
Corporate Presentation October 2016 Exhibit 99.1

Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and assumptions, and actual results may differ substantially from those projected or expected in the forward-looking statements. Forward-looking statements include, but are not limited to: any projections of financial information; any statements about future development, clinical or regulatory events; any statements concerning CymaBay's plans, strategies or objectives; and any other statements of expectation or belief regarding future events. These statements are based on estimates and information available to CymaBay at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from CymaBay's current expectations as a result of many factors including, but not limited to: CymaBay's ability to obtain additional financing to fund its operations; unexpected delays or results in clinical trials; uncertainties regarding obtaining regulatory approvals; uncertainties regarding the ability to protect CymaBay's intellectual property; uncertainties regarding market acceptance of any products for which CymaBay is able to obtain regulatory approval; the effects of competition; and other market and general economic conditions. You should read CymaBay's Annual Report on Form 10-K filed with the SEC on March 29, 2016 and Quarterly Report on Form 10-Q filed with the SEC on August 9, 2016, especially under the caption “Risk Factors,” which is available on the SEC web site at http://www.sec.gov, for a fuller discussion of these and other risks relating to an investment in CymaBay’s common stock. CymaBay assumes no obligation for and does not intend to update these forward-looking statements, except as required by law.
CymaBay Highlights MBX-8025 is a potential novel treatment for serious lipid and liver disorders Primary biliary cholangitis (PBC) Homozygous familial hypercholesterolemia (HoFH) Nonalcoholic steatohepatitis (NASH) Arhalofenate is the first potential drug in the Urate Lowering Anti-Flare Therapy (ULAFT) class for the treatment for gout Reduces gout flares and lowers serum uric acid (sUA) Differentiated target profile established in five Phase 2 studies Good overall and renal safety data in over 1,100 subjects End-of-Phase 2 FDA discussions completed; now Phase 3 ready Near-term, value-driving catalysts Dose-ranging Phase 2 study of MBX-8025 in PBC initiation in 4Q 2016 Partnership for arhalofenate and start of Phase 3 in 2017
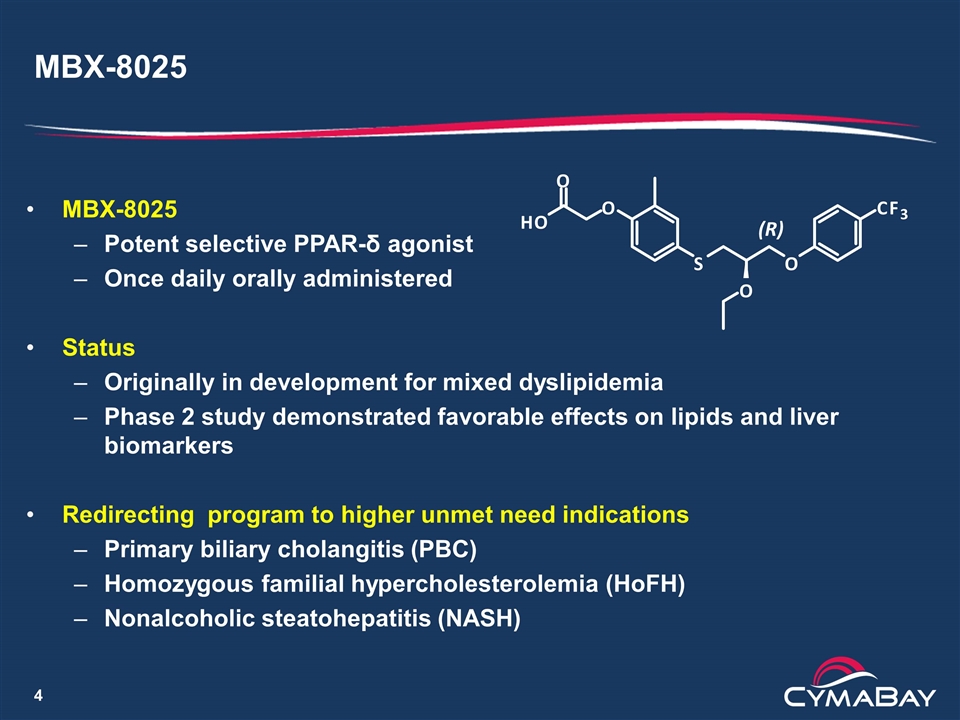
MBX-8025 MBX-8025 Potent selective PPAR-δ agonist Once daily orally administered Status Originally in development for mixed dyslipidemia Phase 2 study demonstrated favorable effects on lipids and liver biomarkers Redirecting program to higher unmet need indications Primary biliary cholangitis (PBC) Homozygous familial hypercholesterolemia (HoFH) Nonalcoholic steatohepatitis (NASH) O H O O S O O C F 3 (R)
Primary Biliary Cholangitis (PBC) Disease pathophysiology Autoimmune disease of the liver affecting primarily women (90%) Characterized by cholestasis (impairment of bile flow), portal inflammation and destruction of the intrahepatic bile ducts Elevated liver function parameters ALP, GGT and total bilirubin Fatigue and pruritus Can progress to fibrosis, cirrhosis and liver failure Incidence Affects 1 in 1,000 women over 40 (~300,000 patients in major markets) Qualifies as an orphan disease in US and EU
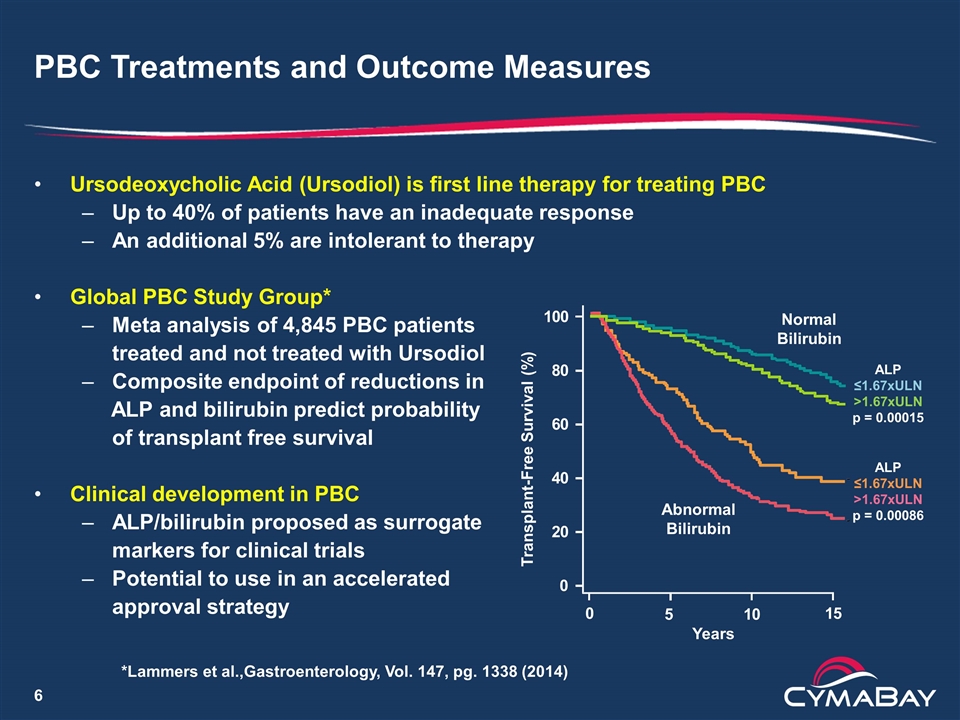
PBC Treatments and Outcome Measures Ursodeoxycholic Acid (Ursodiol) is first line therapy for treating PBC Up to 40% of patients have an inadequate response An additional 5% are intolerant to therapy Global PBC Study Group* Meta analysis of 4,845 PBC patients treated and not treated with Ursodiol Composite endpoint of reductions in ALP and bilirubin predict probability of transplant free survival Clinical development in PBC ALP/bilirubin proposed as surrogate markers for clinical trials Potential to use in an accelerated approval strategy 0 0 20 40 60 80 100 15 Transplant-Free Survival (%) Years 10 5 Normal Bilirubin Abnormal Bilirubin ALP ≤1.67xULN >1.67xULN p = 0.00015 ALP ≤1.67xULN >1.67xULN p = 0.00086 *Lammers et al.,Gastroenterology, Vol. 147, pg. 1338 (2014)
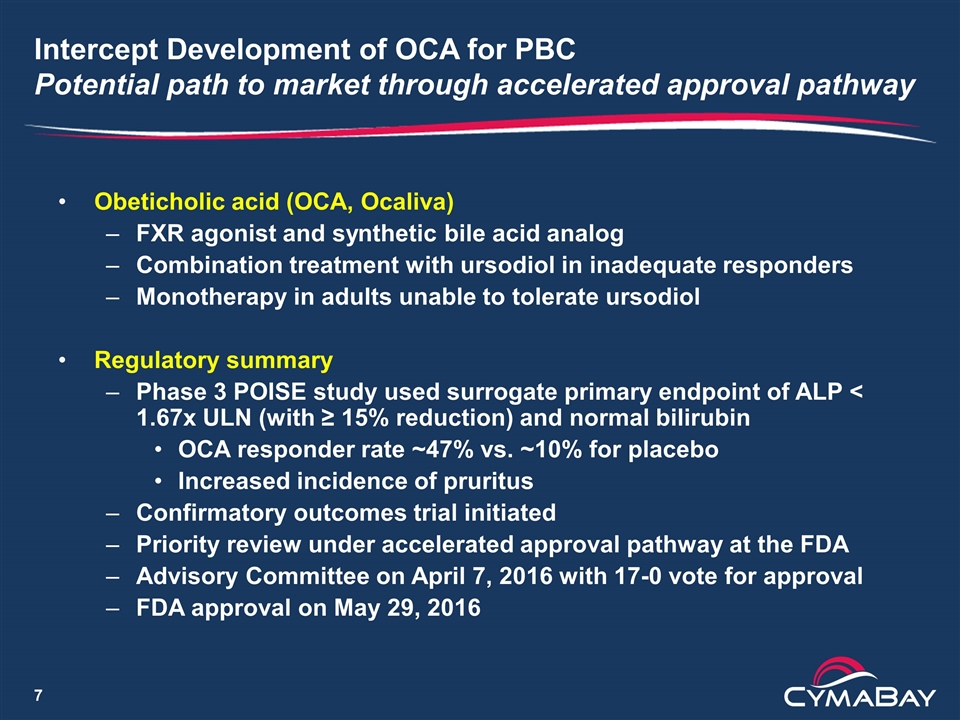
Intercept Development of OCA for PBC Potential path to market through accelerated approval pathway Obeticholic acid (OCA, Ocaliva) FXR agonist and synthetic bile acid analog Combination treatment with ursodiol in inadequate responders Monotherapy in adults unable to tolerate ursodiol Regulatory summary Phase 3 POISE study used surrogate primary endpoint of ALP < 1.67x ULN (with ≥ 15% reduction) and normal bilirubin OCA responder rate ~47% vs. ~10% for placebo Increased incidence of pruritus Confirmatory outcomes trial initiated Priority review under accelerated approval pathway at the FDA Advisory Committee on April 7, 2016 with 17-0 vote for approval FDA approval on May 29, 2016
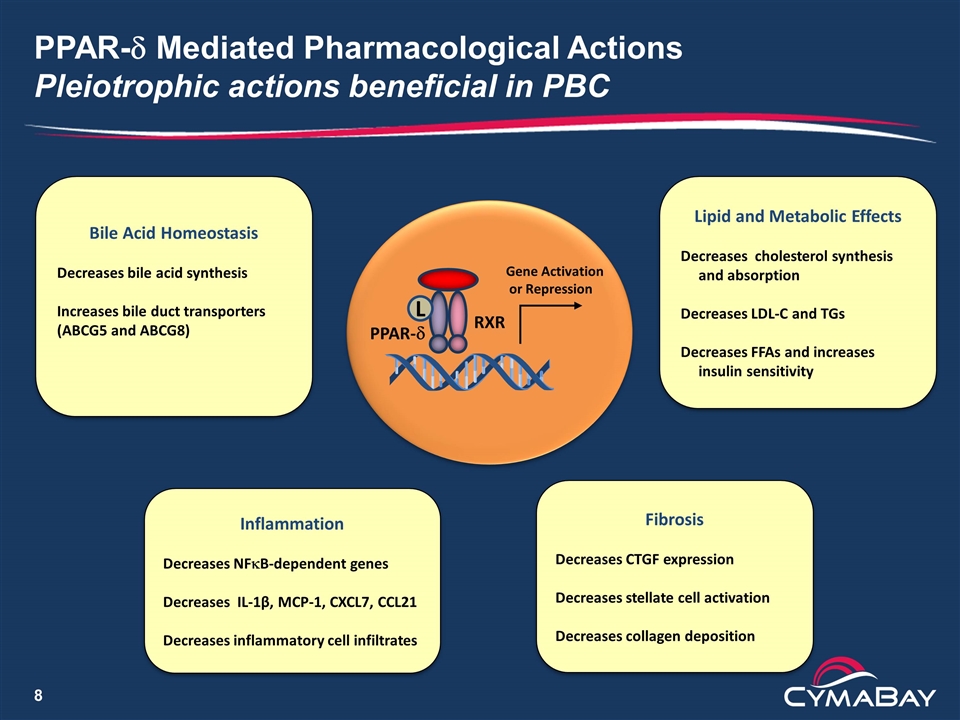
PPAR-d Mediated Pharmacological Actions Pleiotrophic actions beneficial in PBC RXR PPAR-d Gene Activation or Repression Bile Acid Homeostasis Decreases bile acid synthesis Increases bile duct transporters (ABCG5 and ABCG8) Inflammation Decreases NFkB-dependent genes Decreases IL-1β, MCP-1, CXCL7, CCL21 Decreases inflammatory cell infiltrates Fibrosis Decreases CTGF expression Decreases stellate cell activation Decreases collagen deposition L Lipid and Metabolic Effects Decreases cholesterol synthesis and absorption Decreases LDL-C and TGs Decreases FFAs and increases insulin sensitivity
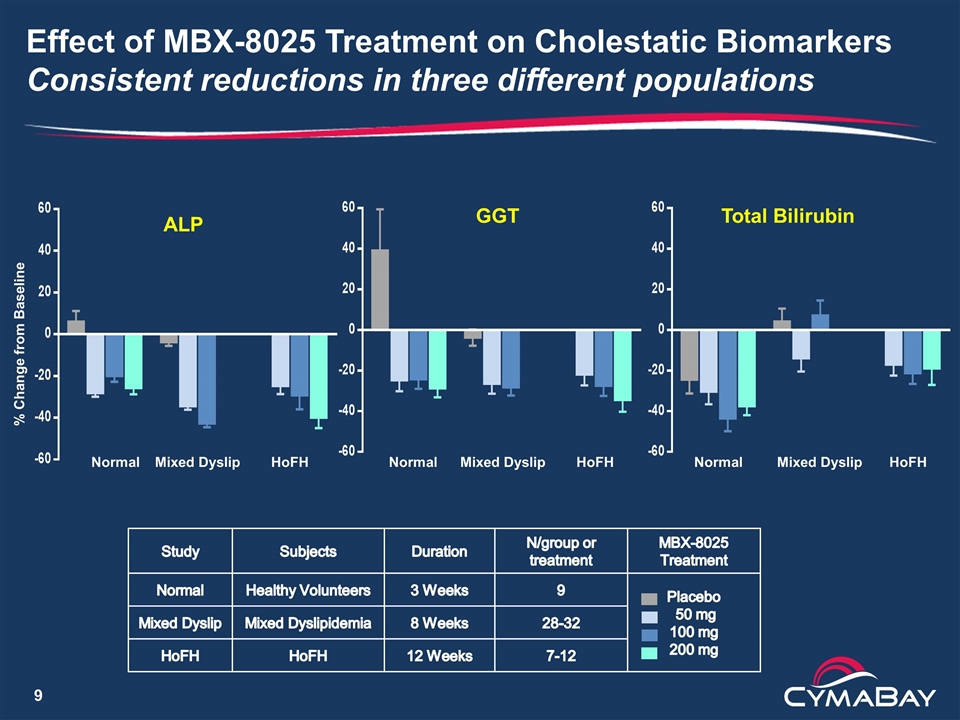
Effect of MBX-8025 Treatment on Cholestatic Biomarkers Consistent reductions in three different populations ALP GGT Total Bilirubin % Change from Baseline Normal Mixed Dyslip HoFH Normal Mixed Dyslip HoFH Normal Mixed Dyslip HoFH Study Subjects Duration N/group or treatment MBX-8025 Treatment Normal Healthy Volunteers 3 Weeks 9 Placebo 50 mg 100 mg 200 mg Mixed Dyslip Mixed Dyslipidemia 8 Weeks 28-32 HoFH HoFH 12 Weeks 7-12
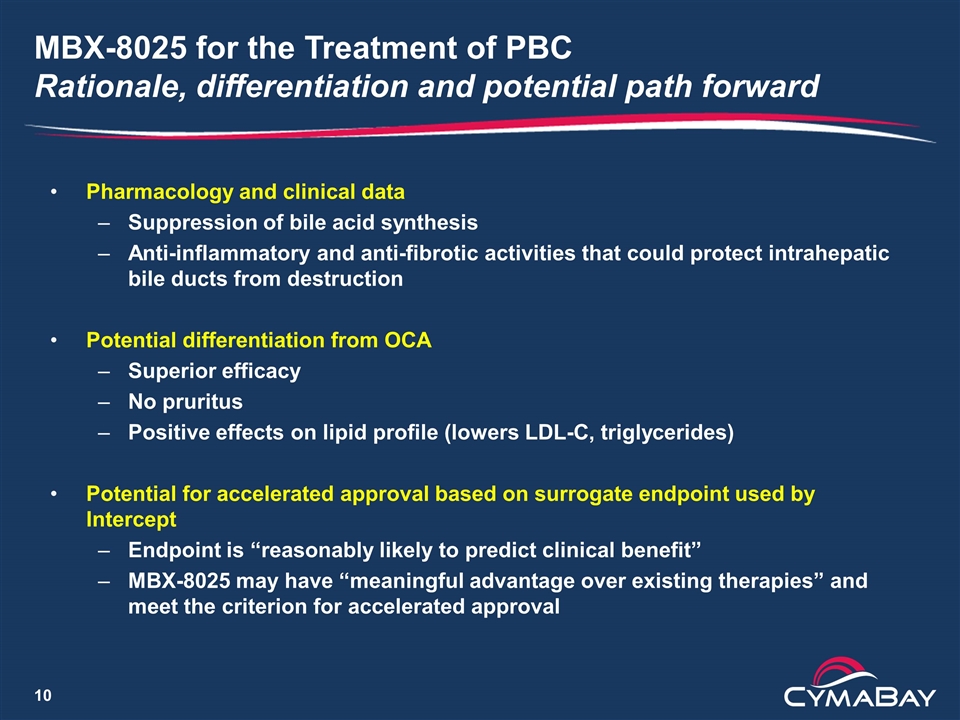
MBX-8025 for the Treatment of PBC Rationale, differentiation and potential path forward Pharmacology and clinical data Suppression of bile acid synthesis Anti-inflammatory and anti-fibrotic activities that could protect intrahepatic bile ducts from destruction Potential differentiation from OCA Superior efficacy No pruritus Positive effects on lipid profile (lowers LDL-C, triglycerides) Potential for accelerated approval based on surrogate endpoint used by Intercept Endpoint is “reasonably likely to predict clinical benefit” MBX-8025 may have “meaningful advantage over existing therapies” and meet the criterion for accelerated approval
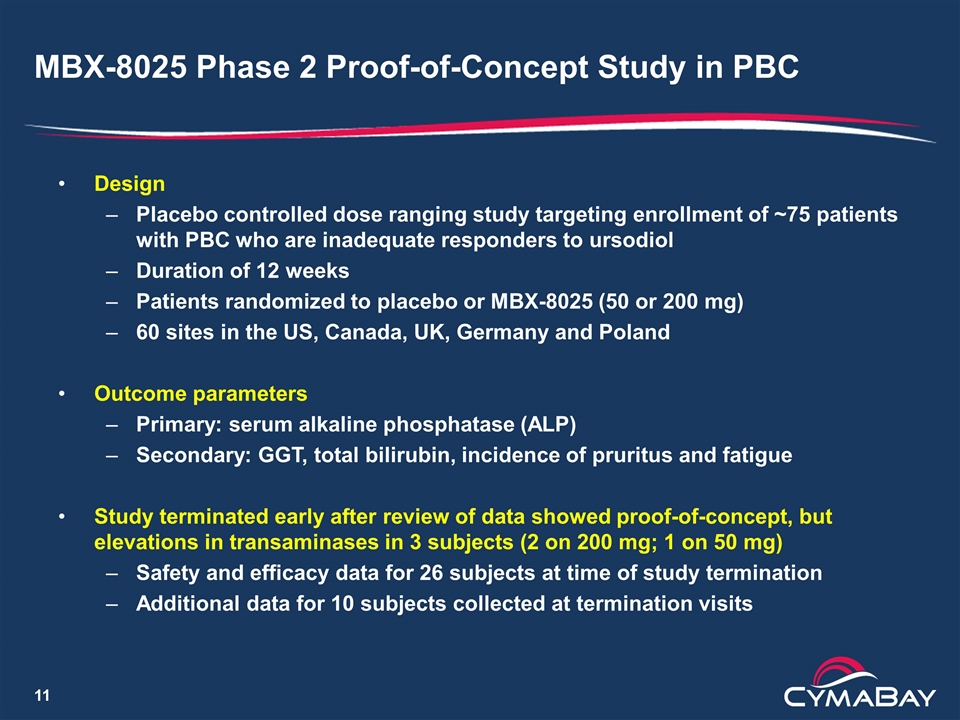
MBX-8025 Phase 2 Proof-of-Concept Study in PBC Design Placebo controlled dose ranging study targeting enrollment of ~75 patients with PBC who are inadequate responders to ursodiol Duration of 12 weeks Patients randomized to placebo or MBX-8025 (50 or 200 mg) 60 sites in the US, Canada, UK, Germany and Poland Outcome parameters Primary: serum alkaline phosphatase (ALP) Secondary: GGT, total bilirubin, incidence of pruritus and fatigue Study terminated early after review of data showed proof-of-concept, but elevations in transaminases in 3 subjects (2 on 200 mg; 1 on 50 mg) Safety and efficacy data for 26 subjects at time of study termination Additional data for 10 subjects collected at termination visits
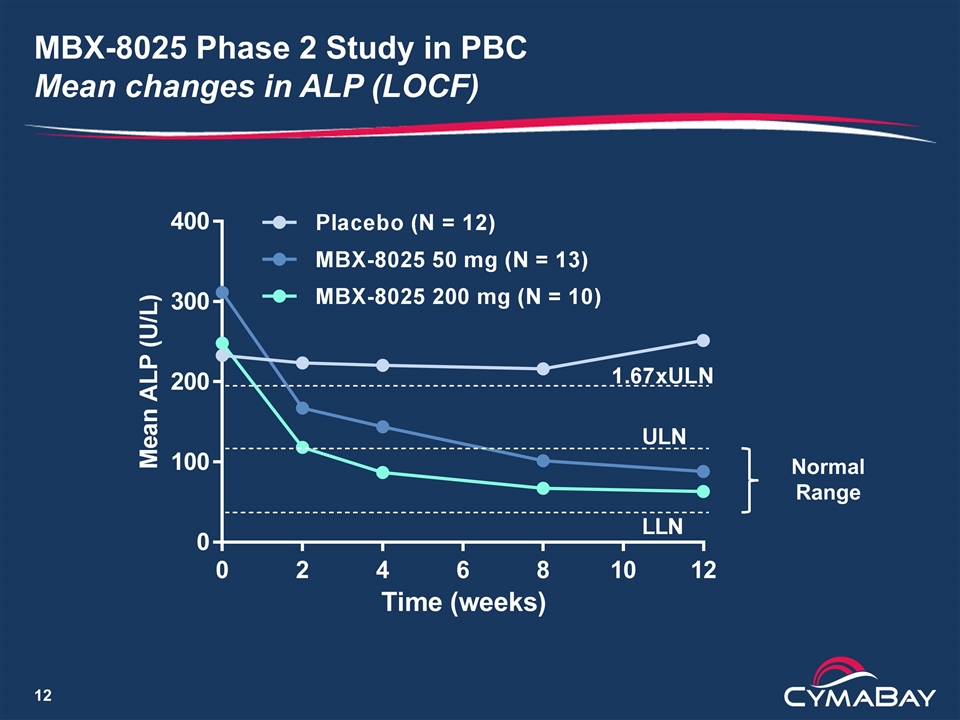
MBX-8025 Phase 2 Study in PBC Mean changes in ALP (LOCF) Normal Range
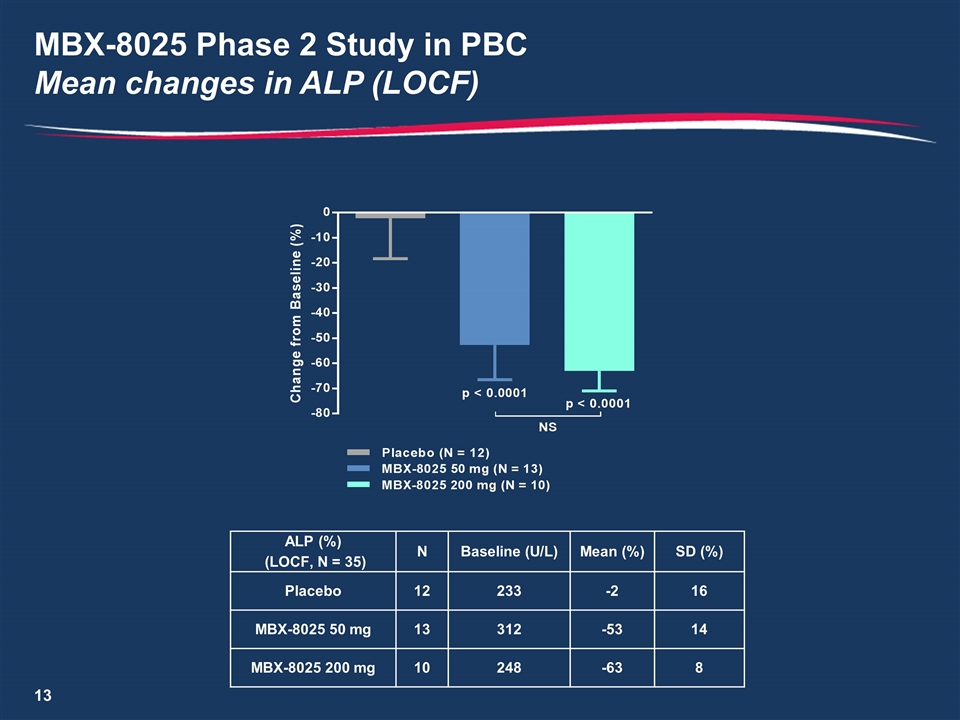
MBX-8025 Phase 2 Study in PBC Mean changes in ALP (LOCF) ALP (%) (LOCF, N = 35) N Baseline (U/L) Mean (%) SD (%) Placebo 12 233 -2 16 MBX-8025 50 mg 13 312 -53 14 MBX-8025 200 mg 10 248 -63 8
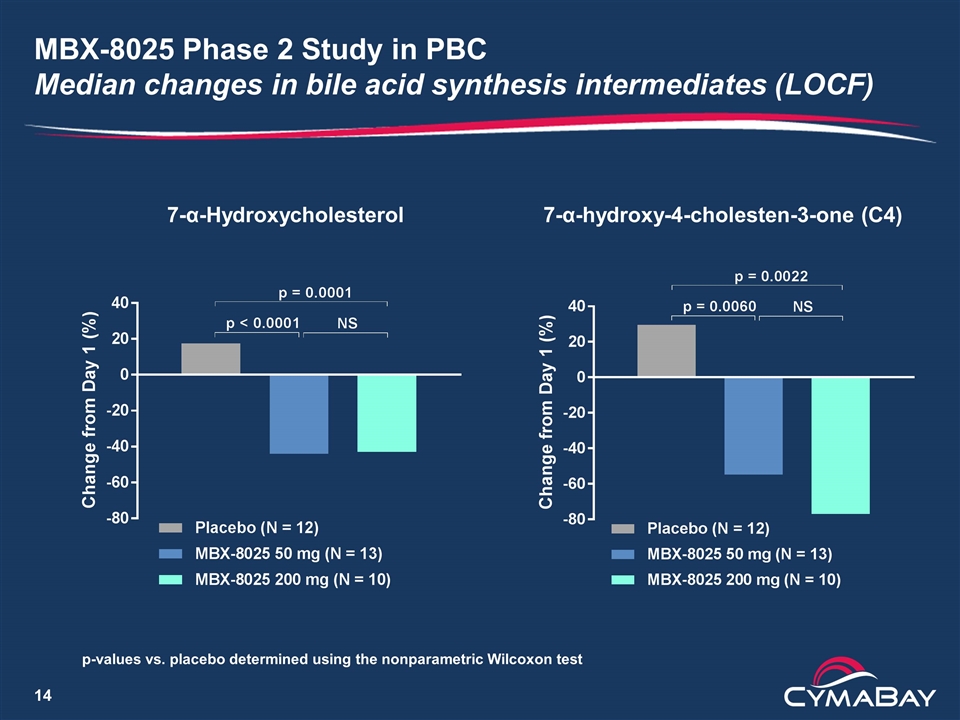
MBX-8025 Phase 2 Study in PBC Median changes in bile acid synthesis intermediates (LOCF) 7-α-Hydroxycholesterol 7-α-hydroxy-4-cholesten-3-one (C4) p-values vs. placebo determined using the nonparametric Wilcoxon test
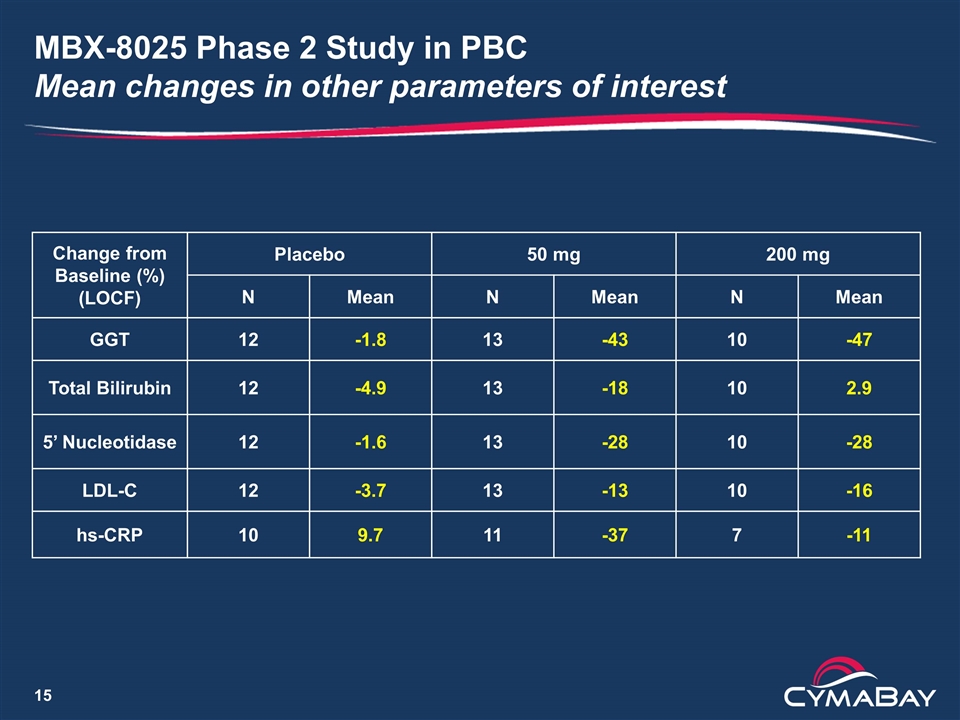
MBX-8025 Phase 2 Study in PBC Mean changes in other parameters of interest Change from Baseline (%) (LOCF) Placebo 50 mg 200 mg N Mean N Mean N Mean GGT 12 -1.8 13 -43 10 -47 Total Bilirubin 12 -4.9 13 -18 10 2.9 5’ Nucleotidase 12 -1.6 13 -28 10 -28 LDL-C 12 -3.7 13 -13 10 -16 hs-CRP 10 9.7 11 -37 7 -11

MBX-8025 Open Label Dose Ranging Phase 2 PBC Study Study objectives Generate data to allow selection of doses for Phase 3 Gather safety data to support registration Study design Open label, dose ranging study in PBC subjects with an inadequate response or intolerant to ursodiol Staggered arms (12 subjects/group) 5, 10 mg groups in parallel 25 mg group after review of safety from 5 and 10 mg groups 8 week treatment phase followed by 18 week extension Subjects can dose adjust in extension phase Primary efficacy outcome is ALP Pharmacokinetics in sub-group of patients
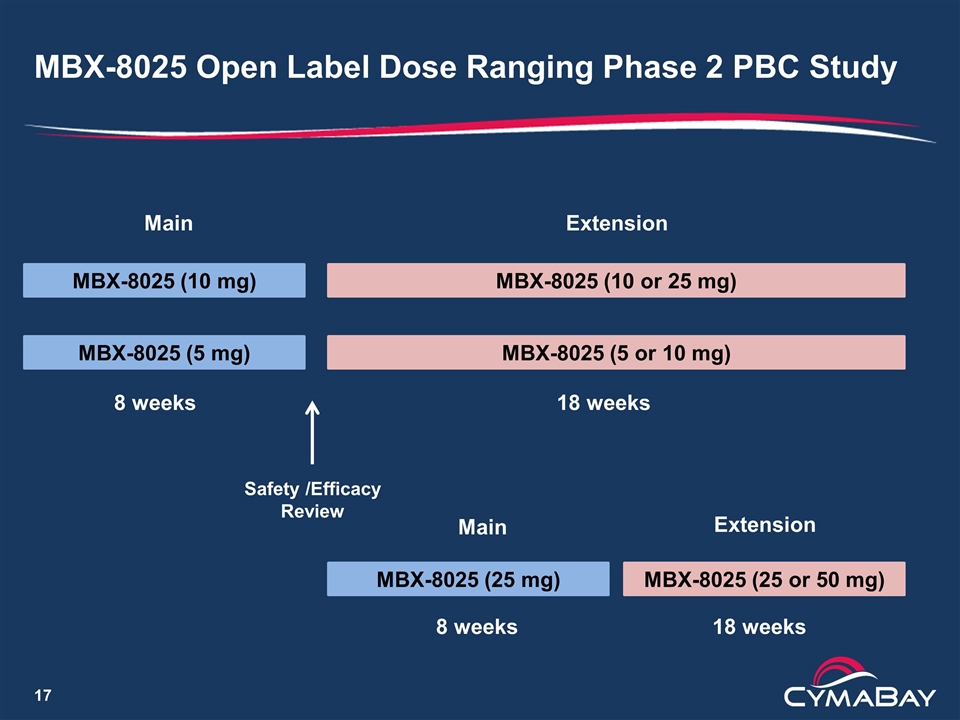
MBX-8025 Open Label Dose Ranging Phase 2 PBC Study MBX-8025 (5 mg) MBX-8025 (10 mg) MBX-8025 (5 or 10 mg) MBX-8025 (25 mg) Safety /Efficacy Review 8 weeks MBX-8025 (10 or 25 mg) 18 weeks MBX-8025 (25 or 50 mg) Main Main Extension Extension 8 weeks 18 weeks
Homozygous Familial Hypercholesterolemia (HoFH) Rare disease of impaired cholesterol metabolism Orphan disease affecting 1 in 1 million Markedly elevated LDL-C (>500 mg/dL) caused by loss-of-function mutations in LDL receptor (LDL-R) genes Cardiovascular disease (MI, stroke) before the age of 20 Mean age of death is in the 30s Therapy is focused on lowering LDL-C Therapies that raise LDL-R activity are minimally effective Statins, bile acid sequestrants, ezetimibe PCSK9 inhibitors reduce LDL-C by ~20% Current therapeutic options LDL apheresis Juxtapid (lomitapide) Kynamro (mipomersen) Repatha (evolocumab) 28 year-old female with cutaneous xanthoma Black Box and REMs Monthly liver testing
MBX-8025 for the Treatment of HoFH Rationale and proof-of-concept study design Rationale MBX-8025 exhibited a strong anti-atherogenic profile in patients with mixed dyslipidemia including reductions in LDL-C Watanabe Heritable Hyperlipidemic rabbit data demonstrate decreases in LDL-C in the setting of low LDL-R activity characteristic of HoFH Orphan drug designation granted by FDA Proof-of-Concept study design Open label dose escalation study in 13 patients Doses are 50, 100 and 200 mg Study duration of 3 months Study conducted in Europe and Canada
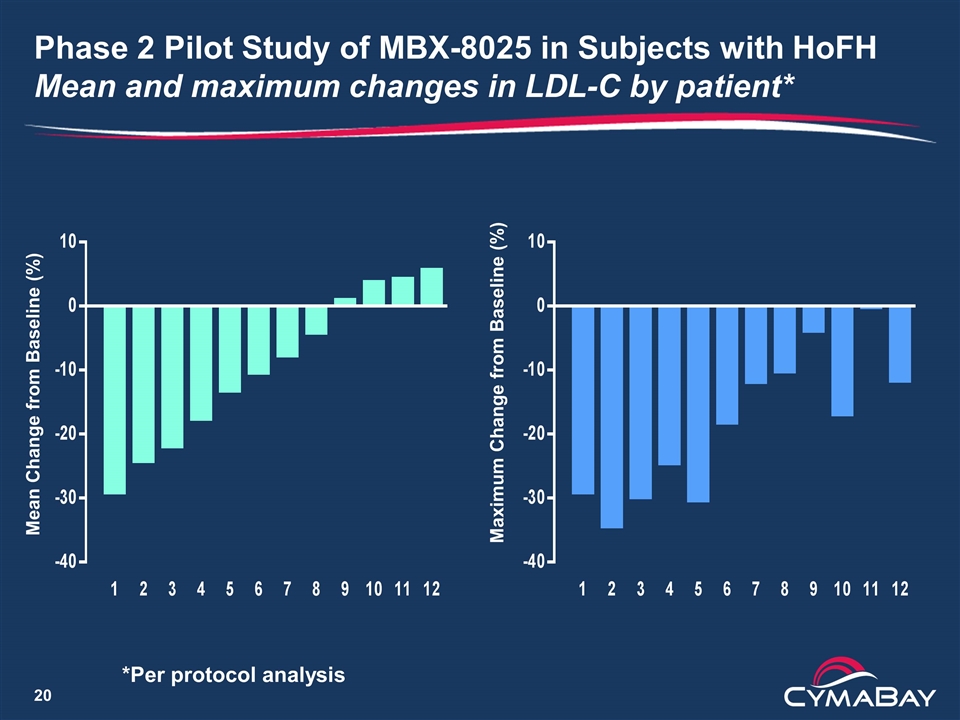
Phase 2 Pilot Study of MBX-8025 in Subjects with HoFH Mean and maximum changes in LDL-C by patient* Maximum Change from Baseline (%) Mean Change from Baseline (%) *Per protocol analysis
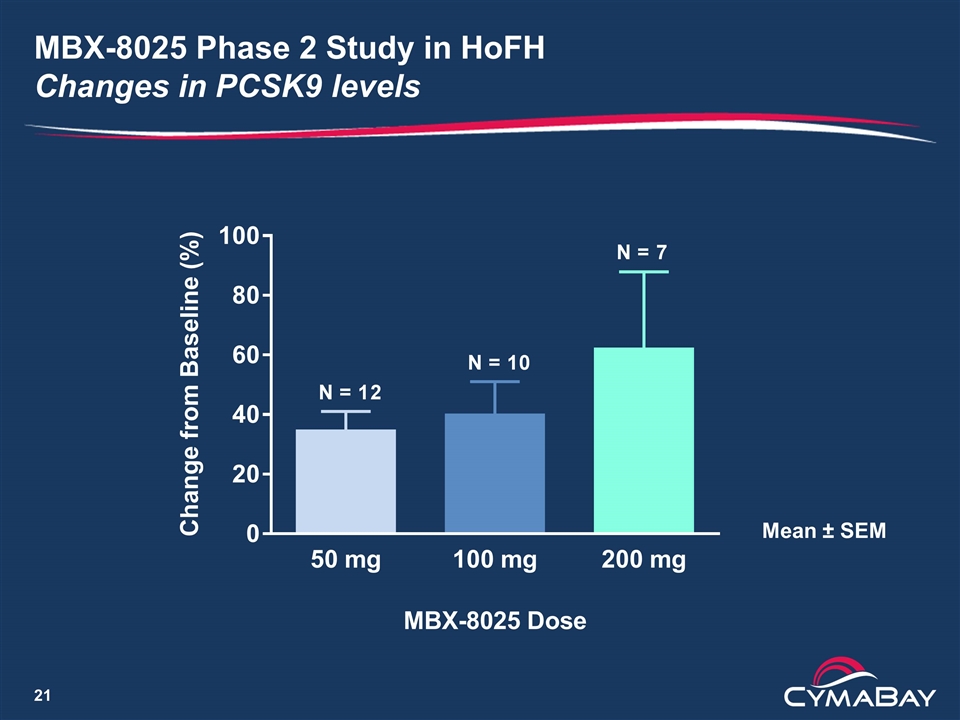
MBX-8025 Phase 2 Study in HoFH Changes in PCSK9 levels Mean ± SEM

Phase 2 Pilot Study of MBX-8025 in Subjects with HoFH Conclusions Summary LDL-C decrease of ≥ 15% for 5/12 patients (42%) is clinically significant Five patients (42%) showed little or no response, lowering the average LDL-C decrease to below commercial viability The increases in PCSK9 may have attenuated the LDL-C lowering Paper presented as a late breaking oral presentation at the European Atherosclerosis Society Congress in Innsbruck Next steps Analysis of other lipid parameters underway A study in combination with PCSK9 inhibitor is under evaluation
Development of MBX-8025 for NASH We have continued to monitor the space Progress of leading programs Regulatory clarity on path to registration Interest in MBX-8025 and the PPAR-δ mechanism Evaluation of MBX-8025 in preclinical models of NASH now achieved Preclinical proof-of-concept Differentiation from OCA Oral presentation at AASLD in November Establish an IP position Recently awarded method of use patent for treatment of NASH (expires 2035)
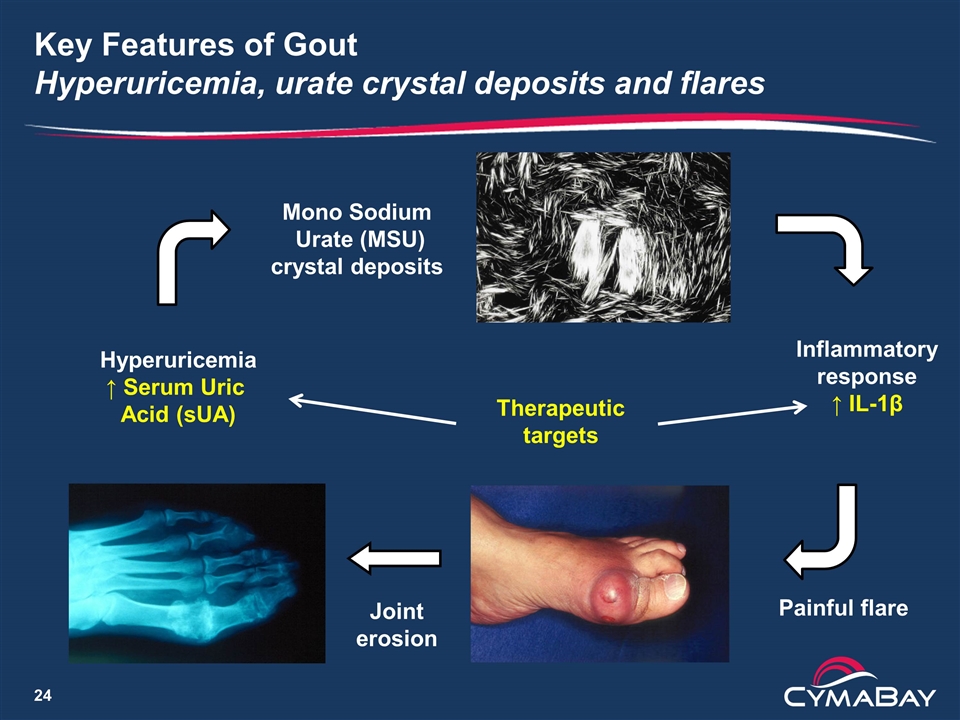
Key Features of Gout Hyperuricemia, urate crystal deposits and flares Hyperuricemia ↑ Serum Uric Acid (sUA) Mono Sodium Urate (MSU) crystal deposits Inflammatory response ↑ IL-1β Painful flare Therapeutic targets Joint erosion
Current Treatment of Gout in US Uric acid Lowering Therapies (ULTs) and anti-inflammatories Treatment paradigm (American College of Rheumatology Guidelines) Anti-inflammatory to treat the flare ULT to reduce sUA and debulk urate burden in tissue First line: xanthine oxidase inhibitor (XOI) Second line: If sUA goal is not reached, add a uricosuric agent Initiation of ULT increases flare risk, requiring colchicine prophylaxis ULTs XOIs: allopurinol (generic), febuxostat (Uloric) Uricosurics: probenecid, lesinurad (Zurampic) Pegloticase (severe treatment failure gout only) Anti-inflammatory drugs Colchicine (Colcrys, Mitigare), NSAIDs, steroids
A Safe and Effective Uricosuric Drug is Needed to Address the sUA Lowering Need of Inadequate Responders Desired characteristics Additional 20% lowering of sUA on top of XOI Good renal safety (no increase in risk of hyperuricosuria or lithiasis) Effective in patients with renal impairment No increase in flares Uricosuric drugs approved in the US Probenecid has significant limitations and is rarely used Lesinurad (Zurampic) label includes precautions and warnings for use Black box warning for acute renal failure Restricted to 200 mg dose in combination with XOIs Not to be used as monotherapy Potential increased risk of flares on initiation of use
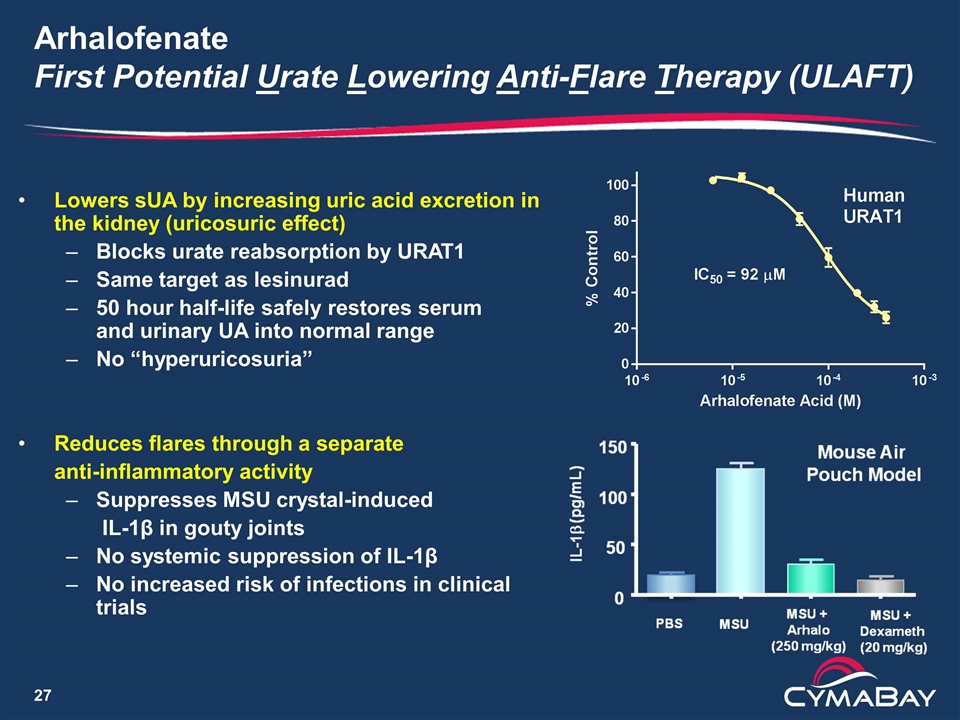
Arhalofenate First Potential Urate Lowering Anti-Flare Therapy (ULAFT) Lowers sUA by increasing uric acid excretion in the kidney (uricosuric effect) Blocks urate reabsorption by URAT1 Same target as lesinurad 50 hour half-life safely restores serum and urinary UA into normal range No “hyperuricosuria” Reduces flares through a separate anti-inflammatory activity Suppresses MSU crystal-induced IL-1β in gouty joints No systemic suppression of IL-1β No increased risk of infections in clinical trials
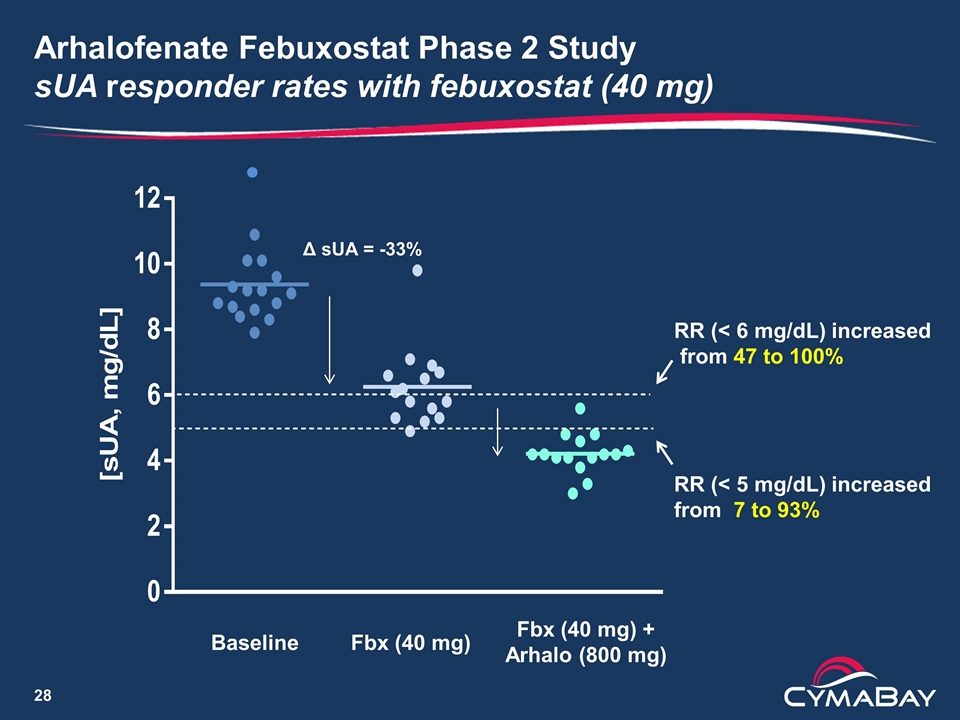
Arhalofenate Febuxostat Phase 2 Study sUA responder rates with febuxostat (40 mg) Δ sUA = -33% Baseline Fbx (40 mg) Fbx (40 mg) + Arhalo (800 mg) RR (< 6 mg/dL) increased from 47 to 100% RR (< 5 mg/dL) increased from 7 to 93%
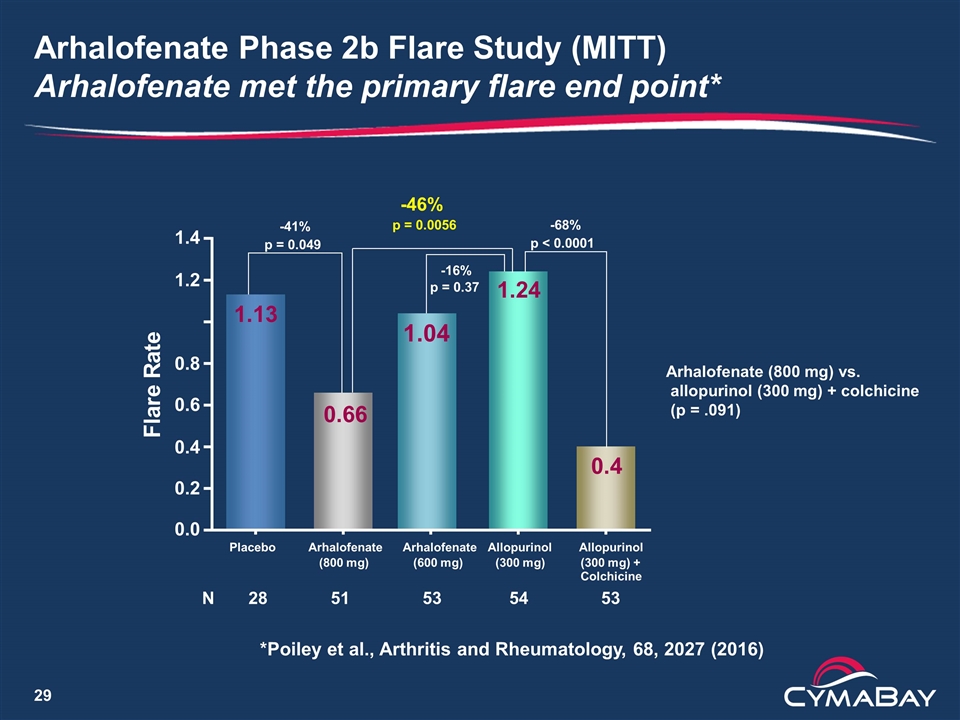
Arhalofenate Phase 2b Flare Study (MITT) Arhalofenate met the primary flare end point* 0.0 0.2 0.4 0.6 0.8 1.2 1.4 F l a r e R a t e N 28 51 53 54 53 0.66 0.4 1.24 1.13 Placebo Arhalofenate (800 mg) Arhalofenate (600 mg) Allopurinol (300 mg) Allopurinol (300 mg) + Colchicine -41% p = 0.049 -46% p = 0.0056 -68% p < 0.0001 -16% p = 0.37 1.04 Arhalofenate (800 mg) vs. allopurinol (300 mg) + colchicine (p = .091) *Poiley et al., Arthritis and Rheumatology, 68, 2027 (2016)
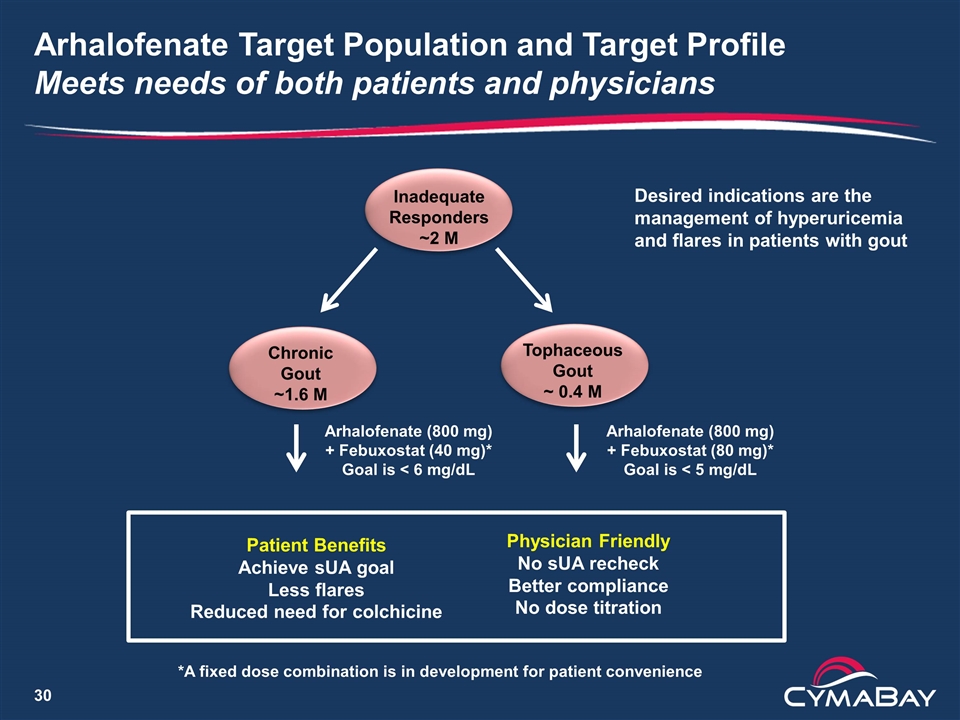
Inadequate Responders ~2 M Arhalofenate (800 mg) + Febuxostat (80 mg)* Goal is < 5 mg/dL Arhalofenate Target Population and Target Profile Meets needs of both patients and physicians Chronic Gout ~1.6 M Tophaceous Gout ~ 0.4 M Arhalofenate (800 mg) + Febuxostat (40 mg)* Goal is < 6 mg/dL *A fixed dose combination is in development for patient convenience Patient Benefits Achieve sUA goal Less flares Reduced need for colchicine Physician Friendly No sUA recheck Better compliance No dose titration Desired indications are the management of hyperuricemia and flares in patients with gout
Arhalofenate Summary Phase 3 ready asset Safety summary from nine Phase 2 clinical studies More than 1,100 subjects exposed for up to 6 months Low incidence of asymptomatic transaminase elevations No creatinine signal or increase in infections Drug materials and preclinical safety package Tablet formulations developed All toxicology and carcinogenicity studies completed and reviewed by FDA Agreement with FDA on Phase 3 program for ULAFT label Patient populations (chronic and tophaceous gout) Endpoints (sUA responder rate and flare rate) Size of safety database (1300 patients at 6 months; 650 at 12 months) Unique profile recognized by rheumatology community Editorial: Neogi and Choi, “Pursuit of a Dual Benefit Antigout Drug: A First Look at Arhalofenate”, Arthritis and Rheumatology, 68, 1793 (2016) CymaBay is in active partnering discussion for US, EU and far east territories
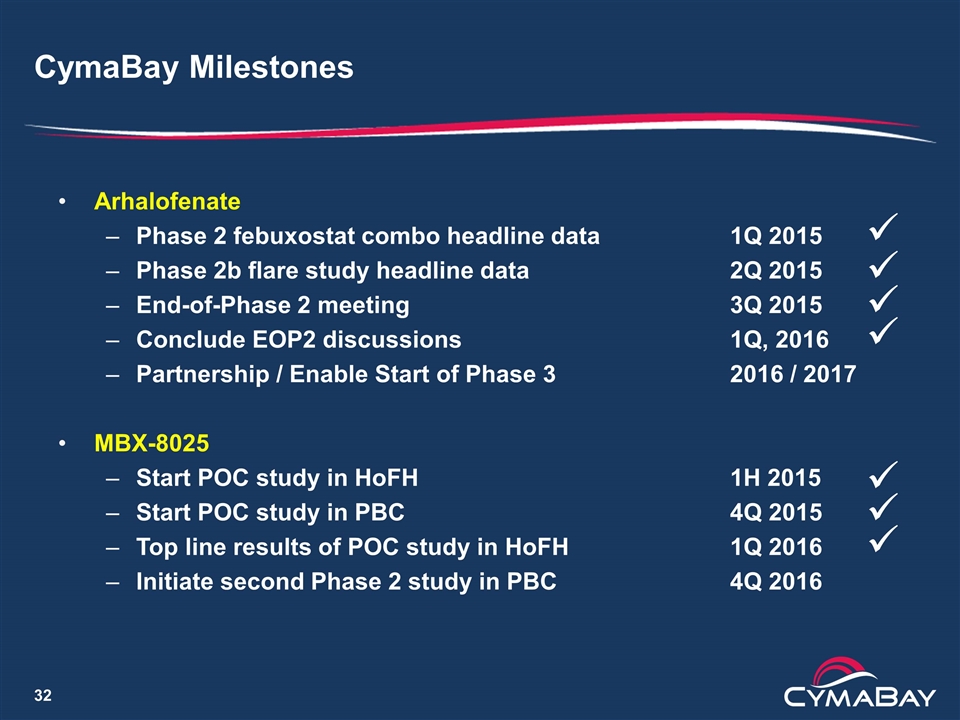
CymaBay Milestones Arhalofenate Phase 2 febuxostat combo headline data1Q 2015 Phase 2b flare study headline data2Q 2015 End-of-Phase 2 meeting3Q 2015 Conclude EOP2 discussions1Q, 2016 Partnership / Enable Start of Phase 3 2016 / 2017 MBX-8025 Start POC study in HoFH1H 2015 Start POC study in PBC 4Q 2015 Top line results of POC study in HoFH1Q 2016 Initiate second Phase 2 study in PBC4Q 2016 ü ü ü ü ü ü ü































