (105.1 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Percentage of scheme liabilities at end of year | | | 29 | % | | | 21 | % | | | 6 | % | | | 5 | % | | | 23 | % |
| | | | | | | | | | | | | | | | | | | | | |
F-50
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The movement in the deficit during the year under FRS 17 would have been:
| | | | | | | | | | | | | | | | | | | | | |
| | | UK | | US | | Norway | | Other | | Total |
| | |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m |
| Deficit in the scheme at the beginning of the year | | | (21.8 | ) | | | (37.8 | ) | | | (3.6 | ) | | | (40.4 | ) | | | (103.6 | ) |
| Movement in the year: | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation | | | — | | | | 5.2 | | | | (0.8 | ) | | | (2.9 | ) | | | 1.5 | |
| Current service cost | | | (10.7 | ) | | | (4.8 | ) | | | (1.6 | ) | | | (2.9 | ) | | | (20.0 | ) |
| Past service costs | | | — | | | | (0.3 | ) | | | — | | | | — | | | | (0.3 | ) |
| Contributions | | | 10.7 | | | | 5.6 | | | | 3.0 | | | | 2.5 | | | | 21.8 | |
| Other finance income/(charge) | | | 2.0 | | | | (2.0 | ) | | | (0.2 | ) | | | (2.4 | ) | | | (2.6 | ) |
| Actuarial loss | | | (78.3 | ) | | | (21.6 | ) | | | (2.0 | ) | | | (3.2 | ) | | | (105.1 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Deficit in the scheme at the end of the year | | | (98.1 | ) | | | (55.7 | ) | | | (5.2 | ) | | | (49.3 | ) | | | (208.3 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Opening related deferred tax asset | | | — | | | | 15.1 | | | | 1.0 | | | | 12.5 | | | | 28.6 | |
| Movement in the year | | | 7.5 | | | | 7.2 | | | | 0.4 | | | | 2.9 | | | | 18.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Closing related deferred tax asset | | | 7.5 | | | | 22.3 | | | | 1.4 | | | | 15.4 | | | | 46.6 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
31 SHARES IN GROUP UNDERTAKINGS
Information relating to subsidiary, joint venture and associated undertakings is given on those that, in the opinion of the Directors, principally affected the profits or assets of the Group.
The primary activity of the majority of the undertakings shown below is the development and manufacture and/or sale of specialized products for research-based biotechnology supply and the diagnosis and treatment of disease. Subsidiary, joint venture, and associated undertakings are listed under the relevant business operation. Where the relevant company serves more than one business it is listed under the dominant business operation.
With the exception of Amersham Buchler GmbH & Co. KG and Amersham Biosciences Limited, the company’s holdings in the following undertakings are represented by ordinary shares. Amersham Buchler GmbH & Co. KG is a partnership in which the Group’s interest is in limited liability form. The Company holds ordinary and non-voting preference shares in Amersham Biosciences Limited in the proportion stated below.
All of the companies listed below operate principally in their country of incorporation or registration and have coterminous year ends.
| | | | | | | | | | | | | |
| | | | | | | Proportion of nominal value |
| | | | | | | of issued shares held |
| | | Country of | |
|
| | | incorporation or | | By the | | By subsidiary |
| | | registration | | Company | | undertakings |
| | |
| |
| |
|
| | | | | | | % | | % |
Holding companies | | | | | | | | | | | | |
| Amersham Health Norge AS | | Norway | | | — | | | | 100 | |
| Acam Overseas Holdings Ltd | | UK | | | — | | | | 100 | |
| Acam Holdings (UK) Ltd | | UK | | | 100 | | | | — | |
| Amersham Benelux BV | | Netherlands | | | — | | | | 100 | |
| Amersham Biosystems AB | | Sweden | | | — | | | | 100 | |
F-51
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | |
Amersham Biosciences | | | | | | | | | | | | |
| Amersham Biosciences Ltd | | UK | | | 61 | | | | 39 | |
| Amersham Biosciences Corp | | USA | | | — | | | | 100 | |
| Amersham Biosciences UK Ltd | | UK | | | — | | | | 100 | |
| Amersham Biosciences Europe GmbH | | Germany | | | — | | | | 100 | |
| Amersham Biosciences AB | | Sweden | | | — | | | | 100 | |
| Amersham Biosciences (SV) Corp | | USA | | | — | | | | 100 | |
| Amersham Biosciences KK | | Japan | | | — | | | | 100 | |
| Amersham Biosciences Ltd | | Hong Kong | | | — | | | | 100 | |
| AG Technology Corp | | USA | | | — | | | | 100 | |
| InnovaSep Technology Corporation | | USA | | | — | | | | 100 | |
Amersham Health | | | | | | | | | | | | |
| Amersham Buchler GmbH & Co. KG | | Germany | | | — | | | | 60 | |
| Amersham Health AS | | Norway | | | — | | | | 100 | |
| Amersham Health | | Ireland | | | — | | | | 100 | |
| Amersham Health Inc | | USA | | | — | | | | 100 | |
| Amersham Health Pte Ltd | | Singapore | | | — | | | | 100 | |
| Nycomed Amersham Sorin Srl | | Italy | | | 100 | | | | — | |
| Amersham Health Ltd | | China | | | — | | | | 80 | |
| Amersham Health Holdings Inc | | USA | | | — | | | | 100 | |
Joint venture | | | | | | | | | | | | |
Amersham Health | | | | | | | | | | | | |
| Nihon Medi-Physics Company Ltd | | Japan | | | 29 | | | | 21 | |
The investment in Nihon Medi-Physics Company Ltd relates to ordinary shares. The shares carry standard pre-emption rights.
A full list of all Group undertakings will be appended to the annual return.
32 NET CASH INFLOW FROM OPERATING ACTIVITIES
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | |
| |
| |
|
| | | £m | | £m | | £m |
Total operating profit before exceptional items | | | 230.5 | | | | 280.8 | | | | 273.2 | |
| Share of operating profit of joint venture and associates | | | (29.8 | ) | | | (26.1 | ) | | | (23.2 | ) |
| Depreciation and amortization | | | 71.9 | | | | 80.0 | | | | 116.4 | |
| Loss on sale of tangible fixed assets | | | 1.1 | | | | 0.3 | | | | 1.5 | |
| Increase/(decrease) in provisions | | | (7.7 | ) | | | 1.3 | | | | (6.8 | ) |
| Increase in stocks | | | (26.8 | ) | | | (12.1 | ) | | | (19.9 | ) |
| (Increase)/ decrease in debtors | | | 11.6 | | | | (16.8 | ) | | | 5.3 | |
| Increase/(decrease) in creditors | | | 4.5 | | | | 34.9 | | | | (1.0 | ) |
| | | |
| | | |
| | | |
| |
Net cash inflow from operating activities before exceptional items | | | 255.3 | | | | 342.3 | | | | 345.5 | |
| Integration costs paid | | | (15.8 | ) | | | — | | | | — | |
| Other exceptional items | | | (14.8 | ) | | | 12.0 | | | | — | |
| | | |
| | | |
| | | |
| |
Net cash inflow from operating activities | | | 224.7 | | | | 354.3 | | | | 345.5 | |
| | | |
| | | |
| | | |
| |
F-52
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
33 ANALYSIS OF NET DEBT
| | | | | | | | | | | | | | | | | |
| | | As at | | | | | | Exchange | | As at |
| | | Jan 1, 2002 | | Cash flow | | movements | | Dec 31, 2002 |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
| Cash at bank and in hand | | | 56.9 | | | | (30.3 | ) | | | 6.1 | | | | 32.7 | |
| Overdrafts | | | (11.1 | ) | | | 4.0 | | | | (0.1 | ) | | | (7.2 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 45.8 | | | | (26.3 | ) | | | 6.0 | | | | 25.5 | |
| Liquid resources | | | 96.5 | | | | (74.5 | ) | | | 0.1 | | | | 22.1 | |
| Loans due within one year | | | (19.7 | ) | | | (14.7 | ) | | | (3.0 | ) | | | (37.4 | ) |
| Loans due after more than one year | | | (148.0 | ) | | | (133.6 | ) | | | 89.1 | | | | (192.5 | ) |
| | | |
| | | |
| | | |
| | | |
| |
Net debt | | | (25.4 | ) | | | (249.1 | ) | | | 92.2 | | | | (182.3 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | |
| | | As at | | | | | | Exchange | | As at |
| | | Jan 1, 2001 | | Cash flow | | movements | | Dec 31, 2001 |
| | | £m | | £m | | £m | | £m |
| | |
| |
| |
| |
|
| Cash at bank and in hand | | | 50.1 | | | | 7.8 | | | | (1.0 | ) | | | 56.9 | |
| Overdrafts | | | (10.9 | ) | | | — | | | | (0.2 | ) | | | (11.1 | ) |
| | | |
| | | |
| | | |
| | | |
| |
| | | | 39.2 | | | | 7.8 | | | | (1.2 | ) | | | 45.8 | |
| Short term deposits and interest bearing investments | | | 4.7 | | | | 91.4 | | | | 0.4 | | | | 96.5 | |
| Loans due within one year | | | (2.0 | ) | | | (17.9 | ) | | | 0.2 | | | | (19.7 | ) |
| Loans due after more than one year | | | (365.4 | ) | | | 223.2 | | | | (5.8 | ) | | | (148.0 | ) |
| | | |
| | | |
| | | |
| | | |
| |
Net debt | | | (323.5 | ) | | | 304.5 | | | | (6.4 | ) | | | (25.4 | ) |
| | | |
| | | |
| | | |
| | | |
| |
34 RECONCILIATION OF NET CASH FLOW TO MOVEMENT IN NET DEBT
| | | | | | | | | | | | | |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
| |
|
Increase/(decrease) in cash in period | | | 0.8 | | | | 7.8 | | | | (26.3 | ) |
| Cash (inflow)/outflow from liquid resources | | | (64.1 | ) | | | 91.4 | | | | (74.5 | ) |
| Cash (inflow)/outflow from reduction/increase in loans and lease finance | | | 70.3 | | | | 205.3 | | | | (148.3 | ) |
| | | |
| | | |
| | | |
| |
| Change in net debt resulting from cash flows | | | 7.0 | | | | 304.5 | | | | (249.1 | ) |
| Exchange movements | | | (31.6 | ) | | | (6.4 | ) | | | 92.2 | |
| | | |
| | | |
| | | |
| |
Movement in net debt in the period | | | (24.6 | ) | | | 298.1 | | | | (156.9 | ) |
| Net debt at January 1 | | | (298.9 | ) | | | (323.5 | ) | | | (25.4 | ) |
| | | |
| | | |
| | | |
| |
Net debt at December 31 | | | (323.5 | ) | | | (25.4 | ) | | | (182.3 | ) |
| | | |
| | | |
| | | |
| |
35 RELATED PARTY DISCLOSURES
Apart from the transactions noted below there were no other material items requiring disclosure.
Transactions with Nihon Medi-Physics Company Limited (NMP)
During 2002 the Group made sales to NMP of £7.6m (2001 — £6.6m, 2000 — £7.5m) and purchases of £0.1m, (2001 — £0.1m, 2000 — £0.1m). At the year end the Group was owed £1.0m (2001 — £1.3m, 2000 — £2.1m) in respect of trading balances.
Transactions with Reviss Services Limited (RSL)
During 1996 the Group disposed of its Puridec business to RSL, then an associated undertaking, for a consideration of £5.9m payable within a seven year period. At December 31, 2002 the group held a 10% trade investment in RSL. During 2002 the Group recharged RSL in relation to the provision of payroll and other services £0.8m (2001 — £1.1m, 2000 — £1.5m) and made purchases of £2.9m (2001 — £4.7m, 2000 —
F-53
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
£4.5m) in respect of normal trading activities. At December 31, 2002 the Group owed RSL £0.2m (2001 — £0.1m, 2000 — £0.2m) and was owed £0.6m, (2001 — £1.3m, 2000 — £2.2m) comprising £0.6m (2001 — £0.6m, 2000 — £0.5m) in respect of other services and £nil, (2001 — £0.7m, 2000 — £1.7m) in relation to the sale of Puridec business.
Transactions with Gyros AB
During 2002 the Group made milestone payments of £nil (2001 — £1.0m, 2000 - £2.8m) to Gyros AB. The Group holds a 9.7% interest in Gyros AB. At December 31, 2002 the Group owed Gyros AB £nil (2001 — £nil, 2000 — £1.1m).
Transactions with Cimarron Software Inc
From January 1, 2002 to May 10, 2002 the Group held a trade investment in Cimarron Software Inc representing 19% of that company’s equity. During the period to May 10, 2002 the Group paid £0.5m (2001 — £1.1m, 2000 — £0.8m) to Cimarron Software Inc in respect of normal trading activities. From May 10, 2002 onwards, Cimarron Software Inc has been consolidated as a subsidiary undertaking.
Transactions with Molecular Staging Inc.
The Group holds a trade investment in Molecular Staging Inc. During 2002, the Group made sales of £nil (2001 — £0.2m, 2000 — £nil) to Molecular Staging Inc, and made purchases of £0.8m (2001 — £0.4m, 2000 — £0.8m), in respect of normal trading activities.
Transactions with Nycomed Pharma
During 2001 the Group made sales to Nycomed Pharma of £2.8m (2000 — £7.0m) and purchased £4.0m (2000 — £2.7m) in respect of normal trading activities to the date of disposal.
Loan to Dr A Carr
The Group has agreed to provide an interest free, secured loan to Dr A Carr, the Chief Executive of Amersham Biosciences, of up to $300,000 to assist in the purchase of a house following his relocation to the US. The loan will be repayable over five years in equal bi-weekly installments or on his ceasing to be employed by the Group, if earlier. The balance due on the loan at December 31, 2002 was $231,000 (2001 — $291,000, 2000 — $162,000). The loan has been repaid since the year end.
Transactions with MU Biotech AB
During the year, the Group made royalty payments to MU Biotech AB, a company of which Professor Uhlen is the sole director and shareholder, of SEK 800,000, with an additional SEK 400,000 accrued payments at December 31, 2002. These payments were made in respect of royalty agreements entered into prior to the appointment of Professor Uhlen as a non-executive director.
36 CONTINGENT LIABILITIES
The Group is involved in various negotiations or disputes of a nature considered typical for its businesses, including those relating to product liability and infringements and claims of intellectual property rights, validity of patents and taxation. Although there can be no assurance regarding the outcome of any of these negotiations or disputes, the Group believes that on the basis of current available information they will not have a material adverse impact on the Group’s financial position.
37 RESTATEMENT OF PRIOR PERIODS
Following the introduction of Financial Reporting Standard 19, ‘Deferred Taxation’ in 2002, the Group has changed its accounting policy with respect to deferred taxation. Under the previous policy, provision was made for deferred taxation, using the liability method, on all material timing differences to the extent that it was probable that an asset or liability would crystallise. Under the revised accounting policy, liabilities will be recognized for most types of timing differences regardless of whether they are anticipated to reverse in the foreseeable future. Deferred taxation assets will be recognized to the extent that it is more likely than not that they will reverse.
F-54
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The adjustments required to shareholders’ funds are set out below:
| | | | | | | | | |
| | | 12 months | | 12 months |
| | | to Dec 31, | | to Dec 31, |
| | | 2001 | | 2000 |
| | | £m | | £m |
| | |
| |
|
| Adjustment to opening shareholders’ funds | | | 8.5 | | | | — | |
| Effect of adoption of FRS 19 at January 1, 2001 | | | — | | | | 12.3 | |
| Reverse deferred tax provision under SSAP 15 | | | 4.5 | | | | — | |
| Deferred tax provision under FRS 19 | | | (9.0 | ) | | | — | |
| Attributable to minority interest – FRS 19 | | | 0.4 | | | | (3.8 | ) |
| | | |
| | | |
| |
| Adjustment to closing shareholders’ funds | | | 4.4 | | | | 8.5 | |
| | | |
| | | |
| |
In the year ended December 31, 2001, deferred tax charged under FRS 19 amounted to £9.0m; this would have been £4.5m under SSAP 15. For the year ended December 31, 2002, deferred tax charged under FRS 19 amounted to £23.1m; this would have been £18.9m under SSAP 15.
F-55
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| 38. | | SUMMARY OF DIFFERENCES BETWEEN UK AND US GENERALLY ACCEPTED ACCOUNTING PRINCIPLES (“GAAP’’) |
The accompanying financial statements have been prepared in accordance with UK GAAP, which differs in certain material respects from US GAAP. Such differences involve methods for measuring the amounts shown in the financial statements, as well as additional disclosures required by US GAAP.
The effect on the Group’s net income and shareholders’ equity of applying the significant differences between UK GAAP and US GAAP is summarized in the tabular reconciliation statement set out below:
Reconciliation of consolidated profit and loss accounts
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | 12 months to | | 12 months to | | | | |
| | | | | | | | | Dec 31, 00 | | Dec 31, 01 | | 12 months to |
| | | | | | | | | (restated) | | (restated) | | Dec 31, 02 |
| | | | | | | | | £m | | £m | | £m |
| | | | | | | | |
| |
| |
|
| Profit attributable to shareholders reported under UK GAAP | | | | | | | 107.0 | | | | 210.7 | | | | 178.7 | |
US GAAP adjustments: | | | | | | | | | | | | | | | | |
| Business combinations: | | | | | | | (118.0 | ) | | | (107.7 | ) | | | (5.6 | ) |
| | Effect of SFAS 142 | | | (i | ) | | | — | | | | — | | | | 36.6 | |
| | Goodwill amortization | | | (i | ) | | | (74.9 | ) | | | (65.1 | ) | | | — | |
| | Amortization of intangible assets | | | (i | ) | | | (41.1 | ) | | | (41.8 | ) | | | (42.2 | ) |
| | Write-off of purchased in-process R&D | | | (i | ) | | | (2.0 | ) | | | — | | | | — | |
| | Revaluation of inventory | | | (i | ) | | | — | | | | (0.8 | ) | | | — | |
| Accounting for purchase of minority interest in Amersham Biosciences: | | | | | | | — | | | | — | | | | (108.2 | ) |
| | Amortization of intangible assets | | | (i | ) | | | — | | | | — | | | | (41.4 | ) |
| | Write-off of purchased in-process R&D | | | (i | ) | | | — | | | | — | | | | (47.7 | ) |
| | Revaluation of inventory | | | (i | ) | | | — | | | | — | | | | (19.1 | ) |
| Investment in Nycomed Pharma | | | (i | ) | | | (2.1 | ) | | | (0.3 | ) | | | — | |
| Disposal of Nycomed Pharma | | (iii) | | | — | | | | (10.4 | ) | | | (1.2 | ) |
| Revenue recognition | | (ii) | | | 7.6 | | | | (2.9 | ) | | | (1.2 | ) |
| Long term incentive plans | | (iv) | | | 0.6 | | | | 0.8 | | | | 0.4 | |
| Financial instruments hedging | | (vi) | | | 17.6 | | | | 5.6 | | | | 66.4 | |
| Stock-based compensation | | (vii) | | | (14.1 | ) | | | (7.9 | ) | | | 2.9 | |
| Pensions | | (viii) | | | (1.8 | ) | | | 0.9 | | | | (4.4 | ) |
| Transfer of assets from former pension plans | | (ix) | | | — | | | | 6.0 | | | | (1.0 | ) |
| Accounting for investment in NMP: | | | | | | | | | | | | | | | | |
| | | Goodwill amortization | | | (i | ) | | | (3.6 | ) | | | (3.6 | ) | | | — | |
| Investments in equity securities | | | (v | ) | | | — | | | | (1.6 | ) | | | (1.2 | ) |
| Other | | | | | | | (1.2 | ) | | | (1.3 | ) | | | (1.8 | ) |
| Deferred taxation: | | | | | | | | | | | | | | | | |
| | Application of full liability method | | (xi) | | | 35.8 | | | | 5.6 | | | | 5.9 | |
| | Tax effect of US GAAP adjustments | | (xi) | | | 30.0 | | | | 20.5 | | | | 29.7 | |
| Cumulative effect of changes in revenue recognition as at January 1, 2000 net of tax | | (ii) | | | (10.8 | ) | | | — | | | | — | |
| Minority interest | | (xii) | | | 17.6 | | | | 10.0 | | | | 1.1 | |
| | | | | | | |
| | | |
| | | |
| |
| Total US GAAP adjustments | | | | | | | (42.4 | ) | | | (86.3 | ) | | | (18.2 | ) |
| | | | | | | |
| | | |
| | | |
| |
| Net income in accordance with US GAAP | | | | | | | 64.6 | | | | 124.4 | | | | 160.5 | |
| | | | | | | |
| | | |
| | | |
| |
| Basic earnings per share in accordance with US GAAP | | | | | | | 10.2p | | | | 19.6p | | | | 23.4p | |
| Diluted earnings per share in accordance with US GAAP* | | | | | | | 10.1p | | | | 19.5p | | | | 23.3p | |
*Options exercisable under the Group’s share option schemes (see note 25) could dilute basic earnings per share in the future.
F-56
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Reconciliation of shareholders’ equity
| | | | | | | | | | | | | | | | | | |
| | | | | | | | 12 months to | | 12 months to | | | | |
| | | | | | | | Dec 31, 00 | | Dec 31, 01 | | 12 months to |
| | | | | | | | (restated) | | (restated) | | Dec 31, 02 |
| | | | | | | | £m | | £m | | £m |
| | | | | | | |
| |
| |
|
| Equity shareholders’ funds reported under UK GAAP | | | | | | | 416.9 | | | | 596.3 | | | | 1,172.0 | |
US GAAP adjustments: | | | | | | | | | | | | | | | | |
| Business combinations: | | | | | | | 1,757.6 | | | | 1,761.6 | | | | 1,829.3 | |
| | Effect of SFAS 142 | | | | | | | — | | | | — | | | | 36.6 | |
| | Goodwill | | | (i | ) | | | 1,240.4 | | | | 1,237.7 | | | | 1,242.5 | |
| | Intangible assets | | | (i | ) | | | 364.3 | | | | 371.8 | | | | 398.1 | |
| | Purchased in-process R&D | | | (i | ) | | | 152.9 | | | | 152.9 | | | | 152.9 | |
| | Revaluation of inventory | | | (i | ) | | | — | | | | (0.8 | ) | | | (0.8 | ) |
| Accumulated amortization: | | | | | | | (558.6 | ) | | | (667.9 | ) | | | (753.2 | ) |
| | Goodwill | | | (i | ) | | | (240.1 | ) | | | (304.8 | ) | | | (308.9 | ) |
| | Intangible assets | | | (i | ) | | | (161.6 | ) | | | (206.2 | ) | | | (287.4 | ) |
| | Purchased in-process R&D written off | | | (i | ) | | | (156.9 | ) | | | (156.9 | ) | | | (156.9 | ) |
| Accounting for purchase of minority interest in Amersham Biosciences: | | | | | | | — | | | | — | | | | (8.6 | ) |
| | Goodwill | | | (i | ) | | | — | | | | — | | | | (391.2 | ) |
| | Intangible assets | | | (i | ) | | | — | | | | — | | | | 422.9 | |
| | Intangible assets — accumulated amortization | | | (i | ) | | | — | | | | — | | | | (40.3 | ) |
| | Purchased in-process R&D | | | (i | ) | | | — | | | | — | | | | 47.7 | |
| | Purchased in-process R&D written off | | | (i | ) | | | — | | | | — | | | | (47.7 | ) |
| Investment in Nycomed Pharma | | | (i | ) | | | 11.0 | | | | 1.5 | | | | — | |
| Long term incentive plan | | (iv) | | | (1.1 | ) | | | (0.3 | ) | | | 0.1 | |
| Stock-based compensation | | (vii) | | | — | | | | 0.6 | | | | 0.2 | |
| Share ownership trust (QUEST) | | (iv) | | | (16.4 | ) | | | (19.5 | ) | | | (16.4 | ) |
| Investments in equity securities | | | (v | ) | | | — | | | | (1.4 | ) | | | (2.4 | ) |
| Financial instruments hedging | | (vi) | | | 13.3 | | | | 12.8 | | | | 32.9 | |
| Pensions | | (viii) | | | (5.8 | ) | | | (26.1 | ) | | | (73.5 | ) |
| Transfer of assets from former pension plans | | (ix) | | | — | | | | 6.0 | | | | 5.0 | |
| Accounting for investment in NMP: | | | | | | | 45.3 | | | | 41.7 | | | | 41.7 | |
| | Step-by-step acquisition/foreign exchange | | | (x | ) | | | 0.1 | | | | 0.1 | | | | 0.1 | |
| | Goodwill | | | (i | ) | | | 76.9 | | | | 76.9 | | | | 76.9 | |
| | Accumulated amortization of goodwill | | | (i | ) | | | (19.9 | ) | | | (23.5 | ) | | | (23.5 | ) |
| | Purchased in-process R&D written off | | | (i | ) | | | (11.8 | ) | | | (11.8 | ) | | | (11.8 | ) |
| Revenue recognition | | (ii) | | | — | | | | (2.9 | ) | | | (3.9 | ) |
| Deferred taxation: | | | | | | | | | | | | | | | | |
| | Application of full liability method | | (xi) | | | 22.7 | | | | 28.7 | | | | 27.6 | |
| | Tax effect of US GAAP adjustments | | (xi) | | | (75.9 | ) | | | (55.7 | ) | | | (142.8 | ) |
| Other | | | | | | | (1.0 | ) | | | (3.1 | ) | | | (3.8 | ) |
| Minority interest | | (xii) | | | (85.2 | ) | | | (75.6 | ) | | | — | |
| Ordinary dividends | | (xiii) | | | 26.9 | | | | 30.3 | | | | 36.0 | |
| | | | | | | |
| | | |
| | | |
| |
| Total US GAAP adjustments | | | | | | | 1,132.8 | | | | 1,030.7 | | | | 968.2 | |
| | | | | | | |
| | | |
| | | |
| |
| Shareholders’ equity in accordance with US GAAP | | | | | | | 1,549.7 | | | | 1,627.0 | | | | 2,140.2 | |
| | | | | | | |
| | | |
| | | |
| |
Figures for the 12 months ended December 31, 2000 and 2001 have been restated following the introduction of FRS 19 as explained in Note 37.
F-57
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Net income for the 12 months to December 31, 2000 and 2001, and shareholders’ equity at December 31, 2000 and 2001 in accordance with US GAAP have been restated as outlined in notes (iv), (vi), (ix) and (xi) below.
The effect of these restatements on the 12 months to December 31, 2000 reduces net income by £8.2m from £72.8m to £64.6m, with a corresponding 1.3p reduction in basic net income per share for the 12 months to December 31, 2000. The restatements have reduced shareholders’ equity at December 31, 2000 by £8.2m, from £1,557.9m to £1,549.7m.
The effect of these restatements on the 12 months to December 31, 2001 increases net income by £16.1m, from £108.3m to £124.4m, with a corresponding 2.5p increase in basic net income per share for the 12 months to December 31, 2001. The restatements have reduced shareholders’ equity by £1.6m, from £1,628.6m to £1,627.0m at December 31, 2001.
| (i) | | Business combinations |
Under both UK GAAP and US GAAP acquisitions are accounted for as purchase business combinations. However, certain differences between UK GAAP and US GAAP in the application of the purchase method of accounting for business combinations are set out below.
Goodwill, intangible and other assets
Both UK GAAP and US GAAP require purchase consideration to be allocated to the net assets acquired at their fair value on the date of acquisition, with the difference between the consideration and the fair value of the identifiable net assets recorded as goodwill. Under the requirements of FRS10 “Goodwill and Intangible Assets”, goodwill arising on acquisitions of subsidiaries, joint ventures, and associates during accounting periods ending on or after December 23, 1998 should be capitalized and amortized over its useful economic life. This treatment of goodwill is similar to its treatment under US GAAP, prior to the introduction of Statement of Financial Accounting Standards (SFAS) 142 “Goodwill and other intangible assets”. The impact of the introduction of SFAS 142 is discussed below. However, because FRS 10 need not be applied retrospectively to goodwill written off against shareholders’ funds in earlier years, there will continue to be differences between the goodwill calculated under UK GAAP and US GAAP. For the purposes of US GAAP, goodwill written off against shareholders’ funds under UK GAAP has been reinstated.
UK GAAP requires an allocation of purchase consideration to intangible assets which are separable from the business. For UK GAAP purposes, intangible assets, other than goodwill, acquired in a business combination, which are not separable and measurable, are not capitalized. US GAAP requires an allocation of consideration to identifiable intangible assets if they arise from contractual or other legal rights. If intangible assets do not arise from contractual or legal rights, the asset is recognized apart from goodwill only if it is separable. The intangible assets are being amortized on a straight-line basis over periods up to 10 years.
Effect of SFAS 142 “Goodwill and other intangible assets”
Under US GAAP, following the introduction of SFAS 142, goodwill and intangible assets with indefinite useful lives are not amortized, but reviewed annually for impairment. The 2002 amortization charge on such goodwill recognized for UK GAAP has been reversed for US GAAP purposes. The impact of the introduction of SFAS 142 on net income per share is analyzed below:
F-58
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | | | |
| | | Dec 31, 00 | | Dec 31, 01 | | 12 months to |
| | | (restated) | | (restated) | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
Net Income | | | | | | | | | | | | |
| Net income as reported under US GAAP | | | 64.6 | | | | 124.4 | | | | 160.5 | |
| Add back: goodwill amortization | | | 89.2 | | | | 80.4 | | | | — | |
| | | |
| | | |
| | | |
| |
Net income under US GAAP adjusted for SFAS 142 | | | 153.8 | | | | 204.8 | | | | 160.5 | |
| | | |
| | | |
| | | |
| |
Net income per share – basic | | | p | | | | p | | | | p | |
| Net income per share as reported under US GAAP | | | 10.2 | | | | 19.6 | | | | 23.4 | |
| Add back: goodwill amortization | | | 14.1 | | | | 12.7 | | | | — | |
| | | |
| | | |
| | | |
| |
Net income per share (basic) as reported under US GAAP adjusted for SFAS 142 | | | 24.3 | | | | 32.3 | | | | 23.4 | |
| | | |
| | | |
| | | |
| |
Net income per share – diluted | | | p | | | | p | | | | p | |
| Net income per share (diluted) as reported under US GAAP | | | 10.1 | | | | 19.5 | | | | 23.3 | |
| Add back: goodwill amortization | | | 14.0 | | | | 12.6 | | | | — | |
| | | |
| | | |
| | | |
| |
Net income per share (diluted) as reported under US GAAP adjusted for SFAS 142 | | | 24.1 | | | | 32.1 | | | | 23.3 | |
| | | |
| | | |
| | | |
| |
Amortization of Intangible assets
The estimated aggregate amortization expense under US GAAP for each of the five succeeding years, is as follows:
| | | | | |
| | | £m |
| | |
|
| 12 months to: | | | | |
| December 31, 2003 | | | 100.9 | |
| December 31, 2004 | | | 94.5 | |
| December 31, 2005 | | | 67.4 | |
| December 31, 2006 | | | 65.4 | |
| December 31, 2007 | | | 61.4 | |
Purchased research and development
Acquired in-process research and development expenditure recognized under US GAAP purchase accounting requirements is written off directly to net income in the year of acquisition: the technological feasibility of such in-process technology not having been established and not having an alternative future use. Acquired research and development is not recognized as a separate asset under UK GAAP.
Purchase of 45% minority interest in Amersham Biosciences
Under UK GAAP, fair value adjustments have been recognized for tangible fixed assets, listed investments and the funded status of pensions. Under US GAAP, additional fair value adjustments have been recognized for separable intangible assets, in-process research and development and the revaluation of inventory.
The book and fair values of the 45% net assets purchased, under US GAAP, are set out below:
F-59
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | |
| | | March 21, 02 |
| | | £m |
| | |
|
| Book value of net assets purchased | | | 143.4 | |
| Net book value of US GAAP goodwill subsumed on purchase | | | (96.5 | ) |
| Fair value adjustment: | | | | |
| - tangible assets | | | 13.4 | |
| - intangible fixed assets | | | 446.2 | |
| - in-process research and development | | | 47.7 | |
| - revaluation of inventory | | | 19.1 | |
| - pensions | | | 0.3 | |
| | | |
| |
| Fair value of net assets published | | | 573.6 | |
| Cost of purchase | | | 708.6 | |
| | | |
| |
| Goodwill arising on purchase (US GAAP) | | | 135.0 | |
| | | |
| |
The intangible assets recognized are being written-off on a straight-line basis over periods up to 10 years. The in-process research and development asset has been written-off to income in the year. Following the introduction of SFAS 142, goodwill amortization recorded under UK GAAP has been reversed for US GAAP purposes.
Investment in Nycomed Pharma
The remaining 29% investment in Nycomed Pharma was being recorded under the cost method of accounting under UK GAAP prior to its disposal as the Company did not exert significant influence over the entity. Under US GAAP, the investment was accounted for using the equity method.
Revaluation of inventory
Under UK GAAP inventory is recognized at replacement cost. Under US GAAP inventory acquired in a business combination is recognized at selling price less a margin for selling.
The introduction by the US Securities and Exchange Commission of its Staff Accounting Bulletin 101 required certain amendments under US GAAP to the revenue recognition policies of the Group. At January 1, 2000 the cumulative effect of these changes in revenue recognition was a charge of £16.5m, together with a corresponding tax credit of £5.7m.
Under UK GAAP, contract revenue is recognized when it is earned and non-refundable and when there are no future obligations under the contract terms. Under US GAAP, more prescriptive criteria are applied to assess whether the culmination of the earnings process has occurred and the Group has a continuing obligation throughout the contract terms. As a result, in the years ending December 31, 2001 and 2002, certain non-refundable fees have been deferred over the term of the contract under US GAAP.
| (iii) | | Disposal of Nycomed Pharma |
In 2001, the Group sold its trade investment in Nycomed Pharma. Under US GAAP, the investment was treated as an associate company resulting in a lower profit on disposal under the equity method of accounting. Certain costs associated with the sale have been recognized in the year ended December 31, 2002.
| (iv) | | Long term incentive plan/share ownership trust |
Under UK GAAP, repurchases of share capital are recorded as fixed asset investments at cost and amortized over the life of the Company’s long-term incentive plan. Under US GAAP, repurchases of share capital are recorded at cost as treasury stock within shareholders’ equity.
F-60
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
During the year ended December 31, 2002, the Group determined that the adjustment to shareholders equity at December 31, 2001 relating to ESOP transactions had been incorrectly calculated. Correspondingly, the adjustment to shareholders’ equity at December 31, 2001 has been restated with a reduction of £3.1m to £19.5m.
| (v) | | Investments in equity securities |
Under UK GAAP, equity securities held for the long-term are recorded in the balance sheet at cost, less any provision for impairment. Under US GAAP, available-for-sale securities are carried at fair (i.e. market) value with unrealized holding gains and losses included as a separate component of equity, net of tax effects. If a decline in an available for sale security is considered other than temporary the loss is regarded as realized and taken to current earnings.
| (vi) | | Financial instruments hedging |
Under US GAAP, the Group does not qualify for hedge accounting, therefore gains and losses arising on derivative instruments have been booked directly to income.
During the year ended December 31, 2002, the Group determined that the adjustment relating to gains and losses arising on financial instruments for the years ending December 31, 2000 and December 31, 2001 had been incorrectly accounted for. Accordingly, the Group has restated the years to December 31, 2000 and December 31, 2001. The adjustment to net income in the year ending December 31, 2000 has reduced by £11.7m, and the adjustment to shareholders’ equity has reduced by £11.7m to £13.3m at December 31, 2000. The adjustment to net income in the year ending December 31, 2001 has correspondingly increased by £21.0m to £5.6m at December 31, 2001, with a cumulative increase to shareholders equity of £3.2m to £12.8m.
| (vii) | | Stock-based compensation |
Under UK GAAP, the Company does not recognize a compensation expense under its share option plans. Under US GAAP, the compensation expense associated with shares issued through the share option plans, in consideration for services received, is recognized as the difference between the quoted market price of the stock, at the measurement date, and the exercise price of the option. Compensation costs, as determined above, are expensed over the period in which the related service is provided. The Group chose to continue applying the recognition provisions of APB 25 under US GAAP. The pro forma disclosures required under SFAS 123 are presented in Note 39(e). Under UK GAAP, National Insurance contributions payable by the Company at the exercise of the options should be accrued over the relevant vesting period. Under US GAAP, such costs must be recognized at the exercise date of the option.
Under UK GAAP, the cost of providing defined benefit pension arrangements is expensed over the average expected service lives of eligible employees on the basis of a constant percentage of current and estimated future earnings. Under US GAAP, SFAS 87Employers’ Accounting for Pensions, requires that the cost is determined based on a comparison of the projected benefit obligation with the market value of the underlying plan assets and other unrecognized gains and losses assessed on an actuarial basis. Principally as a result of this difference in methodology, the Group’s US GAAP pension cost can be significantly different from that determined under UK GAAP and tends to be more sensitive to changing economic conditions. In line with SFAS 87 an additional minimum liability of £65.9m at December 31, 2002, and £21.3m at December 31, 2001, has been recorded, of which £65.0m at December 31, 2002 and £20.1m at December 31, 2001 has been charged to shareholders’ equity.
| (ix) | | Transfer of assets from pension plans |
A transfer of assets was received from former pension plans in 2001. Under US GAAP, the proportion of the transfer relating to current employees of the Group is being spread over the remaining service lives of the employees under SSAP 24. Under US GAAP, this was taken to income in 2001.
During the year ended December 31, 2002, the Group determined that income recognized in the year ending December 31, 2001 relating to the transfer of assets from further pension plans had been incorrectly accounted for under US GAAP. Net income in the year ending December 31, 2001 has been restated and
F-61
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
income recognized on the transfer reduced by £1.0m to £6.0m. The adjustments to shareholders equity at December 31, 2001 has also been restated, with a corresponding reduction of £1.0m to £6.0m.
| (x) | | Accounting for investment in NMP |
Under UK GAAP, the change from the cost method to the equity method in the year ended March 31, 1997, due to the acquisition of an additional interest in NMP, did not require retroactive adjustment to the financial statements. Under US GAAP the investment, results of operations, and shareholders’ equity of the Group have been adjusted retroactively in a manner consistent with the accounting for a step-by-step acquisition of a subsidiary.
Under UK GAAP, provision for deferred taxation is recorded under FRS 19. Except for certain timing differences relating to revaluations, rolled over taxable gains and unremitted income, provision is made for all timing differences that would give rise to a deferred tax liability. Deferred tax assets are recognized to the extent that it is more likely than not they will be recoverable. Under US GAAP, deferred taxation is provided for on a full liability basis. A valuation allowance is established when it is more likely than not that some portion of all of the deferred tax asset will not be realized.
Following the adjustments to net income in the years ending December 31, 2000 and December 31, 2001, as outlined in notes (vi) and (ix), the tax effects of the US GAAP adjustments have been increased by £3.5m to £30.0m in the year ending December 31, 2000, with a corresponding reduction in shareholders’ equity by £3.5m to £75.9m. In the year ending December 31, 2001, the tax effect of the US GAAP adjustments have been reduced by £3.9m to £20.5m, and the adjustment to shareholders’ equity of December 31, 2001 correspondingly reduced by £0.7m to £55.7m.
This amount represents the components of the US GAAP adjustment attributable to Pharmacia Corporation’s 45% interest in Amersham Biosciences. The minority interest was extinguished during the year ended December 31, 2002 on the purchase of the 45% minority interest in Amersham Biosciences that the Group did not already own.
| (xiii) | | Ordinary dividends |
Under UK GAAP, dividends proposed are provided for on the basis of recommendation by the Board of Directors for approval by the shareholders. Under US GAAP, dividends are recorded as a reduction to retained earnings when they have been formally declared and notice given to shareholders.
| (xiv) | | Cash flow information |
Under UK GAAP, the Consolidated Cash Flow Statements are presented in accordance with FRS 1. The Statements prepared under FRS 1 present substantially the same information as that required under US GAAP as interpreted by SFAS 95.
Under UK GAAP, cash comprises cash in hand and at bank (including overnight deposits), net of bank overdrafts. Under US GAAP, cash and cash equivalents include cash and short-term investments with original maturities of three months or less.
Under UK GAAP, cash flows are presented for operating activities; returns on investments and servicing of finance; taxation; capital expenditure and financial investment; acquisitions and disposals; equity dividends paid; management of liquid resources and financing. US GAAP requires the classification of cash flows as resulting from operating, investing and financing activities.
Cash flows under US GAAP in respect of interest received, interest paid, investment income and taxation would be included within operating activities. Capital expenditure and financial investment and cash flows from acquisitions and disposals would be included within investing activities under US GAAP. Dividends paid by subsidiary undertakings to minority interests, equity dividends paid and management of liquid resources would be included within financing activities under US GAAP.
F-62
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Management of liquid resources under UK GAAP represents current asset investments that are held as readily disposable stores of value. Under UK GAAP, short term deposits, interest bearing and other investments are classified within management of liquid resources; under US GAAP short term deposits have been classified as cash and cash equivalents.
A summary of the Group’s operating, investing and financing activities, classified in accordance with US GAAP, is presented below. For purposes of this summary, cash and cash equivalents consist of cash at bank and in hand, and short term deposits.
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Cash provided by operating activities | | | 195.8 | | | | 302.1 | | | | 289.4 | |
| Cash provided by/(used in) investing activities | | | (155.4 | ) | | | 29.6 | | | | (901.6 | ) |
| Cash used in financing activities | | | (60.4 | ) | | | (230.3 | ) | | | 507.4 | |
| | | |
| | | |
| | | |
| |
| Net (decrease) / increase in cash and cash equivalents under US GAAP | | | (20.0 | ) | | | 101.4 | | | | (104.8 | ) |
| Effect of exchange rate changes on cash and cash equivalents | | | (9.1 | ) | | | (0.6 | ) | | | 6.2 | |
| Cash and cash equivalents under US GAAP at the beginning of the year | | | 81.7 | | | | 52.6 | | | | 153.4 | |
| | | |
| | | |
| | | |
| |
| Cash and cash equivalents under US GAAP at the end of the year | | | 52.6 | | | | 153.4 | | | | 54.8 | |
| Less: Investments within three months of maturity when acquired (short term deposits) | | | (2.5 | ) | | | (96.5 | ) | | | (22.1 | ) |
| Add: Overdrafts | | | (10.9 | ) | | | (11.1 | ) | | | (7.2 | ) |
| | | |
| | | |
| | | |
| |
| Cash under UK GAAP at the end of the period | | | 39.2 | | | | 45.8 | | | | 25.5 | |
| | | |
| | | |
| | | |
| |
Consistent with UK GAAP, the cash flows of the overseas subsidiary and associated undertakings are translated at the average exchange rate of the period during which they arise.
| (xv) | | Comprehensive income |
For the Amersham Group the statement of total recognized gains and losses is largely the same as if a statement of comprehensive income had been presented in accordance with SFAS 130, “Reporting Comprehensive Income”. Under US GAAP, the unrealized holding gains and losses on equity securities and the additional minimum pension liability as presented in items (v) and (viii) of the US GAAP reconciliation, would be recognized in the statement of comprehensive income.
Additional Information
Presentation of income from interests in associated undertakings
In accordance with US GAAP, the income from interests in associated undertakings would be presented in the profit and loss account net of tax as follows:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | | £m | | £m | | £m |
| | |
| |
| |
|
| Income from interests in associated undertakings | | | 29.8 | | | | 26.1 | | | | 23.2 | |
| Net interest | | | (0.6 | ) | | | (0.6 | ) | | | (0.6 | ) |
| Tax | | | (13.3 | ) | | | (11.7 | ) | | | (10.1 | ) |
| | | |
| | | |
| | | |
| |
| | | | 15.9 | | | | 13.8 | | | | 12.5 | |
| | | |
| | | |
| | | |
| |
| 39 | | ADDITIONAL US GAAP DISCLOSURE REQUIREMENTS |
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the accounting period. Accounting estimates have been employed in these financial statements to determine reported amounts, including realizability, useful lives of assets and corporate taxes. Actual results could differ from those estimates.
F-63
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| (b) | | Earnings/(loss) per share |
Basic and diluted earnings per share in accordance with US GAAP is calculated as follows:
| | | | | | | | | | | | | |
| | | Net | | | | | | Net earnings |
| | | income | | Shares | | per share |
| | |
| |
| |
|
| | | £m | | m | | p |
Year ended December 31, 2002 | | | | | | | | | | | | |
| Basic earnings per share | | | 160.5 | | | | 684.7 | | | | 23.4p | |
| Effect of potentially dilutive securities – share options | | | | | | | 4.0 | | | | | |
| | | | | | | |
| | | |
| |
| Diluted earnings per share | | | | | | | 688.7 | | | | 23.3p | |
| | | | | | | |
| | | |
| |
Year ended December 31, 2001 | | | | | | | | | | | | |
| Basic earnings per share | | | 124.4 | | | | 634.4 | | | | 19.6p | |
| Effect of potentially dilutive securities – share options | | | | | | | 4.3 | | | | | |
| | | | | | | |
| | | |
| |
| Diluted earnings per share | | | | | | | 638.7 | | | | 19.5p | |
| | | | | | | |
| | | |
| |
Year ended December 31, 2000 | | | | | | | | | | | | |
| Basic earnings per share | | | 64.6 | | | | 630.9 | | | | 10.2p | |
| Effect of potentially dilutive securities – share options | | | | | | | 7.4 | | | | | |
| | | | | | | |
| | | |
| |
| Diluted earnings per share | | | | | | | 638.3 | | | | 10.1p | |
| | | | | | | |
| | | |
| |
F-64
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The net pension cost in accordance with SFAS 87 for the defined benefit plans in the UK, US, Norway and Sweden were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefits |
| | |
|
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | |
| |
| |
|
| | | UK | | Other | | UK | | Other | | UK | | Other |
| | |
| |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m | | £m |
| Change in benefit obligation: | | | | | | | | | | | | | | | | | | | | | | | | |
| Benefit obligation at beginning of period | | | 162.4 | | | | 101.6 | | | | 219.5 | | | | 117.7 | | | | 240.3 | | | | 130.3 | |
| Service cost | | | 4.0 | | | | 5.5 | | | | 5.5 | | | | 6.3 | | | | 10.7 | | | | 6.7 | |
| Interest cost | | | 10.7 | | | | 7.1 | | | | 13.0 | | | | 8.0 | | | | 13.8 | | | | 8.7 | |
| Changes in assumptions | | | — | | | | — | | | | (0.1 | ) | | | (3.9 | ) | | | 11.6 | | | | 11.4 | |
| Actuarial loss/(gain) | | | 42.4 | | | | 3.8 | | | | 5.3 | | | | 3.2 | | | | (4.5 | ) | | | (1.1 | ) |
| Settlements | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Benefits paid | | | — | | | | (0.7 | ) | | | (2.9 | ) | | | (3.1 | ) | | | (3.6 | ) | | | (3.9 | ) |
| Foreign currency translation | | | — | | | | 0.4 | | | | — | | | | 2.1 | | | | — | | | | (3.8 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Benefit obligation at end of period | | | 219.5 | | | | 117.7 | | | | 240.3 | | | | 130.3 | | | | 268.3 | | | | 148.3 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Change in plan assets: | | | | | | | | | | | | | | | | | | | | | | | | |
| Fair value of plan assets at beginning of period | | | 175.6 | | | | 68.6 | | | | 192.8 | | | | 74.4 | | | | 190.6 | | | | 77.1 | |
| Actual return on plan assets | | | 10.3 | | | | 1.0 | | | | (6.3 | ) | | | (2.4 | ) | | | (27.5 | ) | | | (5.5 | ) |
| Employer contributions | | | 6.9 | | | | 3.3 | | | | 7.0 | | | | 6.0 | | | | 10.7 | | | | 7.7 | |
| Transfers | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Settlements | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Benefits paid | | | — | | | | (2.4 | ) | | | (2.9 | ) | | | (2.6 | ) | | | (3.6 | ) | | | (2.8 | ) |
| Foreign currency translation | | | — | | | | 3.9 | | | | — | | | | 1.7 | | | | — | | | | (1.3 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Fair value of plan assets at end of period | | | 192.8 | | | | 74.4 | | | | 190.6 | | | | 77.1 | | | | 170.2 | | | | 75.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Funded status | | | (26.7 | ) | | | (43.3 | ) | | | (49.7 | ) | | | (53.3 | ) | | | (98.1 | ) | | | (73.2 | ) |
| Unrecognized net actuarial (gain)/loss | | | 29.8 | | | | (7.6 | ) | | | 55.4 | | | | (0.4 | ) | | | 98.2 | | | | 19.5 | |
| Unrecognized prior service cost | | | — | | | | — | | | | — | | | | (0.3 | ) | | | — | | | | (0.5 | ) |
| Unrecognized transitional asset | | | 1.5 | | | | (0.4 | ) | | | 1.2 | | | | — | | | | 0.9 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Prepaid/(accrued) benefit cost | | | 4.6 | | | | (51.3 | ) | | | 6.9 | | | | (54.0 | ) | | | 1.0 | | | | (54.2 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Weighted-average assumptions: | | | | | | | | | | | | | | | | | | | | | | | | |
| Discount rate | | | 6.00 | % | | | 6.83 | % | | | 5.75 | % | | | 6.99 | % | | | 5.50 | % | | | 6.49 | % |
| Rates of return on plan assets | | | 7.50 | % | | | 7.71 | % | | | 7.25 | % | | | 7.71 | % | | | 7.25 | % | | | 7.51 | % |
| Salary growth | | | 4.50 | % | | | 4.17 | % | | | 4.25 | % | | | 4.05 | % | | | 4.25 | % | | | 4.03 | % |
| Pension increases | | | 2.50 | % | | | 2.25 | % | | | 2.25 | % | | | 2.26 | % | | | 2.25 | % | | | 2.24 | % |
| Components of net periodic benefit cost: | | | | | | | | | | | | | | | | | | | | | | | | |
| Service cost | | | 3.9 | | | | 5.5 | | | | 5.5 | | | | 6.3 | | | | 10.7 | | | | 6.7 | |
| Interest cost | | | 10.7 | | | | 7.1 | | | | 13.0 | | | | 8.0 | | | | 13.8 | | | | 8.7 | |
| Expected return on plan assets | | | (13.9 | ) | | | (5.9 | ) | | | (14.7 | ) | | | (5.9 | ) | | | (13.8 | ) | | | (5.9 | ) |
| Amortization of prior service cost | | | — | | | | 0.1 | | | | — | | | | 0.1 | | | | — | | | | — | |
| Amortization of transitional asset | | | 0.3 | | | | — | | | | 0.3 | | | | — | | | | 0.3 | | | | — | |
| Recognized net actuarial (gain)/loss | | | — | | | | (0.8 | ) | | | 0.5 | | | | — | | | | 2.0 | | | | (0.1 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Net periodic benefit cost | | | 1.0 | | | | 6.0 | | | | 4.6 | | | | 8.5 | | | | 13.0 | | | | 9.4 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
F-65
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The pension increase assumption for the plans in Norway reflects past practice and is not based on current contractual arrangements.
The assets of each funded plan are invested in domestic and overseas equities, in domestic fixed interest and indexed securities and in property. The asset allocation reflects the maturity profile of each plan. None of the plans’ assets comprise shares in the Company or are leased to the Company.
Amounts recognized in the statement of financial position for the defined benefit schemes in the UK, US, Norway and Sweden include:
| | | | | | | | | | | | | | | | | |
| | | at Dec 31, 01 | | at Dec 31, 02 |
| | |
| |
|
| | | UK | | Other | | UK | | Other |
| | |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m |
| Balance sheet (accrued) benefit cost | | | (14.4 | ) | | | (54.0 | ) | | | (58.7 | ) | | | (61.5 | ) |
| Balance sheet prepaid benefit cost | | | — | | | | — | | | | — | | | | 1.1 | |
| Accumulated other comprehensive income | | | 20.1 | | | | — | | | | 58.8 | | | | 6.2 | |
| Intangible asset | | | 1.2 | | | | — | | | | 0.9 | | | | — | |
| | | |
| | | |
| | | |
| | | |
| |
| Net amount recognized | | | 6.9 | | | | (54.0 | ) | | | 1.0 | | | | (54.2 | ) |
| | | |
| | | |
| | | |
| | | |
| |
In respect of the above plans where the accumulated benefit obligation exceeded the total plan assets, the total projected benefit obligation was £388.1m (2001 — £350.0m), the total accumulated benefit obligation was £327.8m (2001 — £295.8m), and the fair value of plan assets was £216.6m, (2001 - £245.0m).
| (d) | | Financial instruments and related disclosures |
Concentration of credit risk
The Group enters into a variety of financial instruments to reduce its risk associated with fluctuations in interest rates and foreign currencies. The Group does not hold or issue financial instruments for trading purposes. The Group controls credit risk by entering into transactions involving financial instruments only with authorized counter-parties of strong credit quality consisting of a large number of major international financial institutions. Counter-party positions are regularly monitored.
At December 31, 2002, the Group did not consider there to be any significant concentration of credit risk.
Potential concentrations of credit risk to the Group comprise principally cash and short term deposits.
The Group’s minimum acceptable credit rating for counterparties, for the purpose of the investment of surplus cash, is A- from Standard & Poors.
Interest rate risk management
The Group uses interest rate swaps to manage its interest rate exposures on debt and cash positions.
At December 31, 2002, the Group had outstanding interest rate swap agreements to convert fixed rate obligations to floating rates on notional amounts of £31.1m, (2001 — £52m, 2000 — £50m). In addition, the Group had outstanding interest rate swap agreements to convert floating rate obligations to fixed rate on notional amounts of £nil, (2001 — £35m, 2000 — £34m). The table below summarizes, by notional currency, the weighted average interest rates of the Group’s outstanding interest rate swaps.
| | | | | | | | | |
| | | 12 months to | | 12 months to |
| | |
| |
|
| | | Dec 31, 01 | | Dec 31, 02 |
| | |
| |
|
| | | US$ | | US$ |
| | | % | | % |
| Average incoming swap rate | | | 5.01 | | | | 7.09 | |
| Average outgoing swap rate | | | 4.22 | | | | 1.60 | |
F-66
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Swap contracts have a remaining life of up to two years, (2001 — three years).
Foreign exchange risk management
The Group borrows in foreign currencies and enters into foreign exchange contracts to reduce its current balance sheet exposure to foreign exchange rate fluctuations. The Group maintains foreign currency liabilities to hedge some of its foreign currency equity investments and Group net assets. The Group also enters into foreign exchange contracts to reduce the foreign exchange risk on uncommitted anticipated transactions.
The contractual amounts of the Group’s forward foreign exchange contracts were as follows:
| | | | | | | | | | | | | | | | | |
| | | Dec 31, 01 | | Dec 31, 02 |
| | | Forwards | | Forwards |
| | |
| |
|
| | | Buy | | Sell | | Buy | | Sell |
| | |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m |
| U.S. dollars | | | 66.3 | | | | 423.0 | | | | 60.4 | | | | 416.1 | |
| Euro | | | 111.0 | | | | 106.0 | | | | 41.6 | | | | 105.1 | |
| Japanese yen | | | 5.2 | | | | 191.2 | | | | 2.9 | | | | 137.9 | |
| Sterling | | | 332.3 | | | | 96.8 | | | | 265.5 | | | | 159.6 | |
| Danish krona | | | 0.0 | | | | 2.5 | | | | 2.2 | | | | 3.1 | |
| Norwegian kroner | | | 229.6 | | | | 17.1 | | | | 454.8 | | | | 11.9 | |
| Swedish krona | | | 152.7 | | | | 0.0 | | | | 92.2 | | | | 17.8 | |
| Other | | | 3.5 | | | | 36.5 | | | | 3.8 | | | | 39.1 | |
| | | |
| | | |
| | | |
| | | |
| |
| Total | | | 900.6 | | | | 873.1 | | | | 923.4 | | | | 890.6 | |
| | | |
| | | |
| | | |
| | | |
| |
The Company does not obtain collateral or other security to support financial instruments subject to credit risk but monitors the credit standing of the counterparties.
Anticipated transactions are hedged up to three years in advance. Only forward foreign exchange contracts and currency options are used to hedge anticipated transactions.
Gains explicitly deferred at the end of the last three financial periods are:
| | | | | |
| | | £m |
| | |
|
| December 31, 2002 | | | 19.6 | |
| December 31, 2001 | | | 11.1 | |
| December 31, 2000 | | | 0.1 | |
Gains or losses on anticipated transactions are only recognized in the year to which the cover relates. The aggregate transaction gain/(loss) included in net income is as follows:
| | | | | |
| | | £m |
| | |
|
| Year ended December 31, 2002 | | | 10.4 | |
| Year ended December 31, 2001 | | | 14.8 | |
| Year ended December 31, 2000 | | | (5.7 | ) |
F-67
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| (e) | | Group share option schemes |
The rules of the share option schemes and share purchase plan were approved by shareholders at Annual General Meetings held in 1998 (Sharesave and Executive Share Option Scheme 1993), 2000 (Employee Stock Purchase Plan), 2001 (Executive Share Option Scheme 2001) and 2002 (Sharesave). The schemes are subject to limits on share issuance including the requirement to make available not more than 10% of issued share capital over any ten year period.
Certain employees of the Company participate in Amersham sponsored stock option plans. Grants pursuant to the plans are at the market price of Amersham shares at the date of grant and, prior to October 2000, included restrictions on vesting until certain performance targets were met. Unearned compensation expense, which is shown as a separate component of stockholders’ equity, has been recorded pursuant to the intrinsic value approach under APB 25 based on the market value of Amersham stock at the end of each period and is being amortized over the vesting period based upon expectations of meeting these performance targets. In October 2000, the Amersham Board of Directors elected to remove the performance conditions of the options. Thus, the unearned compensation expense became fixed based on the market value of Amersham stock on the date the performance condition was removed, and is being amortized over the remaining vesting period of the options.
Save as you earn scheme (Sharesave)
Under Sharesave, eligible employees (i.e. all UK employees, including Executive Directors) may elect to save specified amounts each month for a period of three, five or seven years. The scheme is subject to a cumulative maximum investment of £250 per month for each individual. Within six months from the end of this period the employee can elect to buy shares at a price approximately 20% below the market price of Amersham shares at the date the employee originally elects to participate in the Scheme or the employee may elect to receive a refund of contributions with interest.
Stock options under Sharesave are as follows:
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (1,279,992 exercisable) | | | 6,363,224 | | | | £2.86 | |
| Granted | | | 810,516 | | | | £4.80 | |
| Exercised | | | (911,382 | ) | | | £1.60 | |
| Lapsed | | | (253,107 | ) | | | £3.21 | |
| | | |
| | | |
| |
Balance at December 31, 2001 (1,370,781 exercisable) | | | 6,009,251 | | | | £3.30 | |
| | | |
| | | |
| |
| Granted | | | 1,084,797 | | | | £4.49 | |
| Exercised | | | (1,397,806 | ) | | | £2.17 | |
| Lapsed | | | (214,744 | ) | | | £3.83 | |
| | | |
| | | |
| |
Balance at December 31, 2002 (1,464,396 exercisable) | | | 5,481,498 | | | | £3.80 | |
| | | |
| | | |
| |
The weighted-average exercise price of options exercisable as at December 31, 2002 and December 31, 2001 are £2.77 and £2.15 respectively.
Executive Share Option Scheme 1993 (ESOS 1993)
Under the ESOS eligible employees may be granted options that shall only be exercisable if certain conditions of performance are satisfied. The Scheme permits the Group to make grants with an exercise price up to 15% below the market value at the date of grant, however, all grants have been made at market value. Options to acquire shares in the Company are awarded on a discretionary basis and the options are normally exercisable between three and ten years from the date of grant. The exercise of the options granted since October 1993 are subject to the attainment of growth in the Group’s earnings per share of at least 6% more than the increase in the Retail Price Index in any three consecutive years prior to the exercise of the option. Under the earlier plan, options were exercisable after three years with no performance condition.
F-68
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (5,330,136 exercisable) | | | 11,544,473 | | | | £4.08 | |
| Granted | | | — | | | | — | |
| Exercised | | | (3,262,198 | ) | | | £3.40 | |
| Lapsed | | | (475,423 | ) | | | £4.38 | |
| | | |
| | | |
| |
Balance at December 31, 2001 (5,165,340 exercisable) | | | 7,806,852 | | | | £4.35 | |
| | | |
| | | |
| |
| Granted | | | — | | | | — | |
| Exercised | | | (1,529,306 | ) | | | £3.74 | |
| Lapsed | | | (140,605 | ) | | | £4.72 | |
| | | |
| | | |
| |
Balance at December 31, 2002 (6,136,941 exercisable) | | | 6,136,941 | | | | £4.49 | |
| | | |
| | | |
| |
The weighted-average exercise price of options exercisable as at December 31, 2002 and December 31, 2001 are £4.49 and £3.79 respectively.
Nycomed Share Option Plan (NSOP)
The Nycomed Share Option Plan was a stock option plan formerly operated by the Nycomed Group for its employees in the USA under which options were granted over Nycomed B ADSs. Under the rules of the Plan all outstanding options became fully exercisable upon the approval of the Offer (made by Amersham International to acquire all of the issued and outstanding Nycomed Securities) by the Nycomed Board on July 1, 1997.
Under the terms of the merger outstanding options over Nycomed B ADSs were converted into options over Amersham B ADSs in accordance with a formula based on the terms of the merger. Each outstanding option over a Nycomed B ADS was converted into an option to purchase 0.6506 Amersham B ADSs. The exercise price of the options was adjusted on conversion by the inverse factor of 0.6506 to preserve the intrinsic value of the option. The conversion formula also reflected the value of the special dividend which Nycomed paid to its shareholders on completion of the merger. All converted options remain exercisable for their full term.
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (212,305 exercisable) | | | 212,305 | | | $ | 3.89 | |
| Granted | | | — | | | | — | |
| Exercised | | | (128,395 | ) | | $ | 3.81 | |
| Lapsed | | | — | | | | — | |
| | | |
| | | |
| |
Balance at December 31, 2001 (83,910 exercisable) | | | 83,910 | | | $ | 4.02 | |
| | | |
| | | |
| |
| Granted | | | — | | | | — | |
| Exercised | | | (83,910 | ) | | $ | 4.02 | |
| Lapsed | | | — | | | | — | |
| | | |
| | | |
| |
Balance at December 31, 2002 (nil exercisable) | | | — | | | | — | |
| | | |
| | | |
| |
The weighted-average exercise price of options exercisable as at December 31, 2002 and December 31, 2001 are $nil and $4.02 respectively.
Amersham US Stock Option Plan (USOP)
During 2000 a new Executive scheme was introduced in North America consistent with US Market practice (the US Stock Option Plan). Under this plan options become exercisable in four equal tranches on the first, second, third and fourth anniversaries of the date of grant. The shares utilized by the plan have been purchased in the market and will not lead to a dilution in share capital. None of the executive directors received option grants under this plan.
F-69
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (651,672 exercisable) | | | 2,606,686 | | | | £6.19 | |
| Granted | | | — | | | | — | |
| Exercised | | | (3,489 | ) | | | £6.23 | |
| Lapsed | | | (198,309 | ) | | | £6.17 | |
| | | |
| | | |
| |
Balance at December 31, 2001 (601,222 exercisable) | | | 2,404,888 | | | | £6.19 | |
| | | |
| | | |
| |
| Granted | | | — | | | | — | |
| Exercised | | | (101,925 | ) | | | £6.19 | |
| Lapsed | | | (331,856 | ) | | | £6.18 | |
| | | |
| | | |
| |
Balance at December 31, 2002 (492,777 exercisable) | | | 1,971,107 | | | | £6.19 | |
| | | |
| | | |
| |
The weighted-average exercise price of options exercisable as at December 31, 2002 and December 31, 2001 are £6.19 at both period ends.
Executive share option scheme 2001 (ESOS 2001)
During 2001 and 2002, shares under ESOS 2001 were awarded to Executive Directors and Senior Managers. Options granted to Senior Managers are exercisable in four equal tranches in the First, Second, Third and Fourth anniversaries of the grant. Options granted to Executive Directors are not normally exercisable until the Third anniversary of the date of grant and to the extent performance conditions have been satisfied. Options granted to Senior Managers worldwide are not subject to performance conditions.
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (nil exercisable) | | | — | | | | — | |
| Granted | | | 6,843,888 | | | | £5.62 | |
| Exercised | | | — | | | | — | |
| Lapsed | | | (89,696 | ) | | | £5.60 | |
| | | |
| | | |
| |
Balance at December 31, 2001 (1,564,621 exercisable) | | | 6,754,192 | | | | £5.62 | |
| | | |
| | | |
| |
| Granted | | | 8,678,506 | | | | £6.94 | |
| Exercised | | | (7,165 | ) | | | £5.60 | |
| Lapsed | | | (617,668 | ) | | | £6.22 | |
| | | |
| | | |
| |
Balance at December 31, 2002 (3,322,163 exercisable) | | | 14,807,865 | | | | £6.37 | |
| | | |
| | | |
| |
The weighted-average exercise price of options exercisable as at December 31, 2002 and at December 31 2001 are £6.37 and £5.62 respectively.
Options for All
During the year, options over 1,000 shares each were granted to all employees in the Group, except for the then four Executive Directors, under the Company’s ‘Options for All’ programme, to mark the new corporate identity of the Group and to reinforce the ‘one company’ strategy. These options were granted under the rules of the Executive Share Option Scheme approved by shareholders at the Annual General Meeting in 2001. The options are not subject to performance conditions and are normally exercisable after three but not more than six years from the date of grant. Options were granted on a tax approval basis as far as possible in those countries where local legislation permitted — UK, US, Ireland, France and Italy. In addition, in those countries where it was not tax efficient to grant options over shares or there were legal constraints, equivalent cash options were granted instead of conventional share options.
F-70
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | |
| | | | | | | Weighted exercise |
| | | Shares under option | | price per option |
| | |
| |
|
Balance at December 31, 2000 (nil exercisable) | | | — | | | | — | |
| Granted | | | 9,298,000 | | | | £6.35 | |
| Exercised | | | — | | | | — | |
| Lapsed | | | (148,000 | ) | | | £6.35 | |
| | | |
| | | |
| |
Balance at December 31, 2001 (nil exercisable) | | | 9,150,000 | | | | £6.35 | |
| | | |
| | | |
| |
| Granted | | | — | | | | — | |
| Exercised | | | — | | | | — | |
| Lapsed | | | (568,648 | ) | | | £6.35 | |
| | | |
| | | |
| |
Balance at December 31, 2002 (nil exercisable) | | | 8,581,352 | | | | £6.35 | |
| | | |
| | | |
| |
Had compensation expense for the Company’s SAYE, its ESOS and its NSOP been determined based upon the fair value at the grant date for awards under these plans consistent with the methodology prescribed under SFAS No. 123,Accounting for Stock-Based Compensation, the Group’s US GAAP net income and earnings per share would have been reduced to the following pro forma amounts:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | |
| |
| |
|
| | | (In £m, except per share data) |
US GAAP Net Income | | | | | | | | | | | | |
| As reported | | | 64.6 | | | | 124.4 | | | | 160.5 | |
| Pro forma | | | 73.4 | | | | 124.1 | | | | 139.6 | |
Basic earnings per share | | | | | | | | | | | | |
| As reported | | | 10.2p | | | | 19.6p | | | | 23.4p | |
| Pro forma | | | 11.6p | | | | 19.5p | | | | 20.4p | |
| | | | | | | | | | | | | |
Diluted earnings per share | | | | | | | | | | | | |
| As reported | | | 10.1p | | | | 19.5p | | | | 23.3p | |
| Pro forma | | | 11.5p | | | | 19.4p | | | | 20.3p | |
The fair value of the options granted are estimated using the Black-Scholes option pricing model with the following assumptions:
| | | | | | | | | | | | | |
| | | 12 months to | | 12 months to | | 12 months to |
| | | Dec 31, 00 | | Dec 31, 01 | | Dec 31, 02 |
| | |
| |
| |
|
| Risk free rate | | | 6.1 | % | | | 5.0 | % | | | 5.0 | % |
| Expected life | | 4.2 years | | 4.4 years | | 4.7 years |
| Expected volatility | | | 35 | % | | | 46 | % | | | 46 | % |
| Underlying dividend yield | | | 1.8 | % | | | 1.25 | % | | | 1.3 | % |
The weighted average fair value of options granted at market price was £3.05 (2001 — £2.31, 2000 — £1.97) and granted at a discount to market price was £2.62 (2001 — £2.98, 2000 — £2.66). The weighted average fair value of grants, issued at 85% of the lesser of market price on grant date and vesting date, based on one ADR, was £8.69, (2001 — £7.66, 2000 — £nil).
No options were issued under the US Stock Option Plan in 2002. The weighted average fair value for options issued under the stock purchase plan in 2001 was £nil, (2000 — £7.82).
F-71
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Deferred taxation (assets)/liabilities on the full provision basis under US GAAP.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | At Dec 31, 01 | | | | | | | | | | | | |
| | | (restated) | | At Dec 31, 02 |
| | |
| |
|
| | | Full | | Valuation | | Net | | Full | | Valuation | | Net |
| | | provision | | allowances | | provision | | provision | | allowances | | provision |
| | |
| |
| |
| |
| |
| |
|
| | | £m | | £m | | £m | | £m | | £m | | £m |
| | | | | | | * | | | | | | | | | | * | | | | |
| Accelerated capital allowances | | | 13.6 | | | | — | | | | 13.6 | | | | 27.0 | | | | — | | | | 27.0 | |
| Capital gains rolled over | | | 6.5 | | | | — | | | | 6.5 | | | | 6.6 | | | | — | | | | 6.6 | |
| Other timing differences | | | (68.2 | ) | | | 31.3 | | | | (36.9 | ) | | | (66.8 | ) | | | 34.2 | | | | (32.6 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | (48.1 | ) | | | 31.3 | | | | (16.8 | ) | | | (33.2 | ) | | | 34.2 | | | | 1.0 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| * | | The valuation allowances relate principally to tax losses and tax allowable goodwill primarily in respect of acquired businesses. |
A valuation allowance is provided to reduce the deferred tax asset to a level which more likely than not will be realized, based on available evidence including historical and projected operating results, estimated reversals of temporary differences and tax planning strategies.
The principal exchange rates used to translate overseas group companies’ financial information were:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Average rate | | Year end rate |
| | |
| |
|
| | | Dec 31 | | Dec 31 | | Dec 31 | | Dec 31 | | Dec 31 | | Dec 31 |
| | | 2002 | | 2001 | | 2000 | | 2002 | | 2001 | | 2000 |
| | |
| |
| |
| |
| |
| |
|
| US dollar | | | 1.51 | | | | 1.44 | | | | 1.51 | | | | 1.61 | | | | 1.45 | | | | 1.49 | |
| Canadian dollar | | | 2.37 | | | | 2.24 | | | | 2.25 | | | | 2.54 | | | | 2.31 | | | | 2.23 | |
| German mark | | | — | | | | 3.16 | | | | 3.22 | | | | — | | | | 3.20 | | | | 3.13 | |
| French franc | | | — | | | | 10.59 | | | | 10.81 | | | | — | | | | 10.75 | | | | 10.50 | |
| Netherlands guilder | | | — | | | | 3.56 | | | | 3.63 | | | | — | | | | 3.61 | | | | 3.53 | |
| Belgian franc | | | — | | | | 65.11 | | | | 66.48 | | | | — | | | | 66.09 | | | | 64.59 | |
| Danish krone | | | 11.81 | | | | 12.03 | | | | 12.28 | | | | 11.42 | | | | 12.18 | | | | 11.94 | |
| Norwegian kroner | | | 11.90 | | | | 12.97 | | | | 13.36 | | | | 11.18 | | | | 13.06 | | | | 13.20 | |
| Swedish krona | | | 14.52 | | | | 14.99 | | | | 13.96 | | | | 14.07 | | | | 15.24 | | | | 14.14 | |
| Italian lira | | | — | | | | 3,125.42 | | | | 3,191.00 | | | | — | | | | 3,172.00 | | | | 3,100.00 | |
| Spanish peseta | | | — | | | | 268.54 | | | | 274.17 | | | | — | | | | 273.00 | | | | 266.00 | |
| Australian dollar | | | 2.77 | | | | 2.81 | | | | 2.63 | | | | 2.85 | | | | 2.84 | | | | 2.68 | |
| Japanese yen | | | 188.00 | | | | 175.58 | | | | 164.00 | | | | 191.00 | | | | 190.00 | | | | 171.00 | |
| Swiss franc | | | 2.33 | | | | 2.44 | | | | 2.57 | | | | 2.23 | | | | 2.42 | | | | 2.44 | |
| Euro | | | 1.59 | | | | 1.61 | | | | 1.65 | | | | 1.54 | | | | 1.64 | | | | 1.60 | |
| (h) | | Impact of recently issued accounting standards |
| |
| | i) | | UK GAAP |
| |
| | | | Retirement Benefits |
| | | | In November 2000, the Accounting Standards Board, (ASB), issued FRS 17Retirement Benefits. The standard will have a significant impact on the way that companies account for pensions and other post retirement benefits in the future. Although the standard does not become mandatory until accounting periods beginning on or after January 1, 2005, it sets down transitional arrangements which require footnote disclosures in the interim period up to full adoption. Transitional disclosures required under the standard are given in note 30 to the accounts. |
F-72
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
Asset Retirement Obligations
In June 2001, the FASB published SFAS 143,Accounting for Asset Retirement Obligations. The standard codifies the accounting for any obligations arising from asset retirements, requiring the fair value of a liability for obligations to be recognized in the period it is incurred. Upon initial recognition of such a liability, entities should capitalize the cost by recognizing an increase in the carrying value of the related tangible fixed asset. The statement is effective for financial years beginning after June 15, 2002.
Costs Associated with Exit or Disposal Activities
In June 2002, the FASB published SFAS 146,Accounting for Costs Associated with Exit or Disposal Activitieswhich addresses financial accounting and reporting for costs associated with exit or disposal activities and nullifies Emerging Issues Task Force (EITF) Issue no 94-3, Liability Recognition for Certain Employee Termination Benefits and other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring). The statement requires that a liability for a cost associated with an exit or disposal activity be recognized when the liability is incurred and can be measured at fair value. The provisions of this statement are effective prospectively for exit or disposal activities initiated after December 31, 2002.
Disclosure Requirements for Guarantees
In November 2002, the FASB published Interpretation No 45, (FIN 45),Guarantors Accounting and Disclosure Requirements for Guarantors, Including Indirect Guarantees of the Indebtedness of Others. FIN 45 elaborates on the existing disclosure requirements for most guarantees, including loan guarantees such as standby letters of credit. FIN 45 will be effective on a prospective basis to guarantees issued or modified after December 31, 2002.
Consolidation of Variable Interest Entities
In January 2003, the FASB published Interpretation No. 46, (FIN 46)Consolidation of Variable Interest Entities.Under FIN 46, certain entities known as “Variable Interest Entities” (VIE) must be consolidated by the “primary beneficiary” of the entity. The primary beneficiary is generally defined as having the majority of the risks and rewards arising from the VIE. For VIE’s in which a significant (but not majority) variable interest is held, certain disclosures are required. The measurement principles of this interpretation will be effective for the Group’s December 31, 2003 financial statements.
The Group is still evaluating the impact of SFAS 143, SFAS 146, FIN 45 and FIN 46.
| 40. | | POST BALANCE SHEET EVENTS |
On February 26, 2003, the Group announced a restructuring of its discovery systems business area. The restructuring programme, which is intended to deliver a more efficient manufacturing cost base and focus R&D on fewer sites, will result in the net loss of approximately 400 jobs. The Group will incur one-off costs in the range of £45-50m, which are expected to result in savings running at the rate of £30-£35m per annum by the end of 2004. The restructuring plans are expected to bring benefits in 2003 moving the business to profitability during 2004.
On April 22, 2003, we announced an intended merger of our brachytherapy business with the urology business of Galil Medical to create a stand-alone marketing company. The combined sales of both businesses would have been approximately £57m, ($90m) in 2002. The new company, which is 75% owned by Amersham, will be accounted for as a joint venture.
F-73
AMERSHAM PLC AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (continued)
The consolidated financial statements and notes thereto set out on pages F-1 to F-74, of which this note forms part, do not comprise statutory accounts within the meaning of Section 240 of the Companies Act 1985 (as amended) insofar as such accounts have to comply with the disclosure and other provisions of that Act. Certain reclassifications and changes in presentation and disclosure have been made to the Group’s statutory accounts in order to conform more closely with the accounting presentation and disclosure requirements applicable in the United States. Statutory accounts for the year ended December 31, 2002, year ended December 31, 2001, and the year ended December 31, 2000, on which the auditors expressed unqualified opinions, have been delivered to the Registrar of Companies for England and Wales.
F-74


