UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
PROXY STATEMENT PURSUANT TO SECTION 14(a) OF
THE SECURITIES EXCHANGE ACT OF 1934
Filed by the Registrantþ
Filed by a Party other than the Registranto
Check the appropriate box:
| o | | Preliminary Proxy Statement |
| |
| o | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| o | | Definitive Proxy Statement |
| |
| þ | | Definitive Additional Materials |
| |
| o | | Soliciting Material Pursuant to § 240.14a-12 |
PENWEST PHARMACEUTICALS CO.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| þ | | No fee required. |
| |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | (1) | | Title of each class of securities to which transaction applies: |
| |
| | (2) | | Aggregate number of securities to which transaction applies: |
| |
| | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | (4) | | Proposed maximum aggregate value of transaction: |
| |
| | (5) | | Total fee paid: |
| o | | Fee paid previously with preliminary materials. |
| |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | (1) | | Amount Previously Paid: |
| |
| | (2) | | Form, Schedule or Registration Statement No.: |
| |
| | (3) | | Filing Party: |
| |
| | (4) | | Date Filed: |
The Company is using the following set of slides in presentations in connection with the proxy solicitation for its 2009 Annual Meeting of Shareholders.

| Focused Strategy to Drive Shareholder Value Jennifer Good President , CEO & Director June 2009 |

| Forward-Looking Statements The matters discussed herein contain forward-looking statements that involve risks and uncertainties, which may cause the actual results in future periods to be materially different from any future performance suggested herein. For this purpose, any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words, "believes," "anticipates," "plans," "expects," "intends," "potential," "appears," "estimates," "projects," "targets," "may," "could," and similar expressions are intended to identify forward-looking statements. Important factors that could cause results to differ materially include: risks relating to the commercial success of Opana ER, including our reliance on Endo Pharmaceuticals Inc. for the commercial success of Opana ER and risks of generic competition, the need for capital; regulatory risks relating to drugs in development, including the timing and outcome of regulatory submissions and regulatory actions; uncertainty of success of collaborations; the timing of clinical trials; whether the results of clinical trials will warrant further clinical trials, warrant submission of an application for regulatory approval of, or warrant the regulatory approval of, the product that is the subject of the trial; whether the patents and patent applications owned by us will protect the Company's products and technology; actual and potential competition; and other risks as set forth under the caption Risk Factors in Penwest's Annual Report on Form 10-Q filed with the Securities and Exchange Commission on May 11, 2009, which risk factors are incorporated herein by reference. The forward-looking statements contained in this press release speak only as of the date of the statements made. Penwest disclaims any intention or obligation to update any forward-looking statements. TIMERx is a registered trademark of Penwest. All other trademarks referenced herein are the property of their respective owners. |
| Information Concerning Participants Information required to be disclosed with regard to the Company's directors, director nominees, officers and employees who, under the rules of the Securities and Exchange Commission (the "SEC"), are considered to be "participants" in the Company's solicitation of proxies from its shareholders in connection with its 2009 Annual Meeting of Shareholders (the "Annual Meeting") may be found in the Company's Proxy Statement for its 2009 Annual Meeting of Shareholders, as filed with the SEC on May 7, 2009 (the "2009 Proxy Statement"). Security holders may obtain a free copy of the 2009 Proxy Statement and other documents (when available) that the Company files with the SEC at the SEC's website at www.sec.gov. Security holders may also obtain a free copy of these documents by writing the Company at Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, CT 06810, attn: Corporate Secretary, or by telephoning the Company at (877) 736-9378. We have circulated a WHITE proxy card together with our definitive proxy statement. We urge shareholders to vote FOR our nominees on the WHITE proxy card and not to sign or return a green, gold or other colored proxy card to the Company. |


| Our Business Drug development company focused on identifying and developing products that address unmet medical needs, primarily for rare disorders of the nervous system A0001 is lead development asset. Also have rights to an additional NCE from Edison Also license our drug delivery technologies and drug formulation expertise for the formulation of product candidates under licensing collaborations Expect quarterly profitability by Q4 '09 and full year profitability for 2010 We have adequate cash to fund us to profitability and do not believe we need to raise capital to fund this business plan |

| 2009 Strategy Focused strategy to drive shareholder value. The Company's plan for 2009 consists of the following key components: Maximize Opana revenues in partnership with Endo Advance A0001 to establish proof of concept for safety and efficacy Monetize the value of the Company's proven drug delivery technologies and drug formulation expertise through additional drug delivery collaborations Aggressively manage the Company's overhead and other costs to ensure that our spending is commensurate with our other priorities |
| 2009 Value Drivers We believe that by pursuing this strategy, we can enter 2010: As a profitable and cash flow positive Company Opana ER contributing at the full royalty rate Additional assets licensed to create incremental long term growth On a path towards demonstrating proof of concept around safety and biological activity for A0001 |

| Opana(r) ER Developed using Penwest's TIMERx technology Marketed by Endo Pharmaceuticals Indication: For the relief of moderate-to-severe pain in patients requiring continuous, around-the-clock opioid treatment for an extended period of time Endo's Opana ER Sales 1st Quarter Sales YTD Sales 2009 $ 40m n/a 2008 33m 143m 21% Product still on a strong growth trajectory |
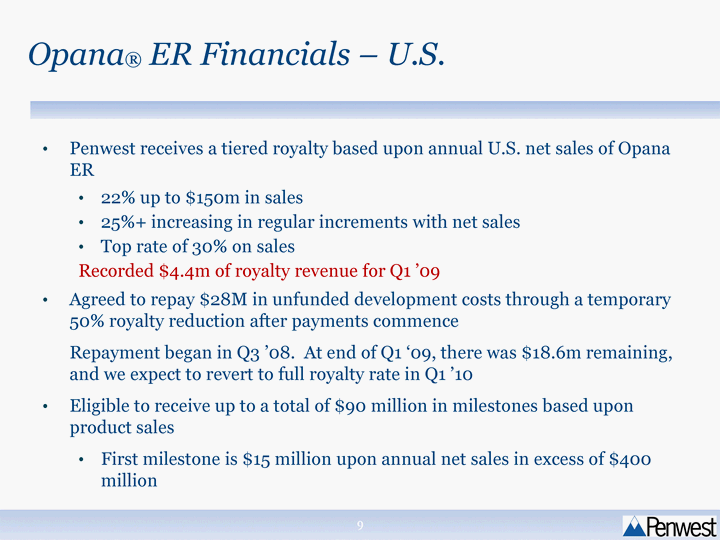
| Penwest receives a tiered royalty based upon annual U.S. net sales of Opana ER 22% up to $150m in sales 25%+ increasing in regular increments with net sales Top rate of 30% on sales Recorded $4.4m of royalty revenue for Q1 '09 Agreed to repay $28M in unfunded development costs through a temporary 50% royalty reduction after payments commence Repayment began in Q3 '08. At end of Q1 '09, there was $18.6m remaining, and we expect to revert to full royalty rate in Q1 '10 Eligible to receive up to a total of $90 million in milestones based upon product sales First milestone is $15 million upon annual net sales in excess of $400 million Opana(r) ER Financials - U.S. |
| Worldwide rights ex-U.S. are jointly owned with Endo Economics to be shared 50/50 Penwest took the business development lead for Opana outside the U.S. in November 2008 Currently seeking licensees in several ex-U.S. territories; we believe a deal can be signed by the end of the first half of this year We believe this is one of the assets that will leave unrealized value on the table if our licensing efforts are not completed Opana(r) ER ex-U.S. |
| Patents - Opana ER Multiple generic filers in the U.S. Penwest and Endo have sued all generic filers First 30-month stay ends June 2010 Latest patent expires in 2013 Multiple additional clinical and formulation patent applications being prosecuted by Penwest and Endo. Beginning in late 2007, this created a major overhang on the Company's stock |
| Development of orphan drugs has risks, but is approachable for a small company: Receptive regulatory environment worldwide Reasonably sized clinical trials Marketing through specialty niche model A0001 Orphan Neurological Diseases Significant unmet medical need |

| A0001-Why We Think It Is Exciting... Compound is alpha-tocopherol quinone Co-enzyme Q class of drug. High dose co-enzyme Q supplementation is proven to have some effect in mitochondrial diseases. A0001 is significantly more bioavailable than co-enzyme Q Marketed in the 70's in France as a compound for hypertension and various myopathies Preclinical assays and Phase I information indicates strong potency and good drug pharmacokinetics Readily available biomarkers that substantiate the energy defect of the disease and allow for assessment of drug benefit |

| 2009 A0001 Development Plan Commenced MAD dosing in Q2; data by end of June Commenced 6 month preclinical tox program; expect data by year end to enable clinical program in patients Initiate Phase IIa trial in H2 '09 with data expected in Q1 '10 The goal of these trials is to establish proof of concept of safety and biological activity, which we believe will be a value creation point for the compound Biological Activity Safety |
| Pursuing technology licensing collaboration with companies utilizing our extended release technologies and formulation expertise Provides stability during the development of our own portfolio Leverages Company asset and capability Covers the salaries of approximately 8 people and a portion of our fixed overhead Good scientific experience for the Company Drug Delivery Technology Licensing Developing product using TIMERx(r). Lifecycle management of a marketed product Developing a generic version of a branded controlled release product Developing product using TIMER(r)x for life cycle management of a marketed product |

| Goals for Drug Delivery - 2009 Sign at least 2 additional deals. We expect that this business at least breaks even on a fully loaded basis with 2 deals a year Advance the 3 compounds currently under development to deliver the targeted formulations to collaborator Maintain upside on drugs from the royalty stream should they hit additional milestones or be marketed We have been receiving royalties from Mylan for nifedipine XL for 10 years with cumulative revenues of approximately $40 million |
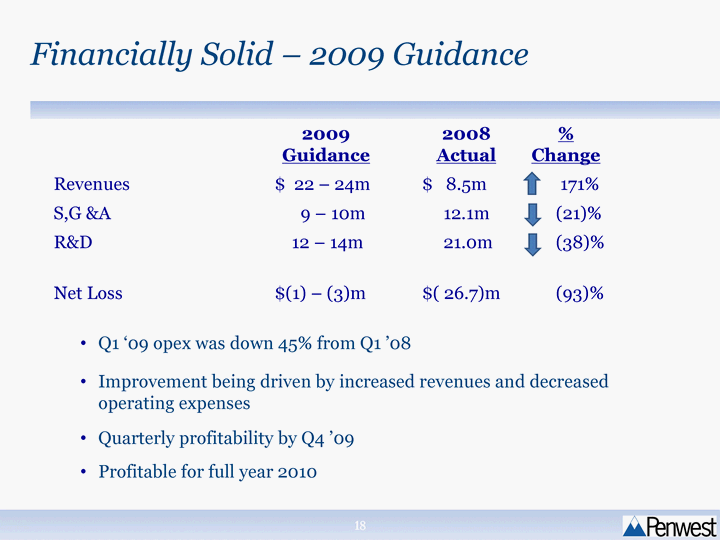
| Financially Solid - 2009 Guidance 2009 Guidance 2008 Actual % Change Revenues $ 22 - 24m $ 8.5m 171% S,G &A 9 - 10m 12.1m (21)% R&D 12 - 14m 21.0m (38)% Net Loss $(1) - (3)m $( 26.7)m (93)% Q1 '09 opex was down 45% from Q1 '08 Improvement being driven by increased revenues and decreased operating expenses Quarterly profitability by Q4 '09 Profitable for full year 2010 |
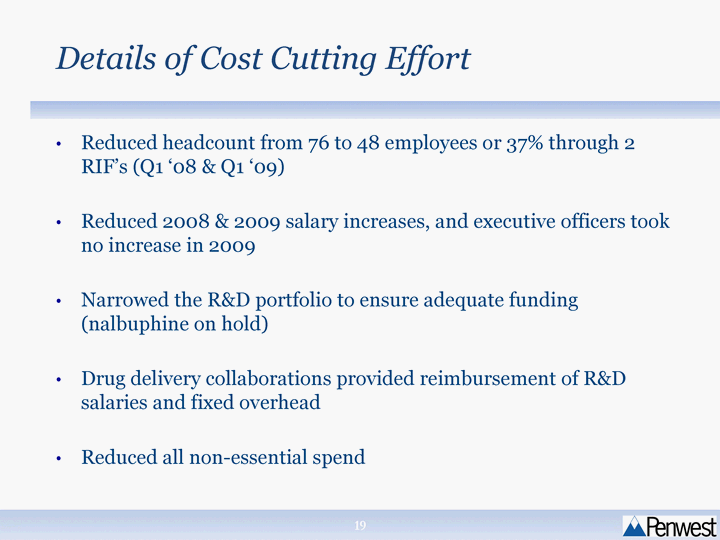
| Details of Cost Cutting Effort Reduced headcount from 76 to 48 employees or 37% through 2 RIF's (Q1 '08 & Q1 '09) Reduced 2008 & 2009 salary increases, and executive officers took no increase in 2009 Narrowed the R&D portfolio to ensure adequate funding (nalbuphine on hold) Drug delivery collaborations provided reimbursement of R&D salaries and fixed overhead Reduced all non-essential spend |
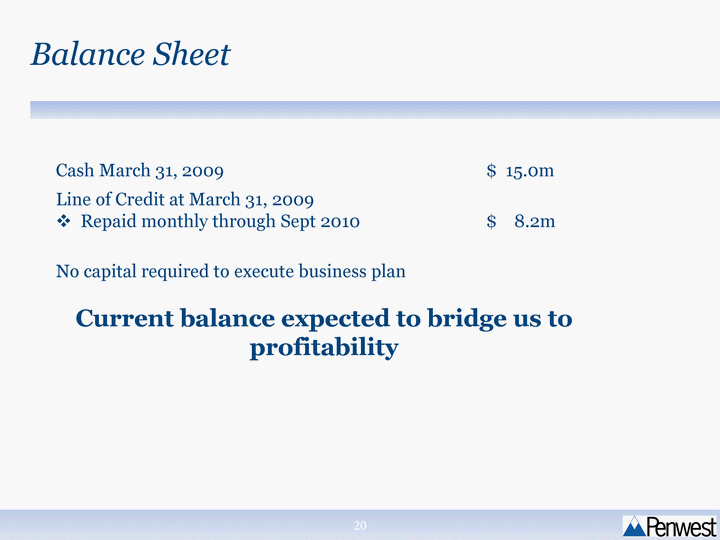
| Balance Sheet Cash March 31, 2009 $ 15.0m Line of Credit at March 31, 2009 Repaid monthly through Sept 2010 $ 8.2m No capital required to execute business plan Current balance expected to bridge us to profitability No capital required to execute business plan Current balance expected to bridge us to profitability No capital required to execute business plan Current balance expected to bridge us to profitability |

| Opana ER Increasing sales by our partner Endo Additional issued patents Ex-U.S. marketing collaborations with at least one signed by end H1 '09 A0001 Complete Phase Ib multiple ascending dose safety study in healthy volunteers with data by end of Q2 '09 Conduct long term preclinical tox program Initiate Phase IIa clinical trial in patients in 2H '09 Select additional NCE from Edison entitled to under agreement Drug Delivery Complete 2 additional technology licensing deals Advance 3 compounds currently under development Financial Continue to aggressively manage costs Manage Company to ensure 2009 financial guidance is met Penwest 2009 Key Milestones |

| Opposing Strategy Vote for Shareholder Value or Vote to Call It Quits Tang/Edelman Strategy: "...immediately winding down substantially all of the Company's operations..." Board unanimously disagrees for the following reasons: Leaves assets on the table which have not realized their full value (Opana ex-U.S. outlicensing, completion of drug development of current technology collaborations, additional drug delivery collaborations) Turn over the card on A0001. Proof of concept of safety and biological activity No quick way to distribute the cash from the Opana asset. Will have to be done on a quarterly basis over the life of the asset. Selling it now would result in a significant discount |

| Opposing Strategy Risk of losing access to the majority of our $91 million of available net operating loss tax carryforwards In 2008, Perceptive triggered a change in control under tax rules, which cost us access to $123 million of NOL's and an additional loss of $7 million in tax credits Under Sec 382, if a Company does not continue its business enterprise at all times during the two years after a change in control, the Company may lose access to NOL's available to offset any taxable income Loss of access to NOL's would result in Opana royalties being immediately taxable! There is risk in dissidents strategy to "immediately wind down substantially all of the Company's operations..." |

| Opposing Strategy Risk of losing our NASDAQ listing because Penwest would be a "public shell" A public shell is defined as "A company with no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents..." Company would likely be delisted, impairing shareholders liquidity in our stock Dissidents have not disputed this fact |

| A Simple Choice for Our Investors: Build Shareholder Value or Call it Quits |

| Why are We Fighting? Strongly believe narrowed strategy for 2009 is right one to build shareholder value in the short-term and prepare the Company for long-term growth Stopping now would prematurely cap potential upside for shareholders May even reduce the value through loss of access to tax credits and loss of NASDAQ listing Dissidents' proposed immediate wind down ignores value/potential value of assets Opana ER ex-US A0001 Drug delivery technology collaborations Dissidents seek to change our bylaws and potentially give veto power and control over important Board decisions to a minority of directors Could stalemate operations |
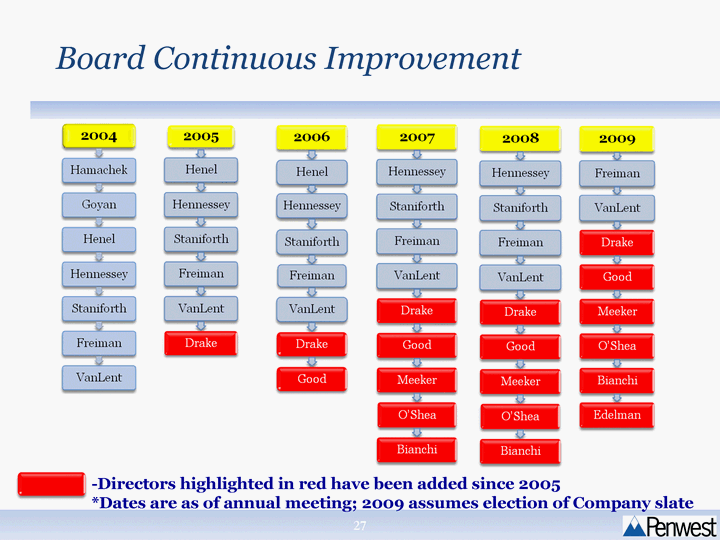
| Board Continuous Improvement Board Continuous Improvement - -Directors highlighted in red have been added since 2005 *Dates are as of annual meeting; 2009 assumes election of Company slate |

| Highly Qualified Board Focused on Building Shareholder Value Since 2005, conscious effort to build a Board of experienced, skilled directors to match the Company's strategy If our nominees are elected, Board will reflect valuable combination of scientific, regulatory, medical, commercial, strategic and financial expertise Only two founding Board members will remain post-election Post election, only three directors will have served for more than three years |

| Board Not Opposed to Considering Alternative Views Nomination of Joe Edelman reflects balanced, reasonable approach Dissident views will be represented in decision-making Facts about Edelman: Long-term Penwest shareholder Has previously offered constructive input about operations and R&D program Interests better aligned with those of other shareholders than Tang Capital nominees |
| Tang's Conduct Raises Serious Concerns Tang's conduct with Penwest raises serious concerns about his suitability to be a director Tang has twice offered in writing to give the Company's directors personally the A0001 asset and business opportunity for their own financial benefit without their having to pay the Company anything Tang proposed to the Company's CEO that a majority of the Board resign, in exchange for severance payments, so that the dissidents could immediately gain complete control of the Company Tang filed three separate lawsuits against the Company (one of which has since been dropped) Superior Court in Washington last week denied dissident's request for preliminary injunction in one of the lawsuits related to proxy vote Tang rejected our offer to discuss a compromise slate of Board seats as a way to avoid this unnecessary and distracting proxy fight - because he wants as much immediate control as he can get |

| Penwest Board is Committed to Good Corporate Governance Penwest's Board is committed to good corporate governance and pay practices while protecting the interests of all shareholders Listens to Shareholders We have recommended that shareholders vote to elect as a director Joseph Edelman, a member of the dissident slate. We believe that this nomination reflects the Company's balanced, reasonable approach to ensuring that the dissidents' views will be represented in its decision making. We have received a signed, written consent from Edelman agreeing to serve on our Board if nominated Compensation is Tied to Performance Our executive compensation program ties a substantial portion of each executive's overall compensation to key company strategic, financial and operational goals, such as new product development initiatives, clinical trial and regulatory progress, intellectual property portfolio development, establishment and maintenance of key strategic relationships, and completion of business development opportunities, as well as our financial and operational performance as measured by adherence to operating budgets approved by our Board. In 2009, the executive officers' salaries were frozen at 2008 levels Rights Plan Implemented to Protect Value We recently adopted a limited-duration (15 months) rights plan which we believe will give the Company an appropriate amount of time to execute its strategy and ensure that all Penwest shareholders have the opportunity to benefit from our initiatives to build shareholder value this year and into 2010. The plan will expire on July 1, 2010, unless shareholders decide to extend it beyond that date |

| By-Law Amendments & Litigation= Seeking Immediate Control The Dissidents' proposals include: A resolution requesting that the Board immediately wind down substantially all of the Company's operations A resolution to amend the Company's by-laws to require approval of 75% or 81% of the directors for certain matters A resolution to amend the Company's by-laws to require the Company to hold its annual meetings on April 30th |
| Conclusion Board unanimously believes that our strategy can build shareholder value in the short-term and prepare the Company for long-term growth by: Maximizing Opana revenues in partnership with Endo Advancing A0001 to establish proof of concept for safety and biological activity Monetizing the value of the Company's proven drug delivery technologies and drug formulation expertise through additional drug delivery collaborations Aggressively managing the Company's overhead and other costs to ensure that our spending is commensurate with our priorities Dissident strategy of winding down doesn't only cap the value for shareholders, but could actually reduce the value through loss of tax credits and NASDAQ listing. |


































