UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934
| | | |
Filed by the Registrantþ | | Filed by a Party other than the Registranto |
Check the appropriate box:
| | o | | Preliminary Proxy Statement |
| |
| | o | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| | þ | | Definitive Proxy Statement |
| |
| | o | | Definitive Additional Materials |
| |
| | o | | Soliciting Material Pursuant to §240.14a-12 |
PENWEST PHARMACEUTICALS CO.
(Name of Registrant as Specified in Its Charter)
Payment of Filing Fee (Check the appropriate box):
| | þ | | No fee required. |
| |
| | o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | 1. | | Title of each class of securities to which transaction applies: |
| |
| | 2. | | Aggregate number of securities to which transaction applies: |
| |
| | 3. | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | 4. | | Proposed maximum aggregate value of transaction: |
| |
| | 5. | | Total fee paid: |
| | o | | Fee paid previously with preliminary materials. |
| |
| | o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | 1. | | Amount previously paid: |
| |
| | 2. | | Form, Schedule or Registration Statement No.: |
| |
| | 3. | | Filing Party: |
| |
| | 4. | | Date Filed: |
 Penwest Pharmaceuticals Co.
Penwest Pharmaceuticals Co.39 Old Ridgebury Road, Suite 11
Danbury, Connecticut 06810-5120
May 3, 2006
Dear Shareholders:
You are cordially invited to attend the annual meeting of shareholders of Penwest Pharmaceuticals Co. to be held on June 7, 2006 at 11:00 a.m. at 39 Old Ridgebury Road, Danbury, Connecticut 06810.
In addition to the items set forth in the accompanying notice of annual meeting of shareholders and proxy statement, we will report on our current activities and will provide an opportunity to discuss matters of interest to you as a shareholder.
We sincerely hope you will be able to attend our annual meeting. However, whether or not you plan to attend, please sign, date and promptly return the enclosed proxy to ensure that your shares are represented. Alternatively, you may also vote your shares over the Internet or by telephone. Please refer to the enclosed proxy for instructions. If you so desire, you may withdraw your proxy and vote in person at our annual meeting.
On behalf of our board of directors, I would like to express our appreciation for your continued interest in Penwest.
| |
| | Very truly yours, |
| |
| |  |
| |
| | Jennifer L. Good |
| | President, Chief Operating Officer
and Chief Financial Officer |
TABLE OF CONTENTS
PENWEST PHARMACEUTICALS CO.
39 Old Ridgebury Road, Suite 11
Danbury, Connecticut 06810-5120
NOTICE OF ANNUAL MEETING OF SHAREHOLDERS
To be held on June 7, 2006
To the Shareholders:
Notice is hereby given that the annual meeting of shareholders of Penwest Pharmaceuticals Co., a Washington corporation, will be held at 39 Old Ridgebury Road, Danbury, Connecticut 06810, on June 7, 2006 at 11:00 a.m., for the following purposes:
| | |
| | 1. | To elect two class III directors for the ensuing three years; |
| |
| | 2. | To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the current fiscal year; and |
| |
| | 3. | To transact such other business as may properly come before the meeting or at any adjournment or postponement thereof. |
Our board of directors has no knowledge of any other business to be transacted at the meeting.
A copy of our annual report to shareholders for the year ended December 31, 2005, which contains financial statements and other information of interest to shareholders, accompanies this notice and the enclosed proxy statement.
Only shareholders of record at the close of business on April 14, 2006 are entitled to notice of, and to vote at, the annual meeting and at any adjournments or postponements thereof.
| |
| | BY ORDER OF THE BOARD OF DIRECTORS |
| |
| |  |
| | Jennifer L. Good |
| | Corporate Secretary |
Danbury, Connecticut
May 3, 2006
IMPORTANT
Whether or not you plan to attend the meeting, please sign, date and return promptly the enclosed proxy in the enclosed envelope, which requires no postage if mailed in the United States. Alternatively, please vote over the Internet or by telephone by following the instructions on the enclosed proxy.Promptly signing, dating and returning the proxy or otherwise voting your shares will save Penwest the additional expense of further solicitation.
PENWEST PHARMACEUTICALS CO.
39 Old Ridgebury Road, Suite 11
Danbury, Connecticut 06810-5120
PROXY STATEMENT
This proxy statement is furnished in connection with the solicitation of proxies by the board of directors of Penwest Pharmaceuticals Co., a Washington corporation, to be voted at our 2006 annual meeting of shareholders to be held at 11:00 a.m. on June 7, 2006, at our principal executive offices at 39 Old Ridgebury Road, Danbury, Connecticut 06810, and at any adjournment or postponement thereof.
The notice of the annual meeting, this proxy statement, the enclosed proxy and our 2005 annual report to shareholders, which contains our annual report on Form 10-K for the year ended December 31, 2005 as filed with the Securities and Exchange Commission, without exhibits, are being mailed to shareholders on or about May 3, 2006. Exhibits to our annual report on Form 10-K will be provided upon written request and payment of an appropriate processing fee. Please address all such requests to Penwest Pharmaceuticals Co., Attention: Corporate Secretary, 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810-5120.
Voting Securities and Votes Required
On April 14, 2006, the record date for determination of shareholders entitled to notice of and to vote at the annual meeting, there were outstanding and entitled to vote an aggregate of 22,800,975 shares of our common stock, $.001 par value per share. Holders of our common stock are entitled to one vote per share.
The holders of a majority of the shares of our common stock issued and outstanding and entitled to vote at the annual meeting shall constitute a quorum for the transaction of business at the annual meeting. Shares of our common stock present in person or represented by executed proxies received by us will be counted for purposes of establishing a quorum at the annual meeting. These shares include shares that are held in “street name” by brokers or nominees who indicate on their proxies that they do not have discretionary authority to vote such shares as to one or more of the matters to be voted upon, which we refer to as broker non-votes, and shares that abstain or do not vote with respect to one or more of the matters to be voted upon.
The two director candidates receiving the largest number of votes cast by shareholders entitled to vote in the election will be elected at the annual meeting. The votes cast by the shareholders entitled to vote favoring the action must exceed the votes cast by the shareholders entitled to vote opposing the action for the ratification of the appointment of our independent registered public accounting firm.
Shares will not be counted as votes in favor of a matter, and will also not be counted as votes cast or shares voting on such matter, if the holder of the shares either withholds the authority to vote for a particular director nominee or nominees, or abstains from voting on a particular matter, or if the shares constitute broker non-votes. Accordingly, withheld shares, abstentions and broker non-votes will have no effect on the election of directors or the ratification of the appointment of our independent registered public accounting firm.
Shareholders may vote by any one of the following means:
| | |
| | • | by mail; |
| |
| | • | by telephone (toll free); |
| |
| | • | over the Internet; or |
| |
| | • | in person at the annual meeting. |
1
To vote by mail, please sign, date and complete the enclosed proxy and return it in the enclosed self-addressed envelope. No postage is necessary if the proxy is mailed in the United States. Instructions for voting by using a toll-free telephone number or over the Internet can be found on your proxy. If you hold your shares through a bank, broker or other nominee, it will give you separate instructions for voting your shares.
All proxies will be voted in accordance with the instructions of the shareholder. If no choice is specified, the proxies will be voted in favor of the matters set forth in the accompanying notice. Any proxy may be revoked by a shareholder at any time before its exercise by delivery of a written revocation or a subsequently dated proxy to our corporate secretary, or by voting in person at the annual meeting. Attendance at the annual meeting will not itself be deemed to revoke a proxy unless the shareholder gives affirmative notice at the annual meeting that the shareholder intends to revoke the proxy and vote in person.
2
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information, as of February 28, 2006, regarding the beneficial ownership of our common stock by:
| | |
| | • | any person known to us to be the beneficial owner of more than five percent of our common stock; |
| |
| | • | each director; |
| |
| | • | each of the following individuals, whom we refer to as our named executive officers: (i) Jennifer L. Good, our current President, Chief Operating Officer and Chief Financial Officer, (ii) our four other most highly compensated executive officers who were serving as executive officers as of December 31, 2005 and (iii) Tod R. Hamachek and Robert J. Hennessey, each of whom served as Chief Executive Officer of our company during 2005; and |
| |
| | • | all of our current directors and executive officers as a group. |
| | | | | | | | | | |
| | | Number of Shares of | | | |
| | | Common Stock | | | Percent of | |
| Name and Address for Beneficial Owners | | Beneficially Owned(1) | | | Class(2) | |
| | | | | | | |
5% Shareholders: | | | | | | | | |
| Balyasny Asset Management LLC | | | | | | | | |
| | 181 West Madison Street, Suite 3600 | | | | | | | | |
| | Chicago, IL 60602 | | | 1,366,731 | (3) | | | 6.1 | % |
| |
| Orbimed Advisors LLC | | | | | | | | |
| | 767 3RD Avenue 30th Floor | | | | | | | | |
| | New York, New York 10017 | | | 1,311,300 | (4) | | | 5.8 | % |
| |
Directors: | | | | | | | | |
| Peter F. Drake | | | 27,905 | | | | * | |
| Paul E. Freiman | | | 98,998 | (5) | | | * | |
| Rolf H. Henel | | | 70,083 | (6) | | | * | |
| Robert J. Hennessey | | | 178,420 | (7) | | | * | |
| John N. Staniforth | | | 103,460 | (8) | | | * | |
| Anne M. VanLent | | | 104,160 | (9) | | | * | |
Other Named Executive Officers: | | | | | | | | |
| Jennifer L. Good | | | 296,450 | (10) | | | 1.3 | % |
| Anand R. Baichwal | | | 193,234 | (11) | | | * | |
| Alan F. Joslyn, Ph.D. | | | 260 | | | | * | |
| Theodor Rozsa | | | — | | | | * | |
| Thomas R. Sciascia | | | 155,238 | (12) | | | * | |
| Tod R. Hamachek | | | 664,507 | (13) | | | 2.9 | % |
| All directors and executive officers as a group (11 persons) | | | 1,228,208 | (14) | | | 5.2 | % |
| | |
| | * | Represents less than 1%. |
| | |
| | (1) | The number of shares beneficially owned by each person or entity is determined under rules promulgated by the Securities and Exchange Commission, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which an individual or group has sole or shared voting power or investment power, although the inclusion of such shares does not constitute an admission that the named shareholder is a direct or indirect beneficial owner of such shares. Unless otherwise indicated, each person or group named in the table has sole voting and investment power (or shares such power with his or her spouse) with respect to all shares of common stock listed as owned by such person or entity. |
| |
| | (2) | Percentage ownership calculations are based on 22,548,894 shares of our common stock outstanding as of February 28, 2006. Any shares that may be acquired by a person or entity upon the exercise of any stock option, warrant or other right within 60 days of February 28, 2006 are deemed to be |
3
| | |
| | | outstanding for the purpose of calculating the percentage of the outstanding common stock owned by such person or entity. These shares, however, are not considered outstanding when computing the percentage ownership of any other person or entity. |
| |
| | (3) | The foregoing information is based solely on a Schedule 13G/ A filed with the Securities and Exchange Commission on February 14, 2006. |
| |
| | (4) | The foregoing information is based solely on a Schedule 13G/ A filed with the Securities and Exchange Commission on February 10, 2006. |
| |
| | (5) | Includes 76,611 shares subject to outstanding stock options held by Mr. Freiman that are exercisable within 60 days following February 28, 2006. |
| |
| | (6) | Includes 56,333 shares subject to outstanding stock options held by Mr. Henel that are exercisable within 60 days following February 28, 2006. |
| |
| | (7) | Includes 146,900 shares subject to outstanding stock options held by Mr. Hennessey that are exercisable within 60 days following February 28, 2006. |
| |
| | (8) | Includes 79,095 shares subject to outstanding stock options held by Dr. Staniforth that are exercisable within 60 days following February 28, 2006. |
| |
| | (9) | Includes 71,871 shares subject to outstanding stock options held by Ms. VanLent that are exercisable within 60 days following February 28, 2006. |
| |
| (10) | Includes 290,583 shares subject to outstanding stock options held by Ms. Good that are exercisable within 60 days following February 28, 2006. |
| |
| (11) | Consists of 193,234 shares subject to outstanding stock options held by Dr. Baichwal that are exercisable within 60 days following February 28, 2006. |
| |
| (12) | Includes 150,750 shares subject to outstanding stock options held by Dr. Sciascia that are exercisable within 60 days following February 28, 2006. |
| |
| (13) | Includes 425,000 shares subject to outstanding stock options held by Mr. Hamachek that are exercisable within 60 days following February 28, 2006. |
| |
| (14) | Includes 1,065,377 shares subject to outstanding options that are exercisable within 60 days following February 28, 2006. |
PROPOSAL 1 — ELECTION OF DIRECTORS
Board of Directors
We have a classified board of directors that currently consists of two class I directors, two class II directors and two class III directors. At each annual meeting of shareholders, directors are elected for a full term of three years to succeed those whose terms are expiring. The term for our class III directors elected at this annual meeting will expire at our 2009 annual meeting of shareholders, the term for our current class I directors will expire at our 2007 annual meeting of shareholders, and the term for our current class II directors will expire at our 2008 annual meeting of shareholders.
Our board, upon the recommendation of the Nominating and Governance Committee, has nominated Robert J. Hennessey and John N. Staniforth for election as class III directors. Mr. Hennessey and Dr. Staniforth are currently our class III directors. The persons named in the enclosed proxy will vote to elect, as class III directors, Mr. Hennessey and Dr. Staniforth, the director nominees named below, unless the proxy is marked otherwise.
Each class III director will be elected to hold office until our 2009 annual meeting of shareholders and until his successor is elected and qualified. Each of the nominees has indicated his willingness to serve, if elected; however, if any nominee should be unable to serve, the person acting under the proxy may vote the proxy for a substitute nominee designated by the board. The board has no reason to believe that either of the nominees will be unable to serve if elected.Our board of directors recommends the election of Robert J. Hennessey and John N. Staniforth as class III directors.
4
Set forth below for each nominee for election as a class III director and for each director whose term of office will continue beyond the date of the annual meeting, are the director’s name and age as of February 28, 2006, his or her principal occupation and business experience during at least the past five years, the names of other publicly held companies in which he or she serves as a director and the year of the commencement of his or her term as one of our directors.
Nominees for Election (Class III Directors)
Robert J. Hennessey, 64, has served as one of our directors since October 1997. Mr. Hennessey served as our President and Chief Executive Officer from February 15, 2005 until November 23, 2005. Mr. Hennessey served as President and Chief Executive Officer of Genome Therapeutics Corporation, a biotechnology company, from March 1993 until his retirement in November 2000, and served as its Chairman of the Board until March 2003. Prior to joining Genome Therapeutics Corporation, Mr. Hennessey was an independent consultant of Hennessey & Associates, Ltd., a strategic consulting firm to biotechnology and healthcare companies. Prior to that, Mr. Hennessey was Senior Vice President of Corporate Development for Sterling Drug, Inc. and also served in various executive assignments at Merck & Co., Inc., SmithKline Beecham PLC and Abbott Laboratories, each a pharmaceutical company. Mr. Hennessey is a director of Repligen, Inc., a biotechnology company, and Oscient Pharmaceuticals (formerly known as Genome Therapeutics). Mr. Hennessey holds an M.A. in Political Science and an A.B. in Liberal Arts from the University of Connecticut.
Dr. John N. Staniforth, 52, has served as one of our directors since December 1998. Dr. Staniforth has served as the Chief Scientific Officer of Vectura, Limited, a biosciences company in the United Kingdom, since September 1999. Dr. Staniforth is an Honorary Professor of the University of Bath in Bath, England. Prior to joining Vectura, Dr. Staniforth was Professor of Pharmaceutics Technology at the University of Bath from 1980 to 1999. Dr. Staniforth serves as scientific advisor to a number of international pharmaceutical companies and has extensive teaching and research experience, chiefly at the University of Bath, Department of Pharmacy, at Rutgers University and Cornell University in the United States, and at Monash University in Australia. His research into powder mixing technology has been widely published, and Dr. Staniforth is the recipient of numerous scientific awards, including the Churchill Fellowship, the Pfizer Medal for Pharmaceutical Research, and the Special Upjohn Award for research in the field of microwave and radio-frequency drying, and has been elected Fellow of the American Association of Pharmaceutical Sciences. Dr. Staniforth was also the recipient of the 2003 AstraZeneca Industrial Achievement Award. Dr. Staniforth has served as a consultant to us since our inception and is the co-inventor of our TIMERx technology.
Class I Directors (Term Expires 2007)
Paul E. Freiman, 71, has served as our Chairman of the Board since February 2005 and as the Lead Director since October 1997. Mr. Freiman has served as the Chief Executive Officer and President of Neurobiological Technologies, Inc., a biotechnology company, since May 1997. Mr. Freiman served as Chairman and Chief Executive Officer of Syntex Corporation, a pharmaceutical company, from 1990 to 1995. Mr. Freiman is also a director of Calypte Biomedical Corporation, a developer of in vitro testing solutions, NeoPharm Inc., a biotechnology company, and Otsuka America Pharmaceuticals Inc., a pharmaceutical company. Mr. Freiman has been chairman of the Pharmaceutical Manufacturers Association of America and has chaired a number of its key committees. He is a graduate of Fordham University with a B.S. in Pharmacy and holds an honorary doctorate from the Arnold & Marie Schwartz College of Pharmacy.
Rolf H. Henel, 68, has served as one of our directors since October 1997. Mr. Henel has served as a consultant to the health care industry as a partner of Naimark & Associates P.C. since September 1994. From 1996 to 1997, Mr. Henel was a director and Chief Operating Officer of Immunomedics, Inc., a biopharmaceutical company. From 1978 to 1993, Mr. Henel served in a variety of positions at American Cyanamid Co., a pharmaceutical company, most recently as President of Cyanamid International — Lederle Division, as well as Vice Chairman of Lederle’s Medical Research Planning Committee. He is a
5
director of SciClone Pharmaceuticals, a biopharmaceutical company, and is a director of Draxis Health, Inc., a specialty pharmaceutical company. Mr. Henel holds an M.B.A. from New York University and a B.A. from Yale University.
Class II Directors (Term Expires 2008)
Dr. Peter F. Drake, 52, has served as one of our directors since April 13, 2005. Dr. Drake is currently the Managing General Partner of Mayflower Partners, a healthcare investment fund. From 1999 to 2002, he served as a Managing Director in the Equity Research Department of Prudential Securities, Inc., following Prudential’s acquisition of Vector Securities International, an investment banking firm co-founded by Dr. Drake in 1988. From 1988 to 1998, Dr. Drake served as Executive Vice President at Vector. Dr. Drake joined the investment banking firm of Kidder, Peabody & Co. as a Biotechnology Analyst in 1983, becoming a partner in 1986. He currently serves on the Board of Directors of Trustmark Insurance Co., a healthcare insurance provider. Dr. Drake received a B.A. in Biology from Bowdoin College, a CBA from the Wharton School of Business at the University of Pennsylvania and a Ph.D. in Biochemistry and Neurobiology from Bryn Mawr College.
Anne M. VanLent, 58, has served as one of our directors since December 1998. Ms. VanLent has served as Executive Vice President and Chief Financial Officer of Barrier Therapeutics, Inc., a specialty pharmaceutical company in the field of dermatology, since May 2002. Prior to joining Barrier, Ms. VanLent served as a principal of The Technology Compass Group, LLC, a healthcare/technology consulting firm, which she founded in October 2001. From mid-1997 through October 2001, Ms. VanLent served as Executive Vice President, Portfolio Management of Sarnoff Corporation, a privately-held research and development company that creates and commercializes electronic, biomedical and information technologies. Her last position with Sarnoff was Executive Vice President, Portfolio Management, overseeing creation of spin-off companies, and patent and licensing activities. Ms. VanLent served as President of AMV Associates, an emerging growth healthcare consulting firm, from March 1994 through August 1997, and as Senior Vice President and Chief Financial Officer of The Liposome Company, Inc., a biotechnology company, from 1985 through 1993. Ms. VanLent currently serves as a director and a member of the audit committee of Integra Life Science Holdings, a medical technology company. Ms. VanLent received a B.A. in Physics from Mount Holyoke College.
Board Independence
Under applicable Nasdaq Stock Market, Inc. Marketplace Rules, a director will qualify as an “independent director” if, in the opinion of the board of directors of the company, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our board has determined that none of Dr. Drake, Messrs. Freiman and Henel and Ms. VanLent has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, and that each of these directors is an “independent director” as defined under the Nasdaq Stock Market, Inc. Marketplace Rules.
Director Nomination Process
The process followed by the nominating and governance committee of our board of directors to identify and evaluate director candidates includes requests to members of the board and others, including the engagement of search firms, for recommendations. The Committee meets from time to time to evaluate biographical information and background material relating to potential candidates, and selected candidates are interviewed by members of the nominating and governance committee and the board.
In considering whether to recommend any particular candidate for inclusion in the board’s slate of recommended director nominees, the nominating and governance committee applies the criteria set forth in our corporate governance guidelines. These criteria include the candidate’s integrity, business acumen, knowledge of our business and industry, age, experience, diligence, conflicts of interest and ability to act in the interests of all shareholders. The nominating and governance committee does not assign specific
6
weights to particular criteria and no particular criterion is a prerequisite for each prospective nominee. We believe that the backgrounds and qualifications of our directors, considered as a group, should provide a composite mix of experience, knowledge and abilities that will allow the board to fulfill its responsibilities.
Shareholders may recommend individuals to the nominating and governance committee for consideration as potential director candidates by submitting their names, together with appropriate biographical information and background materials, and a statement as to whether the shareholder or group of shareholders making the recommendation has beneficially owned more than 5% of our common stock for at least a year as of the date such recommendation is made, to: Nominating and Governance Committee, c/o Corporate Secretary, Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810-5120. Assuming that appropriate biographical and background material has been provided on a timely basis, the nominating and governance committee will evaluate shareholder-recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others. If our board determines to nominate a shareholder-recommended candidate and recommends his or her election, then his or her name will be included in our proxy card for the next annual meeting of shareholders.
Shareholders also have the right under our bylaws to directly nominate director candidates, without any action or recommendation on the part of the nominating and governance committee or the board, by following the procedures set forth under “Shareholder Proposals for 2007 Annual Meeting” below. Candidates nominated by shareholders in accordance with the procedures set forth in the bylaws may not be included in our proxy card for the next annual meeting.
Director Compensation
Under our director compensation program, non-employee directors receive annual fees, meeting fees and equity compensation as follows:
Annual Fees. We pay the following annual fees to our non-employee directors:
| | | | | |
| Annual retainer as a director | | $ | 20,000 | |
| Additional annual retainer for Chairman of the Board | | | 15,000 | |
| Annual retainer for audit committee chair | | | 15,000 | |
| Annual retainer for other audit committee members | | | 5,000 | |
| Annual retainer for other board committee chairs | | | 10,000 | |
| Annual retainer for other board committee members | | | 3,000 | |
We pay these annual retainers in quarterly installments on the first business day of each calendar quarter. Directors may elect to receive these fees in cash, shares of our common stock, or both. The number of shares of common stock issued in lieu of fees is determined by dividing the fees to be paid in stock by the average of the high and low trading price of our common stock on the date of grant. In 2005, we granted an aggregate of 7,929 shares of common stock related to annual fees and meeting fees, as described below, to our non-employee directors at a weighted average fair market value of $13.18 per share pursuant to directors’ elections.
Meeting Fees. We also pay to non-employee directors, in cash or shares of our common stock pursuant to directors’ elections, fees of $1,500 for each board meeting attended in person and fees of between $500 and $1,000 for each board meeting attended telephonically. The number of shares of common stock issued in lieu of fees is determined by dividing the fees to be paid in stock by the average of the high and low trading price of our common stock on the date of grant.
Equity Compensation. On the first business day of each calendar year, we issue to each non-employee director either options to purchase 12,000 shares of our common stock or a grant of 6,000 shares of restricted common stock, as elected by each director. The exercise price of these options equals the fair market value of one share of our common stock on the grant date. Options granted pursuant to this program vest on the first anniversary of the date of grant. Restricted common stock granted pursuant to
7
this program is granted without requiring payment of additional consideration by the director and vests on the first anniversary of the grant date. The vesting of options and of the restricted common stock is subject to acceleration in full upon a change in control of our company.
In addition, upon the date of the initial election of any non-employee director to our board, we grant such non-employee director 20,000 shares of restricted common stock and grant an additional 12,000 shares of restricted common stock generally every four years thereafter. These shares vest in four equal annual installments commencing upon the first anniversary of the date of grant. The vesting of the restricted common stock is subject to acceleration in full upon a change in control of our company.
Expense Reimbursement. We reimburse our non-employee directors for all reasonable expenses incurred in attending meetings of the board of directors and committees of the board.
The following table sets forth the cash and equity compensation received during 2005 by the members of our board of directors who were not our employees:
2005 BOARD OF DIRECTOR COMPENSATION
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Fees | | | | | | | | | Option | | | |
| | | | | Earned or | | | Stock | | | Stock | | | Option | | | Awards | | | |
| | | | | Paid in | | | Awards | | | Awards | | | Awards | | | Exercise | | | All Other | |
| Name | | Total ($)(1) | | | Cash ($) | | | (#)(2) | | | ($)(3) | | | (#) | | | Price ($)(4) | | | Compensation ($) | |
| | | | | | | | | | | | | | | | | | | | | | |
| Peter F. Drake(5) | | $ | 289,096 | | | $ | 7,500 | | | | 21,544 | | | $ | 281,596 | | | | — | | | | — | | | | — | |
| Paul E. Freiman | | | 62,492 | | | | 36,500 | | | | 1,976 | | | | 25,992 | | | | 12,000 | | | $ | 11.90 | | | | — | |
| Rolf H. Henel | | | 44,500 | | | | 44,500 | | | | — | | | | — | | | | 12,000 | | | | 11.90 | | | | — | |
| Robert J. Hennessey(6) | | | 83,767 | | | | 1,500 | | | | 6,836 | | | | 82,267 | | | | — | | | | — | | | | — | |
| John N. Staniforth | | | 127,247 | | | | 7,500 | | | | 1,540 | | | | 20,000 | | | | 12,000 | | | | 11.90 | | | $ | 99,747 | (7) |
| Anne M. VanLent | | | 115,255 | | | | 16,500 | | | | 8,033 | | | | 98,755 | | | | — | | | | — | | | | — | |
| |
| (1) | Includes the value of stock awards granted, but excludes the value of stock option awards granted in 2005. |
| |
| (2) | Includes restricted stock totaling 32,000 shares and unrestricted stock totaling 7,929 shares issued in connection with director compensation discussed previously. |
| |
| (3) | Values based on fair market value of our common stock on the dates of grant. |
| |
| (4) | The exercise price is equal to the average of the high and low prices of our common stock on the date of grant as reported by the NASDAQ National Market. |
| |
| (5) | Dr. Drake joined our board of directors on April 13, 2005. His total compensation includes the value of 20,000 restricted shares granted upon his initial election to our board. |
| |
| (6) | Mr. Hennessey received no compensation for his services as a director between February 15, 2005 and November 23, 2005, during which time he served as our Chief Executive Officer and President. This table excludes all compensation paid to Mr. Hennessey as our Chief Executive Officer and President, which compensation is set forth below under “Executive Compensation.” |
| |
| (7) | Includes (i) $19,747 in royalties payable to Dr. Staniforth pursuant to a royalty agreement between us and Dr. Staniforth and (ii) $80,000 paid to Dr. Staniforth pursuant to a consulting agreement between us and Dr. Staniforth, each of which is described below under “Certain Relationships and Related Transactions.” |
Committees of the Board
Our board of directors has three standing committees — audit, compensation, and nominating and governance. Each committee operates under a written charter that has been approved by the board. Current copies of each committee’s charter are posted on the corporate governance section of our website,www.penwest.com. In addition, a copy of the audit committee charter, as currently in effect, is attached to this proxy statement as Appendix A.
8
The board has determined that all of the current members of the audit, compensation, and nominating and governance committees are independent as defined under the rules of the Nasdaq Stock Market, including, in the case of all members of the audit committee, the additional independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Prior to February 15, 2005, Mr. Hennessey served on the compensation committee and the nominating and governance committee and during this time was an independent director.
Prior to April 13, 2005, we also had an executive committee of the board of directors. The executive committee exercised all powers and authority of the board with certain exceptions as provided under Washington law. The executive committee consisted of Messrs. Freiman and Hennessey and, until his resignation as our Chief Executive Officer and Chairman of the Board on February 14, 2005, Mr. Tod R. Hamachek. The executive committee did not meet during 2005. The board dissolved the executive committee on April 13, 2005.
During 2005, the board met eight times, either in person or by teleconference. In 2005, each director attended at least 75% of the aggregate of the number of board meetings and the number of meetings held by all committees on which he or she then served. Our corporate governance guidelines provide that directors are expected to attend the annual meeting of shareholders. All directors attended the 2005 annual meeting of shareholders.
Audit Committee
The audit committee’s responsibilities include:
| | |
| | • | appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm; |
| |
| | • | overseeing the work of our independent registered public accounting firm, including through the receipt and consideration of certain reports from such firm; |
| |
| | • | reviewing and discussing with management and our independent registered public accounting firm, our annual and quarterly financial statements and related disclosures; |
| |
| | • | coordinating our board of director’s oversight of our internal control over financial reporting and disclosure controls and procedures; |
| |
| | • | reviewing and approving any related party transactions; |
| |
| | • | serving as the qualified legal compliance committee, the purpose of which is to receive, review, investigate and respond to reports from attorneys reporting evidence of material violations; |
| |
| | • | reviewing our risk management policies; |
| |
| | • | establishing policies regarding hiring employees from our independent registered public accounting firm, and procedures for the receipt and retention of accounting related complaints and concerns; |
| |
| | • | meeting independently with our independent registered public accounting firm and management; and |
| |
| | • | preparing the audit committee report required by Securities and Exchange Commission rules included on page 18 of this proxy statement. |
The current members of the audit committee are Dr. Drake, Mr. Henel and Ms. VanLent. Dr. Drake was appointed to the audit committee on April 13, 2005. Until April 13, 2005, Mr. Freiman was a member of the audit committee. Our board of directors has determined that Ms. VanLent, the chair of the audit committee, is an “audit committee financial expert” as defined in Item 401(h) of Regulation S-K. The audit committee met ten times during 2005.
9
Compensation Committee
The compensation committee’s responsibilities include:
| | |
| | • | reviewing and approving all aspects of the compensation of our chief executive officer and other executive officers; |
| |
| | • | overseeing an evaluation of our senior executives; |
| |
| | • | overseeing and administering our cash and equity incentive plans; |
| |
| | • | reviewing and making recommendations to our board of directors with respect to director compensation; |
| |
| | • | periodically reviewing and making recommendations to the board relating to management succession planning; and |
| |
| | • | preparing the compensation committee report required by Securities and Exchange Commission rules included on page 14 of this proxy statement. |
The members of the compensation committee are Dr. Drake, Mr. Freiman and Mr. Henel. Mr. Henel, the chair of the compensation committee, was appointed to the compensation committee on February 15, 2005. Dr. Drake was appointed to the compensation committee on April 13, 2005. Until his appointment as our President and Chief Executive Officer on February 15, 2005, Mr. Hennessey served as the chair of the compensation committee. The compensation committee met five times during 2005.
Nominating and Governance Committee
The nominating and governance committee’s responsibilities include:
| | |
| | • | identifying individuals qualified to become members of the board of directors; |
| |
| | • | recommending to the board the persons to be nominated for election as directors and to each of the board’s committees; |
| |
| | • | developing and recommending to the board corporate governance guidelines; and |
| |
| | • | overseeing an annual self-evaluation of the board to determine if it is functioning effectively. |
The members of the nominating and governance committee are currently Mr. Freiman, the chair of the nominating and governance committee, Mr. Henel and Ms. VanLent. The nominating and governance committee met two times in 2005. Mr. Hennessey served on the nominating and governance committee until his appointment as our president and chief executive officer on February 15, 2005. Mr. Henel was appointed to the nominating and governance committee on April 13, 2005.
10
Executive Compensation
The following table sets forth the total compensation paid by us during 2005, 2004 and 2003 to each of our named executive officers:
Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Long Term | | | |
| | | | | | | | | Compensation | | | |
| | | | | | | | | Awards | | | |
| | | | | | | | | | |
| | | | | Annual Compensation | | | Securities | | | |
| | | | | | | | Underlying | | | All Other | |
| Name and Principal Position | | Year | | | Salary | | | Bonus | | | Options (#) | | | Compensation | |
| | | | | | | | | | | | | | | | |
| Jennifer L. Good | | | 2005 | | | $ | 252,692 | | | $ | 100,000 | | | | 95,000 | | | $ | 9,163 | (1) |
| | President, Chief Operating Officer | | | 2004 | | | | 234,000 | | | | 49,140 | | | | 50,000 | | | | 7,516 | (1) |
| | and Chief Financial Officer | | | 2003 | | | | 213,000 | | | | 71,888 | | | | 50,000 | | | | 7,534 | (1) |
| Thomas R. Sciascia, M.D. | | | 2005 | | | | 263,077 | | | | 69,563 | | | | 35,000 | | | | 9,450 | (1) |
| | Senior Vice President, and Chief | | | 2004 | | | | 255,000 | | | | 44,625 | | | | 30,000 | | | | 9,750 | (1) |
| | Medical Officer | | | 2003 | | | | 232,000 | | | | 54,375 | | | | 36,000 | | | | 8,617 | (1) |
| Alan F. Joslyn, Ph.D. | | | 2005 | | | | 252,500 | | | | 66,938 | | | | 35,000 | | | | 9,450 | (1) |
| | Senior Vice President, Research & | | | 2004 | | | | 121,000 | | | | 50,820 | | | | 100,000 | | | | 20,409 | (2) |
| | Development | | | 2003 | | | | — | | | | — | | | | — | | | | — | |
| Anand R. Baichwal, Ph.D. | | | 2005 | | | | 197,472 | | | | 50,925 | | | | 20,000 | | | | 29,197 | (3) |
| | Senior Vice President, Licensing | | | 2004 | | | | 182,300 | | | | 38,283 | | | | — | | | | 34,868 | (3) |
| | and Chief Scientific Officer | | | 2003 | | | | 182,300 | | | | — | | | | 20,000 | | | | 32,141 | (3) |
| Theodor Rozsa | | | 2005 | | | | 99,000 | (4) | | | 24,063 | | | | 100,000 | | | | 36,820 | (5) |
| | Senior Vice President, Business | | | 2004 | | | | — | | | | — | | | | — | | | | — | |
| | Development | | | 2003 | | | | — | | | | — | | | | — | | | | — | |
| Tod R. Hamachek | | | 2005 | | | | 78,015 | | | | — | | | | — | | | | 834,605 | (6) |
| | Former Chairman of the Board and | | | 2004 | | | | 396,000 | | | | — | | | | 60,000 | | | | 15,344 | (6) |
| | Chief Executive Officer | | | 2003 | | | | 380,000 | | | | 120,000 | | | | 90,000 | | | | 21,921 | (6) |
| Robert J. Hennessey | | | 2005 | | | | 342,700 | | | | — | | | | 125,000 | | | | 4,330 | (1) |
| | Former Chief Executive Officer and | | | 2004 | | | | — | | | | — | | | | — | | | | — | |
| | President | | | 2003 | | | | — | | | | — | | | | — | | | | — | |
| |
| (1) | Consists of matching contributions under the Penwest Pharmaceuticals Co. Savings Plan. |
| |
| (2) | Consists of |
| | |
| | • | $18,664 in relocation reimbursements for 2004; and |
| |
| | • | $1,745 in matching contributions under the Penwest Pharmaceuticals Co. Savings Plan. |
| | |
| | • | $19,747, $25,469 and $23,141, respectively, paid to Dr. Baichwal for 2005, 2004 and 2003 under a royalty agreement between Dr. Baichwal and us, which is described below under “Certain Relationships and Related Transactions;” and |
| |
| | • | $9,450, $9,399 and $9,000, respectively, in matching contributions under the Penwest Pharmaceuticals Co. Savings Plan for 2005, 2004 and 2003. |
| |
| (4) | Mr. Rosza commenced employment with us on July 7, 2005 at an annual salary of $220,000. |
| |
| (5) | Consists of relocation reimbursements. |
| |
| (6) | Consists of: |
| | |
| | • | severance pay of $594,000, payments of $100,743 under Mr. Hamachek’s supplemental executive retirement plan and payments of $139,862 under Mr. Hamachek’s deferred compensation plan for 2005; |
11
| | |
| | • | $15,344 in premiums paid on behalf of Mr. Hamachek for supplemental life and disability insurance plans for each of 2004 and 2003; and |
| |
| | • | $6,577 in matching contributions under the Penwest Pharmaceuticals Co. Savings Plan for 2003. |
Option Grants in Last Fiscal Year
The following table sets forth certain information regarding stock options we granted to our named executive officers during the year ended December 31, 2005:
Option Grants In Last Fiscal Year
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Individual Grants | | | | | |
| | | | | | |
| | | | | Percent of | | | | | Potential Realizable Value | |
| | | Number of | | | Total Options | | | | | at Assumed Annual Rates | |
| | | Securities | | | Granted to | | | | | of Stock Price Appreciation | |
| | | Underlying | | | Employees in | | | Exercise or | | | | | for Option Term(3) | |
| | | Options | | | Fiscal Year | | | Base Price | | | Expiration | | | | |
| Name | | Granted (#) | | | (%)(1) | | | ($/Sh)(2) | | | Date | | | 5% ($) | | | 10% ($) | |
| | | | | | | | | | | | | | | | | | | |
| Jennifer L. Good | | | 45,000 | (4) | | | 7.0 | % | | $ | 10.35 | | | | 2/17/2015 | | | $ | 292,908 | | | $ | 742,286 | |
| | | | 50,000 | (5) | | | 7.8 | % | | | 16.14 | | | | 11/23/2015 | | | | 507,518 | | | | 1,286,150 | |
| Thomas R. Sciascia, M.D. | | | 35,000 | (4) | | | 5.4 | % | | | 10.35 | | | | 2/17/2015 | | | | 227,817 | | | | 577.333 | |
| Alan F. Joslyn, Ph.D. | | | 35,000 | (4) | | | 5.4 | % | | | 10.35 | | | | 2/17/2015 | | | | 227,817 | | | | 577,333 | |
| Anand R. Baichwal, Ph.D. | | | 20,000 | (4) | | | 3.1 | % | | | 10.35 | | | | 2/17/2015 | | | | 130,181 | | | | 329,905 | |
| Theodor Rozsa | | | 100,000 | (4) | | | 15.5 | % | | | 12.13 | | | | 7/14/2015 | | | | 762,849 | | | | 1,933,210 | |
| Tod R. Hamachek(6) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Robert J. Hennessey | | | 100,000 | (7) | | | 15.5 | % | | | 10.10 | | | | 2/16/2015 | | | | 635,184 | | | | 1,609,680 | |
| | | | 25,000 | (8) | | | 3.9 | % | | | 15.78 | | | | 9/08/2015 | | | | 248,099 | | | | 628,731 | |
| |
| (1) | Calculated based on options to purchase an aggregate of 645,000 shares of common stock granted to employees during 2005 under our 2005 Stock Incentive Plan and our 1997 Equity Incentive Plan. |
| |
| (2) | The exercise price is equal to the average of the high and low prices of our common stock on the date of grant as reported by the Nasdaq National Market. |
| |
| (3) | Potential realizable value is based on an assumption that the market price of the stock will appreciate at the stated rate, compounded annually, from the date of grant until the end of the option term. These values are calculated based on rules promulgated by the Securities and Exchange Commission and do not reflect our estimate or projection of future stock prices. Actual gains, if any, on stock option exercises will depend on the future performance of our common stock through the date on which the stock options are exercised. |
| |
| (4) | Options vest in four equal annual installments commencing on the first anniversary of the date of grant. |
| |
| (5) | Options vest in twelve equal monthly installments commencing one month following the date of the grant. |
| |
| (6) | In connection with his resignation from our company, we accelerated the vesting of all of Mr. Hamachek’s outstanding options and extended the period during which he could exercise such stock options to the earlier of two years or until their original expiration date. On December 27, 2005, we agreed with Mr. Hamachek to change the date through which Mr. Hamachek can exercise his stock options from February 14, 2007 to December 31, 2006. |
| |
| (7) | Of the 100,000 shares issuable upon exercise of these options, options to purchase 16,667 shares were cancelled in accordance with their terms on November 23, 2005 upon the resignation of Mr. Hennessey. Options were scheduled to vest in twelve equal monthly installments commencing one month following the date of grant. |
| |
| (8) | These options were cancelled in accordance with their terms on November 23, 2005 upon the resignation of Mr. Hennessey. |
12
Aggregated Option Exercises in 2005 and Fiscal Year-End Option Values
The following table sets forth certain information concerning each exercise of stock options by our named executive officers during 2005, and the number and value of unexercised stock options to purchase shares of our common stock held by each of our named executive officers as of December 31, 2005.
Aggregated Option Exercises in 2005 and Fiscal-Year End Option Values
| | | | | | | | | | | | | | | | | |
| | | | | | | Number of Securities | | | Value of | |
| | | | | | | Underlying Unexercised | | | Exercisable/Unexercised | |
| | | Shares Acquired | | | | | Options at | | | In-the-Money Options at | |
| | | on Exercise | | | Value | | | Fiscal Year-End (#) | | | Fiscal Year-End ($(2) | |
| Name | | (#) | | | Realized ($)(1) | | | Exercisable/Unexercisable | | | Exercisable/Unexercisable | |
| | | | | | | | | | | | | |
| Jennifer L. Good | | | 39,664 | | | $ | 387,043 | | | | 229,666/ | 161,334 | | $ | 2,097,409/ | $928,751 |
| Thomas R. Sciascia, M.D. | | | — | | | | — | | | | 119,250/ | 81,750 | | | 692,985/ | 567,085 |
| Alan F. Joslyn, Ph.D. | | | 8,000 | | | | 56,508 | | | | 17,000/ | 110,000 | | | 143,820/ | 955,450 |
| Theodor Rozsa | | | — | | | | — | | | | —/ | 100,000 | | | —/ | 739,000 |
| Anand R. Baichwal, Ph.D. | | | 8,363 | | | | 44,366 | | | | 193,234/ | 35,000 | | | 2,058,988/ | 281,550 |
| Tod R. Hamachek | | | — | | | | — | | | | 820,501/ | — | | | 9,576,947/ | — |
| Robert J. Hennessey | | | — | | | | — | | | | 146,900/ | — | | | 1,212,588/ | — |
| |
| (1) | Value realized on exercised options is calculated by subtracting the exercise price of such options from the fair market value of our common stock as of the date of exercise. |
| |
| (2) | Value of unexercised options at fiscal year-end is calculated by subtracting the exercise price of such options from the fair market value of our common stock as of December 31, 2005, which was $19.52 per share. |
Employment and Compensation Arrangements
Employment and Termination of Employment Arrangements
On February 14, 2005, we entered into a severance and settlement agreement and release with Tod R. Hamachek, our former Chairman and Chief Executive Officer, in connection with Mr. Hamachek’s resignation. Under this agreement, we agreed to pay Mr. Hamachek severance pay equal to 18 months of base salary, or $594,000, payable in eighteen monthly installments commencing in February 2005, and all premium costs relating to medical insurance continuation coverage for eighteen months. We settled our severance pay obligations to Mr. Hamachek with a lump sum payment made in December 2005. Under the severance and settlement agreement and release, we also agreed to accelerate in full the vesting of all stock options held by Mr. Hamachek and to extend the period during which he could exercise such stock options for up to two years. On December 27, 2005, we agreed with Mr. Hamachek to amend the severance and settlement agreement and release to change the date through which Mr. Hamachek can exercise his stock options from February 14, 2007 to December 31, 2006. Mr. Hamachek is also entitled to benefits under his supplemental executive retirement plan and deferred compensation plan.
Executive Retention Agreements
On December 6, 2005, we entered into executive retention agreements with each of our executive officers, Ms. Good, Drs. Joslyn, Baichwal and Sciascia and Mr. Rozsa. The retention agreements provide that if within twelve months following a change in control of our company, the executive’s employment is terminated by us other than for cause, death or disability or by the executive for good reason, as such terms are defined in the retention agreements:
| | |
| | • | we will continue to pay to the executive his or her base salary for a period, which we refer to as the payment period, that is equal in length to twelve months plus two weeks for each full year during which the executive was employed by us or our predecessors; |
13
| | |
| | • | we will, during the payment period, continue to provide benefits to the executive and his or her family at least equal to those that would have been provided had the executive’s employment not been terminated; provided that our obligation to provide these benefits will terminate when and if the executive subsequently receives the same type of benefits from a new employer; |
| |
| | • | on or before January 15 of the calendar year following the calendar year during which the executive’s employment is terminated, we will make an additional cash payment to the executive equal to the executive’s target bonus for the calendar year in which the change in control occurs, as established in writing by our board of directors; and |
| |
| | • | the vesting of all stock options and restricted stock held by the executive will be accelerated in full, to the extent not already vested, and all shares of stock underlying stock options and all shares of restricted stock will be free of any right of repurchase by us. |
The retention agreements terminate if a change in control of our company does not occur prior to December 31, 2008.
Report of the Compensation Committee on Executive Compensation
We maintain the philosophy that compensation of our executive officers should be directly and materially linked to the long-term results our shareholders receive, including a balanced combination of targets requiring the achievement of short-term operating goals and longer-term strategic objectives.
The executive compensation program consists of base salary, an incentive compensation program based on predetermined objectives and stock–based incentive programs.
Base Salary. The compensation committee uses outside consultants and information contained in third party surveys, such as the Radford Biotechnology Compensation Survey, to identify competitive salary grades and ranges. The compensation committee directs the outside consultants to consider similar sized companies (based on market capitalization, number of employees and revenue size), geographic factors, similar market-related companies and growth profiles of other companies. These competitive standards are reviewed periodically and are targeted towards the 50th percentile of the companies surveyed. In addition, an executive officer’s performance and potential, as well as changes in duties and responsibilities, are factors that may be considered in adjusting base salaries.
Incentive Compensation. Our incentive compensation program provides for an annual cash payout dependent on achieving predetermined goals and objectives. The compensation committee strongly believes that a balanced combination of targets requiring the achievement of short-term operating goals and longer-term strategic objectives translates directly into increasing the long-term value of our common stock. Individual incentive compensation target awards are determined by salary grade and are subject to an adjustment based on individual performance. The highest individual target payout is 30% of an individual’s base salary, and the lowest individual target payout is 25%. Payouts can exceed or be less than targets based on quantitative and qualitative target achievements. For 2005, other than for Ms. Good, executive payouts were paid based on the achievement of 87.5% of our predetermined objectives, which primarily related to advancing our product pipeline. Ms. Good’s payout exceeded the payout of 87.5% due to her individual performance during the year, and her increased duties within the Company.
Stock Based Incentive Programs. The compensation committee strongly encourages all of our executive officers to build an ownership position, over time, in our common stock. All stock options granted to executive officers have been granted at market prices. Options under the stock-based incentive programs offered by us consist of nonstatutory stock options that vest over four years and are granted at levels deemed competitive in the marketplace. In granting stock options, the compensation committee relies on outside consultants and surveys, such as the Radford Survey, to determine competitive levels and considers an individual’s performance for the year. The committee also considers the impact on our financial results under FASB Statement No. 123(R), “Share-Based Payment” of awarding stock options.
14
Chief Executive Officer Compensation. As discussed above, our executive cash compensation program includes base salary, performance-based incentive compensation and stock-based incentive programs. Ms. Good participates in the same incentive compensation programs applicable to the other executive officers. The compensation committee’s objective is to correlate her remuneration with our company’s performance and the achievement of predetermined goals.
In connection with her appointment as President and Chief Operating Officer on November 23, 2005, we increased Ms. Good’s annual base salary to $325,000 and granted to Ms. Good non-statutory stock options to purchase 50,000 shares of common stock under our 2005 Stock Incentive Plan at an exercise price equal to $16.14 per share, the fair market value of the common stock on the date of grant. In determining Ms. Good’s salary, the compensation committee considered the salaries paid by similar sized companies as well as Ms. Good’s experience and her background and knowledge of our Company.
In connection with his appointment as President and Chief Executive Officer on February 15, 2005, we agreed to pay Mr. Hennessey an annual base salary of $400,000 and granted Mr. Hennessey nonstatutory stock options to purchase 100,000 shares of our common stock under our 1997 Equity Incentive Plan at an exercise price equal to $10.10 per share, the fair market value of our common stock on the date of grant. In setting Mr. Hennessey’s salary, the compensation committee considered the salaries paid by similar sized companies and Mr. Hennessey’s background and knowledge of our company and our industry.
Compliance with Internal Revenue Code Section 162(m). Section 162(m) of the Internal Revenue Code of 1986, as amended, generally disallows a tax deduction to a public company for compensation over $1 million paid to its chief executive officer and its four other most highly compensated executive officers. However, qualifying performance-based compensation will not be subject to the deduction limit if certain requirements are met. The compensation committee reviews the potential effect of Section 162(m) periodically and generally seeks to structure the long-term incentive compensation granted to our executive officers in a manner that is intended to avoid disallowance of deductions under Section 162(m). Nevertheless, the compensation committee reserves the right to use its judgment to authorize compensation payments that may be subject to the limit when the compensation committee believes that such payments are appropriate, and in the best interests of us and our shareholders, after taking into consideration changing business conditions and the performance of our employees.
The members of the compensation committee are Dr. Drake and Messrs. Freiman and Henel. Mr. Henel was appointed to the compensation committee as its chair on February 15, 2005. Dr. Drake was appointed to the compensation committee on April 13, 2005. Until his appointment as our President and Chief Executive Officer on February 15, 2005, Mr. Hennessey served as the chair of the compensation committee.
| |
| | By the Compensation Committee of |
| | the Board of Directors of |
| | Penwest Pharmaceuticals Co. |
| |
| | Rolf H. Henel,Chair |
| | Peter F. Drake |
| | Paul E. Freiman |
Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer, and principal accounting officer or controller. The Code of Business Conduct and Ethics is posted on our website, www.penwest.com, and is available without charge upon request to Corporate Secretary, Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810, telephone (877) 736-9378.
15
Information regarding any amendments to, or waivers from, the Code of Business Conduct and Ethics will be posted on our website, www.penwest.com.
Compensation Committee Interlocks and Insider Participation
Since January 1, 2005, the members of the compensation committee of our board of directors have been Mr. Freiman, Mr. Hennessey (until February 15, 2005), Mr. Henel (since February 15, 2005) and Dr. Drake (since April 13, 2005). None of our executive officers served as a director or member of the compensation committee or other committee serving an equivalent function of any other entity, one of whose executive officers served as a director of or member of our compensation committee.
Certain Relationships and Related Transactions
Since January 1, 2005, we have not engaged in any transactions with any of our directors or officers or any individual or entity holding more than 5% of our common stock, or any of their or our affiliates, except as described below.
Under a Recognition and Incentive Agreement, as amended, with Dr. Baichwal, which we refer to as the Baichwal agreement, we are obligated to pay to Dr. Baichwal on an annual basis in arrears (i) one-half of one percent of our “net sales” (as defined in the Baichwal agreement) of “TIMERx material” (as defined in the Baichwal agreement) to third parties, (ii) one-half of one percent of royalties received by us under licenses, collaborations or other exploitation agreements with third parties with respect to the sale, license, use or exploitation by such third parties of products based on or incorporating the TIMERx material, and (iii) one-half of one percent of payments made in lieu of such net sales or royalties and received by us. Such payments cease in the event that Dr. Baichwal’s employment with us is terminated for cause. The Baichwal agreement also contains non-competition and non-solicitation provisions that expire two years after the termination of Dr. Baichwal’s employment with us. Dr. Baichwal is due $19,747 under the Baichwal agreement in connection with amounts earned for 2005, which is payable within 120 days after the end of the year.
Under a Royalty Agreement with Dr. Staniforth, which we refer to as the Staniforth agreement, we are obligated to pay Dr. Staniforth on an annual basis in arrears one-half of one percent of our “net sales” (as defined in the Staniforth agreement) of “TIMERx material” (as defined in the Staniforth agreement) related to the products covered by the TIMERx patents. Dr. Staniforth is due $19,747 in royalties for net sales in 2005 related to the products covered by the TIMERx patents, which is payable within 120 days after the end of the year.
We also are a party to an annual consulting agreement with Dr. Staniforth, pursuant to which we pay Dr. Staniforth $80,000 per year, payable in quarterly payments. The consulting agreement is automatically renewed each year unless canceled by either Dr. Staniforth or us upon thirty days prior notice. Any invention that results from this consulting agreement is legally owned by us.
Section 16(a) Beneficial Ownership Reporting Compliance
The federal securities laws require our directors and executive officers and persons who own more than 10% of our common stock to file with the Securities and Exchange Commission initial reports of ownership and reports of changes in ownership of any of our securities.
To our knowledge, based solely on review of the copies of such reports furnished to us and written representations that no other reports were required during 2005, all of our directors, executive officers and greater-than-10% beneficial owners made all required filings on a timely basis during 2005, except for Dr. Baichwal, who made one late Form 4 filing for a transaction that occurred in March 2005, and Dr. Joslyn, who made one late Form 4 filing for a transaction that occurred in December 2005.
16
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides information, as of December 31, 2005, about our common stock that may be issued upon exercise of options, warrants and rights under all of our equity compensation plans, which consist of our 2005 Stock Incentive Plan, our 1997 Equity Incentive Plan, our 1998 Spin-Off Plan, our 1997 Employee Stock Purchase Plan and a nonstatutory stock option agreement between us and Dr. Joslyn.
| | | | | | | | | | | | | |
| | | (a) | | | (b) | | | (c) | |
| | | | | | | Number of securities | |
| | | | | | | remaining available for | |
| | | | | | | future issuance under | |
| | | Number of securities to | | | Weighted-average | | | equity compensation | |
| | | be issued upon exercise | | | exercise price of | | | plans (excluding | |
| | | of outstanding options, | | | outstanding options, | | | securities reflected in | |
| Plan Category | | warrants and rights | | | warrants and rights | | | column (a)) | |
| | | | | | | | | | |
| Equity compensation plans approved by shareholders | | | 2,923,563 | | | $ | 10.87 | | | | 1,413,804 | |
| Equity compensation plans not approved by shareholders | | | 92,000 | | | | 11.06 | | | | 0 | |
| | | | | | | | | | |
| Total | | | 3,015,563 | | | $ | 10.88 | | | | 1,413,804 | |
Summary of Equity Compensation Plan not Approved by Shareholders
On June 21, 2004, we granted to Dr. Joslyn nonstatutory stock options outside of any equity compensation plan approved by our shareholders, pursuant to a Nonstatutory Stock Option Agreement providing for the purchase of 100,000 shares of our common stock at an exercise price of $11.06 per share. This option vests with respect to 25,000 shares of common stock at the end of each successive one year period following the date of grant until it is fully vested on June 21, 2008, subject to acceleration upon the occurrence of a change in control of our company.
17
Report of the Audit Committee of the Board of Directors
The audit committee has reviewed our audited financial statements for the fiscal year ended December 31, 2005 and has discussed these financial statements with our management and our independent registered public accounting firm.
The audit committee has also received from, and discussed with, our independent registered public accounting firm various communications that our independent registered public accounting firm is required to provide to the audit committee, including the matters required to be discussed by Statement on Auditing Standards 61 (Communication with Audit Committees).
Our independent registered public accounting firm also provided the audit committee with the written disclosures and the letter required by Independence Standards Board Standard No. 1 (Independence Discussions with Audit Committees). The audit committee has discussed with the independent registered public accounting firm their independence from us.
Based on its discussions with management and the independent registered public accounting firm, and its review of the representations and information provided by management and the independent registered public accounting firm, the audit committee recommended to our board of directors that the audited financial statements be included in our annual report on Form 10-K for the year ended December 31, 2005.
| |
| | By the Audit Committee of the Board of Directors |
| | of Penwest Pharmaceuticals Co. |
| |
| | Anne M. VanLent,Chair |
| | Rolf H. Henel |
| | Paul E. Freiman |
18
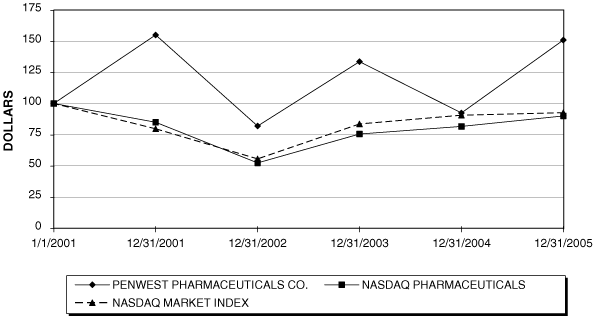
Stock Performance Graph
The following graph compares our cumulative total shareholder return on our common stock from January 1, 2001 through December 31, 2005 with the cumulative total return on (a) the Nasdaq Market Index, U.S. companies, or Nasdaq U.S., and (b) the Nasdaq Pharmaceutical Index, or Nasdaq-Pharmaceutical. The graph assumes that $100 was invested on January 1, 2001 in our common stock and in the stated indices. The comparison assumes that all dividends are reinvested.
COMPARE 5-YEAR CUMULATIVE TOTAL RETURN
AMONG PENWEST PHARMACEUTICALS CO.,
NASDAQ MARKET INDEX AND NASDAQ PHARMACEUTICAL INDEX
ASSUMES $100 INVESTED ON JAN. 1, 2001
ASSUMES DIVIDEND REINVESTED
FISCAL YEAR ENDING DEC. 31, 2005
19
PROPOSAL 2 — RATIFICATION OF THE APPOINTMENT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The audit committee has appointed the firm of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2006. Ernst & Young has been our independent registered public accounting firm since our inception. Although shareholder approval of the appointment of Ernst & Young is not required by law, our board of directors believes that it is advisable to give shareholders an opportunity to ratify this appointment. If this proposal is not approved at the annual meeting, the audit committee will reconsider this appointment.Our board of directors recommends a vote in favor of the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2006.
Representatives of Ernst & Young are expected to be present at the annual meeting. They will have the opportunity to make a statement if they desire to do so and will also be available to respond to appropriate questions from shareholders.
Independent Auditor Fees and Other Matters
The following table summarizes the fees of Ernst & Young LLP, our independent registered public accounting firm, billed to us for each of the last two fiscal years.
| | | | | | | | | | |
| Fee Category | | Fiscal 2005 | | | Fiscal 2004 | |
| | | | | | | |
| Audit Fees(1) | | $ | 429,000 | | | $ | 476,000 | |
| Audit-Related Fees(2) | | | 37,300 | | | | 26,000 | |
| Tax Fees(3) | | | 40,000 | | | | 74,000 | |
| All Other Fees(4) | | | 1,500 | | | | 1,500 | |
| | | | | | | |
| | Total Fees | | $ | 507,800 | | | $ | 577,500 | |
| | | | | | | |
| | |
| | (1) | Audit Fees — Audit fees consist of fees for the audit of our financial statements, the audit of our internal control over financial reporting, the review of the interim financial statements included in our quarterly reports on Form 10-Q, and other professional services provided in connection with statutory and regulatory filings or engagements. |
| |
| | (2) | Audit-Related Fees — Audit-related fees consist of fees for assurance and related services that are reasonably related to the performance of the audit and the review of our financial statements and that are not reported under “Audit Fees.” In 2005 and 2004, these fees principally included fees for audits of our retirement plan. |
| |
| | (3) | Tax Fees — Tax fees consist of fees for tax compliance, tax advice and tax planning services. Tax compliance services, which relate to preparation of original and amended tax returns, claims for refunds and tax payment-planning services, accounted for $40,000 of the total tax fees billed in 2005 and $43,000 of the total tax fees billed in 2004. Tax advice and tax planning services primarily relate to employee benefit plans. |
| |
| | (4) | All Other Fees — In 2005 and 2004, these fees related to a subscription to the Ernst & Young Global Accounting and Auditing Information Tool. |
Pre-Approval Policy and Procedures
The audit committee of our board of directors has adopted policies and procedures relating to the approval of all audit and non-audit services that are to be performed by our independent registered public accounting firm. These policies generally provide that we will not engage our independent registered public accounting firm to render audit or non-audit services unless the service is specifically approved in advance by the audit committee or the engagement is entered into pursuant to one of the pre-approval procedures described below.
20
From time to time, the audit committee may pre-approve specified types of services that are expected to be provided to us by our independent registered public accounting firm during the next twelve months. Any such pre-approval is detailed as to the particular service or type of services to be provided and is also generally subject to a maximum dollar amount.
The audit committee has also delegated to the chair of the audit committee the authority to approve any audit or non-audit services to be provided to us by our independent registered public accounting firm. Any approval of services by the chair of the audit committee pursuant to this delegated authority is reported on at the next meeting of the audit committee.
SHAREHOLDER PROPOSALS FOR 2007 ANNUAL MEETING
Under Rule 14a-8(e)(2) of the Securities and Exchange Commission, shareholder proposals intended for inclusion in next year’s proxy statement must be directed to Corporate Secretary, Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810-5120, and must be received by January 3, 2007.
If a shareholder wishes to present a proposal, other than the nomination of a director, before the 2007 annual meeting of shareholders but has not complied with the requirements for inclusion of such proposal in our proxy materials pursuant to Rule 14a-8 under the Exchange Act, such shareholder must give written notice of such proposal to our Corporate Secretary at the address above not less than 60 days nor more than 90 days prior to the 2007 annual meeting of shareholders. Notwithstanding the foregoing, if we provide less than 70 days notice or prior public disclosure of the date of the meeting to our shareholders, notice by the shareholder must be received by our Corporate Secretary no later than the close of business on the tenth day following the date on which the notice of the meeting was mailed or such public disclosure was made, whichever occurs first. If a shareholder makes timely notification, the holders of the proxies may still exercise discretionary voting authority under circumstances consistent with the Securities and Exchange Commission’s proxy rules. If a shareholder fails to make timely notification but the board of directors determines, at its discretion, to present such proposal at the meeting, the holders of the proxies that management solicits for the 2007 annual meeting of shareholders will have discretionary authority to vote on the proposal. In the case of director nominations by a shareholder, notice from the shareholder of the intent to make a nomination must be received by our Corporate Secretary not later than 120 days in advance of the 2007 annual meeting of shareholders.
COMMUNICATING WITH THE DIRECTORS
Our board of directors will give appropriate attention to written communications that are submitted by shareholders, and will respond if and as appropriate. The Chairman is primarily responsible for monitoring communications from shareholders and for providing copies or summaries to the other directors as he considers appropriate.
Under procedures approved by the board, including a majority of the independent directors, communications are forwarded to all directors if they relate to important substantive matters and include suggestions or comments that the Chairman considers to be important for the directors to know. In general, communications relating to corporate governance and corporate strategy are more likely to be forwarded than communications relating to ordinary business affairs, personal grievances and matters as to which we tend to receive repetitive or duplicative communications.
Shareholders who wish to send communications on any topic to the board should address such communications to Board of Directors, c/o Corporate Secretary, Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810-5120.
21
SOLICITATION OF PROXIES
The proxy card accompanying this proxy statement is solicited by our board of directors. Proxies may be solicited by our officers, directors and other employees, none of whom will receive any additional compensation for their services. We reserve the right to retain other outside agencies for the purpose of soliciting proxies. Solicitations of proxies may be made personally, or by mail, telephone, telegraph, facsimile or messenger. We will pay persons holding shares of common stock in their names or in the names of nominees, but not owning such shares beneficially, such as brokerage houses, banks and other fiduciaries, for the expense of forwarding soliciting materials to their principals. All costs of soliciting proxies will be paid by us.
HOUSEHOLDING OF ANNUAL MEETING MATERIALS
Some banks, brokers and other nominee record holders may be participating in the practice of “householding” proxy statements and annual reports. This means that only one copy of our proxy statement or annual report may have been sent to multiple shareholders in your household. We will promptly deliver a separate copy of either document to you if you call or write us at the following address or phone number: Penwest Pharmaceuticals Co., 39 Old Ridgebury Road, Suite 11, Danbury, Connecticut 06810-5120, Attention: Corporate Secretary, (877) 736-9378. If you would like to receive separate copies of our annual report and proxy statement in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker, or other nominee record holder, or you may contact us at the above address and phone number.
OTHER MATTERS
We are not aware of any other business to be acted upon at the annual meeting. If other business requiring a vote of our shareholders should come before the annual meeting, the holders of the proxies will vote in accordance with their best judgment.
22
Appendix A
PENWEST PHARMACEUTICALS CO.
AUDIT COMMITTEE CHARTER
Adopted April 13, 2005
The purpose of the Audit Committee is to assist the Board of Directors’ oversight of the Company’s accounting and financial reporting processes and the audits of the Company’s financial statements.
| |
| B. | Structure and Membership |
1. Number. The Audit Committee shall consist of at least three members of the Board of Directors.
2. Independence. Except as otherwise permitted by the applicable NASDAQ rules, each member of the Audit Committee shall be independent as defined by NASDAQ rules, meet the criteria for independence set forth in Rule 10A-3(b)(1) under the Exchange Act (subject to the exemptions provided in Rule 10A-3(c)), and not have participated in the preparation of the financial statements of the Company or any current subsidiary of the Company at any time during the past three years. The Audit Committee will monitor its members throughout the year to confirm that they remain independent as required by the NASDAQ rules. The Audit Committee will also consider whether any members of the Audit Committee have relationships with the Company that may create the appearance of a lack of independence, even though such relationships do not technically disqualify the person from being independent.
3. Financial Literacy. Each member of the Audit Committee must be able to read and understand fundamental financial statements, including the Company’s balance sheet, income statement, and cash flow statement, at the time of his or her appointment to the Audit Committee. In addition, at least one member must have past employment experience in finance or accounting, requisite professional certification in accounting, or any other comparable experience or background which results in the individual’s financial sophistication, including being or having been a chief executive officer, chief financial officer or other senior officer with financial oversight responsibilities. Unless otherwise determined by the Board of Directors (in which case disclosure of such determination shall be made in the Company’s annual report filed with the SEC), at least one member of the Audit Committee shall be an “audit committee financial expert” (as defined by applicable SEC rules).
4. Chair. Unless the Board of Directors elects a Chair of the Audit Committee, the Audit Committee shall elect a Chair by majority vote.
5. Compensation. The compensation of Audit Committee members shall be as determined by the Board of Directors. No member of the Audit Committee may receive, directly or indirectly, any consulting, advisory or other compensatory fee from the Company or any of its subsidiaries, other than fees paid in his or her capacity as a member of the Board of Directors or a committee of the Board.
6. Selection and Removal. Members of the Audit Committee shall be appointed by the Board of Directors, upon the recommendation of the Nominating Committee. The Board of Directors may remove members of the Audit Committee from such committee, with or without cause.
| |
| C. | Authority and Responsibilities |
The Audit Committee shall discharge its responsibilities, and shall assess the information provided by the Company’s management and the independent auditor, in accordance with its business judgment. Management is responsible for the preparation, presentation, and integrity of the Company’s financial statements and for the appropriateness of the accounting principles and reporting policies that are used by
A-1
the Company. The independent auditors are responsible for auditing the Company’s financial statements and for reviewing the Company’s unaudited interim financial statements. The authority and responsibilities set forth in this Charter do not reflect or create any duty or obligation of the Audit Committee to plan or conduct any audit, to determine or certify that the Company’s financial statements are complete, accurate, fairly presented, or in accordance with generally accepted accounting principles or applicable law, or to guarantee the independent auditor’s report.
| |
| Oversight of Independent Auditors |
1. Selection. The Audit Committee shall be solely and directly responsible for appointing, evaluating, retaining and, when necessary, terminating the engagement of the independent auditor. The Audit Committee may, in its discretion, seek shareholder ratification of the independent auditor it appoints.
2. Independence. The Audit Committee shall take, or recommend that the full Board of Directors take, appropriate action to oversee the independence of the independent auditor. In connection with this responsibility, the Audit Committee shall obtain and review a formal written statement from the independent auditor describing all relationships between the auditor and the Company, including the disclosures required by Independence Standards Board Standard No. 1. The Audit Committee shall actively engage in dialogue with the auditor concerning any disclosed relationships or services that might impact the objectivity and independence of the auditor. The Audit Committee shall also confirm (a) the regular rotation of the lead audit partner and reviewing partner as required by Section 203 of the Sarbanes-Oxley Act and (b) that the Chief Executive Officer, the Chief Financial Officer and the Controller were not employed by the independent auditor, or if employed, did not participate in any capacity in the audit of the Company, in each case, during the one-audit-year period preceding the date of initiation of the audit, as required by Section 206 of the Sarbanes-Oxley Act.
3. Compensation. The Audit Committee shall have sole and direct responsibility for setting the compensation of the independent auditor. The Audit Committee is empowered, without further action by the Board of Directors, to cause the Company to pay the compensation of the independent auditor established by the Audit Committee.
4. Preapproval of Services. The Audit Committee shall preapprove all audit services to be provided to the Company, whether provided by the principal auditor or other firms, and all other services (review, attest and non-audit) to be provided to the Company by the independent auditor; provided, however, that de minimis non-audit services may instead be approved in accordance with applicable SEC rules.
5. Oversight. The independent auditor shall report directly to the Audit Committee, and the Audit Committee shall have sole and direct responsibility for overseeing the work of the independent auditor, including resolution of disagreements between Company management and the independent auditor regarding financial reporting. In connection with its oversight role, the Audit Committee shall, from time to time as appropriate, receive and consider the reports required to be made by the independent auditor regarding:
| |
| | critical accounting policies and practices; |
| |
| | alternative treatments within generally accepted accounting principles for policies and practices related to material items that have been discussed with Company management, including ramifications of the use of such alternative disclosures and treatments, and the treatment preferred by the independent auditor; and |
| |
| | other material written communications between the independent auditor and Company management. |
The Audit Committee shall also review with the independent auditor the following:
| |
| | any audit problems or difficulties the independent auditor encountered in the course of the audit work and management’s response, including any restrictions on the scope of the independent auditor’s activities or on access to requested information and any significant disagreements with management; |
A-2
| |
| | major issues as to the adequacy of the Company’s internal controls and any special audit steps adopted in light of material control deficiencies; any accounting adjustments that were noted or proposed by the independent auditor but were “passed” (as immaterial or otherwise); |
| |
| | any “management” or “internal control” letter issued, proposed to be issued, by the independent auditor to the Company; |
| |
| | accounting for unusual transactions; and |
| |
| | adjustments arising from audits that could have a significant impact on the Company’s financial reporting process. |
6. Significant Issues; Disagreements. The Audit Committee shall instruct the independent auditor and the senior financial management (including the Chief Financial Officer and Controller) that they should promptly contact the Audit Committee or its Chair about any significant issue or disagreement concerning the Company’s accounting practices or financial statements that is not resolved to their satisfaction. If the Chair is contacted about such an issue, he or she should confer with the independent auditor about the issue and decide whether it is necessary to contact the other members of the Audit Committee prior to the next scheduled meeting of the Committee.
7. Government Correspondence; Published Reports. The Audit Committee shall discuss with management and the independent auditor (a) any recent SEC comments on the Company’s SEC reports that relate to the Company’s financial statements or the Company’s disclosure of financial information, including any resolved or future-compliance comments, and (b) any correspondence with regulators or governmental agencies or published reports that raise material issues regarding the Company’s financial statements or accounting policies.
| |
| Audited Financial Statements |
8. Pre-Audit Meeting. Prior to any audit of the Company’s financial statements, the Audit Committee shall meet with senior financial management and the independent auditor prior to each audit to discuss the planning and staffing of the audit and any issues anticipated to arise from the audit.
9. Review and Discussion. The Audit Committee shall review and discuss with the Company’s management and independent auditor the Company’s audited financial statements, including the matters about which Statement on Auditing Standards No. 61 (Codification of Statements on Auditing Standards, AU §380) requires discussion.
10. Recommendation to Board Regarding Financial Statements. The Audit Committee shall consider whether it will recommend to the Board of Directors that the Company’s audited financial statements be included in the Company’s Annual Report on Form 10-K.
11. Audit Committee Report. The Audit Committee shall prepare an annual committee report for inclusion where necessary in the proxy statement of the Company relating to its annual meeting of security holders.
| |
| Review of Other Financial Disclosures |
12. Independent Auditor Review of Interim Financial Statements. The Audit Committee shall direct the independent auditor to use its best efforts to perform all reviews of interim financial information prior to disclosure by the Company of such information and to discuss promptly with the Audit Committee and the Chief Financial Officer any matters identified in connection with the auditor’s review of interim financial information which are required to be discussed by applicable auditing standards. The Audit Committee shall direct management to advise the Audit Committee in the event that the Company proposes to disclose interim financial information prior to completion of the independent auditor’s review of interim financial information.
A-3
13. Other Financial Information. The Audit Committee shall discuss generally the financial information to be disclosed in the Company’s earnings press releases and in other public disclosures
14. Quarterly Reports. Members of the Audit Committee should review the Company’s unaudited financial statements to be included in the Company’s Quarterly Reports on Form 10-Q prior to filing, including in particular the financial statements and other financial disclosure contained therein.
15. Oversight. The Audit Committee shall coordinate the Board of Directors’ oversight of the Company’s internal control over financial reporting, disclosure controls and procedures and code of conduct. The Audit Committee shall receive and review the reports of the Chief Executive Officer and Chief Financial Officer required by Rule 13a-14 of the Exchange Act.
16. Procedures for Complaints. The Audit Committee shall establish procedures for (i) the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters; and (ii) the confidential, anonymous submission by employees of the Company of concerns regarding questionable accounting or auditing matters.
17. Related-Party Transactions. The Audit Committee shall review all related party transactions on an ongoing basis, and all such transactions must be approved by the Audit Committee.
18. Insider Trading Policy. The Audit Committee is responsible for oversight and monitoring compliance with the Company’s Insider Trading Policy. If the Committee suspects any potential violations, they will be responsible for overseeing the investigation. The Committee will periodically review the Company’s Insider Trading Policy to determine if any changes should be recommended for adoption by the Board of Directors.
19. Investment Policy. The Audit Committee is responsible for an annual review of the Company’s Investment Policy and current treasury practices of the Company.
20. Risk Management. The Audit Committee will be responsible for reviewing the business insurance coverage of the Company on a periodic basis.
21. Additional Powers. The Audit Committee shall have such other duties as may be delegated from time to time by the Board of Directors.
| |
| D. | Procedures and Administration |
1. Meetings. The Audit Committee shall meet as often as it deems necessary in order to perform its responsibilities. The Audit Committee may also act by unanimous written consent in lieu of a meeting. The Audit Committee shall periodically (i) meet separately with the independent auditor and (ii) Company’s management. The Audit Committee shall keep such records of its meetings as it shall deem appropriate. These records shall include minutes of the Audit Committee’s meetings that accurately describe the issues considered by the Committee, the process the Committee used to discuss and evaluate such issues and the Committee’s final determination of how to proceed. The minutes should document the Committee’s consideration of issues in a manner that demonstrates that the Committee acted with due care.
2. Subcommittees. The Audit Committee may form and delegate authority to one or more subcommittees (including a subcommittee consisting of a single member), as it deems appropriate from time to time under the circumstances. Any decision of a subcommittee to preapprove audit, review, attest or non-audit services shall be presented to the full Audit Committee at its next scheduled meeting.
3. Reports to Board. The Audit Committee shall report regularly to the Board of Directors.
4. Charter. At least annually, the Audit Committee shall review and reassess the adequacy of this Charter and recommend any proposed changes to the Board of Directors for approval.
A-4
5. Independent Advisors. The Audit Committee is authorized, without further action by the Board of Directors, to engage such independent legal, accounting and other advisors as it deems necessary or appropriate to carry out its responsibilities. Such independent advisors may be the regular advisors to the Company. The Audit Committee is empowered, without further action by the Board of Directors, to cause the Company to pay the compensation of such advisors as established by the Audit Committee.
6. Investigations. The Audit Committee shall have the authority to conduct or authorize investigations into any matters within the scope of its responsibilities as it shall deem appropriate, including the authority to request any officer, employee or advisor of the Company to meet with the Audit Committee or any advisors engaged by the Audit Committee.
7. Funding. The Audit Committee is empowered, without further action by the Board of Directors, to cause the Company to pay the ordinary administrative expenses of the Audit Committee that are necessary or appropriate in carrying out its duties.
8. Self-Evaluation. The Audit Committee shall annually evaluate its own performance.
E. Qualified Legal Compliance Committee
The Audit Committee shall serve as the “Qualified Legal Compliance Committee” of the Company in accordance with the rules and regulations promulgated under Section 307 of the Sarbanes Oxley Act of 2002 and adopted as Part 205 of the Securities and Exchange Commission’s rules and regulations (the “Rules”). The purpose of the QLCC is to receive, review, investigate and respond to reports from attorneys (both in-house and law firm counsel) reporting evidence of a material violation in accordance with the Rules.
| |
| Authority and Responsibilities |
In connection with its role as the Company’s Qualified Legal Compliance Committee, the Committee shall have the following authority and responsibilities.
9. Inform the Company’s chief executive officer of any report of “evidence of a material violation” as defined by the Rules.
10. Determine whether an investigation is necessary regarding any report of evidence of a material violation by the Company, its officers, directors, employees or agents and, if it determines an investigation is necessary or appropriate, to:
| |
| | (A) Notify the full board of directors of the Company; |
| |
| | (B) Initiate an investigation, to be conducted by outside attorneys; and |
| |
| | (C) Retain such additional expert personnel as the Audit Committee deems necessary. |
11. At the conclusion of any such investigation:
| |
| | (A) Recommend, by majority vote, that the Company implement an appropriate response to evidence of a material violation; and |
| |
| | (B) Inform the chief executive officer and the board of directors of the results of any such investigation under this section and the appropriate remedial measures to be adopted. |
12. Take all other appropriate action, including notifying the Securities and Exchange Commission in the event that the Company fails in any material respect to implement an appropriate response that the Audit Committee has recommended the Company to take.
13. Establish written procedures for the confidential receipt, retention and consideration of reports regarding potential material violations.
A-5
PROXY
For the Annual Meeting of the Shareholders of
Penwest Pharmaceuticals Co.
THIS PROXY IS SOLICITED ON BEHALF OF THE
BOARD OF DIRECTORS OF THE COMPANY
The undersigned, revoking all prior proxies, hereby appoint(s) Jennifer L. Good and Frank P. Muscolo, and each of them, as proxies of the undersigned (with full power of substitution in them and each of them) to attend and represent the undersigned at the Annual Meeting of Shareholders of Penwest Pharmaceuticals Co. (the “Company”) to be held at 39 Old Ridgebury Road, Danbury, Connecticut 06810 on June 7, 2006, at 11:00 a.m., and any adjourned sessions thereof, and there to act and vote as indicated, upon all matters referred to on the reverse side and described in the proxy statement relating to the annual meeting, all shares of Common Stock of the Company which the undersigned would be entitled to vote or act upon, with all powers the undersigned would possess, if personally present at the meeting and at any adjourned sessions thereof. Each of the following matters is being proposed by the Board of Directors of the Company.
IN THEIR DISCRETION, THE PROXIES ARE AUTHORIZED TO VOTE UPON OTHER MATTERS AS PROPERLY MAY COME BEFORE THE MEETING, OR ANY ADJOURNMENT THEREOF.
(Continued and to be signed on the reverse side)
Address Change/Comments
(Mark the corresponding box on the reverse side)
5 FOLD AND DETACH HERE5
| | | | | |
This proxy will be voted in accordance with any directions herein given. If no direction is given, this proxy will be voted for the director nominees and proposal 2. | | Please Mark Here for
Address Change or
Comments
SEE REVERSE SIDE | | o |
| | | |
| 1. | | ELECTION OF DIRECTORS |
| | | Election of Directors: 01 Robert J. Hennessey and 02 John N. Staniforth |
| | | | | |
| | | FOR all nominees | | WITHHOLD |
| | | listed above | | AUTHORITY |
| | | (except as provided | | to vote for all |
| | | to the right) | | nominees listed |
| | | o | | o |
TO WITHHOLD AUTHORITY TO VOTE FOR ANY INDIVIDUAL NOMINEE, PRINT THAT NOMINEE’S NAME ON THE LINE PROVIDED.
| | | | | | | | | |
| | | | | FOR | | AGAINST | | ABSTAIN |
| 2. | | Ratification of the appointment of Ernst & Young LLP as the independent registered public accounting firm for the Company for the current fiscal year: | | o | | o | | o |
| | | | | | | |
| | | I PLAN TO ATTEND THE MEETING o |
| | | | | | | |
| | | Dated: | | | | , 2006 |
| | | | | | | |
| | | | | | | |
| | | Signature
|
| | | | | | | |
| | | Signature if held jointly
|
| | | | | | | |
| | | Attendance of the undersigned at the meeting or at any adjourned session thereof will not be deemed to revoke this proxy unless the undersigned shall affirmatively indicate thereof the intention of the undersigned to vote said shares in person. If the undersigned hold(s) any of the shares of the Company in a fiduciary, custodial or joint capacity or capacities, this proxy is signed by the undersigned in every such capacity as well as individually. |
| | | | | | | |
| | | IMPORTANT — PLEASE SIGN AND RETURN THIS PROXY PROMPTLY. When shares are held by joint tenants, both should sign. When signing as attorney, executor, administrator, trustee or guardian, please give full title as such. If a corporation, please sign in full corporate name by President or other authorized officer. If a partnership, please sign in partnership name by an authorized person. |
5 FOLD AND DETACH HERE5
Vote by Internet or Telephone or Mail
24 Hours a Day, 7 Days a Week
Internet and telephone voting is available through 11:59 PM Eastern Time
the day prior to annual meeting day.
Your Internet or telephone vote authorizes the named proxies to vote your shares in the same manner
as if you marked, signed and returned your proxy card.
Internet
http://www.proxyvoting.com/ppco
Use the Internet to vote your proxy. Have your proxy card in hand when you access the web site.
Telephone
1-866-540-5760
Use any touch-tone telephone to vote your proxy. Have your proxy card in hand when you call.
Mail
Mark, sign and date your proxy card
and return it in the enclosed
postage-paid envelope.
If you vote your proxy by Internet or by telephone, you do NOT need to mail back your proxy card.
 Penwest Pharmaceuticals Co.
Penwest Pharmaceuticals Co.