UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2005; or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 000-29173
DIVERSA CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 22-3297375 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
| 4955 Directors Place, San Diego, California | | 92121 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (858) 526-5000
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 par value
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ Nox
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ Nox
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (Section 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer.
| | | | |
| Large accelerated filer ¨ | | Accelerated filer x | | Non-accelerated filer ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of Act). Yes ¨ Nox
The aggregate market value of the voting stock held by non-affiliates of the Registrant as of June 30, 2005 was $93,480,883 million.*
The number of shares outstanding of the Registrant’s common stock was 46,803,292 as of February 28, 2006.
DOCUMENTS INCORPORATED BY REFERENCE
Designated portions of the Registrant’s definitive Proxy Statement to be filed with the Securities and Exchange Commission (the “Commission”) pursuant to Regulation 14A in connection with the 2006 Annual Meeting of Stockholders to be held on May 11, 2006 (the “2006 Annual Meeting”) are incorporated herein by reference into Part III of this report. Such Proxy Statement will be filed with the Commission not later than 120 days after the Registrant’s year ended December 31, 2005.
| * | Excludes the common stock held by executive officers, directors and stockholders whose ownership exceeded 10% of the common stock outstanding at June 30, 2005. This calculation does not reflect a determination that such persons are affiliates for any other purposes. |
DIVERSA CORPORATION
FORM 10-K
For the Year Ended December 31, 2005
INDEX
Forward Looking Statements
This report contains statements that are “forward-looking” and involve a high degree of risk and uncertainty. These include statements related to investments in our core technologies, investments in our internal product candidates, our ability to enter into additional biodiversity access agreements, the discovery, development, and/or optimization of novel genes, enzymes, and other biologically active compounds, the development and commercialization of products and product candidates, the opportunities in our target markets, the benefits to be derived from our current and future strategic alliances, the benefits to be derived from our recent strategic reorganization, our estimates for charges we expect to record in 2006 related to employee separation and facilities consolidation costs, our plans for future business development activities, our plans for our discontinued programs and products, including our sordarins anti-fungal program and other pharmaceutical programs, and our estimates regarding market sizes and opportunities, as well as our future revenue, product-related revenue, profitability, and capital requirements, all of which are prospective. Such statements are only predictions and reflect our expectations and assumptions as of the date of this report based on currently available operating, financial, and competitive information. The actual events or results may differ materially from those projected in such forward-looking statements. Risks and uncertainties and the occurrence of other events could cause actual events or results to differ materially from these predictions. The risk factors set forth below at pages 25-35 should be considered carefully in evaluating us and our business. These forward-looking statements speak only as of the date of this report. We expressly disclaim any intent or obligation to update these forward-looking statements.
We use market data and industry forecasts throughout this report. We have obtained this information from internal surveys, market research, publicly available information, and industry publications. Industry publications generally state that the information they provide has been obtained from sources believed to be reliable but that the accuracy and completeness of such information is not guaranteed. Similarly, we believe that the surveys and market research we or others have performed are reliable, but we have not independently verified this information. We do not represent that any such information is accurate.
Accentuase, DIVERSA®, Cottonase, DirectEvolution®, DiverseLibrary, GeneReassembly, Gene Site Saturation Mutagenesis, GigaMatrix, GSSM, Luminase, Purifine, Pyrolase, and SingleCell are trademarks of Diversa Corporation. ThermalAce is a trademark of Invitrogen Corporation. Phyzyme is a trademark of Danisco Animal Nutrition. Quantum is a trademark of Syngenta Animal Nutrition. Bayovac® is a registered trademark of Bayer Animal Health. Ultra-Thin is a trademark of Valley Research, inc. This report also refers to trade names and trademarks of other organizations.
PART I
We are a leader in applying proprietary genomic technologies for the rapid discovery and optimization of novel protein-based products, including (a) enzymes, which are catalytic proteins, and (b) antibodies, which are binding proteins. We are directing our integrated portfolio of technologies to the discovery, evolution, and production of commercially valuable molecules with agricultural, industrial, and chemical applications, such as enzymes, as well as optimized antibodies with pharmaceutical applications. While our technologies have the potential to serve many large markets, our key areas of focus for internal product development are alternative fuels, specialty industrial processes, and health and nutrition. In addition to our internal product development efforts, we have formed alliances with market leaders, such as BASF, Cargill Health and Food Technologies, DuPont Bio-Based Materials, Medarex, Merck, and Xoma. We have also formed a broad strategic relationship with Syngenta AG, a world-leading agribusiness company. We have two inactive subsidiaries, Innovase LLC and TNEWCO Inc.
1
We were incorporated in Delaware in December 1992 under the name Industrial Genome Sciences, Inc. In August 1997 we changed our name to Diversa Corporation. In January 2006, following a comprehensive review of our operations, we announced a strategic reorganization designed to focus our resources on advancing our most promising products and product candidates in three key areas: alternative fuels; specialty industrial processes; and health and nutrition. As a result of this decision, we discontinued development of a number of less promising products and programs and reduced our workforce by 83 employees. In the fourth quarter of 2005, we recorded a non-cash impairment charge of $45.7 million, and we expect to record additional charges of $15.0 to $17.0 million in 2006 related to employee separation and facilities consolidation costs.
Our Strategy
The key elements of our strategy are to:
Deploy our technologies across diverse markets. We use our technologies to develop commercial solutions for a broad range of applications within our three focus areas of alternative fuels, specialty industrial processes, and health and nutrition. We believe that this multi-market approach gives us the ability to capitalize on near-term revenue opportunities in lower-risk applications and longer-term opportunities in higher-risk applications.
Commercialize products independently and with partners in agricultural, chemical, and industrial markets. Our technologies can be applied to develop products for a wide range of agricultural, chemical, and industrial applications where development costs are lower and regulatory cycles are shorter compared to those for pharmaceutical products. Our key areas of focus for internal product development in these non-pharmaceutical markets are alternative fuels, specialty industrial processes, and selected areas within health and nutrition focused on animal health and nutrition. In addition to our internal product development efforts, we have formed alliances with numerous partners, including a broad relationship with Syngenta to pursue product opportunities within several of these focus areas. To date, we have commercialized eight products independently and four products with our partners and have multiple late-stage candidates that we expect to be commercialized in the next three years.
Leverage our evolution technologies to develop optimized, second-generation antibody therapeutics through collaborations. We are applying our established evolution technologies to optimize existing antibodies through strategic alliances. We have existing collaboration agreements for the development of optimized therapeutic antibodies with Merck, Medarex, and Xoma. We intend to continue to pursue product development initiatives under these collaborations and to seek additional therapeutic antibody optimization collaborations through which we can pursue additional product development activities. We intend to pursue these opportunities only through collaborations with partners under which we expect our unreimbursed costs to be minimal.
Utilize strategic alliances to enable the development of a broad portfolio of products. In all of our target markets, we have identified key market segments where we intend to develop products through strategic alliances. We have established criteria for entering into such alliances, including: required investment, estimated time to market, regulatory hurdles, infrastructure requirements, and industry-specific expertise necessary for successful commercialization. We believe that these alliances will allow us to utilize our partners’ marketing and distribution networks, share the investment risk, and access additional resources to expand our product portfolio. In entering these agreements, we typically seek to obtain a combination of technology access fees, research support payments, milestone payments, license or commercialization fees, and royalties or profit sharing income from the commercialization of products resulting from these alliances.
Protect and enhance our technology leadership position. We are advantaged relative to our competitors in that we have an end-to-end product solution consisting of access to novel genetic material, several technologies capable of screening more than a billion genes per day, multiple evolution technologies for optimizing enzymes and therapeutic antibodies, and manufacturing capabilities. We have protected our technologies with a substantial intellectual property estate, and we will continue to make investments in developing and protecting these assets.
2
Market Opportunities and Product Development Programs
Our strategy in the industrial enzyme area is to establish a sustainable, high-growth, profitable business that targets high-value applications where we believe our technologies can deliver superior, proprietary solutions and we can achieve attractive gross margins on product sales. Within the optimized therapeutic antibody arena, our strategy is to leverage our proven antibody evolution technologies across a portfolio of pharmaceutical discovery and development programs funded by strategic partners.
Through our independent and collaborative research and development programs, we have developed commercial products across multiple markets as well as a pipeline of product candidates that we expect to launch independently and in collaboration with strategic partners To date, we have commercialized the following products, either independently or in collaboration with our partners: Accentuase™-G enzyme, Bayovac® SRS vaccine for farmed salmon, Cottonase™ enzyme, Cyan Fluorescent Protein, Green Fluorescent Protein, Luminase™ PB-100 enzyme, Quantum™ phytase, Phyzyme™ XP phytase, Pyrolase™ 160 Enzyme, Pyrolase™ 200 Enzyme, ThermalAce™, and Ultra-Thin™ enzyme. Although we have not made any acquisitions specifically related to our current market focus areas, we have evaluated, and expect to continue to evaluate, acquisition opportunities that represent a strategic fit to our capabilities.
We have identified the following three focus areas in which we intend to pursue product opportunities either independently or through collaborations and distribution agreements with third parties:
| | • | | Specialty industrial enzymes; and |
Within each of these three focus areas, we have a combination of (i) products that either we or one of our partners have commercialized, (ii) product candidates that either we or one of our partners are developing, and (iii) research and development programs for the development of new product candidates.
The market opportunities we have identified within each of these focus areas, as well as our strategies for pursuing these opportunities, are discussed below.
Alternative Fuels
Alternative fuels refers to fuels derived from sources other than petroleum. In the past several years, a variety of factors have been contributing, and continue to contribute, to an increasing awareness of and demand for alternatives to petroleum-based fuels. Such factors include, but are not limited to, the following:
| | • | | Macroeconomic factors affecting the global supply of and demand for oil, including significantly increased demand for oil from developing countries whose economies are growing at high rates, such as China and India, coupled with flat or decreasing supplies of oil from stable sources throughout the world; |
| | • | | In the United States and other developed countries throughout the world, significantly and persistently higher prices for gasoline and other petroleum-based products due in large part to the macroeconomic factors discussed above; |
| | • | | In the United States, an increasing number of local, state, and federal policies and initiatives aimed at reducing the dependence on imported sources of oil, particularly oil imported from unstable regions of the world such as the Middle East; |
| | • | | In the United States, increasing visibility from vehicle manufacturers such as General Motors and Ford Motor Company regarding so-called “flexible fuel vehicles,” or FFVs, capable of operating on various blends of gasoline and ethanol, and the production and availability of FFVs throughout the United States; |
3
| | • | | In the United States, an increasing number of fuel stations that sell both gasoline and ethanol or blends of the two fuels. |
Of all of the alternative fuels that are sold or are being developed, the two most significant alternative fuels that present current or future opportunities for us are fuel ethanol and biodiesel fuel.
Fuel Ethanol
Ethanol, or ethyl alcohol, is commonly known as “grain alcohol,” as it is typically produced by extracting or using sugars derived from the starch within a grain source, such as corn kernels, and fermenting the sugars via fermentation to produce ethanol. Ethanol can be used as a fuel source to power combustion engines in an increasing number of different types of vehicles throughout the United States and the rest of the world. Enzymes are currently being sold to large-scale ethanol mills to enhance the efficiency and cost-effectiveness of the ethanol production process. While more traditional, small-scale production systems for manufacturing ethanol have in the past relied on the direct fermentation of the grain by yeast to produce ethanol, modern production systems for manufacturing ethanol at large scale have relied on the application of enzymes to more efficiently convert the starch from grains into sugars that can more readily be converted into ethanol via fermentation.
According to the Renewable Fuels Association (RFA), the national trade association for the United States ethanol industry, as of December 31, 2005, there were 95 ethanol plants in the U.S. having a combined production capacity of more than 4.3 billion gallons of ethanol per year, and there were 29 ethanol plants and nine expansions under construction with a combined annual capacity of more than 1.5 billion gallons of ethanol per year. Between 1980 and 1991, less than 1 billion gallons of ethanol were produced annually in the United States. In 2005, the U.S. ethanol industry produced a record 3.9 billion gallons of fuel ethanol, representing an increase of 15% from 2004 and 121% since 2001. The graph below shows historic U.S. fuel ethanol production from 1980 to 2005.

Source: Renewable Fuels Association; www.ethanolrfa.org
4
Figure 2: The current process for manufacturing bioethanol
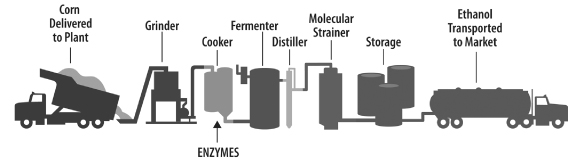
Source: Renewable Fuels Association; Diversa Corporation
We have developed, either independently or through our collaborations, a number of enzyme products and product candidates that may be utilized to convert various sources of starch into sugars that can be used to produce ethanol, commonly referred to as “bioethanol.” The figure above shows the major steps in the process for manufacturing bioethanol from corn kernels, including the step in which enzymes are applied to convert the corn starch to fermentable sugars.
In addition, we have several research and development programs aimed at developing “cocktails” of enzymes to break down more complex starting materials commonly referred to as “biomass”—such as corn stover, wood chips, switchgrass, sugar cane bagasse, and municipal waste materials, for example—into fermentable sugars that could be used to produce ethanol, commonly referred to as “cellulosic ethanol” or “lignocellulosic ethanol.”
Ultra-Thin™ Enzyme
Ultra-Thin™ enzyme is a new, next-generation alpha amylase enzyme designed to significantly improve the efficiency and economics of ethanol production from corn and other starch sources. Distributed by Valley Research, inc. (“Valley Research”), this new product dramatically lowers the viscosity of the corn starch stream and operates at high temperature and at a lower pH than other commercially available enzymes, all of which offers ethanol producers the potential for substantial throughput advantages and cost savings. It works in concert with other enzymes to efficiently convert the starch present in corn and other sources into sugars that can then be processed into ethanol. Ethanol producers have traditionally used other alpha amylase enzymes that do not reduce the starch stream viscosity as efficiently as Ultra-Thin enzyme does and do not operate at an optimal pH, thus limiting plant capacity and requiring costly process adjustments. We manufacture Ultra-Thin enzyme under our agreement with Fermic S.A. de C.V. a U.S. Food and Drug Administration-approved fermentation and synthesis plant located in Mexico City. We estimate that the addressable market for this product is in excess of $50 million in the United States alone and is currently growing at a rate in excess of 10% per year given the significantly increasing demand for ethanol.
Amylase-T
Syngenta has a project in development to produce corn enhanced through biotechnology that expresses high levels of amylase. Using high amylase corn may result in improved process efficiency and possible savings in the cost of ethanol. This transgenic amylase enzyme, which we refer to internally as Amylase-T, was originally developed under our collaboration with Syngenta. We are entitled to receive royalties from Syngenta on sales of products incorporating Amylase-T. We cannot predict with certainty when, if ever, any products incorporating Amylase-T will receive regulatory approval in the United States or any other countries, or whether any such products will be accepted by the intended customers of such products.
5
Biomass-to-Ethanol Programs
We have several research and development programs whose objective involves the production of ethanol from various sources of so-called “biomass.” The figure below shows a general schematic for producing ethanol from sugar, starch, and biomass, together with the associated technologies required for such production.
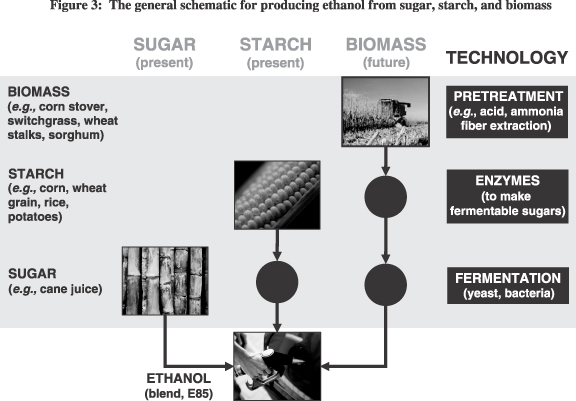
Since 2003, we have been collaborating with DuPont Bio-Based Materials on the development of an integrated corn-based biorefinery (“ICBR”) for the production of ethanol and other value-added chemical products from corn biomass. This multi-year program is being co-funded by the U.S. Department of Energy (“DOE”) and includes within the consortium the National Renewable Energy Lab, or NREL, which is part of the DOE. Our objective under the program is to discover, optimize, and manufacture a “cocktail” of enzymes that can efficiently convert the different components of an entire corn plant, including the stalk, into simple sugars that can then be used to make ethanol and other products.
In 2005, we announced that the performance of the enzymes we developed under the ICBR program with DuPont substantially exceeded the initial targets set by the Department of Energy, triggering a milestone payment to us of over five hundred thousand dollars. The performance of this enzyme mix is already superior to current commercial enzymes for biomass conversion. Accordingly, the next phase of the program is focused on improving the overall economics of the enzyme-based process.
Regarding biomass-to-ethanol opportunities in general, our strategy for generating ethanol from biomass is centered around using our technology platform to develop superior enzymes customized for the starting material and pre-treatment process and then working to produce them cost-effectively. We believe that this approach is much more likely to be ultimately successful than competing approaches which are based primarily on taking existing commercial enzymes and simply trying to produce them at low cost. Given the complexities of the process of producing ethanol from biomass, we believe that the same enzyme cocktail is unlikely to work with each source of biomass, each pre-treatment process, and each fermentation organism; rather, we expect that
6
customized sets of new and improved enzymes—that is, ones that are optimized for the specific source of biomass, the specific pre-treatment process, and the specific fermentation organism—will be required to make biomass-to-ethanol an economic reality.
Biodiesel
Biodiesel is the name of a clean burning alternative fuel that can be produced from renewable resources such as soybeans, canola, and other oilseeds. Biodiesel contains no petroleum, but it can be blended with petroleum diesel to create a biodiesel blend. It can be used in compression-ignition (diesel) engines with little or no modifications. Biodiesel is simple to use, biodegradable, and nontoxic.
Biodiesel’s properties with respect to the operation of diesel engines are similar to diesel fuels that are petroleum-based. Biodiesel has many positive attributes associated with its use, including its similar operating performance compared to conventional diesel fuel and the lack of changes required in facilities and maintenance procedures regarding its handling and use.
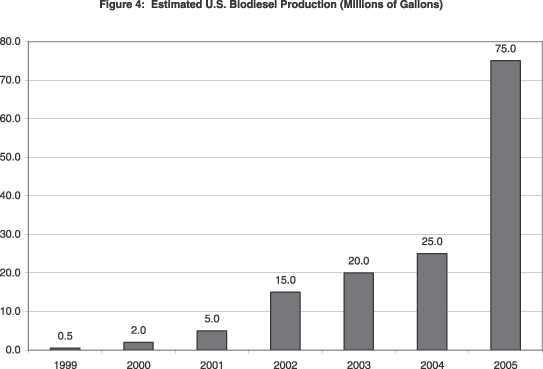
According to the National Biodiesel Board, as of January 2006, there were more than 53 companies that have invested in the development of biodiesel manufacturing plants and that are actively marketing biodiesel, with current production capacity estimated to be 354 million gallons per year. Thirty-five companies have reported that their plants are currently under construction and are scheduled to be completed within the next 18 months. Their combined capacity, if realized, would result in another 278 million gallons per year of biodiesel production. The graph below shows estimated production of biodiesel in the United States from 1999 to 2005.
Source: National Biodiesel Board; www.biodiesel.org
While we developed our Purifine™ enzymes primarily to improve the processing of edible vegetable oils, these enzymes, and / or similar enzymes in our DiverseLibrary™ collection of enzymes, may also improve the refining of biodiesel fuel based on a similar mechanism of action. We are currently investigating the utility of our oil processing enzymes to improve the refining of biodiesel fuels.
7
Specialty Industrial Processes
Within the area of specialty industrial processes, we have identified a number of industrial processing opportunities for high-value enzymes to potentially decrease processing time, improve product quality, lower total processing costs, and/or reduce harmful waste streams. In many cases, these enzymes are intended to replace or reduce the use of commodity chemicals that have been traditionally used in the applicable industrial process.
Pulp and Paper Processing
More than 190 million tons of pulp fiber and 340 million tons of paper and board products are produced annually worldwide. Environmental regulations are becoming increasingly stringent, and the use of harsh chemicals, such as chlorine for bleaching, is no longer preferred in most parts of the world. Substitute chemistries are more expensive, less effective, and more damaging to fiber. Reducing chemical usage in fiber processing through the use of biochemical products can decrease manufacturing and energy costs and environmental impact.
We have developed enzymes to aid in bleaching pulp, which reduce the need to use strong oxidizing chemicals, such as chlorine compounds. These enzymes can reduce the cost of pulp processing both by reducing the amount of oxidizing chemicals required and the expense associated with treating the waste water resulting from the use of these harsh chemicals.
In July 2004, we launched Luminase™ PB-100 enzyme for pulp bleaching enhancement. This product improves the response of pulp fiber to bleaching chemicals, which can reduce the need for harsh bleaching chemicals or enable the customer to make whiter pulp for new products. Decreasing bleach chemicals lowers costs and offers a potential environmental benefit by reducing the amount of waste material requiring removal from pulp mill effluent. In mills, Luminase PB-100 enzyme has outperformed competitive products, demonstrating bleach chemical cost savings of up to 20%. Beginning in late 2004 and continuing through 2005, we initiated several mill trials for our Luminase PB-100 enzyme. We are continuing to run these trials and schedule additional trials in 2006. Additionally, Luminase PB-100 enzyme may produce whiter pulp, potentially extending a customer’s market to new products. We intend to develop line extensions of additional Luminase enzymes that are capable of operating cost-effectively at higher temperature and pH ranges, in order to increase the number of mills that can be addressed by one or more Luminase enzymes, with product launches planned for 2006.
Enhanced Processing of Edible Nutritional Oil
Oils and fats are two of the most abundant and readily available renewable raw materials for use within the food, feed and chemical industries. The fatty acids contained in these lipids are extremely useful precursors for a wide variety of valuable molecules, including animal feed additives, nutritional oils, specialty chemicals and polymers. Current oil processing and oleochemical production processes are generally energy and capital intensive and utilize harsh chemical methods. Major process issues include yield loss, high volume waste streams, high processing costs, and utilization of toxic or environmentally harmful chemicals and solvents. Further, these chemical processes do not allow access to the full value of the feedstock because the processes lack selectivity and control of the fatty acid structure and functionality.
Despite the shortfalls associated with the current chemical processes, biological processes have not been extensively considered due in large part to the weak performance of alternative bioprocesses. Nevertheless, we believe significant opportunities exist to reduce processing costs and enable the utilization of low cost raw materials for higher value chemicals and materials if these limitations can be overcome.
We intend to develop superior enzymes to be employed in both commodity oils processing and also in specialty products such as margarines, cooking oils, and lubricants. Our enzymes will be directed to increasing
8
process efficiency and improving product qualities, such as reducing the cholesterol-causing components in margarine and cooking oils and improving the heat stability of lubricants. The first of such enzymes in development is Purifine™ enzyme, a novel oil processing enzyme designed to increase the yield of oil processing from oil seeds. This product has passed preliminary safety tests, and we expect to receive the results of toxicology studies in the first half of 2006. Assuming satisfactory toxicology results and anticipated timelines for the U.S. regulatory approval process, we anticipate that we could launch Purifine™ enzyme on a commercial basis in mid-2007, assuming it receives regulatory approval. Purifine™ enzyme is expected to minimize chemical usage, improve operating efficiency, and reduce waste by allowing a higher percentage of nutritional oil to be recovered from oil seeds economically.
Fine Chemicals
We are developing enzymes to aid in the manufacture of both fine chemicals, such as chiral pharmaceutical intermediates, as well as other high performance chemicals. These enzymes are used to create manufacturing efficiencies, reduce production costs, and accelerate the generation of new chemical products and processes. Today, we are well positioned to become a leading provider of integrated biological solutions and services to the chemical and pharmaceutical industries through strategic alliances. Historically, we have established collaborative agreements in this area with BASF, Cargill Health and Food Technologies, The Dow Chemical Company, DSM Pharma Products, DuPont Bio-Based Materials, and Givaudan Flavors Corporation. We expect to continue to establish or maintain collaborative relationships for the development of products for fine chemical applications, although we do not currently intend to invest our own financial resources in the development of internal products for these applications. We intend to pursue these opportunities only through collaborations with partners under which we expect our unreimbursed costs to be minimal.
Health and Nutrition Programs
Animal Feed Additives to Enhance Animal Nutrition
Animal feed additives are designed to increase absorption of essential vitamins and minerals, increase nutritional value and animal product yield, and reduce harmful materials in waste. In 2001 alone, over $8 billion was spent on animal feed additives that improve digestibility and increase nutritional value. We are developing several classes of enzymes, including phytases and carbohydrases, for the increased absorption of organic phosphorous and digestibility of carbohydrates, as well as the promotion of weight gain in livestock.
When used as an additive in animal feed applications, phytase enzymes allow higher utilization of naturally occurring phosphorus from the feed, thereby increasing its nutritional value and reducing phosphate pollution. The worldwide market for phytase enzymes was estimated to be more than $200 million in 2005. This growth has been driven by economics as well as regulatory pressure to decrease pollution caused by the phosphate-rich waste from swine and poultry farms that is a leading cause of water pollution. We have developed two phytase products to address this market.
In March 2003, we launched Phyzyme™ XP in collaboration with our partner Danisco Animal Nutrition. The addition of Phyzyme™ XP to animal feed reduces the need for inorganic phosphorus supplementation by approximately 20% and lowers the level of harmful phosphates that are introduced to the environment through animal waste by approximately 30%, resulting in inorganic phosphate cost savings and a significant reduction in environmental pollution. We are responsible for manufacturing Phyzyme XP, and Danisco is responsible for its sales and marketing.
In December 2003, our thermostable Quantum™ phytase, developed under our collaboration with Syngenta, received regulatory approval in Mexico and has subsequently received regulatory approval in other countries, including Brazil. Quantum phytase is currently under regulatory review for sale in the U.S. and several other countries. As reported by Syngenta, the results from more than 50 poultry and swine trials of this product show that Quantum phytase consistently outperforms other commercial phytases in a wide variety of diets. This is the
9
first product we have commercialized with Syngenta, and we believe it is indicative of our ability to deliver differentiated animal nutrition products.
Through our collaboration with Syngenta, we have also developed a next-generation transgenic phytase product candidate, which we refer to internally as Phytase-T, that is intended to be grown directly in corn. This product is intended to be both cost-effective and heat-stable, and it is expected to supplement Quantum phytase. We are entitled to receive royalties from Syngenta on sales of products incorporating Phytase-T. We cannot predict with certainty when, if ever, any products incorporating Phytase-T will receive regulatory approval in the United States or any other countries, or whether any such products will be accepted by the intended customers of such products.
Animal Health Vaccines for Prevention or Treatment of Disease
Over the past several years, we have worked on the development, optimization, and manufacture of vaccines for use in animal health. We have commercialized one vaccine product for farmed salmon, but we do not intend to invest additional resources in the development of additional animal health vaccines.
We formed a collaboration with Bayer Animal Health in 2003 to develop microbially-produced vaccine products initially focused on the prevention of infectious diseases in fish. Our initial product, Bayovac® SRS, is a proprietary and novel subunit recombinant vaccine for farmed salmon. This vaccine product has demonstrated superior protection against salmon rickettsial septicemia (SRS). SRS is the major infectious disease in Chilean aquaculture, typically killing upwards of 20% of untreated farmed salmon, which represent the highest value per pound of all farmed animals. Bayovac® SRS received regulatory approval in September 2004 in Chile, which produces 40% of the world’s farmed salmon, and we recorded our first sales of this product in September 2004.
Therapeutic Antibody Optimization Program
Our antibody optimization program focuses on the application of our technologies to improve existing antibody therapeutics with the objective of creating superior products. Our technologies have the potential to improve the potency, safety, and convenience of antibody therapeutics, as well as to decrease their manufacturing cost.
We believe that applying our directed evolution technologies to antibodies represents an attractive opportunity, because:
| | • | | Biotherapeutics, and in particular antibodies, are an emerging treatment modality that has demonstrated value in treating serious and chronic diseases; |
| | • | | Biotherapeutics are suitable for accelerated clinical development, because they potentially present fewer side effects as a result of their high target specificity; and |
| | • | | The development of improved versions of marketed biopharmaceuticals and / or clinical candidates for validated targets represents a lower-risk development strategy while still targeting highly profitable markets. |
Given the flexibility, comprehensiveness, and controlled nature of our proprietary technology platforms, we are in a unique position to tailor antibody therapeutics to the specific needs of the therapeutic indication. For instance, oncology indications require antibodies with a high degree of specificity and the ability to activate the immune system. On the other hand, antibodies for the treatment of immunological disorders are required to have a high affinity for their target while maintaining a very good side effect profile. Our technology platforms can be used to yield antibodies with the key properties outlined above.
Furthermore, some of the key issues associated with antibody therapeutics are related to their challenges in manufacturability and formulation. Our technology platforms can also potentially address these issues by
10
providing variants that, while maintaining their biological and pharmacological properties, provide better stability and solubility.
Monoclonal Antibody Market
Therapeutic antibodies comprise one of the fastest-growing classes of pharmaceutical products, generating worldwide sales of $10.5 billion in 2004. There are currently18 therapeutic antibodies on the market and over 300 candidates in various stages of clinical development. Currently, antibodies are mainly applied for serious indications in oncology and autoimmune diseases. The use of monoclonal antibodies as an emerging therapeutic modality represents an attractive market opportunity expected tomore than double in worldwide sales by 2010.
Shortcomings in Current Antibody Development
Initially, antibodies were generated using laboratory mice. These murine antibodies were often rejected by patients because they were recognized as foreign proteins, and the patients produced human anti-mouse antibodies, also known as HAMA response. This response reduces the effectiveness of the antibody by neutralizing its activity and clearing the antibody from circulation. HAMA response can also result in significant toxicity with subsequent administration of mouse antibodies.
Subsequent generations of antibodies have been re-engineered in an attempt to address these immunogenic complications, resulting in antibodies that are less mouse-like and more human-like. One such example is chimeric antibodies, which still contain approximately 38% mouse sequences. Some of the antibodies still manifested the HAMA responses initially observed in mouse antibodies. Scientists then developed CDR-grafted, or humanized, antibodies in which mouse sequences were minimized to represent less than 10% of the final antibody. This engineering process commonly results in alteration (reduction) of both affinity and, sometimes, specificity of the antibody.
Fully human antibodies represent the latest development in antibody generation. They are currently generated through the use of transgenic mice and phage display. Lines of transgenic mice have been engineered to carry the human germline genes encoding antibodies, but the following issues may be implicated by the generation of human antibodies using transgenic mice:
| | • | | Transgenic mice do not currently harbor the entire human gene repertoire, and therefore the diversity generated is unlikely to fully represent the human immune system. Similarly, B-cells do not express all potential human antibodies. |
| | • | | The antibody selection and maturation processes in a biological system may result in fewer therapeutically relevant candidates. |
| | • | | From a process perspective, transgenic mouse-based antibody generation requires a hybridoma phase that represents a long and inefficient process, and there are very limited opportunities to influence or direct the process, thereby greatly limiting the ability to engineer antibodies. |
The other approach to screen for fully human antibodies is based on a phage display platform. It is important to emphasize that phage display is a selection tool only. In this sense, the limitations associated with phage display as a screening tool will bias any antibodies selected in the process. These limitations include those antibodies that can effectively be expressed and displayed, potential conformational changes or distortions associated with the display, and the high frequency of repeated hits identified in any given screen.
11
Advantages of Our Antibody Optimization Program
The emerging antibody therapeutic market, in our view, is transitioning from a first generation of products to next generations, where the ability to engineer and build in desired properties will likely be increasingly demanded and likely to provide products with superior performance.
In this context, we believe that our antibody program offers the following value propositions:
| | • | | Medicinal Evolution of Antibody Therapeutics. We employ a robust process that enables the generation of a large number of candidates which can be screened, not only for biological properties, but also for improved process development requirements (e.g., formulation, solubility, and stability). Our antibody generation capability can yield a diverse selection of antibodies that is potentially capable of responding to many disease targets. Our optimization technologies can rapidly create large numbers of variants, which can then be the subject of multiple selections for the variant(s) with the optimal characteristics. |
| | • | | Broad Intellectual Property Protection. We provide the opportunity to create a broad intellectual property position around a given antigen and / or a given antibody by characterizing all potential antibodies naturally produced by the human immune system as well as derivatives with functional properties. |
We intend to continue to develop optimized therapeutic antibodies in collaboration with strategic partners. However, given our focus in the near-term on industrial enzyme product opportunities, we do not intend to invest a significant amount of our own financial resources in the development of optimized antibody therapeutics under such collaborations or under internal programs.
Small Molecule Drug Discovery Program
Our small molecule drug discovery programs have been based on two unique technology platforms, whose competitive properties can be outlined as follow:
High-Throughput Culturing Platform (HTC)
| | • | | HTC provides access to previously uncultured microorganisms by creating nano-environments similar to those encountered in natural habitats. The specific technology and an extensive report on its findings have been published in the Proceedings of the National Academy of Sciences. |
| | • | | Novel isolates can be cultured and assayed for biological activities of interests in a high-throughput manner. |
| | • | | The isolates can be investigated for novel chemical structures by using high-throughput mass spectrometry coupled with proprietary software for compound analysis (MQuest). This chemical screening enables us to analyze more of the metabolites within each organism and to identify novel chemistries that may be broadly applicable to all therapeutic areas. |
Recombinant Natural Product Platform (RNP)
| | • | | We are able to access genetic diversity from the estimated 99% of microbial organisms that account for the Earth’s untapped diversity. |
| | • | | Our patented DNA recovery technologies, together with a suitable expression host, enable us to access previously undiscovered pathways from natural microbial sources and to ensure that those compounds we discover can be expressed in sufficient quantities to be exploited further. |
| | • | | RNP represents a new paradigm in compound discovery and is a highly complementary approach to HTC. We expect the two approaches to accelerate the hit-to-lead process and enable viable options for commercial production of novel compounds discovered through this process. |
12
To date, our program has produced a number of confirmed broad-spectrum hits in the antibacterial / antifungal area. Approximately 4% of these are novel structures, and we have profiled many of these compounds for spectrum, potency, and selectivity in order to prioritize them for potential lead optimization by medicinal chemistry and / or biotransformation.
In January 2006, pursuant to a corporate reorganization, we announced our intention to discontinue further investment in the development of our small molecule programs for human pharmaceuticals. We intend to explore opportunities to sell or out-license these programs to third parties.
Our Technologies and Advantages
Traditional Approaches and Their Limitations
Enzymes have been shown to catalyze more than 3,000 individual chemical reactions. Nearly all of the currently characterized enzymes have been isolated from organisms that were cultured in the laboratory, representing only a small percentage of the billions of species believed to exist. The reasons for this include:
| | • | | Less than 1% of microbial species will grow under standard laboratory conditions; |
| | • | | Enzymes and other bioactive molecules may only be produced at specific times during growth or under specific conditions not present in the lab; |
| | • | | Even when enzymes are found, recovery of the corresponding genes can be difficult. |
Accordingly, biodiversity remains largely untapped.
Once an enzyme or small molecule of interest is discovered, the genetic sequence of the gene or genes encoding it can be studied, and genetic variation can be introduced in an attempt to modify its function through a process of test tube evolution. Genetic variation is generated predominantly by two methods: mutation and recombination. Mutation is the introduction of changes into a gene. Mutation can be achieved by several methods, including forcing the DNA to replicate in a manner that intentionally causes random changes. Mutagenesis has been achieved by randomly introducing single nucleotide changes into a gene in an attempt to alter a single amino acid within the corresponding protein. Random methods have deficiencies that make it virtually impossible to generate all 19 possible amino acid changes at each position within the protein. The best method to generate all amino acid changes at each site requires multiple, appropriately positioned DNA base changes (non-random methods). Historically, on average, three or fewer changes are explored due to deficiencies in mutation and sampling methods. Recombination, or shuffling, the other method for producing genetic variation, is the mixing of two or more related genes to form hybrids. However, the generation of improved variants has, to date, been inefficient and laborious, or has allowed only closely related genes to be recombined.
Once a desired gene is found and optimized, commercial production requires insertion of the gene into a production system or host. Almost all of the current commercial enzymes used in industrial applications today were derived from cultured microorganisms and produced in these or similar organisms referred to as homologous expression. However, genes encoding unique biomolecules may not be able to be expressed and commercially produced in traditional systems. Thus, traditional methods present both the problem of novel biomolecule identification and the challenge of commercial production of any identified biomolecules.
Biodiversity Access
Our discovery program begins with access to biodiversity. Biodiversity can be defined as the total variety of life on earth, including genes, species, ecosystems, and the complex interactions between them. We have collected microbial samples from numerous types of ecosystems represented on earth, including such environments as geothermal and hydrothermal vents, acidic soils and boiling mud pots, alkaline springs, marine and freshwater sediments, savanna grasslands, rainforests, montane and subalpine landscapes, industrial sites,
13
arctic tundra, and dry Antarctic valleys. We have also sampled microbial communities living in close association with insects, arachnids, and nematodes, as well as the symbionts residing within marine sponges and soft corals. All of our samples from the countries within our biodiversity access network have been acquired through agreements that permit broad access to biologically diverse environments within such countries. These agreements are generally with domestic land management agencies and scientific research institutions associated with appropriate government agencies. Our relationships have been founded on the fundamental principles of the Convention on Biological Diversity: (1) conservation of biological diversity; (2) the sustainable use of its resources; and (3) the fair and equitable sharing of the benefits derived from the utilization of genetic resources.
We believe our ability to create expanded libraries using minute samples of genetic material collected from diverse environments is an important factor to our success. Our need to use only small environmental samples results in minimal impact to the surrounding ecosystem, enabling us to enter into formal genetic resource access agreements. In 1997, we signed a Cooperative Research and Development Agreement with Yellowstone National Park, which was the first agreement of its kind for the U.S. National Park Service. To date, we have obtained samples under various access agreements from Alaska, Antarctica, Australia, Bermuda, Costa Rica, Ghana, Hawaii, Iceland, Indonesia, Kenya, Mexico, the Meadowlands Superfund site, Puerto Rico, Russia, the San Diego Zoological Society, South Africa, and Yellowstone National Park. We also access marine and terrestrial samples from Antarctica, as well as deep-sea hydrothermal vents off the shores of Costa Rica and the Pacific Northwest. Many of these samples are taken using deep-sea submersibles or remotely operated vehicles.
We intend to enter into additional agreements to further strengthen our biodiversity access program by expanding the network of countries from which we obtain samples. Using our proprietary techniques to recover the genes from these samples, we have constructed our DiverseLibrary collection. We intend to expand this DiverseLibrary collection, which we estimate currently contains the total genomes of millions of unique microorganisms. We believe that the application of our proprietary technologies to this vast resource of genetic material will provide us with a myriad of product candidates for attractive commercial applications.
Screening
We have developed an array of automated, ultra high-throughput screening technologies and enrichment strategies. Our proprietary rapid screening capabilities are designed to discover novel biomolecules by screening for biological activity, known as expression-based screening, as well as by identifying specific DNA sequences of interest, known as sequence-based screening.
We have developed numerous assays capable of expression-based screening from thousands to over 1 billion clones per day. Our key screening technologies include SingleCell™ screening and high-throughput robotic-based screening. Our ultra high-throughput SingleCell screening system uses Fluorescence Activated Cell Sorting, or FACS, a technology that enables the rapid identification of biological activity within a single cell or individual organism. Our SingleCell screens have been developed to identify clones based on activity or DNA sequences. This system incorporates a laser with multiple wavelength capabilities and the ability to screen up to 50,000 clones per second, or over 1 billion clones per day. Our robotic screening systems use high-density (1536 wells) microtiter plates and are capable of screening and characterizing over 1 million clones per day. If the clone expresses an activity or contains a DNA sequence of interest, we isolate it for further analysis.
We have also developed rapid methods for sequence-based screening for targeted genes directly from purified DNA. One of these methods, genomic biopanning, is a powerful alternative to traditional methods, especially when the gene is toxic or unstable, or when the expression assay is laborious and time consuming. Using our proprietary techniques, it is possible to screen billions of clones per day for DNA sequences of interest.
Because we conduct patented, activity-based screening, we are able to use gene sequences with known function from our proprietary database to identify the function of genes in public databases based on their
14
sequences. These newly identified sequences are then added to the repertoire of proprietary sequences in our own database. As more microbial genomes are sequenced, our ability to associate gene sequence with enzyme function will be enhanced. This sequence database provides us with unique opportunities to find and patent more sequences with similar function and the potential to modify these sequences in order to create optimized catalysts and other biomolecules for various commercial applications.
Our GigaMatrix™ platform is an ultra high-throughput screening platform that is the first system known to utilize plates with a 100,000-well density. Exponentially more efficient than standard 96-, 384-, or 1536-well screening systems, the GigaMatrix platform combines automated robotics and a 100,000-well format contained in the 3.3” x 5” footprint of a standard plate.
The GigaMatrix platform permits rapid screening of genes and gene pathways, and is expected to increase the productivity of our discovery programs for products such as novel enzymes. In 2002, we developed the capability to screen in plates with one million wells and initiated screening in this ultra-high density format.
The GigaMatrix technology, employing over 12,000 wells per square centimeter, greatly expands the amount of molecular diversity that can be screened to discover products. The platform also dramatically reduces equipment and operator time through massively parallel dispensing and reading of biological samples. The GigaMatrix plates, with wells each about the diameter of a human hair, are reusable and require only miniscule volumes of reagents, making them highly cost effective.
Our DirectEvolution® Technologies
The genetic code is structured such that a sequence of three nucleotides defines an amino acid. Nature uses 20 common amino acids in proteins arranged in a sequence, defining the protein structure and activity. Over the course of almost 4 billion years of evolution, nature has sampled countless sequence possibilities to evolve proteins to function optimally within the cell. However, when a protein is removed from its natural cellular environment and used to perform reactions, such as an enzyme used to catalyze a chemical process, its function may not be optimal. Laboratory methods can accelerate the evolutionary process of optimization outside of the cell by creating a large number of variants for screening. In the traditional method for improving proteins, called site-directed mutation, a single site is typically targeted for change based on prior knowledge of the protein structure. Other traditional techniques, including random mutation, typically produce single nucleotide changes which can only access a limited number of alternative amino acids, typically fewer than 3 of the possible 19 alternatives. These methods are limited by their inability to produce all DNA and amino acid sequence variations. Furthermore, the large number of resulting sequences presents formidable screening challenges.
We believe our techniques overcome the limitations of these traditional methods, not only because of our superior screening capabilities, but also by increasing the number and types of sequence variations we can create. Our evolution technologies used to modify the DNA sequence of the genes, our DirectEvolution technologies, include Gene Site Saturation Mutagenesis™ (GSSM™) and Tunable GeneReassembly™. Our GSSM technology is a patented method of creating a family of related genes that all differ from a parent gene by at least a single amino acid change at a defined position. By performing GSSM on a gene encoding a protein, we create all possible single amino acid codon substitutions within that protein, removing the need for prior knowledge about the protein structure and allowing all possibilities to be tested in an unbiased manner. The family of variant genes created using GSSM is then available to be screened for proteins with improved qualities, such as increased ability to work at high temperature, increased reaction rate, resistance to deactivating chemicals, or other properties important in a chemical process. Individual changes in the gene that cause improvements can then be combined to create a single highly improved version of the protein. Additionally, our patented GSSM methodology employs a more comprehensive approach than other methods of site-directed mutation.
In addition to altering single genes using our patented GSSM technique, we use our patented Tunable GeneReassembly technology for the reassembly of related or unrelated genes from two or more different species
15
or strains. Our Tunable GeneReassembly technology recombines multiple genes to create a large population of new gene variants. The new genes created by Tunable GeneReassembly are then screened for one or more desired characteristics. This evolutionary process can be repeated on reassembled genes until new genes expressing the desired properties are identified. Tunable GeneReassembly technologies can be used to evolve properties which are coded for by single or multiple genes. We have received over 20 patents worldwide for our broad portfolio of proprietary processes for evolution, from gene shuffling based on interrupted DNA synthesis, to Tunable GeneReassembly, GSSM, and a number of additional evolution technologies. Further, this suite of multiple, patented evolution technologies successfully overcomes the limitations of traditional shuffling techniques. For instance, unlike widespread shuffling technologies that require highly related gene sequences to achieve successful recombination, our proprietary Tunable GeneReassembly technology also allows unrelated genes to be combined to maximize evolved improvements.
We believe that the ability to selectively apply our GSSM or Tunable GeneReassembly technologies to optimize proteins provides us with a distinct competitive advantage. GSSM is better suited in some situations, for example, in the optimization of a protein’s stability or its immune response characteristics. With respect to stability, applying GSSM may significantly improve temperature tolerance through combining amino acid alterations at defined positions, while maintaining the protein’s overall characteristics, such as specificity. In one program, we have used this technology to improve enzyme stability by a factor of 30,000. Similarly, adverse immune system responses may be avoided by the incremental changes created by GSSM compared to traditional stochastic methods. In contrast, random shuffling technologies that cause block shifts in DNA structure may be more likely to reduce stability and create undesirable immune response characteristics.
Current Alliances and Other Agreements
Our strategy includes pursuing strategic alliances with market leaders in our target markets. In exchange for selected rights to future products, these strategic alliances provide us funding and resources to develop and commercialize a larger product portfolio. In various instances, these strategic alliances allow us to leverage our partners’ established brand recognition, global market presence, established sales and distribution channels, and other industry-specific expertise. The key components of the commercial terms of such arrangements typically include some combination of the following types of fees: exclusivity fees, technology access fees, technology development fees and research support payments, as well as milestone payments, license or commercialization fees, and royalties or profit sharing from the commercialization of any products that result from the alliance. As of December 31, 2005, our strategic partners have provided us approximately $240 million in funding since inception and are committed to additional funding of more than $60 million through 2010, subject to our performance under existing agreements, excluding milestone payments, license and commercialization fees, and royalties or profit sharing.
Collaborative revenue accounted for 63% of total revenue for the year ended December 31, 2005, 73% of total revenue for the year ended December 31, 2004, and 86% of total revenue for the year ended December 31, 2003. As a result of our recent reorganization, we expect to de-emphasize certain collaborations that are not strategic to our current market focus.
To date, we have entered into the following strategic alliances and other agreements:
Research and Development Collaborations
Syngenta
In January 1999, we entered the agricultural biotechnology arena through a strategic alliance with Syngenta Biotechnology, Inc. (formerly Syngenta Agribusiness Biotechnology Research, Inc.), referred to below as Syngenta Biotechnology. This alliance covered a multi-project collaborative research and development agreement to develop products for crop enhancement and improved agronomic performance. Under the terms of
16
the agreement, we utilized our unique discovery and screening technologies to identify and optimize genes and gene pathways for use in transgenic crops. The initial projects focused on new genomic approaches that would provide improved performance and quality traits in crops and enhance production. In conjunction with the transaction, Syngenta Biotechnology purchased 5,555,556 shares of our Series E convertible preferred stock (which converted to shares of our common stock upon completion of our initial public offering), paid a technology access fee, and provided project research funding to us, for aggregate total proceeds of $12.5 million. We recognized the research payments on a percentage of completion basis as research was performed. We recognized the technology access fee in 1999, as all the research required under the collaboration was completed by December 31, 1999. During 2000, we expanded our collaboration agreement with Syngenta Biotechnology to further develop and optimize novel synthesis routes to crop protection chemicals.
In December 1999, we formed a five-year strategic alliance with Syngenta Seeds AG, referred to below as Syngenta Seeds. Through a contract joint venture, named Zymetrics, we pursued opportunities jointly with Syngenta Seeds in the fields of animal feed and agricultural product processing. Both parties shared in the management of the venture and funded a portion of its sales and marketing costs. Under the agreement, Syngenta Seeds received exclusive, worldwide rights in the field of animal feed and project exclusive, worldwide rights in the field of agricultural product processing. Syngenta Seeds agreed to pay us $20.0 million for the rights granted under this agreement. In May 2004, we entered into an agreement with Syngenta that continued the development and commercialization of novel animal feed enzymes beyond the five-year initial term of our 1999 Zymetrics joint venture agreement. Under the agreement, Syngenta has agreed to continue its collaboration with us in the area of animal feed enzymes on an exclusive basis, paid us an exclusivity fee, and committed to continued research and development funding. As of November 30, 2004, the Zymetrics joint-venture related agreements expired in accordance with their terms; however, we are entitled a share of the profits in the form of royalties on any future product sales that result from products developed under these agreements.
In February 2003, we completed a series of transactions with Syngenta Participations AG, or Syngenta, and its wholly-owned subsidiary, Torrey Mesa Research Institute, or TMRI. Under the transactions, the companies formed an extensive research collaboration whereby we are entitled to receive a minimum of $118.0 million in research and development funding over the initial seven-year term of the related research collaboration agreement, and will be eligible to receive certain milestone payments and royalties upon product development and commercialization. Additionally, we acquired certain property and equipment from TMRI and certain licenses from Syngenta to intellectual property rights used in activities conducted at TMRI that primarily involve tools, technologies and methods relating to proteomics, metabolomics, RNA dynamics, and bioinformatics and methods to analyze and link these components of genomics or that primarily relate to TMRI’s fungal program, for use outside of Syngenta’s exclusive field as defined in the related research collaboration agreement. In consideration, we issued to Syngenta and TMRI a total of 6,034,983 shares of common stock and a warrant to purchase 1,293,211 shares of common stock at $22.00 per share that is exercisable for ten years starting in 2008. The total value of the acquisition was approximately $74.0 million, including transaction fees.
The purchase price was allocated, based upon a third party valuation, to tangible assets, consisting primarily of computer and lab equipment, and identifiable intangible assets, consisting of acquired in-process research and development, core technology, and the value associated with the research collaboration agreement. The in-process research and development was recorded as expense upon closing of the transactions. Prior to December 31, 2005, the tangible assets were being depreciated over their estimated useful lives of three to five years, the core technology was being amortized over its estimated life of fifteen years, and the value associated with the research collaboration agreement was being amortized over its estimated useful life of seven years. During the fourth quarter of 2005, in connection with our strategic reorganization, we assessed the carrying values of these assets and technologies on our balance sheet and determined that such assets and technologies were impaired. As a result we have written off the carrying value of these assets and technologies on our balance sheet as of December 31, 2005.
Either party may terminate our research collaboration agreement with Syngenta upon the other party’s material uncured breach or default in the performance of any of its obligations under the research collaboration
17
agreement or in the event the other party becomes subject to voluntary or undismissed involuntary bankruptcy or similar proceedings. In addition, our research collaboration agreement with Syngenta may be terminated by Syngenta in the event that we undergo a change of control while we are performing research under the research collaboration agreement and either the change of control transaction is with or involving any entity that is a competitor of Syngenta or its affiliates or, as a result of the change of control, Syngenta reasonably determines in its sole judgment that such change of control would have an adverse effect on our ability or the ability of the surviving entity to perform the research collaboration agreement’s research program.
In 2002, we entered into a manufacturing agreement with an affiliate of Syngenta to supply commercial quantities of Quantum phytase at a fixed price, determined by a negotiated formula, that is subject to adjustment during the term of the agreement. In addition, we are entitled to receive royalties from Syngenta on their sales of Quantum phytase.
Revenue recognized under the Syngenta agreements was $24.3 million, $36.9 million, and $29.2 million for the years ended December 31, 2005, 2004 and 2003.
DuPont Bio-Based Materials
In 2003, we entered into a six-year alliance with DuPont Bio-Based Materials to discover and develop novel biocatalysts for the production of fuel ethanol, 1,3 propanediol, and other added-value chemicals from renewable resources such as corn and biomass. The program with DuPont, referred to as an “Integrated Corn-Based Biorefinery” program, is part of a grant consortium funded by the U.S. Department of Energy to develop a biorefinery capable of producing high-value chemical products from biomass. DuPont is expecting to receive $19 million in matching funds from the U.S. Department of Energy over four years. Under our collaboration agreement with DuPont regarding this biorefinery program, we have received research funding, as well as milestone payments, and we are entitled to additional milestone payments as well as royalties on any new products developed under the agreement that incorporate our technologies.
Cargill Health and Food Technologies
In 2005, we signed a collaboration agreement with Cargill Health and Food Technologies to discover and develop novel enzymes for the cost-effective production of a proprietary Cargill product. Under the terms of the agreement, we received upfront payments and research funding, and we are entitled to receive milestone payments, license fees, and royalties on products that may be developed under the agreement.
Bayer Animal Health
In December 2003, we formed a collaboration with Bayer Animal Health to develop and market products to prevent infectious diseases in fish. Under the agreement, we collaborated to complete the development and registration of an existing pipeline of microbially-produced vaccine candidates for aquaculture previously developed by a Bayer venture. Under the agreement, we were responsible for developing and manufacturing these microbially-produced vaccine candidates, which were to be marketed and distributed by Bayer in designated countries on an exclusive basis. We completed the registration of and launched commercially the first vaccine product under this agreement, Bayovac®-SRS, in Chile in 2004 and advanced the development of a number of additional vaccine candidates. In January 2006, pursuant to a corporate reorganization, we announced our intention to discontinue further investment in the development of these additional vaccine candidates. We intend to continue to sell Bayovac®-SRS in Chile and to continue to explore opportunities to sell this product in other markets.
Merck & Co., Inc.
In December 2004, we entered into an agreement with Merck & Co., Inc. to collaborate on the development of therapeutic antibodies for a key target by applying our proprietary MedEv™ platform. Under the terms of the
18
agreement, we received an upfront payment and received research funding. In mid-2005, we amended this agreement to provide for additional research and development activities as well as terms for additional research funding, milestone payments, and royalties.
DSM Pharma Chemicals
In December 2003, we entered into a collaborative agreement with DSM Pharma Chemicals to discover and develop biocatalytic solutions designed to simplify and lower the cost of a variety of chemical transformations. Under the terms of the agreement, DSM will identify targeted chemical conversions, we will work to develop appropriate biocatalysts, and DSM will scale-up these processes to manufacture pharmaceutical intermediates and active ingredients. We receive research payments and are entitled to milestones and royalties on products commercialized by DSM.
The Dow Chemical Company
From 1997 to 2000, we entered into a number of collaboration and license agreements with The Dow Chemical Company, or Dow, involving various industrial enzyme research and development programs and products. In June 2000, we formed a 50/50 joint venture with Dow, named Innovase LLC, to develop and commercialize innovative products for the industrial enzyme market segment. Under the various joint venture related agreements, we received exclusivity fees, technology development fees, and research and development payments. We were also required to fund certain operating expenses of the joint venture, excluding certain expenses for which Dow was responsible under the joint venture agreement.
In December 2003, we signed a definitive agreement with Dow to restructure the Innovase joint venture. Under the terms of the restructuring agreement, rights to all products and product candidates developed by Innovase were conveyed to us, and the companies mutually agreed to terminate all exclusivity and non-competition arrangements for the industrial enzyme fields related to the Innovase joint venture. Dow divested its 50% ownership interest to us and paid us $5.0 million. All joint venture related agreements involving Dow and us were terminated.
Revenue recognized under the Dow agreements was $1.8 million and $10.7 million for the years ended December 31, 2004 and 2003. No revenue was recognized under any of the Dow agreements for the year ended December 31, 2005, as such agreements expired prior to such time.
Givaudan S.A.
In November 2004, we entered into a license agreement with Givaudan S.A. for Accentuase™-G enzyme, a novel enzyme product that significantly improves the efficient production of a natural flavor ingredient. As of the date we licensed this enzyme to Givaudan, the new proprietary biocatalytic process incorporating Accentuase-G enzyme had been successfully scaled up by Givaudan for commercial production. Under the terms of the collaborative agreement initiated in January 2002, we received a license fee and became entitled to receive royalties from Givaudan’s sales or use of the flavor ingredient. We also manufacture the enzyme product for Givaudan under a supply agreement entered into concurrently with this license. In 2005, we amended our license agreement with Givaudan to eliminate our right to receive royalties on Givaudan’s sales or use of the flavor ingredient in exchange for a one-time payment.
Government Grants and Contracts
To date we have received grants contracts for more than $25.0 million in funding from a number of government agencies, including the U.S. Department of Defense, the U.S. Department of Energy, and the National Institutes of Health. Revenue related to government grants and contracts was $10.1 million, $10.2 million, and $3.9 million for the years ended December 31, 2005, 2004, and 2003. As a result of our recent reorganization, we expect to de-emphasize grants and contracts that are not strategic to our current market focus.
19
Manufacturing, Supply, and Distribution Agreements
Danisco Animal Nutrition
In May 1996, we entered into a collaboration agreement with Danisco Animal Nutrition (formerly Finnfeeds International Ltd) to jointly identify and develop a novel phytase enzyme that when used as an additive in animal feed applications allows higher utilization of phytic acid phosphates from the feed, thereby increasing its nutritional value. The addition of phytase to animal feed reduces the need for inorganic phosphorus supplementation and lowers the level of harmful phosphates that are introduced to the environment through animal waste, resulting in inorganic phosphate cost savings and a significant reduction in environmental pollution. Following the completion of the initial objectives of our agreement with Danisco, in December 1998, we entered into a license agreement with Danisco to commercialize an enzyme developed under the collaboration agreement. Under the terms of the license agreement, we granted Danisco an exclusive license to manufacture, use, and sell the developed enzyme. In consideration for the license, we are paid a royalty on related product sales made by Danisco equal to 50% of the cumulative profits generated by Danisco on such sales. In March 2003, the FDA approved Phyzyme XP Animal Feed Enzyme, which we developed in collaboration with Danisco. Additionally, we entered into a manufacturing agreement with Danisco to supply commercial quantities of Phyzyme XP at our cost to manufacture such quantities. Revenue recognized from transactions with Danisco, including contract manufacturing performed on behalf of Danisco, was $5.2 million, $2.0 million, and $2.0 million for the years ended December 31, 2005, 2004, and 2003.
Valley Research, inc.
In 2005, we signed, and later amended, a distribution agreement with Valley Research, inc. (“Valley”) covering our Ultra-Thin alpha amylase enzyme and potentially additional enzyme products. Under the amended agreement, we appointed Valley as our exclusive distributor in the United States for Ultra-Thin enzyme for ethanol and high fructose corn sweetener applications, subject to certain limitations, and subject to certain conditions required to be met for such exclusivity to be maintained. The term of this distribution agreement regarding Ultra-Thin enzyme is for a period of five years following regulatory approval of such enzyme by the FDA’s Center for Veterinary Medicine, which approval was obtained on February 24, 2006.
License or Other Acquisition Agreements
In addition to our strategic alliances, we have entered into various agreements whereby we have in-licensed or otherwise acquired patented technologies to supplement our internally developed technologies, the most significant of which we have outlined below.
Terragen Discovery, Inc.
In November 1999, we signed a license agreement with Terragen Discovery Inc., or Terragen, under which we and Terragen agreed to cross license certain technologies. Under the terms of the agreement, we made an initial payment of $2.5 million in 1999 and agreed to make annual payments of $0.1 million to Terragen to maintain the patent rights over the remaining patent life. We capitalized the initial payment as an intangible asset, which through December 31, 2005 was amortized over the sixteen year patent life. During the fourth quarter of 2005, in connection with our strategic reorganization, we assessed the carrying value of this license on our balance sheet and determined that it was impaired. As a result, we have written off the carrying value of the license on our balance sheet as of December 31, 2005.
Xoma Ltd.
In December 2003, we signed a license and product development agreement with Xoma Ltd. Under the terms of the agreement, we received a license to use Xoma’s antibody expression technology for developing antibody products independently and with collaborators, and an option to a license for the production of
20
antibodies under the Xoma patents. We paid an initial license fee and may be required to pay future milestones and royalties. Under the terms of the development portion of the agreement, we and Xoma will combine our respective capabilities to discover and develop antibodies for autoimmune-related diseases. During the fourth quarter of 2005, in connection with our strategic reorganization, we assessed the carrying value of this license on our balance sheet and determined that it was impaired. As a result, we have written off the carrying value of the license on our balance sheet as of December 31, 2005.
Glaxo Wellcome, S.A.
In July 2003, we acquired an antifungal program consisting of preclinical Sordarins compounds from Glaxo Wellcome, S.A. In consideration for the antifungal program, we issued an aggregate of 806,873 shares of our common stock to Glaxo Group Limited, an affiliate of Glaxo Wellcome, S.A. Under the terms of the agreement, we received worldwide rights to the program, which consists of preclinical antifungal compounds and lead candidates for development, marketing, and distribution. Based upon the closing price of our common stock immediately preceding consummation of the transaction, the fair value of the compounds and lead candidates was $8.7 million. As of the acquisition date, these compounds and lead products had not reached technological feasibility and had no alternative future use. Accordingly, we recorded $8.7 million as a write-off of acquired in-process research and development in 2003. In January 2006, pursuant to our corporate reorganization, we announced our intention to discontinue further investment in the development of these lead candidates and additional sordarin antifungal compounds. We intend to explore opportunities to sell or out-license these lead candidates, additional sordarin antifungal compounds, and associated intellectual property, to a third party.
Biodiversity Access Agreements
Through genetic resource access agreements, we have obtained genetic material from Alaska, Antarctica, Australia, Bermuda, Costa Rica, Ghana, Hawaii, Iceland, Indonesia, Kenya, Mexico, the Meadowlands Superfund site, Puerto Rico, Russia, the San Diego Zoological Society, South Africa, and Yellowstone National Park. Pursuant to the terms of these agreements, we have obtained non-exclusive access to collect samples from diverse ecosystems, we own products developed and discoveries made from our use of the samples, and we pay a royalty to the other party on the sale of products derived from the samples. All of these agreements expire in 2008 or earlier, are renewable, and are all subject to earlier termination. If an access agreement terminates and a new agreement is not established, we will not collect any further materials from the specified location; however, we will retain the right to use any samples we have already collected.
Competition
We are a leader in the field of biomolecule discovery and optimization from biodiversity. We are not aware of another company that has the scope and integration of technologies and processes that we have. There are, however, a number of competitors who are competent in various steps throughout our technology process. For example, Codexis, Maxygen, Inc., Evotec, and Xencor have alternative evolution technologies. Integrated Genomics Inc., Myriad Genetics, Inc., and ArQule, Inc. perform screening, sequencing, and/or bioinformatics services. Novozymes A/S and Genencor International Inc. are involved in development, overexpression, fermentation, and purification of enzymes. Abgenix, Inc., Cambridge Antibody Technology, Medarex, Inc., and Morphosys AG are involved in the development of human monoclonal antibodies. There are also a number of academic institutions involved in various phases of our technology process. Many of these competitors have significantly greater financial and human resources than we do.
We believe that the principal competitive factors in our market are access to genetic material, technological experience and expertise, and proprietary position. We believe that we compete favorably with respect to the foregoing factors.
Any products that we develop will compete in multiple, highly competitive markets. Many of our potential competitors in these markets have substantially greater financial, technical, and marketing resources than we do
21
and may succeed in developing products that would render our products or those of our strategic partners obsolete or noncompetitive. In addition, many of these competitors have significantly greater experience than we do in their respective fields. Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner and are technologically superior to, and/or are less expensive than, other products on the market. Current competitors or other companies may develop technologies and products that are more effective than ours. Our technologies and products may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors. The existing approaches of our competitors or new approaches or technology developed by our competitors may be more effective than those developed by us.
Manufacturing Strategy
Our manufacturing strategy is to secure contract manufacturing relationships with qualified third parties possessing sufficient fermentation capacity to meet our commercial production requirements. We add supplemental equipment as required for our specific products, and we place our own technical personnel on site at contract manufacturing facilities to plan and supervise our production. Our employees have significant experience in scale-up and production of fermentation products, including industrial enzymes. We have cleared regulatory requirements for our first commercial enzymes, and we are producing these products at commercial scale in connection with our manufacturing agreement with Fermic, S.A. de C.V. (“Fermic”). We manufacture products for our own sales in addition to products produced under supply agreements for three of our partners. We have our own pilot development facility that is used for developing new products and processes, providing developmental quantities of products for internal and external use, and for producing commercial quantities of smaller-scale specialty products. We will continue to depend on contractual arrangements with third parties to provide the bulk of the capital infrastructure required for large-scale commercial manufacturing.
During 2002, we entered into a manufacturing agreement with Fermic, a U.S. Food and Drug Administration-approved fermentation and synthesis plant located in Mexico City, to provide us with the capacity to produce commercial quantities of certain enzyme products. Based on actual and projected increased product requirements, the agreement was amended in 2004 to provide for additional capacity to be installed over the succeeding four-year period. Under the terms of the agreement, we can cancel the committed purchases with thirty months’ notice provided that the term of the agreement, including the termination notice period, aggregates four years. Pursuant to our agreement with Fermic, we are also obligated to reimburse monthly costs related to manufacturing activities. These costs scale up as our projected manufacturing volume increases. As of December 31, 2005, under this agreement we have made minimum commitments to Fermic of approximately $15.5 million, over the next three years. In addition, under the terms of the agreement, we are required to purchase certain equipment required for fermentation and downstream processing of our products. Through December 31, 2005, we had incurred costs of approximately $10.5 million for equipment related to this agreement. During 2006, we anticipate spending as much as $5.0 million in additional equipment costs related to the manufacturing agreement. As we continue to develop our commercial manufacturing platforms, we will be required to purchase additional capital equipment under this agreement.
Fermic is currently our sole supplier for commercial-scale enzymes. We do not currently depend on any single supplier for the raw materials necessary for the operation of our business. However, we may become dependent on a single supplier in the future.
Government Regulation
All of our products to date have applications other than as regulated drug products. Non-drug biologically derived products are regulated, in the United States, based on their application, by either the United States Food and Drug Administration, or FDA, the Environmental Protection Agency, or EPA, or, in the case of plants and animals, the United States Department of Agriculture, or USDA. In addition to regulating drugs, the FDA also regulates food and food additives, feed and feed additives, and GRAS (Generally Recognized As Safe)
22
substances used in the processing of food. The EPA regulates biologically derived chemicals not within the FDA’s jurisdiction. Although the food and industrial regulatory process can vary significantly in time and expense from application to application, the timelines generally are shorter in duration than the drug regulatory process, ranging from six months to three years.
The European regulatory process for these classes of biologically derived products has undergone significant change in the recent past, as the EU attempts to replace country by country regulatory procedures with a consistent EU regulatory standard in each case. Some country-by-country regulatory oversight remains. Most other regions of the world generally accept either a United States or a European clearance together with a filing of associated data and information for their review of a new biologically derived product.
In the United States, transgenic agricultural products may be reviewed by the FDA, EPA, and USDA, depending on the plant and the trait engineered into it. The regulatory process for these agricultural products can take up to five years of field testing under USDA oversight, and up to another two years for applicable agencies to complete their reviews.
Outside of the United States, scientifically-based standards, guidelines and recommendations pertinent to transgenic and other products intended for the international marketplace are being developed by, among others, the representatives of national governments within the jurisdiction of the standard-setting bodies, including Codex Alimentarius, the International Plant Protection Convention, and the Office des International Epizooties. The use of the existing standard-setting bodies to address concerns about products of biotechnology is intended to harmonize risk-assessment methodologies and evaluation of specific products or classes of products.
Proprietary Rights
Our intellectual property consists of patents, copyrights, trade secrets, know-how, and trademarks. Protection of our intellectual property is a strategic priority for our business. Our ability to compete effectively depends in large part on our ability to obtain patents for our technologies and products, to maintain trade secrets, to operate without infringing the rights of others, and to prevent others from infringing on our proprietary rights. As of December 31, 2005, we owned242 issued patents relating to our technologies and had over 500 patents pending. In addition, as of December 31, 2005, we had in-licensedover 100 patents or patent applications that we believe strengthen our patent position.
The patent positions of biotechnology companies, including our patent position, involve complex legal and factual questions and, therefore, enforceability cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated, or circumvented. We cannot be sure that relevant patents have not been issued that could block our ability to obtain patents or to operate as we would like to. Others may develop similar technologies or duplicate technologies developed by us. There may exist patents in some countries that, if valid, may block our ability to commercialize products in these countries if we are unsuccessful in circumventing or acquiring the rights to these patents. There also may exist claims in published patent applications in some countries that, if granted and valid, may also block our ability to commercialize products in these countries if we are unable to circumvent or license them.
The biotechnology industry is characterized by extensive litigation regarding patents and other intellectual property rights. Many biotechnology companies have employed intellectual property litigation as a way to gain a competitive advantage. Third parties may sue us in the future to challenge our patent rights or claim infringement of their patents. An adverse determination in litigation or interference proceedings to which we may become a party could subject us to significant liabilities to third parties, require us to license disputed rights from third parties, or require us to cease using the disputed technology. We are aware of a significant number of patents and patent applications relating to aspects of our technologies filed by, and issued to, third parties. Should any of our competitors have filed patent applications or obtain patents that claim inventions also claimed by us, we may have to participate in an interference proceeding declared by the relevant patent regulatory agency to determine
23
priority of invention and, thus, the right to a patent for these inventions in the United States. Such a proceeding could result in substantial cost to us even if the outcome is favorable. Even if successful on priority grounds, an interference may result in loss of claims based on patentability grounds raised in the interference. Although patent and intellectual property disputes in the biotechnology area are often settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include ongoing royalties. Furthermore, we cannot be certain that the necessary licenses would be available to us on satisfactory terms, if at all.
We also rely on trade secrets, technical know-how, and continuing invention to develop and maintain our competitive position. We have taken security measures to protect our trade secrets, proprietary know-how and technologies, and confidential data and continue to explore further methods of protection. Our policy is to execute confidentiality agreements with our employees and consultants upon the commencement of an employment or consulting arrangement with us. These agreements generally require that all confidential information developed or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. There can be no assurance that proprietary information will not be disclosed, that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, or that we can meaningfully protect our trade secrets.
An interference proceeding has been declared in the U.S. Patent and Trademark Office between a U.S. patent assigned to us and a pending U.S. patent application owned by another company with an allowable claim directed to optimization of immunomodulatory genetic vaccines. The interference was declared in February 2004. We have received a decision from the Board of Patent Appeals and Interferences in the motions phase, and our core claims have survived. We are currently due to file a brief in the priority phase by March 27, 2006.
Employees
As of December 31, 2005, we had 287 full-time employees, 93 of whom held Ph.D. degrees. Of these employees, 214 were engaged in research and development and 73 were engaged in business development, sales and marketing, finance, and general administration. On January 5, 2006, we announced a corporate reorganization that involved, among other things, a reduction in our workforce. Immediately following this corporate reorganization, we had 204 full-time employees, 61 of whom hold Ph.D. degrees. Of these employees, 147 were engaged in research and development and 57 were engaged in business development, sales and marketing, finance, and general administration. None of our employees is represented by labor unions or covered by collective bargaining agreements. We have not experienced any work stoppages and consider our employee relations to be good.
Investor Information
Financial and other information about us is available on our website (http://www.diversa.com). We make available on our website, free of charge, copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the Securities and Exchange Commission.
24
Except for the historical information contained herein, this Form 10-K contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed here. Factors that could cause or contribute to differences in our actual results include those discussed in the following section, as well as those discussed in Part II, Item 7 entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere throughout this Form 10-K. You should consider carefully the following risk factors, together with all of the other information included in this Form 10-K. Each of these risk factors could adversely affect our business, operating results, and financial condition, as well as adversely affect the value of an investment in our common stock.
We have a history of net losses, we expect to continue to incur net losses, and we may not achieve or maintain profitability.
We have incurred net losses since our inception, including a net loss of approximately $90 million for the year ended December 31, 2005. As of December 31, 2005, we had an accumulated deficit of approximately $290 million. We expect to incur additional losses in 2006 and 2007 as we continue to develop independent products and as a result of our continued investment in our sales and marketing infrastructure to support anticipated growth in product sales. The extent of our future losses will depend, in part, on the rate of growth, if any, in our contract revenue, future product sales at profitable margins, and on the level of our expenses. To date, most of our revenue has been derived from collaborations and grants, and we expect that a significant portion of our revenue for 2006 will result from the same sources.
Future revenue from collaborations is uncertain because our ability to generate revenue will depend upon our ability to maintain our current collaborations, enter into new collaborations and to meet research, development, and commercialization objectives under new and existing agreements. We anticipate that our sales and marketing expenses will remain at comparable levels, or increase, in future periods as we introduce new products and invest in the necessary infrastructure to support an anticipated increased level of product revenues. We will need to generate significant additional revenue to achieve profitability. In order for us to generate revenue, we must not only retain our existing collaborations and/or attract new ones and achieve milestones under them, but we must also develop products or technologies that we or our partners choose to commercialize and that are commercially successful and from which we can derive revenue through sales or royalties. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
Because we are an early stage company developing and deploying new technologies, we may not be able to commercialize our technologies or products, which could cause us to be unprofitable or cease operations.
You must evaluate our business in light of the uncertainties and complexities affecting an early stage biotechnology company. Our existing proprietary technologies are new and in the early stage of development. We may not be successful in the commercial development of these or any further technologies or products. Successful products require significant development and investment, including testing, to demonstrate their cost- effectiveness prior to regulatory approval and commercialization. To date, we have commercialized only eight of our own products, Ultra-Thin™ enzyme, Pyrolase™ 160 enzyme, Pyrolase™ 200 enzyme, Cottonase™ enzyme, Luminase™ PB-100 enzyme, Bayovac® SRS, and blue and green fluorescent proteins. In addition, four of our collaborative partners, Invitrogen Corporation, Danisco Animal Nutrition, Givaudan Flavors Corporation, and Syngenta Animal Nutrition (formerly known as Zymetrics, Inc.), have incorporated our technologies or inventions into their own commercial products from which we have generated and/or can generate royalties. Our products and technologies have generated only modest revenues to date. Because of these uncertainties, our discovery process may not result in the identification of product candidates that we or our collaborative partners will successfully commercialize. If we are not able to use our technologies to discover new materials or products with significant commercial potential, we will not be able to achieve our objectives or build a sustainable or profitable business.
25
We are dependent on our collaborative partners, and our failure to successfully manage our existing and future collaboration relationships could prevent us from developing and commercializing many of our products and achieving or sustaining profitability.
We currently have license agreements, strategic alliance agreements, collaboration agreements, supply agreements, and/or distribution agreements with BASF, Bayer Animal Health, Cargill Health and Food Technologies, DSM Pharma Chemicals, DuPont Bio-Based Materials, Givaudan Flavors Corporation, Medarex, Merck, Valley Research, inc., and Xoma. We have also formed a broad strategic relationship with Syngenta AG, a world-leading agribusiness company. For the year ended December 31, 2005, approximately 45% of our revenue was from affiliates of Syngenta AG, and a major portion of our future committed funding is also to be received from affiliates of Syngenta AG. We expect that a significant portion of any future revenue will be derived from our collaboration agreements. Since we do not currently possess the resources necessary to independently develop and commercialize all of the potential products that may result from our technologies, we expect to continue to enter into, and in the near-term derive additional revenue from, strategic alliance and collaboration agreements to develop and commercialize products. We will have limited or no control over the resources that any strategic partner or collaborator may devote to our partnered products. Any of our present or future strategic partners or collaborators may fail to perform their obligations as expected. These strategic partners or collaborators may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Further, our strategic partners or collaborators may not develop products arising out of our collaborative arrangements or devote sufficient resources to the development, manufacture, marketing, or sale of these products. If any of these events occur, or we fail to enter into or maintain strategic alliance or collaboration agreements, we may not be able to commercialize our products, grow our business, or generate sufficient revenue to support our operations. Our present or future strategic alliance and collaboration opportunities could be harmed if:
| | • | | We do not achieve our research and development objectives under our strategic alliance and collaboration agreements; |
| | • | | We develop products and processes or enter into additional strategic alliances or collaborations that conflict with the business objectives of our strategic partners or collaborators; |
| | • | | We disagree with our strategic partners or collaborators as to rights to intellectual property we develop, or their research programs or commercialization activities; |
| | • | | We are unable to manage multiple simultaneous strategic alliances or collaborations; |
| | • | | Our strategic partners or collaborators become competitors of ours or enter into agreements with our competitors; |
| | • | | Our strategic partners or collaborators become less willing to expend their resources on research and development due to general market conditions or other circumstances beyond our control; |
| | • | | Consolidation in our target markets limits the number of potential strategic partners or collaborators; or |
| | • | | We are unable to negotiate additional agreements having terms satisfactory to us. |
We may not be able to realize any future benefits from the products and programs that we are discontinuing and/or de-emphasizing in connection with the strategic reorganization that we announced in January 2006.
In January 2006, we announced a strategic reorganization designed to focus our resources on programs and products that have the greatest opportunity for success. Accordingly, we elected to discontinue or to exit certain products and programs, many of which we have spent significant amounts of research funds on to date. We will attempt to sell and/or out-license to third parties some of these products and programs, including, but not limited to, our sordarins anti-fungal program, our recombinant natural products program, and our animal health programs. It is possible that we could be unsuccessful in our attempts to sell or out-license these products and/or
26
programs. In the event that we are successful in selling or out-licensing any of these products and/or programs, the structure of such transactions may provide for only future compensation in the event that the third party is ultimately successful in development of the products and/or programs. Accordingly, it is possible that we may not receive any financial benefit from any sale or out license of these products and/or programs.
We do not own equipment with the capacity to manufacture products on a commercial scale. If we are unable to access the capacity to manufacture products in sufficient quantity, we may not be able to commercialize our products or generate significant sales.
We have only limited experience in enzyme manufacturing, and we do not have our own capacity to manufacture products on a commercial scale. We expect to be dependent to a significant extent on third parties for commercial scale manufacturing of our products. We have arrangements with third parties that have the required manufacturing equipment and available capacity to manufacture Ultra-Thin™ enzyme, Phyzyme™ XP, Bayovac® SRS, Quantum™ phytase, Luminase™ PB-100 enzyme, Pyrolase 160 enzyme, Pyrolase 200 enzyme, and Cottonase™ enzyme. While we have our own pilot development facility, we continue to depend on third parties for large-scale commercial manufacturing. Additionally, one of our third party manufacturers is located in a foreign country. Any difficulties or interruptions of service with our third party manufacturers or our own pilot manufacturing facility could disrupt our research and development efforts, delay our commercialization of products, and harm our relationships with our strategic partners, collaborators, or customers.
We have only limited experience in independently developing, manufacturing, marketing, selling, and distributing commercial products.
We intend to pursue some product opportunities independently. We currently have only limited resources and capability to develop, manufacture, market, sell, or distribute products on a commercial scale. We will determine which products to pursue independently based on various criteria, including: investment required, estimated time to market, regulatory hurdles, infrastructure requirements, and industry-specific expertise necessary for successful commercialization. At any time, we may modify our strategy and pursue collaborations for the development and commercialization of some products that we had intended to pursue independently. We may pursue products that ultimately require more resources than we anticipate or which may be technically unsuccessful. In order for us to commercialize more products directly, we would need to establish or obtain through outsourcing arrangements additional capability to develop, manufacture, market, sell, and distribute products. If we are unable to successfully commercialize products resulting from our internal product development efforts, we will continue to incur losses. Even if we successfully develop a commercial product, we may not generate significant sales and achieve profitability.
Ethical, legal, and social concerns about genetically engineered products could limit or prevent the use of our products and technologies and limit our revenue.
Some of our anticipated products are genetically engineered. If we and/or our collaborators are not able to overcome the ethical, legal, and social concerns relating to genetic engineering, our products may not be accepted. Any of the risks discussed below could result in expenses, delays, or other impediments to our programs or the public acceptance and commercialization of products dependent on our technologies or inventions. Our ability to develop and commercialize one or more of our technologies and products could be limited by the following factors:
| | • | | Public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and genetically engineered products, which could influence public acceptance of our technologies and products; |
| | • | | Public attitudes regarding, and potential changes to laws governing, ownership of genetic material which could harm our intellectual property rights with respect to our genetic material and discourage collaborative partners from supporting, developing, or commercializing our products and technologies; and |
27
| | • | | Governmental reaction to negative publicity concerning genetically modified organisms, which could result in greater government regulation of genetic research and derivative products, including labeling requirements. |
The subject of genetically modified organisms has received negative publicity, which has aroused public debate. The adverse publicity could lead to greater regulation and trade restrictions on imports of genetically altered products.
If we are unable to continue to collect genetic material from diverse natural environments, our research and development efforts and our product development programs could be harmed.
We collect genetic material from organisms found in diverse environments. We collect material from government-owned land in foreign countries and in areas of the United States under formal resource access agreements and from private lands under individual agreements with private landowners. If our access to materials under biodiversity access agreements or other arrangements is reduced or terminates, it could harm our internal and our collaborative research and development efforts. We also collect samples from other environments where agreements are currently not required, such as the deep sea. All of our agreements with foreign countries expire in 2008 or earlier, are renewable, and are all subject to earlier termination. We have voluntarily ceased collections of further samples in Yellowstone National Park pending the park’s resolution of collection guidelines.
Our ability to compete may decline if we do not adequately protect our proprietary technologies or if we lose some of our intellectual property rights due to becoming involved in expensive lawsuits or administrative proceedings.
Our success depends in part on our ability to obtain patents and maintain adequate protection of our other intellectual property for our technologies and products in the United States and other countries. The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting their proprietary rights in these foreign countries. These problems can be caused by, for example, a lack of rules and methods for defending intellectual property rights.
Our commercial success depends in part on not infringing patents and proprietary rights of third parties, and not breaching any licenses or other agreements that we have entered into with regard to our technologies, products, and business. The patent positions of biotechnology companies, including our patent position, involve complex legal and factual questions and, therefore, enforceability cannot be predicted with certainty. We will apply for patents covering both our technologies and products as we deem appropriate. Patents, if issued, may be challenged, invalidated, or circumvented. We cannot be sure that relevant patents have not been issued that could block our ability to obtain patents or to operate as we would like. Others may develop similar technologies or duplicate technologies developed by us. There may be patents in some countries that, if valid, may block our ability to commercialize products in these countries if we are unsuccessful in circumventing or acquiring the rights to these patents. There also may be claims in published patent applications in some countries that, if granted and valid, may also block our ability to commercialize products in these countries if we are unable to circumvent or license them.
In 2003, an interference proceeding was declared in the U.S. Patent and Trademark Office in 2004 between a U.S. patent assigned to us and a pending U.S. patent owned by another company with allowable claims directed to optimization of immunomodulatory genetic vaccines. Other than this interference proceeding, we are not currently a party to any litigation with regard to our patent position. However, the biotechnology industry is characterized by extensive litigation regarding patents and other intellectual property rights. Many biotechnology companies have employed intellectual property litigation as a way to gain a competitive advantage. If we became involved in litigation or interference proceedings declared by the United States Patent and Trademark Office, or
28
oppositions or other intellectual property proceedings outside of the United States, to defend our intellectual property rights or as a result of alleged infringement of the rights of others, we might have to spend significant amounts of money. We are aware of a significant number of patents and patent applications relating to aspects of our technologies filed by, and issued to, third parties. Should any of our competitors have filed patent applications or obtained patents that claim inventions also claimed by us, we may have to participate in an interference proceeding declared by the relevant patent regulatory agency to determine priority of invention and, thus, the right to a patent for these inventions in the United States. Such a proceeding, like the one described above, could result in substantial cost to us even if the outcome is favorable. Even if successful on priority grounds, an interference may result in loss of claims based on patentability grounds raised in the interference. The litigation or proceedings could divert our management’s time and efforts. Even unsuccessful claims could result in significant legal fees and other expenses, diversion of management time, and disruption in our business. Uncertainties resulting from initiation and continuation of any patent or related litigation could harm our ability to compete.
An adverse ruling arising out of any intellectual property dispute, including an adverse decision as to the priority of our inventions, would undercut or invalidate our intellectual property position. An adverse ruling could also subject us to significant liability for damages, prevent us from using processes or products, or require us to negotiate licenses to disputed rights from third parties. Although patent and intellectual property disputes in the biotechnology area are often settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include ongoing royalties. Furthermore, necessary licenses may not be available to us on satisfactory terms, if at all.
We may encounter difficulties managing our growth, which could adversely affect our results of operations.
Our strategy includes entering into and working on simultaneous projects across multiple industries. While we do not expect to significantly increase our headcount in the near future, if we add more projects, it may place increased demands on our limited human resources. Our ability to effectively manage our operations, growth, and various projects requires us to continue to improve our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. We may not be able to successfully implement improvements to our management information and control systems in an efficient or timely manner. In addition, we may discover deficiencies in existing systems and controls.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we also rely in part on trade secret protection for our confidential and proprietary information. We have taken security measures to protect our trade secrets and proprietary information. These measures may not provide adequate protection for our trade secrets or other proprietary information. Our policy is to execute confidentiality agreements with our employees and consultants upon the commencement of an employment or consulting arrangement with us. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us shall be our exclusive property. There can be no assurance that proprietary information will not be disclosed, that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, or that we can meaningfully protect our trade secrets. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
29
Many potential competitors who have greater resources and experience than we do may develop products and technologies that make ours obsolete.
The biotechnology industry is characterized by rapid technological change, and the area of biomolecule discovery and optimization from biodiversity is a rapidly evolving field. Our future success will depend on our ability to maintain a competitive position with respect to technological advances. Rapid technological development by others may result in our products and technologies becoming obsolete.
We face, and will continue to face, intense competition. We are not aware of another company that has the scope and integration of technologies and processes that we have. There are, however, a number of companies who compete with us in various steps throughout our technology process. For example, Codexis, Maxygen, Inc., Evotec, and Xencor have alternative evolution technologies. Integrated Genomics Inc., Myriad Genetics, Inc., and ArQule, Inc. perform screening, sequencing, and/or bioinformatics services. Novozymes A/S and Genencor International Inc. are involved in development, overexpression, fermentation, and purification of enzymes. Abgenix, Inc., Cambridge Antibody Technology, Medarex, Inc., and Morphosys AG are involved in the development of human monoclonal antibodies. There are also a number of academic institutions involved in various phases of our technology process. Many of these competitors have significantly greater financial and human resources than we do. These organizations may develop technologies that are superior alternatives to our technologies. Further, our competitors may be more effective at implementing their technologies for modifying DNA to develop commercial products.
Any products that we develop through our technologies will compete in multiple, highly competitive markets. Many of our potential competitors in these markets have substantially greater financial, technical, and marketing resources than we do and may succeed in developing products that would render our products or those of our collaborators obsolete or noncompetitive. In addition, many of these competitors have significantly greater experience than we do in their respective fields. Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner and are technologically superior to and/or are less expensive than other products on the market. Current competitors or other companies may develop technologies and products that are more effective than ours. Our technologies and products may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors. The existing approaches of our competitors or new approaches or technology developed by our competitors may be more effective than those developed by us.
Stringent laws and required government approvals could delay our introduction of products.
All phases, especially the field testing, production, and marketing, of our potential products are subject to significant federal, state, local, and/or foreign governmental regulation. Regulatory agencies may not allow us to produce and/or market our products in a timely manner or under technically or commercially feasible conditions, or at all, which could harm our business.
In the United States, products for our target markets are regulated based on their application, by either the Food and Drug Administration, or FDA, the Environmental Protection Agency, or EPA, or, in the case of plants and animals, the United States Department of Agriculture, or USDA. The FDA regulates drugs, food, and feed, as well as food additives, feed additives, and substances generally recognized as safe that are used in the processing of food or feed. While substantially all of our projects to date have focused on non-human applications of our technologies and products outside of the FDA’s review, in the future we may pursue collaborations for further research and development of drug products for humans that would require FDA approval before they could be marketed in the United States. In addition, any drug product candidates must also be approved by the regulatory agencies of foreign governments before any product can be sold in those countries. Under current FDA policy, our products, or products of our collaborative partners incorporating our technologies or inventions, to the extent that they come within the FDA’s jurisdiction, may be subject to lengthy FDA reviews and unfavorable FDA determinations if they raise safety questions which cannot be satisfactorily answered, if
30
results from pre-clinical or clinical trials do not meet regulatory requirements or if they are deemed to be food additives whose safety cannot be demonstrated. An unfavorable FDA ruling could be difficult to resolve and could prevent a product from being commercialized. Even after investing significant time and expenditures, our collaborators may not obtain regulatory approval for any drug products that incorporate our technologies or inventions. Our collaborators have not submitted an investigational new drug application for any product candidate that incorporates our technologies or inventions, and no drug product candidate developed with our technologies has been approved for commercialization in the United States or elsewhere. The EPA regulates biologically derived chemical substances not within the FDA’s jurisdiction. An unfavorable EPA ruling could delay commercialization or require modification of the production process resulting in higher manufacturing costs, thereby making the product uneconomical. In addition, the USDA may prohibit genetically engineered plants from being grown and transported except under an exemption, or under controls so burdensome that commercialization becomes impracticable. Our future products may not be exempted by the USDA.
The European regulatory process for these classes of biologically derived products has been in a state of flux in the recent past, as the EU attempts to replace country by country regulatory procedures with a consistent EU regulatory standard in each case. Some country-by-country regulatory oversight remains. Other than Japan, most other regions of the world generally find adequate either a United States or a European clearance together with associated data and information for a new biologically derived product.
If we require additional capital to fund our operations, we may need to enter into financing arrangements with unfavorable terms or which could adversely affect the ownership interest and rights of our common stockholders as compared to our other stockholders. If such financing is not available, we may need to cease operations.
We currently anticipate that our available cash resources, short-term investments, receivables, and committed funding from collaborative partners and government grants will be sufficient to meet our capital requirements for the near-term. However, market acceptance of our products could be slower than anticipated, or we may use a significant amount of our cash to successfully develop and market our products.
Our capital requirements depend on several factors, including:
| | • | | The level of research and development investment required to maintain our technology leadership position; |
| | • | | Our ability to enter into new agreements with collaborative partners or to extend the terms of our existing collaborative agreements, and the terms of any agreement of this type; |
| | • | | The success rate of our discovery efforts associated with milestones and royalties; |
| | • | | Our ability to successfully commercialize products developed independently and the demand for such products; |
| | • | | The timing and willingness of strategic partners and collaborators to commercialize our products that would result in royalties; |
| | • | | Costs of recruiting and retaining qualified personnel; and |
| | • | | Our need to acquire or license complementary technologies or acquire complementary businesses. |
If additional capital is required to operate our business, we cannot assure you that additional financing will be available on terms favorable to us, or at all. If adequate funds are not available or are not available on acceptable terms, our ability to fund our operations, take advantage of opportunities, develop products or technologies, or otherwise respond to competitive pressures could be significantly limited. In addition, if financing is not available, we may need to cease operations.
31
If we raise additional funds through the issuance of equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock. If we raise additional funds through the issuance of debt securities, such debt securities would have rights, preferences and privileges senior to holders of common stock and the terms of such debt could impose restrictions on our operations.
We expect that our quarterly results of operations will fluctuate, and this fluctuation could cause our stock price to decline, causing investor losses.
Our quarterly operating results have fluctuated in the past and are likely to do so in the future. These fluctuations could cause our stock price to fluctuate significantly or decline. Revenue in future periods may be greater or less than revenue in the immediately preceding period or in the comparable period of the prior year. Some of the factors that could cause our operating results to fluctuate include:
| | • | | Termination of strategic alliances and collaborations; |
| | • | | The success rate of our discovery efforts associated with milestones and royalties; |
| | • | | The ability and willingness of strategic partners and collaborators to commercialize, market, and sell royalty-bearing products on expected timelines; |
| | • | | Our ability to enter into new agreements with strategic partners and collaborators or to extend the terms of our existing strategic alliance agreements and collaborations, and the terms of any agreement of this type; |
| | • | | Our ability to successfully satisfy all pertinent regulatory requirements; |
| | • | | Our ability to successfully commercialize products developed independently and the demand for such products; and |
| | • | | General and industry specific economic conditions, which may affect our collaborative partners’ research and development expenditures. |
If revenue declines or does not grow as anticipated due to the expiration of strategic alliance or collaboration agreements, failure to obtain new agreements or grants, lower than expected royalty payments, slow market acceptance of our products, or other factors, we may not be able to correspondingly reduce our operating expenses. A large portion of our expenses, including expenses for facilities, equipment and personnel, are relatively fixed. Failure to achieve anticipated levels of revenue could therefore significantly harm our operating results for a particular fiscal period.
Due to the possibility of fluctuations in our revenue and expenses, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance. Our operating results in some quarters may not meet the expectations of stock market analysts and investors. In that case, our stock price would probably decline.
If we lose our key personnel or are unable to attract and retain qualified personnel as necessary, it could delay our product development programs and harm our research and development efforts.
Our success depends to a significant degree upon the continued contributions of our executive officers, management, and scientific staff. If we lose the services of one or more of these people, we may be unable to achieve our business objectives or our stock price could decline. We may not be able to attract or retain qualified employees in the future due to the intense competition for qualified personnel among biotechnology and other technology-based businesses, particularly in the San Diego area. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will adversely
32
affect our ability to meet the demands of our collaborative partners in a timely fashion or to support our internal research and development programs. In particular, our product development programs depend on our ability to attract and retain highly skilled scientists, including molecular biologists, biochemists, and engineers. Although we believe we will be successful in attracting and retaining qualified personnel, competition for experienced scientists and other technical personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms. All of our employees are at-will employees, which means that either the employee or we may terminate their employment at any time.
Our planned activities will require additional expertise in specific industries and areas applicable to the products developed through our technologies. These activities will require the addition of new personnel, including management, and the development of additional expertise by existing management personnel. The inability to acquire these services or to develop this expertise could impair the growth, if any, of our business.
If we engage in any acquisitions, we will incur a variety of costs and may potentially face numerous other risks that could adversely affect our business operations.
If appropriate opportunities become available, we may consider acquiring businesses, assets, technologies, or products that we believe are a strategic fit with our business. We currently have no commitments or agreements with respect to any material acquisitions. If we do pursue such a strategy, we could
| | • | | Issue equity securities which would dilute current stockholders’ percentage ownership; |
| | • | | Incur substantial debt; or |
We may not be able to successfully integrate any businesses, assets, products, technologies, or personnel that we might acquire in the future without a significant expenditure of operating, financial, and management resources, if at all. In addition, future acquisitions might negatively impact our business relations with our collaborative partners. Further, recent accounting changes could result in a negative impact on our results of operations as well as the resulting cost of the acquisition. Any of these adverse consequences could harm our business.
We may be sued for product liability.
We may be held liable if any product we develop, or any product which is made with the use of any of our technologies, causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing, or sale. We currently have limited product liability insurance that may not fully cover our potential liabilities. In addition, if we attempt to obtain additional product liability insurance coverage, this additional insurance may be prohibitively expensive, or may not fully cover our potential liabilities. Inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of products developed by us or our collaborative partners. If we are sued for any injury caused by our products, our liability could exceed our total assets.
We are subject to anti-takeover provisions in our certificate of incorporation, bylaws, and Delaware law and have adopted a shareholder rights plan that could delay or prevent an acquisition of our company, even if the acquisition would be beneficial to our stockholders.
Provisions of our certificate of incorporation, our bylaws and Delaware law could make it more difficult for a third party to acquire us, even if doing so would be beneficial to our stockholders. In addition, we adopted a share purchase rights plan that has anti-takeover effects. The rights under the plan will cause substantial dilution to a person or group that attempts to acquire us on terms not approved by our board of directors. The rights should not interfere with any merger or other business combination approved by our board, since the rights may
33
be amended to permit such an acquisition or may be redeemed by us. These provisions in our charter documents, under Delaware law, and in our rights plan could discourage potential takeover attempts and could adversely affect the market price of our common stock. Because of these provisions, our common stockholders might not be able to receive a premium on their investment.
Our stock price has been and may continue to be particularly volatile because of the industry we are in.
The stock market, from time to time, has experienced significant price and volume fluctuations that are unrelated to the operating performance of companies. The market prices of technology companies, particularly life science companies, have been highly volatile. Our stock has been and may continue to be affected by this type of market volatility, as well as by our own performance. The following factors, among other risk factors, may have a significant effect on the market price of our common stock:
| | • | | Developments in our relationships with current or future strategic partners and collaborators; |
| | • | | Announcements of technological innovations or new products by us or our competitors; |
| | • | | Developments in patent or other proprietary rights; |
| | • | | Our ability to access genetic material from diverse ecological environments and practice our technologies; |
| | • | | Future royalties from product sales, if any, by our collaborative partners; |
| | • | | Fluctuations in our operating results; |
| | • | | Developments in domestic and international governmental policy or regulation; and |
| | • | | Economic and other external factors or other disaster or crisis. |
Concentration of ownership among our existing officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price.
Our officers, directors, and stockholders with at least 5% of our stock together controlled approximately 56.9% of our outstanding common stock as of February 28, 2006. If these officers, directors, and principal stockholders act together, they will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of directors and approval of mergers or other business combination transactions. In addition, as of February 28, 2006, Syngenta and its affiliates controlled approximately 17% of our outstanding common stock, and by themselves will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval. The interests of this concentration of ownership may not always coincide with our interests or the interests of other stockholders. For instance, officers, directors, and principal stockholders, acting together, could cause us to enter into transactions or agreements that we would not otherwise consider. Similarly, this concentration of ownership may have the effect of delaying or preventing a change in control of our company otherwise favored by our other stockholders. This concentration of ownership could depress our stock price.
Future sales of our stock by large stockholders could cause the price of our stock to decline.
A number of our stockholders hold significant amounts of our stock. For example, as of February 28, 2006, Syngenta, our largest stockholder, owned 7,963,593 shares of our common stock, or approximately 17% of our outstanding shares. All of our shares owned by Syngenta are eligible for sale in the public market subject to compliance with the applicable securities laws. We have agreed that, upon Syngenta’s request, we will file one or more registration statements under the Securities Act in order to permit Syngenta to offer and sell shares of our common stock. Sales of a substantial number of shares of our stock by our large stockholders, including Syngenta, in the public market could adversely affect the market price of our stock.
34
We use hazardous materials in our business. Any claims relating to improper handling, storage, or disposal of these materials could be time consuming and costly.
Our research and development processes involve the controlled use of hazardous materials, including chemical, radioactive, and biological materials. Our operations also produce hazardous waste products. We cannot eliminate entirely the risk of accidental contamination or discharge and any resultant injury from these materials. Federal, state, and local laws and regulations govern the use, manufacture, storage, handling, and disposal of these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our total assets. In addition, compliance with applicable environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development, or production efforts.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
None.
Our executive offices and research and development facilities are currently located in adjacent 75,000 and 60,000 square foot buildings in San Diego, California. The facilities are leased through November 2015 and March 2017, respectively. We believe that our facilities are adequate to meet our current requirements. In connection with the corporate reorganization we announced on January 5, 2006, we are consolidating our facilities. We intend to seek a subtenant to occupy the majority of the 60,000 square-foot facility.
| ITEM 3. | LEGAL PROCEEDINGS. |
In December 2002, we and certain of our officers and directors were named as defendants in a class action shareholder complaint filed in the United States District Court for the Southern District of New York, now captioned In re Diversa Corp. Initial Public Offering Sec. Litig., Case No. 02-CV-9699. In the amended complaint, the plaintiffs allege that we and certain of our officers and directors, and the underwriters (the “Underwriters”) of our initial public offering, or IPO, violated Sections 11 and 15 of the Securities Act of 1933, as amended, based on allegations that our registration statement and prospectus prepared in connection with our IPO failed to disclose material facts regarding the compensation to be received by, and the stock allocation practices of, the Underwriters. The complaint also contains claims for violation of Sections 10(b) and 20 of the Securities Exchange Act of 1934, as amended, based on allegations that this omission constituted a deceit on investors. The plaintiffs seek unspecified monetary damages and other relief. This action is related to In re Initial Public Offering Sec. Litig., Case No. 21 MC 92, in which similar complaints were filed by plaintiffs (the “Plaintiffs”) against hundreds of other public companies (collectively, the “Issuers”) that conducted IPOs of their common stock in the late 1990s and 2000 (collectively, the “IPO Cases”). On January 7, 2003, the IPO Case against us was assigned to United States Judge Shira Scheindlin of the Southern District of New York, before whom the IPO Cases have been consolidated for pretrial purposes.
In February 2003, the Court issued a decision denying the motion to dismiss the Sections 11 and 15 claims against us and our officers and directors, and granting the motion to dismiss the Section 10(b) claim against us without leave to amend. The Court similarly dismissed the Sections 10(b) and 20 claims against two of our officers and directors without leave to amend, but denied the motion to dismiss these claims against one officer/director.
In June 2003, Issuers and Plaintiffs reached a tentative settlement agreement and entered into a memorandum of understanding providing for, among other things, a dismissal with prejudice and full release of the Issuers and their officers and directors from all further liability resulting from Plaintiffs’ claims, and the assignment to Plaintiffs of certain potential claims that the Issuers may have against the Underwriters. The
35
tentative settlement also provides that, in the event that Plaintiffs ultimately recover less than a guaranteed sum of $1 billion from the Underwriters in the IPO Cases and related litigation, Plaintiffs would be entitled to payment by each participating Issuer’s insurer of a pro rata share of any shortfall in the Plaintiffs’ guaranteed recovery. In the event, for example, the Plaintiffs recover nothing in judgment against the Underwriter defendants in the IPO Cases and the Issuers’ insurers therefore become liable to the Plaintiffs for an aggregate of $1 billion pursuant to the settlement proposal, the pro rata liability of our insurers, with respect to us, would be $5 million, assuming that 200 Issuers which approved the settlement proposal, and their insurers, were operating and financially viable as of the settlement date. We are covered by a claims-made liability insurance policy that would satisfy our insurers’ pro rata liability described in this hypothetical example.
In June 2004, we executed a settlement agreement with the Plaintiffs pursuant to the terms of the memorandum of understanding. On February 15, 2005, the Court issued a decision certifying a class action for settlement purposes and granting preliminary approval of the settlement subject to modification of certain bar orders contemplated by the settlement. In addition, the settlement is still subject to statutory notice requirements as well as final judicial approval.
On August 31, 2005, the Court reaffirmed class certification and preliminary approval of the modified settlement in a comprehensive order. In addition, the Court approved the form of notice to be sent to members of the settlement classes, which was published and mailed beginning November 15, 2005. The Court has set a final settlement fairness hearing on the settlement for April 24, 2006. The settlement is still subject to statutory notice requirements as well as final judicial approval. We are covered by a claims-made liability insurance policy which we believe will satisfy our insurers’ pro-rata liability under this settlement.
We are also, from time to time, subject to legal proceedings and claims which arise in the normal course of business. In our opinion, the amount of ultimate liability with respect to these actions will not have a material adverse effect on our consolidated financial position, results of operations, or cash flows.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS. |
No matters were submitted to a vote of security holders during the quarter ended December 31, 2005.
36
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
(a) Our common stock is traded on the Nasdaq National Market under the symbol DVSA. The following table sets forth the high and low sale prices for our common stock for the periods indicated, as reported on the Nasdaq National Market. Such quotations represent inter-dealer prices without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| | | | | | |
| | | High
| | Low
|
2005 | | | | | | |
First Quarter | | $ | 8.80 | | $ | 4.96 |
Second Quarter | | | 5.86 | | | 4.65 |
Third Quarter | | | 6.25 | | | 4.55 |
Fourth Quarter | | | 5.91 | | | 4.55 |
| | |
| | | High
| | Low
|
2004 | | | | | | |
First Quarter | | $ | 12.30 | | $ | 7.95 |
Second Quarter | | | 10.52 | | | 8.31 |
Third Quarter | | | 10.26 | | | 7.20 |
Fourth Quarter | | | 9.50 | | | 7.92 |
As of February 28, 2006, there were approximately 168 holders of record of our common stock. We have never declared or paid any cash dividends on our capital stock. On February 28, 2006, the last sale price reported on the Nasdaq National Market for our common stock was $7.91 per share. We currently intend to retain future earnings, if any, for development of our business and, therefore, do not anticipate that we will declare or pay cash dividends on our capital stock in the foreseeable future.
(b) The effective date of our first registration statement, filed on Form S-1 under the Securities Act (No. 333-92853) relating to our initial public offering of common stock, was February 11, 2000. In addition, in accordance with Rule 462(b) under the Securities Act, we filed a subsequent registration statement on Form S-1 (No. 333-30290) that related to the first registration statement that we had filed for our initial public offering of common stock, and the effective date of that subsequent registration statement was February 14, 2000. Under the two registration statements, we sold a total of 8,337,500 shares of our common stock at a price of $24.00 per share to an underwriting syndicate led by Bear, Stearns & Co. Inc., Chase H&Q, and Deutsche Banc Alex. Brown. Of these 8,337,500 shares, 1,087,500 were issued upon exercise of the underwriters’ over-allotment option. The initial public offering resulted in gross proceeds of $200.1 million, $14.0 million of which was applied toward the underwriting discount. Expenses related to the offering totaled approximately $1.6 million. Net proceeds to us were approximately $184.5 million. From the time of receipt through December 31, 2005, approximately $16.6 million of the net proceeds had been used to purchase property and equipment and approximately $102.7 million had been used for general corporate purposes. The balance is invested in cash equivalents and short-term investments.
The equity compensation plan information required by this item is incorporated by reference to Part III, Item 12 entitled “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” in this annual report on Form 10-K.
37
| ITEM 6. | SELECTED FINANCIAL DATA. |
The selected consolidated financial data set forth below with respect to our consolidated statements of operations for the years ended December 31, 2005, 2004, and 2003, and with respect to our balance sheets at December 31, 2005 and 2004 are derived from our consolidated financial statements that have been audited by Ernst & Young LLP, which are included elsewhere in this report, and are qualified by reference to such consolidated financial statements. The consolidated statement of operations data for the years ended December 31, 2002 and 2001 and the balance sheet data as of December 31, 2003, 2002, and 2001 are derived from our audited consolidated financial statements that are not included in this report. The selected financial information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes appearing elsewhere in this report on Form 10-K.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| | | 2002
| | | 2001
| |
| | | (in thousands, except per share data) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Collaborative revenue | | $ | 34,392 | | | $ | 41,897 | | | $ | 41,980 | | | $ | 30,276 | | | $ | 34,936 | |
Grant revenue | | | 10,079 | | | | 10,241 | | | | 3,923 | | | | 1,047 | | | | 910 | |
Product-related revenue | | | 9,832 | | | | 5,412 | | | | 3,056 | | | | 332 | | | | 193 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total revenue | | | 54,303 | | | | 57,550 | | | | 48,959 | | | | 31,655 | | | | 36,039 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Cost of product-related revenue | | | 10,662 | | | | 3,698 | | | | 2,997 | | | | 66 | | | | 30 | |
Research and development | | | 72,276 | | | | 73,405 | | | | 70,657 | | | | 50,096 | | | | 48,042 | |
Write-off of acquired in-process research and development | | | — | | | | — | | | | 19,478 | | | | — | | | | — | |
Selling, general and administrative | | | 12,588 | | | | 11,607 | | | | 12,181 | | | | 10,269 | | | | 10,102 | |
Amortization of acquired intangible assets | | | 2,602 | | | | 2,598 | | | | 2,290 | | | | 156 | | | | 156 | |
Non-cash, stock-based compensation | | | 877 | | | | — | | | | 131 | | | | 701 | | | | 2,544 | |
Asset impairment charges | | | 45,745 | | | | — | | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Total operating expenses | | | 144,750 | | | | 91,308 | | | | 107,734 | | | | 61,288 | | | | 60,874 | |
Operating loss | | | (90,447 | ) | | | (33,758 | ) | | | (58,775 | ) | | | (29,633 | ) | | | (24,835 | ) |
Interest and other income, net | | | 729 | | | | 333 | | | | 1,079 | | | | 1,646 | | | | 9,171 | |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net loss | | | (89,718 | ) | | | (33,425 | ) | | | (57,696 | ) | | | (27,987 | ) | | | (15,664 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Net loss per share, basic and diluted | | $ | (2.04 | ) | | $ | (0.77 | ) | | $ | (1.39 | ) | | $ | (0.79 | ) | | $ | (0.44 | ) |
| | |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Weighted average shares outstanding | | | 44,064 | | | | 43,416 | | | | 41,592 | | | | 35,650 | | | | 35,243 | |
| |
| | | As of December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| | | 2002
| | | 2001
| |
| | | (in thousands) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 65,428 | | | $ | 98,193 | | | $ | 127,483 | | | $ | 163,096 | | | $ | 190,700 | |
Working capital | | | 53,753 | | | | 82,931 | | | | 104,609 | | | | 142,394 | | | | 172,049 | |
Total assets | | | 98,069 | | | | 184,056 | | | | 221,323 | | | | 197,197 | | | | 227,678 | |
Long-term debt, less current portion | | | 6,332 | | | | 8,825 | | | | 10,131 | | | | 11,884 | | | | 12,421 | |
Stockholders’ equity | | | 64,804 | | | | 150,946 | | | | 181,443 | | | | 157,315 | | | | 183,614 | |
38
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
The following discussion of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and the notes to those statements included elsewhere in this report.
Except for the historical information contained herein, the following discussion contains forward-looking statements that involve risks and uncertainties. These statements speak only as of the date on which they are made, and we undertake no obligation to update any forward-looking statement. Forward-looking statements include statements related to investments in our core technologies, investments in our internal product candidates, our future revenues and net losses, and our future capital requirements, all of which are prospective. Such statements are only predictions, and the actual events or results may differ materially from those projected in the forward-looking statements. Factors that could cause or contribute to differences include, but are not limited to, risks involved with our new and uncertain technologies, risks associated with our dependence on patents and proprietary rights, risks associated with our protection and enforcement of our patents and proprietary rights, our dependence on existing collaborations, our ability to enter into and/or maintain collaboration and joint venture agreements, our ability to commercialize products directly and through our collaborators, the timing of anticipated regulatory approvals and product launches, and the development or availability of competitive products or technologies, as well as other risks and uncertainties set forth below and in the section of this report entitled “Risk Factors.”
Overview
We were incorporated in December 1992 and began operations in May 1994. We are a leader in applying proprietary genomic technologies for the rapid discovery and optimization of novel protein-based products, including (a) enzymes, which are catalytic proteins, and (b) antibodies, which are binding proteins. We are directing our integrated portfolio of technologies to the discovery, evolution, and production of commercially valuable molecules with agricultural, industrial, and chemical applications, such as enzymes, as well as optimized antibodies with pharmaceutical applications. While our technologies have the potential to serve many large markets, our key areas of focus for internal product development are alternative fuels, specialty industrial processes, and health and nutrition. In addition to our internal product development efforts, we have formed alliances with market leaders, such as BASF, Cargill Health and Food Technologies, DuPont Bio-Based Materials, Medarex, Merck, and Xoma. We have also formed a broad strategic relationship with Syngenta AG, a world-leading agribusiness company. We have two inactive subsidiaries, Innovase LLC and TNEWCO Inc.
In January 2006, following a comprehensive review of our operations, we announced a strategic reorganization designed to focus our resources on advancing our most promising products and product candidates in three key areas: alternative fuels; specialty industrial processes; and health and nutrition. As a result of this decision, we discontinued development of a number of less promising products and programs and reduced our workforce by 83 employees. In the fourth quarter of 2005, we recorded a non-cash impairment charge of $45.7 million, and we expect to record additional charges of $15.0 to $17.0 million in 2006 related to employee separation and facilities consolidation costs.
We have dedicated substantial resources to the development of our proprietary technologies, which include capabilities for sample collection from the world’s microbial populations, generation of environmental libraries, screening of these libraries using ultra high-throughput methods capable of analyzing more than one billion genes per day, and optimization based on our gene evolution technologies.
In February 2003, we completed a series of transactions with Syngenta. Under the transactions, we formed an extensive research collaboration whereby we are entitled to receive a minimum of $118.0 million in research and development funding over the initial seven-year term of the agreement and will be eligible to receive certain milestone payments and royalties upon product development and commercialization. Additionally, we acquired certain intellectual property rights licenses used in activities that primarily involve tools, technologies and
39
methods relating to proteomics, metabolomics, RNA dynamics, and bioinformatics and methods to analyze and link these components of genomics or that primarily relate to their fungal program, for use outside of Syngenta’s exclusive field as defined in the research collaboration agreement. We also purchased certain property and equipment. As of December 31, 2005, in connection with our strategic reorganization, we assessed the carrying values of these assets and technologies on our balance sheet and determined that such assets and technologies were impaired. As a result, we have written off the carrying value of these assets and technologies on our balance sheet as of December 31, 2005.
In May 2004, we entered into an agreement with Syngenta that strengthened our research and product development alliance and continued the development and commercialization of novel animal feed enzymes beyond the five-year initial term of our 1999 Zymetrics joint venture agreement, which ended in 2004. Under the agreement, Syngenta agreed to continue its collaboration with us in the area of animal feed enzymes on an exclusive basis, paid us an exclusivity fee, and agreed to continued research and development funding.
As of December 31, 2005, Syngenta is committed to pay us more than $50.0 million in additional research funding under our current collaboration.
For the year ended December 31, 2005, total revenues decreased 6% compared to the year ended December 31, 2004, while product-related revenue increased 82% over the same period. We expect that product-related revenue will represent a larger percentage of our total revenues in the future. For 2006, we expect to de-emphasize grant revenue and certain collaborations that are not strategic to our current market focus; however, certain of our partners and funding sources have ongoing obligations to fund our programs, and we have ongoing obligations to provide research and development services under our current agreements. As of December 31, 2005, our strategic partners have provided us with approximately $240.0 million in funding since inception and are committed to additional funding of more than $60.0 million through 2010, subject to our performance under existing agreements, excluding milestone payments, license and commercialization fees, and royalties or profit sharing. Our strategic partners often pay us before we recognize the revenue, and these payments are deferred until earned. As of December 31, 2005, we had deferred revenue totaling $8.8 million.
We have incurred net losses since our inception. As of December 31, 2005, our accumulated deficit, including $45.7 million in non-cash asset impairment charges in 2005, was $290.2 million. Our results of operations have fluctuated from period to period and likely will continue to fluctuate substantially in the future. We expect to incur losses for the next two fiscal years as we continue to develop independent products and as a result of our continued investment in sales and marketing infrastructure intended to strengthen our customer contact and focus, our continued investment in manufacturing facilities necessary to meet increased demand for our products, as well continued research and development expenses for our internal product candidates. Results of operations for any period may be unrelated to results of operations for any other period. In addition, we believe that our historical results are not a good indicator of our future operating results.
Recent Events
On October 5, 2005, Jay M. Short, Ph.D. resigned as our President and Chief Executive Officer, and our Board of Directors appointed Edward T. Shonsey, our then Executive Vice President of Internal Development, as our Chief Executive Officer on an interim basis. Upon his appointment, Mr. Shonsey began working with existing management to review all of our programs and operations with the goal to accelerate the development and commercialization of products which we believe have the greatest potential, while we reduce unnecessary expenditures.
Our management reported the results of this review and its preliminary recommendations to our Board of Directors in December 2005. Our Board of Directors approved our reorganization plan on January 4, 2006, and on January 5, 2006, we announced a strategic reorganization, pursuant to which we plan to focus our resources on advancing our most promising product candidates and programs that have the greatest near-term opportunities. As part of this reorganization, we will eliminate and/or significantly scale back our investments in
40
certain programs and lines of business which are not consistent with our current strategic focus, which is to market and develop commercial products that address needs in the following categories:
| | 2) | Specialty Industrial Processes, and; |
We also announced that we will no longer invest in developing pharmaceutical products which are not partnered.
As a result of these actions, we reduced our workforce by 83 employees and plan to consolidate our facilities. In connection with the reorganization, during the fourth quarter of 2005, we recorded a non-cash impairment charge of $45.7 million, which consisted primarily of the write-off of approximately $43.5 million of previously recorded long-lived intangible assets that are no longer essential to our focus, or we otherwise determined to be impaired under current accounting rules. We expect to incur additional restructuring charges of approximately $15.0 to $17.0 million, primarily in the first quarter of 2006, related to employee separation and facilities consolidation costs.
We believe that these recent actions will enable us to reduce our loss and cash usage while we realize the greatest value from our programs and move toward profitability.
Results of Operations
Years Ended December 31, 2005 and 2004
Revenues
Revenues decreased 6%, or $3.2 million, to $54.3 million for the year ended December 31, 2005 from $57.6 million for the year ended December 31, 2004, attributed primarily to a decrease in collaborative revenue, offset in part by an increase in product-related revenues.
Collaborative revenue decreased 18%, or $7.5 million, to $34.4 million from $41.9 million and accounted for 63% and 73% of total revenue for the years ended December 31, 2005 and 2004. This decrease resulted primarily from the amortization in 2004 of certain one-time fees related to our collaboration with Syngenta. These fees were fully amortized during 2004 and were not repeated in 2005.
Product-related revenue for the year ended December 31, 2005 increased 82% to $9.8 million from $5.4 million for the year ended December 31, 2004. This increase was attributable primarily to increased revenue and profit sharing associated with Phyzyme™ XP phytase sold through our collaboration with Danisco Animal Nutrition, or Danisco, and, to a lesser extent, sales of our Ultra-Thin™ enzyme, which we began selling through our distributor, Valley Research, during the last half of 2005.
Grant revenue remained flat at $10.1 million for the year ended December 31, 2005 as compared to $10.2 million for the year ended December 31, 2004.
Our revenues have historically fluctuated from period to period and likely will continue to fluctuate substantially in the future based upon the timing and composition of funding under existing and future collaboration agreements, regulatory approval timelines for new products, as well as adoption rates of our new and existing commercial products. We anticipate that our revenue mix will continue to shift toward a higher percentage of product-related revenue. For 2006, we expect to de-emphasize grant revenue and certain collaborations that are not strategic to our current market focus.
41
Cost of Product-Related Revenue
Cost of product-related revenue includes both fixed and variable costs, including materials and supplies, labor, facilities and other overhead costs, associated with our product-related revenues. For the year ended December 31, 2005, these costs increased $7.0 million to $10.7 million compared to $3.7 million for the year ended December 31, 2004. This increase resulted from an increase in fixed costs related to expanded manufacturing capacity to support anticipated growth in product-related revenues, an increase in product-related revenues, and, to a lesser extent, charges for inventory obsolescence. Cost of product-related revenue was 108% of product-related revenues in 2005 compared to 68% of product-related revenues for the year ended December 31, 2004. During the fourth quarter of 2005, we generated a positive gross margin of approximately 17%. We expect that the cost of product-related revenue will decrease as a percentage of product-related revenue once we have completed our manufacturing ramp-up and have achieved a scalable volume of product sales. However, our gross margins are dependent upon the mix of product-related sales as the cost of product-related revenue varies from product to product. We expect our gross margins in 2006 to be positively impact by continued growth in sales of Phyzyme XP through our relationship with Danisco. Under our manufacturing agreement, we supply Danisco commercial quantities of Phyzyme XP at our cost. We are paid a royalty on related product sales made by Danisco equal to 50% of the cumulative profits generated by Danisco on such sales. We only began receiving profit share revenue during mid-2005, but expect a full year of profit share revenue during 2006.
Research and Development
Research and development expenses consist primarily of costs associated with internal development of our technologies and our product candidates and costs associated with research activities performed on behalf of our collaborators. We track our researchers’ time by type of project. However, we do not track other research and development costs by project; rather, we track such costs by the type of cost incurred.
For the year ended December 31, 2005, we estimate that approximately 64% of our research and development personnel costs, based upon hours incurred, were spent on research activities funded by our collaborators and grants, and that 36% were spent on internal product and technology development. By comparison, for the year ended December 31, 2004 we estimated that approximately 60% of our research and development personnel costs, based upon hours incurred, were spent on research activities funded by our collaborators and grants, and that 40% were spent on internal product and technology development. Our research and development expenses decreased $1.1 million to $72.3 million for the year ended December 31, 2005 from $73.4 million for the year ended December 31, 2004. This was attributed in large part to a $3.9 million decrease in personnel costs for direct research and development. Our direct research and development staff decreased to 214 at December 31, 2005 from 297 at December 31, 2004. The decrease in staff was primarily due to a realignment of our workforce in early 2005 to more closely focus on the sales and marketing of our new and existing products and the development of other product candidates. The decrease in personnel costs for direct research and development was offset in large part by an increase in outside services costs under our Syngenta collaboration and costs during the first half of 2005 associated with our internal pharmaceutical development programs, as well as an increase in costs to support our internal product development efforts, including increases in laboratory and supplies expense, third party outside services costs, indirect support personnel, depreciation and facilities and overhead-related costs.
42
Research and development direct costs and unallocated costs incurred by type of project during the years ended December 31, 2005 and 2004 were as follows (in thousands):
| | | | | | |
| | | 2005
| | 2004
|
Collaborations: | | | | | | |
Syngenta | | $ | 6,433 | | $ | 7,780 |
Other | | | 4,969 | | | 3,918 |
| | |
|
| |
|
|
Total collaborations | | | 11,402 | | | 11,698 |
Grants | | | 4,573 | | | 5,484 |
Internal development | | | 9,006 | | | 11,698 |
Unallocated | | | 47,295 | | | 44,525 |
| | |
|
| |
|
|
| | | $ | 72,276 | | $ | 73,405 |
| | |
|
| |
|
|
Research and development costs based upon type of cost incurred for the years ended December 31, 2005 and 2004 were as follows (in thousands):
| | | | | | |
| | | 2005
| | 2004
|
Personnel related | | $ | 24,981 | | $ | 28,880 |
Laboratory and supplies | | | 9,609 | | | 9,191 |
Outside services | | | 11,071 | | | 10,331 |
Equipment and depreciation | | | 9,282 | | | 9,147 |
Facilities, overhead and other | | | 17,333 | | | 15,856 |
| | |
|
| |
|
|
| | | $ | 72,276 | | $ | 73,405 |
| | |
|
| |
|
|
We have only recently launched our first products. Additionally, we have several product candidates in various stages of development related to collaborations and grants as well as internally developed products. It can take many years from the initial decision to perform research through development until products, if any, are ultimately marketed. The outcome of research is unknown until each stage of testing is completed, up through and including product trials and regulatory approvals, if needed. Accordingly, we are unable to predict which potential product candidates we may proceed with, the time and costs to complete development, and ultimately whether we will have any products approved by the appropriate regulatory bodies. The various risks associated with our research and development activities are discussed more fully in this report under “Risk Factors.” While we anticipate that we will continue to dedicate significant resources to development of future products, we expect that our total research and development costs will decrease in future periods as we position our Company to focus on near-term commercialization, sales and marketing of products, and reducing our loss and cash usage.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased 8%, or $1.0 million, to $12.6 million for the year ended December 31, 2005 from $11.6 million for the year ended December 31, 2004. This increase was primarily related to personnel costs to support sales and marketing for our new and existing products. We expect our sales and marketing expenses to remain at comparable levels, or increase, in future periods as we introduce new products and invest in the necessary infrastructure to support our anticipated increase in product revenue.
Amortization of Acquired Intangible Assets
We recorded amortization of acquired intangible assets of approximately $2.6 million for each of the years ended December 31, 2005 and 2004, primarily associated with the February 2003 acquisition of intellectual property rights licenses from Syngenta, which we were amortizing over 7 to 15 years. As more fully described below, we recorded an impairment charge related to our intangible assets during the fourth quarter of 2005.
43
Non-Cash, Stock-Based Compensation Charges
We recorded net stock-based deferred compensation of approximately $3.9 million during the year ended December 31, 2005 related to the granting of restricted stock awards to employees. We amortize the deferred compensation balance to expense on a straight-line basis over the vesting period of the awards, which is generally four years. We recorded net expense of $0.8 million related to the amortization of deferred stock-based compensation during the year ended December 31, 2005. We also recorded a charge of approximately $0.1 million during the fourth quarter of 2005 related to the acceleration of vesting on certain restricted shares granted to our former Chief Executive Officer.
Effective for fiscal year 2006, we will implement the provisions of Financial Accounting Standards Board Statement, or FASB, No. 123R, “Share-Based Payment,” which requires us to record a charge to expense equal to the fair value of all options and restricted stock awards issued to employees. We expect to recognize non-cash charges for stock options and restricted stock awards of approximately $2.5 million to $3.0 million in 2006, based on options and restricted stock awards to date. The exact amount of our stock-based compensation charges will fluctuate based on future awards and cancellations of options and restricted stock, as well as variability in our stock price, expected option exercise behavior, and expected option termination.
During the fourth quarter of fiscal 2005, we accelerated the vesting of unvested stock options awarded to all employees and officers under our stock option plan that had exercise prices greater than $10.00. The unvested options to purchase approximately 710,000 shares became fully vested as of December 8, 2005 as a result of this acceleration. These stock options would have all become fully vested before or during 2008. We accelerated these options because the options had exercise prices significantly in excess of then current market value ($5.25 at December 8, 2005), and thus were not fully achieving their original objectives of incentive compensation and employee retention. We expect the acceleration to have a positive effect on employee morale, retention, and perception of value. The acceleration also eliminated future compensation expense we would otherwise recognize in our statements of operations with respect to these options with the implementation of FASB No. 123R, which becomes effective for our fiscal year 2006. The future expense eliminated as a result of the acceleration of the vesting of these options is approximately $1.1 million.
Asset Impairment Charges
During the fourth quarter of 2005, we recorded a $45.7 million impairment charge for activities resulting from management’s strategic decision to reorganize and refocus our resources to advance our most promising product candidates and programs that have the greatest near-term opportunities. As a result, in 2005, we recorded write-downs to the carrying value of tangible and intangible assets considered non-essential to our current focus, or otherwise deemed impaired under the provisions set forth by FASB No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.”
These charges are summarized below (in thousands):
| | | |
| | | Year Ended
December 31,
2005
|
Write-off of intangible assets acquired in connection with fiscal 2003 transactions with Syngenta | | $ | 40,622 |
Excess or idle equipment costs | | | 2,237 |
Write-off of intellectual property licenses | | | 2,886 |
| | |
|
|
Total | | $ | 45,745 |
| | |
|
|
In connection with the decision to reorganize and refocus our resources, in January 2006 we commenced several cost containment measures, including a reduction in workforce of 83 employees and the consolidation of our facilities. Pursuant to FASB No. 146,“Accounting for Costs Associated with Exit or Disposal Activities,” we expect to record restructuring charges of approximately $15.0 to $17.0 million, primarily in the first quarter of 2006, related to these actions.
44
Interest Income, net
Interest income on cash and short-term investments was $2.0 million for the year ended December 31, 2005 compared to $1.8 million for the year ended December 31, 2004. The increase was primarily due to higher average rates of return on our investments, consistent with the increase in short-term interest rates from 2004 to 2005. This increase was offset in large part by a decrease in cash and investment balances during 2005. Interest expense was $1.3 million and $1.7 million for the years ended December 31, 2005 and 2004. The decrease in interest expense was primarily due to lower debt balances in 2005 as compared to 2004.
Other Income (Expense), net
We recorded other income of $0.2 million for the year ended December 31, 2004. Other income is the result of miscellaneous projects undertaken for customers which are not related to our core business. There was no such income recorded for the year ended December 31, 2005.
Provision for Income Taxes
For the years ended December 31, 2005 and 2004, we incurred net operating losses and, accordingly, did not record a provision for income taxes. As of December 31, 2005, we had federal net operating loss carry-forwards of approximately $197.1 million, which begin to expire in 2011 unless utilized. Our net operating loss carry-forwards for state tax purposes were approximately $54.2 million as of December 31, 2005, which will begin to expire in 2006 unless utilized. We also had federal research credits of approximately $5.2 million which will begin to expire in 2011, California research credits of approximately $3.9 million which will carry over indefinitely, and California manufacturer’s investment credits of approximately $0.7 million which will begin to expire in 2010. Our utilization of the net operating losses and credits may be subject to substantial annual limitations pursuant to Section 382 of the Internal Revenue Code, and similar state provisions, as a result of changes in our ownership structure. The annual limitations may result in the expiration of a portion of our net operating loss carry-forwards and credits.
Years Ended December 31, 2004 and 2003
Revenues
Our revenues increased $8.6 million or 18% to $57.6 million for the year ended December 31, 2004 from $49.0 million for the year ended December 31, 2003. This increase was due to an increase of $6.3 million in grant revenue, and an increase in product related-revenue of $2.4 million. Collaborative revenue, which totaled $41.9 million was consistent with 2003 collaborative revenue, and accounted for 73% of total revenue for the year ended December 31, 2004 and 86% of total revenue for the year ended December 31, 2003.
Cost of Product-Related Revenue
For the year ended December 31, 2004, costs of product-related revenue increased $0.7 million to $3.7 million compared to $3.0 million for the year ended December 31, 2003. This increase resulted from higher product-related revenue. Cost of product-related revenue was 68% of product-related revenues in 2004 compared to 98% of product-related revenues for the year ended December 31, 2003.
Research and Development
For the year ended December 31, 2004, we estimate that approximately 60% of our research and development personnel costs, based upon hours incurred, were spent on research activities funded by our collaborators and grants, and that 40% were spent on internal product and technology development. Our research and development expenses increased $2.7 million to $73.4 million for the year ended December 31, 2004 from $70.7 million for the year ended December 31, 2003. The increase was primarily due to an increase of $3.7
45
million for outside services related to legal costs for protection and maintenance of intellectual property as well as costs associated with regulatory and grant activities. This increase was somewhat offset by a decrease in facilities related costs including a $1.6 million reduction in information technology costs.
Costs for research and development personnel for the year ended December 31, 2004 increased by $0.6 million over the year ended December 31, 2003. Our research staff decreased to 279 at December 31, 2004 from 305 at December 31, 2003. The decrease in staff was primarily due to a realignment of our workforce in late 2004 to more closely focus on the sales and marketing of our new and existing products and the development of other product candidates.
Research and development direct costs and unallocated costs incurred by type of project during the years ended December 31, 2004 and 2003 were as follows (in thousands):
| | | | | | |
| | | 2004
| | 2003
|
Collaborations: | | | | | | |
Syngenta | | $ | 7,780 | | $ | 8,444 |
Other | | | 3,918 | | | 2,509 |
| | |
|
| |
|
|
Total collaborations | | | 11,698 | | | 10,953 |
Grants | | | 5,484 | | | 2,903 |
Internal development | | | 11,698 | | | 14,394 |
Unallocated | | | 44,525 | | | 42,407 |
| | |
|
| |
|
|
| | | $ | 73,405 | | $ | 70,657 |
| | |
|
| |
|
|
Research and development costs based upon type of cost incurred for the years ended December 31, 2004 and 2003 were as follows (in thousands):
| | | | | | |
| | | 2004
| | 2003
|
Personnel related | | $ | 28,880 | | $ | 28,250 |
Laboratory and supplies | | | 9,191 | | | 8,808 |
Outside services | | | 10,331 | | | 6,601 |
Equipment and depreciation | | | 9,147 | | | 8,062 |
Facilities and other | | | 15,856 | | | 18,936 |
| | |
|
| |
|
|
| | | $ | 73,405 | | $ | 70,657 |
| | |
|
| |
|
|
Selling, General and Administrative Expenses
Our selling, general and administrative expenses decreased $0.6 million to $11.6 million for the year ended December 31, 2004 from $12.2 million for the year ended December 31, 2003. The decrease was primarily attributable to reduced salary expense associated with employee bonuses and lower administrative costs associated with Zymetrics, Inc., our former contract joint venture with Syngenta, which is now managed exclusively by Syngenta. Included in selling, general and administrative expenses for the year ended December 31, 2004 is $0.5 million in third-party consulting and attestation services related to compliance with Sarbanes-Oxley Section 404.
Amortization of Acquired Intangible Assets
For the year ended December 31, 2004, we recorded amortization of acquired intangible assets of approximately $2.6 million, compared to $2.3 million for the year ended December 31, 2003. The increase was associated with the February 2003 acquisition of intellectual property rights licenses from Syngenta.
46
Non-Cash, Stock-Based Compensation Charges
For the year ended December 31, 2004, we did not incur any amortization charges of deferred compensation, as it was fully amortized at the end of 2003.
Interest Income, net
Interest income on cash and short-term investments was $1.8 million for the year ended December 31, 2004 compared to $3.5 million for the year ended December 31, 2003. The decrease was primarily due to lower average cash balances during 2004. Interest expense was $1.7 million and $1.4 million for the years ended December 31, 2004 and 2003.
Other Income (Expense), net
We recorded other income of $0.2 million for the years ended December 31, 2004 and 2003. Other income is the result of miscellaneous projects undertaken for customers which are not related to our core business.
Equity in Loss of Joint Venture
Our subsidiary, Innovase LLC, was formed in June 2000 and was formerly an industrial enzyme joint venture between us and The Dow Chemical Company (“Dow”). Under the terms of the joint venture agreement, we were required to fund certain operating expenses of Innovase LLC. Our share of the net income or loss of Innovase LLC, excluding certain expenses for which Dow was solely responsible under the joint venture agreement, was recorded utilizing the equity method of accounting. In December 2003, we signed a definitive agreement with Dow to restructure the Innovase joint venture. Under the terms of the restructuring agreement, rights to all products and product candidates developed by Innovase were conveyed to us, and the companies mutually agreed to terminate all exclusivity and non-competition arrangements for the industrial enzyme fields related to the Innovase joint venture. Dow divested its 50% ownership interest to us and paid us $5.0 million. All joint venture related agreements involving Dow and us were terminated.
Costs related to Zymetrics, Inc., our contract joint venture with Syngenta Seeds AG, were recorded as selling, general and administrative expense as incurred, as we did not have an ownership interest in Zymetrics, Inc. Zymetrics is now known as Syngenta Animal Nutrition and managed exclusively by Syngenta.
Provision for Income Taxes
For the years ended December 31, 2004 and 2003, we incurred net operating losses and, accordingly, did not record a provision for income taxes. As of December 31, 2004, we had federal net operating loss carry-forwards of approximately $147.8 million, which begin to expire in 2011 unless utilized. Our net operating loss carry-forwards for state tax purposes were approximately $35.8 million as of December 31, 2004, which will begin to expire in 2006 unless utilized. We also had federal research credits of approximately $5.0 million which will begin to expire in 2011, California research credits of approximately $3.4 million which will carryover indefinitely, and California manufacturer’s investment credits of approximately $0.7 million which will begin to expire in 2010. Our utilization of the net operating losses and credits may be subject to substantial annual limitations pursuant to Section 382 of the Internal Revenue Code, and similar state provisions, as a result of changes in our ownership structure. The annual limitations may result in the expiration of a portion of our net operating loss carry-forwards and credits.
Liquidity and Capital Resources
Since inception, we have financed our business primarily through the sale of common and preferred stock and funding from strategic partners and government grants. As of December 31, 2005, our strategic partners have provided us approximately $240.0 million in funding since inception and are also committed to additional funding of more than more than $60.0 million through 2010, subject to our performance under existing agreements,
47
excluding milestone payments, license and commercialization fees, and royalties or profit sharing. Future committed funding is subject to our performance under existing agreements, and excludes milestone payments, license and commercialization fees, and royalties or profit sharing. Our future committed funding is concentrated within a limited number of collaborators. Our failure to successfully maintain our relationship with these collaborators could have a material adverse impact on our operating results and financial condition.
As of December 31, 2005, we had cash, cash equivalents, and short-term investments of approximately $65.4 million. Our short-term investments as of such date consisted primarily of U.S. Treasury and government agency obligations and investment-grade corporate obligations. Historically, we have funded our capital equipment purchases through available cash, capital leases and equipment financing line of credit agreements.
During 2002, we entered into a manufacturing agreement with Fermic to provide us with the capacity to produce commercial quantities of certain enzyme products. Based on actual and projected increased product requirements, the agreement was amended in 2004 to provide for additional capacity to be installed over the succeeding four year period. Under the terms of the agreement, we can cancel the committed purchases with thirty months’ notice provided that the term of the agreement, including the termination notice period, aggregates four years. Pursuant to our agreement with Fermic, we are also obligated to reimburse monthly costs related to manufacturing activities. These costs scale up as our projected manufacturing volume increases. As of December 31, 2005, under this agreement we have made minimum commitments to Fermic of approximately $15.5 million, over the next three years. In addition, under the terms of the agreement, we are required to purchase certain equipment required for fermentation and downstream processing of the products. Through December 31, 2005, we had incurred costs of approximately $10.5 million for equipment related to this agreement.
We purchased capital equipment totaling $7.3 million during 2005, and financed approximately $5.5 million of these purchases through our existing financing arrangements. We anticipate the cost of capital equipment required to support the ongoing needs of our existing research could be as much as $8.0 million for 2006. We anticipate funding our capital requirements for 2006 with our existing bank credit facility, and with available cash.
Our operating activities used cash of $23.7 million for the year ended December 31, 2005. Our cash used by operating activities consisted primarily of cash used to fund our net loss of $89.7 million, offset in large part by non-cash impairment charges of $45.7 million and depreciation and amortization of $17.7 million.
Our investing activities provided cash of $35.7 million for the year ended December 31, 2005. Our investing activities consisted primarily of a net decrease in short-term investments of $43.0 million to fund operations, partially offset by purchases of property and equipment of $7.3 million.
Our financing activities used cash of $1.9 million for the year ended December 31, 2005. Our financing activities consisted primarily of borrowings under our equipment financing arrangements of $5.5 million and proceeds from the sale of common stock under our Employee Stock Purchase Plan and from the exercise of stock options of $2.6 million, offset by payments on notes payable and capital leases of $10.0 million.
48
The following table summarizes our contractual obligations at December 31, 2005 (in thousands):
| | | | | | | | | | | | | | | |
| | | | | Payments due by period
|
| | | Total
| | Less Than
1 Year
| | 1-3 Years
| | 3-5 Years
| | After 5 Years
|
Contractual Obligations | | | | | | | | | | | | | | | |
Long-term debt | | $ | 14,673 | | $ | 7,882 | | $ | 6,396 | | $ | 395 | | $ | — |
Operating leases | | | 56,410 | | | 4,663 | | | 9,827 | | | 10,515 | | | 31,405 |
Manufacturing costs to Fermic | | | 15,480 | | | 5,940 | | | 9,540 | | | — | | | — |
License and research agreements | | | 1,563 | | | 403 | | | 535 | | | 250 | | | 375 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total Contractual Obligations | | $ | 88,126 | | $ | 18,888 | | $ | 26,298 | | $ | 11,160 | | $ | 31,780 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
During 2006, we anticipate funding as much as $5.0 million in additional equipment costs related to our manufacturing agreement with Fermic. As we continue to develop our commercial manufacturing platforms, we will be required to purchase additional capital equipment under this agreement.
On September 30, 2005 we entered into a $14.6 million Loan and Security Agreement, or the Bank Agreement, with a commercial bank, or the Bank. The Bank Agreement provides for a one-year credit facility for up to $10.0 million in financing for qualified equipment purchases, or the Equipment Advances, in the United States and Mexico, and a $4.6 million letter of credit sub-facility, or the Letter of Credit Sublimit. Borrowings under the Equipment Advances will be structured as promissory notes which are secured by qualified equipment purchases and repaid over 36 to 48 months, depending on the location of the equipment financed. Borrowings will bear interest at the Bank’s prime rate (7.25% at December 31, 2005) plus 0.75%. Amounts outstanding under the Letter of Credit Sublimit are unsecured, and are subject to an annual fee of 1.25%.
The Bank Agreement contains standard affirmative and negative covenants and restrictions on actions by us, including, but not limited to, activity related to our common stock repurchases, liens, investments, indebtedness, and fundamental changes in, or dispositions of, our assets. Certain of these actions may be taken by us with the consent of the Bank. In addition, we are required to meet certain financial covenants, primarily a minimum balance of unrestricted cash, cash equivalents, and investments in marketable securities of $25.0 million, including $15.0 million maintained in accounts at the Bank or its affiliates.
At December 31, 2005, there was approximately $1.6 million in outstanding borrowings under the Equipment Advances and a letter of credit for approximately $4.6 million under the Letter of Credit Sublimit, as required under our facilities leases. We lease approximately 140,000 square feet of space in San Diego, California under two separate operating leases. Under the terms of the leases, we are required to maintain an irrevocable standby letter of credit from a bank in lieu of a cash security deposit. The amount of the letter of credit is based upon certain financial covenants requiring minimum market capitalization or working capital.
On January 5, 2006, we announced a strategic reorganization, pursuant to which we plan to focus our resources on advancing our most promising product candidates and programs that have the greatest near-term opportunities. As part of this reorganization, we will eliminate and/or significantly scale back our investments in certain programs and lines of business which are not consistent with our current strategic focus. As a result, we reduced our workforce by 83 employees and we plan to consolidate our facilities. In connection with the reorganization, during the fourth quarter of 2005, we recorded a non-cash impairment charge of $45.7 million, which consisted primarily of the write-off of approximately $43.5 million of previously recorded long-lived intangible assets that are no longer essential to our focus, or we otherwise determined to be impaired under current accounting rules. We expect to incur additional charges of approximately $15.0 to $17.0 million, primarily in the first quarter of 2006, related to employee separation and facilities consolidation costs. We expect that these charges will result in future cash expenditures of $15.0 to $17.0 million through 2017.
49
We believe that these recent actions will enable us to reduce our loss and cash usage while we realize the greatest value from our programs and move toward profitability. While we expect to reduce our cash usage in 2006, we anticipate that our sales and marketing expenses will remain at comparable levels, or increase, in future periods as we introduce new products and invest in the necessary infrastructure to support an anticipated increased level of product revenue. We will also have cash usage requirements to support working capital needs as we continue to derive more revenue from product sales.
We expect that our current cash and cash equivalents, short-term investments, and funding from existing collaborations and grants will be sufficient to fund our working and other capital requirements for at least the next two years, including amounts required to fund internal research and development and enhancements of our core technologies. This estimate is a forward-looking statement that involves risks and uncertainties. Our future capital requirements and the adequacy of our available funds will depend on many factors, including scientific progress in our research and development programs, the magnitude of those programs, our ability to maintain our collaborations and achieve milestones under them and our ability to establish new collaborative relationships, our ability, alone or with our collaborative partners, to commercialize products successfully, and competing technological and market developments. Therefore, it is possible that we may seek additional financing in the future, whether through private or public equity offerings, debt financings, or collaborations, which may not be available on terms favorable to us, if at all. In addition, if we enter into financing arrangements in the future, such arrangements could dilute our stockholders’ ownership interests and adversely affect their rights.
We do not have any off-balance sheet arrangements.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosures. On an ongoing basis, we evaluate these estimates, including those related to revenue recognition, long-lived assets, accrued liabilities, and income taxes. These estimates are based on historical experience, information received from third parties, and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect the significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We follow the provisions as set forth by current accounting rules, which primarily include the Securities and Exchange Commission’s Staff Accounting Bulletin, or SAB, No. 104, “Revenue Recognition.”
We generally recognize revenue when we have satisfied all contractual obligations and we are reasonably assured of collecting the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue under current accounting rules. In those instances where we have billed our customers or received payment from our customers in advance of recognizing revenue, we include the amounts in deferred revenue on the balance sheet.
We generate revenue from research collaborations generally through funded research, up-front fees to initiate research projects, fees for exclusivity in a field, and milestones. We recognize revenue from research funding on a percentage-of-completion basis, as research hours are incurred under each agreement. We recognize fees to initiate research over the life of the project. We recognize revenue from exclusivity fees over the period of
50
exclusivity. Our collaborations often include contractual milestones. When we achieve these milestones, we are entitled to payment, as defined by the underlying agreements. We recognize revenue for milestone payments when earned, as evidenced by written acknowledgement from the collaborator, provided that (i) the milestone event is substantive and its achievement was not reasonably assured at the inception of the agreement, and (ii) our performance obligations after the milestone achievement will continue to be funded by the collaborator at a level comparable to the level before the milestone achievement.
We recognize revenue from grants as related costs are incurred, as long as such costs are within the funding limits specified by the underlying grant agreements.
We recognize revenue related to the sale of our inventory as we ship or deliver products, provided all other revenue recognition criteria have been met. We recognize revenue from products sold through distributors or other third-party arrangements upon shipment of the products, provided that (a) the price is substantially fixed and determinable at the time of sale; (b) the distributor’s obligation to pay us is not contingent upon resale of the products; (c) title and risk of loss passes to the distributor at time of shipment; (d) the distributor has economic substance apart from that provided by us; (e) we have no significant obligation to the distributor to bring about resale of the products; and (f) future returns can be reasonably estimated. For any sales that do not meet all of the above criteria, revenue is deferred until all such criteria have been met. We include our profit-sharing revenues in product-related revenues on the statement of operations. We recognize profit-sharing revenues during the quarter in which such profit sharing revenues are earned, based on estimates provided by our profit-sharing partner. We adjust these estimates for actual results in the subsequent quarter. We include profit-sharing revenues in product-related revenues on the statement of operations. To date, we have generated a substantial portion of our product-related revenues, including profit-sharing revenues, through our agreements with Danisco.
We sometimes enter into revenue arrangements that include the delivery of more than one product or service. In these cases, we recognize revenue from each element of the arrangement as long as we are able to determine a separate value for each element, we have completed our obligation to deliver or perform on that element and we are reasonably assured of collecting the resulting receivable.
Long-Lived Assets
We review long-lived assets, including leasehold improvements, property and equipment, and acquired intangible assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. This requires us to estimate future cash flows related to these assets. Actual results could differ from those estimates, which may affect the carrying amount of assets and the related amortization expense. As of December 31, 2005, we determined that the carrying amounts of certain of our long-lived assets were impaired, and recorded an impairment charge of $45.7 million.
Income Taxes
We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of their net recorded amounts, an adjustment to the deferred tax assets would increase our income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to income in the period such determination was made. As of December 31, 2005, we had $116.9 million in gross deferred tax assets, which were fully offset by a valuation allowance.
51
Inventories
We value inventory at the lower of cost (first in, first out) or market value and, if necessary, reduce the value by an estimated allowance for excess and obsolete inventories. The determination of the need for an allowance is based on our review of inventories on hand compared to estimated future usage and sales, as well as, judgments, quality control testing data, and assumptions about the likelihood of obsolescence.
Purchase Price Allocation
The purchase price for certain intellectual property rights licenses acquired from Syngenta and certain property and equipment acquired from Torrey Mesa Research Institute, or TMRI, was allocated to the tangible and identifiable intangible assets acquired based on their estimated fair values at the acquisition date. An independent third-party valuation firm was engaged to assist in determining the fair values of in-process research and development, identifiable intangible assets, and certain property, plant, and equipment. Such a valuation requires significant estimates and assumptions, including but not limited to: determining the timing and expected costs to complete the in-process projects, estimating future cash flows from product sales resulting from in-process projects, and developing appropriate discount rates and probability rates by project. We believe the fair values assigned to the assets acquired and liabilities assumed were based on reasonable assumptions. As of December 31, 2005, we assessed the carrying values of these assets on our balance sheet and determined that such assets and technologies were impaired. As a result we have written off the carrying value of these assets on our balance sheet as of December 31, 2005.
Segment Reporting
Effective for fiscal 2005, we determined that we operated in two segments: bioscience products and pharmaceuticals. Pursuant to the provisions of FASB No. 131, “Disclosures about Segments of an Enterprise and Related Information,” we would be required to report separate financial and descriptive information about our reportable operating segments in our Form 10-K for the fiscal year ended December 31, 2005. Operating segments are components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. Generally, financial information is required to be reported on the basis that it is used internally for evaluating segment performance and deciding how to allocate resources to segments. However, as part of the recent strategic reorganization which we commenced in the fourth quarter of 2005 and announced in January 2006, we have determined that our reduced pharmaceutical programs do not qualify as a reportable segment and that the segment disclosures under FASB No. 131 are not applicable. As such, we have not included segment disclosures in the accompanying notes to the condensed consolidated financial statements for year ended December 31, 2005.
Recently Issued Accounting Standards
In December 2004, the FASB issued Statement No. 123R “Share-Based Payment.” FASB No. 123R eliminates, among other items, the use of the intrinsic value method of accounting contained in Accounting Principles Bulletin No. 25, or APB No. 25, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. We expect to apply the “modified prospective” method and will begin expensing amounts related to employee stock options, restricted stock and stock issued under our employee equity incentive plans using the Black-Scholes option pricing model to measure the fair value of stock options granted to employees effective January 1, 2006. The adoption of FASB No. 123R will have a material impact on the our results of operations. Assuming levels of equity awards similar to 2005, the estimated stock-based compensation expense for 2006 may range from approximately $2.5 to $3.0 million. However, the calculation of compensation cost for share-based payment transactions may be significantly different because of the uncertainty of additional equity awards which may be granted, the unpredictability of the fair value of stock options granted and the estimated
52
expected forfeiture rates. As a result, stock-based compensation charges may differ significantly from our current estimates.
During the fourth quarter of fiscal 2005, we accelerated the vesting of unvested stock options awarded to all employees and officers under our stock option plan that had exercise prices greater than $10.00. The unvested options to purchase approximately 710,000 shares became fully vested as of December 8, 2005 as a result of the acceleration. These stock options would have all become fully vested before or during 2008. We accelerated these options because the options had exercise prices significantly in excess of then current market value ($5.25 at December 8, 2005), and thus were not fully achieving their original objectives of incentive compensation and employee retention. The company expects the acceleration to have a positive effect on employee morale, retention, and perception of value. The acceleration also eliminated future compensation expense we would otherwise recognize in our statements of operations with respect to these options with the implementation of FASB No. 123R, which becomes effective for our fiscal year 2006. The future expense eliminated as a result of the acceleration of the vesting of these options is approximately $1.1 million.
In June 2005, the FASB issued Statement No. 154, “Accounting Changes and Error Corrections,” a replacement of APB Opinion No. 20, “Accounting Changes” and Statement No. 3, “Reporting Accounting Changes in Interim Financial Statements.” FASB No. 154 changes the requirements for the accounting for and reporting of changes in accounting principles. Previously, most voluntary changes in accounting principles required recognition via a cumulative effect adjustment within net income in the period of the change. FASB No. 154 requires retrospective application to prior periods’ financial statements, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. FASB No. 154 is effective for accounting changes made in fiscal years beginning after December 15, 2005; however, the statement does not change the transition provisions of any existing accounting pronouncements. We do not believe that the adoption of FASB No. 154 will have a material effect on our consolidated financial position, results of operations or cash flows.
In March 2005, the FASB issued Interpretation, or FIN, No. 47, “Accounting for Conditional Asset Retirement Obligations,” which clarifies that an entity is required to recognize a liability for the fair value of a conditional asset retirement obligation if the fair value can be reasonably estimated even though uncertainty exists about the timing and/or method of settlement. We adopted FIN 47 in 2005. The adoption of FIN No. 47 did not have a material impact on our results of operations and financial condition.
In December 2004, the FASB issued Statement No. 153, “Exchanges of Nonmonetary Assets.” FASB No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. FASB No. 153 is effective for fiscal periods beginning after June 15, 2005. We do not believe that the adoption of FASB No. 153 will have a material effect on our consolidated financial position, results of operations or cash flows.
In November 2004, the Financial Accounting Standards Board issued FASB No. 151, Inventory Costs, which clarifies the accounting for abnormal amounts of idle facility expense, freight, handling costs, and wasted material. FASB No. 151 will be effective for inventory costs incurred during fiscal years beginning after June 15, 2005. We do not believe that the adoption of FASB No. 151 will have a material effect on our consolidated financial position, results of operations or cash flows.
53
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
Our exposure to market risk is limited to interest rate risk and to a lesser extent to foreign currency risk. Our exposure to changes in interest rates relates primarily to the increase or decrease in the amount of interest income we can earn on our investment portfolio and on the increase or decrease in the amount of interest expense we must pay with respect to our various outstanding debt instruments. Our risk associated with fluctuating interest expense is limited to our future financings, including any future equipment financing line of credit agreements, the interest rates under which are expected to be closely tied to market rates. Our risk associated with fluctuating interest income is limited to our investments in interest rate sensitive financial instruments. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. We ensure the safety and preservation of our invested principal funds by limiting default risk, market risk, and reinvestment risk. We mitigate default risk by investing in short-term investment grade securities and limiting the amount invested in any single security. We mitigate market risk by maintaining an average maturity of less than one year for our investments. We mitigate reinvestment risk by investing in securities with varying maturity dates. A hypothetical 100 basis point adverse move in interest rates along the entire interest rate yield curve would have decreased the fair value of our interest sensitive financial instruments at December 31, 2005 and 2004 by $0.2 million and $0.5 million. Declines in interest rates over time will reduce our interest income, while increases in interest rates over time will increase our interest expense. In connection with one of our research collaborations we engage third parties to provide various services. Certain of these services result in obligations that are denominated in other than U.S. dollars. Foreign currency risk is minimized due to the less than material amount of such obligations. Additionally, the collaboration under which such services are performed provides for reimbursement of such costs in U.S. dollars.
54
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Diversa Corporation
We have audited the accompanying consolidated balance sheets of Diversa Corporation as of December 31, 2005 and 2004, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2005. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Diversa Corporation at December 31, 2005 and 2004, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2005, in conformity with U. S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Diversa Corporation’s internal control over financial reporting as of December 31, 2005, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2006 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Diego, California
March 10, 2006
55
DIVERSA CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value)
| | | | | | | | |
| | | December 31,
| |
| | | 2005
| | | 2004
| |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 43,859 | | | $ | 33,796 | |
Short-term investments | | | 21,569 | | | | 64,397 | |
Accounts receivable (including $1,657 and $1,453 from a related party at December 31, 2005 and 2004) | | | 9,012 | | | | 5,771 | |
Other current assets | | | 4,996 | | | | 3,252 | |
| | |
|
|
| |
|
|
|
Total current assets | | | 79,436 | | | | 107,216 | |
Property and equipment, net | | | 18,245 | | | | 27,019 | |
Acquired intangible assets, net | | | — | | | | 48,714 | |
Other assets | | | 388 | | | | 1,107 | |
| | |
|
|
| |
|
|
|
Total assets | | $ | 98,069 | | | $ | 184,056 | |
| | |
|
|
| |
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 4,968 | | | $ | 2,195 | |
Accrued liabilities | | | 6,156 | | | | 7,216 | |
Deferred revenue (including $5,931 and $5,325 from a related party at December 31, 2005 and 2004.) | | | 7,535 | | | | 5,892 | |
Current portion of debt obligations | | | 7,024 | | | | 8,982 | |
| | |
|
|
| |
|
|
|
Total current liabilities | | | 25,683 | | | | 24,285 | |
Notes payable, less current portion | | | 6,332 | | | | 8,825 | |
Deferred revenue, less current portion | | | 1,250 | | | | — | |
| | |
Commitments and contingencies | | | | | | | | |
| | |
Stockholders’ equity: | | | | | | | | |
Preferred stock—$0.001 par value; 5,000 shares authorized, no shares issued and outstanding at December 31, 2005 and 2004 | | | — | | | | — | |
Common stock—$0.001 par value; 90,000 shares authorized, 45,048 and 43,730 shares issued and outstanding at December 31, 2005 and 2004 | | | 45 | | | | 44 | |
Additional paid-in capital | | | 358,307 | | | | 351,736 | |
Deferred compensation | | | (3,130 | ) | | | — | |
Accumulated deficit | | | (290,215 | ) | | | (200,497 | ) |
Accumulated other comprehensive loss | | | (203 | ) | | | (337 | ) |
| | |
|
|
| |
|
|
|
Total stockholders’ equity | | | 64,804 | | | | 150,946 | |
| | |
|
|
| |
|
|
|
Total liabilities and stockholders’ equity | | $ | 98,069 | | | $ | 184,056 | |
| | |
|
|
| |
|
|
|
See accompanying notes.
56
DIVERSA CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
| | | | | | | | | | | | |
| | | Years Ended December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Revenues (including related party revenues of $24,305, $36,889, and $29,164 in 2005, 2004 and 2003): | | | | | | | | | | | | |
Collaborative | | $ | 34,392 | | | $ | 41,897 | | | $ | 41,980 | |
Grant | | | 10,079 | | | | 10,241 | | | | 3,923 | |
Product-related | | | 9,832 | | | | 5,412 | | | | 3,056 | |
| | |
|
|
| |
|
|
| |
|
|
|
Total revenue | | | 54,303 | | | | 57,550 | | | | 48,959 | |
Operating expenses: | | | | | | | | | | | | |
Cost of product-related revenue | | | 10,662 | | | | 3,698 | | | | 2,997 | |
Research and development | | | 72,276 | | | | 73,405 | | | | 70,657 | |
Write-off of acquired in-process research and development | | | — | | | | — | | | | 19,478 | |
Selling, general and administrative | | | 12,588 | | | | 11,607 | | | | 12,181 | |
Amortization of acquired intangible assets | | | 2,602 | | | | 2,598 | | | | 2,290 | |
Non-cash, stock-based compensation | | | 877 | | | | — | | | | 131 | |
Asset impairment charges | | | 45,745 | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total operating expenses | | | 144,750 | | | | 91,308 | | | | 107,734 | |
| | |
|
|
| |
|
|
| |
|
|
|
Loss from operations | | | (90,447 | ) | | | (33,758 | ) | | | (58,775 | ) |
Other income (expense) | | | — | | | | 230 | | | | 227 | |
Equity in loss of joint venture | | | — | | | | — | | | | (1,255 | ) |
Interest income | | | 2,011 | | | | 1,767 | | | | 3,541 | |
Interest expense | | | (1,282 | ) | | | (1,664 | ) | | | (1,434 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net loss | | $ | (89,718 | ) | | $ | (33,425 | ) | | $ | (57,696 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net loss per share, basic and diluted | | $ | (2.04 | ) | | $ | (0.77 | ) | | $ | (1.39 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Shares used in calculating net loss per share, basic and diluted | | | 44,064 | | | | 43,416 | | | | 41,592 | |
See accompanying notes.
57
DIVERSA CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock
| | Additional
Paid-In
Capital
| | Deferred Compensation
| | | Accumulated
Deficit
| | | Accumulated
Other
Comprehensive
Income (Loss)
| | | Total
Stockholders’
Equity
| |
| | | Shares
| | Amount
| | | | | |
Balance at January 1, 2003 | | 35,769 | | $ | 36 | | $ | 265,150 | | $ | (131 | ) | | $ | (109,376 | ) | | $ | 1,636 | | | $ | 157,315 | |
Net loss | | — | | | — | | | — | | | — | | | | (57,696 | ) | | | — | | | | (57,696 | ) |
Change in unrealized loss on available-for-sale securities | | — | | | — | | | — | | | — | | | | — | | | | (1,443 | ) | | | (1,443 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
|
|
|
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (59,139 | ) |
Issuance of common stock under stock plans, net of forfeitures | | 440 | | | — | | | 1,758 | | | — | | | | — | | | | — | | | | 1,758 | |
Issuance of common stock in connection with strategic transactions | | 6,842 | | | 7 | | | 81,371 | | | — | | | | — | | | | — | | | | 81,378 | |
Amortization of deferred compensation | | — | | | — | | | — | | | 131 | | | | — | | | | — | | | | 131 | |
| | |
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at December 31, 2003 | | 43,051 | | | 43 | | | 348,279 | | | — | | | | (167,072 | ) | | | 193 | | | | 181,443 | |
Net loss | | — | | | — | | | — | | | — | | | | (33,425 | ) | | | — | | | | (33,425 | ) |
Change in unrealized loss on available-for-sale securities | | — | | | — | | | — | | | — | | | | — | | | | (530 | ) | | | (530 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
|
|
|
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (33,955 | ) |
Issuance of common stock under stock plans, net of forfeitures | | 679 | | | 1 | | | 3,457 | | | — | | | | — | | | | — | | | | 3,458 | |
| | |
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at December 31, 2004 | | 43,730 | | | 44 | | | 351,736 | | | — | | | | (200,497 | ) | | | (337 | ) | | | 150,946 | |
Net loss | | — | | | — | | | — | | | — | | | | (89,718 | ) | | | — | | | | (89,718 | ) |
Change in unrealized loss on available-for-sale securities | | — | | | — | | | — | | | — | | | | — | | | | 134 | | | | 134 | |
| | | | | | | | | | | | | | | | | | | | | | |
|
|
|
Comprehensive loss | | — | | | — | | | — | | | — | | | | — | | | | — | | | | (89,584 | ) |
Issuance of common stock under stock plans, net of forfeitures | | 1,318 | | | 1 | | | 2,564 | | | — | | | | — | | | | — | | | | 2,565 | |
Non-cash compensation charges | | — | | | — | | | 142 | | | — | | | | — | | | | — | | | | 142 | |
Deferred compensation charges, net of adjustments for forfeitures | | — | | | — | | | 3,865 | | | (3,865 | ) | | | — | | | | — | | | | — | |
Amortization of deferred compensation, net | | — | | | — | | | — | | | 735 | | | | — | | | | — | | | | 735 | |
| | |
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at December 31, 2005 | | 45,048 | | $ | 45 | | $ | 358,307 | | $ | (3,130 | ) | | $ | (290,215 | ) | | $ | (203 | ) | | $ | 64,804 | |
| | |
| |
|
| |
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
See accompanying notes
58
DIVERSA CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | Years Ended December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Operating activities: | | | | | | | | | | | | |
Net loss | | $ | (89,718 | ) | | $ | (33,425 | ) | | $ | (57,696 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 17,732 | | | | 17,964 | | | | 16,823 | |
Write-off of acquired in-process research and development | | | — | | | | — | | | | 19,478 | |
Non-cash asset impairment charges | | | 45,745 | | | | — | | | | — | |
Non-cash, stock-based compensation | | | 877 | | | | — | | | | 131 | |
Net loss on disposals of property and equipment | | | 1,297 | | | | — | | | | — | |
Change in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable, net | | | (3,241 | ) | | | (355 | ) | | | (3,393 | ) |
Other current assets | | | (1,744 | ) | | | (1,793 | ) | | | (322 | ) |
Other assets | | | 719 | | | | (184 | ) | | | (1,561 | ) |
Accounts payable | | | 2,773 | | | | (558 | ) | | | (1,380 | ) |
Accrued liabilities | | | (1,060 | ) | | | 1,387 | | | | 1,432 | |
Deferred revenue | | | 2,893 | | | | (5,422 | ) | | | (761 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash used in operating activities | | | (23,727 | ) | | | (22,386 | ) | | | (27,249 | ) |
Investing activities: | | | | | | | | | | | | |
Purchases of property and equipment | | | (7,286 | ) | | | (7,654 | ) | | | (9,387 | ) |
Purchases of investments | | | (223,015 | ) | | | (42,876 | ) | | | (128,774 | ) |
Sales and maturities of investments | | | 265,977 | | | | 84,925 | | | | 163,338 | |
Other | | | — | | | | — | | | | (270 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash provided by investing activities | | | 35,676 | | | | 34,395 | | | | 24,907 | |
Financing activities: | | | | | | | | | | | | |
Proceeds from equipment financing | | | 5,540 | | | | 9,077 | | | | 8,858 | |
Principal payments on equipment financing obligations | | | (9,991 | ) | | | (11,254 | ) | | | (7,881 | ) |
Net proceeds from issuance of common stock | | | 2,565 | | | | 3,458 | | | | 1,758 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash (used in) provided by financing activities | | | (1,886 | ) | | | 1,281 | | | | 2,735 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net increase in cash and cash equivalents | | | 10,063 | | | | 13,290 | | | | 393 | |
Cash and cash equivalents at beginning of year | | | 33,796 | | | | 20,506 | | | | 20,113 | |
| | |
|
|
| |
|
|
| |
|
|
|
Cash and cash equivalents at end of year | | $ | 43,859 | | | $ | 33,796 | | | $ | 20,506 | |
| | |
|
|
| |
|
|
| |
|
|
|
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Interest paid | | $ | 1,205 | | | $ | 1,541 | | | $ | 1,416 | |
| | |
|
|
| |
|
|
| |
|
|
|
See accompanying notes.
59
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Organization and Summary of Significant Accounting Policies |
The Company
Diversa Corporation (the “Company) was incorporated in December 1992 and began operations in May 1994. The Company is a leader in applying proprietary genomic technologies for the rapid discovery and optimization of novel protein-based products, including (a) enzymes, which are catalytic proteins, and (b) antibodies, which are binding proteins. The Company is directing its integrated portfolio of technologies to the discovery, evolution, and production of commercially valuable molecules with agricultural, industrial, and chemical applications, such as enzymes, as well as optimized antibodies with pharmaceutical applications. While the Company’s technologies have the potential to serve many large markets, the key areas of focus for internal product development are alternative fuels, specialty industrial processes, and health and nutrition.
Recent Events
As more fully described in the accompanying footnotes and prior filings with the Securities and Exchange Commission, on January 5, 2006 the Company announced a strategic reorganization, pursuant to which the Company plans to focus its resources on advancing its most promising product candidates and programs that have the greatest near-term opportunities. As part of this reorganization, the Company will eliminate and/or significantly scale back its investments in certain programs and lines of business which are not consistent with the current strategic focus. As a result, the Company reduced its workforce by 83 employees and plans to consolidate its facilities. In connection with the reorganization, during the fourth quarter of 2005, the Company recorded a non-cash impairment charge of $45.7 million to write off long-lived tangible and intangible assets that are no longer essential to the Company’s focus and determined to be impaired under current accounting rules. The Company expects to incur additional charges in 2006 related to employee separation and facilities consolidation costs (See Note 7—Impairment Charges and Restructuring Activities).
Basis of Consolidation
The consolidated financial statements include the financial statements of Diversa Corporation and those of the Company’s two wholly-owned subsidiaries, which were inactive as of December 31, 2005. All significant inter-company balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers cash equivalents to be only those investments which are highly liquid, readily convertible to cash and which mature within three months from the date of purchase.
Short-term Investments
Based on the nature of the assets held by the Company and management’s investment strategy, the Company’s investments have been classified as available-for-sale. Management determines the appropriate
60
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
classification of debt securities at the time of purchase. Securities classified as available-for-sale are carried at estimated fair value, as determined by quoted market prices, with unrealized gains and losses reported as a separate component of comprehensive income. At December 31, 2005, the Company had no investments that were classified as trading or held-to-maturity as defined by the Financial Accounting Standards Board (“FASB”) Statement No. 115, “Accounting for Certain Investments in Debt and Equity Securities.” The amortized cost of debt securities classified as available-for-sale is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion is included in interest income. Realized gains and losses on sales of available-for-sale securities are computed based upon initial cost adjusted for any other than temporary declines in fair value and are included in interest income. The cost of securities sold is based on the specific identification method. Interest on securities classified as available-for-sale is included in interest income.
Inventories
Inventories, included in other current assets on the accompanying balance sheets, are valued at the lower of cost (first in, first out) or market value and, if necessary, are reduced by an estimated allowance for excess and obsolete inventories. The determination of the need for an allowance is based on management’s review of inventories on hand compared to estimated future usage and sales as well as judgments, quality control testing data, and assumptions about the likelihood of obsolescence. The Company maintained a valuation allowance of $150,000 and zero at December 31, 2005 and 2004.
Concentration of Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents, and short-term investments. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. The Company generally invests its excess cash in U.S. Treasury and government agency obligations and investment-grade corporate securities.
The Company’s accounts receivable consist of amounts due from customers for the sale of products, amounts due from governmental agencies for costs incurred under funded projects, and amounts due from corporate partners under various collaboration agreements. The Company regularly assesses the need for an allowance for potentially uncollectible accounts receivable arising from our customers’ inability to make required payments. The Company has a limited number of accounts receivable and uses the specific identification method as a basis for determining this estimate. Historically, losses related to uncollectible accounts receivable have been minimal. The Company did not maintain an allowance for doubtful accounts for the years ended December 31, 2005 or 2004.
Property and Equipment
Property and equipment are stated at cost and depreciated over the estimated useful lives of the assets (generally three to five years) using the straight-line method. Amortization of leasehold improvements is computed over the shorter of the lease term or the estimated useful life of the related assets. For the years ended December 31, 2005, 2004, and 2003, the Company recorded depreciation expense of $12.5 million, $12.8 million, and $12.6 million, which includes the depreciation of assets under capital leases.
Acquired Intangible Assets
In accordance with Accounting Principles Board Opinion (“APB”) No. 17, “Accounting for Intangible Assets,” the Company’s intangible assets, which all fall into one intangible asset class, are recorded at cost and are amortized over their estimated useful lives, which range from seven to fifteen years. For purposes of
61
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
evaluating impairment of the acquired intangible assets, the Company compares the carrying values and estimated future cash flows of both the acquired assets and the Company’s internally developed technologies on a combined basis. In connection with the Company’s recent strategic reorganization, the Company determined, based on an analysis of estimated future cash flows, that the acquired intangible assets were fully impaired as of December 31, 2005, and recorded an impairment charge totaling $43.5 million to write off the value of these assets (See Note 7— Impairment Charges and Restructuring Activities).
Impairment of Long-Lived Assets
In accordance with FASB No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” if indicators of impairment exist, the Company assesses the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, the Company measures the amount of such impairment by comparing the carrying value of the asset to the present value of the expected future cash flows associated with the use of the asset. In connection with the Company’s recent strategic reorganization, the Company determined, based on an analysis of estimated future cash flows, that the Company’s property and equipment carrying values were impaired as of December 31, 2005, and recorded an impairment charge totaling $2.2 million to write down the value of these assets to their net realizable value (See Note 7—Impairment Charges and Restructuring Activities).
Fair Value of Financial Instruments
Financial instruments, including cash and cash equivalents, short-term investments, accounts receivable, accounts payable, and accrued liabilities, are carried at cost, which management believes approximates fair value because of the short-term maturity of these instruments. The carrying amounts of debt obligations approximate their respective fair values as they bear terms that are comparable to those available under current market conditions.
Revenue Recognition
The Company recognizes revenue when all of its contractual obligations are satisfied and collection of the underlying receivable is reasonably assured, in accordance with Securities and Exchange Commission Staff Accounting Bulletin (“SAB”) No. 104, “Revenue Recognition.” Billings to customers or payments received from customers are included in deferred revenue on the balance sheet until all revenue recognition criteria are met.
Collaborative Revenue
Revenue from research funding under the Company’s collaboration agreements is earned and recognized on a percentage-of-completion basis as research hours are incurred in accordance with the provisions of each agreement. Fees received to initiate research projects are deferred and recognized over the life of the project. Fees received for exclusivity in a field are deferred and recognized over the period of exclusivity. Milestone payments are recognized when earned, as evidenced by written acknowledgement from the collaborator, provided that (i) the milestone event is substantive and its achievement was not reasonably assured at the inception of the agreement, and (ii) the Company’s performance obligations after the milestone achievement will continue to be funded by the collaborator at a level comparable to the level before the milestone achievement.
Grant Revenue
Revenue from grants is recognized as related costs are incurred, as long as such costs are within the funding limits specified by the underlying grant agreements.
62
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Product-Related Revenue
Product-related revenues are recognized at the time of shipment to the customer provided all other revenue recognition criteria have been met. The Company recognizes revenue on product sales through third-party distribution agreements in accordance with the provisions set forth in FASB No. 48,“Revenue Recognition When Right of Return Exists.” Under FASB No. 48, the Company recognizes product revenues upon shipment to distributors, provided that (a) the price is substantially fixed and determinable at the time of sale; (b) the distributor’s obligation to pay the Company is not contingent upon resale of the products; (c) title and risk of loss passes to the distributor at time of shipment; (d) the distributor has economic substance apart from that provided by the Company; (e) the Company has no significant obligation to the distributor to bring about resale of the products; and (f) future returns can be reasonably estimated. For any sales that do not meet all of the above criteria, revenue is deferred until all such criteria have been met.
The Company recognizes profit-sharing revenue during the quarter in which such revenue is earned, based on estimates provided by the Company’s profit-sharing partner. These estimates are adjusted for actual results in the subsequent quarter. Profit-sharing revenue is included in product-related revenue in the statement of operations.
Revenue Arrangements with Multiple Deliverables
The Company sometimes enters into revenue arrangements that contain multiple deliverables. The Company recognizes revenue from such arrangements entered into subsequent to June 30, 2003 in accordance with Emerging Issues Task Force (“EITF”) Issue No. 00-21, “Accounting for Revenue Arrangements with Multiple Deliverables.” This issue addresses the timing and method of revenue recognition for revenue arrangements that include the delivery of more than one product or service. In these cases, the Company recognizes revenue from each element of the arrangement as long as separate value for each element can be determined, the Company has completed its obligation to deliver or perform on that element, and collection of the resulting receivable is reasonably assured.
Research and Development
Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Research and development costs are expensed as incurred.
Cost of Product-Related Revenue
Cost of product-related revenue includes both internal and third-party fixed and variable costs including materials and supplies, labor, facilities and other overhead costs associated with our product-related revenues. The Company expenses the cost of idle manufacturing capacity to cost of product-related revenue as incurred.
Income Taxes
Current income tax expense (benefit) is the amount of income taxes expected to be payable (receivable) for the current year. A deferred income tax asset or liability is computed for the expected future impact of differences between the financial reporting and tax bases of assets and liabilities, as well as the expected future tax benefit to be derived from tax loss and credit carry-forwards. Deferred income tax expense is generally the net change during the year in the deferred income tax assets and liabilities. Valuation allowances are established
63
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized. The effect of tax rate changes is reflected in income tax expense (benefit) during the period in which such changes are enacted. The Company has provided a full valuation allowance against any deferred tax assets.
Stock-Based Compensation
Prior to January 1, 2006, the Company accounted for stock-based compensation according to the provisions of APB No. 25 and related interpretations and did not recognize compensation expense for options granted to employees and non-employee directors with exercise prices equal to or in excess of the fair value of the underlying shares at the date of grant. Effective January 1, 2006, FASB No. 123R requires the Company to measure the cost of employee services received in exchange for equity instruments, such as stock options or restricted stock, based on the grant-date fair-value of the awards. The associated cost must be recognized over the period during which an employee is required to provide service in exchange for the award (usually the vesting period). FASB No. 123R also requires the Company to estimate forfeitures in calculating stock-based compensation expense, rather than recognizing the impact of the forfeitures as they occur, which was previously permitted. The Company will implement FASB No. 123R, on a prospective basis, beginning in the 2006 first quarter, will not restate prior year amounts, and will not record a cumulative effect adjustment upon adoption of FASB No. 123R.
Pro forma information regarding net loss and net loss per share has been determined as if the Company had accounted for its employee stock options and stock purchase plan under the fair value method of FASB No. 123. For 2005, 2004, and 2003, the fair value of these options was estimated using the Black-Scholes model with the following assumptions: risk-free interest rates ranging from 3.58% to 4.51%, 2.26% to 3.46%, and 2.86% to 4.80%; dividend yield of 0%; volatility factor of 51% to 56%, 49% to 65%, and 61%; and a weighted-average expected life of the options of three years. Based on these assumptions, the weighted-average fair value of options granted during 2005, 2004, and 2003 was $2.55, $4.38, and $3.48.
Had the Company determined compensation expense based on fair value in accordance with FASB No. 123, “Accounting for Stock Based Compensation,” net loss and net loss per common share would have been as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Net loss, as reported | | $ | (89,718 | ) | | $ | (33,425 | ) | | $ | (57,696 | ) |
Add: Stock-based compensation expense included in reported net loss | | | 877 | | | | — | | | | 131 | |
Deduct: Total stock-based compensation expense determined under fair value based method for all awards | | | (7,531 | ) | | | (8,420 | ) | | | (8,487 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Pro forma net loss | | $ | (96,372 | ) | | $ | (41,845 | ) | | $ | (66,052 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Basic and diluted net loss per share, as reported | | $ | (2.04 | ) | | $ | (0.77 | ) | | $ | (1.39 | ) |
Pro forma basic and diluted net loss per share | | $ | (2.19 | ) | | $ | (0.96 | ) | | $ | (1.59 | ) |
In connection with the grant of certain stock options to employees, the Company recorded deferred stock compensation, representing the difference between the exercise price and the fair value of the Company’s common stock as estimated by the Company’s management for financial reporting purposes on the date such stock options were granted. Deferred compensation is included as a reduction of stockholders’ equity and is amortized to expense over the vesting period of the options in accordance with FASB Interpretation No. 28, “Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans,” which permits an
64
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
accelerated amortization methodology. As the deferred compensation was fully amortized as of December 31, 2003, no amortization expense for these stock options was recorded during the year ended December 31, 2005 and 2004.
During 2005, the Company issued approximately 726,000 shares of restricted stock to employees and, pursuant to FASB No. 123, recorded stock-based deferred compensation (net of forfeitures) of approximately $3.9 million in connection with the issuance of these shares. The Company amortizes the deferred compensation balance, net of forfeitures, to expense on a straight-line basis over the vesting period of the awards, which is generally four years. The Company recorded net expense of $0.8 million related to the amortization of deferred stock-based compensation during the year ended December 31, 2005. The Company also recorded a non-cash stock-based compensation charge of approximately $0.1 million during the fourth quarter of 2005 related to the acceleration of vesting on approximately 28,000 restricted shares granted to its former Chief Executive Officer. As of December 31, 2005, approximately 138,000 restricted shares had been forfeited, 588,000 were outstanding, and 28,000 were vested. The weighted average fair value of restricted stock awarded during 2005 was $6.99 per share.
During the fourth quarter of fiscal 2005, the Company accelerated the vesting of unvested stock options awarded to all employees and officers under our stock option plan that had exercise prices greater than $10.00. The unvested options to purchase approximately 710,000 shares became fully vested as of December 8, 2005 as a result of the acceleration. These stock options would have all become fully vested before or during 2008. The Company accelerated these options because the options had exercise prices significantly in excess of the then current market value ($5.25 at December 8, 2005), and thus were not fully achieving their original objectives of incentive compensation and employee retention. The Company expects the acceleration to have a positive effect on employee morale, retention, and perception of value. The acceleration also eliminated future compensation expense that would have been recognized in the statements of operations with respect to these options with the implementation of FASB No. 123R, which becomes effective for our fiscal year 2006. The future expense eliminated as a result of the acceleration of the vesting of these options is approximately $1.1 million.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on marketable securities. The Company presents comprehensive income loss in its Consolidated Statements of Stockholders’ Equity.
Net Loss per Share
Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. During 2005, the Company issued approximately 726,000 restricted shares to employees. As of December 31, 2005, there were approximately 588,000 restricted shares outstanding, net of cancellations and forfeitures, and 560,000 outstanding restricted shares were unvested. For purposes of the computation of net loss per share, these unvested shares are considered contingently returnable shares under FASB No. 128,“Earnings Per Share,” and are not considered outstanding common shares for purposes of computing net loss per share until all necessary conditions are met that no longer cause the shares to be contingently returnable. The impact of these unvested shares on weighted average shares outstanding has been excluded for purposes of computing net loss per share.
65
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table presents the calculation of basic and diluted net loss per share (in thousands, except per share data):
| | | | | | | | | | | | |
| | | Years Ended December 31,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Weighted average shares outstanding during the period | | | 44,589 | | | | 43,416 | | | | 41,592 | |
Less: Weighted average unvested restricted shares outstanding | | | (525 | ) | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Weighted average shares used in computing basic and diluted net loss per share | | | 44,064 | | | | 43,416 | | | | 41,592 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net loss | | $ | (89,718 | ) | | $ | (33,425 | ) | | $ | (57,696 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net loss per share, basic and diluted | | $ | (2.04 | ) | | $ | (0.77 | ) | | $ | (1.39 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
The Company has excluded all outstanding stock options and warrants from the calculation of diluted net loss per share because all such securities are anti-dilutive for all applicable periods presented. The total number of shares excluded from the calculations of diluted net loss per share, prior to application of the treasury stock method for options and warrants, was 7.5 million, 9.8 million, and 8.3 million for the years ended December 31, 2005, 2004, and 2003. Such securities, had they been dilutive, would have been included in the computation of diluted earnings per share.
Segment Reporting
As of December 31, 2005, in connection with the Company’s strategic reorganization, the Company determined that it operates in only one segment. Accordingly, no segment disclosures have been included in the accompanying notes to the consolidated financial statements.
Reclassification
Certain prior year amounts have been reclassified to conform to the current year presentation.
Effect of New Accounting Standards
Stock-Based Compensation
In December 2004, the Financial Accounting Standards Board issued Statement No. 123(R),“Share-Based Payment,”which is a revision of FASB No. 123,“Accounting for Stock-Based Compensation.” FASB No. 123(R) supersedes APB No. 25 and amends FASB No. 95, “Statement of Cash Flows.” Generally, the approach in FASB No. 123(R) is similar to the approach described in FASB No. 123. However, FASB No. 123(R) requires all share-based payments to employees, including grants of employee stock options, to be recognized in the income statement based on their fair values. Pro forma disclosure, which has previously been used by the Company, will no longer be an alternative. FASB No. 123(R) permits companies to adopt its requirements using either a “modified prospective” method, or a “modified retrospective” method. Under the “modified prospective” method, compensation cost is recognized in the financial statements beginning with the effective date, based on the requirements of FASB No. 123(R) for all share-based payments granted after that date, and based on the requirements of FASB No. 123 for all unvested awards granted prior to the effective date of FASB No. 123(R). Under the “modified retrospective” method, the requirements are the same as under the “modified prospective” method, but entities are permitted to restate financial statements of previous periods
66
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
based on pro forma disclosures made in accordance with FASB No. 123. The Company adopted FASB No. 123(R) effective January 1, 2006 and will utilize the “modified prospective” method. The Company believes that the adoption of this statement will have a significant impact on its financial statements, and estimates that, based on current stock options and restricted stock awards granted to date, stock-based compensation expense for 2006 will be $2.5 million to $3.0 million.
Other New Accounting Standards
In June 2005, the FASB issued Statement No. 154, “Accounting Changes and Error Corrections,” a replacement of APB Opinion No. 20, “Accounting Changes” and FASB No. 3, “Reporting Accounting Changes in Interim Financial Statements.” FASB No. 154 changes the requirements for the accounting for and reporting of changes in accounting principles. Previously, most voluntary changes in accounting principles required recognition via a cumulative effect adjustment within net income in the period of the change. FASB No. 154 requires retrospective application to prior periods’ financial statements, unless it is impracticable to determine either the period-specific effects or the cumulative effect of the change. FASB No. 154 is effective for accounting changes made in fiscal years beginning after December 15, 2005; however, the statement does not change the transition provisions of any existing accounting pronouncements. The Company does not believe that the adoption of FASB No. 154 will have a material effect on its consolidated financial position, results of operations or cash flows.
In March 2005, the FASB issued Interpretation No. 47, “Accounting for Conditional Asset Retirement Obligations,” which clarifies that an entity is required to recognize a liability for the fair value of a conditional asset retirement obligation if the fair value can be reasonably estimated even though uncertainty exists about the timing and/or method of settlement. The Company adopted FIN 47 in 2005. The adoption of FIN 47 did not have a material impact on the Company’s results of operations and financial condition.
In December 2004, the FASB issued Statement No. 153, “Exchanges of Nonmonetary Assets.” Statement No. 153 addresses the measurement of exchanges of nonmonetary assets and redefines the scope of transactions that should be measured based on the fair value of the assets exchanged. FASB No. 153 is effective for fiscal periods beginning after June 15, 2005. The Company does not believe the adoption of FASB No. 153 will have a material impact on its financial statements.
In November 2004, the Financial Accounting Standards Board issued FASB No. 151, “Inventory Costs,” which clarifies the accounting for abnormal amounts of idle facility expense, freight, handling costs, and wasted material. FASB No. 151 will be effective for inventory costs incurred during fiscal years beginning after June 15, 2005. The Company does not believe the adoption of FASB No. 151 will have a material impact on its financial statements.
67
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Short-term investments consist of the following (in thousands):
| | | | | | | | | | | | | |
| | | Amortized
Cost
| | Unrealized
Gains
| | Unrealized
Losses
| | | Market
Value
|
December 31, 2005 | | | | | | | | | | | | | |
Corporate debt securities | | $ | 12,214 | | $ | 12 | | $ | (132 | ) | | $ | 12,094 |
U.S. Government and agency obligations | | | 9,558 | | | — | | | (83 | ) | | | 9,475 |
| | |
|
| |
|
| |
|
|
| |
|
|
| | | $ | 21,772 | | $ | 12 | | $ | (215 | ) | | $ | 21,569 |
| | |
|
| |
|
| |
|
|
| |
|
|
December 31, 2004 | | | | | | | | | | | | | |
Corporate debt securities | | $ | 23,438 | | $ | 9 | | $ | (97 | ) | | $ | 23,350 |
U.S. Government and agency obligations | | | 41,296 | | | 7 | | | (256 | ) | | | 41,047 |
| | |
|
| |
|
| |
|
|
| |
|
|
| | | $ | 64,734 | | $ | 16 | | $ | (353 | ) | | $ | 64,397 |
| | |
|
| |
|
| |
|
|
| |
|
|
The estimated fair value of available for sale securities, by contractual maturity is as follows at December 31:
| | | | | | | | | | | | |
| | | 2005
| | 2004
|
| | | Amortized
Cost
| | Market
Value
| | Amortized
Cost
| | Market
Value
|
Due in one year or less | | $ | 11,914 | | $ | 11,830 | | $ | 49,244 | | $ | 48,791 |
Due between one and two years | | | 9,858 | | | 9,739 | | | 15,490 | | | 15,606 |
| | |
|
| |
|
| |
|
| |
|
|
| | | $ | 21,772 | | $ | 21,569 | | $ | 64,734 | | $ | 64,397 |
| | |
|
| |
|
| |
|
| |
|
|
At December 31, 2005, all of the Company’s investments mature within three years with an average maturity of approximately five months.
The Company evaluates the realizable value of its short-term investments. When assessing short-term investments for other-than-temporary declines in value, the Company considers such factors as how significant the decline in value is as a percentage of the original cost and how long the market value of the investment has been below its original cost. If events and circumstances indicate that a decline in the value of these assets has occurred, and is other than temporary, the Company records a charge to investment income (expense). The Company has not incurred any such charges for the years ended December 31, 2005, 2004, or 2003.
Investments considered to be temporarily impaired at December 31, 2005 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Less than 12 Months
of Temporary
Impairment
| | | Greater Than 12
Months of Temporary
Impairment
| | | Total Temporary
Impairment
| |
| | | Number of
Investments
| | Fair
Value
| | Unrealized
Losses
| | | Fair
Value
| | Unrealized
Losses
| | | Fair
Value
| | Unrealized
Losses
| |
Corporate debt securities | | 11 | | $ | 6,389 | | $ | (102 | ) | | $ | 2,550 | | $ | (30 | ) | | $ | 8,939 | | $ | (132 | ) |
U.S. Government and agency obligations | | 2 | | | 1,495 | | | (5 | ) | | | 7,933 | | | (78 | ) | | | 9,428 | | | (83 | ) |
| | |
| |
|
| |
|
|
| |
|
| |
|
|
| |
|
| |
|
|
|
| | | 13 | | $ | 7,884 | | $ | (107 | ) | | $ | 10,483 | | $ | (108 | ) | | $ | 18,367 | | $ | (215 | ) |
| | |
| |
|
| |
|
|
| |
|
| |
|
|
| |
|
| |
|
|
|
68
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Gross realized gains from the sale of cash equivalents and marketable securities were zero, $60,000, and $913,000 for the years ended December 31, 2005, 2004, and 2003, respectively. Gross realized losses from the sale of cash equivalents and marketable securities were $140,000, $186,000, and $135,000 for the years ended December 31, 2005, 2004, and 2003, respectively.
Accounts receivable consist of the following (in thousands):
| | | | | | |
| | | December 31,
|
| | | 2005
| | 2004
|
Trade | | $ | 3,382 | | $ | 1,494 |
Grants | | | 1,181 | | | 1,573 |
Collaborators | | | 4,411 | | | 2,698 |
Other | | | 38 | | | 6 |
| | |
|
| |
|
|
| | | $ | 9,012 | | $ | 5,771 |
| | |
|
| |
|
|
Other current assets consist of the following (in thousands):
| | | | | | |
| | | December 31,
|
| | | 2005
| | 2004
|
Inventory: | | | | | | |
Raw Materials | | $ | 544 | | $ | 153 |
Work in Process | | | 106 | | | 510 |
Finished Goods | | | 2,021 | | | 920 |
| | |
|
| |
|
|
Total Inventory | | | 2,671 | | | 1,583 |
Prepaid expenses | | | 1,504 | | | 1,669 |
Other receivables | | | 821 | | | — |
| | |
|
| |
|
|
| | | $ | 4,996 | | $ | 3,252 |
| | |
|
| |
|
|
Property and equipment consist of the following (in thousands):
| | | | | | | | |
| | | December 31,
| |
| | | 2005
| | | 2004
| |
Laboratory equipment | | $ | 46,832 | | | $ | 47,407 | |
Computer equipment | | | 13,695 | | | | 14,013 | |
Leasehold improvements | | | 7,235 | | | | 7,253 | |
Furniture and fixtures | | | 5,392 | | | | 5,107 | |
| | |
|
|
| |
|
|
|
| | | | 73,154 | | | | 73,780 | |
Reserve for asset impairment | | | (1,530 | ) | | | — | |
Accumulated depreciation and amortization: | | | (53,379 | ) | | | (46,761 | ) |
| | |
|
|
| |
|
|
|
| | | $ | 18,245 | | | $ | 27,019 | |
| | |
|
|
| |
|
|
|
Depreciation of property, plant and equipment is provided on the straight-line method over estimated useful lives as follows:
| | |
Laboratory equipment | | 5 years |
Computer equipment | | 3 years |
Furniture and fixtures | | 5 years |
69
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Leasehold improvements are depreciated using the shorter of the estimated useful life or remaining lease term.
Intangible assets consist of the following (in thousands):
| | | | | | | |
| | | December 31,
| |
| | | 2005
| | 2004
| |
Assets acquired in connection with 2003 Syngenta transactions | | $ | — | | $ | 54,882 | |
Intellectual property licenses | | | — | | | 4,286 | |
| | |
|
| |
|
|
|
| | | | — | | | 59,168 | |
Accumulated amortization: | | | — | | | (10,454 | ) |
| | |
|
| |
|
|
|
| | | $ | — | | $ | 48,714 | |
| | |
|
| |
|
|
|
In connection with the Company’s recent strategic reorganization, the Company determined, based on an analysis of estimated future cash flows, that the acquired intangible assets were fully impaired as of December 31, 2005, and recorded an impairment charge totaling $43.5 million to write off the net carrying value of these assets (See Note 7—Impairment Charges and Restructuring Activities). Amortization expense for acquired intangible assets for each of the years ended December 31, 2005 and 2004 was approximately $5.2 million, of which approximately $2.6 million was recorded as a reduction of revenue as it related to the research collaboration. Amortization expense for acquired intangible assets for the year ended December 31, 2003 was approximately $4.5 million, of which approximately $2.2 million was recorded as a reduction of revenue as it related to the research collaboration.
Accrued liabilities consist of the following (in thousands):
| | | | | | |
| | | December 31,
|
| | | 2005
| | 2004
|
Outside services | | $ | 1,213 | | $ | 2,794 |
Vacation | | | 1,320 | | | 1,272 |
Professional fees | | | 764 | | | 928 |
Other employee costs | | | 619 | | | 755 |
Bonuses | | | 897 | | | 150 |
Other | | | 1,343 | | | 1,317 |
| | |
|
| |
|
|
| | | $ | 6,156 | | $ | 7,216 |
| | |
|
| |
|
|
The Company has a number of strategic alliances and relationships, the more significant of which include the following:
Research and Development Collaborations
Syngenta
In 1999, the Company entered into a strategic alliance with Syngenta Biotechnology, Inc. (formerly Syngenta Agribusiness Biotechnology Research, Inc.) (“Syngenta Biotechnology”). Under the agreement, the Company received research funding from Syngenta Biotechnology to conduct multiple independent research
70
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
projects with the intention of identifying and developing biomolecules that meet the scientific specifications of Syngenta Biotechnology. In conjunction with the transaction, Syngenta Biotechnology purchased 5,555,556 shares of Series E convertible preferred stock (which converted to common shares upon completion of the Company’s initial public offering), paid a technology access fee, and provided project research funding to the Company, for aggregate total proceeds of $12.5 million.
Also in 1999, the Company formed a five-year strategic alliance with Syngenta Seeds AG (“Syngenta Seeds”). Through a contract joint venture, named Zymetrics, Inc., the Company and Syngenta Seeds jointly pursued opportunities in the field of animal feed and agricultural product processing. Under the agreement, both parties shared in the management of the venture and funded a portion of the sales and marketing costs of this venture. Under the agreement, Syngenta Seeds received exclusive, worldwide rights in the field of animal feed and project exclusive, worldwide rights in the field of agricultural product processing. Syngenta Seeds agreed to pay $20.0 million for the rights granted under the original agreement, which expired in 2004. In May 2004, the Company entered into an agreement with Syngenta that continued the development and commercialization of novel animal feed enzymes beyond the five-year initial term of the 1999 Zymetrics joint venture agreement. Under the agreement, Syngenta agreed to continue its collaboration with the Company in the area of animal feed enzymes on an exclusive basis, paid the Company an exclusivity fee and committed to additional research and development funding. Research funding is recognized as the research is performed, and milestone payments are recognized as revenue when earned. The Company is entitled to a share of the profits in the form of royalties on any product sales that result from these agreements.
In 2002, the Company entered into a manufacturing agreement with an affiliate of Syngenta to supply commercial quantities of Quantum phytase at a fixed price, determined by a negotiated formula, that is subject to adjustment during the term of the agreement. In addition, the Company is entitled to receive royalties from Syngenta on their sales of Quantum phytase.
During 2003, the Company completed a series of transactions with Syngenta Participations AG (“Syngenta”) and its wholly-owned subsidiary, the Torrey Mesa Research Institute (“TMRI”). Under the transactions, the companies formed an extensive research collaboration whereby the Company is entitled to receive a minimum of $118.0 million in research and development funding over the initial seven-year term of the related research collaboration agreement and will be eligible to receive certain milestone payments and royalties upon product development and commercialization. Additionally, the Company acquired certain intellectual property rights licenses from Syngenta used in activities conducted at TMRI that primarily involve tools, technologies and methods relating to proteomics, metabolomics, RNA dynamics, and bioinformatics and methods to analyze and link these components of genomics or that primarily relate to TMRI’s fungal program, for use outside of Syngenta’s exclusive field as defined in the research collaboration agreement. The Company also purchased certain property and equipment from TMRI and assumed certain miscellaneous liabilities under equipment maintenance contracts.
Upon closing, the Company issued to Syngenta and TMRI a total of 6,034,983 shares of common stock and a warrant to purchase 1,293,211 shares of common stock at $22.00 per share that is exercisable for ten years starting in 2008. The total value of the acquisition was approximately $74.0 million, including transaction fees. The value of the Company’s shares used in determining the purchase price was $11.44 per share. The value of the warrant issued was determined by a third party valuation. The transaction was accounted for as an asset purchase under accounting principles generally accepted in the United States.
71
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes the estimated fair values and useful lives of the assets acquired as of the acquisition date (in thousands):
| | | | | |
| | | Amount
| | Estimated
Useful Life
|
Property and equipment | | $ | 8,211 | | 3–5 years |
In-process research and development | | | 10,766 | | See below |
Identifiable intangible assets: | | | | | |
Core technology | | | 36,632 | | 15 years |
Research collaboration | | | 18,250 | | 7 years |
| | |
|
| | |
Net assets | | $ | 73,859 | | |
| | |
|
| | |
Approximately $10.8 million of the purchase price represented the estimated fair value of projects that, as of the acquisition date, had not reached technological feasibility and had no alternative future use. Accordingly, this amount was immediately expensed in February 2003. In December 2005, in connection with the Company’s strategic reorganization, the Company recorded an impairment charge related to the write-down of the carrying values of assets and technologies acquired as part of the acquisition (See Note 7—Impairment Charges and Restructuring Activities).
Revenue recognized under the Syngenta agreements was $24.3 million, $36.9 million, and $29.2 million for the years ended December 31, 2005, 2004, and 2003.
DuPont Bio-Based Materials
In 2003, the Company entered into a six-year alliance with DuPont Bio-Based Materials to discover and develop novel biocatalysts for the production of fuel ethanol, 1,3 propanediol, and other added-value chemicals from renewable resources such as corn and biomass. The program with DuPont, referred to as an “Integrated Corn-Based Biorefinery” program, is part of a grant consortium funded by the U.S. Department of Energy to develop a biorefinery capable of producing high-value chemical products from biomass. Under our collaboration agreement with DuPont regarding this biorefinery program, the Company has received research funding, as well as milestone payments, and is entitled to additional milestone payments as well as royalties on any new products developed under the agreement that incorporate the Company’s technologies. Revenue recognized under the DuPont alliance was $3.0 million, $2.4 million, and $1.5 million for the years ended December 31, 2005.
DSM Pharma Chemicals
In 2003, the Company entered into a collaborative agreement with DSM Pharma Chemicals to discover and develop biocatalytic solutions designed to simplify and lower the cost of a variety of chemical transformations. Under the terms of the agreement, DSM will identify targeted chemical conversions, the Company will work to develop appropriate biocatalysts, and DSM will scale-up these processes to manufacture pharmaceutical intermediates and active ingredients. The Company will receive research payments and is entitled to milestones and royalties on products commercialized by DSM. Revenue recognized under the DSM Pharma Chemicals agreements was $0.5 million and $1.0 million for the year ended December 31, 2005 and 2004. There was no revenue recognized under these agreements in 2003.
Cargill Health and Food Technologies
In 2005, the Company signed a collaboration agreement with Cargill Health and Food Technologies to discover and develop novel enzymes for the cost-effective production of a proprietary Cargill product. Under the
72
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
terms of the agreement, the Company received upfront payments and research funding, and is entitled to receive milestone payments, license fees, and royalties on products that may be developed under the agreement. Revenue recognized under the Cargill collaboration was $2.1 million for the year ended December 31, 2005.
Merck & Co., Inc.
In December 2004, the Company entered into an agreement with Merck & Co., Inc. to collaborate on the development of therapeutic antibodies for a key target by applying our proprietary MedEv™ platform. Under the terms of the agreement, the Company received an upfront payment and received research funding. In mid-2005, the two parties amended this agreement to provide for additional research and development activities as well as terms for additional research funding, milestone payments, and royalties.
The Dow Chemical Company
In 2000, the Company formed a 50/50 joint venture with Dow, named Innovase LLC (“Innovase”), to develop and commercialize innovative products for certain fields in the industrial enzyme market. Under the various joint venture related agreements, the Company received exclusivity fees, technology development fees, and research and development payments. The Company was amortizing the exclusivity and technology development fees over the period of the agreements. Research funding was recognized as the research was performed. Revenues earned from the exclusivity and technology development fees and from the research activities performed on behalf of Innovase are included in collaborative revenue in the accompanying consolidated statements of operations. The Company was also required to fund certain operating expenses of the joint venture. The Company’s share of the net income (loss) of Innovase, excluding certain expenses for which Dow was solely responsible under the joint venture agreement, was recorded utilizing the equity method of accounting and is included in the accompanying consolidated statements of operations.
During 2003, the Company and Dow signed a definitive agreement to restructure the Innovase joint venture. Under the terms of the restructuring agreement, rights to all products and product candidates developed in conjunction with Innovase were conveyed to the Company, and the companies mutually agreed to terminate all exclusivity and non-competition arrangements for the industrial enzyme fields related to the Innovase joint venture. Dow divested its 50% ownership interest to the Company and paid the Company $5.0 million. All joint venture related agreements involving Dow were terminated. For the year ended December 31, 2003, the Company recorded expenses of $1.3 million related to the Company’s equity in the loss of the joint venture. Revenue recognized under the Dow agreements was $1.8 million and $10.7 million for the years ended December 31, 2004 and 2003. No revenue was recognized under any of the Dow agreements for the year ended December 31, 2005.
Government Grants and Contracts
The Company has received grants contracts and from a number of government agencies, including the U.S. Department of Defense, the U.S. Department of Energy, and the National Institutes of Health. Revenue related to government grants and contracts was $10.1 million, $10.2 million, and $3.9 million for the years ended December 31, 2005, 2004, and 2003.
Manufacturing, Supply and Distribution Agreements
Valley Research, inc.
In 2005, the Company signed, and later amended, a distribution agreement with Valley Research, inc. (“Valley”) covering the Ultra-Thin alpha amylase enzyme and potentially additional enzyme products. Under the
73
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
amended agreement, the Company appointed Valley as its exclusive distributor in the United States for Ultra-Thin enzyme for ethanol and high fructose corn sweetener applications, subject to certain limitations, and subject to certain conditions required to be met for such exclusivity to be maintained. Valley must purchase certain minimum dollar amounts of Ultra-Thin enzyme from the Company during each year of the agreement in order to maintain exclusivity. The term of this distribution agreement regarding Ultra-Thin enzyme is for a period of five years following regulatory approval of such enzyme by the FDA’s Center for Veterinary Medicine, which approval was obtained on February 24, 2006.
Danisco Animal Nutrition
In May 1996, the Company entered into a collaboration agreement with Danisco Animal Nutrition (formerly Finnfeeds International Ltd) to jointly identify and develop a novel phytase enzyme that when used as an additive in animal feed applications allows higher utilization of phytic acid phosphates from the feed, thereby increasing its nutritional value. The addition of phytase to animal feed reduces the need for inorganic phosphorus supplementation and lowers the level of harmful phosphates that are introduced to the environment through animal waste, resulting in inorganic phosphate cost savings and a significant reduction in environmental pollution. Following the completion of the initial objectives of the agreement with Danisco, in December 1998, the Company entered into a license agreement with Danisco to commercialize an enzyme developed under the collaboration agreement. Under the terms of the license agreement, the Company granted Danisco an exclusive license to manufacture, use, and sell the developed enzyme. In consideration for the license, the Company is paid a profit share equal to 50% of the cumulative profits generated by Danisco on such sales. In March 2003, the FDA approved Phyzyme XP Animal Feed Enzyme, which the Company developed in collaboration with Danisco. Additionally, the Company entered into a manufacturing agreement with Danisco to supply commercial quantities of Phyzyme XP at the Company’s cost to manufacture such quantities. Revenue recognized from transactions with Danisco, including contract manufacturing performed on behalf of Danisco, was $5.2 million, $2.0 million, and $2.0 million for the years ended December 31, 2005, 2004, and 2003.
License Agreements
Xoma Ltd.
In 2003, the Company signed a license and product development agreement with Xoma Ltd. Under the terms of the agreement, the Company received a license to use Xoma’s antibody expression technology for developing antibody products independently and with collaborators, and an option to a license for the production of antibodies under the Xoma patents. The Company paid an initial license fee of $2.0 million, which was initially capitalized and was being amortized over the estimated useful life of seven years. Under the agreement, the Company may also be required to pay future milestones and royalties. As of December 31, 2005, in connection with the Company’s strategic reorganization, the Company assessed the carrying value of this license on its balance sheet and determined that it was impaired. As a result, the Company has written off the carrying value of the license on its balance sheet as of December 31, 2005(See Note 7—Impairment Charges and Restructuring Activities). Under the terms of the development portion of the agreement, the Company and Xoma will combine their respective capabilities to discover and develop antibodies for autoimmune-related diseases.
Terragen Discovery, Inc.
In November 1999, the Company signed a license agreement with Terragen Discovery Inc., or Terragen, under which the Company and Terragen agreed to cross license certain technologies. Under the terms of the agreement, the Company made an initial payment of $2.5 million in 1999 and agreed to make annual payments of $0.1 million to Terragen to maintain the patent rights over the remaining patent life. The Company capitalized
74
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
the initial payment as an intangible asset, which through December 31, 2005 was amortized over the sixteen year patent life. As of December 31, 2005, in connection with the Company’s strategic reorganization, the Company assessed the carrying values of this license on our balance sheet and determined that it was impaired. As a result, the Company has written off the carrying value of the license on its balance sheet as of December 31, 2005(See Note 7—Impairment Charges and Restructuring Activities).
Glaxo Wellcome, S.A.
In 2003, the Company acquired an antifungal program consisting of preclinical Sordarins compounds from Glaxo Wellcome, S.A. In consideration for the antifungal program, the Company issued an aggregate of 806,873 shares of its common stock to Glaxo Group Limited, an affiliate of Glaxo Wellcome, S.A. Under the terms of the agreement, the Company received worldwide rights to the program, which consists of preclinical antifungal compounds and lead candidates for development. Based upon the closing price of the Company’s common stock immediately preceding consummation of the transaction, the fair value of the compounds and lead candidates was $8.7 million. As of the acquisition date, these compounds and lead products had not reached technological feasibility and had no alternative future use. Accordingly, the Company recorded $8.7 million as a write-off of acquired in-process research and development in 2003.
Other Agreements
The Company has signed various agreements with research institutions, as well as other commercial entities. Generally, these agreements call for the Company to pay research support, cost reimbursement, and, in some cases, subsequent royalty payments in the event a product is commercialized. The financial impact of these agreements on the Company is not significant.
The Company has entered into various equipment financing line of credit agreements with lenders to finance equipment purchases. Under the terms of the credit agreements, equipment purchases are structured as notes and are to be repaid over periods ranging from 36 to 48 months at interest rates ranging from 6.99% to 9.66%. The notes are secured by the related equipment. As of December 31, 2005, the Company had $8.4 million available under the existing lines of credit.
On September 30, 2005, the Company entered into a $14.6 million Loan and Security Agreement (the “Bank Agreement”) with a commercial bank (the “Bank”). The Bank Agreement provides for a one-year credit facility for up to $10.0 million in financing for qualified equipment purchases in the United States and Mexico (the “Equipment Advances”) and a $4.6 million letter of credit sub-facility (the “Letter of Credit Sublimit”). Borrowings under the Equipment Advances will be structured as promissory notes which are secured by qualified equipment purchases and repaid over 36 to 48 months, depending on the location of the equipment financed. Borrowings will bear interest at the Bank’s prime rate (7.25% at December 31, 2004) plus 0.75%.
The Bank Agreement contains standard affirmative and negative covenants and restrictions on actions by the Company including, but not limited to, activity related to the Company’s common stock repurchases, liens, investments, indebtedness, and fundamental changes in, or dispositions of, the Company’s assets. Certain of these actions may be taken by the Company with the consent of the Bank. In addition, the Company is required to meet certain financial covenants, primarily a minimum balance of unrestricted cash, cash equivalents, and investments in marketable securities of $25.0 million, including $15.0 million maintained in accounts at the Bank or its affiliates.
75
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
At December 31, 2005, there was approximately $1.6 million in outstanding borrowings under the Equipment Advances and a letter of credit for approximately $4.6 million under the Letter of Credit Sublimit, as required under the Company’s facilities leases (See Note 6—Commitments and Contingencies).
As of December 31, 2005 the Company was in compliance with all debt covenants under its various financing agreements. At December 31, 2005, the Company’s future minimum payments under the equipment financing arrangements are as follows (in thousands):
| | | | |
Year ending December 31: | | | | |
2006 | | $ | 7,882 | |
2007 | | | 4,605 | |
2008 | | | 1,791 | |
2009 | | | 395 | |
| | |
|
|
|
Total future minimum payments | | | 14,673 | |
Less amounts representing interest | | | (1,317 | ) |
| | |
|
|
|
Total future minimum principal payments | | | 13,356 | |
Less current portion of debt obligations | | | (7,024 | ) |
| | |
|
|
|
Non-current portion of debt obligations | | $ | 6,332 | |
| | |
|
|
|
| 5. | Related Party Transactions |
Syngenta AG
On February 20, 2003, the Company entered into a research collaboration with Syngenta, a greater-than 10% owner of the Company’s outstanding common stock. Under the collaboration, which was amended in May 2004, the Company is entitled to receive a minimum of $118.0 million in research and development funding over the initial seven-year term of the related research collaboration agreement and is eligible to receive certain milestone payments and royalties upon product development and commercialization (See Note 3—Significant Agreements).
The Company recognized revenue from Syngenta and its affiliates of $24.3 million, $36.9 million, and $29.2 million for the years ended December 31, 2005, 2004, and 2003. Accounts receivable due from Syngenta were $1.7 million and $1.5 million, and deferred revenue associated with Syngenta was $5.9 million and $5.3 million, at December 31, 2005 and 2004.
In connection with the research collaboration with Syngenta, the Company received $0.5 million in rental cost reimbursements from Syngenta during the year ended December 31, 2005, which was recorded as a reduction in rent expense(See Note 6—Commitments and Contingencies).
Notes Receivable from Officers
In February 2000, the Company initiated a loan program for six employees to pay personal tax liabilities resulting from the failure to file Form 83(b) elections with the Internal Revenue Service related to those employees’ exercise of incentive and non-qualified stock options during 1999. This failure to timely file the Form 83(b) elections exposed the employees to significant personal tax liabilities. To limit the tax exposure, the Company elected to accelerate the vesting of the approximately 207,000 unvested options, and agreed to loan the employees up to $1.6 million in full recourse promissory notes. In 2000, the acceleration of the unvested options
76
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
resulted in a non-cash compensation charge to the Company of approximately $4.1 million. Additionally, the Company agreed to directly compensate two individuals a total of approximately $0.2 million in cash. As of December 31, 2005 and 2004, the Company had a remaining loan balance from four individuals aggregating $0.6 million, which amounts are included in other current assets on the accompanying balance sheet. The notes bear interest at 4.94%, are due in April 2006, and contain certain mandatory prepayment provisions.
| 6. | Commitments and Contingencies |
Leases
At December 31, 2005, the Company’s minimum commitments under non-cancelable operating leases were as follows (in thousands):
| | | |
| | | Operating
Leases
|
Year ending December 31: | | | |
2006 | | $ | 4,663 |
2007 | | | 4,837 |
2008 | | | 4,990 |
2009 | | | 5,176 |
Thereafter | | | 36,744 |
| | |
|
|
Total minimum lease payments | | $ | 56,410 |
| | |
|
|
In November 2000, the Company relocated its San Diego operations to a 75,000 square foot facility. In April 2002, the Company occupied an additional 60,000 square foot research and development facility adjacent to its existing office. The operating leases for the Company’s two facilities expire in November 2015 and March 2017.
In March 2002, the Company entered into an agreement to sublease approximately 27,000 square feet of its new facility. The sublease was effective April 1, 2002 with a term of three years. During the year ended December 31, 2003, the Company received $0.3 million of rental income under its sublease, which was recorded as a reduction of rent expense. In August 2003, the Company agreed to terminate the sublease agreement. As a result, the Company did not receive any rental income from this sublease agreement in 2005 or 2004.
During 2005, the Company received $0.5 million of rent reimbursement from Syngenta, a related party (See Note 5—Related Party Transactions).
For the years ended December 31, 2005, 2004, and 2003 rent and administrative service expense under operating leases was approximately $4.6 million, $4.9 million, and $4.5 million, net of rental income. Under the terms of its facilities leases, the Company is required to maintain an irrevocable standby letter of credit from a bank in lieu of a cash security deposit. The amount of the letter of credit is based upon certain financial covenants requiring minimum market capitalization or working capital. As of December 31, 2005, the amount of the letter of credit required was $4.6 million, which has been issued under the Company’s Bank Agreement(See Note 4—Debt). Amounts outstanding under the letter of credit are unsecured, and are subject to an annual fee of 1.25%. The letter of credit expires in September 2006.
Included in property and equipment at December 31, 2004 were assets acquired under capital lease obligations with an original cost of $7.2 million. Related accumulated depreciation and amortization on these
77
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
assets was $7.2 million as of December 31, 2004. The Company had no equipment under capital lease at December 31, 2005.
During 2002, the Company entered into a manufacturing agreement with Fermic, S.A. de C.V. (“Fermic”), a fermentation and synthesis plant located in Mexico City, to provide the Company with the capacity to produce commercial quantities of certain enzyme products. Based on actual and projected increased product requirements, the agreement was amended in 2004 to provide for additional capacity to be installed over the succeeding four year period. Under the terms of the agreement, the Company can cancel the committed purchases with thirty months’ notice provided that the term of the agreement, including the termination notice period, aggregates four years. Pursuant to the agreement with Fermic, the Company is also obligated to reimburse monthly costs related to manufacturing activities. These costs scale up as the projected manufacturing volume increases. As of December 31, 2005, under this agreement the Company had made minimum commitments to Fermic of approximately $15.5 million, over the next three years. In addition, under the terms of the agreement, the Company is required to purchase certain equipment required for fermentation and downstream processing of the products. Through December 31, 2005, the Company had incurred costs of approximately $10.5 million for equipment related to this agreement. During 2006, the Company anticipates spending as much as $5.0 million in additional equipment costs related to the manufacturing agreement. As the Company continues to develop its commercial manufacturing platforms, it will be required to purchase additional capital equipment under this agreement.
The Company relies on Fermic as its sole-source manufacturer for large volumes of commercial enzymes.
Litigation
In June 2004, the Company executed a formal settlement agreement with the plaintiffs in a class action lawsuit filed in December 2002 in a U.S. federal district court (the “Court”). This lawsuit is part of a series of related lawsuits in which similar complaints were filed by plaintiffs against hundreds of other public companies that conducted IPOs of their common stock in 2000 and the late 1990’s. On February 15, 2005, the Court issued a decision certifying a class action for settlement purposes and granting preliminary approval of the settlement subject to modification of certain orders contemplated by the settlement. On August 31, 2005, the Court reaffirmed class certification and preliminary approval of the modified settlement in a comprehensive order. In addition, the Court approved the form of notice to be sent to members of the settlement classes, which was published and mailed beginning November 15, 2005. The Court has set a final settlement fairness hearing on the settlement for April 24, 2006. The settlement is still subject to statutory notice requirements as well as final judicial approval. The Company is covered by a claims-made liability insurance policy which it believes will satisfy the Company’s insurers’ pro-rata liability under this settlement.
| 7. | Impairment Charges and Restructuring Activities |
During the fourth quarter of 2005, the Company recorded a $45.7 million impairment charge for activities resulting from management’s strategic decision to reorganize and refocus the Company’s resources to advance its most promising product candidates and programs that have the greatest near-term opportunities. The Company wrote-off the carrying values of tangible and intangible assets considered non-essential to the Company’s current focus, or otherwise deemed impaired under the provisions set forth by FASB No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.”
��
78
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
These charges are summarized below (in thousands):
| | | |
| | | Year Ended
December 31,
2005
|
Write-off of intangible assets acquired in connection with fiscal 2003 transactions with Syngenta | | $ | 40,622 |
Excess or idle equipment costs | | | 2,237 |
Write-off of intellectual property licenses | | | 2,886 |
| | |
|
|
Total | | $ | 45,745 |
| | |
|
|
In connection with the decision to reorganize and refocus the Company’s resources, in January 2006 the Company commenced several cost containment measures, including a reduction in workforce of 83 employees and the consolidation of its facilities. Pursuant to FASB No. 146,“Accounting for Costs Associated with Exit or Disposal Activities,” the Company expects to record charges of approximately $15.0 to $17.0 million, primarily in the first quarter of 2006, related to these actions.
| 8. | Concentration of Business Risk |
During the years ended December 31, 2005, 2004, and 2003, the Company had collaborative research agreements that accounted for 63%, 73%, and 86% of total revenue. Including revenue generated from DuPont (See Note 3—Significant Agreements), the Company derived, directly or indirectly, approximately 24%, 22%, and 11%, of its revenue from agencies of the United States Government in 2005, 2004, and 2003.
A relatively small number of customers and collaboration partners historically have accounted for a significant percentage of the Company’s revenue. Revenue from significant customers and / or collaboration partners as a percentage of total revenue was as follows:
| | | | | | | | | |
| | | 2005
| | | 2004
| | | 2003
| |
Customer A | | 45 | % | | 64 | % | | 60 | % |
Customer B | | 10 | % | | 4 | % | | 4 | % |
Customer C | | — | % | | 3 | % | | 22 | % |
Accounts receivable from four significant customers comprised approximately 21%, 18%, 15%, and 12% of accounts receivable at December 31, 2005. Accounts receivable from four significant customers comprised approximately 25%, 17%, 11%, and 10% of accounts receivable at December 31, 2004. Accounts receivable derived directly or indirectly from agencies of the U.S. Government, including accounts receivable from DuPont (See Note 3—Significant Agreements), comprised 13% and 44% of total accounts receivable at December 31, 2005 and 2004.
Revenue by geographic area was as follows (in thousands):
| | | | | | | | | |
| | | For the years ended December 31,
|
| | | 2005
| | 2004
| | 2003
|
North America | | $ | 20,119 | | $ | 16,378 | | $ | 17,741 |
South America | | | 1,583 | | | 1,302 | | | — |
Europe | | | 32,001 | | | 39,870 | | | 31,218 |
Asia | | | 600 | | | — | | | — |
| | |
|
| |
|
| |
|
|
| | | $ | 54,303 | | $ | 57,550 | | $ | 48,959 |
| | |
|
| |
|
| |
|
|
79
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Shareholder Rights Plan
On December 13, 2000, the Board of Directors of the Company approved the adoption of a shareholder rights plan (the “Rights Plan”). Under the Rights Plan, the Board of Directors declared a dividend of one right to purchase one one-hundredth of a share of Series A junior participating preferred stock (a “Right”) for each share of Company common stock outstanding as of December 22, 2000. The exercise price of each Right is $125.00.
Initially, the Rights trade with the Company’s common stock and are not separately transferable. However, subject to certain exceptions, the Rights will become exercisable (i) at such time that a person (or group of affiliated persons) acquires beneficial ownership of 15% or more of the outstanding Company common stock (an “Acquiring Person”) or (ii) on the tenth business day after a person or entity commences, or expresses an intention to commence, a tender or exchange offer that would result in such person acquiring 15% or more of the outstanding Company common stock. In December 2002, in connection with the Company’s entering into a series of agreements with Syngenta and Torrey Mesa Research Institute, the Company amended the Rights Plan to provide that, with respect to Syngenta and its affiliates and associates, the threshold will be 22% rather than 15% for the aggregate beneficial ownership of the Company’s common stock that their holdings may not exceed without the Rights becoming exercisable.
In the event a person becomes an Acquiring Person, each Right held by all persons other than the Acquiring Person will become the right to acquire one share of Company common stock at a price equal to 50% of the then-current market value of the Company common stock. Furthermore, in the event an Acquiring Person effects a merger of the Company, each Right will entitle the holder thereof to purchase one share of common stock of the Acquiring Person or the Acquiring Person’s ultimate parent at a price equal to 50% of the then-current market value of the Acquiring Person’s or the Acquiring Person’s ultimate parent’s common stock.
The Board of Directors can redeem the Rights at any time prior to a person becoming an Acquiring Person at a redemption price of $0.01 per Right. In addition, the Board of Directors may, after any time a person becomes an Acquiring Person, exchange each Right for one share of common stock of the Company. The Rights will expire on December 12, 2010 if not redeemed prior to such date.
| 10. | Stock Option Plans and Warrants |
Non-Employee Directors’ Stock Option Plans
2005 Non-Employee Directors’ Equity Incentive Plan
In March 2005, the Board of Directors of the Company (“Board”) adopted the Company’s 2005 Non-Employee Directors’ Equity Incentive Plan (“Directors’ Plan”), and reserved a total of 600,000 shares for issuance thereunder. The number of shares available for issuance under the Directors’ Plan will automatically increase on the first trading day of each calendar year, beginning with the 2006 calendar year and continuing through and including calendar year 2015, by an amount equal to the excess of (i) the number of shares subject to stock awards granted during the preceding calendar year, over (ii) the number of shares added back to the share reserve during the preceding calendar year pursuant to expirations, terminations, cancellations forfeitures and repurchases of previously granted awards. However this automatic annual increase shall not exceed 250,000 shares in any calendar year.
The Board adopted the Directors’ Plan as the primary equity incentive program for the Company’s non-employee directors in order to secure and retain the services of such individuals, and to provide incentives
80
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
for such persons to exert maximum efforts for the success of the Company. The Directors’ Plan replaced the 1999 Non-Employee Directors’ Stock Option Plan. As of December 31, 2005, there were approximately 170,000 shares outstanding under the Directors’ Plan.
Employee Stock Option and Stock Purchase Plans
1999 Employee Stock Purchase Plan
In December 1999, the Board of Directors adopted the 1999 Employee Stock Purchase Plan (the “Purchase Plan”). As of December 31, 2005, a total of 1,437,000 shares of the Company’s common stock have been reserved for issuance under the Purchase Plan. The Purchase Plan permits eligible employees to purchase common stock at a discount, but only through payroll deductions, during defined offering periods. The price at which stock is purchased under the Purchase Plan is equal to 85% of the fair market value of the common stock on the first or last day of the offering period, whichever is lower. The Purchase Plan provides for annual increases of shares available for issuance under the Purchase Plan.
1997 Equity Incentive Plan
In August 1997, the Company adopted the 1997 Equity Incentive Plan (the “1997 Plan”), which provides for the granting of incentive or non-statutory stock options, stock bonuses, and rights to purchase restricted stock to employees, directors, and consultants as administered by the Board of Directors. Unless terminated sooner by the Board of Directors, the 1997 Plan will terminate in August 2007.
The incentive and non-statutory stock options are granted with an exercise price of not less than 100% and 85%, respectively, of the estimated fair value of the underlying common stock as determined by the Board of Directors. The 1997 Plan allows the purchase of restricted stock at a price that is not less than 85% of the estimated fair value of the Company’s common stock as determined by the Board of Directors.
Options granted under the 1997 Plan vest over periods ranging up to four years and are exercisable over periods not exceeding ten years. As of December 31, 2005, the aggregate number of shares which may be awarded under the 1997 Plan is 12,983,000, with 2,914,000 available for grant.
Restated 1994 Employee Incentive and Non-Qualified Stock Option Plan
The Restated 1994 Employee Incentive and Non-Qualified Stock Option Plan (the “1994 Plan”) provided for the granting of incentive or non-qualified stock options to employees and consultants as administered by the Board of Directors. The incentive stock options were granted with an exercise price of not less than the estimated fair value of the underlying common stock as determined by the Board of Directors. The non-qualified stock options were granted with an exercise price of not less than $0.03.
Options granted under the 1994 Plan vest over periods ranging up to four years and are exercisable over periods not exceeding ten years. Options to purchase 952,000 shares have been granted under the 1994 Plan, and 2,000 options remain outstanding related to the 1994 Plan. In August 1997, the 1994 Plan was terminated, and there are no options available for future grant.
81
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Information with respect to all of the Company’s stock option plans is as follows (in thousands, except per share data):
| | | | | | |
| | | Shares
| | | Weighted-
Average
Exercise Price
Per Share
|
Balance at January 1, 2003 | | 5,206 | | | $ | 11.20 |
Granted | | 2,077 | | | $ | 7.83 |
Exercised | | (194 | ) | | $ | 1.48 |
Cancelled | | (162 | ) | | $ | 13.32 |
| | |
|
| | | |
Balance at December 31, 2003 | | 6,927 | | | $ | 10.42 |
Granted | | 2,802 | | | $ | 9.80 |
Exercised | | (323 | ) | | $ | 4.11 |
Cancelled | | (944 | ) | | $ | 10.42 |
| | |
|
| | | |
Balance at December 31, 2004 | | 8,462 | | | $ | 10.45 |
Granted | | 658 | | | $ | 6.58 |
Exercised | | (366 | ) | | $ | 2.34 |
Cancelled | | (1,215 | ) | | $ | 11.51 |
| | |
|
| | | |
Balance at December 31, 2005 | | 7,539 | | | $ | 10.34 |
| | |
|
| | | |
At December 31, 2005, options to purchase 6,704,423 shares were exercisable, and approximately 3,549,882 shares remain available for grant.
A further detail of the options outstanding as of December 31, 2005 is set forth as follows (in thousands, except per share data):
| | | | | | | | | | |
Range of
Exercise Prices
| | Options
Outstanding
| | Weighted-
Average
Remaining
Life in Years
| | Weighted- Average
Exercise Price Per Share
| | Options
Exercisable
| | Weighted-
Average
Exercise Price
Per Share of
Options
Exercisable
|
$ 0.42 – $ 2.02 | | 1,035 | | 2.8 | | $ 0.96 | | 1,035 | | $ 0.96 |
$ 2.03 – $ 7.63 | | 1,486 | | 7.5 | | $ 6.99 | | 942 | | $ 7.33 |
$ 7.64 – $13.80 | | 3,263 | | 7.7 | | $ 9.62 | | 2,973 | | $ 9.70 |
$13.81 – $88.63 | | 1,755 | | 5.1 | | $20.02 | | 1,754 | | $19.98 |
| | |
| | | | | |
| | |
$ 0.42 – $88.63 | | 7,539 | | 6.4 | | $10.34 | | 6,704 | | $10.71 |
| | |
| | | | | |
| | |
Restricted Shares
During 2005, the Company issued, under the 1997 Equity Incentive Plan, approximately 726,000 shares of restricted stock to employees, which vest over four years. As of December 31, 2005, approximately 138,000 restricted shares had been forfeited, 588,000 were outstanding, and 28,000 were vested.
Options Granted to Non-Employees
The Company has granted non-qualified stock options to certain non-employees in connection with consulting agreements. During the year ended December 31, 2003, the Company recorded non-cash, stock-based
82
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
compensation charges of approximately $0.1 million associated with these grants, and no charges during the years ended December 31, 2005 and 2004.
Warrants
In connection with the closing of a series of transactions with Syngenta Participations AG in February 2003, the Company issued to Syngenta a warrant to purchase 1,293,00 shares of common stock at $22 per share that is exercisable for ten years starting in 2008.
As of December 31, 2005, warrants to purchase 1,331,000 shares of common stock at exercise prices ranging from $0.03 to $38.39 per share were outstanding. Warrants to purchase 38,000 were exercisable as of December 31, 2005. The warrants expire at various dates through 2018.
Common Stock Reserved for Future Issuance
At December 31, 2005, the Company has reserved shares of common stock for future issuance as follows (in thousands):
| | |
Employee Stock Purchase Plan | | 216 |
Stock Option Plans | | 3,550 |
Warrants | | 1,331 |
| | |
|
| | | 5,097 |
| | |
|
The Company has a 401(k) plan which allows participants to defer a portion of their income through contributions. Such deferrals are fully vested and are not taxable to the participant until distributed from the plan upon termination, retirement, permanent disability, or death. The Company matches a portion of the employee contributions and may, at its discretion, make additional contributions. The Company made cash contributions of approximately $0.7 million during each of the years ended December 31, 2005, 2004, and 2003.
83
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Significant components of the Company’s deferred tax assets are shown below. A valuation allowance of $116.9 million and $79.8 million has been recognized to offset the deferred tax assets at December 31, 2005 and 2004 as realization of such assets is uncertain. The following table sets forth the detail of the Company’s deferred taxes (in thousands):
| | | | | | | | |
| | | As of December 31,
| |
| | | 2005
| | | 2004
| |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | $ | 72,101 | | | $ | 53,771 | |
Federal and state tax credits | | | 8,203 | | | | 7,739 | |
Deferred revenue | | | 3,580 | | | | 2,401 | |
Depreciation and amortization | | | 23,718 | | | | 9,266 | |
Allowance and accrued liabilities | | | 2,242 | | | | 2,533 | |
Capitalized research and development | | | 7,030 | | | | 4,081 | |
| | |
|
|
| |
|
|
|
Total deferred tax assets | | | 116,874 | | | | 79,791 | |
Valuation allowance | | | (116,874 | ) | | | (79,791 | ) |
| | |
|
|
| |
|
|
|
Net deferred tax assets | | $ | — | | | $ | — | |
| | |
|
|
| |
|
|
|
At December 31, 2005, the Company has federal and California net operating loss carry-forwards of approximately $197.1 million and $54.2 million, respectively. The federal net operating loss carry-forwards will begin to expire in 2011 unless utilized. The California net operating loss carry-forwards will begin to expire in 2006 unless utilized. The Company also has federal research credits of approximately $5.2 million which begin to expire in 2011, California research credits of approximately $3.9 million which will carryover indefinitely, and California manufacturer’s investment credits of approximately $0.7 million, which will begin to expire in 2010.
Pursuant to Sections 382 and 383 of the Internal Revenue Code, annual use of the Company’s net operating loss and credit carry-forwards may be limited due to cumulative changes in ownership of more than 50%.
84
DIVERSA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 13. | Selected Quarterly Data (Unaudited) |
The following tables set forth certain unaudited quarterly information for each of the eight fiscal quarters in the two year period ended December 31, 2005. This quarterly information has been prepared on a consistent basis with the audited consolidated financial statements and, in the opinion of management, includes all adjustments which management believes are necessary for a fair presentation of the information for the periods presented. Our quarterly operating results may fluctuate significantly as a result of a variety of factors, and operating results for any quarter are not necessarily indicative of results for a full fiscal year or future quarters.
| | | | | | | | | | | | | | | | |
2005 Quarter Ended
| | Dec. 31
| | | Sep. 30
| | | June 30
| | | Mar. 31
| |
| | | (in thousands, except per share data) | |
Total revenue | | $ | 14,516 | | | $ | 12,773 | | | $ | 14,185 | | | $ | 12,829 | |
Operating expenses (1) | | | 69,527 | | | | 24,872 | | | | 25,396 | | | | 24,955 | |
Net loss | | | (54,688 | ) | | | (12,067 | ) | | | (10,977 | ) | | | (11,986 | ) |
Basic and diluted net loss per common share | | | (1.23 | ) | | | (0.27 | ) | | | (0.25 | ) | | | (0.27 | ) |
| | | | |
2004 Quarter Ended
| | Dec. 31
| | | Sep. 30
| | | June 30
| | | Mar. 31
| |
| | | (in thousands, except per share data) | |
Total revenue | | $ | 18,136 | | | $ | 12,896 | | | $ | 14,721 | | | $ | 11,797 | |
Operating expenses | | | 24,269 | | | | 22,149 | | | | 22,778 | | | | 22,112 | |
Net loss | | | (6,026 | ) | | | (9,229 | ) | | | (8,015 | ) | | | (10,155 | ) |
Basic and diluted net loss per common share | | | (0.14 | ) | | | (0.21 | ) | | | (0.18 | ) | | | (0.24 | ) |
| (1) | Includes a non-cash asset impairment charge of $45.7 million during the fourth quarter of 2005 |
85
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures. Under the supervision and with the participation of our management, including our Chief Executive Officer (CEO), who is our principal executive officer, and Chief Financial Officer (CFO), who is our principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rules 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K.
There are inherent limitations in the effectiveness of any internal control, including the possibility of human error and the circumventions or overriding of controls. Consequently, even effective internal controls can only provide reasonable assurances with respect to any disclosure controls and procedures and internal control over financial statement preparation and presentation.
Management’s Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2005 based on the framework inInternal Control—Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework inInternal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2005, and that no material weaknesses have been identified.
Our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2005 has been audited by Ernst &Young LLP, an independent registered public accounting firm, as stated in their attestation report which is included herein.
Changes in Internal Control over Financial Reporting. We do not believe that any of these changes has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. Our CEO and CFO also evaluated whether a change had occurred in our internal controls over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. Based on such evaluation, such officers have concluded that there was no change in our internal control over financial reporting that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Subsequent to December 31, 2005, we implemented a strategic reorganization of our business, including a reduction in workforce of 83 employees. We do not believe that any of these changes has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
86
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Board of Directors and Stockholders
Diversa Corporation
We have audited management’s assessment, included in the accompanying “Management’s Report on Internal Control Over Financial Reporting” that Diversa Corporation maintained effective internal control over financial reporting as of December 31, 2005, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Diversa Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that Diversa Corporation maintained effective internal control over financial reporting as of December 31, 2005, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, Diversa Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2005, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets as of December 31, 2005 and 2004, and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2005 of Diversa Corporation and our report dated March 10, 2006 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Diego, California
March 10, 2006
87
| ITEM 9B. | OTHER INFORMATION. |
Not applicable.
PART III
| ITEM 10. | DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT. |
The information required by this item with respect to executive officers and directors is incorporated by reference from the information under the captions of “Election of Directors,” “Executive Officers,” and “Security Ownership of Certain Beneficial Owners and Management—Section 16(a) Beneficial Ownership Reporting Compliance” contained in the proxy statement to be filed with the SEC pursuant to Regulation 14A within 120 days following the fiscal year ended December 31, 2005 (“proxy statement”) in connection with our 2006 annual meeting of stockholders. The text of the Company’s Code of Business Conduct and Ethics is posted on the Company’s Internet website and may be accessed at the following Internet address: http://www.diversa.com/corpinfo/corpgove/codecond.asp.
There have been no material changes to the procedures under which security holders may recommend nominees to the Company’s Board of Directors.
| ITEM 11. | EXECUTIVE COMPENSATION. |
The information required by this item is incorporated by reference to the information under the captions “Election of Directors—Compensation of Directors,” “Executive Compensation” (through and including the information under the caption “Executive Compensation—Employment Agreements”) and “Report of the Compensation Committee of the Board of Directors on Executive Compensation—Compensation Committee Interlocks and Insider Participation” contained in the proxy statement for our 2006 annual meeting of stockholders.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information required by this item is incorporated by reference to the information under the caption “Security Ownership of Certain Beneficial Owners and Management” contained in the proxy statement for our 2006 annual meeting of stockholders.
The equity compensation plan information required by this item is incorporated by reference to the information under the caption “Equity Compensation Plan Information” contained in the proxy statement for our 2006 annual meeting of stockholders.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS. |
The information required by this item is incorporated by reference to the information under the caption “Certain Transactions” contained in the proxy statement for our 2006 annual meeting of stockholders.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES. |
The information required by this item is incorporated by reference to the information under the captions entitled “Report of the Audit Committee of the Board of Directors” and “Independent Auditors’ Fees” contained in the proxy statement for our 2006 annual meeting of stockholders.
88
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
(a)(1) Index to Consolidated Financial Statements
(a)(2) Financial Statement Schedules: All schedules have been omitted because they are not applicable or required, or the information required to be set forth therein is included in the Consolidated Financial Statements or notes thereto included in Item 8 (“Financial Statements and Supplementary Data”).
(a)(3) Index to Exhibits—See (b) below.
(b) Exhibits
| | |
Exhibit
Number
| | Description of Exhibit
|
| 2.1 | | Transaction Agreement dated as of December 3, 2002 among Syngenta Participations AG, Torrey Mesa Research Institute and the Company.(11) |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation.(1) |
| |
| 3.2 | | Amended and Restated Bylaws.(1) |
| |
| 4.1 | | Form of Common Stock Certificate of the Company.(2) |
| |
| 4.2 | | Rights Agreement by and between the Company and American Stock Transfer and Trust Company, as Rights Agent, dated as of December 13, 2000 (including the Form of Certificate of Designation of Series A Junior Participating Preferred Stock attached thereto as Exhibit A, the Form of Right Certificate attached thereto as Exhibit B, and the Summary of Rights to Purchase Preferred Shares attached thereto as Exhibit C).(3) |
| |
| 4.3 | | Amendment to Rights Agreement by and between the Company and American Stock Transfer and Trust Company, as Rights Agent, dated as of December 2, 2002.(12) |
| |
| 4.4 | | The Company’s Certificate of Designation of Series A Junior Participating Preferred Stock.(3) |
| |
| 4.5 | | Warrant issued by the Company to Syngenta Participations AG.(11) |
| |
| 4.6 | | Registration Rights Agreement dated as of December 3, 2002 among Syngenta Participations AG, Torrey Mesa Research Institute, Syngenta Seeds AG and the Company.(11) |
| |
| 4.7† | | Registration Rights Agreement dated as of July 18, 2003 by and between Glaxo Group Limited and Diversa Corporation.(13) |
| |
| 10.1 | | Form of Indemnity Agreement entered into between the Company and its directors and executive officers.(4) |
| |
| 10.2* | | 1994 Employee Incentive and Non-Qualified Stock Option Plan, as amended.(4) |
89
| | |
Exhibit
Number
| | Description of Exhibit
|
| |
| 10.3* | | Form of Stock Option Agreement under the 1994 Employee Incentive and Non-Qualified Stock Option Plan.(4) |
| |
| 10.4* | | 1997 Equity Incentive Plan.(10) |
| |
| 10.5* | | Form of Stock Option Grant Notice and Stock Option Agreement under the 1997 Equity Incentive Plan.(4) |
| |
| 10.6* | | 1999 Non-Employee Directors’ Stock Option Plan.(4) |
| |
| 10.7* | | Form of Stock Option Grant Notice and Related Stock Option Agreement under the 1999 Non-Employee Directors’ Stock Option Plan.(4) |
| |
| 10.8* | | 1999 Employee Stock Purchase Plan.(4) |
| |
| 10.9† | | Amended and Restated Stockholders’ Agreement by and among the Company and the Stockholders identified therein, dated January 25, 1999.(4) |
| |
| 10.10 | | Form of Warrant Agreement to purchase Series A Preferred Stock (with schedule of holders attached).(4) |
| |
| 10.11 | | Form of Warrant Agreement to purchase Common Stock (with schedule of holders attached).(4) |
| |
| 10.12 | | Form of Warrant Agreement to purchase Common Stock (with schedule of holders attached).(4) |
| |
| 10.13 | | Multi-Tenant Office R&D Building Lease by and between the Company and Sycamore/San Diego Investors, dated September 24, 1996.(4) |
| |
| 10.14 | | Master Lease Agreement by and between the Transamerica Business Credit Corporation and the Company, dated April 4, 1997.(4) |
| |
| 10.15† | | License Agreement by and between the Company and The Dow Chemical Company, dated July 20, 1997 and July 22, 1997.(4) |
| |
| 10.16† | | Collaborative Research Agreement by and between the Company and The Dow Chemical Company, dated July 20, 1999 and July 22, 1999.(4) |
| |
| 10.17† | | License Agreement by and between the Company and Finnfeeds International Limited, dated December 1, 1998.(4) |
| |
| 10.18† | | Collaboration Agreement by and between the Company and Novartis Agribusiness Biotechnology Research, Inc., dated January 25, 1999, as amended.(4) |
| |
| 10.19† | | Stock Purchase Agreement by and between the Company and Novartis Agribusiness Biotechnology Research, Inc., dated January 25, 1999.(4) |
| |
| 10.20† | | Collaboration Agreement by and between the Company and Rhone-Poulenc Animal Nutrition S.A., dated June 28, 1999.(4) |
| |
| 10.21† | | License Agreement by and between the Company and Invitrogen Corporation, dated March 29, 1999.(4) |
| |
| 10.22† | | License Agreement by and between the Company and Mycogen Corporation, dated December 1997, as amended on March 6, 1998 and December 19, 1997.(4) |
| |
| 10.23† | | Patent Cross-License Agreement by and between the Company and Terragen Discovery Inc., dated November 18, 1999.(4) |
| |
| 10.24† | | Joint Venture Agreement by and between the Company and Novartis Seeds AG, dated December 1, 1999.(4) |
90
| | |
Exhibit
Number
| | Description of Exhibit
|
| 10.25† | | Research Lease by and between the Company and One Cell Systems, Inc., dated February 16, 1999.(4) |
| |
| 10.26† | | Research and Development Agreement by and between the Company and Novartis Enzymes, Inc., dated December 1, 1999.(4) |
| |
| 10.27* | | Employment Offer Letter to Patrick Simms, dated February 3, 1997.(4) |
| |
| 10.28* | | Employment Offer Letter to Jay Short, dated August 30, 1994.(4) |
| |
| 10.29* | | Employment Offer Letter to Karin Eastham, dated April 2, 1999.(4) |
| |
| 10.30* | | Employment Offer Letter to William H. Baum, dated July 31, 1997.(4) |
| |
| 10.31* | | Separation Agreement by and between the Company and Terrance J. Bruggeman, effective as of April 12, 1999.(4) |
| |
| 10.32* | | Separation Agreement by and between the Company and Kathleen H. Van Sleen, effective as of May 10, 1999.(4) |
| |
| 10.33* | | Letter Agreement by and between the Company and Jay M. Short, Ph.D., dated June 25, 1998.(4) |
| |
| 10.34 | | Lease Agreement, dated February 11, 2000, by and between the Company and KR—Gateway Partners, LLC.(1) |
| |
| 10.35 | | Lease Agreement, dated February 11, 2000, by and between the Company and KR—Gateway Partners, LLC.(1) |
| |
| 10.36† | | Limited Liability Company Agreement of New Venture LLC, dated June 29, 2000(5) |
| |
| 10.37† | | Industrial Enzymes Research Agreement by and between New Venture LLC and the Company, dated June 29, 2000.(5) |
| |
| 10.38† | | Industrial Enzyme License Agreement by and between New Venture LLC and the Company, dated June 29, 2000.(5) |
| |
| 10.39† | | Addendum to the “Collaboration Agreement” between Novartis Agribusiness Biotechnology Research, Inc. and the Company, dated September 1, 2000.(6) |
| |
| 10.40† | | Master Collaboration Agreement by and between the Company and The Dow Chemical Company, effective as of September 30, 2000.(7) |
| |
| 10.41† | | Project Agreement by and between The Dow Chemical Company and the Company, effective as of September 30, 2000.(7) |
| |
| 10.42† | | Collaboration Agreement by and between the Company, Glaxo Research and Development Limited, and Glaxo Group Limited, made as of December 8, 2000.(7) |
| |
| 10.43† | | Drug Discovery, Development, and License Agreement between the Company and IntraBiotics Pharmaceuticals, Inc. dated January 6, 2001.(8) |
| |
| 10.44† | | Release Agreement between the Company and IntraBiotics Pharmaceuticals, Inc. dated July 27, 2001.(9) |
| |
| 10.45† | | Amended and Restated Research Collaboration Agreement dated as of January 3, 2003 between the Company and Syngenta Participations AG. (11) |
| |
| 10.46 | | Intellectual Property Rights License dated as of December 3, 2002 between the Company and Syngenta Participations AG. (11) |
| |
| 10.47† | | Asset Sale Agreement made as of July 18, 2003 by and between Glaxo Wellcome, S.A. and the Company.(13) |
91
| | |
Exhibit
Number
| | Description of Exhibit
|
| |
| 10.48† | | License Agreement dated December 29, 2003 by and between Xoma Ireland Limited and the Company. (14) |
| |
| 10.49† | | Transition Agreement dated May 28, 2004 by and between the Company, Zymetrics, Inc., Syngenta Seeds AG, and Syngenta Participations AG. (15) |
| |
| 10.50† | | Amendment to Amended and Restated Research Collaboration Agreement dated May 28, 2004 between the Company and Syngenta Participations AG. (15) |
| |
| 10.51* | | Letter Agreement by and between the Company and Karin Eastham dated April 6, 2004 (16) |
| |
| 10.52* | | Employment Offer Letter, dated October 11, 2004, between the Company and Gary W. Noon. (16) |
| |
| 10.53* | | Employment Offer Letter, dated November 11, 2004, between the Company and Anthony E. Altig. (17) |
| |
| 10.54* | | Employment Offer Letter, dated March 31, 2005, between the Company and Jeffrey G. Black. (18) |
| |
| 10.55 | | Loan and Security Agreement by and between the Company and Comerica Bank dated September 30, 2005. (19) |
| |
| 10.56* | | Separation Agreement by and between the Company and Jay M. Short, Ph.D. dated October 26, 2005. (20) |
| |
| 10.57+† | | Distribution Agreement dated January 1, 2005 by and between Valley Research, inc. and the Company. |
| |
| 10.58+† | | Amendment to Distribution Agreement by and between Valley Research, inc. and the Company, effective as of August 1, 2005. |
| |
| 23.1+ | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1 | | Power of Attorney. Reference is made to page 94. |
| |
| 31.1+ | | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities and Exchange Act of 1934, as amended. |
| |
| 31.2+ | | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities and Exchange Act of 1934, as amended. |
| |
| 32+ | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| * | Indicates management or compensatory plan or arrangement. |
| (1) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended March 31, 2000, filed with the Securities and Exchange Commission on May 12, 2000, and incorporated herein by reference. |
| (2) | Filed as an exhibit to the Company’s Registration Statement on Form S-1 (No. 333-92853) originally filed with the Securities and Exchange Commission on December 21, 1999, as amended, and incorporated herein by reference. |
| (3) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 15, 2000, and incorporated herein by reference. |
| (4) | Filed as an exhibit to the Company’s Registration Statement on Form S-1 (No. 333-92853) originally filed with the Securities and Exchange Commission on December 16, 1999, as amended, and incorporated herein by reference. |
| (5) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended June 30, 2000, filed with the Securities and Exchange Commission on August 14, 2000, and incorporated herein by reference. |
92
| (6) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended September 30, 2000, filed with the Securities and Exchange Commission on November 14, 2000, and incorporated herein by reference. |
| (7) | Filed as an exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2000, filed with the Securities and Exchange Commission on March 30, 2001 and incorporated herein by reference. |
| (8) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended March 31, 2001 filed with the Securities and Exchange Commission on May 15, 2001 and incorporated herein by reference. |
| (9) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended June 30, 2001, filed with the Securities and Exchange Commission on August 14, 2001, and incorporated herein by reference. |
| (10) | Filed as part of the Company’s Definitive Proxy Statement on Schedule 14A (File No. 000-29173) filed on April 6, 2001, and incorporated herein by reference. |
| (11) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on January 6, 2003, and incorporated herein by reference. |
| (12) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 4, 2002, and incorporated herein by reference. |
| (13) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended June 30, 2003, filed with the Securities and Exchange Commission on August 14, 2003, and incorporated herein by reference. |
| (14) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on March 12, 2004, and incorporated herein by reference. |
| (15) | Filed as an exhibit to the Company’s Quarterly Report Form 10-Q for the quarter ended June 30, 2004, filed with the Securities and Exchange Commission on August 6, 2004, and incorporated herein by reference. |
| (16) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on October 22, 2004 and incorporated herein by reference. |
| (17) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 18, 2004 and incorporated herein by reference. |
| (18) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on April 26, 2005 and incorporated herein by reference. |
| (19) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on October 6, 2005 and incorporated herein by reference. |
| (20) | Filed as an exhibit to the Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission on October 28, 2005 and incorporated herein by reference. |
| † | Confidential treatment has been granted with respect to portions of this exhibit. A complete copy of the agreement, including the redacted terms, has been separately filed with the Securities and Exchange Commission. |
93
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
DIVERSA CORPORATION |
| |
By: | | /S/ ANTHONY E. ALTIG |
| | | Anthony E. Altig |
| | | Senior Vice President, Finance and Chief Financial Officer |
Date: March 15, 2006
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Anthony E. Altig and Edward T. Shonsey, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them or their or his substitute or substituted, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signatures
| | Title
| | Date
|
| | |
/S/ EDWARD T. SHONSEY
Edward T. Shonsey | | Chief Executive Officer, (Principal Executive Officer) | | March 14, 2006 |
| | |
/S/ ANTHONY E. ALTIG
Anthony E. Altig | | Senior Vice President, Finance and Chief Financial Officer (Principal Financial Officer) | | March 14, 2006 |
| | |
/S/ JEFFREY G. BLACK
Jeffrey G. Black | | Chief Accounting Officer (Principal Accounting Officer) | | March 14, 2006 |
| | |
/S/ JAMES H. CAVANAUGH, PH.D.
James H. Cavanaugh, Ph.D. | | Director | | March 14, 2006 |
| | |
/S/ FERNAND J. KAUFMANN, PH.D.
Fernand J. Kaufmann, Ph.D. | | Director | | March 14, 2006 |
| | |
/S/ PETER JOHNSON
Peter Johnson | | Director | | March 14, 2006 |
94
| | | | |
Signatures
| | Title
| | Date
|
| | |
/S/ MARK LESCHLY
Mark Leschly | | Director | | March 14, 2006 |
| | |
/S/ MELVIN I. SIMON, PH.D.
Melvin I. Simon, Ph.D. | | Director | | March 14, 2006 |
| | |
/S/ CHERYL WENZINGER
Cheryl Wenzinger | | Director | | March 14, 2006 |
95



