UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(RULE 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934 (Amendment No. )
Filed by the Registrant þ
Filed by a Party other than the Registrant o
Check the appropriate box:
o Preliminary Proxy Statement
oConfidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
o Definitive Proxy Statement
þ Definitive Additional Materials
þ Soliciting Material Pursuant to §240.14a-12
PENWEST PHARMACEUTICALS CO.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| þ | | No fee required. |
| |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | (1) | | Title of each class of securities to which transaction applies: |
| |
| | | | |
| |
| | (2) | | Aggregate number of securities to which transaction applies: |
| |
| | | | |
| |
| | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | | | |
| |
| | (4) | | Proposed maximum aggregate value of transaction: |
| |
| | | | |
| |
| | (5) | | Total fee paid: |
| |
| | | | |
| o | | Fee paid previously with preliminary materials: |
| |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | (1) | | Amount Previously Paid: |
| |
| | | | |
| |
| | (2) | | Form, Schedule or Registration Statement No.: |
| |
| | | | |
| |
| | (3) | | Filing Party: |
| |
| | | | |
| |
| | (4) | | Date Filed: |
| |
| | | | |
The Company is using the following set of slides in presentations in connection with the proxy solicitation for its 2010 Annual Meeting of Shareholders.

| Focused Business Plan and Execution: Driving Shareholder Value NASDAQ: PPCO June 2010 |

| Forward-Looking Statements The matters discussed herein contain forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties, which may cause the actual results in future periods to be materially different from any future performance suggested herein. For this purpose, any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words, "believes," "anticipates," "plans," "expects," "intends," "potential," "appears," "estimates," "projects," "targets," "may," "could," and similar expressions are intended to identify forward-looking statements. Important factors that could cause results to differ materially include the following: risks relating to the commercial success of Opana(r) ER , including our reliance on Endo Pharmaceuticals Inc. for the commercial success of Opana(r) ER , risks of generic competition and risks that Opana(r) ER will not generate the revenues anticipated; the need for capital; regulatory risks relating to drugs in development, including the timing and outcome of regulatory submissions and regulatory actions with respect to A0001; whether the results of clinical trials will be indicative of the results of future clinical trials and will warrant further clinical trials, warrant submission of an application for regulatory approval of, or warrant the regulatory approval of, the product that is the subject of the trial; whether the patents and patent applications owned by us will protect the Company's products and technology; actual and potential competition; and other risks as set forth under the caption Risk Factors in Penwest's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 10, 2010, which risk factors are incorporated herein by reference. The forward-looking statements contained in this presentation speak only as of the date of the statements made. Penwest disclaims any intention or obligation to update any forward-looking statements, and these statements should not be relied upon as representing the Company's estimates or views as of any date subsequent to the date of this presentation. TIMERx (r) is a registered trademark of Penwest. All other trademarks referenced herein are the property of their respective owners. |

| Information Concerning Participants Information required to be disclosed with regard to the Company's directors, director nominees, officers and employees who, under the rules of the Securities and Exchange Commission (the "SEC"), are considered to be "participants" in the Company's solicitation of proxies from its shareholders in connection with its 2010 Annual Meeting of Shareholders (the "Annual Meeting") may be found in the Company's Definitive Proxy Statement for its 2010 Annual Meeting of Shareholders, as filed with the SEC on May 17, 2010 (the "2010 Proxy Statement"). Shareholders may obtain a free copy of the 2010 Proxy Statement and other documents (when available) that the Company files with the SEC at the SEC's website at www.sec.gov. Shareholders may also obtain a free copy of these documents by writing the Company at: Penwest Pharmaceuticals Co., Attention: Corporate Secretary, 2981 Route 22, Suite 2, Patterson, New York 12563. We have circulated a WHITE proxy card together with the 2010 Proxy Statement. We urge shareholders to vote FOR our nominees on the WHITE proxy card and not to sign or return a gold or other colored proxy card to the Company. |

| Penwest at a critical point; needs steady experienced hand Now is not the time for change Current team has been successful implementing its plan Worked cooperatively with Tang and Edelman since their election and seriously considered their viewpoints Focused business plan is delivering performance - and shareholder value Tang and Edelman don't have any new answers, other than additional headcount cuts Fight is about control - who leads Penwest at Board and CEO level Overview of Issues in Proxy Contest |

| Penwest Proxy Contest - What a Difference a Year Makes Last year's contest We presented our 2009 focused business plan Explained why we thought we could build shareholder value in short term and long term Wind down did not make sense We were proven right! Achieved our 2009 goals Stock appreciated 102% from 12/31/08 to 12/31/09 and 138% through 4/23/10 (day before the proxy contest announced) Tang and Edelman now recognize our assets may have value as evidenced by their plan |

| 102% 138% Execution of focused Plan has created value for our shareholders 2009/2010 Stock Performance Dec 31, 2008 Dec 31, 2009 June 7, 2010 Date prior to Opana Settlement April 23, 2010 date prior to announcement of proxy contest 26% 26% June 8, 2010 Opana Patent Settlement |

| Business Plan and Execution |
| Penwest - Highlights of Progress Over Past Year Posted 3 consecutive quarters of profitability, beginning with Q3 '09 Guided for full year profitability in 2010 Continued maximizing the value of Opana(r) ER with Endo Pharmaceuticals through: Ex-U.S. licensing collaboration Generic patent litigation and patent prosecution - settled with all four parties Advanced development of A0001 through Phase I and initiated two Phase IIa trials Grew drug delivery business by signing additional collaborations as well as successfully achieving development milestone Continued cost cutting efforts Board announced its intent to authorize a special cash dividend of $0.50 - $0.75 per share in the fourth quarter of 2010; intends to consider additional cash dividends as the Company's cash resources warrant |
| Company focused on 3 key business areas: Opana(r) ER- treatment for chronic, moderate-to-severe pain A0001 - being developed for Friedreich's Ataxia and MELAS Drug delivery technology - licensing and formulation work utilizing our proprietary TIMERx(r) extended release technology |
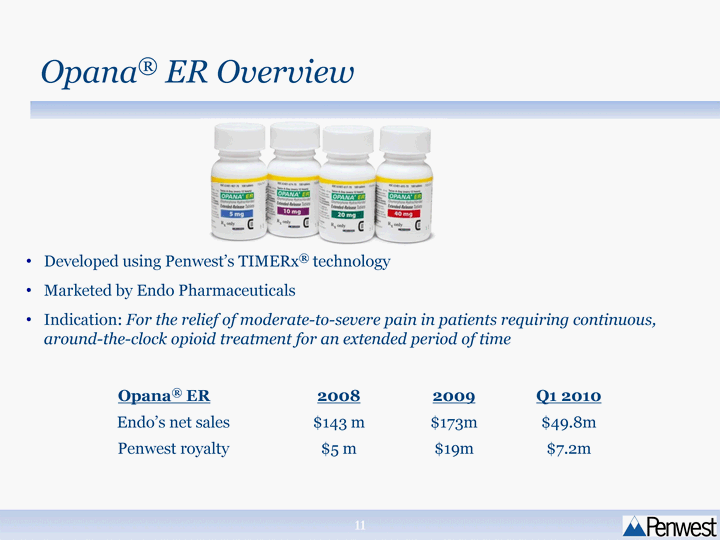
| Developed using Penwest's TIMERx(r) technology Marketed by Endo Pharmaceuticals Indication: For the relief of moderate-to-severe pain in patients requiring continuous, around-the-clock opioid treatment for an extended period of time Opana(r) ER 2008 2009 Q1 2010 Endo's net sales $143 m $173m $49.8m Penwest royalty $5 m $19m $7.2m Opana(r) ER Overview |
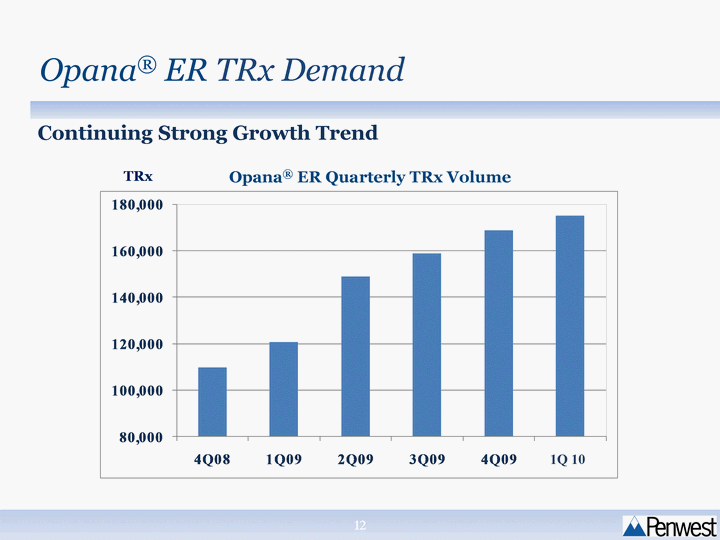
| Opana(r) ER TRx Demand Continuing Strong Growth Trend TRx Opana(r) ER Quarterly TRx Volume 1Q 10 |
| Opana(r) ER Patent Status Multiple generic filers in the U.S. Original filers: IMPAX, Actavis, Sandoz, Barr Recent filers: Watson, Roxanne (running 21/2 years behind) Original litigation settled 5, 10, 20, 40 mg IMPAX first to file IMPAX can launch January 1, 2013, others can launch after 180 days exclusivity 7.5, 15 mg Actavis first to file Actavis can launch July 2011, others can launch these strengths after 180 days exclusivity |
| Penwest receives tiered royalty based on annual U.S. net sales of Opana(r) ER 22% up to $150m in sales 25%+ increasing in regular increments with net sales Top rate of 30% on sales Recorded $7.2m of royalty revenue for Q1 '10 Agreed to repay $28M in unfunded development costs through a temporary 50% royalty reduction after payments commenced Completed this obligation during Q1 '10; full royalty rates going forward As part of the IMPAX settlement, renegotiated rates Through Dec 2012 - 22% cap. Adjustment in Q4 2012 to repay $7.3 m 2013 - 20% cap. Adjustment in Q4 2013 to repay $700,000 Opana(r) ER Financials - U.S. |
| Worldwide rights ex-U.S. jointly owned with Endo Economics to be shared 50/50 In June '09, announced deal with Valeant for Canada, Australia and New Zealand Seeking collaborations in Europe, South America and Asia Opana(r) ER Outside U.S. |
| A0001: ^ tocopherolquinone Coenzyme Q class of drugs, with improved bioavailability Marketed in the '70's in France for hypertension and myopathy Preclinical assays suggest strong potency and Phase I demonstrated good drug pharmacokinetics Targeting development in two mitochondrial disorders: Friedreich's Ataxia MELAS Syndrome |

| A0001: Friedreich's Ataxia Currently no approved therapies in U.S. or Europe Severe genetic disorder Degeneration of nerve and muscle tissue, loss of muscle control Impaired movements, muscle wasting Thickening of heart walls (cardiomyopathy) Caused by reduced level of fraxtaxin (key protein in mitochondria) Onset 5-15 years, reduced life expectancy Estimate approximately 20,000 patients in North America and Europe Chronic disorder, requires life-long treatment |
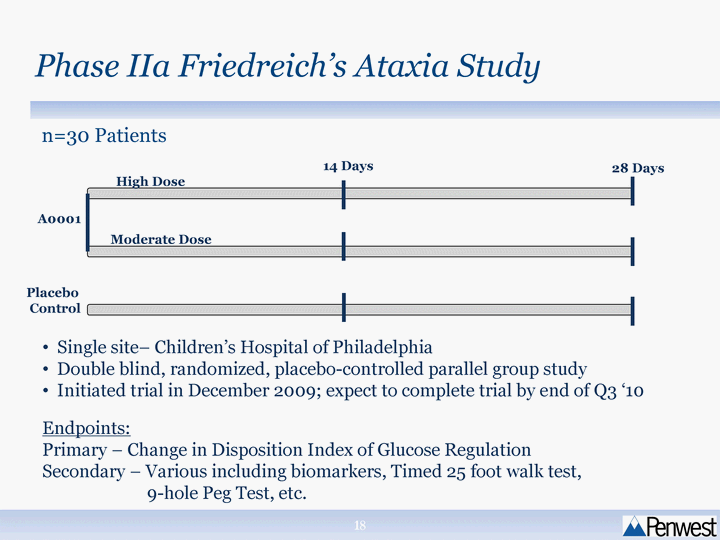
| Phase IIa Friedreich's Ataxia Study 14 Days 28 Days High Dose Moderate Dose A0001 Placebo Control Single site- Children's Hospital of Philadelphia Double blind, randomized, placebo-controlled parallel group study Initiated trial in December 2009; expect to complete trial by end of Q3 '10 Endpoints: Primary - Change in Disposition Index of Glucose Regulation Secondary - Various including biomarkers, Timed 25 foot walk test, 9-hole Peg Test, etc. n=30 Patients |
| A0001: MELAS Syndrome Currently no approved therapies in U.S. or Europe Rare, progressive, genetic neurodegenerative disorder Involves multiple system organs including CNS, skeletal, muscle, eye and cardiac muscle Presentation typically occurs with stroke-like symptom Onset typically 4-15 years of age Estimate 20,000 - 25,000 patients in North America and Europe Chronic disorder, requires life-long treatment |
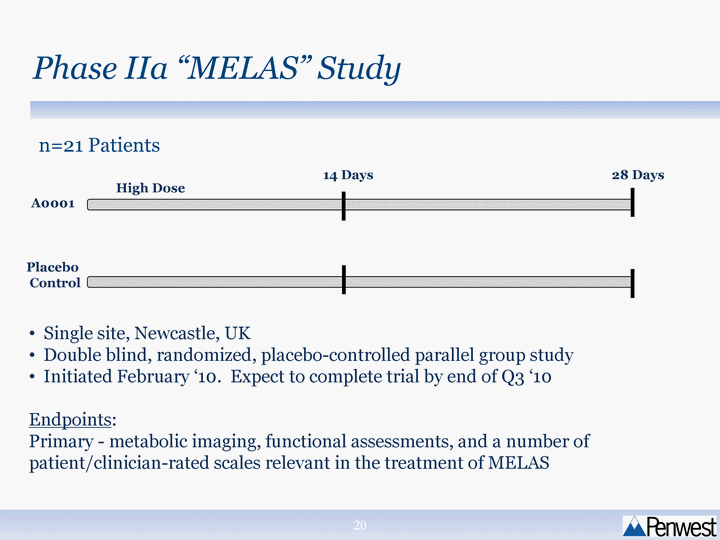
| Phase IIa "MELAS" Study 14 Days 28 Days High Dose A0001 Placebo Control Single site, Newcastle, UK Double blind, randomized, placebo-controlled parallel group study Initiated February '10. Expect to complete trial by end of Q3 '10 Endpoints: Primary - metabolic imaging, functional assessments, and a number of patient/clinician-rated scales relevant in the treatment of MELAS n=21 Patients |
| A0001: Development To Date & Plan Completed Phase I safety program Completed 6-month preclinical tox program Established initial safety in animals and healthy volunteers Initiated Phase IIa trials in Friedreich's Ataxia and MELAS Expect to complete studies by end of Q3 '10 In parallel with development, we will be exploring potential licensing opportunities for A0001 in anticipation of the completion of Phase IIa trials Phase IIa Safety |

| Significant unmet medical need; supports pricing Market exclusivity through orphan drug protection (U.S. 7 years, Europe 10 years) Receptive regulatory environment worldwide Well-organized medical communities through patient advocacy groups Established Initial Proof of Safety Seeking Proof of Biological Activity Good preclinical science supporting efficacy in targeted diseases A0001: Why are we excited? Proven Business Model in Orphan Diseases We believe Phase IIa data could boost value of A0001; we will stop development if data does not support continuing |

| Oral controlled release technology based on a natural gum matrix. Dual-delivery system which can release drugs or isomers at two different rates. Releases drug at the desired time and site in the GI tract. Releases drug in upper GI tract. Our oral controlled release technologies can be customized to deliver drugs via a wide range of desired release profiles Valuable technology successfully used with Opana(r) ER Drug Delivery Technologies |
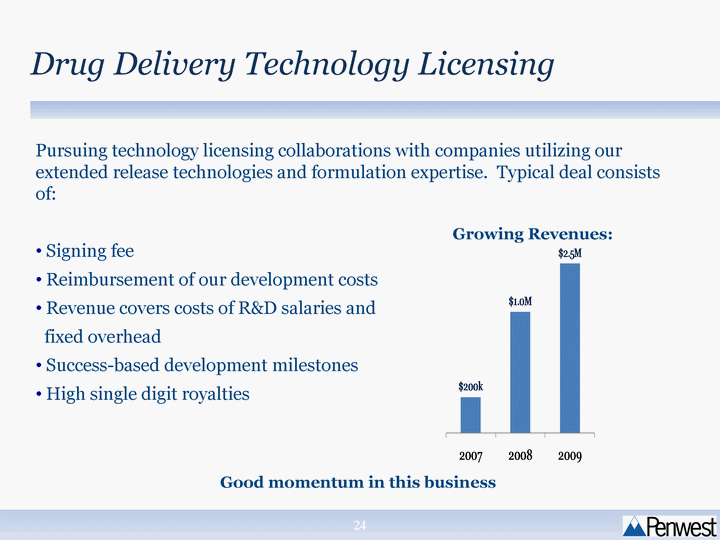
| Pursuing technology licensing collaborations with companies utilizing our extended release technologies and formulation expertise. Typical deal consists of: Signing fee Reimbursement of our development costs Revenue covers costs of R&D salaries and fixed overhead Success-based development milestones High single digit royalties Drug Delivery Technology Licensing Good momentum in this business Growing Revenues: |
| Current Drug Delivery Collaborations Signed 4 collaborations using TIMERx (r) for lifecycle management of marketed products Signed 2 deals in 2009 October 2009 - announced successful proof of principal in Phase I study of formulation under first Otsuka deal Several formulations currently in ongoing clinical trials Alvogen, Inc Signed multi-drug generics agreement in April 2010 Companies will select up to 5 compounds to develop over next two years Work underway on first compound |

| Working closely with Endo to maximize the value of Opana(r) ER Completing both Phase IIa trials of A0001, analyzing the data and making a "go/no-go" decision Exploring potential licensing opportunities for A0001 in anticipation of the completion of the Phase IIa trials Growing our drug delivery business both by completing formulation work on compounds under development and by signing additional deals Continuing to aggressively manage expenses to ensure our costs are appropriate given our priorities 2010 Business Plan |

| Cost Cutting Efforts and Financial Overview |

| Details of Cost Cutting Effort Reduced headcount from 76 to 38 employees, or 50%, as we have focused the business plan Consolidated two office locations into one Narrowed the R&D portfolio to most promising opportunity in A0001 Drug delivery collaborations provide reimbursement of R&D salaries and fixed overhead Eliminated all non-essential spend |
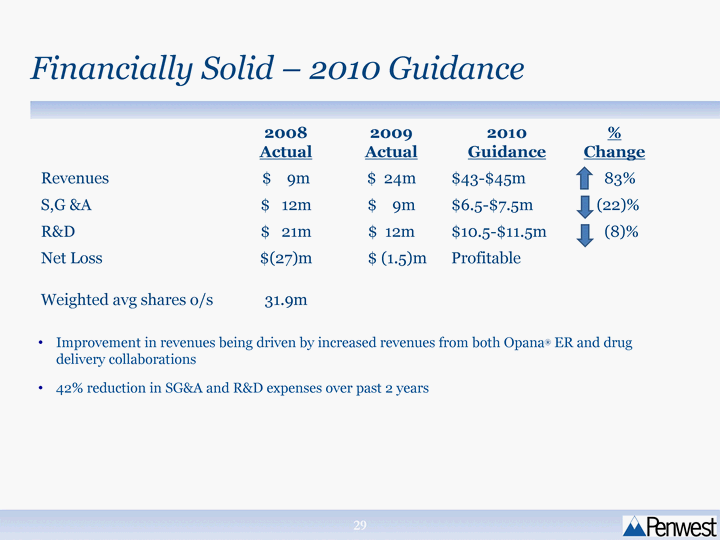
| Financially Solid - 2010 Guidance 2008 Actual 2009 Actual 2010 Guidance % Change Revenues $ 9m $ 24m $43-$45m 83% S,G &A $ 12m $ 9m $6.5-$7.5m (22)% R&D $ 21m $ 12m $10.5-$11.5m (8)% Net Loss $(27)m $ (1.5)m Profitable Weighted avg shares o/s 31.9m Improvement in revenues being driven by increased revenues from both Opana(r) ER and drug delivery collaborations 42% reduction in SG&A and R&D expenses over past 2 years |

| Balance Sheet - March 31, 2010 Cash $ 11.5m Line of Credit to be repaid by Sept 2010 $ 2.7m No capital required to execute business plan in 2010 Board announced intent to authorize a special cash dividend of $0.50 - $0.75/share in Q4 '10 following repayment of line of credit No capital required to execute business plan in 2010 Board announced intent to authorize a special cash dividend of $0.50 - $0.75/share in Q4 '10 following repayment of line of credit No capital required to execute business plan in 2010 Board announced intent to authorize a special cash dividend of $0.50 - $0.75/share in Q4 '10 following repayment of line of credit |

| Board Focus on Shareholder Value |

| Board Focus on Shareholder Value 2007 Focused effort to bring on new Board members; 50% of 2009 Board is new Jan. 2009 Announced focused business plan in response to challenging economic conditions and shareholder concerns Jan.-Dec. 2009 Significantly reduced headcount and expenses to support more focused business plan Q1 2010 Announced intent to declare special cash dividend of $0.50-$0.75 per share in Q4 2010 Board keenly focused on execution of this plan Board keenly focused on execution of this plan |
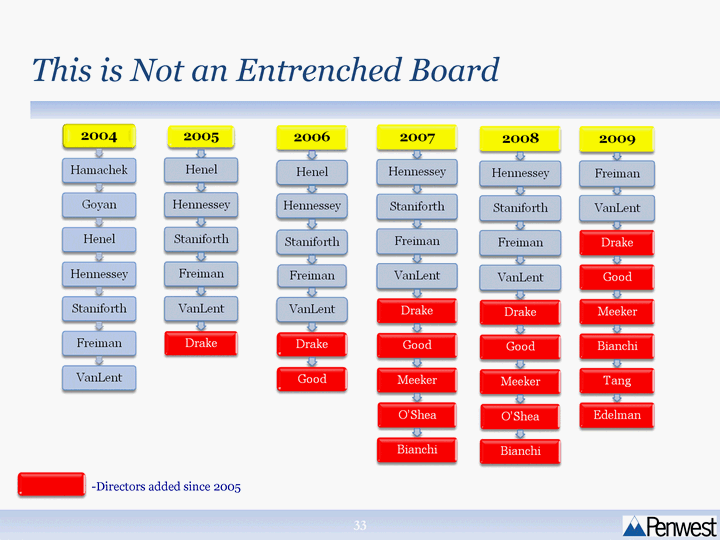
| This is Not an Entrenched Board This is Not an Entrenched Board - -Directors added since 2005 |

| Strong Balanced Working Board Directors have diverse skills including various functional expertise from the industry as well as representation of shareholders' perspective Board members are independent of each other Checks and balances exist Represents the perspectives of all shareholders Since Tang and Edelman joined the Board, the Board has worked cooperatively and seriously considered their input in its deliberations and decisions |

| 2010 Nominating & Governance Committee Conclusions |

| 2010 Nomination Process Tang and Edelman nominated 3 individuals for consideration Conducted thorough and fair review of each candidate Interviewed 2 of 3 candidates in person Offered compromise proposals - Tang and Edelman rejected N&G Committee: Anne VanLent, Chair David Meeker Peter Drake 3 directors independent of this year's election |
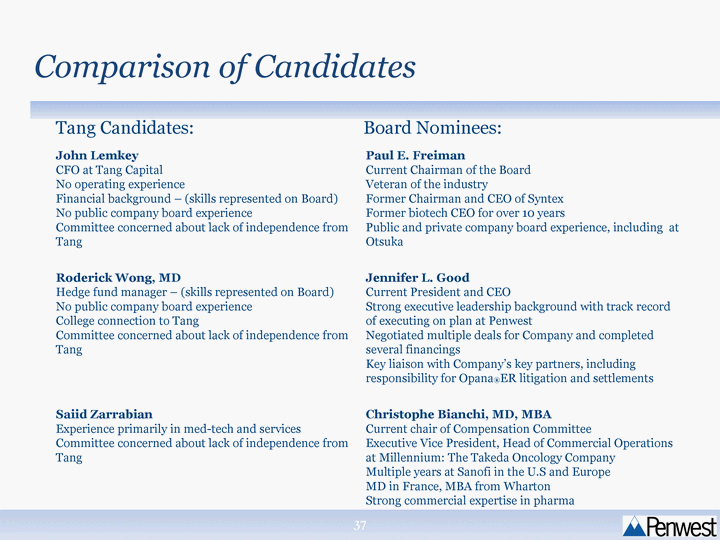
| John Lemkey CFO at Tang Capital No operating experience Financial background - (skills represented on Board) No public company board experience Committee concerned about lack of independence from Tang Paul E. Freiman Current Chairman of the Board Veteran of the industry Former Chairman and CEO of Syntex Former biotech CEO for over 10 years Public and private company board experience, including at Otsuka Roderick Wong, MD Hedge fund manager - (skills represented on Board) No public company board experience College connection to Tang Committee concerned about lack of independence from Tang Jennifer L. Good Current President and CEO Strong executive leadership background with track record of executing on plan at Penwest Negotiated multiple deals for Company and completed several financings Key liaison with Company's key partners, including responsibility for Opana(r)ER litigation and settlements Saiid Zarrabian Experience primarily in med-tech and services Committee concerned about lack of independence from Tang Christophe Bianchi, MD, MBA Current chair of Compensation Committee Executive Vice President, Head of Commercial Operations at Millennium: The Takeda Oncology Company Multiple years at Sanofi in the U.S and Europe MD in France, MBA from Wharton Strong commercial expertise in pharma Comparison of Candidates Tang Candidates: Board Nominees: |

| Nominating & Governance Committee Recommendations Committee recommended Board slate of Paul Freiman, Jennifer Good and Christophe Bianchi Recommendation based on: Concern regarding the independence of the Tang and Edelman slate Board nominees had more expertise and experience |


| Disappointing to be here again a year later We achieved our goals for 2009 - track record of execution Good momentum on deliverables in 2010 Stock price has risen significantly. Shareholders have been rewarded Executing on a plan that is similar to the plan they are now proposing Critical decisions/actions upcoming Need right team making these decisions and taking actions Other shareholders have expressed deep concerns with Tang and Edelman control Execution risk Loss of checks and balances and independence Election of Board nominees preserves an equation that is working |

| Disruptive to the Company Proxy contest has been very costly for the Company and its shareholders Difficult on existing partnerships and negotiations of new collaborations Disruptive to morale of employees who are focused on executing against our plan |

| Tang and Edelman want Control! Tang and Edelman nominees less qualified; less likely to represent the interests of all shareholders Tang and Edelman have no alternative plan Risk of disrupting execution of focused plan that is working -- proxy suggestion of management change and employee downsizing |











































